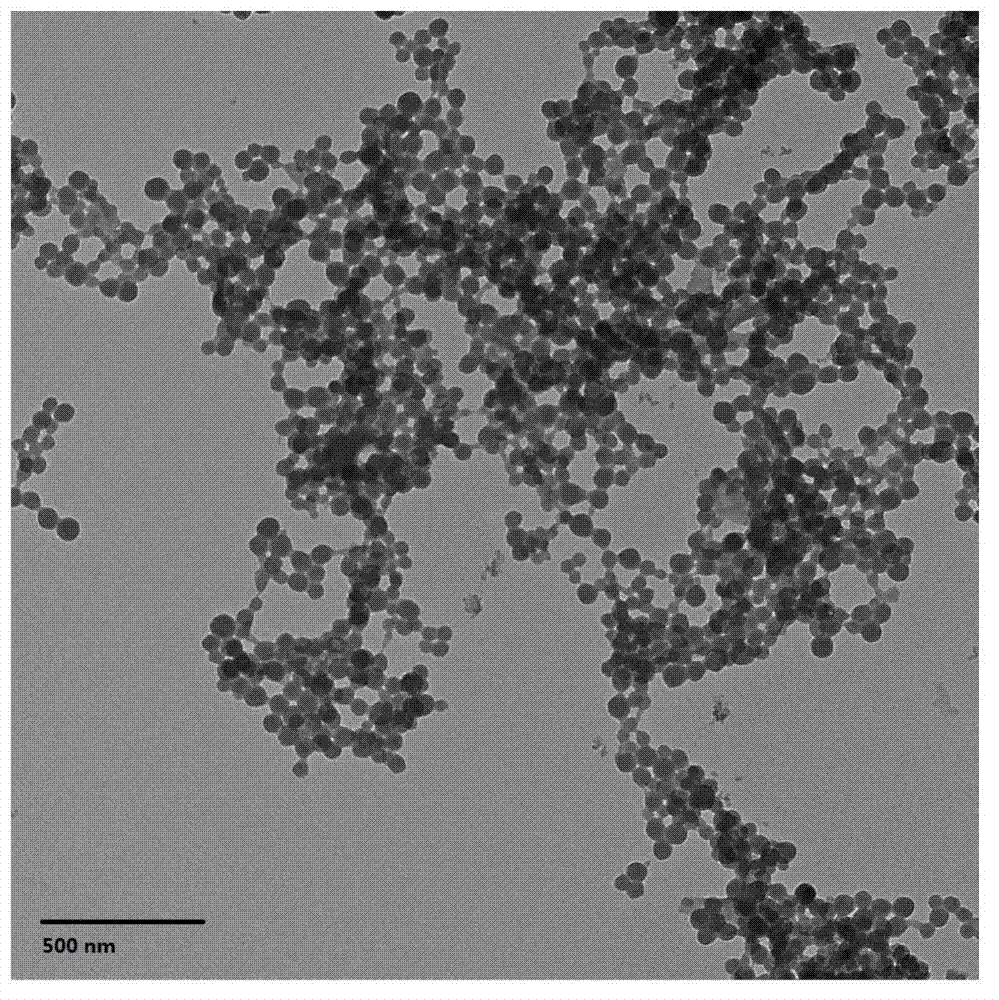
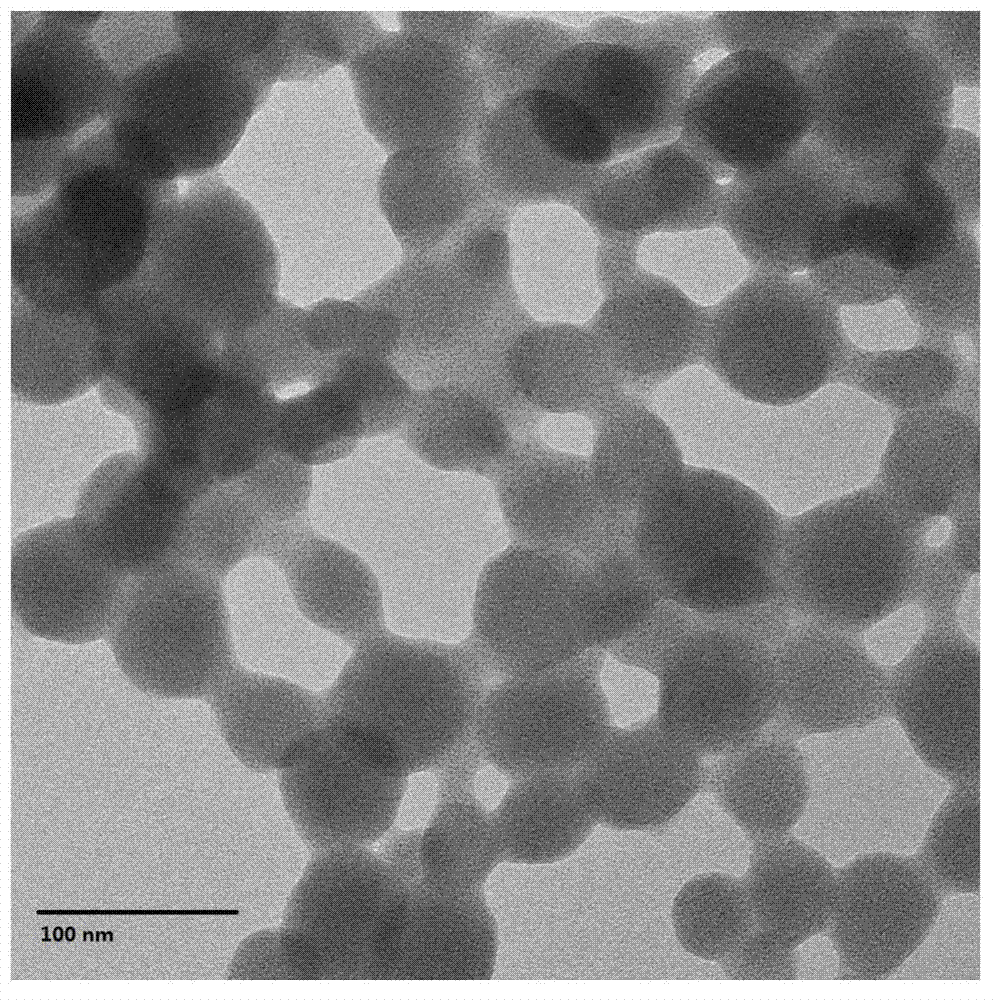
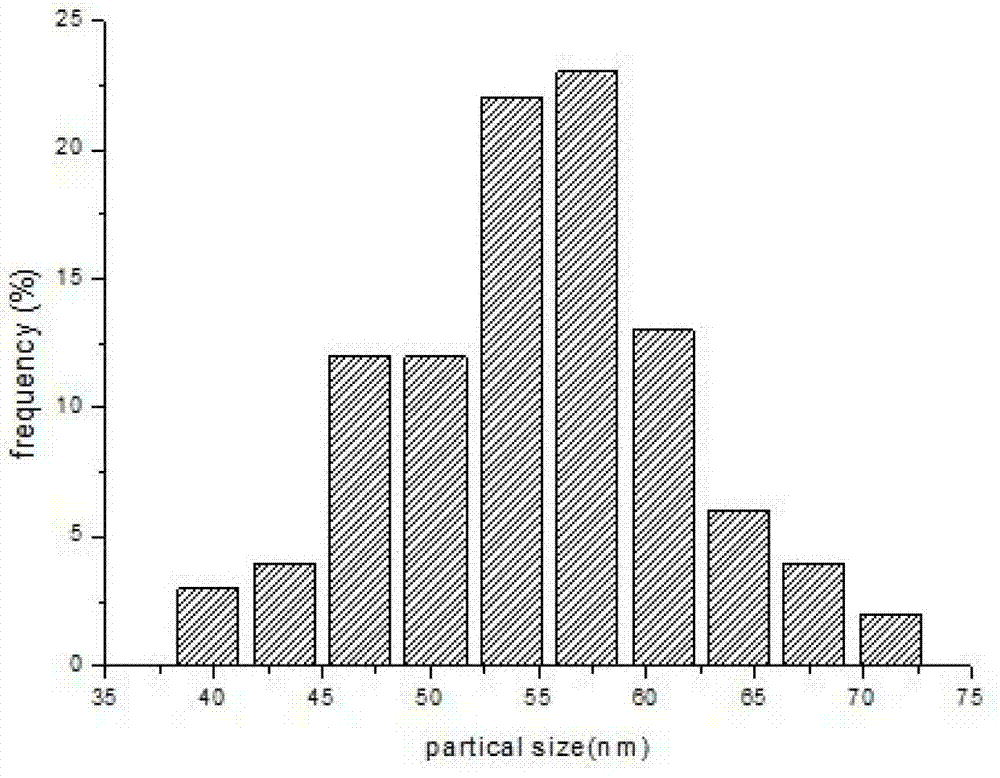
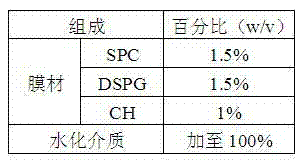
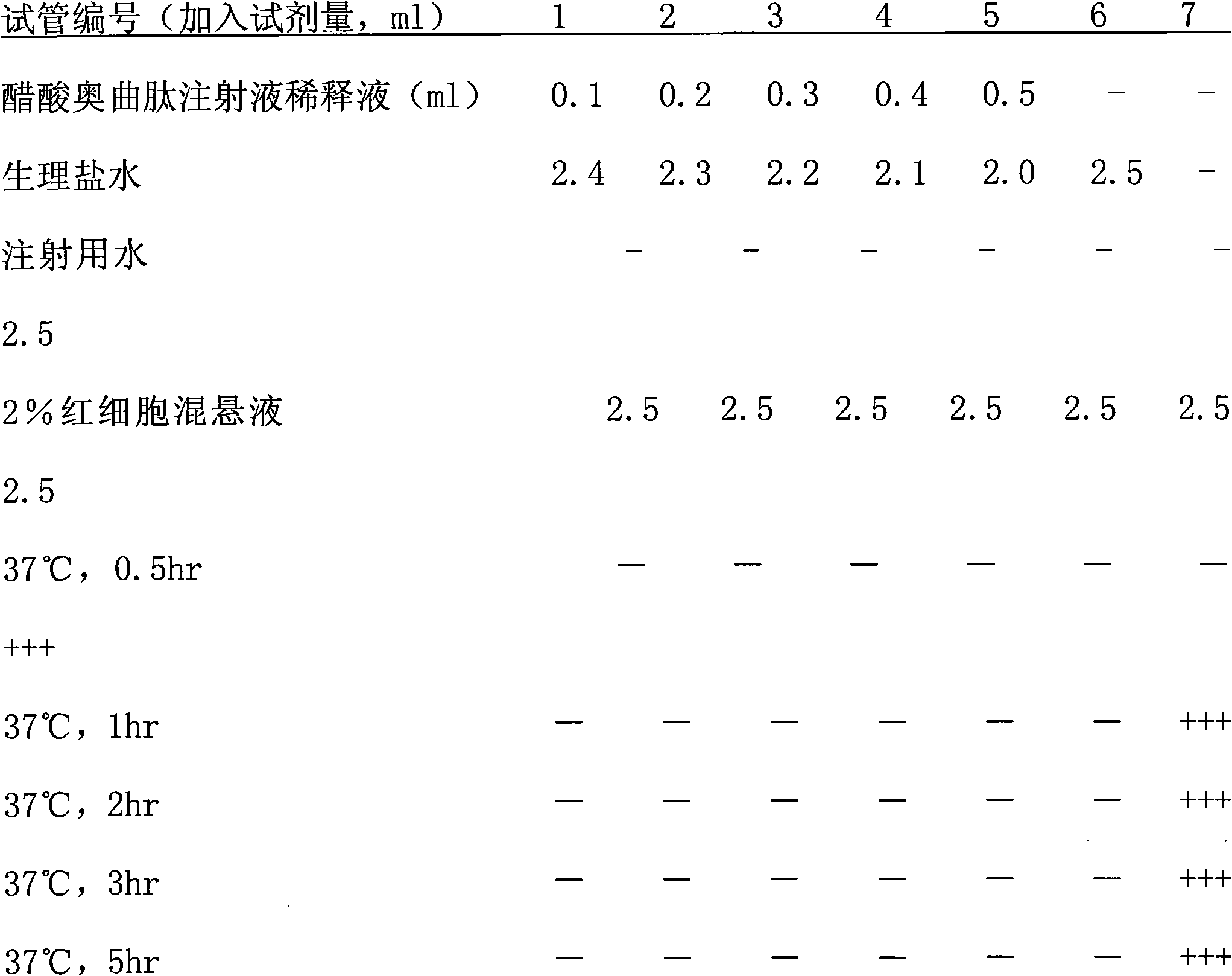
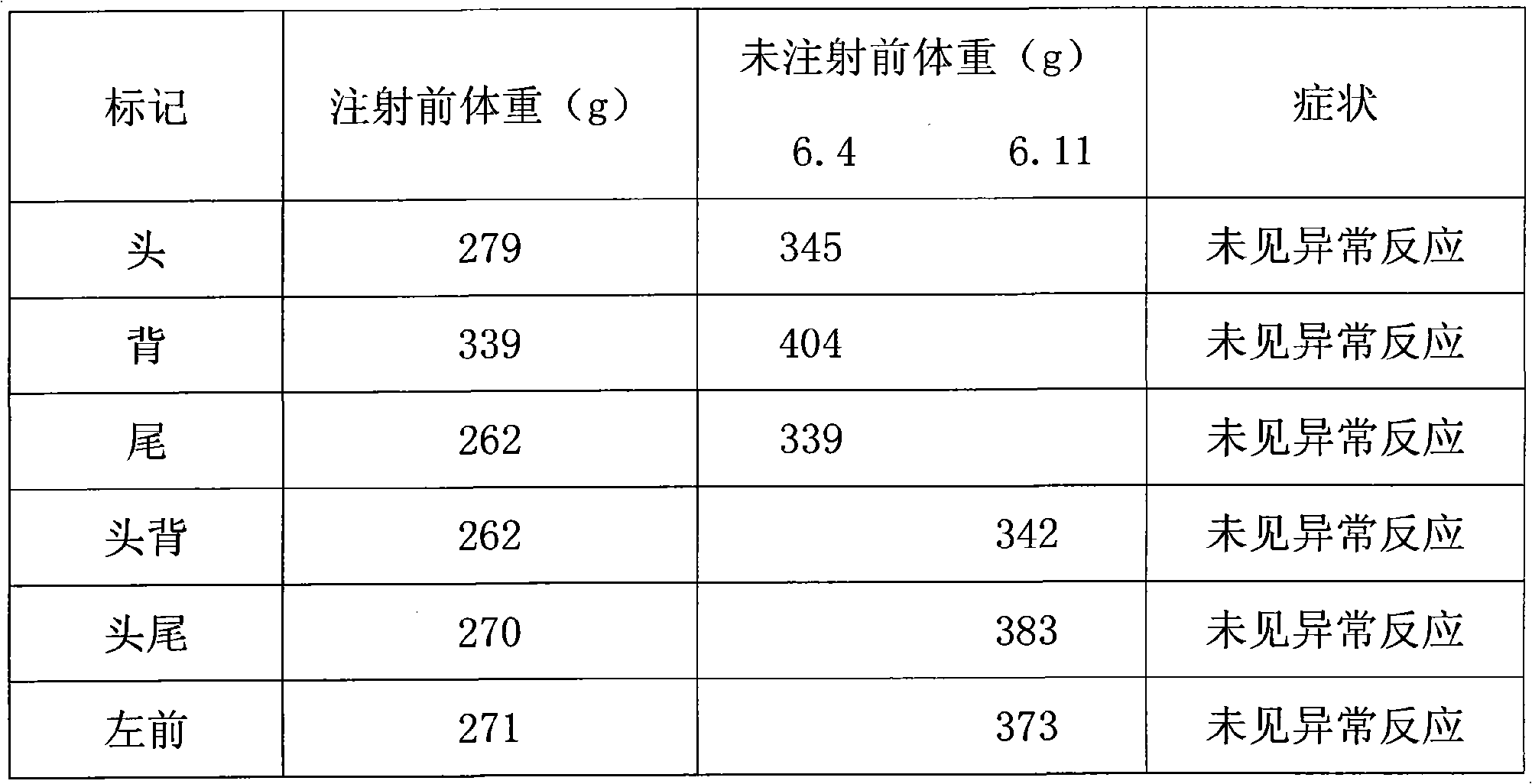
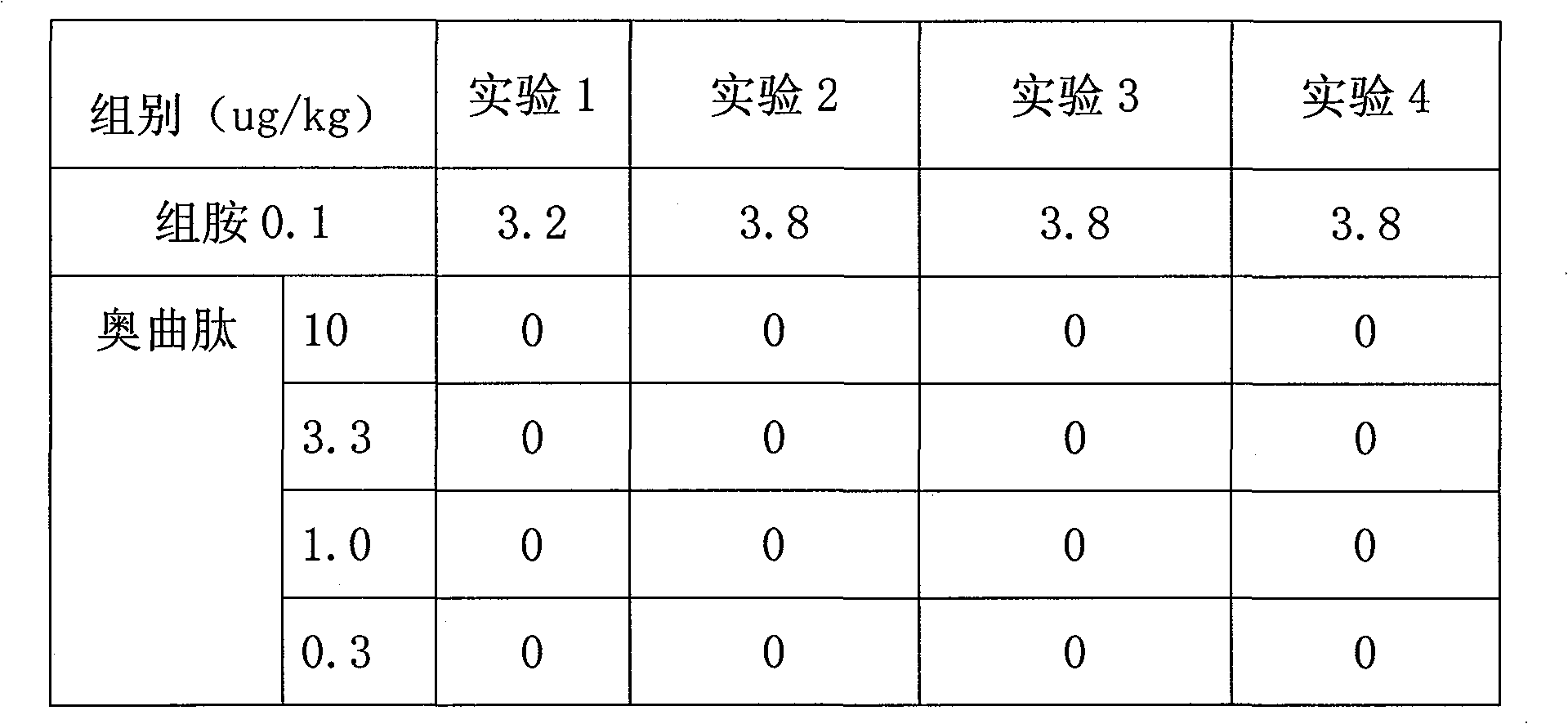
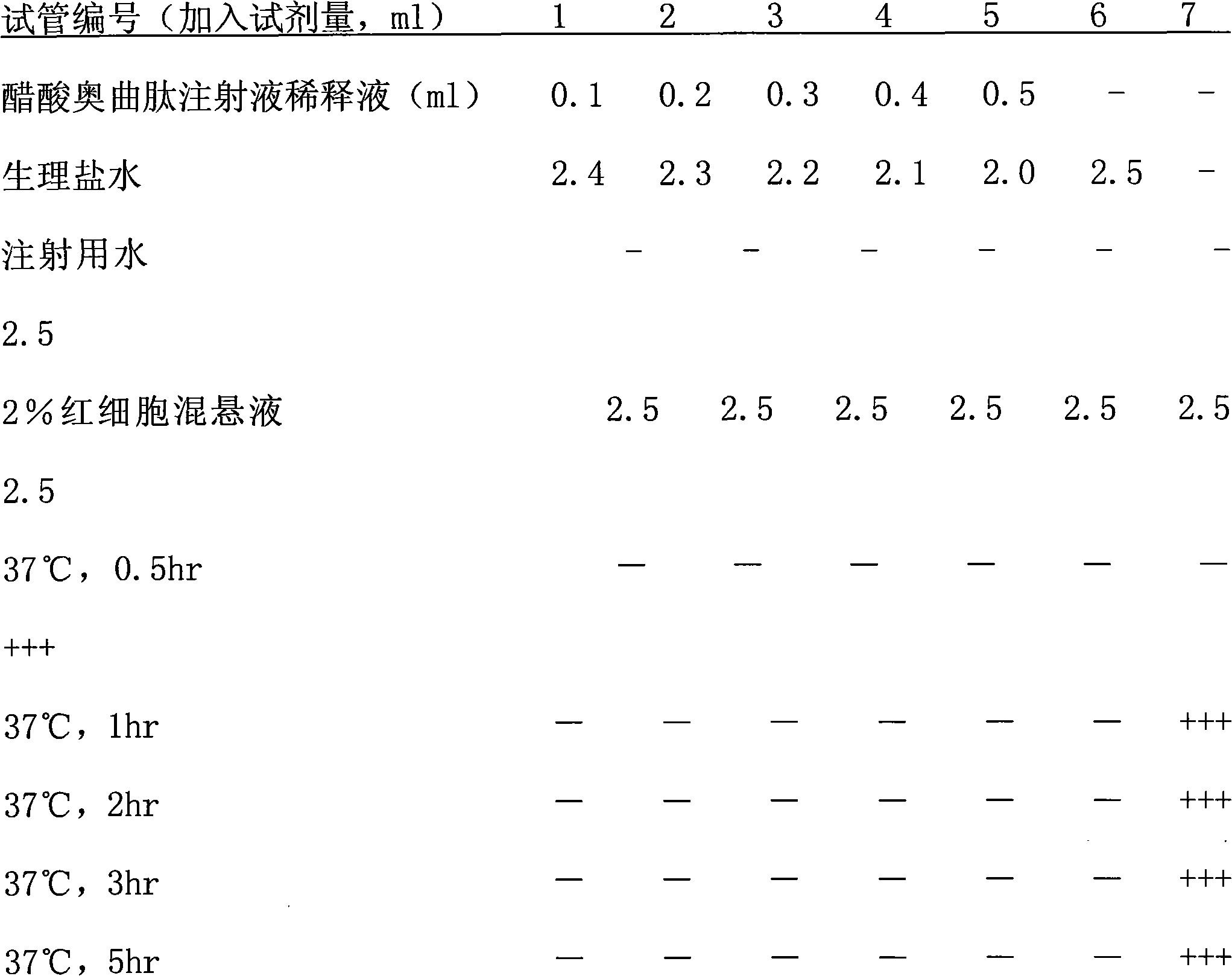
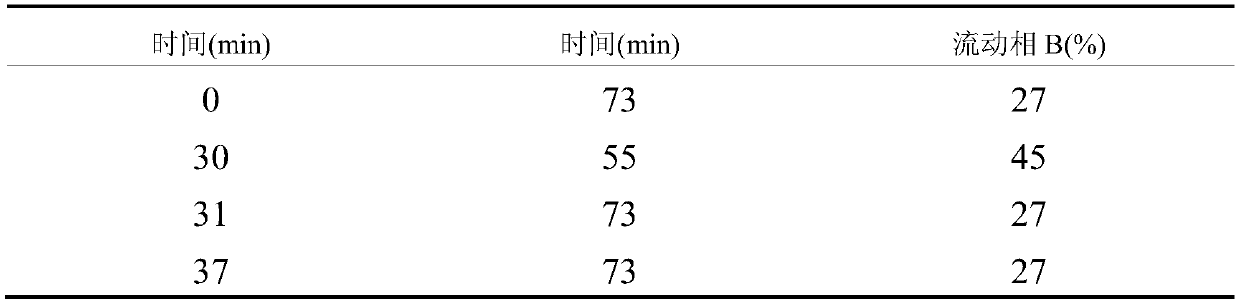
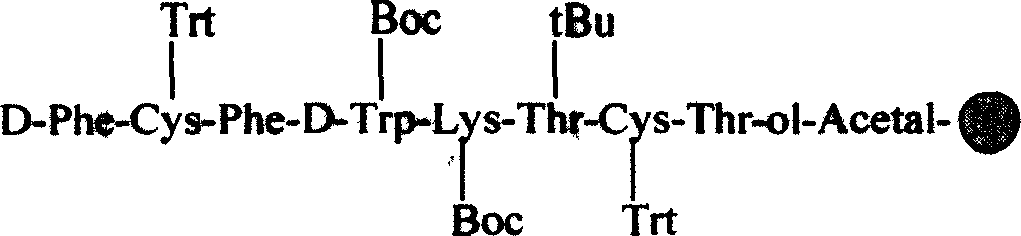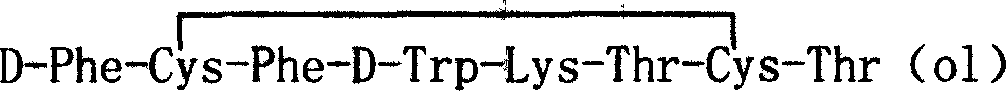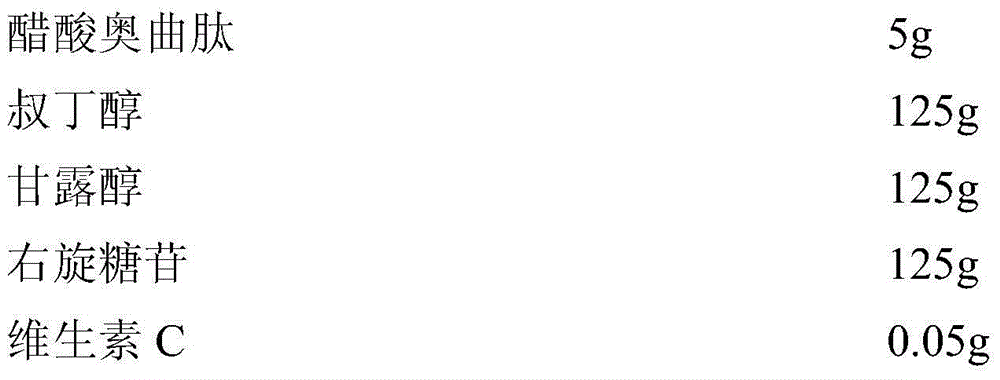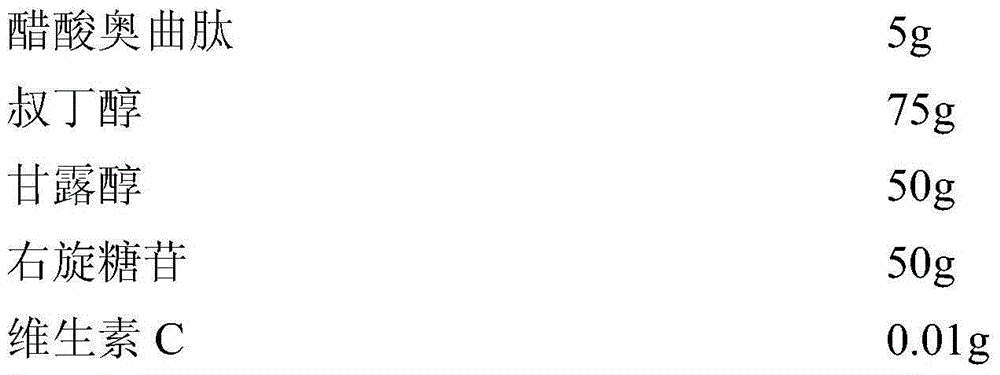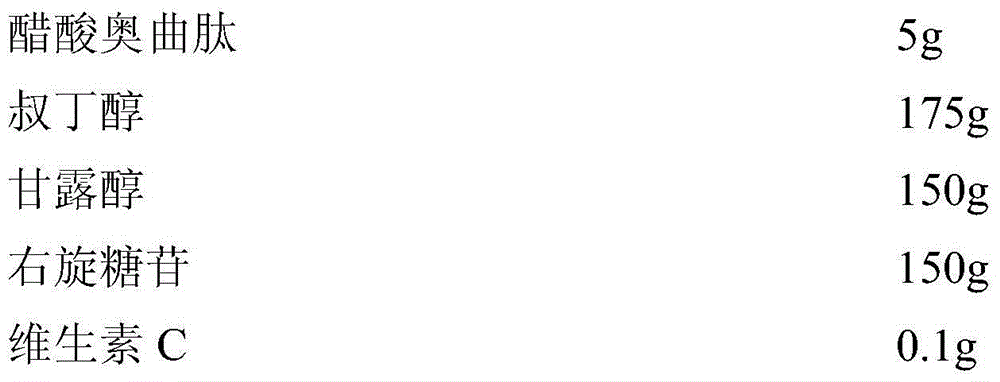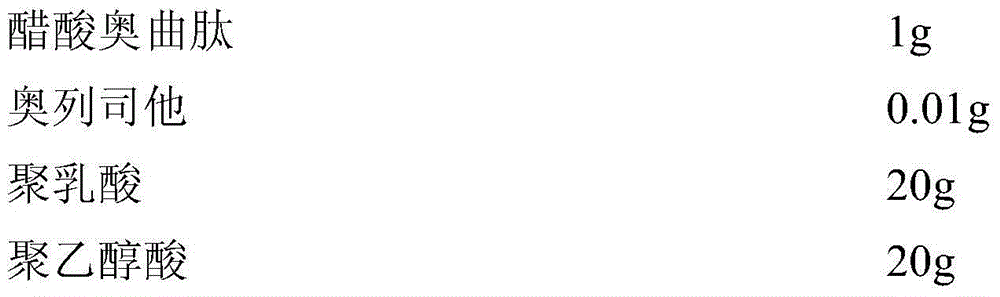Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "Octreotide acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
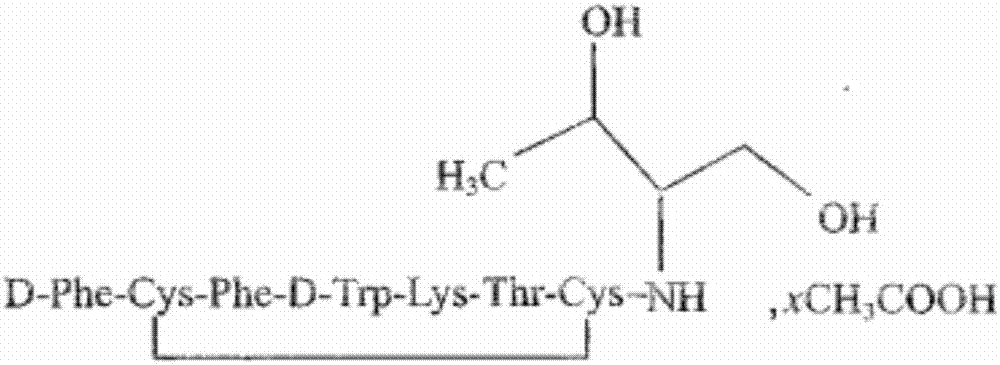
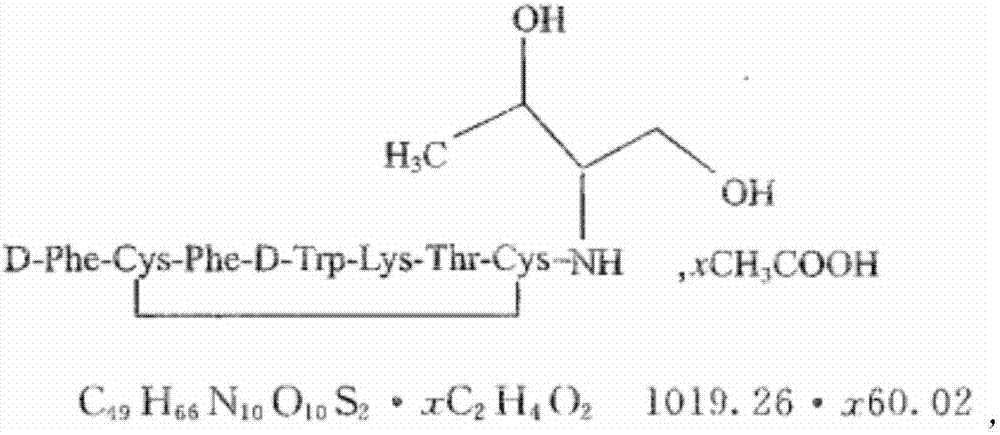
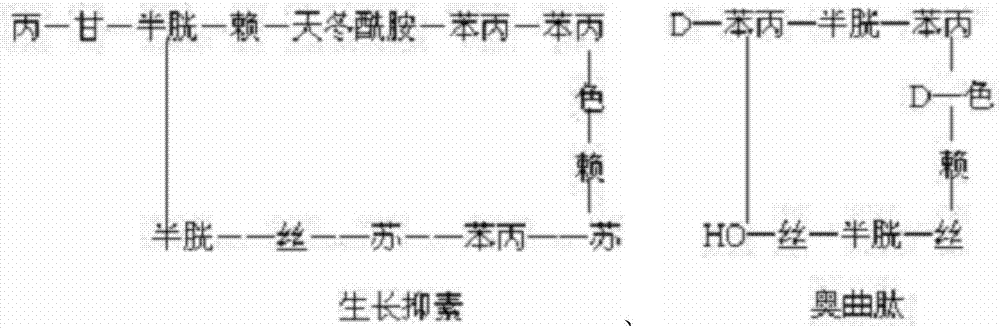
The acetate salt of a synthetic long-acting cyclic octapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin. Octreotide is a more potent inhibitor of growth hormone, glucagon, and insulin than somatostatin. Similar to