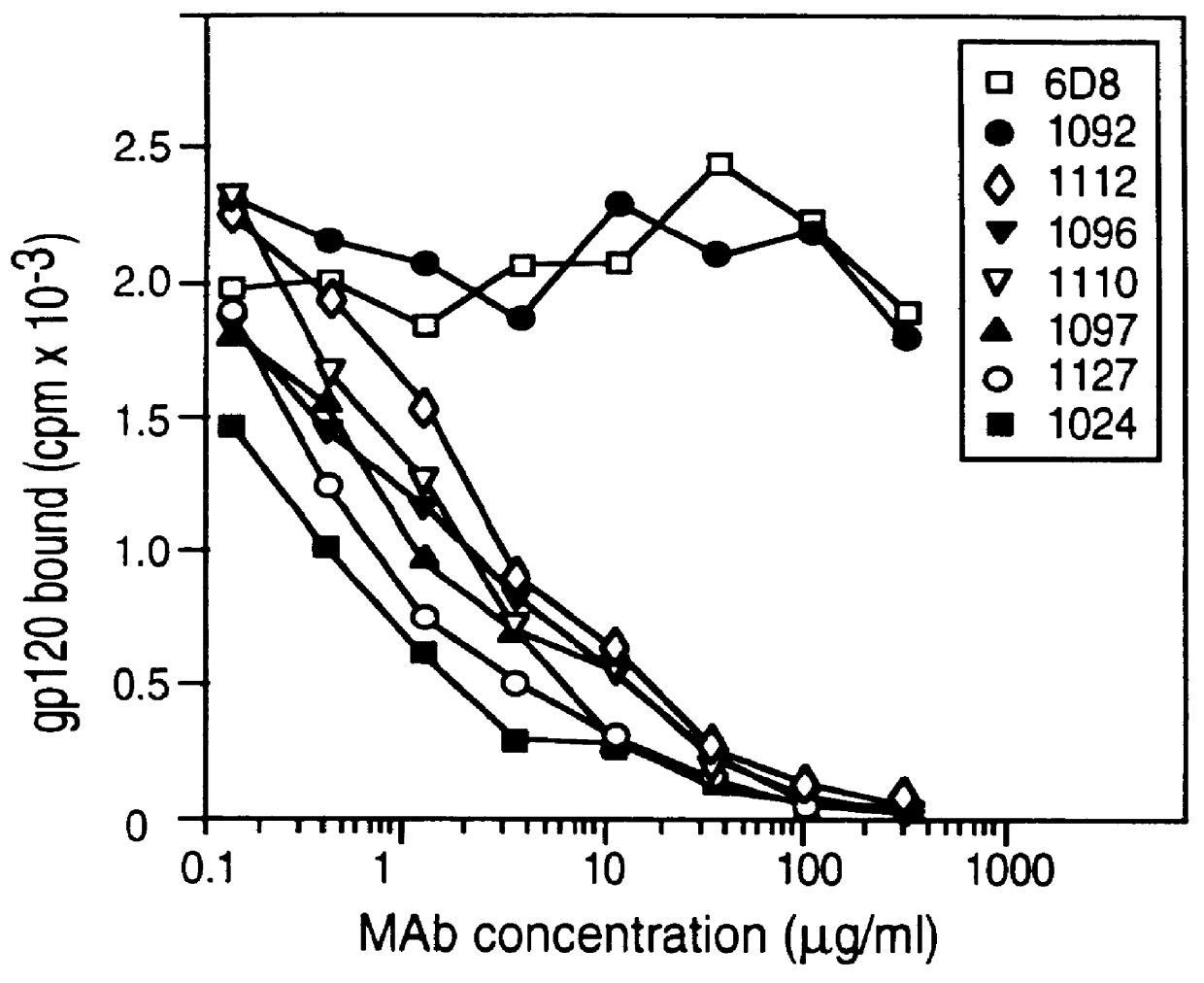
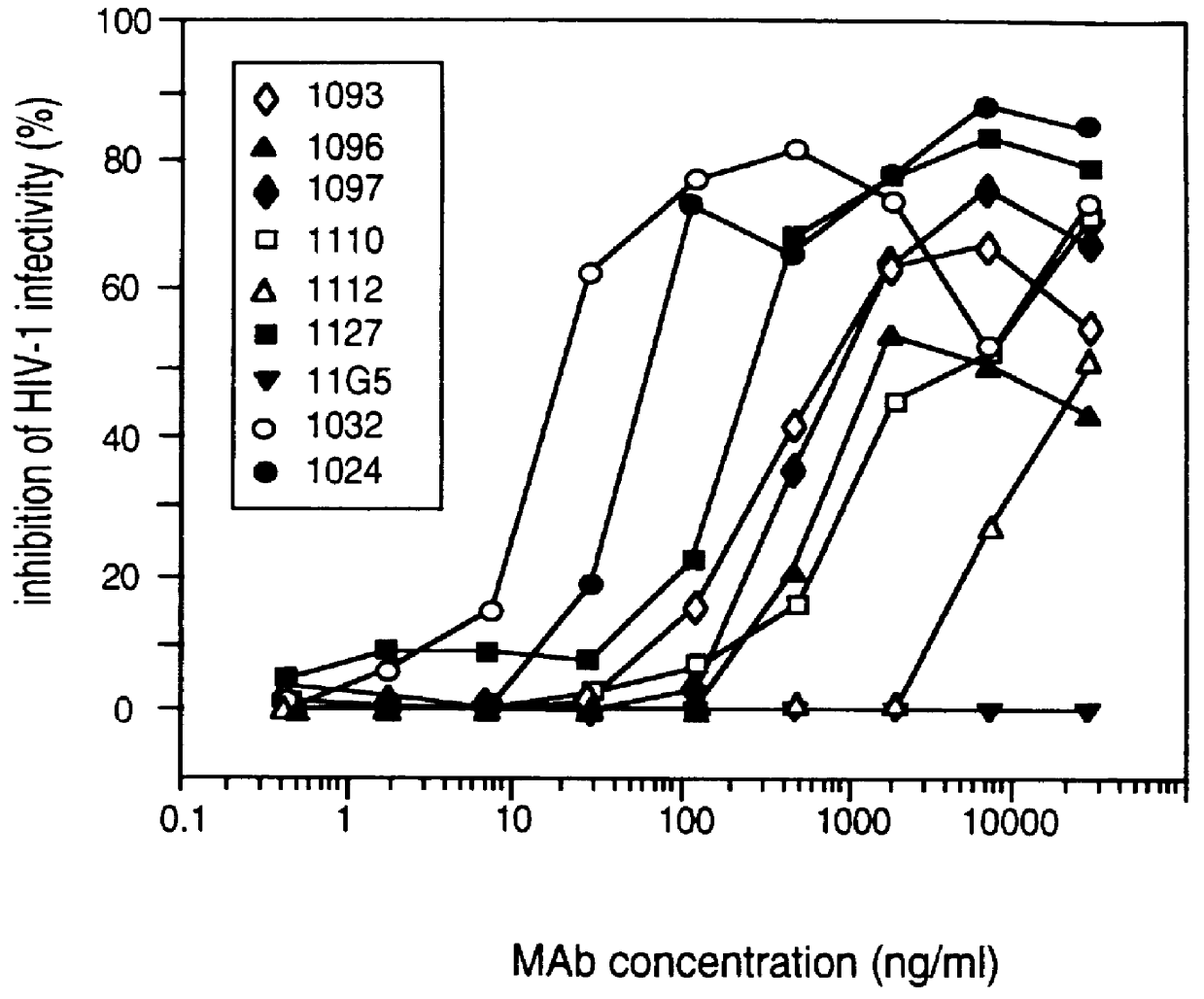
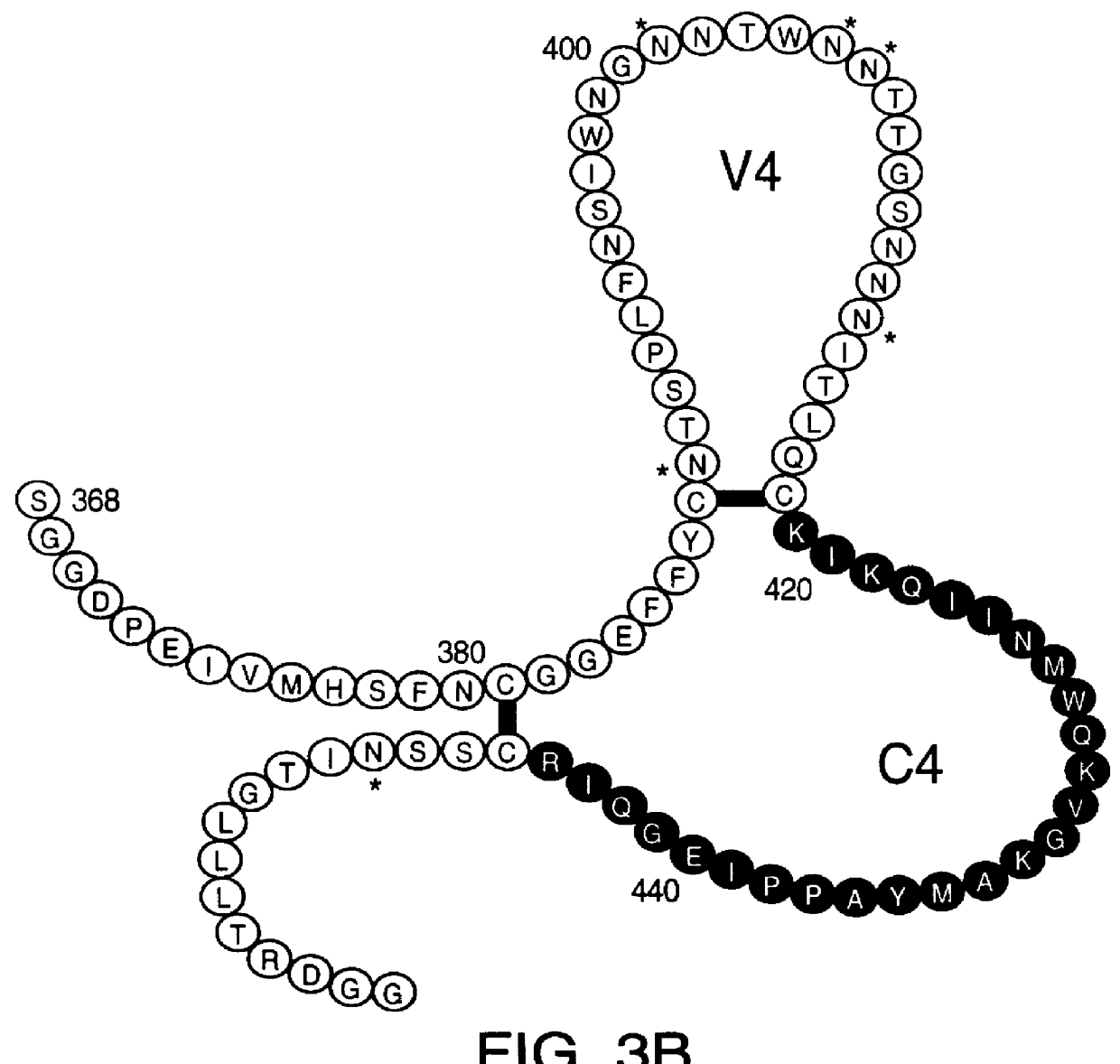
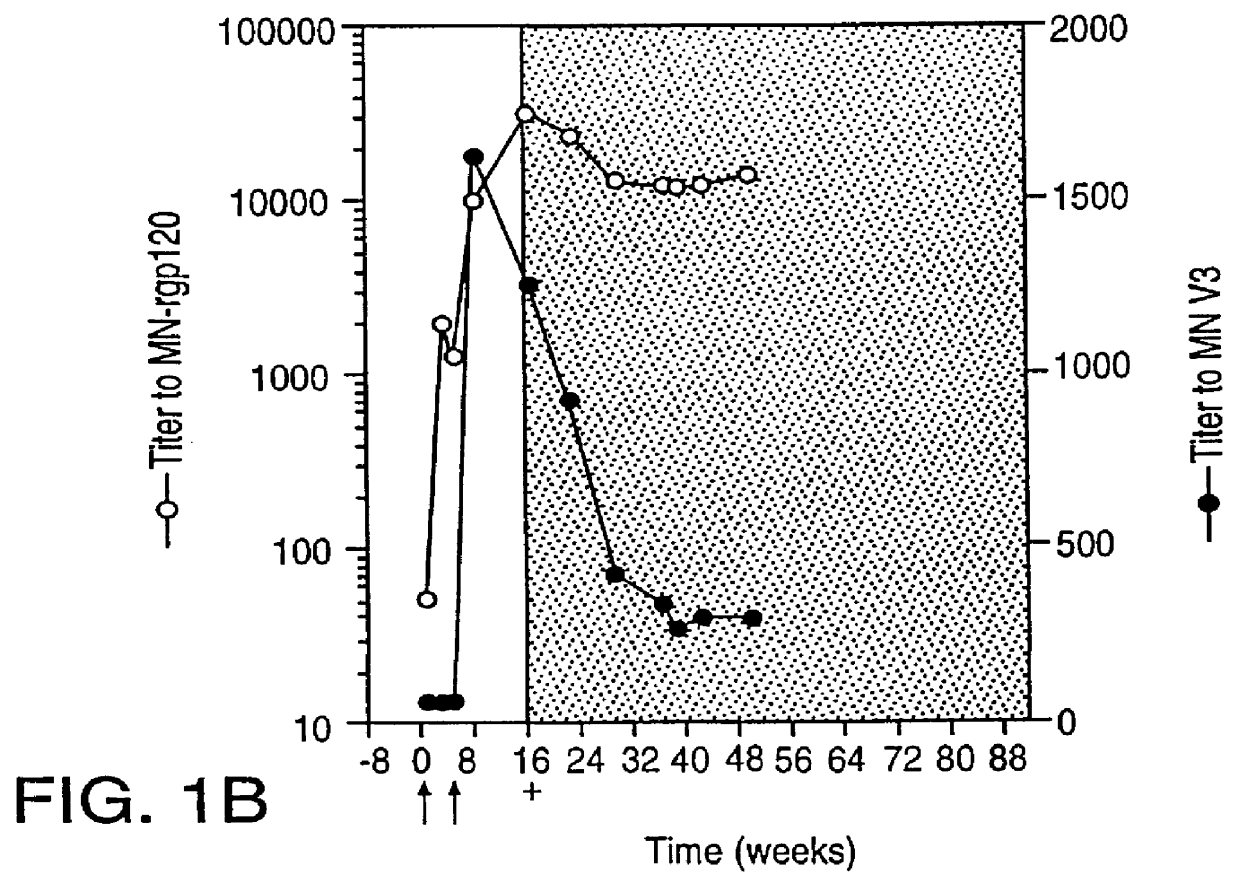
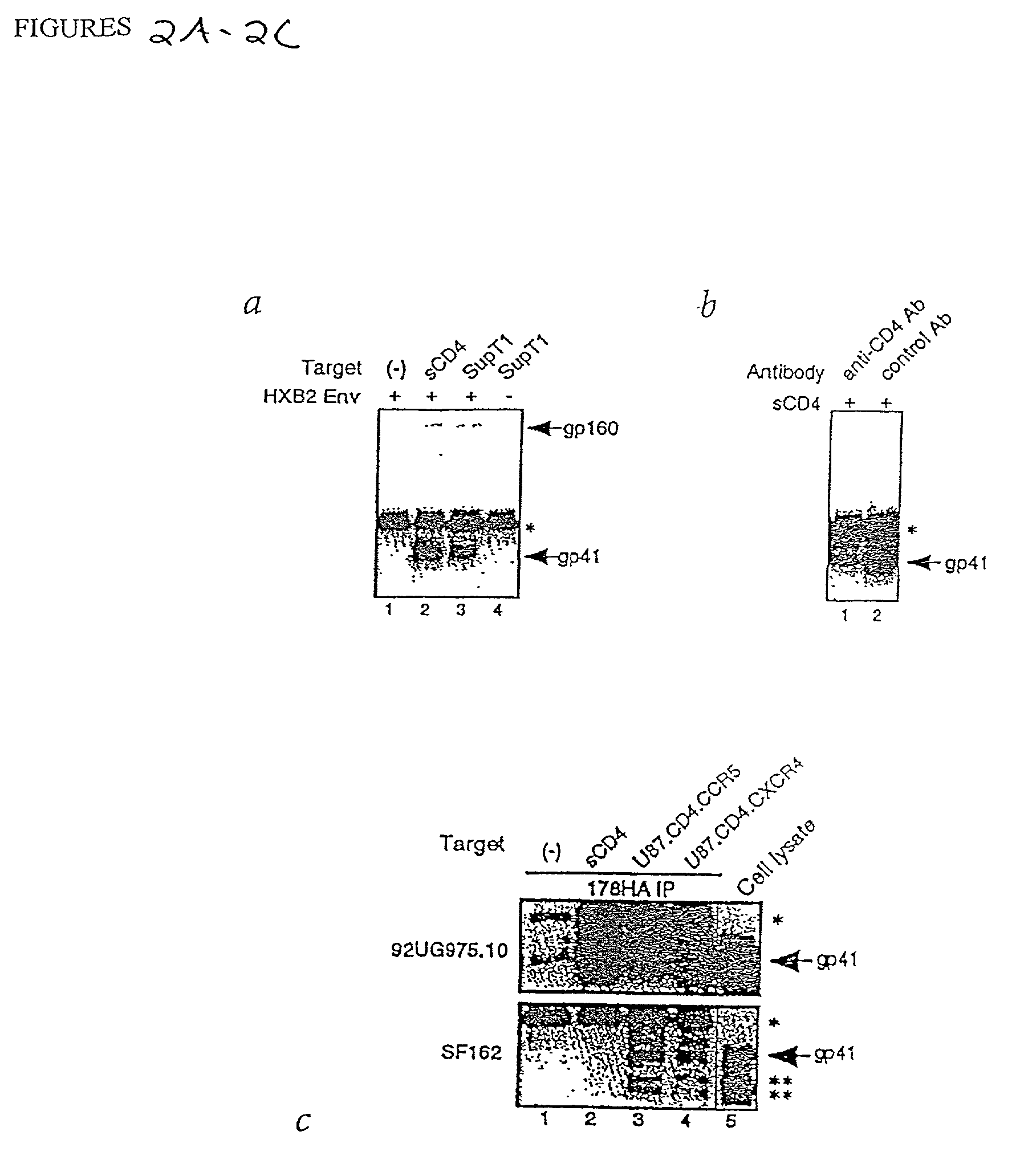
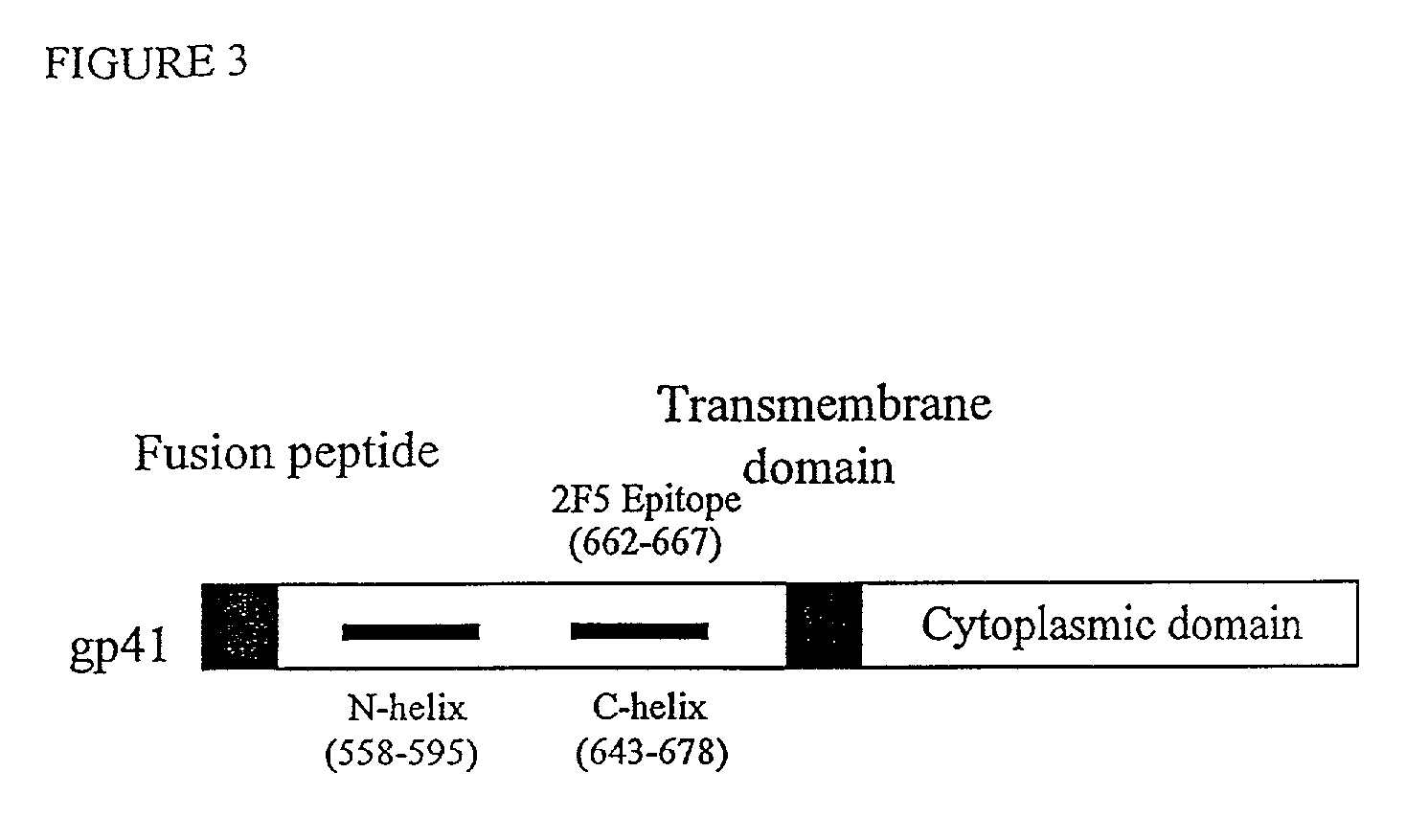
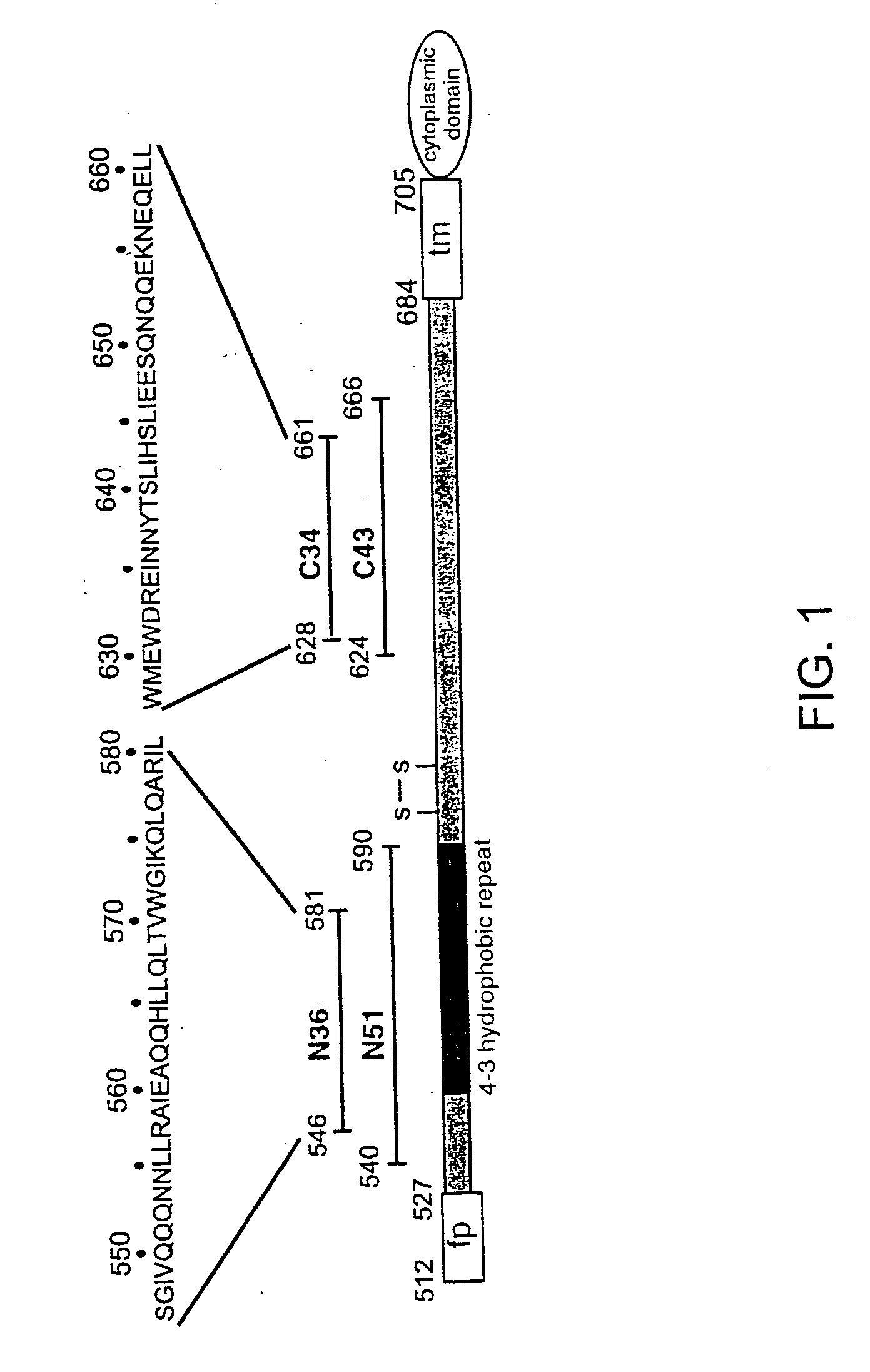
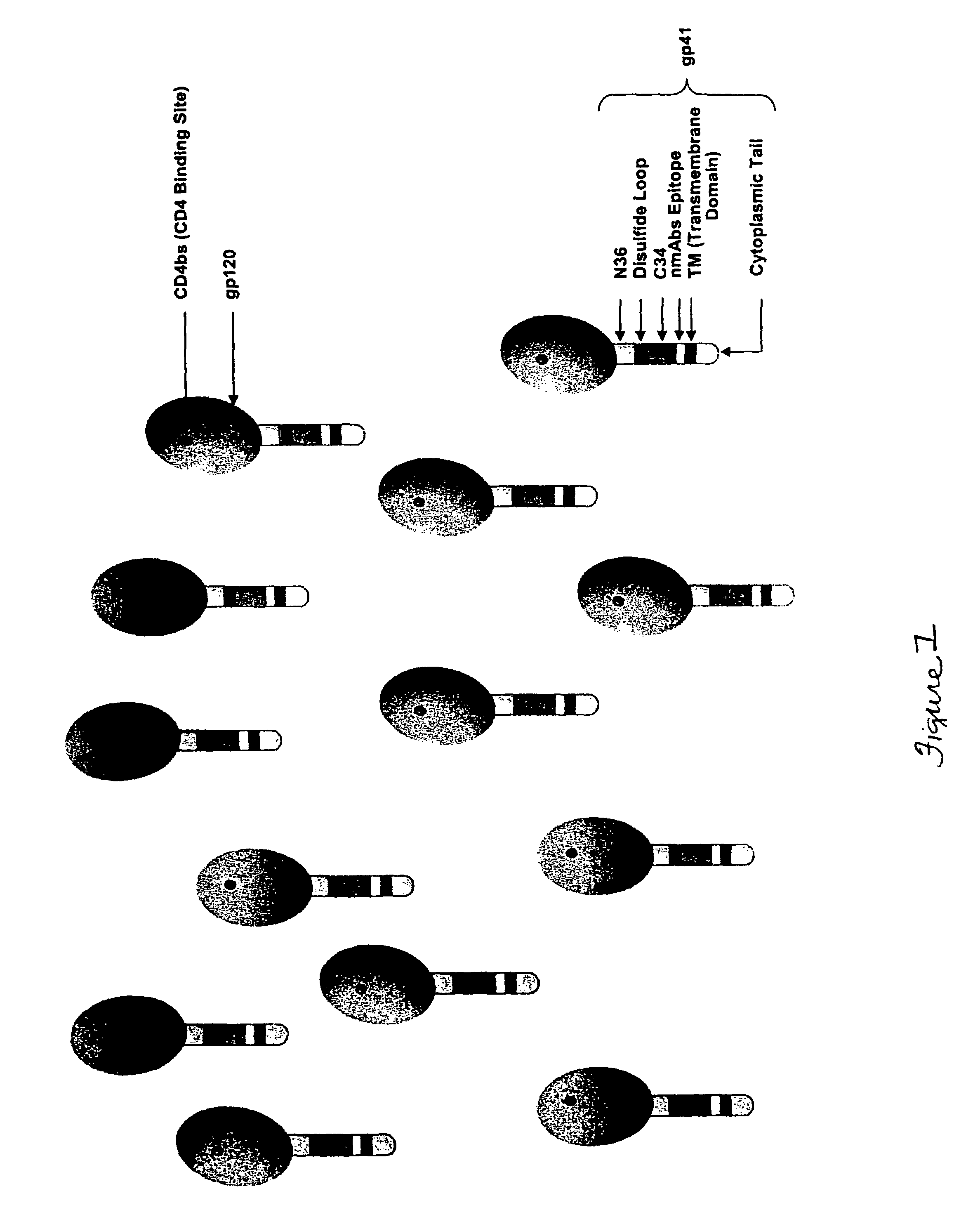
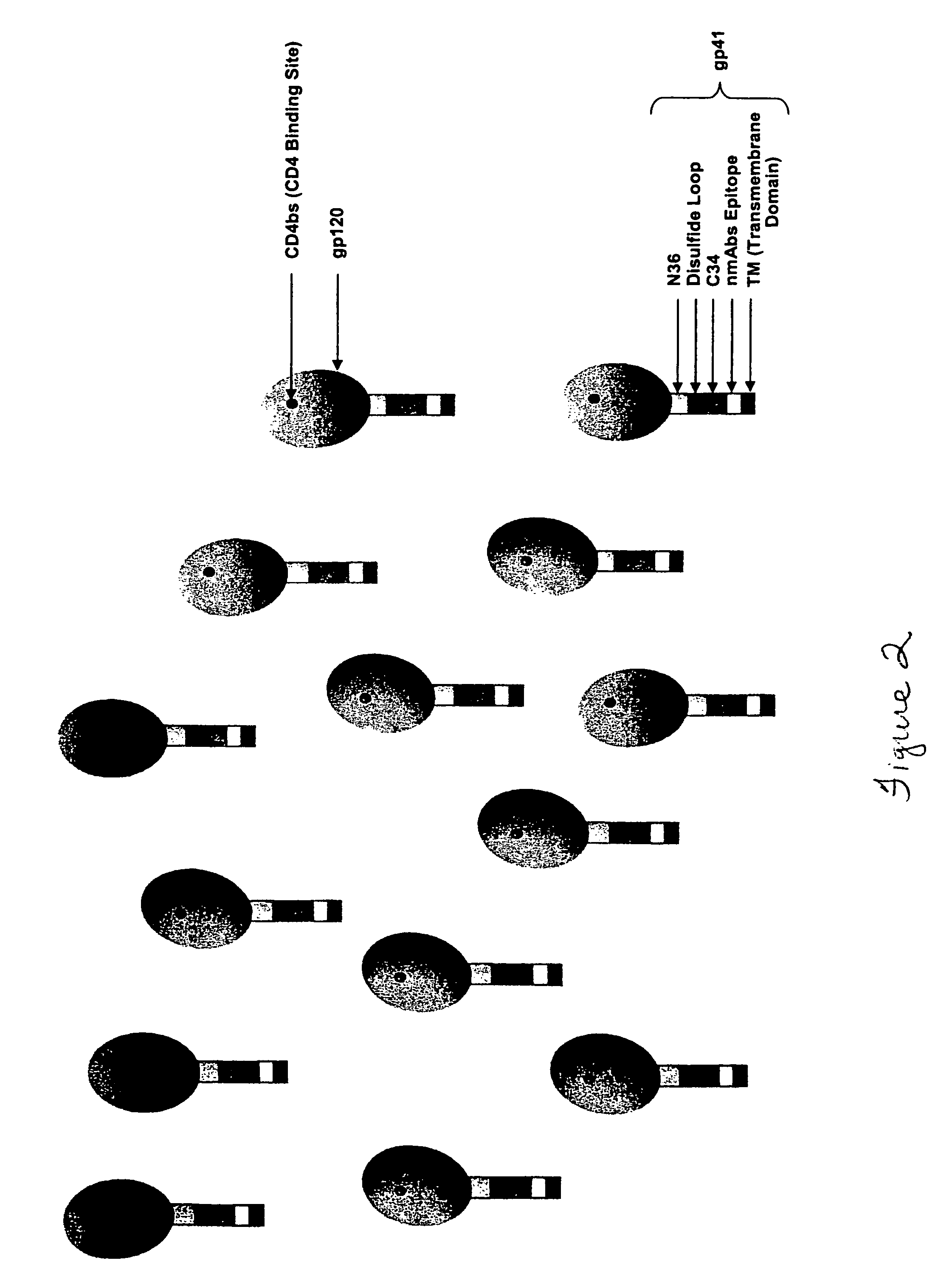
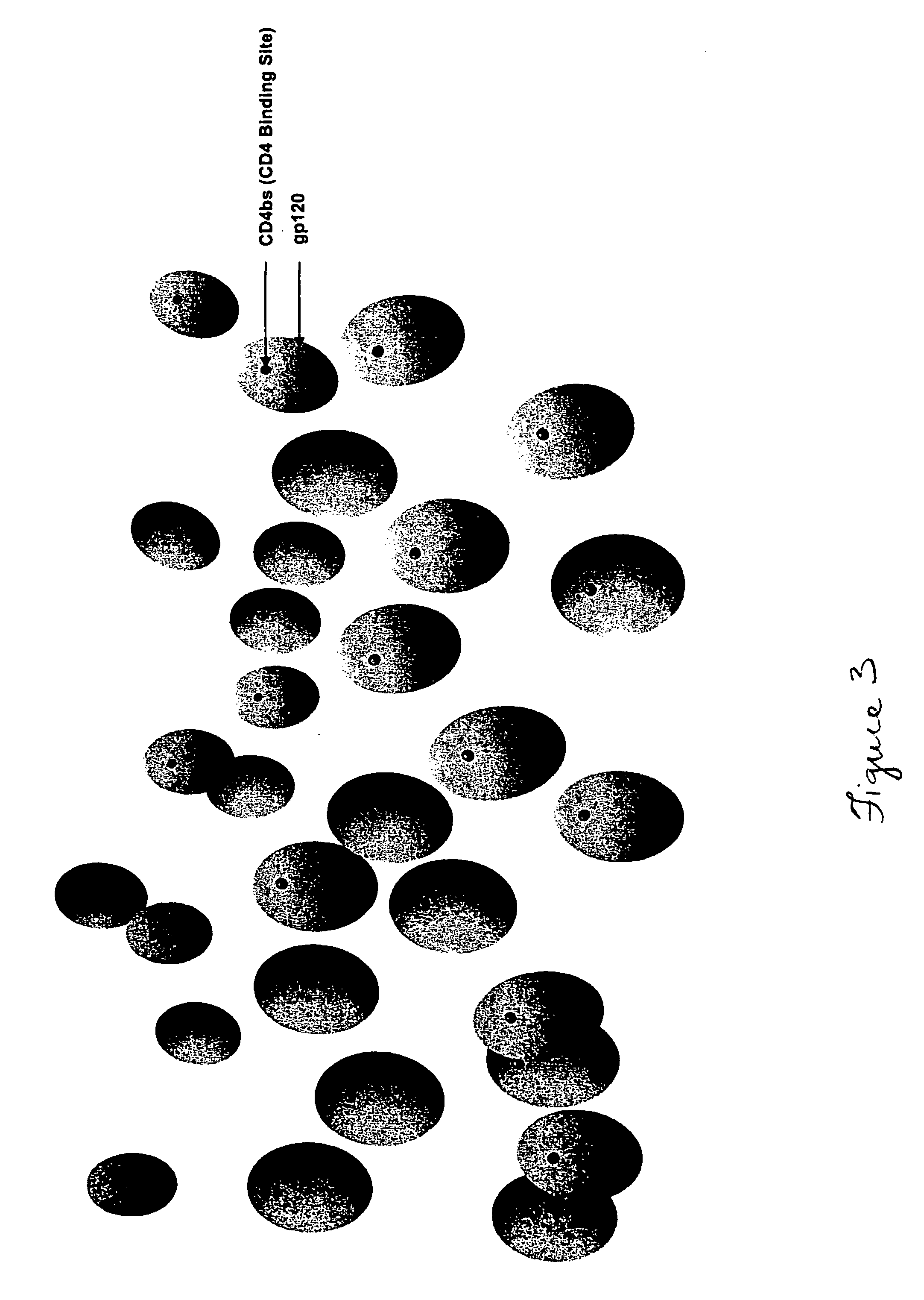
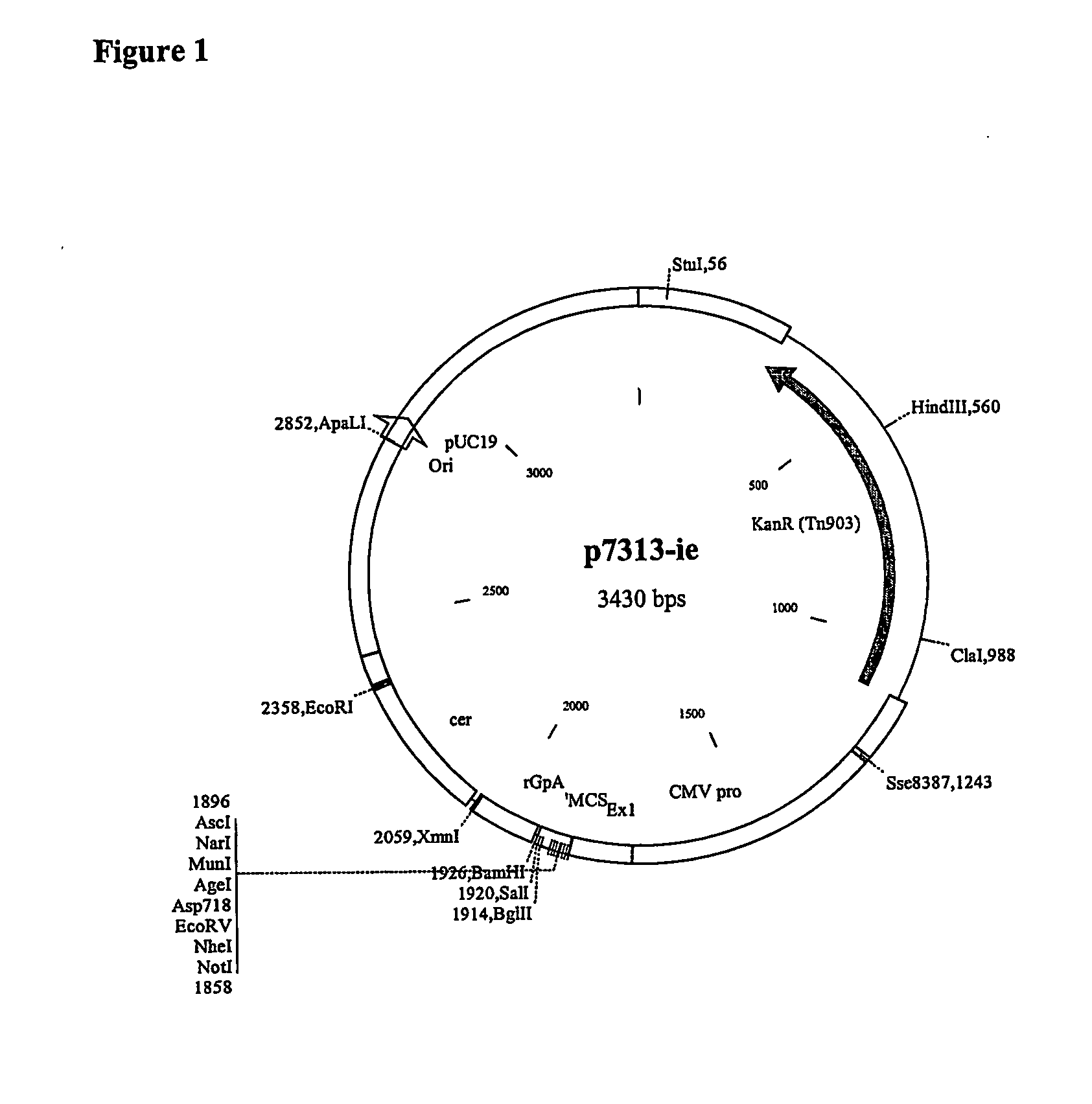
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Hiv envelope" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
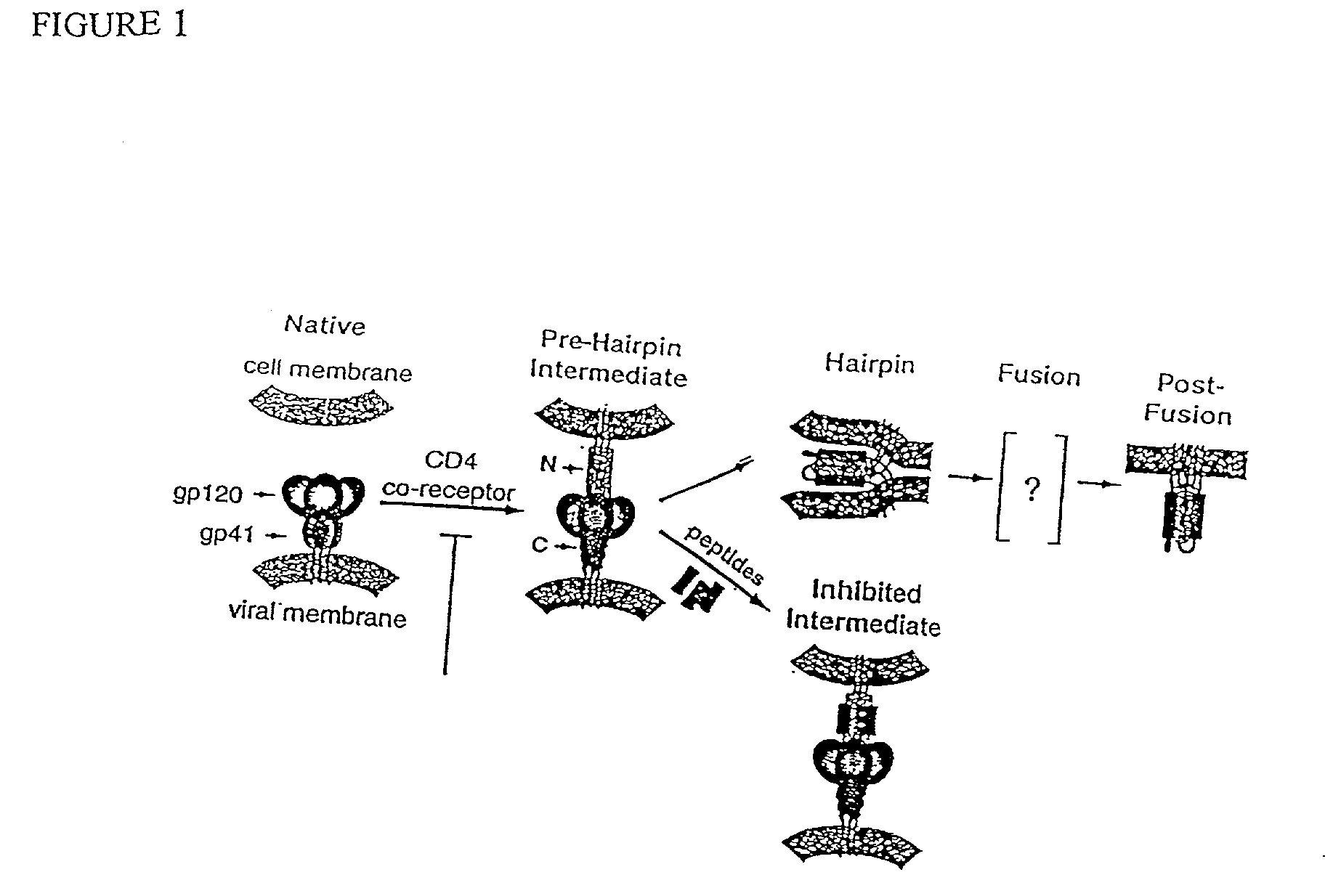
The HIV virus envelope is a derivative of the plasma membrane of a host cell, obtained via budding. When HIV attempts to enter a cell, interactions between cell surface molecules and viral envelope proteins allow the envelope to fuse with the cell membrane.
