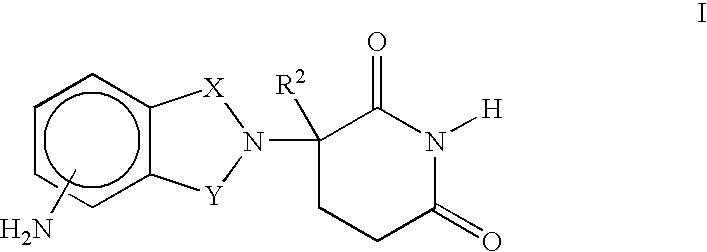
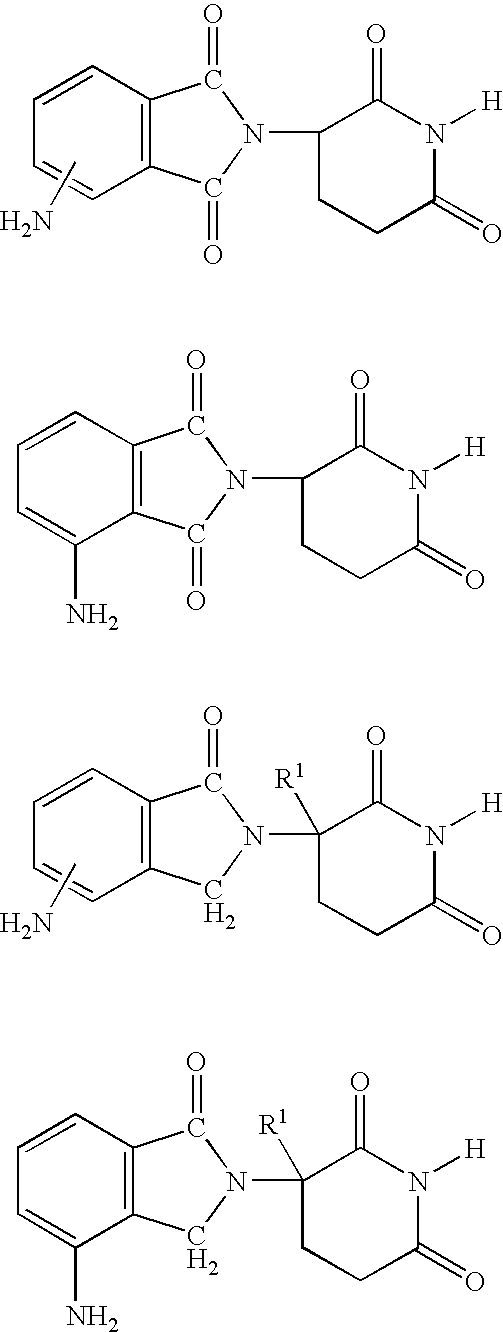
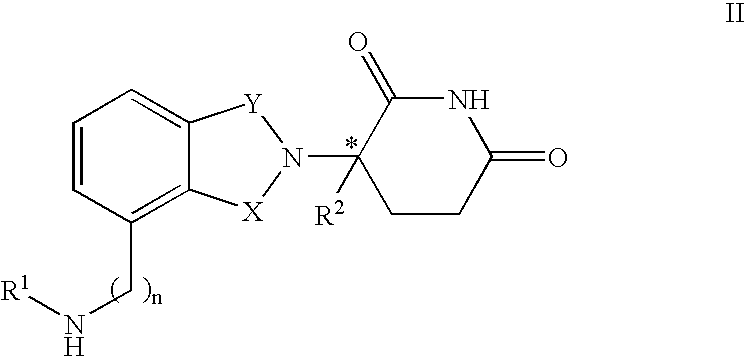
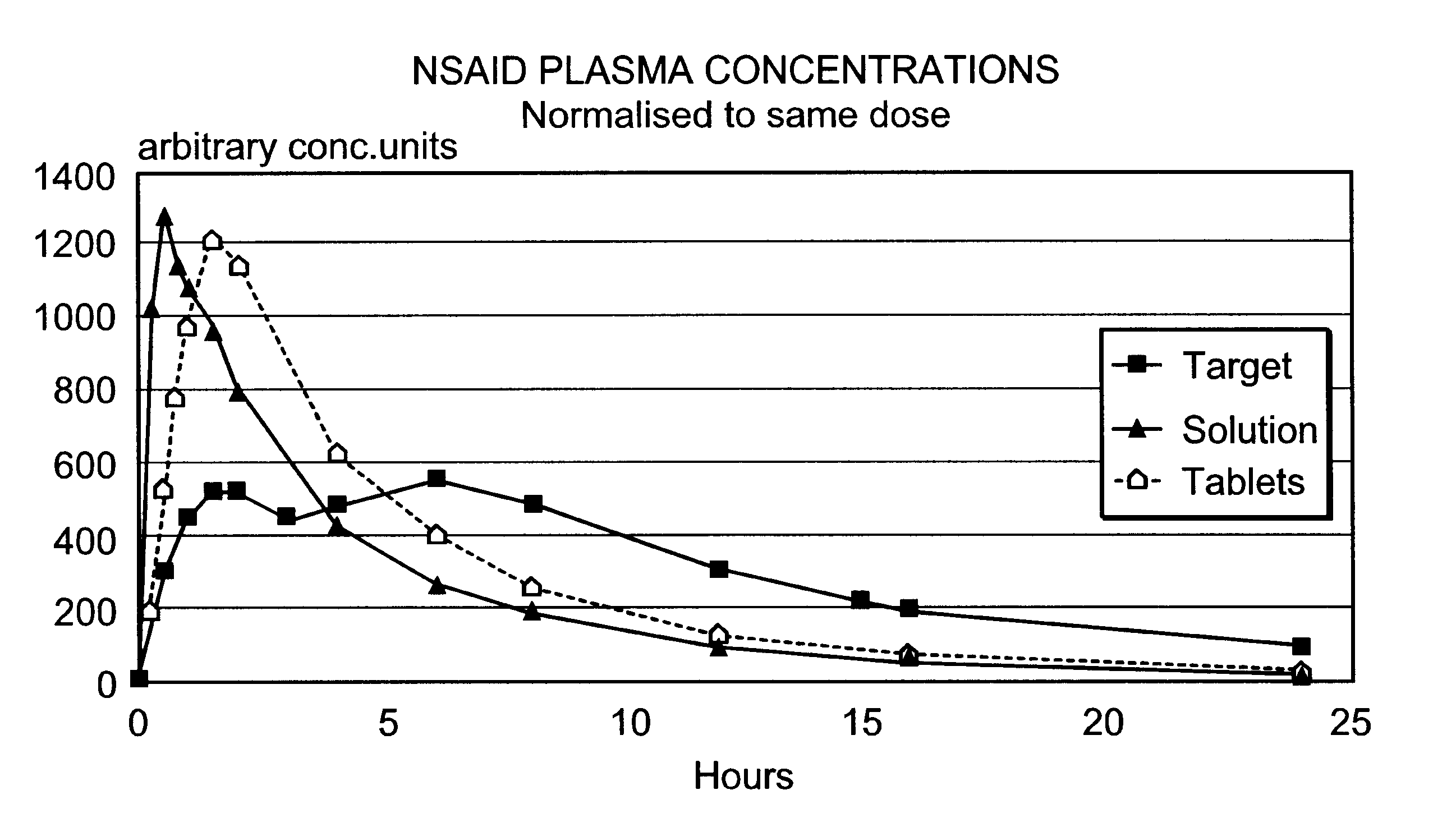
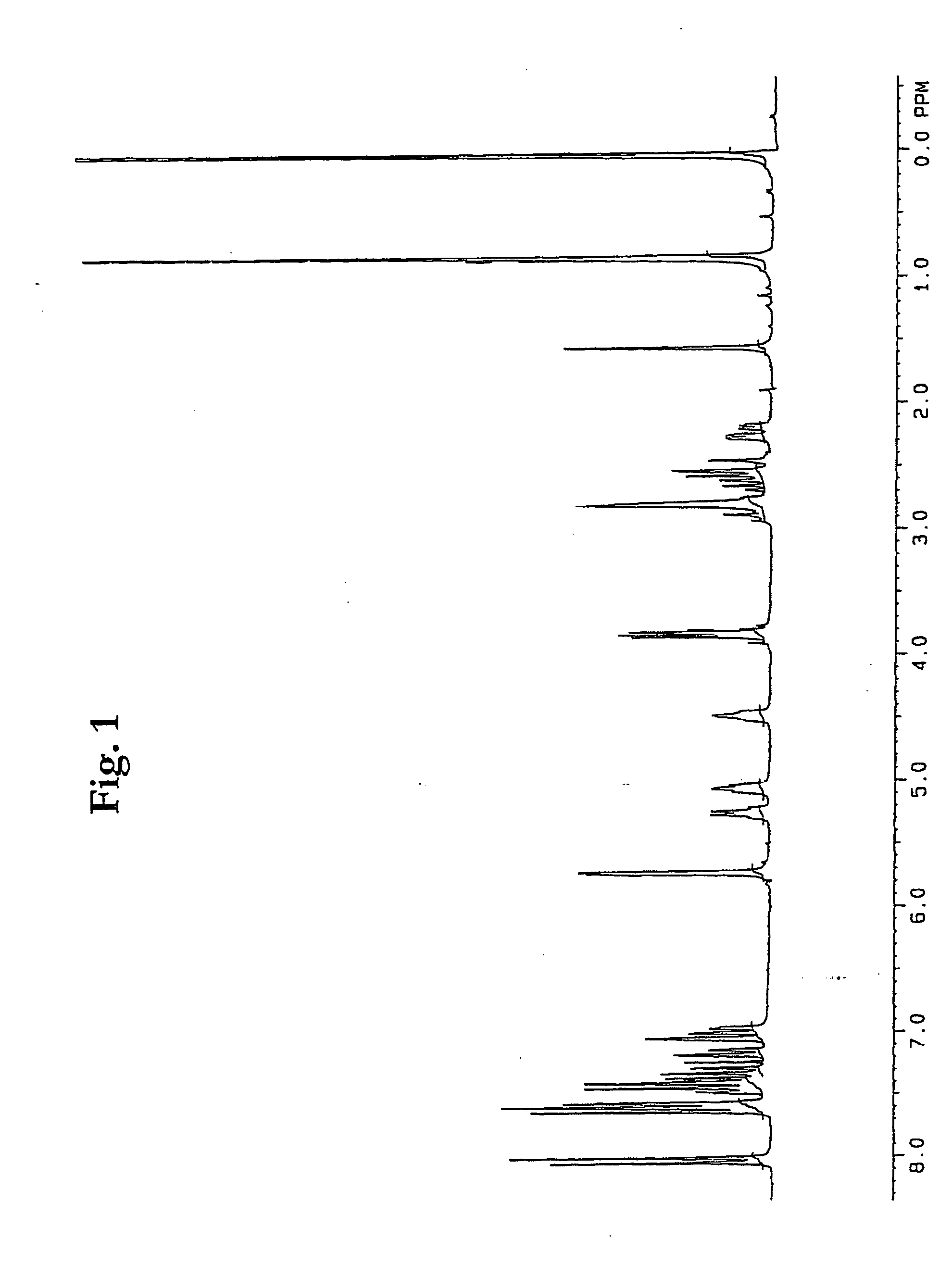
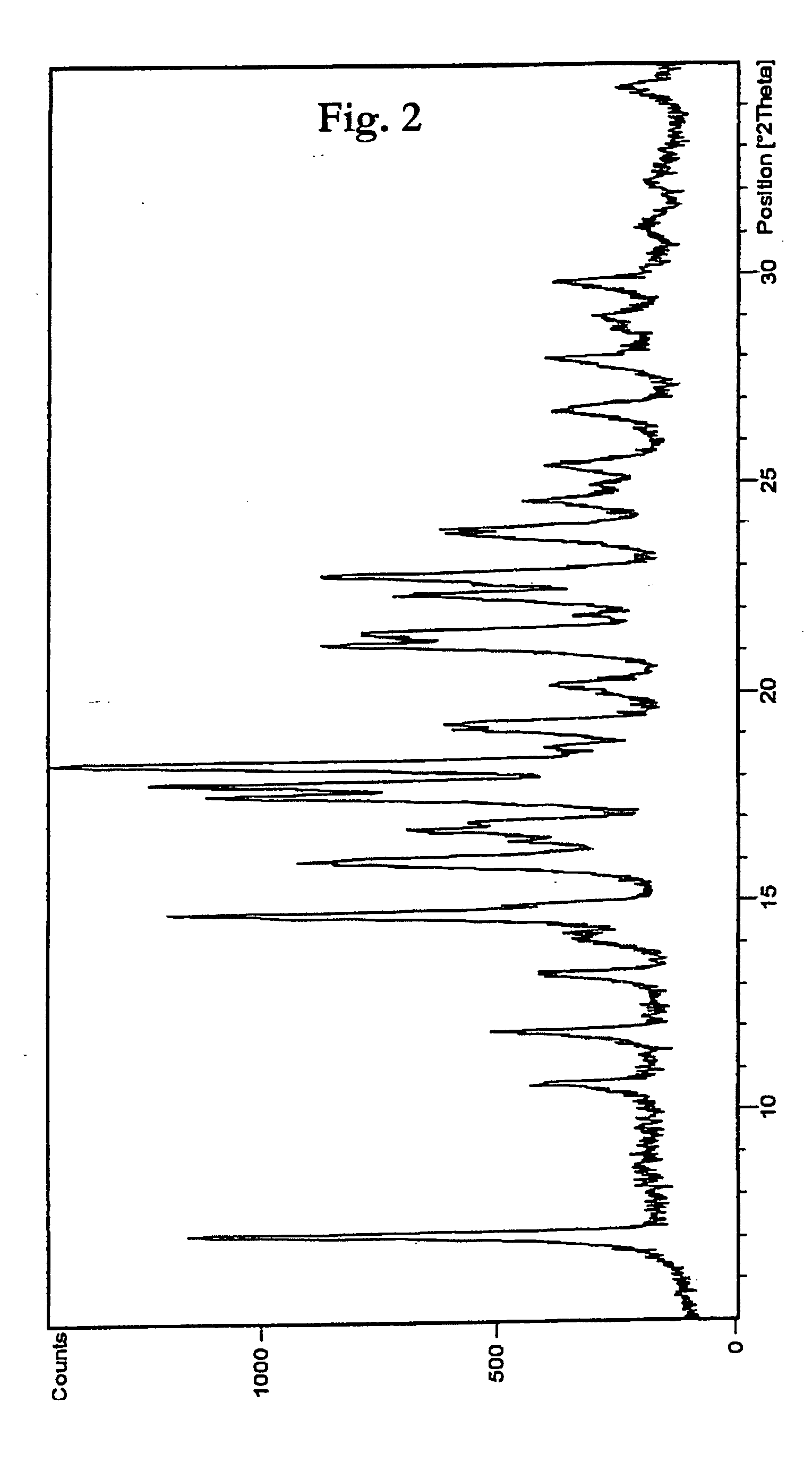
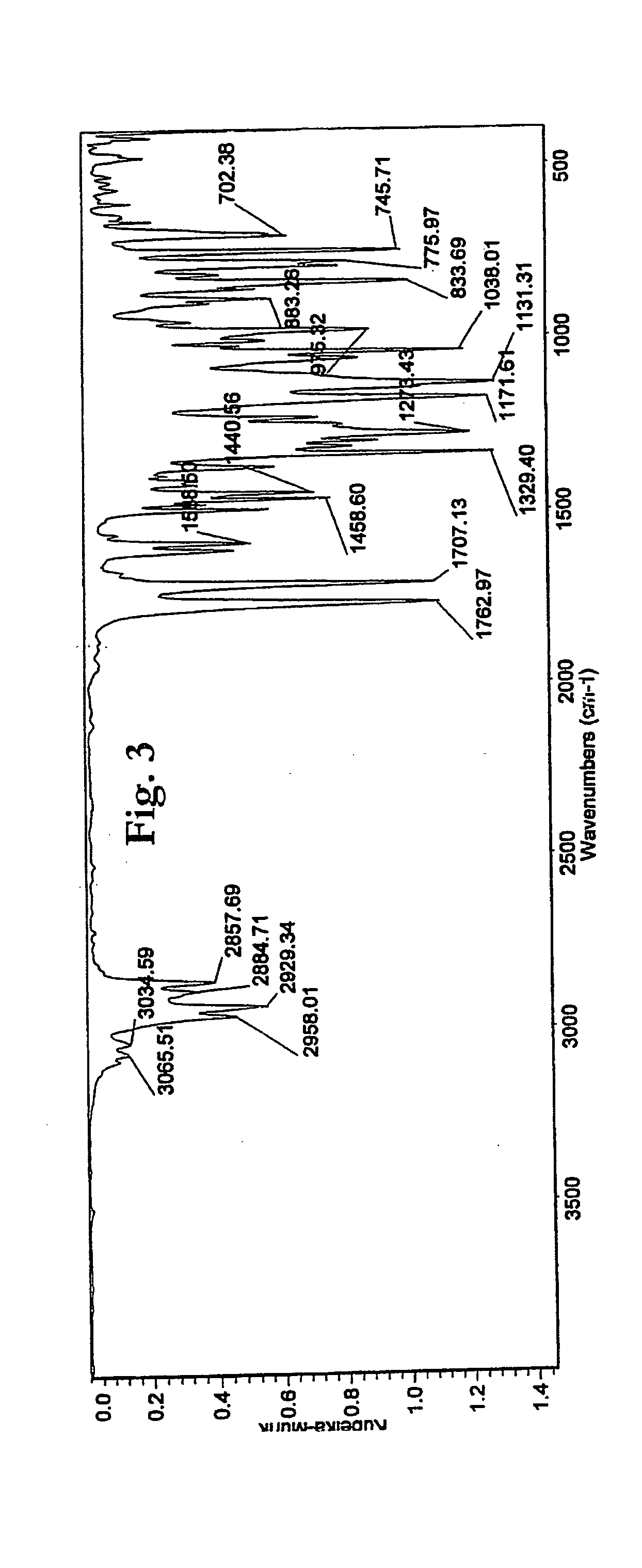
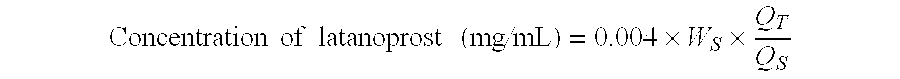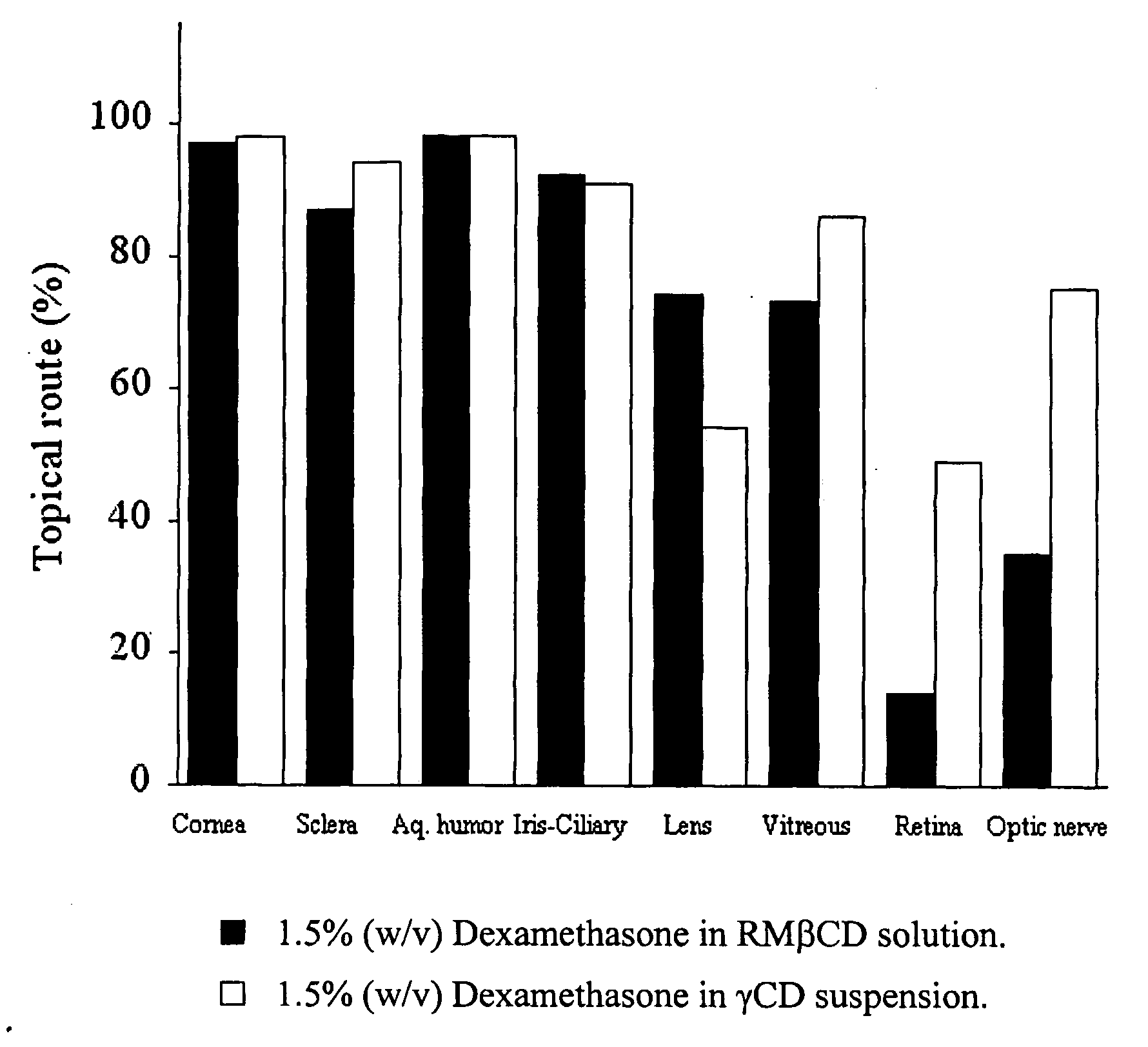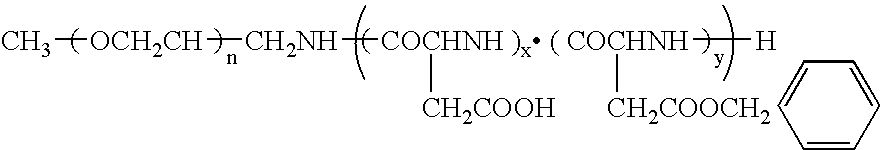Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
849results about "Elcosanoid active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
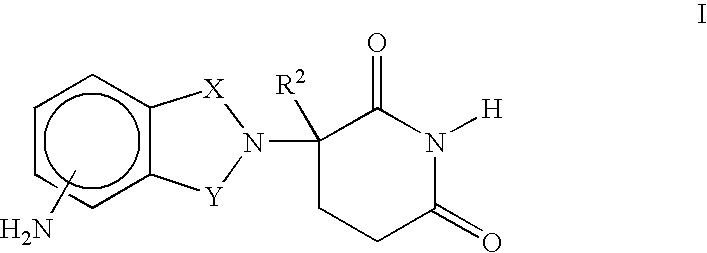
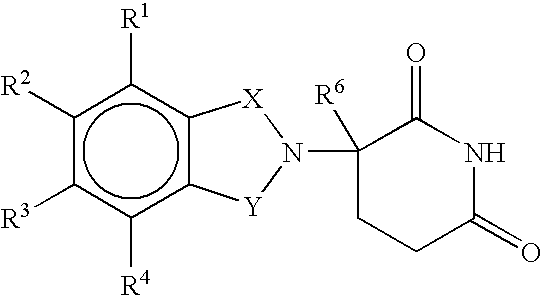
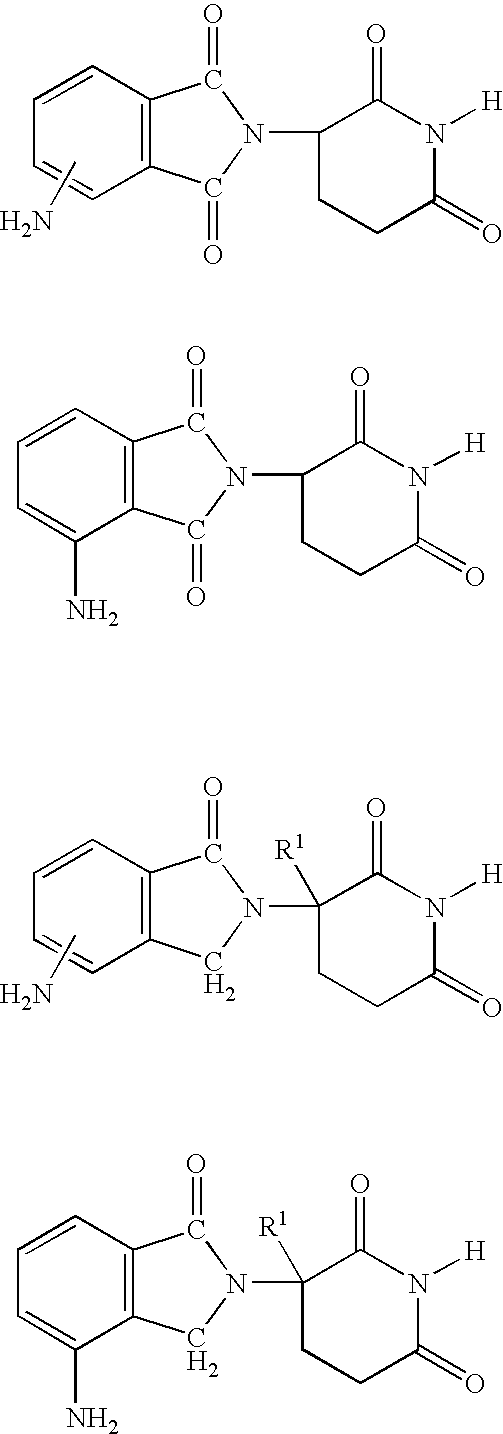
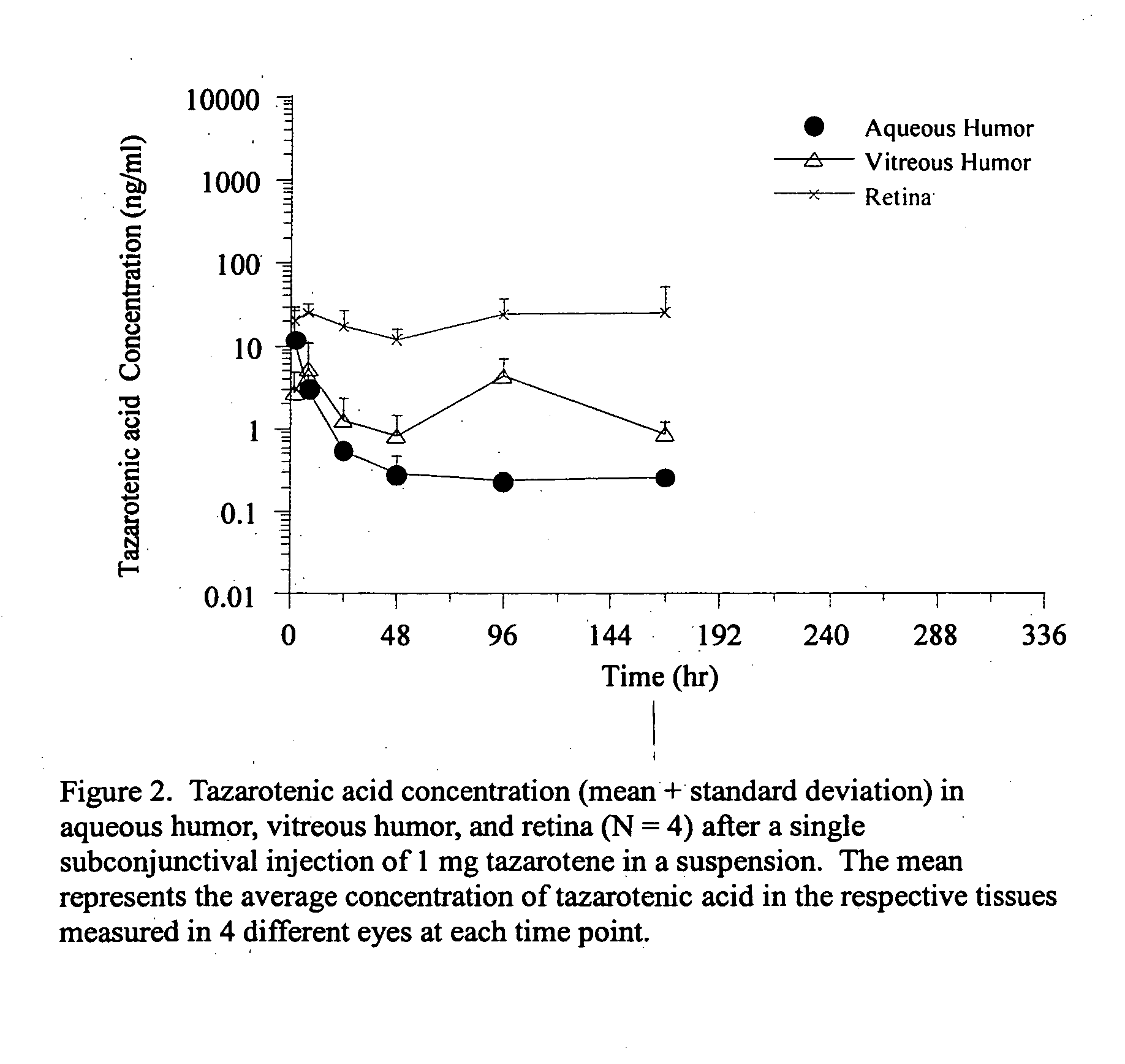
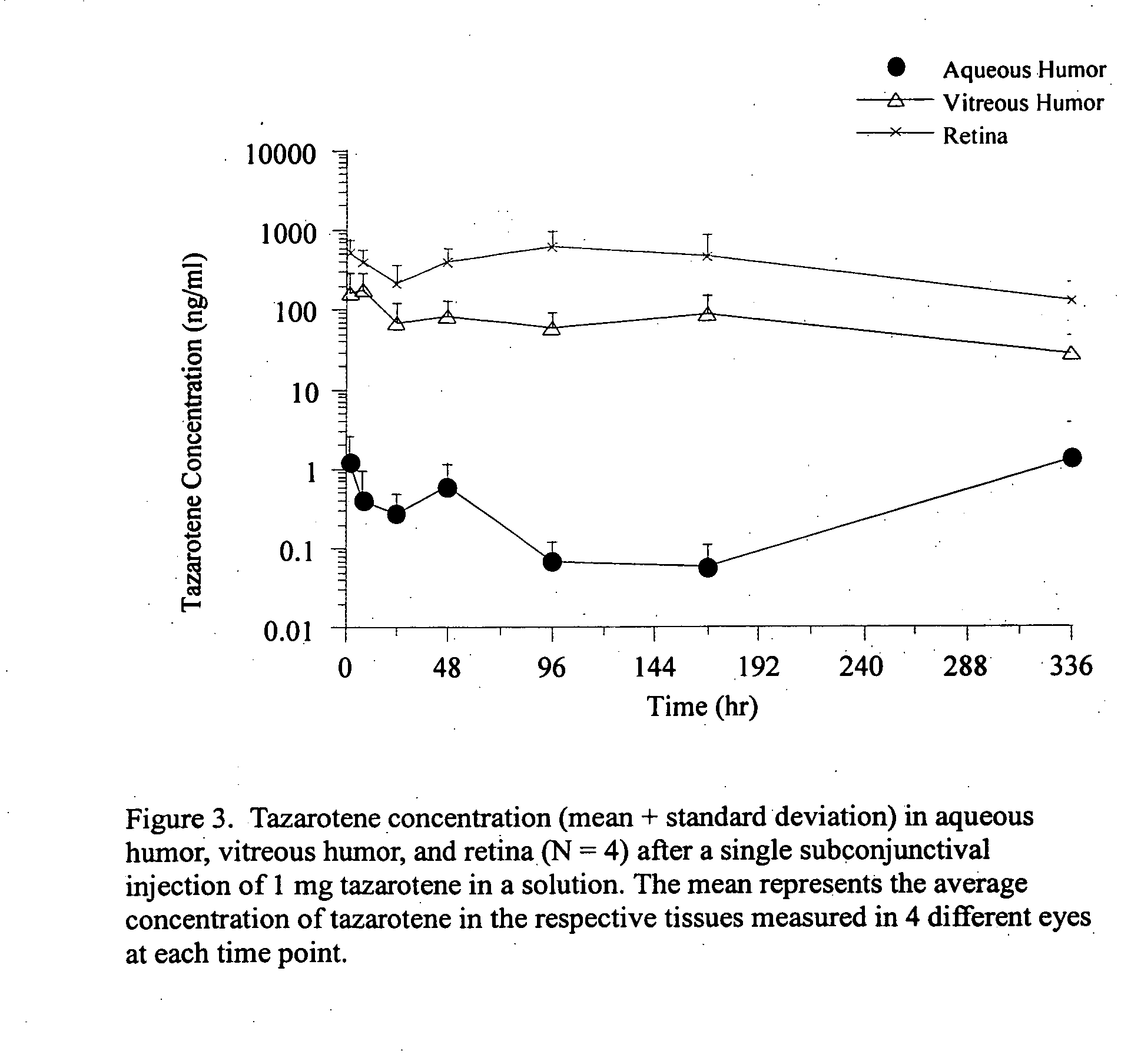
Methods of using and compositions comprising immunomodulatory compounds for treatment and management of macular degeneration
InactiveUS20040091455A1Extension of timeUseful in treatingBiocideSenses disorderActive agentSurgical procedures
Methods of treating, preventing and / or managing macular degeneration are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or surgery. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
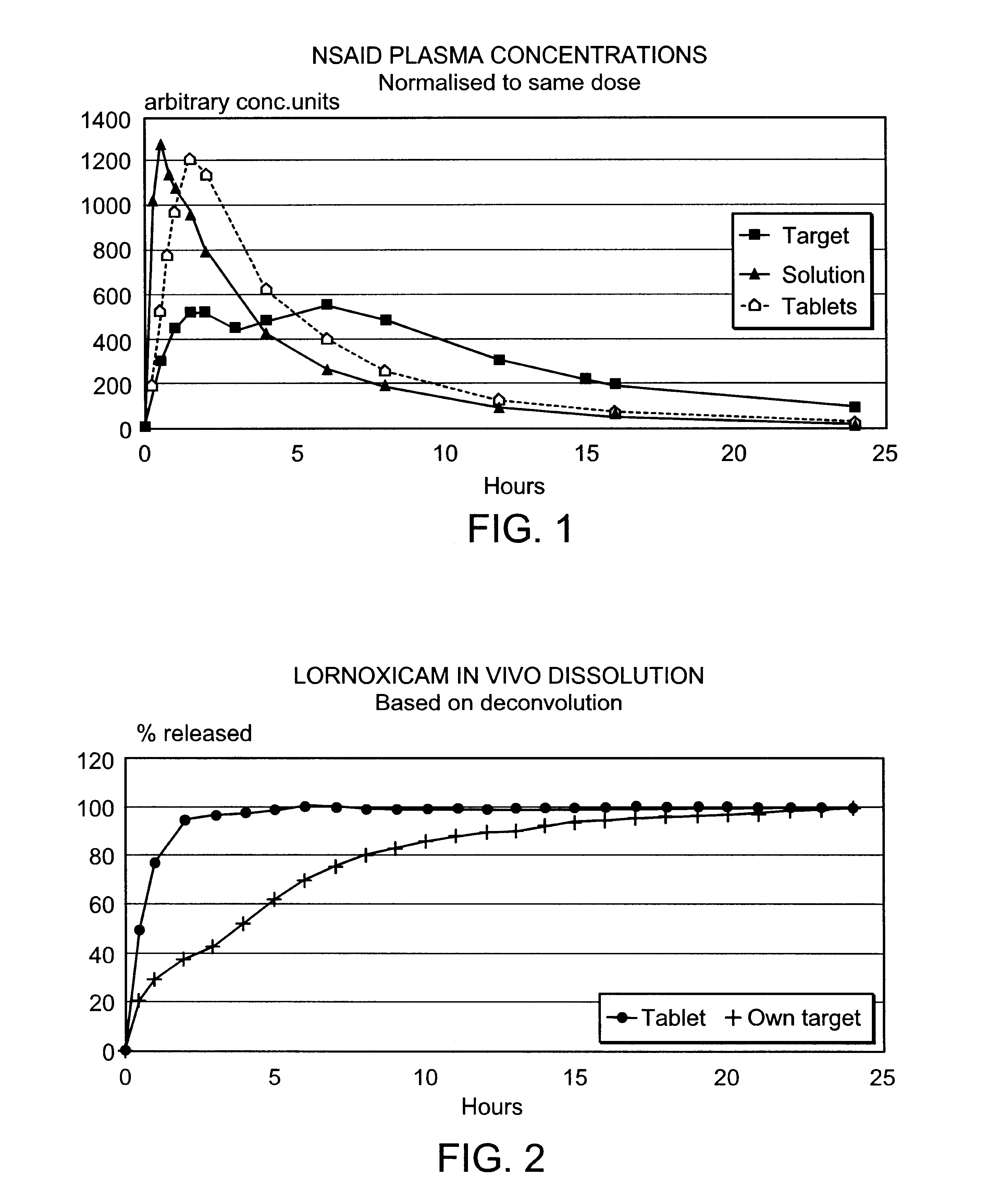
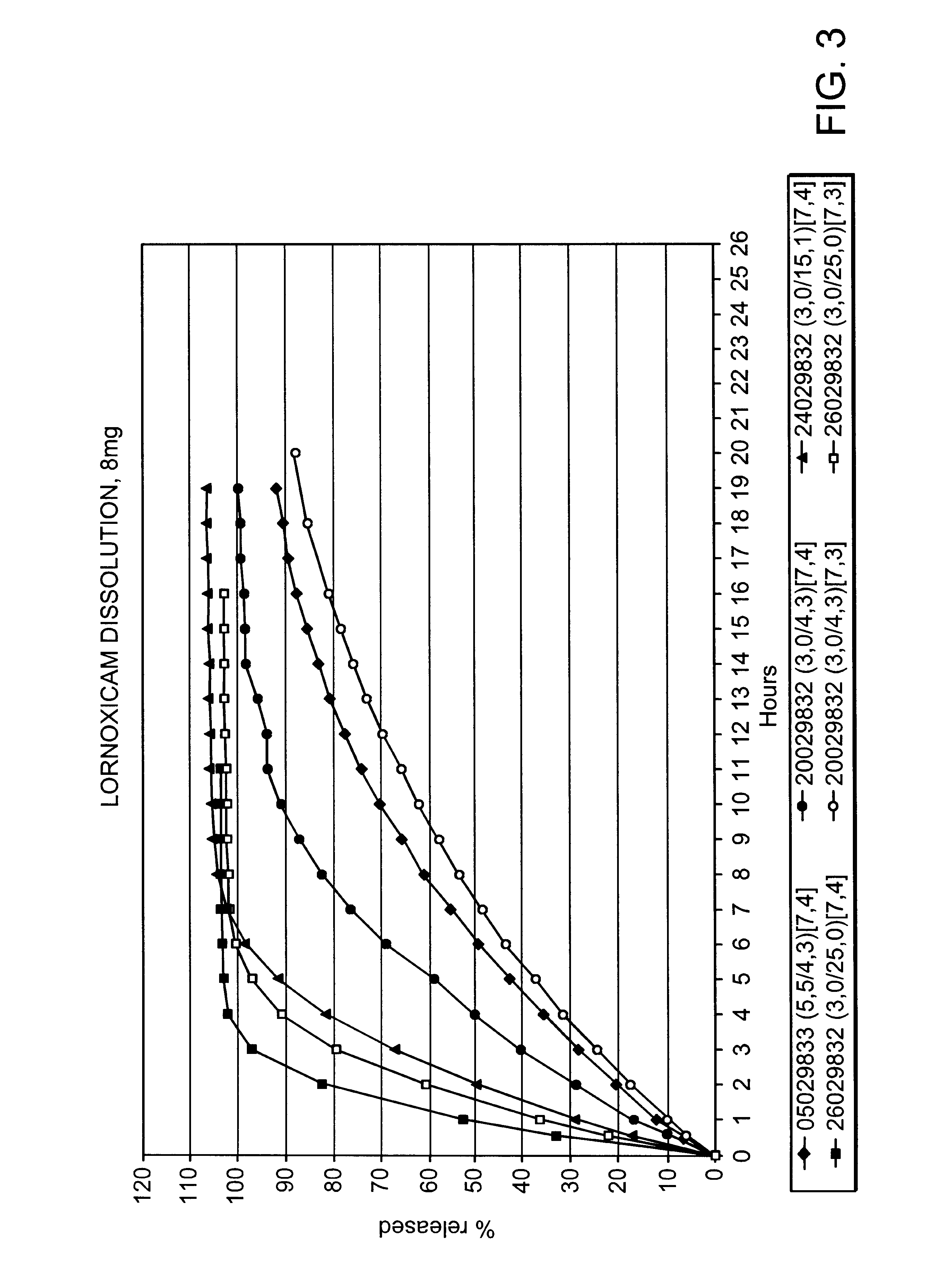
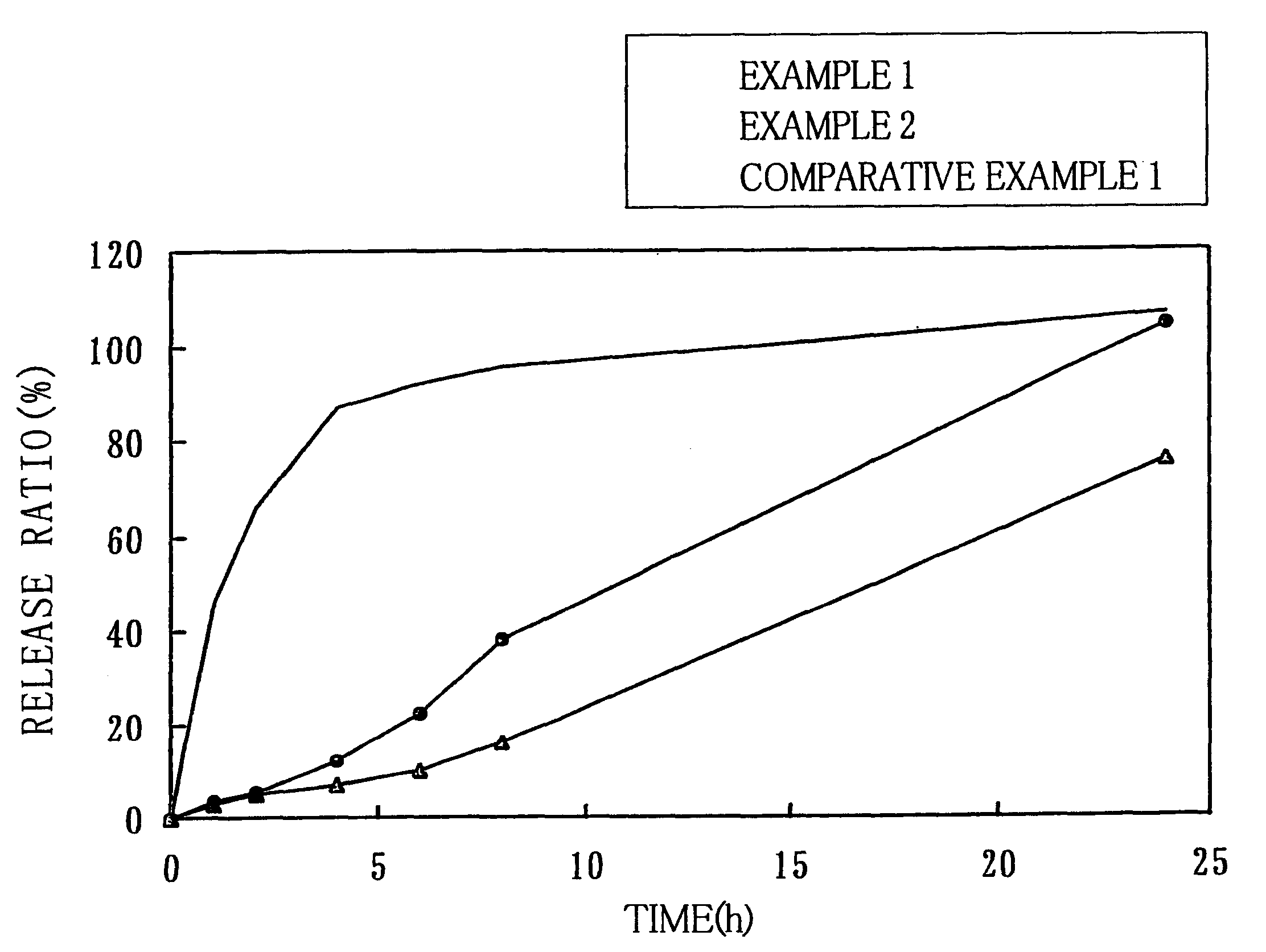
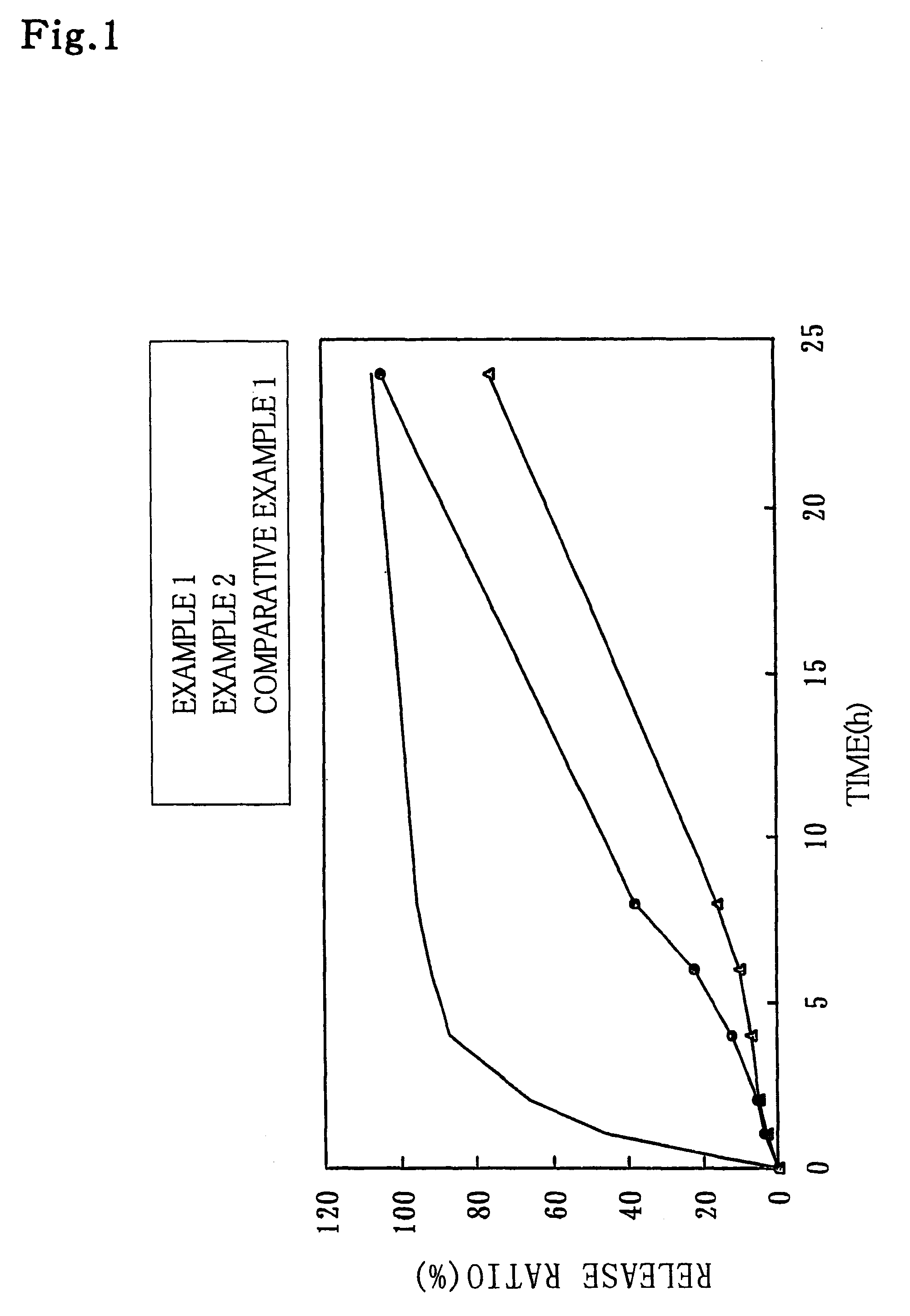
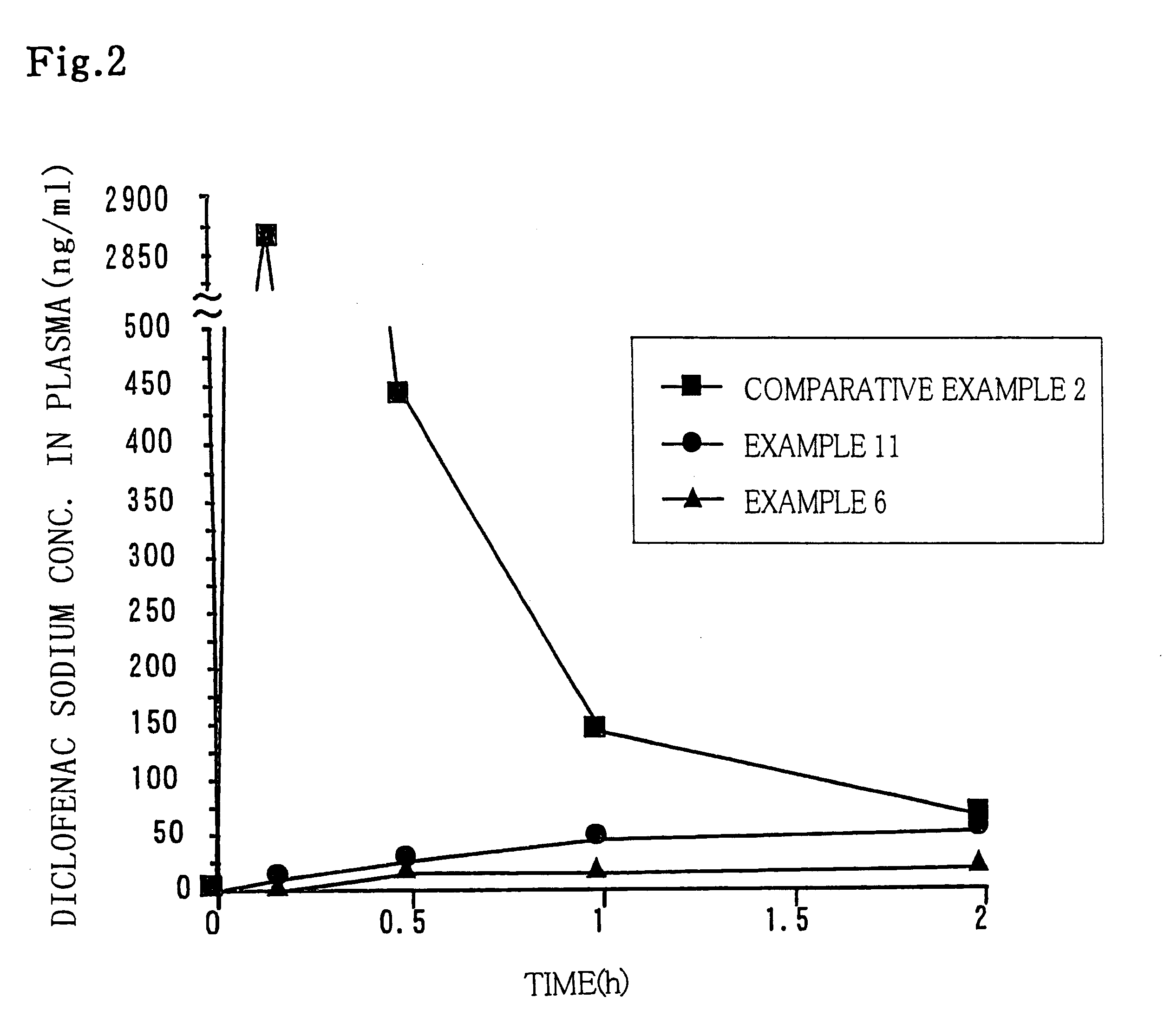
Modified release multiple-units compositions of non-steroid anti-inflammatory drug substances (NSAIDs)
InactiveUS6599529B1Keep low levelQuick releasePowder deliveryNervous disorderNon steroid anti inflammatory drugTherapeutic effect
An oral pharmaceutical modified release multiple-units composition for the administration of a therapeutically and / or prophylactically effective amount of a non-steroid anti-inflammatory drug substance to obtain both a relatively fast onset of the therapeutic effect and the maintenance of a therapeutically active plasma concentration for a relatively long period of time is disclosed.
Owner:TAKEDA PHARMA AS +1
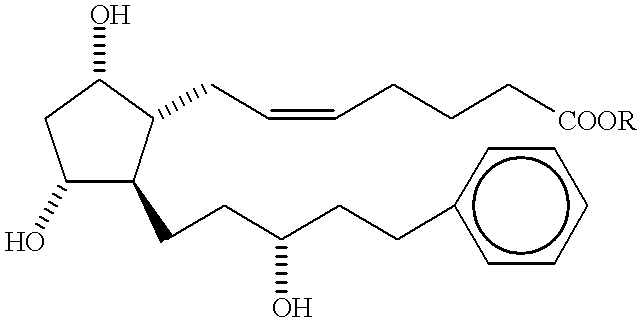
Method of treating macular degeneration with a prostaglandin derivative
InactiveUS6225348B1Reduce elevated intraocular pressureReduce morbidityBiocideElcosanoid active ingredientsProstaglandin F2alphaProstaglandin PGE
