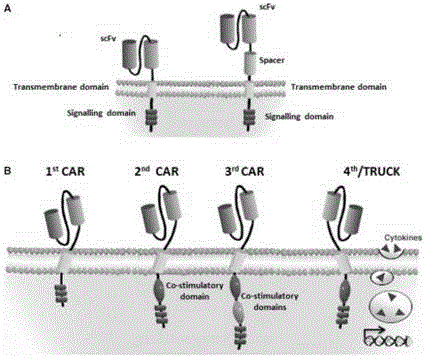
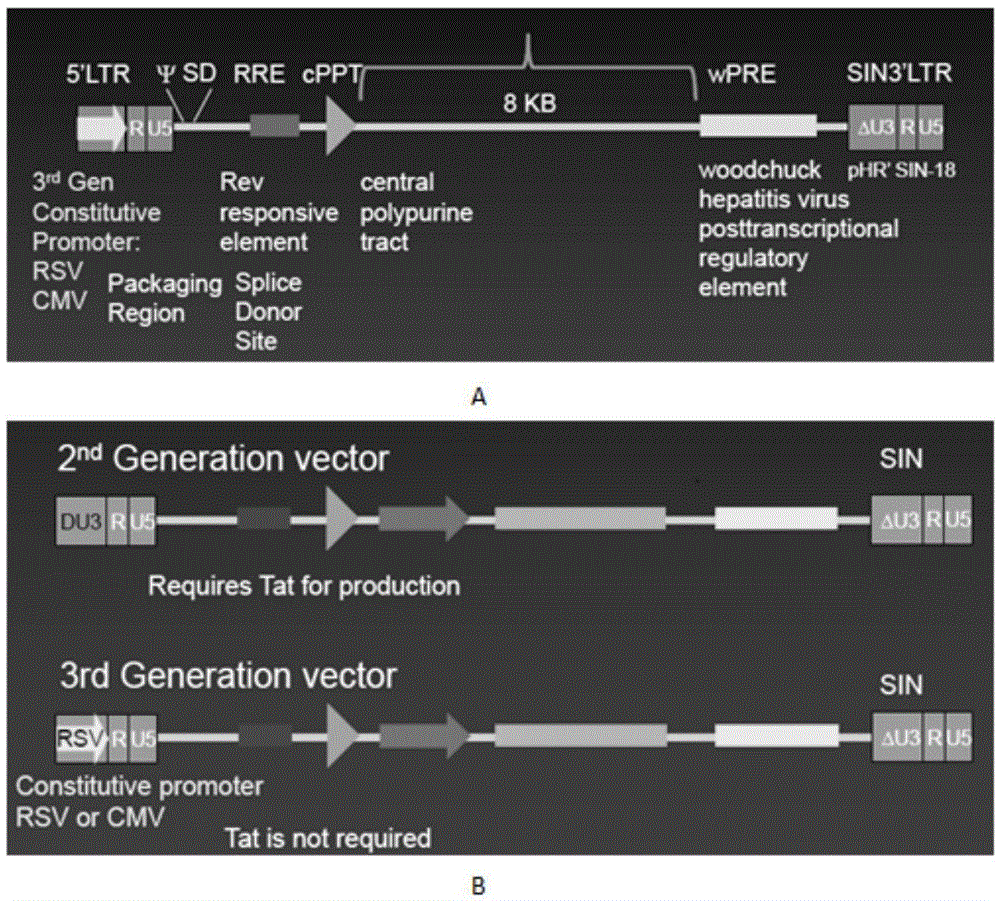
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
935 results about "Lentivirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lentivirus (lente-, Latin for "slow") is a genus of retroviruses that cause chronic and deadly diseases characterized by long incubation periods, in the human and other mammalian species. The best known lentivirus is the Human Immunodeficiency Virus (HIV), which causes AIDS. Lentiviruses are also hosted in apes, cows, goats, horses, cats, and sheep. Recently, lentiviruses have been found in monkeys, lemurs, Malayan flying lemur (neither a true lemur nor a primate), rabbits, and ferrets. Lentiviruses and their hosts have worldwide distribution. Lentiviruses can integrate a significant amount of viral cDNA into the DNA of the host cell and can efficiently infect nondividing cells, so they are one of the most efficient methods of gene delivery. Lentiviruses can become endogenous (ERV), integrating their genome into the host germline genome, so that the virus is henceforth inherited by the host's descendants.
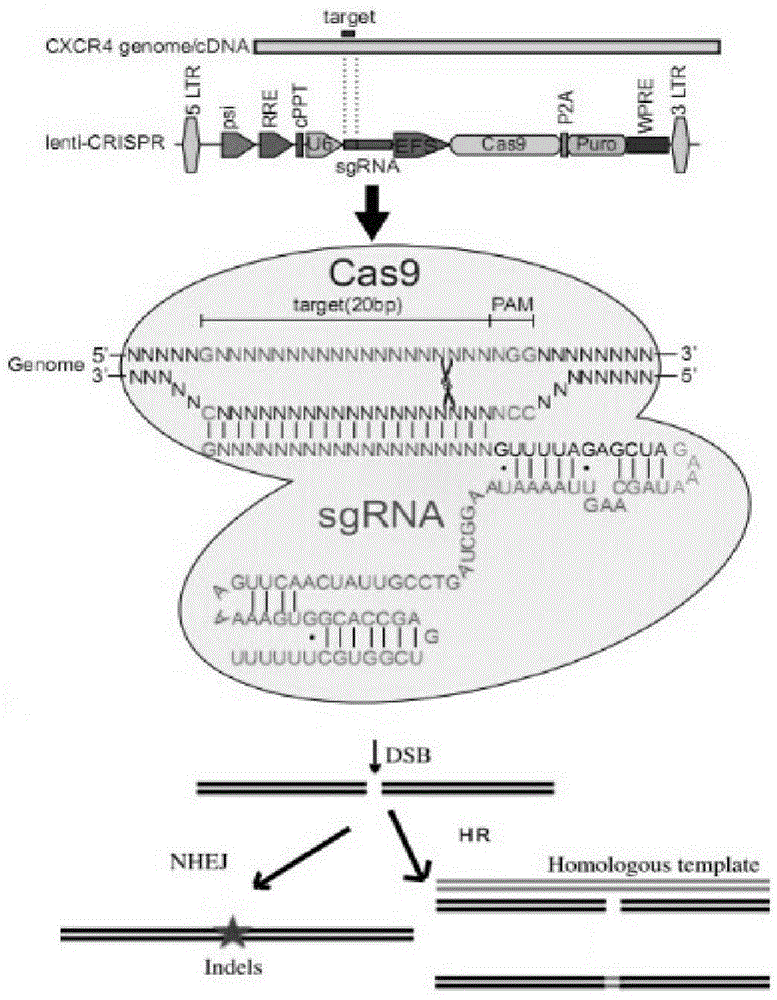
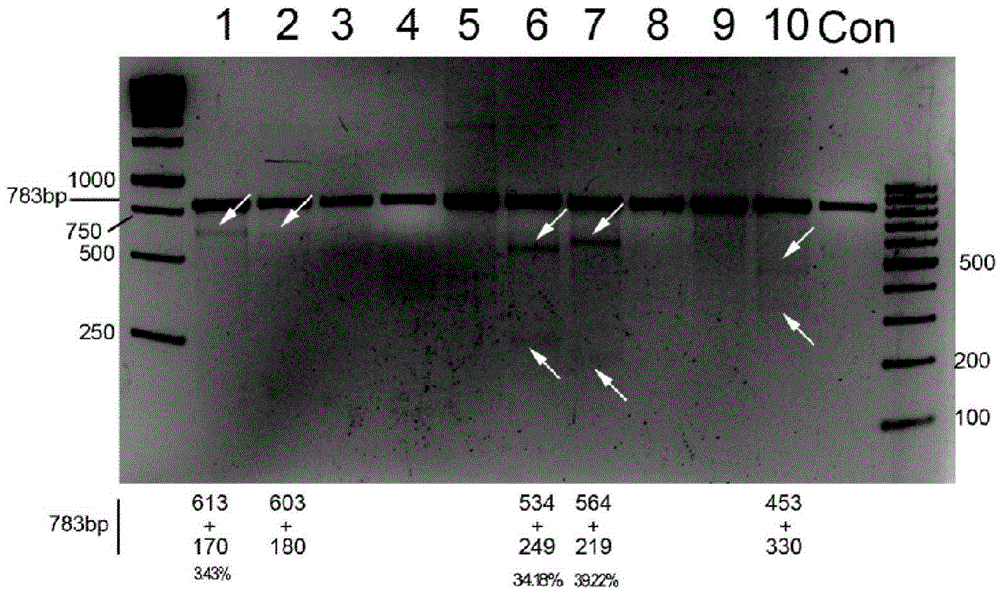
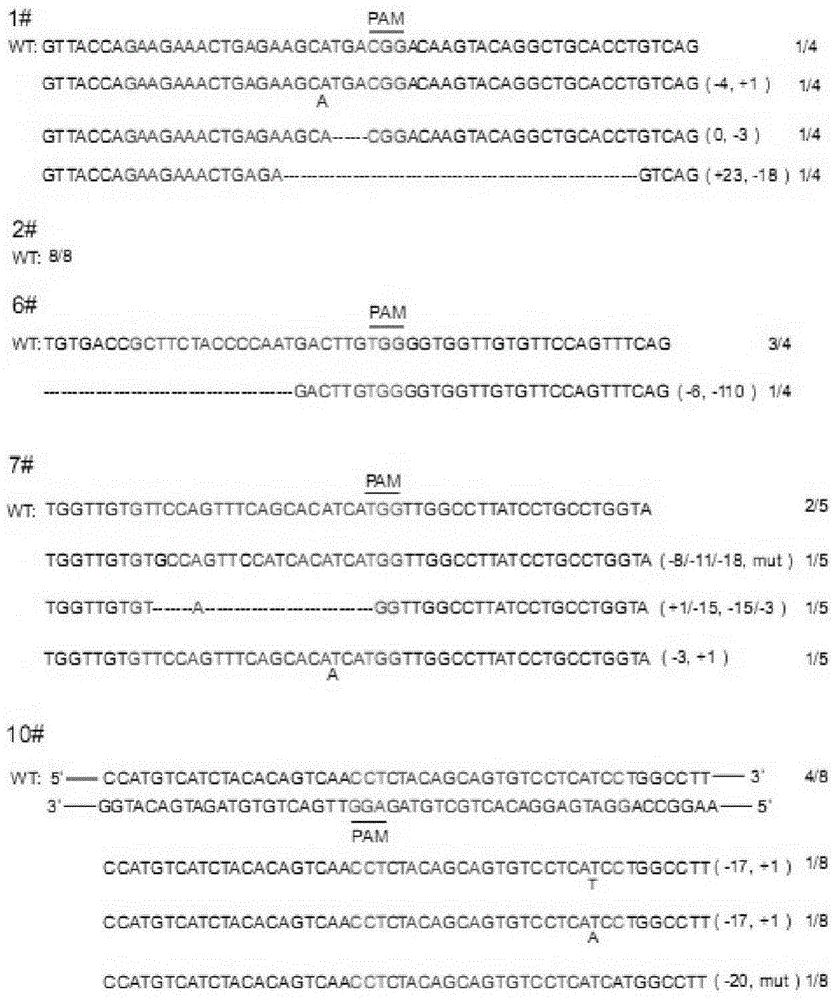
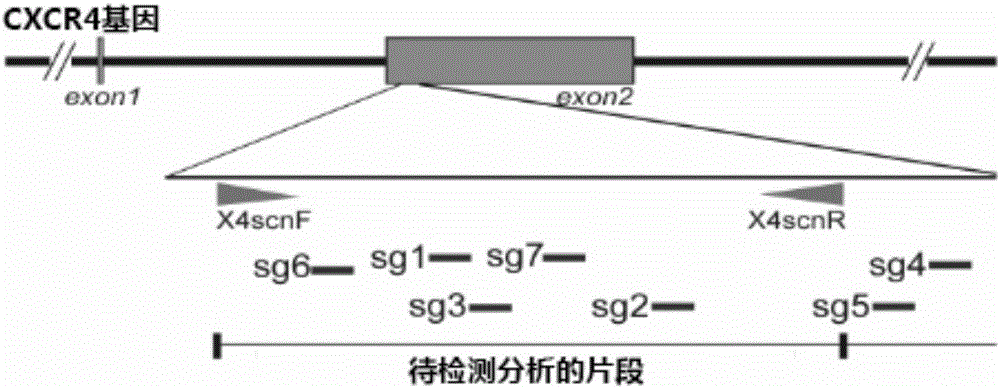
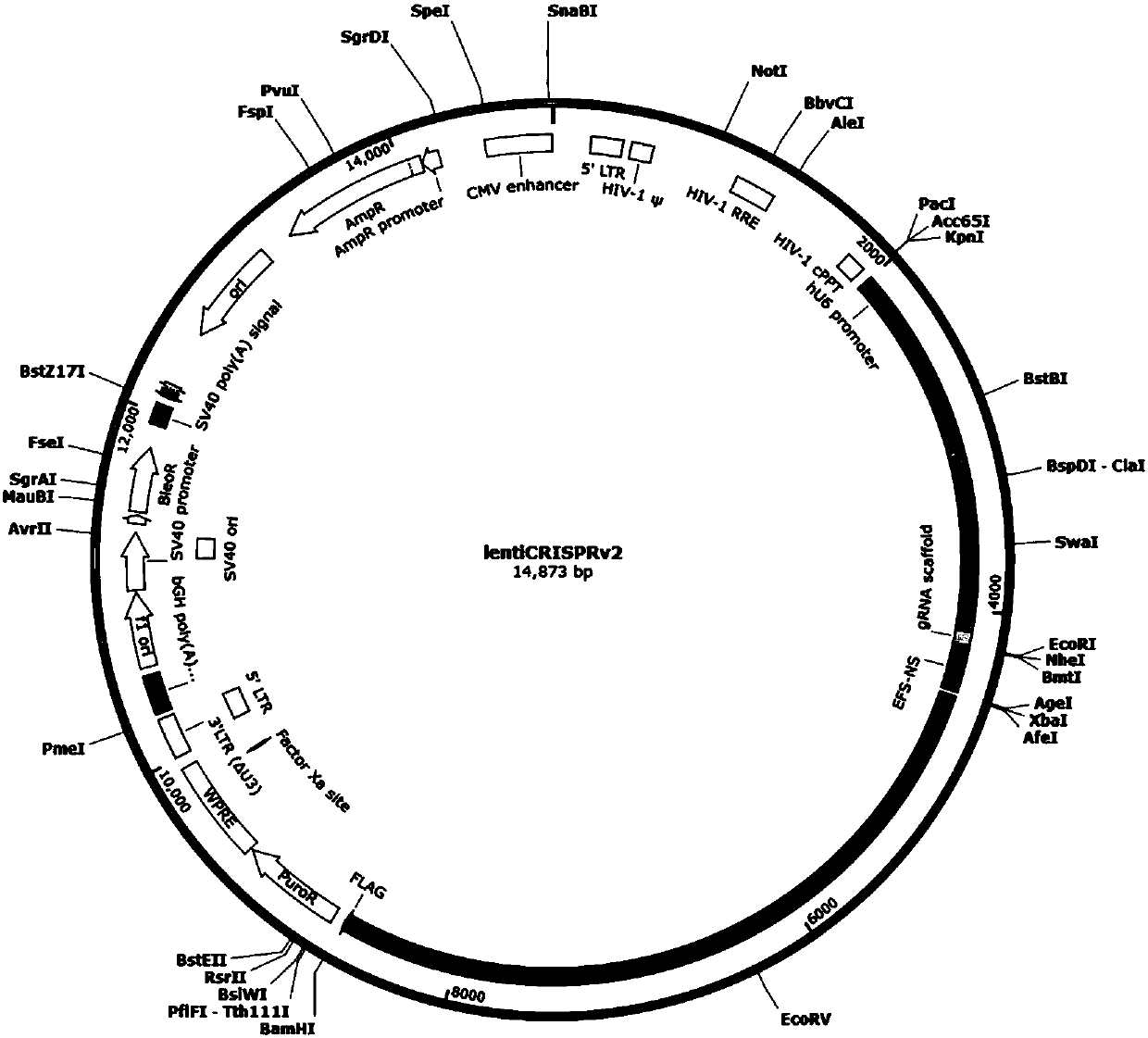
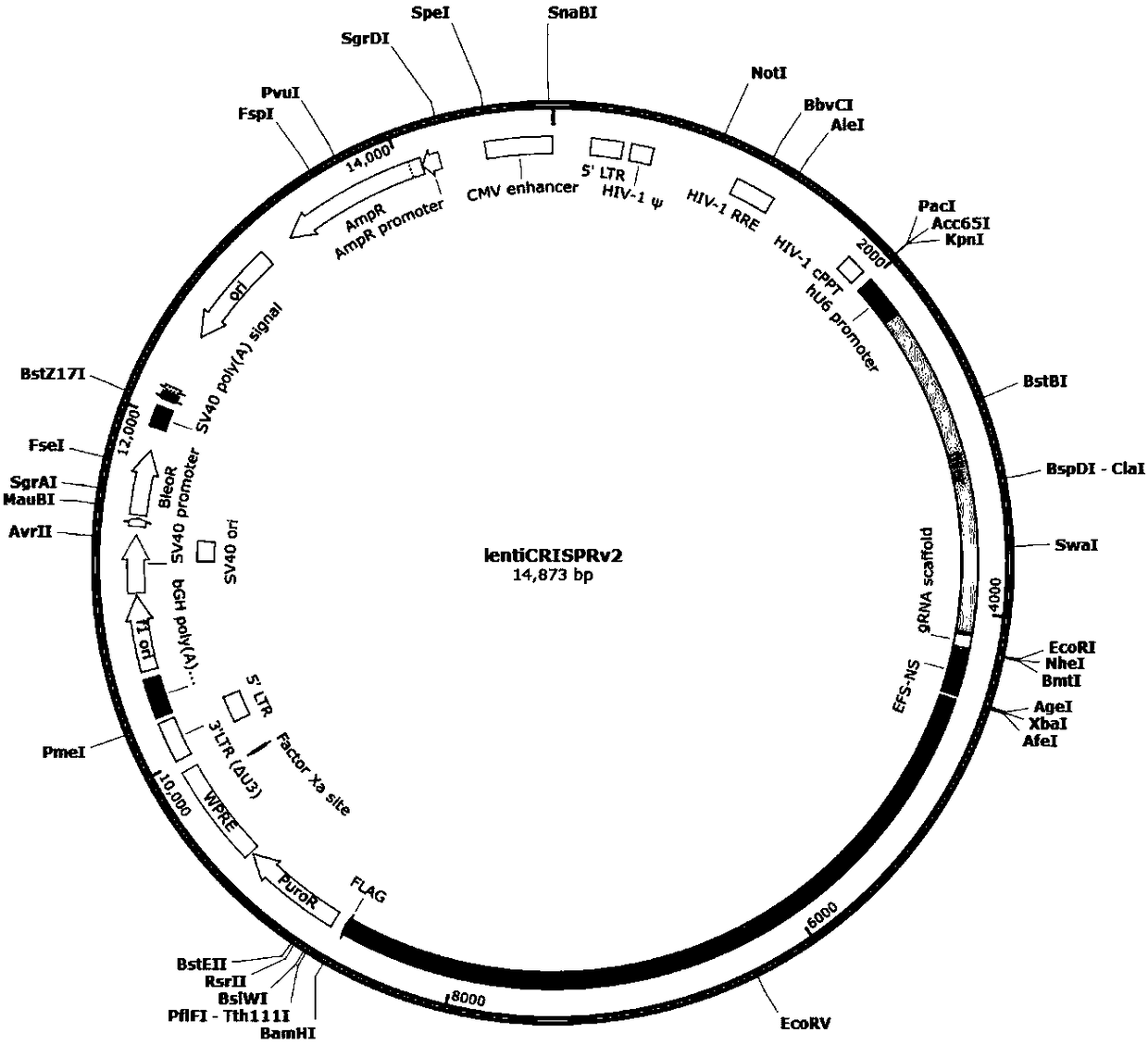
CRISPR/Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and lentivirus of CRISPR/Cas9 recombinant lentiviral vector
ActiveCN104480144AAvoid or delay intrusionInhibit the spread of infectionGenetic material ingredientsAntiviralsEnzyme digestionCXCR4
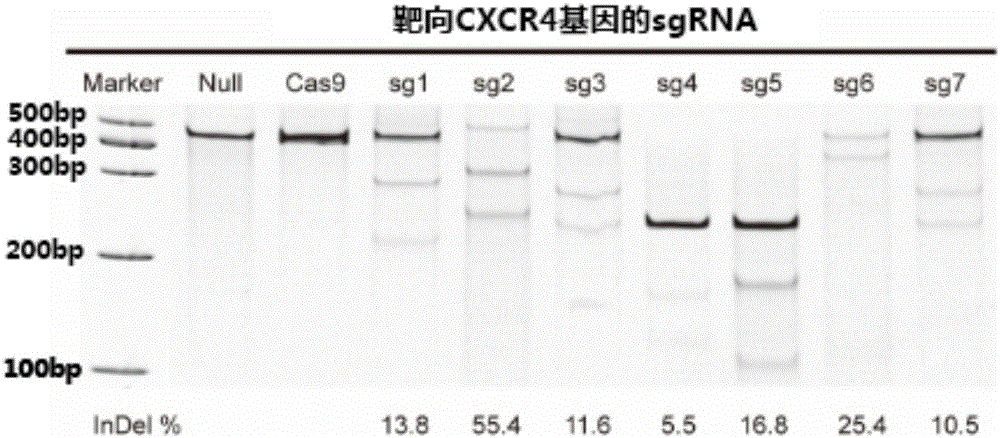
The invention belongs to the field of pharmaceutical and biological engineering, and relates to a CRISPR / Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and a lentivirus of the CRISPR / Cas9 recombinant lentiviral vector. The recombinant lentiviral vector is prepared by carrying out enzyme digestion on a lentiviral vectorlentiCRISPR by BsmBI and connecting into a BsmBI cohesive end-containing CXCR4 specific target sequence to recombine; the obtained CRISPR / Cas9 recombinant lentiviral vector is capable of mutating gene sequences at four different loci of ahuman immunodeficiency virusco-receptor CXCR4 and themutatuin rate is high and up to 25-75%. The cells transformed by the recombinant lentiviral vector cannot be infected by the human immunodeficiency virus. Compared with theRNAi-Knockdown, ZFN and TALEN technologies, the method has higher efficiency of suppressing the human immunodeficiency virus replication; the system is rapid to construct, simple and low in cost, is capable of preventing the invasion of the human immunodeficiency virus and is suitable for human immunodeficiency virus gene therapy.
