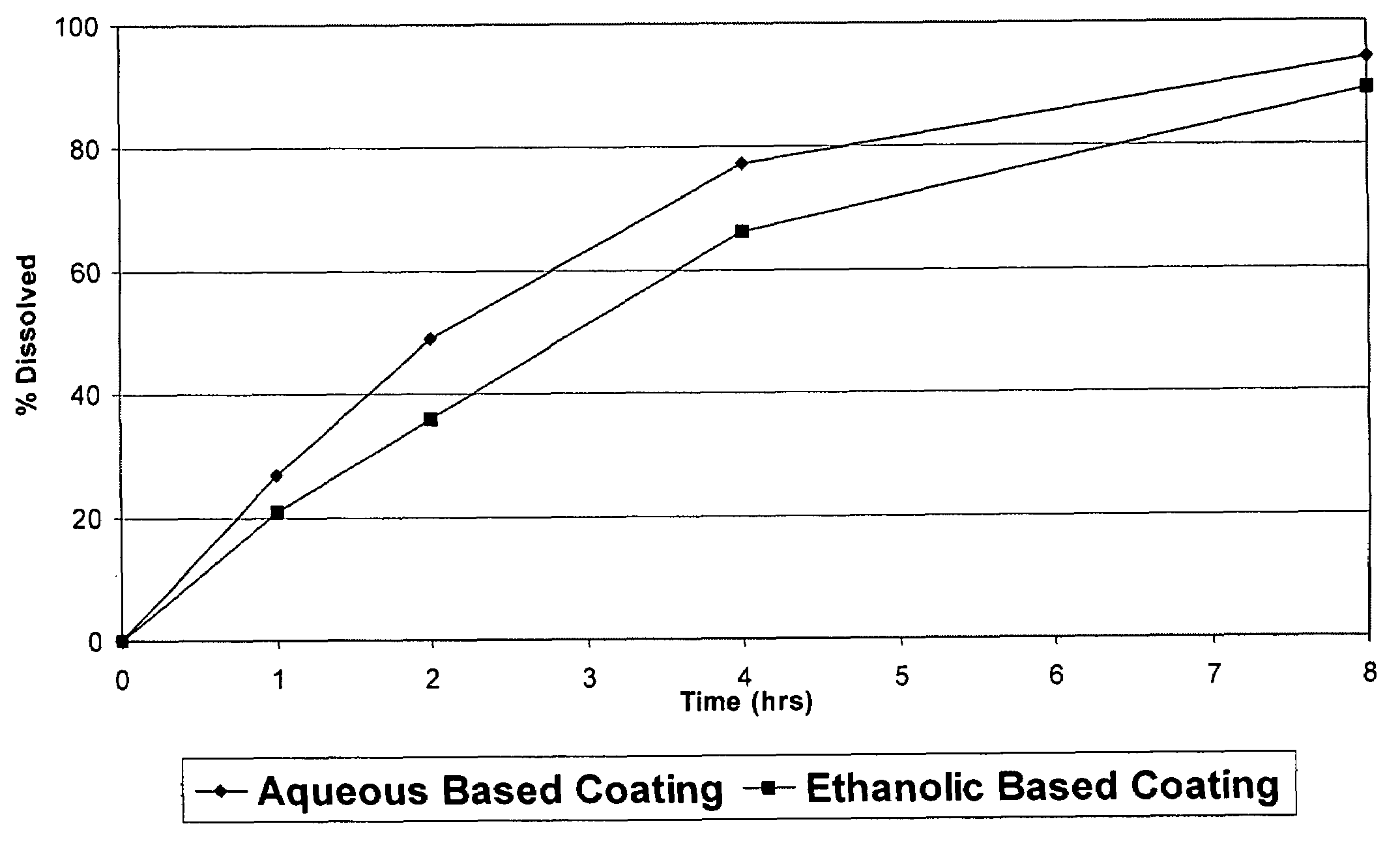
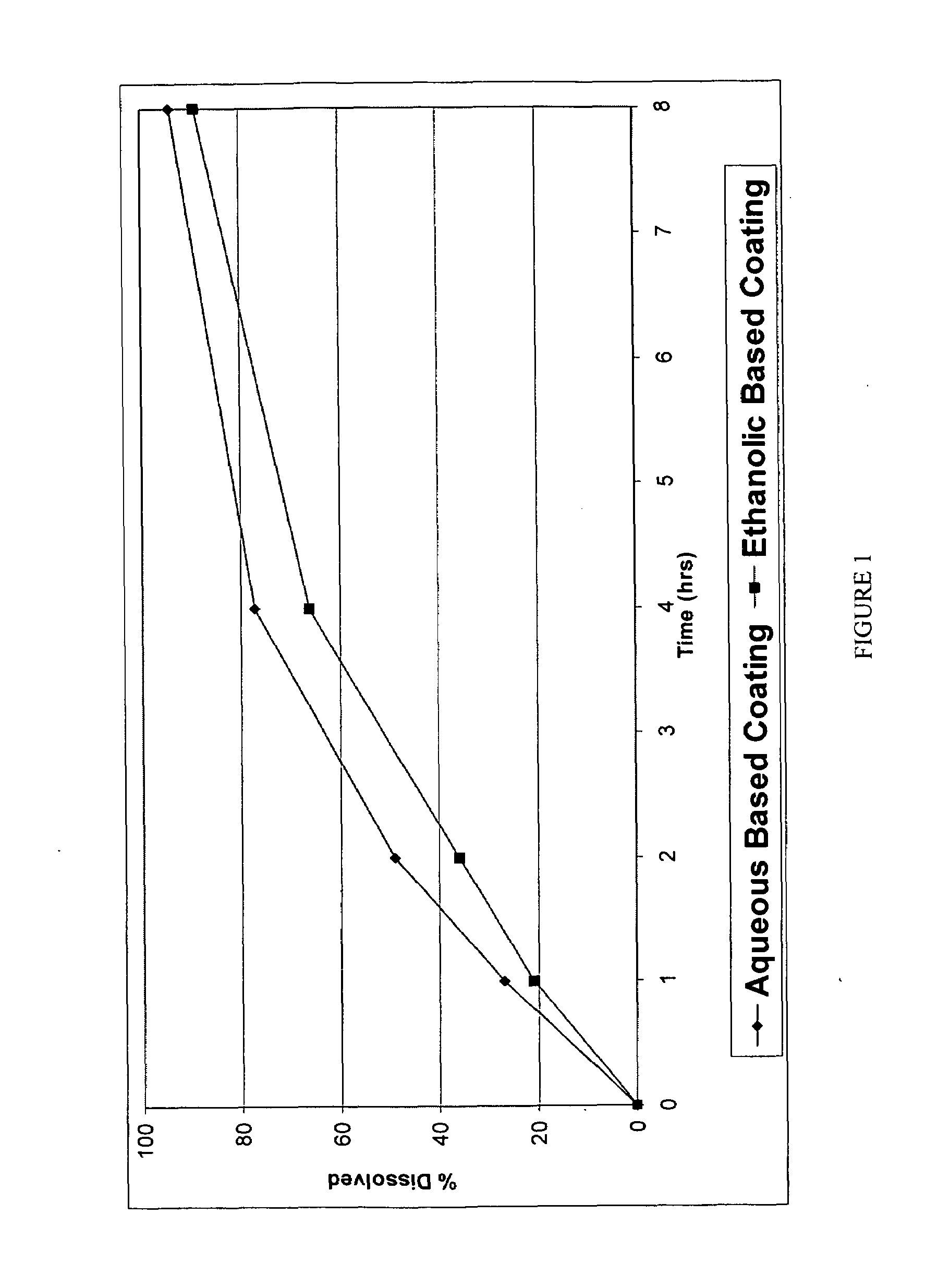
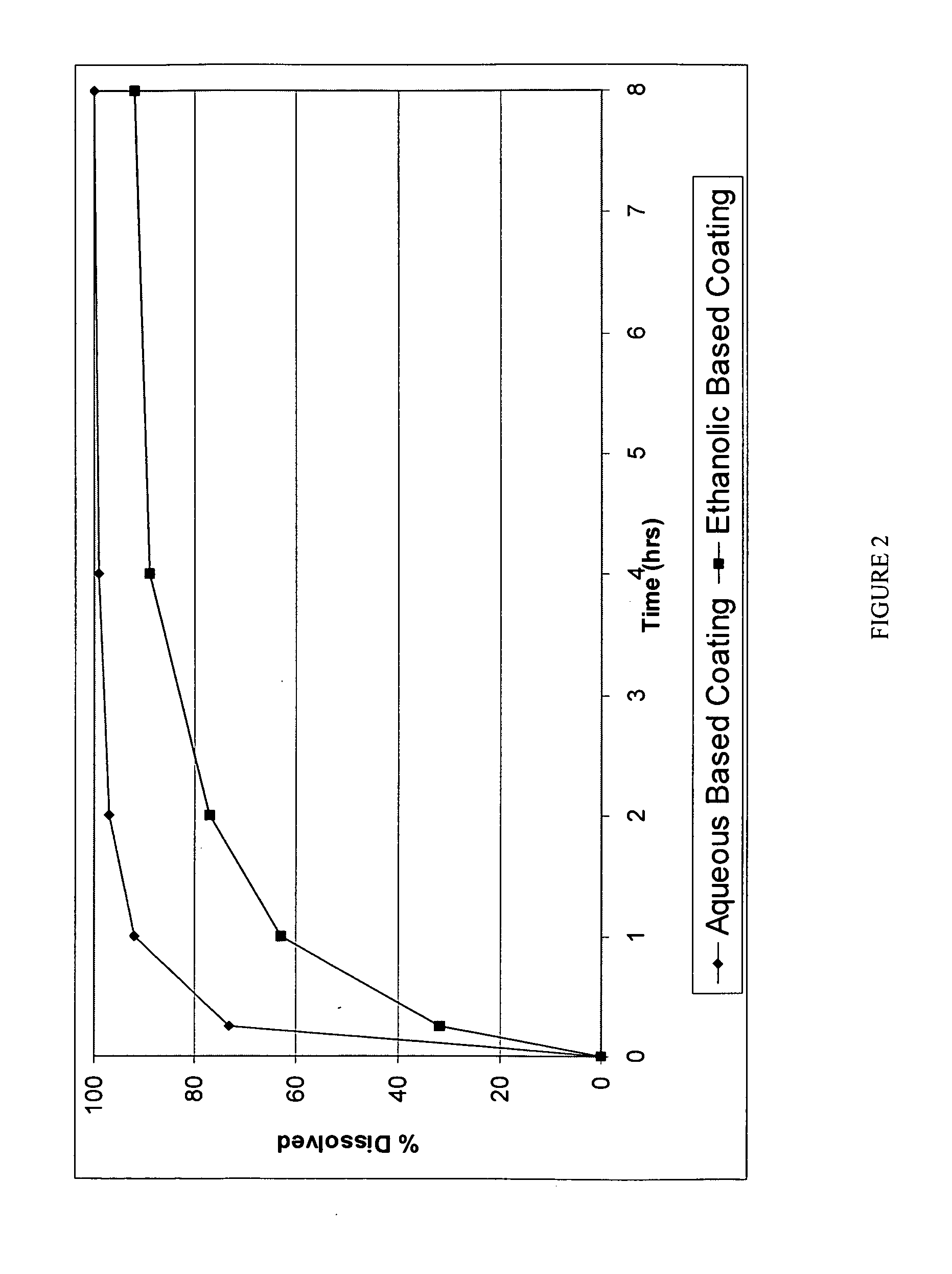
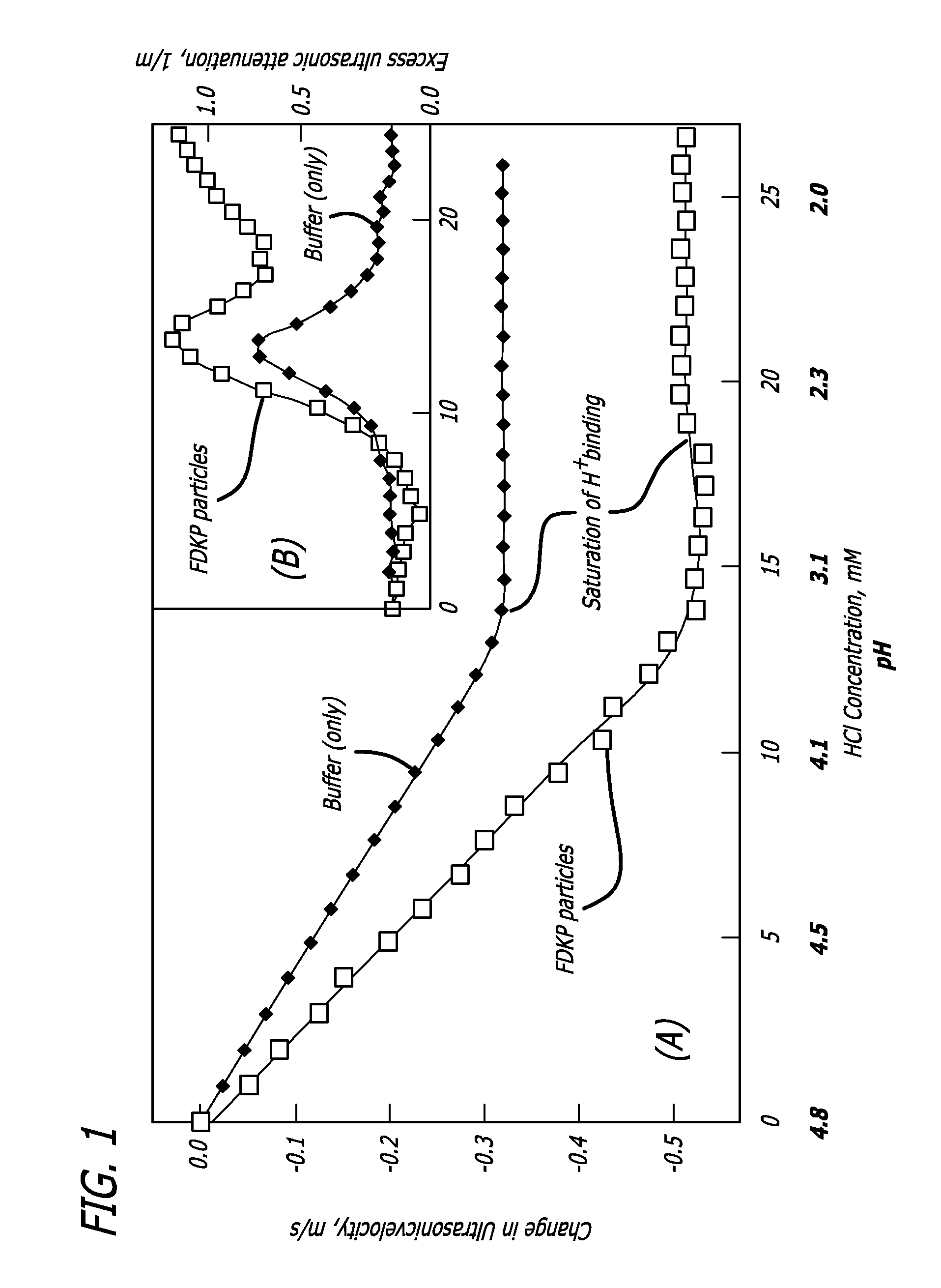
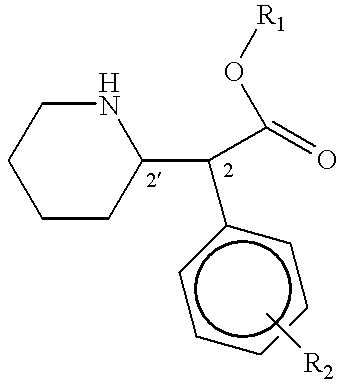
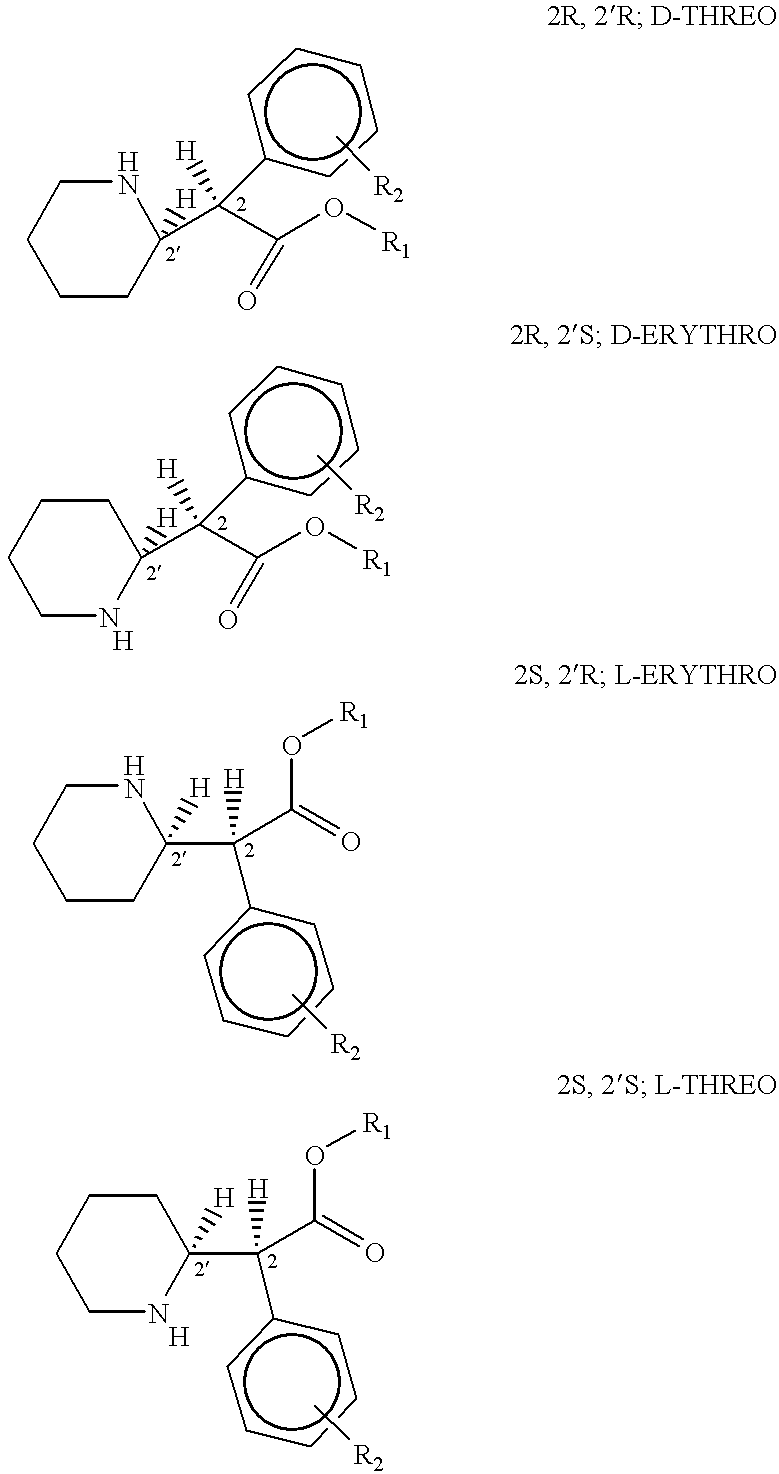
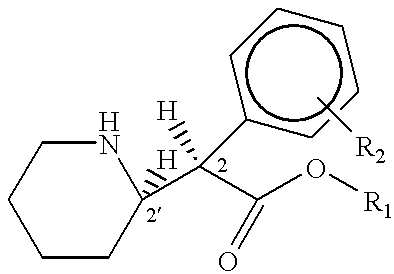
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
703 results about "Drug formulations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
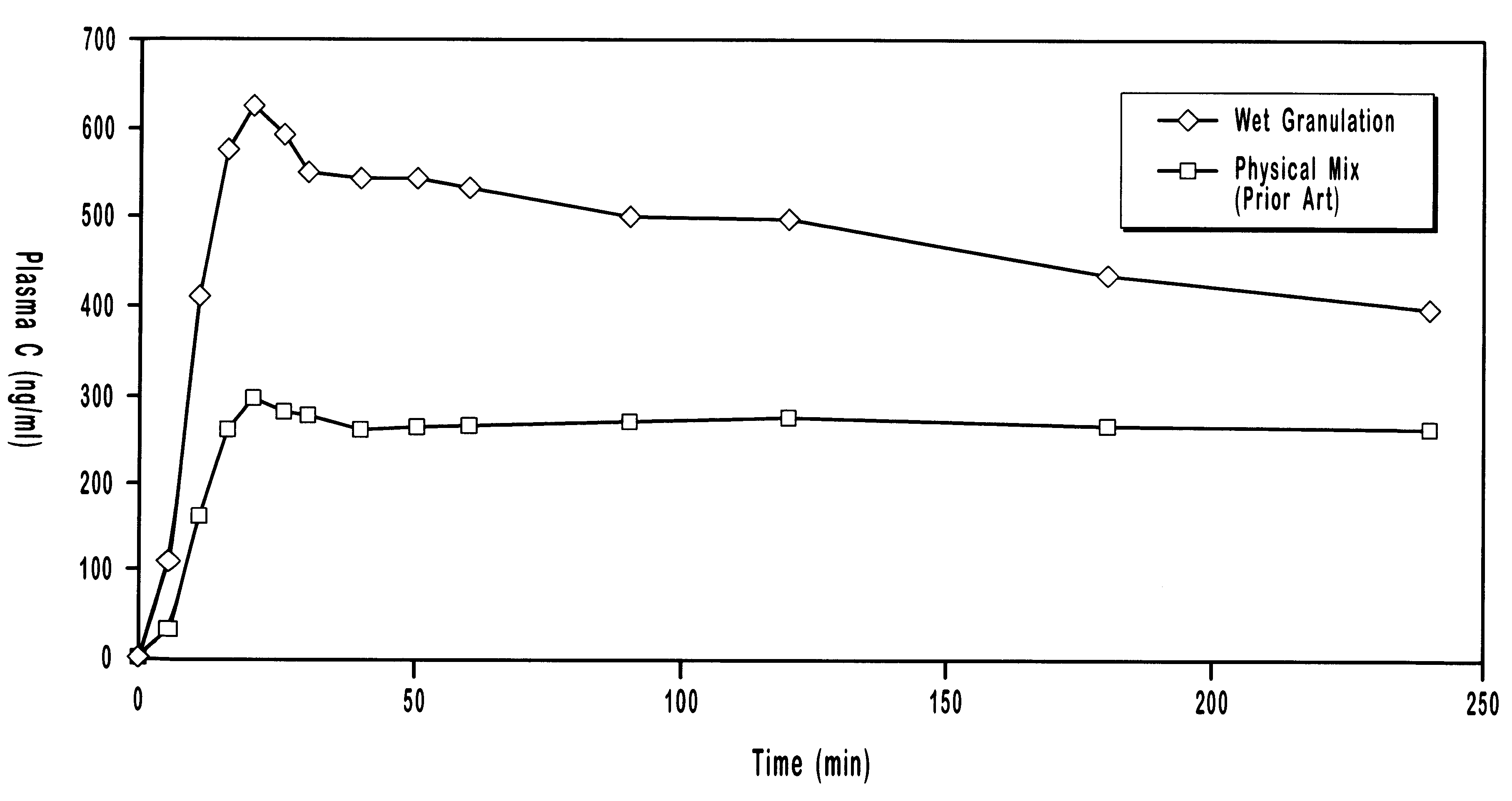
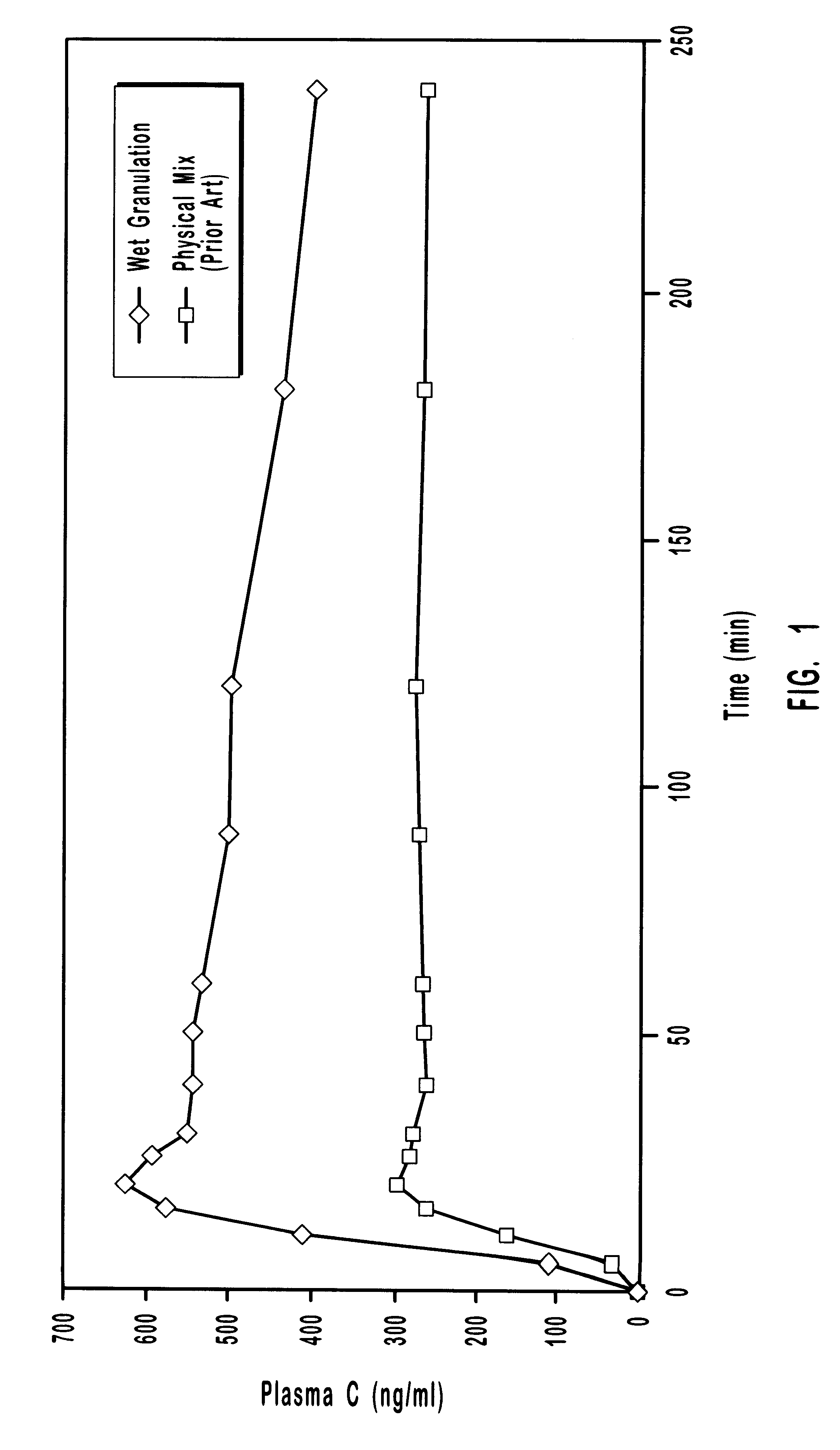
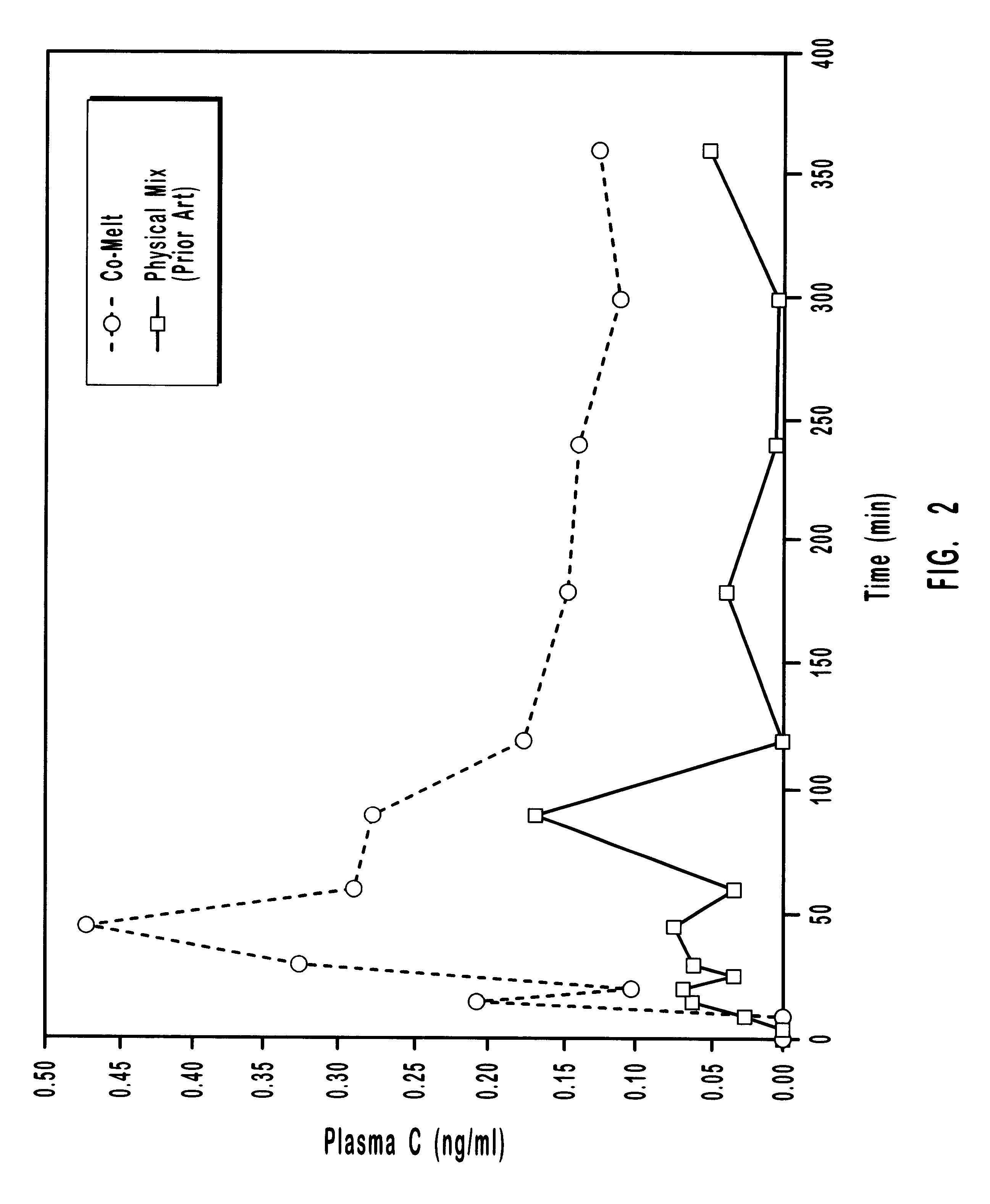
Pharmaceutical formulation is the process of combining various chemical substances with the active drug to form a final medicinal product, which is called a drug mixture or drug formulation. A drug formulation can be given to the patient in various forms like solid, semisolid or liquid.
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Dosage form comprising liquid formulation
InactiveUS6174547B1Improve oral bioavailabilityImprove bioavailabilityCapsule deliveryEmulsion deliveryPharmaceutical formulationDosage form
Owner:ENCINAL PHARMA INVESTMENTS
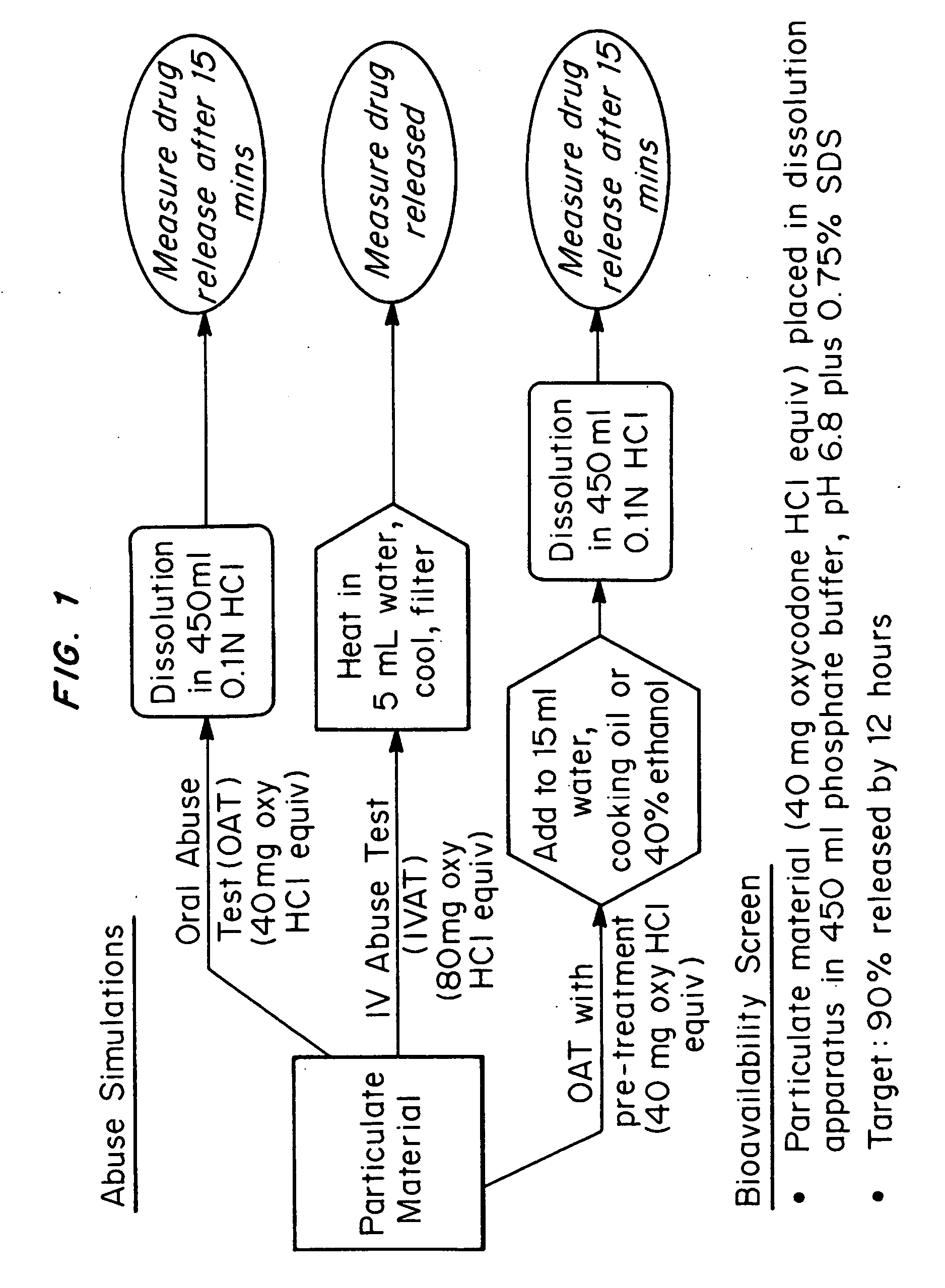
Drug formulations having reduced abuse potential
InactiveUS20040228802A1Prevent extractionReduce potential for abuseBiocidePowder deliverySide effectDrug formulations
Drug formulations having reduced abuse potential which contain one or more of (1) a bittering agent, (2) a bright deterrent / indicator dye and (3) fine insoluble particulate matter. The bittering agent and dye are in a form which does not affect proper administration of the drug, but the bittering agent creates a bitter side effect when the dosage form is crushed or chemically extracted and nasally, orally, buccally