Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
15349 results about "Virology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virology is the study of viruses – submicroscopic, parasitic particles of genetic material contained in a protein coat – and virus-like agents. It focuses on the following aspects of viruses: their structure, classification and evolution, their ways to infect and exploit host cells for reproduction, their interaction with host organism physiology and immunity, the diseases they cause, the techniques to isolate and culture them, and their use in research and therapy. Virology is considered to be a subfield of microbiology or of medicine.
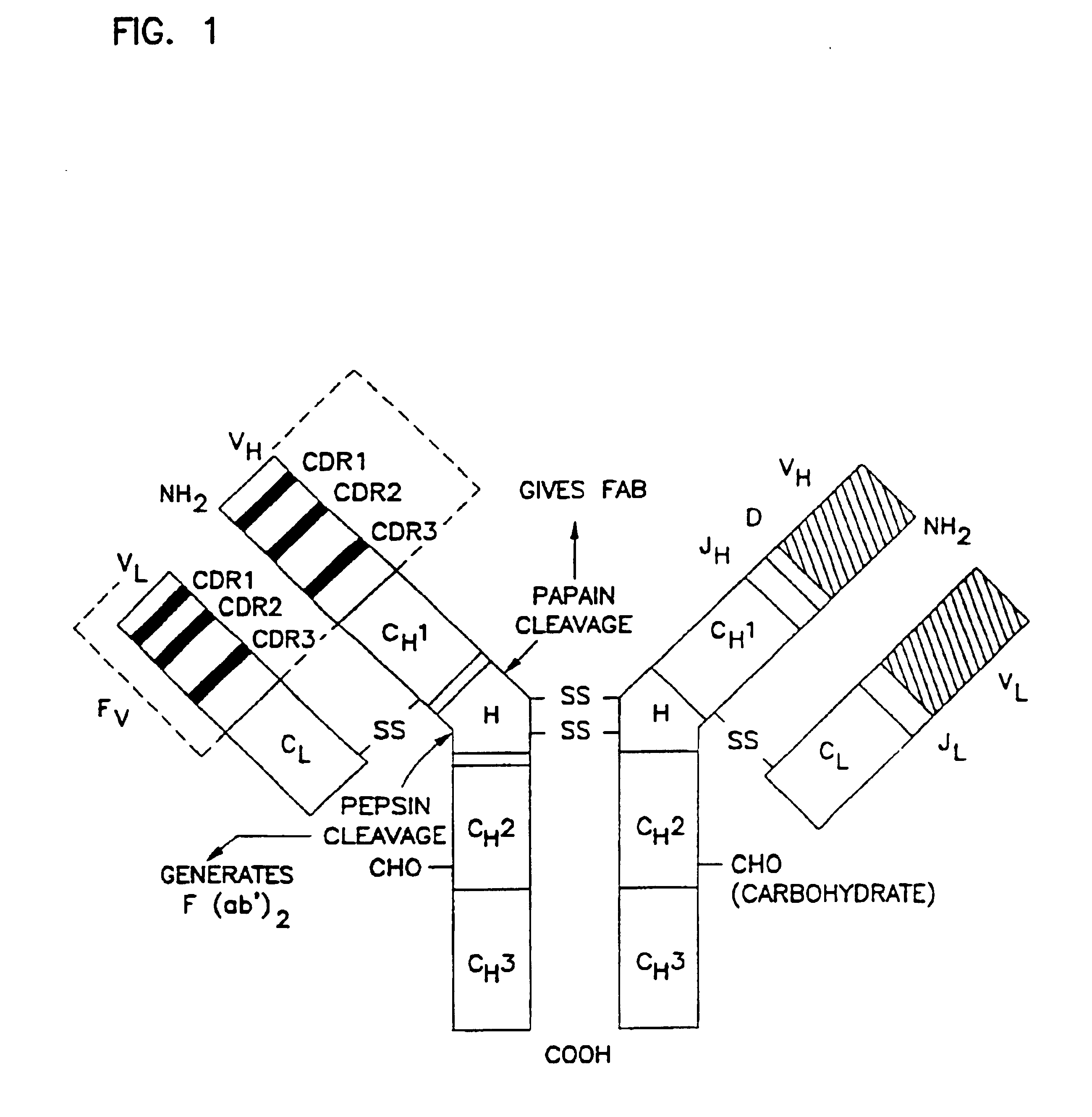
Humanization of antibodies
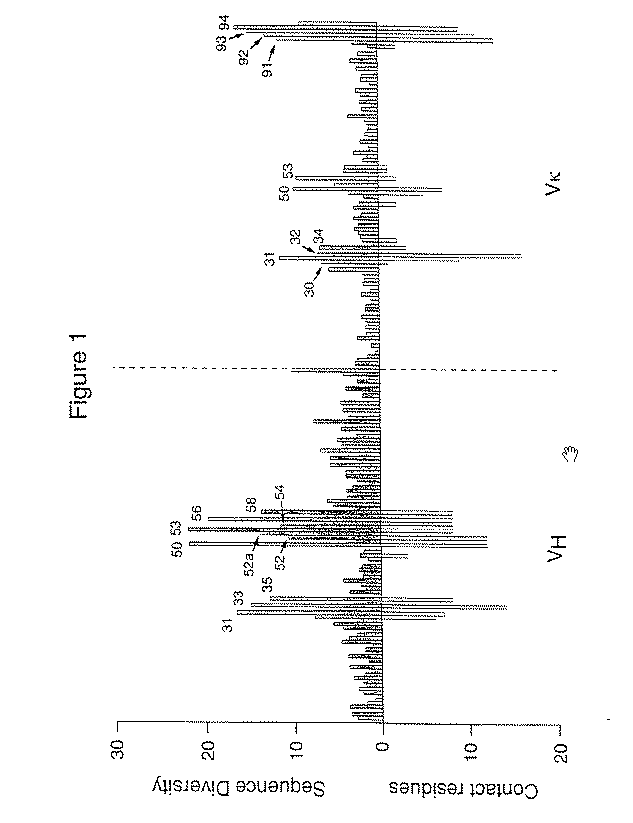
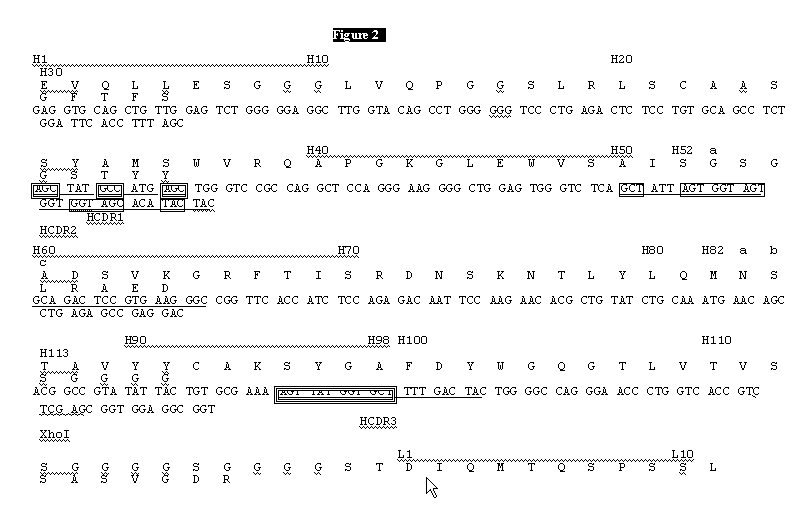
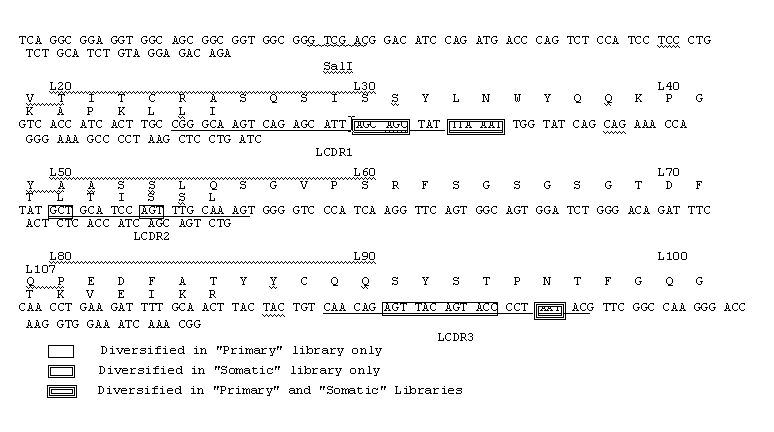
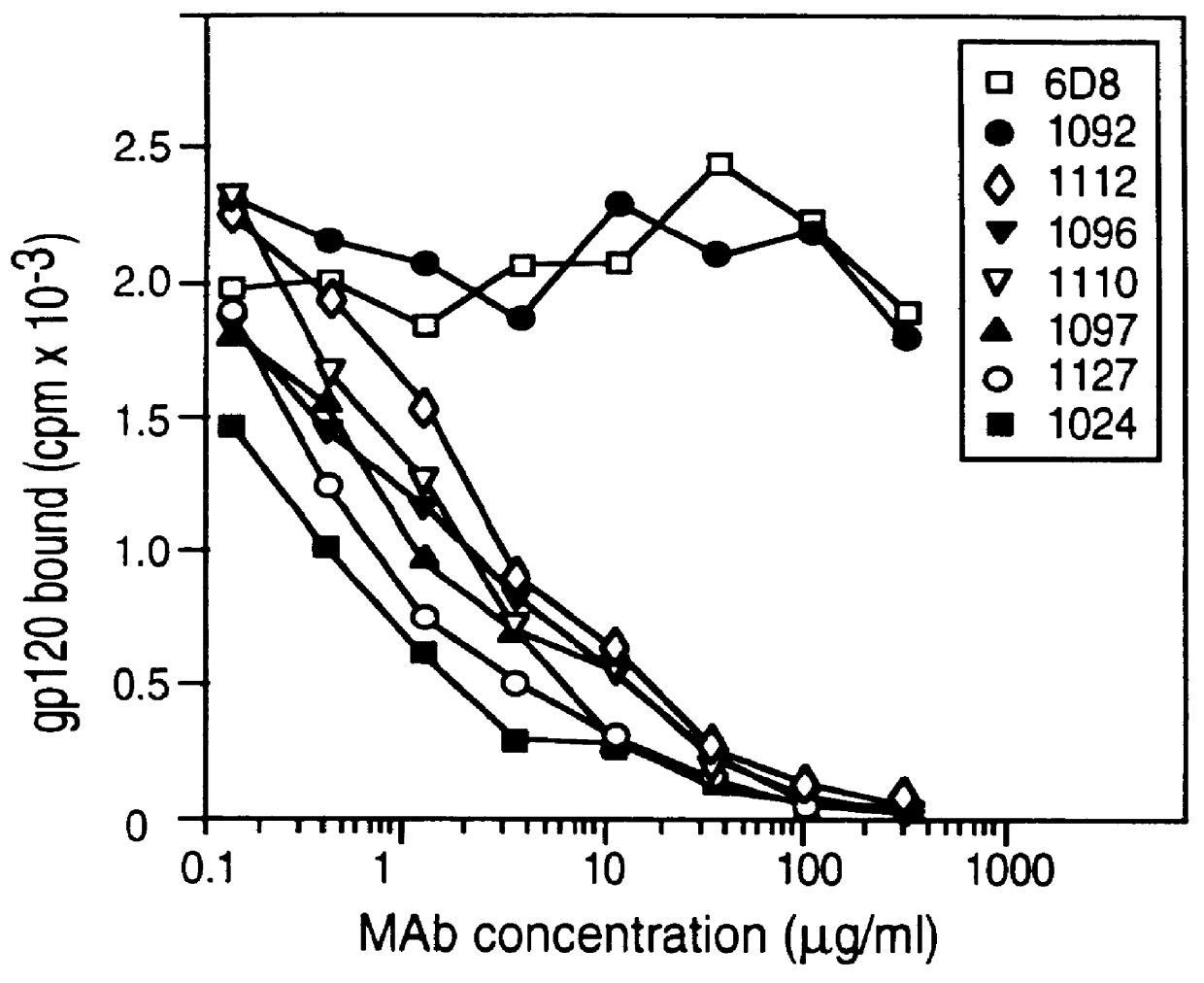
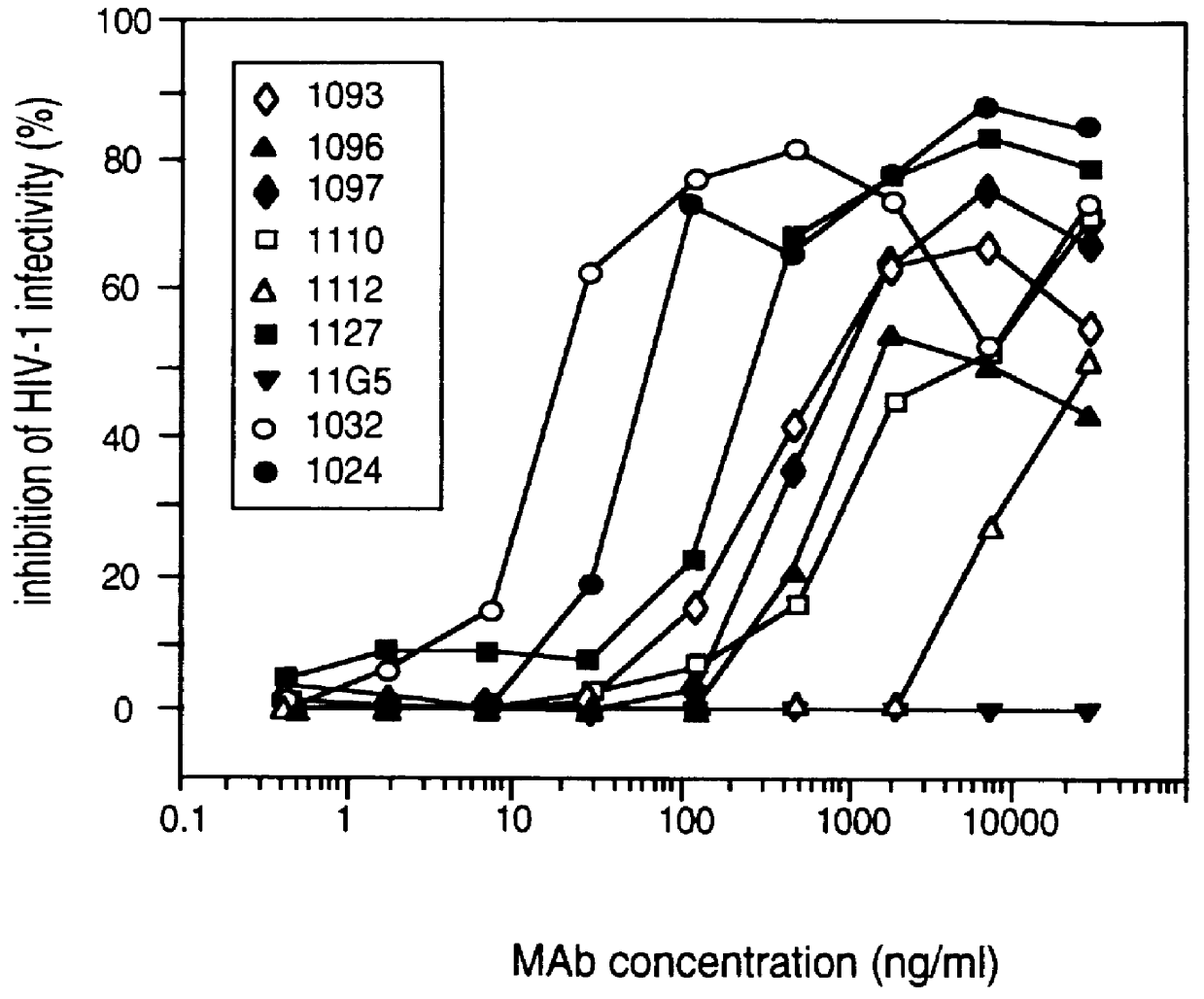
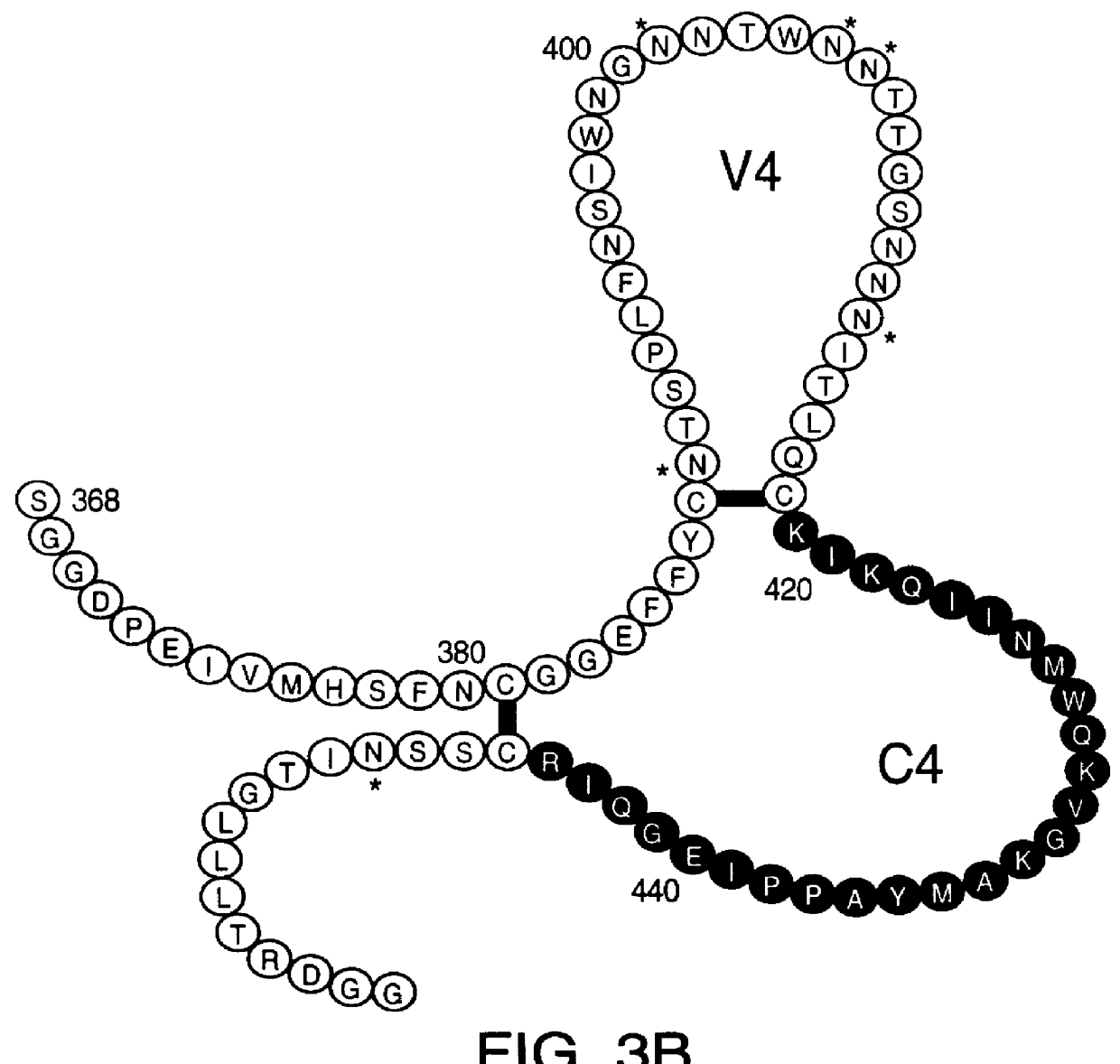
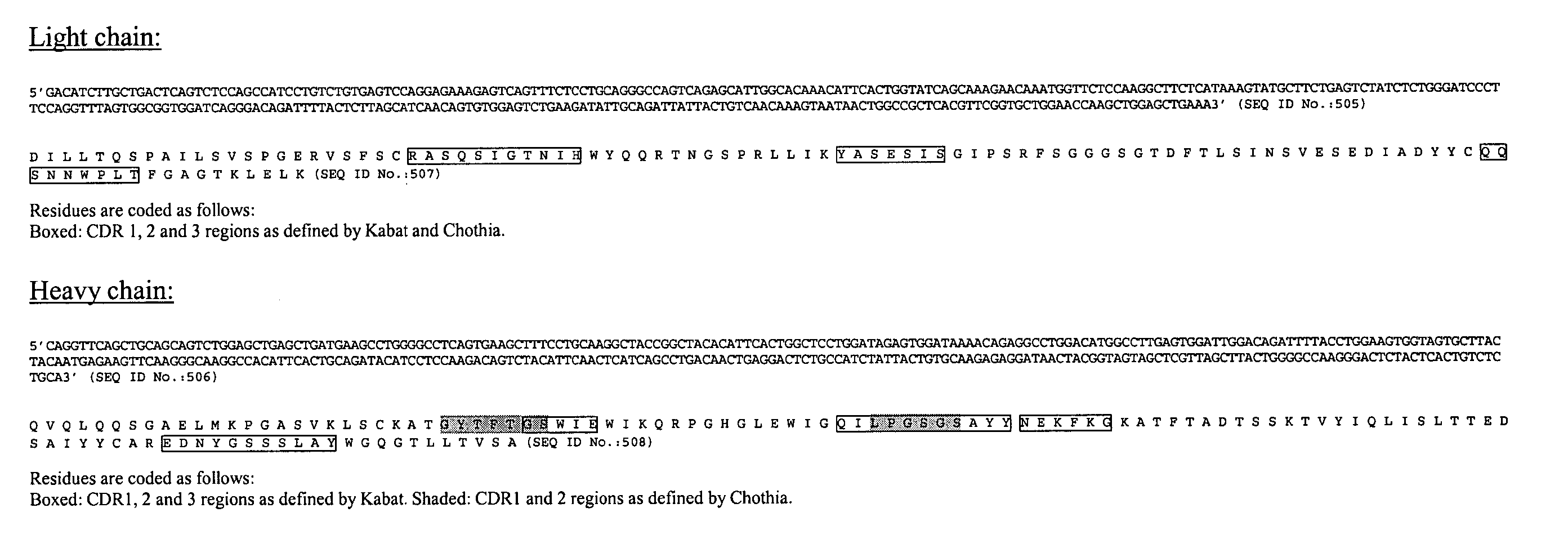
InactiveUS20050042664A1Limited diversityFast and less labor intensive productionHybrid immunoglobulinsMicrobiological testing/measurementAntigen bindingHumanized antibody
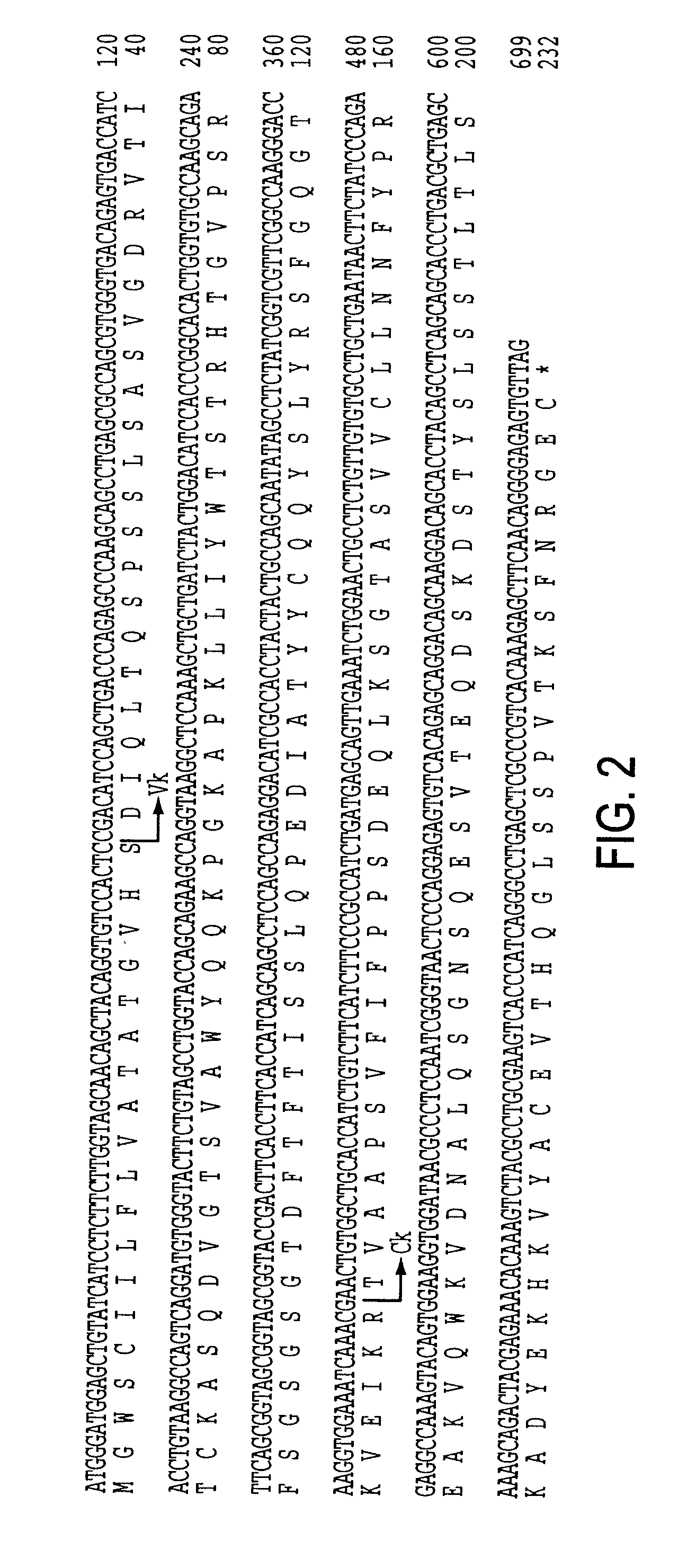
The present invention provides methods of re-engineering or re-shaping an antibody from a first species, wherein the re-engineered or re-shaped antibody does not elicit undesired immune response in a second species, and the re-engineered or re-shaped antibody retains substantially the same antigen binding-ability of the antibody from the first species. In accordance with the present invention, a combinatorial library comprising the CDRs of the antibody from the first species fused in frame with framework regions derived from a second species can be constructed and screened for the desired modified antibody. In particular, the present invention provides methods utilizing low homology acceptor antibody frameworks for efficiently humanizing an antibody or a fragment thereof. The present invention also provides antibodies produced by the methods of the invention.