A method of treating age-related macular degeneration in an eye is described, comprising contacting the eye with a therapeutic amount of a prostaglandin F2alpha derivative. The preferred prostaglandin F2alpha derivative is latanoprost, and the preferred dosage is between about 1.5 mug and about 4.5 mug per day per eye.
Owner:PAULSEN ALFRED W
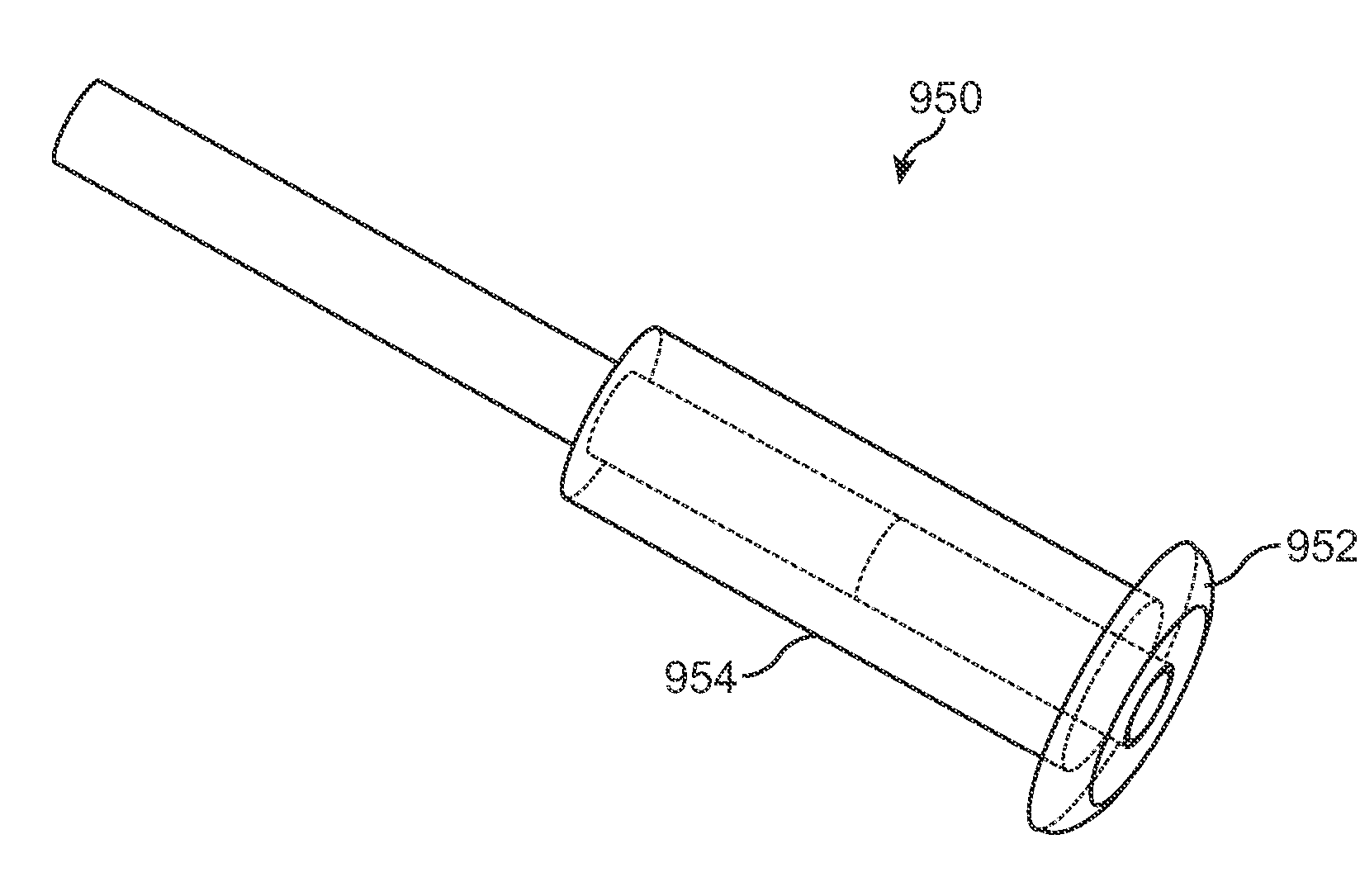
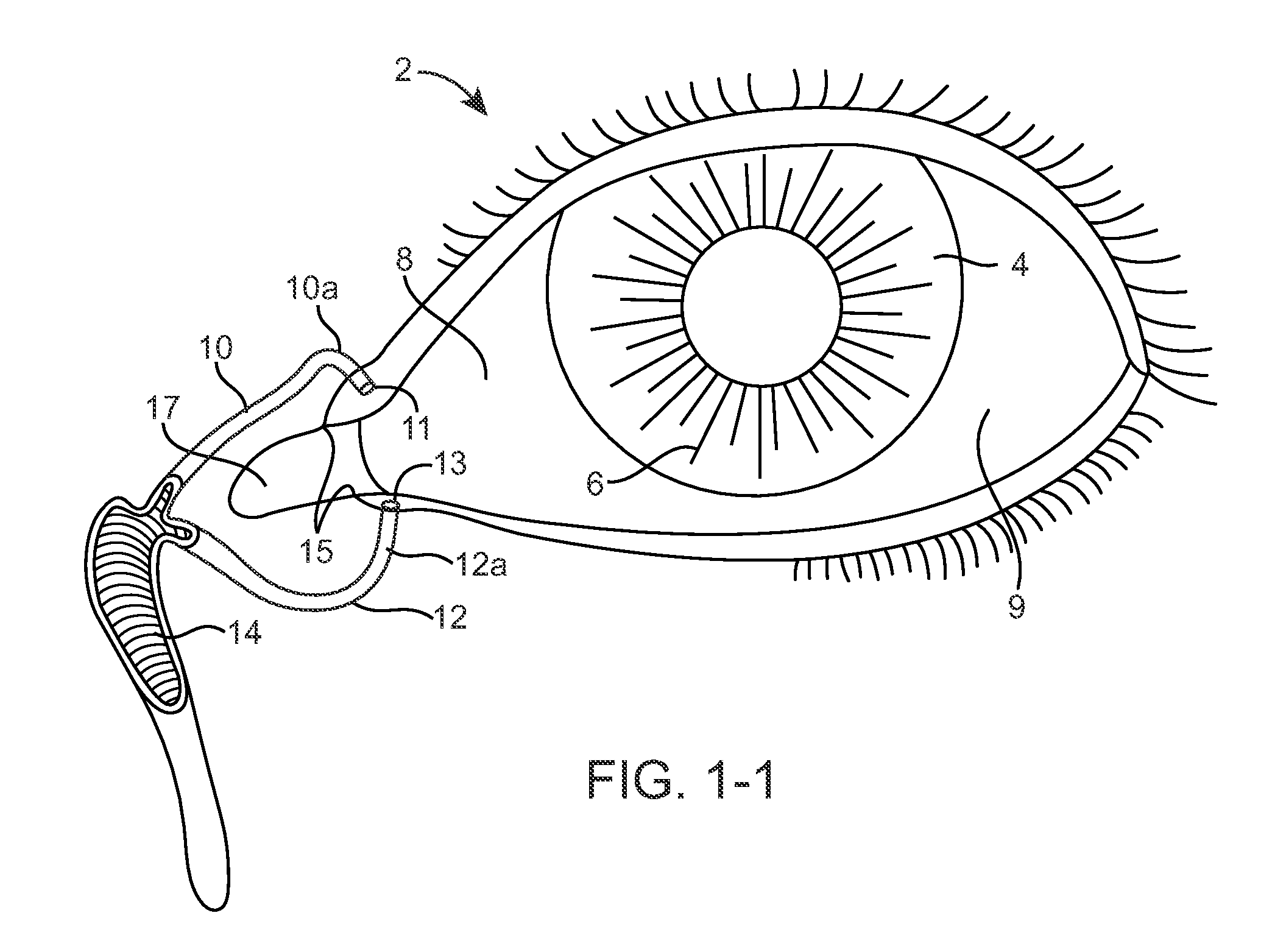
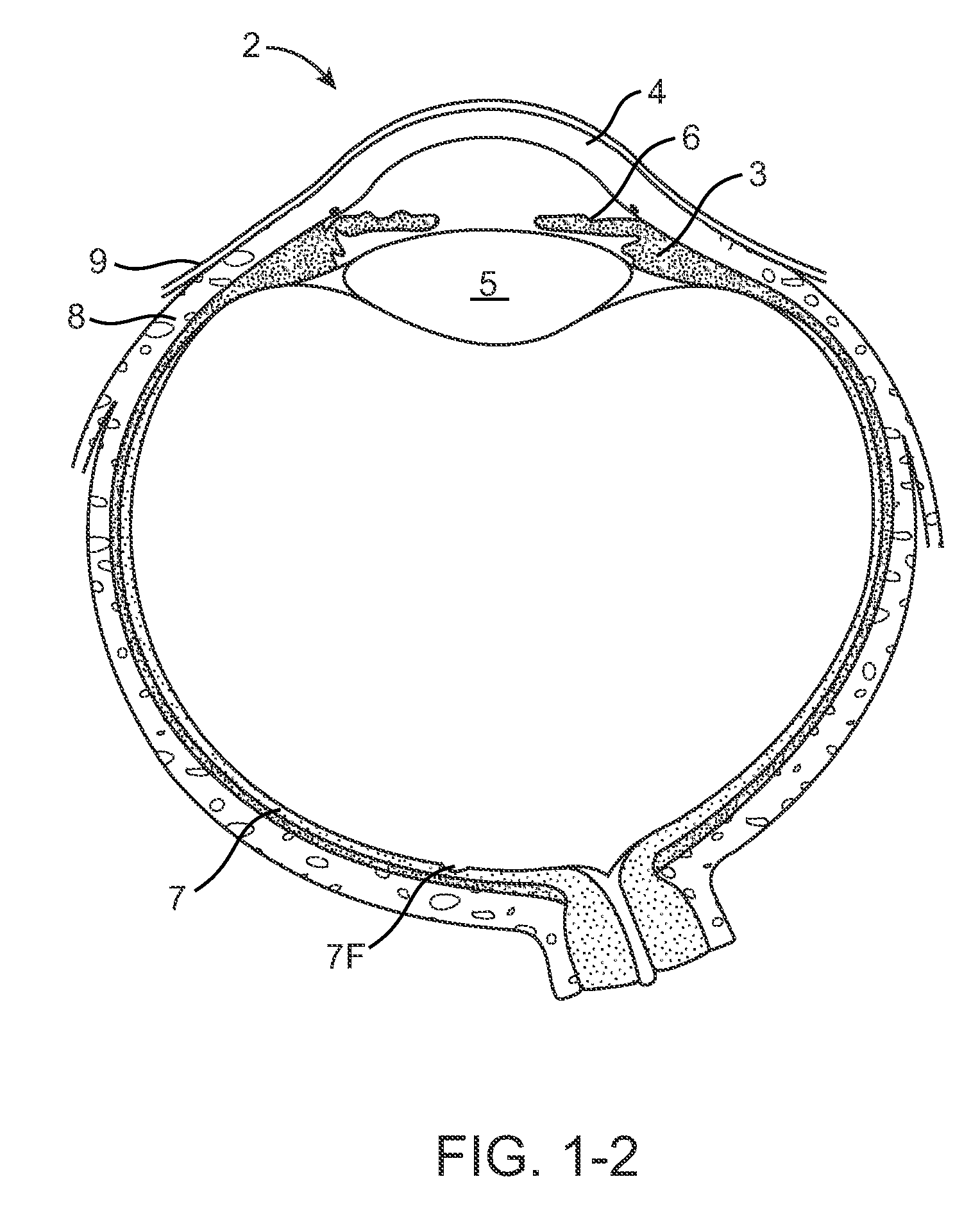
Nasolacrimal Drainage System Implants for Drug Therapy
ActiveUS20070243230A1Reduce deliveryAvoid flowAntibacterial agentsSenses disorderShape-memory alloyImplanted device
Implant devices, systems and methods for insertion into a punctum of a patient optionally comprises a drug core and a sheath body disposed over the drug core. The drug core includes a therapeutic agent deliverable into the eye, and the sheath defines at least one exposed surface of the drug core. The exposed surface(s) of the drug core may contact a tear or tear film fluid and release the therapeutic agent at therapeutic levels over a sustained period when the implant is implanted for use. The implant may include a retention element to retain the drug core and sheath body near the punctum, optionally comprising a shape memory alloy that can resiliently expand. An occlusive element may be attached to the retention element to at least partially occlude tear flow through the canalicular lumen.
Owner:MATI THERAPEUTICS
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
ActiveUS20070269487A1Avoid expulsionInhibition releaseAntibacterial agentsPowder deliveryEffective treatmentBiomedical engineering
Owner:MATI THERAPEUTICS
Composition and methods for treatment of neurological disorders and neurodegenerative diseases
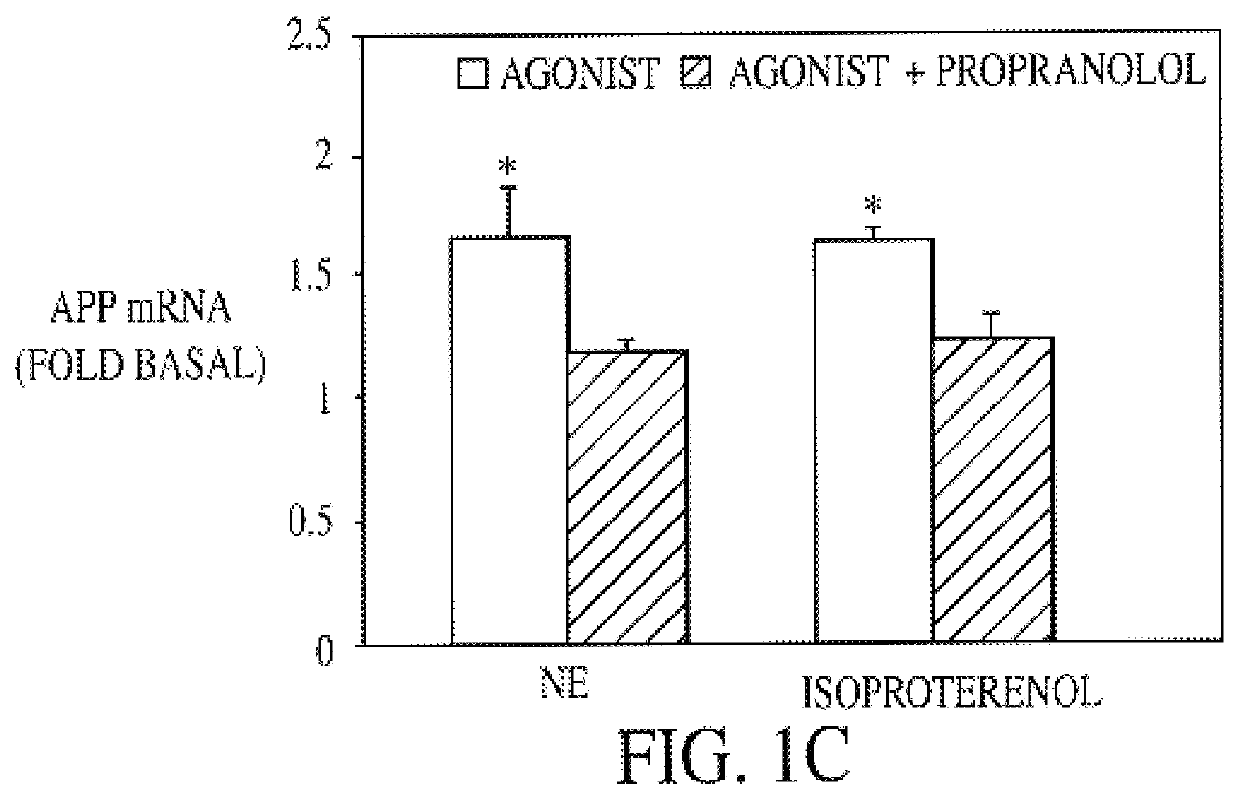
InactiveUS6187756B1Increased formationPromote activationBiocideElcosanoid active ingredientsDiseaseGlial fibrillary acidic protein
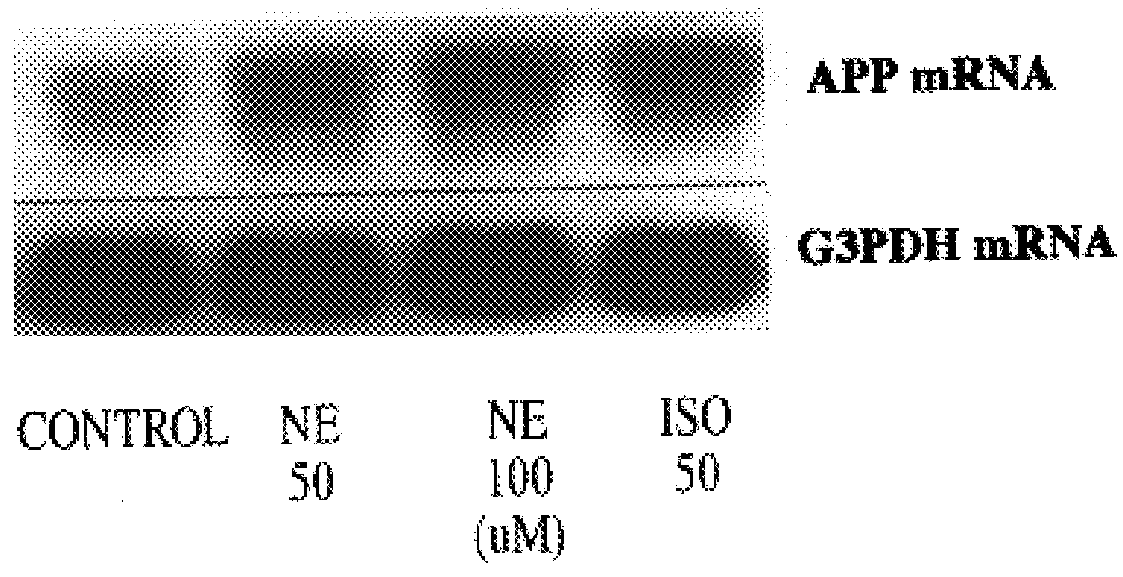
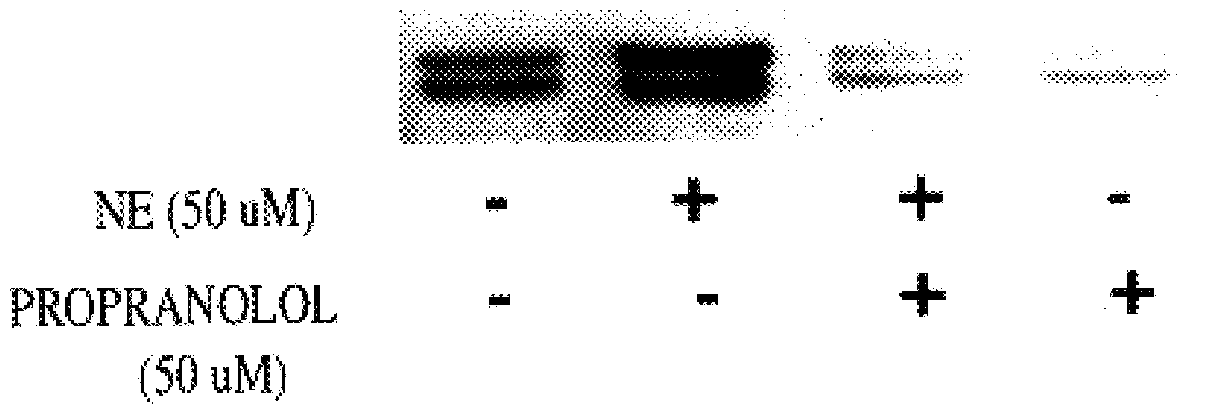
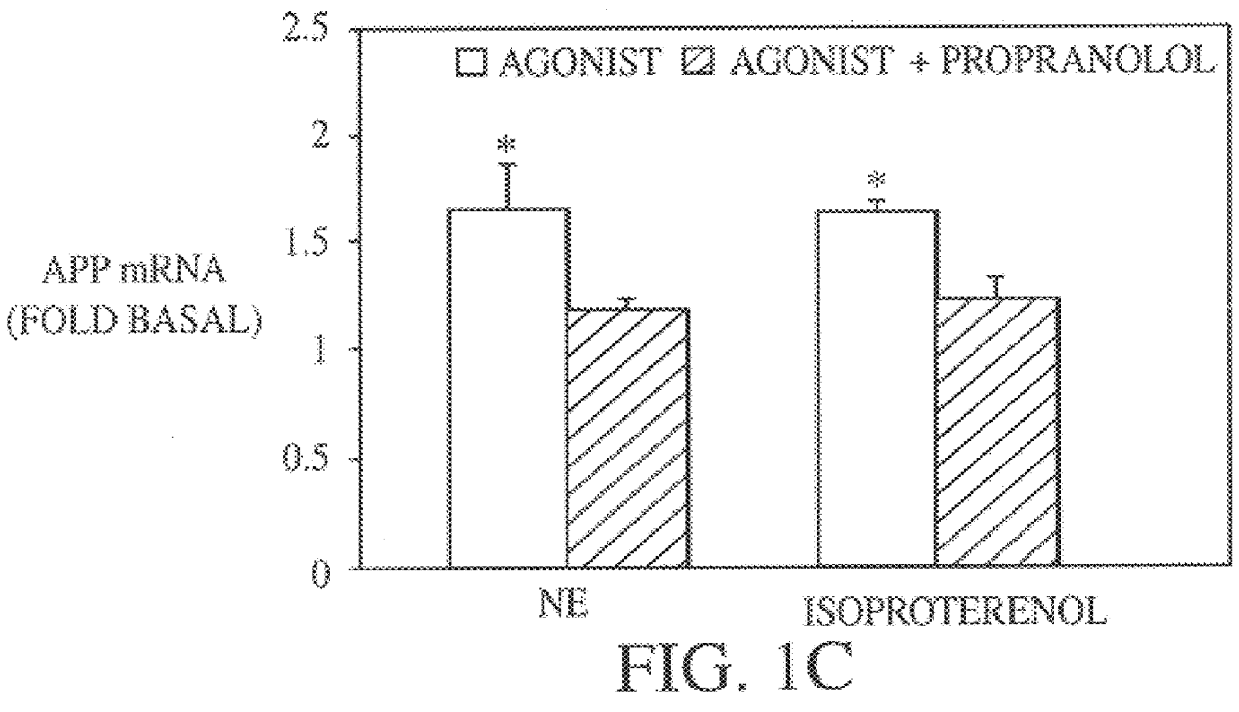
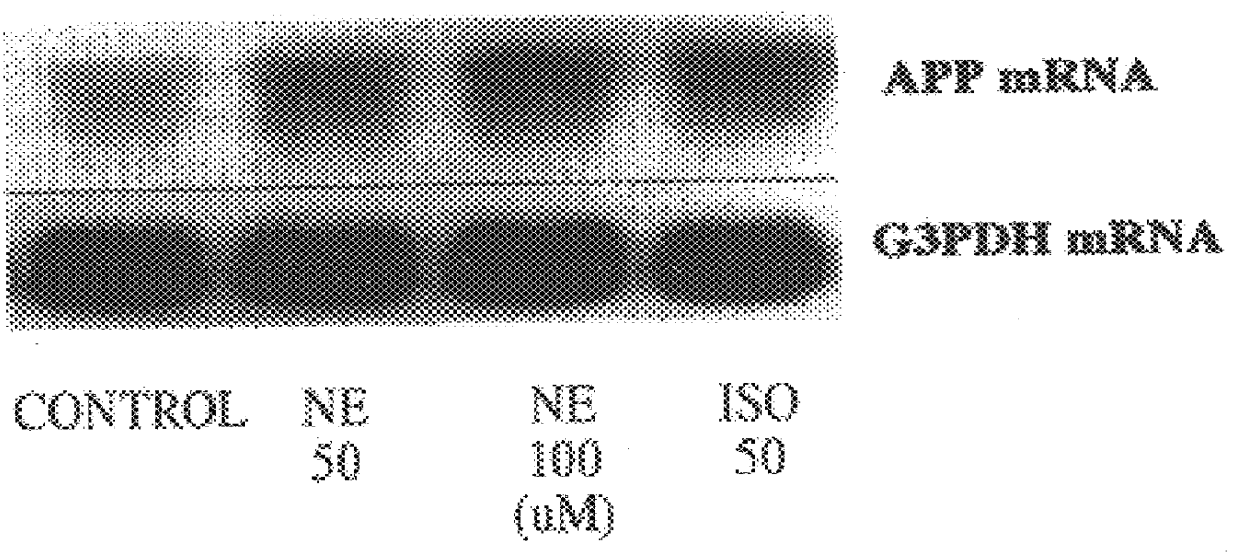
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Compounds and methods for delivery of prostacyclin analogs
Owner:UNITED THERAPEUTICS CORP
