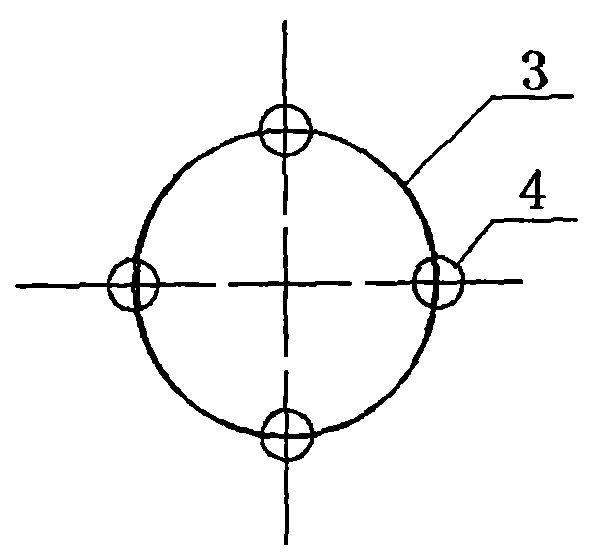Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
484 results about "Biliary tract" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
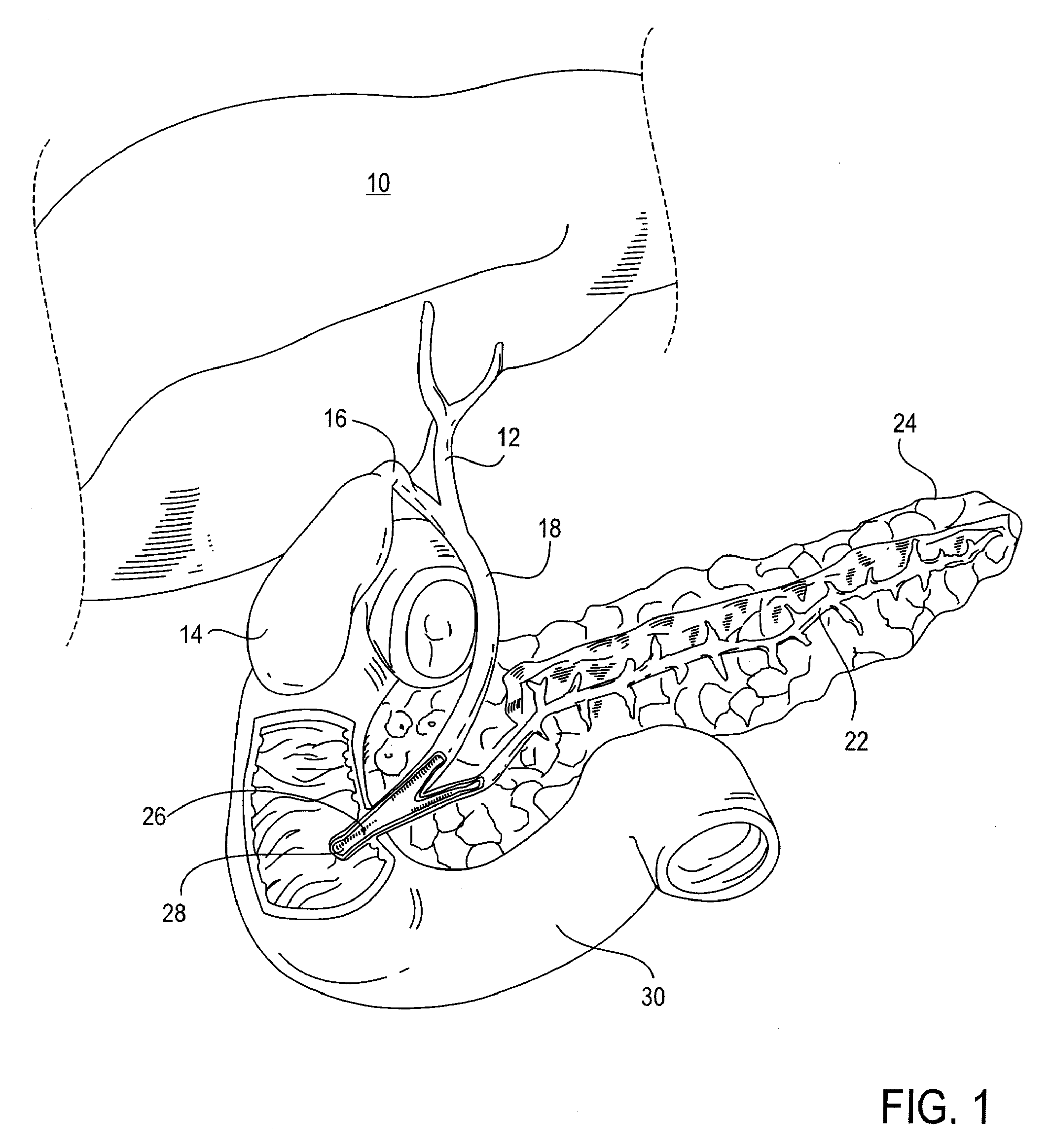
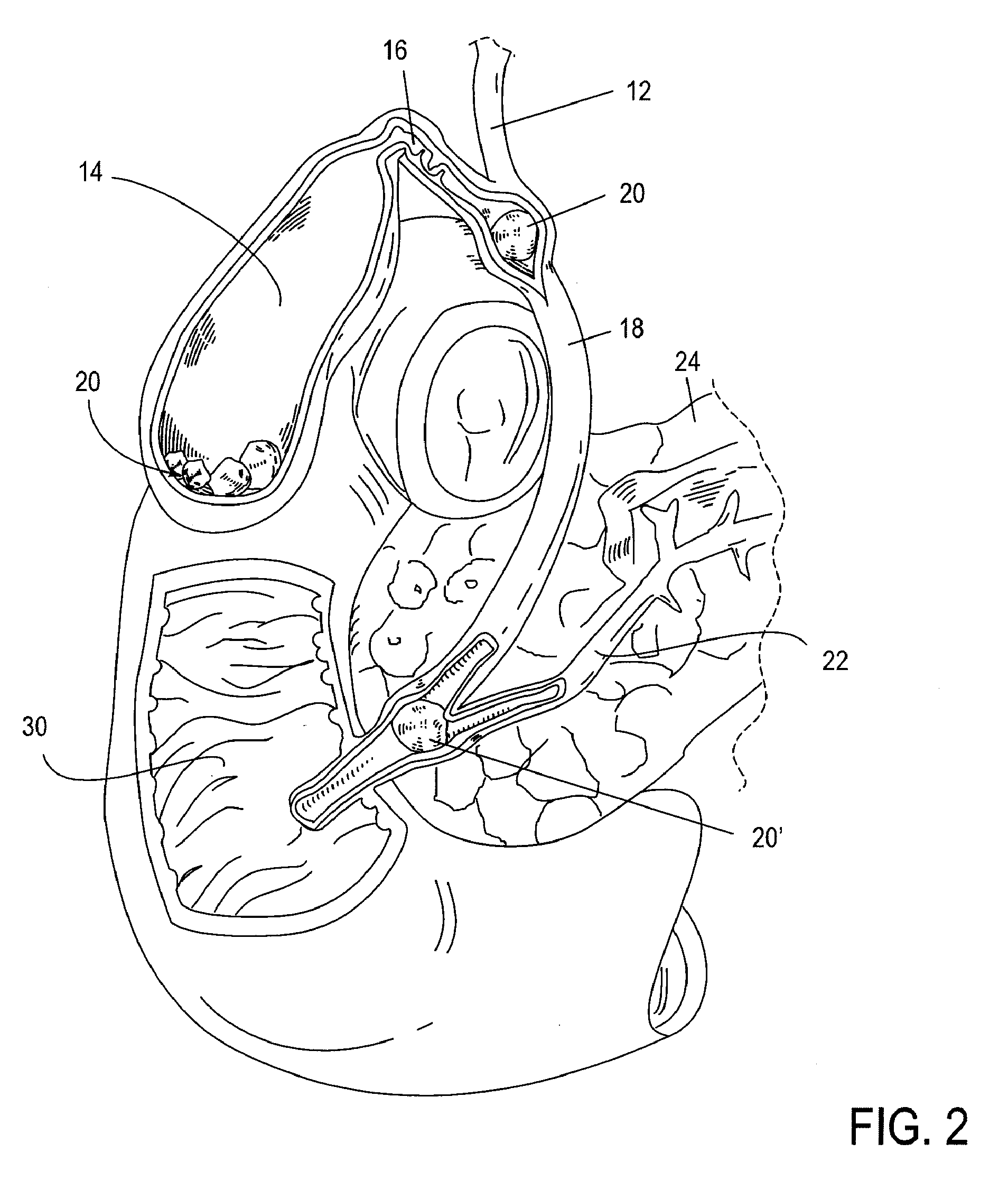
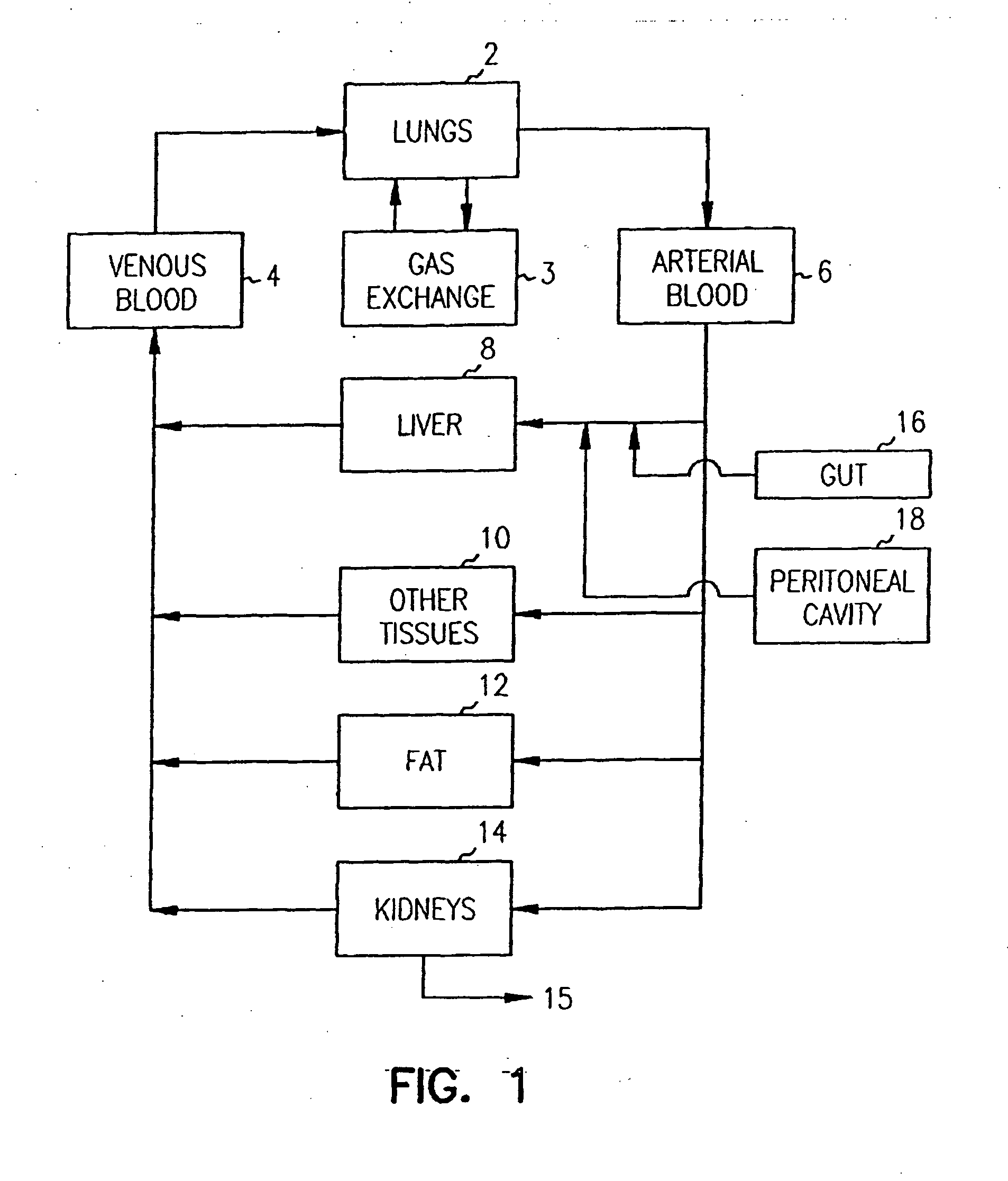
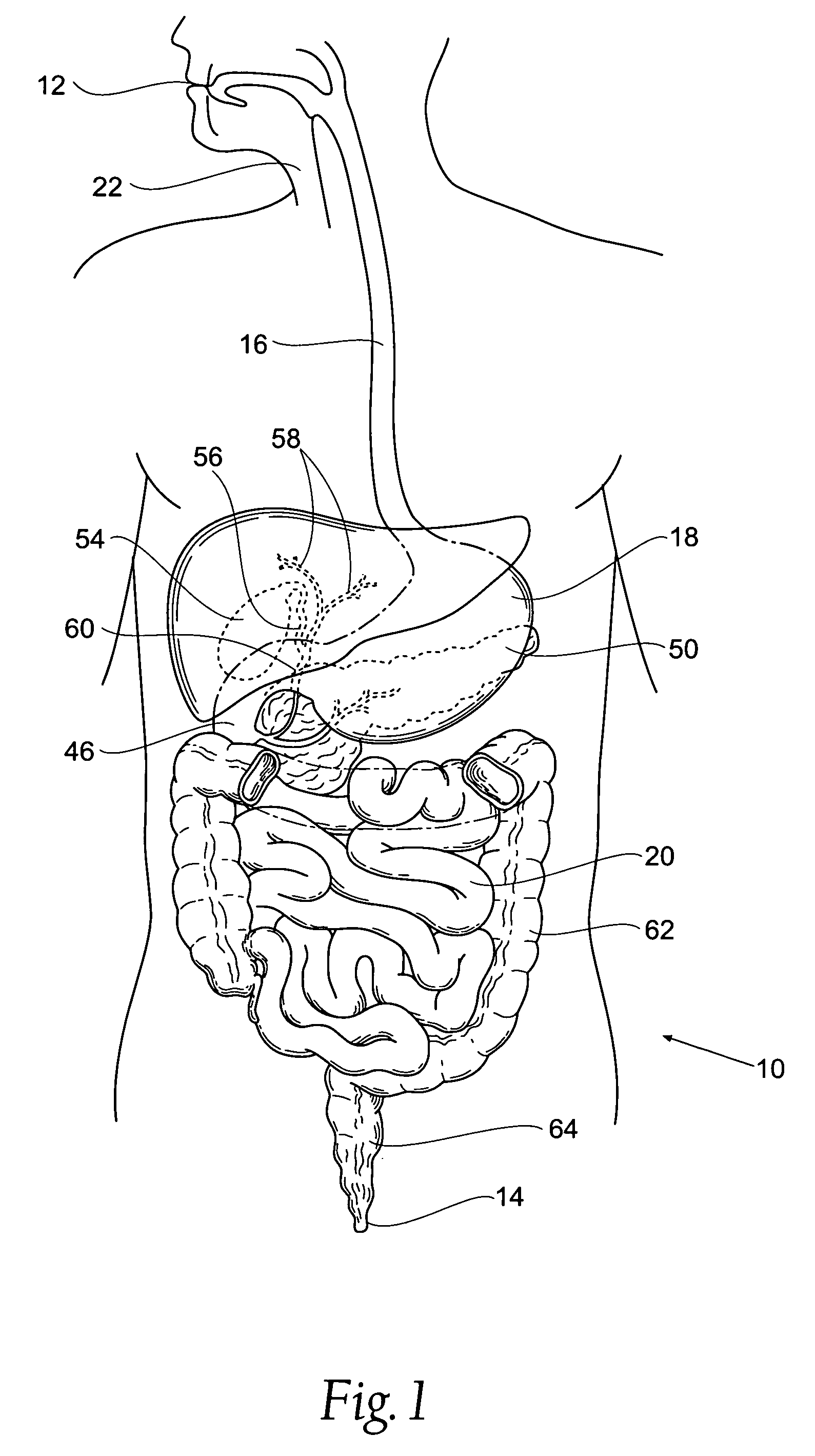
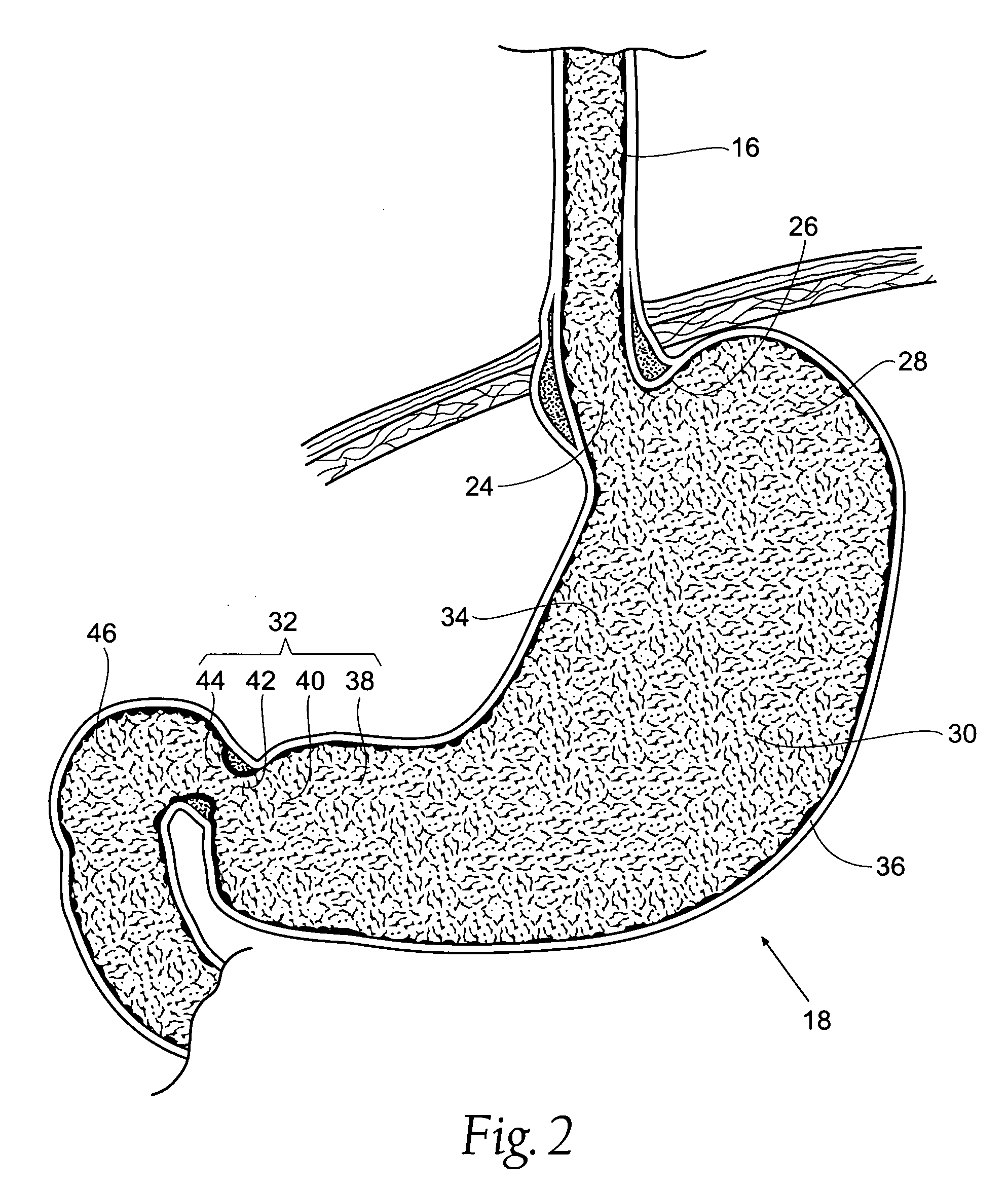
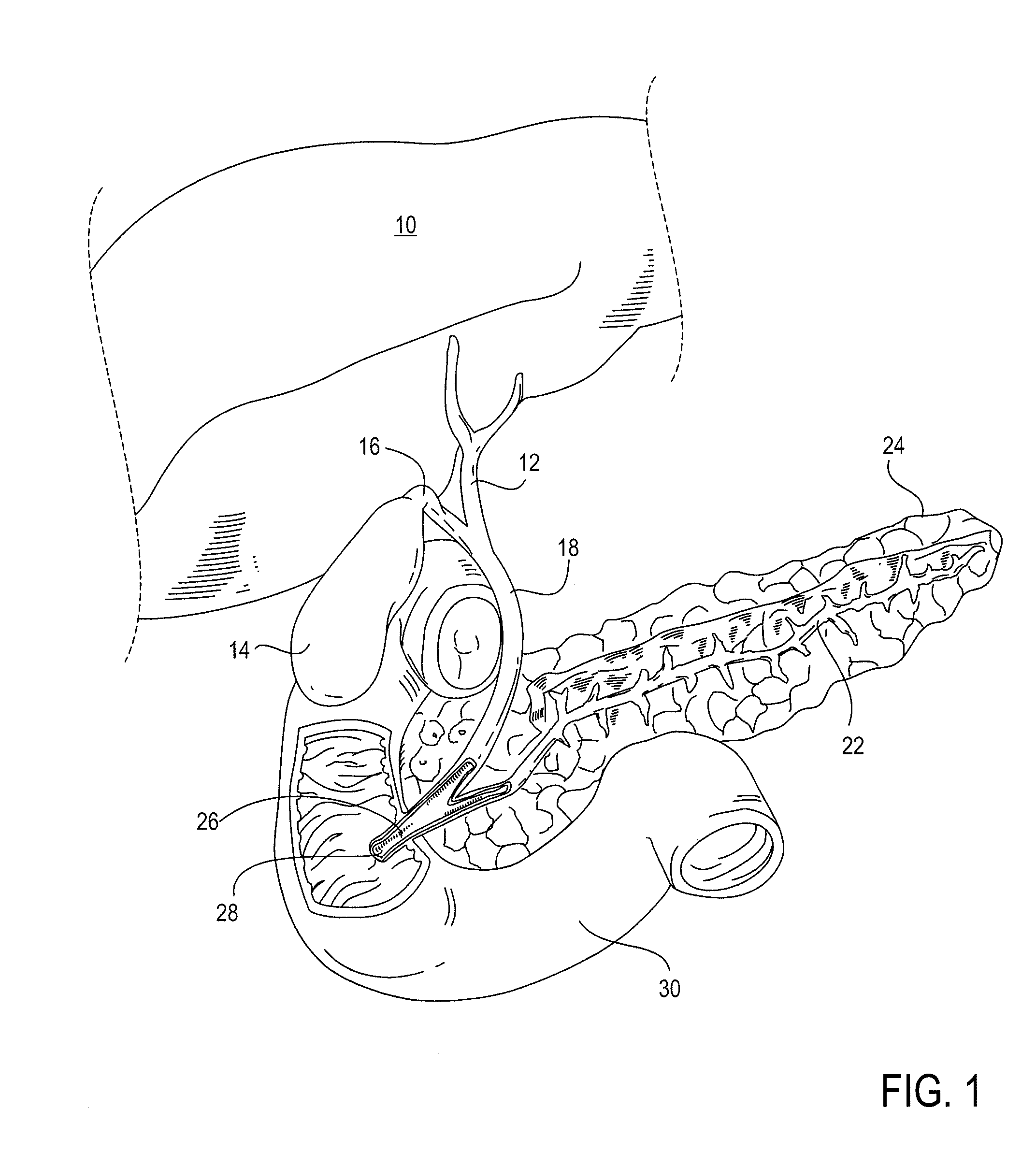
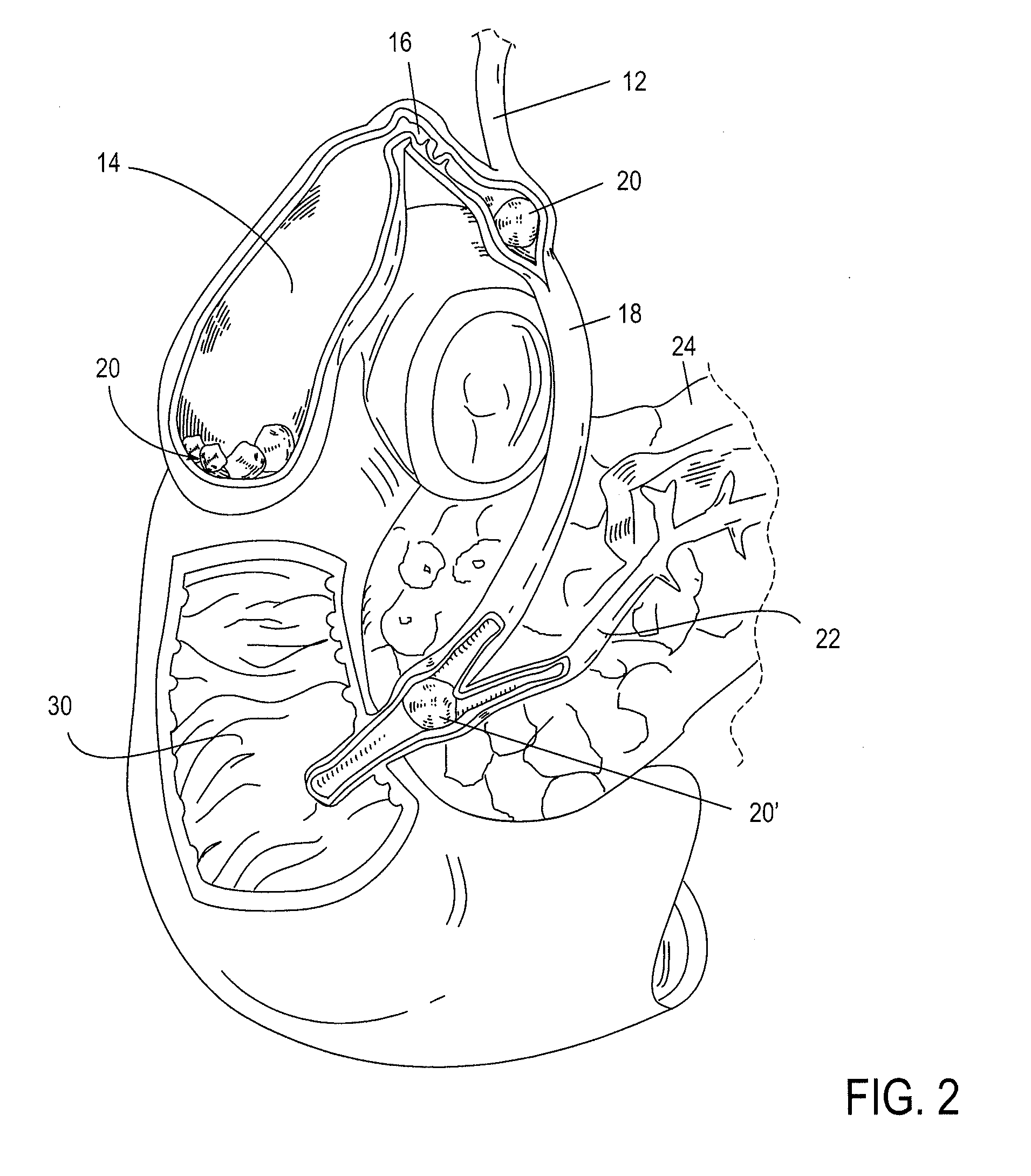
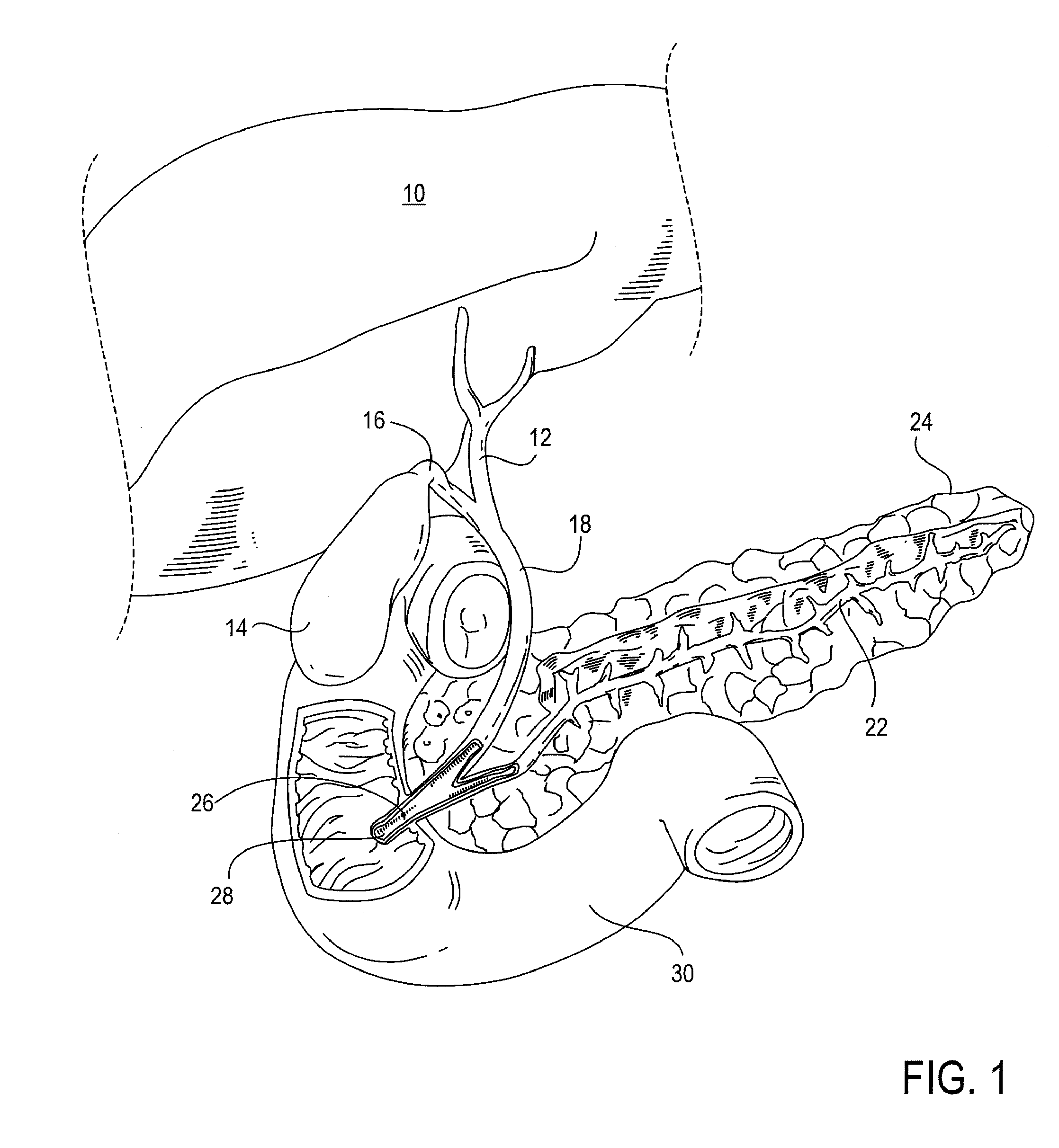
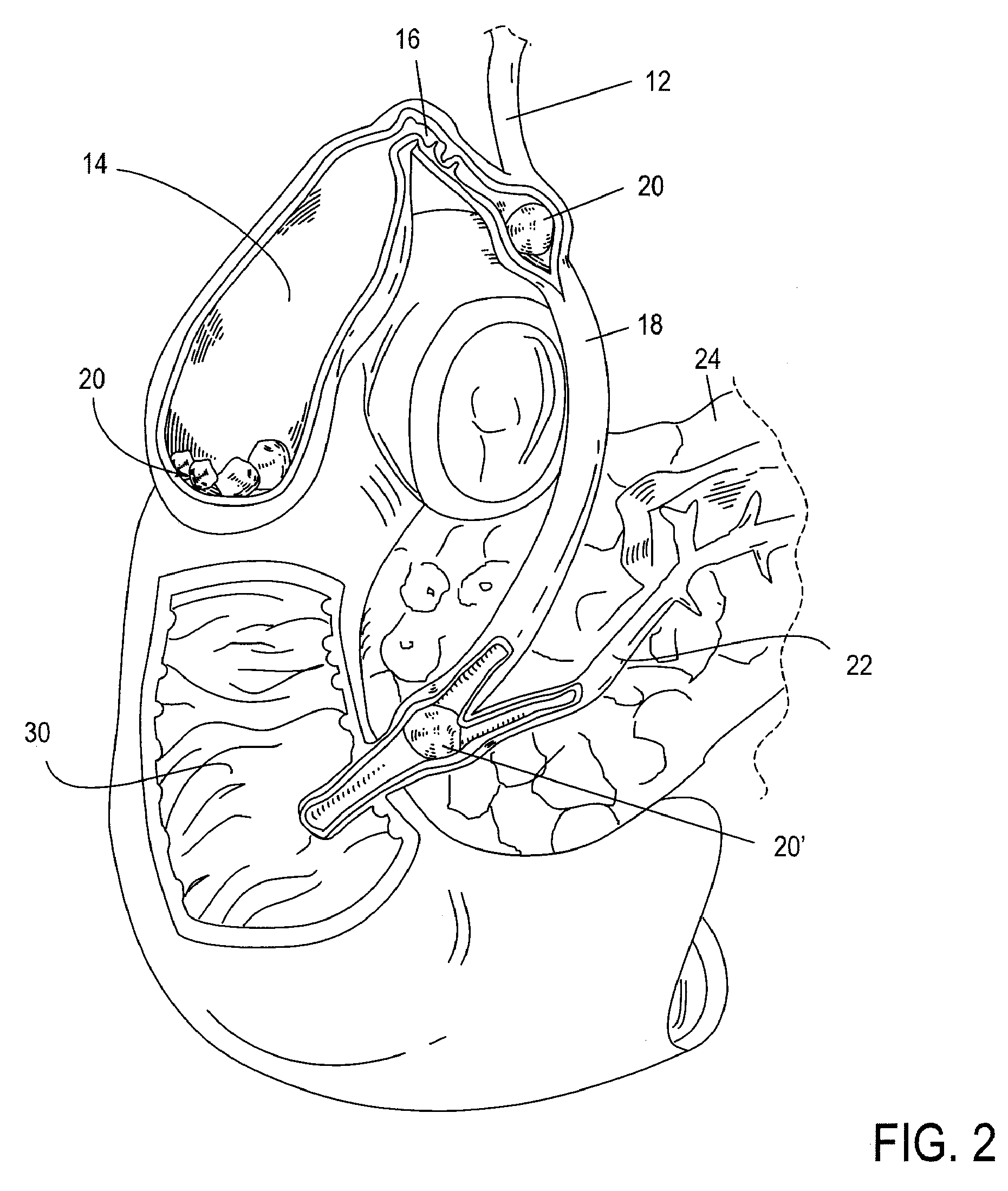
The biliary tract, (biliary tree or biliary system) refers to the liver, gall bladder and bile ducts, and how they work together to make, store and secrete bile. Bile consists of water, electrolytes, bile acids, cholesterol, phospholipids and conjugated bilirubin. Some components are synthesised by hepatocytes (liver cells), the rest are extracted from the blood by the liver.
