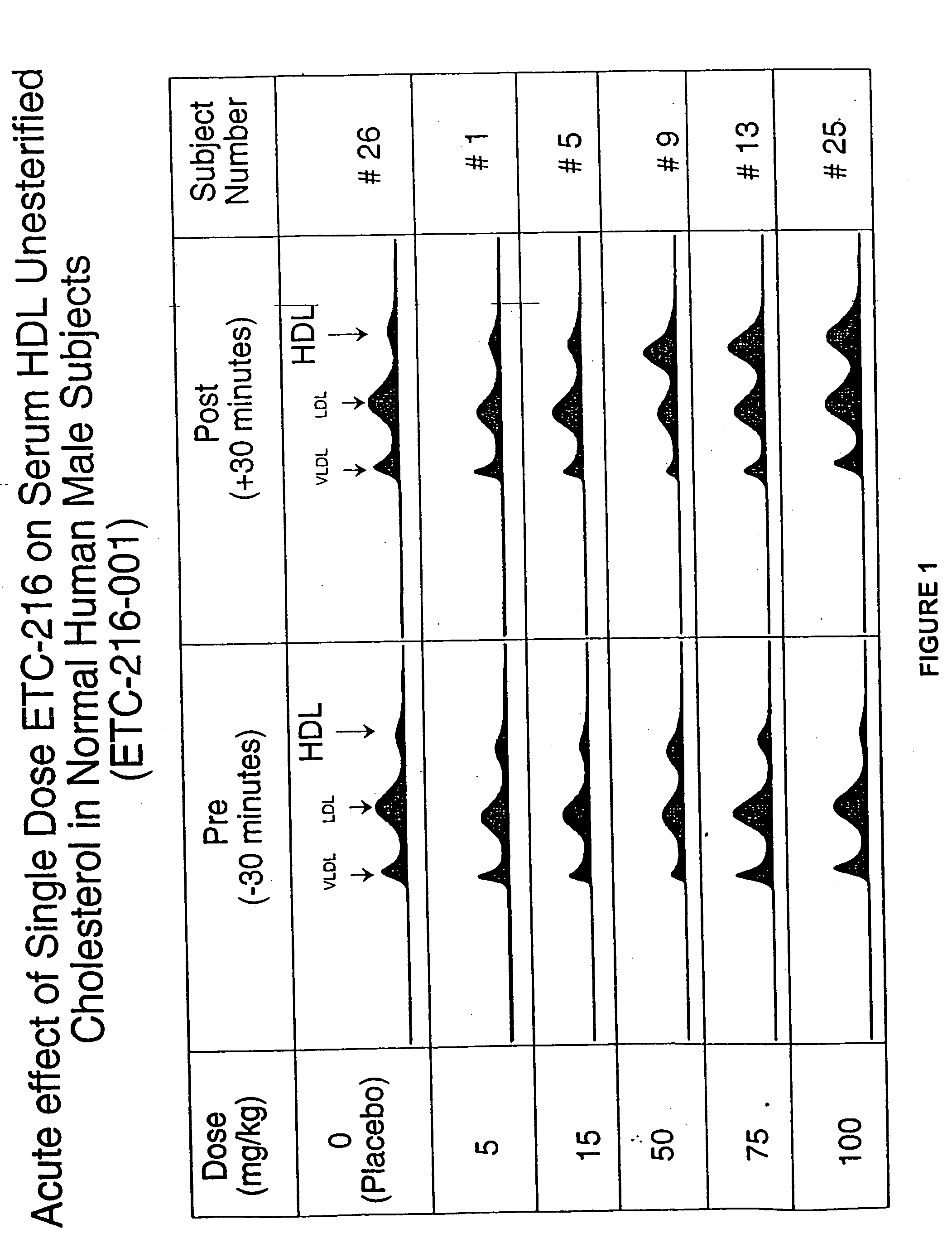
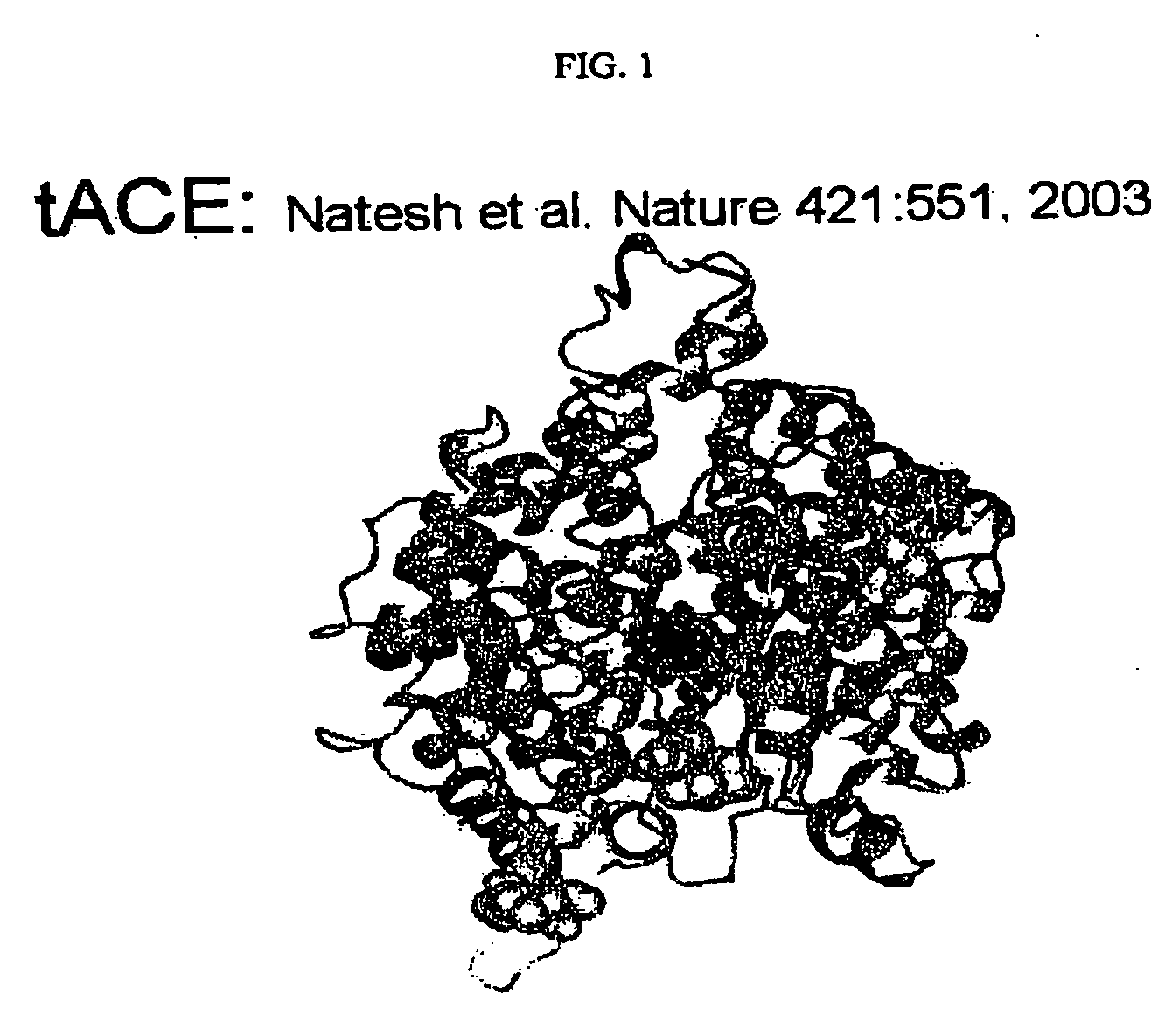
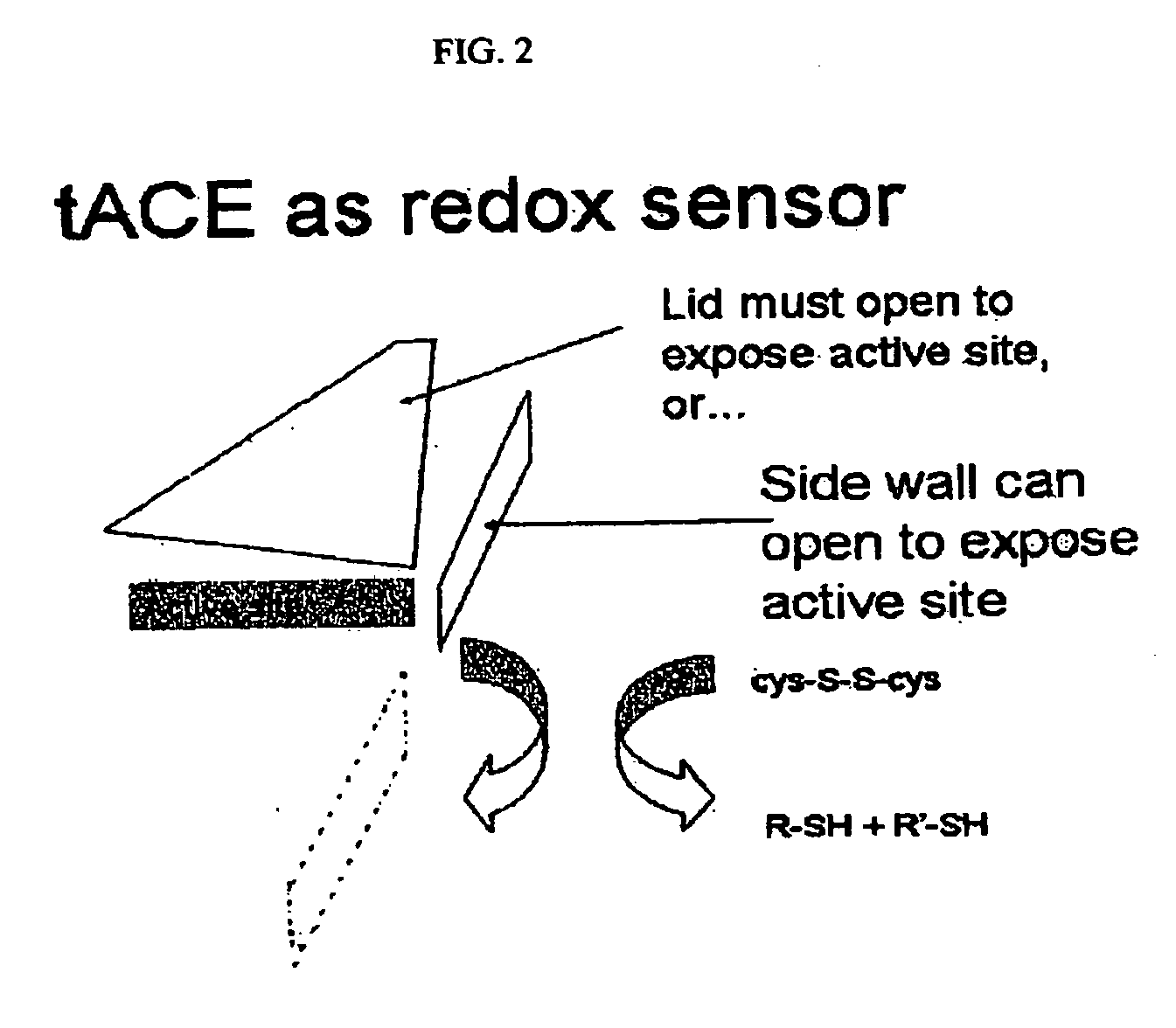
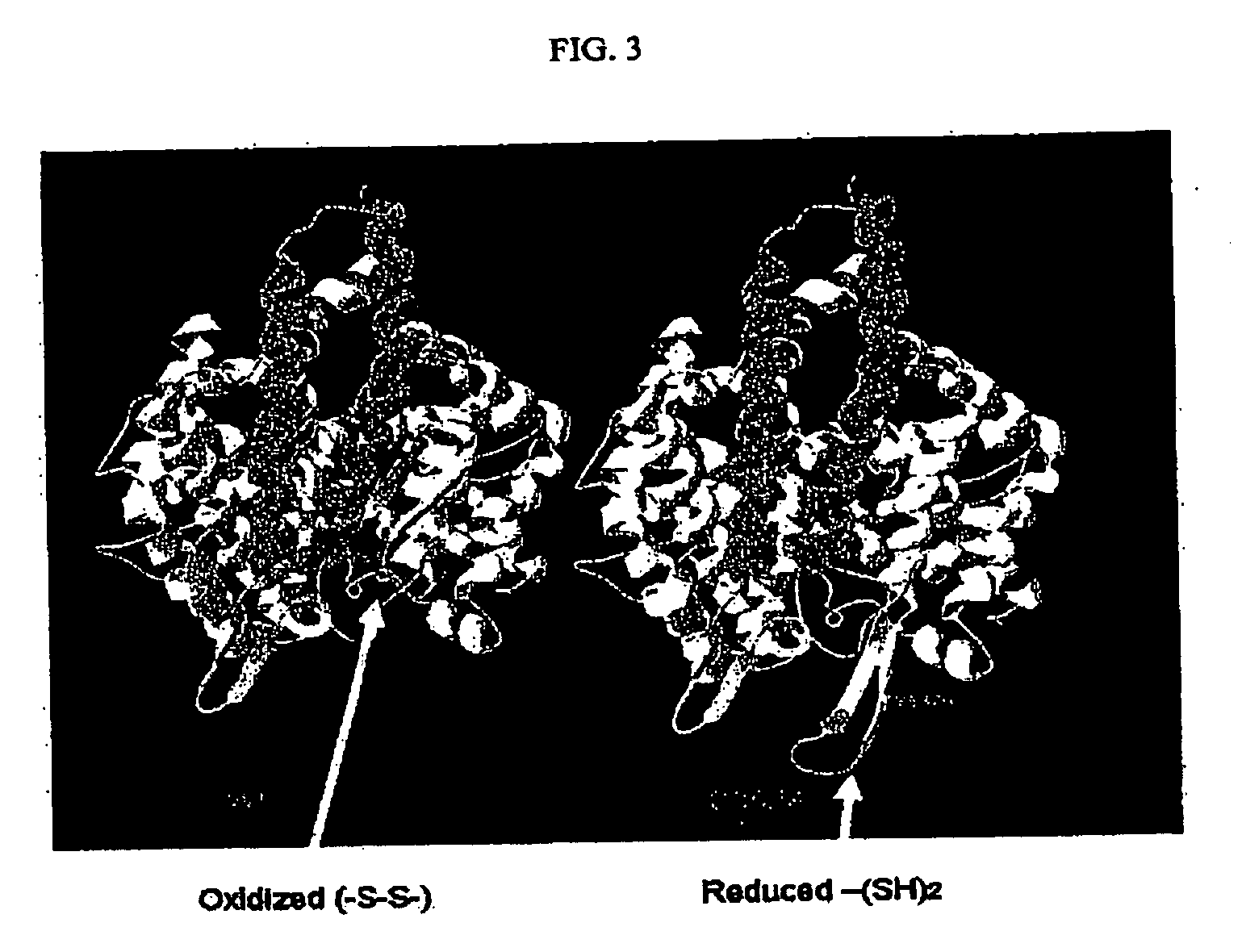
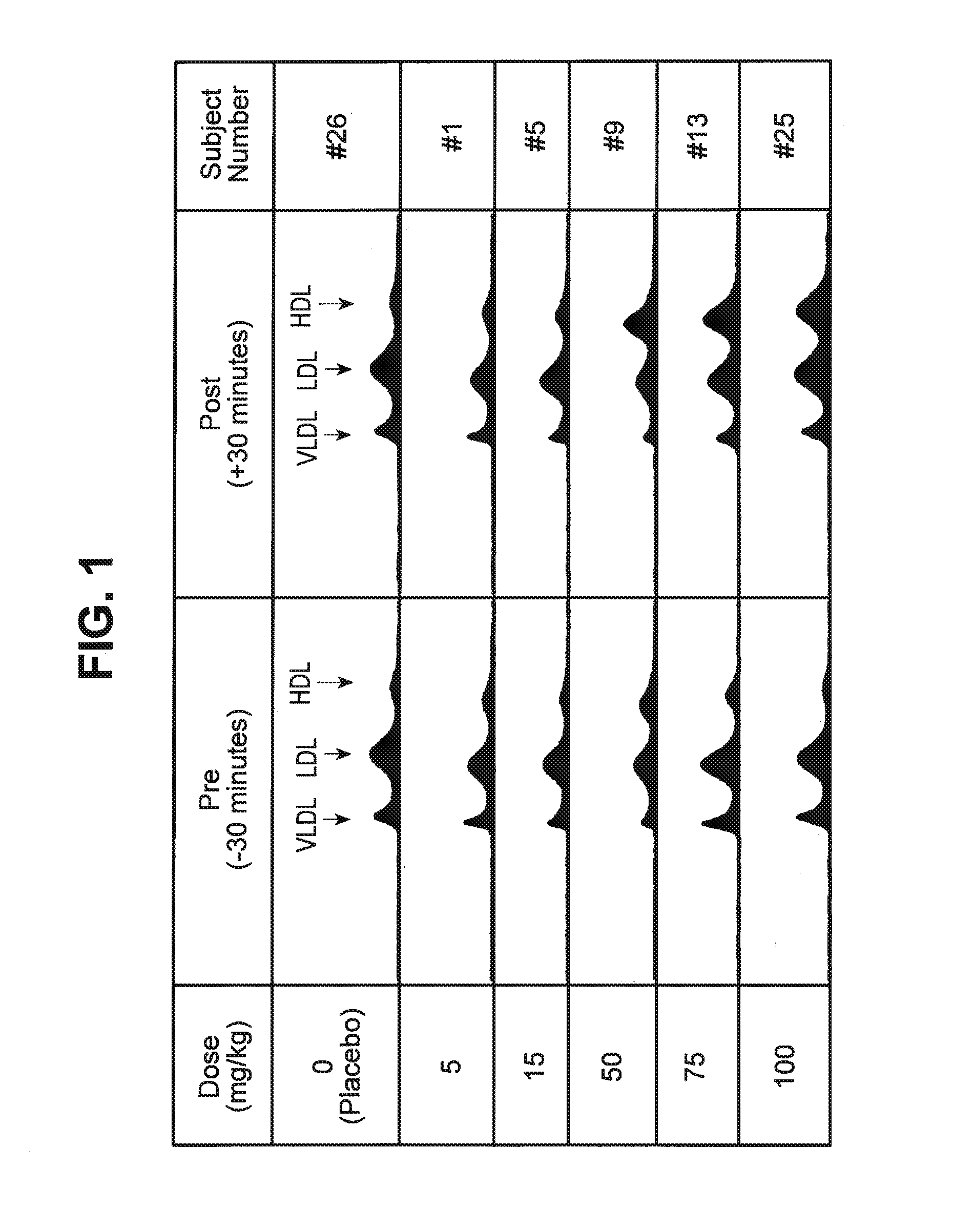
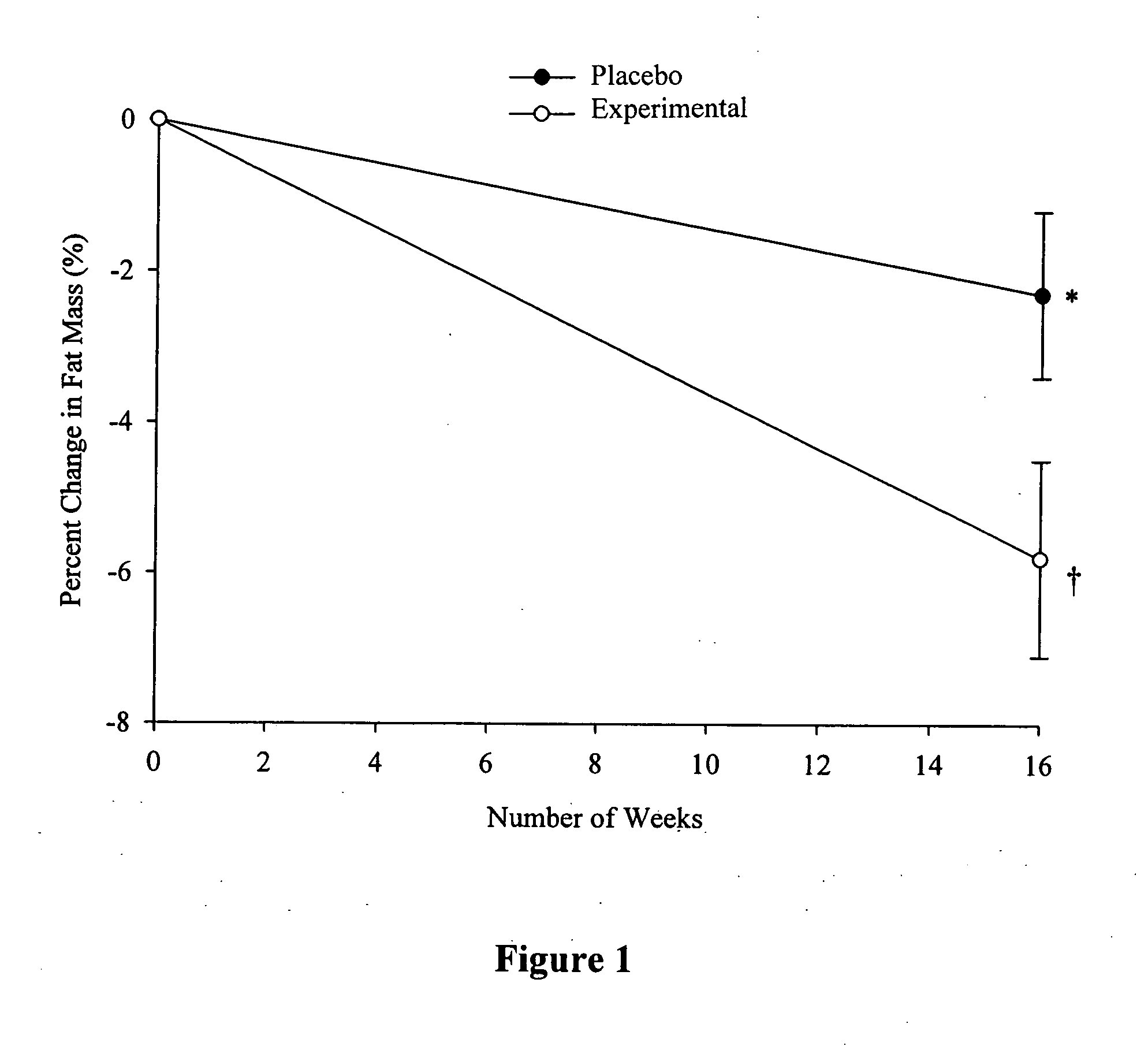
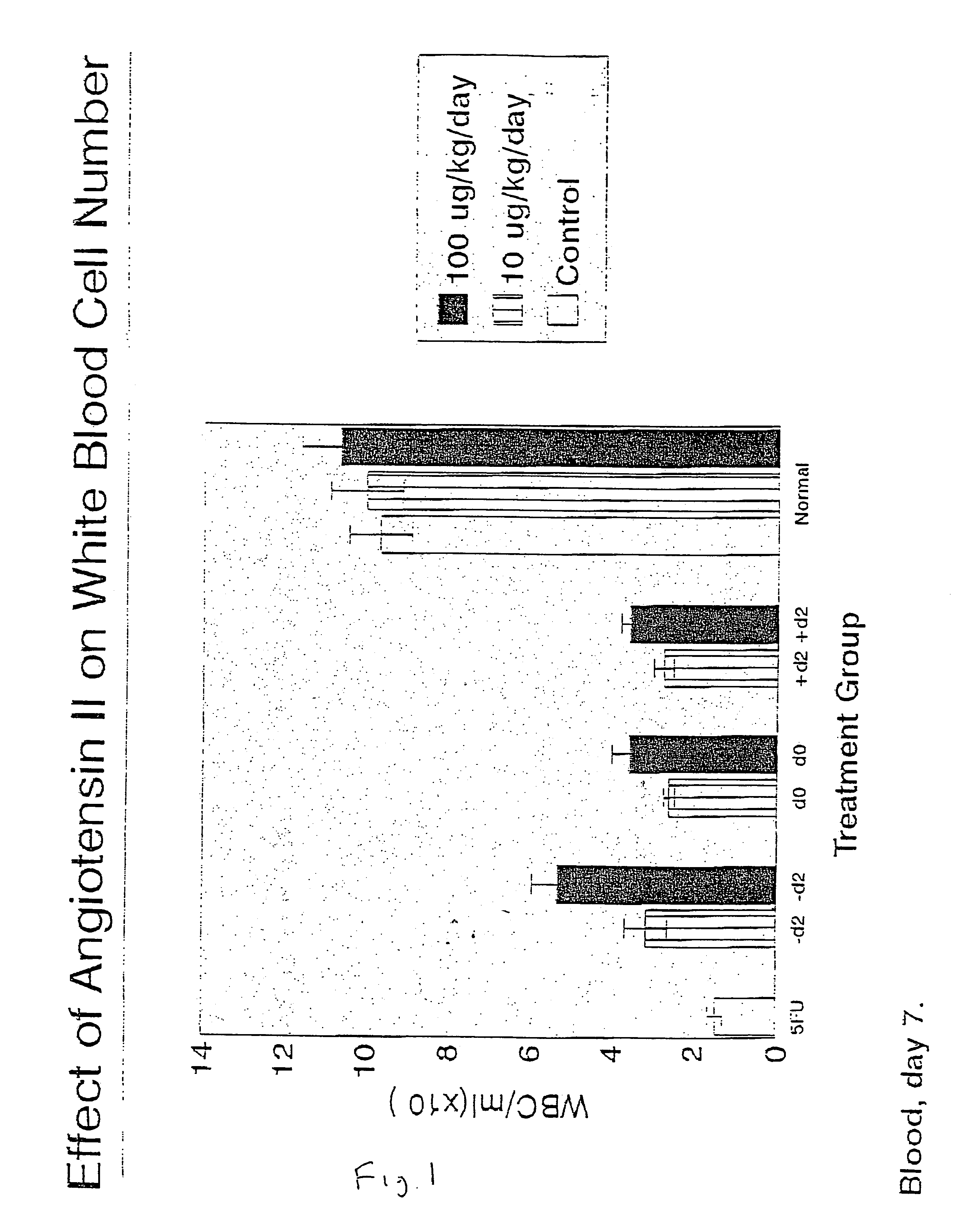
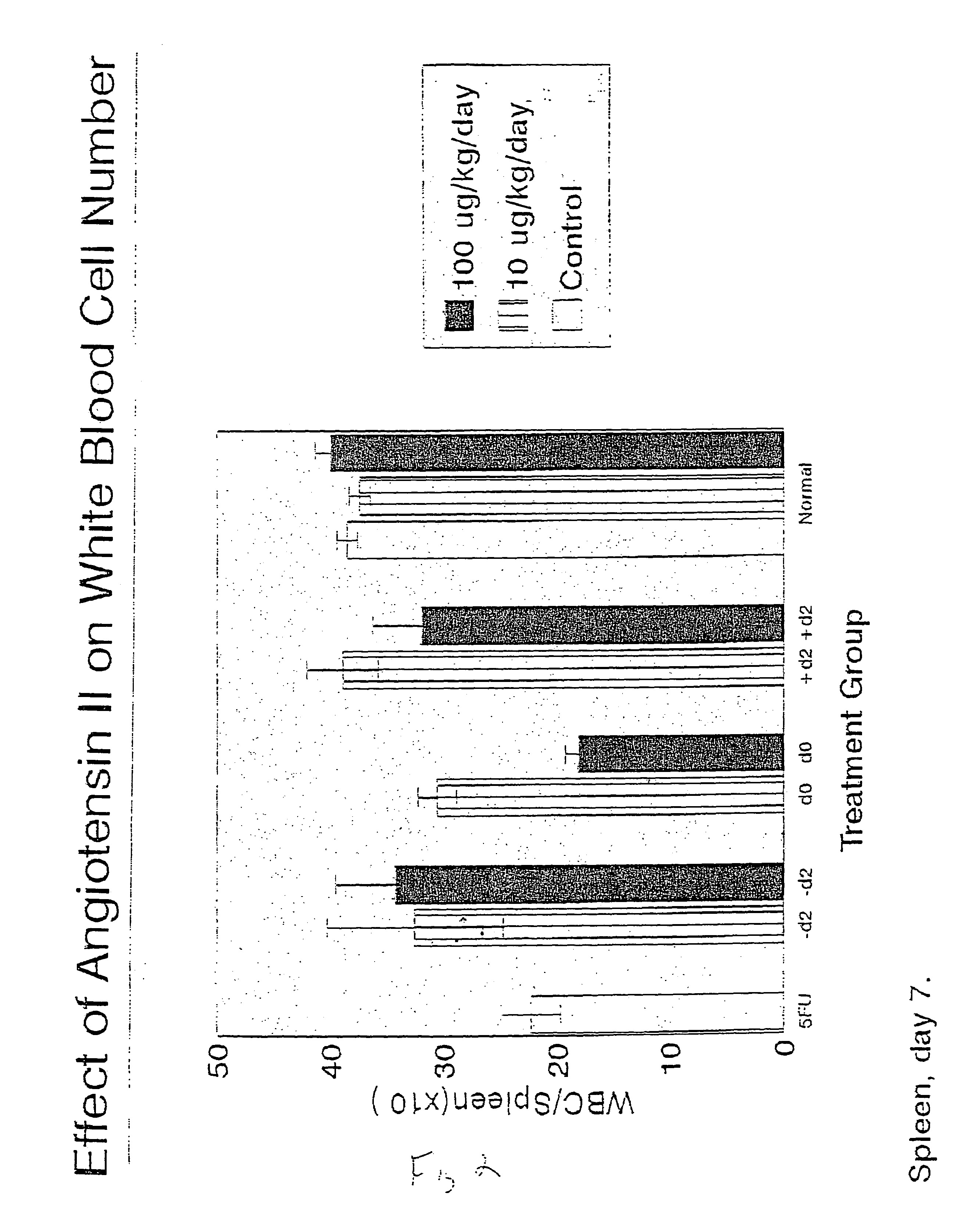
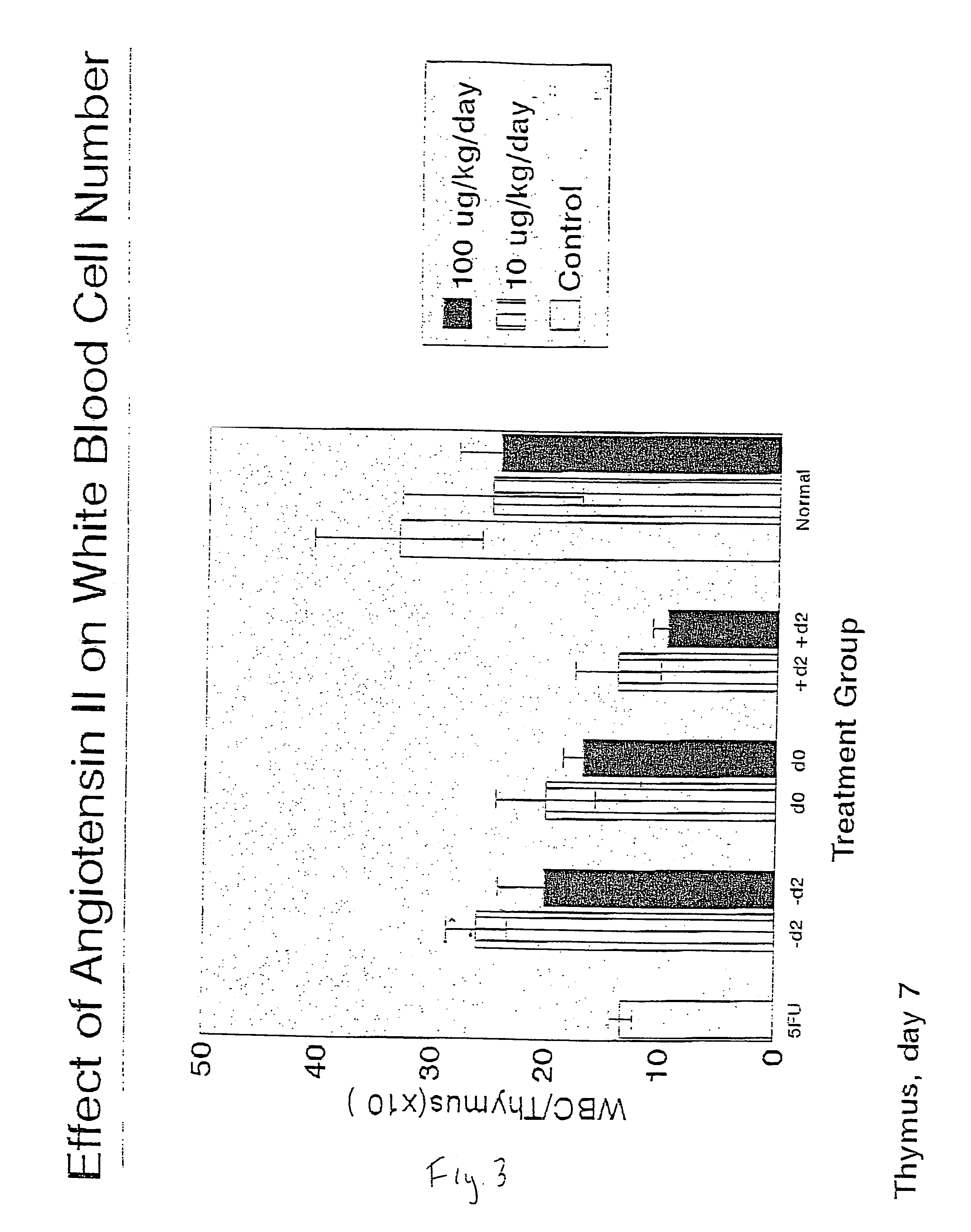
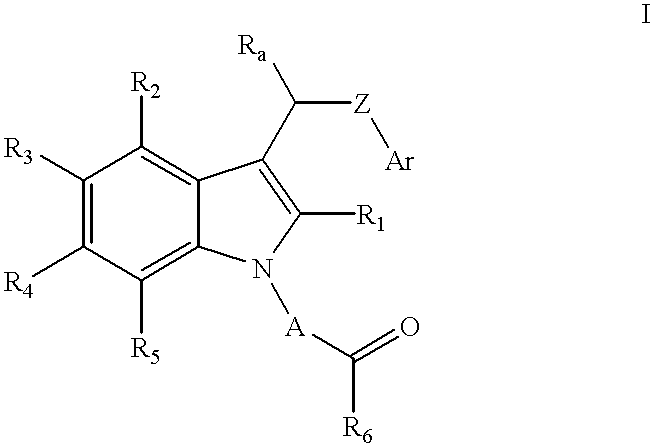
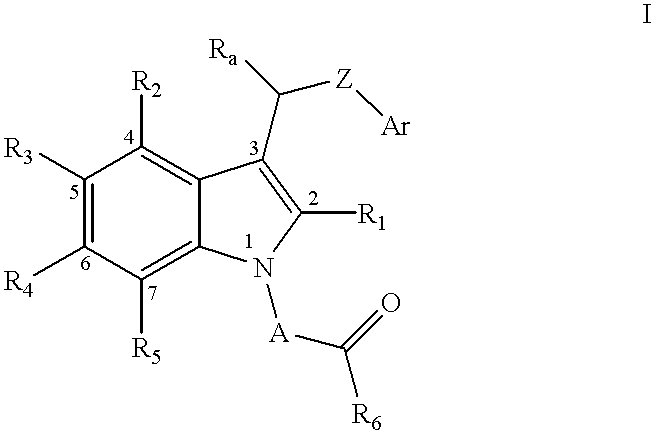
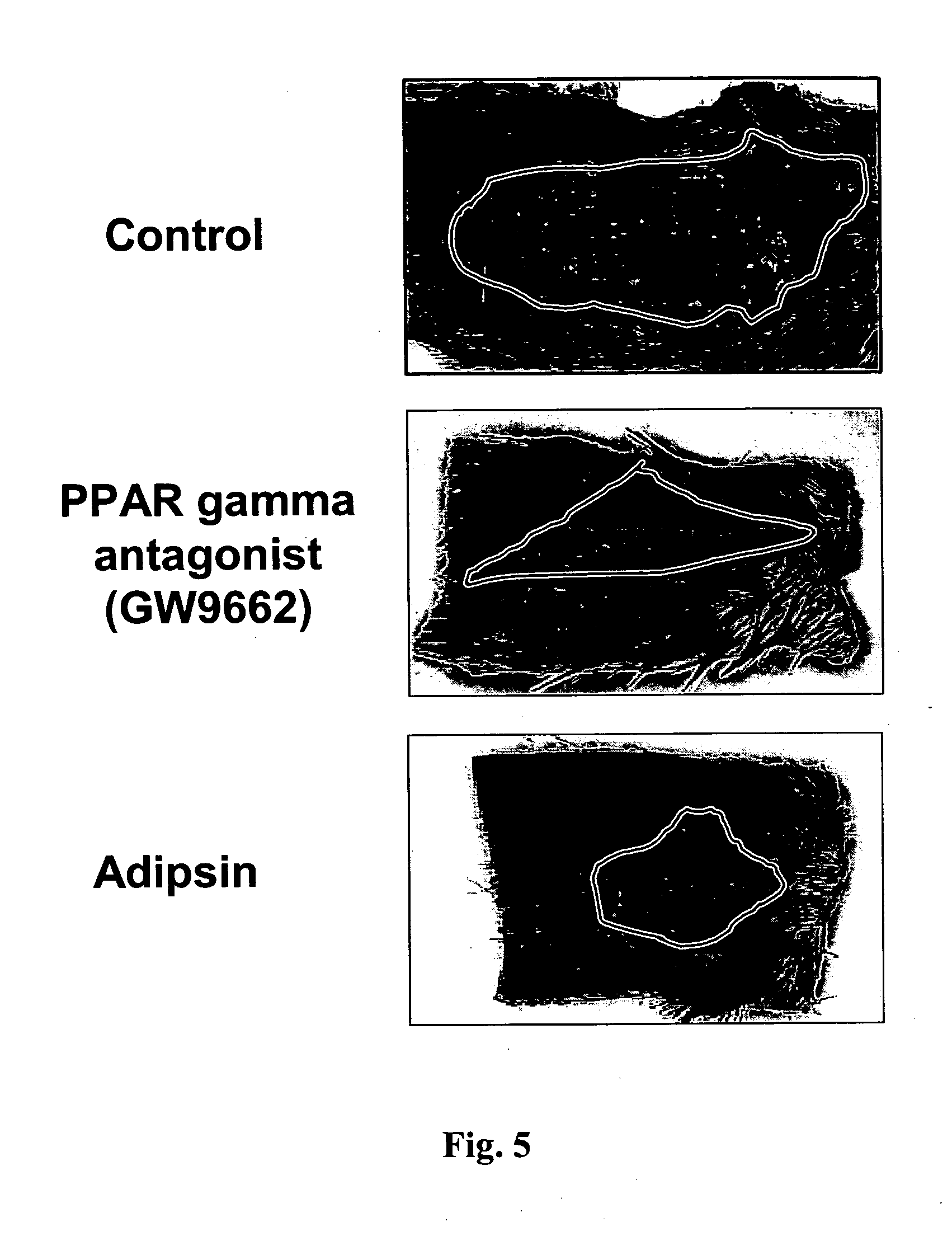
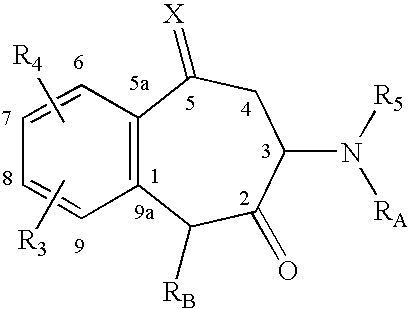
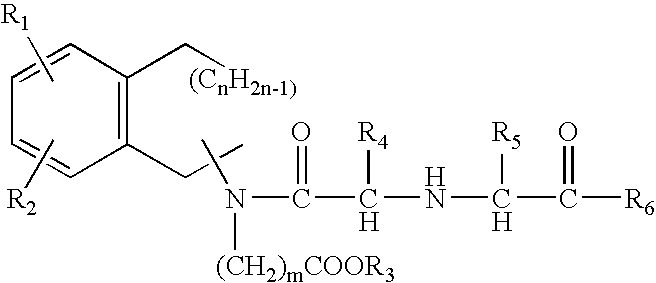
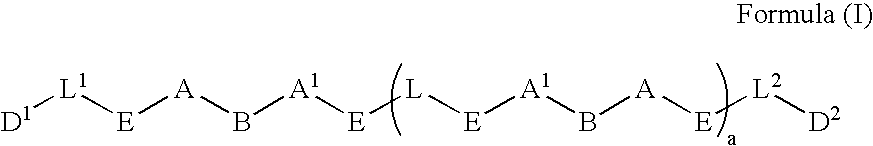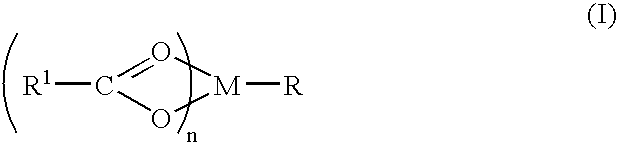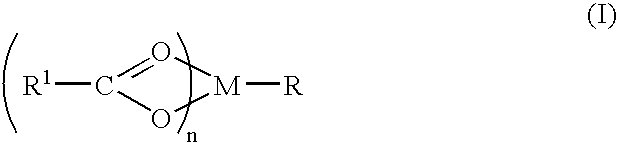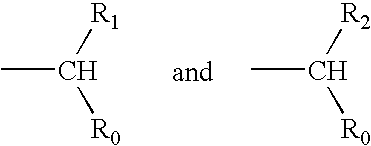Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
158results about "Angiotensins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
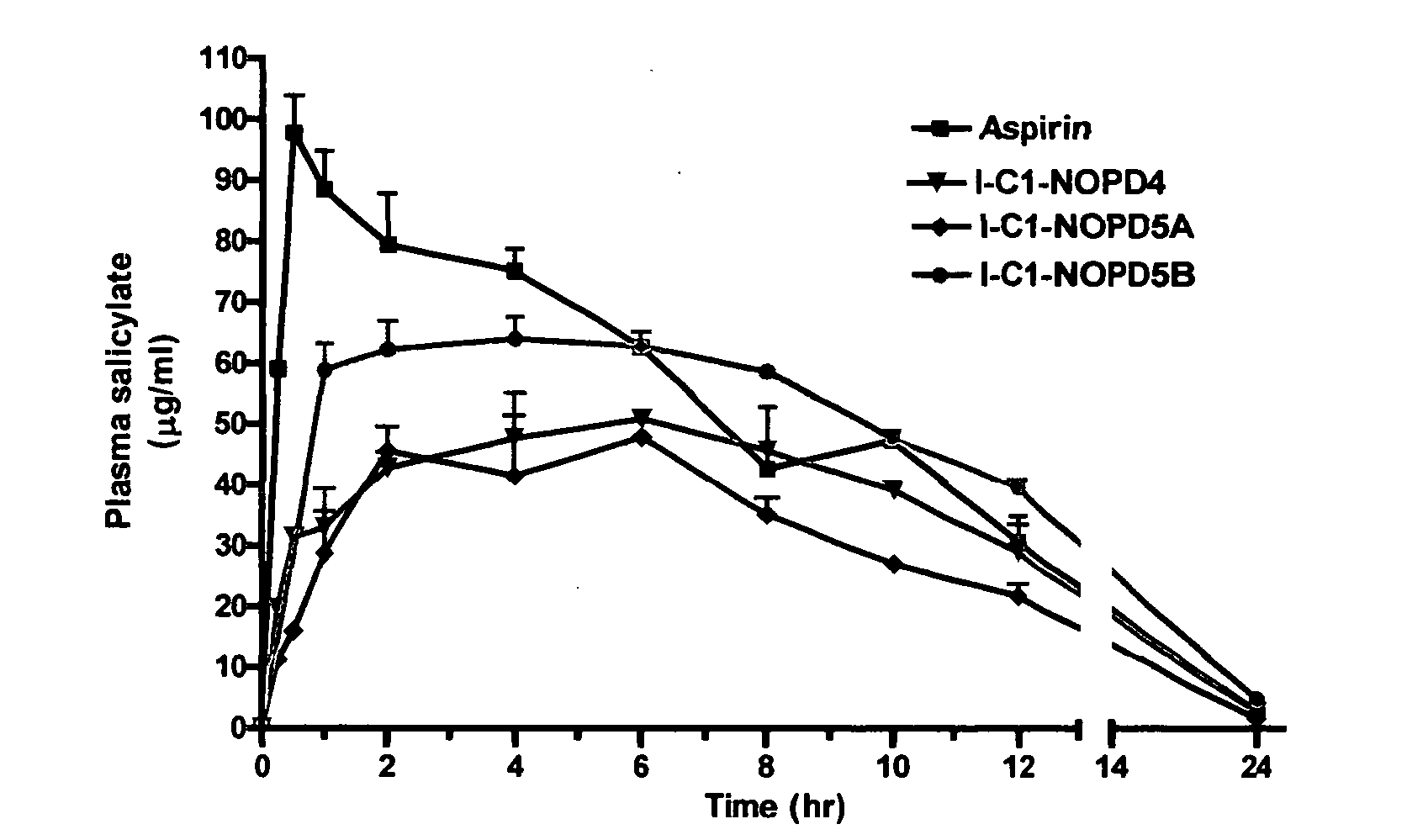
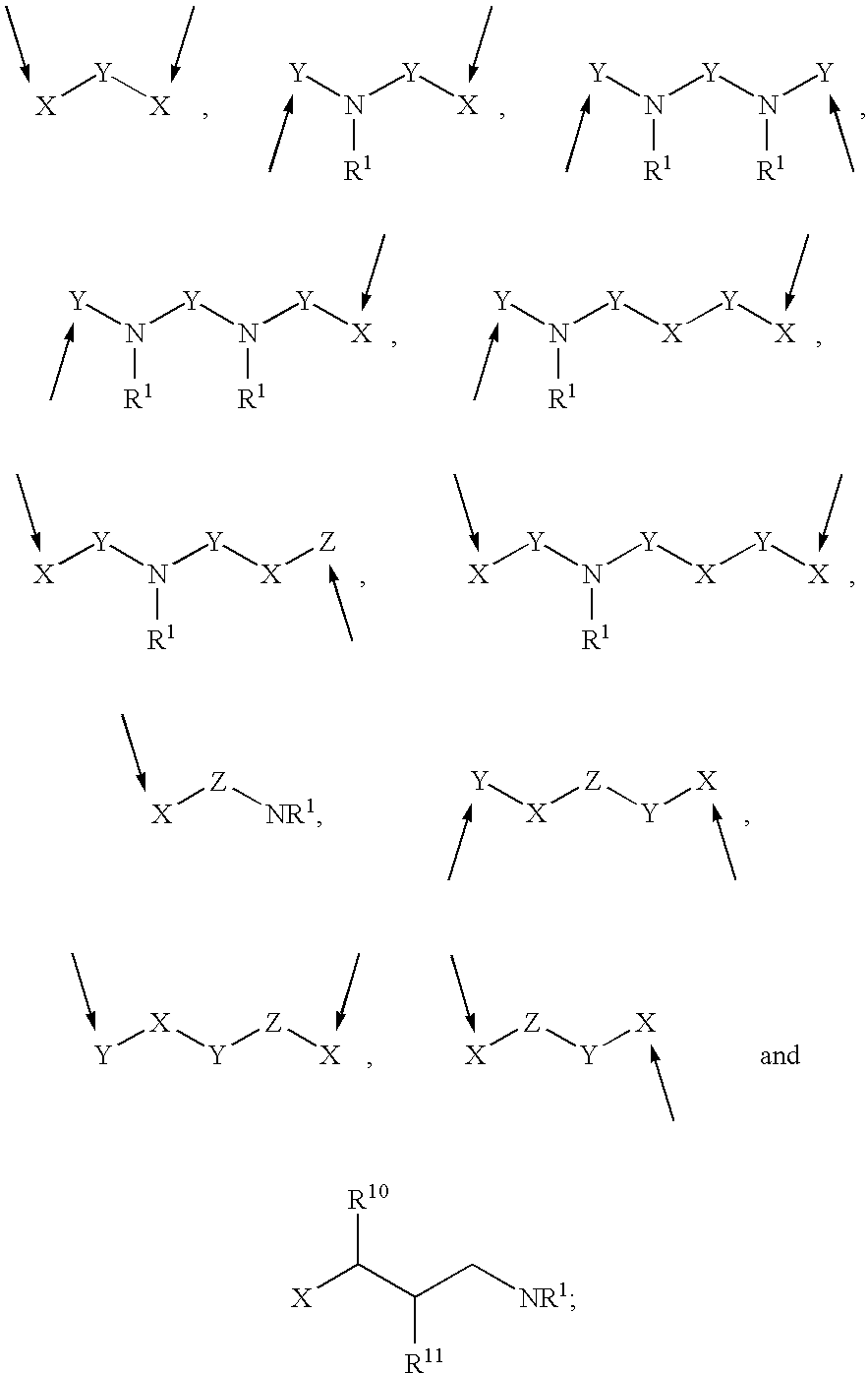
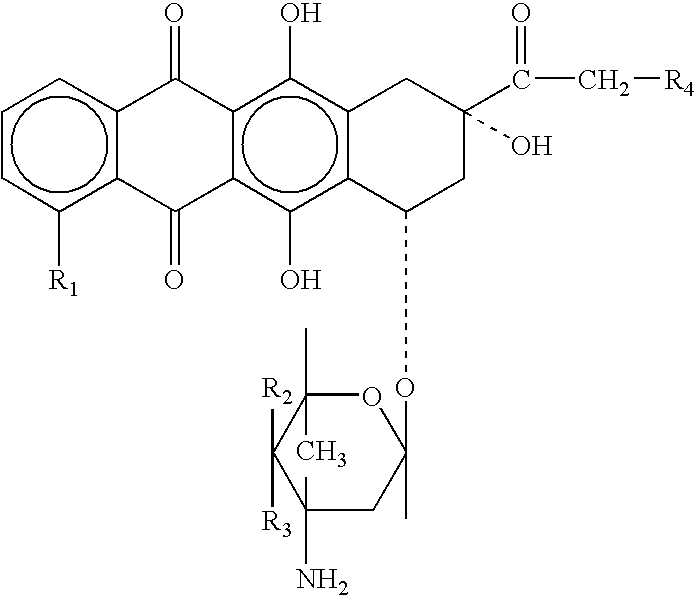
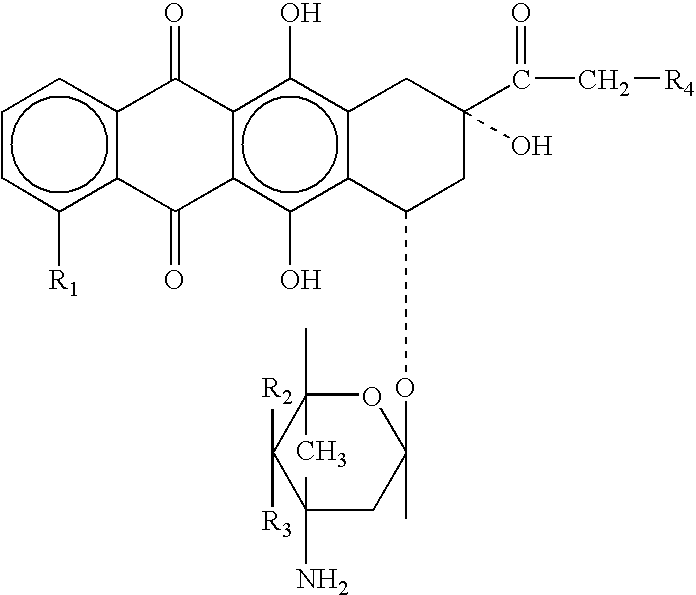
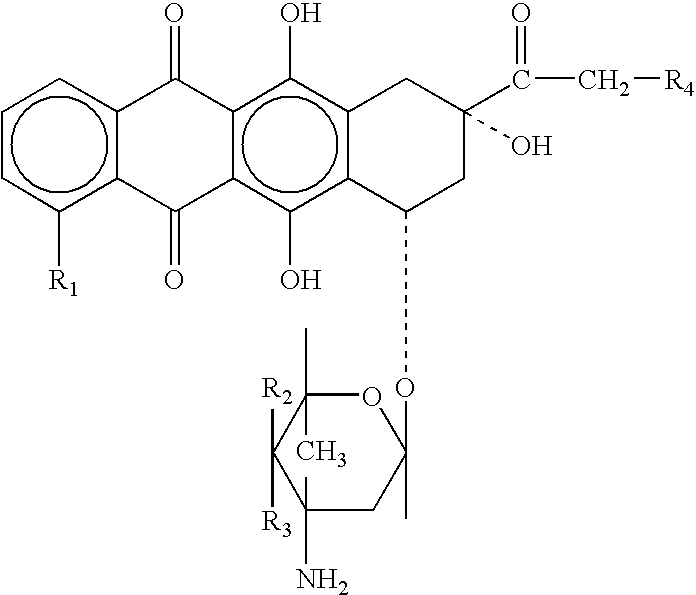
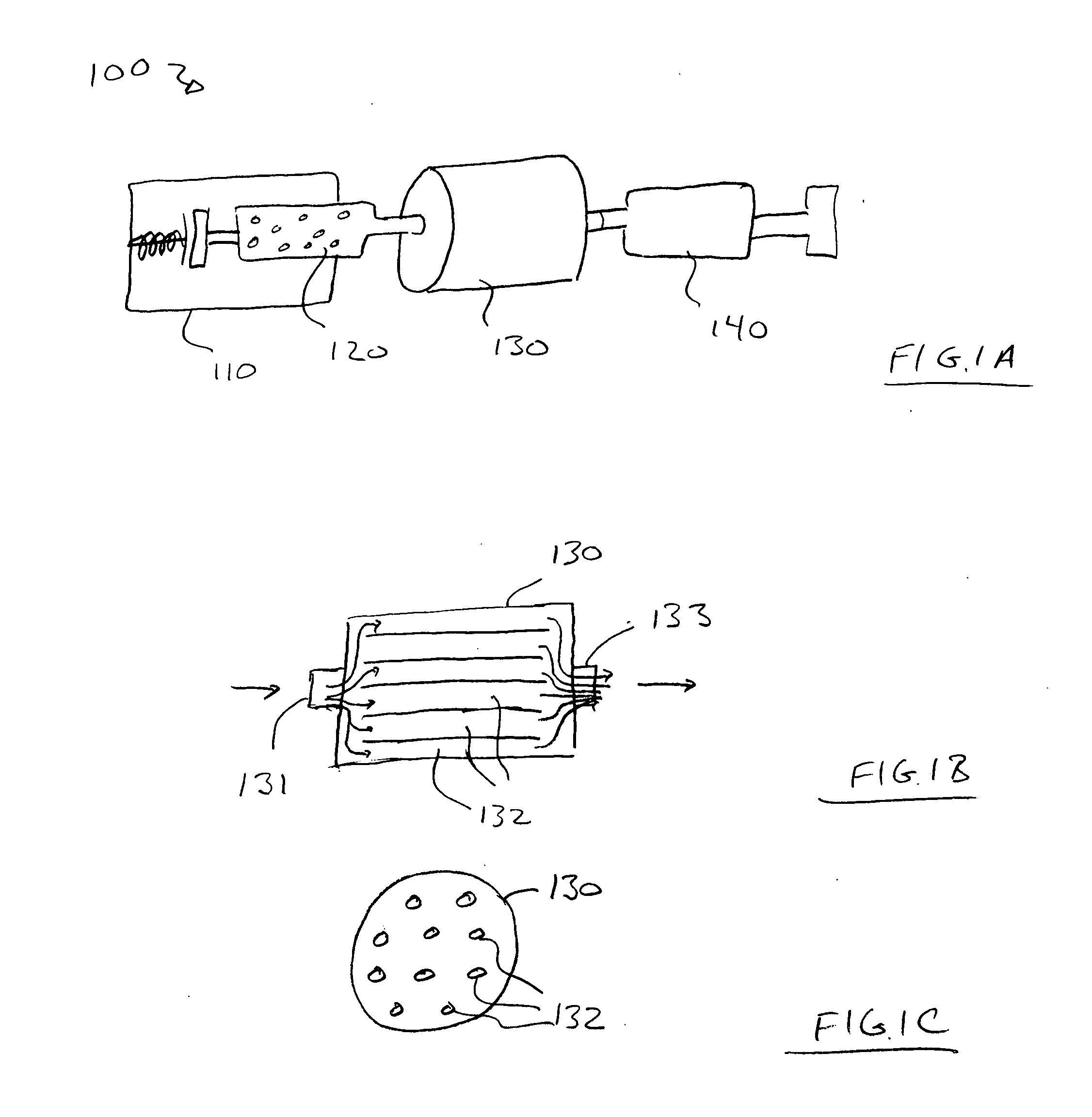
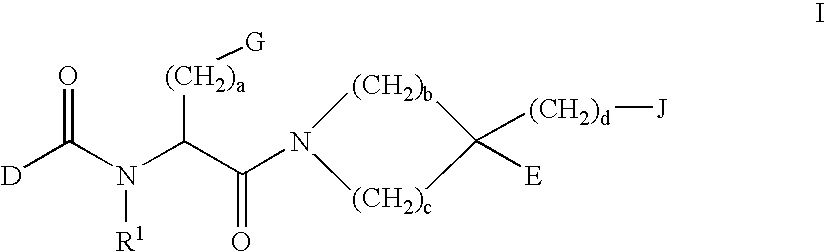
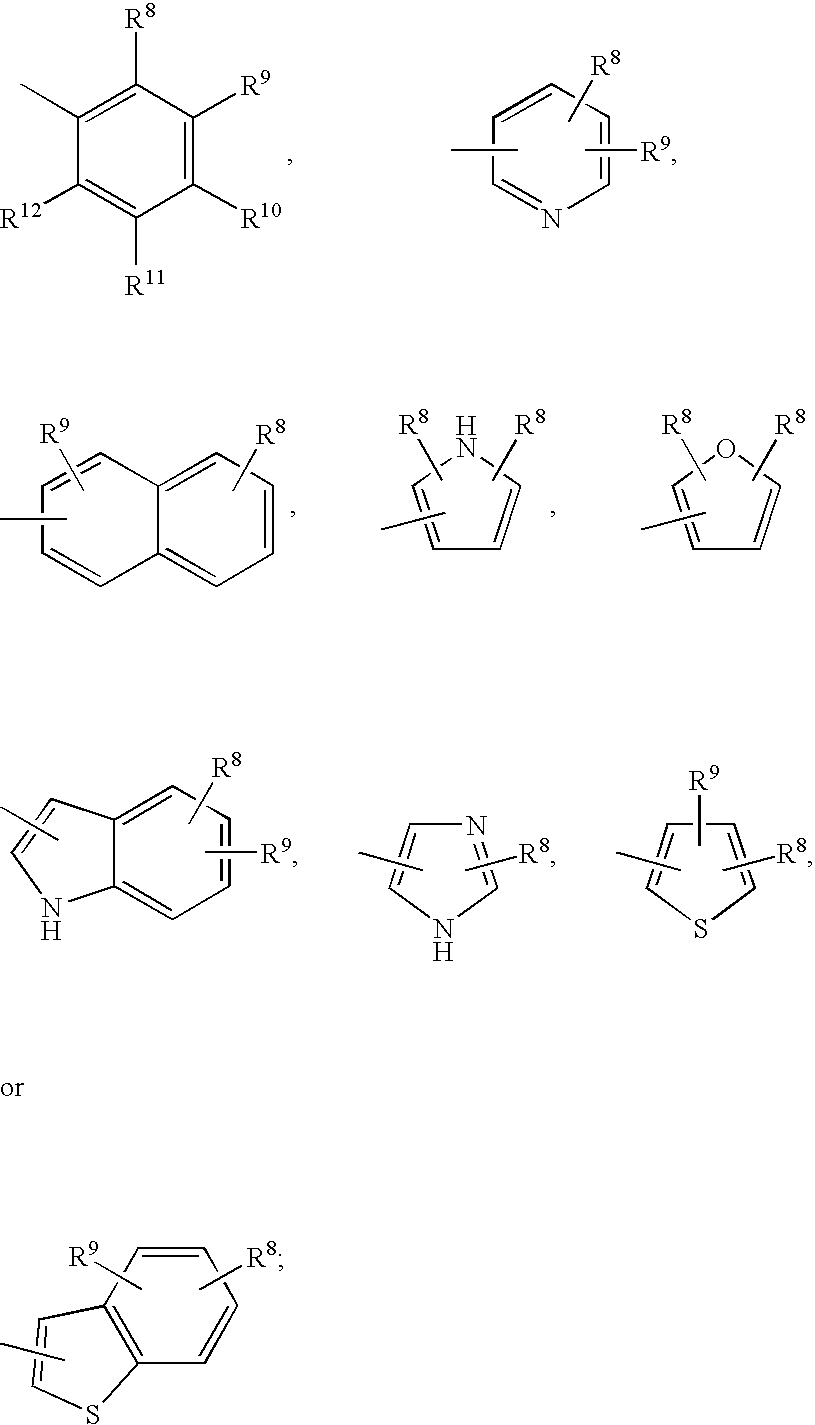
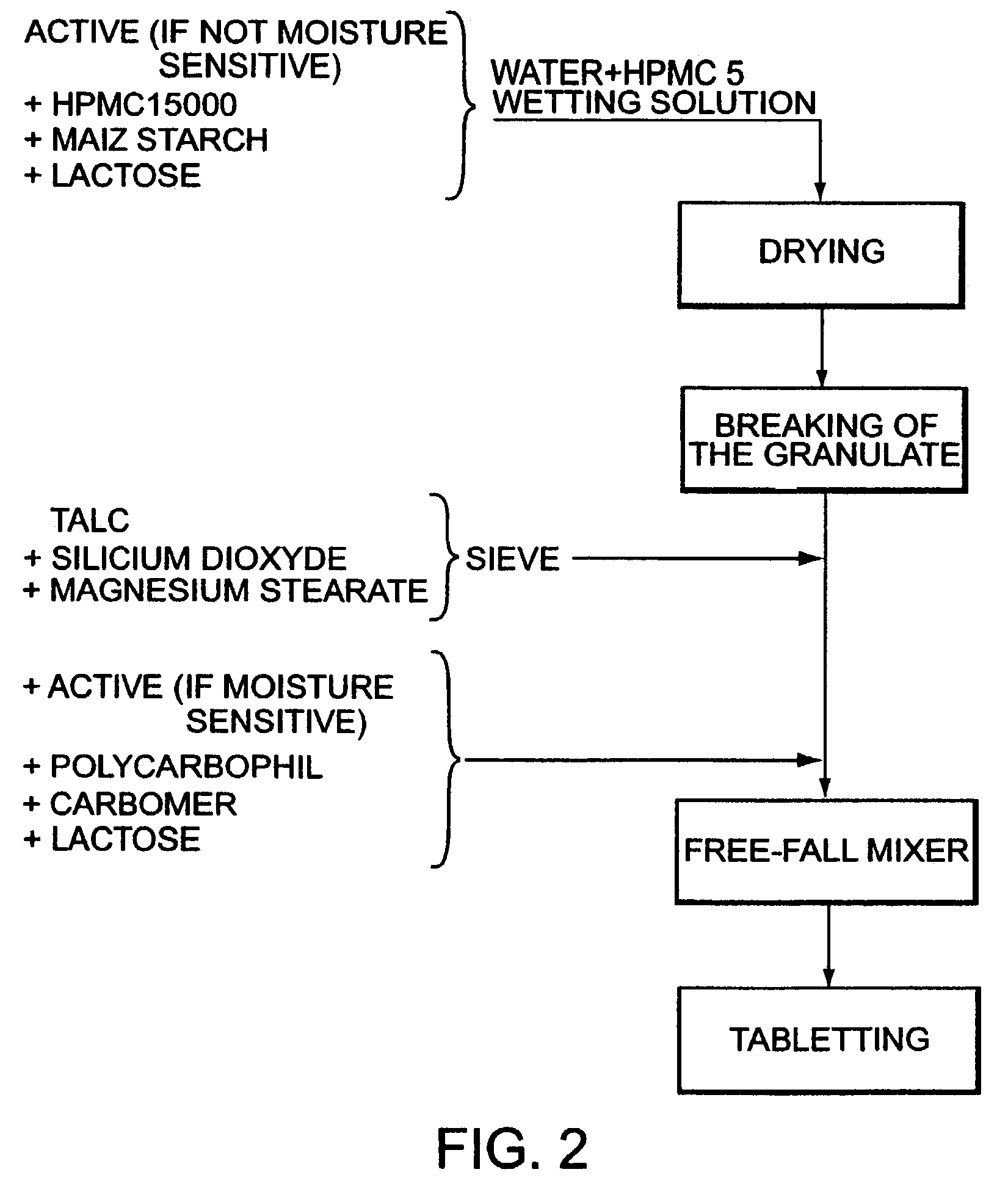
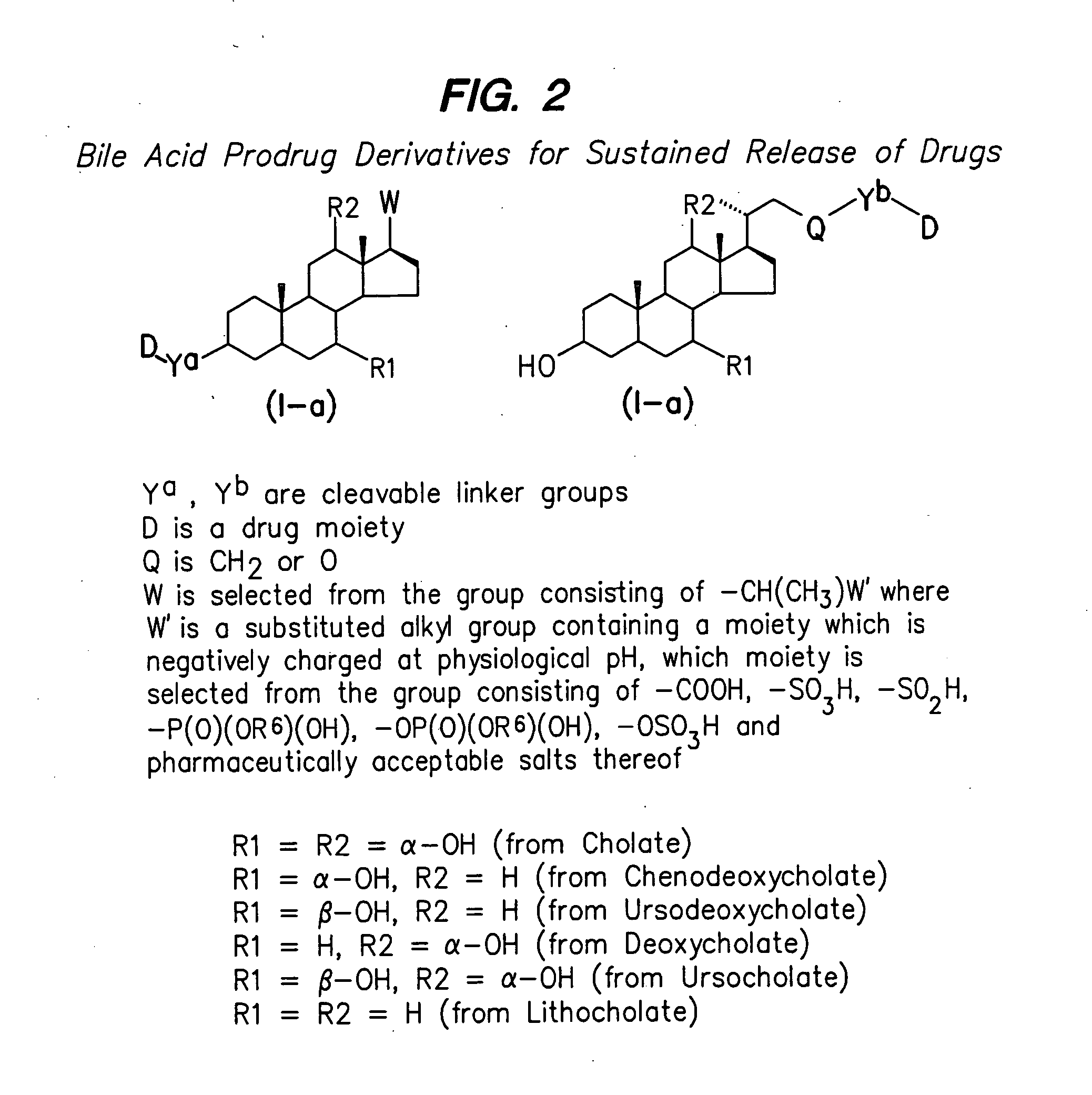
Prodrugs containing novel bio-cleavable linkers
Owner:PIRAMAL ENTERPRISES LTD
Non-aqueous single phase vehicles and formulations utilizing such vehicles
InactiveUS20050008661A1Increase stickinessReduce occlusionPowder deliveryPeptide/protein ingredientsEngineeringSolvent
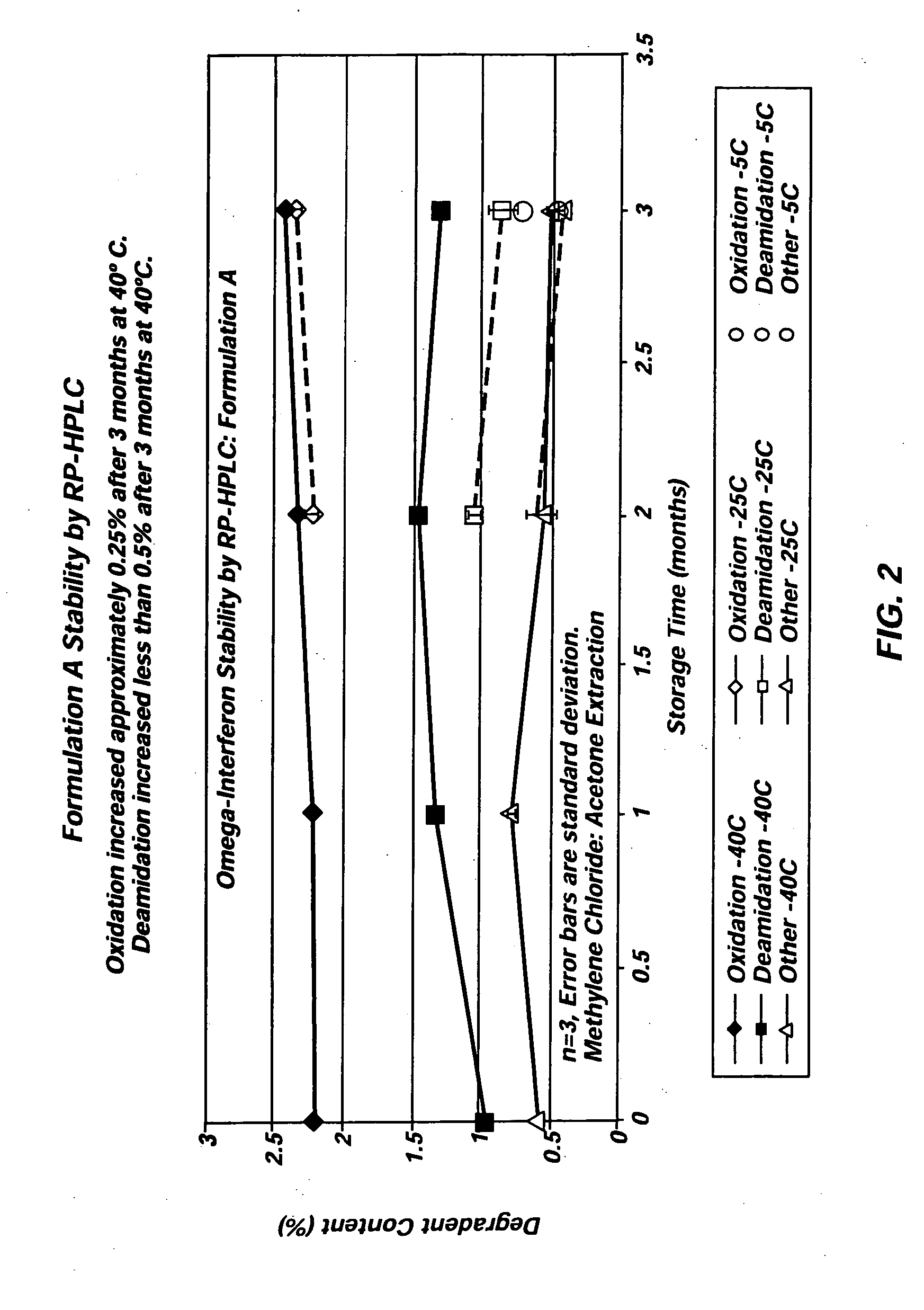