HIV envelope polypeptides
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
HIV envelope polypeptides and vaccine
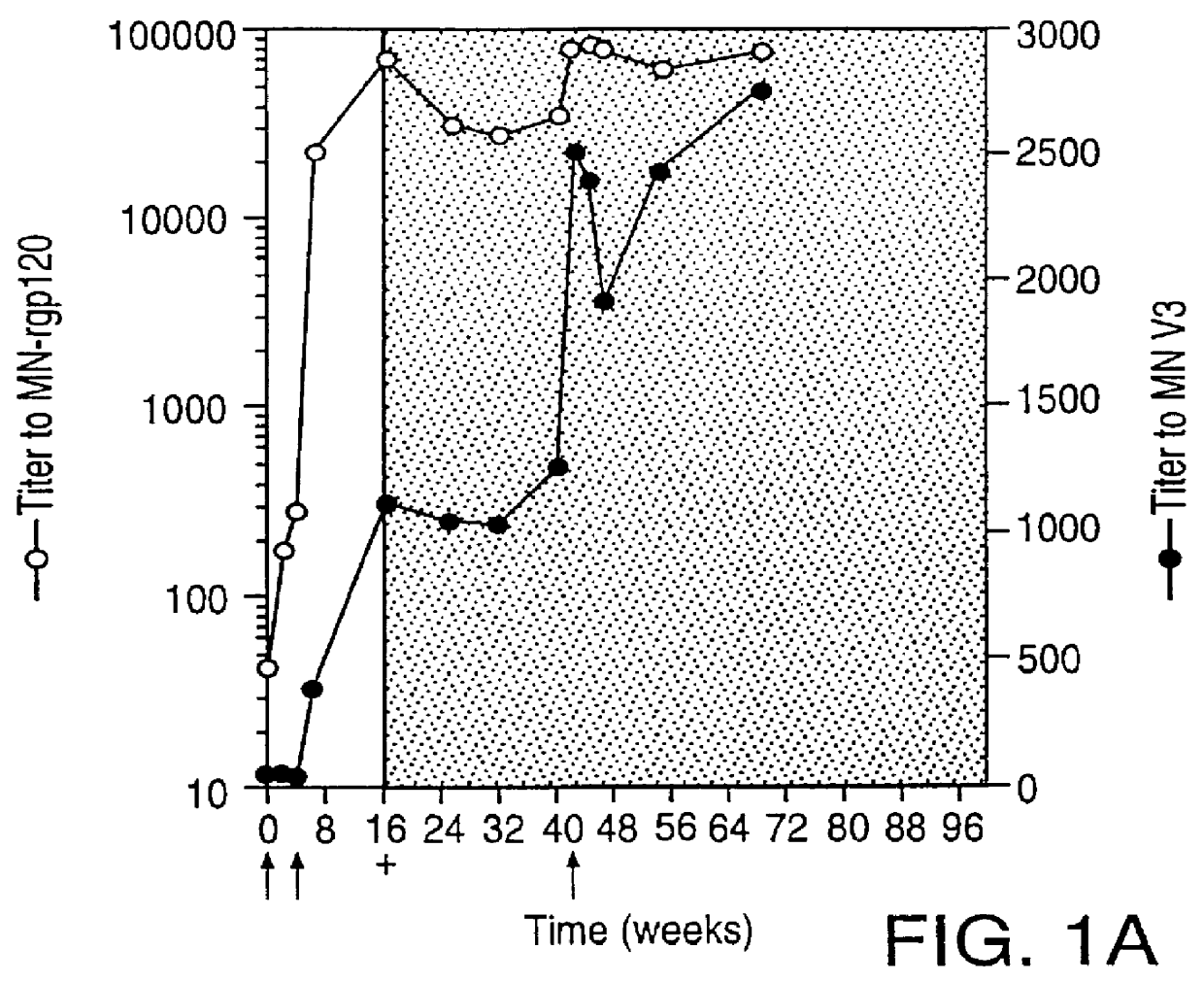
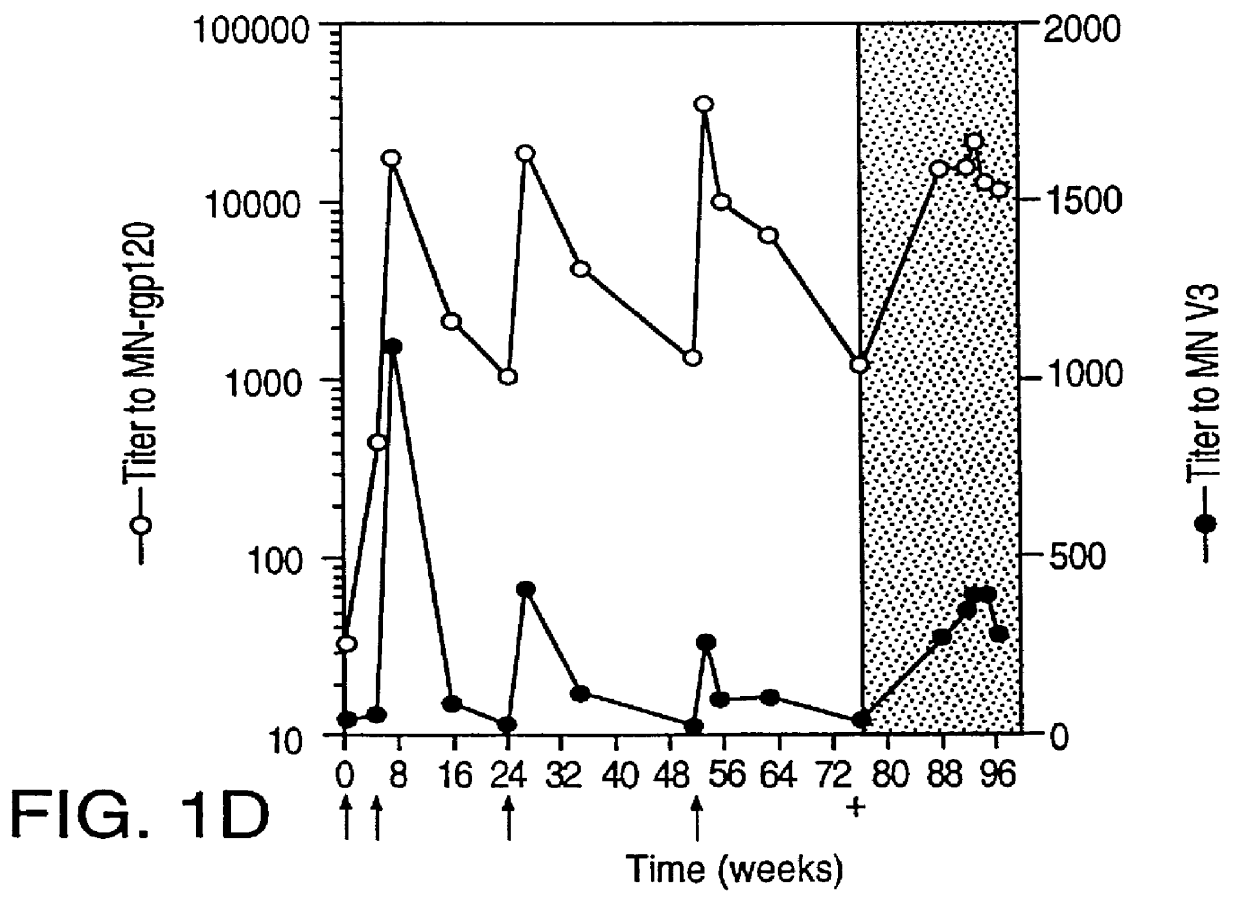
Oligonucleotide sequences encoding gp120 polypeptides from breakthrough isolates of vaccine trials using MN-rgp120 and the encoded gp120 polypeptides are provided. Use of the gp120 polypeptides from one or more of the isolates in a subunit vaccine, usually together with MN-rgp120, can provide protection against HIV strains that are sufficiently different from the vaccine strain (e.g.; MN-rgp120) that the vaccine does not confer protection against those strains. Antibodies induced by the polypeptides are also provided.