or sublingually administered and the dye produces a bright color when crushed and contacted. The fine insoluble particulate matter hinders extraction of the drug from the dosage form and, when crushed, can deter intravenous injection because of the presence of the insoluble particles or hinder injection by blocking an intravenous needle. The bright color of the dye, when extracted, also has a psychologically deterrent effect on intravenous abusers.
Owner:SUPERNUS PHARM INC
Method and package for storing a pressurized container containing a drug
Owner:GLAXO SMITHKLINE LLC
Methods and apparatus for drug delivery involving phase changing formulations
InactiveUS20020004063A1Cleanly peeled from the skinMaintenance of such surfaceSalicyclic acid active ingredientsAnaestheticsPharmaceutical formulationSoft solids
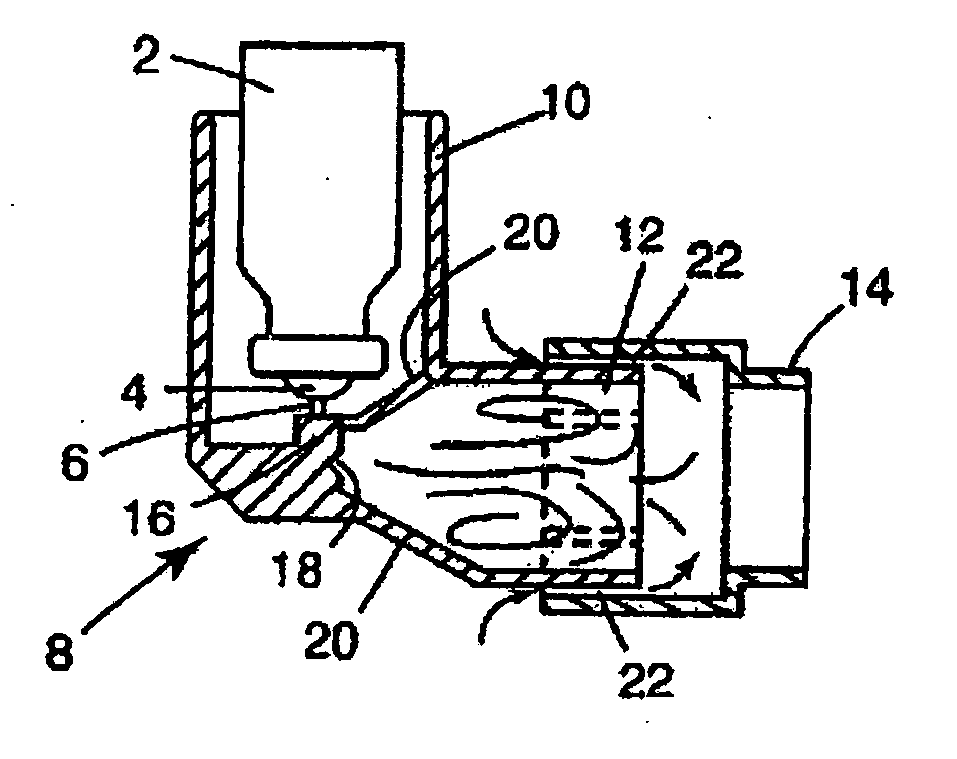
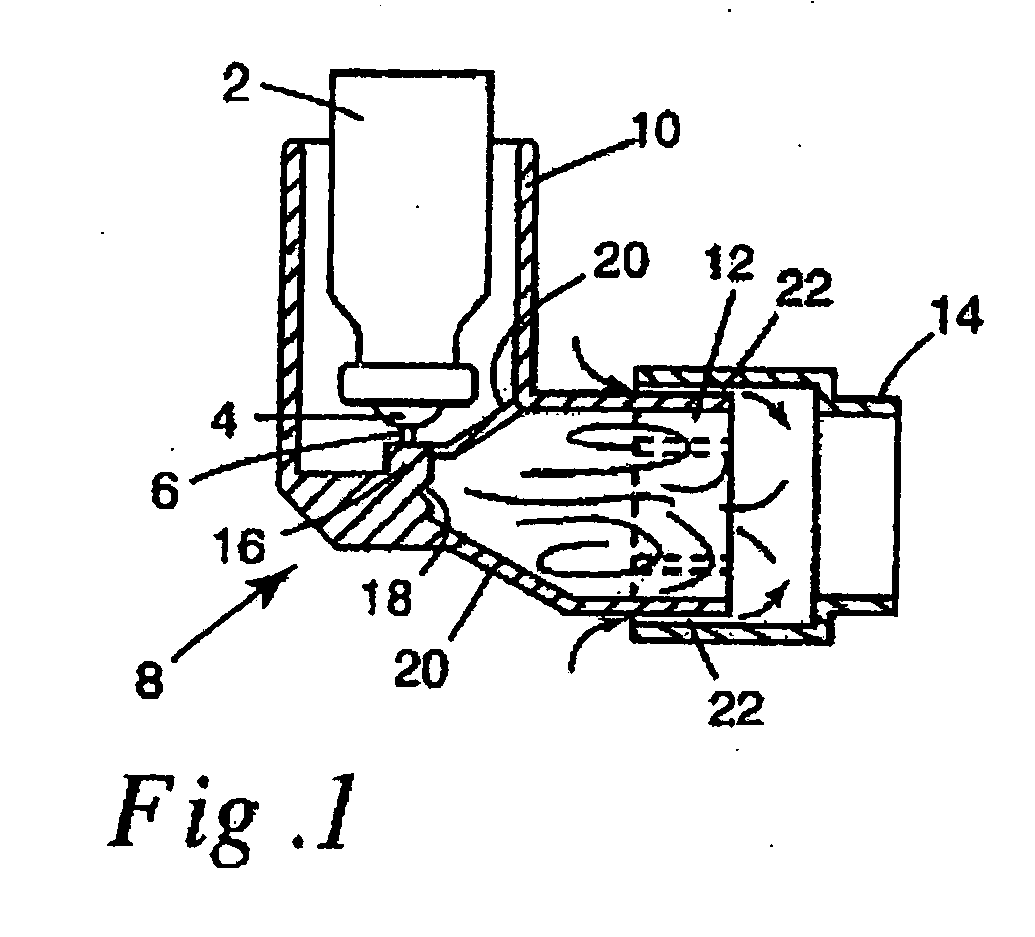
This invention relates to an apparatus and method of drug delivery on a human body surface. The formulation comprises a drug, a conversion agent capable of converting the formulation from a less solid phase to a coherent, soft, solid phase, and a vehicle medium or carrier for the drug and conversion agent. The drug formulation is applied to this human body surface in its less than solid phase and is subsequently converted to a soft solid phase while the drug is being delivered through the human body surface. After delivery of the drug is complete, the soft solid formulation can be removed or peeled from the body surface as a coherent solid formulation. The drug formulation provides control over drug delivery rates and allows the formulation to be removed without leaving a messy, residual formulation on the body surface.
