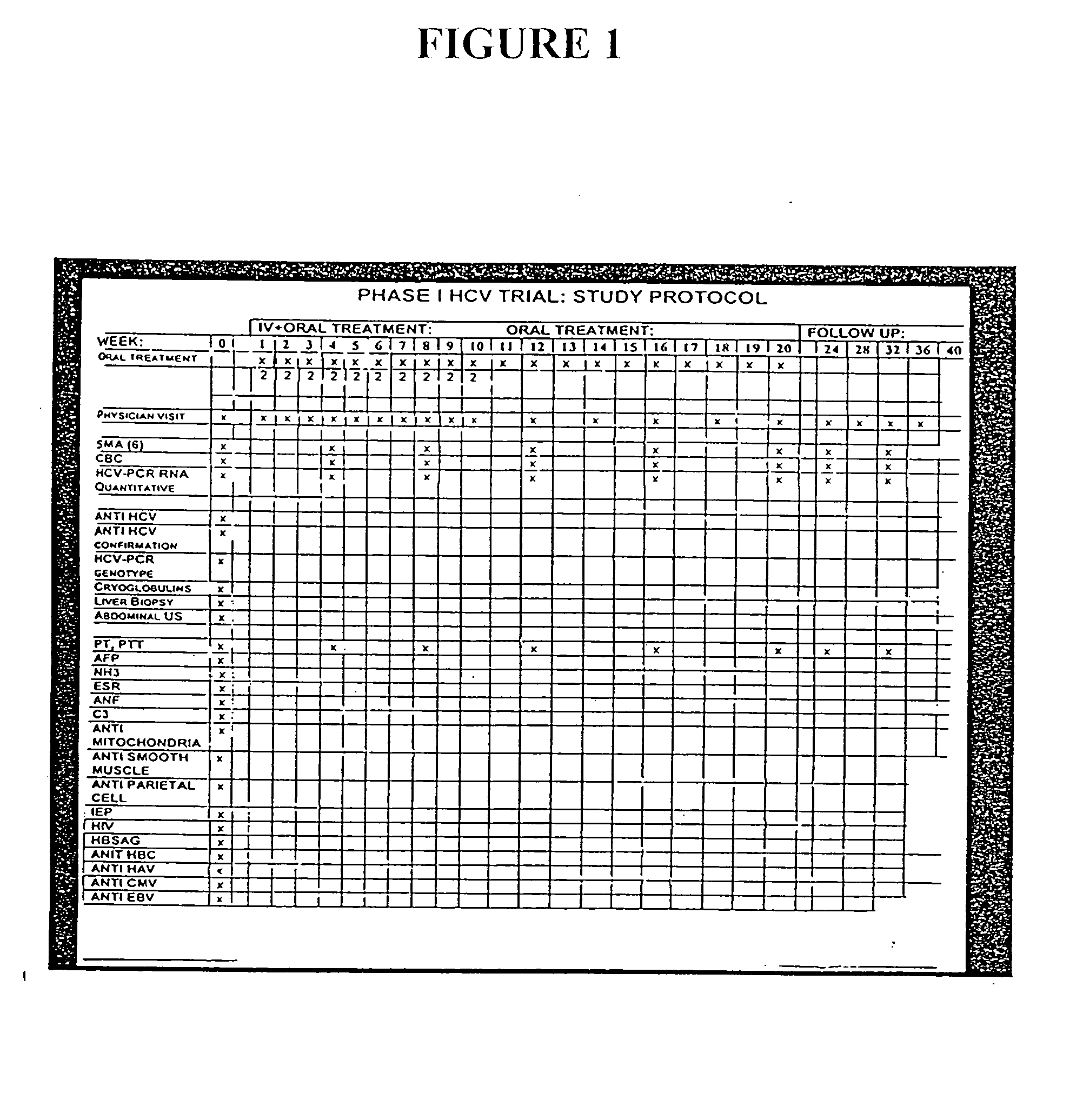
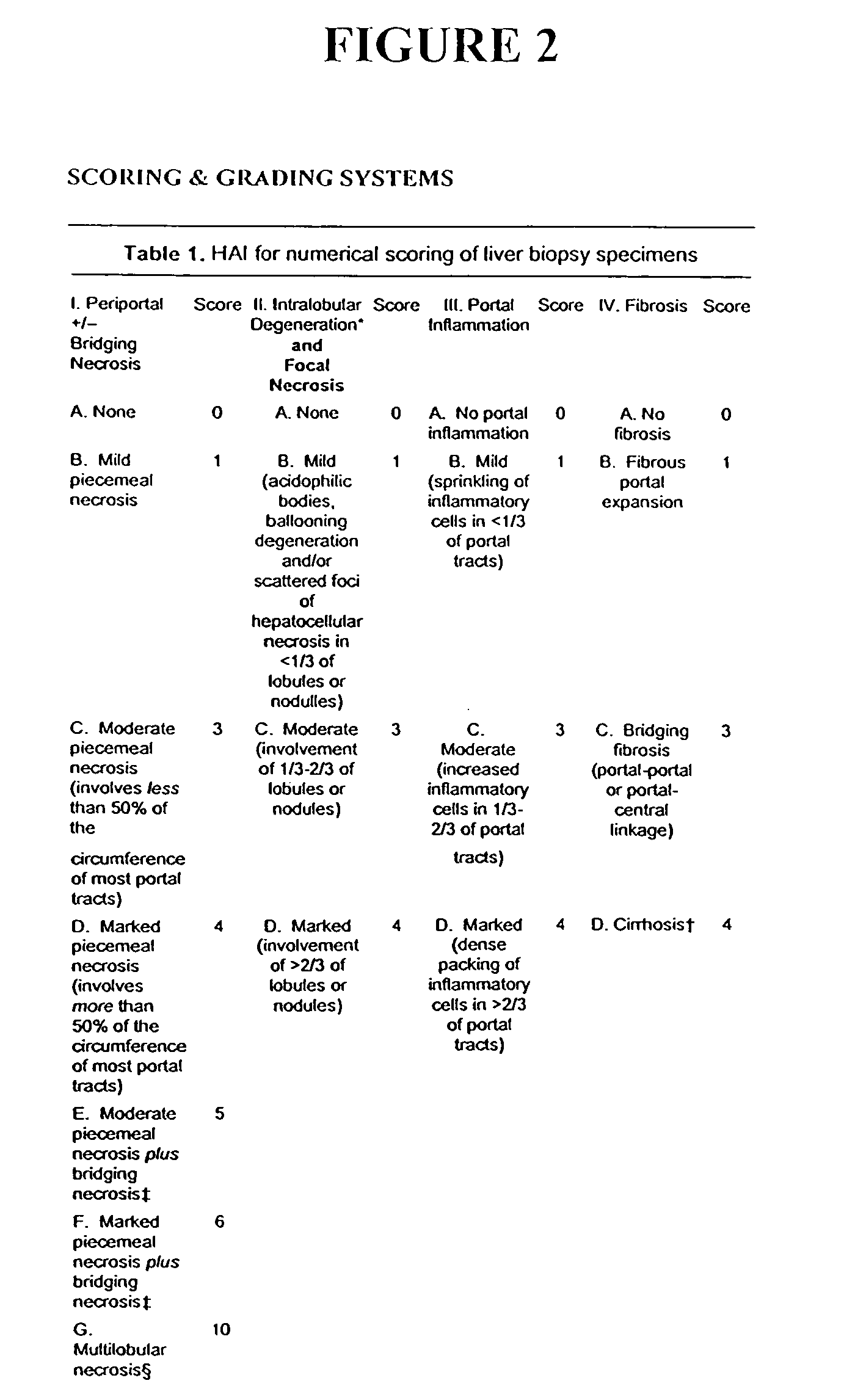
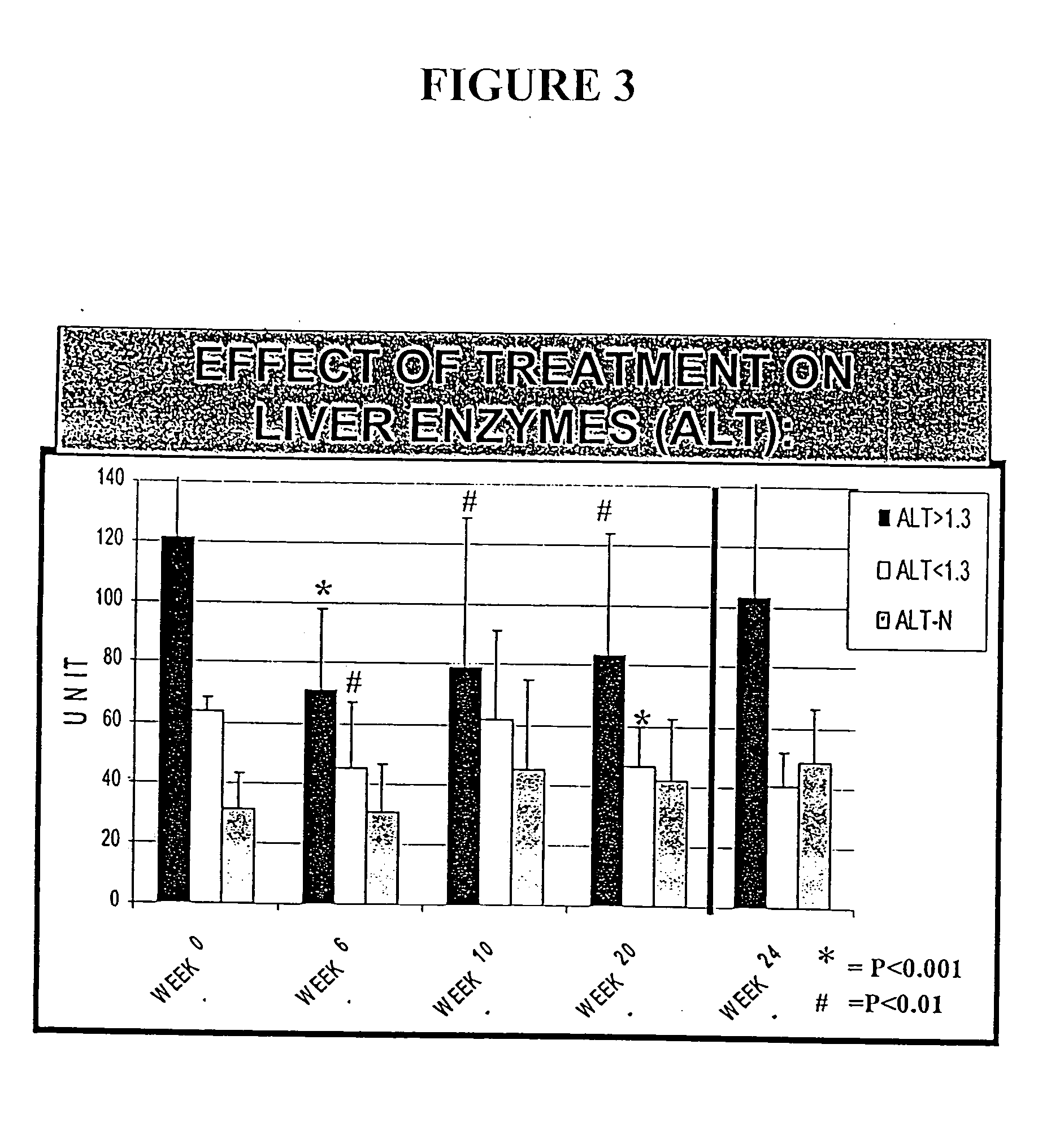
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Chronic viral hepatitis C" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This long-lasting liver infection is caused by the hepatitis C virus. It begins as an acute hepatitis that starts within the first 6 months of exposure to the virus. For most people who get it -- up to 85% -- the illness moves into a long-lasting stage. This is called a chronic hepatitis C infection.
