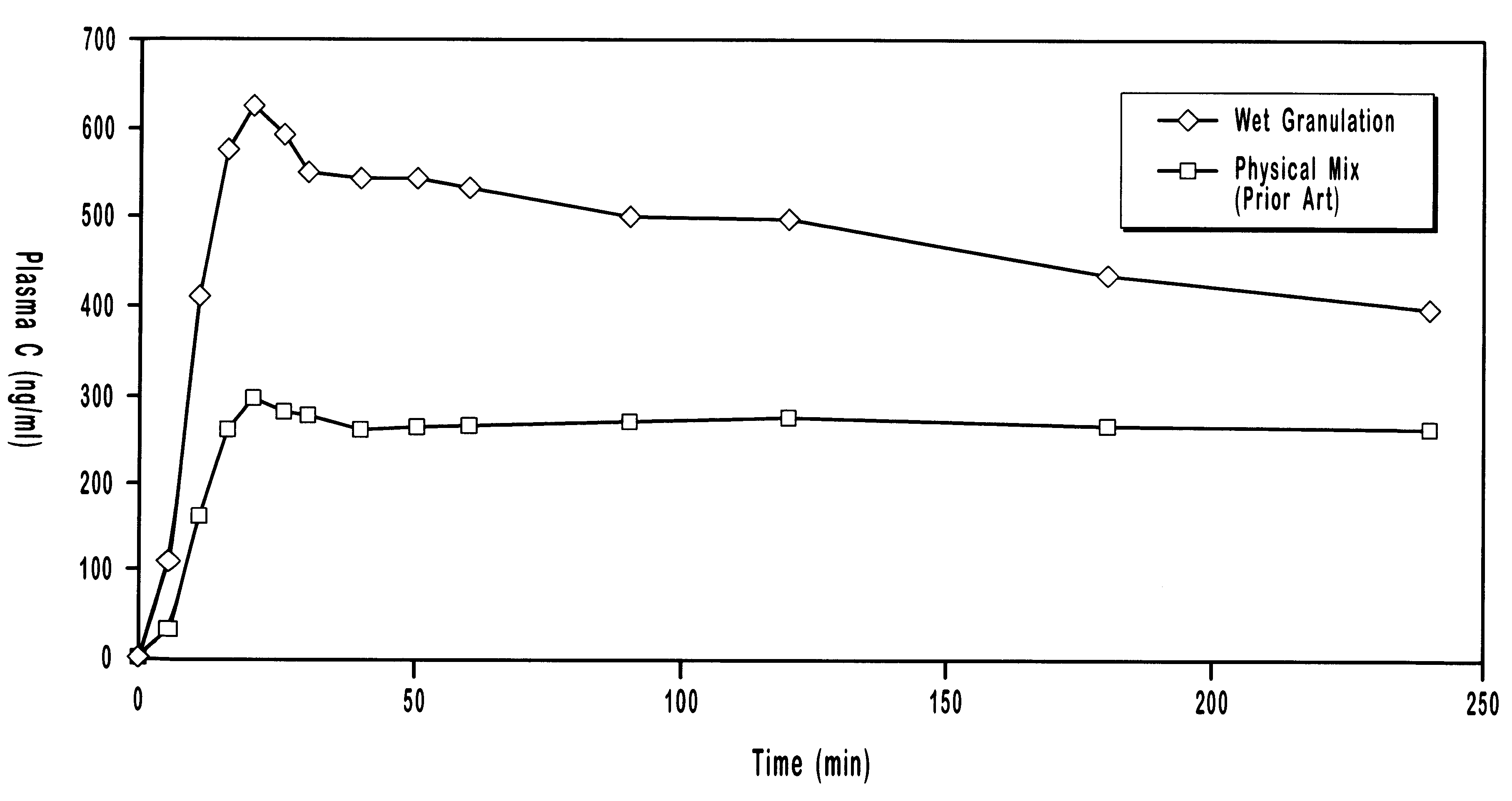
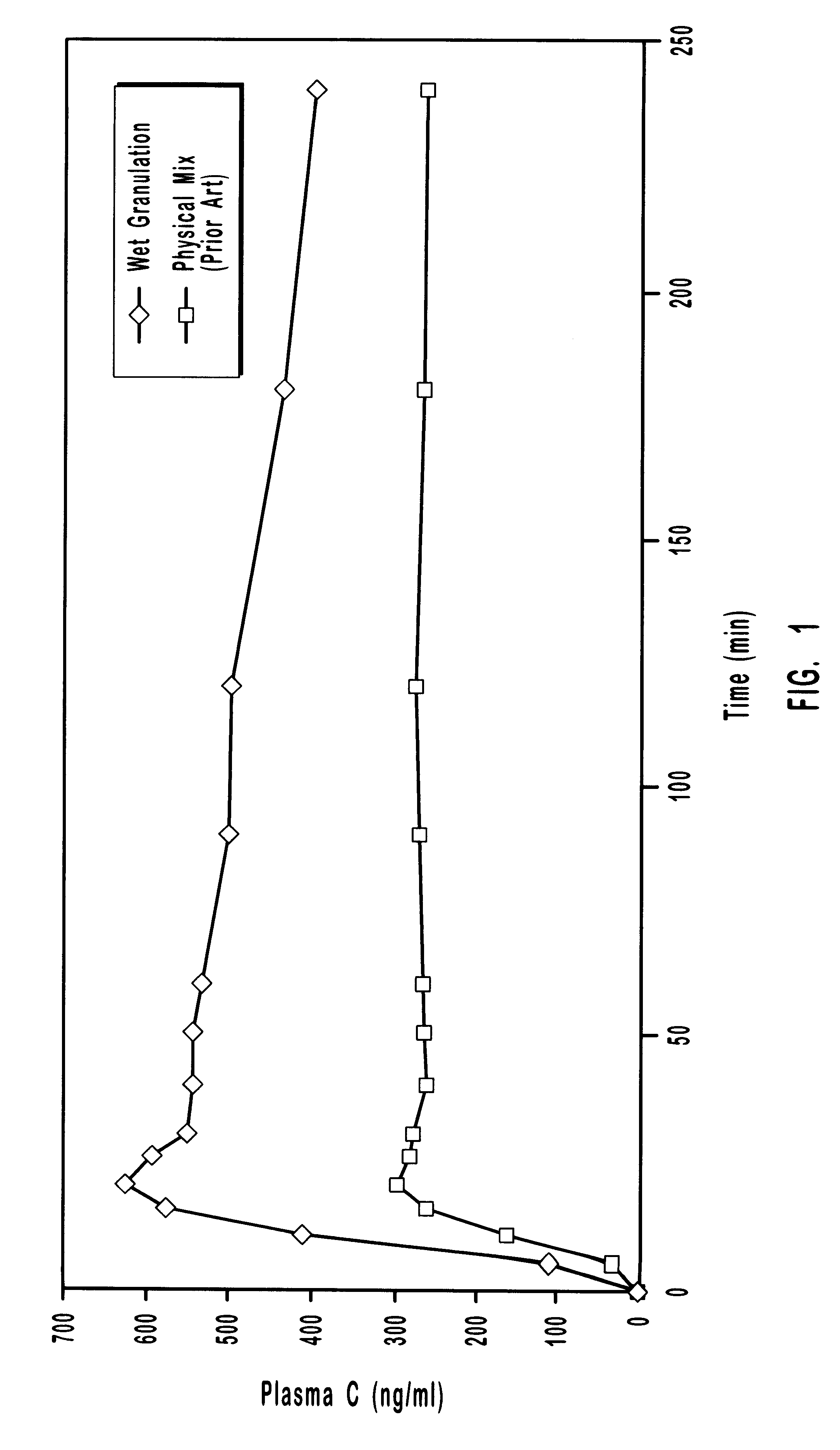
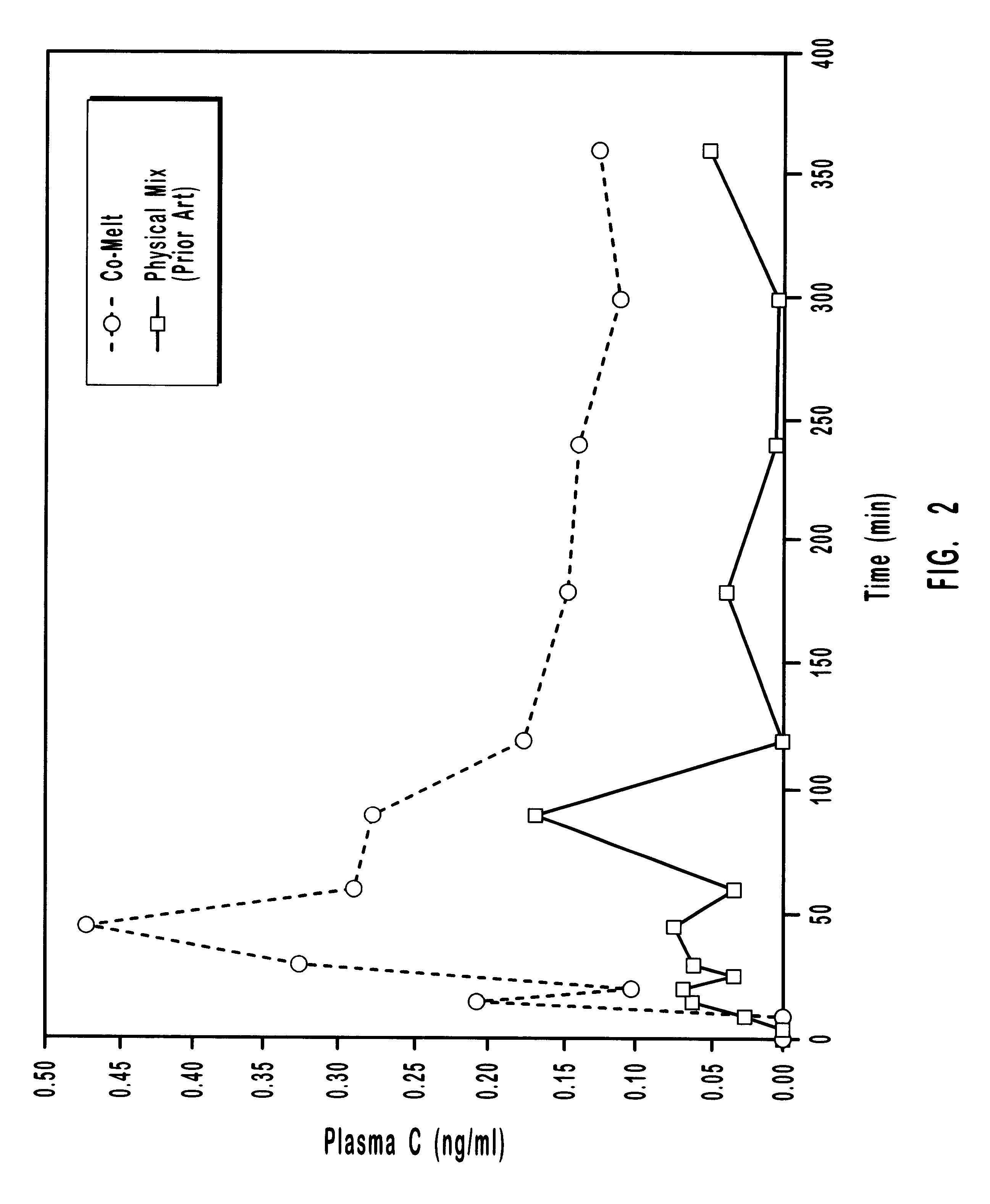
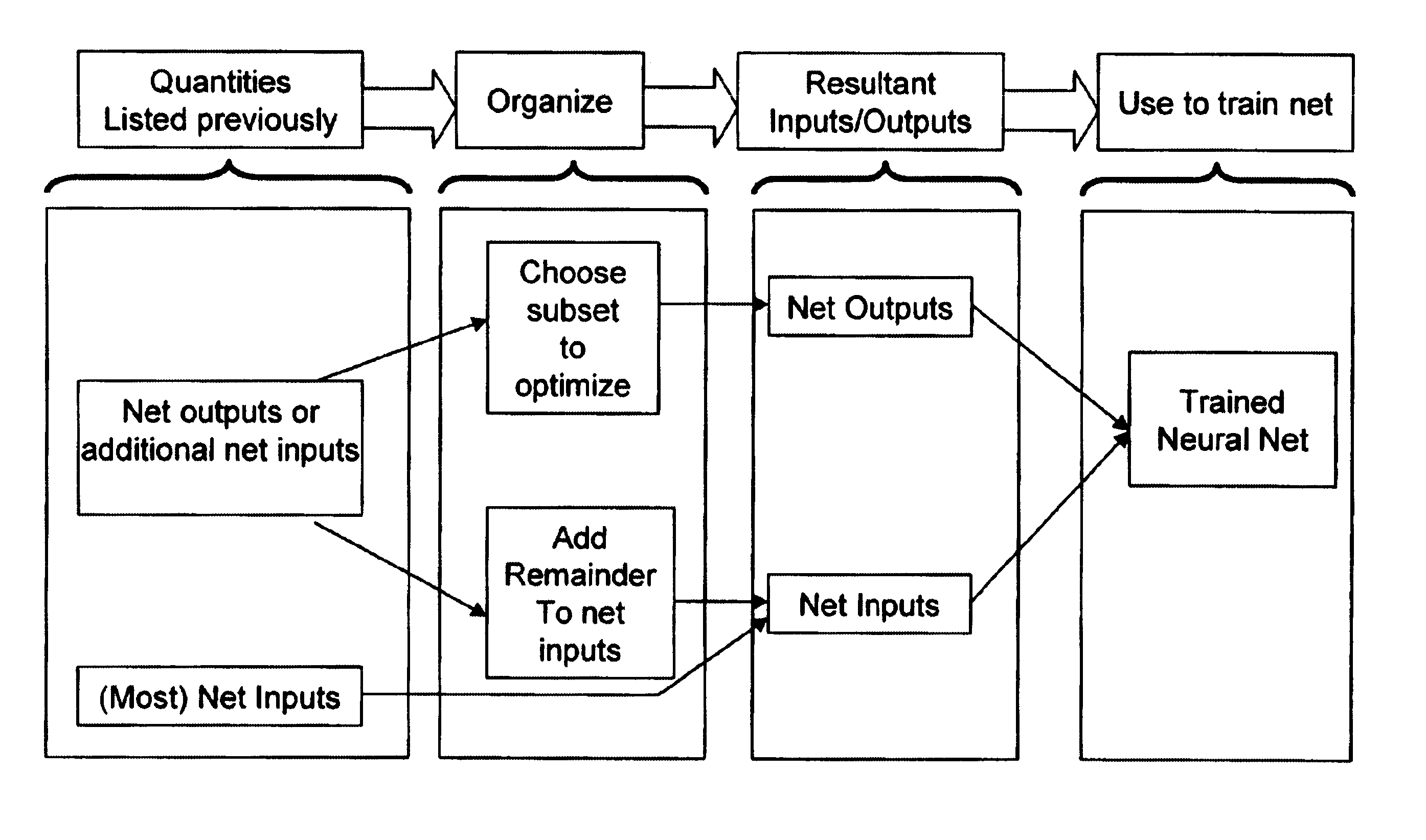
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
658 results about "Drug dosages" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
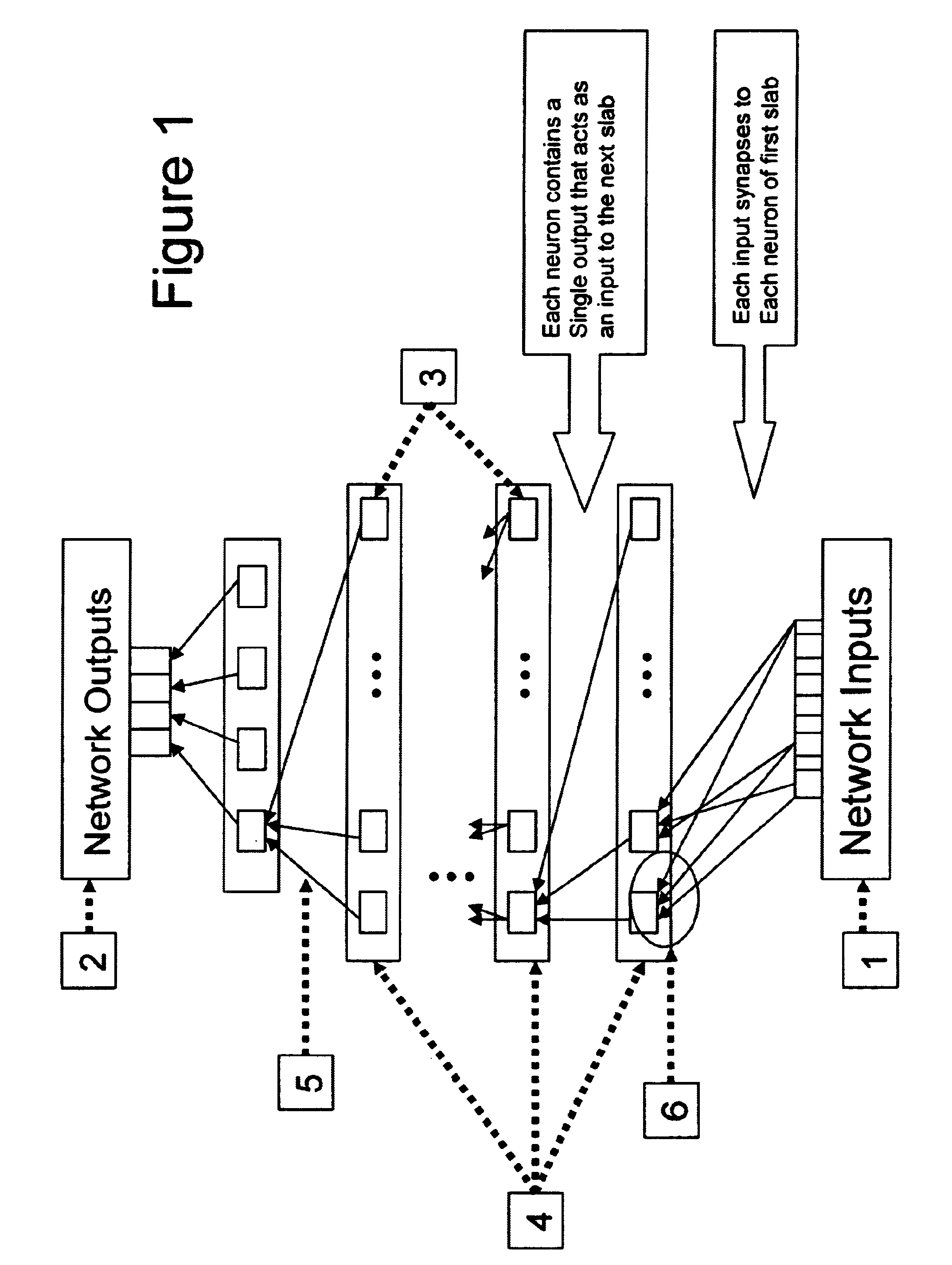
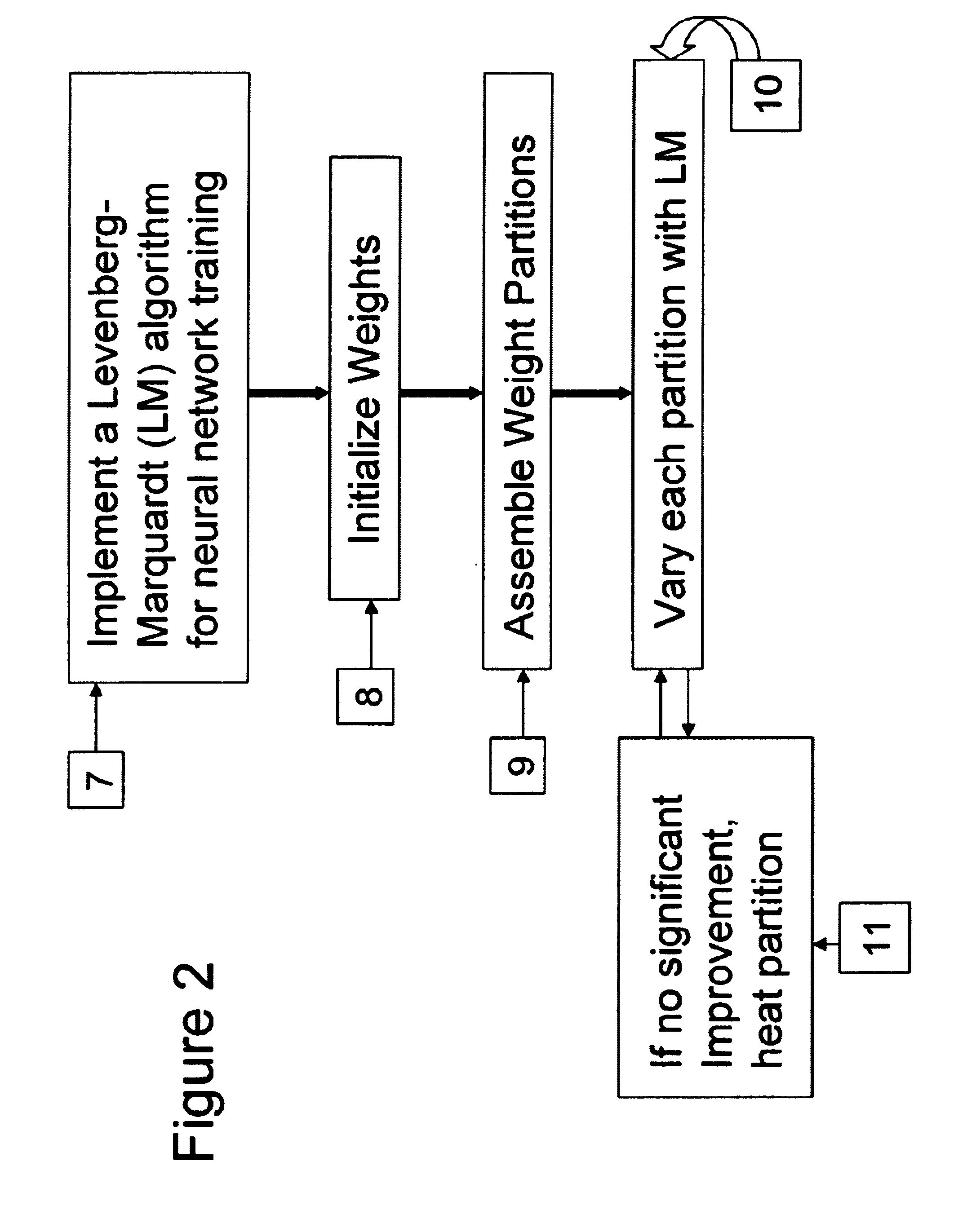
Neural network drug dosage estimation
InactiveUS6658396B1Improve accuracyGood precisionDrug and medicationsBiological neural network modelsNerve networkPatient characteristics
Neural networks are constructed (programmed), trained on historical data, and used to predict any of (1) optimal patient dosage of a single drug, (2) optimal patient dosage of one drug in respect of the patient's concurrent usage of another drug, (3a) optimal patient drug dosage in respect of diverse patient characteristics, (3b) sensitivity of recommended patient drug dosage to the patient characteristics, (4a) expected outcome versus patient drug dosage, (4b) sensitivity of the expected outcome to variant drug dosage(s), (5) expected outcome(s) from drug dosage(s) other than the projected optimal dosage. Both human and economic costs of both optimal and sub-optimal drug therapies may be extrapolated from the exercise of various optimized and trained neural networks. Heretofore little recognized sensitivities-such as, for example, patient race in the administration of psychotropic drugs-are made manifest. Individual prescribing physicians employing deviant patterns of drug therapy may be recognized. Although not intended to prescribe drugs, nor even to set prescription drug dosage, the neural networks are very sophisticated and authoritative "helps" to physicians, and to physician reviewers, in answering "what if" questions.