Owner:CRESCITA THERAPEUTICS INC
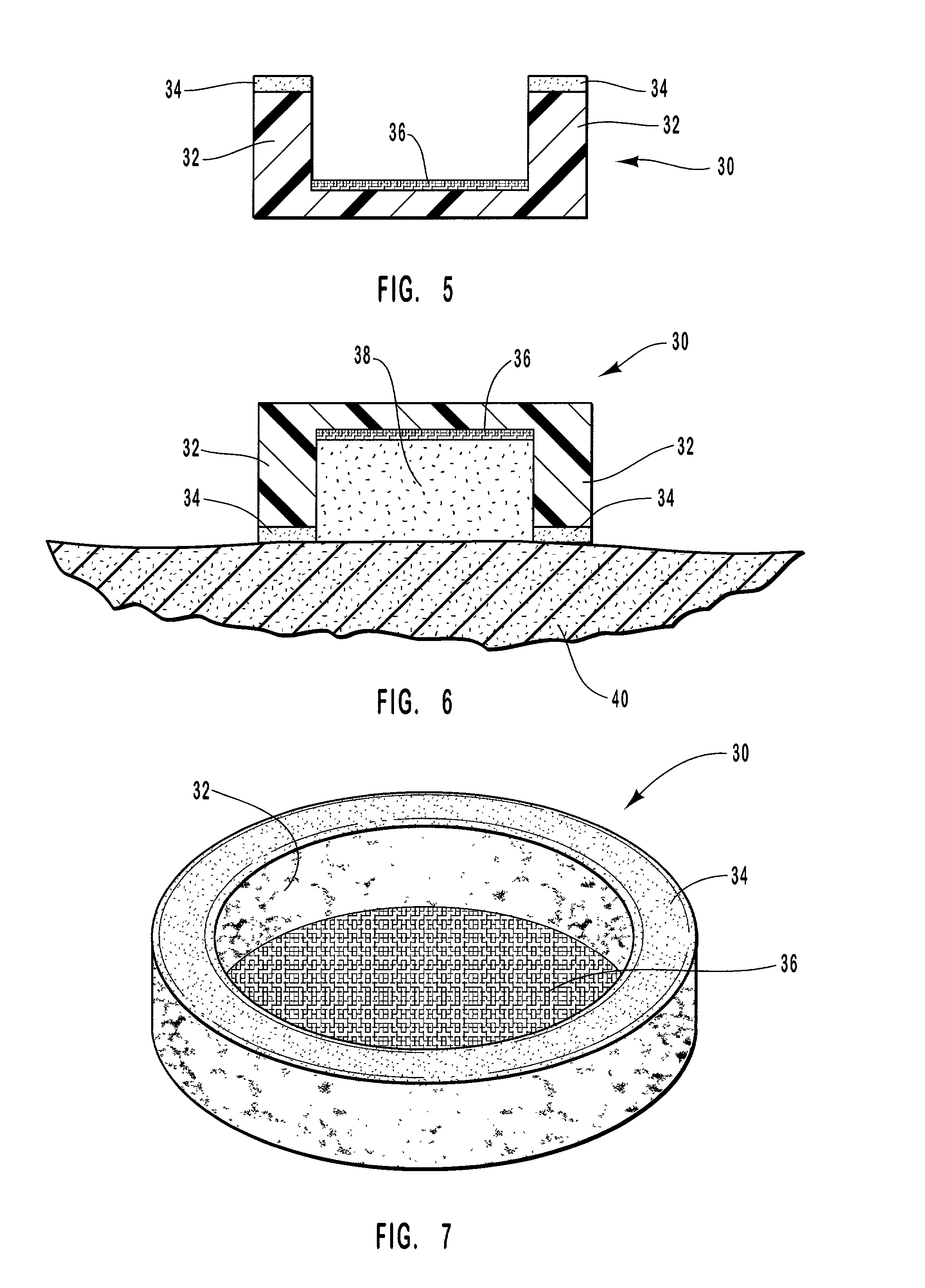
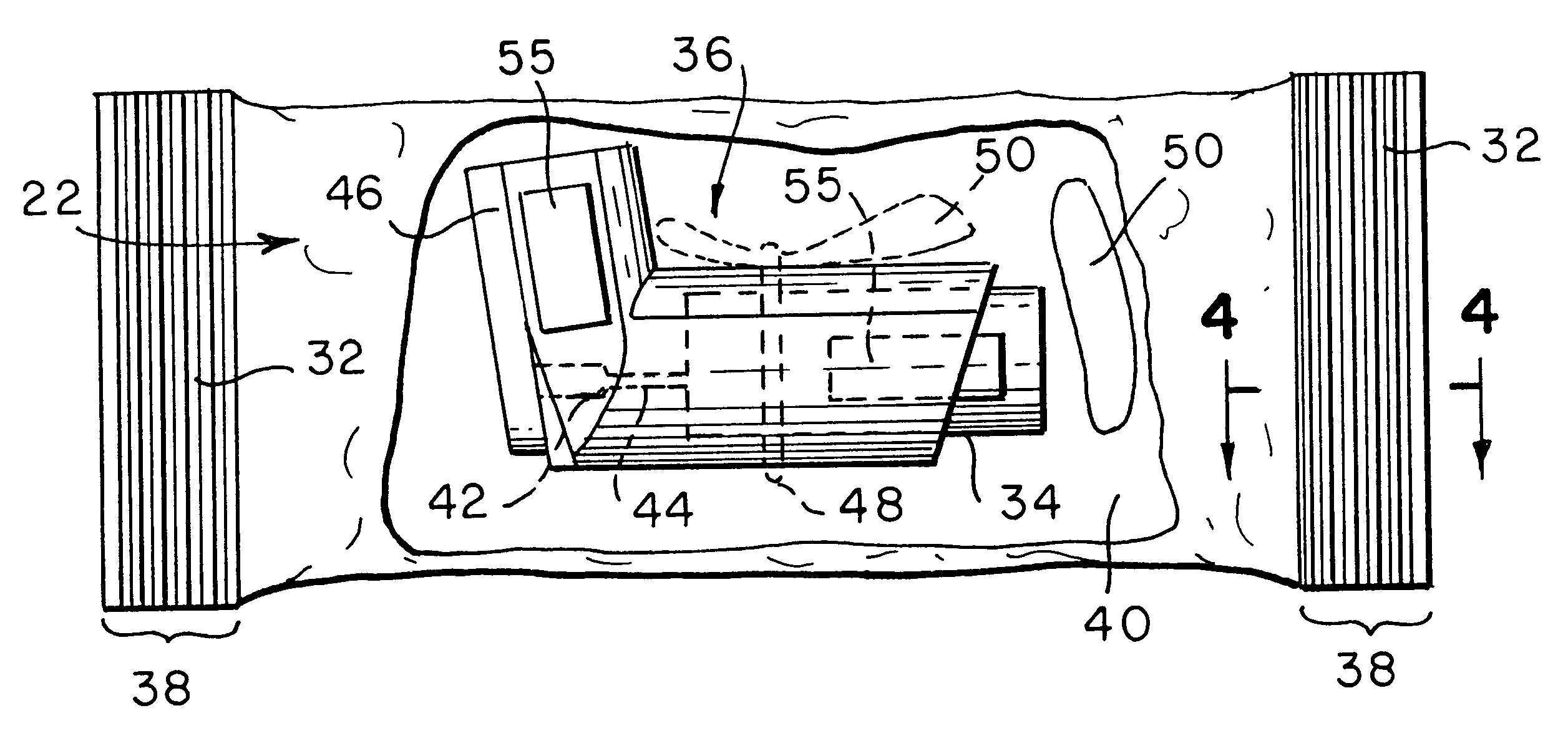
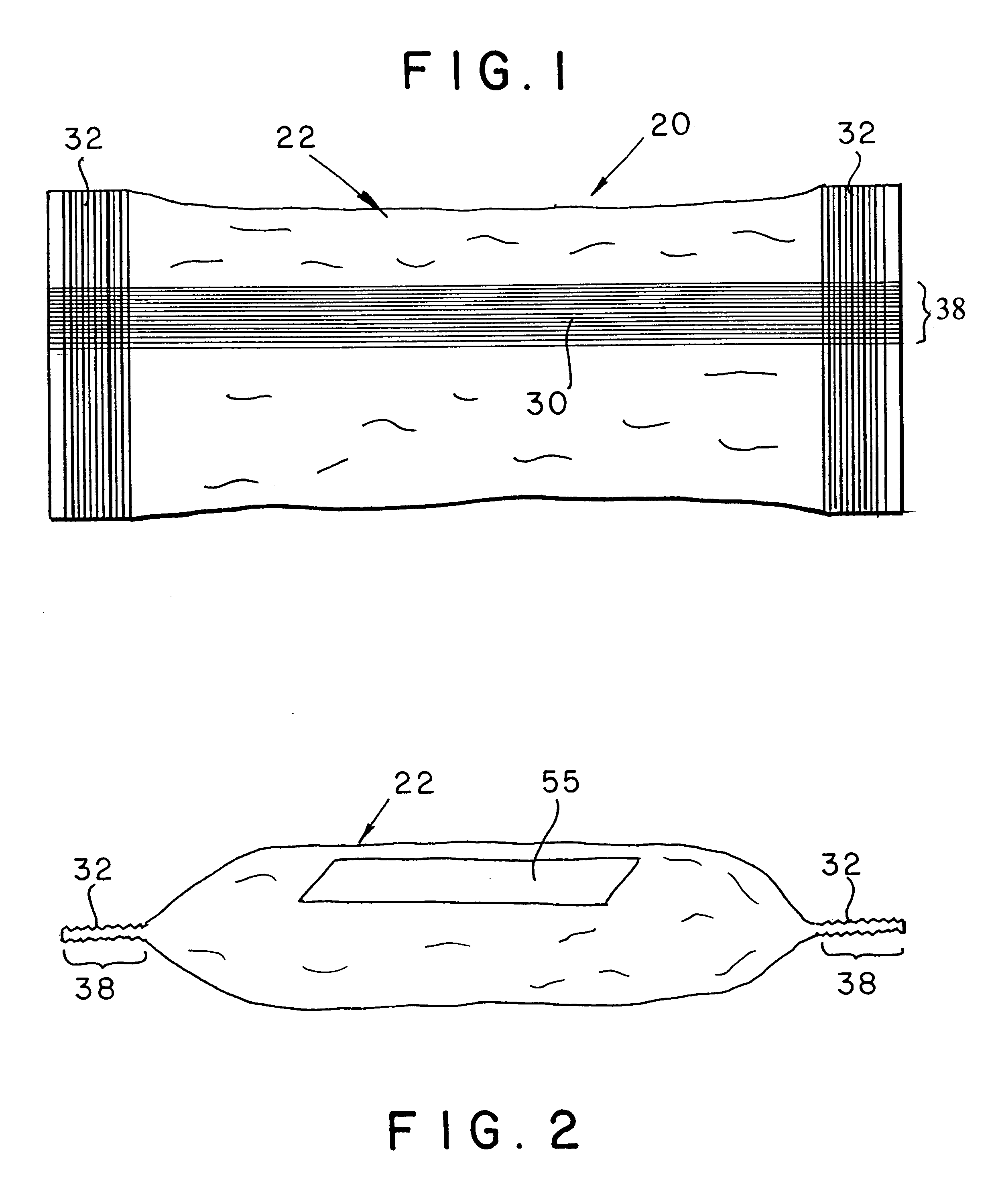
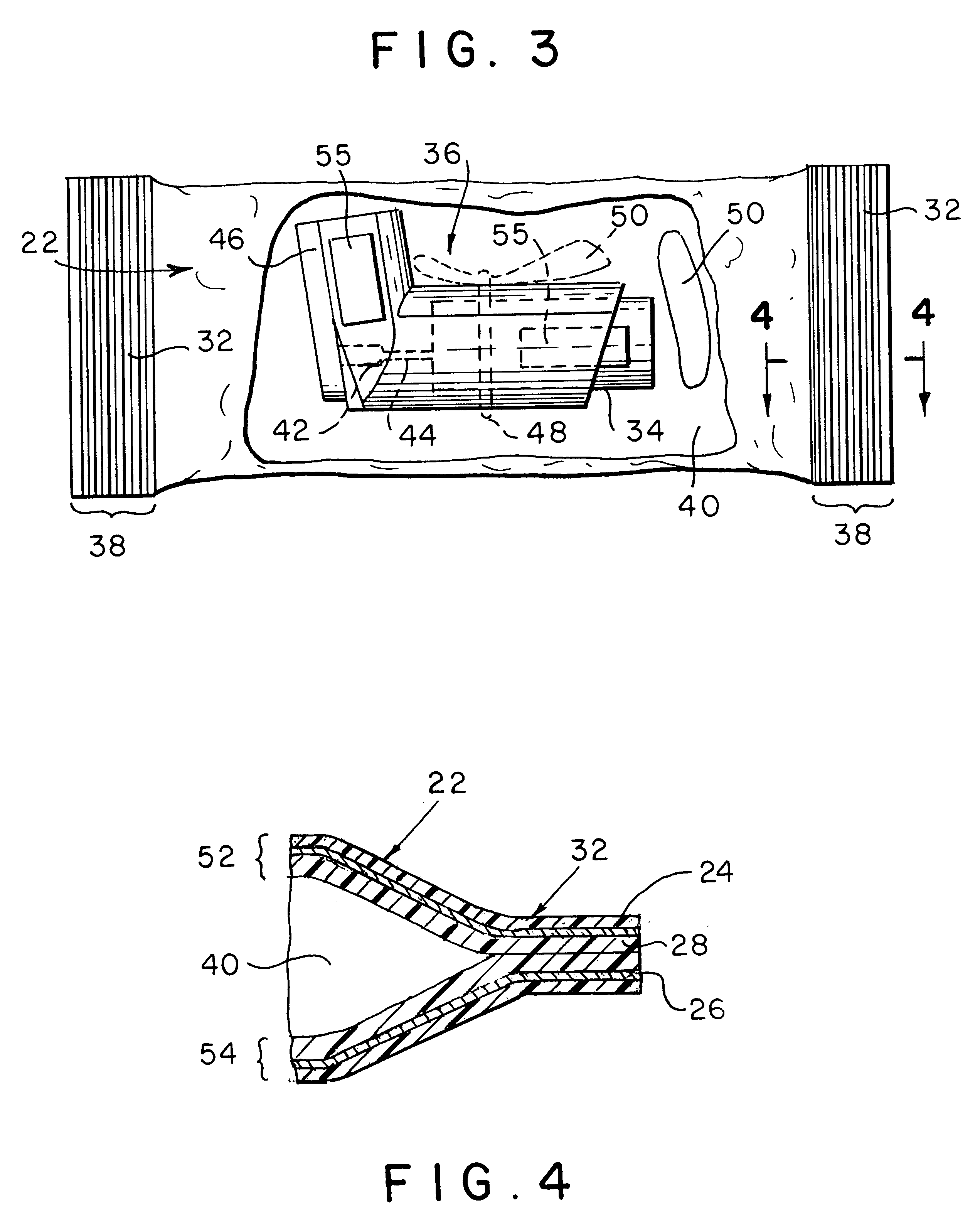
Apparatus and formulations for suprachoroidal drug delivery
InactiveUS20070202186A1Avoid traumaMinimally-invasive deliveryBiocidePowder deliveryPosterior regionPharmaceutical formulation
Drug formulations, devices and methods are provided to deliver biologically active substances to the eye. The formulations are delivered into scleral tissues adjacent to or into the suprachoroidal space without damage to the underlying choroid. One class of formulations is provided wherein the formulation is localized in the suprachoroidal space near the region into which it is administered. Another class of formulations is provided wherein the formulation can migrate to another region of the suprachoroidal space, thus allowing an injection in the anterior region of the eye in order to treat the posterior region.
Owner:CLEARSIDE BIOMEDICAL
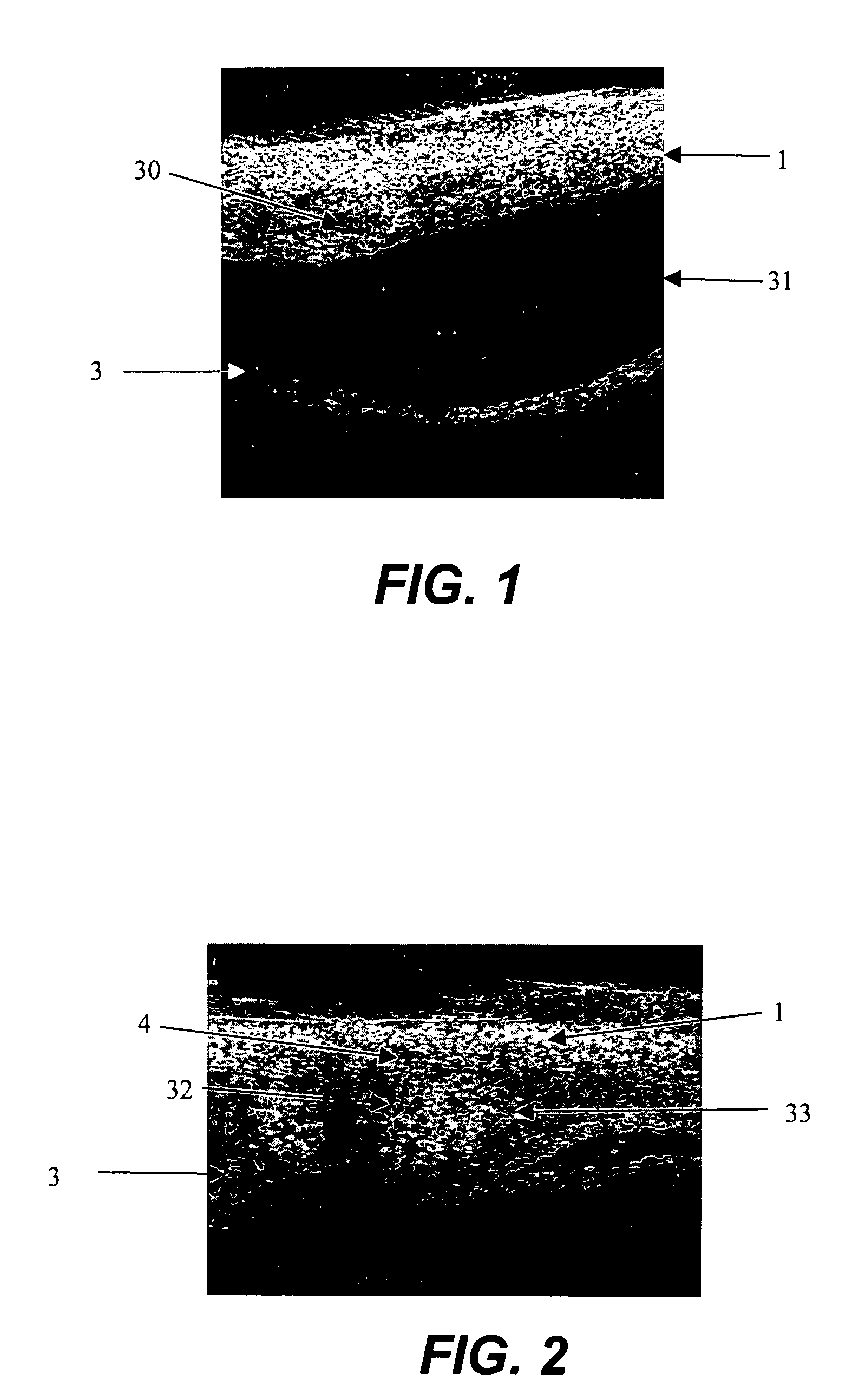
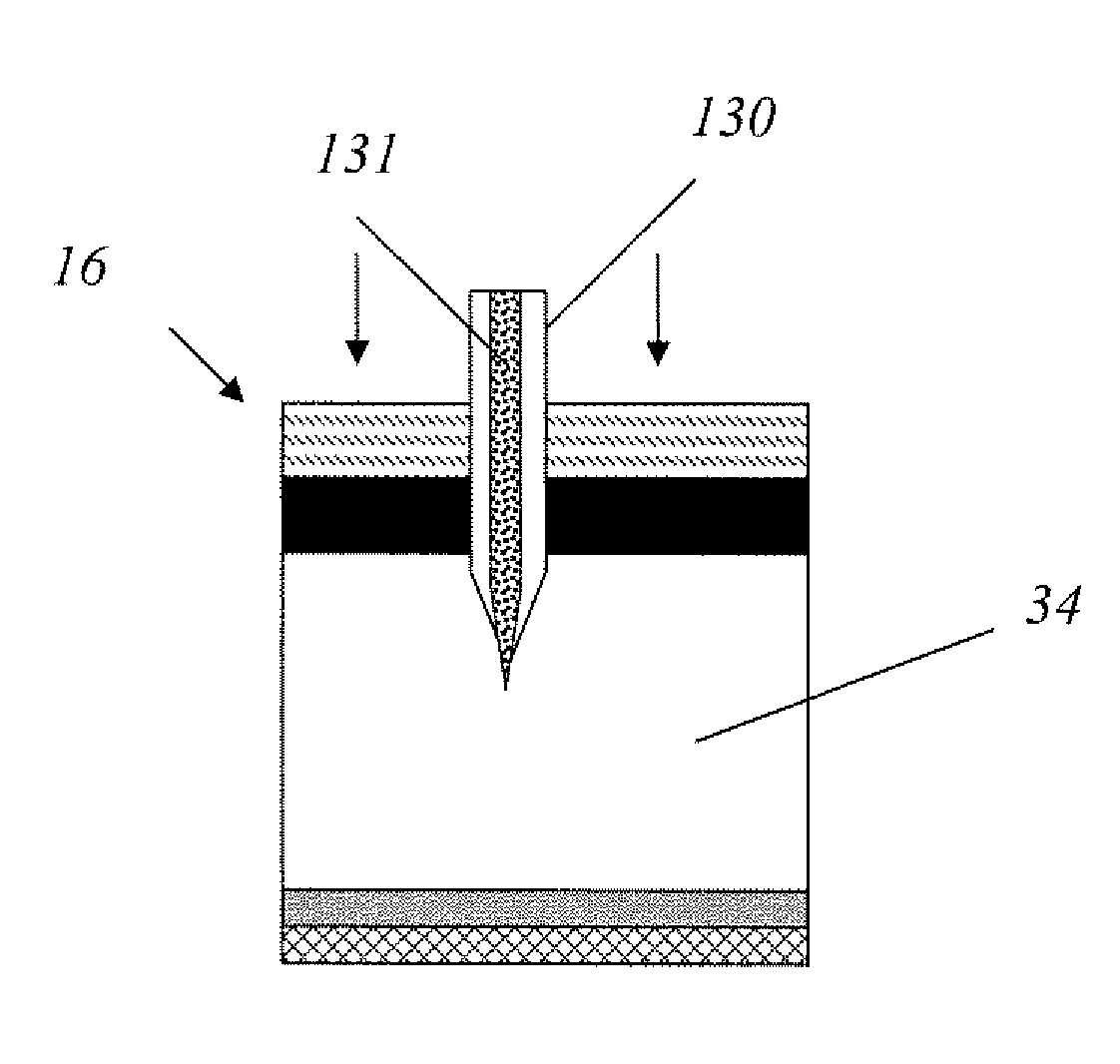
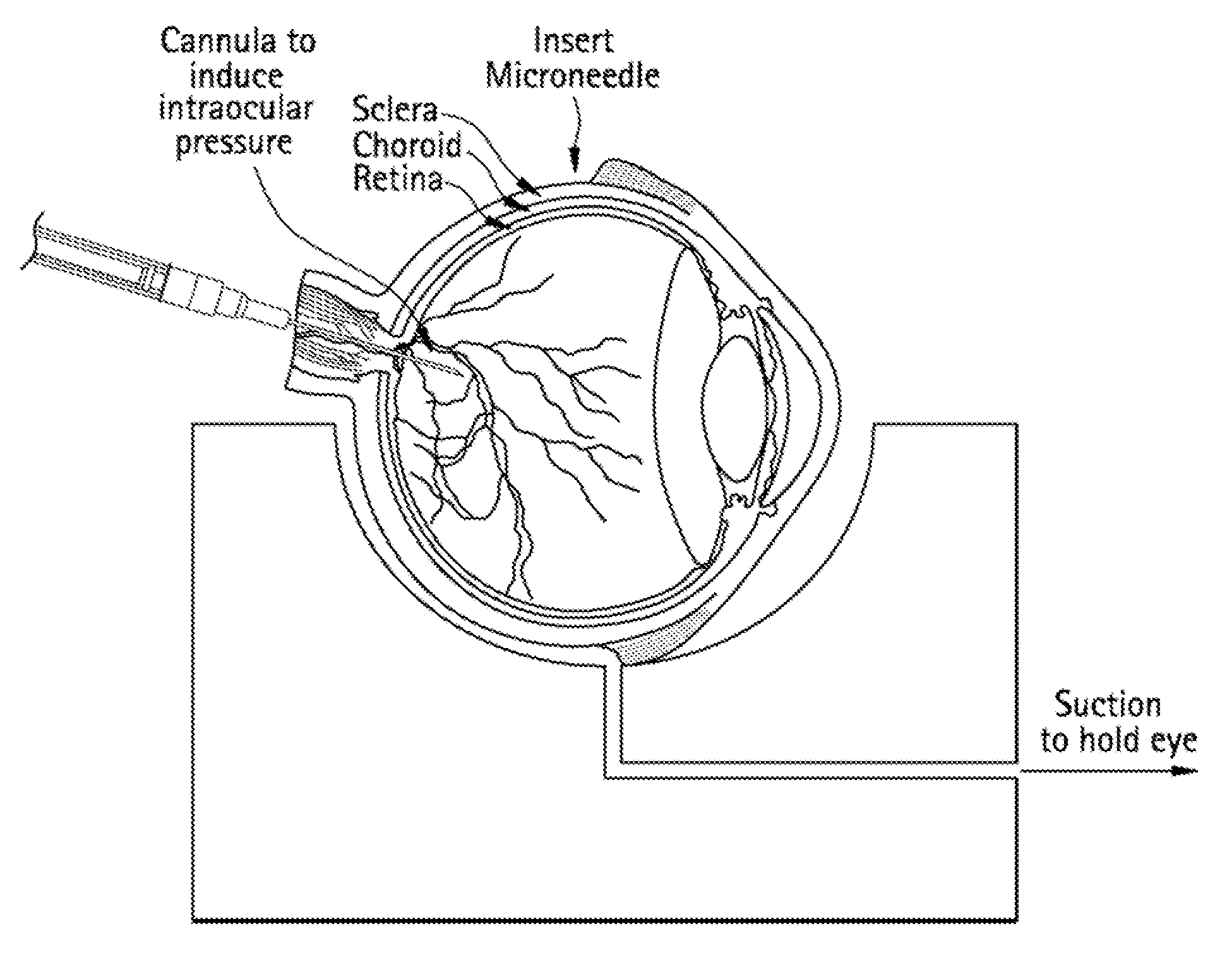
Method for drug delivery to ocular tissue using microneedle
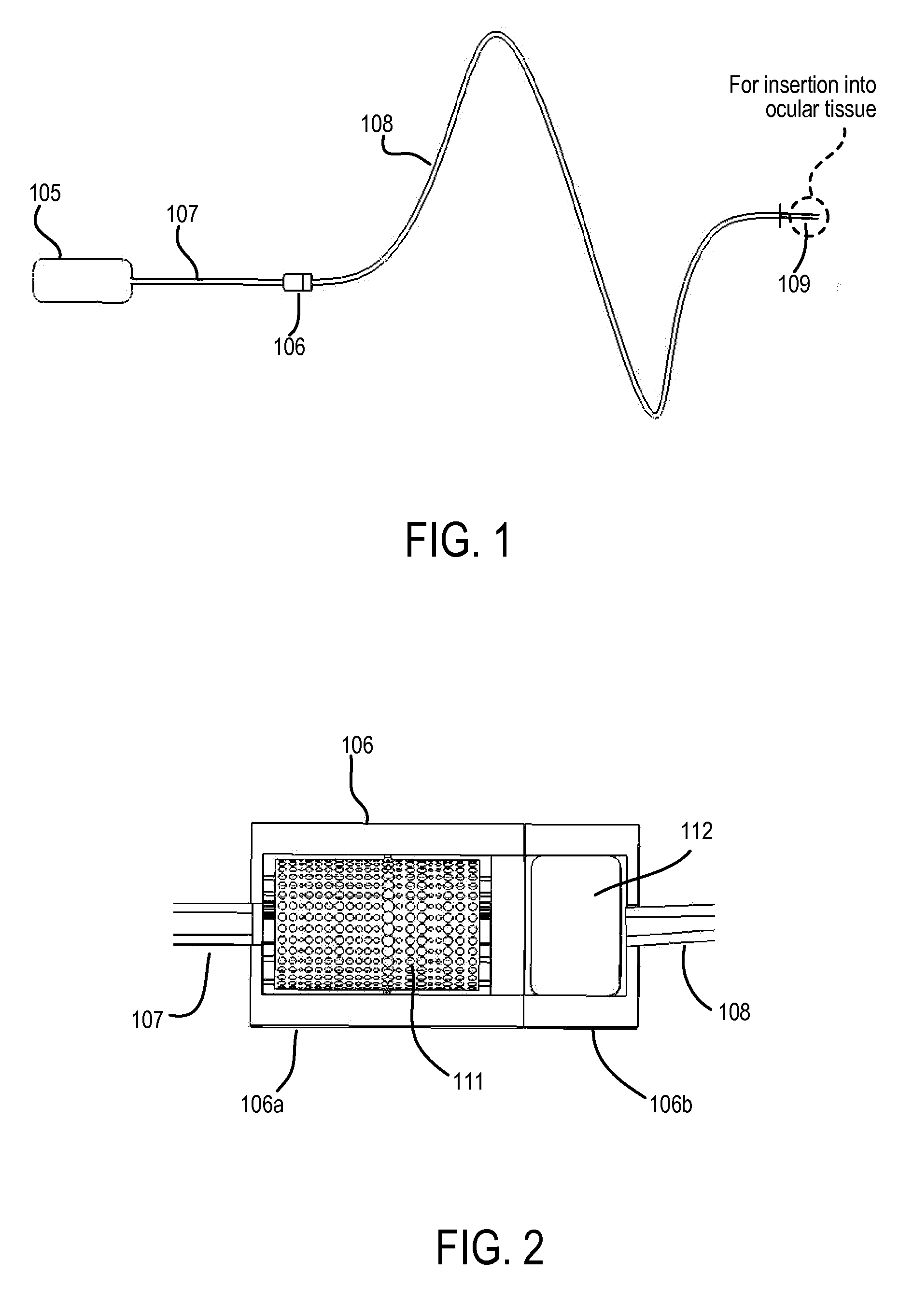
Methods and devices are provided for administering a drug to a patient's eye. The methods include (a) inserting a hollow microneedle into the sclera or corneal stroma without penetrating across the sclera or corneal stroma; and (b) infusing a fluid drug formulation through the microneedle and into the sclera or cornea. It further may include partially retracting the microneedle before infusion to enhance delivery. Alternatively, the methods may include (a) inserting a solid microneedle into the sclera or corneal stroma without penetrating across the sclera or corneal stroma, wherein the solid microneedle comprises a first quantity of a drug formulation and inserting causes the solid microneedle to form a pocket in the sclera or corneal stroma; and (b) releasing the drug formulation into the pocket to form a drug depot, whereby a drug is released from the depot. The methods and devices may include an array of multiple microneedles.
