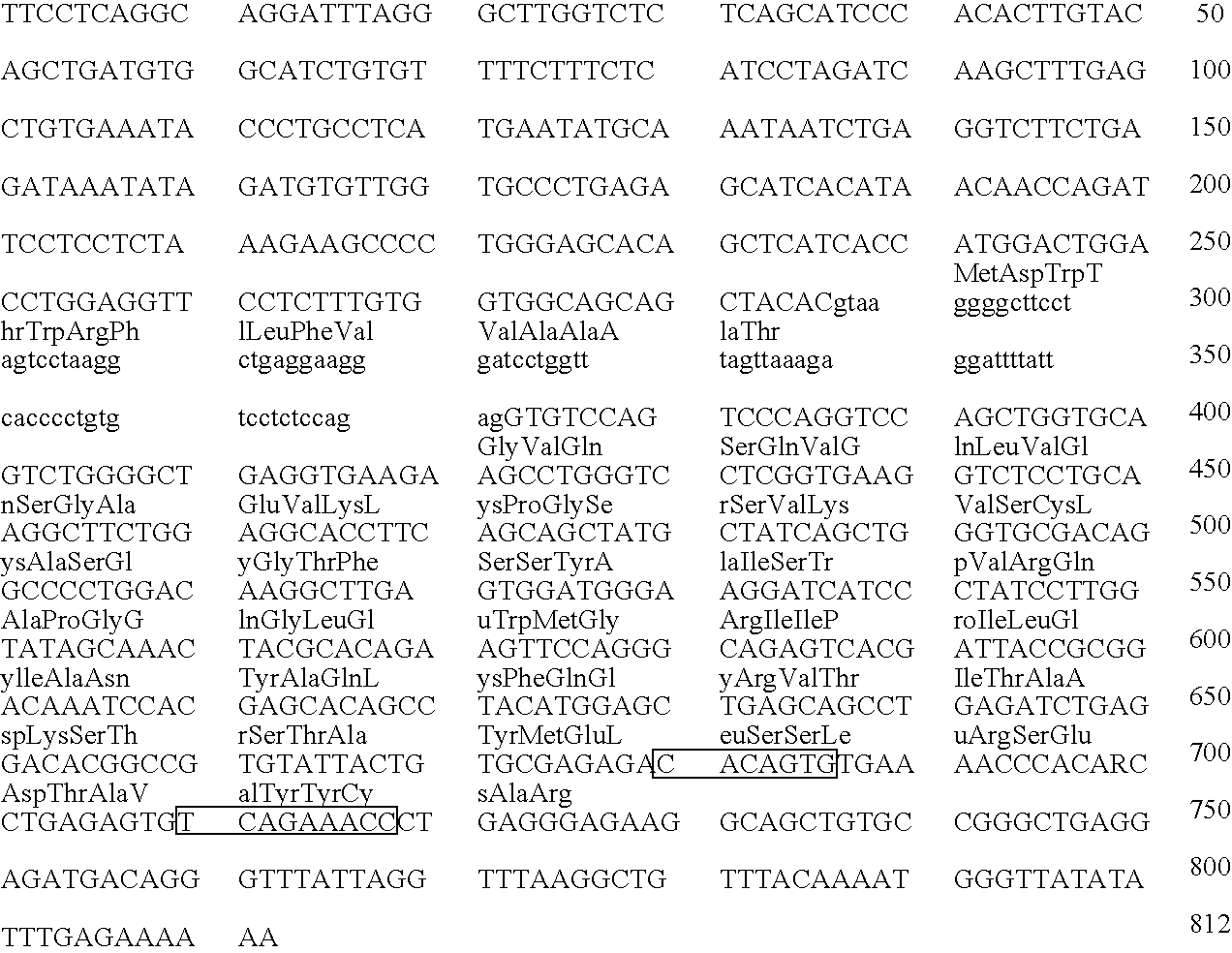
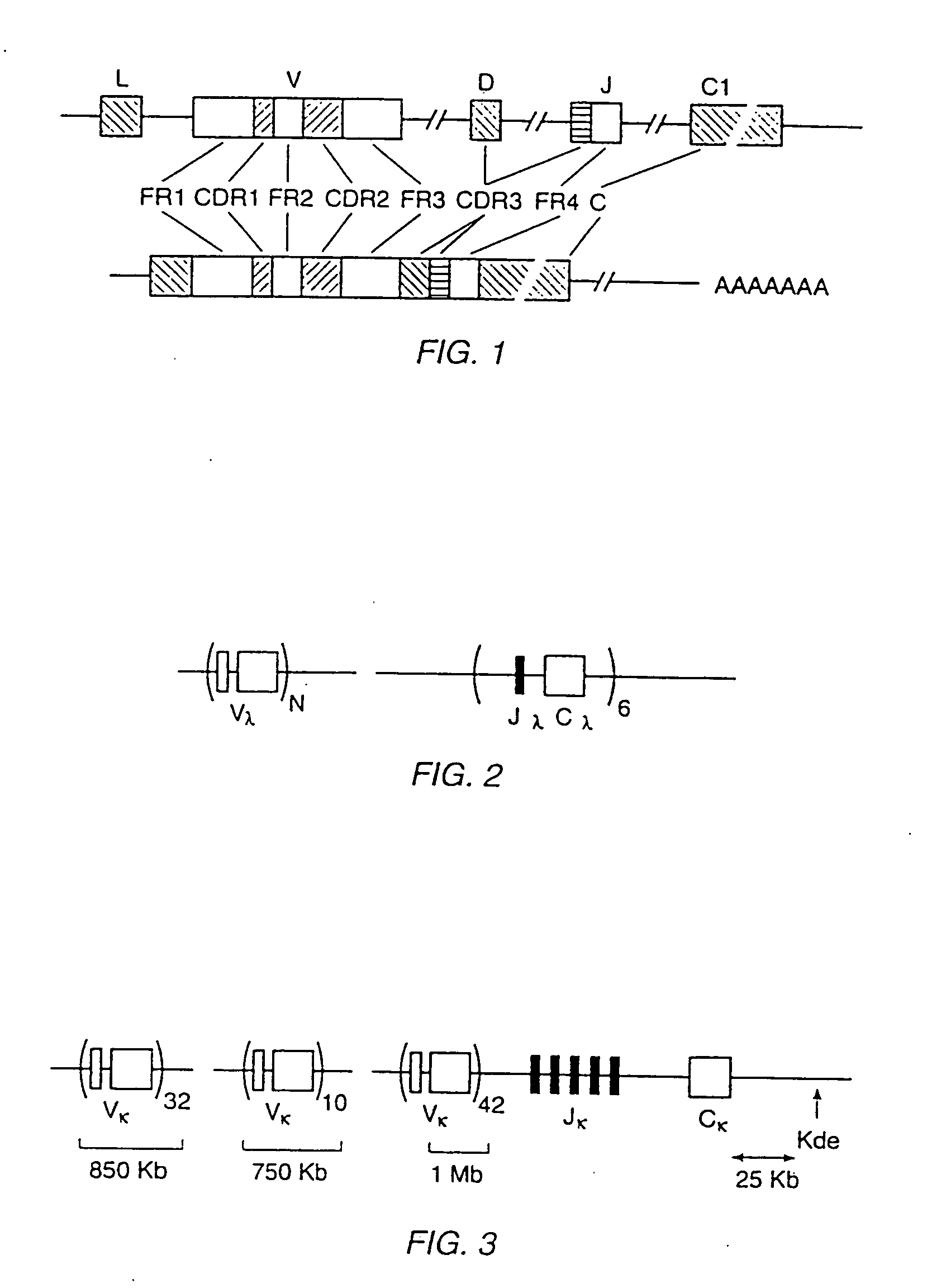
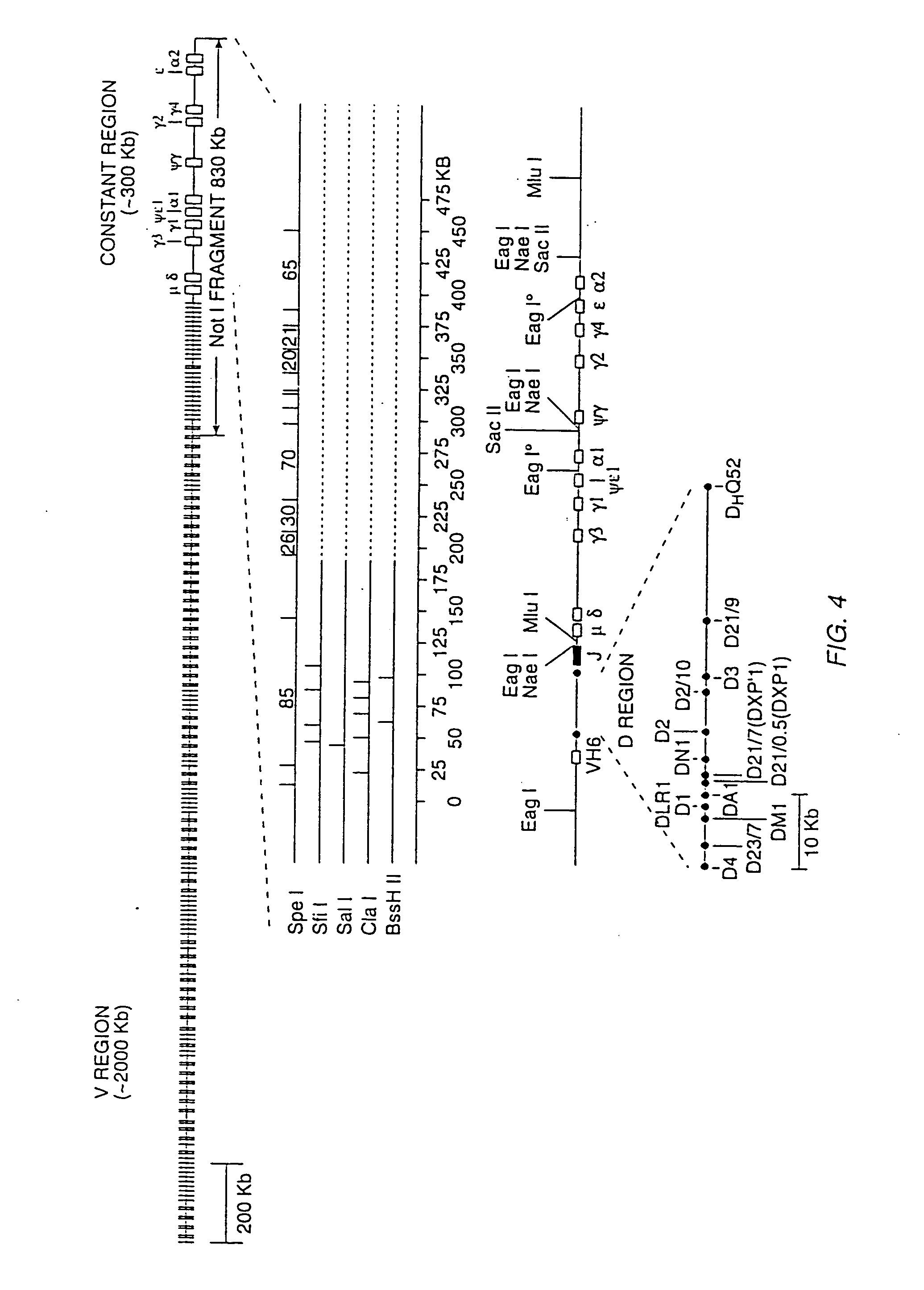
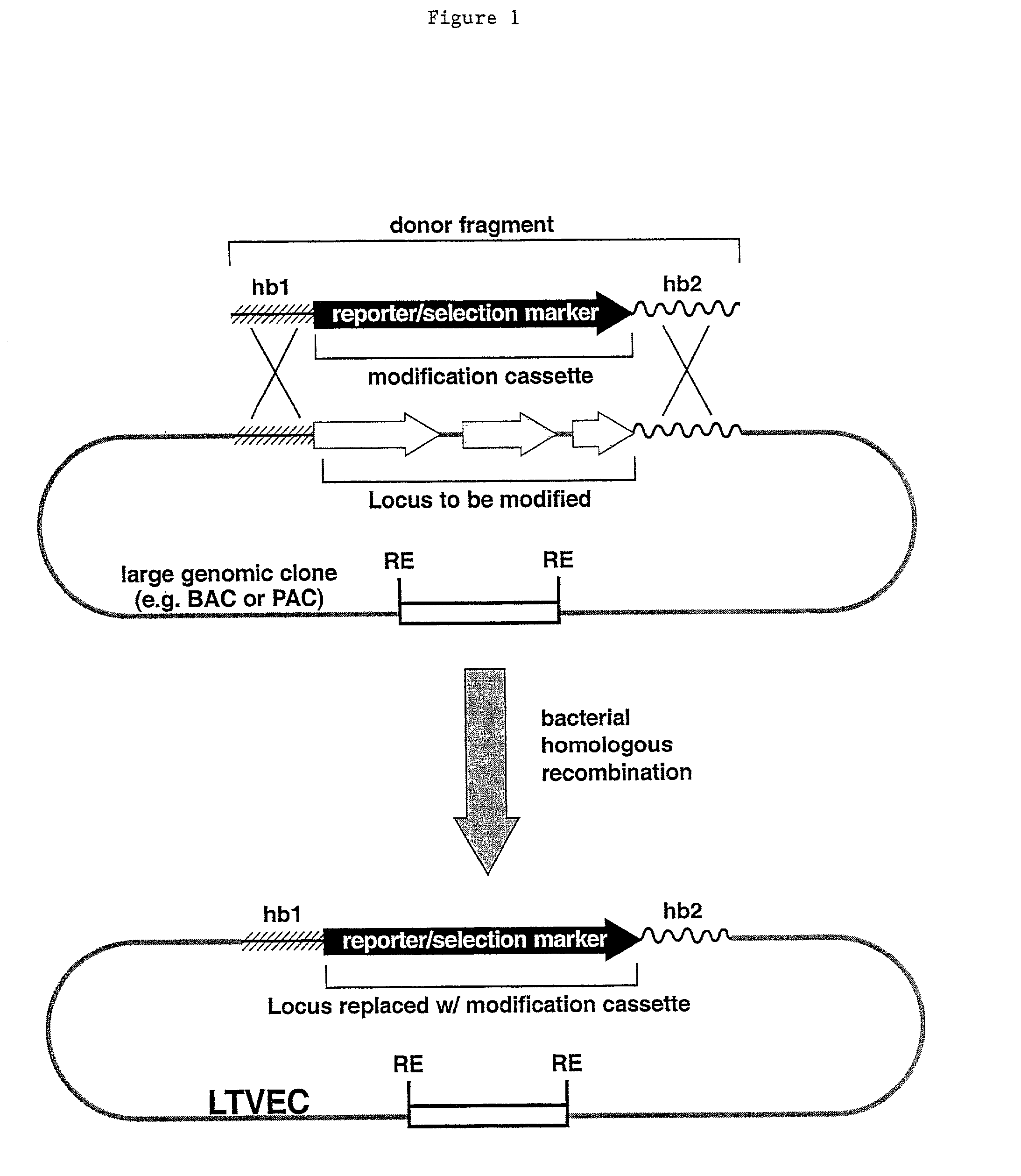
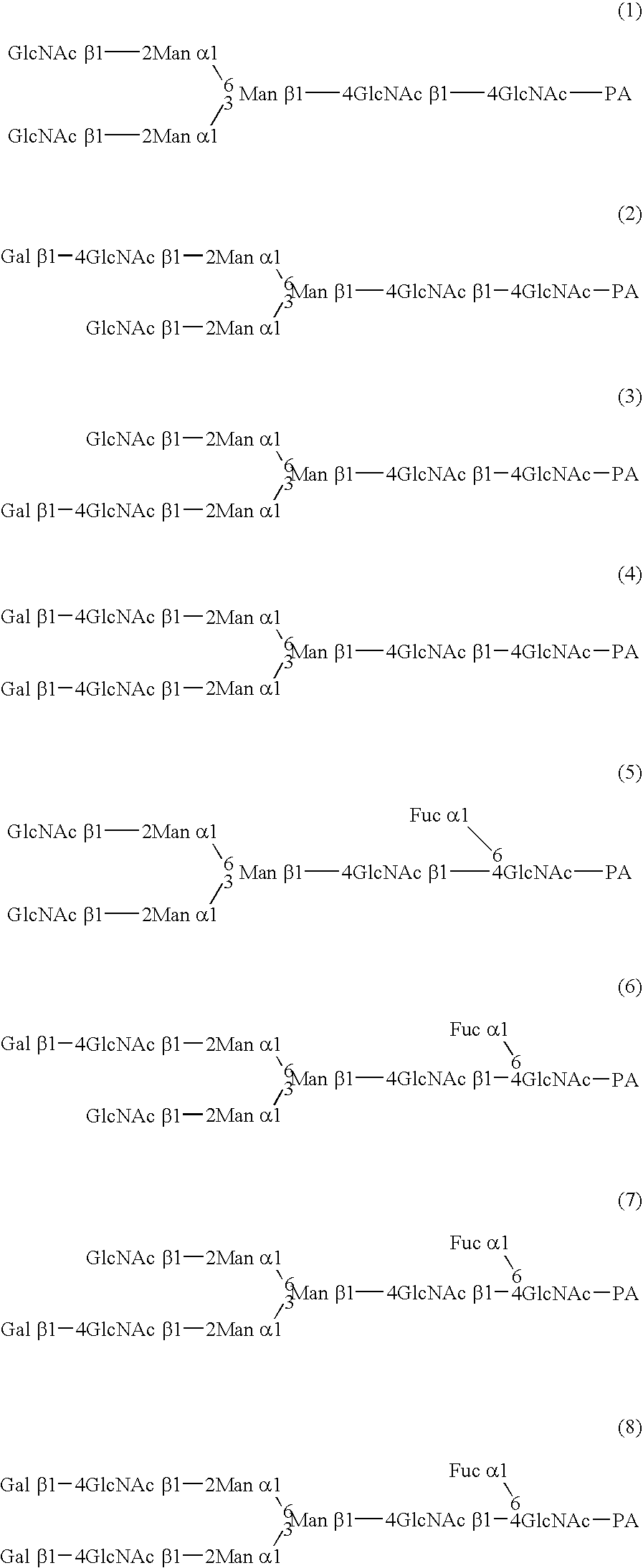Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
20784results about "Animal husbandry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
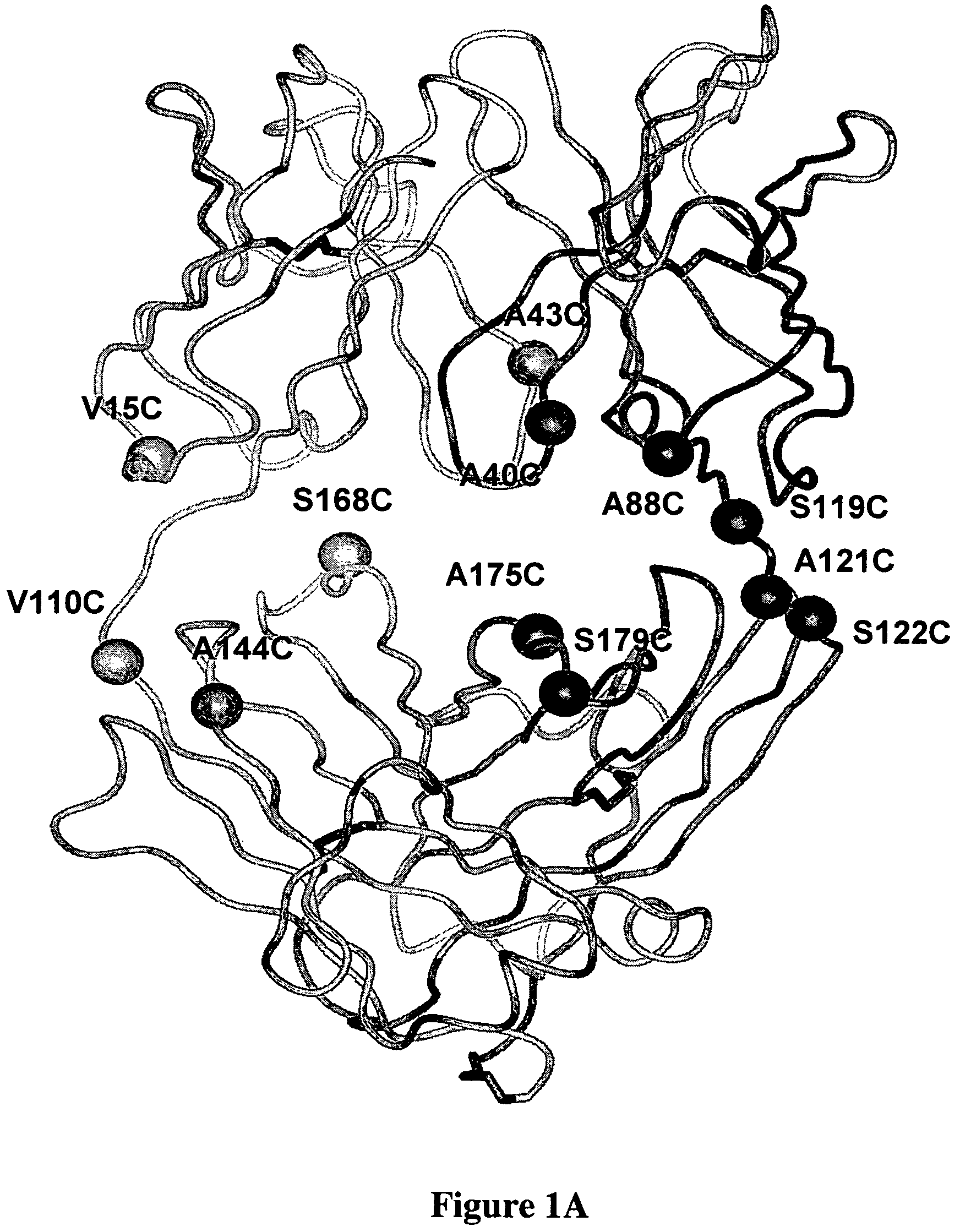
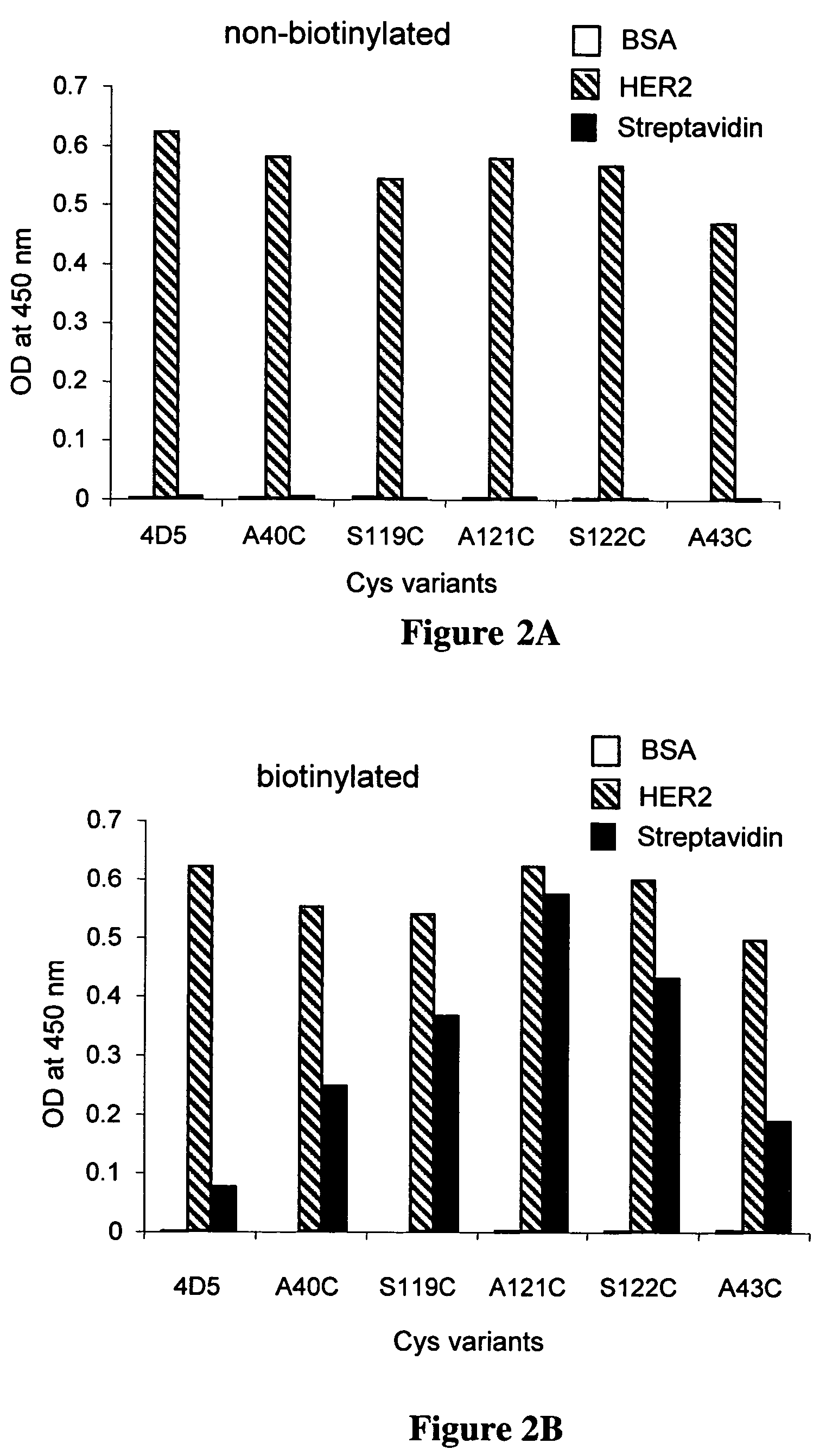
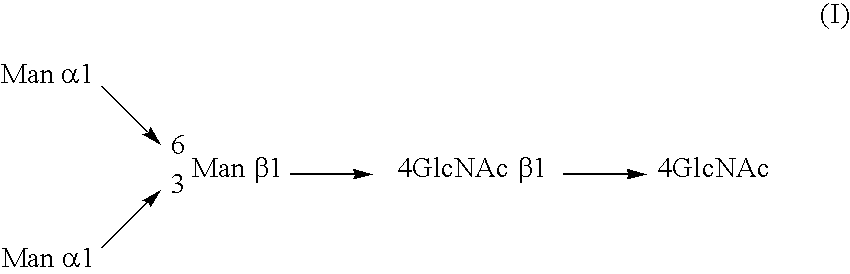
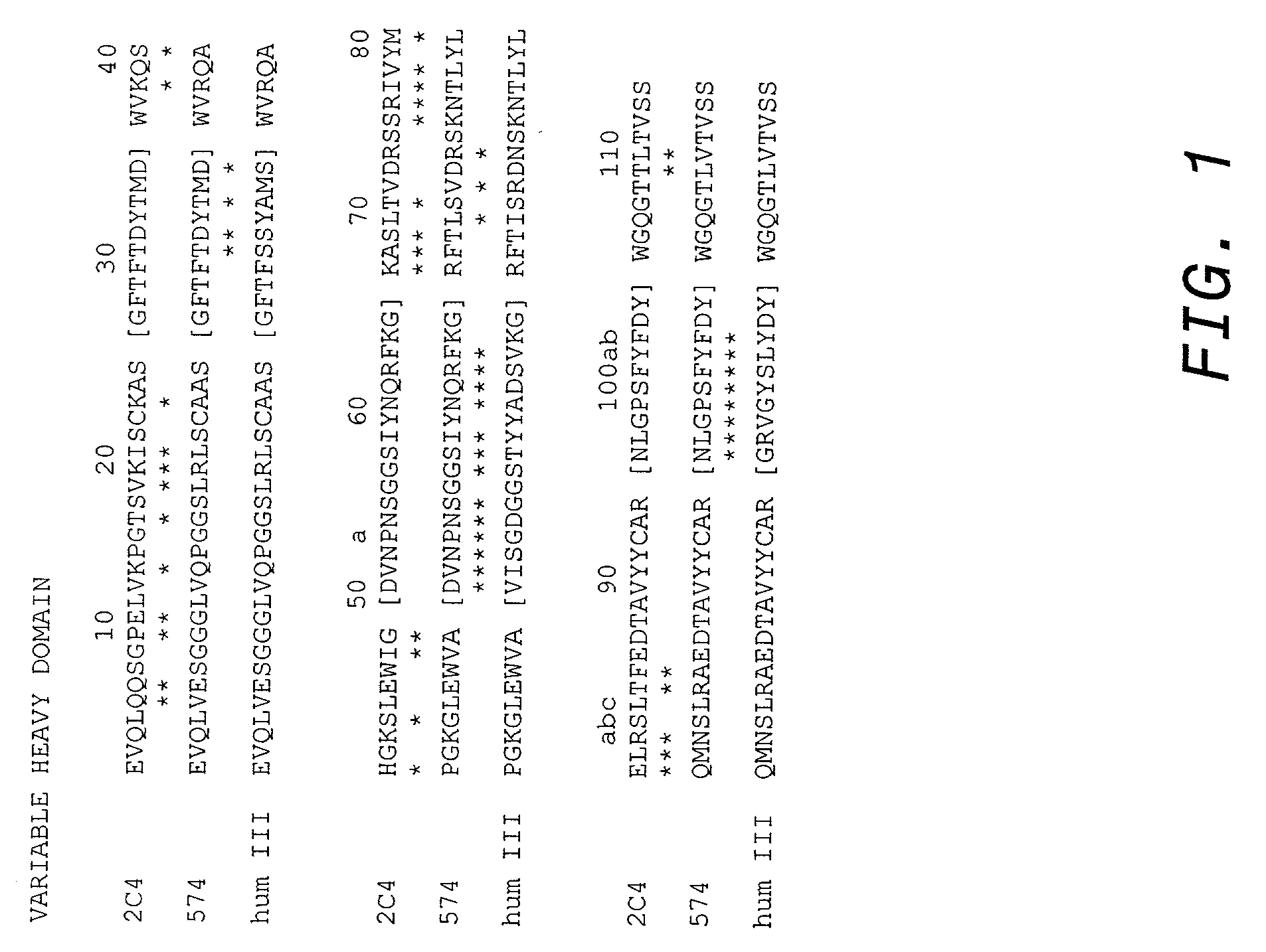
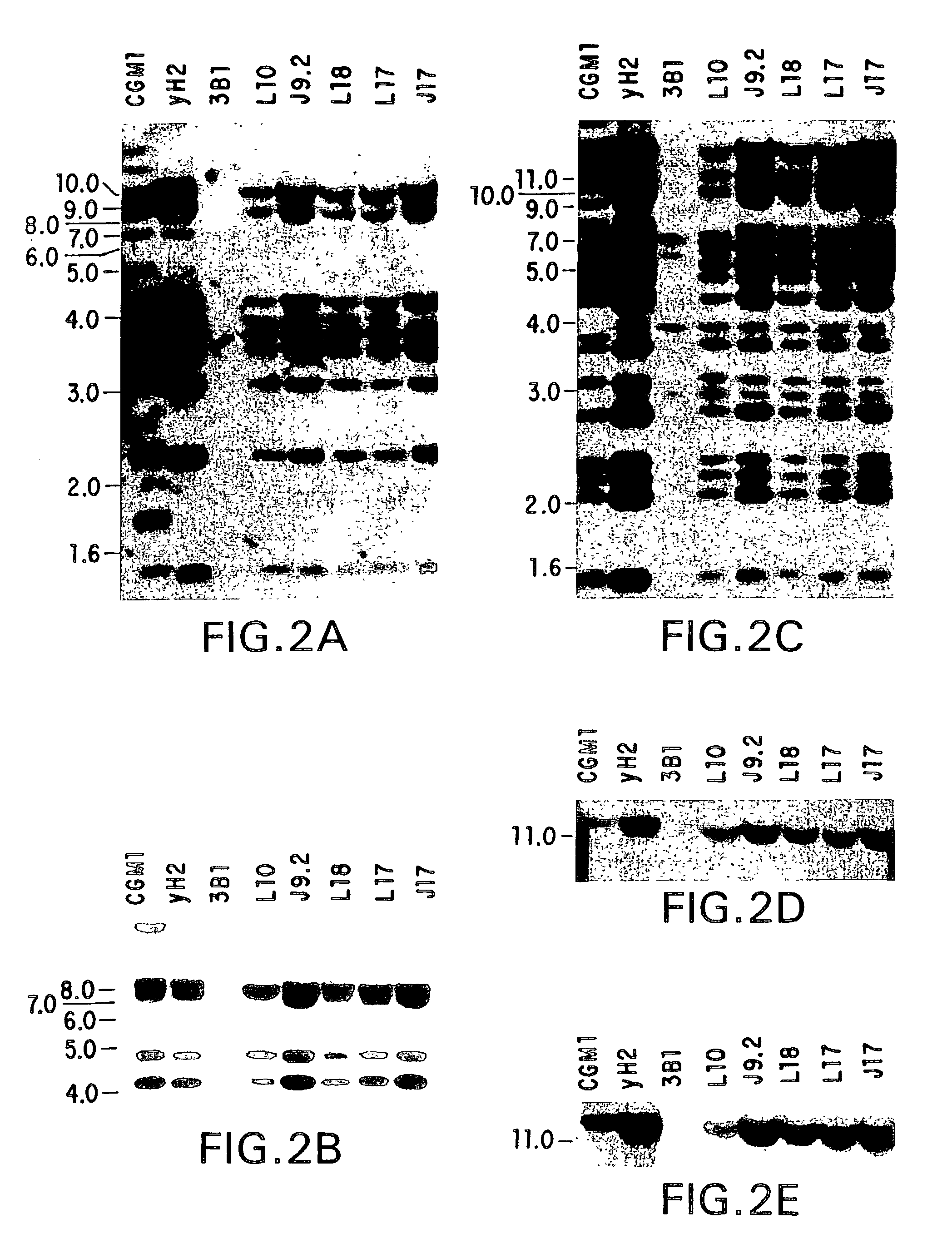
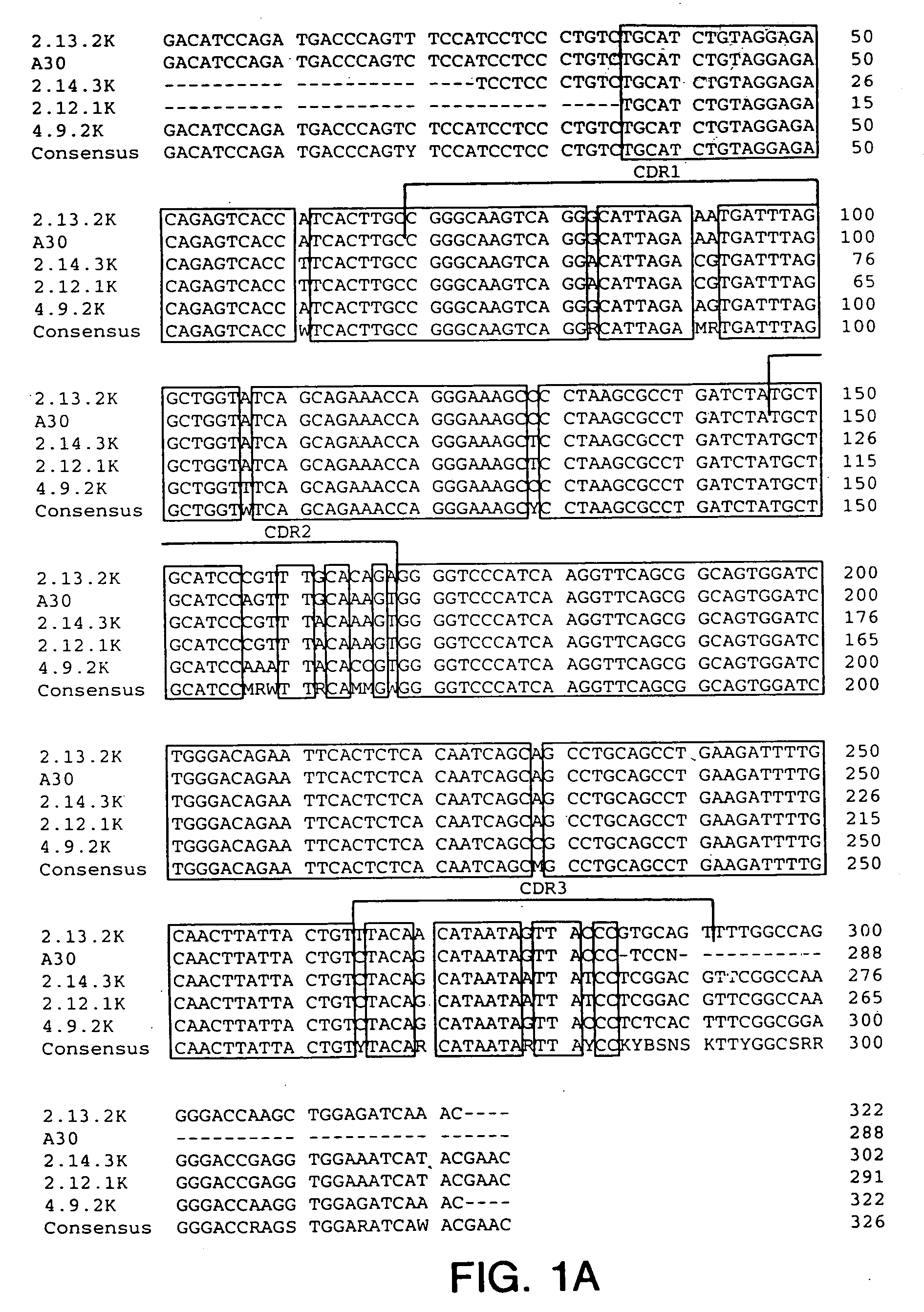