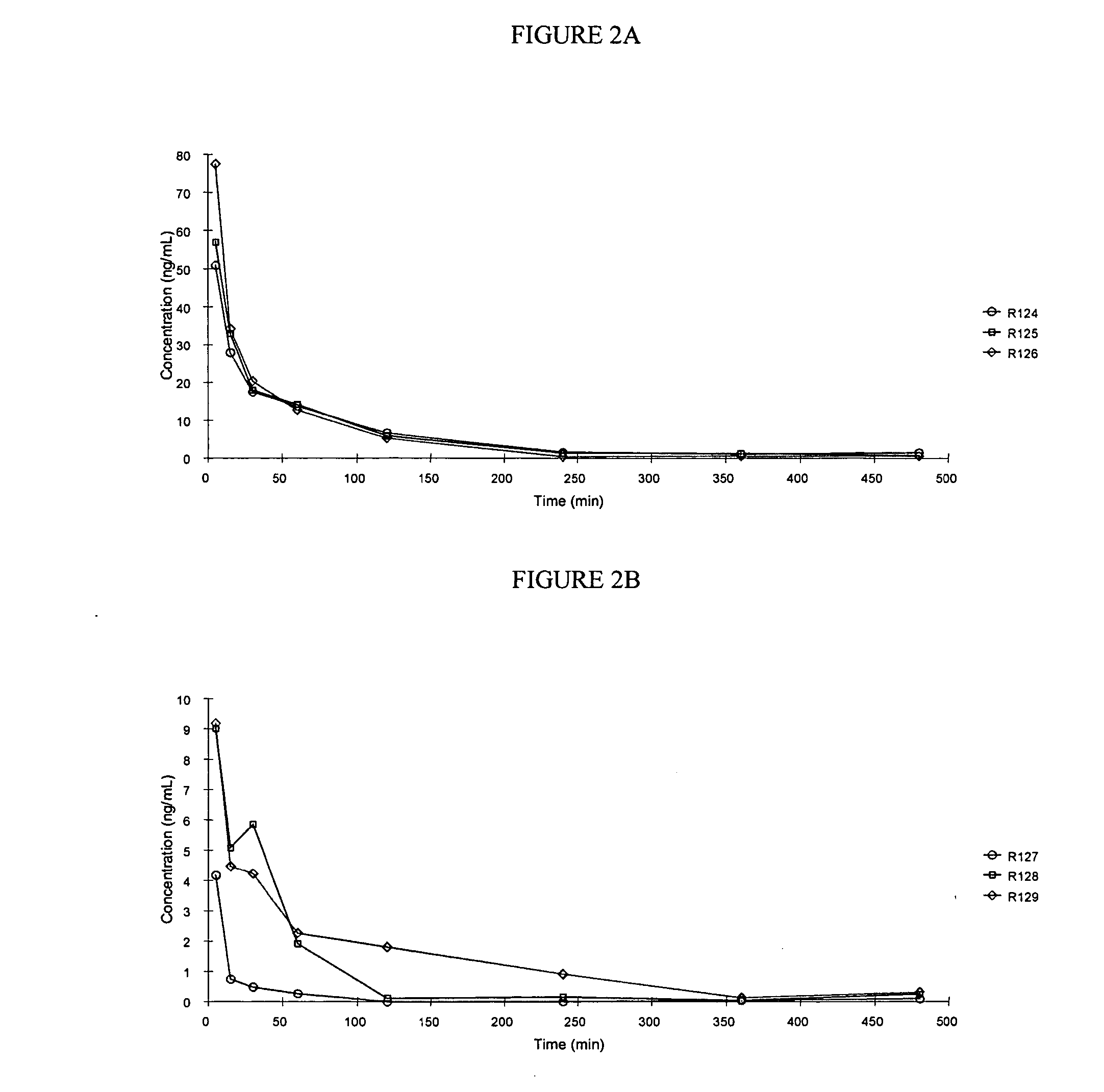
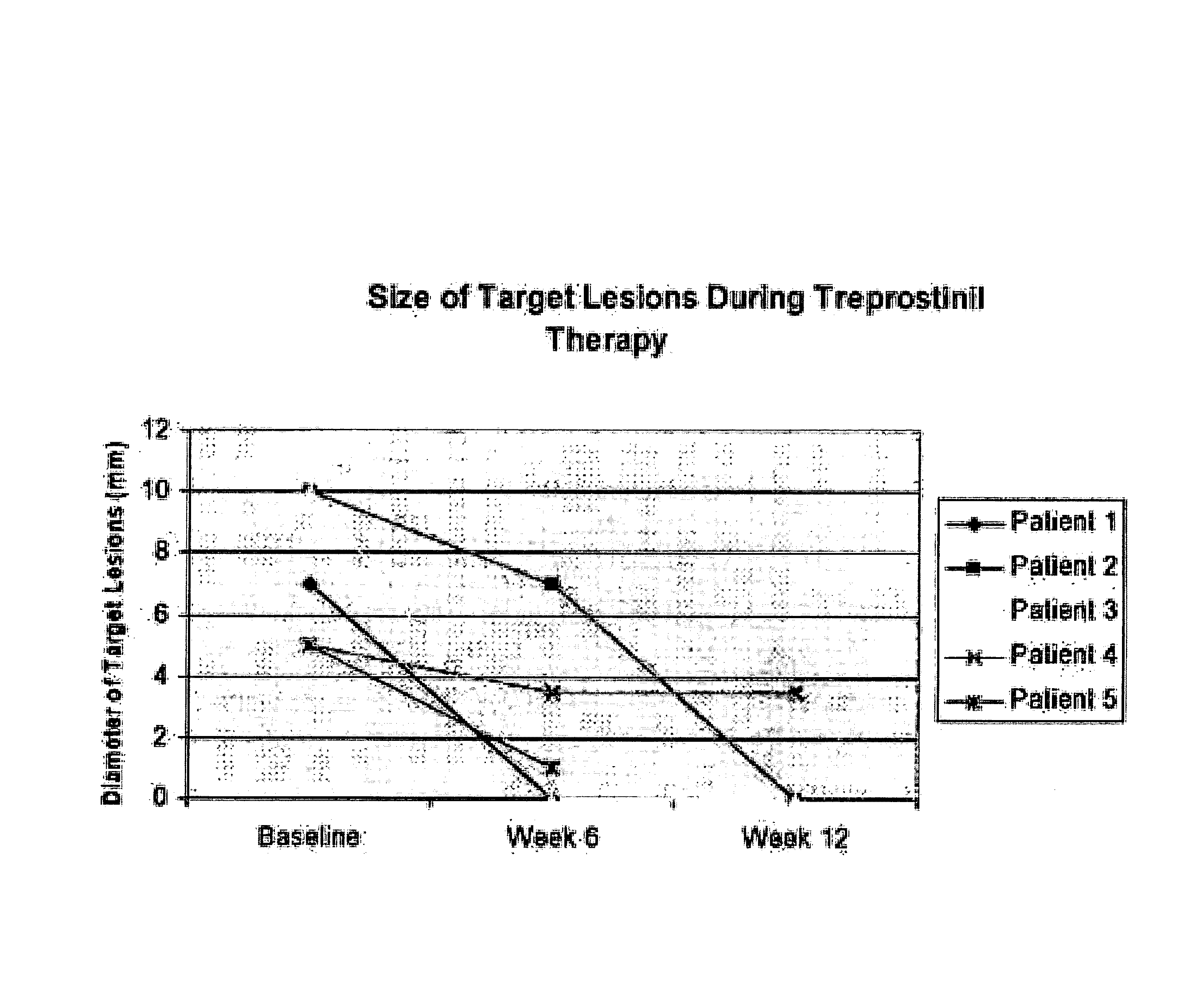
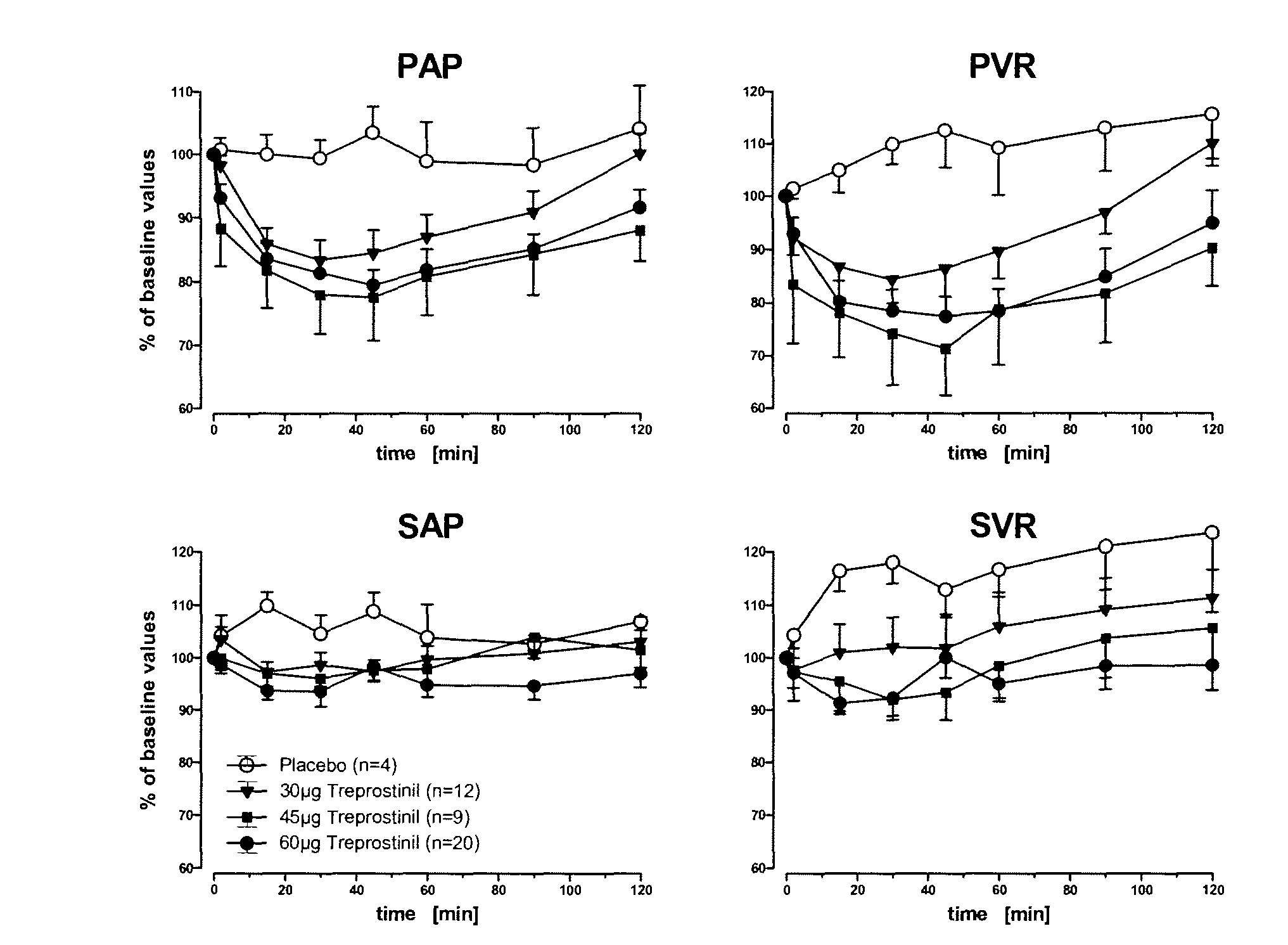
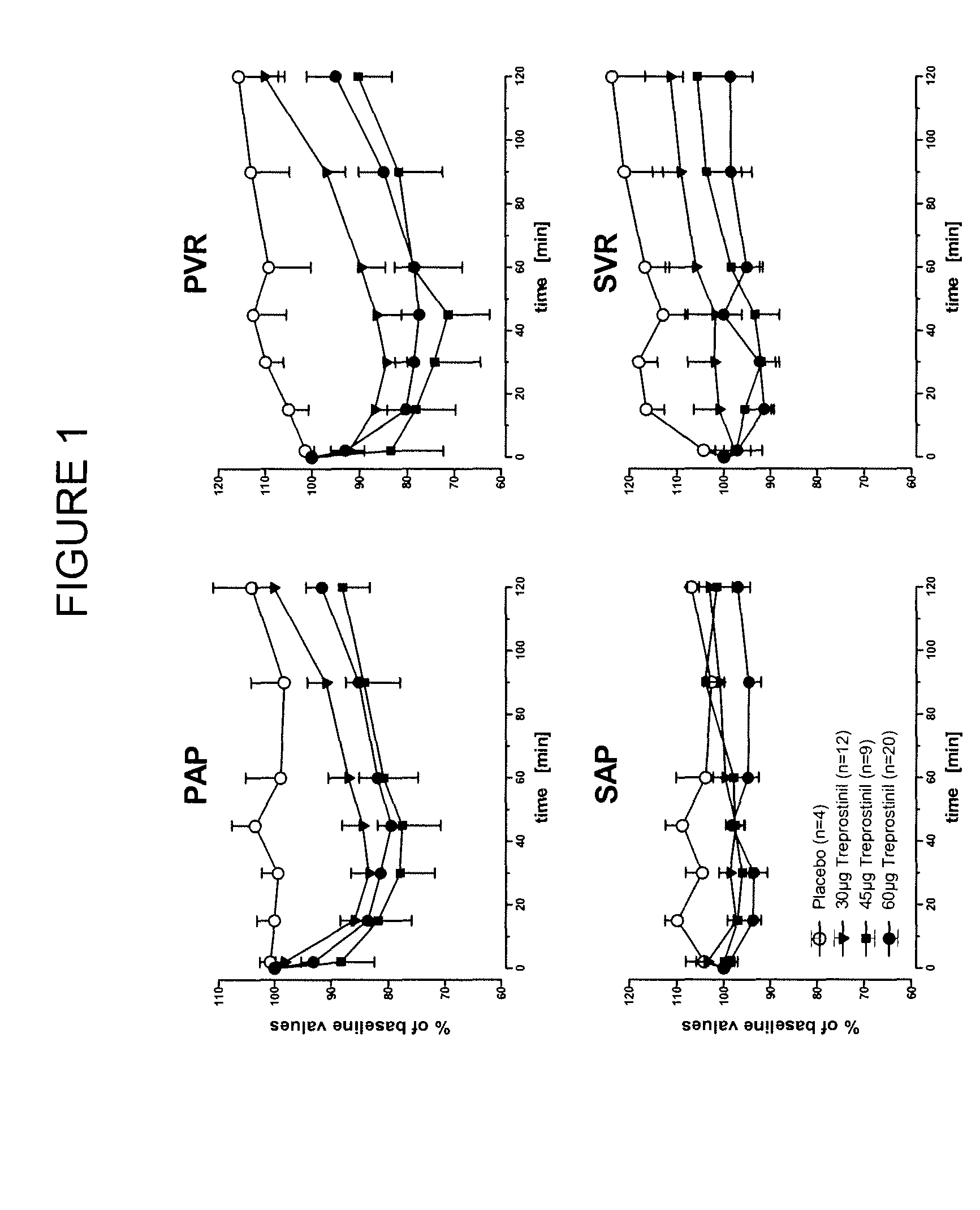
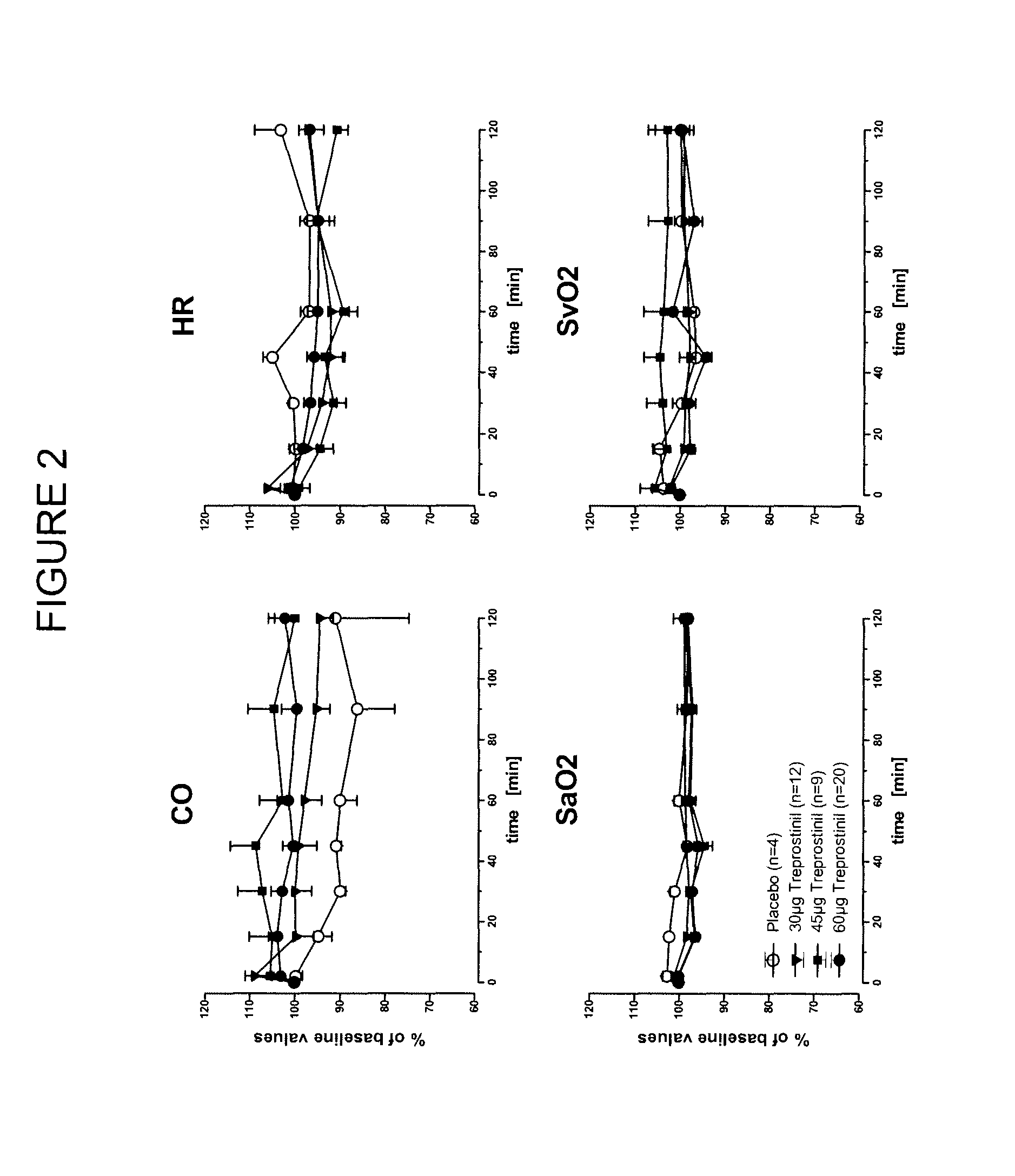
Use of treprostinil to treat and prevent ischemic lesions
ActiveUS20050165111A1Reduce occurrenceReduce the numberBiocideNervous disorderTreprostinilIschemic lesion
The present invention describes novel methods for using Treprostinil or its derivative, or a pharmaceutically acceptable salt thereof, for the treatment and / or prevention of ischemic lesions, such as digital ulcers, in subjects with scleroderma (including systemic sclerosis), Buerger's disease, Raynaud's disease, Raynaud's phenomenon and / or other conditions that cause such lesions. The invention also relates to kits for treatment and / or prevention of ischemic lesions, comprising an effective amount of Treprostinil or its derivative, or a pharmaceutically acceptable salt thereof.
Owner:UNITED THERAPEUTICS CORP
Treprostinil administration using a metered dose inhaler
Treprostinil can be administered using a metered dose inhaler. Such administration provides a greater degree of autonomy to patients. Also disclosed are kits that include a metered dose inhaler containing a pharmaceutical formulation containing treprostinil.
Owner:UNITED THERAPEUTICS CORP
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of pulmonary hypertension
InactiveUS20050239842A1Lengthening time of remissionBiocideNervous disorderActive agentLung transplantation
Methods of treating, preventing and managing pulmonary hypertension are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate (e.g., hydrate), stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, surgery and / or lung transplantation. Specific second active agents are capable of reducing pulmonary artery pressure. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Use of a virucidal hand lotion to prevent the spread of rhinovirus colds
InactiveUS6034133AEfficient killingSynergistic rhinovirus killing capacityBiocideElcosanoid active ingredientsRhinovirusCommon cold
Frequent application of a virucidal hand lotion containing malic acid, citric acid, and a C1-6 alcohol will prevent the hand-to-hand transmission of rhinoviruses and reduce the incidence of the "common cold" caused by rhinoviruses.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
