Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1192 results about "Oral mucosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
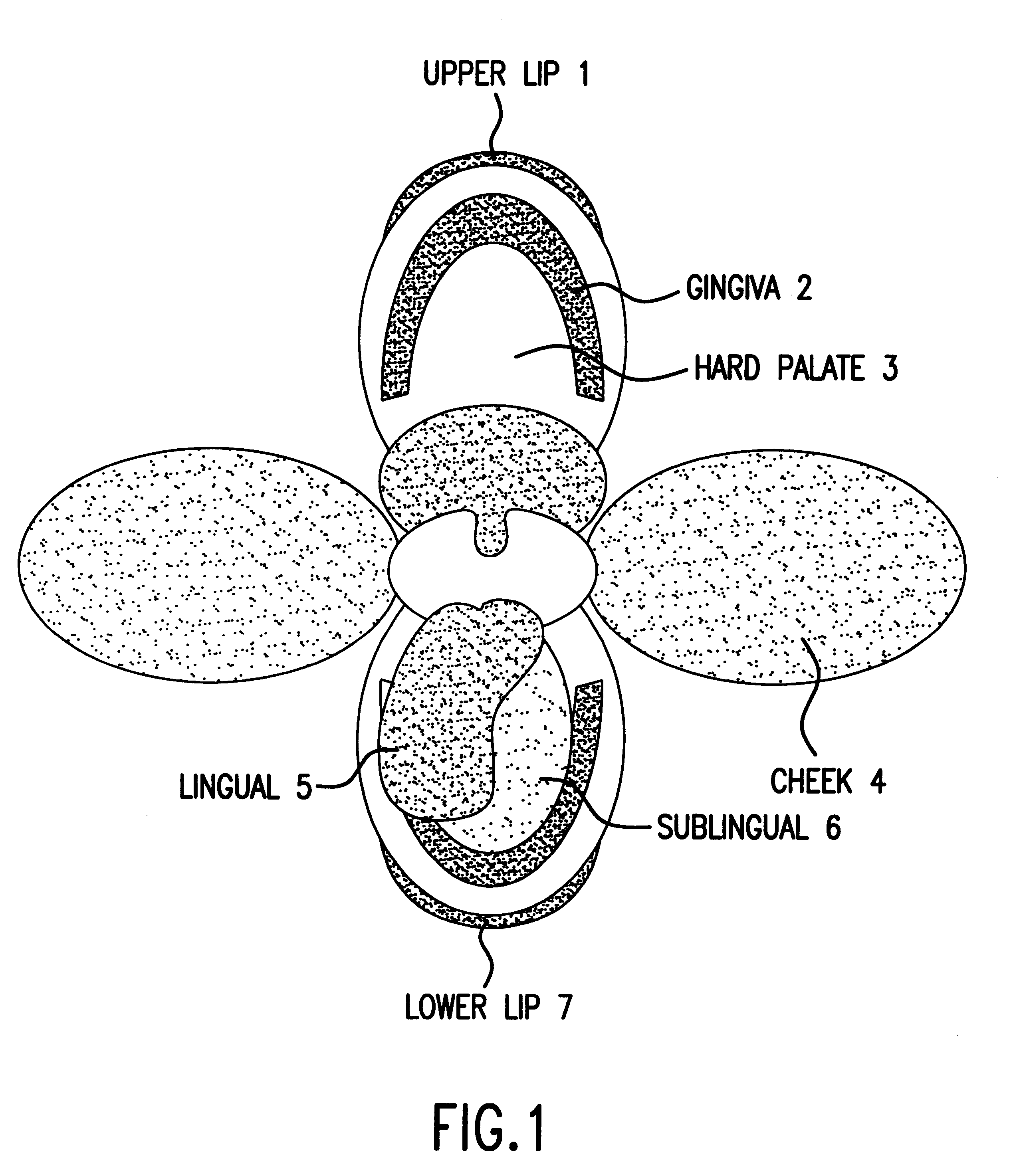
The oral mucosa is the mucous membrane lining the inside of the mouth. It comprises stratified squamous epithelium, termed "oral epithelium", and an underlying connective tissue termed lamina propria. The oral cavity has sometimes been described as a mirror that reflects the health of the individual. Changes indicative of disease are seen as alterations in the oral mucosa lining the mouth, which can reveal systemic conditions, such as diabetes or vitamin deficiency, or the local effects of chronic tobacco or alcohol use. The oral mucosa tends to heal faster and with less scar formation compared to the skin. The underlying mechanism remains unknown, but research suggests that extracellular vesicles might be involved.
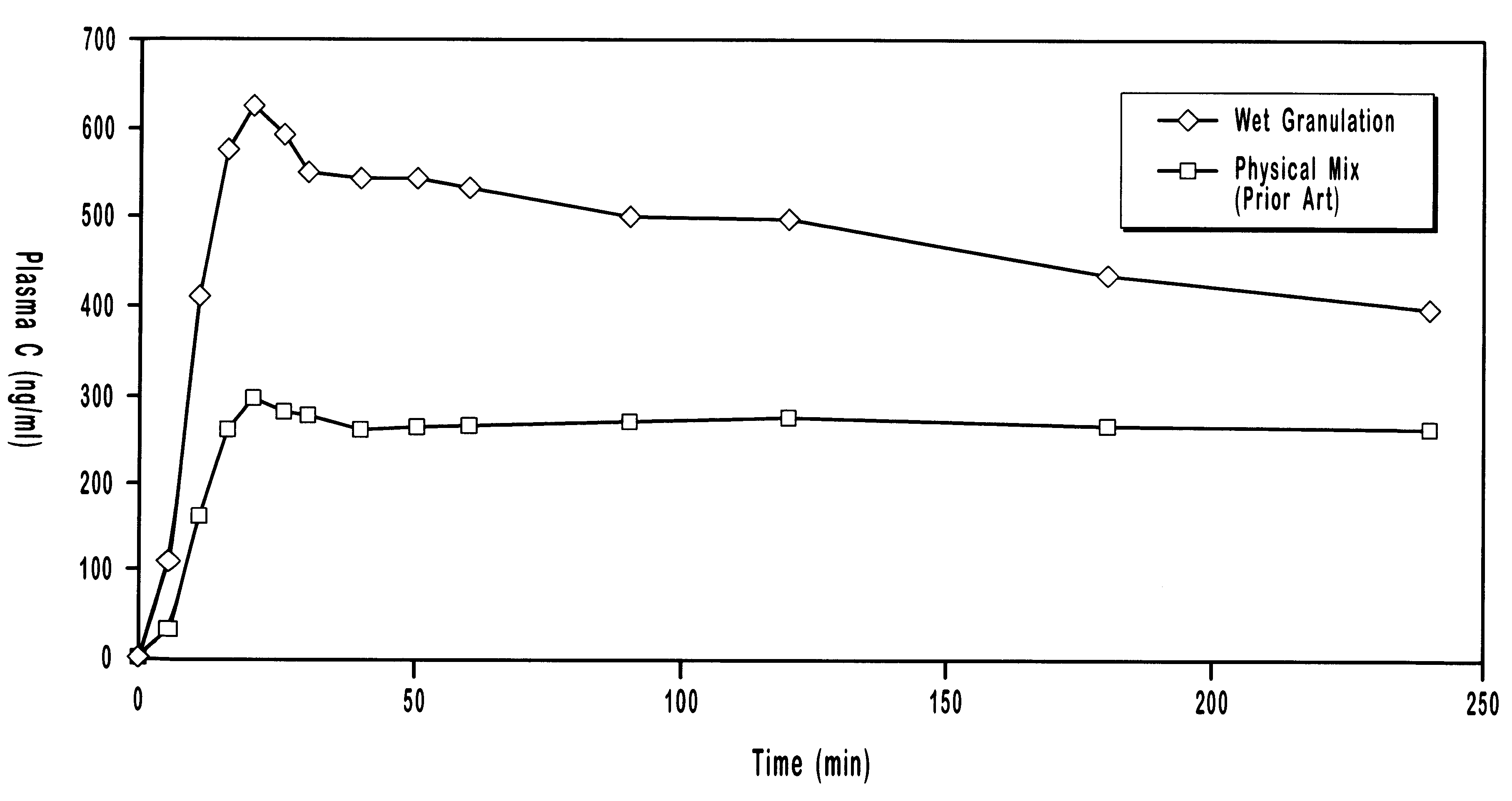
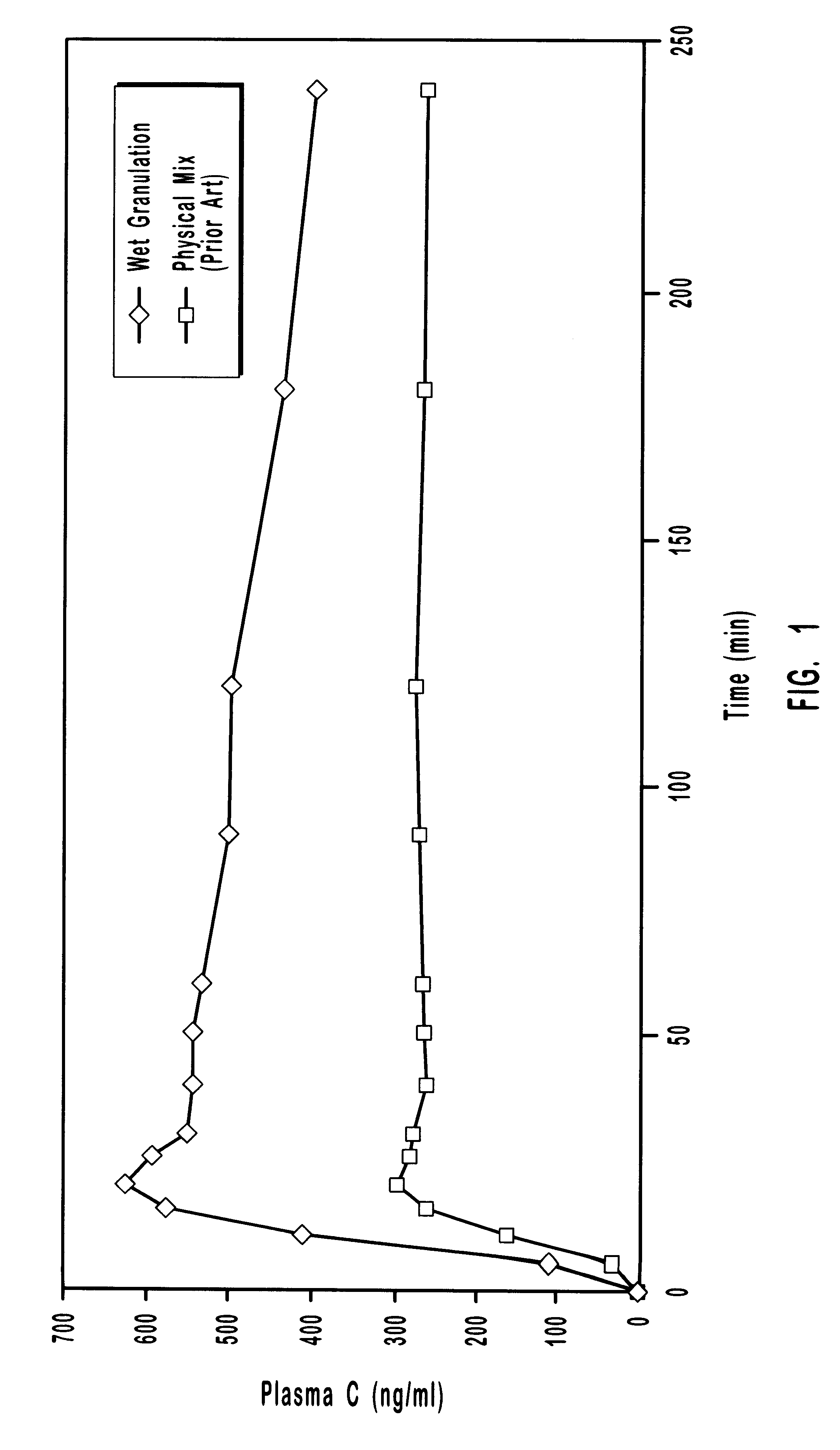
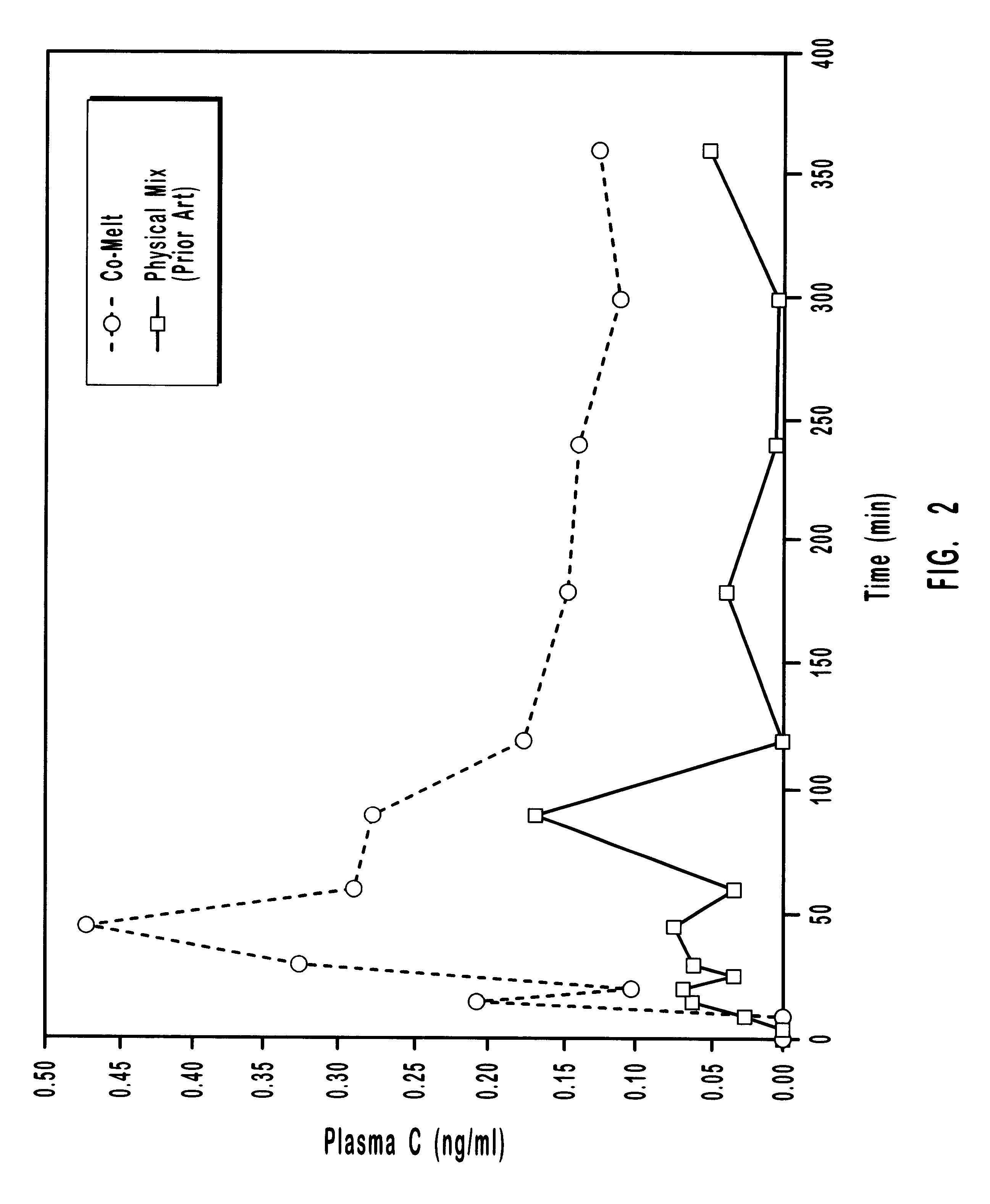
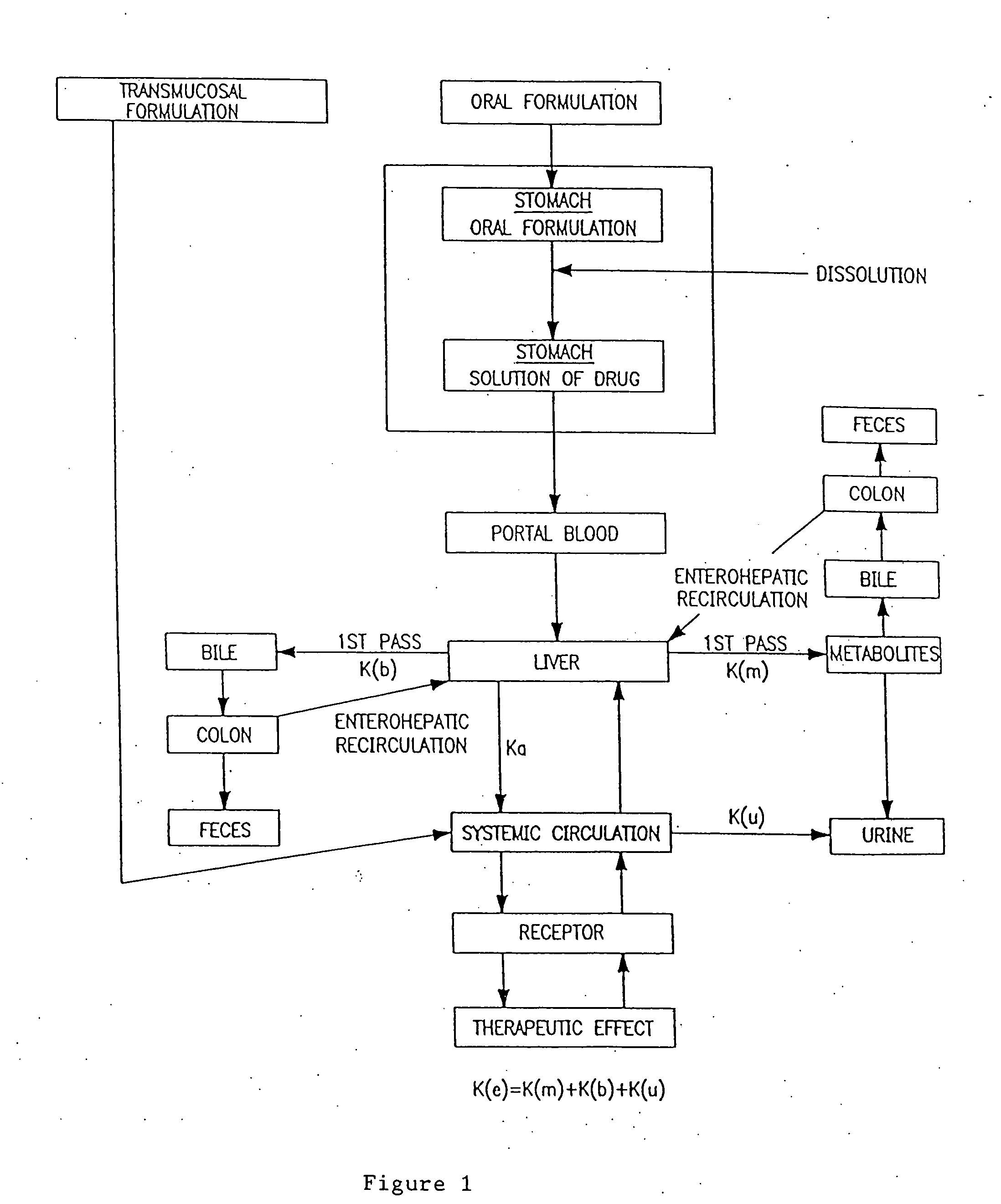
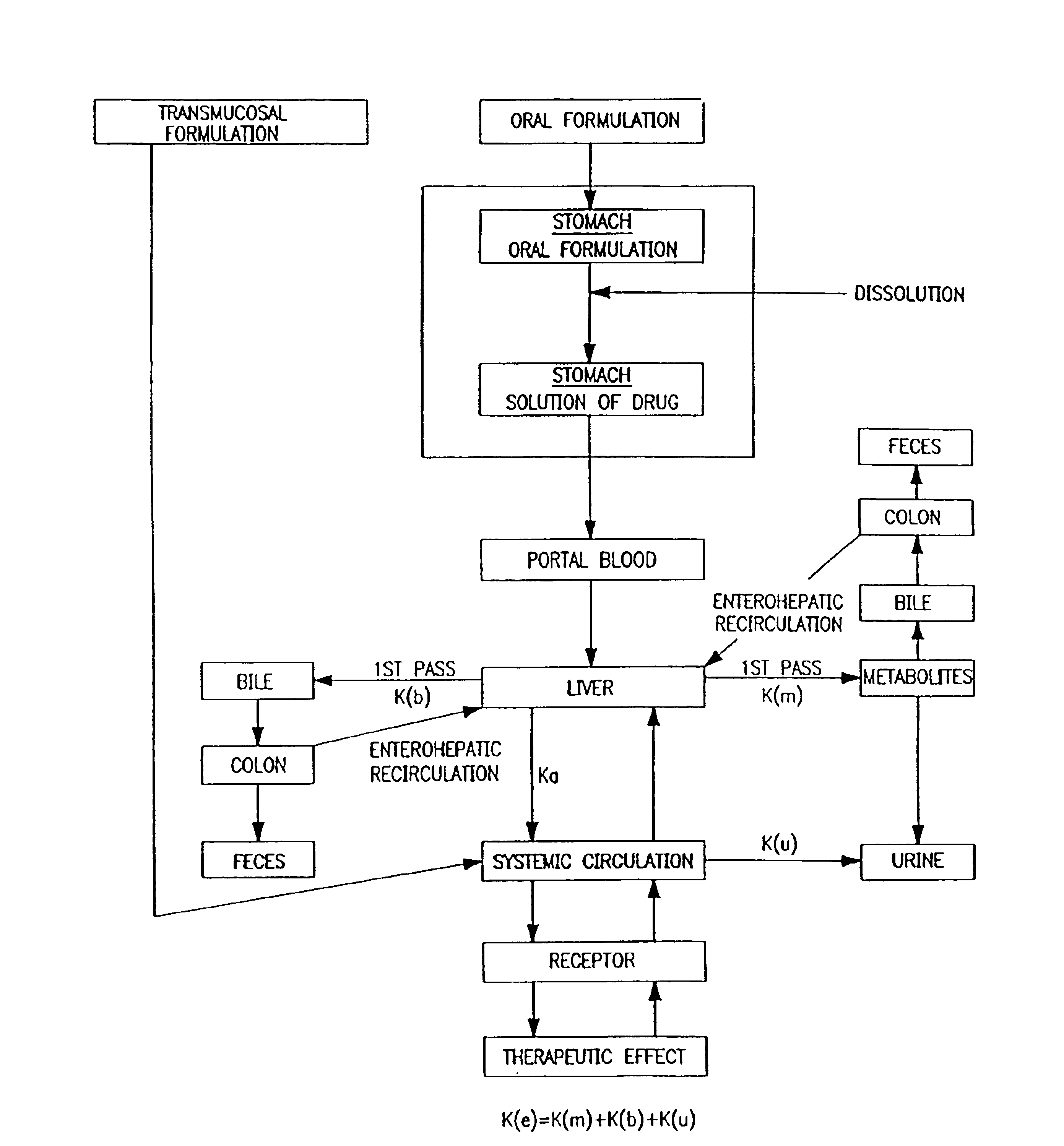
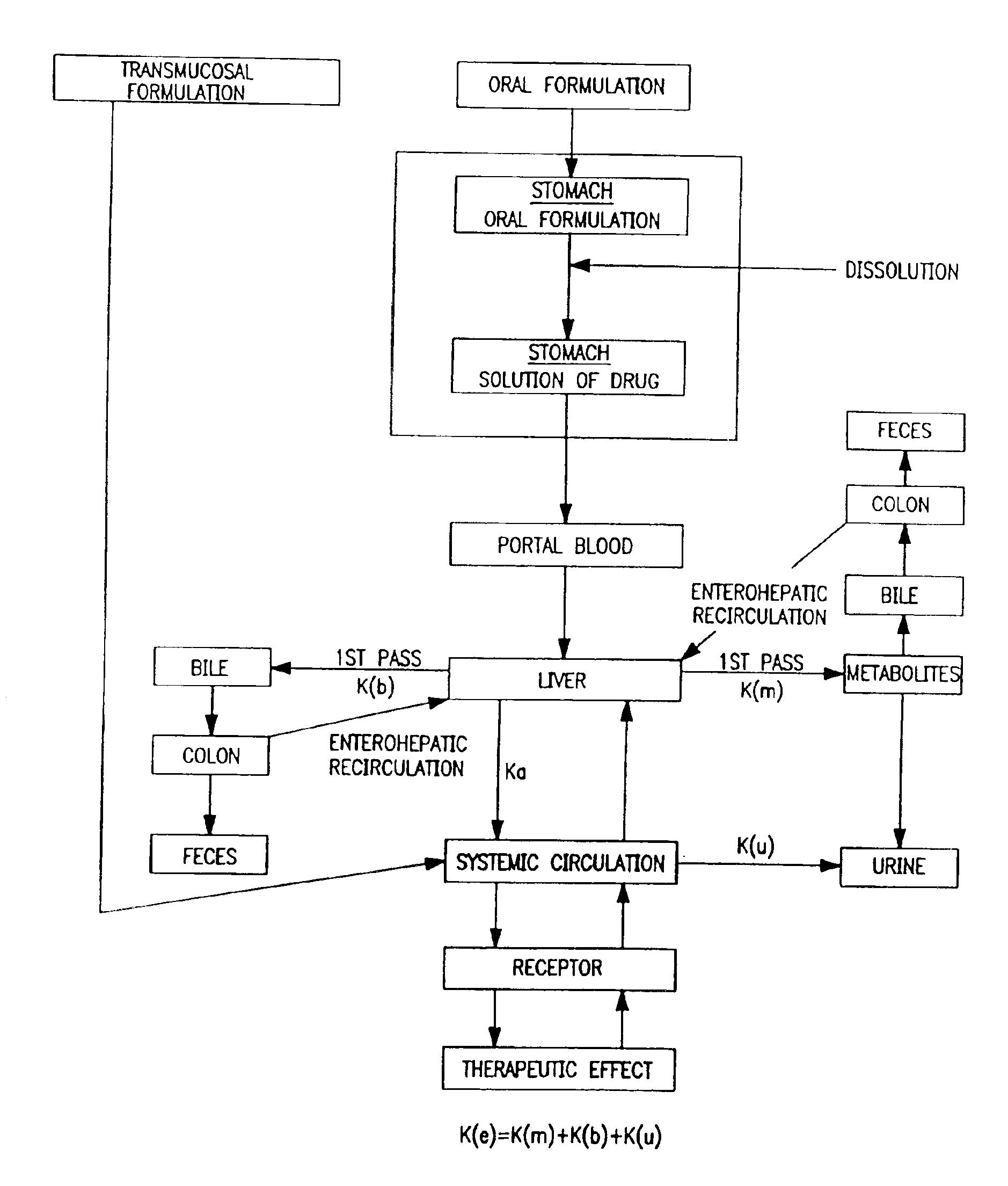