Owner:MEDIMMUNE LLC
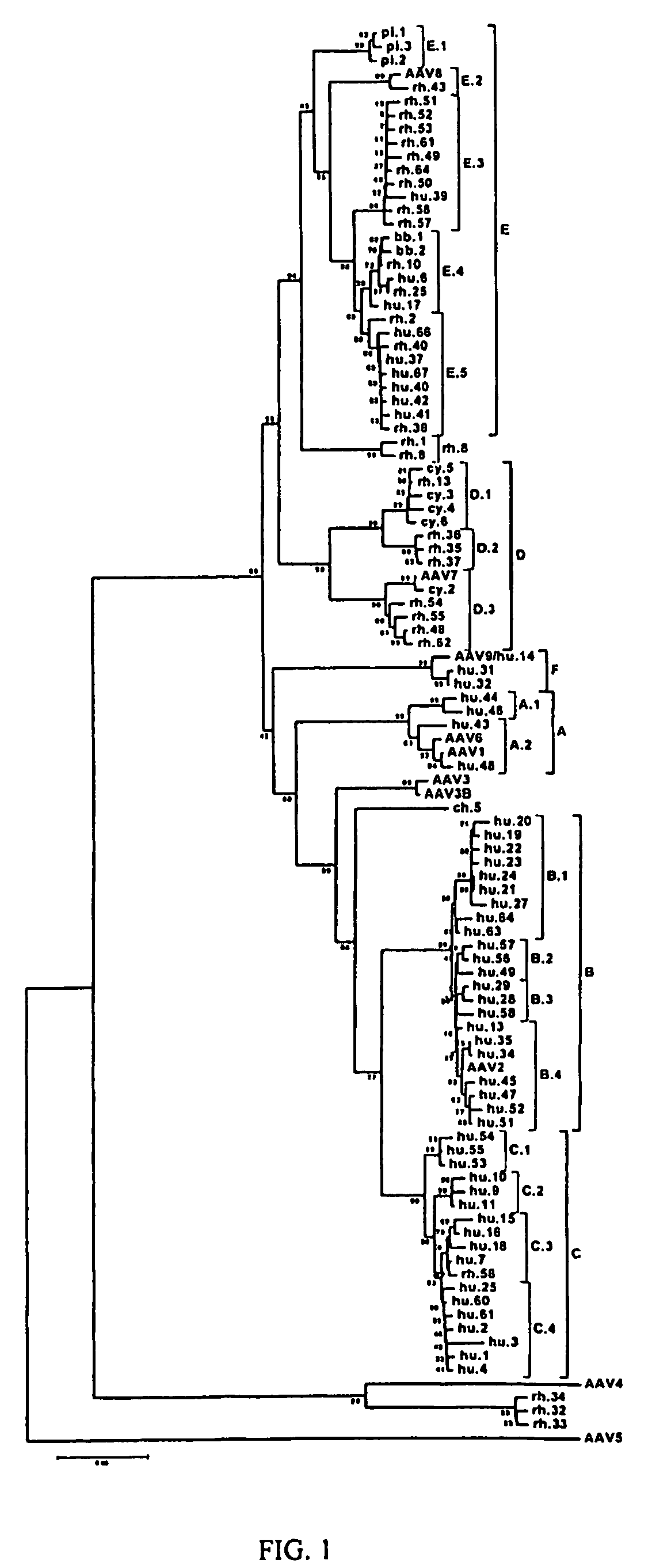
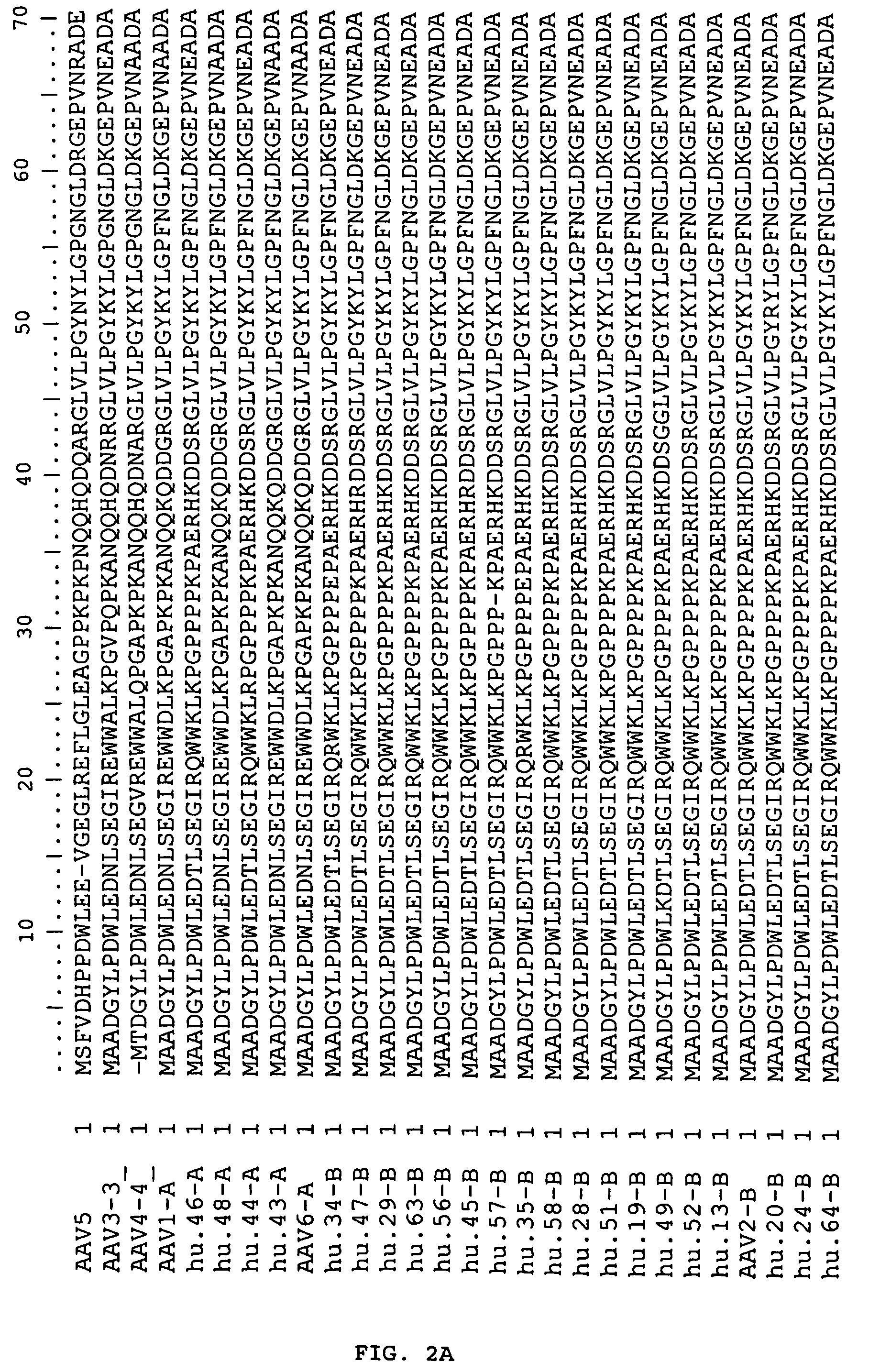
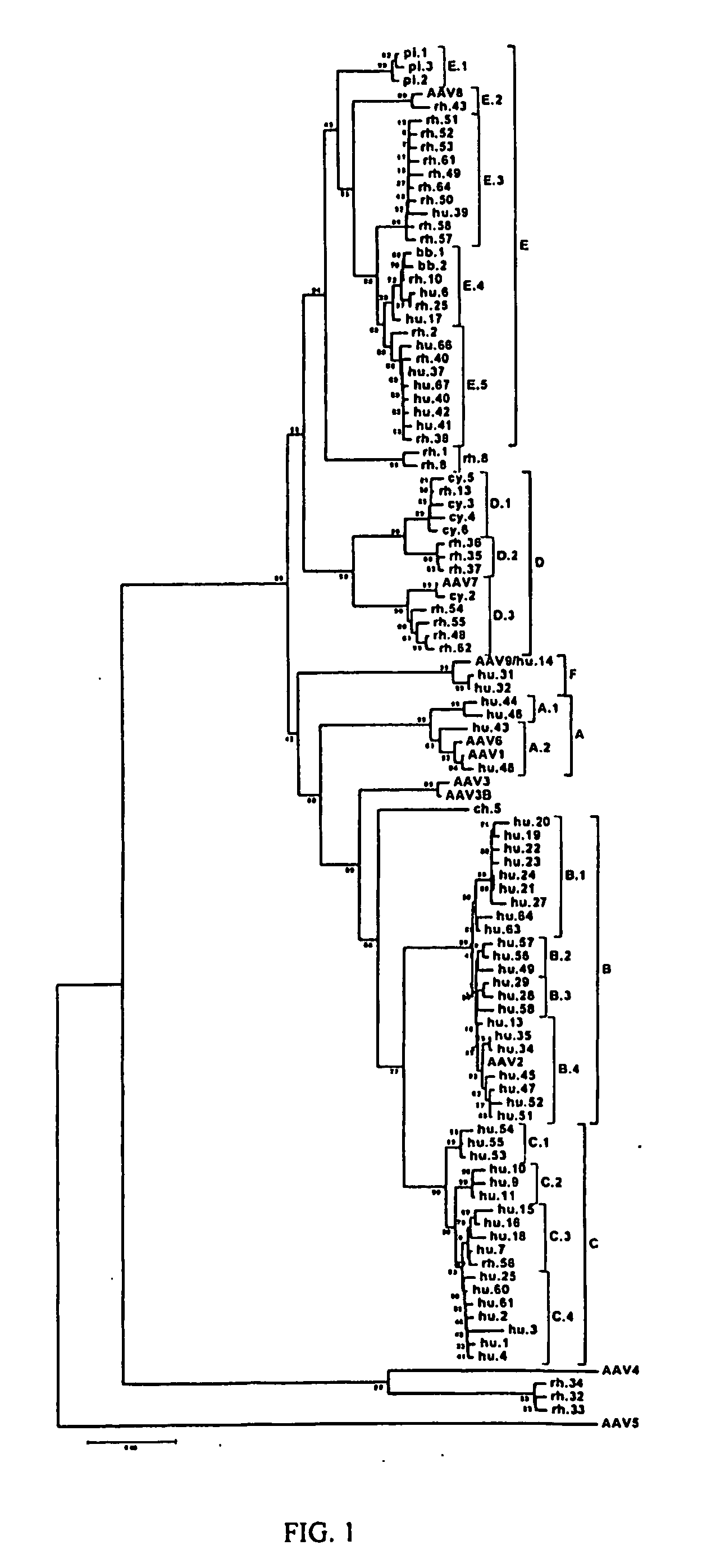
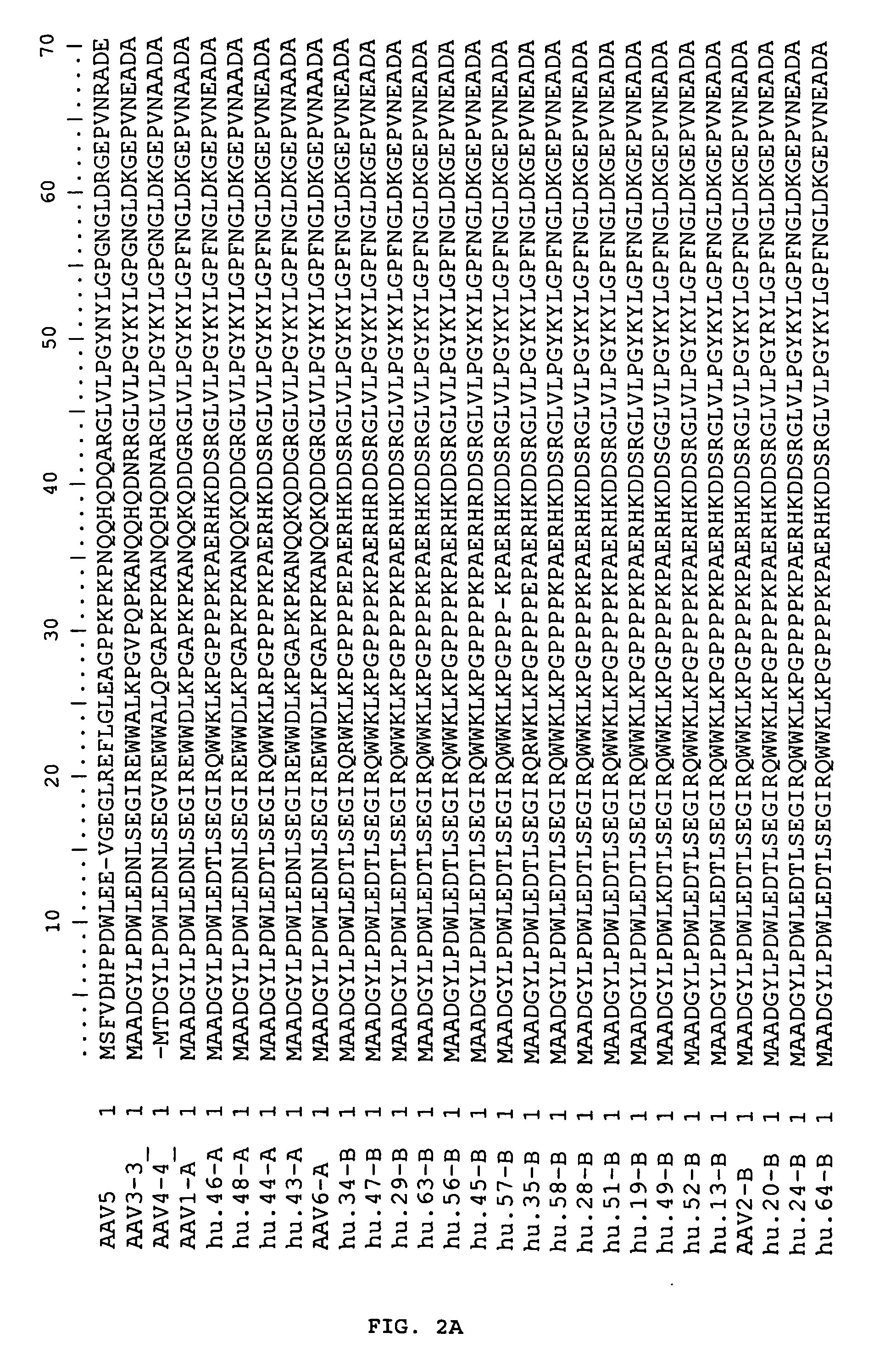
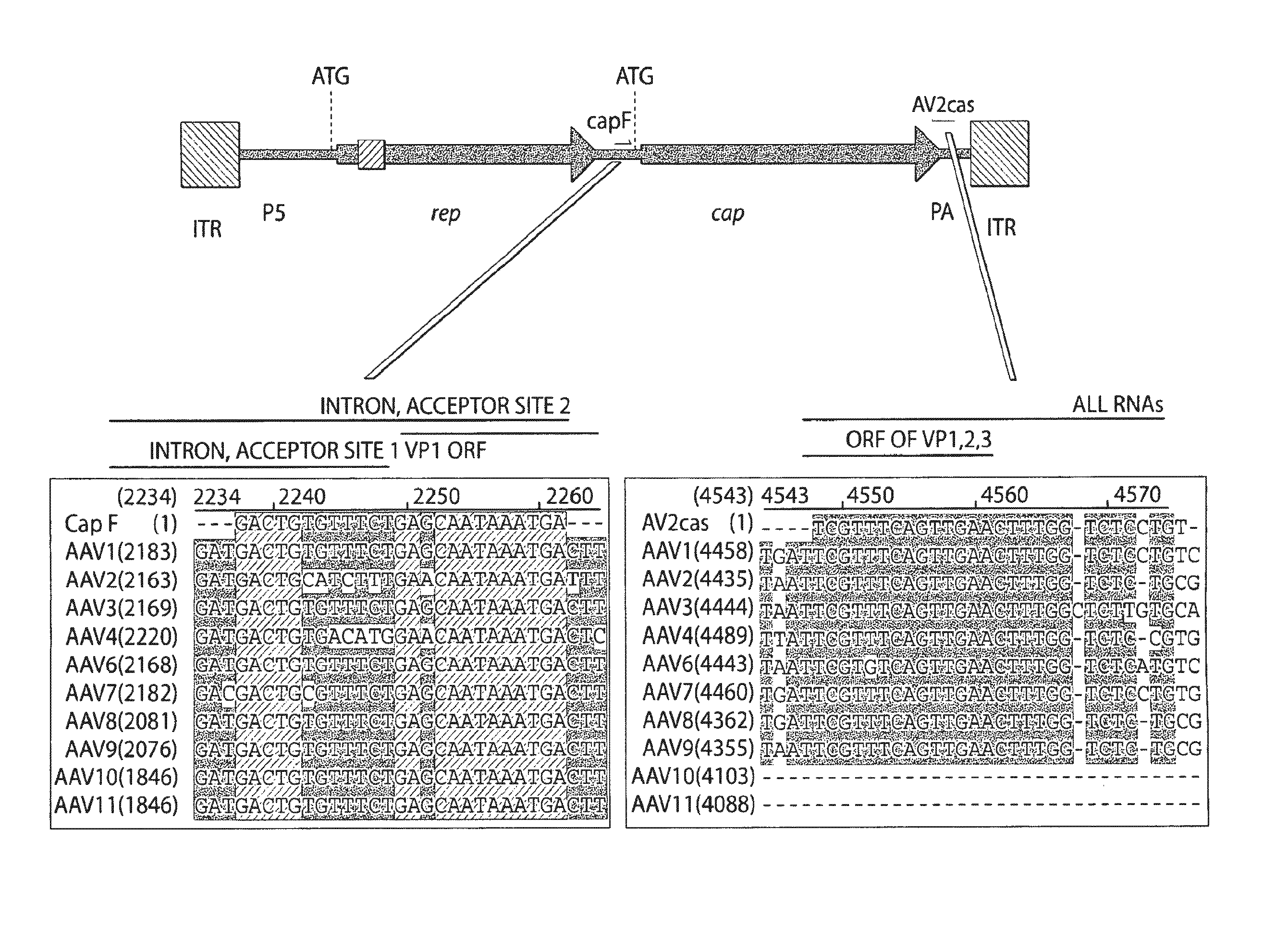
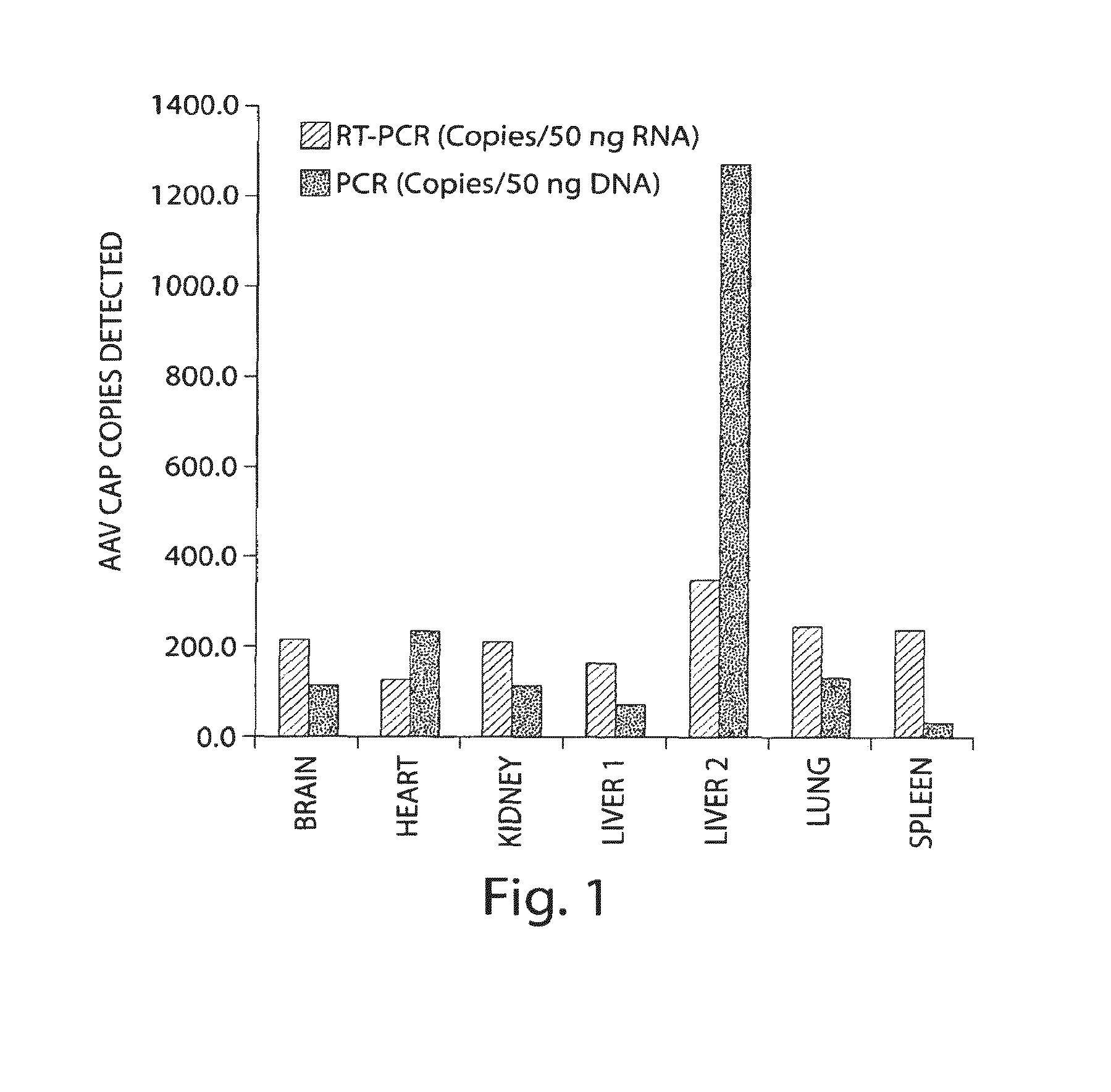
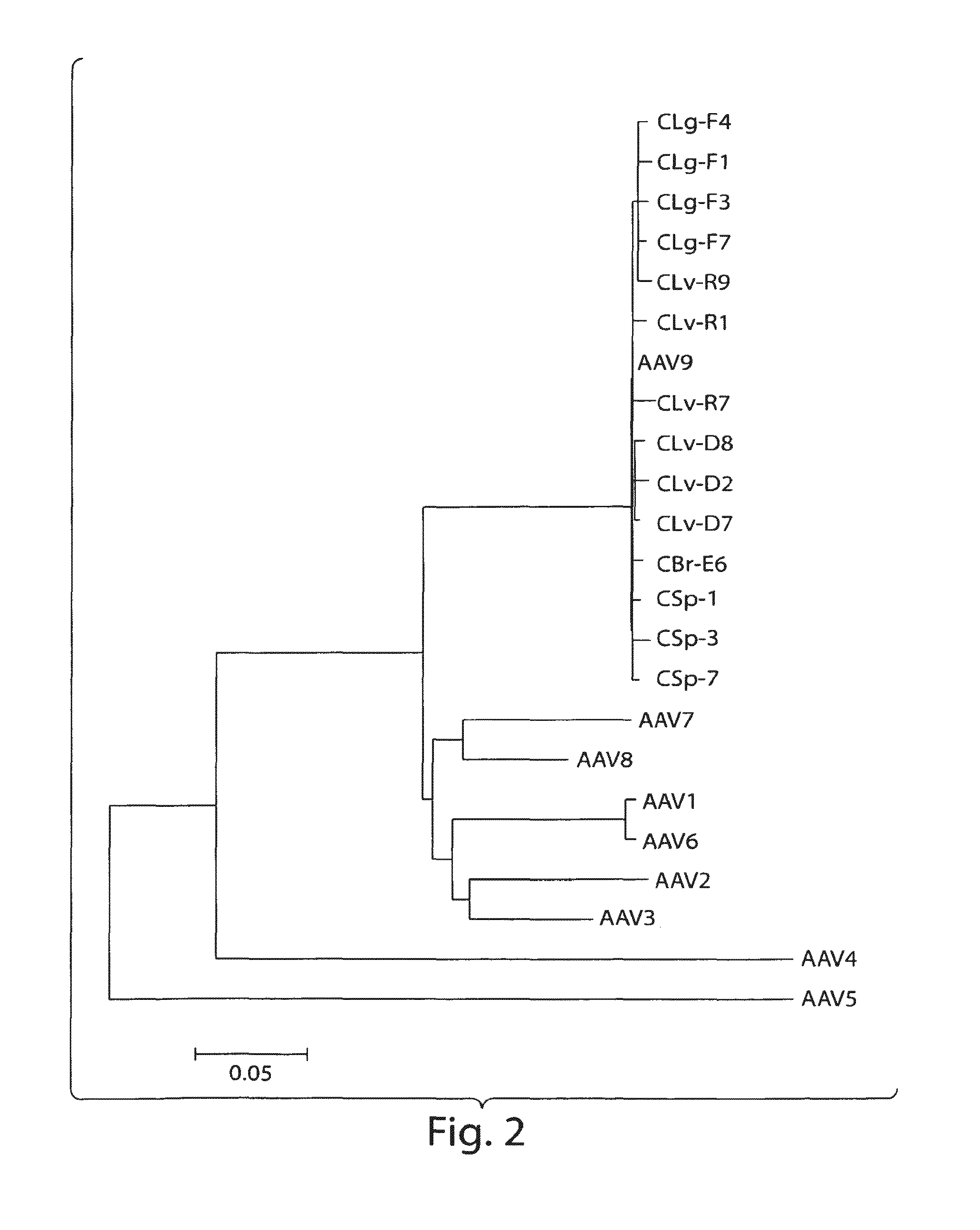
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
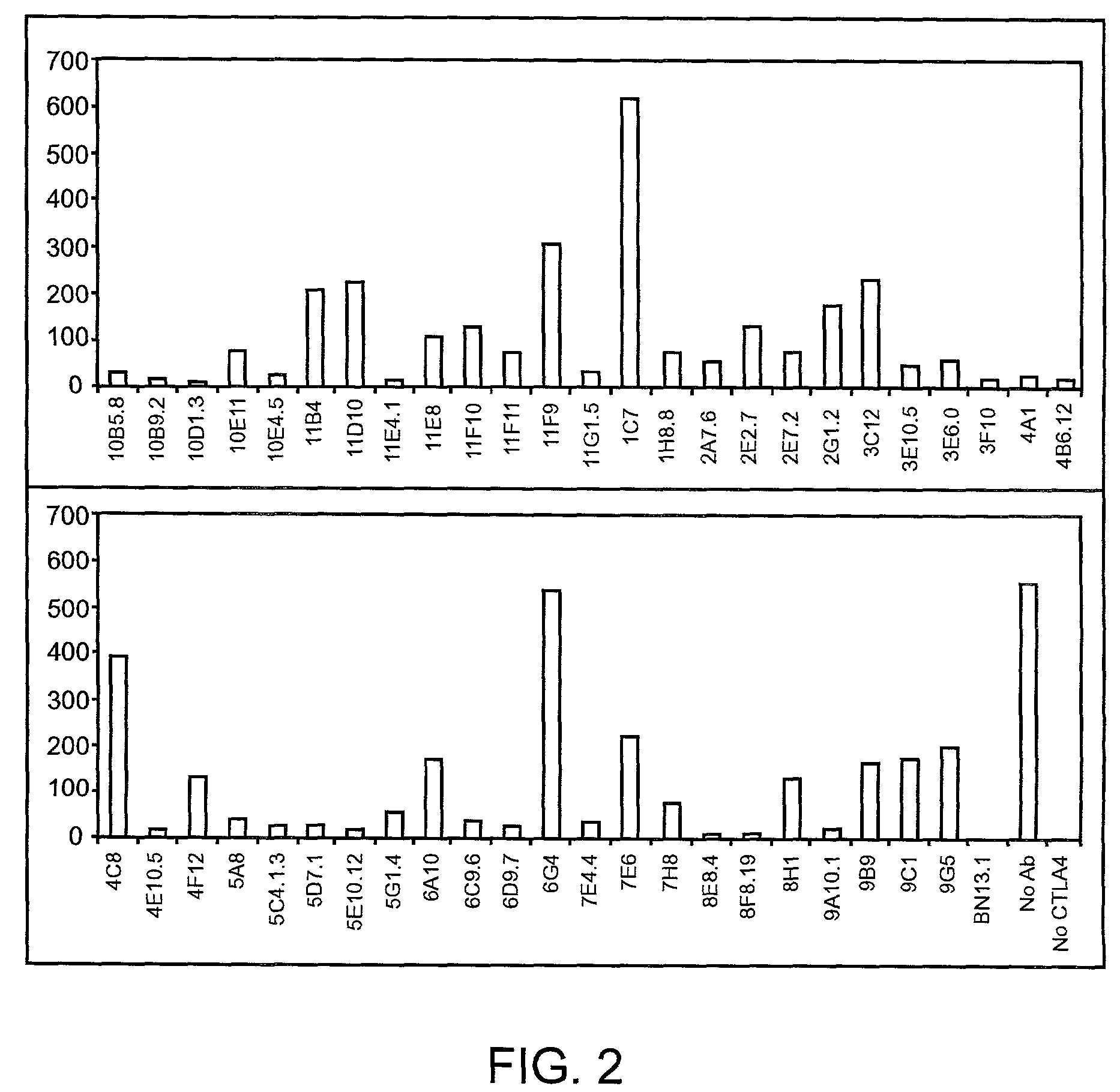
Human CTLA-4 antibodies and their uses
InactiveUS7605238B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesVirology
The present invention provides human sequence antibodies against human CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
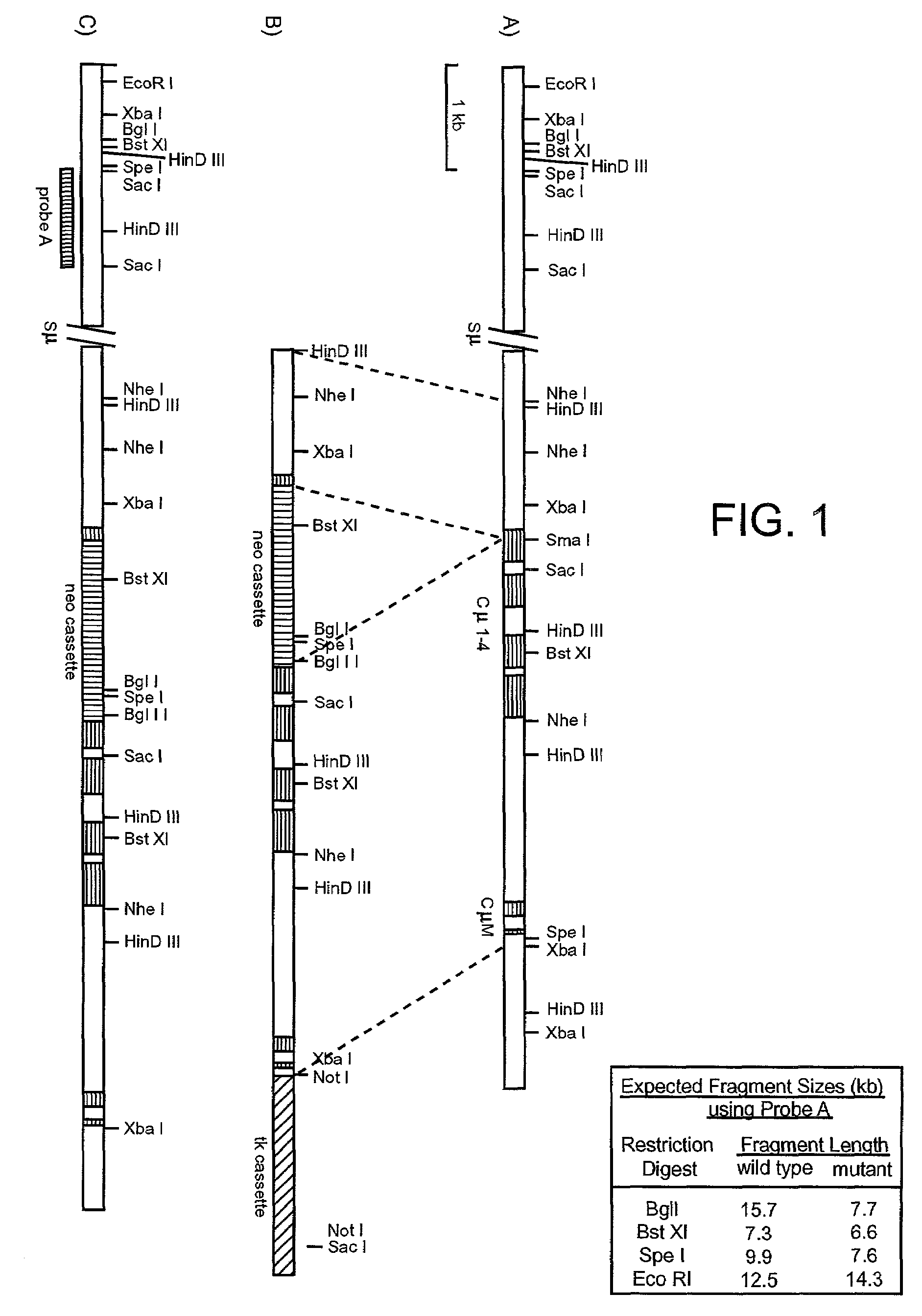
Transgenic non-human animals for producing chimeric antibodies
InactiveUS20060015957A1Inhibit expressionEasy to switchImmunoglobulinsGenetic engineeringAntigenHuman animal
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
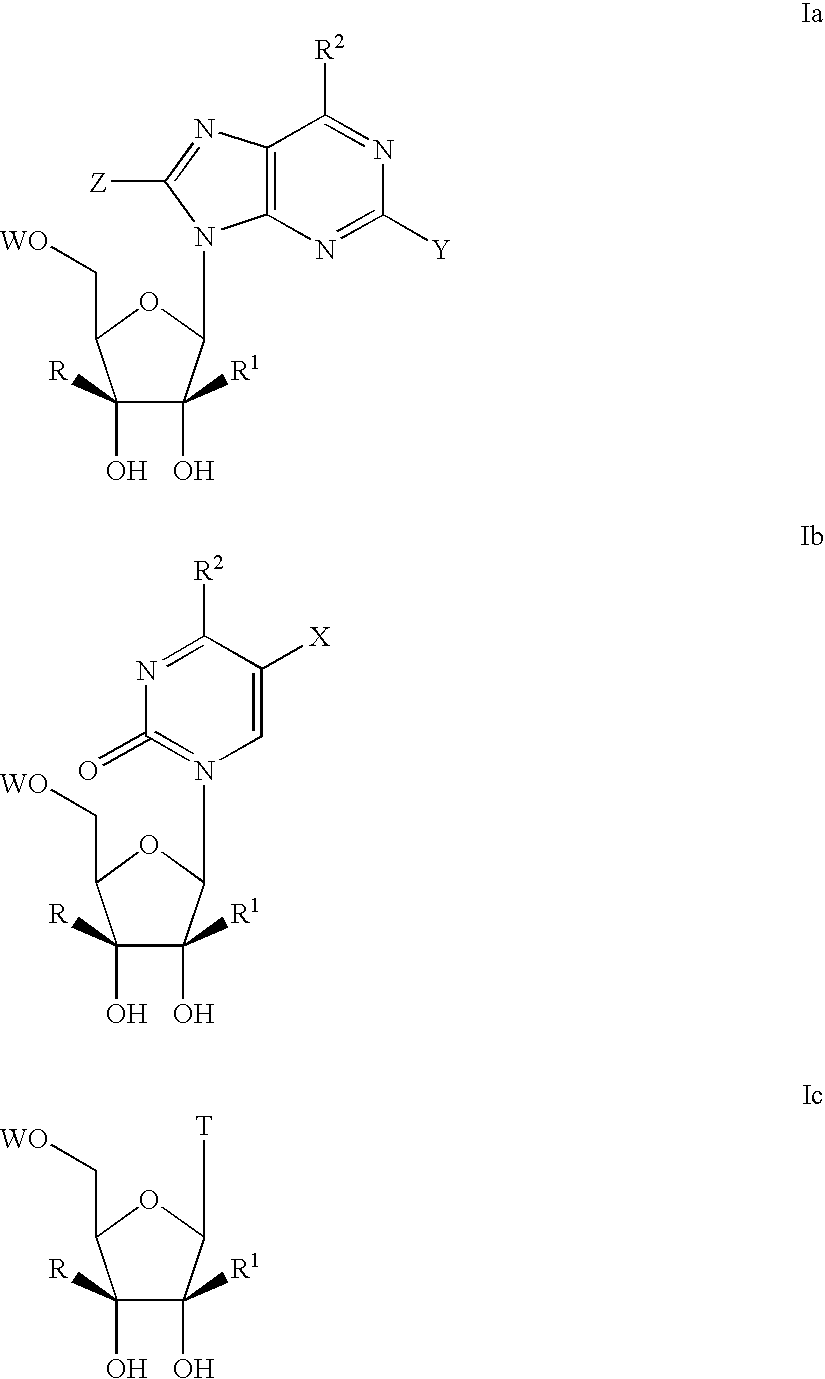
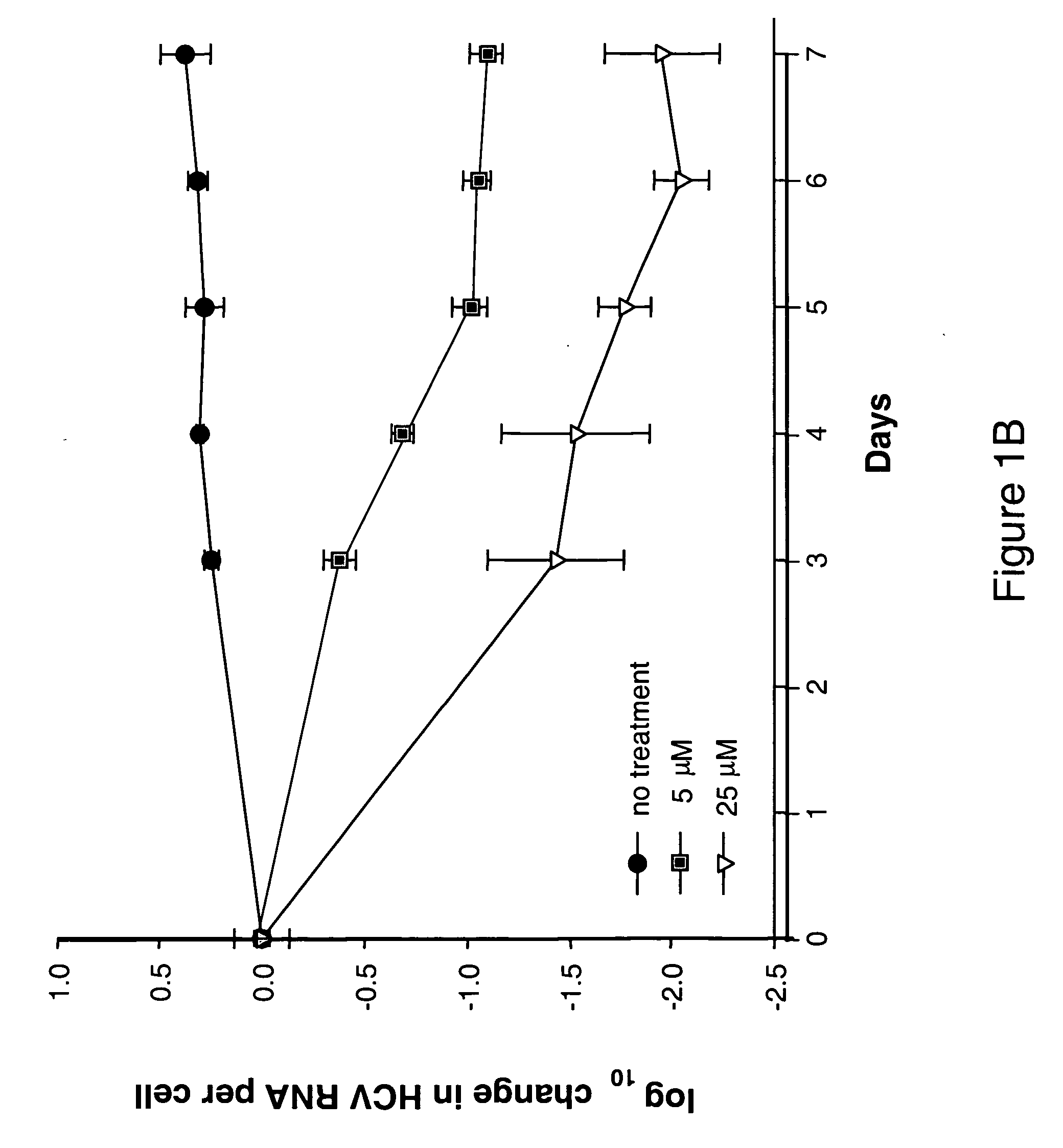
Nucleoside derivatives for treating hepatitis C virus infection
Owner:GENELABS TECH INC
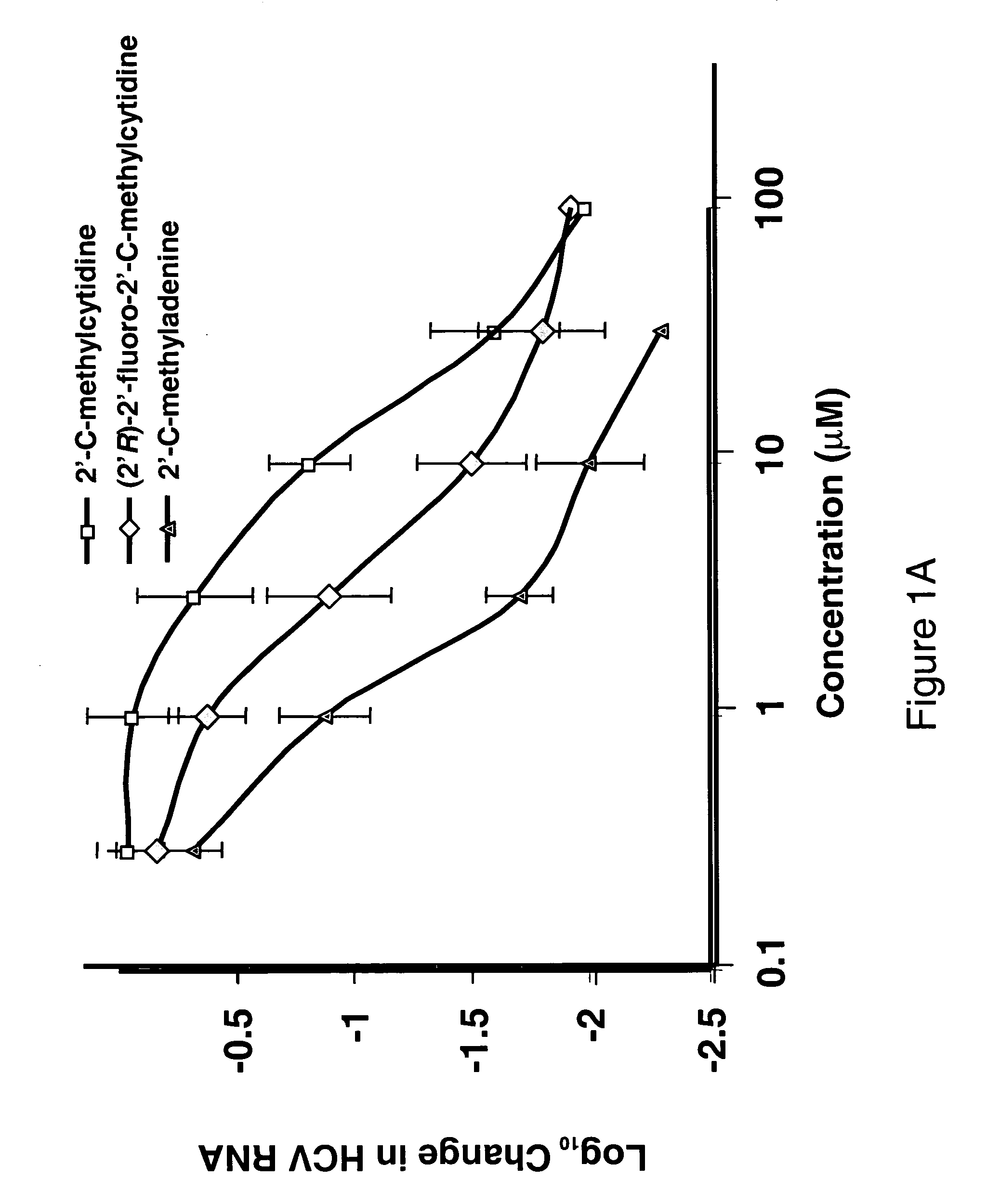
Modified fluorinated nucleoside analogues
ActiveUS20050009737A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
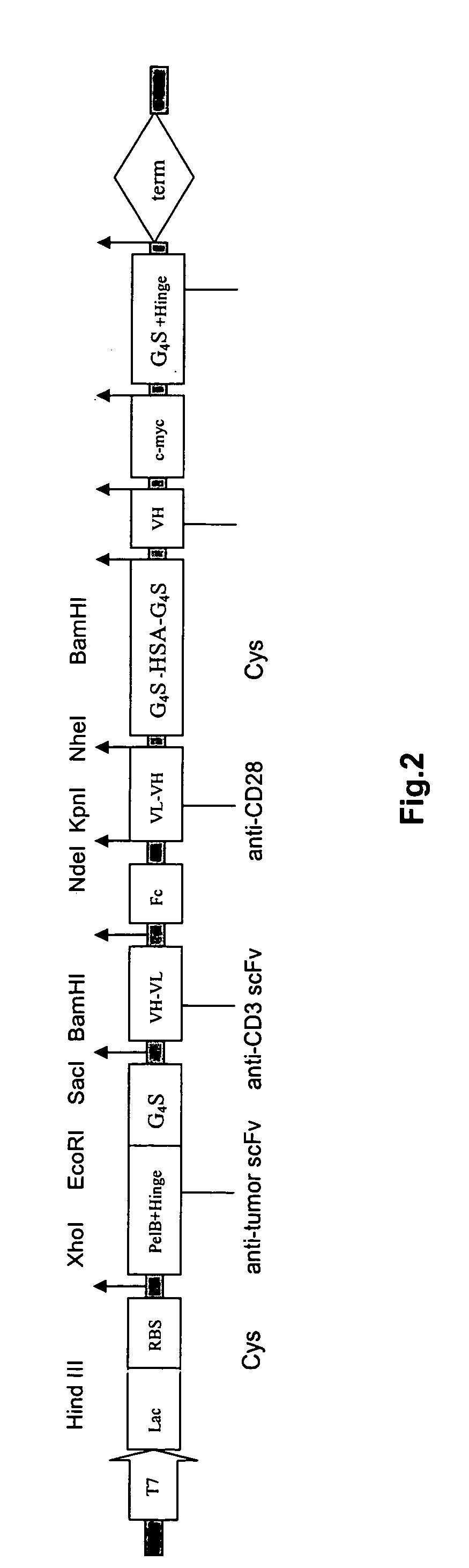
Cyclic single-chain trispecific antibody
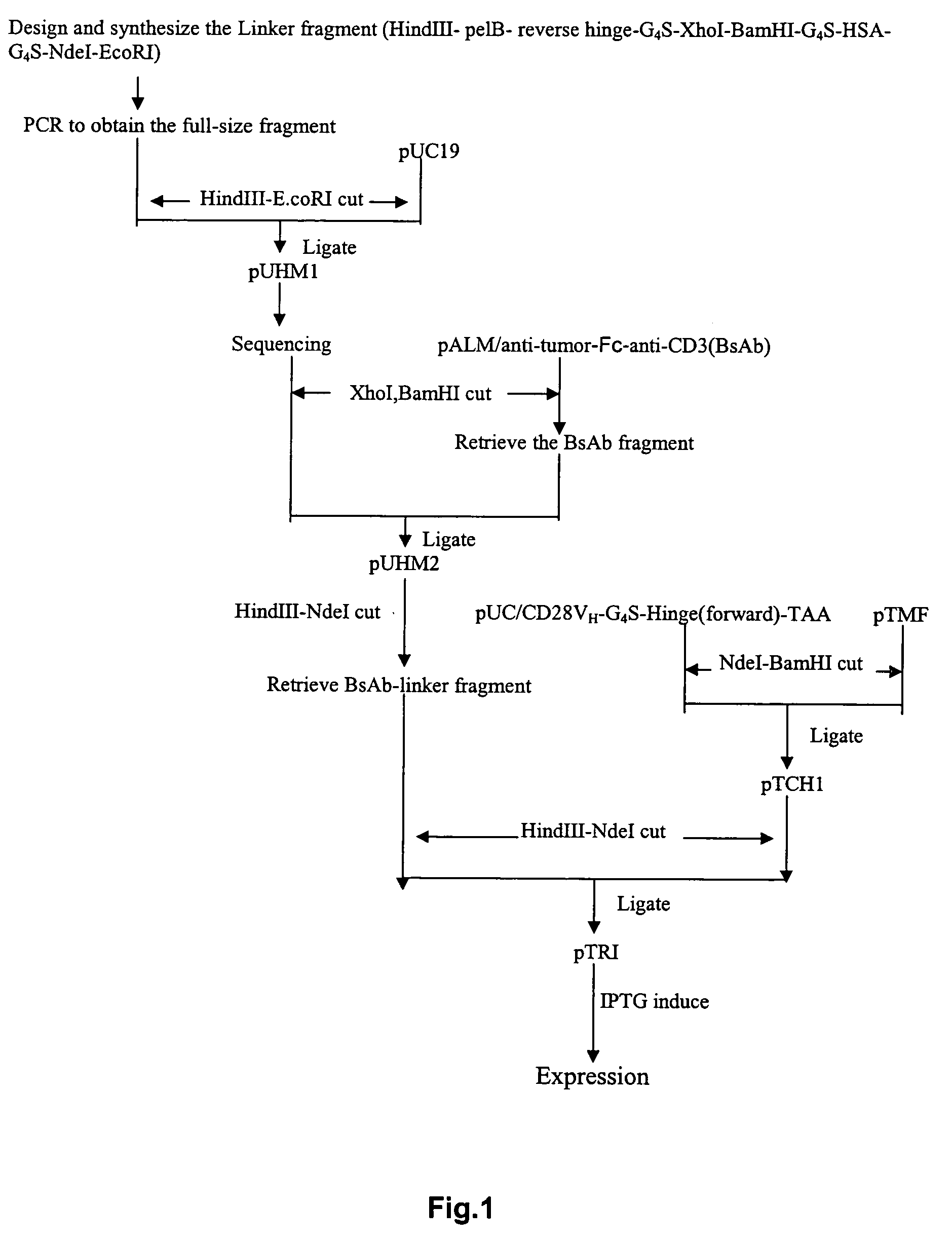
The invention provides a cyclic single-chain trispecific antibody against human tumor. It comprises three parts. The first part is an anti-tumor Fab antibody, an anti-tumor single-domain antibody or an scFv. The second part is a reshaped Fab antibody against human CD3, a reshaped single-domain antibody