The present invention includes materials and methods for providing vehicles useful for providing drug formulations that address the potential drawbacks of known nonaqueous formulations. In particular, the present invention includes nonaqueous vehicles that are formed using a combination of polymer and solvent that results in a vehicle that is miscible in water. The nonaqueous vehicles facilitate the formulation of drug formulations that are stable over time, even when stored at, or exposed to, elevated temperatures. Moreover, the miscible vehicles of the present invention allow the preparation of drug formulations that work to reduce the occurrence of partial or complete occlusions of the delivery conduits included in delivery devices used to administer the drug formulations.
Owner:DURECT CORP
Non-aqueous single phase vehicles and formulations utilizing such vehicles
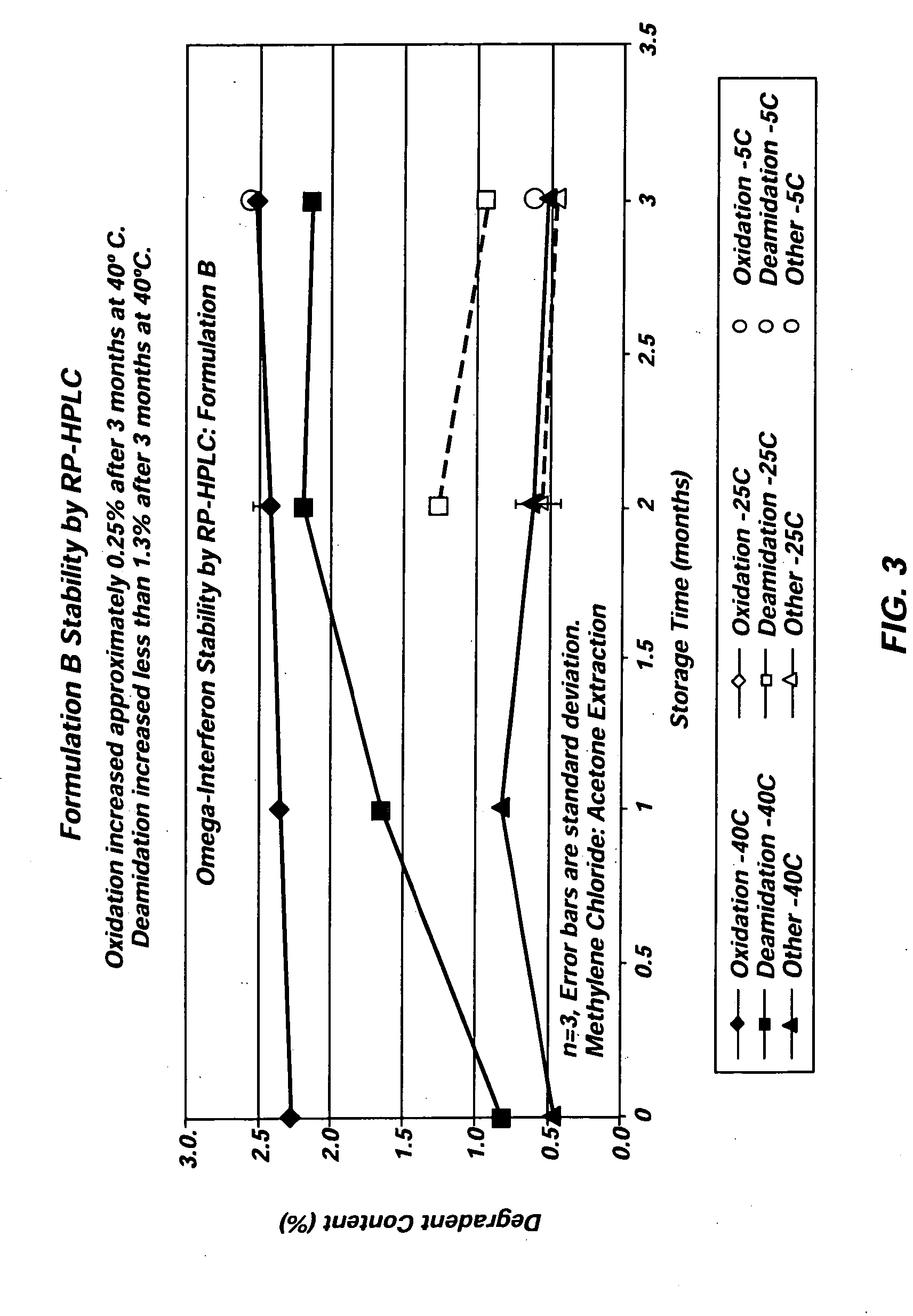
InactiveUS20050276856A1Decrease in levelLower Level RequirementsPowder deliveryPeptide/protein ingredientsMedicineDelivery vehicle
Owner:DURECT CORP
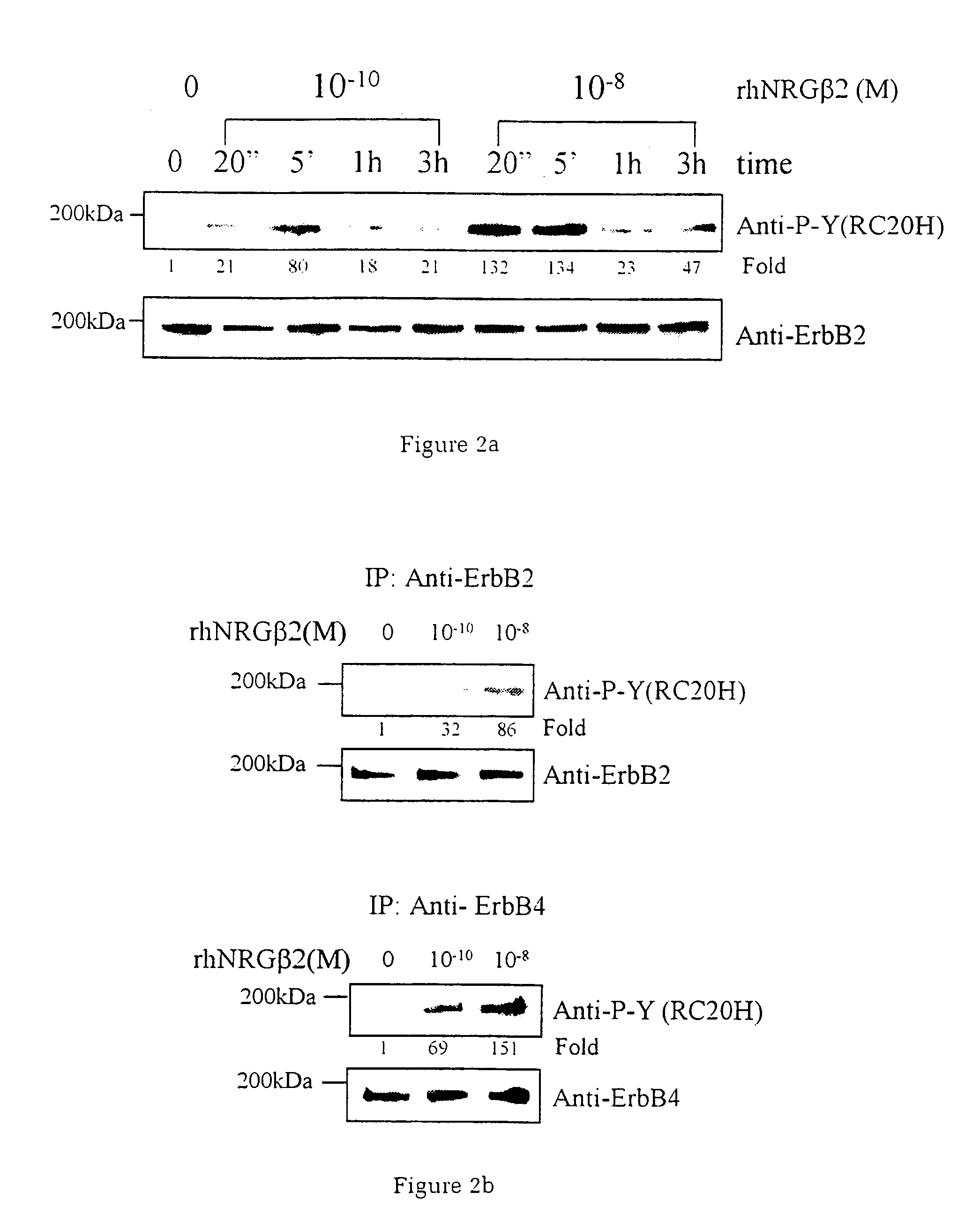
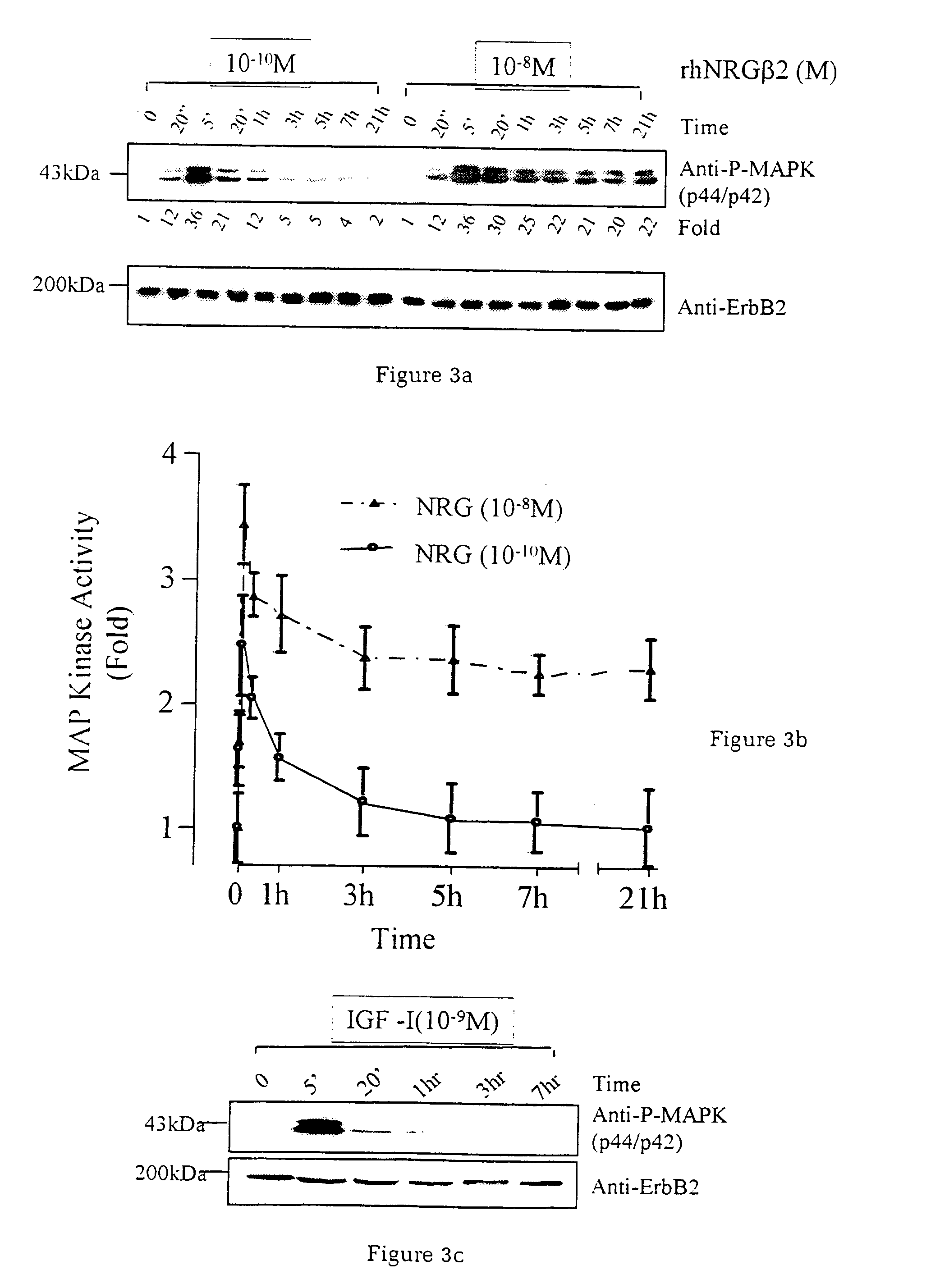
Neuregulin based methods and compositions for treating cardiovascular diseases
ActiveUS20060019888A1Prevent and reduce and delay cardiac toxicityAvoid toxicityAntibacterial agentsBiocideNeuregulinInternal medicine
The present invention relates to compositions and methods for preventing, treating or delaying various cardiovascular diseases or disorders in mammals, particularly in humans. More particularly, the present invention provides for compositions and methods for preventing, treating or delaying various cardiovascular diseases or disorders using, inter alia, a neuregulin protein, or a functional fragment thereof, or a nucleic acid encoding a neuregulin protein, or a functional fragment thereof, or an agent that enhances production and / or function of said neuregulin.