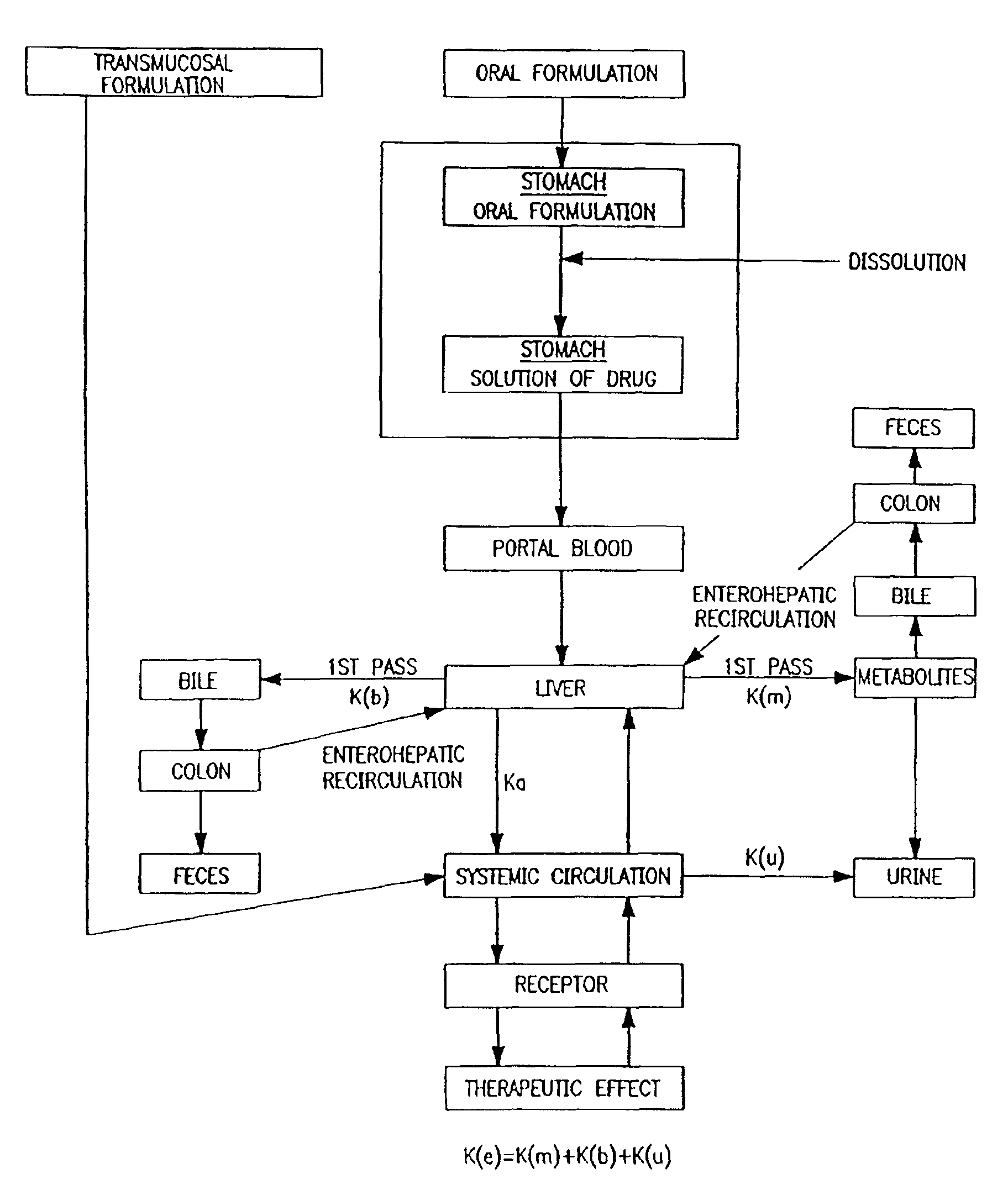
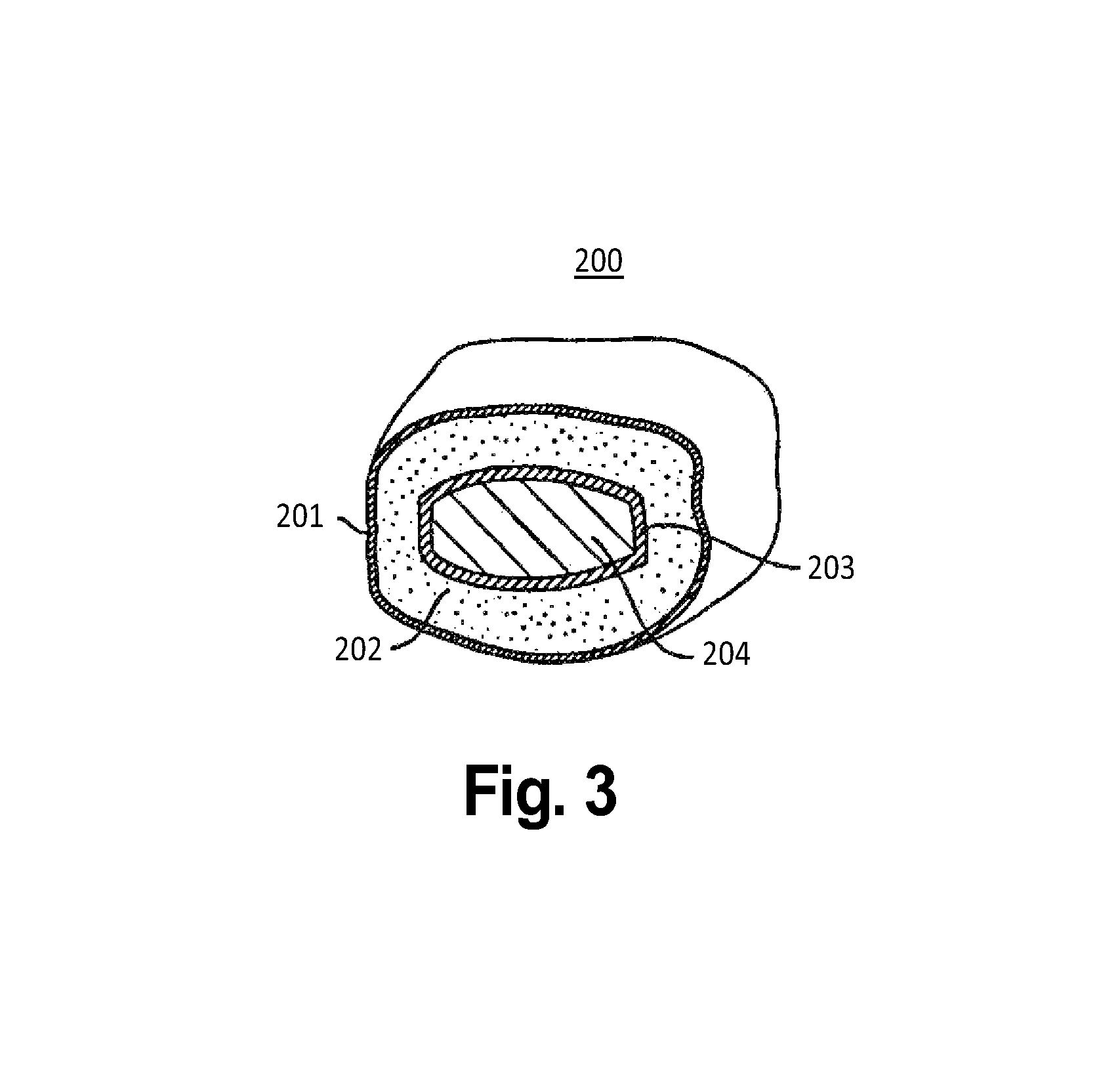
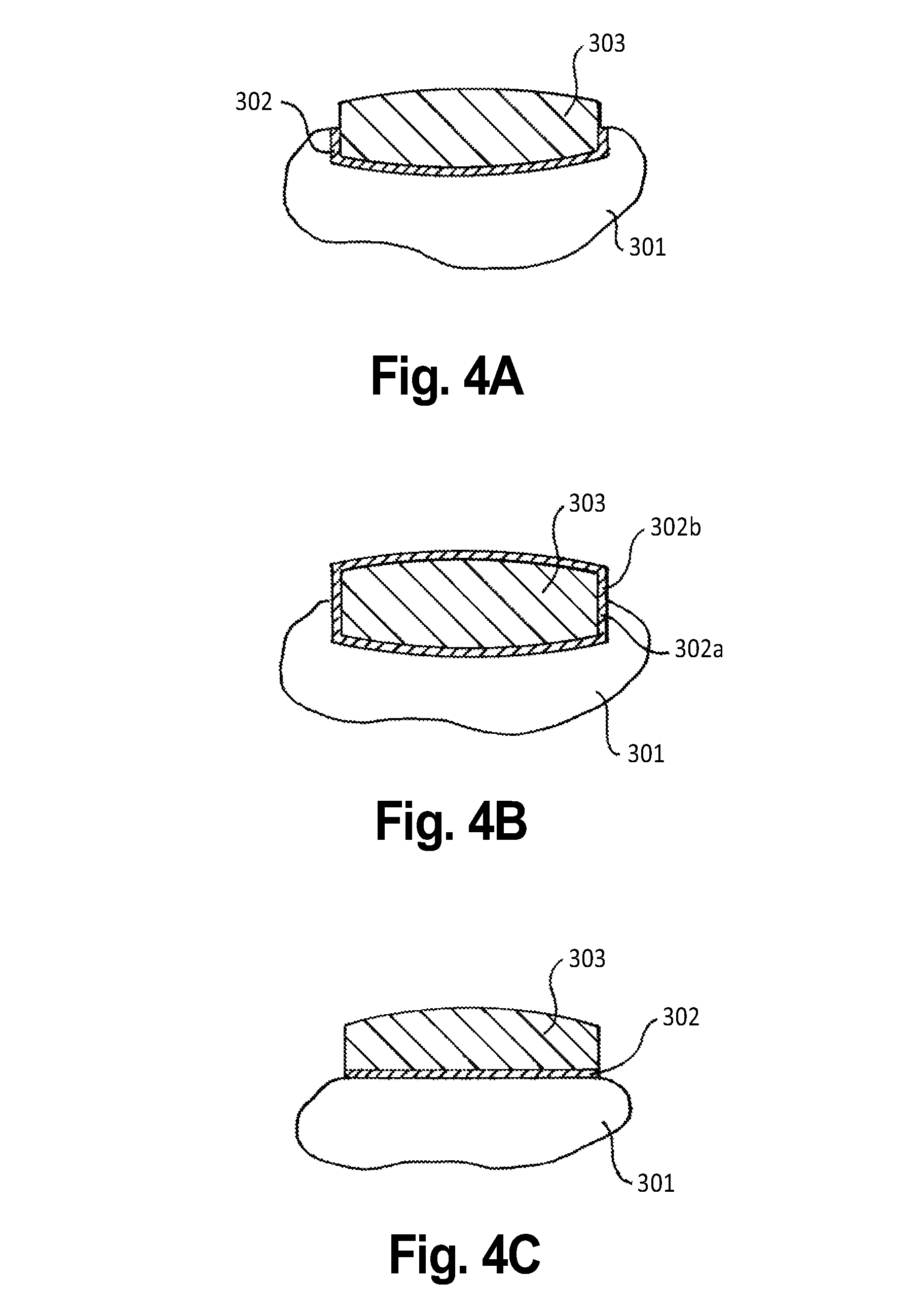
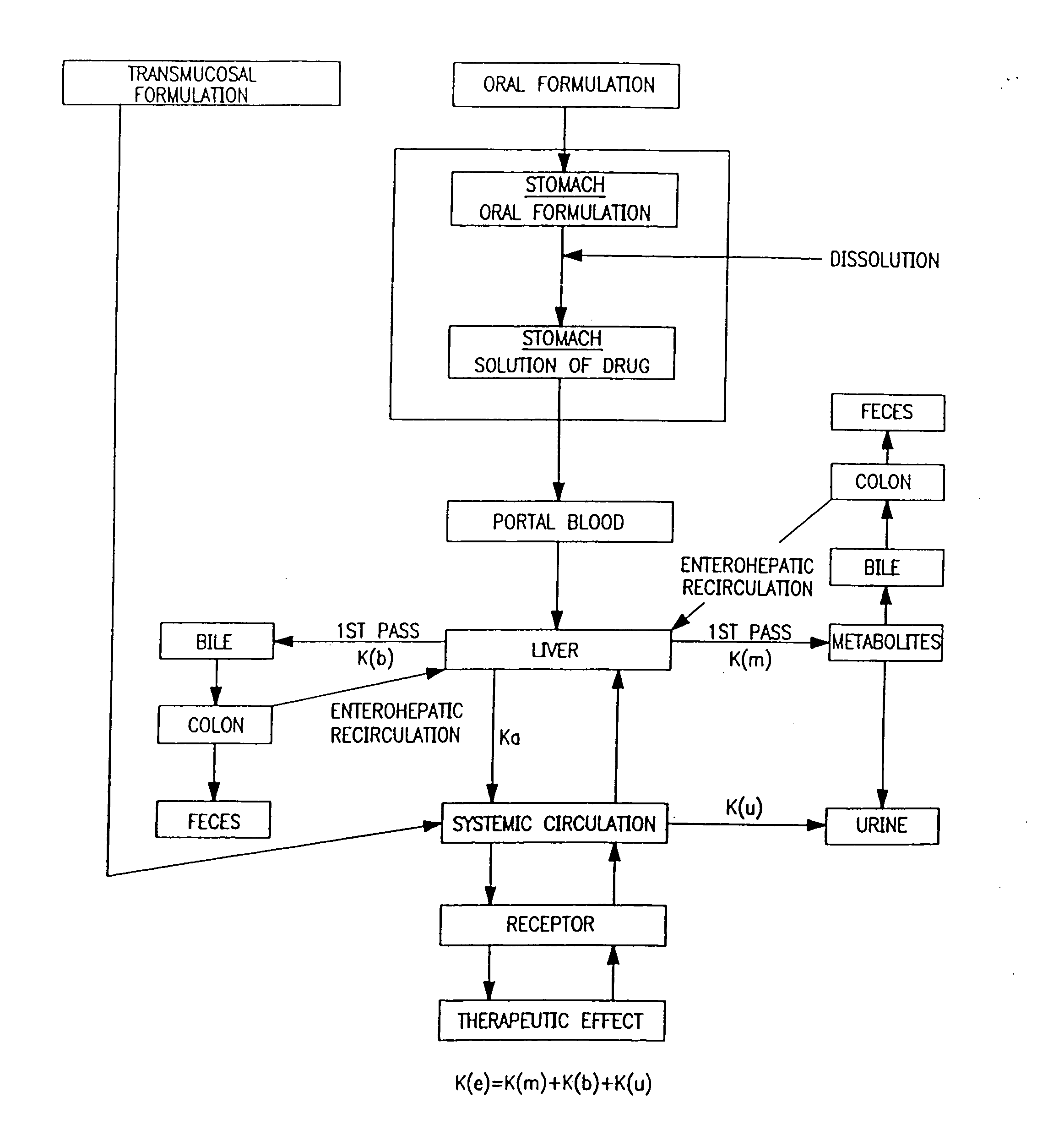
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Compositions and methods for mucosal delivery
Mucosal surface-coat-forming film dosage units containing a water-soluble hydrocolloid, an effective dose of an active agent and a mucosal adhesion enhancer; wherein the active agent is encapsulated within a polymer which is chemically or physically distinct from the hydocolloid; wherein the mucosal adhesion enhancer is a starch graft copolymer; wherein the film exhibits a dry tack value of less than 3.5 g, a wet tack of greater than 35 g, a gelation temperature that is greater than 70° C. for a 2% polymer solution, a dry film thickness of less than 20 mil, a water content of 0.5 to 10%, a tensile strength greater than 1500 psi, a modulus in the range of 35,000 to 300,000 psi, a % elongation of less than 20%, a tear probagation resistance of 0.001 to 1 N, and a dissolution time in the range of 1 to 600 seconds upon application to an oral mucosal surface.
Owner:THALLIUM HLDG CO LLC
Oral transmucosal delivery of drugs or any other ingredients via the inner buccal cavity
InactiveUS6210699B1Avoid irritationAvoid the tasteAdhesive dressingsPill deliverySide effectAdditive ingredient
A device and method for the oral transmucosal delivery of active substances to the oral cavity utilizing an unplasticized polyvinyl pyrrolidone polymer (PVP) as the primary mucoadhesive. The device is applied and adheres to the mucosa of the oral cavity without causing side effects or leaving an unpleasant taste. Preferably the device is a bilayer tablet having a mucoadhesive layer and an overlying active substance containing layer. The mucoadhesive layer may contain PVP as the only adhesive or may be combined with other hydrophilic polymeric substances. The active layer also contains a hydrophilic polymer carrier. The layers in the device dissolve and release the active substance to the oral cavity and is particularly adapted for the delivery of substances active in the oral cavity such as breath fresheners and substances to combat dry mouth. It is also useful for the delivery of ionic drugs such as peptides.
