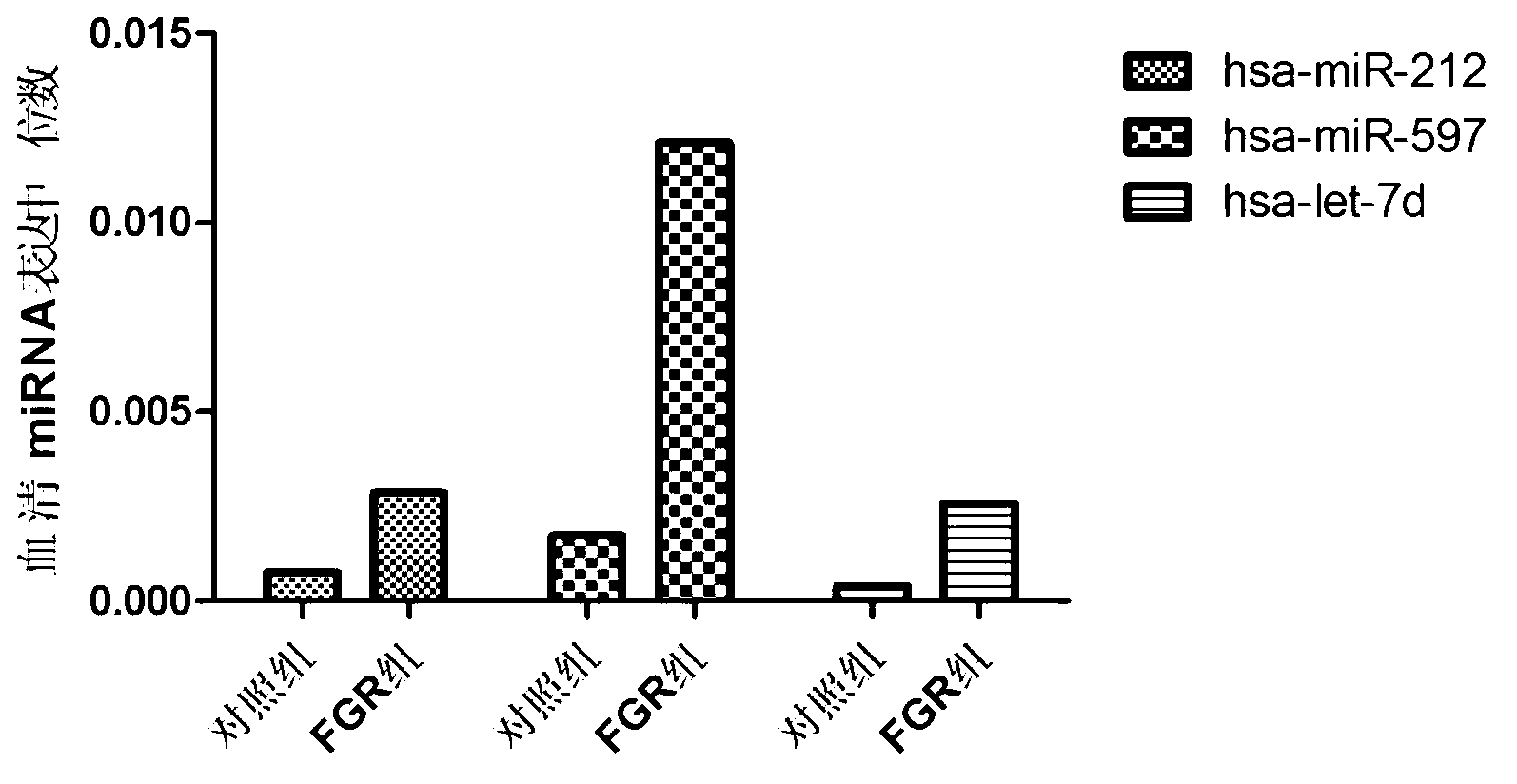Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63 results about "Fetal growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
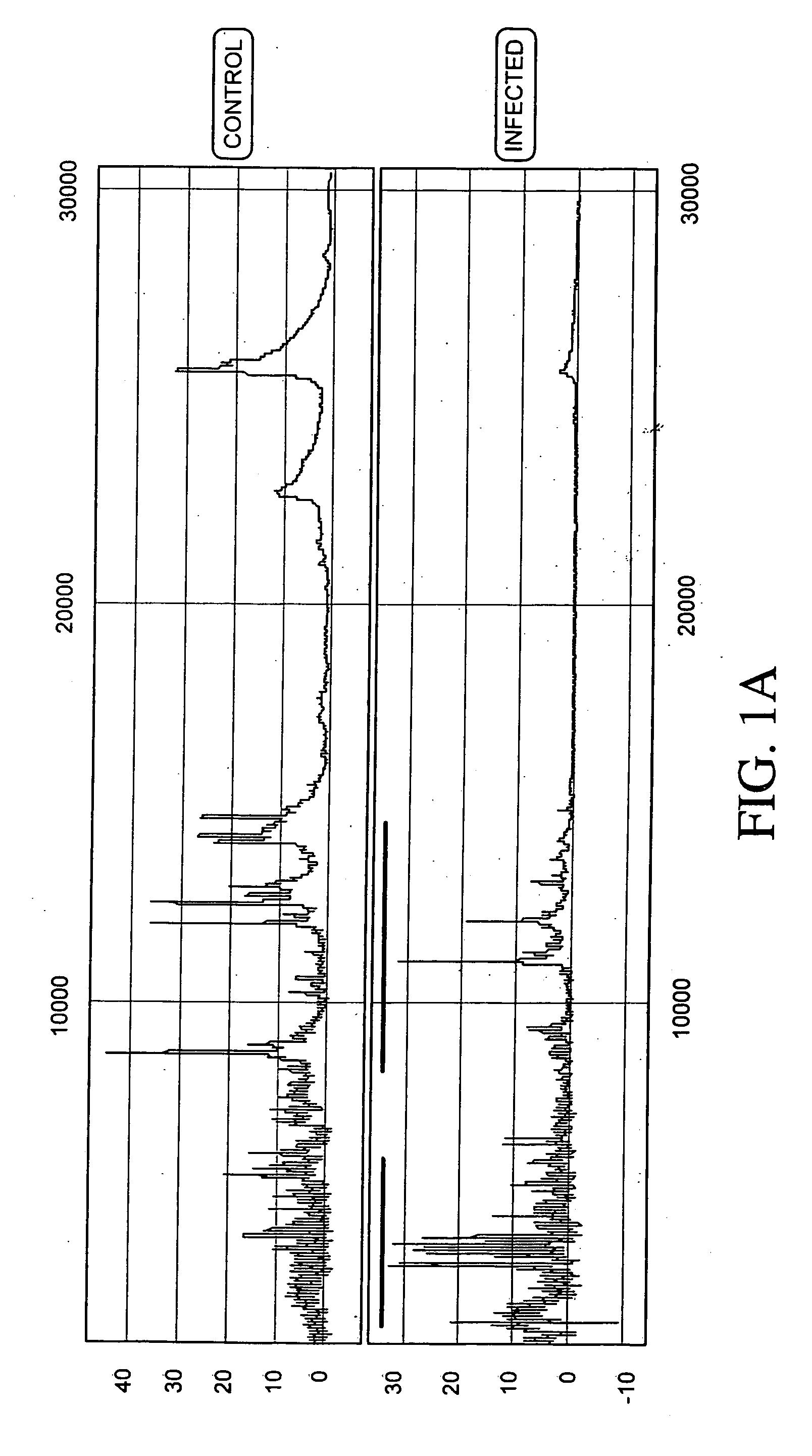
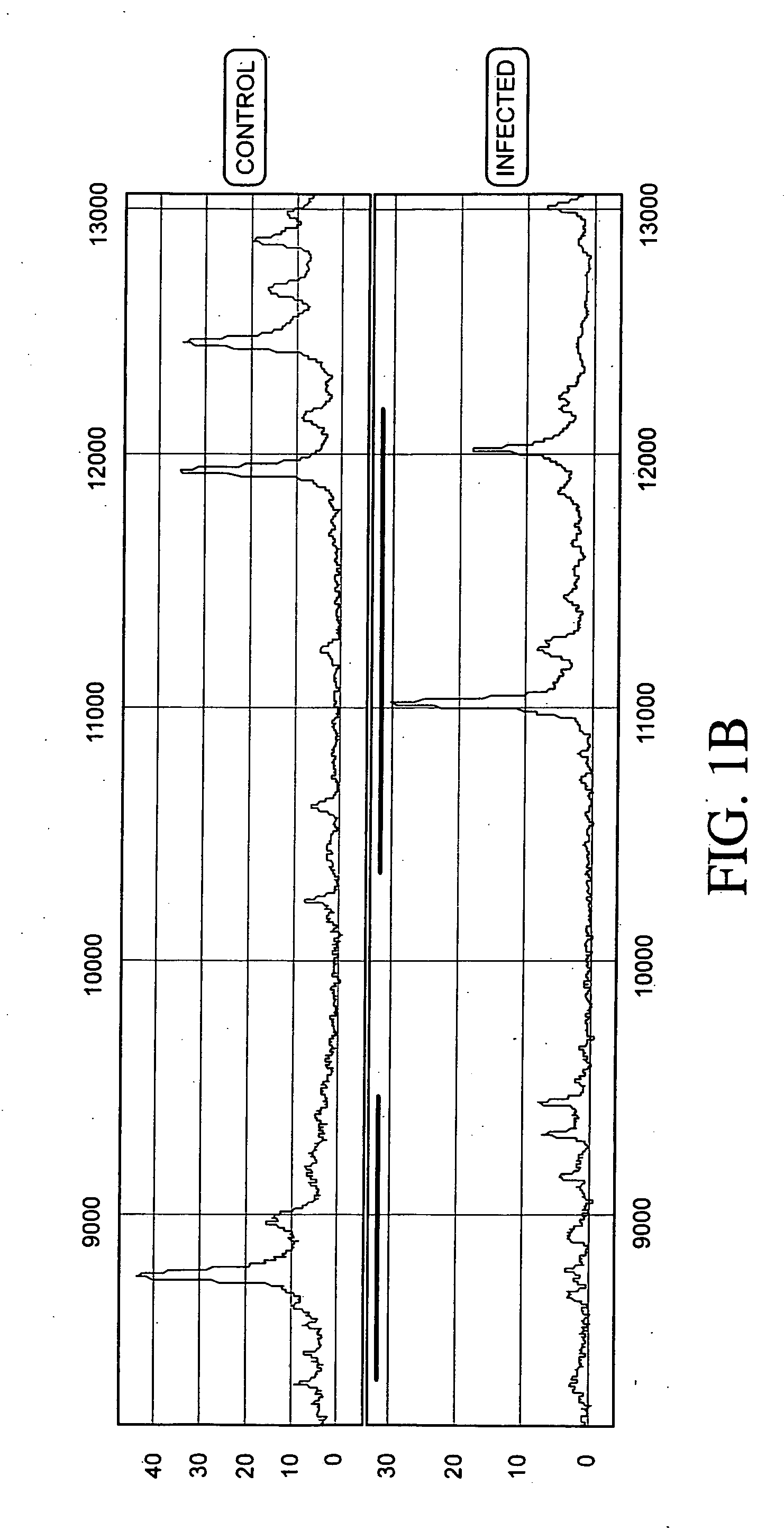
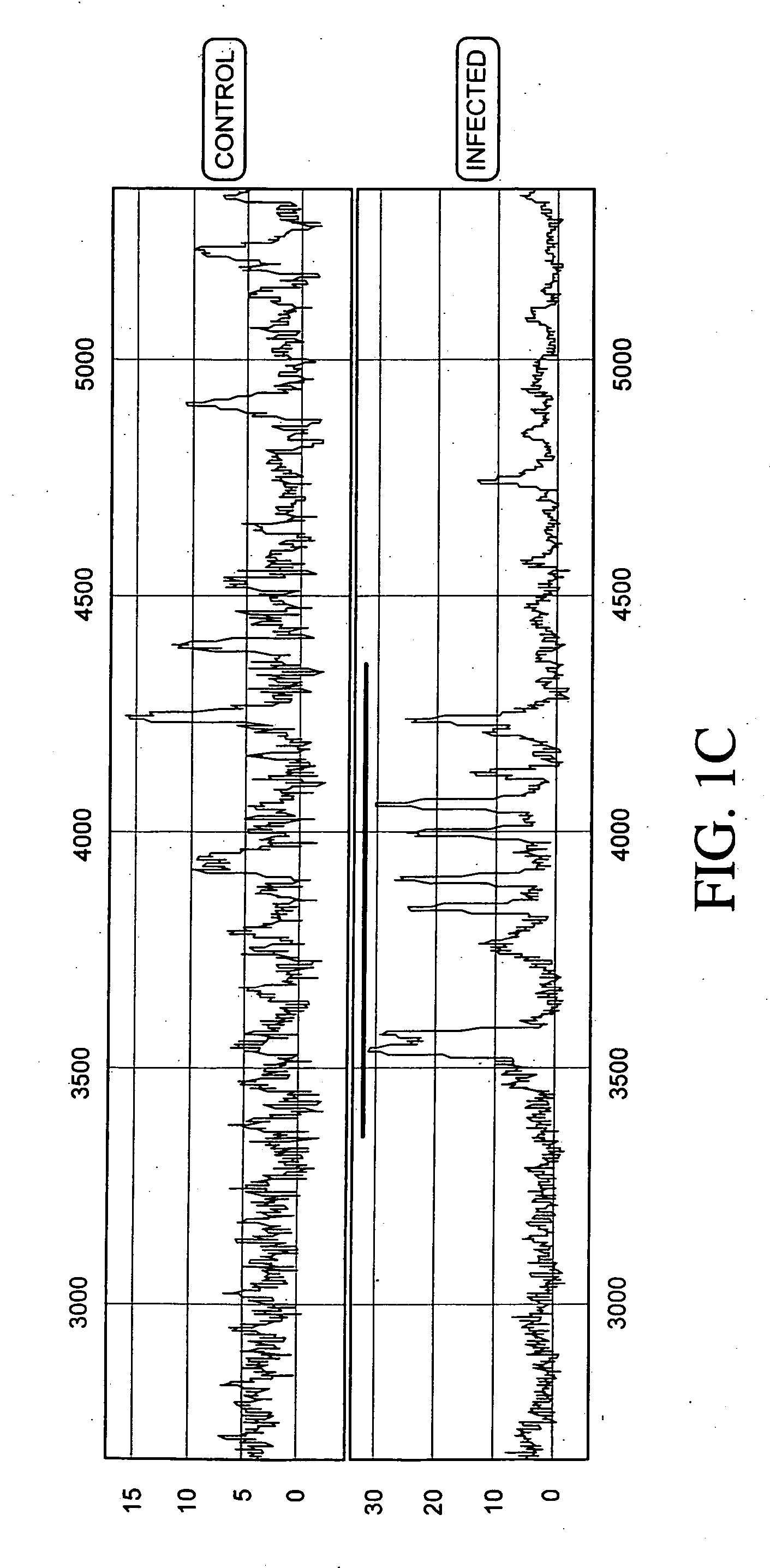
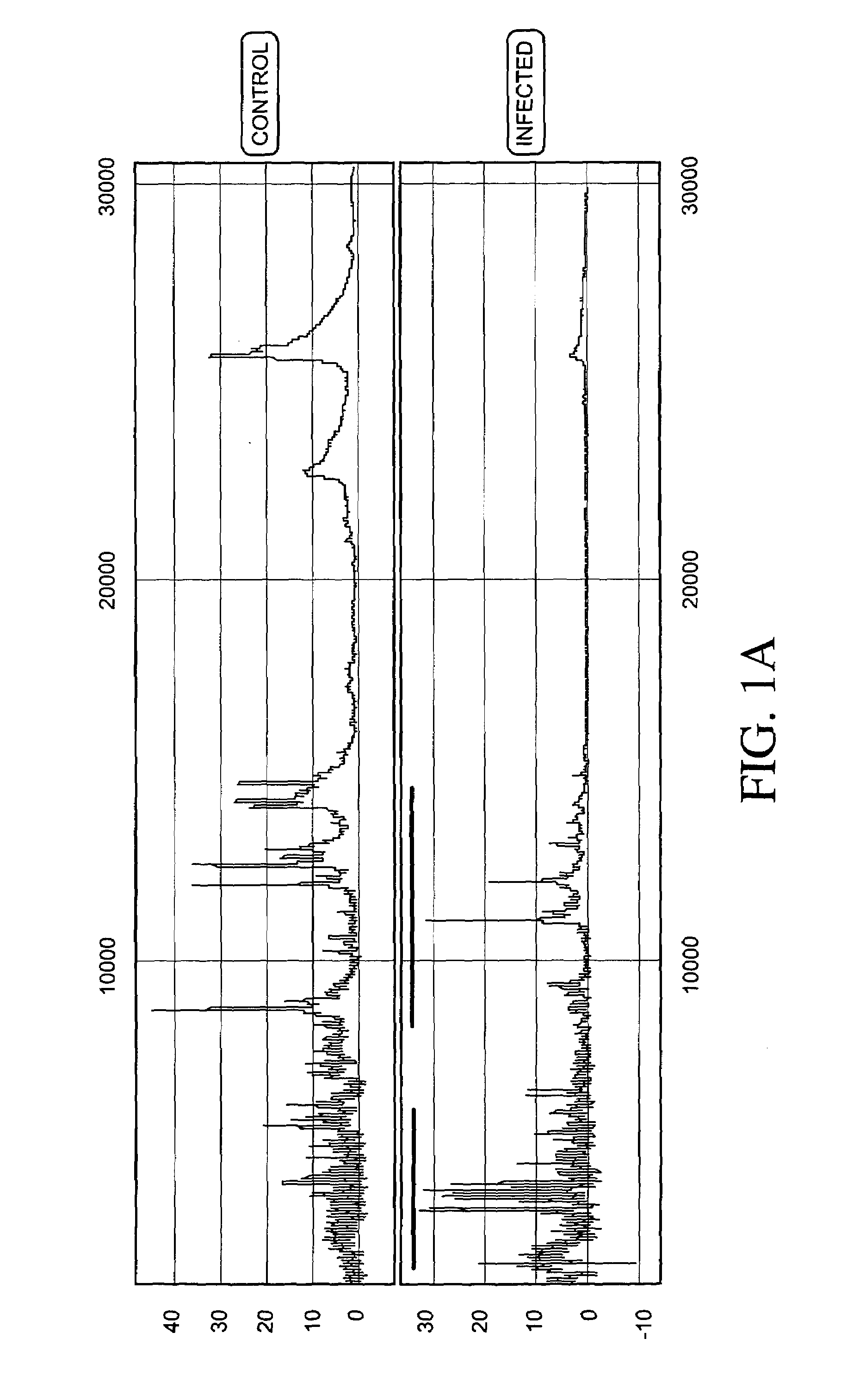
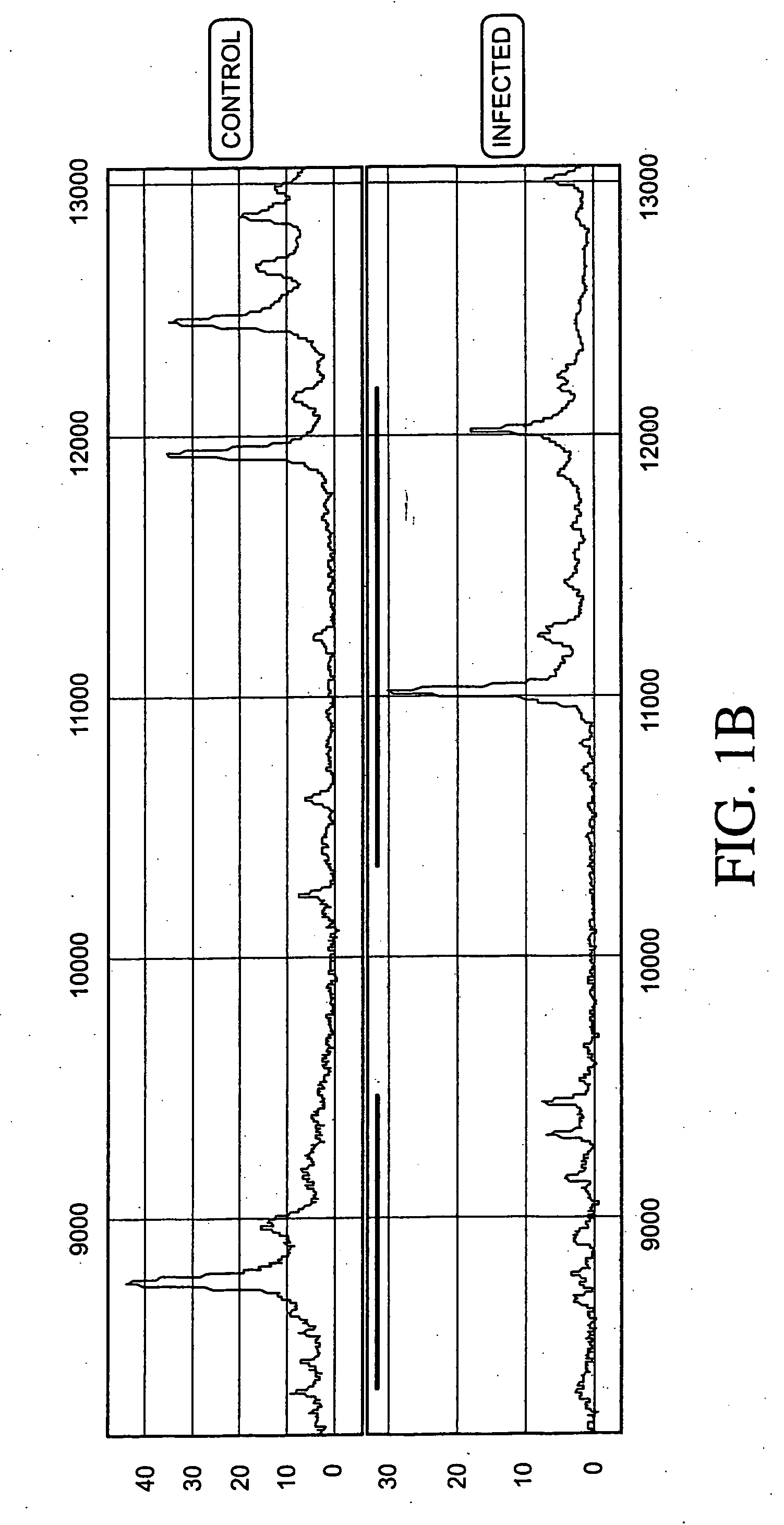
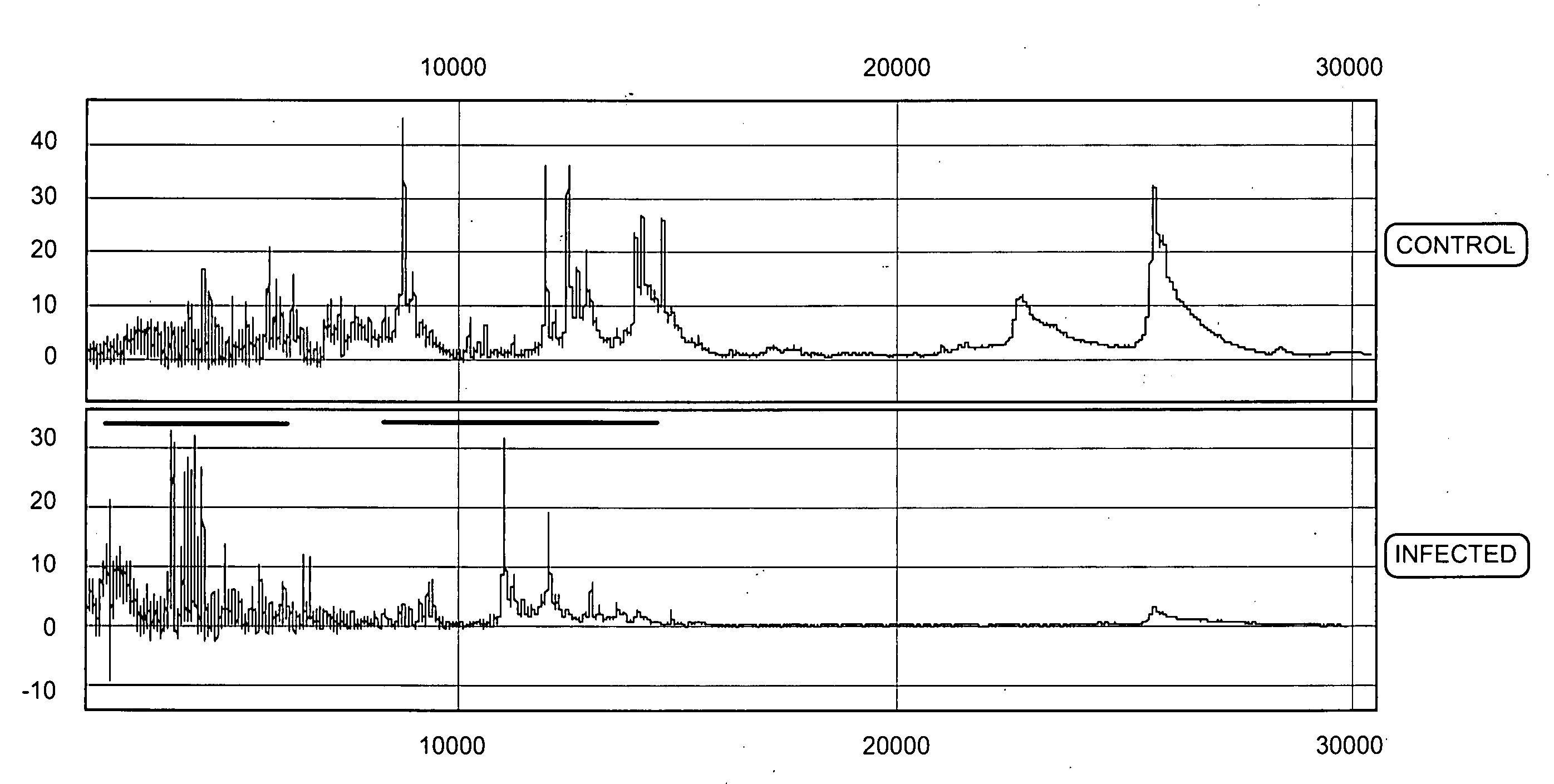
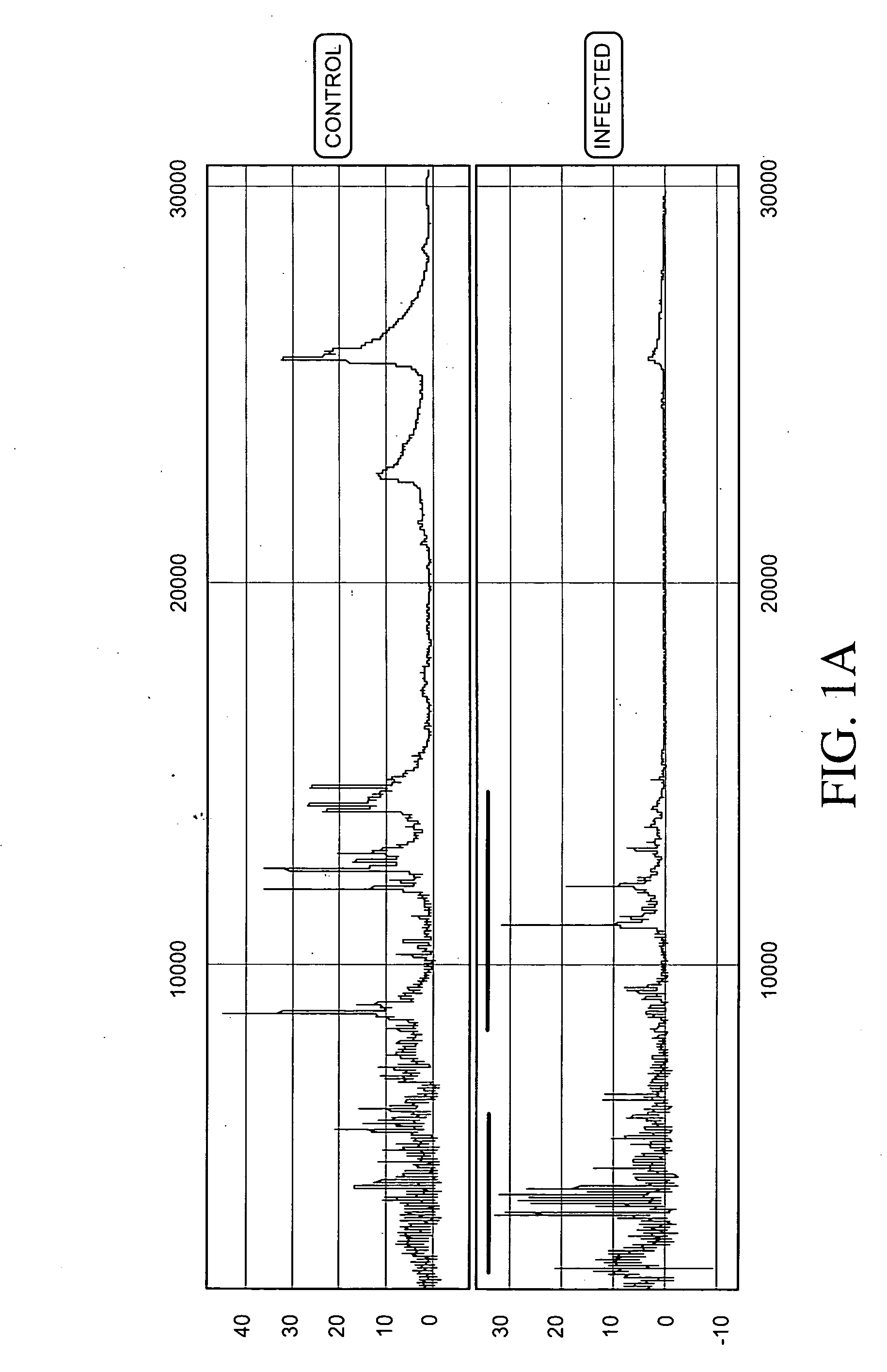
Proteomic analysis of biological fluids
ActiveUS20070161125A1Eliminate needMicrobiological testing/measurementAnalogue computers for chemical processesDiseaseGynecology
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns a comprehensive proteomic analysis of human amniotic fluid (AF) and cervical vaginal fluid (CVF), and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, pre-term labor, and / or chromosomal defects. The invention further concerns the identification of biomarkers and groups of biomarkers that can be used for non-invasive diagnosis of various pregnancy-related disorders, and diagnostic assays using such biomarkers.