somatostatin, this agent also suppresses the luteinizing hormone response to gonadotropin-releasing hormone, decreases splanchnic blood flow, and inhibits the release of serotonin, gastrin, vasoactive intestinal peptide (VIP), secretin, motilin, pancreatic polypeptide, and thyroid stimulating hormone. Check for http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=38866&idtype=1 active clinical trials or http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=38866&idtype=1&closed=1 closed clinical trials using this agent. (http://nciterms.nci.nih.gov.libproxy1.nus.edu.sg/NCIBrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&code=C53447 NCI Thesaurus)
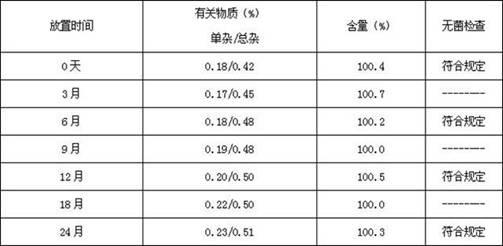
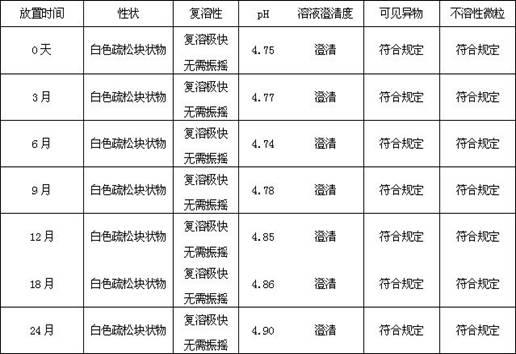
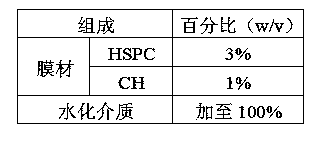
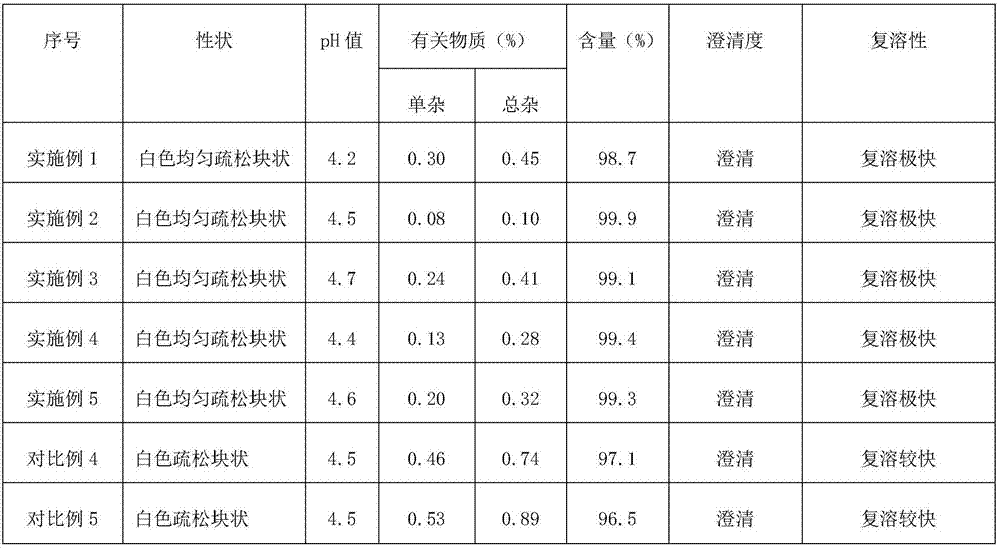
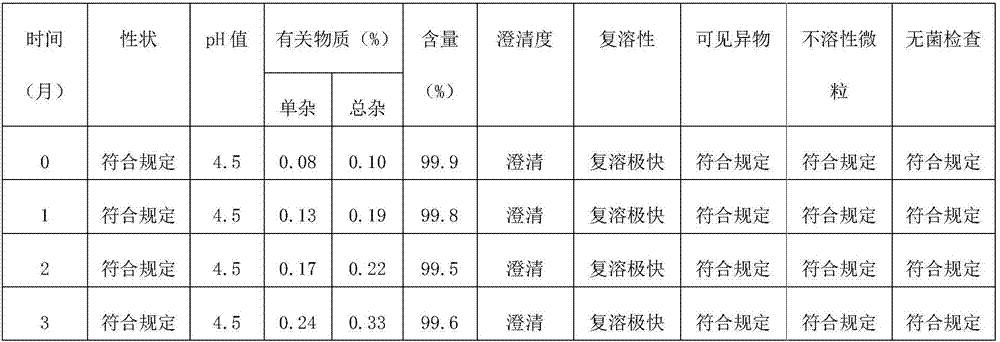
Octreotide acetate freeze-dried combination for injection and preparation method thereof
ActiveCN102526700AImprove stabilityConvenient transportation and distributionPeptide/protein ingredientsDigestive systemSodium bicarbonateSolubility