Owner:GENENTECH INC
Fusion proteins for HIV therapy
InactiveUS20120121597A1Improved potency and breadth against HIVImprove barrier propertiesHybrid immunoglobulinsAntibody mimetics/scaffoldsHiv envelopeBinding site
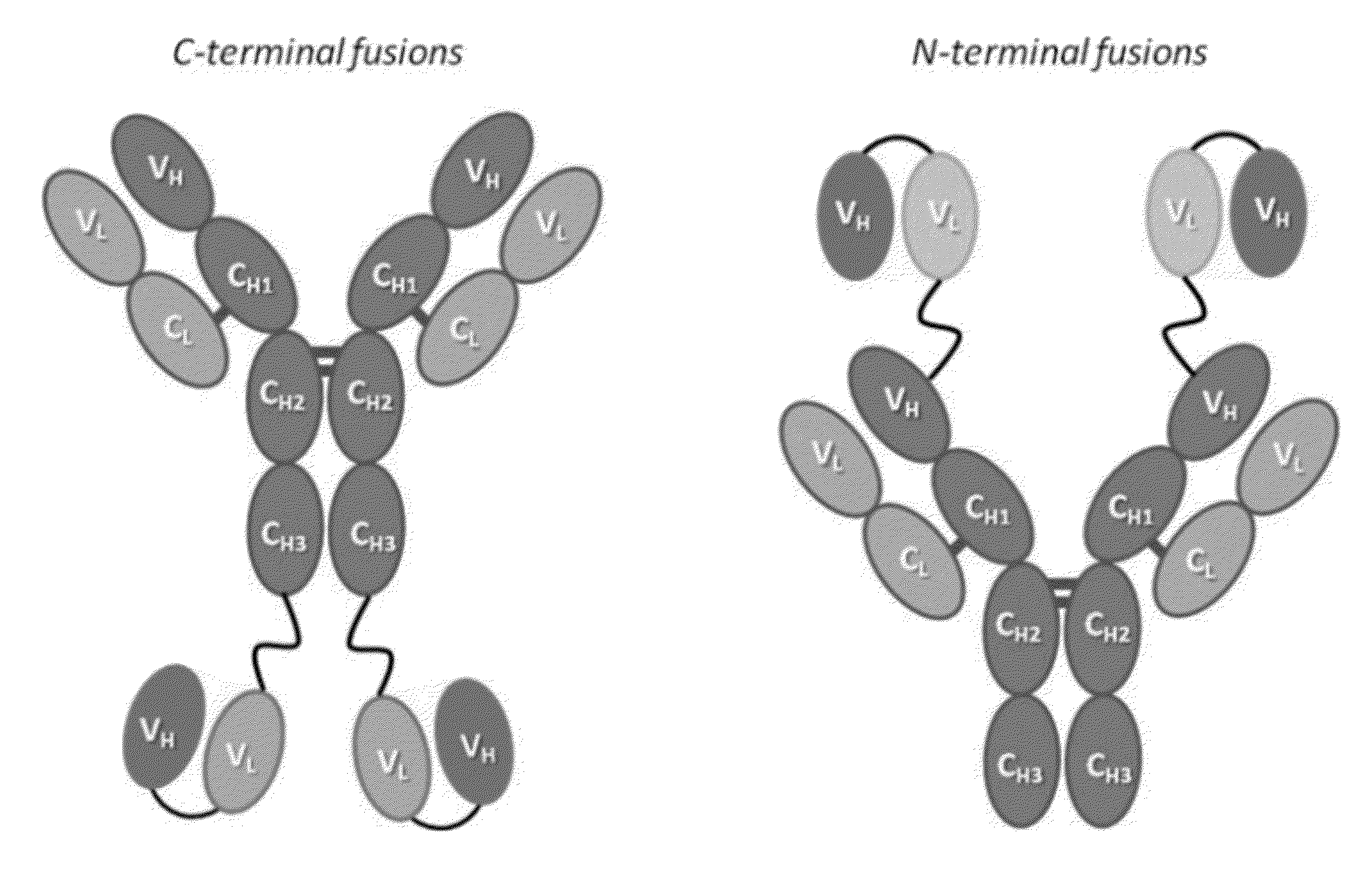
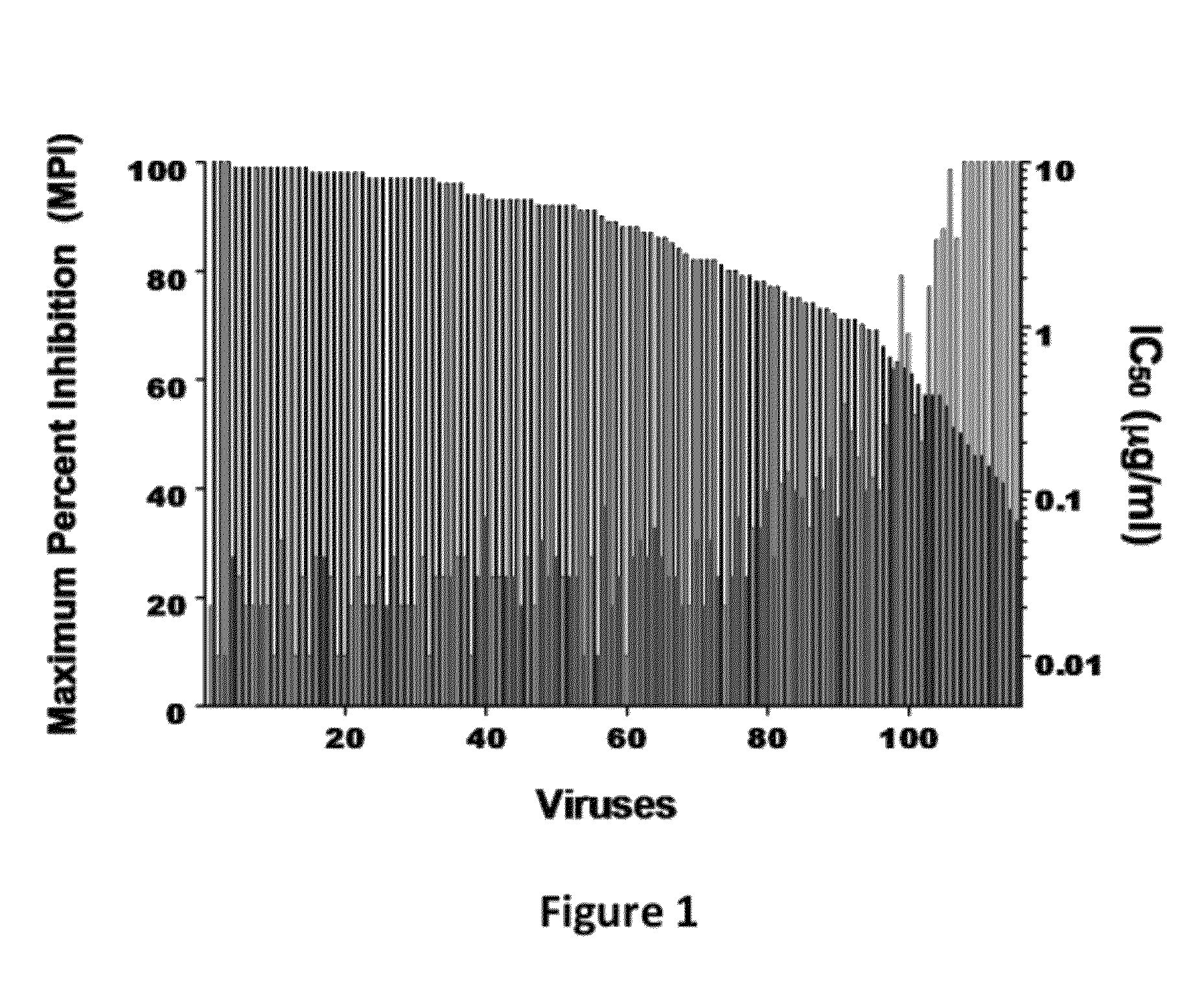
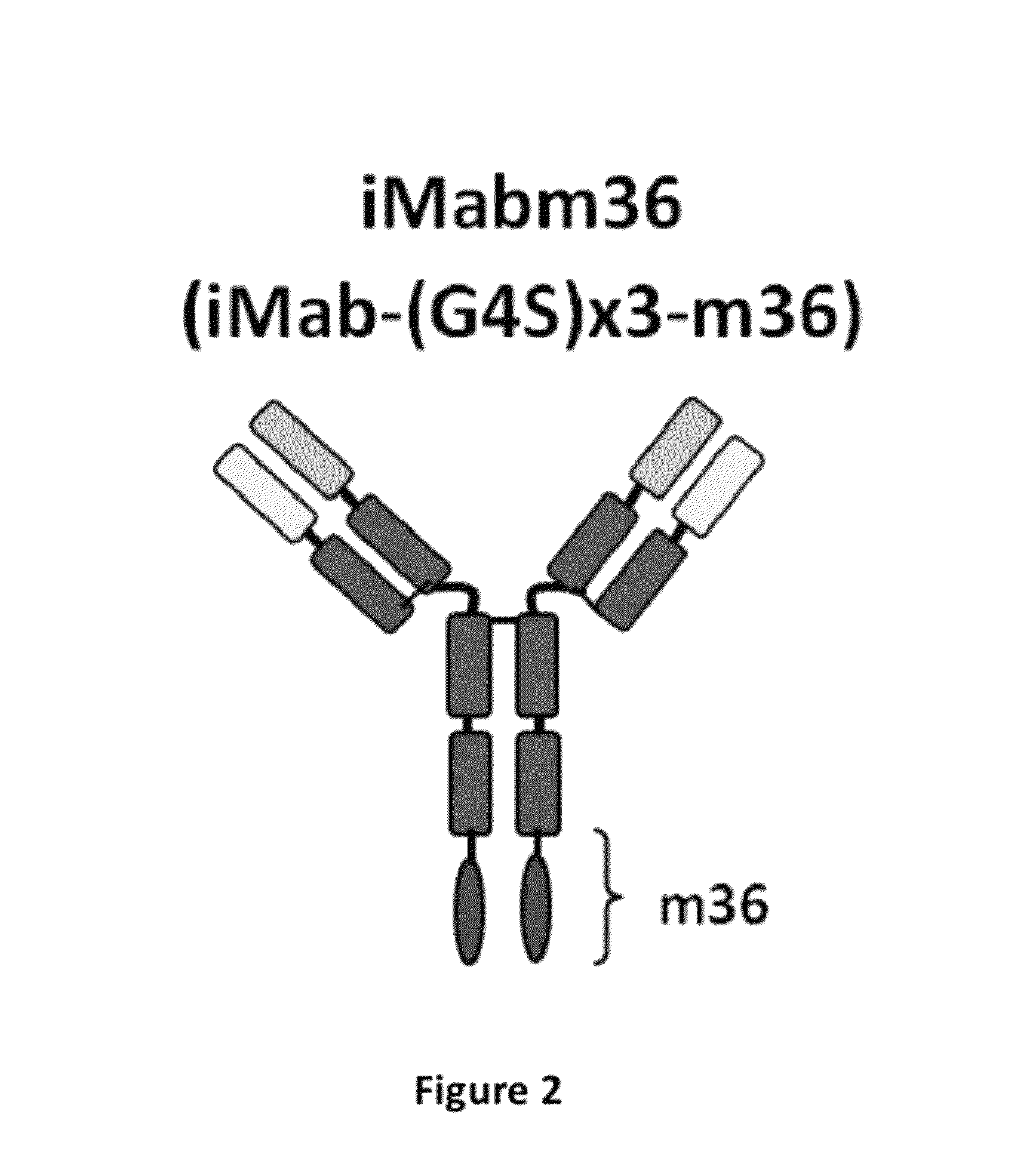
Disclosed herein are fusion antibodies created to provide both an antigen-binding site that targets the CD4 receptor and an antigen-binding site that targets the HIV envelope. The fusion antibodies disclosed herein provide improved potency and breadth against HIV as compared to monospecific antibodies, and additionally provide high barrier against viral resistance. Also disclosed are pharmaceutical formulations and therapeutic methods utilizing such fusion proteins.
Owner:THE ROCKEFELLER UNIV
HIV envelope polypeptides
A method for the rational design and preparation of vaccines based on HIV envelope polypeptides is described. In one embodiment, the method for making an HIV gp120 subunit vaccine for a geographic region comprises determining neutralizing epitopes in the V2 and / or C4 domains of gp120 of HIV isolates from the geographic region and selecting an HIV strain having gp120 a neutralizing epitope in the V2 or C4-domain which is common among isolates in the geographic region. In a preferred embodiment of the method, neutralizing epitopes for the V2, V3, and C4 domains of gp120 are determined. At least two HIV isolates having different neutralizing epitopes in the V2, V3, or C4 domain are selected and used to make the vaccine. The invention also provides a multivalent HIV gp120 subunit vaccine. A DNA sequence encoding gp120 from preferred vaccine strains of HIV, GNE8 and GNE16, expression constructs comprising the GNE8-gp120 and GNE16-gp120 encoding DNA under the transcriptional and translational control of a heterologous promoter, and isolated GNE8-gp120 and GNE16-gp120 are also described.
Owner:GENENTECH INC
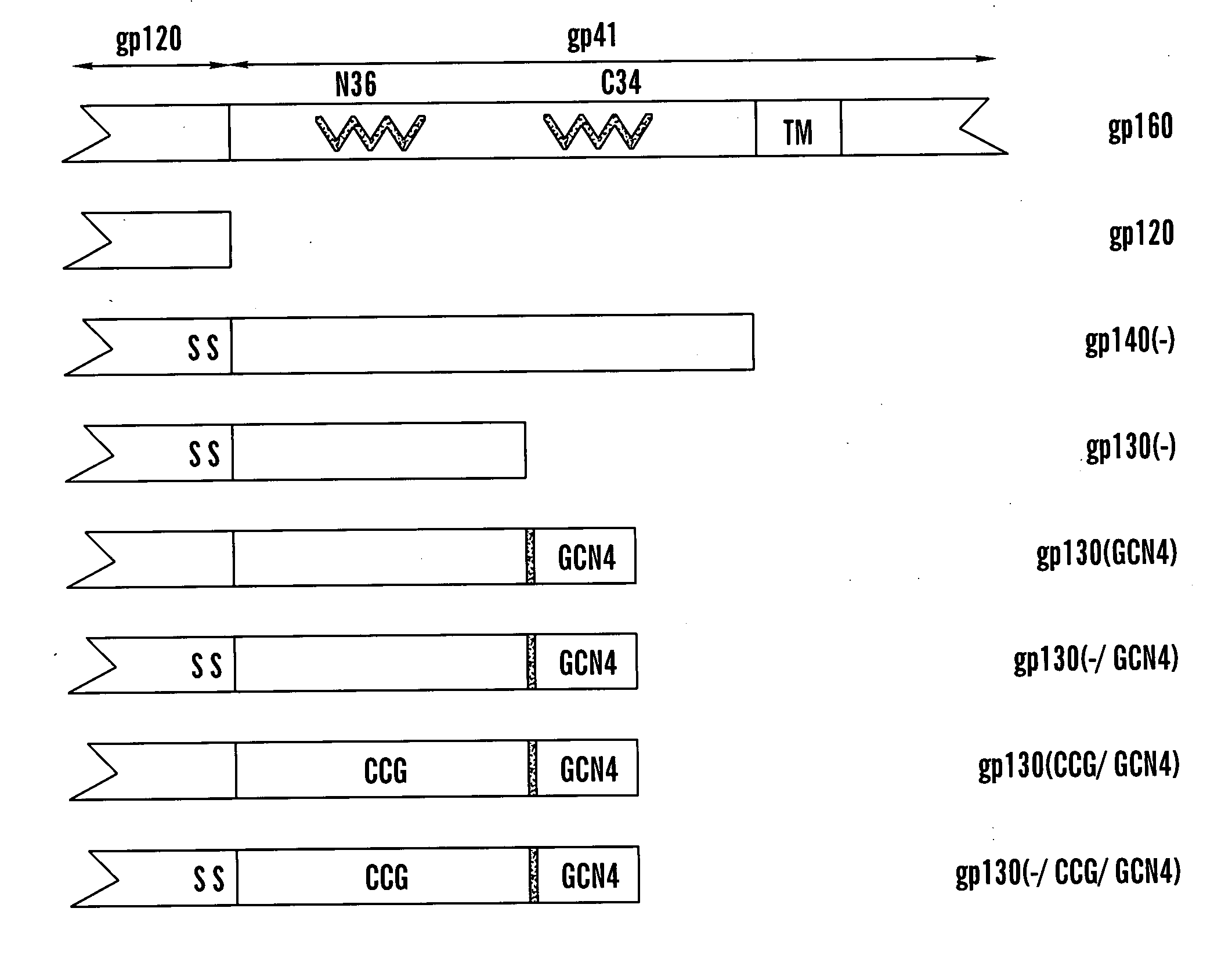
Stabilized soluble glycoprotein trimers
InactiveUS6911205B2Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
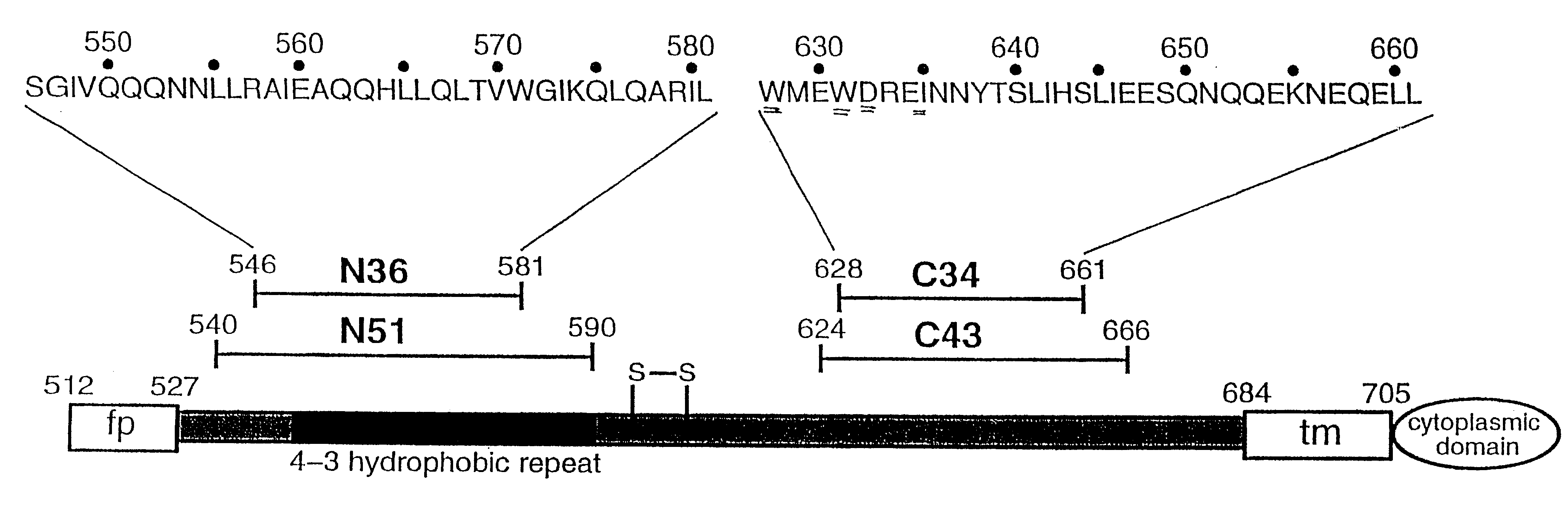
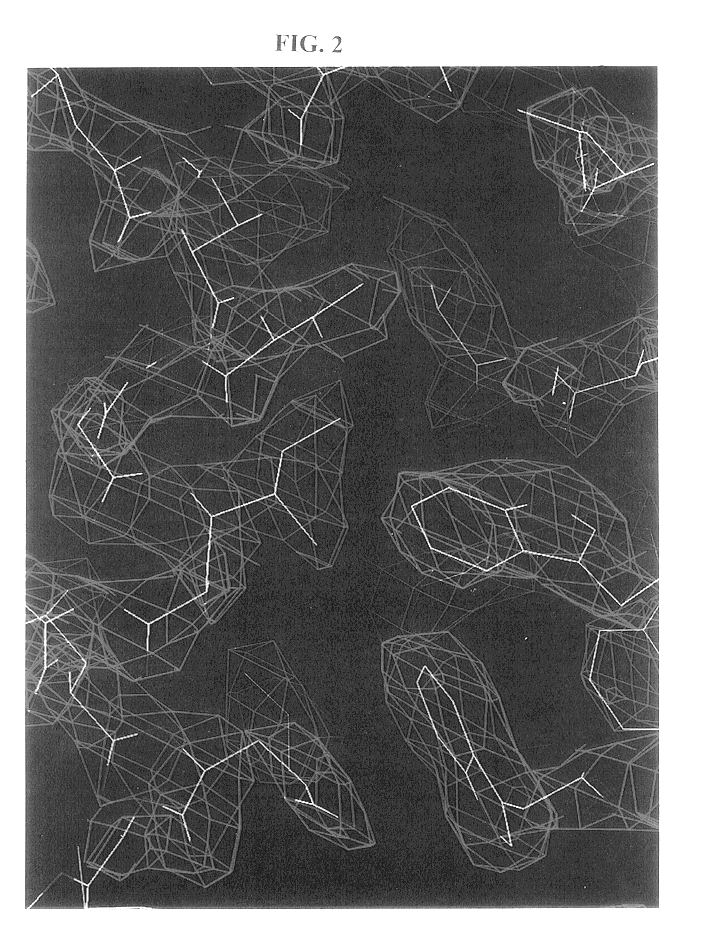
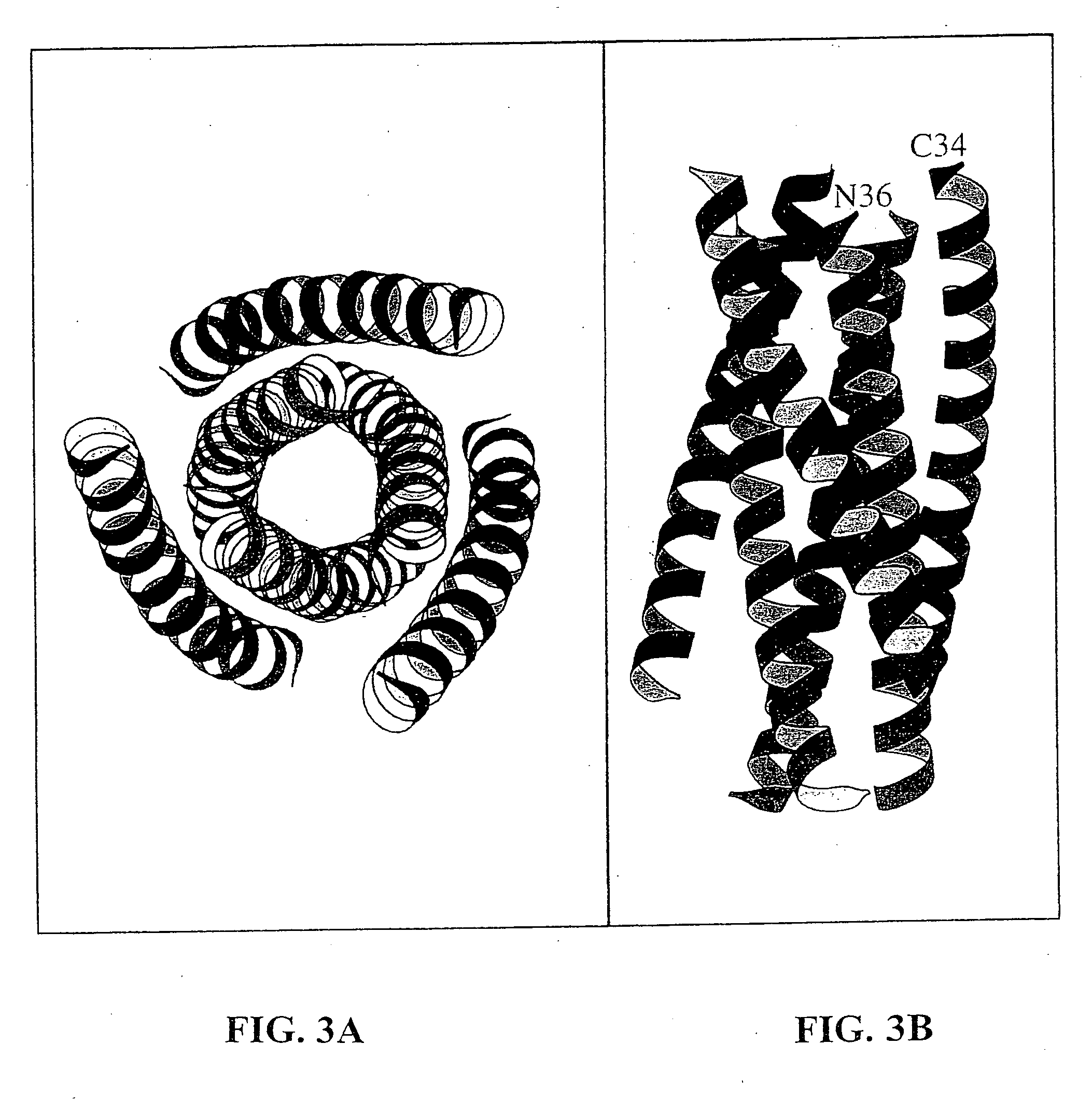
Core structure of gp41 from the HIV envelope glycoprotein
InactiveUS6506554B1Increase infectivityHigh activityPeptide/protein ingredientsMicrobiological testing/measurementHiv envelopeDesigning drug
Described are the crystal structure of the alpha-helical domain of the gp41 component of HIV-1 envelope glycoprotein which represents the core of fusion-active gp41, methods of identifying and designing drugs which inhibit gp41 function and drugs which do so.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Stabilized soluble glycoprotein trimers
