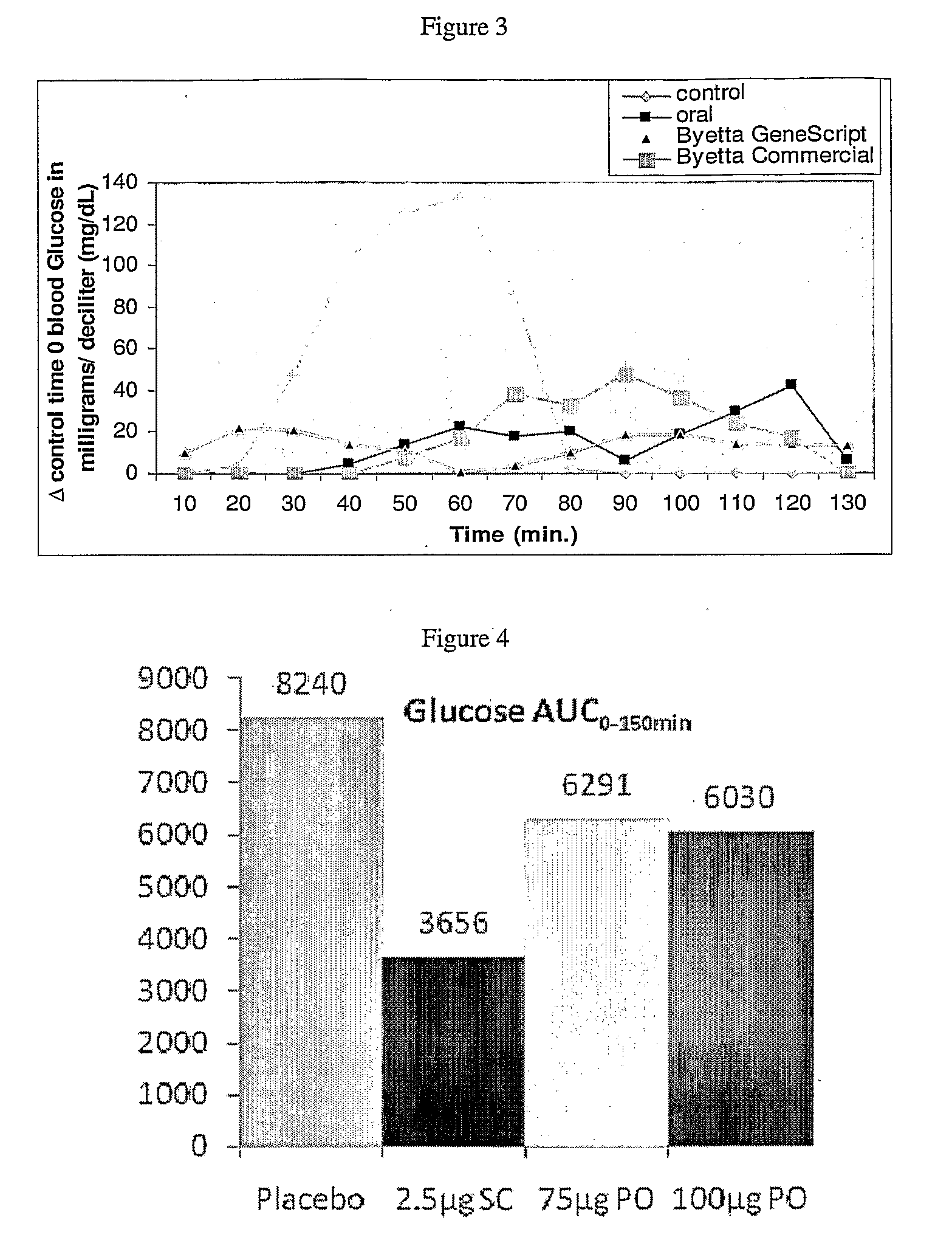
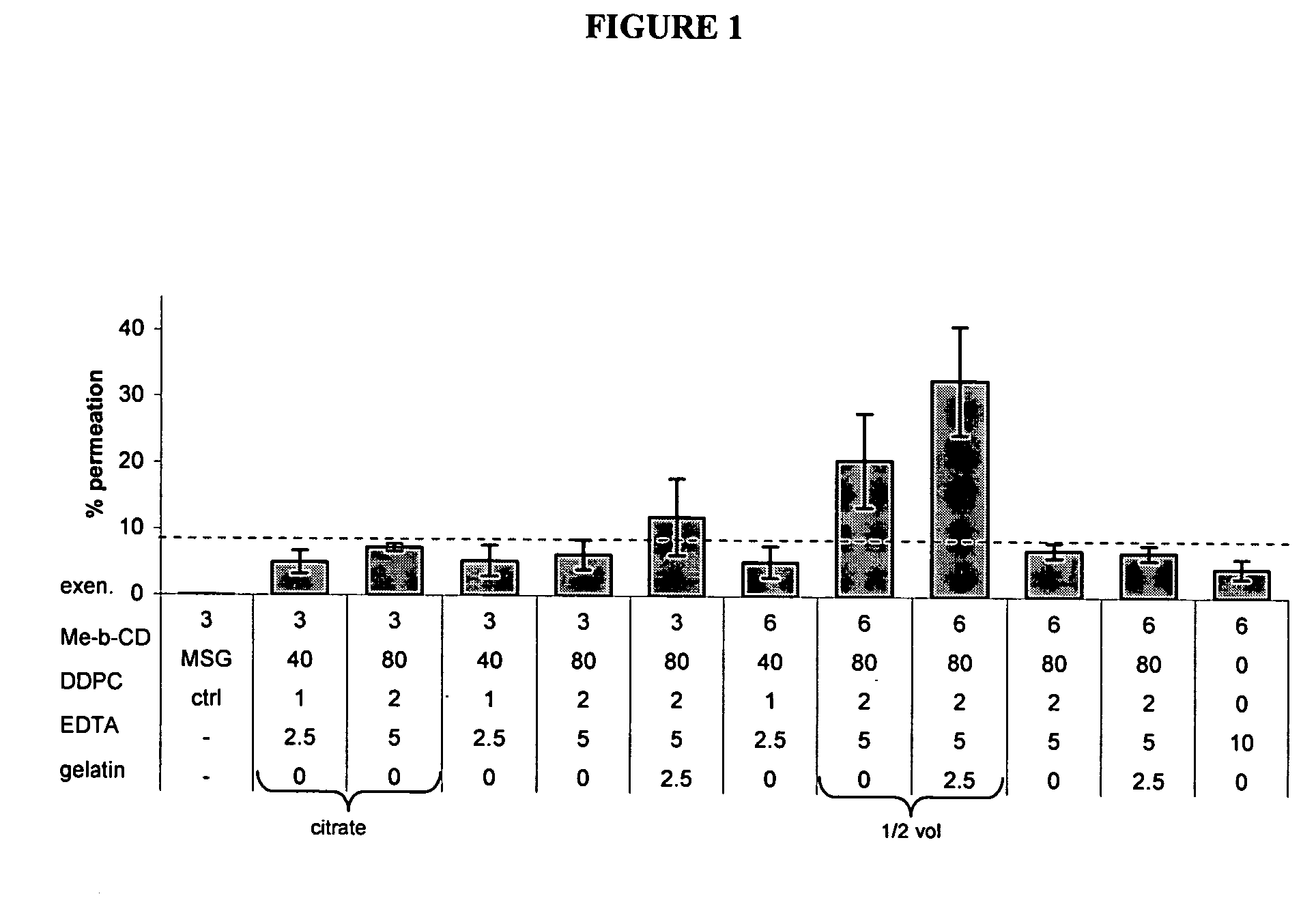
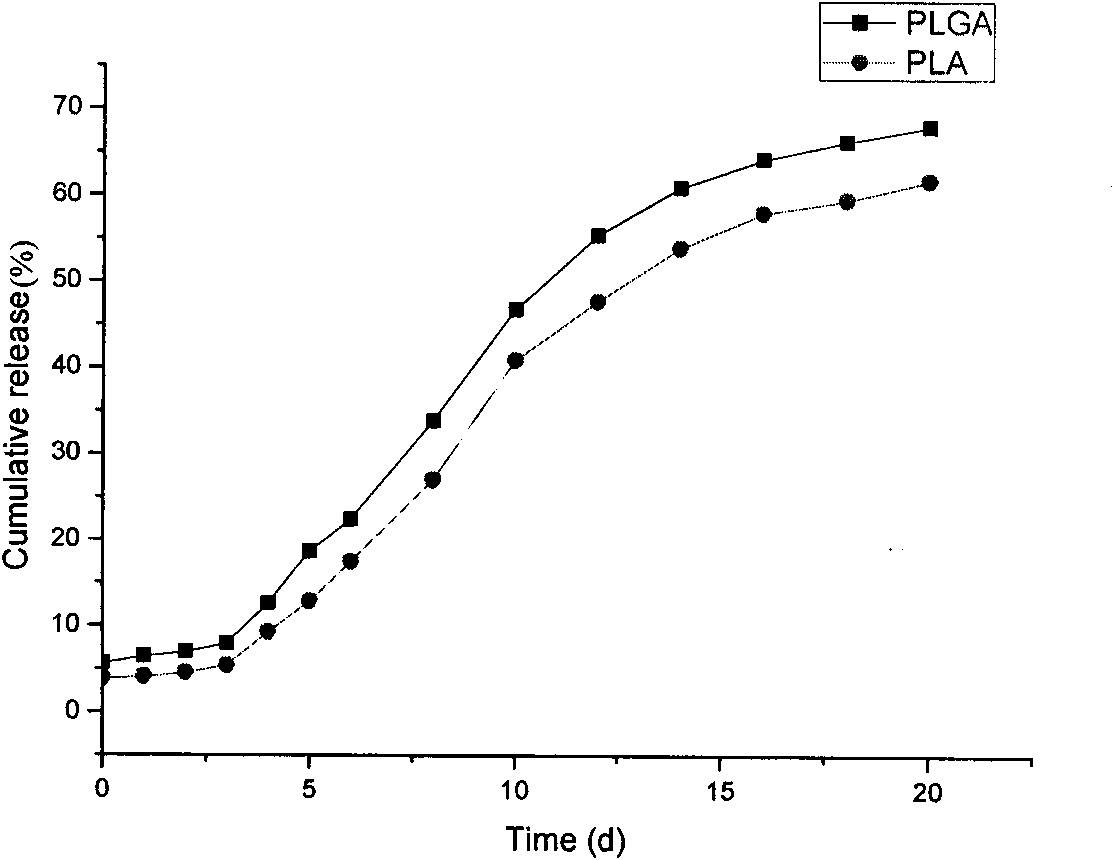
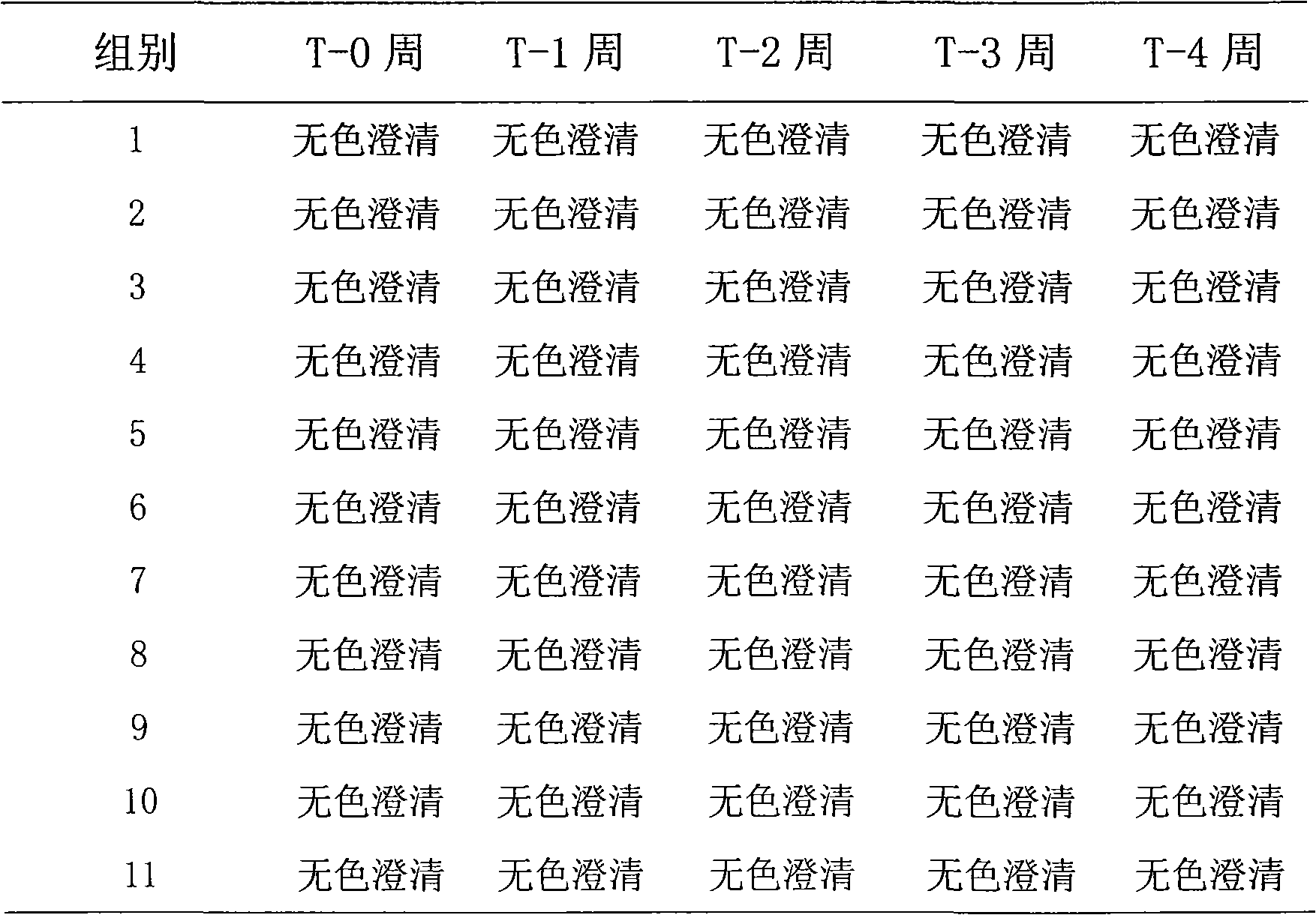
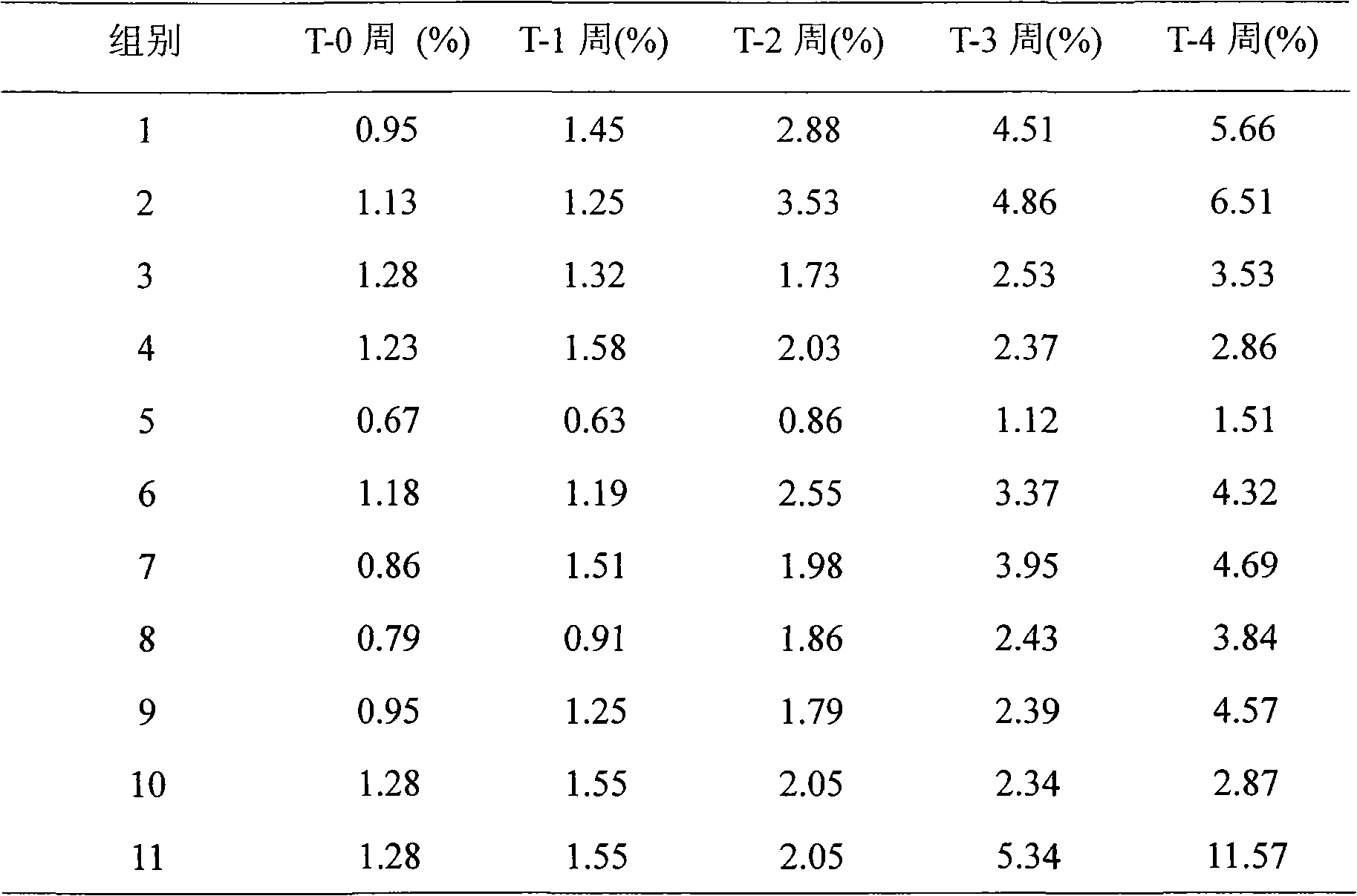
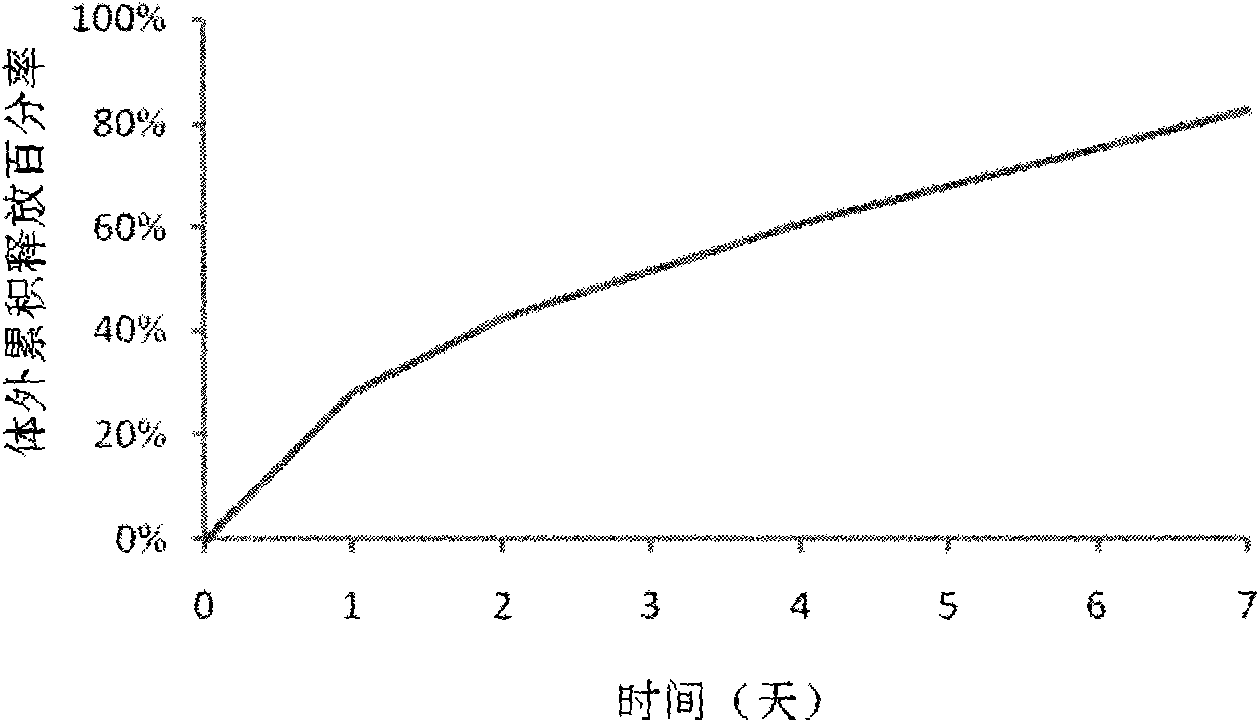
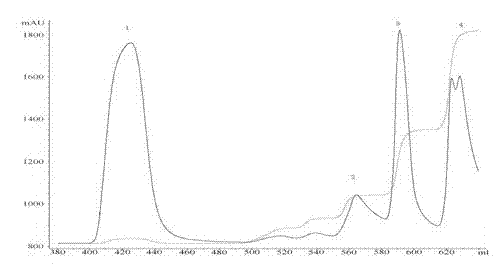