Owner:PREDICTION SCI
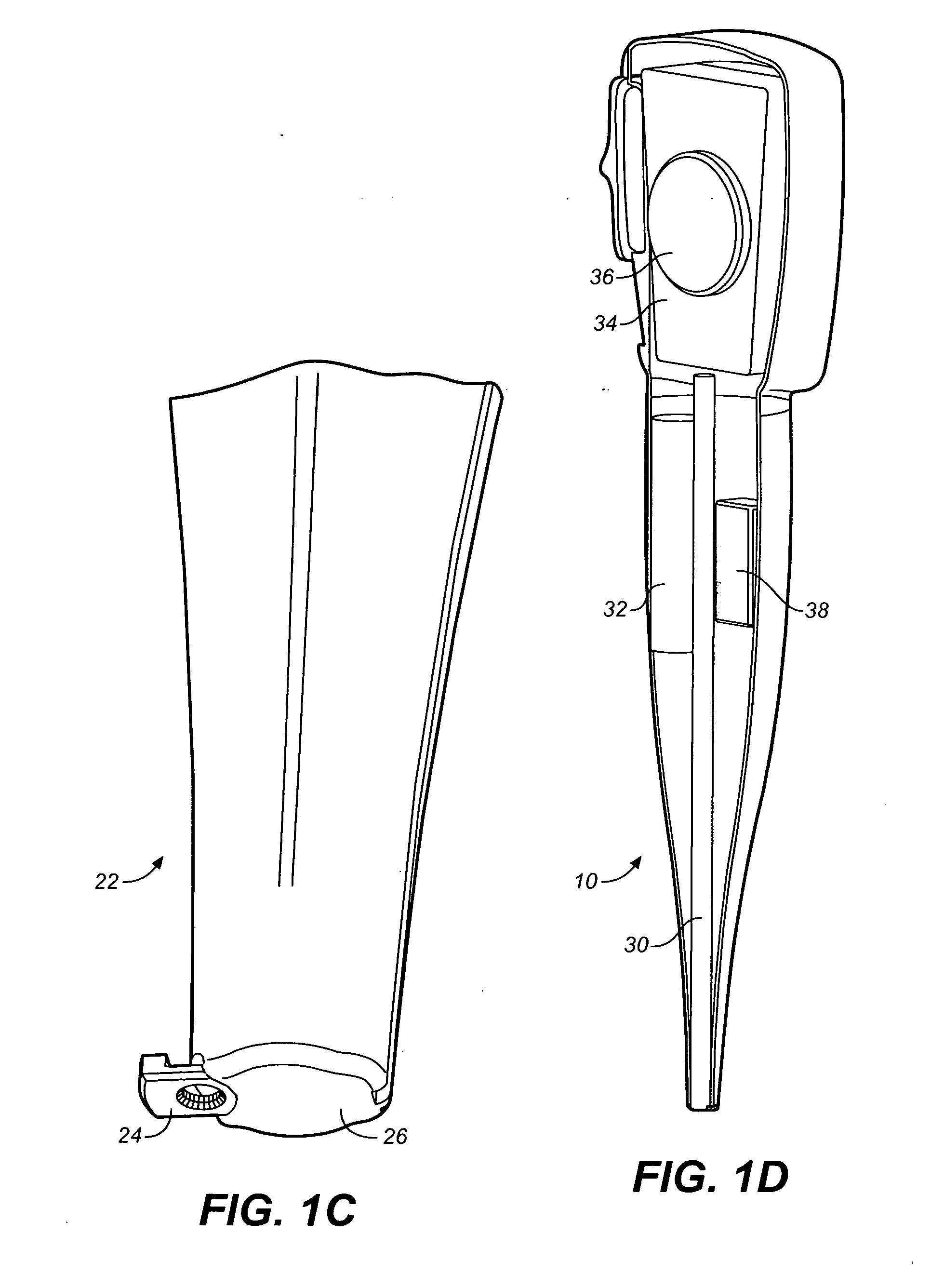
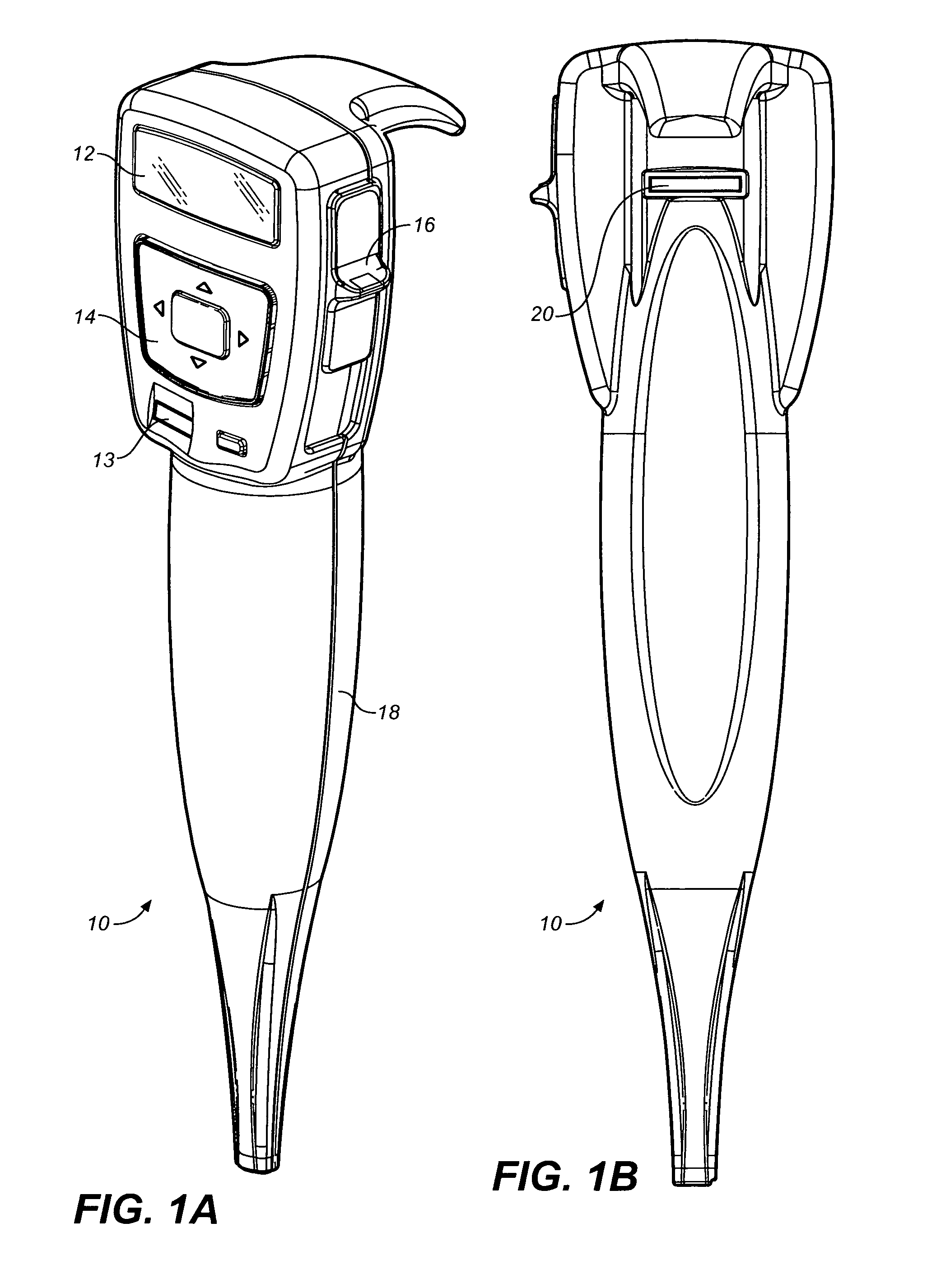
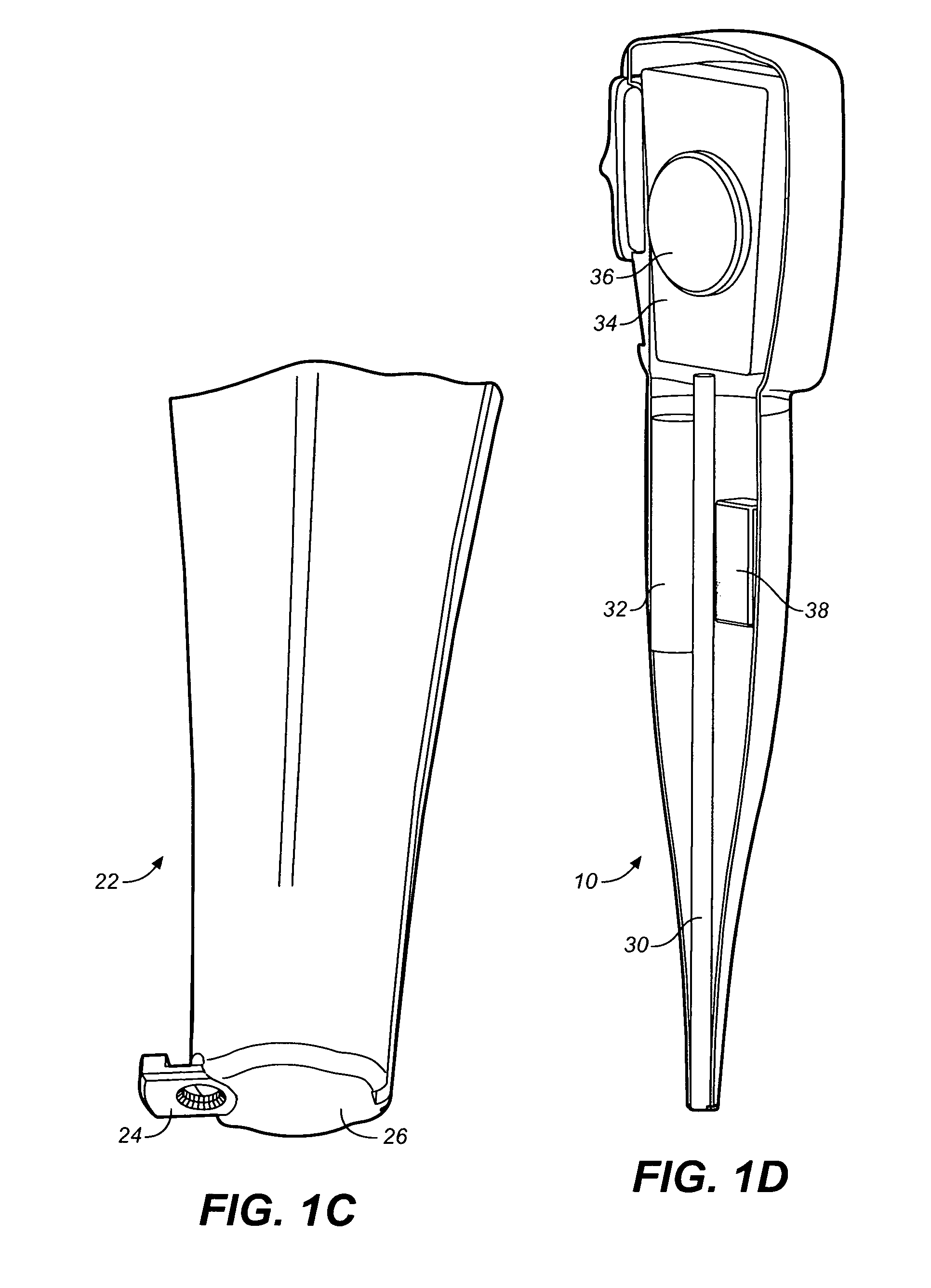
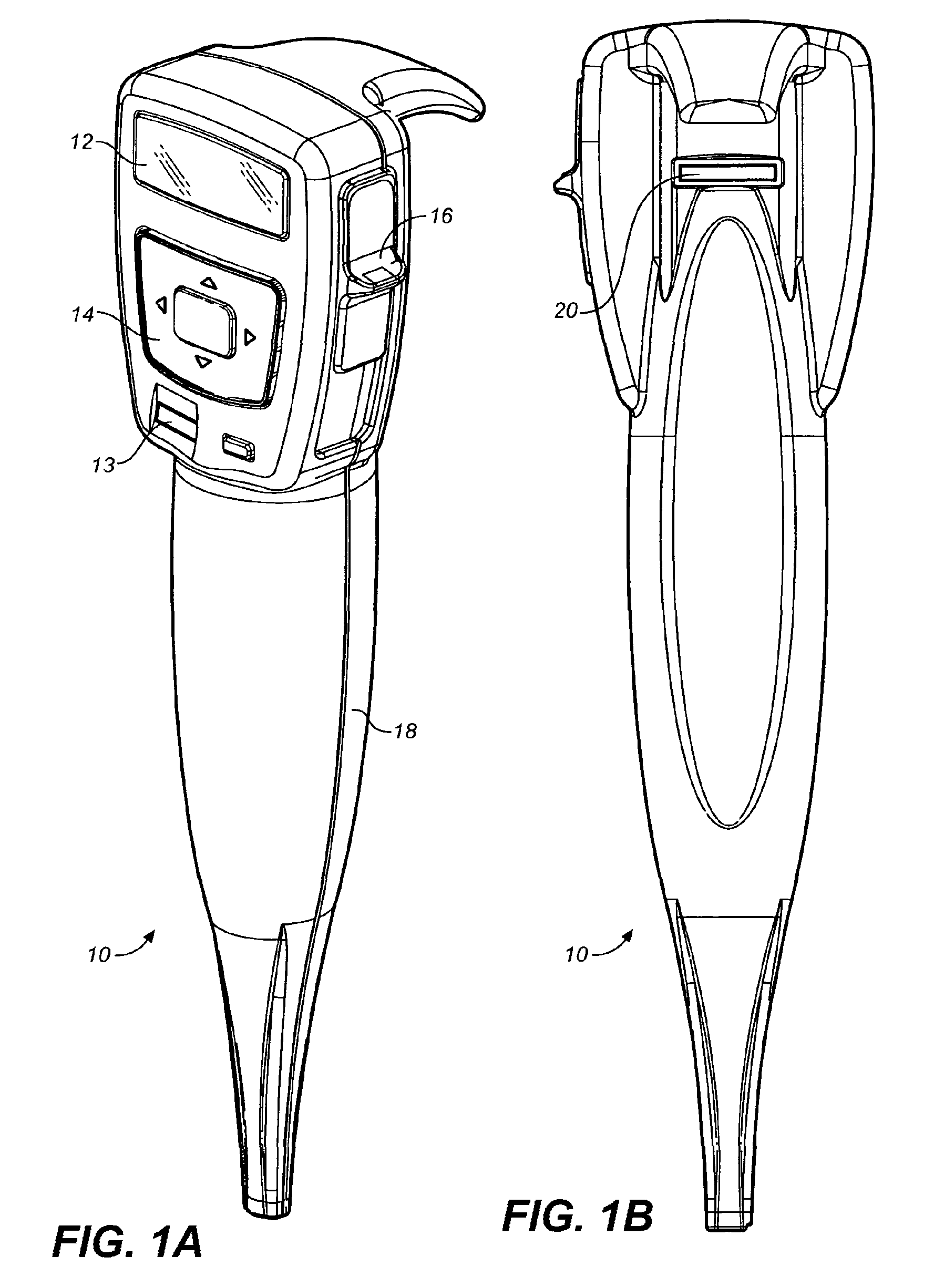
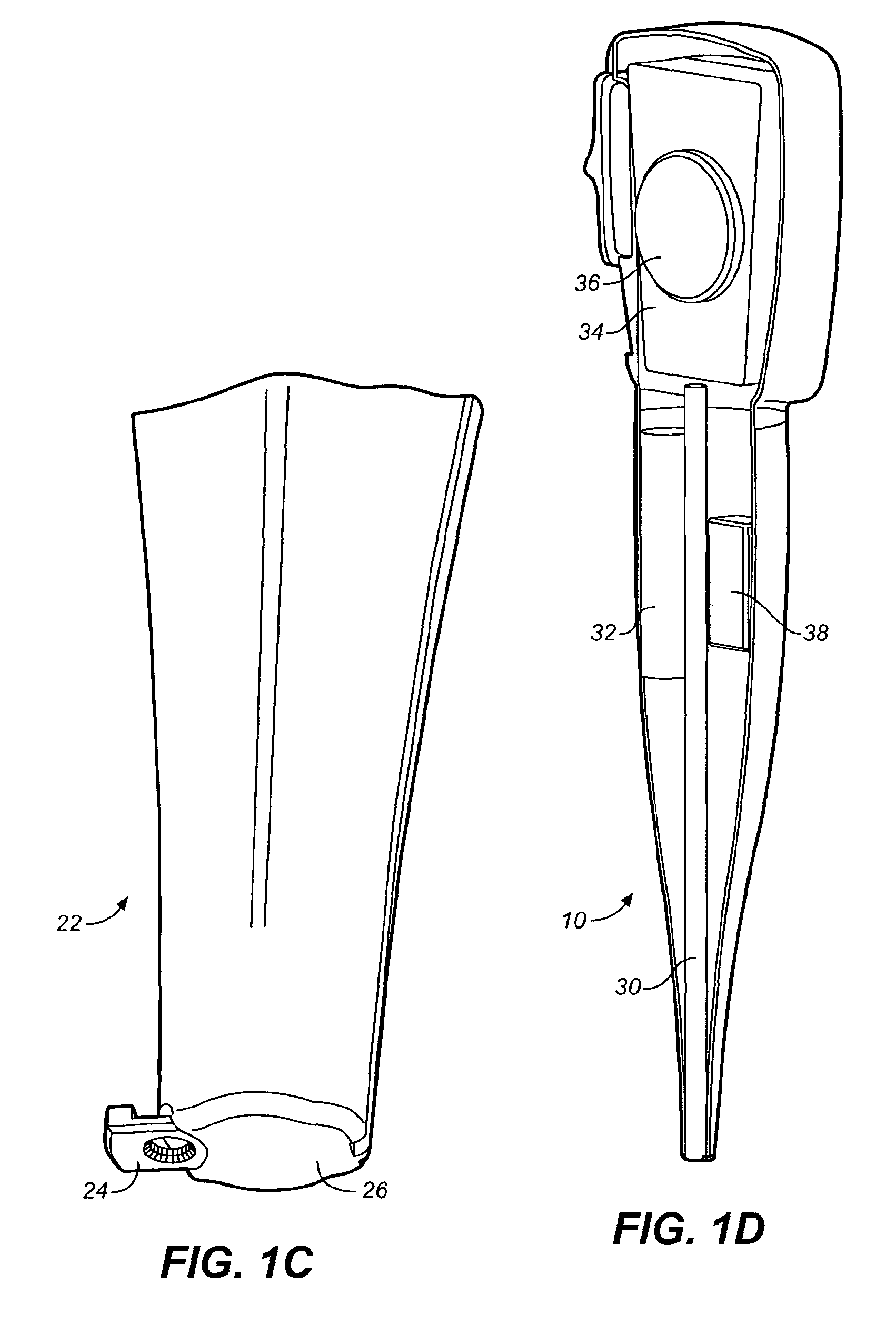
Drug storage and dispensing devices and systems comprising the same
ActiveUS20070186923A1Minimizing saliva influxPowdered material dispensingDrug and medicationsDrug StorageBiomedical engineering
Drug storage and dispensing devices for dispensing a drug dosage form to a patient are disclosed. The dispensing device has a programmable lock-out feature for locking the dispensing device and is capable of detecting the identity of a user. The invention further provides a method for the treatment of subject, by administering to the subject a drug dosage form using a dispensing device of the invention.
Owner:ACEIRX PHARM INC
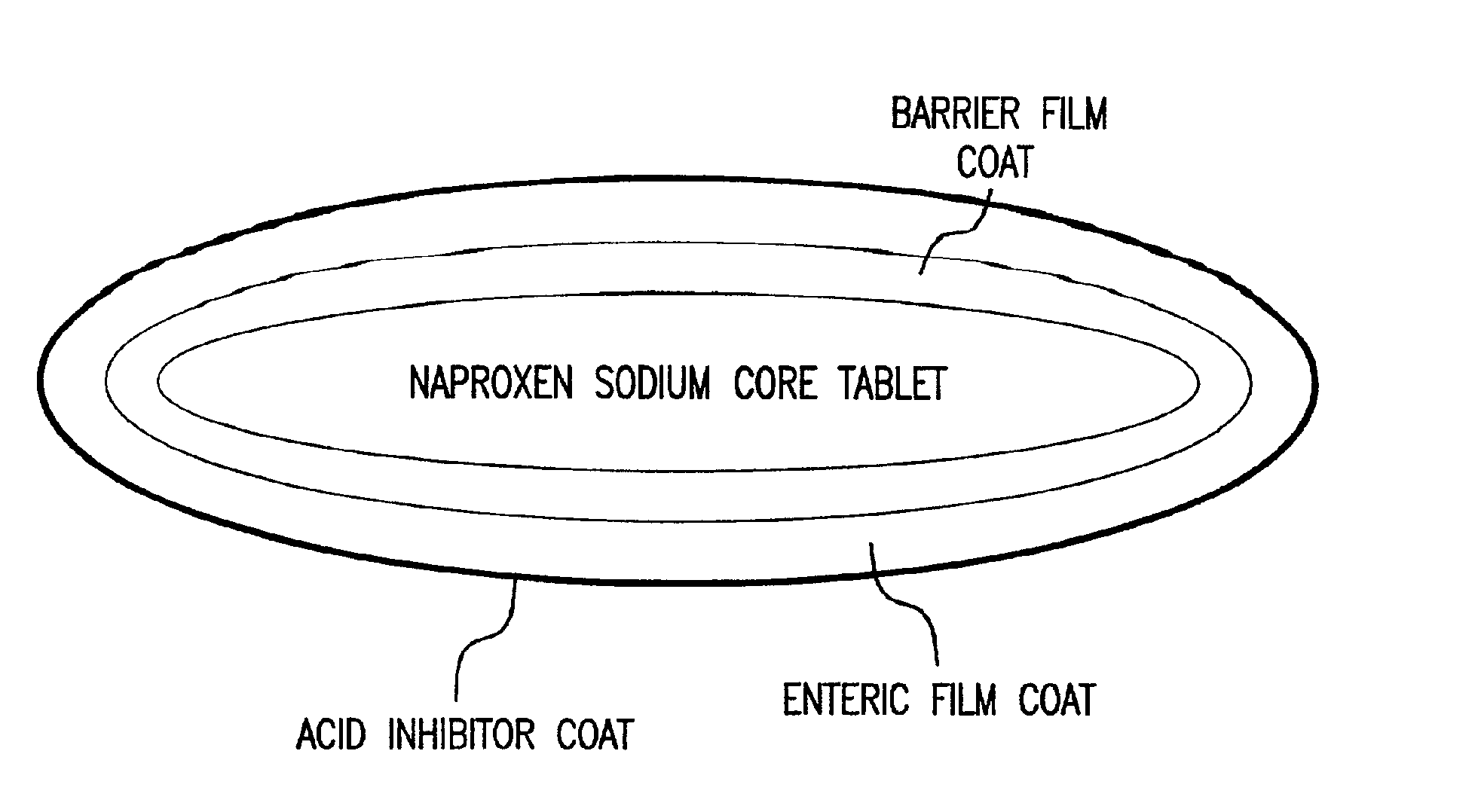
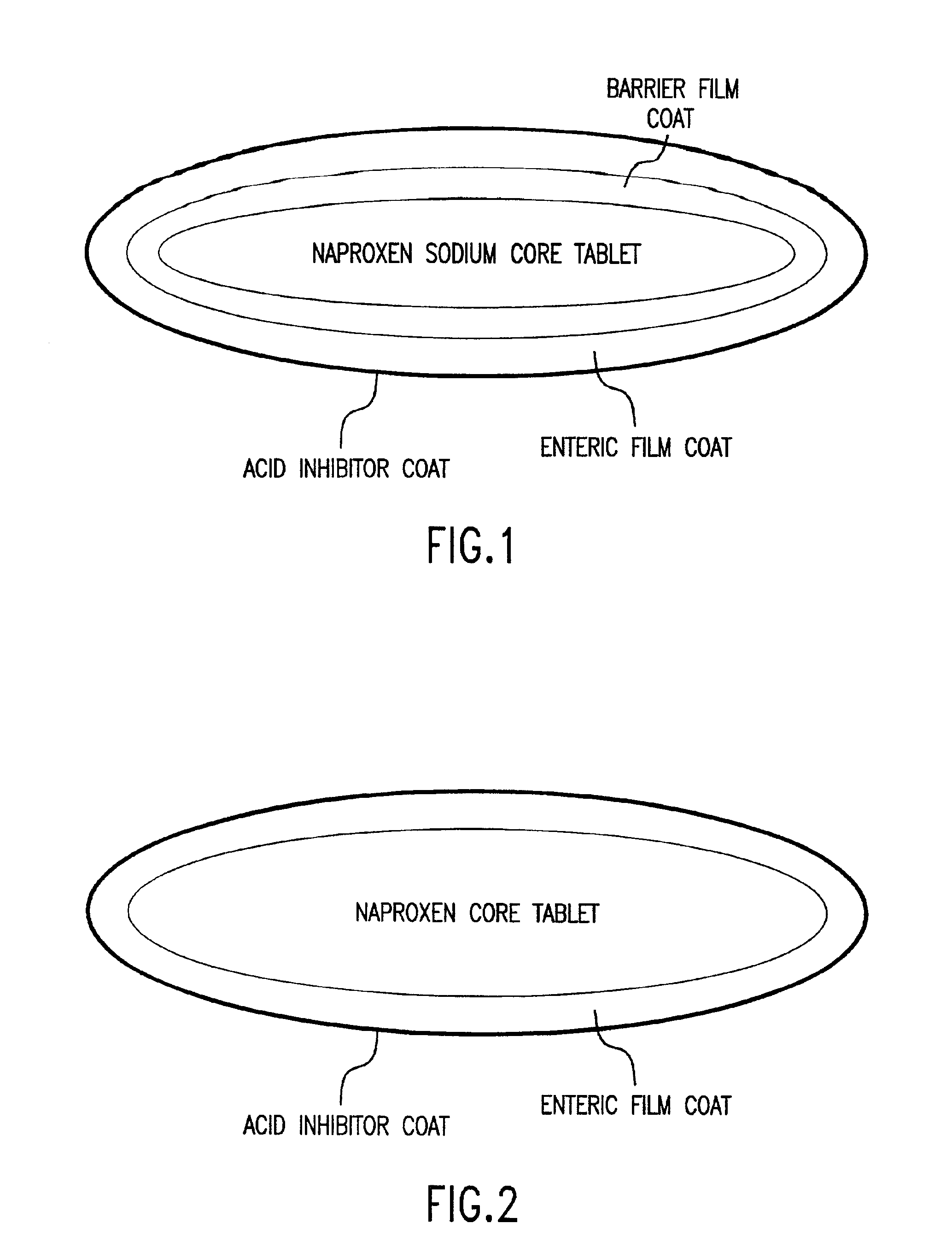
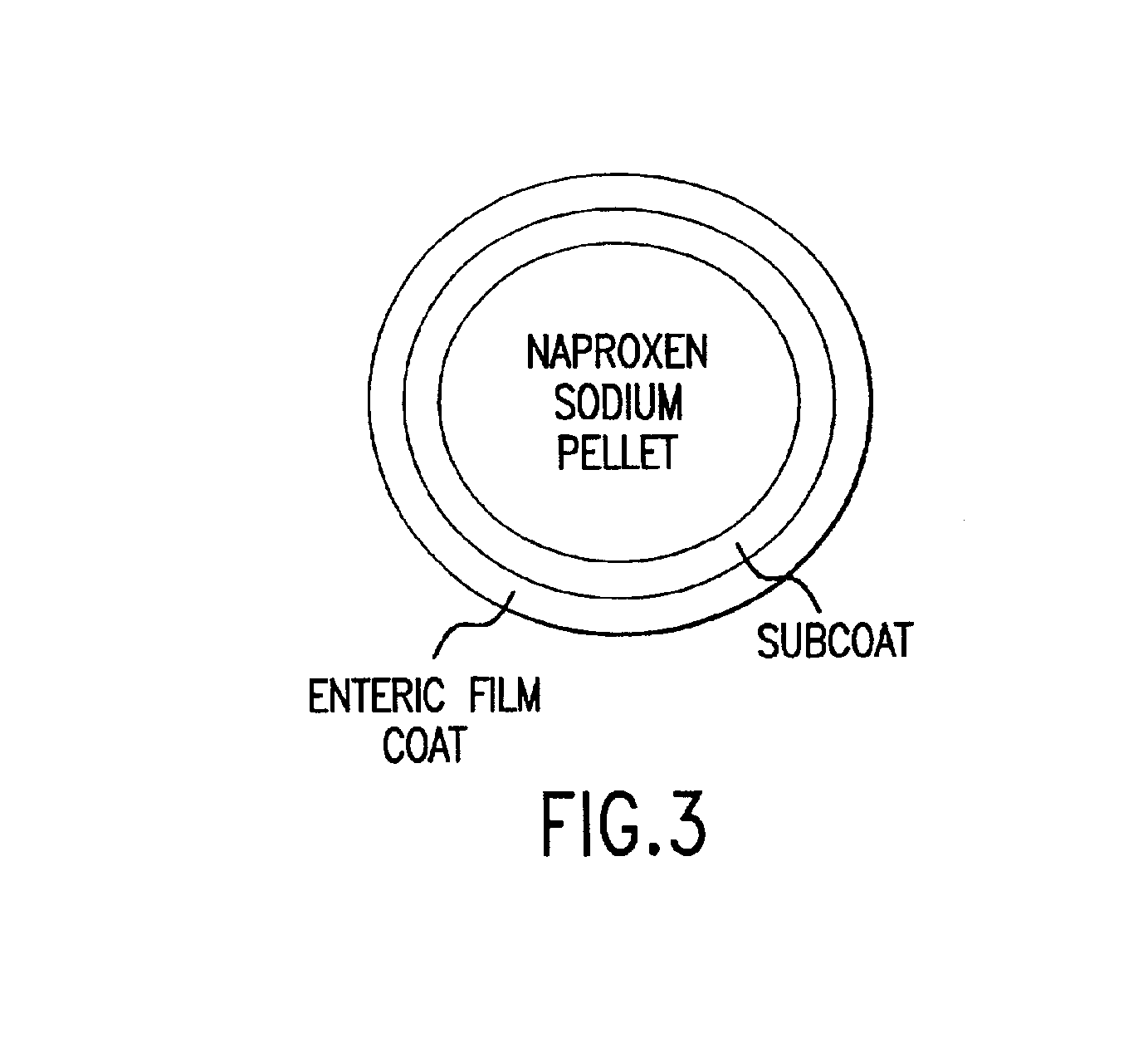
Pharmaceutical compositions for the coordinated delivery of NSAIDs
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO
Oral devices and methods for controlled drug release
Drug dosage forms, which are housed in oral devices, and methods for controlled drug release are provided. The oral devices are permanently or removably inserted in the oral cavity and refilled or replaced as needed. The controlled drug release may be passive, based on the dosage form, or electronically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled release may be any one of the following: release in accordance with a preprogrammed schedule, release at a controlled rate, delayed release, pulsatile release, chronotherapeutic release, closed-loop release, responsive to a sensor's input, release on demand from a personal extracorporeal system, release in accordance with a schedule specified by a personal extracorporeal system, release on demand from a monitoring center, via a personal extracorporeal system, and release in accordance with a schedule specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted by an electrotransport mechanism. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen.
