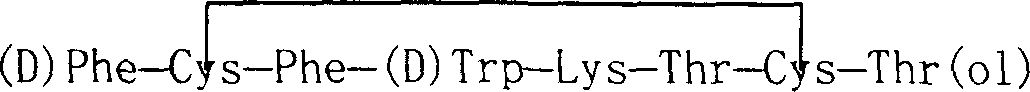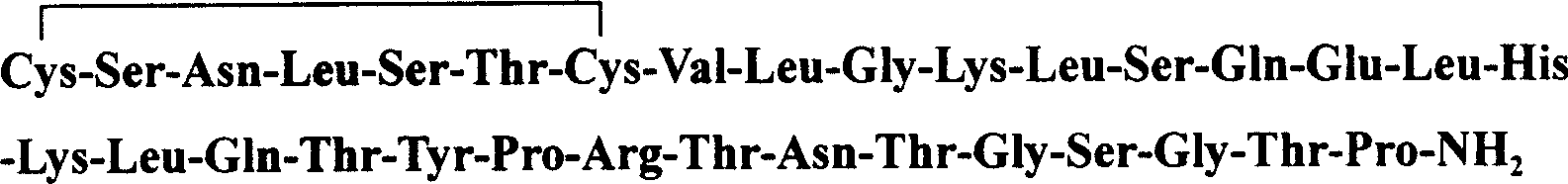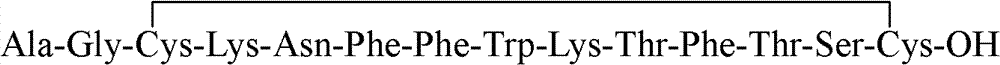Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58results about How to "With large-scale production capacity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
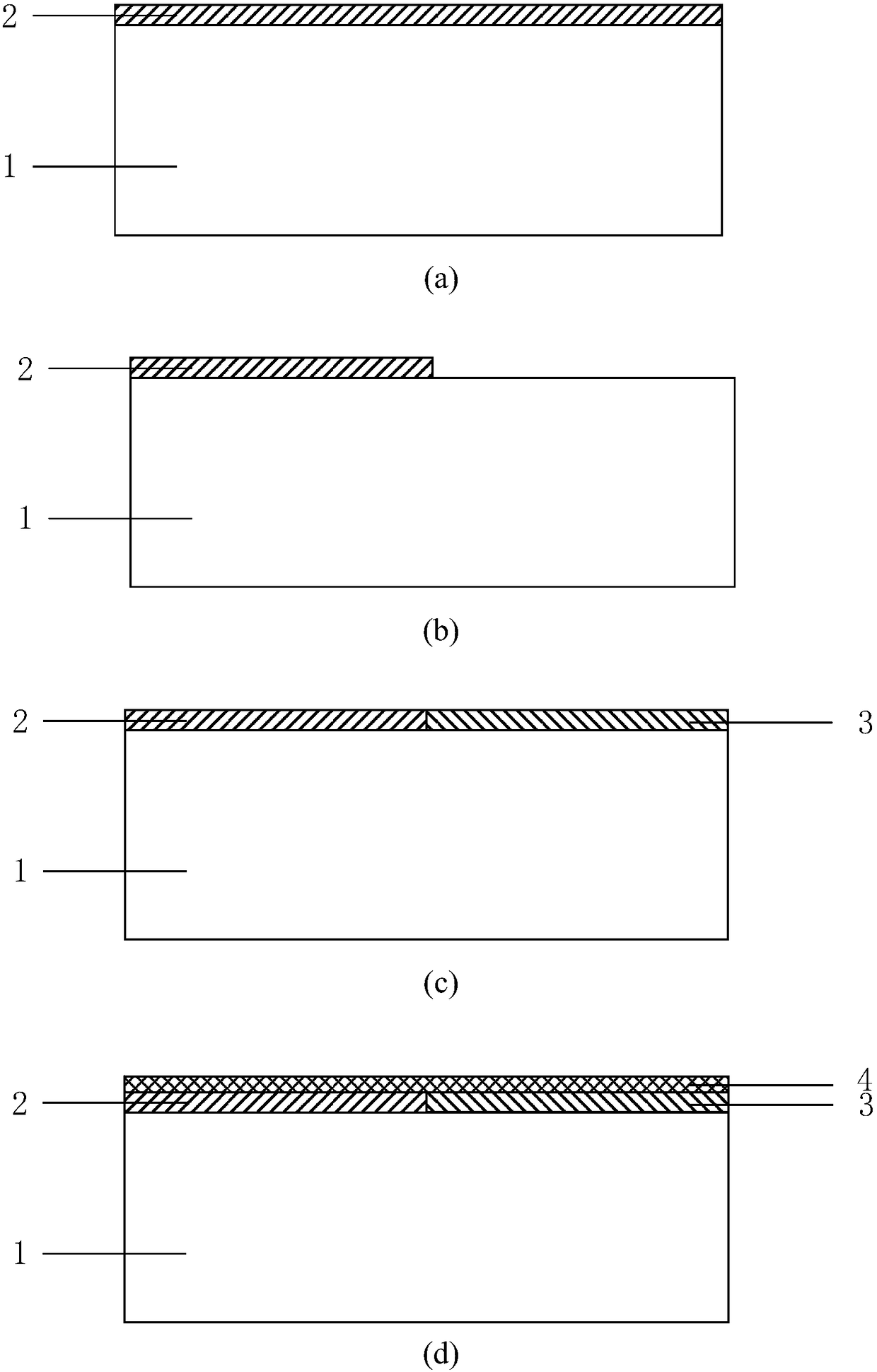
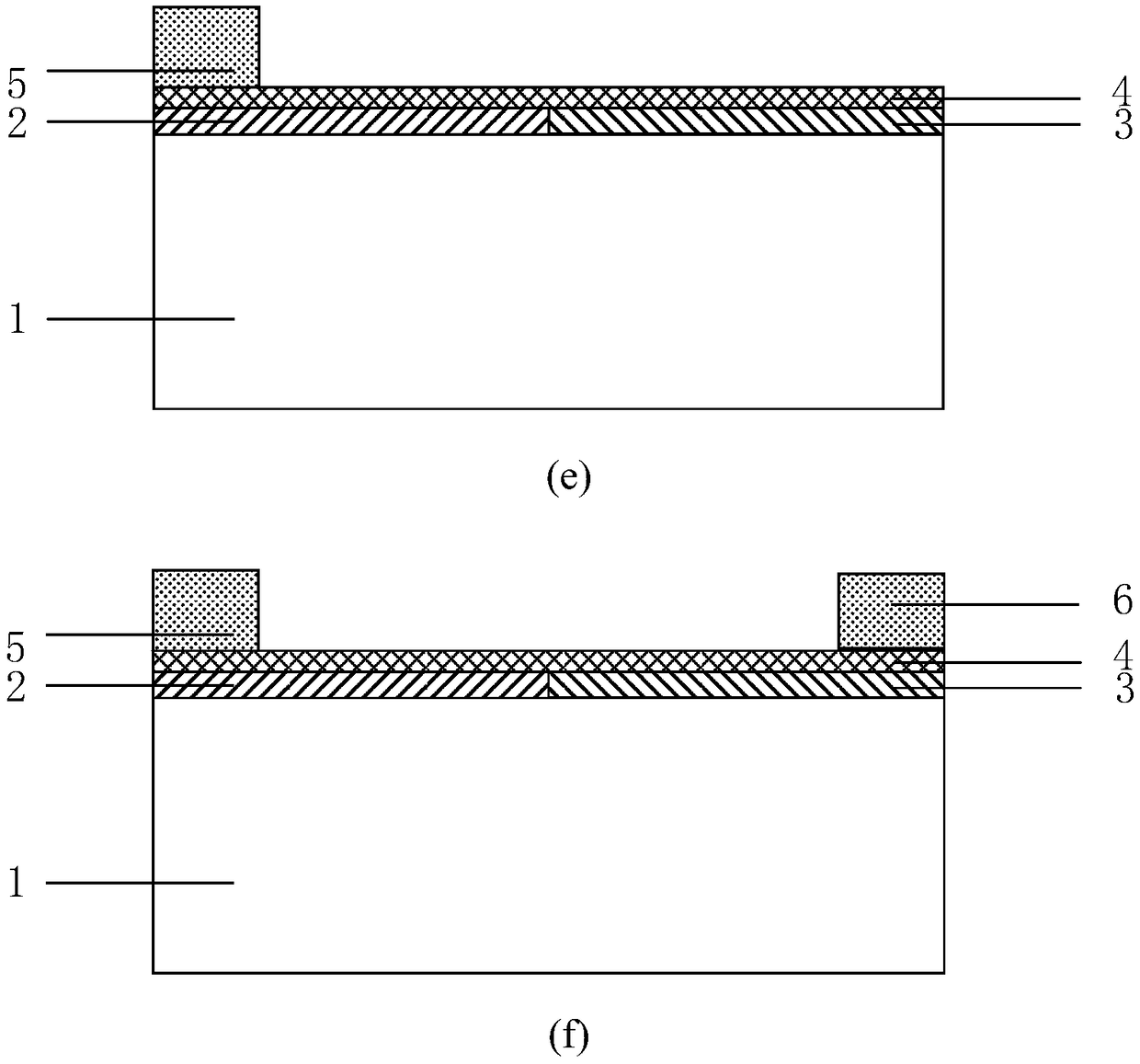
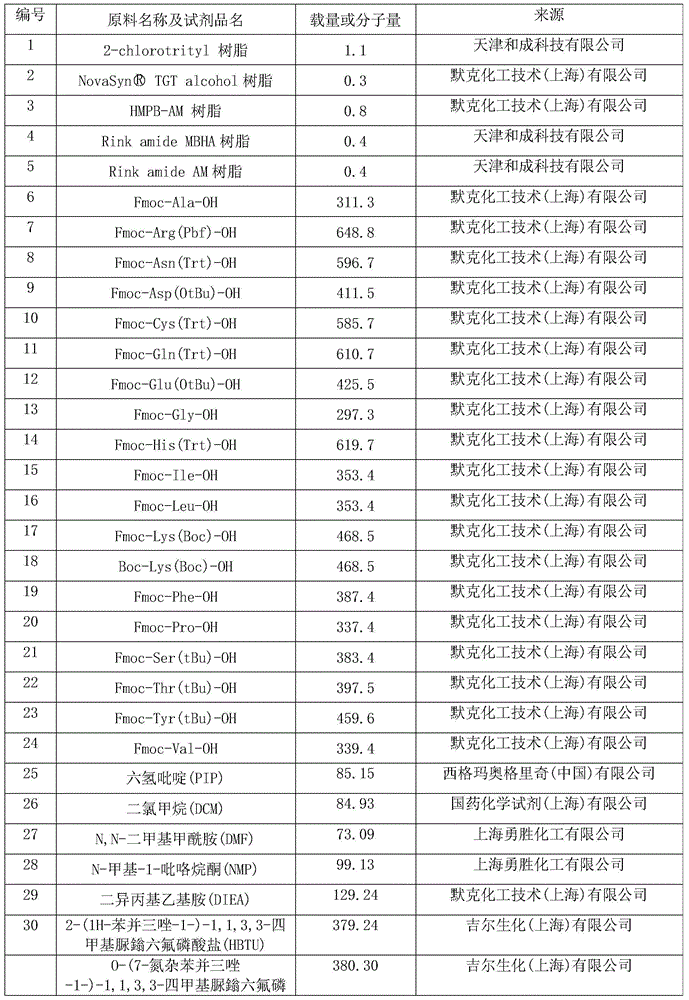
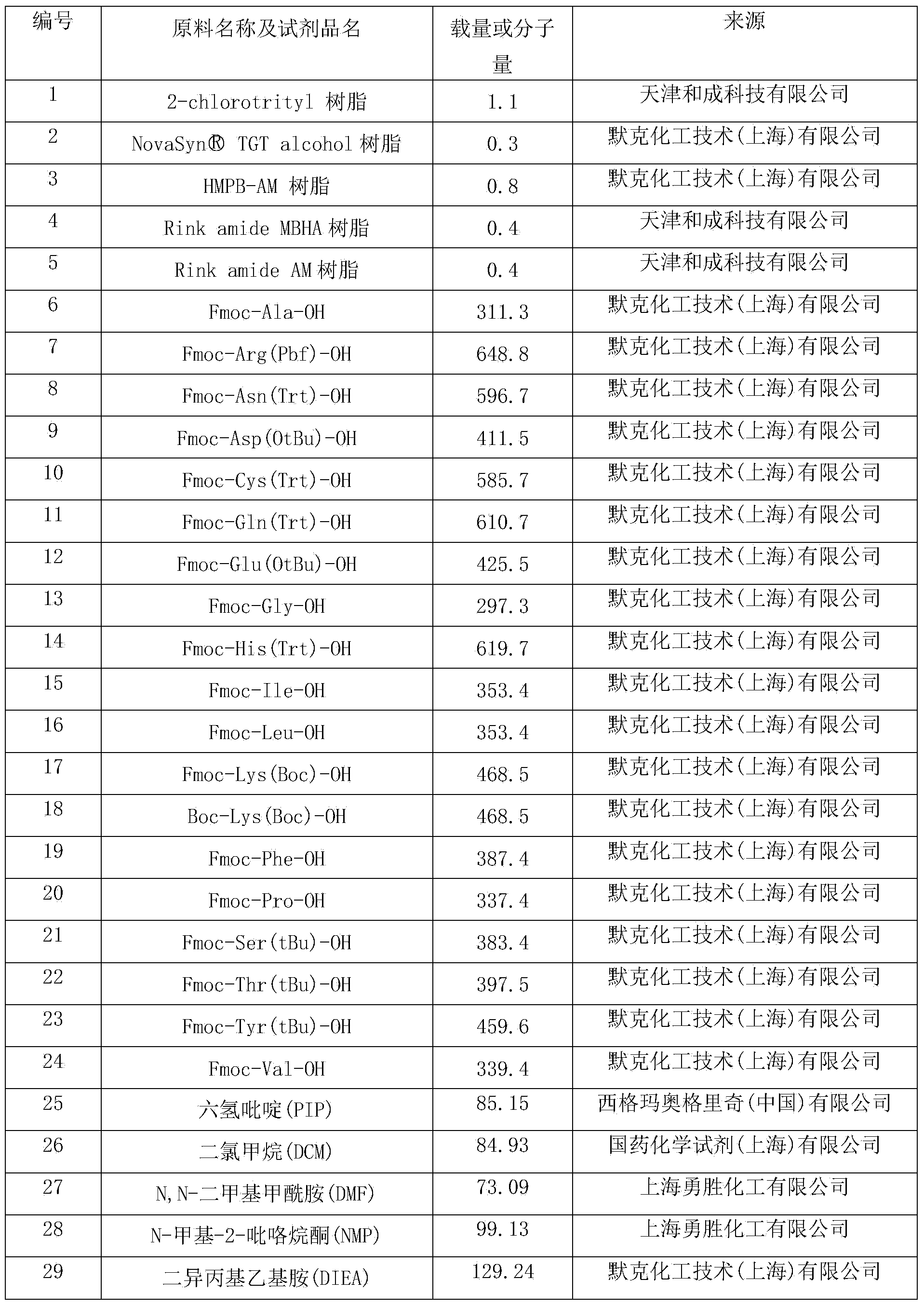
Preparation method of synthesizing bivalirudin from solid phase polypeptide
ActiveCN101033249AConvenient sourceReduce usagePeptide-nucleic acidsPeptide preparation methodsSide chainWang resin
This invention discloses a preparation method of solid-phase peptide synthesizing bivalirudin. It includes the following steps: taking any one of triphenyl methyl chloride resin, 4-methyl-triphenyl methyl chloride resin, 4-methoxy-triphenyl methyl chloride resin, 2-chlorine-triphenyl methyl chloride resin, or Wang resin as the starting raw materials, connecting amino acids in turn according to the method of solid-phase synthesis, to get a protective 28-peptide resin, removing Fmoc-protective group in turn, side-chain protecting group and cutting the peptide to get a crude, then purifying the crude through C18 (or C8) high-pressure column to get bivalirudin exquisite article. In this invention, the peptide yield of every step is more than 99%, and the total yield is 14%.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for synthesizing triptorelin from solid phase polypeptide
ActiveCN101357936AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionSide chainFreeze-drying
The invention discloses a preparation method of solid phase peptide synthesis triptorelin, which includes the following steps: with Rink Amide AM resins or Rink Amide MBHA resins as starting materials, amino acids with protective groups are sequentially connected according to solid phase synthesis, so as to obtain protective decapeptide resins, and meanwhile crude products are obtained by sequentially removing Fmoc-protective groups and synchronously removing side-chain protective groups and cutting peptides, and triptorelin elaborate products are prepared after the crude products are separated and purified by C18 (or C8 ) column and freeze-dried. The preparation method is stable in technology, convenient in raw and auxiliary material sources, short in production cycle, high in yield, stable in quality, low in production cost and high in transpeptidase yield. Besides, as the preparation method avoids using poisonous reagents, such as hydrogen fluoride, and the like, the pollution of three wastes is low, purification yield is over 25 percent and each step of transpeptidase yield is above 98 percent; the yield after cutting peptides is 78.8 percent and the total yield is 25.4 percent.