Owner:WUHAN UNIV
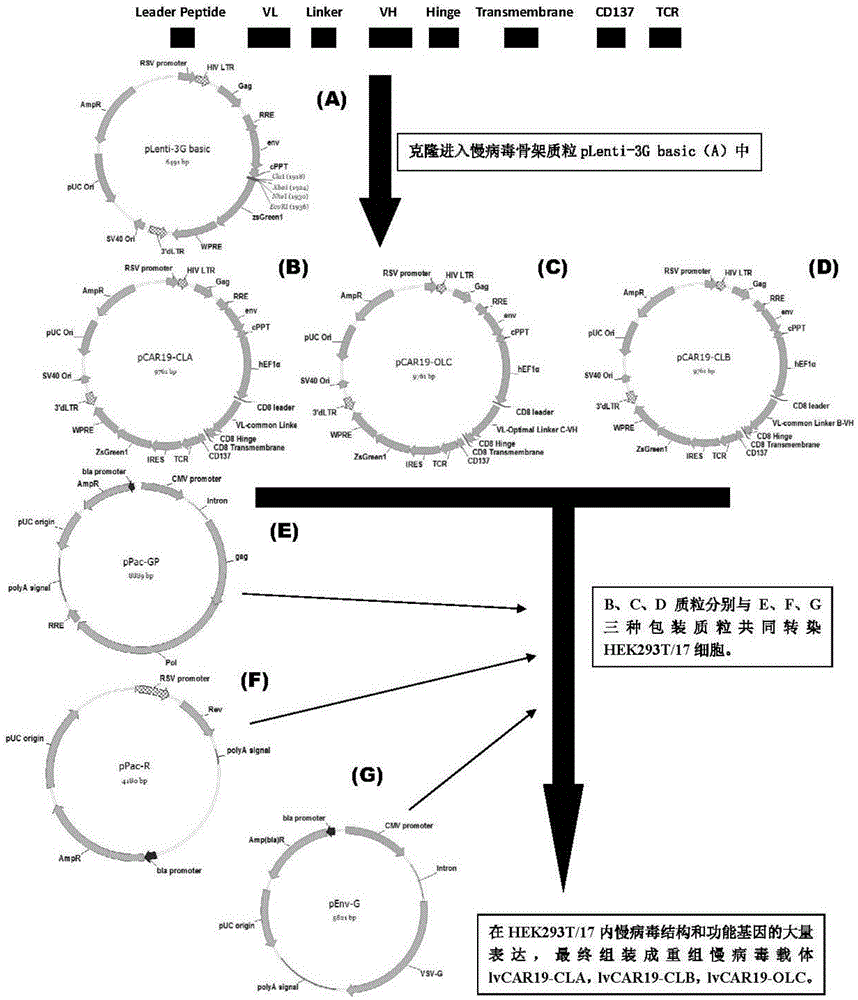
CAR-T transgene vector based on replication defective recombinant lentivirus and construction method and application of CAR-T transgene vector
ActiveCN105602992ASignificant effectPromote secretionGenetic material ingredientsFermentationEucaryotic cellAmpicillin
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Method for expression of small antiviral RNA molecules within a cell
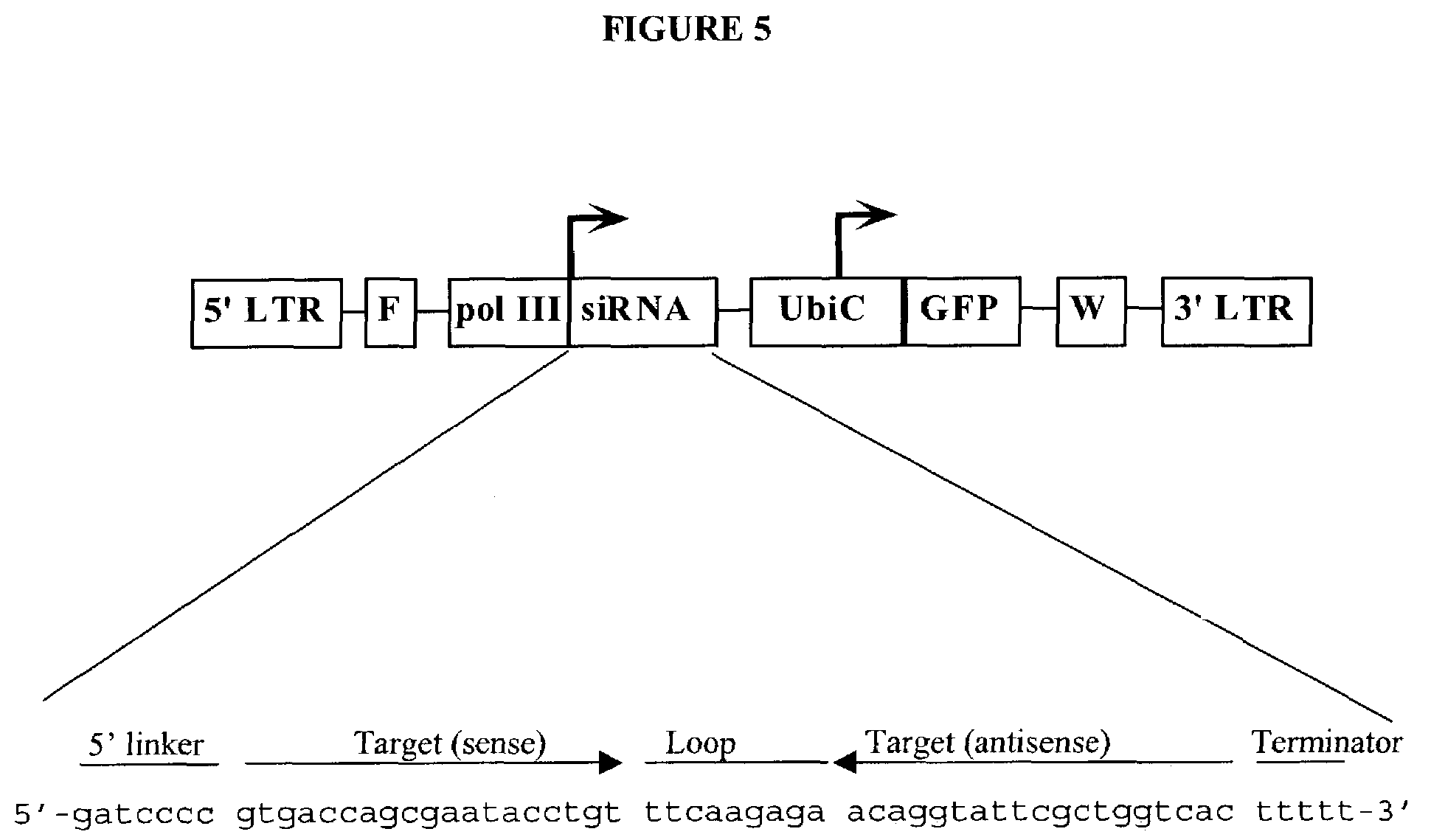
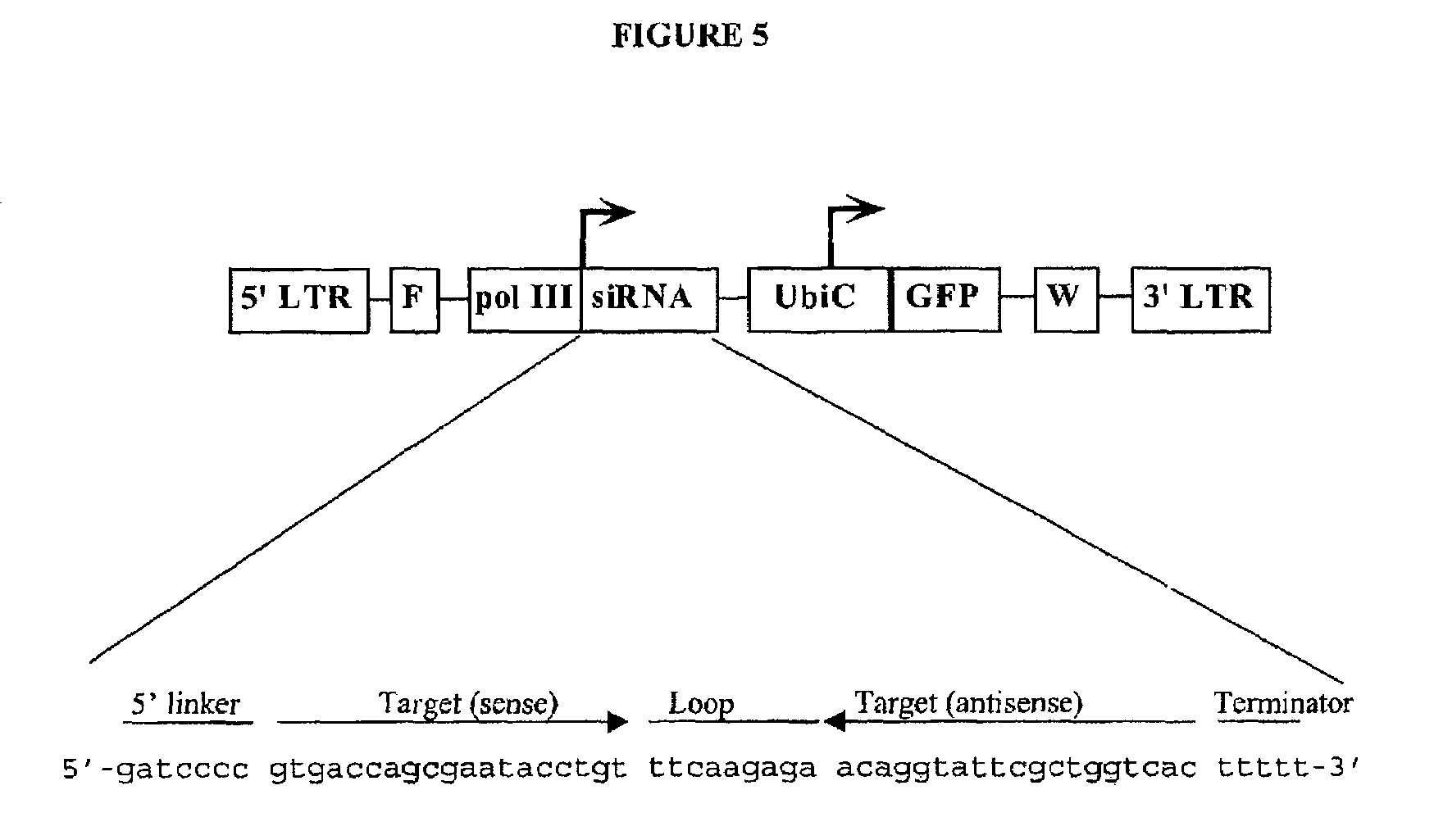
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector. The methods can be used to express double stranded RNA complexes. Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell, that interfere with a viral life cycle by down regulating either the viral genome, a viral genome transcript, or a host cell that. In another aspect the invention provides methods for treating patients having suffering from infection, particularly infection with HIV. In a further aspect, the invention provides methods for producing siRNA encoding lentivirus where the siRNA activity may interfere with the lentiviral life cycle.
Owner:CALIFORNIA INST OF TECH +1
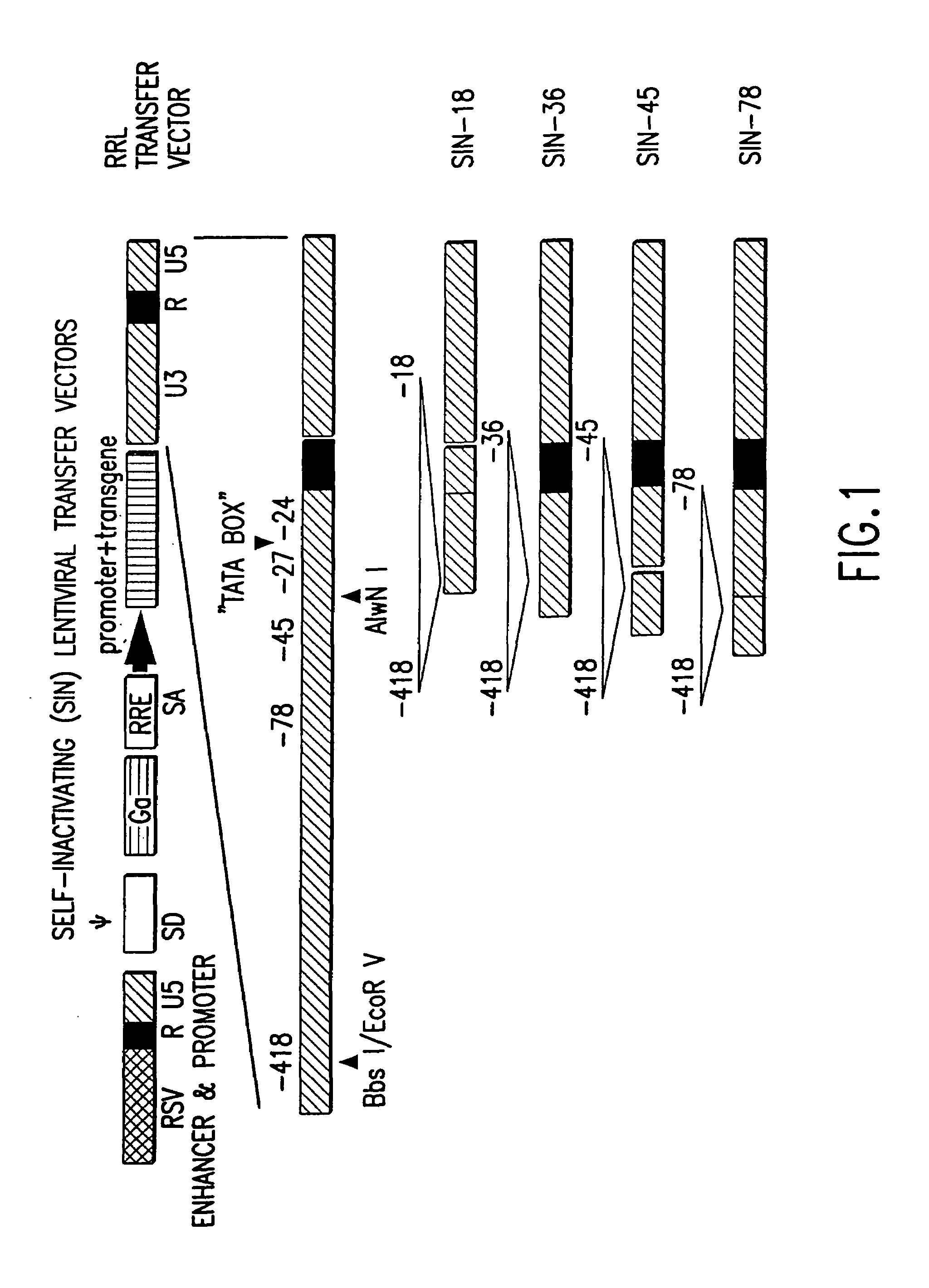
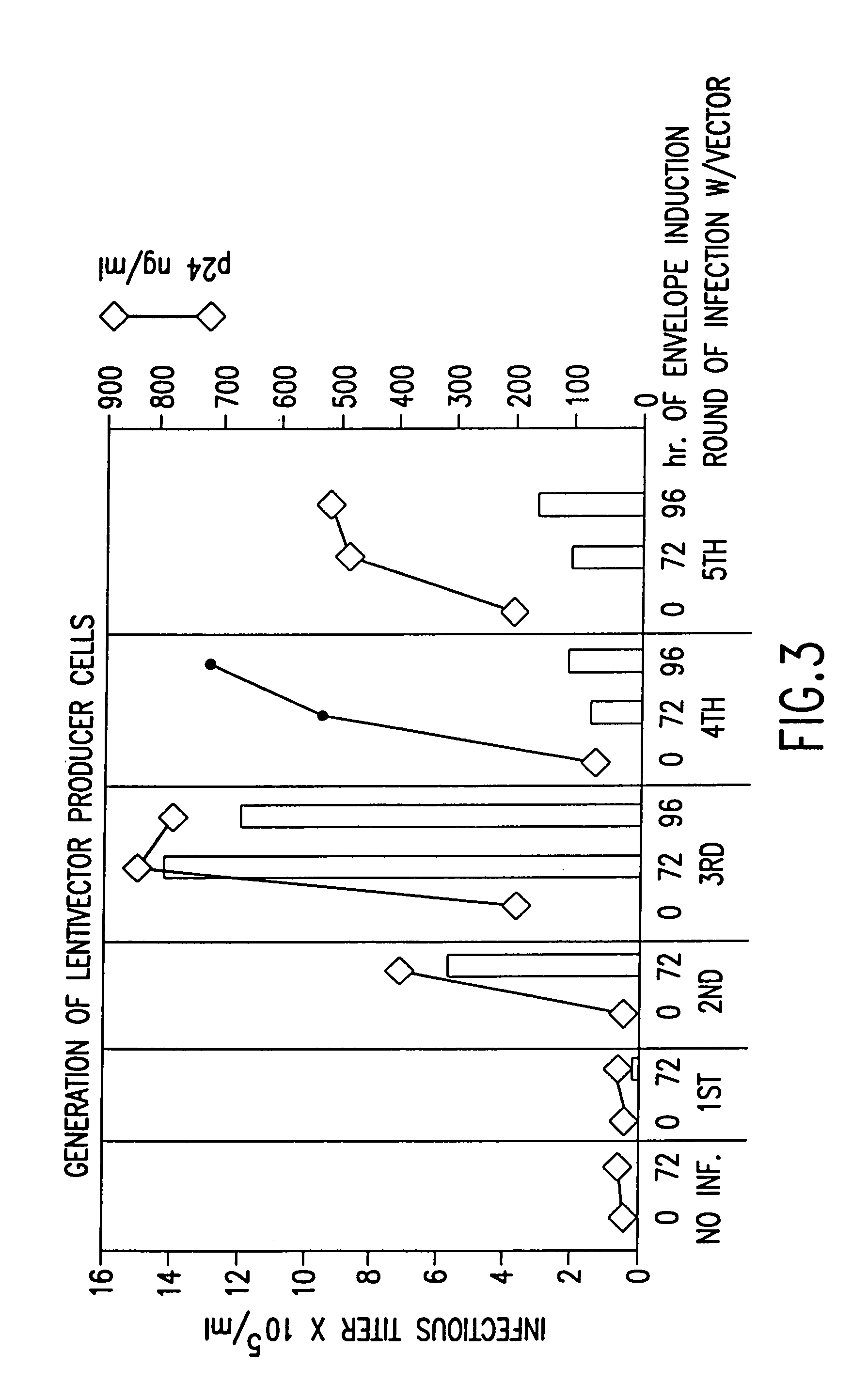
Method and means for producing high titer, safe, recombinant lentivirus vectors
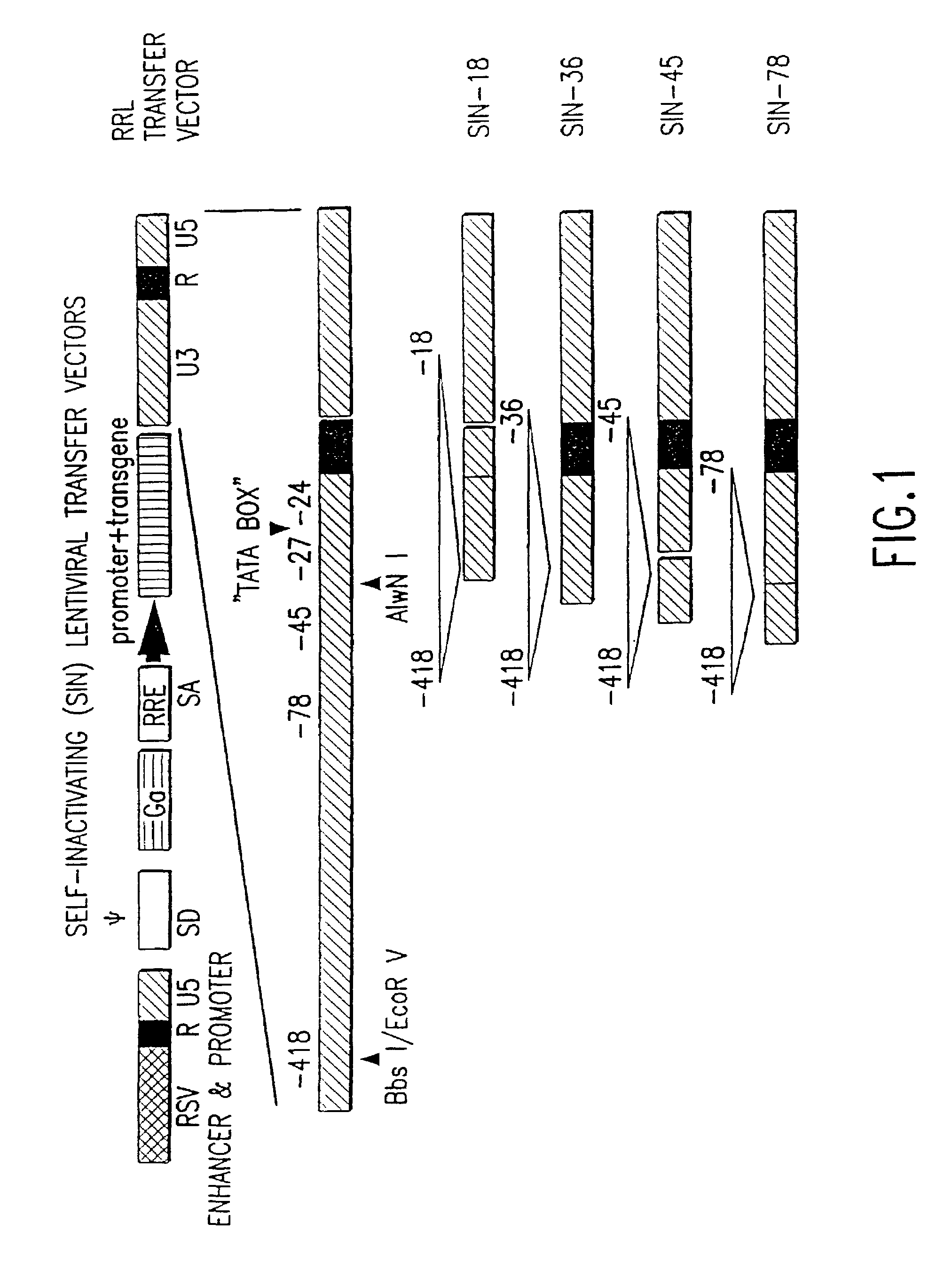
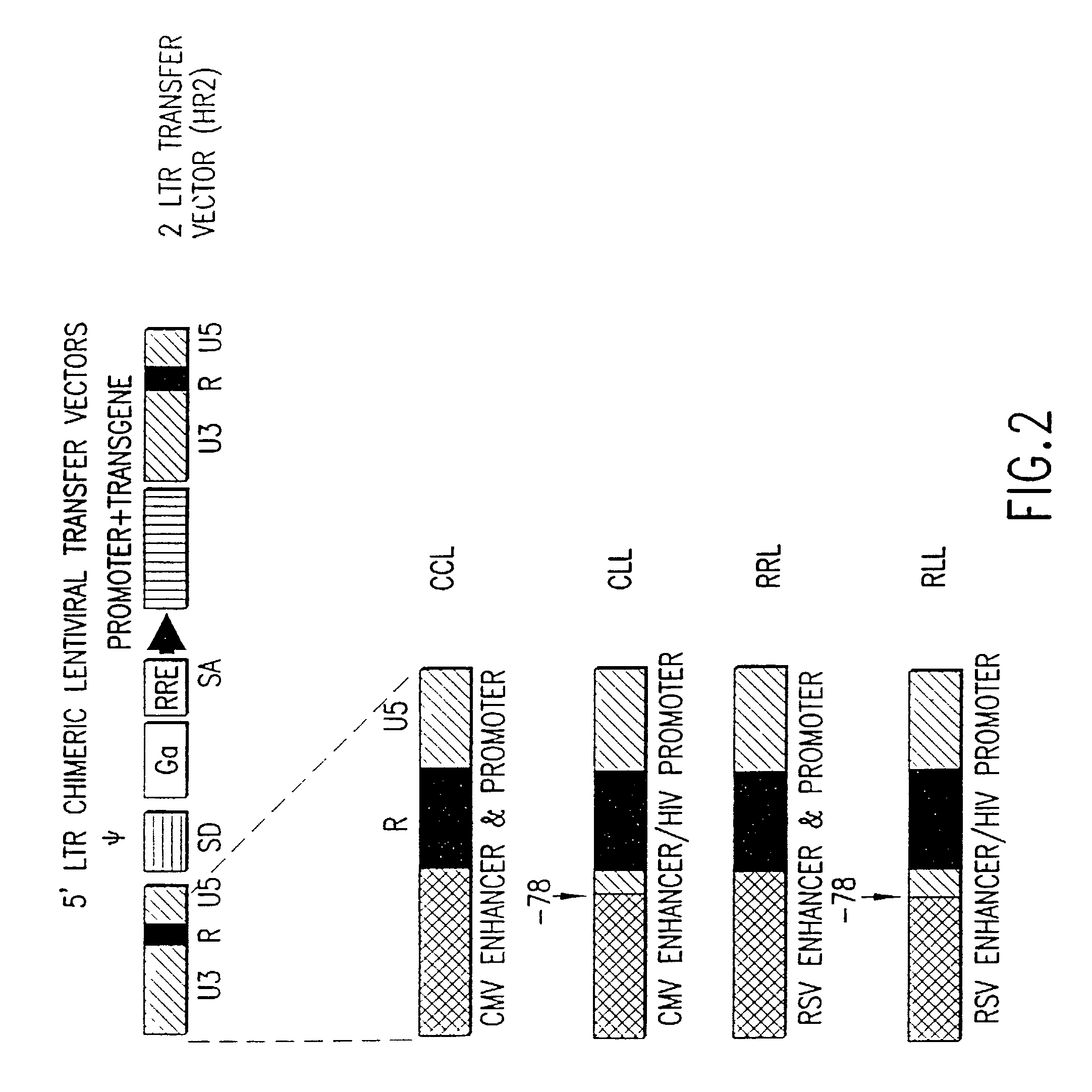
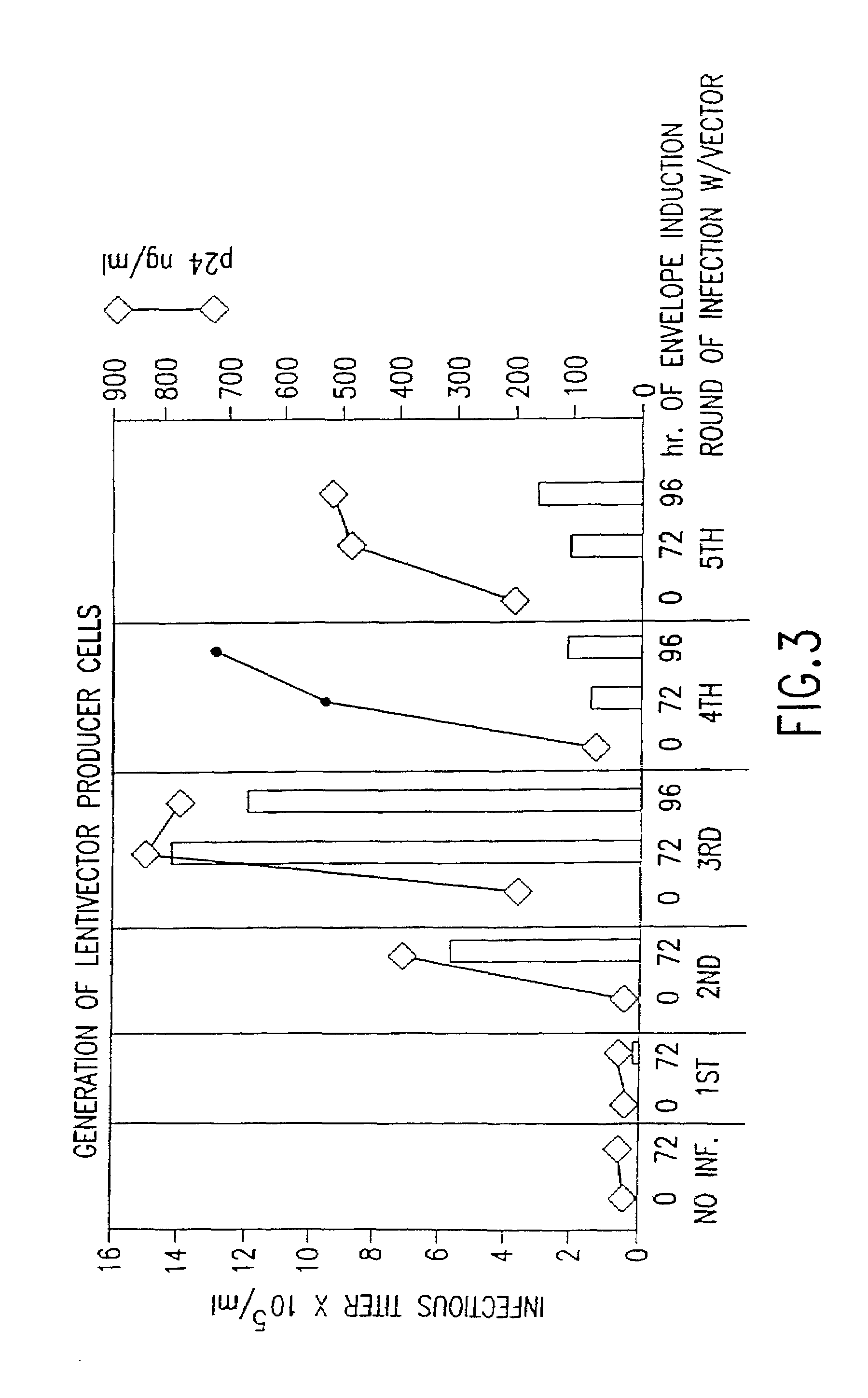
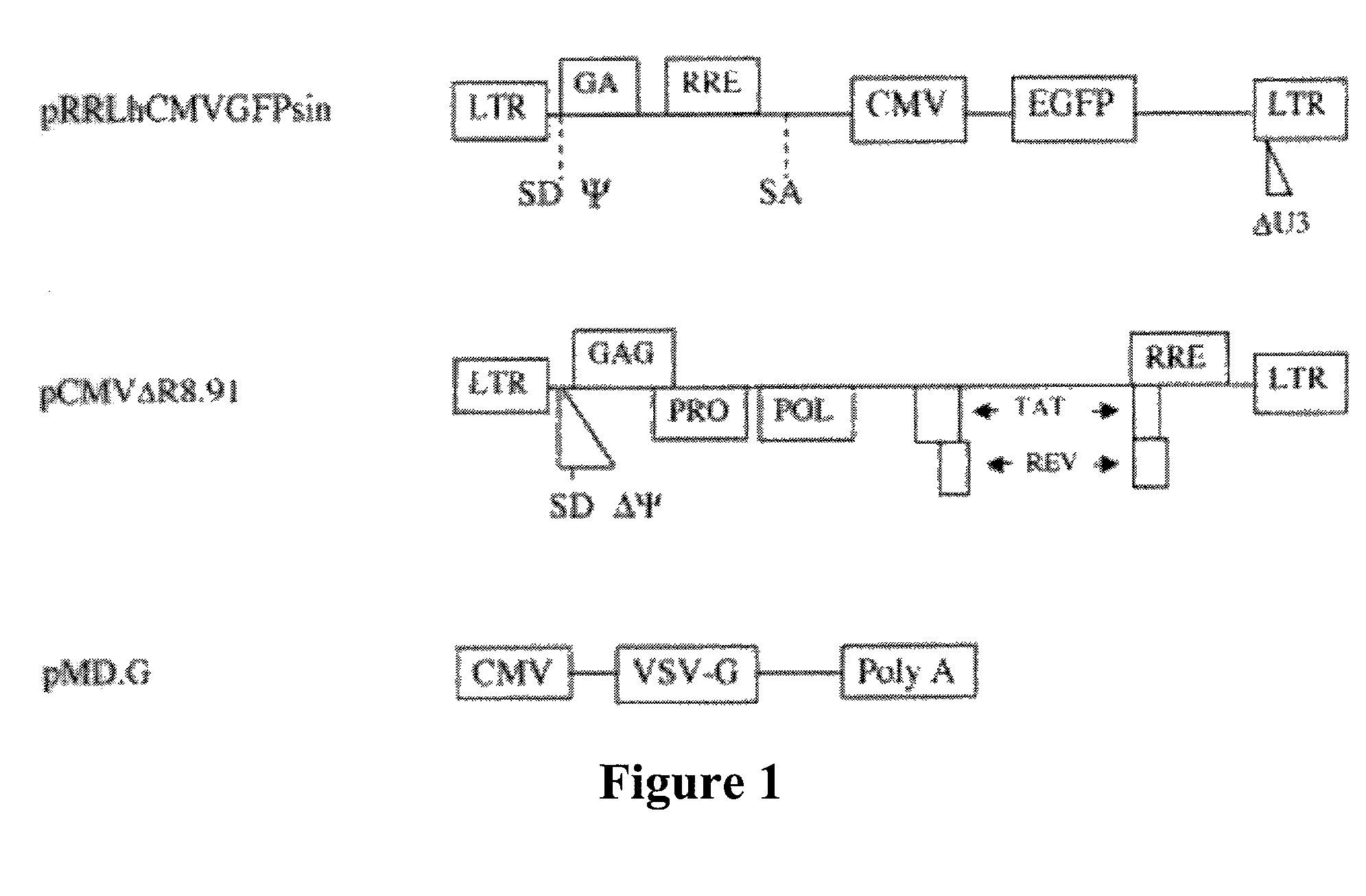
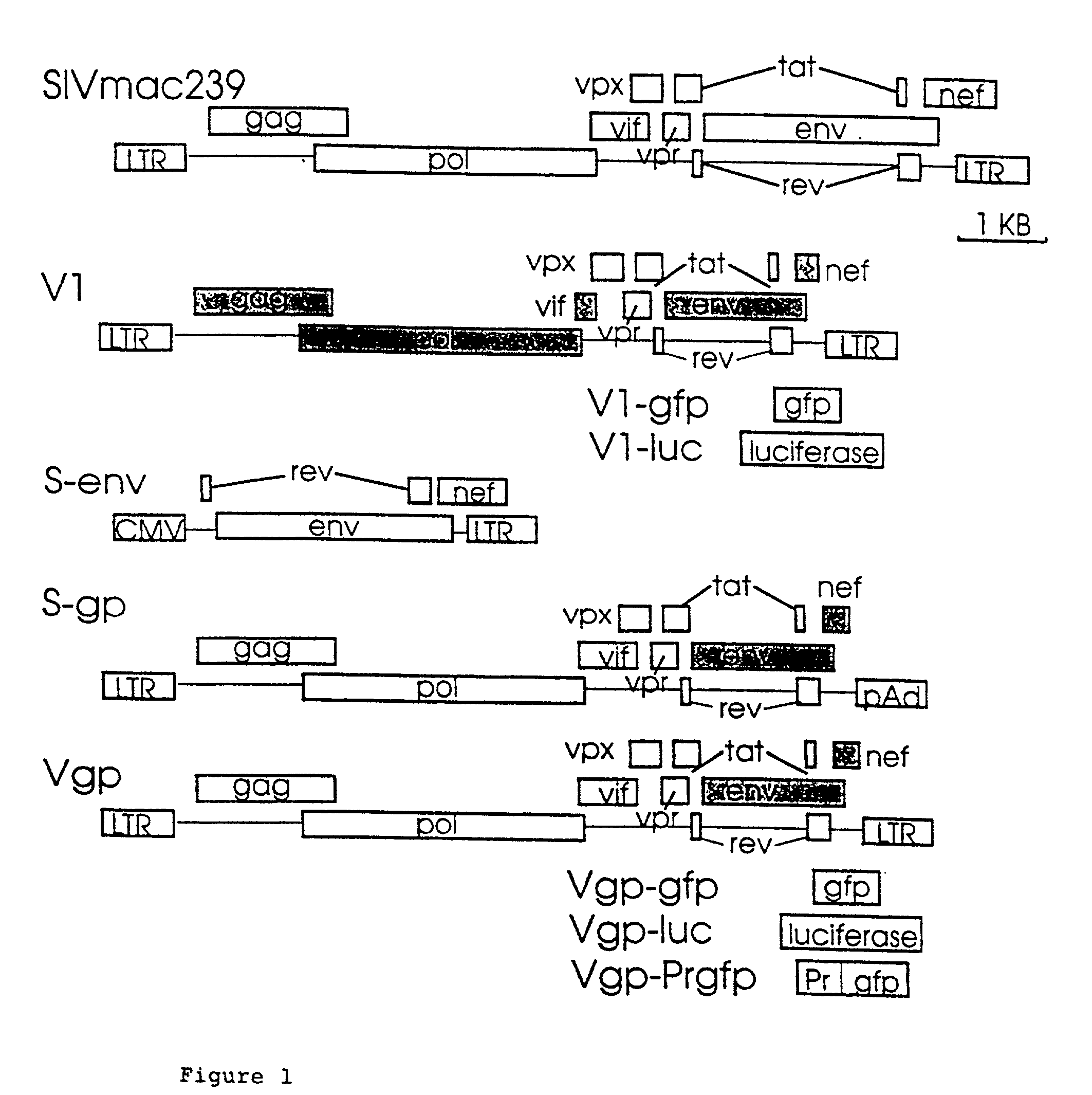
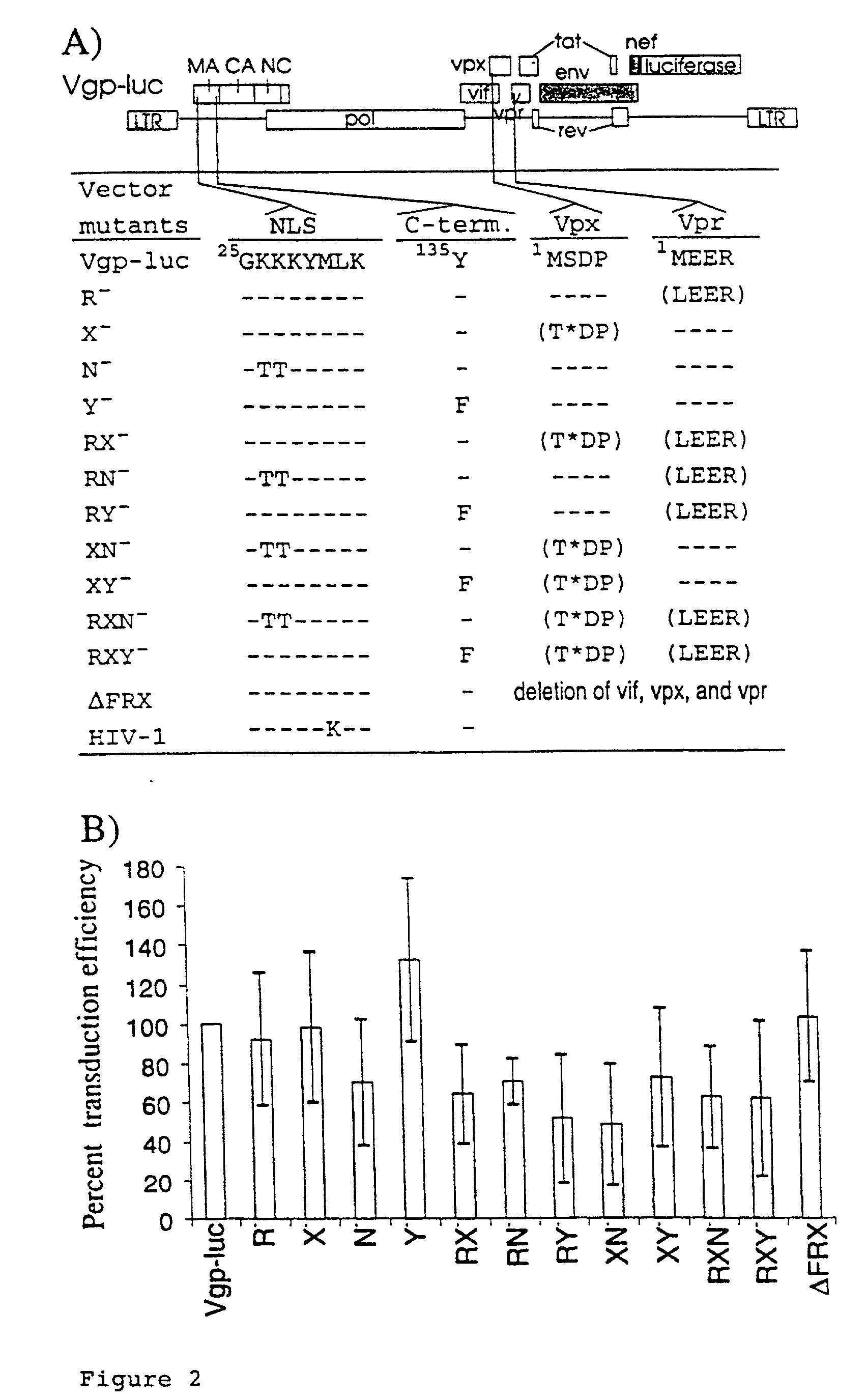
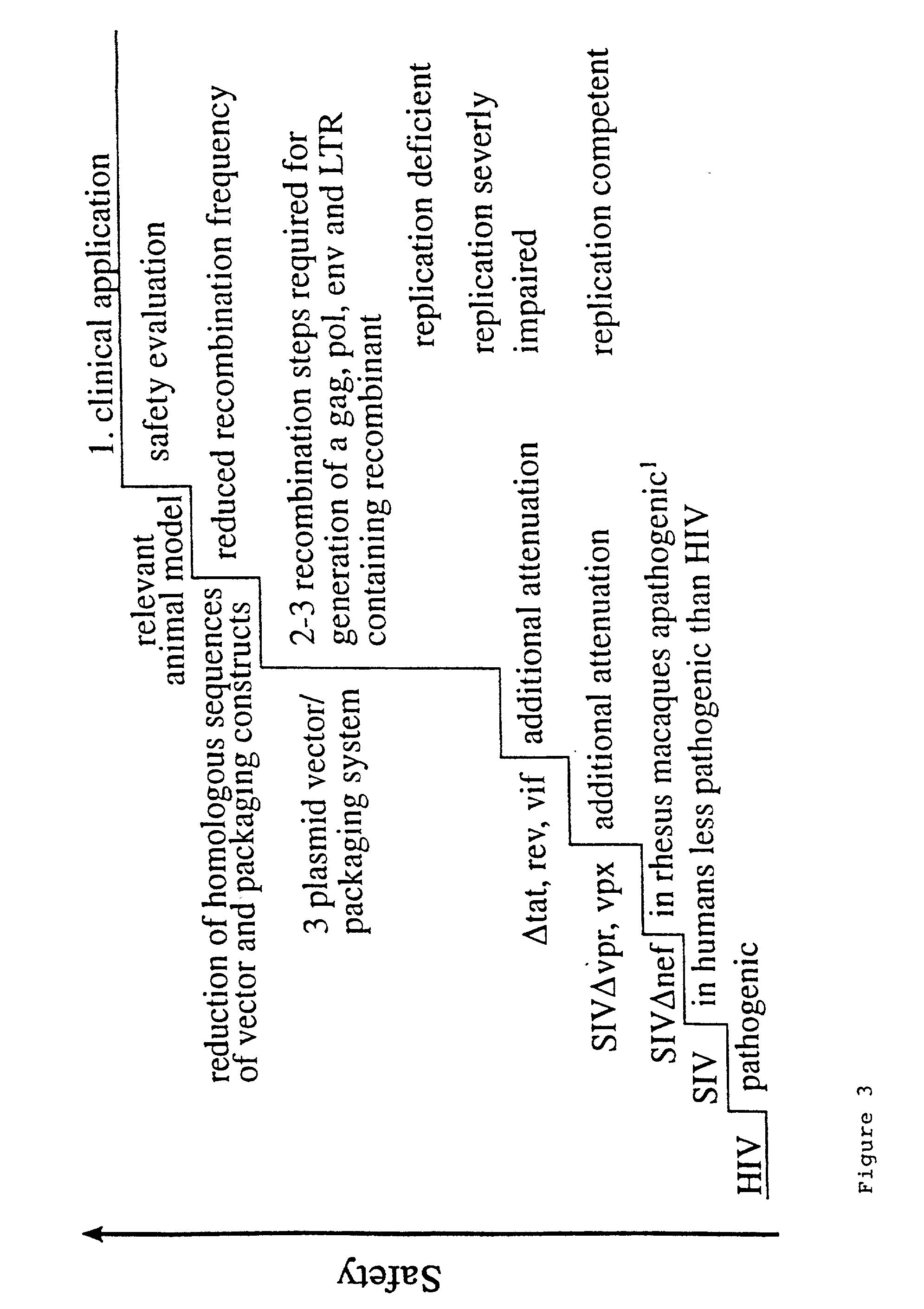
Lentiviral vectors modified at the 5′ LTR or both the 5′ and 3′ LTR are useful in the production of recombinant lentivirus vectors (See the Figure). Such vectors can be produced in the absence of a functional tat gene. Multiple transformation of the host cell with the vector carrying the transgene enhances virus production. The vectors can contain inducible or conditional promoters.
Owner:MILTENYI BIOTEC B V & CO KG
Method and means for producing high titer, safe, recombinant lentivirus vectors