Owner:WOLFAF ANDY +1
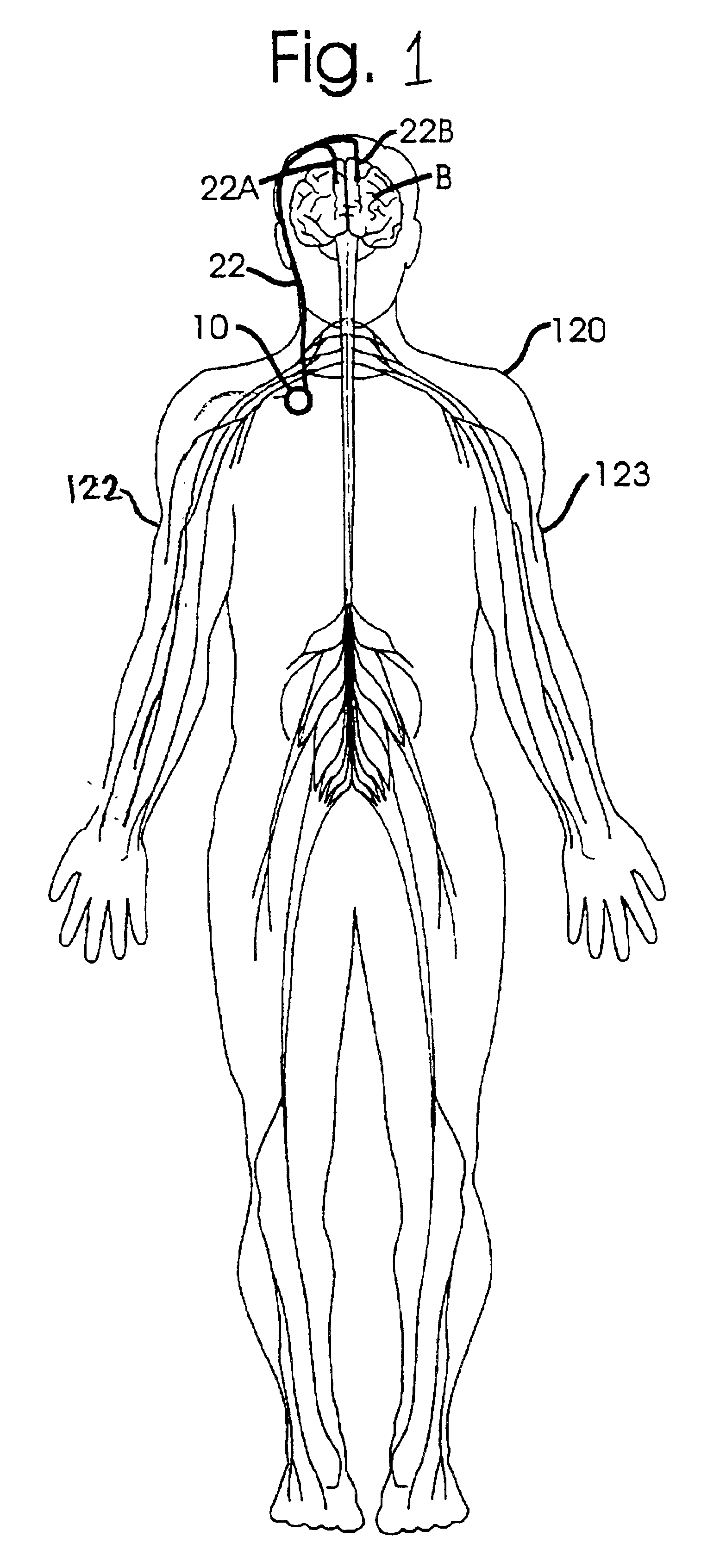
Therapeutic treatment of disorders based on timing information
Disclosed are techniques for operation of neurostimulation or drug delivery devices to stop treatment therapy during times when the patient does not need to be treated. Advantageously, the present invention reduces battery usage and / or drug dosage during periods when treatment therapy need not be provided. Further, the present invention slows or reduces the tolerance the patient may develop from the electrical stimulation or treatment therapy. In one embodiment, the present invention includes a timer or a real time clock for shutting off the device during periods when the patient is sleeping in accordance with a preset schedule. The present invention preferably turns off after the patient has fallen asleep and right before the patient has awakened. Alternatively, the invention may include a sensor for sensing conditions indicative of whether the patient is awake or asleep. This sensed information may also be used to determine whether the treatment therapy should be delivered or stopped.
Owner:MEDTRONIC INC
System and method for therapeutic drug monitoring
InactiveUS20050054942A1Accurate assessmentCost-effective and frequentNervous disorderElectrotherapyNoseEnvironmental health
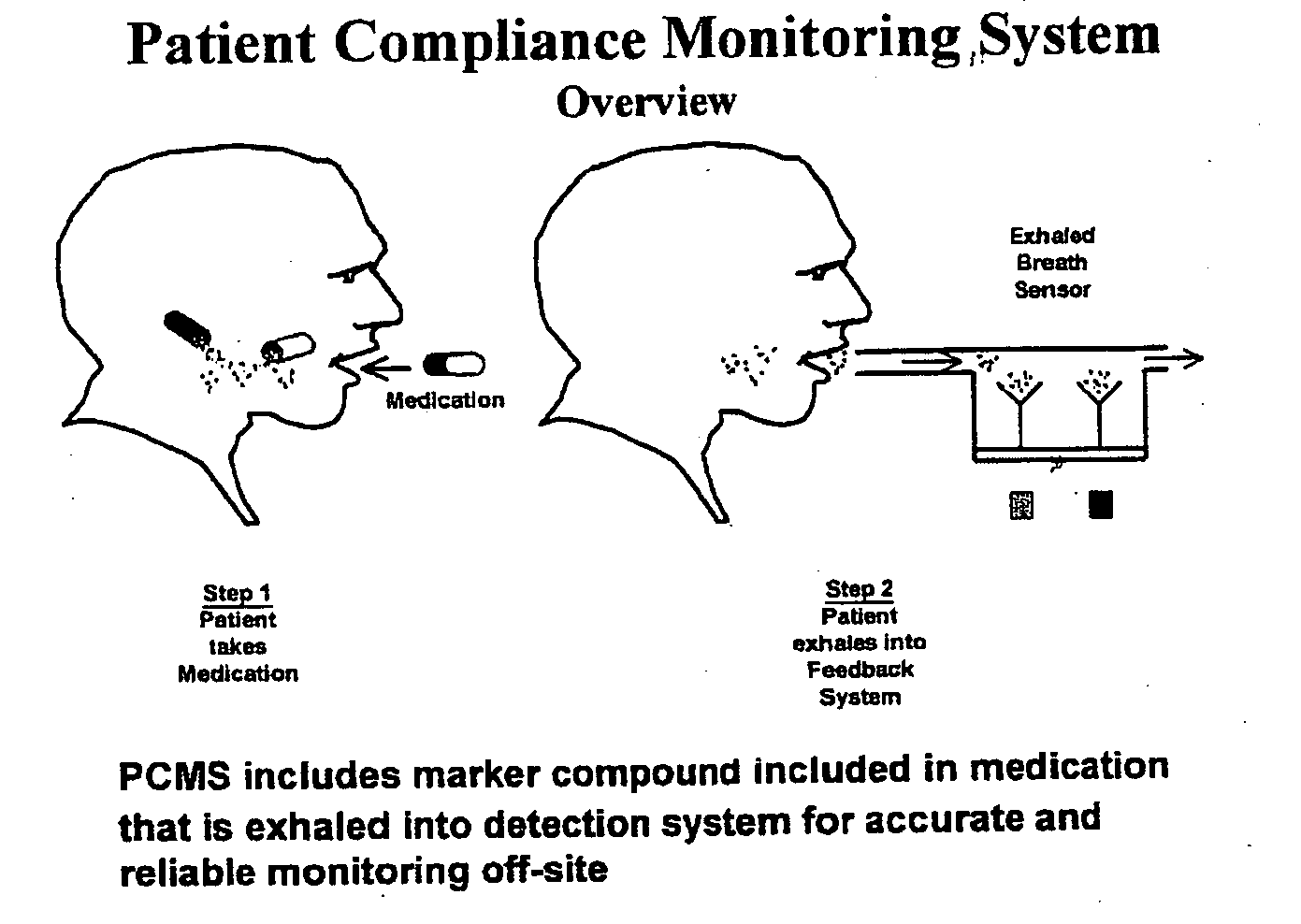
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA
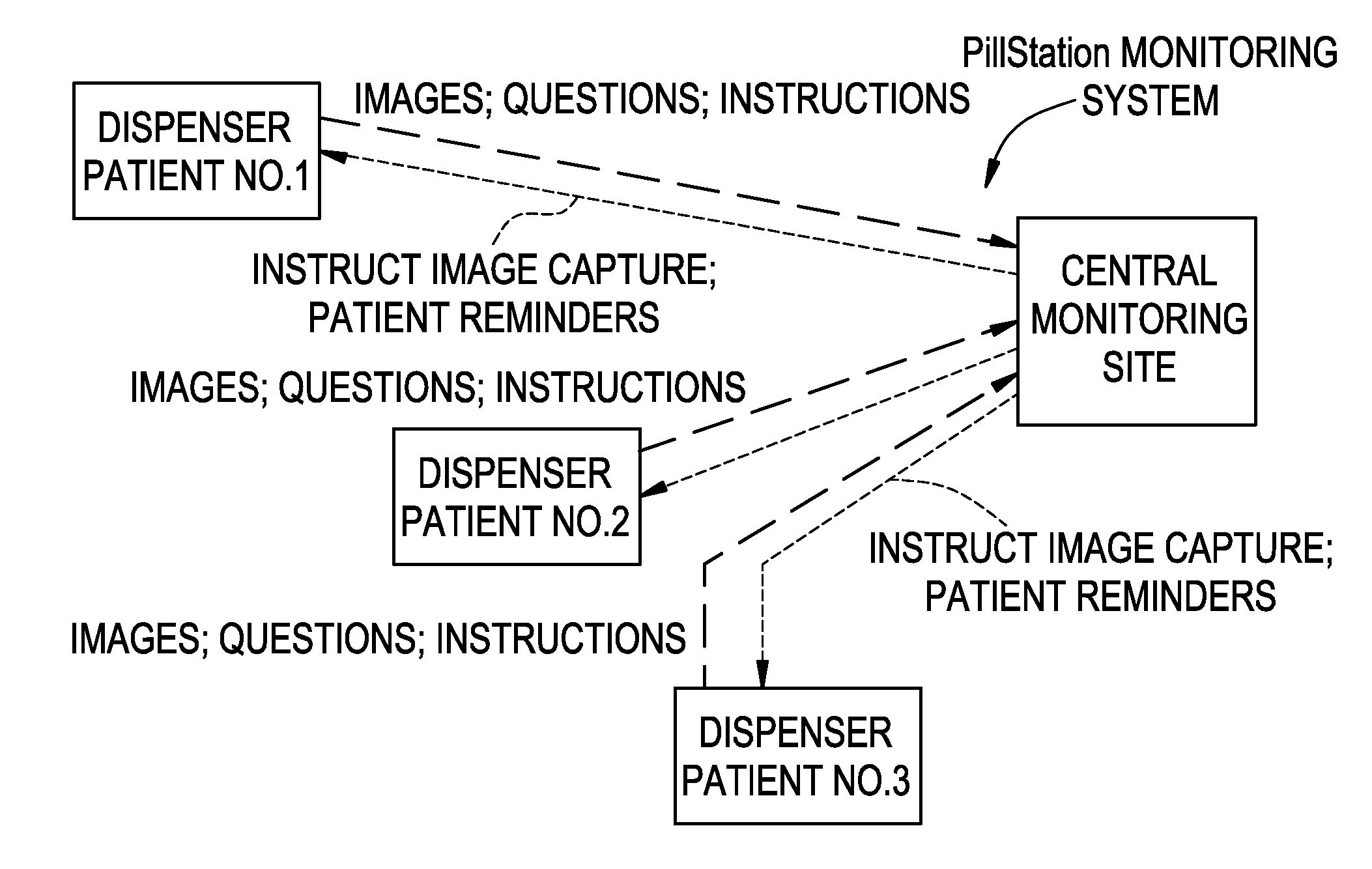
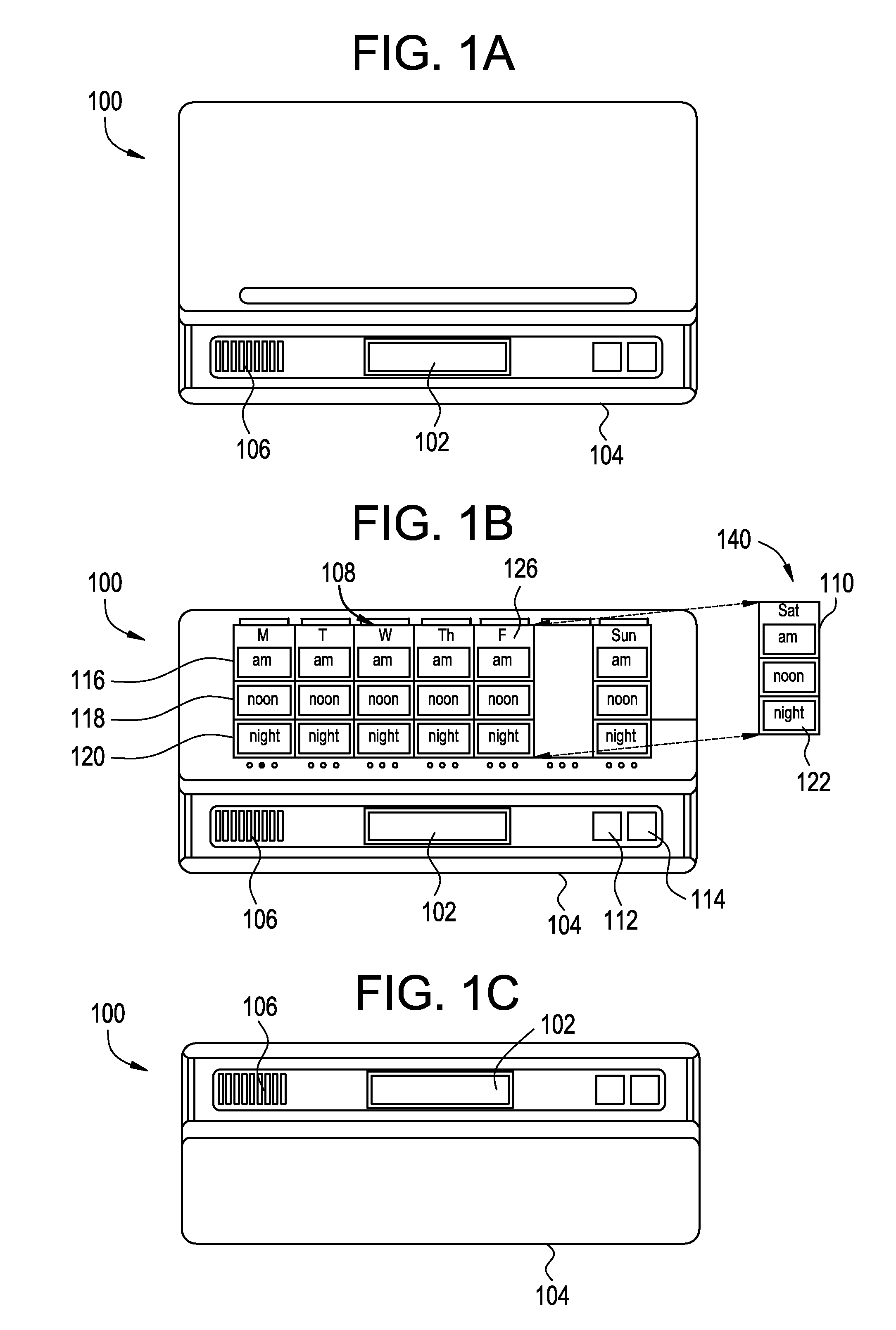
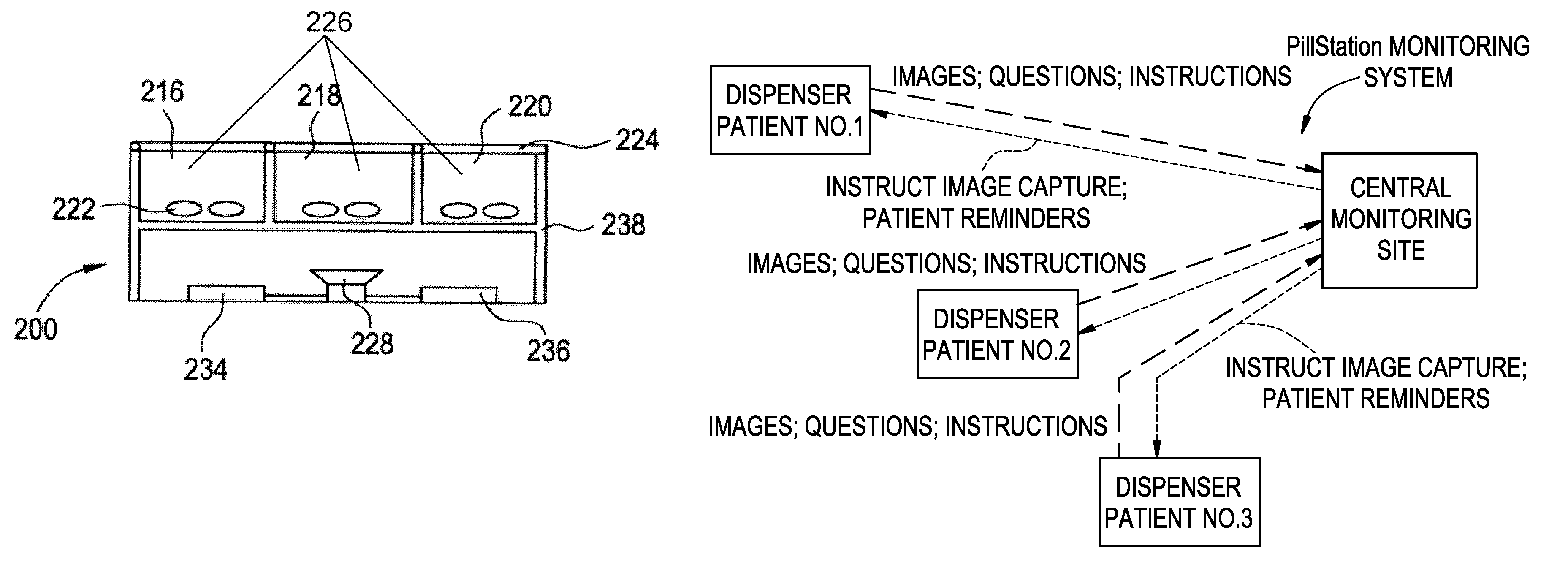
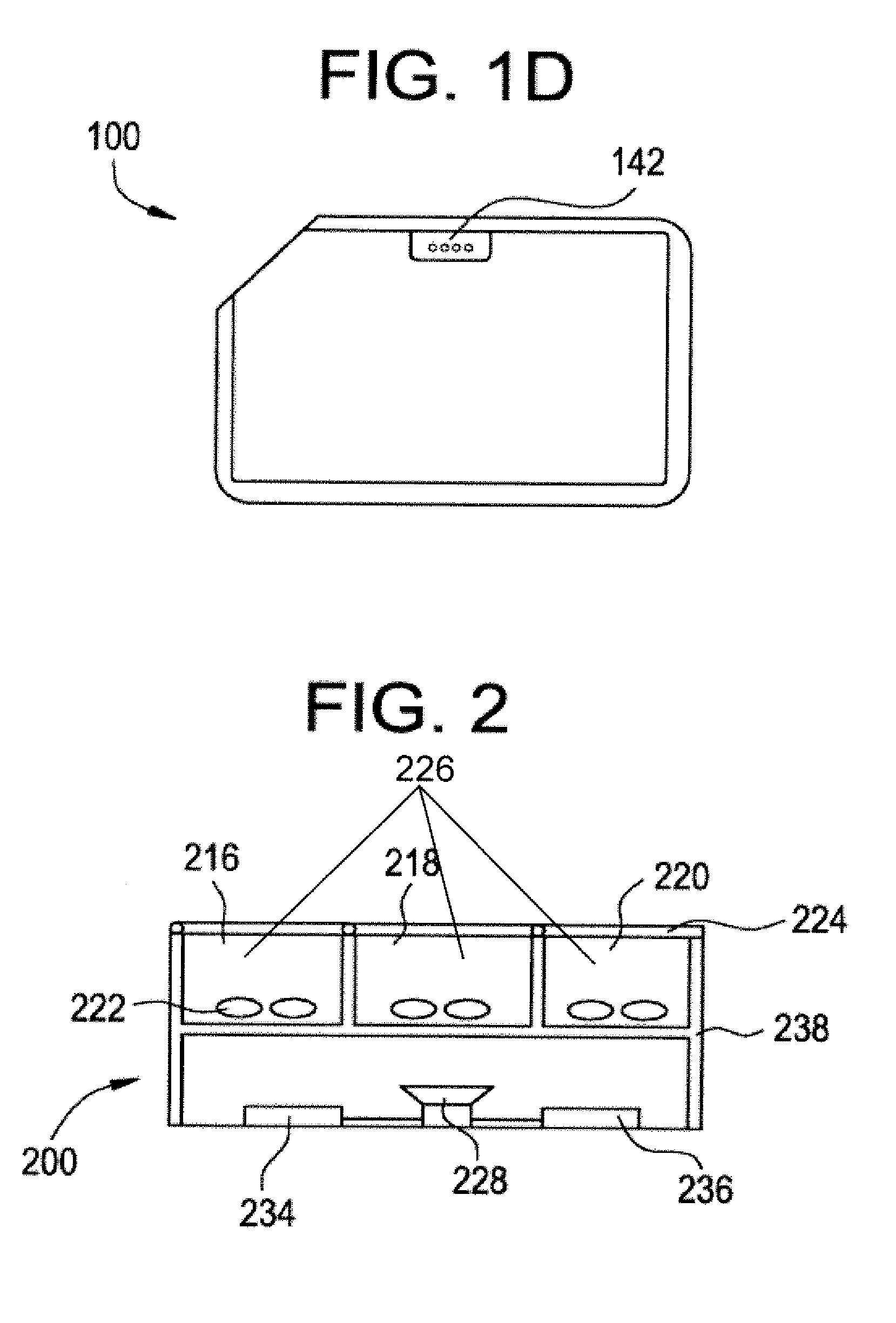
Medication Dispenser with Integrated Monitoring System
ActiveUS20080119958A1Drug and medicationsPharmaceutical containersInterior spaceMedication Dispenser
Devices, systems, and methods are provided for remote visualization of the storage compartments in a medication dispenser device, to monitor a patient's compliance with a medication dosage schedule and for verifying the proper loading of medication into the patient's medication dispenser device. The device may include a plurality of storage compartments, each having an interior space for storing at least one medication or medication reminder marker; an image capturing device (e.g., a camera) positionable to capture an image of the interior space of each storage compartment; and a communications module for electronically transmitting the captured image to a central monitoring station.
Owner:RXADVANCE
System and method for improving compliance of a medical regimen
InactiveUS6305377B1Reduce violationsBetter addressData processing applicationsDrug and medicationsRegimenElectronic communication
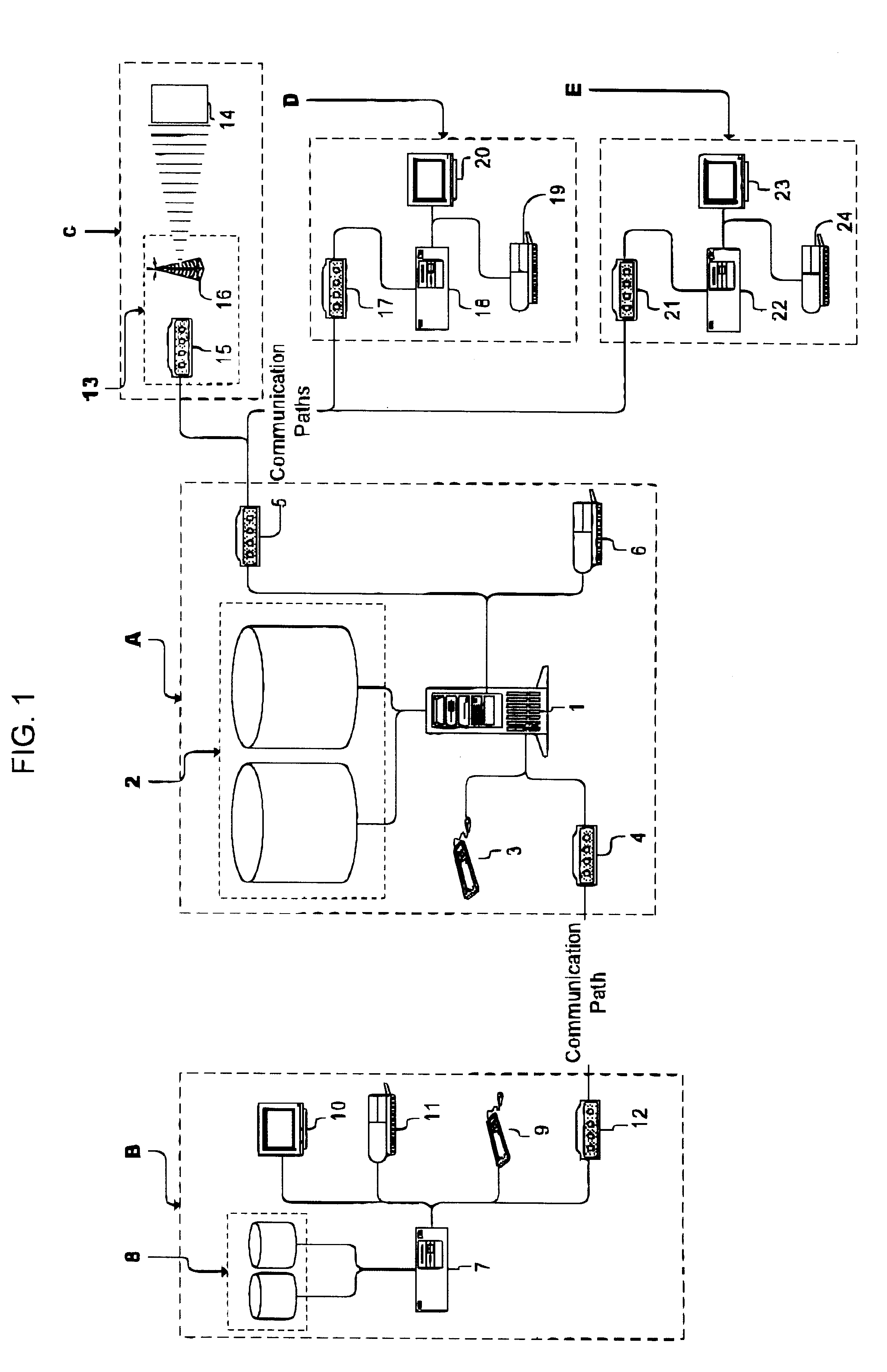
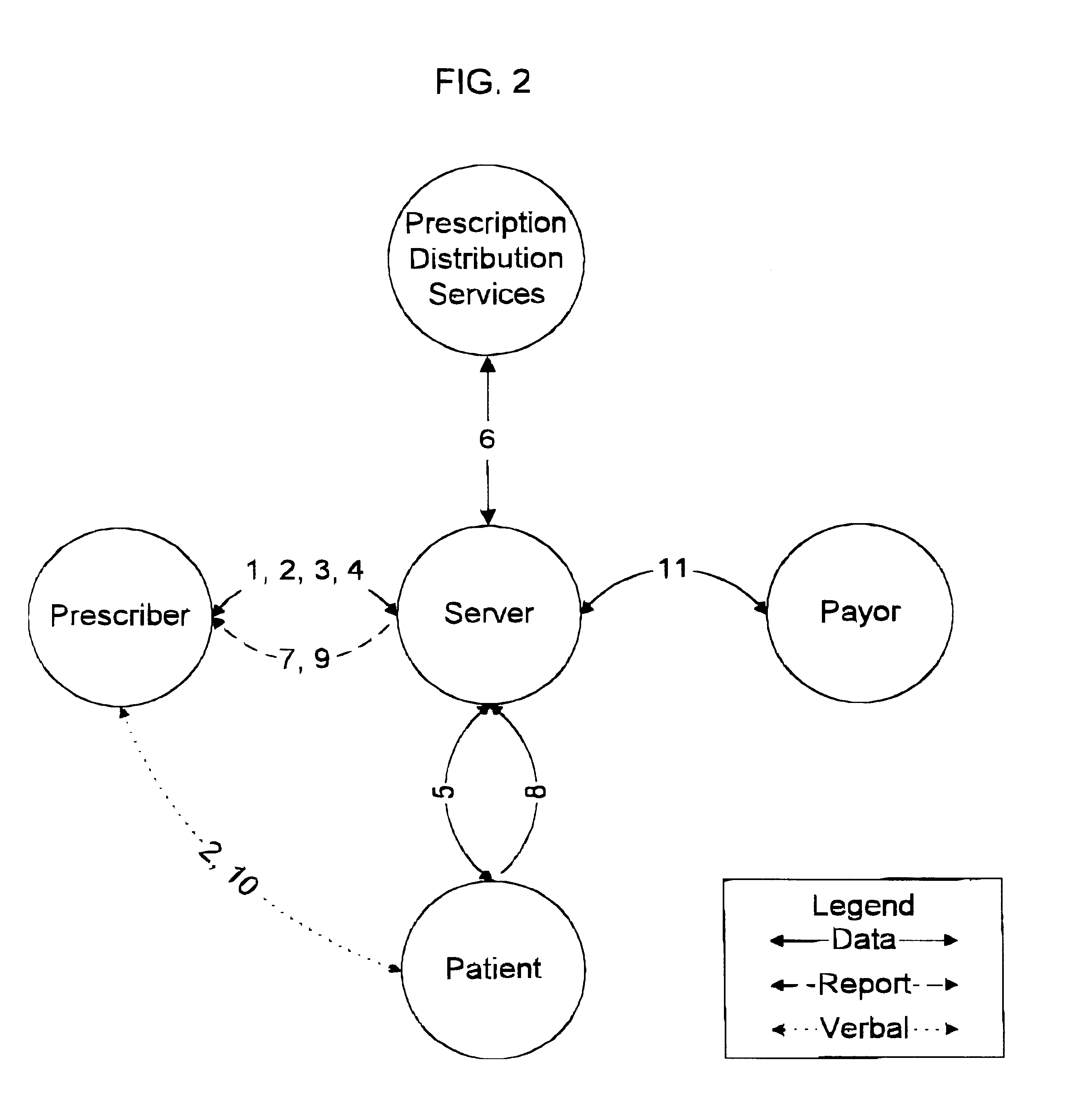
A system and method for improving compliance by a patient with a medical regimen that has been prescribed to the patient wherein utilizing computer and electronic communication systems, patient data is compared to pharmaceutical data to verify prescribed drug dosage and prescribed medication duration are within acceptable limits, any abnormalities found by the comparisons are reported to the treating physician who may then alter the medical regimen before authorizing dispensing of the prescribed drugs and providing drug taking information to the patient, and upon authorization being issued, the patient is scheduled to receive reminder notifications prior to the prescribed time for the prescribed drugs to be administered, as well as automatically notifying the prescription distribution service to deliver the prescribed drugs to the patient, and notifying the payor service to pay for the prescribed drugs.