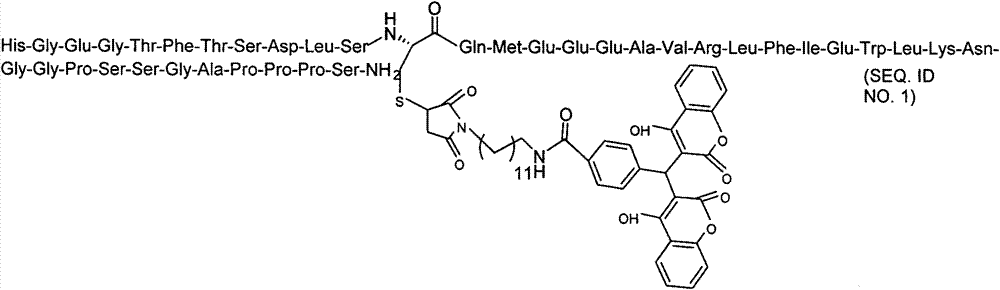
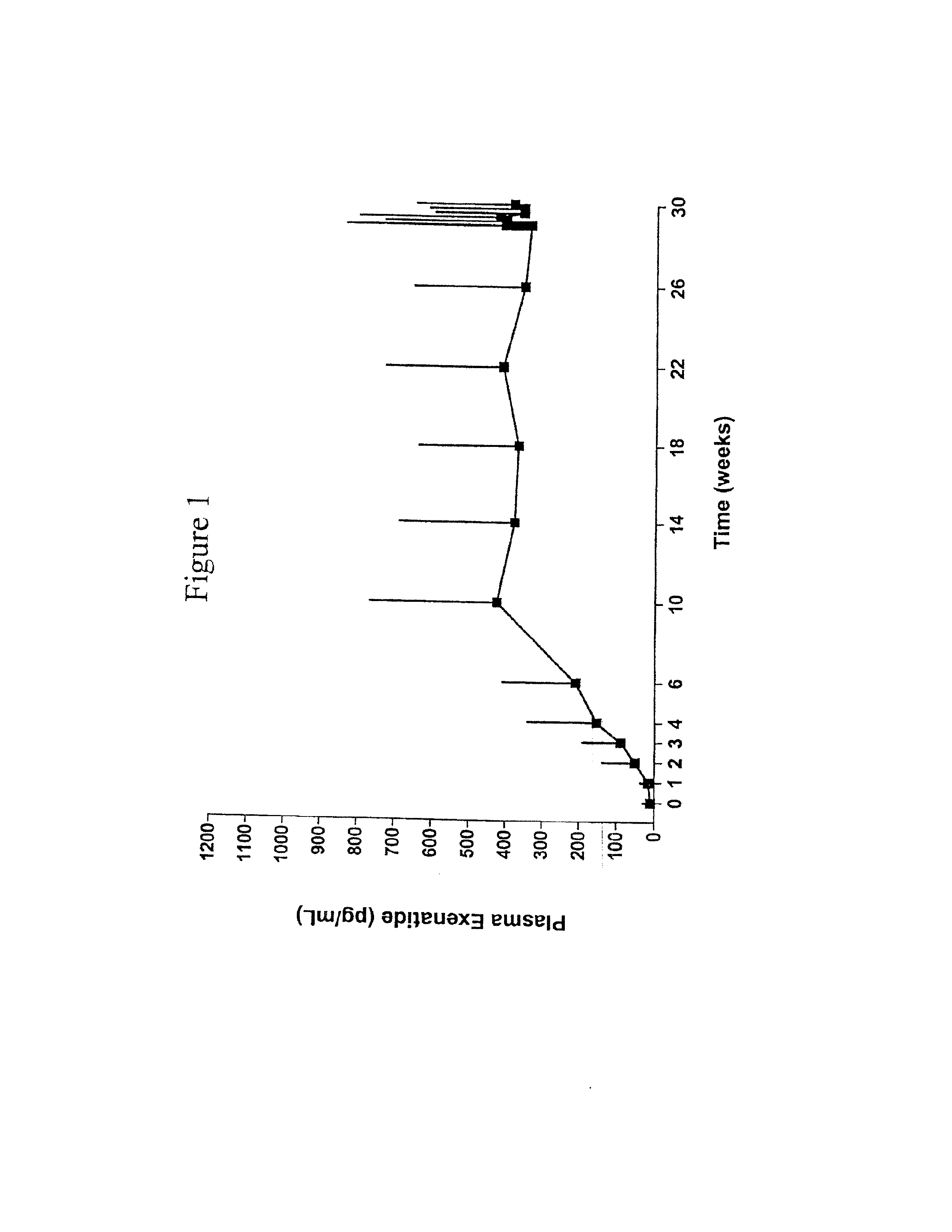
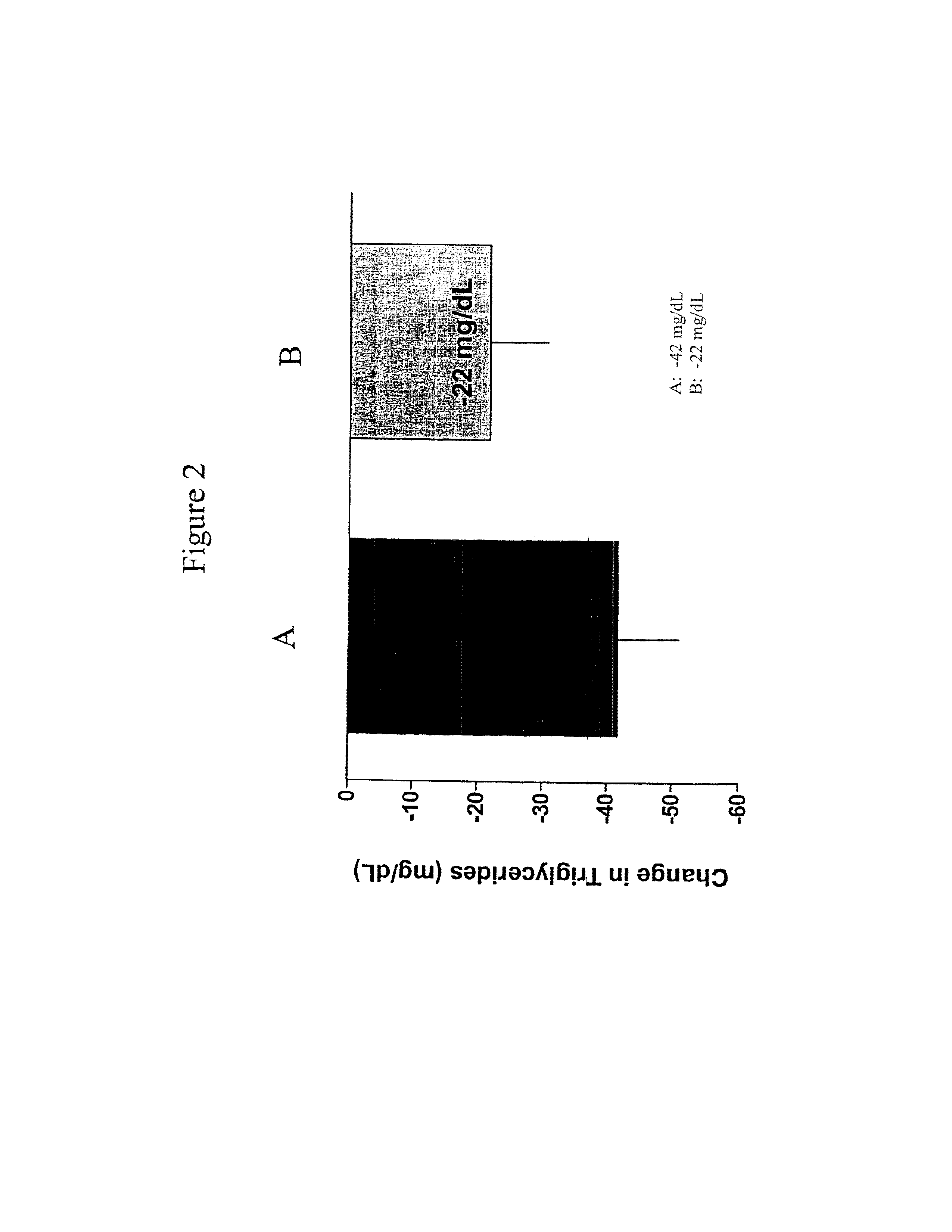
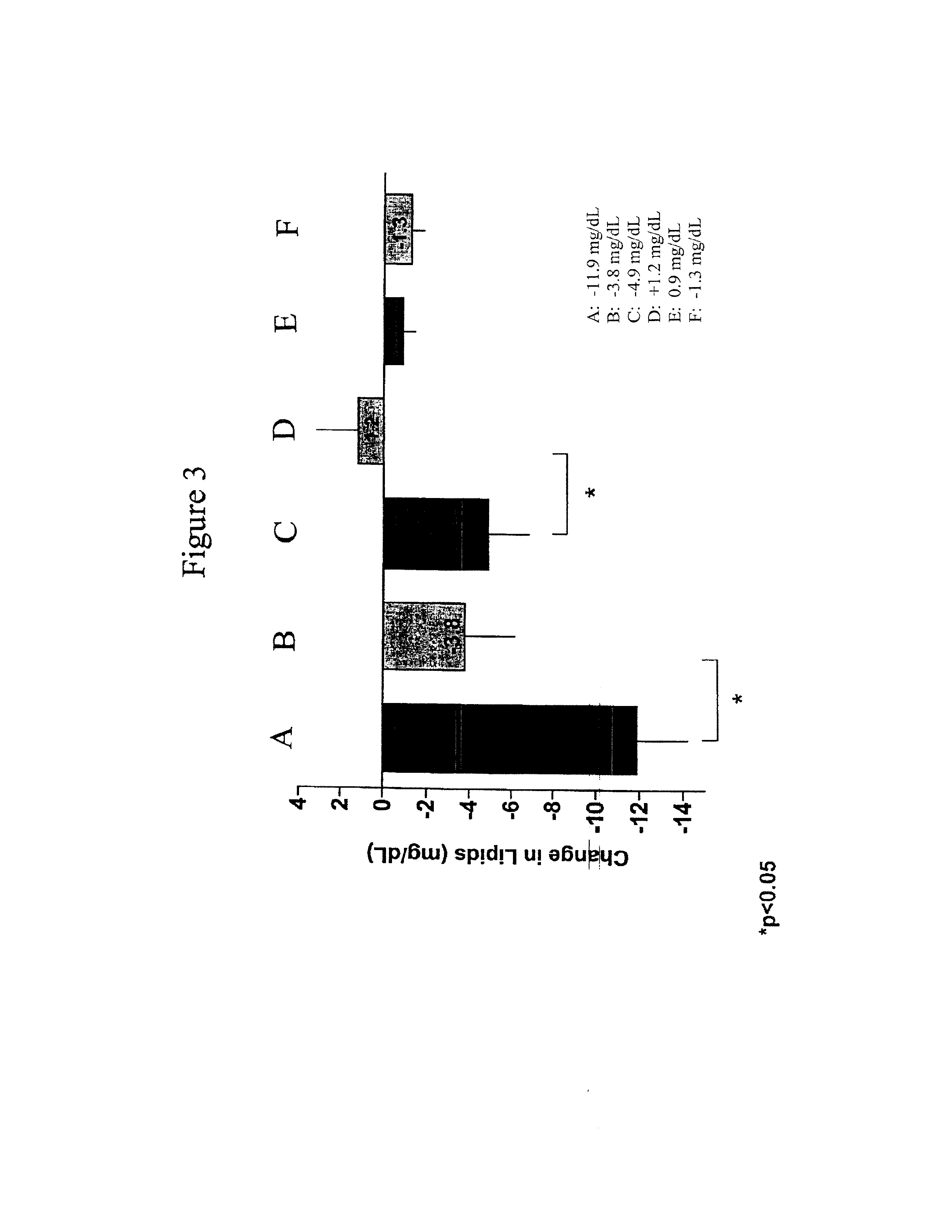
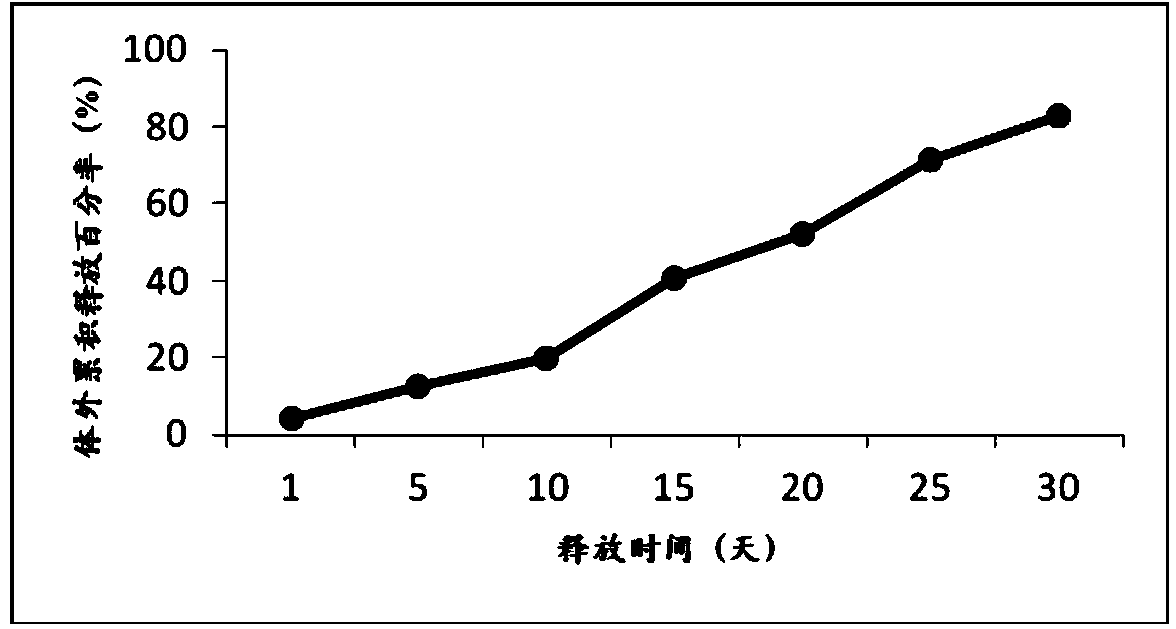
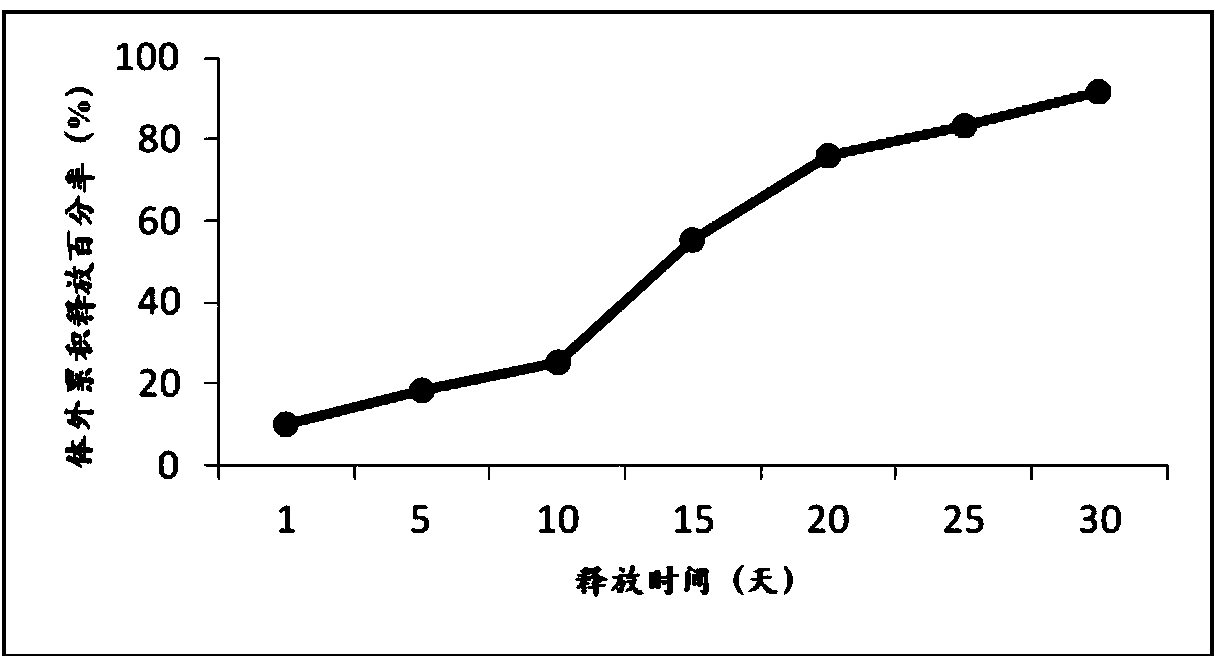
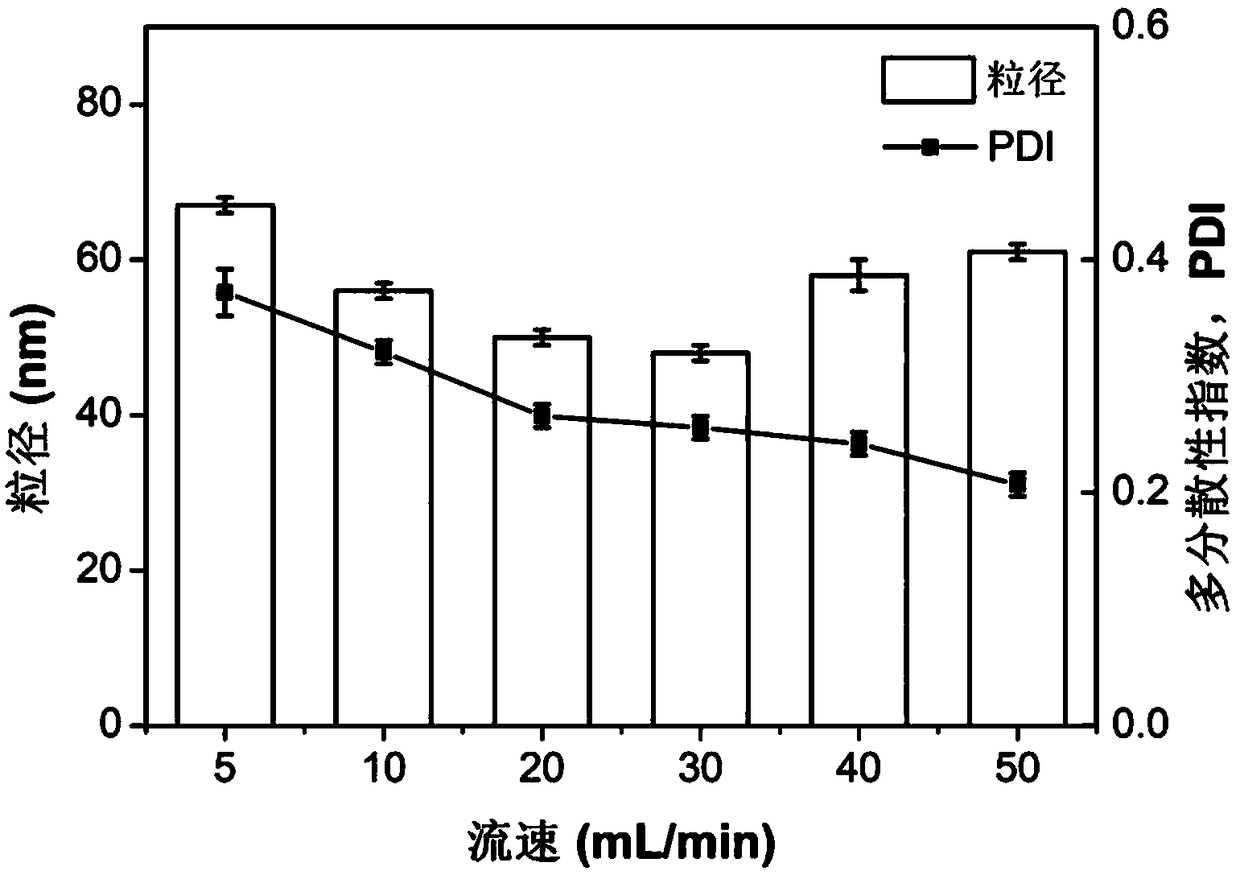