Owner:SHANGHAI SOHO YIMING PHARMA
Preparation method of synthesizing octriotide from solid phase polypeptide
ActiveCN1923849AConvenient sourceReduce usagePeptidesBulk chemical productionHydrogen fluorideSide chain
The invention discloses an aoqu-peptide preparing method of solid-phase polypeptide, which comprises the following steps: adopting 2-chloride-trityl resin, 4-methyl trityl resin or 4-methoxyl trityl resin as raw material; connecting amino acid with protective group according to solid-phase synthetic method; obtaining protected octapeptide resin; removing Fmoc-protective group sequently; stripping side-chain protective group; cutting peptide to obtain reduced aoqu-peptide; oxidizing through air under pH 7-11 condition; separating and purifying rought product through C18 (C8) column to produce exquisite.
Owner:SHANGHAI SOHO YIMING PHARMA
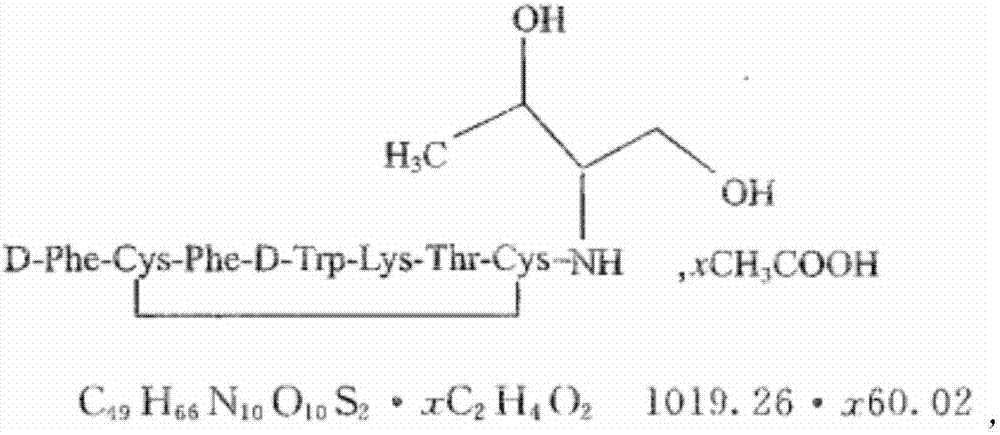
Solid-phase synthesis method of ularitide
InactiveCN103145827AEasy to operateProcess stabilityPeptide preparation methodsDepsipeptidesSide chainAmino acid
The invention relates to a method for synthesizing ularitide by a solid phase and mainly solves the technical problem that a suitable industrial method for preparing the ularitide does not exist. The solid-phase synthesis method disclosed by the invention mainly comprises the following steps of: 1) taking Fmoc-Tyr(tbu)-Wang resin as starting resin; gradually coupling each protected amino acid by adopting a Fmoc solid-phase synthesis method to synthesize a peptide chain tree which is wholly protected by a side chain; 2) cutting the peptide chain tree which is wholly protected by the side chain by utilizing a cutting reagent; and cracking 32 peptide from the resin and removing a side chain protective group to obtain a straight-chain peptide coarse product of the ularitide; and 3) dissolving the straight-chain peptide coarse product of the ularitide in water and carrying out air oxidization to form a ring to obtain the coarse peptide of the ularitide. The method disclosed by the invention has the advantages of large-scale production capability, simplicity in operation, stability of process and low production cost; and the total yield exceeds 20%.
