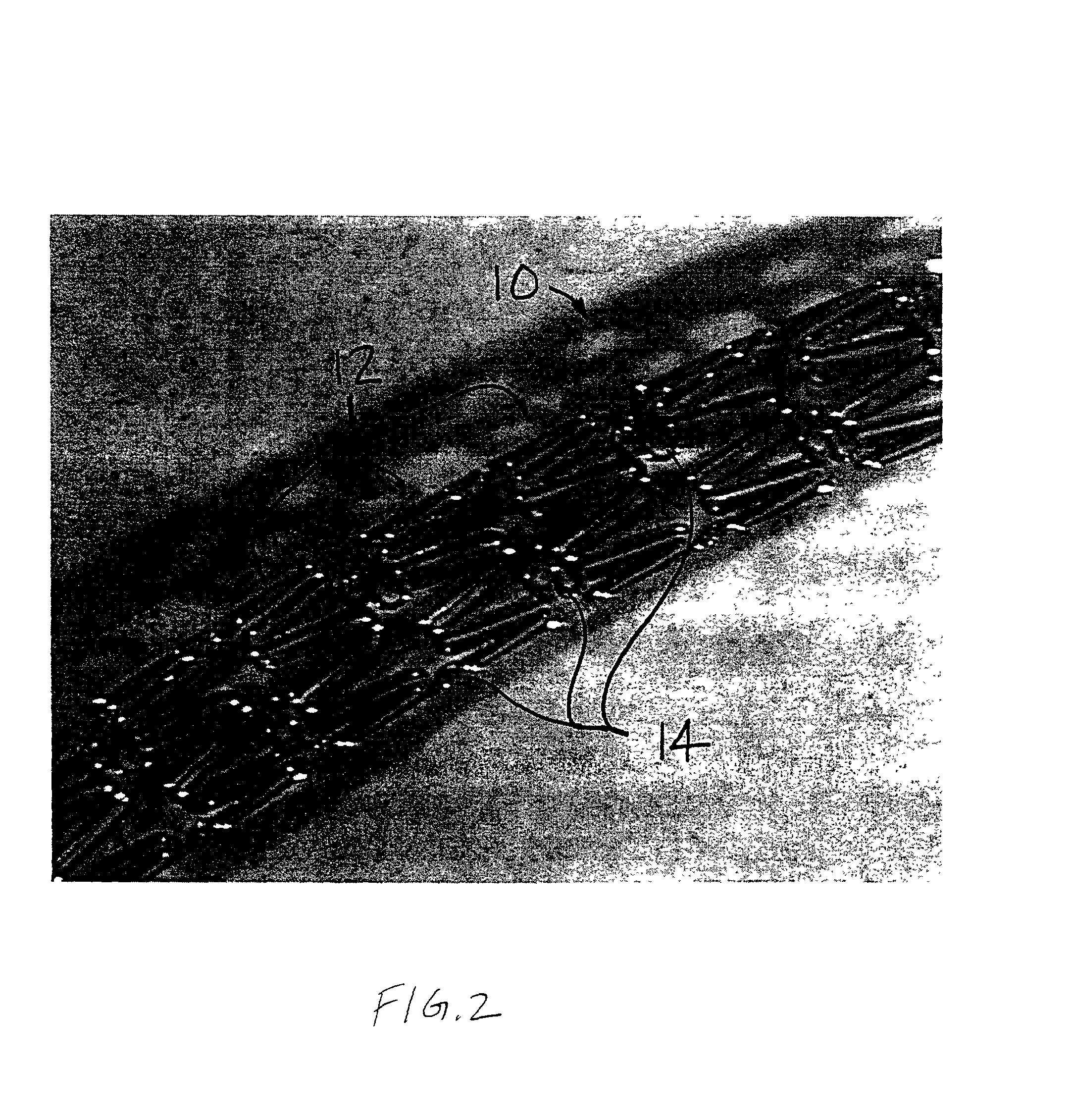
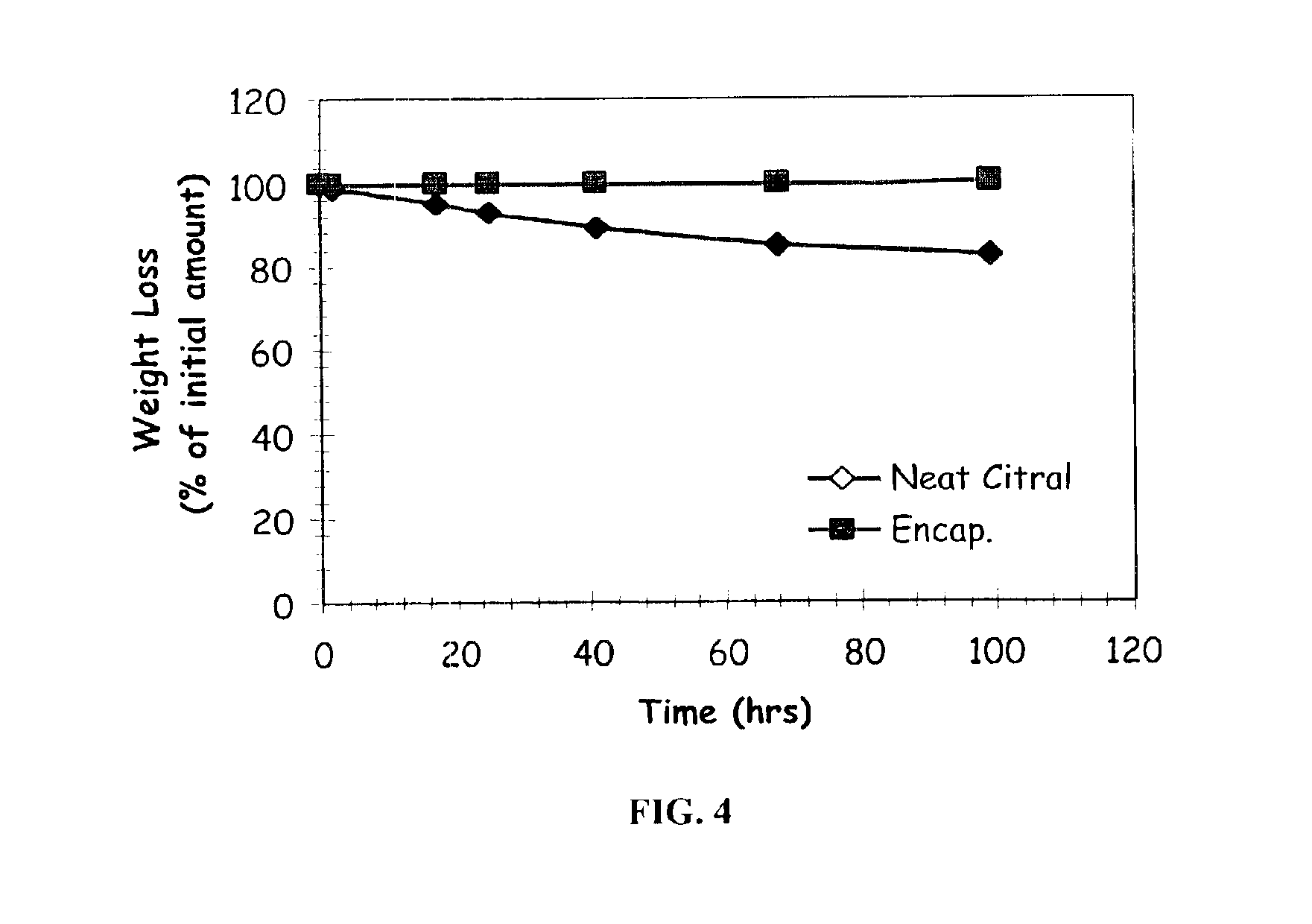
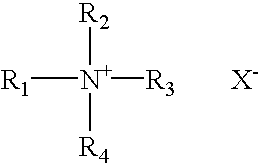
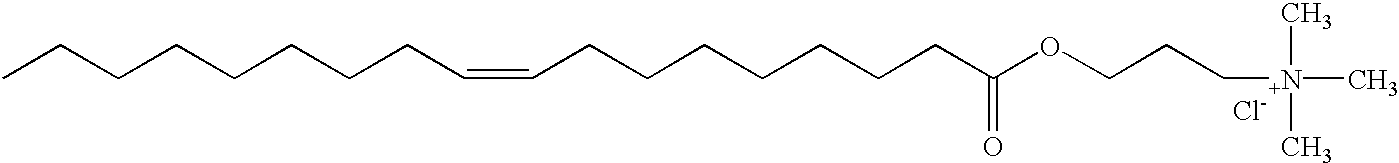
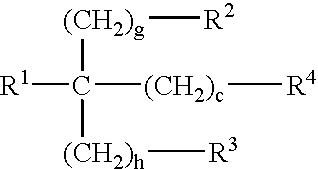Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
444results about How to "Increase release rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
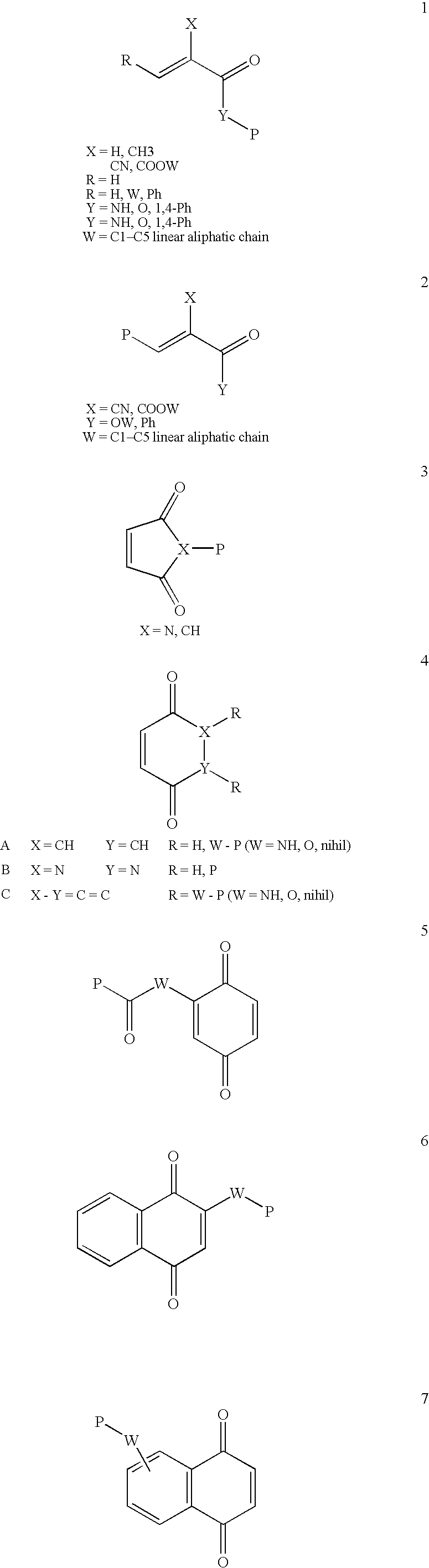
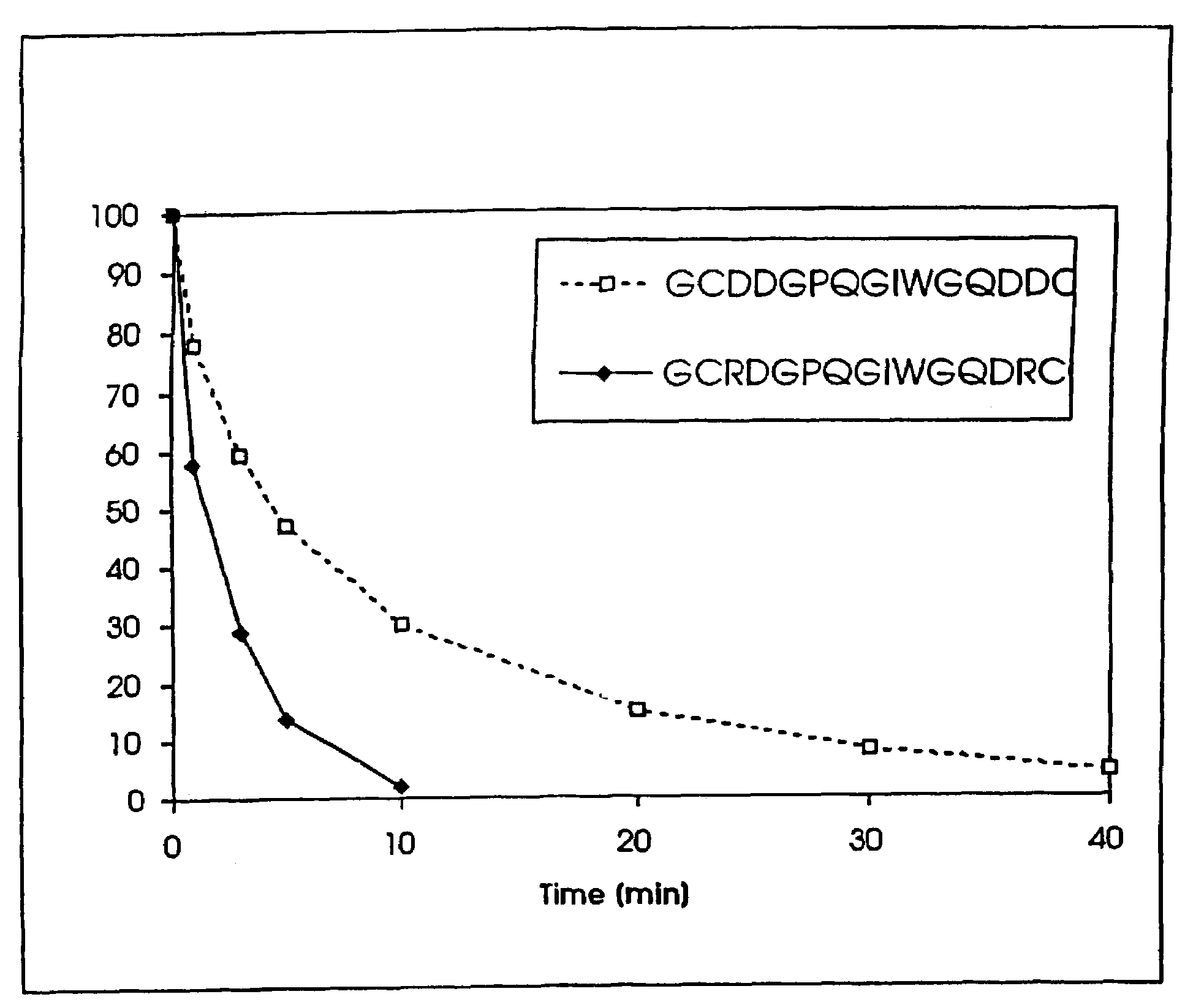
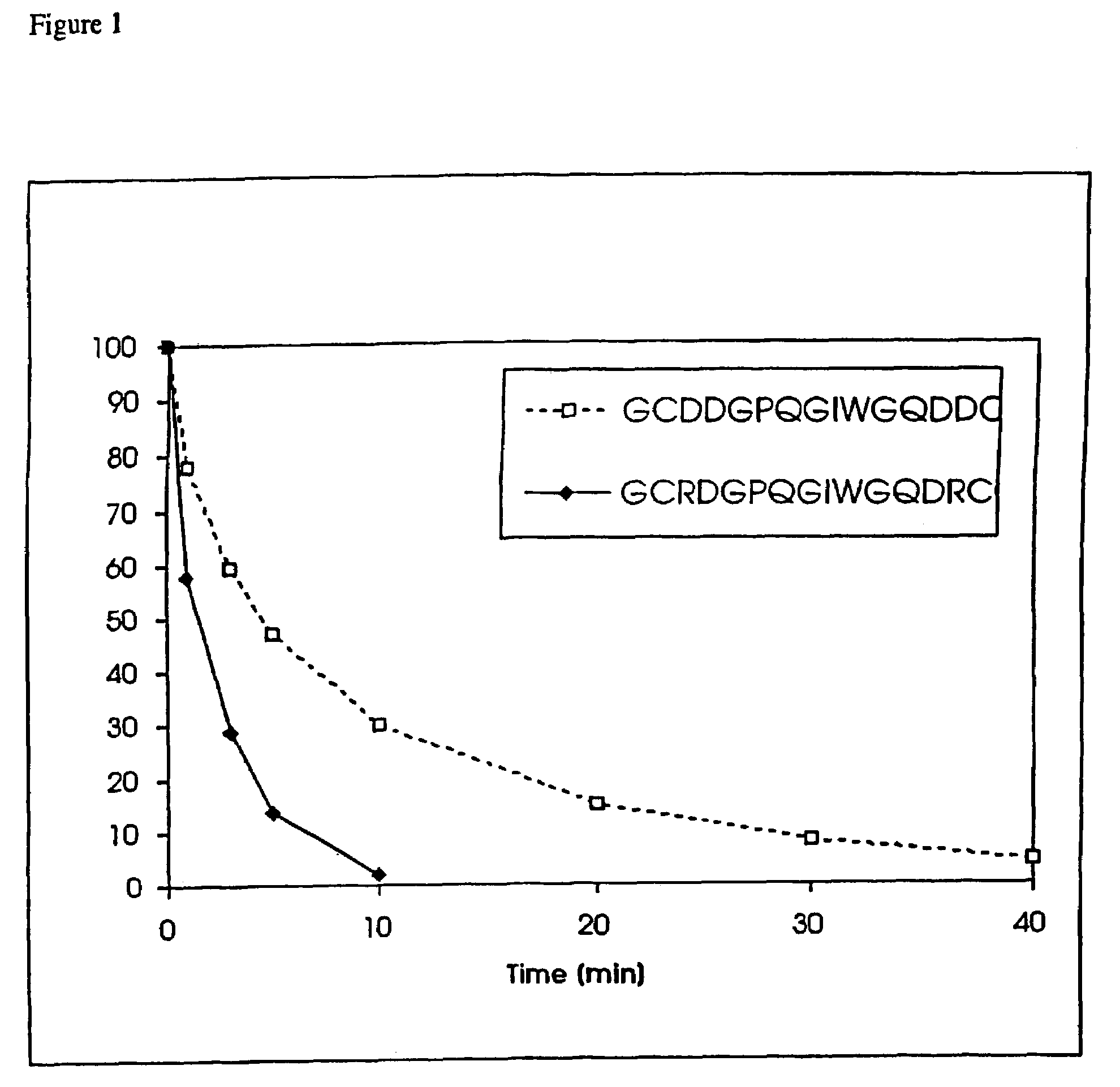
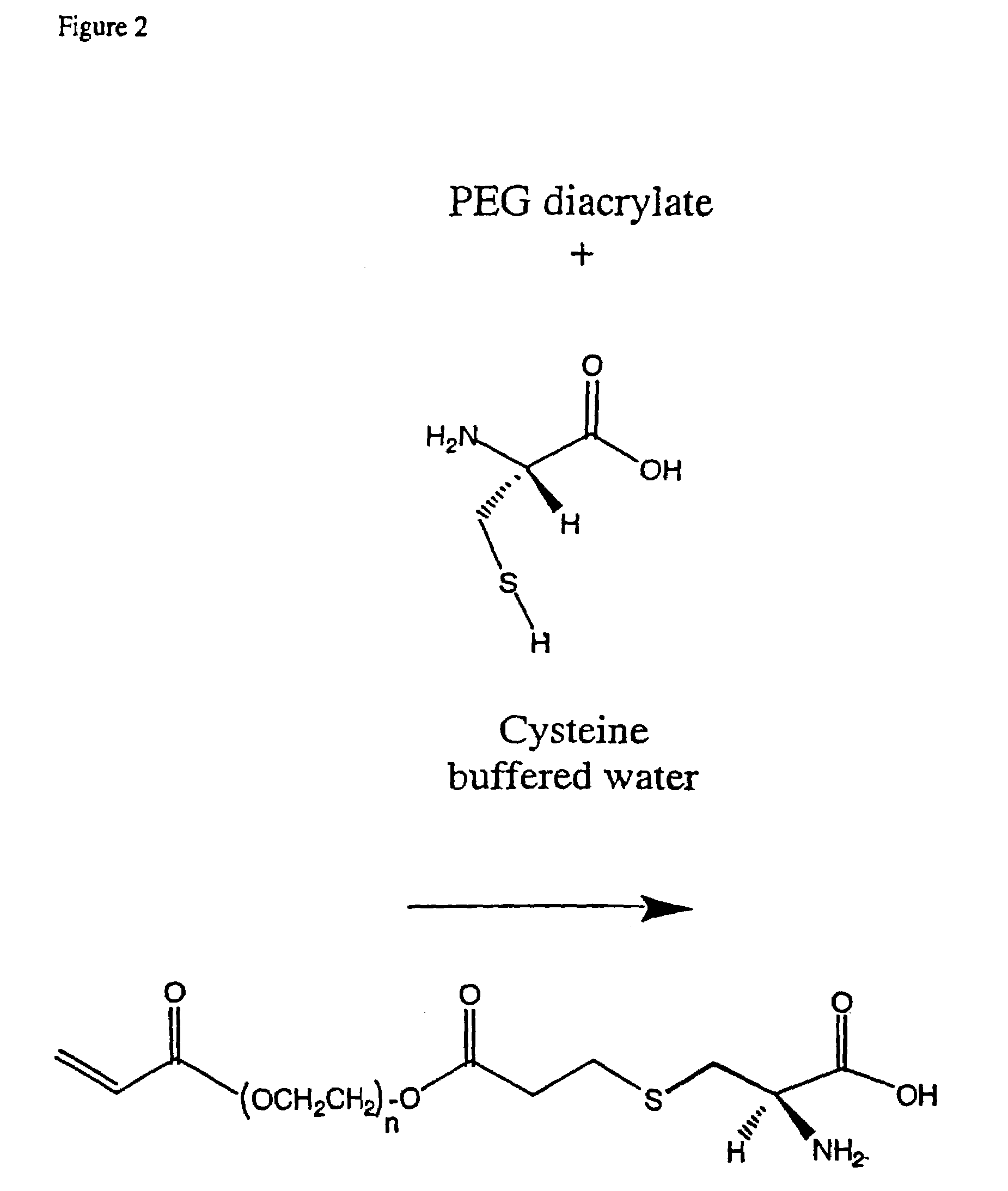
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS6958212B1Reducing and delaying onsetGood water solubilitySugar derivativesPeptide/protein ingredientsBiological materialsPolymer
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:ETH ZZURICH +1
Percutaneous delivery system
InactiveUS6211250B1Good substantivityAppropriate thicknessAntibacterial agentsAntimycoticsActive agentPharmacology
The invention relates to a substantially homogenous liquid composition capable of percutaneous delivery of one or more physiologically active agents, the composition including a rate modulating polymer, a volatile solvent and at least one physiologically active agent, said rate modulating polymer being selected to enable modulation of the rate of delivery of said physiologically active agent. Methods of percutaneous delivery of active agents and of prophylactic or therapeutic antimicrobial, antifungal or antiviral treatment using the compositions of the invention are also described.