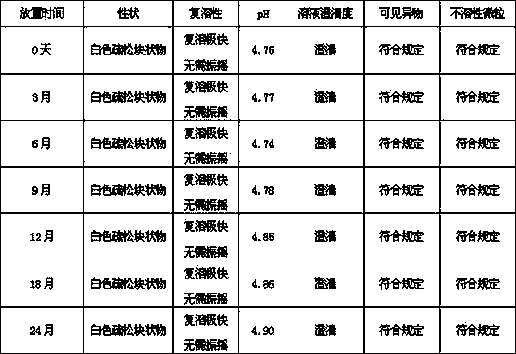
The invention provides an octreotide acetate freeze-dried combination for injection, which comprises octreotide acetate, mannitol and a proper amount of buffer substances, wherein the mass ratio of the octreotide acetate to the mannitol is 1:(450-500); and the buffer substances are lactic acid and sodium bicarbonate and can be tartaric acid and sodium tartrate. The invention also provides a preparation method of the composition. The composition provided by the invention is produced by adopting an aseptic technique; by striving to make the technological breakthrough in optimal pH range and optimal preparation temperature in a liquid medicine preparation process, the product stability is improved; and finally, a freeze-dried product is prepared. By adopting a quick-freezing mode for pre-freezing, a finished product has low related substance content and good re-solubility, can be preserved at room temperature for two months and can be refrigerated for two years, thereby facilitating the product transportation and delivery. By clinically matching with a solution, the combination has good stability and low local injection irritation. The invention provides a safe and effective octreotide acetate freeze-dried combination for injection, which has good and controllable quality, for clinics.
Owner:西藏嘉信景天药业有限公司 +1
Method for improving stability of polypeptide active pharmaceutical ingredients
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Solid-phase synthesis process of octreotide acetate
ActiveCN1569890AIncrease reaction rateRapid responsePowder deliveryPeptide/protein ingredientsAutoxidationOctreotide acetate
The invention discloses the solid-phase synthesis process of octreotide acetate which consists of, bonding acetal product with macromolecular resin, merging the bonded products with protection amino acid residue in sequence to obtain octapeptide resin, cutting the octapeptide from the resin to obtain aqueous solution, carrying out naturally oxidation in air to obtain Octreotide, charging glacial acetic acid into Octreotide aqueous solution, and freeze-drying.