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
Cardiac muscle function and manipulation
InactiveUS7226907B1Augment PE-mediated cardiac muscle cell differentiationImprove heart functionPeptide/protein ingredientsGenetic material ingredientsCardiomyocyte growthNeuregulin
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
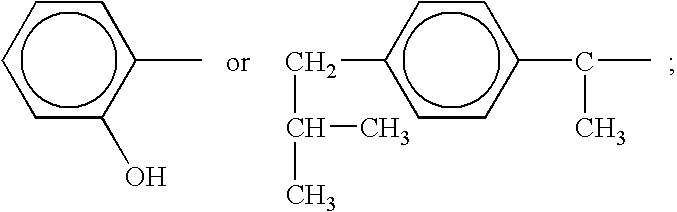
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
Medical devices having nanoporous bonding layers
The present invention relates generally to medical devices with therapy eluting components and methods for making same. More specifically, the invention relates to implantable medical devices having at least one porous layer, and methods for making such devices, and loading such devices with therapeutic agents. A mixture or alloy is placed on the surface of a medical device, then one component of the mixture or alloy is generally removed without generally removing the other components of the mixture or alloy. In some embodiments, a porous layer is adapted for bonding non-metallic coating, including drug eluting polymeric coatings. A porous layer may have a random pore structure or an oriented or directional grain porous structure. One embodiment of the invention relates to medical devices, including vascular stents, having at least one porous layer adapted to resist stenosis or cellular proliferation without requiring elution of therapeutic agents. The invention also includes methods, devices, and specifications for loading of drugs and other therapeutic agents into nanoporous coatings.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
