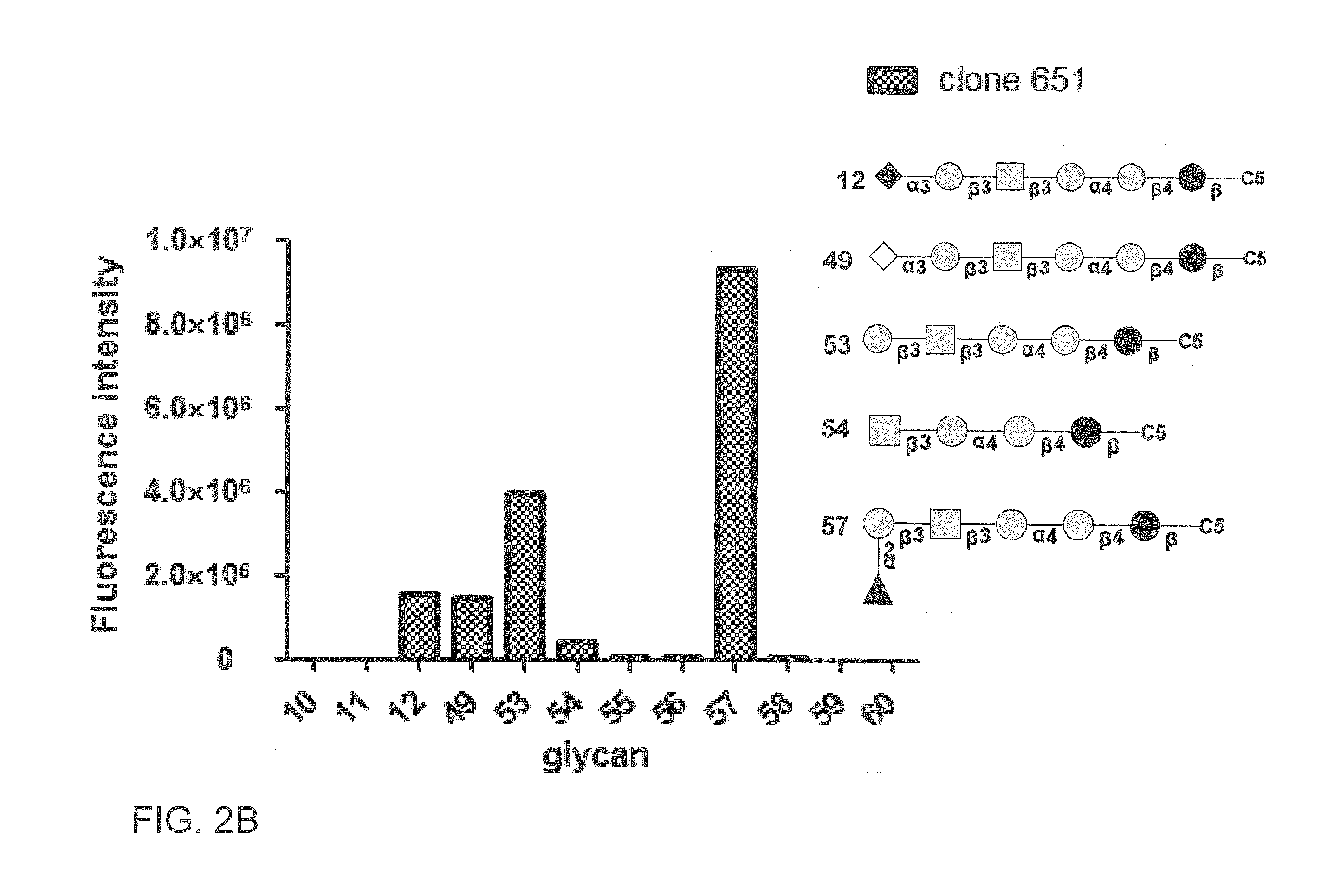
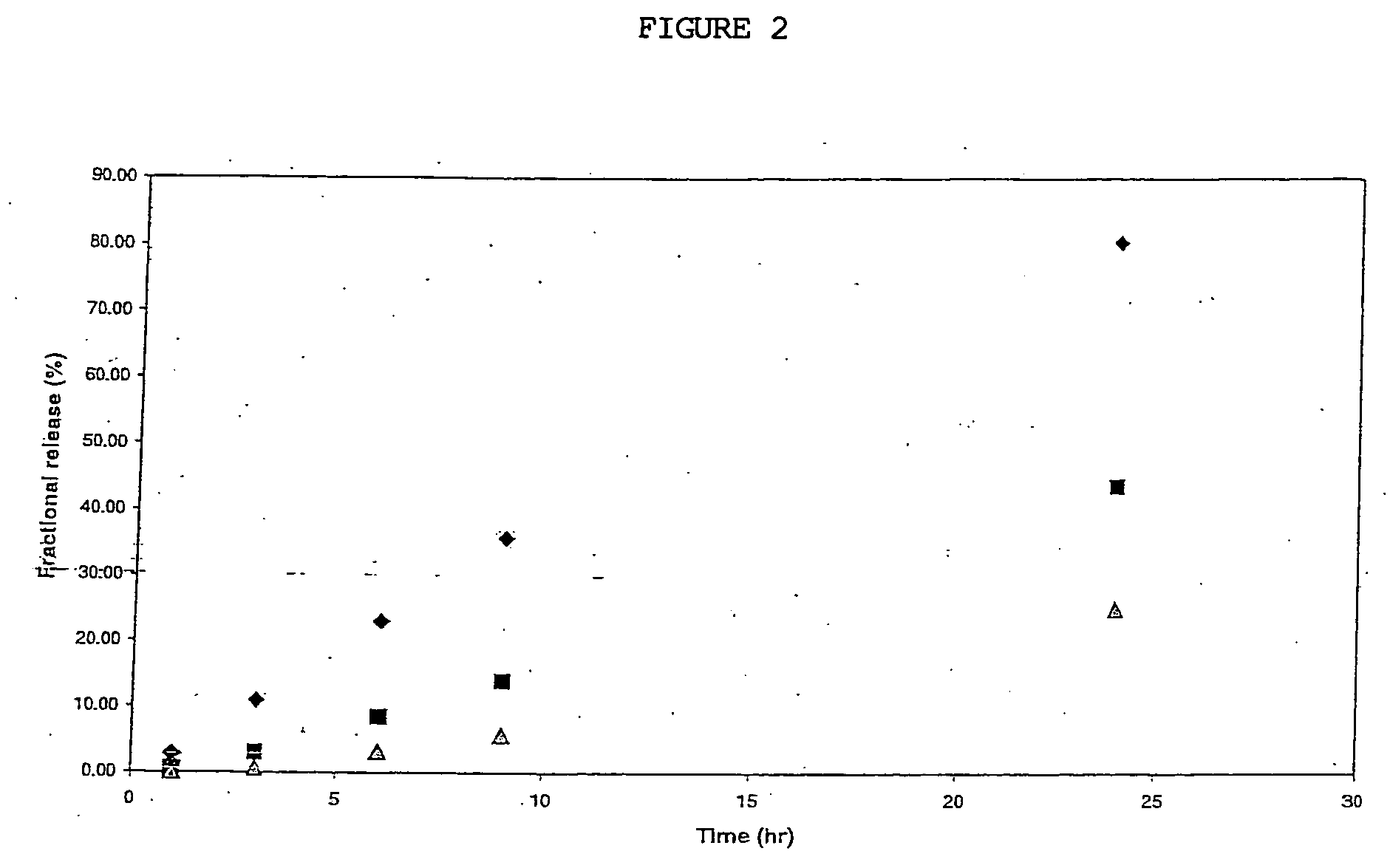
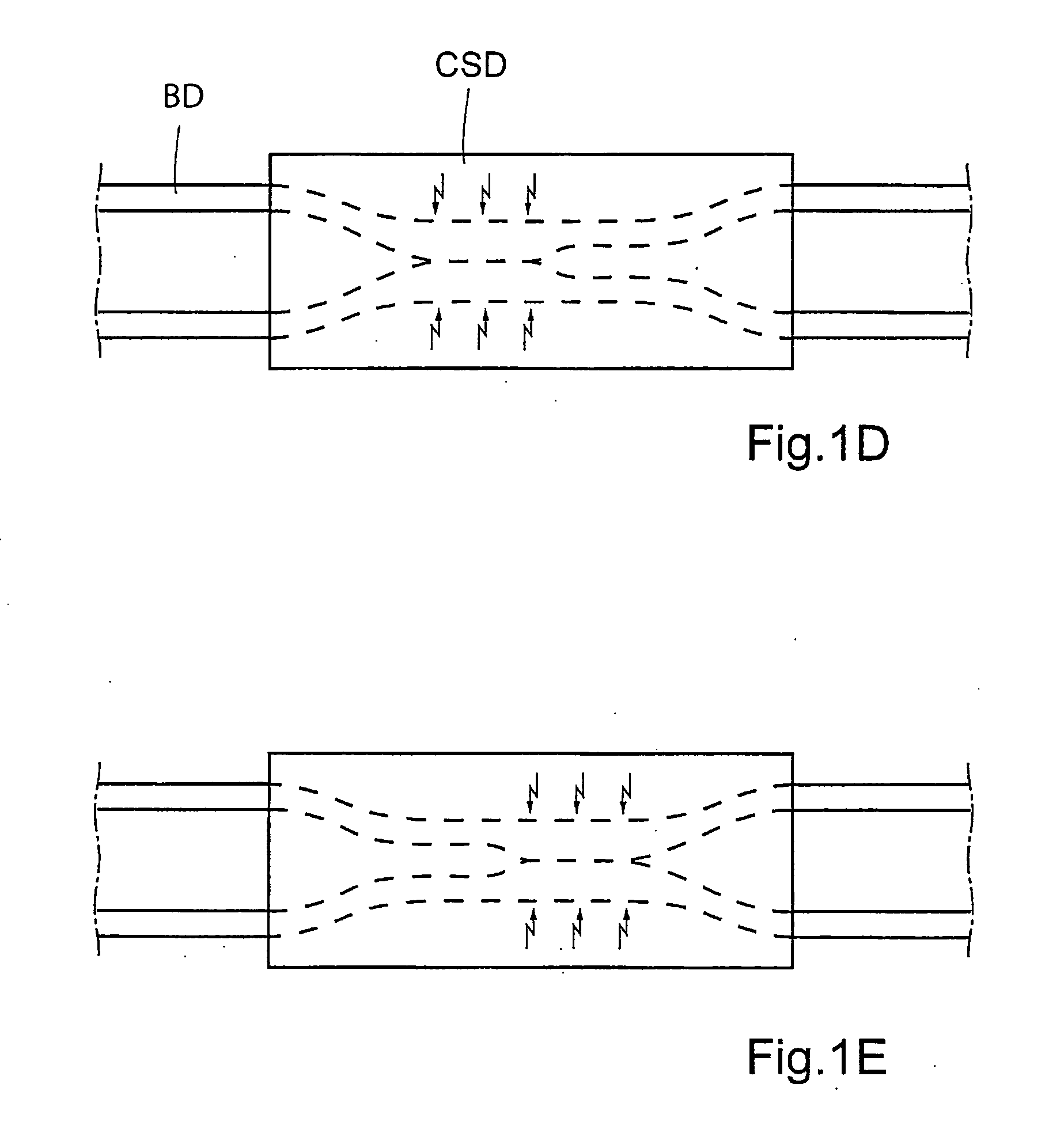
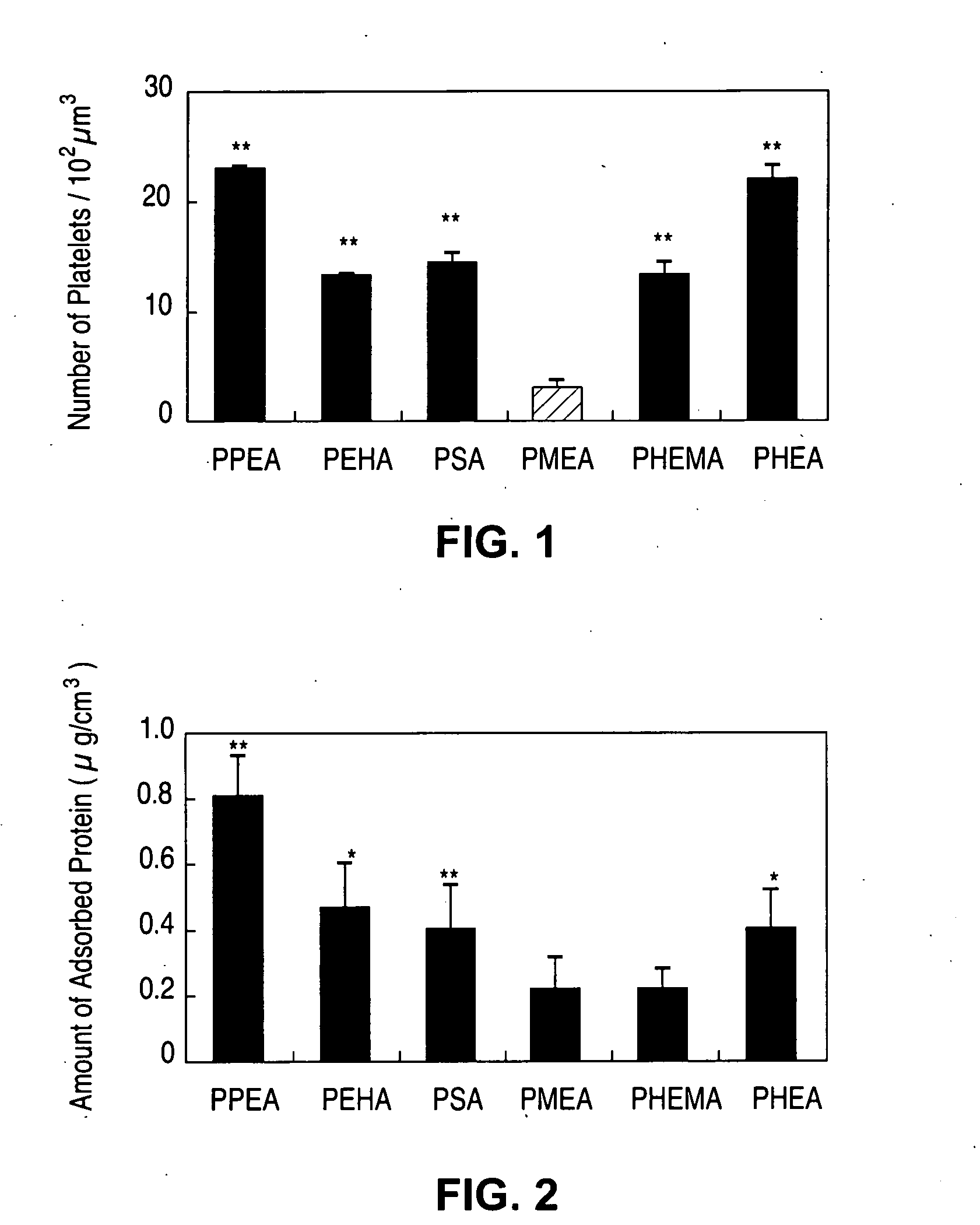
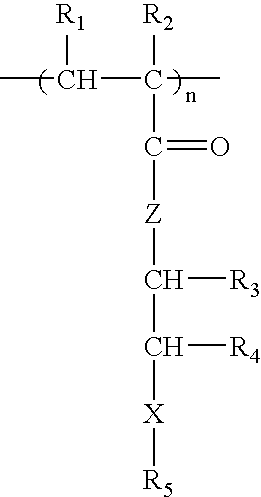
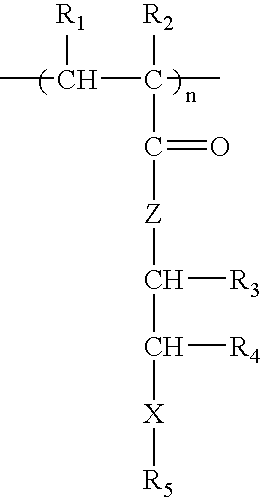
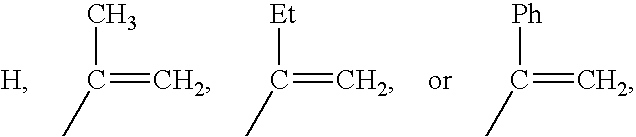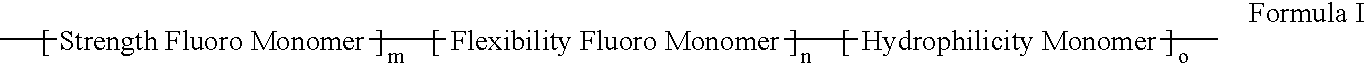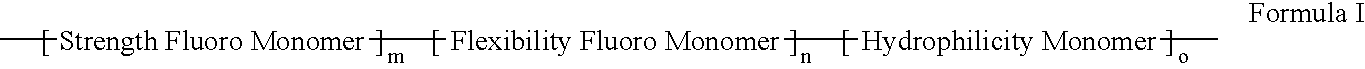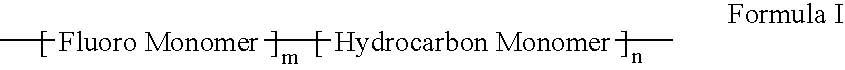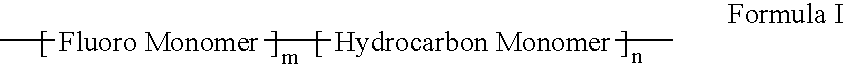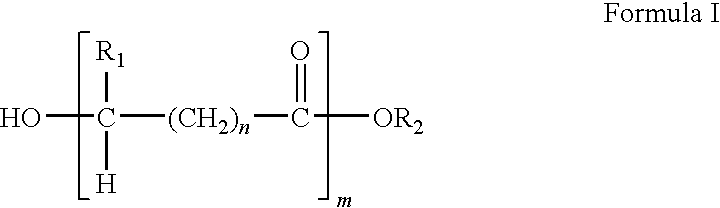Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
432 results about "Bile duct" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A bile duct is any of a number of long tube-like structures that carry bile, and is present in most vertebrates. Bile, required for the digestion of food, is secreted by the liver into passages that carry bile toward the hepatic duct, which joins with the cystic duct (carrying bile to and from the gallbladder) to form the common bile duct, which opens into the intestine.
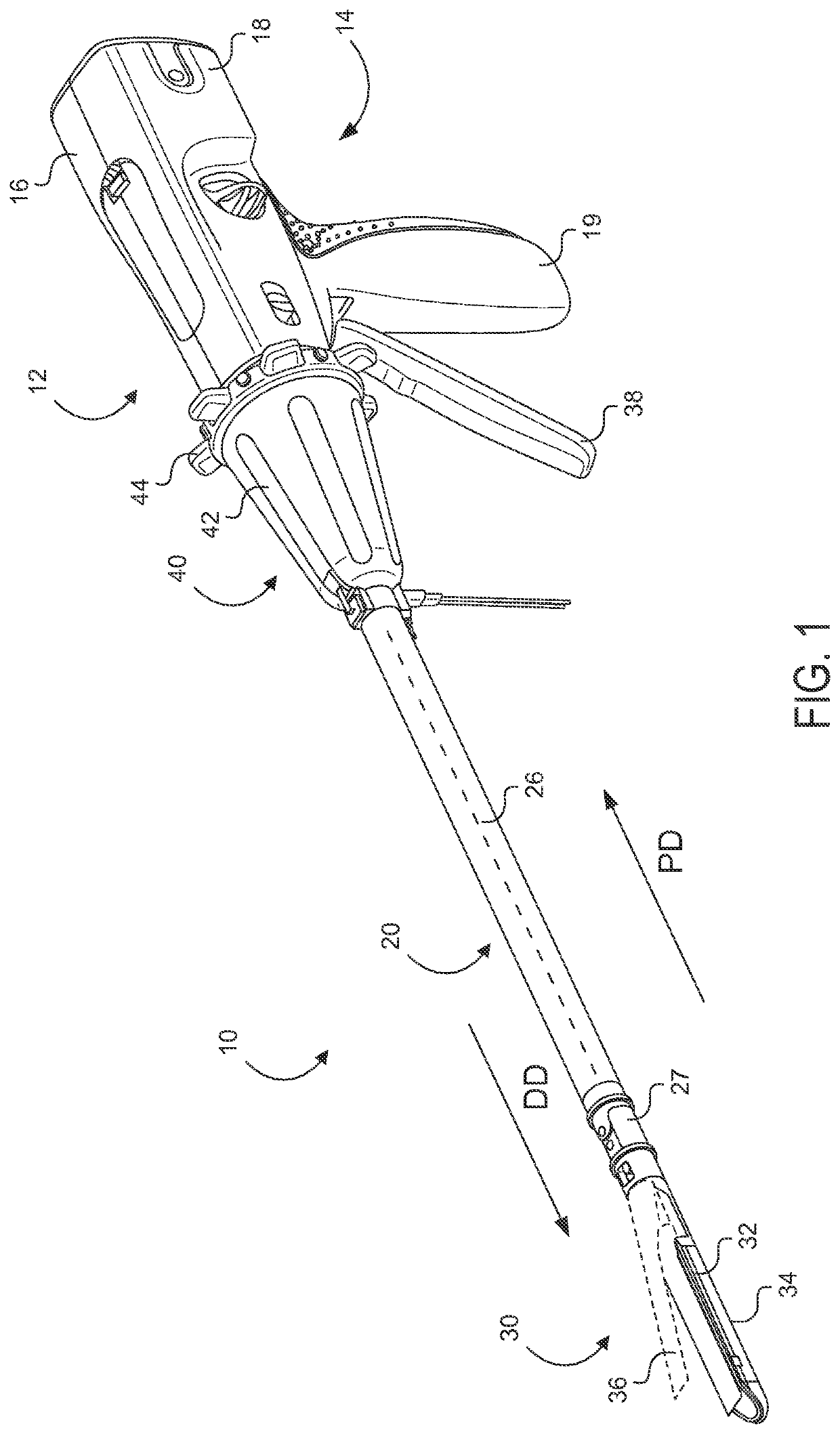
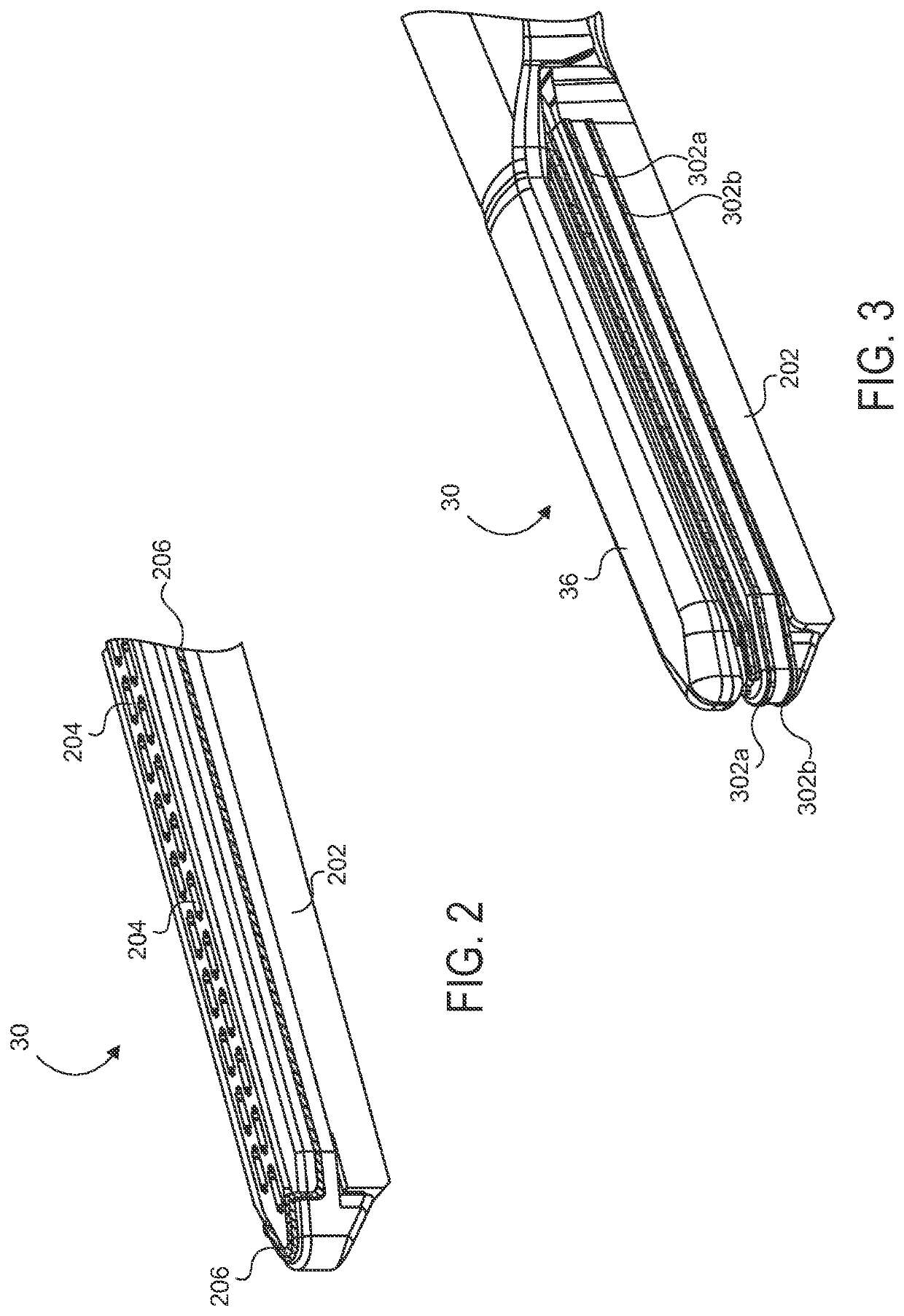
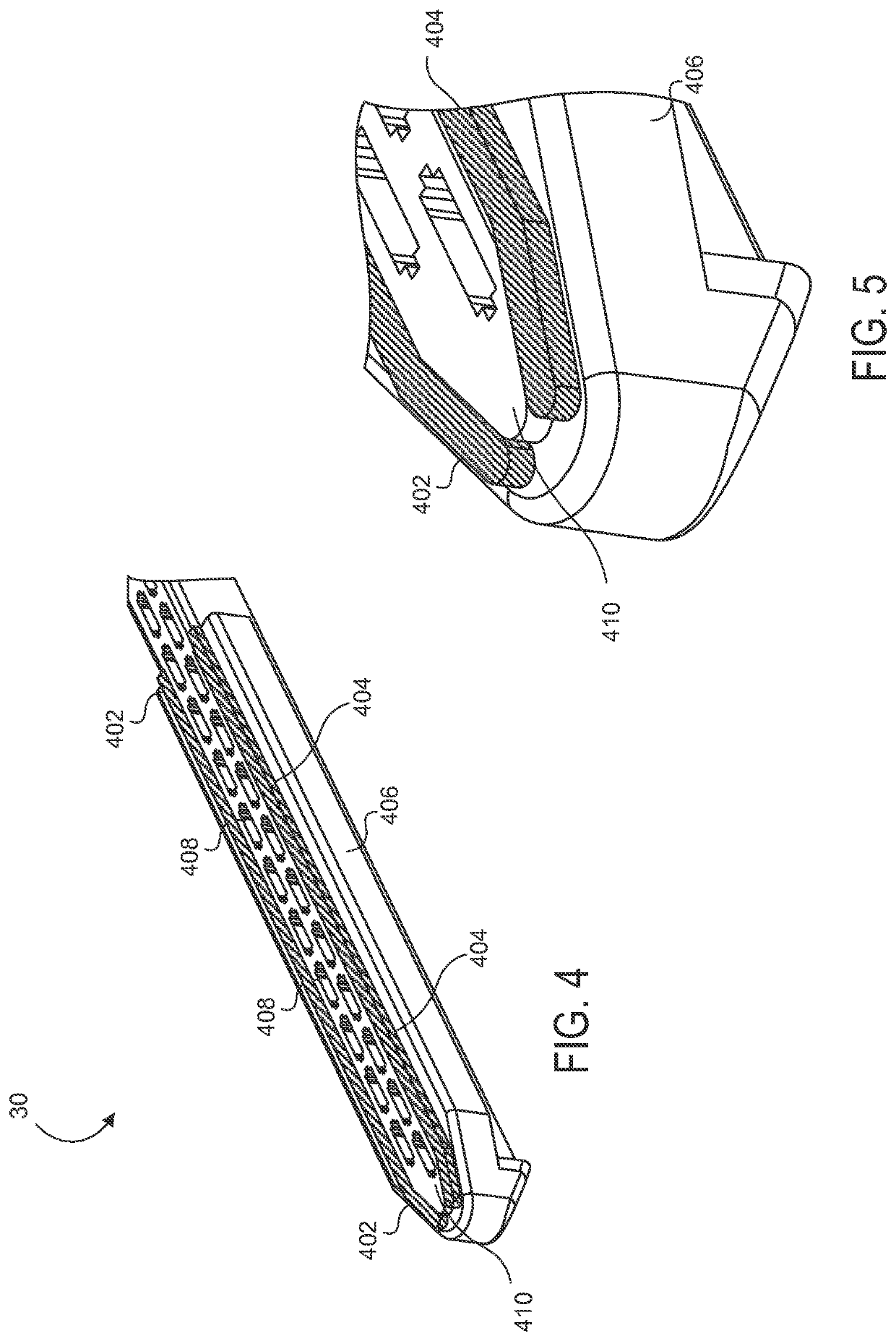
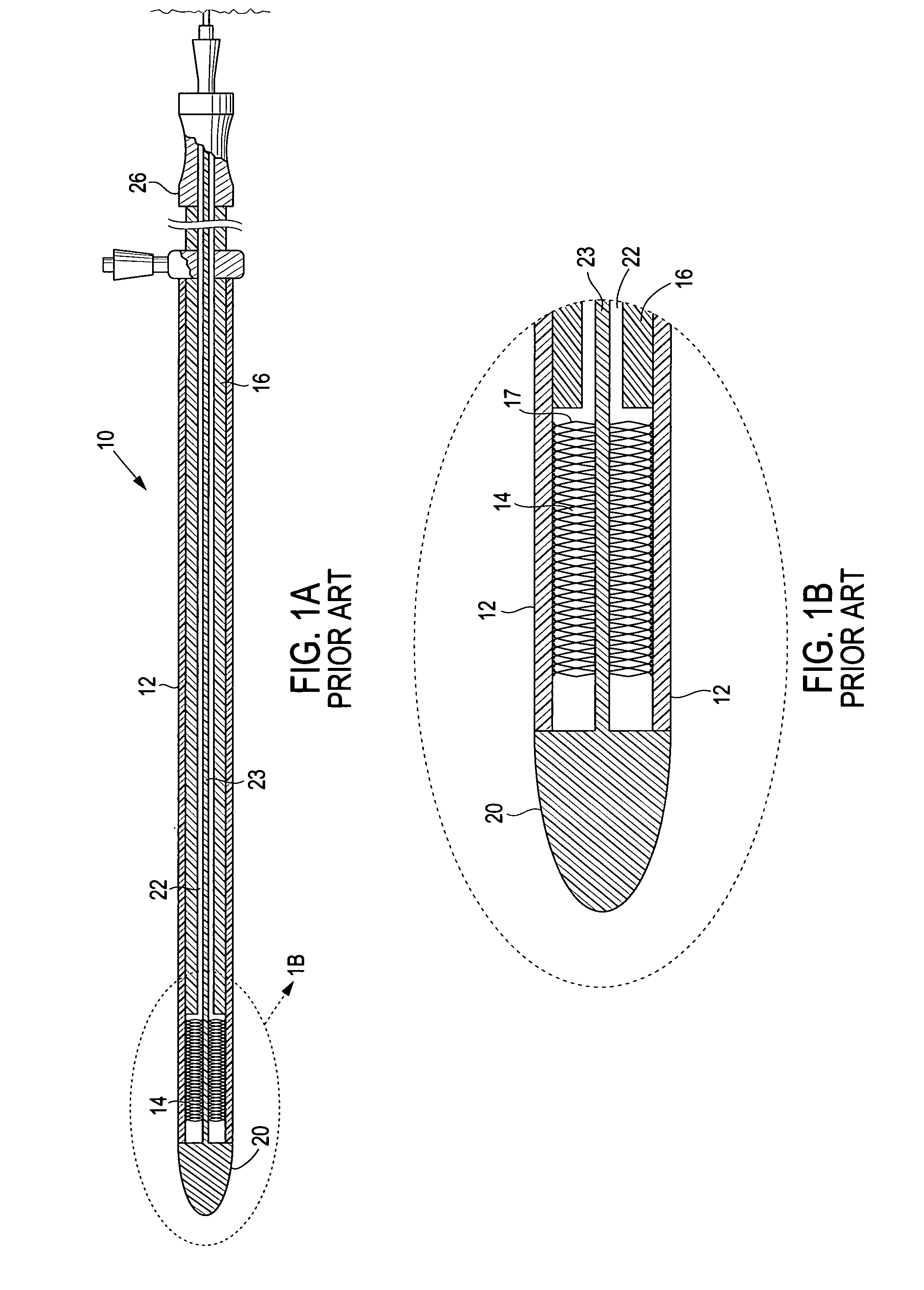