Owner:SINOPHARM A THINK PHARMA
Water phase preparation method for chain platinum nanosphere by taking octreotide acetate as template
InactiveCN102784924ASimple processReaction is easy to controlNanotechnologyOctreotide acetatePlatinum tetrachloride
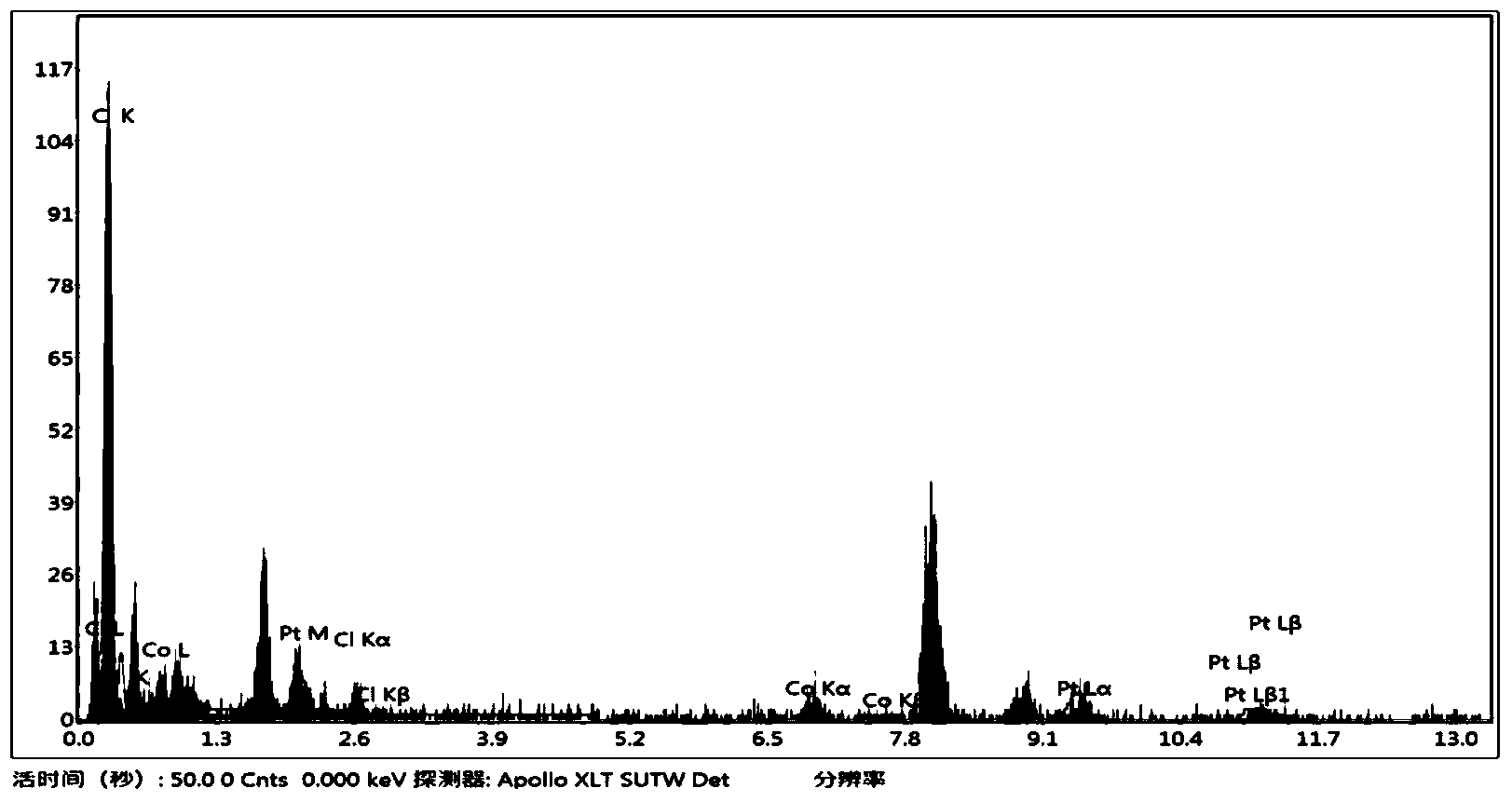
The invention discloses a water phase preparation method for chain platinum nanosphere by taking octreotide acetate as a template. The method mainly comprises the following steps: regulating octreotide acetate into acid solution with a pH of 2 to 3 using hydrochloric acid or alkaline solution with a pH of 8 to 10 using sodium hydroxide; adding platinum tetrachloride solution into the obtained solution at a mole ratio of 1:8-12, placing the mixed solution into a water-bathing constant temperature vibrator, and incubating the mixed solution for 20-26 h at the temperature of 13-25 DEG C at a speed of 10-200 rpm; and dropwise adding reducing agent dimethylborane into the incubated solution to turn the light yellow color of the incubated solution into black gray color, that is, obtaining an octreotide acetate-chain platinum nanosphere, wherein the mole ratio of dimethylborane to octreotide acetate is 1:45-55. The water phase preparation method has the advantages of simple process, low cost, availability, easiness in reaction control, high yield and the like.
Owner:YANSHAN UNIV
Method for preparing hammer-shaped palladium nanoparticle by utilizing octreotide acetate as template
InactiveCN103203461AExcellent biological templateSimple molecular structureOctreotide acetateSodium borohydride
Owner:YANSHAN UNIV
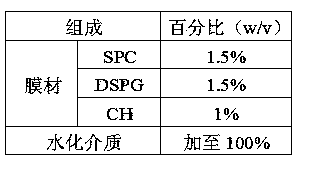
Octreotide acetate preparation and preparation method thereof
InactiveCN102525927AFull appearanceNo significant change in encapsulation efficiencyPeptide/protein ingredientsDigestive systemOctreotide preparationFreeze-drying
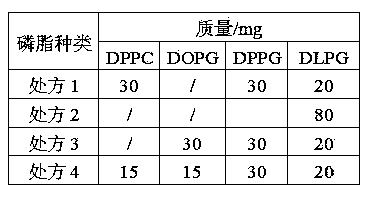
The invention belongs to the field of medicament preparations, and discloses an octreotide acetate lipidosome precursor and a preparation method thereof. The precursor lipidosome contains octreotide acetate, negatively-charged phospholipid and a cryoprotectant, and can contain an appropriate quantity of other lipids including phosphatidylchline and cholesterol; components such as an antioxidant, a pH regulating agent and the like can be added as required; the molar ratio of the octreotide acetate to the negatively-charged phospholipid is smaller than 1:1; and the mass ratio of the negatively-charged phospholipid to the cryoprotectant is 1:1-1:10. In the invention, a tert-butyl alcohol-water cosolvent freeze-drying method is adopted. The entrapment rate of an octreotide acetate lipidosome / micelle obtained by hydrating a freeze-dried product can be over 50 percent, an octreotide acetate lipidosome / micelle obtained by hydrating the freeze-dried product has high stability, and the problem of difficulty in entrapping a protein polypeptide medicament during preparation of a lipidosome / micelle preparation is solved. The preparation method is simple and practicable, and is suitable for industrial mass production.
Owner:SHENYANG PHARMA UNIVERSITY
Method for preparing octreotide and octreotide acetate
ActiveCN103965291AAvoid the problem of excessive volume and diluted concentrationSuitable for continuous productionOxytocins/vasopressinsAngiotensinsOctreotide acetateDesalination
The invention discloses a method for preparing octreotide and octreotide acetate. The method for preparing octreotide comprises the following steps: sequentially performing reverse phase purification and reverse phase desalination on an octreotide crude product solution by using a high performance liquid reversion phase chromatography method, wherein the packing of the high performance liquid reversion phase chromatography method is a poly styrene-divinyl benzene (PS-DVB) copolymer. The method integrates reverse phase purification and reverse phase desalination, novel application of the copolymer packing, namely, the styrene-divinyl benzene, is designed, and octreotide and octreotide acetate can be prepared in a large scale.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Method for manufacturing strepto-shaped cobalt platinum alloy by using octreotide acetate as template
InactiveCN104014810ASimple molecular structureEasy to analyzeNanotechnologyWater bathsOctreotide acetate
A method for manufacturing strepto-shaped cobalt platinum alloy by using octreotide acetate as a template mainly comprises the steps that the octreotide acetate is dissolved through pH2-3 hydrochloric acid, the octreotide acetate is regulated into an acid solution, a cobalt chloride solution and a platinum chloride solution which are the same in amount are simultaneously added in the regulated solution with the mole ratio of 1:10-20, the solution is then put in a water bath constant temperature oscillator, and hatching is carried out for 20-26 hours under the temperature of 13-25 DEG C at 100-200 rpm.; then, the reducing agent sodium borohydride is added in the hatched solution at a time so that the hatched solution is changed into dark black from light yellow, and the strepto-shaped cobalt platinum alloy is obtained, wherein the mole ratio of the reducing agent sodium borohydride and the octreotide acetate is 1:25-35. The method for manufacturing the strepto-shaped cobalt platinum alloy by using the octreotide acetate as the template has the advantages that the manufacturing technology is simple, conditions are mild, the strepto-shaped cobalt platinum alloy is cheap and easy to obtain, the reaction is easy to control, the productivity is high, and mass production is easy to achieve.
Owner:YANSHAN UNIV
Polypeptide synthesis method for octreotide acetate
ActiveCN103351426AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFluoroacetic acidAceric acid
The invention relates to a polypeptide synthesis method for octreotide acetate. The method comprises the following steps of: taking chloromethyl resin as a starting raw material, preparing a cesium salt from Boc-Thr(tBu)-OH, sequentially connecting amino acids with protecting groups according to a solid-phase synthesis method so as to obtain protected octapeptide resin, meanwhile, removing Boc protecting groups by sequentially using HCl / isopropyl alcohol, carrying out peptide connecting reaction in a manner of taking DIC and HOBT as condensing agents, carrying out reduction by using palladium carbon / hydrogen gas, meanwhile, cutting off peptide chains so as to obtain reduced octreotide, introducing air at the Ph of 7.8-9 so as to cyclize disulfide linkages, then, obtaining a crude octreotide product, and carrying out separation and purification through a C18 column, thereby preparing a fine octreotide acetate product. The method disclosed by the invention has the advantages that threoninol and Fmoc-threoninol are not adopted, the production cost is very low, the method has large-scale production capacity, the process is stable, the raw and auxiliary materials are convenient to obtain, the production cycle is short, the yield of connected peptide is high, the quality is stable, the use of highly-toxic reagents, such as hydrogen fluoride, trifluoroacetic acid and the like, is avoided, and the pollution caused by waste gas, waste water and waste residues is little.
Owner:SHANGHAI SOHO YIMING PHARMA
Octreotide injection
InactiveUS20140213984A1Peptide/protein ingredientsPharmaceutical delivery mechanismOctreotide acetateOctreotide Injection
Owner:SUN PHARMA INDS
Octreotide acetate freeze-dried powder injection for injection and preparation method thereof
ActiveCN102416001AImprove solubilityPowder deliveryPeptide/protein ingredientsSolubilityOctreotide acetate
The invention discloses octreotide acetate freeze-dried powder injection, which takes octreotide acetate as an active ingredient, mannitol as a freeze-dried excipient and citric acid as a regulating agent, and is characterized in that the pH value of the octreotide acetate freeze-dried powder injection is 5.5 to 5.7. The octreotide acetate freeze-dried powder injection prepared by the method can dissolve quickly, and the solubility of the injection is high.