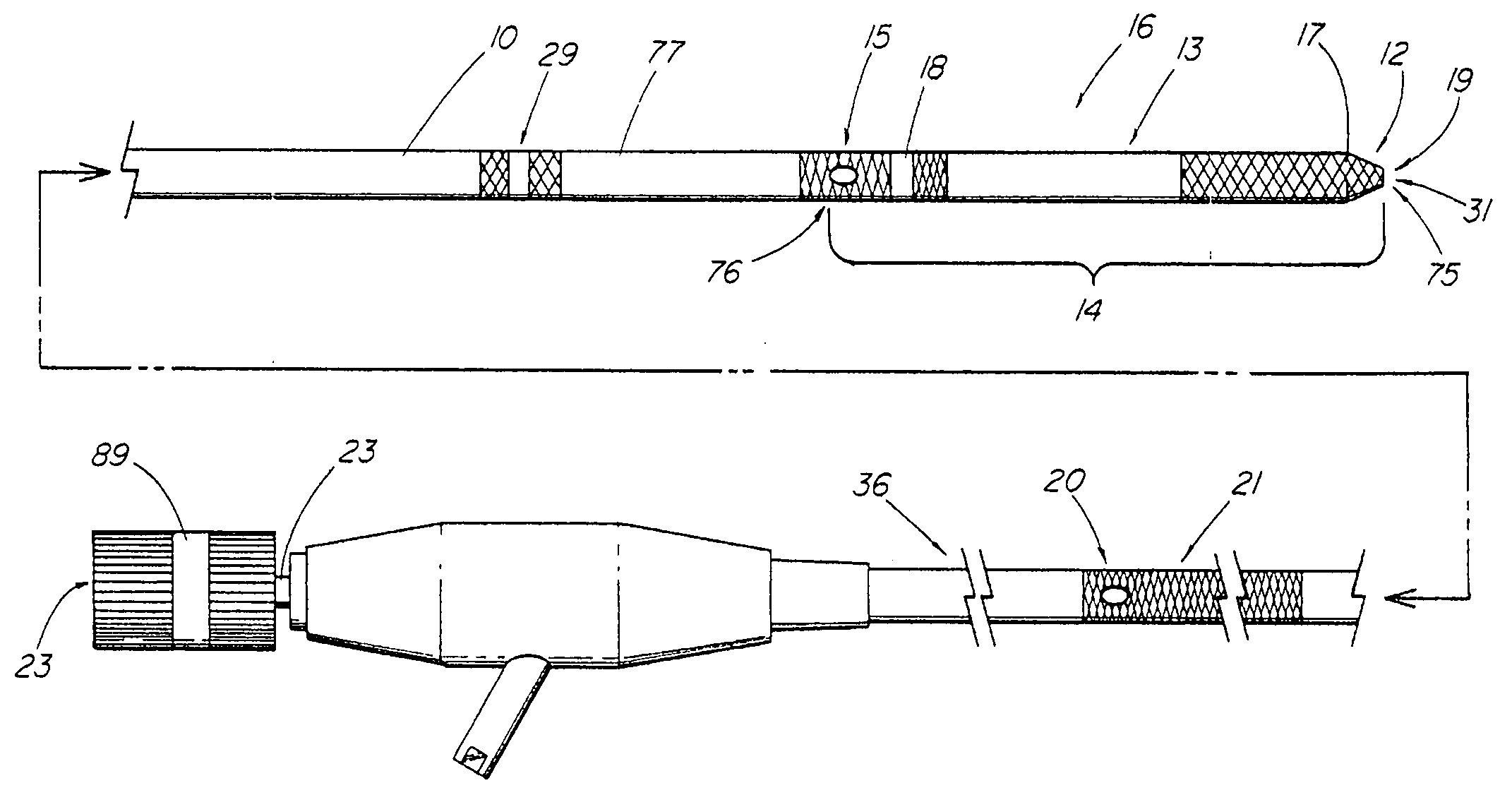
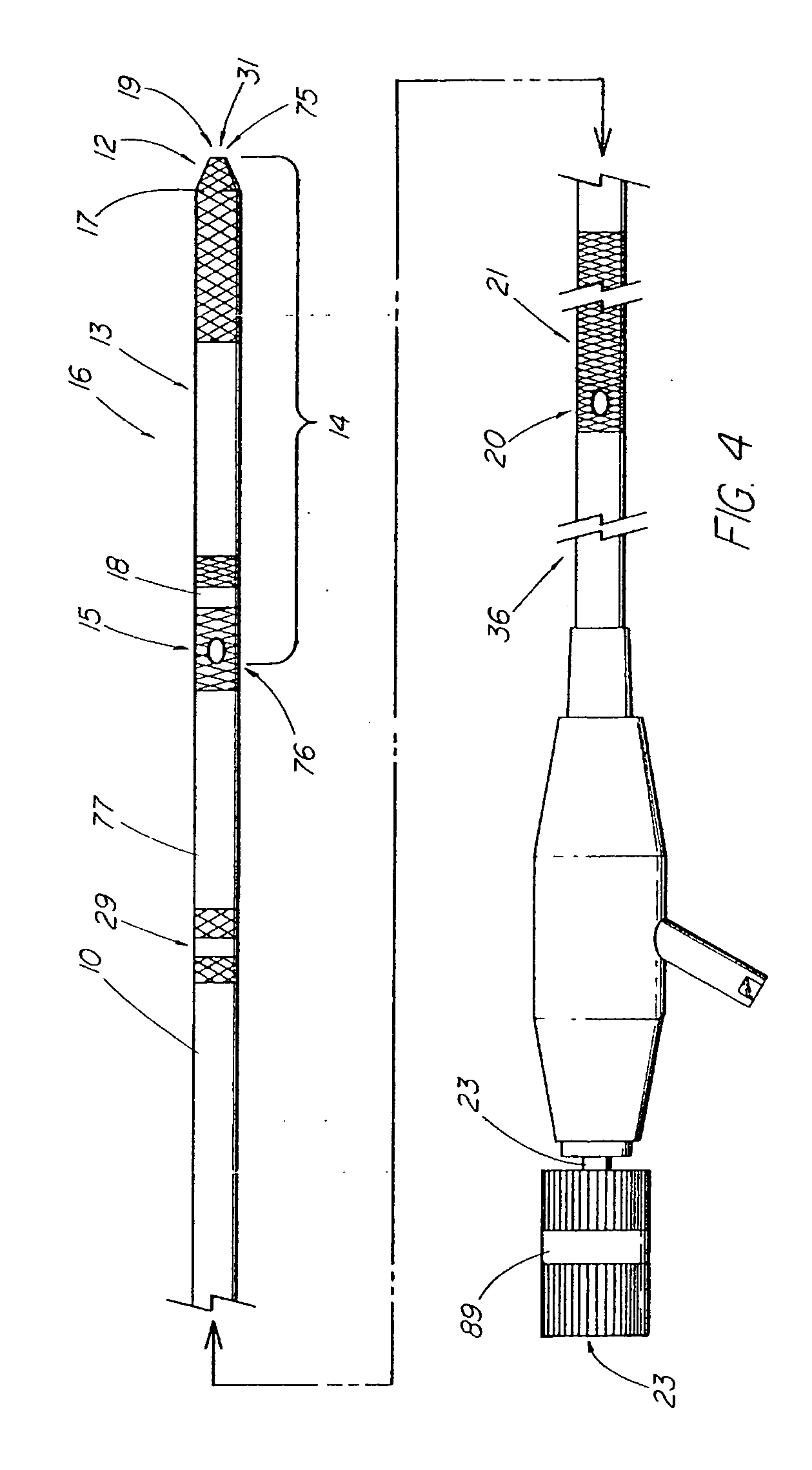
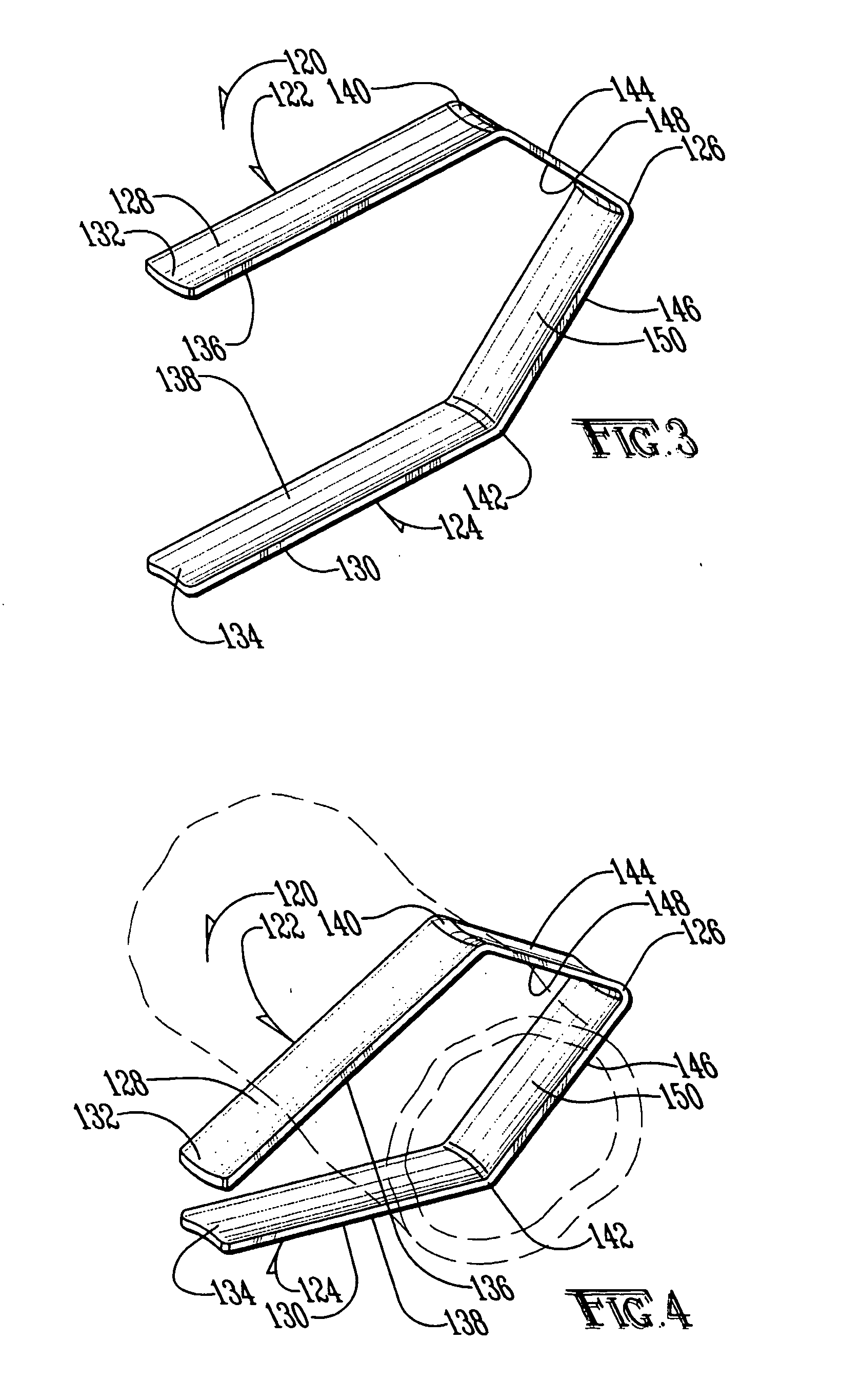
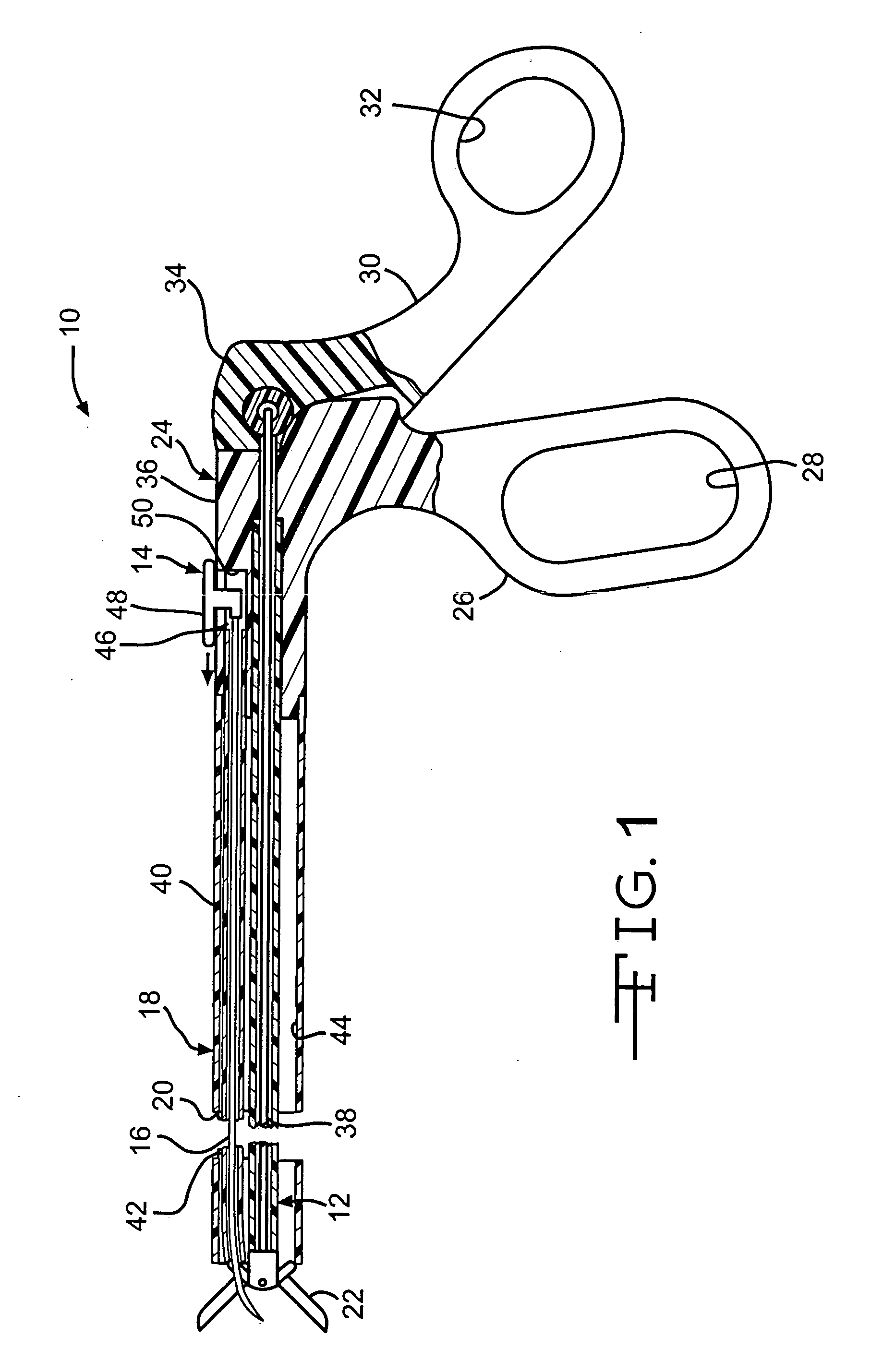
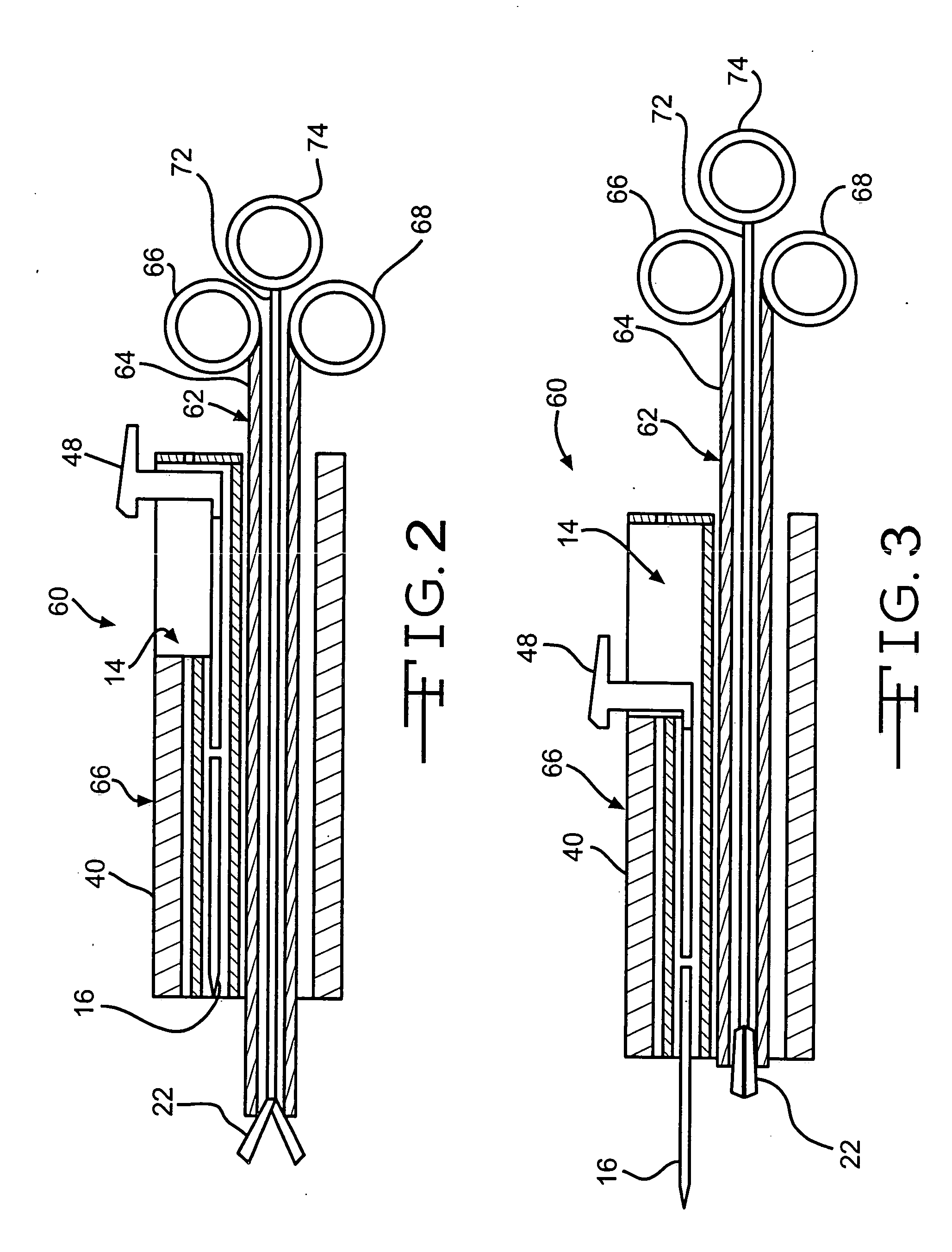
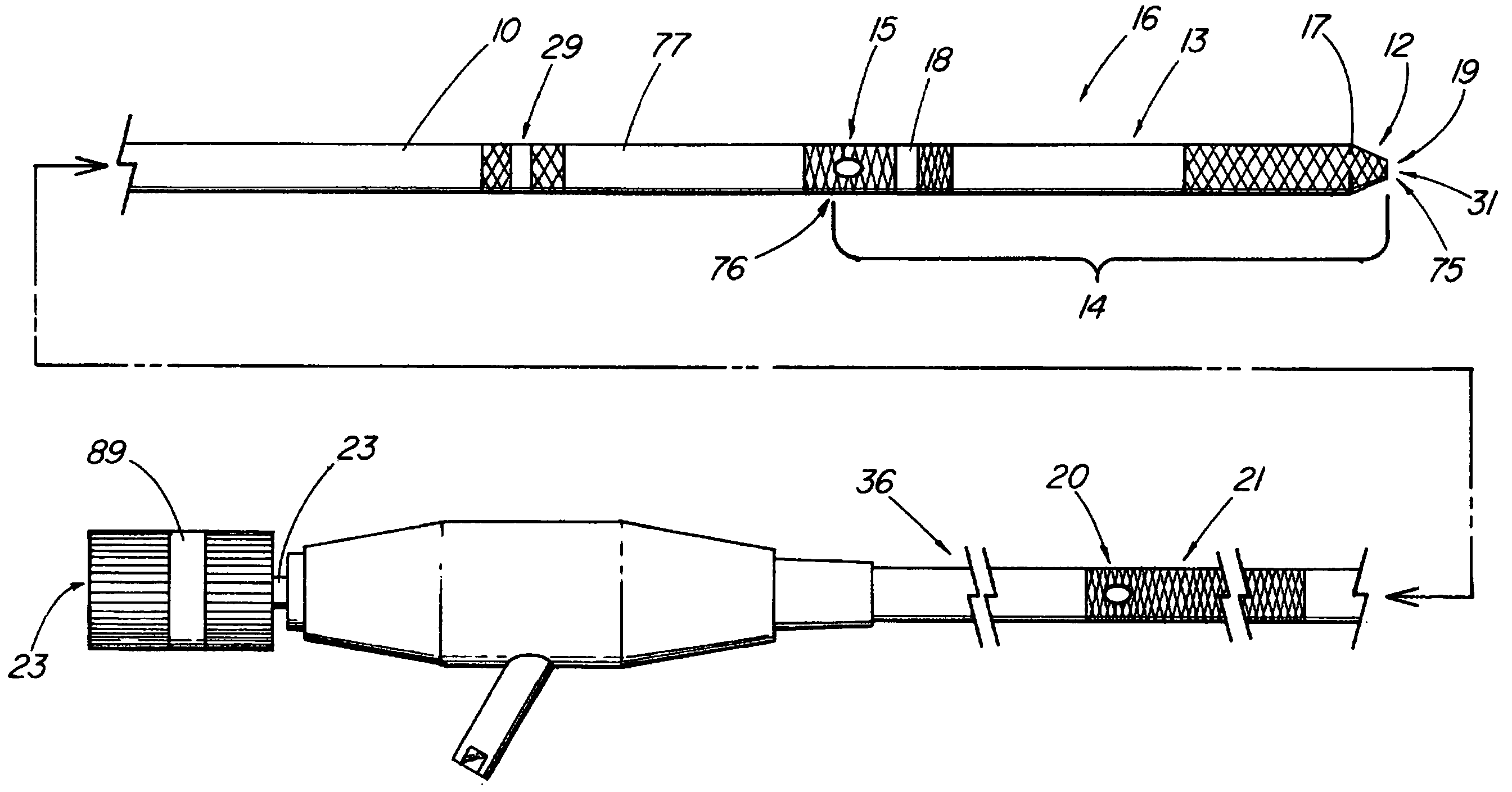
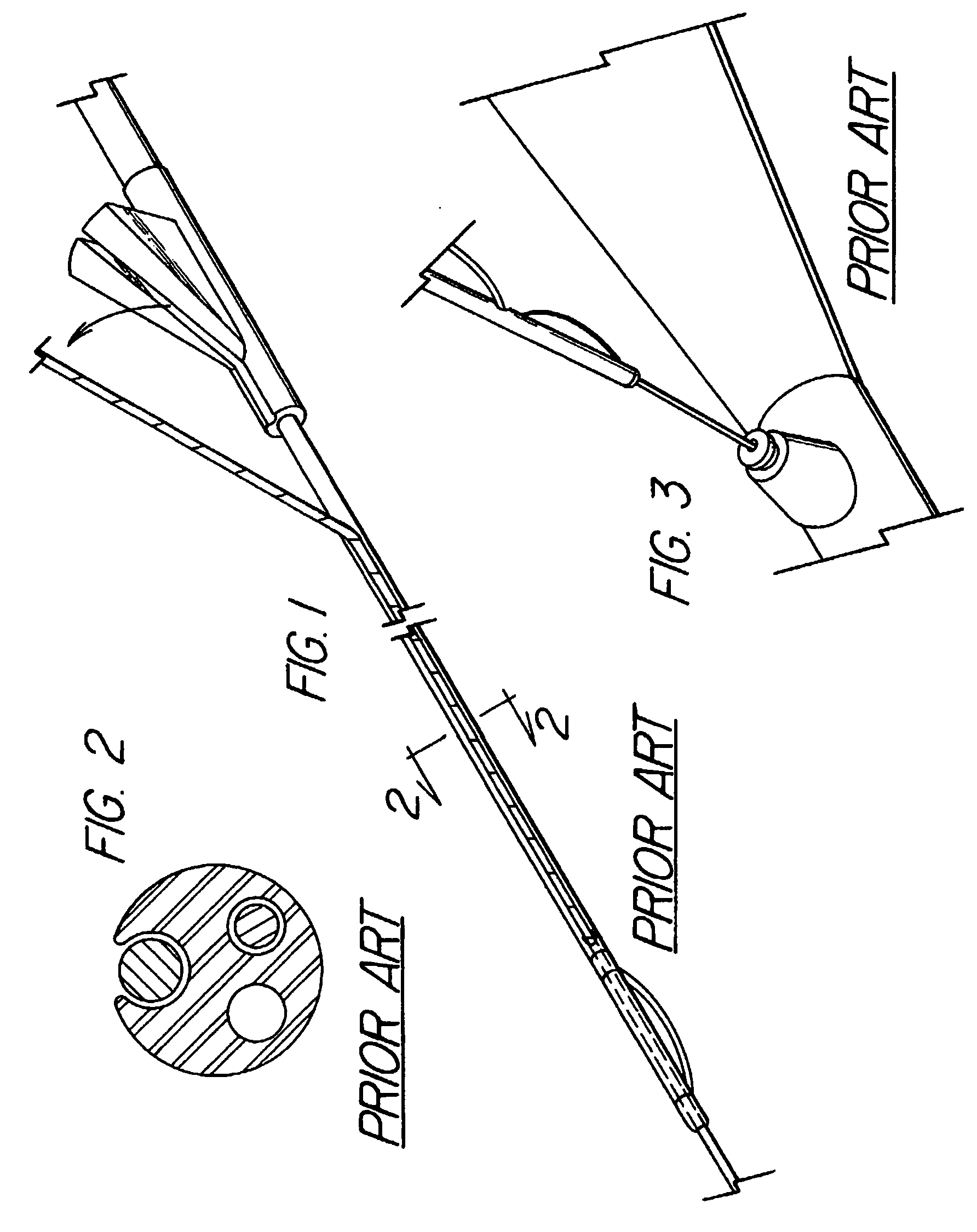
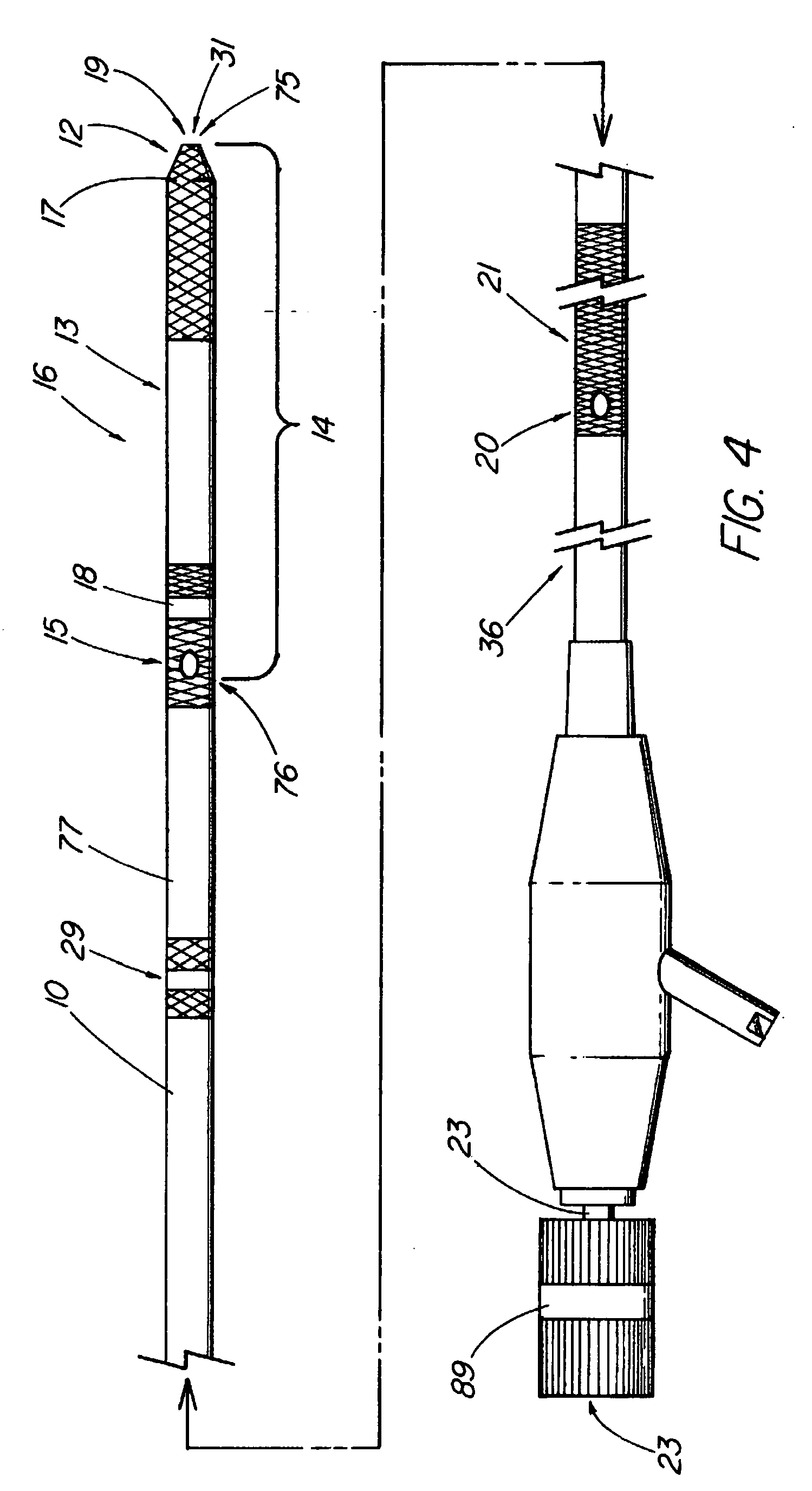
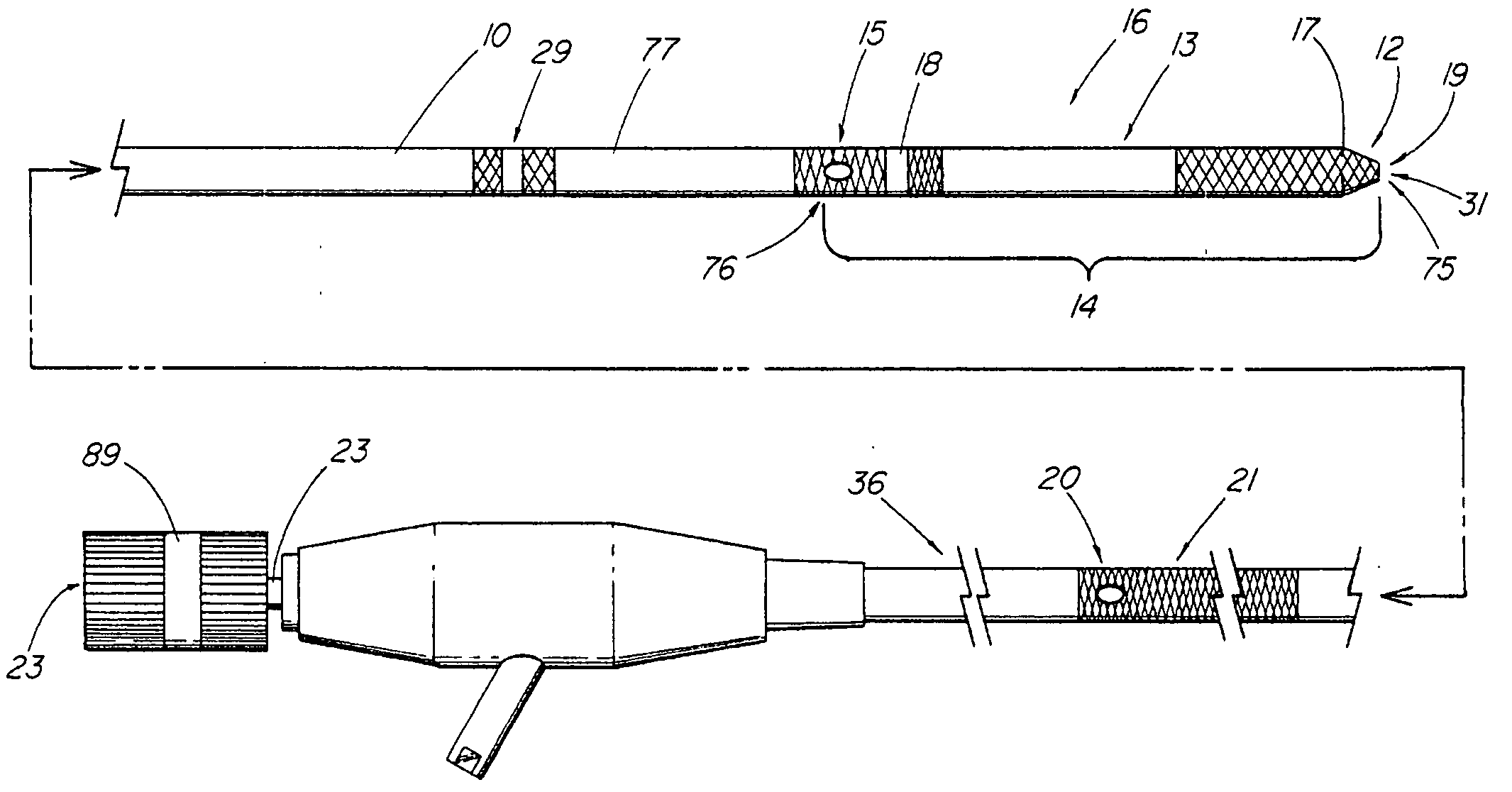
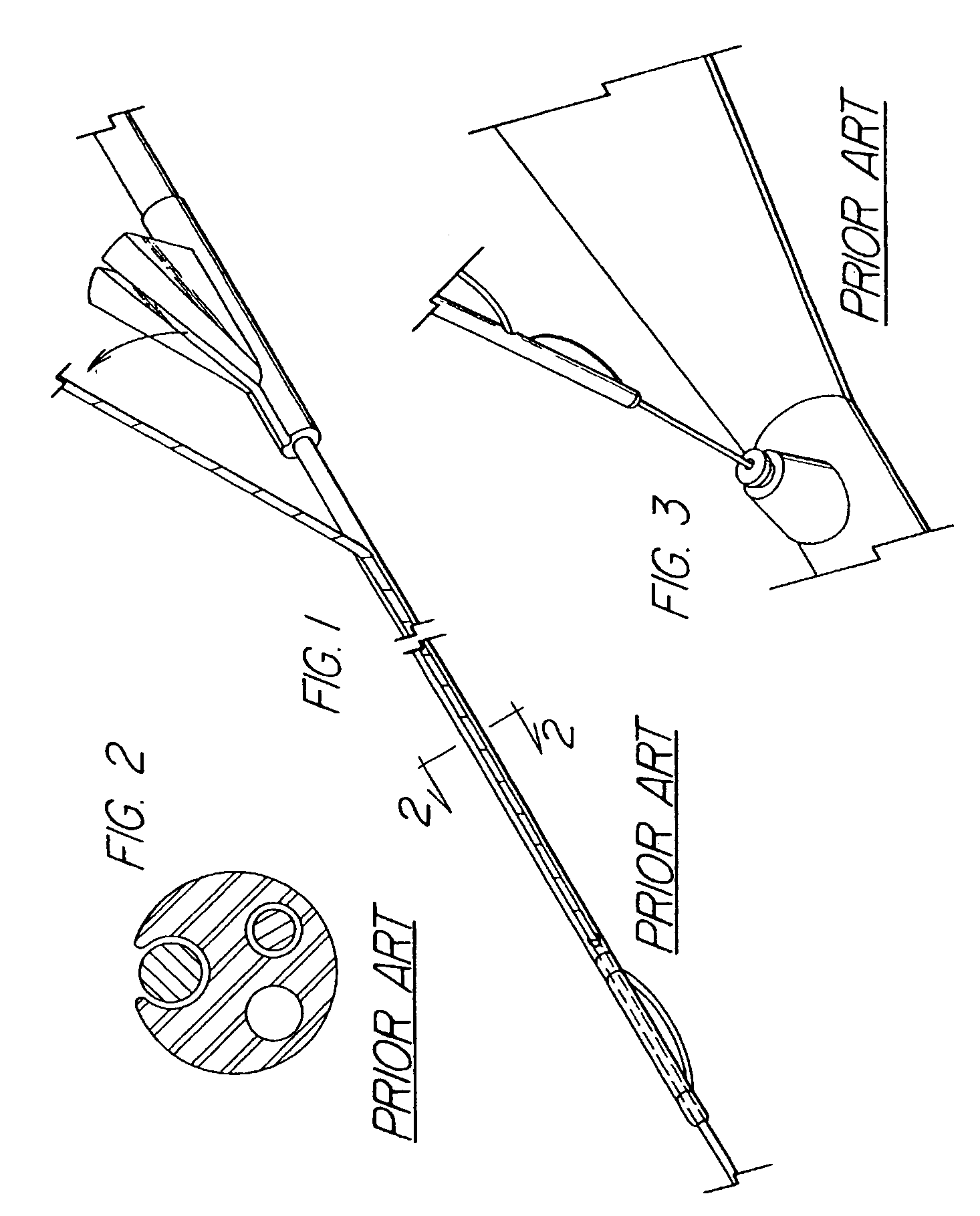
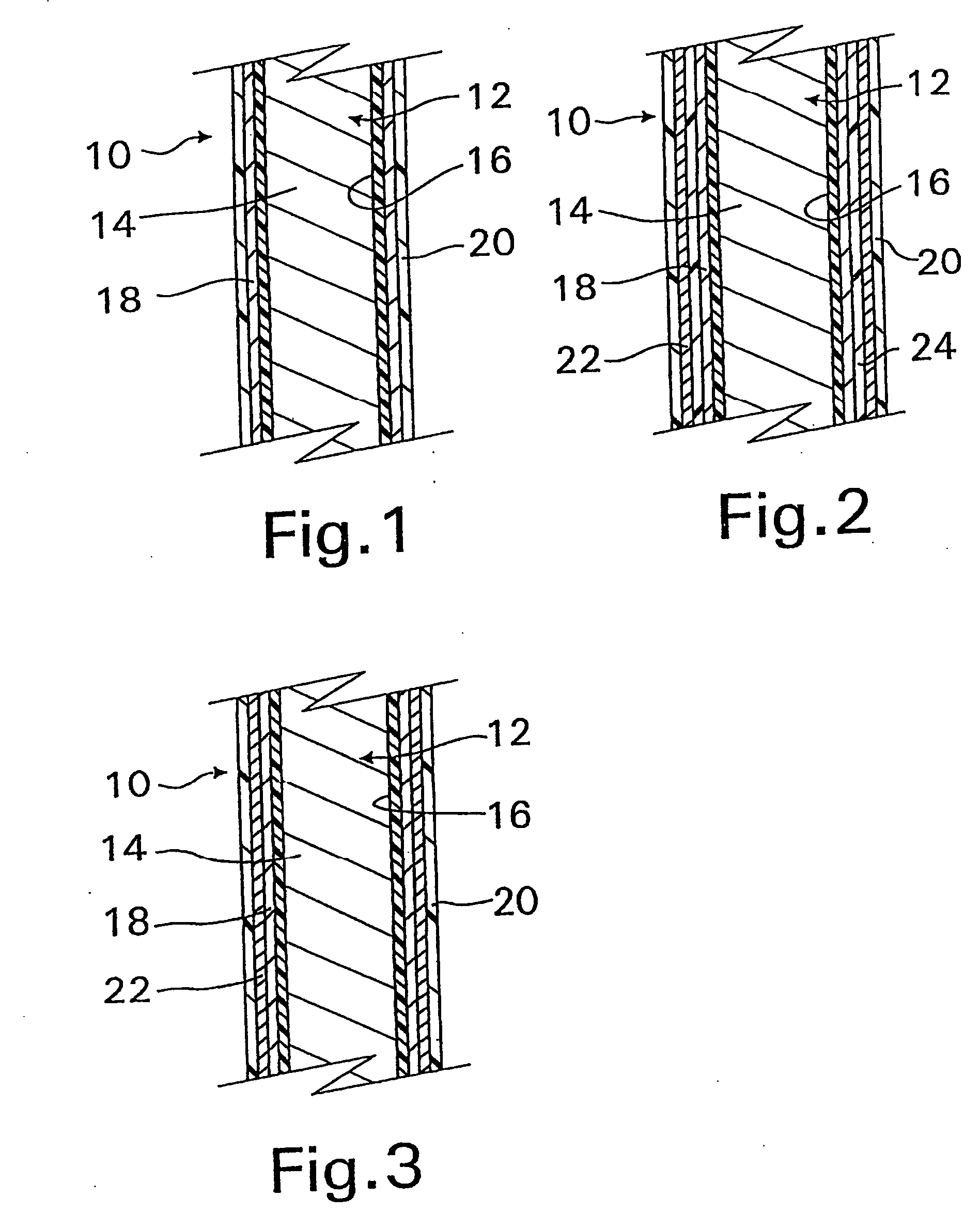
System and method for introducing multiple medical devices
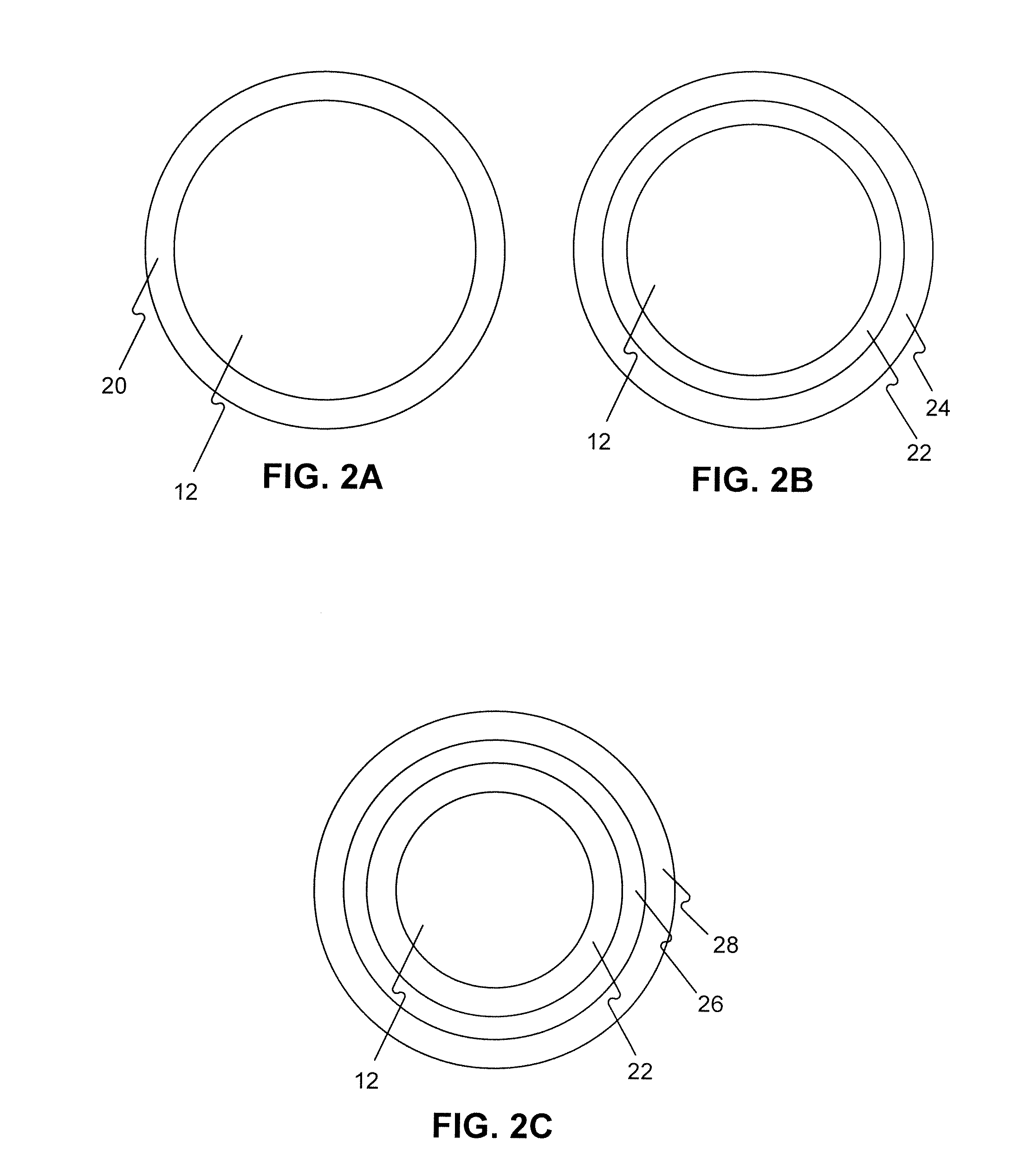
ActiveUS20050059990A1Timely controlSatisfy safety performance requirementsStentsGuide needlesDilatorBiliary tract
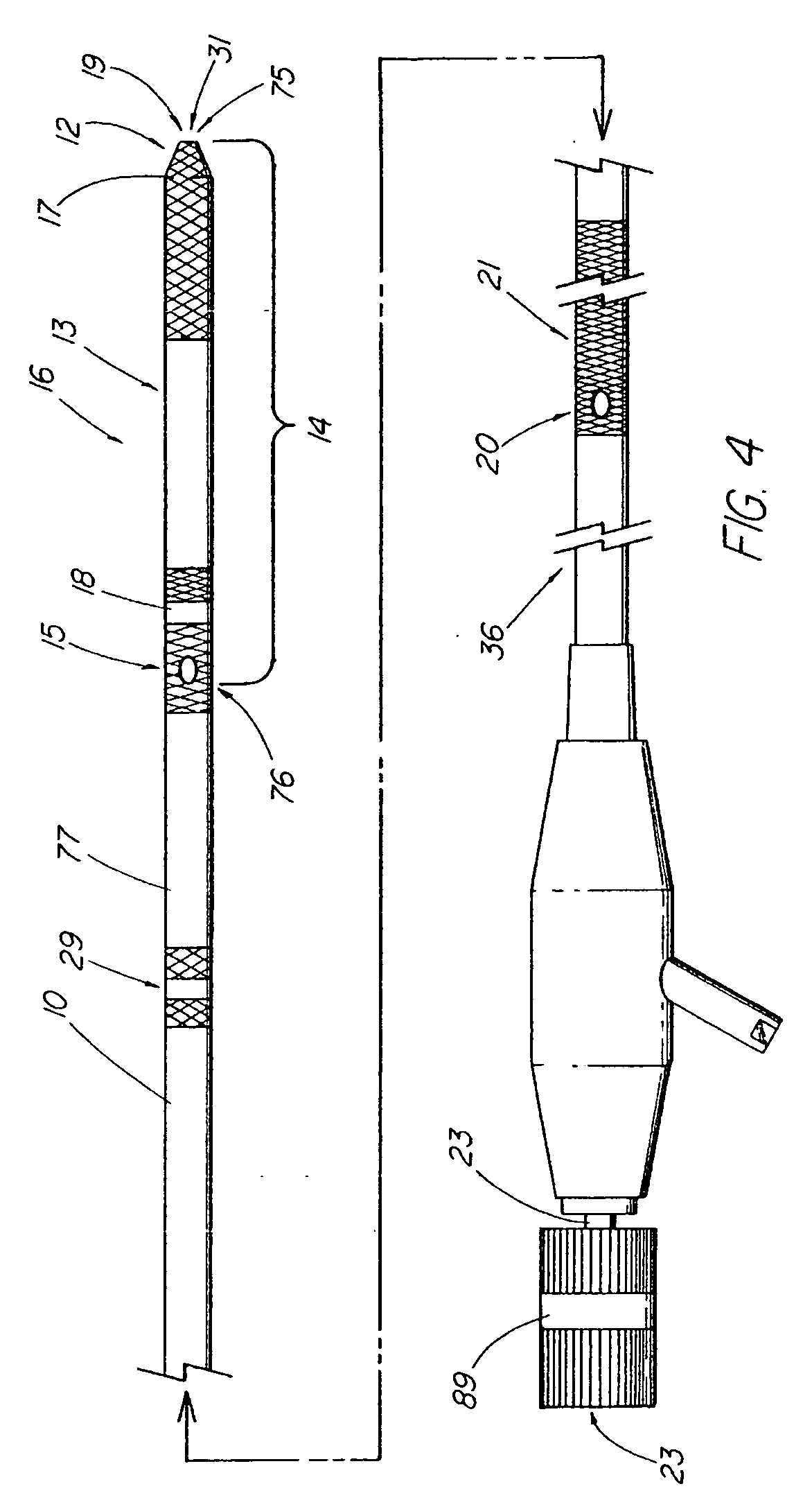
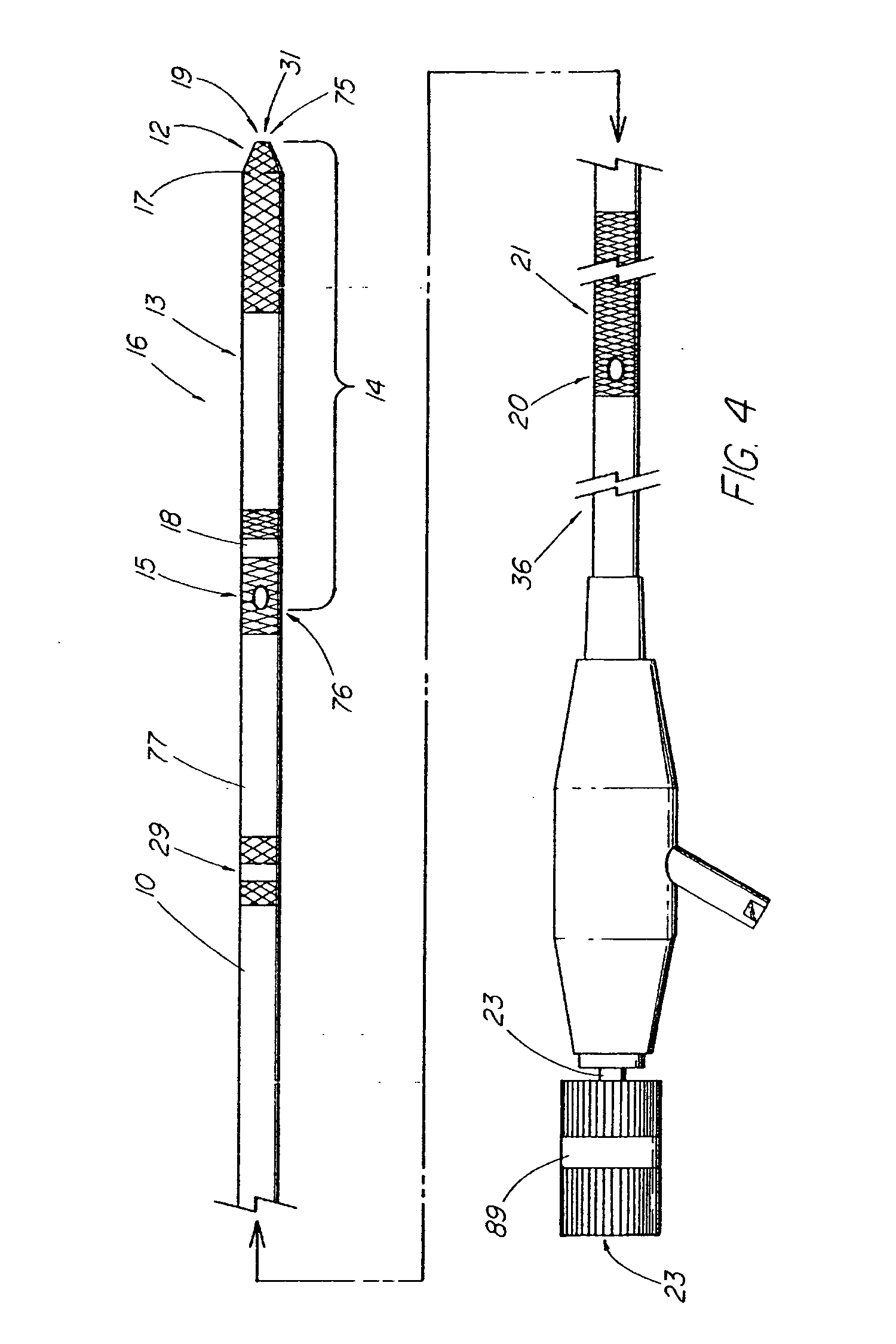
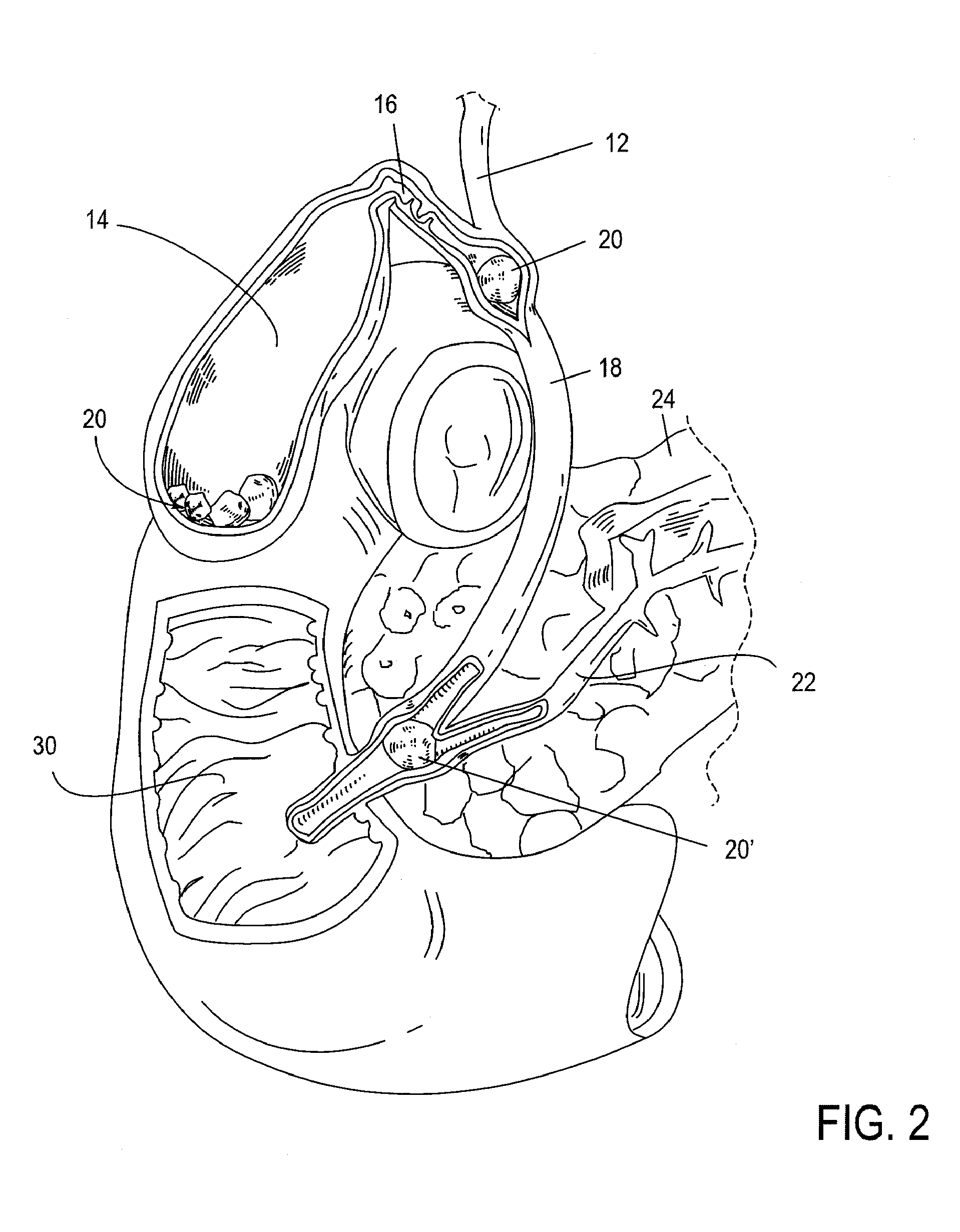
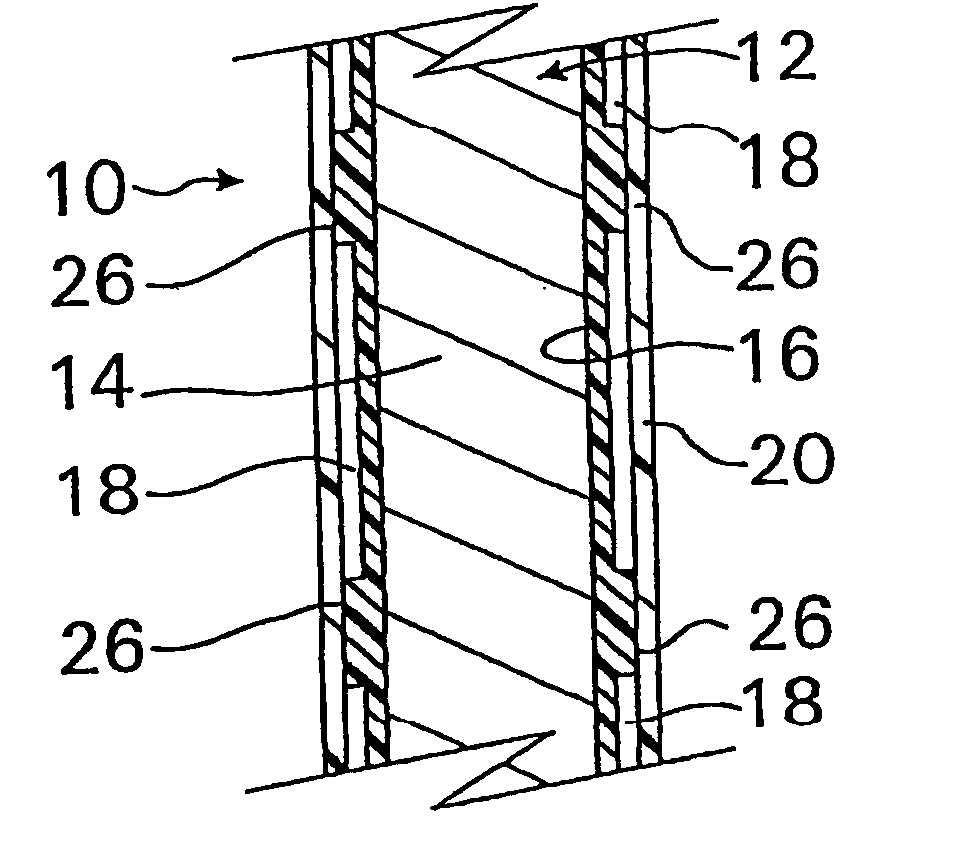
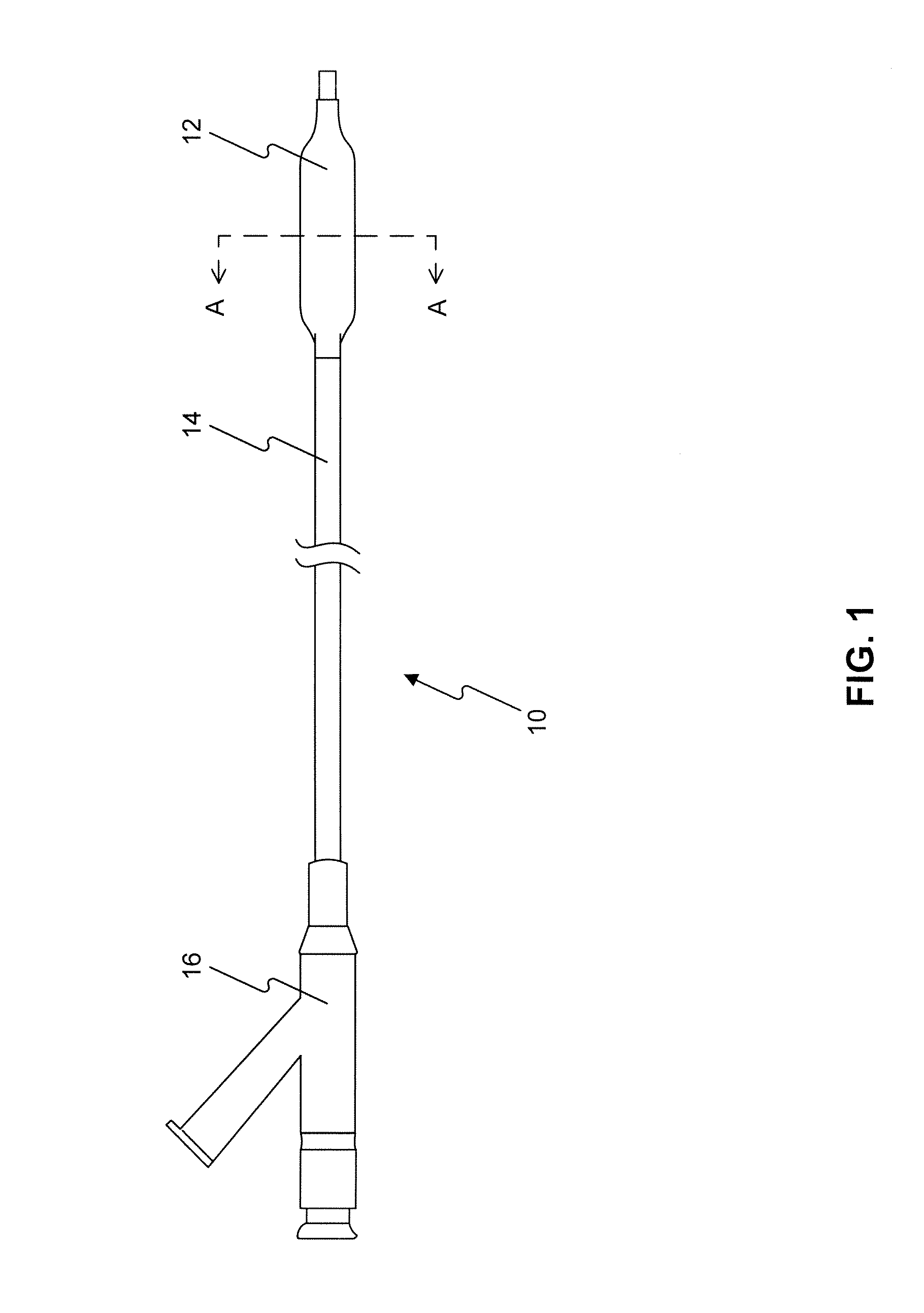
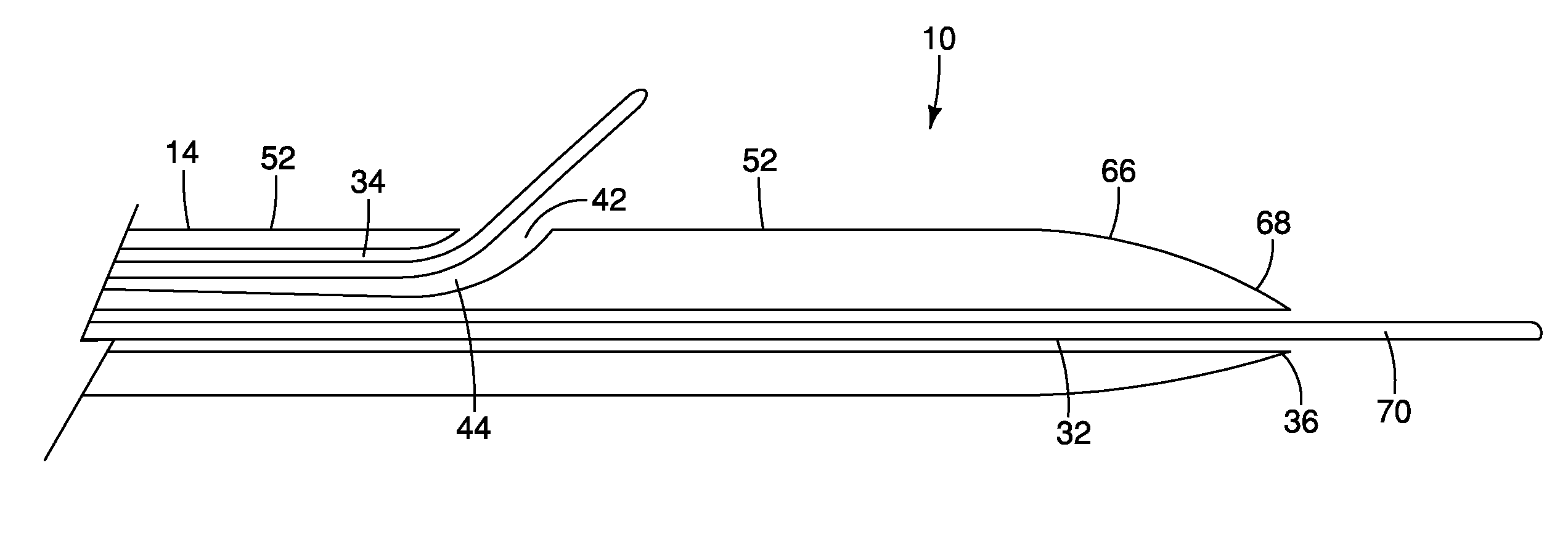
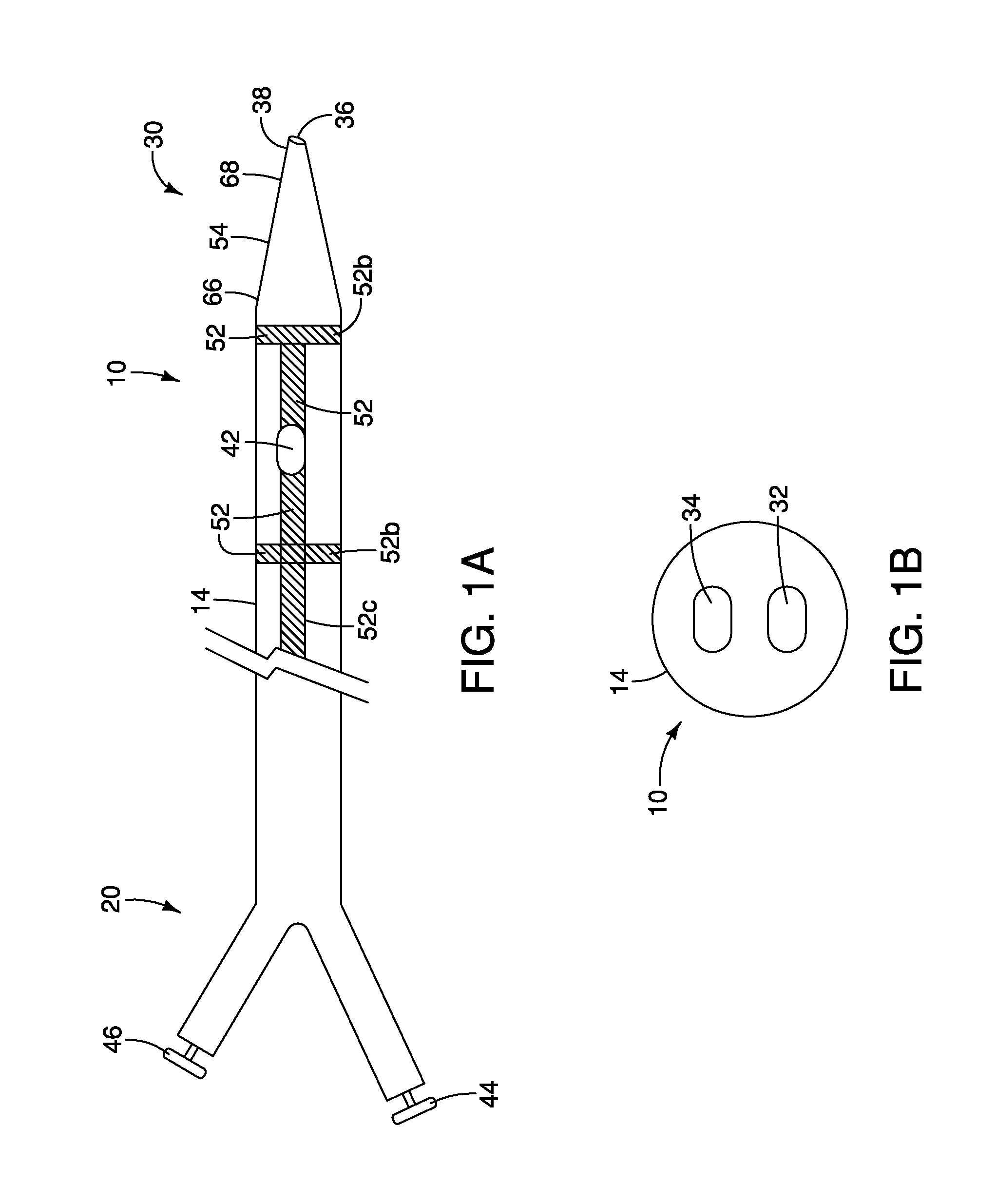
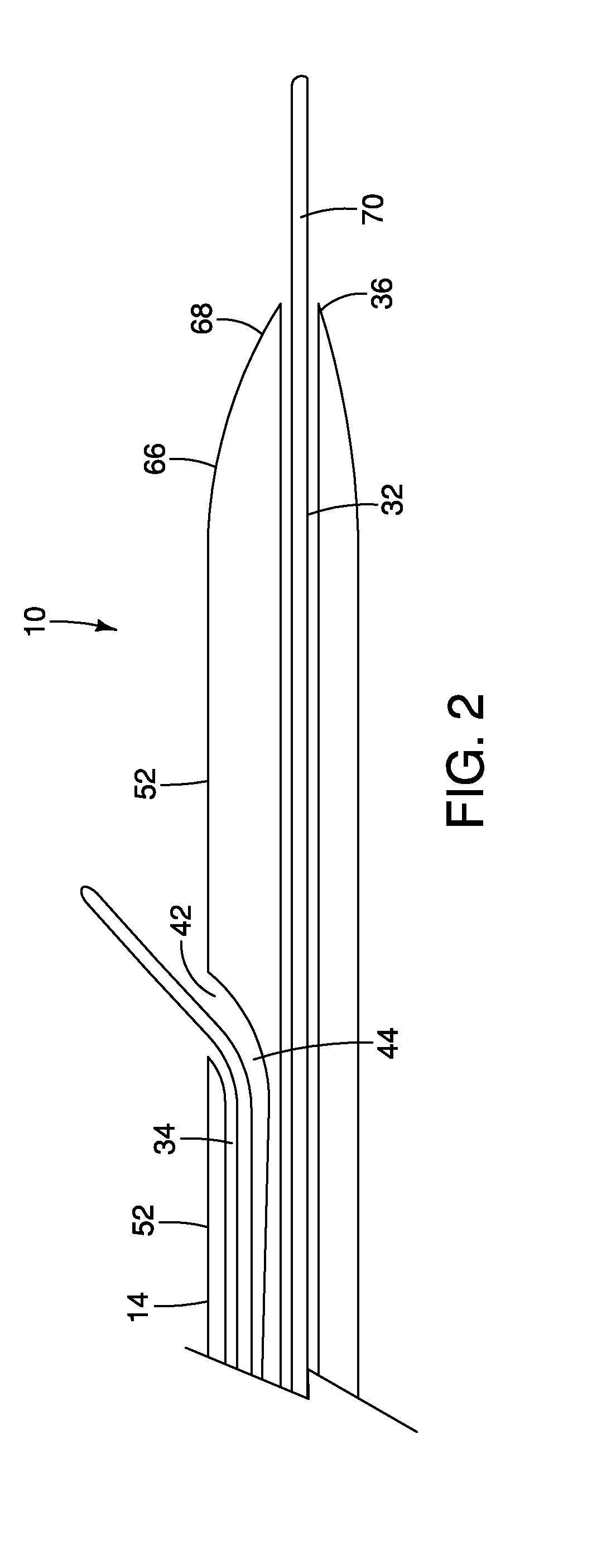
A method and apparatus for introducing a first elongate medical device and short wire guide that are coupled together into a work site and remotely disconnecting them within the work site such that a secondary device comprising a catheter member can be introduced over the wire guide to the work site, and / or a second wire guide can be introduced to the work site via a passageway of the primary access device. A separating member may be provided to remotely separate the wire guide from the elongate medical device. A system of indicia, such as radiopaque or viewable markers, permits the operator to monitor the relative alignment of the devices within the work site to determine when uncoupling has occurred. In one example of the method, a wire guide and primary access device (e.g., a sphincterotome) is coupled to the wire guide and introduced via a duodenoscope into the biliary system. After performing a first medical operation, the devices are uncoupled with the wire guide being left within the biliary system such that a secondary access device, such as a balloon, biopsy device, stent delivery catheter, dilator, etc., can be introduced to perform a second medical operation without a traditional over-the-wire exchange being required. In another example of the method, a prosthesis, such as a valve or stent, is placed within the work site coupled to a wire guide which is remotely disconnected within the work site and a secondary device, such as a dilation balloon or second prosthesis, is introduced into the work site after the first delivery system is removed.