Owner:GL BIOCHEM SHANGHAI +2
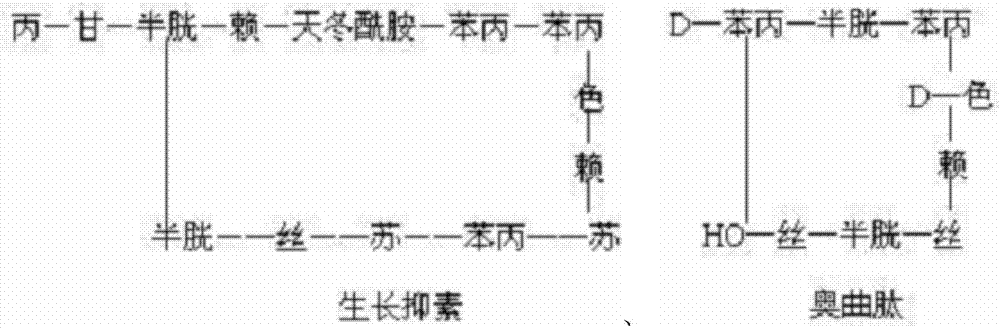
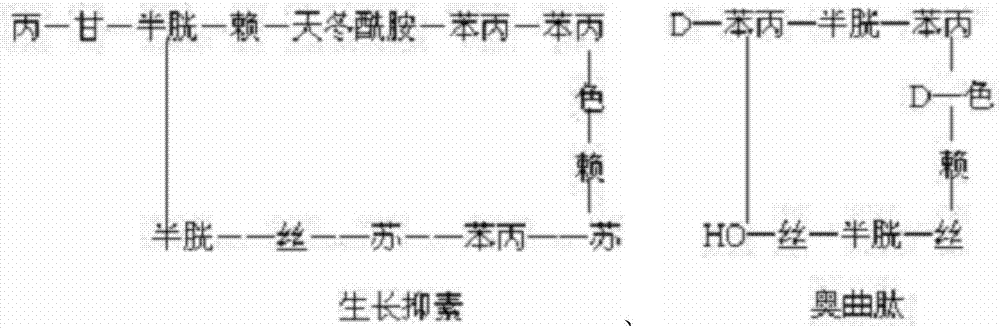
Preparation method of synthesizing growth chalone from solid phase polypeptide
InactiveCN1923851AConvenient sourceReduce usageSomatostatinsPeptide preparation methodsHydrogen fluorideSomatomedin
The invention discloses a chalone preparing method of solid-phase polypeptide, which comprises the following steps: adopting 2-chloride-trityl resin, 4-methyl trityl resin or 4-methoxyl trityl resin as raw material; connecting amino acid with protective group according to solid-phase synthetic method; obtaining protected octapeptide resin; removing Fmoc-protective group sequently; stripping side-chain protective group; cutting peptide to obtain reduced aoqu-peptide; oxidizing through air under pH 7-11 condition; separating and purifying rought product through C18 (C8) column to produce exquisite.
Owner:SHANGHAI SOHO YIMING PHARMA +1
Electrode, electrolyte thin layer and preparation method thereof
InactiveCN112467074AReduce contentGood solid contactFinal product manufactureCell electrodesSolid state electrolyteManufacturing technology
The invention relates to the technical field of secondary batteries, in particular to an electrode, an electrolyte thin layer and a preparation method thereof. An electrode is prepared from a halide solid electrolyte material, the solid electrolyte material is LiaMXb, M is one or more of Al, Ga, In, Sc, Y and La series, X is one or more of F, Cl and Br, a is greater than or equal to 0 and less than or equal to 10, and b is greater than or equal to 1 and less than or equal to 13. According to the invention, the ionic conductivity, the chemical / electrochemical stability and the plasticity can beremarkably improved; and in the manufacturing process of the electrode and the electrolyte, inert atmosphere protection is not needed, the method is very compatible with a traditional electrode manufacturing technology, the process is simple, the cost is low, the large-scale production capacity is extremely achieved, and therefore the commercial application value is extremely achieved.
Owner:CHINA AUTOMOTIVE BATTERY RES INST CO LTD +1
Polypeptide synthesis method for octreotide acetate
ActiveCN103351426AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFluoroacetic acidAceric acid
The invention relates to a polypeptide synthesis method for octreotide acetate. The method comprises the following steps of: taking chloromethyl resin as a starting raw material, preparing a cesium salt from Boc-Thr(tBu)-OH, sequentially connecting amino acids with protecting groups according to a solid-phase synthesis method so as to obtain protected octapeptide resin, meanwhile, removing Boc protecting groups by sequentially using HCl / isopropyl alcohol, carrying out peptide connecting reaction in a manner of taking DIC and HOBT as condensing agents, carrying out reduction by using palladium carbon / hydrogen gas, meanwhile, cutting off peptide chains so as to obtain reduced octreotide, introducing air at the Ph of 7.8-9 so as to cyclize disulfide linkages, then, obtaining a crude octreotide product, and carrying out separation and purification through a C18 column, thereby preparing a fine octreotide acetate product. The method disclosed by the invention has the advantages that threoninol and Fmoc-threoninol are not adopted, the production cost is very low, the method has large-scale production capacity, the process is stable, the raw and auxiliary materials are convenient to obtain, the production cycle is short, the yield of connected peptide is high, the quality is stable, the use of highly-toxic reagents, such as hydrogen fluoride, trifluoroacetic acid and the like, is avoided, and the pollution caused by waste gas, waste water and waste residues is little.
Owner:SHANGHAI SOHO YIMING PHARMA
Solid phase polypeptide synthesis preparation method for salcatonin
ActiveCN1865283AReduce usageWith large-scale production capacityCalcitoninsPeptide preparation methodsSolid phasesSide chain
The invention discloses a new salmon calcimar preparing method of Fmoc-strategy solid-phase polypeptide, which comprises the following steps: a. adopting Fmoc-Rink Amide MBHA or Rink Amide AM resin to connect the 32-piptide resin protected by kinds of amino acid after eluting Fmoc-protection sequently; b. eluting Fmoc-protective group sequently; c. removing side-chain protective group and cutting peptide synchronously to obtain reduced crude product; d. making crude product to do oxidation reaction through (7.5-10.0 pH) air; proceeding inverse-phase HPLC separation and purifying to produce fine salmon calcimar. The invention possesses scale producing capacity, which improves the quality, obtaining rate, and total obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
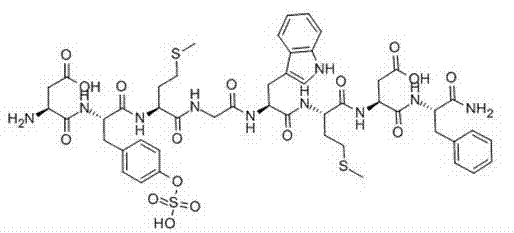
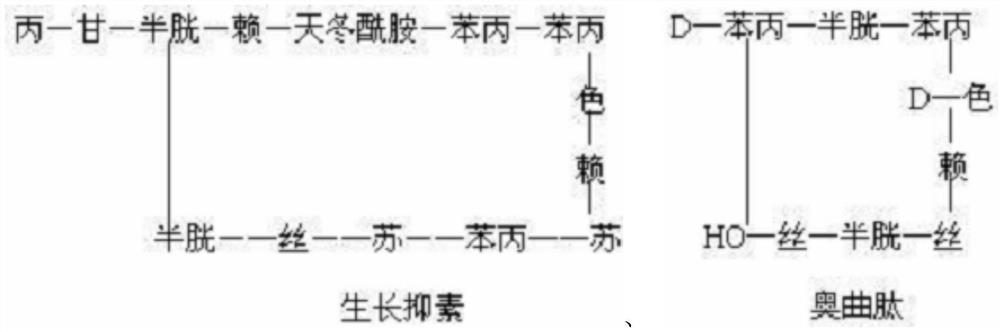
Method for preparing somatostatin through solid-phase peptide synthesis
InactiveCN102952175AWith large-scale production capacityProcess stabilityPeptide preparation methodsBulk chemical productionAlcoholSide chain
The invention provides a method for preparing somatostatin through solid-phase peptide synthesis. The method comprises the steps that: 2-chloro triphenyl alcohol resin is adopted as an initial raw material; amino acids with protective groups are sequentially connected with a solid-phase synthesis method; Fmoc- protective groups are sequentially removed; a peptide grafting reaction is carried out with TBTU and HOBT as condensing agents; after protected reduction-type 14-peptide resin is obtained, side-chain protective group removing and peptide cutting are carried out simultaneously, such that reduction-type somatostatin is obtained; the reduction-type somatostatin is oxidized by using hydrogen peroxide under a pH of 7-9, such that a somatostatin crude product is obtained; separation purification is carried out by using C18 high-performance liquid column; lyophilization is carried out, such that somatostatin refined product is obtained. The method provided by the invention has the advantages of capability of large-scale production, stable process, low production cost, reduced three-waste, reduced by-product, stable quality, low production cost, and high market competitiveness.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for preparing immune seven peptides
Owner:程云 +1
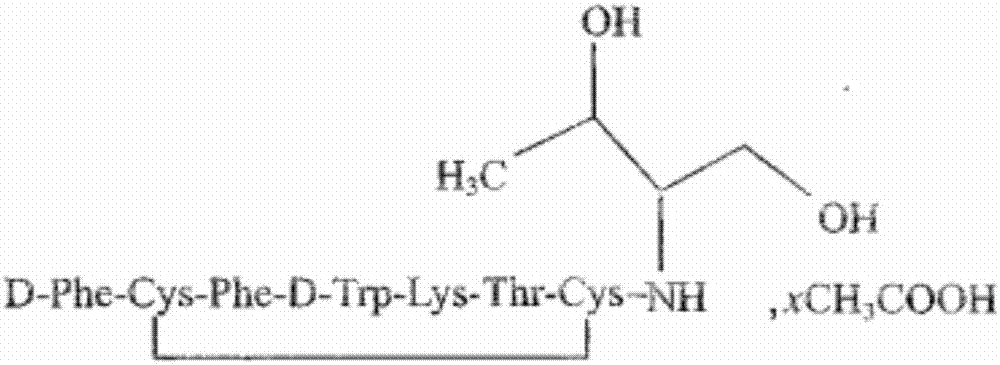
Preparation of octreotide acetate and octreotide acetate injection pharmaceutical composition
ActiveCN106866788AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemDisulfide bondSolid-phase synthesis
The invention relates to preparation of octreotide acetate and an octreotide acetate injection pharmaceutical composition. Specifically, the invention relates to a method for preparing the octreotide acetate; the method comprises the following steps: taking merrifield resin as a starting raw material and preparing Boc-Thr(tBu)-OH into cesium salt; sequentially connecting amino acid with protecting groups according to a solid-phase synthesis method to obtain protected octapeptide resin; removing Boc-protecting groups in sequence by utilizing HCl / isopropyl alcohol and carrying out peptide linking reaction by utilizing a condensing agent; reducing with palladium-carbon / hydrogen; meanwhile, chopping off a peptide chain to obtain reduced octreotide; ventilating air under the condition that the pH (Potential of Hydrogen) is 8 to 9 to form a ring by a disulfide bond, so as to obtain an octreotide crude product; separating and purifying the octreotide crude product through a C18 column to prepare refined octreotide. The invention further relates to the octreotide acetate injection pharmaceutical composition. The method for preparing the octreotide acetate and the octreotide acetate injection pharmaceutical composition, provided by the invention, have the excellent technical effects shown in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
Thin-layer self-control static solid fermentation integrated optimizing method and device thereof
InactiveCN103966084ALarge capacityIncrease the areaBioreactor/fermenter combinationsBiological substance pretreatmentsAutomatic controlAir volume
The invention relates to a thin-layer self-control static solid fermentation integrated optimizing method and a device thereof. To solve the problems existing in the prior art, the method comprises the following steps: paving solid fermentation materials, which have been sterilized and inoculated, on a multi-layer movable slat fermentation bed in a sterile fermentation room to form a thin layer at a time, automatically controlling the sterile air volume, temperature and humidity to carry out solid fermentation according to the different fermentation phases, starting the driving device of the multi-layer movable slat fermentation bed to move the materials out of the fermentation room after the fermentation of the solid materials is finished, and finally carrying out a product post-treatment. The device comprises a sterile air adjusting system, a movable bacterium sterilizing and inoculating tank, a spiral paving machine, a multi-layer movable slat fermentation bed, and a sterile fermentation room. The method and device have the following advantages: (1) the hyphae of zymocyte are not easy to break, the growth of zymocyte is good, and the device is especially good for the enzyme-producing and enzymolysis solid fermentation; (2) the massive production bottleneck of thin-layer static solid fermentation is broken down, the industrial scale-production degree is high, the equipment investment is little, and the production efficiency is high.