InactiveUS20050106177A1Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
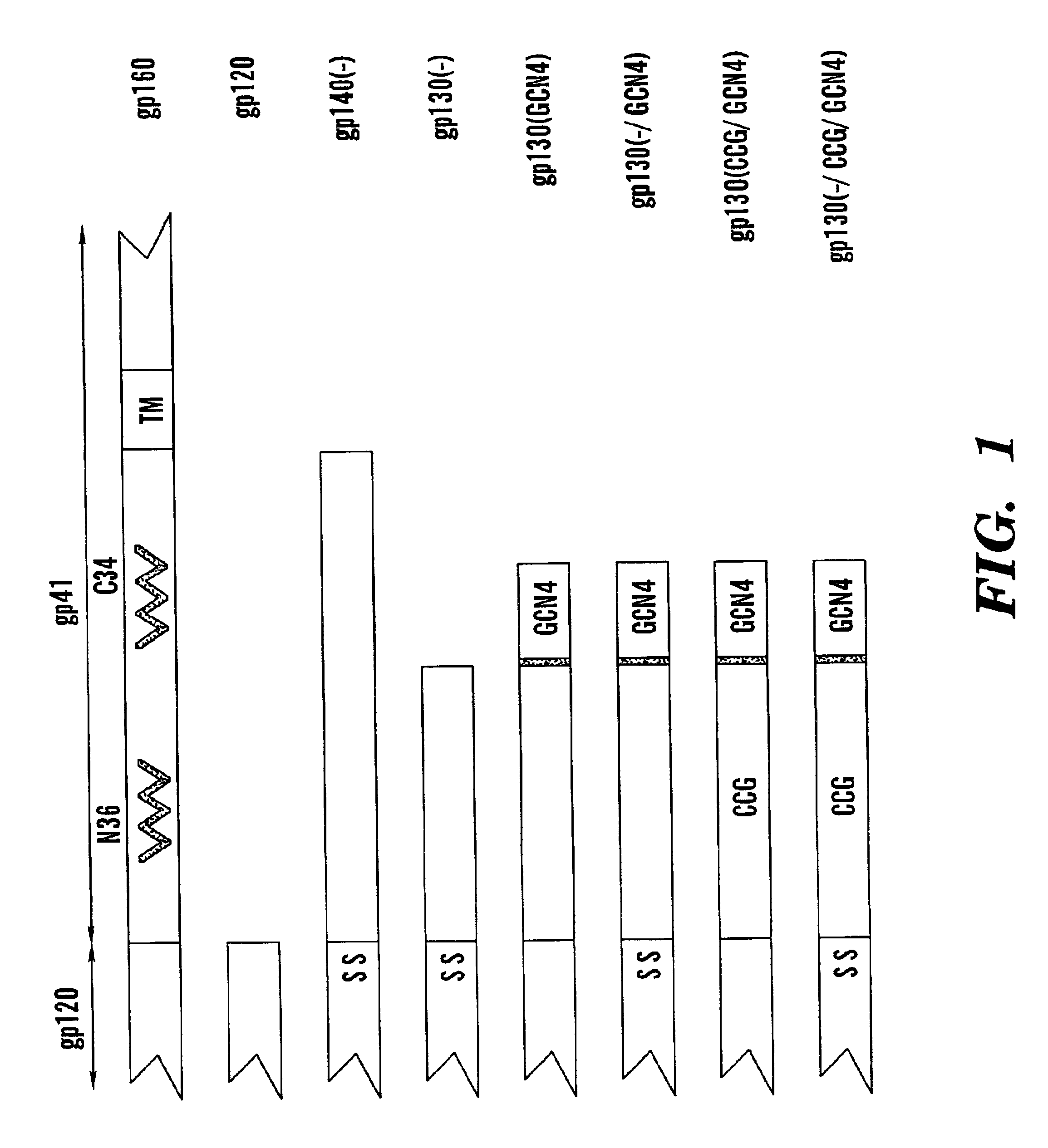
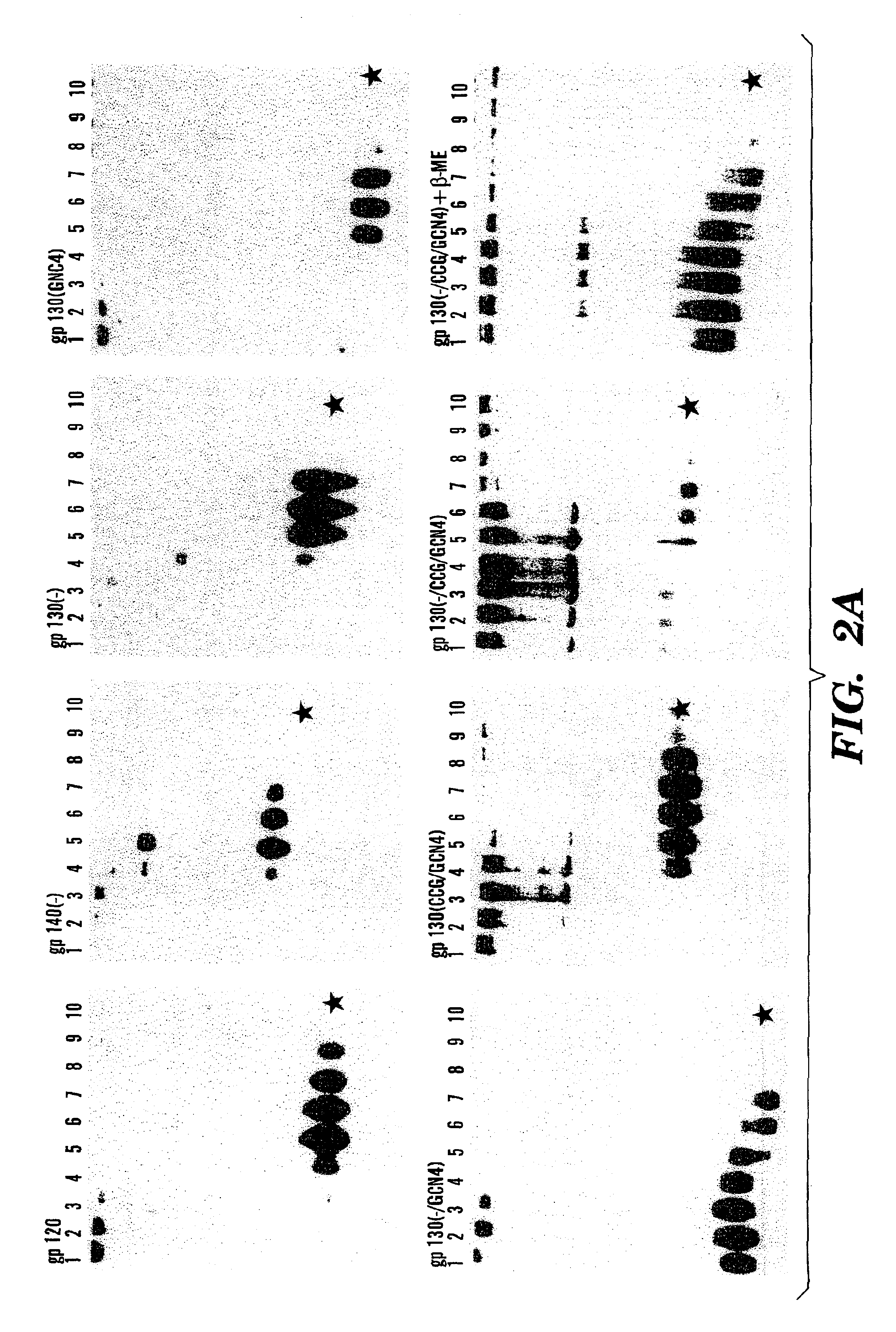
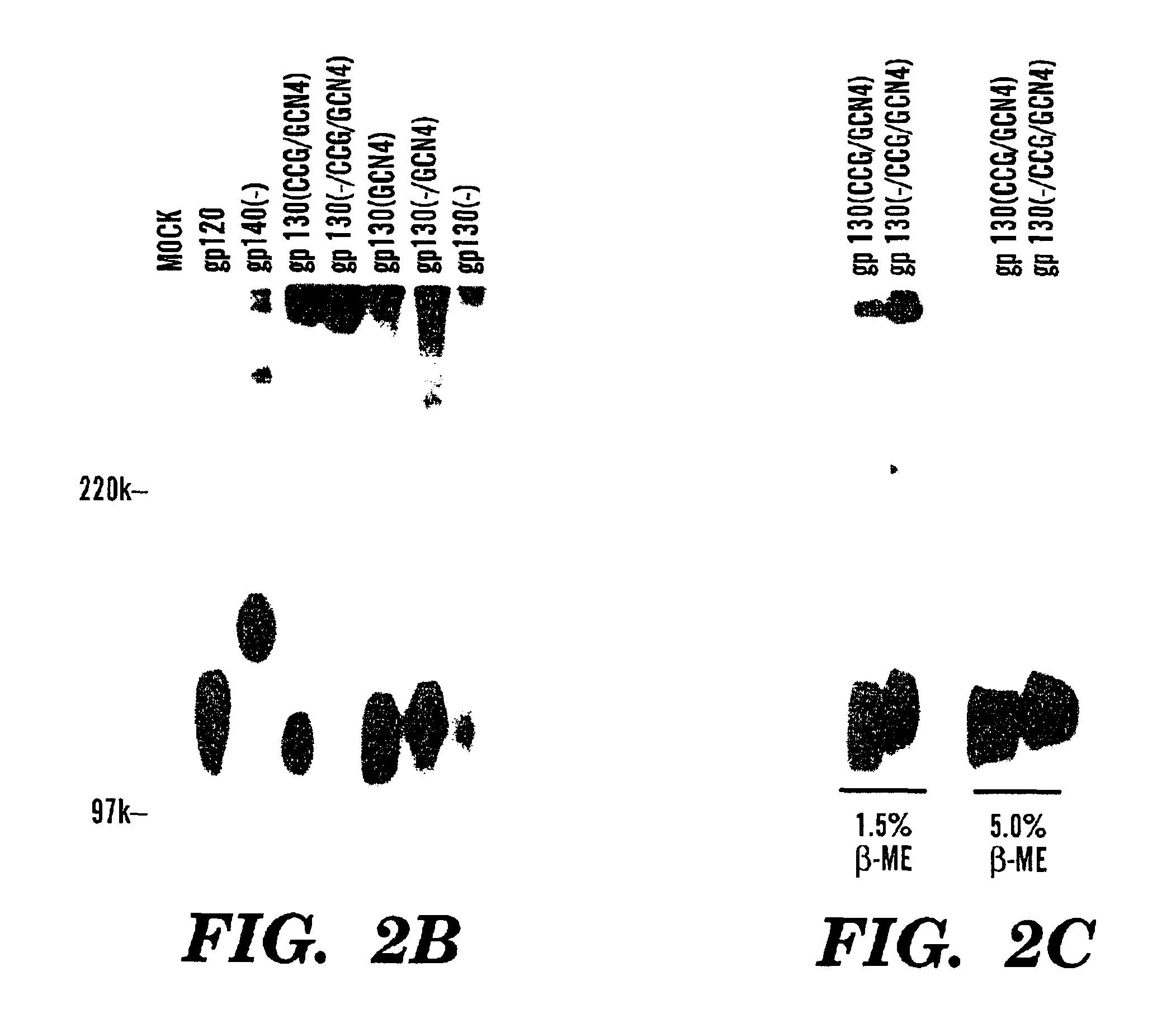
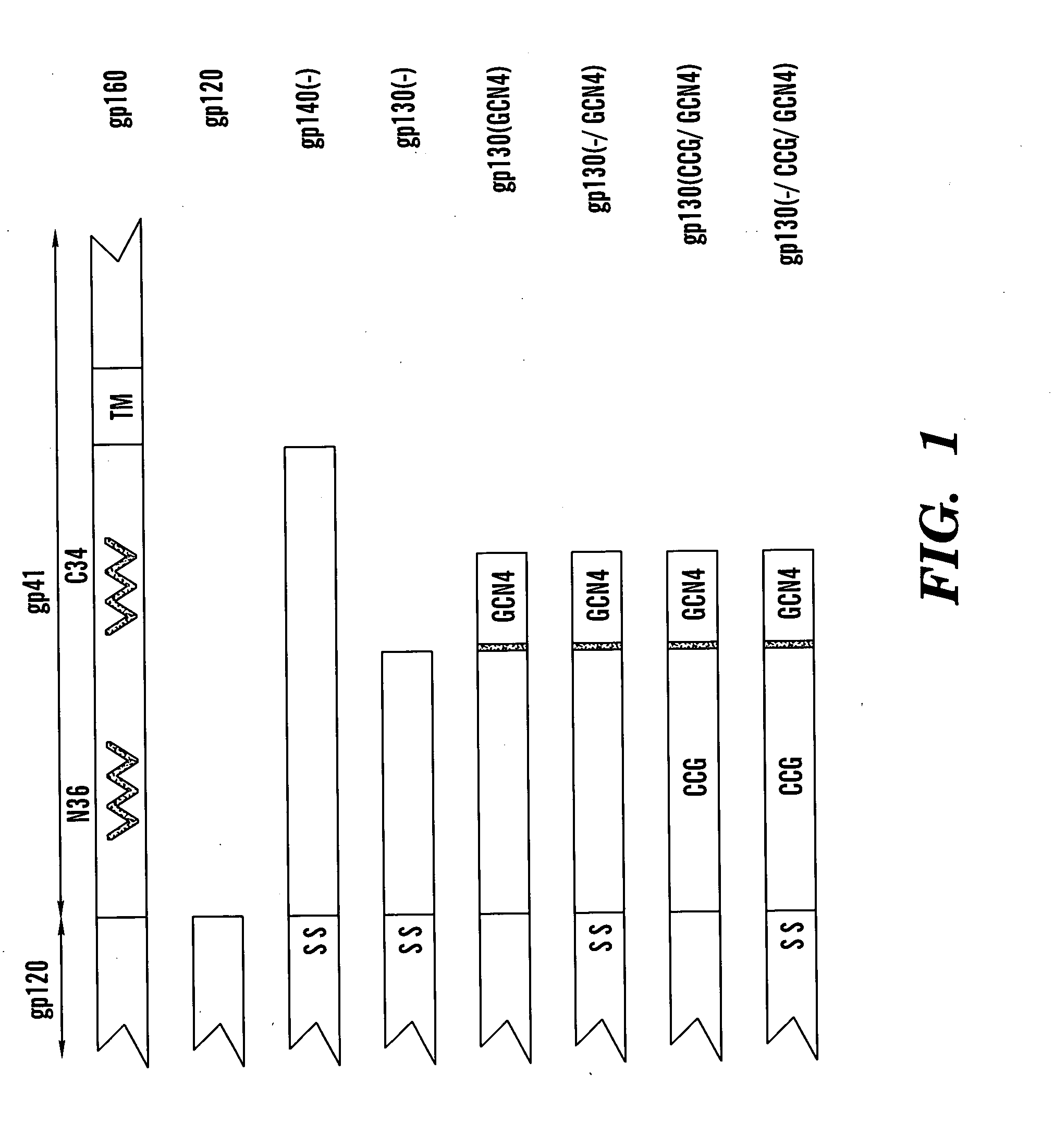
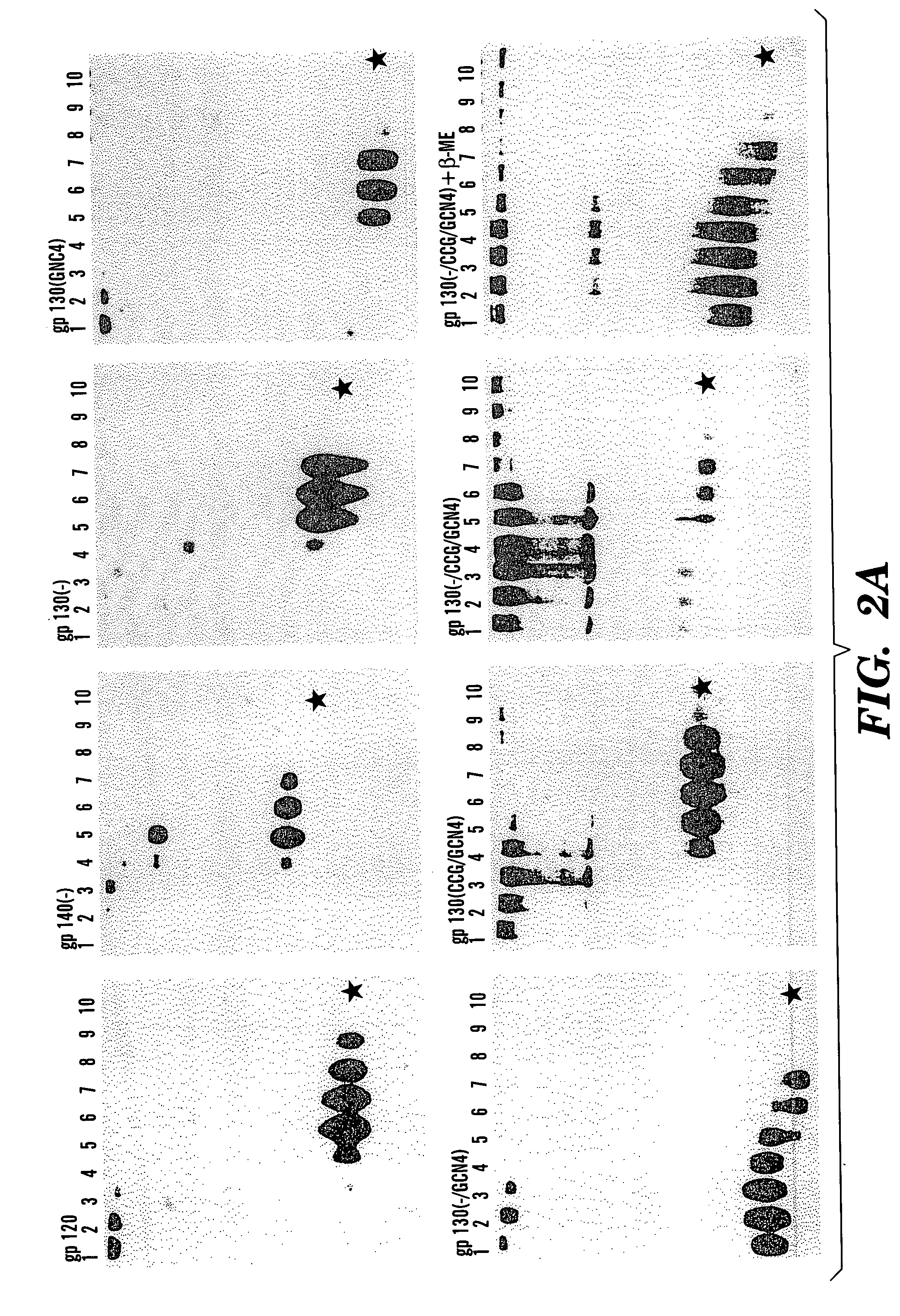
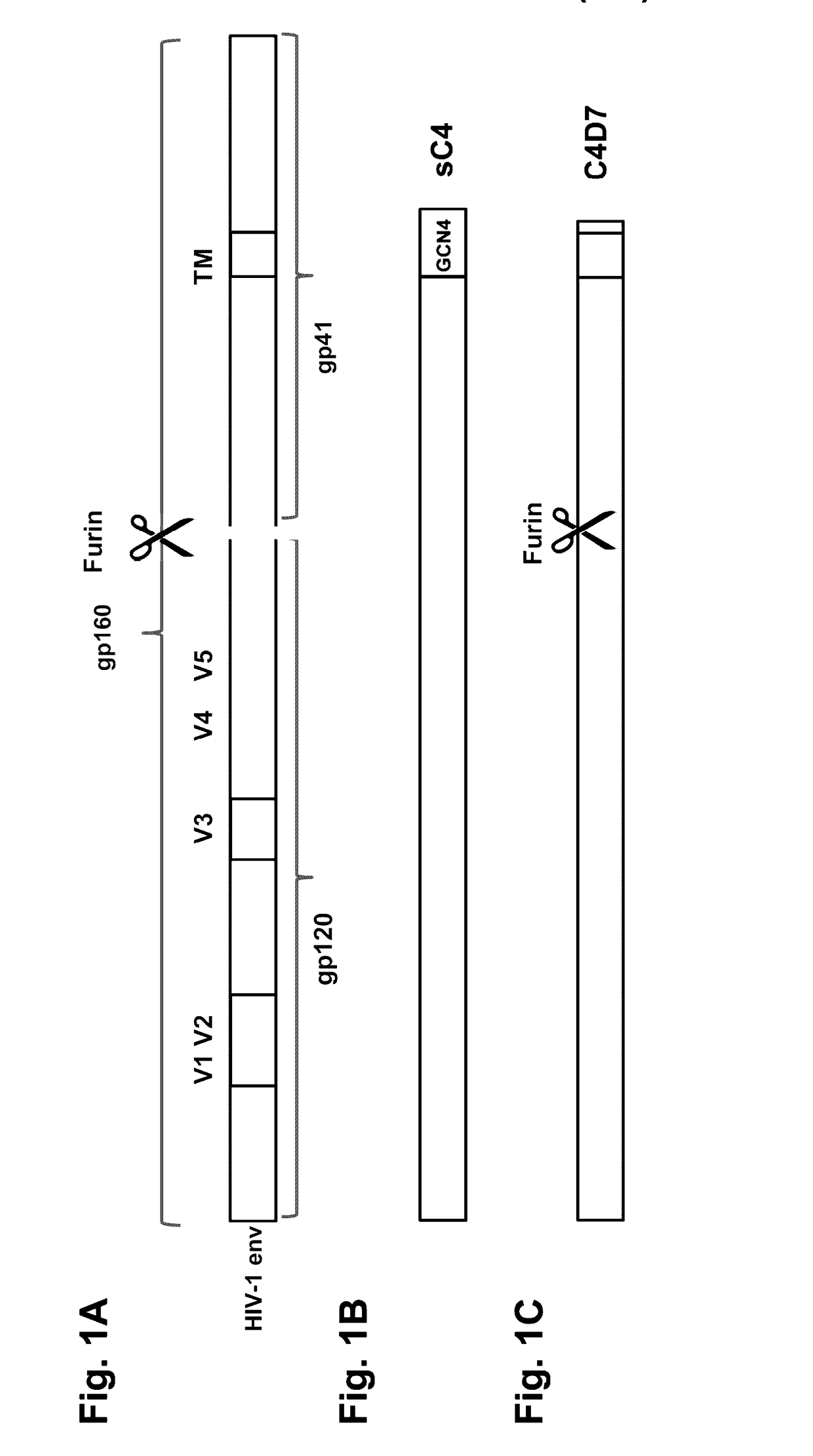
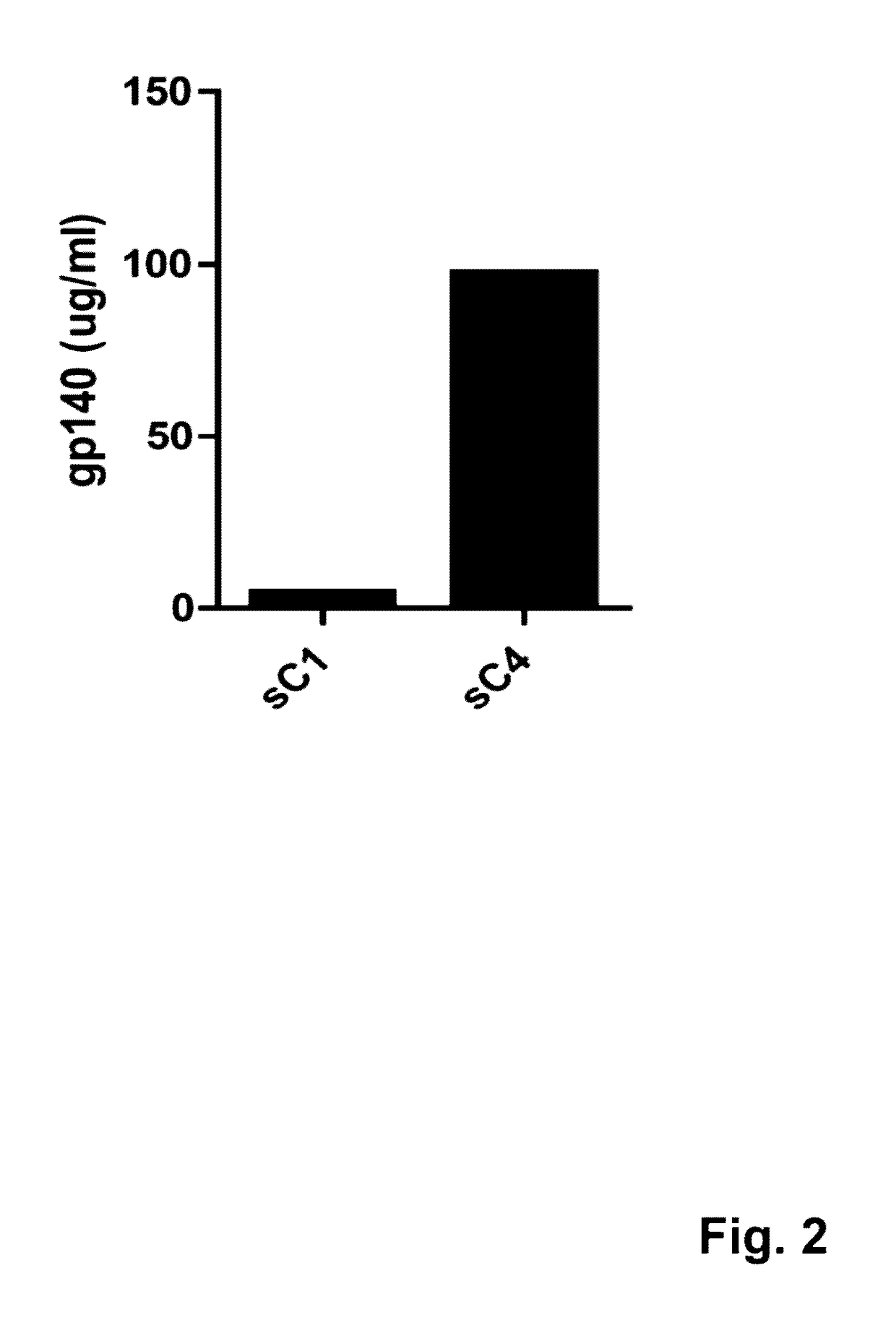
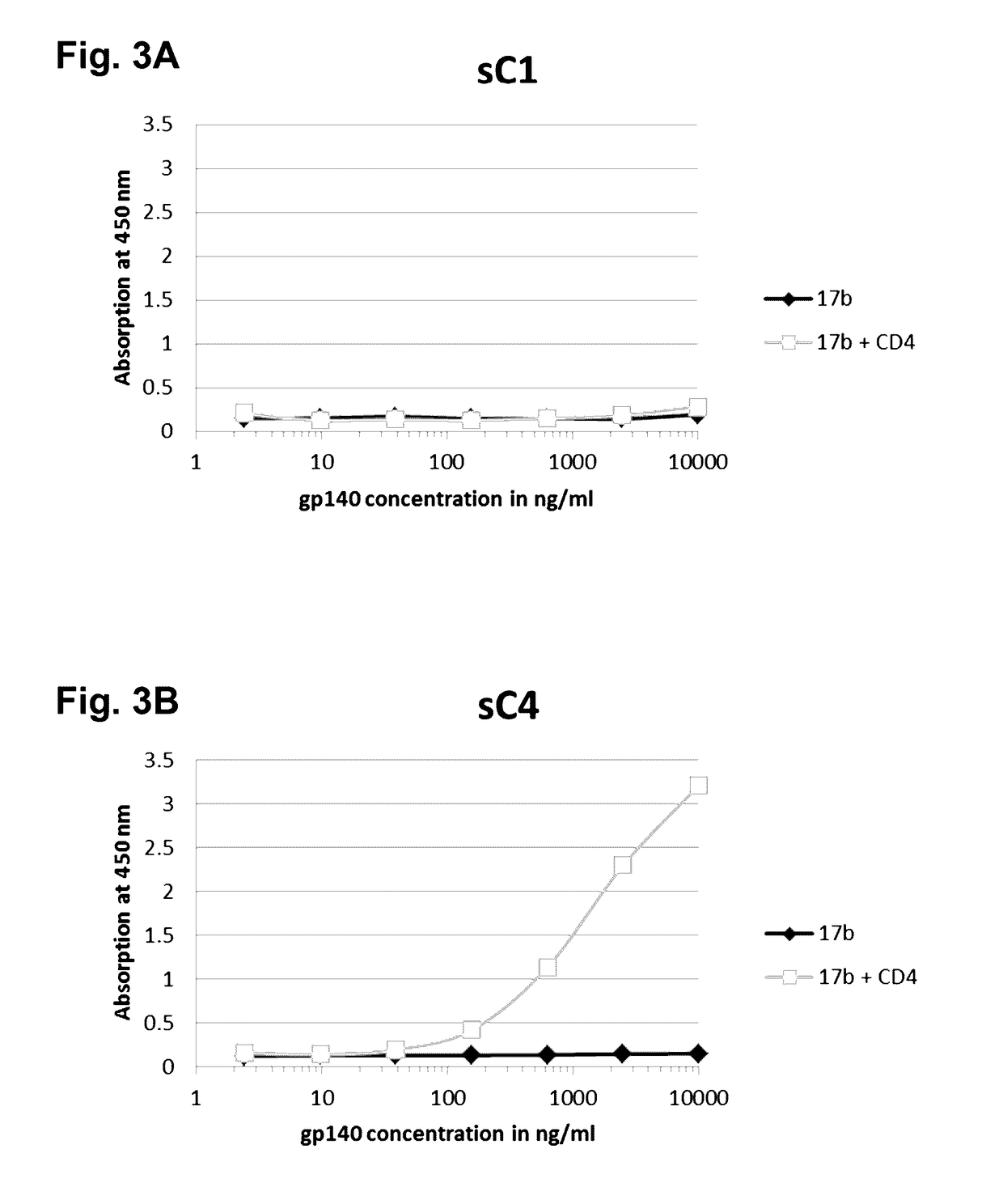
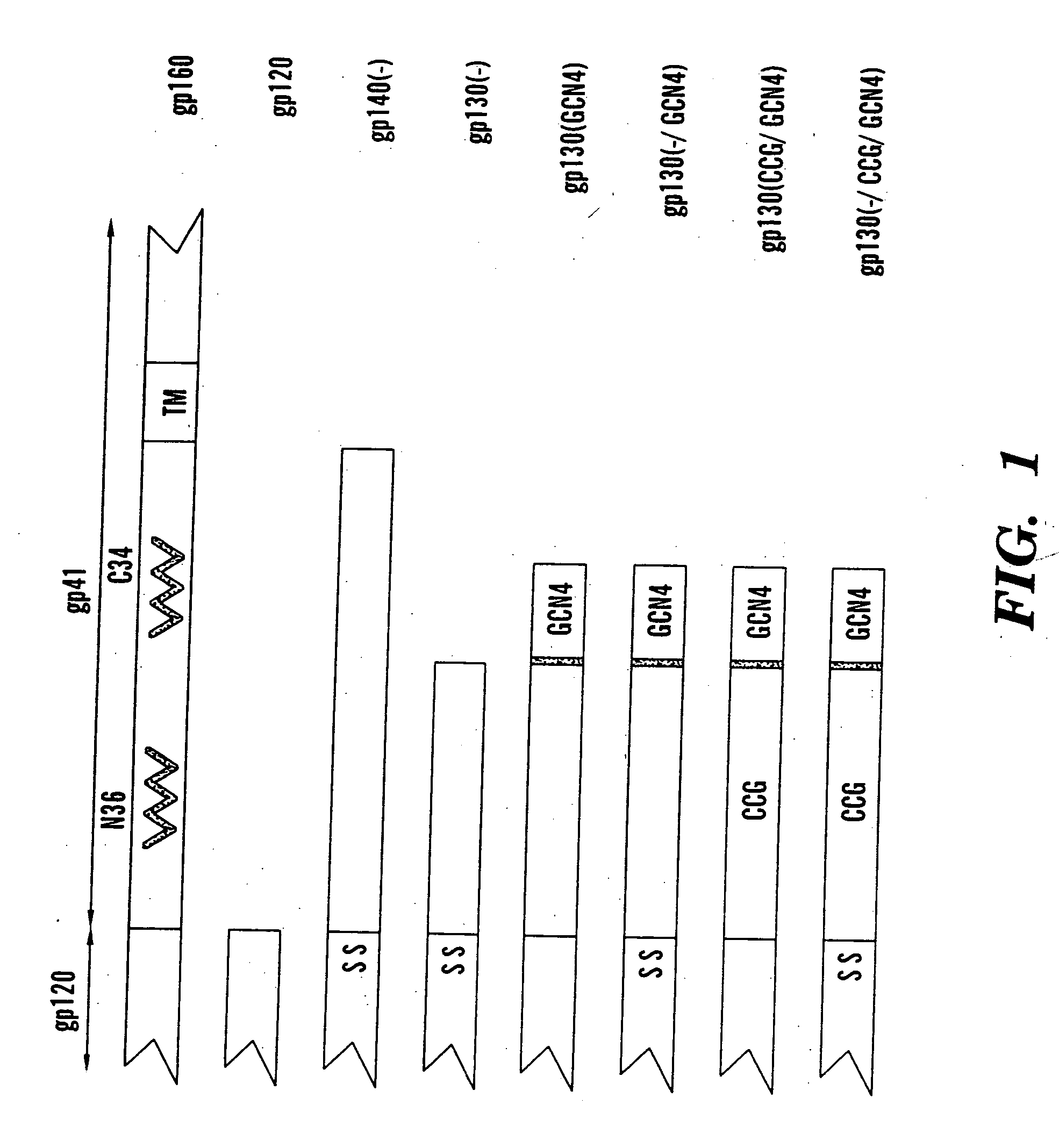
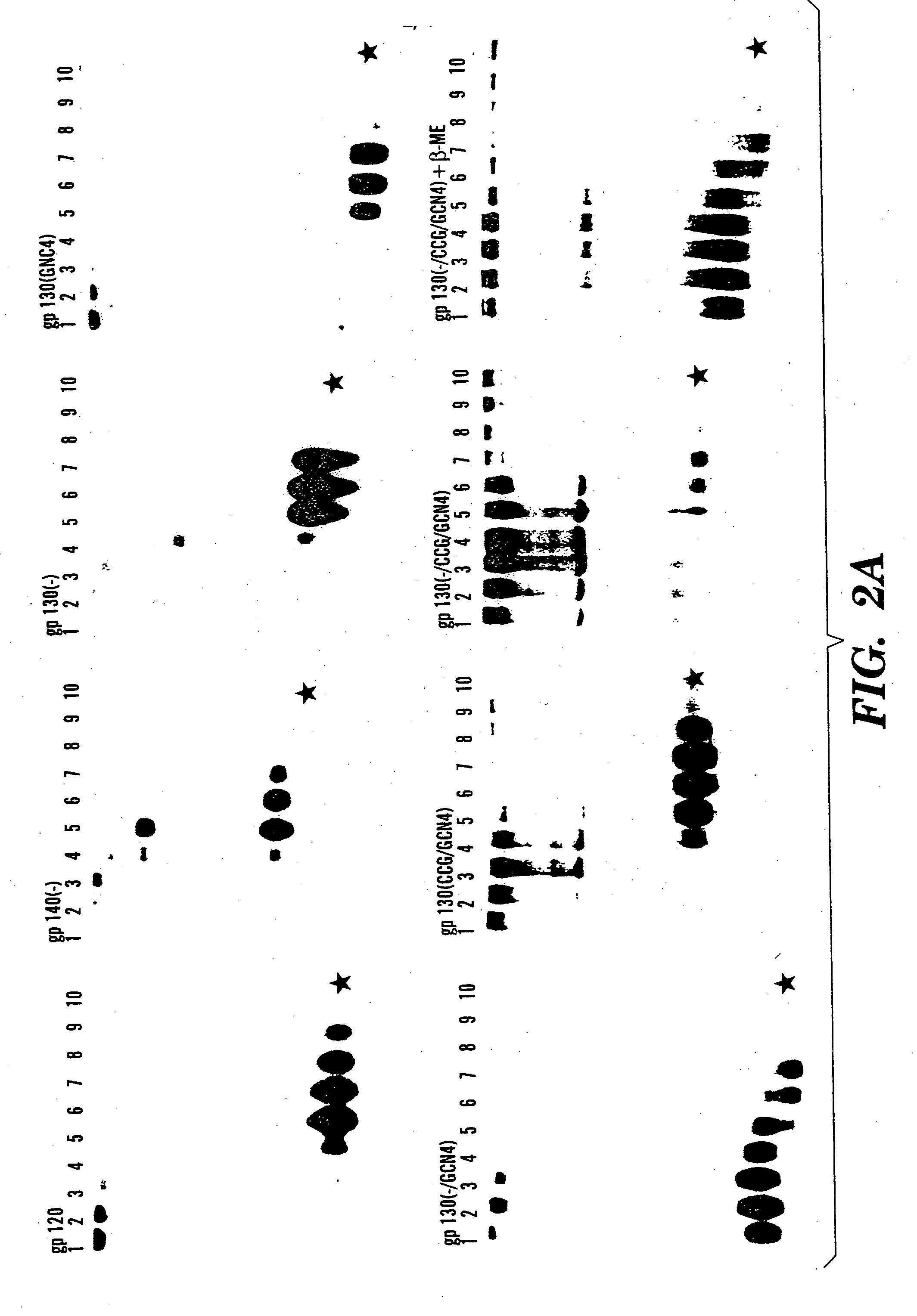
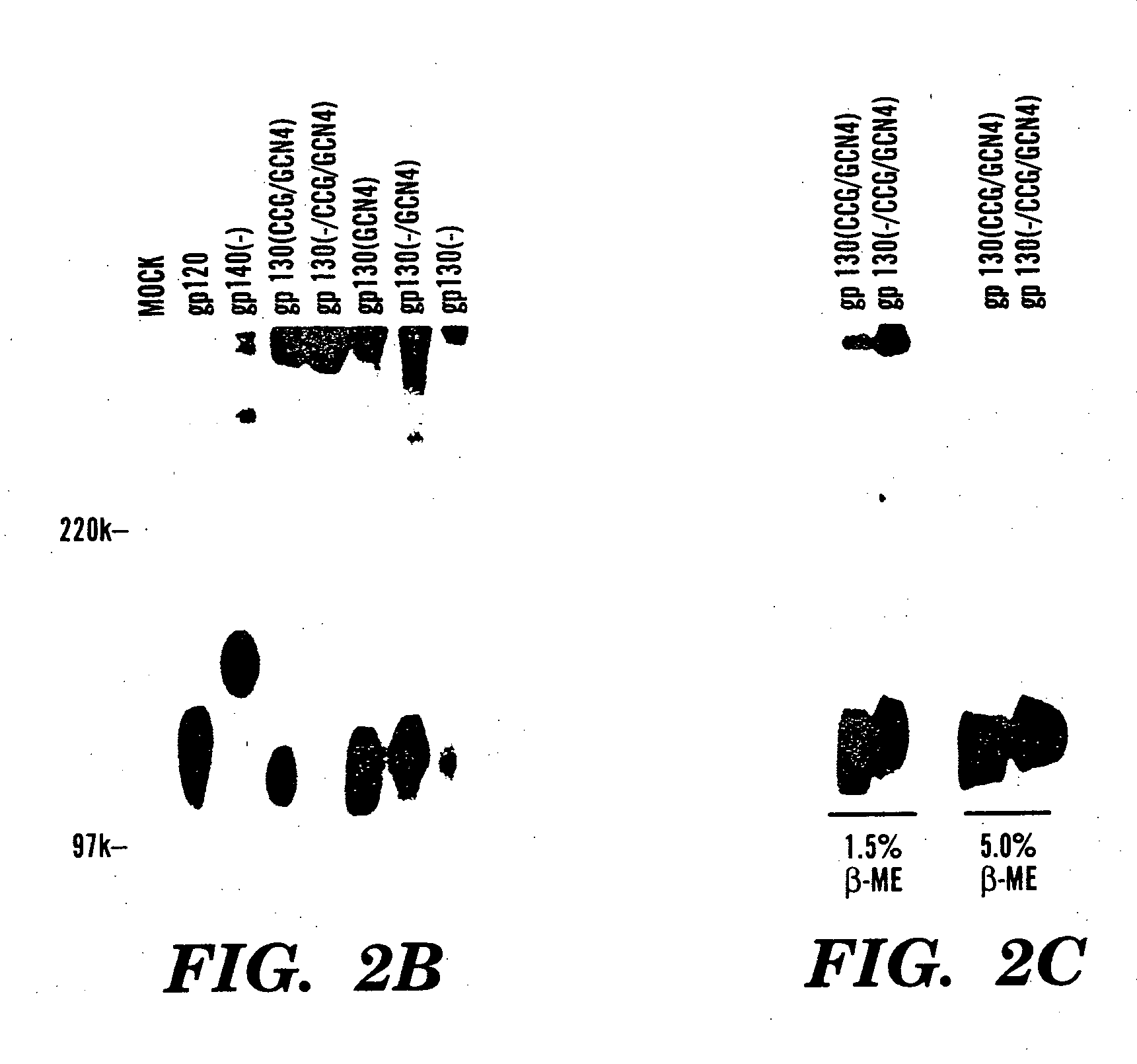
The present application is directed to stabilized HIV envelope glycoprotein trimers. The trimers are stabilized by introducing trimeric motifs, preferably the GCN4 coiled coil or the fibritin trimeric domain, at certain sites, for example in the gp41 ectodomain. These stabilized trimers or DNA molecules encoding such trimers can be used to generate an immunogenic reaction. The trimers can also be used in assays to screen for molecules that interact with them—and to identify molecules that interact with specific sites.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Vaccine
InactiveUS7655235B2Reduce and prevent glycosylationGlycosylation can be reduced and preventedAntibody mimetics/scaffoldsVirus peptidesDNA vaccinationHeterologous
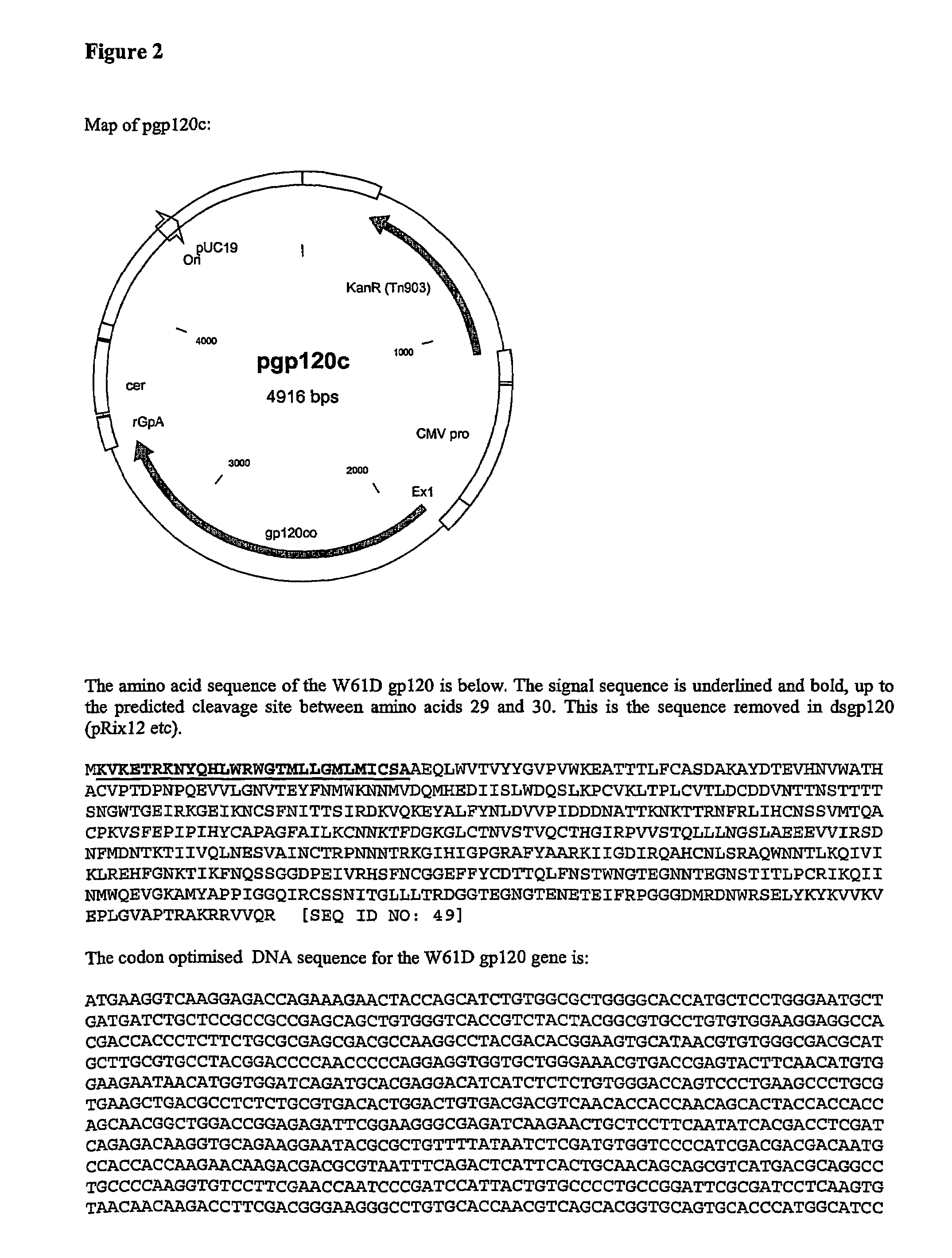
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative, which is non-glycosylated when expressed in a mammalian target cell, operably linked to a heterologous promoter. Preferably the HIV envelope molecule, such as gp120 or gp140 or gp160, lacks a functional secretion signal. It may be fused to additional HIV proteins such as Nef, Gag, RT or Tat.
Owner:GLAXO GRP LTD
Human immunodeficiency virus antigens, vectors, compositions, and methods of use thereof
ActiveUS20170165355A1Improved cell surface expressionImproved expression stabilityViral antigen ingredientsVirus peptidesAntigenHiv envelope
Synthetic HIV envelope proteins, vectors and compositions thereof, and methods for inducing protective immunity against human immunodeficiency virus (HIV) infection are described. Viral expression vectors encoding the synthetic HIV envelope proteins can be used in vaccines to provide improved protective immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV
Fusion antibodies for HIV therapy
InactiveUS8637024B2Improved potency and breadth against HIVImprove barrier propertiesHybrid immunoglobulinsPeptide/protein ingredientsHiv envelopeBinding site
Owner:THE ROCKEFELLER UNIV
Stabilized soluble glycoprotein trimers
InactiveUS20050220817A1Stabilize trimerSimplifies isolationAntibody mimetics/scaffoldsVirus peptidesHiv envelopeGp41