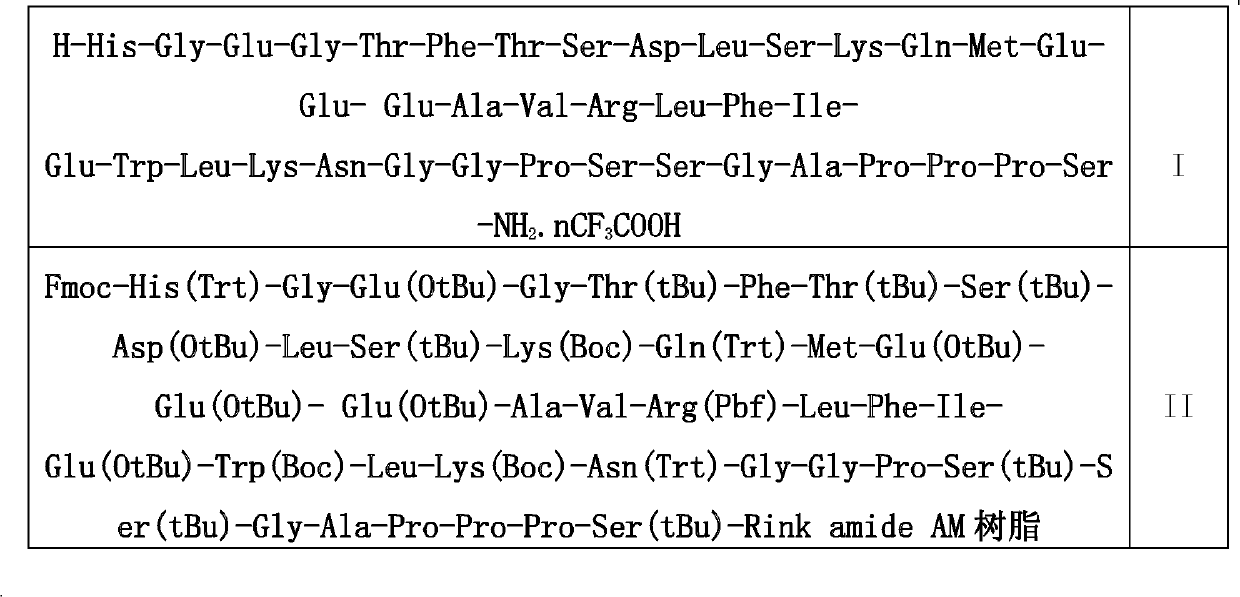
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
183 results about "Exenatide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Exenatide is used with a proper diet and exercise program to control high blood sugar. It is used by people with type 2 diabetes.