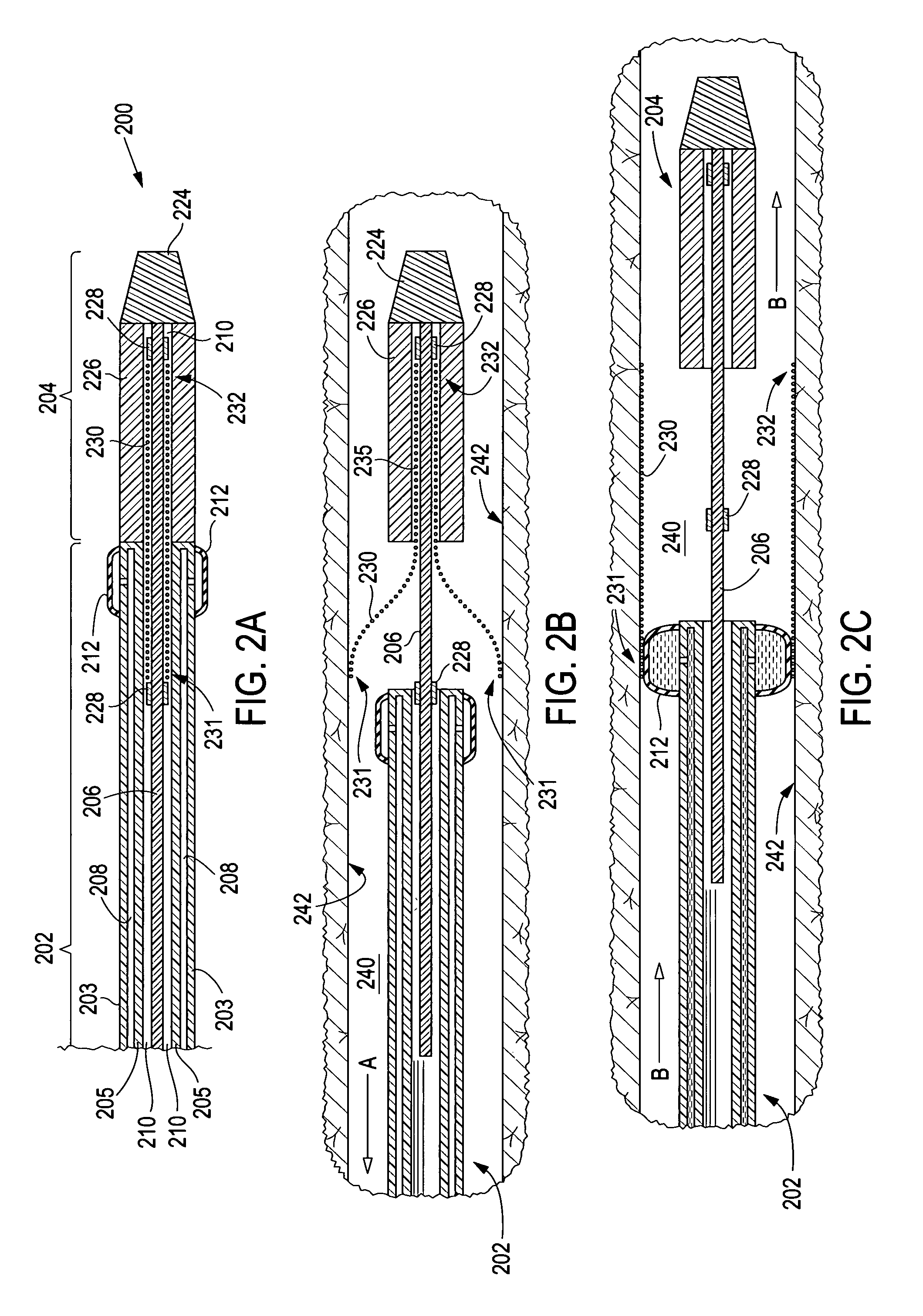
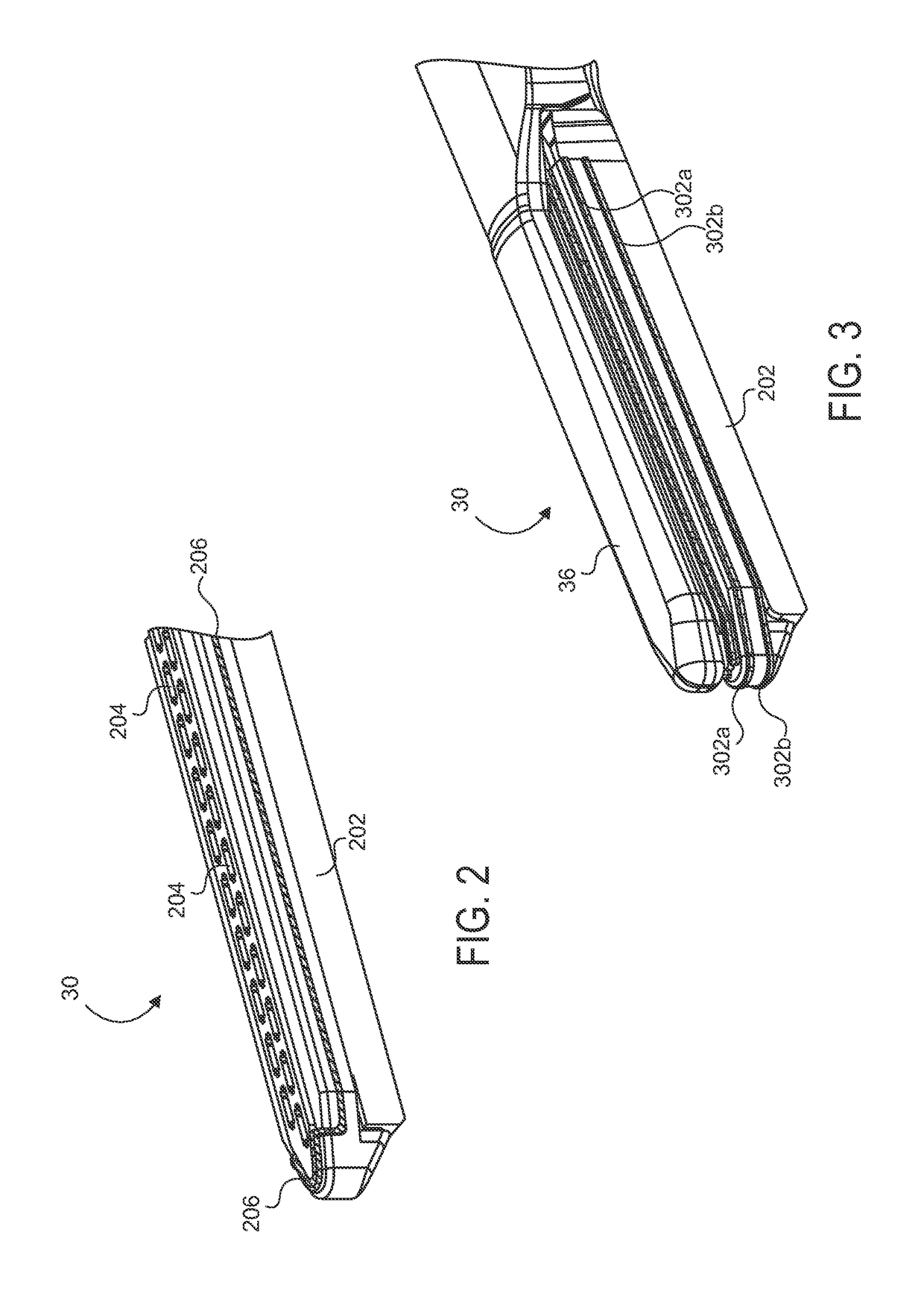
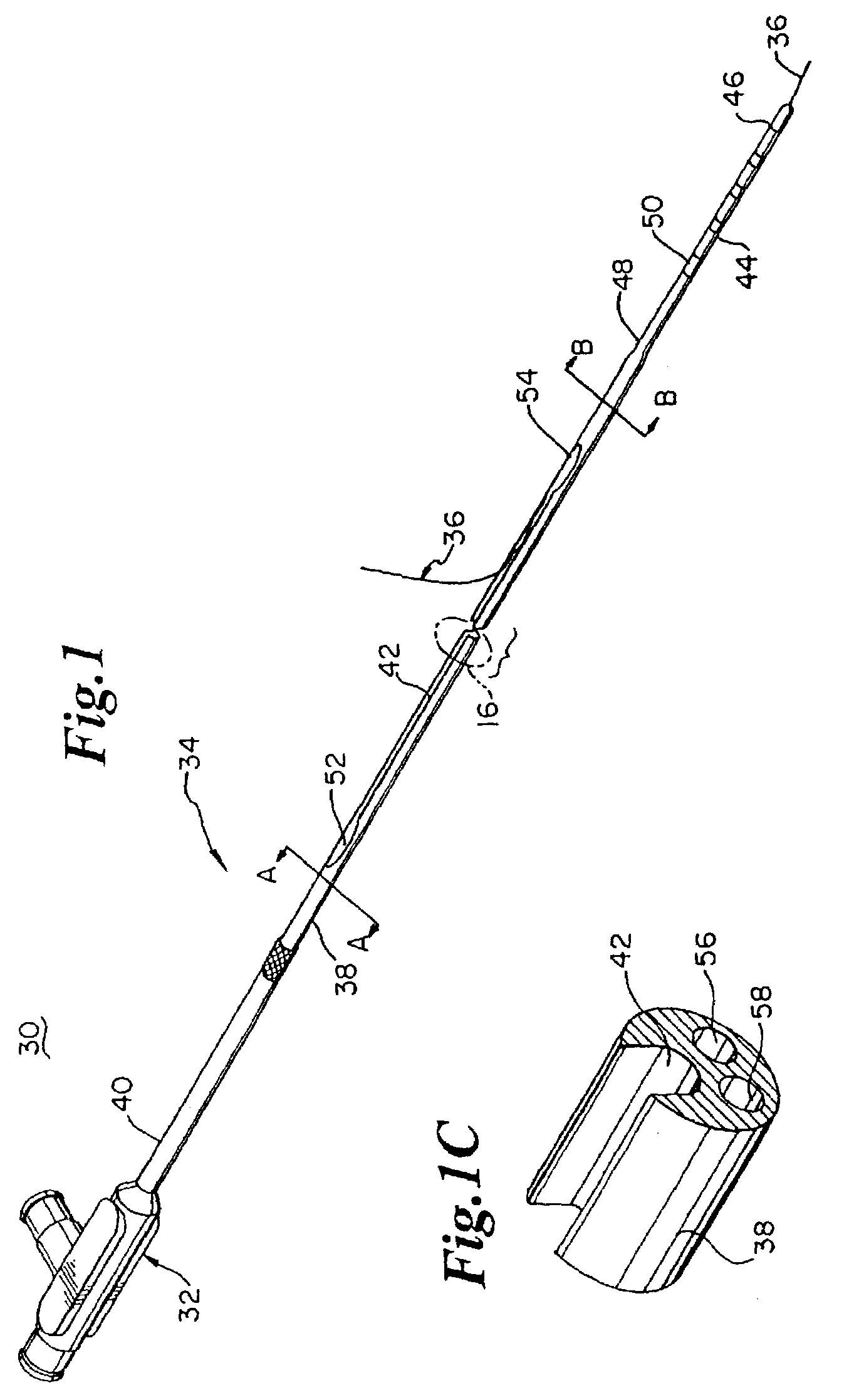
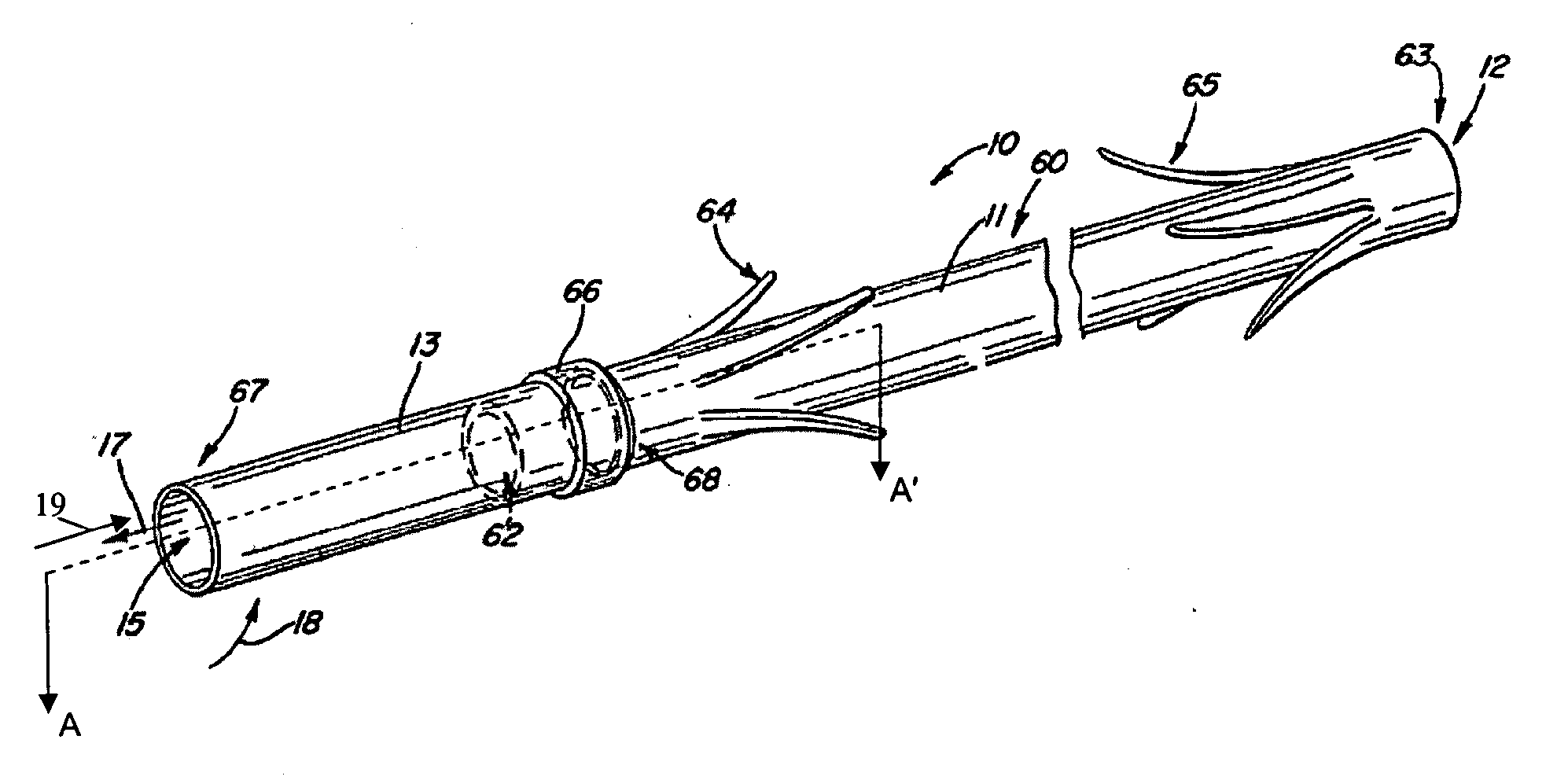
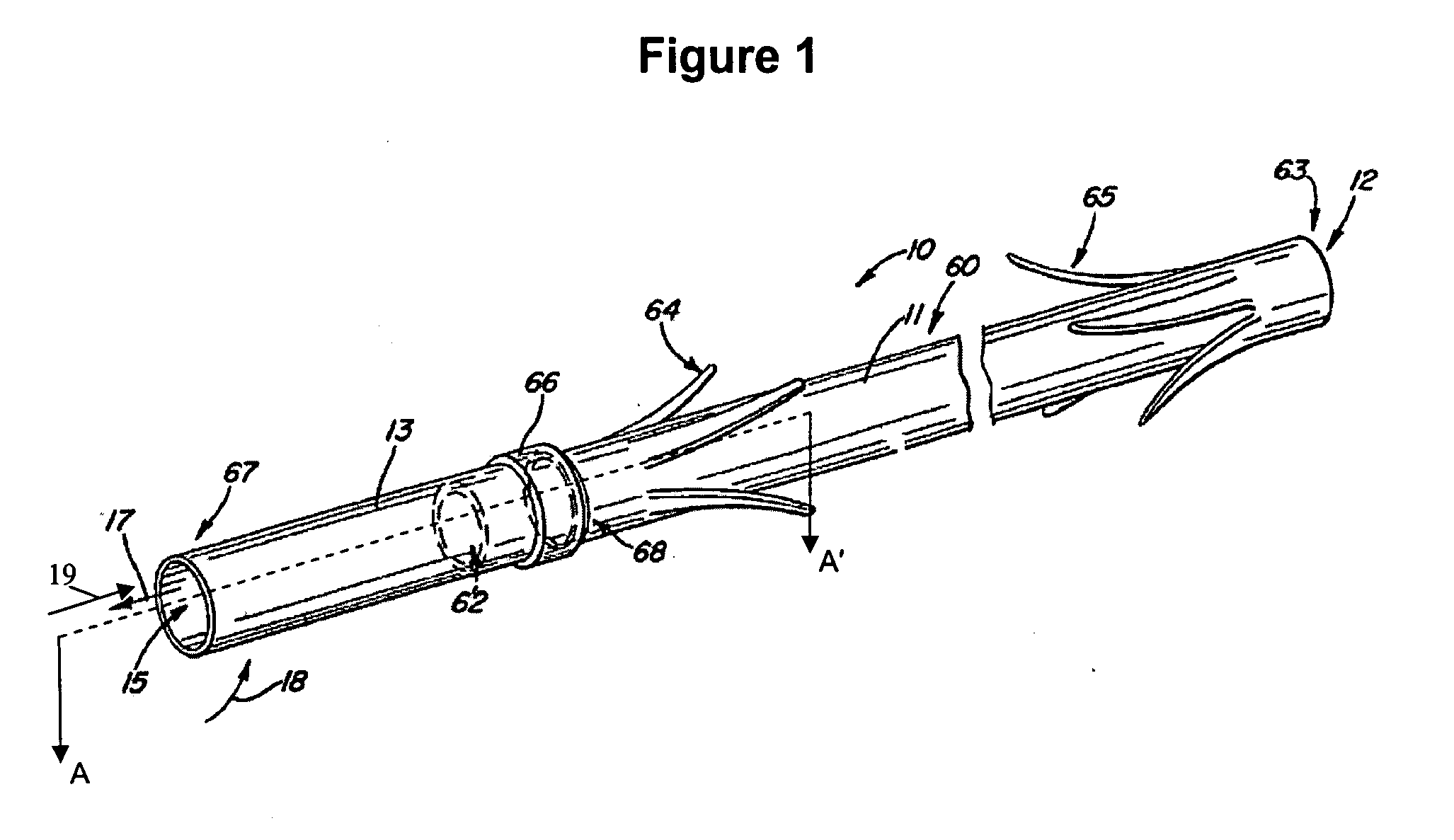
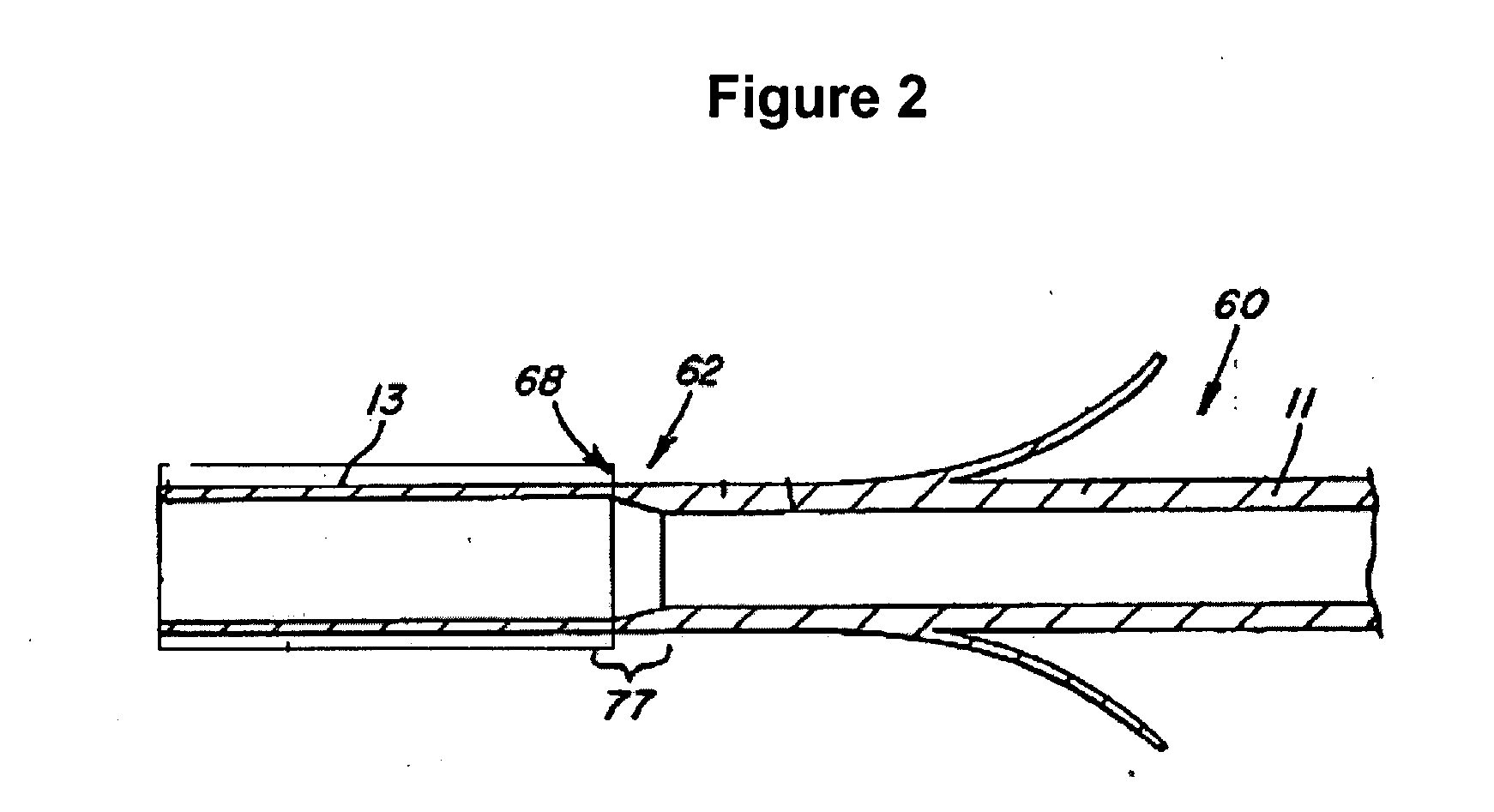
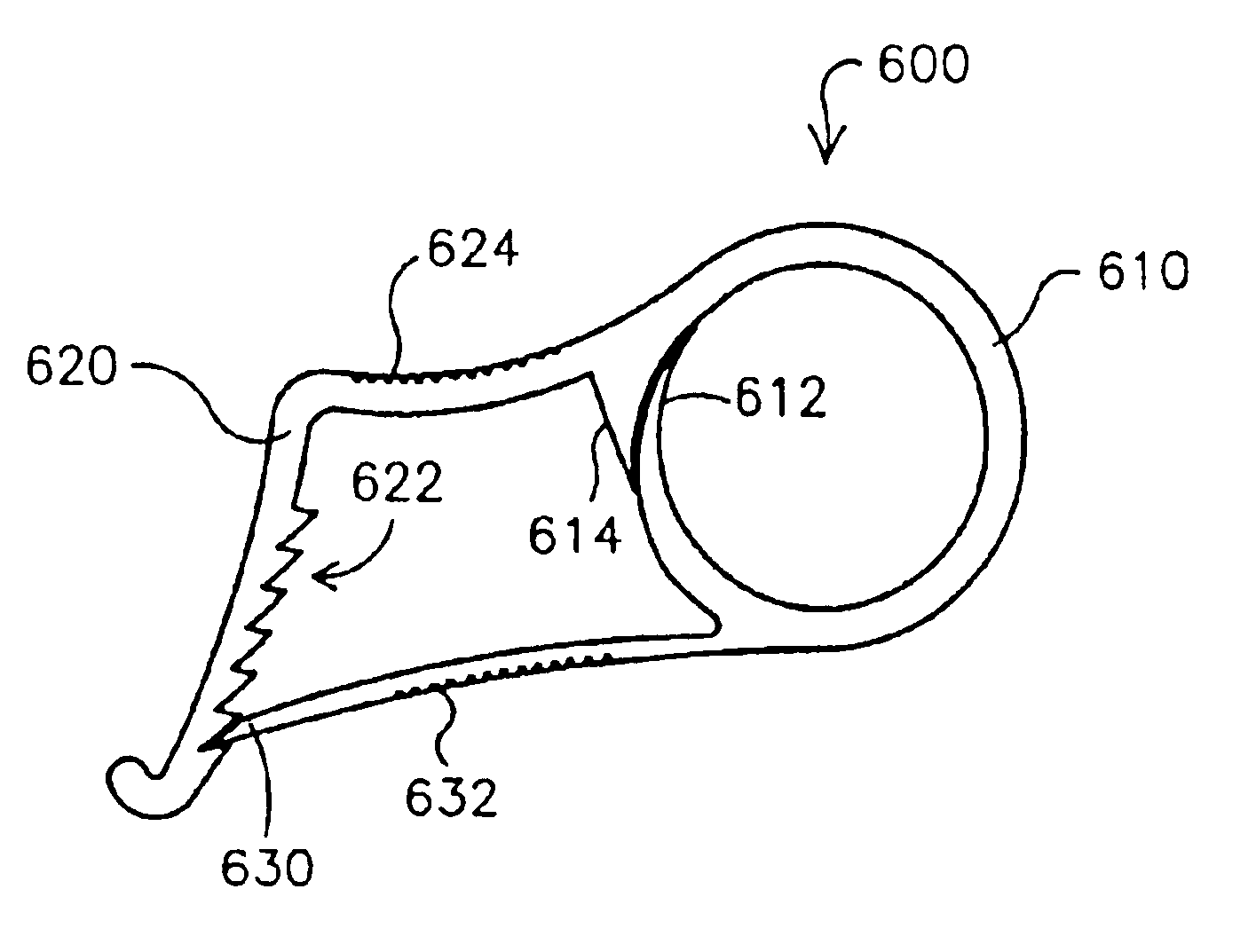
Electrode wiping surgical device
Aspects of the present disclosure include a surgical device comprising electrodes on the sides of an end of an effector to aide in sealing during various surgical procedures, such as a liver resection. During a sealing procedure, the surgeon may wipe the surgical site with the end effector, causing the electrodes to touch the fractured area. Electrosurgical energy may be applied to the electrodes during the wiping, causing coagulation of smaller vessels, such as tiny blood vessels and bile ducts in the parenchyma of the liver. In some cases, due to the nature of some smaller vessels, electrosurgical energy should be delicately applied to cause sealing but to avoid overly damaging the remaining tissue. In some embodiments, the thin design of the electrodes allows for an appropriate amount of electrosurgical energy to be applied to the fractured area.