Owner:COOK MEDICAL TECH LLC
Surgical marker clip and method for cholangiography
A surgical marker clip and method for enhancing the safe performance of a cholangiography and cholecystectomy is disclosed. The clips are configured to frictionally engage the outer surface of the duct and are retained in place by light clamping force without damaging the duct. Placement of the clips allows a physician to visually isolate the common bile duct from the cystic duct during laparoscopic procedures which reduces bile duct injury typically caused by misidentification or visual misperception of the anatomy during the procedure.
Owner:DUFF MICHAEL
Device and method for intralumenal anastomosis
Owner:ETHICON ENDO SURGERY INC
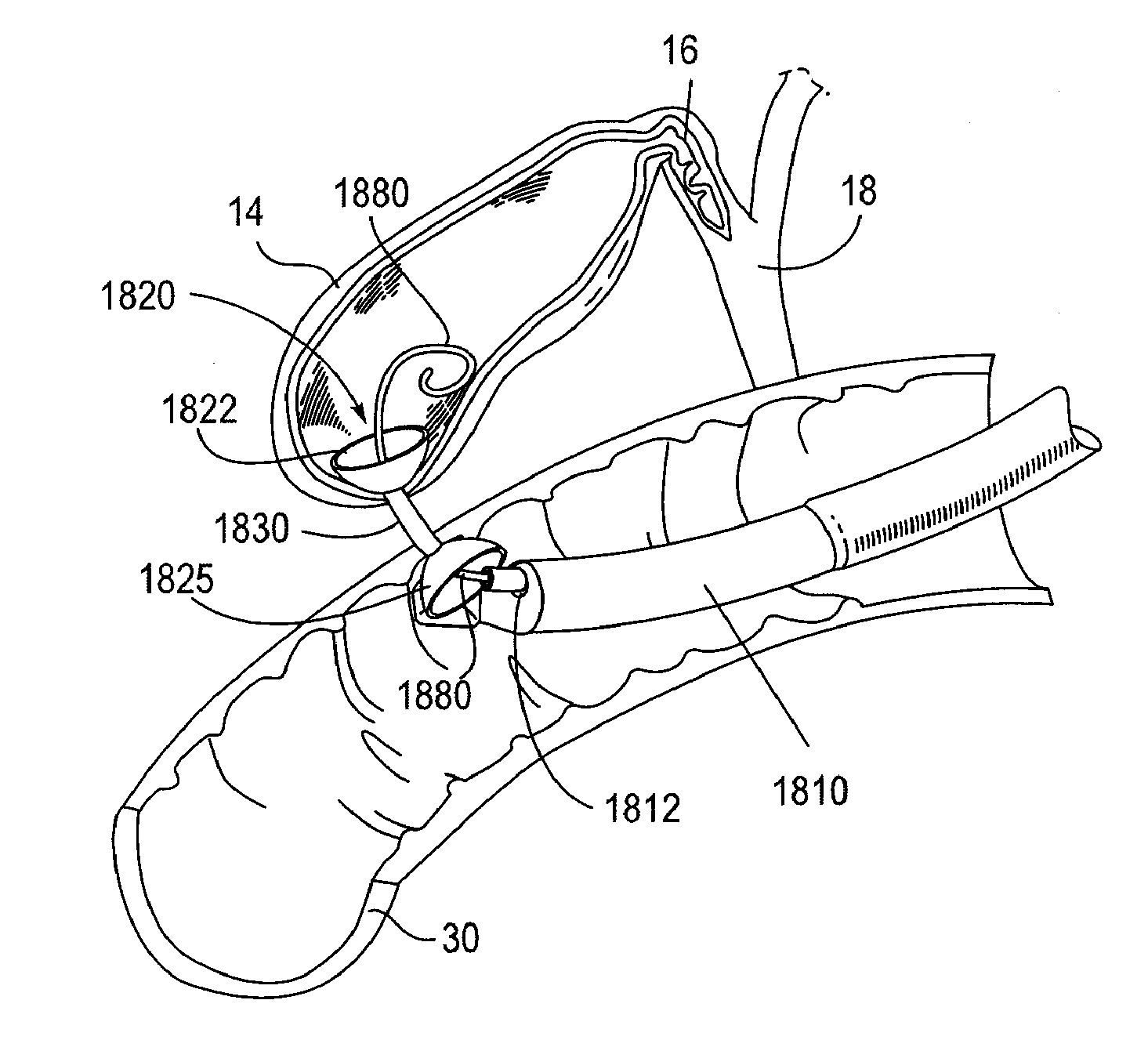
Biliary Shunts, Delivery Systems, Methods of Using the Same and Kits Therefor
InactiveUS20090143713A1Adjustable lengthPrevent leakageBalloon catheterHeart valvesDiseaseBiliary tract
The application discloses devices, systems, kits and methods for treating biliary disease. Device comprise, for example, a component configured for deployment between a gallbladder and location within a gastrointestinal tract of a patient which has a proximal end and a distal end with a lumen extending therethrough. A method of deploying the device can be achieved by, for example, creating a duct or fistula between a gallbladder lumen and a portion of a gastrointestinal tract; and providing for drainage from the gallbladder to the gastrointestinal tract.
Owner:TREUS MEDICAL
System and method for introducing multiple medical devices
A method and apparatus for introducing a first elongate medical device and short wire guide that are coupled together into a work site and remotely disconnecting them within the work site such that a secondary device comprising a catheter member can be introduced over the wire guide to the work site, and / or a second wire guide can be introduced to the work site via a passageway of the primary access device. A system of indicia, such as radiopaque or viewable markers, permit the operator to monitor the relative alignment of the devices within the work site to determine when uncoupling has occurred. In one example of the method, a wire guide and primary access device (e.g., a sphincterotome) is coupled to the wire guide and introduced via a duodenoscope into the biliary system. After performing a first medical operation, the devices are uncoupled with the wire guide being left within the biliary system such that a secondary access device, such as a balloon, biopsy device, stent delivery catheter, dilator, etc., can be introduced to perform a second medical operation without a traditional over-the-wire exchange being required. In another example of the method, a prosthesis, such as a valve or stent, is placed within the work site coupled to a wire guide which is remotely disconnected within the work site and a secondary device, such as a dilation balloon or second prosthesis, is introduced into the work site after the first delivery system is removed.
Owner:COOK MEDICAL TECH LLC +1
System for introducing multiple medical devices
InactiveUS20050070794A1Timely controlSatisfy safety performance requirementsStentsGuide needlesDilatorBiliary tract
Owner:WILSONCOOK MEDICAL
System and method for introducing a prosthesis
InactiveUS20050070821A1Timely controlSatisfy safety performance requirementsStentsGuide needlesDilatorBiliary tract