Owner:CHONGQING Y S F PHARMA
Octreotide acetate sterile injection powder preparation and preparation method thereof
InactiveCN104689297AExtended shelf lifeImprove stabilityPowder deliveryPeptide/protein ingredientsOctreotide acetateVitamin C
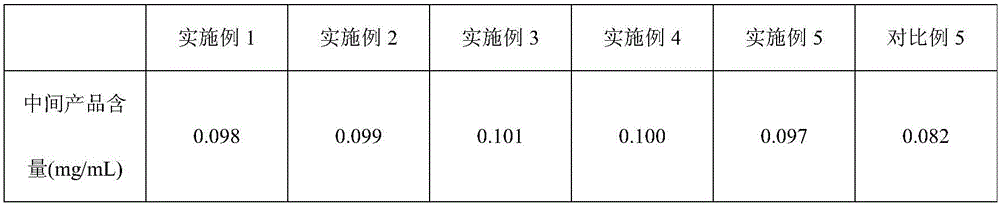
The invention relates to an octreotide acetate sterile injection powder preparation and a preparation method thereof. The octreotide acetate sterile injection powder preparation is prepared by octreotide acetate, TBA (Tert Butyl Alcohol), mannitol, dextran and a vitamin C. The preparation method comprises the following steps: 1) adding the vitamin C, the mannitol, the dextran, the TBA and the octreotide acetate into purified water sequentially, and stirring to completely dissolve; 2) filling a prepared liquid medicine in an ampoule bottle for freeze-drying. According to the octreotide acetate sterile injection powder preparation provided by the invention, the guarantee period can be greatly improved, the stability can be improved, and the preparation method is simple.
Owner:陈卓杰
Octreotide acetate injection and preparation process thereof
ActiveCN106668830AMature technologyQuality is easy to controlPeptide/protein ingredientsDigestive systemSodium bicarbonateOctreotide acetate
The invention belongs to the field of pharmaceutical preparations and provides an octreotide acetate injection and a preparation process thereof. According to the octreotide acetate injection provided by the invention, each 50000mL of the injection is prepared from the following components: 5.0g of octreotide acetate, 169.3g of lactic acid, 2200.0g to 2300.0g of mannitol, a proper amount of sodium hydrogen carbonate and the balance of injection water; the pH (Potential of Hydrogen) value of the octreotide acetate injection is 4.0 to 4.2. The preparation process provided by the invention is carried out under a sterile condition, and the pH value and temperature of the solution are controlled; a special material preparation system, and steps of magnetically stirring and carrying out secondary sterilization and filtering are adopted. The prepared injection can be preserved at room temperature for 6 months and can be refrigerated and preserved for 4 years; the octreotide acetate injection has good safety and the product is convenient to convey and distribute. The process provided by the invention is developed, has controllable quality and relatively low energy consumption and is economical and practical. The safe, effective and high-quality octreotide acetate injection is provided for clinical utilization.
Owner:武汉人福药业有限责任公司
Production of acetooxdralpeptide
A process for preparing the octretide acetate includes such steps as loading Boc-Thr(Ac)-Wang resin in reactor, adding the liquid mixture of TFA and dichloromethane, reacting, washing, adding the liquid mixture of triethane and dichloromethane, adding Fmoc-Cys(Trt)-OH, TBTU and HOBt, dissolving in peptide conjugating agent, testing amino radical to show negative, adding end closing agent and cap removing agent, washing, adding Fmoc-Thr(Trt)-OH, TBTU and HOBt, dissolving in peptide conjugating agent, testing amino radical to show negative, adding end closing agent and cap removing agent, washing, adding Fmoc-Thr(tBu)-OH, TBTU, HOBt, Fmoc-Lys(Boc)-OH, TBTU and HOBt, dissolving, adding Fmoc-D-Trp(Boc)-OH, TBTU and HOBt, dissolving in peptide conjugating agent, adding Fmoc-Phe-OH, TBTU and HOBt, washing, dewatering, adding Fmoc-Cys(Trt)-OH, TBTU, HOBt, Fmoc-D-Phe-OH, TBTU and HOBt, drying to obtain octapeptide resin, further processing, and freez drying. Its advantage is low cost.
Owner:JIANGSU SOHO INTERNATIONAL GROUP CORPORATION
Octreotide acetate injection
InactiveCN101647773AAchieve their goalsPeptide/protein ingredientsPharmaceutical delivery mechanismOctreotide acetateMedicine
The invention discloses an octreotide acetate injection comprising the following components of octreotide acetate, mannite, tween-80 and citric acid which are in weight ratio of 1.2:8-12:0.001-0.0015:0.1-0.5. The octreotide acetate injection has good stability, can be placed at normal temperature for 6 months and has no need to protect from light.
Owner:李红力
Octreotide acetate injection
InactiveCN101647773BAchieve their goalsPeptide/protein ingredientsPharmaceutical delivery mechanismOctreotide acetateMedicine
The invention discloses an octreotide acetate injection comprising the following components of octreotide acetate, mannite, tween-80 and citric acid which are in weight ratio of 1.2:8-12:0.001-0.0015:0.1-0.5. The octreotide acetate injection has good stability, can be placed at normal temperature for 6 months and has no need to protect from light.
Owner:李红力
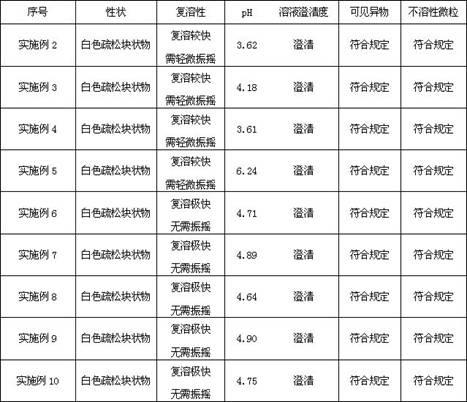
Injection octreotide acetate lyophilized composition and preparation method thereof
ActiveCN103932996AQuality improvementReduce typesPowder deliveryPeptide/protein ingredientsOctreotide acetateTromethamine
The invention discloses an injection octreotide acetate lyophilized composition and a preparation method thereof. The lyophilized composition comprises octreotide acetate, tromethamine and maleic acid. The preparation method comprises the following steps: weighing octreotide acetate and tromethamine, adding into injection water which is cooled to 10-20 DEG C, and stirring to dissolve; regulating the pH of the liquid medicine to be 4.0-5.0 with maleic acid, filtering, filling and lyophilizing. Compared with the prior art, the lyophilized composition is stable in quality, few in auxiliary varieties and simple in preparation process.
Owner:南京易亨制药有限公司
Preparation of octreotide acetate and octreotide acetate injection pharmaceutical composition
ActiveCN106866788AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemDisulfide bondSolid-phase synthesis
The invention relates to preparation of octreotide acetate and an octreotide acetate injection pharmaceutical composition. Specifically, the invention relates to a method for preparing the octreotide acetate; the method comprises the following steps: taking merrifield resin as a starting raw material and preparing Boc-Thr(tBu)-OH into cesium salt; sequentially connecting amino acid with protecting groups according to a solid-phase synthesis method to obtain protected octapeptide resin; removing Boc-protecting groups in sequence by utilizing HCl / isopropyl alcohol and carrying out peptide linking reaction by utilizing a condensing agent; reducing with palladium-carbon / hydrogen; meanwhile, chopping off a peptide chain to obtain reduced octreotide; ventilating air under the condition that the pH (Potential of Hydrogen) is 8 to 9 to form a ring by a disulfide bond, so as to obtain an octreotide crude product; separating and purifying the octreotide crude product through a C18 column to prepare refined octreotide. The invention further relates to the octreotide acetate injection pharmaceutical composition. The method for preparing the octreotide acetate and the octreotide acetate injection pharmaceutical composition, provided by the invention, have the excellent technical effects shown in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
Octreotide acetate freeze-dried combination for injection and preparation method thereof
ActiveCN102526700BImprove stabilityConvenient transportation and distributionPeptide/protein ingredientsDigestive systemSodium bicarbonateSolubility
Owner:西藏嘉信景天药业有限公司 +1
Octreotide acetate tablet and preparation method thereof
InactiveCN104667258AExtended shelf lifeMedication conveniencePeptide/protein ingredientsPill deliveryOctreotide acetateMedicine
The invention relates to an octreotide acetate tablet and a preparation method thereof. The octreotide acetate tablet is prepared from octreotide acetate nanoparticle and pharmaceutically acceptable tablet auxiliary materials, wherein the octreotide acetate nanoparticle comprises octreotide acetate, an enzyme inhibitor and a high polymer material. The octreotide acetate tablet provided by the invention is capable of significantly prolonging the medicine action time, and is convenient to medicate in comparison with an injection dosage form; the compliance of patients is improved; and meanwhile, the octreotide acetate tablet wraps a high-molecular compound and exists in a solid form, so that the quality guarantee period of the octreotide acetate is significantly prolonged.
Owner:陈卓杰
Octreotide acetate freeze-dried powder injection for injection and preparation method thereof
ActiveCN102416001BImprove solubilityPowder deliveryPeptide/protein ingredientsOctreotide acetateEthylic acid
The invention discloses octreotide acetate freeze-dried powder injection, which takes octreotide acetate as an active ingredient, mannitol as a freeze-dried excipient and citric acid as a regulating agent, and is characterized in that the pH value of the octreotide acetate freeze-dried powder injection is 5.5 to 5.7. The octreotide acetate freeze-dried powder injection prepared by the method can dissolve quickly, and the solubility of the injection is high.
Owner:CHONGQING Y S F PHARMA
Octreotide acetate injection pharmaceutical composition and octreotide acetate
ActiveCN106860854AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemOctreotide acetateSodium bicarbonate
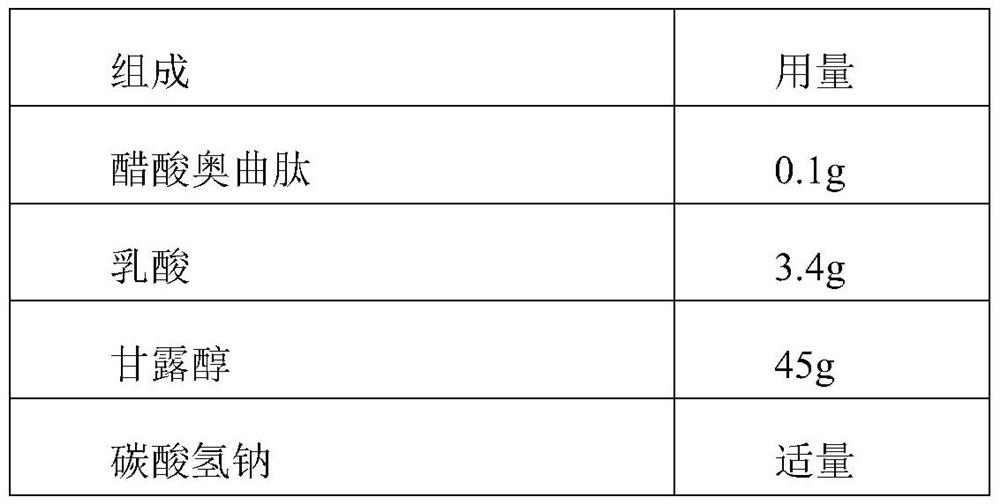
The invention relates to an octreotide acetate injection pharmaceutical composition and octreotide acetate. Specifically, the octreotide acetate injection pharmaceutical composition provided by the invention is prepared from octreotide acetate containing 0.1g of octreotide, 40g to 50g of mannitol, 3g to 4g of lactic acid, a proper amount of sodium hydrogen carbonate which is added until the pH (Potential of Hydrogen) is 3.7 to 4.7 and a proper amount of injection water which is added until the total amount is 1000ml and the like. The invention further relates to an octreotide acetate product and a preparation method thereof. The octreotide acetate injection pharmaceutical composition and the octreotide acetate, provided by the invention, have the excellent technical effects shown in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
Octreotide acetate preparation and preparation method thereof
InactiveCN102525927BFull appearancePeptide/protein ingredientsDigestive systemOctreotide preparationFreeze-drying
The invention belongs to the field of medicament preparations, and discloses an octreotide acetate lipidosome precursor and a preparation method thereof. The precursor lipidosome contains octreotide acetate, negatively-charged phospholipid and a cryoprotectant, and can contain an appropriate quantity of other lipids including phosphatidylchline and cholesterol; components such as an antioxidant, a pH regulating agent and the like can be added as required; the molar ratio of the octreotide acetate to the negatively-charged phospholipid is smaller than 1:1; and the mass ratio of the negatively-charged phospholipid to the cryoprotectant is 1:1-1:10. In the invention, a tert-butyl alcohol-water cosolvent freeze-drying method is adopted. The entrapment rate of an octreotide acetate lipidosome / micelle obtained by hydrating a freeze-dried product can be over 50 percent, an octreotide acetate lipidosome / micelle obtained by hydrating the freeze-dried product has high stability, and the problem of difficulty in entrapping a protein polypeptide medicament during preparation of a lipidosome / micelle preparation is solved. The preparation method is simple and practicable, and is suitable for industrial mass production.