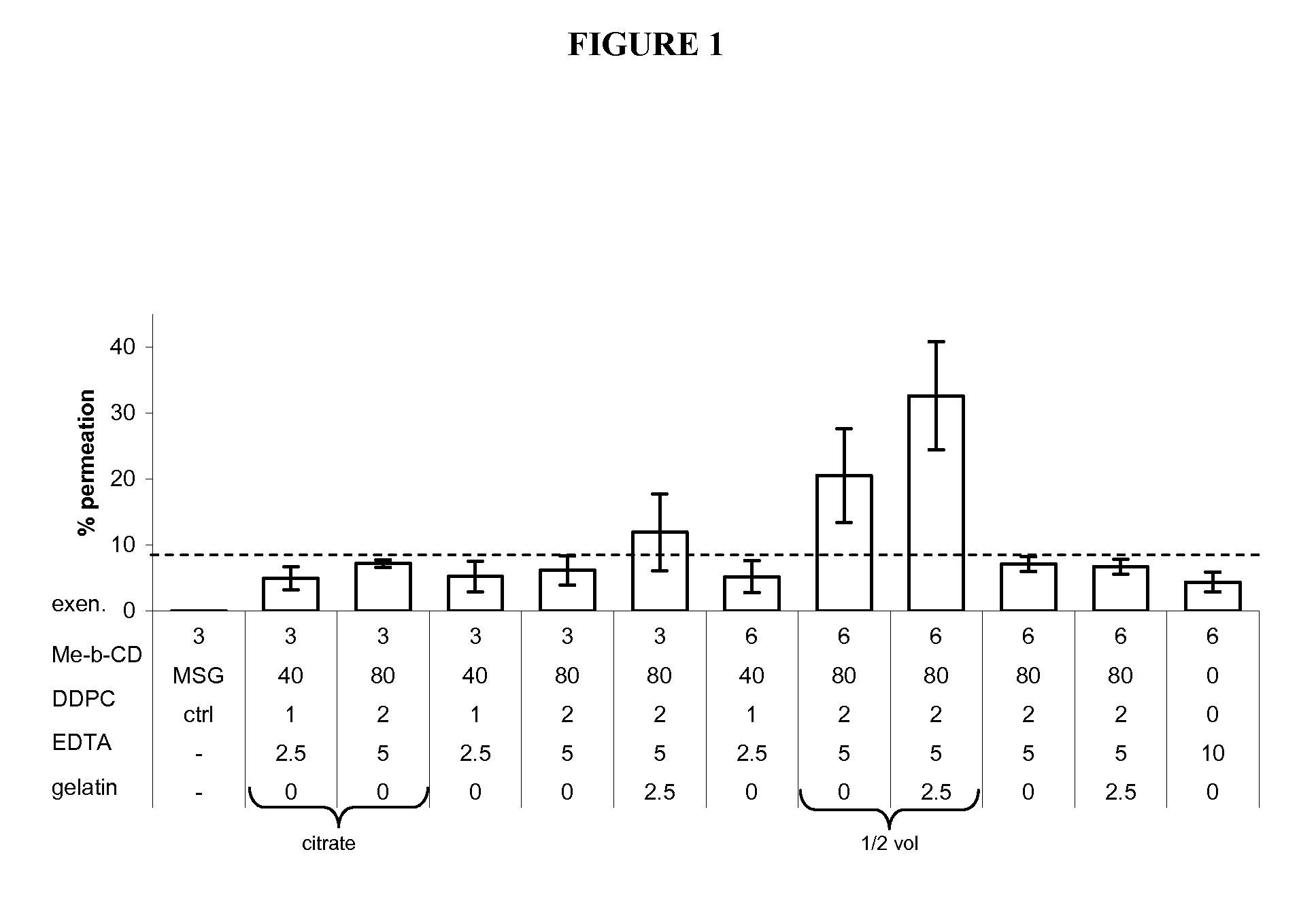
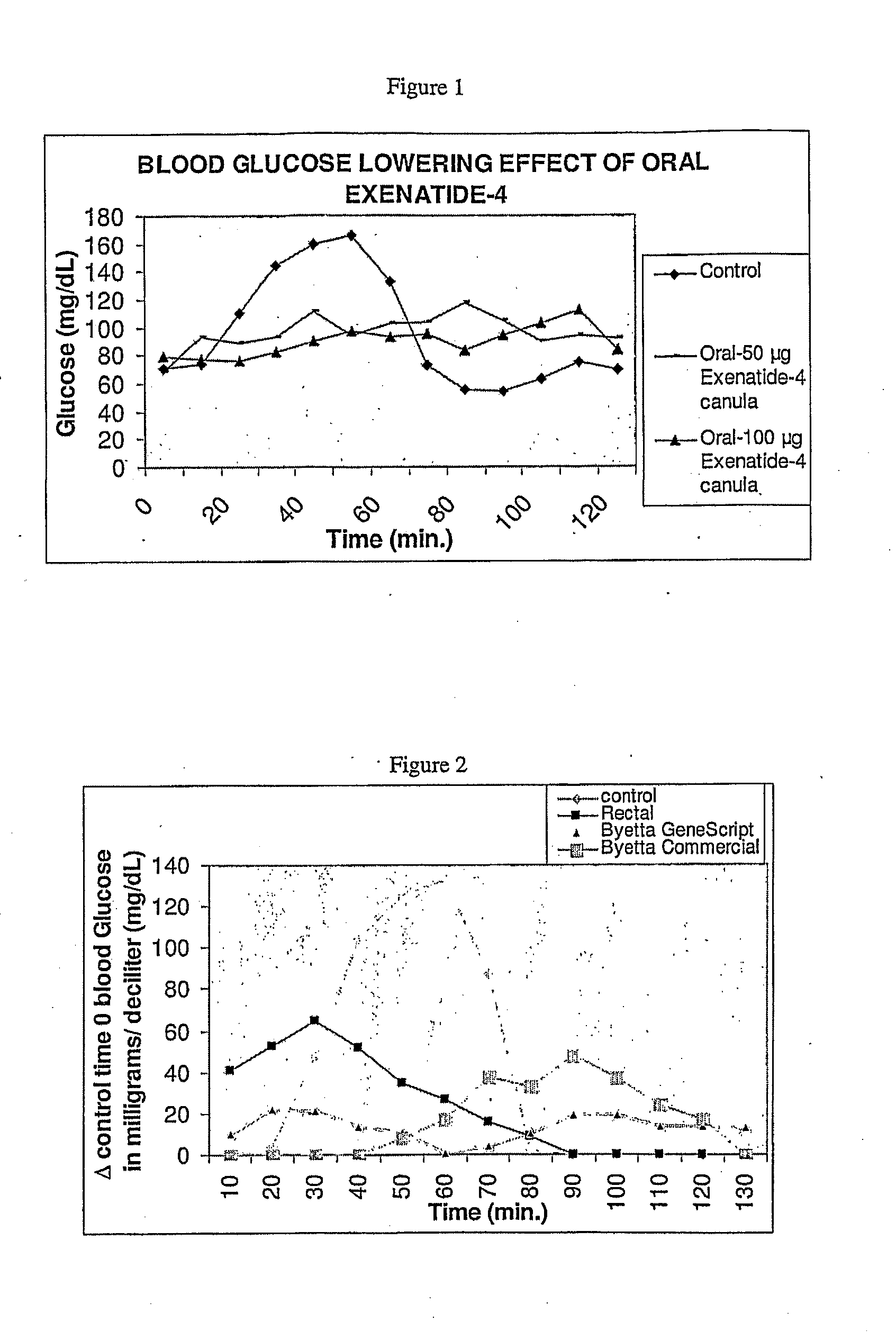
Methods and compositions for oral administration of exenatide
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
Method of treatment of a metabolic disease using intranasal administration of exendin peptide
Owner:NASTECH PHARMA
Polypeptide medicament sustained release microsphere or microcapsule preparation with uniform grain size and preparation method thereof
ActiveCN101559041ASmall particle sizeRegulated release ratePeptide/protein ingredientsMetabolism disorderMicrosphereMedicine
The invention discloses a polypeptide medicament sustained release microsphere or a microcapsule preparation with uniform grain size, a preparation method thereof and application. The average grain size of the microsphere or the microcapsule preparation is between 50 nanometers and 100 microns, and the grain size distribution coefficient CV value is less than 20 percent. The polypeptide medicament has a definite structure, has functions of therapy or adjuvant therapy of type-2 diabetes, and is preferably one or more of GLP-1, Exenatide, Exendin-4 and derivatives and analogs thereof. The microsphere or the microcapsule preparation uses a microsphere or a microcapsule with uniform grain size as a substrate to prepare the polypeptide medicament into a sustained release preparation through an embedding mode, and by changing the grain size of the microsphere or the microcapsule, the sustained release cycle is adjustable between one week and one month, and the microsphere or the microcapsule preparation can be applied to the therapy or the adjuvant therapy of the type-2 diabetes and body weight control. Besides, the microsphere or the microcapsule preparation has the advantages of simple preparation process and mild preparation course, and can protect the biological activity of the embedded polypeptide medicament.
Owner:辉粒药业(苏州)有限公司
Pharmaceutical preparation containing exenatide
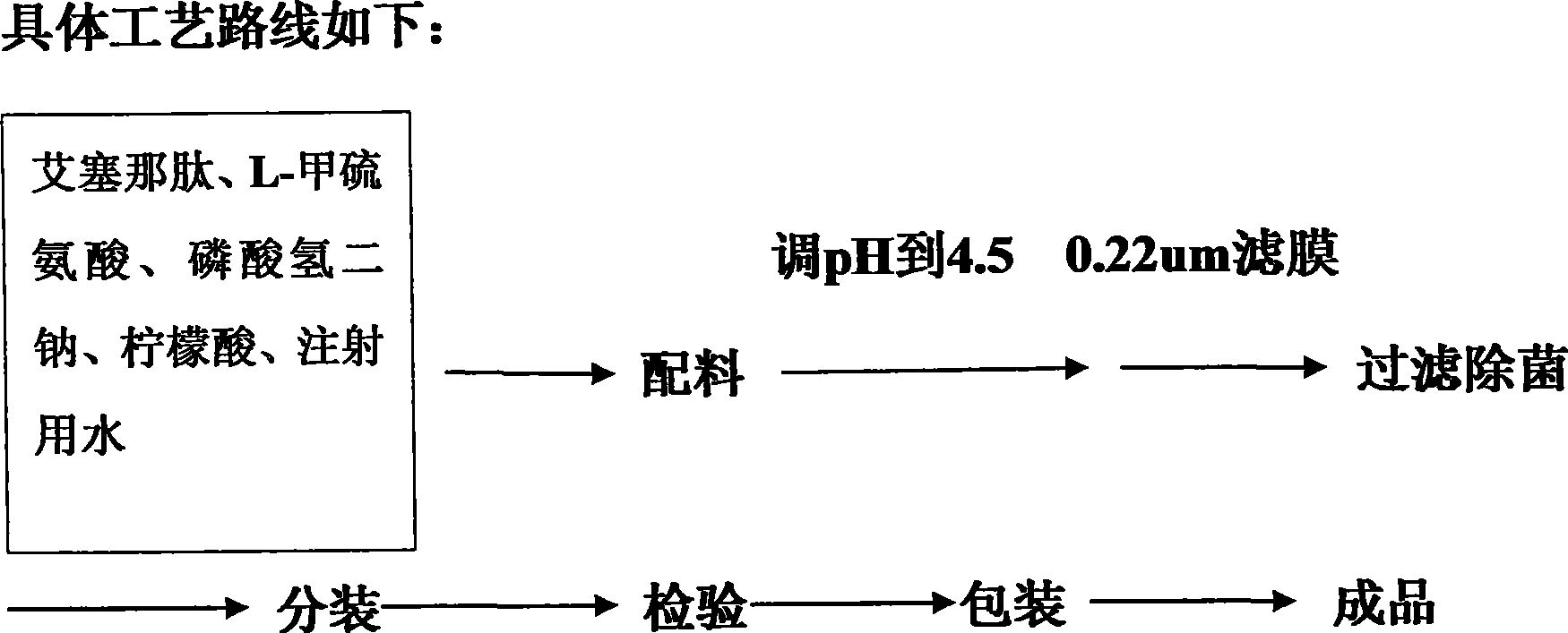
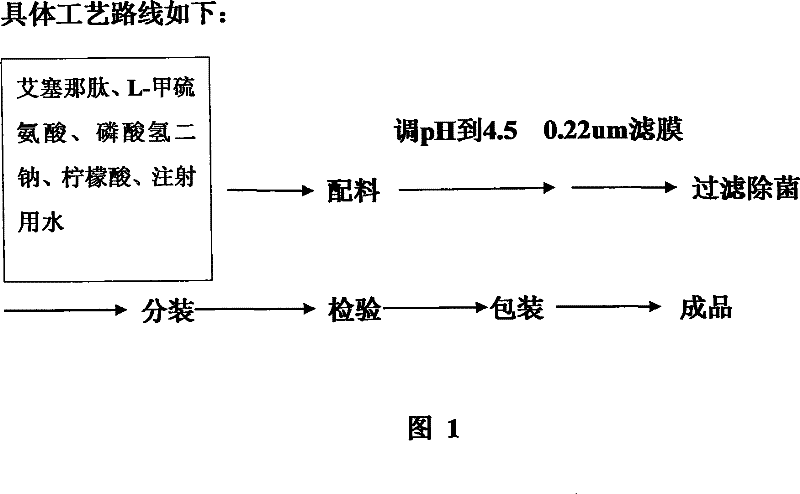
The invention discloses a pharmaceutical preparation containing exenatide. Besides the active constituent exenatide, the pharmaceutical preparation further contains 10-100mm of buffer with pH being 3-7 and adjuvants for enhancing stability of the exenatide. The invention has the advantages of preparing an exenatide preparation which is applicable to clinical application, in particular to injection and can be stably kept by adding a plurality of human acceptable constituents.
