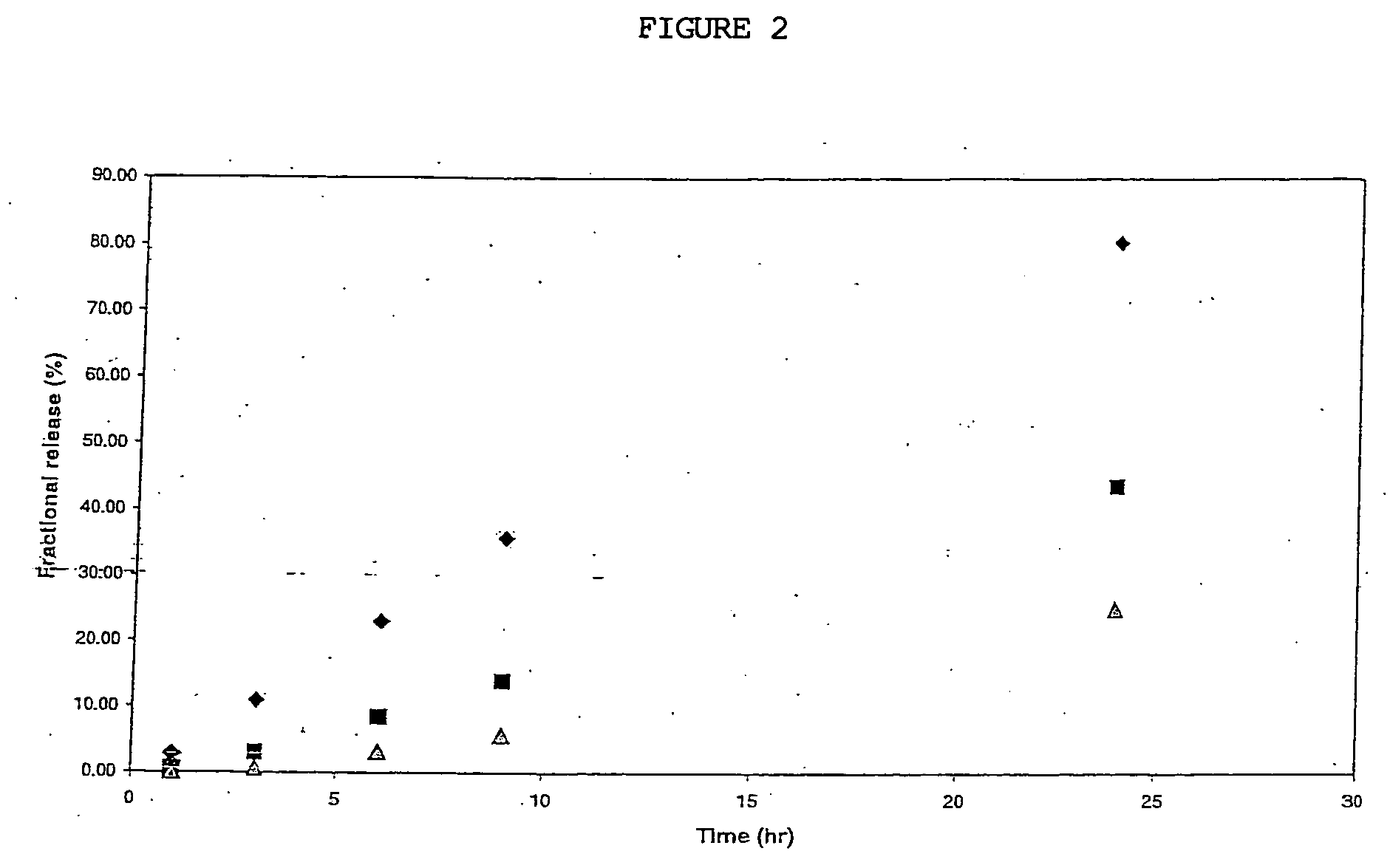
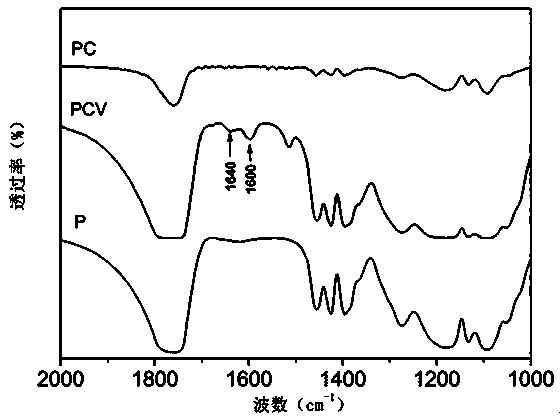
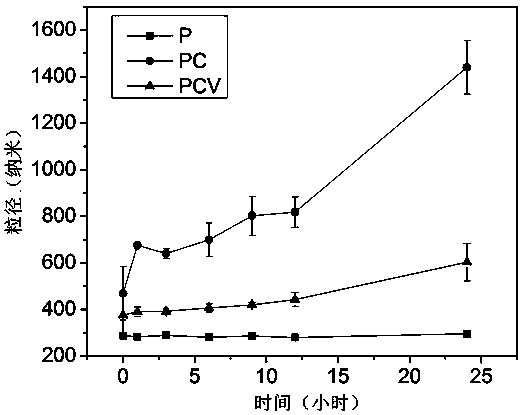
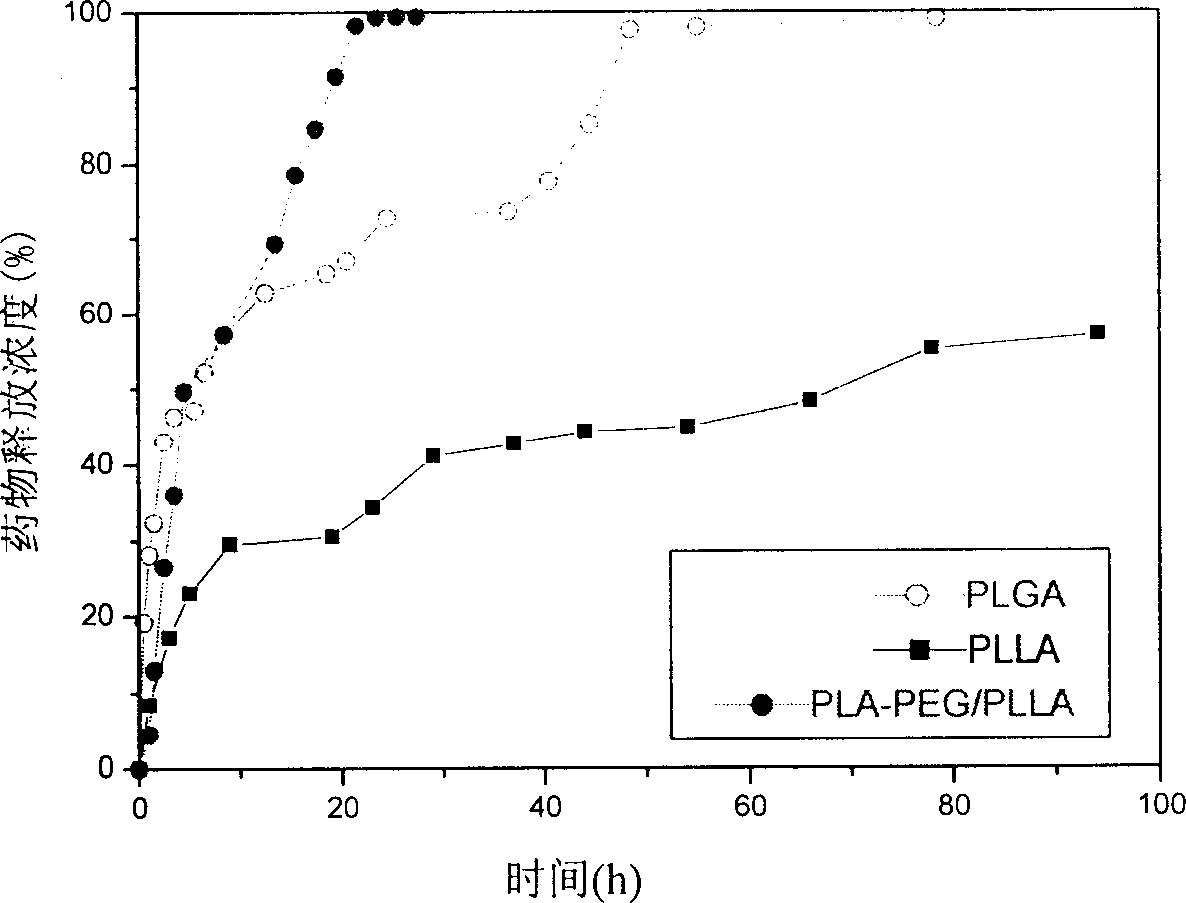
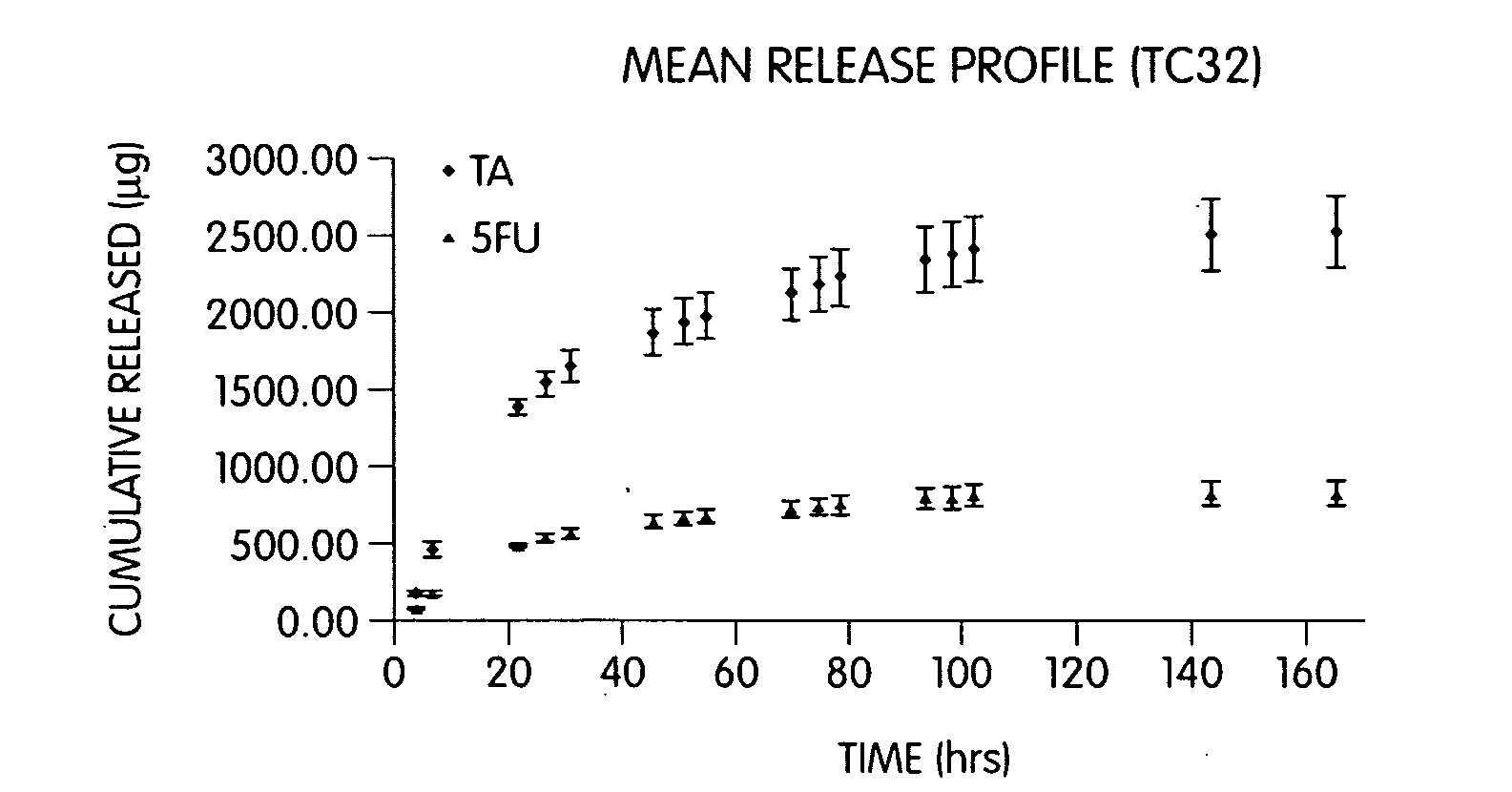
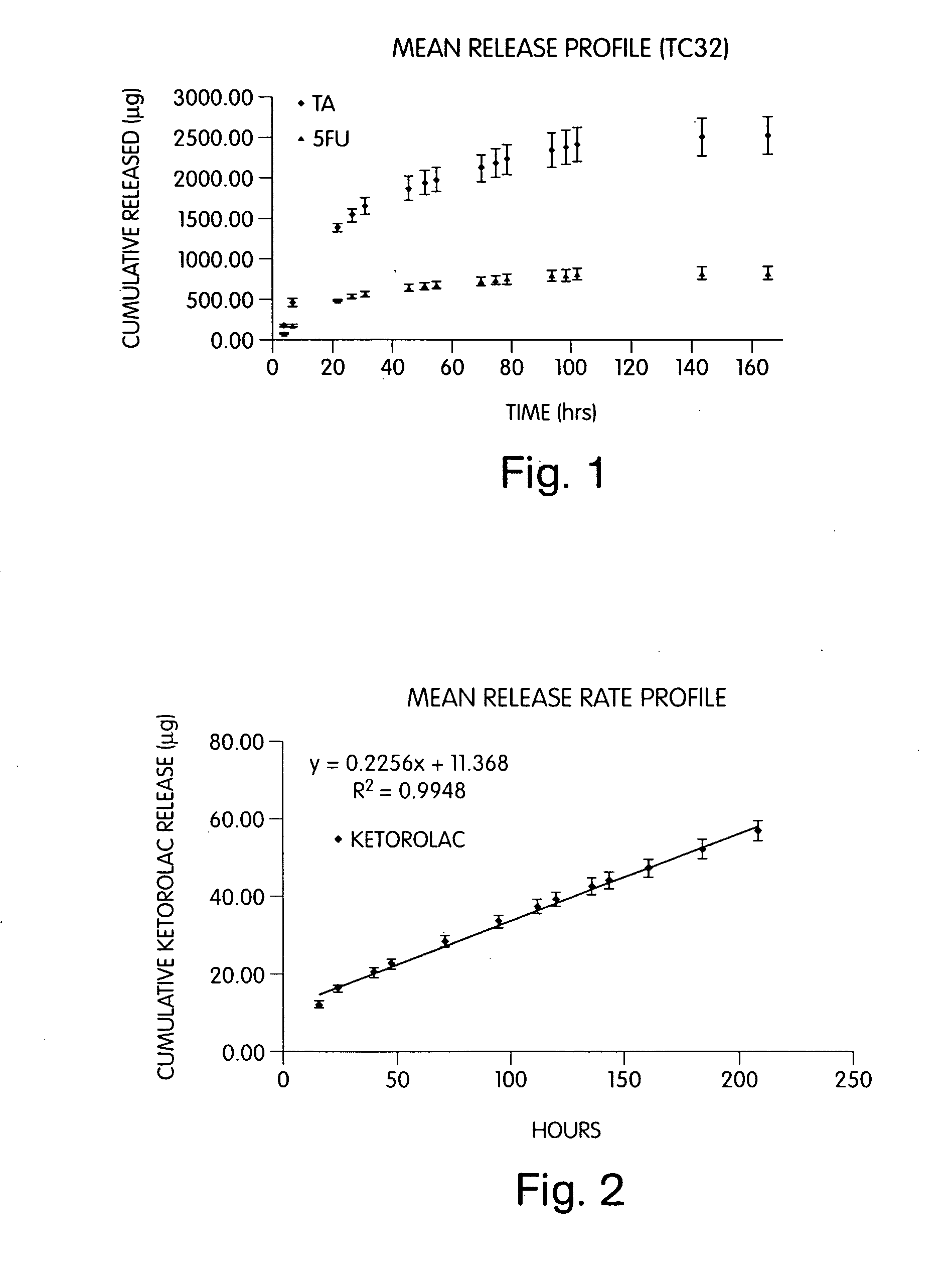
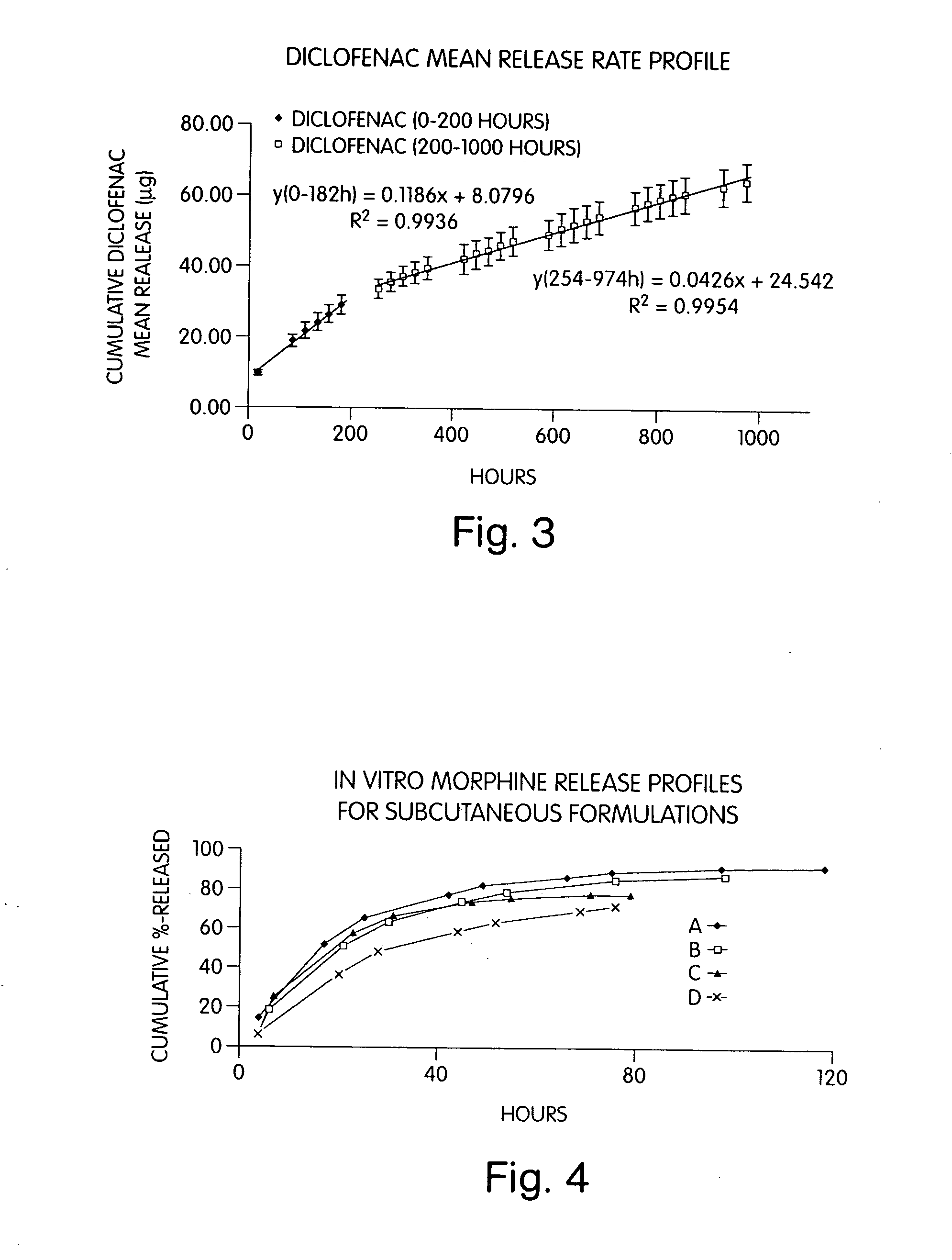
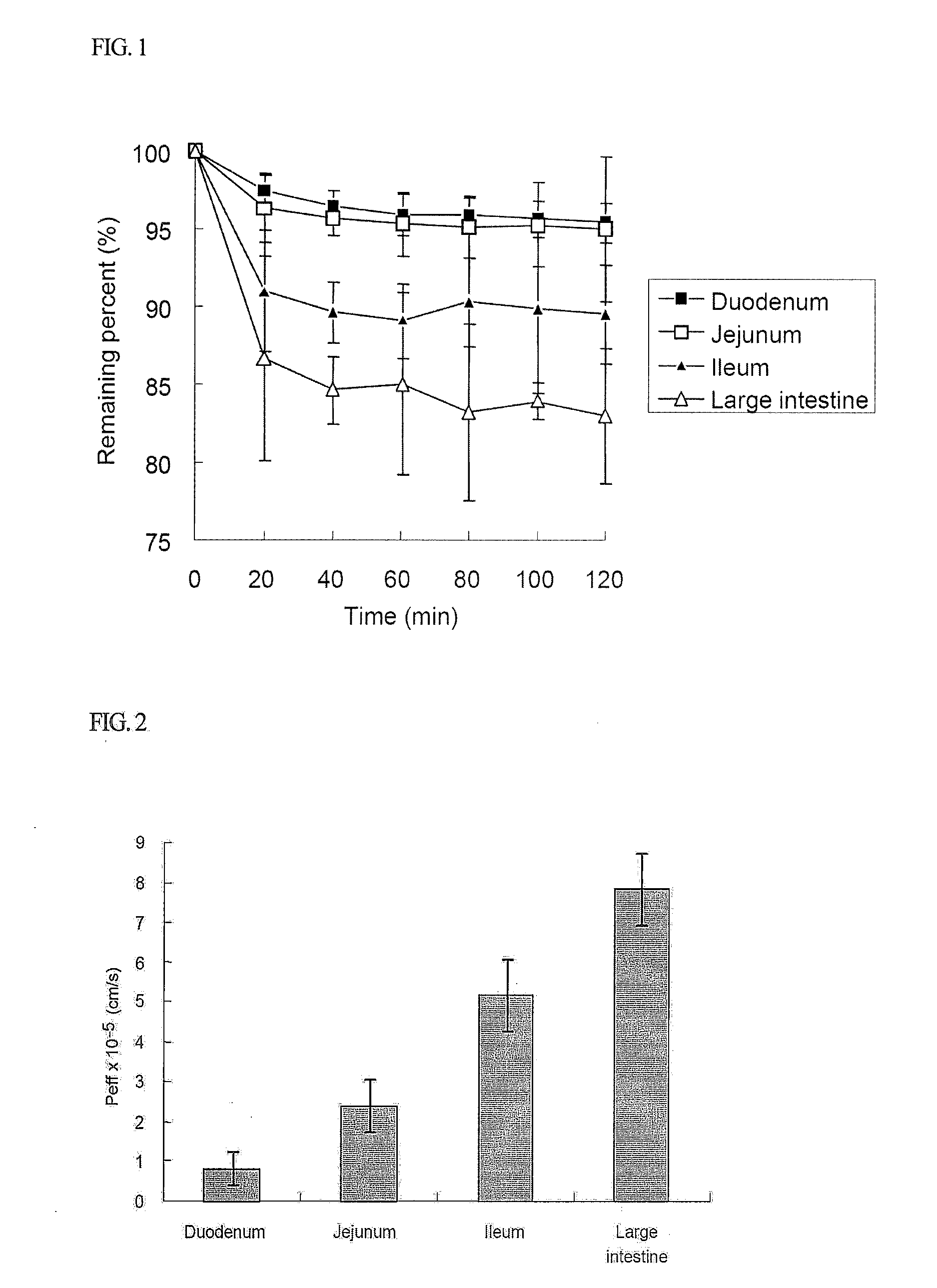
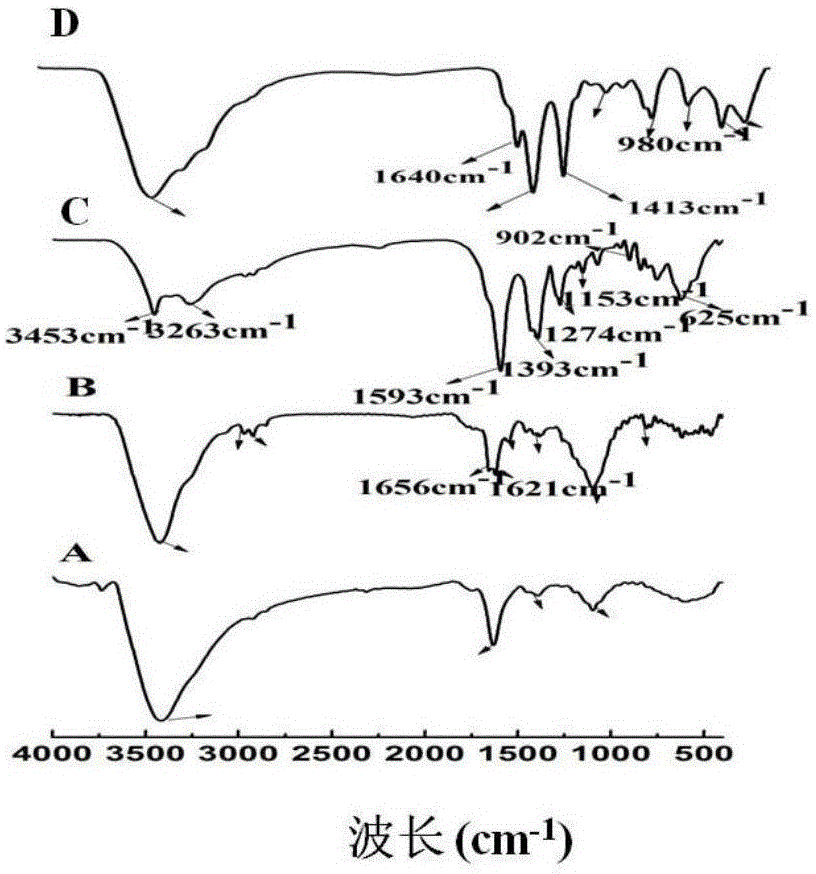
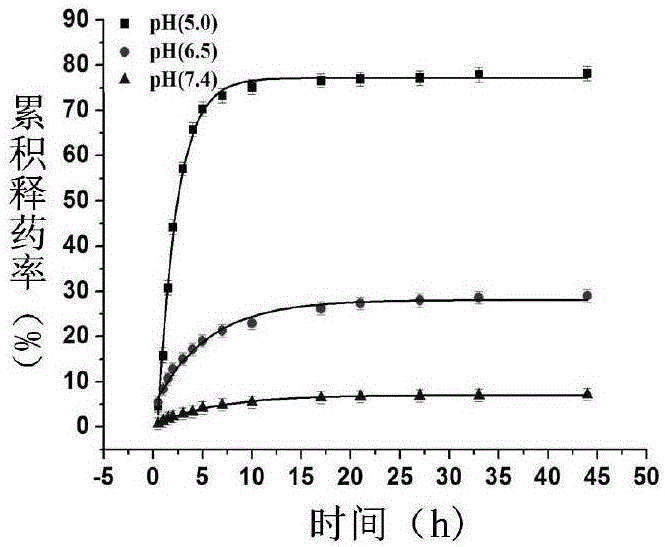
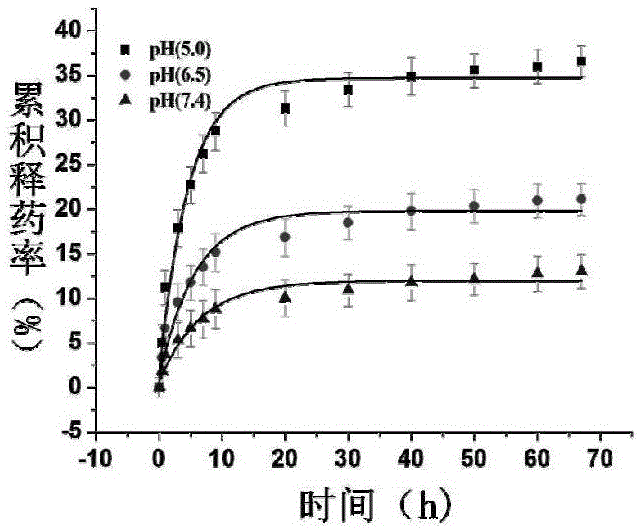
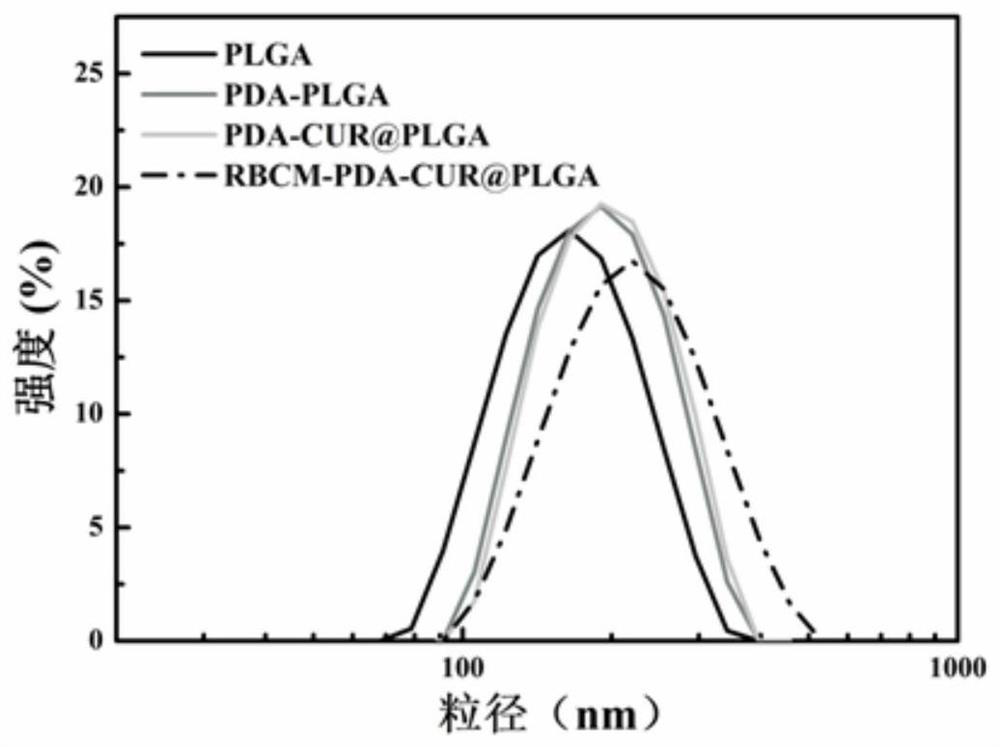
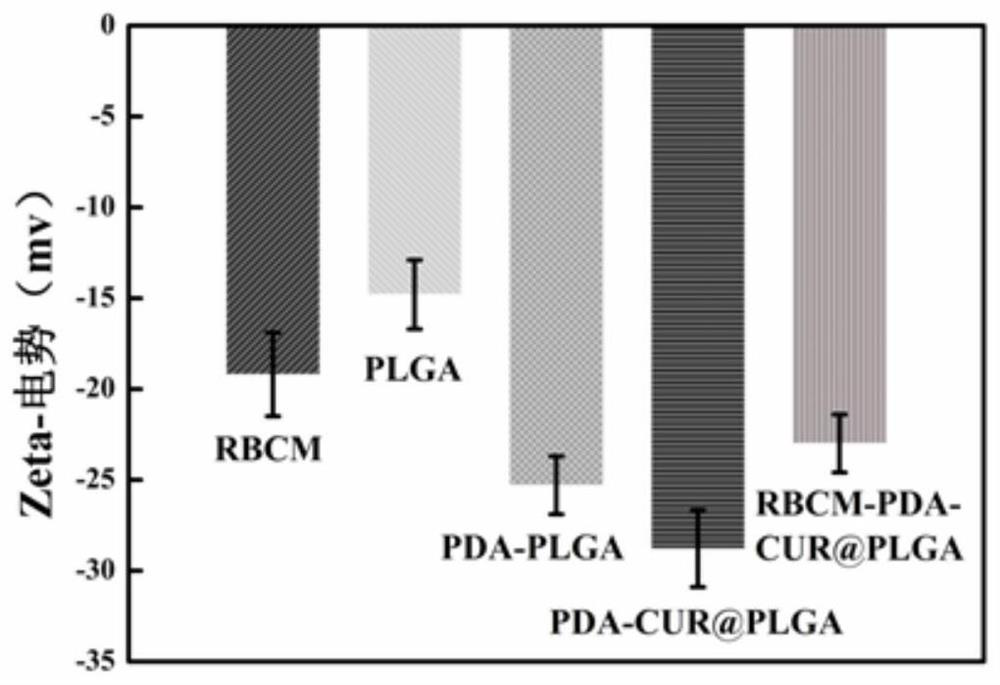
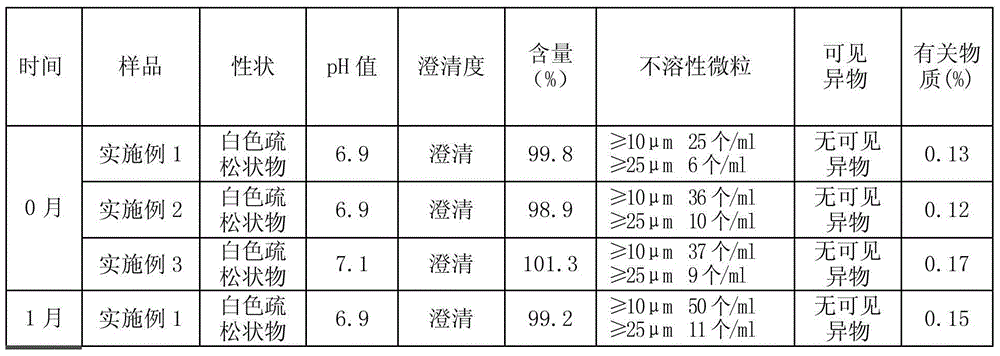
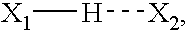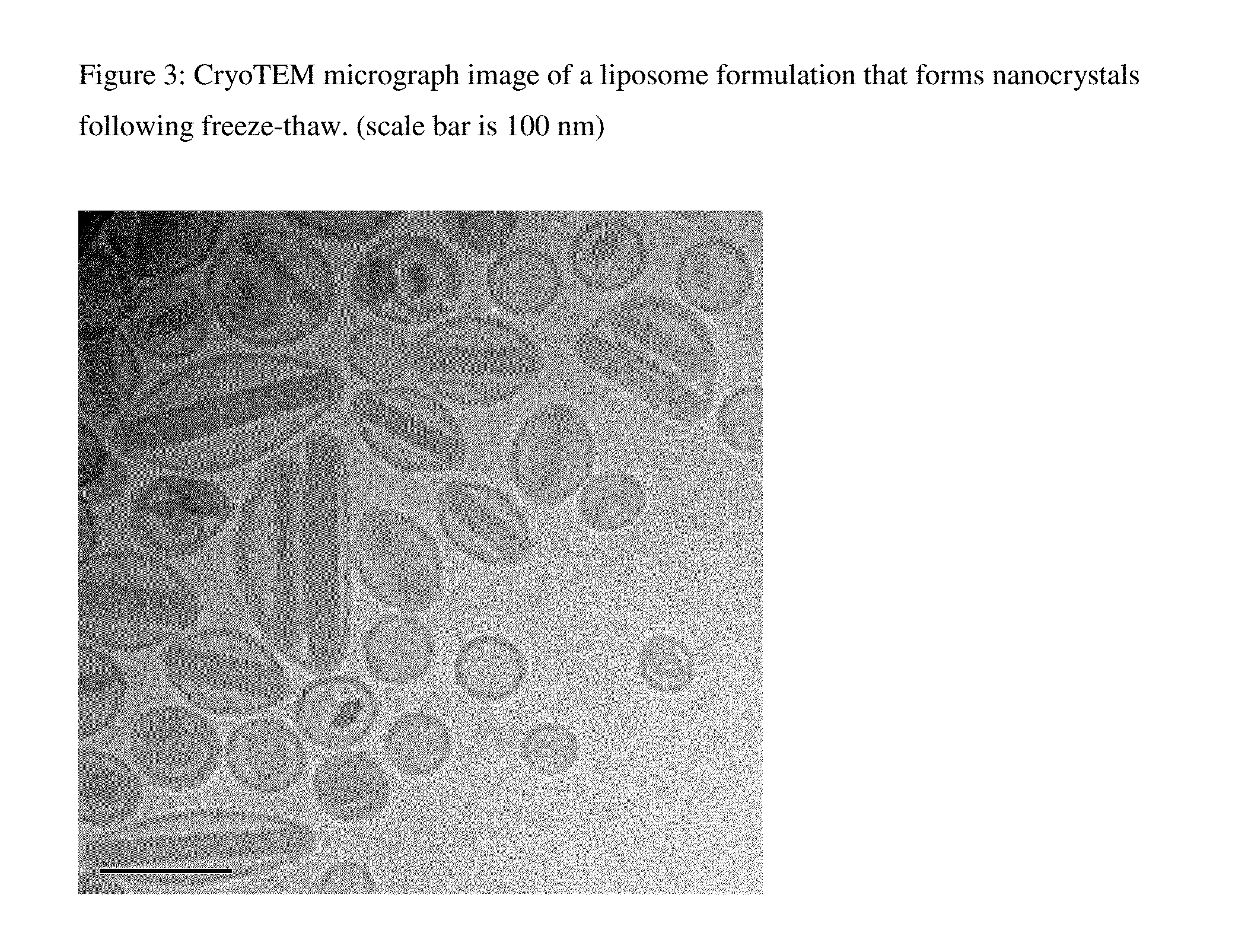Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49results about How to "Increase drug release rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Poly(ester amide) filler blends for modulation of coating properties