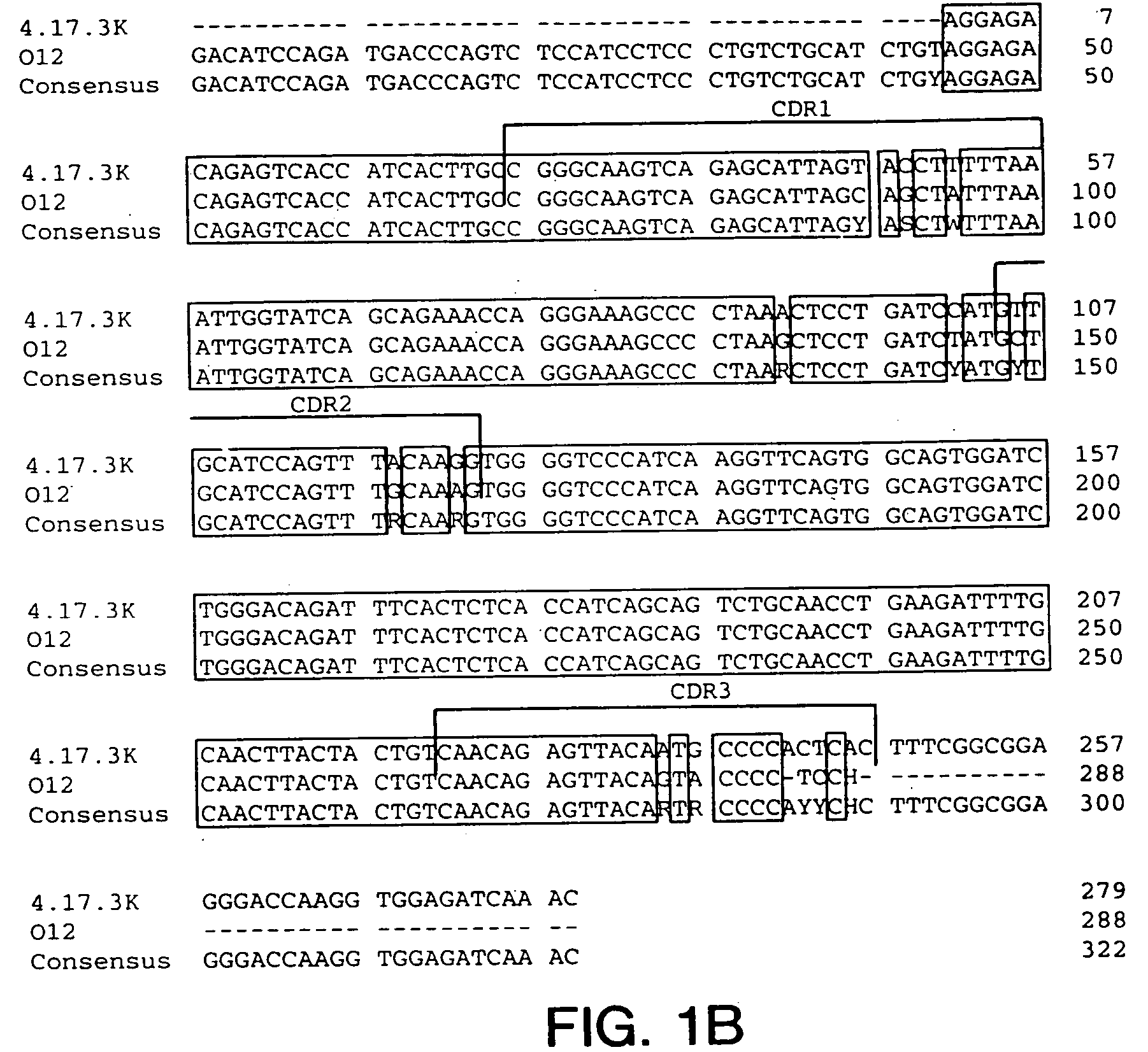
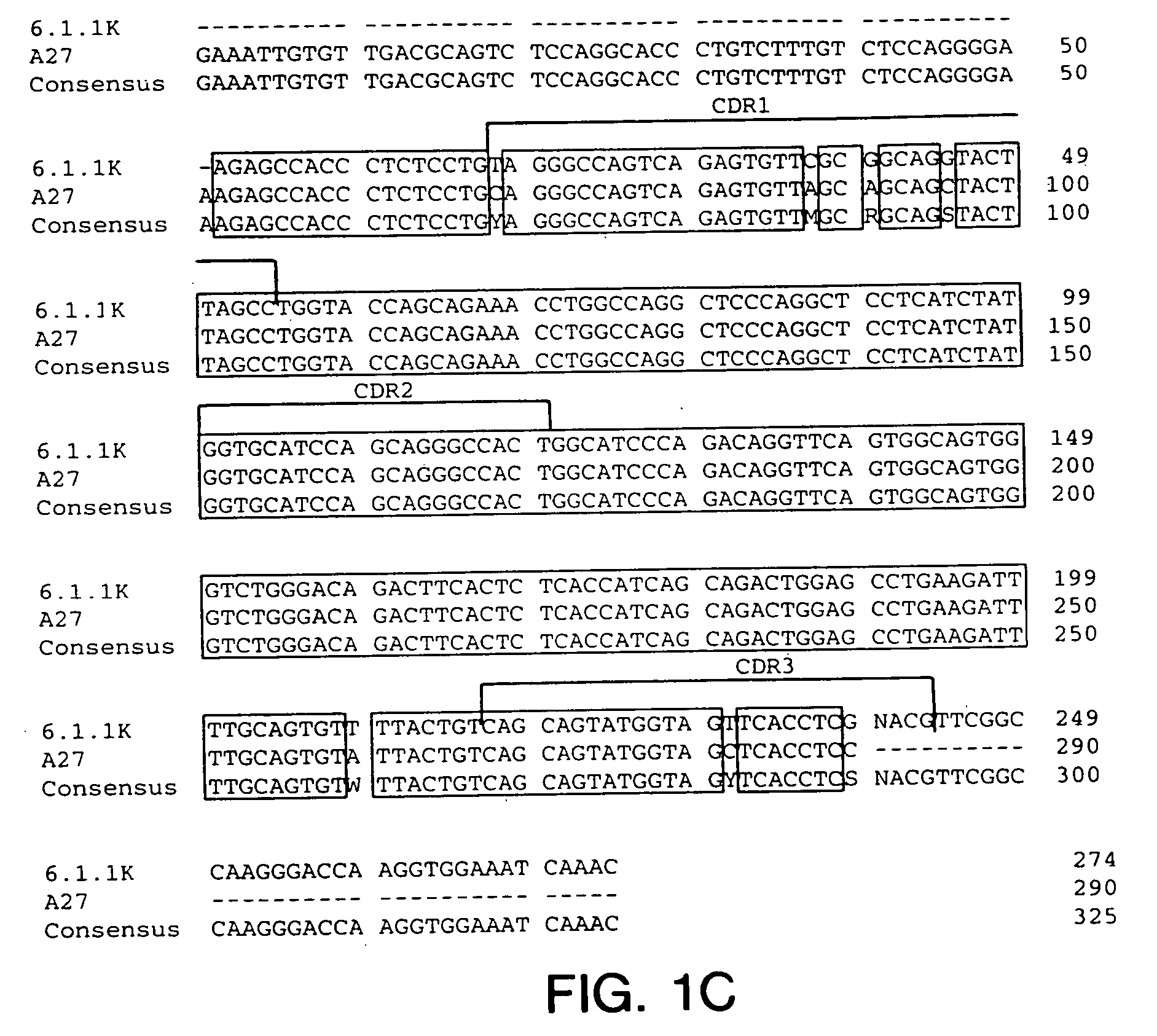
Cysteine engineered antibodies and conjugates
Owner:GENENTECH INC
Cells of which genome is modified
InactiveUS20040110704A1Raise the ratioDecreased and deleted activityAntibacterial agentsAntipyreticGlycosideN-Acetylglucosamine
A cell in which genome is modified so as to have a more decreased or deleted activity of an enzyme relating to modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain than its parent cell, and a process for producing an antibody composition using the cell.