Owner:SHENYANG PHARMA UNIVERSITY
Octreotide acetate freeze-dried powder injection for injection and preparation method thereof
InactiveCN107510837AImprove stabilityExtended storage timePowder deliveryPeptide/protein ingredientsOctreotide acetateSodium bicarbonate
The invention discloses an octreotide acetate freeze-dried powder injection for injection, which comprises octreotide acetate, mannitol, trehalose, L-cysteine, lactic acid and sodium bicarbonate, wherein the mass ration of the octreotide acetate to the mannitol to the trehalose to the L-cysteine is 1 to (200 to250) to (50 to 100) to (5 to 10). According to the octreotide acetate freeze-dried powder injection for injection provided by the invention, by adding the mannitol, the trehalose and the L-cysteine with a certain ratio to combine a freeze-drying protective additive, the stability of octreotide acetate is greatly improved, and the storage time of the octreotide acetate is prolonged.
Owner:国药集团成都信立邦生物制药有限公司
Method for preparing cube-shaped silver nano box with octreotide acetate as template
ActiveCN106735289ASimple molecular structureEasy to analyzeMaterial nanotechnologyTransportation and packagingHeat fusionTumor cells
Provided is a method for preparing a cube-shaped silver nano box with octreotide acetate as a template. The method mainly comprises the steps that an octreotide acetate acid solution with the concentration being 0.1 mM to 0.3 mM is used for carrying out constant temperature treatment for 10 min to 30 min at the temperature of 60 DEG C to 90 DEG C; 1-3% ascorbic acid and a 0.9-1.2 mM silver nitrate solution are mixed, treatment is carried out for 30 s to 1 min at ultrasonic waves of 60 W to 90 W, and a colloidal solution of silver is obtained; the octreotide acetate solution, the colloidal solution of silver and the silver nitrate solution are mixed according to the volume ratio of 1:1:(1-3) and placed in an oscillator, and incubation is carried out for 20 h to 50 h at the temperature of 15 DEG C to 25 DEG C; and a sodium borohydride water solution is dropwise added, the molar ratio of the sodium borohydride water solution to silver nitrate is (8-12):1, reacting is carried out at the temperature of 20 DEG C to 30 DEG C till the color of the solution turns into ash black, and the cube-shaped silver nano box is prepared. The preparation process is simple, conditions are mild, cost is low, the morphology is controllable, and the metal load rate is high; and tumor cell heat fusion death can be induced through photothermal conversion under near-infrared light irradiation, and the effect of treating tumors is achieved.
Owner:YANSHAN UNIV
Octreotide injection
InactiveUS20130303453A1Peptide/protein ingredientsPharmaceutical delivery mechanismHypodermoclysisOctreotide acetate
The present invention provides a sterile solution comprising octreotide acetate in a pharmaceutically acceptable vehicle, wherein solution is present as a reservoir in a multiple dose pen injection device, the device being adapted to subcutaneously inject a portion of the said reservoir in multiple daily doses and further being adapted to provide multiple portions of the said solution, while the reservoir remains sterile.
Owner:SUN PHARMA INDS
Preparation method and packaging method of octreotide acetate injection
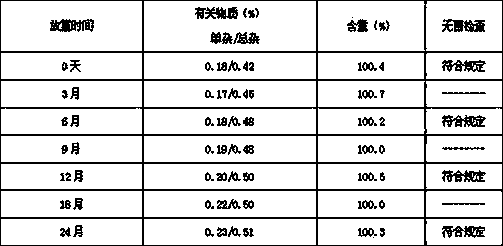
InactiveCN113350276AImprove stabilityMaximum single impurity lowPeptide/protein ingredientsDigestive systemOctreotide acetatePhysical chemistry
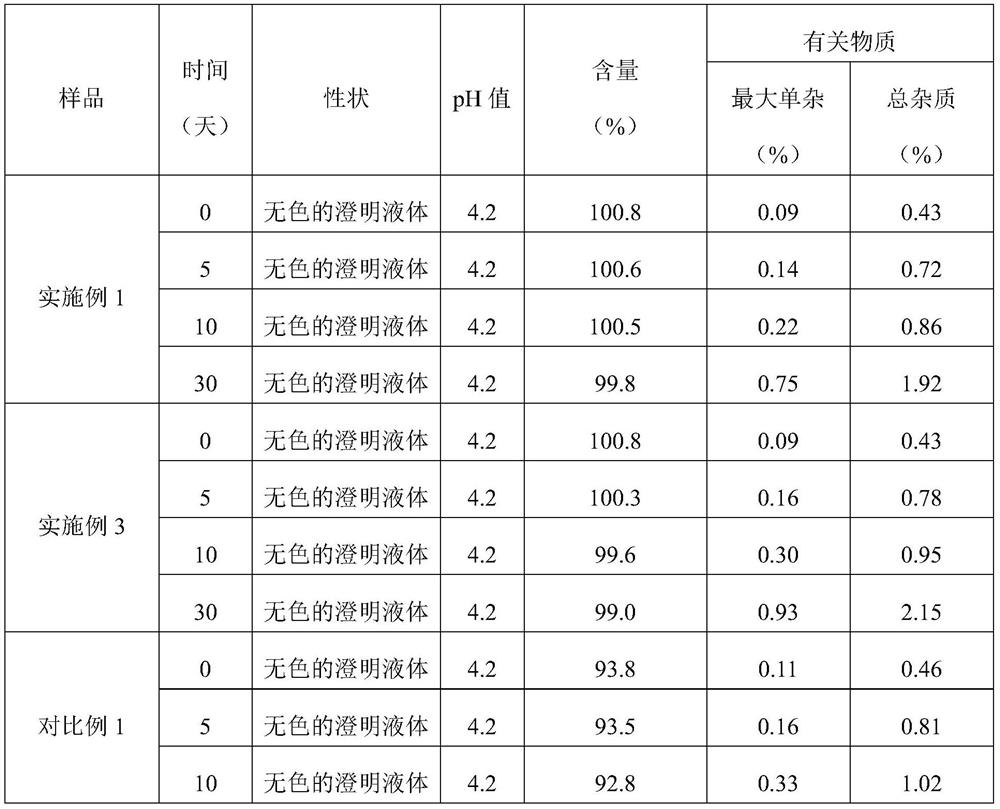