Owner:HOLOGIC INC
Proteomic analysis of biological fluids
ActiveUS7191068B2Eliminate needMicrobiological testing/measurementBiological testingDiseaseFetal growth
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns the identification of the proteome of amniotic fluid (multiple proteins representing the composition of amniotic fluid) and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, or chromosomal defects.
Owner:HOLOGIC INC +1
gRNA target sequences for endogenous overexpression of 1ncRNA-XIST and application thereof
ActiveCN107513531AImprove efficiencyFermentationVector-based foreign material introductionFetal growthCell migration
The invention discloses gRNA target sequences for endogenous overexpression of 1ncRNA-XIST, a CRISPR / dCas9 lentivirus system and application thereof. The gRNA target sequences are respectively as shown in SED ID No. 1, SED ID No. 2 and SED ID No. 3. The CRISPR / dCas9 lentivirus system comprises the gRNA target sequences for endogenous overexpression of 1ncRNA-XIST. A method for screening stable strains according to the characteristics that lentivirus must be integrated into a host genome is cooperated with CRISPR / dCas9 to realize the endogenous overexpression of the large fragment gene 1ncRNA-XIST, so that the defect of incapability of stably expressing the large fragment gene 1ncRNA-XIST in a traditional method is overcome, the efficient overexpression stable cell strain of the large fragment gene 1ncRNA-XIST can be obtained in a short time, or cells obtained by screening can stably express target genes, thereby obtaining stably silenced 1ncRNA-XIST downstream specific gene cell strain. The gRNA target sequences and the CRISPR / dCas9 lentivirus system have important guiding significance on the research of the function of 1ncRNA-XIST in the trophocyte migration and the effect of 1ncRNA-XIST in the proliferation disorder and fetal growth restriction process.