against human CD3 or a reshaped scFv against human CD3. The third part is a reshaped Fab antibody against human CD28, a reshaped single-domain antibody against human CD28 or a reshaped scFv against human CD28. The present invention also offers the DNA sequence coding for this trispecific antibody, expression vectors containing this DNA sequence and host cells (E. coli) containing the vectors.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Monoclonal antibodies detection methods for enzymes that confer resistance to 2,4-dichlorophenoxyacetic acid in plants
ActiveUS8460891B2Animal cellsImmunoglobulins against cytokines/lymphokines/interferonsMonoclonal antibodyDioxygenase
Owner:CORTEVA AGRISCIENCE LLC
Heteromultimeric molecules
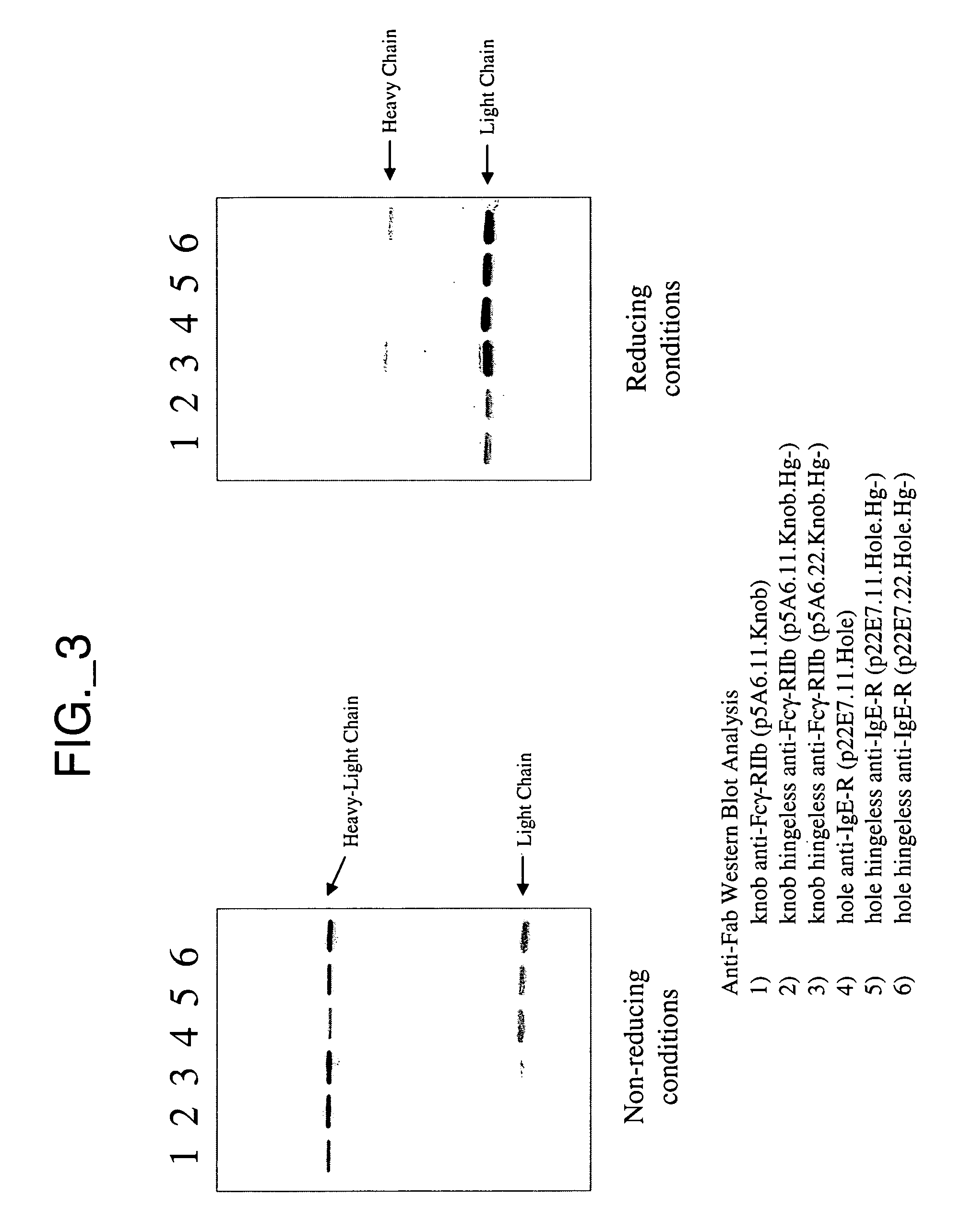
InactiveUS20060204493A1Facilitates heterodimerizationHigh yieldAntipyreticAnalgesicsVirologyAntibody
Owner:GENENTECH INC
Small Molecule Modulators of HIV-1 Capsid Stability and Methods Thereof
InactiveUS20130165489A1Inhibiting and suppressing and preventing viral infectionBiocideOrganic chemistryViral infectionVirology
The present invention includes a method of inhibiting, suppressing or preventing a viral infection in a subject, comprising administering to the subject a pharmaceutical composition comprising one or more of the compounds useful within the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
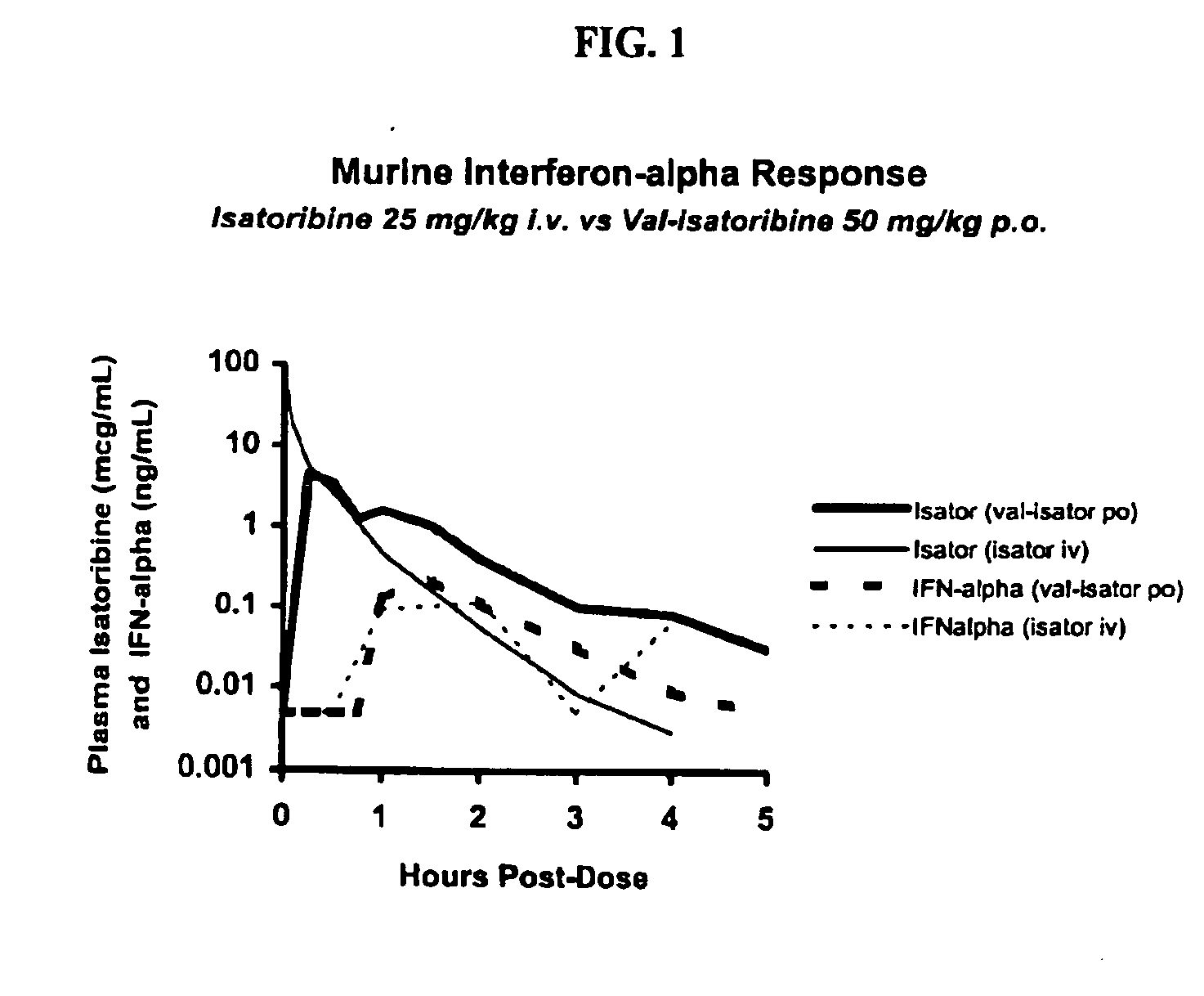
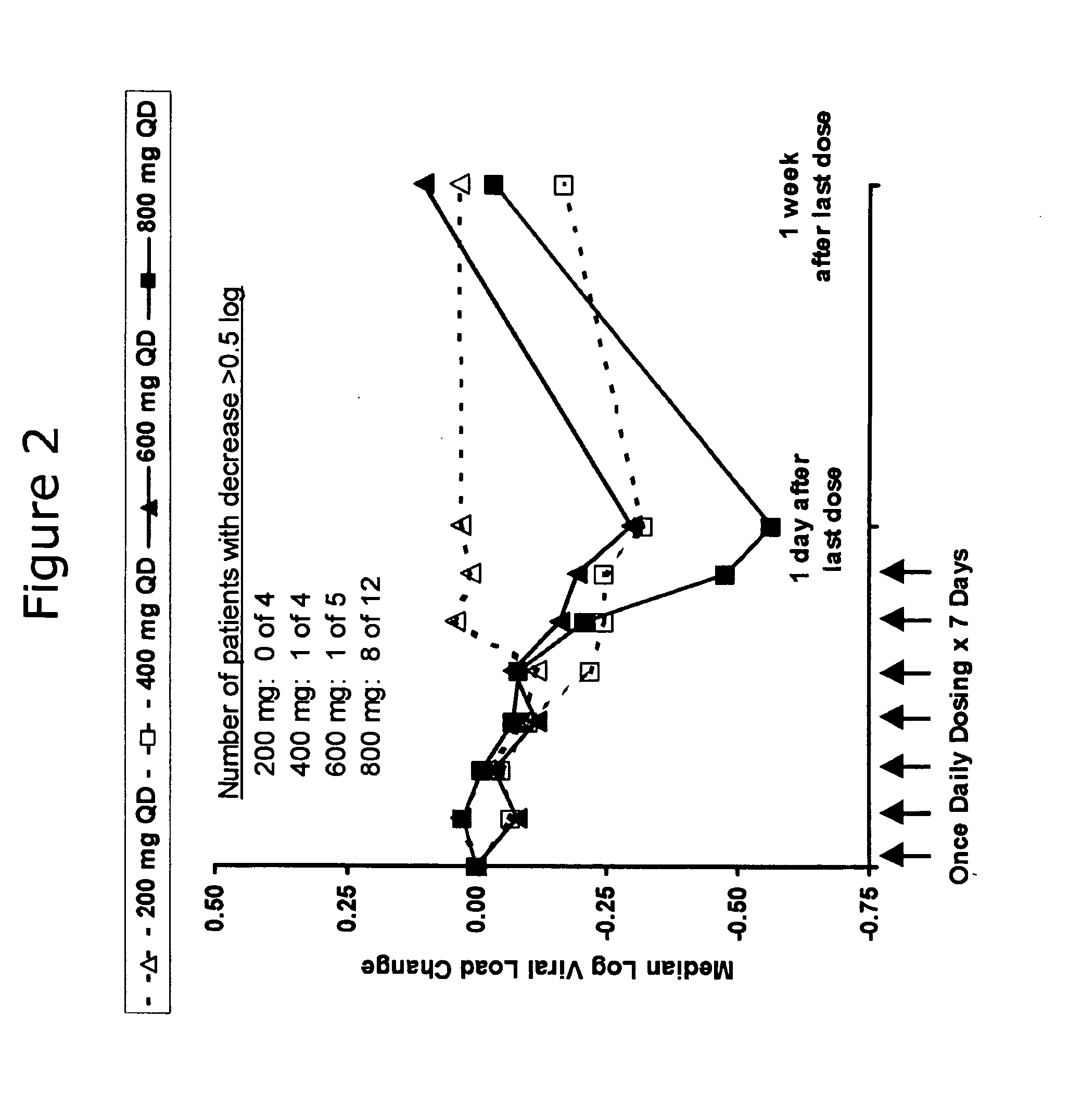
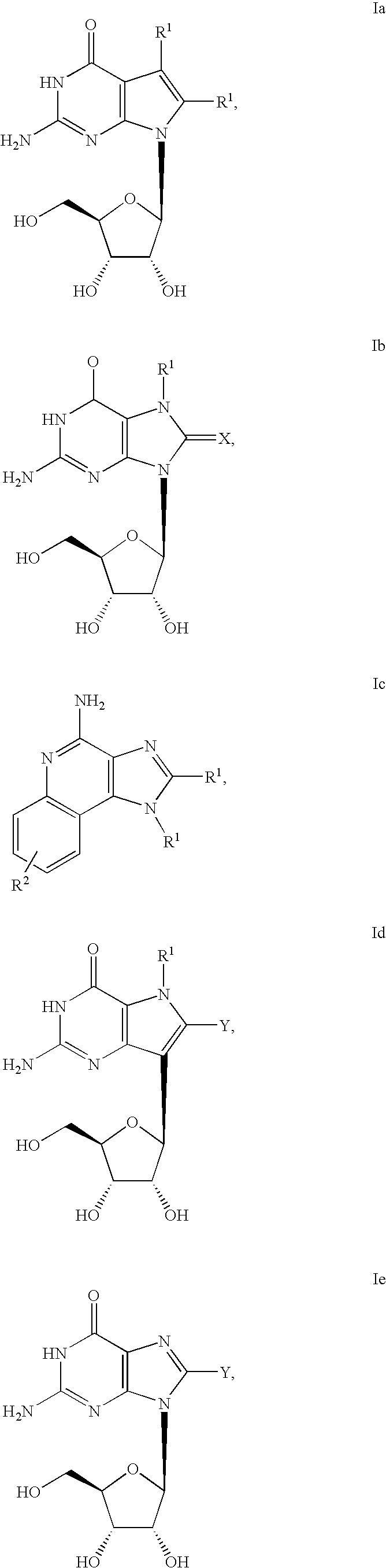
Administration of TLR7 ligands and prodrugs thereof for treatment of infection by hepatitis C virus
