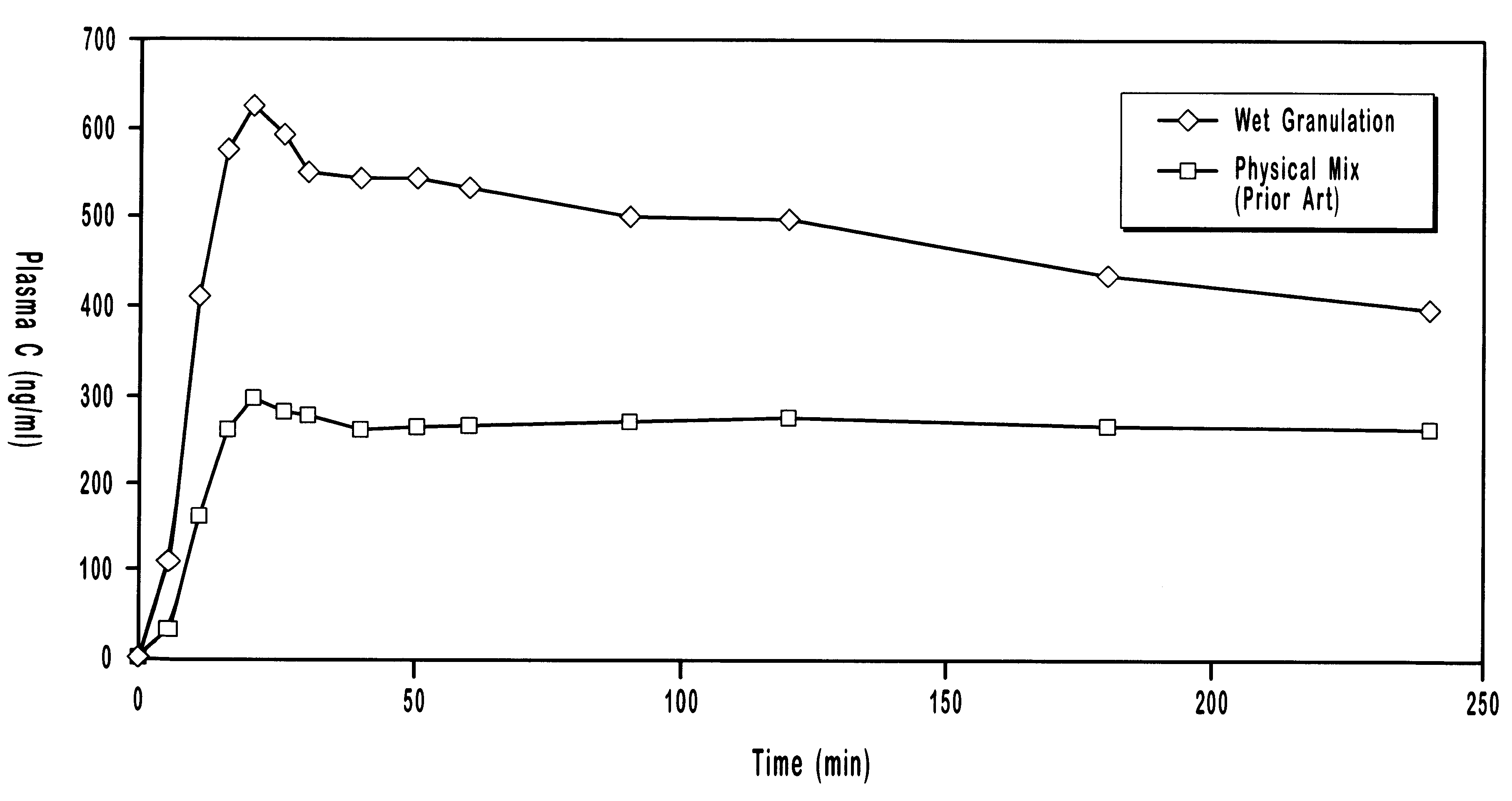
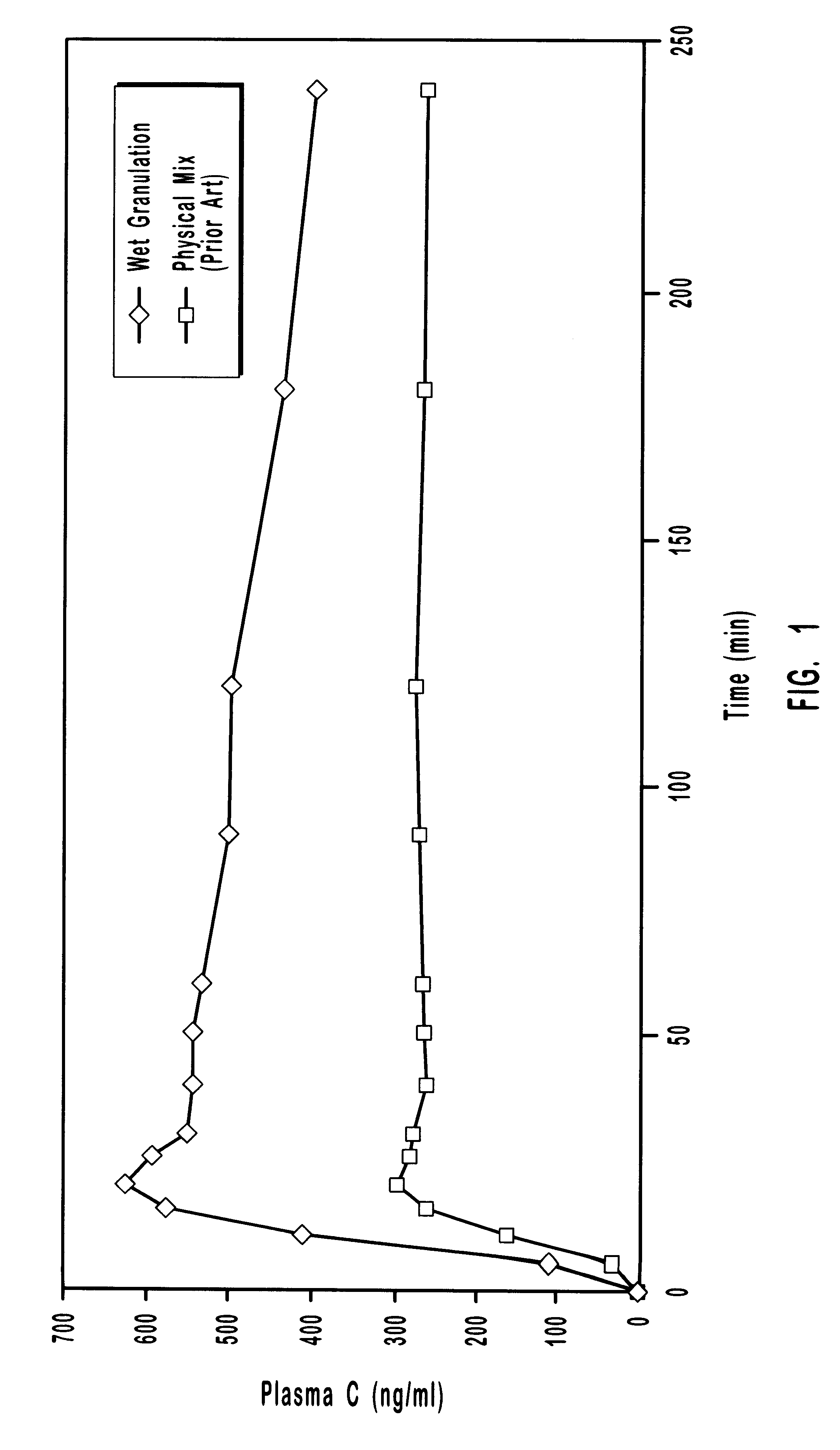
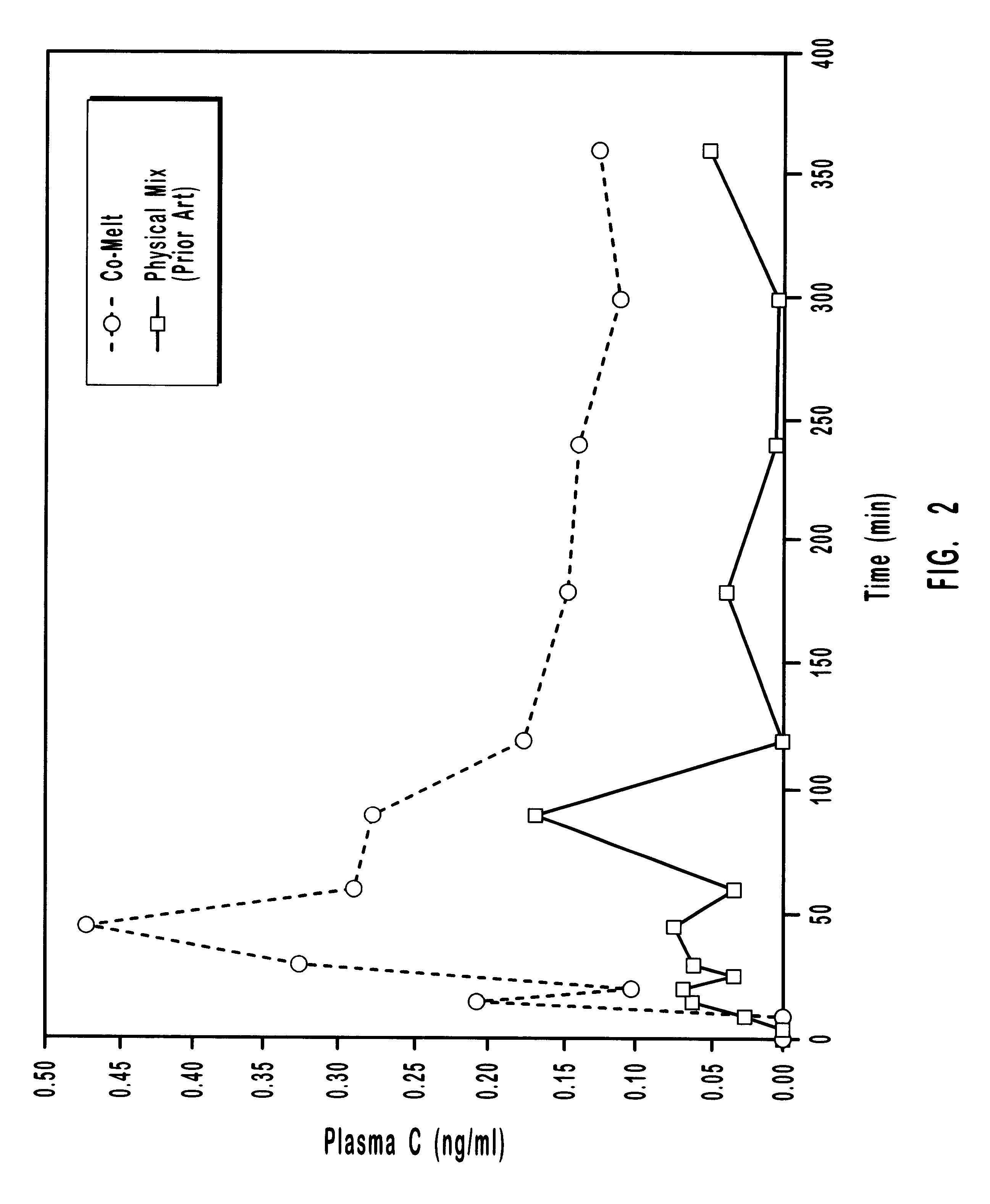
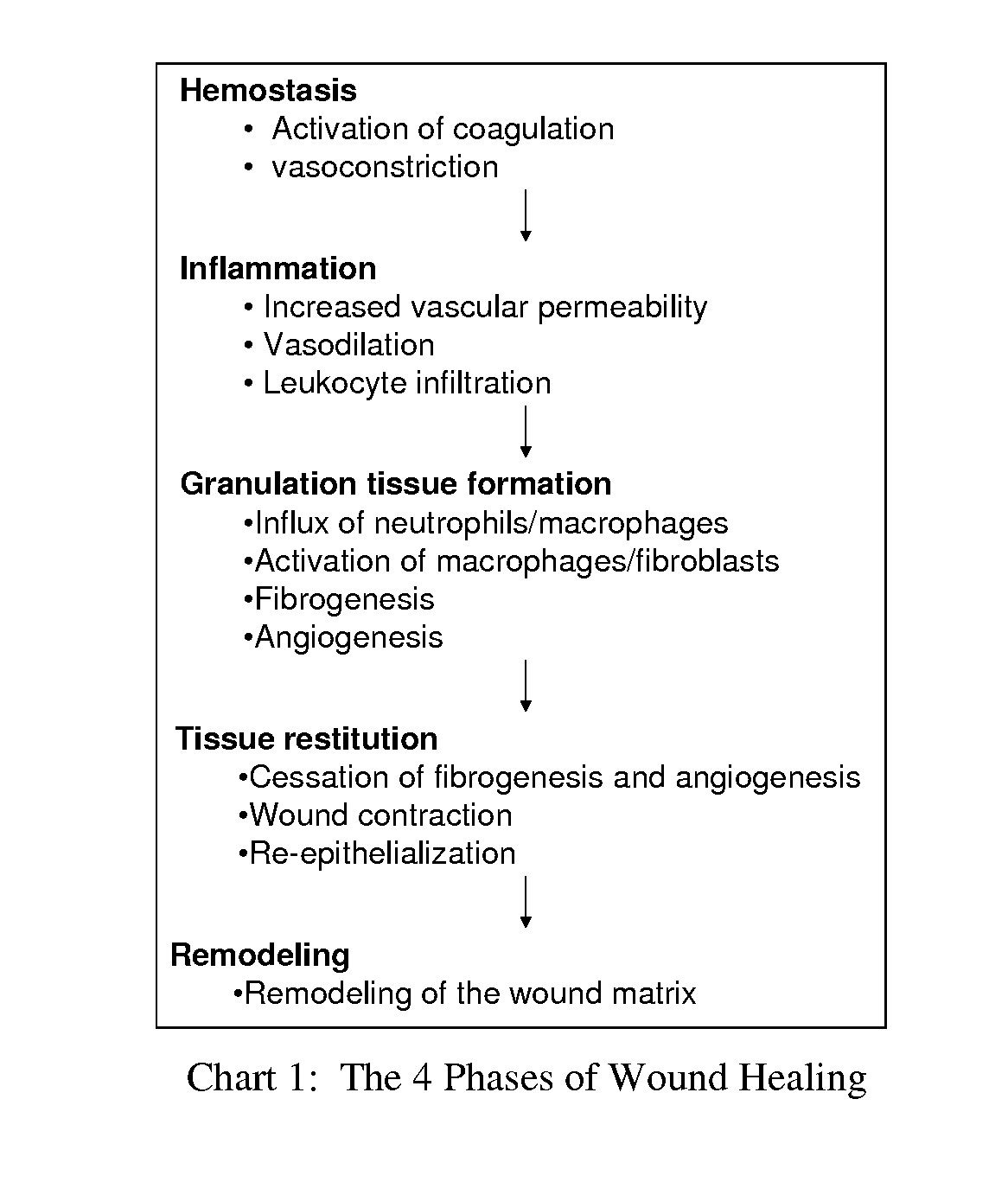
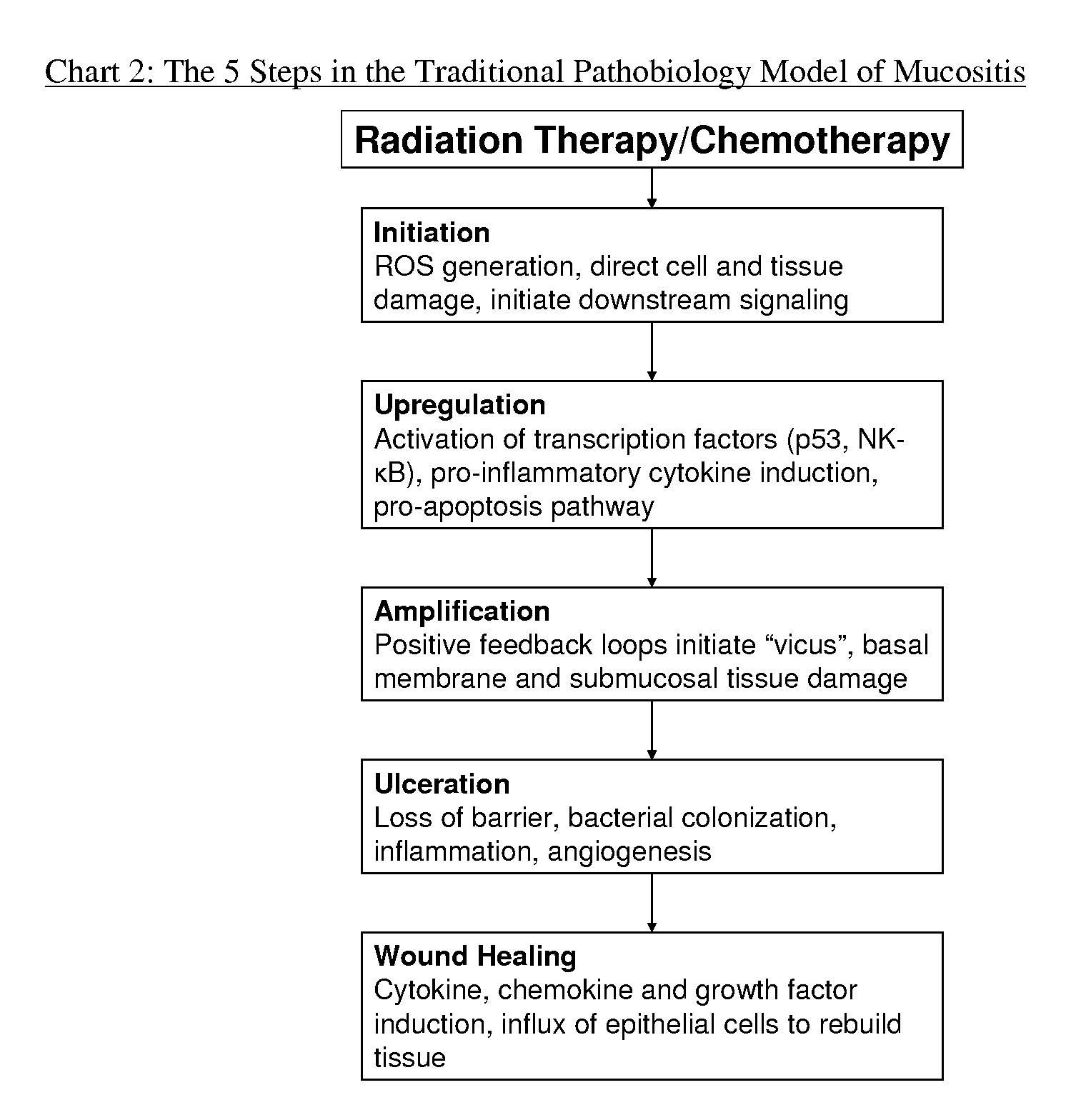
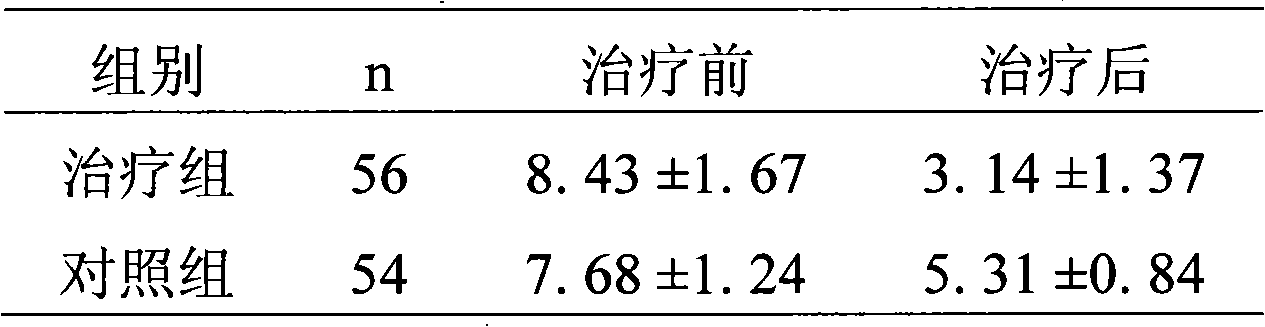
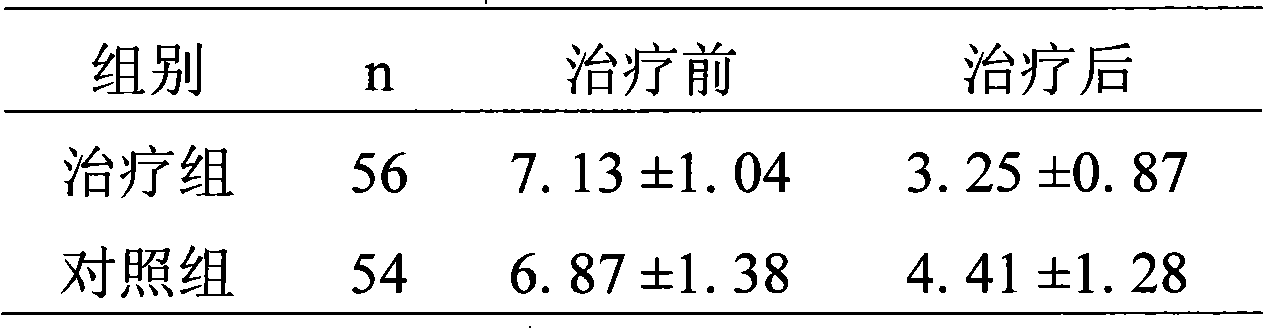
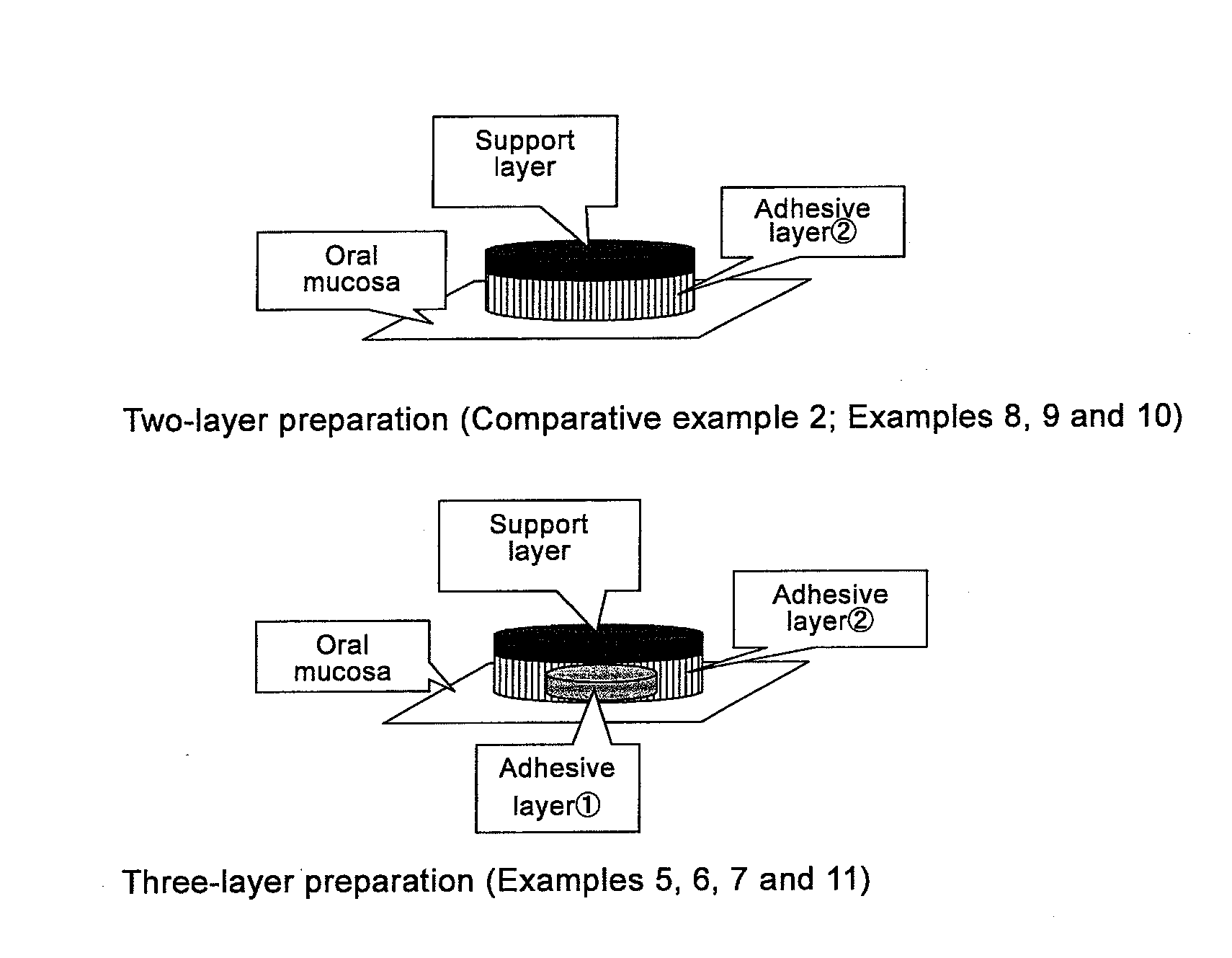
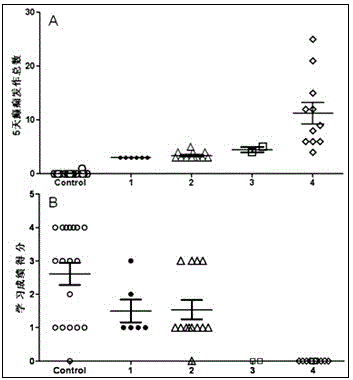
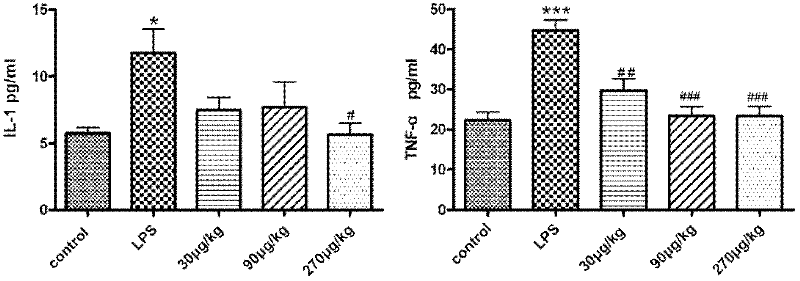
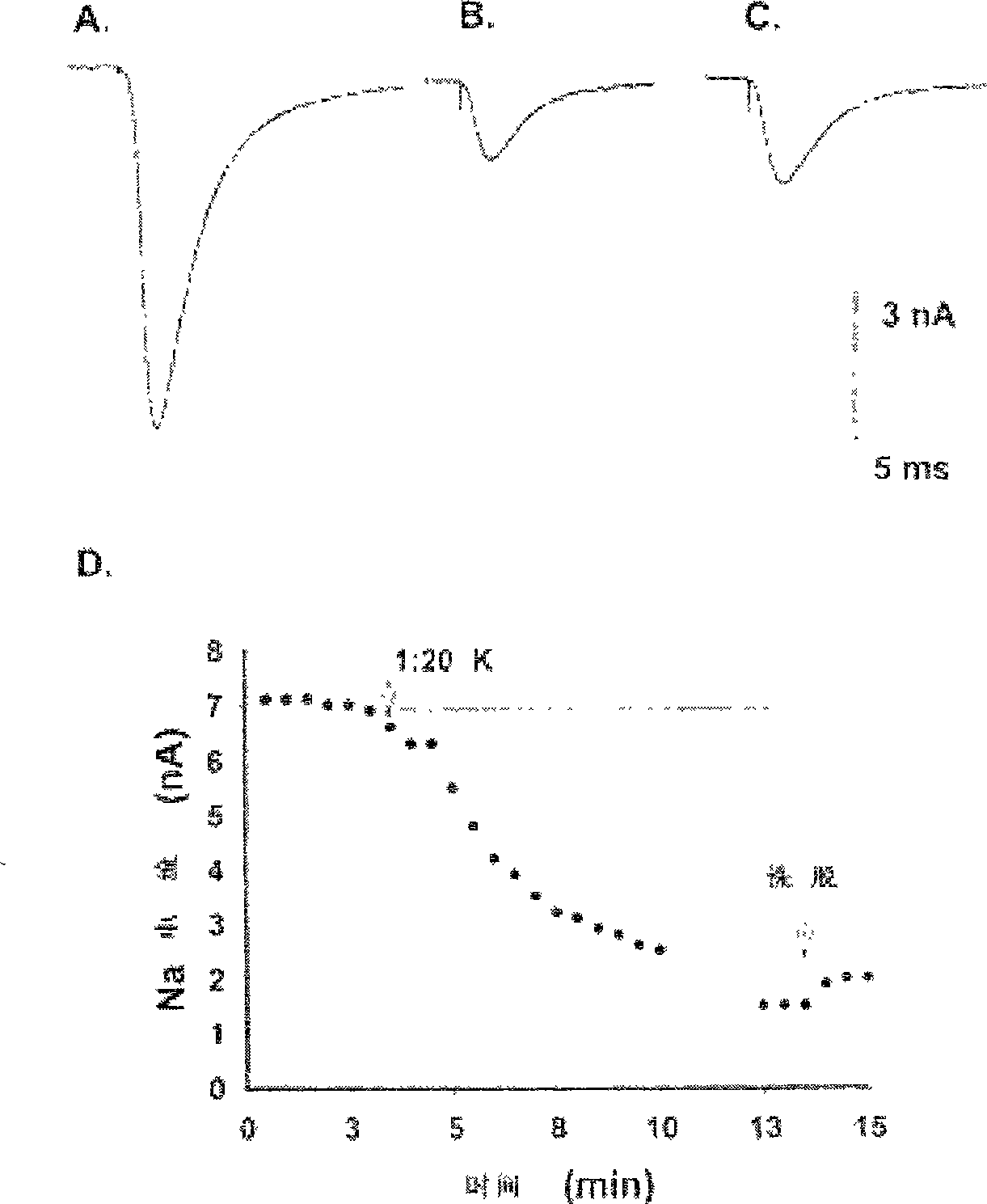
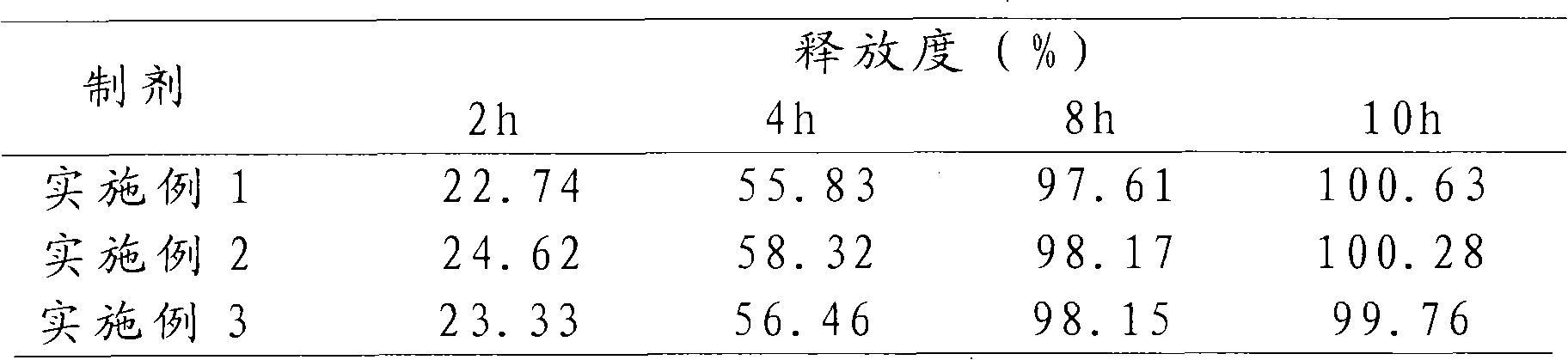
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
122 results about "Oral mucous membrane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Compositions and methods for mucositis and oncology therapies
In alternative embodiments, this invention provides compositions and methods for treating cancer or any condition caused by dysfunctional cells, side effects from treatments for cancer or any condition caused by dysfunctional cells, e.g., mucositis