Owner:KYOWA HAKKO KOGYO CO LTD
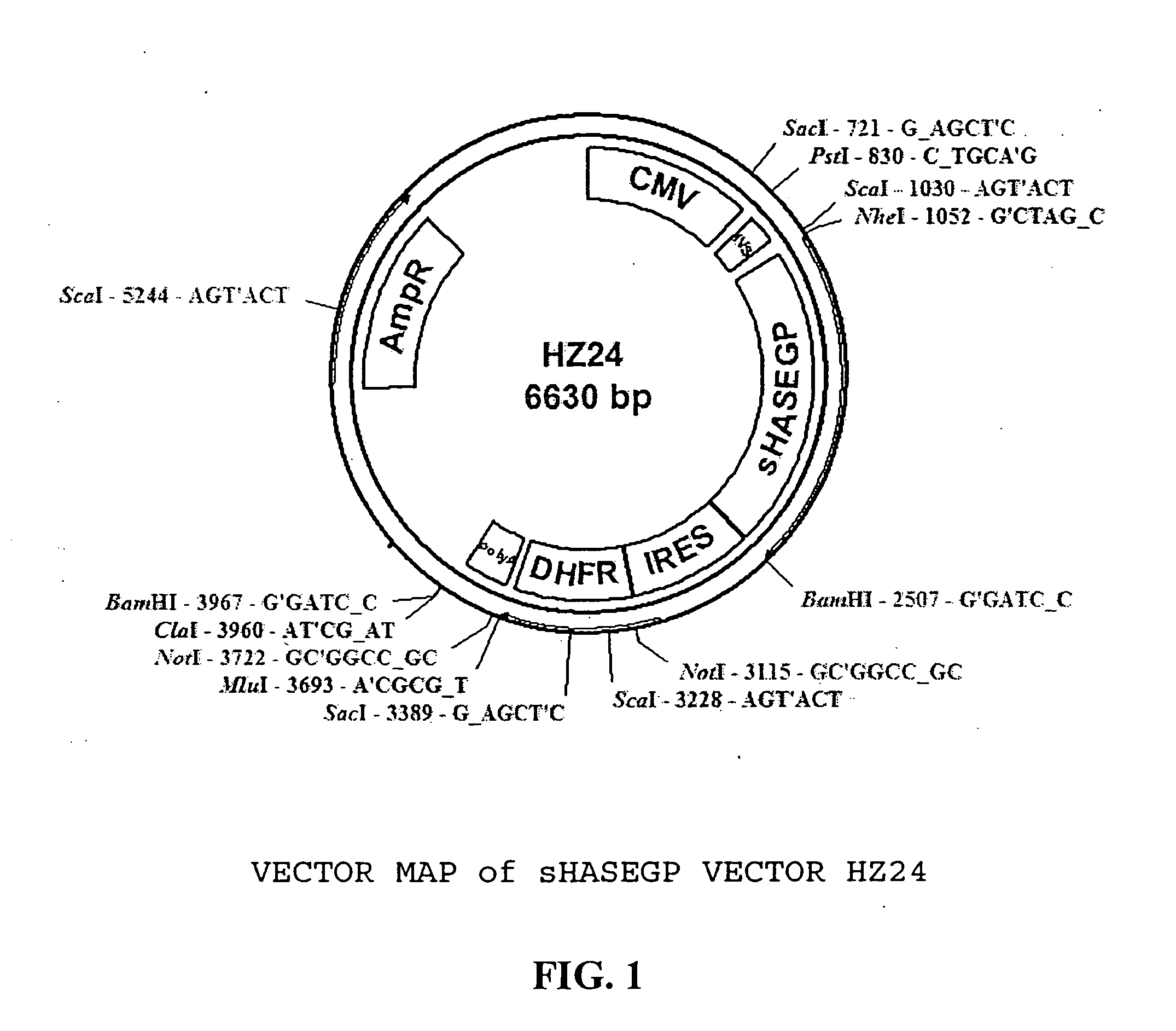
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
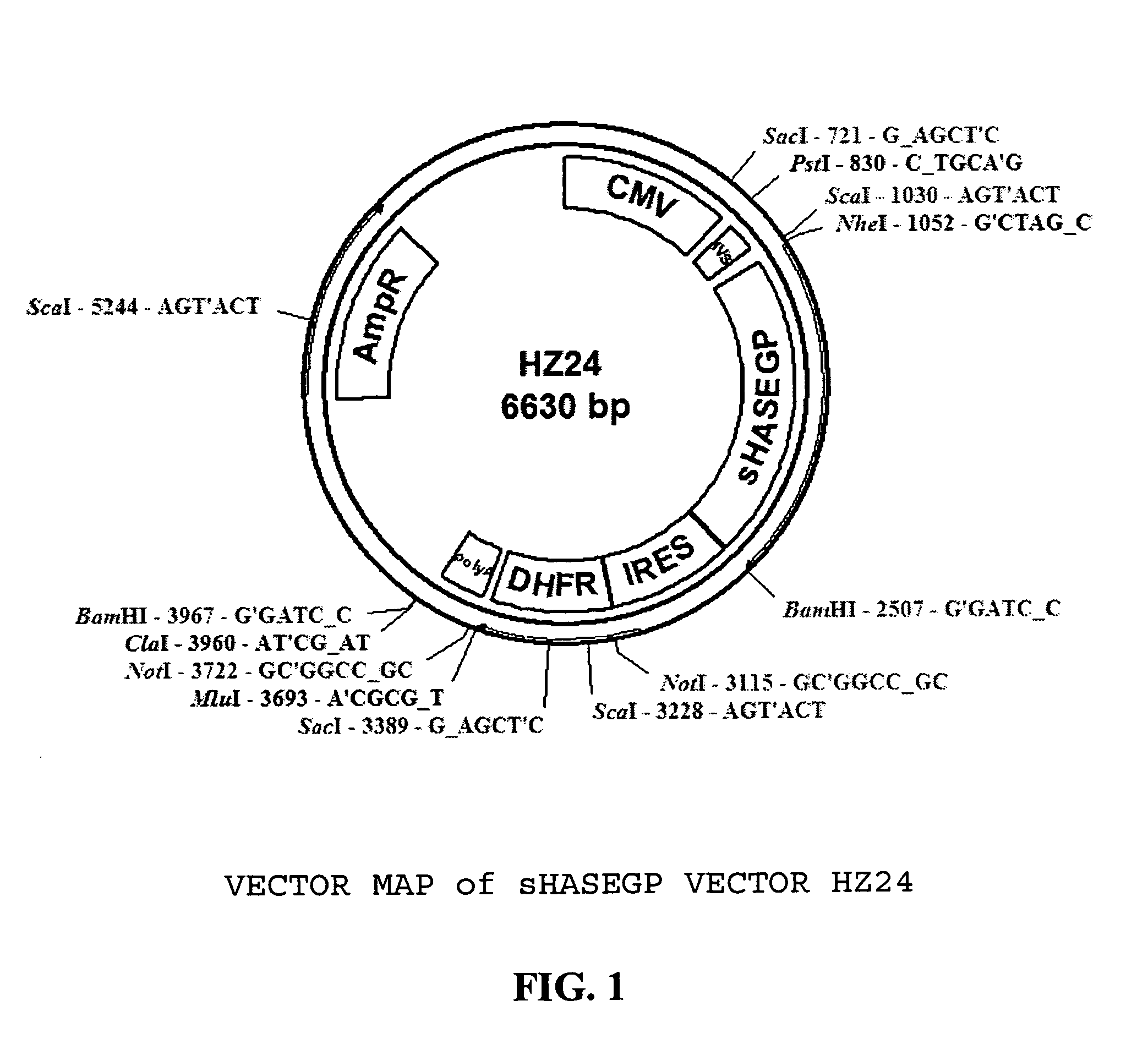
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
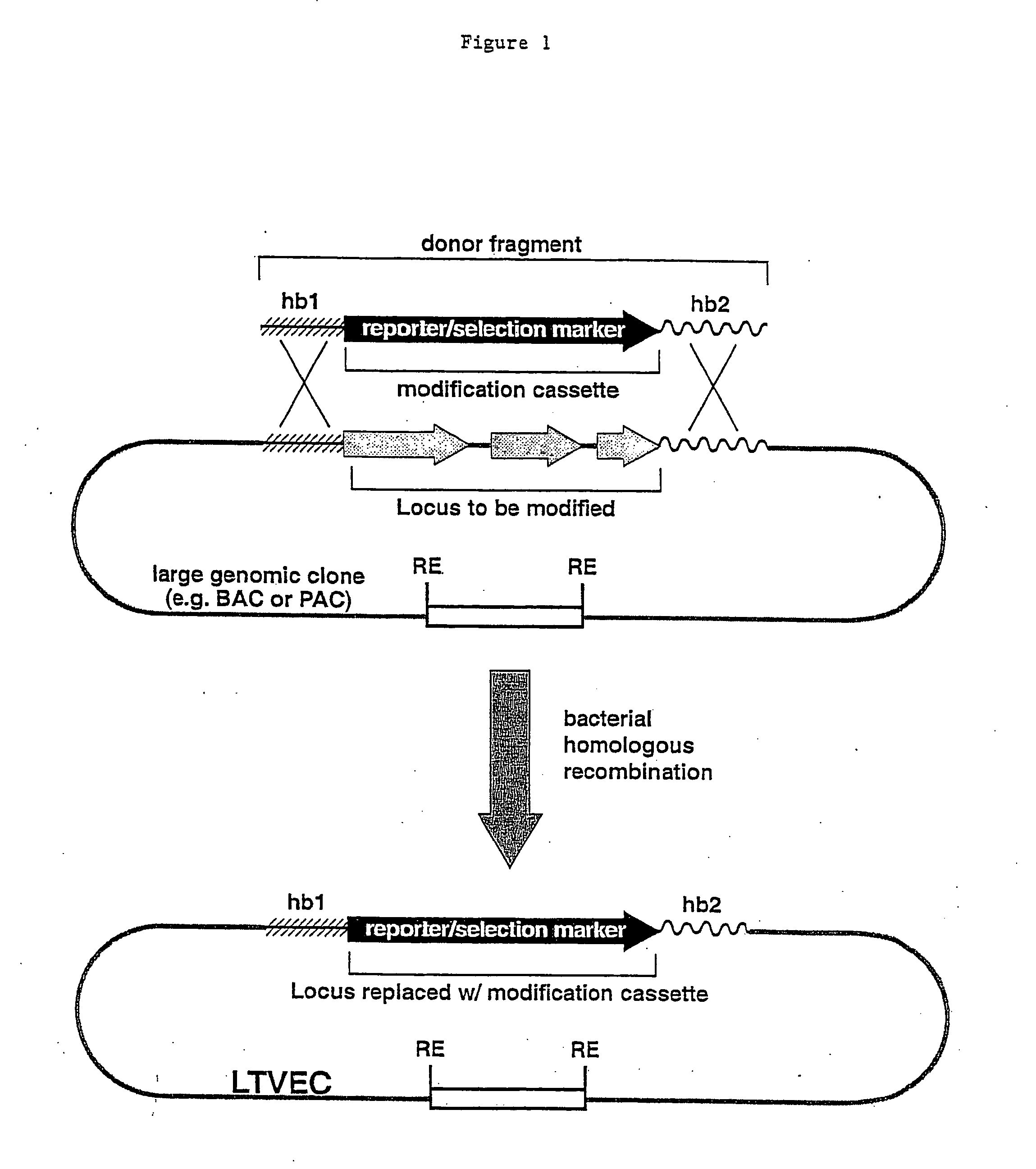
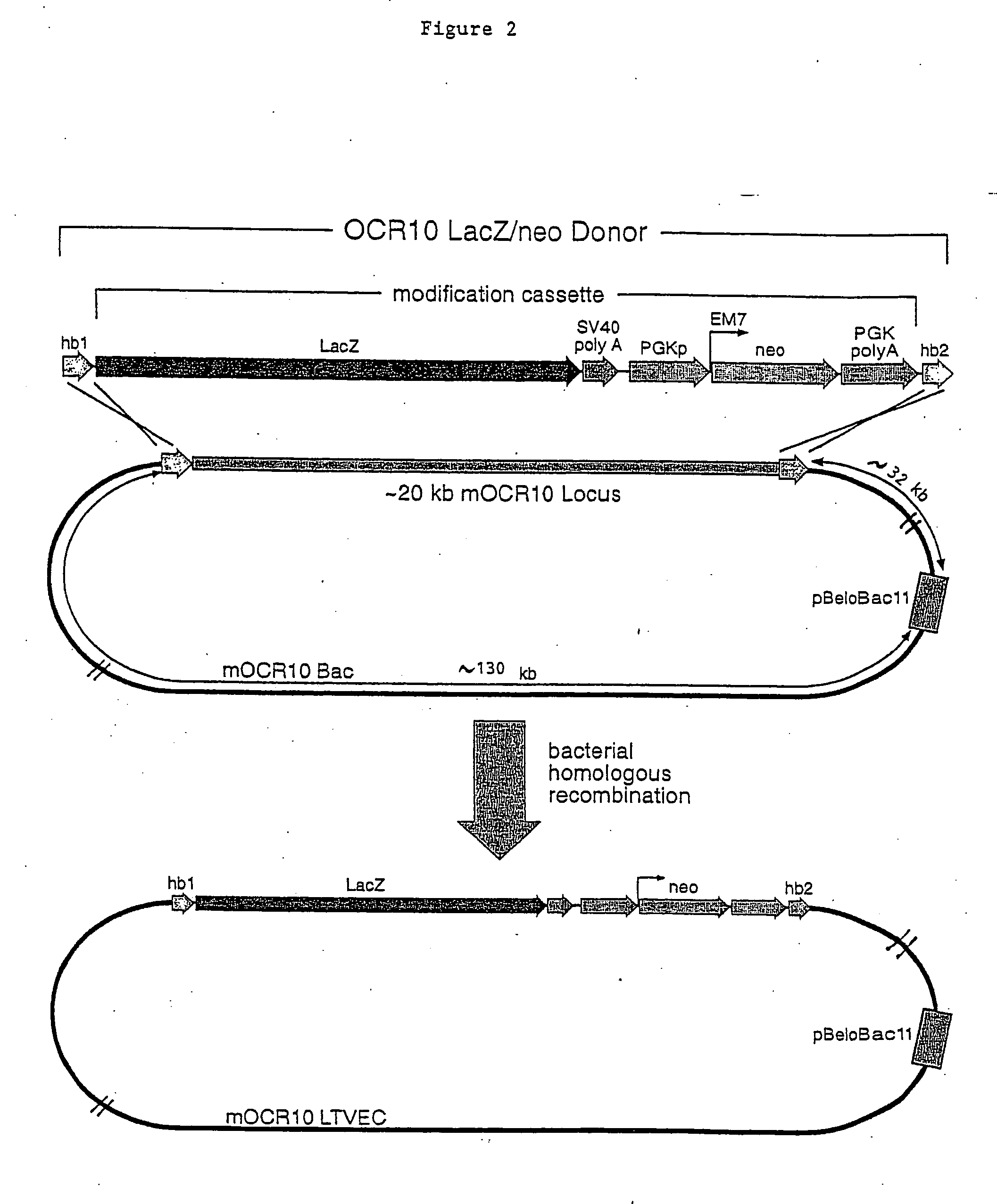
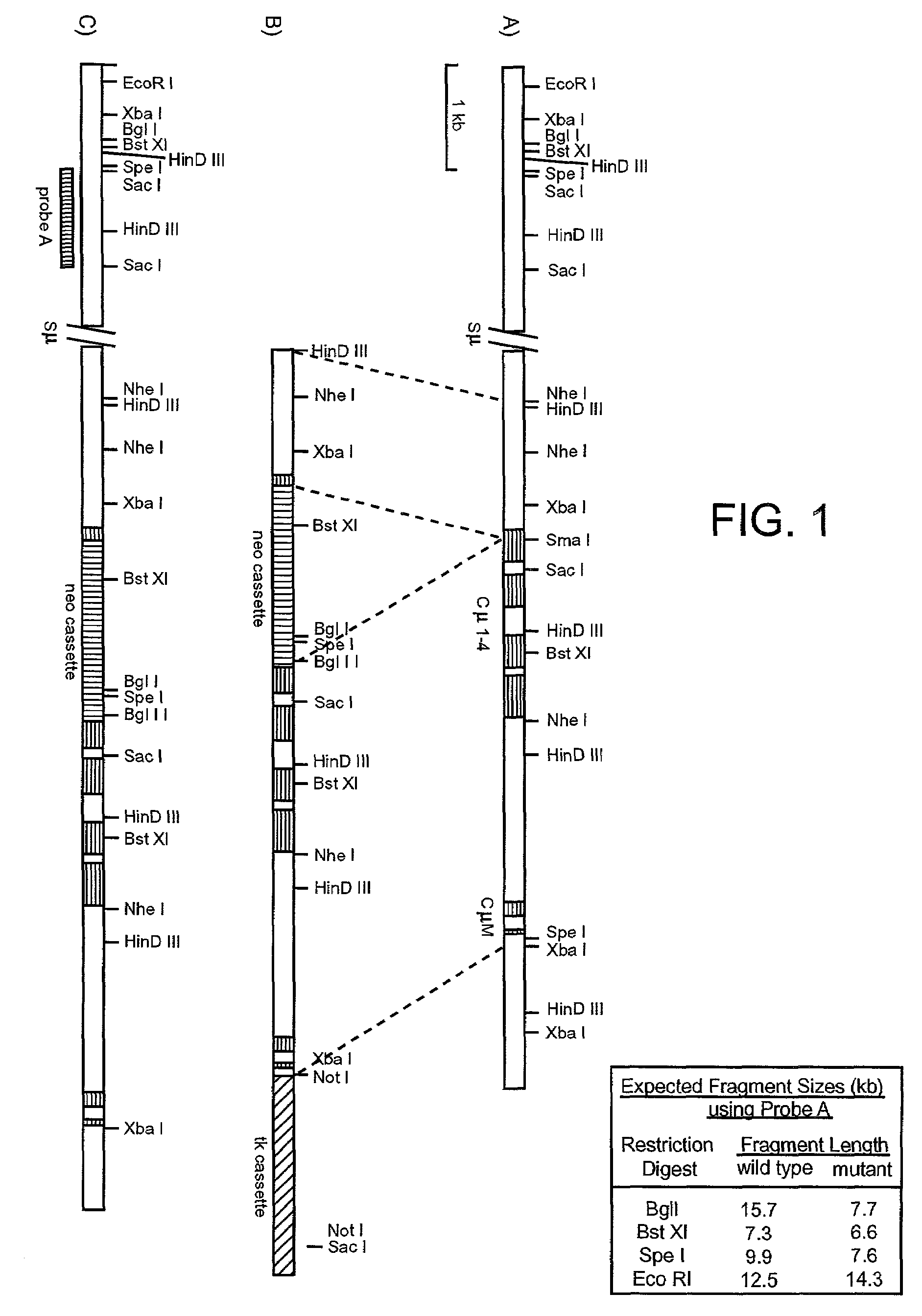
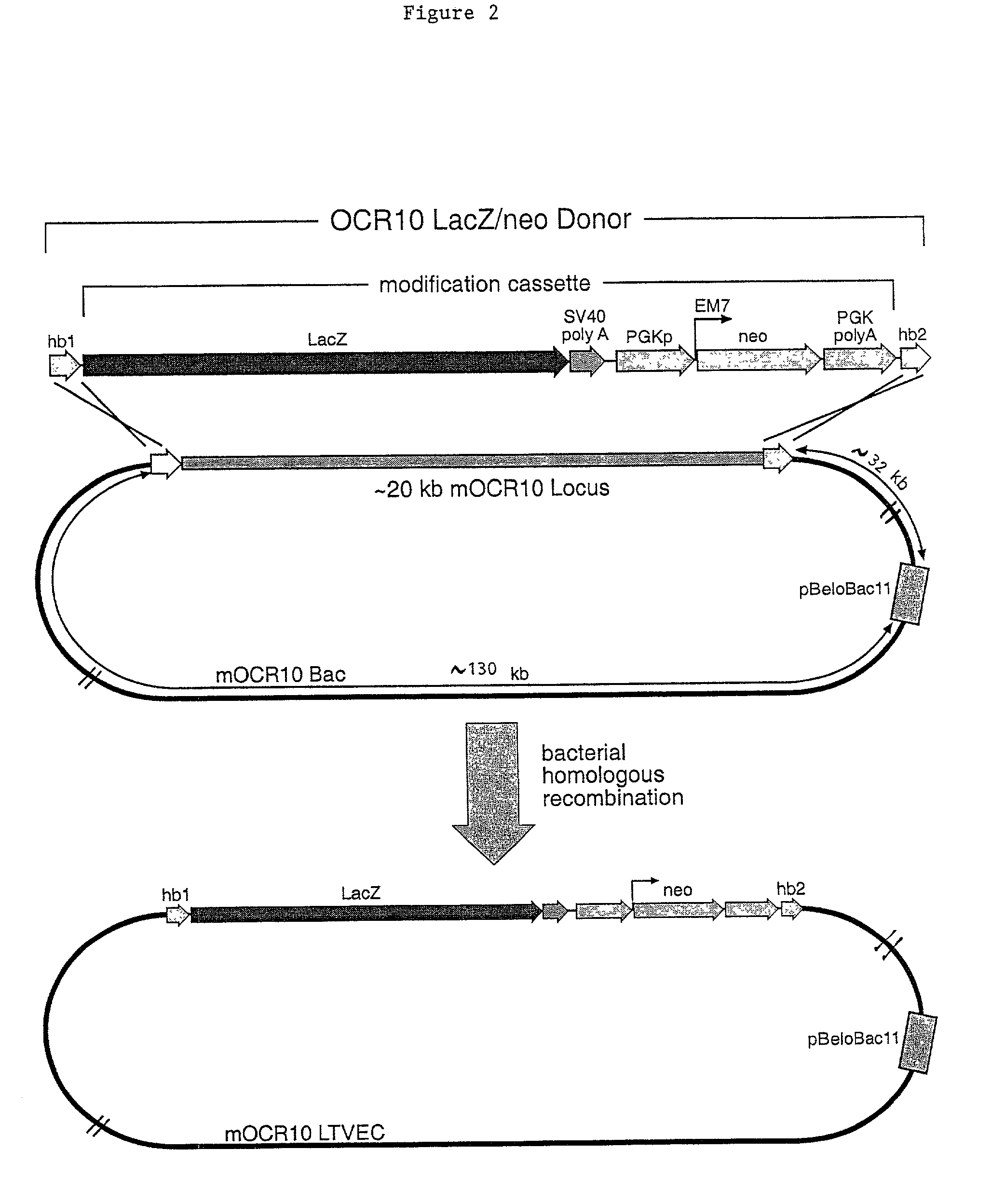
Methods of modifying eukaryotic cells
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous gene(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
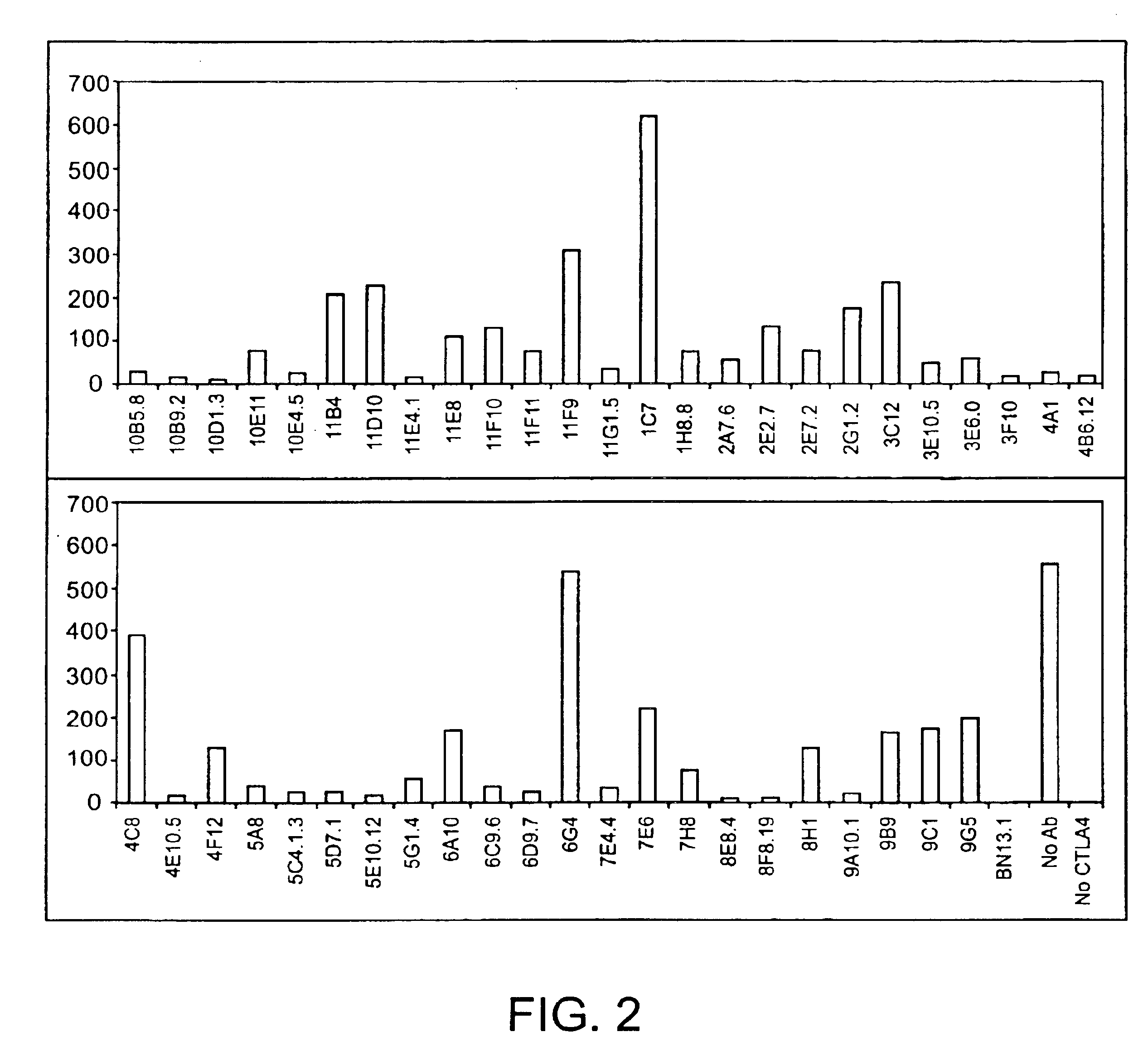
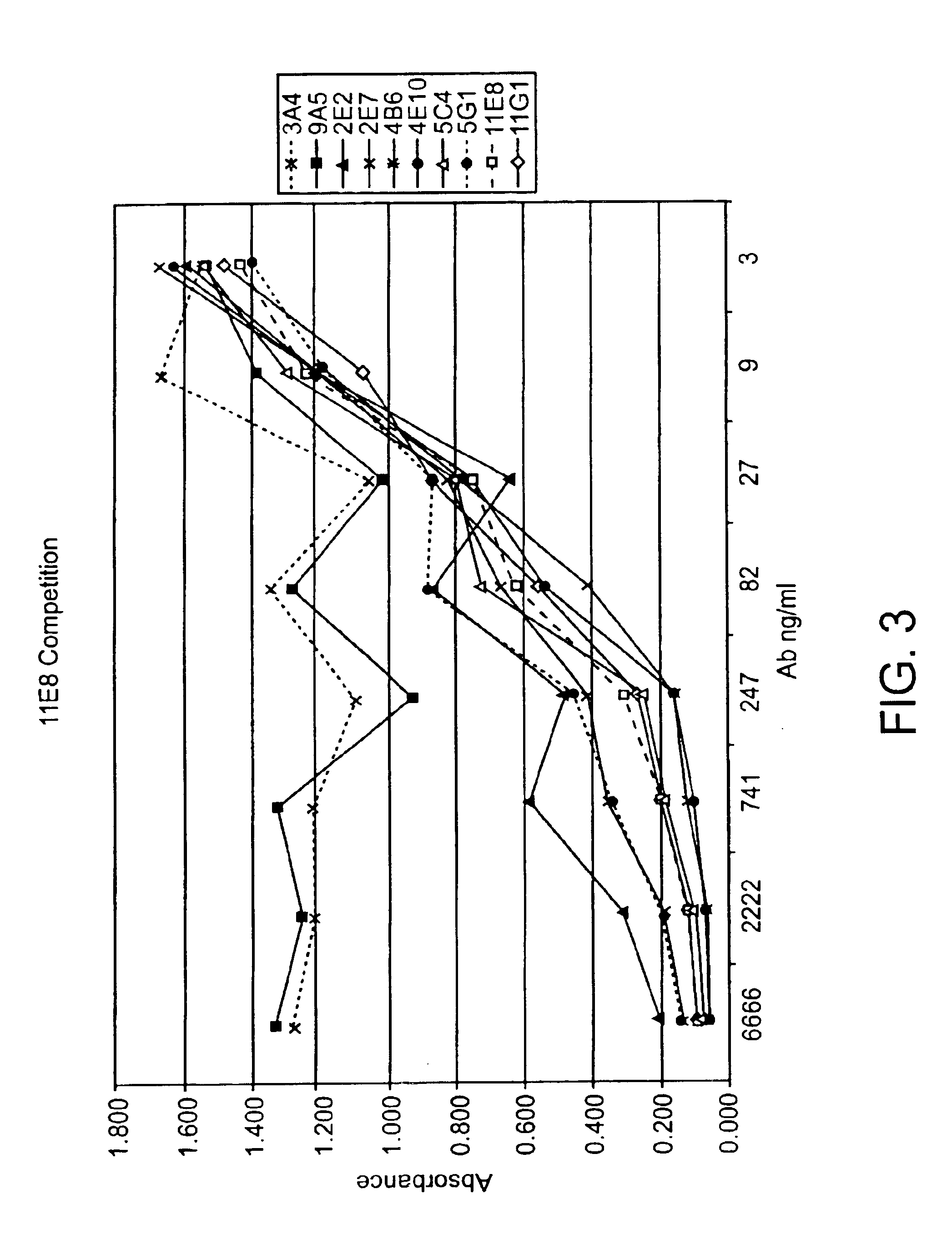
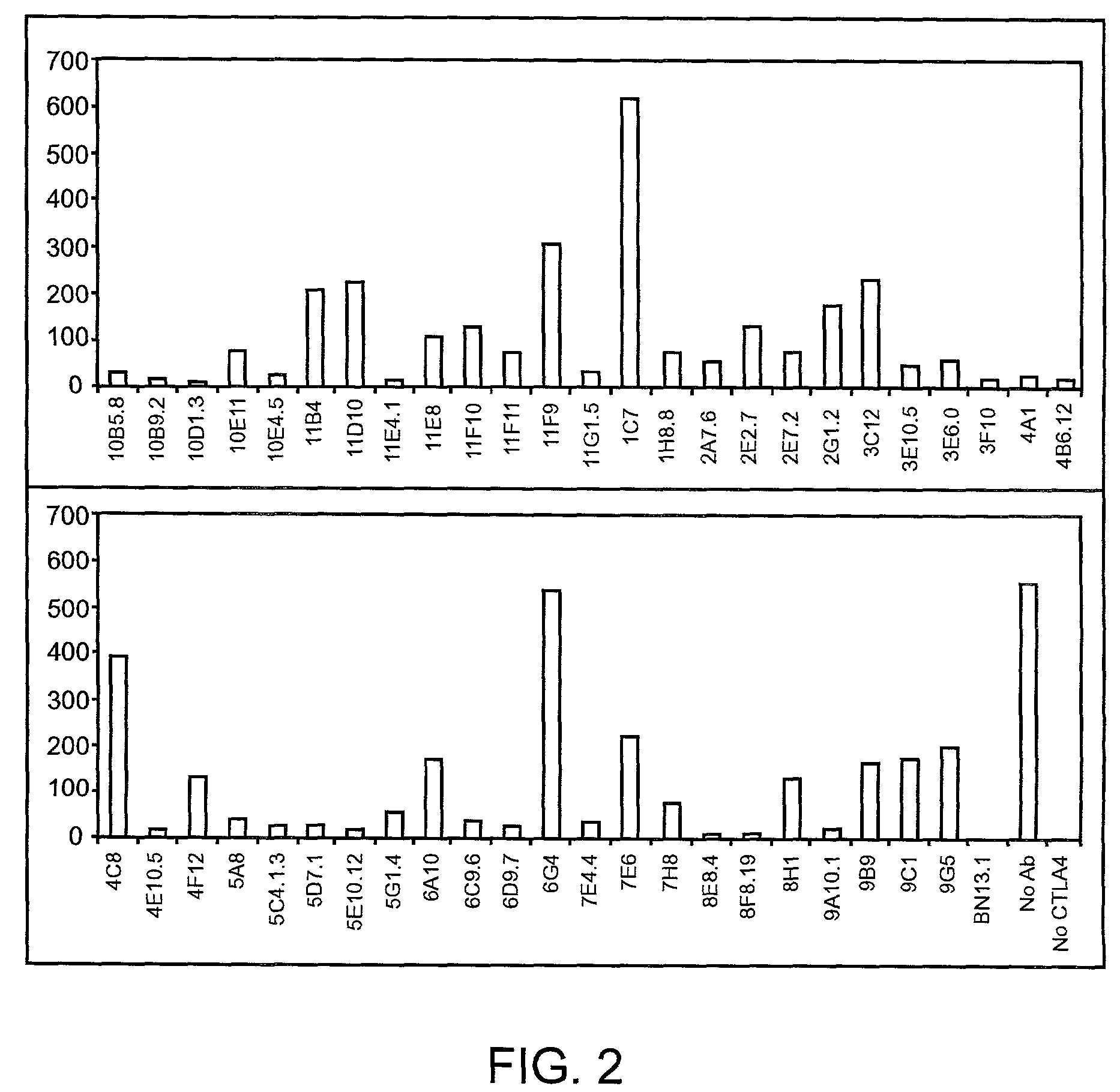
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
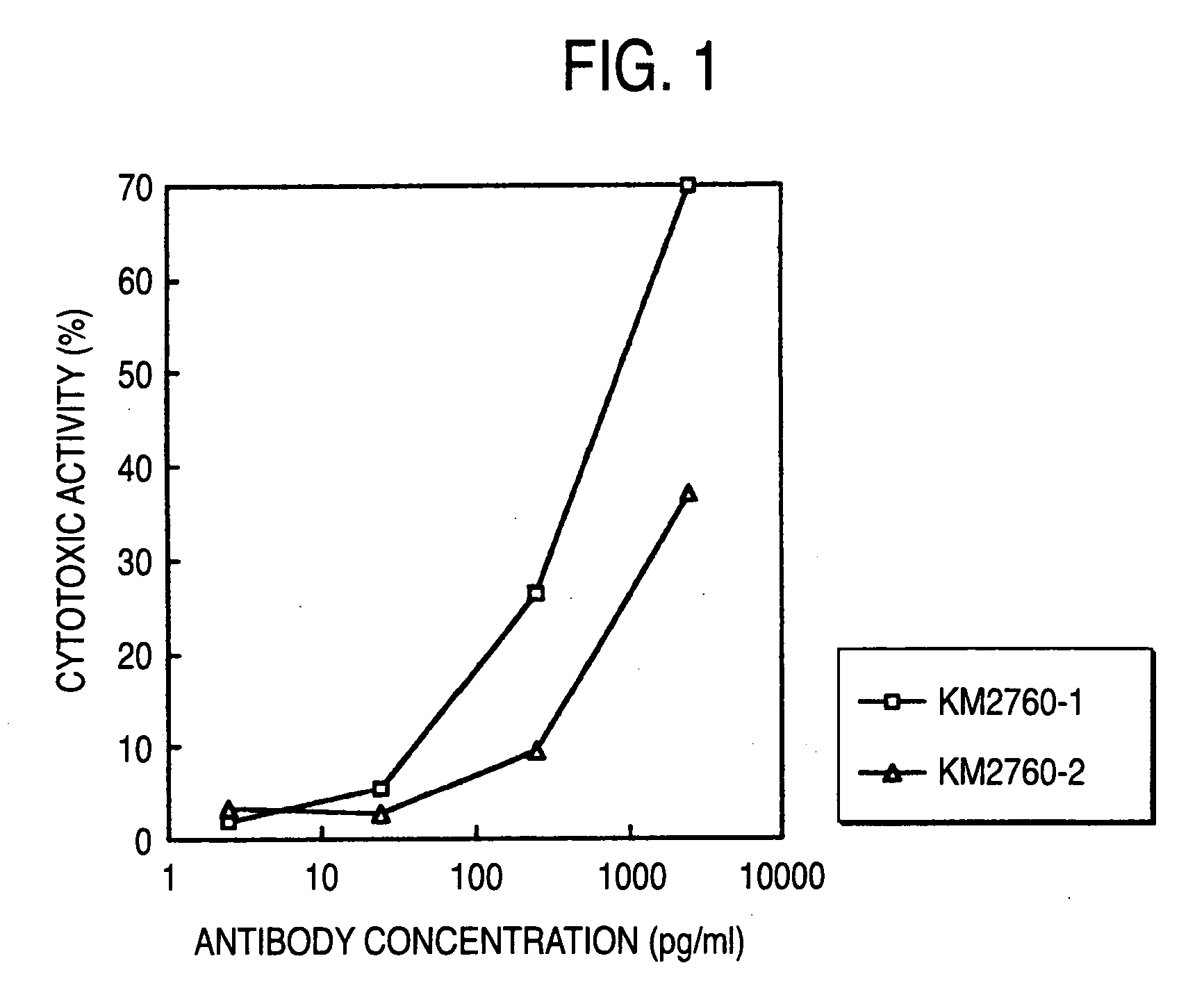
Cells producing antibody compositions with increased antibody dependent cytotoxic activity
The present invention relates to a cell for the production of an antibody molecule such as an antibody useful for various diseases having high antibody-dependent cell-mediated cytotoxic activity, a fragment of the antibody and a fusion protein having the Fc region of the antibody or the like, a method for producing an antibody composition using the cell, the antibody composition and use thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Receptor specific transepithelial transport of therapeutics
InactiveUS6030613AEffective strategyImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
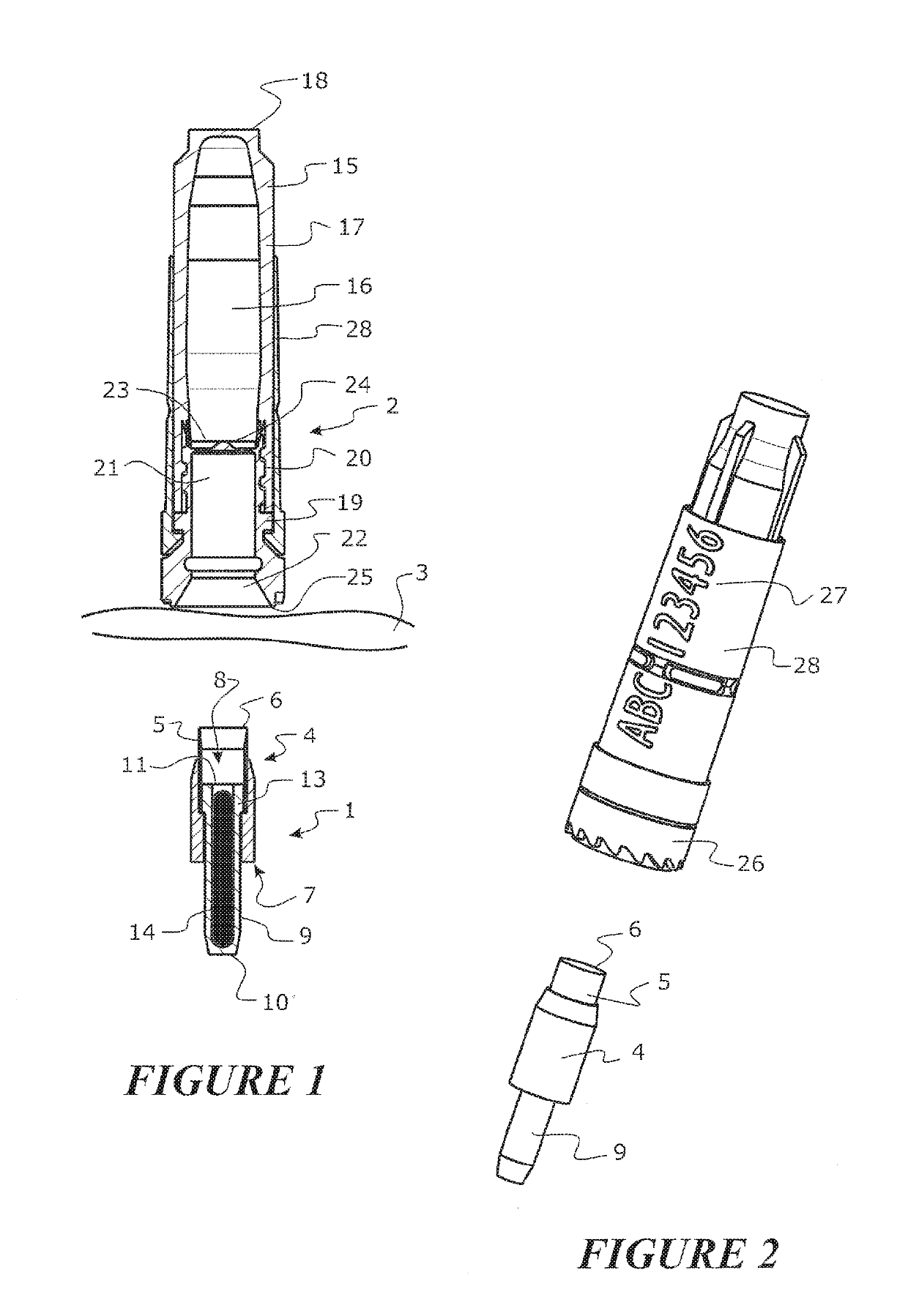
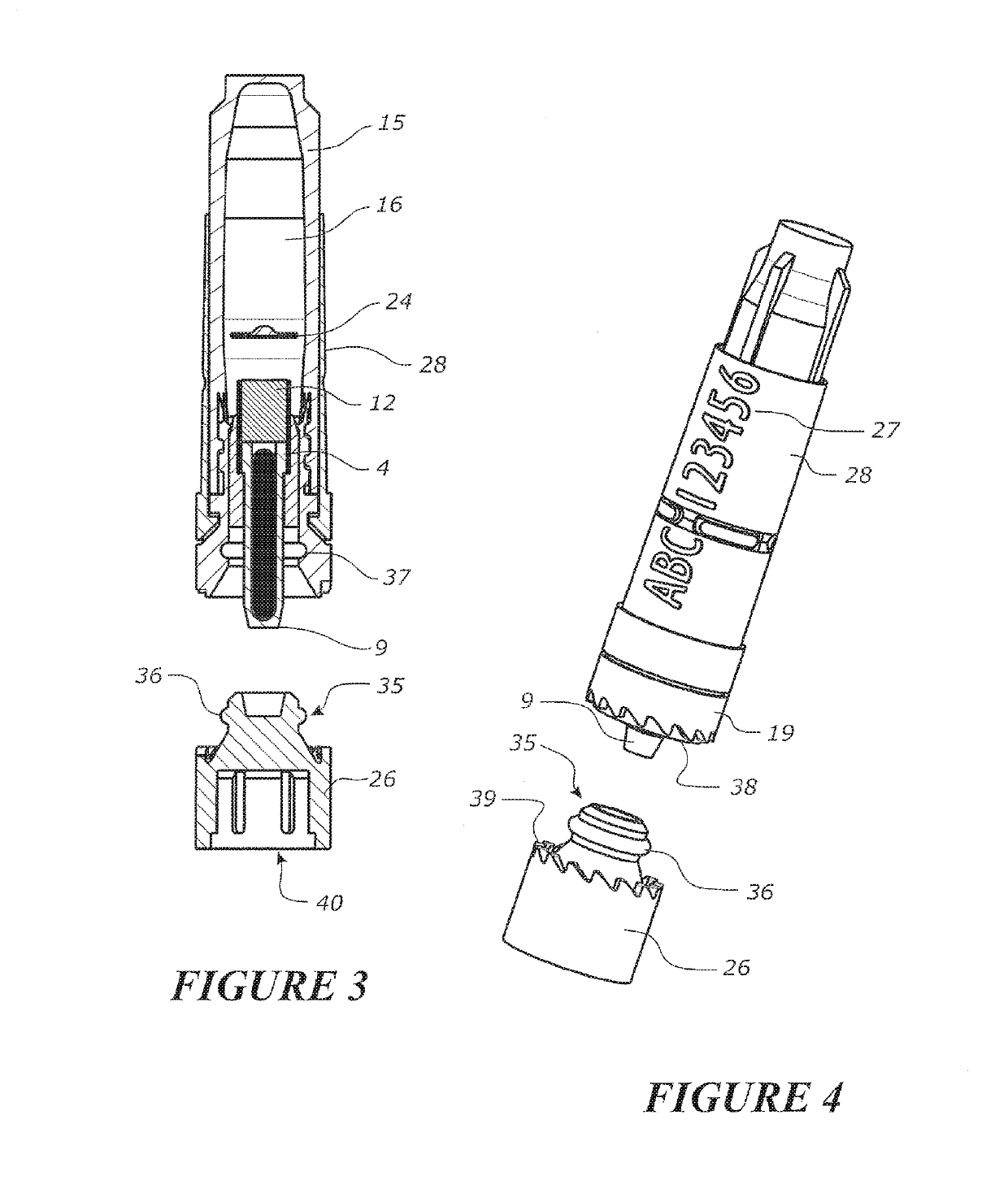
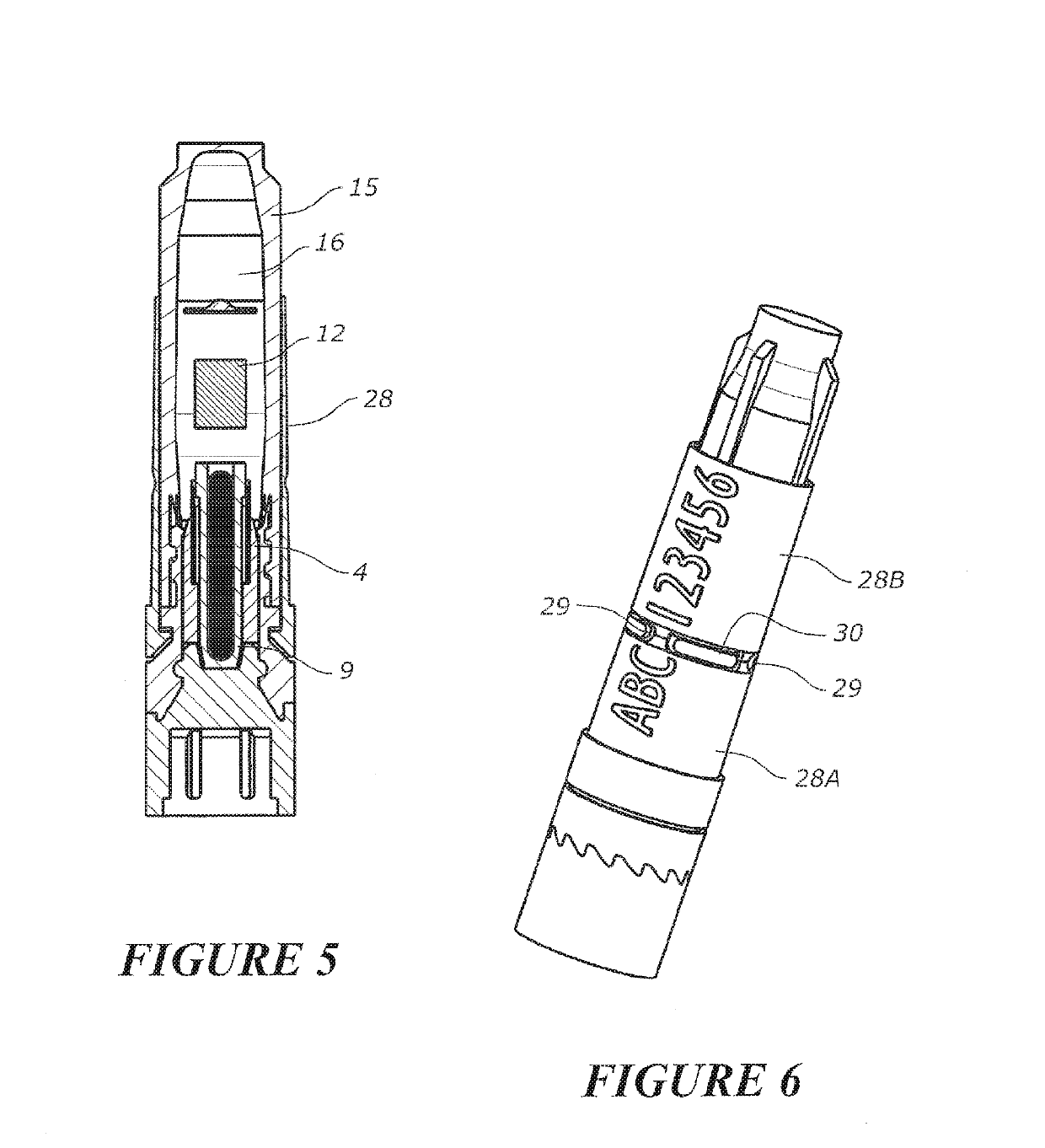
Tool for collecting a sample of animal tissue
ActiveUS9955954B2Reduce riskSurgical needlesVaccination/ovulation diagnosticsEngineeringMechanical engineering