InactiveUS7083981B2Rapid productionHigh titer recombinantGenetic material ingredientsVirus peptidesLentivirusViral vector
Lentiviral vectors modified at the 5′ LTR or both the 5′ and 3′ LTR's are useful in the production of recombinant lentivirus vectors. Such vectors can be produced in the absence of a functional tat gene. Multiple transformation of the host cell with the vector carrying the transgene enhances virus production.
Owner:MILTENYI BIOTEC TECH
ALVAC/FIV constructs
InactiveUS7255862B1Improve security levelViral antigen ingredientsGenetic material ingredientsFeline immunodeficiency virusHeterologous
Recombinants containing and expressing lentivirus, retrovirus or immunodeficiency virus DNA and methods for making and using the same are disclosed and claimed. In an exemplified embodiment, attenuated recombinant viruses containing DNA encoding a feline immunodeficiency virus epitope such as an antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinants can be NYVAC or ALVAC recombinants. The DNA can encode at least one of: Env, Gag, Pol, or combinations thereof such as Gag and Pol or protease or Env, Gag and Pol or protease. The recombinants and gene products therefrom and antibodies generated by them have several preventive, therapeutic and diagnostic uses. DNA from the recombinants are useful as probes or, for generating PCR primers or for immunization. The immunogenicity and protective efficacy of immunization protocols involving ALVAC-FIV and priming with a recombinant canarypox virus ALVAC-FIV vaccine followed by a booster immunization with inactivated FIV-infected celled vaccine (ICV) was evaluated against FIV challenge in cats and the protocol was shown to effectively induce FIV-specific protective immune responses. Further, it was found that immunized cats were fully protected from an initial challenge with a slightly heterologous FIV strain (50CID50) and were partially protected from a second challenge with a distinctly heterologous FIV strain (75CID50) given eight months after the initial challenge without any intervening booster.
