Non-aqueous single phase vehicles and formulations utilizing such vehicles
a single-phase vehicle, non-aqueous technology, applied in the direction of peptides, cardiovascular disorders, drug compositions, etc., can solve the problems of partial or complete occlusion of the delivery conduit, the difficulty of delivering beneficial agents that include biomolecular material over an extended period of time using an implantable drug delivery system, etc., to achieve high viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
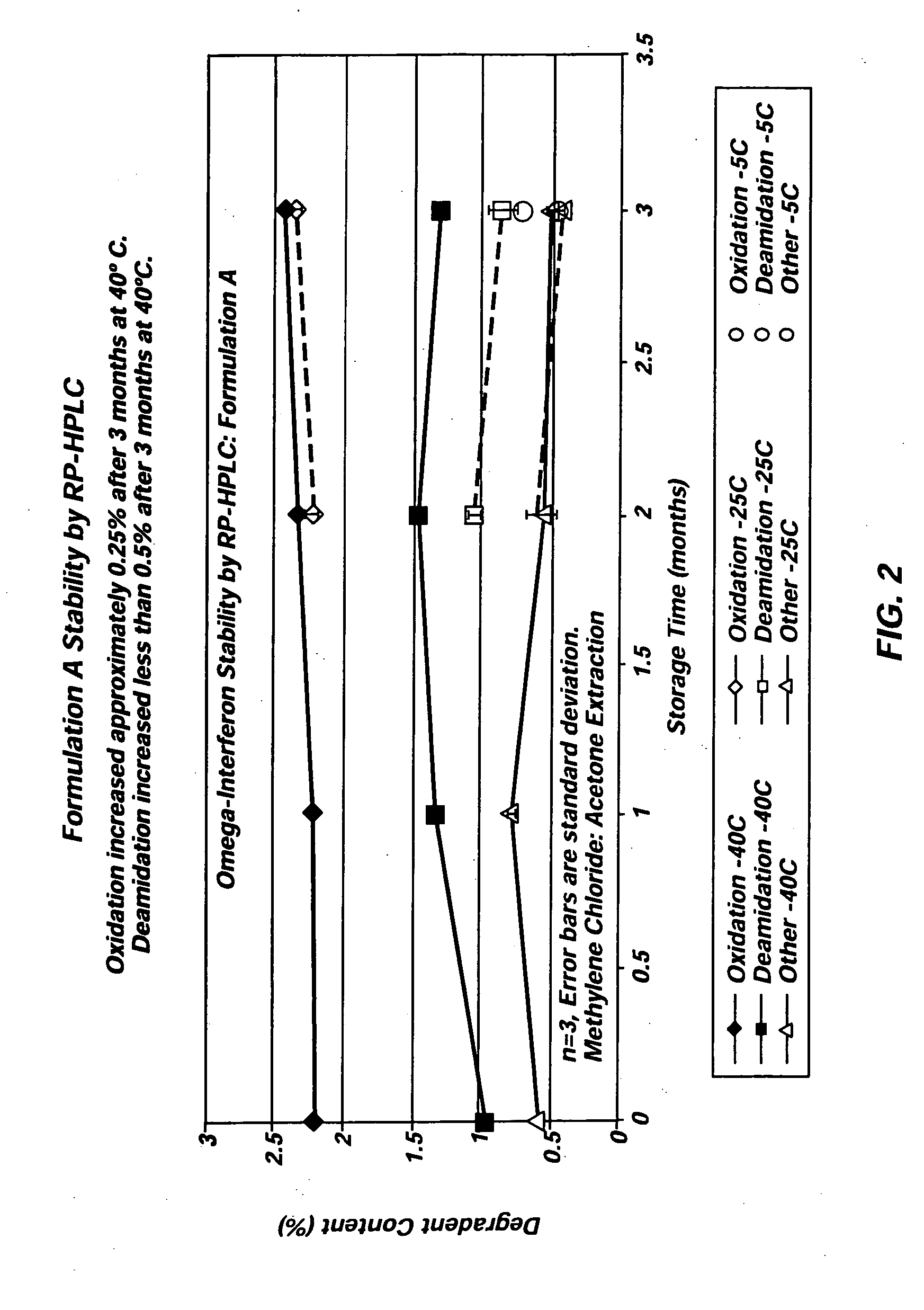
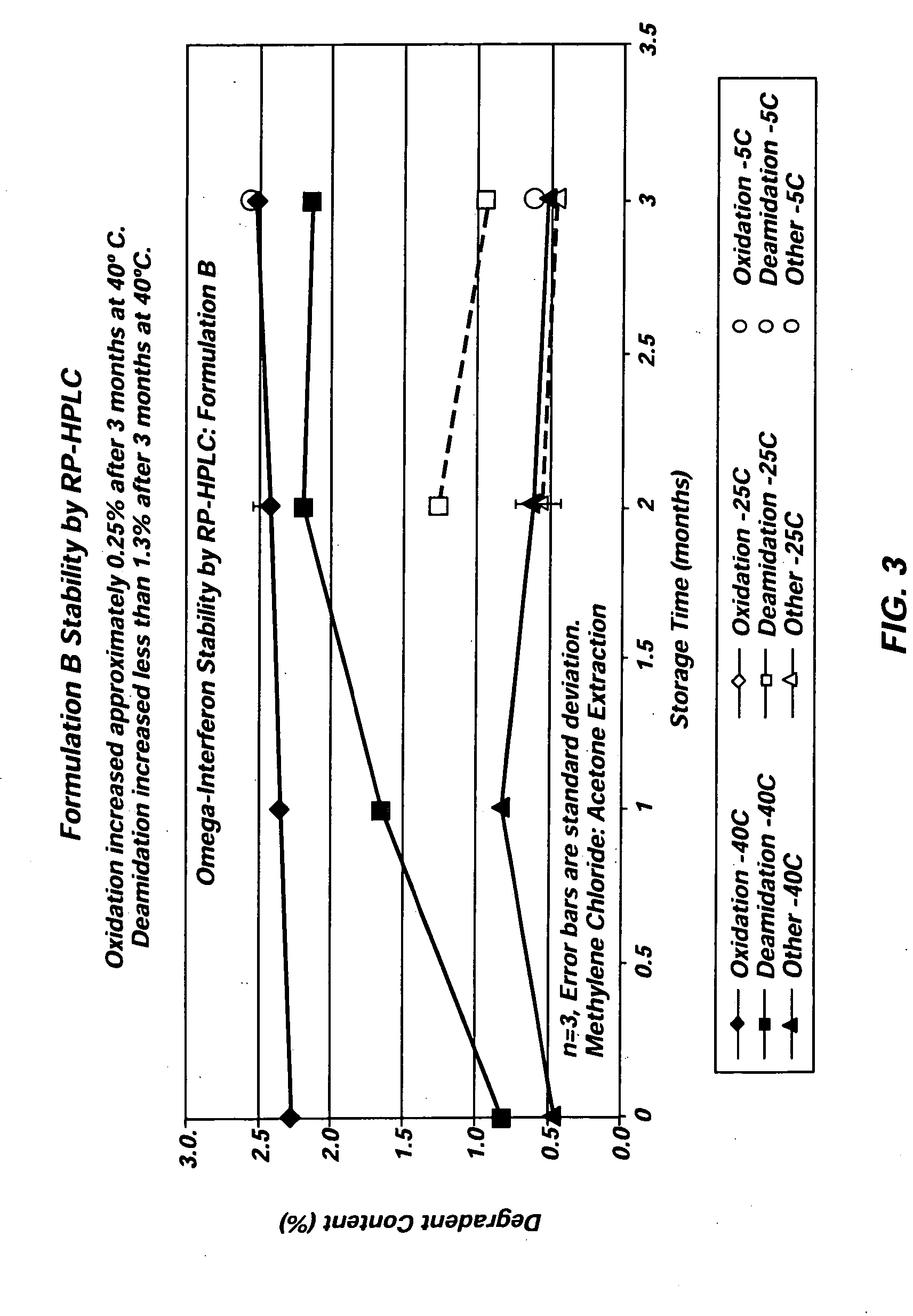
[0031] Three different exemplary vehicles according to the present invention were produced using Glycofurol (“GF”) and polyvinylpyrrolidone (“PVP”). The PVP included in each of the three vehicles was obtained from BASF (17 pf) and had a molecular weight below 18,000 MW. The first vehicle included 42% (wt / wt) GF and 58% (wt / wt) PVP. The second vehicle included 40% (wt / wt) GF and 60% (wt / wt) PVP, and the third vehicle included 50% (wt / wt) GF and 50% (wt / wt) PVP. In each instance, the vehicles were created by first charging the raw materials into a mixer. The taw materials were then blended at about 60° C. under vacuum (about −27 in Hg) for two hours to achieve a single-phase vehicle. Each of the three vehicles was miscible with water in all proportions.