Owner:STIEFEL RESEARCH AUSTRALIA PTY LTD
Apparatus and methods for controlled substance delivery from implanted prostheses
InactiveUS20020082685A1Improve drug delivery efficiencyReduce lossesStentsSurgeryPercent Diameter StenosisControl substances
The present invention provides improved devices and methods for inhibiting restenosis and hyperplasia after intravascular intervention. In particular, the present invention provides luminal prostheses which allow for programmed and controlled substance delivery with increased efficacy to selected locations within a patient's vasculature to inhibit restenosis. The luminal delivery prosthesis comprises a scaffold which is implantable within a body lumen and means on the scaffold for releasing a substance from the scaffold. The substance is released over a predetermined time pattern comprising an initial phase wherein the substance delivery rate is below a threshold level and a subsequent phase wherein the substance delivery rate is above a threshold level.
Owner:ALTAI MEDICAL TECH
Multi component controlled release system for oral care, food products, nutraceutical, and beverages
InactiveUS6887493B2Improve bioavailabilityImprove stabilityCosmetic preparationsPowder deliveryActive agentMicrosphere
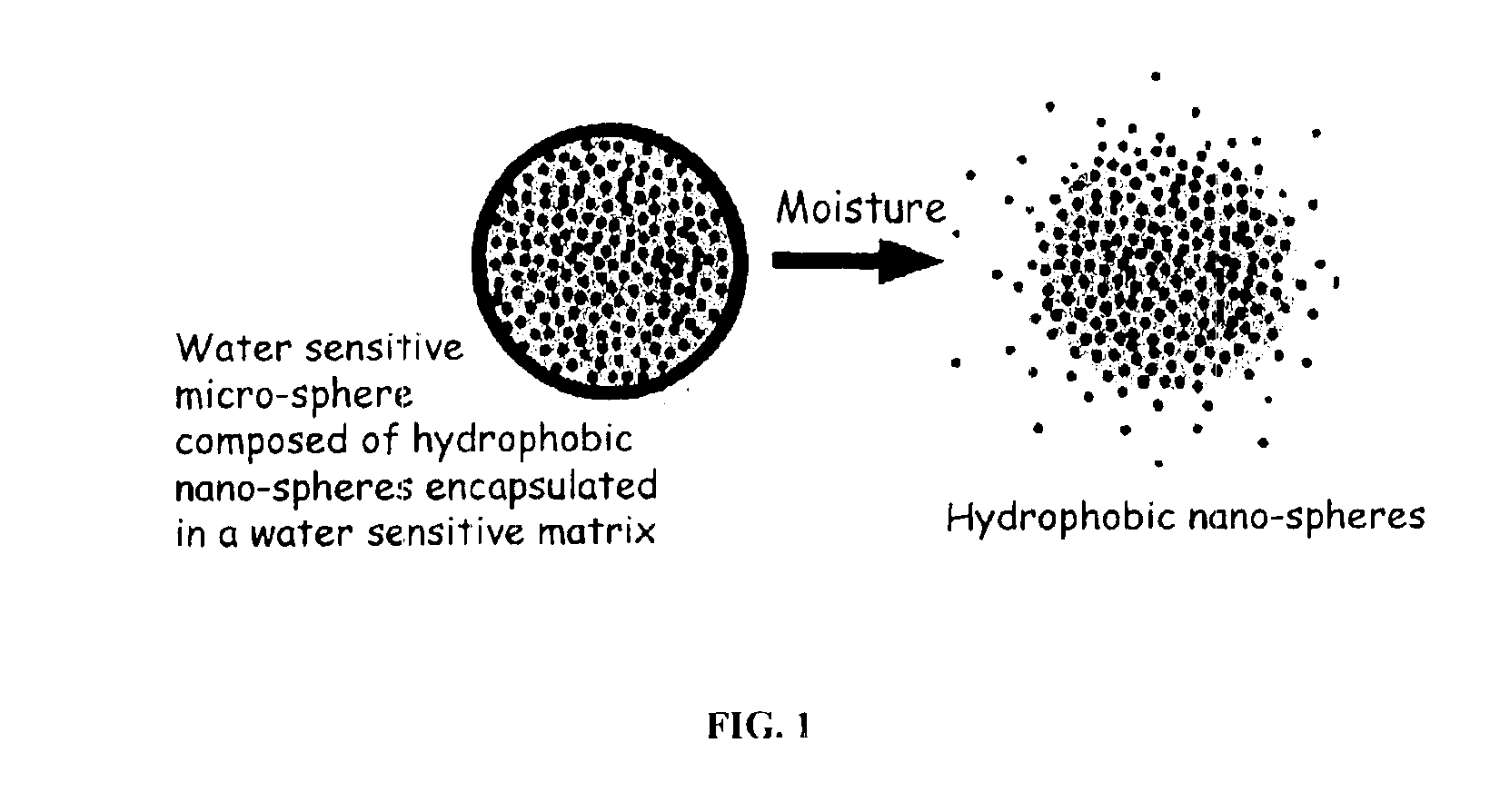
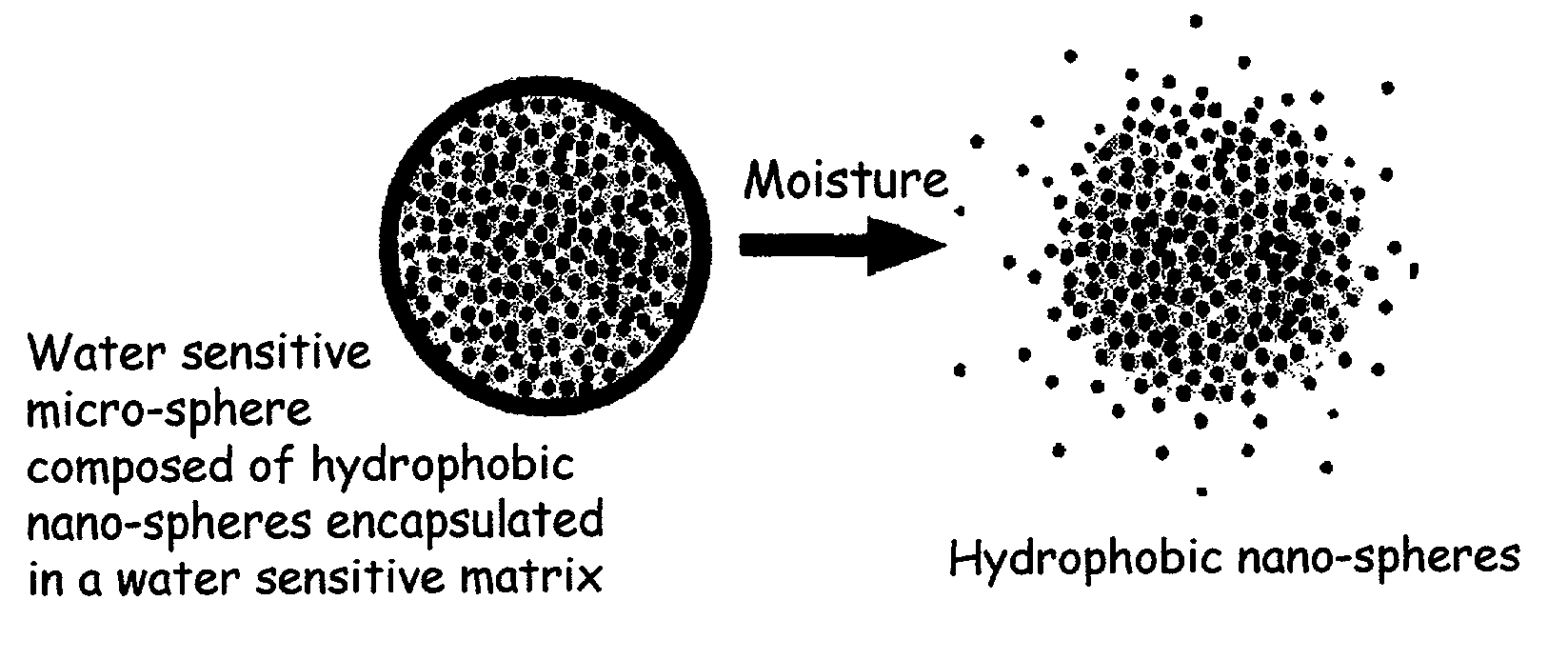
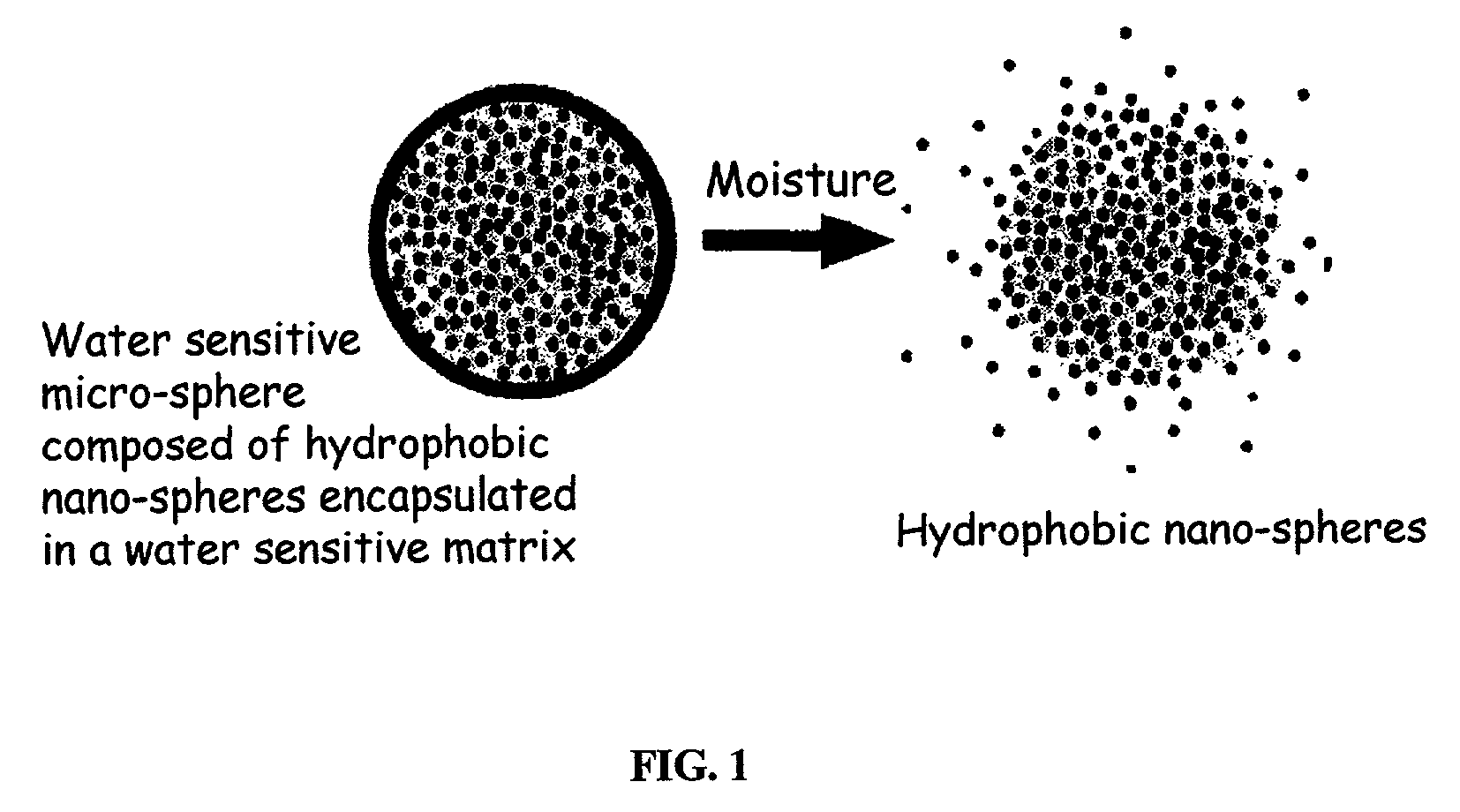
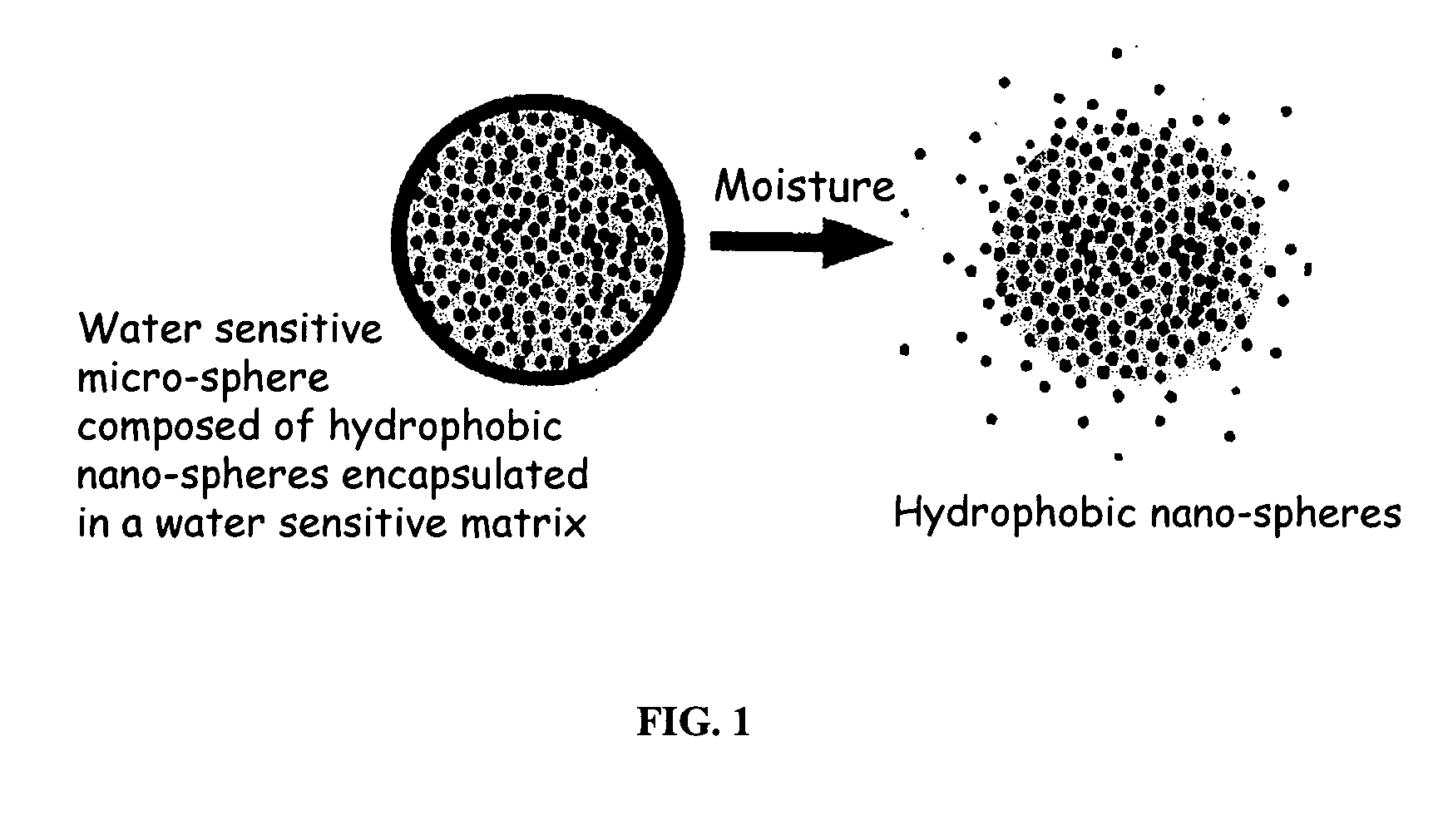
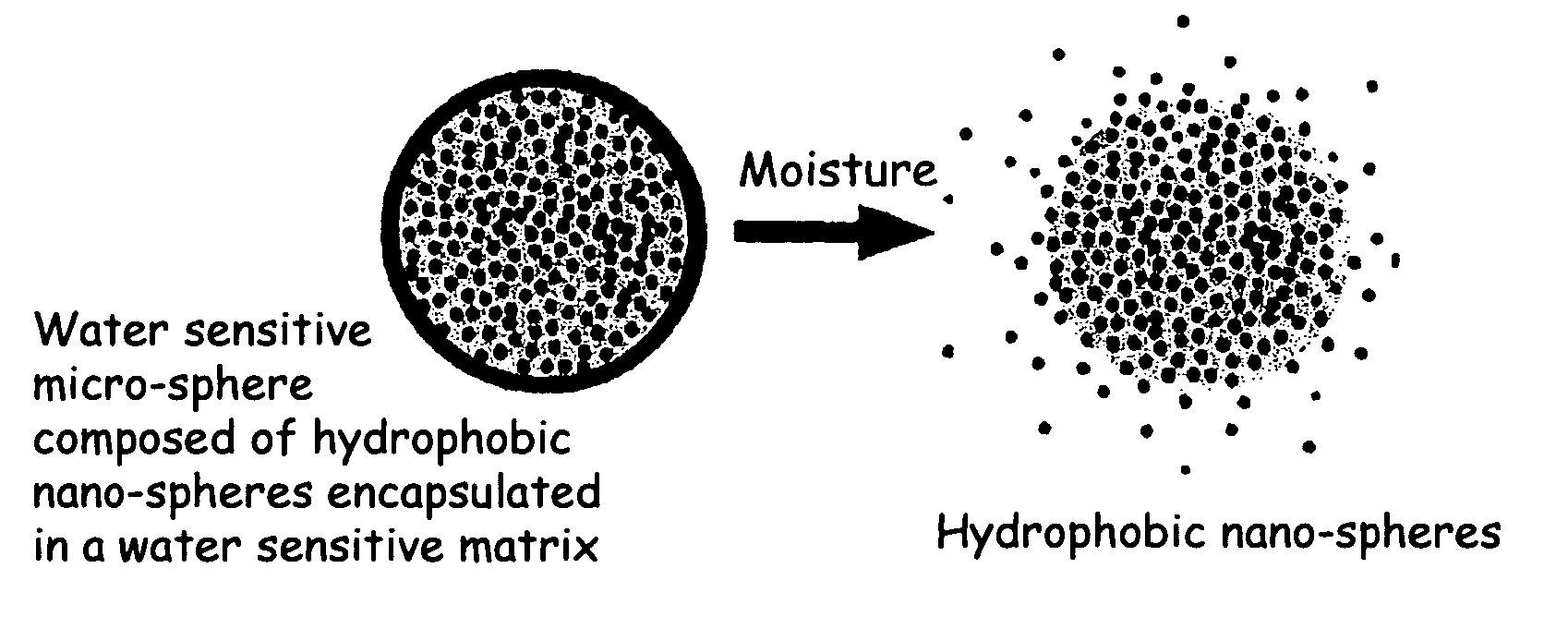
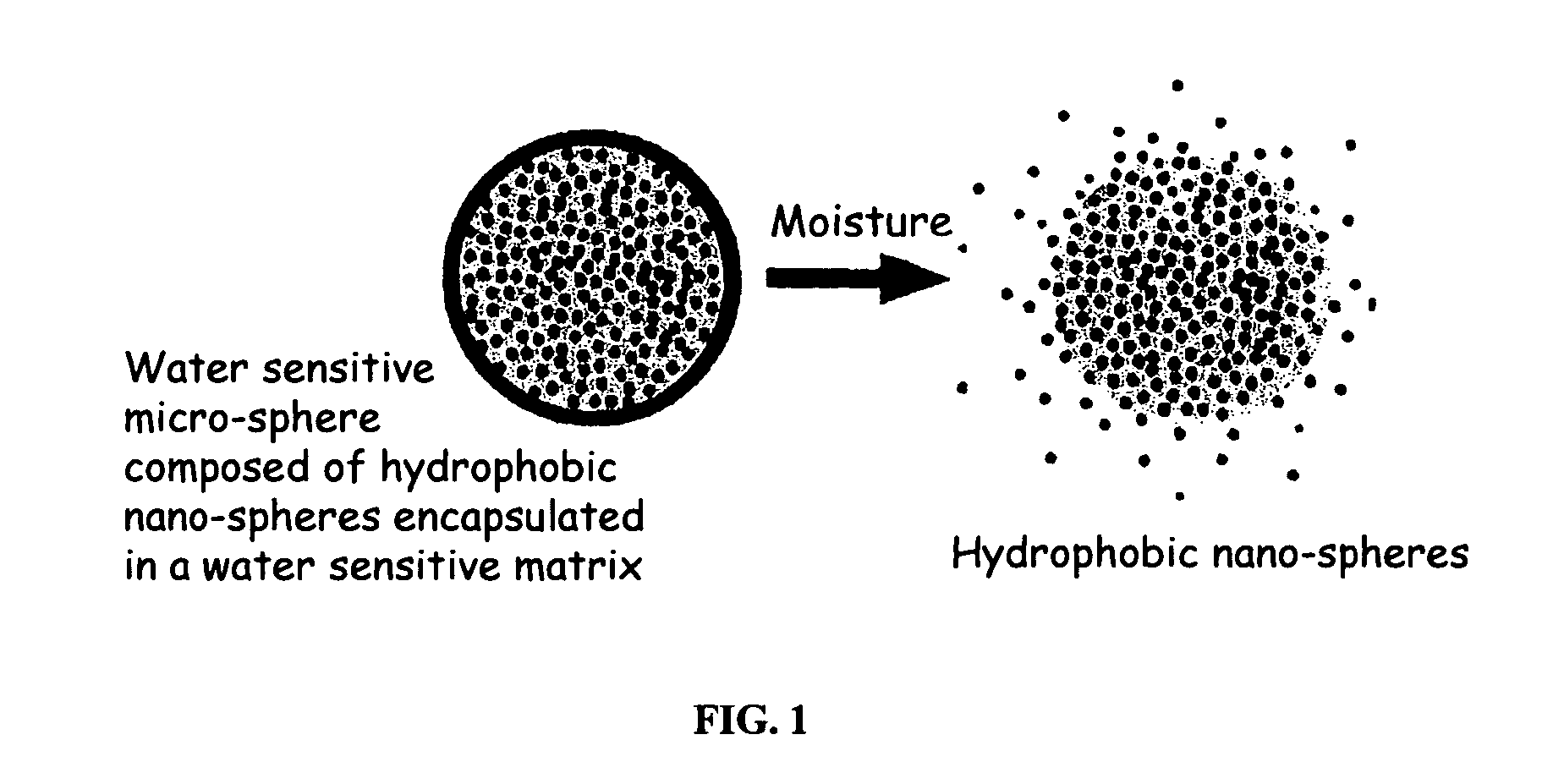
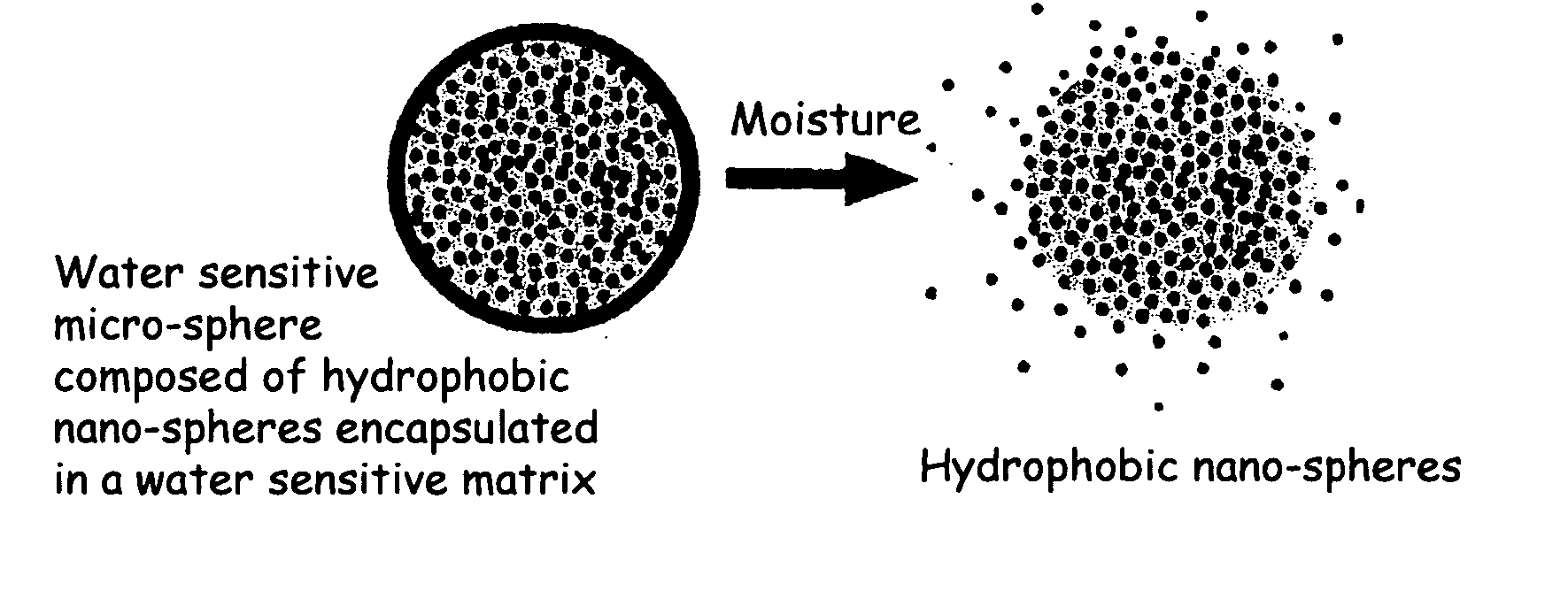
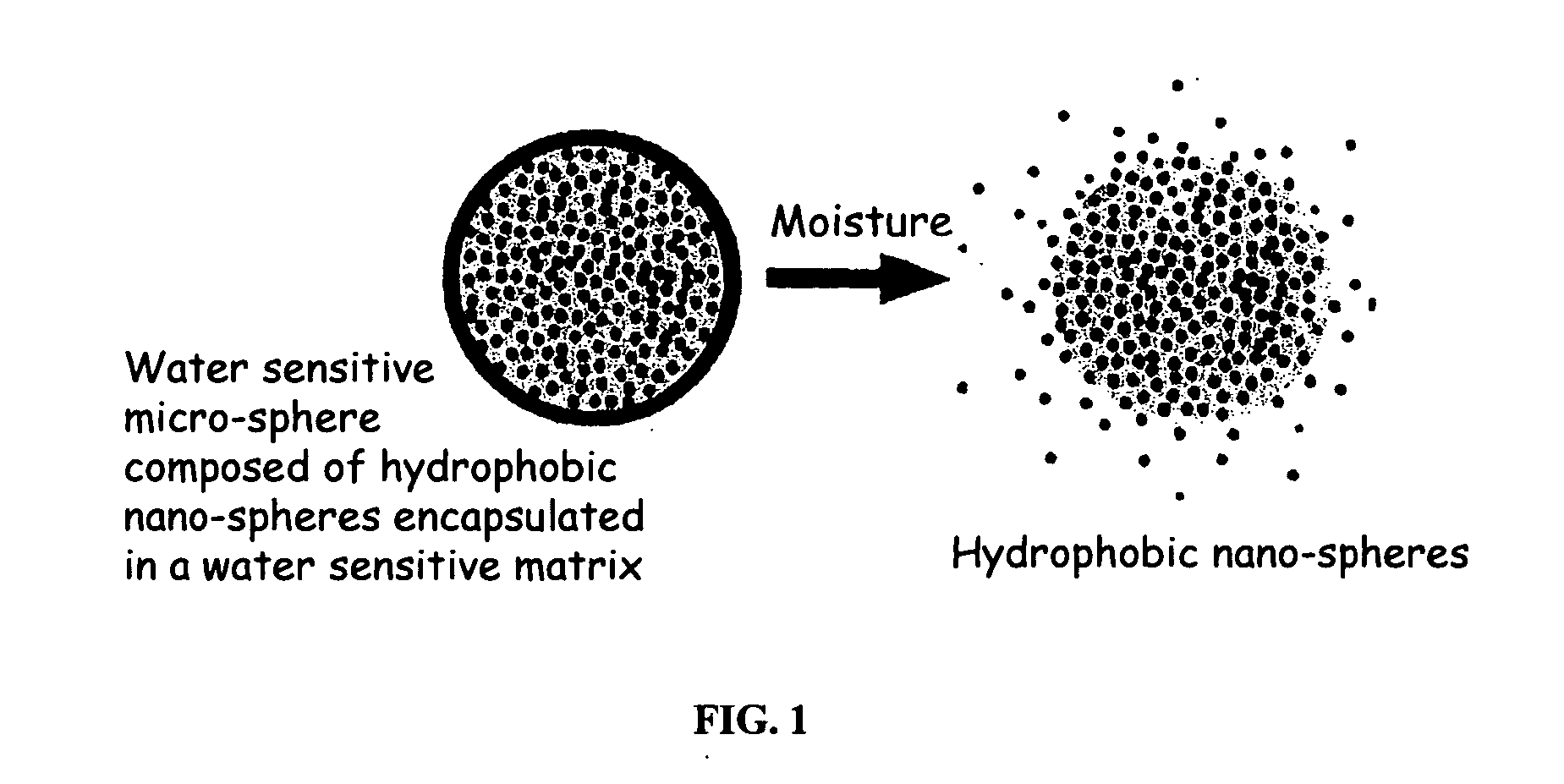
The present invention relates to an improved controlled release system that can encapsulate different flavors, sensory markers, and active ingredients, or combinations of flavors, sensory markers and various active ingredients and release multiple active ingredients in a consecutive manner, one after the other. The controlled delivery system of the present invention is substantially free-flowing powder formed of solid hydrophobic nanospheres that are encapsulated in a moisture sensitive microspheres. The flavors, and active ingredients encapsulated in the hydrophobic nanospheres, in the water sensitive microsphere, or in both the nano and the microsphere. The flavors and active ingredients encapsulated in the nanospheres can be the same or different from those encapsulated in the microspheres. The encapsulation of different flavors or active agents in the various components of the system, such as nanospheres and microspheres, provides flavor transition (change in flavor character) during the use of the products. The controlled release system of the present invention enhances the stability and bioavailability of wide range of flavors, sensory markers, and other active ingredients, prolong their residence time in the oral cavity, control their release characteristics, and prolong the sensation of flavors and other sensory markers in the mouth to provide long lasting organoleptic perception or long lasting mouthfeel. The invention further relates oral care, food products, and beverages comprising the controlled release system of the present invention.
Owner:SHEFER ADI +1
Drug eluting implant
InactiveUS20070141106A1Increase release rateControl releaseSuture equipmentsStentsBiomedical engineeringMedical systems
The present invention provides a medical system for the administration of a pharmaceutical agent in vivo to a patient. The medical system includes a medical implant positionable in a body of a patient. A pharmaceutical agent in disposed on the medical implant and at least partially coated with a reactive coating. The reactive coating act to controls the release of the pharmaceutical agent. An energy unit is provided for transmitting an energy signal to the reactive coating, wherein the reactive coating reacts to the energy signal to increase the release rate of the pharmaceutical agent.
Owner:P TECH
Compositions and method for targeted controlled delivery of active ingredients and sensory markers onto hair, skin, and fabric
InactiveUS6979440B2Increase depositionHigh cationic charge densityCosmetic preparationsCationic surface-active compoundsActive agentBULK ACTIVE INGREDIENT
The present invention is a controlled delivery system that can be incorporated in hair, skin, and fabric care products such as shampoos, conditioners, hair styling products, lotions, creams, liquid laundry detergents, fabric softener, and other hair, skin, and fabric care products to effectively deliver a broad range of active agents and sensory markers onto the hair, skin, and fabric. The system also prolongs the release rate of the active agents or sensory markers over an extended period of time, or provides heat triggered release of the active agents and yields a high impact fragrance “burst” upon blow drying the hair, ironing the fabric, or other types of heat treatment. The controlled delivery system of the present invention is a nano-sphere, having an average sphere diameter of from about 0.01 microns to about 10 microns. The nano-sphere comprises hydrophobic materials, cationic conditioning agent or, cationic conditioning agent in conjunction with a cationic charge booster to assist in adhering the spheres onto hair, skin, and fabric. The invention further relates to a controlled delivery system where the release rate of the active ingredients is synchronized with that of a sensory marker to convey to the consumer the product performance.