Owner:VIROGENETICS
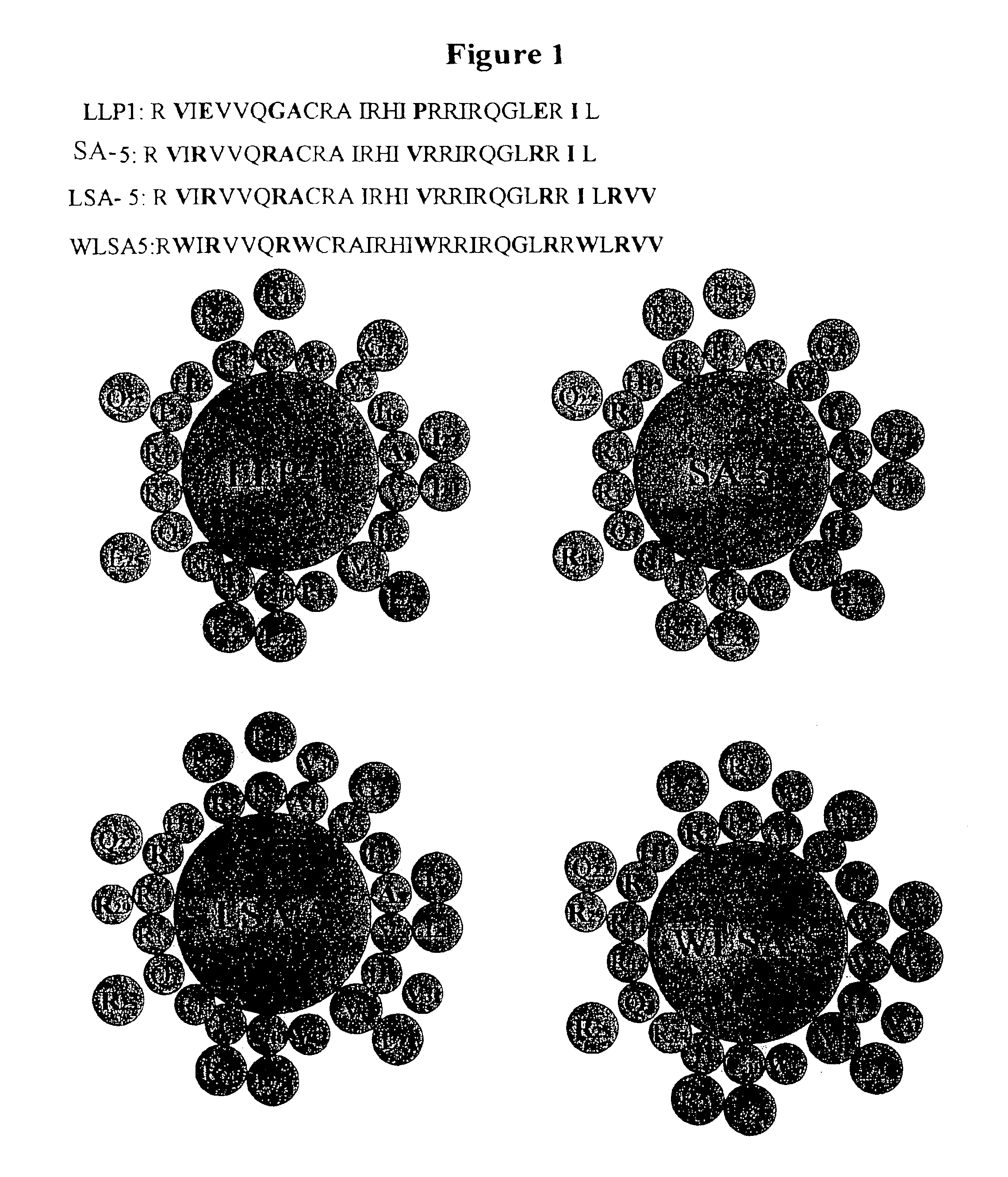
Virus derived antimicrobial peptides
InactiveUS6887847B2Lowering molar concentration requiredMinimal toxicityAntibacterial agentsBiocideBacteroidesLytic peptide
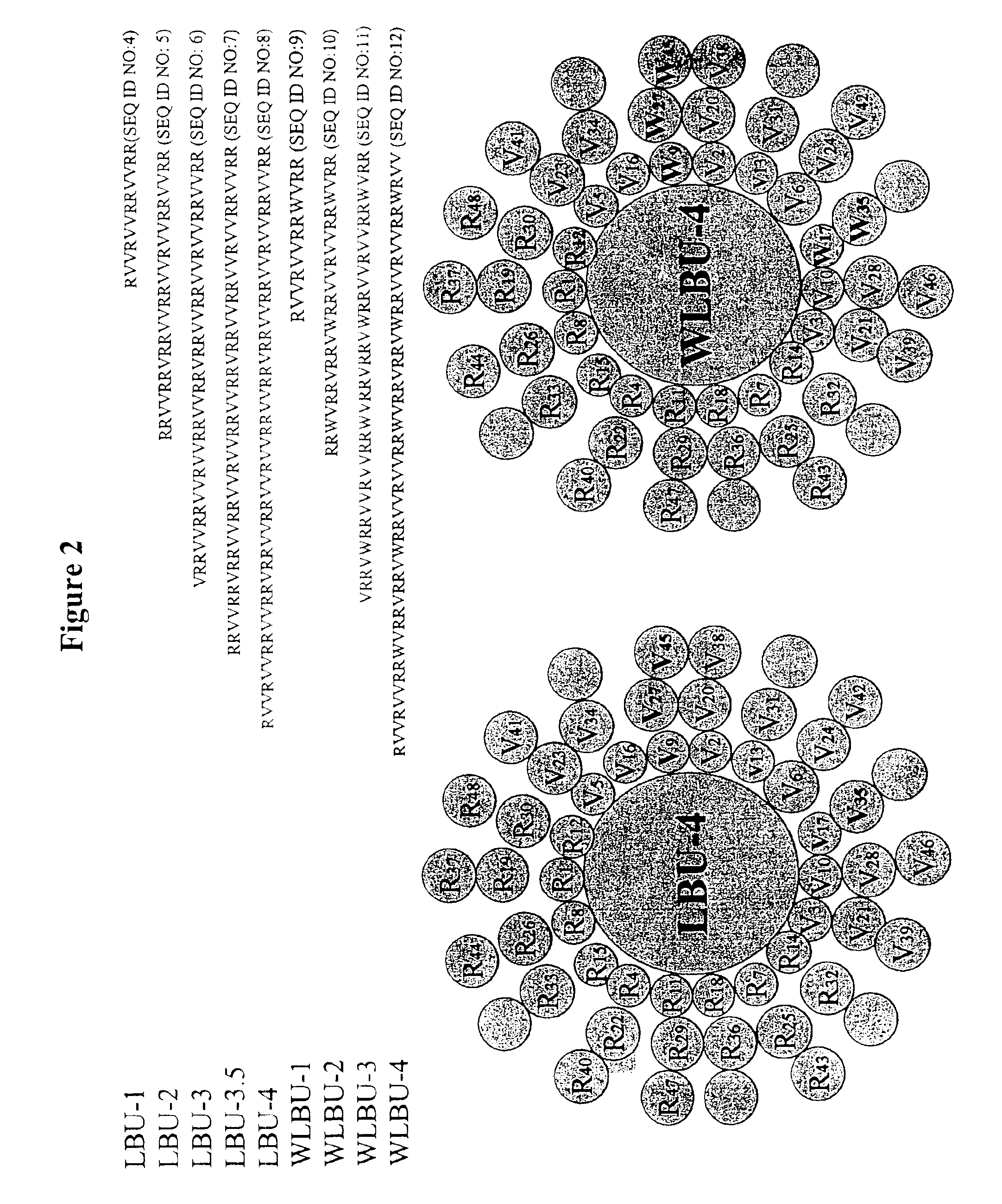
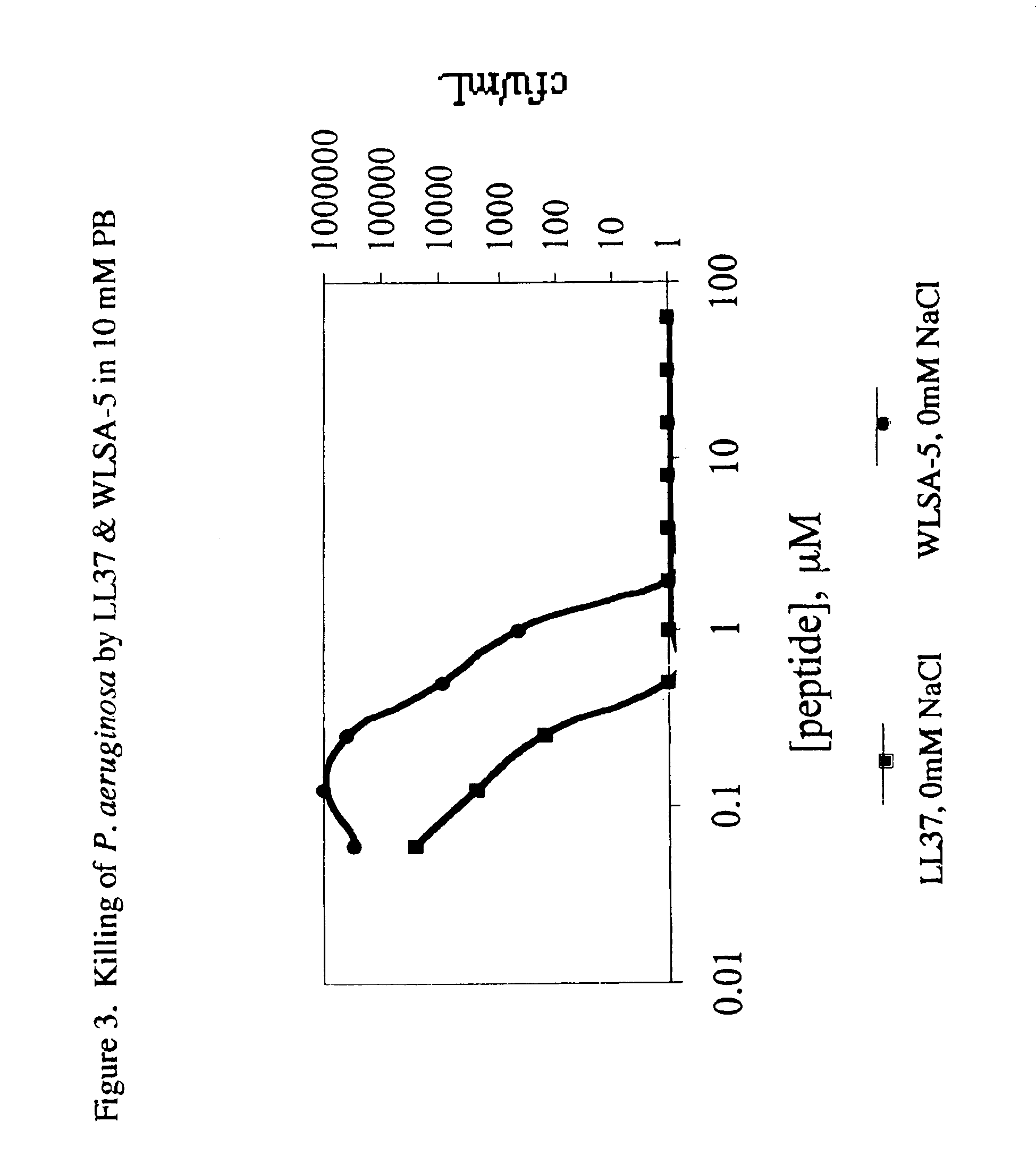
The invention is directed to peptides having antimicrobial activity (antimicrobial peptides). The antimicrobial peptides of the present invention are analogs of the Lentivirus Lytic Peptide 1 (LLP1) amino acid sequence. The invention is further directed to peptides referred to as the Lytic Base Unit (LBU) peptides derived from the LLP1 analogs, also having antimicrobial activity. In addition, the present invention is also directed to methods of using the peptides in a variety of contexts, including the treatment or prevention of infectious diseases. The antimicrobial LLP1 analog peptides and the LBU peptides (collectively eLLPs) may be highly active under high salt conditions and in biologic fluids. In addition, the eLLPs are effective when presented either in soluble form, or when attached to a solid surface. Furthermore, the peptides of the present invention are selectively active against a wide variety of bacterial pathogens and exhibit minimal toxicity to eukaryotic cells in vitro and in vivo.
Owner:UNIVERSITY OF PITTSBURGH
CRISPR/SaCas9 system for gene therapy of AIDS
InactiveCN105567688AImprove efficiencyPrevent intrusionGenetic material ingredientsAntiviralsVector systemLentivirus
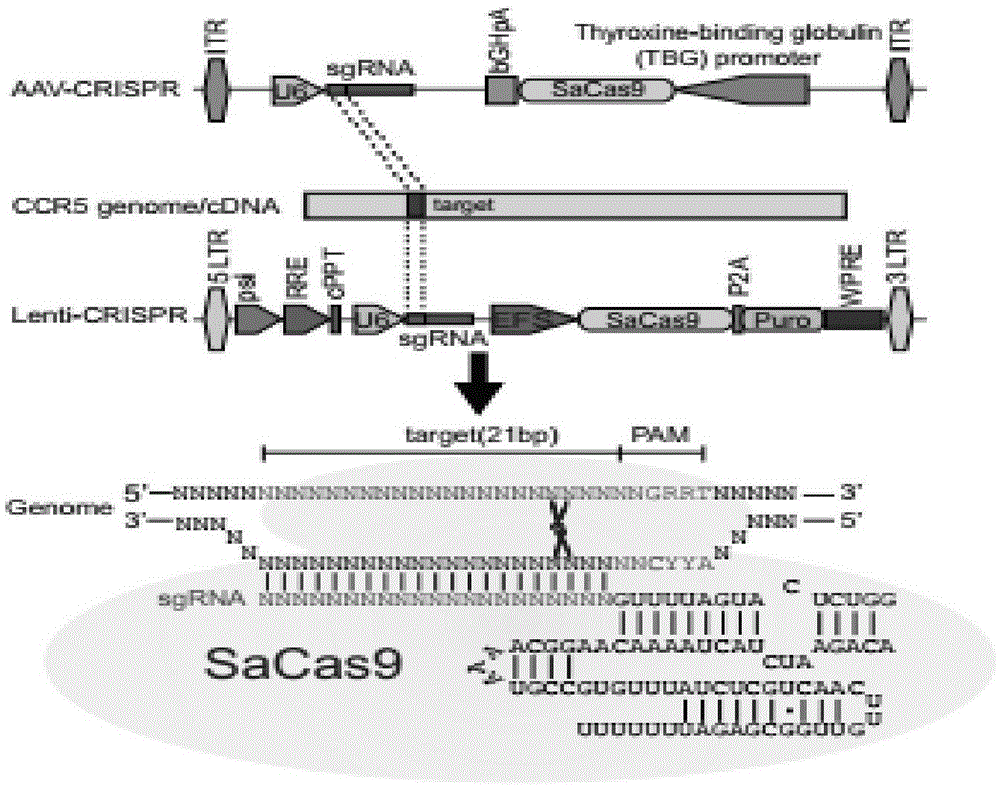
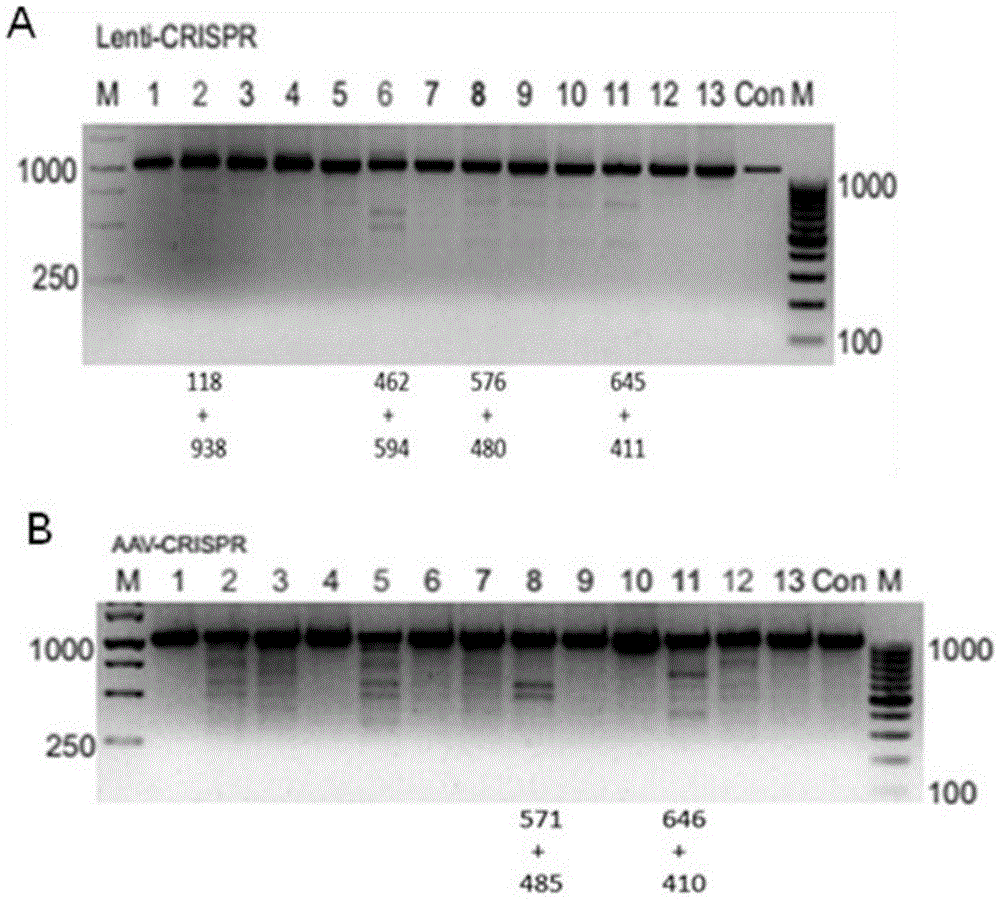
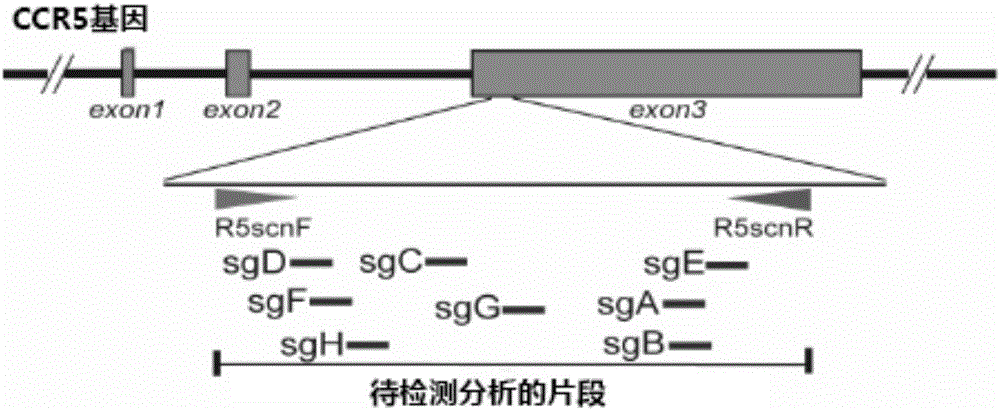
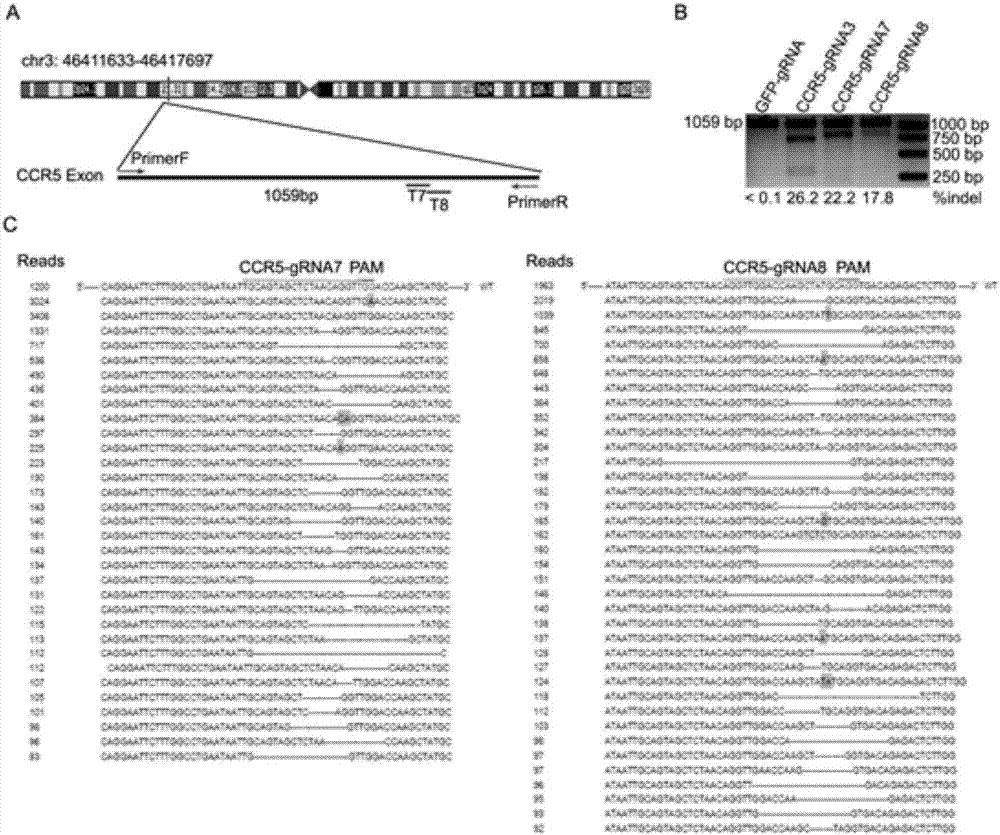
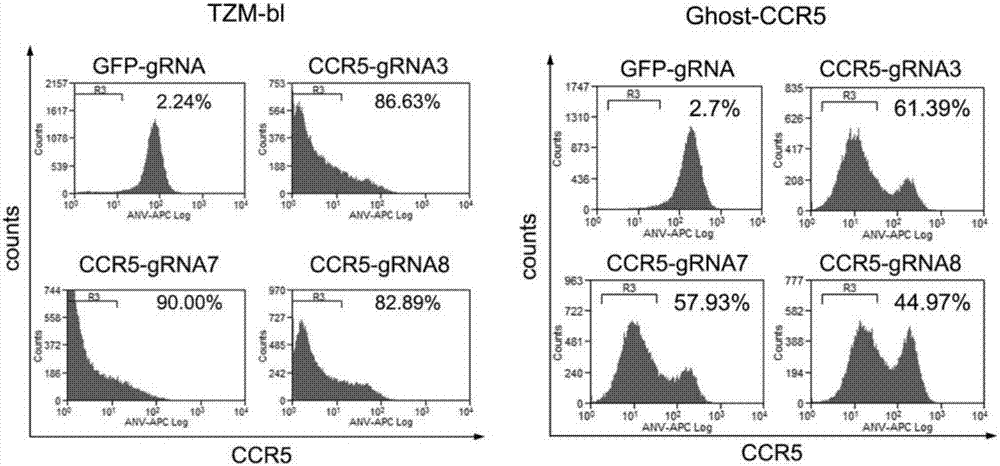
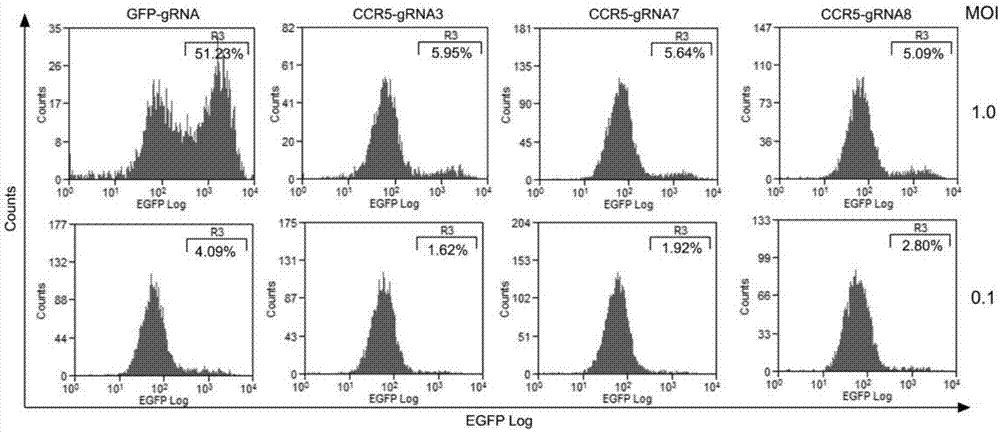
The invention belongs to the field of gene engineering, and particularly relates to a method for specifically knocking out CCR5 genes of human bodies through CRISPR / SaCas9 and sgRNA used for specifically targeting CCR5 genes. The invention provides the method for specifically knocking out the CCR5 genes of the human bodies through CRISPR / SaCas9 and sgRNA used for specifically targeting the CCR5 genes and relates to a CRISPR / SaCas9 recombinant lentivirus and adeno-associated virus vector system and application. CRISPR / SaCas9 recombinant lentivirus and adeno-associated virus vectors can express SaCas9-RNA aiming at the CCR5 target spot of one same gene of the human bodies and rhesus monkeys, therefore, the application range is wider, and the efficiency is higher. The preparation method is easy to construct, the targeting efficiency is high, and a novel technology is provided for the gene therapy of HIV.
