Synthesis of exenatide through solid phase fragment method
A technology of exenatide and solid-phase synthesis, which can be applied in the direction of peptides, specific peptides, hormone peptides, etc., and can solve the problems of incomplete amino acid coupling reaction, long peptide, and long synthesis period.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
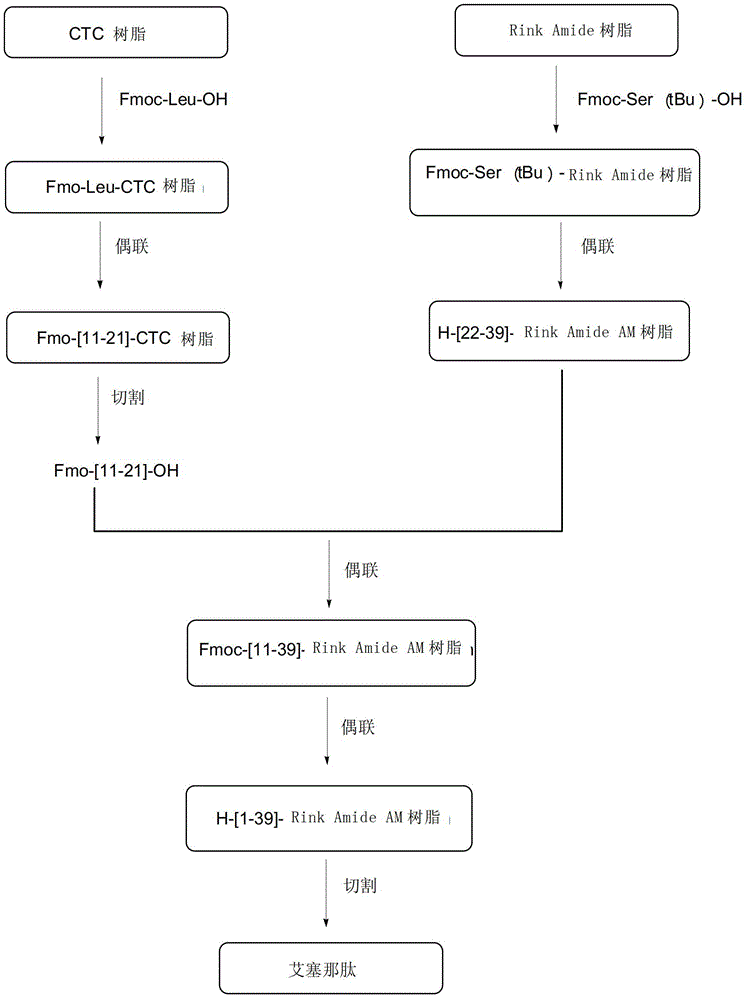
[0041] Specifically, the exenatide solid-phase synthesis method provided by the invention comprises the following steps:
[0042] In the first step, peptide resin fragments (formula II and formula III) are prepared in solid phase respectively;
[0043] In the second step, the formula III is cut to obtain a polypeptide fragment with fully protected side chains (formula IV);
[0044] In the third step, the polypeptide fragment (formula IV) with fully protected side chains is coupled to the peptide resin (formula II), and Fmoc is removed to obtain the polypeptide resin (formula V);
[0045] The fourth step is to sequentially couple the remaining ten protected amino acids by solid-phase synthesis, and de-Fmoc to obtain Exenatide-Rink Amide Resin (Formula VI);
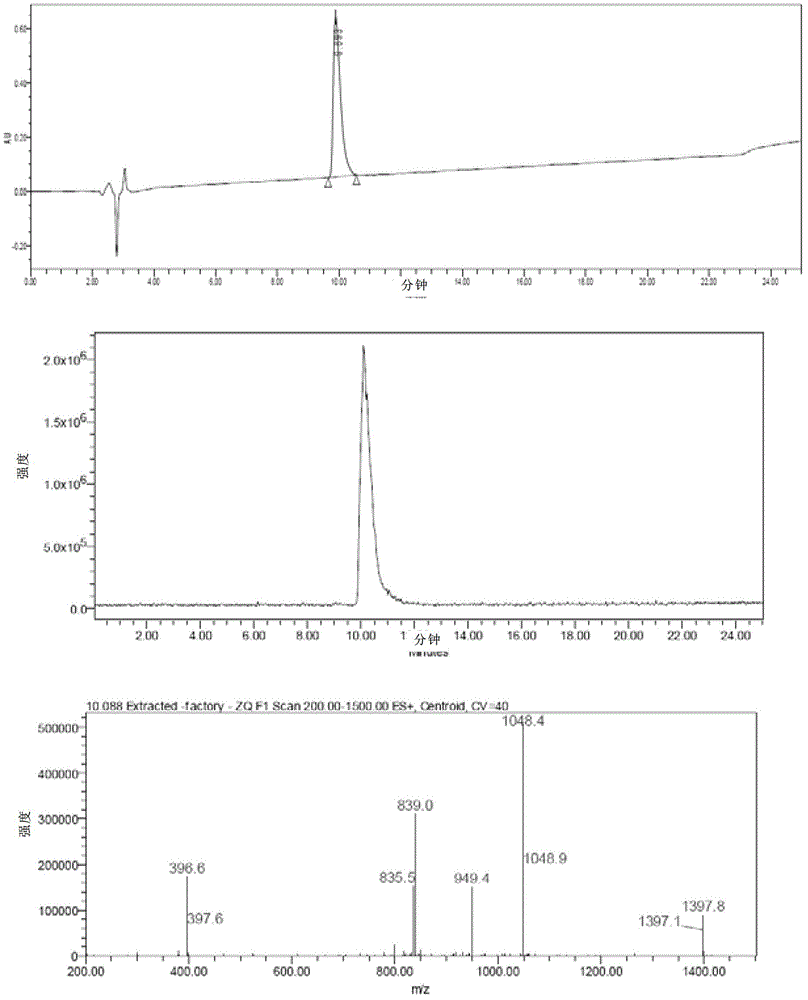
[0046] In the fifth step, the polypeptide on the polypeptide resin represented by formula VI is separated from the resin to obtain exenatide represented by formula I.
[0047]The synthesis of the peptide resin fragment sh...
Embodiment 1
[0079] Peptide resin H-[22-39]-Rink Amide AM resin with protective groups on the side chain
[0080] 1: Synthesis of Fmoc-Ser(tBu)-Rink Amide AM resin
[0081] (1) Fmoc-Rink Amide AM resin (manufactured by Tianjin Nankai Hecheng Technology Co., Ltd., with a substitution degree of 0.8mmol / g, 10g) was put into a solid-phase reaction column, washed twice with DMF, and swelled in DMF for 30 minutes.