Owner:GEORGIA TECH RES CORP +1
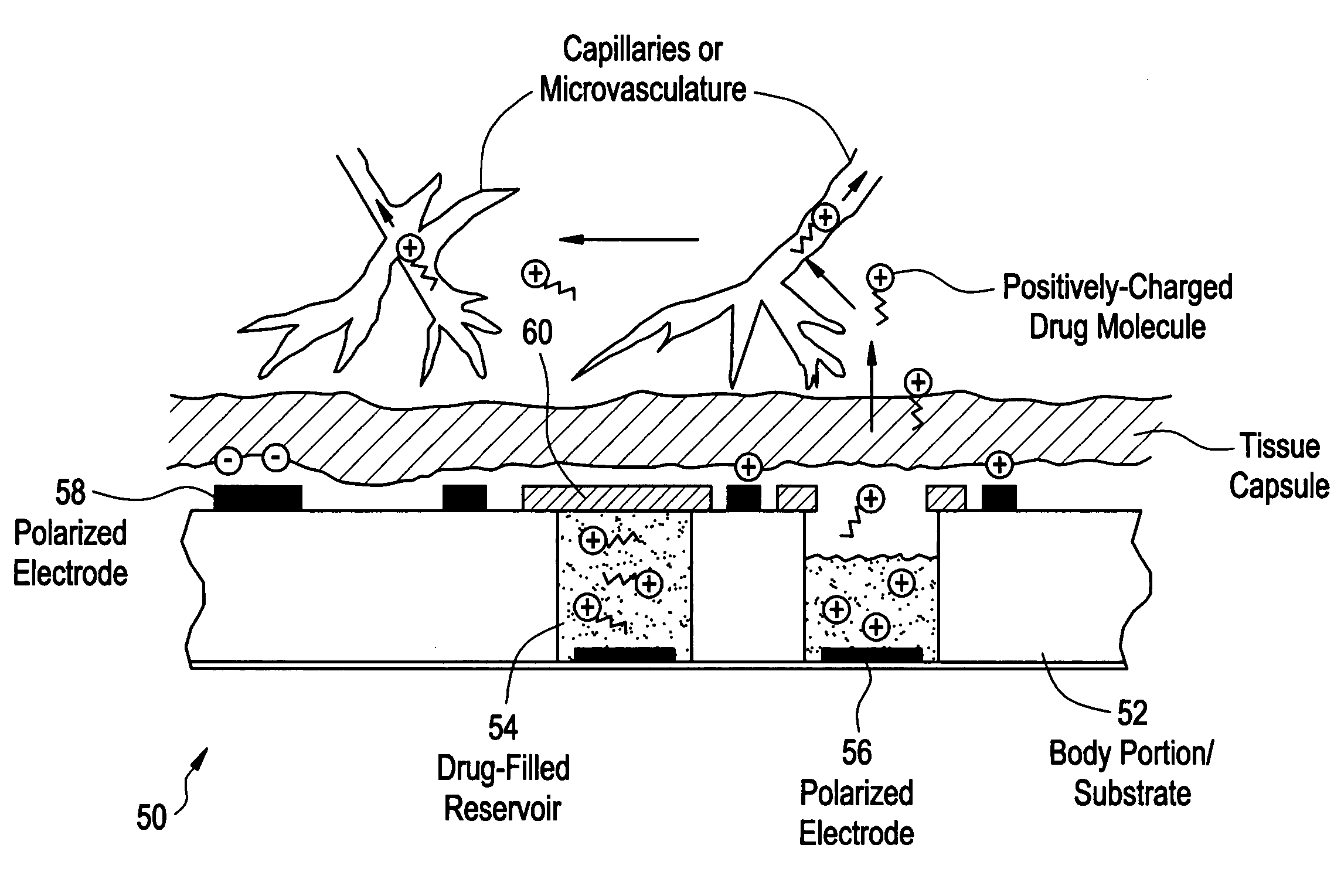
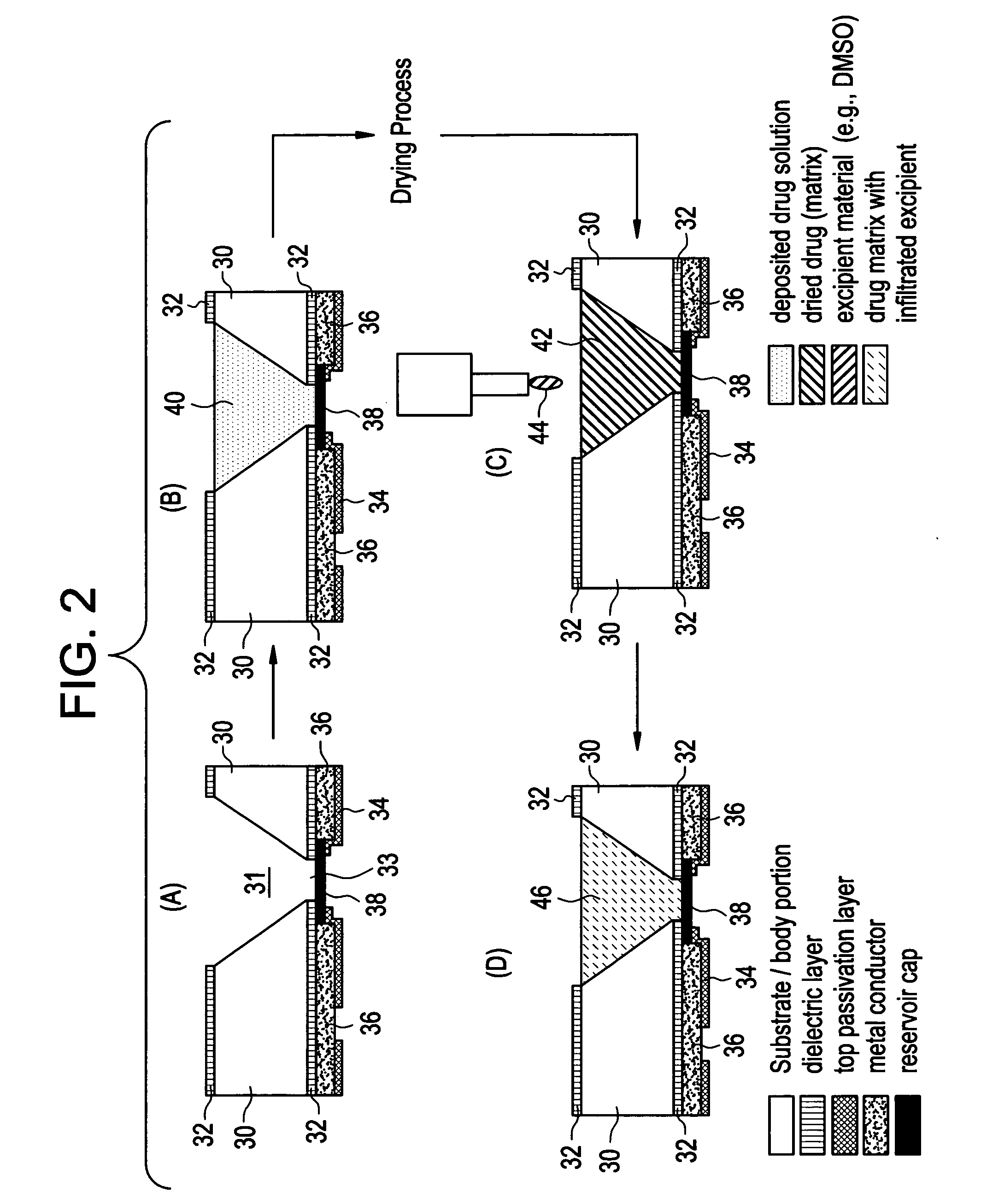
Devices and methods for measuring and enhancing drug or analyte transport to/from medical implant
InactiveUS20050267440A1Easy to transportEfficient propulsionElectrocardiographyAntipyreticControlled drugsAnalyte
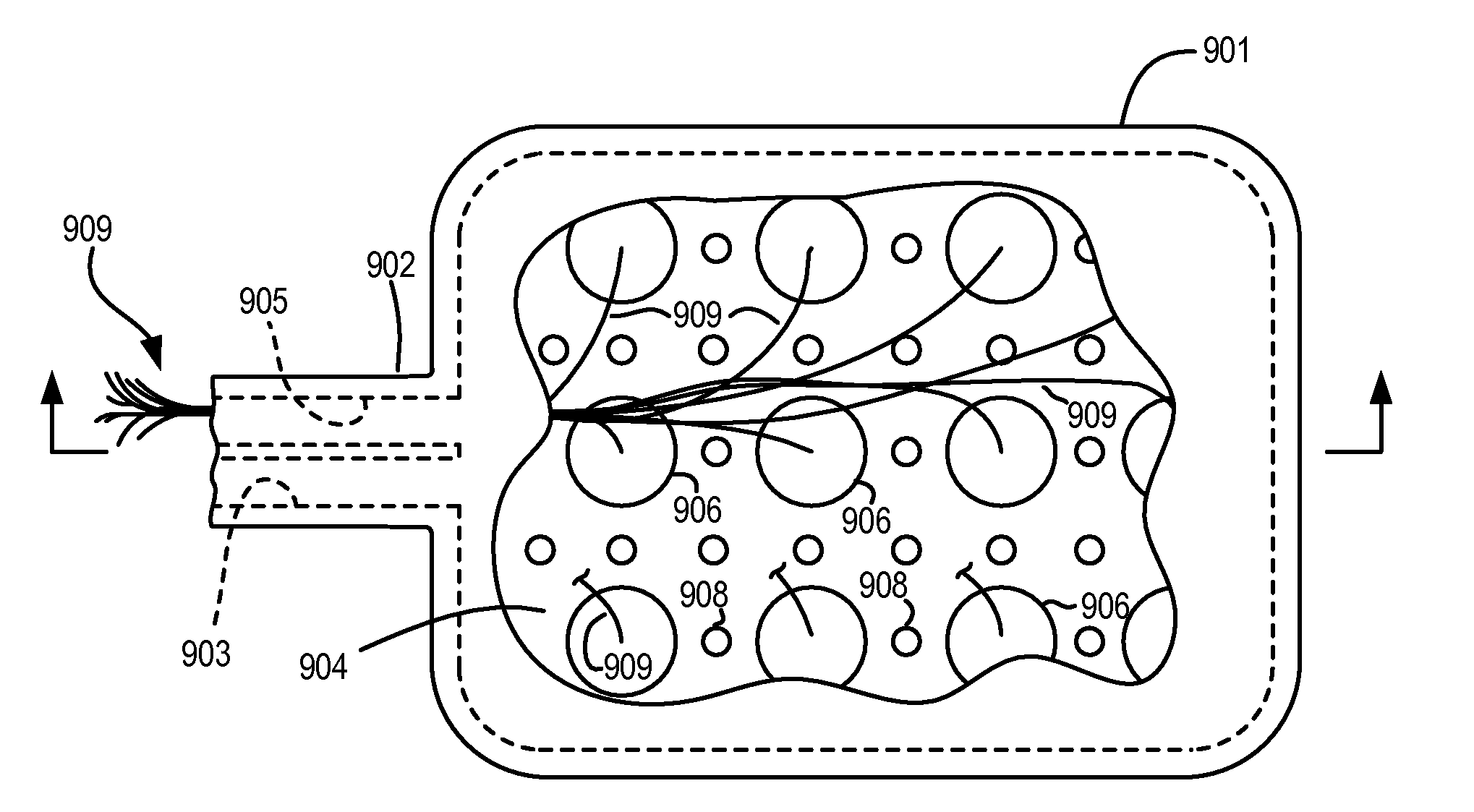
Methods and devices are provided for enhancing mass transport through any fibrous tissue capsule that may form around an implanted medical device following implantation. Methods and devices are also provided to enhance vascularization around the implanted device, which also will aid in mass transport to / from the device. The device preferably comprises multiple reservoirs containing (i) a drug formulation for short- or long-term, controlled drug delivery, (ii) sensors for sensing an analyte in the patient, or (iii) a combination thereof.