The present application is directed to stabilized HIV envelope glycoprotein trimers. The trimers are stabilized by introducing trimeric motifs, preferably the GCN4 coiled coil or the fibritin trimeric domain, at certain sites, for example in the gp41 ectodomain. These stabilized trimers or DNA molecules encoding such trimers can be used to generate an immunogenic reaction. The trimers can also be used in assays to screen for molecules that interact with them—and to identify molecules that interact with specific sites.
Owner:DANA FARBER CANCER INST INC
Hiv envelope-cd4 complexes and hybrids
Env-CD4 complexes and hybrids are disclosed that expose cryptic epitopes that are important in virus neutralization. Methods of diagnosis, treatment and prevention using the polynucleotides and polypeptides are also provided.
Owner:NOVARTIS AG +1
Method for generating immunogens that elicit neutralizing antibodies against fusion-active regions of HIV envelope proteins
The current invention relates to methods of generating immunogens that elicit broadly neutralizing antibodies which target regions of viral envelope proteins such as the gp 120 / gp41 complex of HIV-1. More specifically, the current invention involves using stabilizing peptides modeling the alpha-helical regions of the ectodomain of the HIV-1 transmembrane protein to stabilize fusion-active intermediate structures which can be used as vaccine immunogens.
Owner:PANACOS PHARMACEUTICALS INC
Core structure of gp41 from the HIV envelope glycoprotein
Described are the crystal structure of the α-helical domain of the gp41 component of HIV-1 envelope glycoprotein which represents the core of fusion-active gp41, methods of identifying and designing drugs which inhibit gp41 function and drugs which do so.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Vaccine
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative fused to an additional HIV protein selected from a non-structural protein or capsid protein or fragment or immunogenic derivative thereof. Preferably the HIV envelope molecule is gp120 and preferred fusions include one or more of HIV Nef, Gag, RT or Tat. Preferably the HIV envelope molecule is non-glycosylated in mammalian cells.
Owner:GLAXO GRP LTD
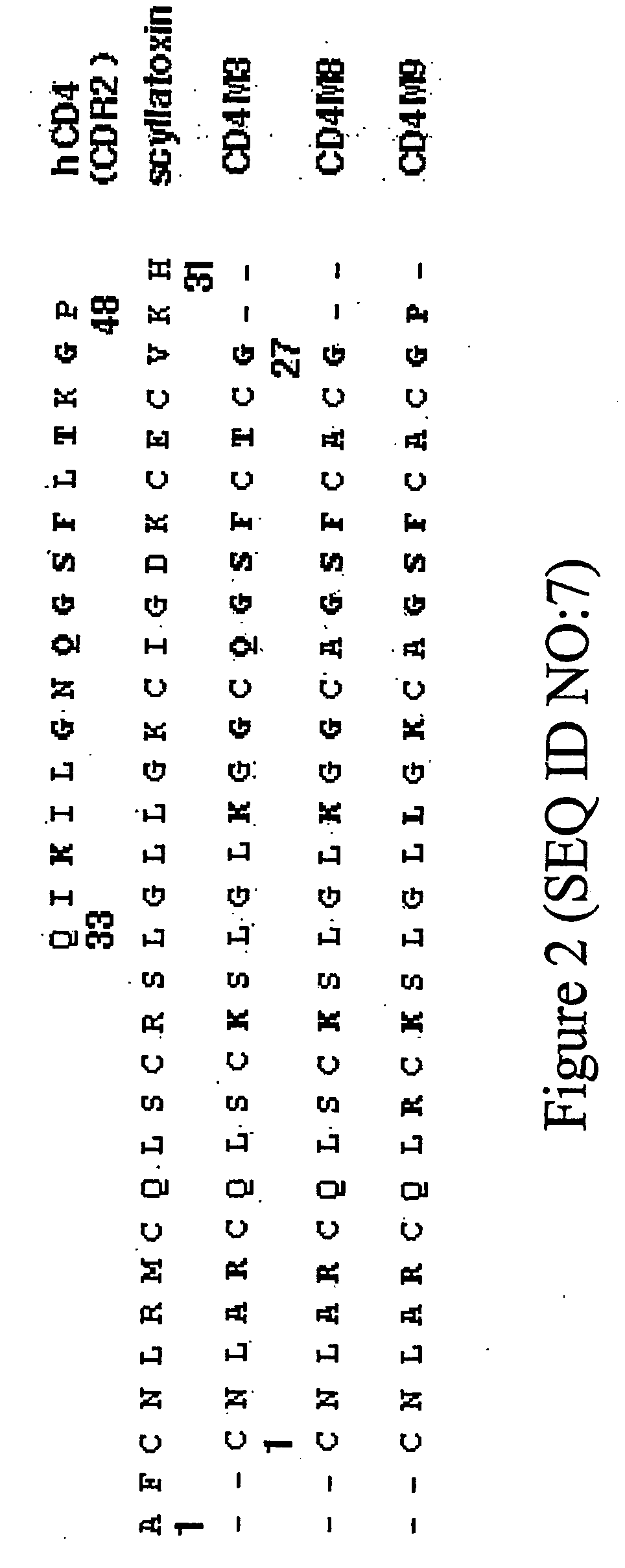
Webbed immunogens comprising recombinant human immunodeficiency virus (HIV) envelope glycoproteins and the M9 scorpion toxin
HIV envelope immunogens that display multivalent epitopes are provided. The immunogens are aggregated, “webbed” HIV envelope immunogens in which native envelope structures are stabilized due to interactions with multimeric derivatives of M9 scorpion toxin.