Owner:WUXI MATERNAL & CHILD HEALTH HOSPITAL
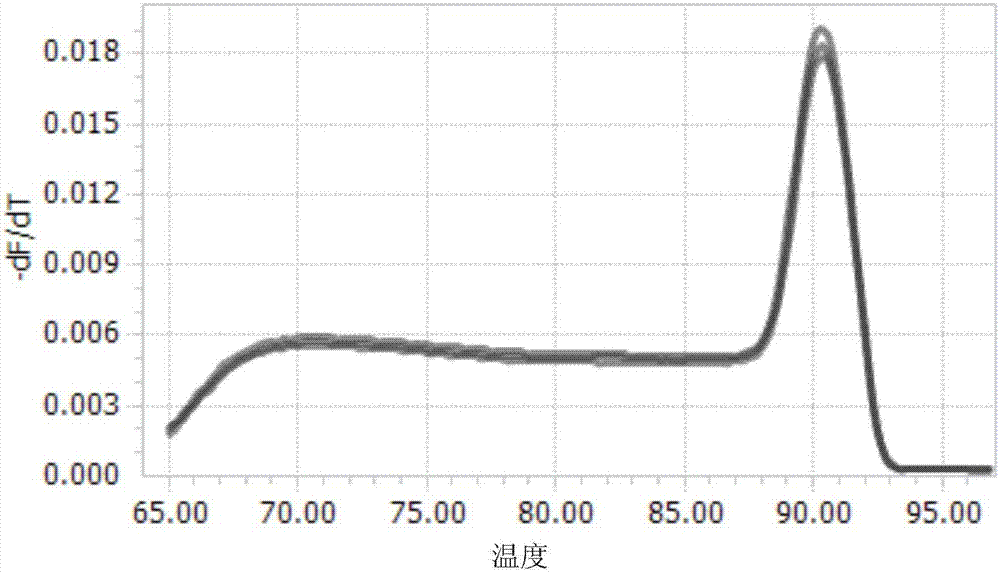
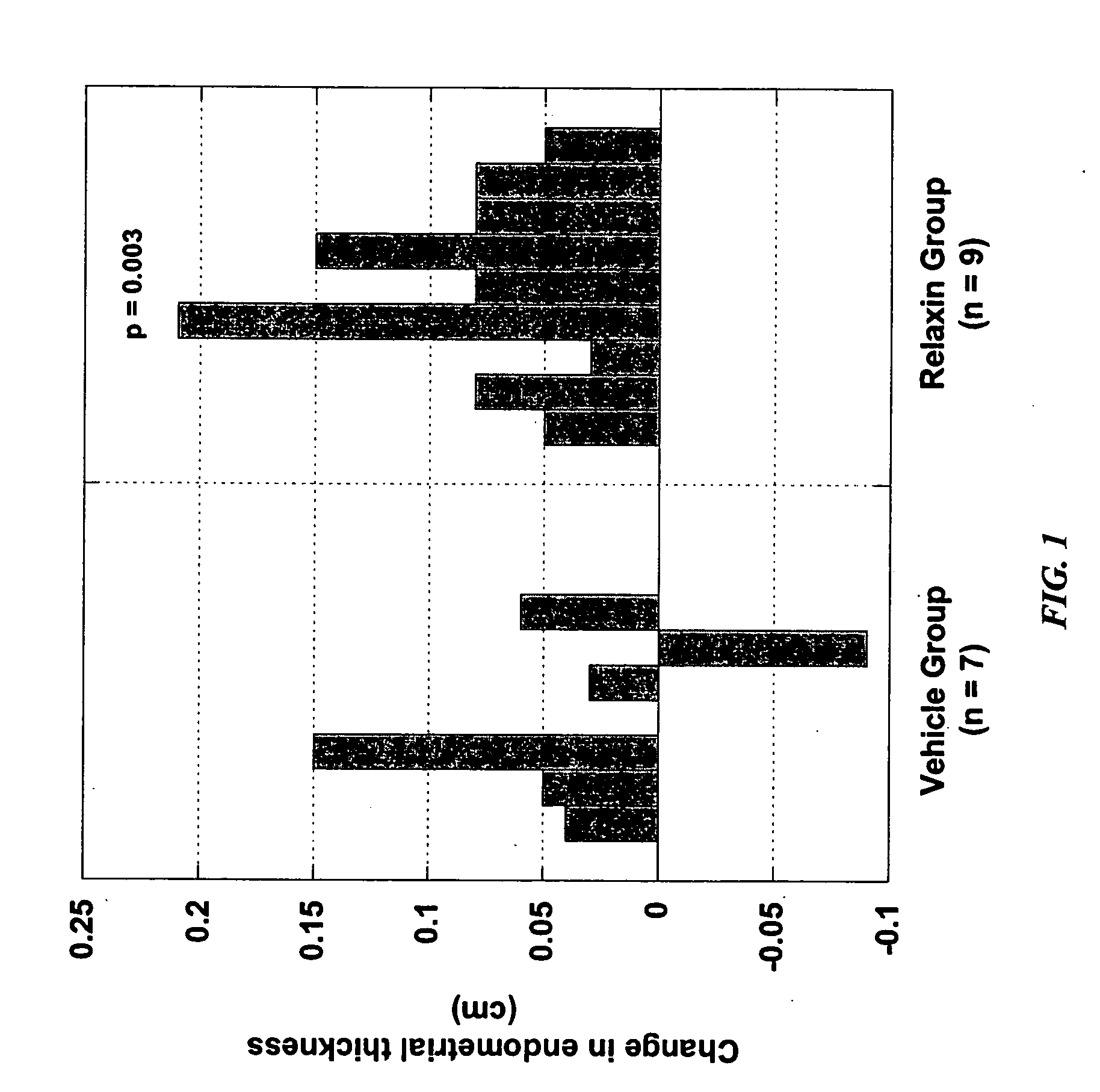
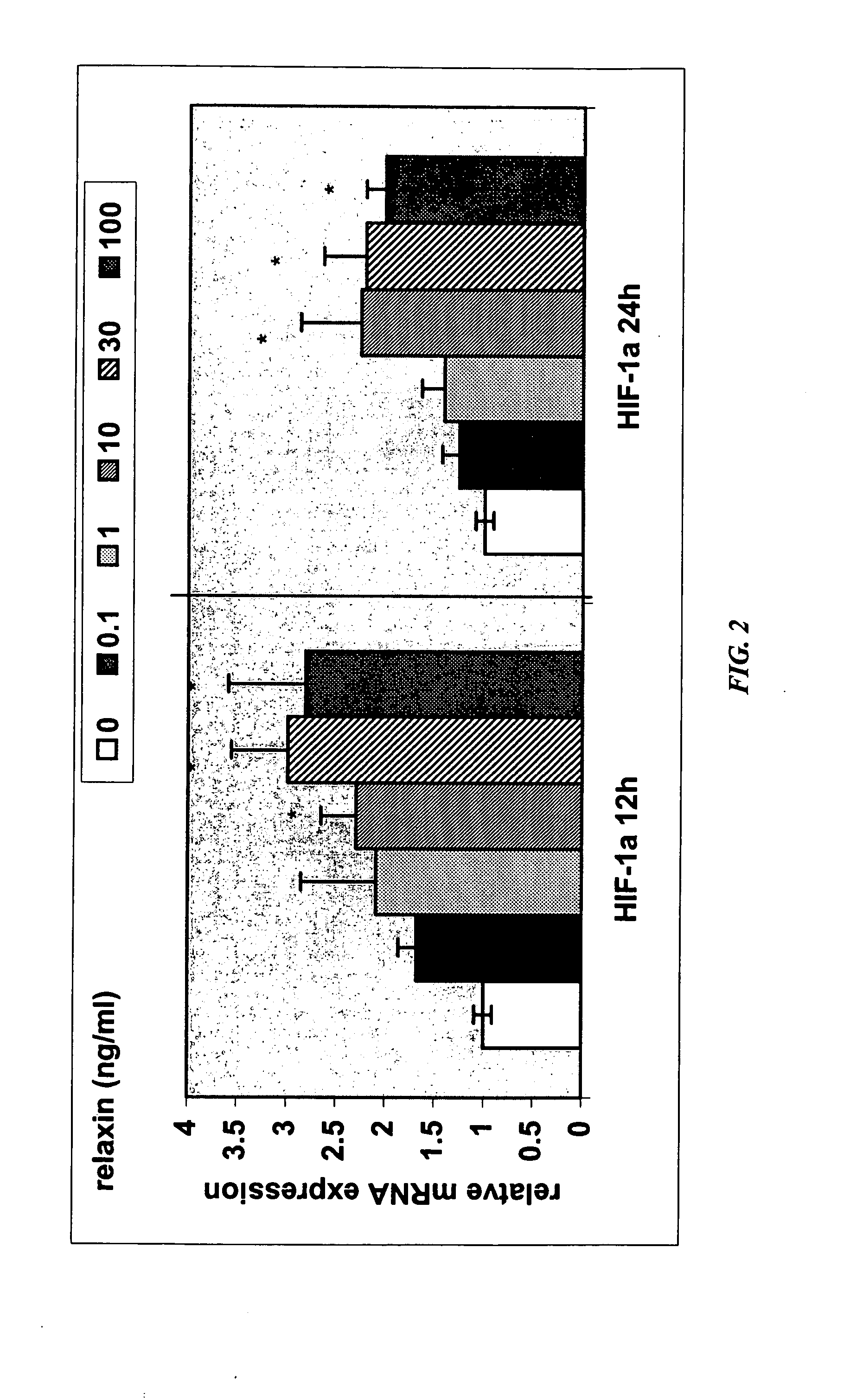
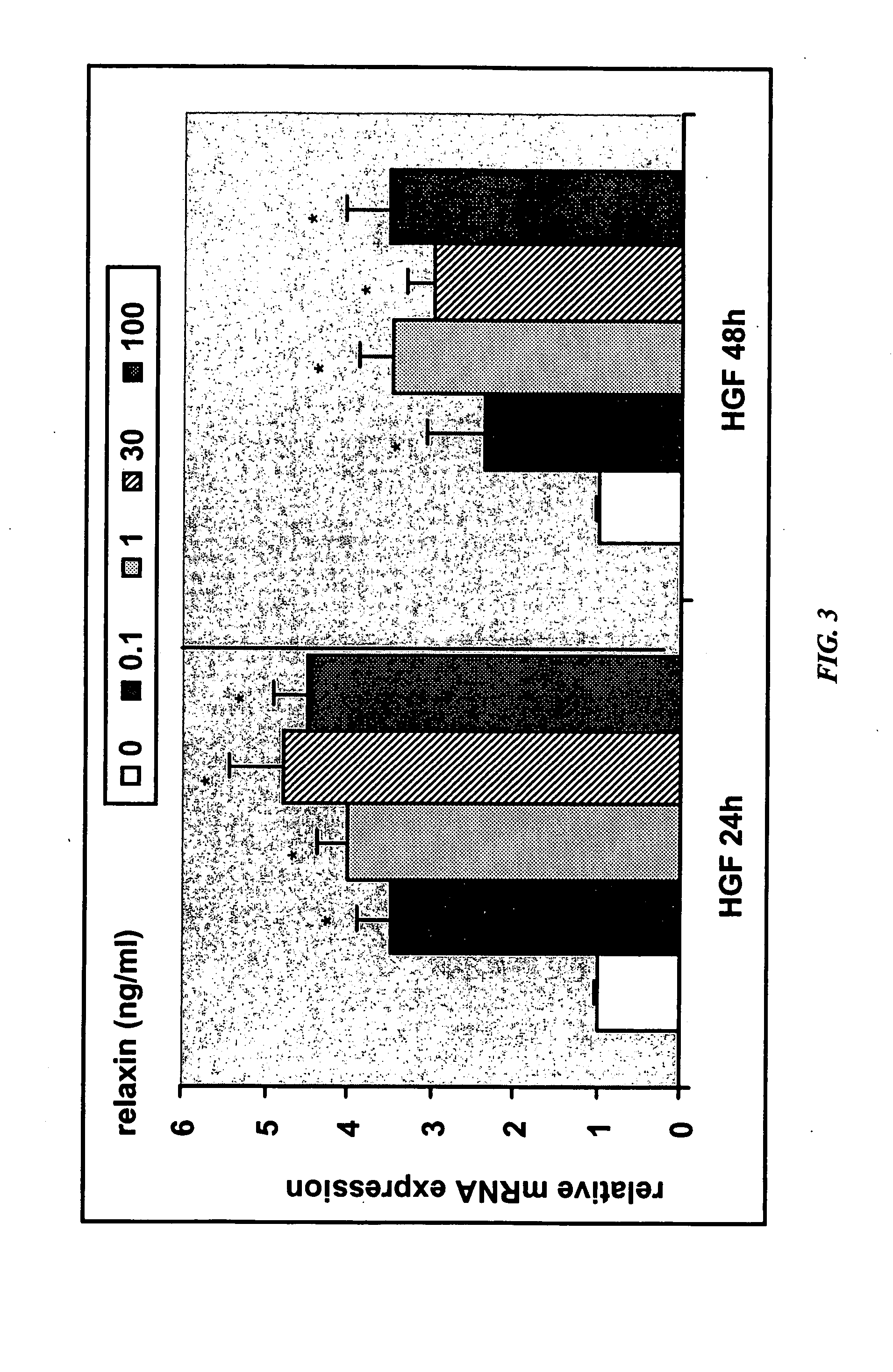
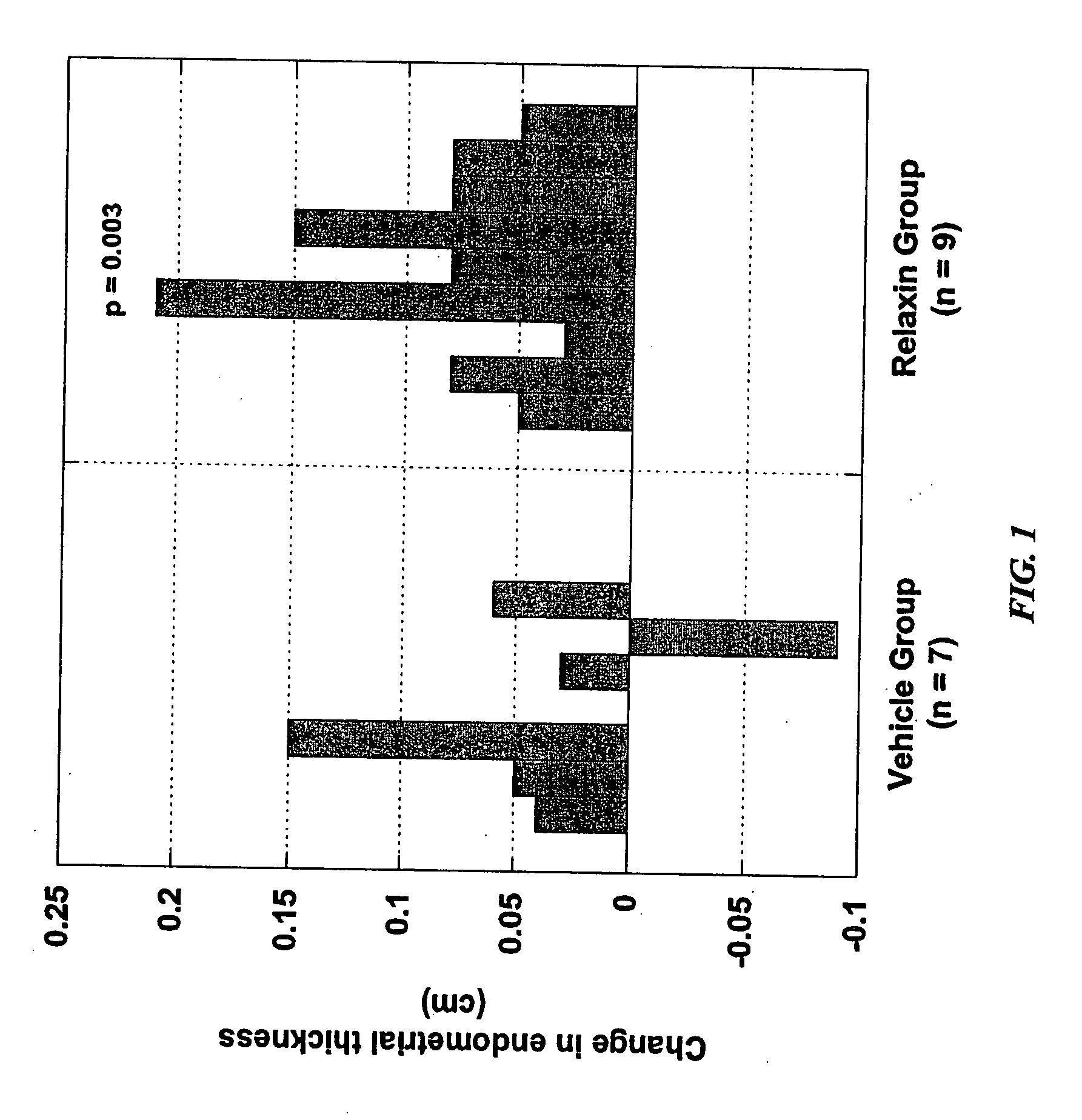
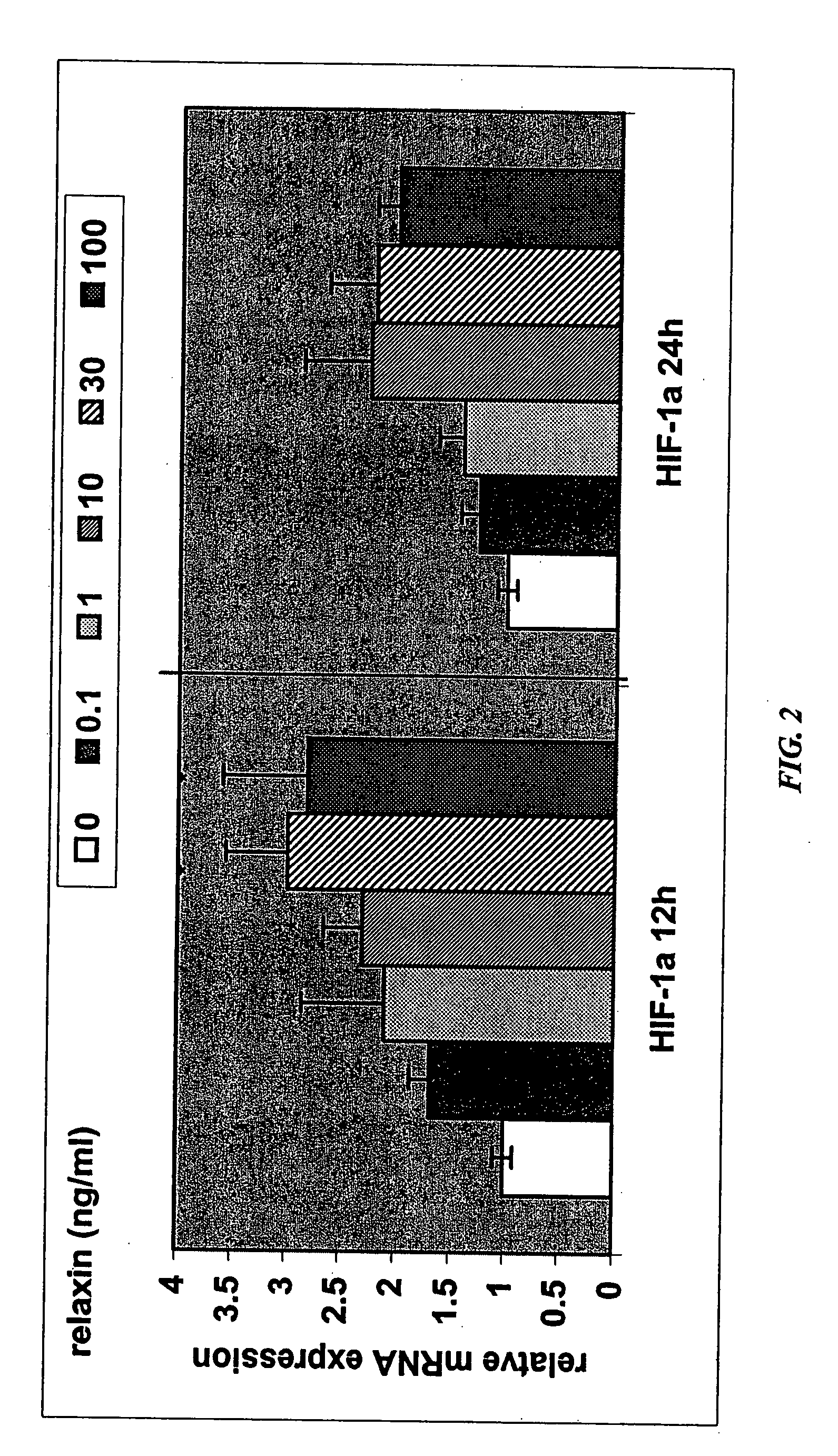
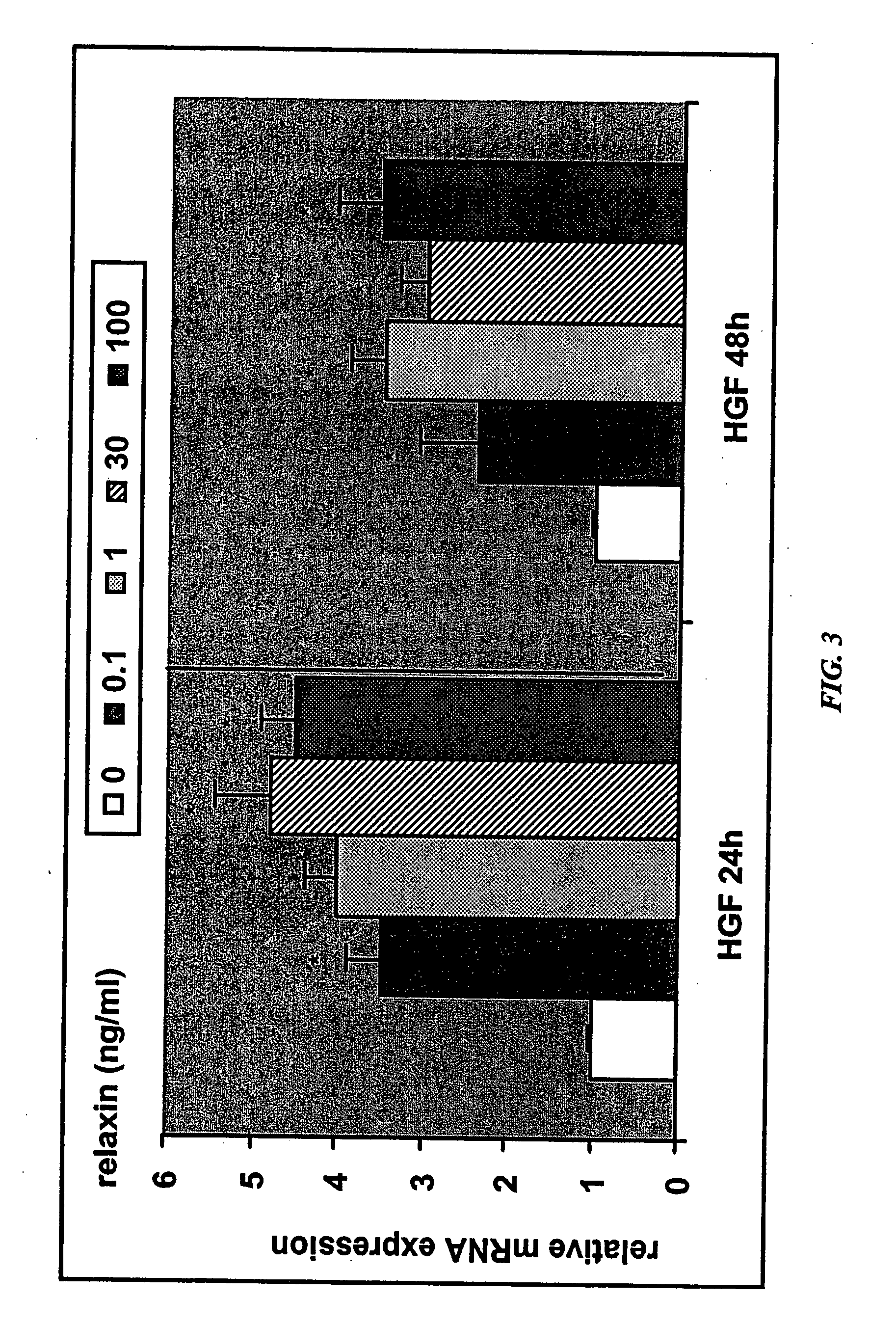
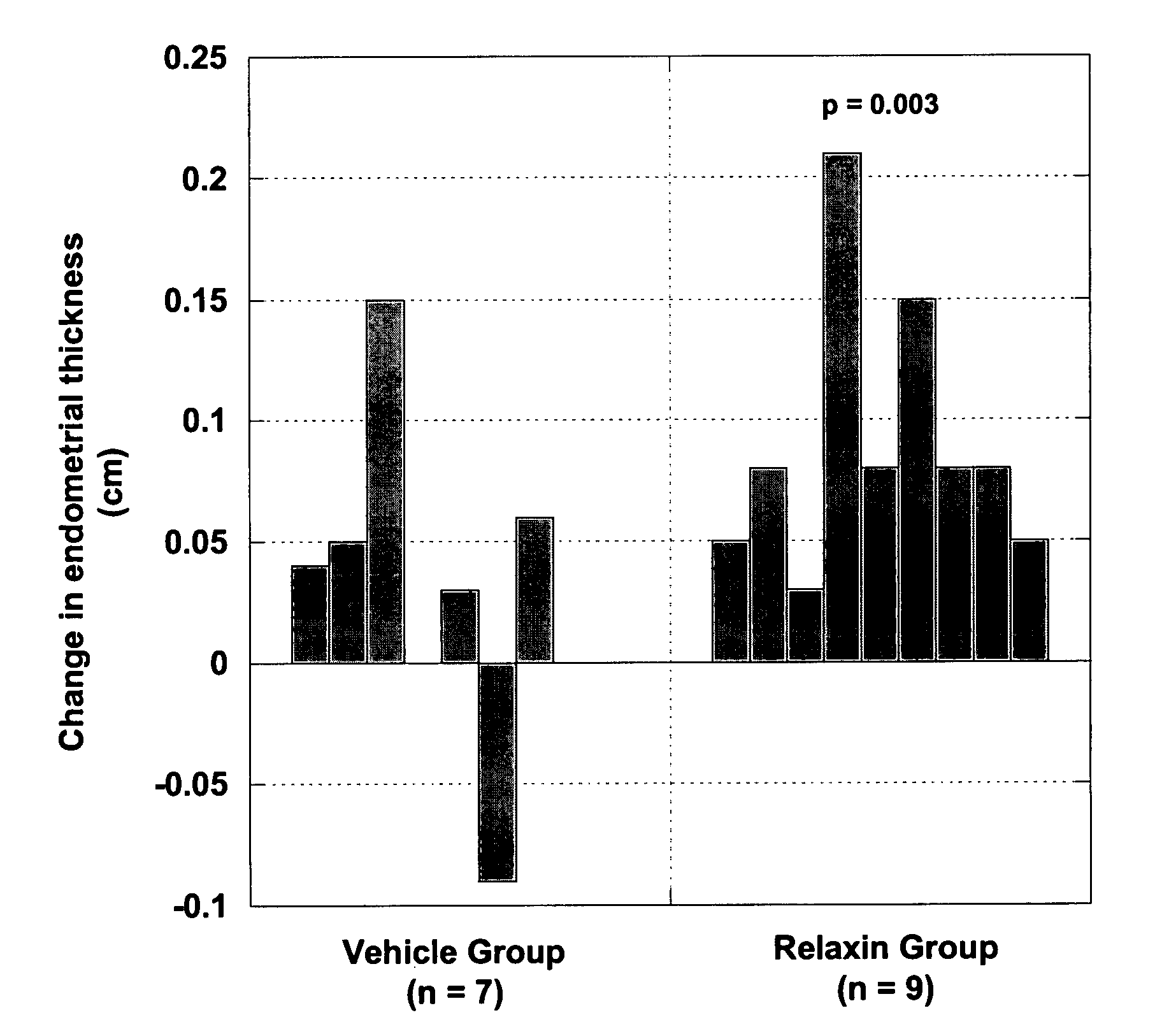
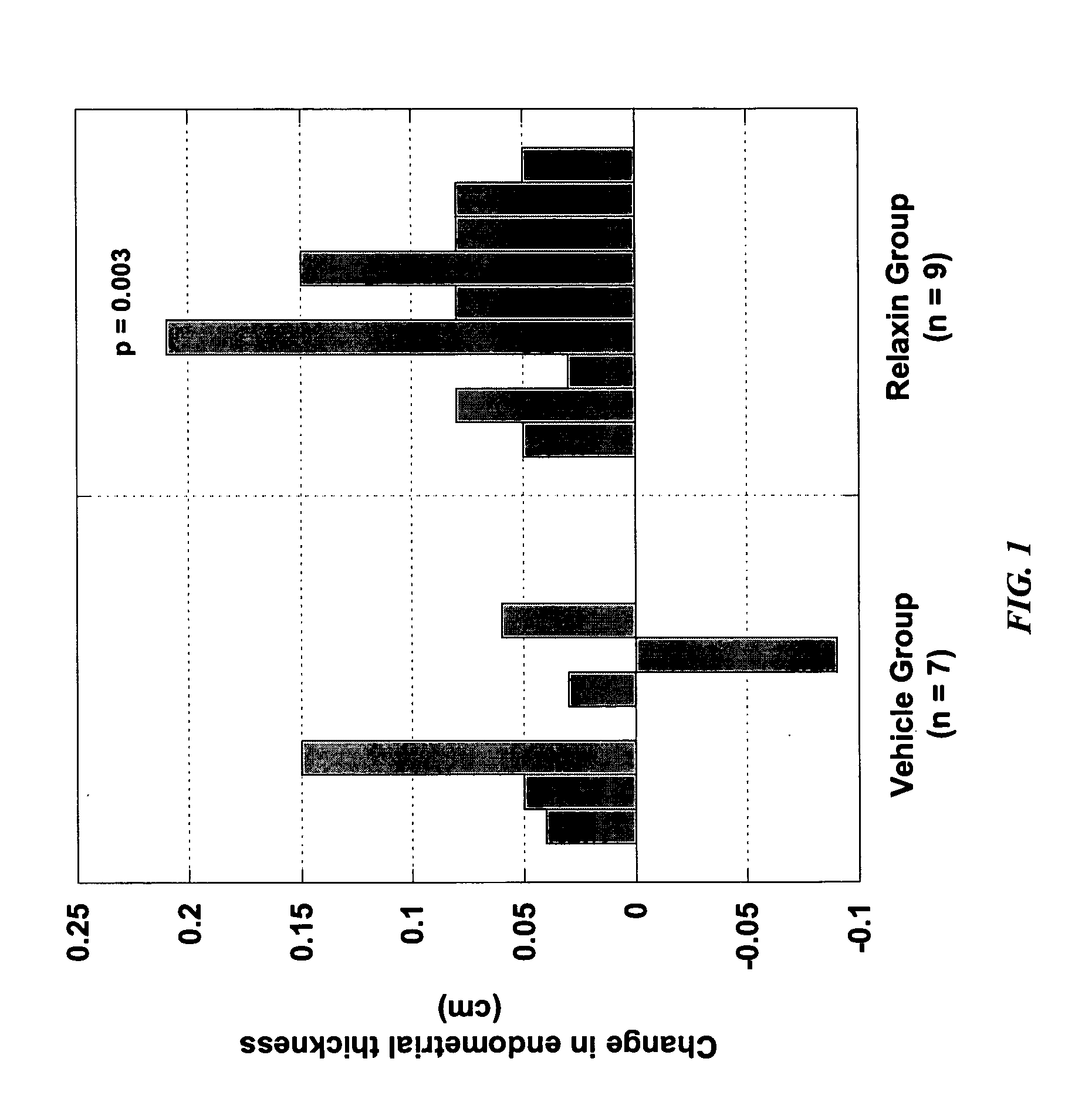
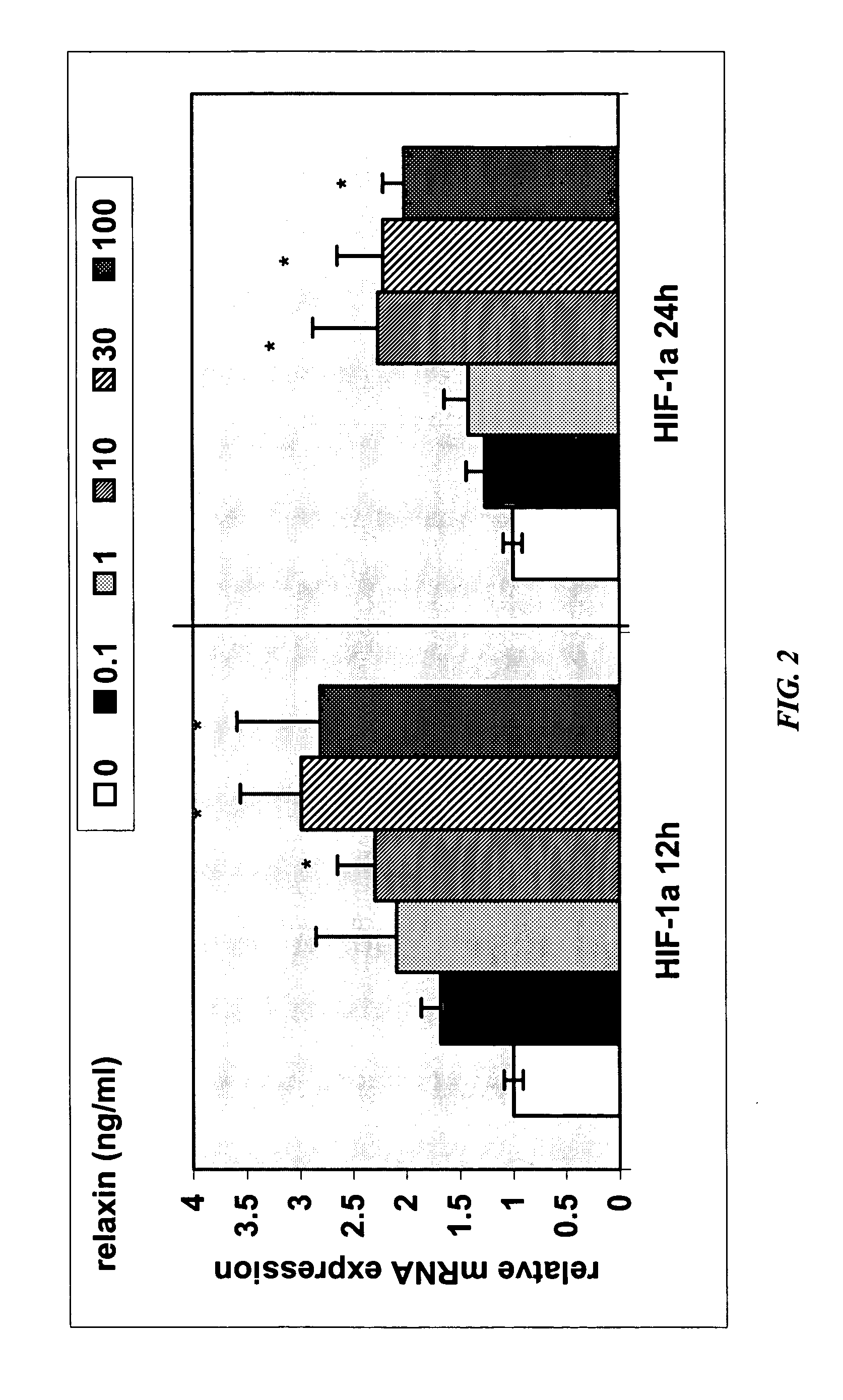
Methods and compositions for control of fetal growth via modulation of relaxin
InactiveUS20060247172A1Increase probabilitySufficient amountPeptide/protein ingredientsMetabolism disorderDiseaseRelaxin-3
The invention relates to the method for treatment, diagnosis and prevention of diseases related to fetal growth and placental insufficiency and comprises methods including inhibiting or increasing relaxin synthesis, relaxin receptor synthesis, relaxin binding to the relaxin receptor, and relaxin receptor activity. The invention also relates to screening assays to identify compounds that modulate relaxin and / or relaxin receptor activity. The invention further relates to gene therapy methods utilizing relaxin and relaxin-related sequences for the treatment and prevention of diseases related to fetal growth and placental insufficiency.