The invention discloses a preparation method and a packaging method of an octreotide acetate injection. The preparation method comprises the following steps: (1) mixing lactic acid with water for injection; (2) mixing mannitol with the mixture obtained in the step (1); (3) adjusting the pH value of the mixture obtained in the step (2), and then adding octreotide acetate; (4) filtering to obtain an octreotide acetate injection; and (5) adding the water for injection to the total amount. The stability of the octreotide acetate injection can be effectively improved, so that the octreotide acetate injection still has stable pH value and octreotide acetate total amount and low maximum single impurity and total impurity percentage after being stored for 5 days and 10 days. The packaging method comprises the step of filling carbon dioxide, the stability of the octreotide acetate injection under the conditions of high temperature and illumination can be improved, and after the octreotide acetate injection is stored for 5 days, 10 days and 30 days, the high octreotide acetate content and the low maximum single impurity and total impurity percentage can still be kept.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
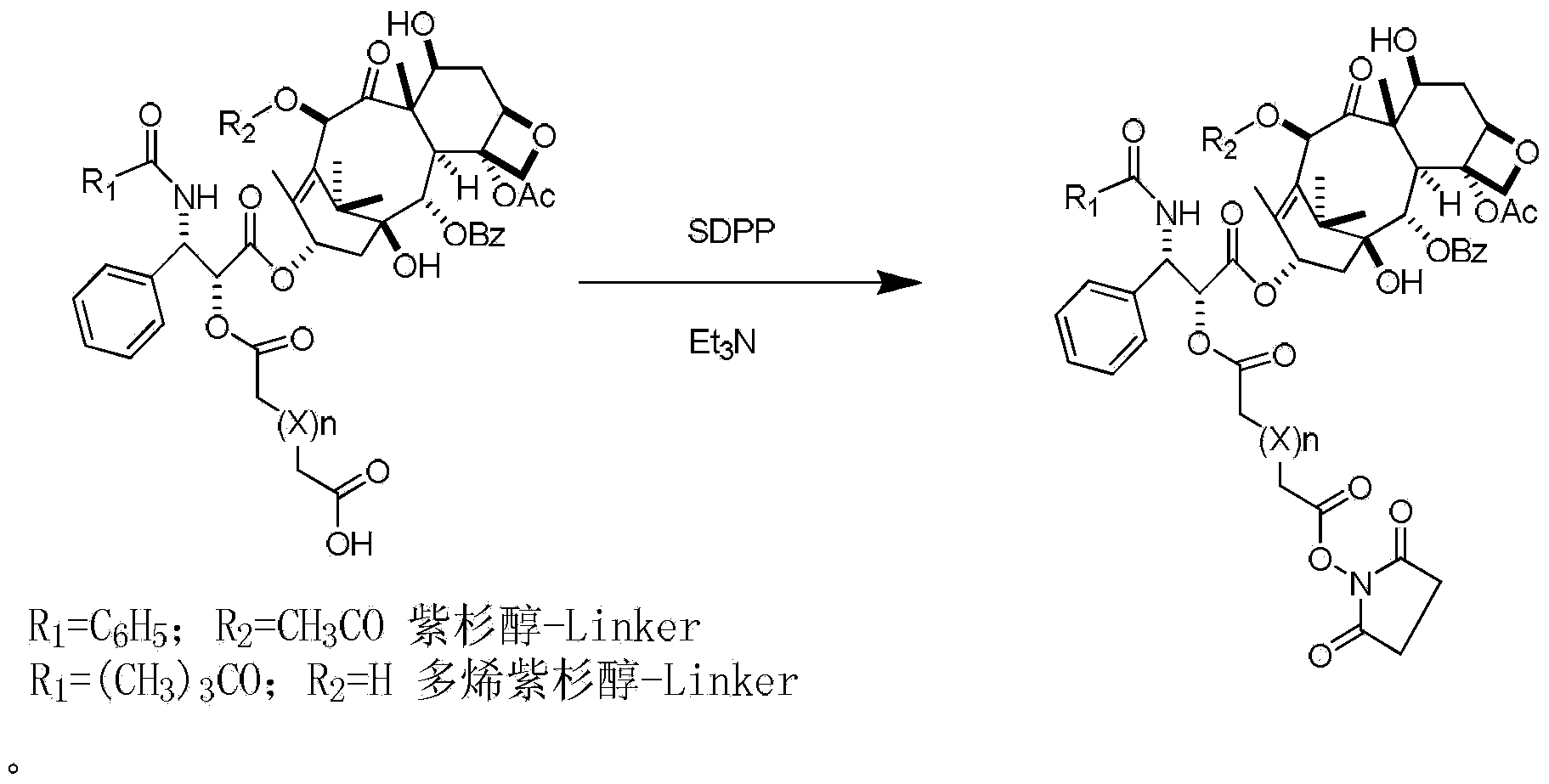
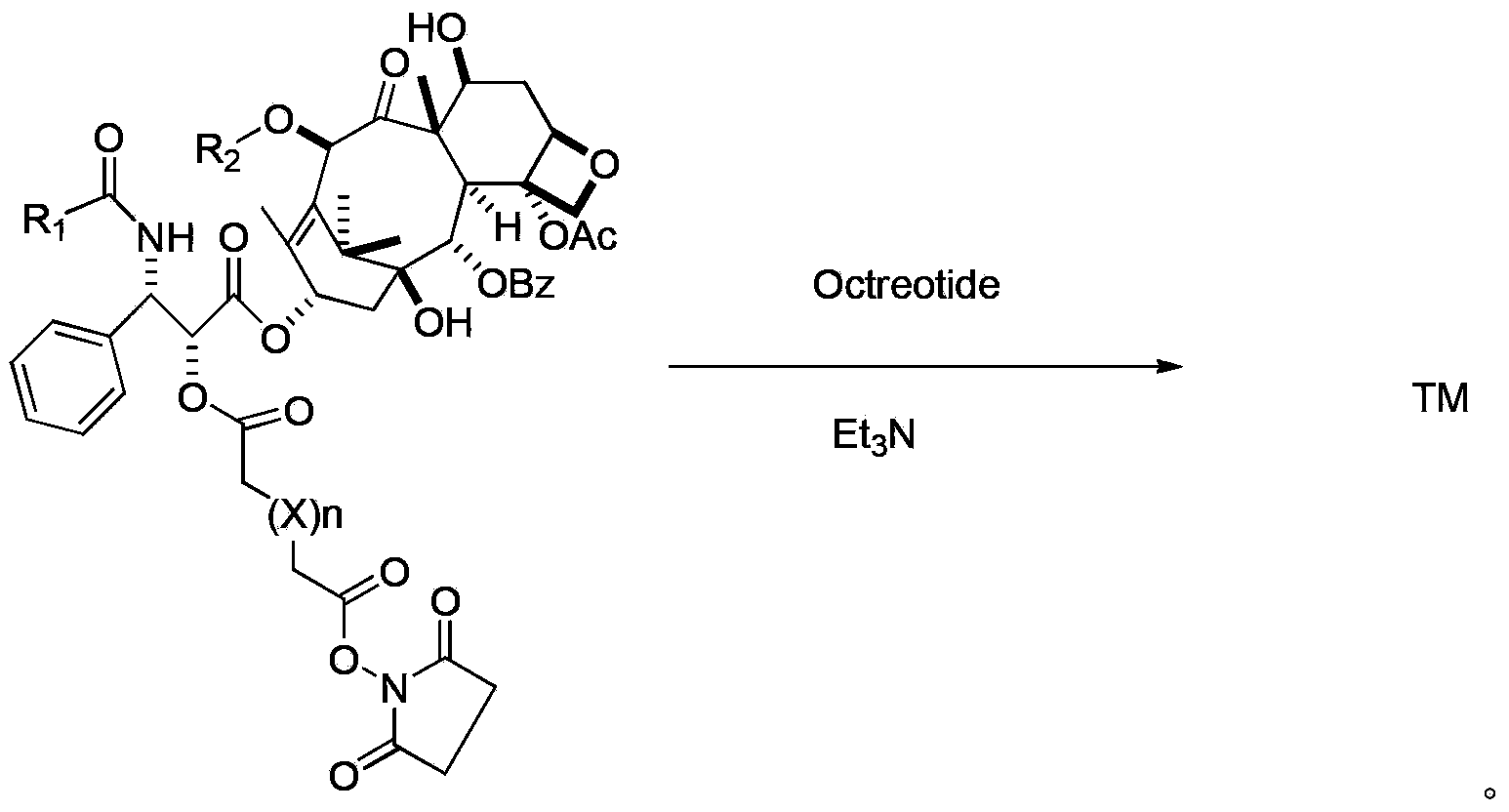
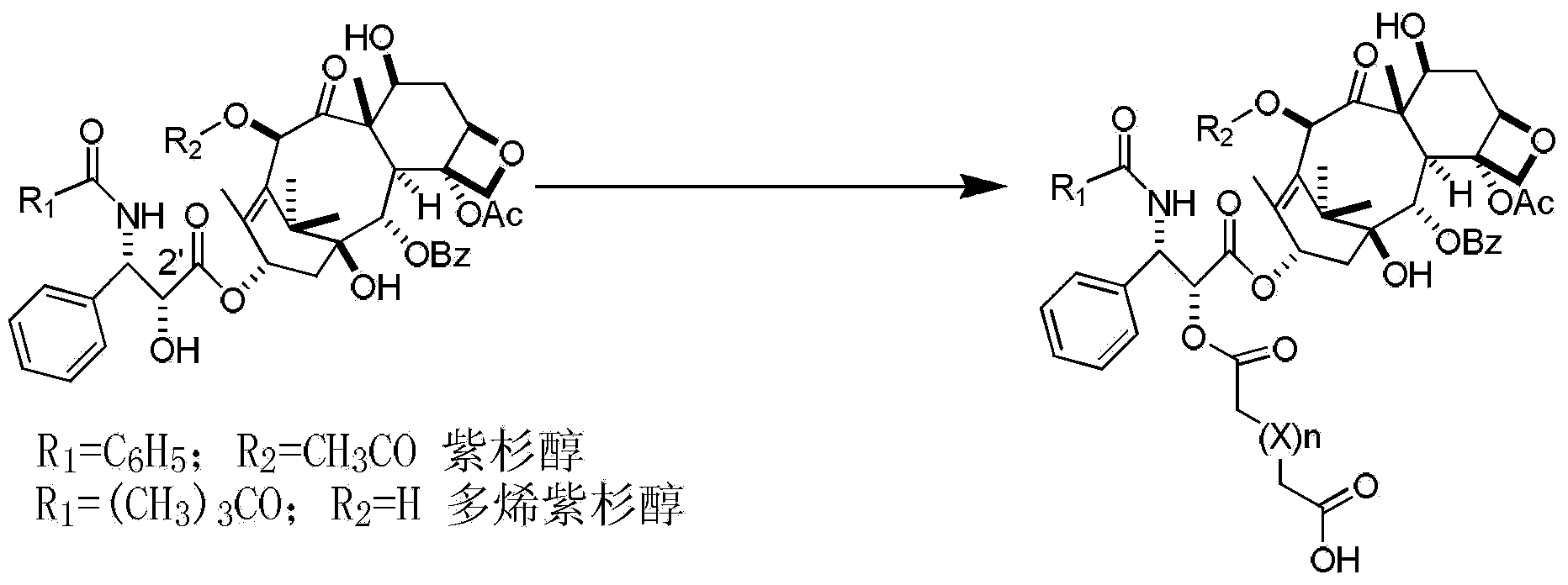
Preparation method of paclitaxel or docetaxel octreotide conjugate
The invention relates to a preparation method of a paclitaxel or docetaxel octreotide conjugate. The method comprises the steps that: 1, in an aprotic solvent, paclitaxel-2'-linker sequence acid or docetaxel-2'-acid linker sequence and diphenyl N-succinimidyl phosphate (SDPP) are subjected to the catalysis of tertiary amine, such that N-hydroxysuccinimide paclitaxel-2'-linker sequence ester or N-hydroxysuccinimide docetaxel-2'-linker sequence ester is obtained; 2, in an aprotic solvent, N-hydroxysuccinimide paclitaxel-2'-linker sequence ester or N-hydroxysuccinimide docetaxel-2'-linker sequence ester and octreotide are conjugated under the catalysis of tertiary amine, such that paclitaxel or docetaxel octreotide conjugate is obtained. The invention assists in solving problems of impossibility of purchasing octreotide resin compositions and expensive catalysts. The conjugate provided by the invention can be synthesized in large batches by using raw materials in the market and a cheap reagent diphenyl N-succinimidyl phosphate (SDPP).
Owner:SOUTHEAST UNIV
Octreotide acetate injection and preparation process thereof
ActiveCN106668830BMature technologyQuality is easy to controlPeptide/protein ingredientsDigestive systemOctreotide acetateEthylic acid
The invention belongs to the field of pharmaceutical preparations and provides an octreotide acetate injection and a preparation process thereof. According to the octreotide acetate injection provided by the invention, each 50000mL of the injection is prepared from the following components: 5.0g of octreotide acetate, 169.3g of lactic acid, 2200.0g to 2300.0g of mannitol, a proper amount of sodium hydrogen carbonate and the balance of injection water; the pH (Potential of Hydrogen) value of the octreotide acetate injection is 4.0 to 4.2. The preparation process provided by the invention is carried out under a sterile condition, and the pH value and temperature of the solution are controlled; a special material preparation system, and steps of magnetically stirring and carrying out secondary sterilization and filtering are adopted. The prepared injection can be preserved at room temperature for 6 months and can be refrigerated and preserved for 4 years; the octreotide acetate injection has good safety and the product is convenient to convey and distribute. The process provided by the invention is developed, has controllable quality and relatively low energy consumption and is economical and practical. The safe, effective and high-quality octreotide acetate injection is provided for clinical utilization.
Owner:武汉人福药业有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com