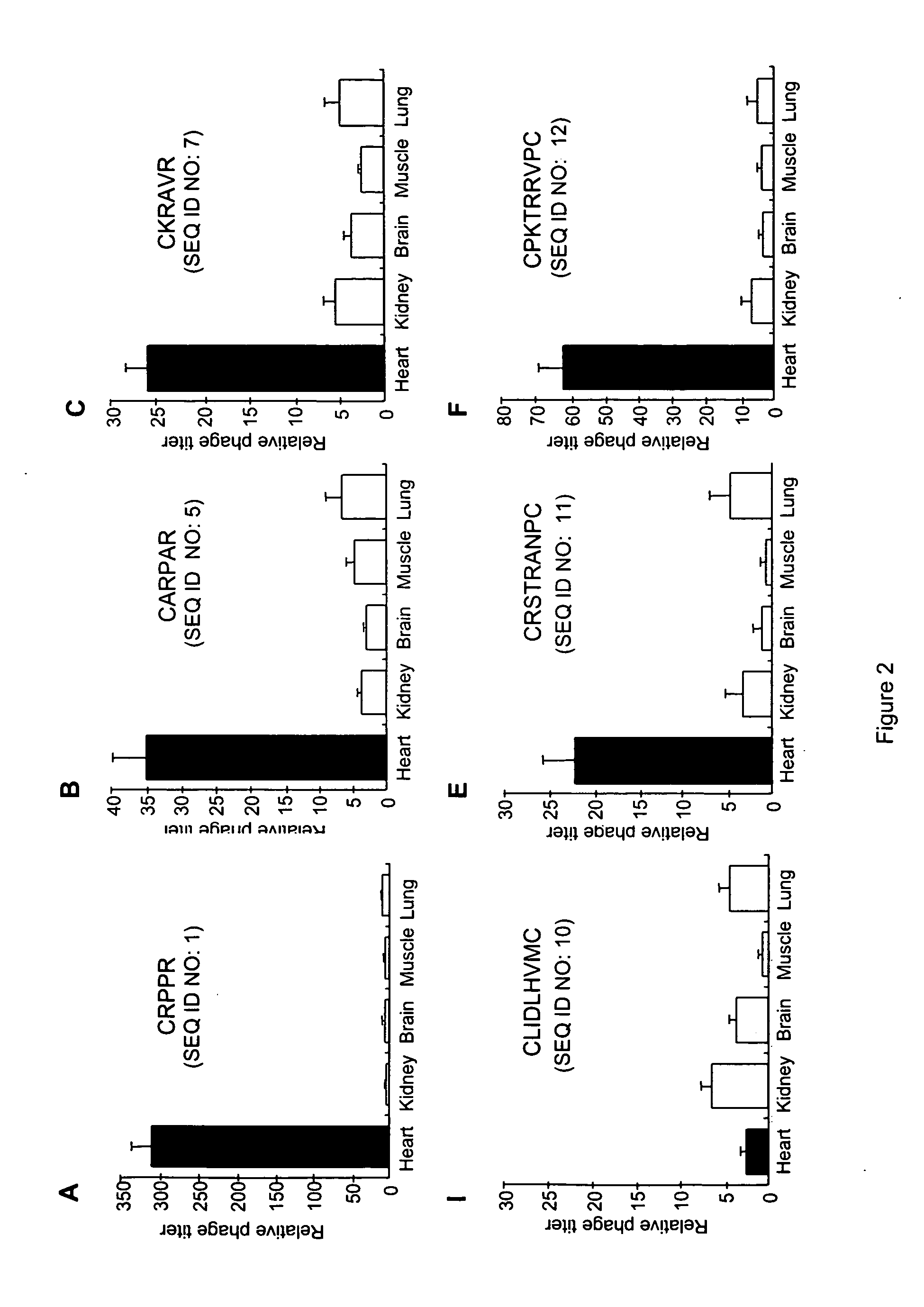
Peptides that selectively home to heart vasculature and related conjugates and methods
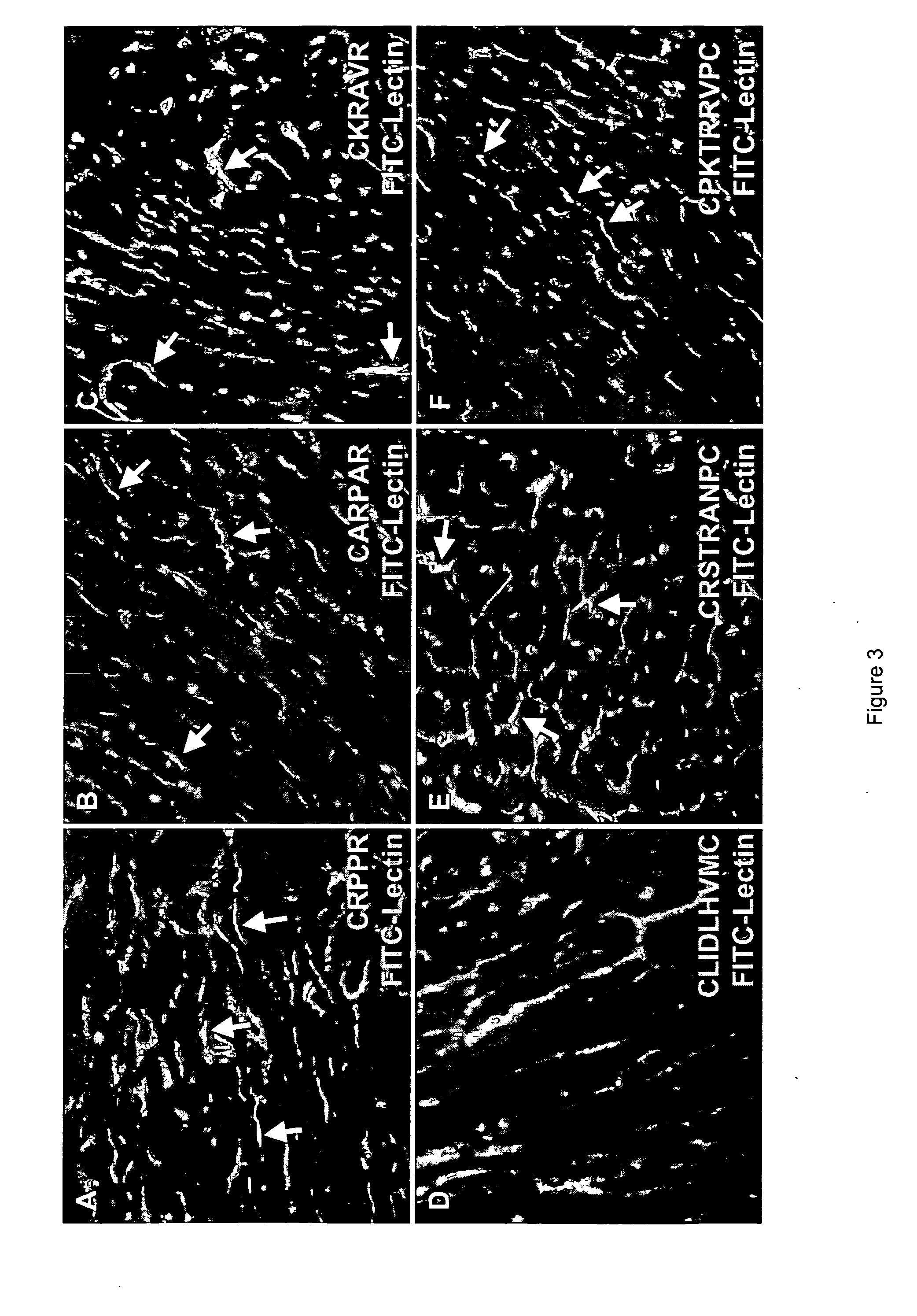
The present invention provides a variety of isolated peptides and peptidomimetics, which can be useful, for example, in constructing the conjugates of the invention or, where the peptide itself has biological activity, in unconjugated form as a therapeutic for treating any of a variety of cardiovascular diseases as described below. Thus, the present invention provides an isolated peptide or peptidomimetic which has a length of less than 60 residues and includes the amino acid sequence CRPPR (SEQ ID NO: 1) or a peptidomimetic thereof. The invention further provides an isolated peptide or peptidomimetic which has a length of less than 60 residues and includes the amino acid sequence CARPAR (SEQ ID NO: 5) or a peptidomimetic thereof, or amino acid sequence CPKRPR (SEQ ID NO: 6) or a peptidomimetic thereof.
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
Therapeutic methods utilizing naturally derived bio-active complexes and delivery systems therefor
InactiveUS6303588B1Restore lipid membraneHigh oxygen utilizationCosmetic preparationsBiocideMedicineNormal cell
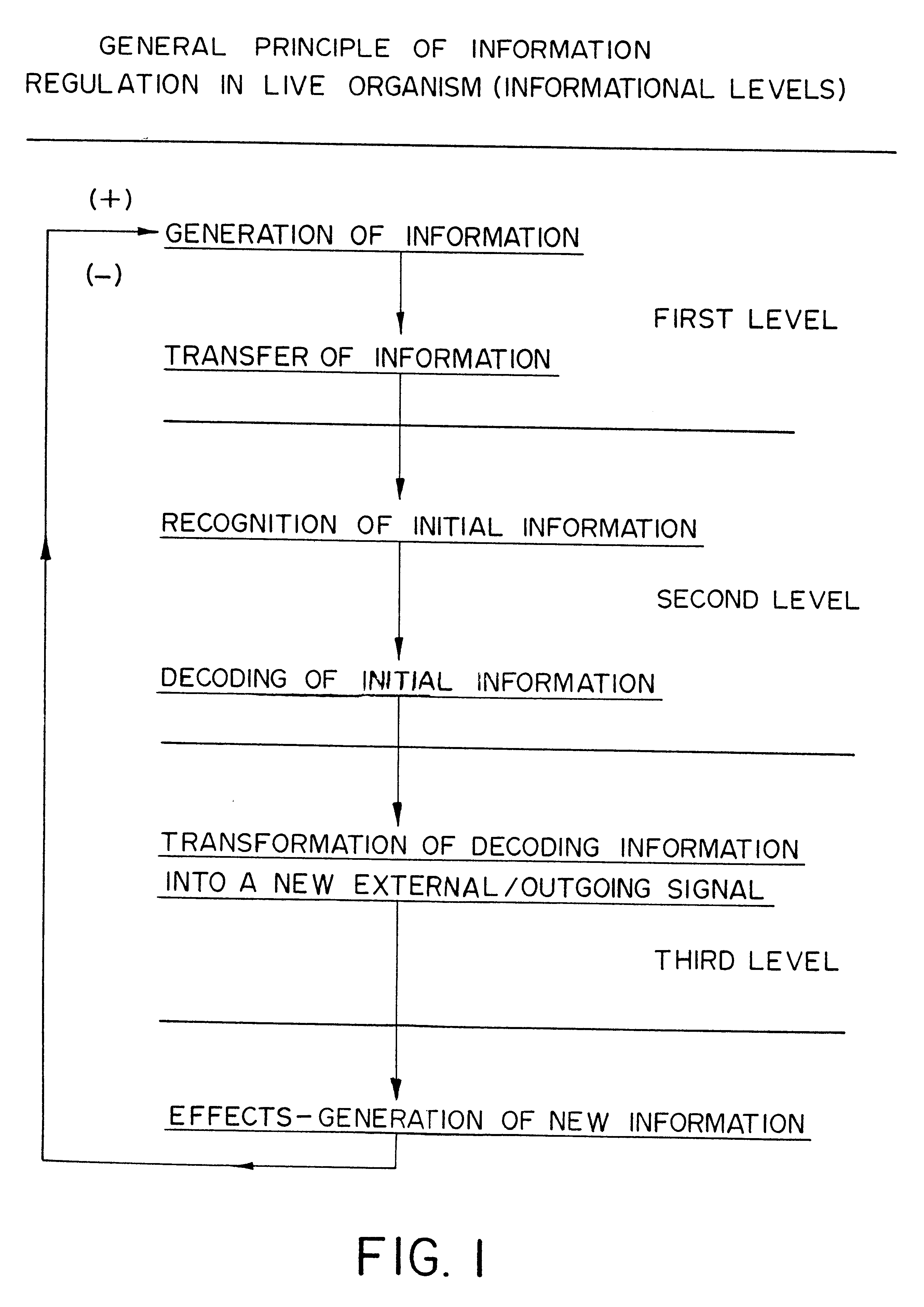
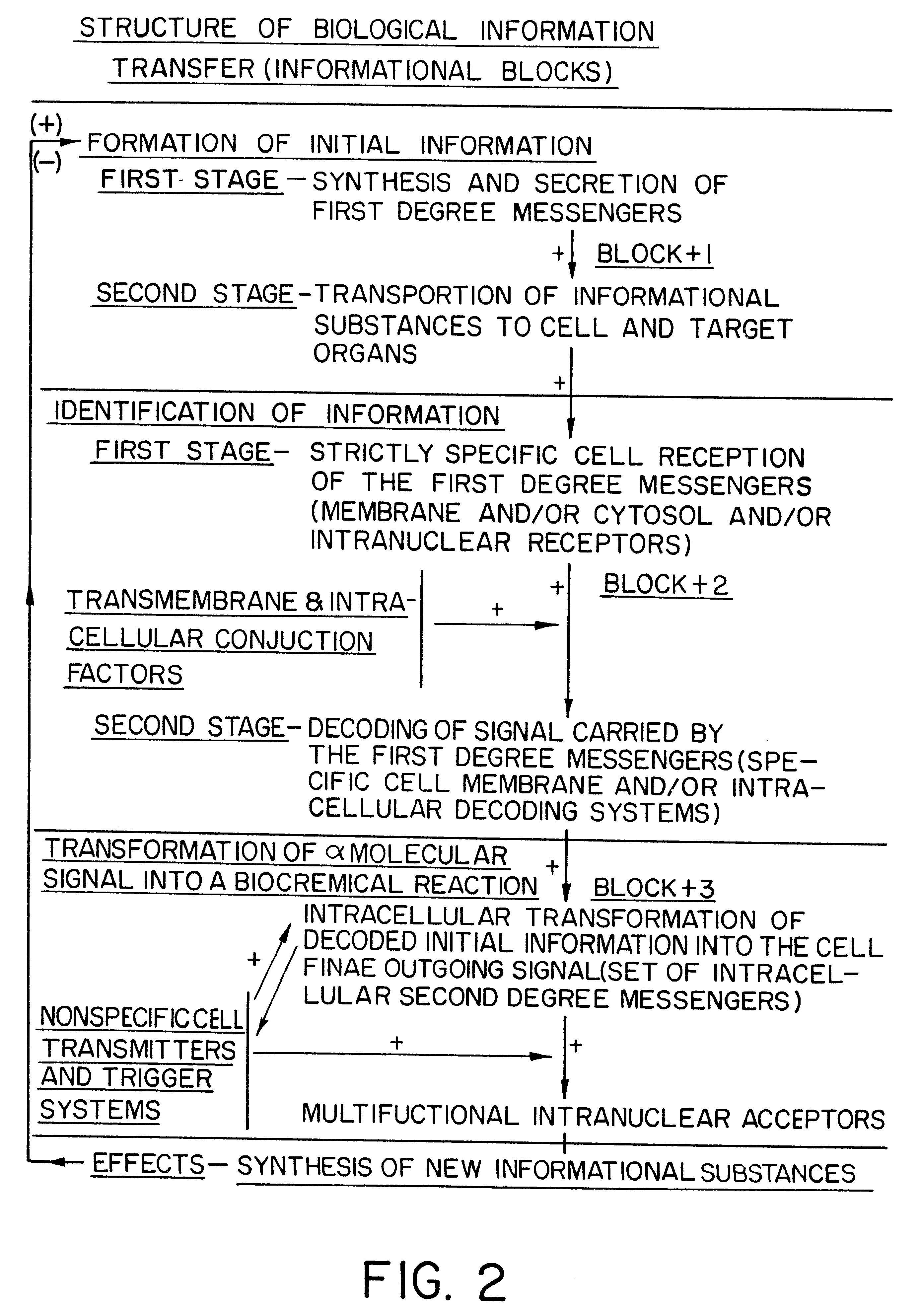
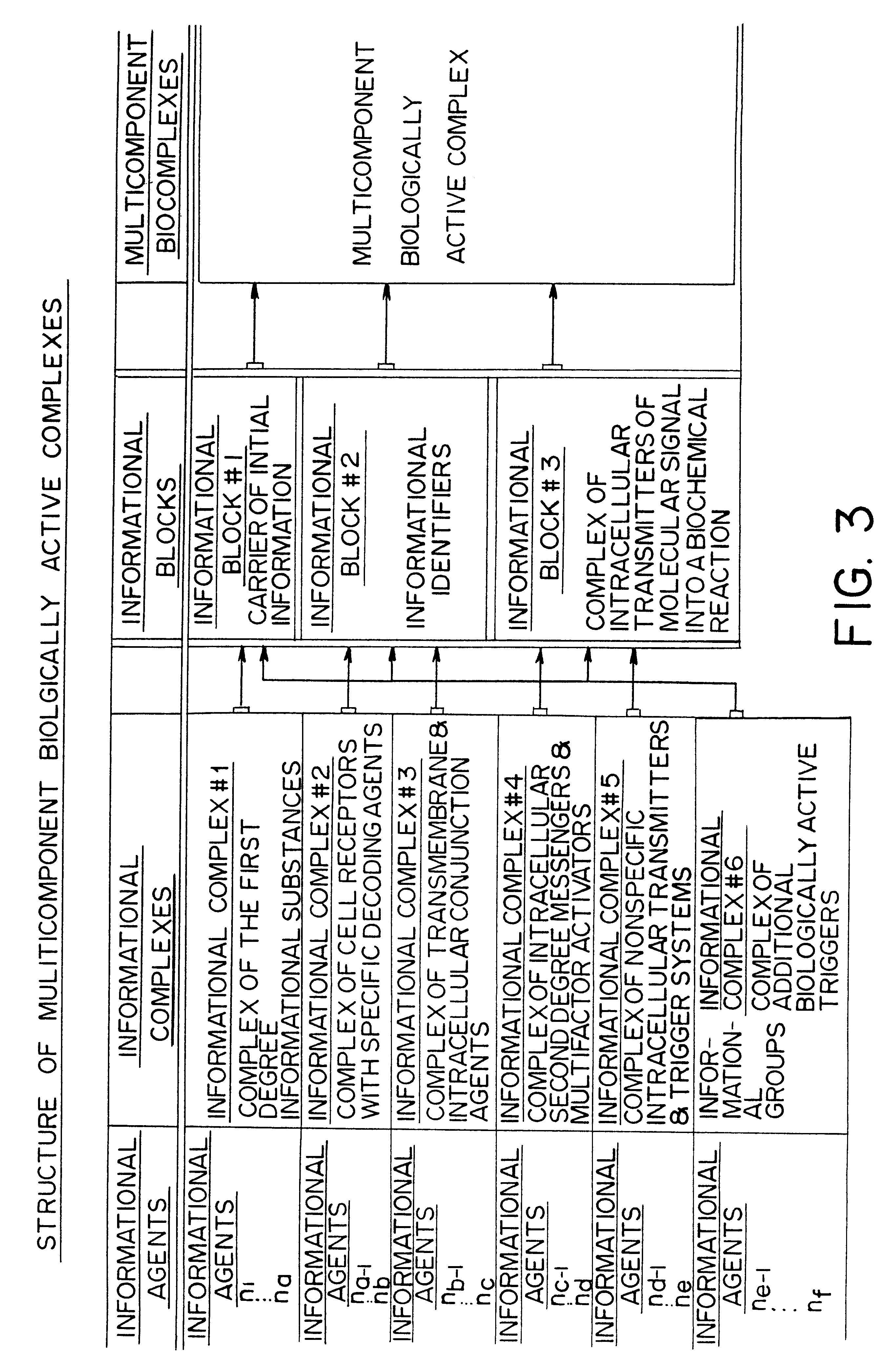