InactiveUS20060093842A1Improve stabilityIncrease drug release rateOrganic active ingredientsNervous disorderAbnormal tissue growthPEA polymer
Provided herein is a PEA polymer blend and coatings or implantable devices formed therefrom. The PEA polymer blend is formed of a PEA polymer and a material capable of hydrogen bonding with the PEA. The PEA polymer blend can form a coating on an implantable device, one example of which is a stent. The coating can optionally include a biobeneficial material and / or optionally with a bioactive agent. The implantable device can be used to treat or prevent a disorder such as one of atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, and combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Polylactic acid-hydroxyacetic acid copolymer nano-drug carrier as well as preparation method and application thereof
ActiveCN104001178AImprove intake capacityEliminate side effectsNanomedicinePharmaceutical non-active ingredientsCrosslinked chitosanMicrosphere
The invention provides a PLGA (polylactic acid-hydroxyacetic acid) nano-drug carrier which is composed of PLGA nano microsphere kernel and an anillic aldehyde crosslinked chitosan housing. A preparation method of the polylactic acid-hydroxyacetic acid copolymer nano-drug carrier is as follows: dispersing a PLGA organic phase which takes dichloromethane and alcohol as a mixed solvent in a water phase to prepare PLGA microspheres by taking PVA (polyvinyl acetate) as an emulsifier by virtue of an one-off emulsion process; then, adding the PLGA microspheres into chitosan liquor, so that the chitosan is adsorbed on the surfaces of the PLGA microspheres, and then adding the chitosan on the surfaces of anillic aldehyde crosslinked PLGA microspheres. The product has a certain pH environmental responsiveness, can realize controlled release of the drug according to in-vivo pH environmental changes, is high in stability, strong in up-taking capacity for microsphere-coated medicaments by the cells, and has very good application prospect in the drug carrier for treating tumors.
Owner:SUN YAT SEN UNIV
Method to enhance drug release from a drug-releasing material
A method of enhancing the drug release rate from a composite material. The composite material includes a synthetic, bioabsorbable polymer matrix and a drug particle phase dispersed therein. The release rate of the drug from the polymer matrix is enhanced by orienting the composite material. The drug release rate of the oriented composite material is greater than the drug release rate from an otherwise comparable non-oriented composite material.