Owner:SALVONA
Encapsulation of sensitive liquid components into a matrix to obtain discrete shelf-stable particles
InactiveUS7201923B1Improve hydrophobicityPrevent and delays penetration of waterPowder deliveryBiocideSolid particleHeat sensitive
A liquid encapsulant component which contains an active, sensitive encapsulant, such as a live microorganism or an enzyme dissolved or dispersed in a liquid plasticizer is admixed with a plasticizable matrix material. The matrix material is plasticizable by the liquid plasticizer and the encapsulation of the active encapsulant is accomplished at a low temperature and under low shear conditions. The active component is encapsulated and / or embedded in the plasticizable matrix component or material in a continuous process to produce discrete, solid particles. The liquid content of the liquid encapsulant component provides substantially all or completely all of the liquid plasticizer needed to plasticize the matrix component to obtain a formable, extrudable, cuttable, mixture or dough. Removal of liquid plasticizer prior to extrusion is not needed to adjust the viscosity of the mixture for formability. Release of an active component from the matrix may be delayed or controlled over time so that the active component is delivered when and where it is needed to perform its intended function. Controlled release, discrete, solid particles which contain an encapsulated and / or embedded component such as a heat sensitive or readily oxidizable pharmaceutically, biologically, or nutritionally active component are continuously produced without substantial destruction of the matrix material or encapsulant.
Owner:GENERAL MILLS INC
Multi component controlled release system for anhydrous cosmetic compositions
InactiveUS7115282B2Facilitated releaseProcess stepBiocideCosmetic preparationsActive agentProlonged release
The present invention relates to an improved controlled release system that can be incorporated into anhydrous cosmetic formulations and can encapsulate different types of fragrances, flavors, active ingredients, or combinations of fragrances, flavors, and various active ingredients. The controlled delivery system of the present invention is substantially free-flowing powder formed of solid hydrophobic nano-spheres that are encapsulated in a moisture sensitive micro-spheres. The fragrances, flavors, and active ingredients encapsulated in the nano-spheres can be the same or different from those encapsulated in the micro-sphere. The encapsulation of one or more fragrances, flavors or active agents in the various components of the system, such as nano-spheres and micro-spheres, provides odor or flavor transition (change in odor character or change in flavor character) during the use of the product, in response to moisture, such as wetting the lips, perspiration, and the like. The incorporation of the controlled release system of the present invention into anhydrous cosmetic formulations was found to provide moisture triggered release, as well as prolonged release of the fragrances, flavors, and active ingredients that are encapsulated in the solid hydrophobic nano-spheres over an extended period of time.
Owner:SALVONA
Compositions and methods for treatment of tumors and metastatic diseases
InactiveUS6406689B1Stimulate immune responseSimple and reliable to useBiocideSnake antigen ingredientsDiseaseActive immunization
Owner:FALKENBERG FR W
Osmotically driven active agent delivery device providing an ascending release profile
ActiveUS7241457B2Increase surface areaReduce effective thicknessPressure infusionOsmotic deliveryActive agentWater flow
In one aspect, the present invention is directed to an osmotic pump that automatically provides an ascending release rate of active agent as the osmotic pump functions in an environment of operation and may be designed for implantation within a desired animal or human subject. An osmotic pump according to the present invention includes a reservoir, a rate controlling membrane, an expandable osmotic composition, an active agent formulation and an exit orifice. Once administered to an environment of operation, water passes through the rate controlling membrane and into the osmotic composition, which causes the osmotic composition to expand and expel the active agent formulation through the exit orifice at a rate that is directly proportional to the rate at which water passes through the rate controlling membrane. An osmotic pump according to the present invention permits the flow of water through the rate controlling membrane to increase automatically without the need for manipulation of the osmotic pump after administration. As the flow of water through the rate controlling membrane increases, the rate at which active agent is delivered from the osmotic pump will also increase proportionally.