Owner:PORTWOOD MICHAEL T +1
Dual drug dosage forms with improved separation of drugs
InactiveUS20050013863A1Reduces and prevents any migrationReduce penetrationCoatingsBlood disorderMedicineImmediate release
Drug tablets that include a prolonged-release core and an immediate-release layer or shell are prepared with a thin barrier layer of drug-free polymer between the prolonged-release and immediate-release portions of the tablet. The barrier layer is penetrable by gastrointestinal fluid, thereby providing full access of the gastrointestinal fluid to the prolonged-release core, but remains intact during the application of the immediate-release layer, substantially reducing or eliminating any penetration of the immediate-release drug into the prolonged-release portion.
Owner:DEPOMED SYST INC
Marker detection method and apparatus to monitor drug compliance
InactiveUS20050233459A1Accurate assessmentPatient complianceDiagnostic recording/measuringSensorsNoseEnvironmental health
The present invention includes systems and methods for monitoring therapeutic drug concentration in blood by detecting markers, such as odors, upon exhalation by a patient after the drug is taken, wherein such markers result either directly from the drug itself or from an additive combined with the drug. In the case of olfactory markers, the invention preferably utilizes electronic sensor technology, such as the commercial devices referred to as “artificial” or “electronic” noses or tongues, to non-invasively monitor drug levels in blood. The invention further includes a reporting system capable of tracking drug concentrations in blood (remote or proximate locations) and providing the necessary alerts with regarding to ineffective or toxic drug dosages in a patient.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Medication dispenser with integrated monitoring system
Devices, systems, and methods are provided for remote visualization of the storage compartments in a medication dispenser device, to monitor a patient's compliance with a medication dosage schedule and for verifying the proper loading of medication into the patient's medication dispenser device. The device may include a plurality of storage compartments, each having an interior space for storing at least one medication or medication reminder marker; an image capturing device (e.g., a camera) positionable to capture an image of the interior space of each storage compartment; and a communications module for electronically transmitting the captured image to a central monitoring station.
Owner:RXADVANCE
Method and system for modulating energy expenditure and neurotrophic factors
InactiveUS20080046012A1Symptoms improvedImprove performanceElectrotherapyImplantable ElectrodesRegimen
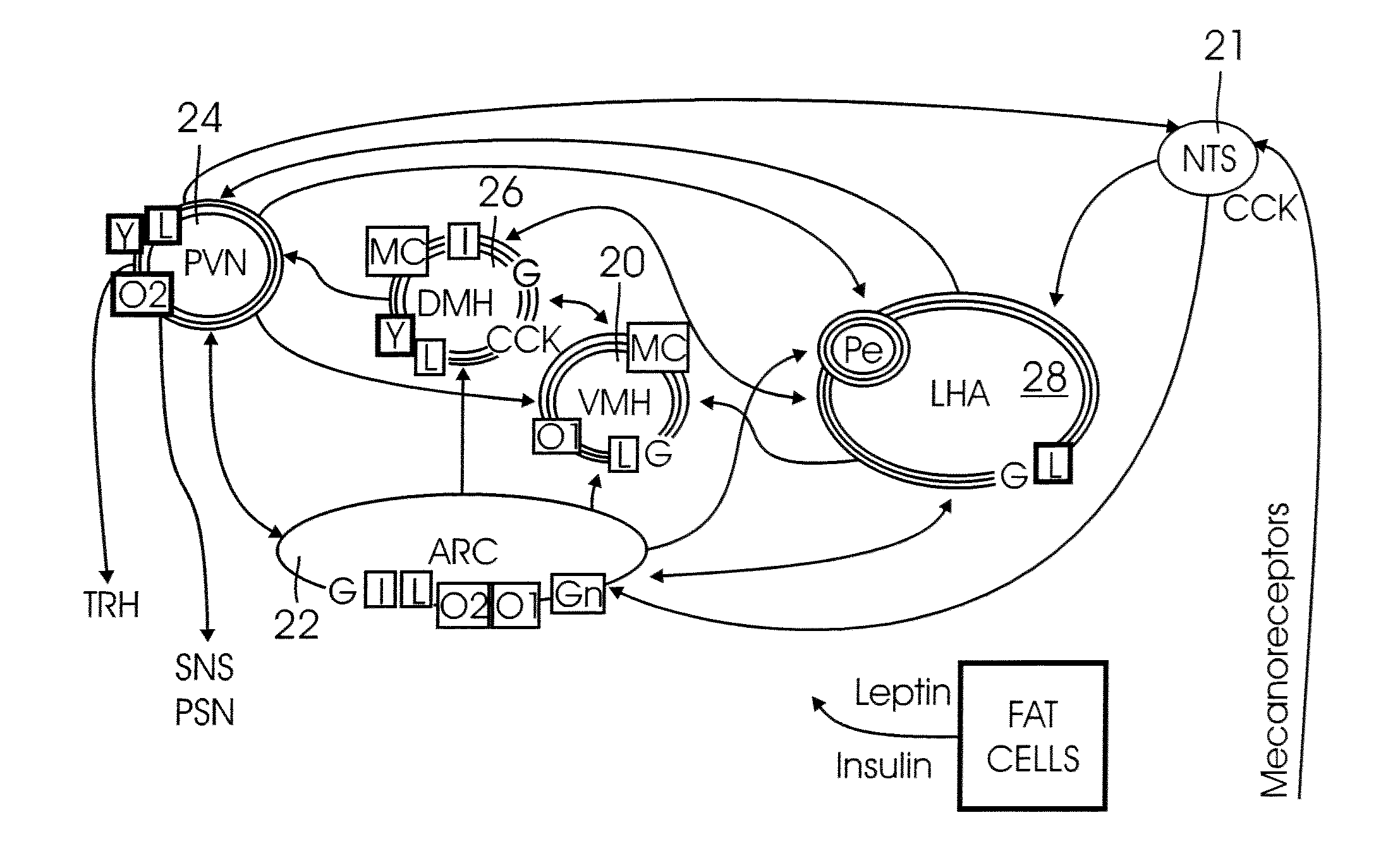
A method system for modulating the energy expenditure and / or the expressed brain-derived neurotrophic factor (BDNF) in the brain of an individual is performed by a system that includes a control device that generates a stimulation pattern from a predetermined set of stimulation parameters, and that converts the stimulation pattern into a stimulation signal. A stimulation signal delivery mechanism, configured for implantation into a selected part of the brain, receives the stimulation signal from the control device and delivers the signal to the selected part of the brain. The stimulation signal may be an electrical signal delivered by a brain-implantable electrode, or a chemical signal in the form of a drug dosage regimen delivered by an implantable micropump under the control of the control device. Modulation of the energy expenditure and / or BDNF is achieved by the stimulation of the hypothalamus, either directly or indirectly, by the stimulation signal.
Owner:RGT UNIV OF CALIFORNIA
Suspending vehicles and pharmaceutical suspensions for drug dosage forms
InactiveUS20060216242A1Process stabilityPeptide/protein ingredientsAerosol deliverySUSPENDING VEHICLESolvent
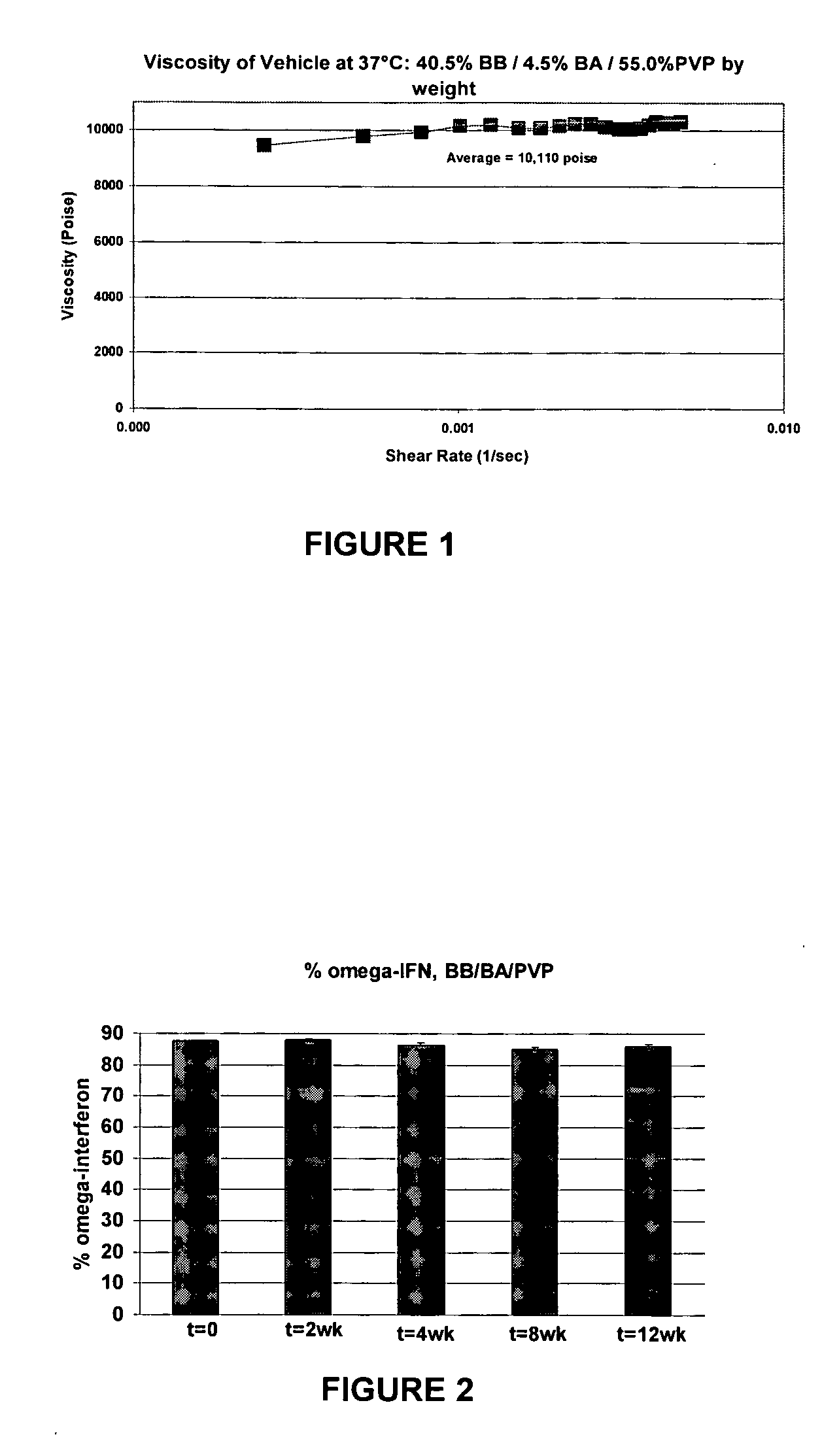
Suspending vehicles and pharmaceutical suspensions that include a biocompatible polymer that can be combined with a hydrophobic solvent and a hydrophilic solvent to provide vehicles and suspensions that are substantially free of stiff gels upon contact with an aqueous medium are provided. Vehicles and suspensions remain flowable out of a pump-driven dosage form over the life of the dosage form. Such vehicles and suspensions are also biocompatible, suitable for creating and maintaining drug suspensions, and capable of providing stable drug formulations.
Owner:DURECT CORP
Methods and apparatus for delivering a drug influencing appetite for treatment of eating disorders
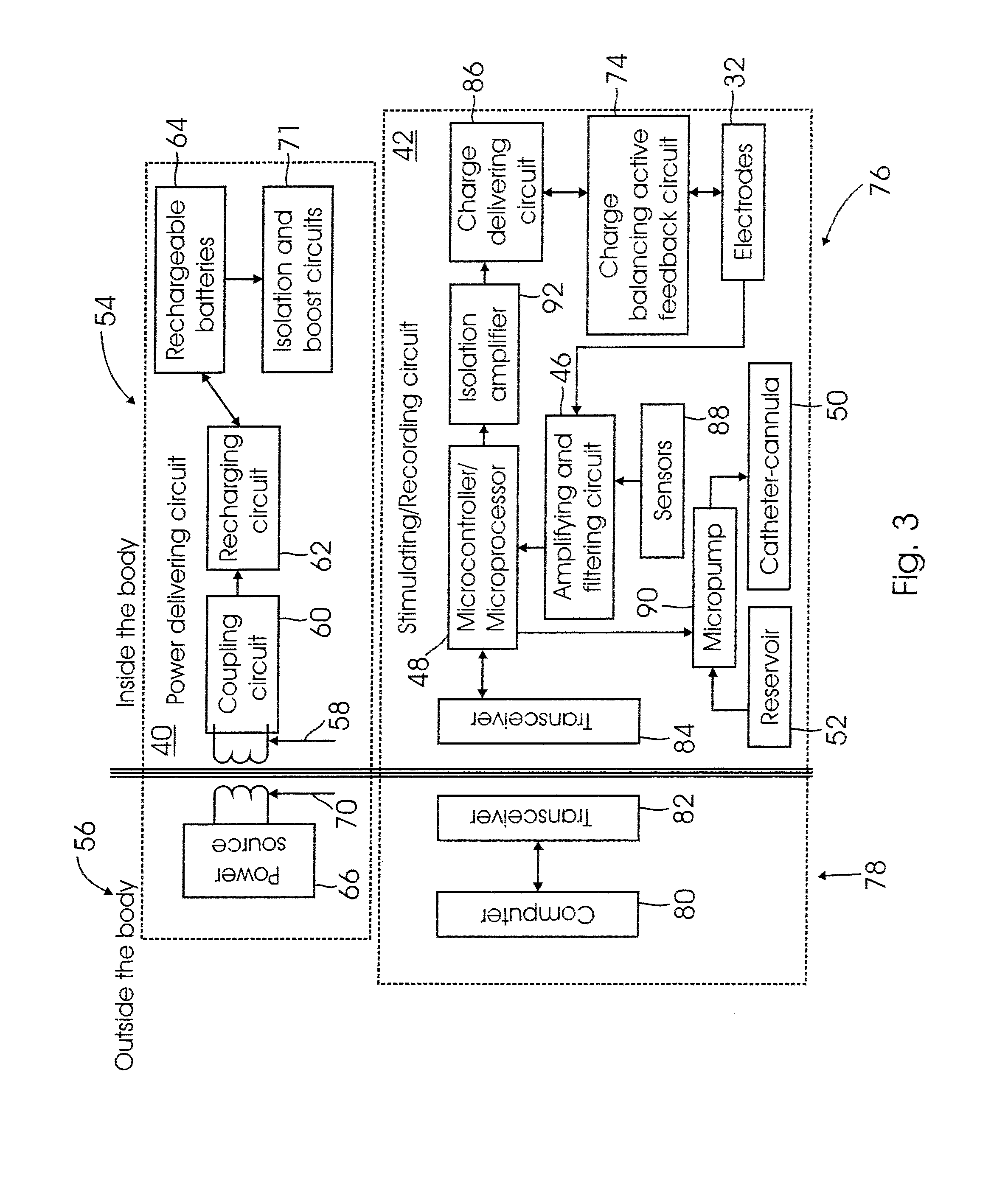
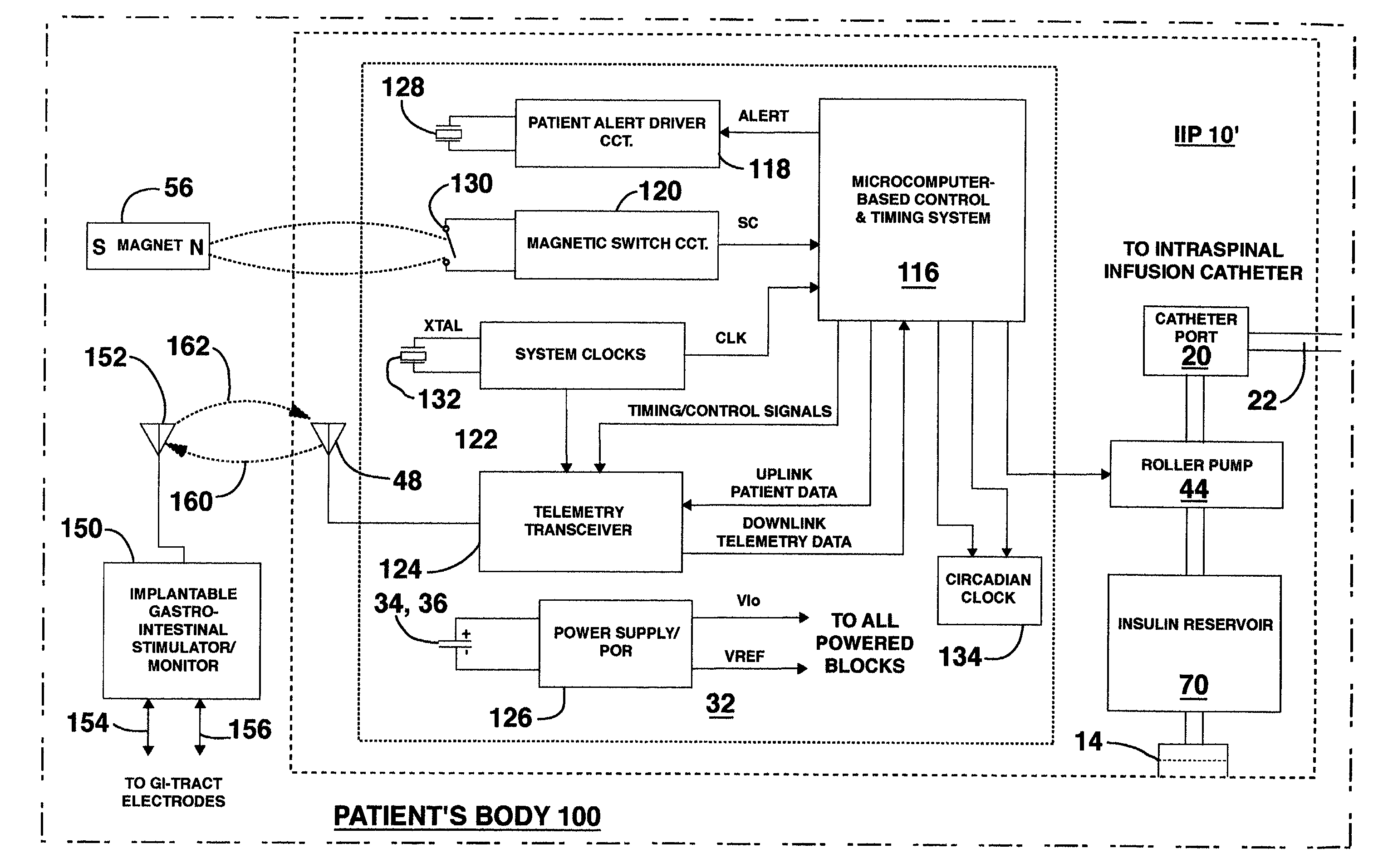
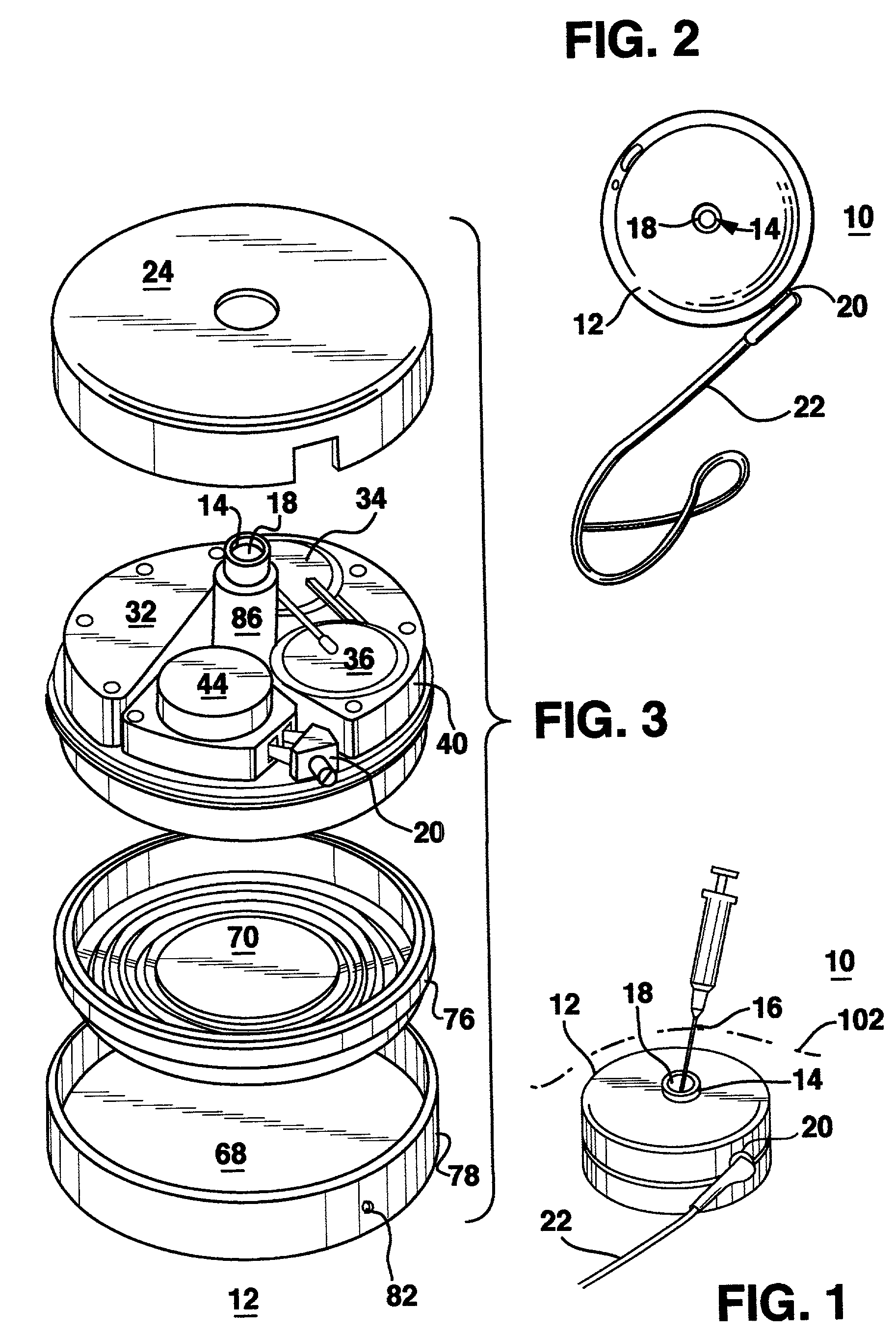
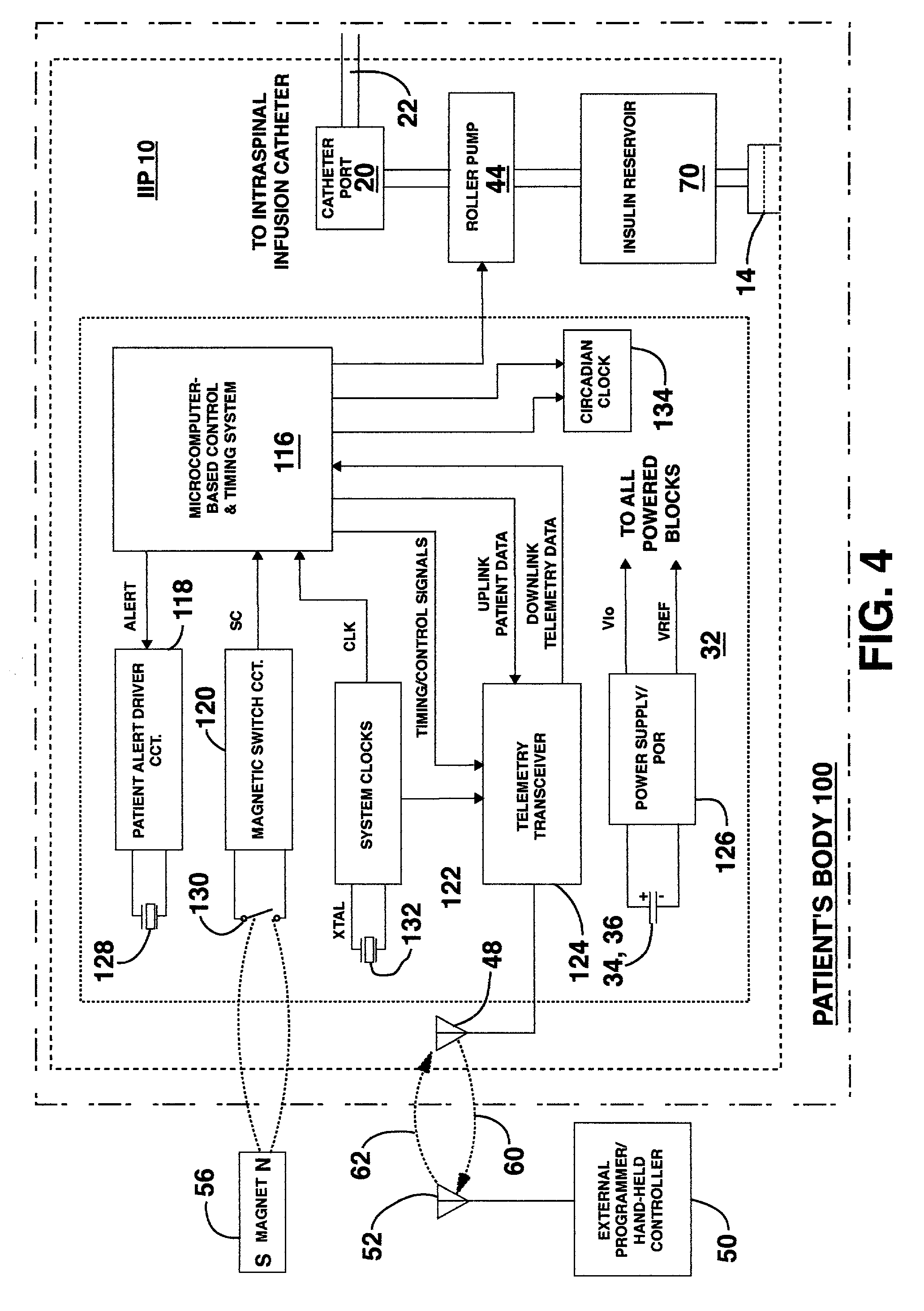
Methods and systems for treating patients suffering from eating disorders, e.g. obesity, through the dispensation of a drug by an implantable infusion pump (IIP) delivering drug into the cerebral spinal fluid (CSF) at a site of the intrathecal space in amounts and at times effective to suppress the patient's appetite through interaction of the drug transported through the CSF with receptors in the brain. Delivery of a programmed drug dosage is preferably by one of time-out of programmed time(s) of day, a command received from the patient, or a trigger signal developed from a sensed GI tract signal accompanying peristalsis.