Owner:WUHAN UNIV
Application of virus-mediated Cpf1 protein in CRISPR/Cpf1 gene editing system
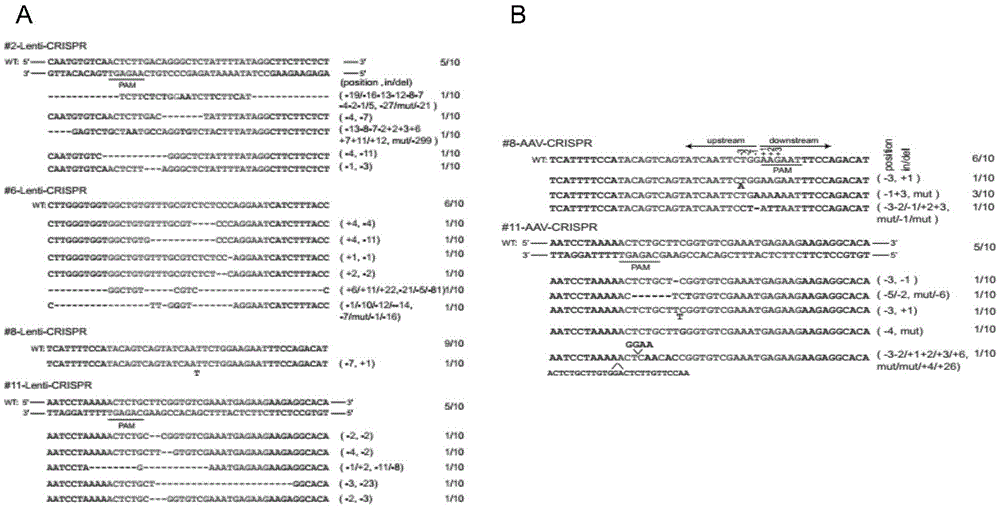
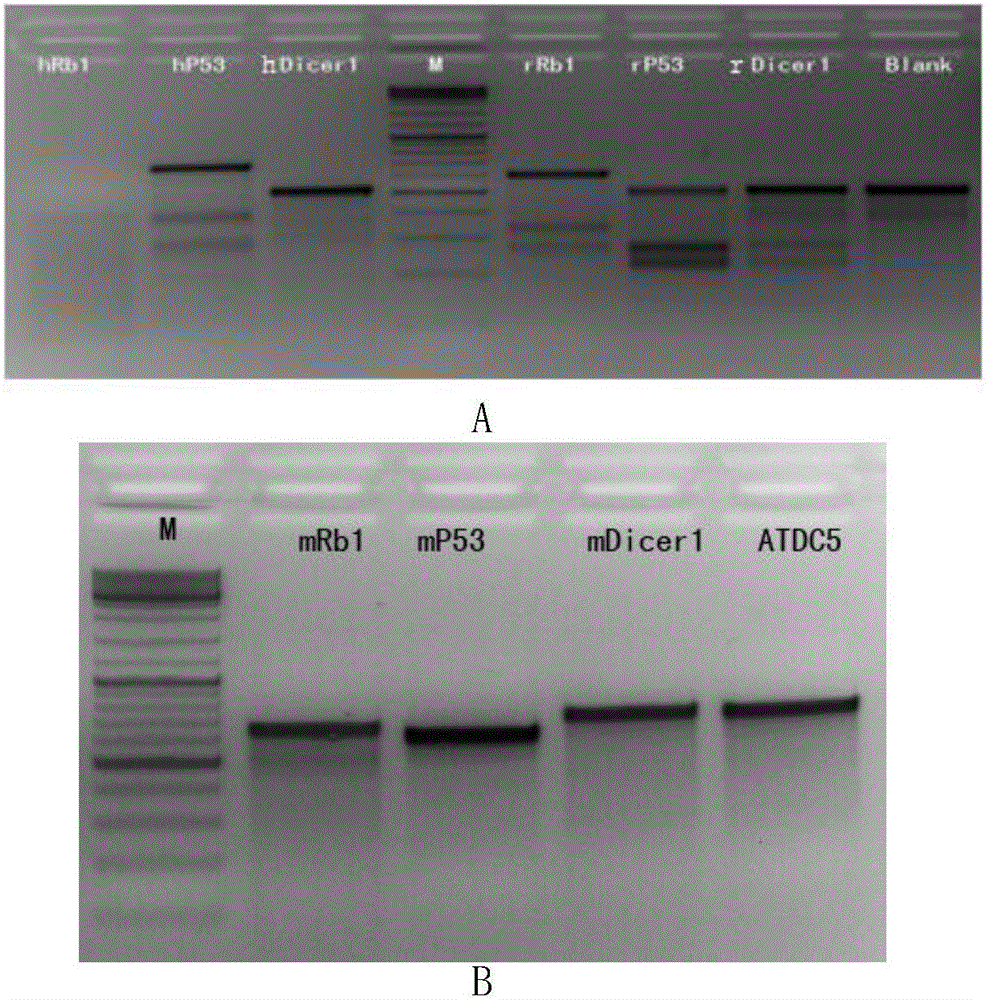
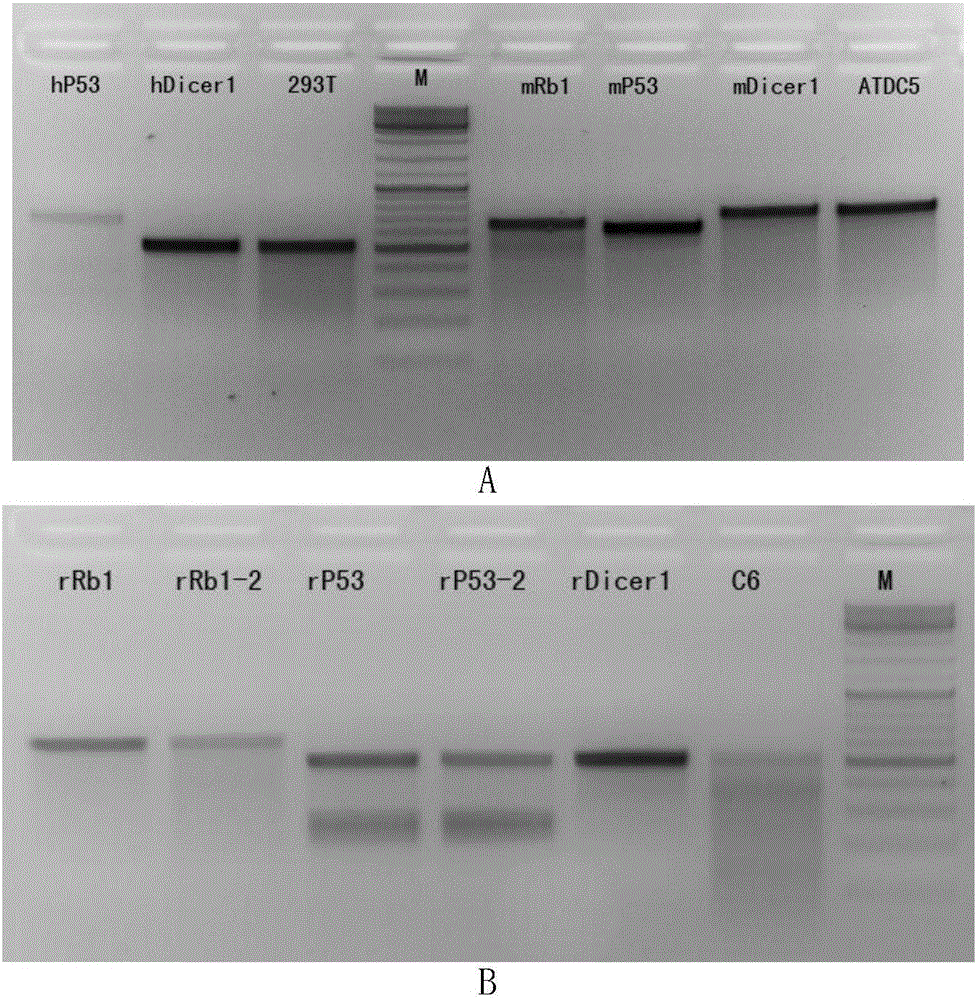
The invention discloses an application of virus-mediated Cpf1 protein in a CRISPR / Cpf1 gene editing system. The CRISPR / Cpf1 gene editing system provided by the invention consists of a recombinant virus or a recombinant vector. The recombinant virus is capable of expressing a Cpf1 gene; and the Cpf1 gene is capable of encoding the Cpf1 protein. The recombinant vector comprises a virus genome and the Cpf1 gene. The virus is either lentivirus or adenovirus. Experiments prove that virus-mediated FnCpf1 protein or AsCpf1 protein causes mutation of an hRb1 gene, an hP53 gene, an hDicer1 gene, an mRb1 gene, an mP53 gene, an mDicer1 gene, an rRb1 gene, an rP53 gene and an rDicer1 gene in the CRISPR / Cpf1 gene editing system. Therefore, the virus-mediated Cpf1 protein is applicable to gene editing in the CRISPR / Cpf1 gene editing system; and the Cpf1 protein has an important application value.
Owner:SHANGHAI GENEPHARMA CO LTD
Glycosylated modified primate lentivirus envelope polypeptides
InactiveUS6908617B1Improving immunogenicityPeptide/protein ingredientsViral antigen ingredientsAdjuvantBinding site
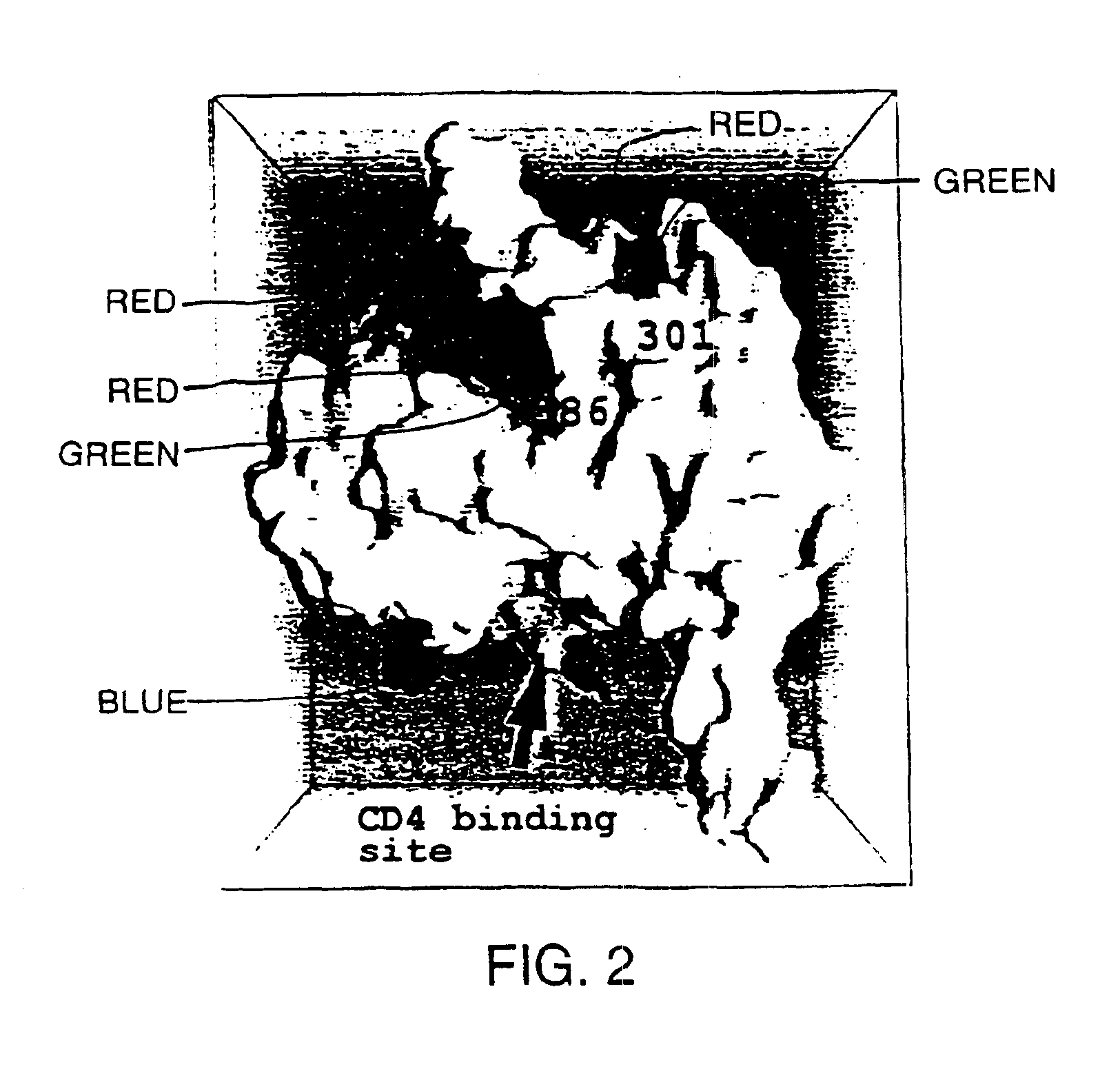
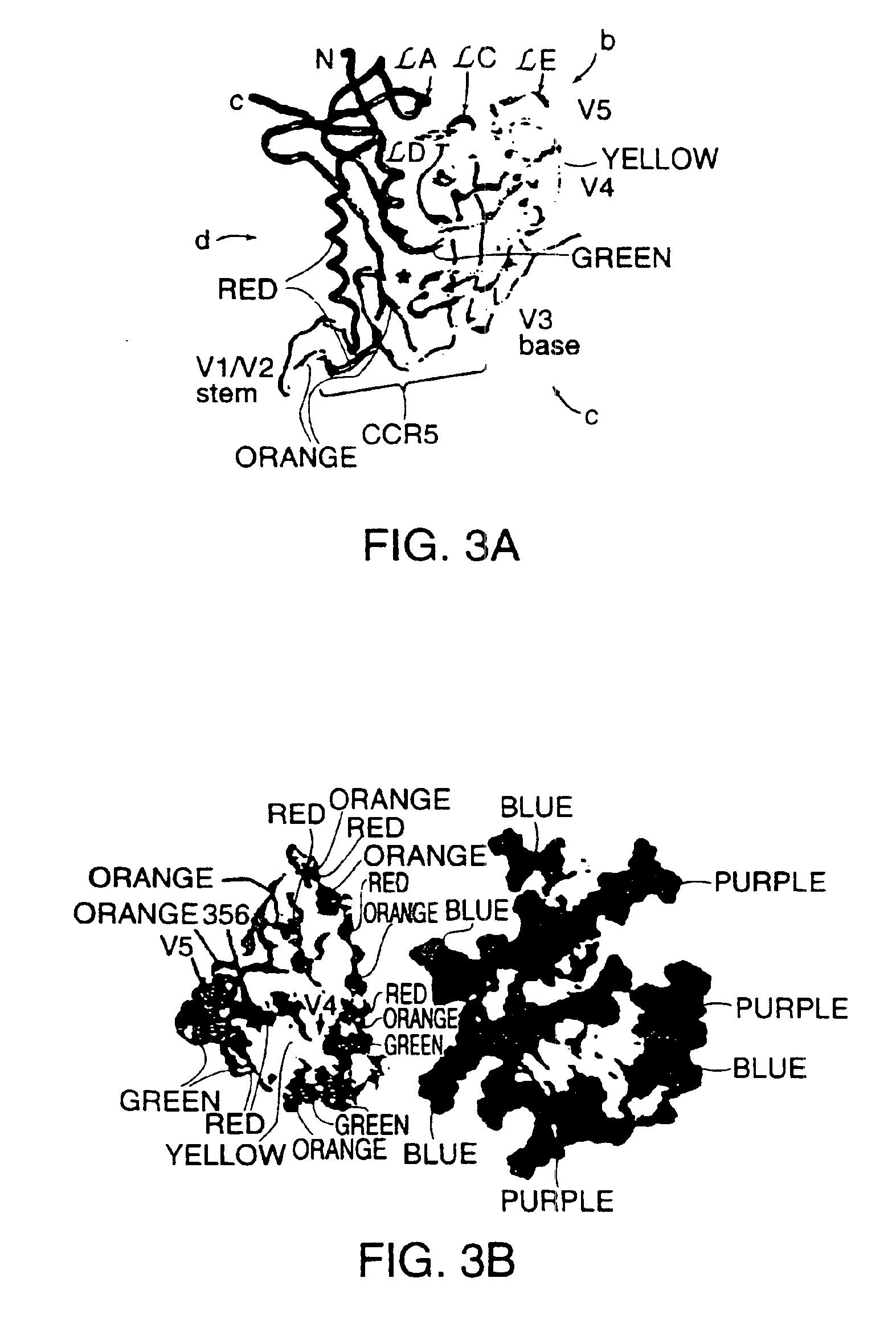
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has at least two of the glycosylation sites proximal to the CD4 binding site or chemokine receptor site altered so that the alteration prevents glycosylation at that site or where glycosylation sites distal to these sites have been derivatized with a molecular adjuvant, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has both changes. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp 120.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
sgRNA (singleguide Ribonucleic Acid), lentiviral vector constructed by the same and application thereof
InactiveCN106801056AEffective knockoutExcellent anti-virus infection abilityCell receptors/surface-antigens/surface-determinantsAntiviralsTreatment fieldHIV receptor
The invention relates to the field of gene therapy, in particular relates to sgRNA (singleguide Ribonucleic Acid), as well as a lentiviral vector constructed by the same and application thereof and specifically relates to sgRNA with SIVmac1A11 lentivirus as a framework to express SpCas9 protein and gene specificity. The sgRNA is applied to treating human and simian AIDS (Acquired Immune Deficiency Syndrome). A nucleotide sequence of the sgRNA is shown as SEQ ID NO.1 to 2. According to the sgRNA disclosed by the invention, a current most efficient CRISPR / Cas9 gene editing tool is utilized, a designed CXCR4 / CCR5 gene sgRNA locus has gene knockout activity superior to other loci reported by existing research, and the sgRNA is applied to gene therapy of SIV infected rhesus monkeys for the first time. Compared with ZFN and TALEN, the sgRNA has the advantages of being convenient to operate, low in cost and the like.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Humanized PD-L1 tumor cell line, animal model with same and application of humanized PD-L1 tumor cell line and animal model
ActiveCN105950560ASpeed up the processLethalCompounds screening/testingCell receptors/surface-antigens/surface-determinantsPD-L1Wilms' tumor
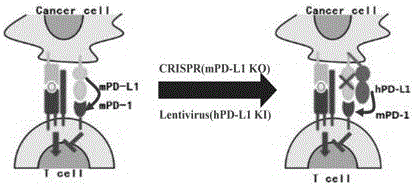
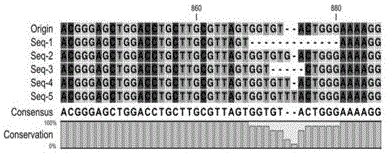
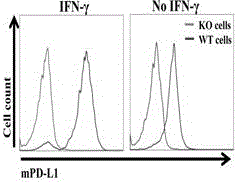
The invention provides a humanized PD-L1 tumor cell line MC-38-hPD-L1, a builtanimal tumor model with the same and a method for constructing the humanized PD-L1 tumor cell line. The method particularly includes knocking out animal-origin PD-L1 by the aid of CRISPR-CAS9; carrying out amplification and cultivation to obtain knocked-out cell banks; extracting DNA (deoxyribonucleic acid) and carrying out PCR (polymerase chain reaction) amplification; recycling and cloning amplification products; carrying out over-expression on human-origin PD-L1 in MC-38 cell lines of mPD-L1 KO by the aid of lentivirus systems; packaging lentivirus and screening Puromycin to obtain the humanized MC-38 cell line of PD-L1. The humanized PD-L1 tumor cell line, the animal tumor model and the method have the advantages that as shown by results, high killing efficiency and multiplication capacity are obviously presented by tumor infiltration CD8 T lymphocytes after antibody treatment is carried out, tumor infiltration Treg cells can be obviously inhibited after antibody treatment is carried out, and accordingly the method is proved to be effective and feasible from the aspect of molecular mechanisms.
Owner:SUZHOU INST OF SYST MEDICINE
Lentivirus vectors for gene transfer to alveolar epithelial cells
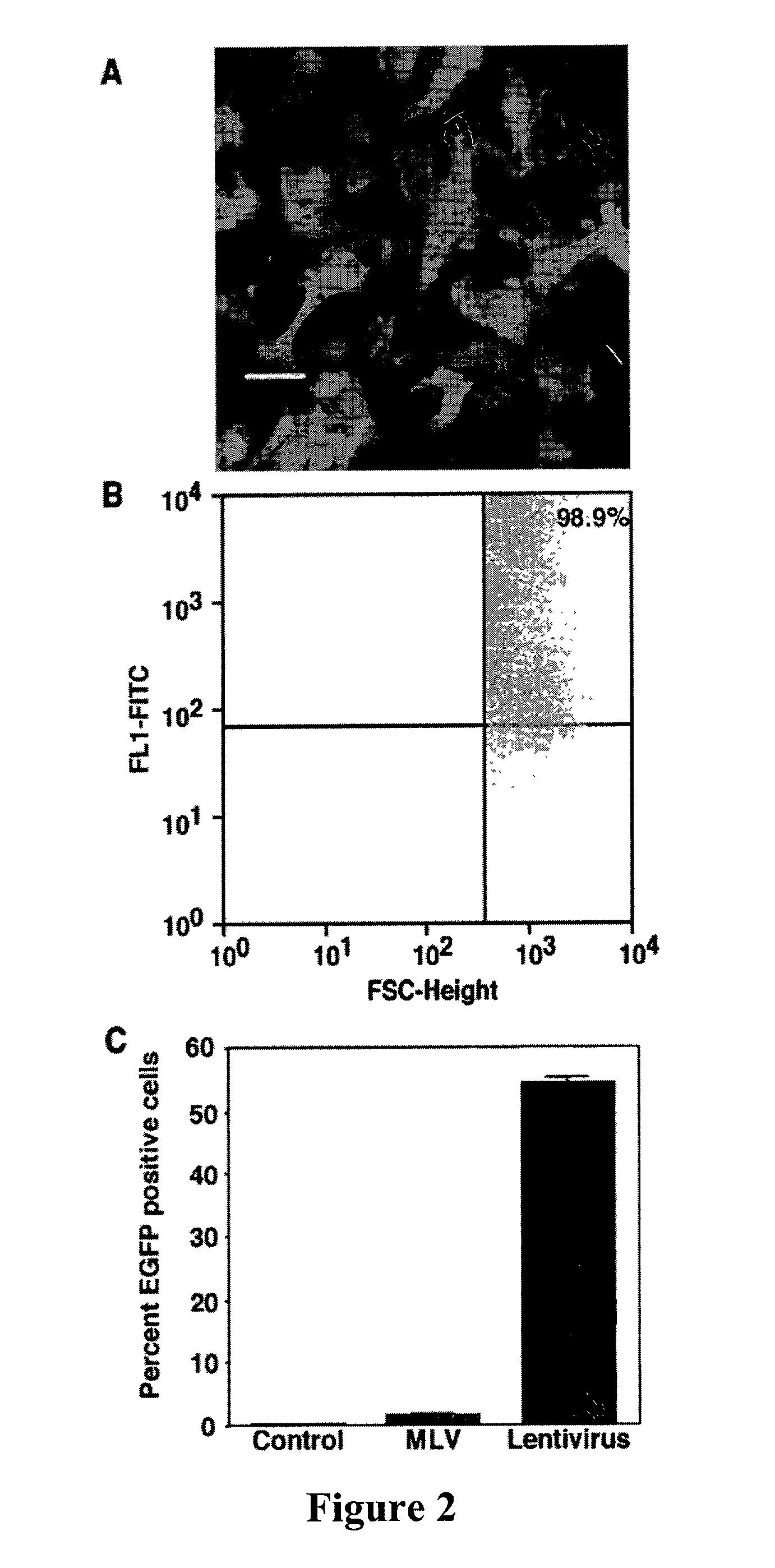
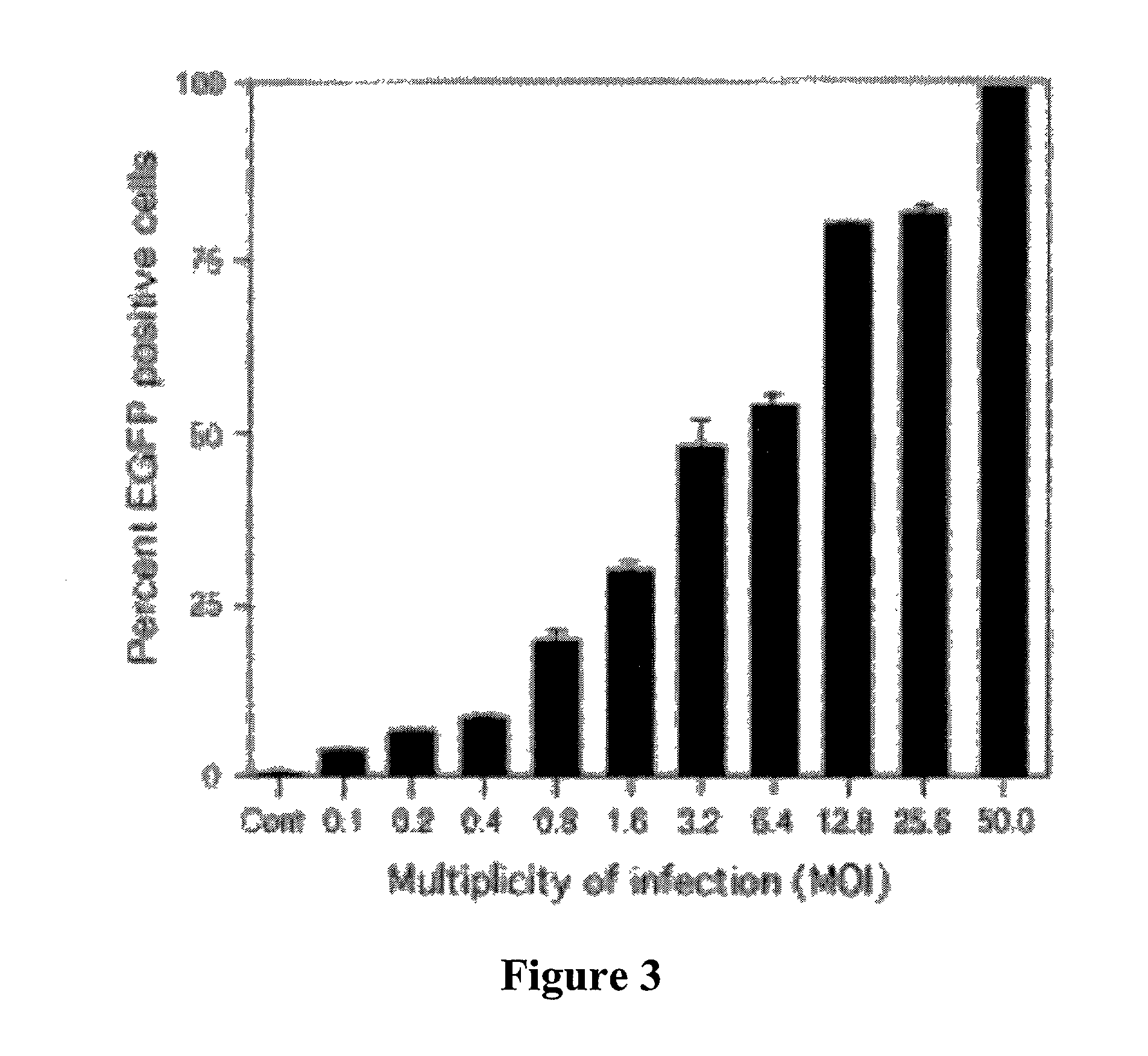
The present invention demonstrates that VSV-G-pseudotyped lentivirus vectors efficiently transduce AEC in primary culture and in vivo with transduction favored by virus application from the apical side. Transduction efficiency in AEC increased with increasing MOI and greatly exceeded that achieved with a similarly pseudotyped MLV retrovirus vector. The present invention also demonstrates the successful in vivo transfer of genes through lentivirus vector transduction. Mammals injected with lentivirus vector via the trachea expressed the reporter protein in alveolar epithelial cells within 48 to 72 hours after infection.