Owner:INTARCIA THERAPEUTICS INC
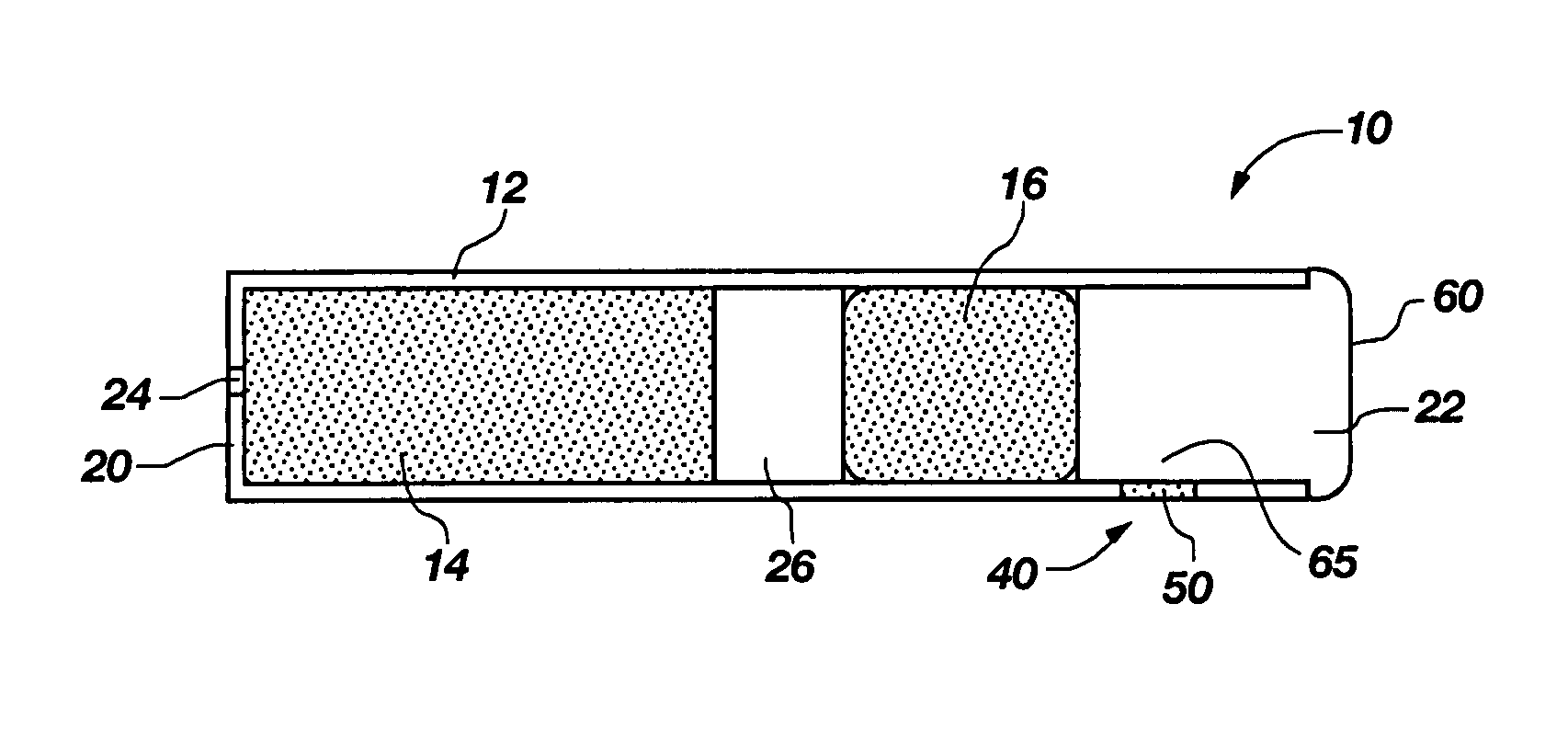
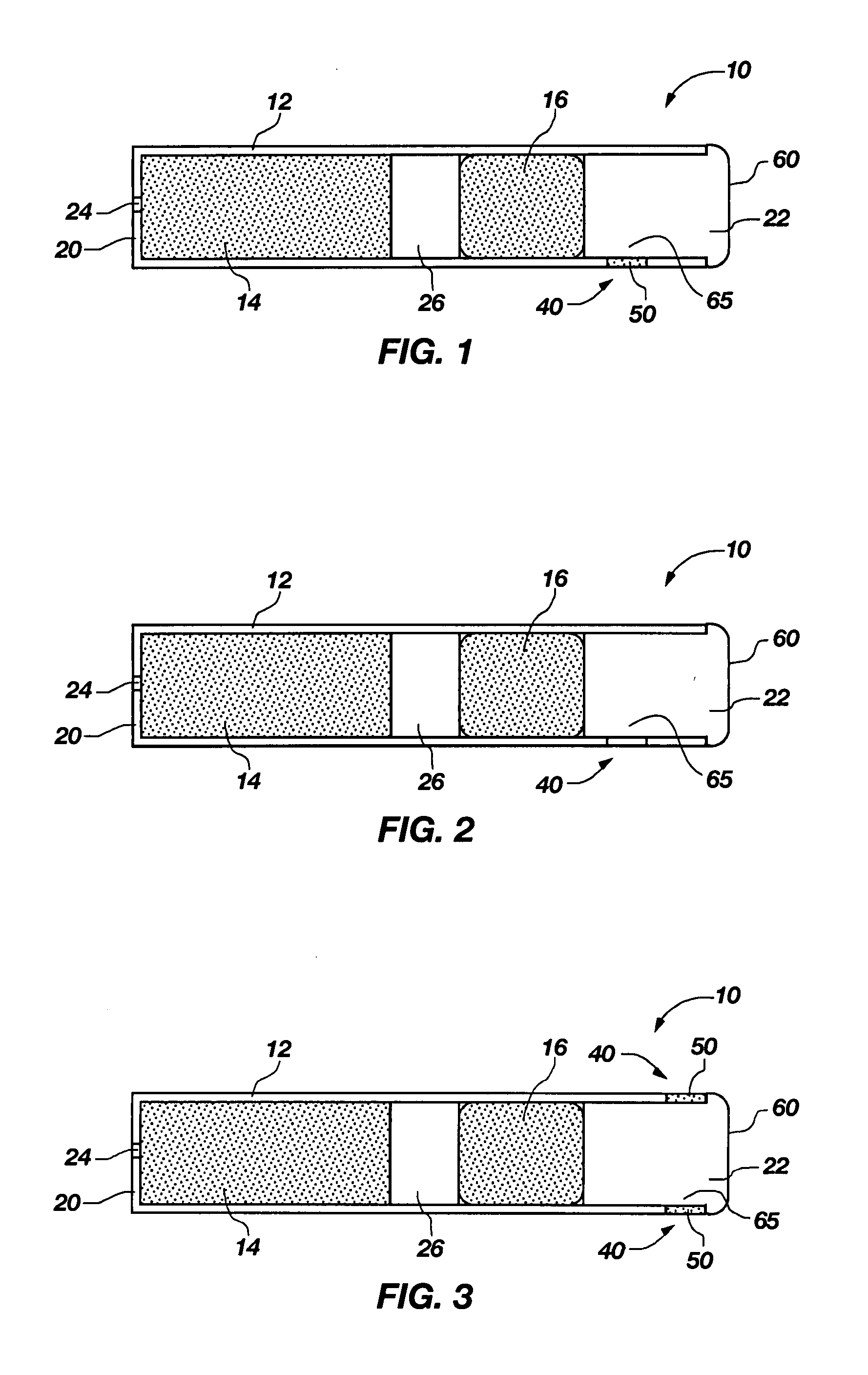
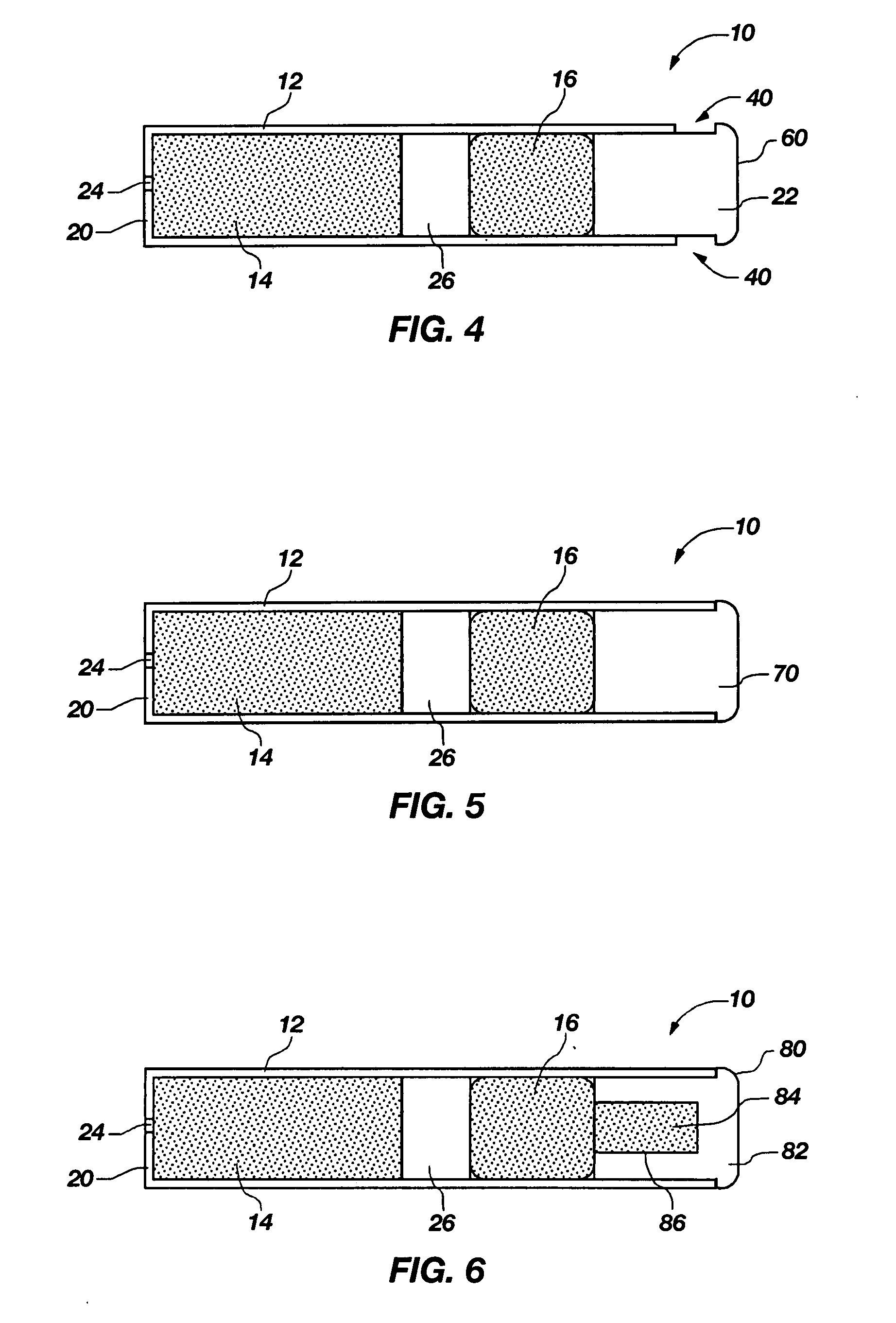
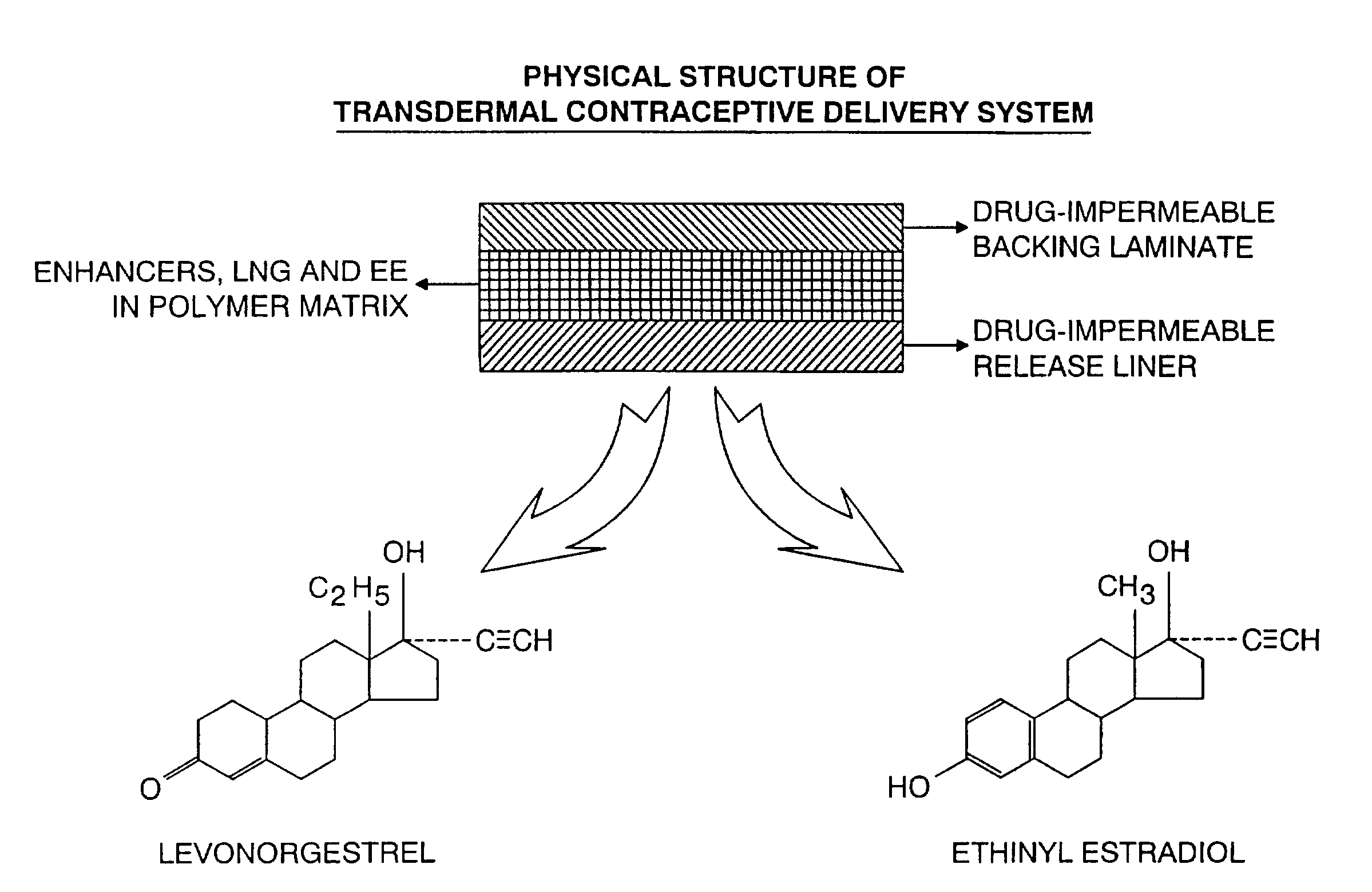
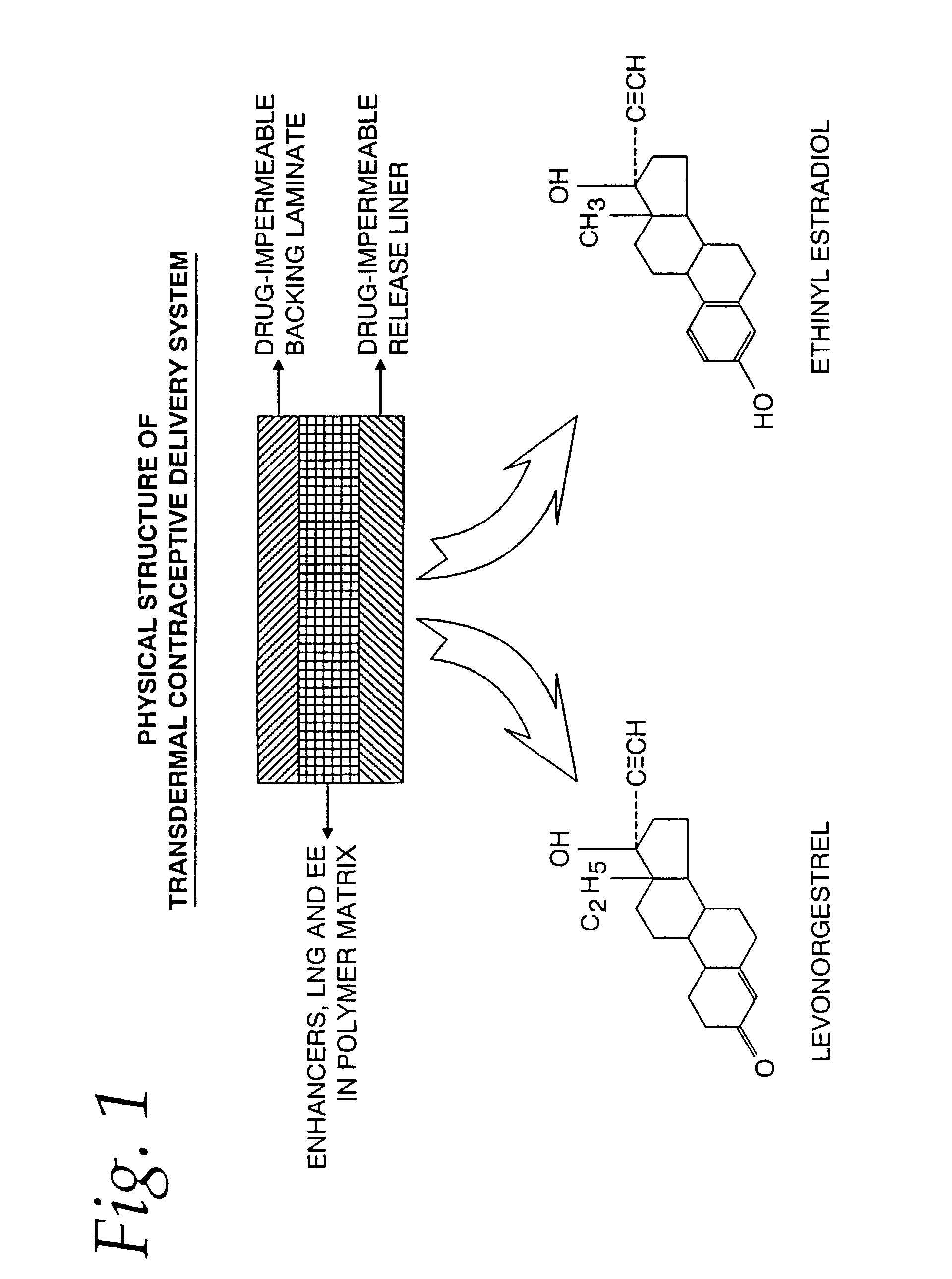
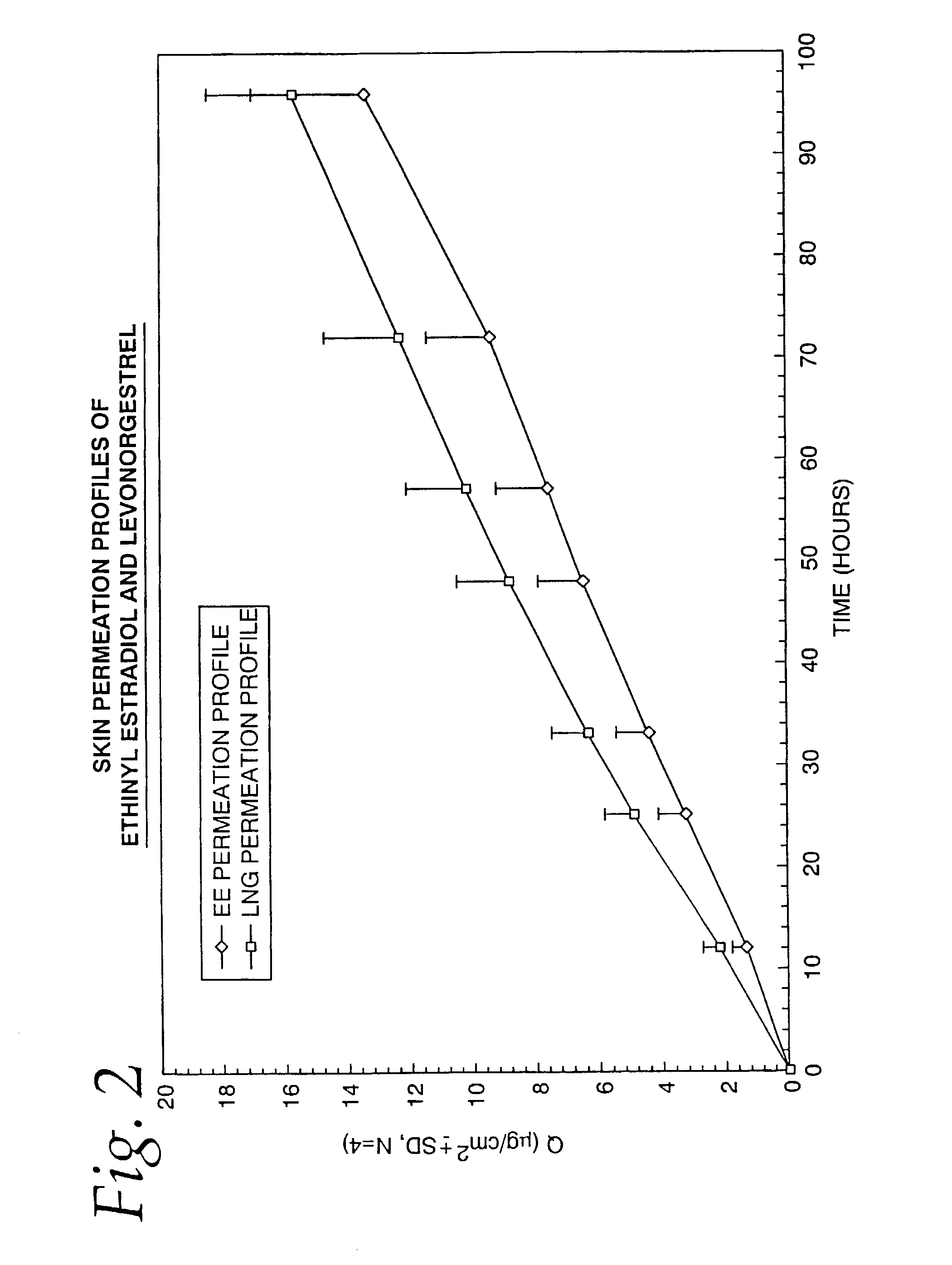
Transdermal contraceptive delivery system and process
InactiveUS7045145B1Cut skinReduce concentrationOrganic active ingredientsAdhesive dressingsObstetricsAdhesive
A transdermal contraceptive delivery system (TCDS) for fertility control in women is described. It comprises a backing layer, an adjoining layer of a solid absorption adhesive polymer matrix in which effective daily doses of an estrogen and a progestin are dispersed and released for transdermal absorption. Presently preferred is the use of the synthetic estrogen, ethinyl estradiol, and the synthetic progestin, levonorgestrel. Along with these two steroidal contraceptive agents, a combination of several chemical skin permeation enhancing agents, including capric acid, blended at specific weight ratios, ranging from 2:1:1:0.8 to 6:1:1:0.8, are homogeneously dispersed in the adhesive polymer matrix. The invention also provides a method of fertility control utilizing the transdermal contraceptive delivery system.