Owner:MEDTRONIC INC
Drug delivery apparatus and method for automatically reducing drug dosage
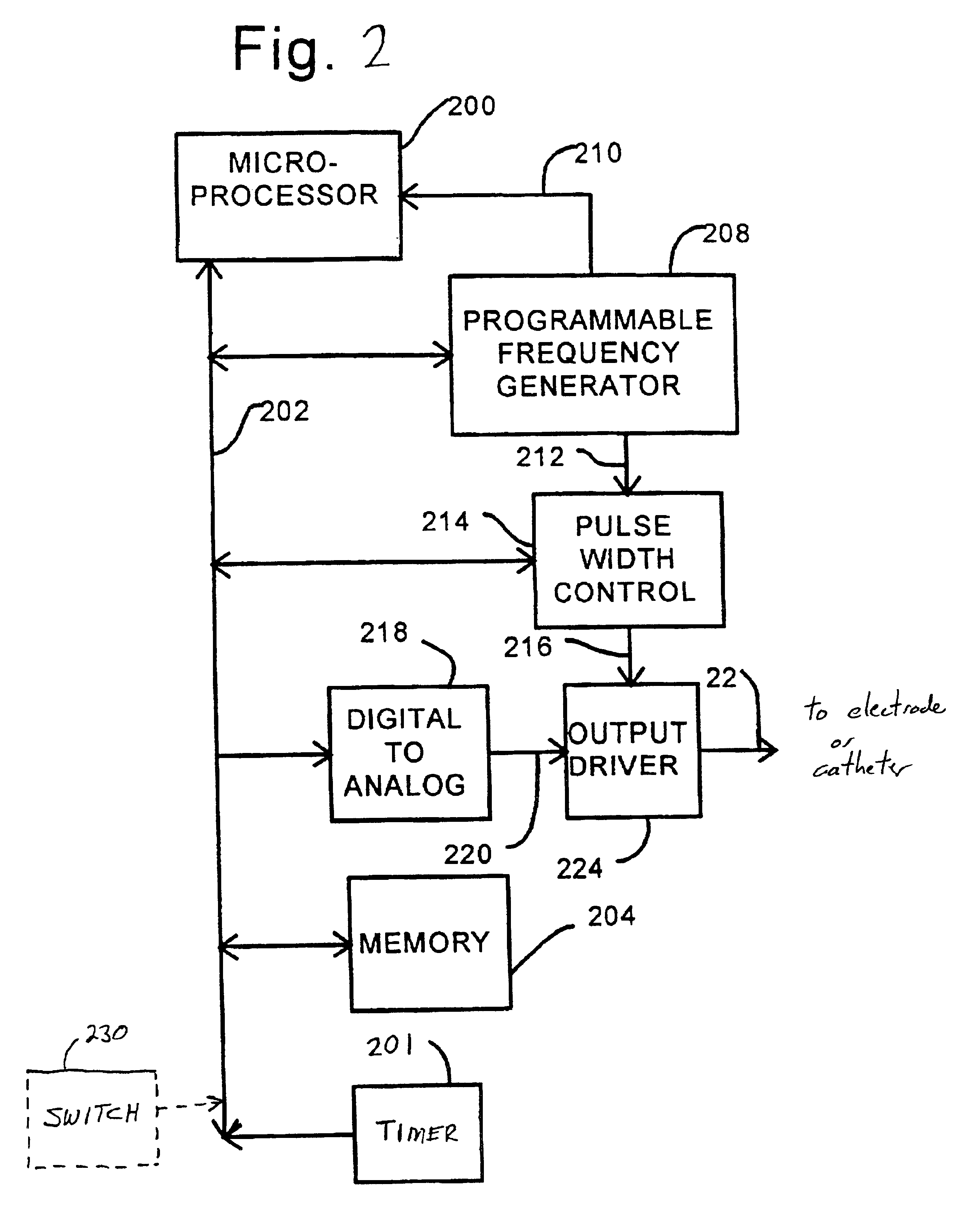
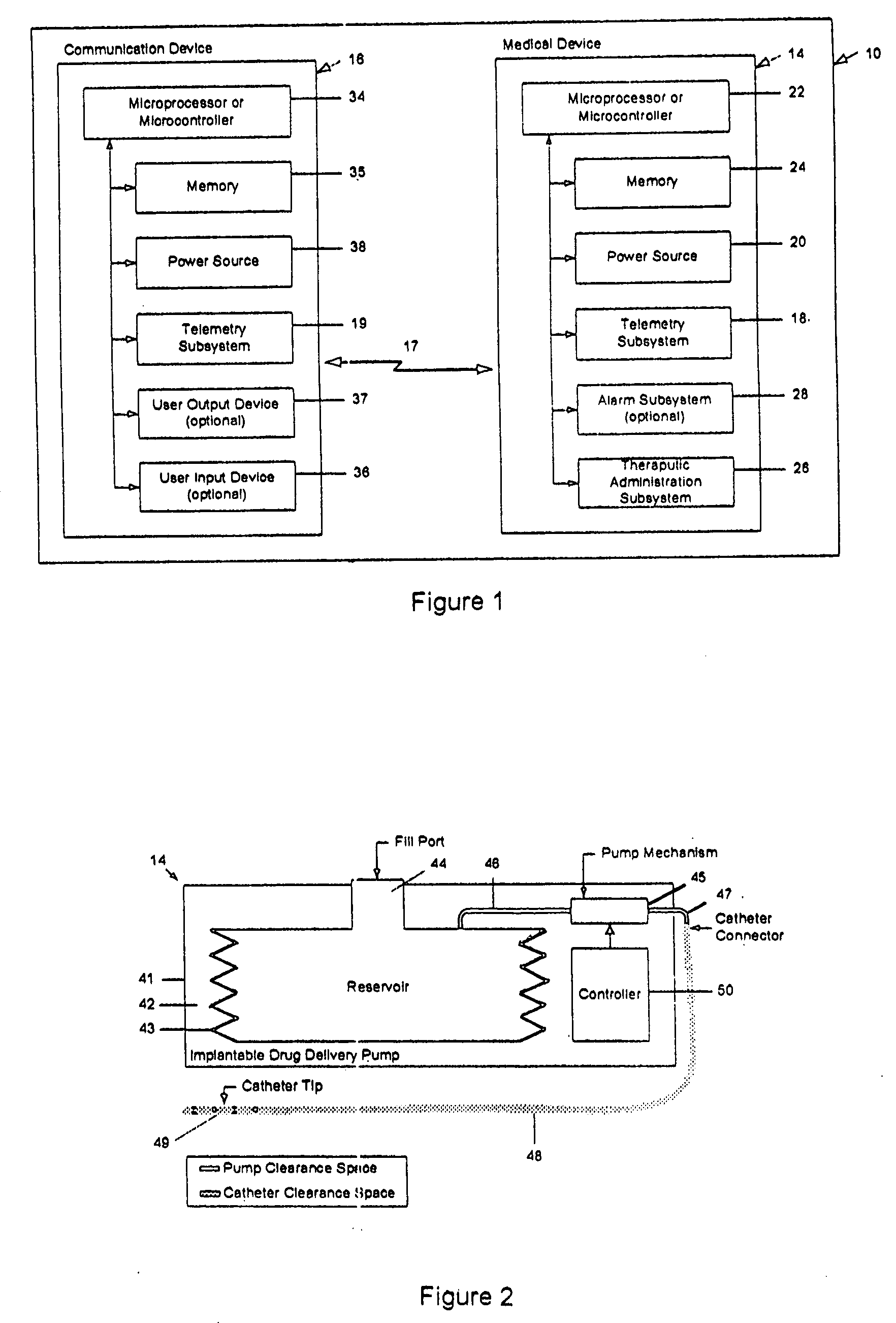
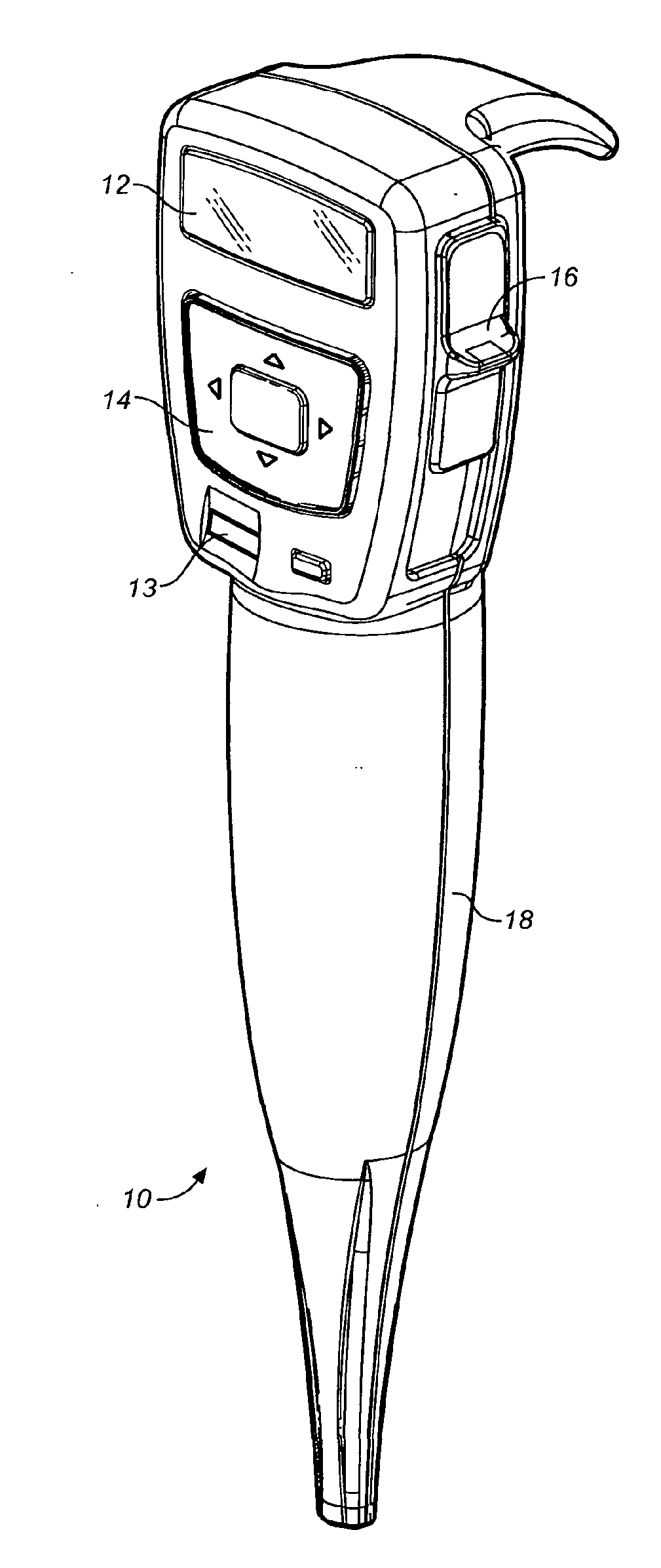
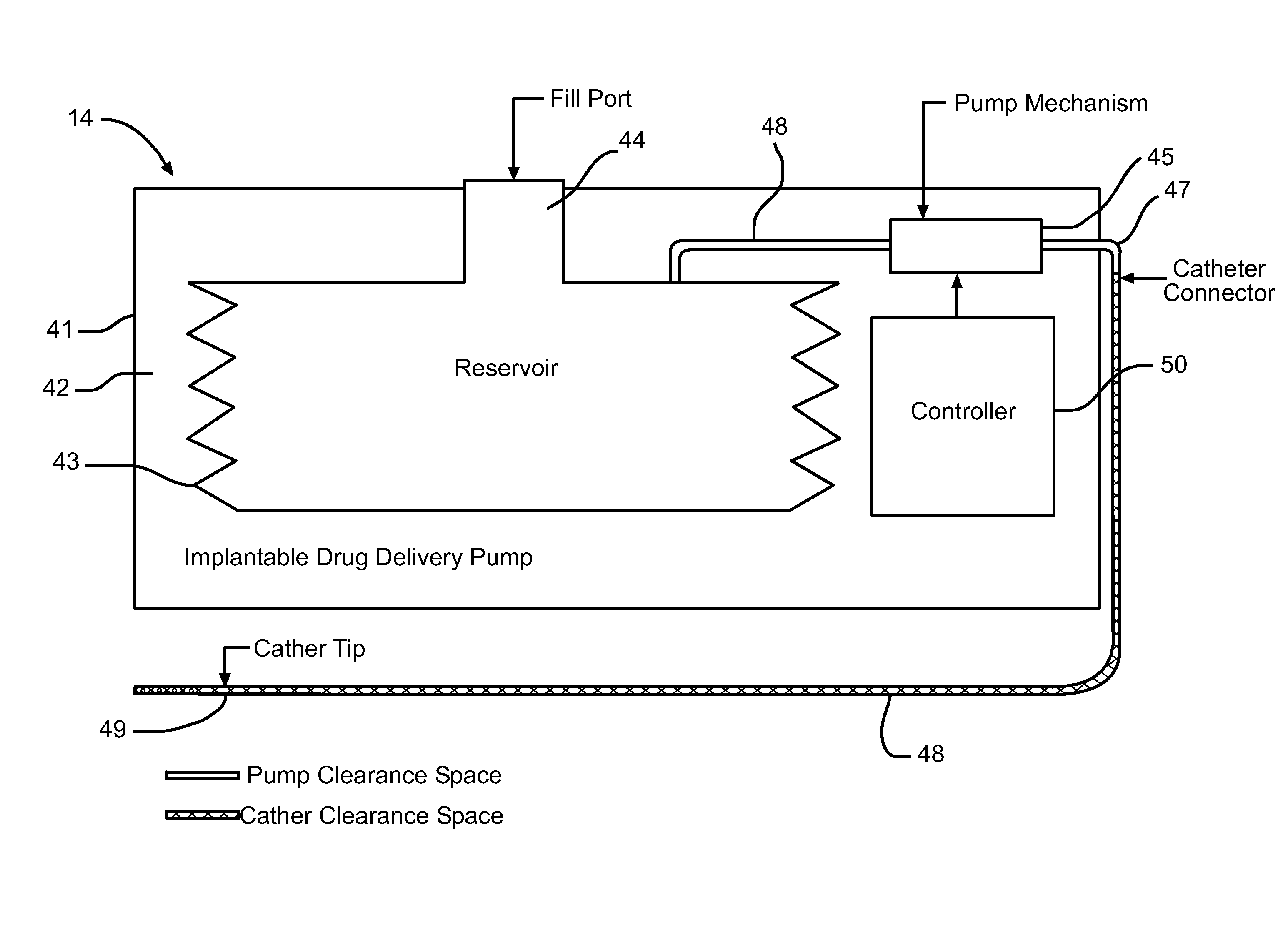
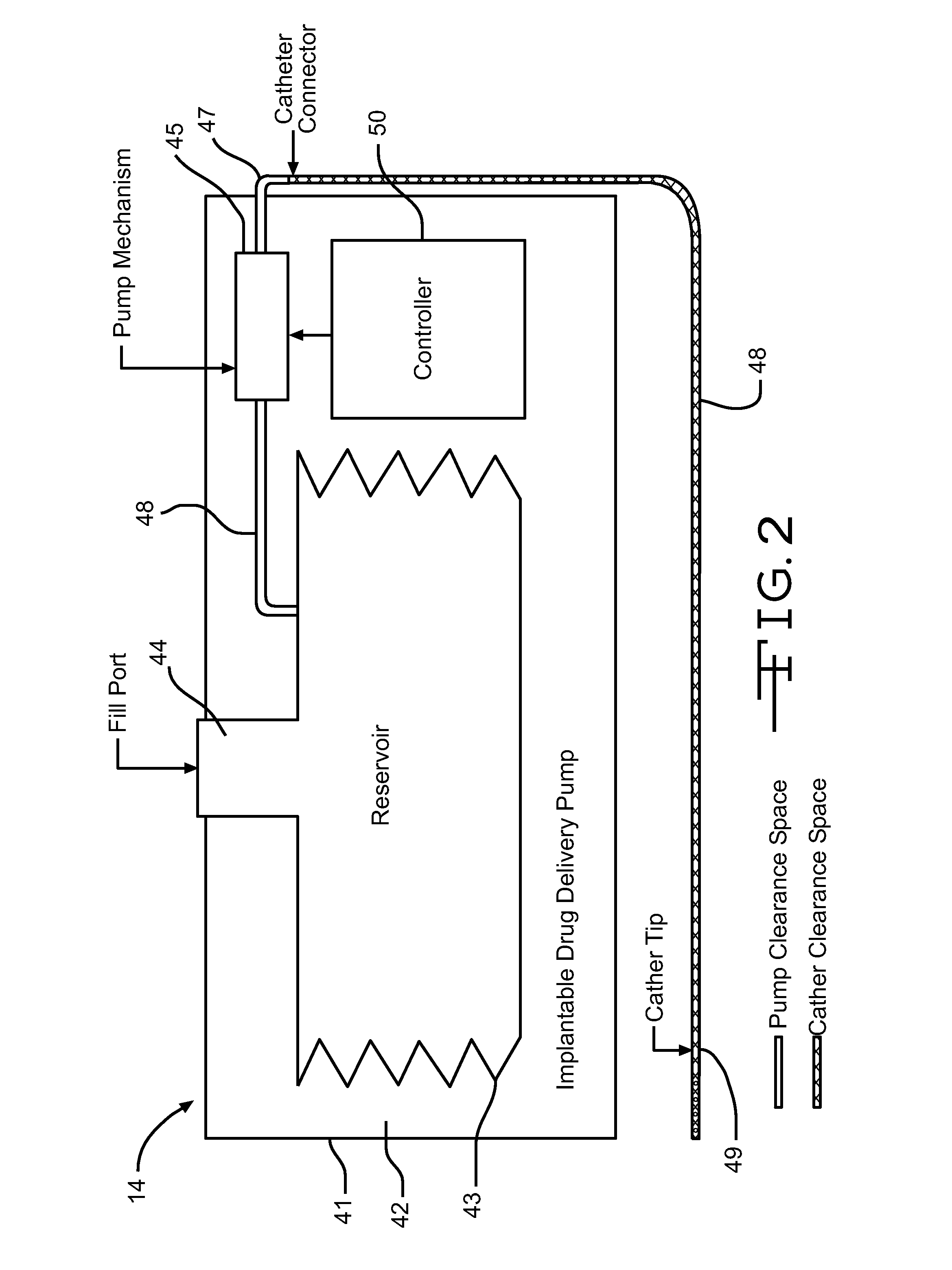
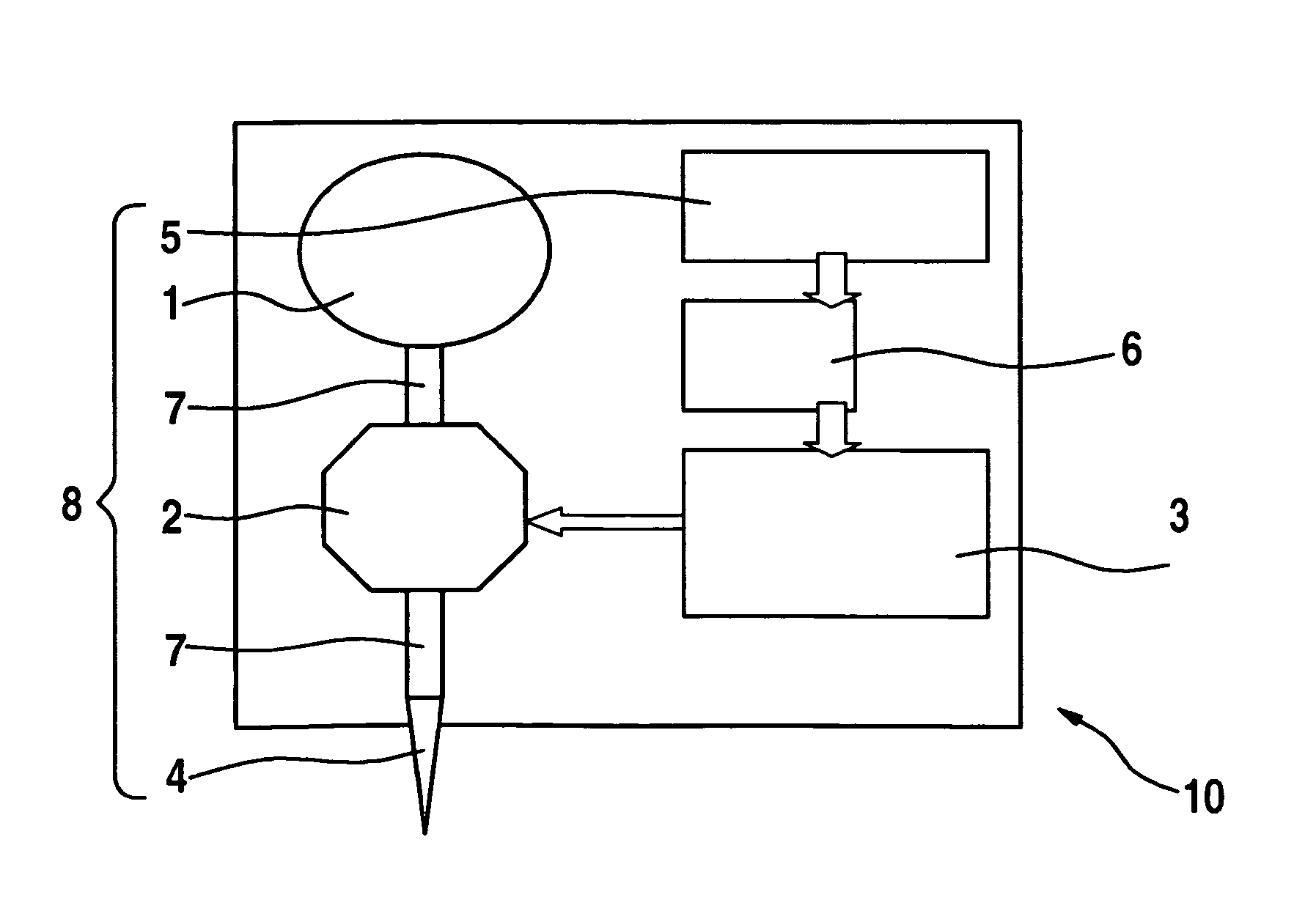
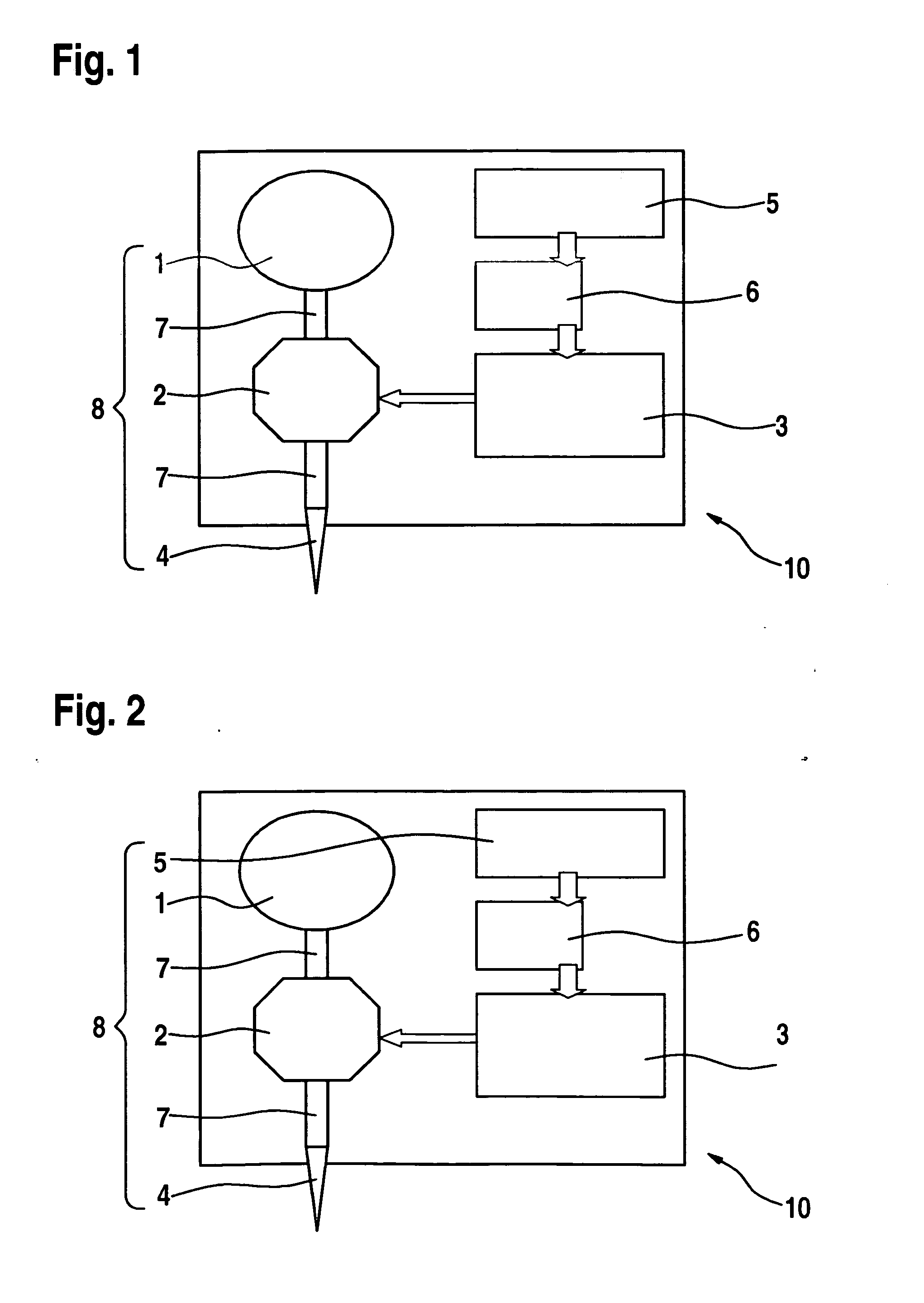
InactiveUS20060047270A1Reduce probabilityMedical devicesPressure infusionMicrocontrollerDrug reservoir
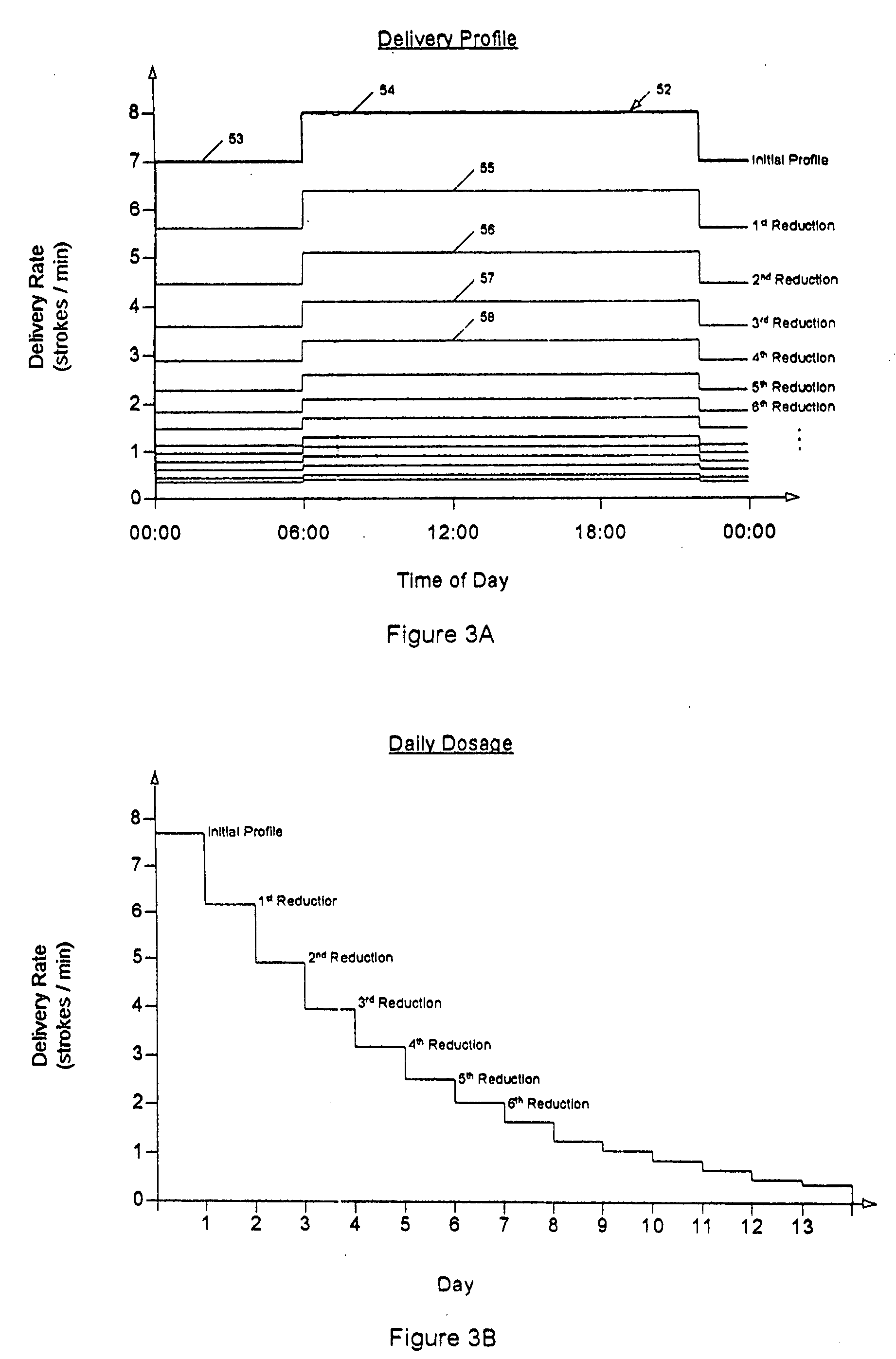
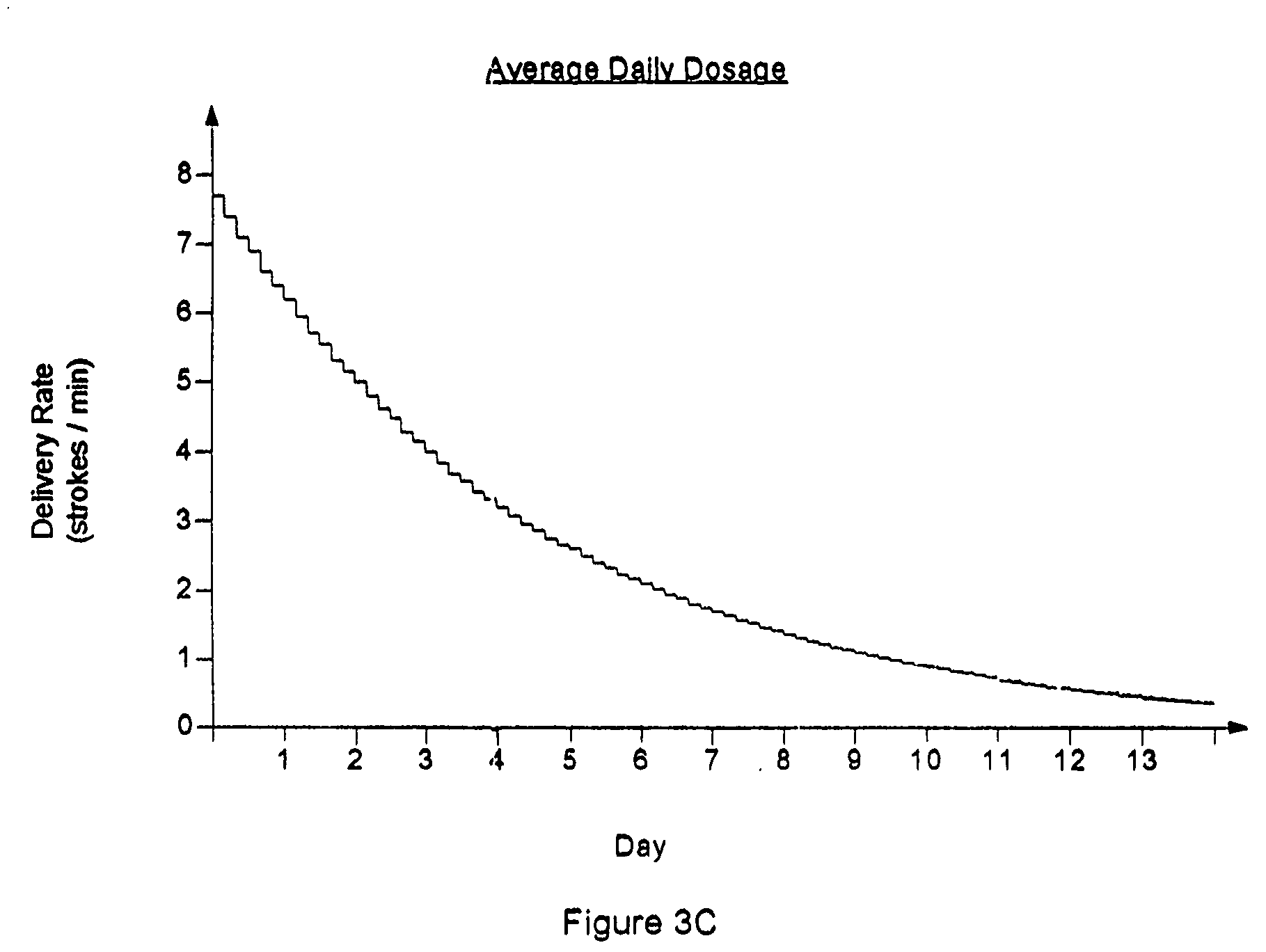
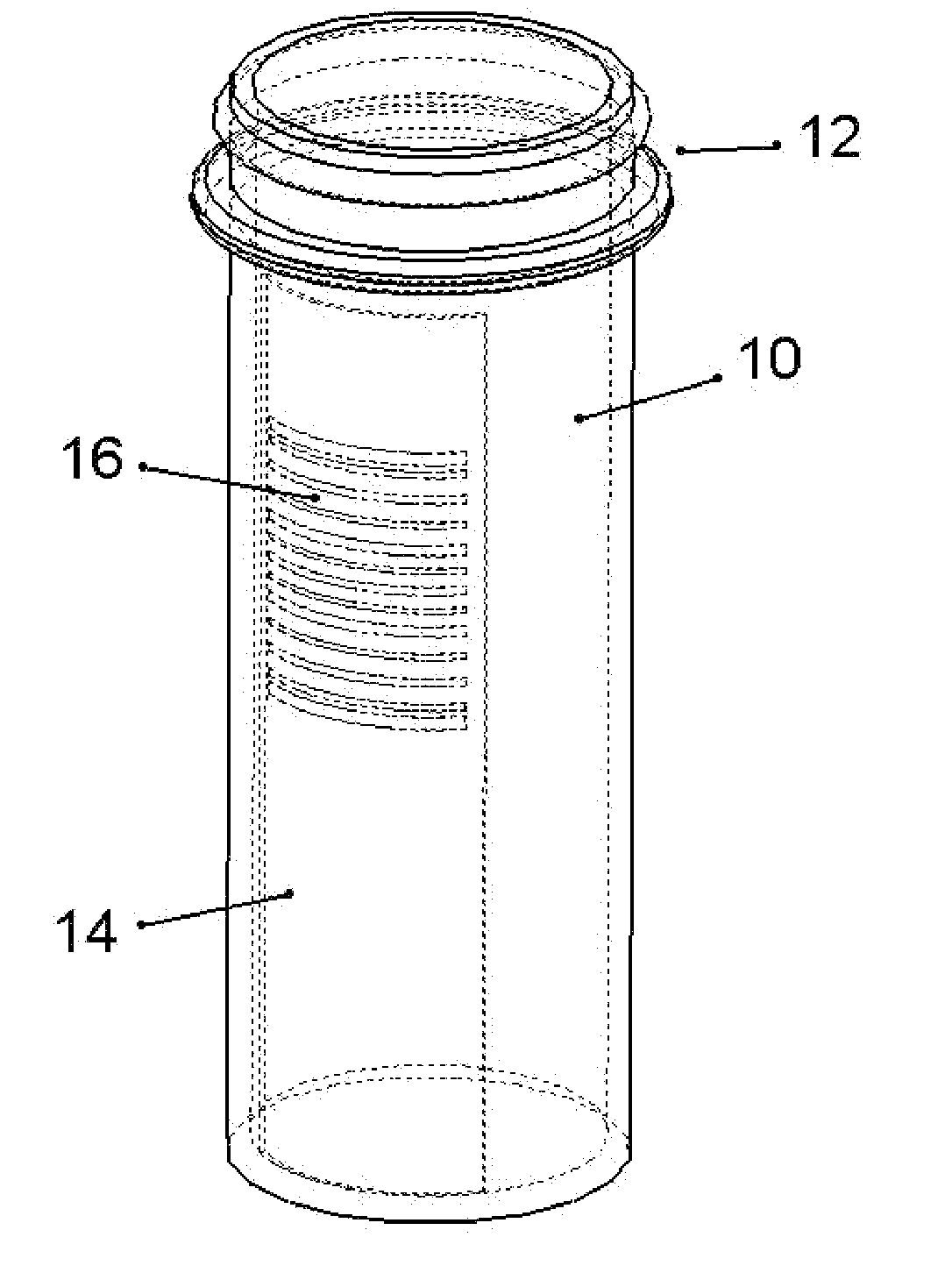
A drug delivery device which includes a fluid drug reservoir, a catheter, a controllable fluid transfer device, e.g., a pump mechanism or valve, and a drug delivery control means. The drug delivery control means comprises a controller, e.g., a