Plant extracts for the preparation of pharmaceutical compositions for the treatment of hepatitis
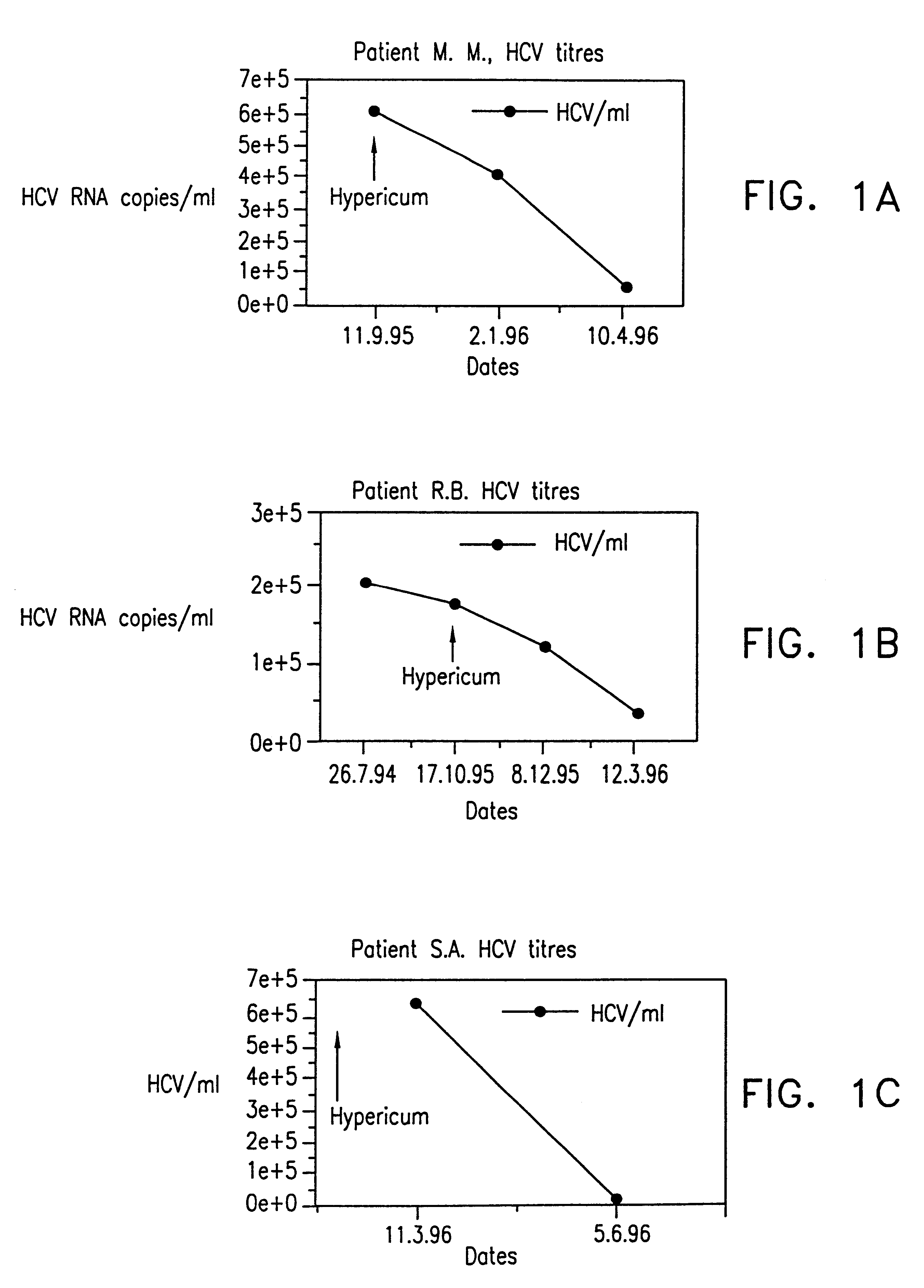
The use of extracts from the plant Hypericum perforatum in the preparation of pharmaceutical compositions for the treatment of hepatitis C, chronic hepatitis C and related viruses, said pharmaceutical compositions comprising at least one extract of the plant Hypericum perforatum and optionally in addition, one or more pharmaceutically acceptable inactive components, selected from, carriers, coatings, diluents and adjuvants.
Owner:LAVIE DAVID +1
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS20050123628A1Reduce oxidative stressReduce lipid peroxidationBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
Drug for reducing side effects in ribavirin interferon combination therapy
InactiveUS20060088502A1Eliminate side effectsReduces side effects found in ribavirin/IFNBiocideElcosanoid active ingredientsChronic viral hepatitis CSide effect
There is provided a drug for reducing side effects, anemia in particular, in combination therapy of chronic hepatitis C with ribavirin and interferon, which contains as the active ingredient at least one member selected from the group consisting of eicosapentaenoic acid (EPA) and pharmaceutically acceptable salts and esters thereof.
Owner:MOCHIDA PHARM CO LTD
Yeast-based therapeutic for chronic hepatitis C infection
InactiveUS7439042B2Reduces and prevents HCV infectionSsRNA viruses positive-senseViral antigen ingredientsChronic viral hepatitis CYeast
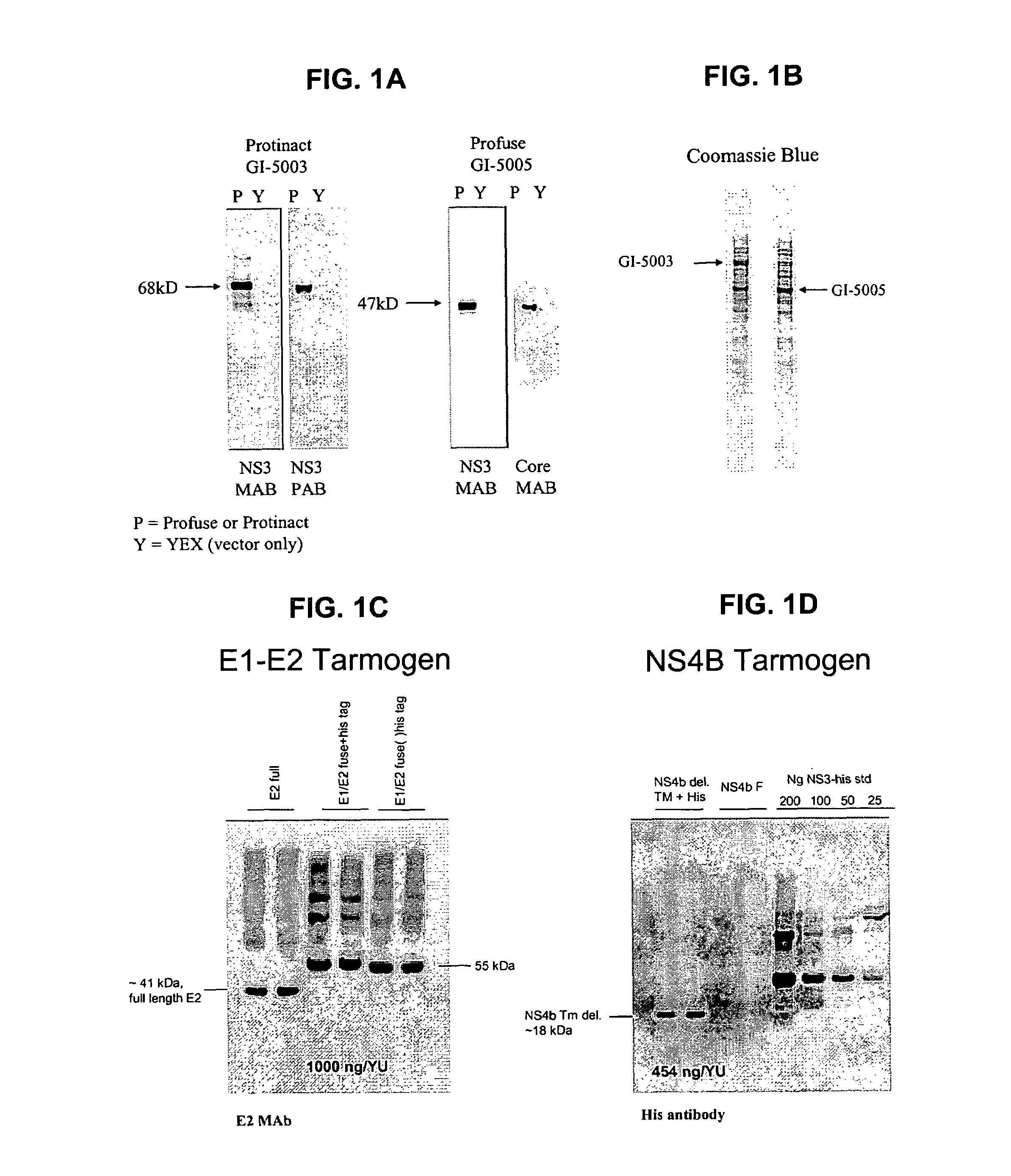
Disclosed are compositions, including vaccines, and methods for vaccinating an animal against hepatitis C virus (HCV) and for treating or preventing hepatitis C viral infection in an animal. The invention includes a variety of novel HCV fusion proteins that can be used directly as a vaccine or in conjunction with a yeast-based vaccine vehicle to elicit an immune response against HCV in an animal. The invention also includes the use of the HCV fusion gene and protein described herein in any diagnostic or therapeutic protocol for the detection and / or treatment or prevention of HCV infection.
Owner:GLOBE IMMUNE INC
HCV combination therapy
InactiveUS6849254B1Ameliorate ribavirin-related hemolysisLow viral-RNABiocidePeptide/protein ingredientsChronic viral hepatitis CHemolysis
Methods of treating patients having susceptible viral infections, especially chronic hepatitis C infection by administering to said patient a therapeutically effective amount of a combination therapy of interferon-alfa and ribavirin for a time sufficient to lower HCV-RNA in association with a therapeutically effective amount of an antioxidant for a time sufficient to ameliorate ribavirin-related hemolysis are disclosed.