Owner:AGILE THERAPEUTICS
Probiotic composition having acid-resistant enteric coating
InactiveUS20070059296A1Improve digestibilityImproving resistance against diseaseOrganic active ingredientsBiocideBiotechnologyTalc
A probiotic composition essentially comprises 15 to 20 wt % of milk powder, 25 to 30 wt % of corn starch, 8 to 15 wt % of modified starch (capsul), 10 to 15 wt % of ethylcellulose, 5 to 15 wt % of bacterial broth, and 10 to 15 wt % of talc. The probiotic composition is microencapsulated to form a plurality of microencapsule coated with an acid-resistant enteric coating for improving the enteric acid-resistance, the probiotic survival rate, the antimicrobial property, the stability, the moisture-proof property, and the mobility of the probiotic composition preventing from coagulation in a moist environment and for being used as an additive applied to livestock feed.
Owner:BION TECH INC
Coated implantable medical device
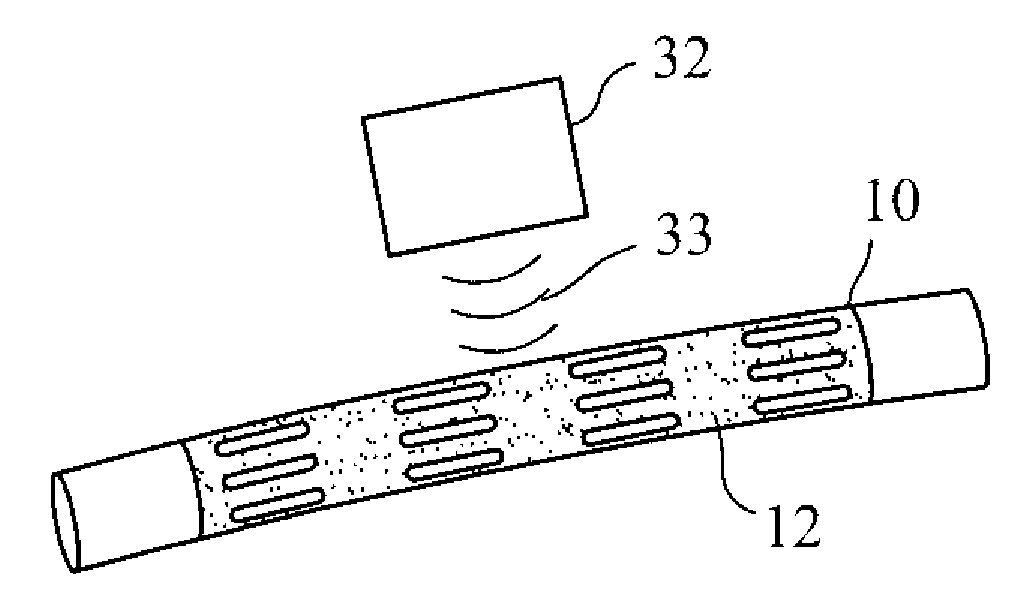
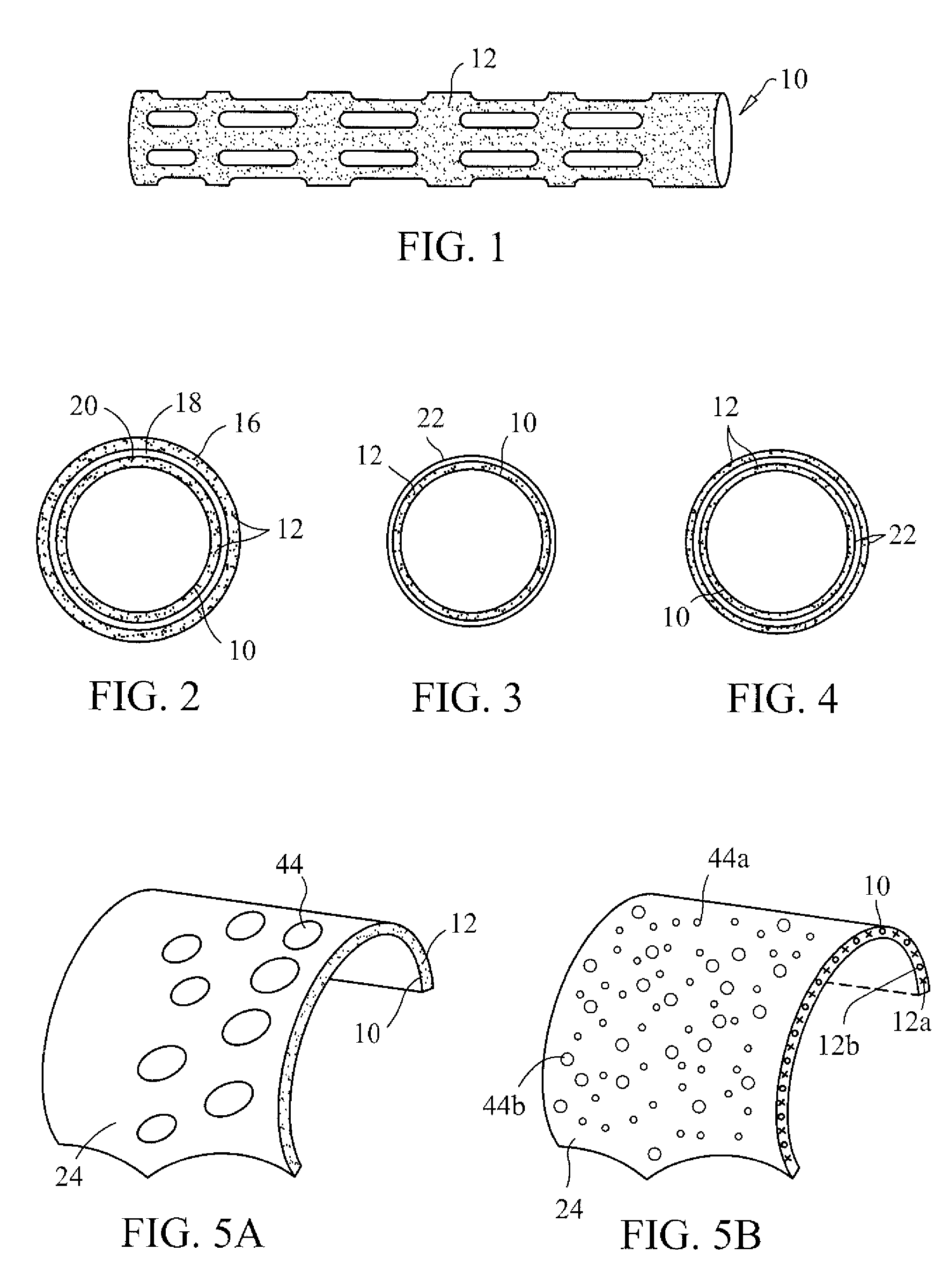
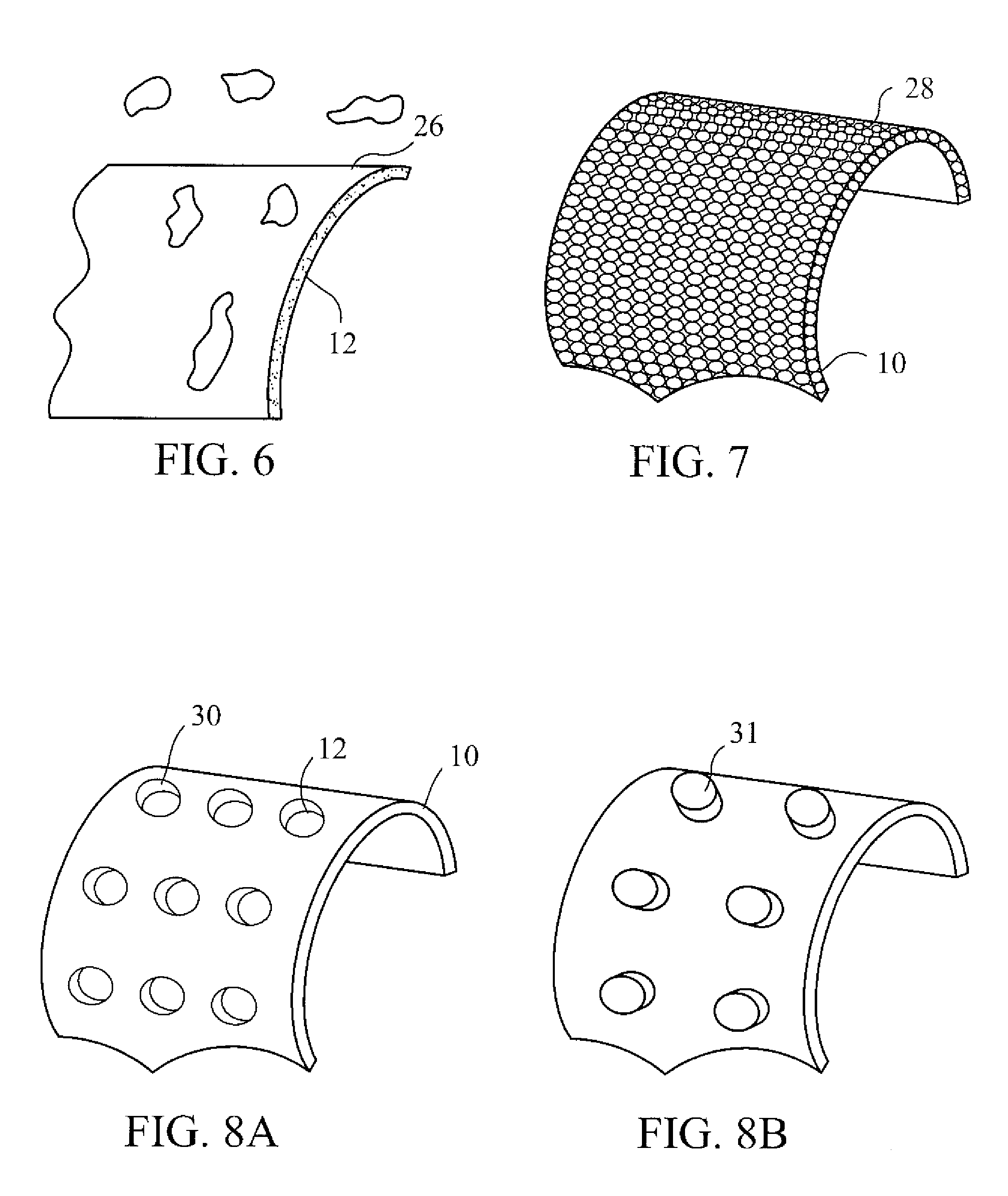
InactiveUS20070168012A1Prevent degradationIncrease release rateHeart valvesSurgeryBiomedical engineeringPorous layer
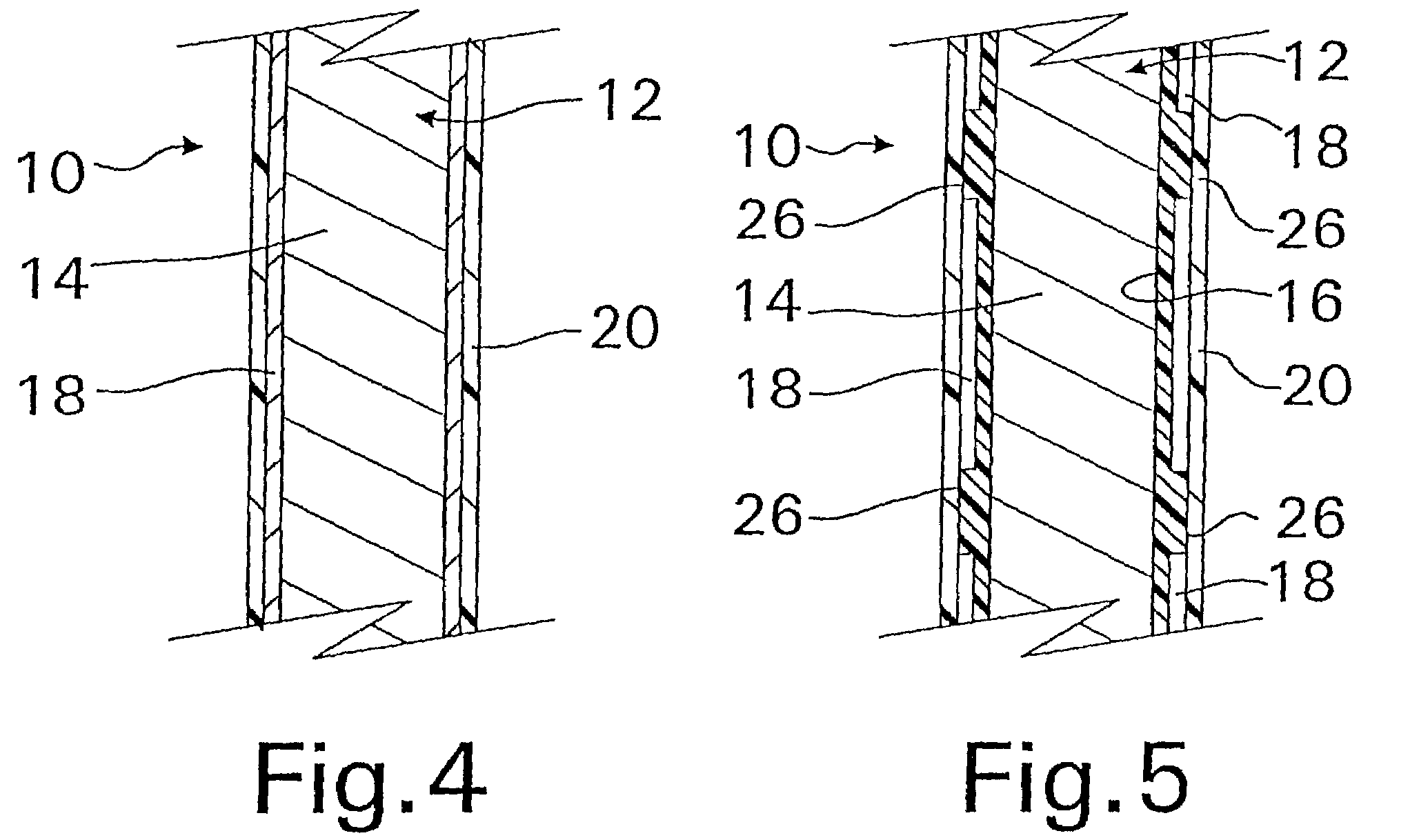
Methods of making coated implantable medical devices are provided. The methods include positioning a first layer comprising a bioactive on at least a portion of a structure, and positioning at least one porous layer over the first layer. The at least one porous layer has a thickness adequate to provide a controlled release of the bioactive.
