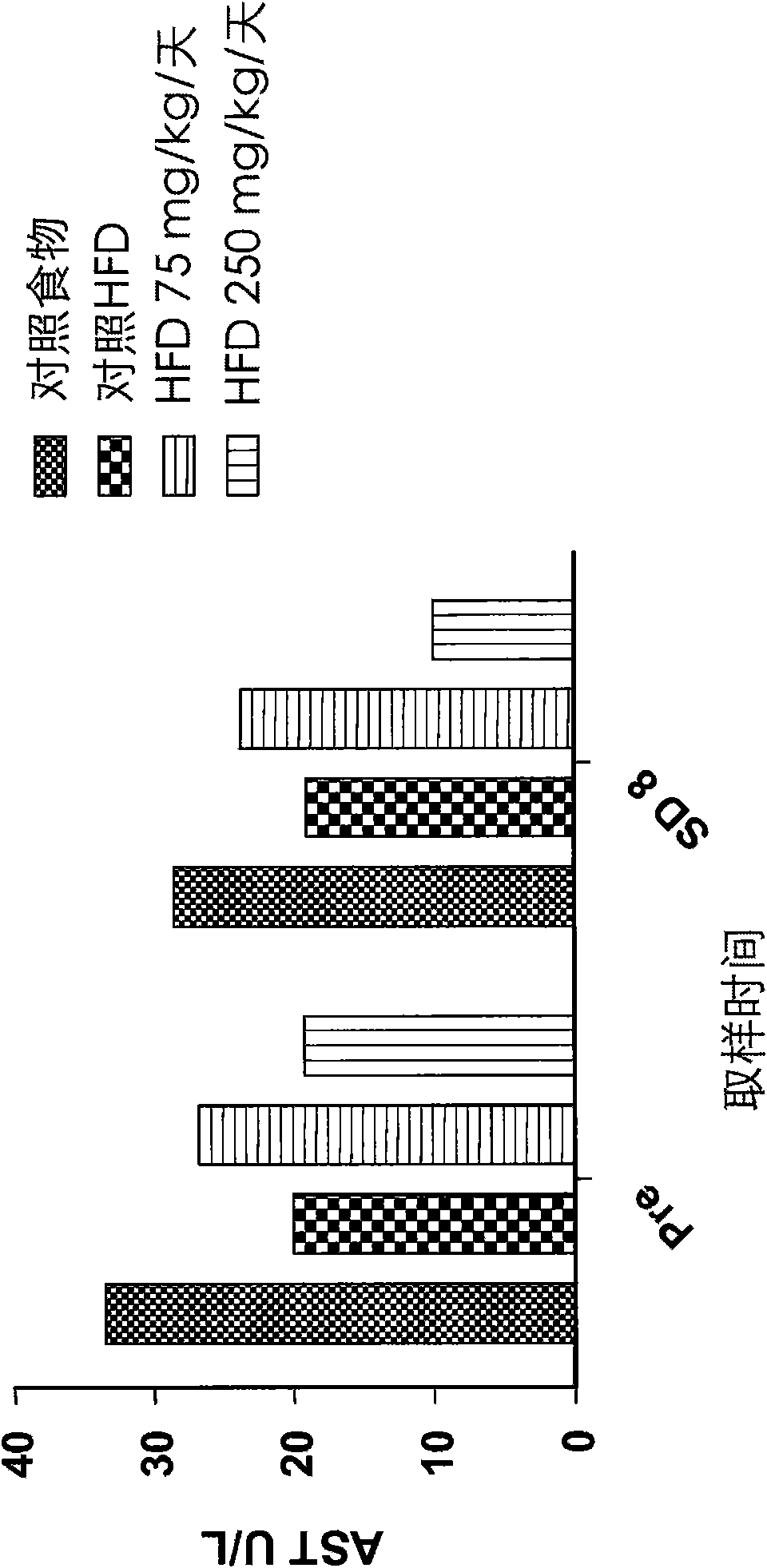
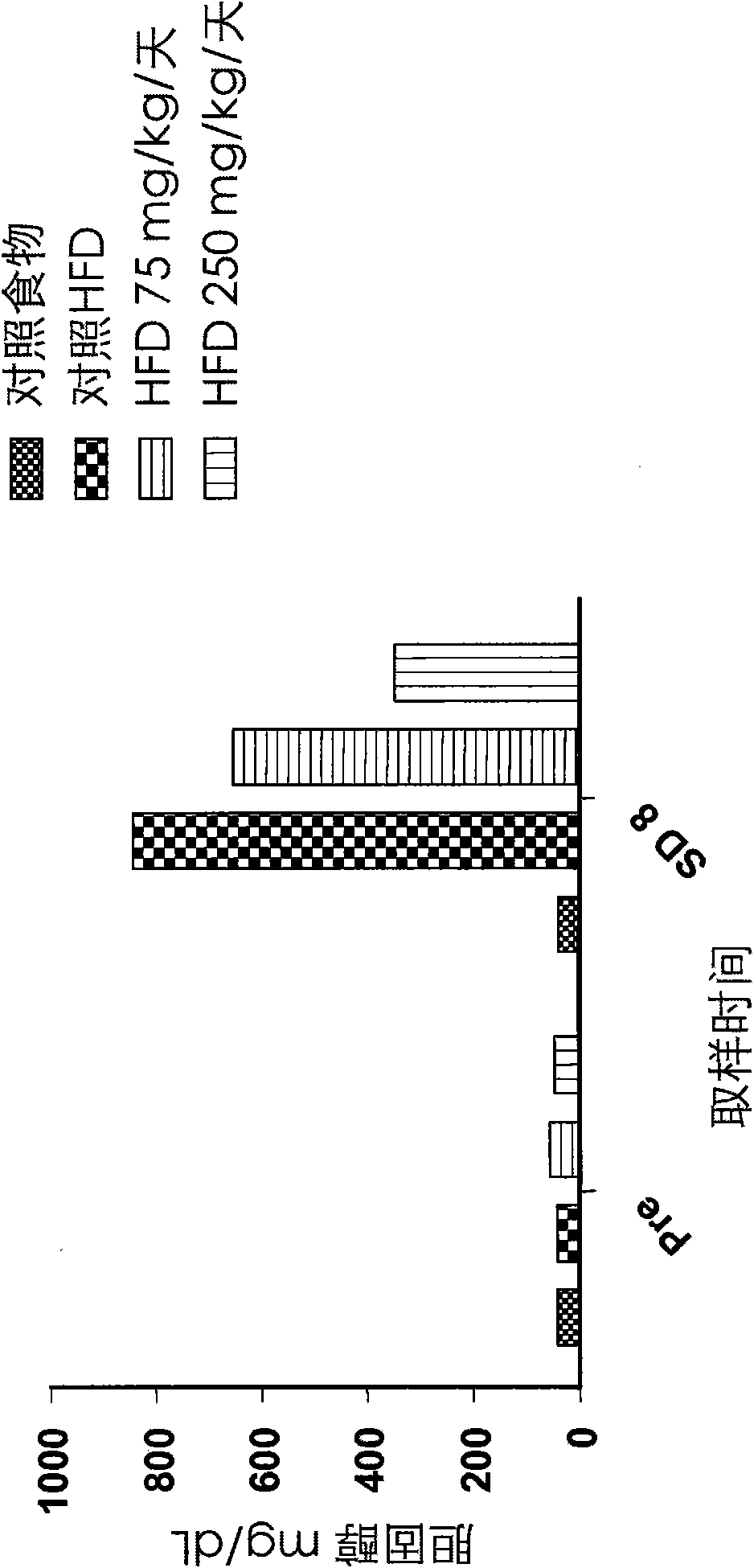
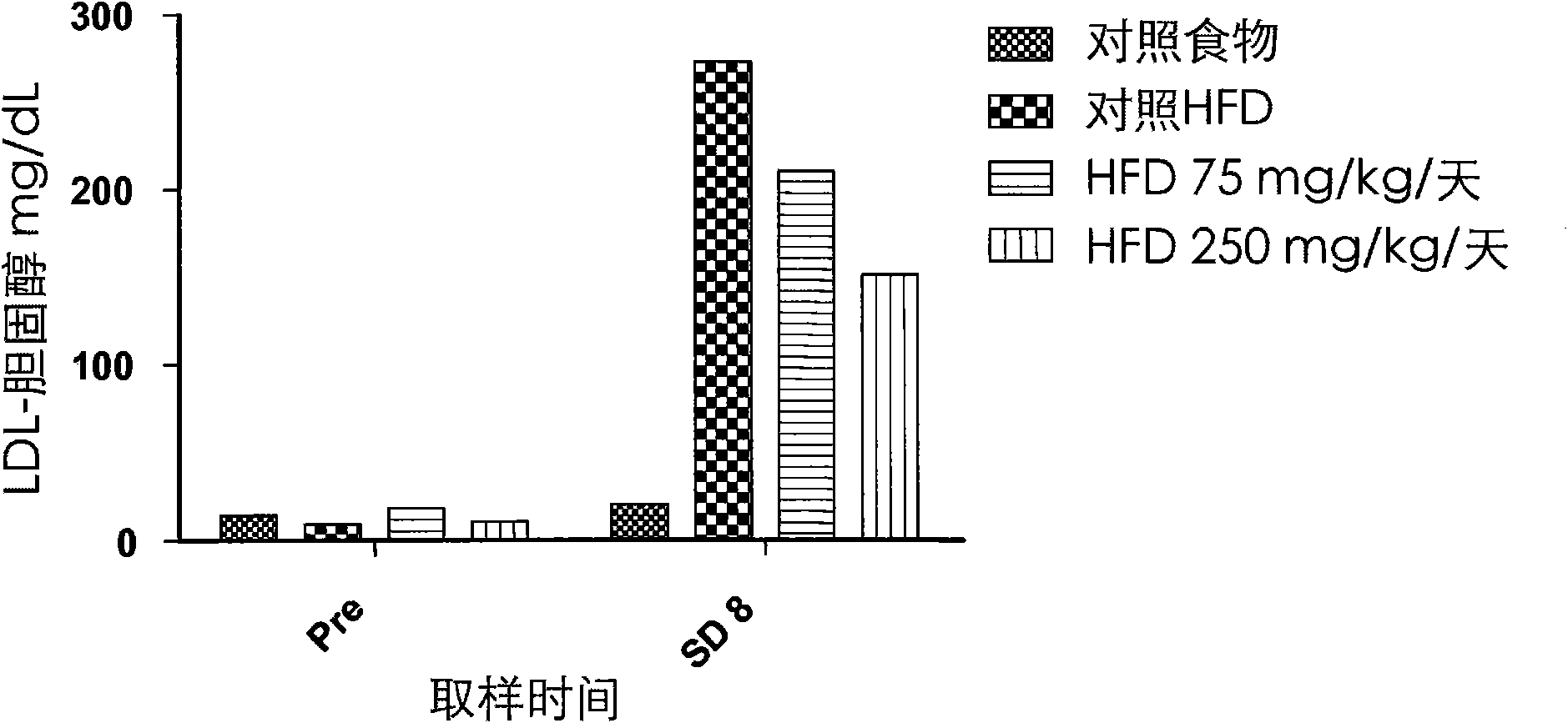
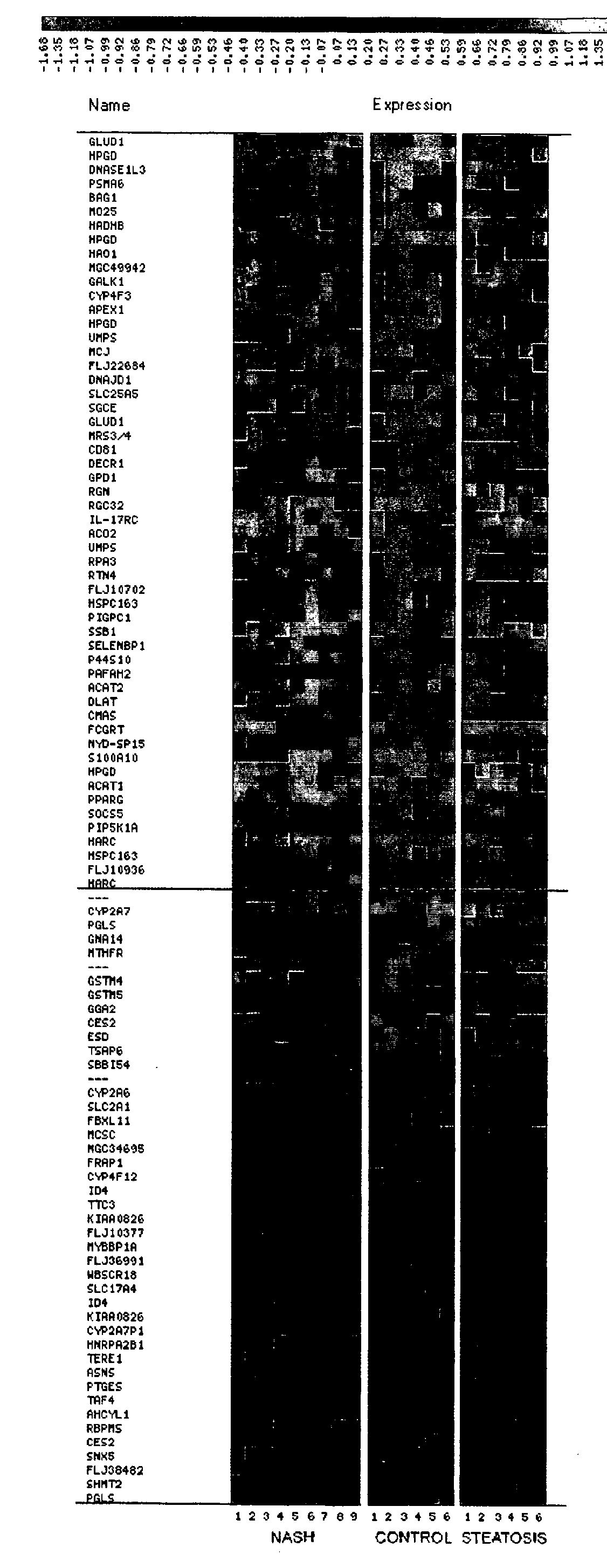
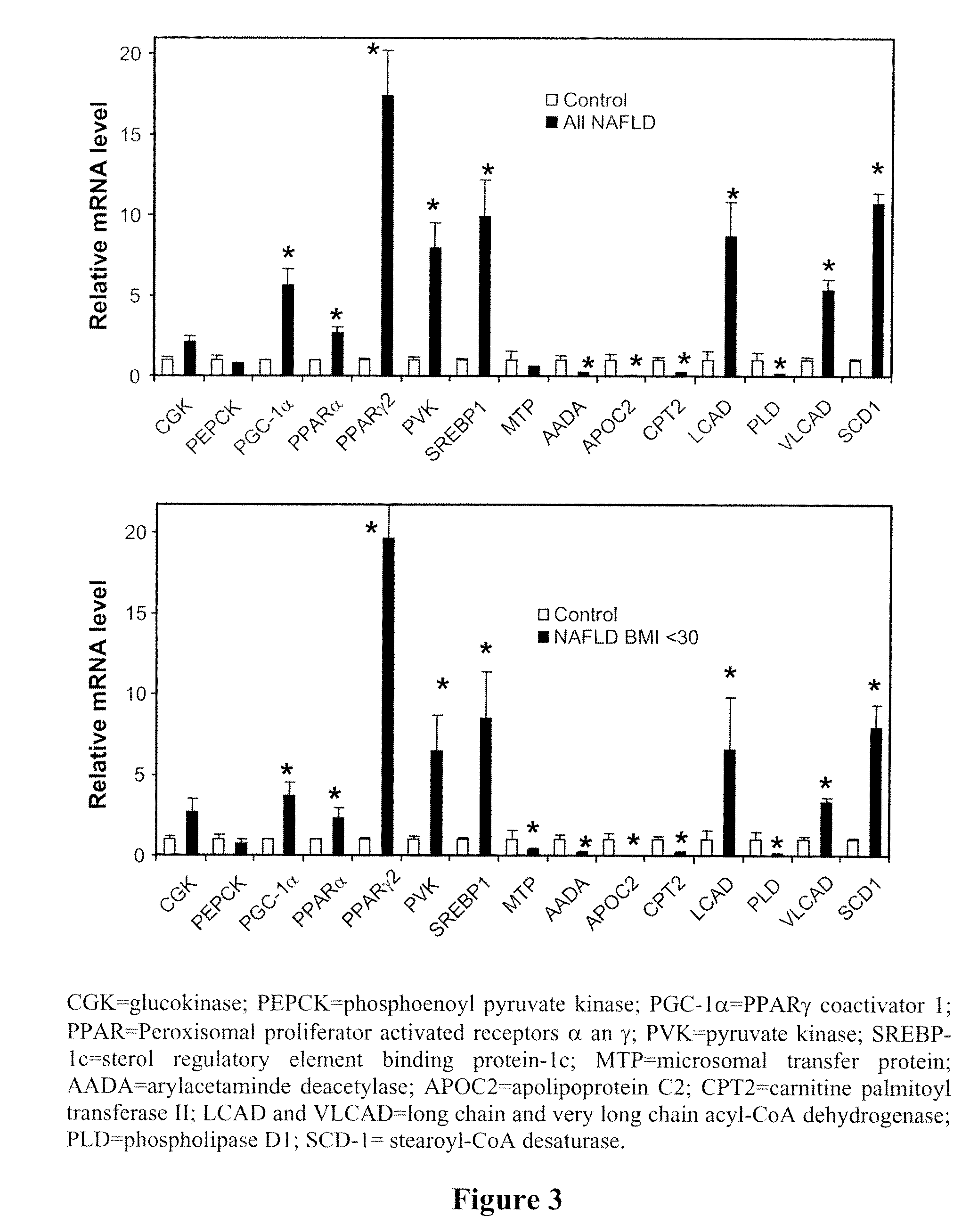
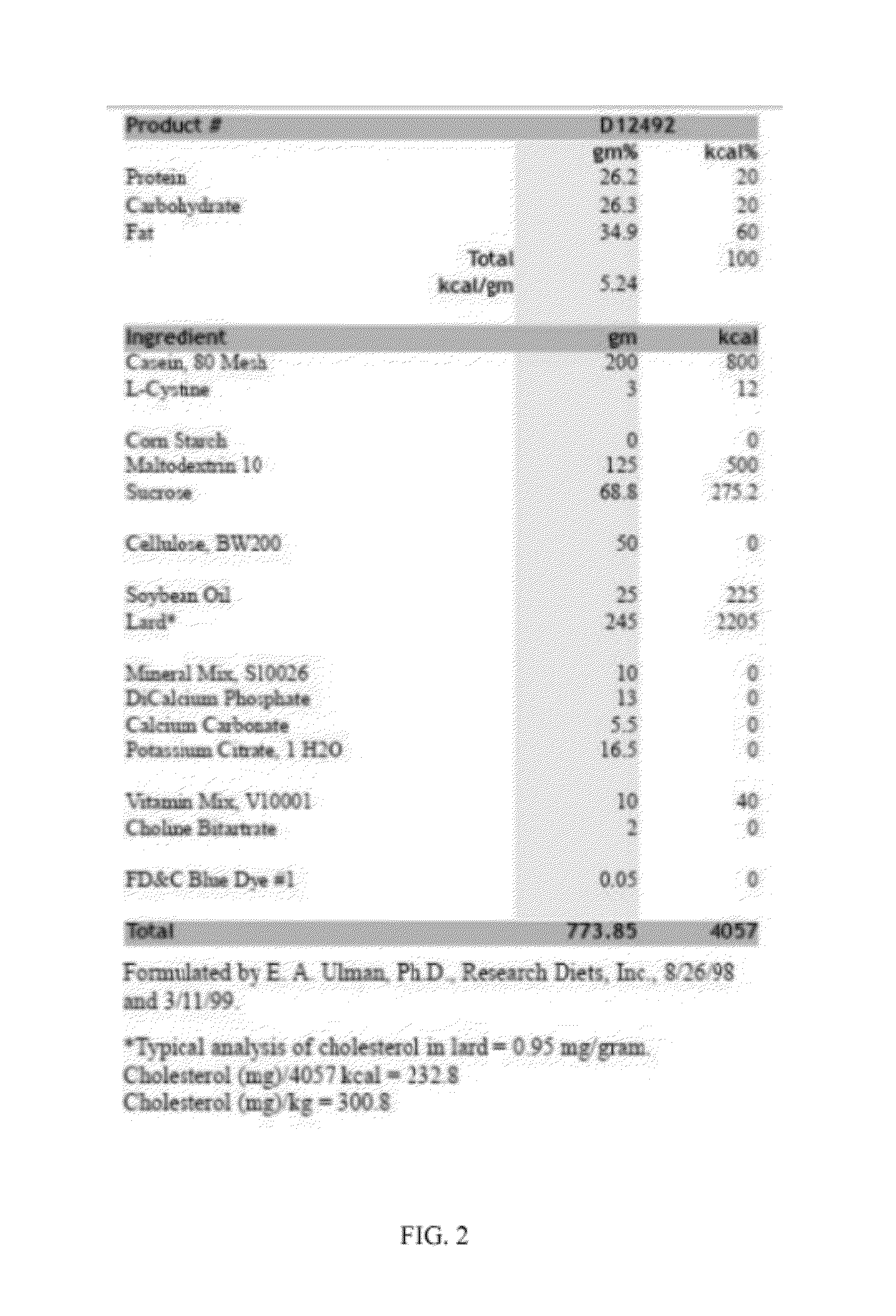
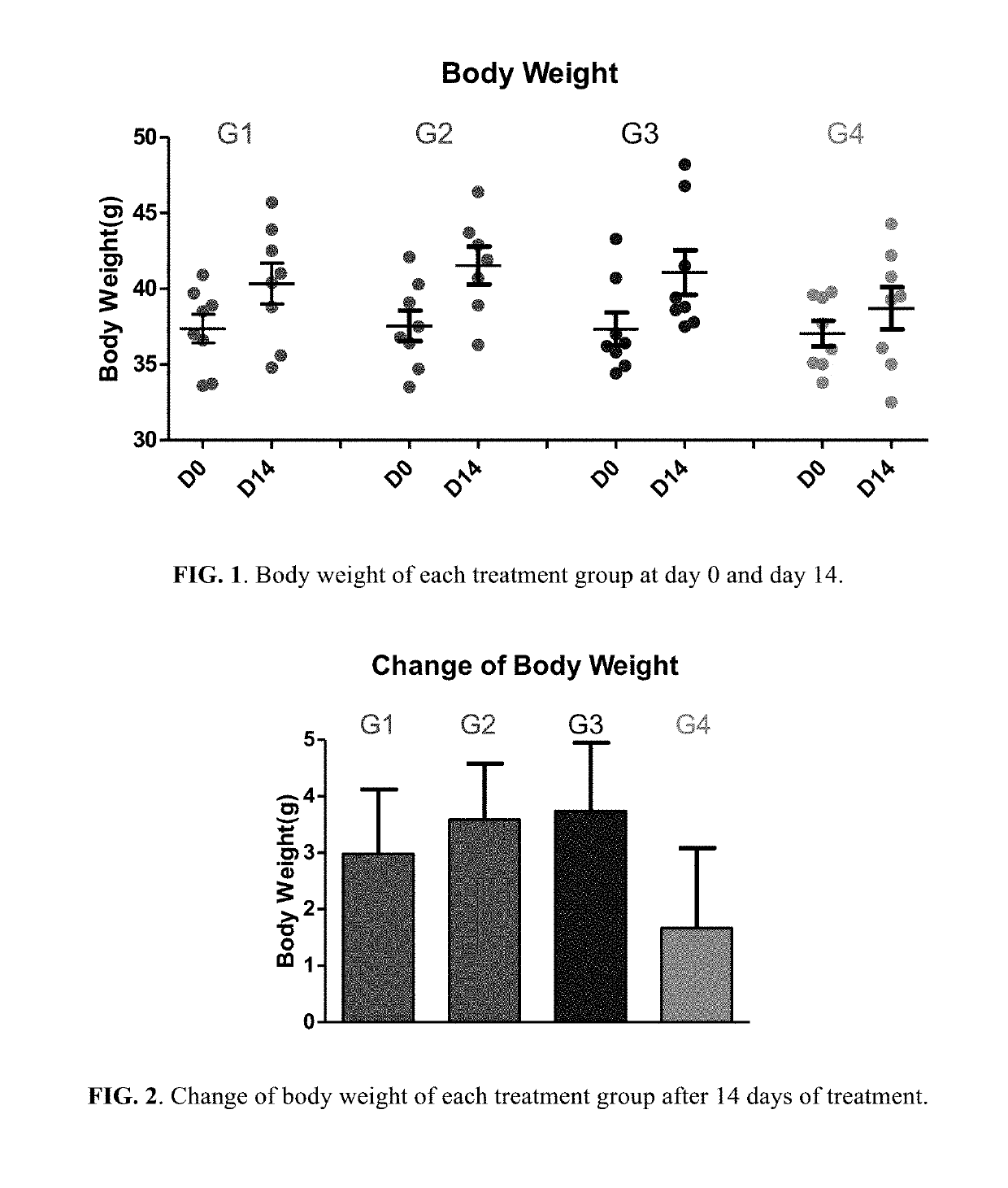
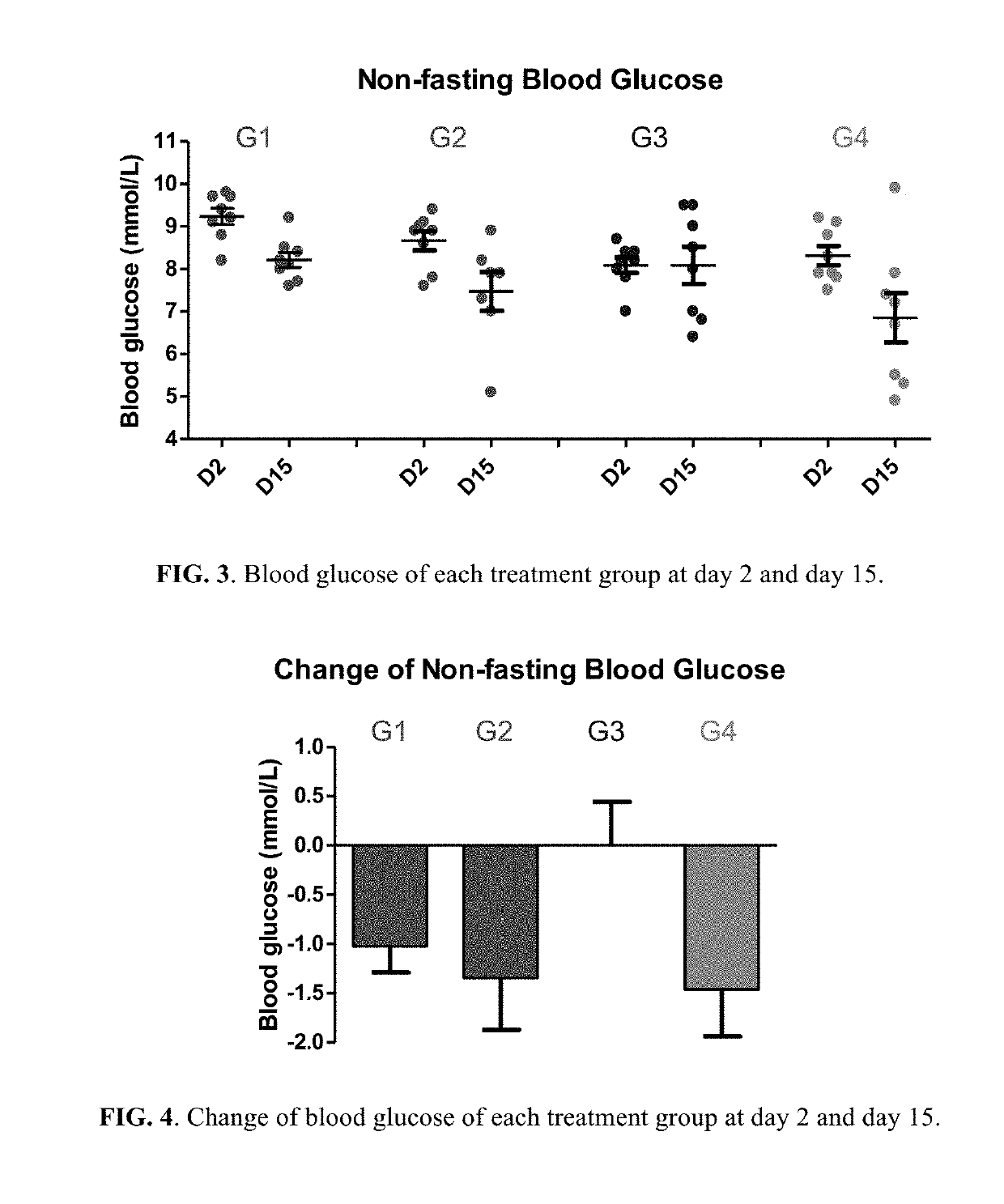
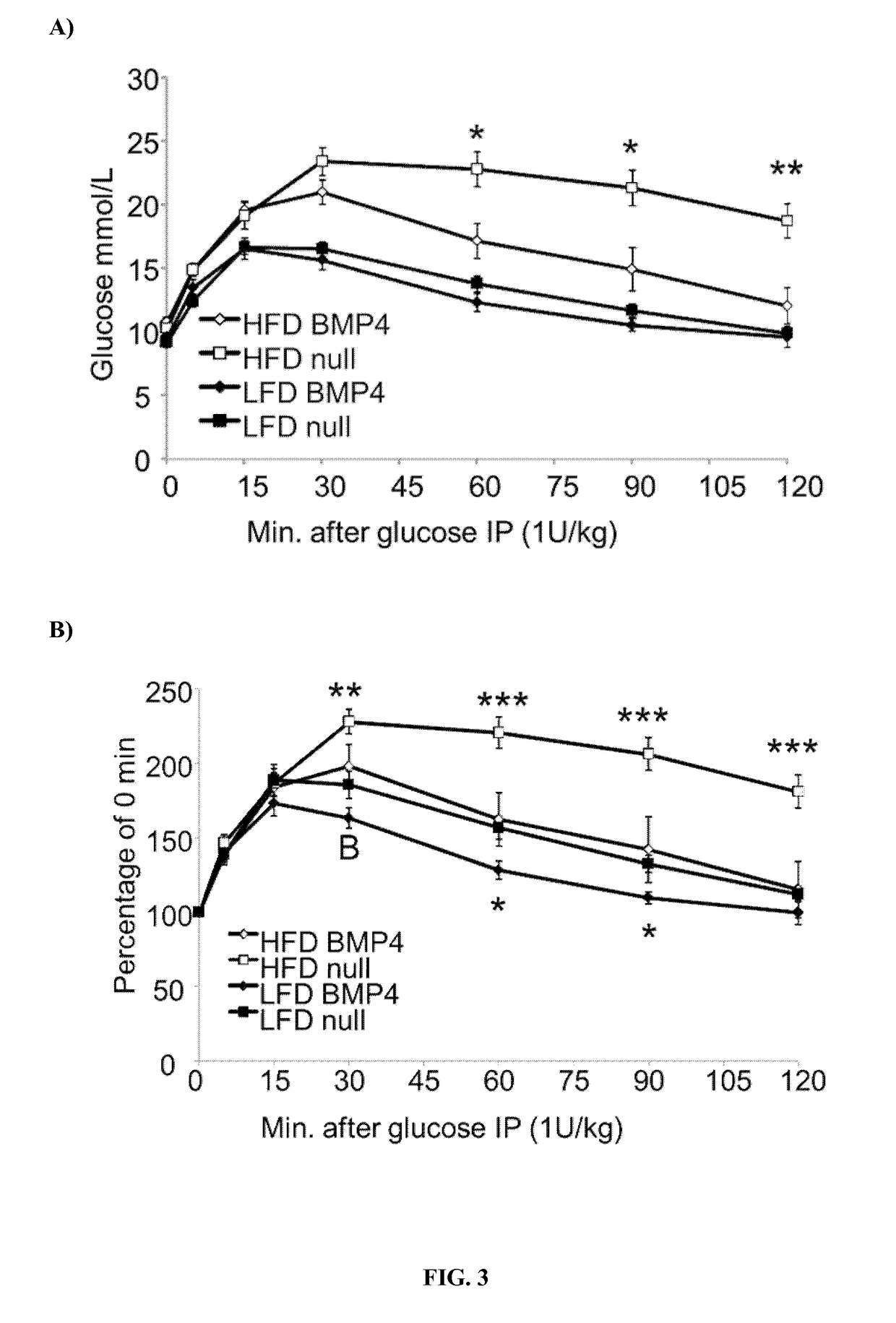