therapies (e.g., for oral mucositis; digestive mucositis; esophageal mucositis; intestinal mucositis). In alternative embodiments, the invention provides cytoprotection products that may be used either alone or in combination with other medical therapies such as cancer chemotherapies and radiation therapies.
Owner:VICUS THERAPEUTICS
Bioadhesive drug formulations for oral transmucosal delivery
Bioadhesive drug formulations that adhere to an oral mucosal membrane of a subject are provided together with single dose applicators and devices for delivering the drug formulations to the oral mucosa, and methods for using the same.
Owner:VERTICAL PHARMA
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Traditional Chinese medicinal powder and preparation method thereof
ActiveCN102526124AChange physical propertiesReduce fishy smellPowder deliveryPlant ingredientsMedicinal herbsFiber
The invention relates to a traditional Chinese medicinal powder. The traditional Chinese medicinal powder comprises the following traditional Chinese medicinal herbs in part by weight: 0.5-1 part of medicinal framework material, a medicinal core material which is 1-5 times of the medicinal framework material, and 0-30 parts of compound medicinal material, wherein the medicinal framework material and the compound medicinal material are medicinal materials which contain fiber as a prescription raw material or have stable physical and chemical properties and stability, low moisture absorption, small irritation; and the medicinal core material is the medicinal material which contains volatile, easily-oxidized and unstable traditional Chinese medicinal active components, or is easy to absorb moisture and irritant to oral mucous membrane or gastrointestinal mucous membrane or is poor in taste and color. The traditional Chinese medicinal powder can change the physical properties of medicinal particles and can reduce the loss of easily-oxidized components and the volatility of volatile components; the moisture absorption of the medicinal powder can be improved; the hydrophilicity (wettability) of the medicinal powder can be increased; the mixing uniformity of multiple materials can be improved; the irritation of the medicine to the oral mucous membrane or the gastrointestinal mucous membrane, and the stench of the medicine can be reduced; and the mouthfeel and the color of the medicine can be improved.
Owner:四川厚德医药科技有限公司
Film comprising nitroglycerin
The present invention is related to the composition and methods of manufacture of orally-dissolvable, edible films as a vehicle for the non-invasive administration of nitroglycerin and / or other active drugs through the mucosal tissues of the oral cavity. The films include a water soluble film-forming polymer such as pullulan. Methods for producing the films are also disclosed.