Owner:HANGZHOU JIUYUAN GENE ENG
Medicinal preparation containing exenatide
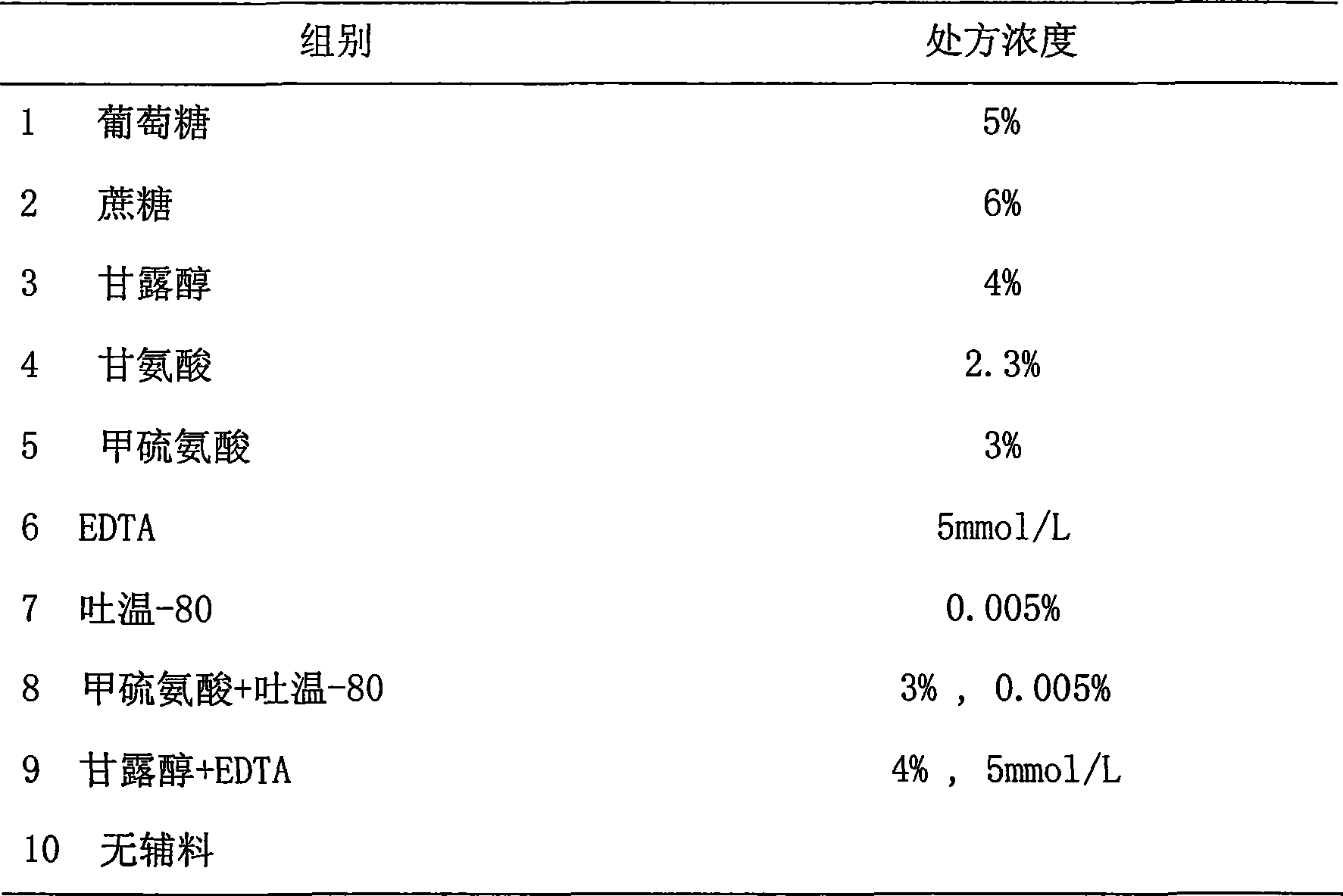
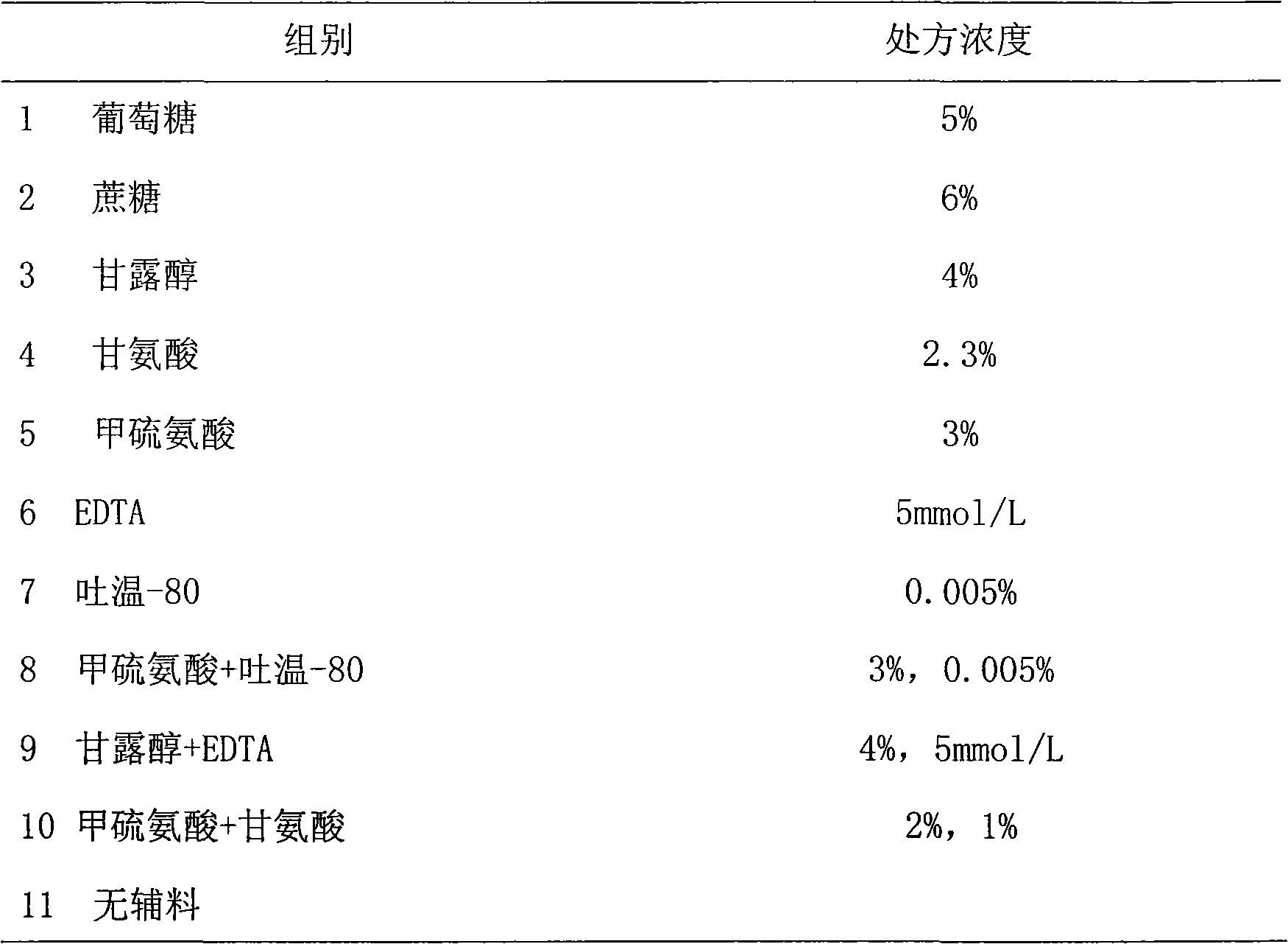
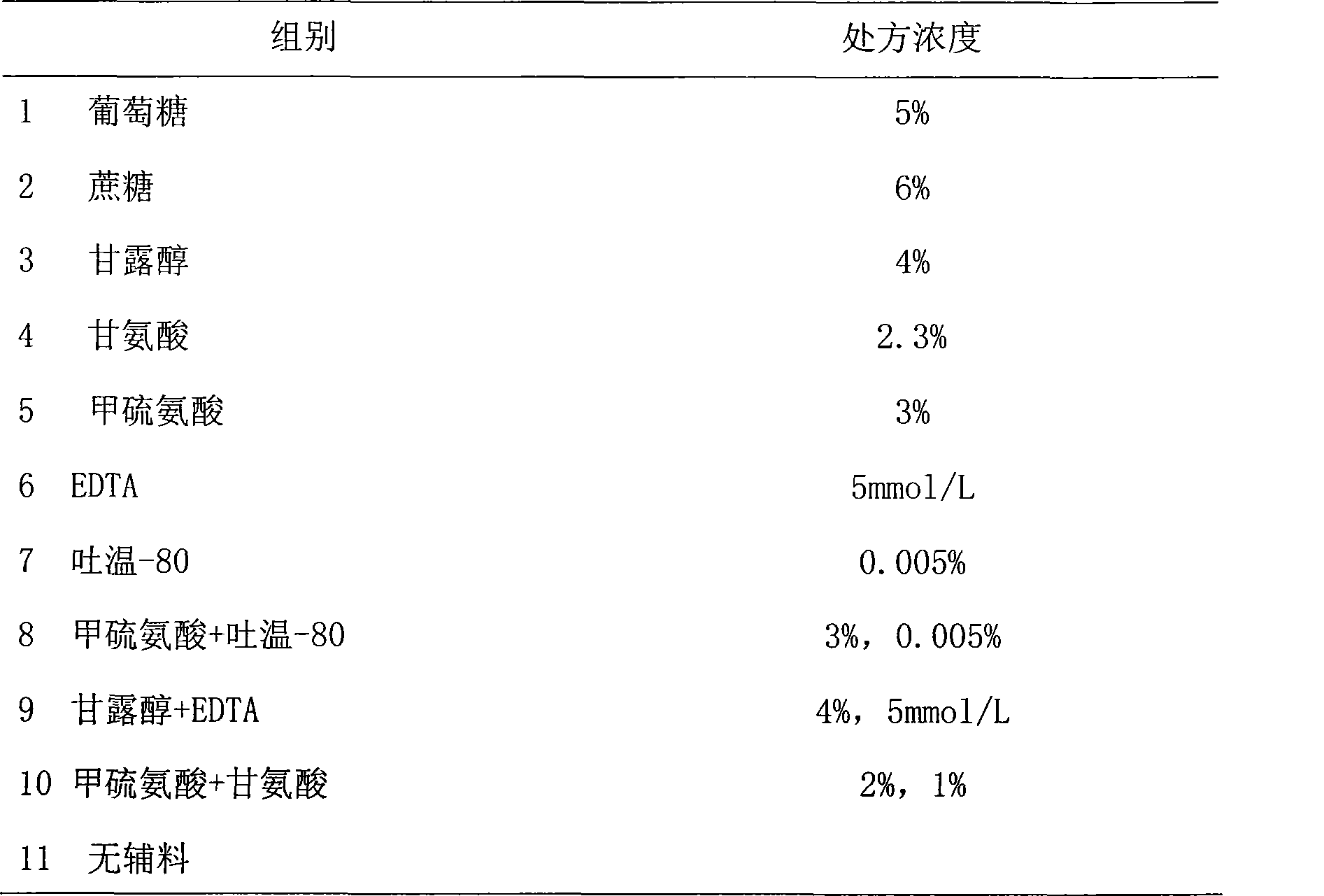
The invention provides a medicinal preparation containing exenatide suitable for multi-administration, which contains exenatide, buffer solution, pharmaceutically acceptable accessory and preservative, wherein the buffer solution can keep the pH value of the preparation in an aqueous solution state at 3.0 to 7.0; the accessory may be one or combination of glucose, sucrose, methionine, mannitol or glycine; and the preservative is selected from benzoic acid, sodium benzoate, potassium sorbate or acetone chloroform. The medicinal preparation has the advantages that the stability of physicochemical and biological activities of the exenatide is enhanced by adding a few components capable of being accepted by the human body, and then a preparation suitable for clinical use, particularly injection use is prepared.
Owner:HANGZHOU JIUYUAN GENE ENG
Exenatide release microsphere preparation, preparation method and application thereof
InactiveCN101658496AProlong the action timeEffective plasma concentrationPeptide/protein ingredientsMetabolism disorderAcetic acidTreatment effect
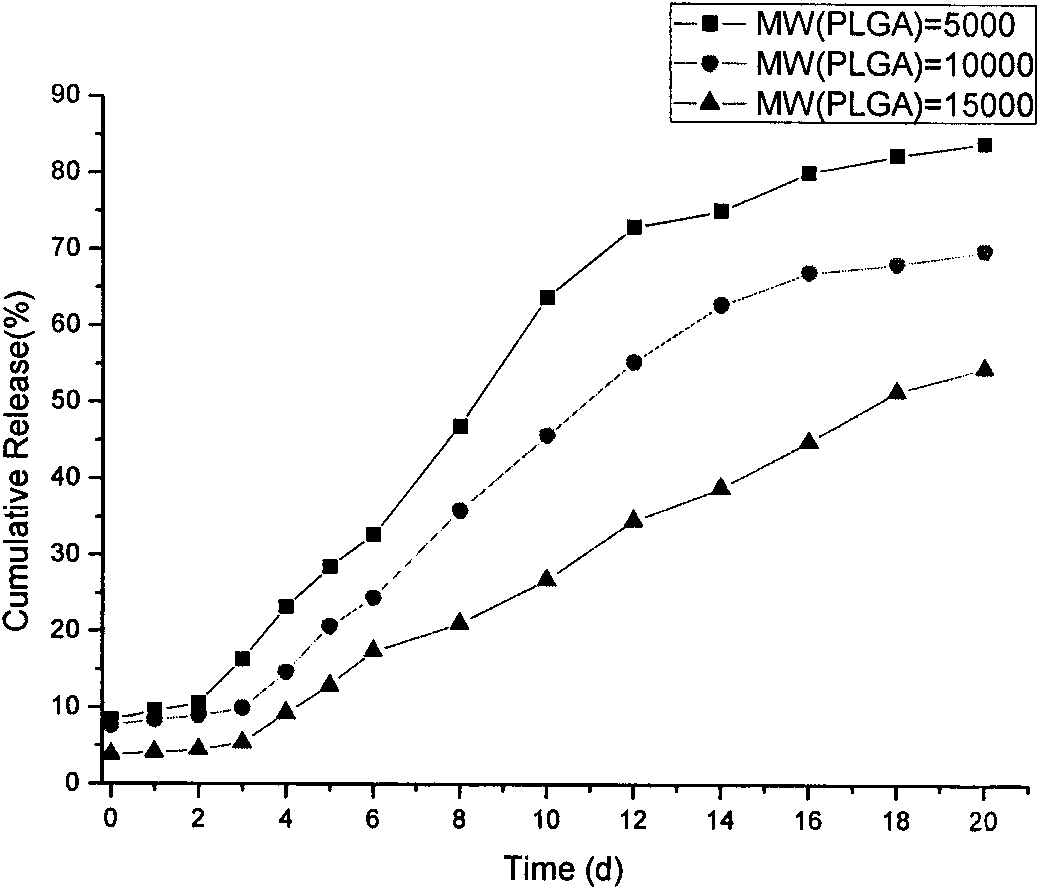
The invention discloses an Exenatide release microsphere preparation mainly comprising the following components in percentage by weight: 0.5-10 percent of Exenatide and 85-99 percent of polylactic acid-glycolic acid copolymer. The invention also discloses a preparation method and an application of the Exenatide release microsphere preparation. The Exenatide release microsphere preparation not onlycan prolong the action time of the Exenatide in the body and reduce the medicine application frequency, but also can maintain the effective blood-medicine concentration of the Exenatide and improve the treatment effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
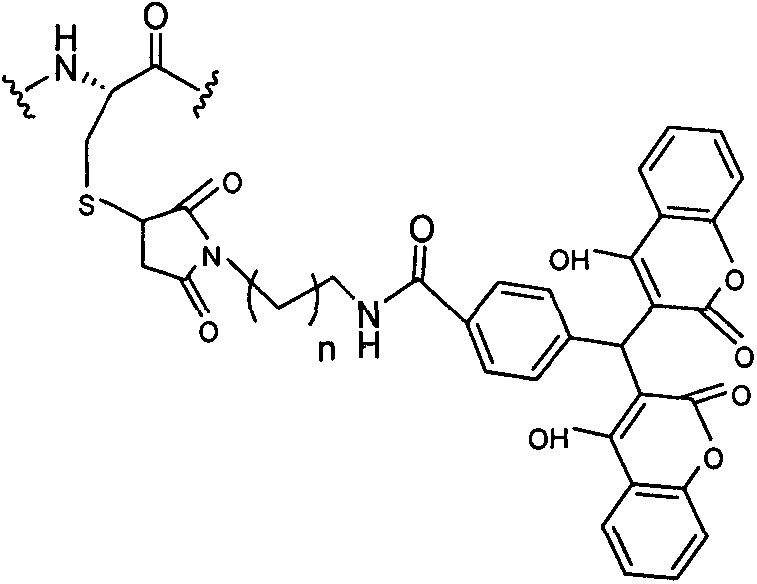
Pegylated exenatide ramification and use thereof
InactiveCN102827270AReduce clearanceExtended half-lifeHormone peptidesPeptide/protein ingredientsClearance rateMotility
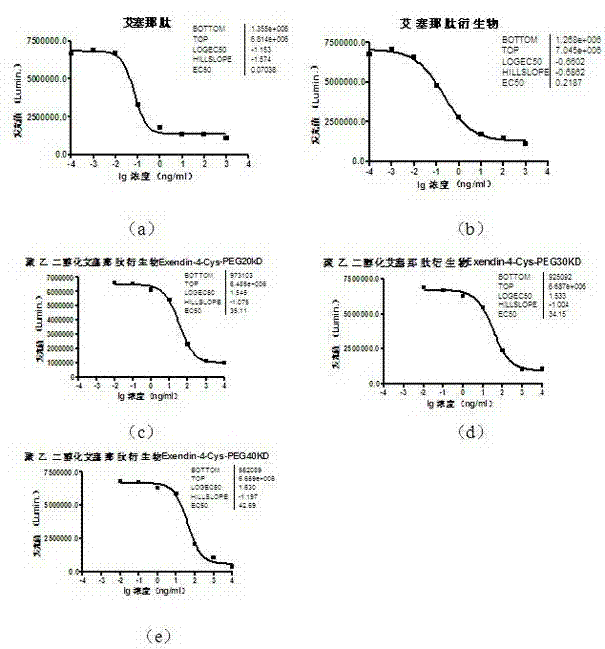
The invention provides a pegylated exenatide ramification (Exendin-4-Cys-PEG (polyethylene glycol)) and the use of the pegylated exenatide ramification, belonging to the technical field of the preparation and the application of a polypeptide drug. A preparation method of the pegylated exenatide ramification provided by the invention comprises the following steps of: introducing a cysteine at the C-end of exenatide, and coupling maleic acylamino polyethylene glycol, so that the pegylated exenatide ramification can be formed. The pegylated exenatide ramification provided by the invention has slow clearance rate in the blood circulation and prolonged half-life period, and can be used for preparing the related illness treatment drugs for reducing the blood sugar, reducing the ingestion, reducing the gastrointestinal emptying, increasing the beta cell quantity or reducing the gastrointestinal motility.