Owner:BIORETEC
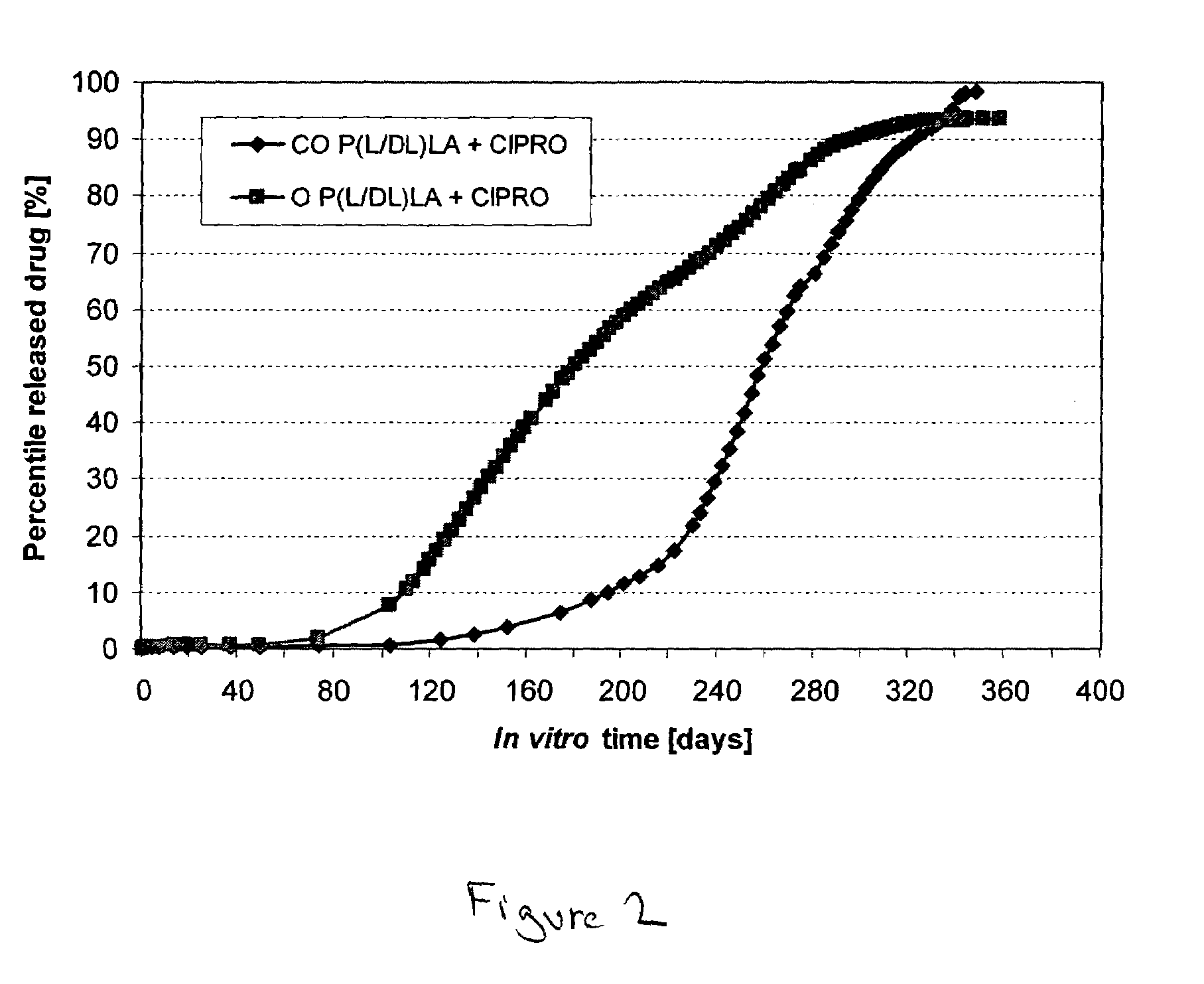
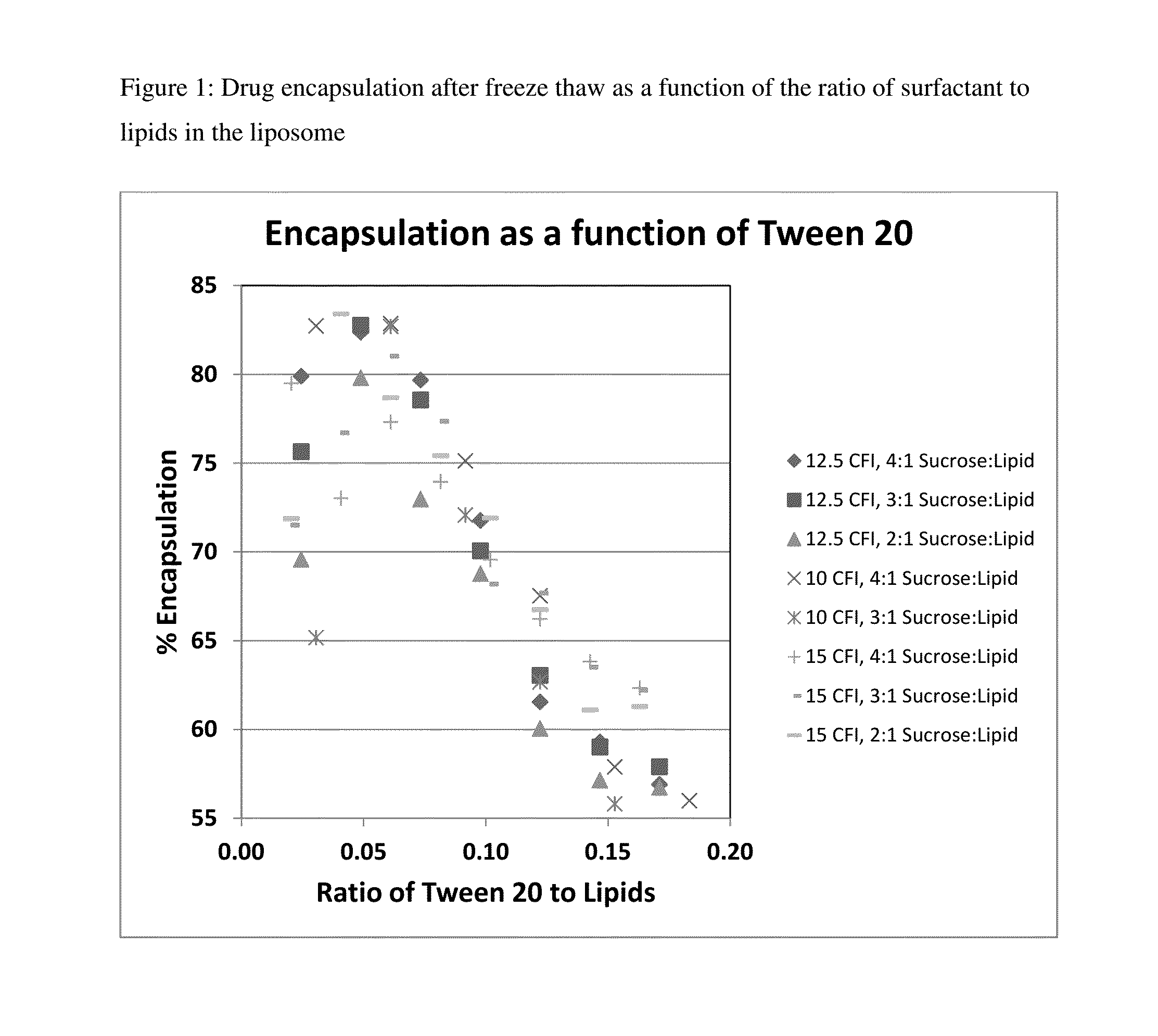
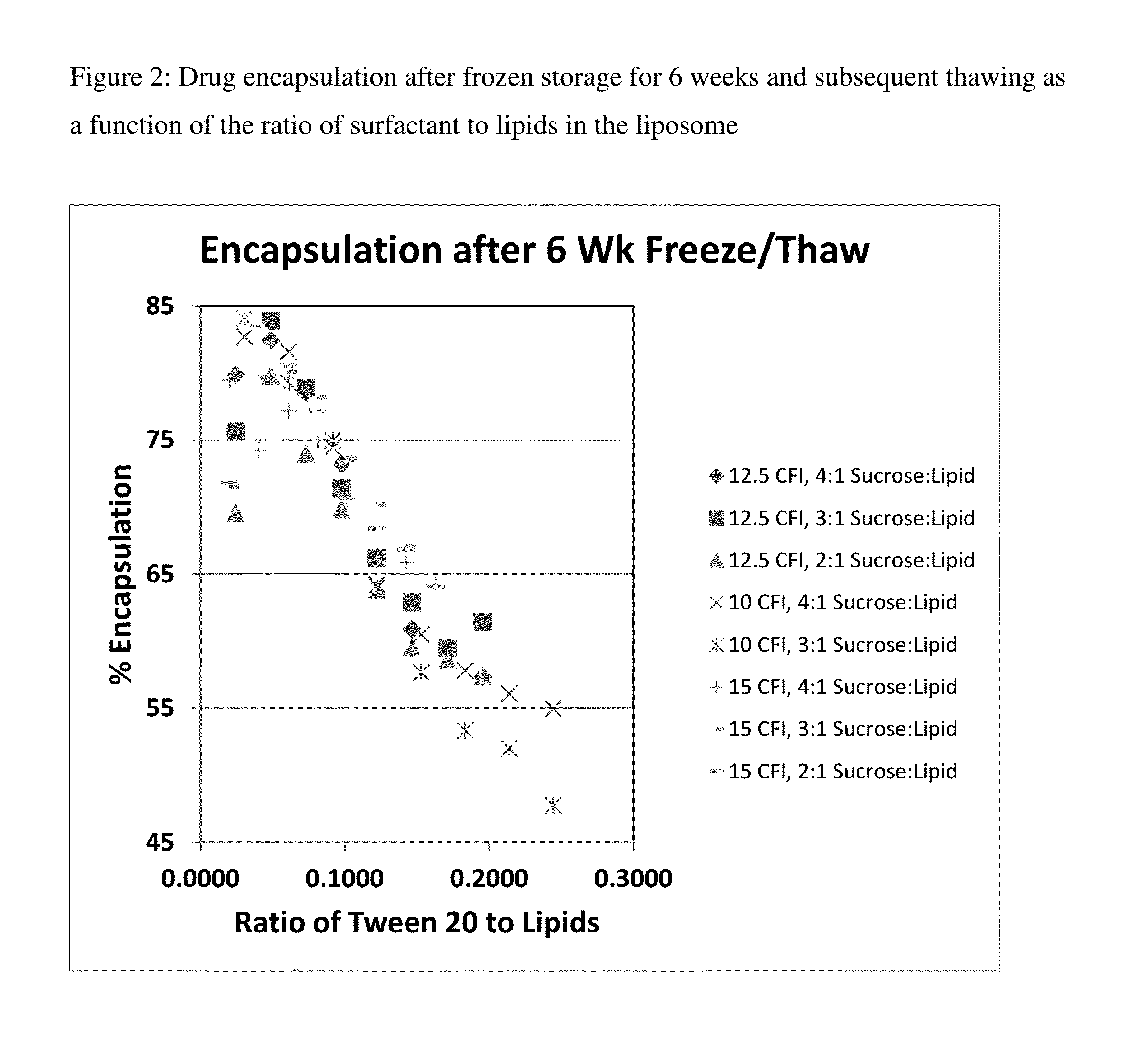
Novel liposomal formulations that form drug nanocrystals after freeze-thaw
InactiveUS20150283076A1Increase drug release rateGood curative effectOrganic active ingredientsDispersion deliveryFreeze thawingNanocrystal
Methods for formulating a liposome comprised of a surfactant and a cryopreservative that can be frozen for long term stability, and upon thawing provides an immediate and sustained release delivery profile. Specific liposome formulations include anti-infectives and delivery of such for treatment of respiratory tract infections and other medical conditions, and devices and formulations used in connection with such are described.