Owner:CILAG GMBH INT
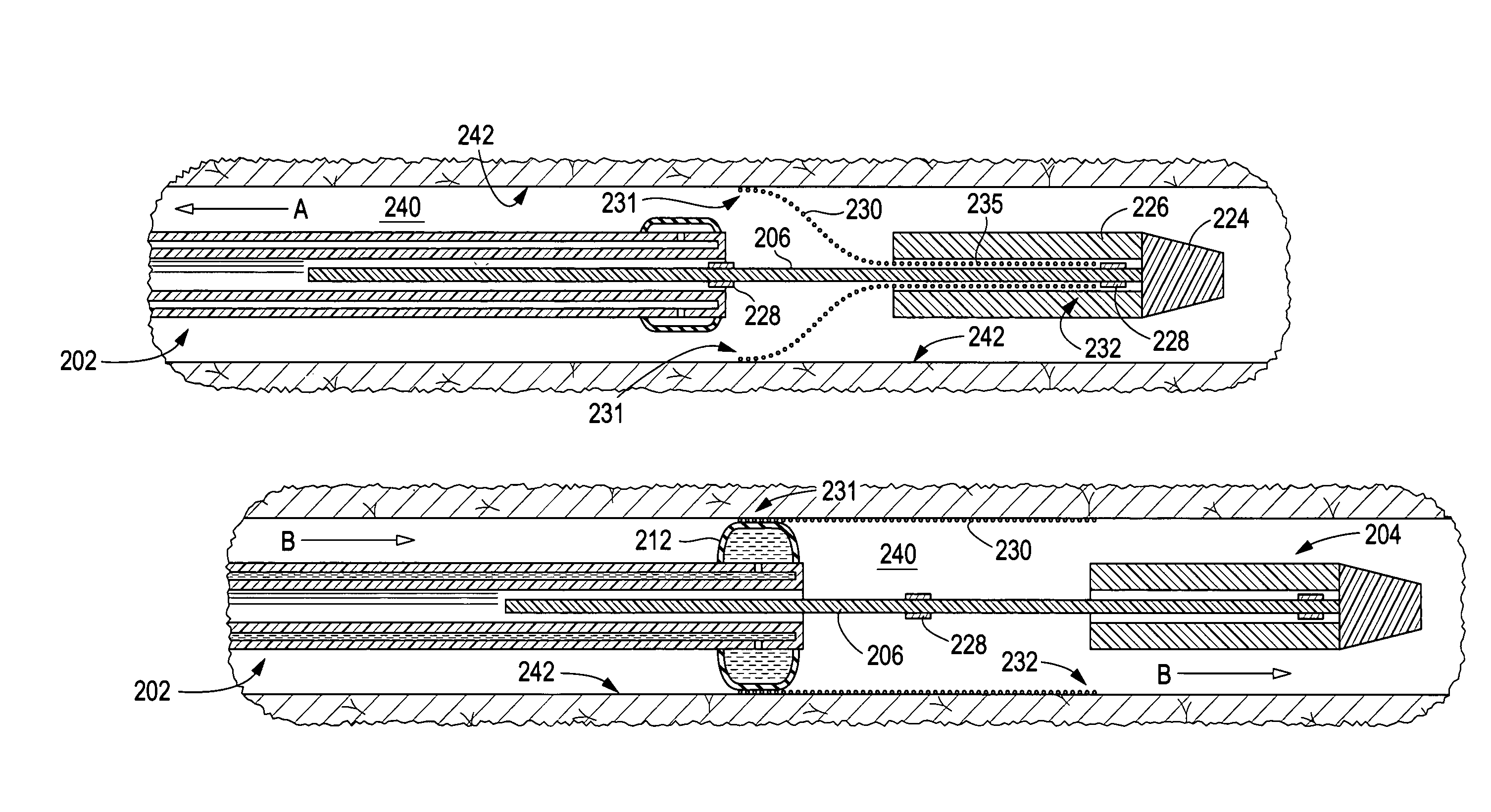
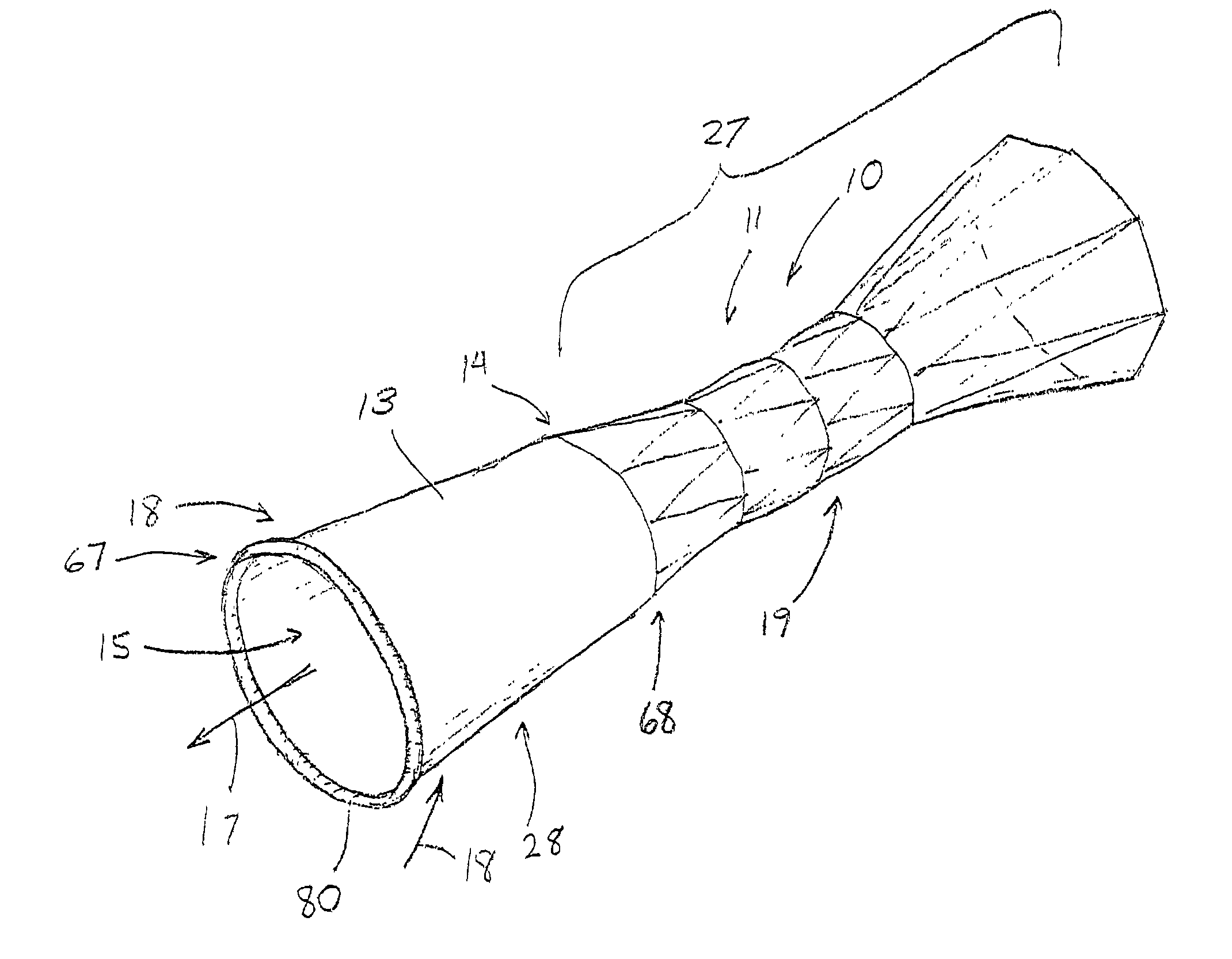
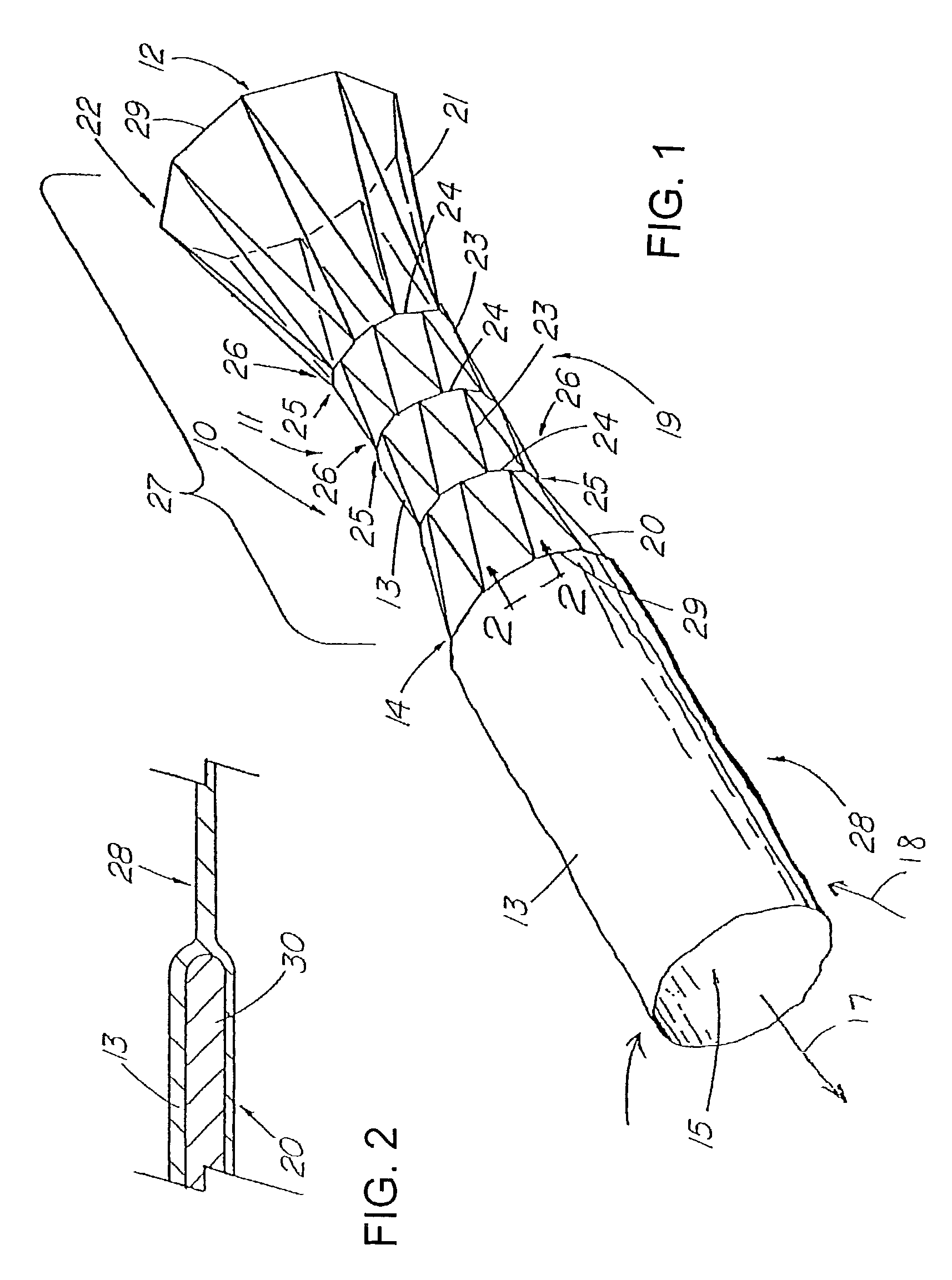
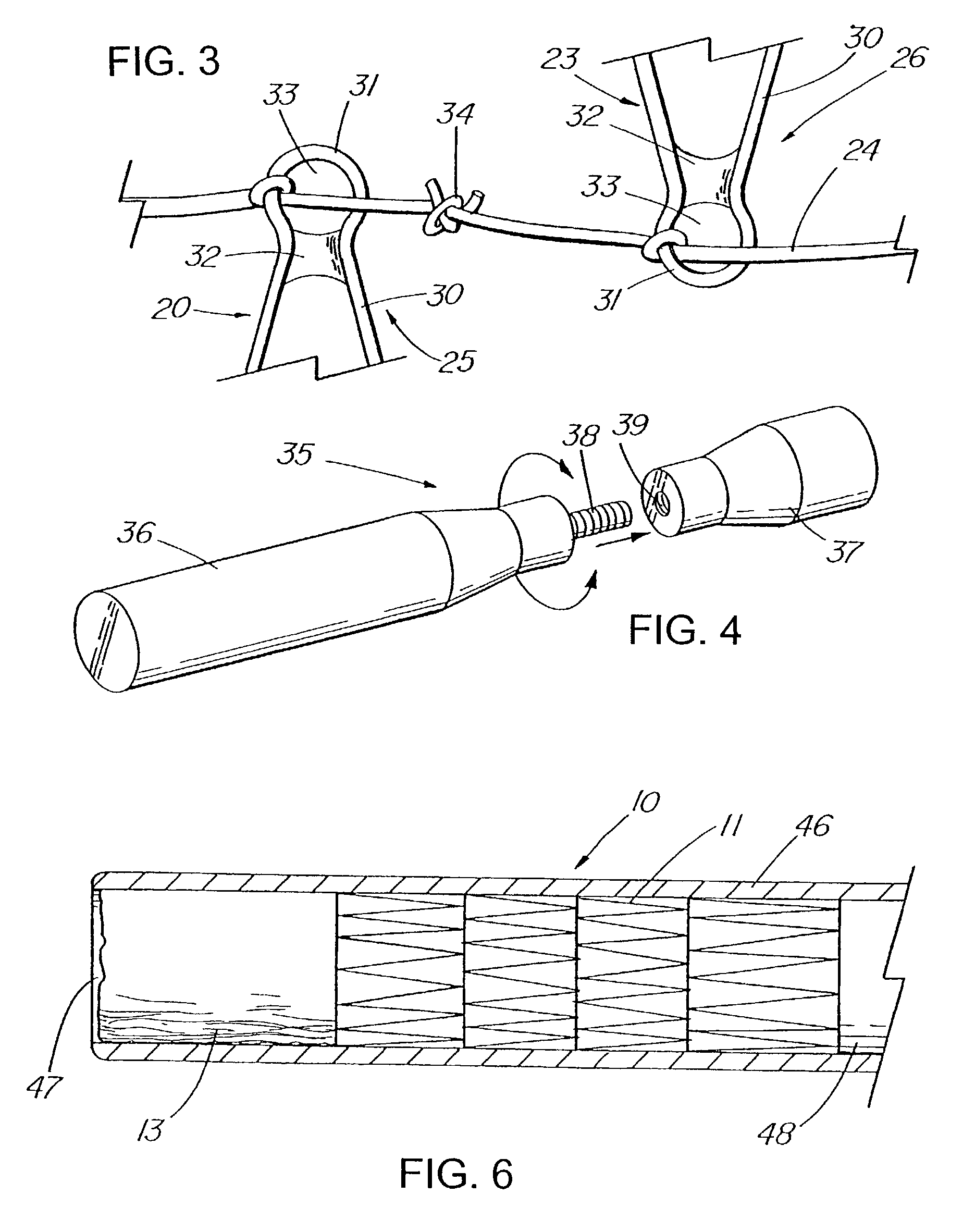
Apparatus and method for deployment of an endoluminal device
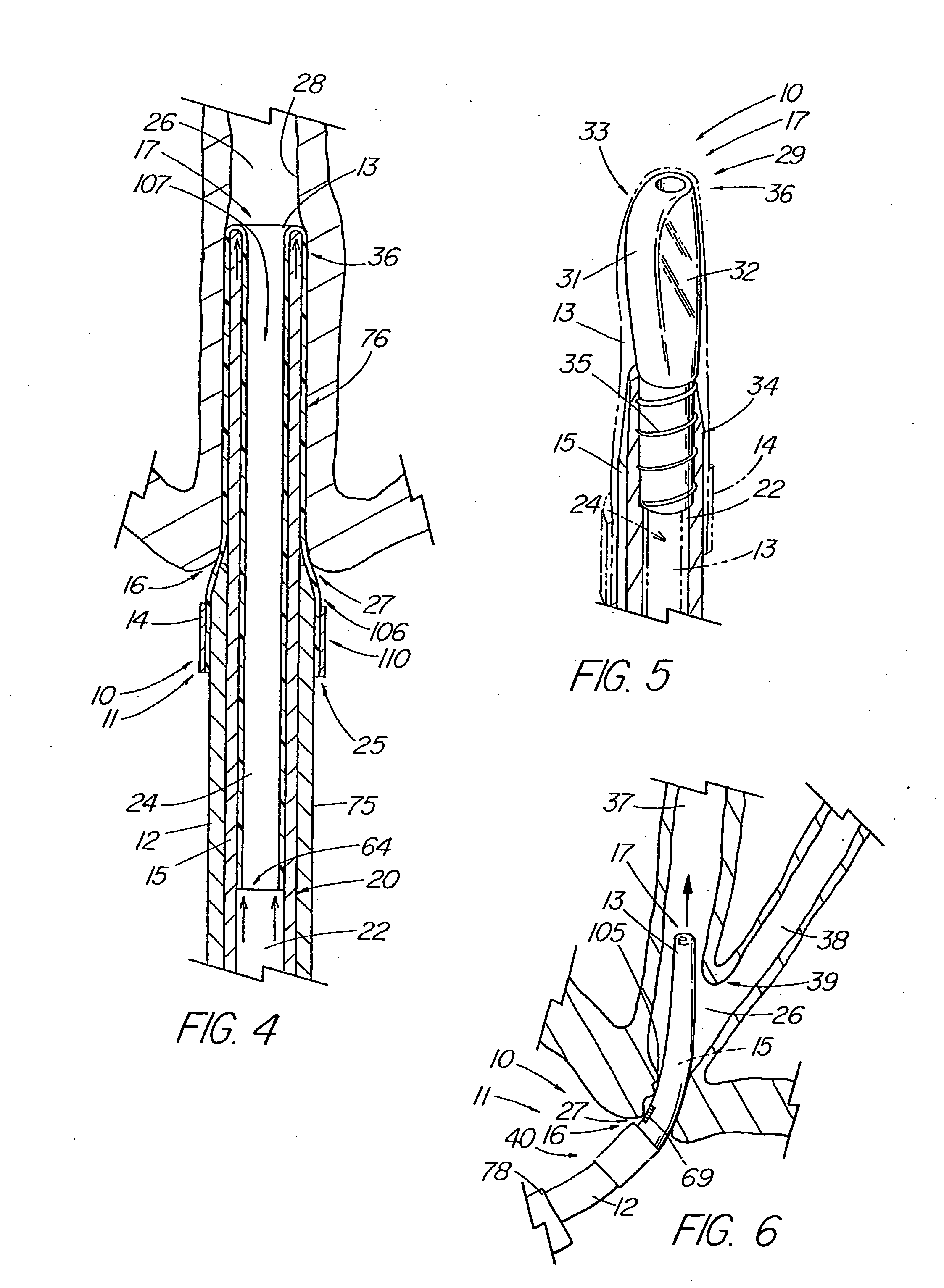
An introducer deploys an endoluminal device in a distal location from a proximal location. The introducer comprises a retrograde portion, an anterograde portion axially moveable relative to the retrograde portion, a shaft having a distal tip and an anterograde sheath attached to the distal tip, and an inflatable balloon mounted radially outside the retrograde portion for anchoring the device during deployment from its proximal end to its distal end. The retrograde portion may comprise bilumen tubing having an external wall, an internal wall that defines a central lumen radially inward of the internal wall, and an annular lumen in fluid communication with the balloon defined between the external wall and the internal wall.
Owner:LIFESHIELD SCI
Electrode wiping surgical device
Aspects of the present disclosure include a surgical device comprising electrodes on the sides of an end of an effector to aide in sealing during various surgical procedures, such as a liver resection. During a sealing procedure, the surgeon may wipe the surgical site with the end effector, causing the electrodes to touch the fractured area. Electrosurgical energy may be applied to the electrodes during the wiping, causing coagulation of smaller vessels, such as tiny blood vessels and bile ducts in the parenchyma of the liver. In some cases, due to the nature of some smaller vessels, electrosurgical energy should be delicately applied to cause sealing but to avoid overly damaging the remaining tissue. In some embodiments, the thin design of the electrodes allows for an appropriate amount of electrosurgical energy to be applied to the fractured area.