Owner:ACADERM
Bagged mouth-containing smokeless tobacco product with cocoa flavor
The invention discloses a bagged mouth-containing smokeless tobacco product with a cocoa flavor. The bagged mouth-containing smokeless tobacco product is characterized by being prepared by taking a tobacco material and a cocoa product as major ingredients and adding seasoning auxiliary materials; the bagged mouth-containing smokeless tobacco product comprises the following components in percentage by weight: 30 to 70 percent of tobacco material, 10 to 40 percent of cocoa product, 1 to 7 percent of cocoa flavor reinforcing agent, 1 to 8 percent of sweetening agent, 1 to 10 percent of flavoring agent and 0 to 5 percent of freshening agent. The product has the pure cocoa flavor and a little smoke flavor or the smoke flavor is slighter. When using the bagged mouth-containing smokeless tobacco product, a user puts the bagged mouth-containing smokeless tobacco product between the side part of the upper lip of an oral cavity and the gum, and the components can be dissolved by spittle and absorbed by the mouth mucosa without being chewed. By the bagged mouth-containing smokeless tobacco product, the flavor species of homemade smokeless tobacco products are enriched; the bagged mouth-containing smokeless tobacco product smells good and can supply the satisfaction similar to the conventional cigarette product to a tobacco customer, so that the tobacco customer can get proper energy; stimulation of the bagged mouth-containing smokeless tobacco product to the oral cavity is lower than that of the common mouth-containing cigarette, and the after taste is a little sweet and makes the tobacco customer comfortable; therefore, the bagged mouth-containing smokeless tobacco product is suitable for the consumption psychology and the taste characteristic of customers in China, cannot produce smokes and has no environmental smoke harm.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Pharmaceutical product for intraoral delivery of nicotine comprising trometamol as buffering agent
InactiveCN101484188AImprove buffering effectOrganic active ingredientsNervous disorderOral mucous membraneCrohn's disease
A pharmaceutical oral formulation for delivering nicotine in any form to a subject by transmucosal uptake in the oral cavity comprising nicotine in any form, wherein said oral formulation is buffered with at least trometamol. Also contemplated is a method for the oral delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as methods for manufacturing the oral formulation, the use of the oral formulation for obtaining transmucosal uptake of nicotine in the oral cavity of a subject, and use of nicotine for the production of an oral formulation for the treatment of a disease selected from the group consisting of tobacco or nicotine dependence, Alzheimer's disease, Crohn's disease, Parkinson's disease, Tourette's syndrome, ulcerous colitis and post-smoking-cessation weight control.
Owner:MCNEIL AB
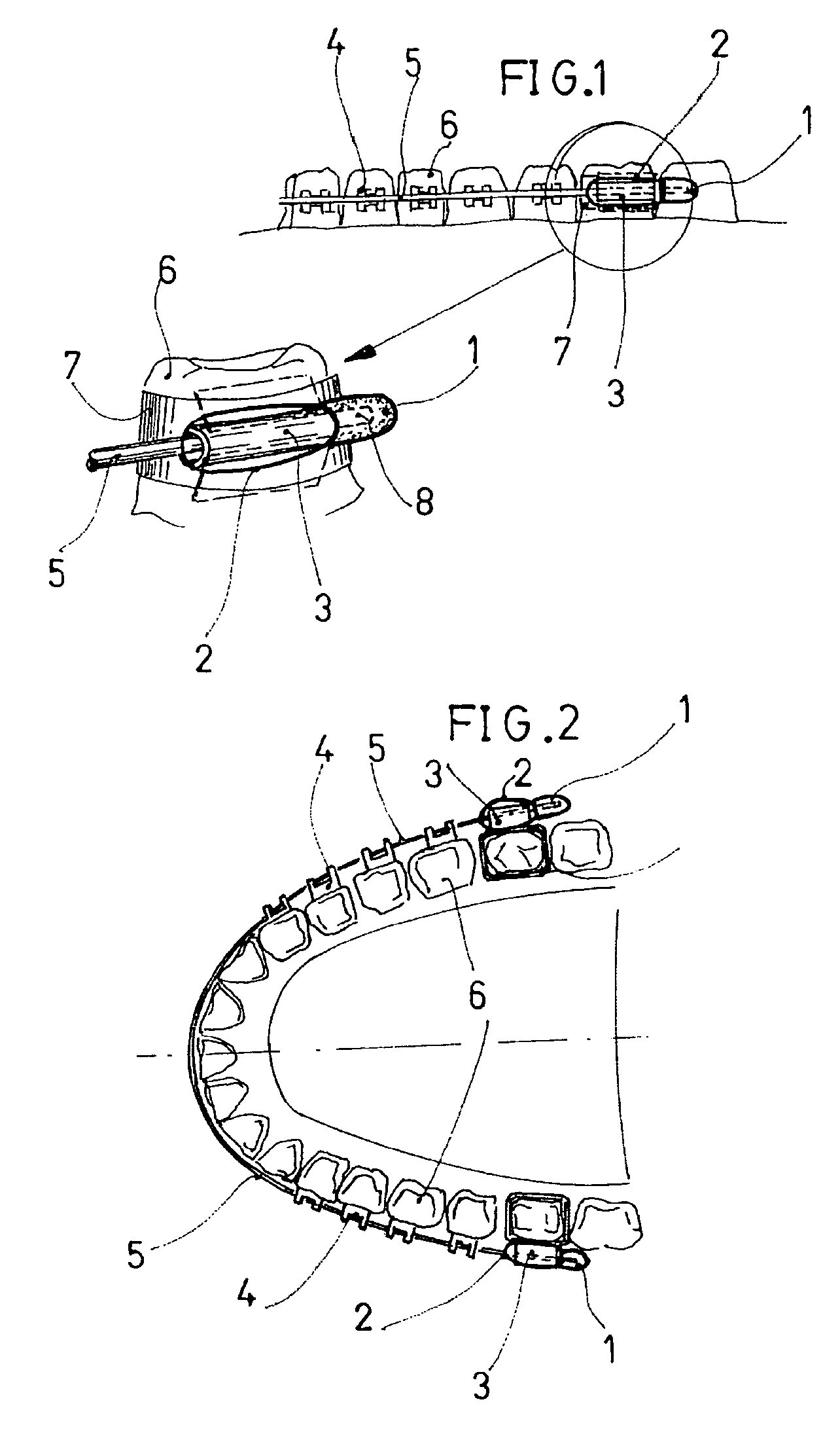
Oral mucous membrane protector for orthodontic applications
An oral mucous membrane protector for orthodontic appliances formed of a protective element or member arranged on the distal end of the archwire that projects from the molar tube, covering or enveloping the archwire and fixing it to the molar tube by means of an elastic ring joined to the protective element. The protective element may be reinforced on the bottom thereof so as to prevent tearing or perforation.
Owner:DE SALAZAR VINAS MARIA PILAR
Biological medical membrane and method of preparing the same
InactiveCN101278948AProtection from damageImprove nutrient metabolismDigestive systemDermatological disorderOral lichen planusSlurry
The invention discloses a biological type drug membrane and a preparation method thereof. The main components of the biological type drug membrane are leaven and additives including film-forming material, plasticizer, aromatic and so on. The making process flow of the drug membrane is as follows: firstly the slurry of the film-forming material is prepared; inactivated leaven and the additives are added and evenly mixed, and then the processes of deaeration, coating, drying, deciduation and packing are carried out. Compared with the prior art, by adopting the leaven as the main component, the drug membrane not only plays a role of protecting a wound surface but also improves the nutrient metabolism of oral mucosa, protects various cell injuries and enhances body immunity. In addition, the drug membrane has the characteristics of small volume and light weight and is also more convenient for being easy to be packed, stored, transported, carried over and used. The biotype-drug membrane is mainly used for treating various congestion, erosion and ulcerous lesion. And the biological type drug membrane has good effect on treating recurring Aphthous ulcer and oral lichen planus (congestive and erosive type).
Owner:DALIAN UNIV
Agent For Oral Mucosal Administration
InactiveUS20110150974A1Superior saliva secretion promotingReduce allocationBiocideOrganic chemistryArylHydrogen atom
A medicament used for prophylactic and / or therapeutic treatment of xerostomia, which is in the form for oral mucosal administration comprising a spirooxathiolane quinuclidine derivative represented by the following general formula (I) or an acid addition salt thereof:[Formula 1](wherein R1 and R2 may be the same or different, and independently represent a hydrogen atom, an alkyl group, a cyclopentyl group, a cyclohexyl group, a monoaryl- or diaryl-substituted methylol group, or an aryl-substituted alkyl group) as an active ingredient.