InactiveUS20050054590A1Reduce sensitivityAvoid spreadingBiocideDigestive systemHepatitis c viralSide effect
This invention relates to methods for treating or preventing hepatitis C virus infections in mammals using Toll-Like Receptor (TLR)7 ligands and prodrugs thereof. More particularly, this invention relates to methods of orally administering a therapeutically effective amount of one or more prodrugs of TLR7 ligands for the treatment or prevention of hepatitis C viral infection. Oral administration of these TLR7 immunomodulating ligands and prodrugs thereof to a mammal provides therapeutically effective amounts and reduced undesirable side effects.
Owner:ANDADYS PHARMA INC
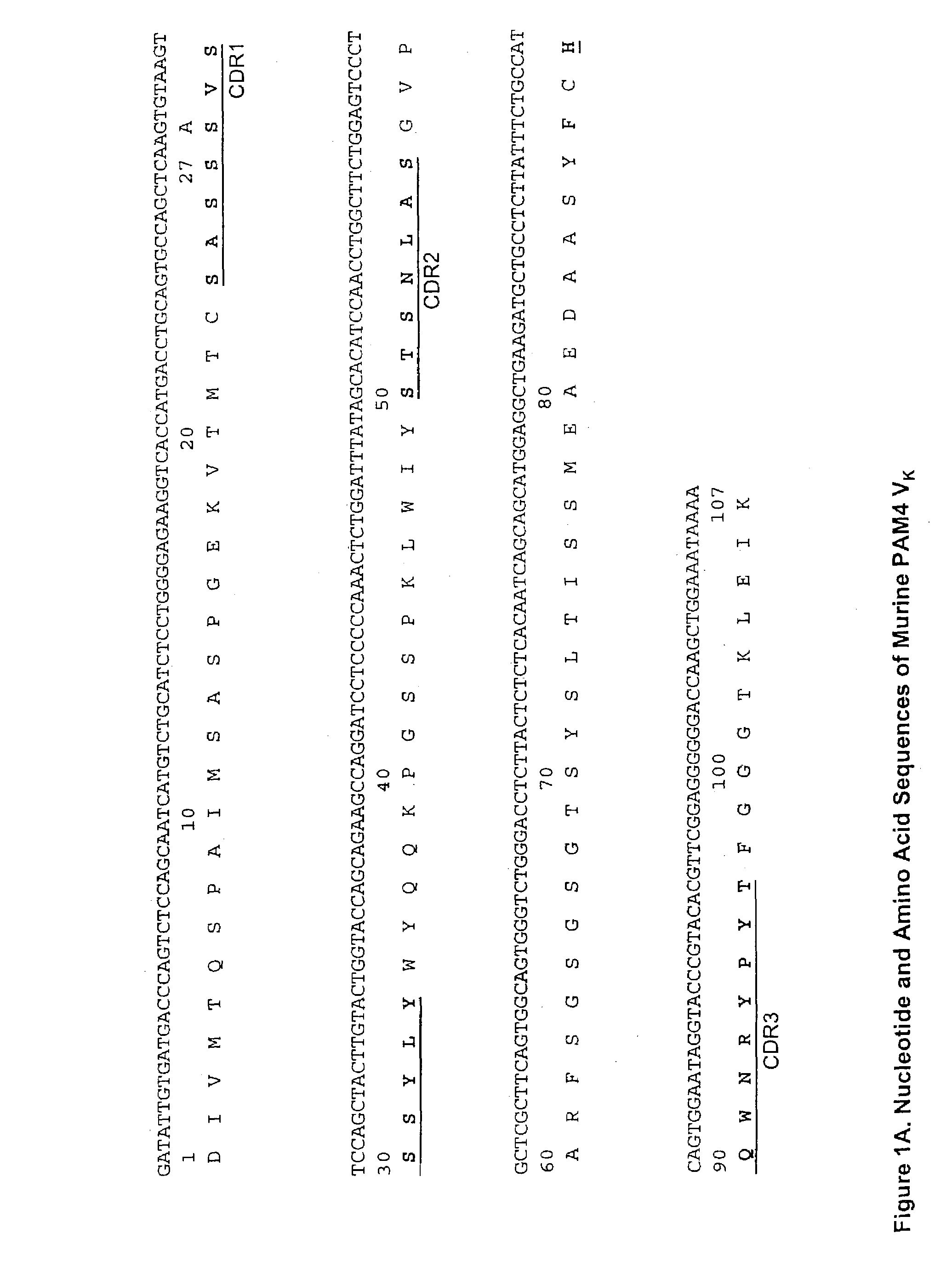
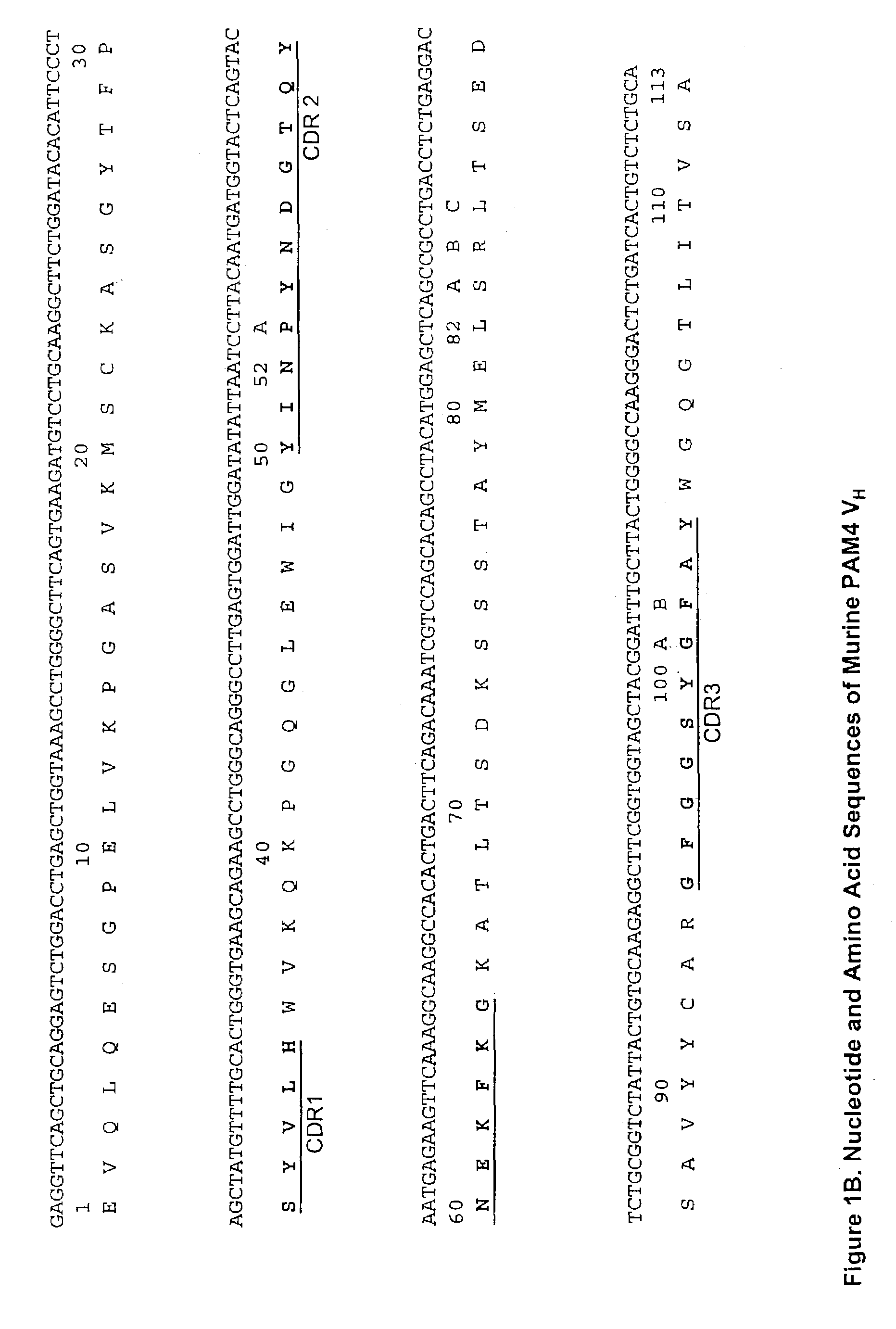
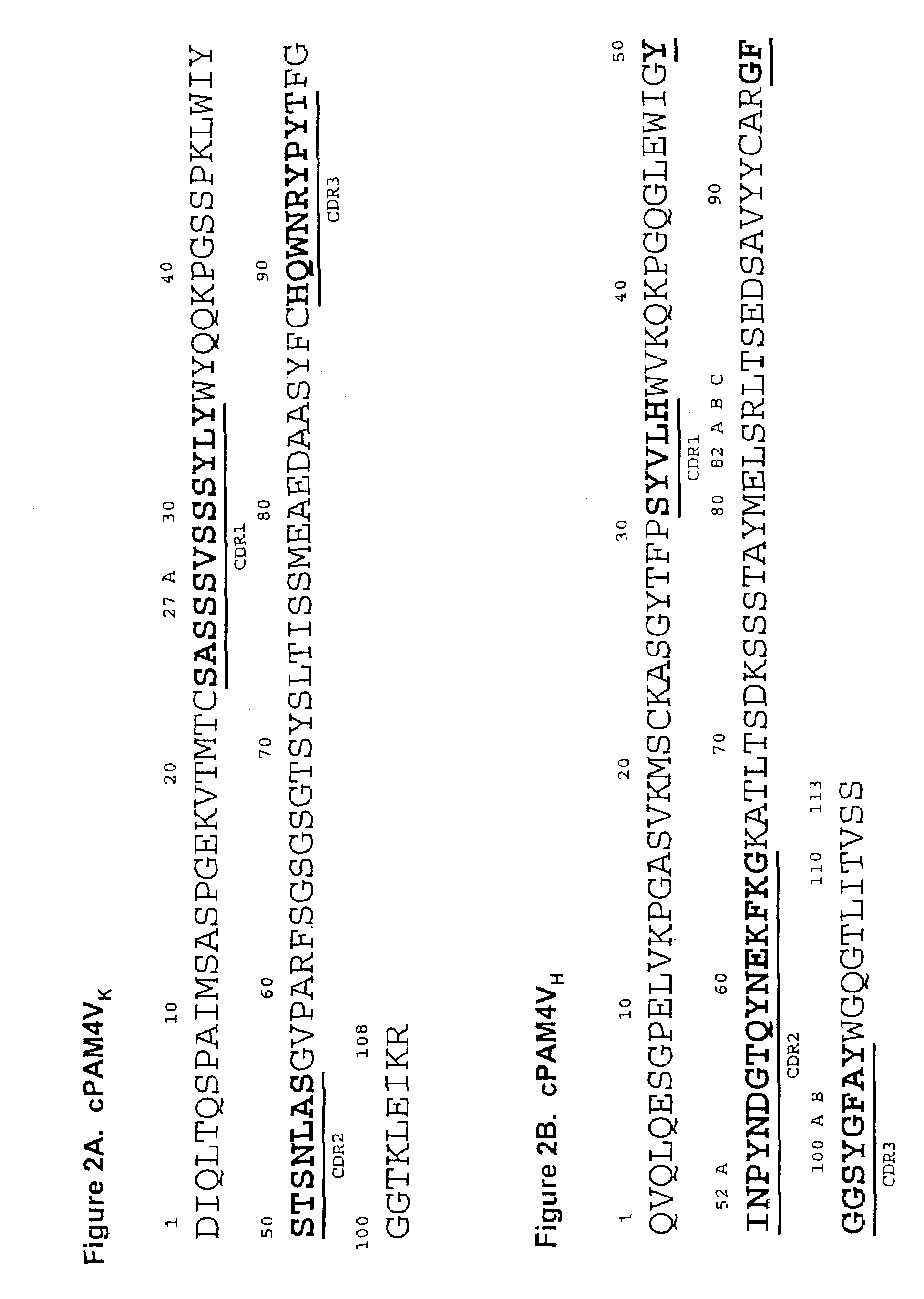
Monoclonal antibody hPAM4
This invention relates to monovalent and multivalent, monospecific antibodies and to multivalent, multispecific antibodies. One embodiment of these antibodies has one or more identical binding sites where each binding site binds with a target antigen or an epitope on a target antigen. Another embodiment of these antibodies has two or more binding sites where these binding sites have affinity towards different epitopes on a target antigen or different target antigens, or have affinity towards a target antigen and a hapten. The present invention further relates to recombinant vectors useful for the expression of these functional antibodies in a host. More specifically, the present invention relates to the tumor-associated antibody designated PAM4. The invention further relates to humanized and human PAM4 antibodies, and the use of such antibodies in diagnosis and therapy.