Owner:UNIV OF SOUTHERN CALIFORNIA
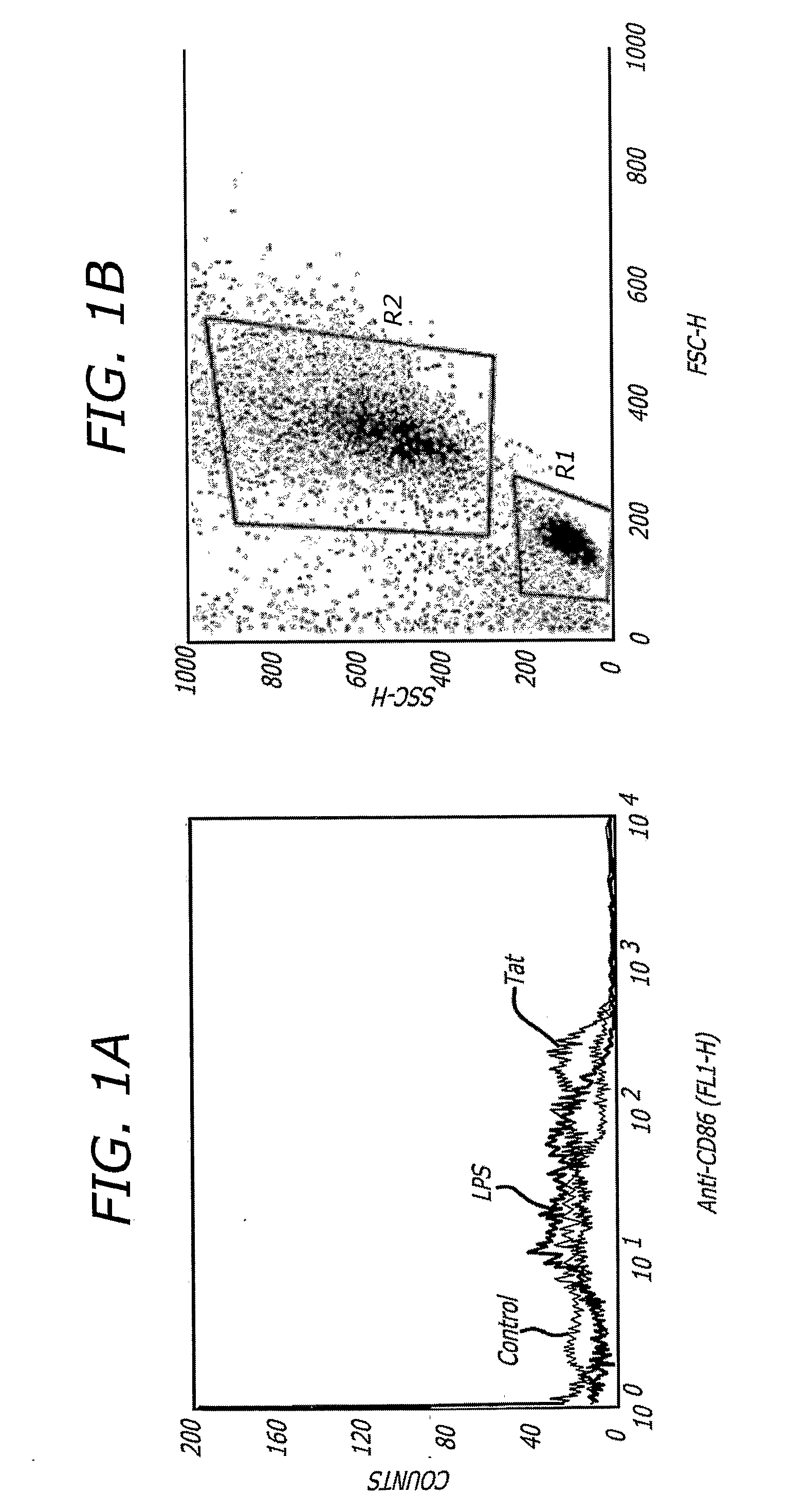
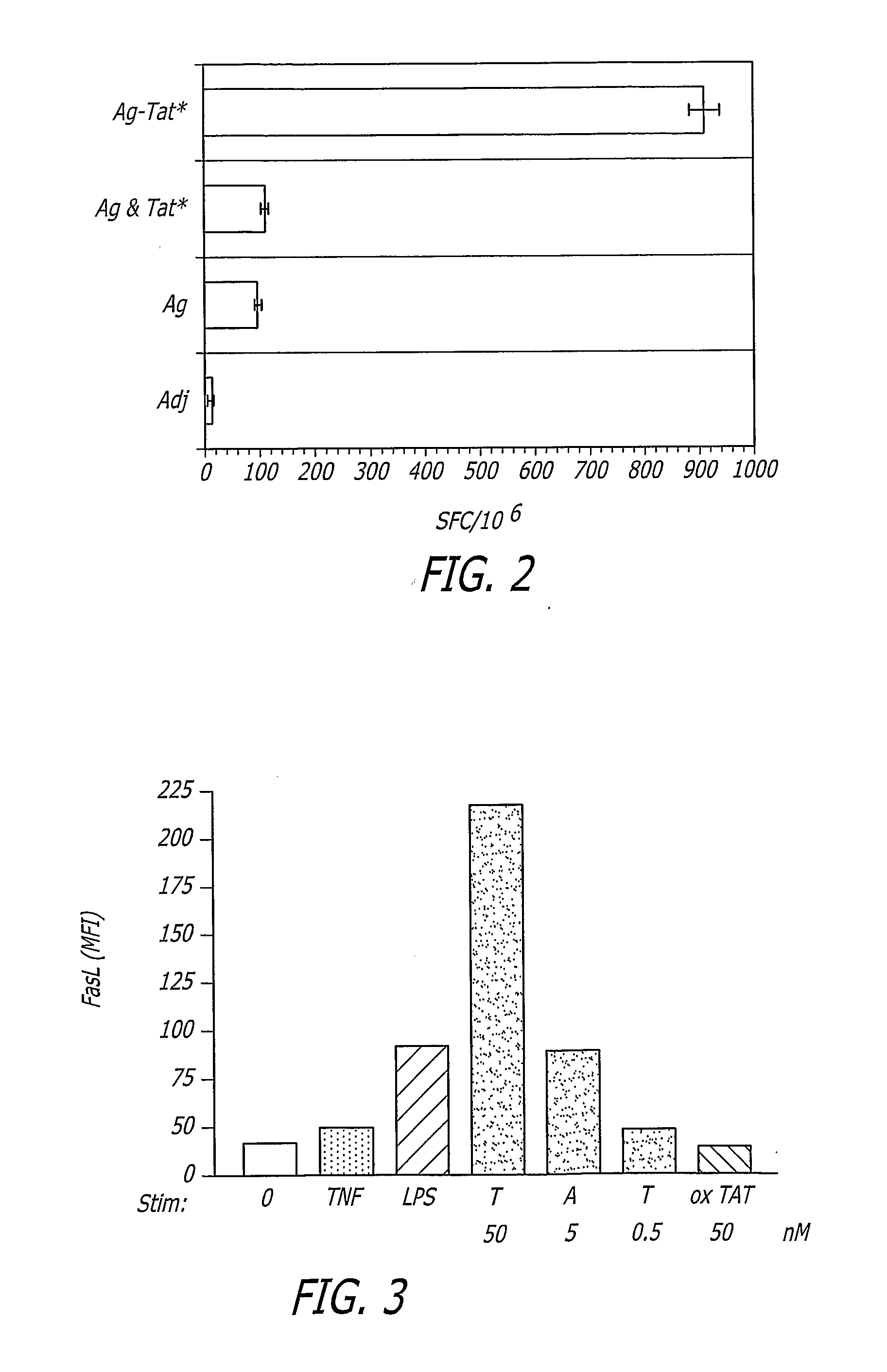
Tat-Based vaccine Compositions and Methods of Making and Using Same
A Tat-based vaccine composition comprising at least one antigen coupled to at least one immunostimulatory lentivirus trans-activator of transcription (Tat) molecule wherein the antigen is a cancer antigen an infectious disease antigen or a fragment thereof and methods to treat disease by administering the Tat-based vaccine composition. An additional Tat-based vaccine composition comprising immunostimulatory lentivirus Tat is provided.
Owner:CHERRY MED +1
CRISPR (clustered regularly interspaced short palindromic repeat)/Cas9 Recombinant lentiviral vector containing gRNA sequence specifically targeting CCR5 and application thereof
ActiveCN107312798AAvoid infectionInfection efficiency dropsOrganic active ingredientsGenetic material ingredientsRandom mutationLentivirus
The invention discloses a CRISPR (clustered regularly interspaced short palindromic repeat) / Cas9 recombinant lentiviral vector containing gRNA sequence specifically targeting CCR5 and application thereof. A lentivirus of the CRISPR / Cas9 recombinant lentiviral vector containing gRNA sequence specifically targeting CCR5 gene Delta 32 region is constructed that the lentivirus can introduce cells into a CRISPR / Cas9 system specific to CCR5, double-chain breakage occurs to a specific site of CCR5 gene, a random mutation is introduced to a breakage site after repairing by means of nonhomogeneous recombinant terminal binding, and the mutation rate reaches 90% and above. As gRNA is a nonhomogeneous region of CCR5 and CCR2, detection shows that the missing efficiency of the two gRNAs is lower than 0.2%. Cells modified via the recombinant lentivirus have significantly decreased efficiency of HIV (human immunodeficiency virus) infection. The system is quick to construct, simple and low in price, and is applicable to gene therapy of acquired immune deficiency syndrome.
Owner:WUHAN UNIV
gRNA target sequences for endogenous overexpression of 1ncRNA-XIST and application thereof
ActiveCN107513531AImprove efficiencyFermentationVector-based foreign material introductionFetal growthCell migration
The invention discloses gRNA target sequences for endogenous overexpression of 1ncRNA-XIST, a CRISPR / dCas9 lentivirus system and application thereof. The gRNA target sequences are respectively as shown in SED ID No. 1, SED ID No. 2 and SED ID No. 3. The CRISPR / dCas9 lentivirus system comprises the gRNA target sequences for endogenous overexpression of 1ncRNA-XIST. A method for screening stable strains according to the characteristics that lentivirus must be integrated into a host genome is cooperated with CRISPR / dCas9 to realize the endogenous overexpression of the large fragment gene 1ncRNA-XIST, so that the defect of incapability of stably expressing the large fragment gene 1ncRNA-XIST in a traditional method is overcome, the efficient overexpression stable cell strain of the large fragment gene 1ncRNA-XIST can be obtained in a short time, or cells obtained by screening can stably express target genes, thereby obtaining stably silenced 1ncRNA-XIST downstream specific gene cell strain. The gRNA target sequences and the CRISPR / dCas9 lentivirus system have important guiding significance on the research of the function of 1ncRNA-XIST in the trophocyte migration and the effect of 1ncRNA-XIST in the proliferation disorder and fetal growth restriction process.
Owner:WUXI MATERNAL & CHILD HEALTH HOSPITAL
CRISPR-Cas9 targeting knockout of human breast cancer cell RASSF2 gene and specific sgRNA of RASSF2 gene
The invention discloses CRISPR-Cas9 targeting knockout of human breast cancer cell RASSF2 gene and specific sgRNA of RASSF2 gene. According to the method, the sgRNA of specific targeting RASSF2 gene is obtained, wherein the base sequence of the sgRNA is represented by SEQ ID NO.1; construction of the sgRNA of RASSF2 gene into a lentiviral vector system is carried out, wherein the lentiviral vectorsystem contains Cas9 protein; and at last, human breast cancer cell MDA-MB-231 is infected with the CRISPR-Cas9 lentivirus containing the sgRNA so as to obtain a cell strain with obviously reduced RASSF2 protein expression level. The operation and the steps are simple; the sgRNA targeting performance is excellent; cutting efficiency on RASSF2 gene is high; the constructed CRISPR-Cas9 lentiviral vector system is high in knockout efficiency, and is capable of realizing specific knockout of RASSF2 gene to obtain human breast cancer cells without RASSF2 gene, so that powerful instrument is provided for study on the action mechanisms of RASSF2 in breast cancer celles.
Owner:OBIO TECH SHANGHAI CORP LTD
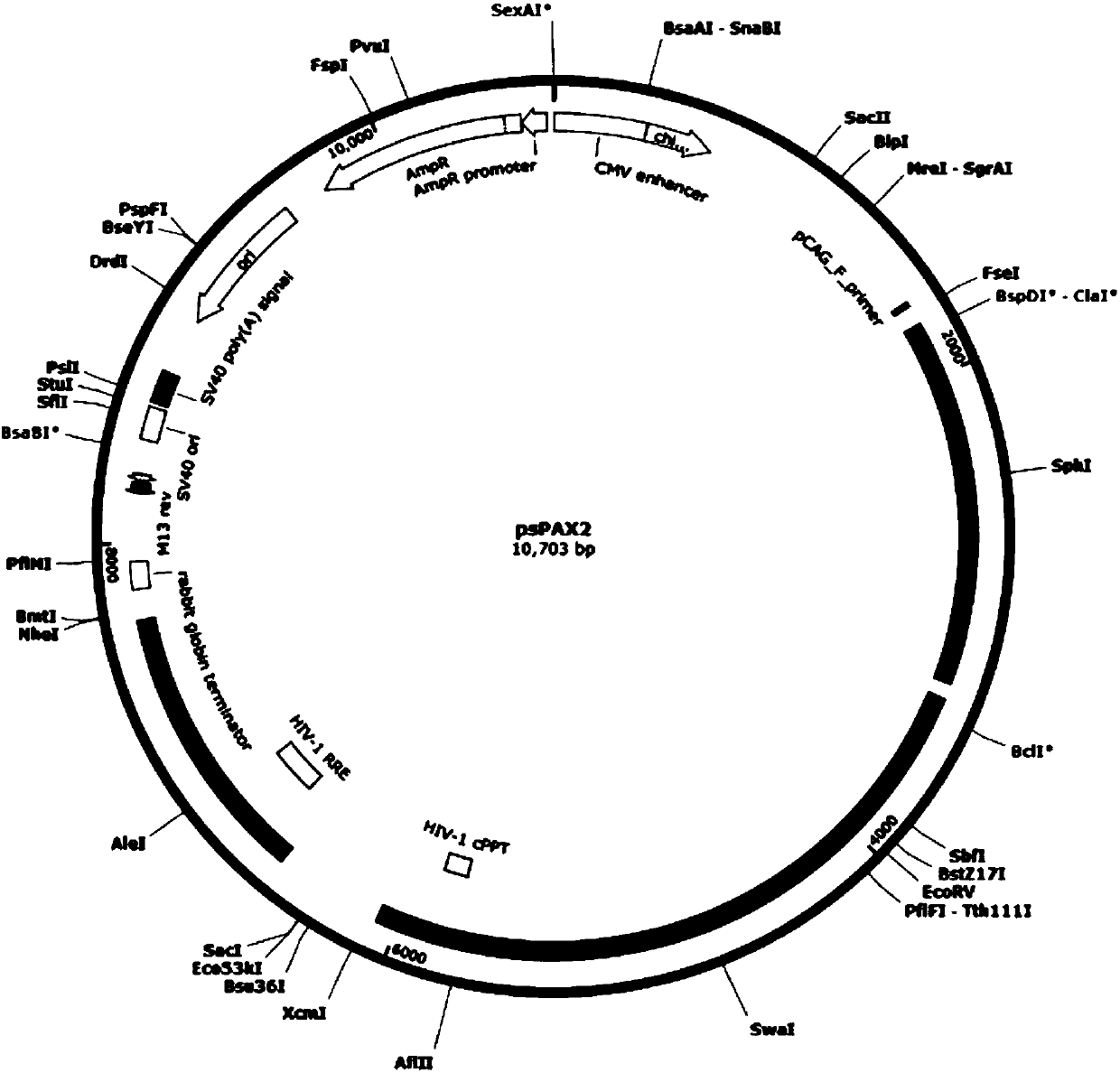
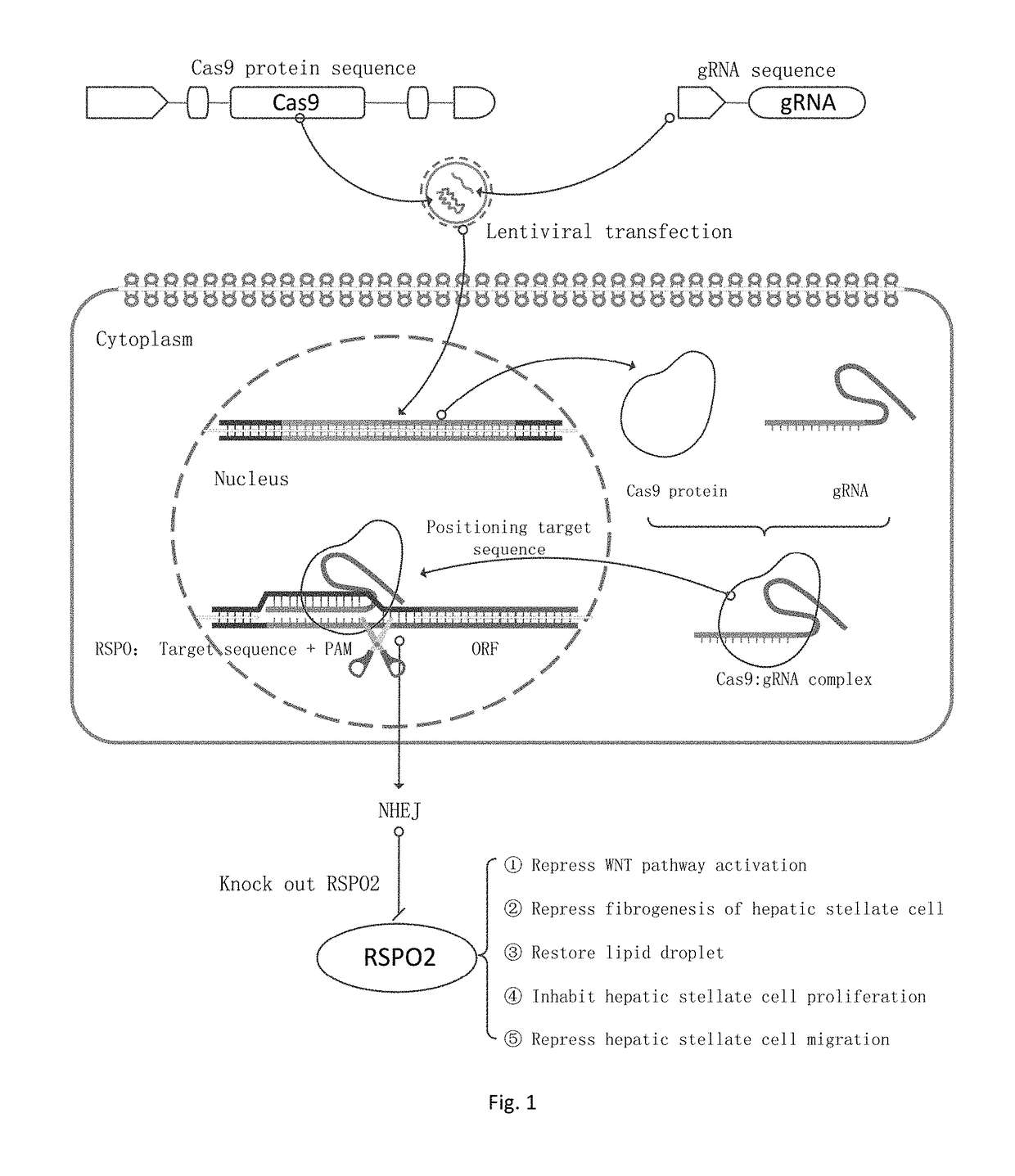
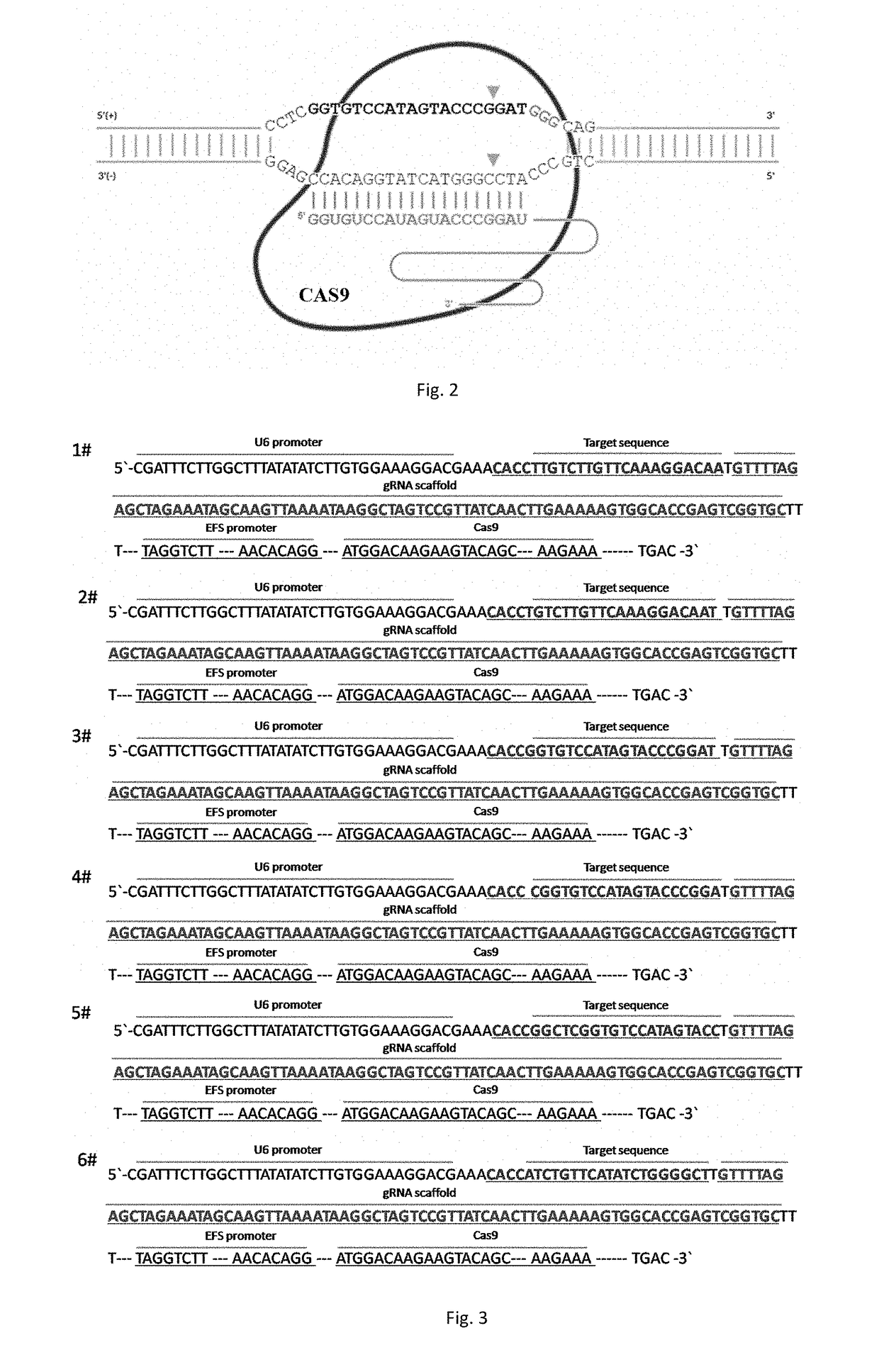
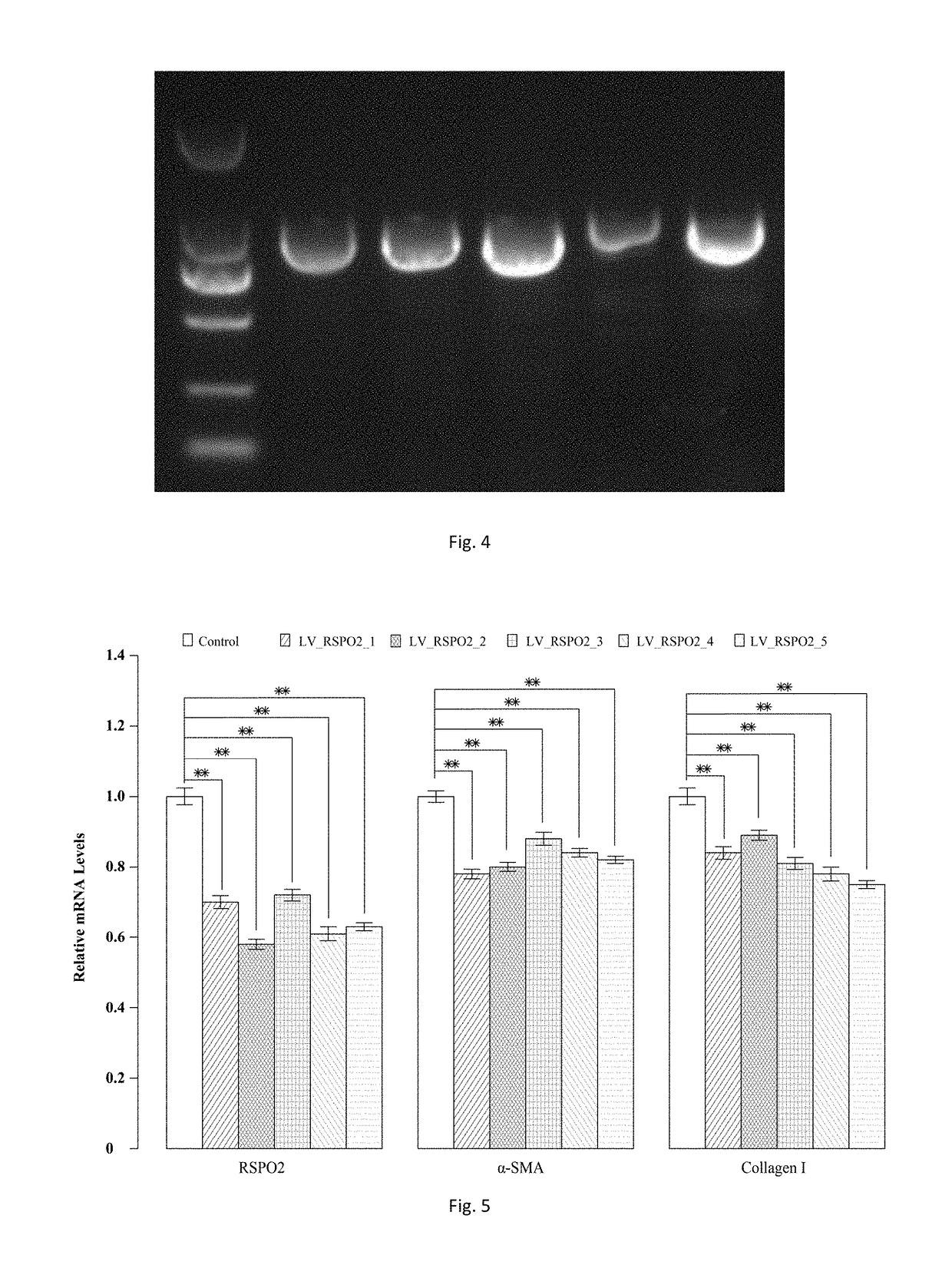
sgRNA and knockout method of human RSPO2 gene targeted with CRISPR-Cas9 specificity and application thereof
InactiveUS20180245066A1Effectively promoting recoveryStable intracellular transcriptionHydrolasesPeptidesLentivirusVirus
A method for knocking out a human RSPO2 gene targeted with CRISPR-Cas9 specificity includes steps of: 1) designing the sgRNA of the human RSPO2 gene targeted; and 2) constructing a CRISPR-Cas9 recombinant lentivirus vector for knocking out the RSPO2 gene. A method for preparing a lentiviral-packaged system for knocking out a human RSPO2 gene targeted with CRISPR-Cas9 specificity includes steps of: 1) designing the sgRNA of the human RSPO2 gene targeted; 2) constructing a CRISPR-Cas9 recombinant lentivirus vector for knocking out the RSPO2 gene; and 3) processing the CRISPR-Cas9 recombinant lentivirus vector for knocking out the sgRNA of the human RSPO2 gene with lentiviral packaging, so as to obtain the lentiviral-packaged system.