Owner:MICROCHIPS INC
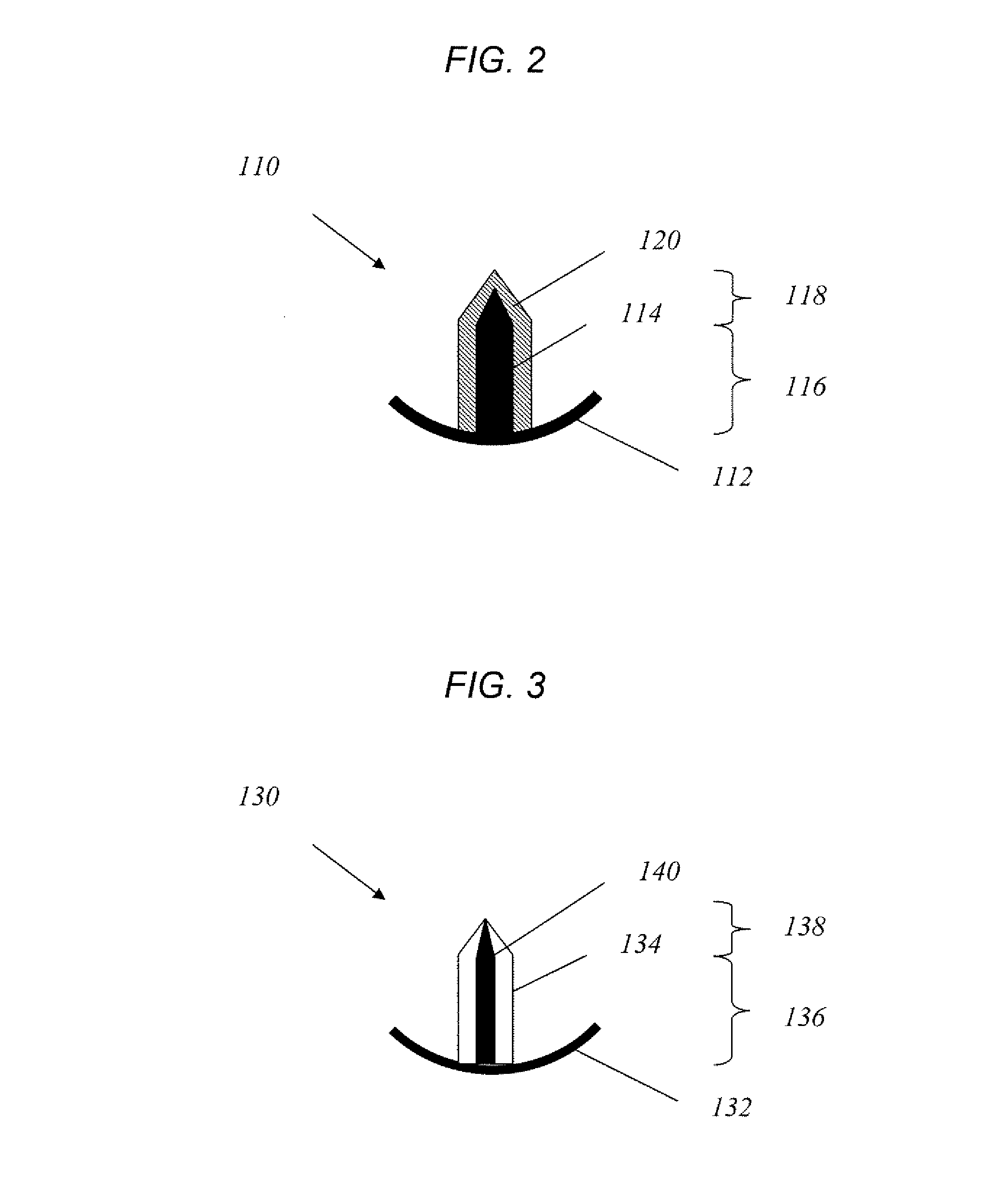
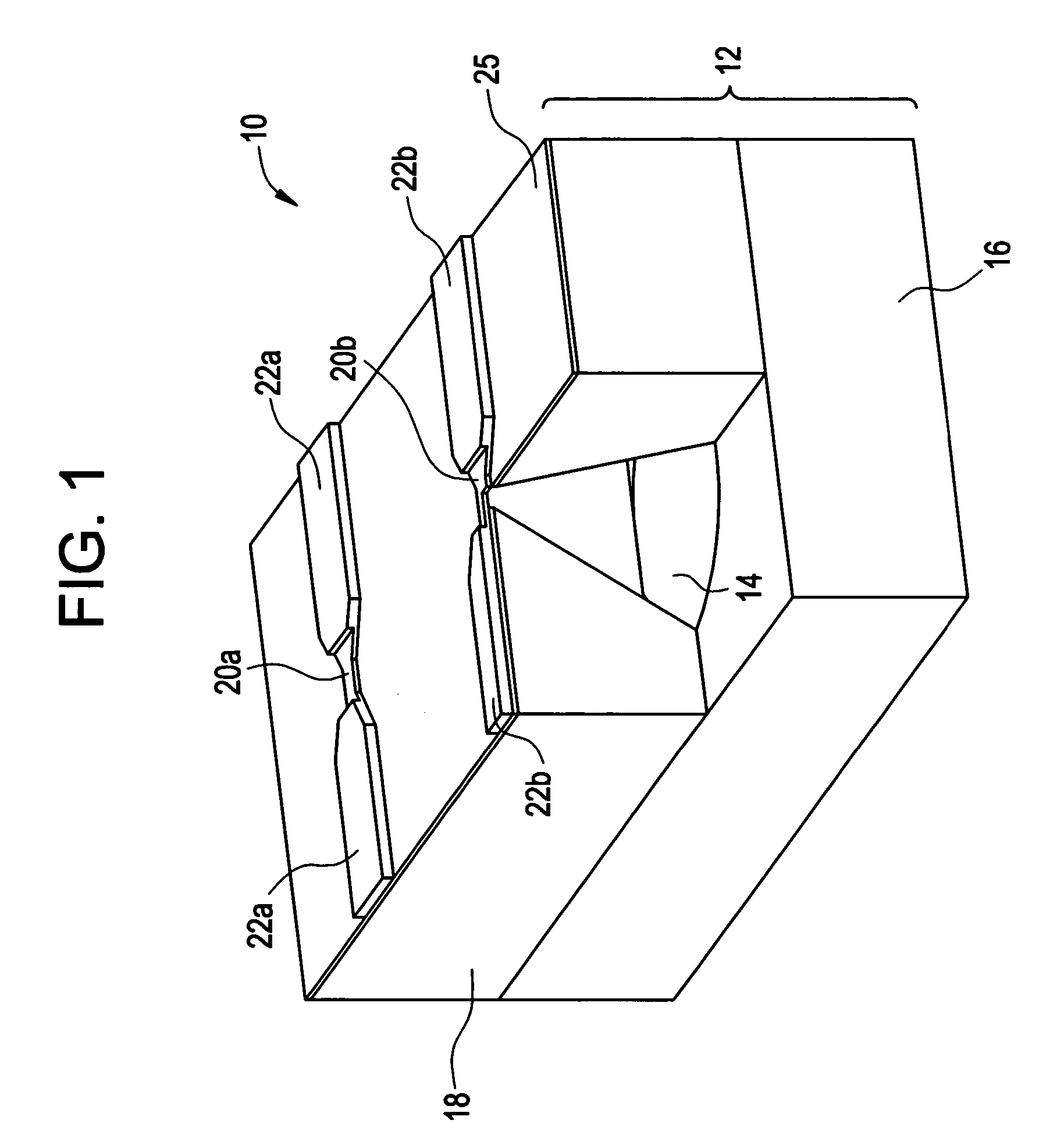
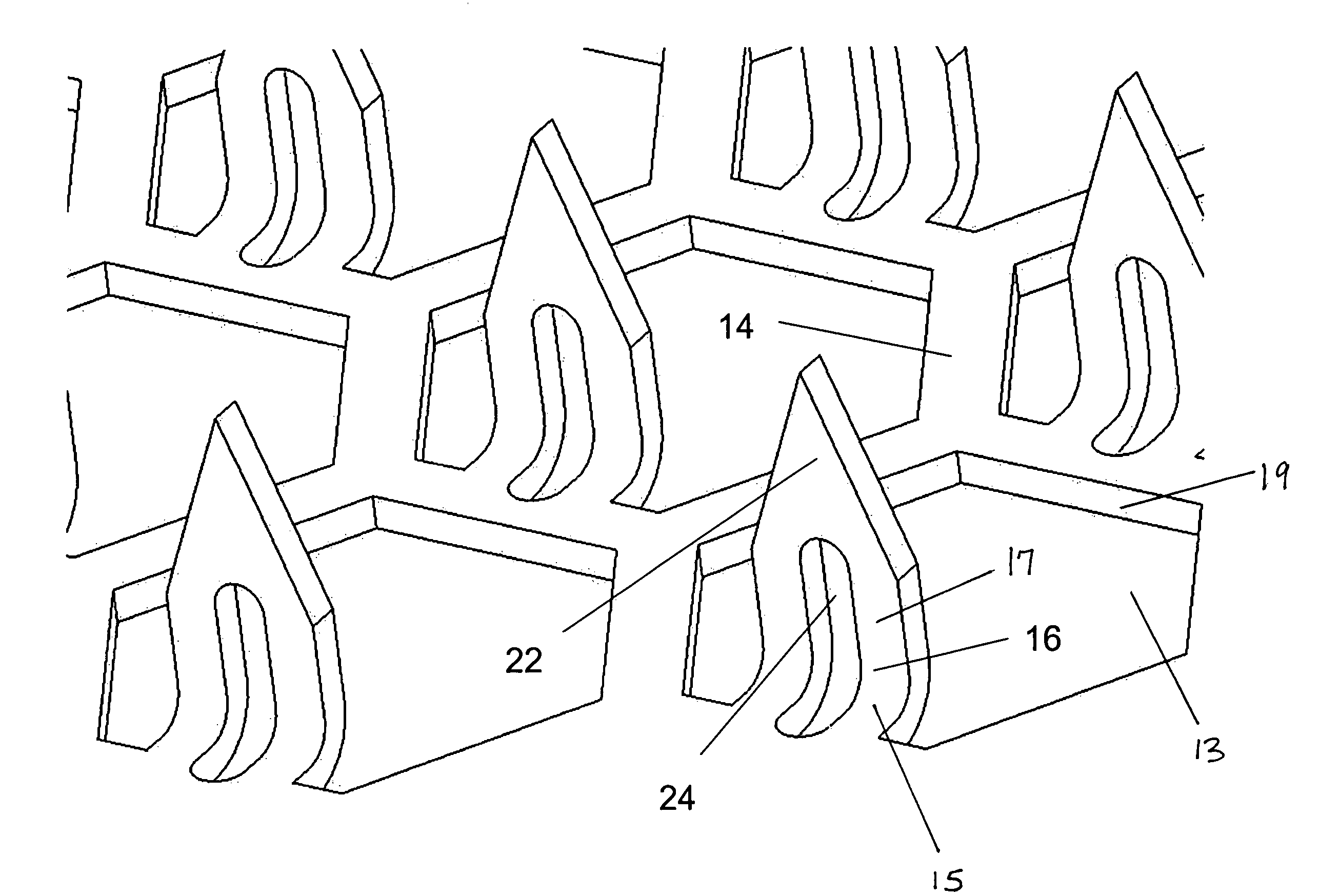
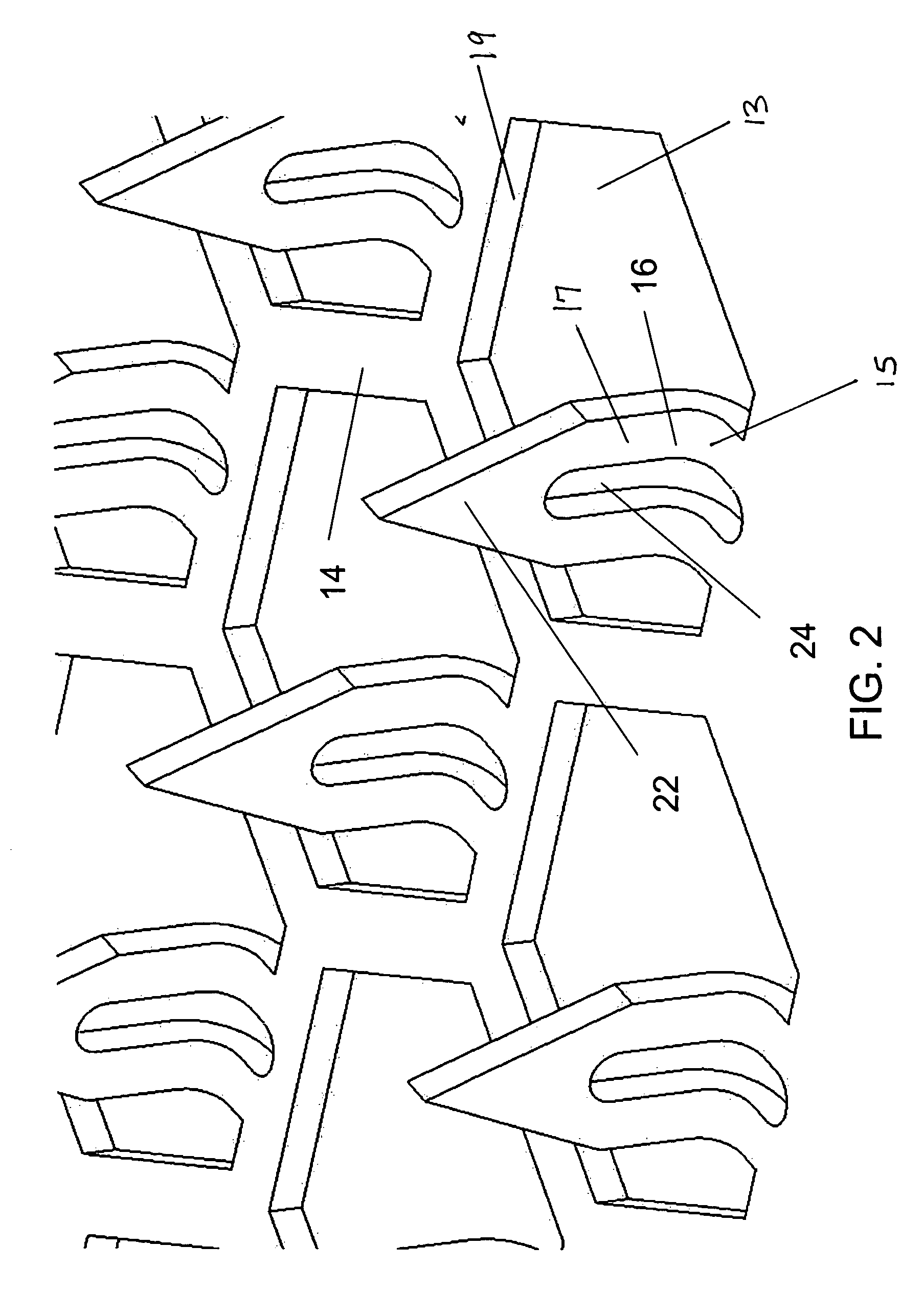
Microneedle array, patch, and applicator for transdermal drug delivery
Microneedle arrays and drug delivery devices are provided for transdermally delivering a drug formulation to a patient. The microneedle array device includes a substantially planar substrate having an array of apertures; and a plurality of microneedles projecting at angle from the planar substrate, the microneedles having a base portion integrally connected to the substrate, a tip end portion distal to the base portion, and body portion therebetween, wherein each microneedle has at least one channel extending substantially from the base portion through at least a part of the body portion, the channel being open along at least part of the body portion and in fluid communication with at least one of the apertures in the substrate. In a preferred embodiment, each microneedle has a substantially rectangular cross-sectional shape and the channel is open to two opposing surfaces of the microneedle.
Owner:YUZHAKOV VADIM V
Method and package for storing a pressurized container containing a drug
InactiveUS6179118B1Reduce manufacturing complexityReduce manufacturing costWrappersDiagnosticsWater vaporWaste management
Owner:GLAXO SMITHKLINE LLC
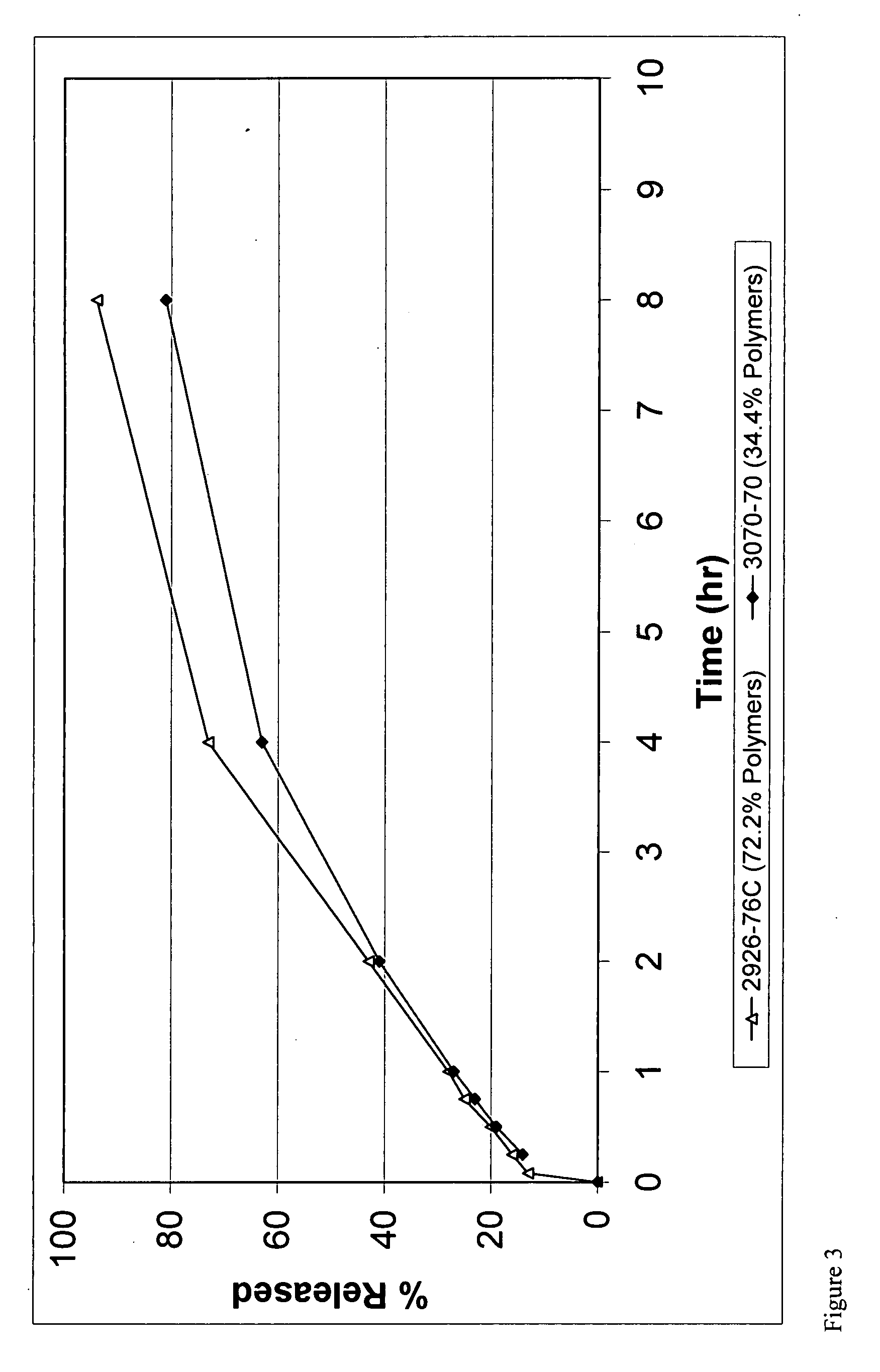
Abuse resistant drug formulation
A pharmaceutical composition may include a coated particulate which may include at least one active pharmaceutical ingredient, particularly one susceptible to abuse by an individual. The coated particles may include a fat / wax and have improved controlled release and / or crush resistance. Method of making these coated particulate and dosage forms therewith are also described.
Owner:CIMA LABS
Method of Drug Formulation Based on Increasing the affinity of Crystalline Microparticle Surfaces for Active Agents
Methods are provided for coating crystalline microparticles with an active agent by altering the surface properties of the microparticles in order to facilitate favorable association on the microparticle by the active agent. Type of surface properties that are altered by the disclosed methods include by electrostatic properties, hydrophobic properties and hydrogen bonding properties.
Owner:MANNKIND CORP
Non-aqueous single phase vehicles and formulations utilizing such vehicles
InactiveUS20050008661A1Increase stickinessReduce occlusionPowder deliveryPeptide/protein ingredientsEngineeringSolvent
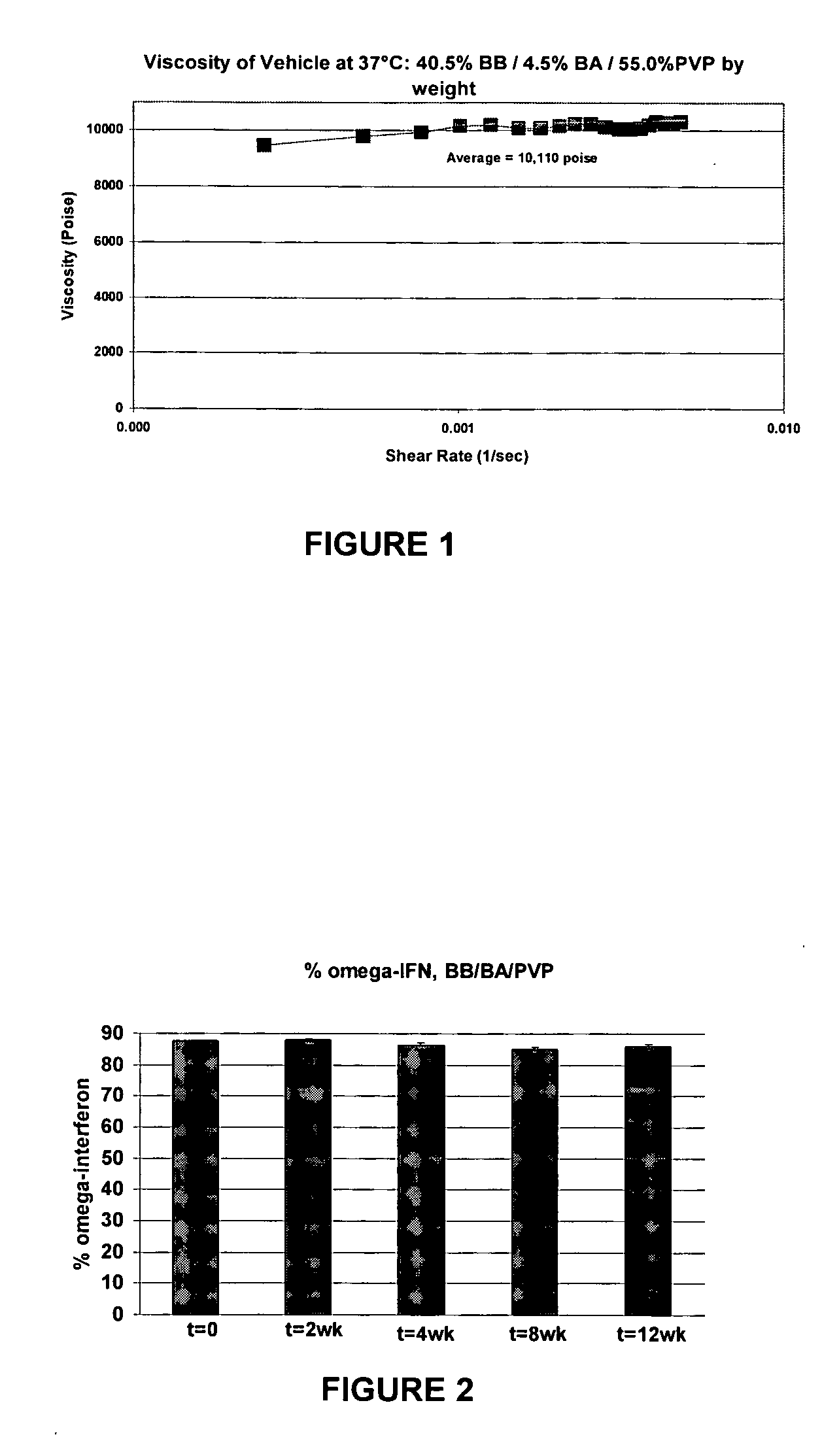
The present invention includes materials and methods for providing vehicles useful for providing drug formulations that address the potential drawbacks of known nonaqueous formulations. In particular, the present invention includes nonaqueous vehicles that are formed using a combination of polymer and solvent that results in a vehicle that is miscible in water. The nonaqueous vehicles facilitate the formulation of drug formulations that are stable over time, even when stored at, or exposed to, elevated temperatures. Moreover, the miscible vehicles of the present invention allow the preparation of drug formulations that work to reduce the occurrence of partial or complete occlusions of the delivery conduits included in delivery devices used to administer the drug formulations.
Owner:DURECT CORP
Phenidate drug formulations having diminished abuse potential
InactiveUS6355656B1High serum levelHigh pharmaceutical efficacy levelBiocideOrganic chemistrySide effectMedicine
Phenidate drug formulations are provided having reduced potential for drug abuse. Dosage forms for treating Attention Deficit Disorder, Attention Deficit Hyperactivity Disorder, AIDS Dementia Complex and cognitive decline in HIV-AIDS are provided which minimize drug hypersensitivity, toxicity, side effects, euphoric effect, and drug abuse potential. Such dosage forms comprise <SMALLCAPS>D< / SMALLCAPS>-threo stereoisomer of a phenidate in the substantial absence of all other stereoisomers.