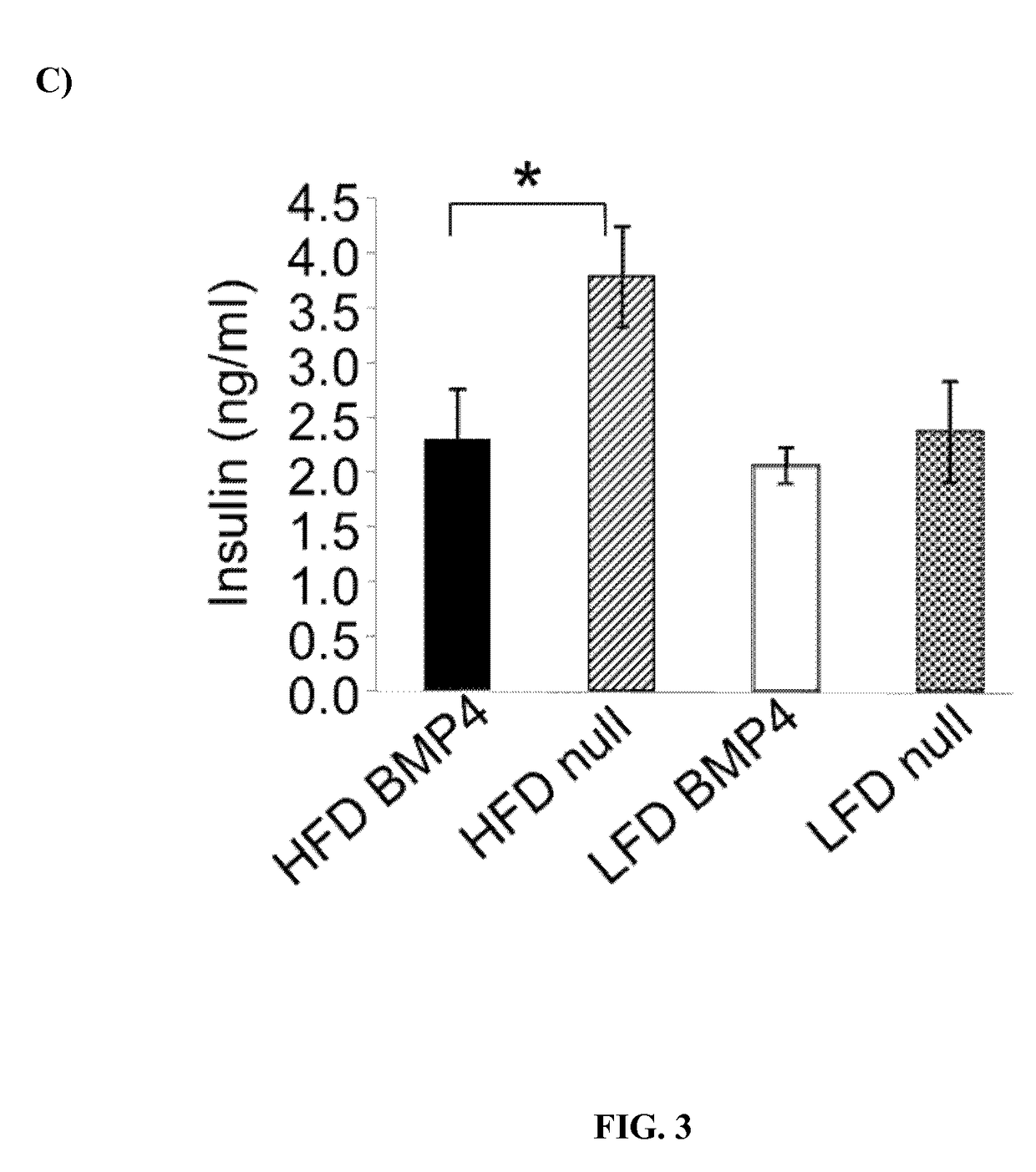
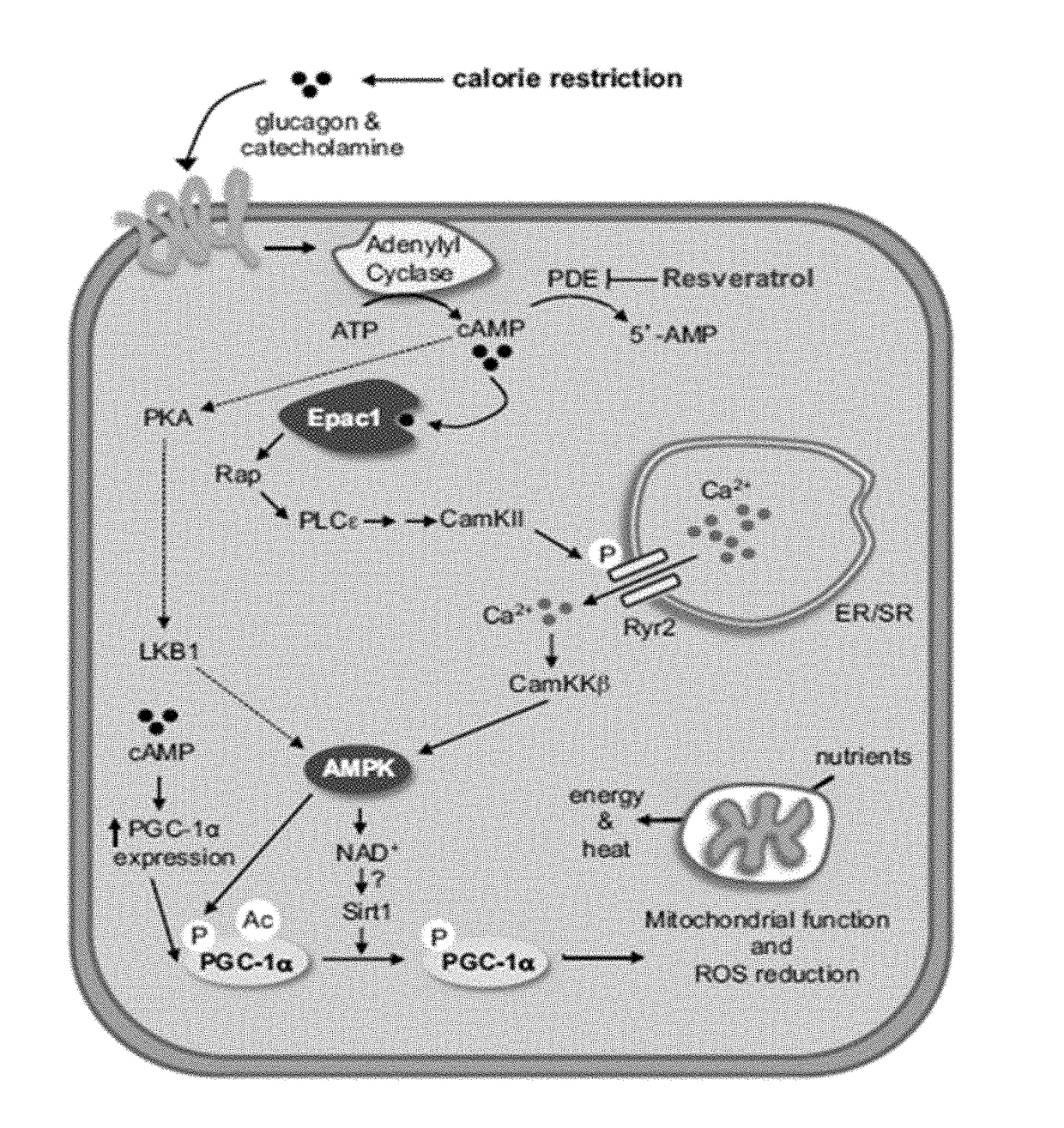
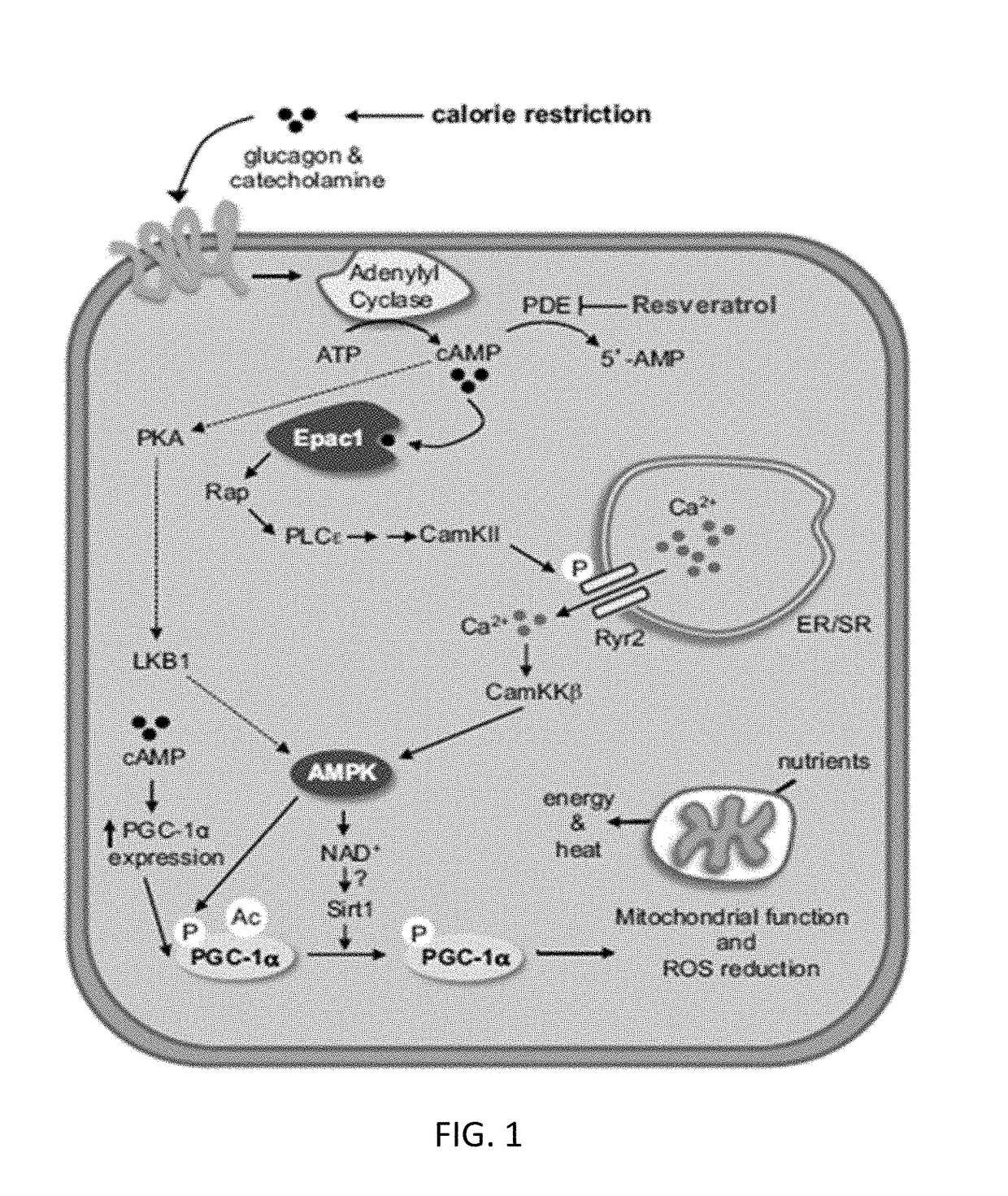
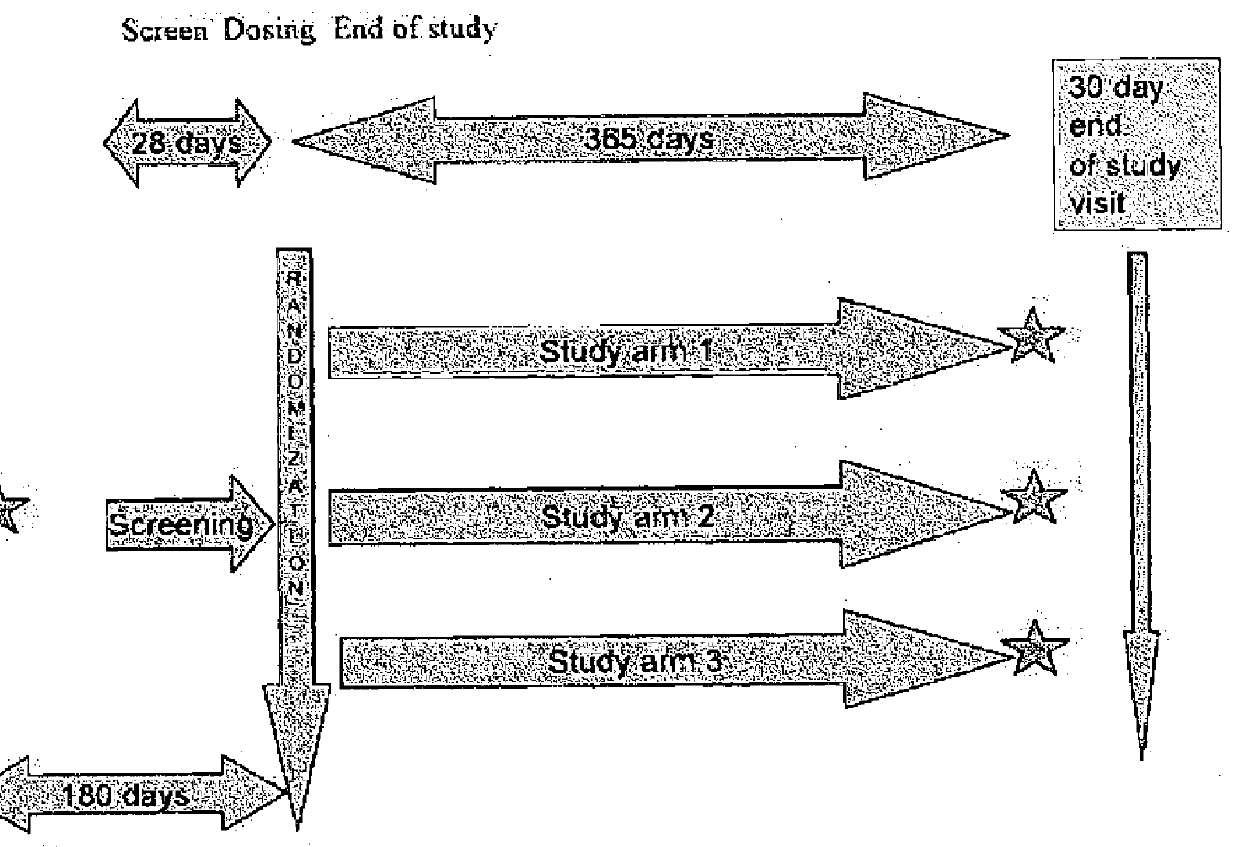
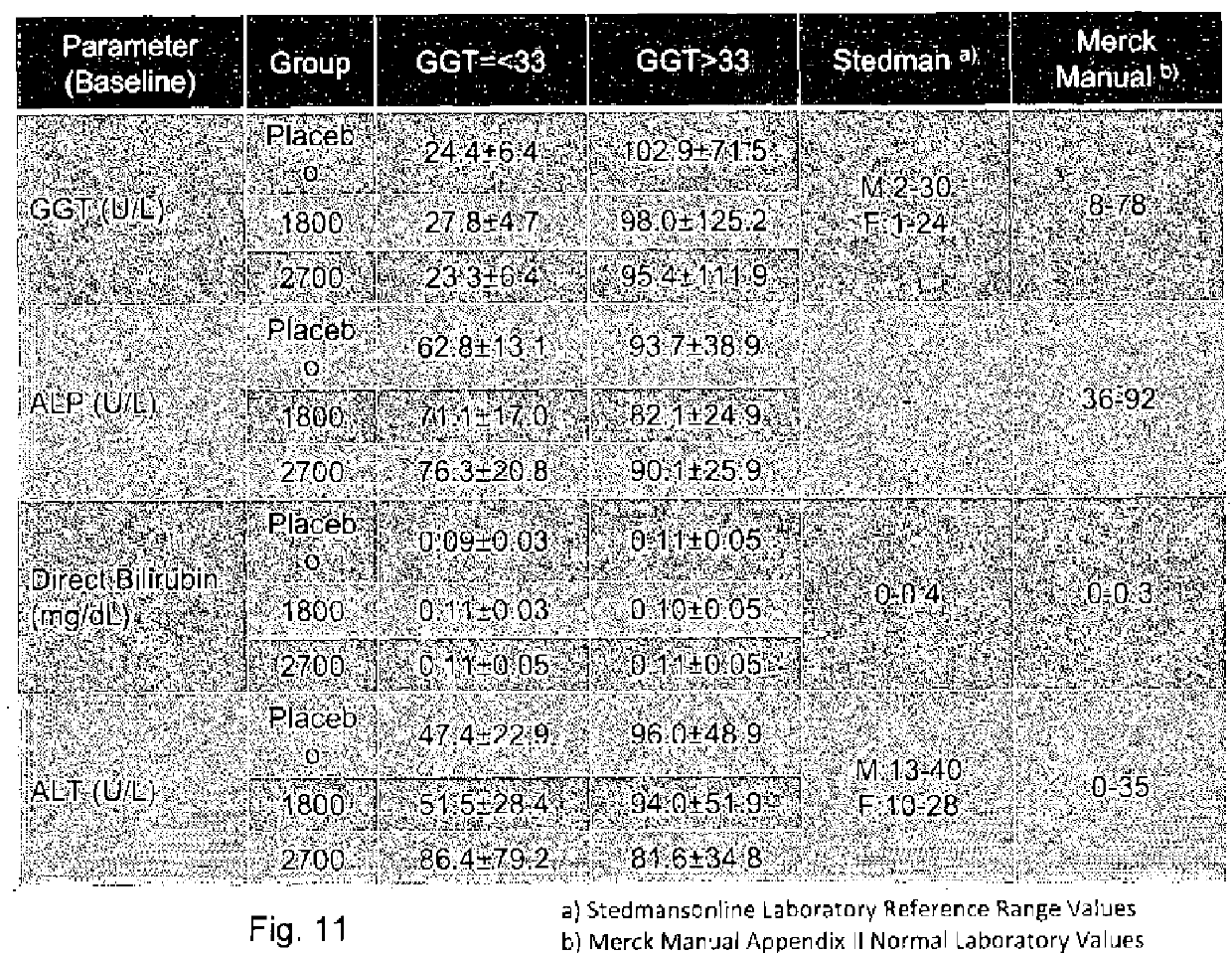
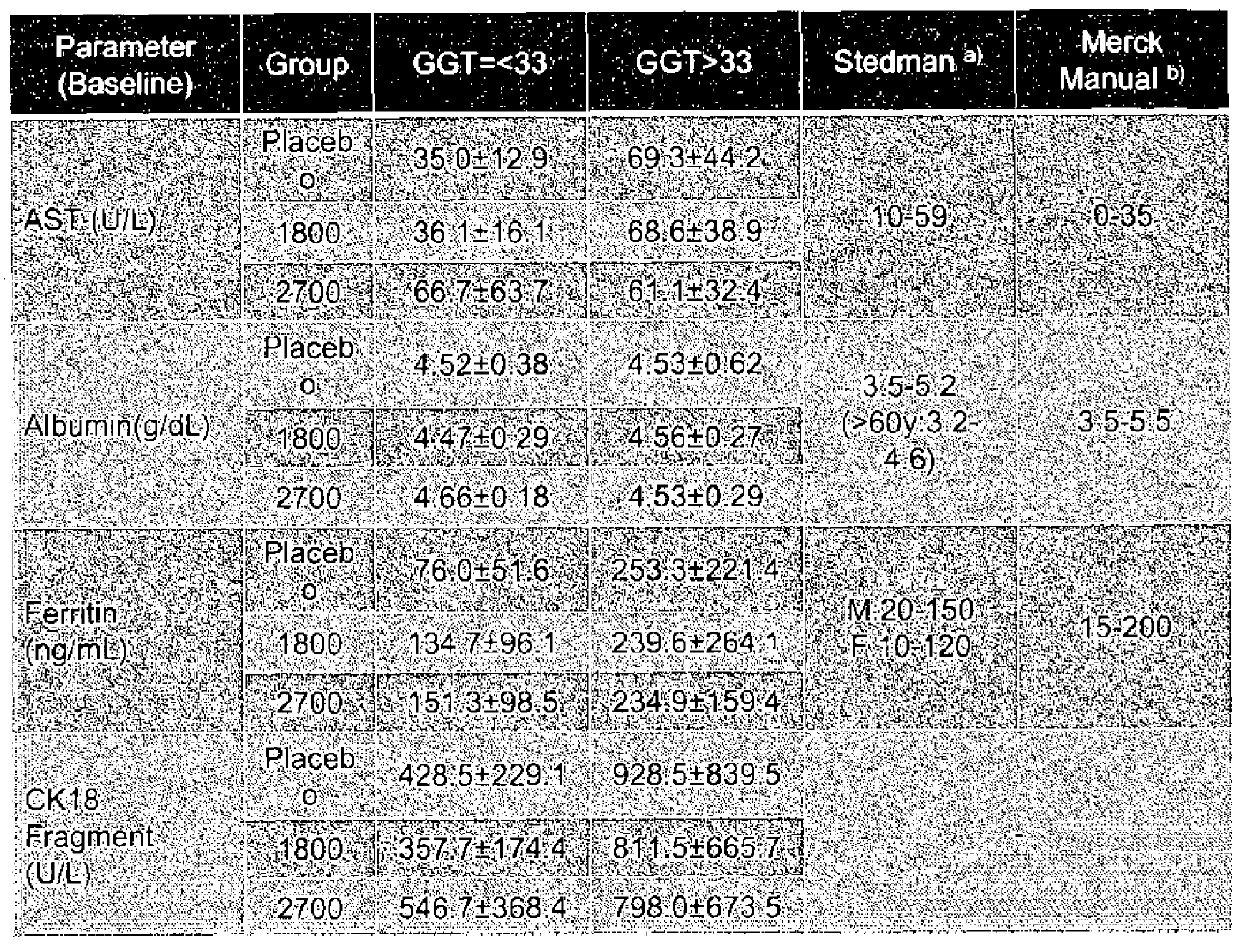
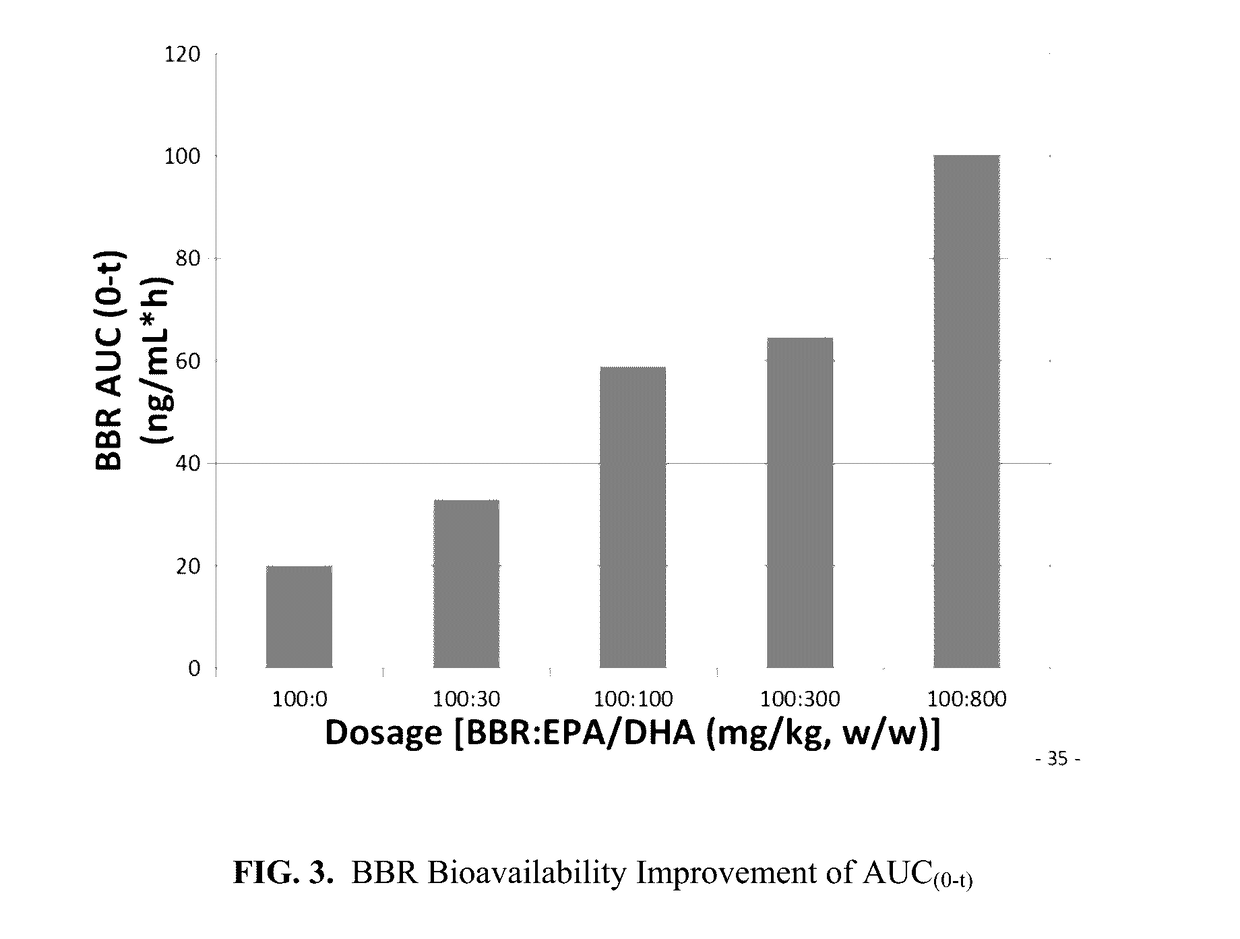
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
313 results about "Alcoholic steatohepatitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alcoholic Steatohepatitis is a chronic, progressive liver disease characterized by thickening and scarring (fibrosis) of the liver as well as possible death (necrosis) of the liver tissue, brought on by excessive, prolonged alcohol use. Alcohol consumption of more than 60-80 ml per day for men or 40-50 ml per day for women is considered toxic.