Owner:IMMUNOMEDICS INC
AAV's and uses thereof
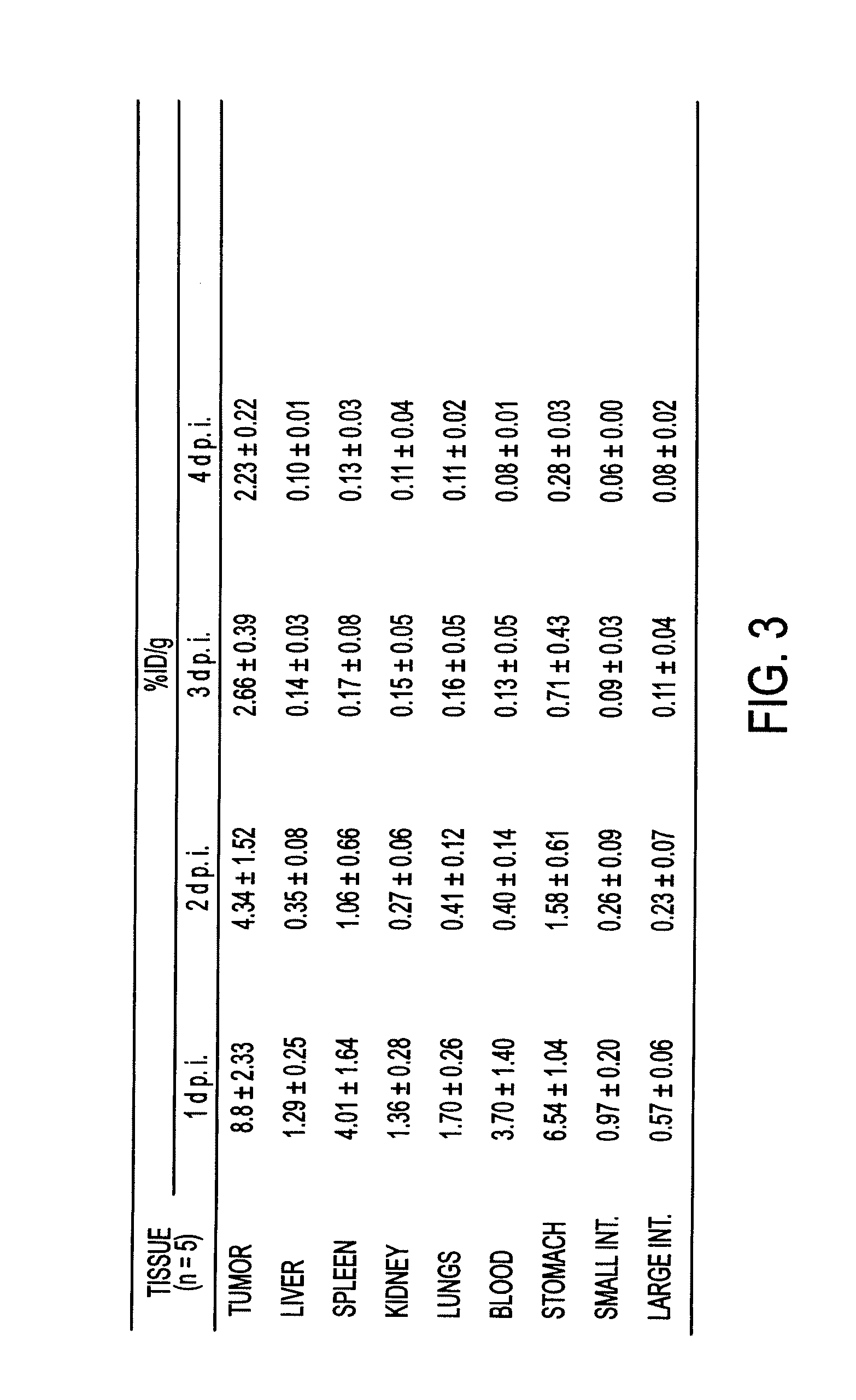
The invention in some aspects relates to recombinant adeno-associated viruses having distinct tissue targeting capabilities. In some aspects, the invention relates to gene transfer methods using the recombinant adeno-associate viruses. In some aspects, the invention relates to isolated AAV capsid proteins and isolated nucleic acids encoding the same.
Owner:UNIV OF MASSACHUSETTS
Single cell bar-coding for antibody discovery
Provided herein are methods and composition for immune repertoire sequencing and single cell barcoding for heavy and IgL pairing if antibodies.
Owner:ABVITRO LLC
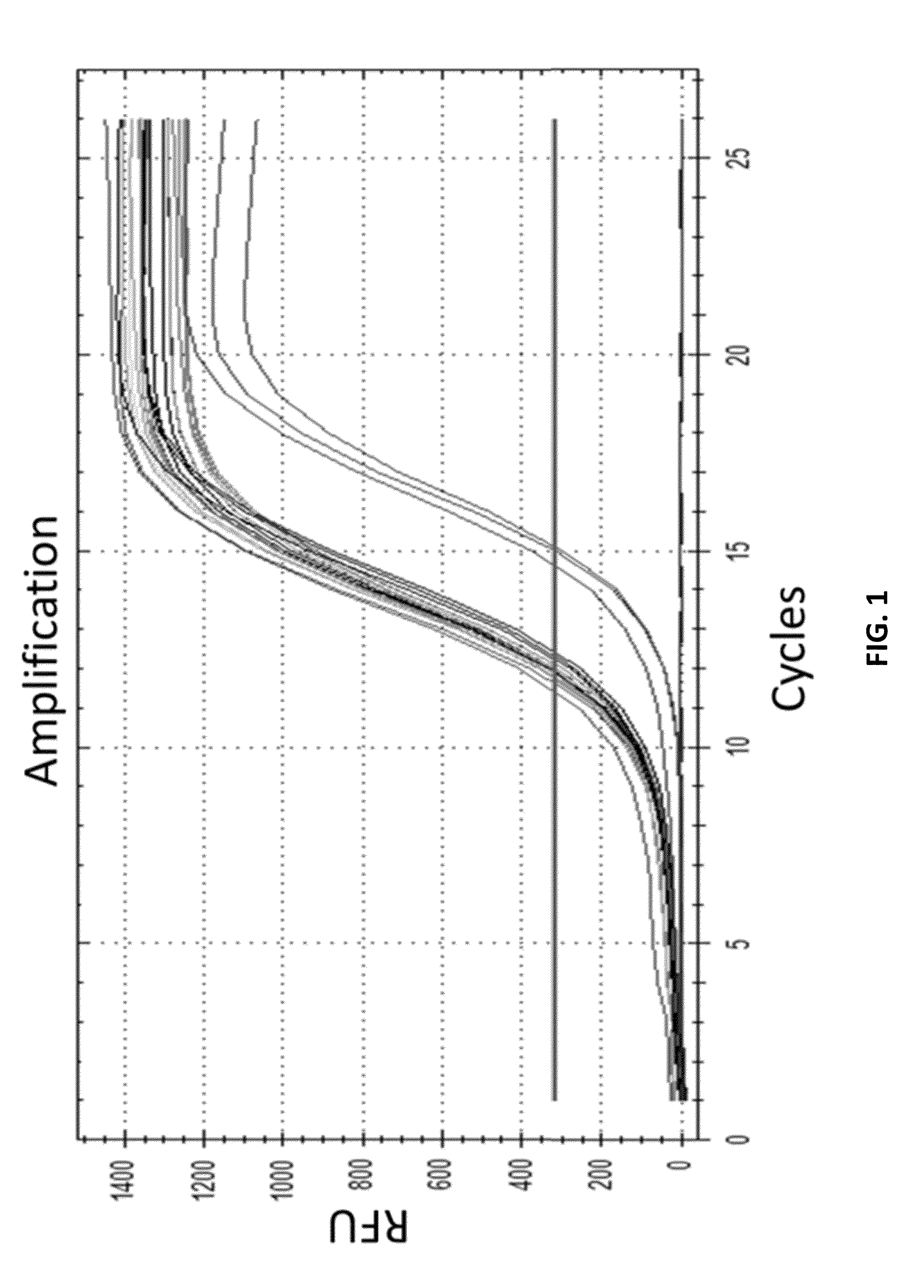
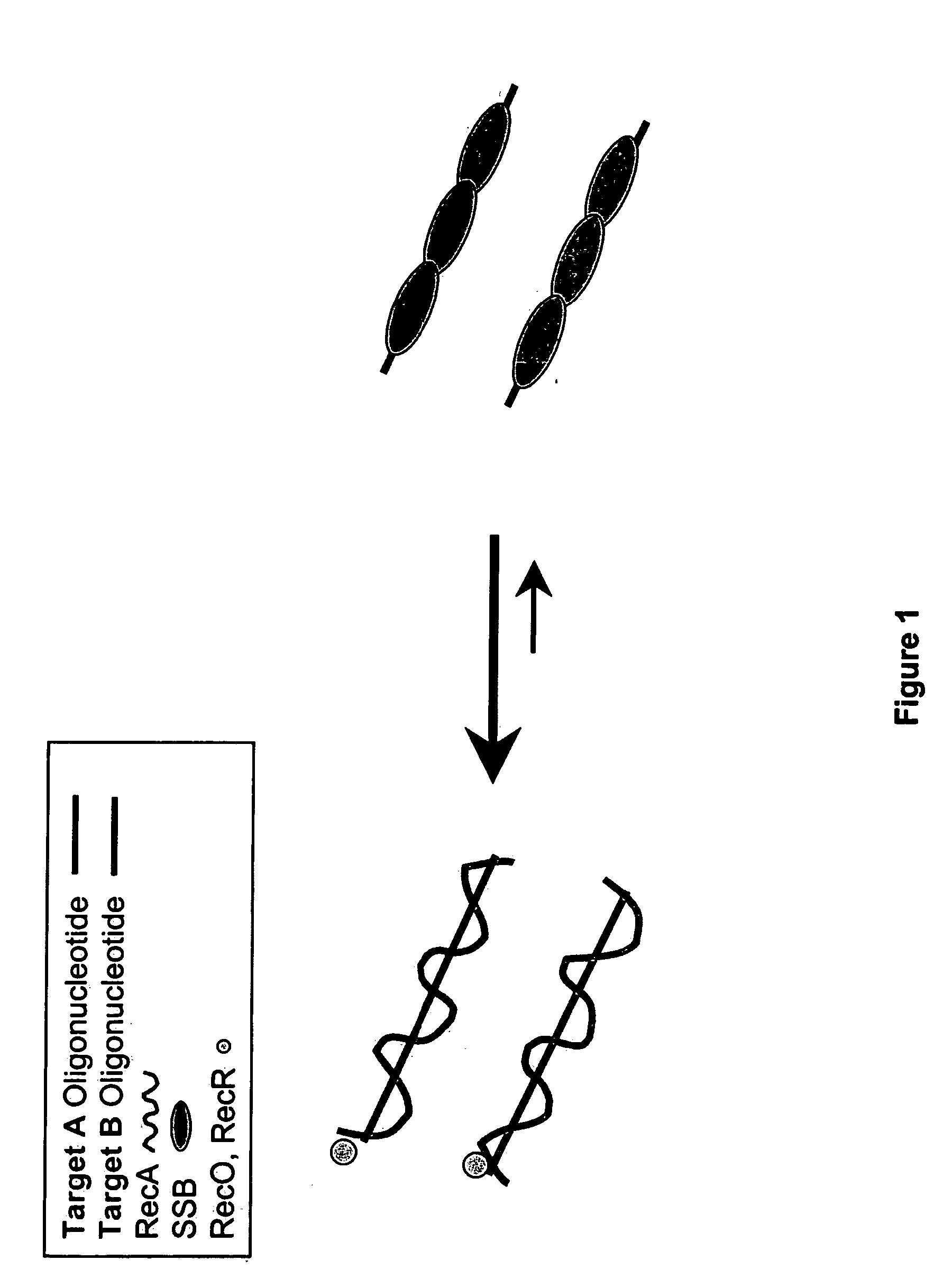
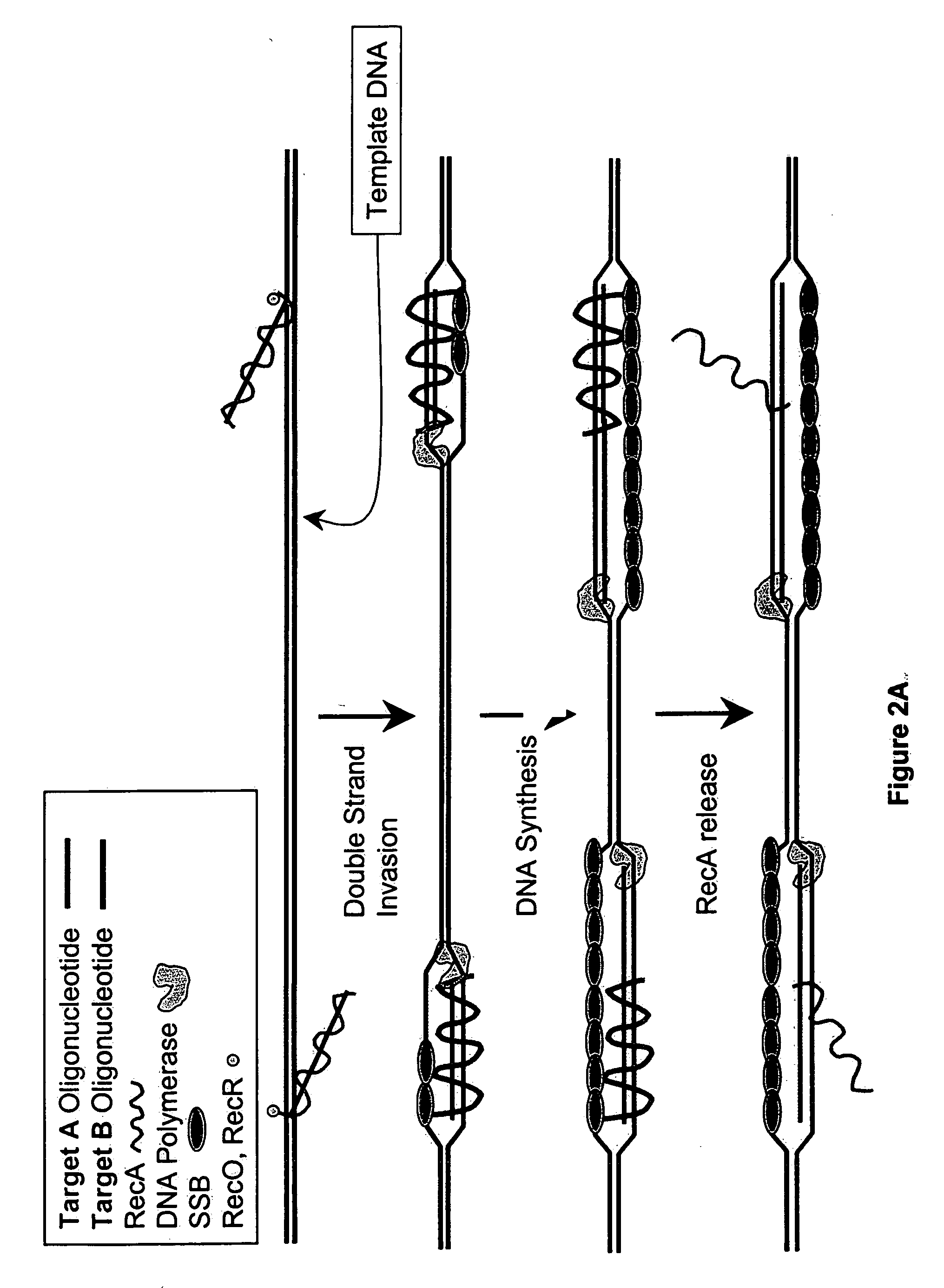
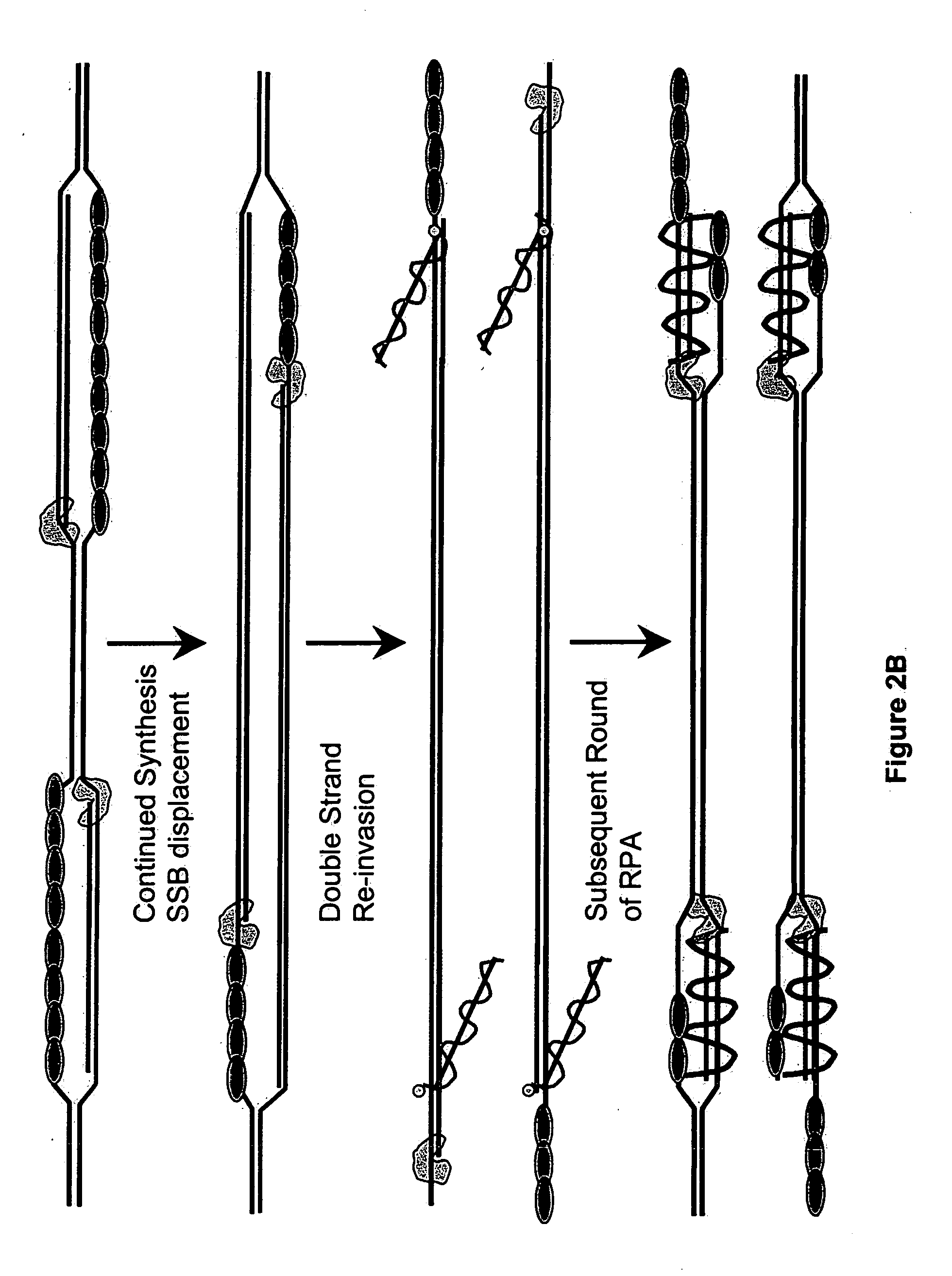
Recombinase polymerase amplification
ActiveUS20050112631A1HydrolasesMicrobiological testing/measurementRecombinase Polymerase AmplificationSingle strand
This disclosure describe three related novel methods for Recombinase-Polymerase Amplification (RPA) of a target DNA that exploit the properties of recombinase and related proteins, to invade double-stranded DNA with single stranded homologous DNA permitting sequence specific priming of DNA polymerase reactions. The disclosed methods have the advantage of not requiring thermocycling or thermophilic enzymes. Further, the improved processivity of the disclosed methods may allow amplification of DNA up to hundreds of megabases in length.
Owner:ABBOTT DIAGNOSTICS SCARBOROUGH INC
Generation of heavy-chain only antibodies in transgenic animals
InactiveUS20090307787A1Maximizing numberIncrease diversityHybrid cell preparationImmunoglobulinsHeavy chainMammal
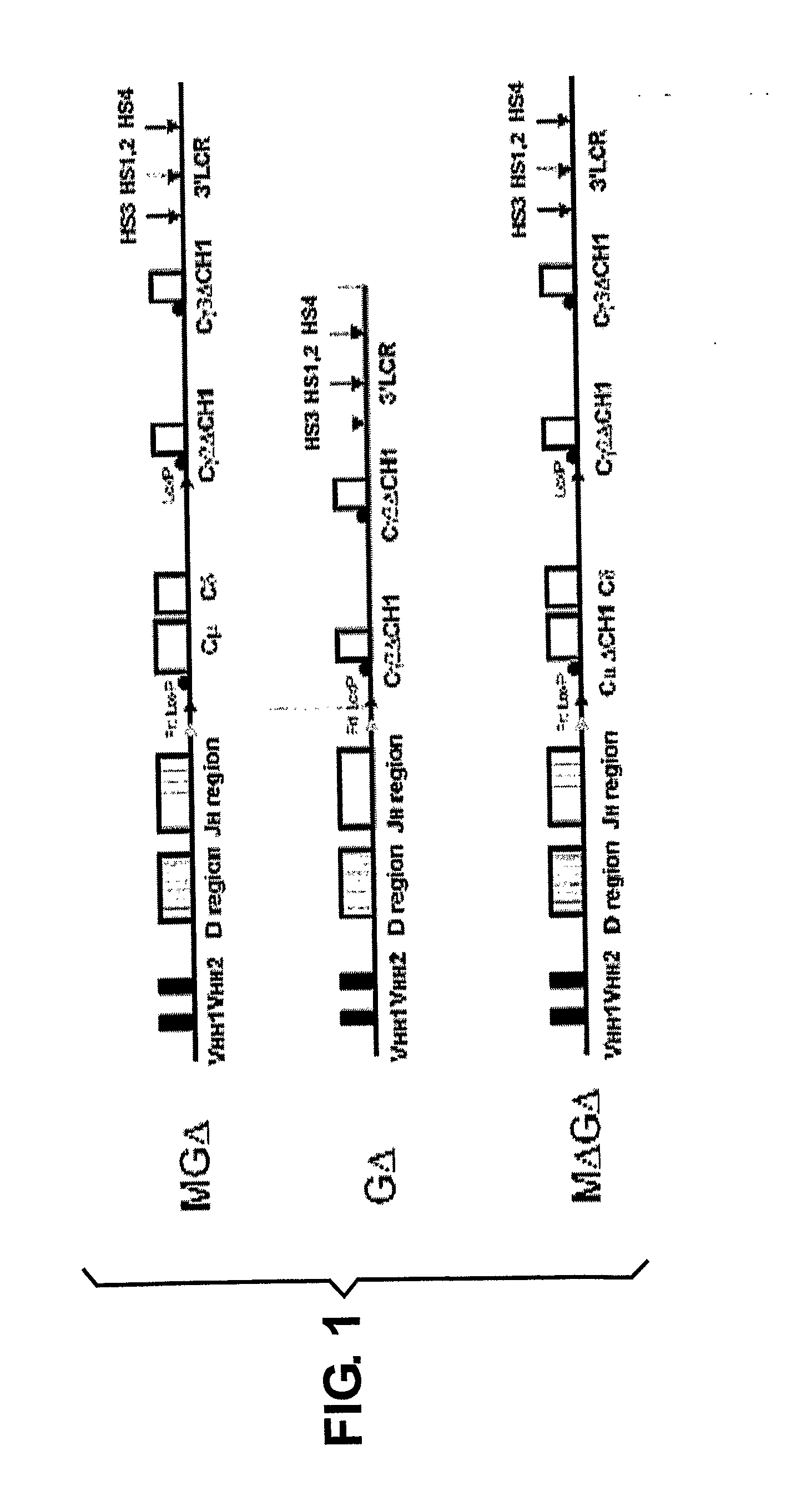
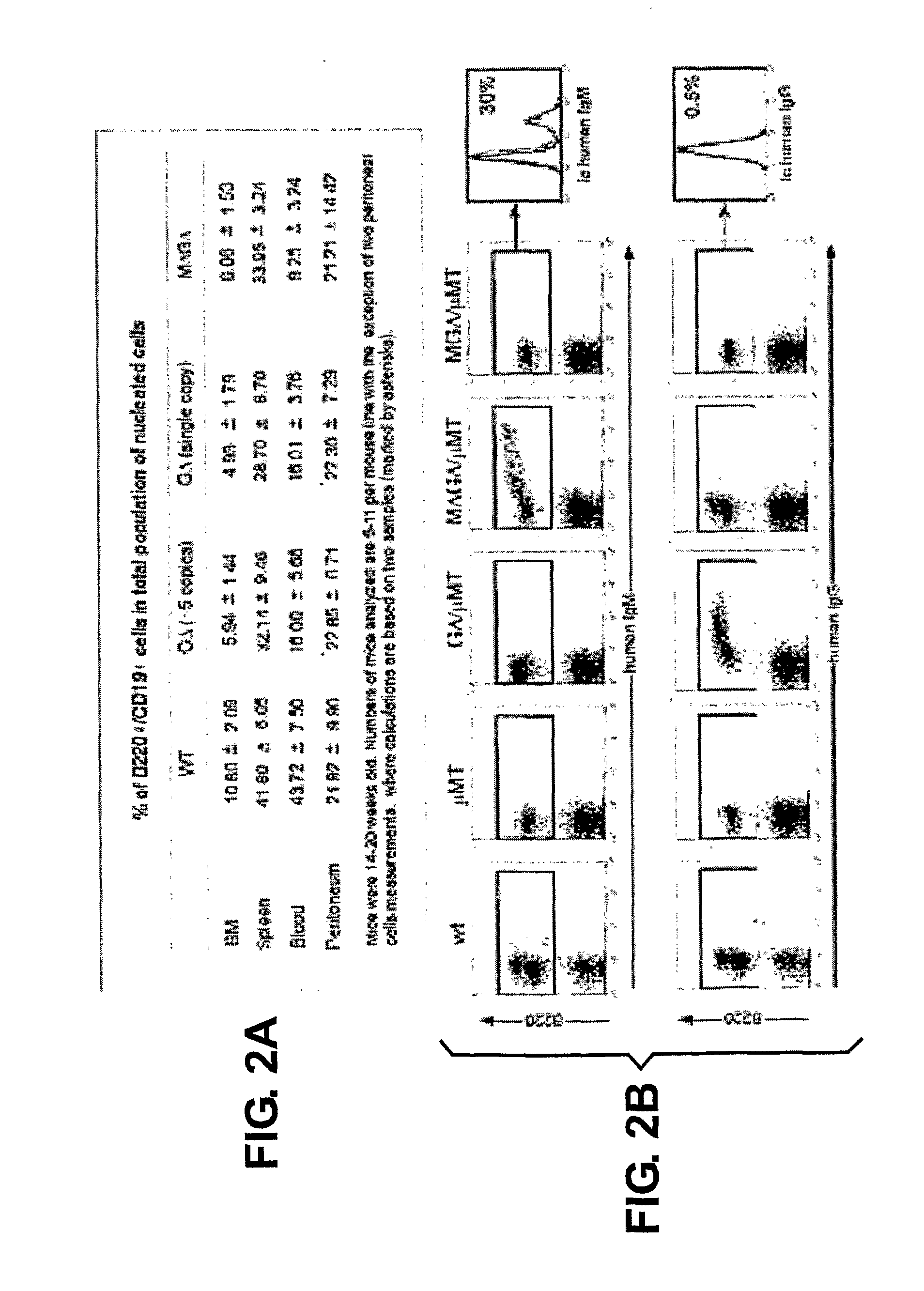
The present invention relates to a method for the generation of VH heavy chain-only antibodies in a transgenic non-human mammal. In particular, the present invention relates to a method for the production of a VH heavy chain-only antibody in a transgenic non-human mammal comprising the step of expressing more than one heterologous VH heavy chain locus in that mammal.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
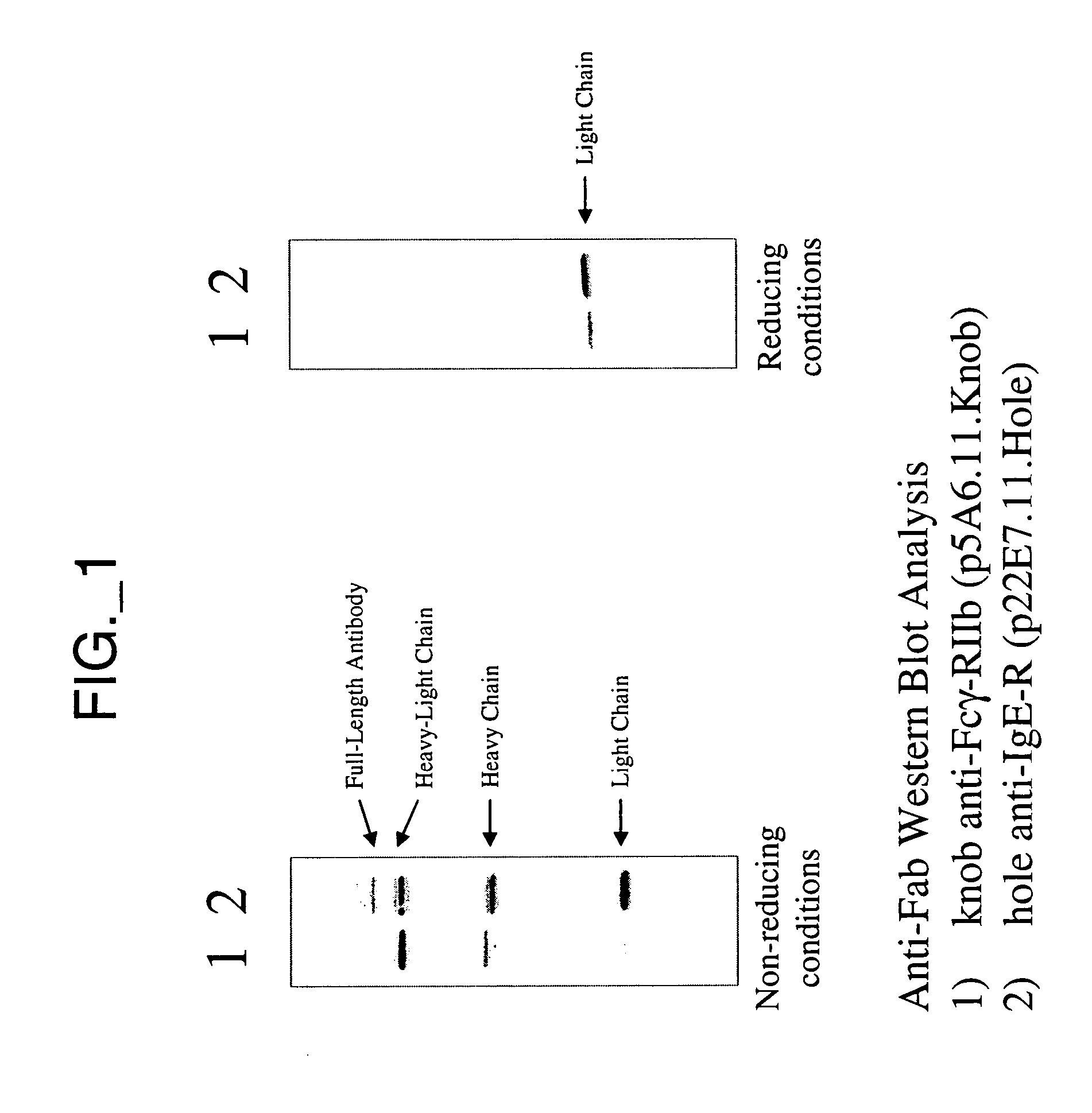
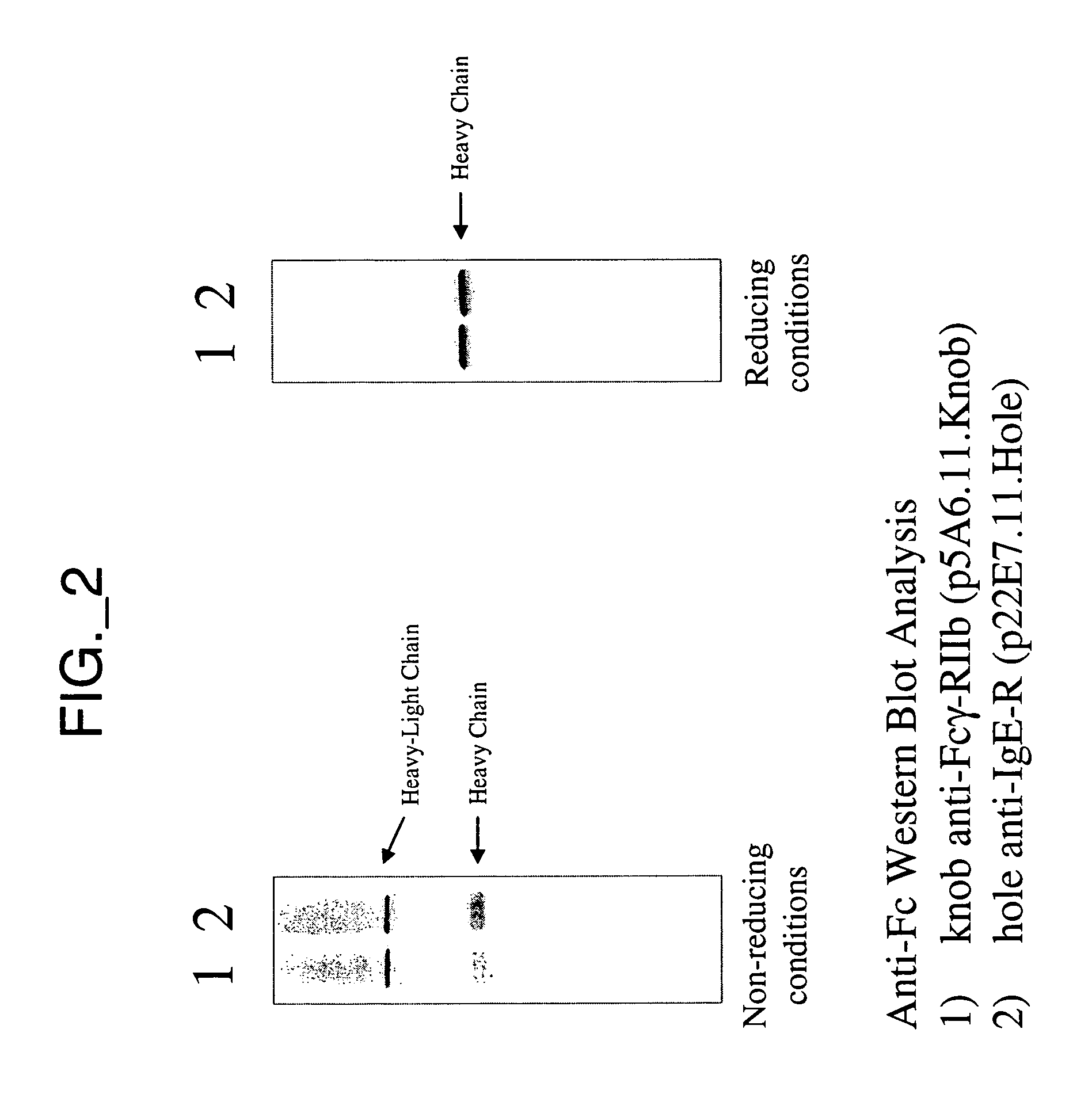
Bispecific Antibody Point Mutations for Enhancing Rate of Clearance
A mutant bispecific antibody that includes (a) a human hinge constant region from IgG having one or more amino acid mutations in the CH2 domain, (b) two scFvs; and (c) two Fvs has been constructed. This type of antibody displays enhanced clearance, which has been found to be particularly useful in the context of pre-targeting methods.