[0082] (2) Drain the solution, and remove Fmoc twice with 20% piperidine in DMF at room temperature for 10 minutes and 20 minutes respectively.
[0083] (3) Drain the solution and wash the resin six times with DMF.
[0084] (4) Dissolve Fmoc-Ser(tBu)-OH (9.20g) and HOBt (4.86g) in DMF (30mL) and DCM (30mL), add DIPCDI (7.52ml), and pre-react in an ice bath for 10 minutes.
[0085] (5) Add the above-mentioned reaction solution into a solid-phase reactor, stir it mechanically, and react at room temperature for 3 hours. The ninhydrin test shows that the resin is colorless and transpar...
Embodiment 2
[0096] Peptide resin Fmoc-[11-21]-CTC resin with protective groups on the side chain
[0097] 1. Synthesis of Fmoc-Leu-CTC resin
[0098] Weigh 30 g of CTC resin with a substitution degree of 1.1 mmol / g, add it to a solid-phase reactor, and wash it twice with DMF. Weigh 17.49g of Fmoc-Leu-OH, add 200mL of DMF to dissolve, then add 20.20mL of DIPEA, stir for 5min, add to the solid phase reactor, and react for 2 hours. The reaction solution was drained and washed three times with DMF. Add a mixture of 60mL methanol, 180mL DMF and 15mL DIPEA, and block for 30min. Wash 3 times with DMF, twice with DCM, twice with methanol, and drain. 43.07 g of Fmoc-Leu-CTC resin was obtained, and the degree of substitution was 0.75 mmol / g after measurement.
[0099] 2. Synthesis of Fmoc-[11-21]-CTC resin
[0100] (1) Put 43.07g of Fmoc-Leu-CTC resin into the solid phase reactor, wash with DMF three times, and swell with DMF for 20 minutes
[0101] (2) Drain the solution, add 2% (g / mL) HOBt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com