Owner:CILAG GMBH INTERNATIONAL
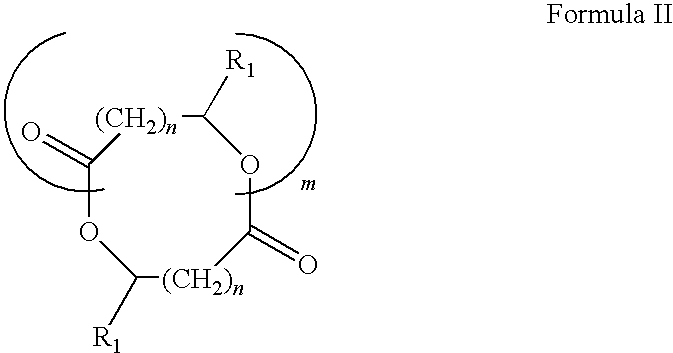
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
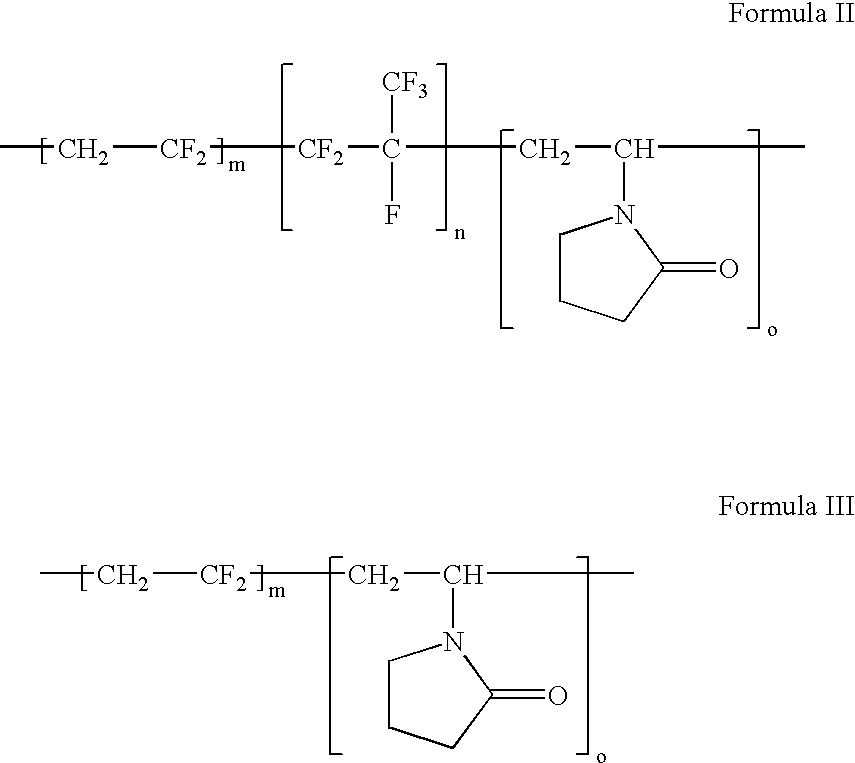
Polymers of fluorinated monomers and hydrophilic monomers
ActiveUS20060047095A1Improve propertiesProvide flexibilityFibre treatmentSurgeryDiseasePolymer science
A polymer of fluorinated monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains a polymer of fluorinated monomers and another biocompatible polymer. The polymer of fluorinated monomers or polymer blend described herein and optionally a bioactive agent can form a coating on an implantable device such as a drug-delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Devices and methods for disruption and removal of luminal occlusions
InactiveUS7618434B2Effective disruptionQuantity minimizationCannulasDilatorsIntestinal structureUrethra
The subject invention pertains to an elastic sheath, device, and methods for disrupting and / or removing occlusive material from lumens, particularly biological lumens, such as the vasculature, ureter, urethra, fallopian tubes, bile duct, intestines, and the like. The subject invention provides for effective disruption and removal of occlusive material, such as a thrombus, from the body lumen with minimal risk of injury to the lumen wall. Advantageously, the invention can be used to achieve a high degree of removal while minimizing the amount of occlusive material that is released into the body lumen. The subject invention further pertains to methods for disrupting and removing occlusive material from a biological lumen. In another aspect, the present invention concerns a device useful as an in vitro model of luminal occlusion and methods for using the device to test the efficacy of devices and methods for treating luminal occlusions.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
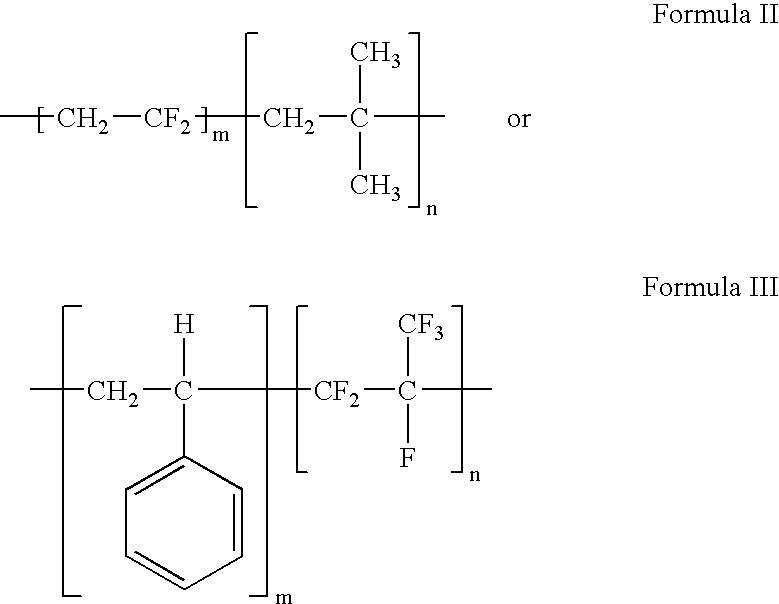
Polymers of fluorinated monomers and hydrocarbon monomers
InactiveUS20060134165A1Provide mechanical strengthGive flexibilityStentsSurgeryAbnormal tissue growthPolymer science
A polymer of fluorinated monomers and hydrocarbon monomers is provided. It is also provided a polymer blend that contains a polymer formed of fluorinated monomers and hydrocarbon monomers and another biocompatible polymer. The polymer or polymer blend described herein and optionally a bioactive agent can form an implantable device such as a stent or a coating on an implantable device such as a drug-delivery stent, which can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Prosthesis having a sleeve valve