Owner:MERCK SHARP & DOHME CORP
Immunotherapy for chronic hepatitis c virus infection
InactiveUS20110256098A1Increase the number ofReduce in quantitySsRNA viruses positive-sensePeptide/protein ingredientsChronic viral hepatitis CInterferon therapy
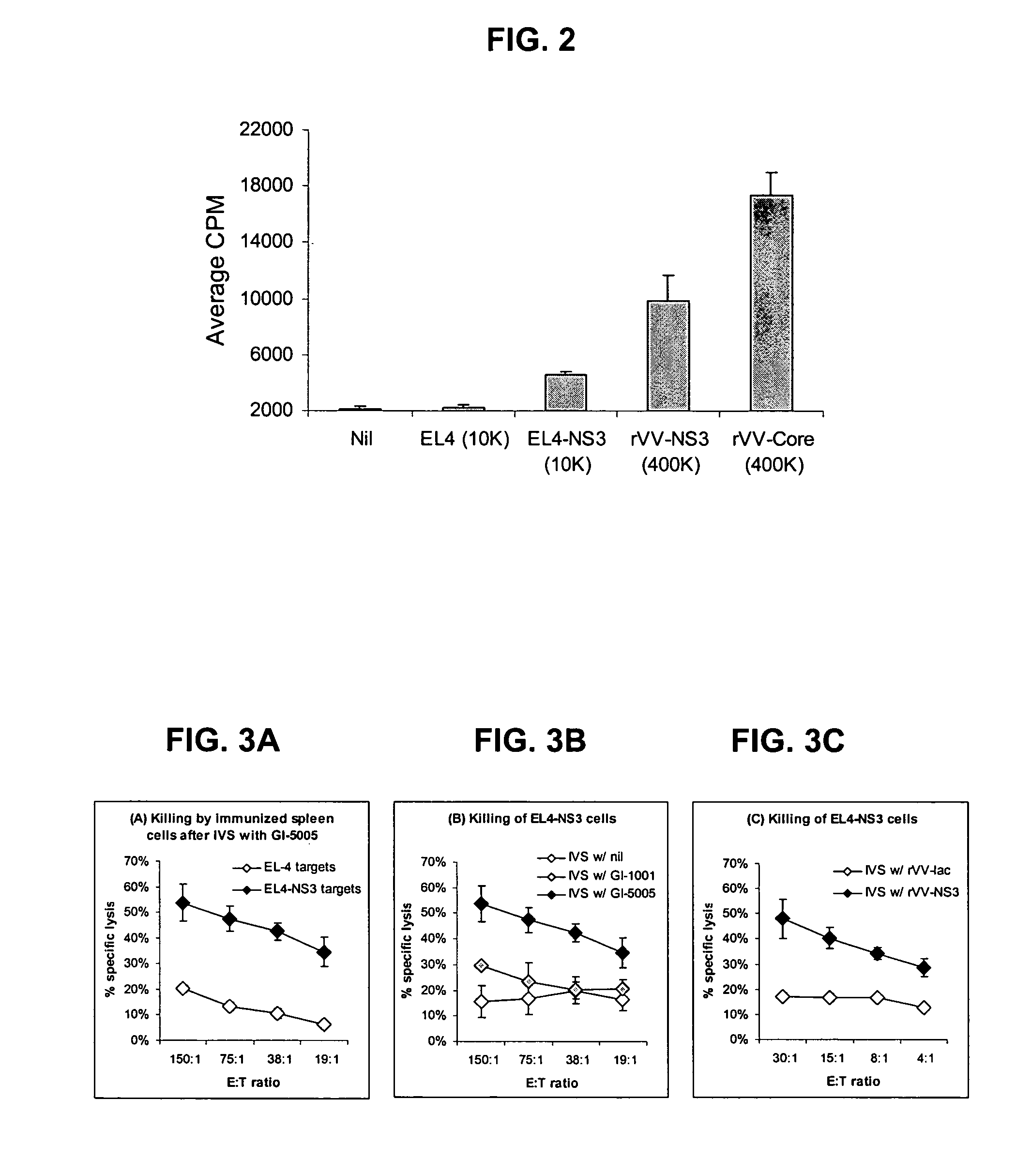
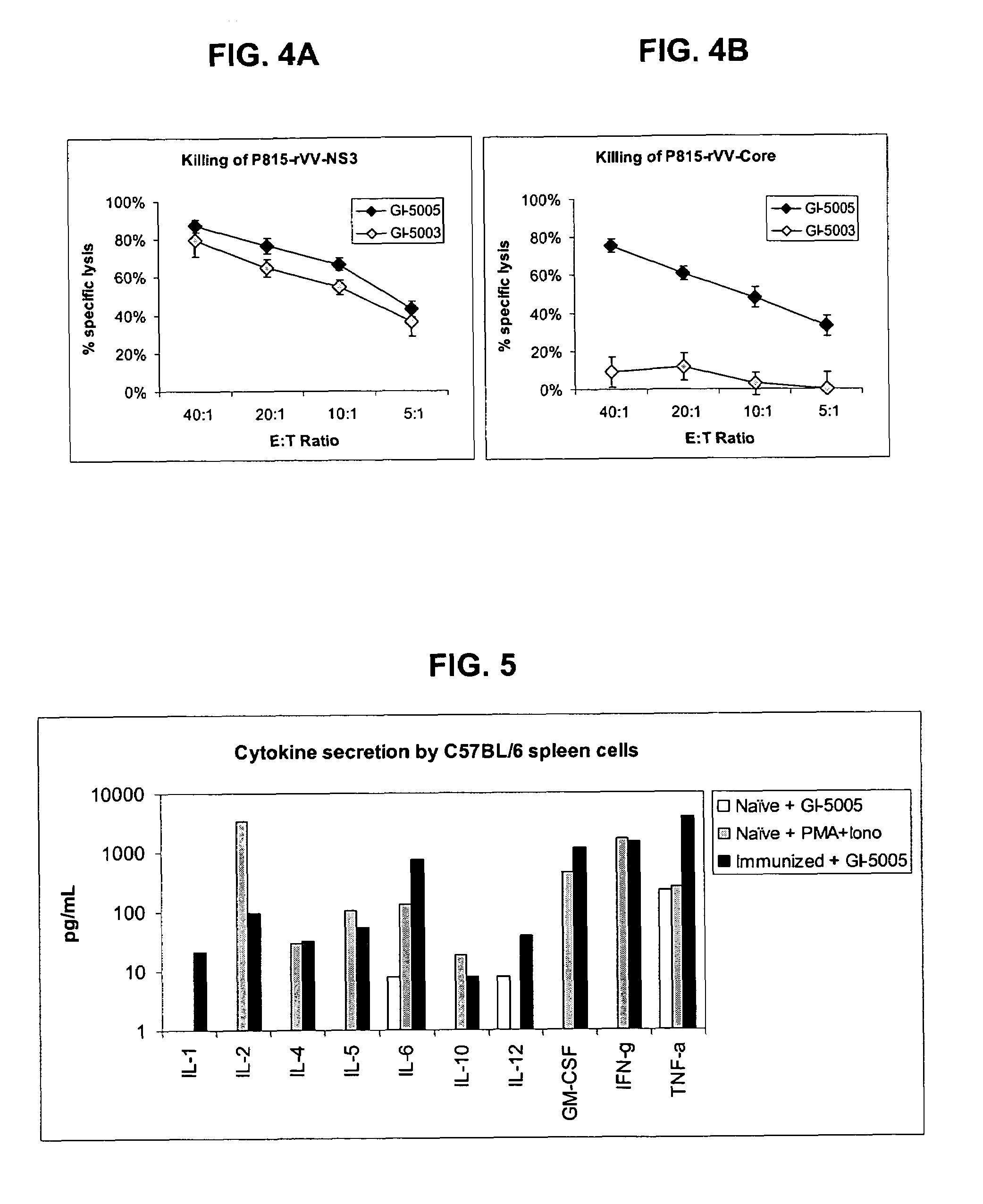
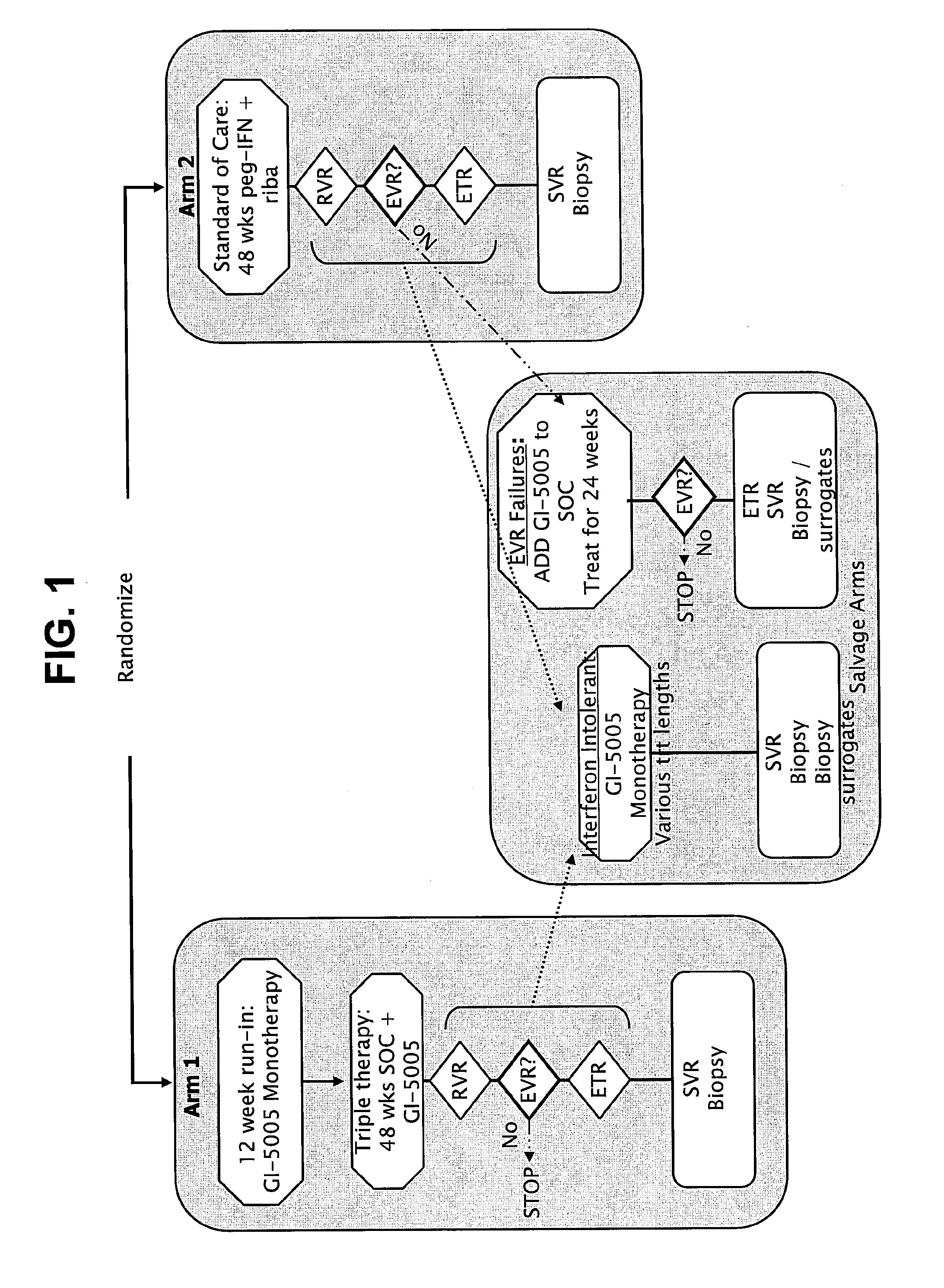
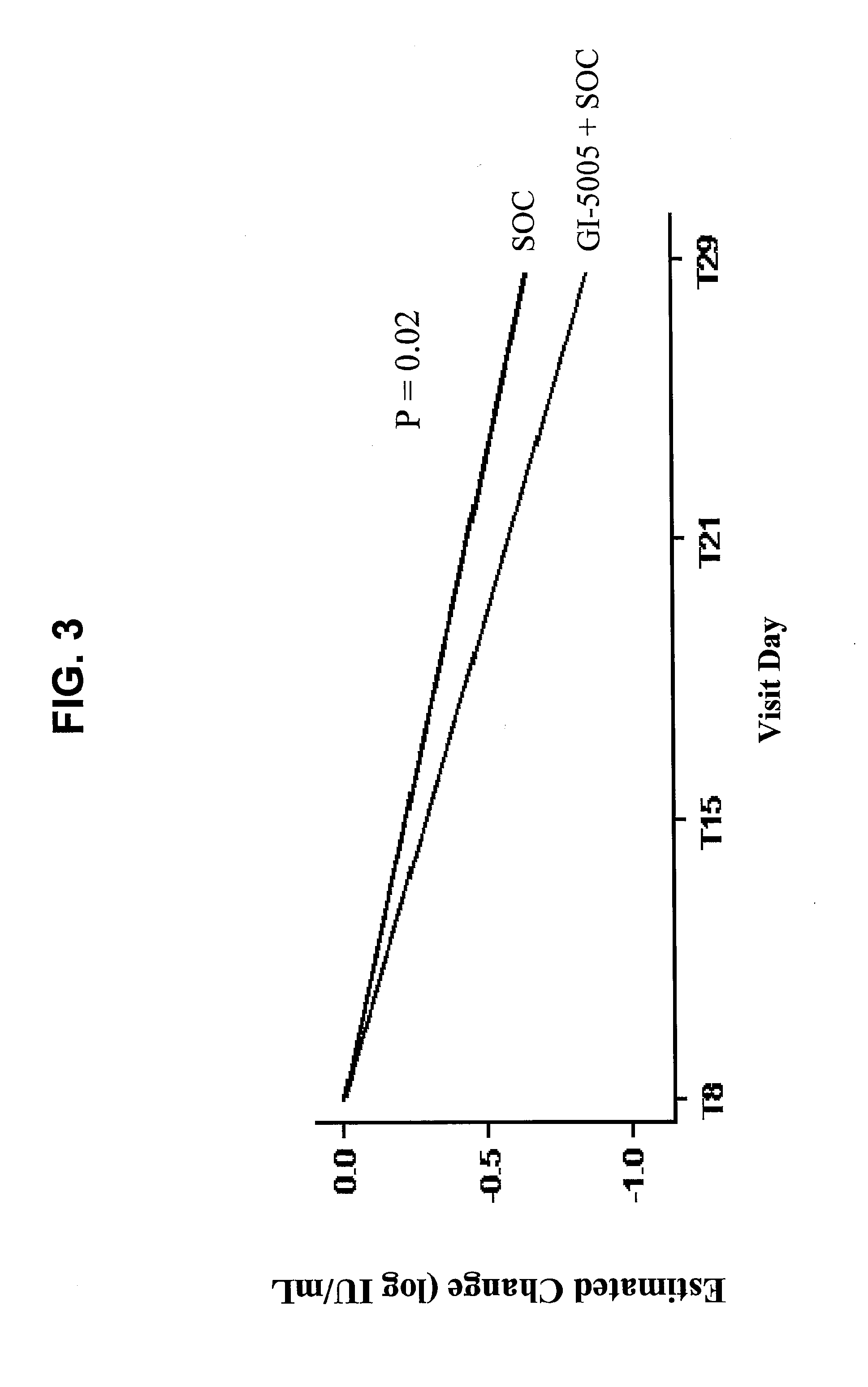
Disclosed are uses of immunotherapeutic compositions in combination with Standard of Care (SOC), or interferon therapy combined with anti-viral therapy, for the improved treatment of chronic hepatitis C virus (HCV) infection and related conditions, including liver function. The compositions, kits and uses of the invention, as compared to the use of SOC therapy alone: improves the rate of early response to therapy as measured by early virologic markers (e.g., RVR and EVR), enlarges the pool of patients who will have sustained responses to therapy over the long term, offers shortened courses of therapy for certain patients, enables “rescue” of patients who are non-responders or intolerant to SOC therapy, improves liver function and / or reduces liver damage in patients, and enables the personalization of HCV therapy for a patient, which can result in dose sparing, improved patient compliance, reduced side effects, and improved long term therapeutic outcomes.