Owner:CORTHERA INC
Methods and compositions for control of fetal growth via modulation of relaxin
InactiveUS20080108572A1Increase probabilitySufficient amountPeptide/protein ingredientsMetabolism disorderDiseaseRelaxin-3
The invention relates to the method for treatment, diagnosis and prevention of diseases related to fetal growth and placental insufficiency and comprises methods including inhibiting or increasing relaxin synthesis, relaxin receptor synthesis, relaxin binding to the relaxin receptor, and relaxin receptor activity. The invention also relates to screening assays to identify compounds that modulate relaxin and / or relaxin receptor activity. The invention further relates to gene therapy methods utilizing relaxin and relaxin-related sequences for the treatment and prevention of diseases related to fetal growth and placental insufficiency.
Owner:CORTHERA INC
Proteomic analysis of biological fluids
InactiveUS20090055099A1Eliminate needBiological testingSpecial data processing applicationsDiseaseObstetrics
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns the identification of the proteome of amniotic fluid (multiple proteins representing the composition of amniotic fluid) and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, or chromosomal defects.
Owner:HOLOGIC INC
Methods and compositions for control of fetal growth via modulation of relaxin
InactiveUS20060247163A1Increase probabilitySufficient amountBiocidePeptide/protein ingredientsDiseaseRelaxin-3
The invention relates to the method for treatment, diagnosis and prevention of diseases related to fetal growth and placental insufficiency and comprises methods including inhibiting or increasing relaxin synthesis, relaxin receptor synthesis, relaxin binding to the relaxin receptor, and relaxin receptor activity. The invention also relates to screening assays to identify compounds that modulate relaxin and / or relaxin receptor activity. The invention further relates to gene therapy methods utilizing relaxin and relaxin-related sequences for the treatment and prevention of diseases related to fetal growth and placental insufficiency.