example 2
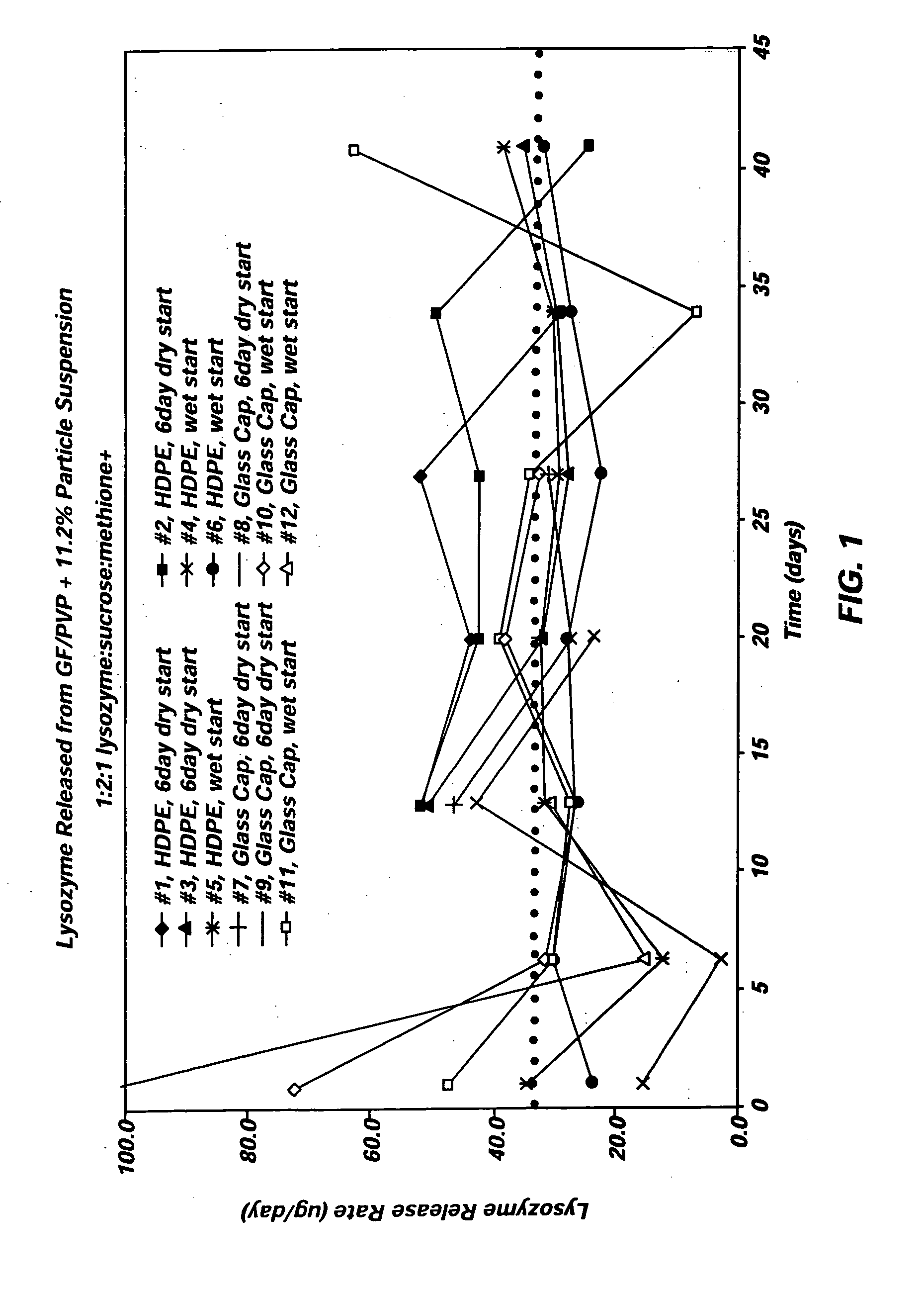
[0032] A lysozyme formulation according to the present invention was manufactured using the second vehicle of Example 1 and dry, particulate lysozyme material. The lysozyme particles used in the formulation included 1 part lysozyme to two parts sucrose, and 1 part methionine, and the particles were spray dried from a solution including a 25 mM citrate buffer. The simulated drug formulation included 11.2% (wt / wt) lysozyme. The lysozyme formulation was prepared by loading appropriate amounts of the vehicle and the lysozyme particles into a mixer. The particles and vehicle were then blended at about 60° C. under vacuum (about −27 in Hg) until a formulation having a substantially uniform suspension of lysozyme particles was achieved.
example 3
[0033] The deliverability of the lysozyme formulation of Example 2 was evaluated using two groups of six osmotic pumps. The osmotic pumps were designed to deliver the lysozyme formulation at 1.5 μl / day over a three-month period of time, providing a targeted lysozyme release rate of 35 μg / day. To evaluate the release rate performance provided by the lysozyme formulation, the osmotic pumps were introduced into an aqueous media that included a phosphate buffer system (PBS) and was maintained at 37° C.
[0034] The first group of 6 osmotic pumps was prepared using the following components: [0035] Reservoir: Titanium alloy [0036] Piston:. C-flex [0037] Lubricant: silicone medical fluid [0038] Osmotic Composition: two osmotic tablets (40 mg osmotic engine tablets formed using 76.4% NaCl, 15.5% sodium carboxymethyl cellulose, 6% povidone, 0.5% Mg Stearate, and 1.6% water)+PEG 400 filler [0039] Semipermeable Membrane: polyurethane polymer, injection molded to desired plug shape [0040] Diffusi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com