Owner:ALLFLEX EURO SAS
Human CTLA-4 antibodies and their uses
InactiveUS7605238B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesVirology
The present invention provides human sequence antibodies against human CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
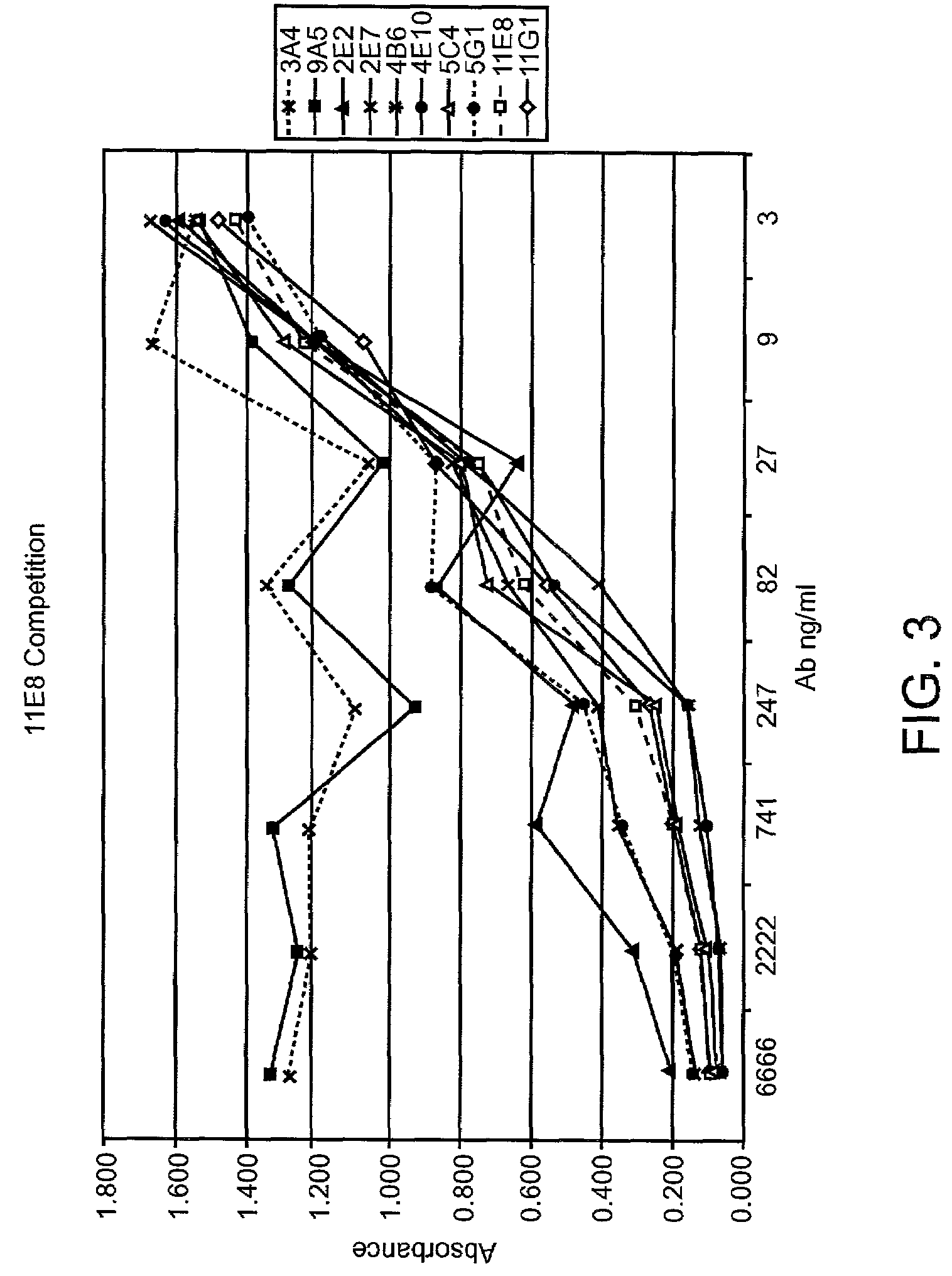
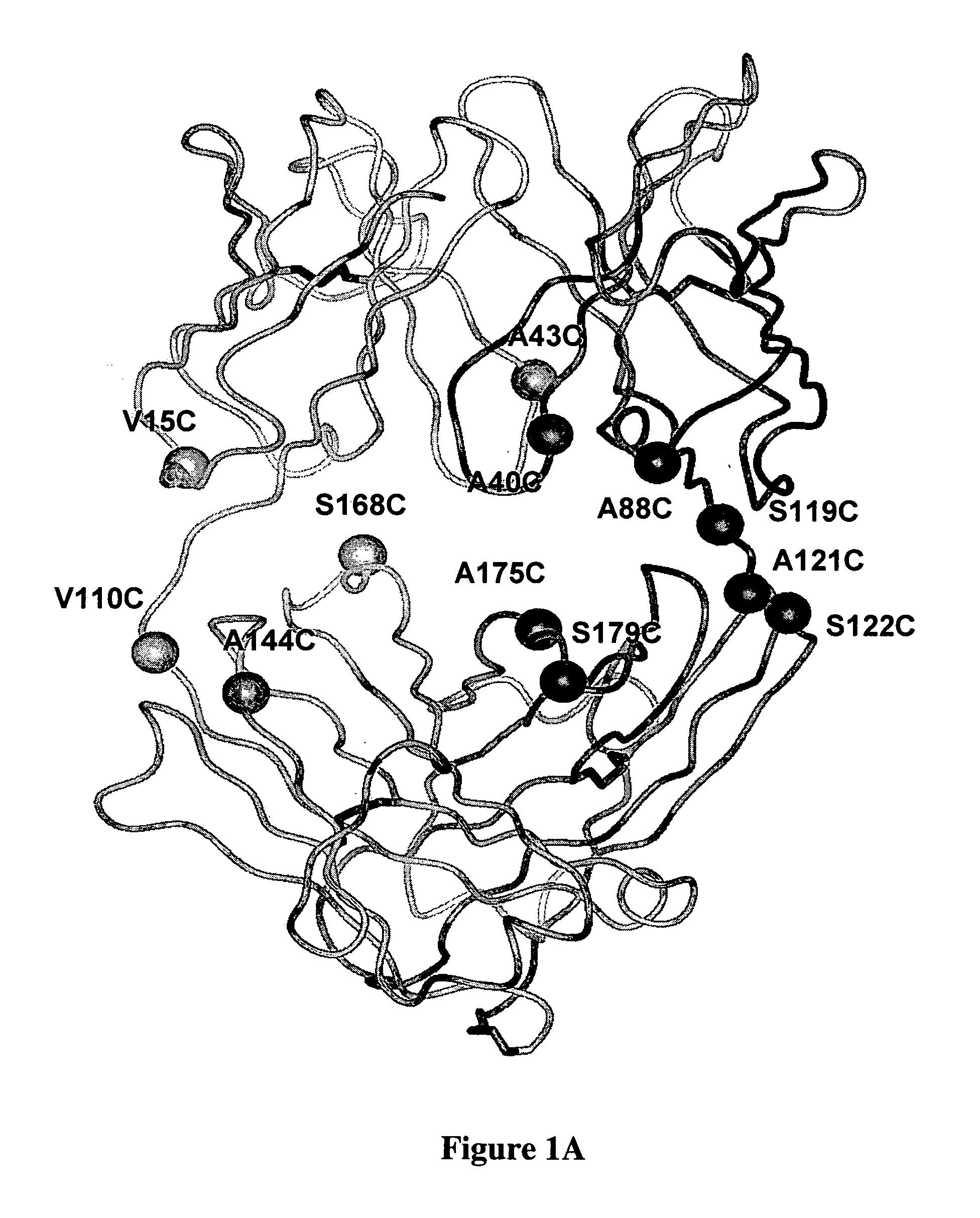
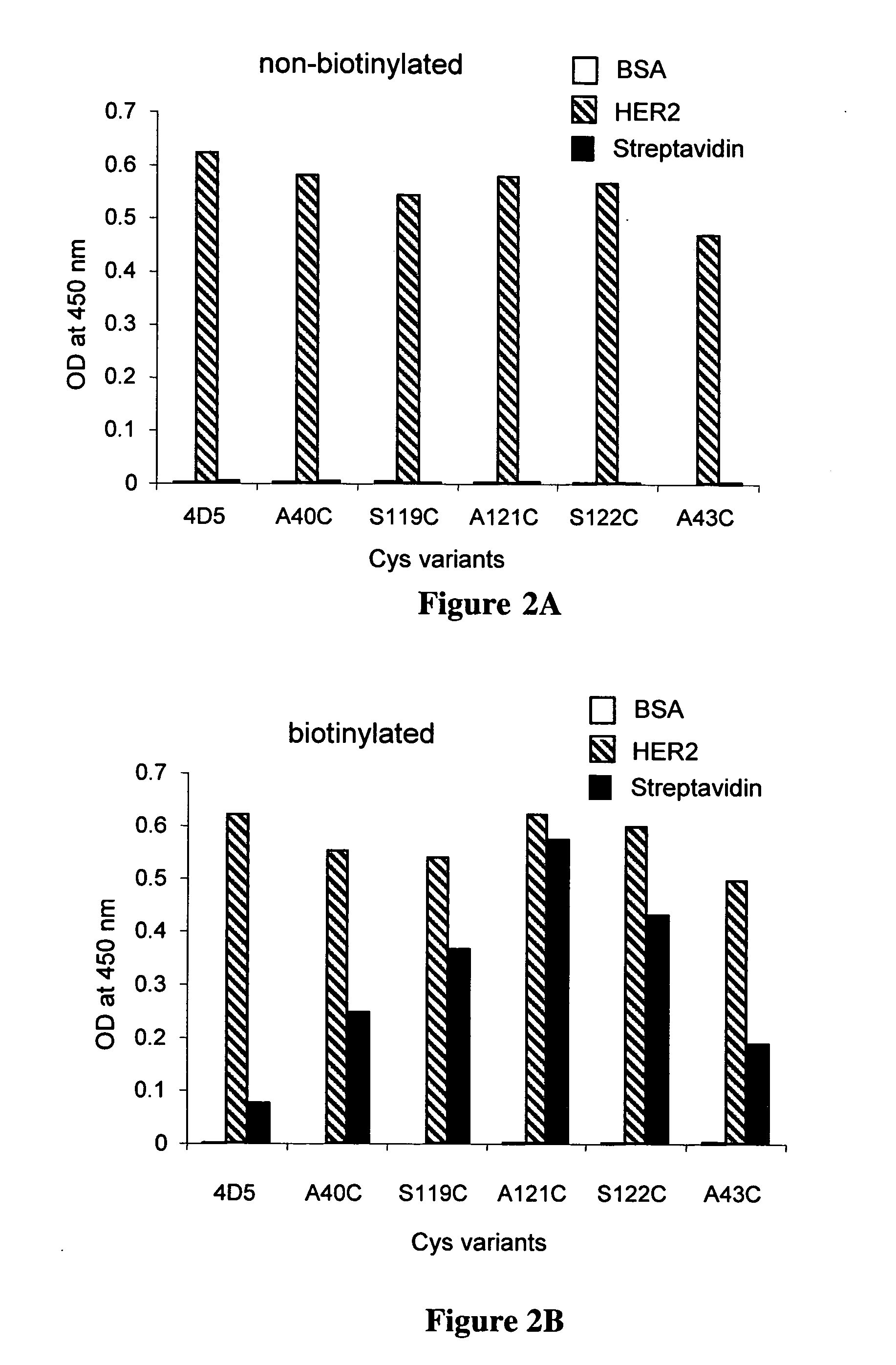
Cysteine engineered antibodies and conjugates
ActiveUS20070092940A1Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkCysteine thiolate
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab), optionally with an albumin-binding peptide (ABP) sequence, are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered antibody-drug conjugates having Formula I: Ab-(L-D)p I where p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Nucleic acids and polypeptides of invertebrate TWIK channels and methods of use
Tandem pore domain weak inward rectifying K+ (TWIK) channel nucleic acids and proteins that have been isolated from Drosophila melanogaster and Leptinotarsa are described. The TWIK channel nucleic acids and proteins can be used to genetically modify metazoan invertebrate organisms, such as insects, coelomates, and pseudocoelomates, or cultured cells, resulting in TWIK channel expression or mis-expression. The genetically modified organisms or cells can be used in screening assays to identify candidate compounds which are potential pesticidal agents or therapeutics that interact with TWIK channel proteins. They can also be used in methods for studying TWIK channel activity and identifying other genes that modulate the function of, or interact with, the TWIK channel gene.
Owner:EXELIXIS PHARMA