A method and apparatus for introducing a first elongate medical device and short wire guide that are coupled together into a work site and remotely disconnecting them within the work site such that a secondary device comprising a catheter member can be introduced over the wire guide to the work site, and / or a second wire guide can be introduced to the work site via a passageway of the primary access device. A system of indicia, such as radiopaque or viewable markers, permits the operator to monitor the relative alignment of the devices within the work site to determine when uncoupling has occurred. In one example of the method, a wire guide and primary access device (e.g., a sphincterotome) is coupled to the wire guide and introduced via a duodenoscope into the biliary system. After performing a first medical operation, the devices are uncoupled with the wire guide being left within the biliary system such that a secondary access device, such as a balloon, biopsy device, stent delivery catheter, dilator, etc., can be introduced to perform a second medical operation without a traditional over-the-wire exchange being required. In another example of the method, a prosthesis, such as a valve or stent, is placed within the work site coupled to a wire guide which is remotely disconnected within the work site and a secondary device, such as a dilation balloon or second prosthesis, is introduced into the work site after the first delivery system is removed.
Owner:WILSONCOOK MEDICAL
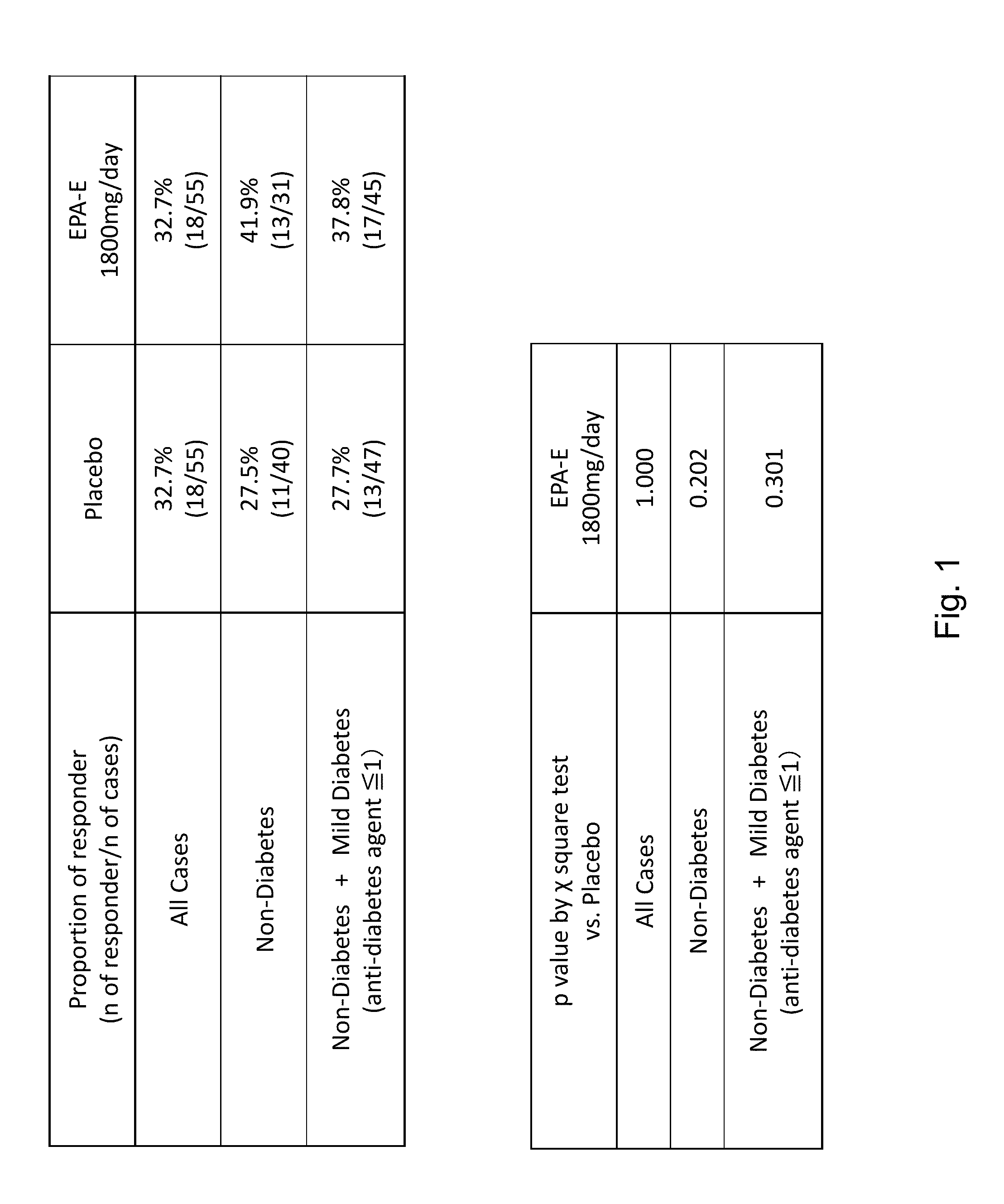
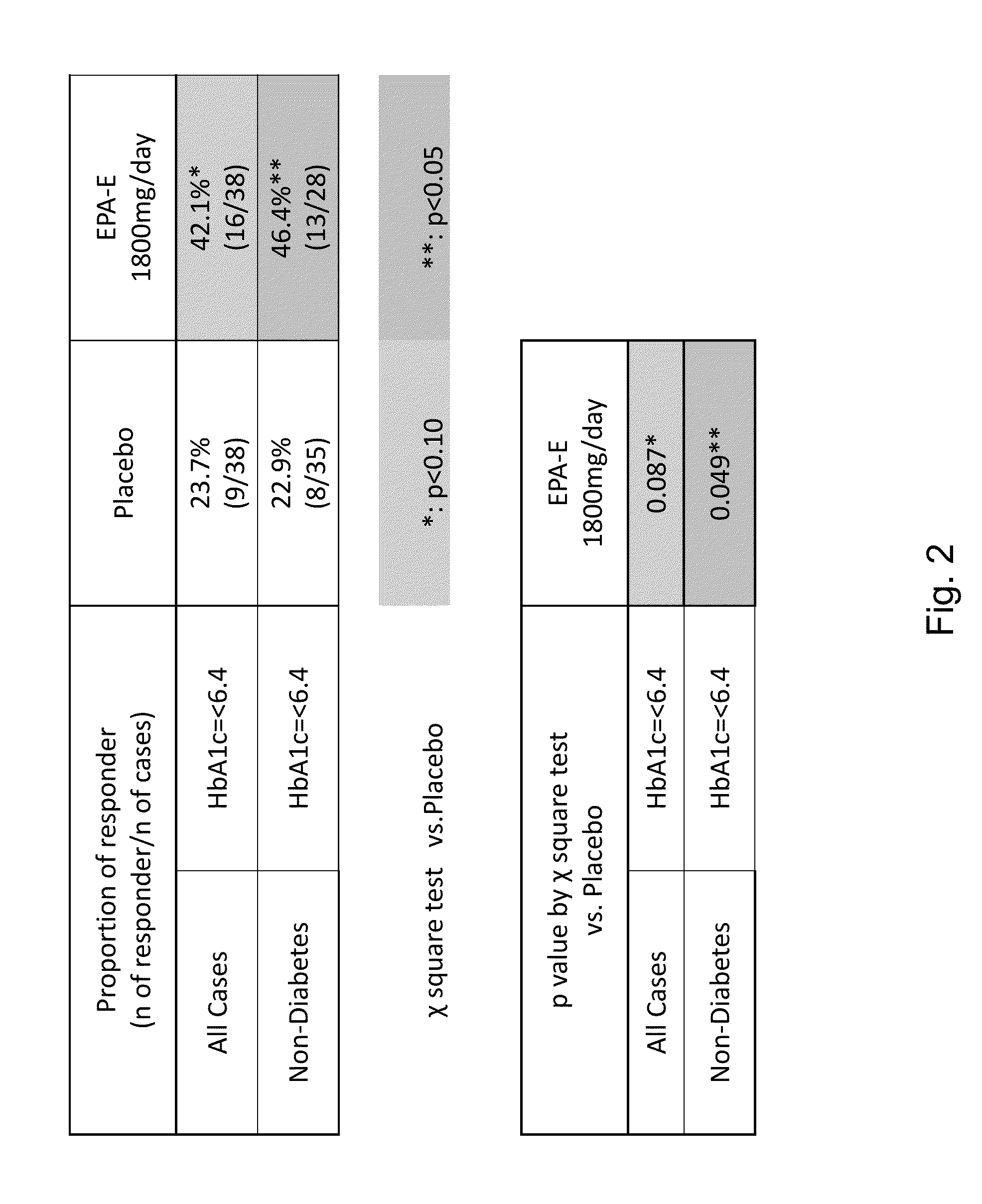
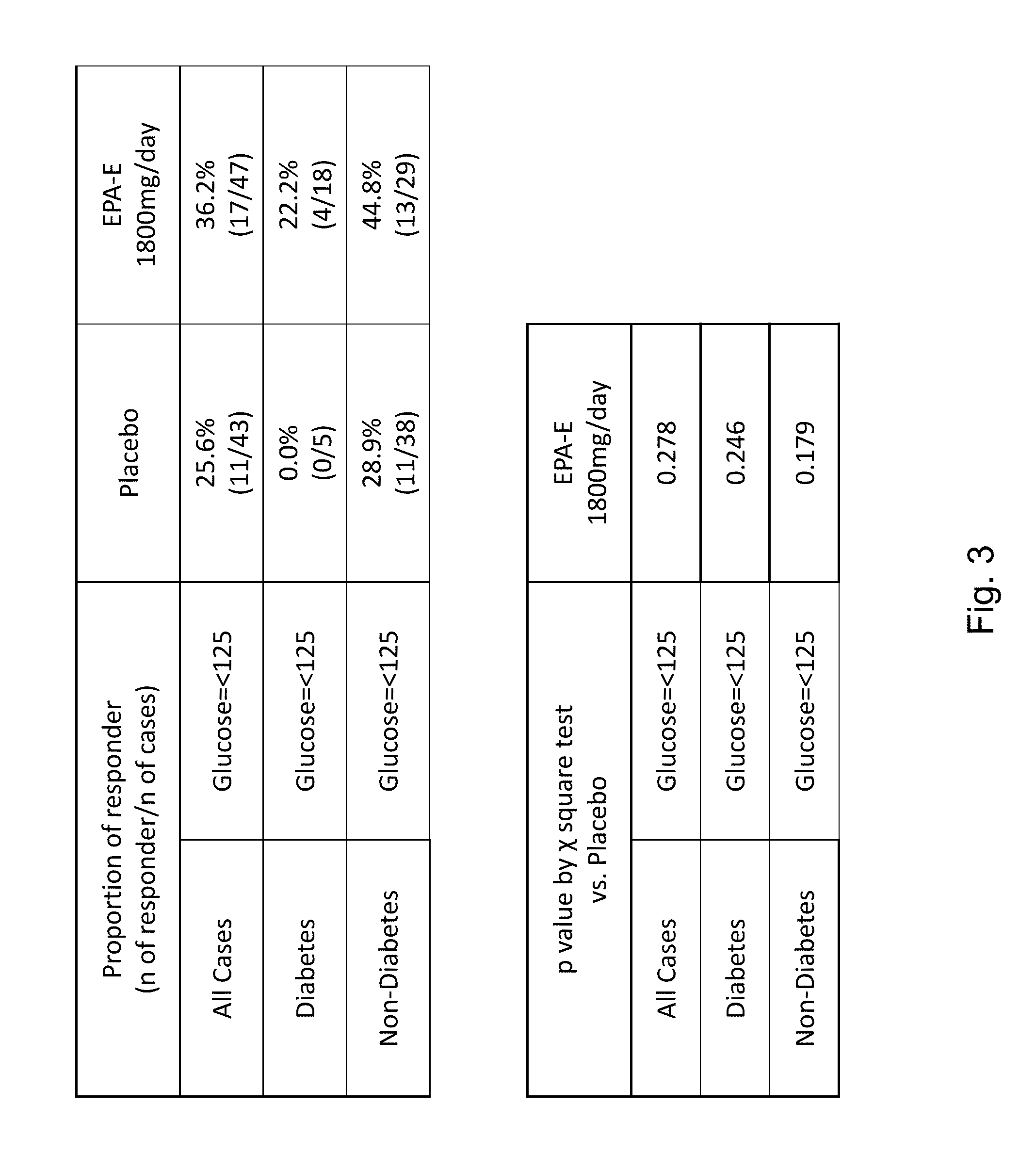
Compositions and methods for treating non-alcoholic steatohepatitis
ActiveUS20150051143A1Improve steatosisImproving lobular inflammation conditionBiocidePeptide/protein ingredientsBiliary tractFatty acid
The disclosure provides for a method for treating a fatty liver disorder in a subject in need thereof, comprising selecting a subject having or suspected of having a fatty liver disease or disorder, wherein the subject is non diabetic, pre-diabetic, mildly diabetic, or has normal or substantially normal biliary tract function; and administering a therapeutically effective amount of a pharmaceutical composition comprising ethyl eicosapentanoate (EPA-E). In some cases EPA-E present may be at least 40% by weight in total of the fatty acids and their derivatives.
Owner:AMARIN PHARMA IRELAND
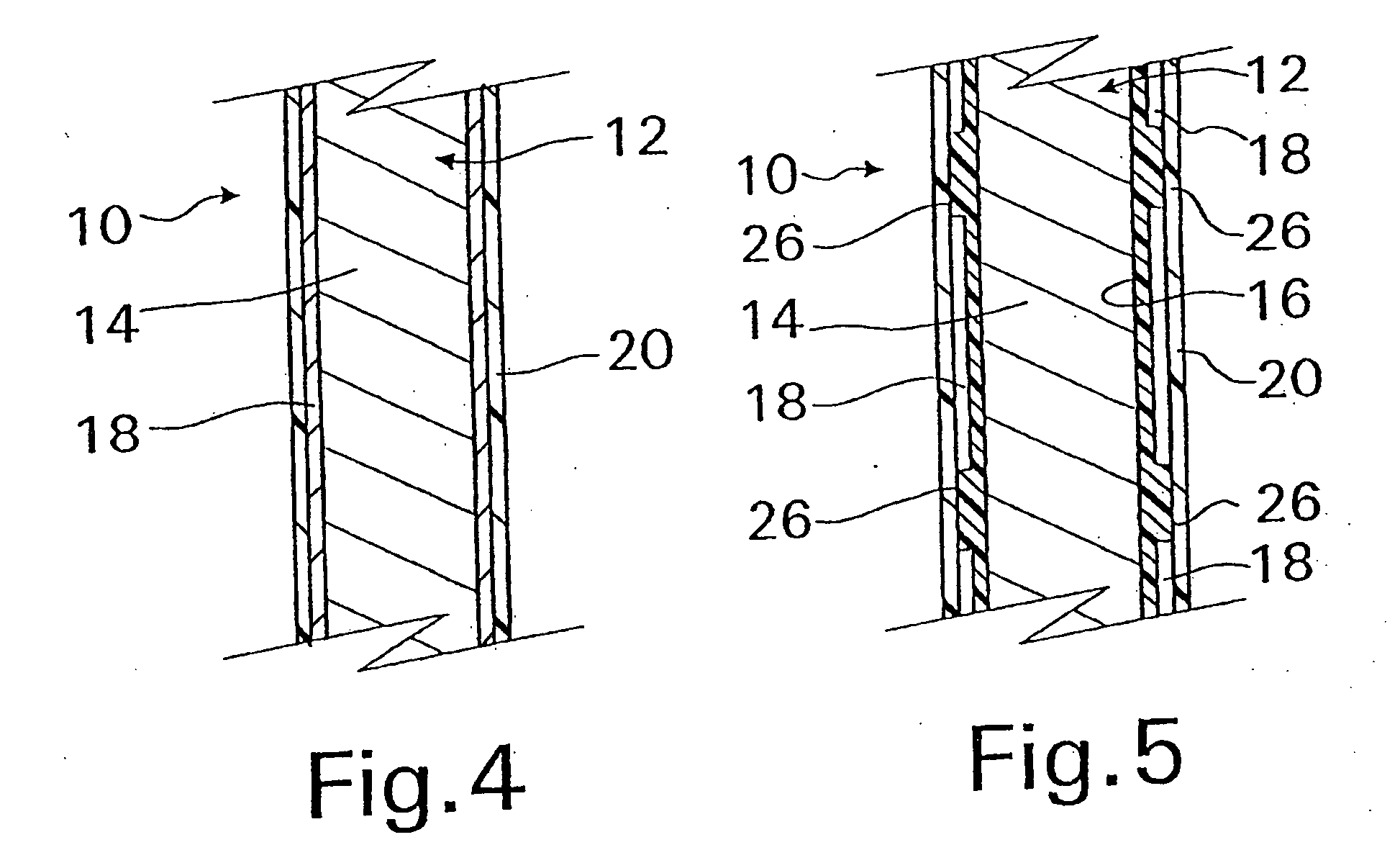
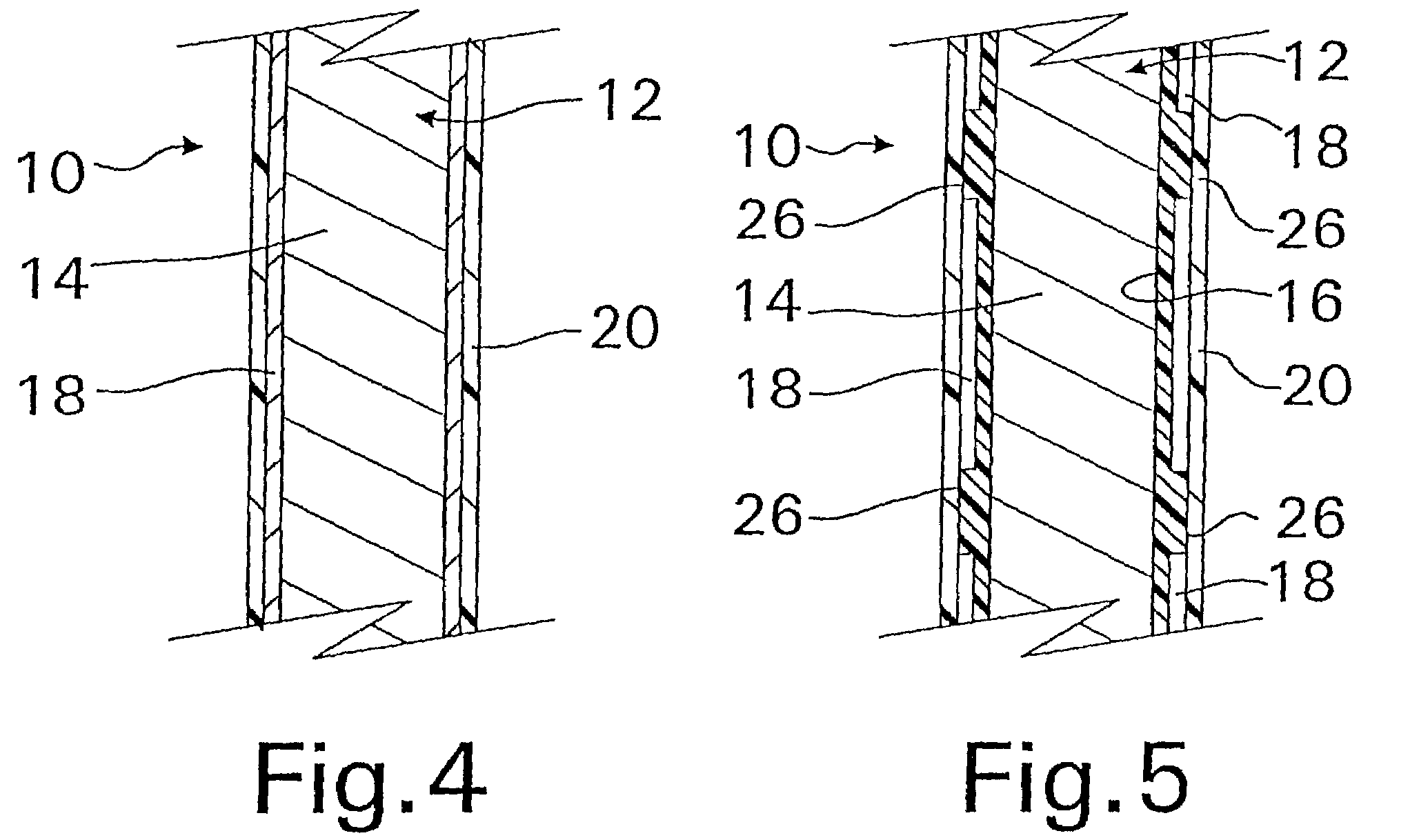
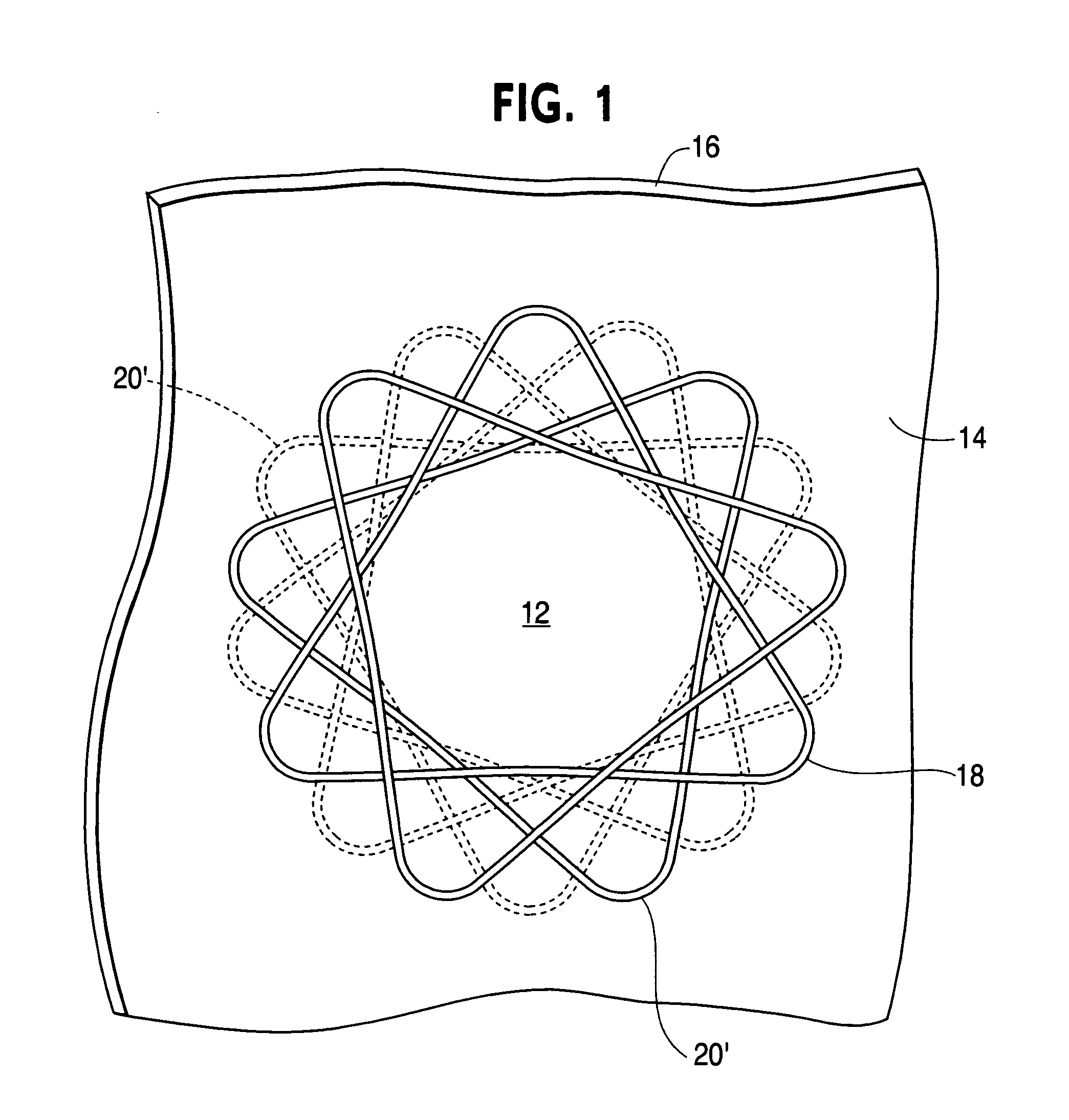
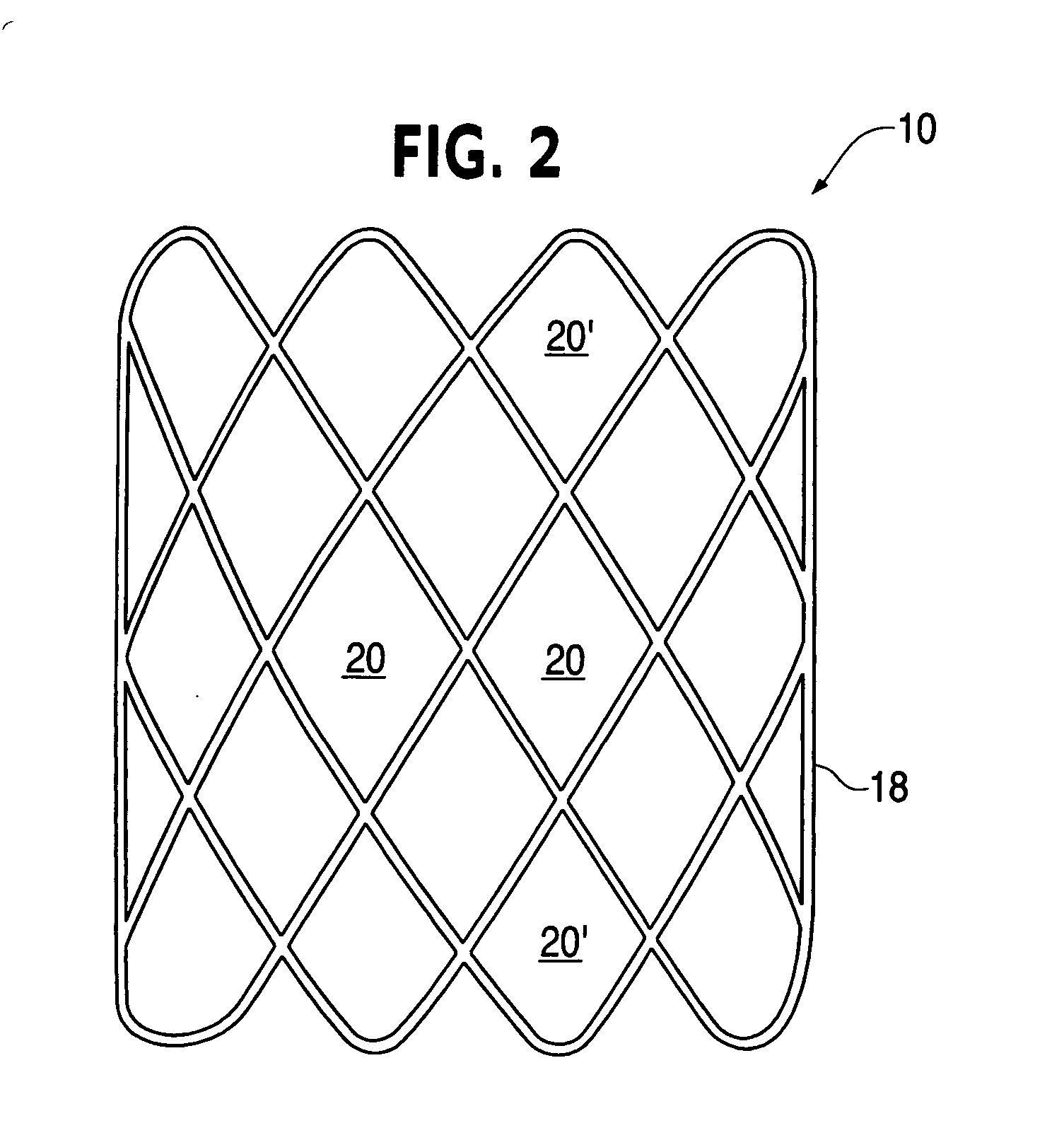
Biodegradable double stent
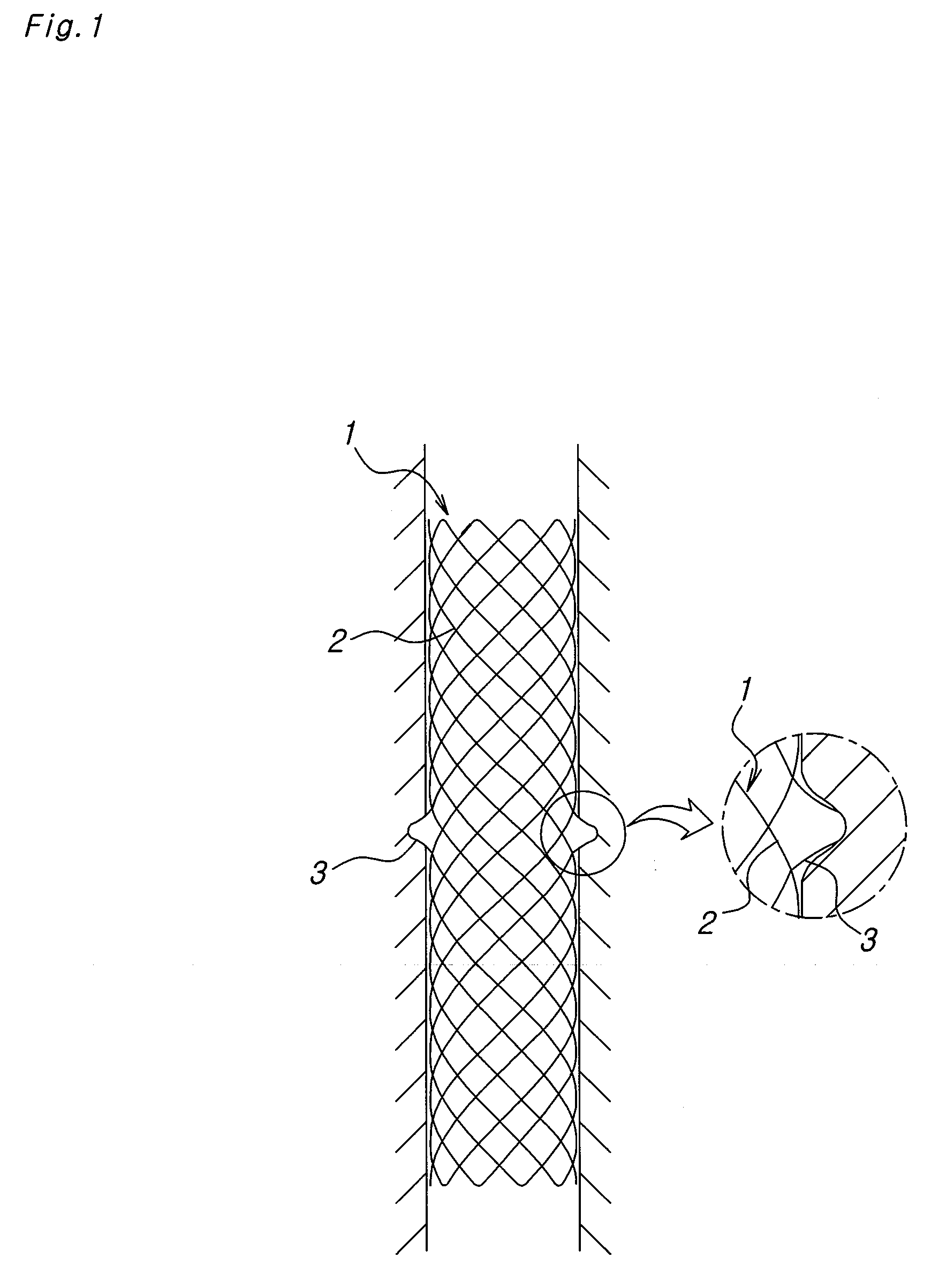
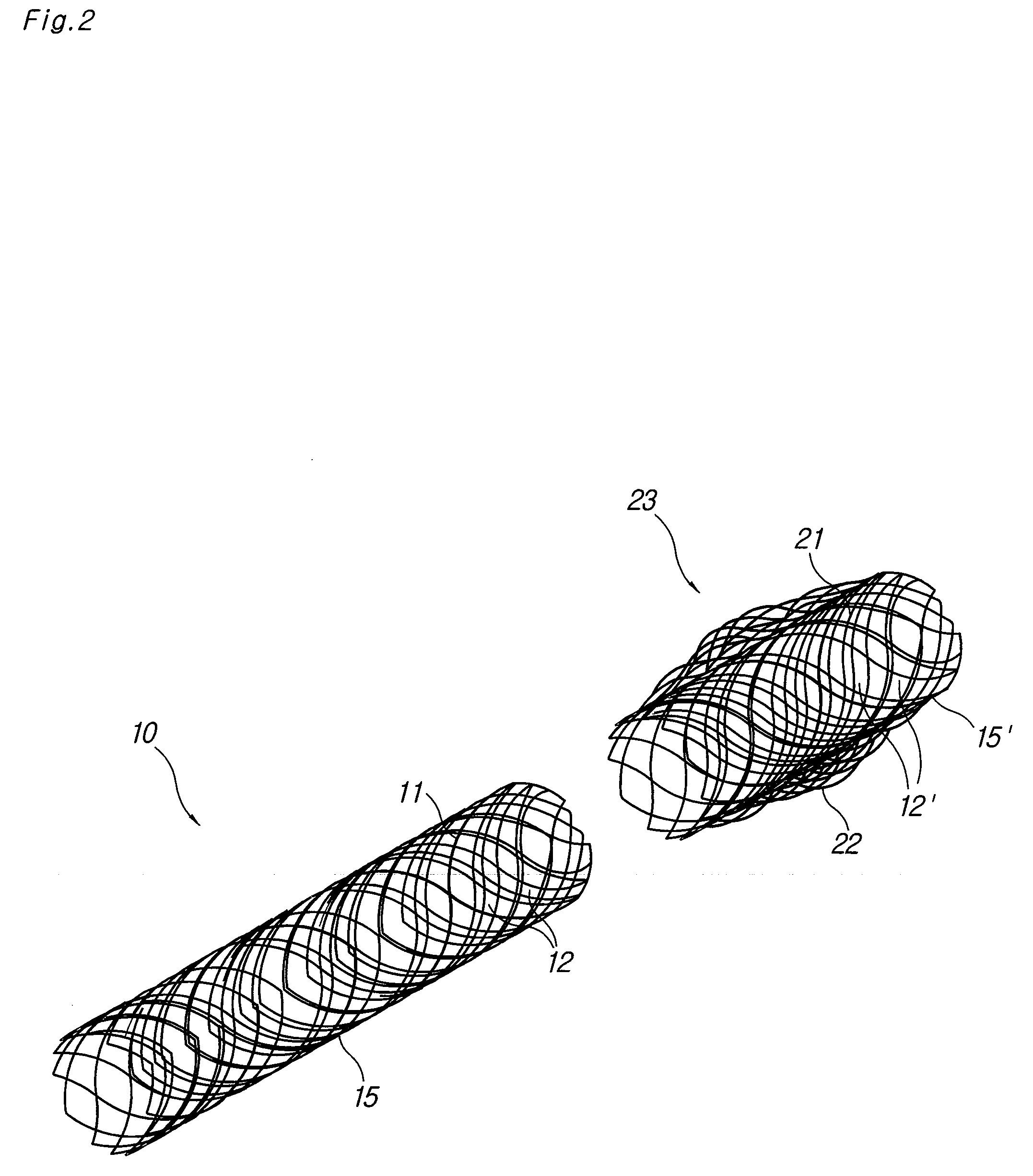
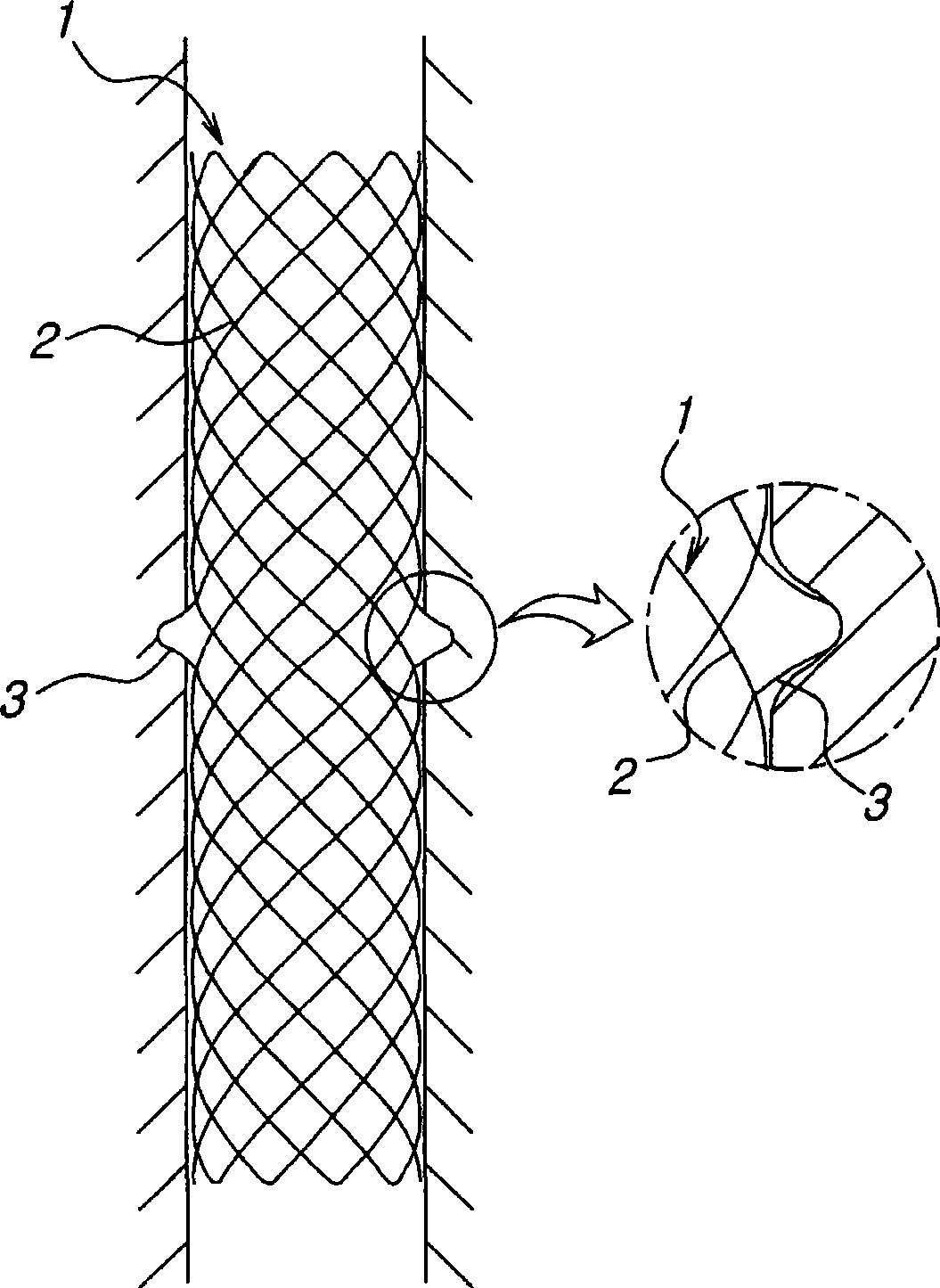
A biodegradable double stent is used for various organs such as a biliary tract, an esophagus, an airway and a ureter. A biodegradable stent having a hollow cylindrical body woven out of a separate wire made of biodegradable polymer so as to have a plurality of rhombic spaces is fixed to an intermediate portion of a primary stent having a cylindrical body. When administered into the organ, the biodegradable stent has its original function of expanding the organ, and is firmly supported in the inner wall of a narrowed passage of the organ by pressurizing the inner wall of the narrowed passage of the organ. After a predetermined time period has elapsed, the biodegradable stent is gradually degraded away by bodily fluids, thereby enabling the primary stent to be easily removed from the organ.
Owner:TAEWOONG MEDICAL CO LTD +1
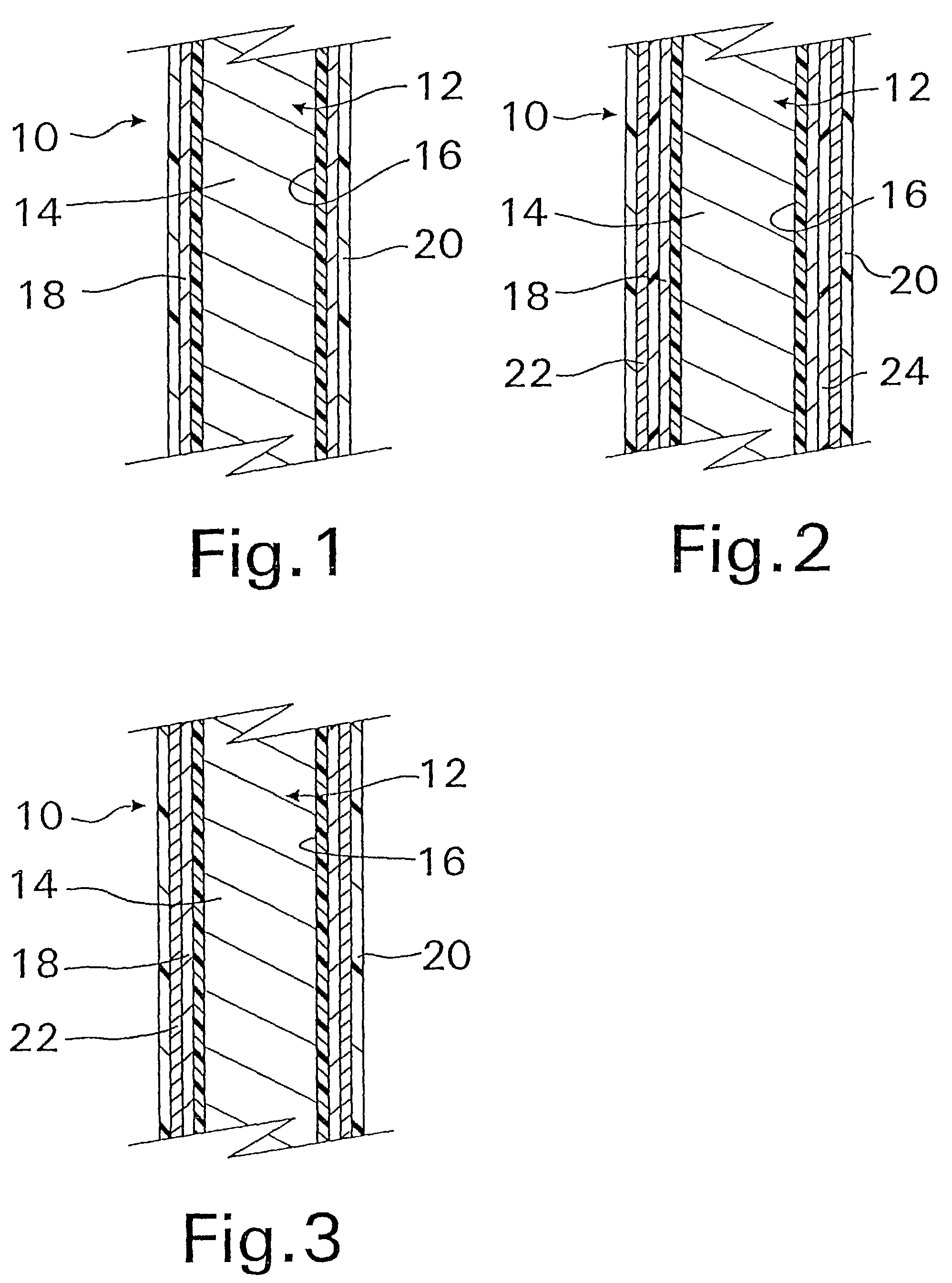
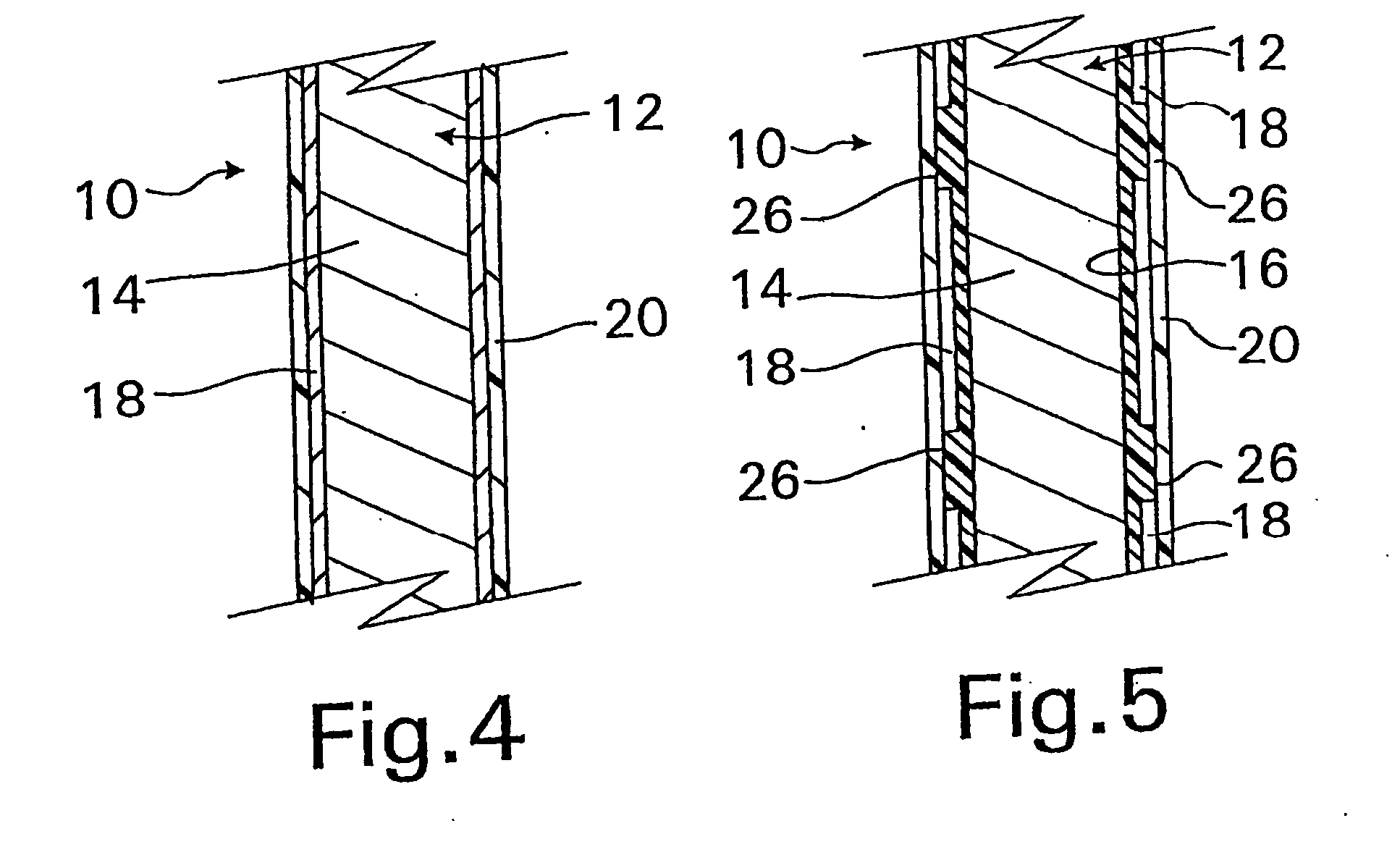