Methods are disclosed for correcting biological information transfer in a patient in need of such therapy which comprise administration to a patient of a composition comprising a therapeutically effective amount of a biocomplex comprising at least one bioactive agent from each of the three informational blocks of biological information transfer, each agent being present in an amount sufficient to correct the biological information transfer of the patient under treatment and resulting in the resumption of normal cell metabolism, said amount being less than the buffering amount of said agent; together with a carrier therefor.
Owner:DANIELOV MICHAEL M
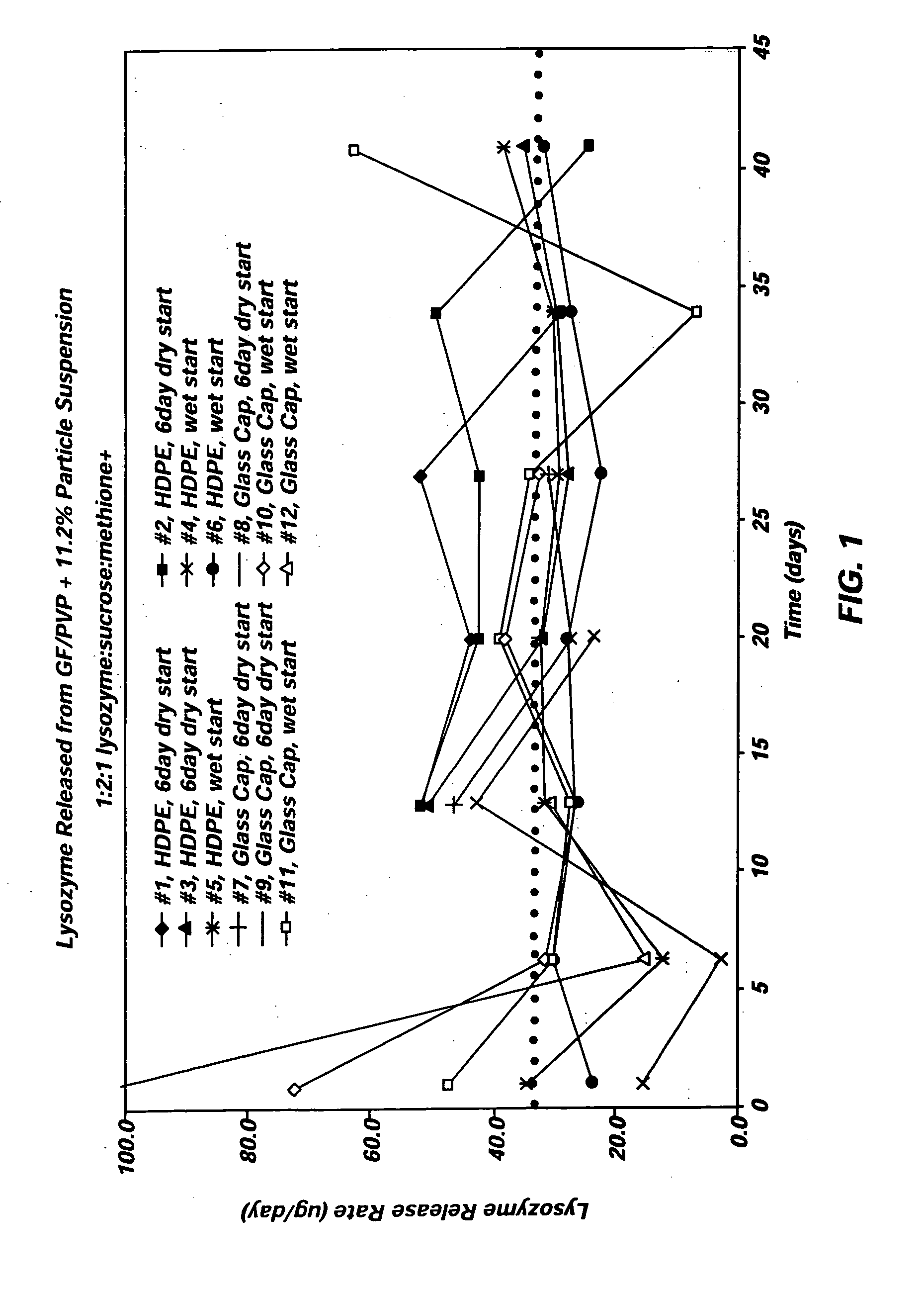
Combination Degradable and Non-Degradable Matrices for Active Agent Delivery
The present invention relates to relates to combination degradable and non-degradable matrices and related methods. In an embodiment, the invention includes an active agent delivery matrix including a degradable polymer network, a non-degradable polymer network, the non-degradable polymer network interspersed within the degradable polymer network, and an active agent. In an embodiment, the invention includes an active agent elution control matrix including a degradable polymer; and a non-degradable polymer interspersed with the degradable polymer. In an embodiment, the invention includes a method of making an active agent delivery matrix including mixing a degradable polymer with a first solvent to form a degradable polymer solution; mixing a non-degradable polymer with a second solvent to form a non-degradable polymer solution; and simultaneously depositing the degradable polymer solution and the non-degradable polymer solution onto a substrate.