Owner:徐少云
Process of preparing calf thymus alphal
ActiveCN101033248AReduce usageWith large-scale production capacityPeptide preparation methodsAnimals/human peptidesAcetic anhydrideSide chain
The invention discloses a new technology to prepare calf thymus alpha 1 (natural existing in thymus cells of calf and other animals), which uses Fmoc-strategy solid phase method, including the following steps: a. taking any one of Rink Amide PEGA resin, Rink Amide AM resin, Rink Amide MBHA resin or Rink Amide PEGA as the starting material, using the amino acid protected by Fmoc as the monomer to hang resin, connecting amino acids in turn to get a protective 28-peptide resin according to the method of solid-phase synthesis, b. using acetic anhydride to close heads during the process in turn, c. removing Fmoc-protective group in turn, d. synchronizing the removal of side-chain protecting group and the cut of peptide to get a crude, e. purifying the crude with antiphase HPLC to get thymosin alpha 1.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for synthesizing cholecystokinin octapeptide by combining solid phase method and liquid phase method
ActiveCN102775471AReduce pollutionReduce investmentPeptide preparation methodsFreeze-dryingSynthesis methods
The invention relates to a preparation method of cholecystokinin octapeptide, in particular to a method for synthesizing the cholecystokinin octapeptide by combining a solid phase method and a liquid phase method. The method mainly solves the technical problem that the existing synthesis method is troublesome in separation of intermediate products, long in preparation period, apt to produce by-products in reaction, high in cost, low in yield and the like. The technical scheme is that the synthesis method comprises the following steps of: (1) synthesizing L-aspartyl-4-tertiary butyl ester-benzene propanamide by using the liquid phase method; (2) synthesizing cholecystokinin octapeptide full-protection fragments by using the solid phase method; (3) carrying out weak acid cutting on the full-protection fragments; (4) carrying out liquid phase condensation on the full-protection fragments and dipeptide fragments to obtain full-protection cholecystokinin octapeptide; (5) cutting, adding the full-protection cholecystokinin octapeptide in cutting fluid for cutting, and then adding ice diethyl ether for sediment to obtain cholecystokinin octapeptide crude products; and (6) purifying the crude products through high-phase liquid chromatogram, preparing, rotatably steaming, carrying out freeze-drying to obtain the cholecystokinin octapeptide competitive products. The method is used for preparing the cholecystokinin octapeptide.
Owner:GL BIOCHEM SHANGHAI +2
Octreotide acetate injection pharmaceutical composition and octreotide acetate
ActiveCN106860854AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemOctreotide acetateSodium bicarbonate
The invention relates to an octreotide acetate injection pharmaceutical composition and octreotide acetate. Specifically, the octreotide acetate injection pharmaceutical composition provided by the invention is prepared from octreotide acetate containing 0.1g of octreotide, 40g to 50g of mannitol, 3g to 4g of lactic acid, a proper amount of sodium hydrogen carbonate which is added until the pH (Potential of Hydrogen) is 3.7 to 4.7 and a proper amount of injection water which is added until the total amount is 1000ml and the like. The invention further relates to an octreotide acetate product and a preparation method thereof. The octreotide acetate injection pharmaceutical composition and the octreotide acetate, provided by the invention, have the excellent technical effects shown in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
Homogenous PN (positive-negative) junction on basis of two-dimensional semiconductor materials and method for preparing homogenous PN junction
ActiveCN108807553AImprove stabilityEnhanced tunneling electric fieldSemiconductor/solid-state device manufacturingSemiconductor devicesSemiconductor materialsWork function
The invention discloses a homogenous PN (positive-negative) junction on the basis of two-dimensional semiconductor materials and a method for preparing the homogenous PN junction. By the aid of the homogenous PN junction and the method, the problems of N-type doping on semiconductor materials with low Fermi energy levels and P-type doping on semiconductor materials with high Fermi energy levels due to the fact that electrons can be transferred to the materials with the low Fermi energy levels from the existing two-dimensional semiconductor materials with the high Fermi energy levels when the two types of semiconductor materials with different work functions are perpendicularly stacked can be solved. The homogenous PN junction and the method have the advantages that the homogenous abrupt PNjunction can be formed in the two-dimensional semiconductor materials by the aid of the doping method, band tails can be prevented from being led into forbidden bands, and the homogenous PN junctionand the method have important significance in electronic device application; the doping method is free of crystal lattice damage due to ion collision, the stability can be greatly enhanced, processesfor preparing the homogenous PN junction are simple, and the homogenous PN junction and the method are easy to popularize to large-scale production.
Owner:PEKING UNIV
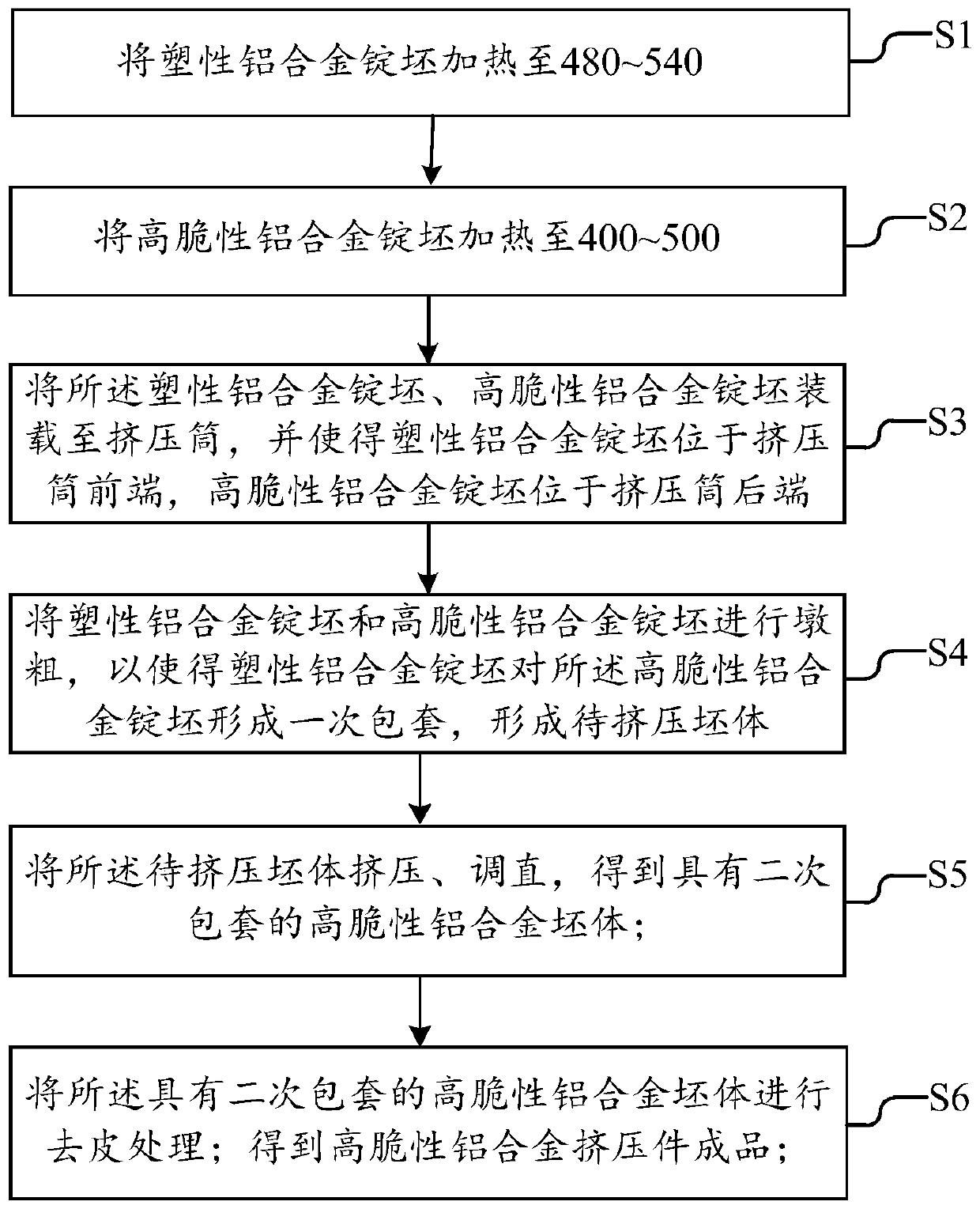
Extrusion method for high brittleness aluminum alloy and high brittleness aluminum alloy extrusion part
ActiveCN110434187AWith large-scale production capacityImprove extrusion yieldExtrusion control devicesIngotMaterials science
The invention discloses an extrusion method for a high brittleness aluminum alloy. The extrusion method comprises the following steps: heating and loading a plastic aluminum alloy ingot blank and a high brittleness aluminum alloy ingot blank to an extrusion barrel, wherein the plastic aluminum alloy ingot blank is located at the front end of the extrusion barrel and the high brittleness aluminum alloy ingot blank is located at the back end of the extrusion barrel; and carrying out upsetting, extruding, straightening and peeling to obtain a high brittleness aluminum alloy extrusion part product. By placing the plastic aluminum alloy ingot blank in front of the high brittleness aluminum alloy ingot blank and controlling the preheating temperatures of the plastic aluminum alloy ingot blank and the high brittleness aluminum alloy ingot blank, in the extruding process, an extrusion die is not in contact with the high brittleness aluminum alloy ingot blank, so that the extruding rate of finished products is increased. The process is simple, a package sheath is not manufactured separately, and the process can be fused with an existing common extrusion process, so that the high brittlenessaluminum alloy extrusion process has a large-scale production capacity.
Owner:GUANGDONG JMA ALUMINUM PROFILE FACTORY GRP +1
Solid phase polypeptide synthesis preparation method for leuprorelin
ActiveCN1865280BConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionHydrogen fluorideLeuprorelin
The invention discloses a bright-ala-ruilin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Wang resin or CTC resin as original material to connect amino with protective group to produce protective nonapeptide resin; removing Fmoc-protective group sequently; proceeding side-chain protective group synchronizingly and cutting peptide; connecting ethylaminethrough ethylamine-to-HOBT to produce crude product; proceeding separation and purifying through C18 (or C8) pillar to produce fine bright-ala-ruilin. The invention avoids the utility of poisonous agent, which improves the purifying, peptide connecting and obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
Octreotide acetate injection drug composition and application thereof
ActiveCN109432394AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemDrugChemistry
The invention relates to an octreotide acetate injection drug composition and application thereof. The octreotide acetate injection drug composition comprises 0.1g of octreotide acetate (by octreotide), 40-50g of mannitol, 3-4g of lactic acid, an appropriate amount of sodium hydrogen carbonate with the pH adjusted to 3.7-4.7, 1000ml of water for injection, and the like. The invention also discloses an octreotide acetate product and a preparation method thereof. The invention further relates to a method of determining impurities, such as oxidatively degraded impurities, in the octreotide acetate or the composition thereof. The octreotide acetate injection drug composition displays excellent technical effects as described in the description, for example, bulk drugs have higher production efficiency in the preparation process than those in the prior art, the drug loss during injection preparation is significantly reduced, and the growth rate of the impurities in long-term stability reserved samples is significantly lower than that in the prior art.