Owner:WATSON PHARMA INC +1
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
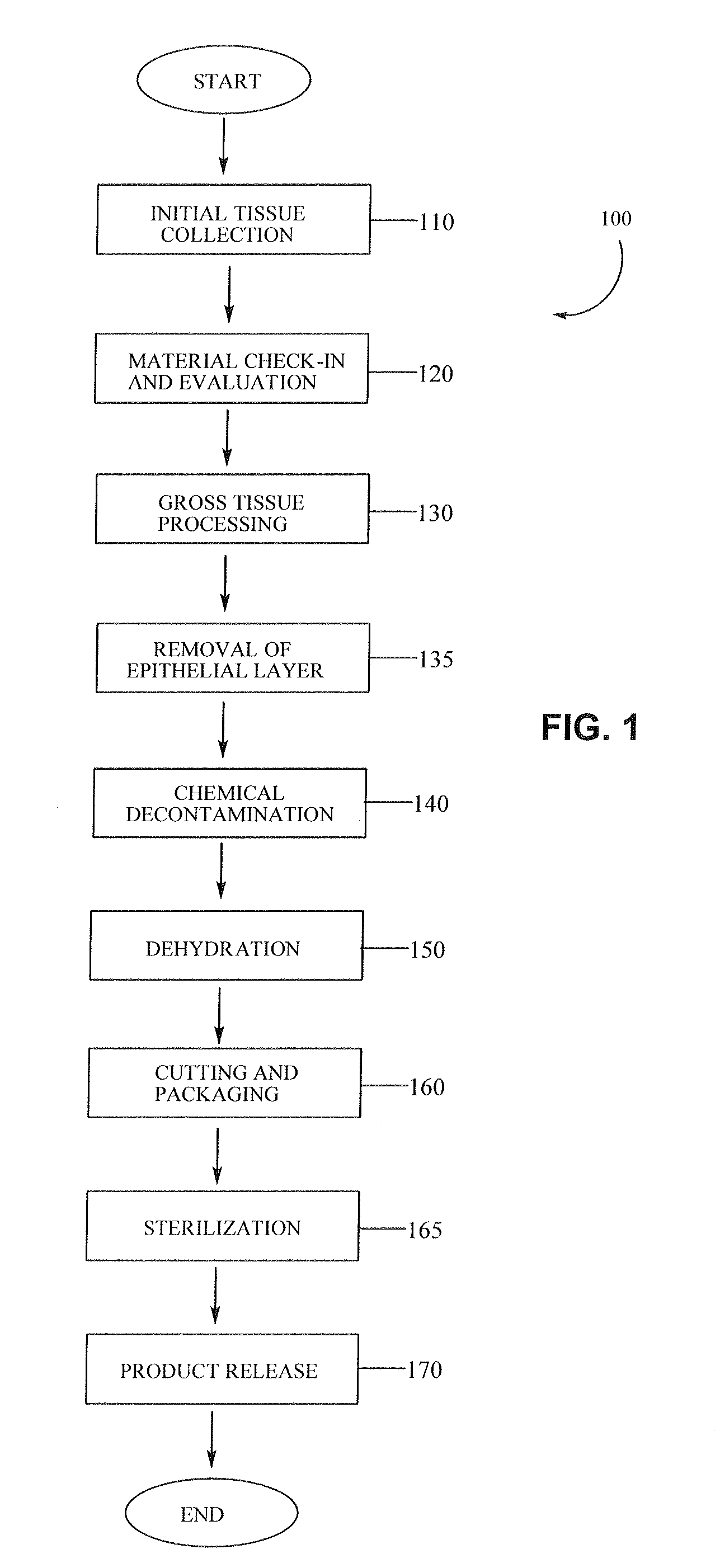
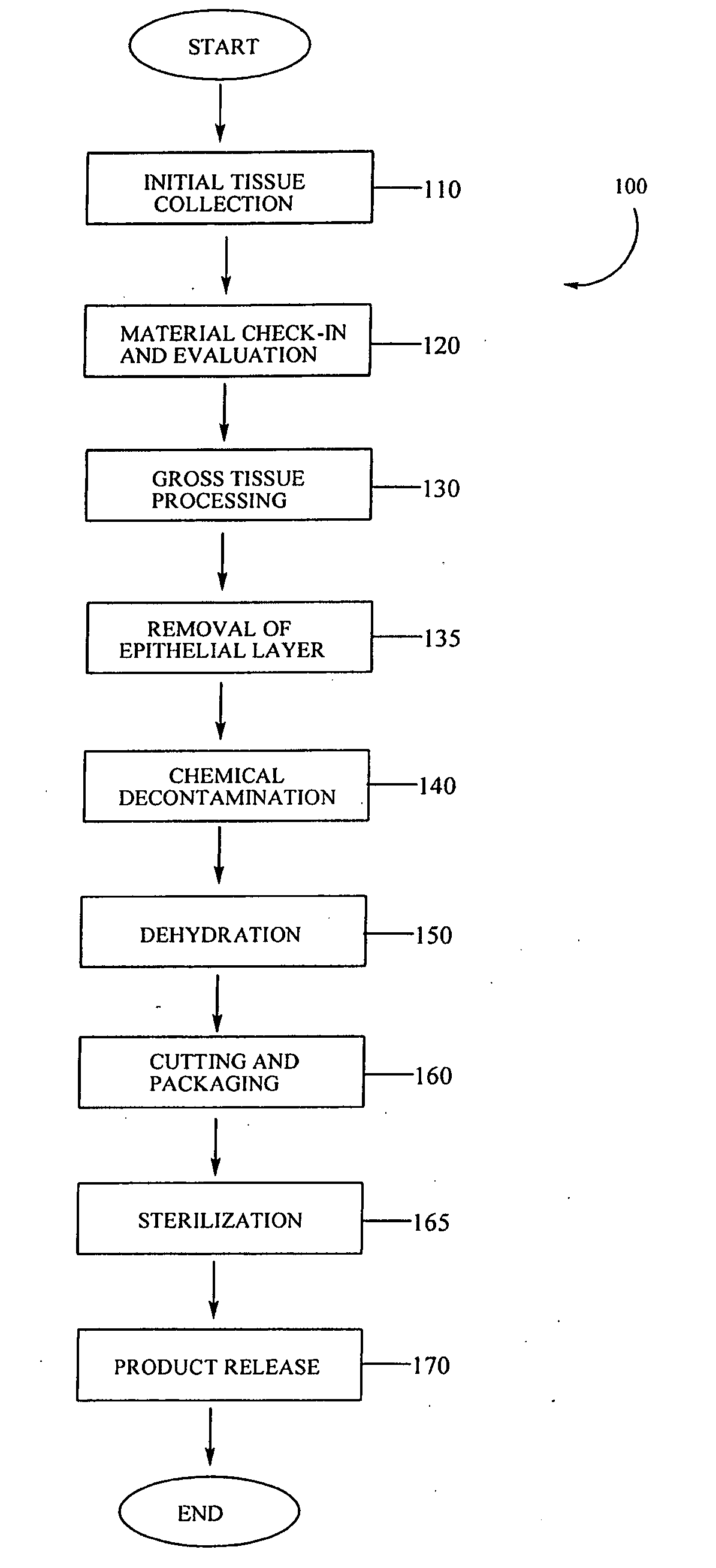
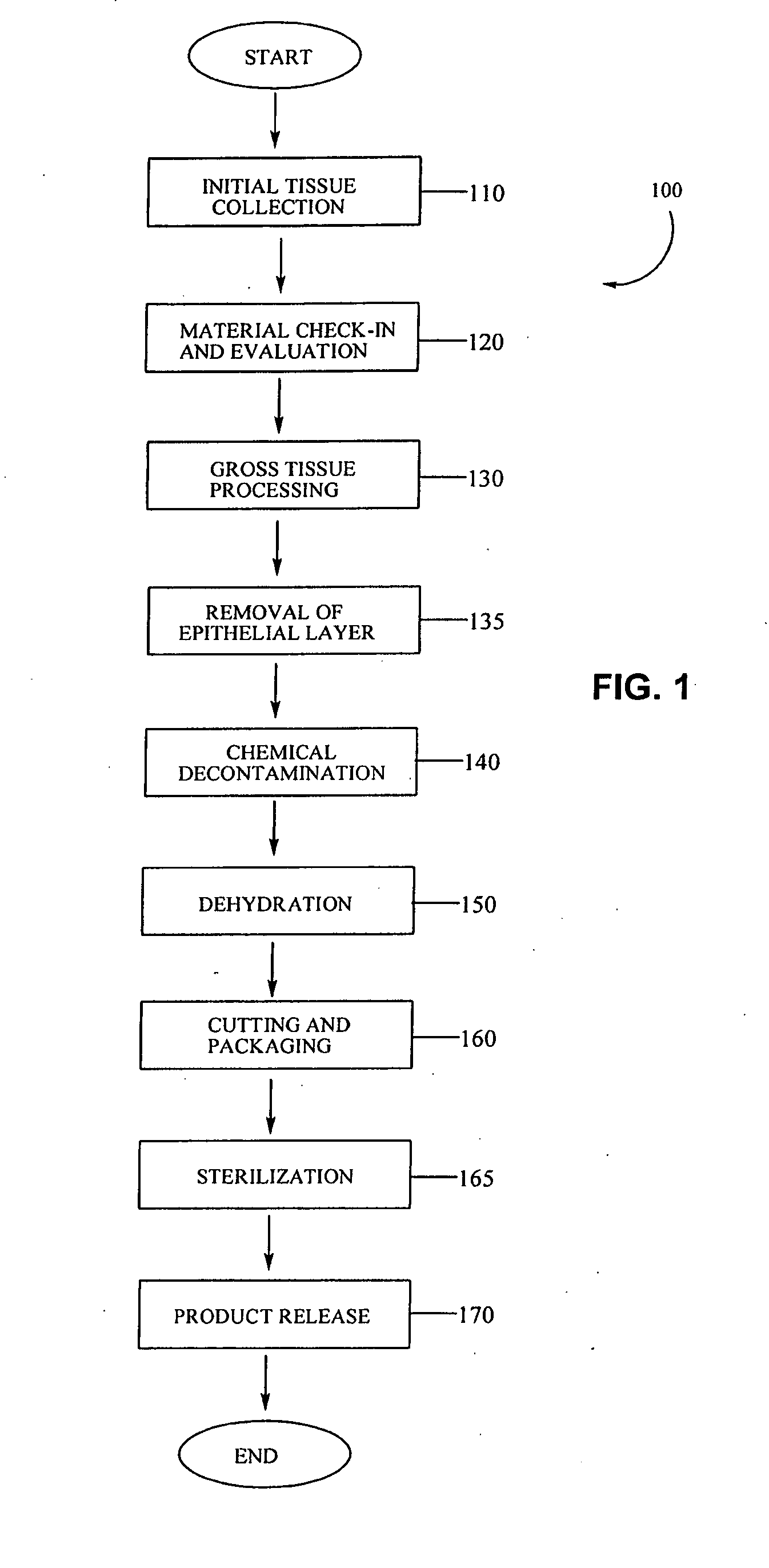
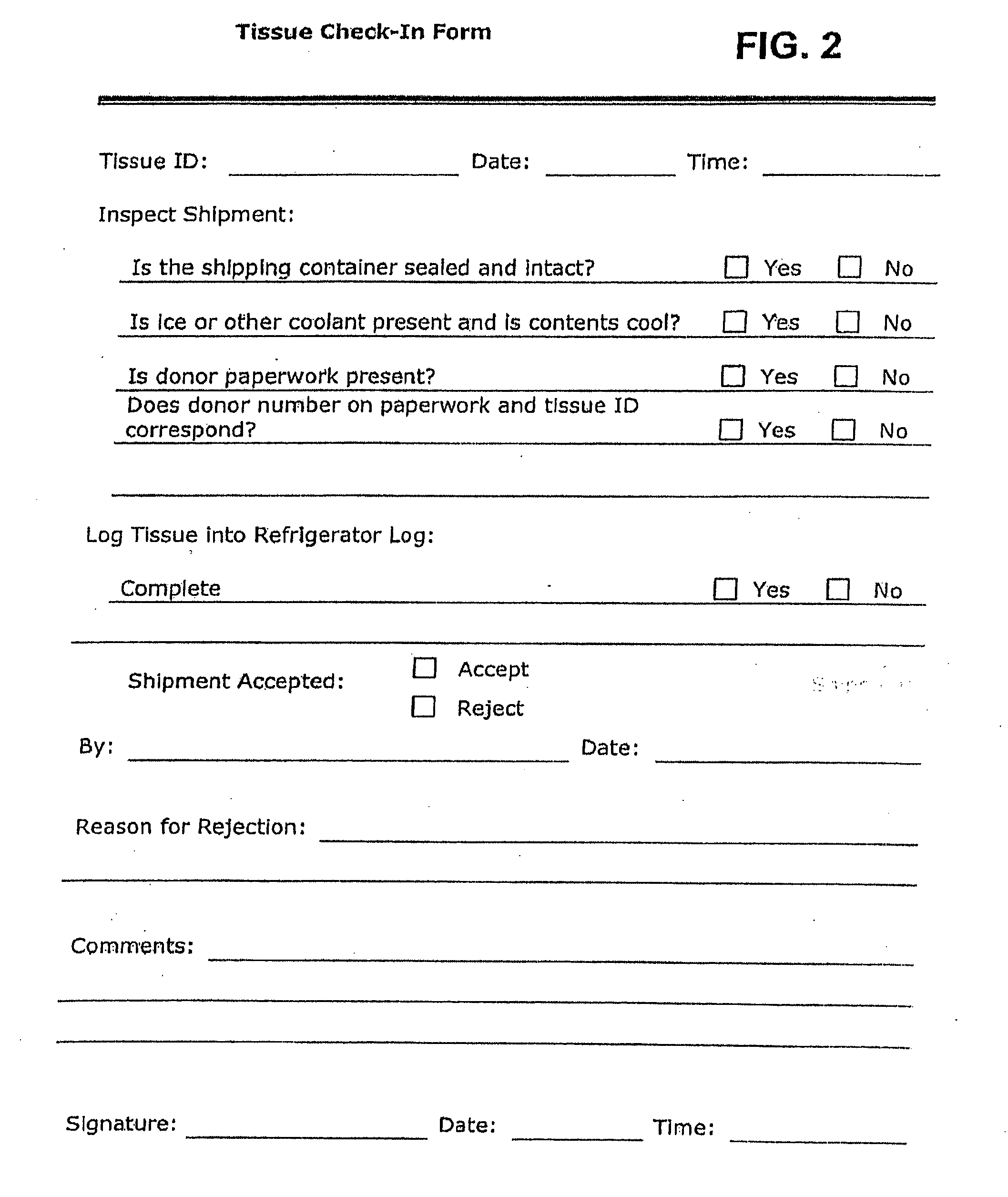
Placental tissue grafts and improved methods of preparing and using the same
Described herein are tissue grafts derived from the placenta. The grafts are composed of at least one layer of amnion tissue where the epithelium layer has been substantially removed in order to expose the basement layer to host cells. By removing the epithelium layer, cells from the host can more readily interact with the cell-adhesion bio-active factors located onto top and within of the basement membrane. Also described herein are methods for making and using the tissue grafts. The laminin structure of amnion tissue is nearly identical to that of native human tissue such as, for example, oral mucosa tissue. This includes high level of laminin-5, a cell adhesion bio-active factor show to bind gingival epithelia-cells, found throughout upper portions of the basement membrane.