Disclosed is a pressure sensitive prosthesis that includes a tubular member having a passageway extending therethrough and a sleeve attached about one end of the tubular member. The sleeve functions as a one-way valve to permit fluid flowing through the sleeve lumen in a first direction and under a first pressure, while collapsing in response to fluid flowing in a second direction when the pressure thereof exceeds that of the first direction or pressure. One aspect of the invention includes an esophageal anti-reflux expandable prosthesis wherein the sleeve is adapted to invert back through the tubular stent frame to permit belching or vomiting (fluid or materials under a third, significantly higher pressure). Another aspect of the invention includes a tubular drainage stent, such as a biliary or urethral stent in which the sleeve opens to permit passage of fluids, then collapses to prevent retrograde flow.
Owner:COOK MEDICAL TECH LLC
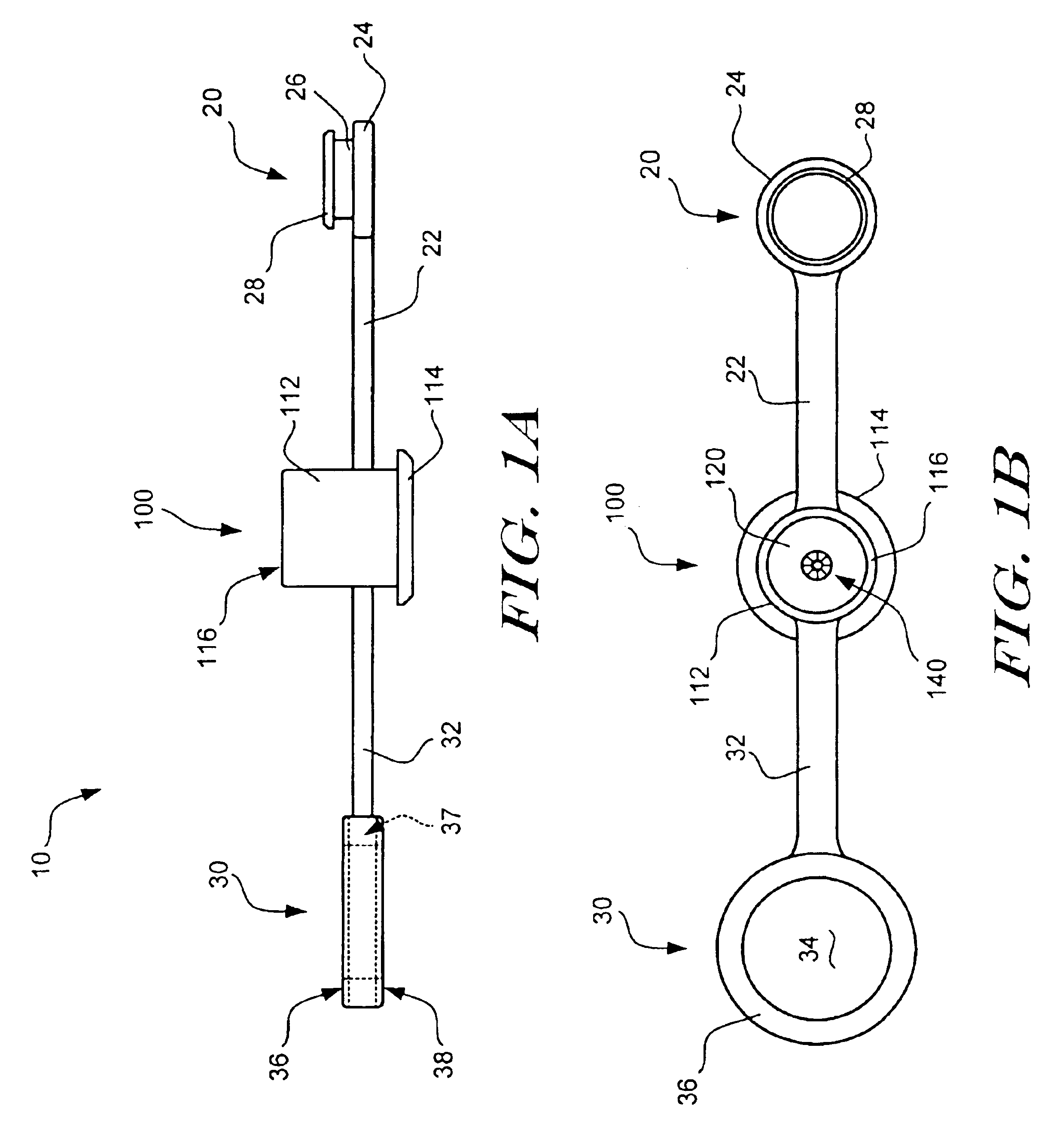
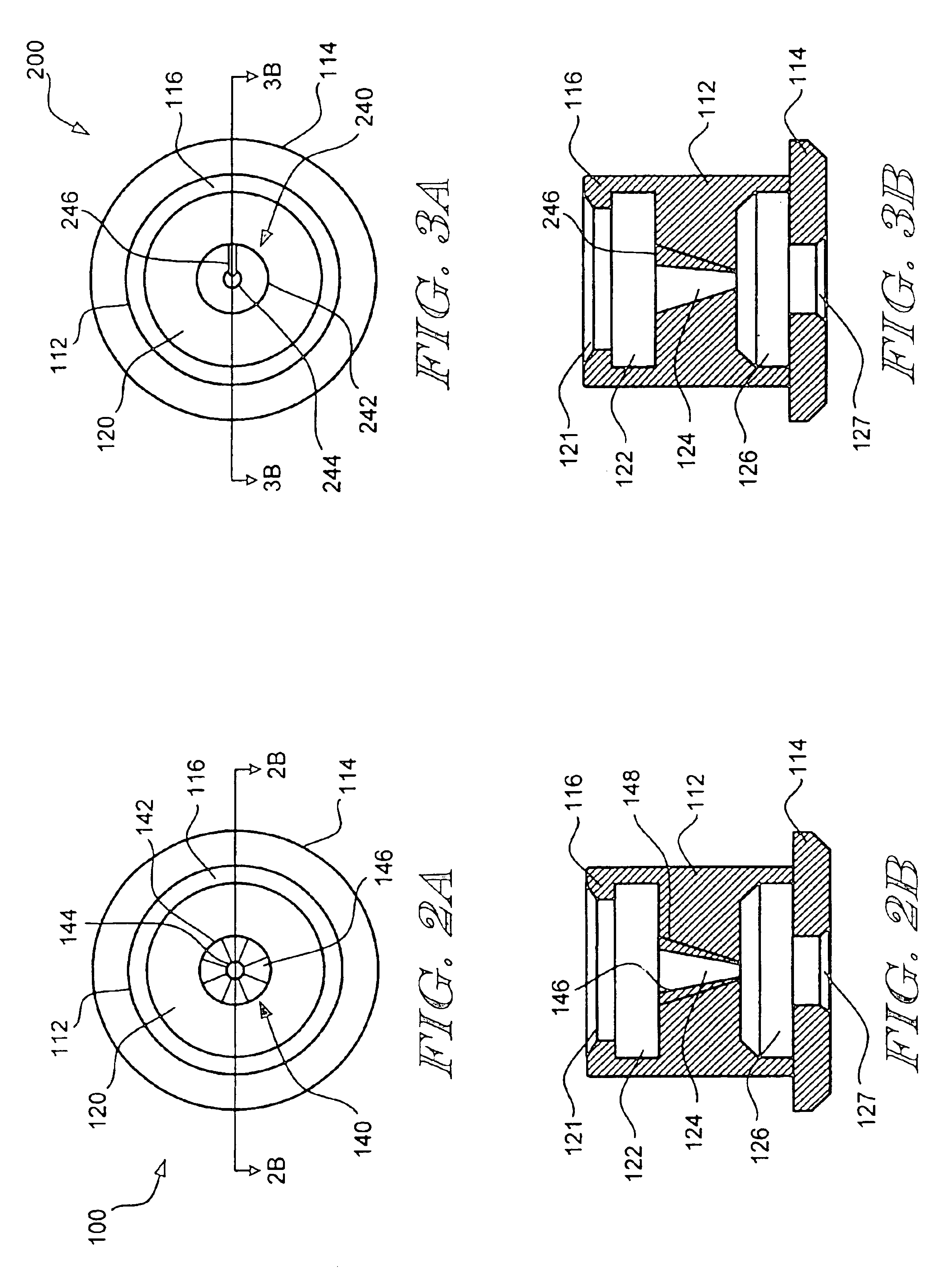
Rapid exchange catheter with detachable hood
A single operator exchange biliary catheter having a common distal lumen. The biliary catheter includes an elongate shaft having a proximal portion defining an ancillary lumen and a distal portion defining a common guidewire and ancillary lumen. The common distal lumen reduces the profile of the distal portion of the shaft. The elongate shaft also includes a proximal guidewire port disposed between the proximal end of the shaft and the distal end of the shaft to facilitate single operator use. A seal may be disposed adjacent the proximal guidewire port to thereby seal the port. Preferably, the shaft includes a single lumen distal portion and a bi-lumen proximal portion. The single lumen distal portion of the shaft may be curved and may include a tapered or spherically shaped distal tip.
Owner:SCI MED LIFE SYST
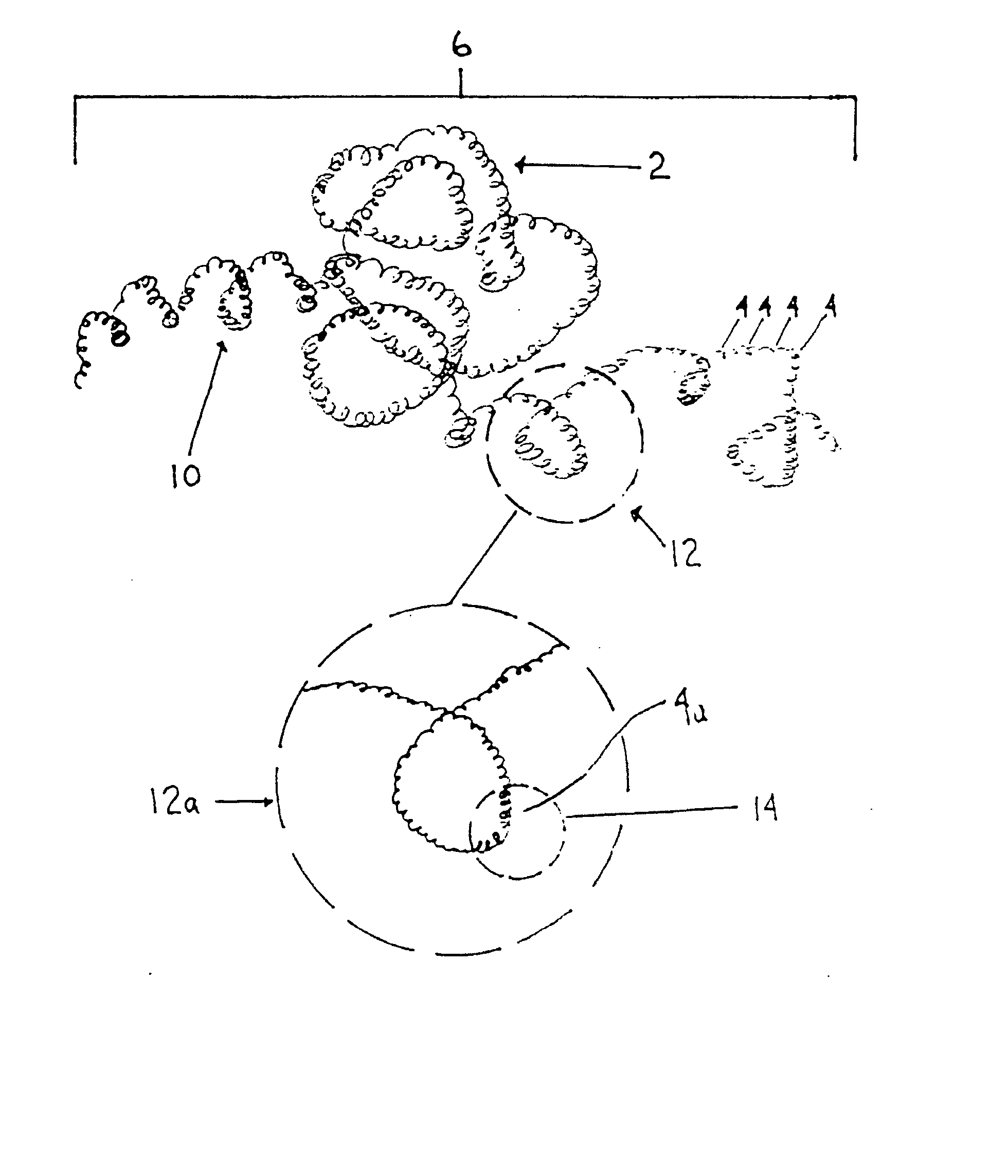
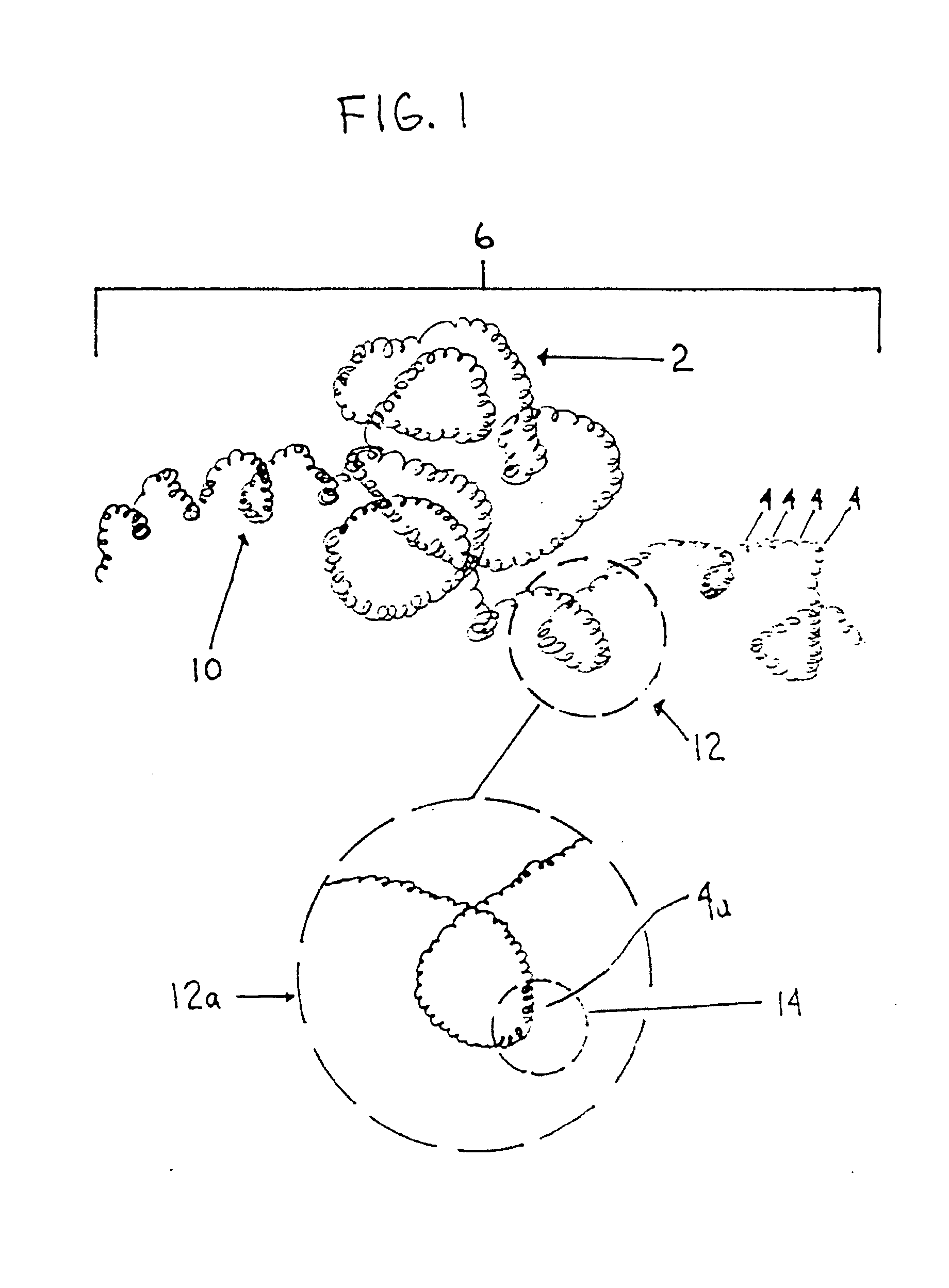
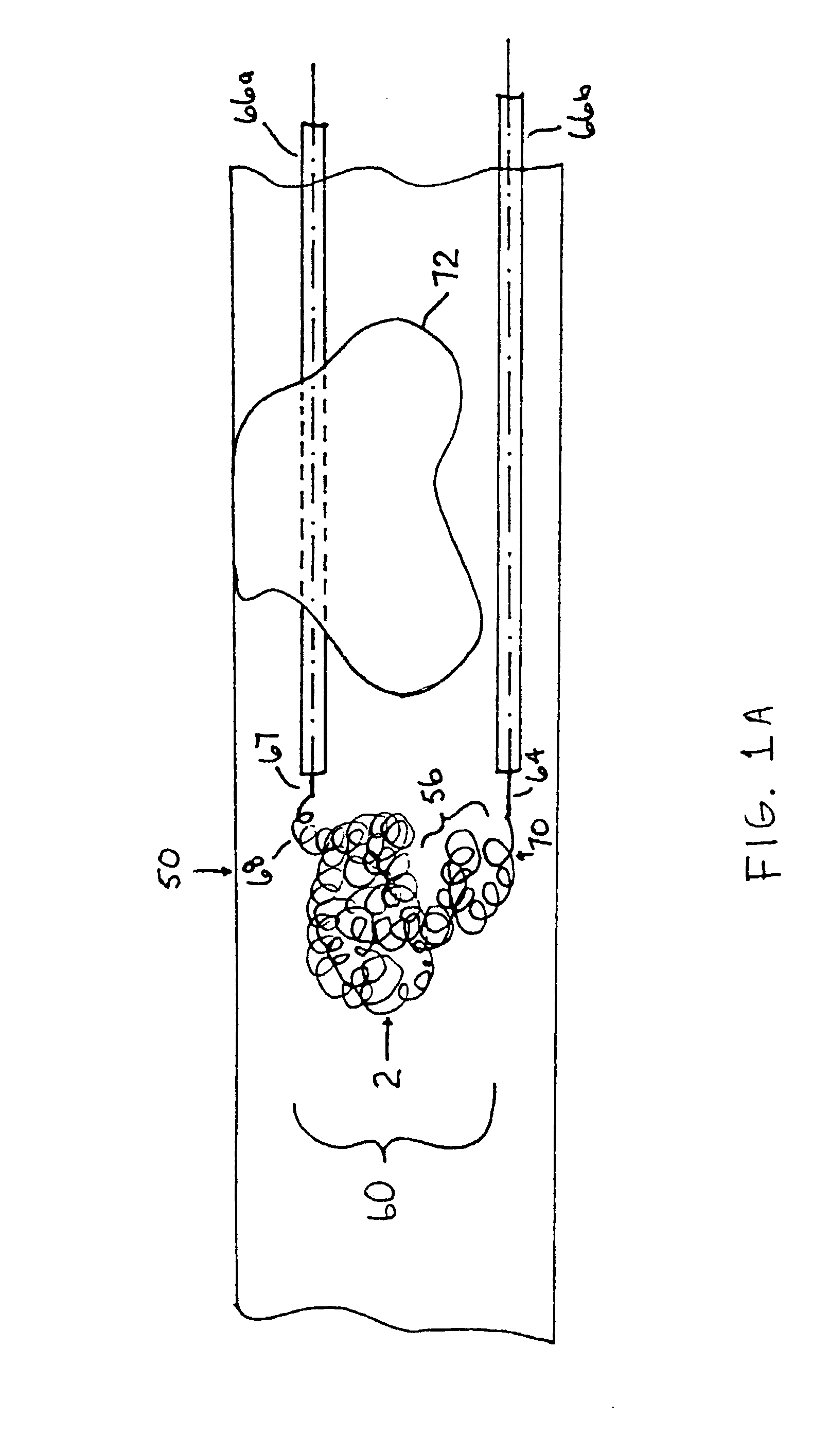
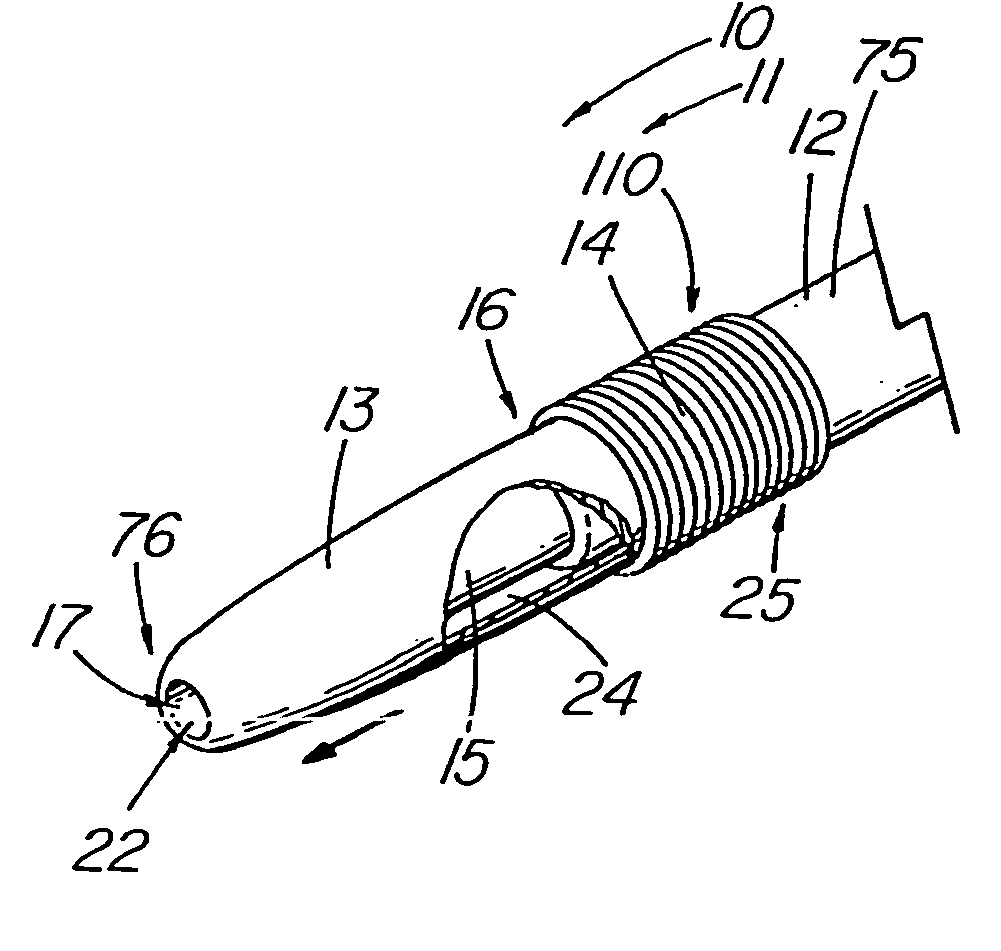
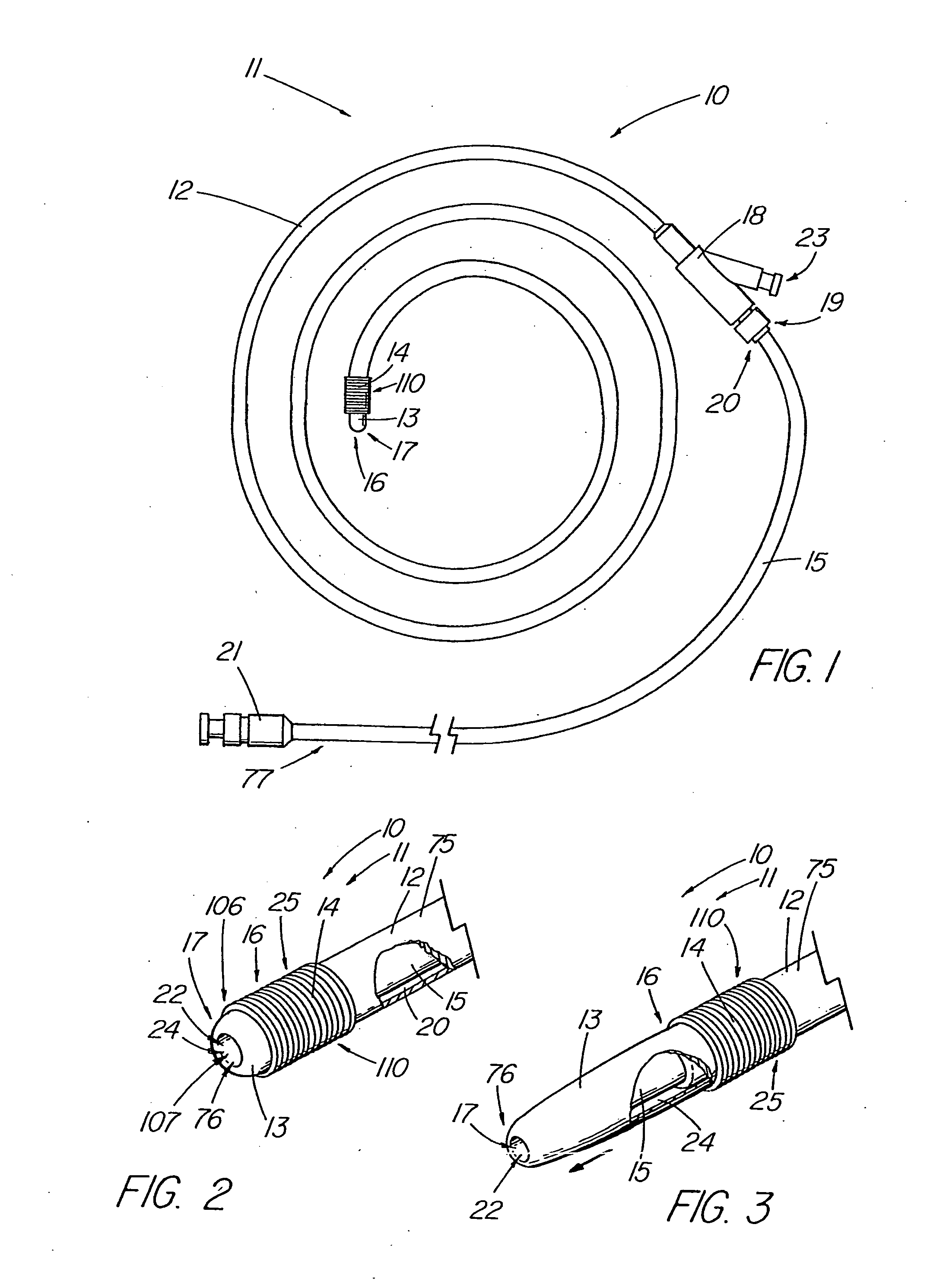
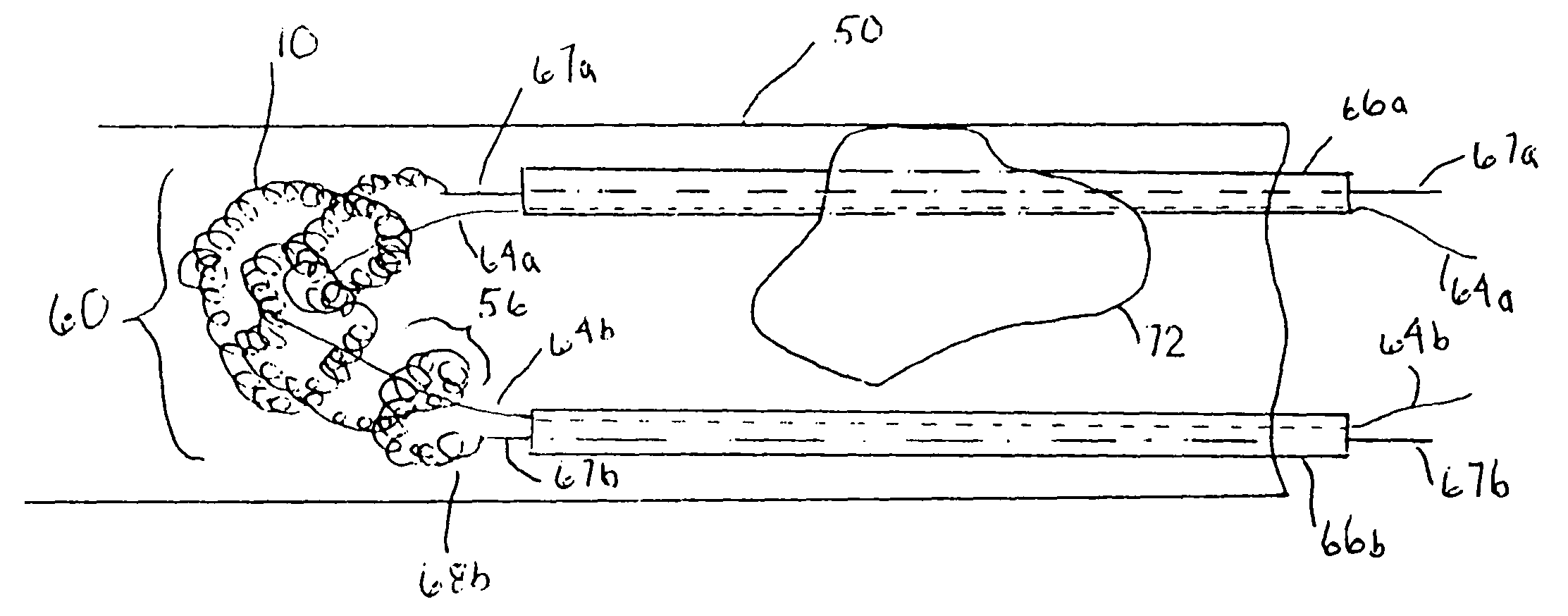
Thrombus removal system and process
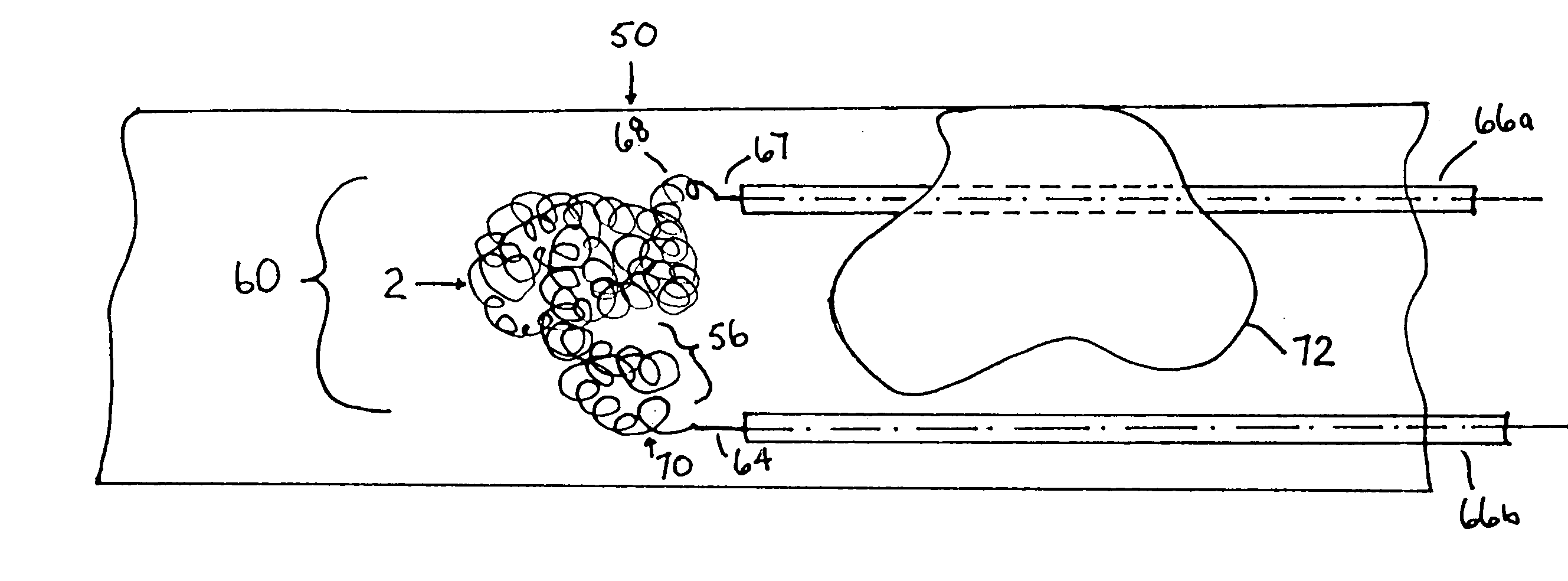
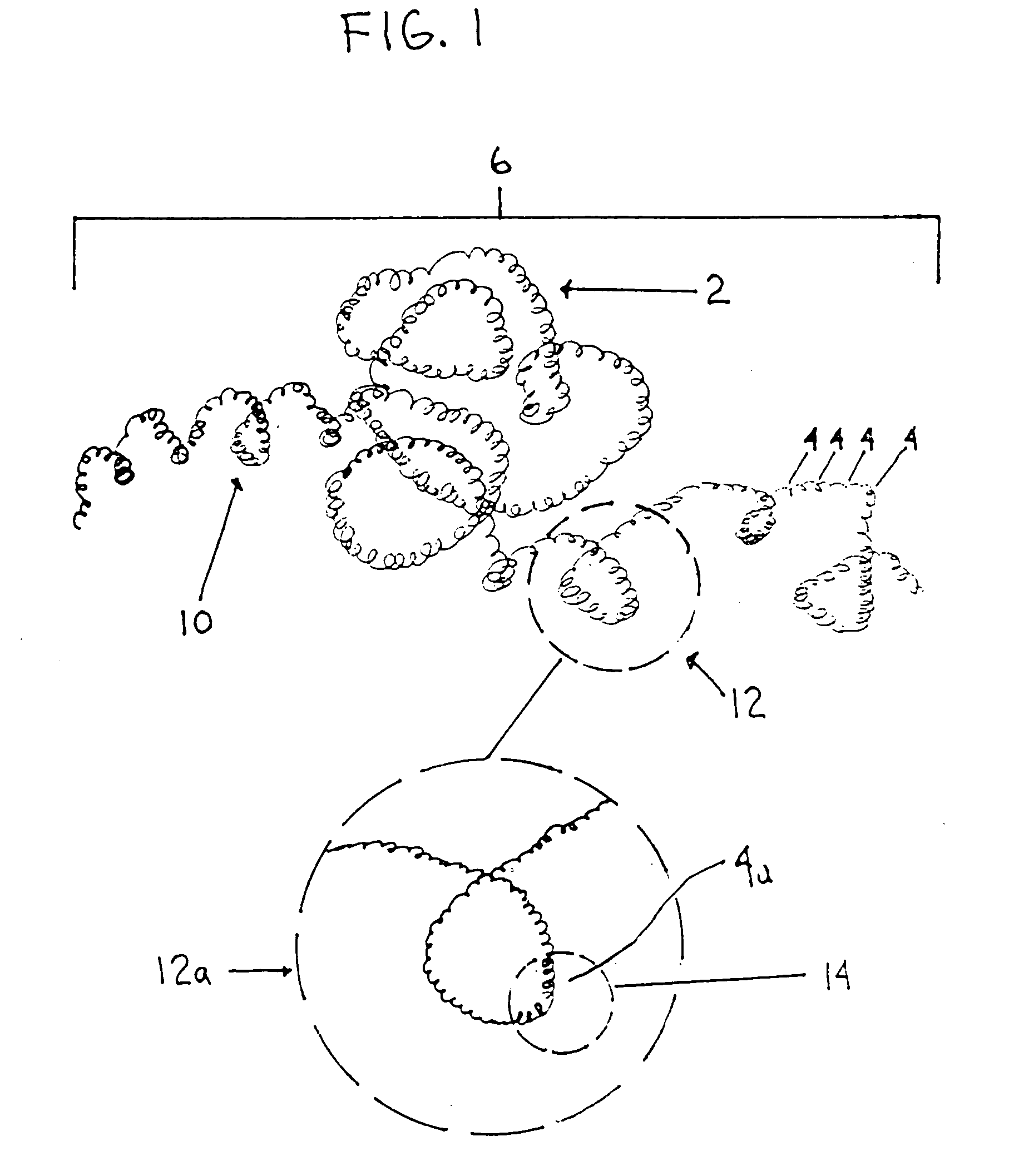
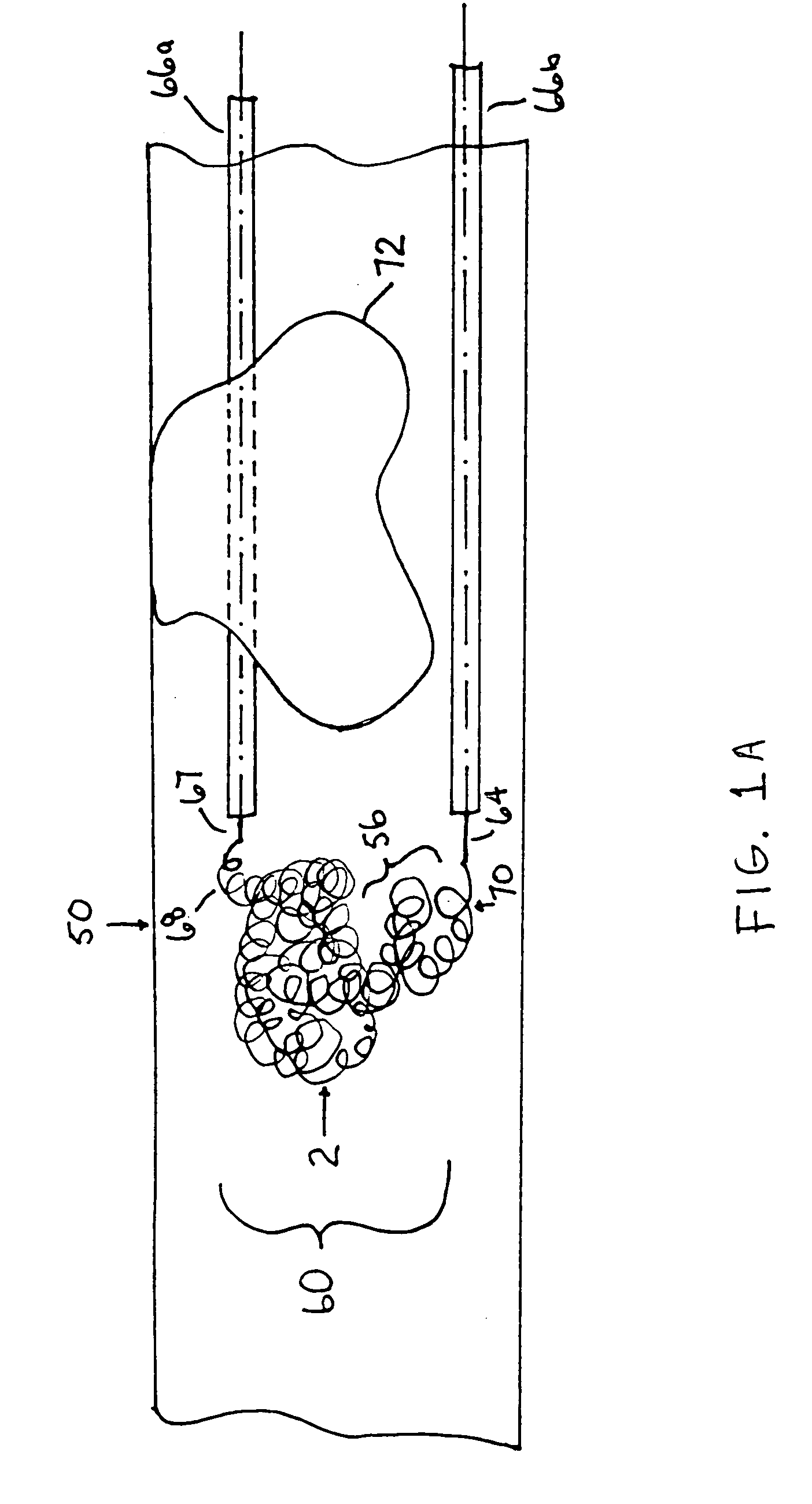
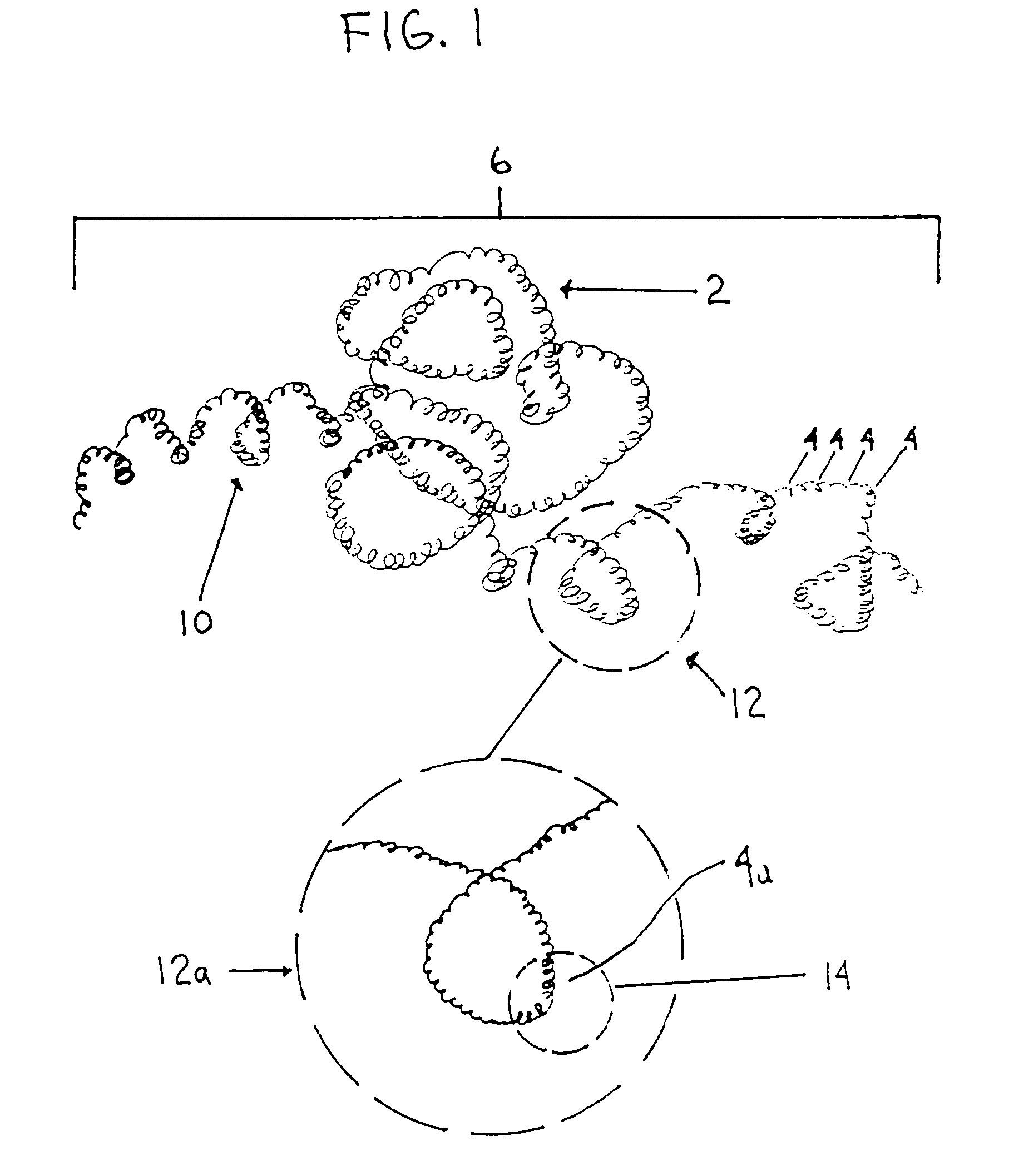
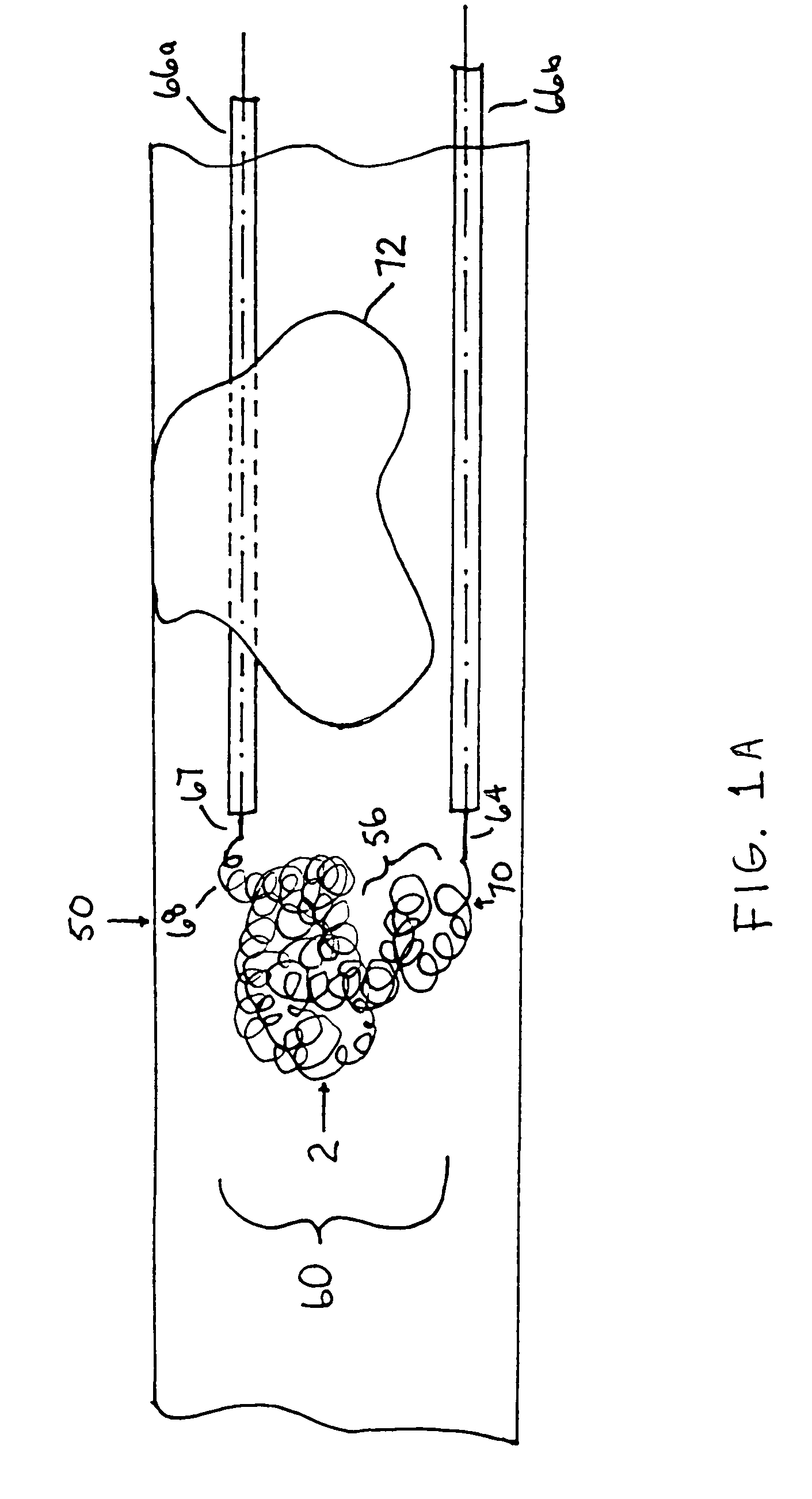
A device capable of capturing and facilitating the removal of a thrombus in blood vessels (or stones in biliary or urinary ducts, or foreign bodies) uses a soft coil mesh with the aid of a pull wire or string to engage the surface of a thrombus, and remove the captured thrombus. The soft coil mesh is formed by an elongated microcoil element that forms the helical elements of a macrocoil element. The microcoil element provides a relatively elastic effect to the helical elements forming the macrocoil and allows for control of gripping forces on the thrombus while reducing non-rigid contact of the device with arterial walls. The use of multiple coil mesh elements, delivered through a single lumen or multiple lumens, preferably with separate control of at least one end of each coil, provides a firm grasp on a distal side of a thrombus, assisting in non-disruptive or minimally disrupted removal of the thrombus upon withdrawal of the device.