Owner:SURMODICS INC
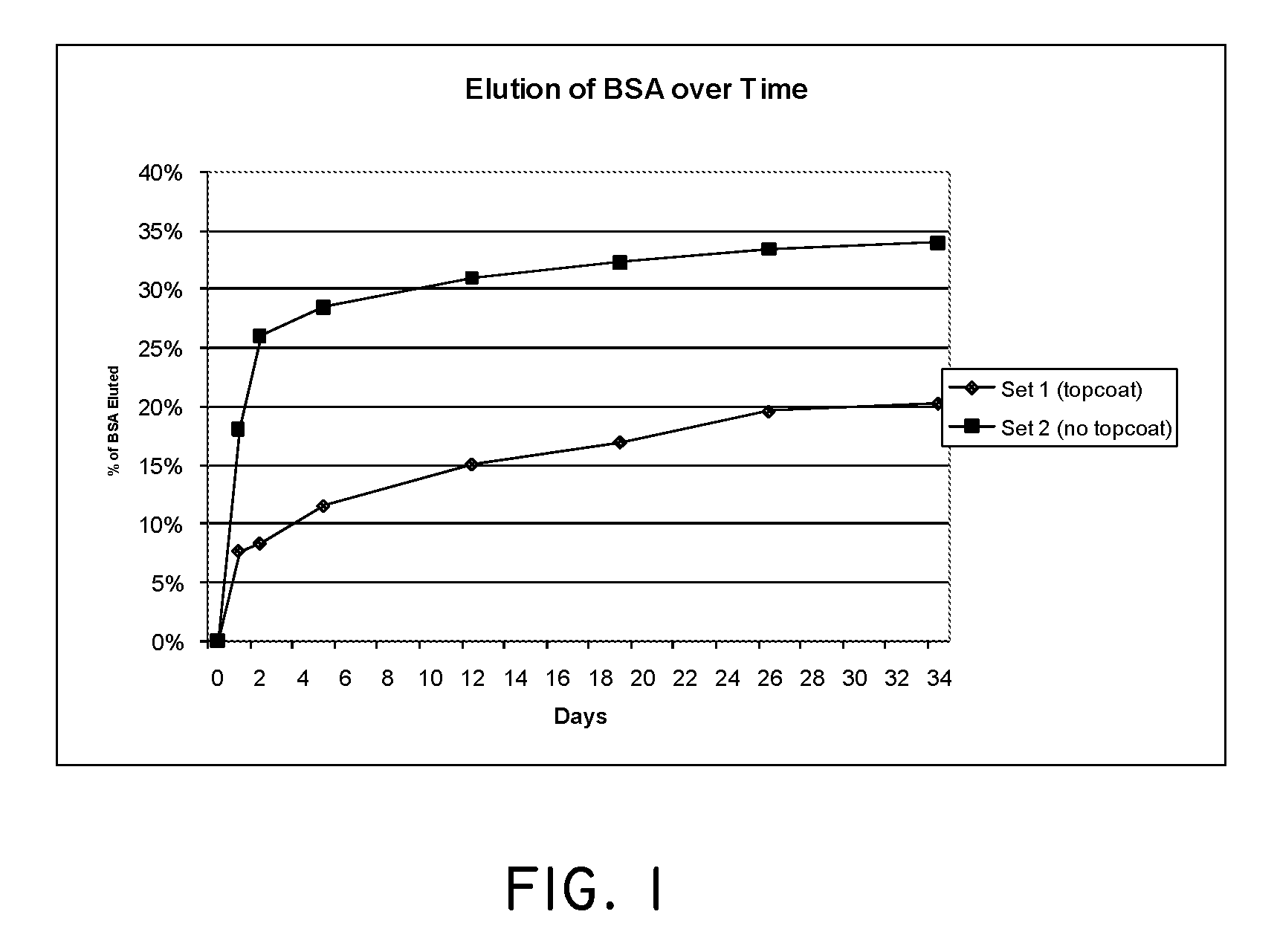
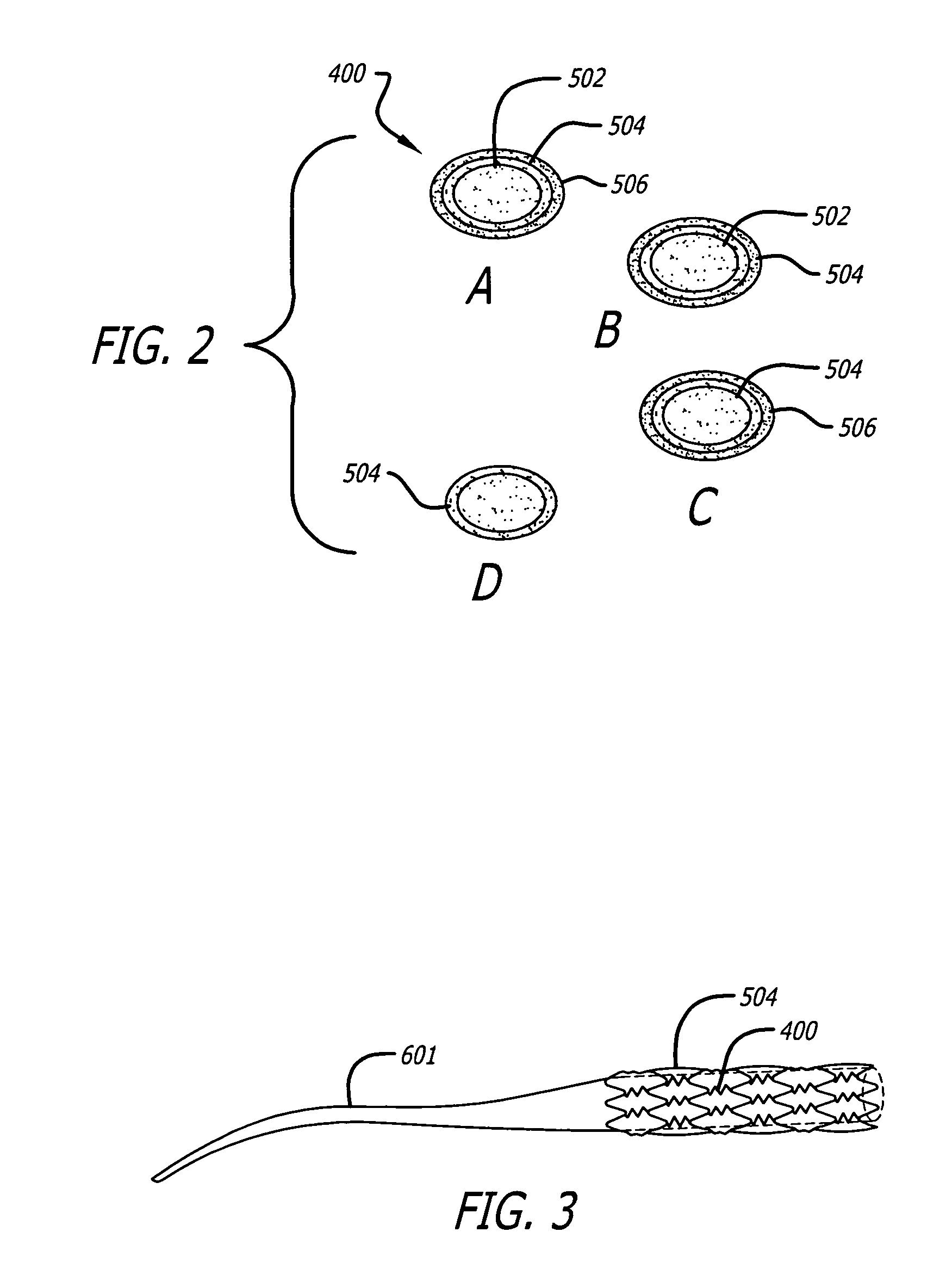
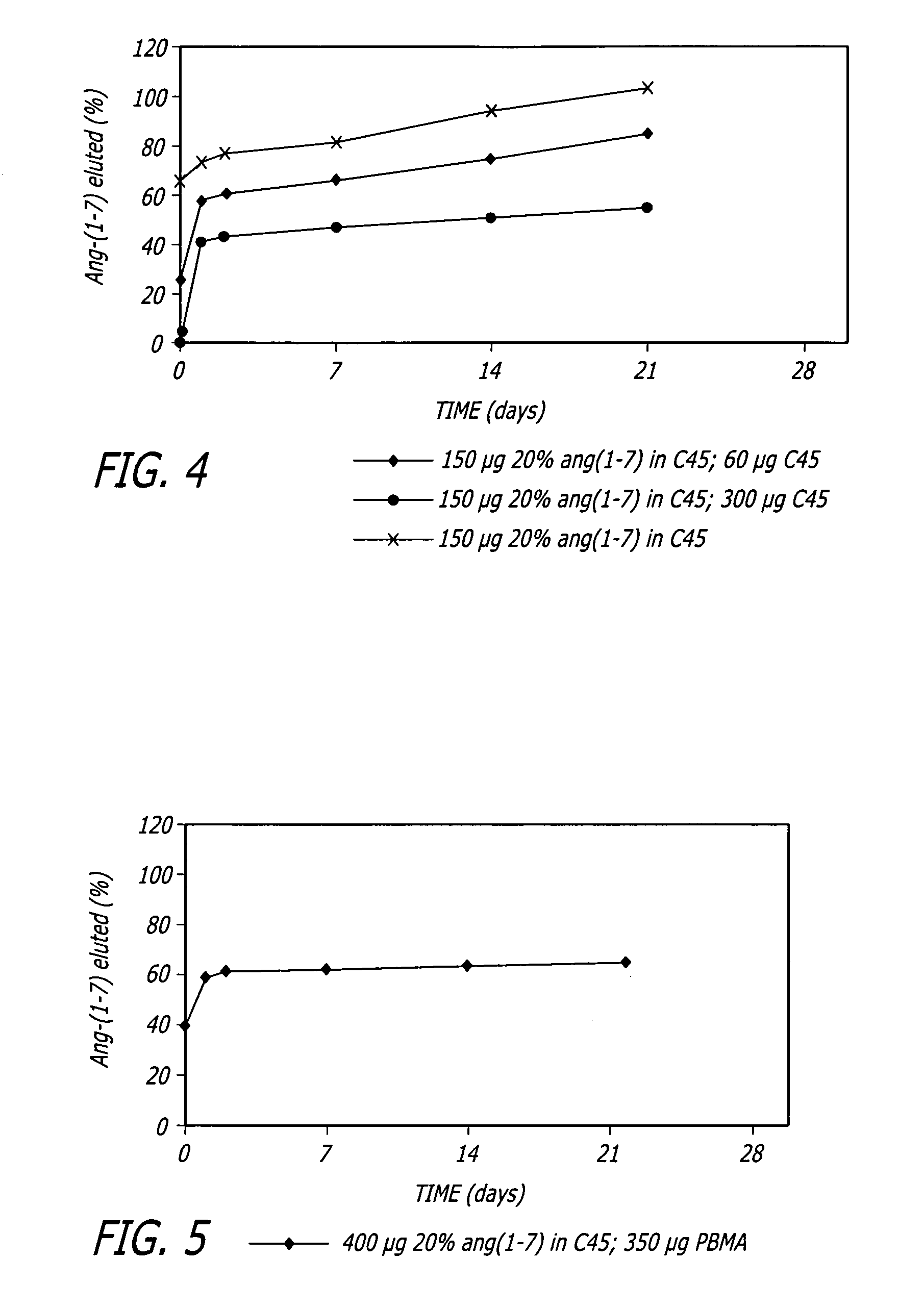
Angiotensin-(1-7) eluting polymer-coated medical device to reduce restenosis and improve endothelial cell function
InactiveUS7176261B2Function increasePrevent restenosisAngiotensinsPeptide/protein ingredientsVascular endotheliumPercent Diameter Stenosis
Medical devices with polymer coatings designed to control the release of angiotensin-(1-7) receptor agonists from medical devices are disclosed. The present application also discloses providing vascular stents with angiotensin-(1-7) receptor agonist-containing controlled-release coatings. Methods for treating or inhibiting post-stent implantation restenosis as well as improving vascular endothelial function in patients are also provided.
Owner:MEDTRONIC INC
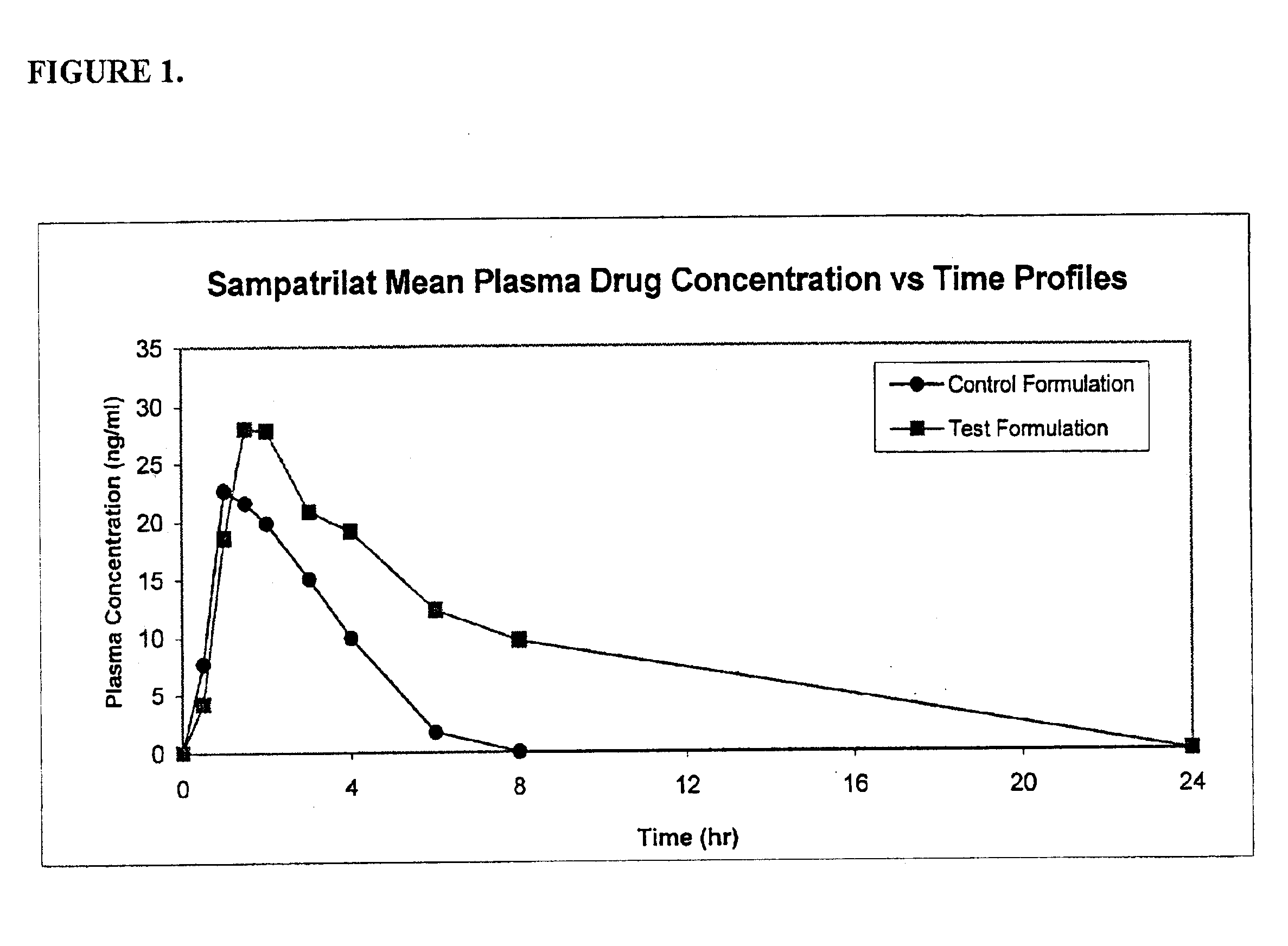
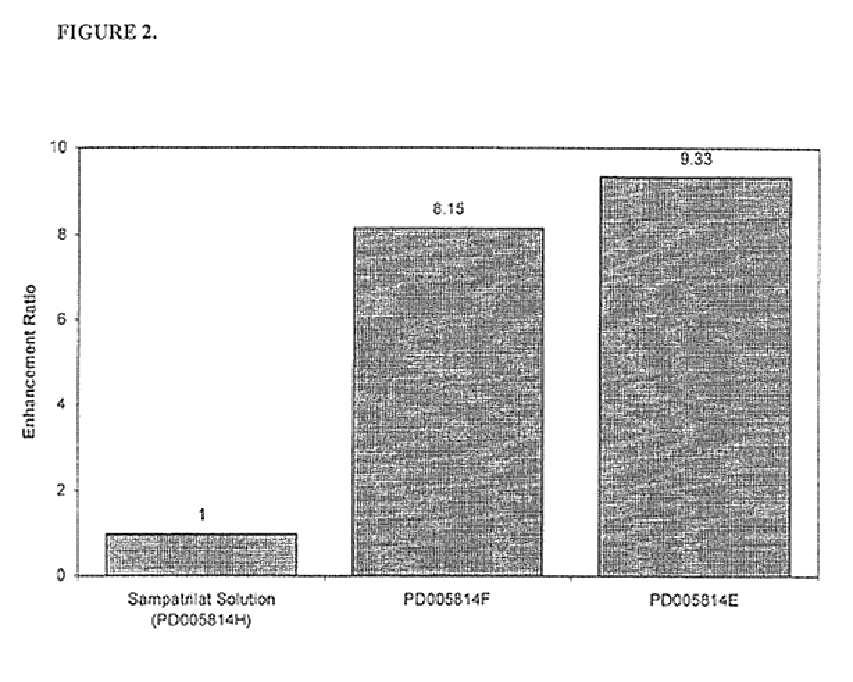
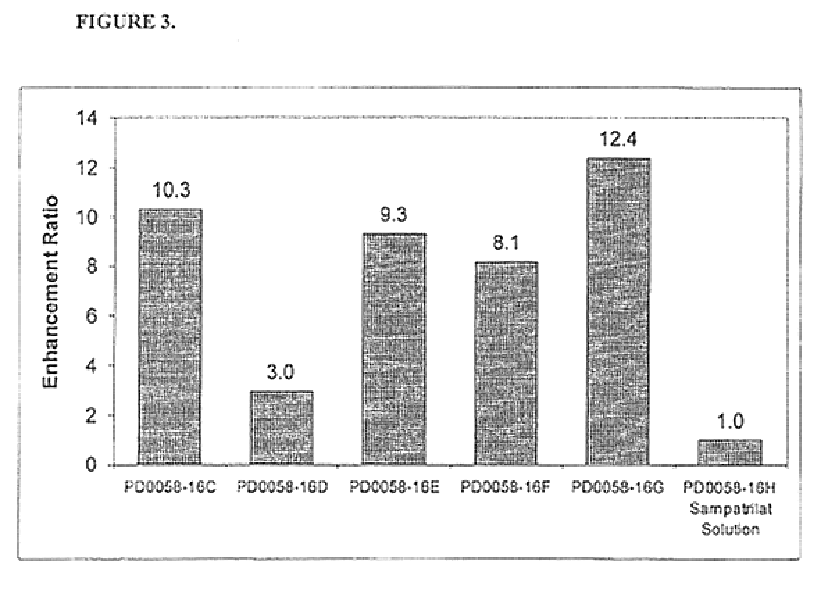
Pharmaceutical compositions including ACE/NEP inhibitors and bioavailability enhancers
InactiveUS6890918B2Enhance the oral bioabsorption of saidBiocideAngiotensinsAngiotensin-converting enzymeOrganic acid
A pharmaceutical composition comprising an inhibitor of angiotensin converting enzyme and neutral endopeptidase, such as sampatrilat, and at least one bioavailability enhancer such as an organic acid, e.g., ascorbic acid. Such a composition has improved systemic bioavailability.