Transgenic non-human animals for producing chimeric antibodies
InactiveUS20060015957A1Inhibit expressionEasy to switchImmunoglobulinsGenetic engineeringAntigenHuman animal
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
Methods of modifying eukaryotic cells
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous gene(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
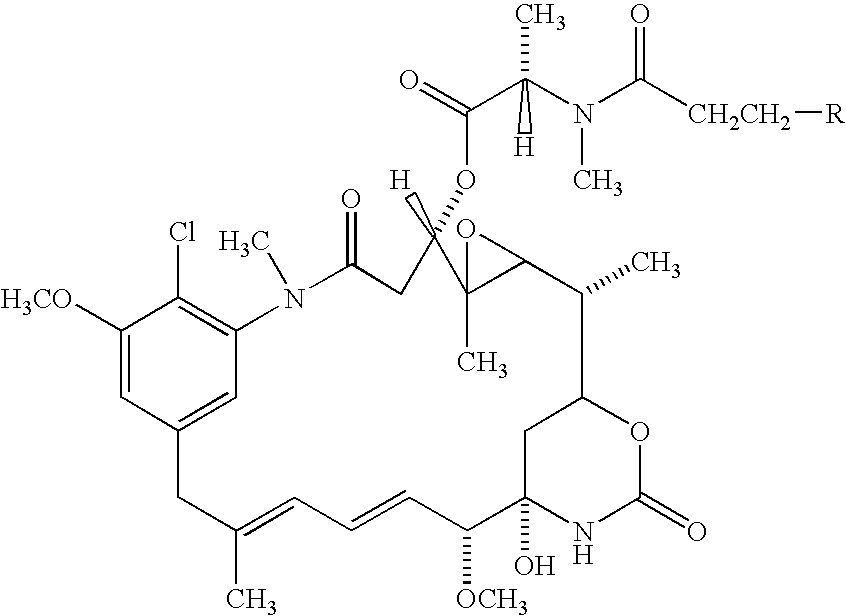
Methods of treatment using anti-ErbB antibody-maytansinoid conjugates
ActiveUS7097840B2Superior clinical activityBetter objective response rateOrganic active ingredientsPharmaceutical delivery mechanismMedicineCancer therapy
The application concerns methods of treatment using anti-ErbB receptor antibody-maytansinoid conjugates, and articles of manufacture suitable for use in such methods. In particular, the invention concerns ErbB receptor-directed cancer therapies, using anri-ErbB receptor antibody-maytansinoid conjugates.
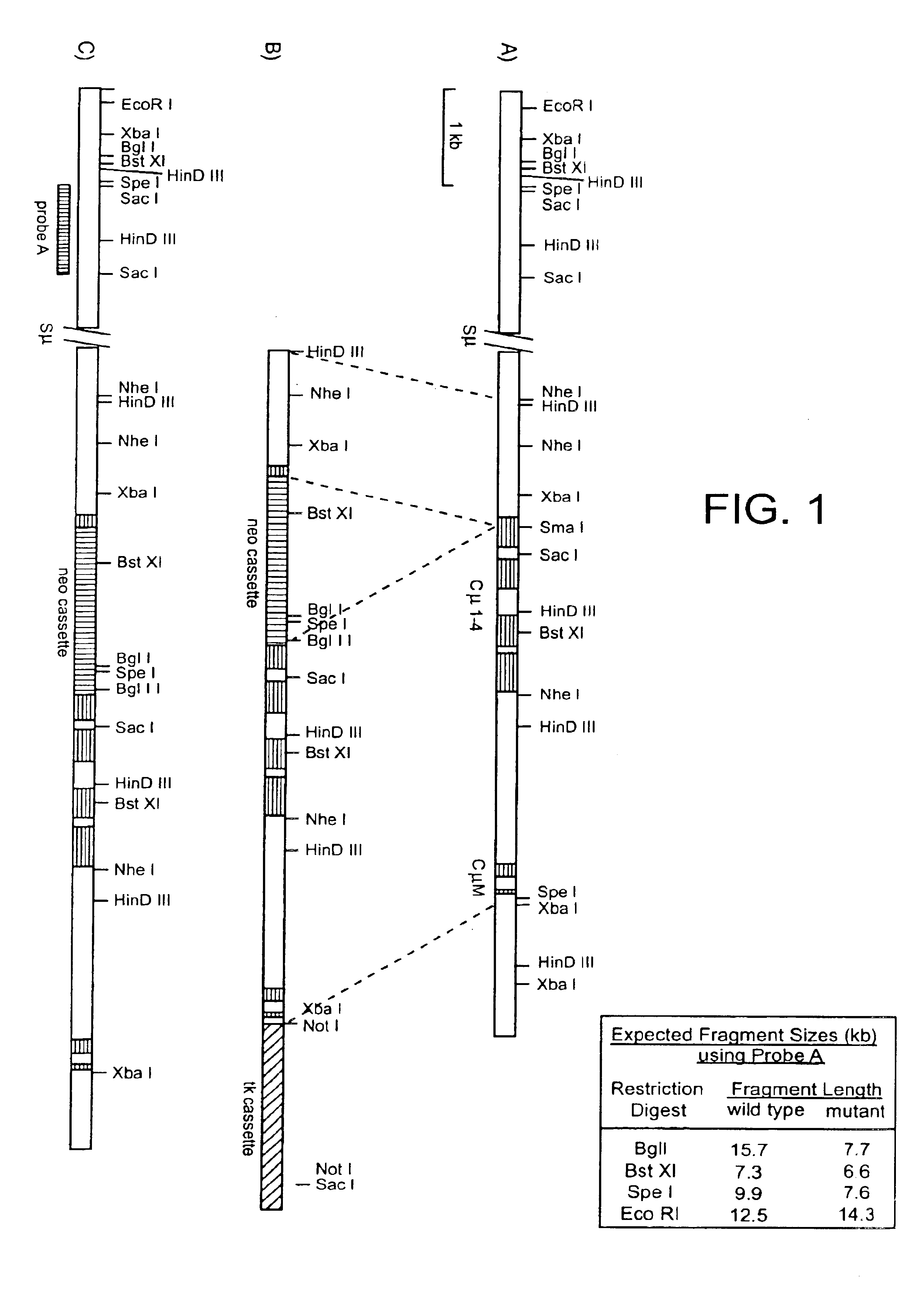
Targeted modification of rat genome
Compositions and methods are provided for modifying a rat genomic locus of interest using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Compositions and methods for generating a genetically modified rat comprising one or more targeted genetic modifications in their germline are also provided. Compositions and methods are provided which comprise a genetically modified rat or rat cell comprising a targeted genetic modification in the rat interleukin-2 receptor gamma locus, the rat ApoE locus, the rat Rag2 locus, the rat Rag1 locus and / or the rat Rag2 / Rag1 locus. The various methods and compositions provided herein allows for these modified loci to be transmitted through the germline.