Owner:NEXGEN MEDICAL SYST
Medical device having a sleeve valve with bioactive agent
InactiveUS20080086214A1Inhibitory contentInhibition formationStentsBile ductsActive agentSleeve valve
Medical devices for implantation in a body vessel are provided. A medical device can be configured as a drainage stent adapted for placement in a bodily passageway. The drainage stent preferably includes a drainage lumen extending longitudinally through the drainage stent, and a sleeve defining a collapsible lumen in fluid flow communication with the drainage lumen. The sleeve may function as a one-way valve and preferably includes a biodeposition-reducing bioactive agent, such as an antibiotic or antimicrobial agent. The medical device may be configured as a biliary or pancreatic stent.
Owner:WILSONCOOK MEDICAL
Thrombus removal system and process
A device capable of capturing and facilitating the removal of a thrombus in blood vessels (or stones in biliary or urinary ducts, or foreign bodies) uses a soft coil mesh with the aid of a pull wire or string to engage the surface of a thrombus, and remove the captured thrombus. The soft coil mesh is formed by an elongated microcoil element that forms the helical elements of a macrocoil element. The microcoil element provides a relatively elastic effect to the helical elements forming the macrocoil and allows for control of gripping forces on the thrombus while reducing non-rigid contact of the device with arterial walls. The use of multiple coil mesh elements, delivered through a single lumen or multiple lumens, preferably with separate control of at least one end of each coil, provides a firm grasp on a distal side of a thrombus, assisting in non-disruptive or minimally disrupted removal of the thrombus upon withdrawal of the device.
Owner:NEXGEN MEDICAL SYST
Introducer apparatus with eversible sleeve
InactiveUS20060173422A1Control expansionReduce patient discomfortGuide needlesStentsNasal passageNasal passages
Disclosed is an introducer apparatus comprising an sleeve fixation mechanism or introducer member, such as a catheter, introducer, or ring-like structure, which is attached to a protective sleeve comprising a thin flexible material such a polymeric film. The sleeve is inverted into the passageway of a second member, such as a catheter, feeding tube, introducer, etc., that is advanced through the passageway of the introducer member and is introduced into a bodily passage of a patient, such as the bile duct, nasal passages, colon, etc. The sleeve everts from the passageway of the second member during its advancement to lay down a friction-reducing surface. The sleeve prevents frictional contact between the second member and delicate or sensitive tissues.
Owner:COOK MEDICAL TECH LLC
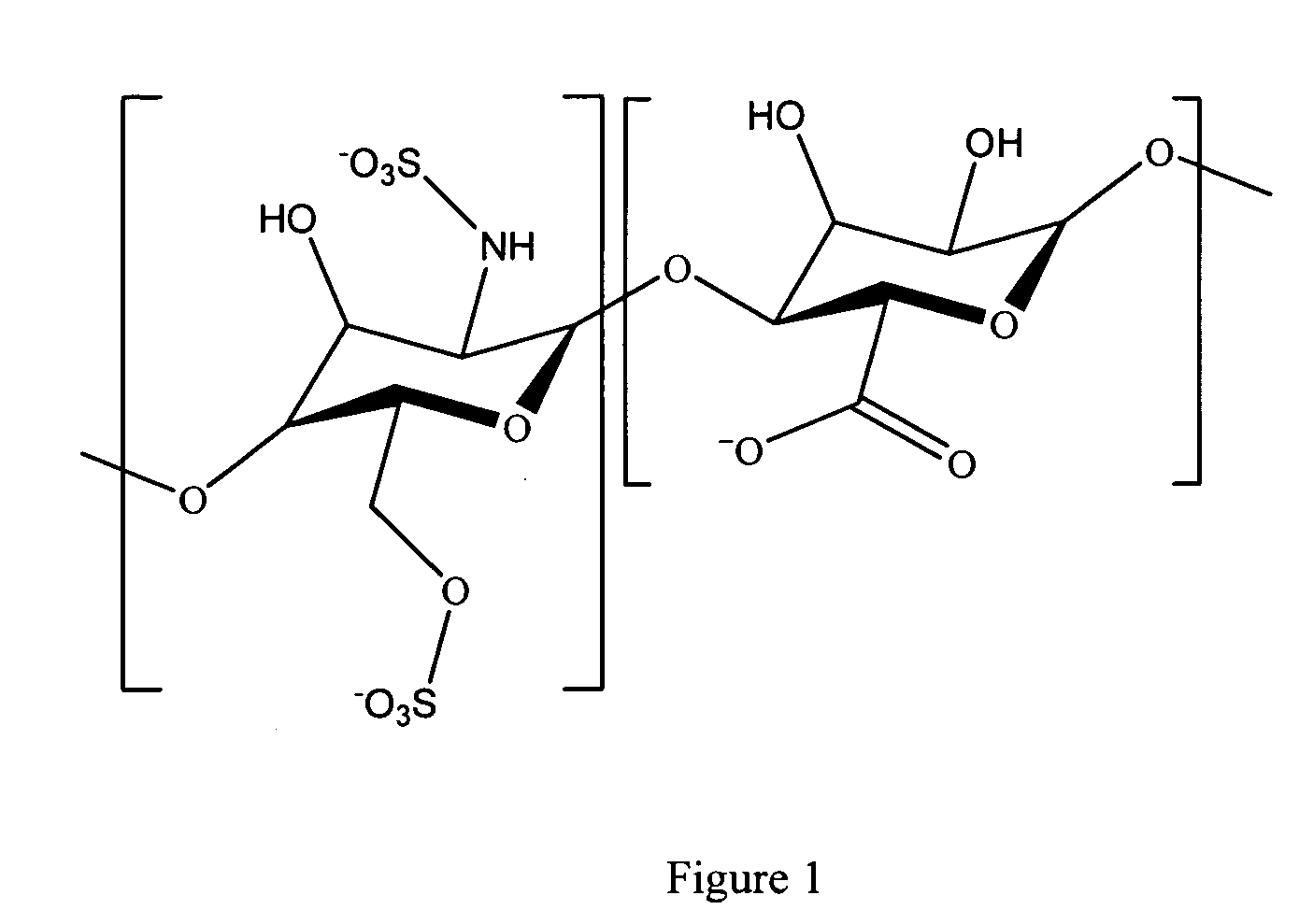
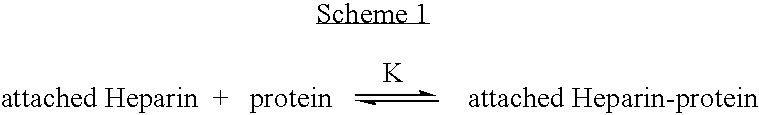
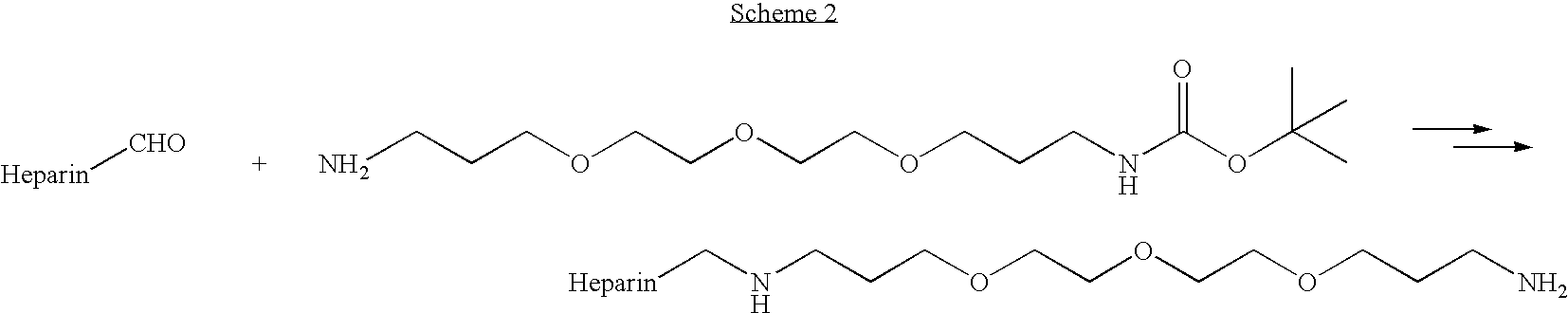
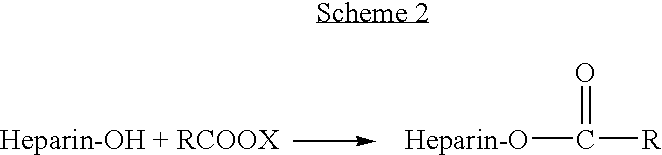
Antifouling heparin coatings
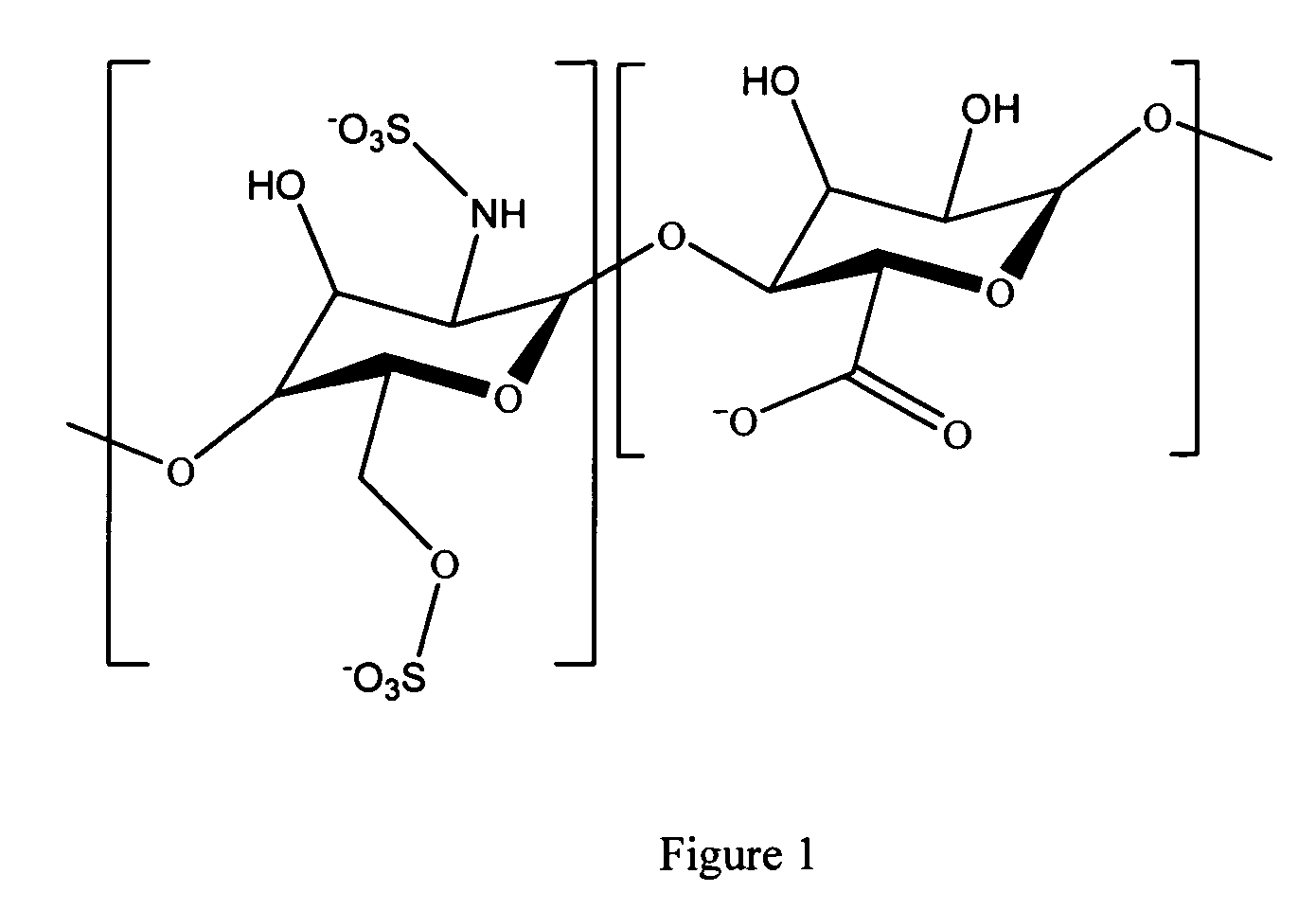
A medical device comprising a coating thereon comprising a biocompatible polymer and heparin is provided herein. Heparin is coupled with the biocompatible polymer via a spacer having a grouping that renders a binding site of the heparin molecule accessible by a binding protein. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Plasticizers for coating compositions
A biocompatible plasticizer useful for forming a coating composition with a biocompatible polymer is provided. The coating composition may also include a biobeneficial polymer and / or a bioactive agent. The coating composition can form a coating on an implantable device. The implantable device can be used to treat or prevent a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
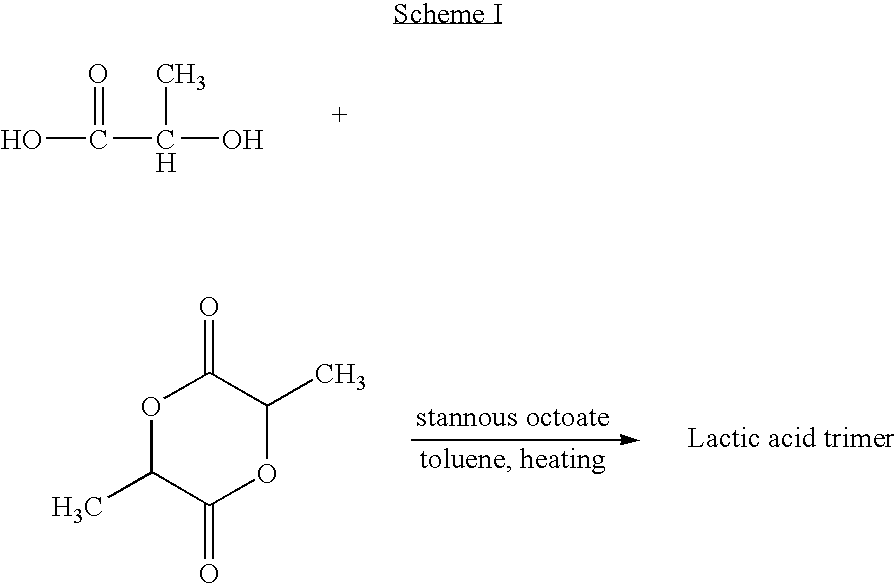
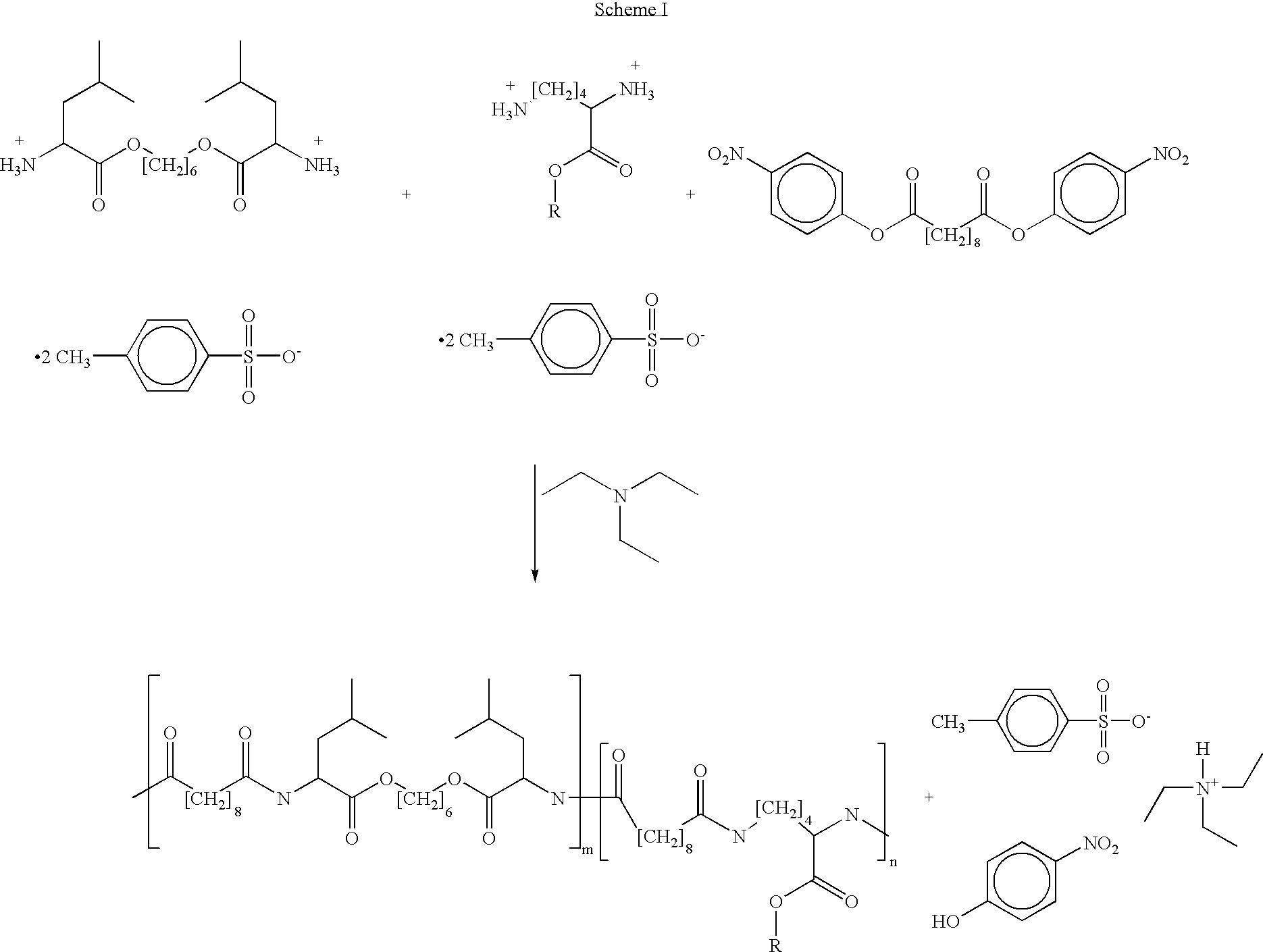
Blends of poly(ester amide) polymers
ActiveUS7166680B2Improved stability and drug release rate and mechanical characteristicReduce degradationSurgeryCatheterDiseasePEA polymer
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Fluid seal for endoscope
An endoscope seal that effectively inhibits the egress of fluids from the working channel of an endoscope when an elongate device having a non-circular shaft is disposed therein. The endoscope seal includes a body portion having a proximal end adapted for insertion of an elongate device such as a rapid exchange biliary catheter, a distal end adapted for connection to the proximal end of an endoscope, a lumen extending therethrough which is adapted to receive the elongate device and to provide access to the working channel of the endoscope, and a means for conforming to the non-circular shaft of the elongate device to inhibit the flow of fluid from the working channel of the endoscope. The conforming means may, for example, comprise one or more protrusions extending radially inward in the lumen of the body portion, a sealing material such as a surgical foam that is disposed in the lumen of the body portion, or a sealing mandrel. Whether a single protrusion, a plurality of protrusions, a sealing material or a sealing mandrel is utilized, the present invention provides endoscope seals that readily seal about elongate devices having either circular or non-circular profiles.