Owner:CORTHERA INC
Treatment or prevention of hypertensive disorders of pregnancy or fetal growth retardation
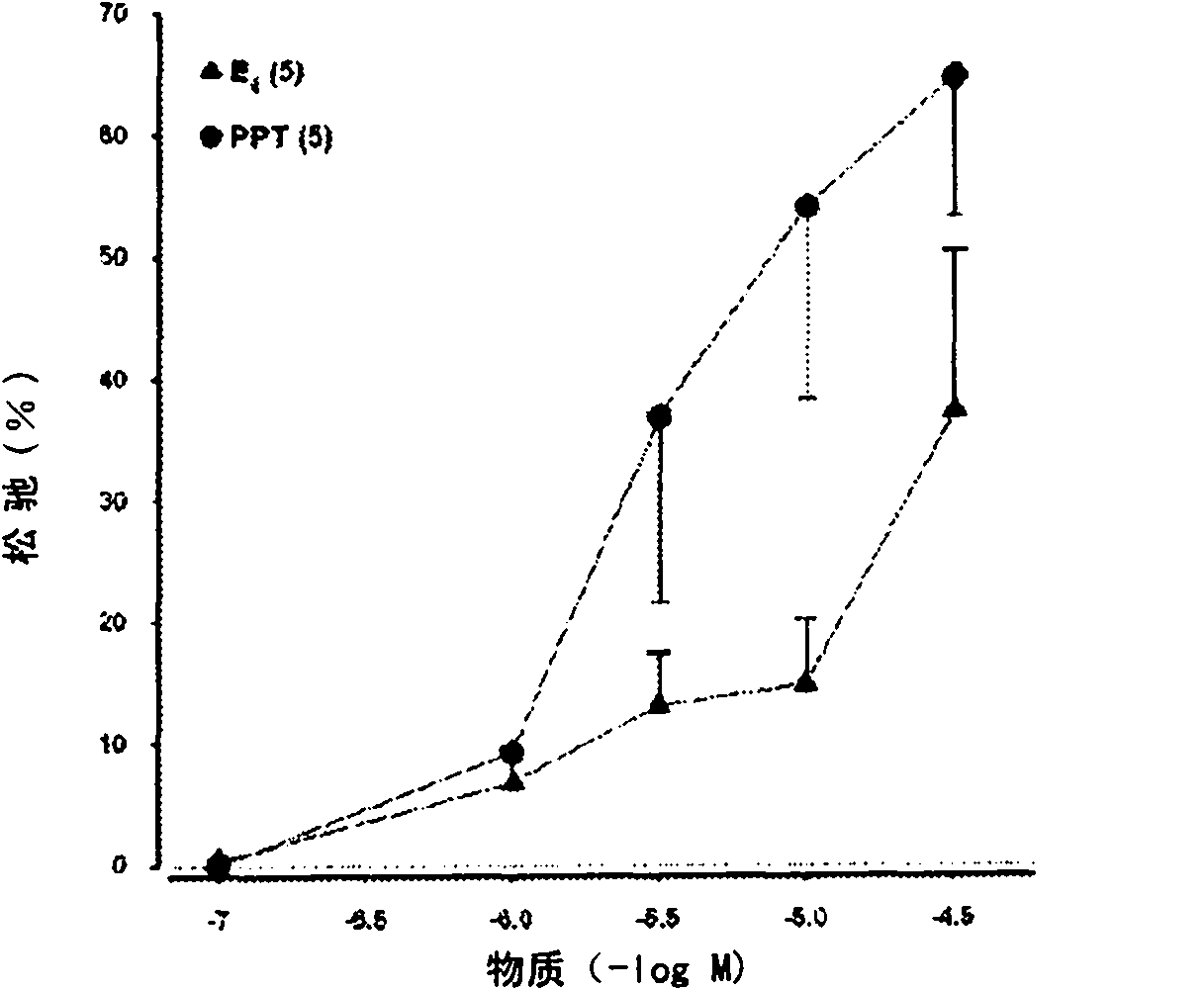
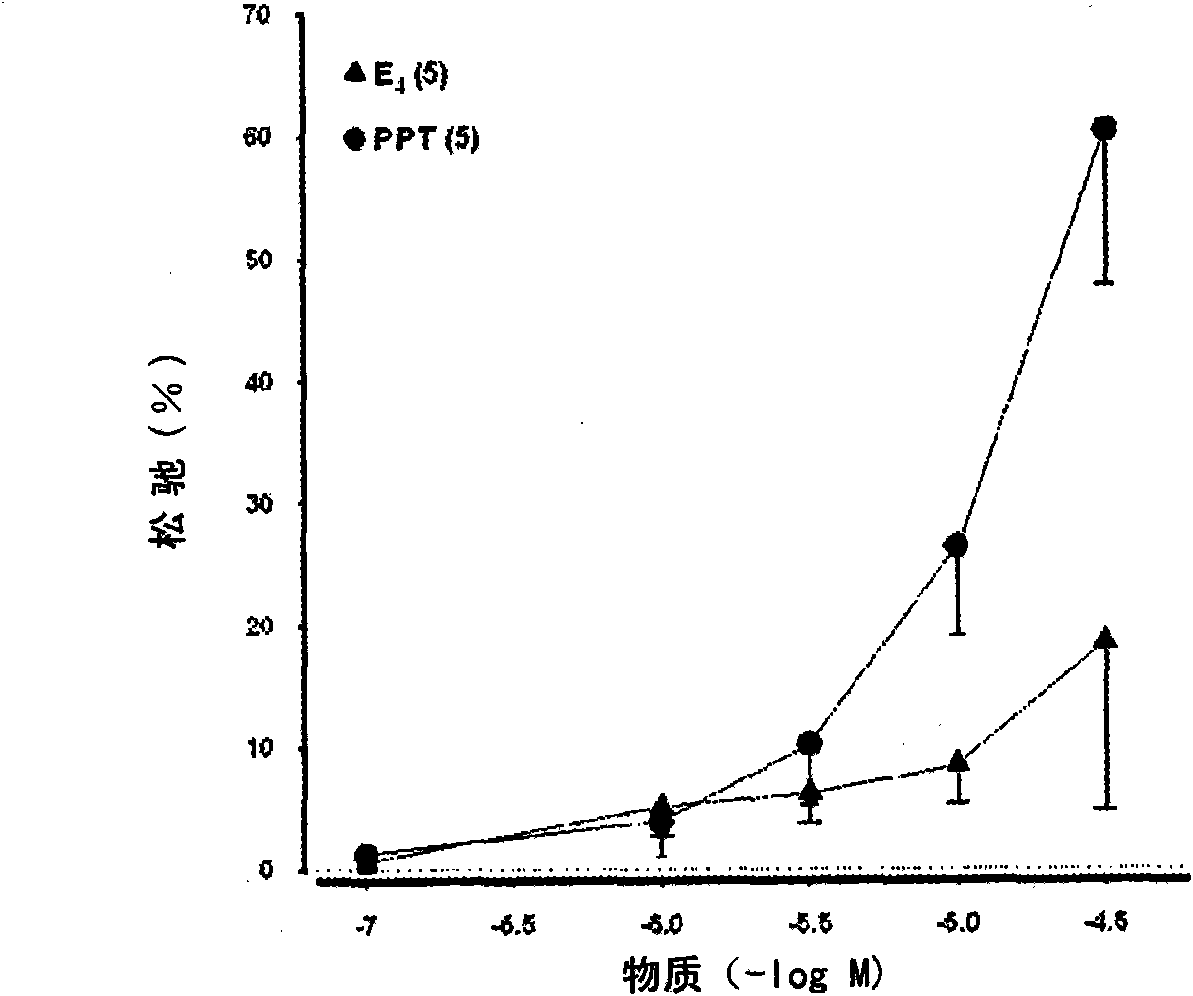
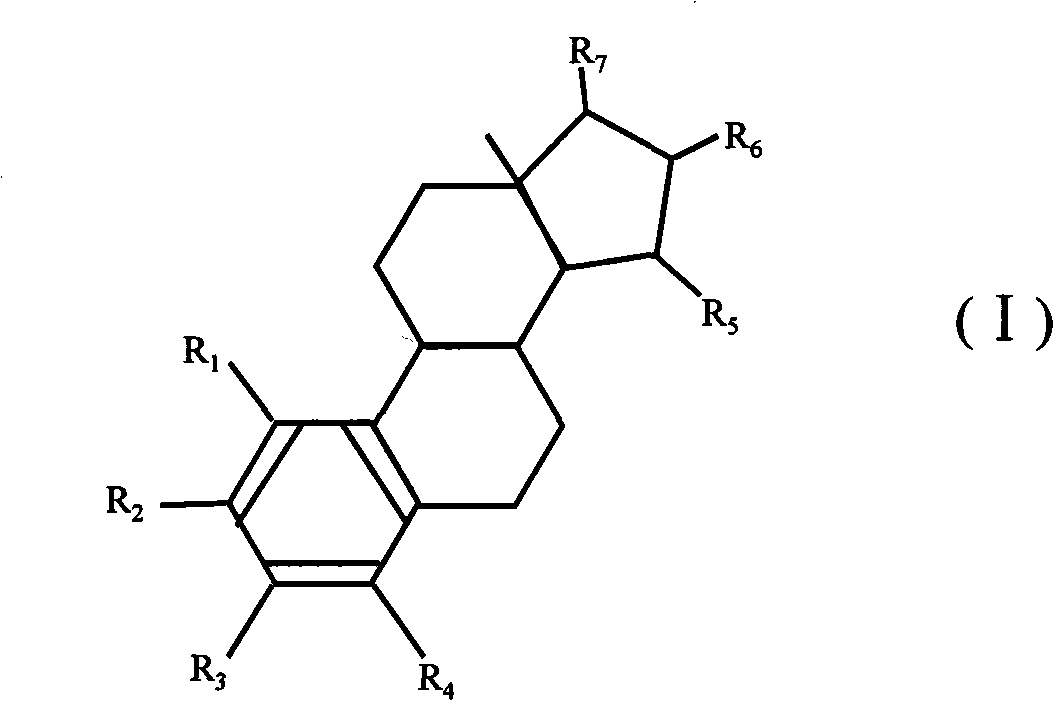
ActiveUS8518923B2High trafficPrevent and reduce blood pressureOrganic active ingredientsSexual disorderObstetricsFetal growth
The present invention relates to the use of a steroid in the manufacture of a pharmaceutical composition for use in the therapeutic or prophylactic treatment of a hypertensive disorder of pregnancy (HDP) or fetal growth retardation, said treatment comprising administering to a female mammal a steroid selected from the group consisting of: substances represented by the following formula (formula I) in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors of such substances; and mixtures of one or more of the aforementioned substances and / or precursors.