InactiveUS20090092654A1Avoid expulsionInhibition releaseAntibacterial agentsBiocideEffective treatmentBioabsorbable polymer
An implant for insertion into a punctum of a patient comprises a body. The body has a distal end, a proximal end, and an axis therebetween. The distal end of the body is insertable distally through the punctum into the canalicular lumen. The body comprises a therapeutic agent included within an agent matrix drug core. Exposure of the agent matrix to the tear fluid effects an effective therapeutic agent release into the tear fluid over a sustained period. The body has a sheath disposed over the agent matrix to inhibit release of the agent away from the proximal end. The body also has an outer surface configured to engage luminal wall tissues so as to inhibit expulsion when disposed therein. In specific embodiments, the agent matrix comprises a non-bioabsorbable polymer, for example silicone in a non-homogenous mixture with the agent.
Owner:MATI THERAPEUTICS
12-Aryl prostaglandin analogs
Compounds comprisingare disclosed, wherein Y, A, X, R, D, and n are as described.A compound comprising a prostaglandin EP2 selective agonist wherein the ω-chain comprises a substituted phenyl, wherein at least one substituent consists of hydrocarbyl or non-linear hydroxyhydrocarbyl is also disclosed herein.Methods, compositions, and medicaments related thereto are also disclosed.
Owner:ALLERGAN INC
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6043224AIncreased formationPromote activationBiocideElcosanoid active ingredientsGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta -adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta -adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta -adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta -adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Method for treating ocular hypertension and glaucoma
Provided is a method for treating ocular hypertension and glaucoma with reduced side effects such as keratoconjunctive disorders and macular edema, which comprises administering an ophthalmic composition comprising latanoprost as an active ingredient thereof to a subject in need of said treatment, wherein the ophthalmic composition contains substantially no benzalkonium chloride.