Owner:INT AIDS VACCINE INITIATIVE INC
Vaccine
InactiveUS20070042977A1Reduce and prevent glycosylationGlycosylation can be reduced and preventedAntibody mimetics/scaffoldsGenetic material ingredientsDNA vaccinationHeterologous
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative, which is non-glycosylated when expressed in a mammalian target cell, operably linked to a heterologous promoter. Preferably the HIV envelope molecule, such as gp120 or gp140 or gp160, lacks a functional secretion signal. It may be fused to additional HIV proteins such as Nef, Gag, RT or Tat.
Owner:GLAXO GROUP LTD
Synthetic human immunodeficiency virus (HIV) envelope antigen, vectors, and compositions thereof
ActiveUS10369214B2High expressionImprove stabilityViral antigen ingredientsVirus peptidesAntigenHiv envelope
Synthetic HIV envelope proteins, vectors and compositions thereof, and methods for inducing protective immunity against human immunodeficiency virus (HIV) infection are described. Viral expression vectors encoding the synthetic HIV envelope proteins can be used in vaccines to provide improved protective immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV
Immunoenhancer-linked oligomeric HIV envelope peptides
Owner:NEW YORK BLOOD CENT
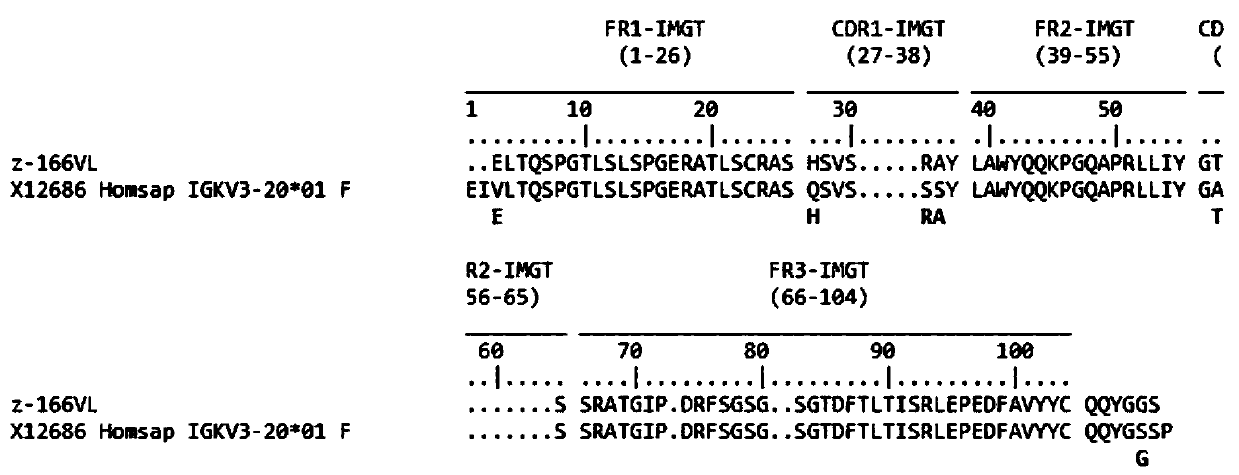
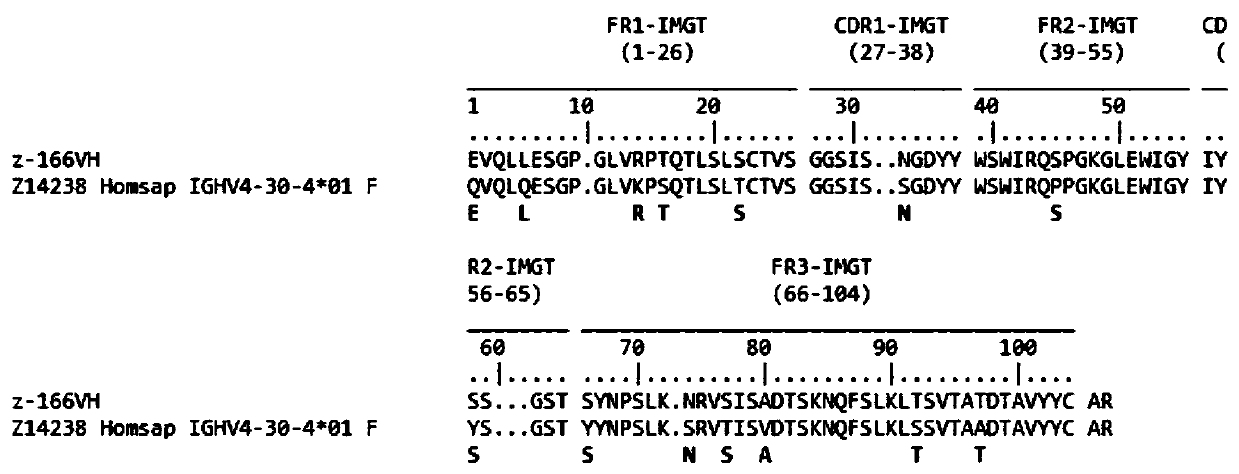
Human anti-HIV gp 120 specific antibody Z166 and application method thereof
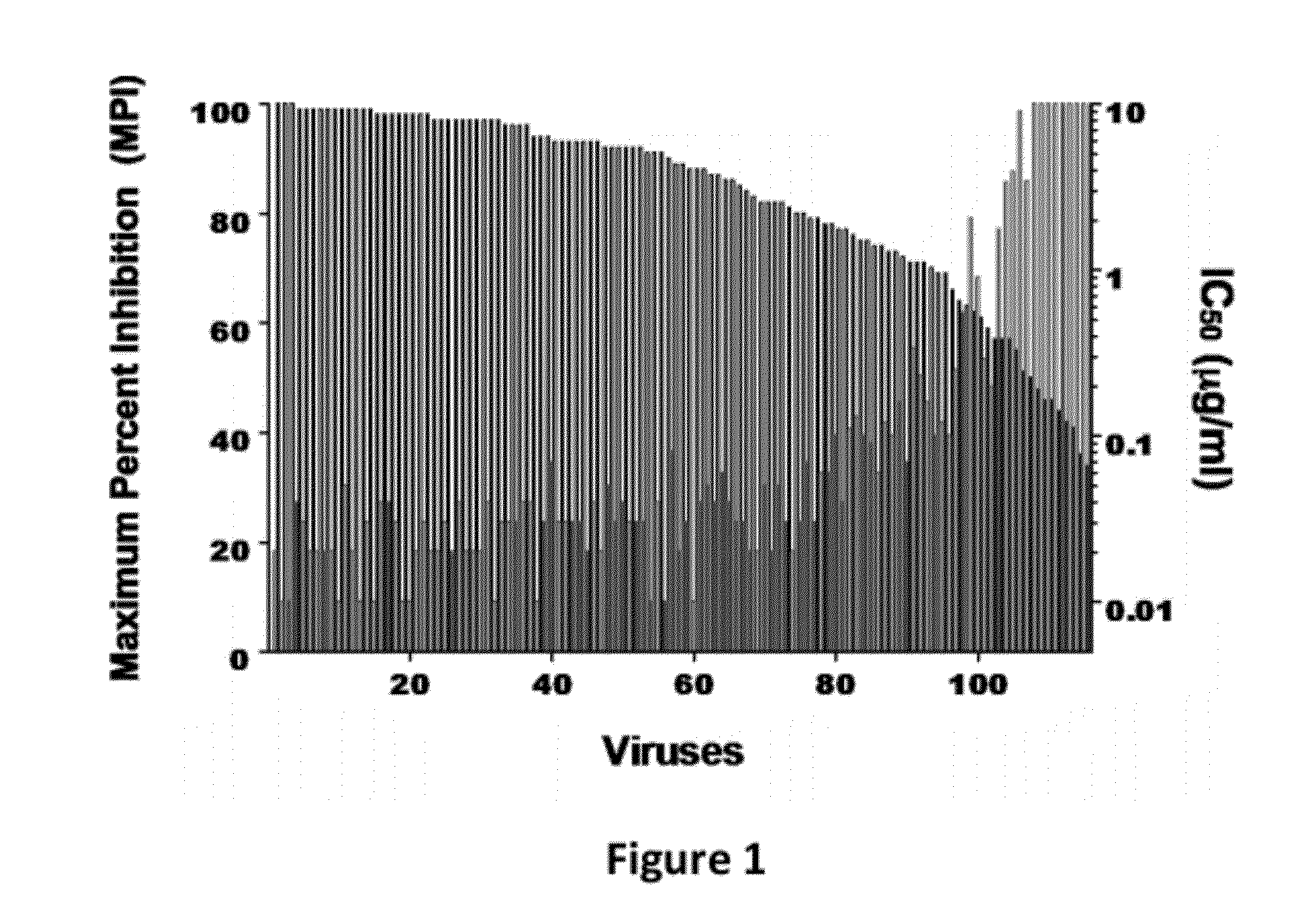
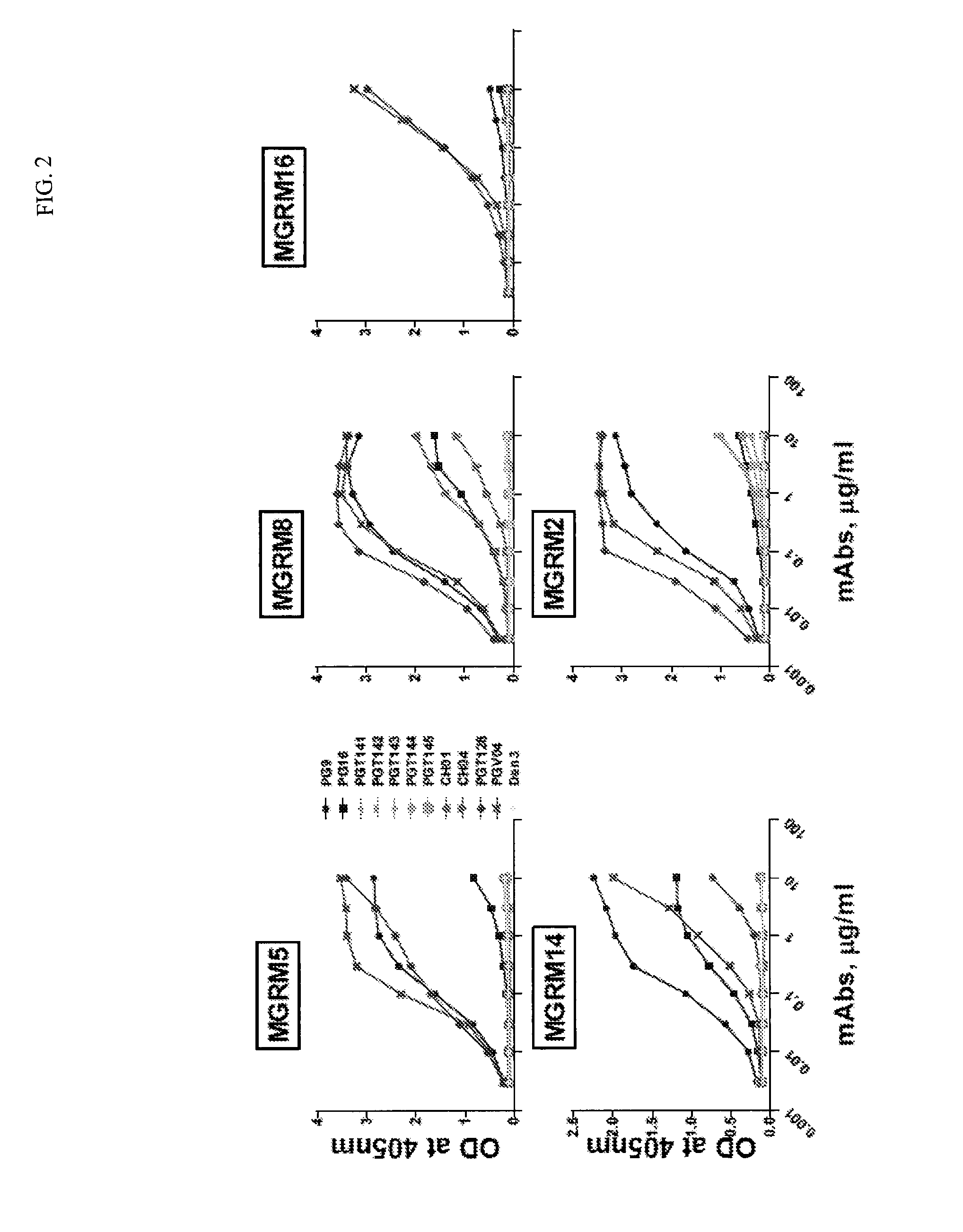
The present invention discloses to a human anti-HIV gp 120 specific antibody Z166, provides amino acid sequences of the antibody and an active fragment thereof, provides coding genes of the antibody or the active fragment thereof, and simultaneously provides application methods of the antibody and the active fragment thereof in preparing medicines for treating or preventing HIV infection diseasesand application methods in preparing HIV diagnosis or detection reagents. The antibody Z166 can effectively neutralize a plurality of HIV virus subtypes, is used for treating HIV infected patients, effectively mediates killing of effector cells to HIV infected cells, can also mediate the killing (antibody-dependent cell-medicated cytotoxicity, ADCC) of the effector cells to an HIV envelope proteinstable expression cell line TF 228, and keeps a killing percentage of 30% or above when antibody concentration is 0.2 [mu]g / ml or more. The provided antibody Z166 can be used for treating the HIV infected patients and is suitable for antibody medicines based on all treatments of diseases caused by HIV based on the Z166.
Owner:GUIZHOU MEDICAL UNIV
Methods of identifying novel HIV-1 immunogens
The present application relates to identifying one or more components of HIV envelope glycoprotein which bind to broadly neutralizing antibodies, which may be utilized as research tools for developing HIV-1 vaccine immunogens, antigens for crystallization and / or for identifying of broad neutralizing antibodies.