Owner:GRIFOLS
Nanometer fiber slow-releasing system and its prepn process and application
InactiveCN1739491AAvoid complicationsHas an anti-inflammatory responsePeptide/protein ingredientsAntipyreticFiberSurgical operation
The present invention relates to medicine material technology, and is especially one kind of antiphlogistic medicine and growth factor slow releasing system and its preparation process and application. The prepared nanometer fiber medicine carrying film may be used in surgical operation to resist inflammation as skin tissue engineering rack. As post-operational adhesion resisting film, the present invention is biodegradable and biocompatible and has antiphlogistic effect. At the same time, medicine is combined with growth factor in controlled release speed and used in skin tissue engineering to prevent inflammation and to release growth factor promoting skin cell regeneration and healing simultaneously. The present invention has wide application foreground in post-operational adhesion prevention and skin tissue engineering.
Owner:TONGJI UNIV
Preparing method for growth factor slow-releasing system for tissue repair
InactiveCN1584143AEfficient use ofLarge specific surface areaArtifical filament manufactureFiberTissue repair
The invention relates to electric filature to produce a kind of nano fiber growth gene slow-release system. This invention is compounded by biology decomposably polymer material and uses electric filature to produce a kind of nano fiber laxyly release system which contains growth gene. The method not only is operated easily and has a simple working procedure, but also can use the growth gene effectively in reducing the absent tissues. The laxly release system can emit growth gene steadily in body and avoids the lost of active of growth gene. More over, it can promote increasing of cells and tissue recovery. The production can not only be mad into the piece of velamen to transplant in the absent tissues for cure, but also be made into tissue engineering shelves to plant on cells and also be fostered out of body to increase cells for the tissue's renovation and then be transplanted in body to repair tissues.
Owner:TSINGHUA UNIV
Lansoprazole nano-particle frozen preparation for injection and preparation method thereof
ActiveCN102198106APromote absorptionImprove bioavailabilityPowder deliveryOrganic active ingredientsLansoprazoleSulfite salt
The invention discloses a lansoprazole nano-particle frozen preparation for injection capable of simultaneously improving the stability and dissolubility, and a preparation method thereof. The preparation comprises the following components in parts by weight: 20-40 parts of lansoprazole, 5-50 parts of dextran, 5-40 parts of sodium sulfite, 5-60 parts of solubilizer, 10-100 parts of nano-carrier material and 10-100 parts of freeze drying excipient. The preparation method comprises the steps of: adding the dextran, the solubilizer and the sodium sulfite into a liquid preparation tank, adding water for injection and stirring until dissolved, regulating the pH value, adding the lansoprazole and the nano-carrier material, continuing to stir evenly, adding the freeze drying excipient and stirring until dissolved, supplementing the water for injection to the full dose, decoloring, finely filtering, subpackaging and freeze drying to obtain the frozen preparation.
Owner:WUHAN PUSHENG PHARMA
Polymeric gel delivery system for pharmaceuticals
InactiveUS20090010986A1Increased hydration rateIncrease drug release rateBiocideSenses disorderBody fluidAdduct
Implantable, injectable, insertable, or otherwise administrable compositions that form hydrogels when implanted, injected, inserted, or administered into or onto living tissues comprise a pharmaceutically effective compound wherein the pharmaceutically effective compound is a codrug, or pharmaceutically acceptable salt or prodrug thereof in admixture with a hydrogel-forming compound. The pharmaceutically effective compound may be any compound that is soluble in bodily fluids, or that forms bodily fluid-soluble adducts when exposed to bodily fluids. Exemplary compounds include analgesic, anti-inflammatory and antibiotic compounds. The hydrogel-forming compound is a biologically tolerated substance that forms a hydrogel upon exposure to bodily fluids, such as the interstitial fluid surrounding or within a joint.
Owner:PSIVIDA US INC
Biodegradable multiple targeting hydrogel orientated to eolon as well as its preparation and application
InactiveCN1480219AIncrease drug release rateOvercome the shortcoming of slow single enzymatic hydrolysis ratePharmaceutical non-active ingredientsCross-linkBiodegradable hydrogels
A biodegradable hydrogel with multiple target location function in colon is prepared from the aqueous solution of konjak glucomannan and acrylic through graft copolymerizing under the action of redox trigger and azo compound as cross-linking agent. It can be used as the carrier of protein polypeptide medicine for locating in the colon and releasing the medicine.
Owner:WUHAN UNIV
Preparation method of tumor targeted photothermal therapy nanocarrier and application
ActiveCN107375928ASimple and fast manufacturing methodMild conditionsOrganic active ingredientsEnergy modified materialsTumor targetDrug release rate
The invention discloses a preparation method of a tumor targeted photothermal therapy nanocarrier. The method comprises steps as follows: with GO as a carrier, loading MoS2 on GO with a hydrothermal method, and performing PEG modification to obtain the GO-MoS2 composite carrier. A composite of GO and MoS2 is taken as a phototherapeutic drug carrier for the first time, the preparation method is simple and convenient, and conditions are mild; the prepared GO-MoS2 drug-carrying composite has good photothermal conversion characteristic and higher drug carrying ratio which is higher than or equal to 80%. The drug release rate of the GO-MoS2 drug-carrying composite is 78% under laser power of 1.8 W / cm<2> in the presence of NIR laser. Compared with a conventional photothermal therapy nano-drug carrier, the GO-MoS2 drug-carrying composite has higher photo-thermal conversion rate due to synergistic effect of the two materials.
Owner:BEIJING UNIV OF CHEM TECH
Pharmaceutical composition containing naphthoquinone-based compound for intestine delivery system
InactiveUS20100062065A1Enhanced solubilizationImprove wettabilityBiocideSenses disorderNaphthoquinonePharmaceutical drug
Provided is an oral pharmaceutical composition with improved bioavailability and pharmacokinetic properties of a drug, by increasing a bioabsorption rate and an in vivo retention time of an active ingredient via intestine-targeted formulation of a particular naphthoquinone-based compound, or a pharmaceutically acceptable salt, prodrug, solvate or isomer thereof, as an active ingredient.
Owner:MAZENCE INC +1
Preparation and application of nano diamond drug with high load and pH capable of controlling release of adriamycin
InactiveCN105106970AGood biocompatibilityChemically stablePowder deliveryPharmaceutical non-active ingredientsDoxorubicinTarget drug
The invention provides preparation and application of a nano diamond drug with high load and pH capable of controlling release of adriamycin. H2N-PEG-COOH is firstly used for decorating a nano diamond, an ND-PEG-COOH carrier is synthesized, adriamycin is physically absorbed under the condition of Na-citrate and natrium aceticum to obtain target drug nano diamond-polyethylene glycol-adriamycin / sodium citrate (ND-PEG-DOX / Na3Cit) or nano diamond-polyethylene glycol-adriamycin / natrium aceticum (ND-PEG-DOX / NaAc). In vitro drug release experiments, MTT experiments and experiments that flow cytometry tests cell taking in nano diamond drug show that ND-PEG-DOX / Na3Cit and ND-PEG-DOX / NaAc can induce a cancer cell to apoptosis, and the nano diamond drug can be applied in preparation of antineoplastic drugs.
Owner:SHANXI UNIV
Pharmaceutical composition for the treatment and prevention of cardiac disease
Provided is a pharmaceutical composition for the treatment and prevention of cardiac diseases, containing (a) a therapeutically effective amount of a compound represented by Formula 1 or 2 or a pharmaceutically acceptable salt, prodrug, solvate or isomer thereof, and (b) a pharmaceutically acceptable carrier, diluent or excipient or any combination thereof.
Owner:KWAK TAEHWAN +1
Preparation method of collagen cladded carbon nano-tube composite material
InactiveCN107158394AHighlight substantive featuresImprove adhesionInorganic non-active ingredientsCarbon nanotubesBiocompatibility TestingDrug carrier
The invention discloses a preparation method of a collagen cladded carbon nano-tube composite material and relates to a carbon nano-tube material. The preparation method is a preparation method for preparing the collagen cladded carbon nano-tube composite material by in-situ cladding a collagen layer on the surface of a functionalized carbon nano-tube by combing a magnetic liquid phase stirring method and a hydrogel method. The preparation method comprises the following steps of preparing carbon nano-tube-hydroxyapatite composite powder; preparing the functionalized carbon nano-tube; and preparing the collagen cladded carbon nano-tube composite material by the technical method of combing the magnetic liquid phase stirring method and a hydrogel method. The defects that the carbon nano-tube is easily agglomerated, uniform dispersion of components is difficult and production efficiency is low in the preparation method of a collagen-carbon nano-tube composite material in the prior art are overcome; and when the prepared collagen-carbon nano-tube composite material is used as a medicine carrying material, the detects that the biocompatibility is still relatively poor, medicine-carrying and medicine-releasing abilities are poor, and hidden danger of toxin is not thoroughly eliminated generally are overcome.