Owner:SUCAMPO USA
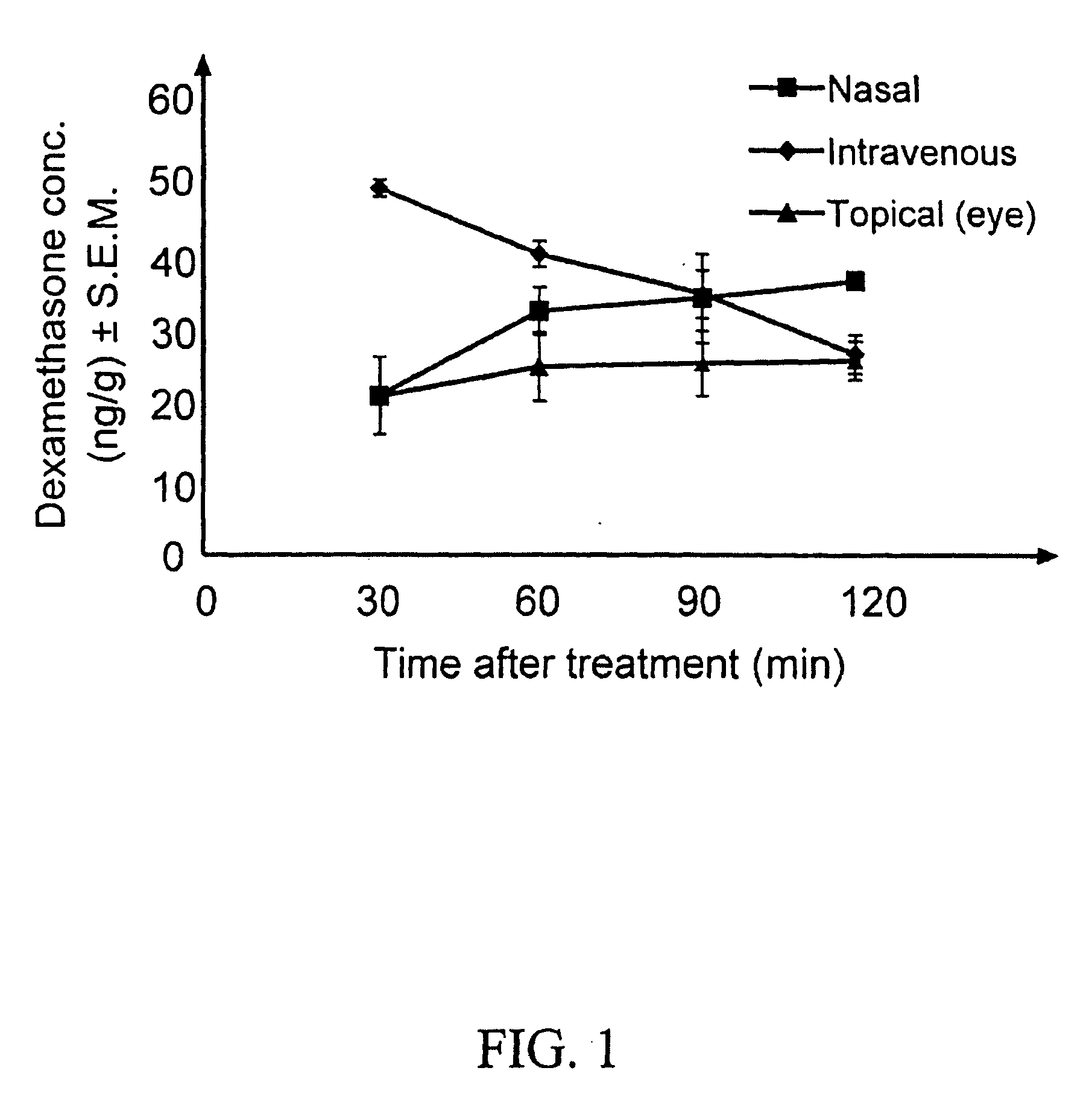
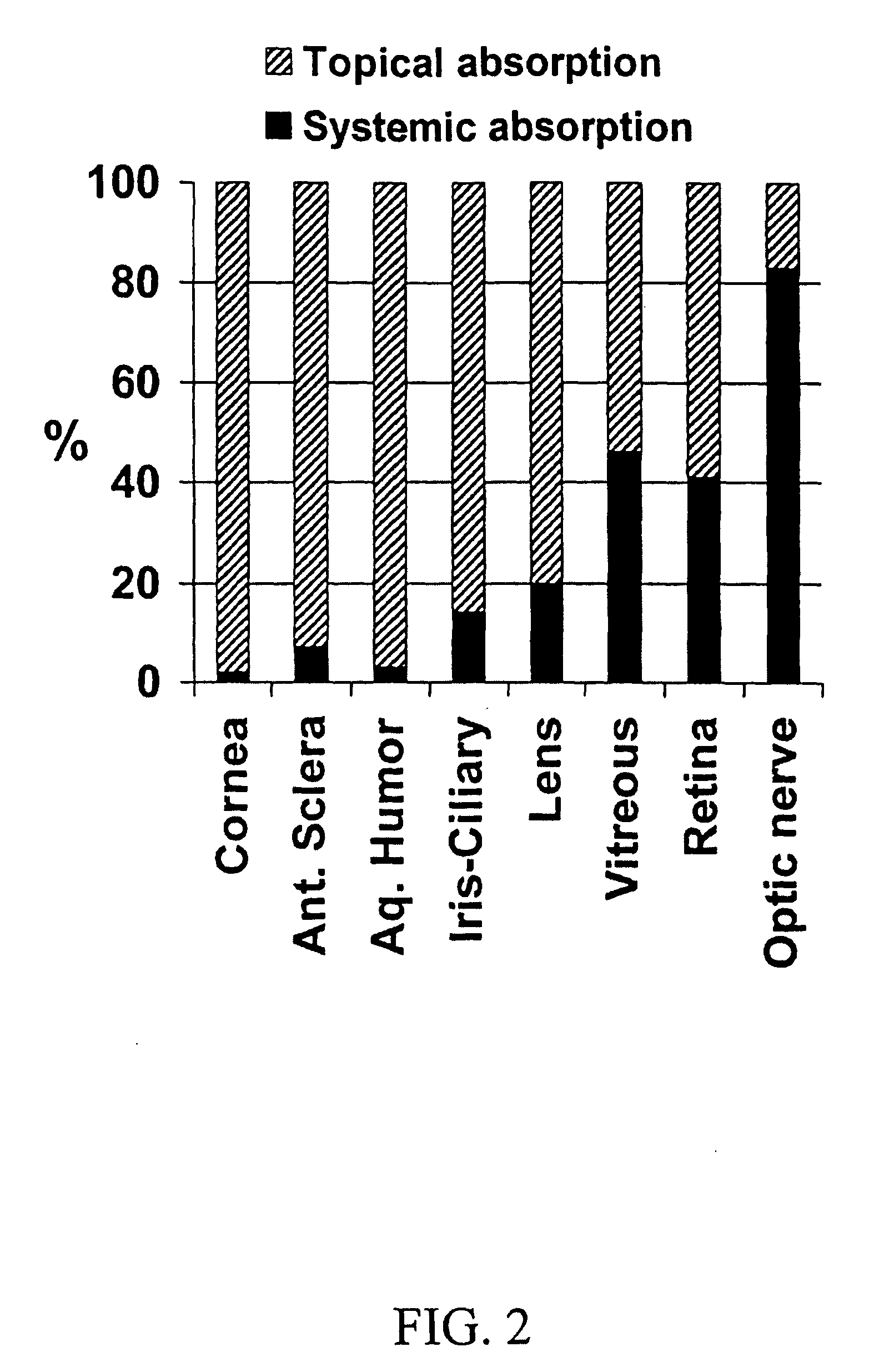
Cyclodextrin nanotechnology for ophthalmic drug delivery
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
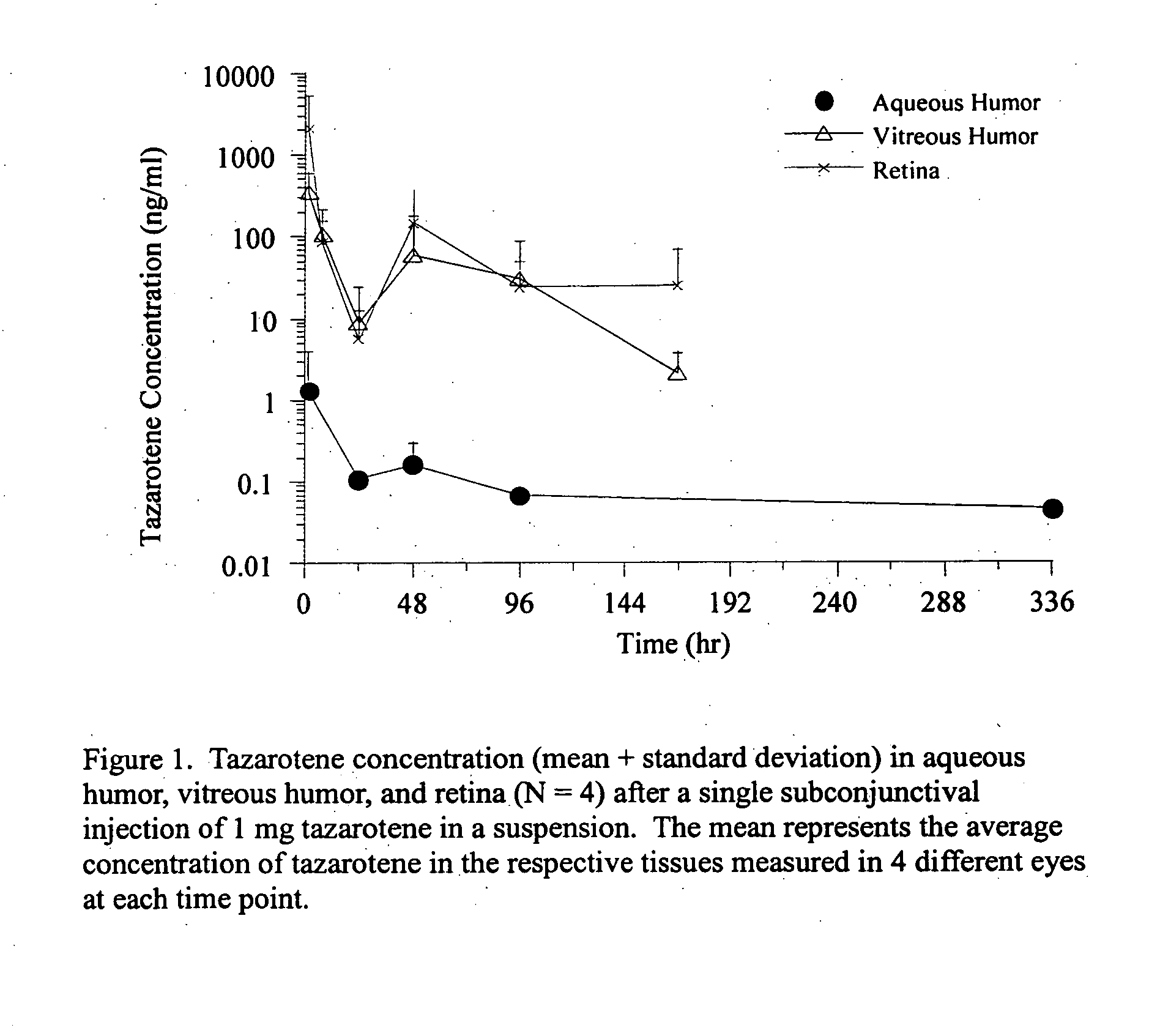
Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug
InactiveUS20050009910A1Prolong the action timeIncrease concentrationBiocideSenses disorderConjunctivaEster prodrug
The present invention relates to method of sustained-delivery of an active drug to a posterior part of an eye of a mammal to treat or prevent a disease or condition affecting said mammal, wherein said disease or condition can be treated or prevented by the action of said active drug upon said posterior part of the eye, comprising administering an effective amount of an ester prodrug of the active drug subconjunctivally or periocularly. Preferably, the active drug is more than about 10 times as active as the prodrug. Other aspects of this invention deal with the treatment of certain diseases by the periocular or subconjunctival delivery of an ester prodrug, and certain pharmaceutical products containing ester prodrugs for periocular or subconjunctival administration.
Owner:ALLERGAN INC
Method and apparatus for connective tissue treatment
InactiveUS7789841B2Promote healingWide applicabilityUltrasound therapyElcosanoid active ingredientsConnective tissueSurgery
The invention relates to methods and apparatus for therapeutically treating connective tissue or increasing vascularization in tissue using ultrasound. More particularly, the present invention relates to methods and apparatus which use ultrasound to stimulate growth or healing, or to treating pathologies, of connective tissue, or to increase vascularization in ischaemic or grafted tissue using ultrasound.
Owner:EXOGEN
Method and apparatus for connective tissue treatment
InactiveUS8123707B2Useful in treatmentUltrasound therapyElcosanoid active ingredientsConnective tissueSurgery