Owner:SCI MED LIFE SYST
Thrombus removal system and process
Owner:NEXGEN MEDICAL SYST
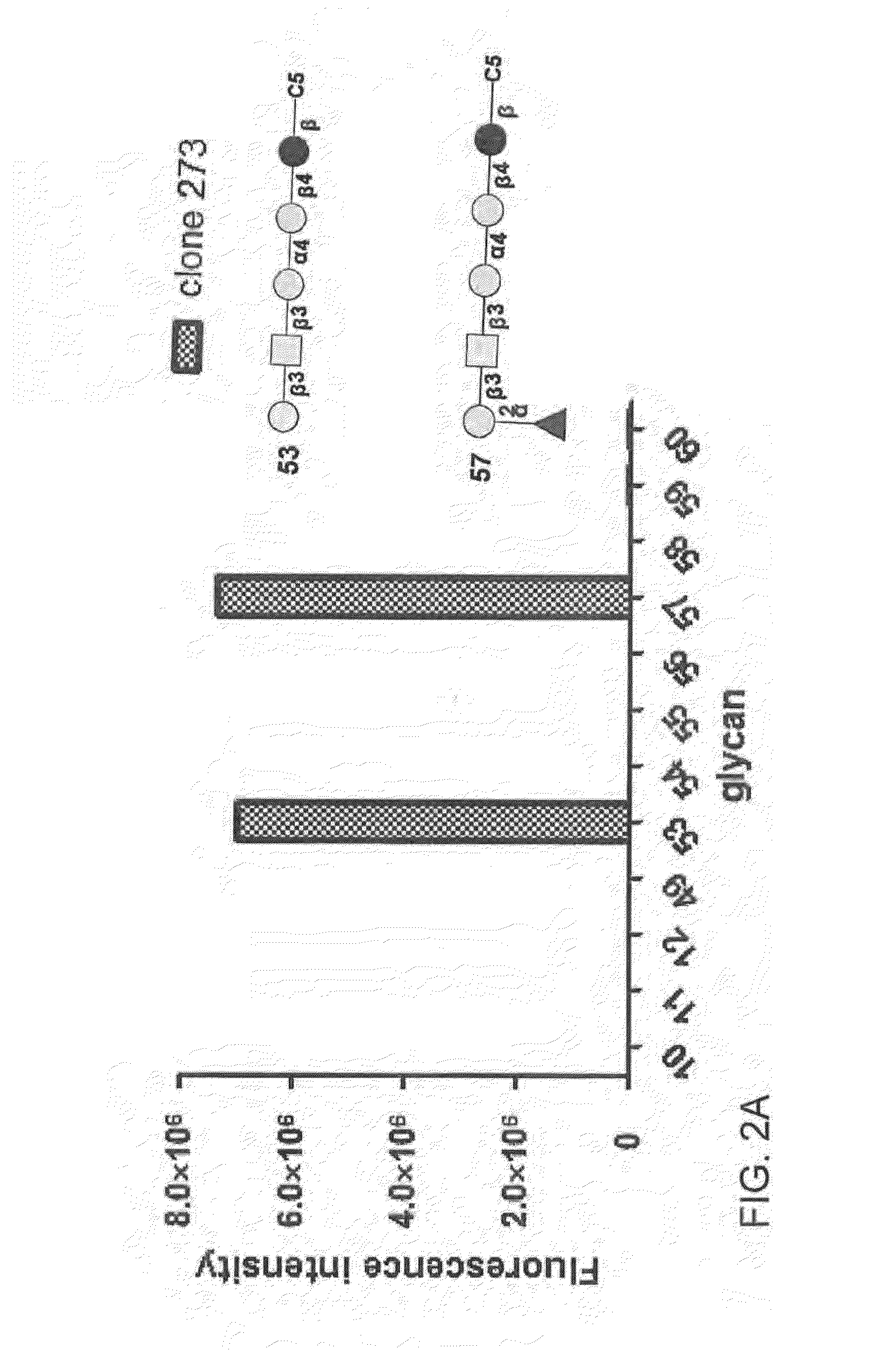
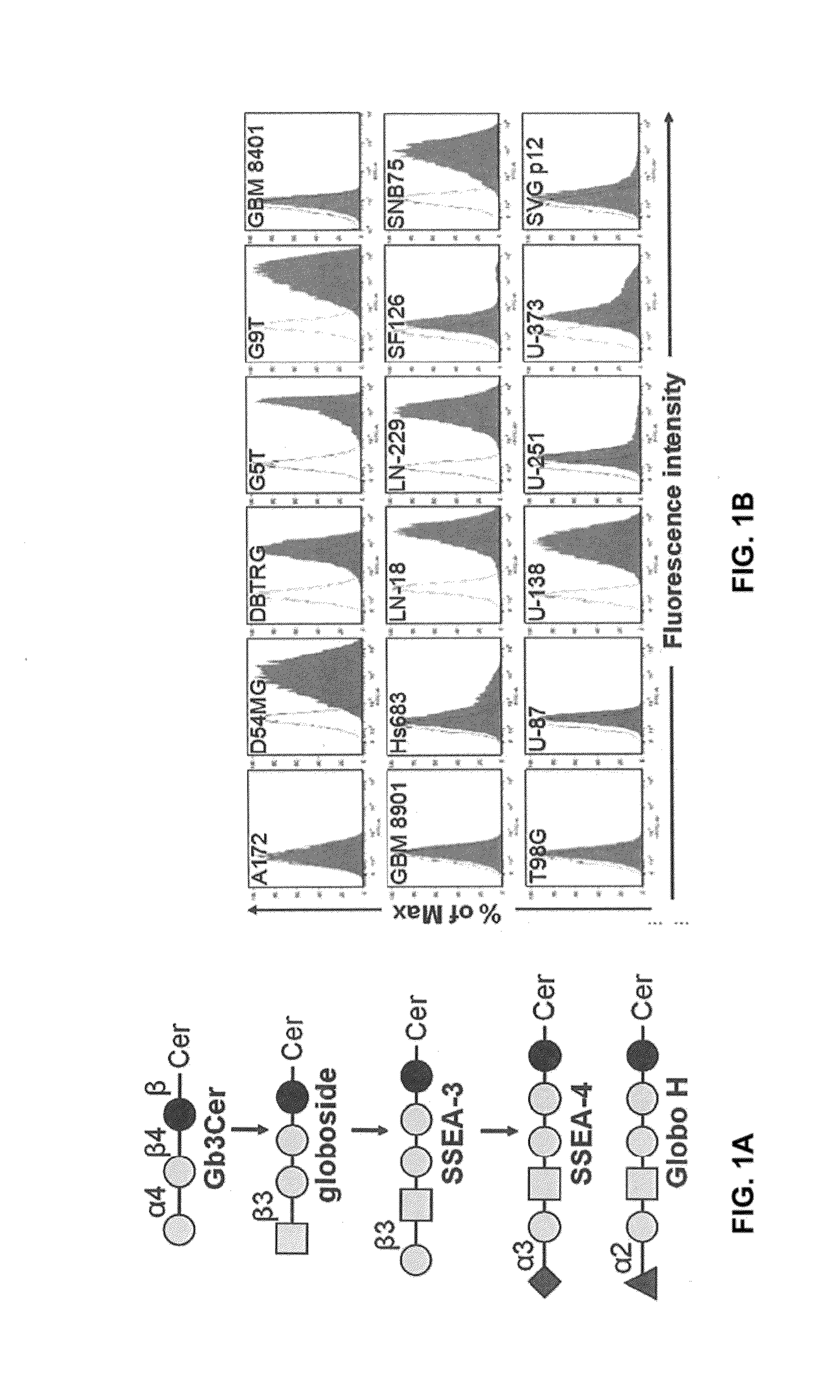
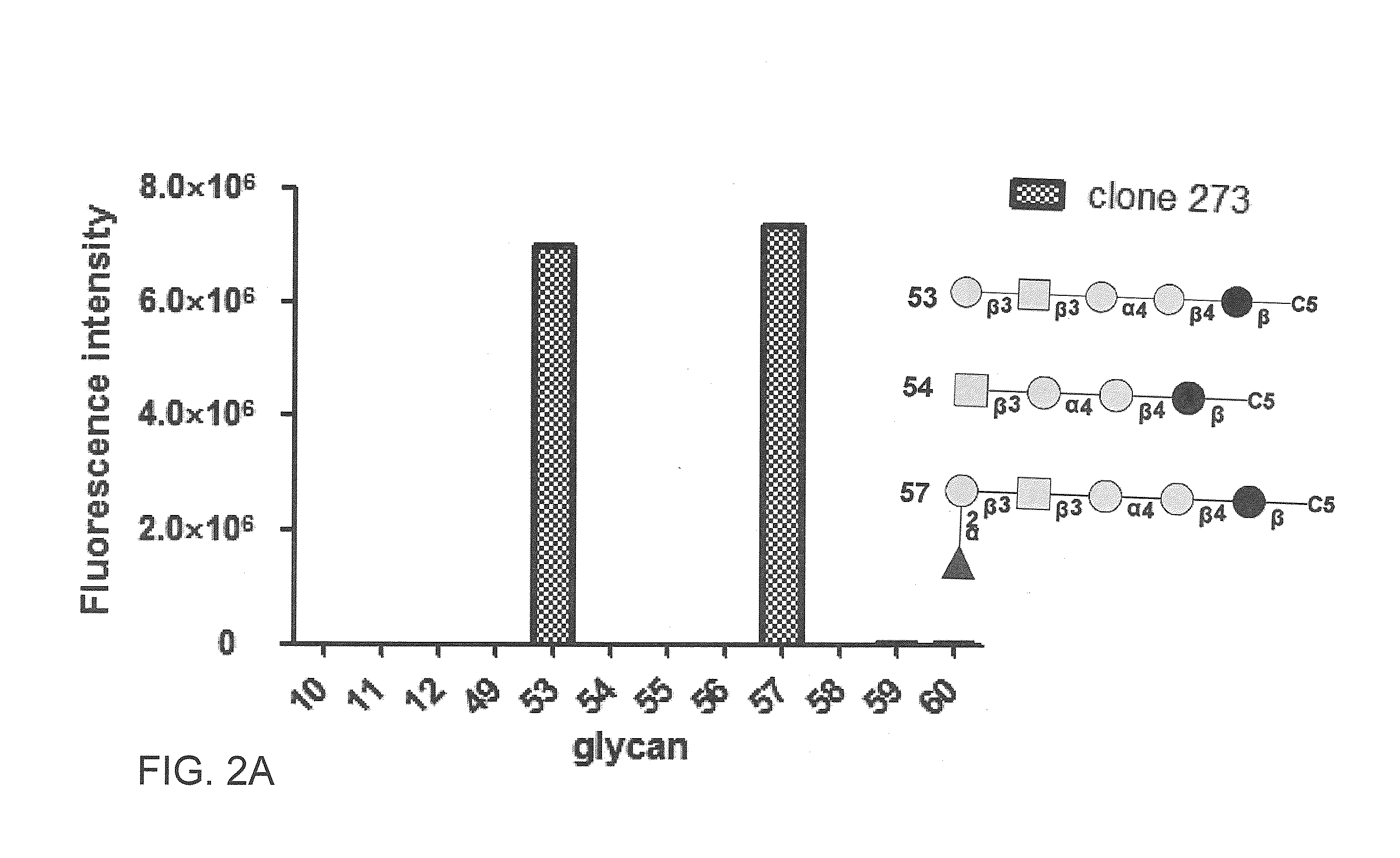
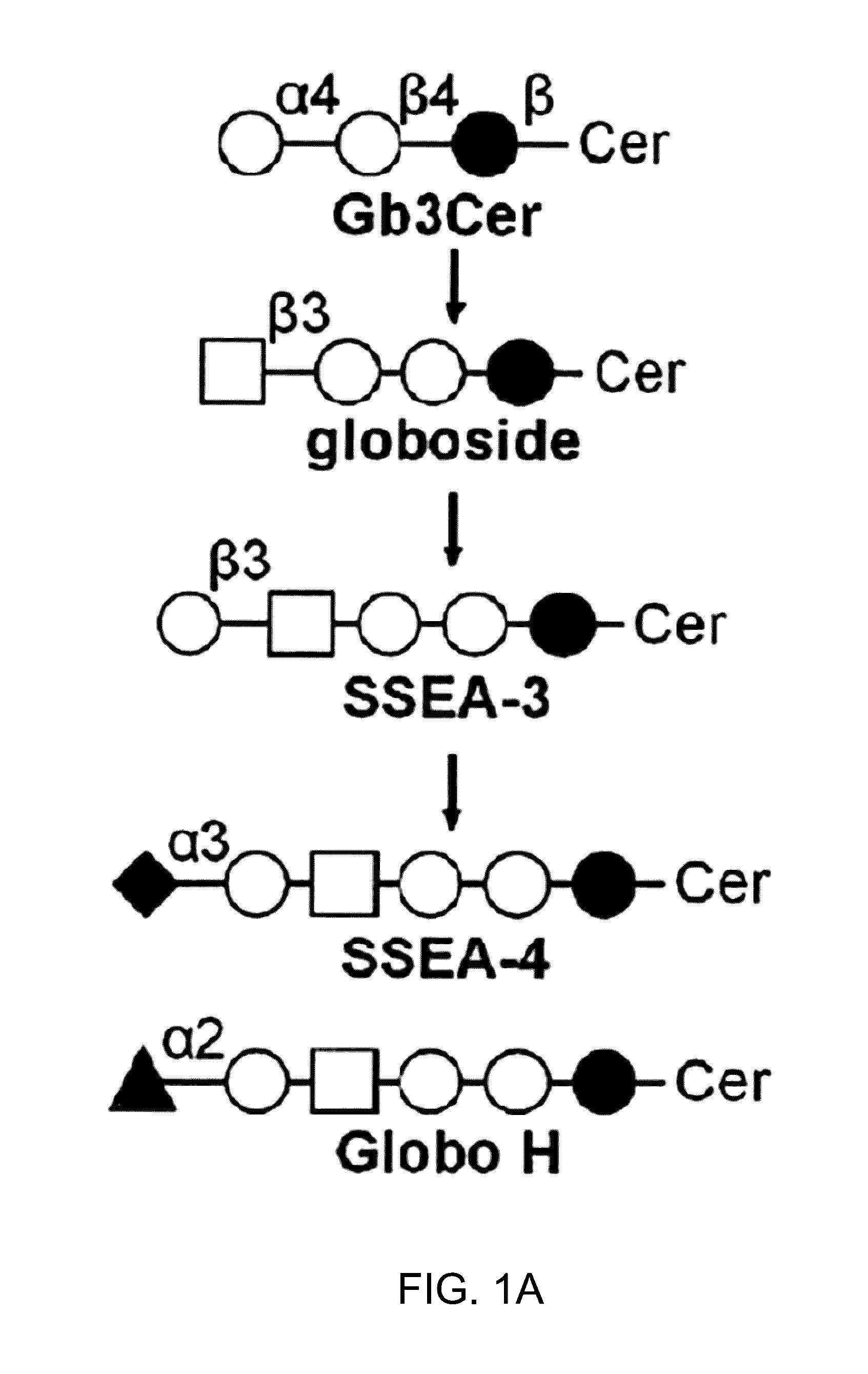
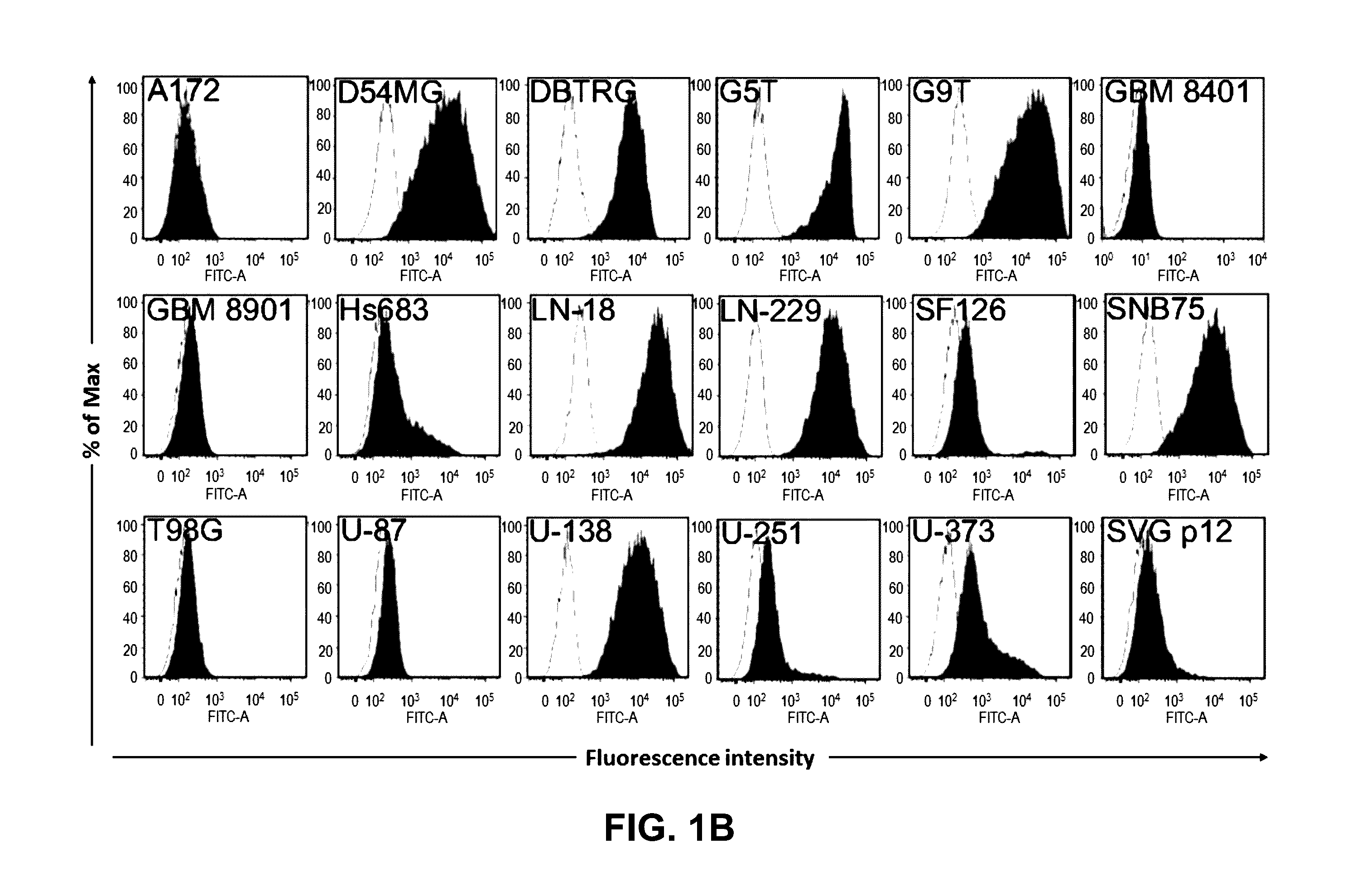
Compositions and methods for treatment and detection of cancers
ActiveUS20160102151A1Shrink tumorElimination of malignant cellLibrary screeningImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellAntigen Binding Fragment
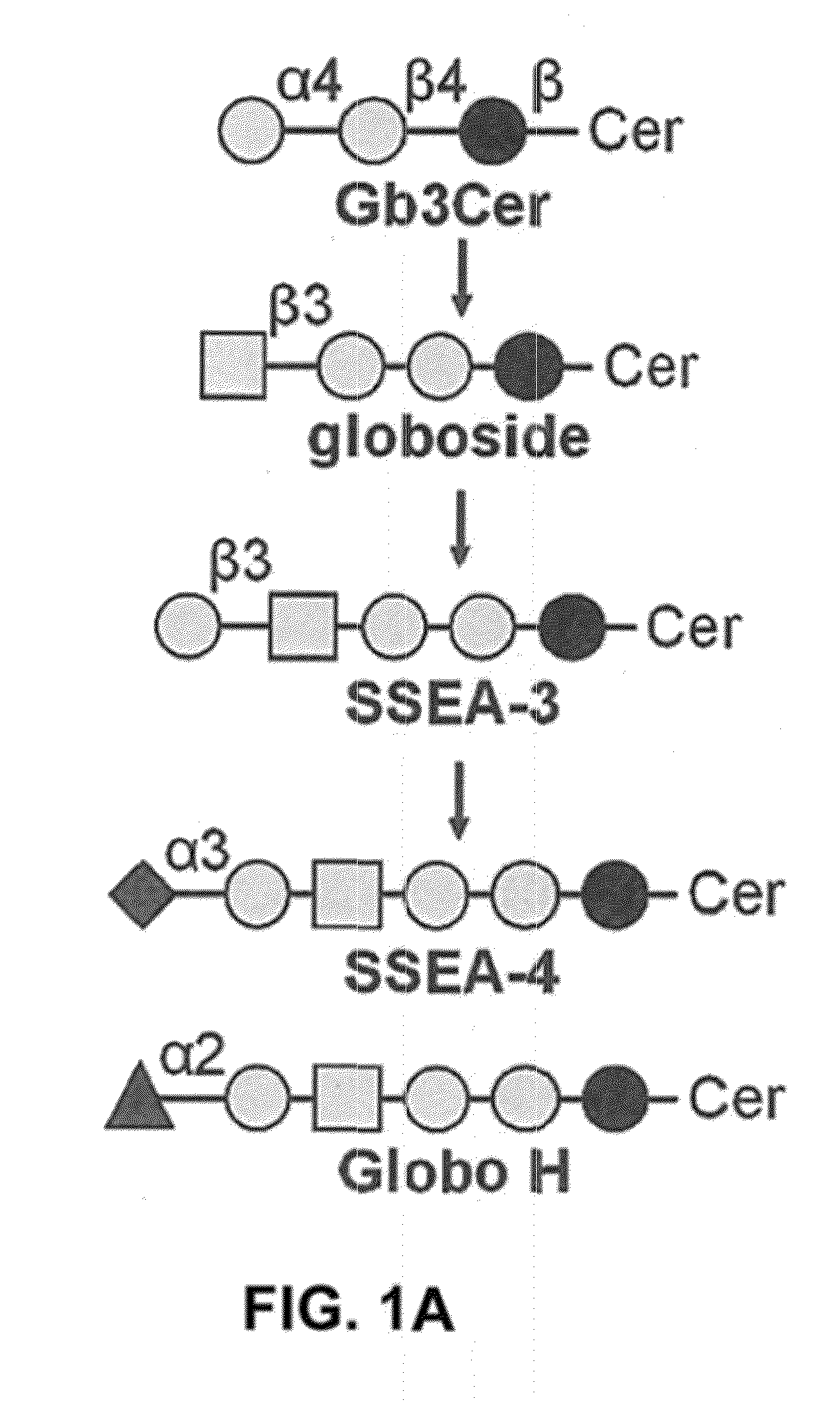
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to globo H, SSEA3, and SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, skin, bone, lungs, breast, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervical, ovarian, and / or prostate cancer.
Owner:ACAD SINIC
End-capped poly(ester amide) copolymers
Provided herein is an end-capped poly(ester amide) PEA) polymer and the method of making the polymer. The PEA polymer is substantially free of active amino end groups and / or activated carboxyl groups. The PEA polymer can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
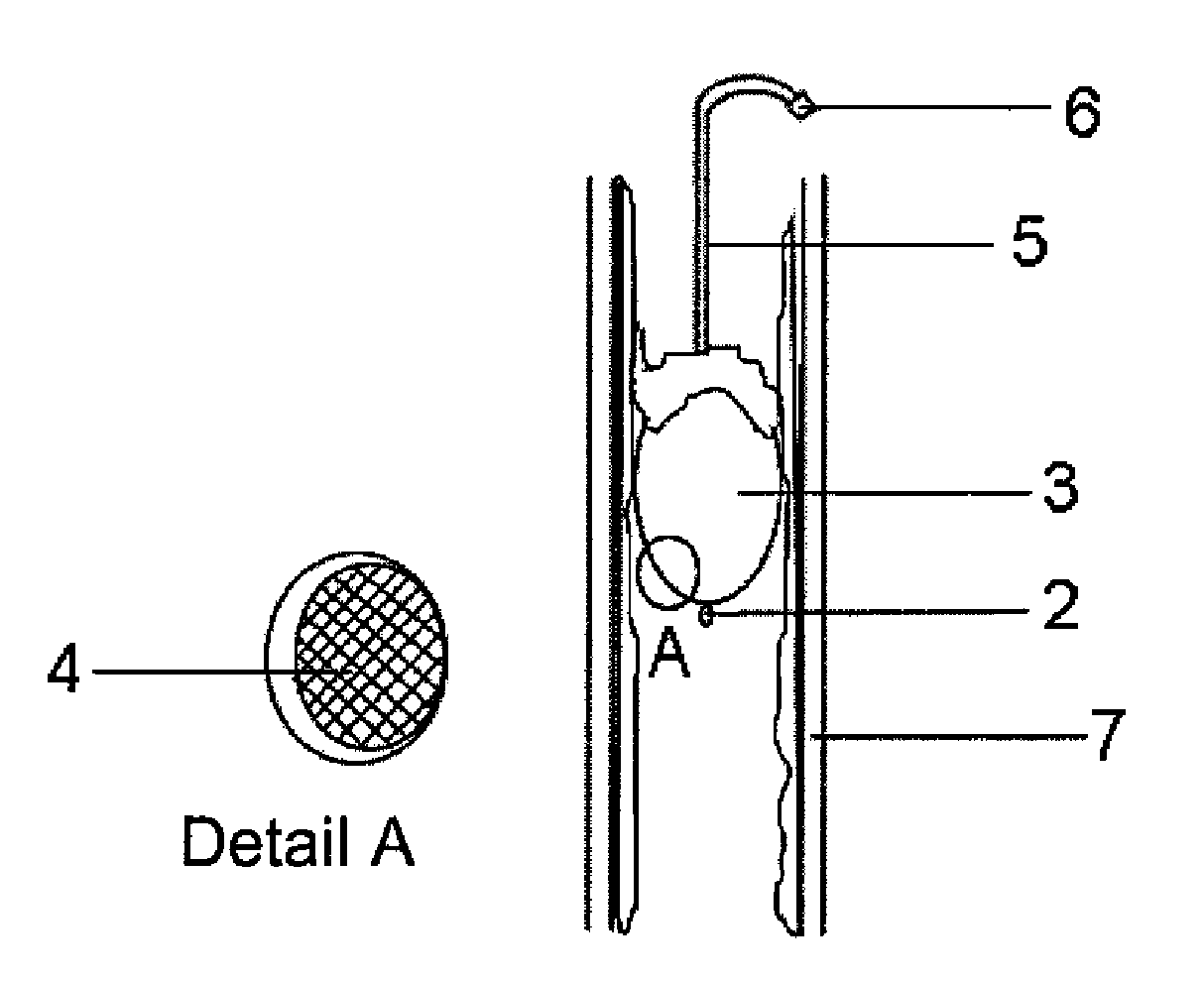
Flexible and Rigid Catheter Resector Balloon
InactiveUS20080171985A1Eliminate riskEasy to applyStentsBalloon catheterOesophageal tubeEndovascular occlusion
The present invention relates to resector balloons (1) employed in treating endoluminal-endobronchial tumoral lesions and endovascular occlusions encountered in blood vessels and in other hollow tube-like organs (7), such as trachea, windpipe, food pipe, urinary tract, bile ducts. Said resector balloon (1) is composed of a resection tip (2); a resection part (3) that is swollen or inflated in such tube-like organs (7) and is displaced or moved back and forth therein to provide tumor resection; a hardening surface (4) provided on the outer surface of said resection part (3) to shave and destroy such tumoral tissues; a catheter section (5) providing access to an endoluminal site; and an injection terminal (6) capable to inflate said resection part (3) by injecting air or fluid.
Owner:Y K K SAGLIK HIZMETLERI LIMITED SIRKETI
Blends of poly(ester amide) polymers
Provided herein is a poly(ester amide) (PEA) polymer blend and a polymeric coating containing the PEA polymer blend. The PEA polymer blend has a Tg above the Tg of poly(ester amide benzyl ester) (PEA-Bz) or the Tg of poly(ester amide TEMPO). The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Compositions and methods for treatment and detection of cancers
ActiveUS20150344551A1Shrink tumorEliminate the problemImmunoglobulins against animals/humansAntibody ingredientsCancer cellAntigen Binding Fragment
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, lung, breast, mouse, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervix, ovary, and / or prostate cancer.
Owner:ACAD SINIC
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
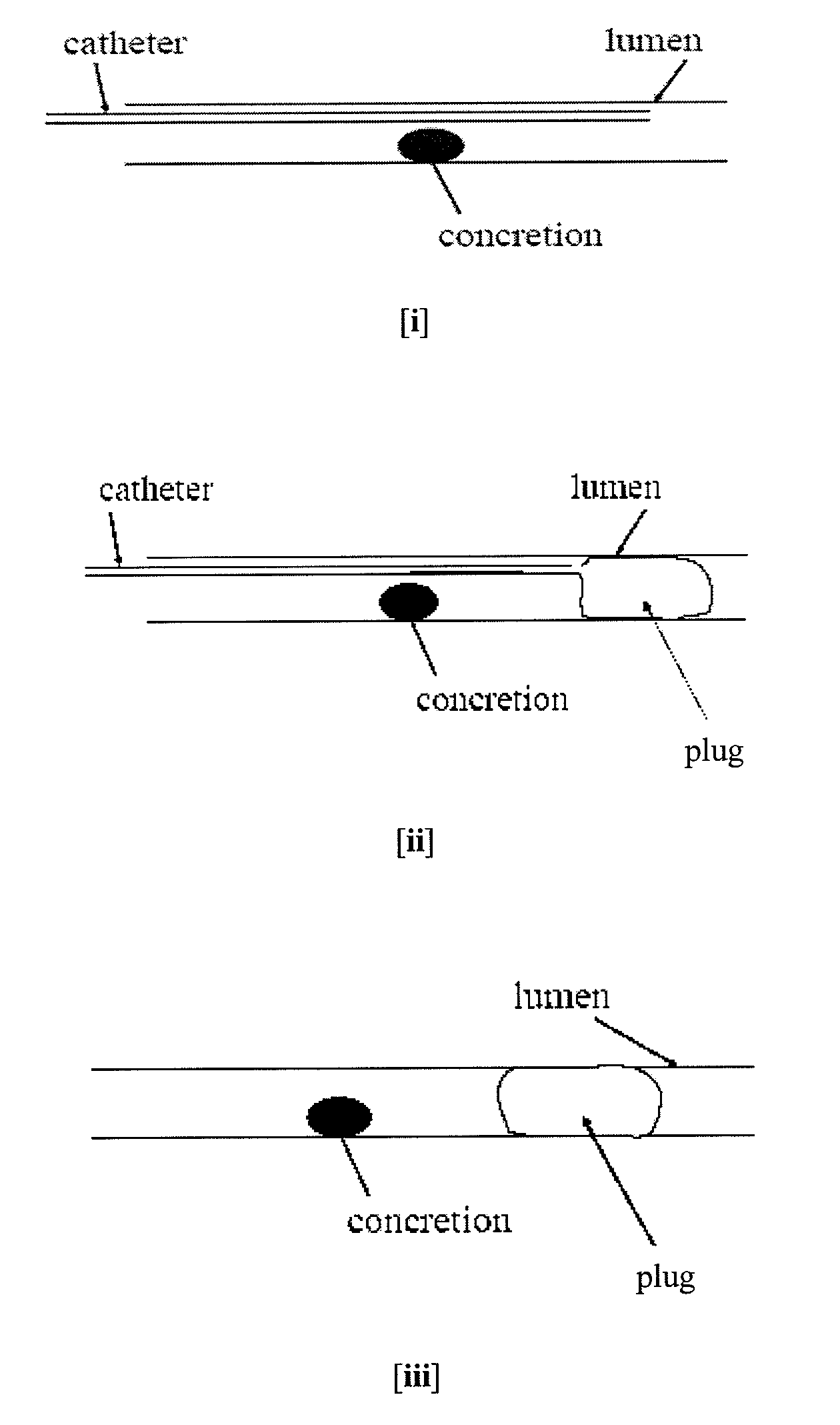
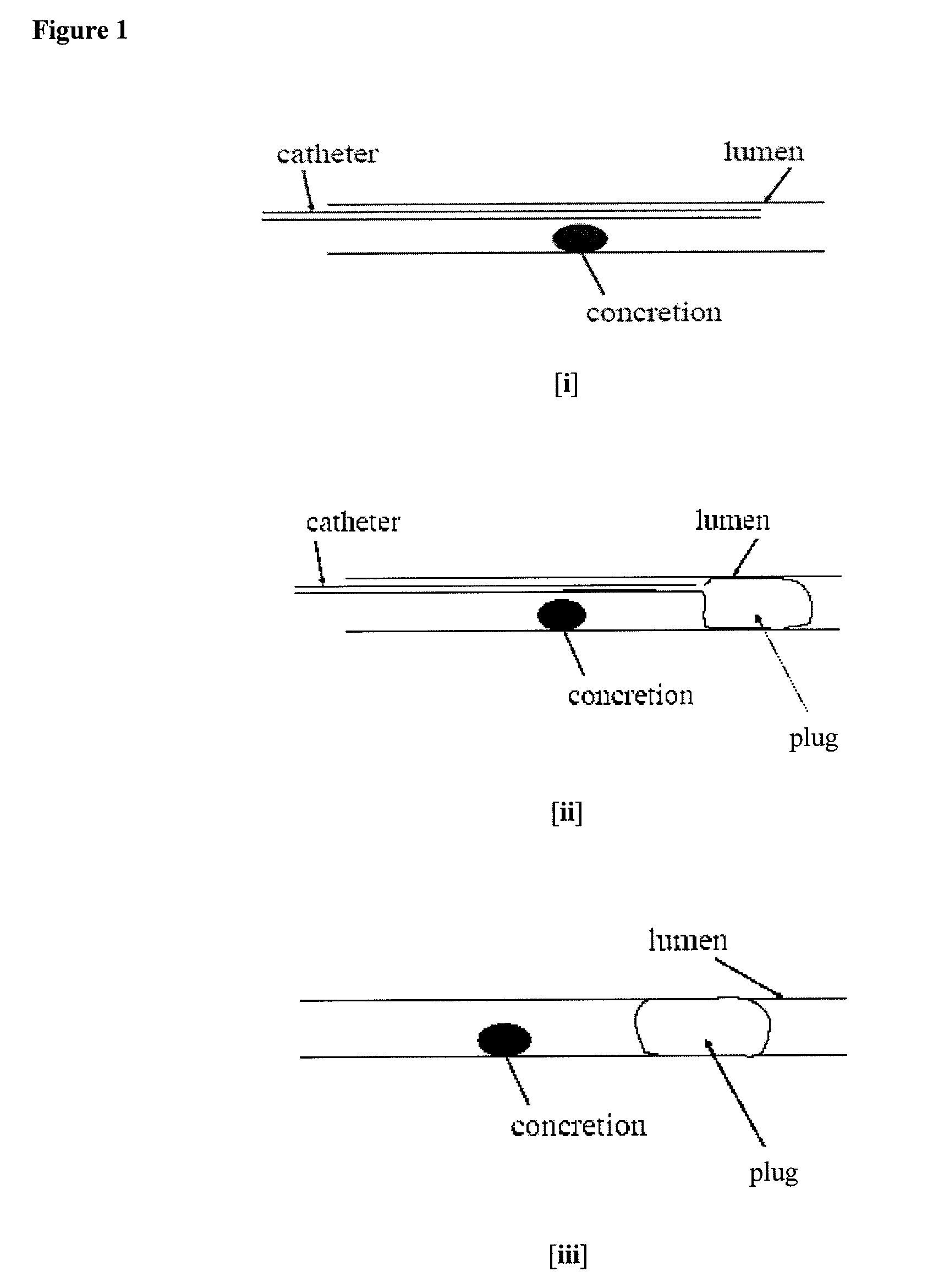
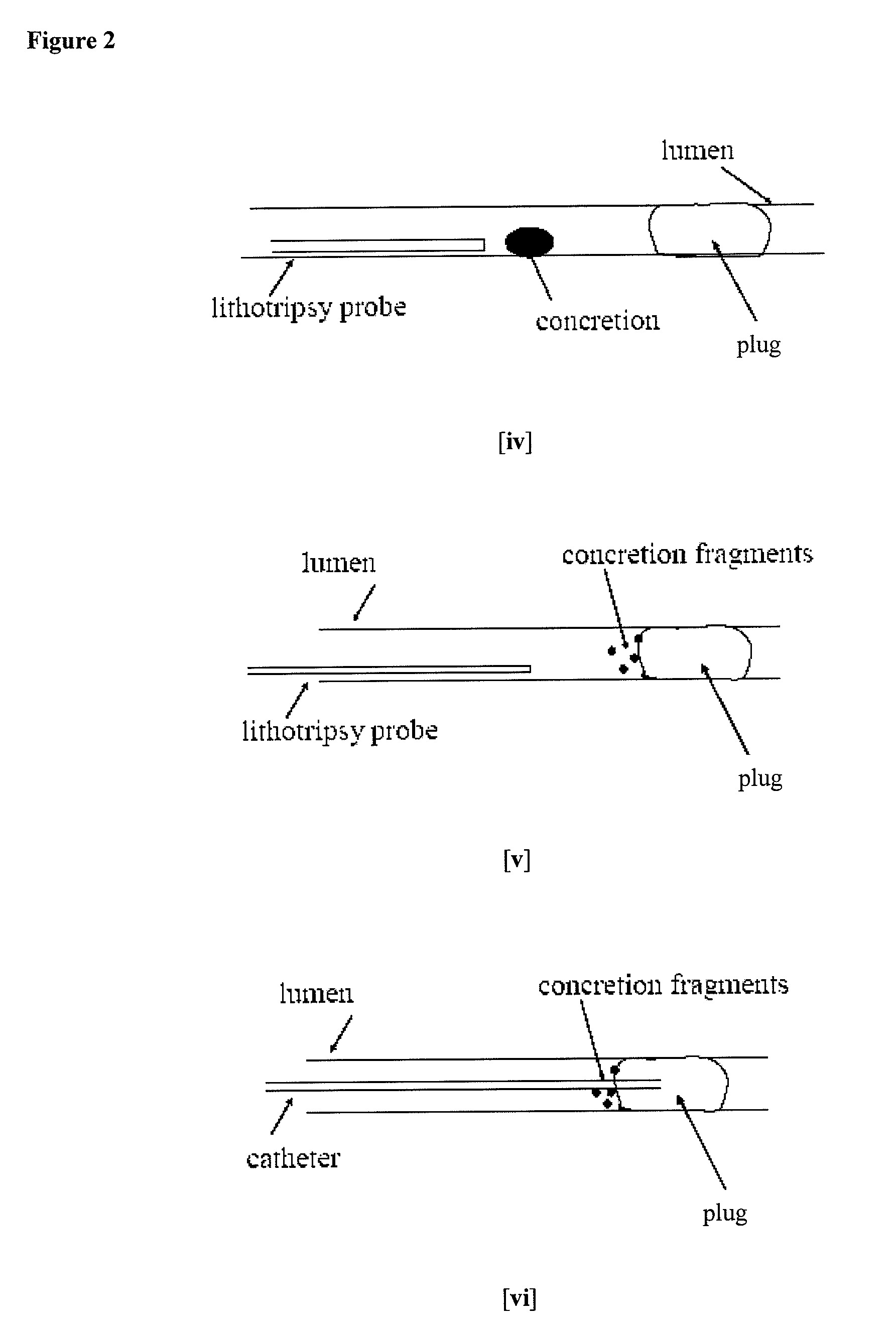
Methods for Preventing Retropulsion of Concretions and Fragments During Lithotripsy
InactiveUS20080103481A1Reduce risk of damageConvenient treatmentIn-vivo radioactive preparationsDigestive systemConcretionBody tissue
One aspect of the present invention provides a method for the treatment of lithiasis, which mitigates the risk of damage to surrounding body tissue when removing a calculi (e.g., biological concretions, such as urinary, biliary, and pancreatic stones) that obstructs or may otherwise be present within a body's anatomical lumen. In one embodiment, the instant invention provides a method of using a polymer plug to occlude a lumen distal to a calculi, whereby calculi fragments resulting from lithotripsy are prevented from traveling up the lumen. In certain embodiments, a dual lumen catheter is utilized to inject two solutions proximal to the calculi, the mixing of said solutions causing a polymer plug to form.