Owner:MIMEDX GROUP
Rapid acting drug delivery compositions
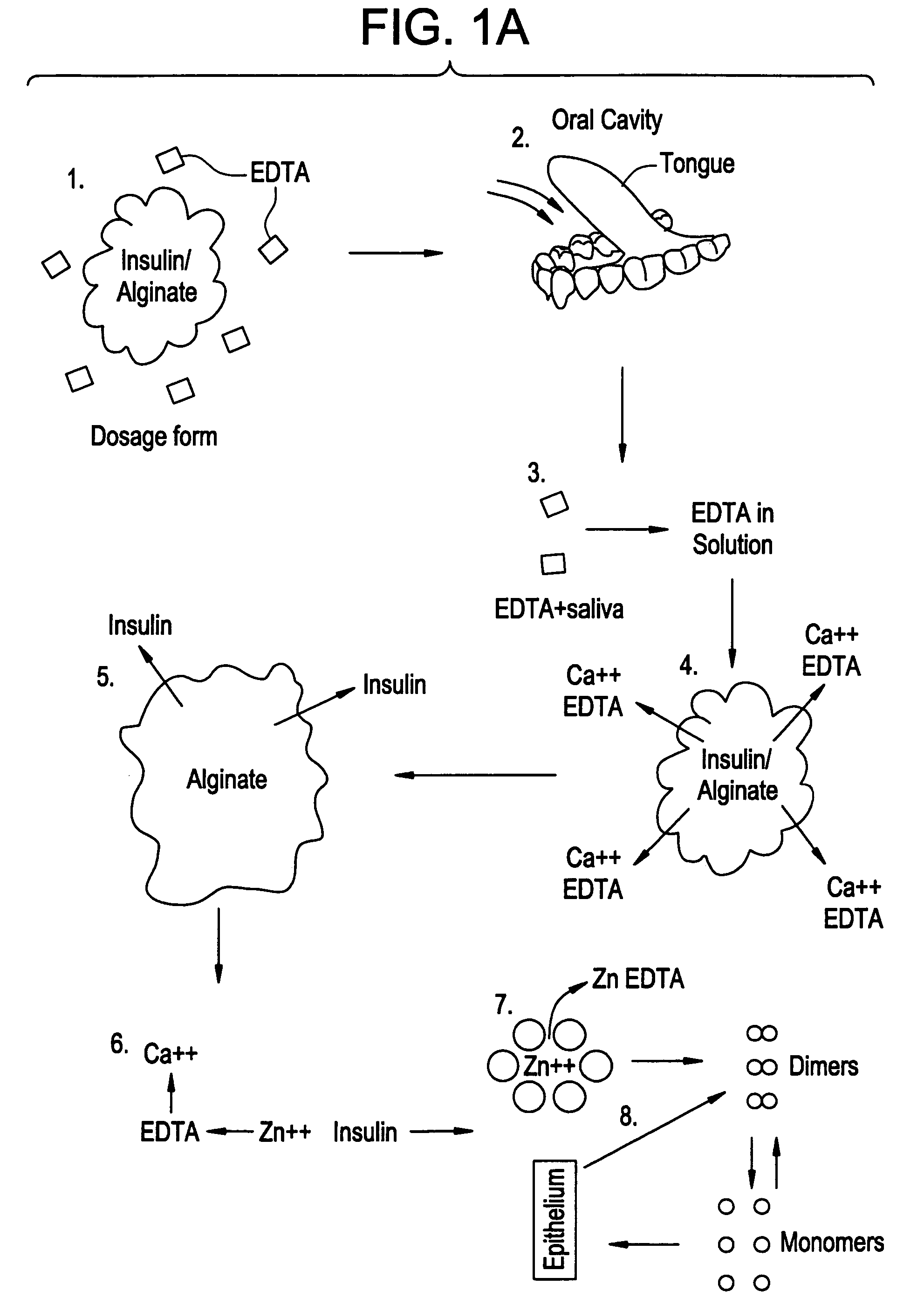
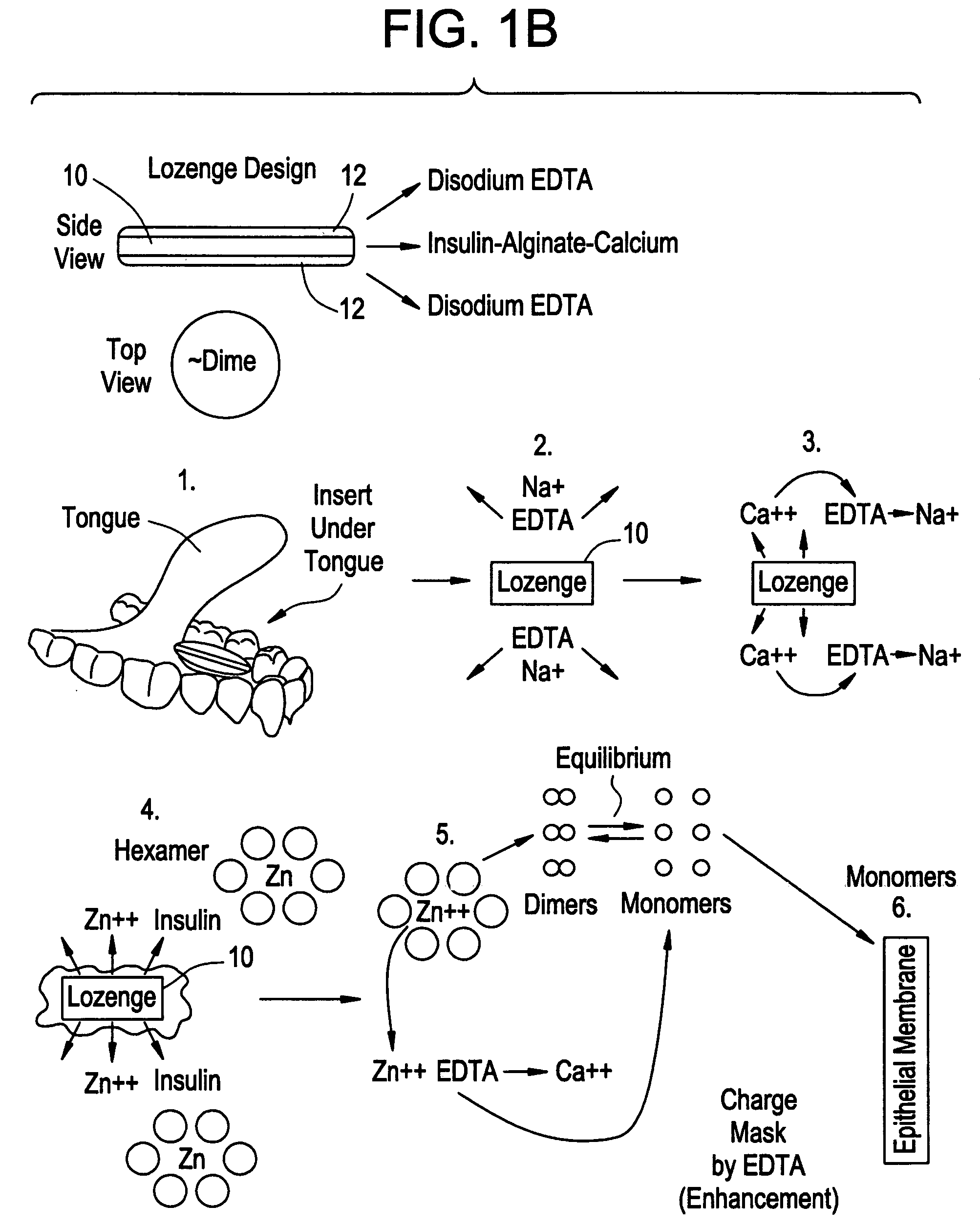
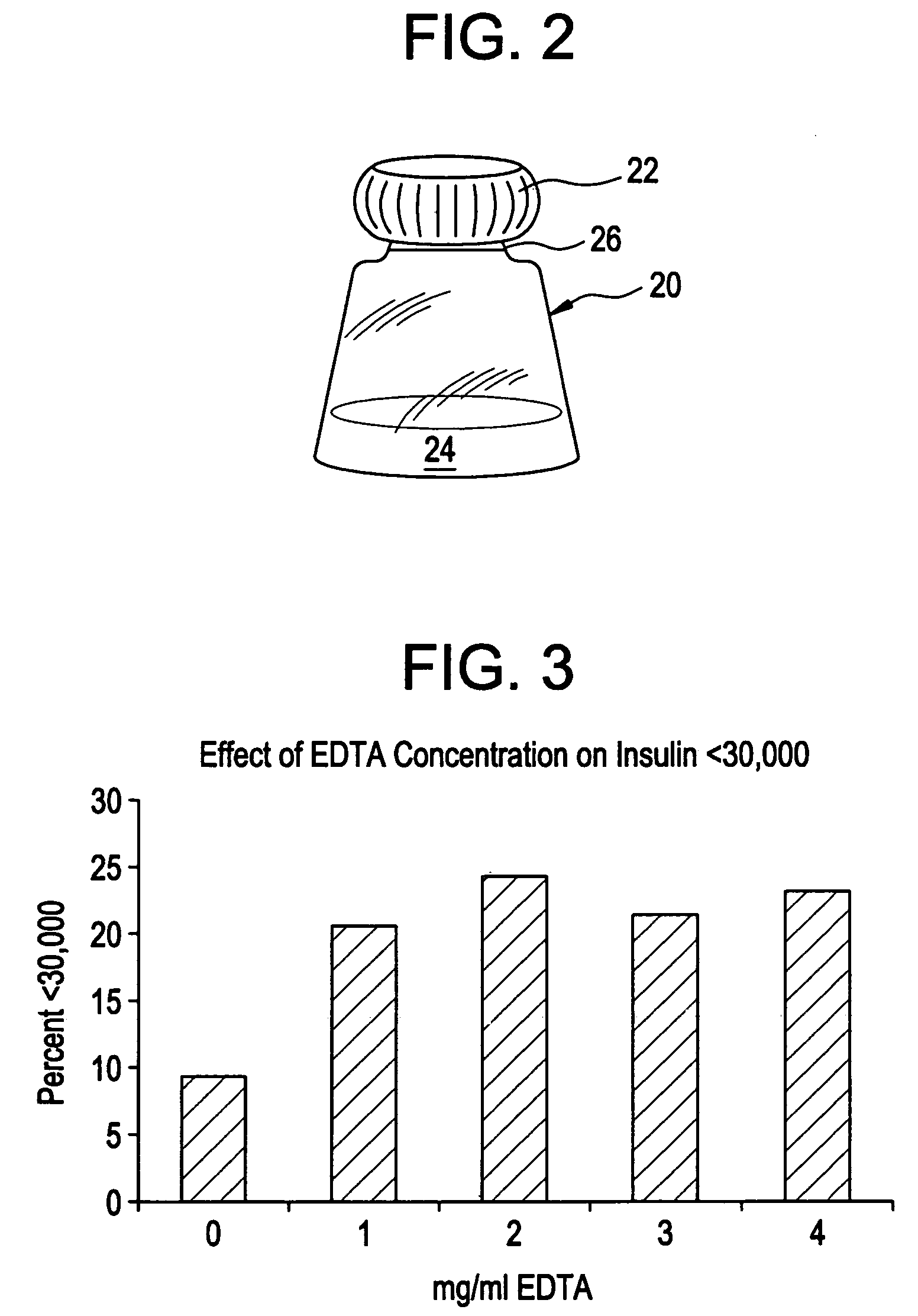
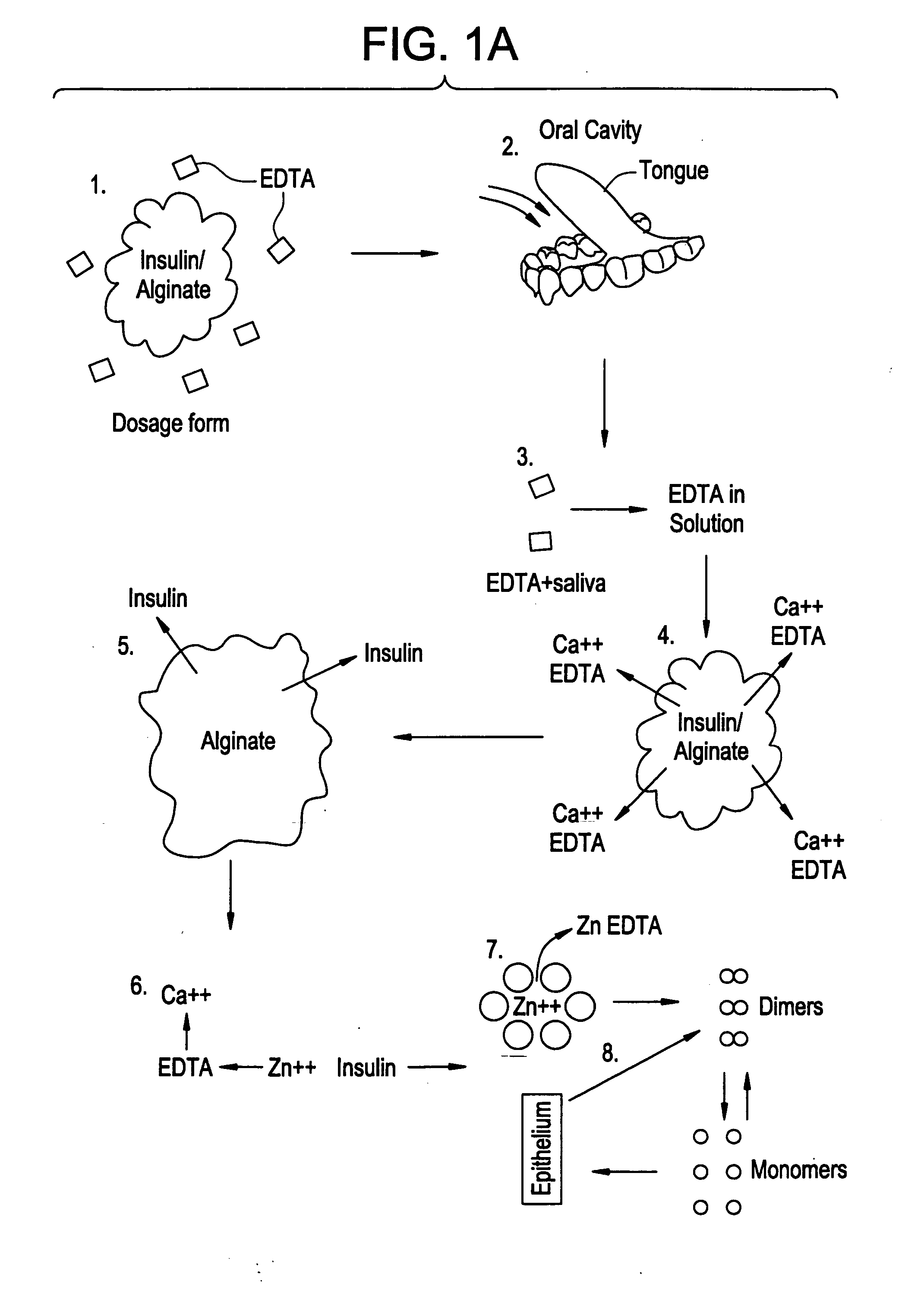
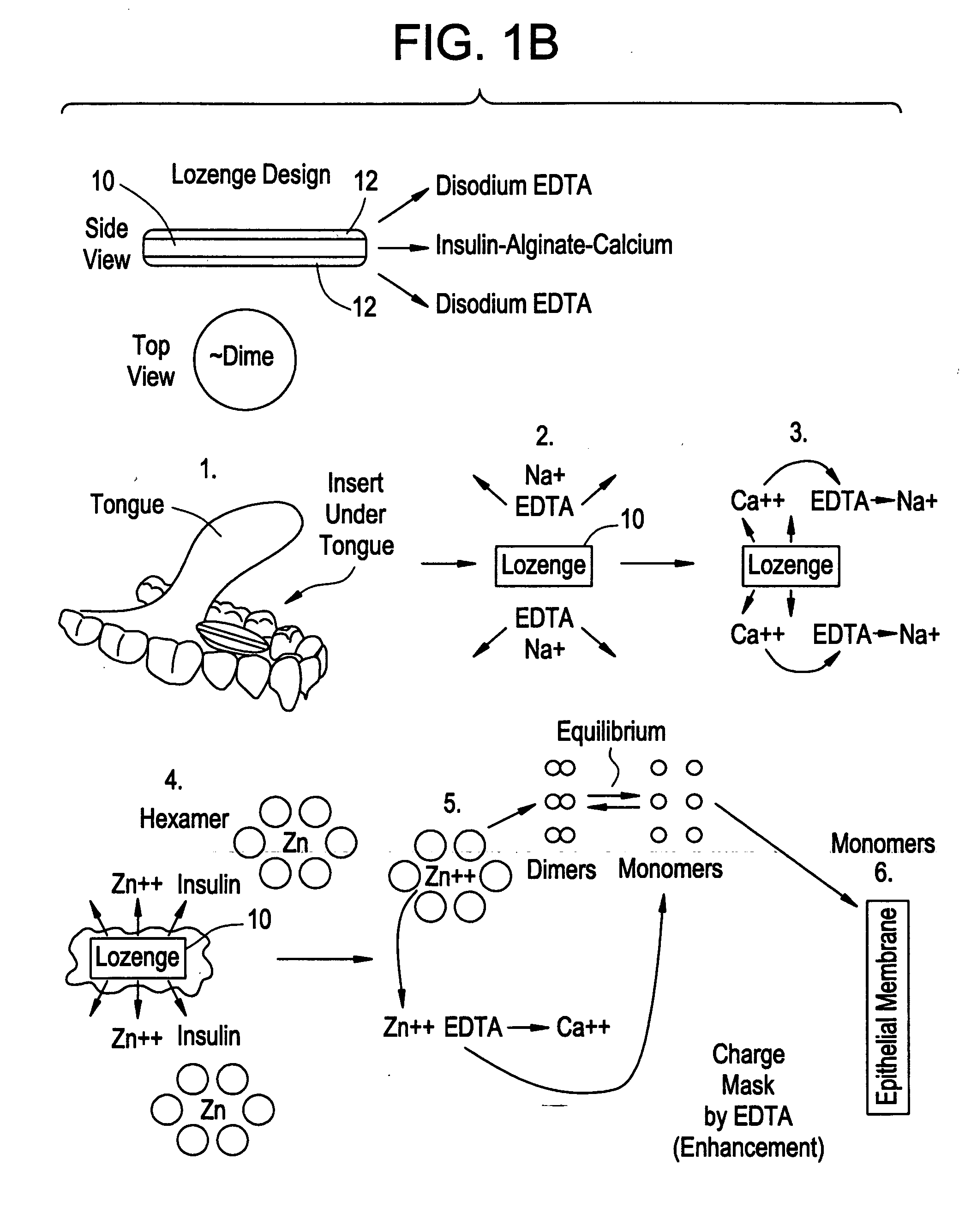
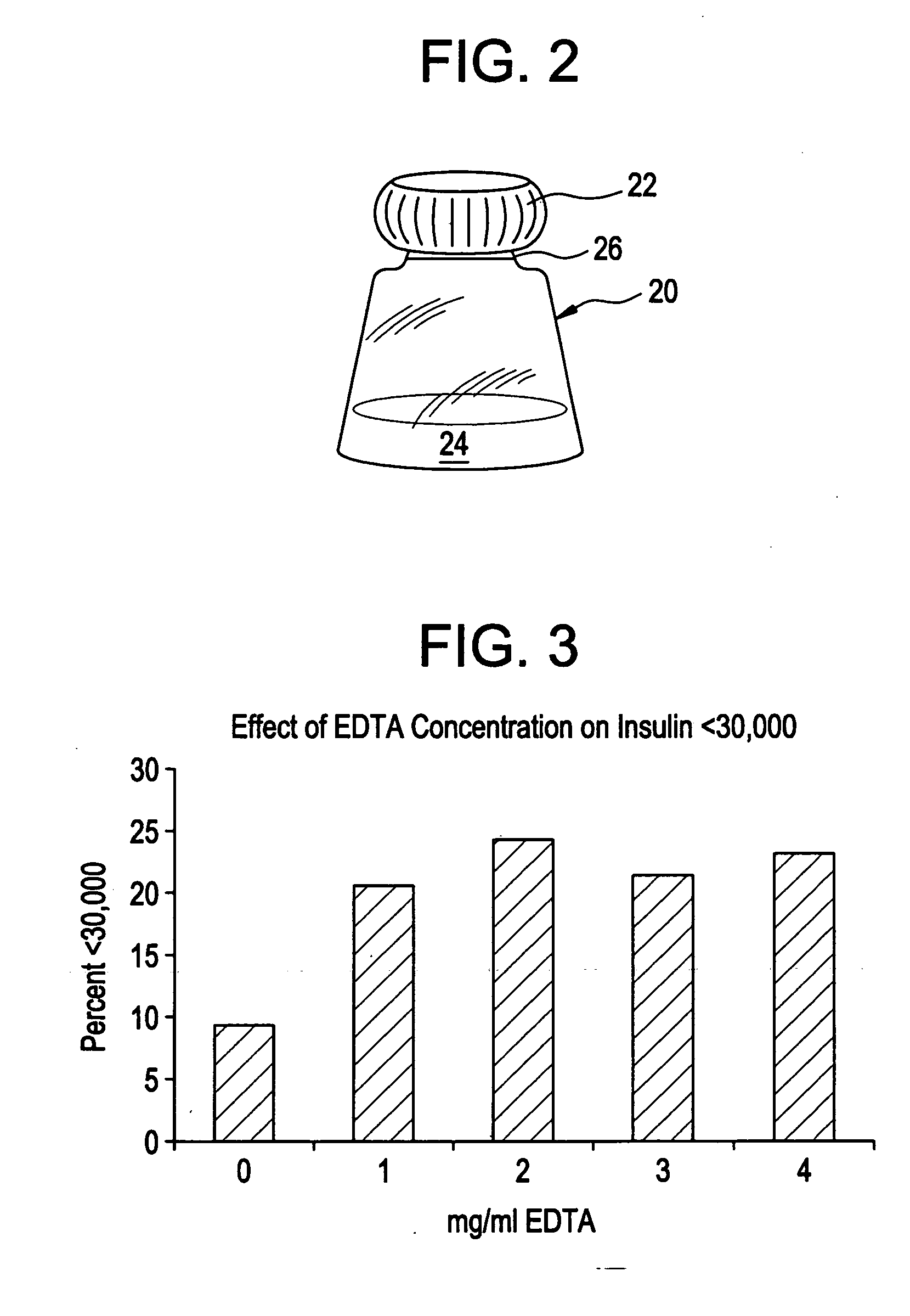
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
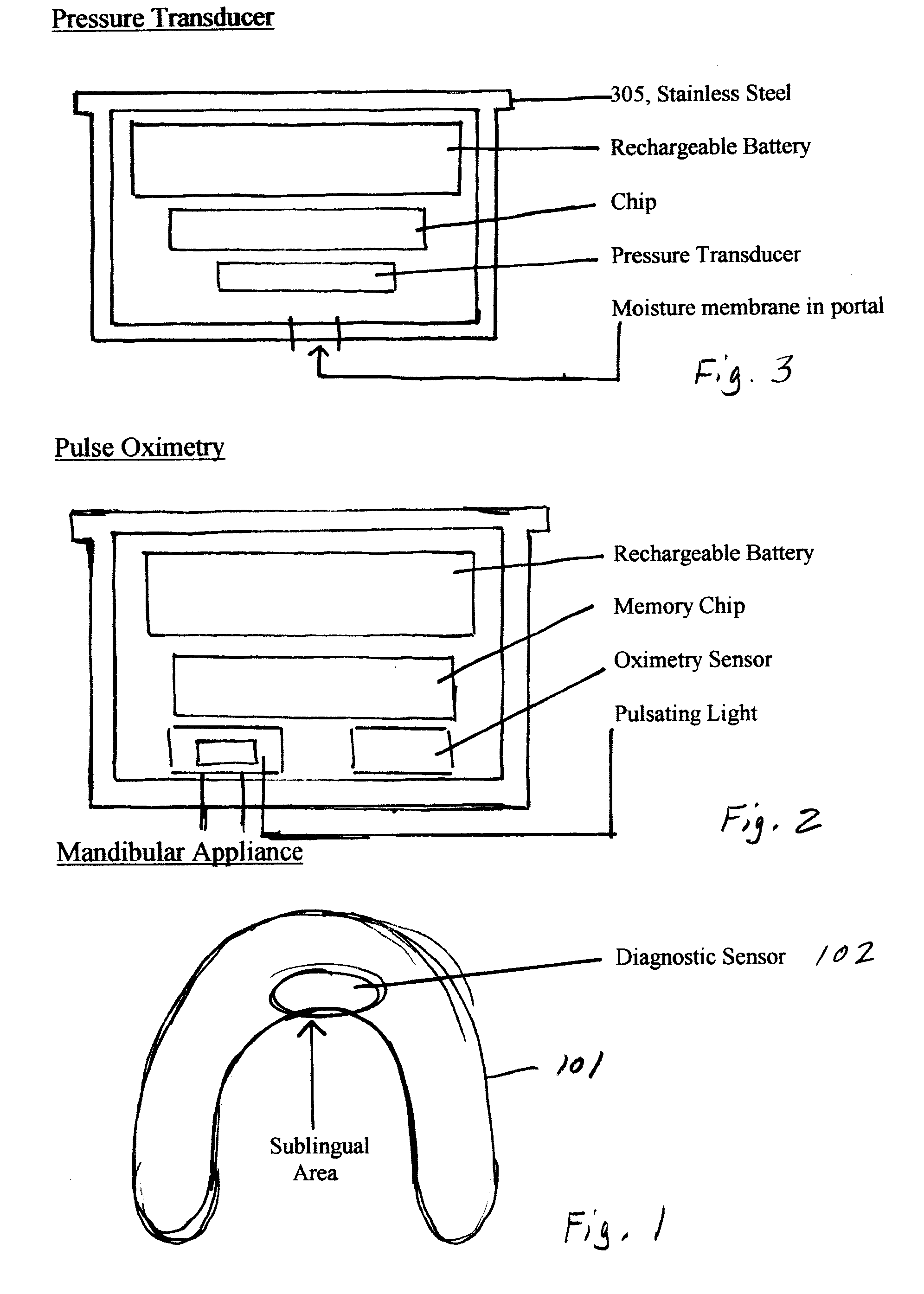
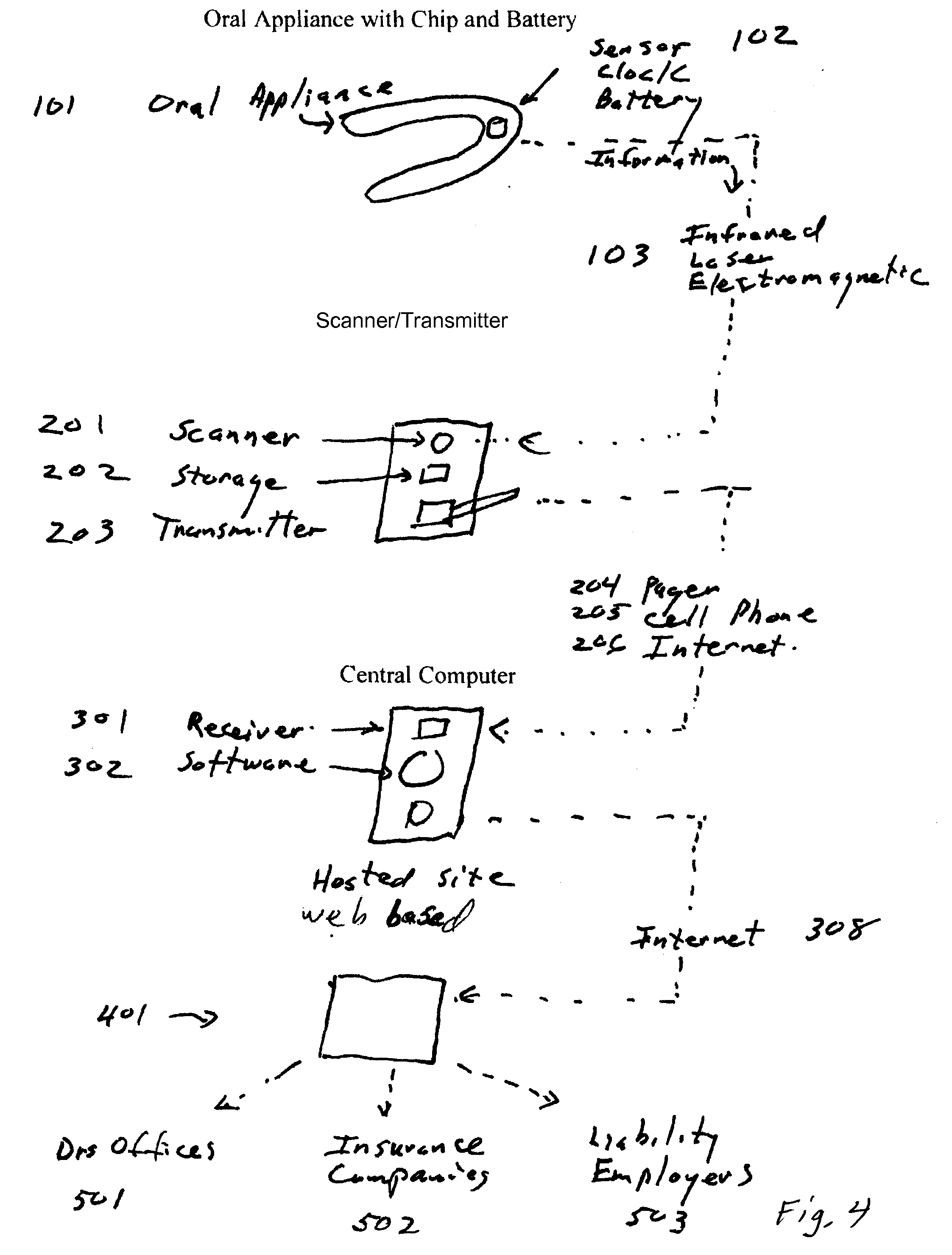
Oral appliance compliance monitoring system
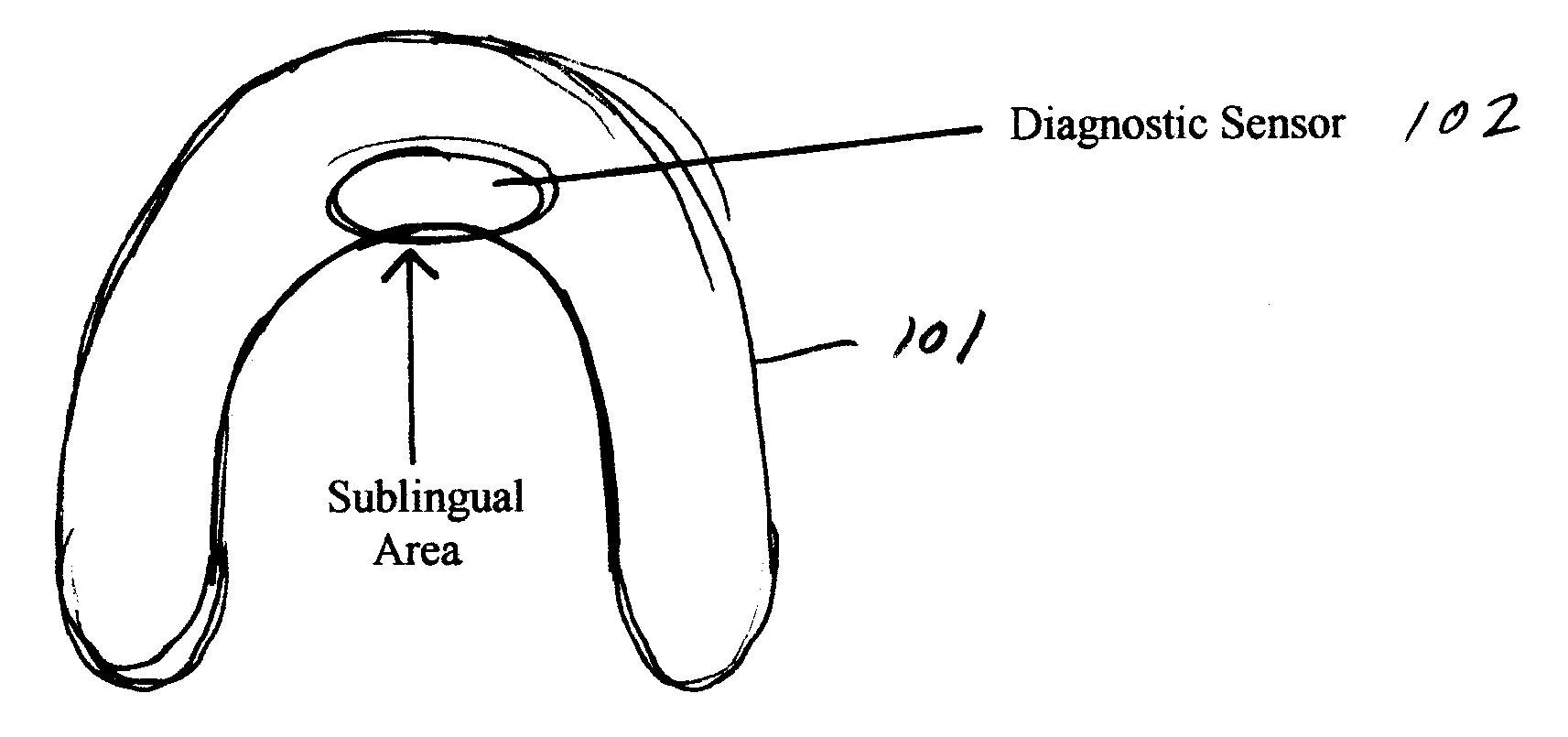
An oral appliance compliance monitoring system and method comprises an oral appliance suitable for wearing in a patient's oral cavity during sleeping periods, the oral appliance having one or more sensors measuring a variety of conditions such as oxygen saturation levels in the oral cavity mucosa. The data generated by the sensor is continuously transmitted to a local scanner which is in communication with a central computer. The computer interprets the data to determine if the patent is wearing the oral appliance in compliance with a prescribed treatment regimen for breathing-related sleep disorders. Remotely located computers are authorized to receive the streamed data to enable remote monitoring of compliance in real time by a plurality of patients with treatment regimens.