The invention relates to methods and apparatus for therapeutically treating connective tissue or increasing vascularization in tissue using ultrasound. More particularly, the present invention relates to methods and apparatus which use ultrasound to stimulate growth or healing, or to treating pathologies, of connective tissue, or to increase vascularization in ischaemic or grafted tissue using ultrasound.
Owner:EXOGEN
Biodegradable polymers for lowering intraocular pressure
InactiveUS20130071349A1Lower eye pressureSenses disorderElcosanoid active ingredientsAcetic acidMedicine
The present invention provides a method of treating glaucoma, the method comprising the step of placing a polymer in an eye of a patient, which biologically degrades over a period of time to release biodegradants, which are effective to lower the intraocular pressure of the patient, thereby treating glaucoma. Said polymer is preferably selected from the group consisting of polymers of lactic acid, glycolic acid and / or mixtures thereof. More preferably the polymer is a copolymer of lactic acid and glycolic acid, e.g. a copolymer comprising from 50 to 100% lactic acid and from 0 to 50% glycolic acid, by weight.
Owner:ALLERGAN INC
Method for preparing a polymer micelle pharmaceutical preparation containing drug for injection
InactiveUS20060057219A1Improve efficiencyThe preparation method is simple and easyHeavy metal active ingredientsPowder deliveryOrganic solventWater soluble drug
A production method of a preparation containing drug-encapsulating polymer micelles is provided, which comprises dissolving a hydrophilic-hydrophobic block copolymer and a sparingly water-soluble drug in a volatile organic solvent, then removing the solvent, and stirring the residue with water at a temperature not higher than 30° C. to dissolve the drug-encapsulating polymer micelles into the water.
Owner:NANOCARRIER
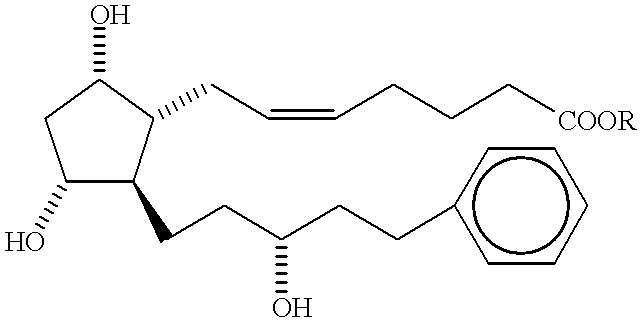
Process for the preparation of prostaglandin derivatives
InactiveUS20050209337A1High yieldDesired purityBiocideElcosanoid active ingredientsMedicinal chemistryCrystallization
Owner:FINETECH PHARMA
Treatment of female sexual dysfunction with vasoactive intestinal polypeptide agonists
InactiveUS7226910B2Good for healthImprove the lubrication effectBiocidePeptide/protein ingredientsObstetricsActive agent
Owner:VIVUS
Drug preparations for treating sexual dysfunction