Owner:IMMUNOMEDICS INC
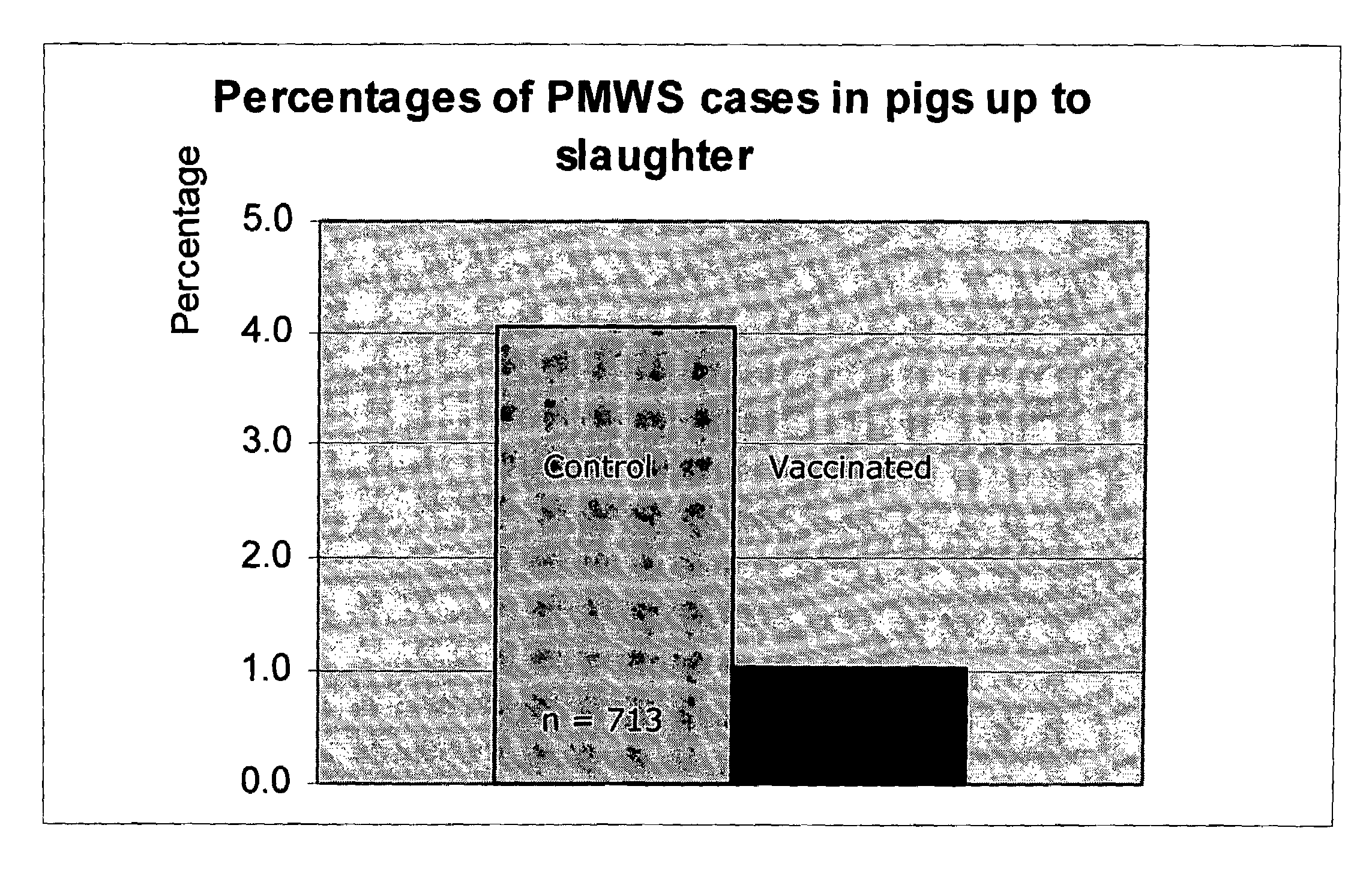
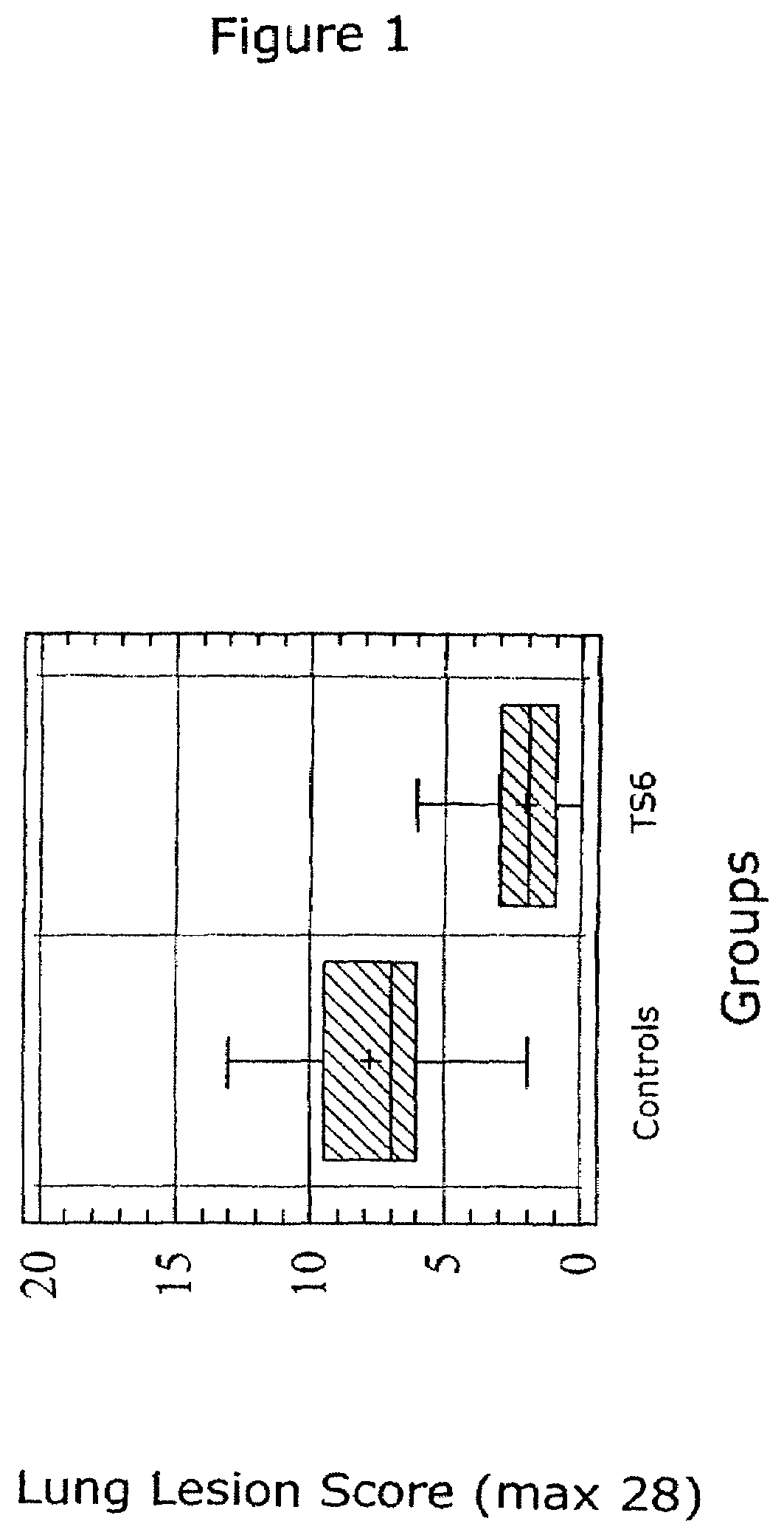
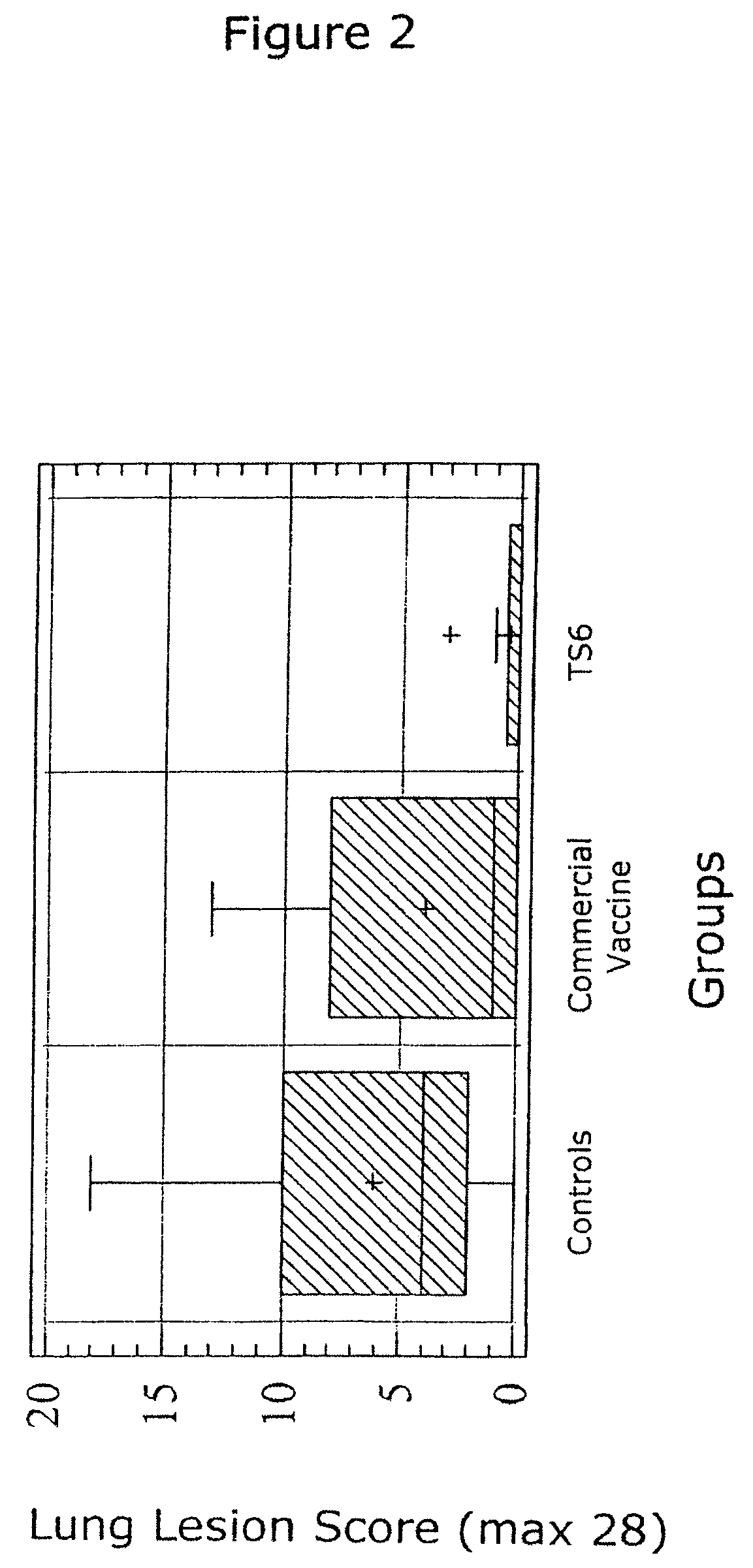
Vaccine formulations
ActiveUS7371395B2Improve stabilityStable and safe and easily administrableSsRNA viruses negative-senseAntibacterial agentsPlasmidBacilli
Owner:MERIAL INC
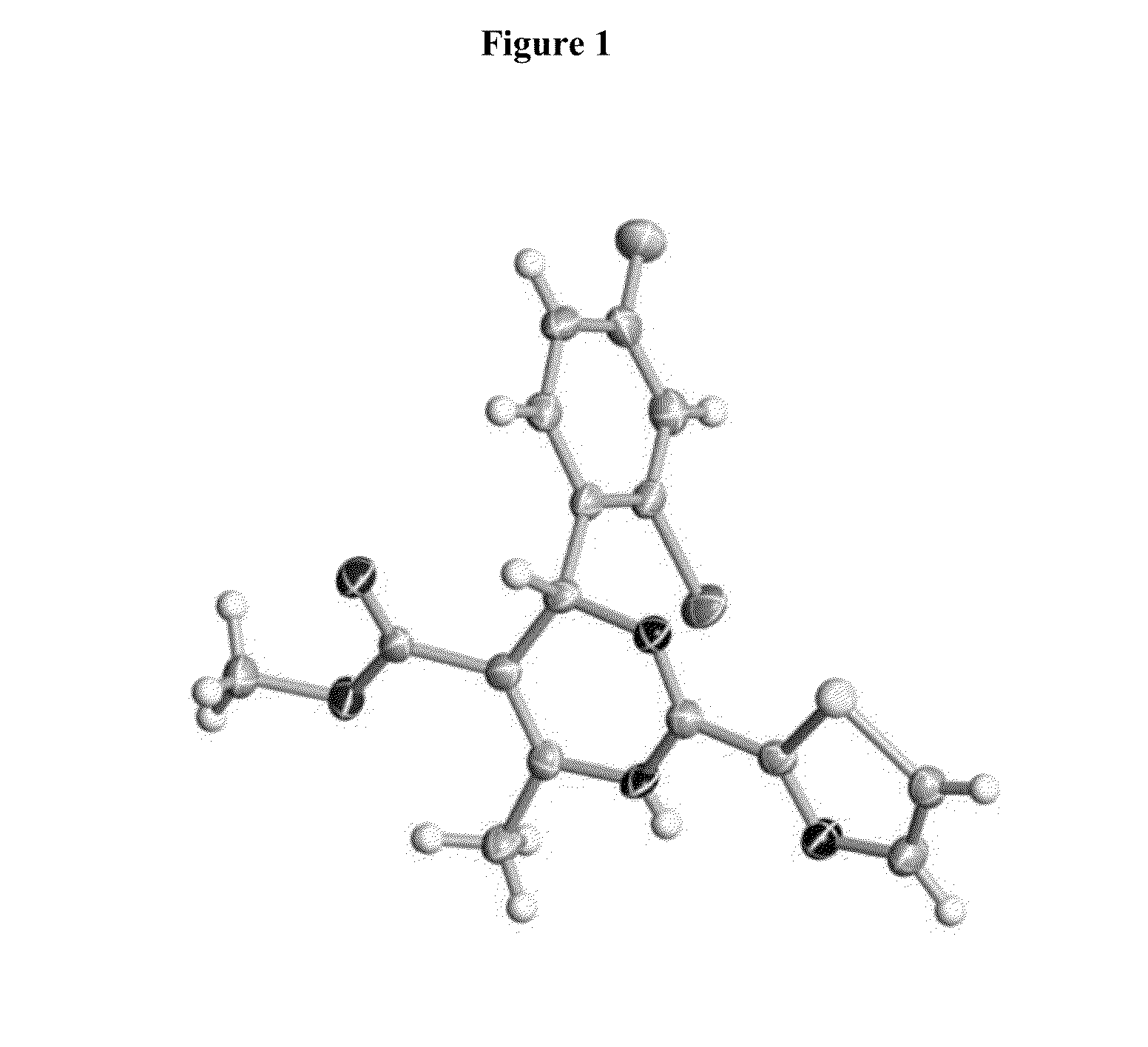
SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR
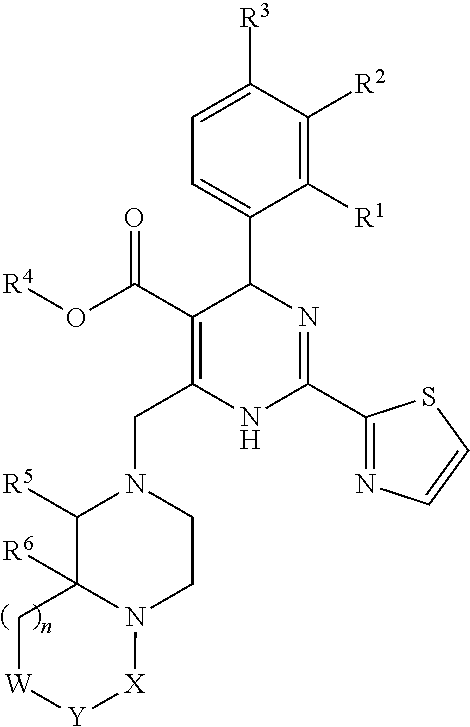
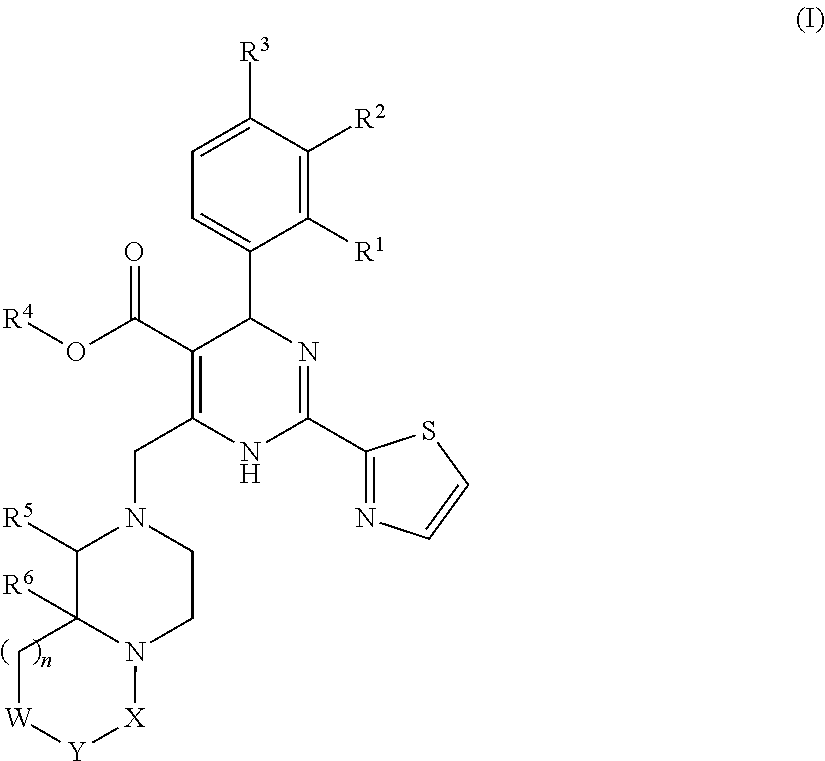
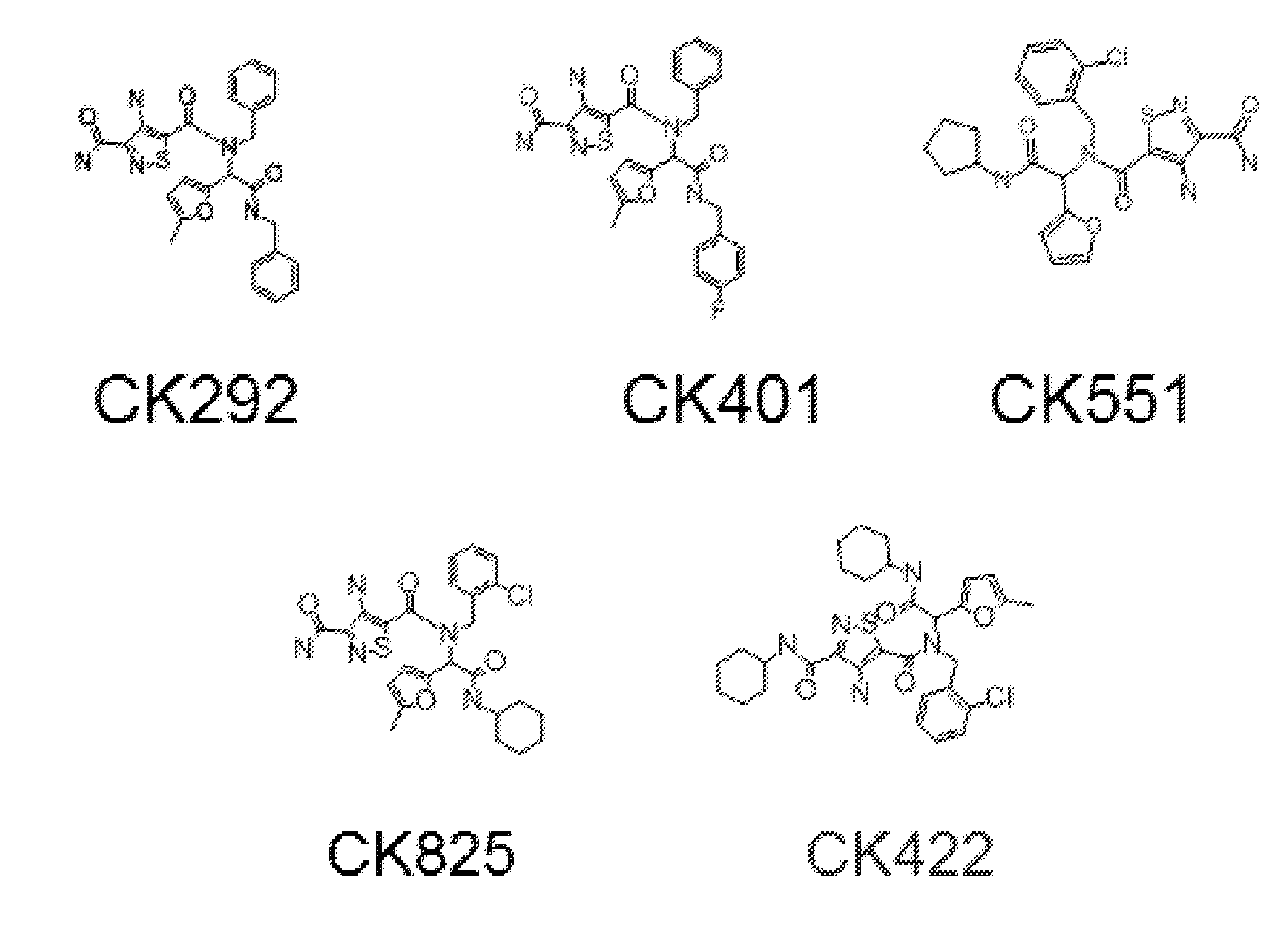
InactiveUS20140221380A1Strong inhibitory activityLess side effectsBiocideOrganic chemistryPyrazinePharmaceutical drug
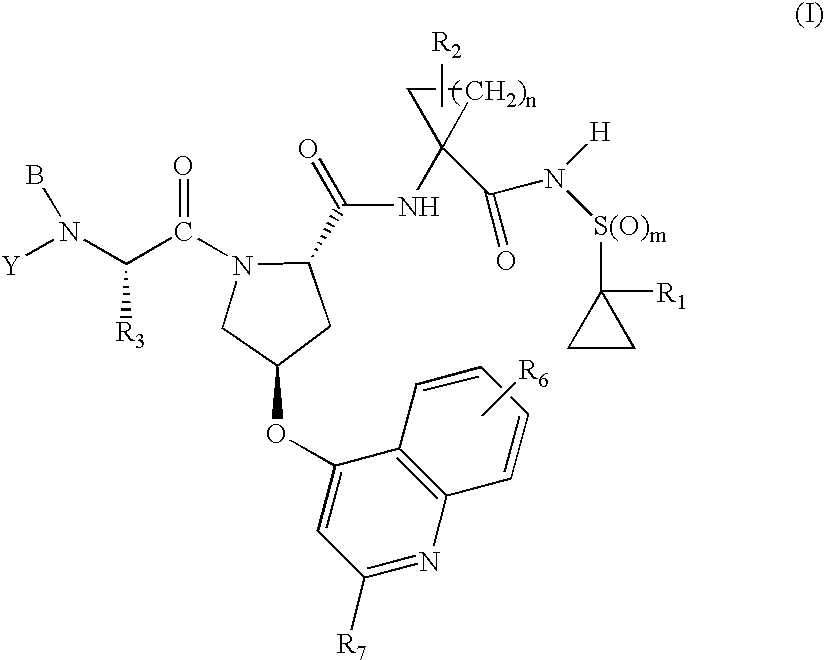
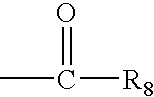
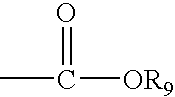
[Summary][Problem] Provided is a substituted spiropyrido[1,2-a]pyrazine derivative or a pharmaceutically acceptable salt thereof, which is useful as an anti-HIV agent.[Solving Means] The present invention relates to a compound represented by the following formula [I] or [II] or a pharmaceutically acceptable salt thereof:wherein each symbol is as defined in the specification.