Owner:REGENERON PHARM INC
Method and device for withdrawing biological samples
InactiveUS6659338B1Eliminate disadvantagesExtensive automationWithdrawing sample devicesSurgeryWork cycleBiomedical engineering
The invention relates to a method and device for withdrawing biological samples. The device has a receptacle which can receive one or several covers for sample containers, another receptacle which can receive one or several sample containers, and a mechanism. Said mechanism joins the covers and containers together during a working cycle in which the biological sample is withdrawn either through the cover or the sample container to a test capsule.
Owner:CAISLEY INT
Methods and Compositions for the Targeted Modification of a Genome
Compositions and methods are provided for modifying a genomic locus of interest in a eukaryotic cell, a mammalian cell, a human cell or a non-human mammalian cell using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Further methods combine the use of the LTVEC with a CRISPR / Cas system. Compositions and methods for generating a genetically modified non-human animal comprising one or more targeted genetic modifications in their germline are also provided.
Owner:REGENERON PHARM INC
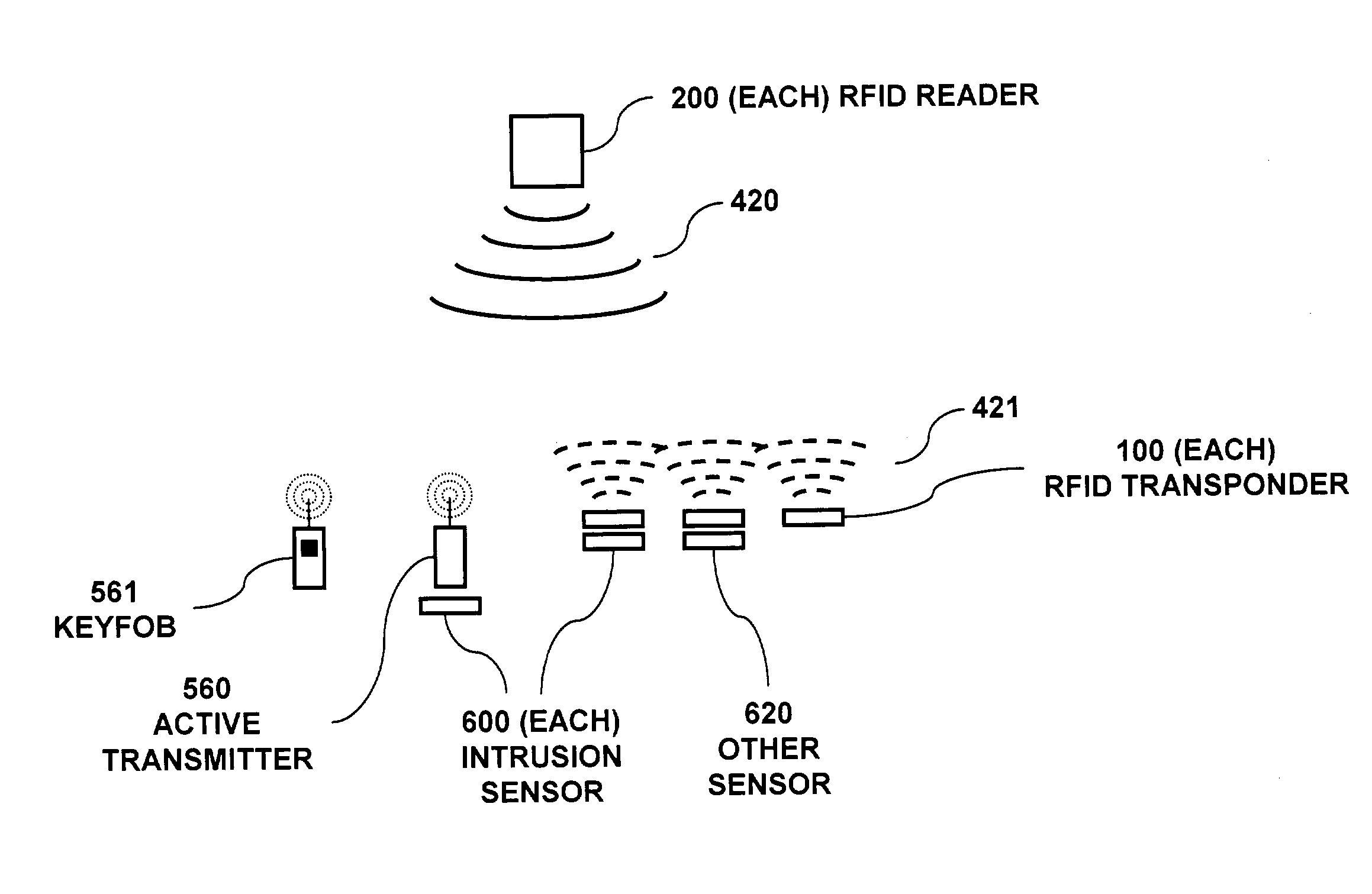
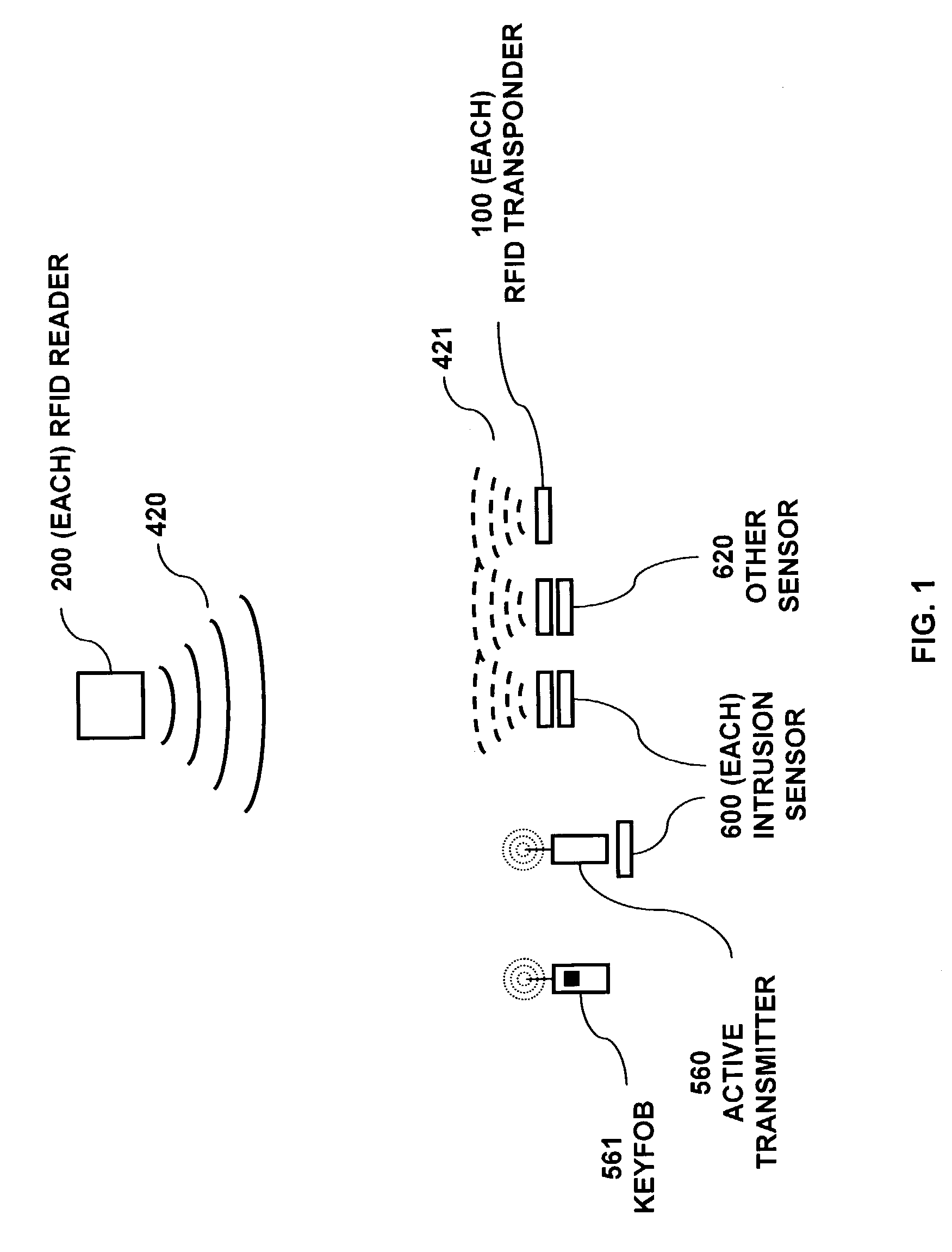
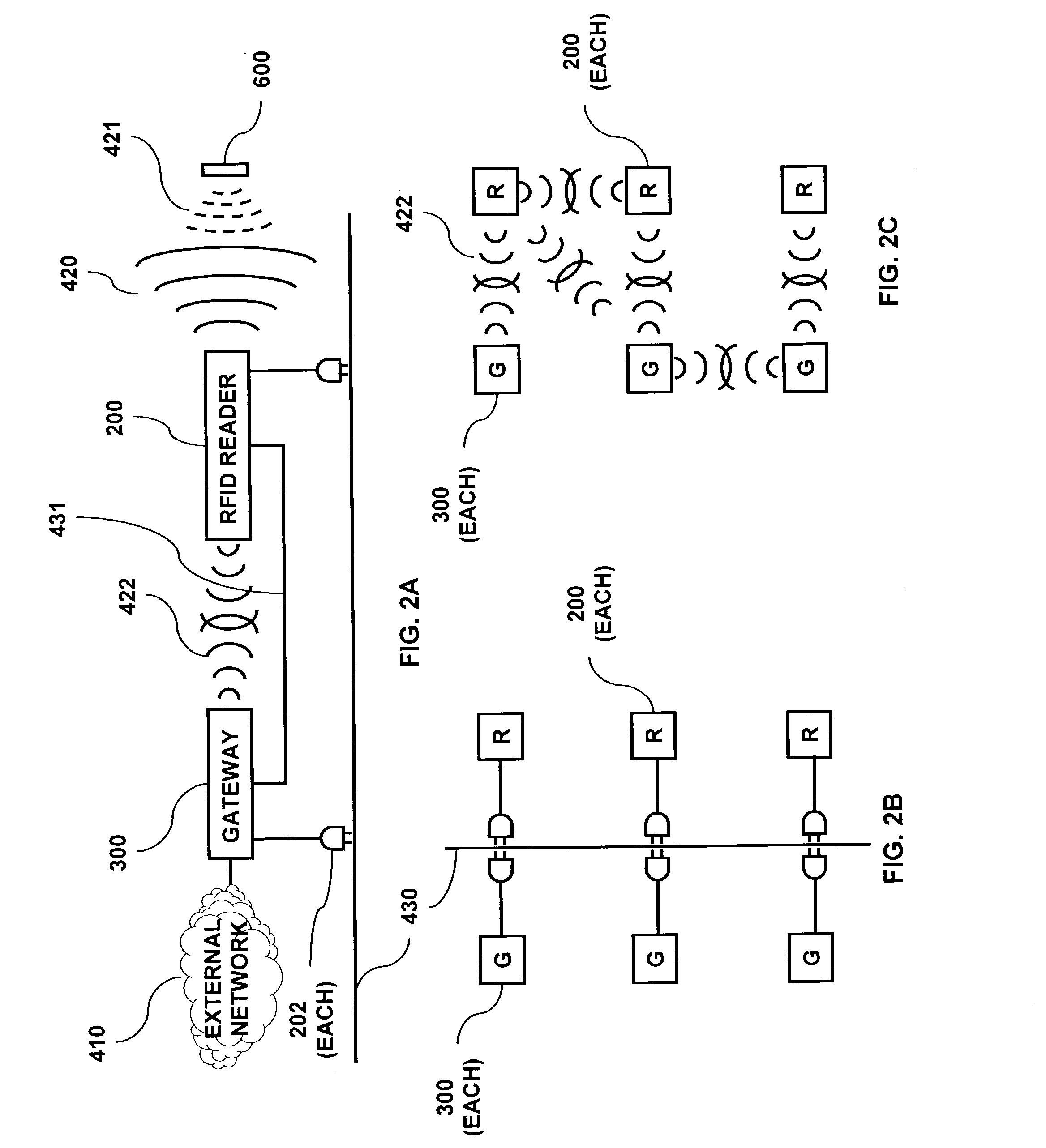
RFID based security network
InactiveUS7019639B2Improve reliabilityLow costBurglar alarm by openingFrequency-division multiplex detailsNetwork Communication ProtocolsElectric power
A security network for a building using at least one RFID reader to communicate with at least one RFID transponder to provide the radio link between each of a number of openings and a control function capable of causing an alert in the event of an intrusion. A gateway provides an interface between the security network and various external networks. The control function can be located in either or both of the RFID reader and the gateway. The RFID transponder is connected to an intrusion sensor. The gateway can communicate with the RFID reader using active RF communications, power-line communications protocol, or hardwire connection. The RFID transponder can contain an energy store. The RFID reader contains means for transferring power to an RFID transponder for the purpose of charging any energy store. The security network can contain more than one RFID reader.
Owner:ADT US HLDG INC
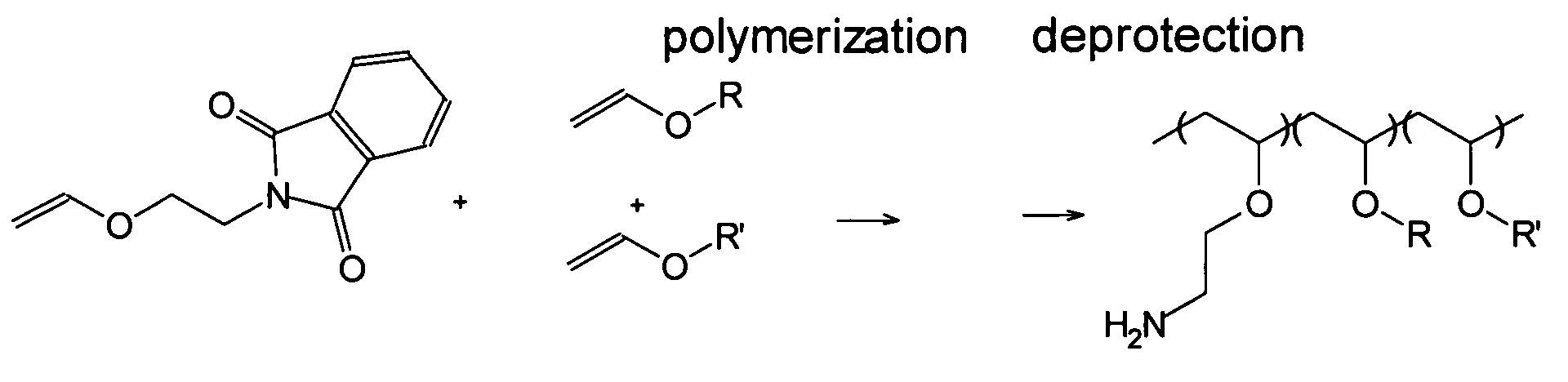
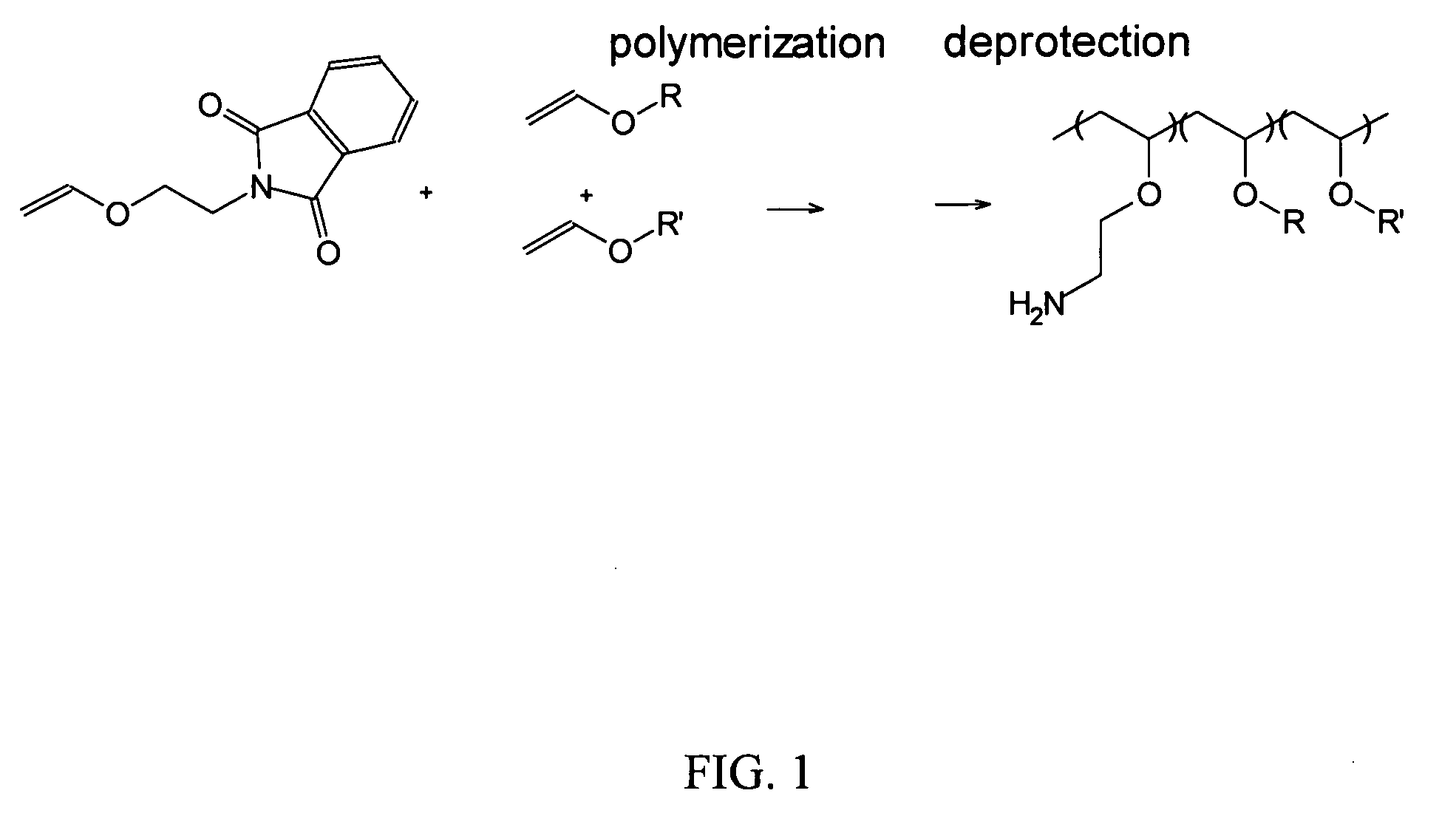
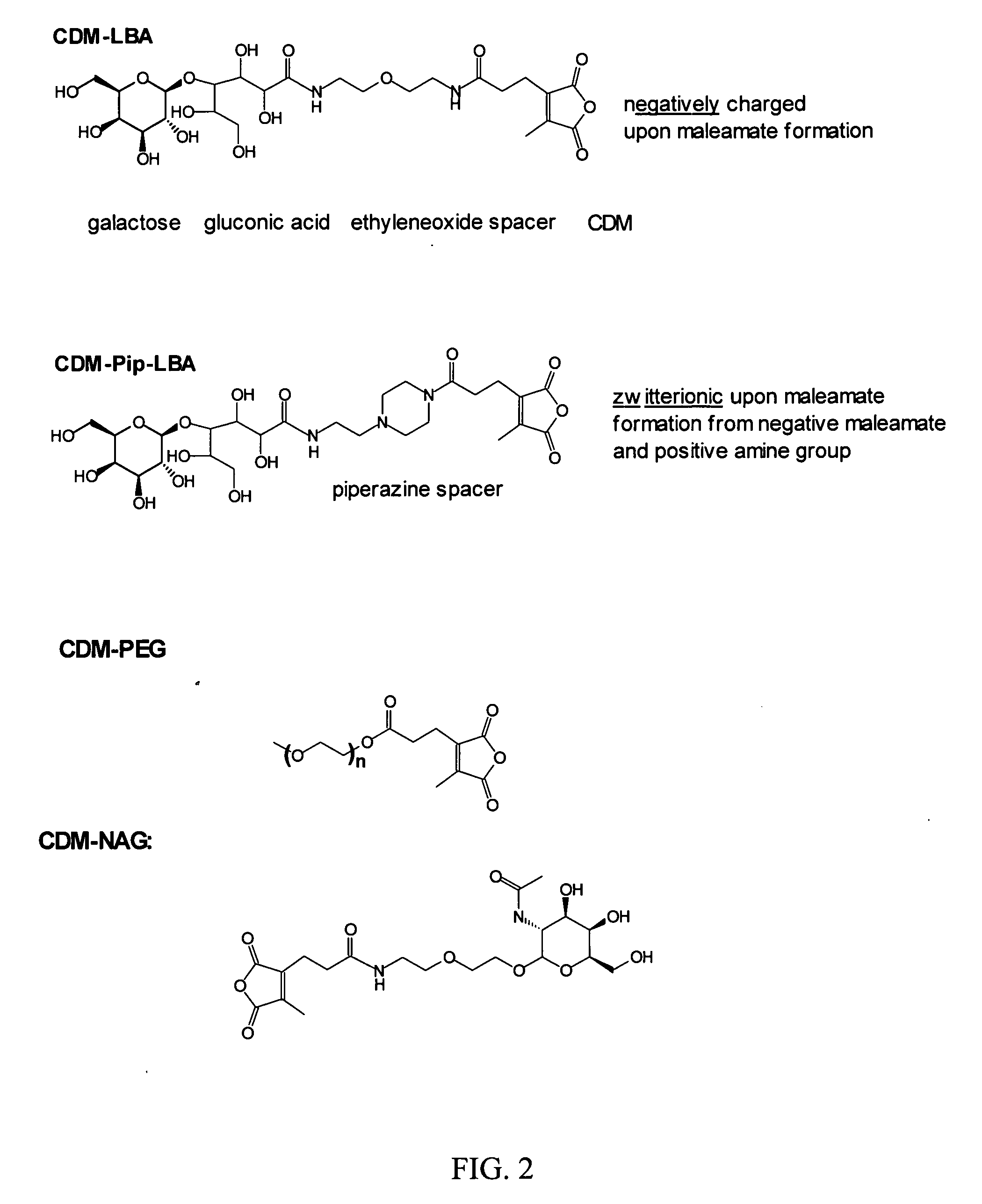
Polyconjugates for In Vivo Delivery of Polynucleotides
ActiveUS20080152661A1Reduce aggregationEnhances transfection activityPowder deliveryPeptide/protein ingredientsDelivery vehicleNucleotide
The present invention is directed to compounds, compositions, and methods useful for delivering polynucleotides or other cell-impermeable molecules to mammalian cells. Described are polyconjugates systems that incorporate targeting, anti-opsonization, anti-aggregation, and transfection activities into small biocompatible in vivo delivery vehicles. The use of multiple reversible linkages connecting component parts provides for physiologically responsive activity modulation.