Owner:GLOBE IMMUNE INC
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS7078064B2Reduce peroxidationReduce stressBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
Polyalkylene polymer compounds and uses thereof
ActiveUS20050107277A1Limit its therapeutic usefulnessMaintain and improve therapeutic benefit of such therapyNervous disorderPeptide/protein ingredientsChronic viral hepatitis CPolyethylene glycol
The invention relates to novel polyalkylene glycol compounds and methods of using them. In particular, compounds comprising a novel polyethylene glycol conjugate are used alone, or in combination with antiviral agents to treat a viral infection, such as chronic hepatitis C.
Owner:BIOGEN IDEC MA INC
Yeast-based therapeutic for chronic hepatitis c infection
InactiveUS20080107671A1Reduces and prevents HCV infectionBiocideSsRNA viruses positive-senseChronic viral hepatitis CYeast
Disclosed are compositions, including vaccines, and methods for vaccinating an animal against hepatitis C virus (HCV) and for treating or preventing hepatitis C viral infection in an animal. The invention includes a variety of novel HCV fusion proteins that can be used directly as a vaccine or in conjunction with a yeast-based vaccine vehicle to elicit an immune response against HCV in an animal. The invention also includes the use of the HCV fusion gene and protein described herein in any diagnostic or therapeutic protocol for the detection and / or treatment or prevention of HCV infection.
Owner:GLOBE IMMUNE INC
Ribavirin-interferon alfa combination therapy for eradicating detectable HCV-RNA in patients having chronic hepatitis C infection
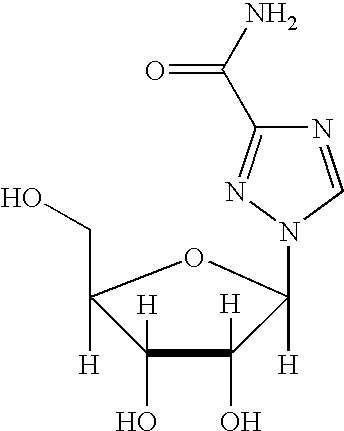
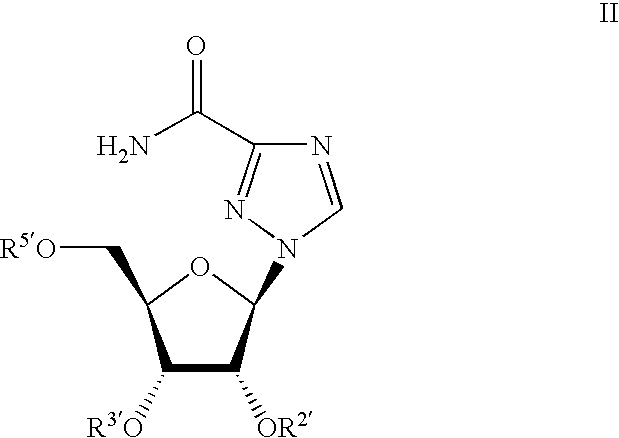
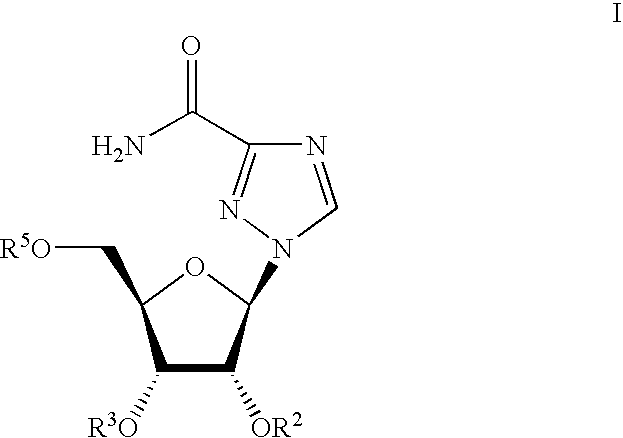
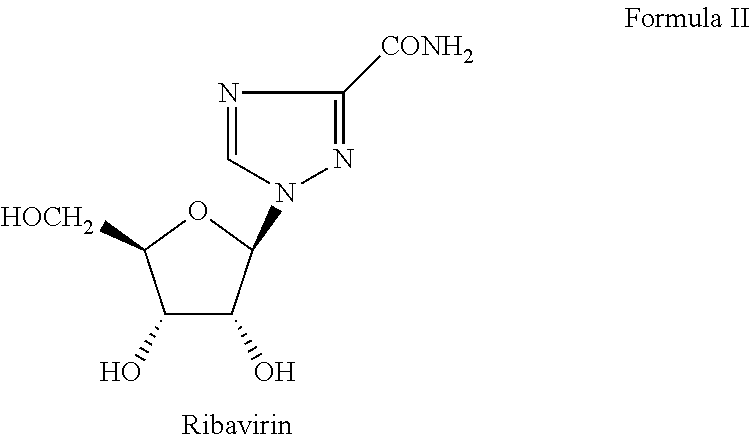
Ribavirin derivatives represented by the formula II, pharmaceutical compositions containing them as well as methods of using the ribavirin derivatives represented by the formula II for the treatment of susceptible viral infections, for example, chronic hepatitis C infections administrating, the ribavirin derivatives being represented by formula II are disclosed.
Owner:SCHERING CORP
Plant extracts for the preparation of pharmaceutical compositions for the treatment of hepatitis
The use of extracts from the plant Hypericum perforatum in the preparation of pharmaceutical compositions for the treatment of hepatitis C, chronic hepatitis C and related viruses, said pharmaceutical compositions comprising at least one extract of the plant Hypericum perforatum and optionally in addition, one or more pharmaceutically acceptable inactive components, selected from, carriers, coatings, diluents and adjuvants.