Owner:CHENGDU TIANTAISHAN PHARMA
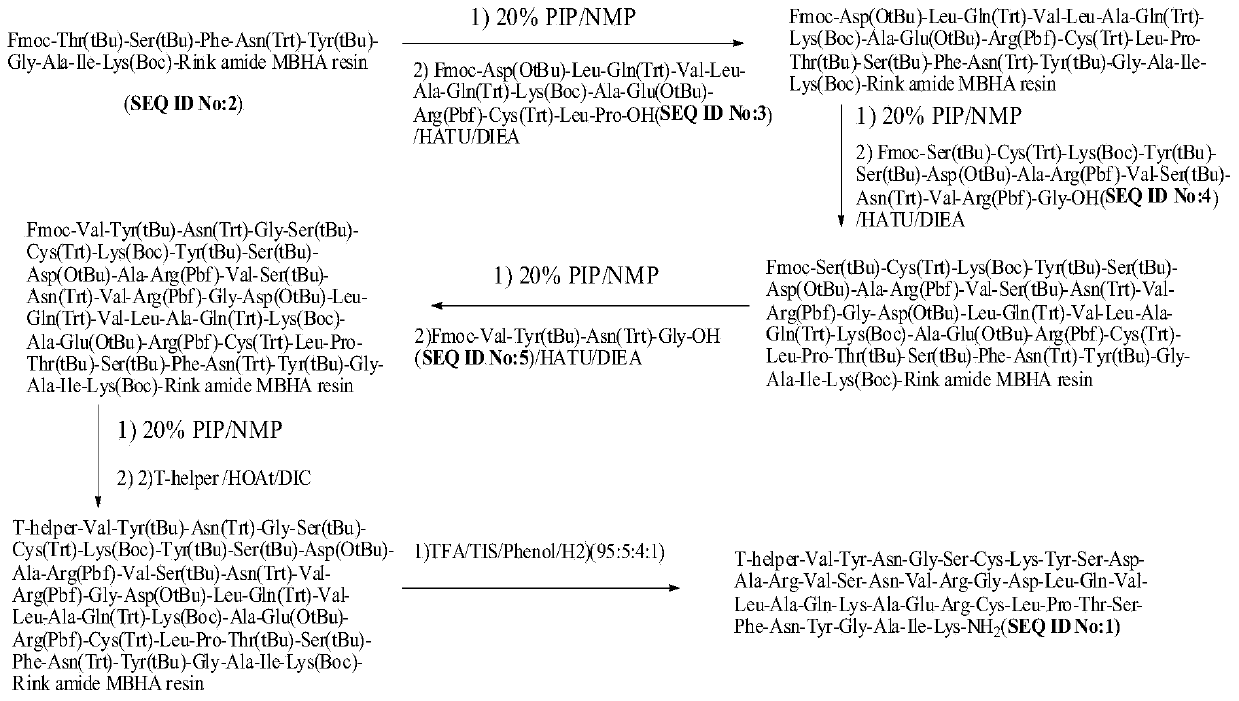
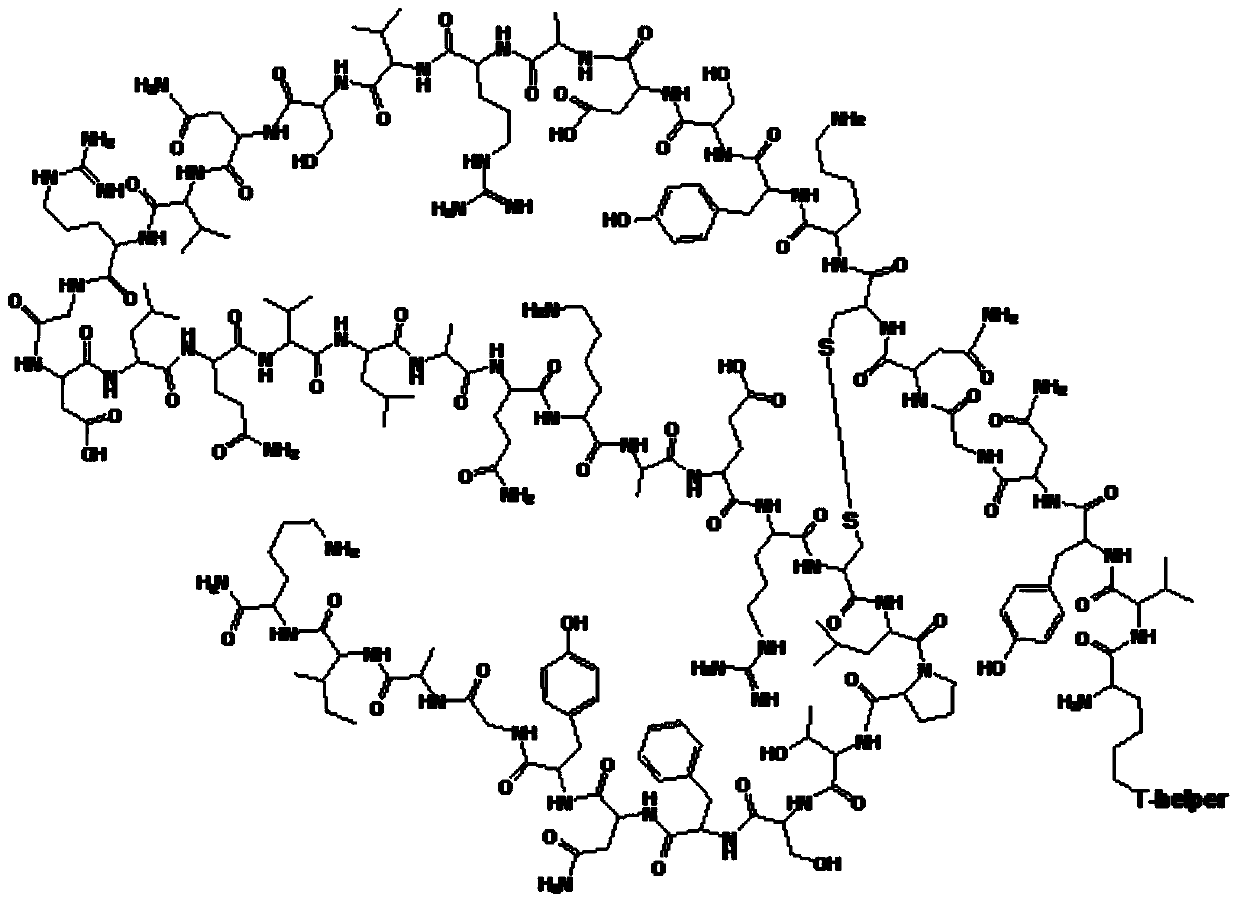
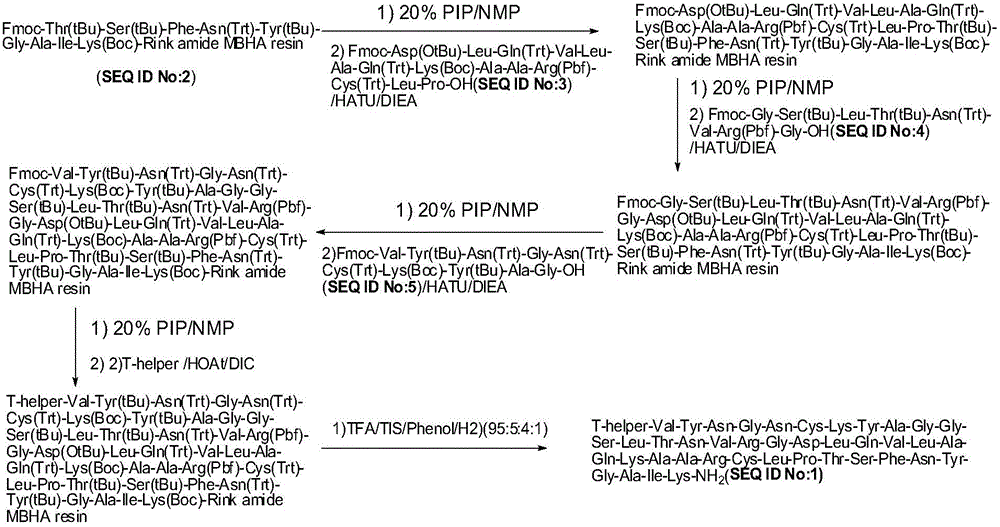
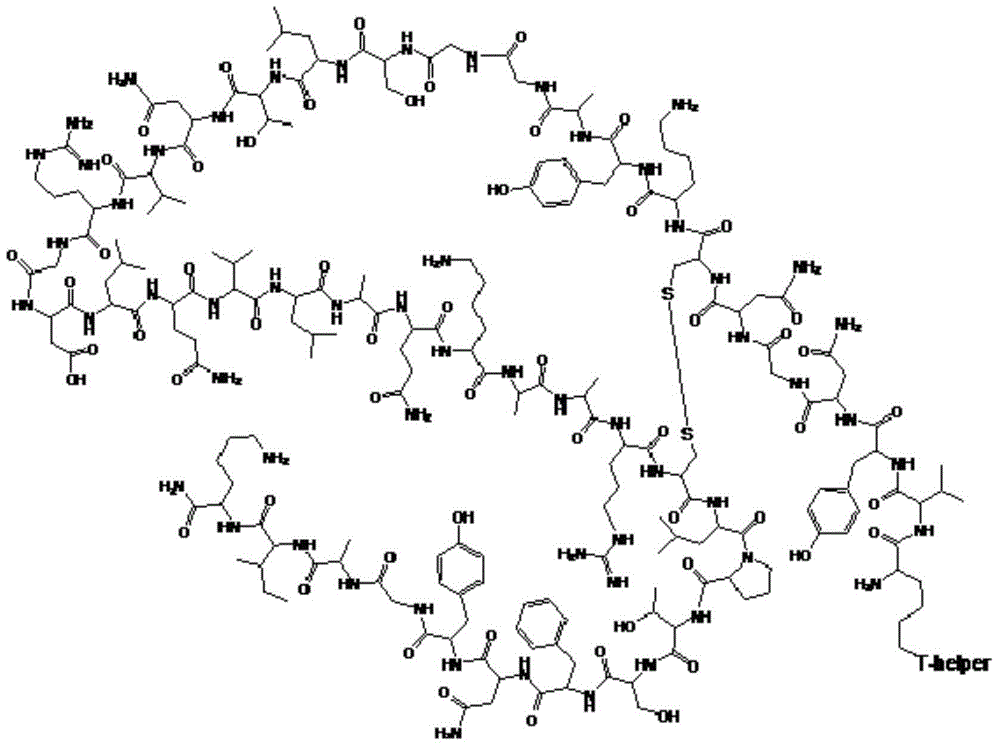
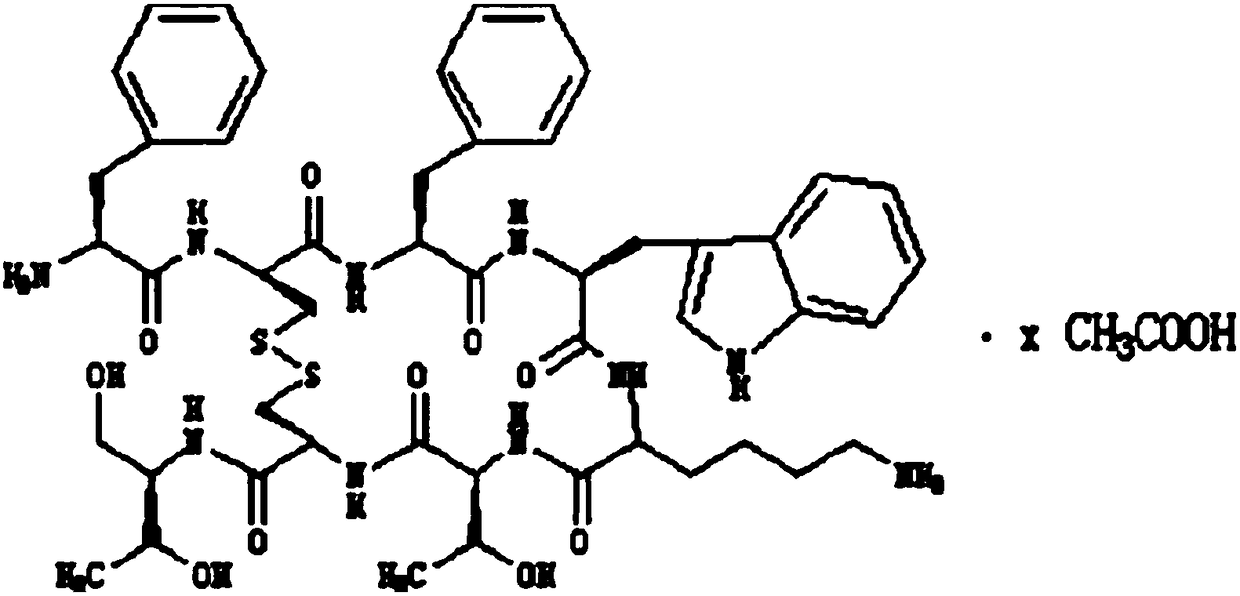
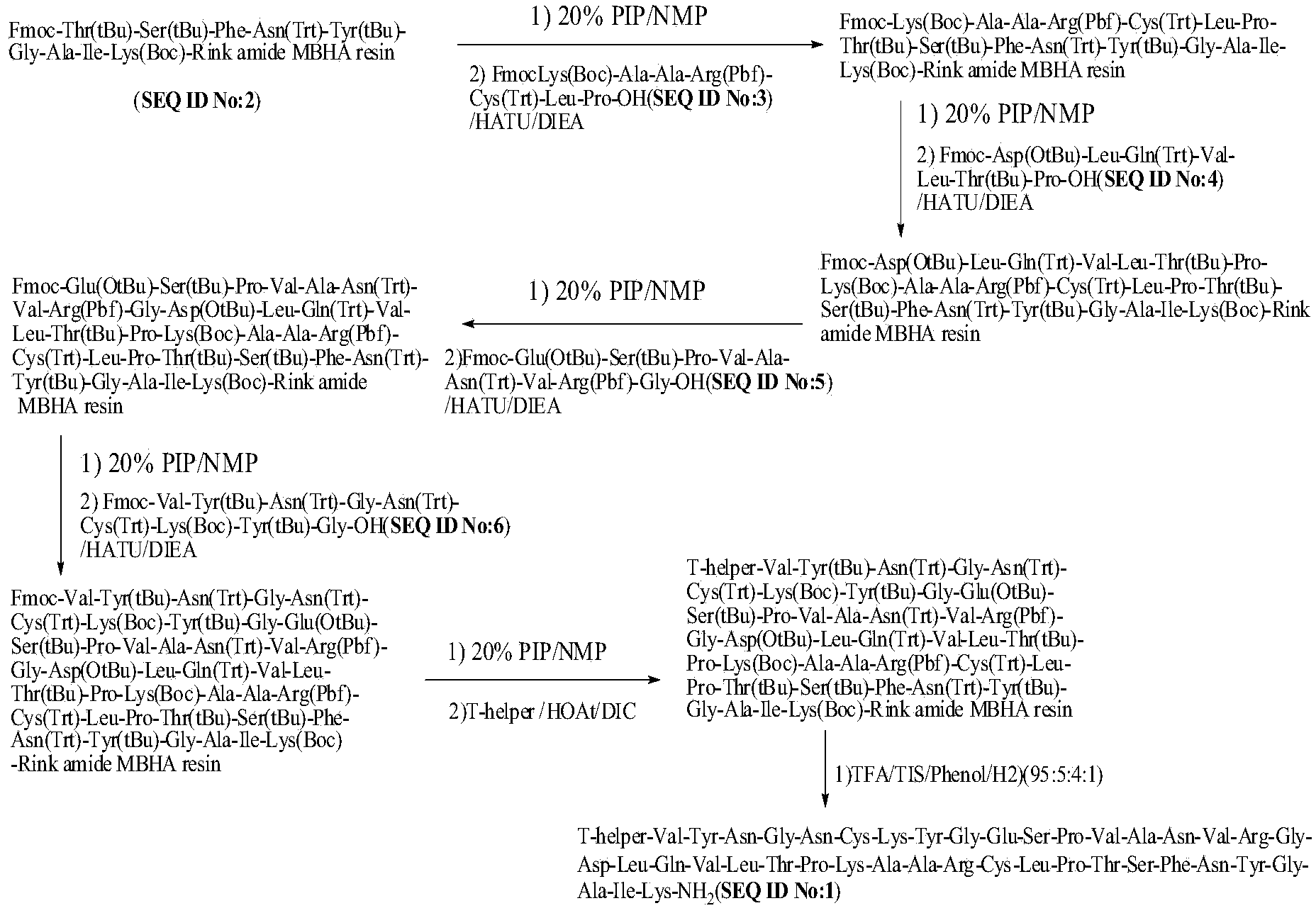
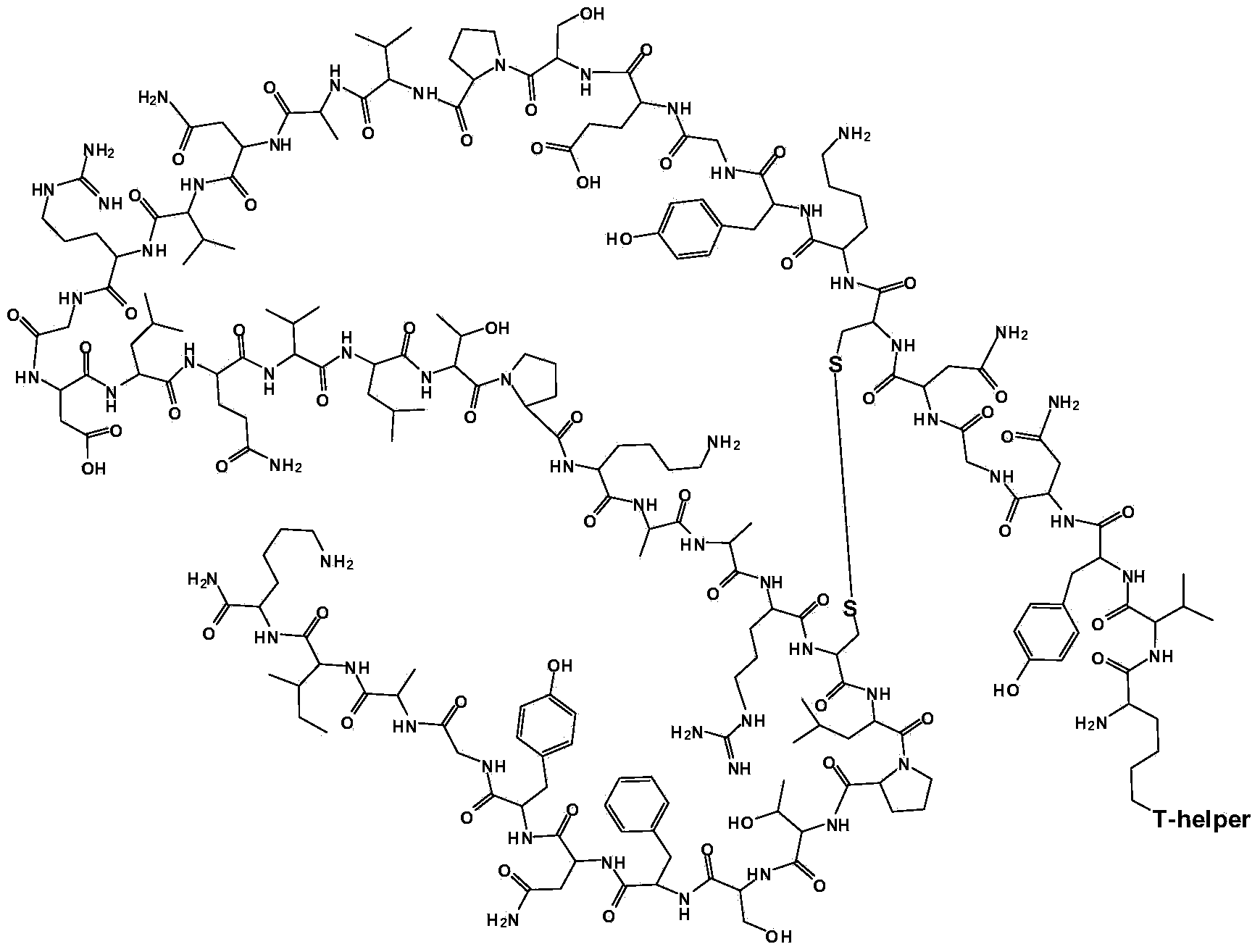
Method for preparing synthetic peptide antigen 2700 of swine O-type foot and mouth disease through solid-phase fragment process
ActiveCN104277097AWith large-scale production capacityProcess stabilitySsRNA viruses positive-senseVirus peptidesPeptide antigen9-fluorenylmethoxycarbonyl
The invention provides a method for preparing a synthetic peptide antigen 2700 of a swine O-type foot and mouth disease through a solid-phase fragment process. The method comprises the steps of with resin as a starting raw material, sequentially connecting 9-fluorenylmethoxycarbonyl protective amino acid to prepare a protected peptide fragment, meanwhile sequentially removing a Fmoc group, carrying out linker reaction by using a condensing agent, and cutting off the protected peptide fragment by using dilute acid or weak acid; connecting the fragment and 4-(4'-dimethoxy-9-fluorenylmethoxyaminomethyl)-phenyloxymethyl resin step by step, and then, connecting the fragment and a T helper cell epitope; removing a protecting group and the resin by using concentrated acid or strong acid to obtain a crude peptide of the synthetic peptide antigen 2700; and purifying by using ion exchange resin and a tangential flow film packaging system, and removing small molecules and salt through a concentration system to obtain the synthetic peptide antigen 2700. The method provided by the invention has the characteristics of stable process, short production period, convenience in obtaining raw and auxiliary materials, few wastewater, waste gas and waste residues, low production cost, high yield and the like.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Octreotide acetate injection pharmaceutical composition and octreotide acetate
ActiveCN106860854BConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemSodium bicarbonateOctreotide acetate
The invention relates to a pharmaceutical composition of octreotide acetate injection and octreotide acetate. Specifically, the pharmaceutical composition of octreotide acetate injection involved in the present invention consists of: 0.1 g of octreotide acetate calculated as octreotide, 40-50 g of mannitol, 3-4 g of lactic acid, an appropriate amount of sodium bicarbonate to adjust the pH to 3.7-4.7, water for injection Appropriate amount is added to 1000ml etc. The invention also relates to an octreotide acetate product and a preparation method thereof. The present invention presents excellent technical effects as described in the specification.