Owner:无锡和邦生物科技有限公司
Method for preparing exenatide
InactiveUS20130289241A1Simple stepsEasy to operatePeptide/protein ingredientsPeptide preparation methodsCombinatorial chemistryExenatide
A method for preparing exenatide by solid-phase synthesis, including: 1) mixing an Fmoc-Rink amide AM resin with a deprotecting agent to obtain a Rink amide AM resin; 2) condensing an Fmoc-Ser(tBu)-OH with the Rink amide AM resin to obtain an Fmoc-Ser(tBu)-Rink amide AM resin; 3) repeating the Fmoc deprotection and the condensation between an amino acid and a polypeptide on the resin, and condensing an amino acid with a polypeptide on the resin from the C-terminal to the N-terminal, to form a polypeptide resin; and 4) separating the polypeptide and the resin on the polypeptide resin.
Owner:SHANGHAI AMBIOPHARM
Synthesis and application of exenatide joined with polyethylene glycol
InactiveCN102532303AImprove in vivo stabilityExtended half-lifeHormone peptidesPeptide/protein ingredientsPharmaceutical SubstancesHistidine residue
The invention relates to exenatide joined with polyethylene glycol. The structural general formula of exenatide is (I), mPEG represents residue of methoxy polyethylene glycol, the selected value of n is 0-3, R represents an exenatide molecule from which an amino is removed, the amino is of lysine residue or of N-end histidine residue in the exenatide molecule, and the molecular weight of the exenatide joined with polyethylene glycol is 9,800-110,000. The invention further relates to a preparation method of the compound and application of the compound in preparing medicines for curing type 2 diabetes mellitus. The exenatide joined with polyethylene glycol has the advantages of being long in half-life period and good in stability and hypoglycemic effect.
Owner:CHEMFUTURE PHARMATECH JIANGSU
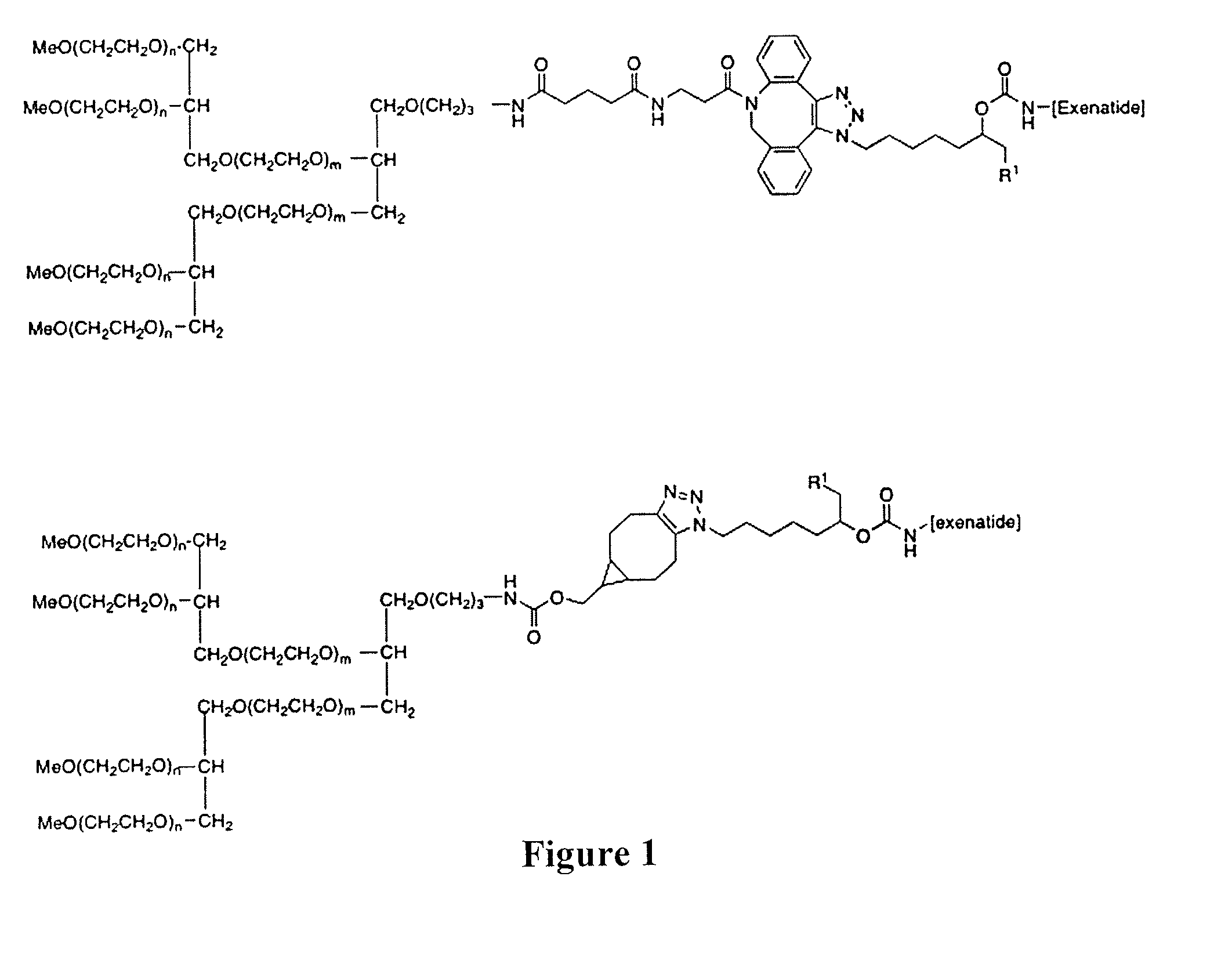
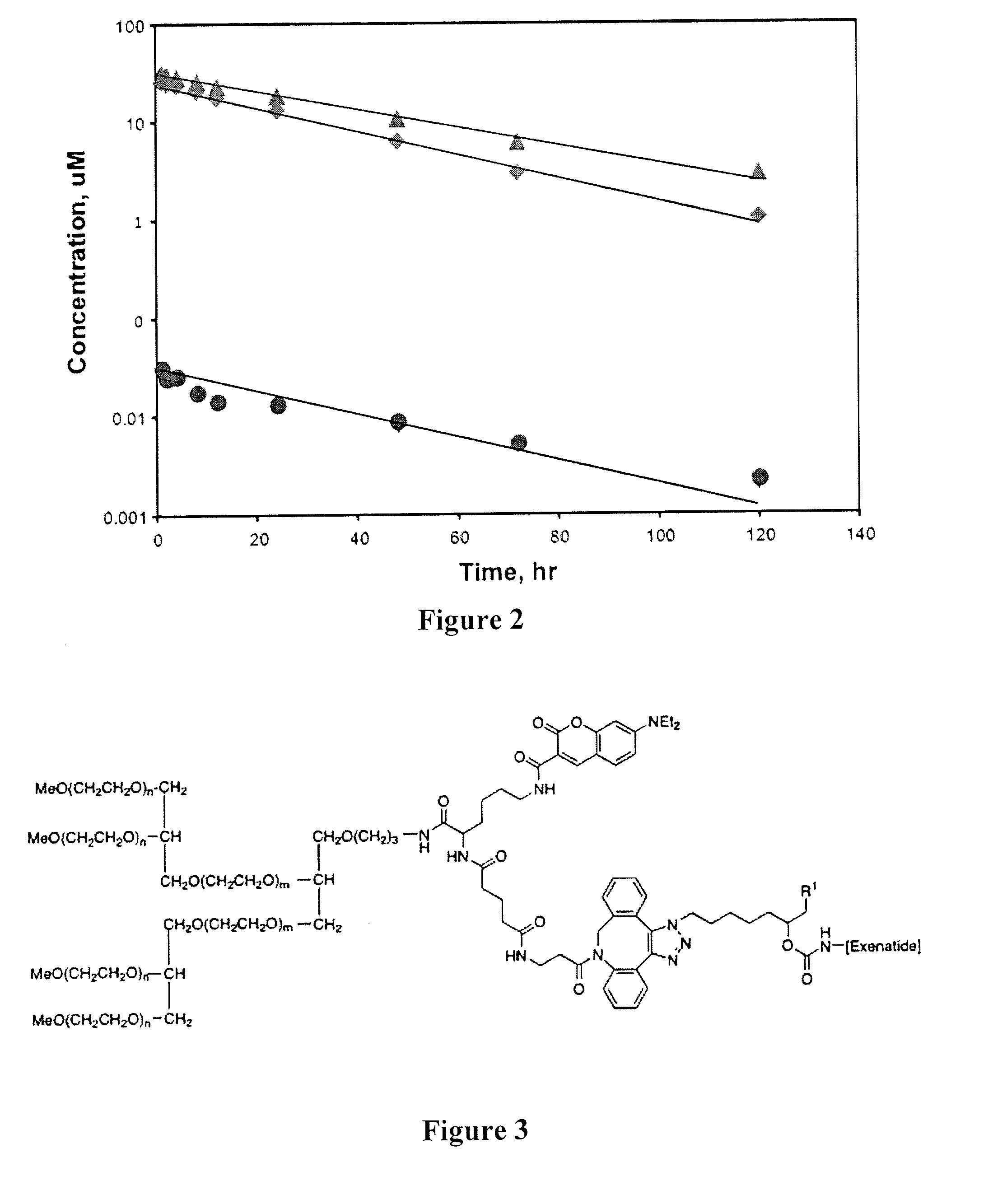
Peg conjugates of exenatide
Slow release forms of exenatide wherein exenatide is releasably linked to polyethylene glycol carriers are disclosed.
Owner:PROLYNX LLC
Method for preparing Exenatide
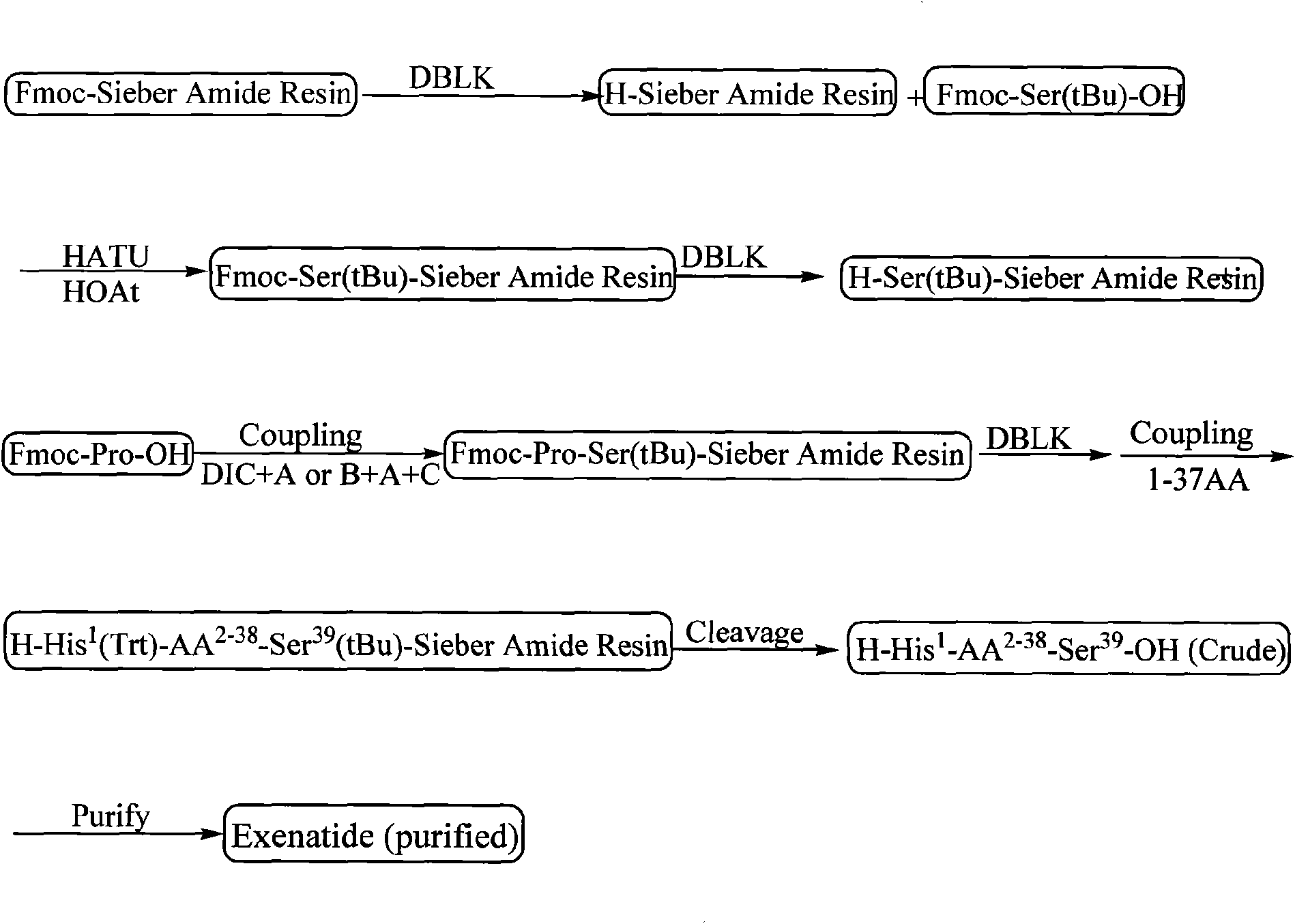
ActiveCN101538324AWith large-scale production capacityReduce investmentMetabolism disorderPeptide preparation methodsFreeze-dryingSide chain
The invention discloses a method for preparing Exenatide, which comprises the following steps of: 1) using Fmoc-Ser(tBu)-OH and Sieber Amide resin with substitutability of 0.1 mmol / g to 0.8 mmol / g as starting material, and obtaining Fmoc-Ser(tBu)-Sieber Amide resin; 2) linking amino acids with protective groups in sequence by adopting a coupling way, synthesizing and obtaining Exenatide-Sieber Amide resin with full-protective lateral chains; and 3) conducting pyrolysis to the resin to remove the protective groups, precipitating with ether and obtaining crude peptides; and conducting high pressure liquid phase purification and freeze drying to the crude peptides and obtaining the Exenatide. The technology for preparing the Exenatide is characterized by simple reaction operation, easy post treatment, high yield, low cost and the like, and has considerable economical and practical value and wide application prospect.