Owner:LAVIE DAVID +1
Methods for diagnosis and intervention of hepatic disorders
ActiveUS20140067276A1Rapid assessmentIn-vivo radioactive preparationsMedical automated diagnosisDiseaseChronic viral hepatitis C
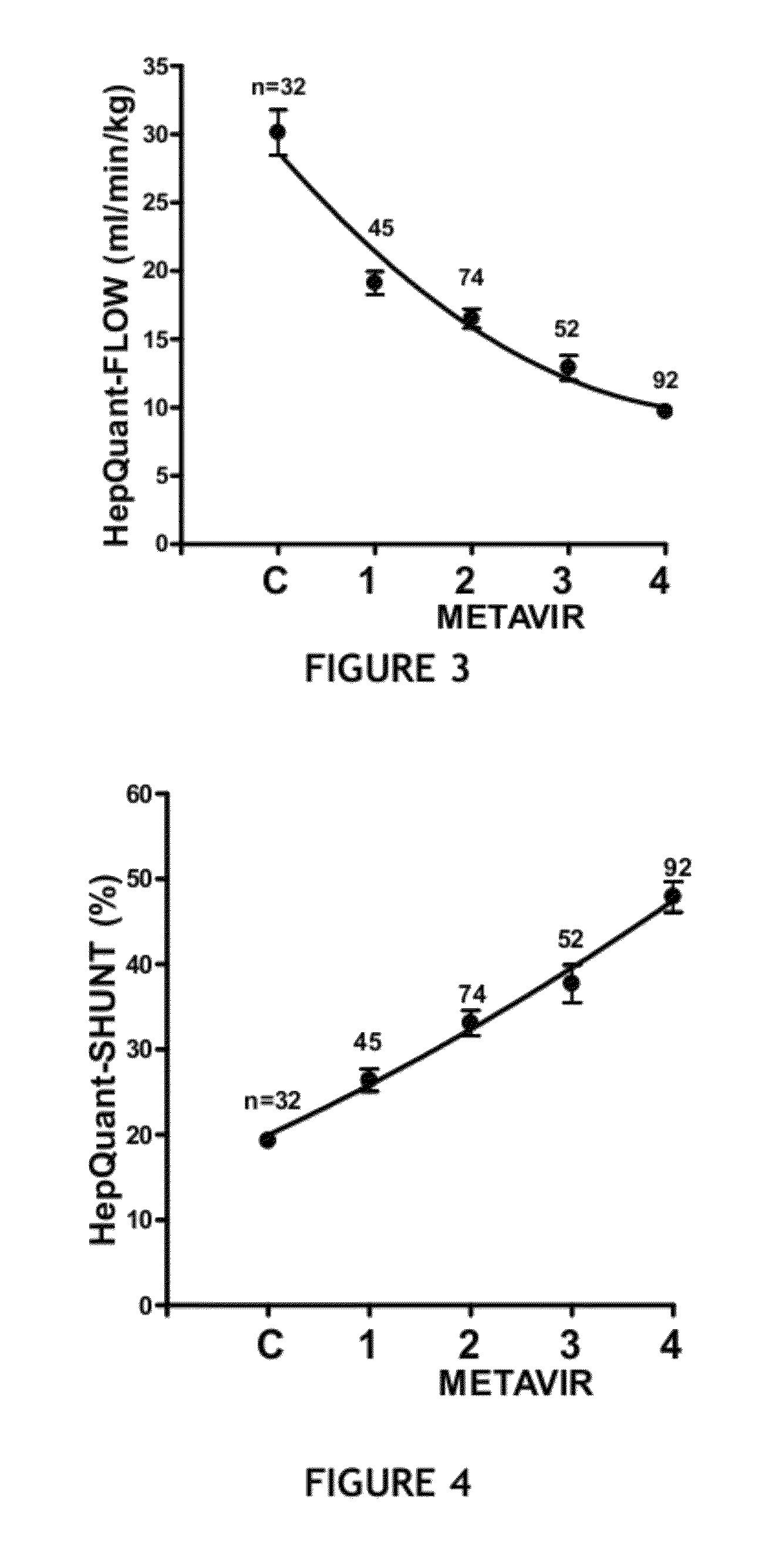
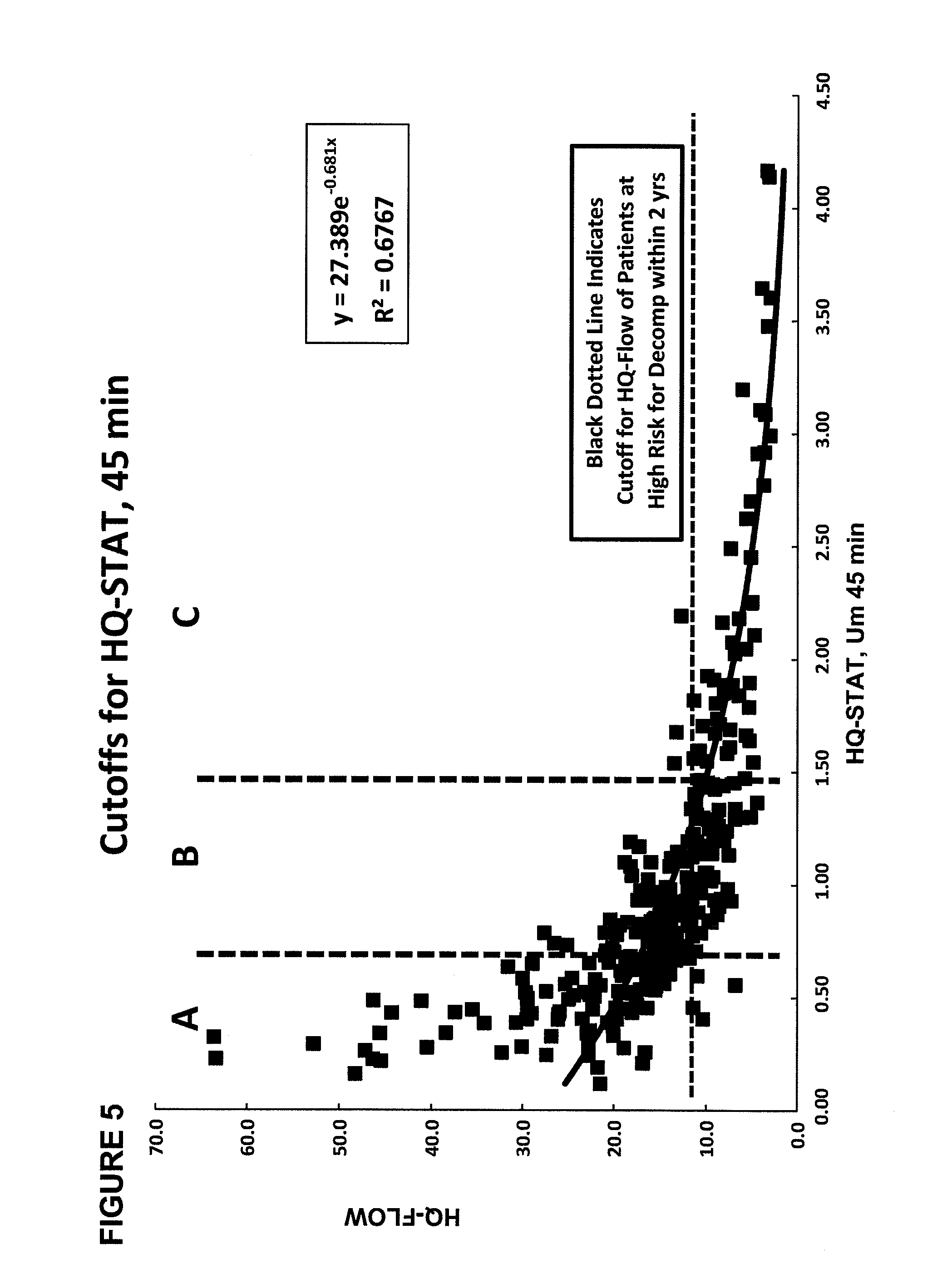
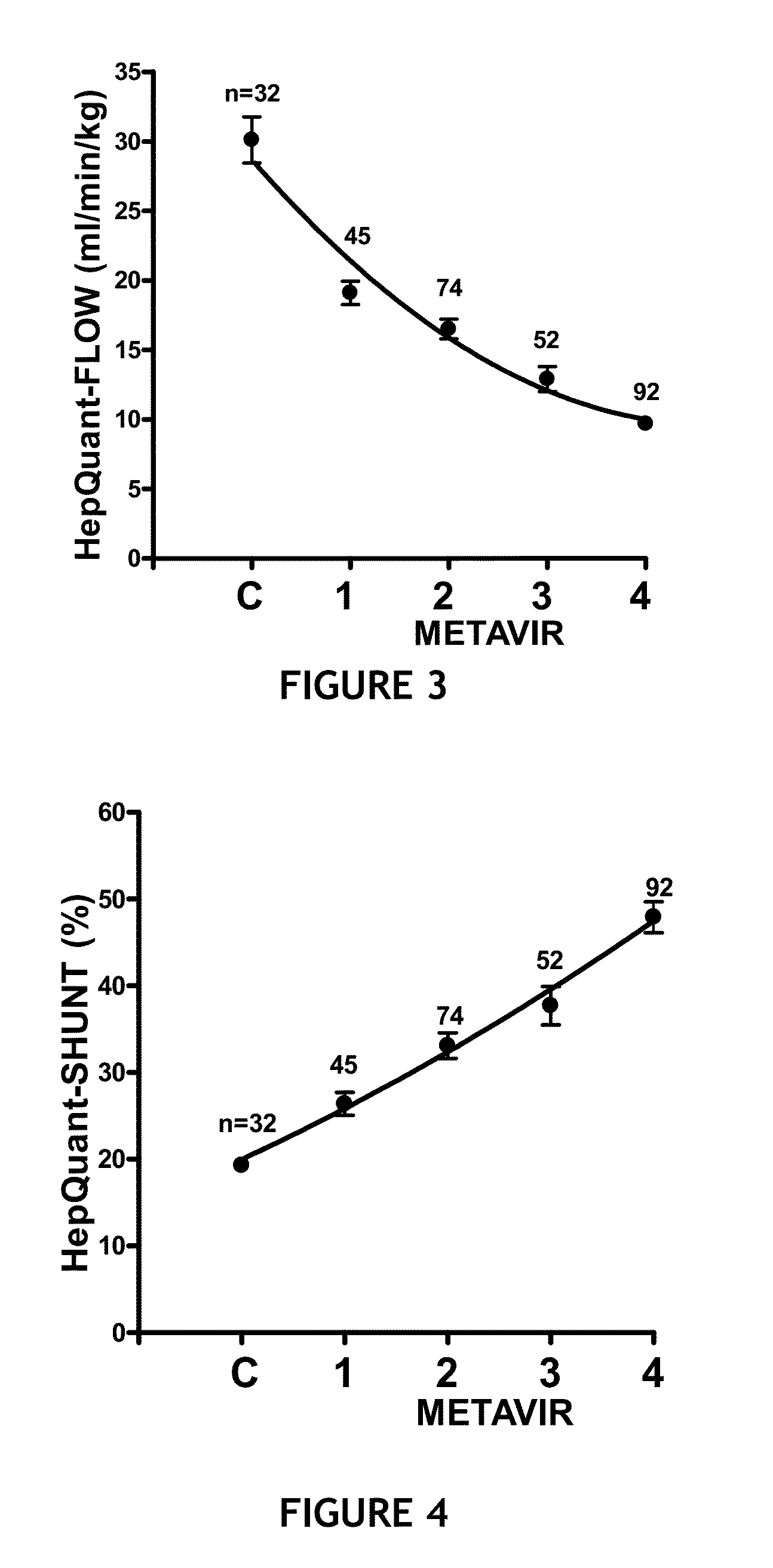
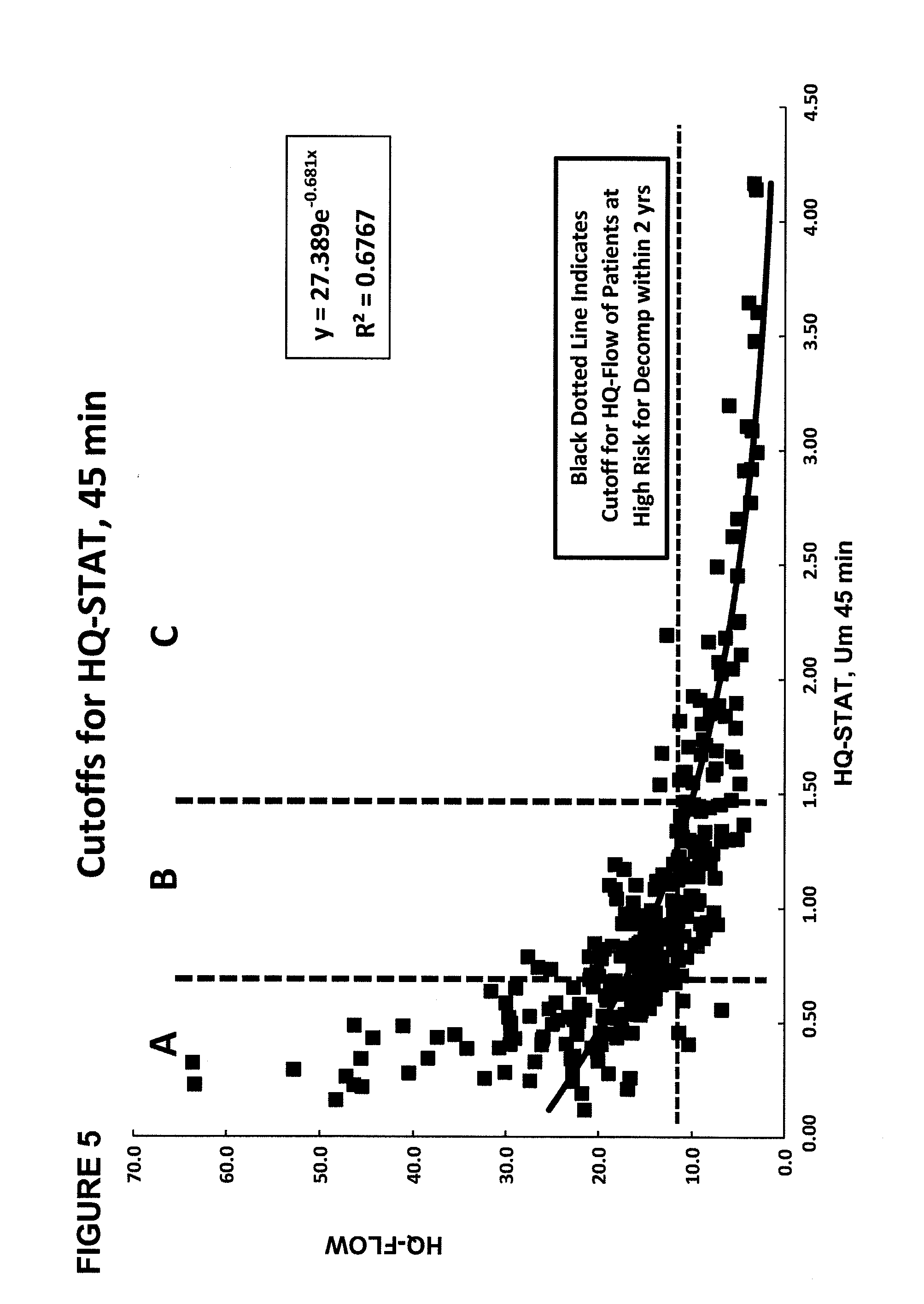
The present disclosure concerns methods of administering and detecting a distinguishable agent in a sample from and assessing the condition of an organ in a subject. In a particular embodiment, the present invention concerns methods of detecting and comparing the cholate shunt, in a subject, preferably in a subject with chronic hepatitis C. In certain embodiments, the methods may comprise obtaining a sample from a subject such as a blood or saliva sample after administering an oral and intravenous dose of a distinguishable agent such as cholate and analyzing the sample clearance of the distinguishable agent from the subject and comparing the clearance levels in order to assess hepatic health. In another embodiment, the methods may comprise analyzing a sample from a subject for the presence of a distinguishable agent such as cholate and applying information obtained from analyzing the presence of the distinguishable agent to determine a treatment for a medical condition of the subject.