microprocessor or microcontroller which is operable to automatically reduce the rate of drug delivery over a certain reduction interval (e.g., multiple days) from an initial dosage value to a final dosage value.
Owner:INFUSION SYST
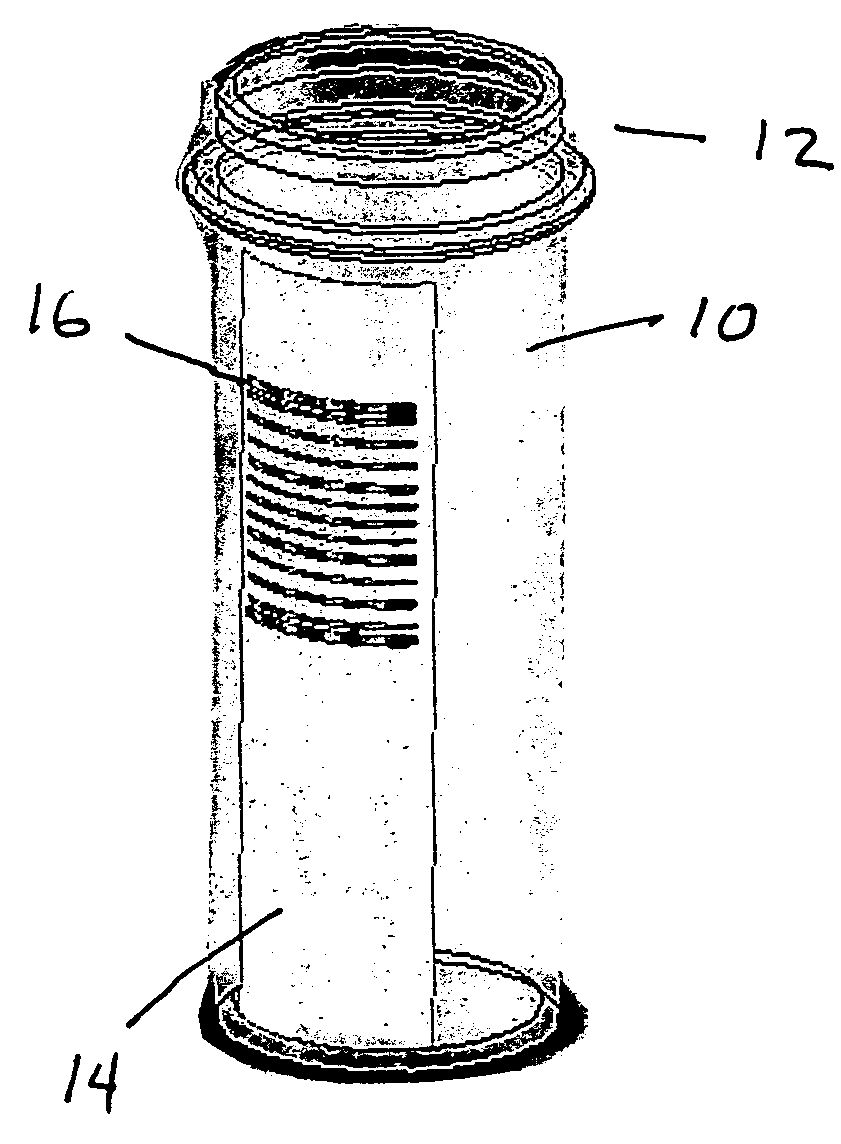
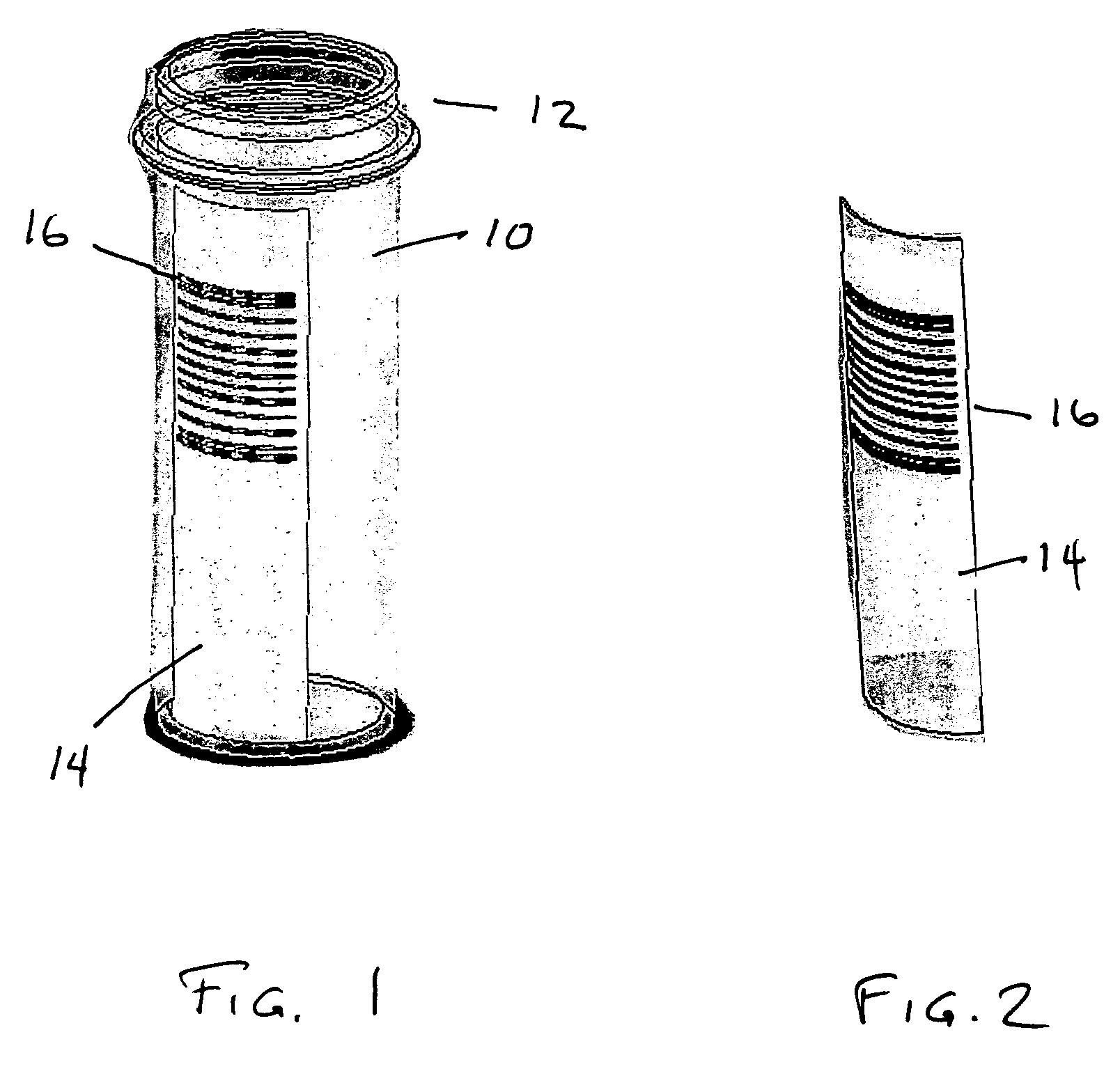
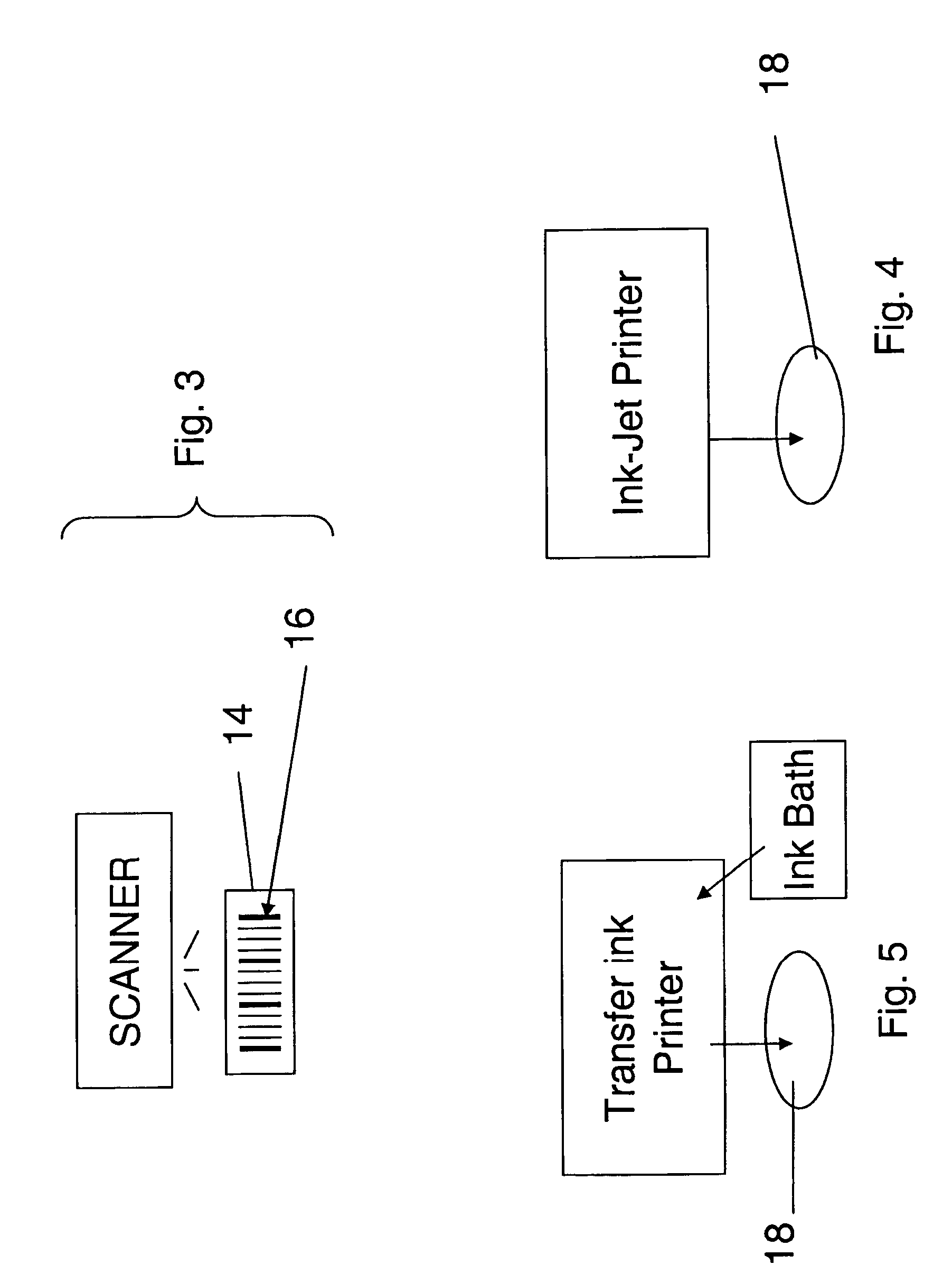
Pill printing identification
InactiveUS20080290168A1Co-operative working arrangementsCharacter and pattern recognitionBarcodeEngineering
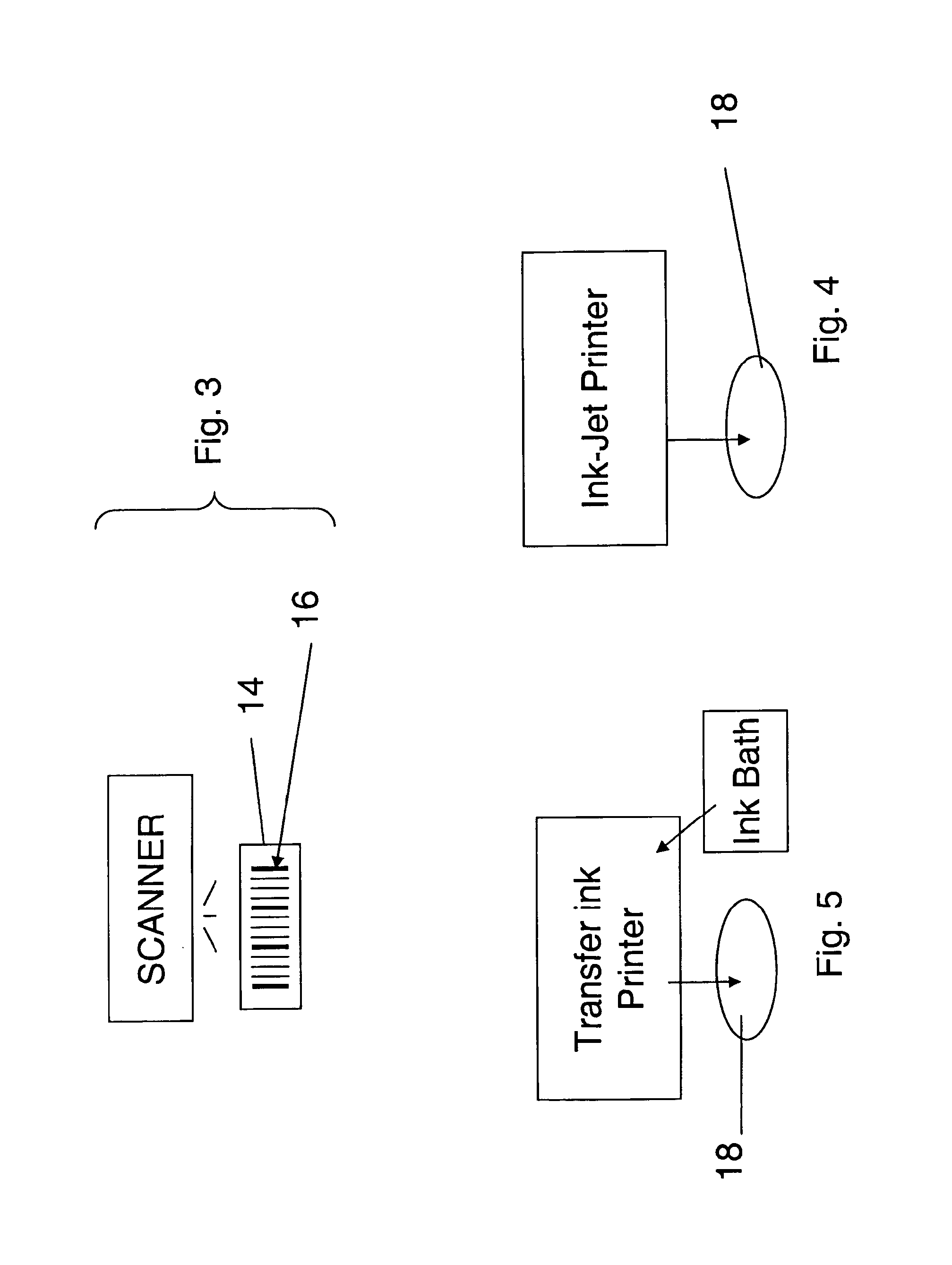
Drug dosages and bar code labels are within a tamperproof, sealed container. A printer is configured to mark a drug dosage with an imprint in a form of a machine readable bar code. A method and product are provided to enable both the source of manufacture and distributor to be identified based on scanning a barcode from a label.