Owner:HYBIO PHARMA
Stable exenatide sustained-release microsphere preparation and preparation method thereof
ActiveCN102198103AImprove technical effectImprove stabilityPeptide/protein ingredientsMetabolism disorderMicrosphereLactide
The invention provides a stable exenatide sustained-release microsphere preparation and a preparation method thereof. The preparation contains 0.1 to 12.5 mass percent of exenatide and 77.5 to 99 percent of glycolide and lactide copolymer. The preparation method comprises the following steps: (a) dissolves glycolide and lactide copolymer in an organic solvent to form an oil phase, wherein the organic solvent may be one or the combination of dichloromethane and ethyl acetate; (b) dissolving exenatide, a protective agent and a suspending aid in water to form an internal water phase; (c) mixing the internal water phase and the oil phase to form emulsion; and (d) after the organic solvent violates, obtaining exenatide-containing sustained-release microspheres. As the formula composition of the stable exenatide sustained-release microsphere preparation is reasonable, the stable exenatide sustained-release microsphere preparation is prepared by common sustained-release microsphere technique, and the sustained-release period of the sustained-release microspheres is as long as 7 to 35 days. The technical effect is better when the exenatide sustained-release microspheres are prepared by a multiple emulsion (water-oil-water) method.
Owner:HYBIO PHARMA
Modified peptides and proteins
InactiveUS20140309168A1High binding affinityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityDisease
Provided are compounds and methods of making compounds containing two or three groups derived from a peptide, such as enfuvirtide or exenatide, covalently bound to a linker. The compounds may contain polyethylene glycol groups to enhance solubility and pharmacokinetic properties. The compounds are useful for the treatment of diseases or conditions subject to treatment with the parent peptide, such as HIV and AIDS in the case of enfuvirtide, or diabetes in the case of exenatide.
Owner:AMIDE BIO
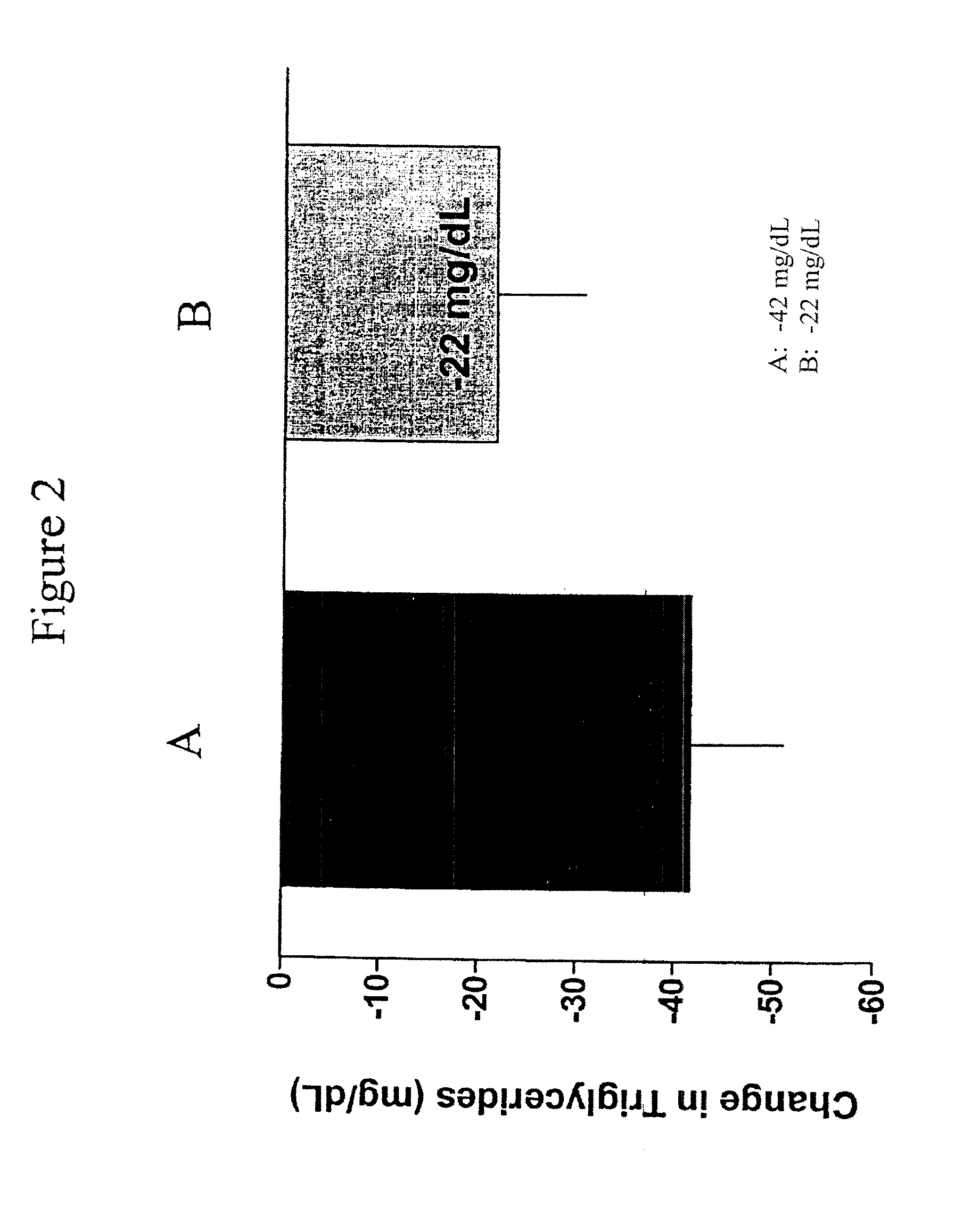
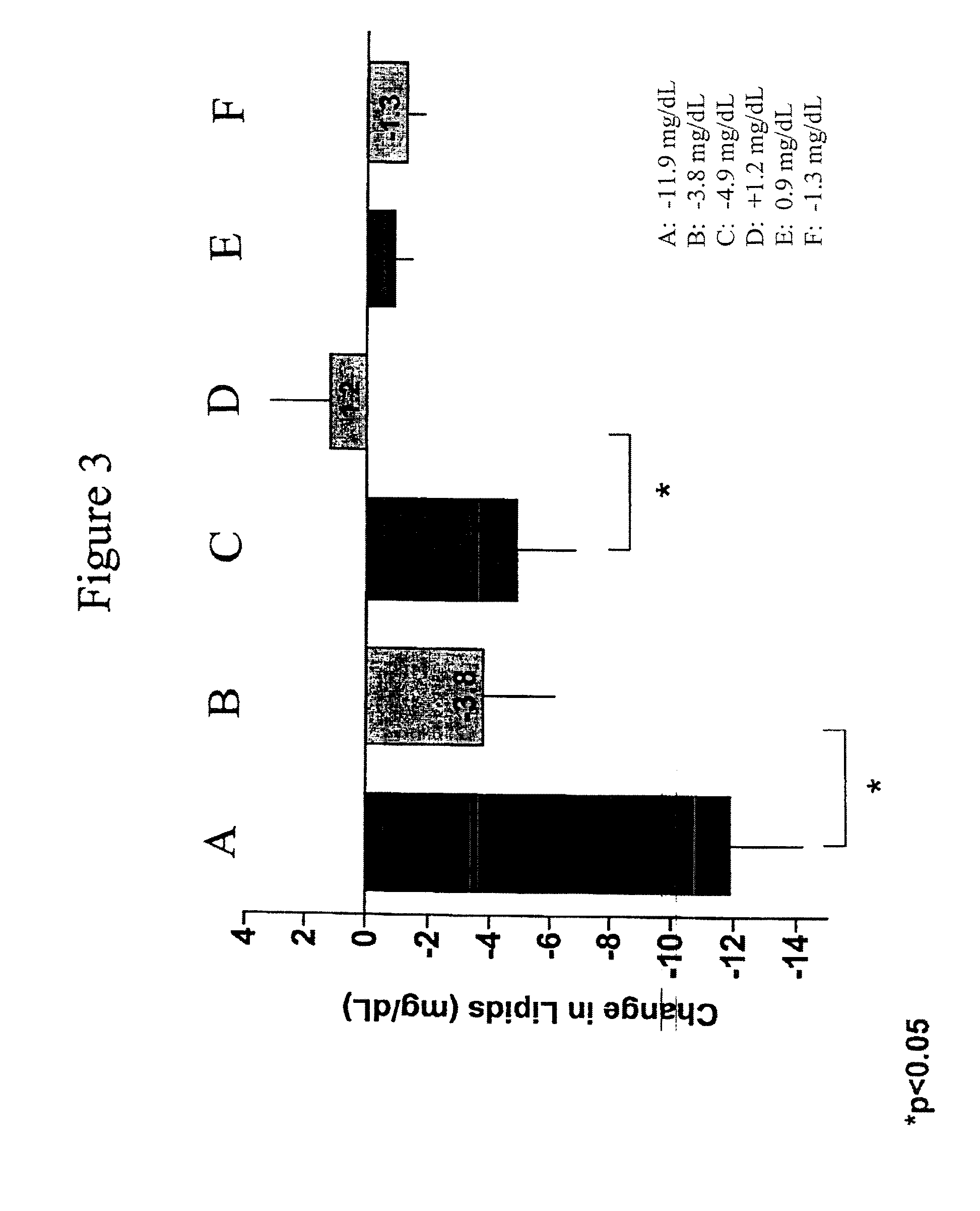
Exendins to lower cholesterol and triglycerides
InactiveUS20110263496A1Lower cholesterol levelsStable statePeptide/protein ingredientsMetabolism disorderDyslipidemiaSucrose
Provided herein are pharmaceutical formulations containing exendins, exendin agonists, or exendin analog agonists that are administered at therapeutic plasma concentration levels over a sustained period of time to lower total cholesterol levels; to lower LDL-cholesterol levels; to lower triglyceride levels; to treat dyslipidemia; to treat and slow the progression of atherosclerosis; and to treat, prevent, and reduce the risk of heart attacks and strokes in patients. In the pharmaceutical formulations and methods of the invention, the exendin may be exendin-4, an exendin-4 agonist, or an exendin-4 analog agonist. The pharmaceutical formulations may be polymer-based pharmaceutical formulations that may be administered once weekly. An exemplary pharmaceutical formulation comprises 5% (w / w) of exenatide, about 2% (w / w) of sucrose, and about 93% (w / w) of a poly(lactide-co-glycolide) polymer, wherein the poly(lactide-co-glycolide) polymer is in the form of microshperes encapsulating the exenatide.
Owner:ASTRAZENECA PHARMA LP
Nasal cavity drop for treating diabetes and preparation method thereof
InactiveCN101601646AAvoid painAvoid inconveniencePeptide/protein ingredientsMetabolism disorderNasal cavityDiabetes mellitus
The invention discloses a nasal cavity drop for treating diabetes and a preparation method. The nasal cavity drop comprises solvent and accessories, and is characterized in that the solvent contains a compound of calcium phosphate nano particles and exenatide, the weight ratio of the calcium phosphate nano particles to the exenatide in the compound is 1-10:0.1-10, and the content of the exenatide in the solvent is 0.01 to 0.5 milligram per milliliter. The preparation method for the nasal cavity drop comprises the following steps: mixing aqueous solutions of calcium salt, phosphate, citrate and exenatide to form the compound of the calcium phosphate nano particles and the exenatide, adding the accessories which are frequently used for aqueous agent into the mixture, detecting and adjusting the pH of the solution, and finally forming the nasal cavity drop, wherein the weight ratio of the calcium phosphate nano particles to the exenatide in the compound is 1-10:0.1-10, and the content of the diluted exenatide in the solvent is 0.01 to 0.5 milligram per milliliter.
Owner:南京凯瑞尔纳米生物技术有限公司
Method of treatment of a metabolic disease using intranasal administration of exendin peptide
Owner:AMYLIN PHARMA INC
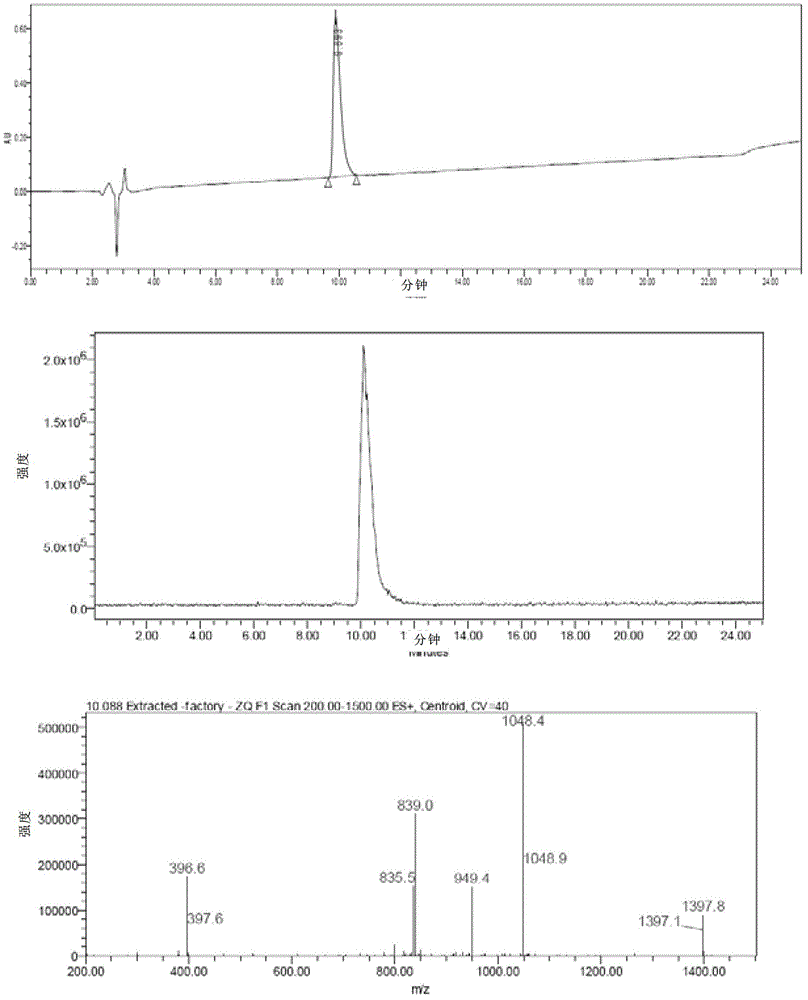
Method for purifying Exenatide
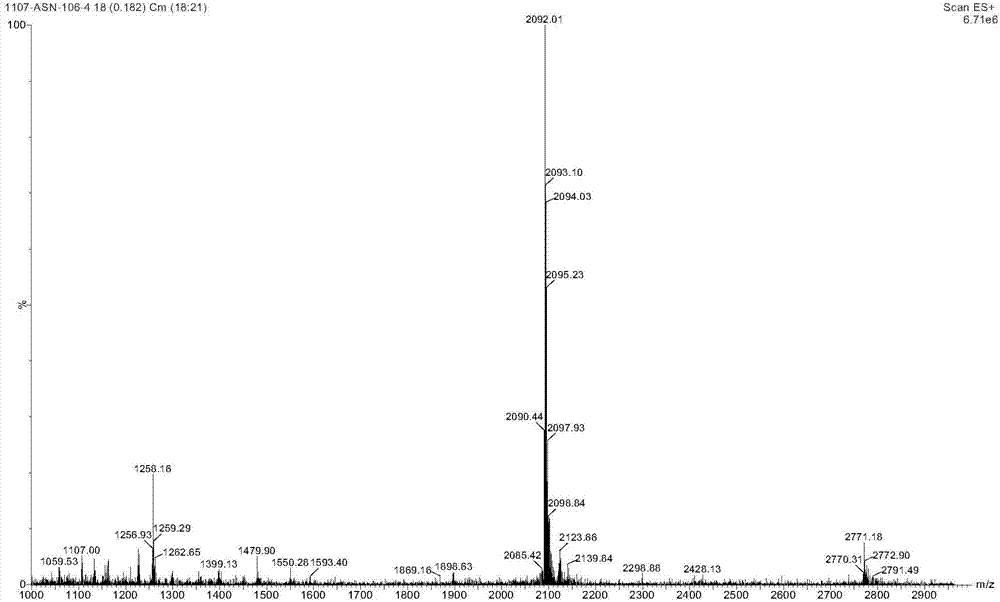
The invention discloses a method for purifying Exenatide, which comprises the following steps of: 1) dissolving crude peptides obtained by solid phase synthesis with water for injection; 2) conducting gradient elution and purification with the fixed phase being reversed-phase silica gel column of tetraalkylsilane bonded silica, octalkylsilane bonded silica or octadecylsilane bonded silica, and the phase A of a mobile phase being phosphate buffer solution and the phase B of the mobile phase being chromatographic grade acetonitrile, and collecting the peptide solution at a target peak value; and 3) converting the high-purity peptides after purification into acetate by using an anion exchange method. The method which is applicable to the industrialized purification of Exenatide uses reversed phase high-performance liquid chromatography for purifying Exenatide, and uses the anion exchange method for converting the high-purity peptides after purification into acetate, thus not only being capable of obtaining the refined peptides with PLC purity higher than 98.0 percent, but also realizing large-scale production and meeting the requirements of high purity, high yield and industrialization.