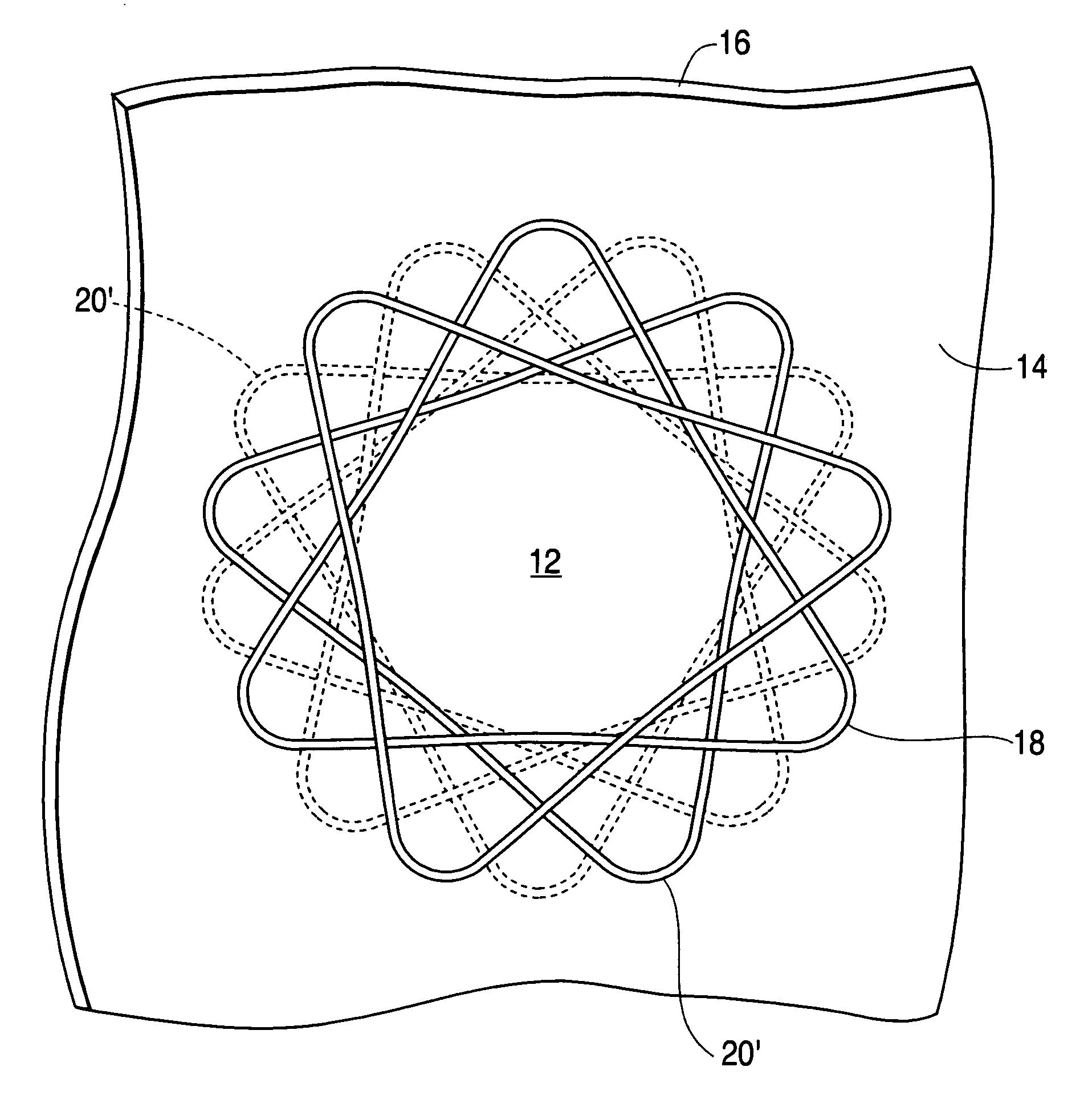
Coated implantable medical device
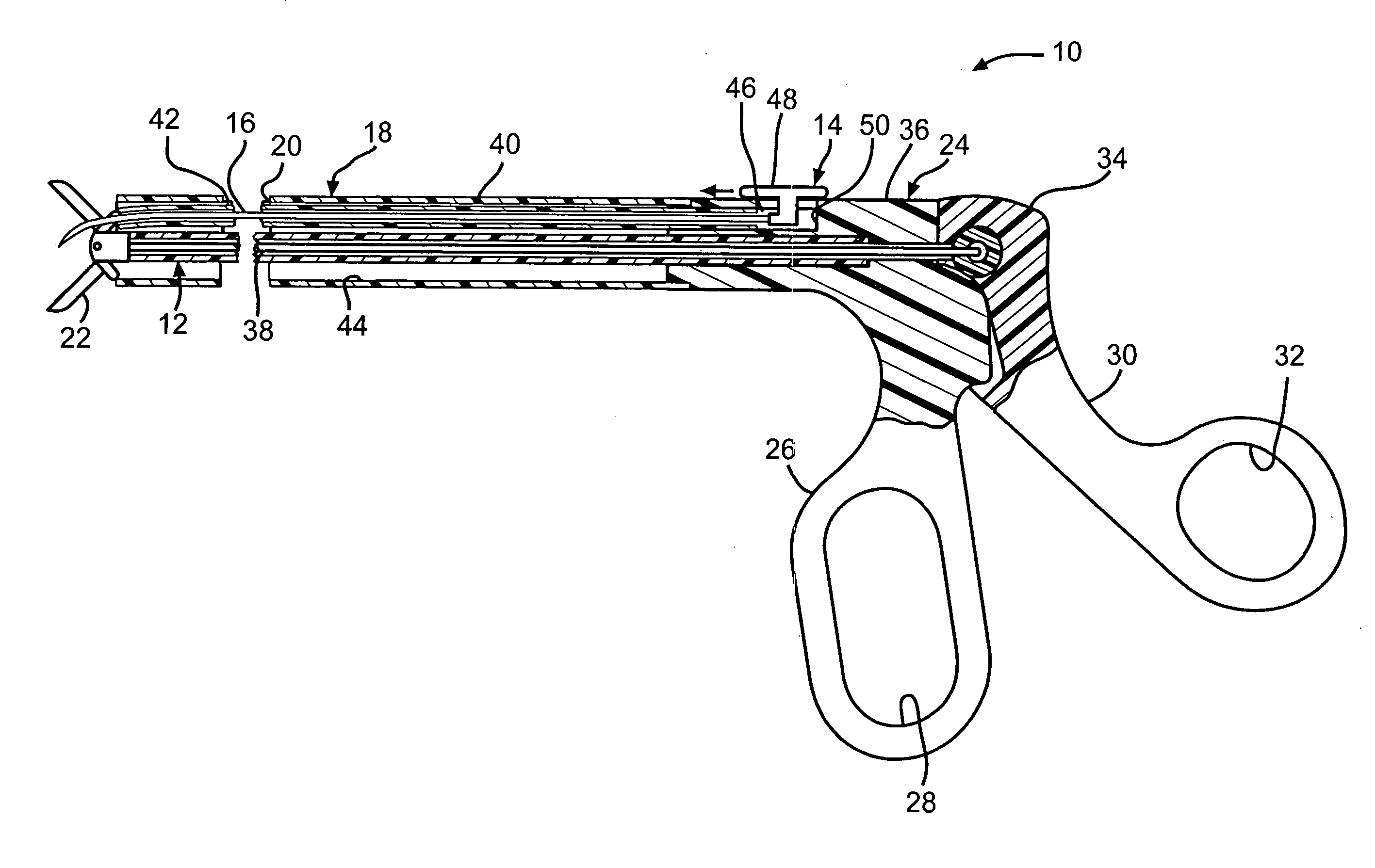
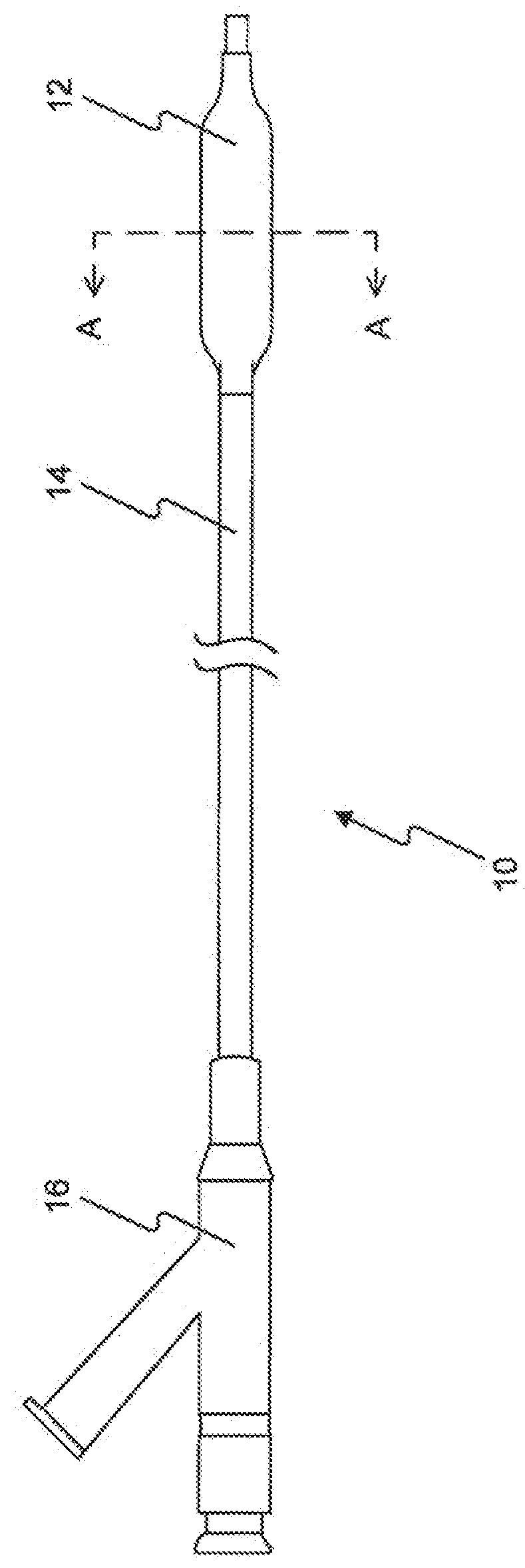
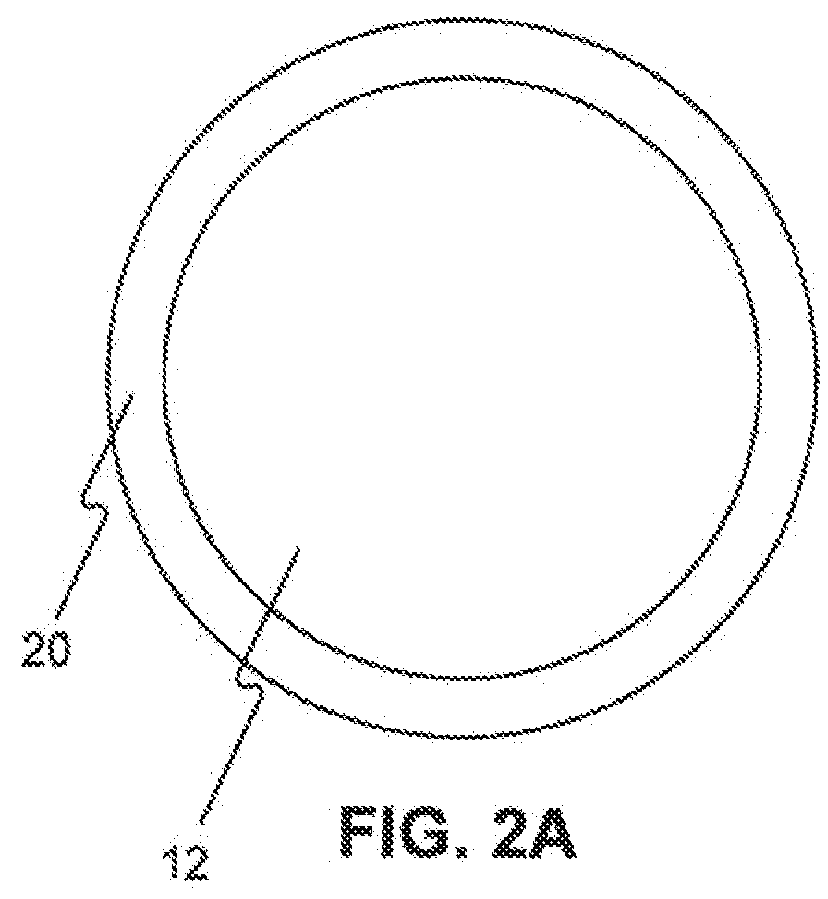
InactiveUS20070050010A1Prevent degradationIncrease release rateStentsIn-vivo radioactive preparationsParyleneGas phase
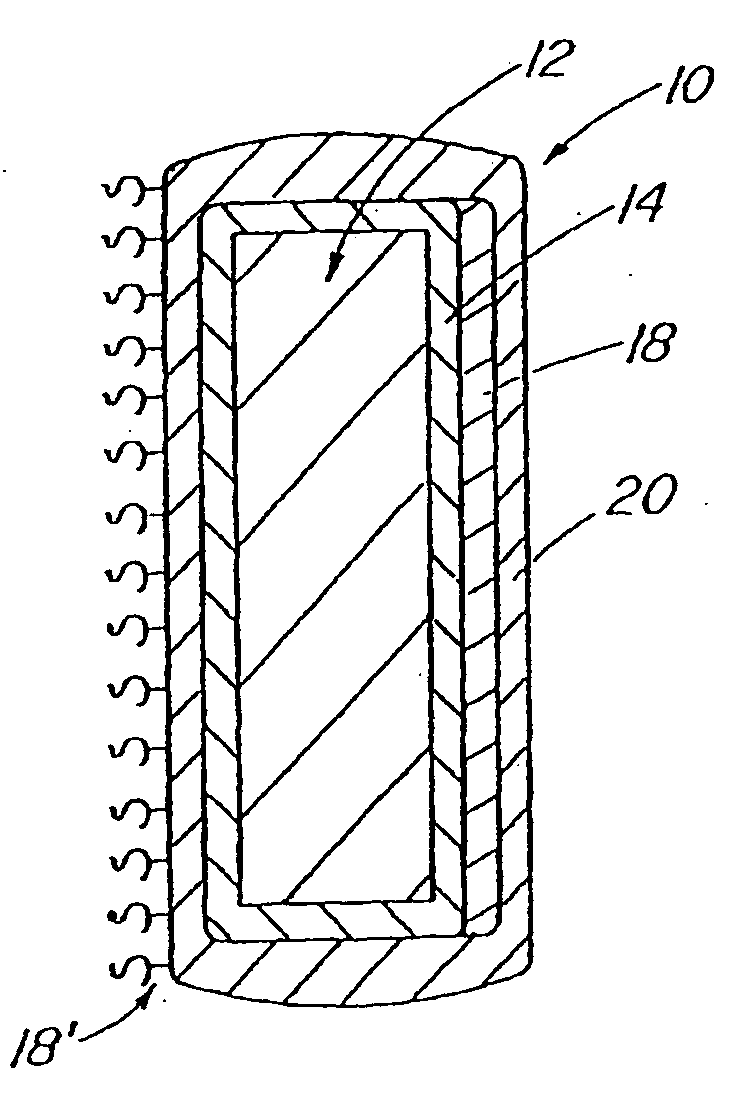
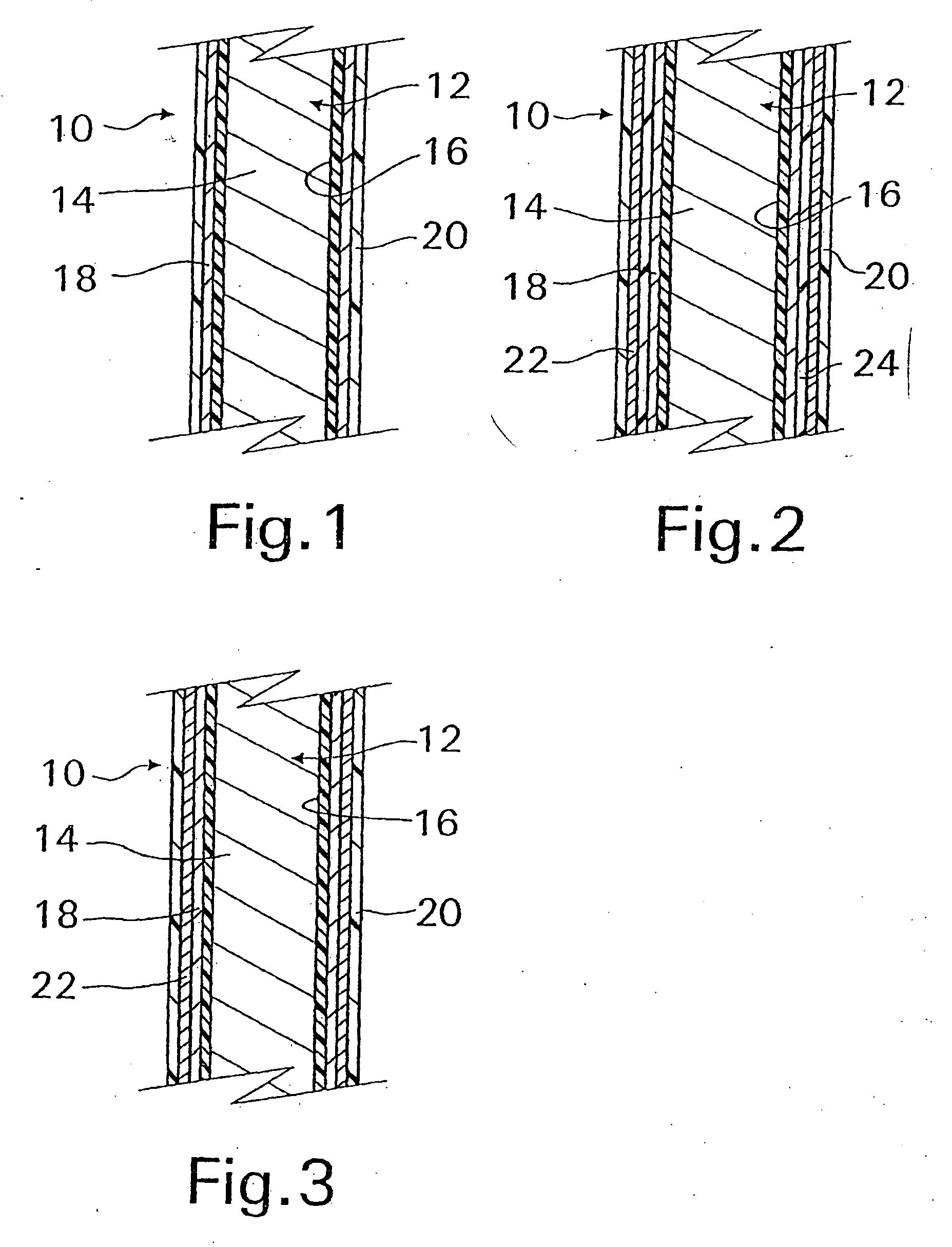
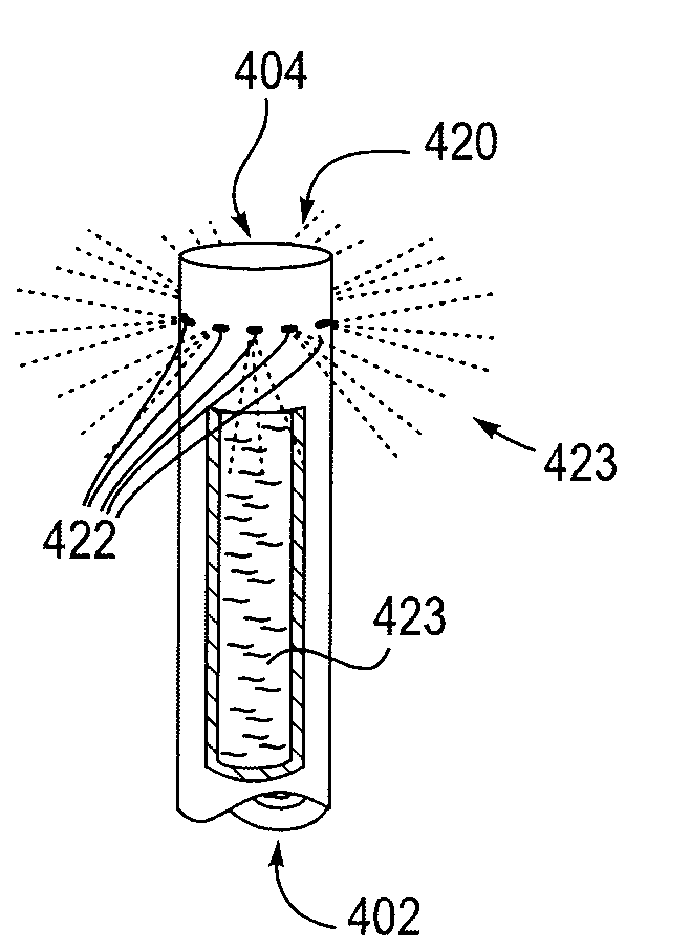
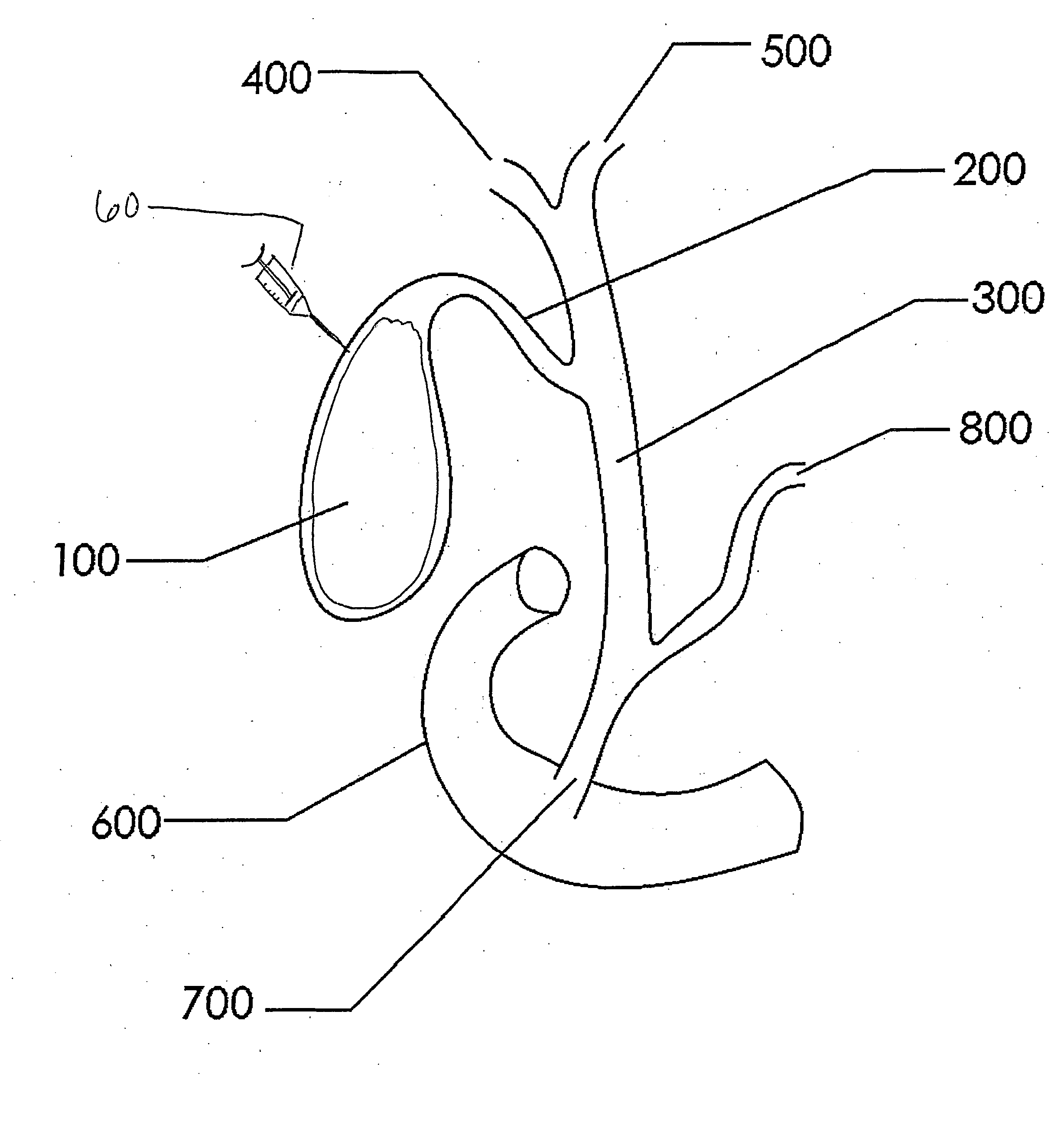
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Biliary barrier
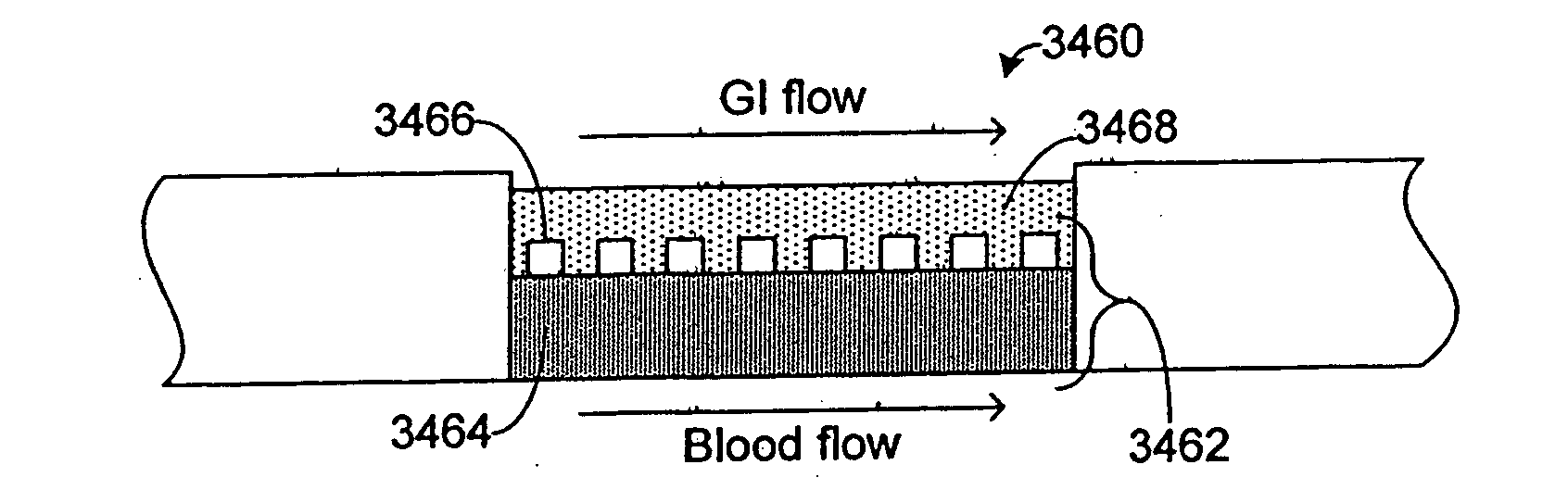
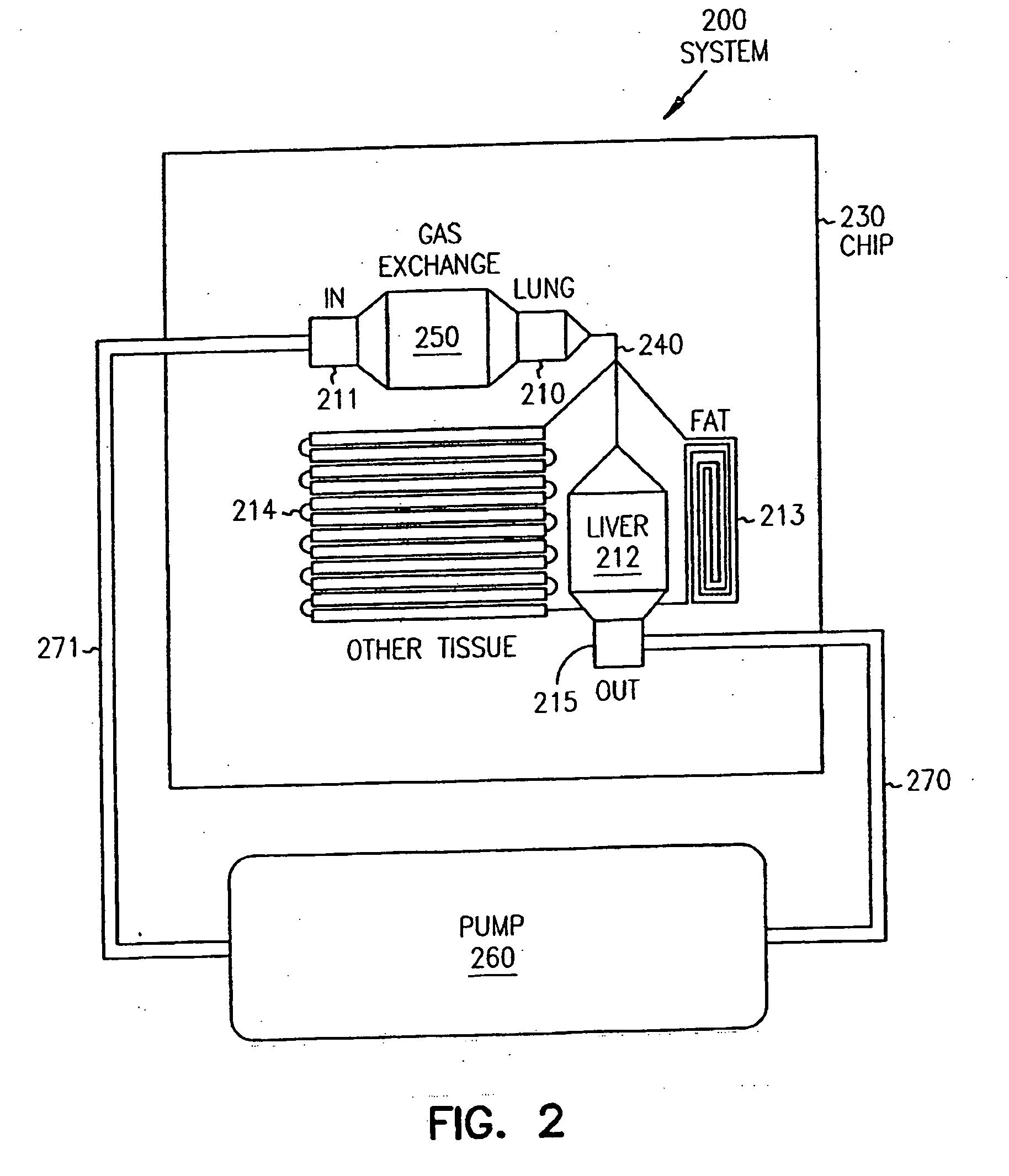
InactiveUS20070048727A1Increased toxicityIncrease valueBioreactor/fermenter combinationsBiological substance pretreatmentsDrug compoundMedicine
Systems and methods are disclosed for microscale pharmacokinetics. Various organs and their interactions with drug compounds can be simulated in vitro by use of microscale compartments that can be interconnected by microscale channels. Cells or cellular materials associated with the organs can be cultured in such compartments to allow interactions with drug compounds in one or more fluidic flows. Such fluidic systems can include, by way of examples, gastrointestinal flow, blood flow, bile flow, urinary flow, and brain fluid flow. Interactions between fluidic systems can be simulated by a microscale permeable member. In one example, blood-biliary interaction can be simulated by a microscale permeable material having hepatocytes bound to a permeable substrate via a binder.
Owner:CORNELL RES FOUNDATION INC
Polyhydroxylated bile acids for treatment of biliary disorders
The invention provides, in part, polyhydroxylated bile acids for treating biliary disorders, for example, biliary disorders arising out of cholestasis of portal hypertension. The invention also provides, in part, polyhydroxylated bile acids for stimulating bile flow. New compounds 2α,3α,7α,12α-tetrahydroxy-5β-cholanoic acid and 3α.4α,7α,12α-tetrahydroxy-5β-cholanoic acid are disclosed, uses thereof and synthesis thereof.
Owner:QING BILE THERAPEUTICS
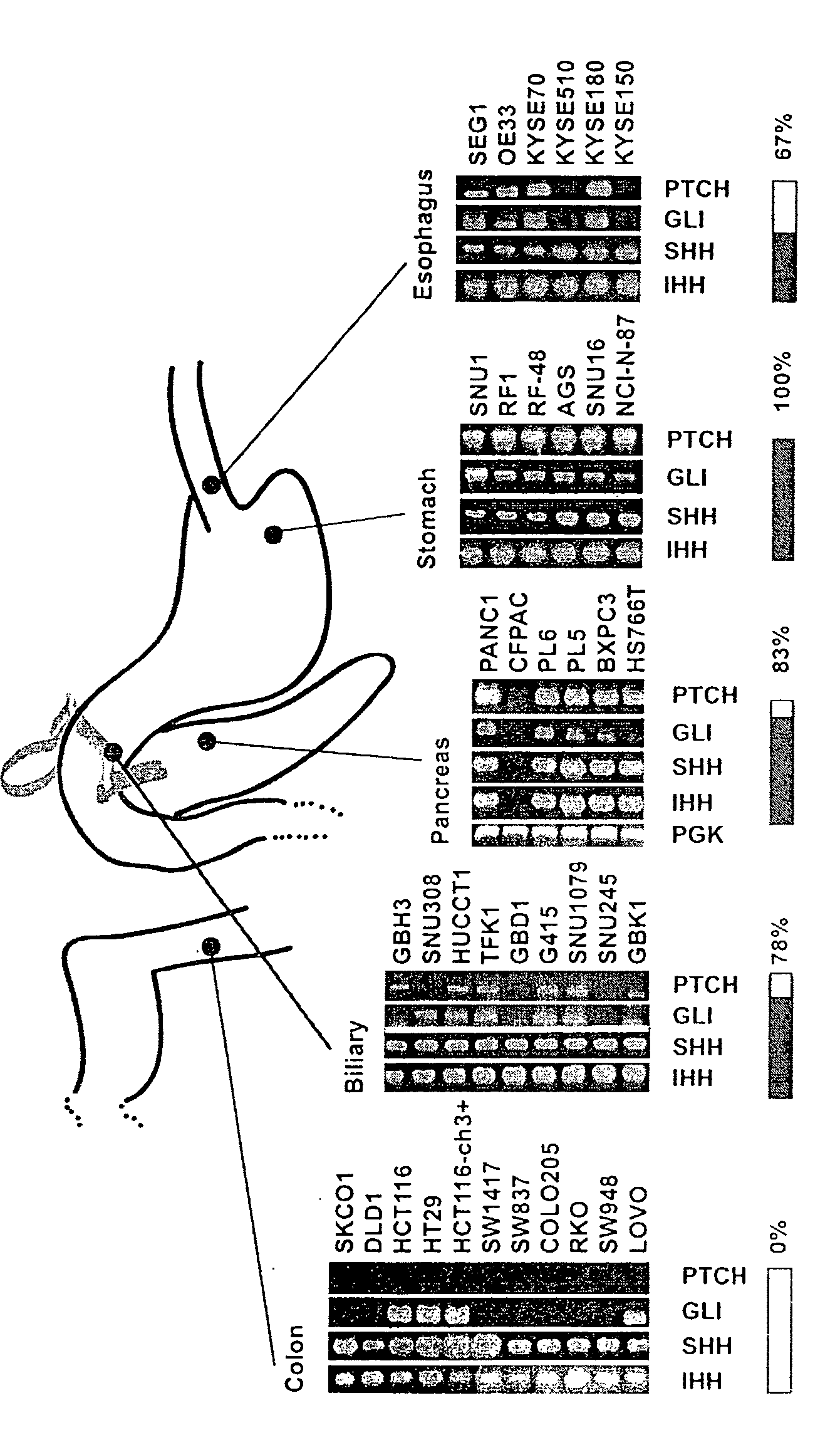
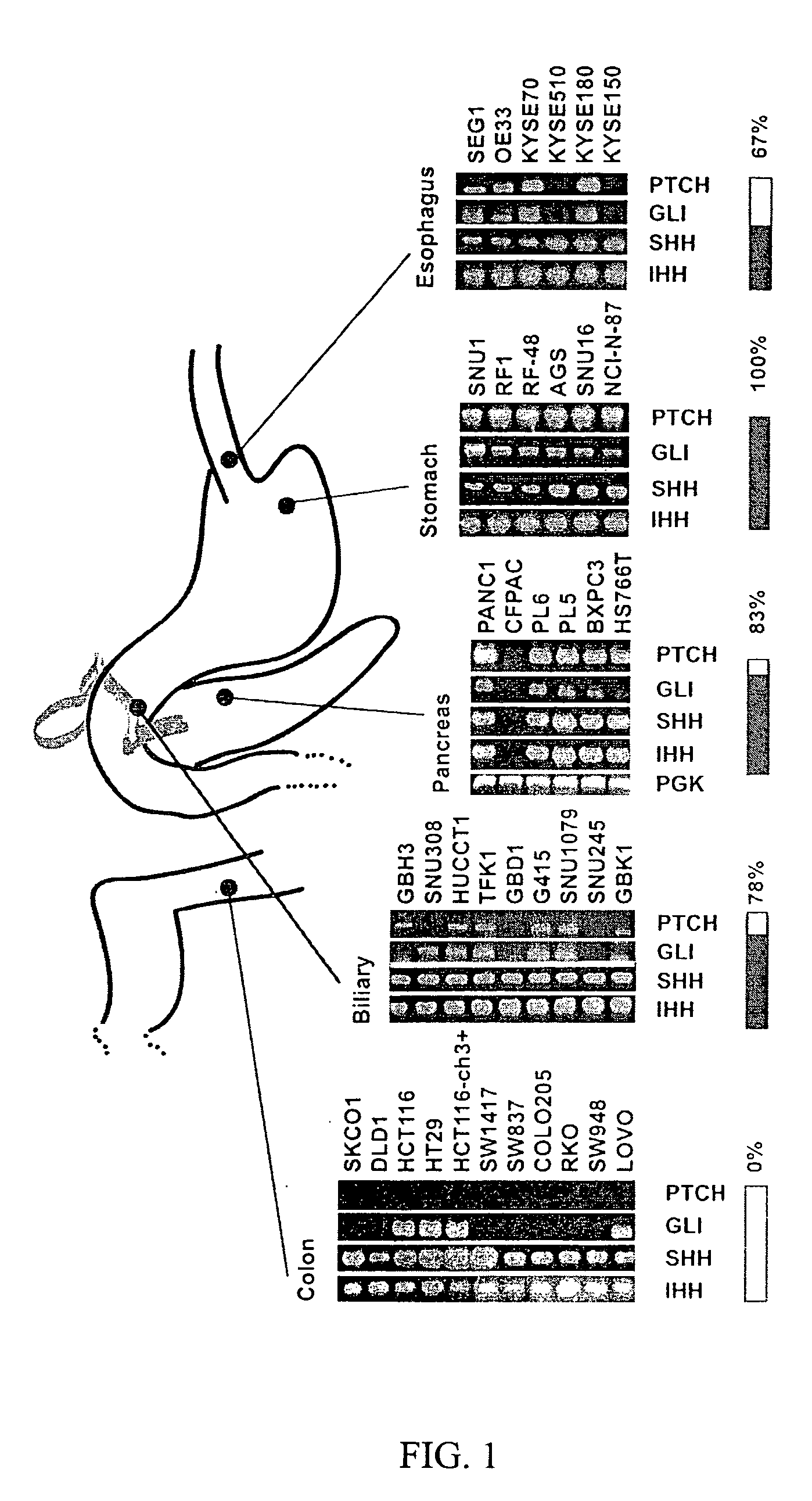
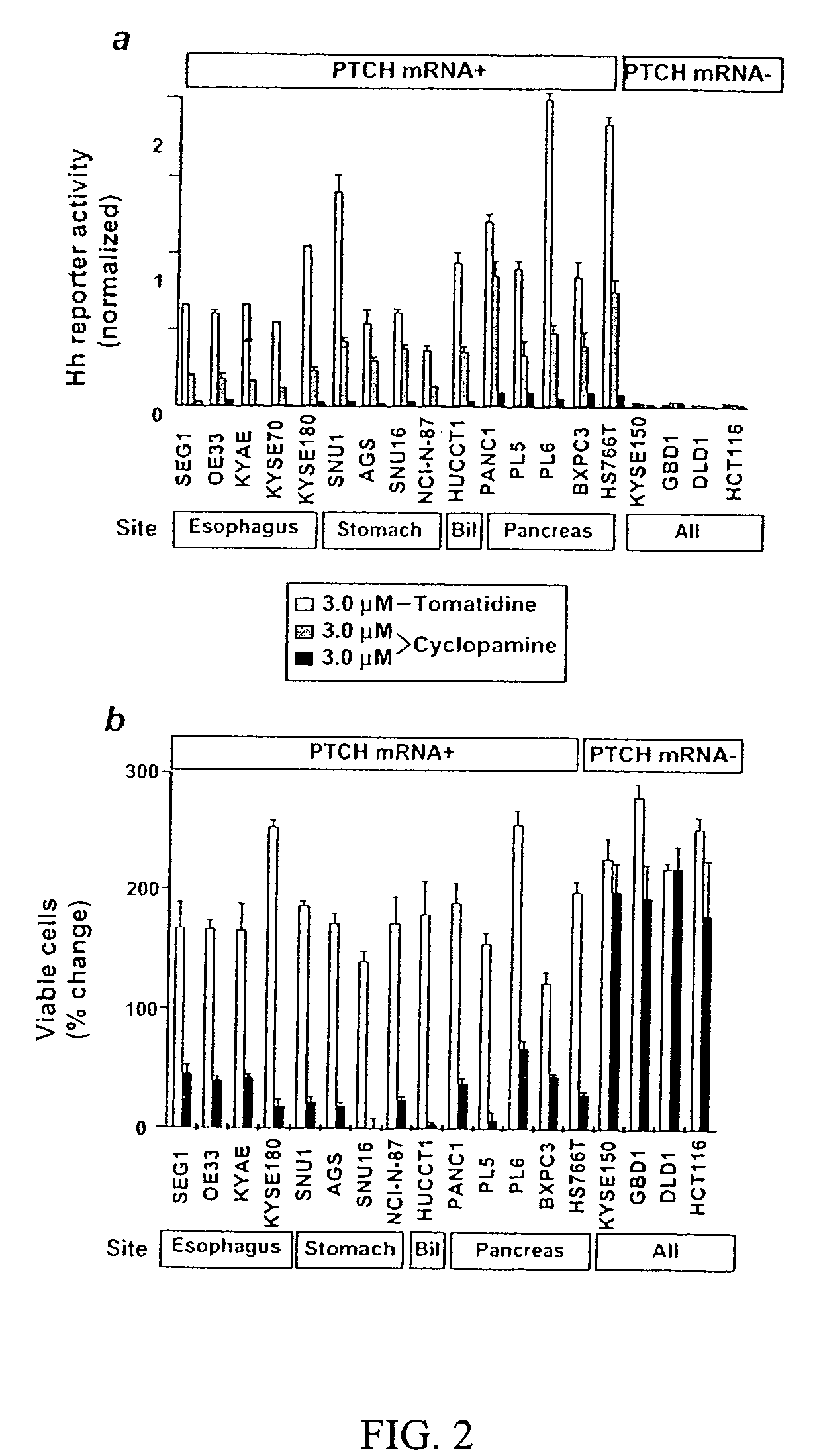
Elevated Hedgehog Pathway Activity In Digestive System Tumors, And Methods Of Treating Digestive Sytem Tumors Having Elevated Hedgehog Pathway Activity
InactiveUS20080118493A1High throughput formatImprove throughputCompound screeningApoptosis detectionAbnormal tissue growthCancer cell
Elevated Hedgehog (Hh) pathway activity, including ligand stimulated Hh pathway activity, was detected in digestive tract cancers, including esophagus, stomach, biliary tract, and pancreatic cancer, and determined to be associated with growth and proliferation of the cancer cells. Accordingly, methods are provided for treating a digestive tract cancer associated with elevated Hh pathway activity by reducing or inhibiting the Hh pathway activity. Also provided are methods of determining the responsiveness of a digestive tract tumor to treatment with an Hh pathway antagonist.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Coated implantable medical device
InactiveUS7550005B2Prevent degradationIncrease release rateStentsHeart valvesParylenePlasma deposition
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Systems and methods for treating obesity and other gastrointestinal conditions
InactiveUS20090118699A1Reduce morbidityIncrease satietyElectrotherapyPeptide/protein ingredientsReflexREFLEX DECREASE