Compositions and methods for treating non-alcoholic steatohepatitis
ActiveUS20150051143A1Improve steatosisImproving lobular inflammation conditionBiocidePeptide/protein ingredientsBiliary tractFatty acid
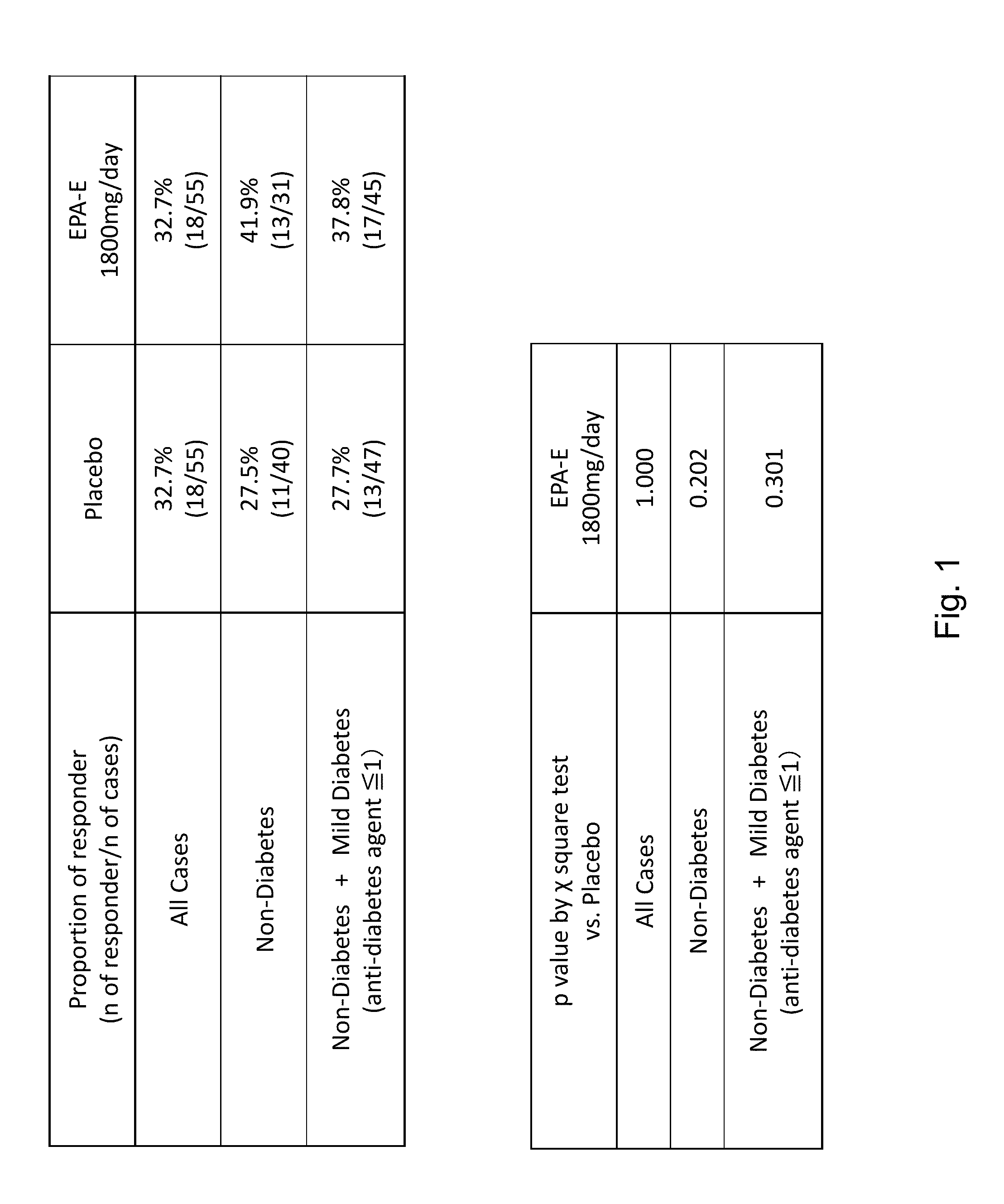
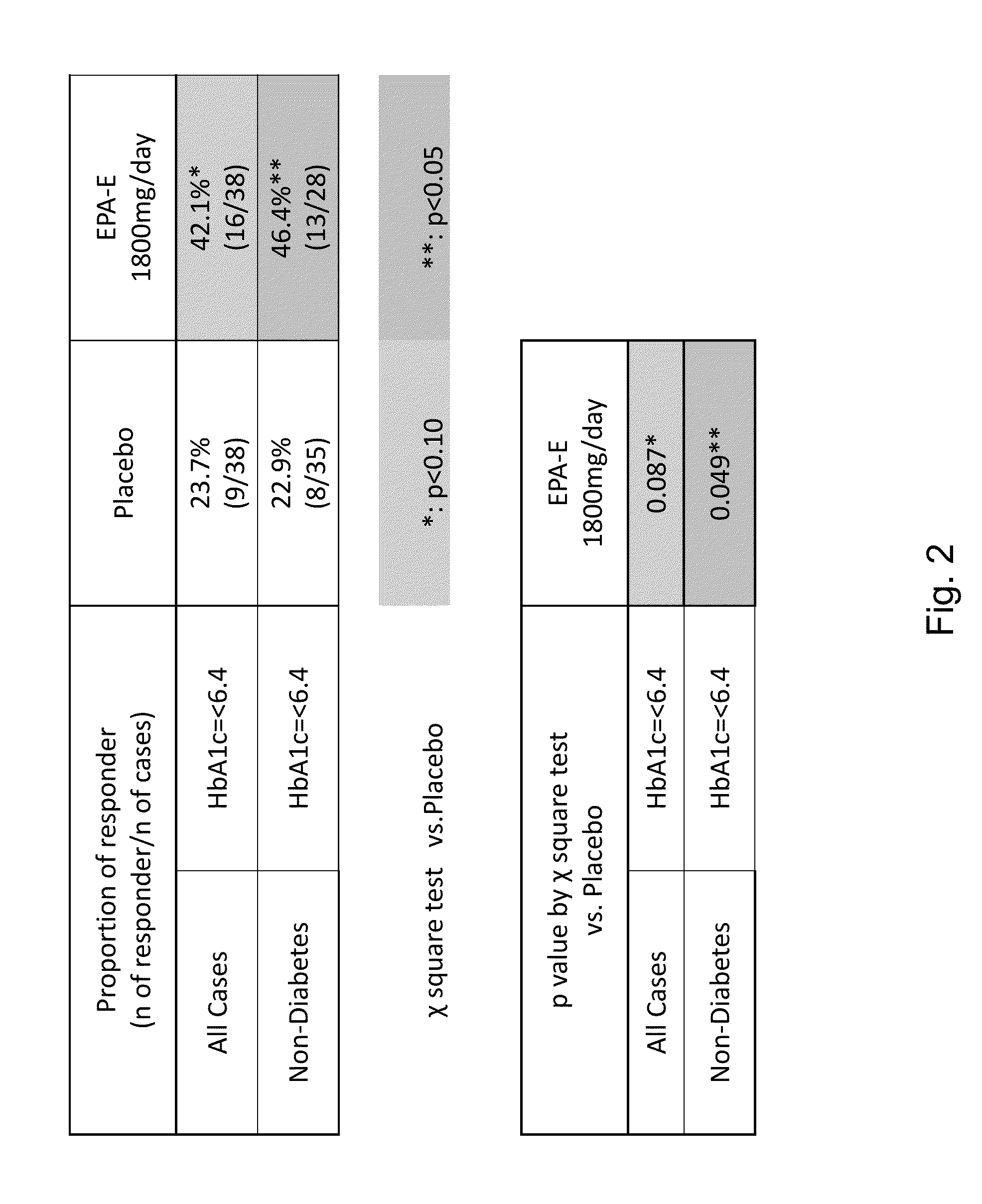
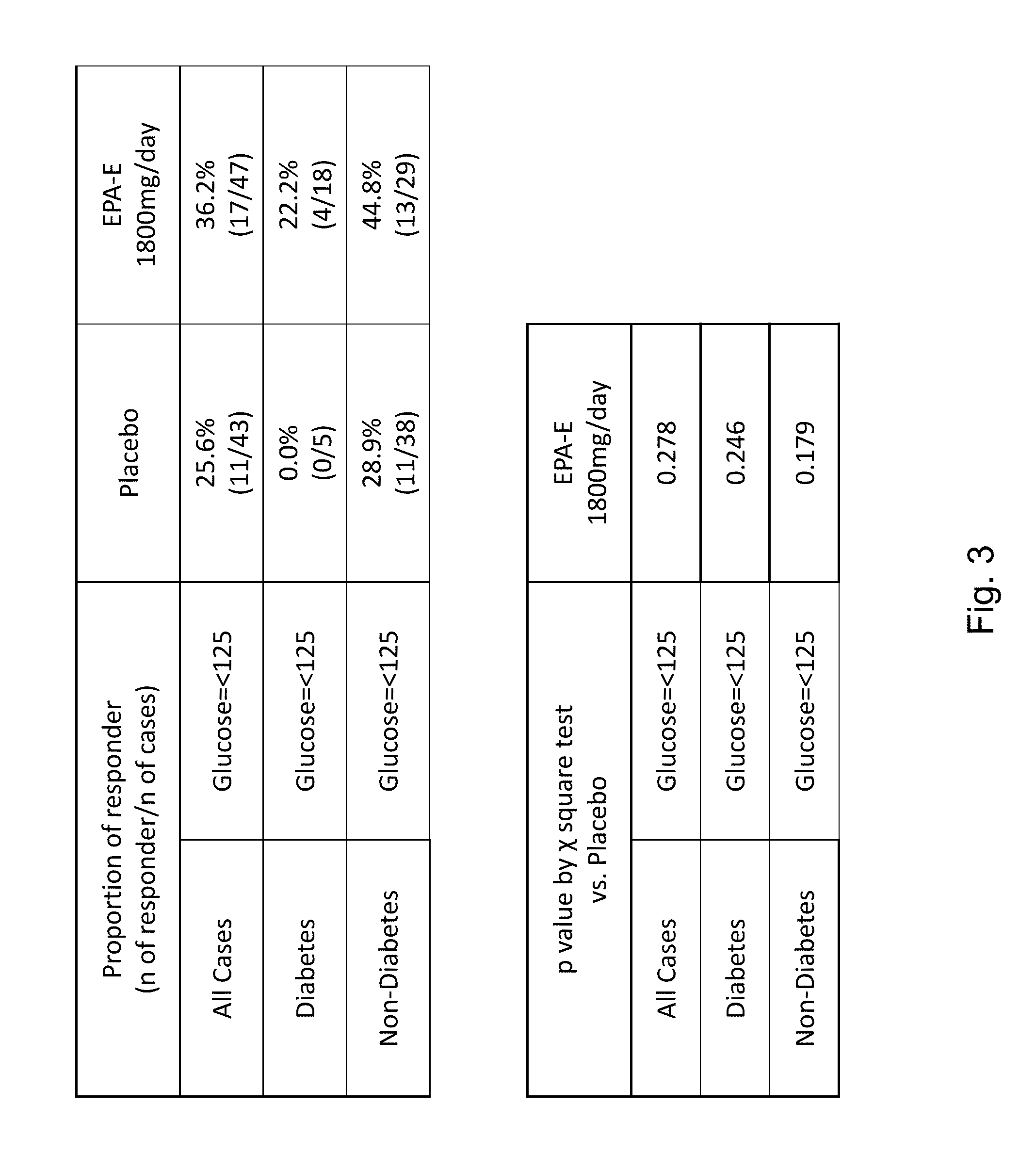
The disclosure provides for a method for treating a fatty liver disorder in a subject in need thereof, comprising selecting a subject having or suspected of having a fatty liver disease or disorder, wherein the subject is non diabetic, pre-diabetic, mildly diabetic, or has normal or substantially normal biliary tract function; and administering a therapeutically effective amount of a pharmaceutical composition comprising ethyl eicosapentanoate (EPA-E). In some cases EPA-E present may be at least 40% by weight in total of the fatty acids and their derivatives.
Owner:AMARIN PHARMA IRELAND
Marker associated with non-alcoholic steatohepatitis
ActiveUS20120231471A1Easy to detectEase of evaluationOrganic active ingredientsMicrobiological testing/measurementPediatricsNon alcoholic
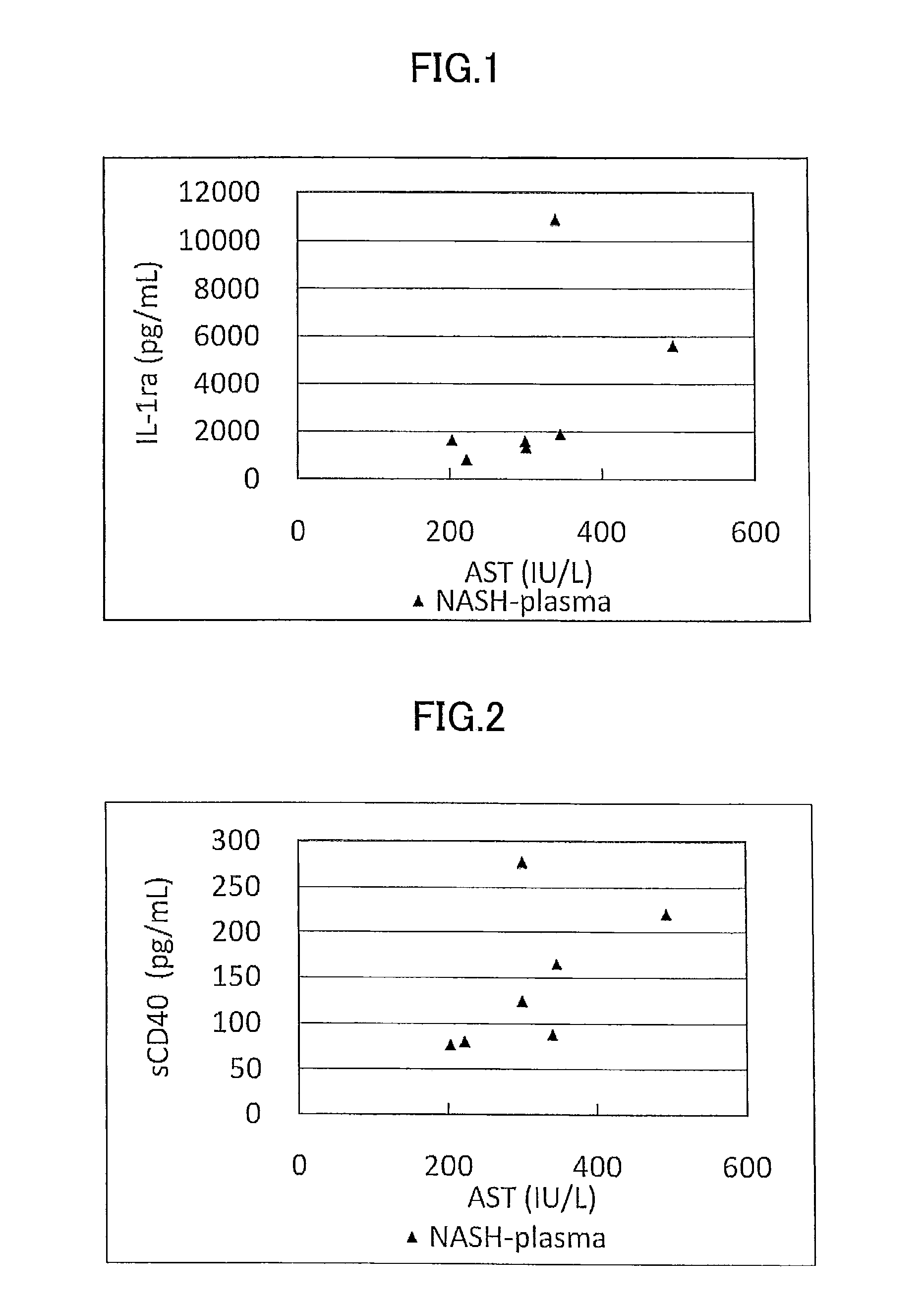
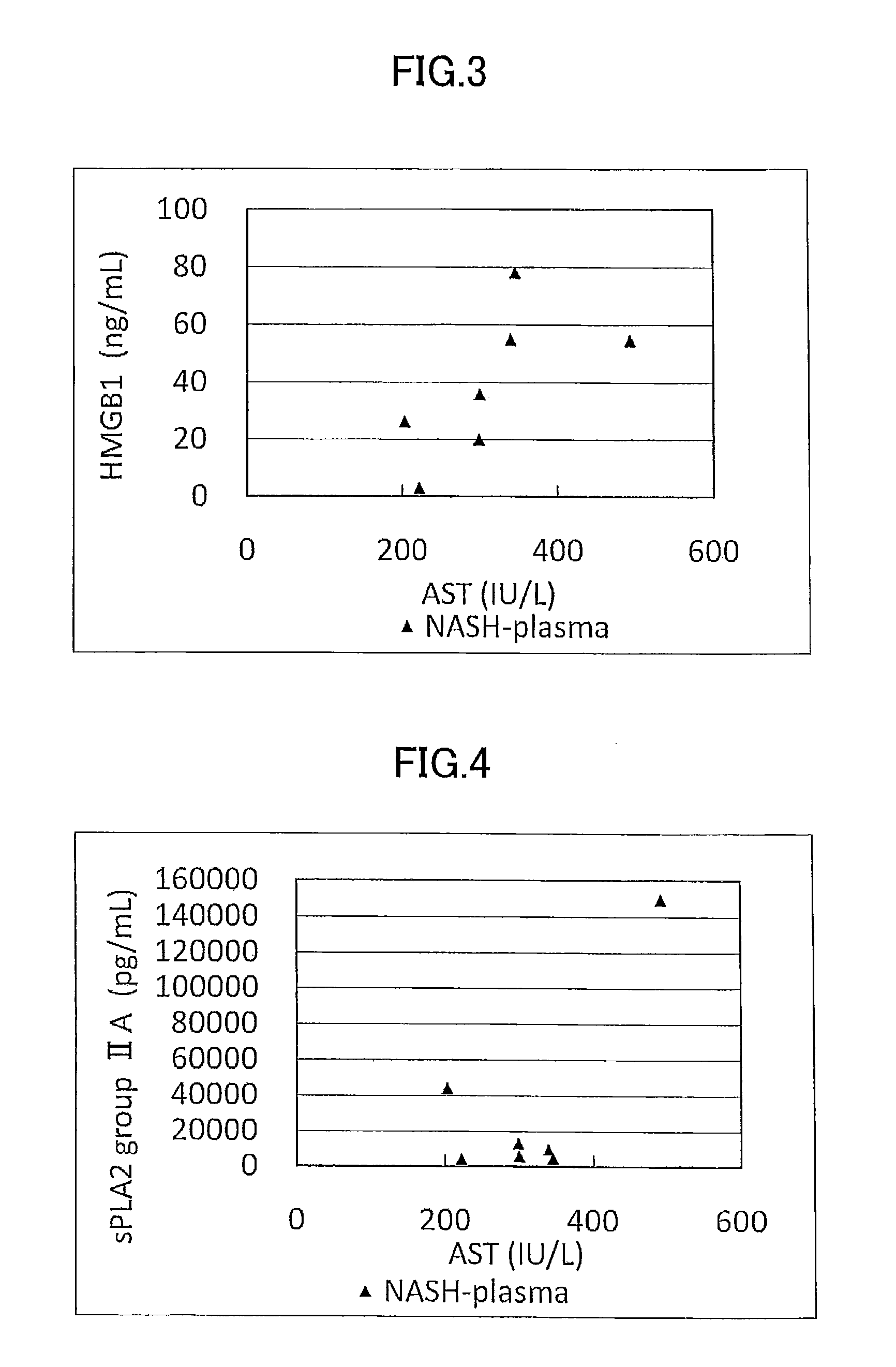
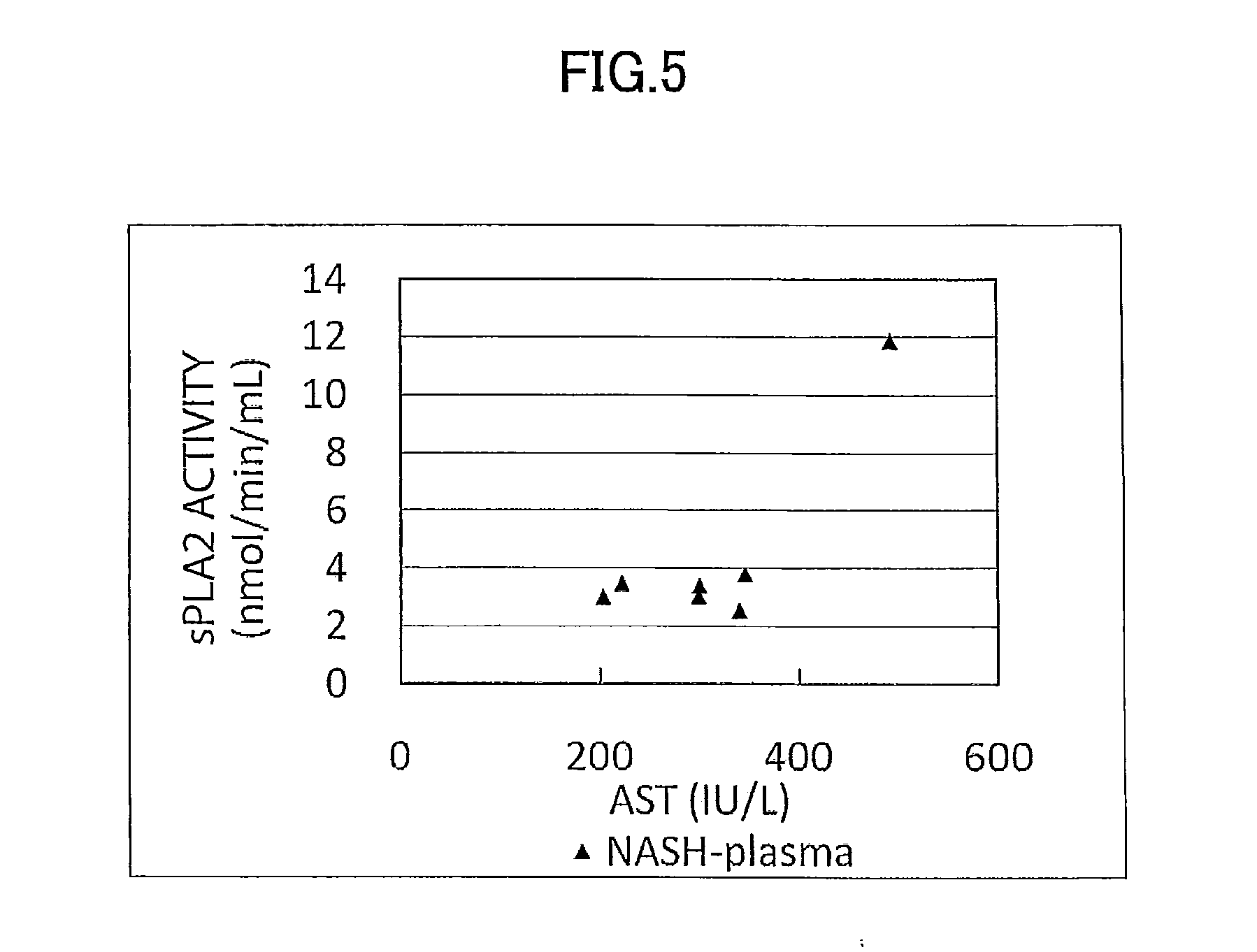
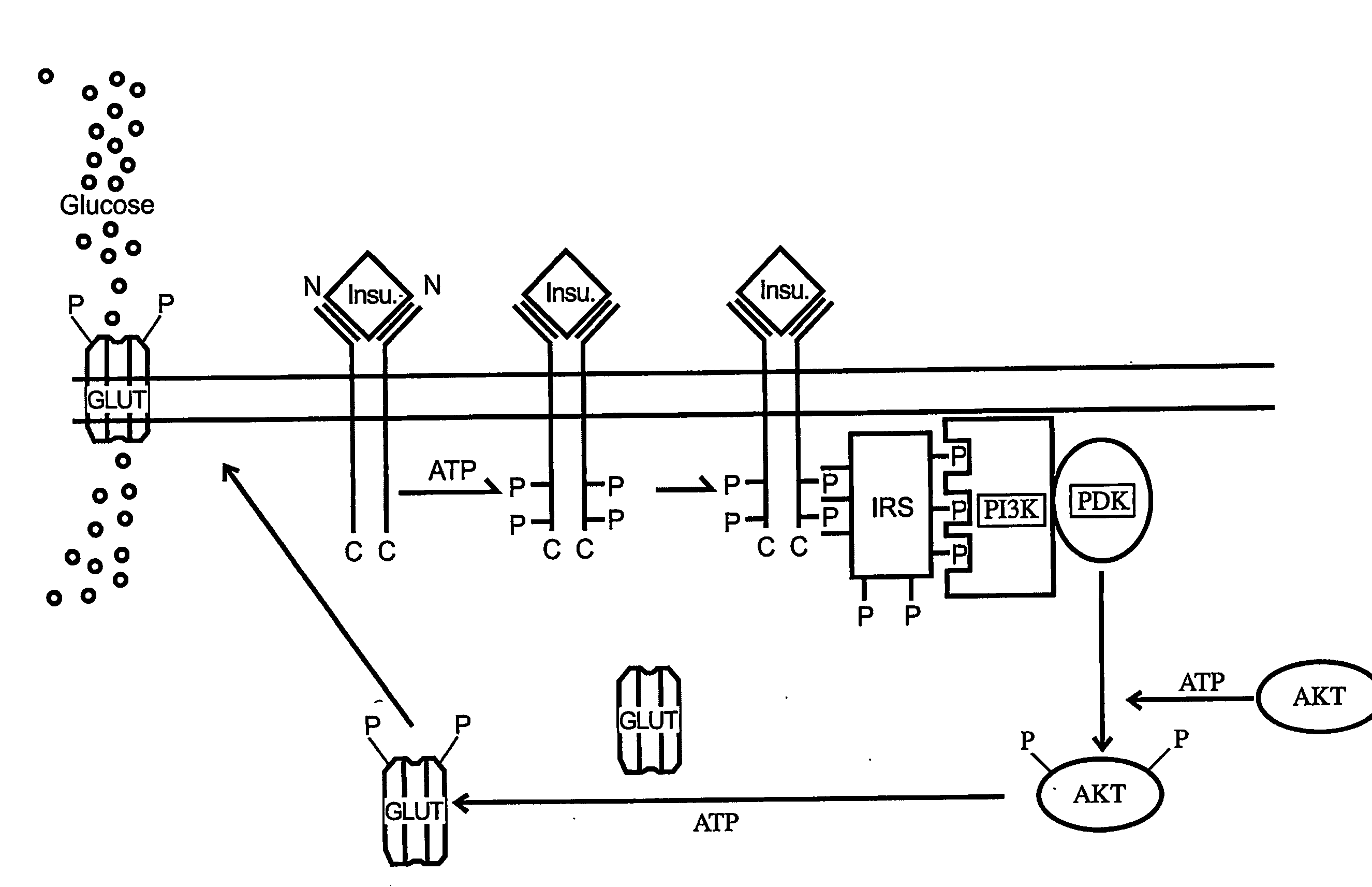
Disclosed is a novel NASH marker for use in a method for detecting NASH or evaluating the severity of NASH, which utilizes at least one factor selected from the group consisting of an IL-1 receptor antagonist, sCD40, HMGB1, sPLA2 group IIA and an sPLA2 activity as the marker. Also disclosed is a method for detecting NASH or evaluating the severity of NASH in a subject, which utilizes the marker.