Owner:DUHAMEL JAMES BRIAN +2
Stable Lozenge Compositions Providing Rapid Release of Nicotine
InactiveUS20100004294A1Quick releaseIncrease concentrationBiocideNervous disorderCelluloseFast release
Compositions comprising nicotine, which compositions provide a rapid release of nicotine. Nicotine is present in the form of a nicotine-cellulose combination. The compositions are designed for administration to the oral cavity where the nicotine is rapidly released from the composition and available for absorption through the oral mucosa. The compositions are lozenges and have excellent storage stability.
Owner:NICONOVUM AB
Generally linear effervescent oral fentanyl dosage form and methods of administering
Fentanyl containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than know oral formulation and have advantages in terms of cost and side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CIMA LABS
Diindolylmethane-based compositions and methods of use thereof for promoting oral mucosal and bone health
InactiveUS20060264497A1Improve bioavailabilityEffective oral systemic useBiocideCosmetic preparationsDiseaseDental flossing
The present invention includes compositions and methods for the treatment and prevention of oral mucosal disorders and for promotion of bone health. In particular, the present invention describes new therapeutic and preventative uses for 3,3′-diindolylmethane (DIM), or a DIM-related indole, alone or in combination with anti-inflammatory agents and / or antibacterial agents, to treat oral mucosal disorders and promote bone health. The compositions of the invention are used to prevent and reverse oral mucosal disorders and bone loss (osteopenia and osteoporosis) associated with aging and chronic inflammation. Oral mucosal disorders include Periodontitis, gingivitis and related oral mucosal inflammation. Formulations of the compositions of the invention include capsules, tablets, toothpastes, oral gels, mouthwashes, mouth rinses, lozenges, chewing gum, dental floss, and dental topical formulations, and fortified foods.
Owner:BIORESPONSE
Generally linear effervescent oral fentanyl dosage form and methods of administering
Fentanyl containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than know oral formulation and have advantages in terms of cost and side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CEPHALON INC
Film comprising active drugs
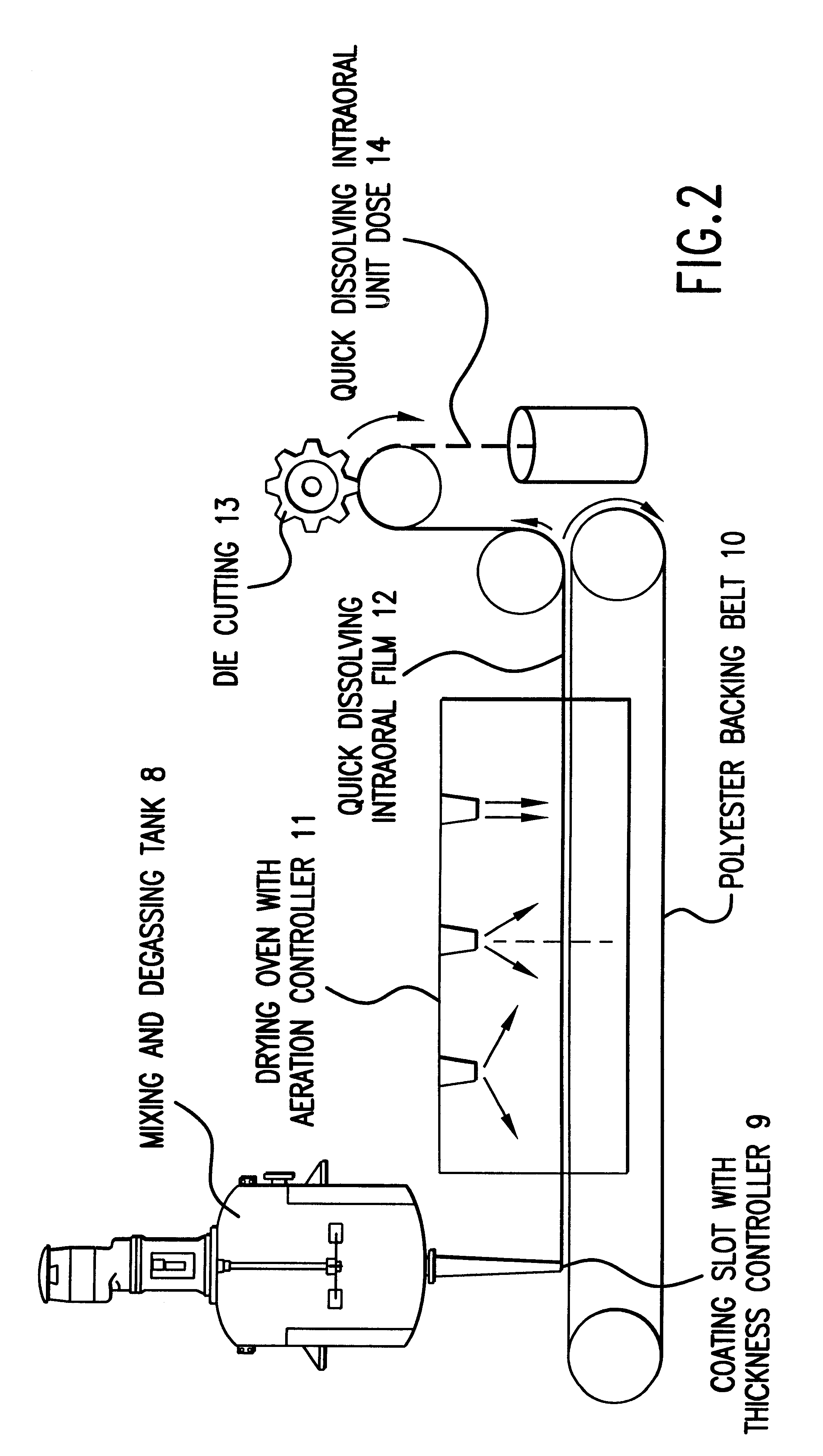
The present invention is related to the composition and methods of manufacture of orally-dissolvable, edible films as a vehicle for the non-invasive administration of active drugs through the mucosal tissues of the oral cavity. The films include a water soluble film-forming polymer such as pullulan. Methods for producing the films are also disclosed.
Owner:MAIBACH TODD
Effervescent oral fentanyl dosage form and methods of administering fentanyl
Fentanyl-containing dosage forms and methods using same are described. These dosage forms include substantially less fentanyl by weight than known oral formulation and have advantages in terms of reduced cost and reduced side effects. These dosage forms are intended for oral administration of fentanyl across the oral mucosa.
Owner:CEPHALON INC
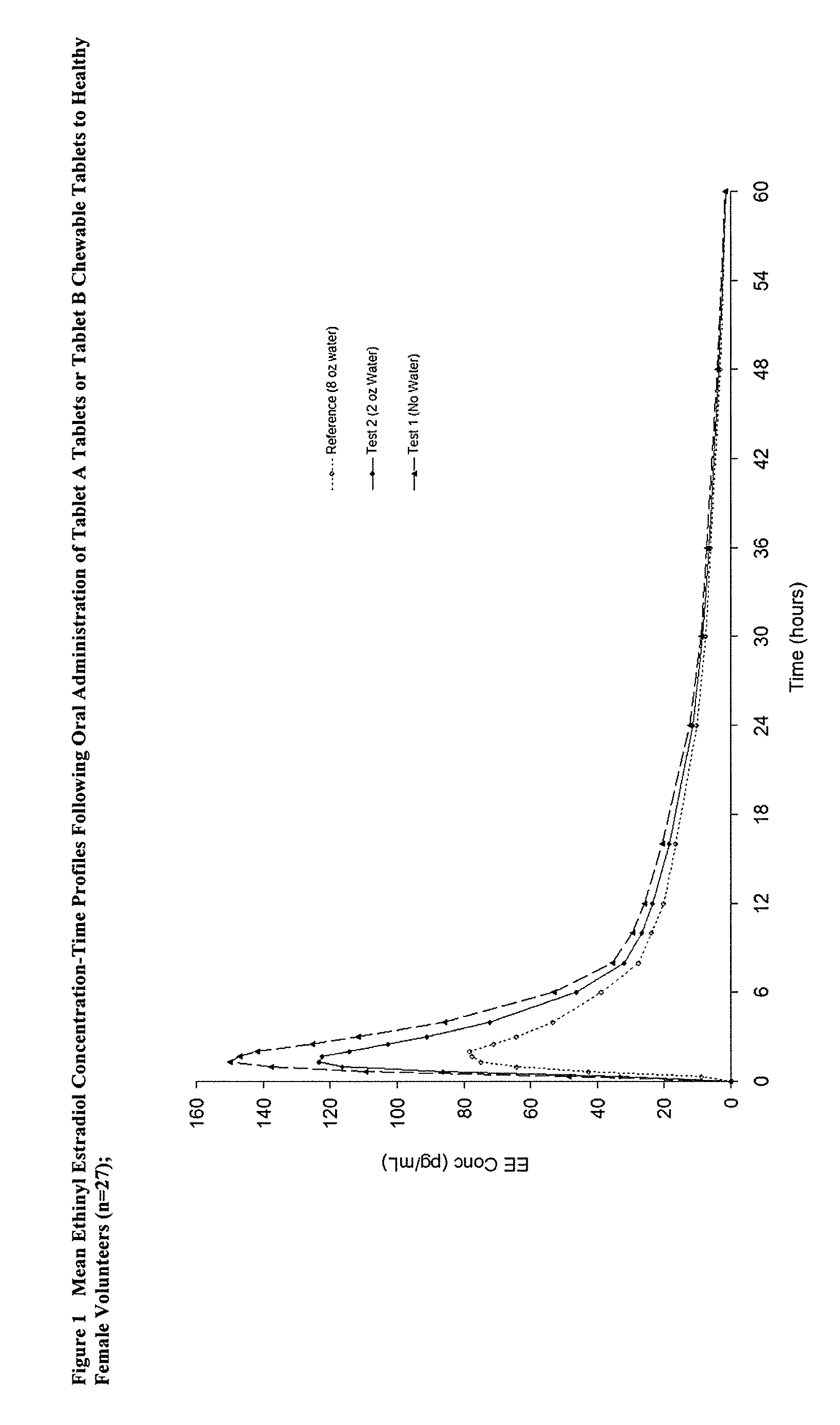
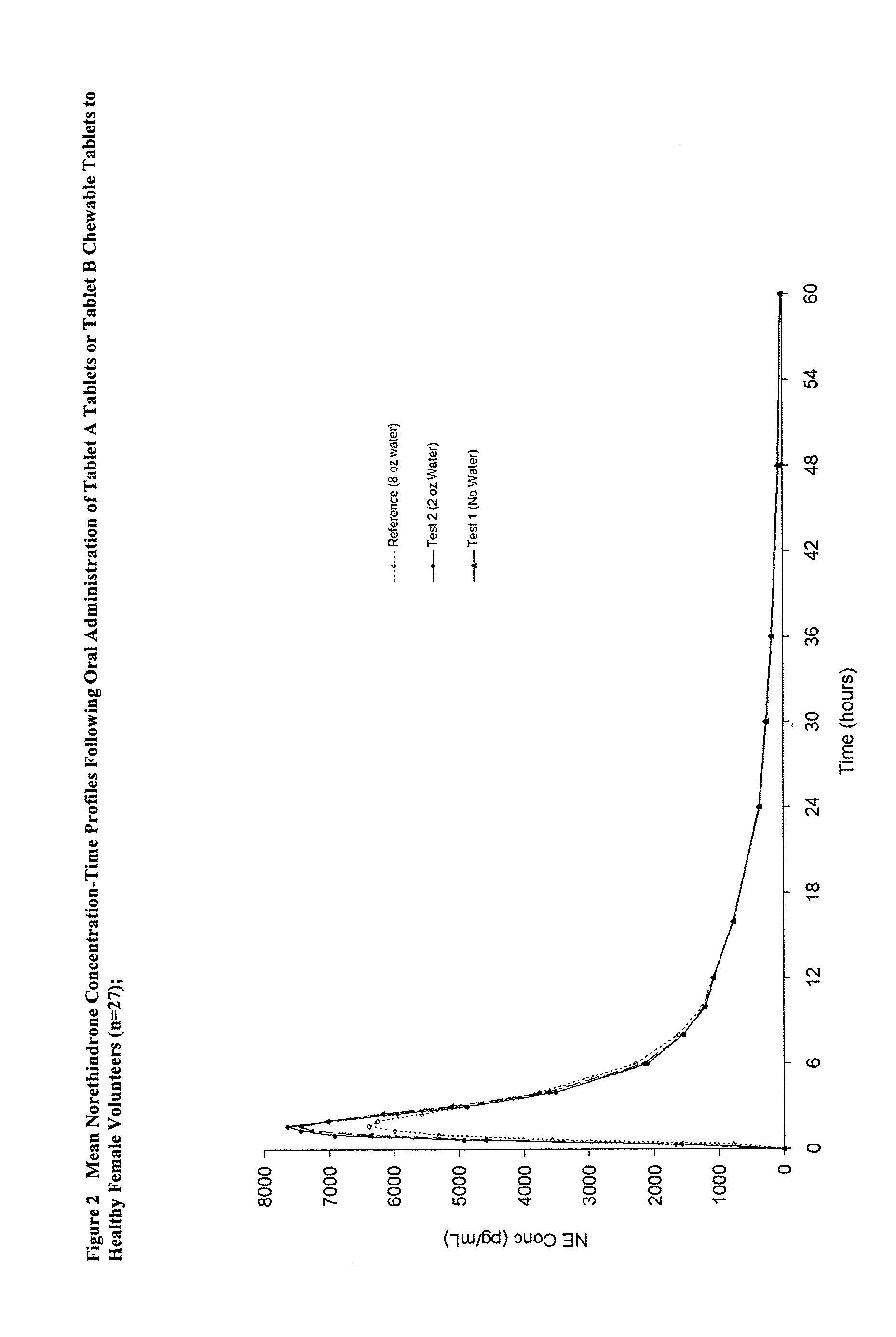
Methods to administer ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20070286819A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPill deliveryHormone replacementBioavailability
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:WARNER CHILCOTT CO LLC
Placental tissue grafts and improved methods of preparing and using the same
Described herein are tissue grafts derived from the placenta. The grafts are composed of at least one layer of amnion tissue where the epithelium layer has been substantially removed in order to expose the basement layer to host cells. By removing the epithelium layer, cells from the host can more readily interact with the cell-adhesion bio-active factors located onto top and within of the basement membrane. Also described herein are methods for making and using the tissue grafts. The laminin structure of amnion tissue is nearly identical to that of native human tissue such as, for example, oral mucosa tissue. This includes high level of laminin-5, a cell adhesion bio-active factor show to bind gingival epithelia-cells, found throughout upper portions of the basement membrane.