Owner:DAIICHI PHARMA CO LTD +1
Lysozyme oral care health-care fluid and preparation method thereof
ActiveCN104983600AEasy to solveGood treatment effectAntibacterial agentsCosmetic preparationsBiotechnologyMouth care
The invention discloses lysozyme oral care health-care fluid and a preparation method thereof. The lysozyme oral care health-care fluid comprises 0.001-10% of lysozyme, 0.01-10% of tea polyphenol, 0.001-0.1 ppm of vitamin B12, 0.1-10% of freshener, 1-20% of sweetening agents, 0.1-6% of solubilizing agents, 0.01-0.6% of scented water and 43.4-98.77% of purified water. The 0.01-10% of lysozyme, 0.01-10% of tea polyphenol and 1-20% of sweetening agents are sequentially dissolved in a stainless steel container according to a proportion, slightly stirred and sufficiently and evenly mixed, so that fluid A is obtained through preparation; the 0.1-10% of freshener and 0.01-0.6% of scented water are sequentially dissolved in the 0.1-6% of solubilizing agents according to a proportion, slightly stirred and sufficiently and evenly mixed, so that fluid B is obtained through preparation. The fluid B is slowly poured into the fluid A and stirred slightly, and the 0.001-0.1 ppm of vitamin B12 is added and sufficiently and evenly mixed. The evenly mixed material is filtered, packaged and subjected to filling, so that the health-care fluid is obtained. The lysozyme oral care health-care fluid is obvious in effect, main functional components are natural extracts and do not contain alcohol, and the lysozyme oral care health-care fluid is mild in taste and capable of effectively improving the micro-ecological environment of the oral cavity without stimulating the oral mucosa while meeting the requirements for cleaning the oral cavity and freshening breath.
Owner:HUBEI SHENDI AGRI SCI & TECH CO LTD
Application of composition to the preparation of antiepileptic drug
ActiveCN103599115AAddress serious deficienciesImprove antiepileptic effectNervous disorderHydroxy compound active ingredientsFructose phosphateOral mucous membrane
The invention provides the application of a 1,6-fructose diphosphate sodium salt, fructose and / or borneol composition to the preparation of an antiepileptic drug. The composition consists of 1,6-fructose diphosphate sodium salt and fructose, or 1,6-fructose diphosphate sodium salt and borneol combination, or 1,6-fructose diphosphate sodium salt, fructose and borneol. The composition provided by the invention can improve in vivo stability of 1, 6 1,6-fructose diphosphate, in order to maintain high level of 1,6-fructose diphosphate in the brain for a long time and make the 1,6-fructose diphosphate sodium salt exert antiepileptic effect for a long time. The composition provided by the invention particularly employs chewable tablets, orally disintegrating tablets and dispersible tablets as dosage forms for the antiepileptic drug, and the dosage forms can be directly absorbed in oral mucosa, avoid hepatic first-pass effect and further increase the level of 1,6-fructose diphosphate in the brain, thereby further improving the antiepileptic efficacy of the drug.
Owner:ZHEJIANG UNIV
Oral loratadine disintegrating tablet and its prepn
ActiveCN1739513AReduce stimulationQuick effectPill deliveryImmunological disordersDiseaseOrally disintegrating tablet
The present invention belongs to the field of medicine technology, and is especially oral loratadine disintegrating tablet for treating allergic diseases and its preparation process. The oral loratadine disintegrating tablet includes loratadine as effective medicine component, and excipient mixture comprising disintegrating agent, stuffing, soluble polyol and penetrant. The oral loratadine disintegrating tablet consists of loratadine 20-50 wt%, disintegrating agent 5-15 wt%, stuffing 10-30 wt%, soluble polyol 30-60 wt%, and penetrant 1-5 wt%. The oral loratadine disintegrating tablet, after being disintegrated fast, can cover gastrointestinal mucous membrane widely, and has fast acting, no first pass effect, high bioavailability, and convenient taking.
Owner:HAINAN PULIN PHARMA +1
Application of cobrotoxin in preparing medicine for treating arthritis
InactiveCN101648000AImprove pathological changesImprove joint swellingPeptide/protein ingredientsAntipyreticOral mucous membraneOral medication
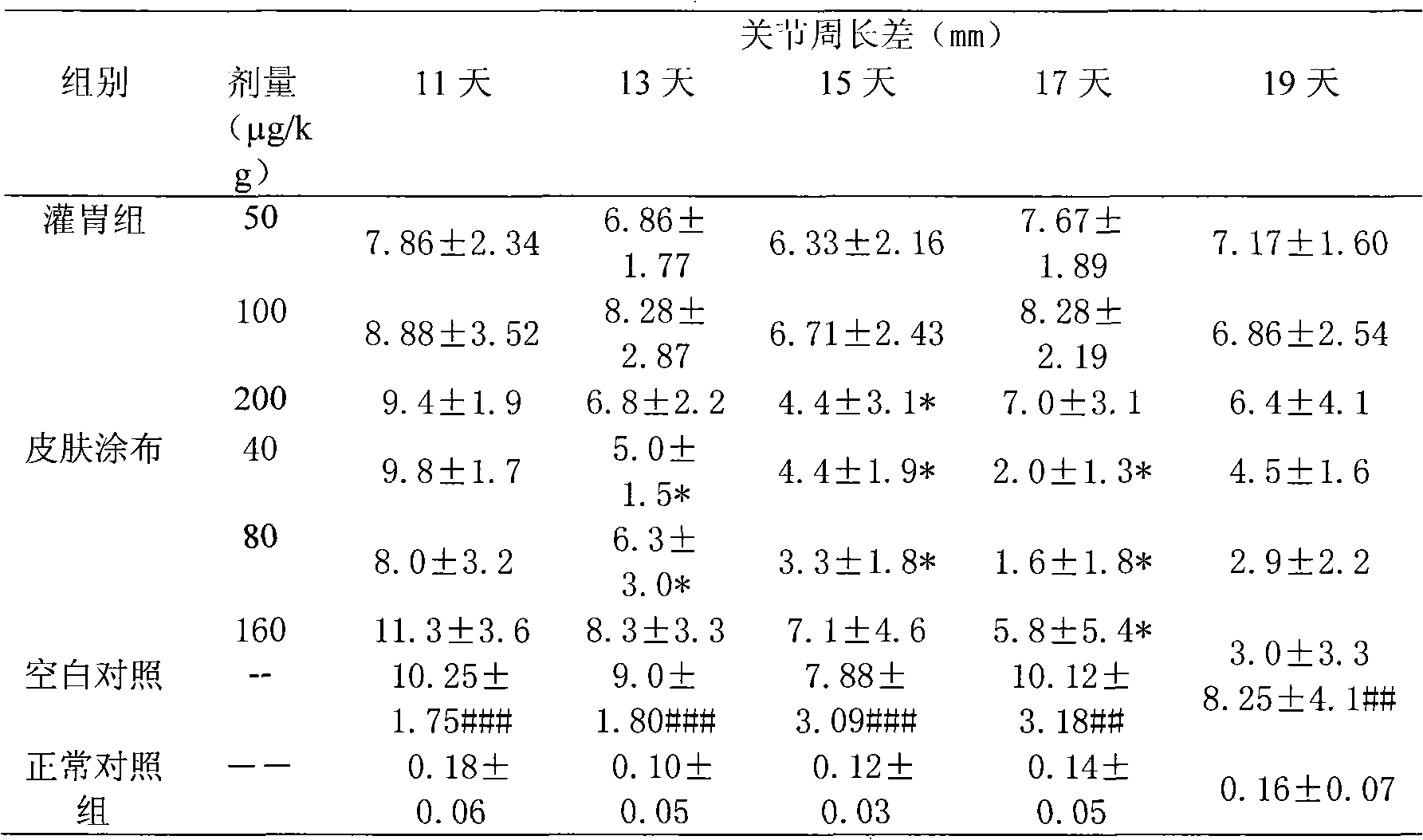
The invention discloses an application of cobrotoxin in preparing a medicine for treating arthritis. Cobrotoxin is a neurotoxin purified from Chinese cobra-venom. People research discovers that the cobrotoxin in the range of 1-600mug / kg has obvious effect on treating rheumatoid arthritis, can relieve the symptoms of arthrocele, arthralgia and the like, and has the advantages of small dose, safety,capability of oral administration, injectability, administration through oral mucous membrane, nasal mucous membrane and skin, and the like.
Owner:ZHEJIANG JINGXIN PHARMA
Ectocornea-like sheet and method of constructing the same
InactiveUS20050003532A1Reduce differentiationHigh divisional potentialEye implantsArtificial cell constructsDiseaseOral mucosal epithelial cell
It is intended to provide a transplantation material applicable to ocular surface diseases with a need for ectocornea transplantation (i.e., an ectocornea-like sheet). Oral mucosal epithelial cells are inoculated onto an amnion and then cultured in the coexistence of supporter cells. When a layered structure of the oral mucosal epithelial cells is formed, the outermost layer is brought into contact with air, thereby inducing differentiation. Thus, an ectocornea-like sheet having an oral mucosal epithelial cell layer on the amnion is obtained.
Owner:AMNIOTEC +1
Pharmaceutical dosage form for mucosal delivery
InactiveCN1627938AImprove sensory qualityEasy to stayOrganic active ingredientsPharmaceutical non-active ingredientsGellan gumBuccal use
The present invention provides a pharmaceutical tablet, which tablet includes a tablet core that can be disintegrated in the oral cavity and an excipient coating adhered thereon, wherein the coating contains gellan gum. The tablet is suitable for oral administration, for example, the drug contained in the core of the tablet is at least partially absorbed through the patient's oral mucosa and delivered to the patient.