Owner:HEBEI UNIV OF TECH
Nano ganciclovir freeze-drying preparation for injection and preparation method thereof
ActiveCN103340830AAvoid local irritation side effectsImprove tolerancePowder deliveryAntiviralsAlkalinityActivated carbon
The invention discloses a nano ganciclovir freeze-drying preparation for injection and a preparation method thereof. The nano ganciclovir freeze-drying preparation for injection comprises the following components in parts by weight: 100-400 parts of ganciclovir, 10-50 parts of dextran 40, 5-50 parts of solubilizer, 10-100 parts of nano carrier material and 10-80 parts of freeze-drying skeleton agent. The preparation method comprises the following steps of: sequentially adding dextran 40, solubilizer, ganciclovir, nano carrier material and freeze-drying skeleton agent into water for injection to be dissolved, filtering a solution step by step, and carrying out freeze-drying, thus a freeze-drying preparation is obtained. According to the preparation method, no activated carbon is introduced, thus a risk that damage is done to a human body when activated carbon particles are introduced into the preparation as the activated carbon is used is avoided; besides, pH of the nano ganciclovir freeze-drying preparation for injection is 6-8 and close to that of plasma, and local irritation produced to the human body owning to overhigh alkalinity is avoided.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Chitose base self-assembly nano microcapsule preparation method and its drug release regulation method
InactiveCN1839805AAdd a second powerful ultrasoundIncreased release ratePharmaceutical non-active ingredientsMicrocapsulesEvaporationDrug release
The invention discloses a process for preparing chitosan-based self-assembled nano microcapsule and the pharmaceutical release and regulation method, wherein the nano pharmaceutical microcapsule is prepared through using N,N-dialkylated chitosan as the capsule material, employing modified retrograde evaporation method according to the principle of the self-assembly technique, i.e. carrying out a second supersonic operation before centrifugal separation. The obtained microcapsule has a dimension distribution between 100-250nm.
Owner:HUAQIAO UNIVERSITY
Preparation method of exenatide microneedle
PendingCN112641931ANo needle breakage problemUniform drug loadingPeptide/protein ingredientsMetabolism disorderAqueous solutionBiomedical engineering
The invention discloses a preparation method of an exenatide microneedle. The preparation method comprises the following steps of: pouring an aqueous solution of a polymer material containing exenatide on a mould with a micropore matrix, filling all micropores with the polymer solution, performing freezing and thawing cycle on the mould poured with the exenatide solution for multiple times, and finally performing film uncovering and drying. Exenatide has the defects that the half-life period is relatively short, exenatide is administrated twice a day, tolerance needs to be established through multiple times of small-dose injection, and much inconvenience and pain are brought to patients due to frequent injection. An exenatide phase conversion microneedle transdermal patch not only can realize minimally invasive non-injection administration of exenatide, but also is relatively safe, more effective and convenient to use, improves the compliance of a patient, and is convenient for the patient to establish tolerance.
Owner:SHANGHAI JIAO TONG UNIV
Pharmaceutical compositions of co-crystals of tramadol and coxibs
ActiveCN102946871AIncrease dissolution rateImprove stabilityNervous disorderOrganic chemistryCelecoxibTramadol
Owner:ESTEVE PHARMA SA
Reduction response type ABC type segmented polymer as well as preparation method and application thereof
InactiveCN108676156AImprove stabilityReduce leakageOrganic active ingredientsPharmaceutical non-active ingredientsSide chainLactide
The invention discloses a reduction response type ABC type segmented polymer as well as a preparation method and application thereof, and belongs to the technical field of a reduction response type medicine carrier. The hydrophilic monomethyl ether polyethylene glycol (mPEG), hydrophobic lactide (LA) and allyl glycidyl ether (AGE) are selected to be used as raw materials; then, sulfydryl-olefin light click reaction and ring opening polymerization are combined to synthetize a polymer mPEG-b-PAGESH-b-PLLA with hydrosulphonyl groups at the side chain; then, the self assembly is performed to forma micelle; under the existence of hydrogen peroxide, hydrosulphonyl groups are oxidized into disulfide bonds; finally, the reduction response type crosslinking micelle with high stability is synthesized. The crosslinking micelle with the disulfide bonds is favorable for reducing the medicine leakage and initial fast release; in addition, the reduction response performance is also realized in the pathological change positions; under the existence of reducing agents DTT and glutathione, the medicine release behavior of the crosslinking micelle is obviously accelerated, so that the treatment effect is improved.
Owner:HENAN NORMAL UNIV
Methods of forming microparticle coated medical device
InactiveUS8361539B2Increase load capacityIncrease drug release rateSurgeryPretreated surfacesChemical solutionInsertion stent
A drug-loaded microparticle is applied to a medical device for subsequent application to biological tissues. A method of formulating a drug-loaded microparticle and applying it to the surface of a medical device, such as a stent, is disclosed. The drug-loaded microparticle is formulated by combining a drug with various chemical solutions. Specified sizes of the microparticles and amounts of drug(s) contained within the microparticles may be varied by altering the proportions of the chemicals / solutions. In addition to various drugs, therapeutic substances and radioactive isotopes may also be loaded into the microparticles. The drug-loaded microparticle are suspended in a polymer solution forming a polymer matrix. The polymer matrix may be applied to the entire surface or only selected portions of the medical device via dipping, spraying or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
Divalproex sodium enteric-coated tablet core as well as preparation method and application thereof
ActiveCN103230380ALow hygroscopicityImprove stabilityNervous disorderPharmaceutical non-active ingredientsCoated tabletsMagnesium stearate
The invention relates to a divalproex sodium enteric-coated tablet core as well as a preparation method and an application thereof. The divalproex sodium enteric-coated tablet core is used for dry granulation. The divalproex sodium enteric-coated tablet core comprises the following components: 20-70 percent of divalproex sodium, 10-70 percent of filling agent, 0.5-20 percent of disintegrating agent, 5-20 percent of antisticking agent, 0.5-10 percent of talcum powder and 0.5-5 percent of magnesium stearate, wherein the antisticking agent is selected from one or a combination of silicon dioxide, superfine silica powder and silicon dioxide precipitate. The divalproex sodium enteric-coated tablet core prepared by using the preparation method is complete in release in vitro, higher in in-vitro dissolution speed and release rate and high in quality and has better safety and safety. The dry granulation method has the advantages of simple, convenient and feasible process, low cost, easiness in mass production, environmental friendliness and the like.
Owner:BEIJING SIHUAN PHARMA +2
Radix salviae miltiorrhizae gel patch for external use
InactiveCN108078960ANon-irritatingIncrease drug release rateAntibacterial agentsOrganic active ingredientsSalvia miltiorrhizaDrug release rate
The invention discloses a radix salviae miltiorrhizae gel patch for external use. The radix salviae miltiorrhizae gel patch comprises medical nonwoven fabric, a gel layer and an anti-sticking layer, wherein the gel layer is radix salviae miltiorrhizae gel, and the radix salviae miltiorrhizae gel is prepared from the following formula components by weight percent: 0.8-1.2% of tanshinone, 18-22% ofcarbomer, 45-50% of hydroxypropyl methyl cellulose, 15-20% of polyvinylpyrrolidone, 2-5% of a cross-linking agent, 1-3% of a surface active agent, 0.8-1.5% of a cosurfactant and 0.5-0.8% of a humidifying agent. According to the radix salviae miltiorrhizae gel patch, the carbomer is used as a gel substrate, the hydroxypropyl methyl cellulose and the polyvinylpyrrolidone which are taken as a penetration enhancer, the cross-linking agent and the surface active agent are added, and all the components are evenly mixed by adopting ultrasonic treatment, so that the drug release rate is increased; furthermore, the radix salviae miltiorrhizae gel patch for external use is free from irritation to skin.