Owner:CHENGDU TIANTAISHAN PHARMA
Preparation method of synthesizing octriotide from solid phase polypeptide
ActiveCN1923849BConvenient sourceReduce usagePeptidesBulk chemical productionHydrogen fluorideSide chain
The invention discloses an aoqu-peptide preparing method of solid-phase polypeptide, which comprises the following steps: adopting 2-chloride-trityl resin, 4-methyl trityl resin or 4-methoxyl trityl resin as raw material; connecting amino acid with protective group according to solid-phase synthetic method; obtaining protected octapeptide resin; removing Fmoc-protective group sequently; strippingside-chain protective group; cutting peptide to obtain reduced aoqu-peptide; oxidizing through air under pH 7-11 condition; separating and purifying rought product through C18 (C8) column to produce exquisite.
Owner:SHANGHAI SOHO YIMING PHARMA
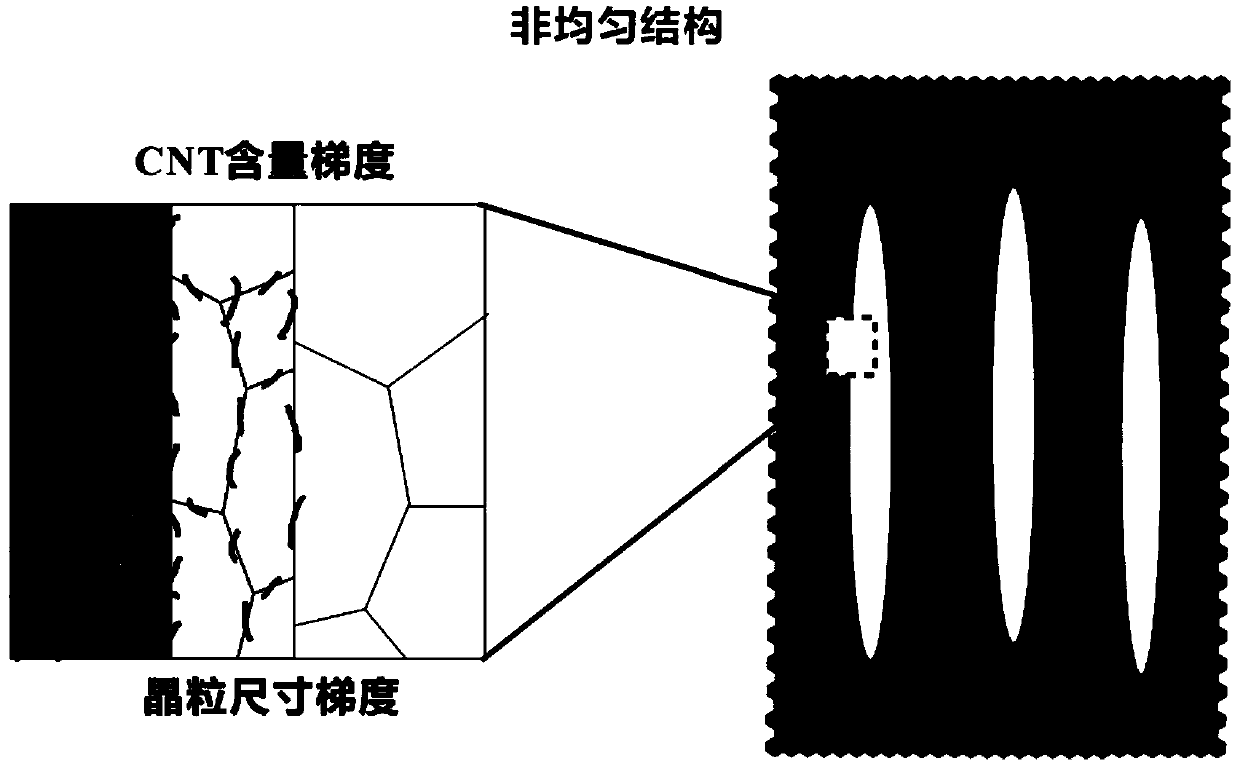
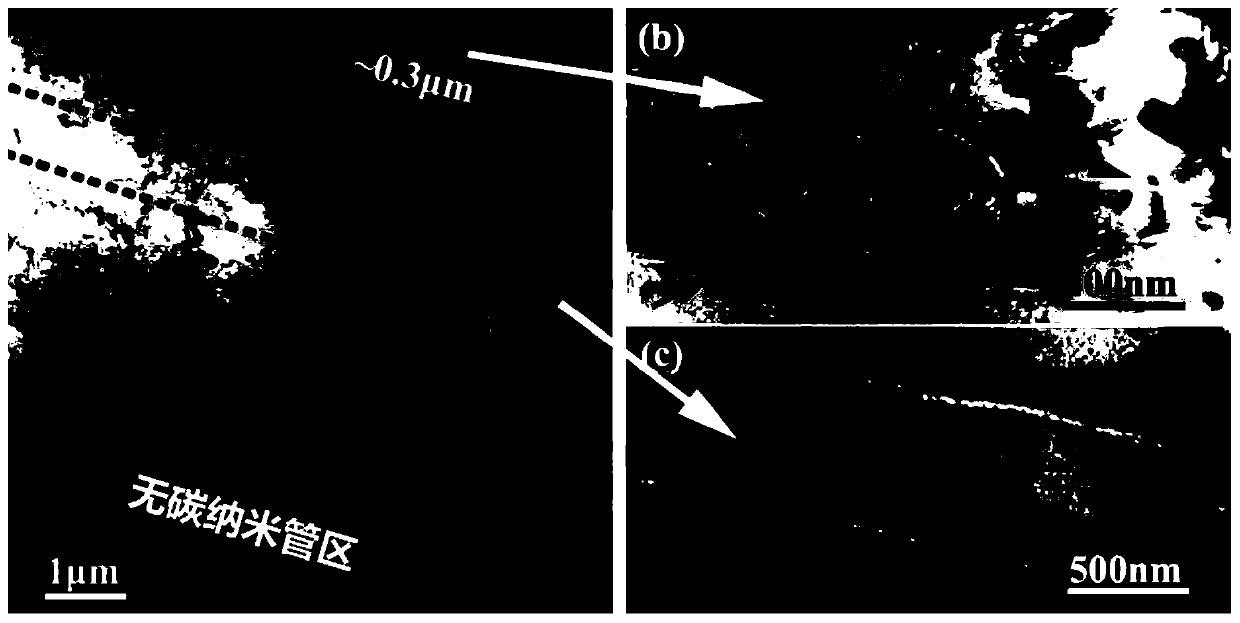
High-toughness carbon nanotube reinforced aluminum-based composite material with non-uniform structure and preparation method thereof
The invention discloses a high-toughness carbon nanotube reinforced aluminum-based composite material with a non-uniform structure and a preparation method thereof, and belongs to the technical fieldof composite material preparation. The preparation method comprises the following steps: pre-grinding high-content carbon nanotube / aluminum composite material powder in a ball milling process, addinglow-content carbon nanotube / aluminum composite material powder at set intervals, performing ball milling, and finally adding aluminum alloy powder, so that gradient change of the content of carbon nanotubes is formed in a composite material micro-area under the action of cold welding. In addition, the later added composite material powder is short in ball milling time and small in grain refinementdegree, so that grain size gradient distribution of the micro-area is formed. The powder is subjected to subsequent densification and secondary processing to obtain the final composite material, andthe toughness of the composite material is far higher than that of a composite material with a uniform structure.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Method for preparing pig O-type foot-and-mouth disease synthetic peptide antigen 2800 by using solid-phase fragment method
ActiveCN104311673AAvoid the risk of wrong connectionIncrease production capacityPeptide preparation methodsHybrid peptidesPeptide antigenCoating system
The invention provides a method for preparing pig O-type foot-and-mouth disease synthetic peptide antigen 2800 by using a solid-phase fragment method. The method comprises the following steps: by taking resin as an initial raw material, sequentially connecting amino acid with 9-fluorene methoxycarbonyl group protection, preparing a protected peptide fragment, sequentially removing 9-fluorene methoxycarbonyl groups in the period, performing peptide connection reaction by using a condensating agent, and cutting by using diluted acid or weak acid so as to obtain the protected peptide fragment; gradually connecting the fragment with 4-(4'-dimethoxy-9-fluorene methoxyamine methyl)-phenoxymethyl resin, and further connecting with T-auxiliary cell epitope; cutting off the protection groups and the resin by using acid so as to obtain synthetic peptide antigen 2800 coarse peptide; purifying by using ion exchange and a tangential flow membrane coating system, and concentrating to remove micromolecules and salt, thereby obtaining the synthetic peptide antigen 2800. The method provided by the invention has the characteristics of being stable in process, short in production period, convenient in obtaining raw / auxiliary materials, small in waste, low in production cost, high yield and the like.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Method for preparing octreotide acetate
ActiveCN109187804AConvenient sourceReduce usageComponent separationPeptide preparation methodsDisulfide bondImpurity
The invention relates to a method for preparing octreotide acetate, in particular to a method for preparing the octreotide acetate. The method comprises the following steps of preparing a cesium saltfrom Boc-Thr(tBu)-OH by taking merrifield resin as an initial raw material, sequentially connecting amino acids with protection groups to obtain protection octapeptide resin according to a method of solid phase synthesis, sequentially stripping a Boc-protection group by employing HC1 / isopropyl alcohol, and performing peptide grafting reaction by employing a condensing agent; reducing with palladium carbon / hydrogen, and simultaneously cutting a peptide chain to obtain reduced octreotide; allowing the reduced octreotide, an oxidizing agent and an additive to react to generate an octreotide roughproduct with looped disulfide bond; and performing separation and purification on the octreotide rough product with a C18 column to prepare an octreotide acetate refined product. The invention also relates to an octreotide acetate injection medium composition and a quality detection method thereof. The method shows an excellent technological effect; and compared with a traditional method, the method has the advantages of high production efficiency, high yield and a few impurities.