Owner:MIMEDX GROUP +1
Coated chewing gum product and method of making
InactiveUS7163705B2Promote absorptionContainers for annular articlesChewing gumSodium bicarbonateWater soluble
A method for producing a coated chewing gum product with accelerated absorption of medicaments through oral mucosa, as well as the chewing gum product so produced, is obtained by using a xylitol or sorbitol coating, or by adding a water-soluble alkaline material, such as a bicarbonate salt, to the chewing gum center, a coating on the center, or both. Coatings made with sorbitol or xylitol or gum centers that include sodium bicarbonate are particularly preferred.
Owner:WM WRIGLEY JR CO
Buccal, polar and non-polar sprays containing propofol
InactiveUS20050002867A1Fast absorptionRapid onsetBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
Buccal, polar and non-polar spray or capsule containing drugs for treating disorders of the central nervous system
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:SUDA
Buccal, polar and non-polar spray or capsule
InactiveUS6998110B2Rapid onsetFast absorptionBatteries circuit arrangementsAerosol deliverySolventPharmacology
Owner:SUDA
Method for preparing epithelium of autologous cornea
ActiveCN1916166ARepair and Restoration FunctionsGood effectArtificial cell constructsVertebrate cellsOral mucosaTissue engineering
This invention discloses a method for preparing auto-corneal epithelia. The method comprises: (1) performing primary culture of cells of patients' oral mucosa tissue or corneal limbal tissue for 3-15 days; (2) performing proliferation sub-culture for 7-30 days; (3) using fibrinogen as the supporting material to construct tissue engineering corneal epithelia together with the proliferation sub-cultured cells. The method can avoid the problems of immunological rejection, pollution, ununiform cell growth caused by the biological support, and slow degradation faced by presents techniques. The preparation of auto-corneal in this invention is not restricted by corneal epithelia damage, and suitable corneal is available for patients at any situation.
Owner:北京智慧细胞生物科技有限公司
Buccal, polar and non-polar spray or capsule containing drugs for treating pain
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:SUDA
Combination tablet with chewable outer layer
ActiveUS8404275B2Improve the level ofAbsorbed more rapidlyBiocideAnimal repellantsDosing regimenSide effect
A pharmaceutical composition in the form of a combination tablet is described. The tablet has a rapidly absorbed component that enters the circulation by traversing the buccal mucosa, oral mucosa and combinations thereof, and a more slowly absorbed component that is swallowed. The therapeutic agent in the swallowed portion is absorbed across the gastric mucosa. The combination tablet may be modified, by varying the specific combinations of excipients, fillers, and the like to effect distinct release rates. In addition, the rapid and slow components may have identical or different therapeutic agents depending on the application to a specific medical condition. One embodiment of the combination tablet includes a prostaglandin inhibitor in the rapidly absorbed component in order to mitigate the side effects of immediate release niacin that is in the slow absorbing component. Such combination compositions will increase patient compliance with various dosing regimens due to the resultant decrease in the number of tablets that a patient would need to take on a daily basis.
Owner:VITALS
Buccal, polar and non-polar spray or capsule containing cardiovascular or renal drugs
InactiveUS20050025713A1Fast absorptionRapid onsetElcosanoid active ingredientsAerosol deliverySolventBioactive compound
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide biologically active compounds for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, active compound, and optional flavoring agent; formulation II: aqueous polar solvent, active compound, optionally flavoring agent, and propellant; formulation III: non-polar solvent, active compound, and optional flavoring agent; and formulation IV: non-polar solvent, active compound, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Composition of Rapid Disintegrating Direct Compression Buccal Tablet
InactiveUS20090263476A1Facilitate drug deliveryReduce deliveryBiocidePill deliveryAlkaline earth oxidesPh gradient
A composition of a rapidly disintegrating buccal dosage form containing a drug, at least one non-effervescent base such as an alkali metal or alkaline earth metal oxide or hydroxide, and a disintegrant. The base regulates the pH gradient to deliver the drug to the buccal, sublingual or oral mucosal membranes at a desired rate of absorption. The composition is micronized for uniform distribution, and the drug is converted from ionized form to unionized form, without the use of an effervescent agent.
Owner:NAVINTA
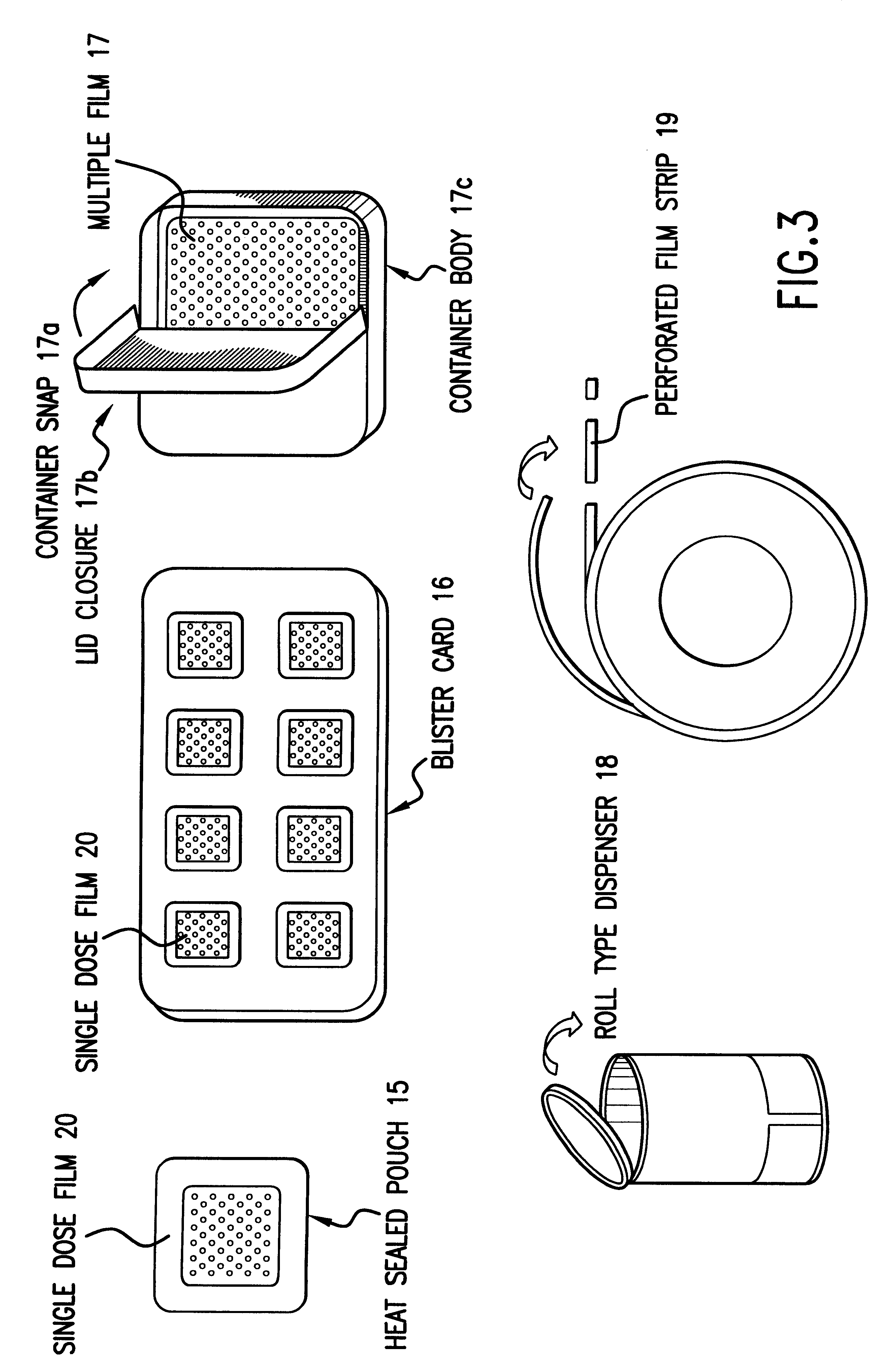
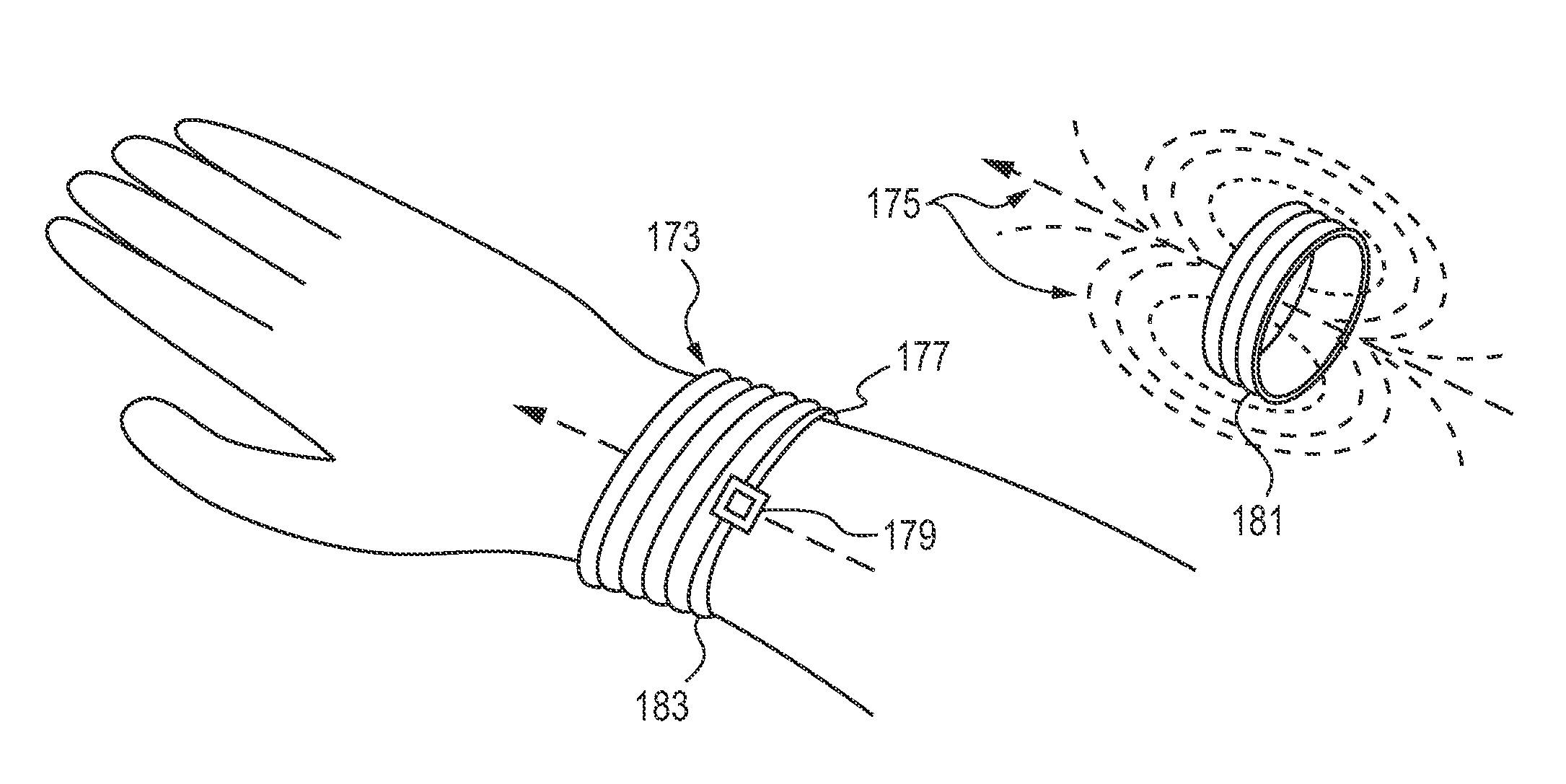
Storage and Dispensing Devices for Administration of Oral Transmucosal Dosage Forms
ActiveUS20100253476A1Drug and medicationsNear-field in RFIDBiomedical engineeringPatient identification
Dispensing devices and systems for oral transmucosal administration of small volume drug dosage forms to the oral mucosa are provided. The dispensing device may be a single dose applicator (SDA), or an electromechanical device comprising a means for patient identification such as a wrist worn RFID tag and annular bidirectional antenna together with a lock-out feature.
Owner:VERTICAL PHARMA
Compositions and methods for mucositis and oncology therapies
In alternative embodiments, this invention provides compositions and methods for treating cancer or any condition caused by dysfunctional cells, side effects from treatments for cancer or any condition caused by dysfunctional cells, e.g., mucositis therapies (e.g., for oral mucositis; digestive mucositis; esophageal mucositis; intestinal mucositis). In alternative embodiments, the invention provides cytoprotection products that may be used either alone or in combination with other medical therapies such as cancer chemotherapies and radiation therapies.
Owner:VICUS THERAPEUTICS
Biological Tissue Sheet, Method Of Forming The Same And Transplantation Method By Using The Sheet
InactiveUS20080039940A1Raise security concernsSimple structureSkin implantsEpidermal cells/skin cellsConjunctival EpitheliumCuticle
A biological tissue sheet which is expected as exerting a favorable therapeutic effect and a high safety in transplantation. The biological tissue sheet formed by (a) preparing in vivo-derived cells; (b) sowing the in vivo-derived cells on amniotic membrane; and (c) culturing and proliferating the in vivo-derived cells in the absence of any xenogeneic animal cells. As the cells of a biological origin, for example, cells originating in corneal epithelium, conjunctival epithelium, skin epidermis, hair follicle epithelium, oral mucosa, respiratory tract mucosa, or intestinal tract mucosa.
Owner:KOUJI HASHIMOTO +1
Buccal, polar and non-polar spray containing sumatriptan
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide sumatriptan for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, sumatriptan, and optional flavoring agent; formulation II: aqueous polar solvent, sumatriptan, optionally flavoring agent, and propellant; formulation III: non-polar solvent, sumatriptan, and optional flavoring agent; formulation IV: non-polar solvent, sumatriptan, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, sumatriptan, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, sumatriptan, optional flavoring agent, and propellant.
Owner:DUGGER HARRY A III +1
Film comprising therapeutic agents
The present invention is related to the composition and methods of manufacture of orally-dissolvable, edible films as a vehicle for the non-invasive administration of nitroglycerin, as well as other therapeutic agents either with or without nitroglycerin, through the mucosal tissues of the oral cavity. The films include a water soluble film-forming polymer such as pullulan. Methods for producing the films are also disclosed.
Owner:ACADERM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com