Owner:JIAXING NO 1 HOSPITAL
CRISPR-Cas9 targeted and knockout SLC30A1 gene and specific sgRNA of gene
The invention discloses a CRISPR-Cas9 targeted and knockout human breast cancer cell SLC30A1 gene and the specific sgRNA of the gene. Firstly, sgRNA for specific targeting of the SLC30A1 gene is obtained, and a base sequence of the sgRNA is shown in SEQ ID NO.1; secondly, the sgRNA of the SLC30A1 gene is constructed to a lentivirus vector system containing Cas9 proteins; finally, CRISPR / Cas9 lentivirus containing the sgRNA is infected with a human breast cancer cell MDA-MB-231 to obtain a cell strain of which the SLC30A1 protein expression level is obviously reduced. The CRISPR-Cas9 targeted and knockout human breast cancer cell SLC30A1 gene and the specific sgRNA of the gene disclosed by the invention have the advantages of simple operation steps, good sgRNA targetability and high cuttingefficiency on the SLC30A1 gene; in addition, the constructed CRISPR / Cas9 lentivirus system has the advantage of high knockout efficiency and can specifically knock out the SLC30A1 gene so as to obtain the human breast cancer cell having the SLC30A1 gene knocked out; therefore, a powerful tool is provided for further researching an action mechanism of the SLC30A1 in breast cancer cells.
Owner:OBIO TECH SHANGHAI CORP LTD
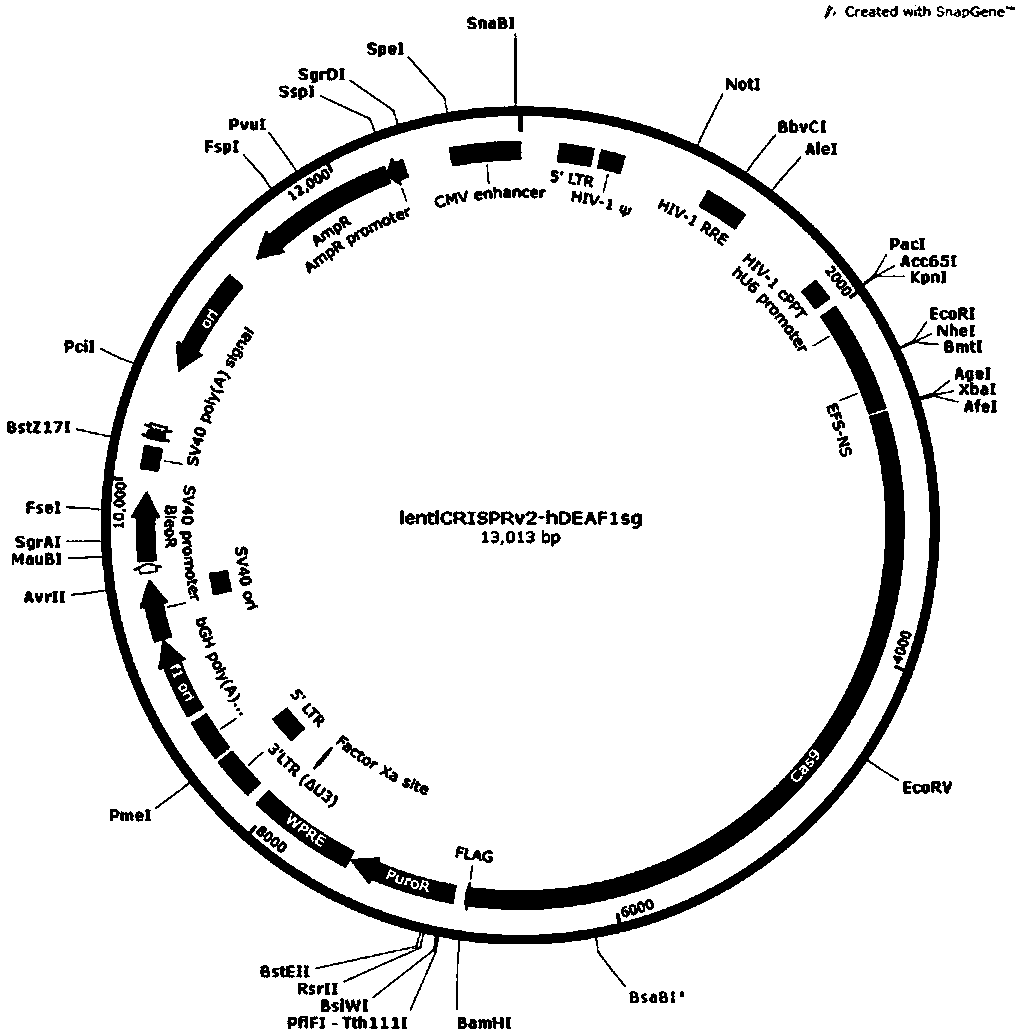
CRISPR-Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene and specific sgRNA thereof
InactiveCN108396027AConvenient researchGenetically modified cellsStable introduction of DNAVector systemLentivirus
The invention discloses a CRISPR / Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene and specific sgRNA thereof. The CRISPR / Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene and the specific sgRNA thereof are characterized in that: firstly, sgRNA of a second exon of the specific targeted DEAF1 gene is obtained and the base sequence of the sgRNA is shown as SEQ ID NO.1; secondly, the sgRNA of the DEAF1 gene is constructed into a lentiviral vector system, which contains Cas9 protein; finally, the CRISPR / Cas9 lentivirus containing the sgRNA is infected with human colorectal carcinoma cell HT-29 cell, so that a cell strain of which DEAF1 protein expression level is obviously reduced is obtained. The CRISPR / Cas9 targeted knockout human colorectal carcinoma cell DEAF1 gene disclosed by the invention has the advantages of simple operation steps, good sgRNA target ability and high cutting efficiency for the DEAF1 gene; in addition, the constructed CRISPR / Cas9 lentiviral vector system has the advantage of high knockout efficiency and can specifically knock out the DEAF1 gene to obtain the human colorectal carcinoma cells knocking out the DEAF1 gene, and therebya powerful tool is provided for further studying an action mechanism of DEAF1 in the colorectal carcinoma cells.
Owner:OBIO TECH SHANGHAI CORP LTD
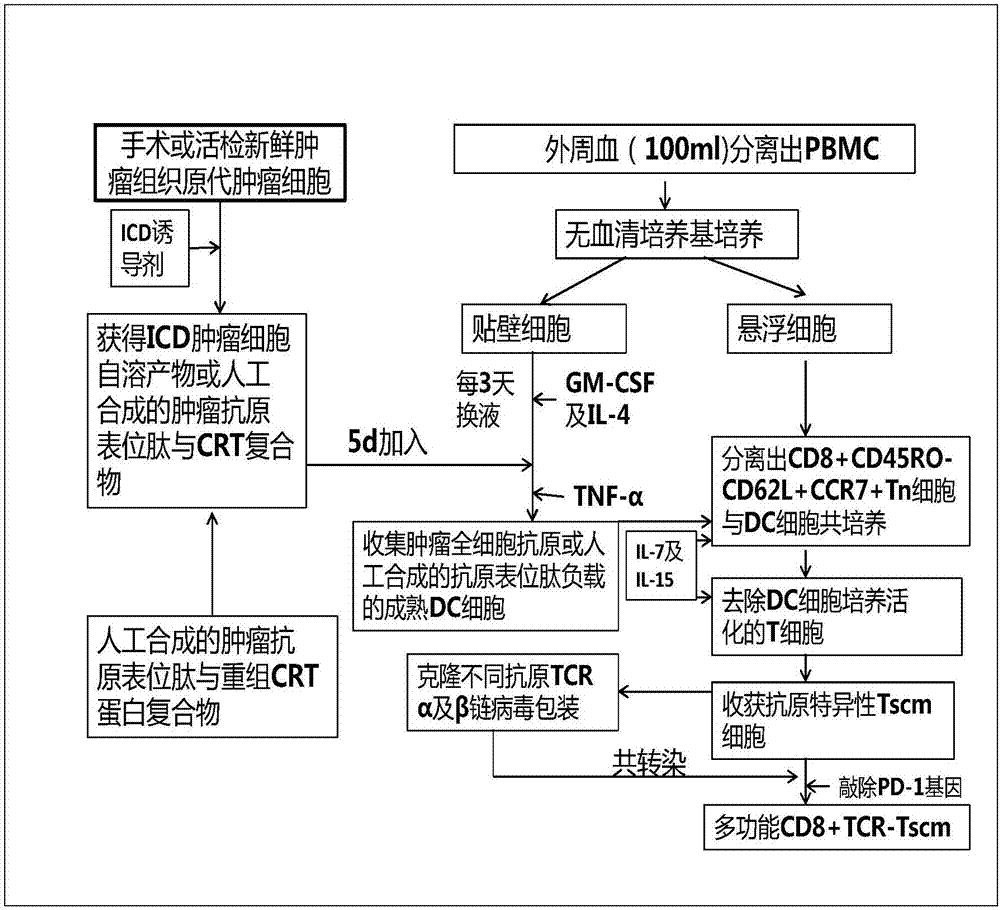
Preparation method and use of TCR gene modified CD8+T memory stem cell
InactiveCN107541498AOvercoming heterogeneityIncrease intakeMammal material medical ingredientsAntineoplastic agentsCell specificDendritic cell
The invention belongs to the technical fields of immunology and tumor therapy, and relates to a preparation method and a use of a TCR gene modified CD8+T memory stem cell. The method comprises the following steps: co-incubating a tumor antigen and an immature dendritic cell to obtain a specific tumor antigen-supported mature dendritic cell, co-culturing the specific tumor antigen-supported maturedendritic cell and a CD8+ naive T cell, and adding a stem cell differentiation inhibitor to promote the generation of a CD8+ stem cell-like memory T lymphocyte; separating the CD8+ stem cell-like memory T lymphocyte; and cloning a T cell receptor gene (TCR) for specifically identifying a specific antigenic epitope, and carrying out lentivirus packaging or retroviral vector cotransfection on an autologous CD8+ Tscm cell to in-vitro prepare a CD8+ TCR-Tscm cell specific for different tumor specific antigens. The CD8+ TCR-Tscm cell prepared by the method has the advantages of overcoming of the tumor heterogeneity, high specificity, few adverse reactions, and highly-efficient and lasting tumor preventing and treating effects.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
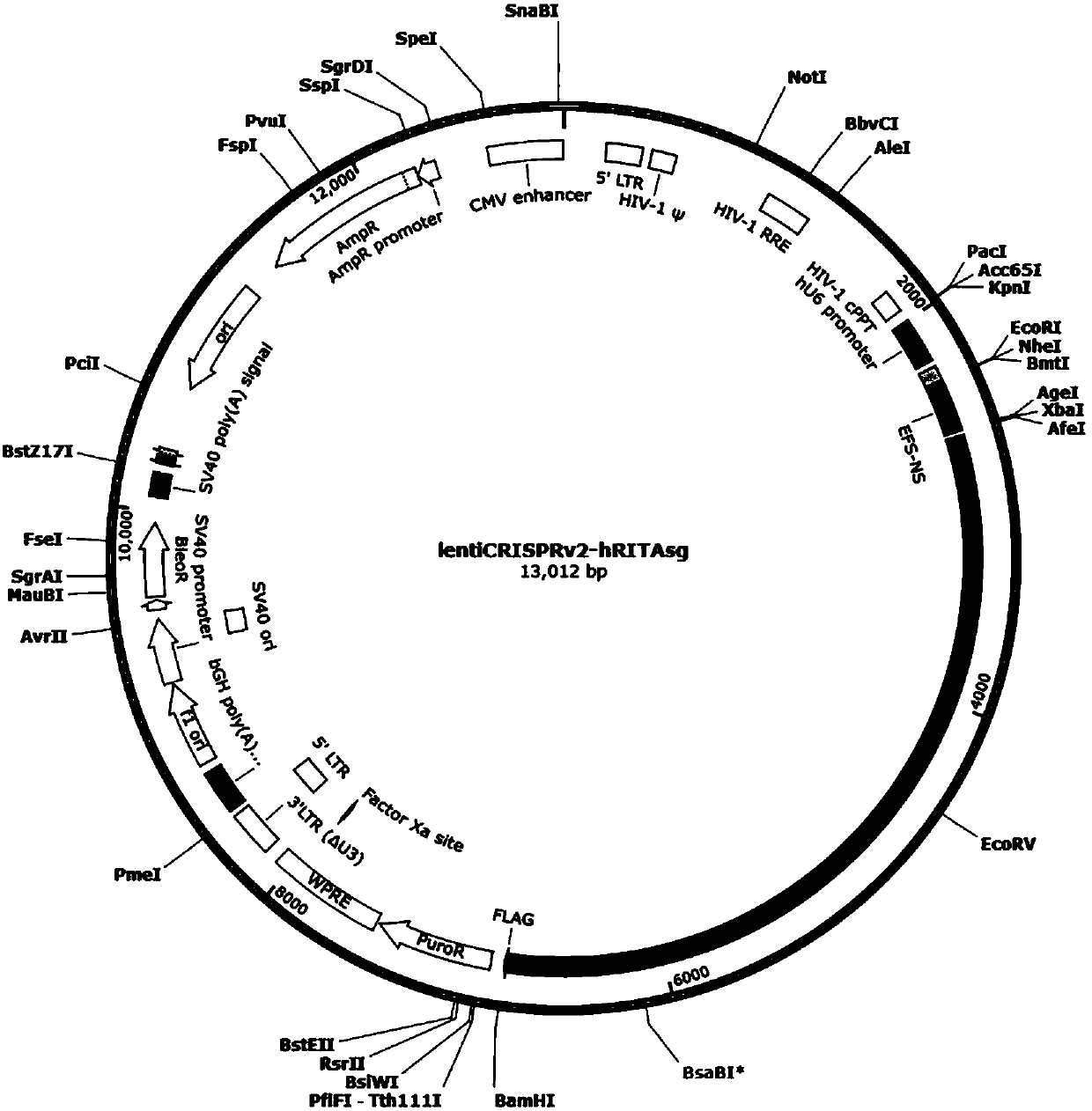
CRISPR/Cas9 targeted knockout human intestinal cancer cell RITA gene and specific sgRNA thereof
InactiveCN107893075AConvenient researchGenetically modified cellsNucleic acid vectorIntestinal CancerLentivirus
The present invention discloses CRISPR / Cas9 targeted knockout human intestinal cancer cell RITA gene and specific sgRNA thereof. First, sgRNA specifically targeted to a second exon of the RITA gene isobtained, and the base sequence of the sgRNA is as shown in SEQ ID NO. 1; secondly, a sgRNA lentiviral vector system of the RITA gene is constructed, and the sgRNA lentiviral vector system contains Cas9 protein; and finally human intestinal cancer HT-29 cells are infected with CRISPR / Cas9 lentivirus containing the sgRNA to obtain a cell line which is significantly reduced in RITA protein expression level. The invention has the advantages of simple operation steps, good sgRNA targeting property, and high RITA gene cutting efficiency; furthermore, the constructed CRISPR / Cas9 lentivirus system has the advantage of high knock-out efficiency and can specifically knock out the RITA gene to obtain RITA gene-knockout human intestinal cancer cells, and a powerful tool for the further study of theaction mechanism of RITA in the intestinal cancer cells is provided.