Owner:COOK MEDICAL TECH LLC
Coated implantable medical device
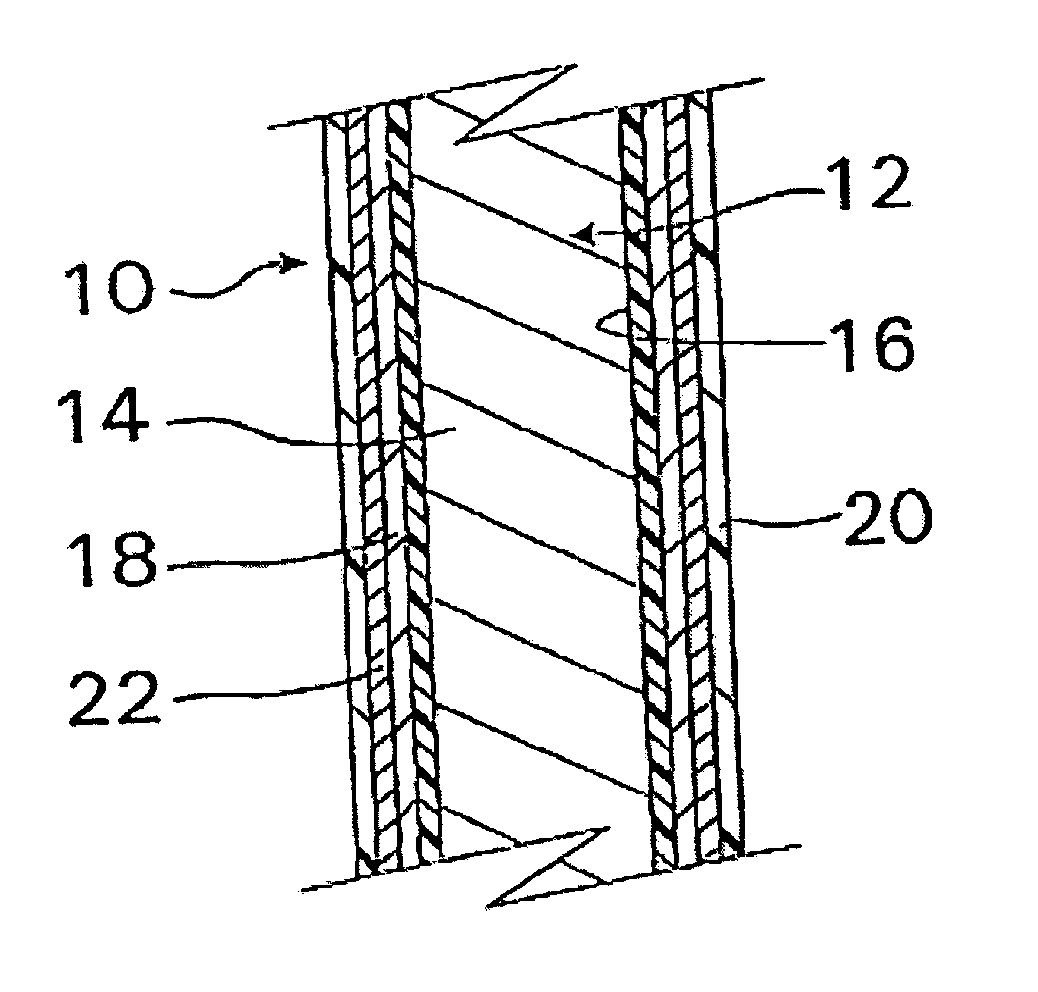
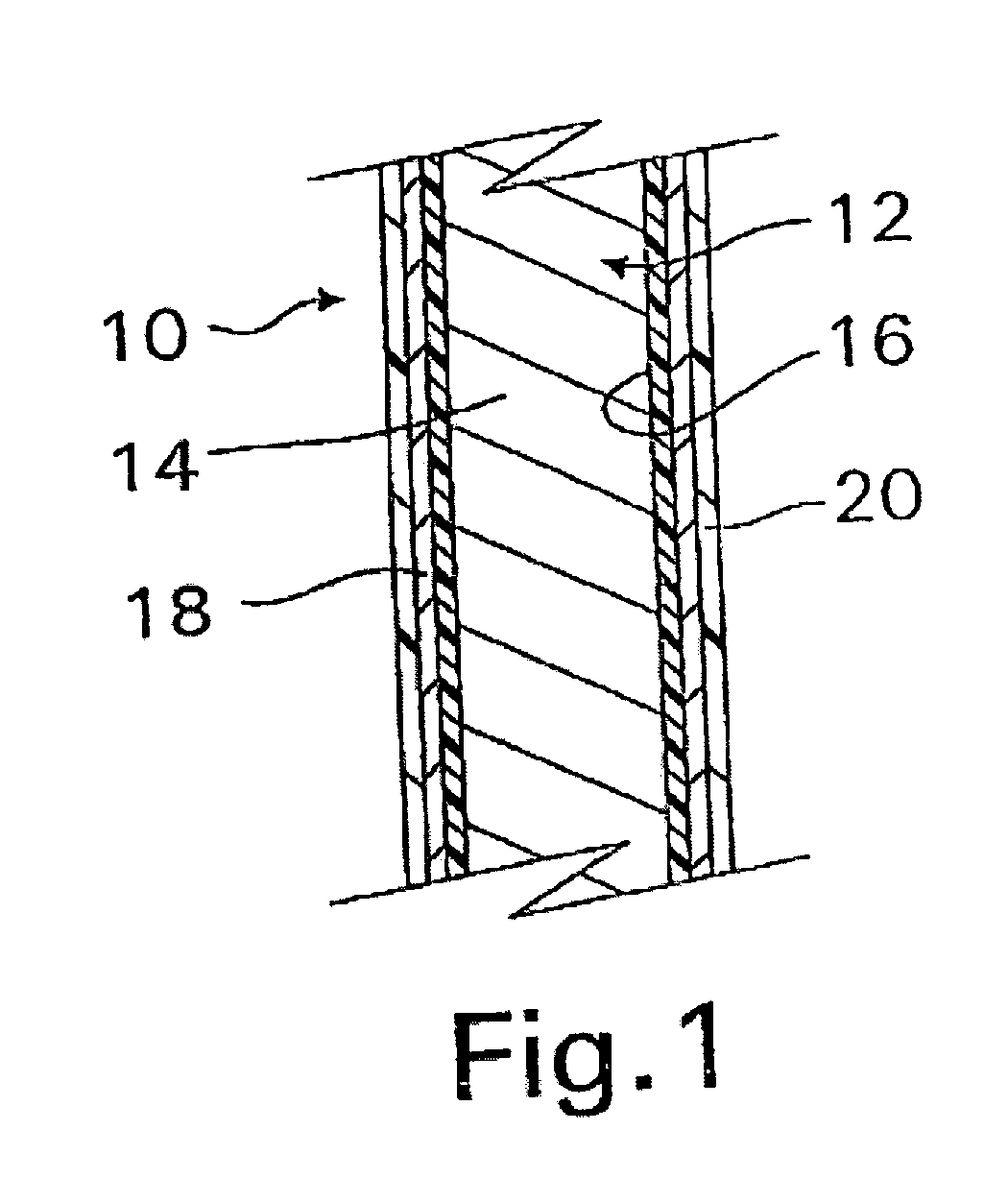
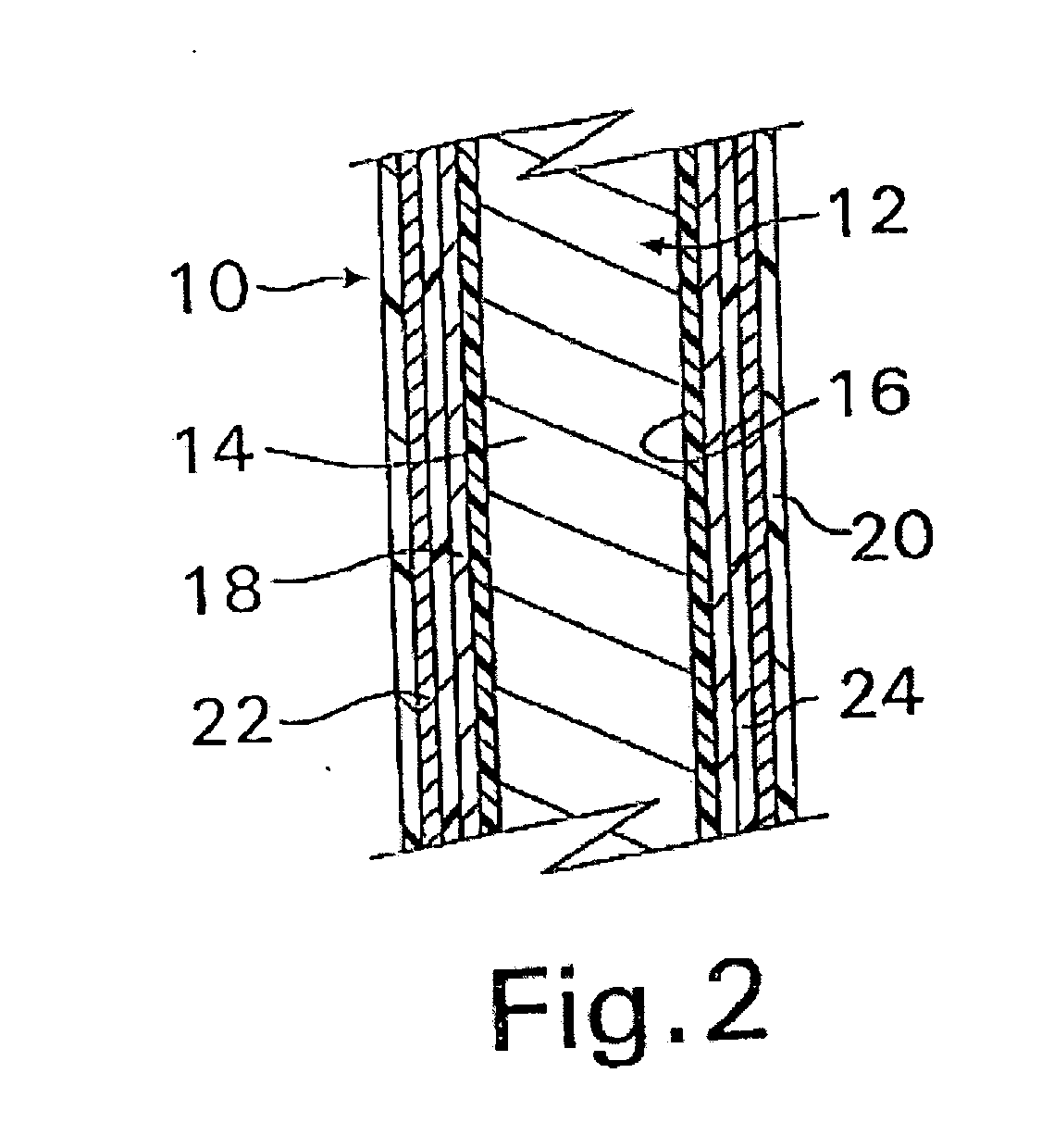
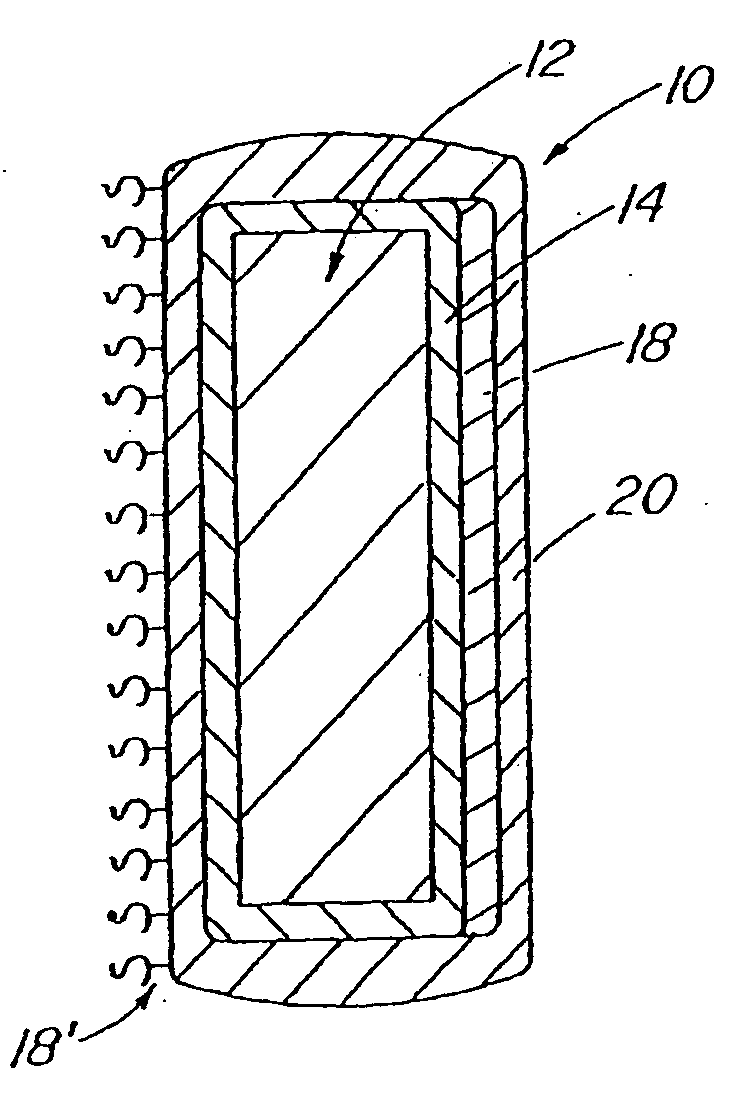
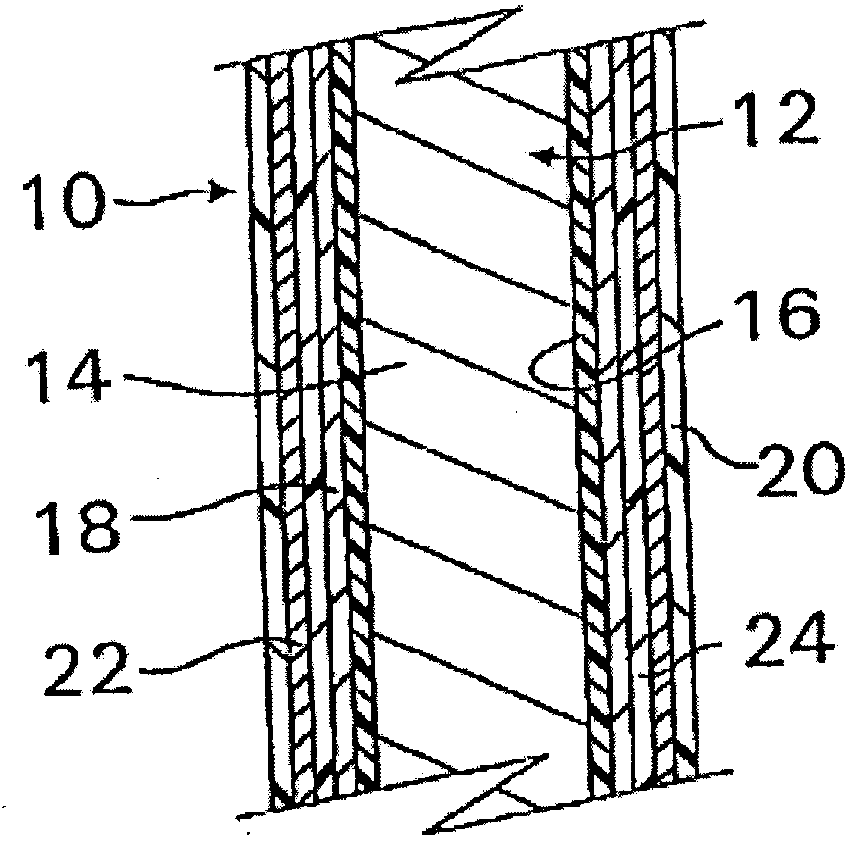
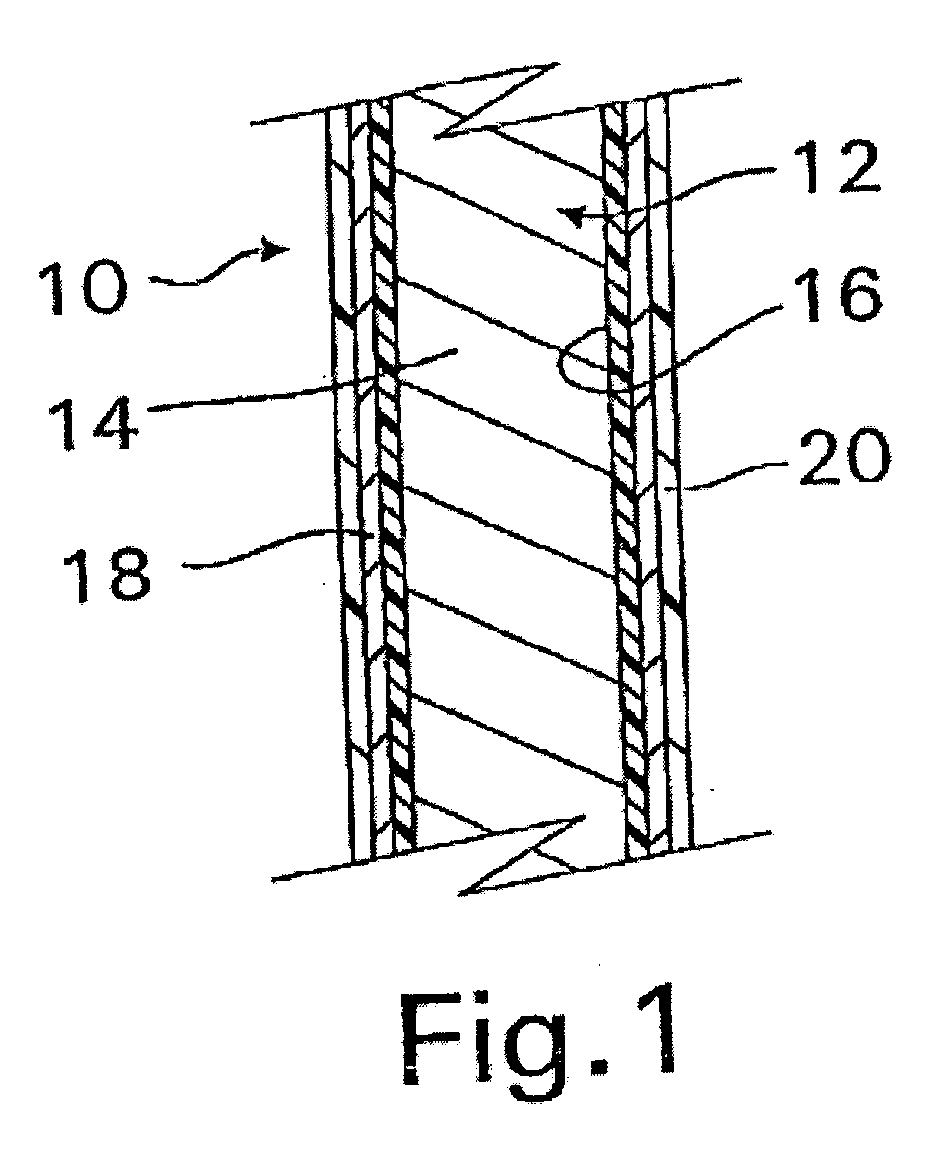
InactiveUS20070050010A1Prevent degradationIncrease release rateStentsIn-vivo radioactive preparationsParyleneGas phase
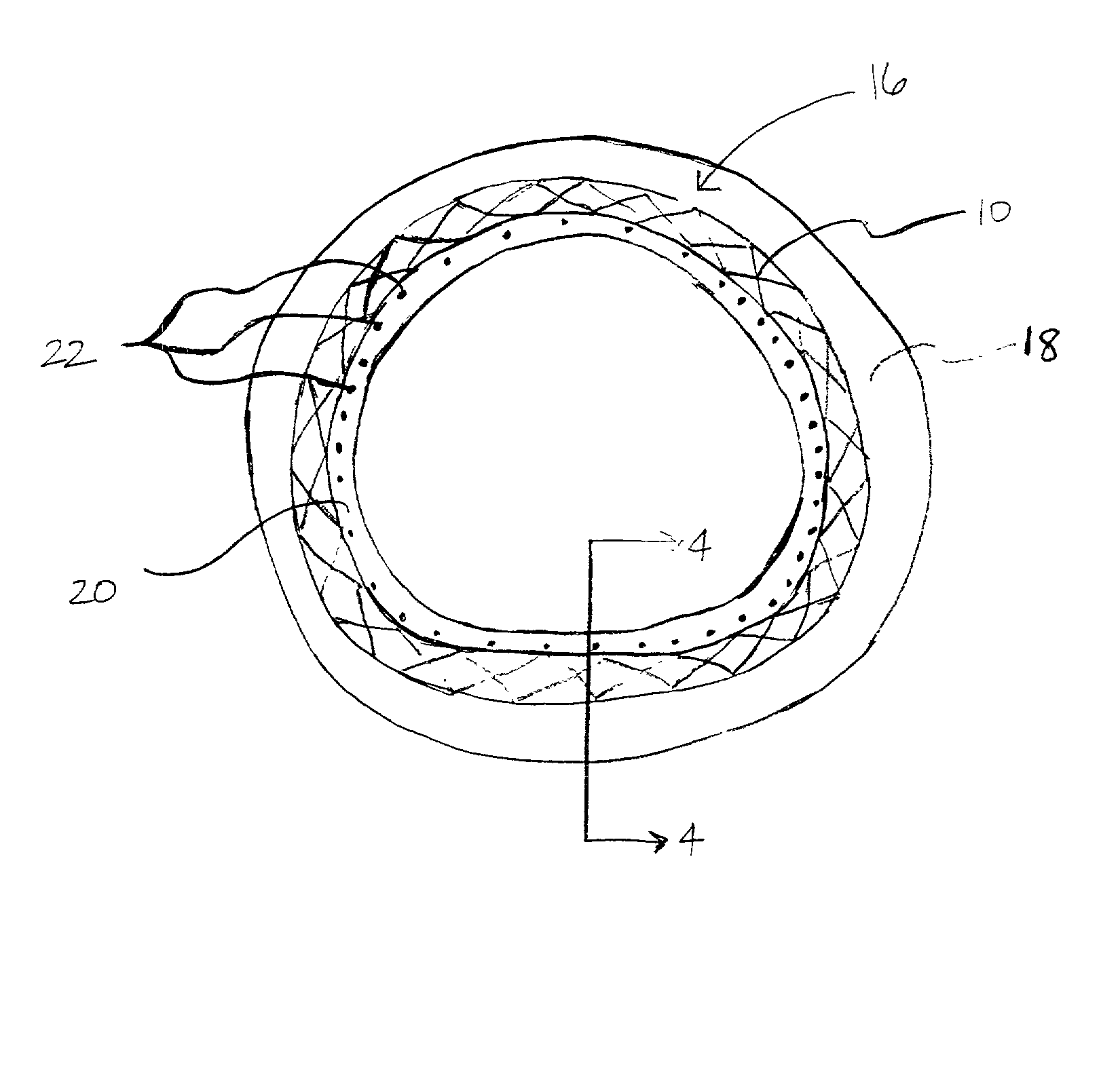
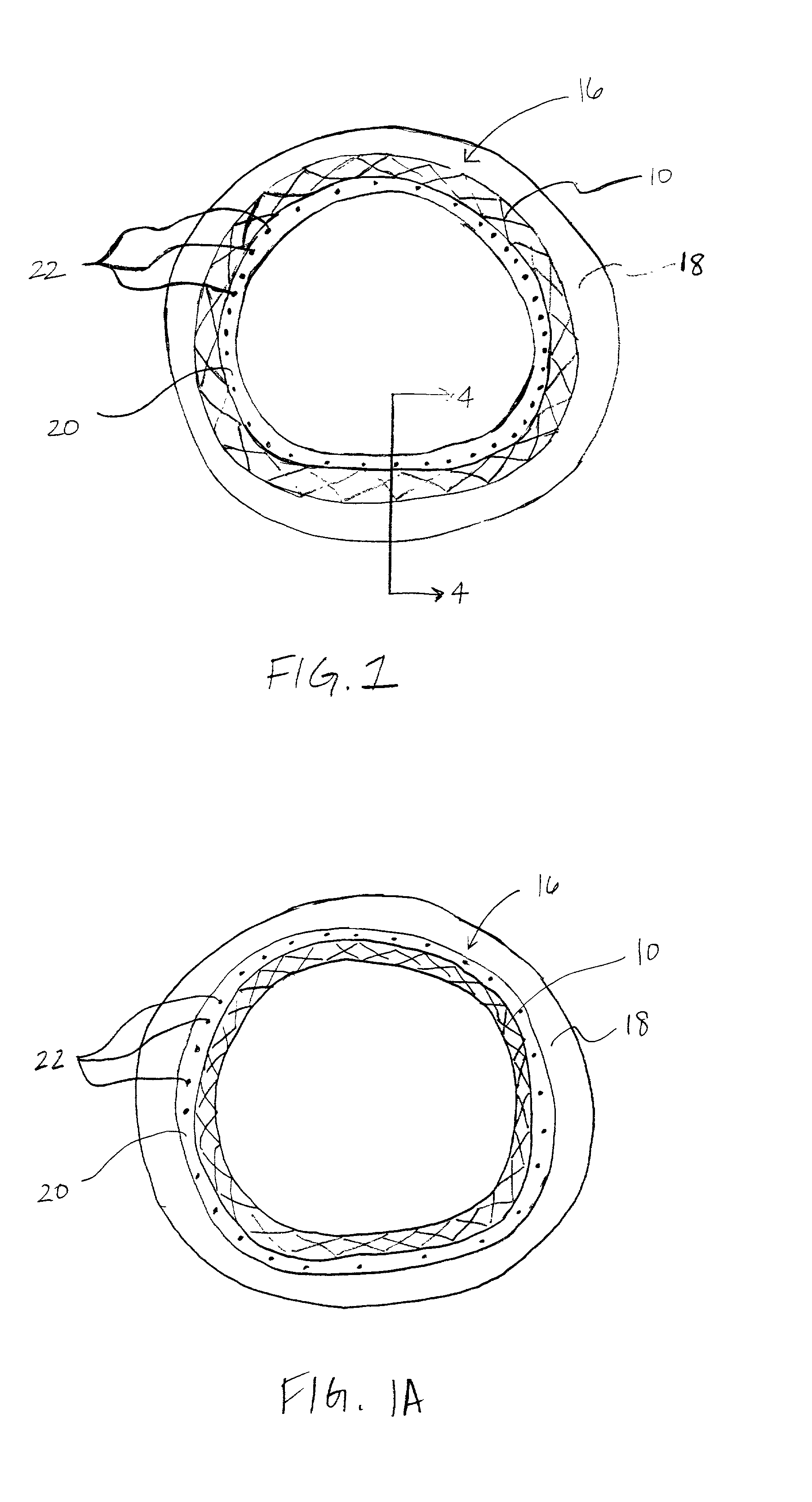
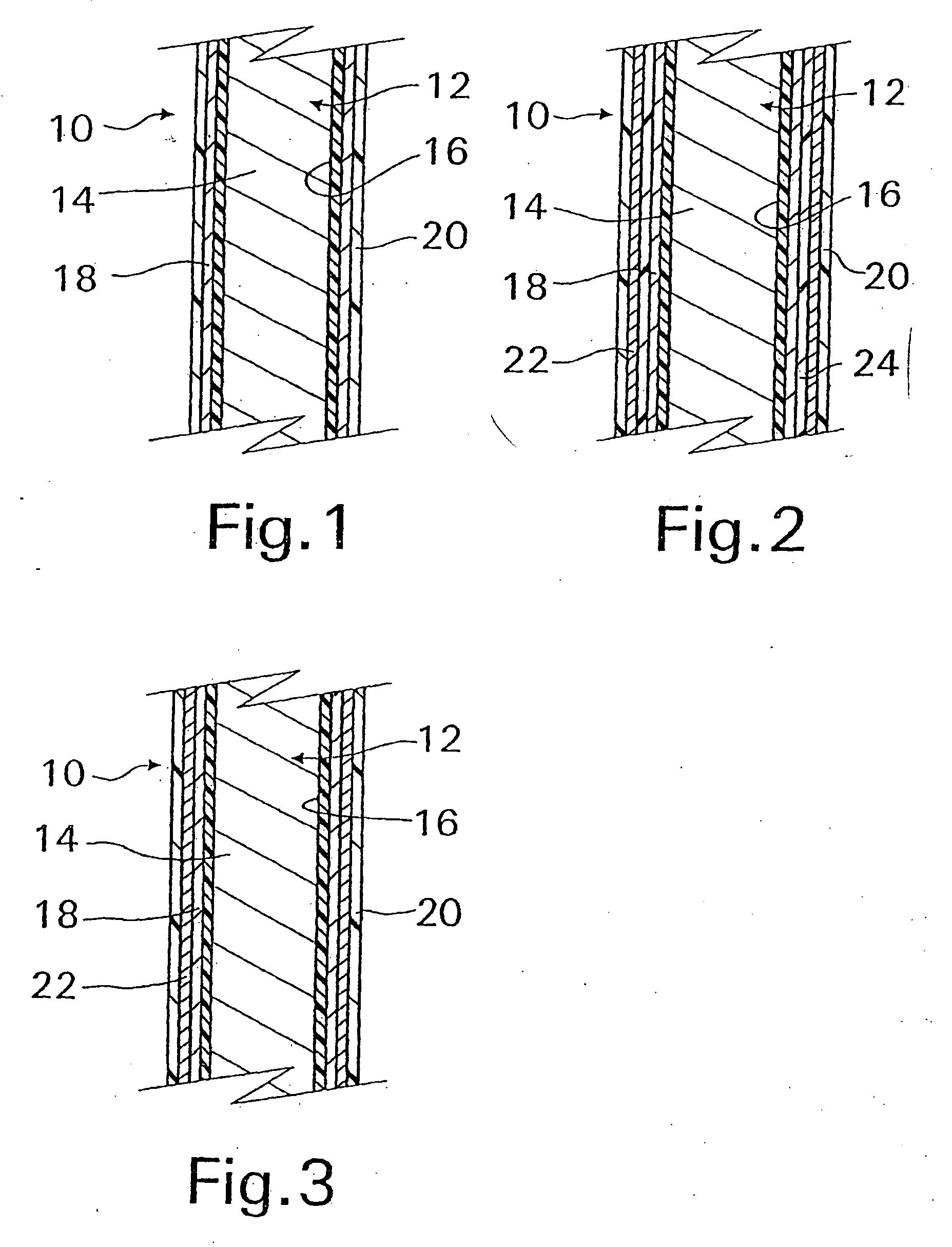