Owner:CELGENE CORP
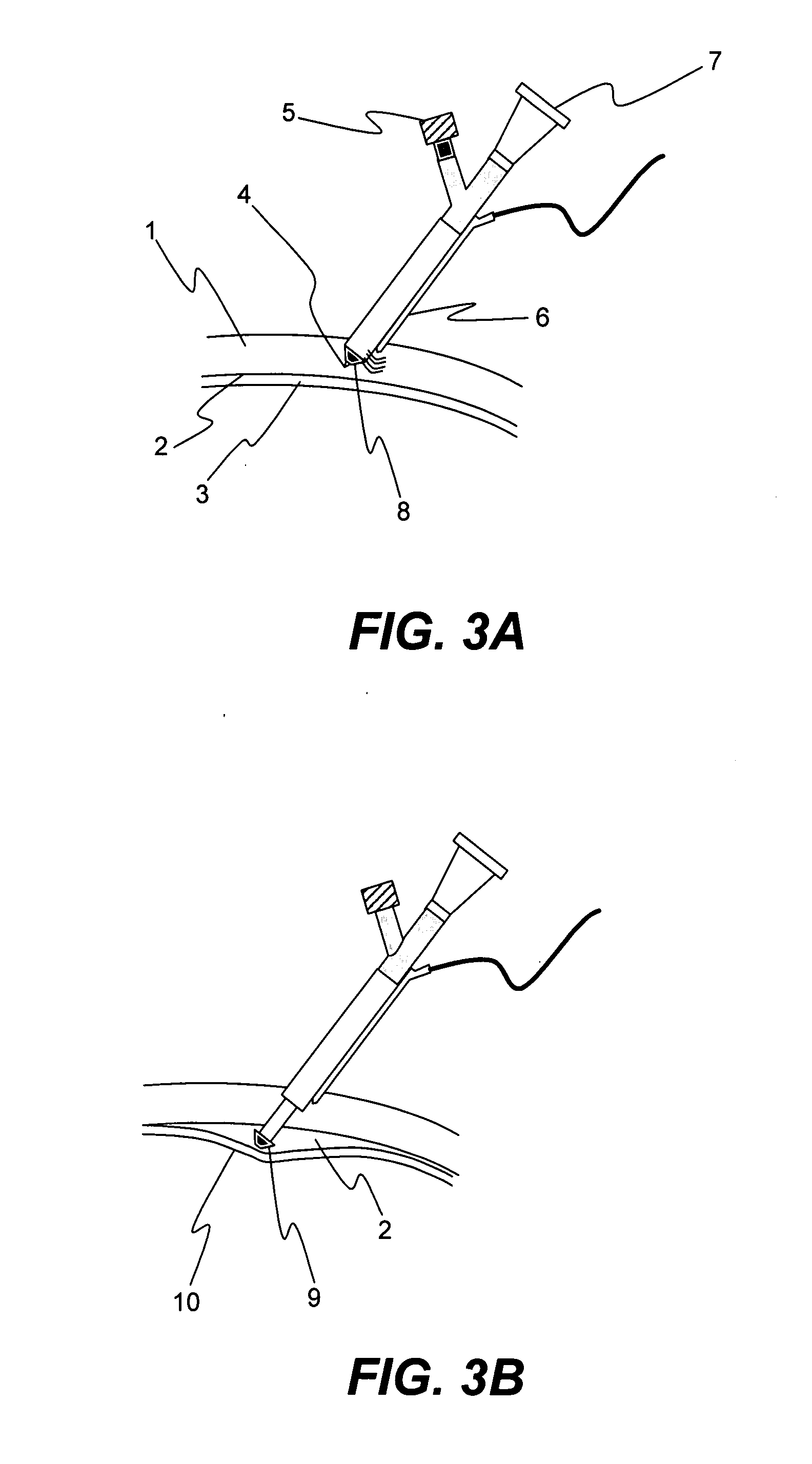
Microneedles and Methods for Microinfusion
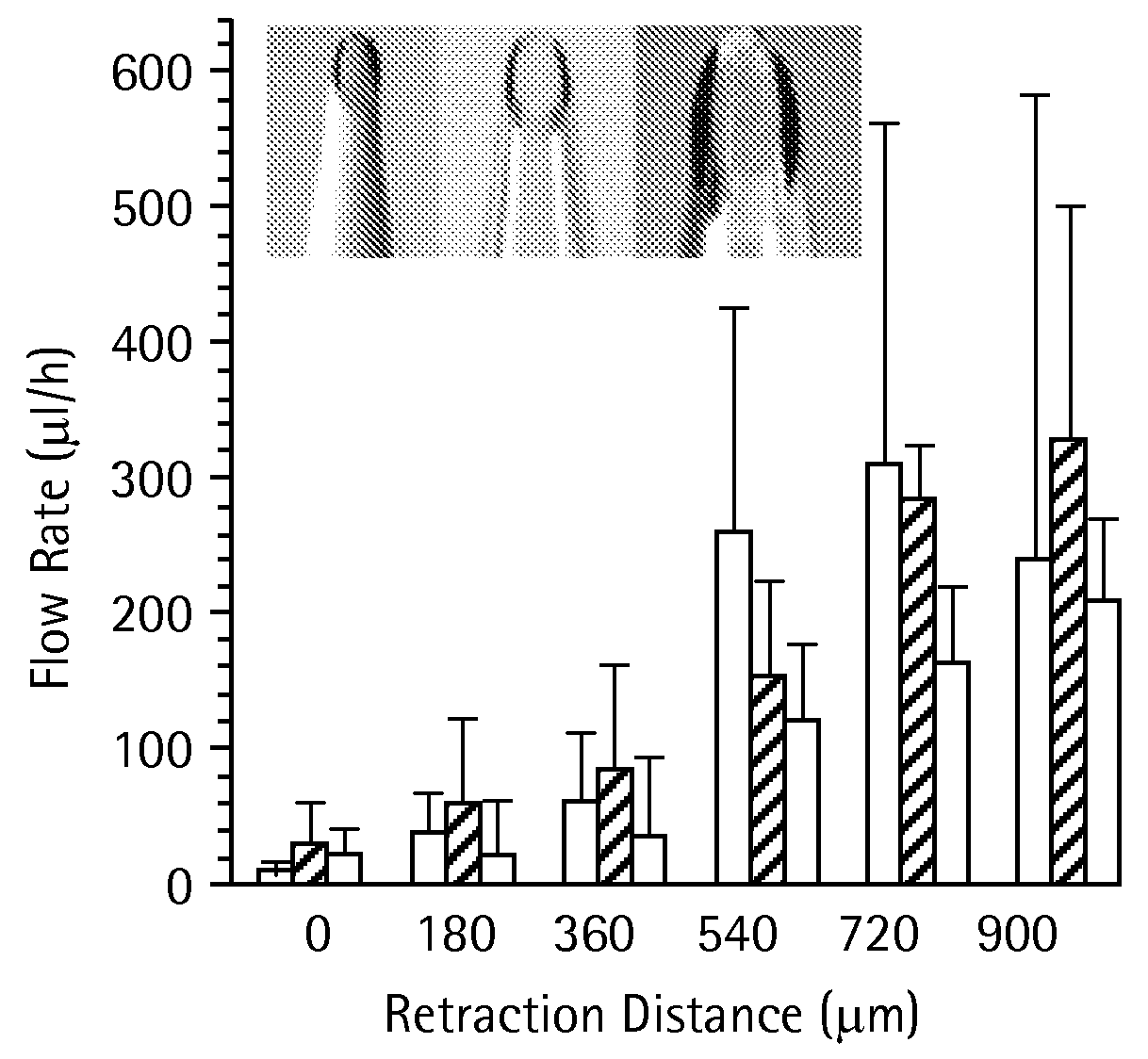
Methods and devices are provided for delivering a drug to or withdrawing a fluid from a biological tissue, such the skin, sclera, cornea, and conjunctiva. One method includes the steps of inserting at least one microneedle into the biological tissue; partially retracting the at least one microneedle from the tissue; and then delivering at least one drug formulation into the biological tissue via the partially retracted at least one microneedle. The microneedle deforms and penetrates the biological tissue during the insertion step, and the retraction step at least partially relaxes the tissue deformation while maintaining at least part of the tissue penetration, facilitating drug delivery or fluid withdrawal.
Owner:GEORGIA TECH RES CORP
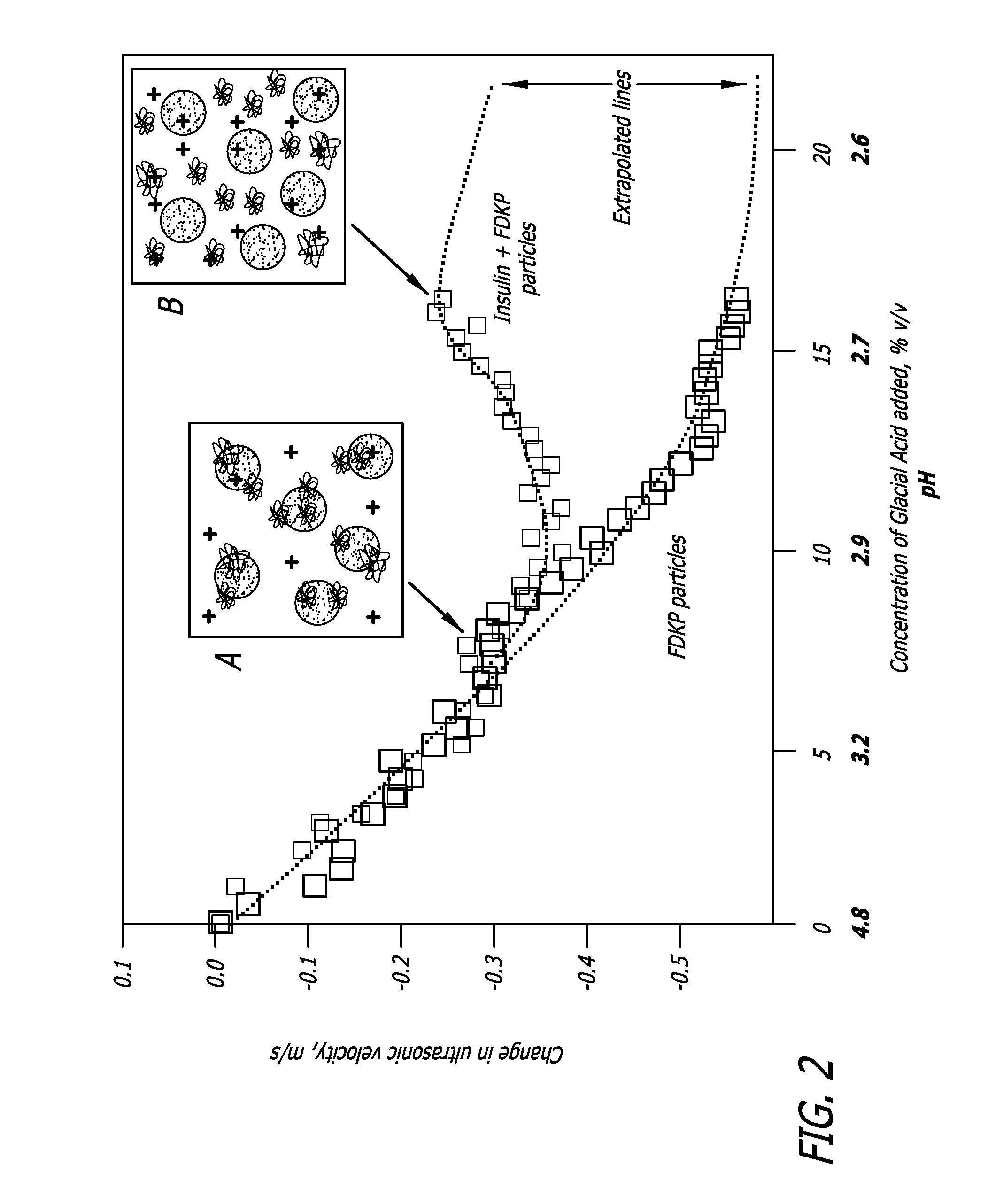
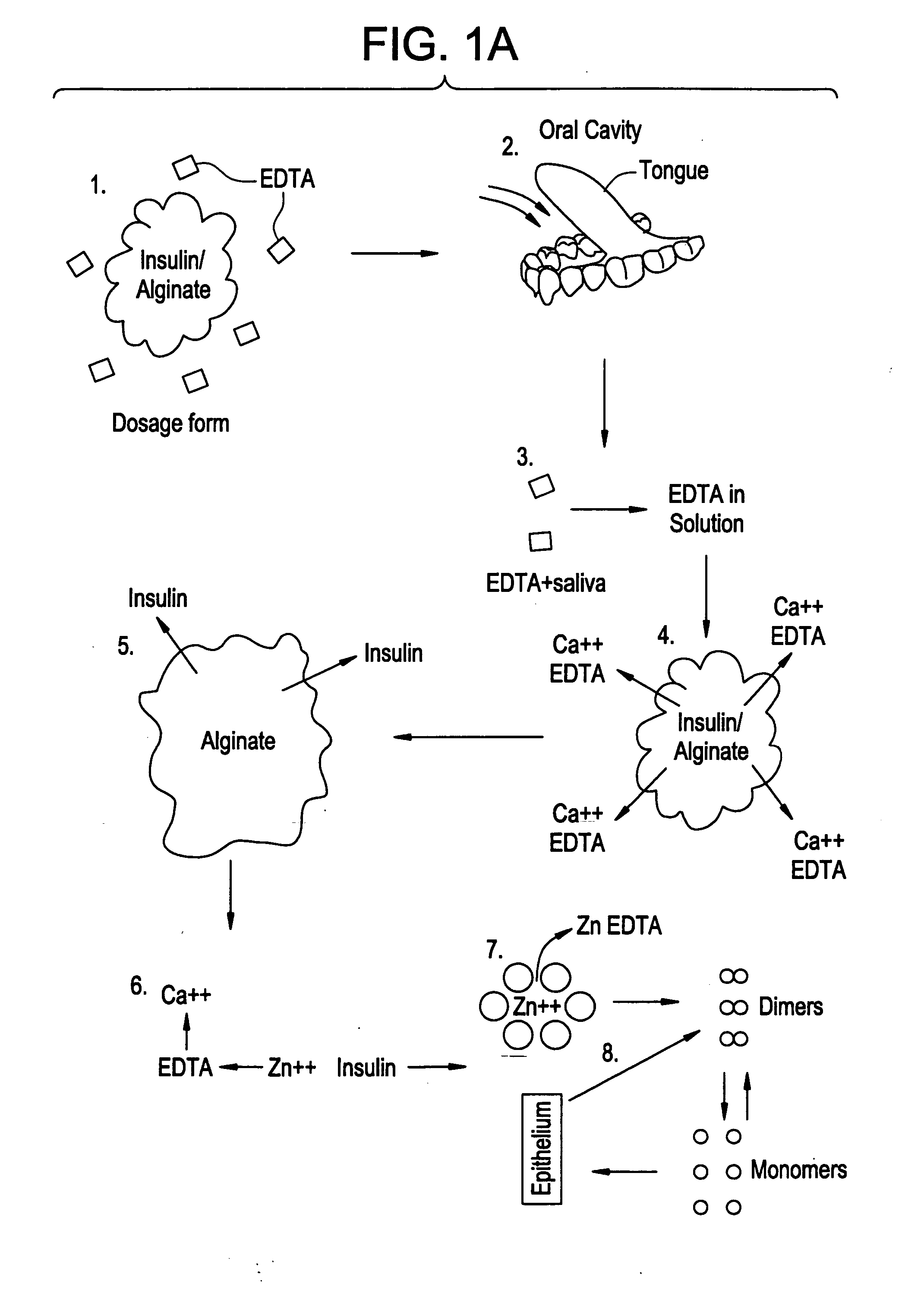