Owner:ARROWHEAD MADISON
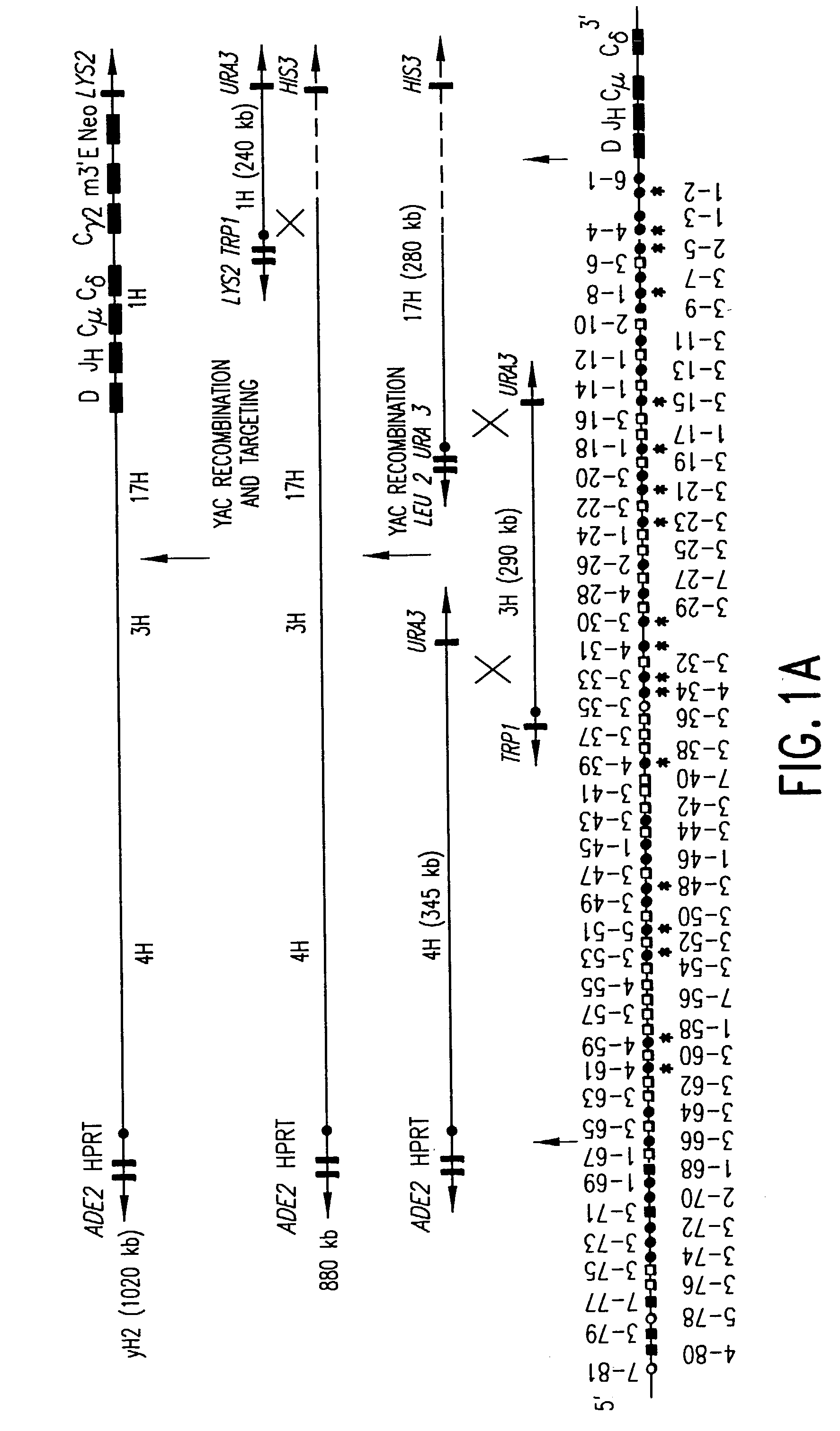
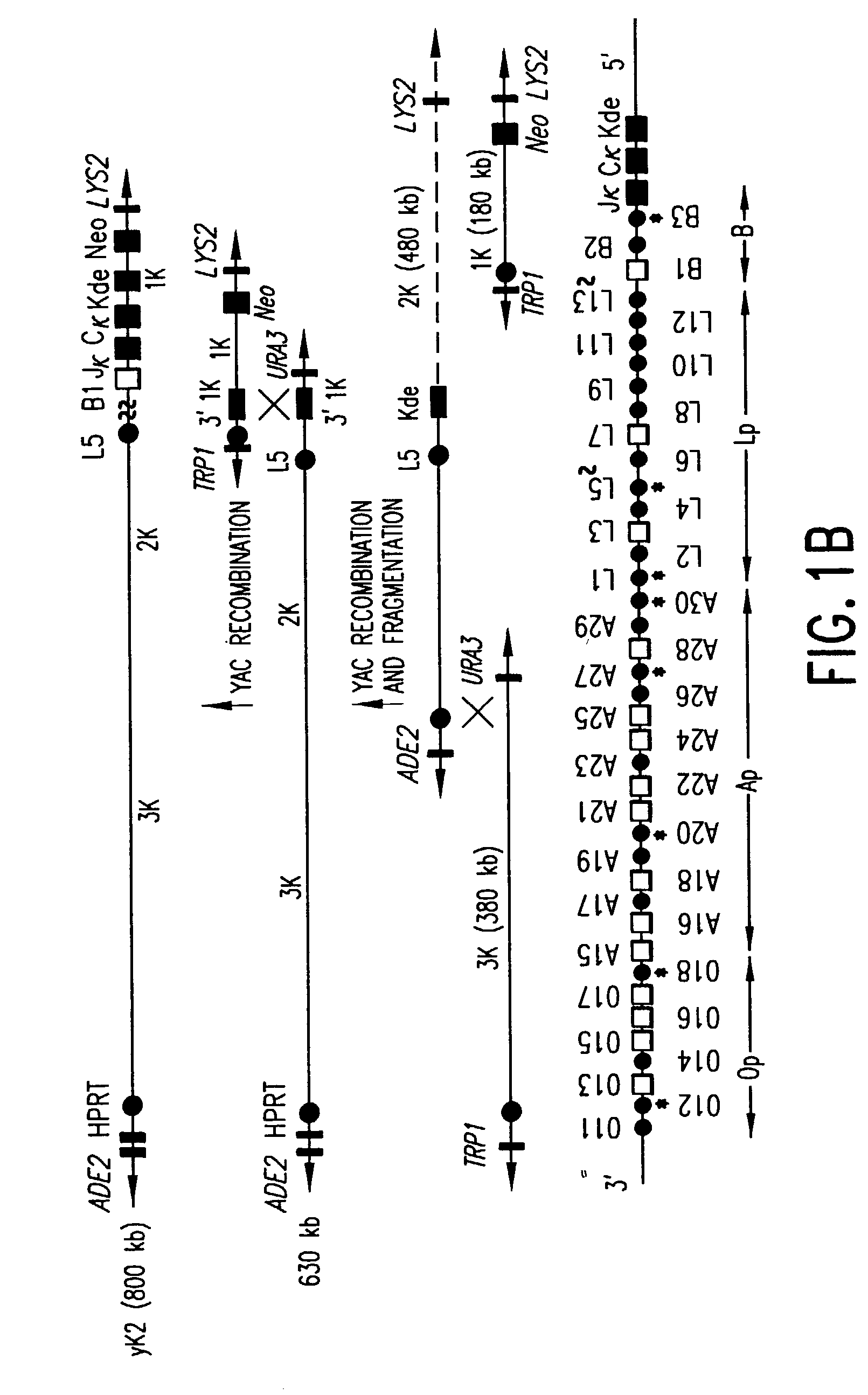
Transgenic mammals having human Ig loci including plural VH and VK regions and antibodies produced therefrom
InactiveUS7064244B2Reduced development and maturation of B-cellsEfficient productionAntipyreticAnalgesicsHuman animalMammal
The present invention relates to transgenic non-human animals that are engineered to contain human immunoglobulin gene loci. In particular, animals in accordance with the invention possess human Ig loci that include plural variable (VH and Vκ) gene regions. Advantageously, the inclusion of plural variable region genes enhances the specificity and diversity of human antibodies produced by the animal. Further, the inclusion of such regions enhances and reconstitutes B-cell development to the animals, such that the animals possess abundant mature B-cells secreting extremely high affinity antibodies.
Owner:ABQENIX INC
Biological sampler, collector and storage container
The invention relates to a storage container to receive and store a biopsy sample of an organism, held by a biopsy sample collector. The storage container comprises a container body defining a containment region with an open or openable end. The storage container further comprises a container cap located at the open end. The container cap is able to be removed from the container body by displacement in a direction (herein after “removal direction”) away from said containment region. The cap includes a passage leading to the containment region the passage able to receive a sample retaining sample collector. The storage container additionally comprises a tamper evident sleeve about at least part of the cap and the container body. The sleeve comprises of two parts that are frangibly connected. The frangible connection is in a manner such that upon displacement of the cap in the removal direction, a first part of the sleeve travels with said cap and a second part of the sleeve is prevented from movement with the first part by said container body to thereby separate the first and second parts. The first and second parts may each include information matched to each other.
Owner:SNPSHOT TRUSTEE
Antibodies to insulin-like growth factor I receptor
Owner:AMGEN FREMONT INC +1
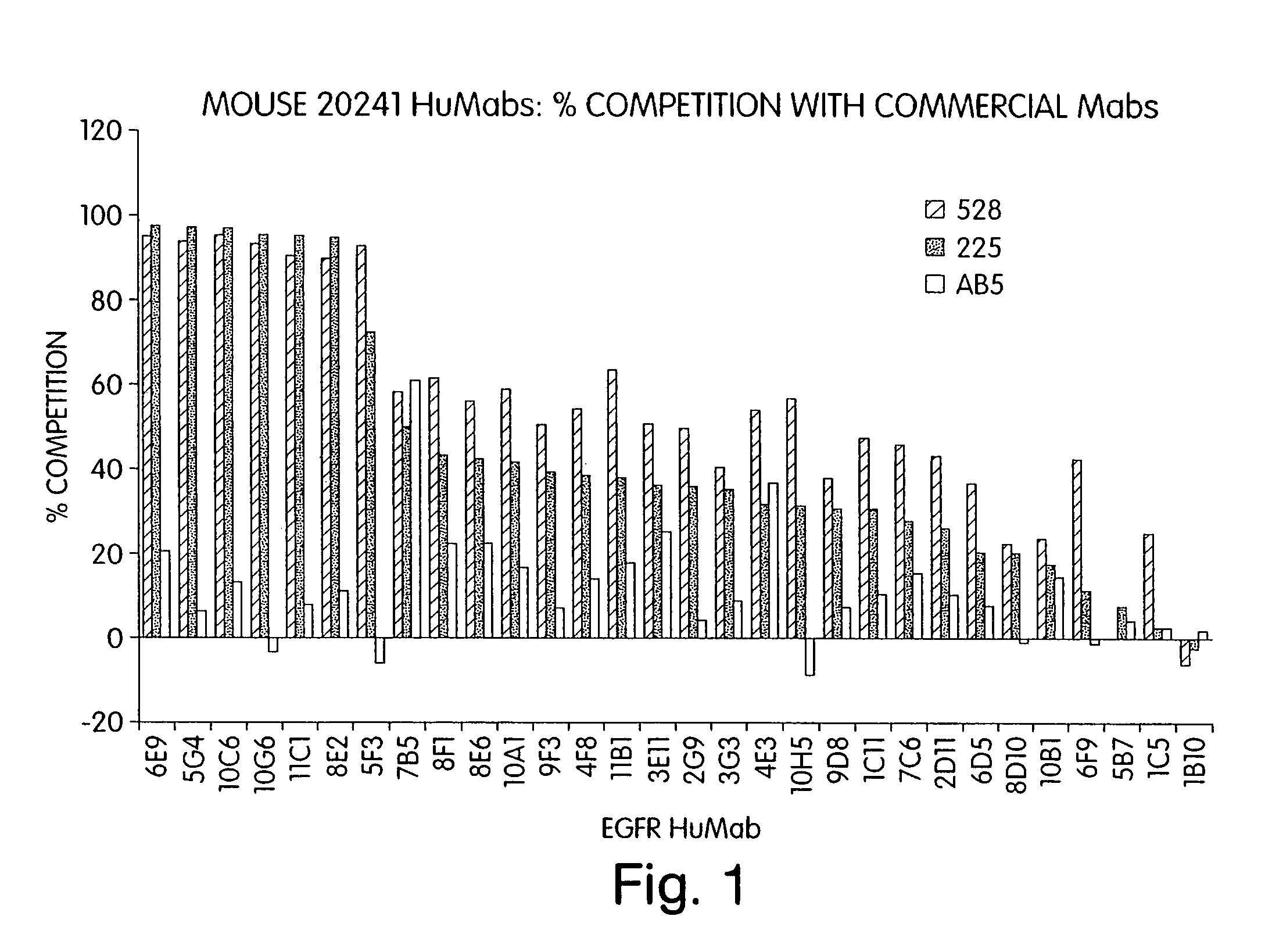
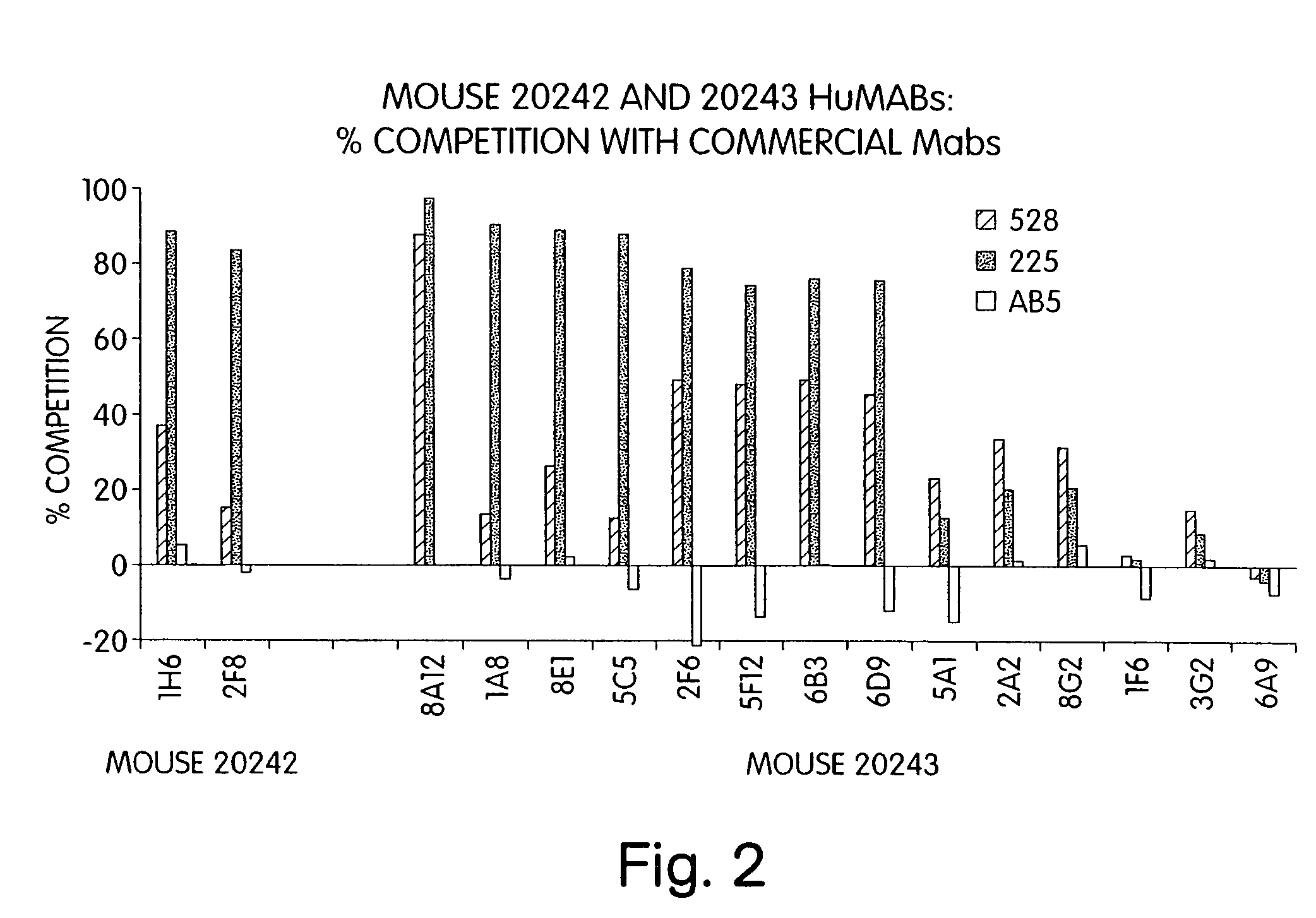
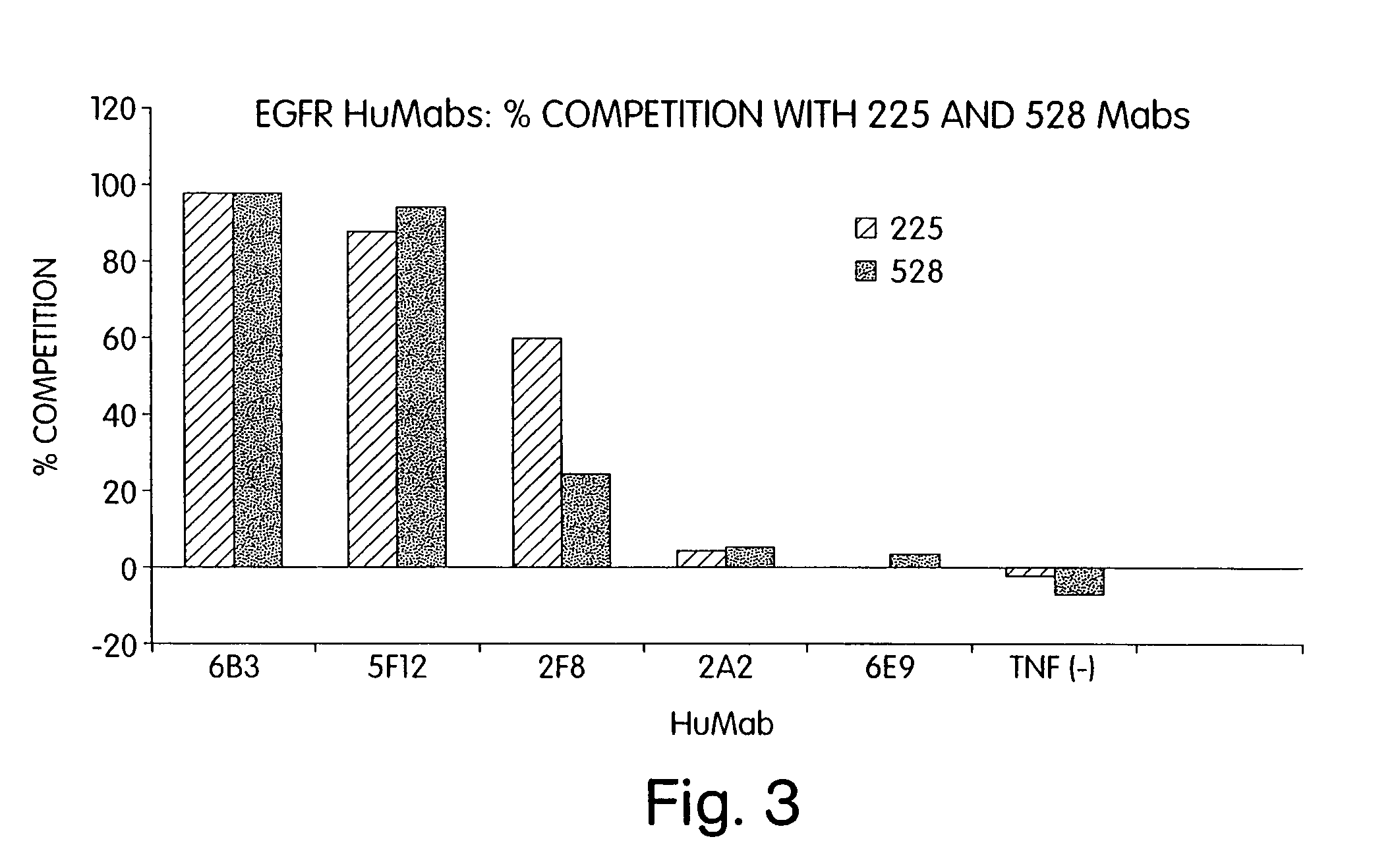
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
Rodent HER2 tumor model
The invention concerns HER<HIL><PDAT>2< / BOLD><PDAT>-transgenic non-human mammals, animal models for screening drug candidates for the treatment of diseases and disorders associated with the overexpression of HER<HIL><PDAT>2< / BOLD><PDAT>. In particular, the invention concerns animal models designed to test drug candidates for the treatment of HER<HIL><PDAT>2< / BOLD><PDAT>-overexpressing cancers, including breast cancer, that are not responding or poorly responding to current treatments.< / PTEXT>
Owner:SAN VALLEY SYST +1
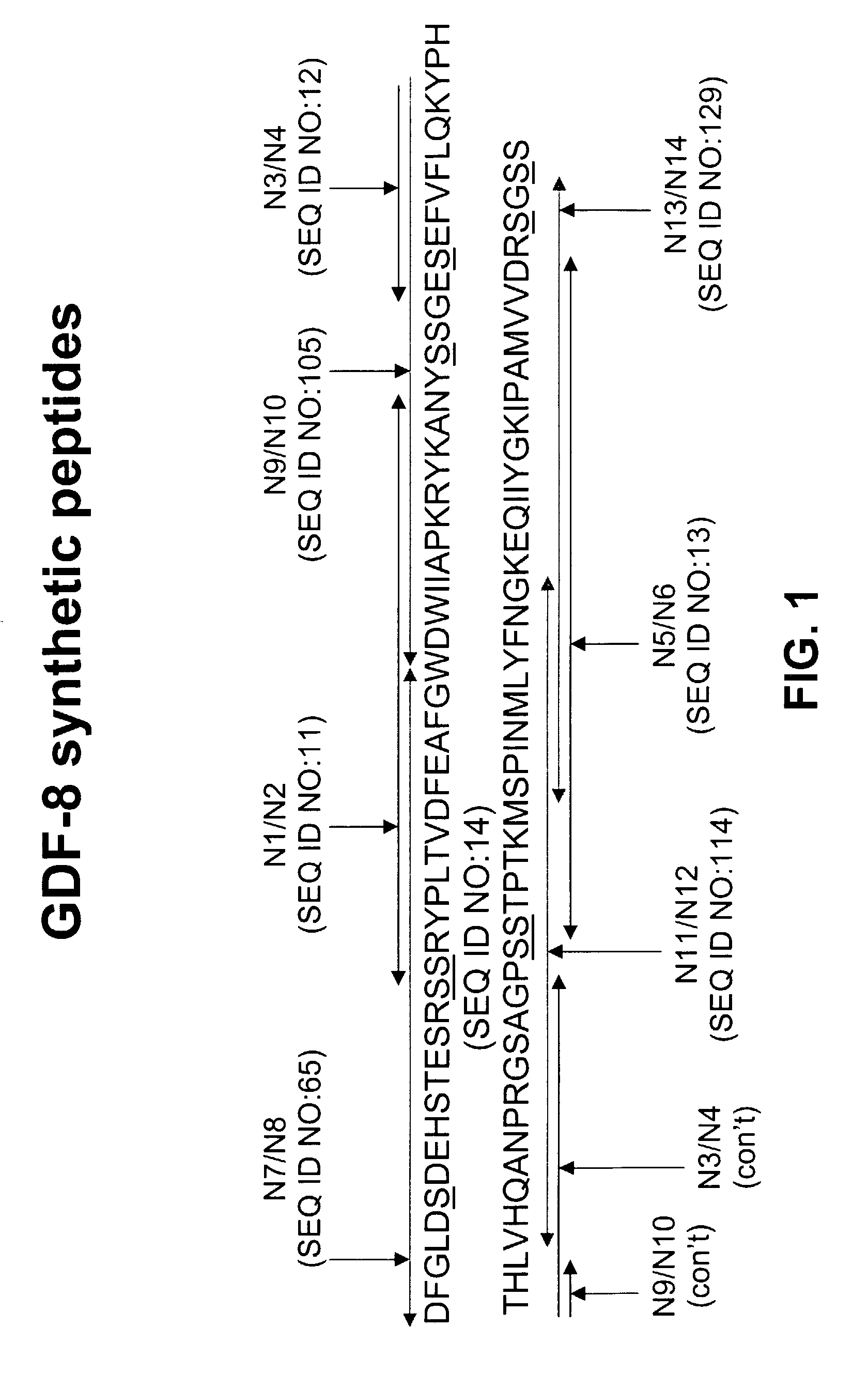