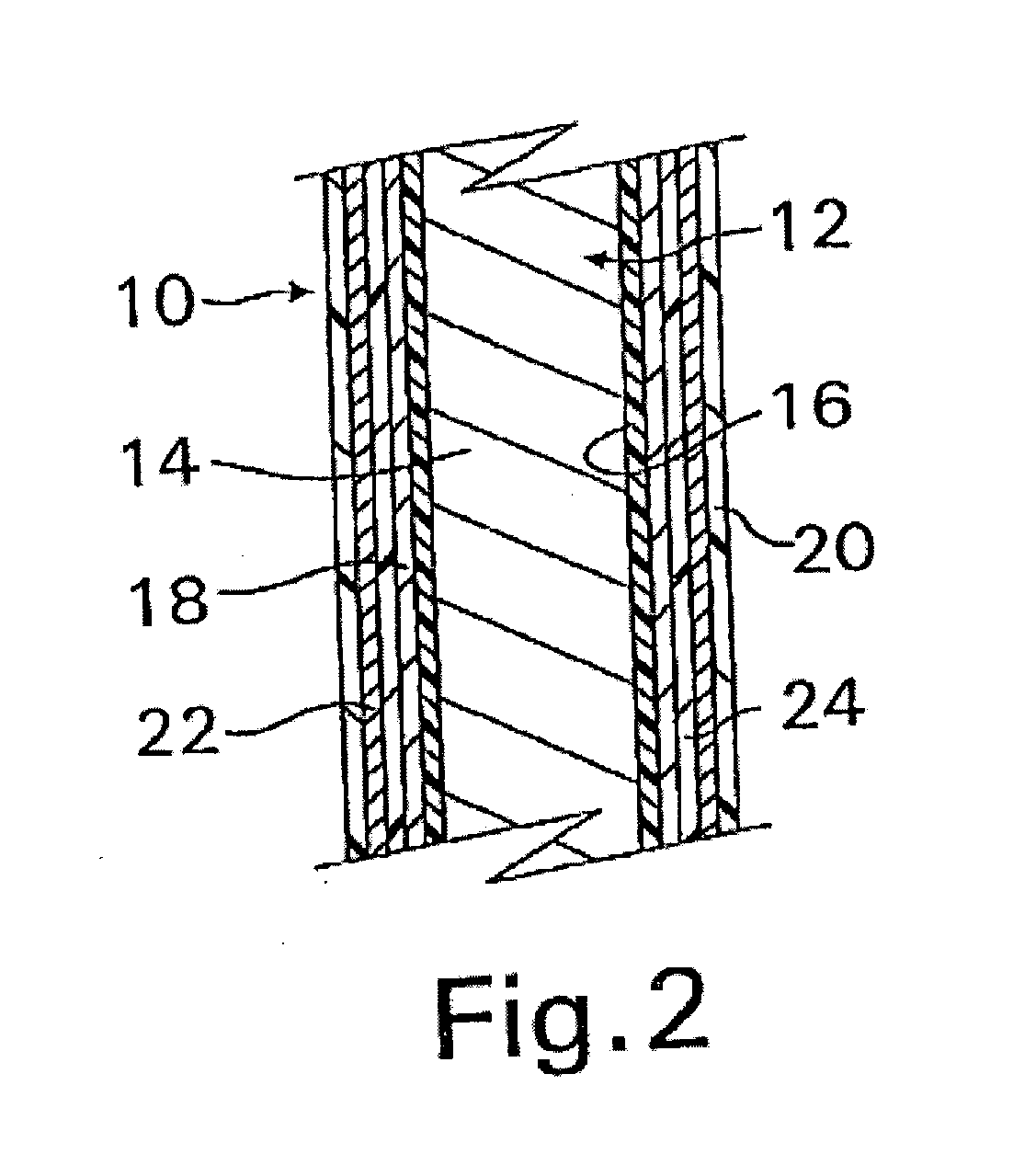
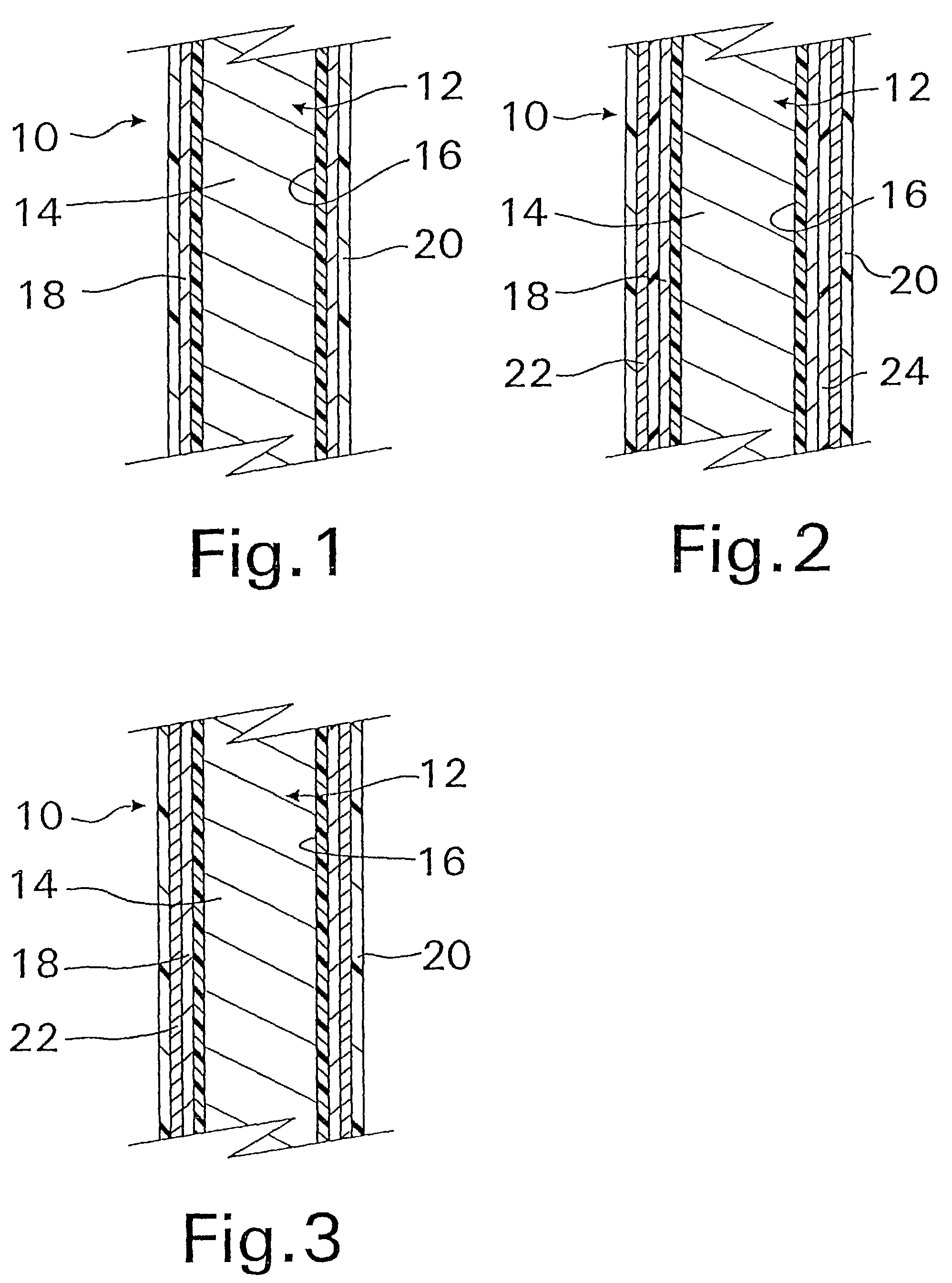
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
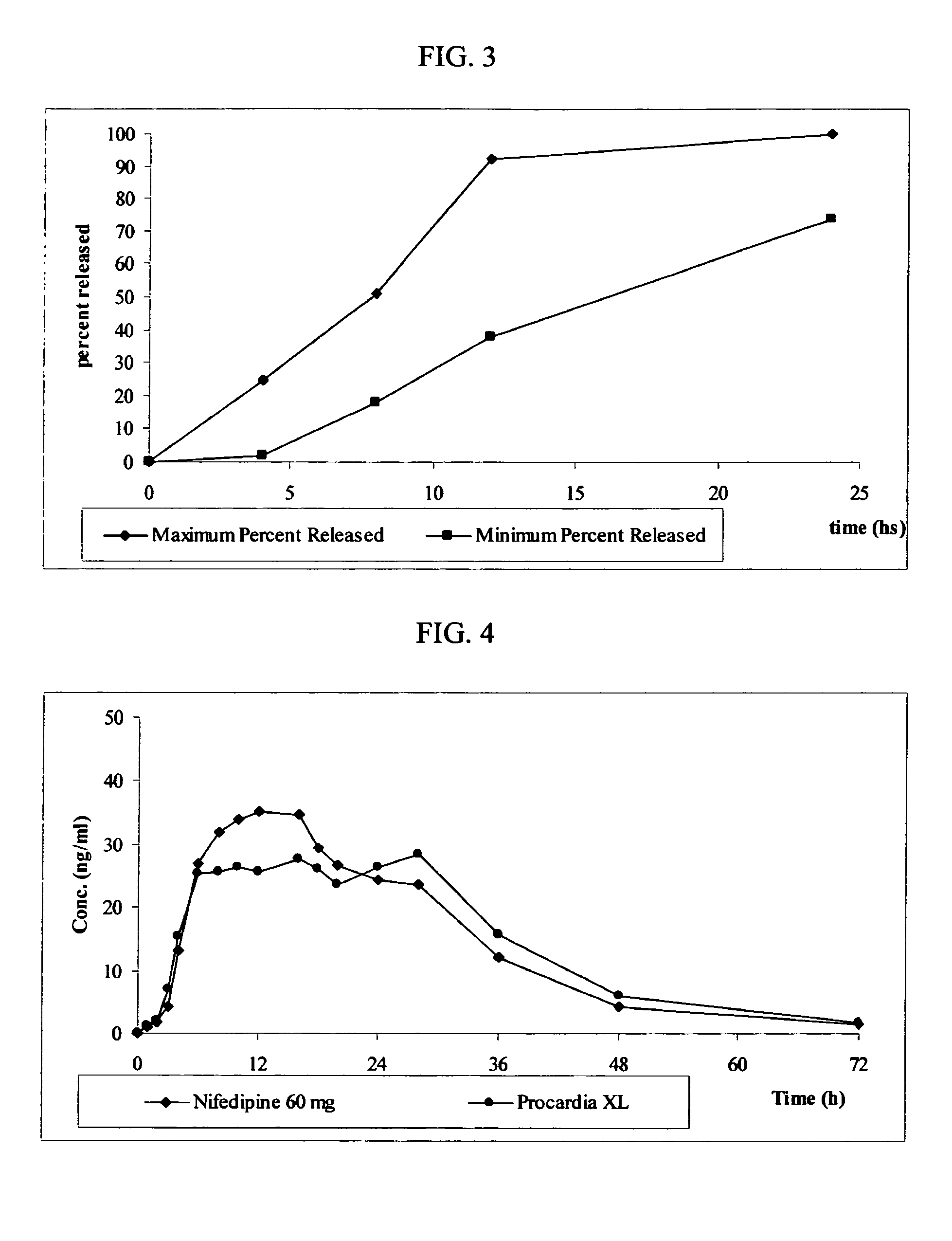
Oral Dosage Forms with Therapeutically Active Agents In Controlled Release Cores and Immediate Release Gelatin Capsule Coats
InactiveUS20080026052A1Increase release rateIncrease ratingsBiocideNervous disorderActive agentGelatin capsule
The present invention relates to oral dosage form with active agents in controlled release cores and in immediate release gelatin capsule coats.