Owner:UNIV OF COLORADO THE REGENTS OF
1-arylsulfonyl-1H-benzimidazole derivative and preparation method and application thereof
InactiveCN102558067AHas anti-HCV activityOrganic active ingredientsOrganic chemistryChronic viral hepatitis CBenzimidazole derivative
The invention relates to a 1-arylsulfonyl-1H-benzimidazole derivative and a preparation method and application thereof, belonging to the field of chemical medicine. The 1-arylsulfonyl-1H-benzimidazole derivative has a structure shown by formula I in the specification. According to the invention, the 1-arylsulfonyl-1H-benzimidazole derivative is obtained based on massive screening, and has anti-HCV activity; and a new option is provided for the development and application of a medicine for resisting chronic hepatitis C.
Owner:SICHUAN UNIV
DNA methylation marker in DNA of host T cells and peripheral blood mononuclear cells (PBMCs) of cancer patients
According to the present invention, it is found that a DNA methylation marker exists in the DNA of host T cells and peripheral blood mononuclear cells (PBMCs) of cancer patients. The invention discloses CG IDs derived from PBMCs DNA, wherein the clinical progression staging of hepatocellular carcinoma (HCC) and chronic hepatitis can be predicted through the methylation level of the CG IDs in the DNA of PBMCs or T cells. The present invention further discloses a kit for predicting HCC by using the identified CG IDs, and applications of a pyrosequencing DNA methylation experiment, a receiver operating characteristic (ROC) experiment, a penalized regression experiment and hierarchical clustering analysis in prediction of HCC. According to the present invention, any one of technicists in the field can obtain the DNA methylation marker for any cancers and the clinical progression staging of any cancers; and the DNA methylation marker (CG IDs) is used for diagnosing cancers, different clinical progression staging of cancers, the response of patients undergoing treatment to treatment, and chronic hepatitis B or chronic hepatitis C.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV +1
Method for assessment of hepatic function and portal blood flow
ActiveUS8961925B2In-vivo radioactive preparationsPharmaceutical containersChronic viral hepatitis CPortal vein flow
A method for estimating portal blood flow and hepatic function in a subject is provided. In one example, the STAT test is an in vitro simplified, convenient test intended for screening purposes that can reasonably estimate the portal blood flow from a single blood sample taken 60 minutes after orally administered deuterated-cholate. The test can be administered to a patient having, or suspected of having, Chronic Hepatitis C, Primary Sclerosing Cholangitis (PSC), Non-Alcoholic Fatty Liver Disease (NAFLD), or any chronic liver disease.
Owner:UNIV OF COLORADO THE REGENTS OF
Method for assessment of hepatic function and portal blood flow
InactiveUS20150204842A1Pharmaceutical containersMedical packagingChronic viral hepatitis CPortal vein flow
A method for estimating portal blood flow and hepatic function in a subject is provided. In one example, the STAT test is an in vitro simplified, convenient test intended for screening purposes that can reasonably estimate the portal blood flow from a single blood sample taken 60 minutes after orally administered deuterated-cholate. The test can be administered to a patient having, or suspected of having, Chronic Hepatitis C, Primary Sclerosing Cholangitis (PSC), Non-Alcoholic Fatty Liver Disease (NAFLD), or any chronic liver disease.
Owner:UNIV OF COLORADO THE REGENTS OF
Method for treating apathy syndrome
InactiveUS20100048634A1Improve frontal and executive cognitive functionBiocideNervous disorderChronic viral hepatitis CRisk stroke
The present invention provides a method of treating apathy syndrome in a human subject. The human subject is first evaluated to determine whether one or more behavioral characteristics of apathy are observed. If such characteristics are observed, the subject is treated with a 2-oxopyrrolidine compound, such as nefiracetam, piracetam, aniracetam, pramiracetam, nebracetam, fasoracetam, levetiracetam, or oxiracetam, in an amount effective to produce an improvement in such apathy characteristics. The present invention is useful in treating apathy in a subject suffering from conditions associated with or characterized by frontal-subcortical dysfunction. The present invention is also useful in treating apathy in a subject suffering from a stroke, Alzheimer's disease, Parkinson's disease, traumatic brain injury, depression, schizophrenia, chronic hepatitis C infection, or HIV infection.
Owner:CHA ALBERT
HCV (hepatitis C virus) poly-epitope peptide vaccine and application thereof
ActiveCN102757484AImproving immunogenicityImprove immunityVirus peptidesAntiviralsChronic viral hepatitis CPeptide vaccine
The invention discloses an HCV (hepatitis C virus) poly-epitope peptide vaccine and application thereof. The HCV poly-epitope peptide vaccine comprises the following poly-epitope polypeptides: poly-epitope polypeptide of which the amino acid residue sequence is disclosed as SEQ.ID.NO.1, or poly-epitope polypeptide of which the amino acid residue sequence is disclosed as SEQ.ID.NO.2. The polypeptide VAL-44 HCV can induce the specific T cell immune response, including CD4 cells and CD8 cells; and the T cells can quickly generate IFN-gamma and IL-2 after being subjected to specific antigenic stimulation. The HCV polypeptide vaccine can stimulate 3 of 10 chronic HCV infected persons PBMC to secrete IFN-gamma. The HCV poly-epitope polypeptide can be an ideal HCV preventive / curative candidate vaccine.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Polyalkylene polymer compounds and uses thereof
ActiveUS8017733B2Organic detergent compounding agentsNervous disorderChronic viral hepatitis CPolyethylene glycol
The invention relates to novel polyalkylene glycol compounds and methods of using them. In particular, compounds comprising a novel polyethylene glycol conjugate are used alone, or in combination with antiviral agents to treat a viral infection, such as chronic hepatitis C.
Owner:BIOGEN MA INC
Polyethylene glycol-interferon alpha conjugates for therapy of infection
InactiveCN1261806AEffective treatmentReduce or eliminate side effectsPeptide/protein ingredientsAntiviralsChronic viral hepatitis CDisease
A method comprising administering a PEG12000-IFN alpha conjugate to an individual afflicted with a viral infection susceptible of treatment with interferon alpha, preferably chronic hepatitis C, is disclosed.
Owner:SCHERING AG
Composition for treating hepatitis C
ActiveUS7381435B2Useful in treatmentControl progressBiocideDigestive systemChronic viral hepatitis CPumpkin seed
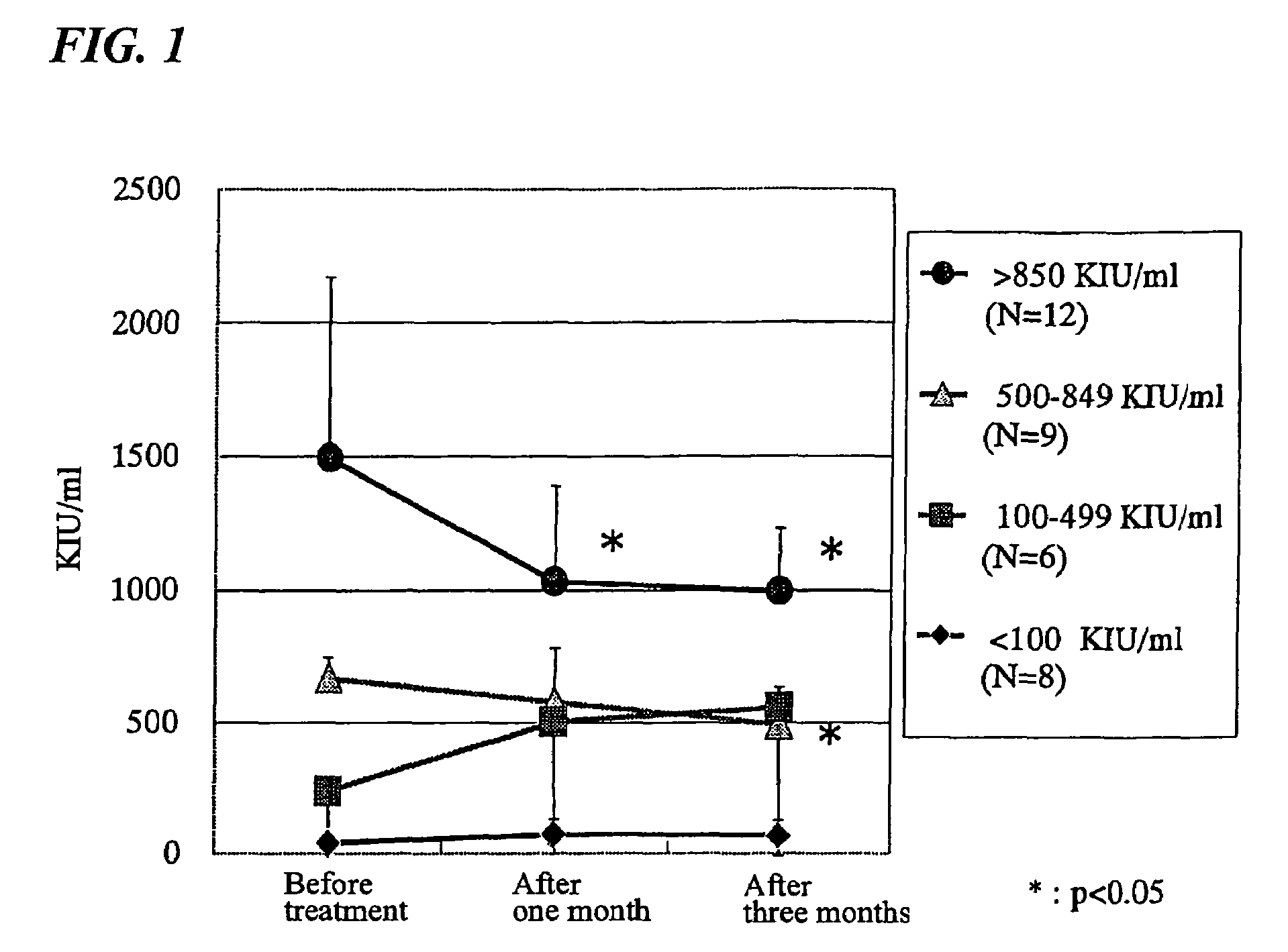
By administering a composition comprising pumpkin seed, safflower, plantain and honeysuckle, subjective symptoms (for example, general malaise and abdominal swelling) of a patient with chronic hepatitis C can be eliminated and, moreover, objective symptoms diagnosed by a medical doctor (for example, liver enlargement and palm erythema) can be relieved or eliminated. From 1 to 3 months after the administration of the composition, a significant decrease in hepatitis C virus RNA level is gradually observed. Therefore, the above composition is useful at least as a composition for treating chronic hepatitis C. In particular, it is advantageous in treating a chronic hepatitis C patient showing a high chronic hepatitis C virus RNA level.