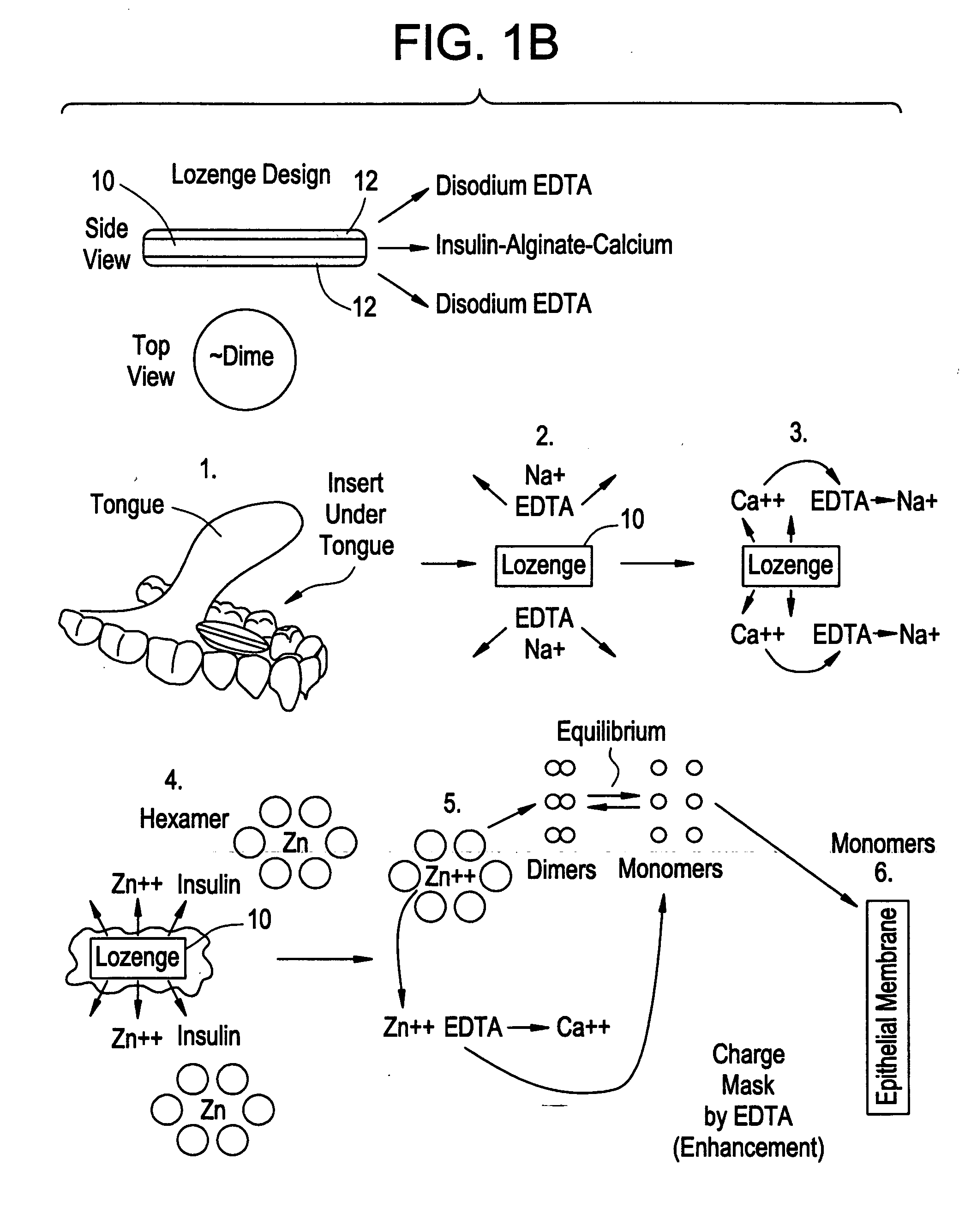
Rapid acting drug delivery compositions
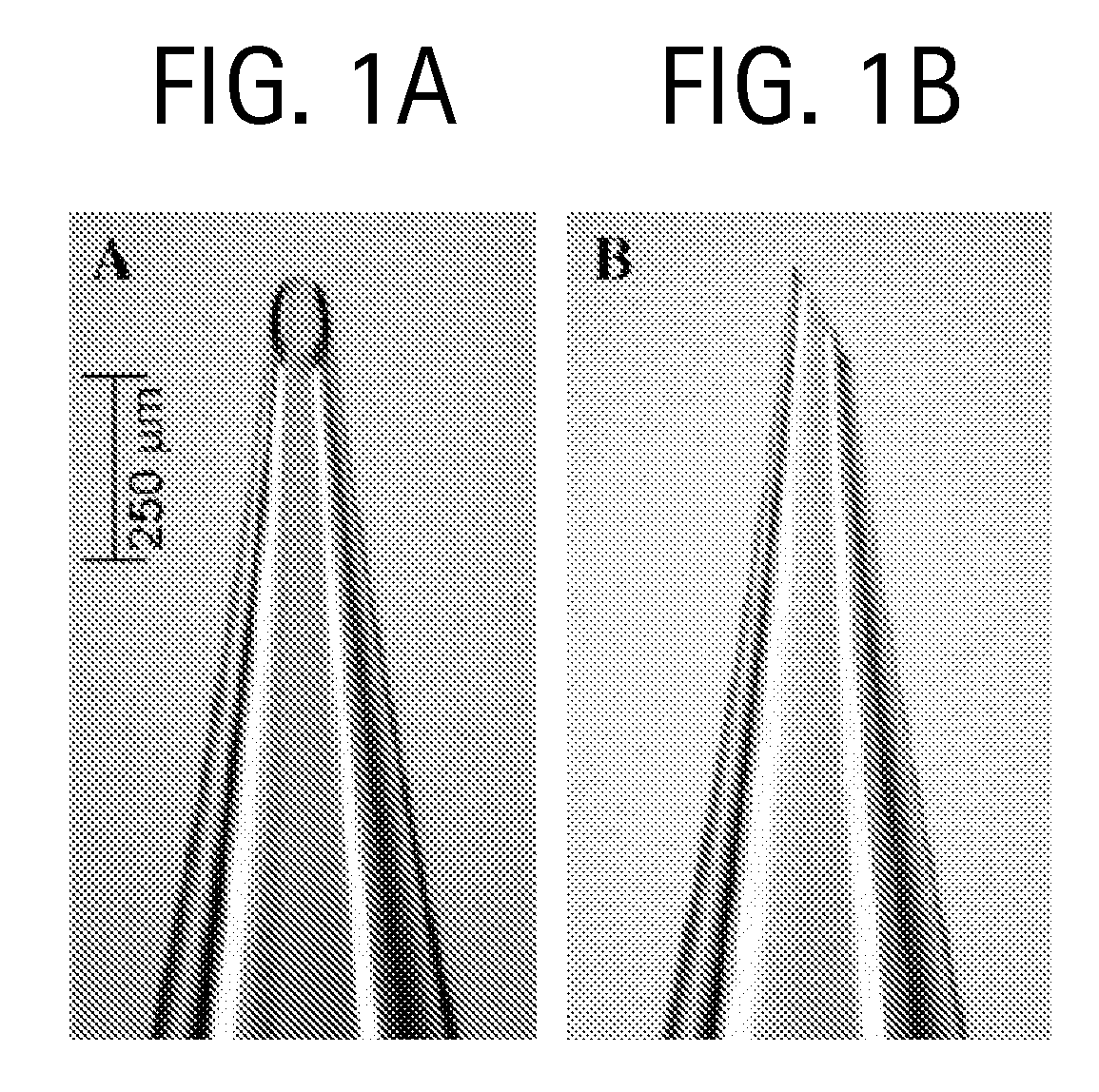
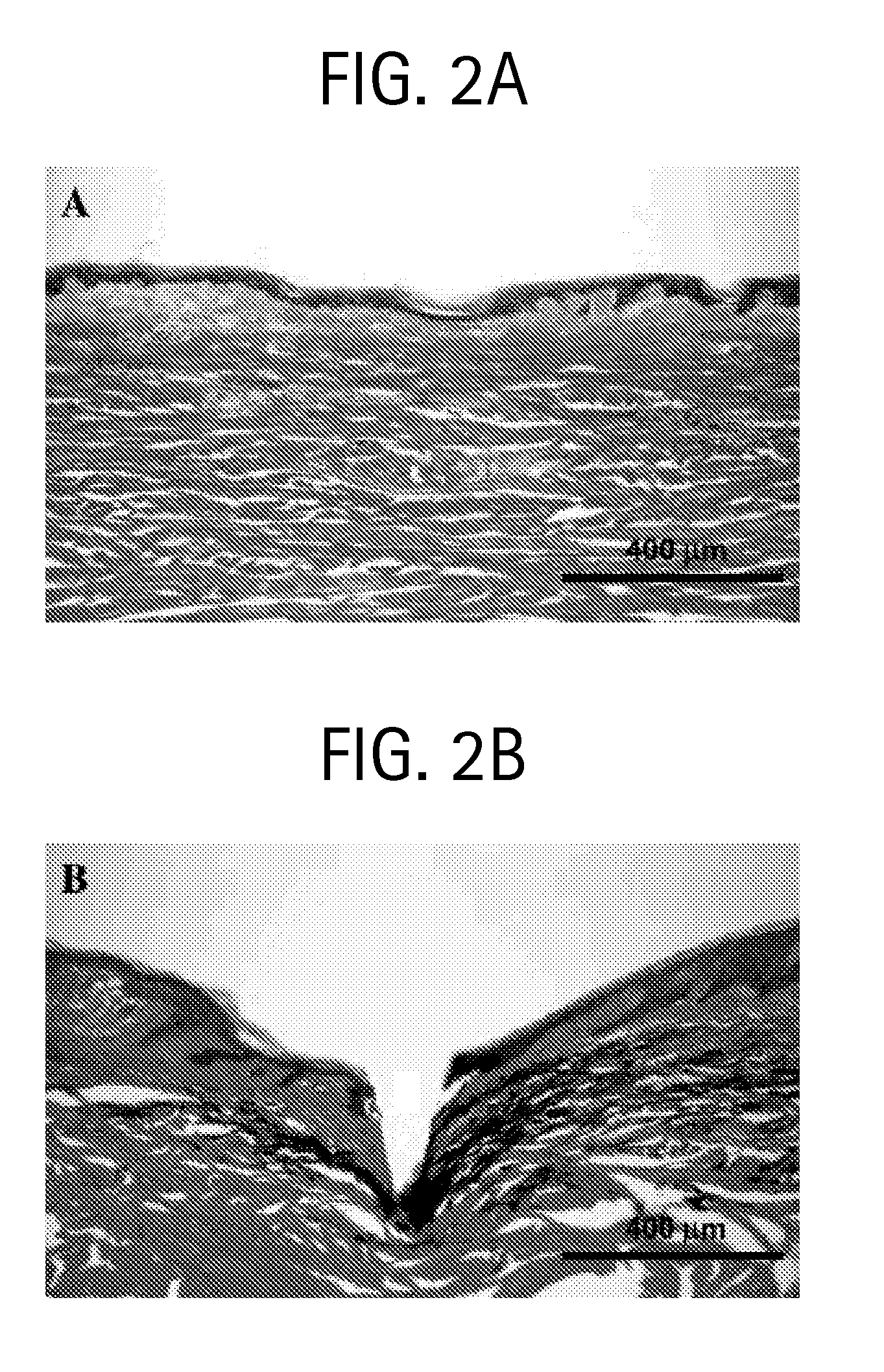
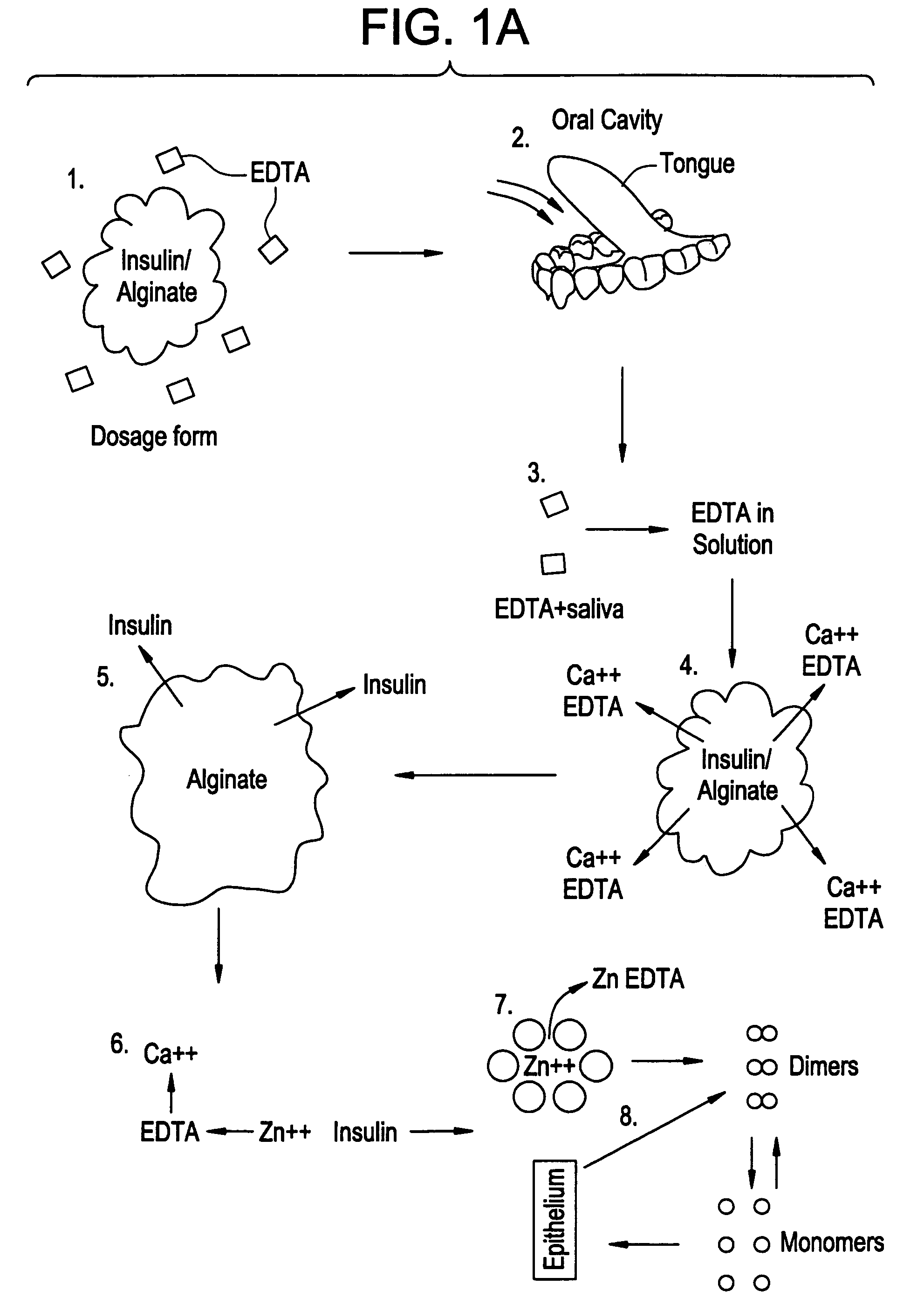
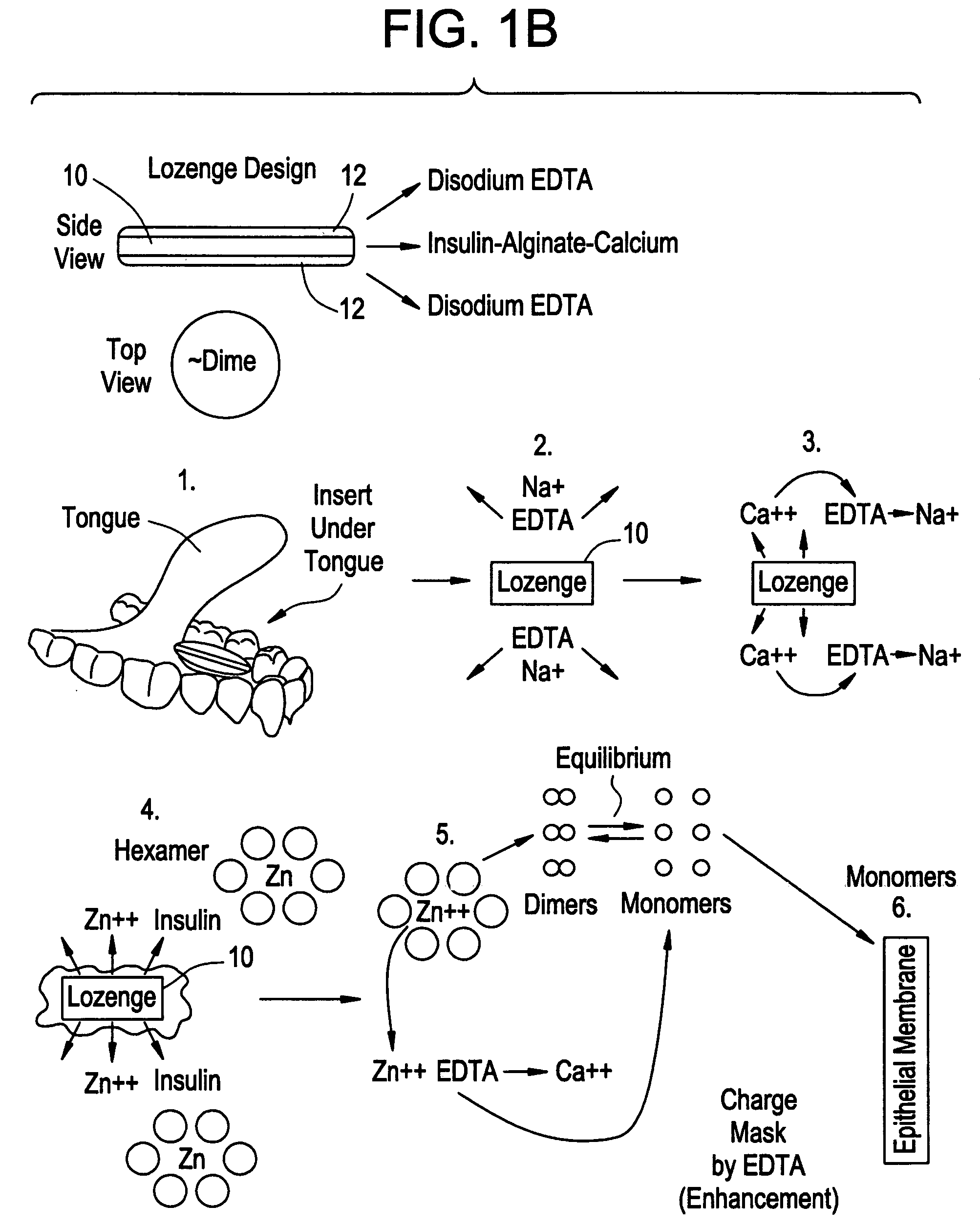
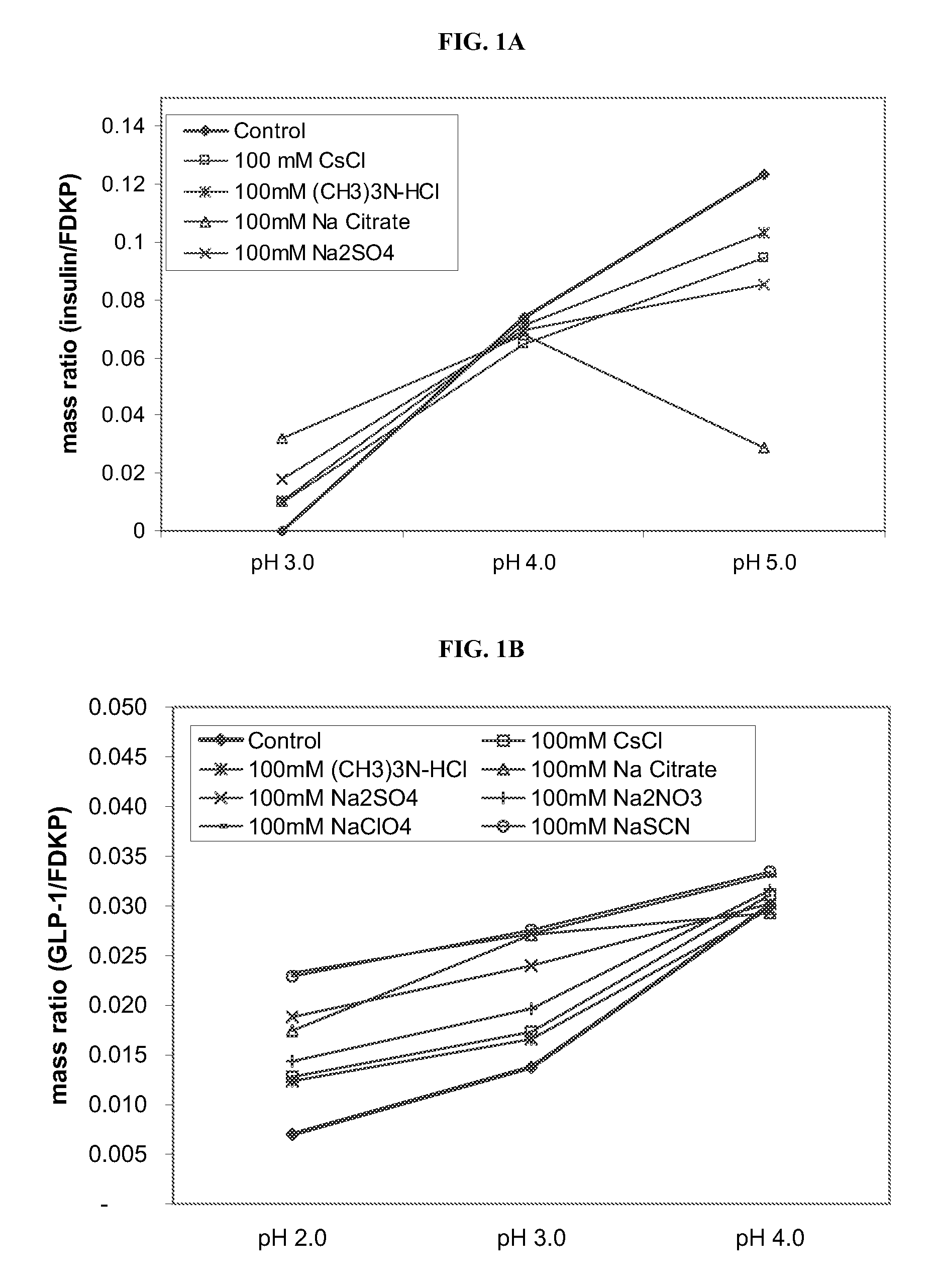
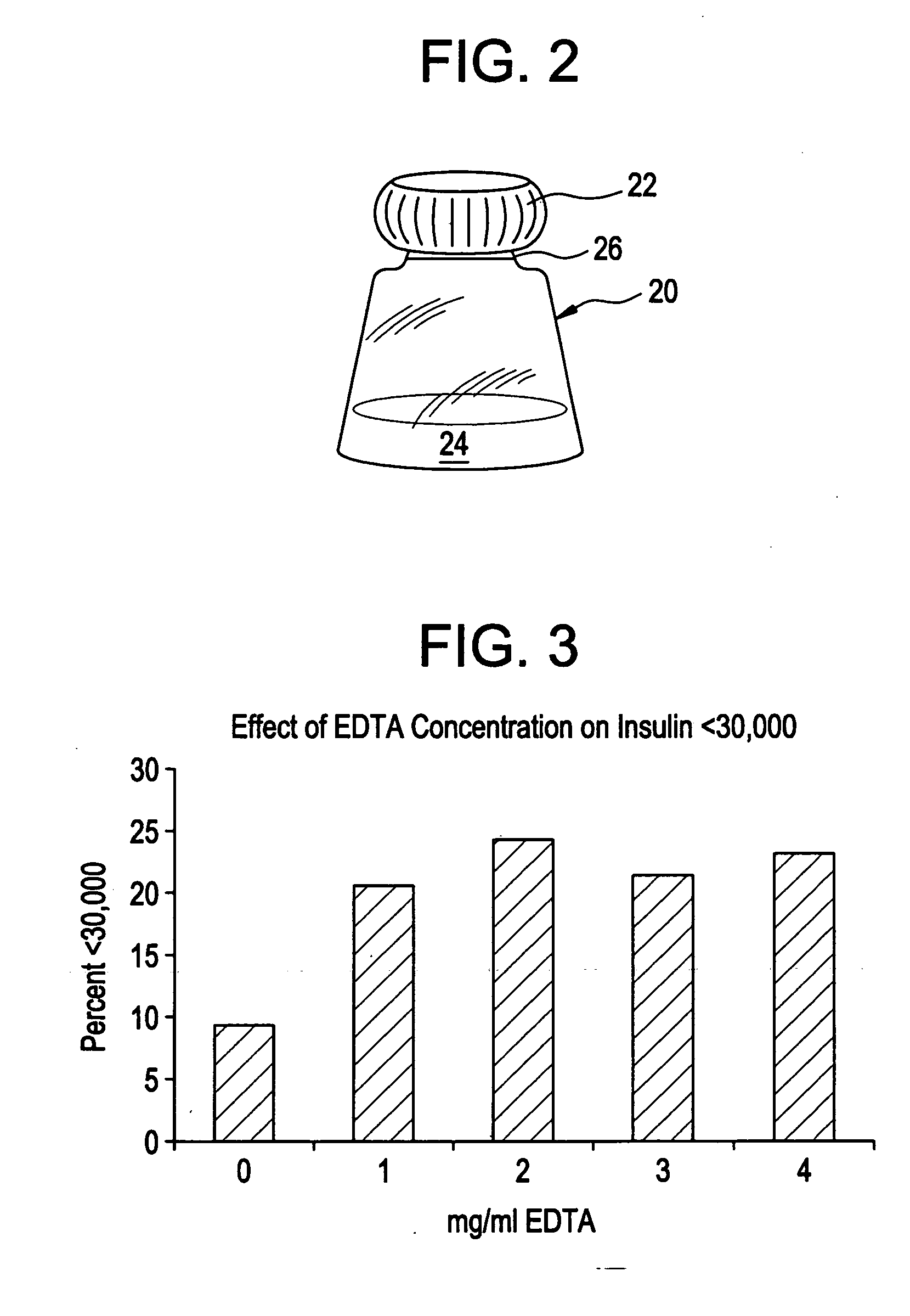
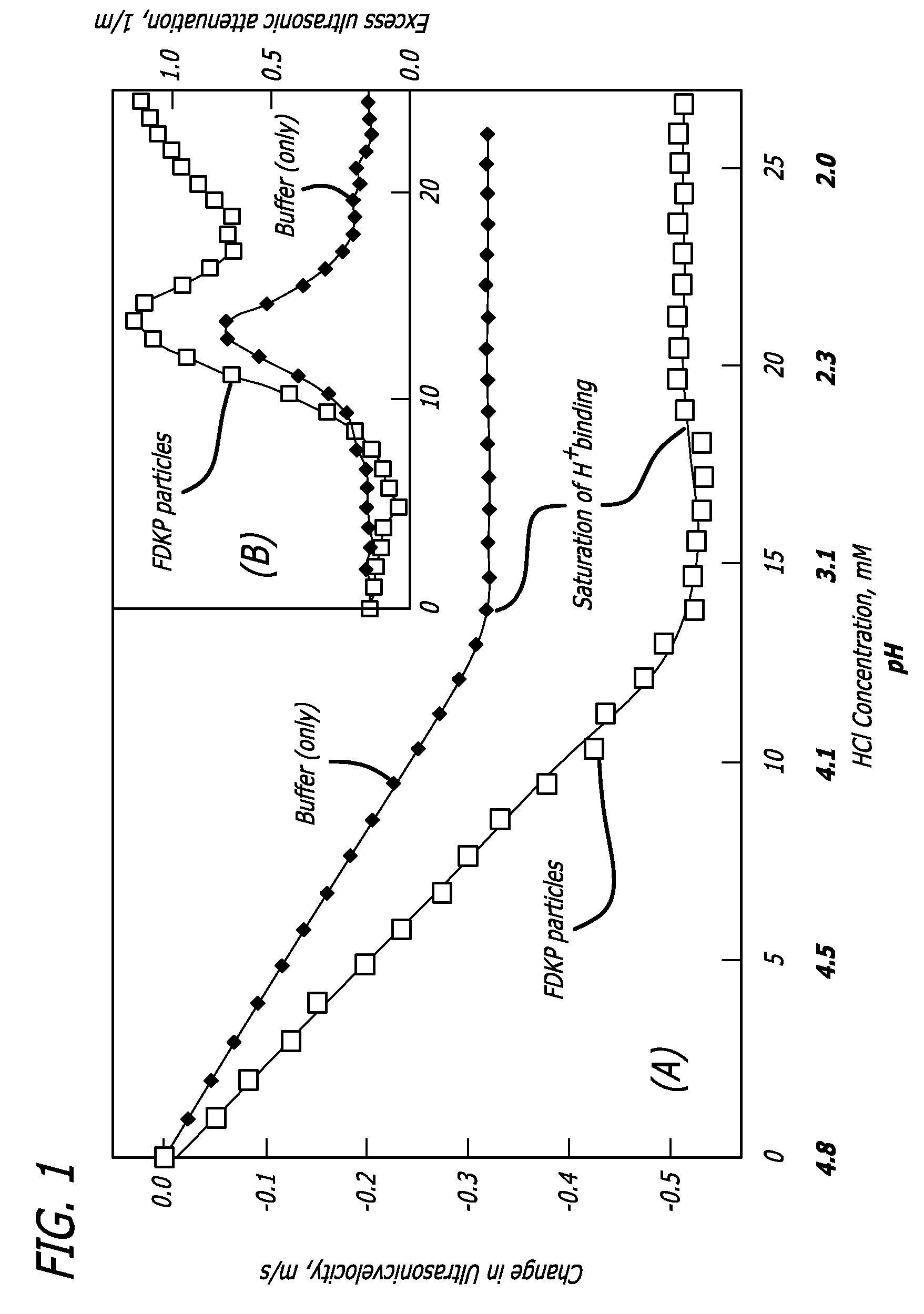
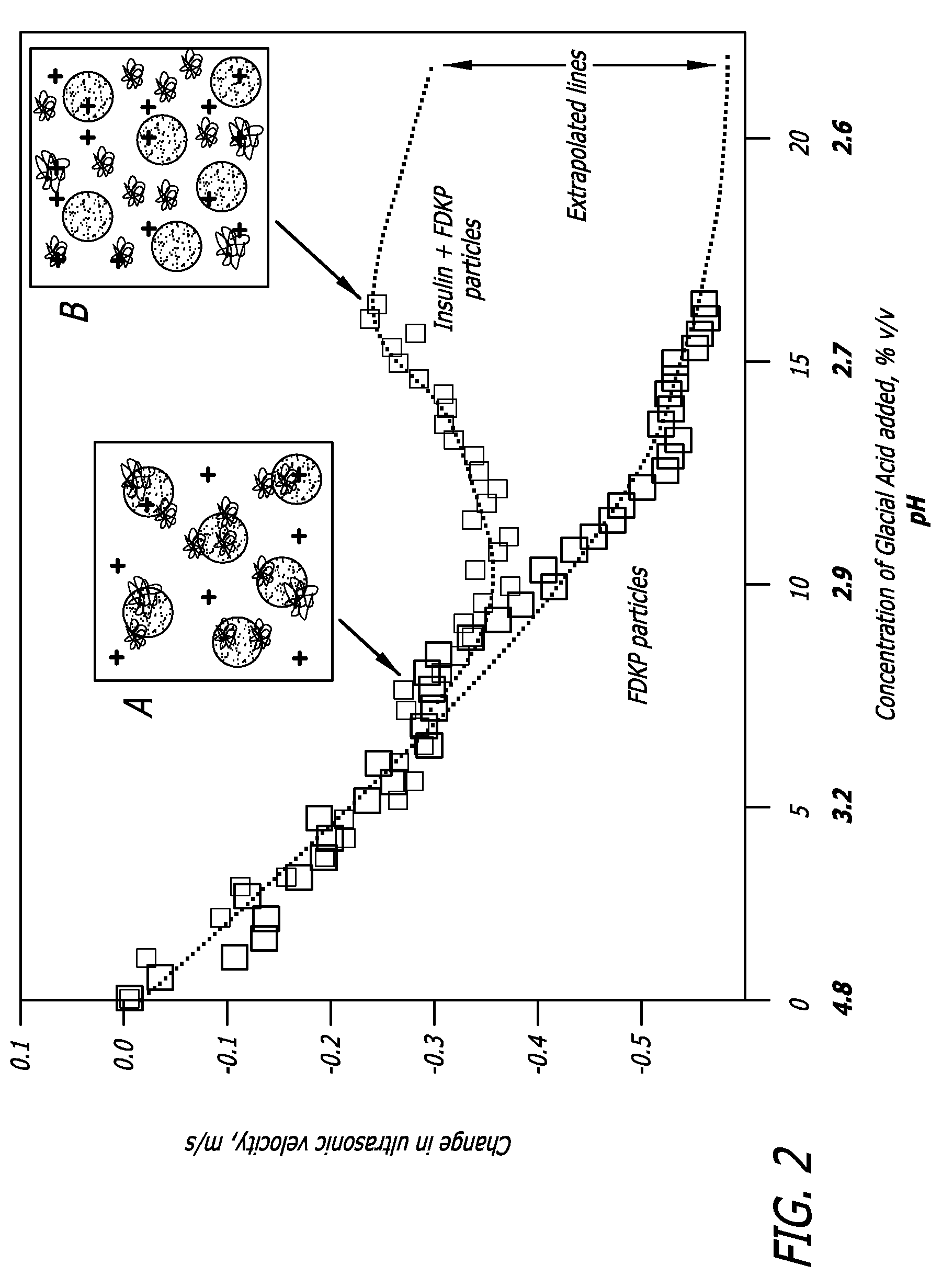
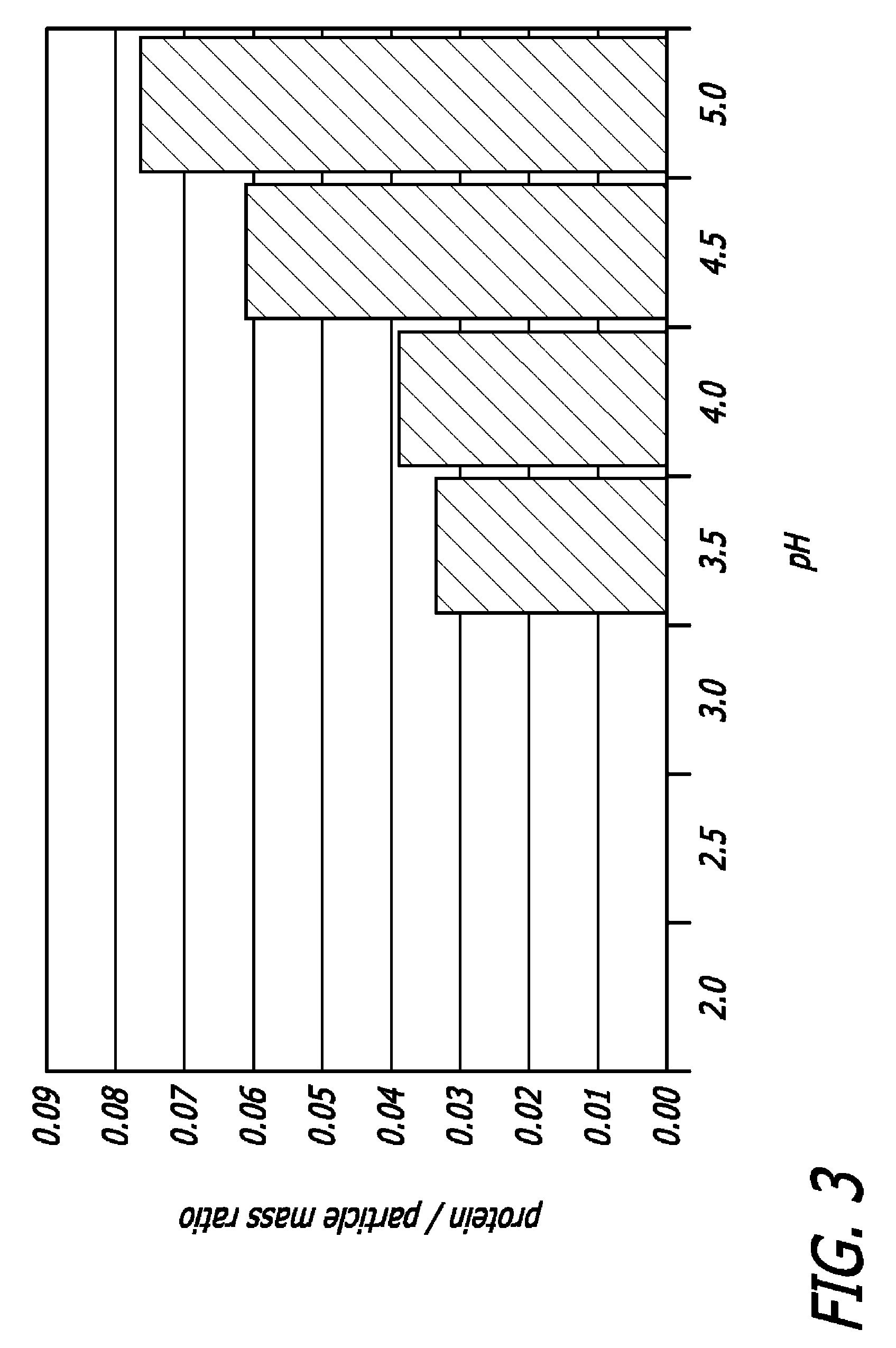
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Method of Drug Formulation Based on Increasing the Affinity of Active Agents for Crystalline Microparticle Surfaces
ActiveUS20070059374A1Reduce solubilityPromote associationPowder deliveryOrganic active ingredientsActive agentMicroparticle
Methods are provided for promoting the adsorption of an active agent to microparticles by modifying the structural properties of the active agent in order to facilitate favorable association to the microparticle.