Owner:成都天台山制药股份有限公司
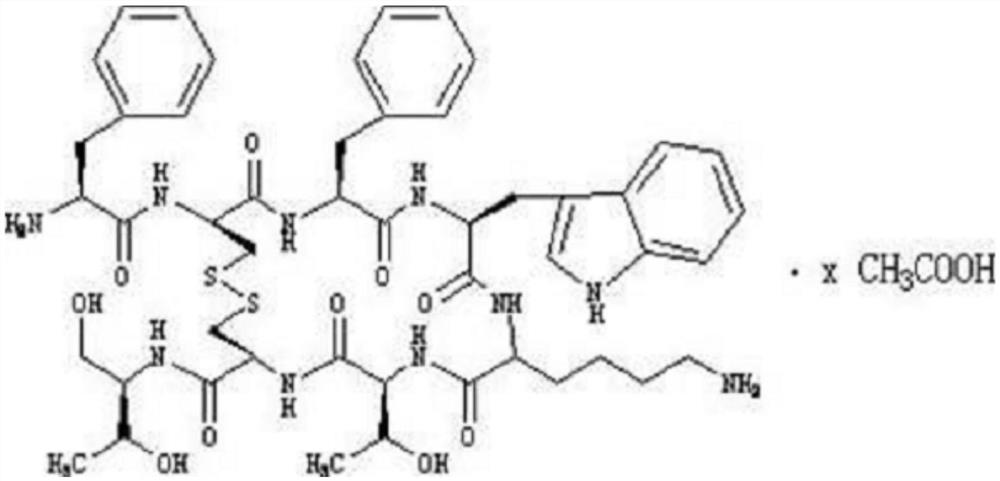
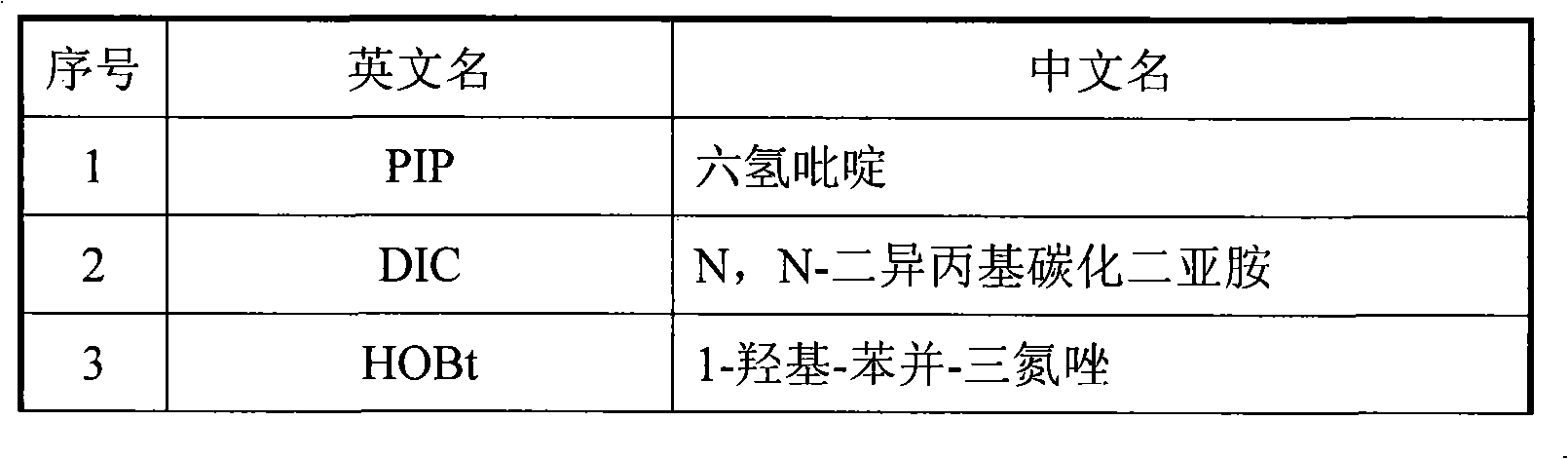
Method for solid phase polypeptide synthesis of hexarelin
ActiveCN101314612ALarge-scale production capacityProcess stabilityPeptide preparation methodsBulk chemical productionSolid phasesChromatography column
The invention relates to a preparation method of hexarelin, and mainly solves the technical problems with long synthetic period and applications of hypertoxic substances in the prior synthetic method. The method for synthesizing the hexarelin by adopting the solid phase polypeptide synthetic method comprises the steps as follows: Fmoc-Linker-AM-Resin or Fmoc-Linker-MBHA-Resin is adopted as the initial raw material; amino acids with Fmoc protective groups are sequentially connected according to the solid phase polypeptide synthetic method to obtain a protected six peptide resin, while the Fmoc protective groups are sequentially removed and a peptide synthetic reaction is carried out in the presence of one of DIC / HOBt, DIC / HOAt, BOP / HOBt, BOP / HOAt, HBTU / HOBt, HBTU / HOAt, HATU / HOBt, HATU / HOAt or HCTU / HOBt as a condensing agent; side chain protective groups removal and peptide cutting are carried out synchronously to obtain a coarse product; the coarse product is subjected to separation and purification to obtain the hexarelin which is further subjected to lyophilization to obtain hexarelin acetate or trifluoroacetate product. The method is applicable to the large-scale hexarelin production.
Owner:GL BIOCHEM SHANGHAI
Method for preparing CW7213 by polypeptide solid-phase synthesis
InactiveCN102174101AReduce usageConvenient sourceMetabolism disorderPeptide preparation methodsSide chainWang resin
The invention provides a method for preparing CW7213 by polypeptide solid-phase synthesis, which comprises the following steps of: with Fmoc-Gly-WANG resin as an initial raw material, Fmoc protected amino acid as a monomer and HBTU (Benzotriazole-N,N,N'N'-hexafluorophosphate) / HoBT (1-Hydroxybenzotrizole) as a condensing agent, charging nitrogen in a sand core funnel for gradually connecting amino acids to obtain protected 24 peptide resin; removing Fmoc protecting groups in sequence; synchronously removing side chain protecting groups and cutting peptide and adding diethyl ether for sedimenting to obtain a crude product of CW7213; subjecting the crude product to separation and purification by a C18 column; and carrying out freezing and drying to obtain a product (comprising medically available salts, such as acetate, trifluoroacetate, and the like). The method provided by the invention has the advantages of stable and mature process, convenience of raw and auxiliary material sources, high peptide yield, less pollution and convenience in industrialized construction and can avoid the use of virulent reagents, such as hydrogen fluoride, and the like.
Owner:CHINA PHARM UNIV
Method for preparing synthetic peptide antigen 2600 of swine O-type foot and mouth disease through solid-phase fragment process
ActiveCN104277098AWith large-scale production capacityProcess stabilitySsRNA viruses positive-senseVirus peptidesPeptide antigenStrong acids
The invention provides a method for preparing a synthetic peptide antigen 2600 of a swine O-type foot and mouth disease through a solid-phase fragment process. The method comprises the steps of with resin as a raw material, sequentially connecting 9-fluorenylmethoxycarbonyl protective amino acid to prepare a protected peptide fragment, meanwhile, sequentially removing a 9-fluorenylmethoxycarbonyl protecting group, carrying out linker reaction by using a condensing agent, and cutting off the protected peptide fragment by using dilute acid or weak acid; connecting the fragment and 4-(4'-dimethoxy-9-fluorenylmethoxyaminomethyl)-phenyloxymethyl resin step by step, and then, connecting the fragment and a T helper cell epitope; removing the protecting group and the resin by using concentrated acid or strong acid to obtain a crude peptide of the synthetic peptide antigen 2600; and purifying by using ion exchange resin and a tangential flow film packaging system, and removing small molecules and salt through a concentration system to obtain the synthetic peptide antigen 2600. The method provided by the invention has the characteristics of stable process, short production period, convenience in obtaining raw and auxiliary materials, few wastewater, waste gas and waste residues, low production cost, high yield and the like.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Method for solid phase polypeptide synthesis of hexarelin
ActiveCN101314612BConvenient sourceWith large-scale production capacityPeptide preparation methodsBulk chemical productionSide chainTrifluoroacetic acid
The invention relates to a preparation method of hexarelin, and mainly solves the technical problems with long synthetic period and applications of hypertoxic substances in the prior synthetic method. The method for synthesizing the hexarelin by adopting the solid phase polypeptide synthetic method comprises the steps as follows: Fmoc-Linker-AM-Resin or Fmoc-Linker-MBHA-Resin is adopted as the initial raw material; amino acids with Fmoc protective groups are sequentially connected according to the solid phase polypeptide synthetic method to obtain a protected six peptide resin, while the Fmocprotective groups are sequentially removed and a peptide synthetic reaction is carried out in the presence of one of DIC / HOBt, DIC / HOAt, BOP / HOBt, BOP / HOAt, HBTU / HOBt, HBTU / HOAt, HATU / HOBt, HATU / HOAtor HCTU / HOBt as a condensing agent; side chain protective groups removal and peptide cutting are carried out synchronously to obtain a coarse product; the coarse product is subjected to separation and purification to obtain the hexarelin which is further subjected to lyophilization to obtain hexarelin acetate or trifluoroacetate product. The method is applicable to the large-scale hexarelin production.
Owner:GL BIOCHEM SHANGHAI
Integrated flexible sensor based on sandwich type spinning film and manufacturing method
PendingCN112923954AImprove conductivityHigh strengthNanosensorsConverting sensor output electrically/magneticallySemiconductor materialsComposite film
The invention relates to an integrated flexible sensor based on a sandwich type electrostatic spinning film and a manufacturing method. The integrated flexible sensor solves problems that in the prior art, sensors produced from metal and semiconductor materials are poor in flexibility and complex in production process. The integrated flexible sensor is made of the flexible composite material, the flexibility is good, and the production process is simple. The sensor comprises an upper conductive layer and a lower conductive layer, an insulating layer is arranged between the upper conductive layer and the lower conductive layer, the upper conductive layer, the lower conductive layer and the insulating layer adopt a composite film, the composite film comprises a flexible substrate, and MXene and silver nanowires as conductive components are dispersed in the flexible substrate of the upper conductive layer and the lower conductive layer. And at least one electrode is led out from the surfaces of the upper conducting layer and the lower conducting layer. The manufacturing method comprises the following steps: (1) preparing MXene and silver nanowires; (2) preparing a flexible matrix liquid; (3) preparing an electrostatic spinning precursor solution and performing electrostatic spinning; and (4) assembling the sandwich type integrated flexible sensor.
Owner:XIAN TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com