Owner:PHARMACIA CORP
Application of allograft acellular dermal matrix in oral diseases
The invention relates to application of an allograft acellular dermal matrix in oral diseases, in particular to application of the allograft acellular dermal matrix in the repairing of cleft lip and / or alveolar cleft, cleft palate and oral mucous membrane defect, the repairing of periodontal defect and gingival recession, the repairing of nasal mucosa, tooth implantation, the repairing of furcation involvement and the prevention of complications such as dry socket after impacted tooth extraction, and belongs to the technical field of biological medical material tissue engineering. The allograft acellular dermal matrix is a product improved by a process, the mechanical performance of the product is suitable for surgery, and the sewing strength of the product is 16-18N; the allograft acellular dermal matrix is free of immunological rejection, capable of fast inducing tissue regeneration, soft in texture after implantation, free of contour feeling and good in in-vivo compatibility and hasa stable stent and template effect.
Owner:BEIJING JAYYALIFE BIOTECH CO LTD
Application of physically-modified cobra venom in preparation of medicament for treating pulmonary fibrosis
InactiveCN102526114ALow toxicityImprove efficacyReptile material medical ingredientsRespiratory disorderDiseaseCobra venom
The invention discloses application of cobra venom in preparation of a medicament for treating lung inflammatory disease and pulmonary fibrosis. Animal experiment proves that Chinese cobra venom can alleviate acute endotoxin poisoning symptom within a range of 10-3000mug / kg, particularly has obvious therapeutic action on lung inflammatory disease caused by endotoxin, and can be used for alleviating inflammatory cell infiltration, alveolar septum thickening, and focal atelectasis and emphysema. Repeated small dose of endotoxin can result in pulmonary fibrosis, the cobra venom can significantly alleviate pulmonary fibrosis symptom and reduce alveoli damage and collagen hyperplasia. Naturally-modified Chinese cobra venom and physically-modified Chinese cobra venom have similar actions, but the physically-modified Chinese cobra venom has better action. Used for treating lung inflammatory disease, the Chinese cobra venom has the advantages of low dose, safety, capability of being orally administrated, injected, and administered through oral mucous membrane and skin and the like.
Owner:SUZHOU RENBEN PHARMA
Insulin spray for oral cavity and its prepn process
InactiveCN1335182AIncrease concentrationIncrease contact areaPowder deliveryPeptide/protein ingredientsPhosphateOil phase
The present invention relates to medicine and its production and aims at raising biological utilization of oral absorption and strengthening the stability of the preparation. The insulin spray for oral cavity contains insulin 10000-70000 U, soybean lecithin 5-50 g and propylene glycol 25-80 g as well as borneol 1.2-10g, absolute ethyl alcohol 1-15 ml and phenol 2-5 g, the rest is pH 6.8-7.8 buffering phosphate solution, in each 1000 ml of microemulsion. The preparation process includes mixing soybean lecithin with propylene glycol, borneol and ethanol solution, addition of phenol containing buffering phosphate solution, supersonic treatment to obtain emulsified oil phase, the dissolution of insulin in the buffering solution, further mixing and supersonic treatment.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Method for producing pure mountain ginseng lozenge
InactiveCN103463151AGood effectShorten the production cyclePill deliveryFood shapingDistilled waterGINSENG EXTRACT
The invention provides a method for producing pure mountain ginseng lozenge, and relates to an improvement of health product, and the producing method comprises the following steps: 1. drying and crushing mountain ginseng, sieving mountain ginseng powder with a 80-800 mesh sieve, and mixing mountain ginseng powder and distilled water according to feed liquid weight ratio 1:20, extracting for three times, each of which lasts for 18 min or more, filtering, merging filtrates, and drying residue; 2. condensing the filtrate below 60 DEG C till the relative density of the extract is 1.10-1.15; 3. mixing the dried residue and the extract, adding distilled water and diluting till the relative density is 1.3-1.4, preparing the mixed mountain ginseng extract and the residue into wet particles, and drying at 68-75 DEG C; 4. compacting the wet particles into tablets by rotary type tablet press. The invention has the advantages that the oral mucosa and the oral gastrointestinal tract absorption pathway are combined, thereby the active ingredients in the mountain ginseng powder can be rapidly absorbed by human body, and the original flavour of the mountain ginseng is maintained, and the health care function is improved, moreover, the dosage is greatly reduced, and the utilization rate of the rare resource is increased.
Owner:吉林省和升圆生物科技有限公司
Preparation for novel administration route of antiarrhythmic medicament
InactiveCN101467968AEasy to administerQuick effectAerosol deliveryAmine active ingredientsDiseaseOral mucous membrane
The invention relates to a formulation with novel administration route for the antiarrhythmic medicaments, comprising antiarrhythmic active drugs, and one or more additives of solvent, latent solvent, antioxidant or surfactant, which are packaged in the containers having a spray device. The formulation is capable of being used for the nasal, oral or sublingual administrations through the pulmonary administration or agents, which is more convenient than injection administration, faster in effect than oral administration to avoid unnecessary prolongation of effect after the onset of diseases.
Owner:ZHEJIANG ZHENGFANG PHARMA TECH
Support steel alloy material for dentistry
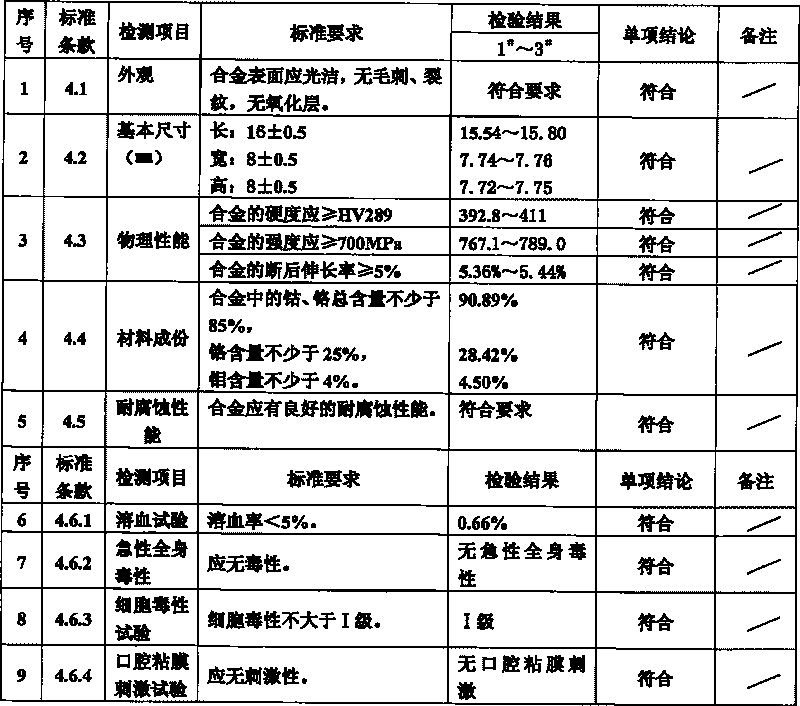
ActiveCN101693966AGood biocompatibilityImprove corrosion resistanceImpression capsDentistry preparationsOral mucous membraneWhole body
A support steel alloy material for dentistry belongs to the technical field of dentistry materials. Chemical elements and weight percentages of the support steel alloy material include that cobalt takes 62%-64%, chromium takes 28%-30%, molybdenum takes 4%-5%, titanium takes 0.3%-1.2%, silicon takes 0.2%-1.3%, iron takes 0.2%-1.0%, manganese takes 0.2%-1.3% and carbon takes 0.1%-1.2%. The support steel alloy material for dentistry provided by the technical scheme of the invention is fine in biocompatibility with human bodies, has fine corrosion resistance, has hemolysis rate lower than 5%, does not have acute systemic toxicity, has cytotoxicity which is not higher than I level, and does not have oral mucous membrane irritation or delayed contact sensitization, and then the support steel alloy material has perfect safety. In addition, because the support steel alloy material is 767.1-789 in tensile strength ob(MPa), 5.36-5.44 in elongation (%) and 392.8-411 in Vickers hardness, the support steel alloy material has appropriate strength, hardness and wear resistance and fine processing property, and can be adaptable to soft and hard tissue inside orals.