Owner:RESPIRATORY DIAGNOSTICS
High-simulation laparoscopic surgery simulated training device
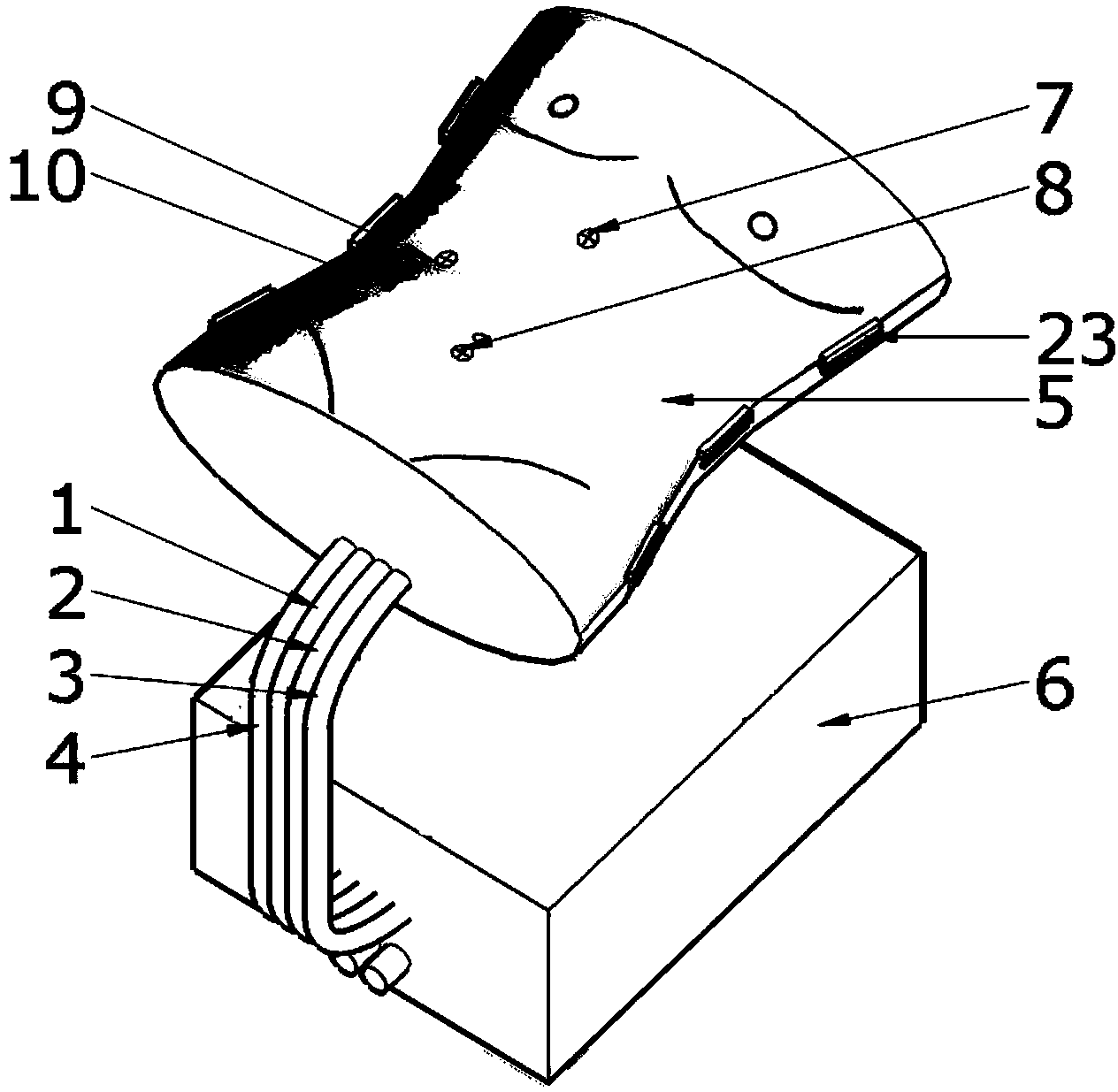
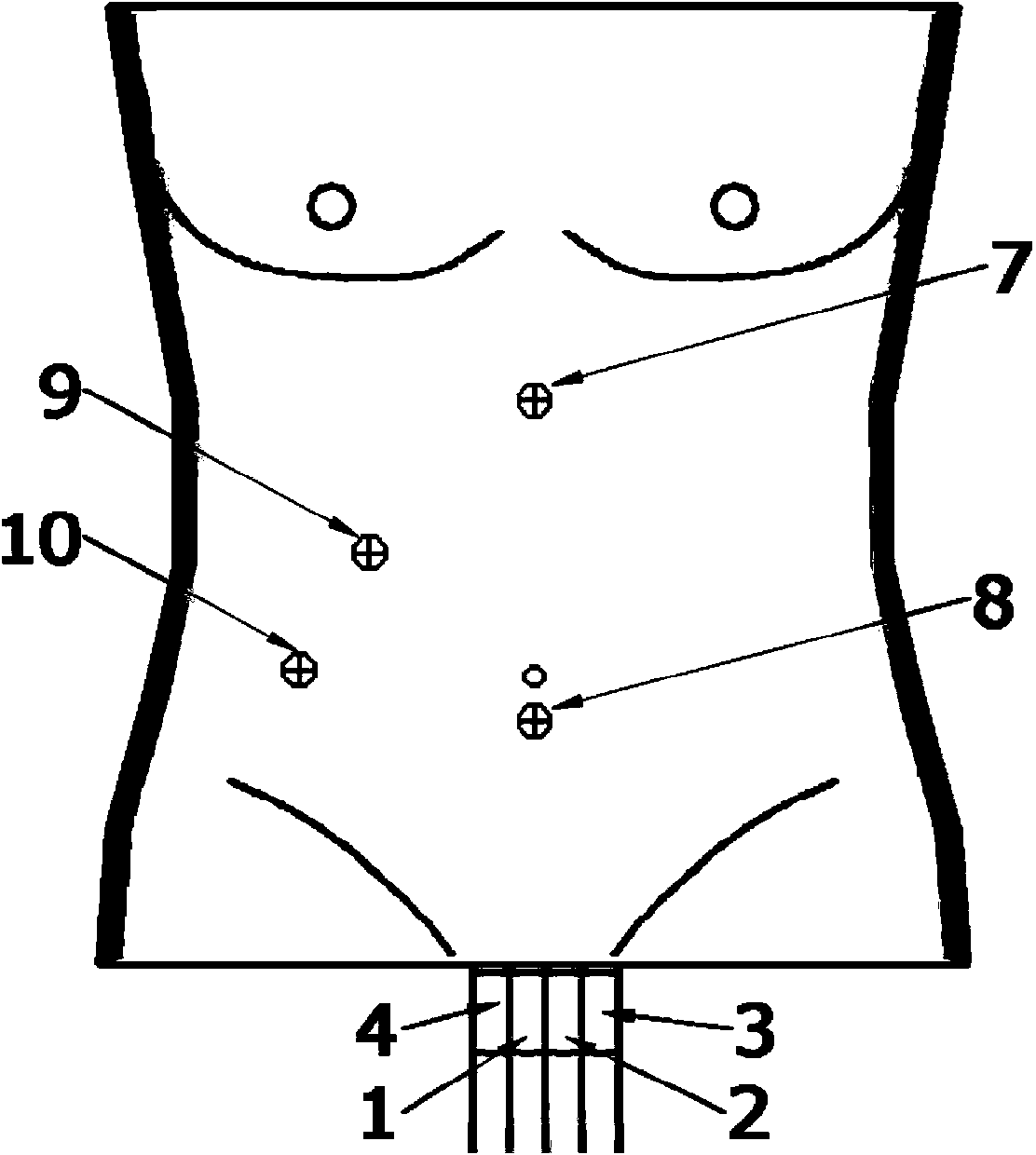
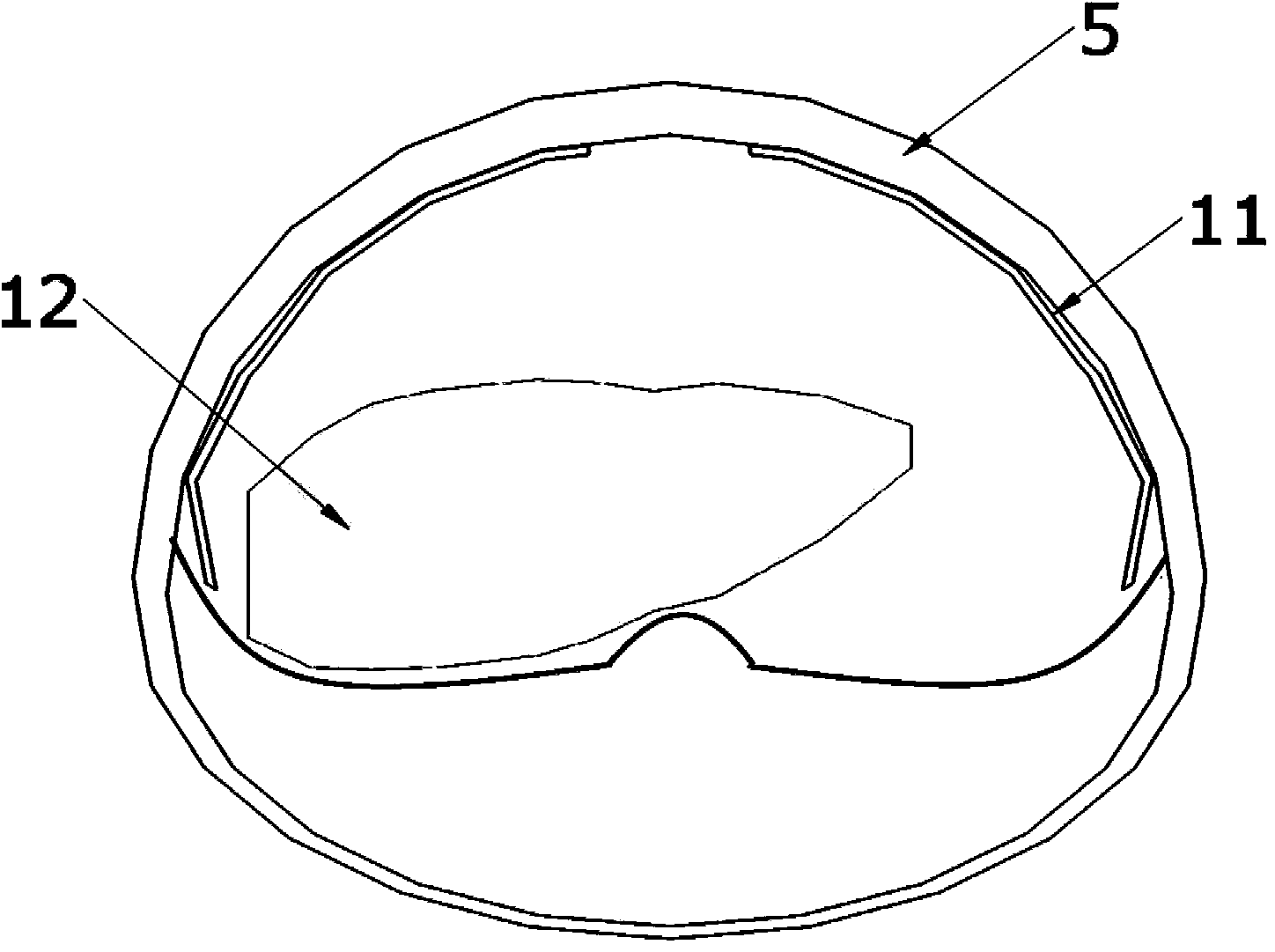
A high-simulation laparoscopic surgery simulated training device comprises a simulated human abdomen unit, an animal liver and a simulated canal system, wherein the simulated human abdomen unit is used for simulating the human abdomen skin through silica gel and composed of anterior abdominal wall skin and back skin which can be separated from each other, the anterior abdominal wall skin and the back skin are connected through sealing buckles, and thus the seal performance of the whole simulated human abdomen is guaranteed; the animal liver is arranged in the simulated human abdomen unit; the simulated canal system is arranged in the simulated human abdomen unit and connected with the animal liver. According to the high-simulation laparoscopic surgery simulated training device, the abdominal skin is simulated through sealing silica gel, inflation is performed through a poking clip to form the pneumoperitoneum, the human blood circulation in the physiological state is achieved through a pumping set, the real animal liver is anastomosed with the postcava, the portal vein, the hepatic artery and the biliary tract of a human simulator training system through the postcava, the portal vein, the hepatic artery and the biliary tract, and thus various simple or complex laparoscopic surgery training items can be carried out.
Owner:XIAN MAGNETIC MEDICAL TECH CO LTD
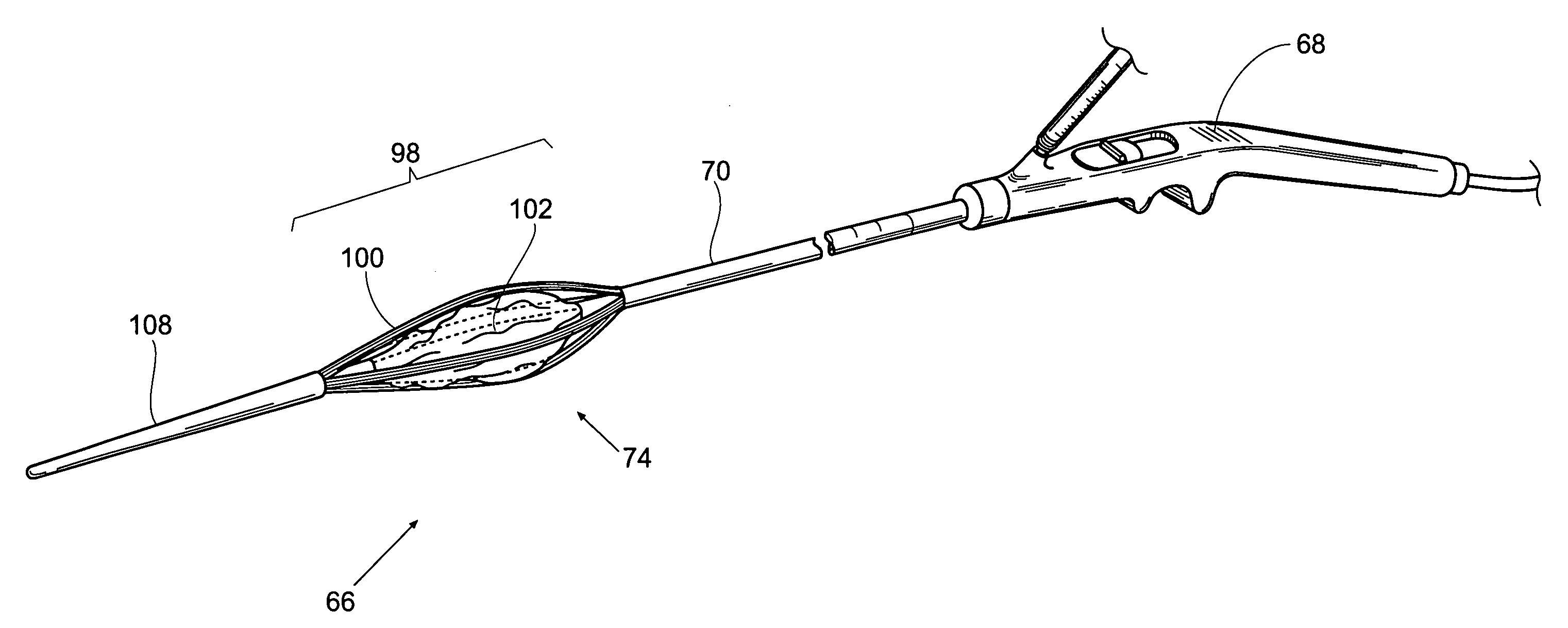
Biliary shunts, delivery systems, and methods of using the same
The application discloses devices, delivery tools, systems, and methods for treating biliary disease. Device comprise, for example, a component configured for deployment between a gallbladder and location within a gastrointestinal tract of a patient which has a proximal end and a distal end with a lumen extending therethrough. A method of deploying the device can be achieved by, for example, creating a duct or fistula between a gallbladder lumen and a portion of a gastrointestinal tract; and providing for drainage from the gallbladder to the gastrointestinal tract.
Owner:TREUS MEDICAL
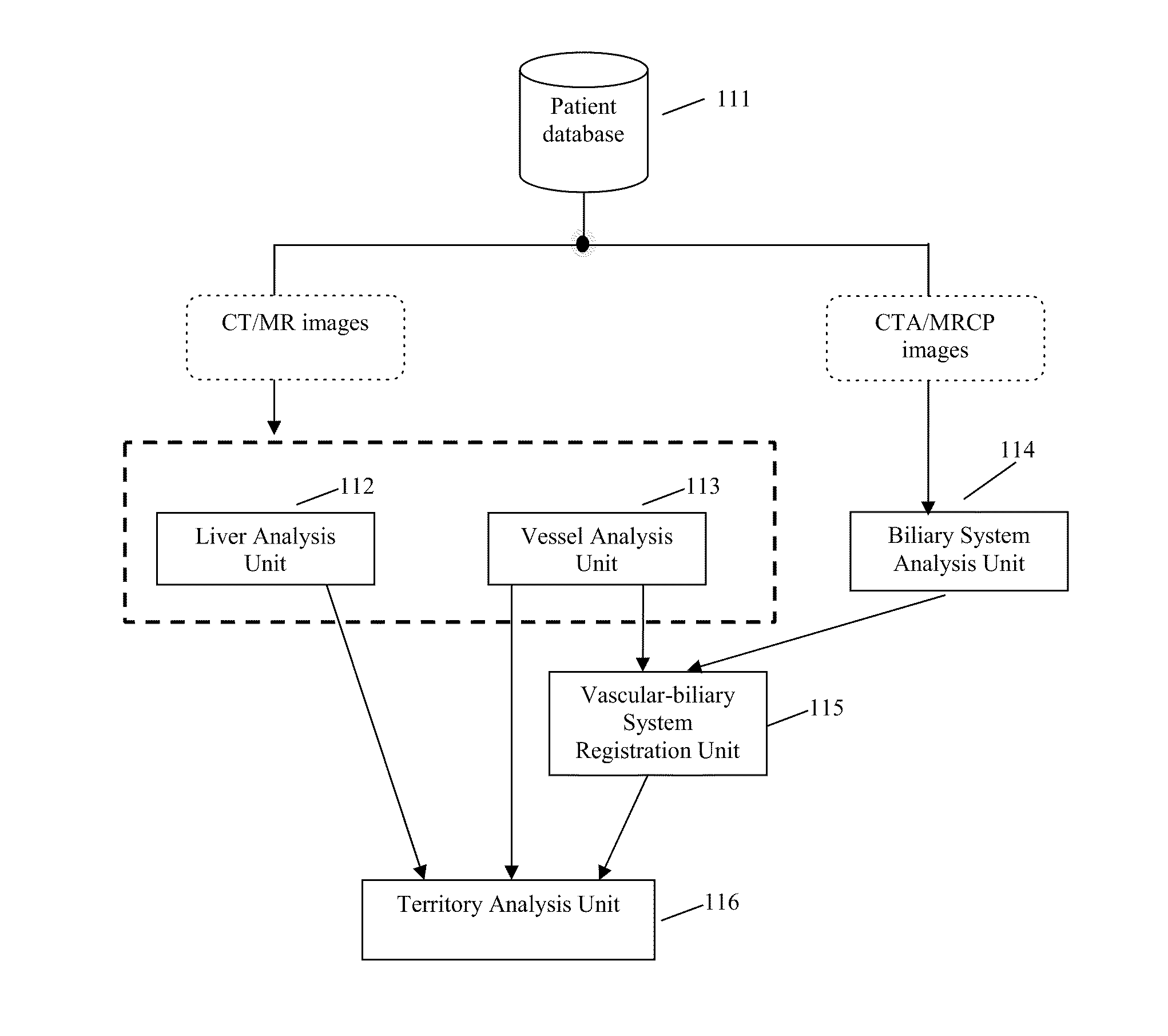
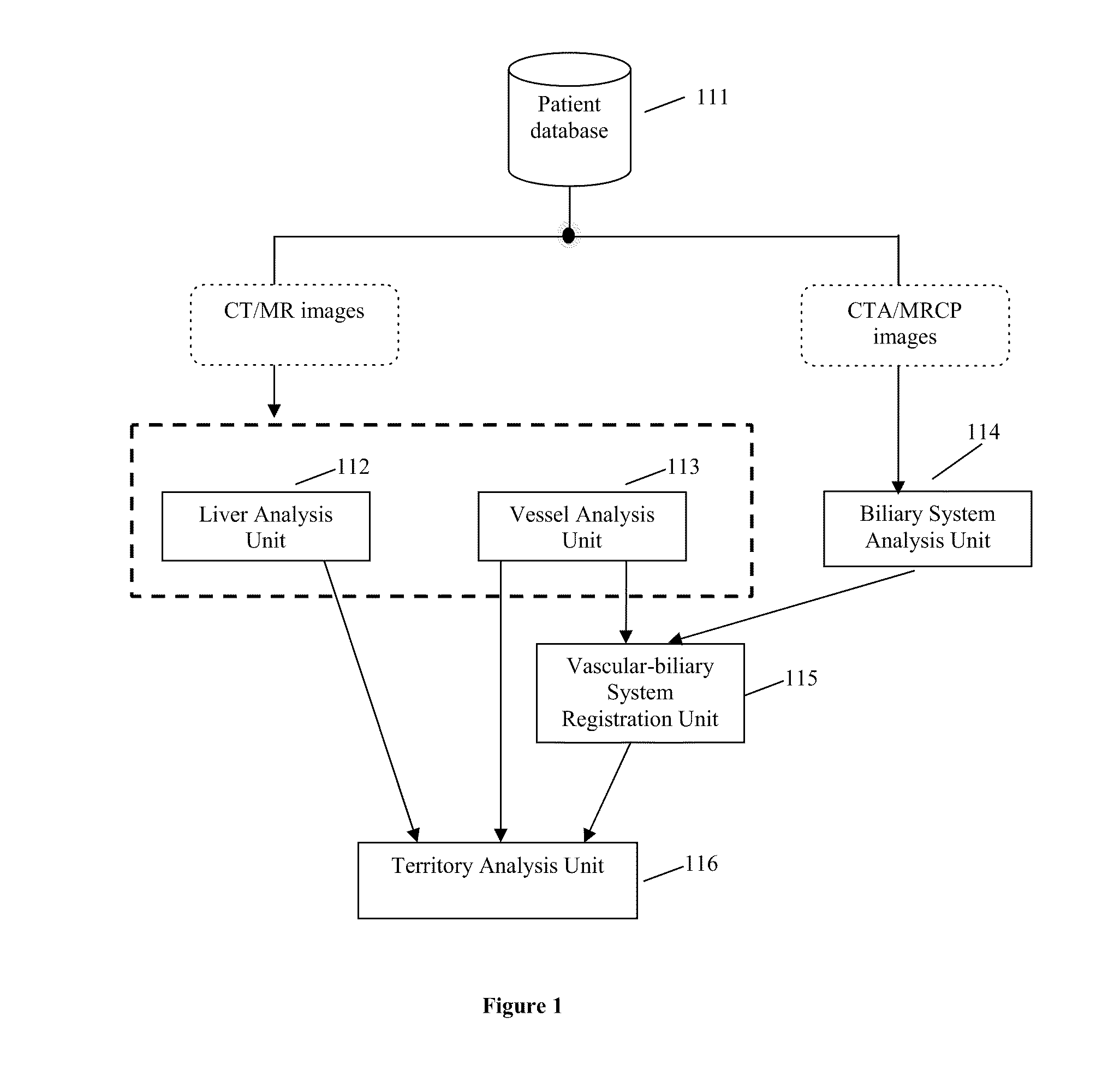
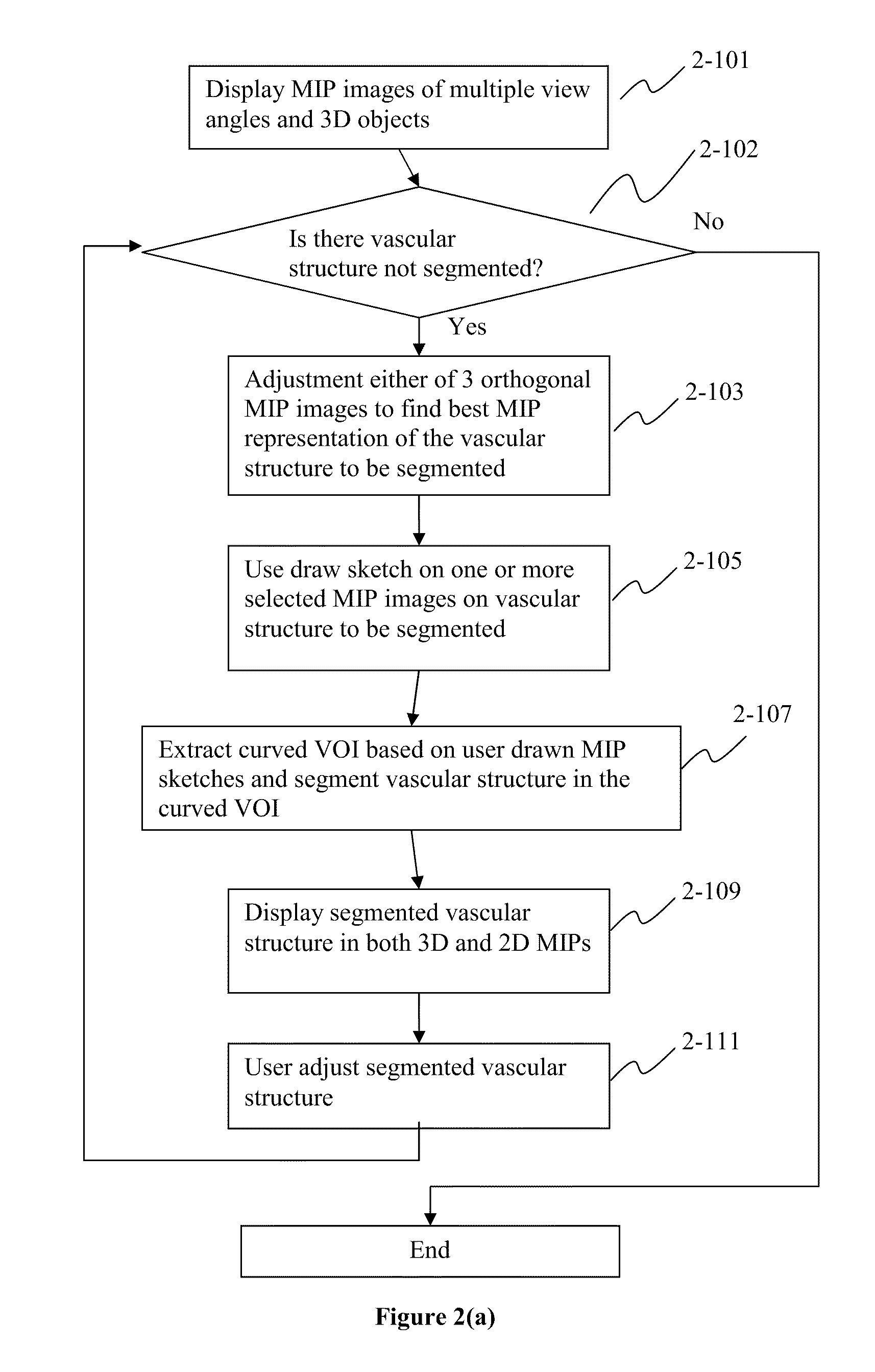
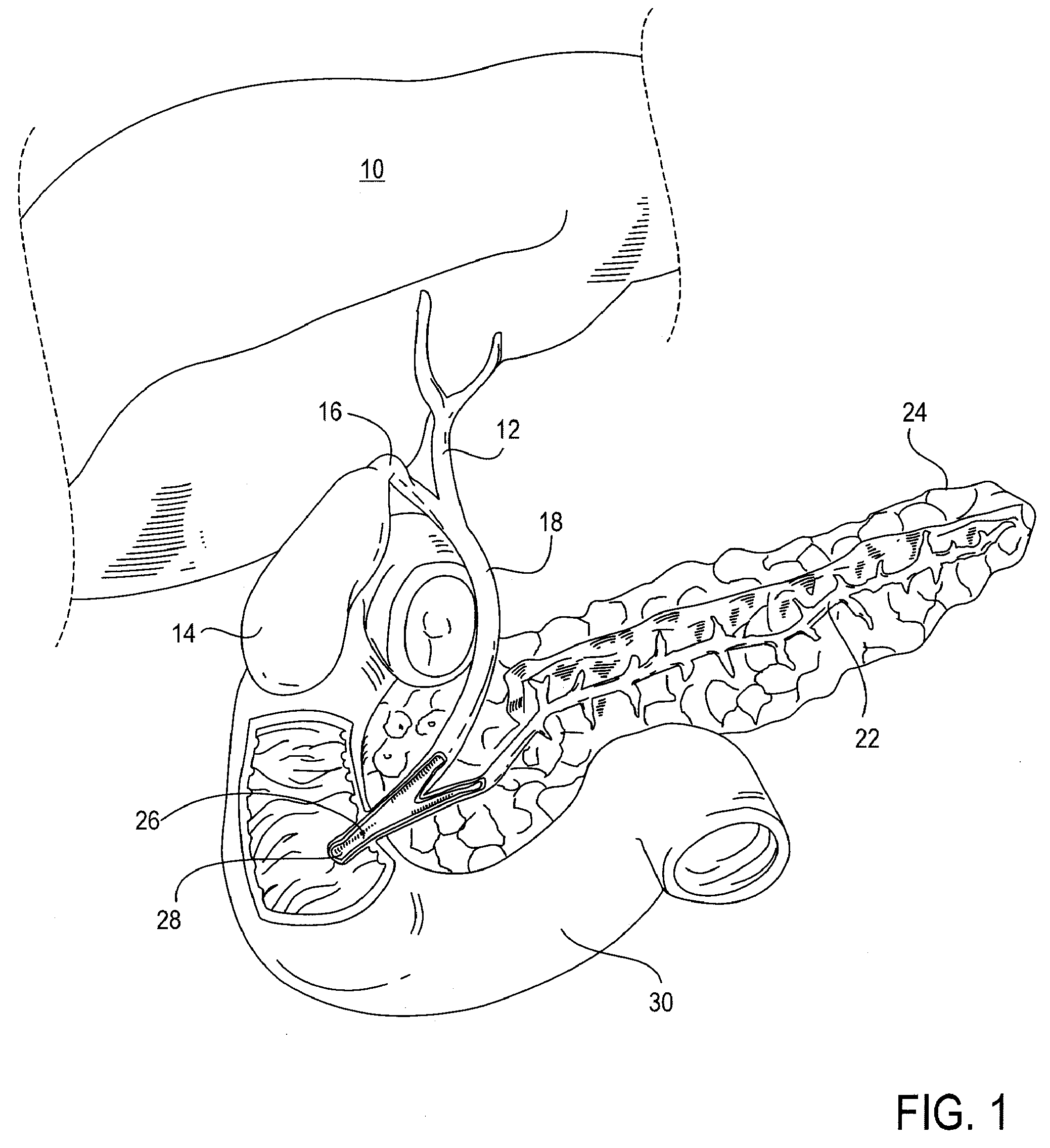
Method, system, apparatus, and computer program product for interactive hepatic vascular and biliary system assessment
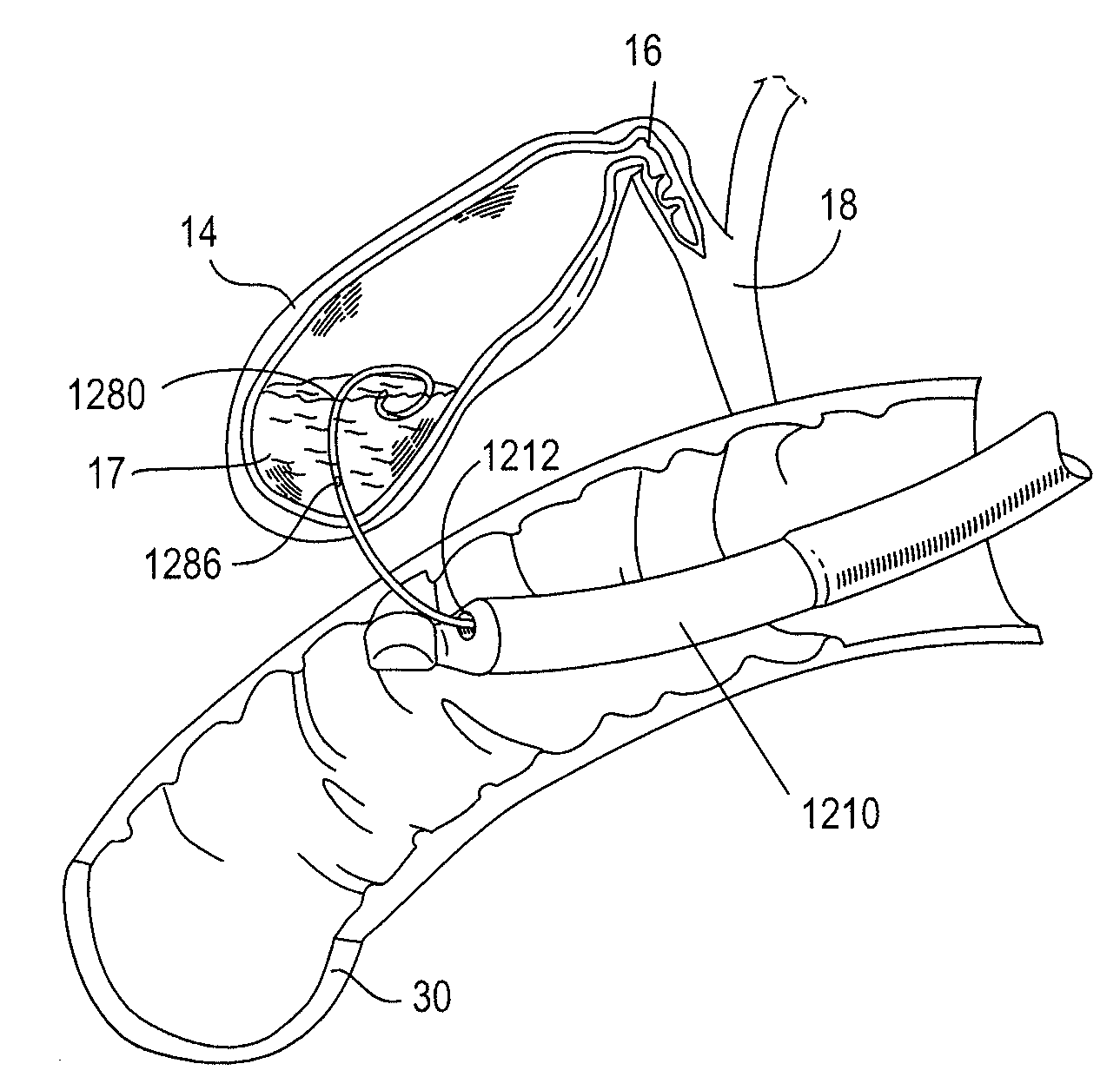
A procedure for image segmentation on three-dimensional (3D) medical images, and a system, apparatus, and computer program that operate in accordance with the procedure. The procedure includes generating a projection including an anatomic structure, tracing a curve corresponding to the anatomic structure, extracting a curved volume of interest based on the curve and the projection, and extracting a segmentation of the anatomic structure. Also provided is a procedure for aligning anatomic structures in images, and a system, apparatus, and computer program that operate in accordance with the procedure. The procedure includes determining part of a biliary system in a first image, determining part of a hepatic portal vein or a hepatic artery in a second image, determining a gallbladder in the images, determining a cost function, and aligning the biliary system and the hepatic portal vein or hepatic artery by maximizing the cost function.