Owner:AMARIN PHARMA IRELAND
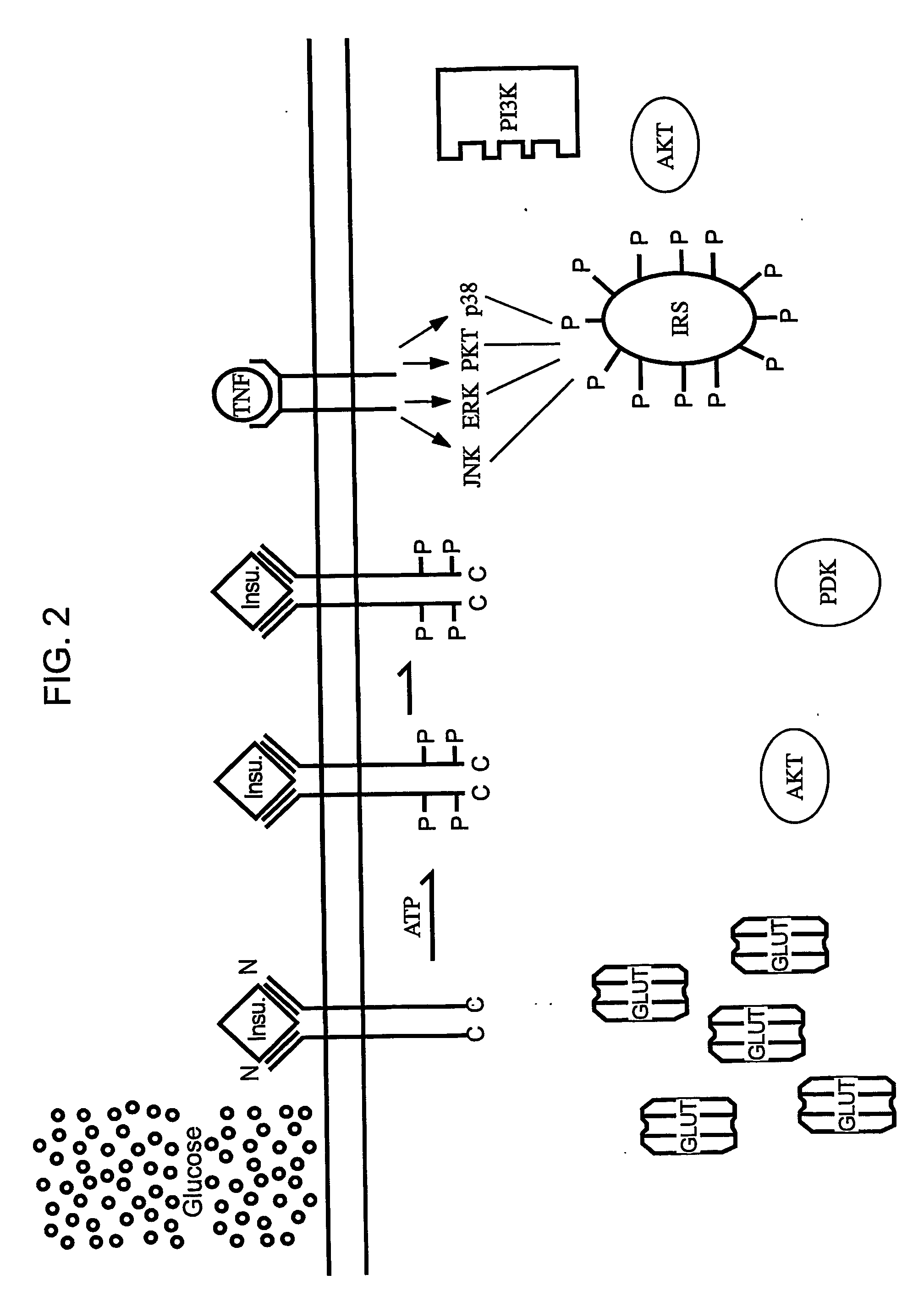
Methods of Treating Tnf-Mediated Disorders
The present invention provides methods of treating TNF-α-mediated disorders, the methods generally involving administering to an individual in need thereof effective amounts of pirfenidone or a pirfenidone analog and a second therapeutic agent that reduces TNF-α synthesis or that reduces TNF-α binding to a TNF receptor. The present invention further provides methods for treating non-alcoholic steatohepatitis, the method generally involving administering to an individual in need thereof an effective amount of pirfenidone. The present invention further provides methods of treating end-stage or advanced Type II diabetes, the methods generally involving administering to an individual in need thereof effective amounts of pirfenidone and insulin.
Owner:INTERMUNE INC
Thyromimetics for the Treatment of Fatty Liver Diseases
InactiveUS20090232879A1Low in fatPreventing and treating and ameliorating fatty liver diseaseBiocideMetabolism disorderSteatosisReceptor
The present invention is directed toward the use of thyromimetic compounds that are thyroid receptor ligands, pharmaceutically acceptable salts thereof, and to prodrugs of these compounds for preventing, treating, or ameliorating fatty liver diseases such as steatosis, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis.
Owner:METABASIS THERAPEUTICS INC
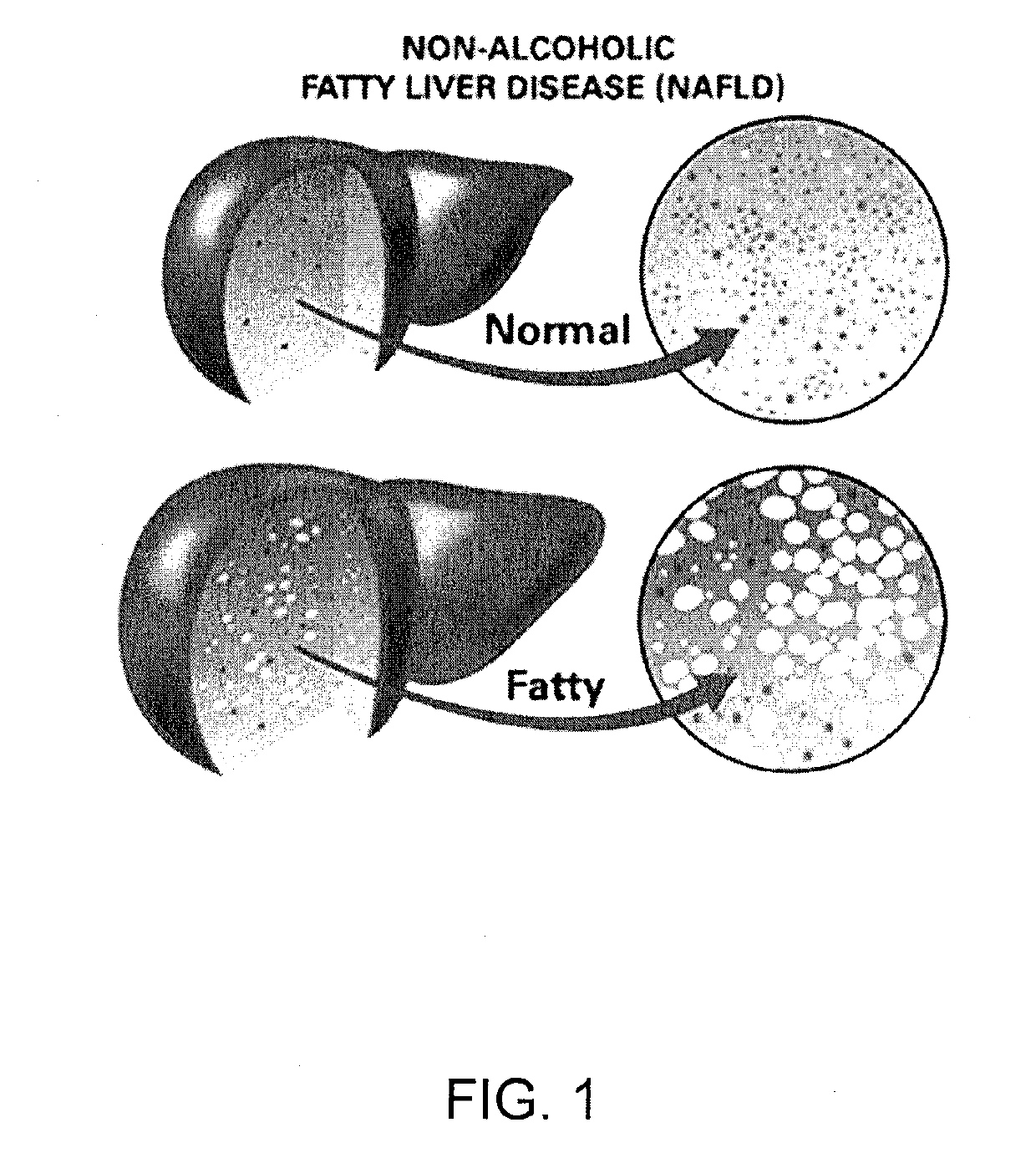
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
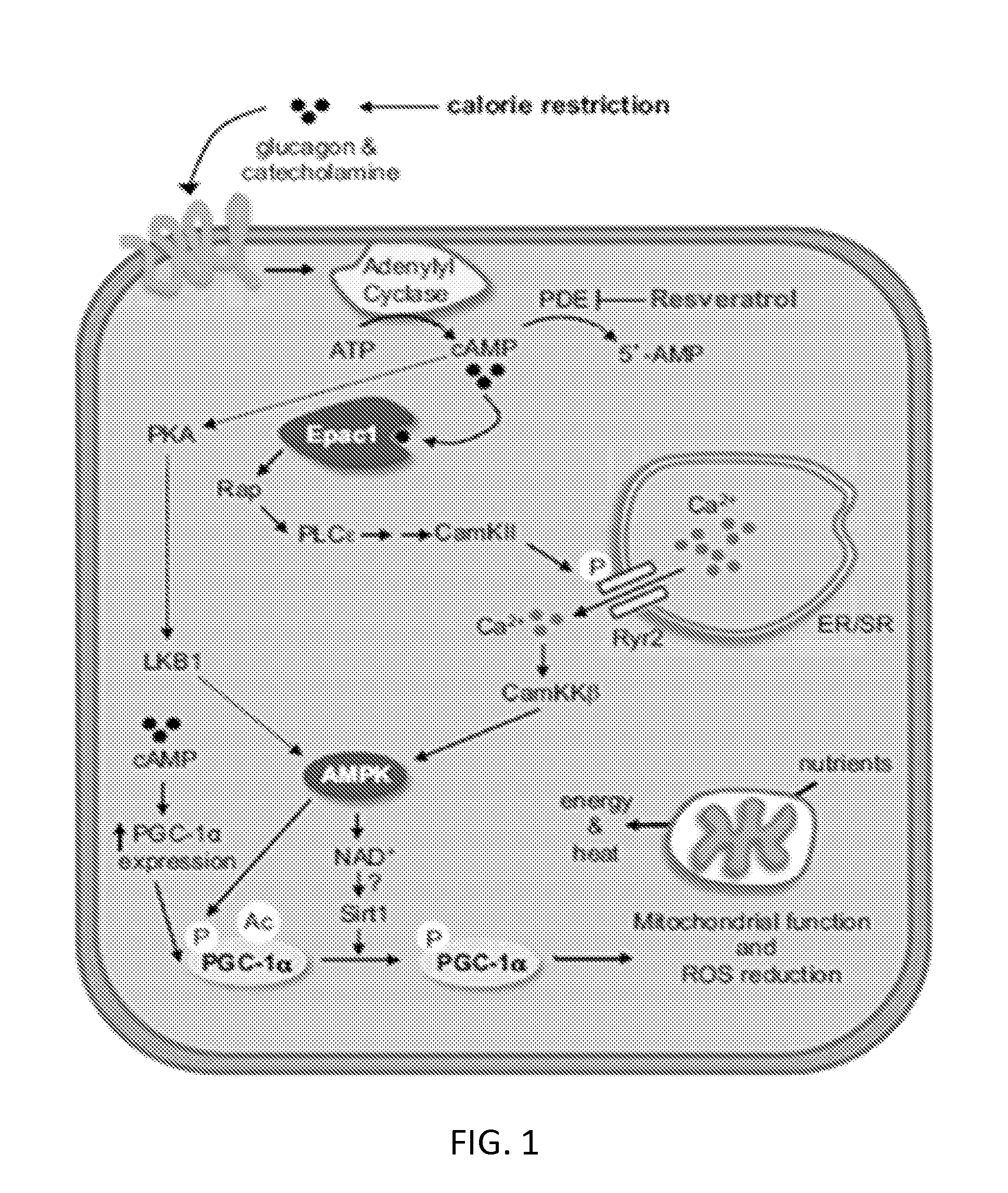
Compositions and methods for the reduction or prevention of hepatic steatosis
ActiveUS20160067201A1Lower Level RequirementsReduction of non-alcoholic steatohepatitis (NASH)BiocidePeptide/protein ingredientsSteatosisMetabolite
Methods useful for reducing or preventing non-alcoholic steatohepatitis or hepatic steatosis are provided herein. Such methods may comprise administering to a subject in need thereof a sirtuin pathway activator and / or PDE5 inhibitor alone or in combination with an amount of a branched amino acid in free amino acid form, or a metabolite thereof. Also provided herein are compositions and kits for practicing any of the methods described herein.
Owner:NUSIRT SCI
Markers of non-alcoholic fatty liver disease (nafld) and non-alcoholic steatohepatitis (nash) and methods of use thereof
Novel methods for assessing the level of triglycerides in the liver of a subject are described, comprising determining the amount of a lipid metabolite in a sample from a body fluid of the subject. The methods may be used, for example, in diagnosing and monitoring liver disorders such as steatosis, NAFLD and NASH.
Owner:METABOLON
Methods of treating non-alcoholic steatohepatitis (nash) using cysteamine products
The disclosure relates, in general, to treatment of fatty liver disorders comprising administering compositions comprising cysteamine products. The disclosure provides administration of enterically coated cysteamine compositions to treat fatty liver disorders, such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).
Owner:RGT UNIV OF CALIFORNIA
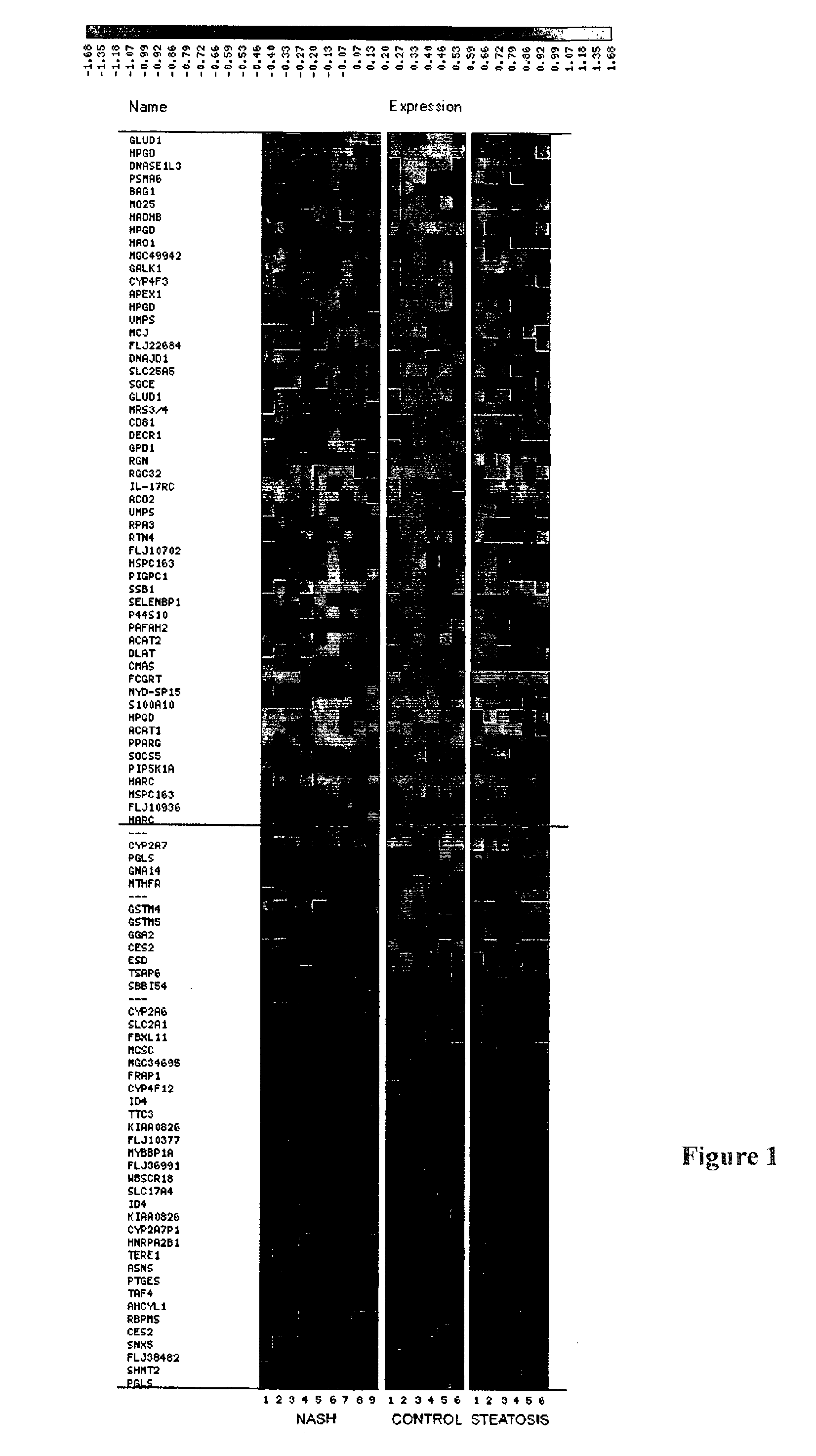
Diagnostic and Prognostic Methods of Non-Alcoholic Steatohepatitis (Nash)
A set of genes that are expressed differentially in normal and NASH-predisposed liver, thus constituting the genomic signature of NASH, is provided. The set of genes can be used to discriminate between normal and NASH-predisposed liver tissues. Accordingly, diagnostic assays for classification of pathological grades, prediction of the pathology outcome, selecting and monitoring treatment regimens and monitoring disease progression / regression also are provided.