Owner:INT AIDS VACCINE INITIATIVE +1
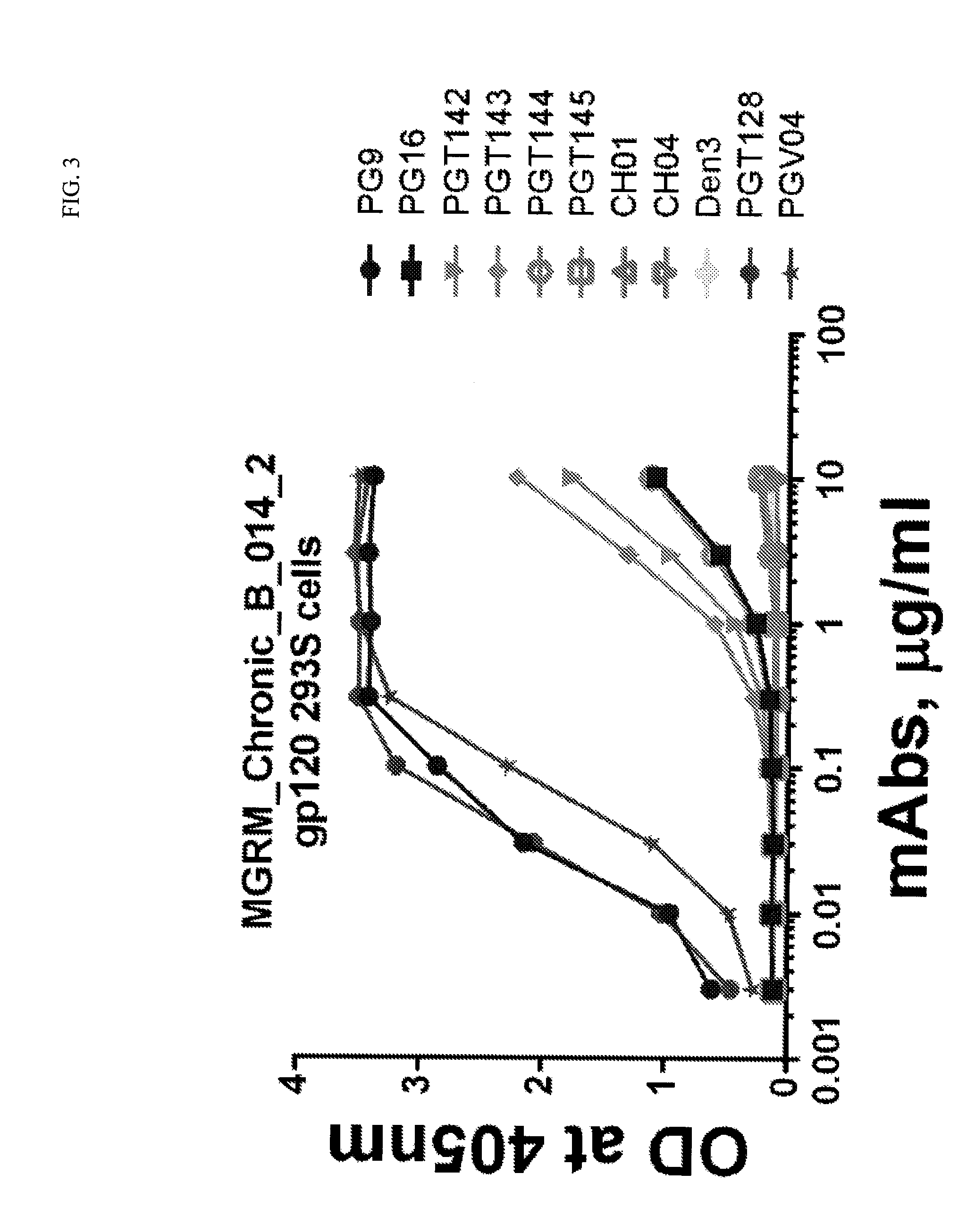
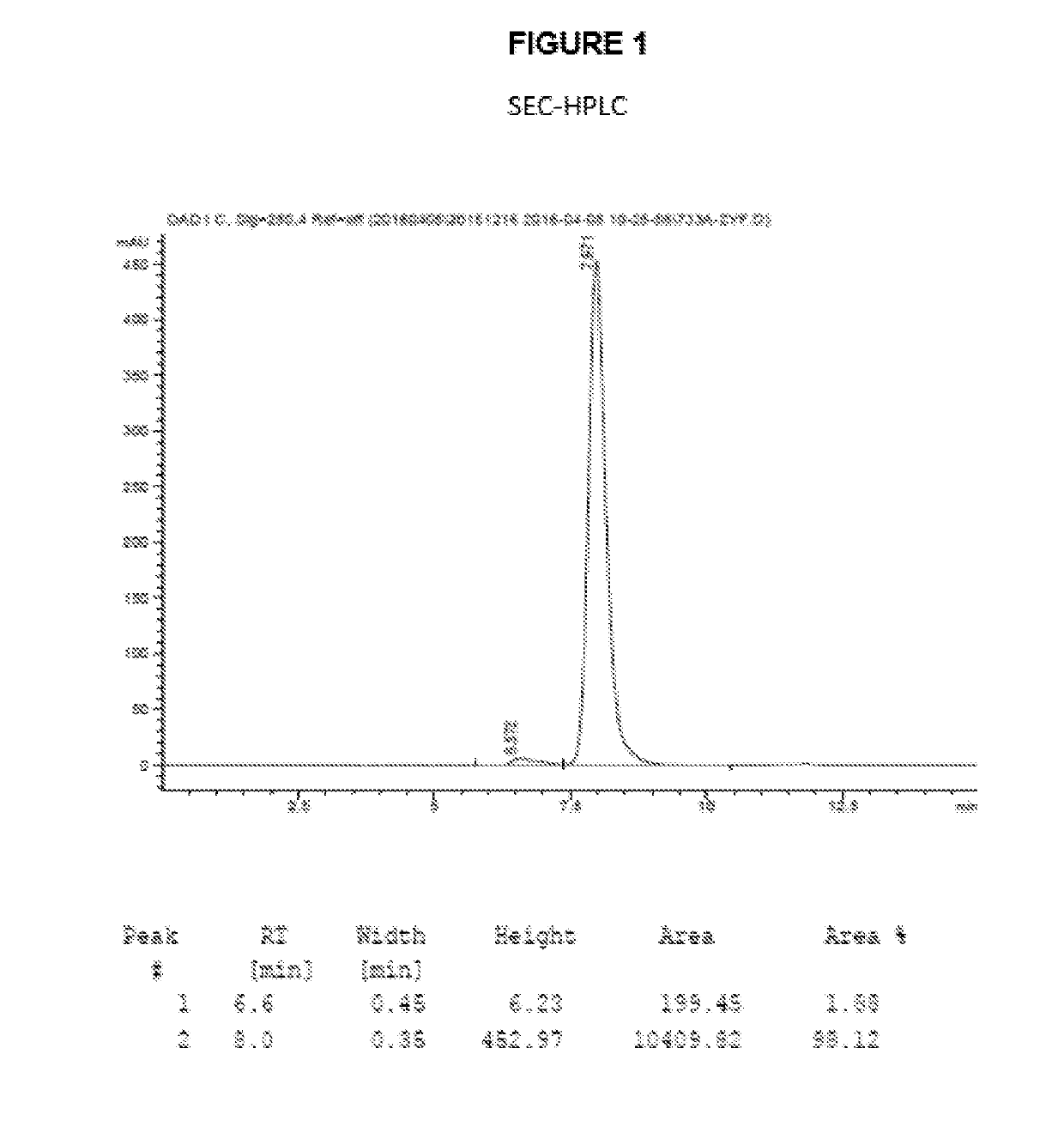
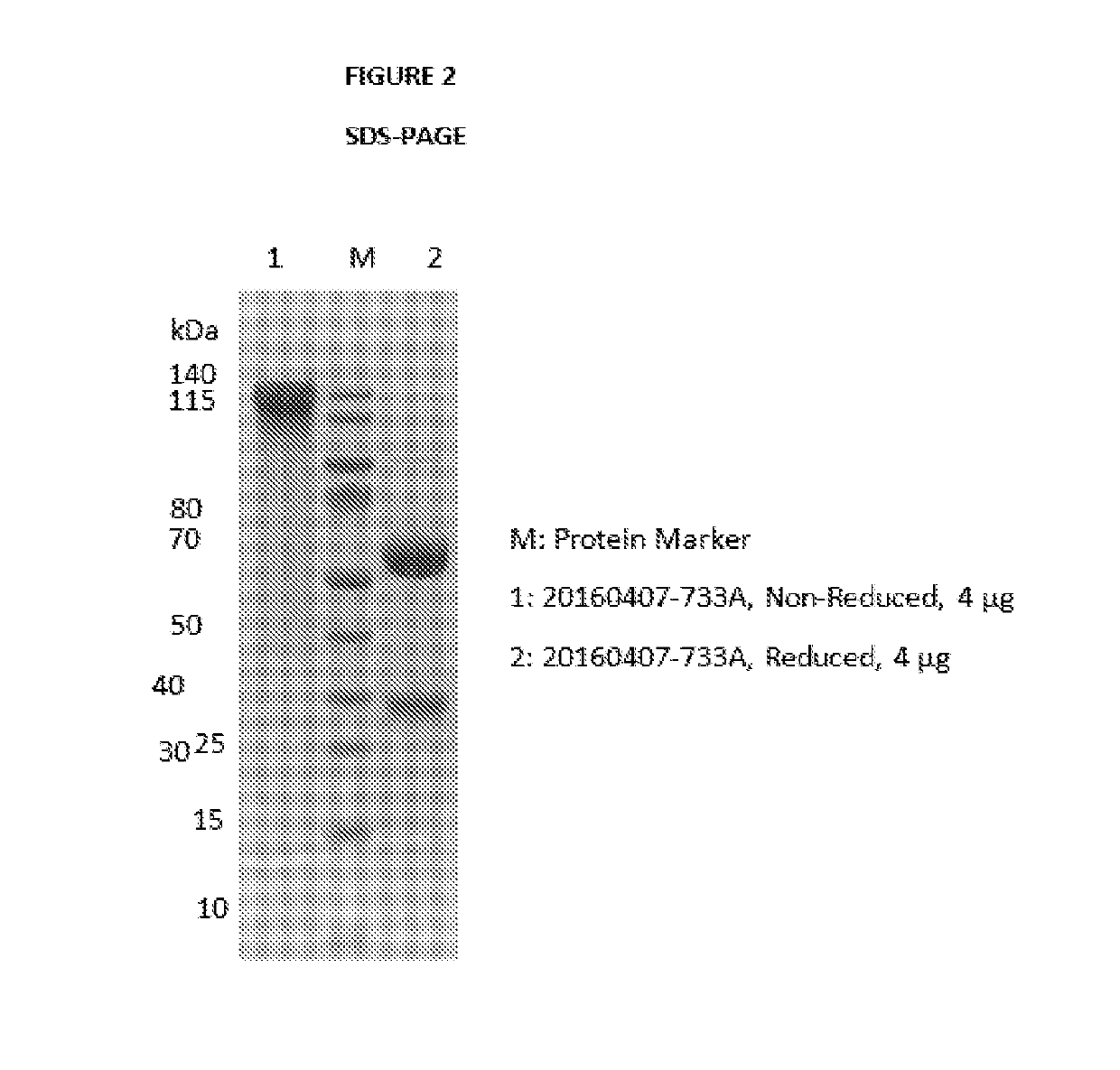
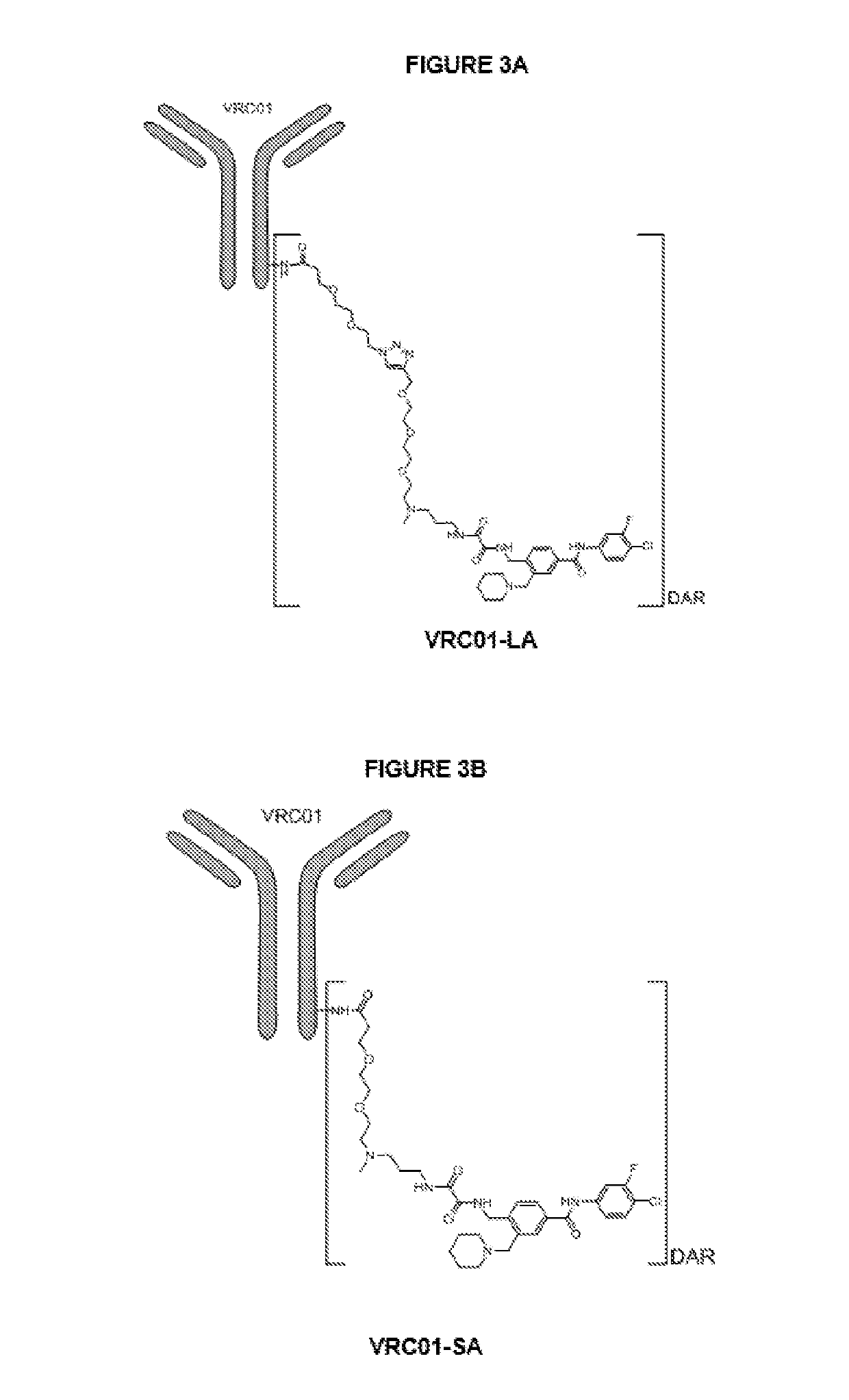
Antibody-drug conjugates and therapeutic methods using the same
InactiveUS20190328900A1Organic active ingredientsImmunoglobulins against virusesDrug specific IgEHiv envelope
The invention discloses an antibody-drug conjugate of Formula (I):Ab-[L-Dn]x (I)wherein:Ab comprises a broadly neutralizing anti-HIV antibody;L comprises a linker molecule covalently bonded to said broadly neutralizing anti-HIV antibody;D comprises one or more drugs comprising an HIV therapeutic compound covalently bonded to said linker molecule L, wherein said one or more broadly neutralizing anti-HIV antibodies Ab specifically bind to an HIV envelope glycoprotein and said one or more drugs D specifically bind to an HIV envelope glycoprotein;n is selected from 1-4; andx is selected from 1-12.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
Methods for inducing an immune response against Human Immunodeficiency Virus (HIV) in HIV-infected subjects undergoing antiretroviral therapy (ART) are described. The methods include administering an adenovirus vector primer vaccine and either a Modified Vaccinia Ankara virus (MVA) vector booster vaccine or adenovirus booster vaccine in combination with isolated HIV envelope polypeptides.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Trimer stabilizing HIV envelope protein mutations
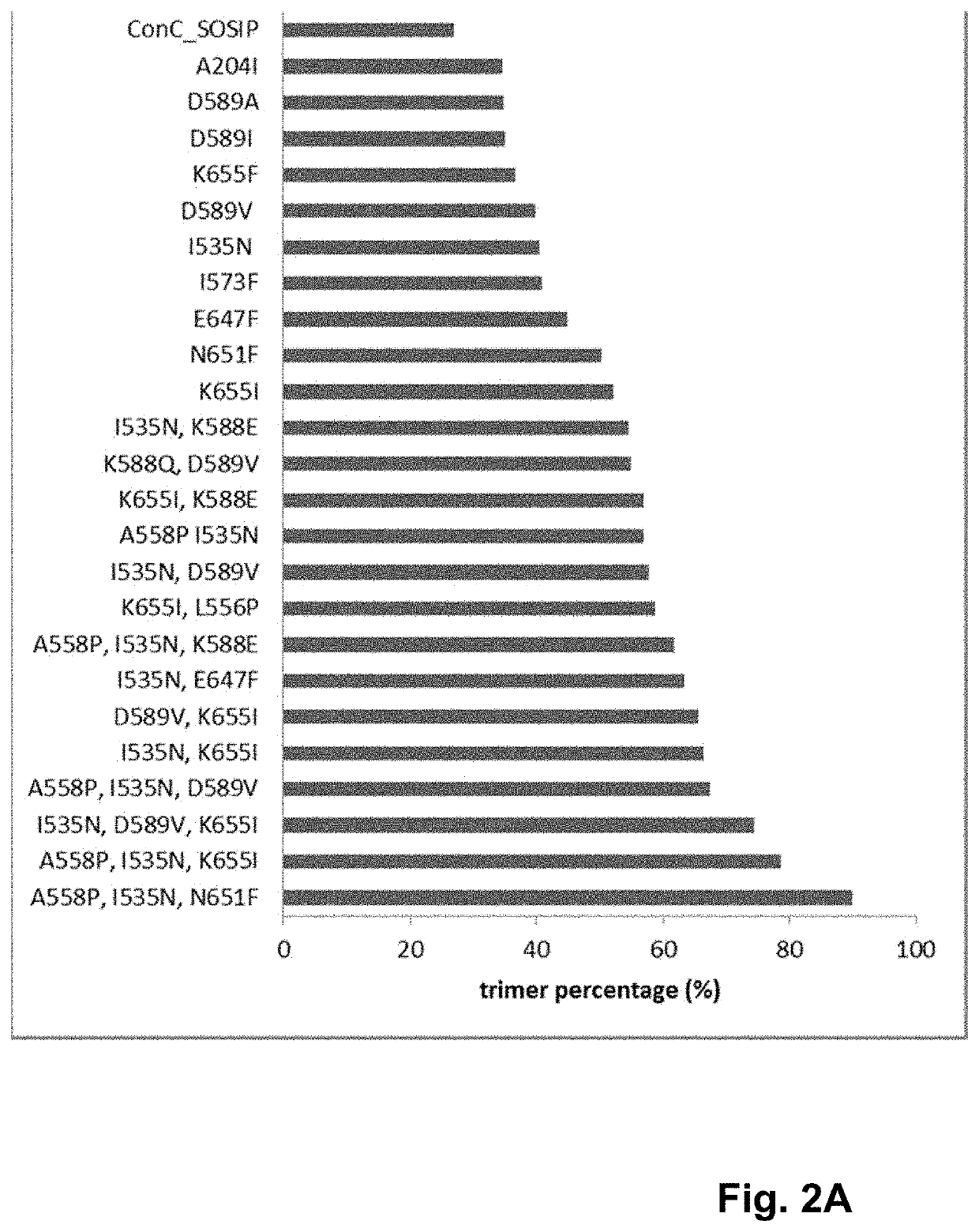
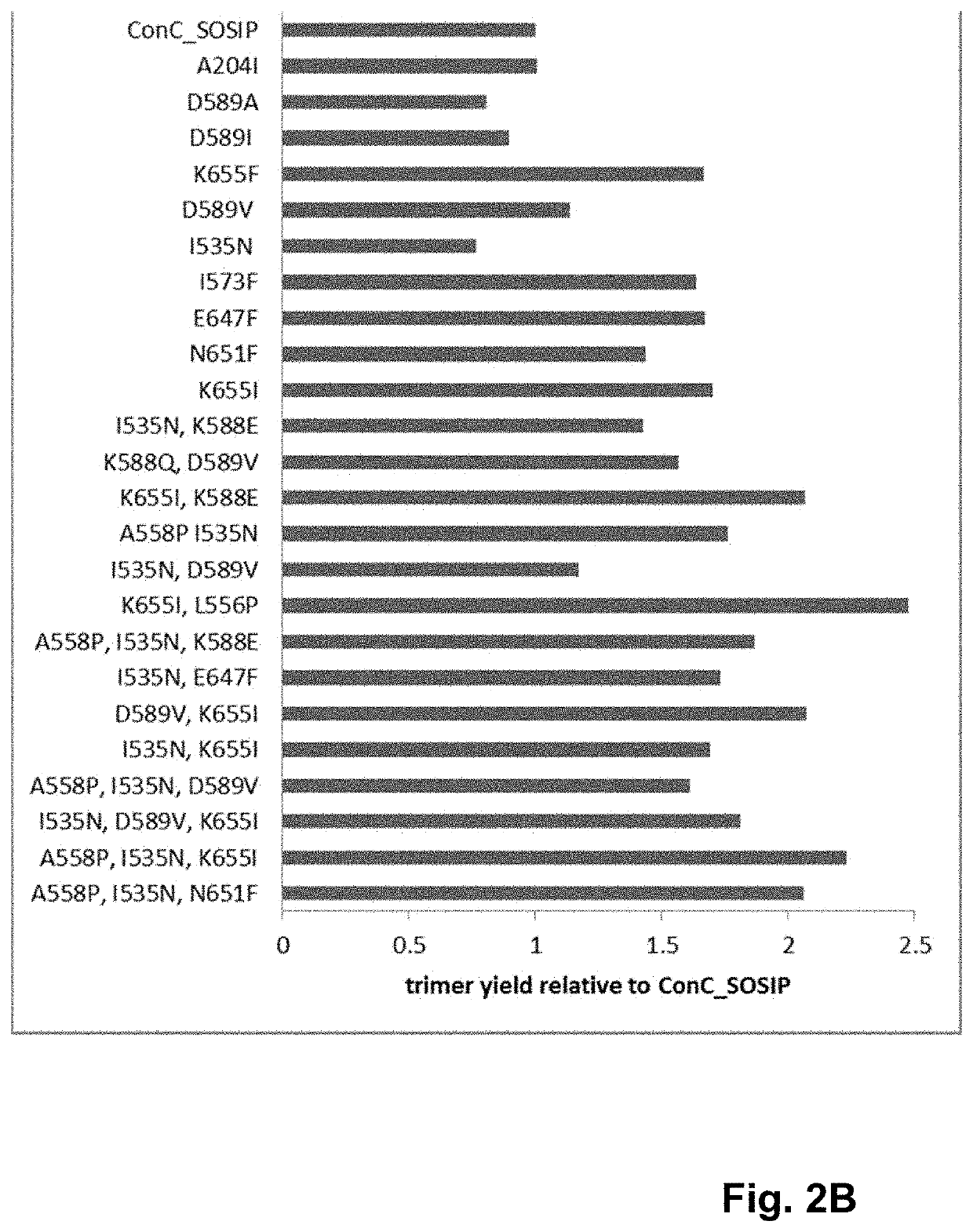
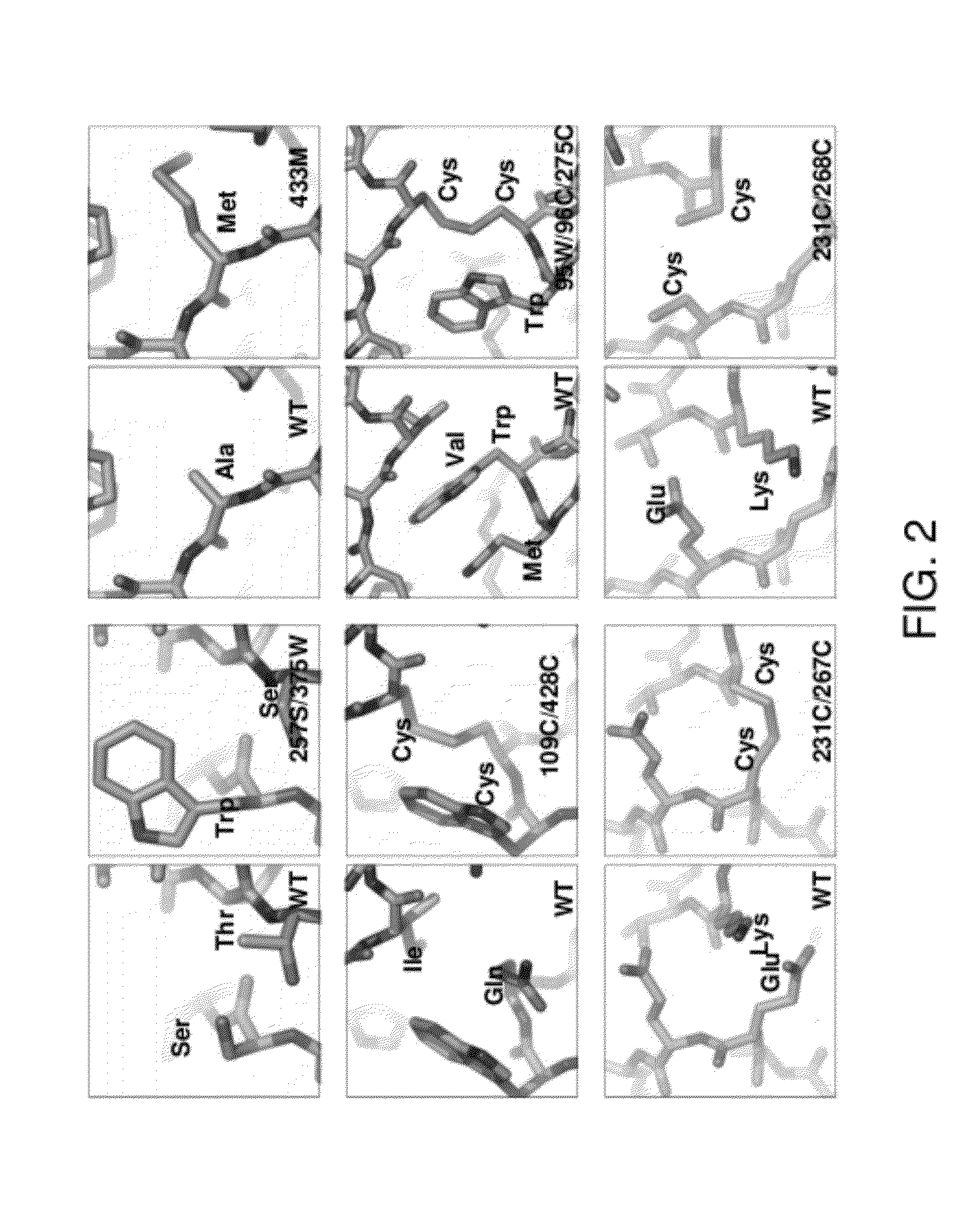
ActiveUS10793607B2Optimize folding and stability of HIV-Increase opportunitiesViral antigen ingredientsVirus peptidesHiv envelopeAmino acid substitution
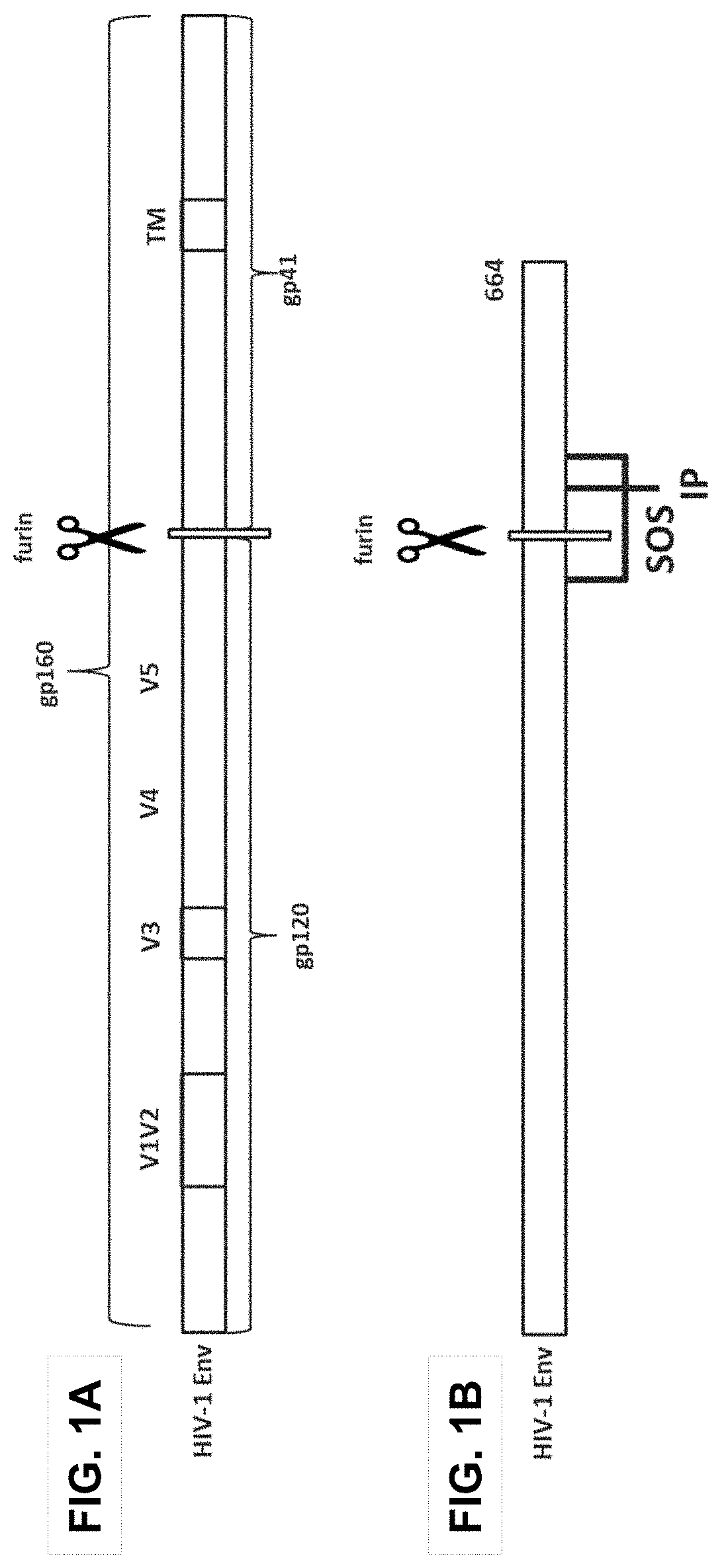
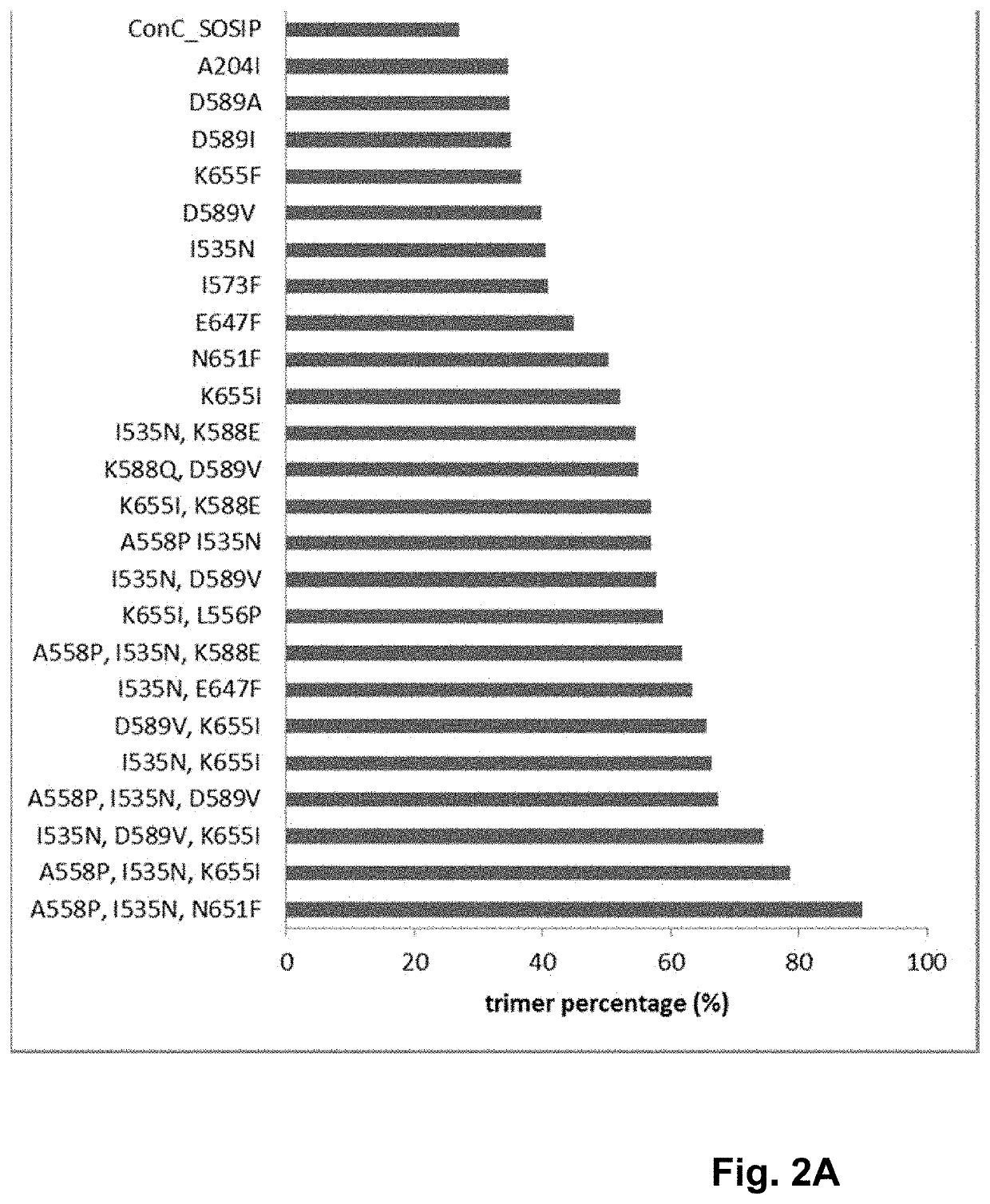
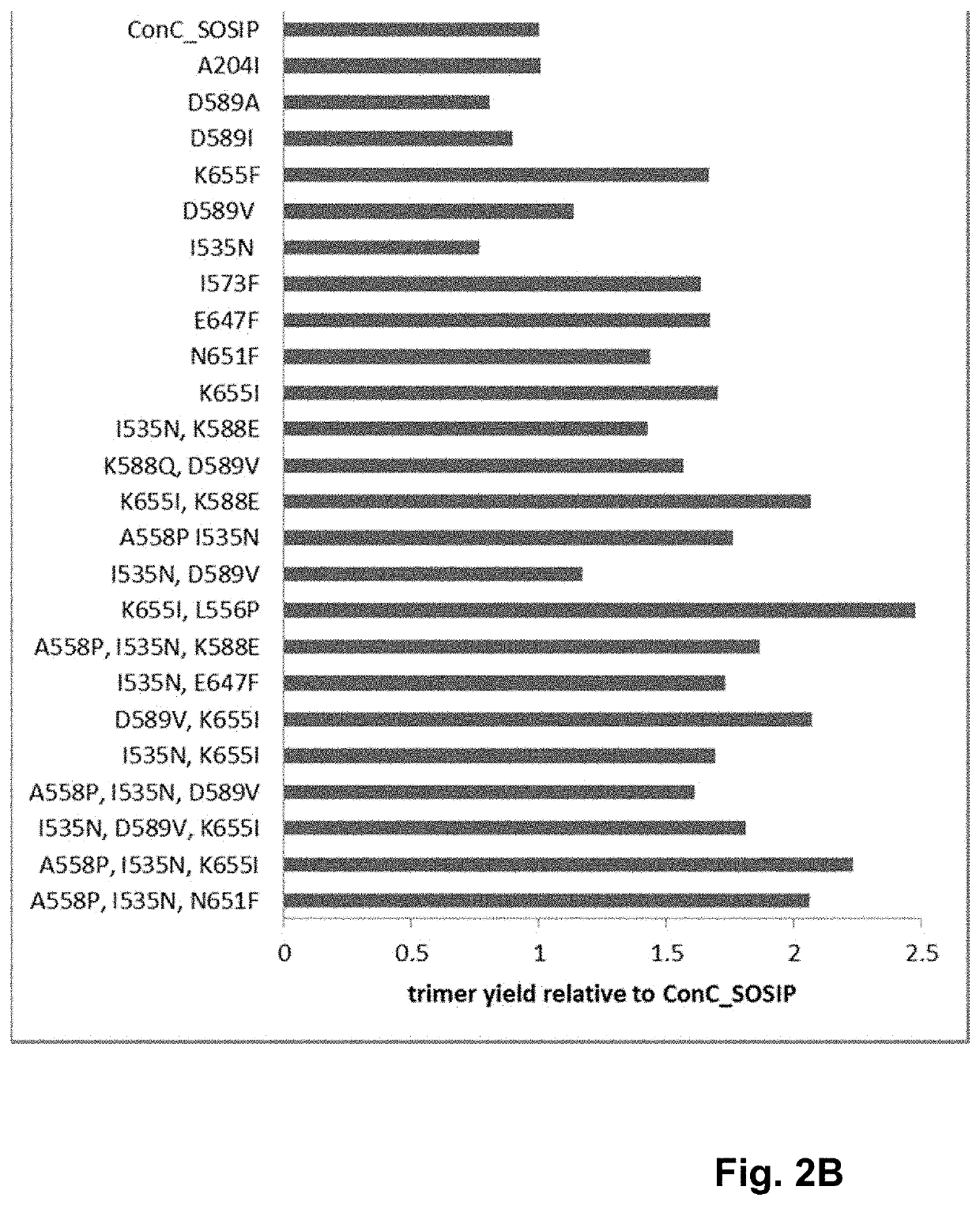
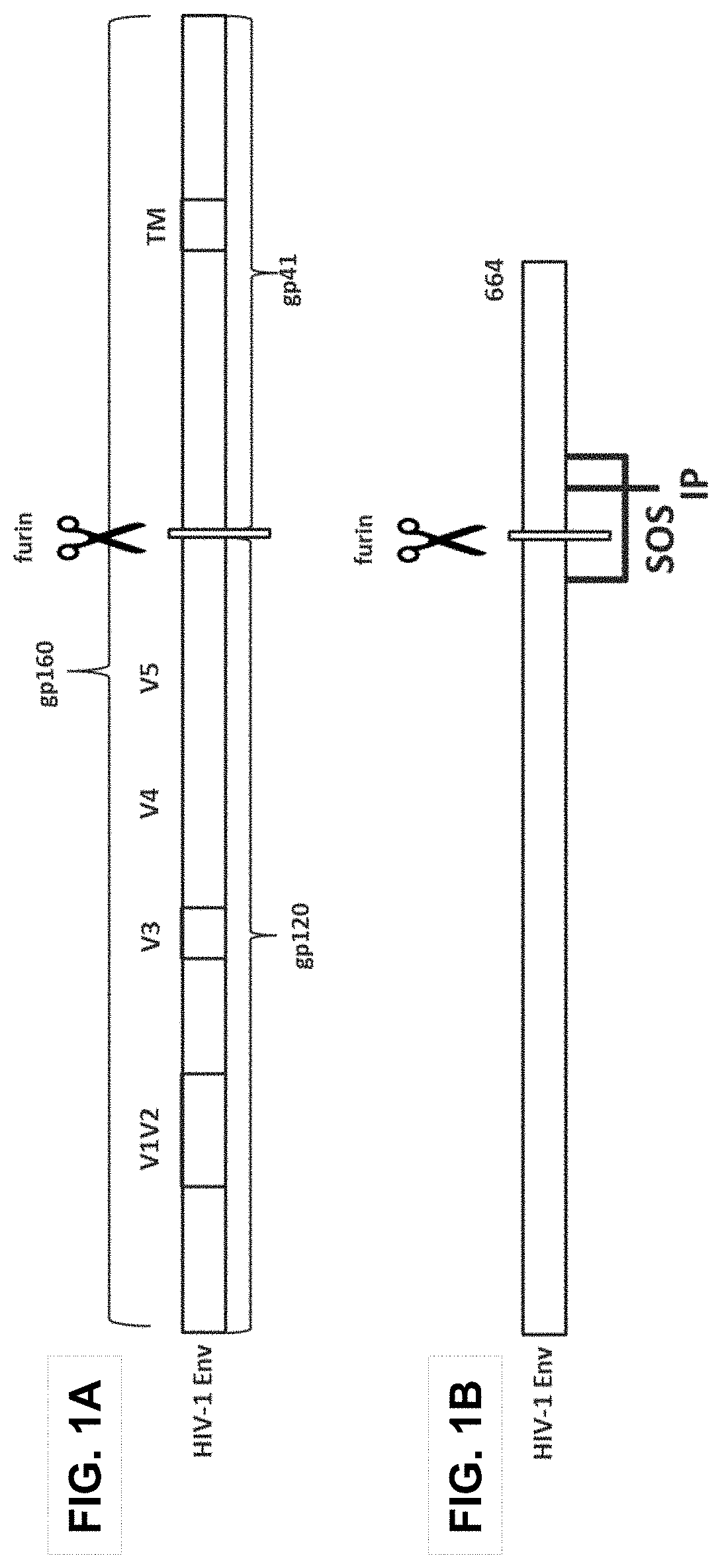
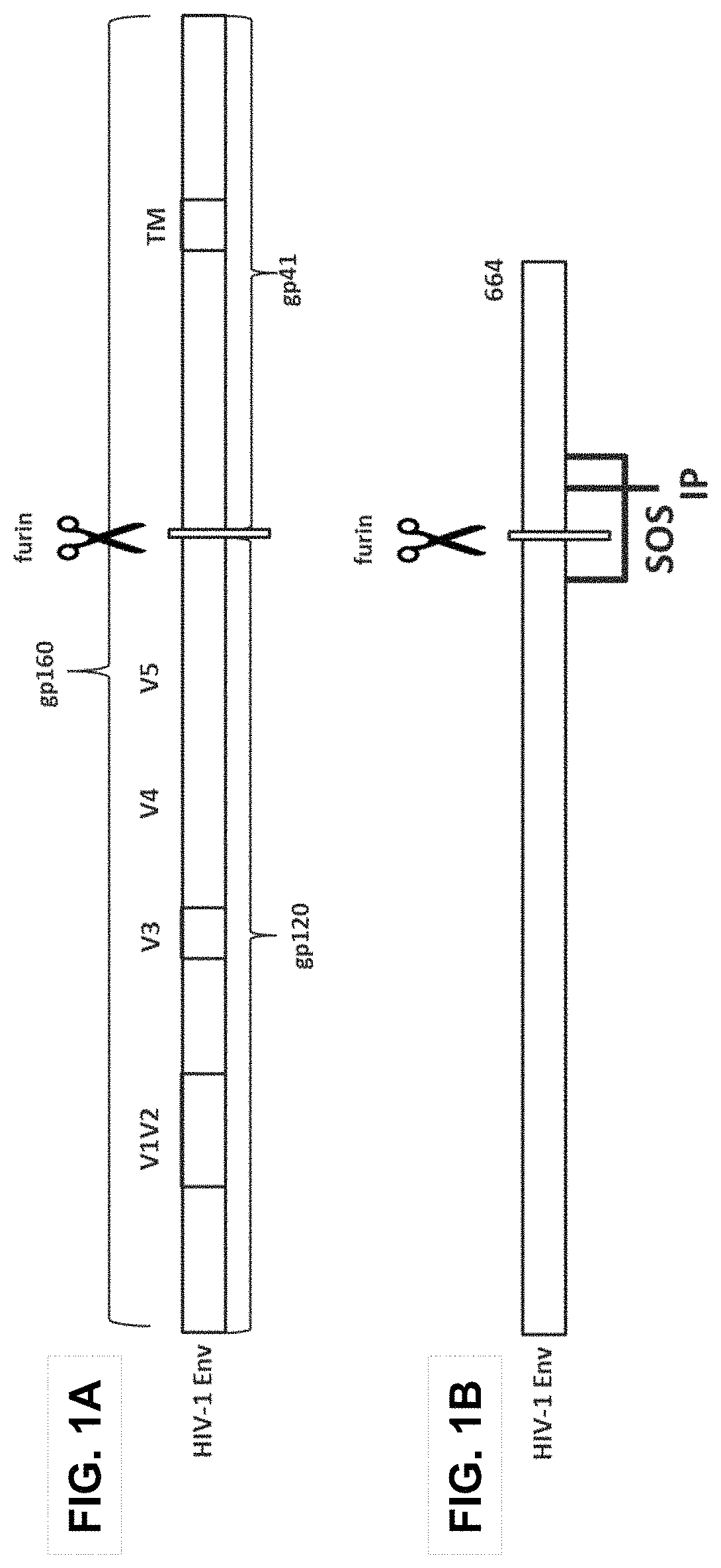
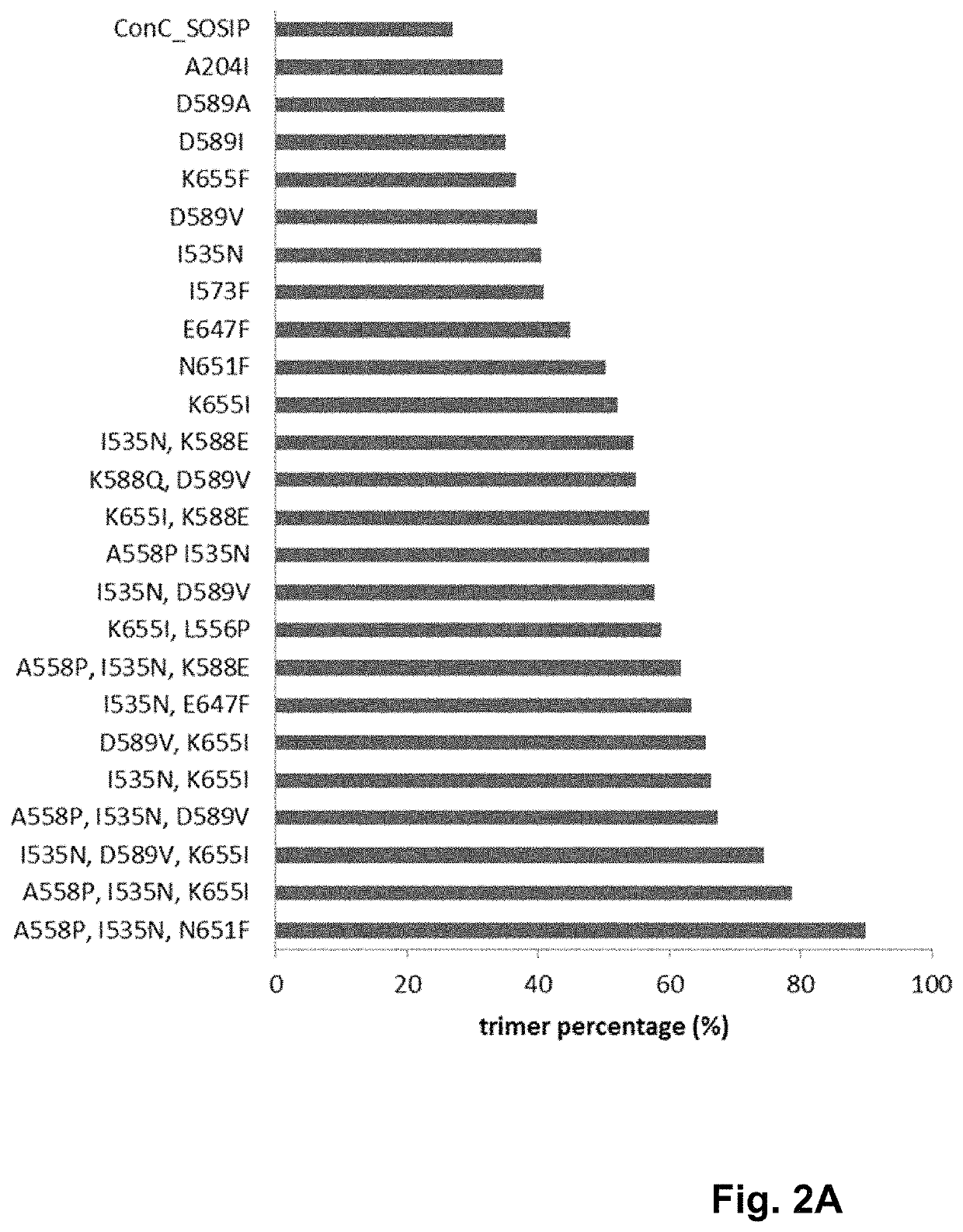
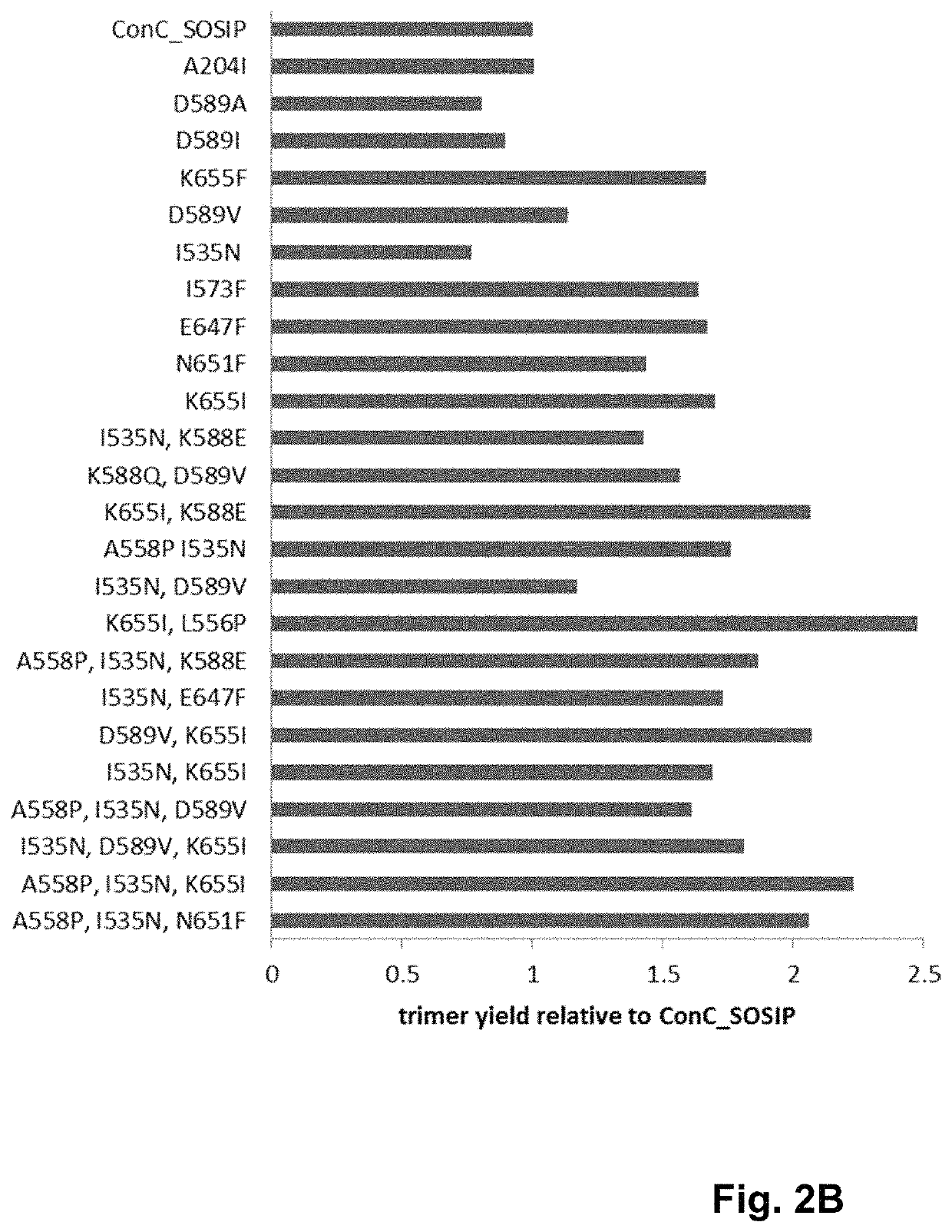
Human immunodeficiency virus (HIV) envelope proteins having mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins have certain amino acid substitutions at specified positions in the envelope protein sequence. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield as compared to an HIV envelope protein that does not have one or more of the indicated amino acid substitutions. Also provided are nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, nucleic acid, and vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
Trimer stabilizing HIV envelope protein mutations
ActiveUS10968254B2Optimize folding and stability of HIV-Increase opportunitiesViral antigen ingredientsVirus peptidesHiv envelopeTrimer