Owner:ORIGINAL IMAGE
Method for predicting therapeutic effect on chronic hepatitis c
InactiveUS20120238459A1Improve reliabilityThe method is simple and convenientMicrobiological testing/measurementDigestive systemChronic viral hepatitis CMedicine
A method for predicting therapeutic effect of combination therapy with peginterferon and ribavirin in chronic hepatitis C therapy, comprising:a drug-sensitivity marker detection step of detecting a drug-sensitivity marker in a biological sample; anda drug-sensitivity evaluation step of evaluating drug sensitivity of the biological sample on the basis of an expression level of the drug-sensitivity marker, whereinthe biological sample is a cell or tissue derived from liver, andthe drug-sensitivity marker comprises at least one microRNA selected from the group consisting of microRNAs shown in SEQ ID NOs: 1 to 37.
Owner:MURAKAMI YOSHIKI
Gene chip for chronic hepatitis C outcome prediction in chronic hepatitis C interferon treatment
InactiveCN102485907ARelieve painLow cost of treatmentNucleotide librariesMicrobiological testing/measurementChronic viral hepatitis CInterferon therapy
The invention relates to a gene chip for chronic hepatitis C outcome prediction in chronic hepatitis C interferon treatment. The gene chip comprises 12 gene fragments of ISG56, ISG20 and the like. Through the gene chip, in an early stage of chronic hepatitis C interferon treatment on patients or before the chronic hepatitis C interferon treatment on patients, interferon treatment long-term effects can be predicted. Therefore, the gene chip is conducive to optimization and selection of a chronic hepatitis C patient therapeutic schedule, reduces patient pain and treatment costs, and is convenient for detection and clinical application.
Owner:PEOPLES HOSPITAL PEKING UNIV
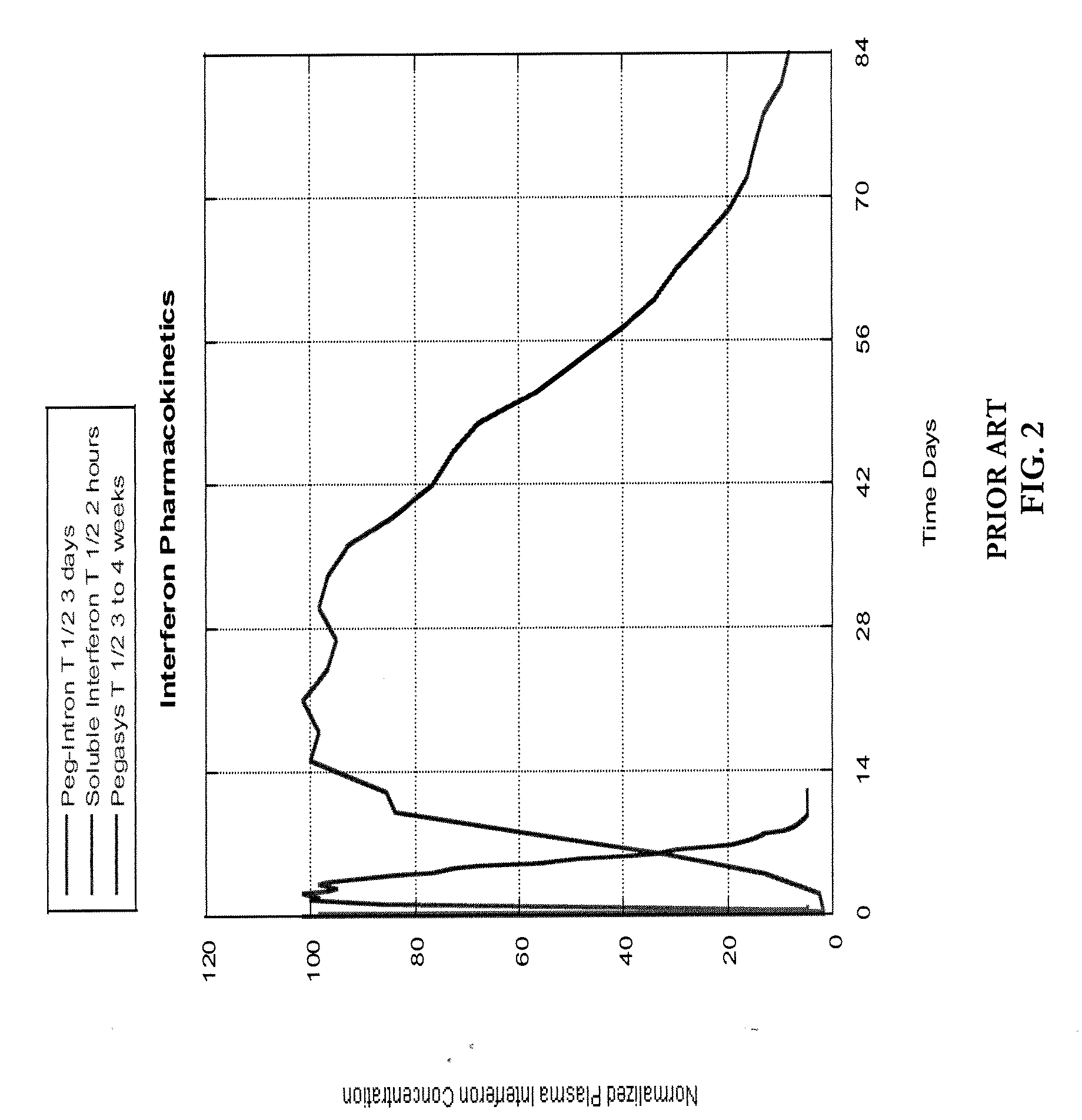
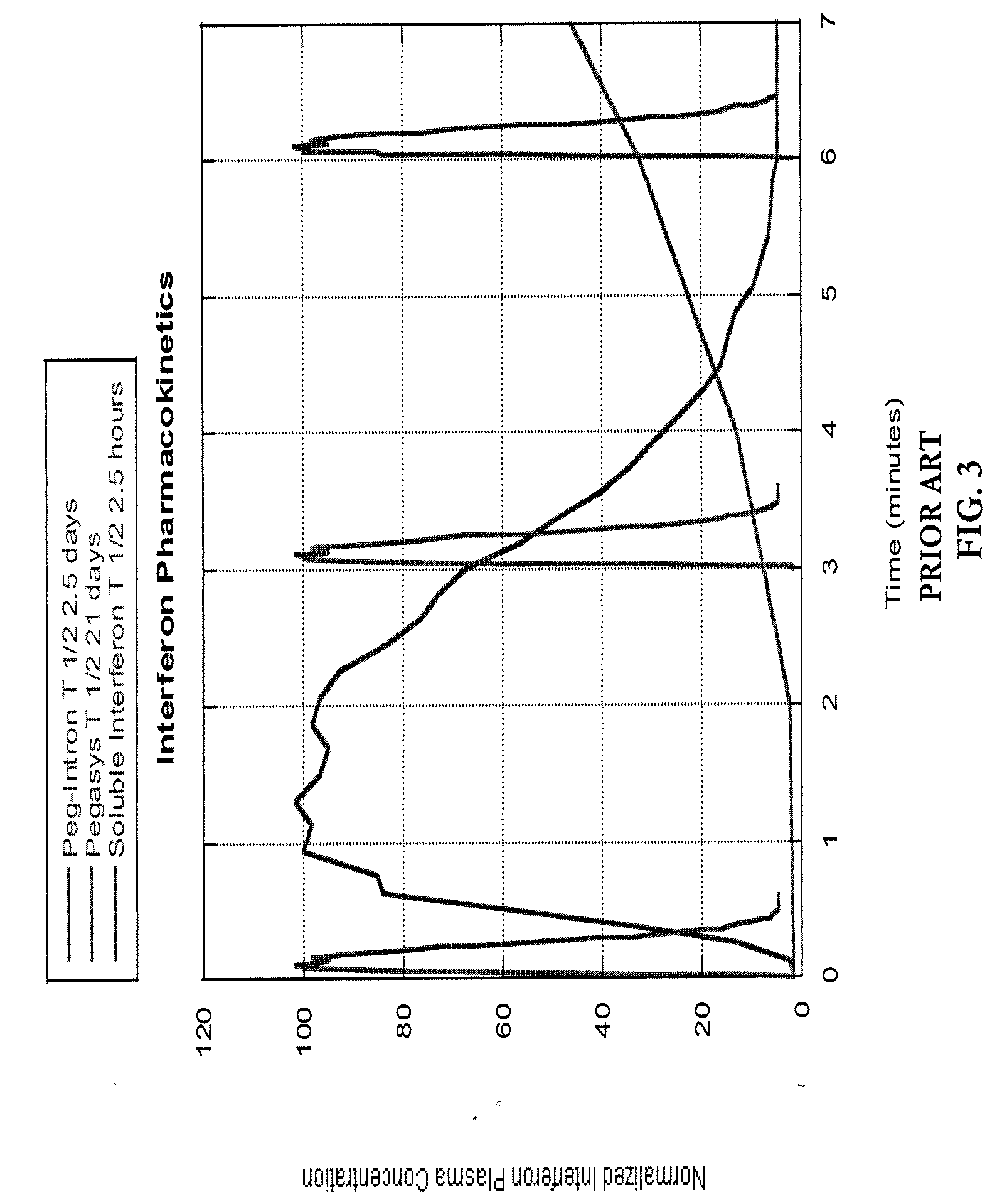
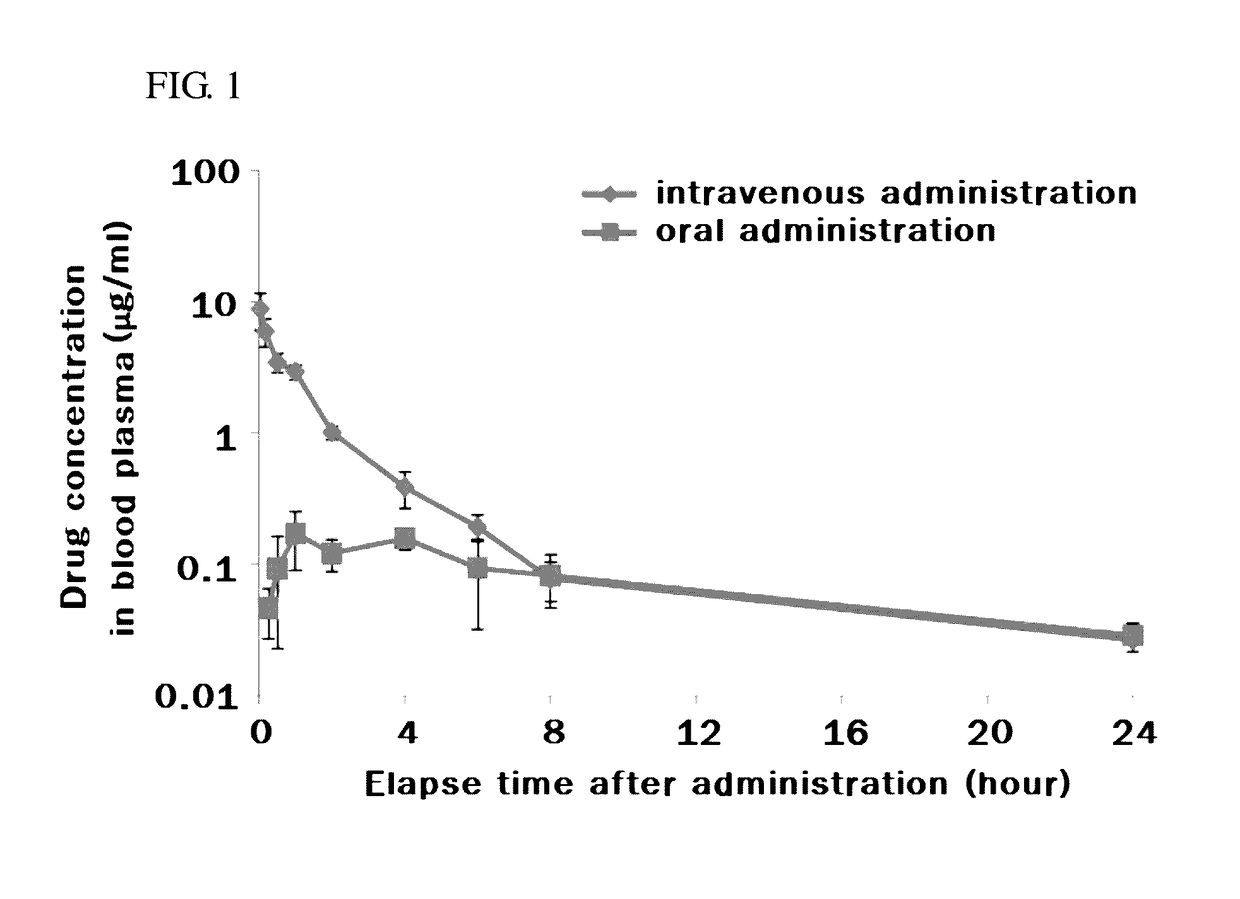
Pharmacokinetic control for optimized interferon delivery
InactiveUS20110270212A1Organic active ingredientsPeptide/protein ingredientsChronic viral hepatitis COncology
Methods and devices for treating patients having chronic hepatitis C infection so as to eradicate detectable HCV-RNA and / or inhibit the emergence of a drug resistant HCV variant are disclosed. Certain methods of the invention involve the use of a continuous infusion pump in a multiphasic combination therapy using a therapeutically effective amount of a small molecule inhibitor such as ribavirin and a therapeutically effective amount of interferon-α.