Owner:ONE WAY LIVER GENOMICS
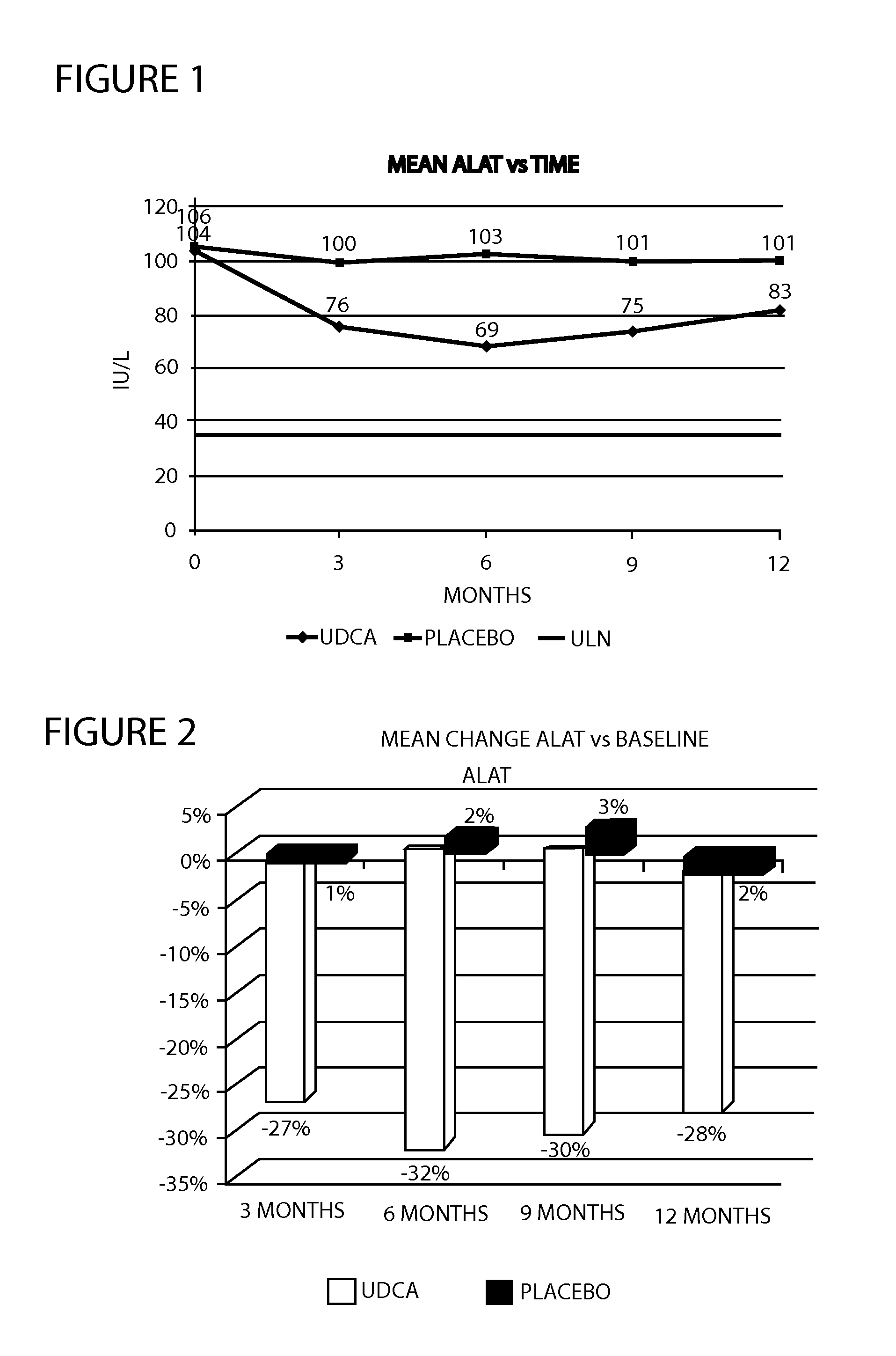
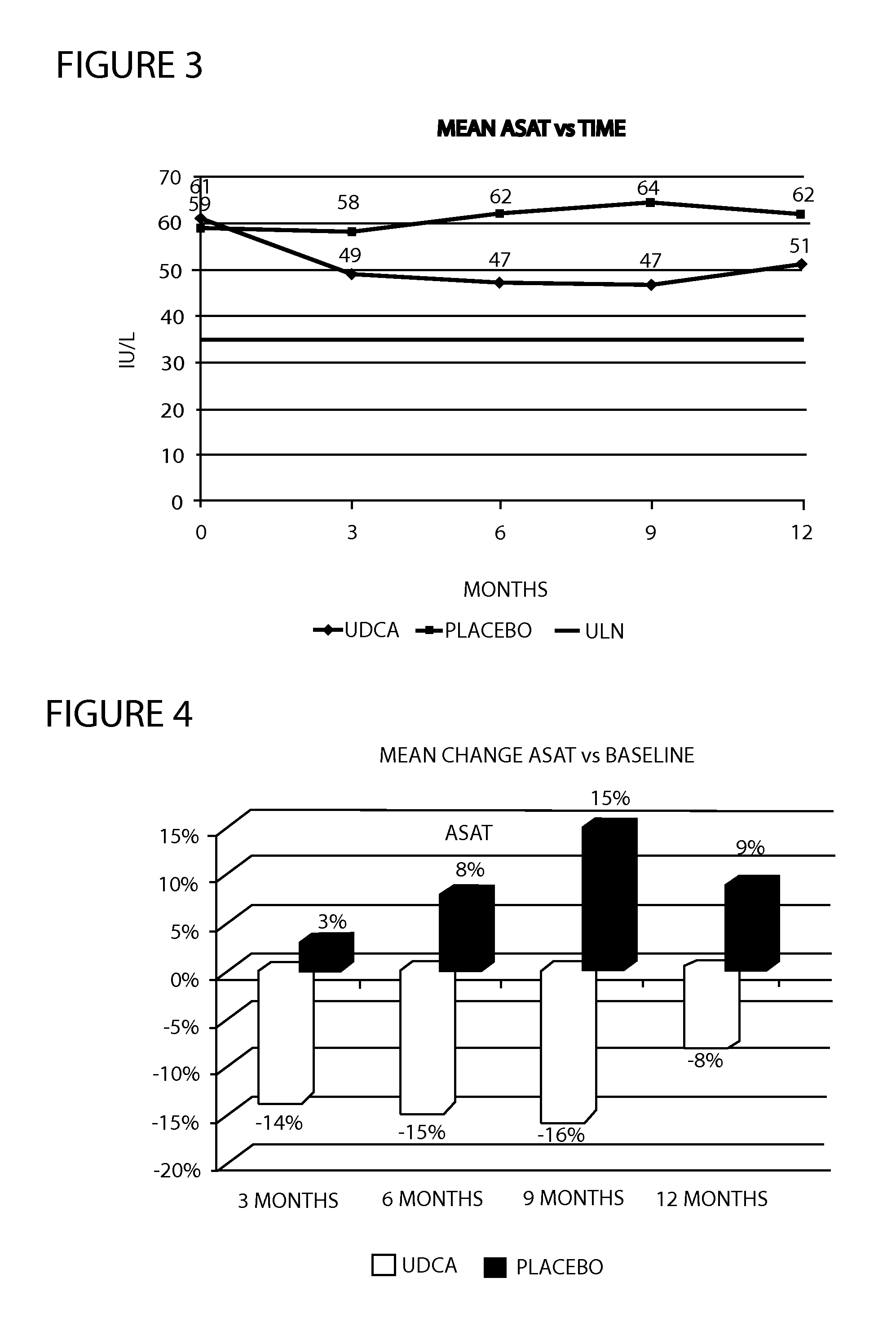
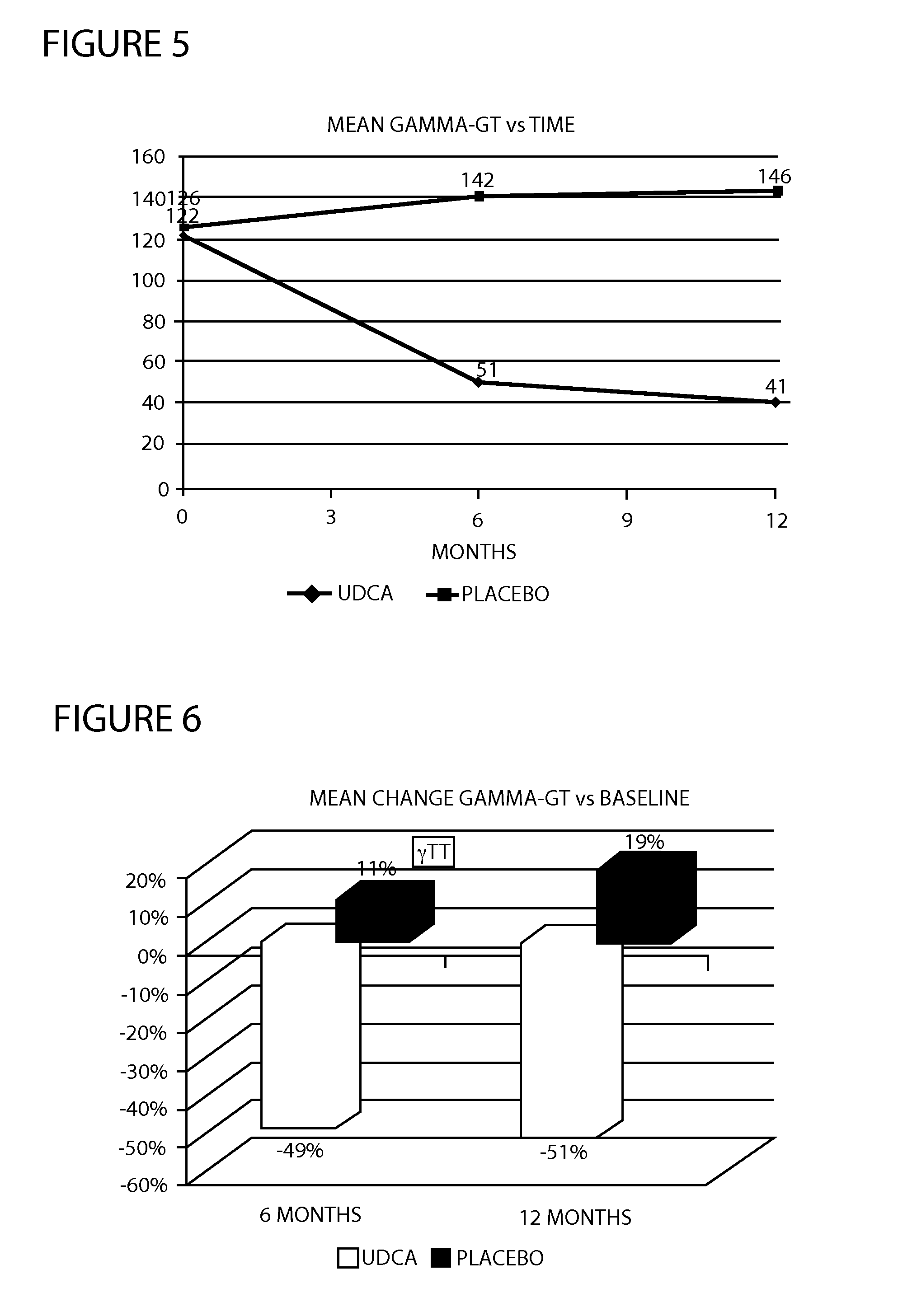
Method of treating nonalcoholic steatohepatitis with elevated doses of ursodeoxycholic acid
InactiveUS20120071451A1Reduces fibrosis level levelReduces level liver inflammation levelOrganic active ingredientsBiocideBlood sugarNonalcoholic steatohepatitis
The present invention is directed to a method for the treatment of nonalcoholic steatohepatitis (NASH) by administering an elevated dose of ursodeoxycholic acid (UDCA), or a pharmaceutically acceptable salt thereof, to a patient in need of such treatment, wherein the patients demonstrate a significantly improved glycemic profile during treatment.
Owner:APTALIS PHARMA CANADA
Methods for diagnosing and predicting non-alcoholic steatohepatitis (NASH)
The invention provides a panel of genes useful for diagnosing non-alcoholic steatohepatitis (NASH). The invention also provides a method of diagnosing NASH in non-invasive assays based on the expression of particular genes in a panel of NASH-related genes. Methods of treatment for NASH and compositions for treating NASH are also provided.
Owner:GEISINGER CLINIC
Use of leptin for the treatment of fatty liver diseases and conditions
ActiveUS20110212889A1Liver inflammation is reducedDecrease triglyceride accumulationHormone peptidesPeptide/protein ingredientsSteatosisLeptin Deficiency
The invention generally relates to the use of leptin in the treatment of a leptin-responsive disease or condition in a non-lipodystrophic subject. More particularly, the invention is directed to the use of leptin in the treatment of a fatty liver disease in a non-lipodystrophic subject with a relative leptin deficiency. The invention includes methods for the treatment of nonalcoholic steatohepatitis (NASH), alcoholic fatty liver disease (AFLD), and nonalcoholic fatty liver disease (NAFLD) in a non-lipodystrophic subject. The invention includes the treatment of conditions ranging from ectopic lipid accumulation (steatosis) to cirrhosis.
Owner:RGT UNIV OF MICHIGAN
Composition and method for treating nonalcoholic steatohepatitis
Nonalcoholic steatohepatitis (NASH) is a disease of the liver characterized by inflammation and damage to the liver cells. Typically, steatohepatitis involves inflammation of the liver related to fat accumulation, and mimics alcoholic hepatitis but is observed in patients who seldom or never consume alcohol. Nonalcoholic steatohepatitis can lead to serious liver damage, and ultimately cirrhosis. The present invention provides methods and compositions useful for the treatment or alleviation of nonalcoholic steatohepatitis and the pharmaceutical formulations for their administration to a human. Specifically, compositions comprised of lecithin, antioxidants and vitamin B complex are administered parenterally, most preferably by oral administration. Specific therapeutic formulations include admixtures of these compounds and specific dosage formulations include daily oral administrations of these compounds in tablet or powder forms.
Owner:VIVA AMERICA MARKETING
Methods of treatment of fatty liver disease by pharmacological activation of cholinergic pathways
A method of treating a fatty liver disease in a subject. The method comprises administering to the subject an effective amount of a cholinergic pathway stimulating agent, wherein the fatty liver disease is selected from non-alcoholic fatty liver (NAFL), alcoholic fatty liver (AFL), non-alcoholic steatohepatitis (NASH), alcoholic steatohepatitis (ASH), NASH-associated liver fibrosis, ASH-associated liver fibrosis, non-alcoholic cirrhosis, and alcoholic cirrhosis.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
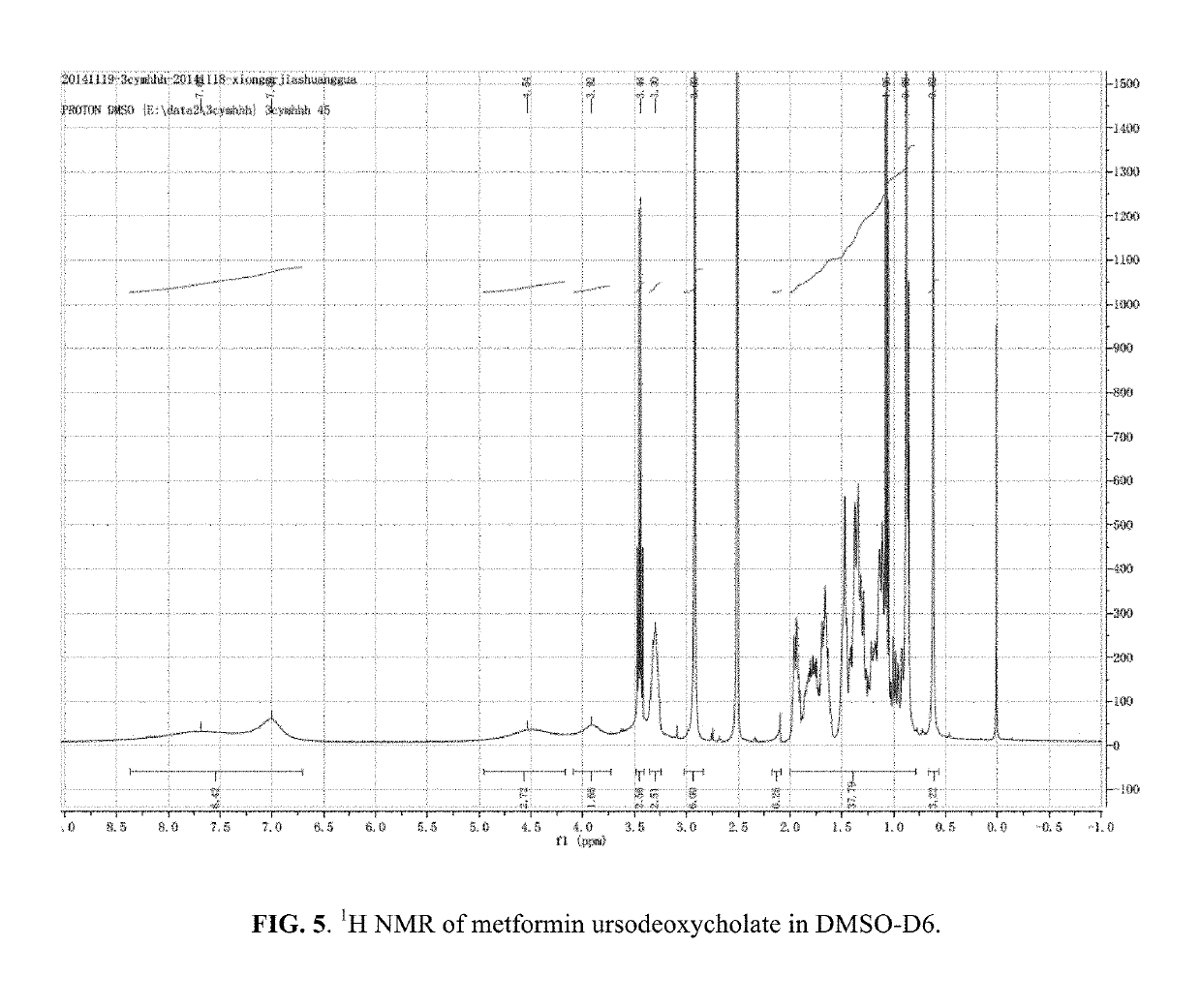
Berberine salts, ursodeoxycholic salts and combinations, methods of preparation and application thereof
Owner:SHENZHEN HIGHTIDE BIOPHARM
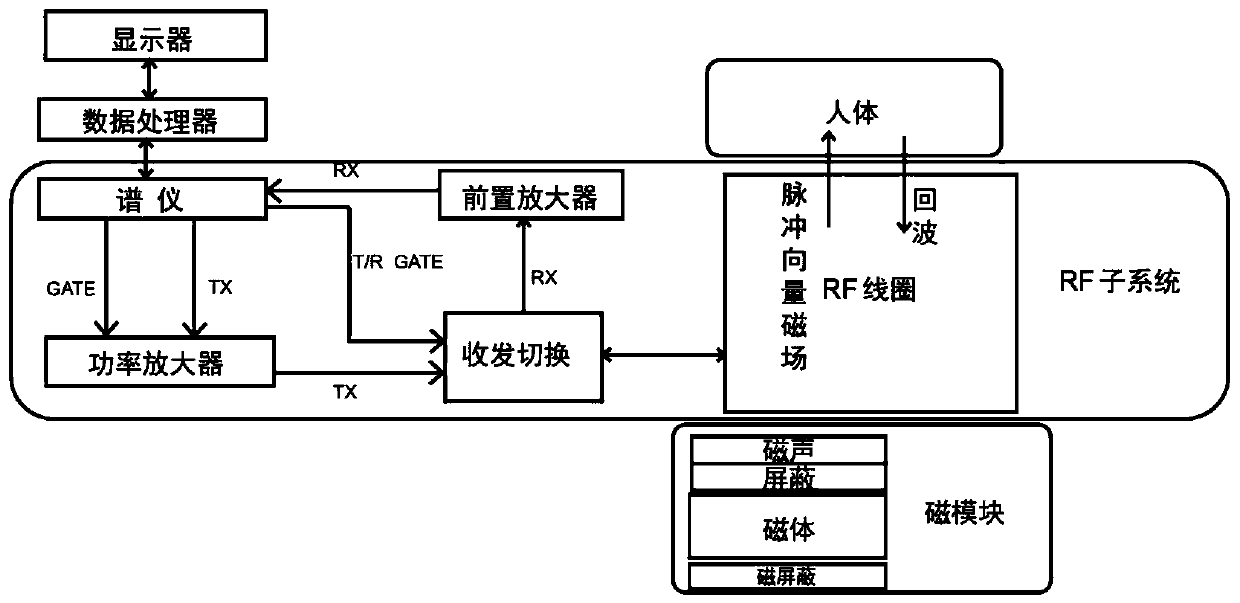
Organ fat non-invasive quantitative detection system based on magnetic resonance principle
InactiveCN110780248ANo damageAccurate quantitative detectionMeasurements using NMR spectroscopyDiagnostic recording/measuringDiseaseLow field nuclear magnetic resonance
The invention discloses an organ fat non-invasive quantitative detection system based on a magnetic resonance principle. The organ fat non-invasive quantitative detection system is characterized in that a radio frequency (RF) subsystem, a portable magnet module and a data processing and display module are adopted to jointly construct a low-field nuclear magnetic resonance system. When the low-field nuclear magnetic resonance system acts on a human body, organs, tissues and cells are not damaged and wounded. Accurate, non-invasive and safe organ fat quantitative detection is achieved, and the organ fat non-invasive quantitative detection system has the advantages of being light, easy to carry, high in cost performance, accurate in quantification and the like. The organ fat non-invasive quantitative detection system is convenient to operate, is not limited by the qualification of operators, achieves one-key detection within a few minutes, is used for rapidly and economically screening related diseases such as NAFLD, metabolic syndrome, non-alcoholic steatohepatitis (NASH) and the like, and has a wide application range. The defects in the prior art are overcome.