Owner:南通今日高科技新材料股份有限公司
Antibacterial paste for killing or inhibiting helicobacter pylori in oral cavity and preparation method thereof
PendingCN111714625AKill PotentPotent lysozymeAntibacterial agentsCosmetic preparationsBiotechnologyFlos chrysanthemi
The invention discloses antibacterial paste for killing or inhibiting helicobacter pylori in the oral cavity and a preparation method thereof. The antibacterial paste comprises the following raw materials in parts by mass: 1 to 1.5 parts of lysozyme, 0.5 to 2.5 parts of lactoferrin, 0.5 to 2 parts of frankincense extract, 0.5 to 2 parts of herba ecliptae extract, 0.5 to 2 parts of minor radix buplenri extract, 0.5 to 2 parts of radix sophorae flavescentis extract, 0.5 to 2 parts of radix isatidis extract, 0.5 to 2 parts of fructus forsythiae extract, 0.5 to 2 parts of radix glycyrrhizae extract, 0.5 to 2 parts of radix stemonae extract, 0.5 to 2 parts of herba hedyotis extract, 0.5 to 2 parts of aloe extract, 0.5 to 2 parts of flos chrysanthemi extract, 0.5 to 2 parts of stevia rebaudianaextract, 0.5 to 2 parts of bletilla striata extract, 0.772.30 parts of aminocaproic acid and the balance of a toothpaste matrix. According to the antibacterial paste capable of killing or inhibiting the helicobacter pylori in the oral cavity provided by the invention, the oral antibacterial paste has the strong-effect lysozyme and frankincense oil, and plays a role in strongly killing the helicobacter pylori for dissolving and degrading helicobacter pylori cell membranes and reducing the adhesiveness of the helicobacter pylori cell membranes and oral mucosa.
Owner:湖南大海医药集团有限公司
Functional toothpaste containing natural Chinese herbal medicine components
InactiveCN104127359ATo promote metabolismInhibition of energy conversionCosmetic preparationsMetabolism disorderSenna LeavesOral mucous membrane
The invention provides functional toothpaste containing natural Chinese herbal medicine components, and the functional toothpaste has a long-term and effective auxiliary effect in controlling body weight. The functional toothpaste is prepared from 85-90wt% of toothpaste body matrix and 10-15wt% of Chinese herbal medicine extracts, wherein the Chinese herbal medicine extracts are prepared from the following raw materials in parts by weight: 30-200 parts of hawthorn, 30-180 parts of platycodon grandiflorum, 20-120 parts of poria, 20-120 parts of rhizoma alismatis, 15-100 parts of angelica sinensis, 15-90 parts of folium sennae, 10-70 parts of green tea, 10-70 parts of puerarin, 10-60 parts of raw rhubarb and 10-60 parts of mulberries. The toothpaste can permeate through mouth mucosa and blood capillaries to further adjust nerves, fat and glycogen of a human body, inhibit absorption of fat by cells and promote metabolism so as to realize the function of inhibiting put-on weight. The functional toothpaste is free from any toxic and side effects.
Owner:柯金表
Almotriptan-containing buccal adhesive tablet as well as preparation method and application thereof
InactiveCN102106836AAvoid first pass effectReduce incidenceOrganic active ingredientsNervous disorderOral mucous membraneBlood concentration
The invention provides an almotriptan-containing buccal adhesive tablet. The tablet consists of a medicine-containing adhesive part and a waterproof protection part, wherein the medicine-containing adhesive part comprises the following components in percentage by weight: 1 to 30 percent of almotriptan, 12 to 85 percent of adhesive agent, and 5 to 30 percent of excipient; and the waterproof protection part comprises the following components in percentage by weight: 1 to 15 percent of film-forming agent, 0.1 to 10 percent of plasticizer, and 0.001 to 3 percent of pigment. The invention also provides a method for preparing the almotriptan-containing buccal adhesive tablet. The medicine-containing adhesive part of the buccal adhesive tablet is in directly contact with an oral mucosa, and the medicament is directly absorbed to enter systemic circulation through the oral mucosa, thus the influence on gastrointestinal tract and pass effect of liver are avoided, and the occurrence of adverse reaction is reduced. Furthermore, the buccal adhesive tablet provided by the invention can be adhered to the oral mucosa for a long time, so that stable blood concentration is maintained, and the bioavailability is improved.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Mouthwash Composition for Managing Oral Mucositis, Process and Methods Thereof
The present disclosure is in relation to a herbal cleansing composition comprising extracts from five plants namely Emblica officinalis, Terminalia chebula, Terminalia bellerica, Glycyrrhiza glabra and Azadiracta indica. Further, the present disclosure also relates to a process of obtaining said composition and applications thereof, wherein the composition is preferably used as an oral wash for delaying the onset of oral mucositis in cancer patients undergoing radiation treatment.
Owner:RAJIV GANDHI CENT FOR BIOTECH +1
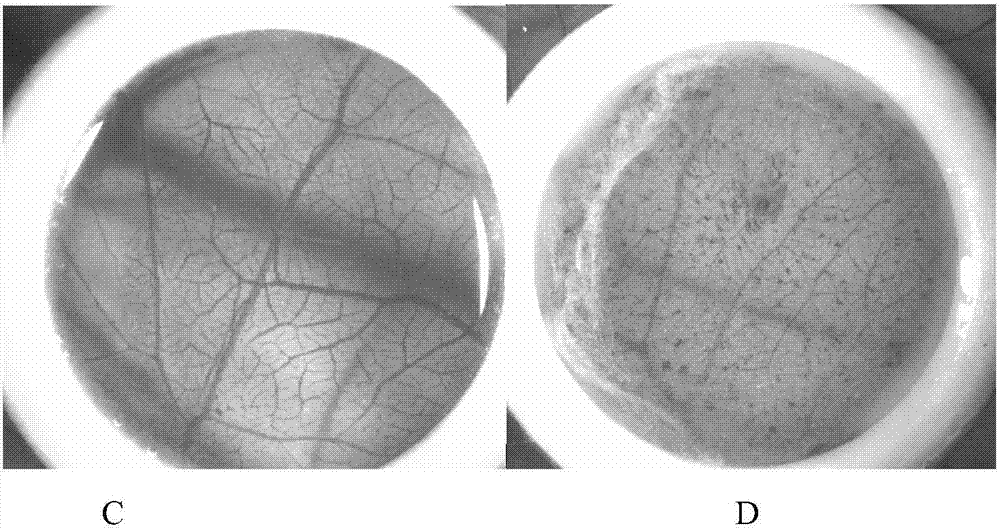
Method for detecting and evaluating conjunctiva irritation and mucous membrane irritation by utilizing chick embryo blood vascular system
The invention discloses a method for detecting and evaluating conjunctival irritation and mucous membrane irritation by using chicken embryo vascular system, comprising the following steps: (1) preparation of chorioallantoic membrane; (2) conjunctival irritation test; (3) sticking mold Stimulation test; (4) prediction model and classification, the present invention uses the standardized chicken embryo as the test system, and its vascular system at different stages of development is similar to the human eye conjunctiva and oral submucosa tissue, and the chicken embryo has a high degree of standardization, stable source and batch The difference between blood vessels is small, the vascular system has biological activity and function, the test system is simple and cheap, no special equipment is required, and the detection method is fast, sensitive and universal. The invention can detect and evaluate the conjunctival irritation and mucous membrane irritation of the substance to be tested from both qualitative and quantitative aspects. The method of the present invention can also replace living animals and human volunteers, and be directly used for the detection of conjunctival irritation and mucosal irritation of substances such as chemicals or cosmetics, and rapid screening, classification and identification thereof.
Owner:秦瑶 +1
Compositions for Oral Transmucosal Delivery of Metformin
InactiveUS20090069437A1Great tasteLow costOrganic active ingredientsBiocideOral mucous membranePharmaceutical drug
The invention relates to oral transmucosal pharmaceutical compositions comprising metformin or a pharmaceutically acceptable salt thereof, methods of using the compositions to treat various conditions, including diabetes, methods of preparing the compositions, and preparations for use in making the compositions.
Owner:GLUSKIN ANNA E +1
Soft steel alloy material for dentistry
InactiveCN101693965AGood biocompatibilityImprove corrosion resistanceImpression capsDentistry preparationsOral mucous membraneManganese
A soft steel alloy material for dentistry belongs to the technical field of dentistry materials. Chemical elements and weight percentages of the soft steel alloy material include 65%-70% of nickel, 8%-10% of chromium, 5%-6% of copper, 3.5%-5.5% of tin, 3.5%-4.5% of molybdenum, 2.5%-3.5% of titanium, 2%-3% of silicon, 1.5%-2% of iron, 1.0%-1.3% of manganese and 1.0%-1.2% of carbon. The soft steel alloy material provided by the technical scheme of the invention is fine in biocompatibility with human bodies, has fine corrosion resistance, has hemolysis rate lower than 5%, does not have acute systemic toxicity, has cytotoxicity which is not higher than I level, and does not have oral mucous membrane irritation or delayed contact sensitization, and then the soft steel alloy material has perfect safety. In addition, because the soft steel alloy material has 589.4-696.7 tensile strength ob(MPa), 4.34-4.05 elongation (%) and 342-392 Vickers hardness, the soft steel alloy material has appropriate strength, hardness and wear resistance and fine processing property, and can be adaptable to soft and hard tissue inside orals.
Owner:南通今日高科技新材料股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com