Human immunodeficiency virus (HIV) envelope proteins having specified mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield. Also provided are particles displaying the HIV envelope proteins, nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, particles, nucleic acid, or vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
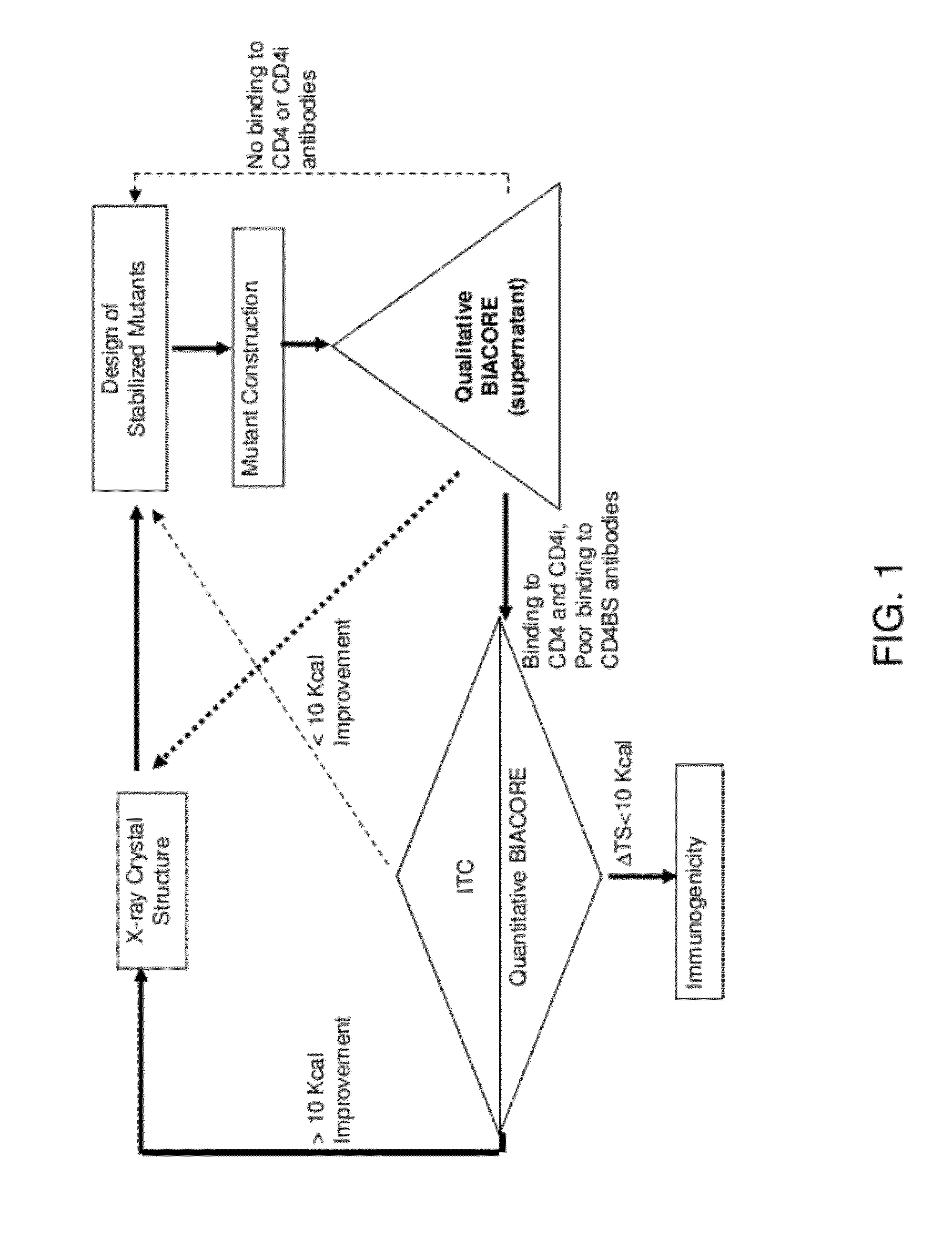
Conformationally stabilized HIV envelope immunogens
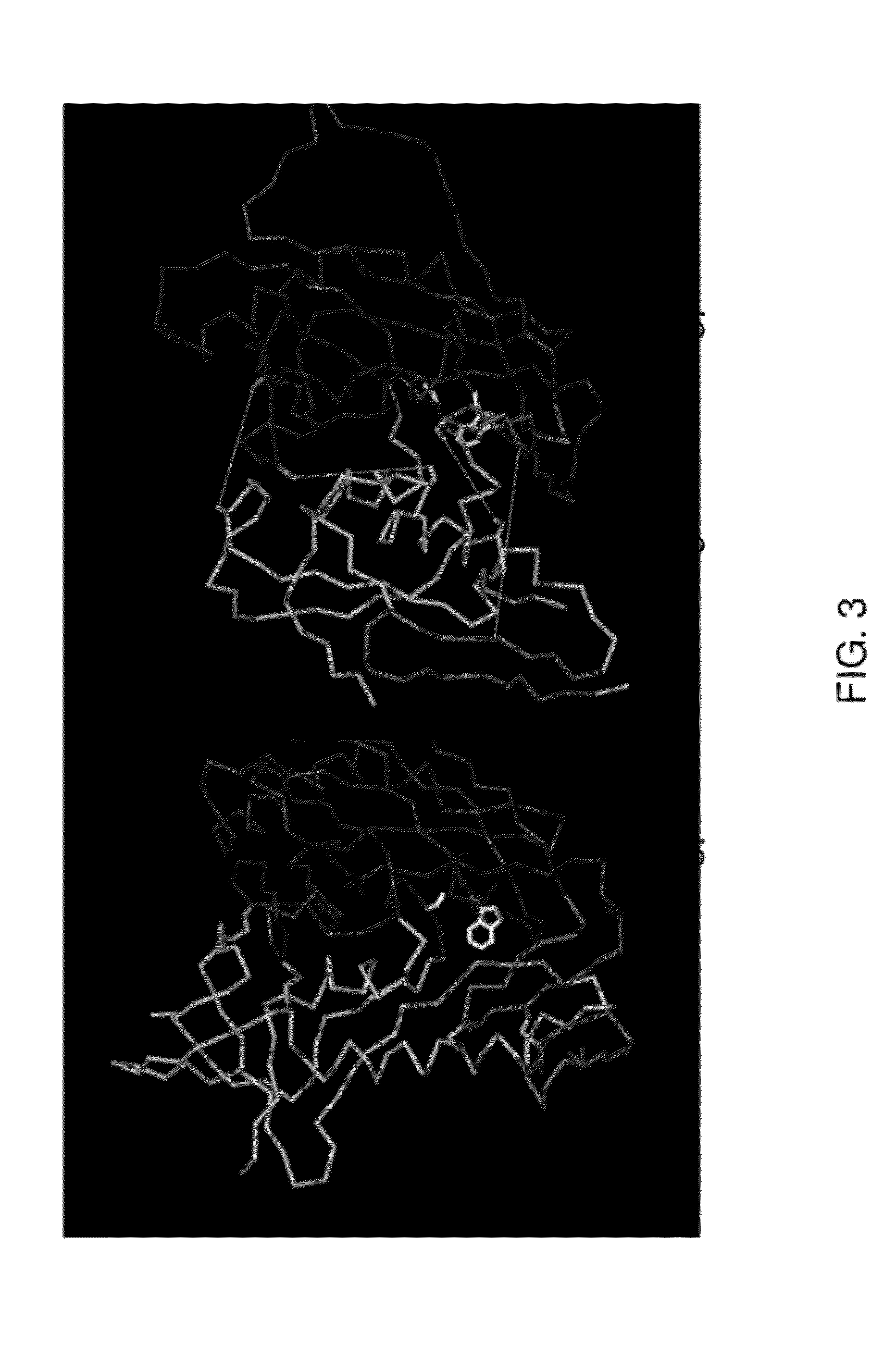
Stabilized forms of gp120 polypeptide, nucleic acids encoding these stabilized forms, vectors comprising these nucleic acids, and methods of using these polypeptides, nucleic acids, vectors and host cells are disclosed. Crystal structures and computer systems including atomic coordinates for stabilized forms of gp120, and gp120 with an extended V3 loop, and methods of using these structures and computer systems are also disclosed.
Owner:UNITED STATES OF AMERICA +1
Compositions and methods for treating hiv/aids with immunotherapy
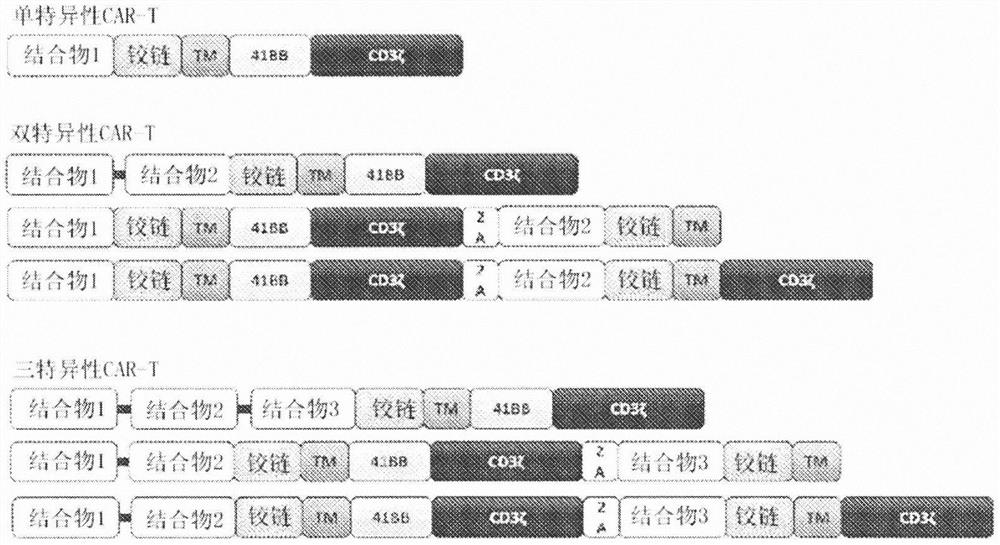
Chimeric antigen receptors (CARs) containing HIV envelope antigen binding domains are disclosed. Nucleic acids, recombinant expression vectors, host cells, antigen binding fragments, and pharmaceutical compositions, relating to the CARs are also disclosed. Methods of treating or preventing HIV-infection in a subject, and methods of making CAR T cells are also disclosed. Results of treating or preventing HIV-infection, and results of making CAR T cells are also disclosed.
Owner:LENTIGEN TECH INC
Vaccine
InactiveUS20060142221A1Reduce removalReduce and prevent glycosylationAntibody mimetics/scaffoldsGenetic material ingredientsDNA vaccinationHIV Proteins
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative fused to an additional HIV protein selected from a non-structural protein or capsid protein or fragment or immunogenic derivative thereof. Preferably the HIV envelope molecule is gp120 and preferred fusions include one or more of HIV Nef, Gag, RT or Tat. Preferably the HIV envelope molecule is non-glycosylated in mammalian cells.
Owner:GLAXO GROUP LTD
CD4-independent HIV envelope proteins as vaccines and therapeutics
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Trimer stabilizing HIV envelope protein mutations
ActiveUS11365222B2Optimize folding and stability of HIV-Increase opportunitiesViral antigen ingredientsVirus peptidesHiv envelopeAmino acid substitution
Human immunodeficiency virus (HIV) envelope proteins having mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins have certain amino acid substitutions at specified positions in the envelope protein sequence. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield as compared to an HIV envelope protein that does not have one or more of the indicated amino acid substitutions. Also provided are nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, nucleic acid, and vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com