Owner:JAPAN TOBACCO INC
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Method to screen phage display libraries with different ligands
InactiveUS20040038291A2Immunoglobulin superfamilyLibrary screeningPolyphageImmunoglobulin superfamily
Abstract of the Disclosure The present invention relates to methods for selecting repertoires of polypeptides using generic and target ligands. In particular, the invention relates to a library comprising a repertoire of polypeptides of the immunoglobulin superfamily, wherein the members of the repertoire have a known main chain conformation.
Owner:DORMANTIS LTD
Novel 6-fused heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis B virus infection
Owner:F HOFFMANN LA ROCHE INC
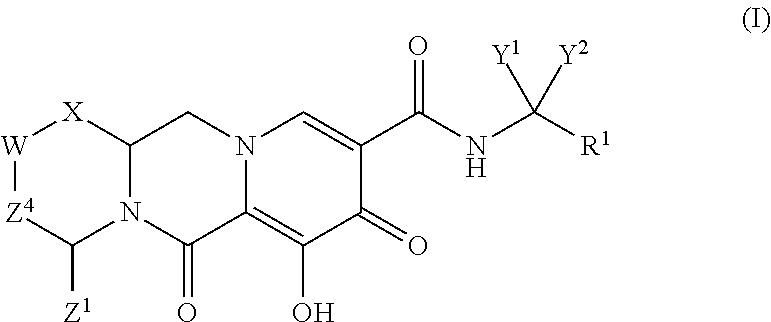
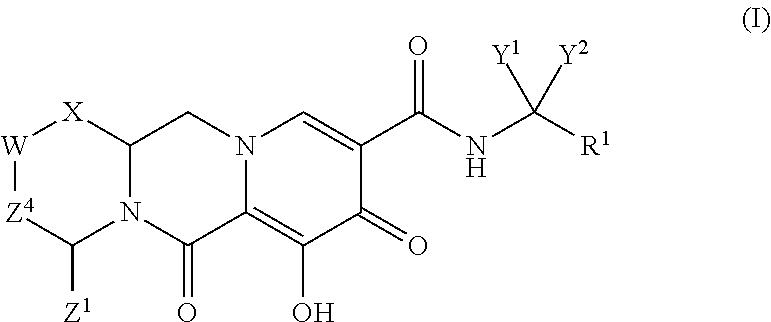
Polycyclic-carbamoylpyridone compounds and their pharmaceutical use
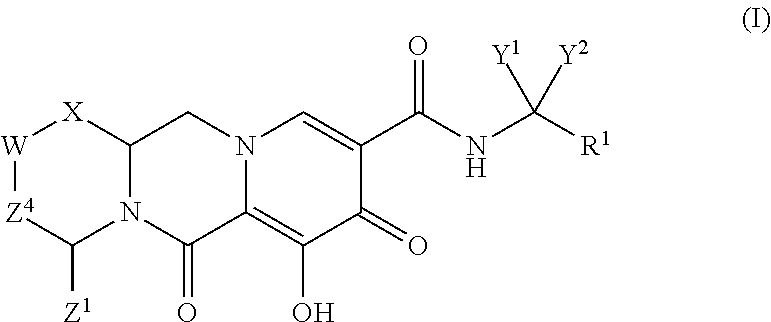
ActiveUS20140221356A1Inhibitory activityReduce HIV replicationBiocideOrganic chemistryImmunodeficiency virusAcyl group
Compounds for use in the treatment of human immunodeficiency virus (HIV) infection are disclosed. The compounds have the following Formula (I):including stereoisomers and pharmaceutically acceptable salts thereof, wherein R1, X, W, Y1, Y2, Z1, and Z4 are as defined herein. Methods associated with preparation and use of such compounds, as well as pharmaceutical compositions comprising such compounds, are also disclosed.
Owner:GILEAD SCI INC
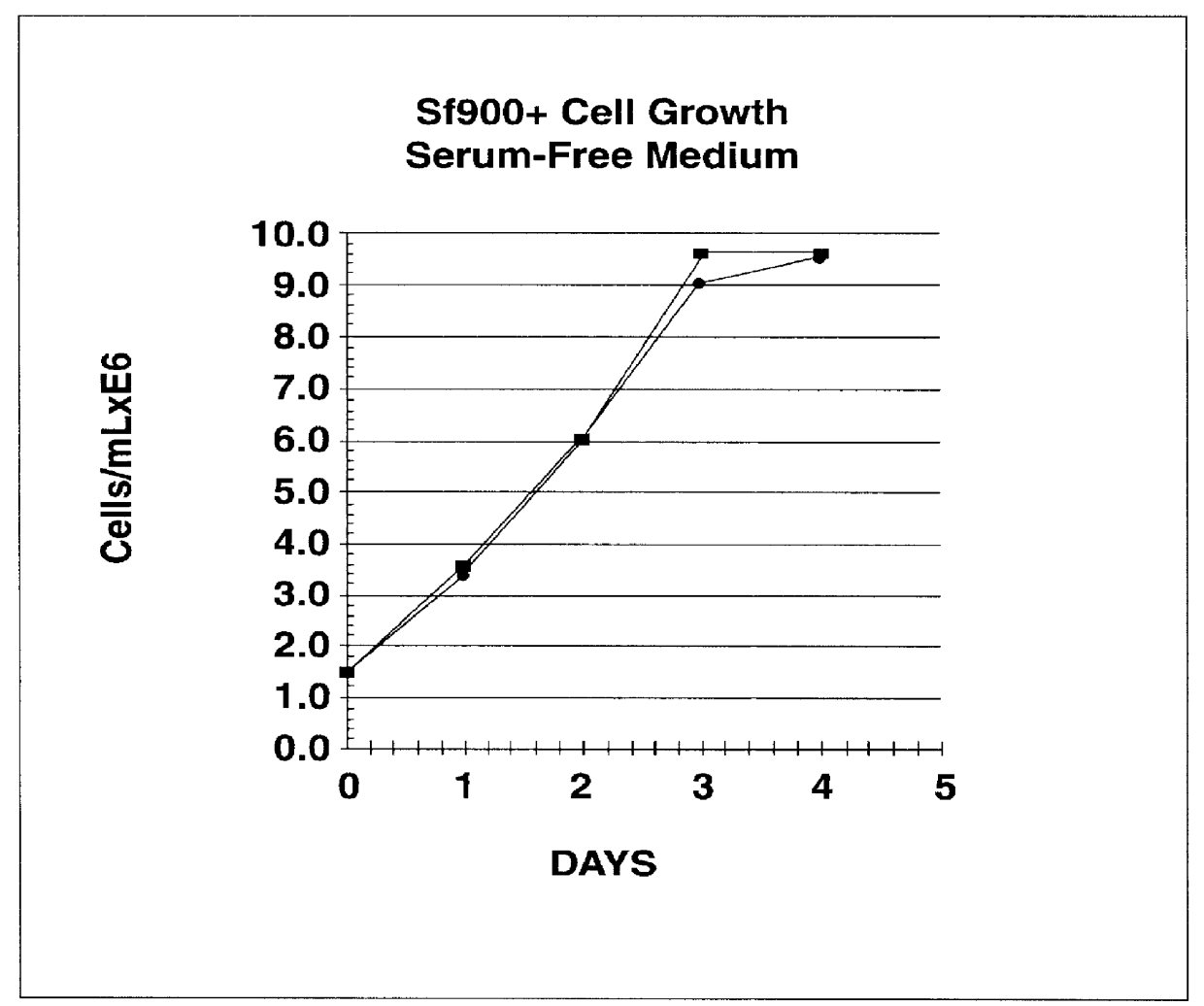
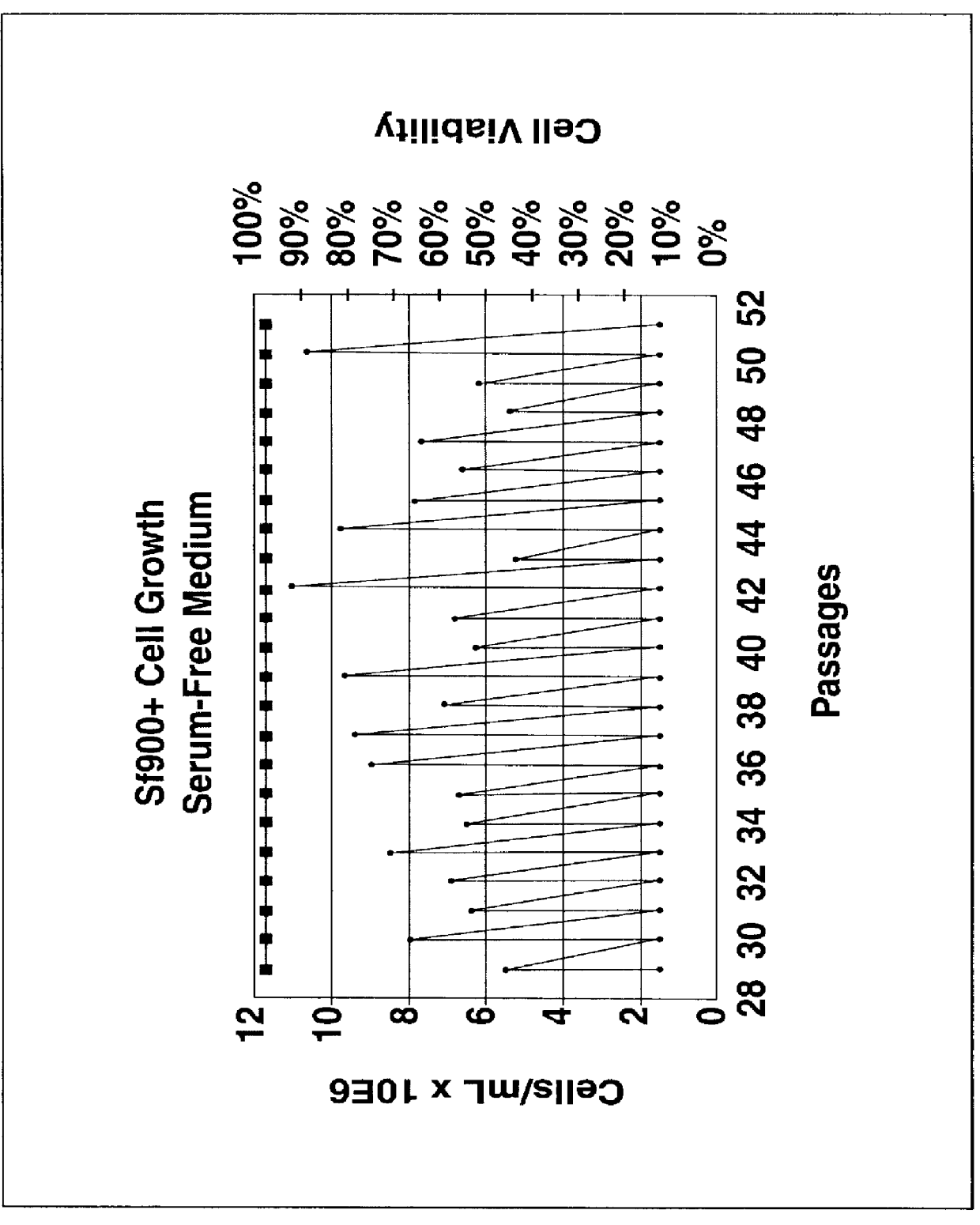
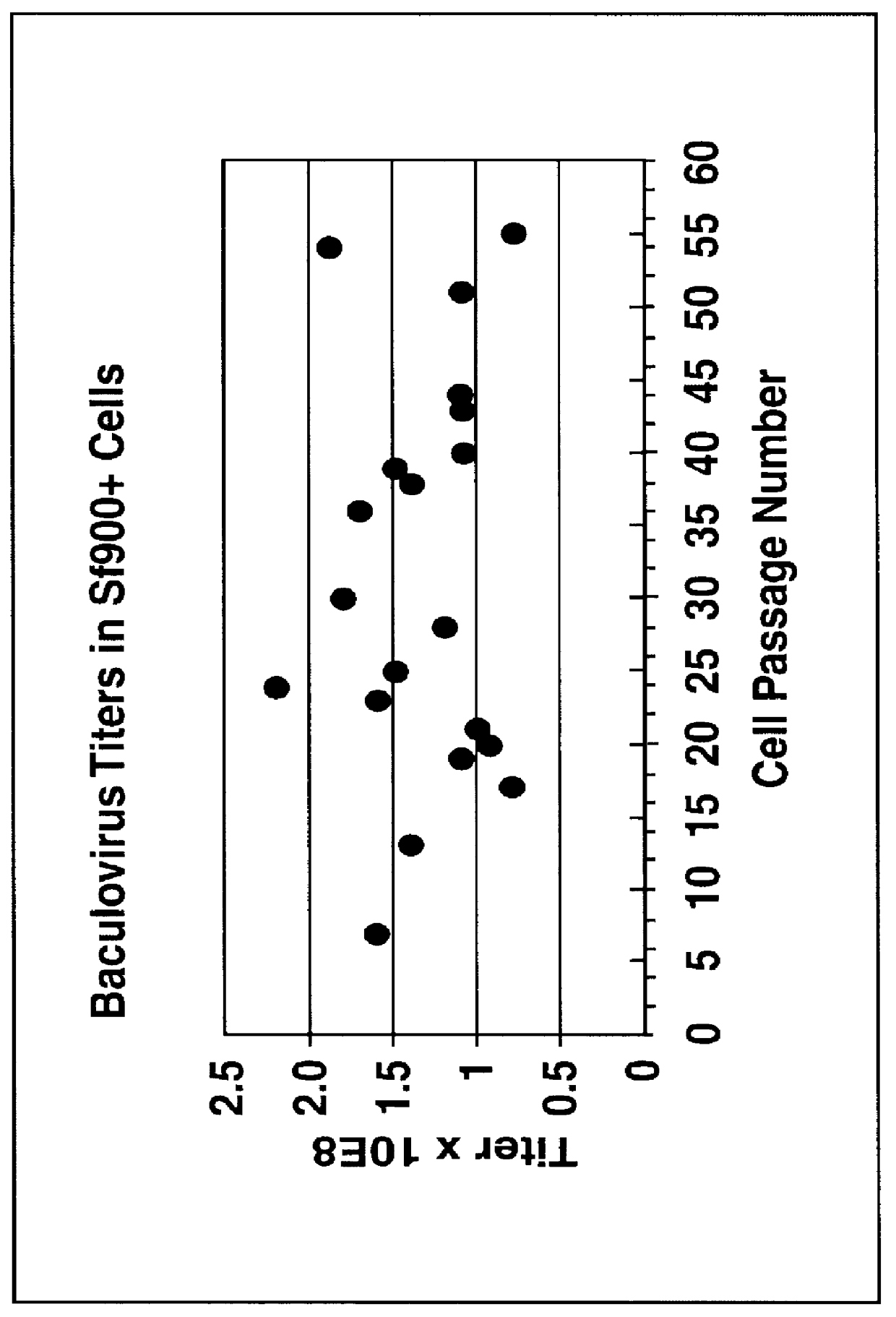
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Method for the preparation of purified HCV RNA by exosome separation
InactiveUS7198923B1Simple preparation processPromote progressSsRNA viruses positive-senseMicrobiological testing/measurementExosomeBlood plasma
Owner:GRIFOLS WORLDWIDE OPERATIONS +1
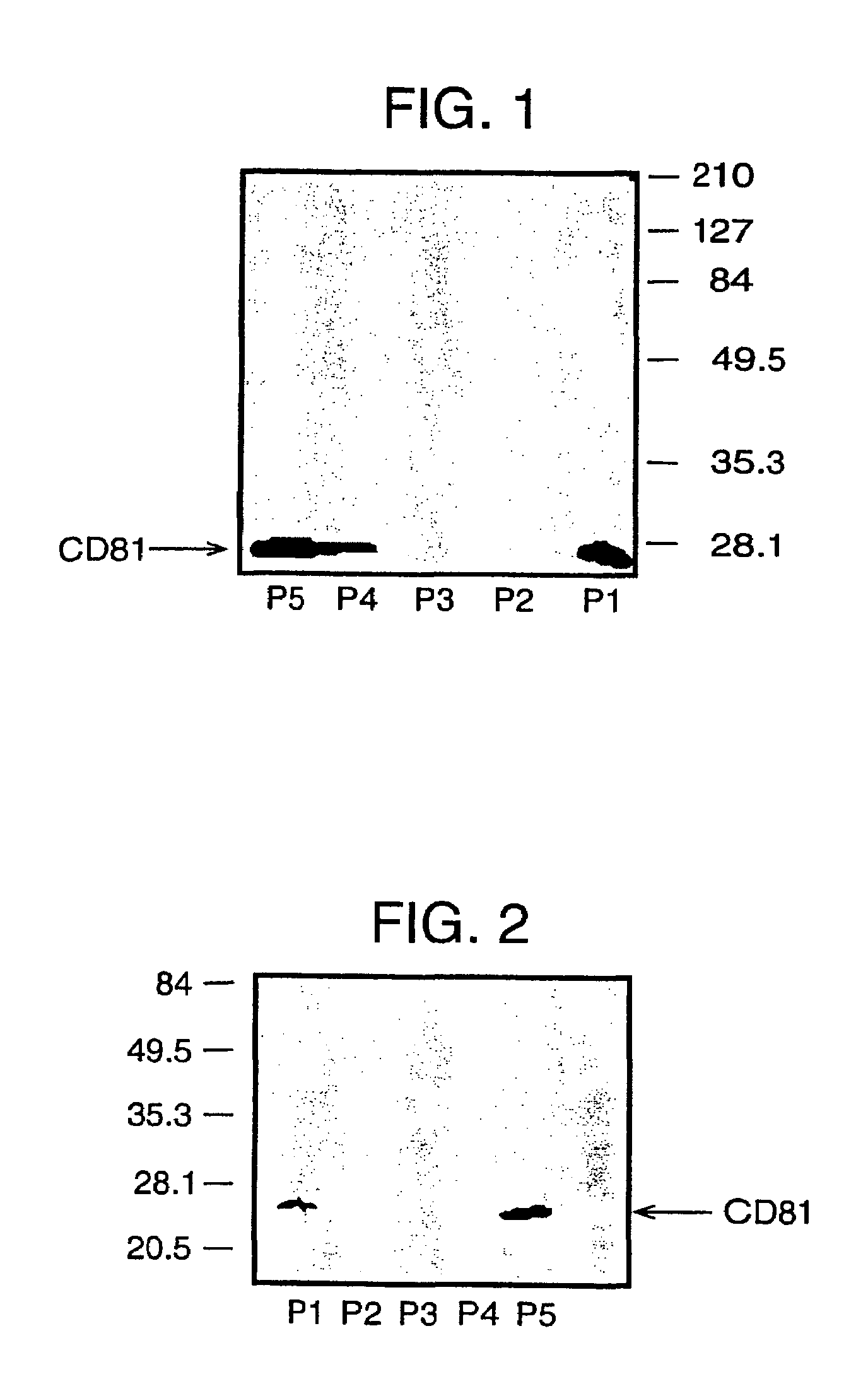
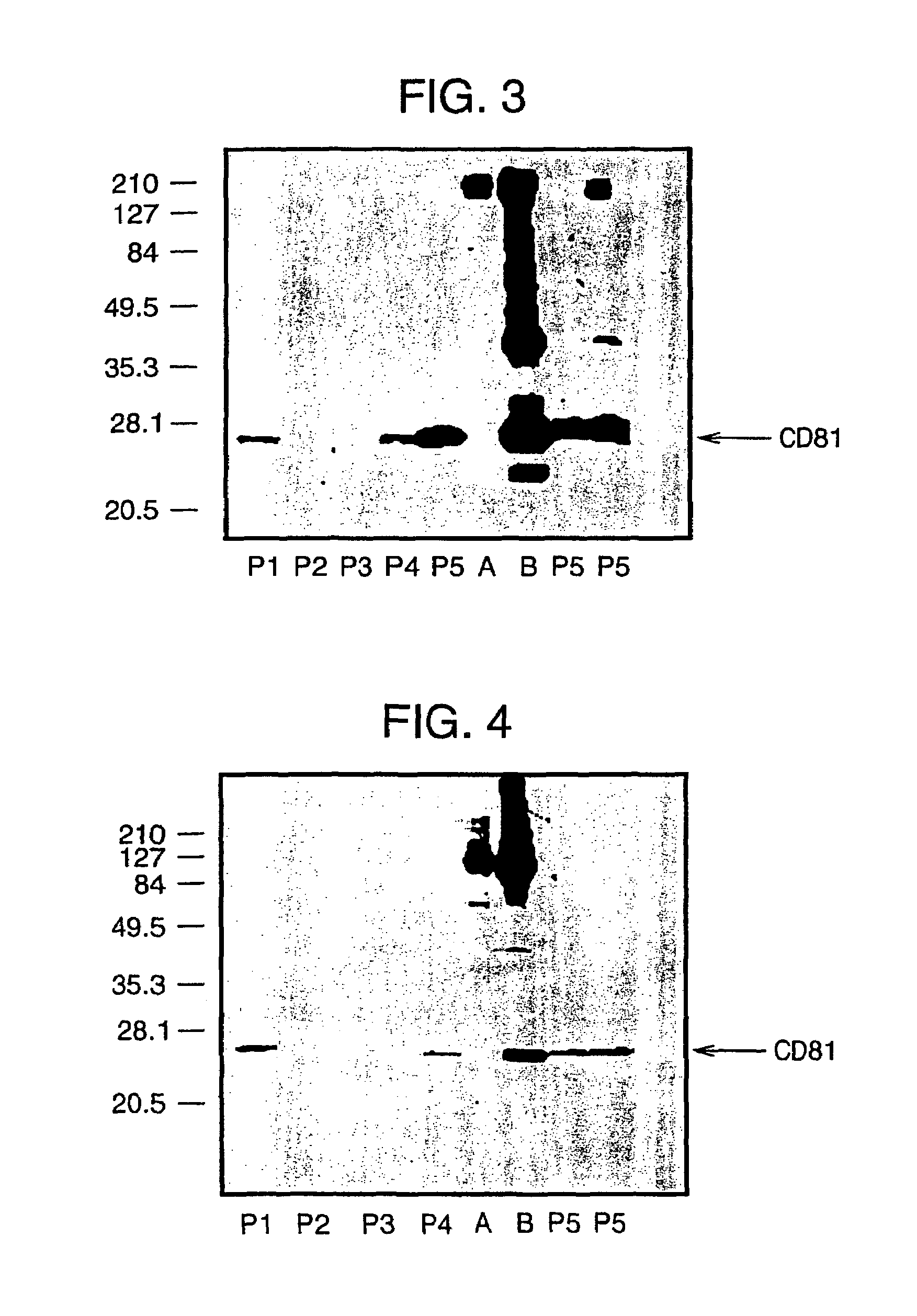
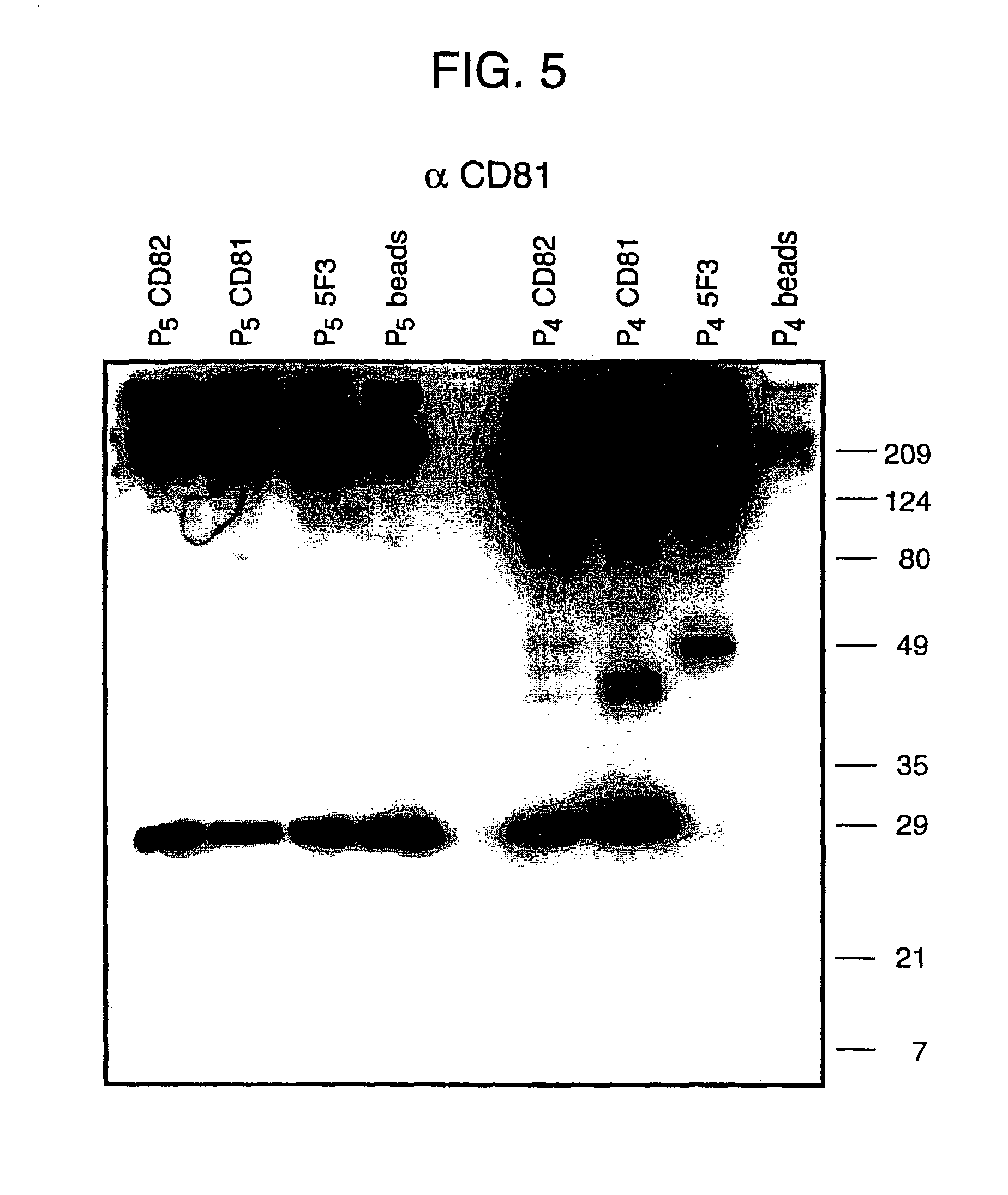
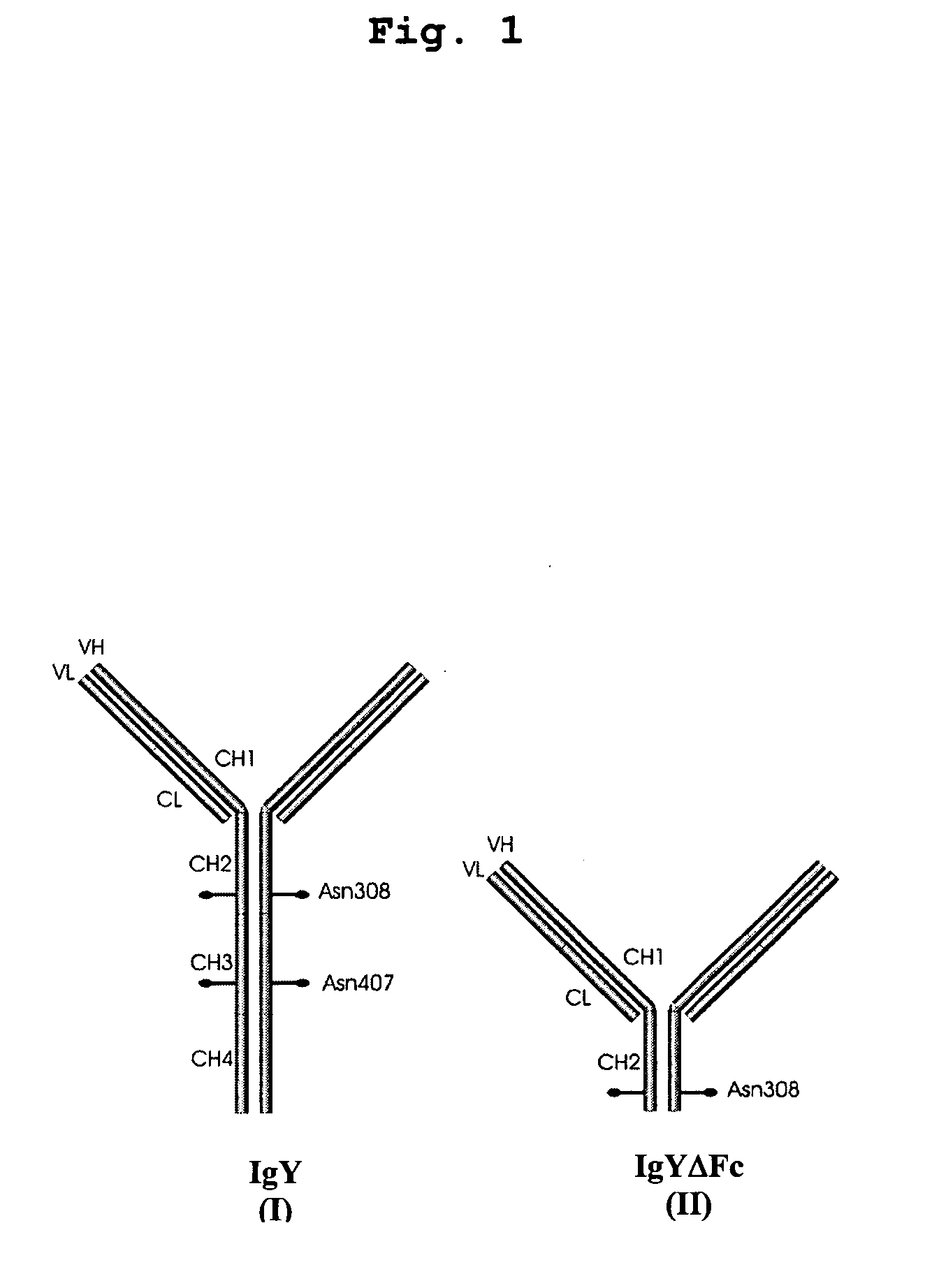
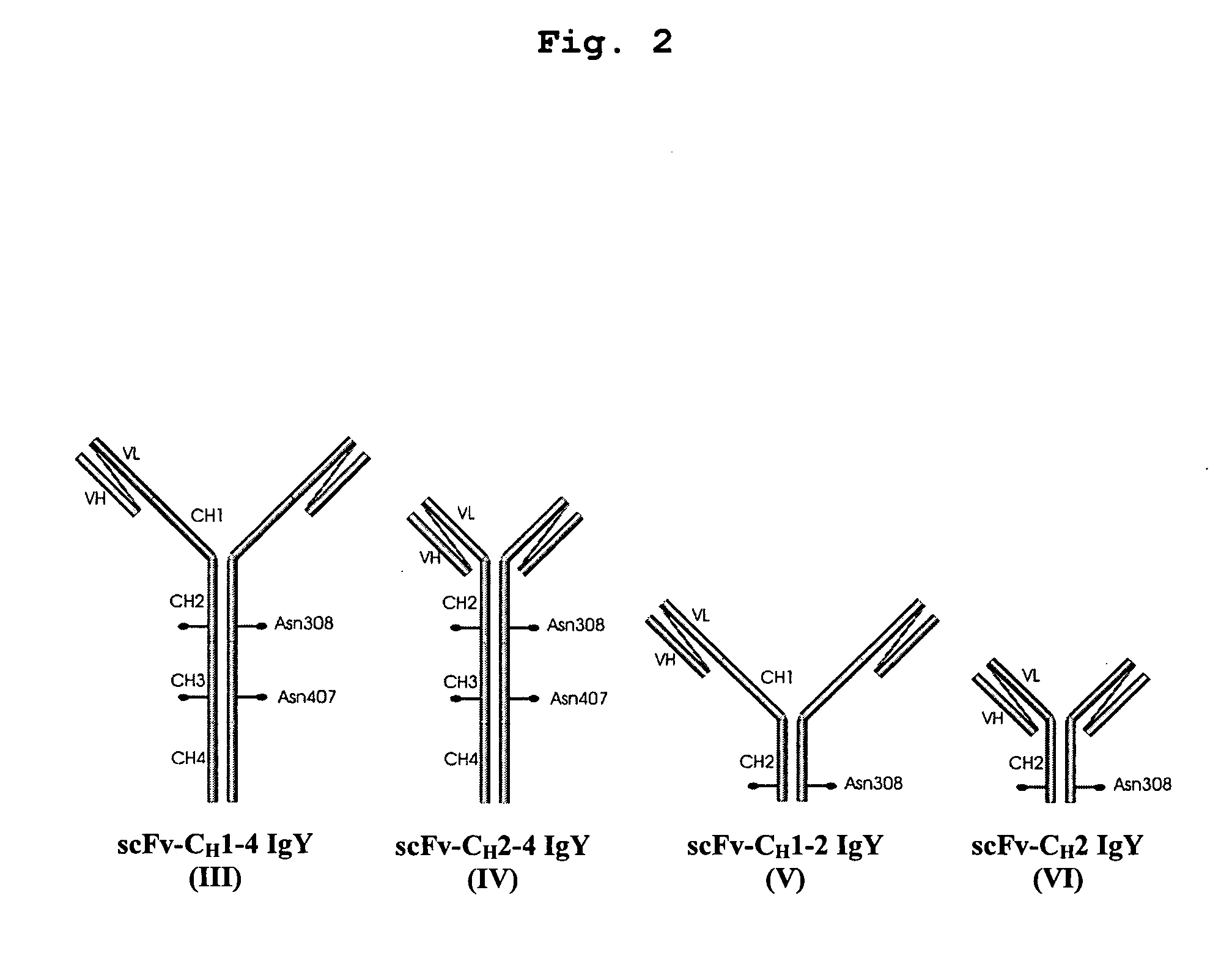
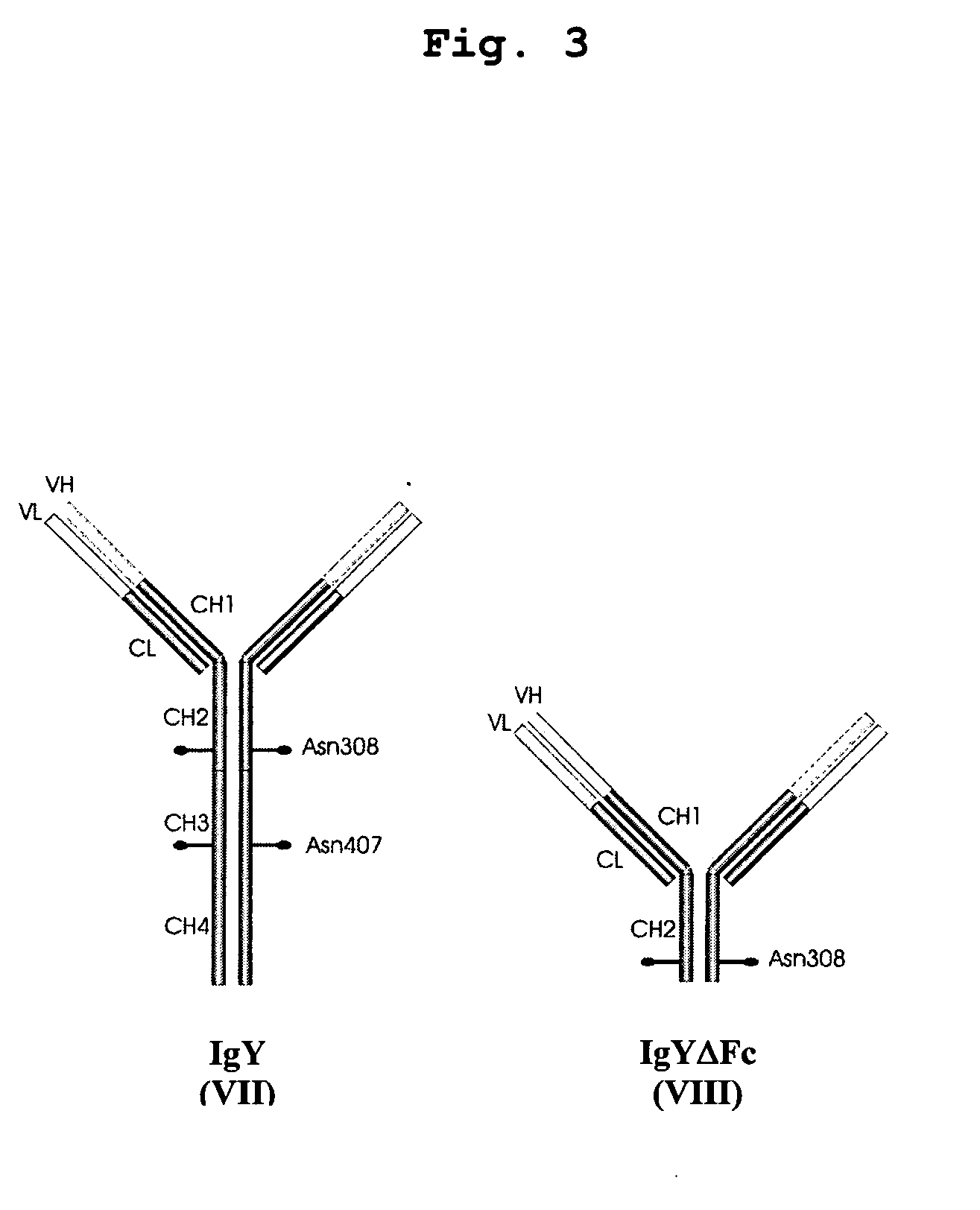
Bivalent IgY antibody constructs for diagnostic and therapeutic applications
InactiveUS20070141049A1Improve the immunityIncrease contentImmunoglobulins against virusesAntibody ingredientsHeavy chainMammal
This invention relates to the field of recombinant antibody technology. It provides novel recombinant IgY antibody constructs for diagnostic and therapeutical applications. The bivalent antibody constructs display a heterotetrameric or homodimeric format stabilized by disulfide bonds. The constant heavy chain domains CH2-CH4 are partly or completely of avian origin, whereas the VH, VL, CL, and CH1 domains as well as the