Owner:EDDA TECH
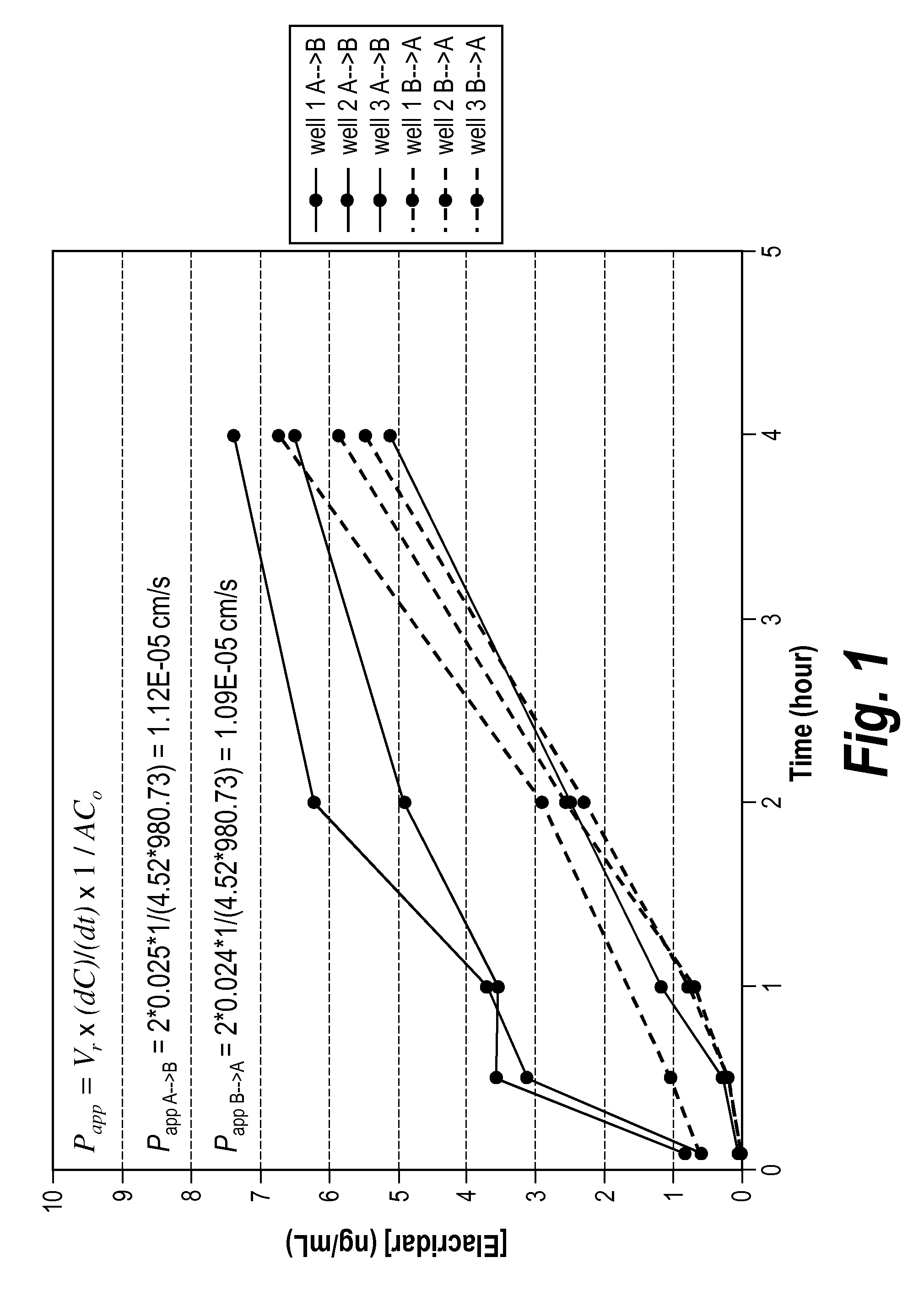
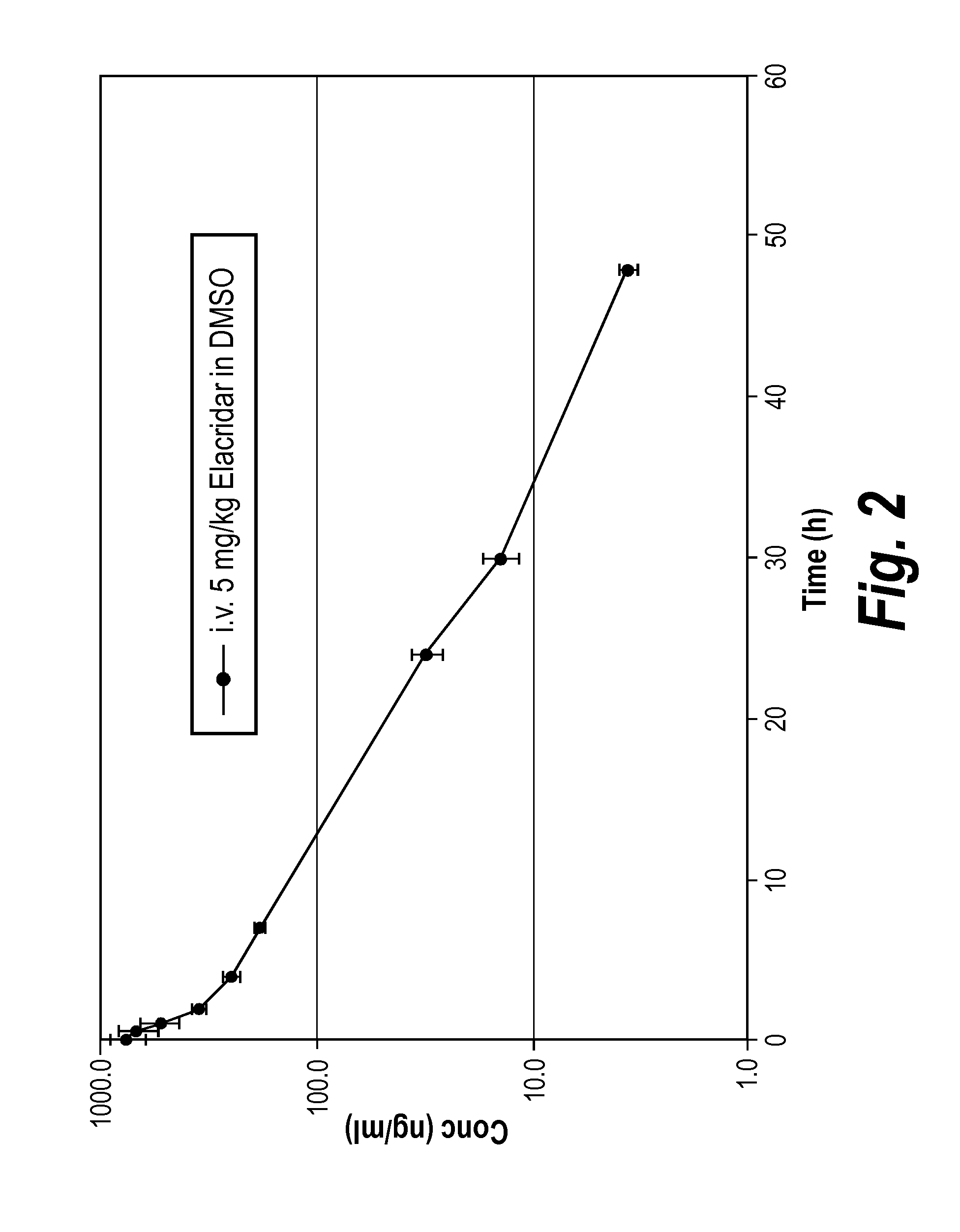
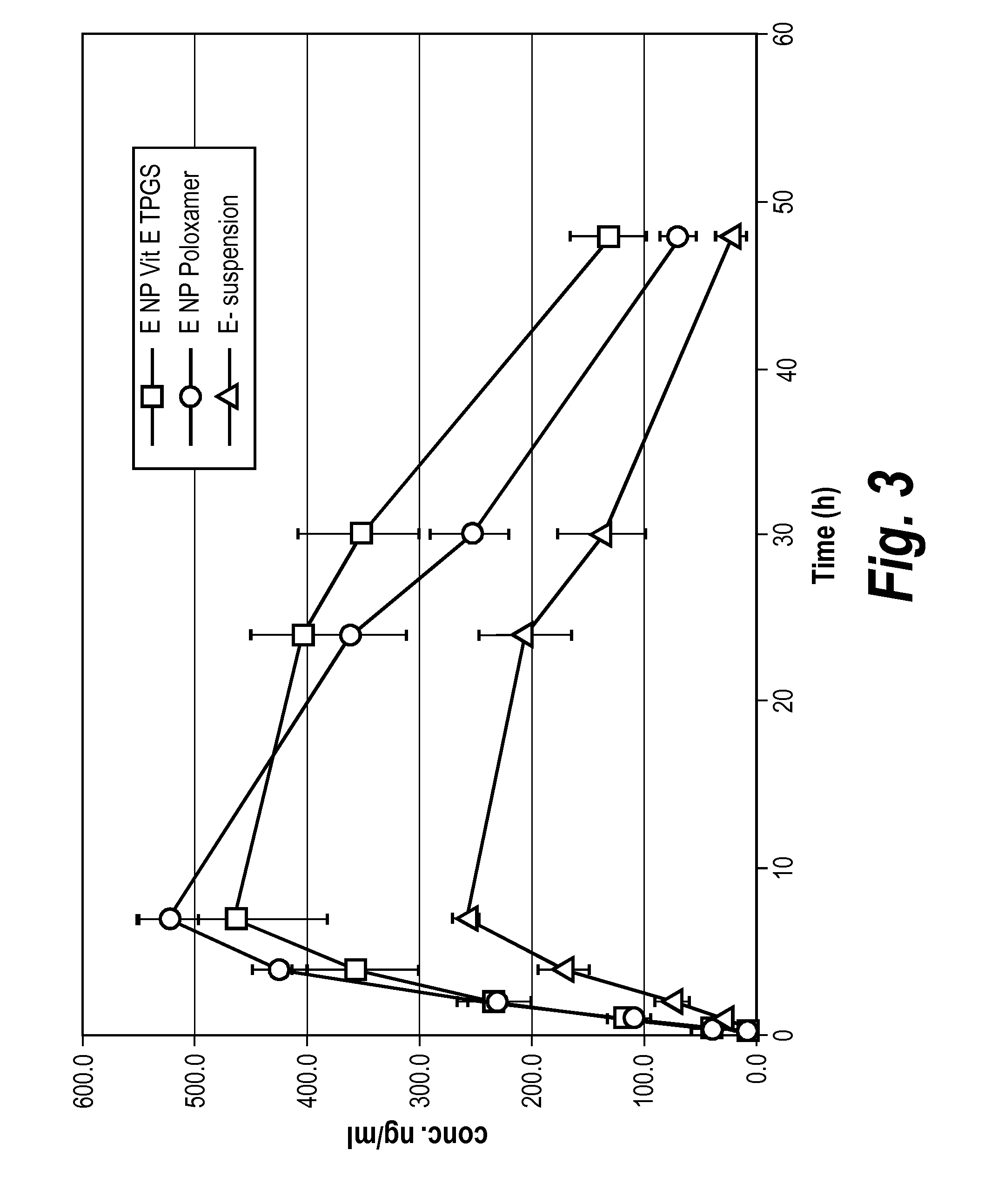
Efflux inhibitor compositions and methods of treatment using the same
InactiveUS20140235631A1Improve blood-brain barrier permeabilityBiocidePowder deliveryIntestinal structureHematopoietic cell
The present invention relates to efflux inhibitor compositions and methods of using these agents for treating conditions where the activity of efflux transporter proteins (e.g., Breast Cancer Resistance Protein (BCRP) and P-Glycoprotein (P-GP)) inhibit effective delivery of a therapeutic agent to a target tissue (e.g., brain, spinal cord, nerves, cerebrospinal fluid, testis, eyeballs, retina, inner ear, placenta, mammary gland, liver, biliary tract, kidney, intestines, lung, adrenal cortex, endometrium, hematopoietic cells, and / or stem cells).
Owner:IZUMI TECH +1
Methods, Devices, Kits and Systems for Defunctionalizing the Gallbladder
The application discloses devices, systems, kits and methods for treating biliary disease. Device comprise, for example, a component configured for deployment to a lumen of a gallbladder or gallbladder duct which has a proximal end and a distal end with a lumen extending therethrough and a fluid or gas delivery apparatus at its distal end.
Owner:TREUS MEDICAL
Coated implantable medical device
A coated implantable medical device 10 includes a structure 12 adapted for introduction into the vascular system, esophagus, trachea, colon, biliary tract, or urinary tract; at least one coating layer 16 posited on one surface of the structure; and at least one layer 18 of a bioactive material posited on at least a portion of the coating layer 16, wherein the coating layer 16 provides for the controlled release of the bioactive material from the coating layer. In addition, at least one porous layer 20 can be posited over the bioactive material layer 18, wherein the porous layer includes a polymer and provides for the controlled release of the bioactive material therethrough. Preferably, the structure 12 is a coronary stent. The porous layer 20 includes a polymer applied preferably by vapor or plasma deposition and provides for a controlled release of the bioactive material. It is particularly preferred that the polymer is a polyamide, parylene or a parylene derivative, which is deposited without solvents, heat or catalysts, and merely by condensation of a monomer vapor.
Owner:COOK MEDICAL TECH LLC
Methods, Devices, Kits and Systems for Defunctionalizing the Cystic Duct
The application discloses devices, systems, kits and methods for treating biliary disease. Devices comprise, for example, a component configurable for deployment between within a cystic duct of a patient which has a proximal end and a distal end. In some embodiments, a lumen may also extend therethrough.
Owner:TREUS MEDICAL
Biodegradable double stent
A biodegradable double stent is used for various organs such as a biliary tract, an esophagus, an airway and a ureter. A biodegradable stent having a hollow cylindrical body woven out of a separate wire made of biodegradable polymer so as to have a plurality of rhombic spaces is fixed to an intermediate portion of a primary stent having a cylindrical body. When administered into the organ, the biodegradable stent has its original function of expanding the organ, and is firmly supported in the inner wall of a narrowed passage of the organ by pressurizing the inner wall of the narrowed passage of the organ. After a predetermined time period has elapsed, the biodegradable stent is gradually degraded away by bodily fluids, thereby enabling the primary stent to be easily removed from the organ.
Owner:TAEWOONG MEDICAL CO LTD +1
Medicinal composition for treating cholecystitis
InactiveCN100998843ANo side effectsWith preventionDigestive systemPlant ingredientsMedicineLiver function
A Chinese medicine for preventing and treating cholecystitis, cholelithiasis and the inflammation of biliary tract, and improving the function of gallbladder and liver is prepared from 23 Chinese-medicinal materials including tackahoe, capejasmine fruit, white peony root, cyprus tuber, etc.
Owner:曹国胜
Drug Coated Balloon Catheters for Nonvascular Strictures
ActiveUS20150273117A1Facilitated releasePromote absorptionBalloon catheterAntipyreticEsophageal stenosisBiliary tract
Embodiments of the present invention provide a method for treatment of nonvascular body lumen strictures such as benign prostatic hyperplasia (BPH), urethral strictures, ureteral strictures, prostate cancer, esophageal strictures, sinus strictures, biliary tract strictures, asthma and chronic obstructive pulmonary disease (COPD). The method involves delivering, preferably via drug coated balloon catheters, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues) and one or more additives.
Owner:UROTRONIC
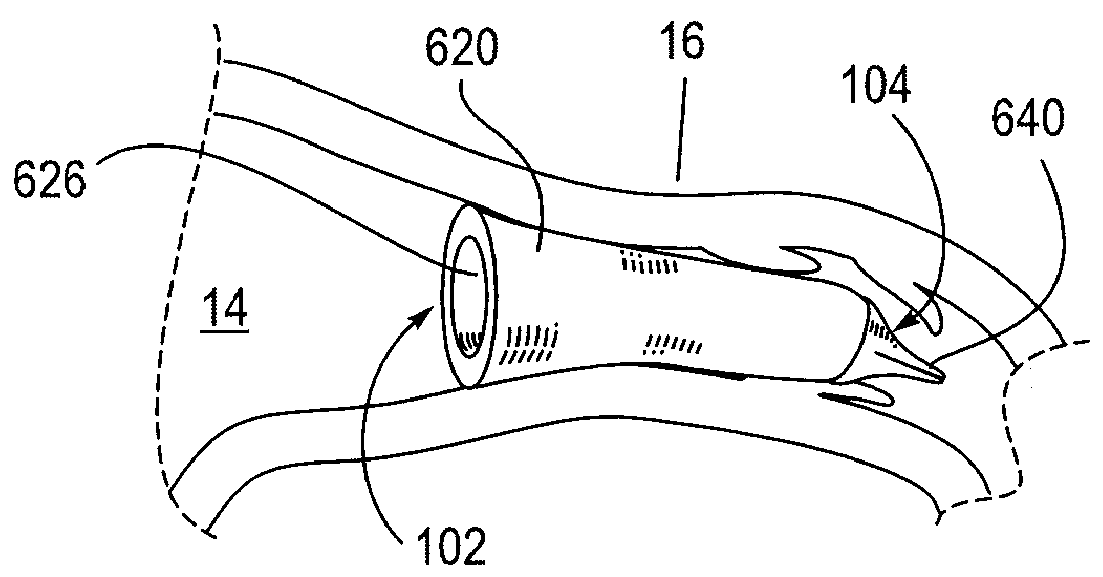
Biliary System Catheter
A catheter system and a method for accessing the bile duct are provided. The catheter system includes an elongate shaft having a proximal portion and a distal portion. The catheter also includes a first lumen extending at least partially through the elongate shaft, a distal portion of the first lumen extending generally parallel to a longitudinal axis of the elongate shaft and a distal port operably connected to the first lumen. The catheter system also includes a second lumen extending at least partially through the elongate shaft and a side port operably connected to the second lumen, the side port positioned proximal to the distal port and operably connected to the second lumen. The second lumen includes an angled wall portion adjacent to the side port. A marker is included on the distal portion of the elongate shaft, the marker configured to indicate the position of the side port.
Owner:COOK MEDICAL TECH LLC
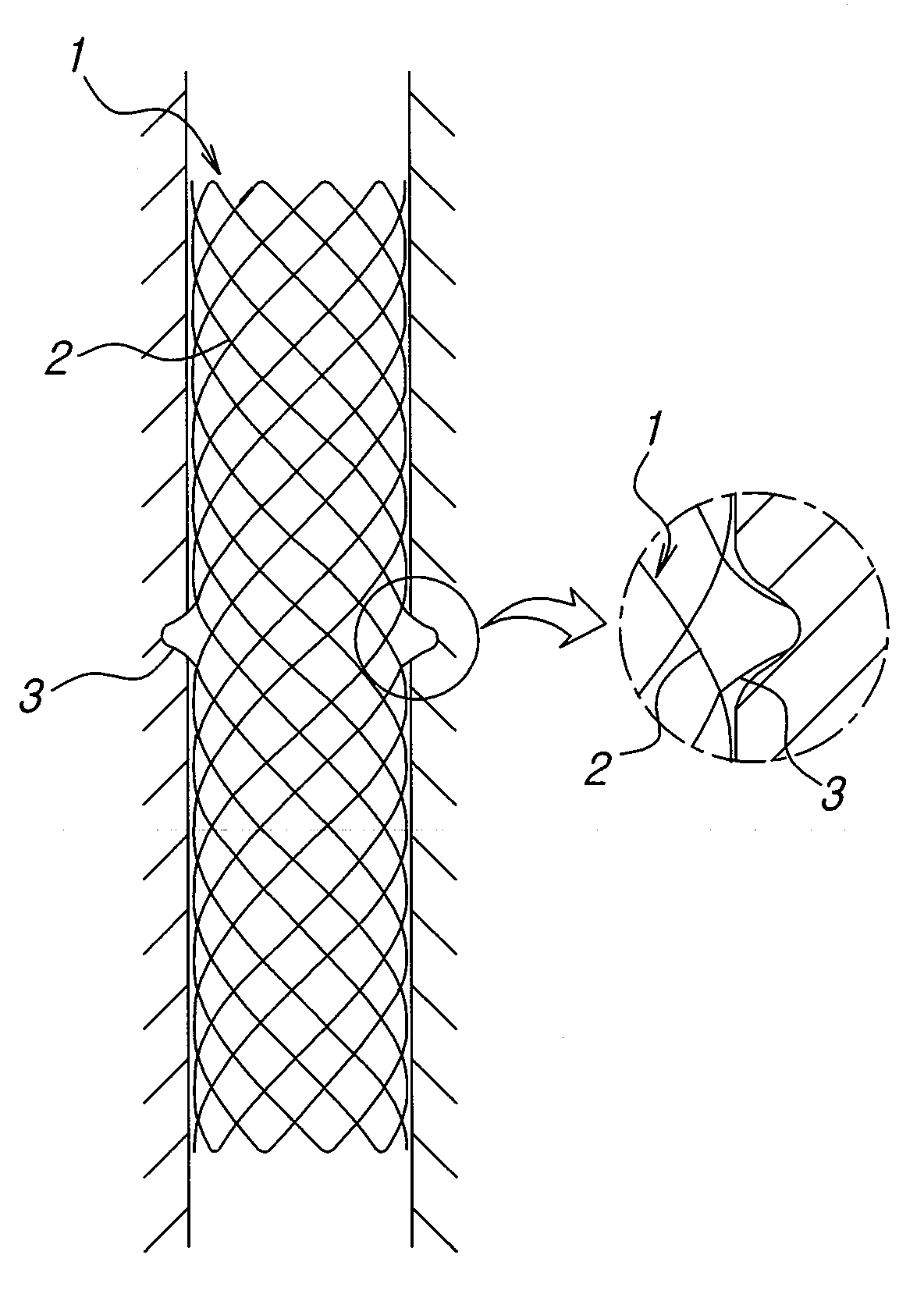
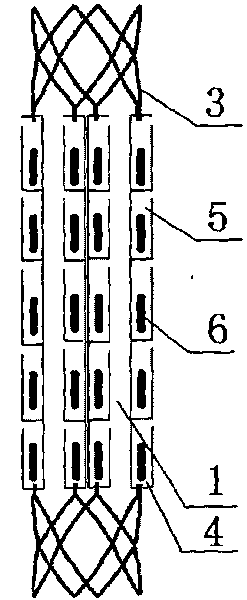
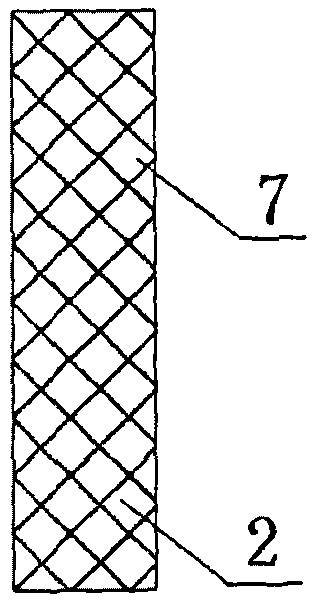
Biliary tract radiation treatment stent
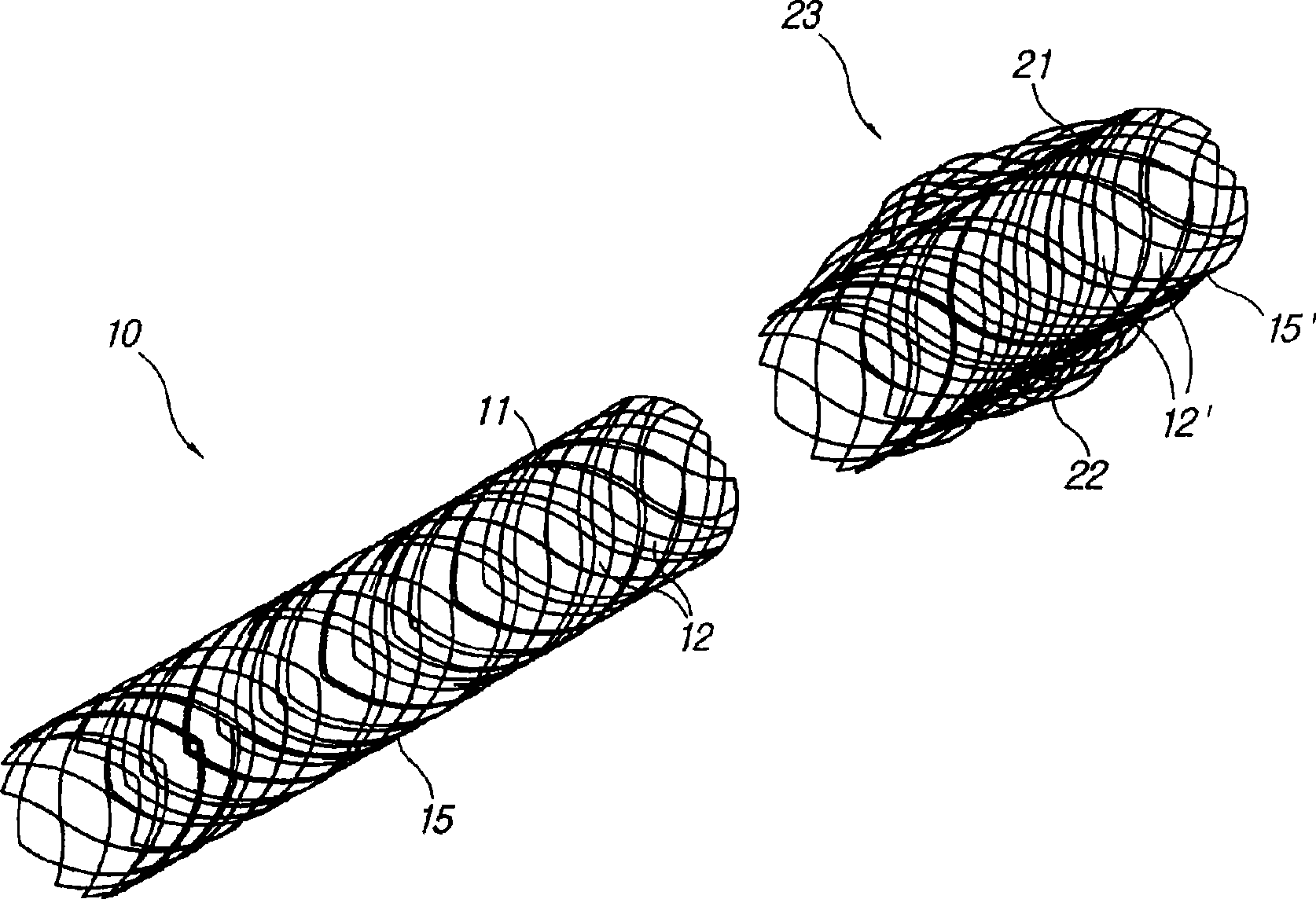
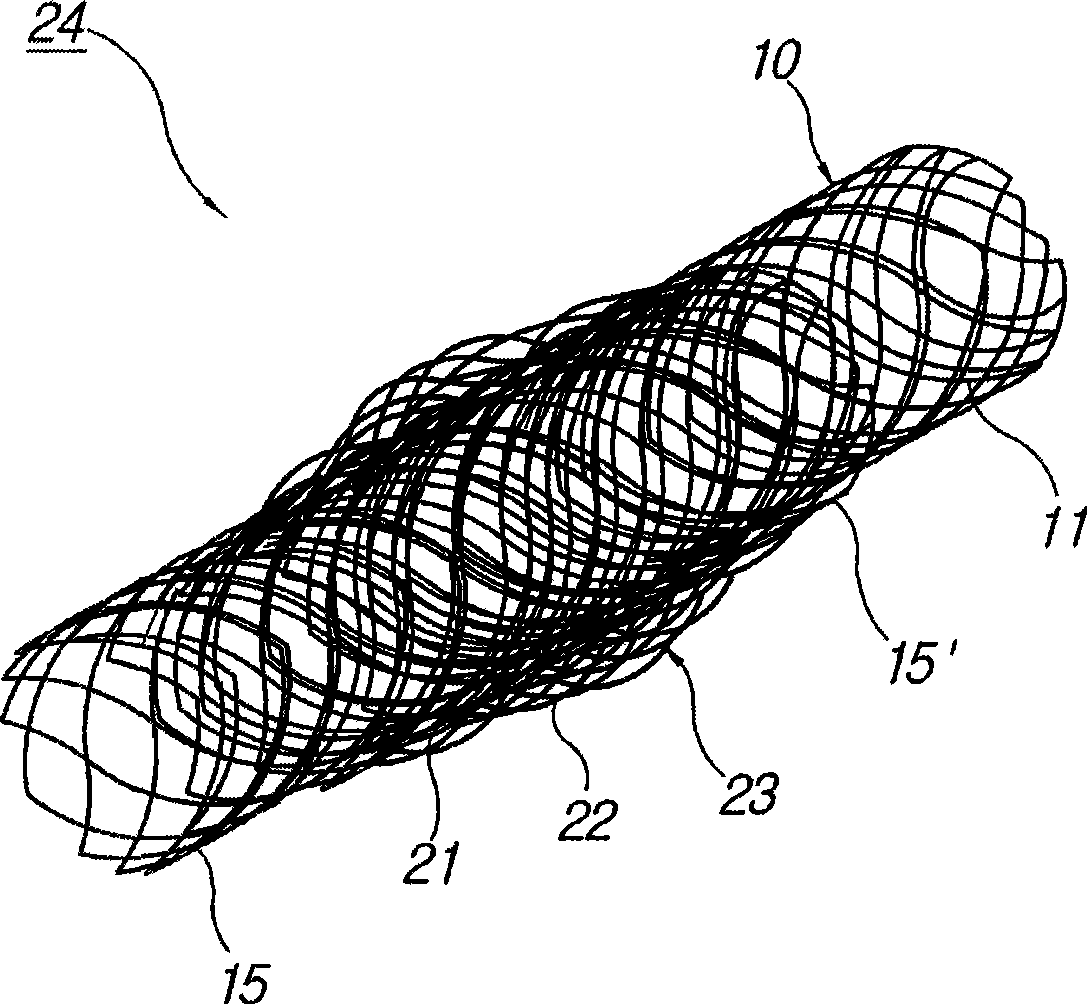
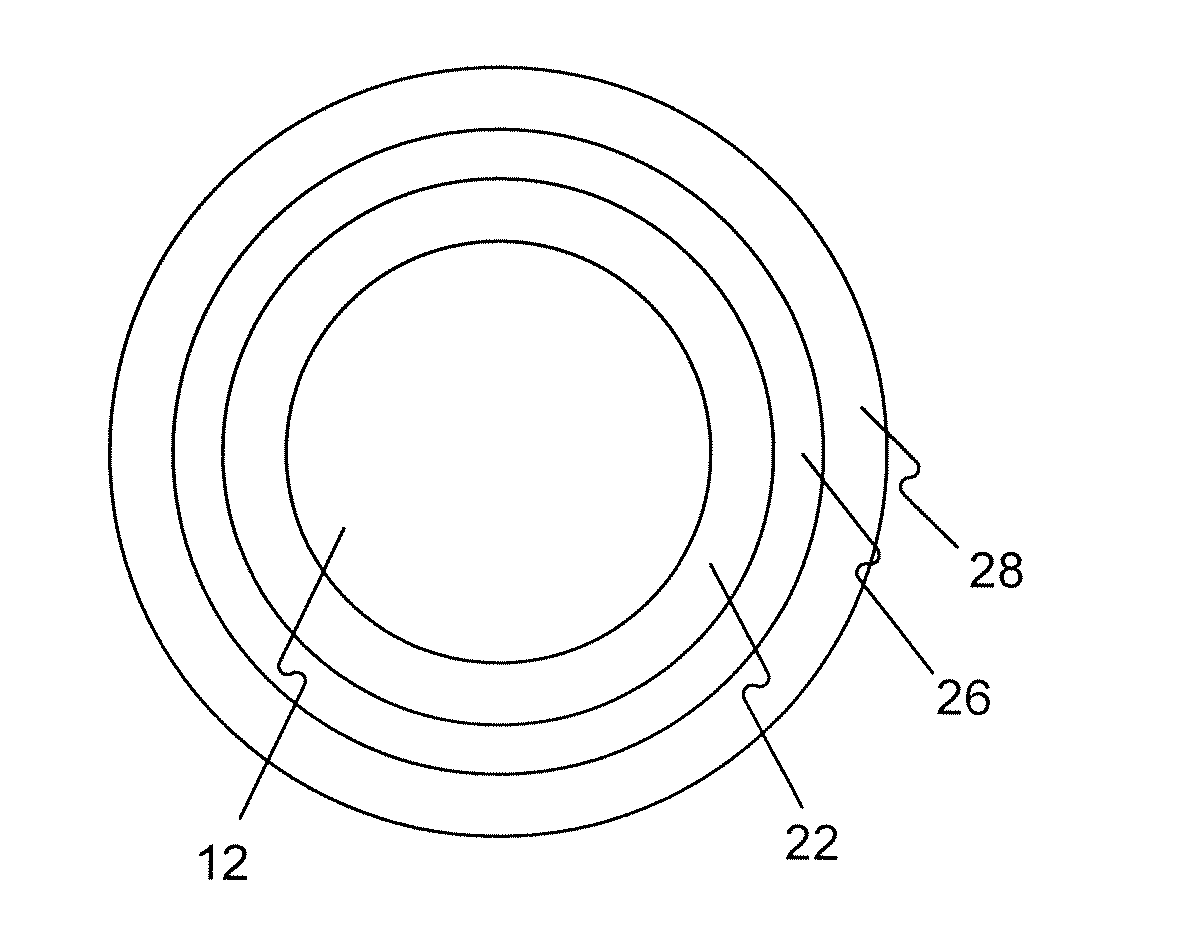
The invention relates to a biliary tract radiation treatment stent which is characterized by comprising an outer stent (1) and an inner stent (2), wherein the main part of the inner stent (2) is expanded in the outer stent (1) in a use state, the main body of the inner stent (2) is a columnar grid framework structure (7) formed by weaving nickel and titanium wires; and the main body of the outer stent (1) is a mesh framework structure (3) formed by weaving nickel and titanium wires, and a radiation particle filling bag (4) is arranged on the surface of the mesh framework structure (3). The invention has the advantages of simple and novel structure, minimally invasive procedure, low risk, little pain of patients, good treatment effect and less complications.
Owner:MICRO TECH (NANJING) CO LTD +1
Anastomotic device
InactiveUS20060264986A1Permit ease of reloadingSurgical needlesEndoscopic cutting instrumentsStapling procedureNose
Owner:PARK MEDICAL LLC
Drug coated balloon catheters for nonvascular strictures
ActiveUS20180104383A1Facilitated releaseImprove permeabilityOrganic active ingredientsBalloon catheterBiliary tractObstructive Pulmonary Diseases
Embodiments of the present invention provide a method of treating a stricture in a nonvascular body lumen such as urethral strictures, benign prostatic hyperplasia (BPH) strictures, ureteral strictures, esophageal strictures, sinus strictures, and biliary tract strictures. Embodiments of the present invention provide a method for treating at least one of benign prostatic hyperplasia (BPH), prostate cancer, asthma, and chronic obstructive pulmonary disease (COPD). The method can include delivering, for example, via drug coated balloon catheters, anti-inflammatory and anti-proliferative drugs (e.g., rapamycin, paclitaxel, and their analogues) and one or more additives.
Owner:UROTRONIC
Methods and devices for treating obesity
Methods and devices for treating obesity are provided. The consumption of calorie dense, lipid rich, or fatty foods is discouraged through the modulation of a subjects gallbladder function or output. Disclosed are devices and methods for delivering devices within the gallbladder and associated ducts and vasculature; other methods involve implanting devices on or around the gallbladder and associated ducts and vasculature. Further treatments involve the use of energy, surgery, or chemicals to alter the function of the gallbladder and biliary system.
Owner:BOYAJIAN TOM +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com