Owner:SUPERNUS PHARM INC
Intracoronary device and method of use thereof
InactiveUS20070003528A1Function increaseExtended stayBiocidePeptide/protein ingredientsBiological bodyBiochemistry
Engraftment of therapeutic cells and agents to a target site in an organism is enhanced by mechanical, chemical and biological methods and systems.
Owner:ABBOTT CARDIOVASCULAR
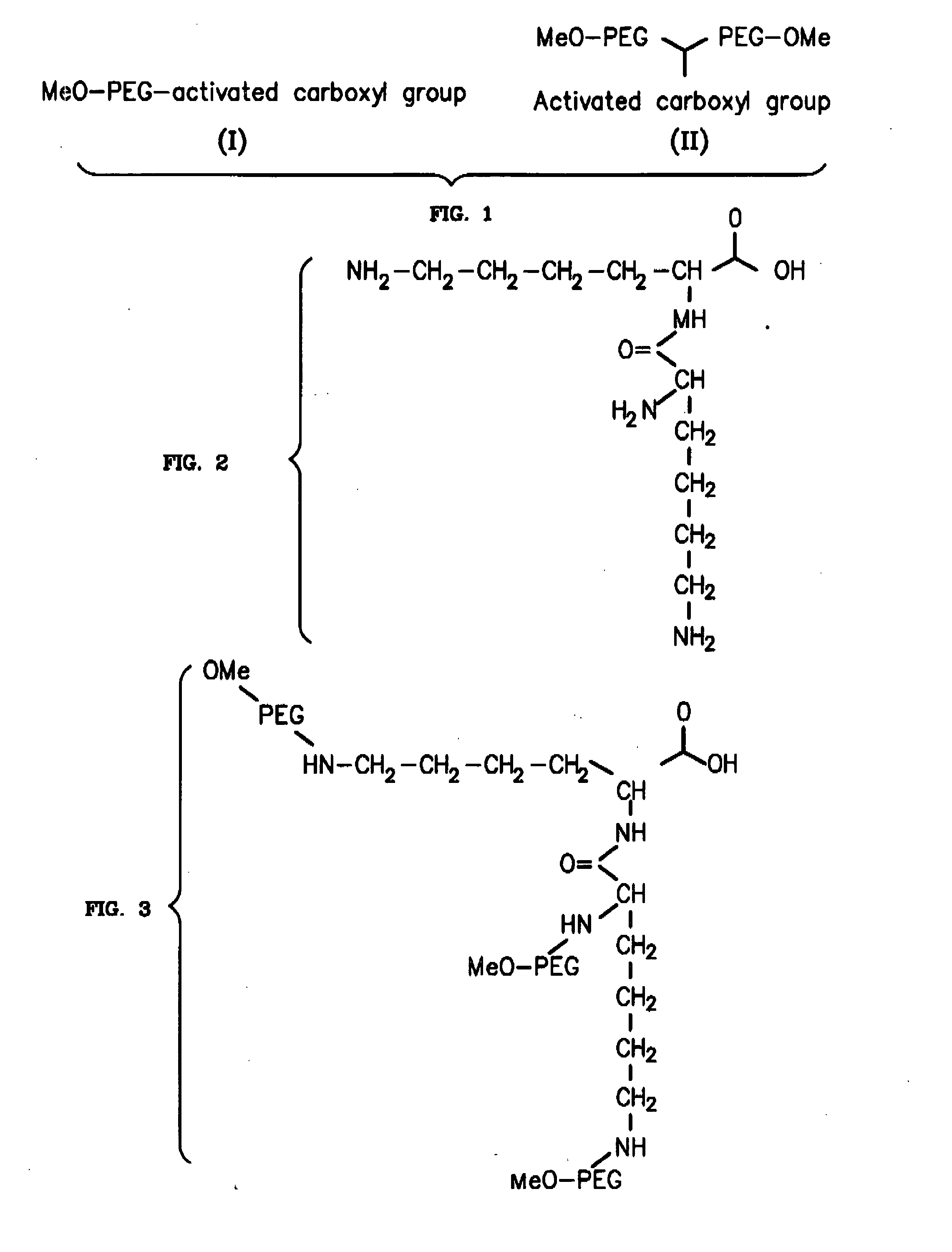
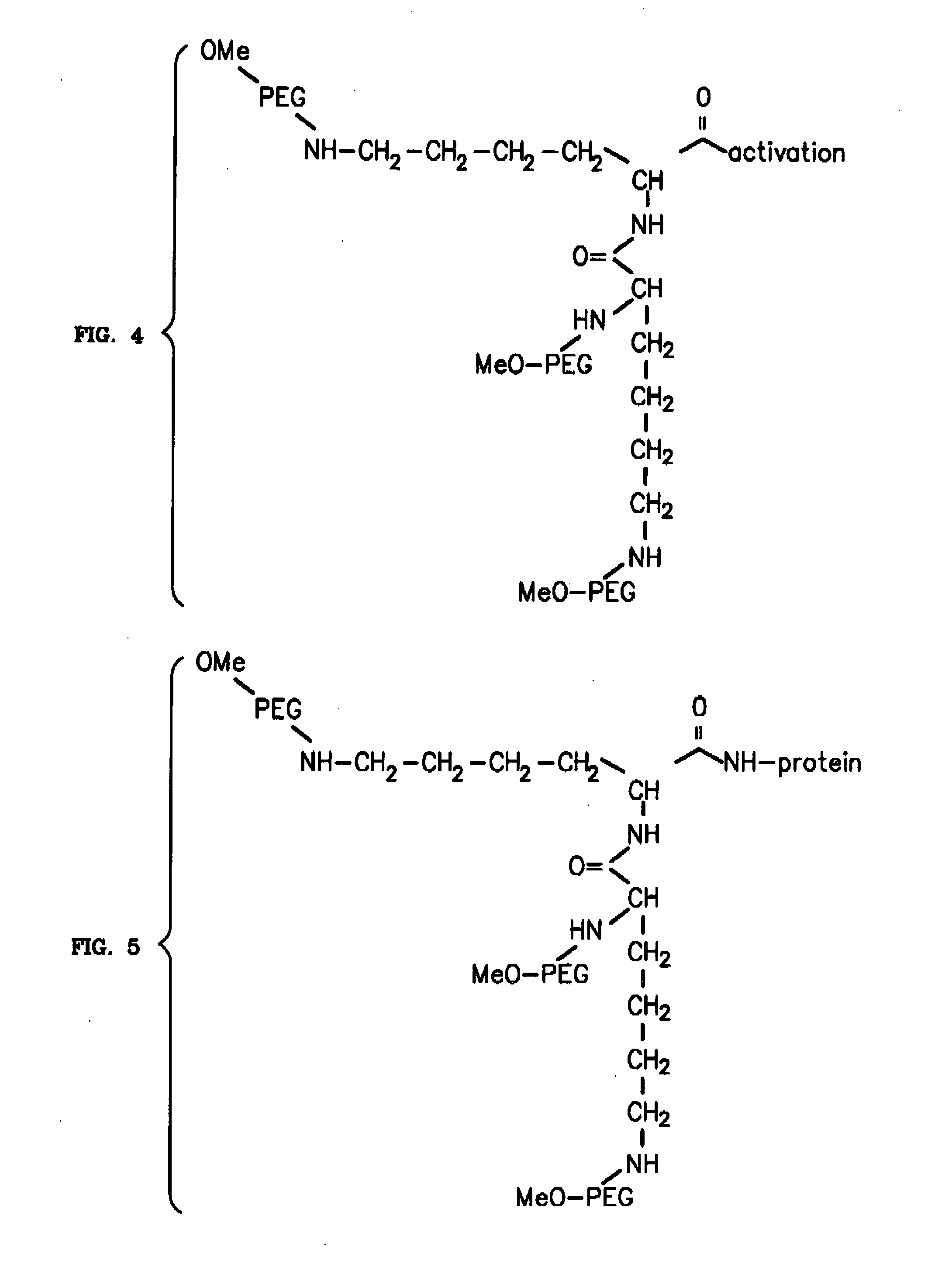
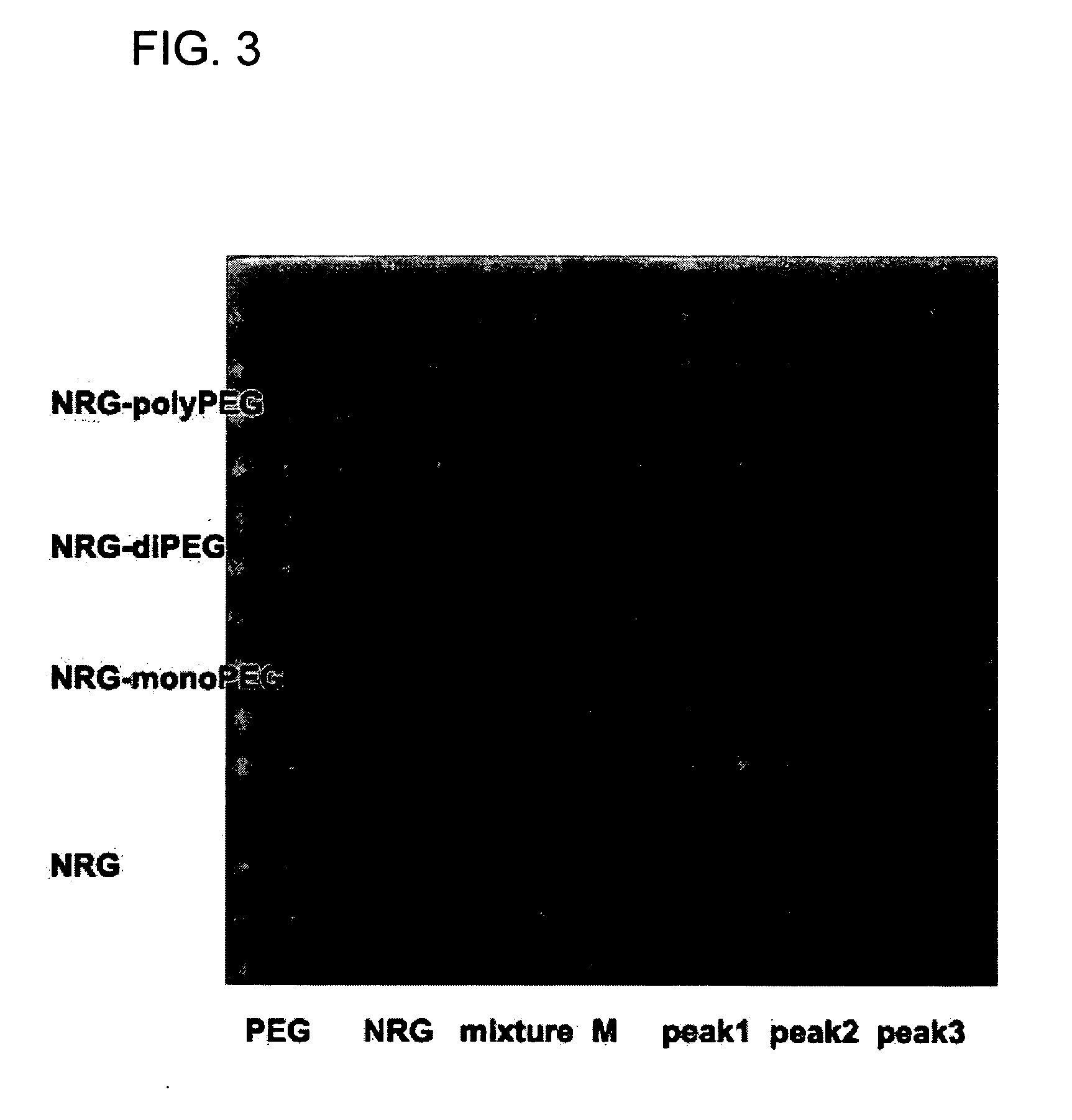
Novel PEGylation agent
InactiveUS20070184015A1Reduce compliancePromote degradationPeptide/protein ingredientsCalcitoninsHalf-lifeBlood plasma
To address the issue of degradation by enzymatic reactions to proteins and peptides, polyethylene glycol (PEGylation) of the proteins and peptides has been established. PEGylated proteins and peptides have increased plasma half-lives and reduced immunogenicity. To further improve and extend the plasma half-life of desired protein or peptide therapeutics, a novel branched molecule of PEG possessing three PEGs with a single point of attachment is designed in this invention disclosure.
Owner:HAHN SOONKAP
Pharmaceutical formulations, methods, and dosing regimens for the treatment and prevention of acute coronary syndromes
InactiveUS20050142180A1Reduction of atherosclerotic plaqueInhibition of disease progressionPeptide/protein ingredientsReceptors for hormonesRegimenApolipoprotein A-I Milano
The invention provides methods and formulations for treating and preventing acute coronary syndromes. The methods of the instant invention provide safe and effective doses of an Apolipoprotein A-I Milano: phospholipid complex to reduce and stabilize atherosclerotic plaque. Pharmaceutical formulations of the Apo A-I Milano:phospholipid complexes are also provided.
Owner:PFIZER INC
Use of angiotensin receptor blockers (ARBs) to treat diseases associated with excess ACE
Disclosed are methods of treating neuropsychiatric diseases, neuromuscular diseases, viral infections, neurodegenerative diseases and ricin poisoning using angiotensin II receptor blockers.
Owner:MOSKOWITZ DAVID W
Extended release of neuregulin for improved cardiac function
InactiveUS20070190127A1Good effectReduce adverse side effectsSenses disorderNervous disorderNeuregulinMedicine
The present invention provides extended release compositions comprising neuregulin for preventing, treating or delaying various diseases or disorders. The present invention also provides methods for preventing, treating or delaying various diseases or disorders by extended release of neuregulin.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
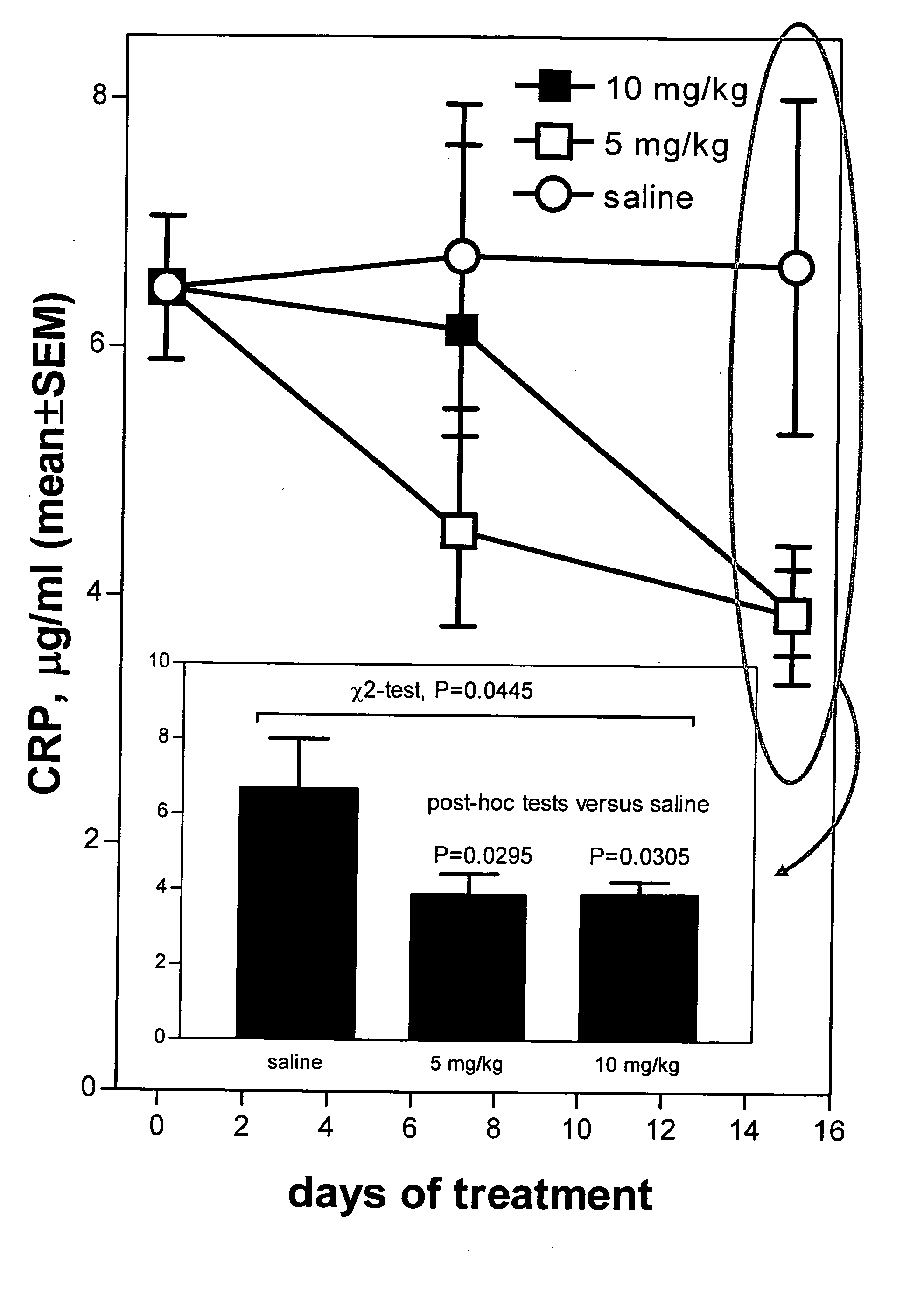
Method of reducing C-reactive protein using growth hormone secretagogues
The present invention relates to a method of reducing C-reactive protein in a subject in need of treatment thereof, wherein the subject is at risk of having or the subject has already had a vascular event or suffering from an inflammatory disease or disorder. In one embodiment, the vascular event is a cardiovascular event (e.g., myocardial infarction). In another embodiment, the vascular event is a cerebrovascular event (e.g., stroke (such as transient ischemic attacks (TIAs)). In yet another embodiment the vascular event is a peripheral vascular event (e.g., intermittent claudication). The method comprises administering a therapeutically effective amount of at least one growth hormone secretagogue compound or a pharmaceutically acceptable salt, hydrate or solvate thereof. The growth hormone secretagogue can be coadministered with a second growth hormone secretagogue, HMG CoA reductase inhibitor, an ACAT inhibitor, a CETP inhibitor, an anti-inflammatory agent, an ACE inhibitor, a Beta blocker, a cholesterol absorption inhibitor, a nicotonic acid, a fibric acid derivative, a bile acid sequestering agent or a combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
Compositions for nasal administration of pharmaceuticals
Compositions for nasal administration, which comprise a pharmaceutical, a physiologically active peptide, or a peptide-related compound, and as the carrier thereof, crystalline cellulose with a specific particle diameter and / or partially pregelatinized starch are provided. Such compositions improve the in vivo absorption efficiency of pharmaceuticals.
Owner:SHIN NIPPON BIOMEDICAL LAB
Pharmaceutical formulations, methods, and dosing regimens for the treatment and prevention of acute coronary syndromes
InactiveUS7435717B2Reduce riskReduced stabilityPeptide/protein ingredientsReceptors for hormonesRegimenApolipoprotein A-I Milano
The invention provides methods and formulations for treating and preventing acute coronary syndromes. The methods of the instant invention provide safe and effective doses of an Apolipoprotein A-I Milano:phospholipid complex to reduce and stabilize atherosclerotic plaque. Pharmaceutical formulations of the Apo A-I Milano:phospholipid complexes are also provided.
Owner:PFIZER INC
Compositions and methods for treatment of body weight conditions
InactiveUS20050106218A1Control weight gainGood for weight lossBiocideDispersion deliveryWeight gainingNutrition supplementation
A nutritional supplement composition having therapeutically effective amounts of milk minerals including calcium, a protein source including κ-casein fragment 106-169, and enzyme-inhibiting peptides is provided for the treatment of body weight conditions. The nutritional supplement composition is administered in amounts effective for limiting weight gain and / or enhancing weight loss, as well as promoting overall good health, in the treatment of body weight conditions, including overweight and obesity.
Owner:GLANBIA NUTRITIONALS IRELAND
Method for promoting hematopoietic and mesenchymal cell proliferation and differentiation
InactiveUS7118748B1Increasing hematopoietic cell survivalReducing and preventing side effectPeptide/protein ingredientsGenetic material ingredientsCell culture mediaAngiogenesis growth factor
The present invention provides methods, improved cell culture medium and kits for promoting hematopoietic and mesenchymal stem and lineage-specific cell proliferation and differentiation by growth in the presence of angiotensinogen, angiotensin I (Al), AI analogues, AI fragments and analogues thereof, angiotensin II (AII), AII analogues, AII fragments or analogues thereof or AII AT2 type 2 receptor agonists, either alone or in combination with other growth factors and cytokines.
Owner:UNIV OF SOUTHERN CALIFORNIA
Treating neoplasms with neurotoxin
InactiveUS7709440B2Inhibit transferSuppression of squeeze effectBiocidePeptide/protein ingredientsCancer cellAutoimmune responses
The present invention provides a method of treating a cancer using a neurotoxin, preferably Botulinum toxin (“BTX”). The application of a neurotoxin around a cancer acts to decrease the contractile forces of the muscles surrounding a neoplasm which normally squeeze cancer cells through efferent channels leaving the cancer vicinity to distant sites. Also, the application of the toxin at sites distant from the cancer enhances cellular and humoral immunologic functions which further contributes to cancer cell death and spread. Following administration of botulinum toxin around and distant to a cancer, it is noticed that local, regional, and distant spread is reduced or eliminated. Immunomodulation with botulinum toxin is also valuable in treating other disease that may or may not be associated with cancers, such as viral-induced growths, viral conditions, fungal disease, chronic wounds, graft versus host disease, autoimmune disease, and HIV.
Owner:TOXCURE
Compositions containing a substituted indolealkanoic acid and an angiotensin converting enzyme inhibitor
Disclosed are methods of reducing serum glucose and triglyceride levels and for inhibiting angiogenesis, the methods comprising administration of substituted indolealkanoic acids to patients in need of such treatment. Also disclosed are such compounds useful in the treatment of angiogenesis, hyperglycemia, hyperlipidemia and chronic complications arising from diabetes mellitus. Also disclosed are pharmaceutical compositions containing the compounds. Further, disclosed are combinations of an angiotensin converting enzyme inhibitor and a substituted indole acetic acid.