Owner:SCHOENHARD GRANT L
Multi component controlled release system for oral care, food products, nutraceutical, and beverages
InactiveUS20050112235A1Extended stayImprove bioavailabilityCosmetic preparationsPowder deliveryMicrosphereActive agent
The present invention relates to an improved controlled release system that can encapsulate different flavors, sensory markers, and active ingredients, or combinations of flavors, sensory markers and various active ingredients and release multiple active ingredients in a consecutive manner, one after the other. The controlled delivery system of the present invention is substantially free-flowing powder formed of solid hydrophobic nanospheres that are encapsulated in a moisture sensitive microspheres. The flavors, and active ingredients encapsulated in the hydrophobic nanospheres, in the water sensitive microsphere, or in both the nano and the microsphere. The flavors and active ingredients encapsulated in the nanospheres can be the same or different from those encapsulated in the microspheres. The encapsulation of different flavors or active agents in the various components of the system, such as nanospheres and microspheres, provides flavor transition (change in flavor character) during the use of the products. The controlled release system of the present invention enhances the stability and bioavailability of wide range of flavors, sensory markers, and other active ingredients, prolong their residence time in the oral cavity, control their release characteristics, and prolong the sensation of flavors and other sensory markers in the mouth to provide long lasting organoleptic perception or long lasting mouthfeel. The invention further relates oral care, food products, and beverages comprising the controlled release system of the present invention.
Owner:SHEFER ADI +1
Multi component controlled delivery system for soap bars
InactiveUS7208460B2Increase depositionImprove skinPowder deliverySoap detergents with organic compounding agentsControlled releaseMicrosphere
Owner:SALVONA IP
Microcapsules having activated release of core material therein
InactiveUS20050277549A1Increase ratingsIncrease release rateBiocideAnimal repellantsPolymer chemistryAqueous dispersion
Owner:MONSANTO TECH LLC
Implantable medical device with bioabsorbable coating
InactiveUS20070150047A1Prevent degradationIncrease release rateStentsSurgeryControlled releasePorous layer
Methods of making coated implantable medical devices are provided. The methods include positioning a first layer comprising a bioactive on at least a portion of a structure, and positioning at least one porous layer over the first layer. The at least one porous layer has a thickness adequate to provide a controlled release of the bioactive.
Owner:MED INST INC +1
Multi component controlled delivery system for soap bars
InactiveUS20050065047A1Increase depositionHigh densitySoap detergents with organic compounding agentsCosmetic preparationsControlled releaseMicrosphere
The present invention relates to an improved controlled delivery system that can be incorporated in soap bars to enhance deposition of active ingredients and sensory markers onto skin. The carrier system also provides controlled release or prolonged release of these actives from the skin over an extended period of time. The controlled delivery system of the present invention comprises substantially free-flowing, powder formed of solid hydrophobic, positively charged, nanospheres of encapsulated active ingredients, that are encapsulated in moisture sensitive microspheres. The high cationic charge density of the nanosphere improves deposition of active ingredients onto skin. The high cationic charge density on the nanosphere surface is created by incorporating a cationic conditioning agent into the solid hydrophobic matrix of the nanospheres, by incorporating a cationic charge “booster” in the moisture sensitive microsphere matrix, or by using a cationic conditioning agent in the nanosphere matrix in conjunction with a cationic charge “booster” in the microsphere matrix. The invention also pertains to soap products comprising the controlled release system of the present invention.
Owner:SALVONA IP
Oral dosage forms with therapeutically active agents in controlled release cores and immediate release gelatin capsule coats
InactiveUS20110287093A1Increase release rateOrganic active ingredientsNervous disorderControlled releaseImmediate release
The present invention relates to oral dosage form with active agents in controlled release cores and in immediate release gelatin capsule coats.
Owner:SCHOENHARD GRANT L
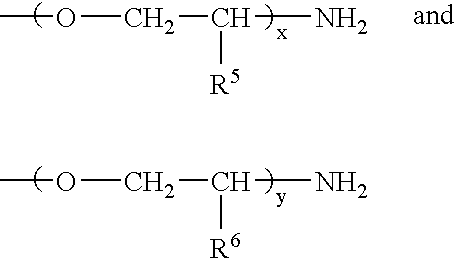
Transdermal delivery of hormones without the need of penetration enhancers
ActiveUS20050142175A1High plasma levelInhibit ovulationBiocideOrganic active ingredientsEthinyl oestradiolHormones regulation
The present invention relates to a patch comprising a drug-containing layer with low content of hormones, such as gestodene, and optionally an estrogen (e.g. ethinyl estradiol). Upon administering the patch to a woman, plasma levels of at least 1.0 ng / ml of Gestodene is achieved at steady state conditions without the need of incorporating penetration enhancers or permeation enhancers in the drug-containing layer. Satisfactorily plasma levels of the hormones is also achieved throughout a period of at least 1 week, making the patch applicable for being used in female contraception with the concept of administering the patch ones weekly.
Owner:LUYE PHARMA SWITZERLAND AG
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS7291673B2Reducing and delaying onsetGood water solubilityBiocideSurgical adhesivesPolymerControlled delivery
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:UNIV ZURICH +1
Coated implantable medical device
InactiveUS7550005B2Prevent degradationIncrease release rateStentsHeart valvesParylenePlasma deposition
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Low burst polymers and methods to produce polymer
ActiveUS20100292195A1Increase release rateReduced initial burstAntibacterial agentsBiocideDrug biological activityCopolymer
A PLG copolymer material, termed a PLG(p) copolymer material, adapted for use in a controlled release formulation for a bioactive material is provided, wherein the formulation exhibits a reduced “initial burst” effect when introduced into the tissue of a patient in need thereof. A method of preparation of the PLG copolymer material is also provided, as are methods of use.
Owner:TOLMAR INC
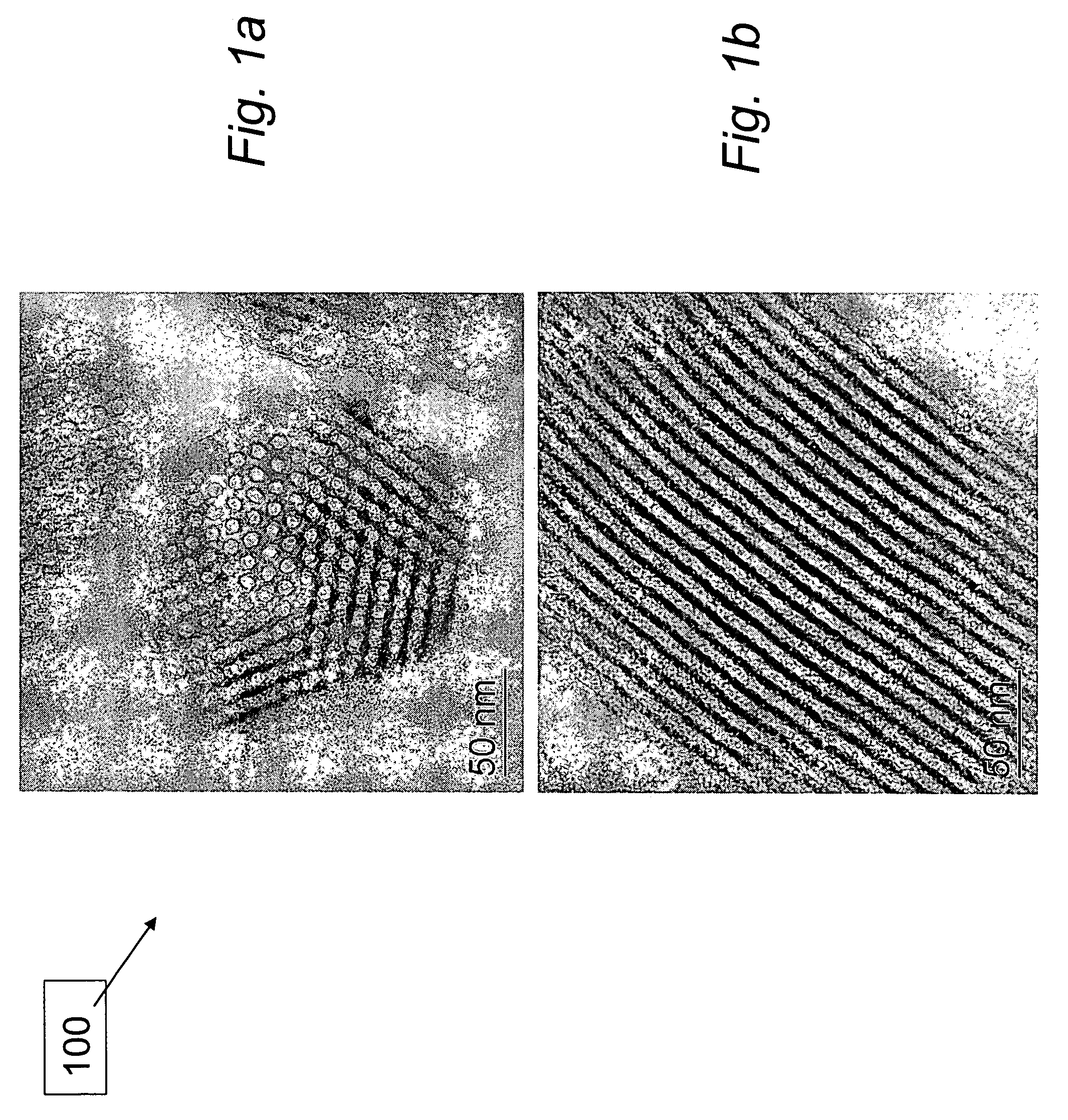
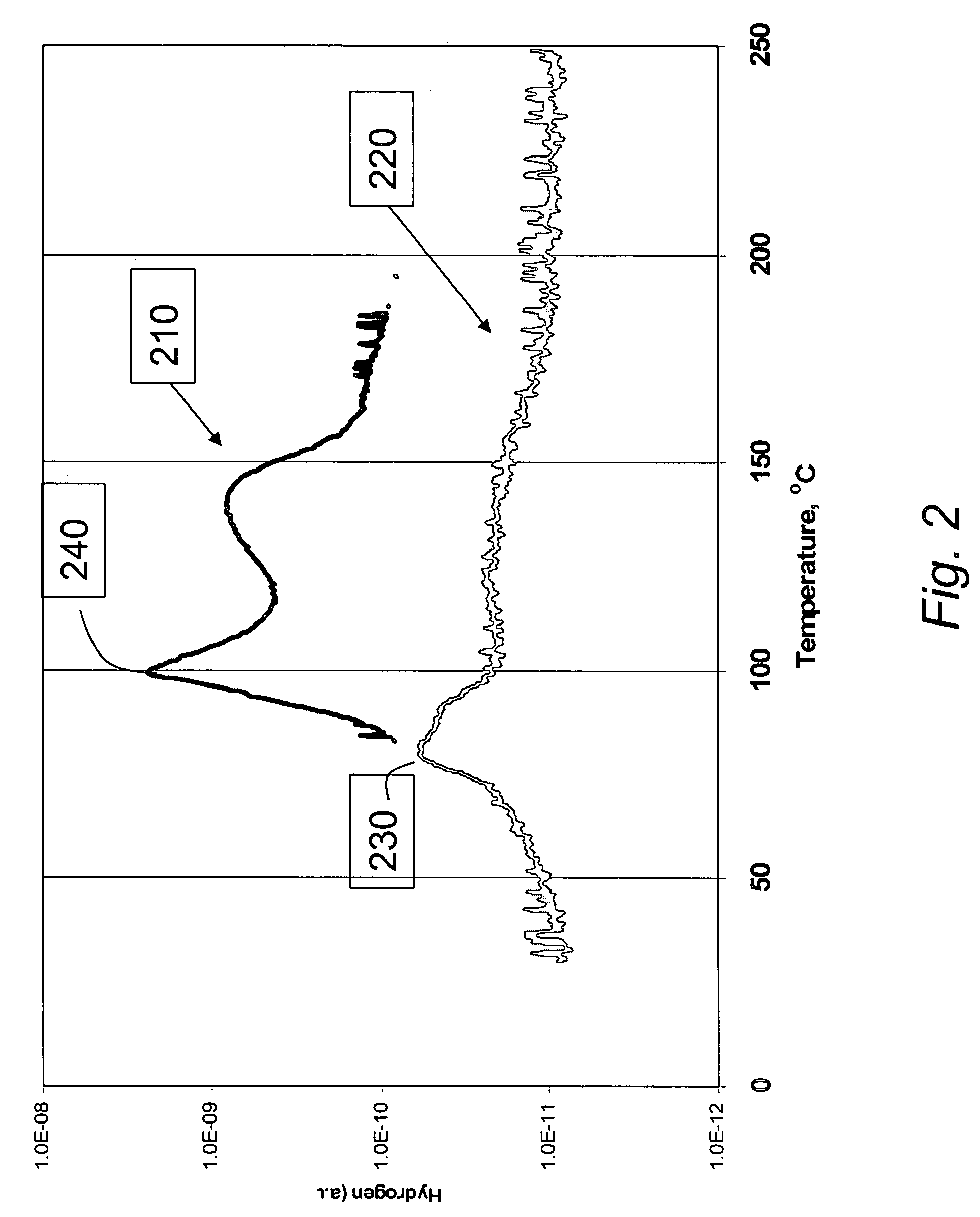
Materials for storage and release of hydrogen and methods for preparing and using same
ActiveUS7316788B2Minimize undesirableHigh purityAlkali/alkaline-earth/beryllium/magnesium hydridesOther chemical processesHydrogenFast release
The invention relates to materials for storing and releasing hydrogen and methods for preparing and using same. The materials exhibit fast release rates at low release temperatures and are suitable as fuel and / or hydrogen sources for a variety of applications such as automobile engines.
Owner:BATTELLE MEMORIAL INST
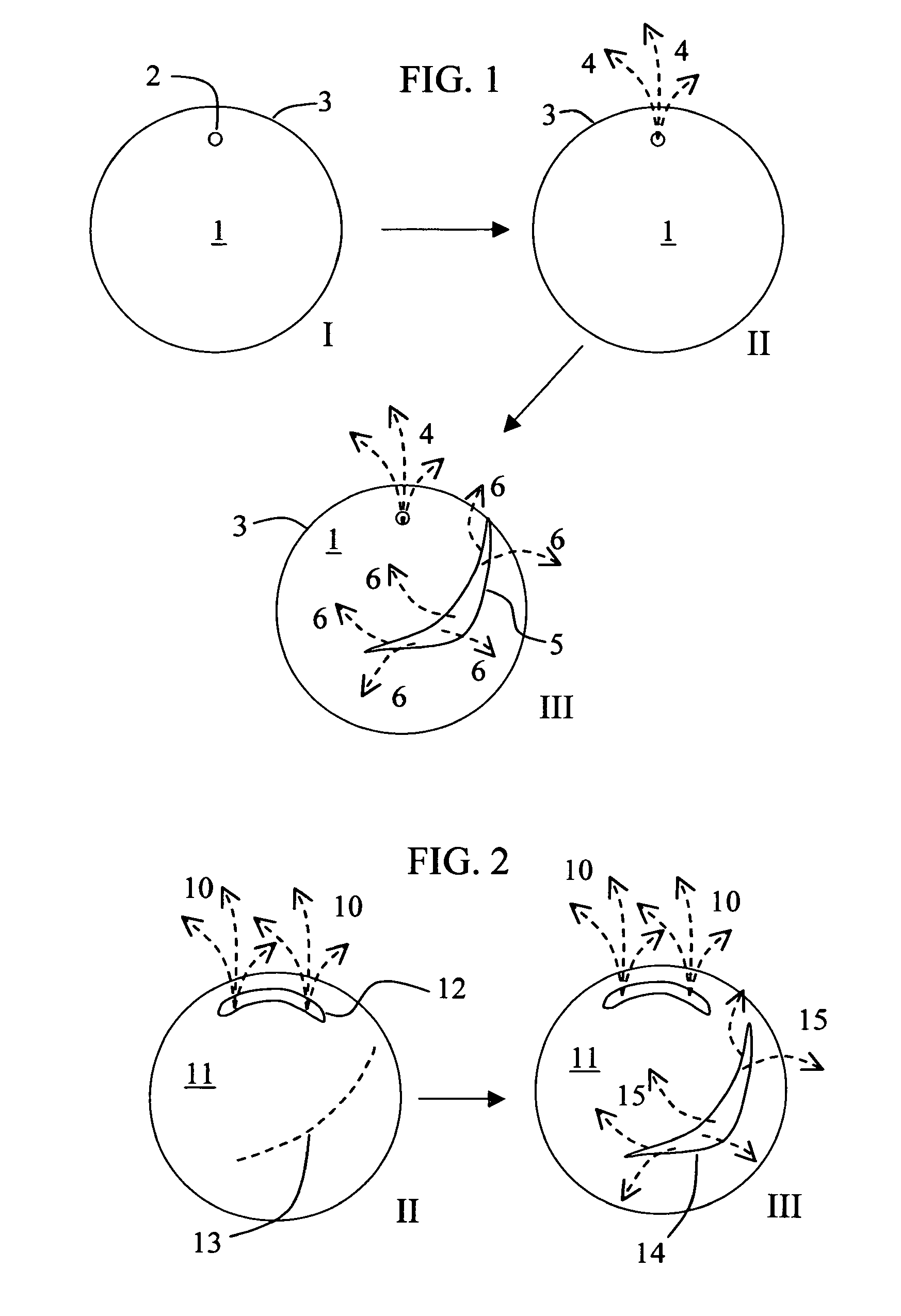
Rupturing controlled release device having a preformed passageway
ActiveUS8029822B2Increase release rateLarge particle sizePill deliveryCapsule deliveryControlled releaseMedicine
The present invention provides a simple and improved osmotic device that is capable of providing a controlled release of active agent contained in the core first through a preformed passageway and then through an in situ formed second passageway into an environment of use. One or both of the passageways optionally increases in size during use of the osmotic device. The preformed passageway and / or the second passageway increase the release rate of the active agent, enable the release of large particles containing active agent, and / or enable the release of active agents that are substantially insoluble in the environment of use. By virtue of the in situ formation of the second aperture, the device is able to release a greater overall percentage of active agent than it would release in absence of the second aperture.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Ocular therapeutic agent delivery devices and methods for making and using such devices
ActiveUS7658364B2Increase release rateReached efficientlyOrganic active ingredientsSenses disorderDual modeOcular implant
Owner:UNITED STATES OF AMERICA
Compositions and method for targeted controlled delivery of active ingredients and sensory markers onto hair, skin and fabric
InactiveUS20050176598A1Increase depositionHigh cationic charge densityCosmetic preparationsOrganic detergent compounding agentsActive agentLitter
The present invention relates to a controlled delivery system that can be incorporated in liquid, as well as, dry granular, or powder, household products, such as dishwashing detergents, surface cleaners, deodorizers, animal litters and cleaning wipes The system also prolongs the release rate of the active agents over an extended period of time, or provides heat triggered release of the active agents and yields a high impact fragrance “burst” upon heat treatment. The controlled delivery system of the present invention is a nano-sphere, having an average sphere diameter of from about 0.01 microns to about 10 microns. The nano-sphere comprises hydrophobic materials, cationic conditioning agent or, cationic conditioning agent in conjunction with a cationic charge booster to assist in adhering the spheres onto a surface.
Owner:BERGQUIST CATHARINE J +2
Encapsulation of sensitive liquid components into a matrix to obtain discrete shelf-stable particles
InactiveUS20070141096A1Avoids destructionImprove hydrophobicityBiocideCosmetic preparationsChemistryPlasticizer
A liquid encapsulant component which contains an active, sensitive encapsulant, such as a live microorganism or an enzyme dissolved or dispersed in a liquid plasticizer is admixed with a plasticizable matrix material. The matrix material is plasticizable by the liquid plasticizer and the encapsulation of the active encapsulant is accomplished at a low temperature and under low shear conditions. The active component is encapsulated and / or embedded in the plasticizable matrix component or material in a continuous process to produce discrete, solid particles. The liquid content of the liquid encapsulant component provides substantially all or completely all of the liquid plasticizer needed to plasticize the matrix component to obtain a formable, extrudable, cuttable, mixture or dough. Removal of liquid plasticizer prior to extrusion is not needed to adjust the viscosity of the mixture for formability. Release of an active component from the matrix may be delayed or controlled over time so that the active component is delivered when and where it is needed to perform its intended function. Controlled release, discrete, solid particles which contain an encapsulated and / or embedded component such as a heat sensitive or readily oxidizable pharmaceutically, biologically, or nutritionally active component are continuously produced without substantial destruction of the matrix material or encapsulant.
Owner:GENERAL MILLS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com