Owner:MANNKIND CORP
Methods and devices for the treatment of ocular diseases in human subjects
InactiveUS20150258120A1Reduce in quantityReduce severityOrganic active ingredientsPowder deliveryDiseaseMicroparticle
Methods and devices are provided for targeted non-surgical administration of a drug formulation to the suprachoroidal space (SCS) of the eye of a human subject for the treatment of a posterior ocular disorder or a choroidal malady. In one embodiment, the method comprises inserting a hollow microneedle into the eye at an insertion site and infusing a drug formulation through the inserted microneedle and into the suprachoroidal space of the eye, wherein the infused drug formulation flows within the suprachoroidal space away from the insertion site during the infusion. In one embodiment, the fluid drug formulation comprises drug nanoparticles or microparticles.
Owner:CLEARSIDE BIOMEDICAL
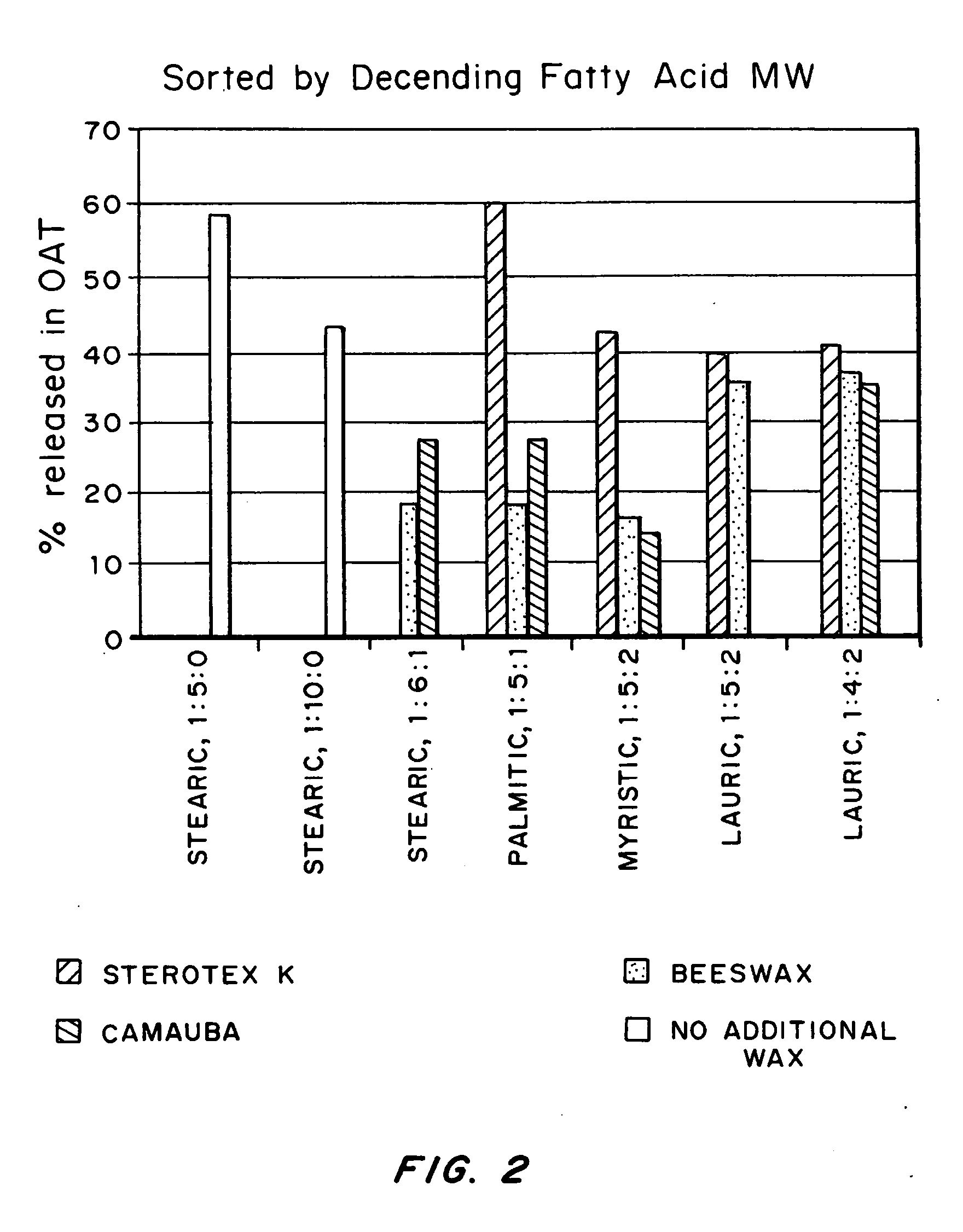
Abuse resistant drug formulation
A pharmaceutical composition may include a granulate which may include at least one active pharmaceutical ingredient susceptible to abuse by an individual mixed with at least two materials, a first material that is substantially water insoluble and at least partially alcohol soluble and a second material that is substantially alcohol insoluble and at least partially water soluble, wherein the active pharmaceutical ingredient and the two materials are granulated in the presence of water and alcohol. The composition may also include a coating on the granulate exhibiting crush resistance which may have a material that is deposited on the granulate using an alcohol based solvent. The composition further comprises a second particle comprising a fat / wax. The present invention also includes a coated granulate, various dosage forms of the composition, as well as methods of production and tableting.
Owner:CIMA LABS +1
Abuse-deterrent drug formulations
ActiveUS20050281748A1Reduce the possibilityImprove lipophilicityTelevision system detailsPowder deliveryImmediate releaseActive agent
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Methods and apparatus for drug delivery involving phase changing formulations
InactiveUS6528086B2Cleanly peeled from the skinMaintenance of such surfaceBiocideAdhesive dressingsHuman bodyPharmaceutical formulation
This invention relates to an apparatus and method of drug delivery on a human body surface. The formulation comprises a drug, a conversion agent capable of converting the formulation from a less solid phase to a coherent, soft, solid phase, and a vehicle medium or carrier for the drug and conversion agent. The drug formulation is applied to this human body surface in its less than solid phase and is subsequently converted to a soft solid phase while the drug is being delivered through the human body surface. After delivery of the drug is complete, the soft solid formulation can be removed or peeled from the body surface as a coherent solid formulation. The drug formulation provides control over drug delivery rates and allows the formulation to be removed without leaving a messy, residual formulation on the body surface.
Owner:CRESCITA THERAPEUTICS INC
Microneedle array, patch, and applicator for transdermal drug delivery
Microneedle arrays and drug delivery devices are provided for transdermally delivering a drug formulation to a patient. The microneedle array device includes a substantially planar substrate having an array of apertures; and a plurality of microneedles projecting at angle from the planar substrate, the microneedles having a base portion integrally connected to the substrate, a tip end portion distal to the base portion, and body portion therebetween, wherein each microneedle has at least one channel extending substantially from the base portion through at least a part of the body portion, the channel being open along at least part of the body portion and in fluid communication with at least one of the apertures in the substrate. In a preferred embodiment, each microneedle has a substantially rectangular cross-sectional shape and the channel is open to two opposing surfaces of the microneedle.
Owner:YUZHAKOV VADIM V
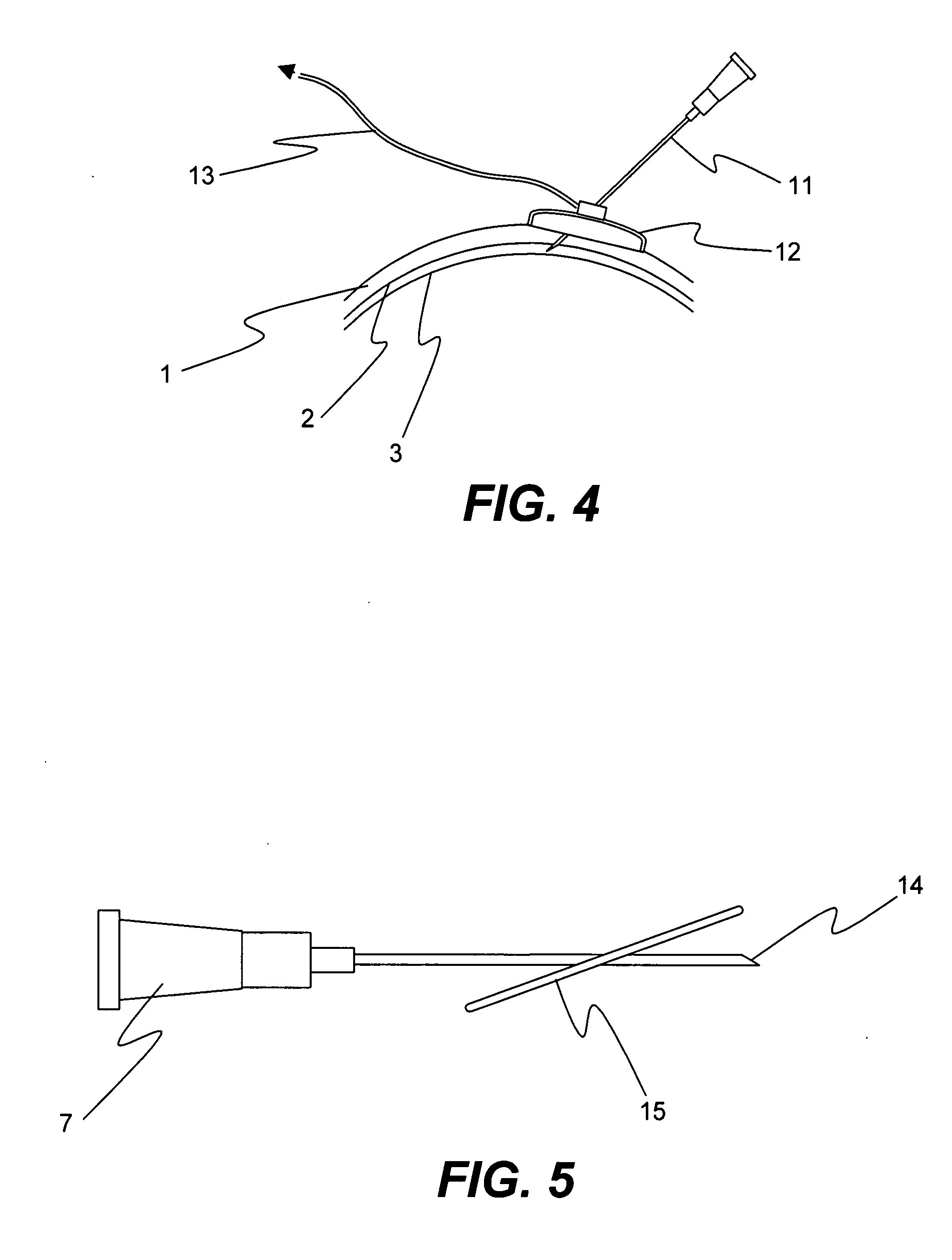
Methods and Devices for Drug Delivery to Ocular Tissue Using Microneedle
Methods and devices are provided for targeted administration of a drug to a patient's eye. In one embodiment, the method includes inserting a hollow microneedle into the sclera of the eye at an insertion site and infusing a fluid drug formulation through the inserted microneedle and into the suprachoroidal space of the eye, wherein the infused fluid drug formulation flows within the suprachoroidal space away from the insertion site during the infusion. The fluid drug formulation may flow circumferentially toward the retinochoroidal tissue, macula, and optic nerve in the posterior segment of the eye.
Owner:GEORGIA TECH RES CORP +1
Injectable sustained release delivery devices
ActiveUS20040176341A1Organic active ingredientsCosmetic preparationsControlled releasePolymer coatings
An injectable drug delivery device includes a core containing one or more drugs and one or more polymers. The core may be surrounded by one or more polymer outer layers (referred to herein as "coatings," "skins," or "outer layers"). In certain embodiments, the device is formed by extruding or otherwise preforming a polymeric skin for a drug core. The drug core may be co-extruded with the skin, or inserted into the skin after the skin has been extruded, and possibly cured. In other embodiments, the drug core may be coated with one or more polymer coatings. These techniques may be usefully applied to fabricate devices having a wide array of drug formulations and skins that can be selected to control the release rate profile and various other properties of the drugs in the drug core in a form suitable for injection using standard or non-standard gauge needles. The device may be formed by combining at least one polymer, at least one drug, and at least one liquid solvent to form a liquid suspension or solution wherein, upon injection, such suspension or solution under goes a phase change and forms a gel. The configuration may provide for controlled release of the drug(s) for an extended period.
Owner:EYEPOINT PHARMA INC
Method for drug delivery to ocular tissue using microneedle
Methods and devices are provided for administering a drug to a patient's eye. The methods include (a) inserting a hollow microneedle into the sclera or corneal stroma without penetrating across the sclera or corneal stroma; and (b) infusing a fluid drug formulation through the microneedle and into the sclera or cornea. It further may include partially retracting the microneedle before infusion to enhance delivery. Alternatively, the methods may include (a) inserting a solid microneedle into the sclera or corneal stroma without penetrating across the sclera or corneal stroma, wherein the solid microneedle comprises a first quantity of a drug formulation and inserting causes the solid microneedle to form a pocket in the sclera or corneal stroma; and (b) releasing the drug formulation into the pocket to form a drug depot, whereby a drug is released from the depot. The methods and devices may include an array of multiple microneedles.
Owner:GEORGIA TECH RES CORP +1
Method of drug formulation based on increasing the affinity of crystalline microparticle surfaces for active agents
Methods are provided for coating crystalline microparticles with an active agent by altering the surface properties of the microparticles in order to facilitate favorable association on the microparticle by the active agent. Type of surface properties that are altered by the disclosed methods include by electrostatic properties, hydrophobic properties and hydrogen bonding properties.
Owner:MANNKIND CORP
Suspending vehicles and pharmaceutical suspensions for drug dosage forms
InactiveUS20060216242A1Process stabilityPeptide/protein ingredientsAerosol deliverySUSPENDING VEHICLESolvent
Suspending vehicles and pharmaceutical suspensions that include a biocompatible polymer that can be combined with a hydrophobic solvent and a hydrophilic solvent to provide vehicles and suspensions that are substantially free of stiff gels upon contact with an aqueous medium are provided. Vehicles and suspensions remain flowable out of a pump-driven dosage form over the life of the dosage form. Such vehicles and suspensions are also biocompatible, suitable for creating and maintaining drug suspensions, and capable of providing stable drug formulations.
Owner:DURECT CORP
Medicament formulations
ActiveUS20060269484A1Low and negligible contribution to ozone depletionBiocideNervous disorderMedicineCompound (substance)
Disclosed are medicinal compositions, and devices, methods and systems which use same, comprising a propellant and at lease one medicinally active compound, said propellant comprising at least one fluoroolefin having at least two but less than seven carbon atoms.
Owner:HONEYWELL INT INC
Devices, Systems and Methods for Ophthalmic Drug Delivery
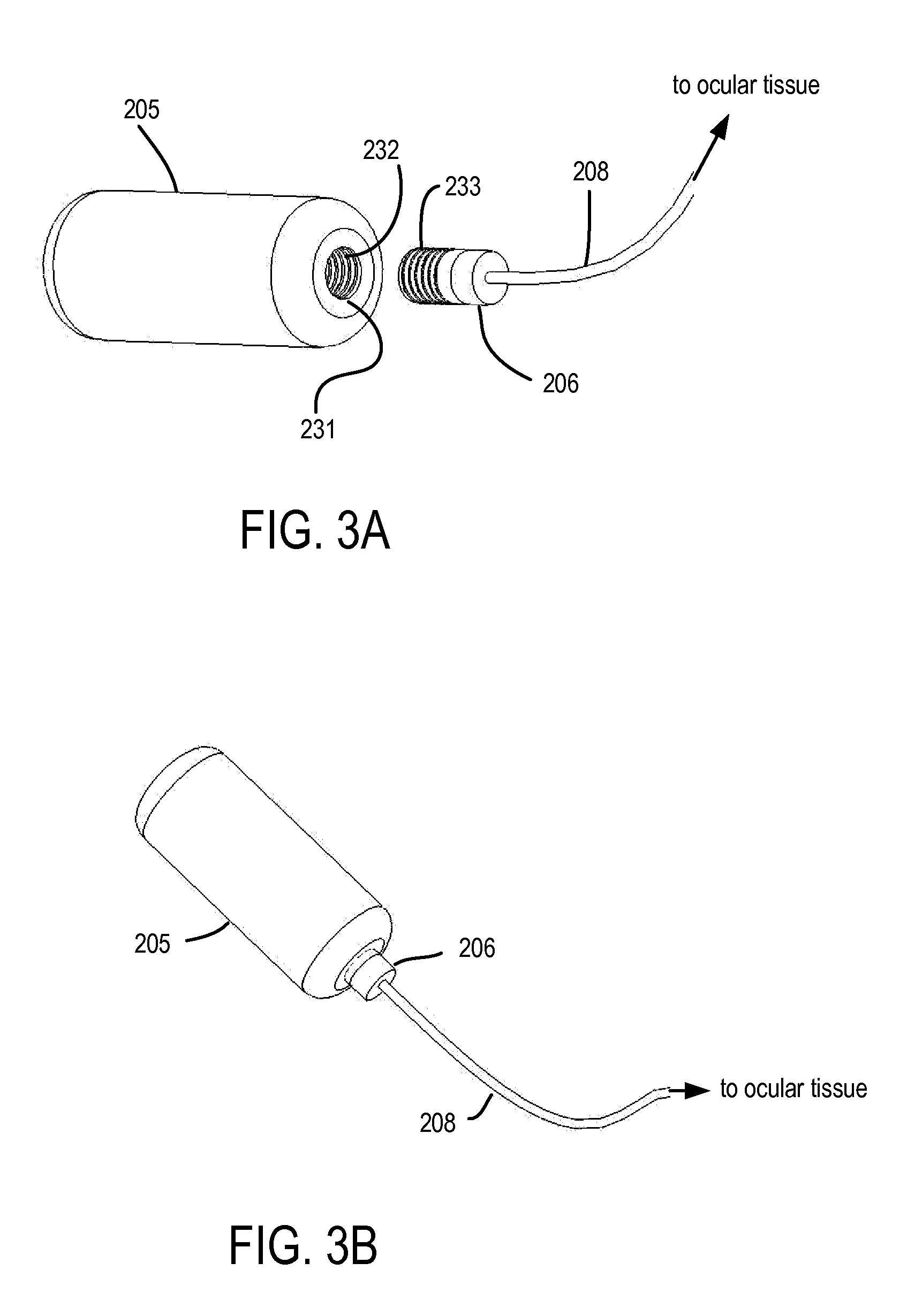
Devices, systems and techniques for delivering drugs to an ocular tissue are described. In at least some embodiments, a terminal component (e.g., a needle or open end of a catheter) is implanted in an ocular tissue and used to deliver one or more drugs. The delivered drugs may come from a source which is also implanted, or may be introduced from an external source (e.g., via a port). Both solid and liquid drug formulations can be used. Ocular implants can alternatively include a thin film coating that releases a drug into an ocular tissue.
Owner:NEUROSYSTEC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com