Owner:ALINEA PHARMA
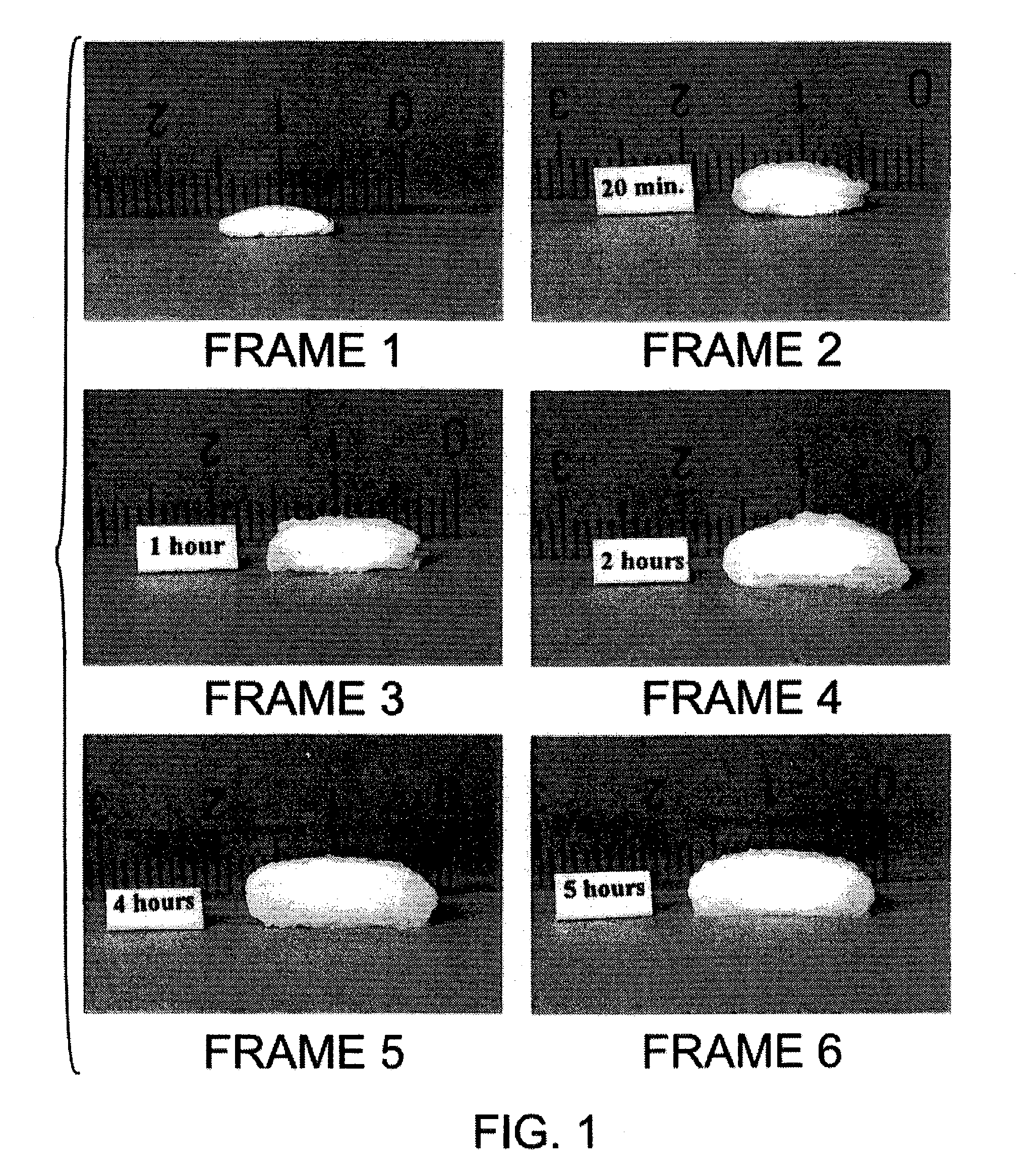
Bioadhesive progressive hydration tablets
A bioadhesive controlled, extended release progressive hydration composition wherein the active ingredient may be protected from water or the surrounding environment, thereby protecting it from metabolism or from other degradation caused by moisture, enzymes, or pH effects, and making it bioavailable only at a controlled rate. The active ingredient may be protected from moisture during the manufacturing process, as necessary or desired, and more importantly may be protected from moisture and the immediate septic environment until well after the patient has applied the composition, and then only at a slow and controlled rate. It is by this process of progressive hydration that the active ingredient remains protected for many hours after administration. It is also by the process of progressive hydration that controlled and sustained release is achieved because only that part of the active ingredient that is the hydrated (aqueous) fraction of the composition is available for absorption (bioavailable).
Owner:JUNIPER PHARMA INC
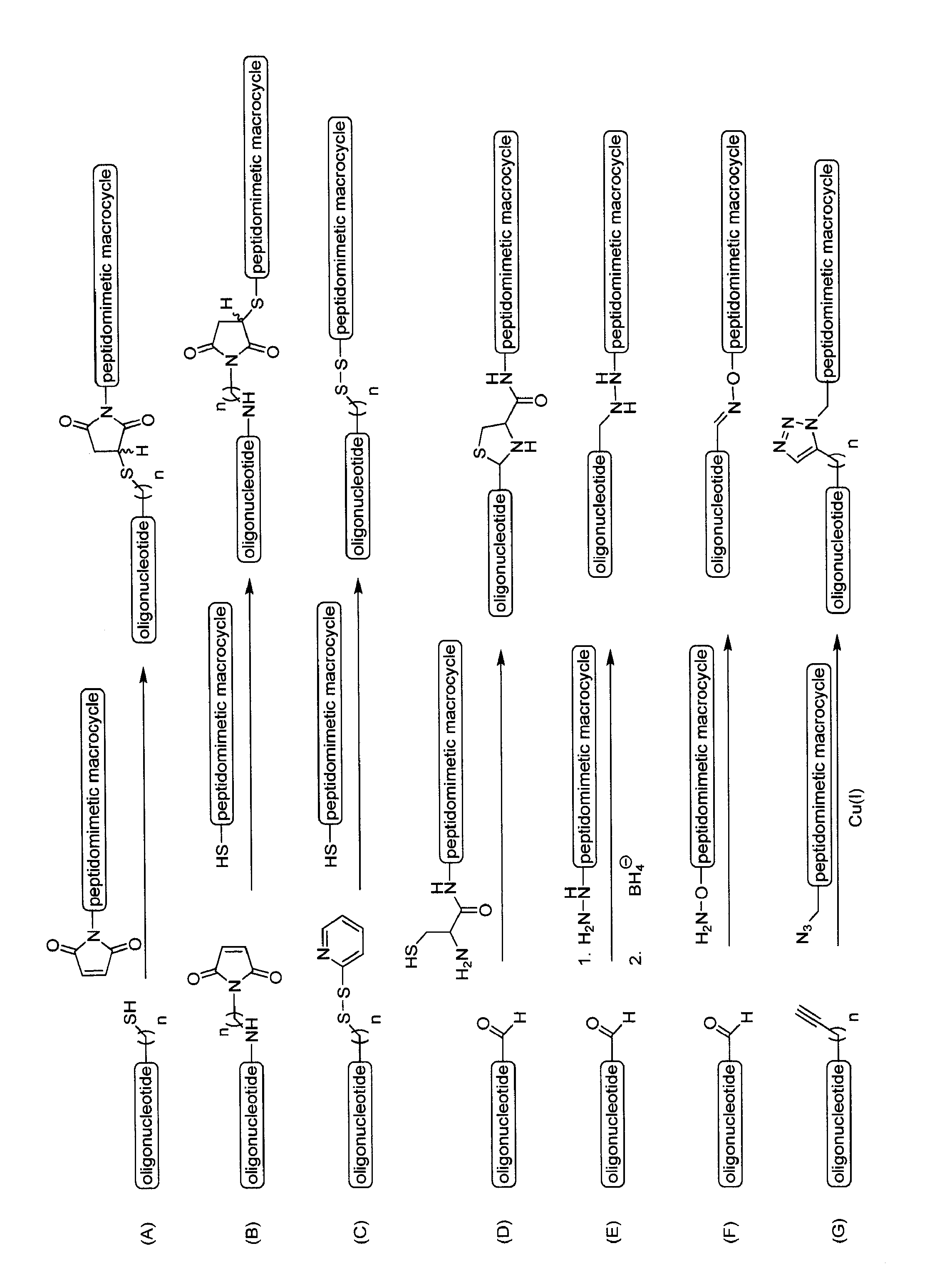
Compositions and methods for enhancing cellular transport of biomolecules
The present invention discloses compositions and methods for delivery of biomolecules into cells. Compositions comprise peptidomimetic macrocycles complexed or conjugated to biomolecules such as nucleic acids.
Owner:NASH HUW M
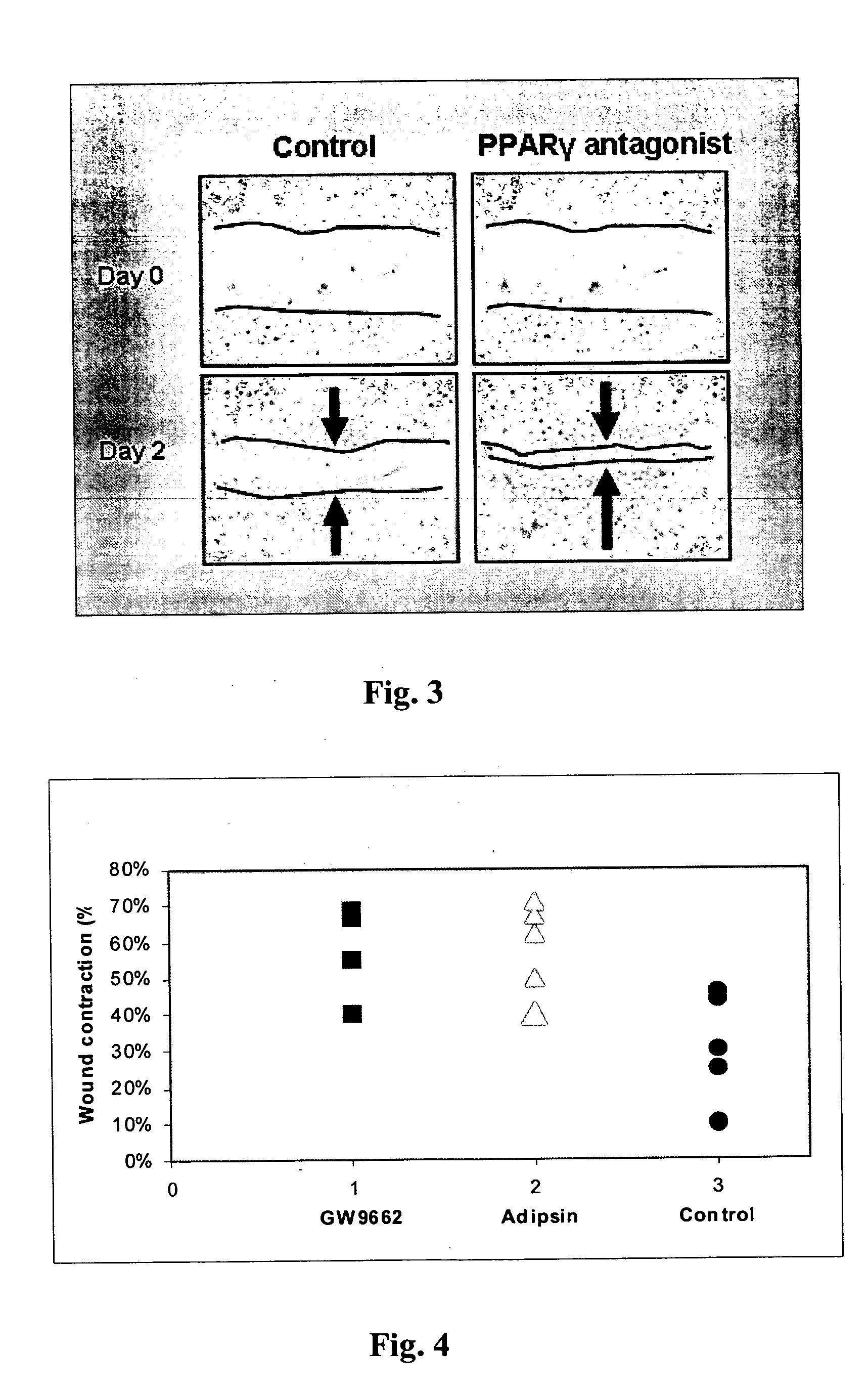
Methods for accelerating wound healing by administration of adipokines
Methods for inducing or accelerating a healing process of a damaged skin or skin wounds are described. The methods include administering to the skin cells colonizing the damaged skin or skin wound a therapeutically effective amount of an adipokine, an adipocyte or preadipocyte modulator, adipocytes, preadipocytes, or stem cells, or transforming the skin cells colonizing the damaged skin or skin wound such as to express and secrete an adipokine, thereby inducing or accelerating the healing process of the damaged skin or skin wound.
Owner:HEALOR LTD
Conjoint administration of morphogens and ACE inhibitors in treatment of chronic renal failure
InactiveUS20050272649A1Preventing delaying needReducing necessary frequencyBiocidePeptide/protein ingredientsRenal disorderMorphine
The present invention provides reagents and methods for the treatment, and pharmaceuticals for use in the prevention and / or treatment, of chronic renal failure and other renal disorders in subjects (particularly mammalian subjects) renal replacement therapy. The methods involve the conjoint administration of ACE (Angiotensin-Converting Enzyme) inhibitors or Angiotensin II Receptor Antagonists (AIIRAs) with one or more OP / BMP family of proteins (morphogens, or inducers of morphogens, or agonists of the corresponding morphogen receptors, etc.). The invention also provides methods for implantation of renal cells induced with the conjoint administration of ACE inhibitors or AIIRAs with those morphogens.
Owner:BARNES JEWISH HOSPITAL +1
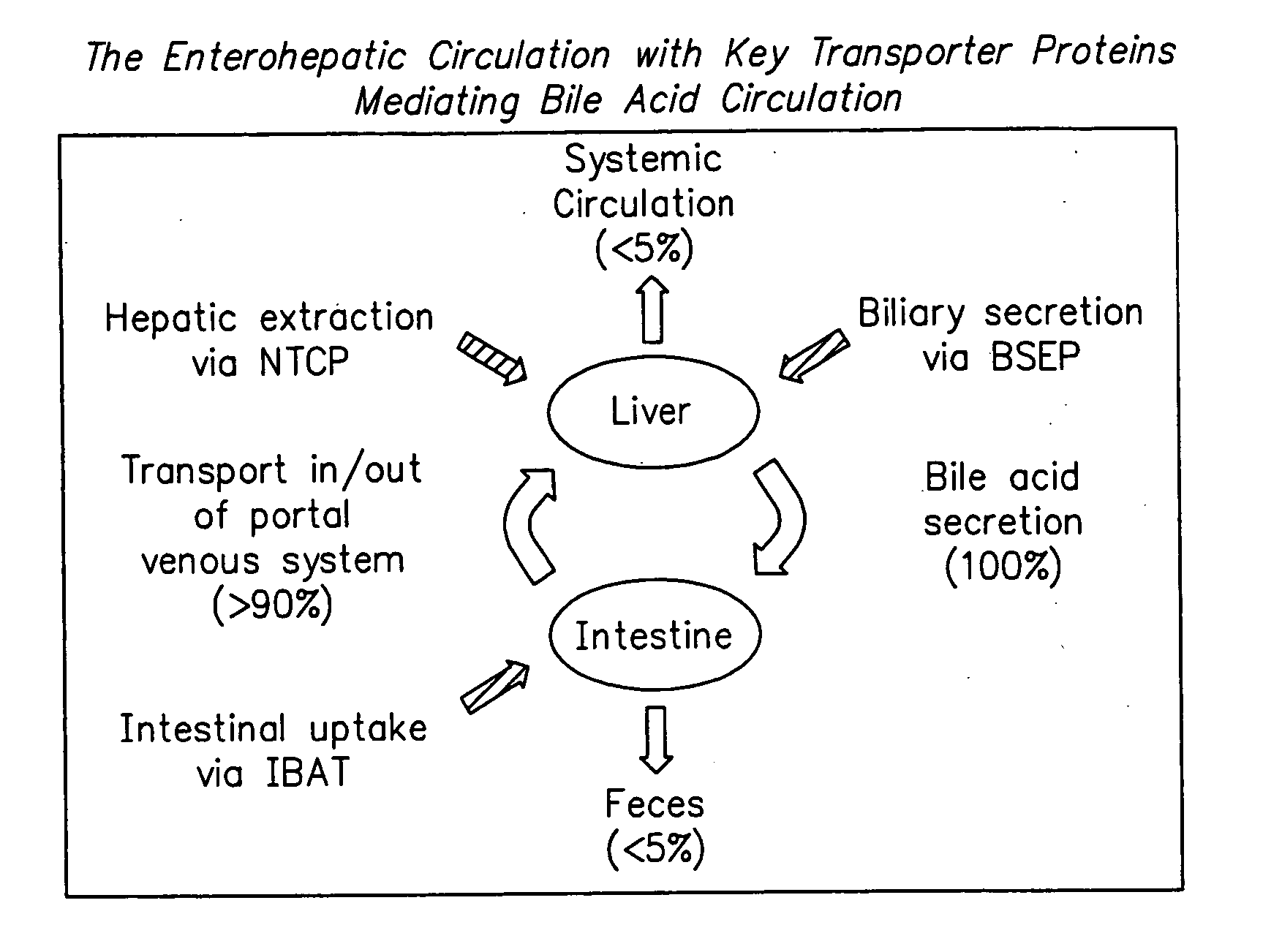
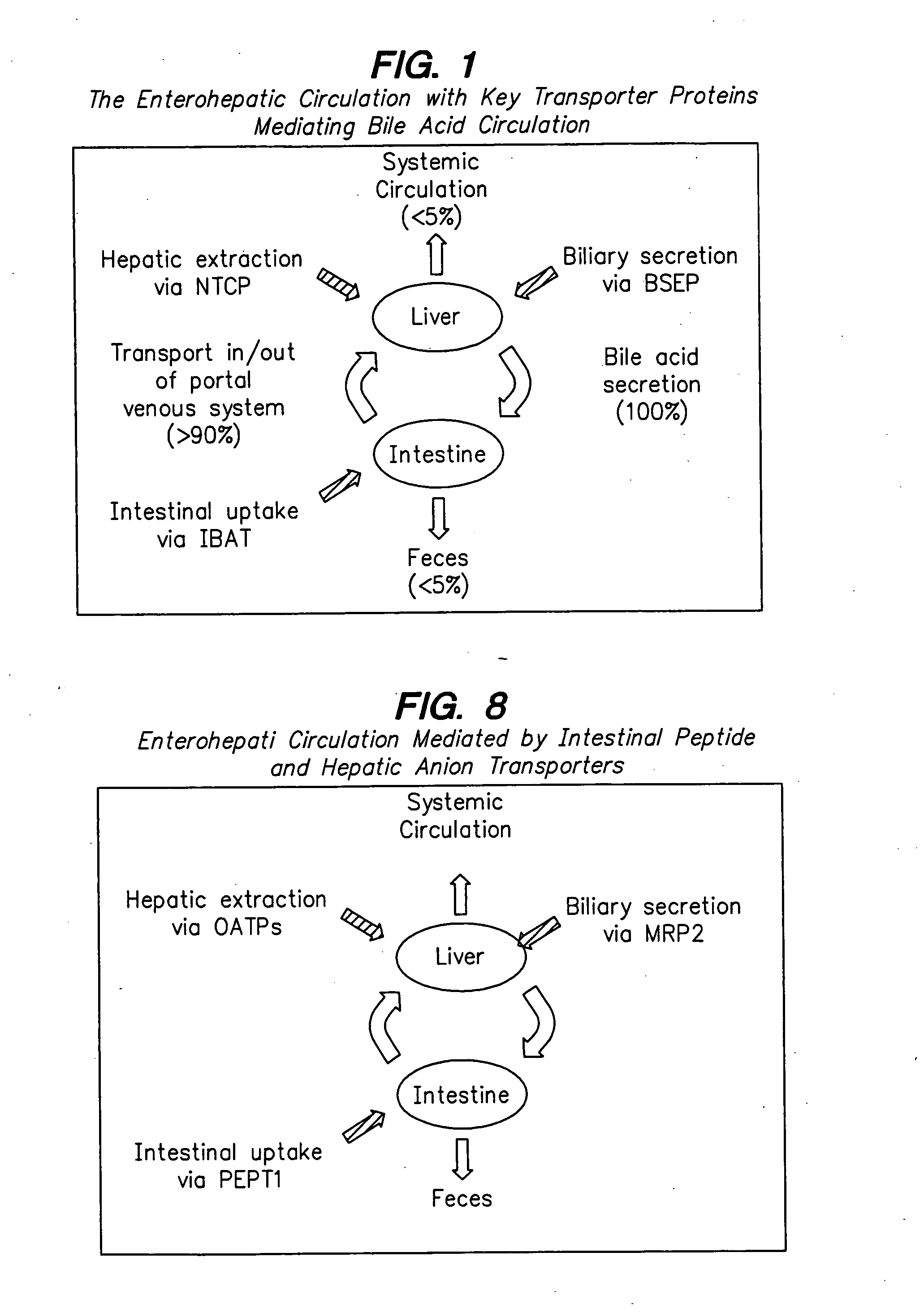
Compounds for sustained release of orally delivered drugs
InactiveUS20050054559A1Sustained releaseAntibacterial agentsOrganic active ingredientsWhole bodySystemic blood
Disclosed are methods for providing sustained systemic blood concentrations of orally delivered drugs. Still further, disclosed are compounds and pharmaceutical compositions that are used in such methods.
Owner:XENOPORT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com