Owner:MARVEL STONE HEALTHCARE CO LTD
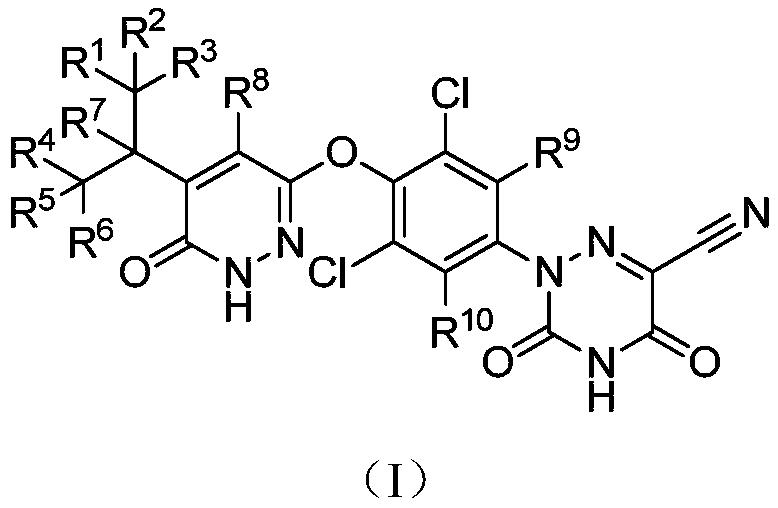
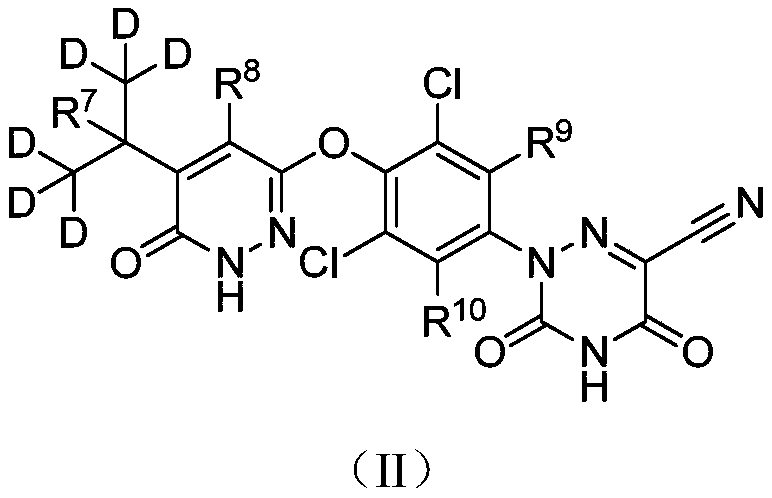
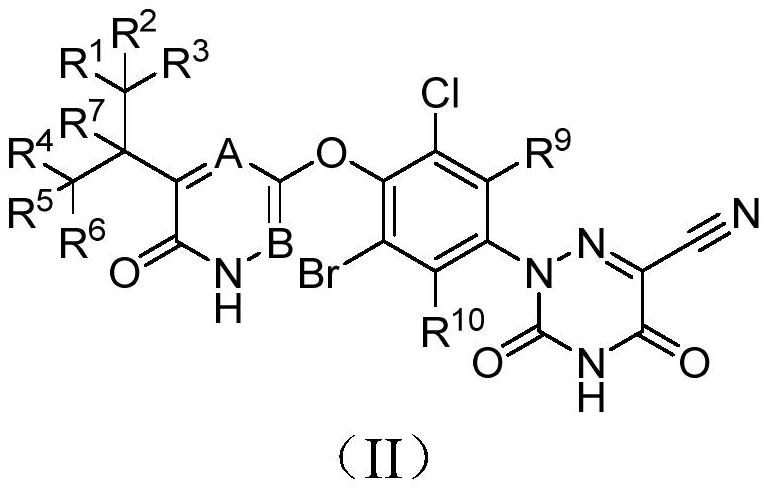
Deuterated MGL-3196 compound and application thereof
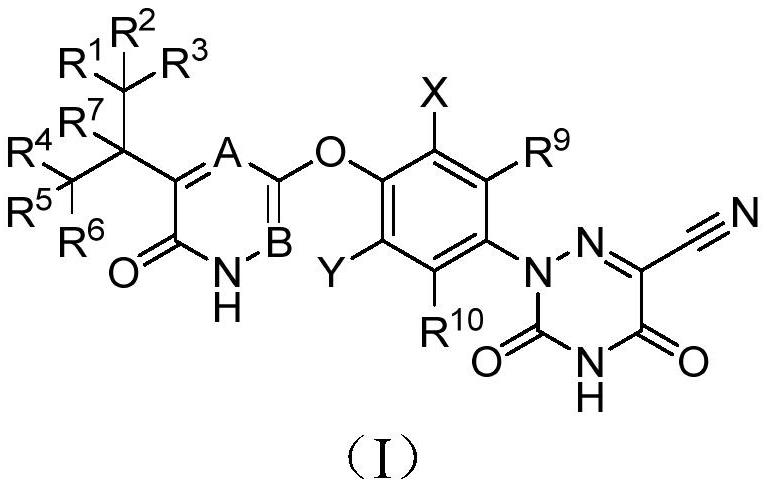
ActiveCN110627773AExcellent agonistic activityGood metabolic stabilityMetabolism disorderOrganic chemistry methodsDyslipidemiaBeta-Adrenergic Agonist
The present invention discloses a compound represented by formula (I), or an optical isomer, a pharmaceutically acceptable salt, a prodrug, a hydrate or a solvate thereof. In the formula (I), R1-R10 are respectively and independently selected from H, D, and are not all H. Compared with a non-deuterated control compound MGL3196, the compound or the optical isomer, the pharmaceutically acceptable salt, the prodrug, the hydrate or the solvate of the compound provided by the invention have the advantages that the agonistic activity on thyroid hormone receptor beta (THR-beta) is better; half-life period is longer, clearance rate is higher, metabolic stability and pharmacokinetic properties are better, and the application prospect in preparation method of THR-beta agonists, and medicines used for treating THR-beta agonist adaptation diseases including dyslipidemia, hypercholesterolemia, non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD) is promising.
Owner:HINOVA PHARM INC
Method of diagnosing non-alcoholic steatohepatitis (nash) using molecular markers
InactiveUS7314720B2Ease of evaluationBioreactor/fermenter combinationsBiological substance pretreatmentsAntioxidant proteinMitochondrial ATPase
The invention relates to a method of diagnosing non-alcoholic steatohepatitis (NASH) using molecular markers. The inventive method consists in detecting and quantifying, in vitro in a hepatic tissue sample, the levels of a protein which can be used as a NASH molecular marker and which is selected from apolipoprotein A1, sub-unit β of the mitochondrial ATPase, leukotriene A4 hydrolase, keratin 18, guanidine acetate N-methyltransferase, superoxide dismutase, albumin, antioxidant protein 2 (isoform 1), prohibitin 1, methionine adenosyl transferase, long-chain acyl CoA dehydrogenase, selenium binding protein, antioxidant protein 2 (isoform 2), and combinations of same. The invention further consists in comparing the results obtained with the normal values of said proteins in healthy hepatic tissue. Said method can be used to diagnose NASH and / or to assess a patient's potential risk of developing NASH.
Owner:ONE WAY LIVER GENOMICS
Combinations For Treatment Of NASH/NAFLD And Related Diseases
The combination of (S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-N-(tetrahydrofuran-3-yl)pyrimidine-5-carboxamide or pharmaceutically acceptable salt thereof, and 4-(4-(1-Isopropyl-7-oxo-1,4,6,7-tetrahydrospiro[indazole-5,4′-piperidine]-1′-carbonyl)-6-methoxypyridin-2-yl)benzoic acid or pharmaceutically acceptable salt thereof, for treatment of diseases, including non-alcoholic steatohepatitis (NASH), in mammals are described herein.
Owner:PFIZER INC
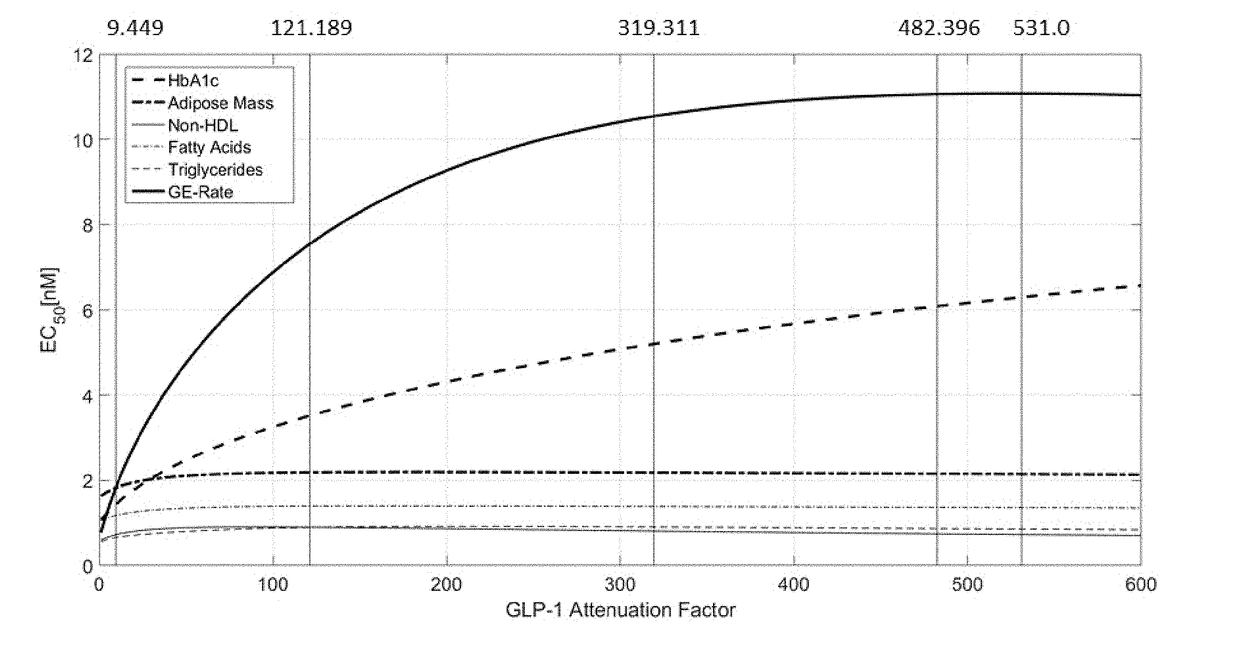
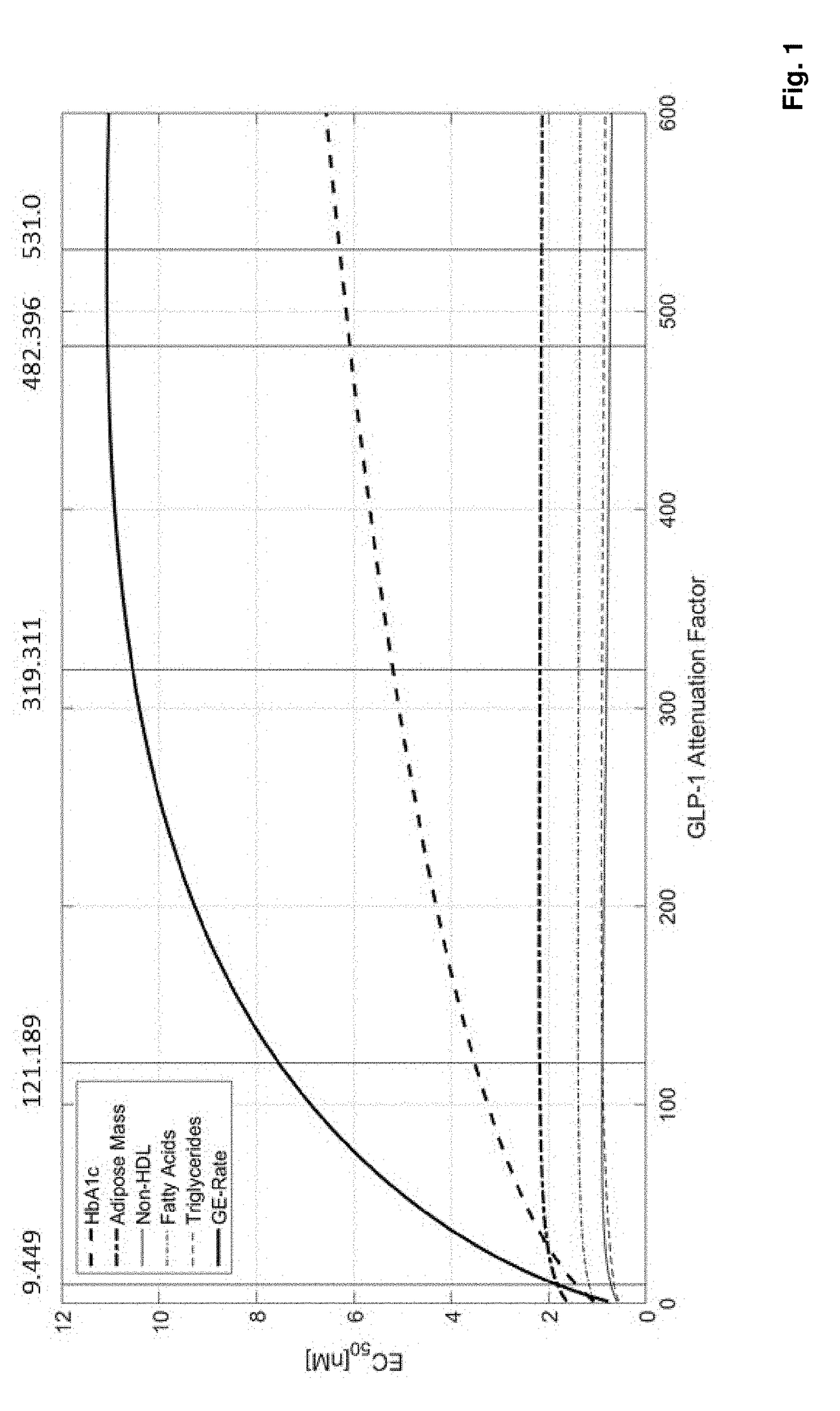
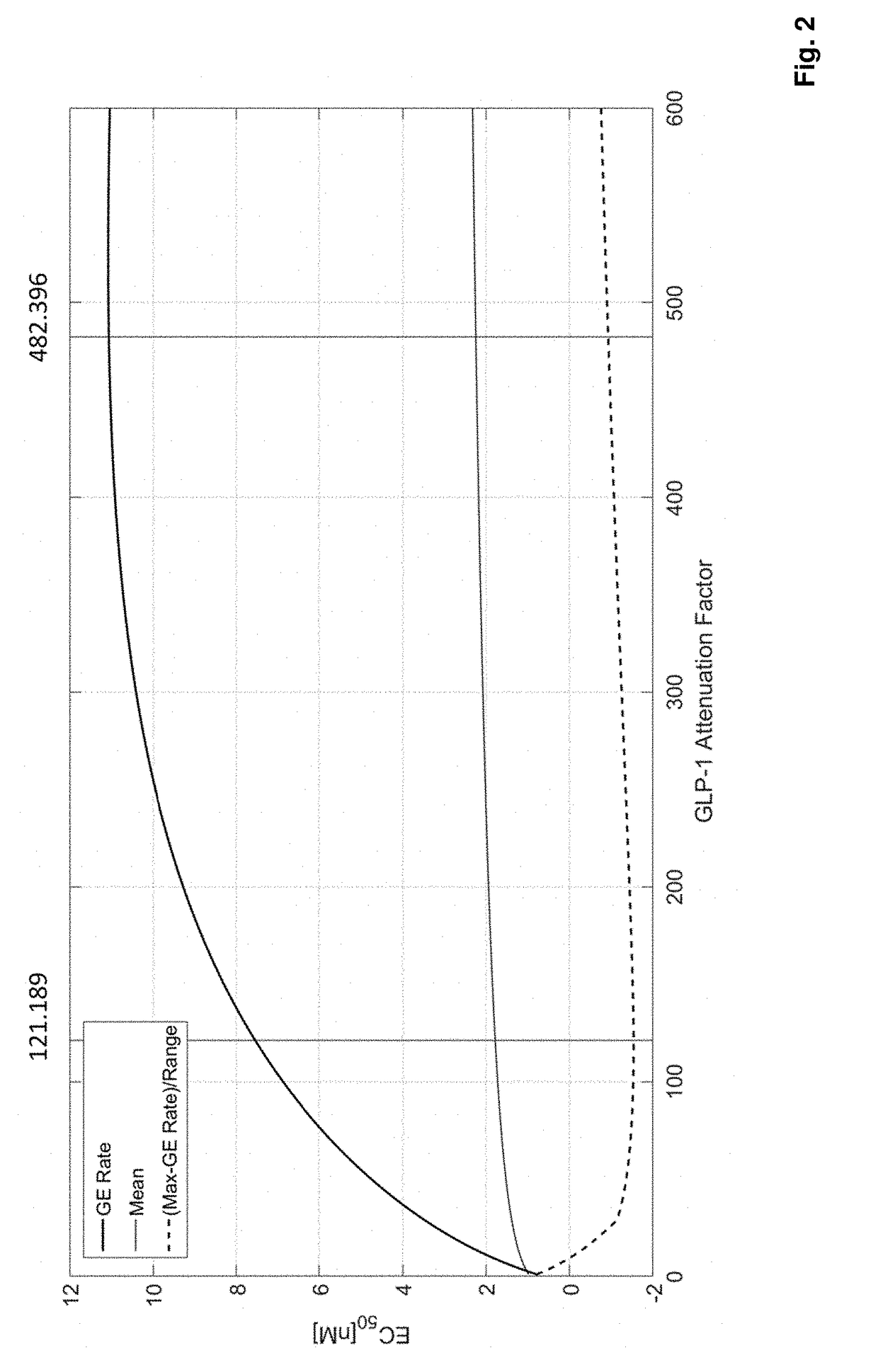
Fgf21 compound / glp-1r agonist combinations with optimized activity ratio
ActiveUS20180236037A1Glycemic control in overweightPeptide/protein ingredientsMetabolism disorderAcute hyperglycaemiaDyslipidemia
The present invention relates to combinations, pharmaceutical compositions and fusion molecules comprising an FGF21 (fibroblast growth factor 21) compound and a GLP-1R (glucagon-like peptide-1 receptor) agonist with optimized GLP-1R agonist / FGF21 compound activity ratio. It further relates to their use as medicaments, in particular for the treatment of obesity, being overweight, metabolic syndrome, diabetes mellitus, diabetic retinopathy, hyperglycemia, dyslipidemia, Non-Alcoholic SteatoHepatitis (NASH) and / or atherosclerosis.