Topical gelled compositions comprising a drug for treating sexual dysfunction dispersed within a polymer matrix and methods and treatments using said compositions.
Owner:GLYCOBIOSCI +1
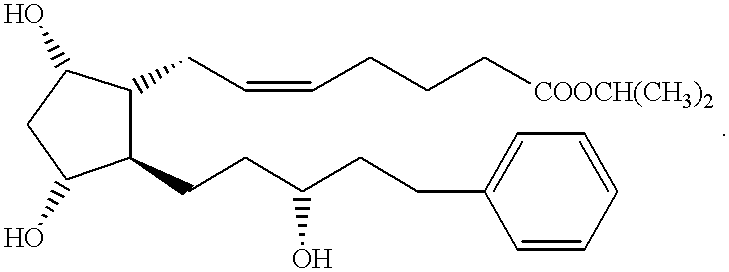
Compounds and methods for delivery of prostacyclin analogs
ActiveUS20050085540A1Improve oral bioavailabilityBiocideSenses disorderThrombusArterial Vasodilation
This invention pertains generally to prostacyclin analogs and methods for their use in promoting vasodilation, inhibiting platelet aggregation and thrombus formation, stimulating thrombolysis, inhibiting cell proliferation (including vascular remodeling), providing cytoprotection, preventing atherogenesis and inducing angiogenesis. Generally, the compounds and methods of the present invention increase the oral bioavailability and circulating concentrations of treprostinil when administered orally. Compounds of the present invention have the following formula:
Owner:UNITED THERAPEUTICS CORP
Method for treating peripheral vascular diseases
The present invention provides a method for treating peripheral vascular diseases in a mammalian subject, which comprises administering to the patient in need thereof an effective amount of 11-deoxy-prostaglandin compound.
Owner:SUCAMPO
Drug for reducing side effects in ribavirin interferon combination therapy
InactiveUS20060088502A1Eliminate side effectsReduces side effects found in ribavirin/IFNBiocideElcosanoid active ingredientsChronic viral hepatitis CSide effect
There is provided a drug for reducing side effects, anemia in particular, in combination therapy of chronic hepatitis C with ribavirin and interferon, which contains as the active ingredient at least one member selected from the group consisting of eicosapentaenoic acid (EPA) and pharmaceutically acceptable salts and esters thereof.
Owner:MOCHIDA PHARM CO LTD
Sustained release medicinal compositions
InactiveUS6328979B1Improve hydrophobicityGood sustained release effectElcosanoid active ingredientsAerosol deliverySolubilityAcid derivative