Owner:GENZYME CORP
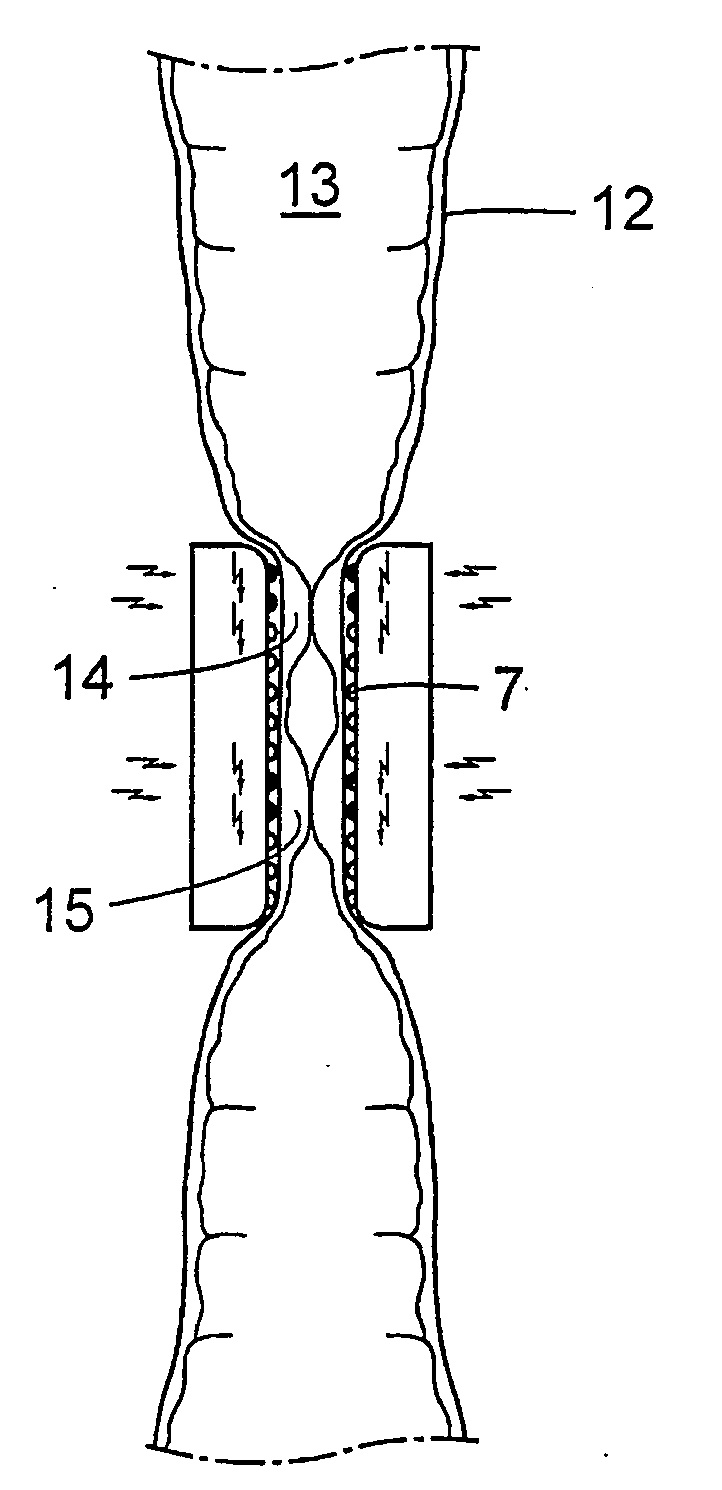
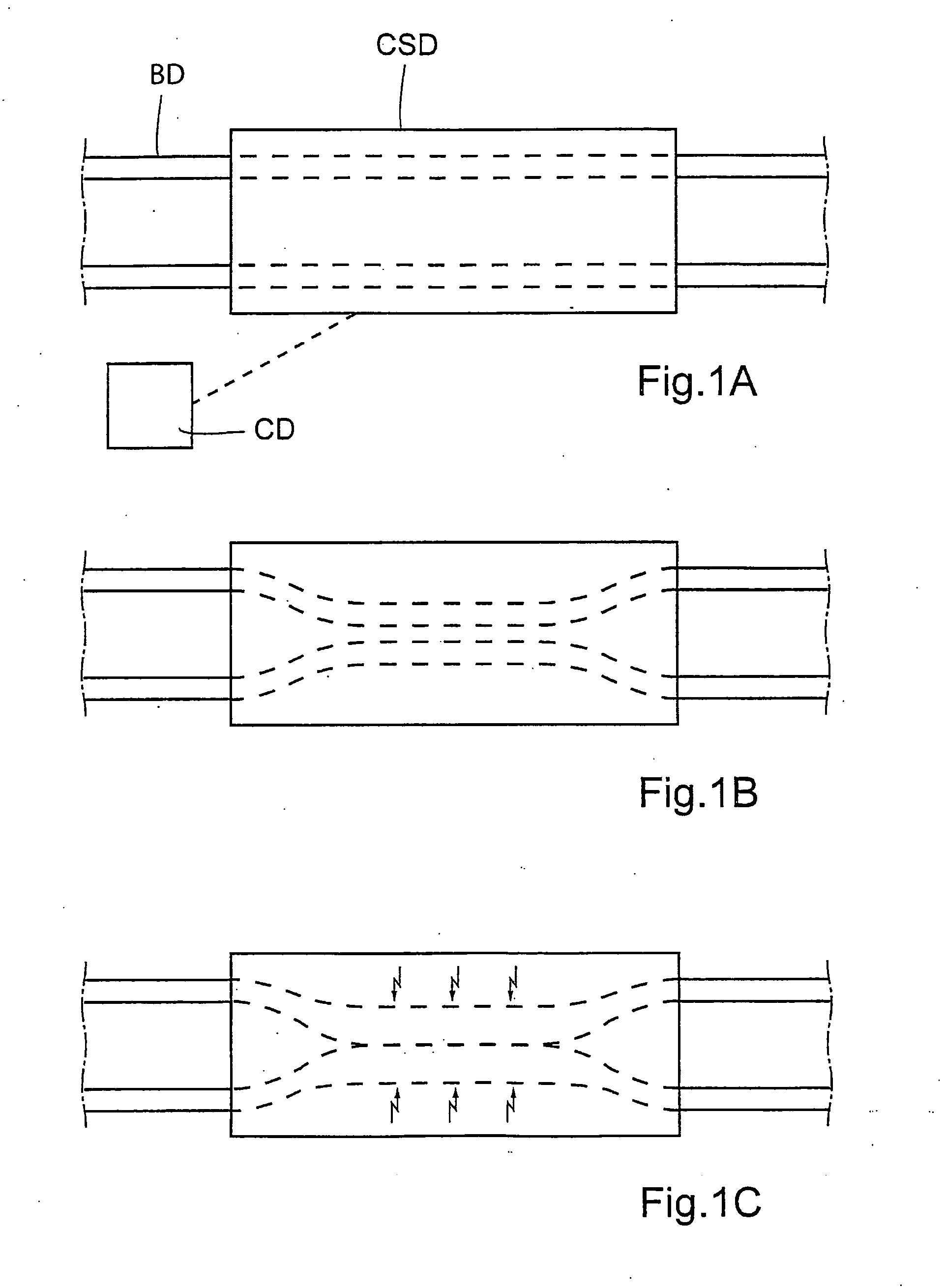
Method for the treatment of gallstones
There is provided a method for controlling the movement of bile and / or gall stones in the biliary duct. The method comprises gently constricting (i.e., without substantially hampering the blood circulation in the tissue wall) at least one portion of the tissue wall to influence the movement of bile and / or gallstones in the biliary duct, and stimulating the constricted wall portion to cause contraction of the wall portion to further influence the movement of bile and / or gallstones in the biliary duct. The method can be used for restricting or stopping the movement of bile and / or gallstones in the biliary duct, or for actively moving the fluid in the biliary duct, with a low risk of injuring the biliary duct.
Owner:FORSELL PETER
Implantable devices formed of non-fouling methacrylate or acrylate polymers
Implantable devices formed of or coated with a material that includes a polymer having a non-fouling acrylate or methacrylate polymer are provided. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
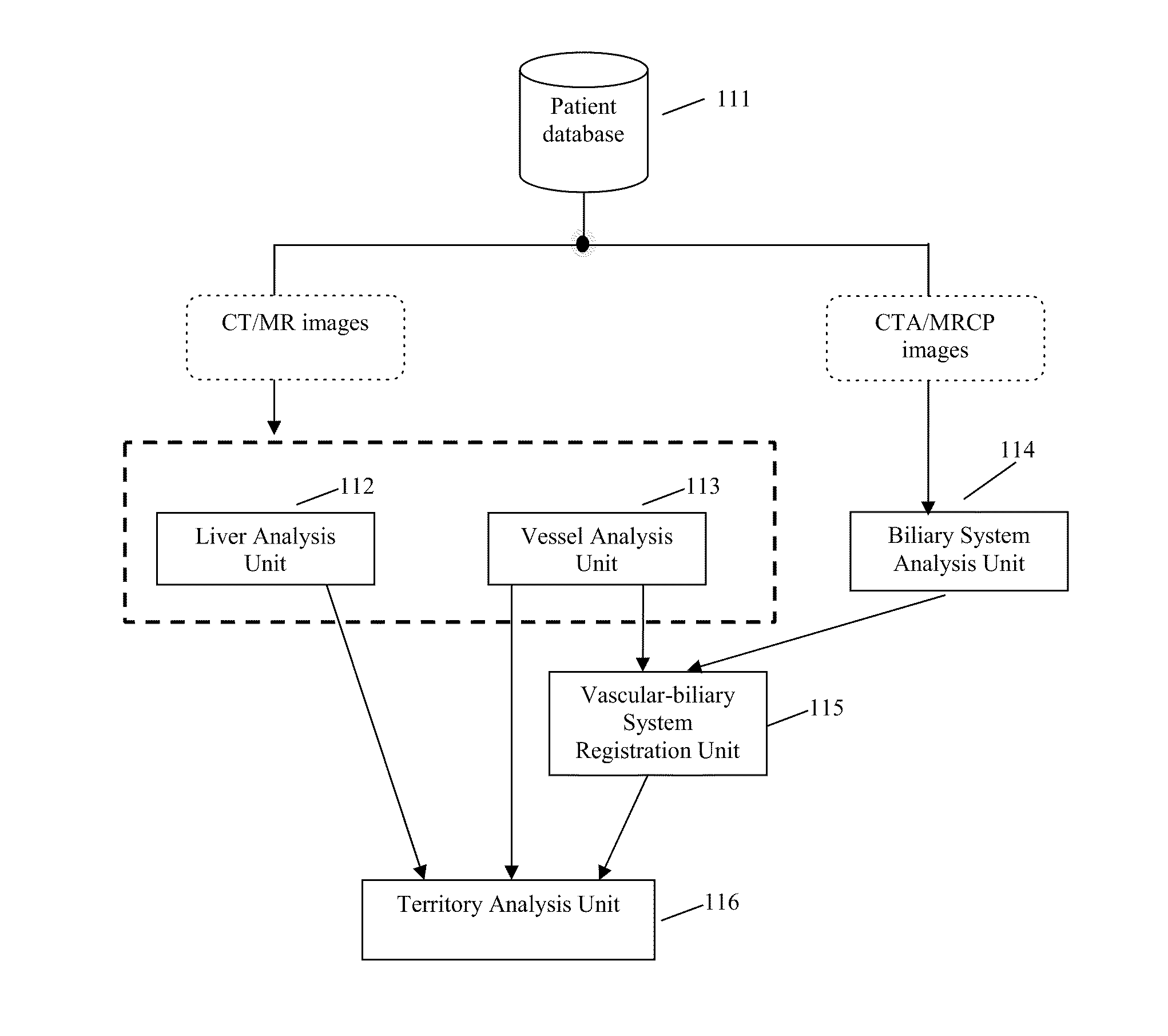
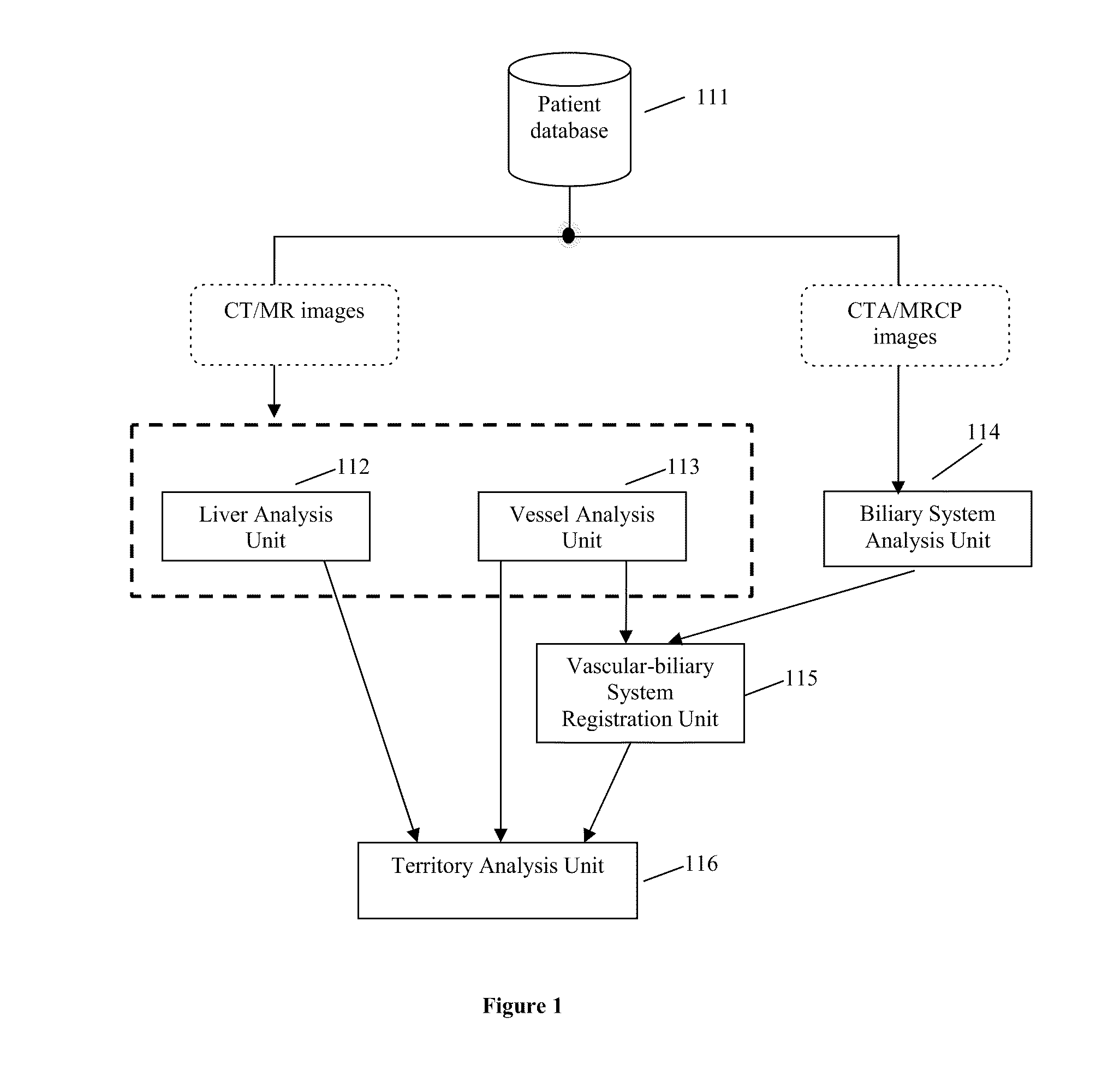
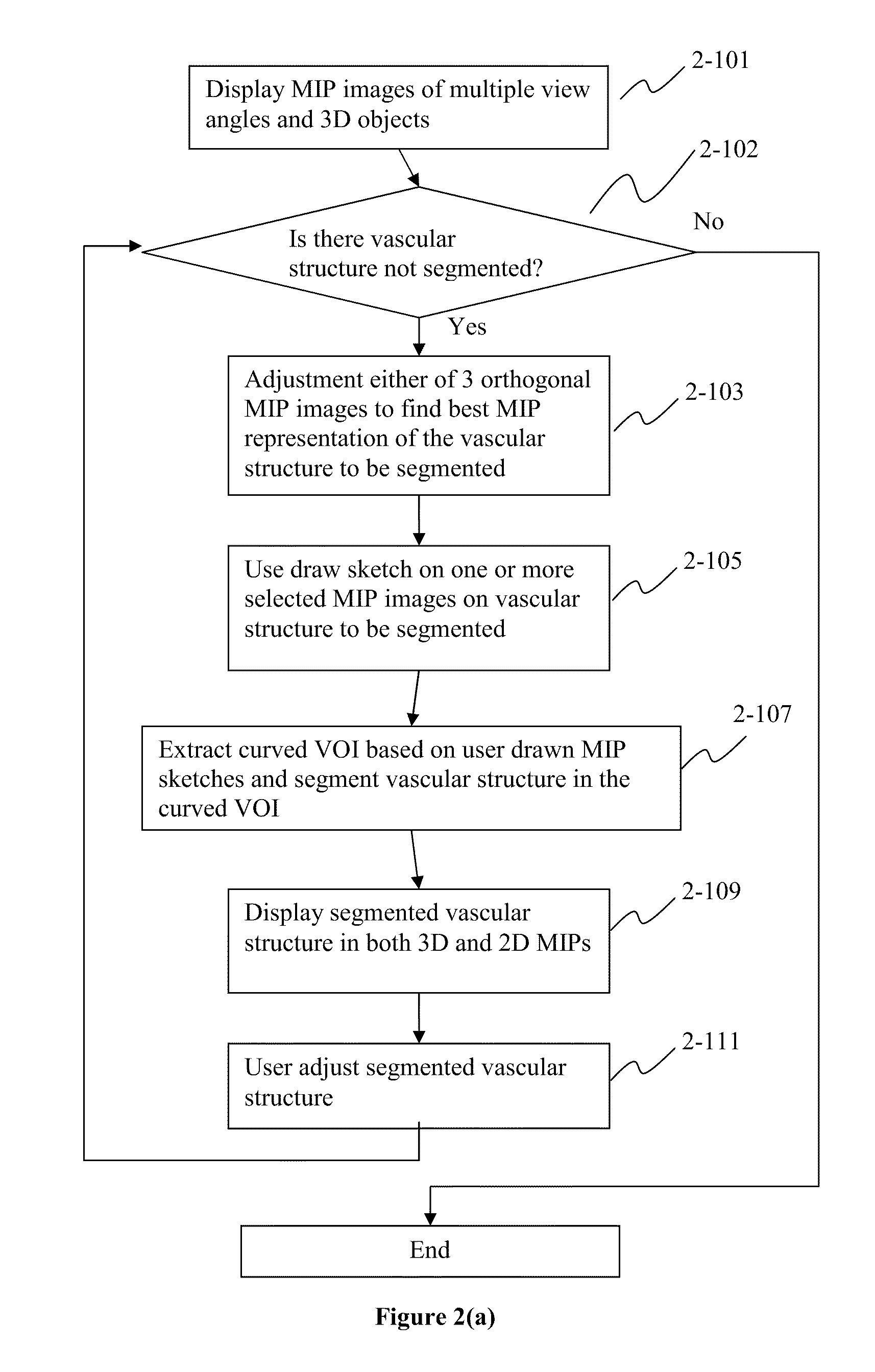
Method, system, apparatus, and computer program product for interactive hepatic vascular and biliary system assessment
A procedure for image segmentation on three-dimensional (3D) medical images, and a system, apparatus, and computer program that operate in accordance with the procedure. The procedure includes generating a projection including an anatomic structure, tracing a curve corresponding to the anatomic structure, extracting a curved volume of interest based on the curve and the projection, and extracting a segmentation of the anatomic structure. Also provided is a procedure for aligning anatomic structures in images, and a system, apparatus, and computer program that operate in accordance with the procedure. The procedure includes determining part of a biliary system in a first image, determining part of a hepatic portal vein or a hepatic artery in a second image, determining a gallbladder in the images, determining a cost function, and aligning the biliary system and the hepatic portal vein or hepatic artery by maximizing the cost function.
Owner:EDDA TECH
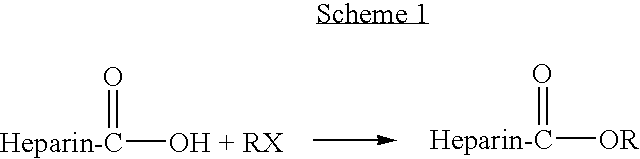
Heparin prodrugs and drug delivery stents formed therefrom
InactiveUS20060014720A1Organic active ingredientsPeptide/protein ingredientsDiseasePercent Diameter Stenosis
A prodrug comprising a heparin and a drug is provided. The prodrug can be used to form a coating on a medical device. The prodrug can also be used with a polymeric material to form a coating on a medical device. The polymeric material can be a hydrophobic polymer, a hydrophilic polymer, a non-fouling polymer, or combinations thereof. The medical device can be implanted in a human being for the treatment of a disease such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Compositions and methods for treatment and detection of cancers
ActiveUS20160289340A1Useful in treatmentEnhanced ADCC activityImmunoglobulins against animals/humansAntibody ingredientsCancer cellAntigen Binding Fragment
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to globo H, SSEA3, and SSEA-4 are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as those in brain, skin, bone, lungs, breast, esophagus, stomach, liver, bile duct, pancreas, colon, kidney, cervical, ovarian, and / or prostate cancer.
Owner:ACAD SINIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com