Owner:SULLIVAN SCOTT LINDSAY +1
Pharmaceutical compositions for the coordinated delivery of NSAIDs
InactiveUS20050249811A1Improve complianceReduce in quantityBiocideAntipyreticGastrointestinal InjuryArthritis
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO +1
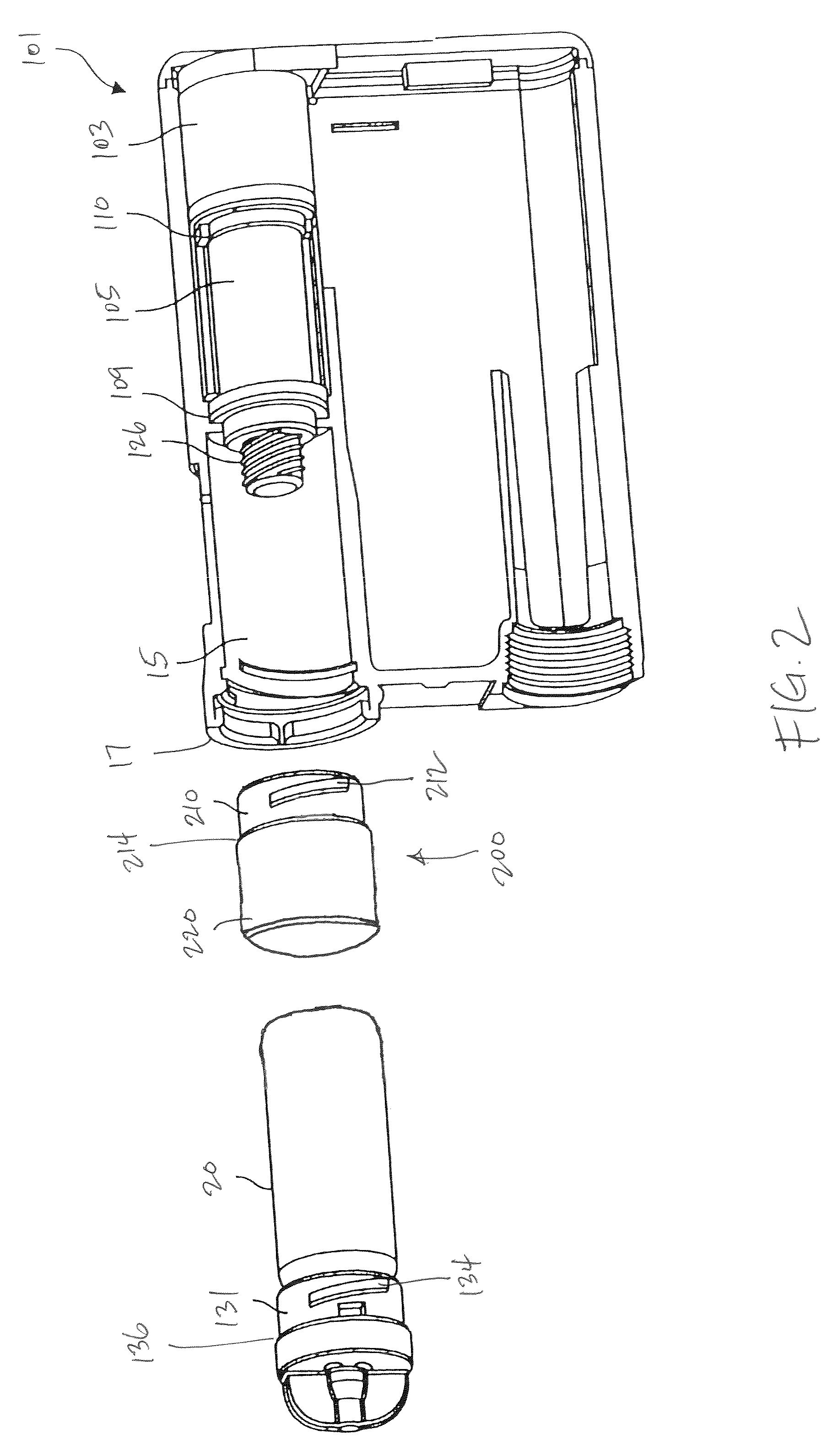
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Systems and methods for administration of small volume sufentanil drug dosage forms to the sublingual mucosa of a subject using a device are disclosed. The dispensing device includes a lock-out feature and a means to retard or prevent saliva and / or moisture ingress such that the drug dosage forms in the device remain dry prior to administration.
Owner:ACEIRX PHARM INC
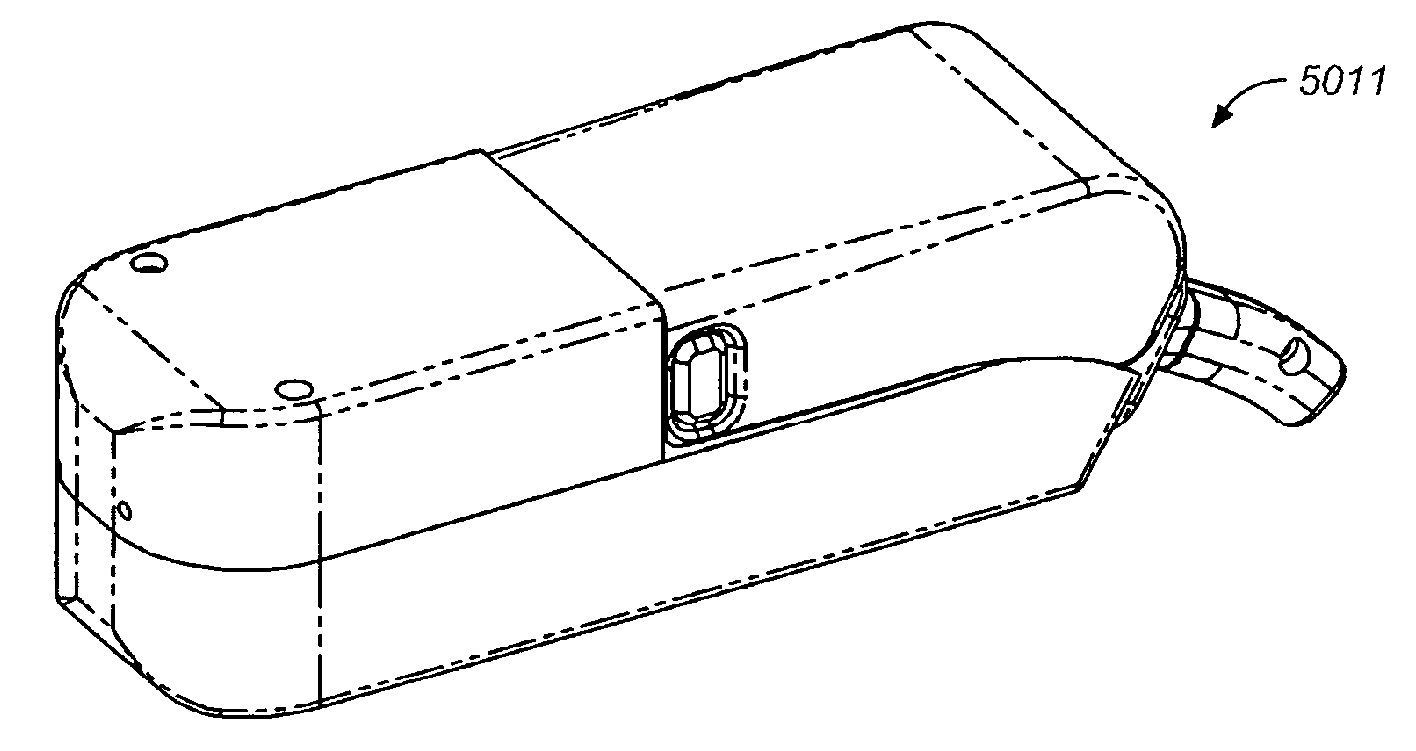
Drug dispensing device with flexible push rod
ActiveUS8357114B2Minimizing saliva influxReduced responsePowdered material dispensingDrug and medicationsDrug dispensingNonlinear channel
Drug storage and dispensing devices for dispensing a drug dosage form are disclosed. Devices having a housing defining a nonlinear passageway and a flexible push rod to move within the nonlinear passageway are disclosed. Devices having a flexible push rod coupled to a rotation actuating member at at an end portion thereof are also disclosed.
Owner:ACEIRX PHARM INC
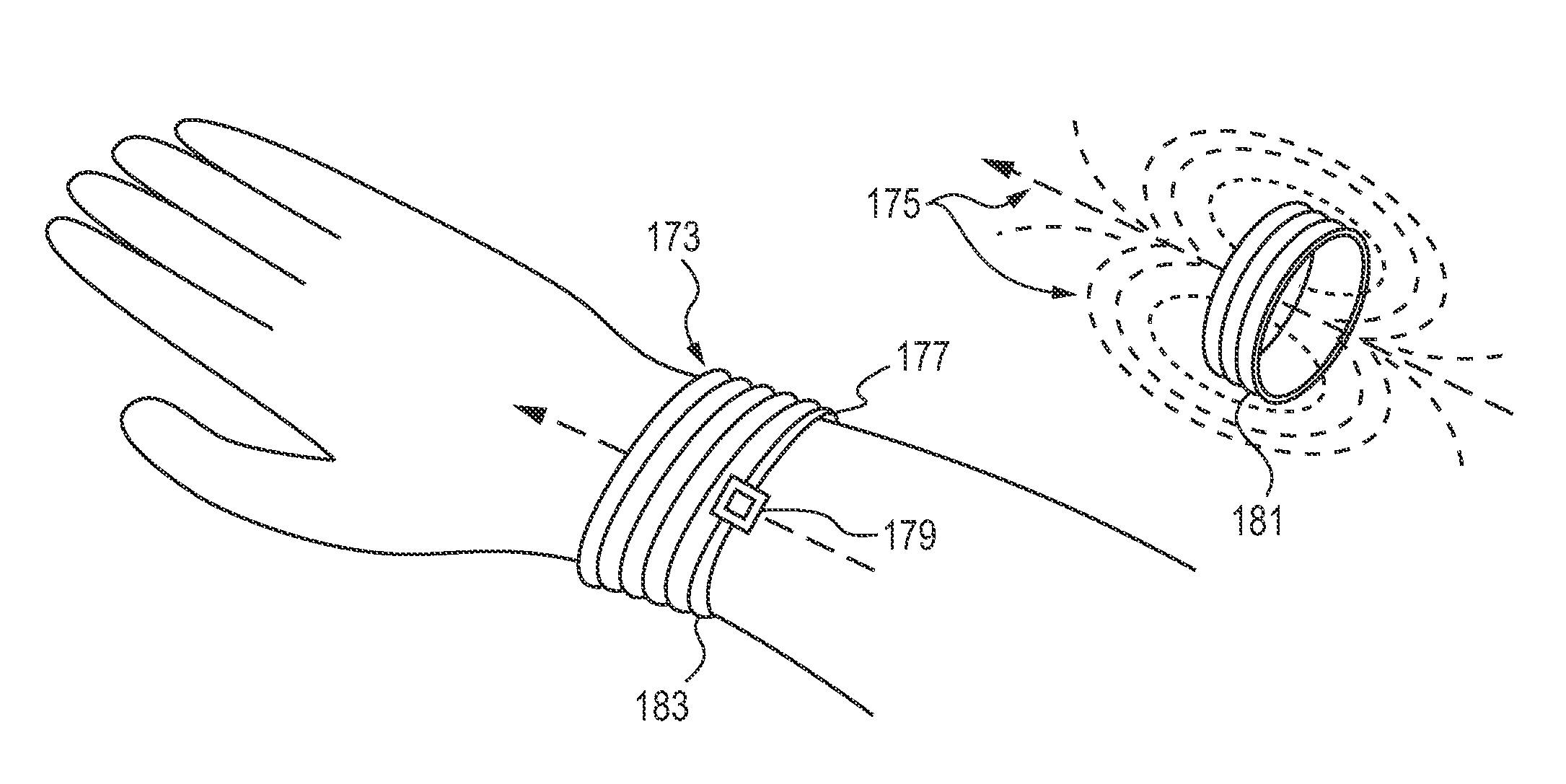
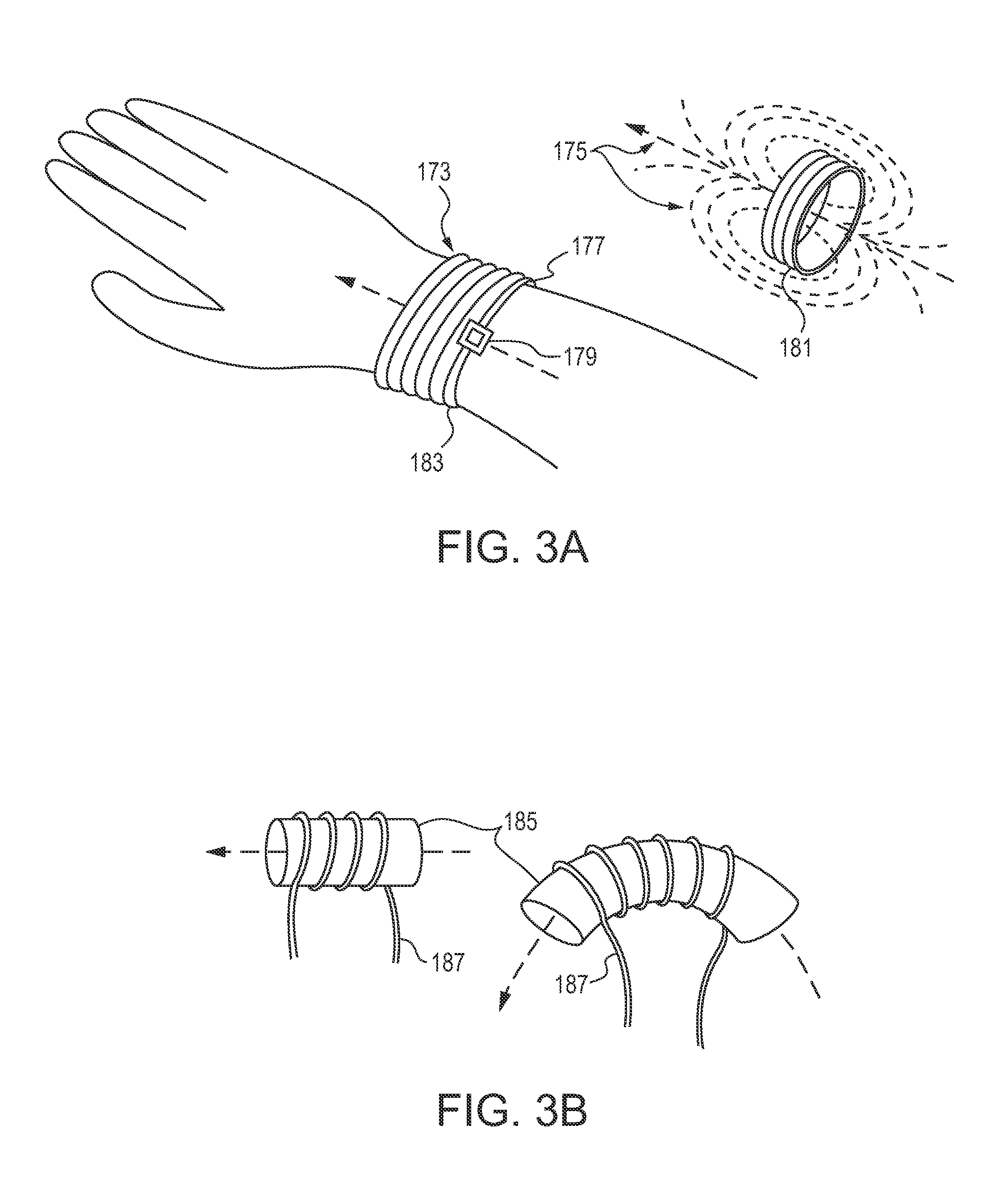
Storage and Dispensing Devices for Administration of Oral Transmucosal Dosage Forms
ActiveUS20100253476A1Drug and medicationsNear-field in RFIDBiomedical engineeringPatient identification
Dispensing devices and systems for oral transmucosal administration of small volume drug dosage forms to the oral mucosa are provided. The dispensing device may be a single dose applicator (SDA), or an electromechanical device comprising a means for patient identification such as a wrist worn RFID tag and annular bidirectional antenna together with a lock-out feature.
Owner:VERTICAL PHARMA
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Owner:ACEIRX PHARM INC
Methods for administering small volume oral transmucosal dosage forms using a dispensing device
Systems and methods for administration of small volume sufentanil drug dosage forms to the sublingual mucosa of a subject using a device are disclosed. The dispensing device includes a lock-out feature and a means to retard or prevent saliva and / or moisture ingress such that the drug dosage forms in the device remain dry prior to administration.
Owner:ACEIRX PHARM INC
Drug Delivery Apparatus and Method for Automatically Reducing Drug Dosage
InactiveUS20080172044A1Reduce probabilityMedical devicesPressure infusionMicrocontrollerDrug reservoir
Owner:INFUSION SYST
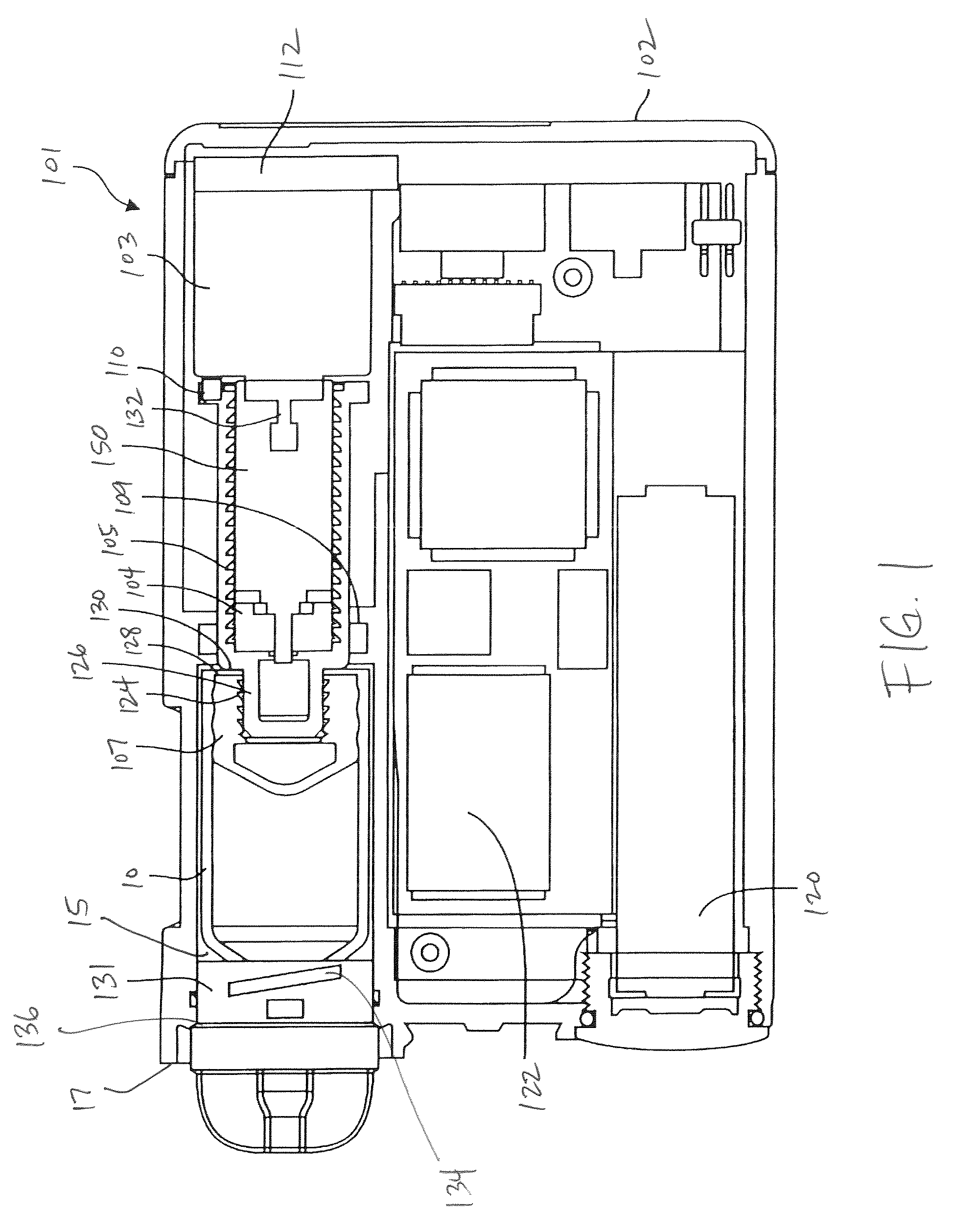
Medication dosing device for administering a liquid medication
InactiveUS20110190702A1Easy to manufactureGuaranteed cost-effective operationAutomatic syringesMedical devicesMedication doseControl system
A medication dosing device for administering a liquid medication, having a housing, the housing having a medication reservoir, a medication dosing unit, a drive for conveying the medication, an administration needle, a power supply unit, and an electrical control system. The medication reservoir, the medication dosing unit, and the administration needle are jointly situated in a unit which may be inserted .into the housing and removed from the housing. The medication dosing device has the advantage that it may be easily manufactured and cost-effectively operated.
Owner:ROBERT BOSCH GMBH
Dosage form for treating gastrointestinal disorders
InactiveUS20060165797A1Quick reliefPrevent relapseBiocideDigestive systemGastrointestinal disorderSecreted substance
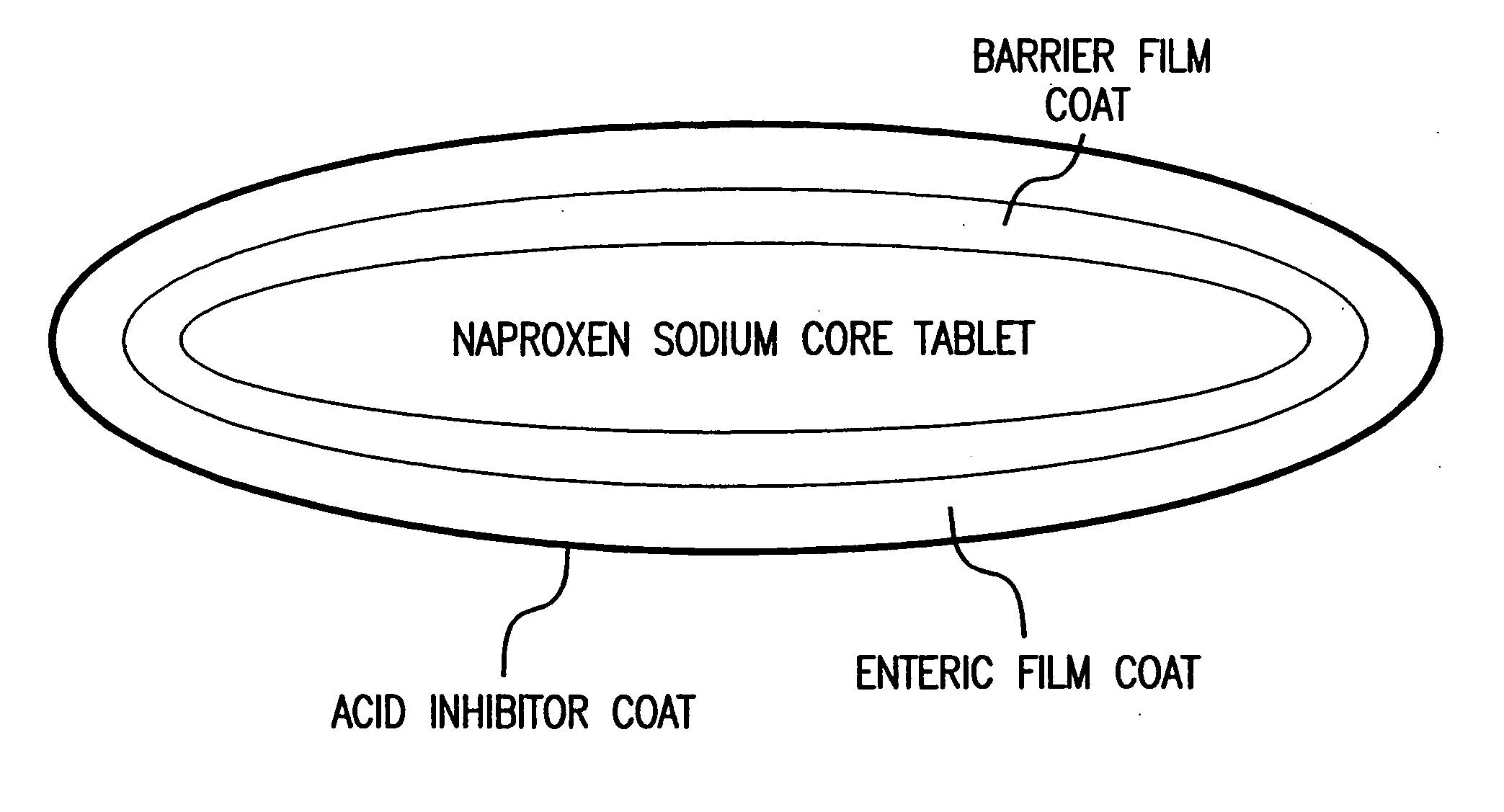
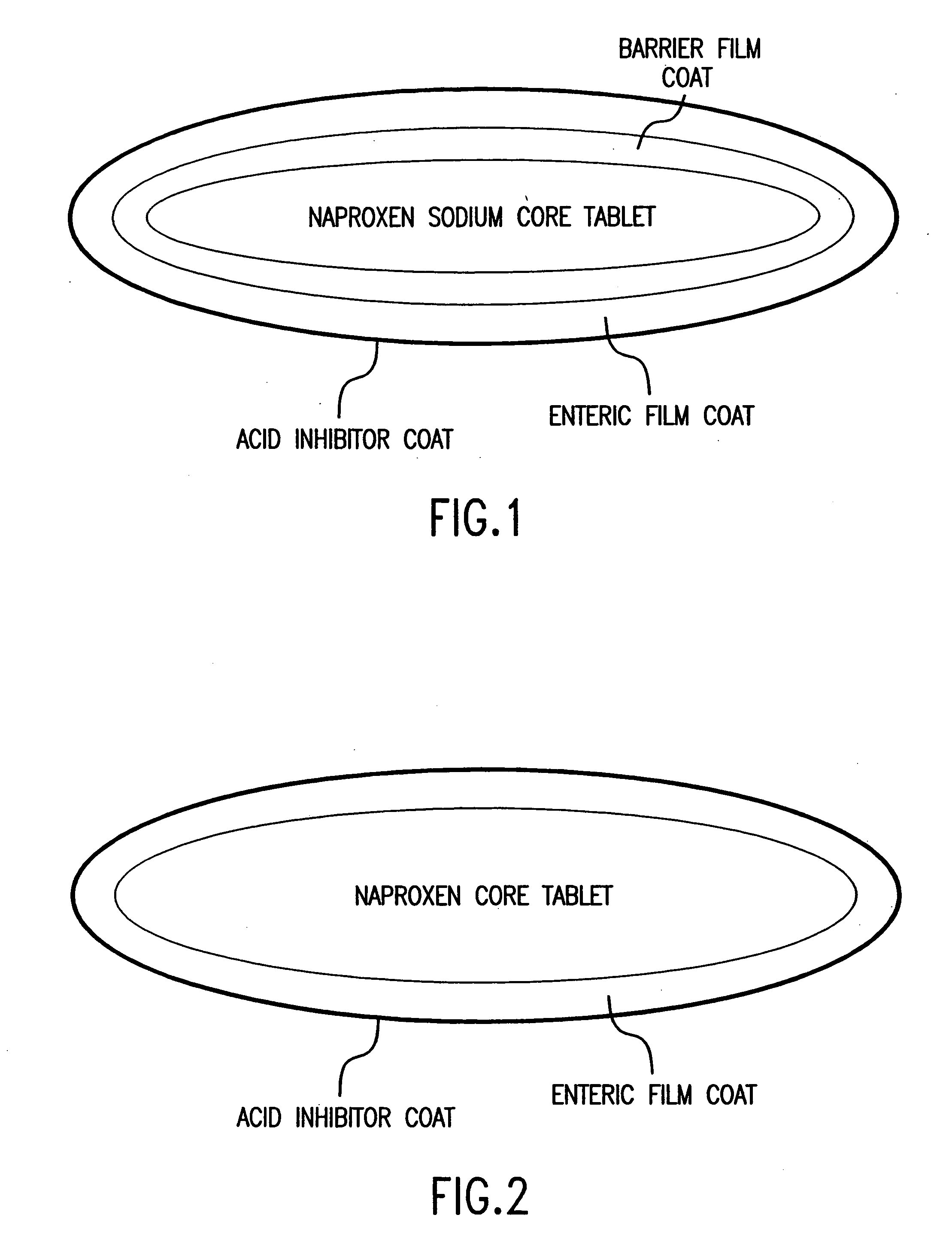
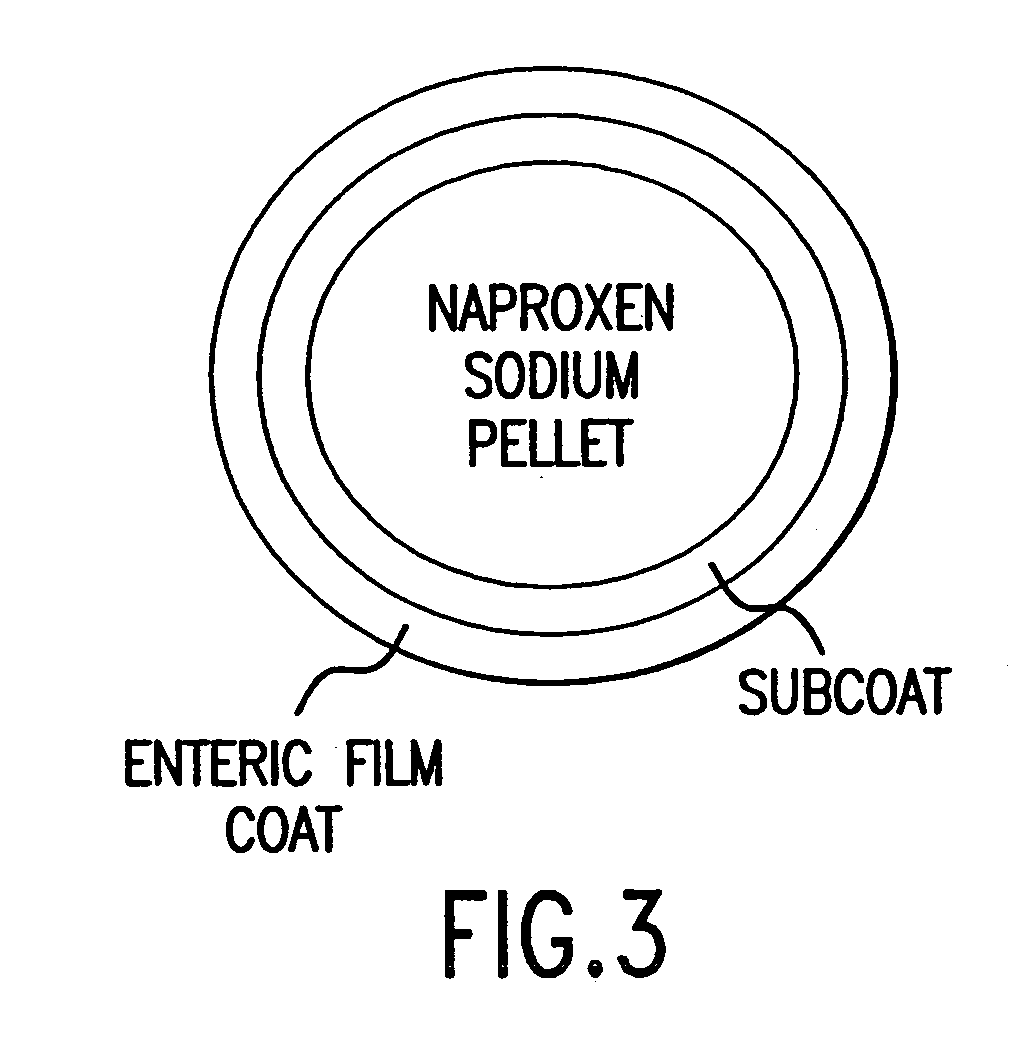
The present invention is directed to drug dosage forms that can be used to treat diseases characterized by abnormal gastric acid secretion. The dosage forms have a core containing a proton pump inhibitor surrounded by an enteric coating or multiple particles containing proton pump inhibitor, each particle being surrounded by an enteric coating. The enteric coating delays the release of drug until the surrounding pH has risen. The tablets also include an outer coating that contains either a proton pump inhibitor or an H2 blocker. The outer coating is designed to rapidly dissolve in a patient's stomach.