The present invention relates to sustained-release pharmaceutical compositions for ionic pharmaceutically active substances (excluding ionic prostanoic acid derivatives) containing ionic compounds having opposite charges to those of the active substances and increasing hydrophobicity of the active substances. More specifically, the invention relates to sustained-release pharmaceutical compositions comprising the ionic pharmaceutically active substances and the ionic compounds having opposite charges to those of the active substances and increasing hydrophobicity of the active substances that contain hydrophobic groups in the molecule thereof. The pharmaceutical composition of the invention can exhibit excellent sustained release effect of the active substance, irrespective of water solubility possessed by the ionic pharmaceutically active substances.
Owner:ASTELLAS PHARMA INC
Immunostimulatory nucleic acids for the treatment of asthma and allergy
InactiveUS20060154890A1Avoid allergiesPrevent asthmaBiocideElcosanoid active ingredientsTime scheduleAllergy
The invention involves administration of an immunostimulatory nucleic acid alone or in combination with an asthma / allergy medicament for the treatment or prevention of asthma and allergy in subjects. The combination of drugs are administered in synergistic amounts or in various dosages or at various time schedules. The invention also relates to kits and compositions concerning the combination of drugs.
Owner:COLEY PHARM GRP INC
Omega3 fatty acid compound preparation
ActiveUS20130115284A1Suppress modification and insolubilizationStabilize compoundBiocideElcosanoid active ingredientsDecompositionCombinatorial chemistry
Provided is a compound preparation including at least one selected from the group consisting of ω3 polyunsaturated fatty acids and pharmaceutically acceptable salts and esters thereof and at least one selected from the group consisting of statin compounds and pharmaceutically acceptable salts thereof. The compound preparation is in a form of a soft capsule having a capsule coating with a pH of 7.0 to 9.5. The compound preparation suppresses the decomposition of the statin compounds and / or the modification / insolubilization of the capsule coating. A medical use for the compound preparation, a method of manufacturing the compound preparation and a method of using the compound preparation are also provided.
Owner:MOCHIDA PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com