Owner:HYBIO PHARMA
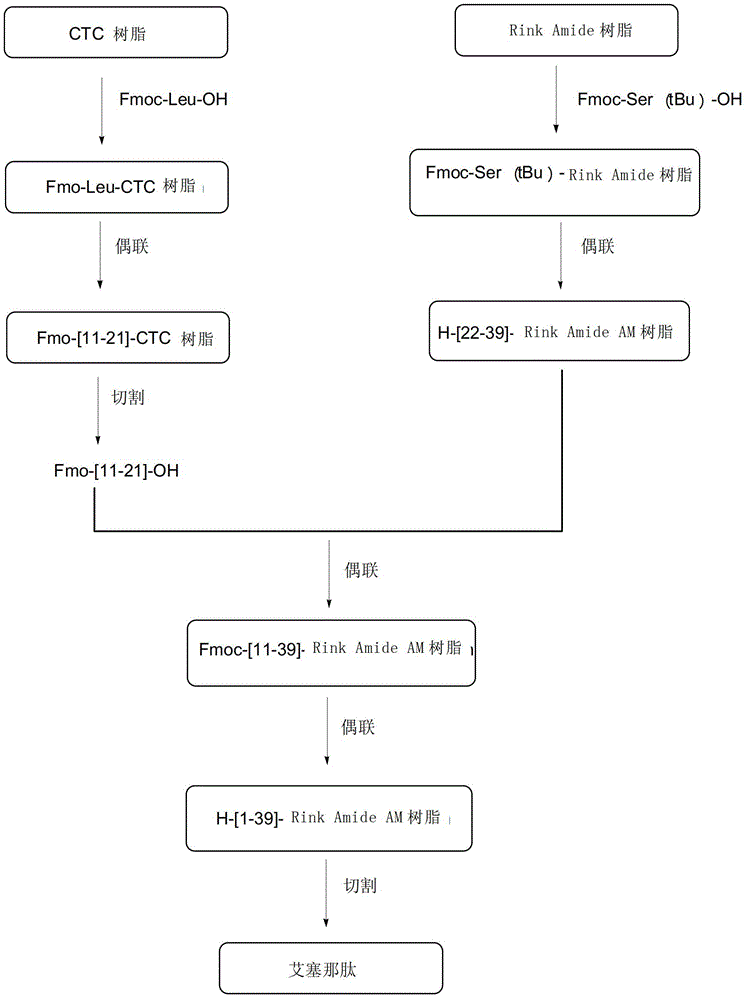
Method for synthesizing Exenatide from solid phase polypeptide
ActiveCN101357938AConvenient sourceReduce usagePeptide preparation methodsDepsipeptidesSide chainFreeze-drying
The invention discloses a preparation method of solid phase peptide synthesis Exenatide which includes the following steps: taking Rink Amide resins, Rink Amide AM resins or Rink Amide MBHA resins as starting materials, amino acids with Fmoc protective groups are sequentially connected, so as to obtain protective thirty-nine peptide resins; and meanwhile, after thirty-nine peptide resins are obtained by sequentially removing Fmoc-protective groups and transpeptidase reactions by condensing agents, acellular side-chain protective groups and cutting peptides are carried out sychronously to obtain Exenatide crude products, and then products (comprising medical salts and free alkali, such as acetates, trifluoroacetate, etc.) are obtained after Exenatide crude products are separated and purified by C18 or C8 column and freeze-dried. The preparation method has the advantages of stable technology, conventient raw and auxiliary material sources, short production cycle, low production cost, few three wastes, high yield, stable yield, stable quality, low production cost and high yield.
Owner:SHANGHAI SOHO YIMING PHARMA
Methods and compositions for oral administration of exenatide
ActiveUS20130195939A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsSkeletal disorderDiabetes mellitusOral medication
Owner:ORAMED
Synthesis of exenatide through solid phase fragment method
ActiveCN103333237AImprove efficiencyShort synthesis cycleHormone peptidesPeptide preparation methodsRink amide resinSide chain
The invention discloses a solid phase synthesis preparation method of exenatide represented by a formula I, wherein a Fmoc-resin and a deprotection agent are mixed to obtain a deprotection resin, a Fmoc-amino acid and a deprotection resin are subjected to condensation to obtain a Fmoc-amino acid-resin, and Fmoc protection removal and condensation of the polypeptides on the Fmoc-amino acid and the resin are repeated to carry out condensation of the amino acid and the polypeptide on the resin according to a C terminal-to-N-terminal sequence to form a polypeptide resin. The method is characterized by comprising the following steps: (1) respectively forming polypeptide resin fragments represented by a formula II and a formula III; (2) cutting the fragment represented by the formula III to obtain a polypeptide fragment with complete side chain protection and represented by a formula IV; (3) conjugating the polypeptide fragment with complete side chain protection and represented by the formula IV to the polypeptide resin fragment represented by the formula II, and removing Fmoc to obtain a polypeptide resin represented by a formula V; (4) sequentially conjugating the remaining 10 Fmoc-amino acids, and removing Fmoc to obtain an exenatide-Rink Amide resin represented by a formula VI; and (5) separating the polypeptide and the resin on the polypeptide resin represented by the formula VI to obtain the exenatide represented by the formula I.
Owner:HAINAN SHUANGCHENG PHARMA
Long-effecting exenatide (Exendin-4) analogue and application thereof
ActiveCN107141348AGood hypoglycemic effectChemically stablePeptide/protein ingredientsMetabolism disorderSynthesis methodsPharmacologic action
The invention relates to a long-effecting exenatide (Exendin-4) analogue and a synthetic method thereof. The Exendin-4 is modified to obtain the Exendin-4 analogue with longer pharmacologic action time, the synthesis of target polypeptide is quickly realized through an orthogonally protection strategy solid-phase synthesis method, and a crude product is purified and freeze-dried to obtain the Exendin-4 analogue.
Owner:CHINA PHARM UNIV
Exenatide short peptide simulation peptide and application thereof in preparing medicament for curing diabetes
InactiveCN101215324APeptide/protein ingredientsMetabolism disorderDiabetes mellitusComputational chemistry
The invention provides an exenatide short peptide and imitation peptide, which transforms the structure of exenatide, and is a novel compound. The invention further discloses a preparation process of exenatide short peptide and imitation peptide, which is suitable to be produced in industrialization. The invention further discloses an application on the preparation therapeutic medicaments of diabetes.
Owner:JILIN UNIV
Exendins To Lower Cholesterol And Triglycerides
InactiveUS20130172250A1Stable stateReduce riskPeptide/protein ingredientsMetabolism disorderDyslipidemiaSucrose
Provided herein are pharmaceutical formulations containing exendins, exendin agonists, or exendin analog agonists that are administered at therapeutic plasma concentration levels over a sustained period of time to lower total cholesterol levels; to lower LDL-cholesterol levels; to lower triglyceride levels; to treat dyslipidemia; to treat and slow the progression of atherosclerosis; and to treat, prevent, and reduce the risk of heart attacks and strokes in patients. In the pharmaceutical formulations and methods of the invention, the exendin may be exendin-4, an exendin-4 agonist, or an exendin-4 analog agonist. The pharmaceutical formulations may be polymer-based pharmaceutical formulations that may be administered once weekly. An exemplary pharmaceutical formulation comprises 5% (w / w) of exenatide, about 2% (w / w) of sucrose, and about 93% (w / w) of a poly(lactide-co-glycolide) polymer, wherein the poly(lactide-co-glycolide) polymer is in the form of microshperes encapsulating the exenatide.
Owner:ASTRAZENECA PHARMA LP
Improved method for preparing exenatide sustained release microspheres
InactiveCN103585114AReduce volatile lossIncrease dissolution ratePeptide/protein ingredientsGranular deliverySolubilityPolymer dissolution
The invention belongs to the field of pharmaceutic preparations, and discloses an improved method for preparing exenatide sustained release microspheres and exenatide sustained release microspheres prepared by the method. In the method of the invention, after being dissolved in dichloromethane, a polymer is mixed with methanol solution;after quicker dissolving of the polymer is guaranteed, exenatide or exenatide and protective agent are added, so that the dissolving speed of the exenatide and the polymer is quickened, the contact time of the exenatide with organic solvent and the volatilization loss of solvent in the polymer dissolving process are reduced, and the solubility of the exenatide in oil phase is largely improved. The method improves the drug loading capacity, reduces the degradation of drugs in the preparation process, simplifies the process, and shortens the preparation period.
Owner:HYBIO PHARMA
Polypeptide or protein nanoparticles based on hydrogen-bonded complexation, and preparation method and application thereof
InactiveCN109224081AKeep alive functionImprove encapsulation efficiencyPowder deliveryPeptide/protein ingredientsRecombinant salmon calcitoninPolyvinylpyrrolidone
The invention discloses a polypeptide or protein nanoparticle based on hydrogen-bonded complexation and a preparation method and application thereof. The nanoparticles comprise a polyphenol compound carrier such as tannic acid as a hydrogen bond donor, a polypeptide or a protein drug such as a glucagon-like peptide as a hydrogen bond acceptor 1 analog (exenatide), salmon calcitonin or insulin, etc., and chitosan, polyvinylpyrrolidone or hyaluronic acid, etc. coated on that surface of the particle as stabilizer and / or functional molecule. The nanoparticles of the invention have extremely high encapsulation efficiency and drug loading capacity, and the prepared therapeutic polypeptide or protein drug nanoparticles are suitable for oral delivery or subcutaneous injection application, and havegreat application prospect.
Owner:SUN YAT SEN UNIV
Method for preparing exenatide
ActiveCN102174082ASimple stepsEasy to operatePeptide preparation methodsCombinatorial chemistryExenatide
The invention discloses a method for preparing polypeptide solid-phase synthesized exenatide. The method comprises the following steps of: (1) mixing Fmoc-Rink amide AM resin with a protection removing agent to obtain Rink amide AM resin; (2) condensing Fmoc-Ser(tBu)-OH and the Rink amide AM resin to obtain Fmoc-Ser(tBu)-Rink amide AM resin; (3) repeating the Fmoc protection removal in the step (1) and condensation of amino acid and polypeptide on the resin in the step (2) according to a solid-phase synthesis method and condensing the amino acids from C end to N end and the polypeptide on the resin in the sequence of Ser to His to form the polypeptide resin shown as the formula II; and separating the polypeptide and resin on the polypeptide resin shown as the formula II to obtain the exenatide shown as the formula I.
Owner:SHANGHAI AMBIOPHARM
Pharmaceutical preparation containing exenatide
The invention discloses a pharmaceutical preparation containing exenatide. Besides the active constituent exenatide, the pharmaceutical preparation further contains 10-100mm of buffer with pH being 3-7 and adjuvants for enhancing stability of the exenatide. The invention has the advantages of preparing an exenatide preparation which is applicable to clinical application, in particular to injection and can be stably kept by adding a plurality of human acceptable constituents.
Owner:HANGZHOU JIUYUAN GENE ENG
Solid-phase fragment synthetic method of exenatide
ActiveCN103613656AReduce accumulationHigh synthesis efficiencyHormone peptidesPeptide preparation methodsCombinatorial chemistrySolid-phase synthesis
The invention discloses a solid-phase fragment synthetic method of exenatide. The solid-phase fragment synthetic method comprises the following steps: dividing 39 amino acids of the exenatide into 6 sections of full-protection fragments; firstly respectively synthesizing the 6 sections of the full-protection fragments; sequentially carrying out solid-phase fragment assembling and connection on the 6 sections of the full-protection fragments to obtain exenatide resin; then cutting to obtain an exenatide crude product; and purifying the crude product to obtain an exenatide product. According to the solid-phase fragment synthetic method of the exenatide, the problems that the existing solid-phase exenatide synthesizing process is complicated, is low in efficiency and difficult to purify and scale are solved; the synthesizing efficiency is improved and connection steps are simplified greatly; the accumulation of impurities is reduced; the invention provides a novel method for realizing the large-scale synthesis of the exenatide, improving the product purity and reducing the purification difficulty.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Method for low-cost purification of exenatide
ActiveCN103613655APlay a role in desalinationReduce difficultyHormone peptidesPeptide preparation methodsDesalinationSilica gel
The invention discloses a method for low-cost purification of exenatide. The method comprises the steps of firstly, performing initial purification on a large quantity of exenatide crude products by using a strong cation exchange column by adopting an efficient liquid phase chromatography so as to remove most of impurities in the exenatide crude products and provide convenience for refining purification from two aspects of quality and quantity, then performing desalination by using a reverse phase polymer column, and then performing refining purification by using a C18 reverse phase silica gel column. By adopting the method, the production cost is reduced, the exenatide with the concentration of more than 98% can be obtained, and the requirements of low cost, high yield and industrialization for purifying exenatide can be met.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Medicinal preparation containing exenatide
The invention provides a medicinal preparation containing exenatide suitable for multi-administration, which contains exenatide, buffer solution, pharmaceutically acceptable accessory and preservative, wherein the buffer solution can keep the pH value of the preparation in an aqueous solution state at 3.0 to 7.0; the accessory may be one or combination of glucose, sucrose, methionine, mannitol or glycine; and the preservative is selected from benzoic acid, sodium benzoate, potassium sorbate or acetone chloroform. The medicinal preparation has the advantages that the stability of physicochemical and biological activities of the exenatide is enhanced by adding a few components capable of being accepted by the human body, and then a preparation suitable for clinical use, particularly injection use is prepared.
Owner:HANGZHOU JIUYUAN GENE ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com