hinge region may be of avian origin or derived from any other species. The invention allows to combine the advantages of IgY antibodies with those of established mammalian monoclonal antibodies. IgY antibody constructs comprising nonglycosylated IgY constant heavy chain domains allow to reduce unwanted interactions with C-type lectins, e.g., in human sera. Furthermore, chimeric IgY antibody containing mammalian VH, VL, CL, and CH1 domains as well as a mammalian hinge region provide a higher molecular stability than IgY antibodies in acidic conditions and, thereby, are especially suited for peroral therapeutic applications.
Owner:PLS DESIGN
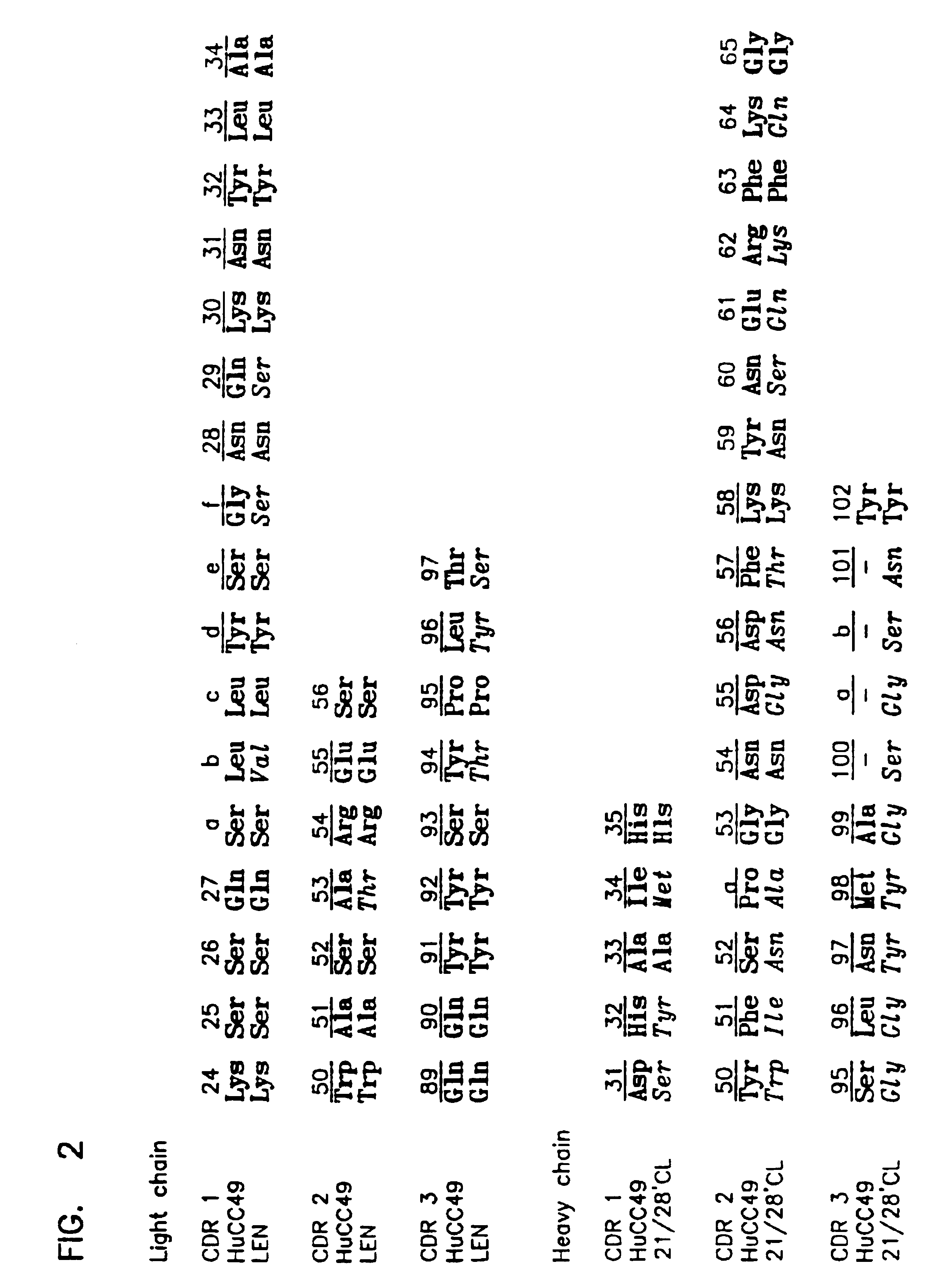
Variants of humanized anti carcinoma monoclonal antibody cc49
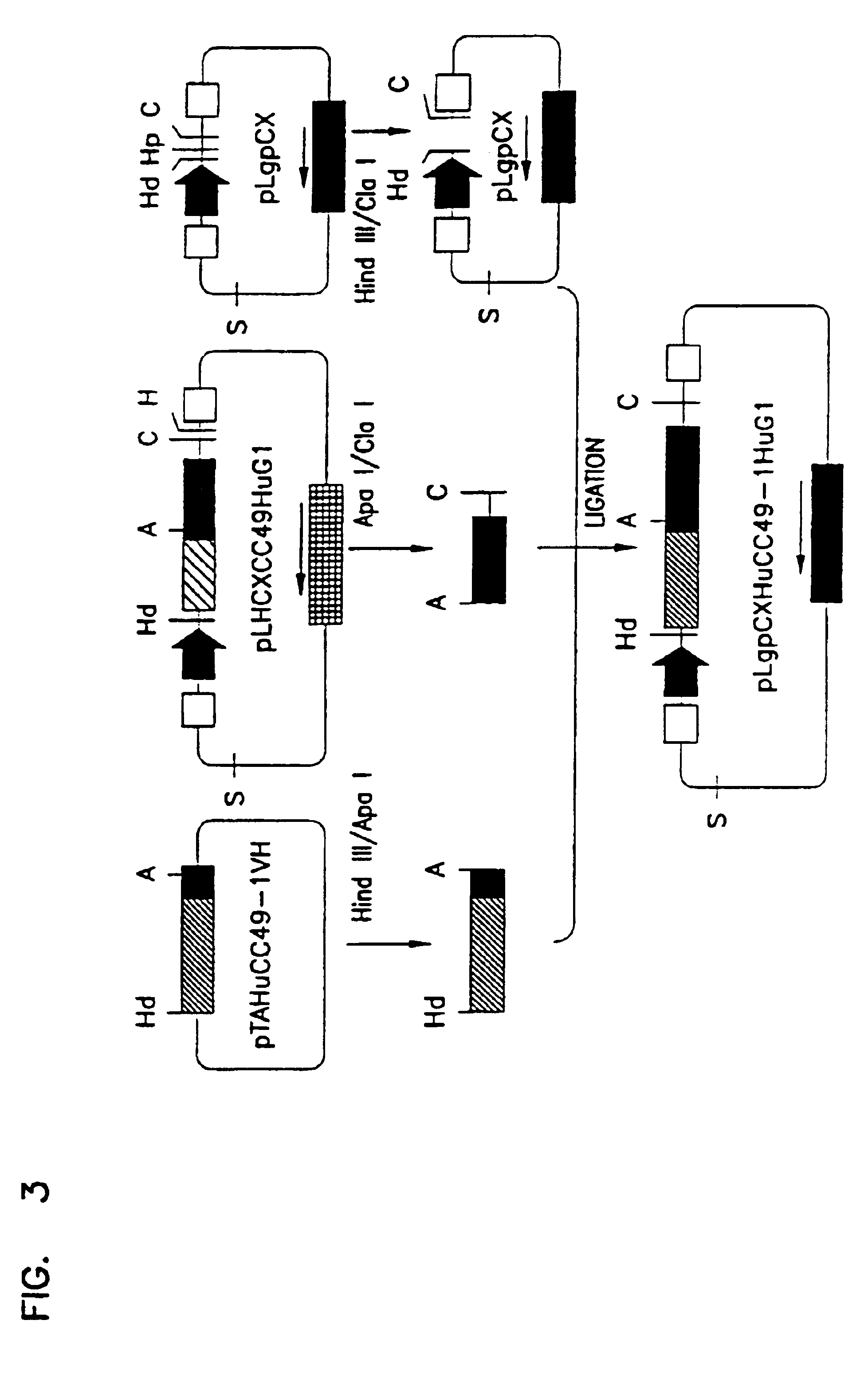
InactiveUS6818749B1Elicit adverse responseVirusesPeptide/protein ingredientsComplementarity determining regionHeavy chain
The invention is directed towards mouse-human chimeric variants of CC49 monoclonal antibodies with minimal murine content. A first aspect of the invention provides CDR variants of humanized monoclonal antibody (HuCC49) in which less than all six (three heavy chain and three light chain) Complementarity Determining Regions (CDRs) of CC49 are present. A second aspect of the invention provides SDR variants of humanized monoclonal antibody (HuCC49) in which only Specificity Determining Regions (SDRs) of at least one CDR from CC49 are present. The invention is also directed towards biotechnological methods of making the variants and therapeutic methods of using the variants.
Owner:UNITED STATES OF AMERICA
Substituted cycloalkyl P1′ hepatitis C virus inhibitors
InactiveUS6878722B2Inhibit functioningEffective treatmentBiocideDipeptide ingredientsHepacivirusVirology
The present invention relates to tripeptide compounds, compositions and methods for the treatment of hepatitis C virus (HCV) infection. In particular, the present invention provides novel tripeptide analogs, pharmaceutical compositions containing such analogs and methods for using these analogs in the treatment of HCV infection.
Owner:BRISTOL MYERS SQUIBB CO
HIV envelope polypeptides
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






![SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaca0958-e088-450b-a622-0be890c729fb/US20140221380A1-20140807-C00001.png)
![SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaca0958-e088-450b-a622-0be890c729fb/US20140221380A1-20140807-C00002.png)
![SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR SUBSTITUTED SPIROPYRIDO[1,2-a]PYRAZINE DERIVATIVE AND PHARMACEUTICAL USE OF SAME AS HIV INTEGRASE INHIBITOR](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/eaca0958-e088-450b-a622-0be890c729fb/US20140221380A1-20140807-C00003.png)