Owner:SANOFI SA
Compositions and methods for treating non-alcoholic steatohepatitis
ActiveUS9486433B2No worseningImproving the NAS scoreOrganic active ingredientsDigestive systemEthyl IcosapentateNon alcoholic
Compositions and method are disclosed comprising ethyl icosapentate for use in treatment of non-alcoholic steatohepatitis (NASH).
Owner:AMARIN PHARMA IRELAND
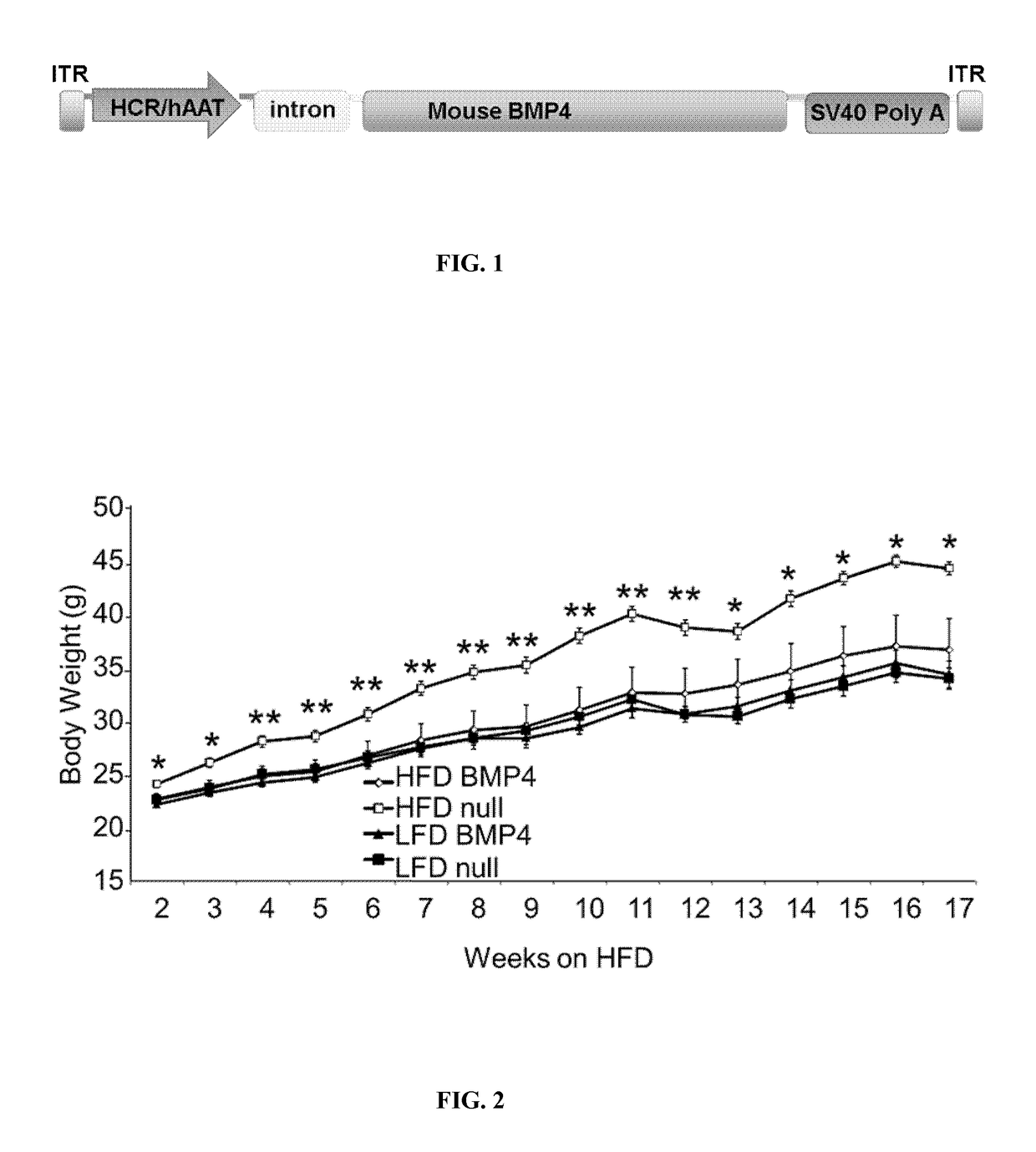
Adeno-Associated Viral Vectors for the Gene Therapy of Metabolic Diseases
The present invention discloses adeno-associated viral vectors useful in gene therapy methods for the treatment of obesity, insulin resistance, type 2 diabetes, liver cirrhosis and non-alcoholic fatty liver disease (NAFLD) / non-alcoholic steatohepatitis (NASH). The invention also relates to polynucleotides, plasmids, vectors and methods for the production of such adeno-associated viral vectors. The invention also relates to pharmaceutical compositions comprising said vectors.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA
Compositions and methods for the reduction or prevention of non-alcoholic steatohepatitis (NASH)
InactiveUS20170239253A1Lower Level RequirementsReduction of non-alcoholic steatohepatitis (NASH)Disease diagnosisPill deliveryMetabolitePhosphodiesterase 5 inhibitor
Methods useful for sustaining a reduction of non-alcoholic steatohepatitis are provided herein. Such methods may comprise administering to a subject in need thereof a sirtuin pathway activator and / or PDE5 inhibitor alone or in combination with an amount of a branched amino acid in free amino acid form, or a metabolite thereof. Also provided herein are compositions and kits for practicing any of the methods described herein.
Owner:NUSIRT SCI
Experimental animal model for converting non-alcoholic steatohepatitis into liver cancer
The invention belongs to the field of biotechnology, and relates to an experimental animal model for transforming nonalcoholic steatohepatitis into liver cancer. In particular, a large and mouse model of nonalcoholic chronic steatohepatitis (NASH) and chronic steatohepatitis developing into chronic liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) can be used for chronic fatty Liver, steatohepatitis, liver fibrosis, liver cirrhosis, hepatocellular carcinoma, metabolic diseases, diabetes, in vivo experiments of related mechanisms of diabetic complications, new drug development, and non-alcoholic steatohepatitis (NASH), liver It has extremely high application value in the study of the pathogenesis of fibrosis and liver cancer and in drug development.
Owner:凯斯艾生物科技(苏州)有限公司
Application of nicotinamide riboside in preparing drugs for treating non-alcoholic steatohepatitis
InactiveCN104622886AIncrease useHelp preventOrganic active ingredientsDigestive systemNicotinamide ribosideLiver fibrosis
The invention relates to an application of nicotinamide riboside in preparing drugs for treating non-alcoholic steatohepatitis. According to the application, a non-alcoholic steatohepatitis animal model is adopted to prove that the nicotinamide riboside is capable of reducing the liver lipid content, the liver inflammations and the liver fibrosis degree of the animal model and prompt that the nicotinamide riboside can be applied to preparing the drugs for treating the non-alcoholic steatohepatitis. The application of the nicotinamide riboside is expanded, a new way is provided for treating the non-alcoholic steatohepatitis, and therefore, the non-alcoholic steatohepatitis can be prevented and prognosed favorably.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
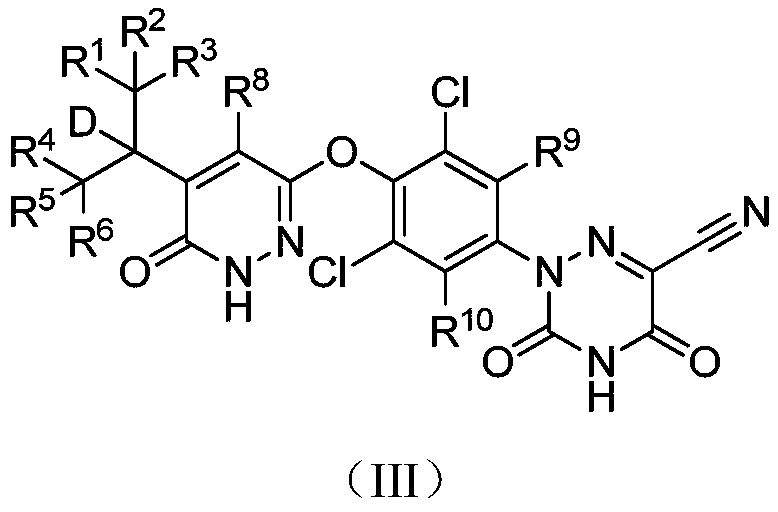
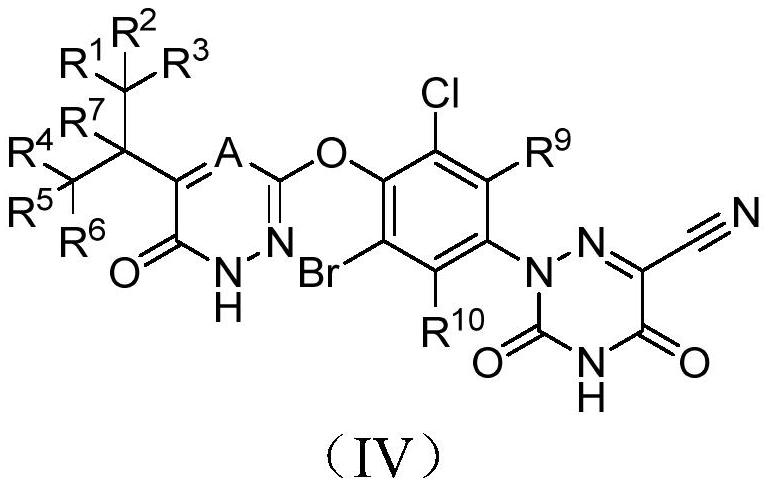
Halogen-substituted phenyl ether compound and application thereof
ActiveCN112409340AHigh activityHigh selectivityMetabolism disorderOrganic chemistry methodsDyslipidemiaPhenyl Ethers
The invention discloses a halogen-substituted phenyl ether compound and an application thereof. Specifically provided are a compound represented by formula (I) or an optical isomer, a pharmaceuticallyacceptable salt, a prodrug, a hydrate or a non-aqueous solvate thereof. The experiments prove that compared with a control compound MGL-3196, the compound shown in the formula (I), which is obtainedthrough a specific substitution position and a specific substitution type, has better agonistic activity on THR-beta, and the selectivity on THR-beta / THR-alpha is remarkably improved. In addition, thecompound provided by the invention also has significantly improved pharmacokinetic properties. The compound provided by the invention has excellent application prospects in preparation of THR-beta agonists and medicines for treating indications (including dyslipidemia, hypercholesterolemia, non-alcoholic steatohepatitis and non-alcoholic fatty liver diseases) suitable for the THR-beta agonists.
Owner:HINOVA PHARM INC
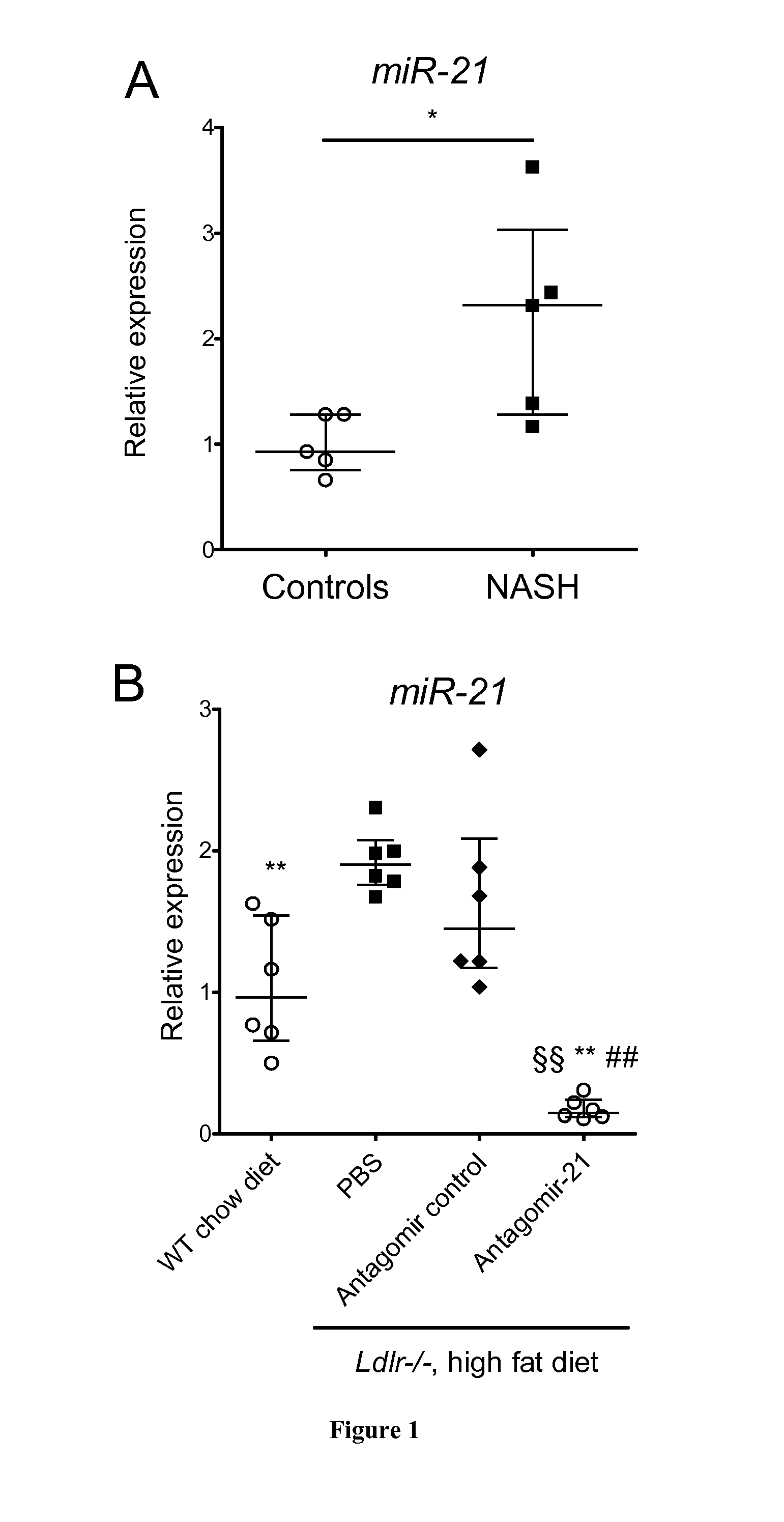
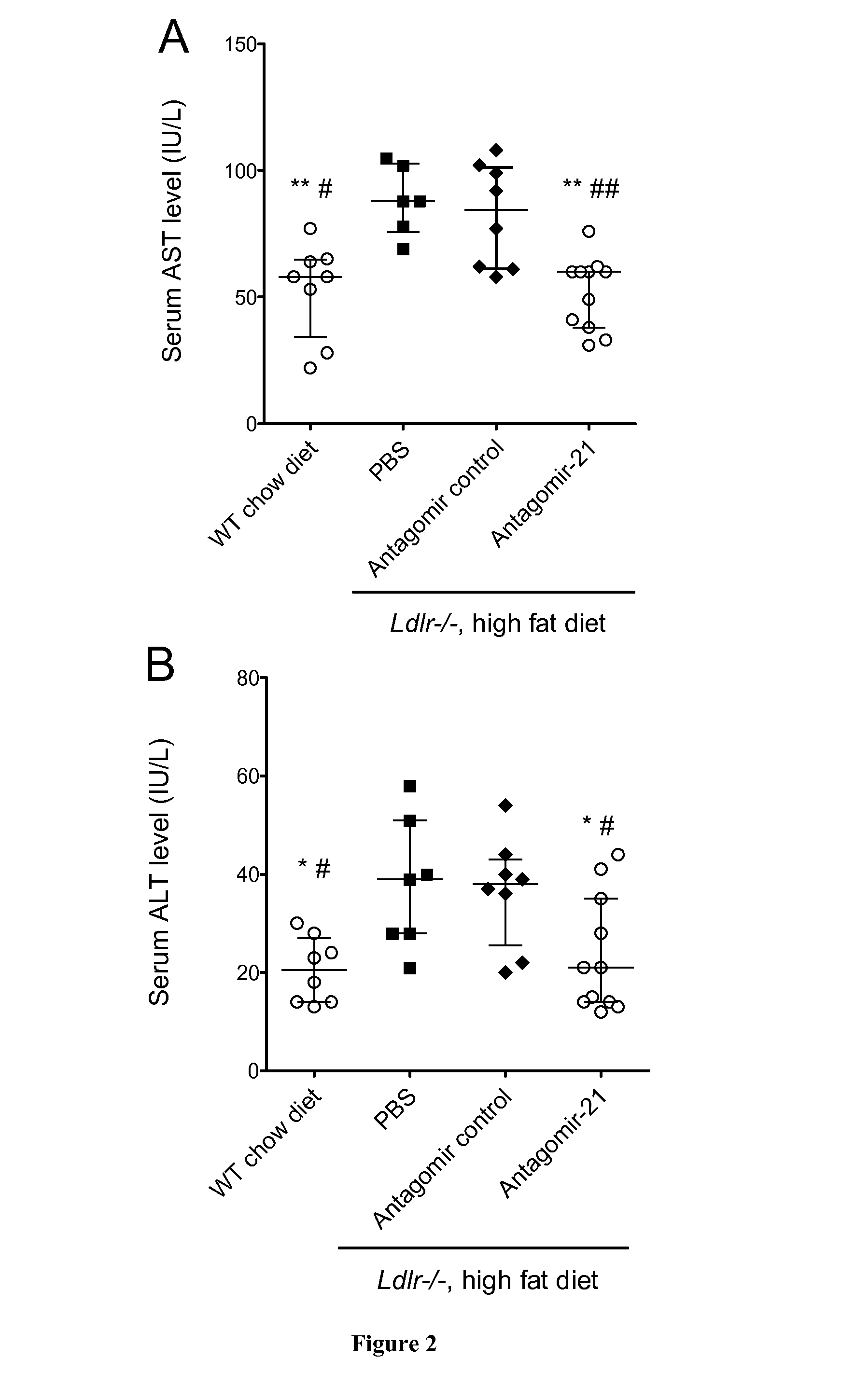
Methods for the Treatment of Nonalcoholic Steatohepatitis
InactiveUS20150057332A1Reduce in quantityLessen liver damageOrganic active ingredientsGenetic material ingredientsCancer researchNonalcoholic steatohepatitis
The present invention relates to the treatment of nonalcoholic steatohepatitis (NASH), in particular to a compound that inhibits miR-21 expression for use in the treatment of nonalcoholic steatohepatitis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Compositions and methods for treating non-alcoholic steatohepatitis
The disclosure provides for a method for treating a fatty liver disease or disorder in a subject in need thereof, comprising selecting a subject having or suspected of having a fatty liver disease or disorder, wherein the subject is non diabetic, pre-diabetic, mildly diabetic; has normal or substantially normal biliary tract function; or has non or early stage hepatocyte apoptosis; and administering a therapeutically effective amount of a pharmaceutical composition comprising EPAs.
Owner:AMARIN PHARMA IRELAND
Pharmaceutical compositions of berberine with epa and dha, and methods thereof
The invention provides various novel compositions of berberine in combination with pharmacologically active EPA and DHA, and related methods of their use in treating various diseases or disorders. The pharmaceutical compositions of the invention are useful in treating and / or preventing various diseases or disorders, including metabolic diseases or disorders such as dyslipidemia, hyperglycemia, hypertriglyceridemia, hyperlipidemia, diabetic dyslipidemia, diabetic hyperlipidemia, dyslipidemia in statin-intolerance patients, diabetes, diabetic complications, hypercholesterolemia, or obesity. Additionally, the pharmaceutical compositions of the invention are useful in treating and / or preventing atherosclerosis, heart diseases, neurodegenerative diseases, inflammation, cancers, as well as various liver diseases or disorders, such as fatty liver, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, cholestatic liver diseases or graft-versus-host disease of the liver. Furthermore, the pharmaceutical compositions of the invention are useful in improving liver functions in chronic viral associated liver diseases and alcohol-related liver diseases.
Owner:SHENZHEN HIGHTIDE BIOPHARM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com