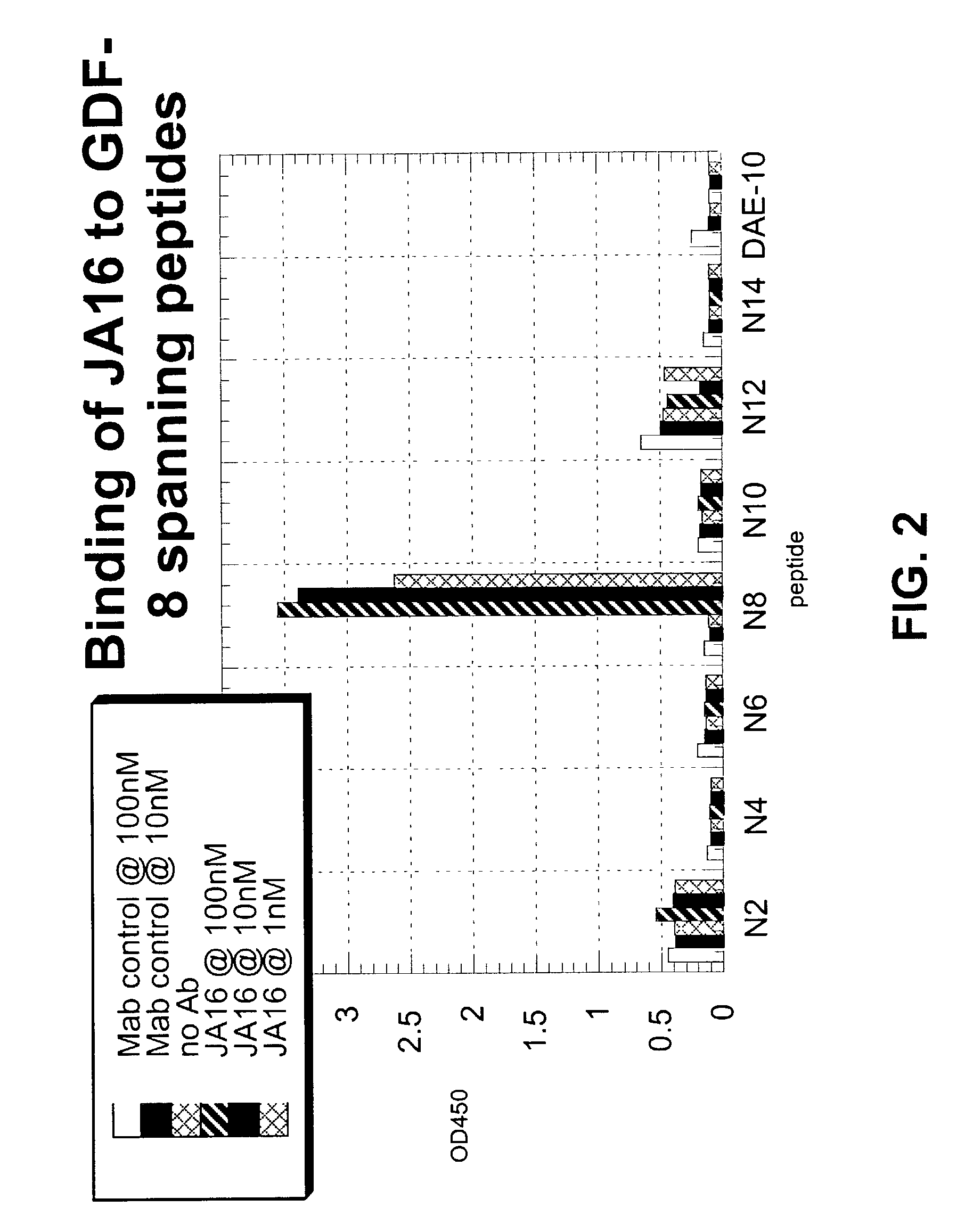
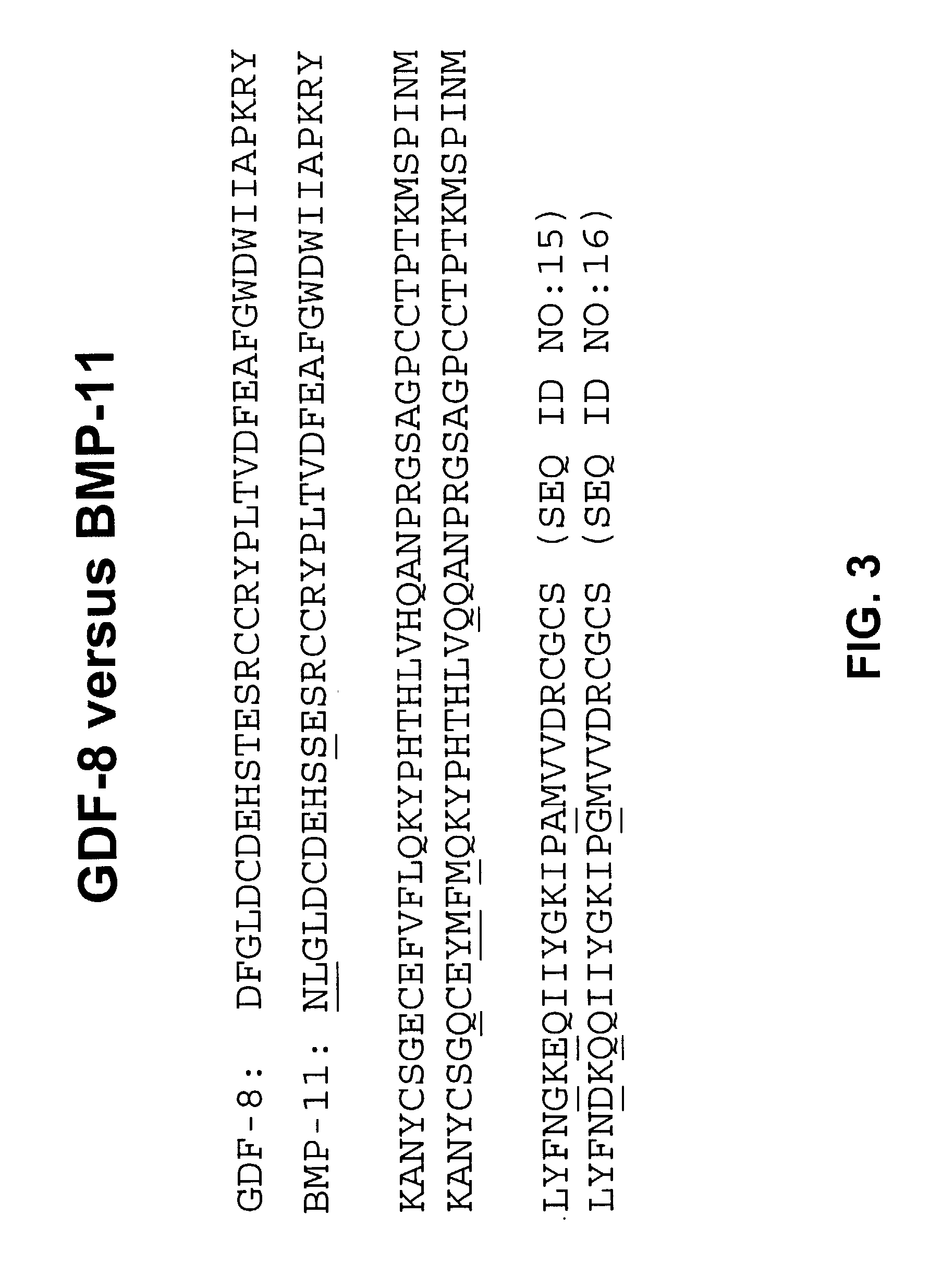
Antibody inhibitors of GDF-8 and uses thereof
ActiveUS7320789B2Reduction in one or more of the biological activitiesReduced activityNervous disorderMuscular disorderAntibody inhibitorAntibody fragments
The disclosure provides novel antibodies against growth and differentiation factor-8 (GDF-8), including antibody fragments, which inhibit GDF-8 activity in vitro and in vivo. The disclosure also provides methods for diagnosing, preventing, or treating degenerative disorders of muscle, bone, or insulin metabolism.
Owner:WYETH LLC
Identification tag and related identification tag system
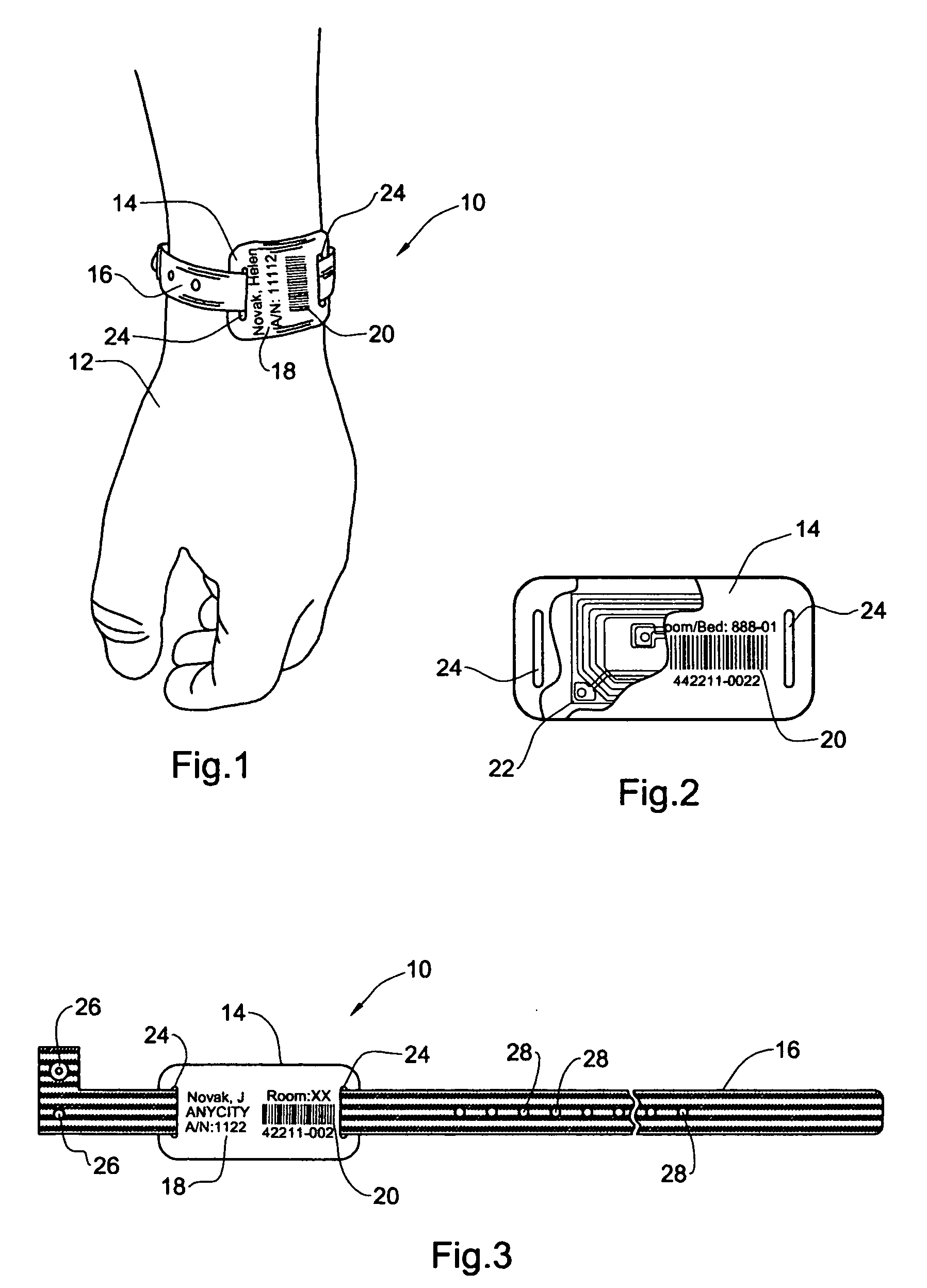
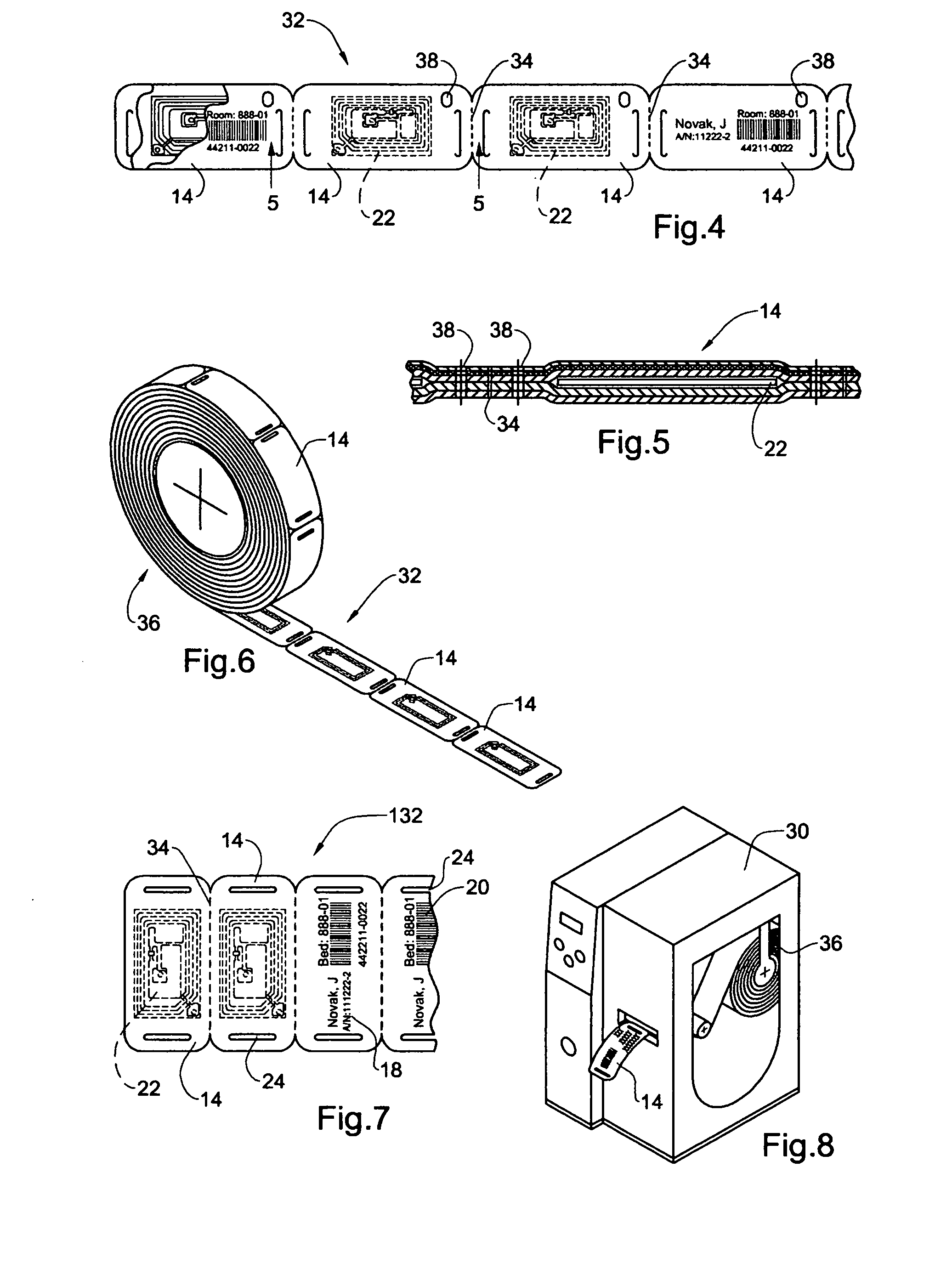
InactiveUS20050108912A1Easy and fast assemblyExtensive and relatively flatStampsPerson identificationEngineeringBearing surface
An identification tag and related tag system are provided for securely mounting the identification tag onto a selected wearer or object, such as mounting onto a wearer's wrist or the like. The identification tag is adapted to receive wearer-related identification and other information in human readable and / or machine readable form, and for slide-fit assembly onto an elongated flexible strap such as a wristband or bracelet for mounting onto the selected wearer or object. In one preferred form, the identification tag includes a radio frequency identification (RFID) circuit adapted for communicating wearer-related information with a remote reader. The tag system, including the identification tag assembled with the flexible strap, is particularly useful with a small wearer or object, such as an infant, to provided a relatively extensive and relatively flat information-bearing surface area.
Owner:PRECISION DYNAMICS CORPORATION
Transgenic mouse allergy models and methods for their use
InactiveUS6118044AImprove import efficiencySufficient amountMicrobiological testing/measurementTissue cultureAntibody typesAllergy
Transgenic mice which constitutively express an antibody-type molecule encoded by the transgene and which has an IgE heavy chain constant region and is specific for a pre-defined antigen, provide an allergic reaction to that antigen without prior sensitization and are useful as allergy models.
Owner:SANKYO CO LTD +1
Pharmaceutical composition for treatment of diseases caused by IL-6 production
Pharmaceutical compositions for prevention or treatment of diseases caused by interleukin-6 production, comprising an antibody to interleukin-6 receptor (IL-6R antibody). As the IL-6R antibody, an antibody of animals other than the human such as mice, rats, etc., a chimeric antibody between these and a human antibody, a reshaped human antibody, etc. may be used. The pharmaceutical compositions are useful for prevention or treatment of diseases caused by interleukin-6 production such as plasmacytosis, anti-IgGl-emia, anemia, nephritis, etc.
Owner:KISHIMOTO TADAMITSU +2
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com