Owner:MEDTRONIC INC
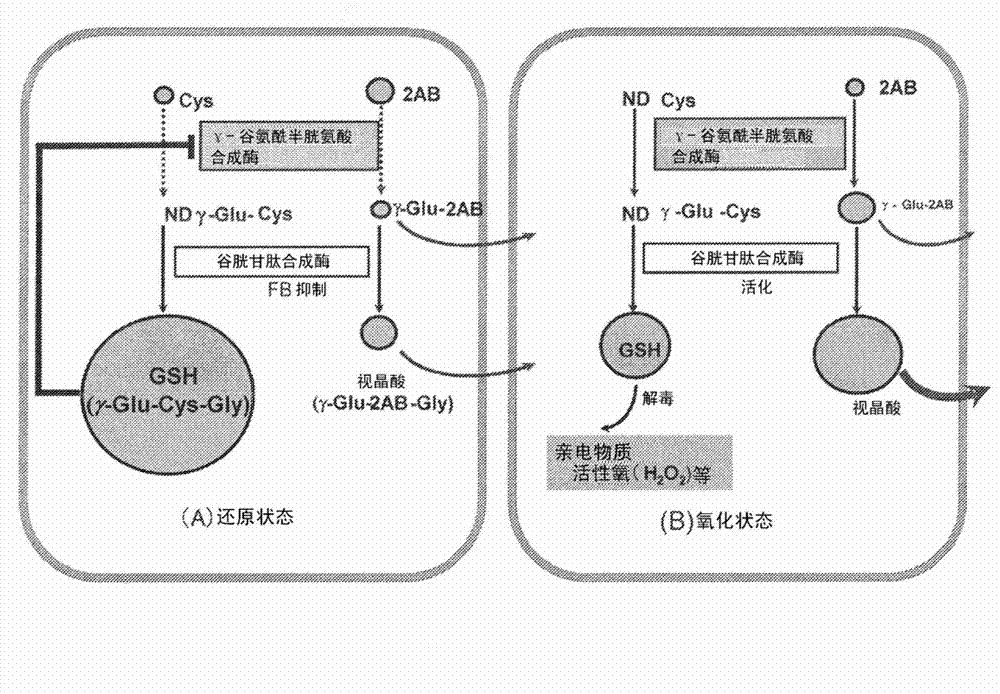
Liver disease marker, method and apparatus for measuring same, and test method for pharmaceutical preparation
ActiveCN102971632ARapid determinationDisease diagnosisBiological testingChronic viral hepatitis CSimple steatosis
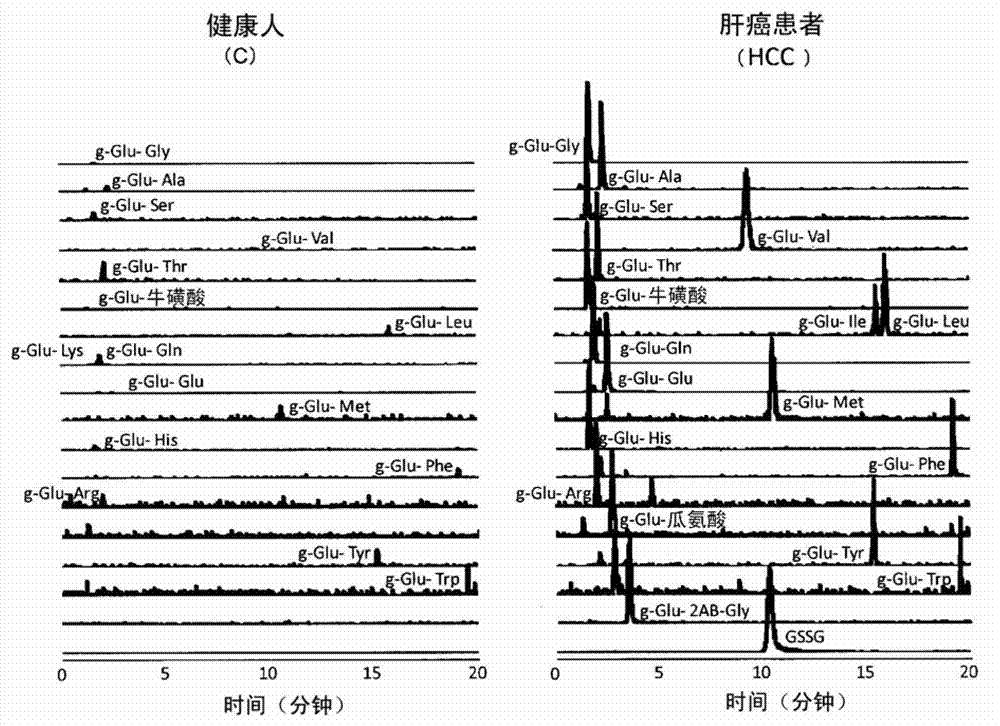
Disclosed is a method for promptly identifying a liver disease. A normal person (C) or a liver disease such as drug-induced liver injury (DI), asymptomatic hepatitis B carrier (AHB), chronic hepatitis B (CHB), hepatitis C with persistently normal ALT (CNALT), chronic hepatitis C (CHC), cirrhosis type C (CIR), hepatocellular carcinoma (HCC), simple steatosis (SS), or non-alcoholic steatohepatitis (NASH) is identified by measuring the concentration of a gamma-Glu-X (wherein X represents an amino acid or an amine) peptide or the level of AST or ALT in blood and carrying out, for example, a multiple logistic regression (MLR) based on the measured value.
Owner:KEIO UNIV
Methods for diagnosis and intervention of hepatic disorders
ActiveUS9639665B2Rapid assessmentMedical automated diagnosisDisease diagnosisChronic viral hepatitis CVein
The present disclosure concerns methods of administering and detecting a distinguishable agent in a sample from and assessing the condition of an organ in a subject. In a particular embodiment, the present invention concerns methods of detecting and comparing the cholate shunt, in a subject, preferably in a subject with chronic hepatitis C. In certain embodiments, the methods may comprise obtaining a sample from a subject such as a blood or saliva sample after administering an oral and intravenous dose of a distinguishable agent such as cholate and analyzing the sample clearance of the distinguishable agent from the subject and comparing the clearance levels in order to assess hepatic health. In another embodiment, the methods may comprise analyzing a sample from a subject for the presence of a distinguishable agent such as cholate and applying information obtained from analyzing the presence of the distinguishable agent to determine a treatment for a medical condition of the subject.
Owner:UNIV OF COLORADO THE REGENTS OF
Immunotherapy for chronic hepatitis C virus infection
InactiveUS8728489B2High frequencyReduce in quantityBiocideFungiChronic viral hepatitis CInterferon therapy
Disclosed are uses of immunotherapeutic compositions in combination with Standard of Care (SOC), or interferon therapy combined with anti-viral therapy, for the improved treatment of chronic hepatitis C virus (HCV) infection and related conditions, including liver function. The compositions, kits and uses of the invention, as compared to the use of SOC therapy alone: improves the rate of early response to therapy as measured by early virologic markers (e.g., RVR and EVR), enlarges the pool of patients who will have sustained responses to therapy over the long term, offers shortened courses of therapy for certain patients, enables “rescue” of patients who are non-responders or intolerant to SOC therapy, improves liver function and / or reduces liver damage in patients, and enables the personalization of HCV therapy for a patient, which can result in dose sparing, improved patient compliance, reduced side effects, and improved long term therapeutic outcomes.
Owner:GLOBE IMMUNE INC
Pharmaceutical combinations of sofosbuvir and ribavirin
InactiveUS20170112867A1Organic active ingredientsAntiviralsChronic viral hepatitis CHepatocellular carcinoma
This invention is a novel pharmaceutical composition comprising sofosbuvir and ribavirin and at least one pharmaceutically acceptable excipient for use in the treatment of hepatitis C virus infections, chronic hepatitis C (CHC), hepatocellular carcinoma or patients with end-stage liver disease awaiting liver transplantation.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Benzidine derivative, method for preparing same, and pharmaceutical composition containing benzidine derivative for treating liver disease caused by hepatitis C virus
The disclosed compounds have antiviral activity against C-type virus, an optical isomer thereof, a pharmaceutically acceptable salt thereof, a method for preparing the same, and a pharmaceutical composition containing the same as an active ingredient for preventing or treating liver disease caused by hepatitis C virus. The benzidine derivative according to the present invention has excellent antiviral activity against hepatitis C virus and exhibits excellent medicinal activity in the living body, and thus the pharmaceutical composition containing the same as an active ingredient can be useful as a pharmaceutical composition for preventing or treating liver disease, such as acute hepatitis C, chronic hepatitis C, cirrhosis, or hepatocellular carcinoma, caused by C-type virus.
Owner:SEOUL NAT UNIV R&DB FOUND +1
Tolerance and chronic hepatitis C virus
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com