Owner:ANHUI AIKEER PHARMA CO LTD
Chitosan microcapsule modified acrylic bone cement and preparation method thereof
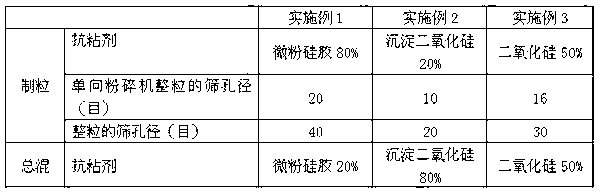
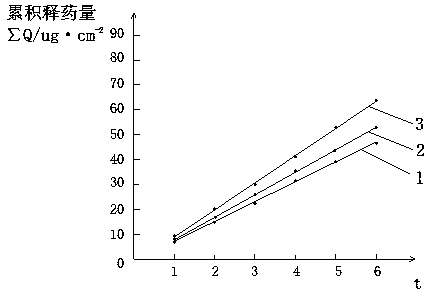
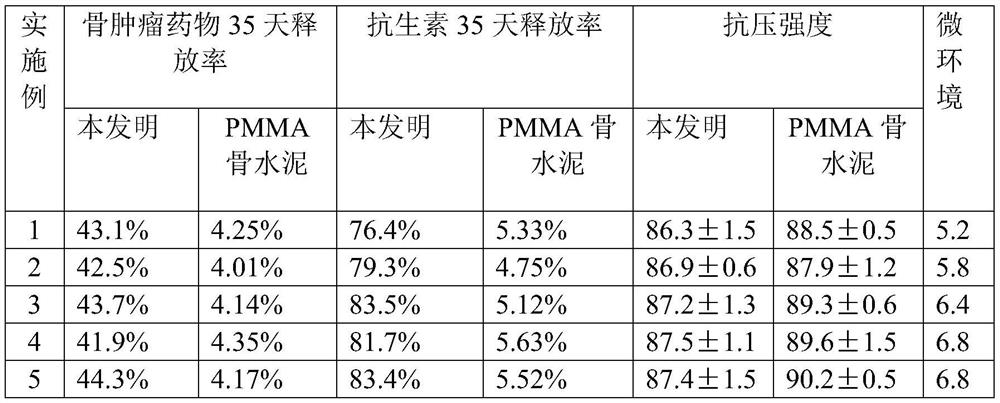
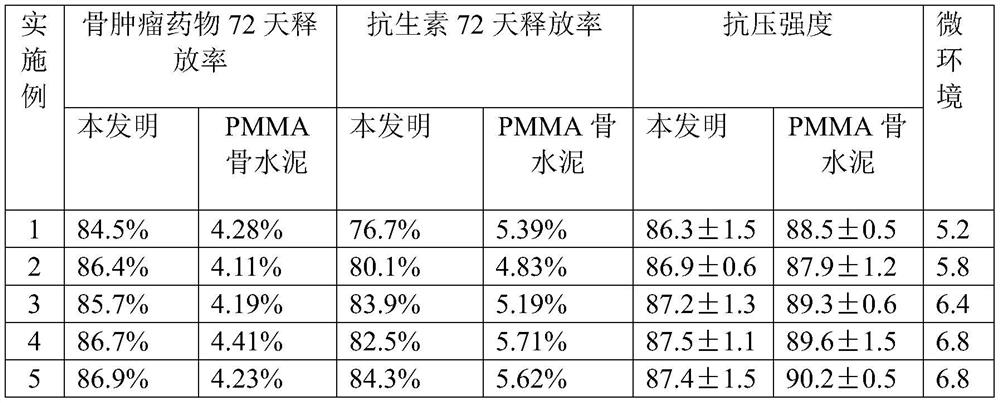
ActiveCN113384747AWater-swellableHigh drug loadingTissue regenerationMicrocapsulesDrug release ratePolymethyl methacrylate
The invention discloses chitosan microcapsule modified acrylic bone cement. The chitosan microcapsule modified acrylic bone cement is formed by mixing a solid phase and a liquid phase, wherein the solid phase comprises a bone tumor drug loaded chitosan microcapsule, an antibiotic loaded water-swellable P (MMA-AA) copolymer nano-microsphere and polymethyl methacrylate; the liquid phase comprises methyl methacrylate, an accelerant and a polymerization inhibitor. The invention further discloses a preparation method of the chitosan microcapsule modified acrylic bone cement. The preparation method comprises the following steps of: uniformly mixing the bone tumor drug loaded chitosan microcapsule, the antibiotic loaded water-swellable P (MMA-AA) copolymer nano-microsphere and polymethyl methacrylate to obtain the solid phase; uniformly mixing methyl methacrylate, the accelerant and the polymerization inhibitor to obtain the liquid phase; and uniformly mixing the solid phase and the liquid phase. The chitosan microcapsule modified acrylic bone cement has a remarkable value for treating postoperative bone tumors, and has relatively high drug loading capacity, drug release rate and drug accumulated release amount under the condition that the compressive strength is basically unchanged.
Owner:XIAN UNIV OF TECH
Drug loading system for loading docetaxel based on oxidized single-walled carbon nanohorns
InactiveCN105879042AGood dispersionNot easy to attractOrganic active ingredientsPharmaceutical non-active ingredientsDispersityDocetaxel-PNP
The invention relates to a method for modifying oxidized single-walled carbon nanohorns by virtue of povidone, in particular to a novel drug loading system for loading docetaxel based on a single-walled carbon nanohorn-povidone compound which is built up by virtue of a nano-precipitation method. Through non-covalent modification of the povidone on the single-walled carbon nanohorns, the dispersity and the stability of the single-walled carbon nanohorns in water and a phosphate buffer solution can be effectively improved. On the basis of a [pi]-[pi] effect, by loading an anti-tumor drug, namely the docetaxel, with the compound single-walled carbon nanohorn-povidone, the water insolubility of the docetaxel is effectively improved and biocompatibility is improved; and the drug loading system is high in drug loading rate and significant in sustained-release effects, and the application of the single-walled carbon nanohorns in the field of nano-biomedicine is expanded.
Owner:CHINA PHARM UNIV
Aesculin nano-suspension gel and preparation method and application thereof
ActiveCN110538137ASolve the problems of low solubility and large dosageIncrease drug release rateOrganic active ingredientsAerosol deliverySolubilitySkin permeability
The invention discloses aesculin nano-suspension gel and a preparation method and application thereof. Aesculin and a stabilizer are made into an aesculin nano-suspension agent, a gel matrix, a moisturizer and a pH regulator are further added, and the aesculin nano-suspension gel is prepared. According to the aesculin nano-suspension gel prepared by combining a precipitation method with a microjethigh-pressure homogenization method, the drug solubility and skin permeability can be increased, transmembrane transport of the aesculin nano-suspension gel can be promoted, the administration dosageis decreased, the toxic and side effects are reduced, the better therapeutic effect can be exerted in the body advantageously, and the ability to pass through a lipid bilayer is improved.
Owner:科贝园(北京)医药科技有限公司
A polylactic acid-glycolic acid copolymer nano drug carrier and its preparation method and application
ActiveCN104001178BImprove adhesionEnhanced cellular uptakeNanomedicinePharmaceutical non-active ingredientsCrosslinked chitosanMicrosphere
The invention provides a PLGA (polylactic acid-hydroxyacetic acid) nano-drug carrier which is composed of PLGA nano microsphere kernel and an anillic aldehyde crosslinked chitosan housing. A preparation method of the polylactic acid-hydroxyacetic acid copolymer nano-drug carrier is as follows: dispersing a PLGA organic phase which takes dichloromethane and alcohol as a mixed solvent in a water phase to prepare PLGA microspheres by taking PVA (polyvinyl acetate) as an emulsifier by virtue of an one-off emulsion process; then, adding the PLGA microspheres into chitosan liquor, so that the chitosan is adsorbed on the surfaces of the PLGA microspheres, and then adding the chitosan on the surfaces of anillic aldehyde crosslinked PLGA microspheres. The product has a certain pH environmental responsiveness, can realize controlled release of the drug according to in-vivo pH environmental changes, is high in stability, strong in up-taking capacity for microsphere-coated medicaments by the cells, and has very good application prospect in the drug carrier for treating tumors.
Owner:SUN YAT SEN UNIV
Preparation and application of nanodiamond drug with high loading and pH-controlled release of doxorubicin
InactiveCN105106970BGood biocompatibilityChemically stablePharmaceutical non-active ingredientsAntineoplastic agentsSodium acetatePh control
The invention provides the preparation and application of a nano-diamond drug with high load and pH-controlled release of doxorubicin. The present invention first uses H2N-PEG-COOH to modify nano-diamonds, synthesizes ND-PEG-COOH carrier, and then physically adsorbs doxorubicin under the condition of sodium citrate or sodium acetate to obtain the target drug nano-diamond-polyethylene glycol-doxorubicin Sodium citrate (ND‑PEG‑DOX / Na3Cit) or nanodiamond‑polyethylene glycol‑doxorubicin / sodium acetate (ND‑PEG‑DOX / NaAc). Through in vitro drug release experiments, MTT experiments and flow cytometry cell uptake nano-drug experiments, it was shown that ND-PEG-DOX / Na3Cit and ND-PEG-DOX / NaAc can induce tumor cell apoptosis, which can be used in the preparation of anti-tumor drugs in the application.
Owner:SHANXI UNIV
Polymeric gel delivery system for pharmaceuticals
InactiveUS20120195934A1Increased hydration rateIncrease drug release rateAntibacterial agentsBiocideBody fluidAdduct
Implantable, injectable, insertable, or otherwise administrable compositions that form hydrogels when implanted, injected, inserted, or administered into or onto living tissues comprise a pharmaceutically effective compound wherein the pharmaceutically effective compound is a codrug, or pharmaceutically acceptable salt or prodrug thereof in admixture with a hydrogel-forming compound. The pharmaceutically effective compound may be any compound that is soluble in bodily fluids, or that forms bodily fluid-soluble adducts when exposed to bodily fluids. Exemplary compounds include analgesic, anti-inflammatory and antibiotic compounds. The hydrogel-forming compound is a biologically tolerated substance that forms a hydrogel upon exposure to bodily fluids, such as the interstitial fluid surrounding or within a joint.
Owner:PSIVIDA US INC
A kind of erythrocyte membrane-wrapped polydopamine-coated drug-loaded plga material and its preparation and application
ActiveCN110898029BGood biocompatibilityProduce toxicityEnergy modified materialsKetone active ingredientsRed blood cellCell membrane
The invention relates to a drug-loaded PLGA material coated with red blood cell membrane-wrapped polydopamine and its preparation and application. The red blood cell membrane, PLGA, TPGS, and DA used are all biomaterials with very good biocompatibility and will not cause toxicity to the body. The preparation method has simple operation and mild reaction conditions.
Owner:河北英治医疗器械科研有限公司
Nano ganciclovir freeze-drying preparation for injection and preparation method thereof
ActiveCN103340830BAvoid local irritation side effectsImprove tolerancePowder deliveryAntiviralsActivated carbonAlkalinity
The invention discloses a nano ganciclovir freeze-drying preparation for injection and a preparation method thereof. The nano ganciclovir freeze-drying preparation for injection comprises the following components in parts by weight: 100-400 parts of ganciclovir, 10-50 parts of dextran 40, 5-50 parts of solubilizer, 10-100 parts of nano carrier material and 10-80 parts of freeze-drying skeleton agent. The preparation method comprises the following steps of: sequentially adding dextran 40, solubilizer, ganciclovir, nano carrier material and freeze-drying skeleton agent into water for injection to be dissolved, filtering a solution step by step, and carrying out freeze-drying, thus a freeze-drying preparation is obtained. According to the preparation method, no activated carbon is introduced, thus a risk that damage is done to a human body when activated carbon particles are introduced into the preparation as the activated carbon is used is avoided; besides, pH of the nano ganciclovir freeze-drying preparation for injection is 6-8 and close to that of plasma, and local irritation produced to the human body owning to overhigh alkalinity is avoided.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com