Owner:OBIO TECH SHANGHAI CORP LTD
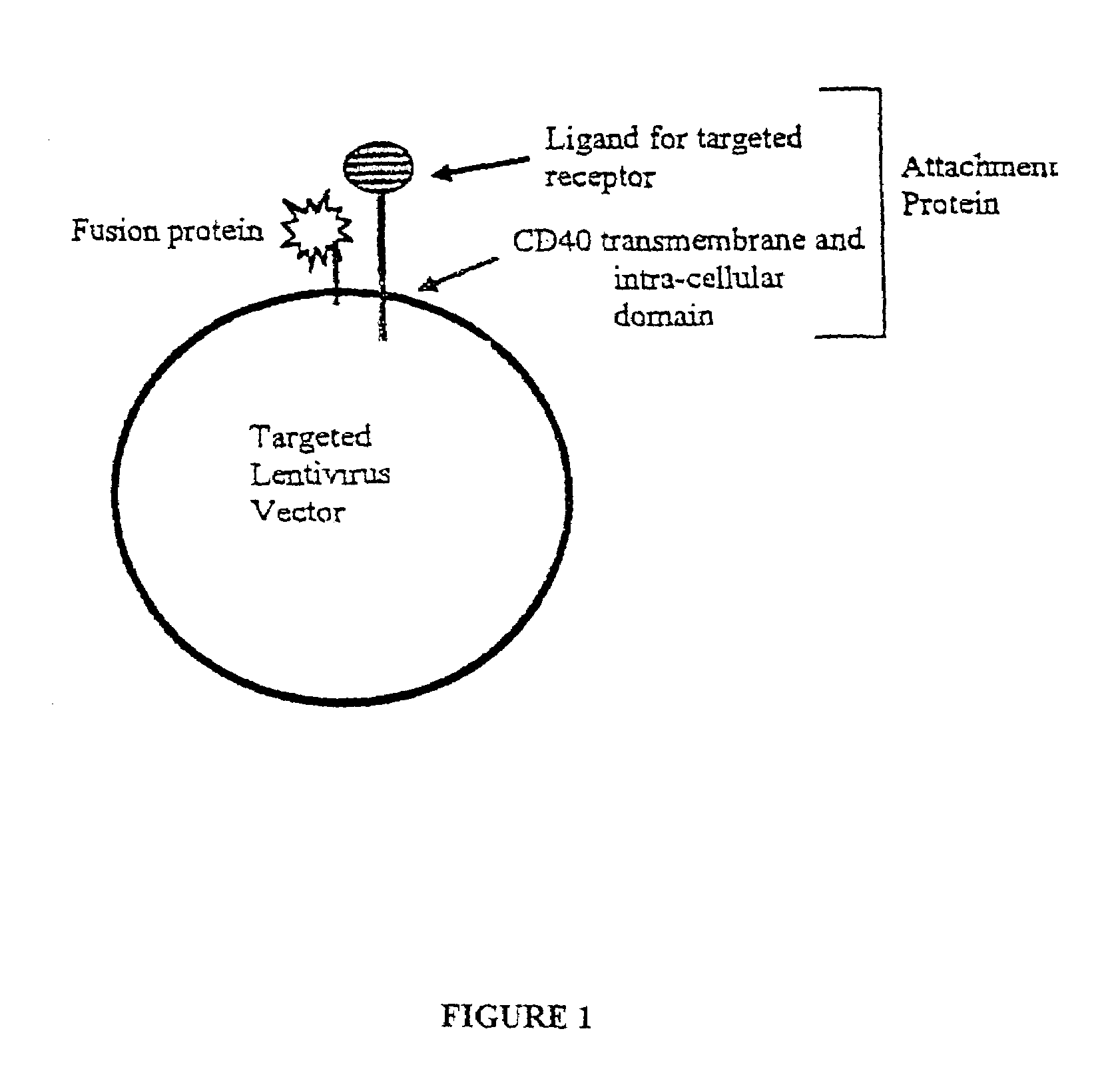
Compositions and methods for tissue specific targeting of lentivirus vectors
This disclosure provides lentiviral vectors containing an attachment incompetent fusogenic polypeptide and a heterologous targeting polypeptide. Also provided are lentiviral packaging constructs, lentiviral packaging systems, and lentiviral gene delivery systems. Finally, methods of transducing a cell and methods of targeting a gene to a cell or tissue using the disclosed lentiviral vectors and systems are also provided.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Stabilized primate lentivirus envelope glycoproteins
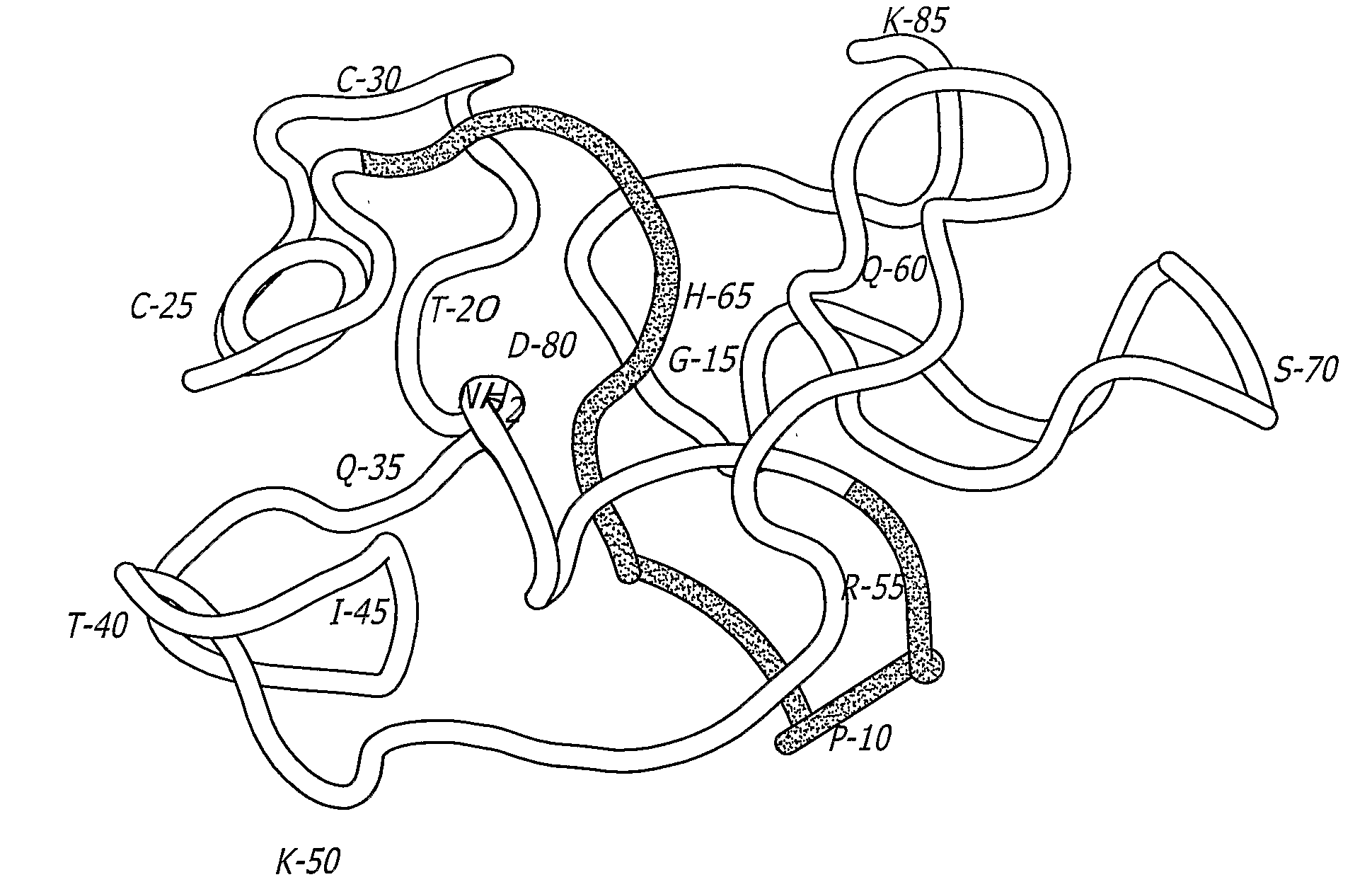
InactiveUS7048929B1Improve stabilityImproving immunogenicityAntibody mimetics/scaffoldsVirus peptidesEnv GlycoproteinsDisulfide bond
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has cysteine amino acid residues introduced to create disulfide bonds, a cavity is filled with hydrophobic amino acids, a Proresidue is introduced at a defined turn structure of the protein, or the hydrophobicity is increased across the interface between different domains, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has more than one of those characteristics. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp120.
Owner:DANA FARBER CANCER INST INC +1
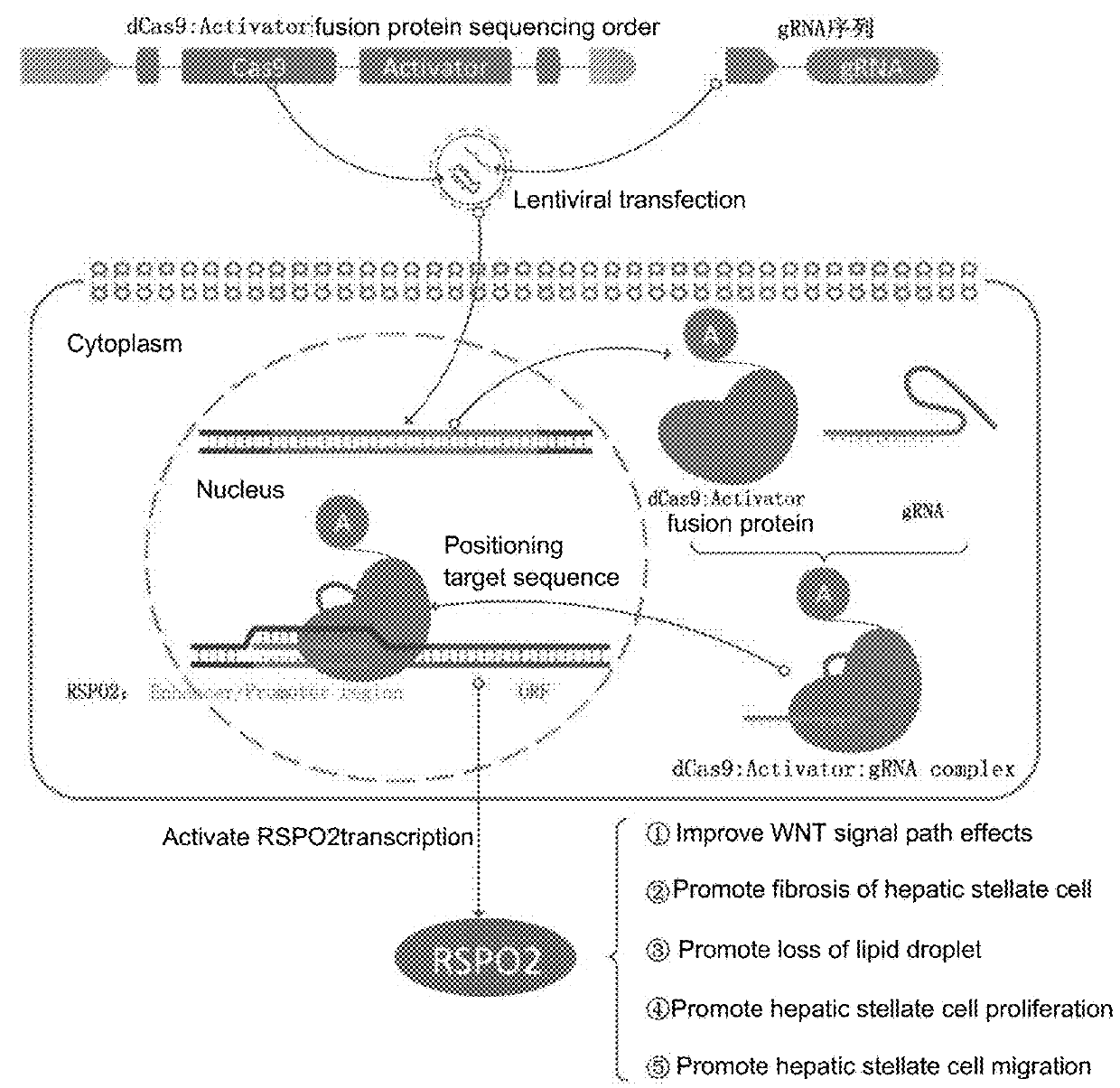
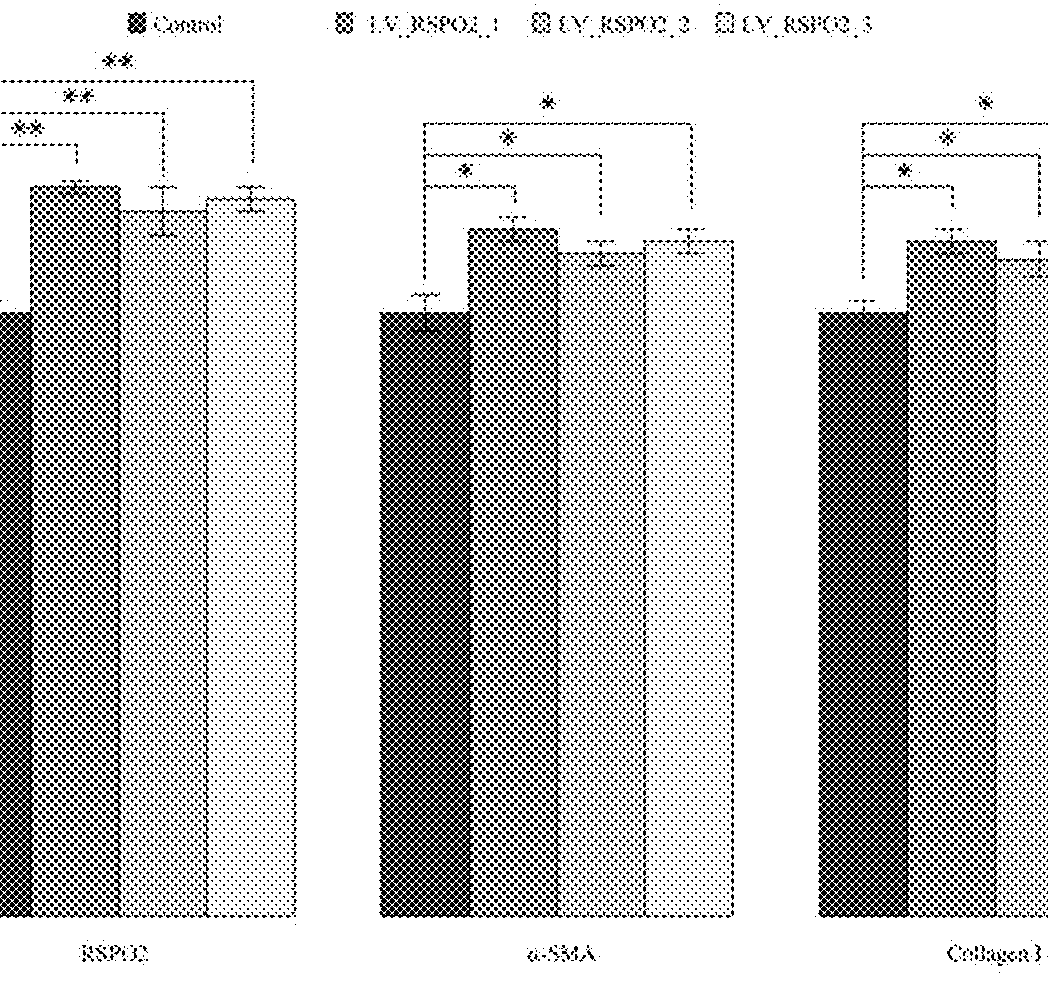
sgRNA and method for specifically activating human RSPO2 gene with CRISPR-Cas9 and application thereof
A method of constructing a specific CRISPR-Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats associated) to activate a RSPO2 (R-spondin 2) gene is disclosed in the present invention. The method comprises the following steps: designing a sgRNA (single guide RNA) of a specifically targeted human RSPO2 gene; constructing a CRISPR-Cas9 recombinant lentivirus vector of a specifically activated RSPO2 gene; and lentiviral packaging a CRISPR-Cas9 system of the specifically activated RSPO2 gene. The CRISPR-Cas9 system designed by the present invention activates the RSPO2 target gene expression and promotes the activation of the hepatic stellate cell.
Owner:JIAXING NO 1 HOSPITAL
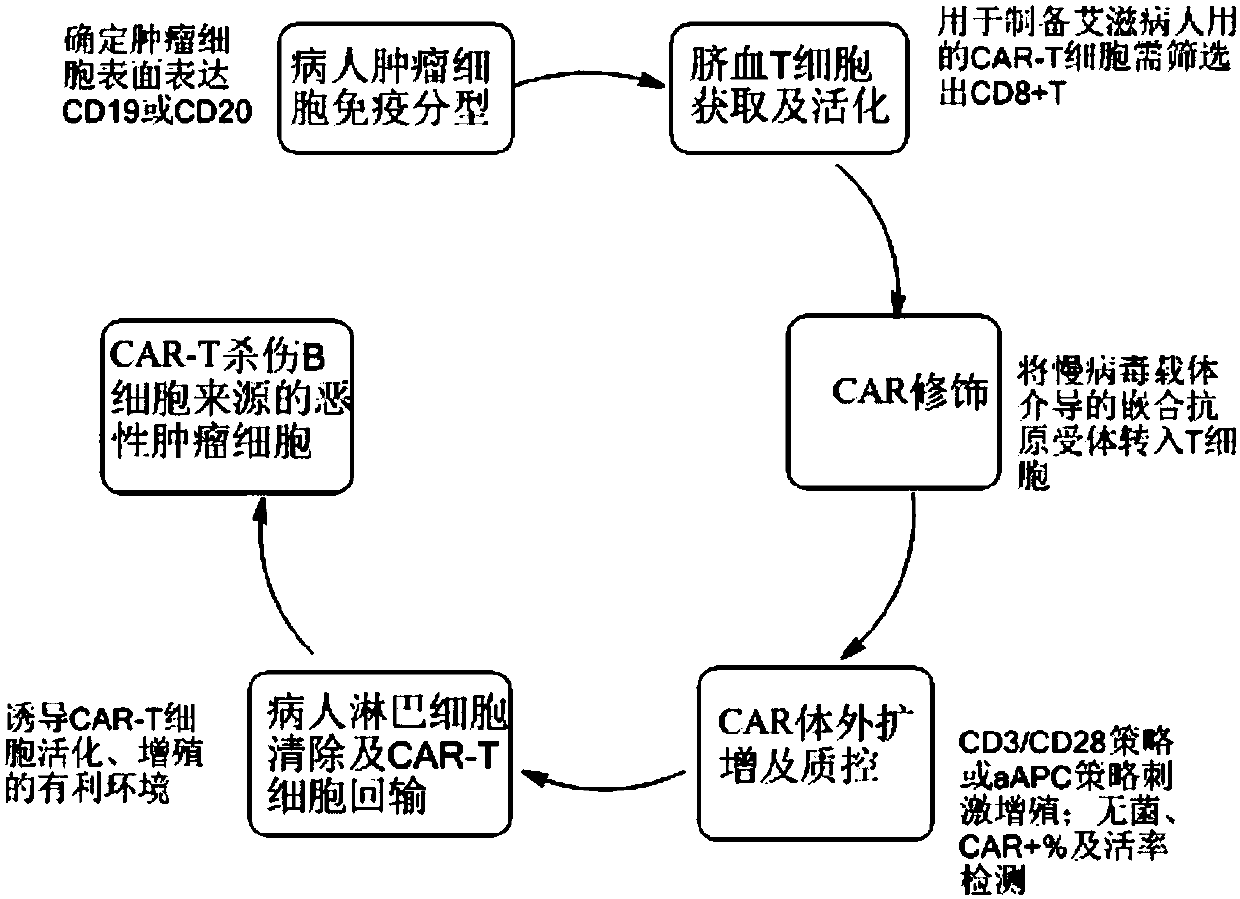
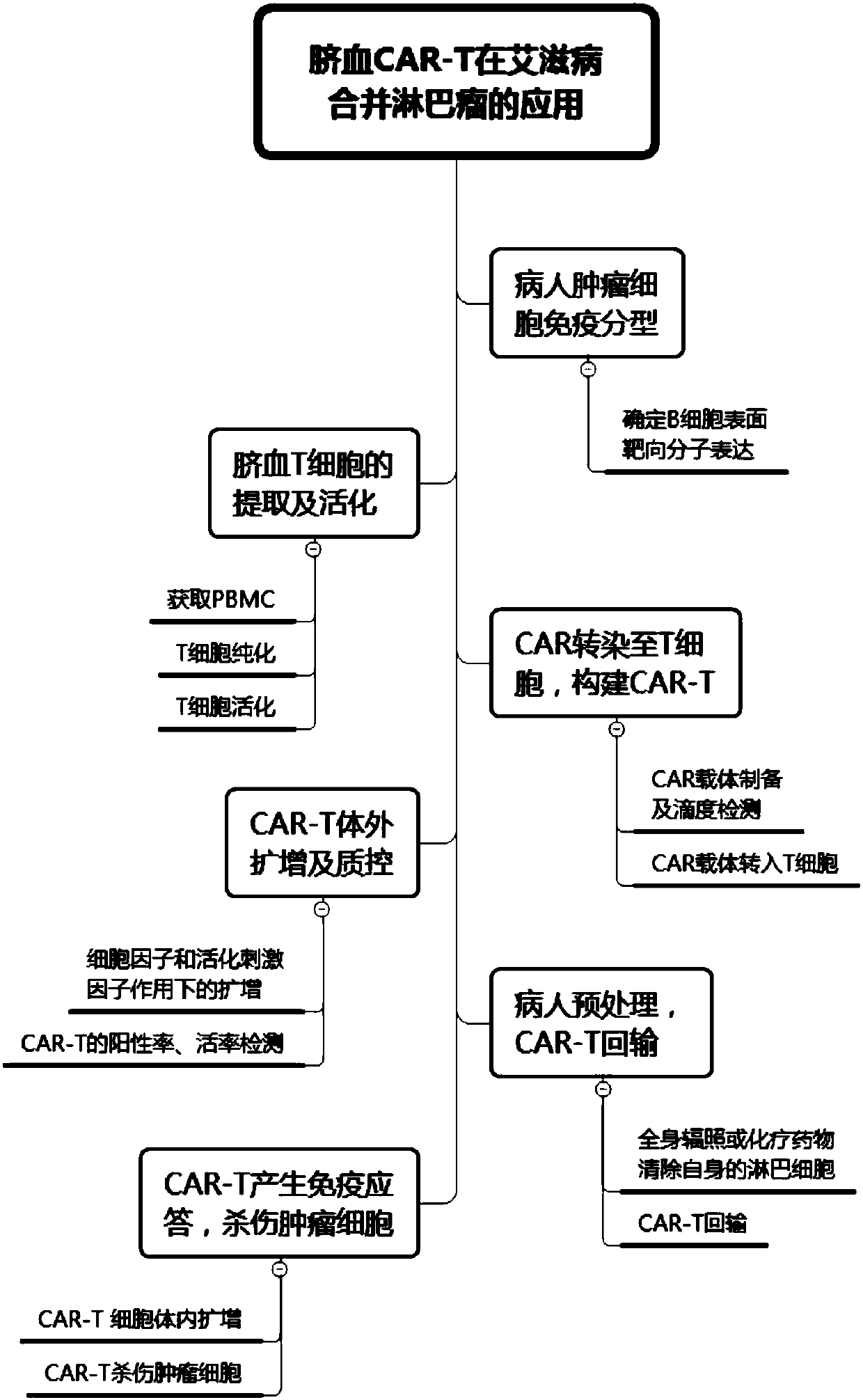
CAR-T cell for treating AIDS-associated lymphoma, and preparation method and application thereof
PendingCN107904259ALow storm riskSmall storm riskGenetically modified cellsAntiviralsAIDS-related lymphomaCord blood stem cell
The invention discloses a preparation method of a CAR-T cell for treating AIDS-related lymphoma. A CD8+ T cell is used to produce the CAR-T cell, and the CD8-T cell derives from a cord blood T cell. The method includes the following steps: preparing cord blood mononuclear cells from cord blood, removing tumors and other cells by using a human T cell purification kit to obtain T cells, and carryingout separation by using magnetic beads to obtain the CD8+ T cell; activating the CD8+ T cell by using an appropriate medium and appropriate stimulation conditions; transferring CAR to the CD8+ T cellby using lentivirus to prepare the CAR-T cell; and amplifying the CAR-T cell in vitro by using cytokines and activating stimulators to achieve the desired effective dose. The invention also relates to the CAR-T cell prepared by the method, and an application thereof. The CAR-T cell prepared in the invention has a good cell activity and a high proliferation speed, and reduces the attack risk of GVHD; and only the CD8+T cell of the umbilical blood T cells of AIDS patients is used to prepare the CAR-T cell for, so the risk of input CAR-T infected with in-vivo HIV is reduced.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Construction and application of farnesyl pyrophosphoric acid synthetase RNA (Ribonucleic Acid) interference recombinant lentivirus vector
InactiveCN101805750AOvercoming No Commercial AntibodyOvercoming low transfection efficiencyMetabolism disorderGenetic material ingredientsDiseaseFhit gene
The invention provides the construction for a farnesyl pyrophosphoric acid synthetase RNA (Ribonucleic Acid) interference recombinant lentivirus vector, which comprises the following steps of: sieving the most effective target sequence of an FDS (farnesyl diphosphate synthase) gene RNAi (RNA interference) in a tool cell 293T cell, synthesizing the double-stranded DNA of the most effective target sequence, connecting to a pGCSIL-GFP vector and successfully constructing the recombinant vector through enzyme cutting, sequencing and identification. Researches indicate that the constructed RNA interference vector LV-sh-FDS can downwards modulate the expression of an FDS mRNA (Messenger RNA) level in a neonatal rat cardiac myocyte, simultaneously can downwards modulate the expression of myocardial hypertrophy markers such as cell areas and marker genes beta-MHC (Myosin Heavy Chain) and BNP (Brain Natriuretic Peptide), additionally can effectively inhabit the activity of RhoA while downwards modulating the FDS, can be applied in preparing medicaments for treating myocardial hypertrophy diseases and also can be applied in preparing medicaments for cholesterol metabolic control.
Owner:ZHEJIANG UNIV
CRISPR-Cas9 for targeting knockout of human intestinal cancer cell CNR1 gene, and specific sgRNA thereof
InactiveCN108588071AFacilitate the study of the mechanism of actionStable introduction of DNAFermentationIntestinal CancerLentivirus
The invention discloses a CRISPR-Cas9 for targeting knockout of a human intestinal cancer cell CNR1 gene, and specific sgRNA sequences thereof. Firstly, an sgRNA specifically targeting CNR1 gene second exon is obtained, and base sequences of the sgRNA are as shown in SEQ ID NO. 1; secondly, the sgRNA of the CNR1 gene is constructed to a lentiviral vector system, and the lentiviral vector system contains Cas9 protein; and finally, a CRISPR / Cas9 lentivirus containing the sgRNA is infected with human intestinal cancer HT-29 cells, and a cell strain with CNR1 protein expression level significantlybeing reduced is obtained. The operation steps are simple, good sgRNA targeting is achieved, and the cutting efficiency to the CNR1 gene is high; and in addition, the constructed CRISPR / Cas9 lentiviral system has the advantage of high knockout efficiency, and can specifically knock out the CNR1 gene, human intestinal cancer cells with the CNR1 gene being knocked out is obtained, and therefore a powerful tool for further study of the mechanism of action of CNR1 in intestinal cancer cells is provided.
Owner:OBIO TECH SHANGHAI CORP LTD
Lentivirus based vector and vector system
InactiveUS20020123471A1Broaden spectrumEfficient infectionBiocideNervous disorderVector systemLentivirus
The present invention relates to retroviral vectors which will infect and confer efficient gene transfer to non-dividing cells including the cells of the central nervous system. The vector system of the present invention is useful as a gene transfer vehicle for gene therapy, i.e. of the central nervous system.
Owner:BAVARIAN NORDIC RES INST AS
Method for expression of small antiviral RNA molecules with reduced cytotoxicity within a cell
Owner:CALIFORNIA INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com