Owner:POZEN INC
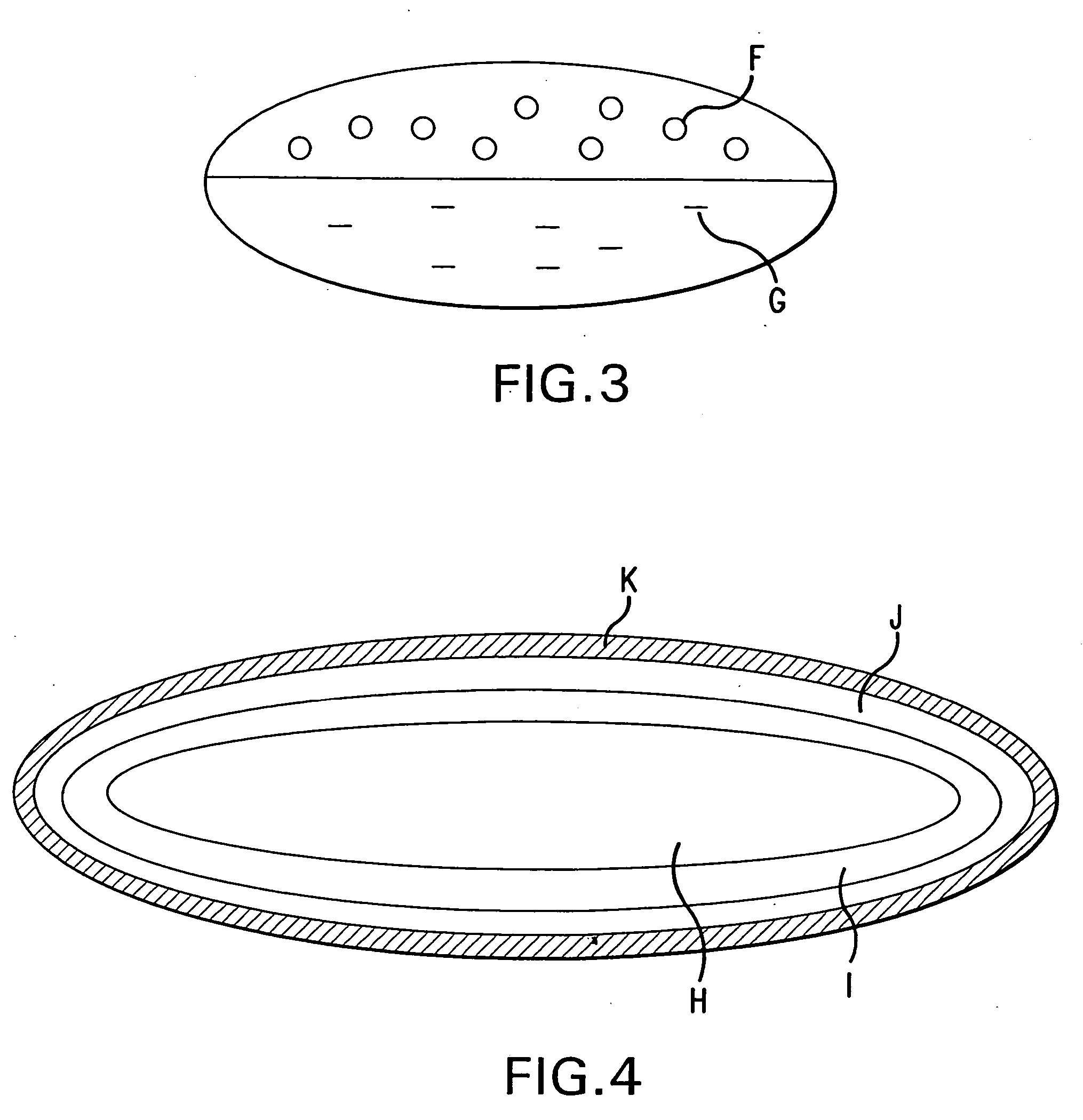
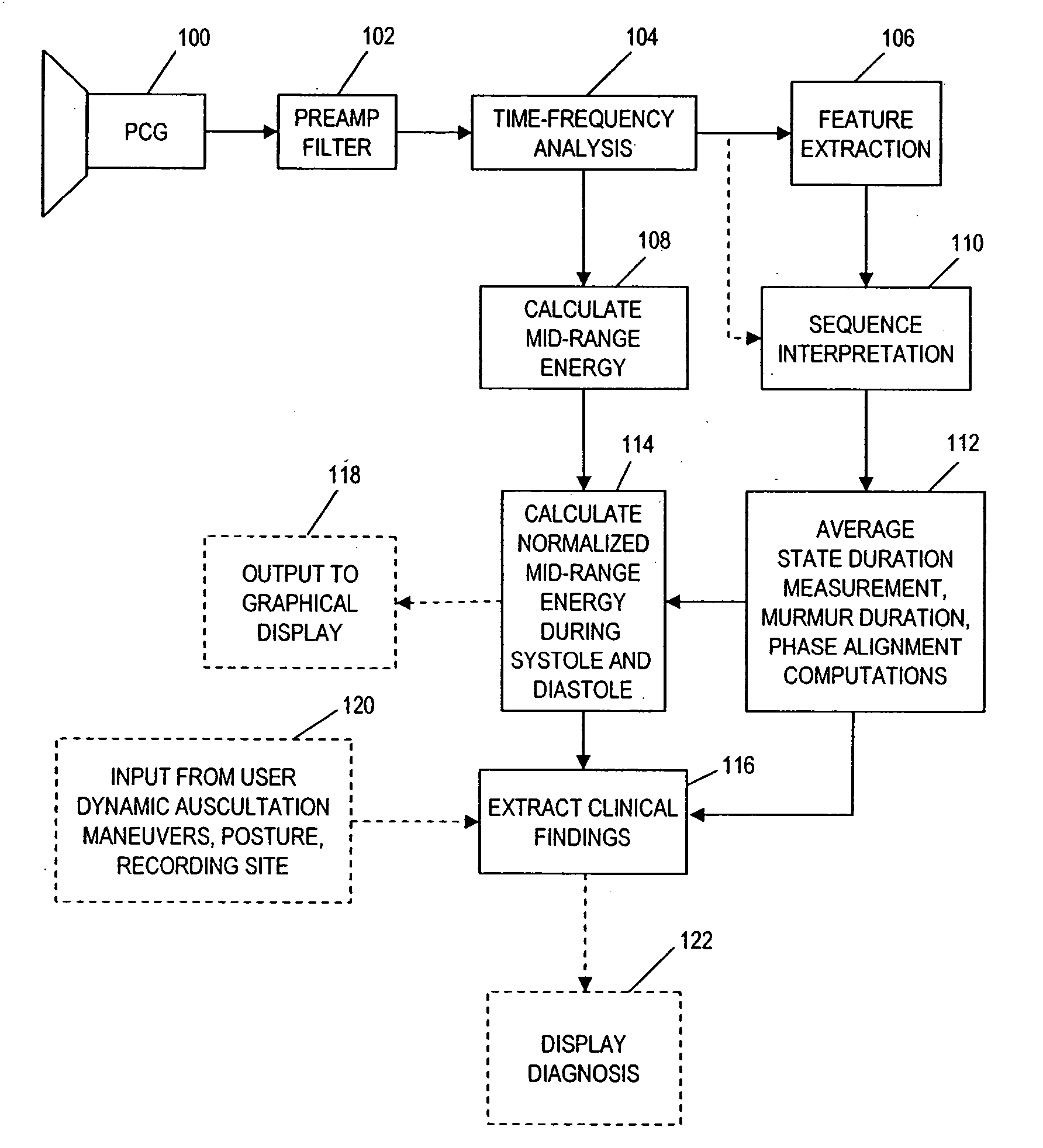
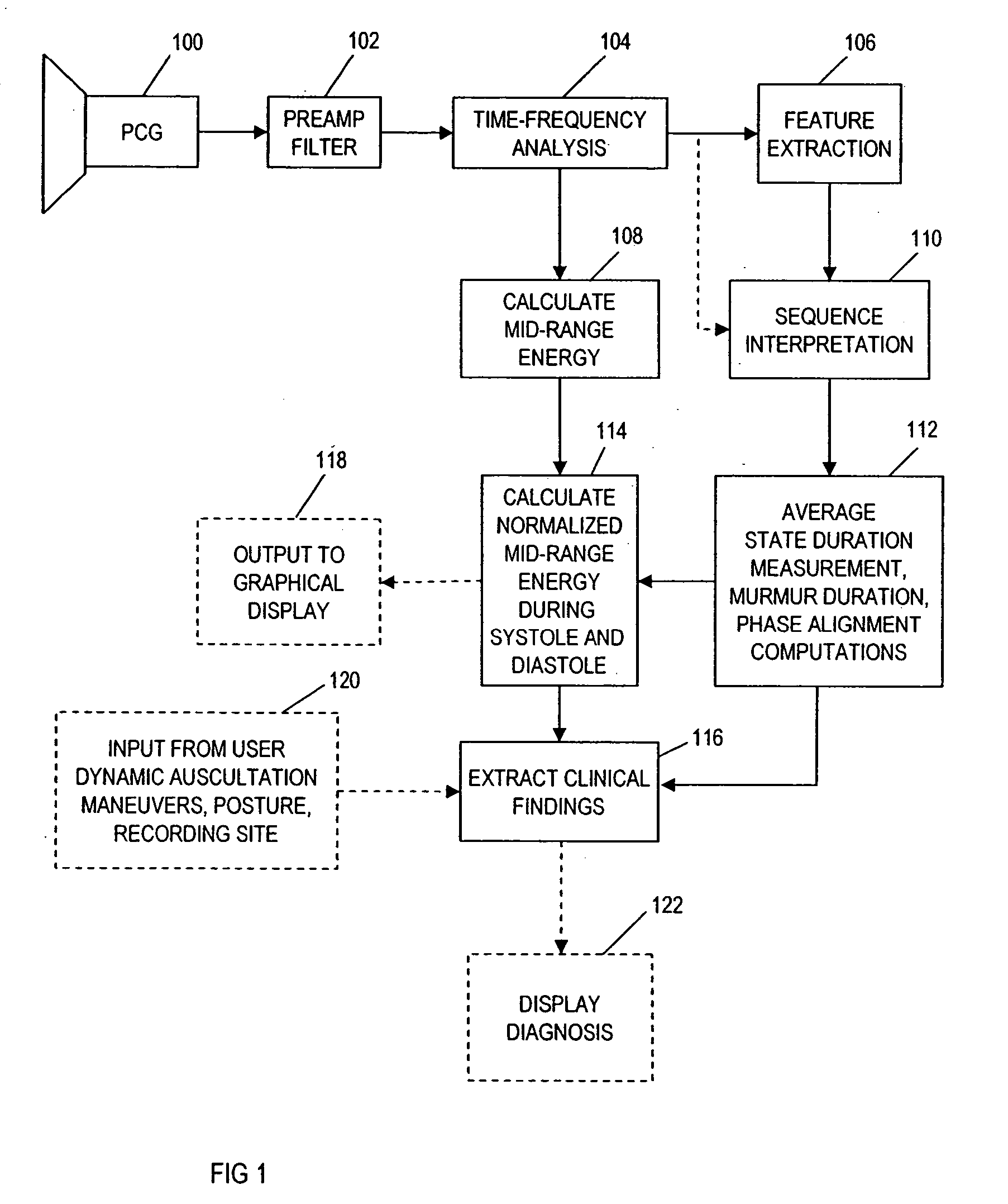
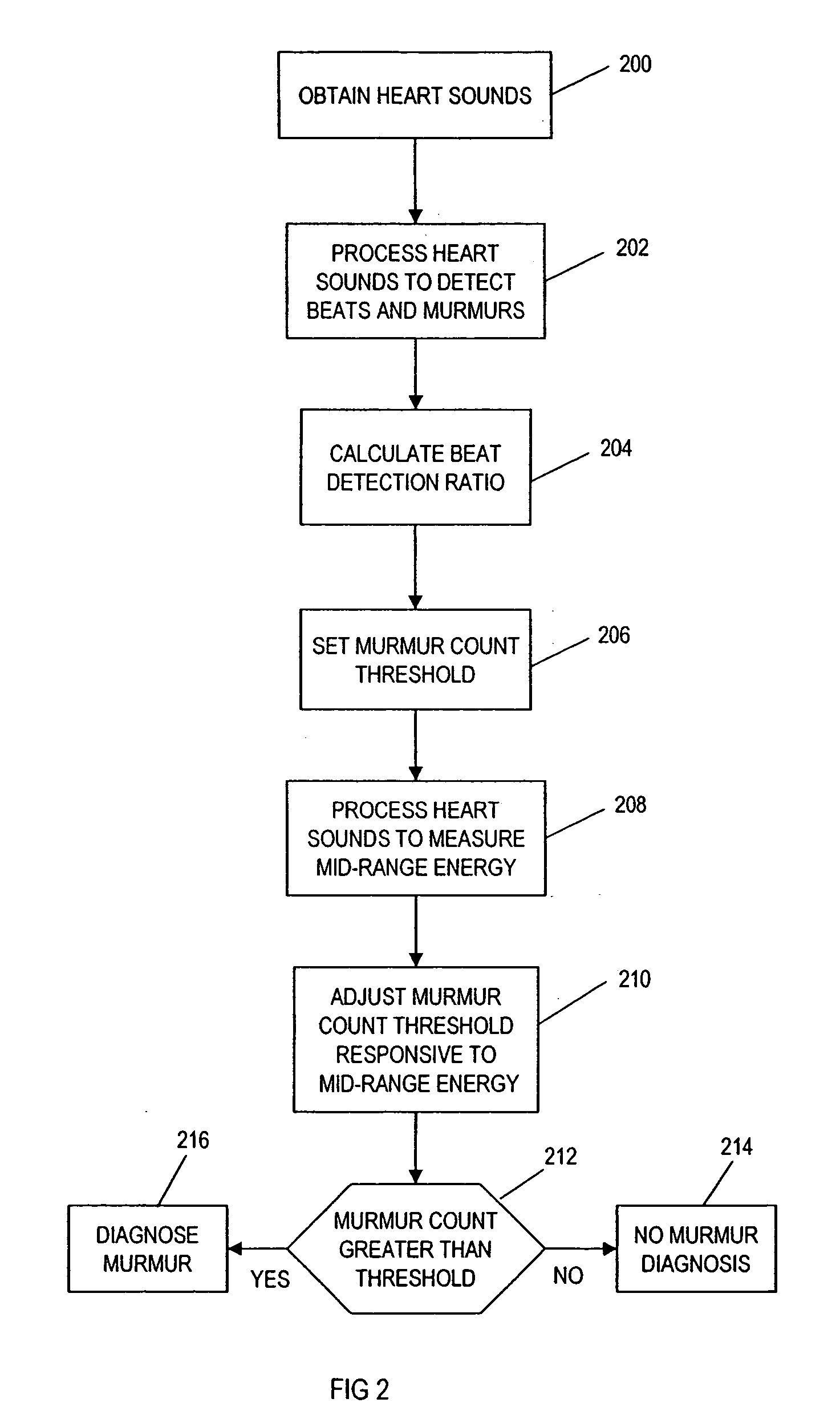
Computer-assisted detection of systolic murmurs associated with hypertrophic cardiomyopathy
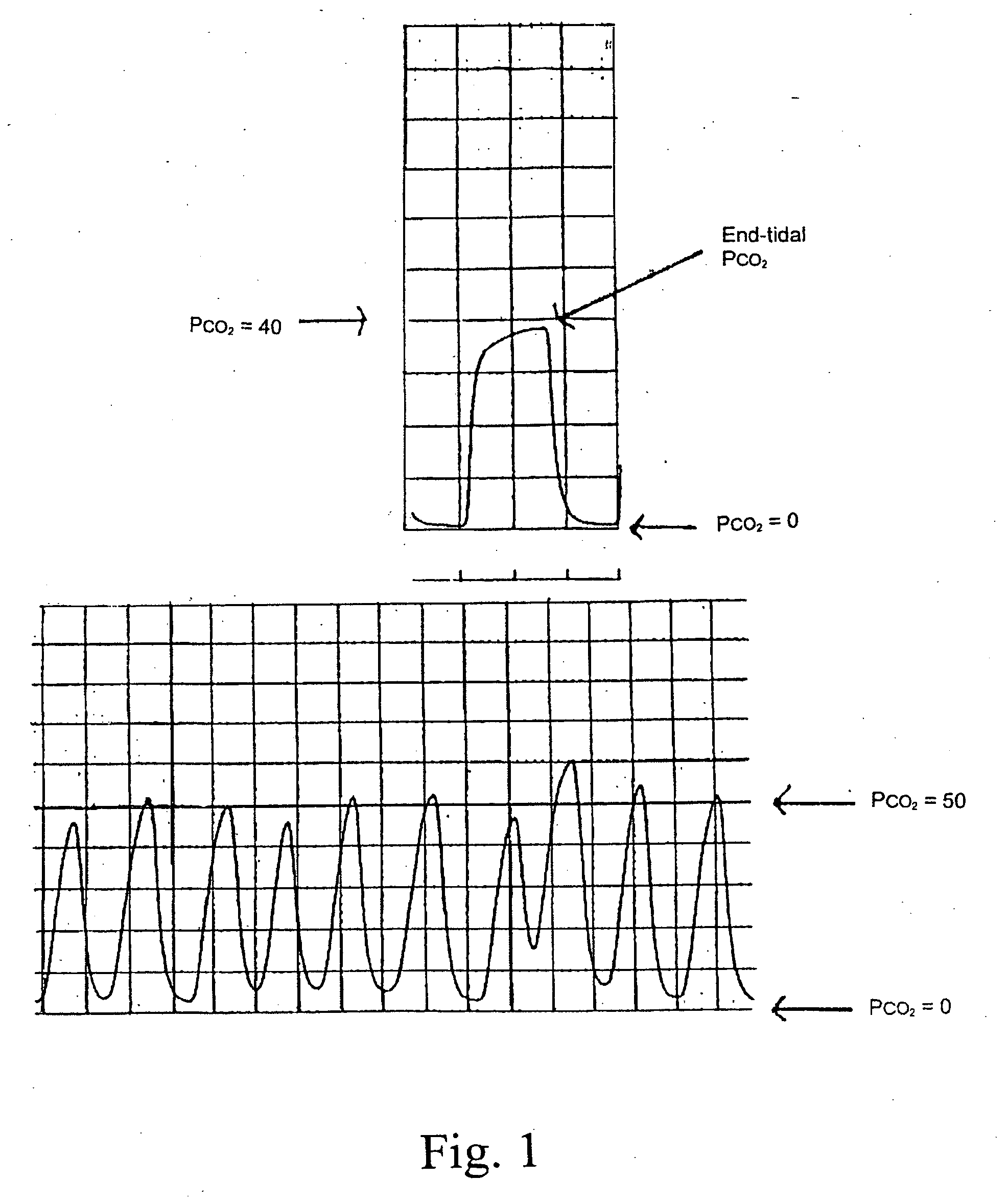
A method for assisting in the diagnosis of heart murmurs. The method calculates a normalized measure of mid-range energy for at least one systolic or diastolic interval in a sequence of heartbeats and displays the mid-range energy measure of the systolic and / or diastolic interval in a graphical form. The sequence of heartbeats is also processed to detect and diagnose heart murmurs by adjusting a murmur count threshold responsive to the mid-range energy. The method may be used to diagnose hypertrophic cardiomyopathy and to determine effective therapeutic drug dosage or therapeutic device setting.
Owner:ZARGIS MEDICAL
Pill printing and identification
InactiveUS7370797B1Co-operative working arrangementsCharacter and pattern recognitionBarcodeEngineering
Drug dosages and bar code labels are within a tamperproof, sealed container. A printer is configured to mark a drug dosage with an imprint in a form of a machine readable bar code. A method and product are provided to enable both the source of manufacture and distributor to be identified based on scanning a barcode from a label.
Owner:HESS ROBERT J +1
Reservoir compartment adapter for infusion device
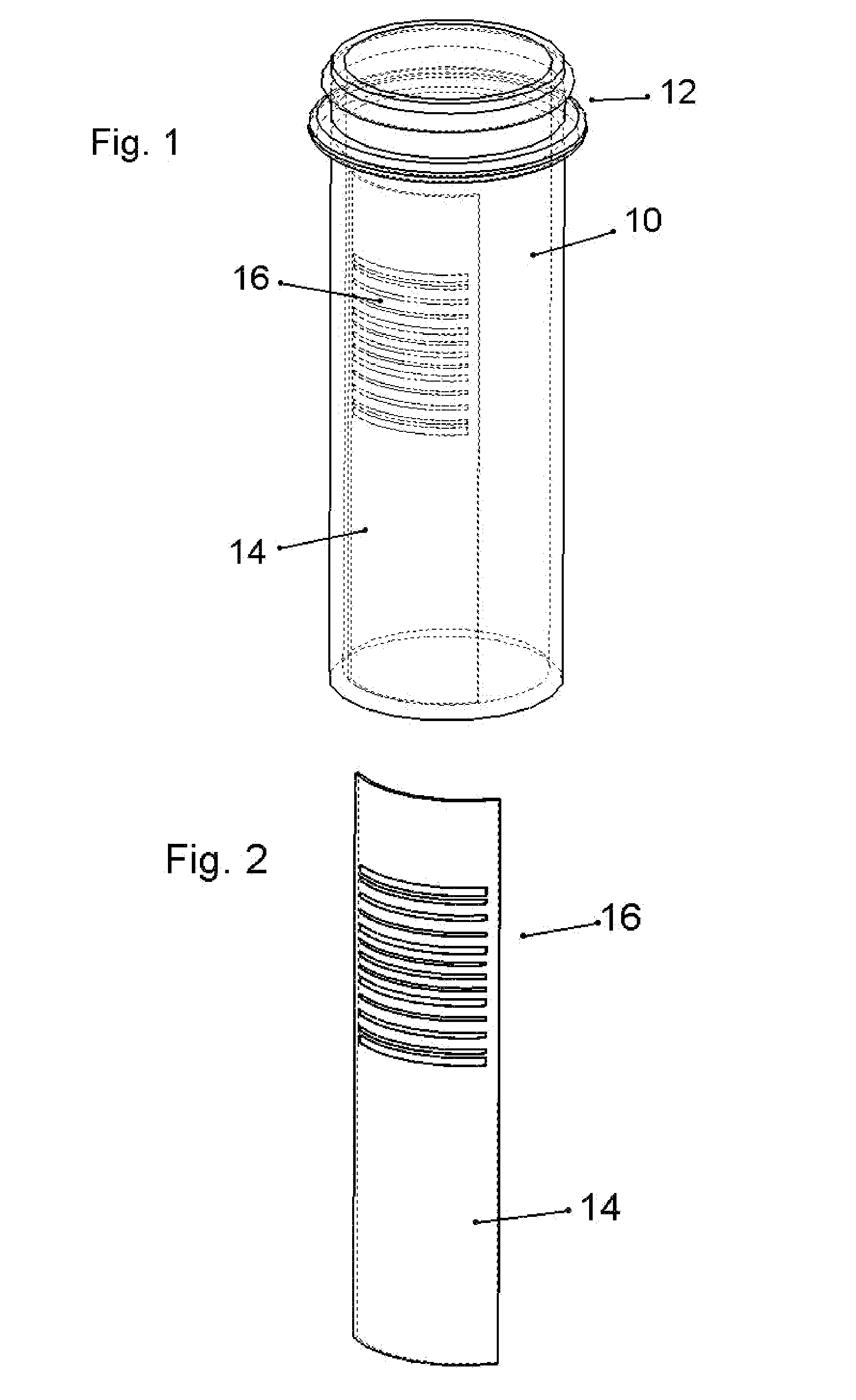
The present invention provides a reservoir compartment adapter for use with a fluid delivery device. The adapter includes a first end adapted for coupling with a fluid delivery device, a second end adapted for coupling with a connector, and a structure between the first end and the second end including an interior space for receiving the reservoir, wherein an extended reservoir compartment for accommodating the reservoir is adapted to be formed when the first end is coupled to the fluid delivery device, and the reservoir is adapted to be secured in the extended reservoir compartment when the connector is coupled to the second end. The adapter allows a user of a delivery device accommodating reservoirs of a certain size to manage periods where increased medication dosage is needed without the burden of carrying a larger delivery device for accommodating reservoirs filled with the increased dosage.
Owner:MEDTRONIC MIMIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com