Owner:ESTETRA SRL
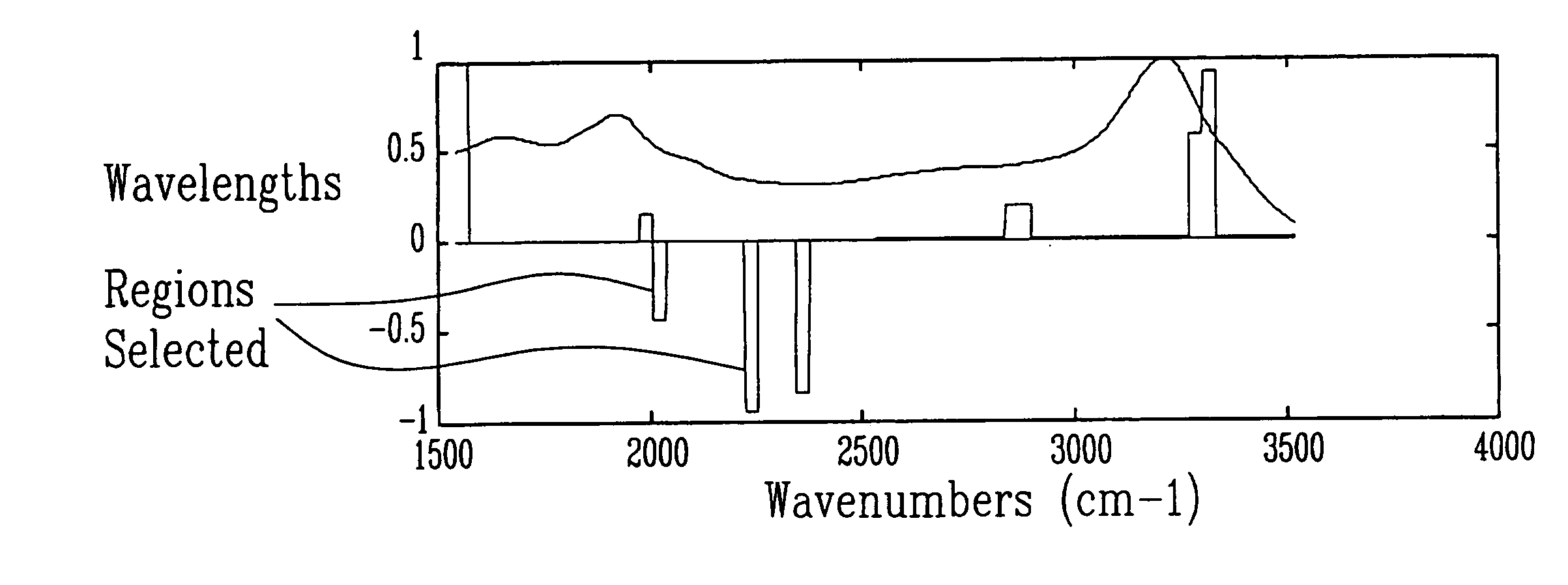
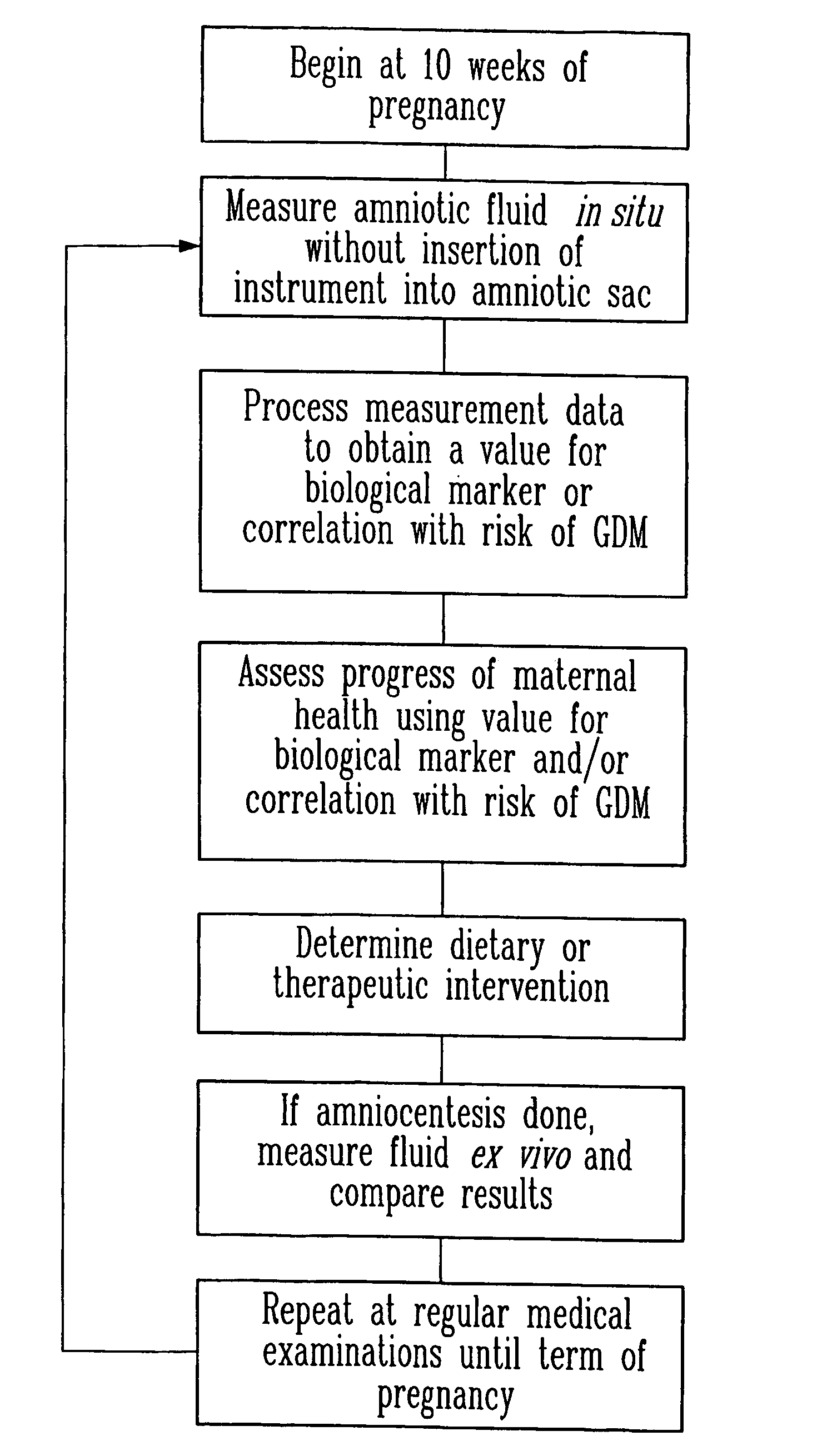
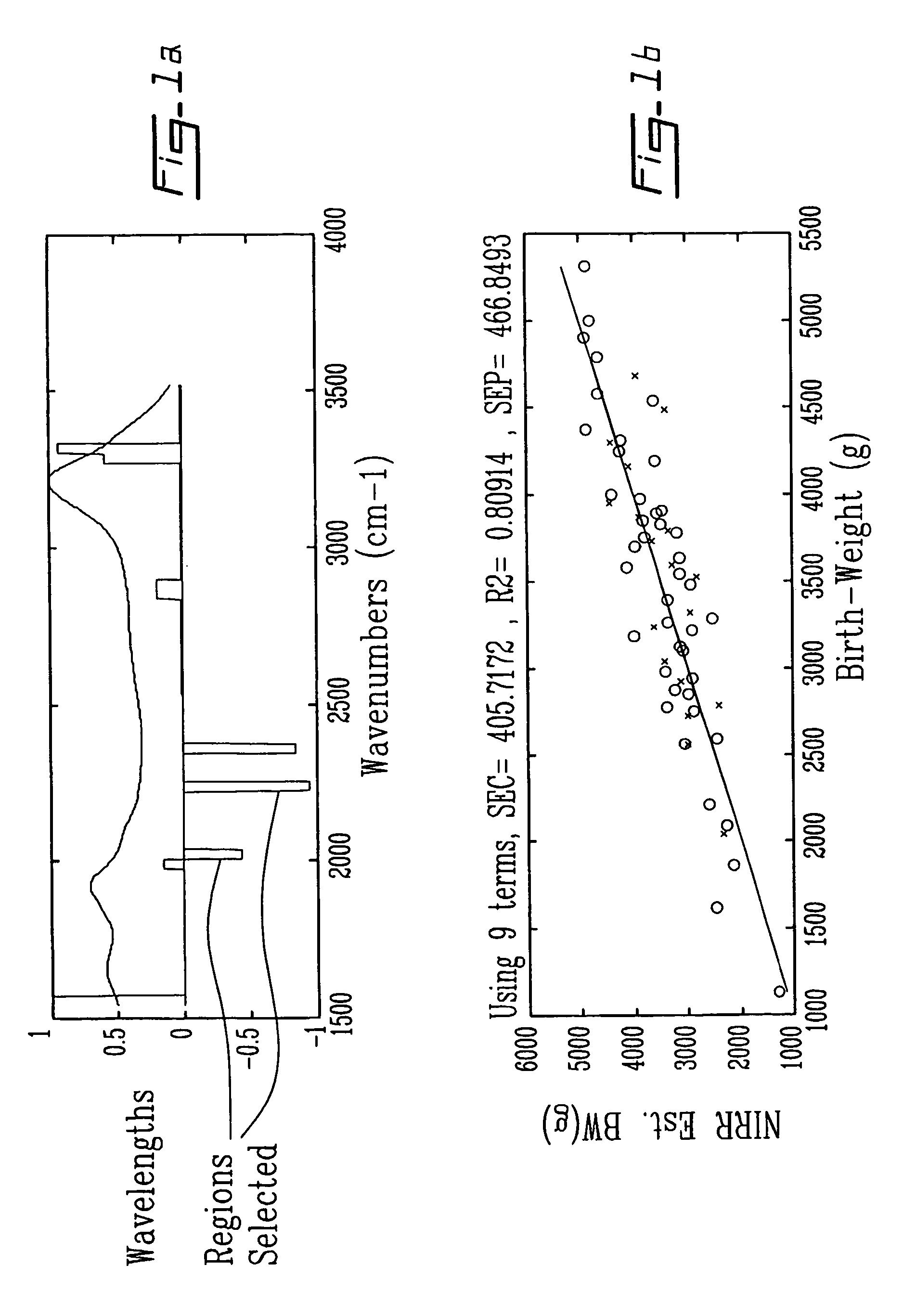
Method and apparatus for analyzing amniotic fluid
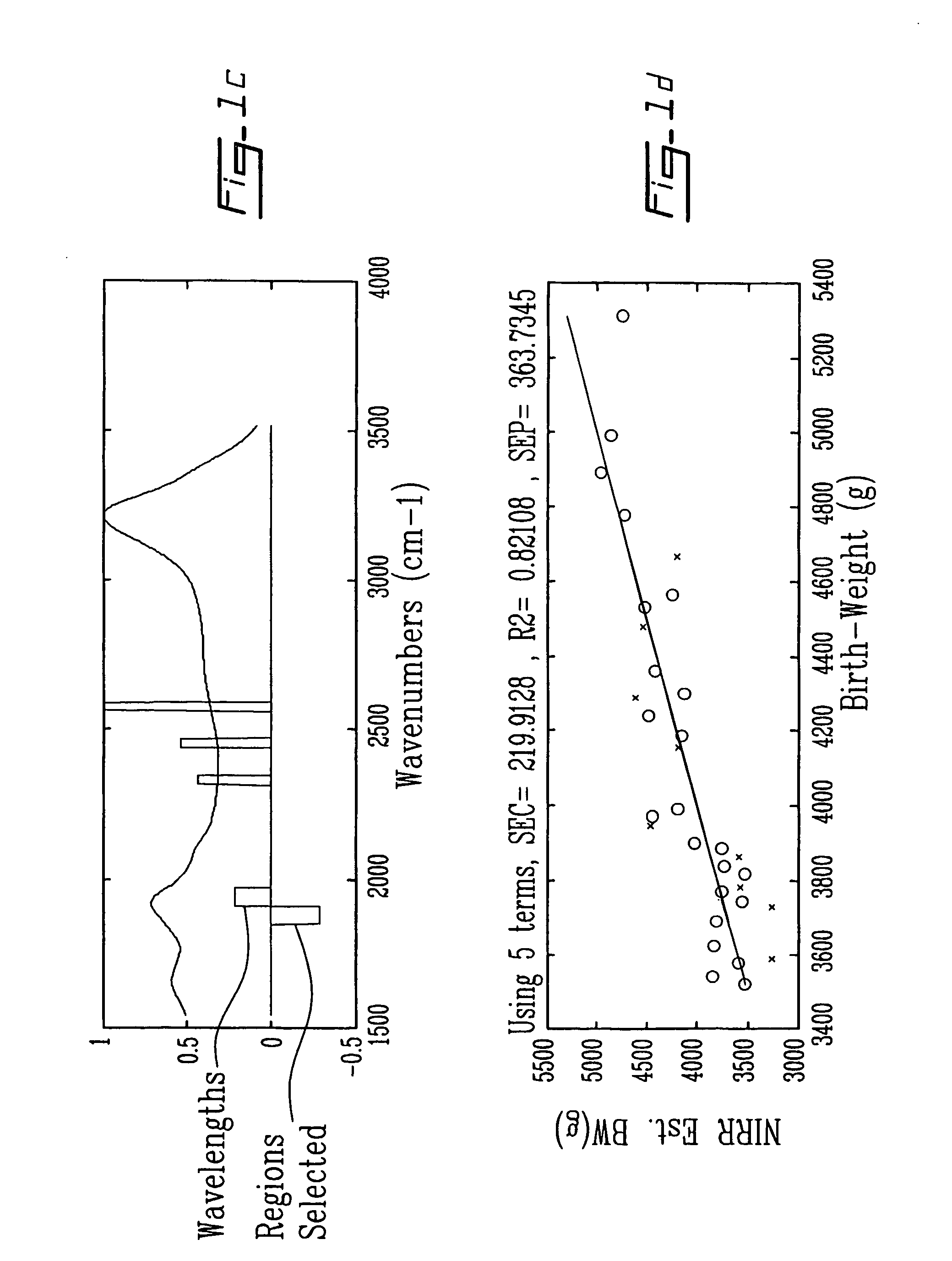
InactiveUS20060247536A1Easy to analyzeImprove accuracyHealth-index calculationDiagnostics using spectroscopyDiseaseObstetrics
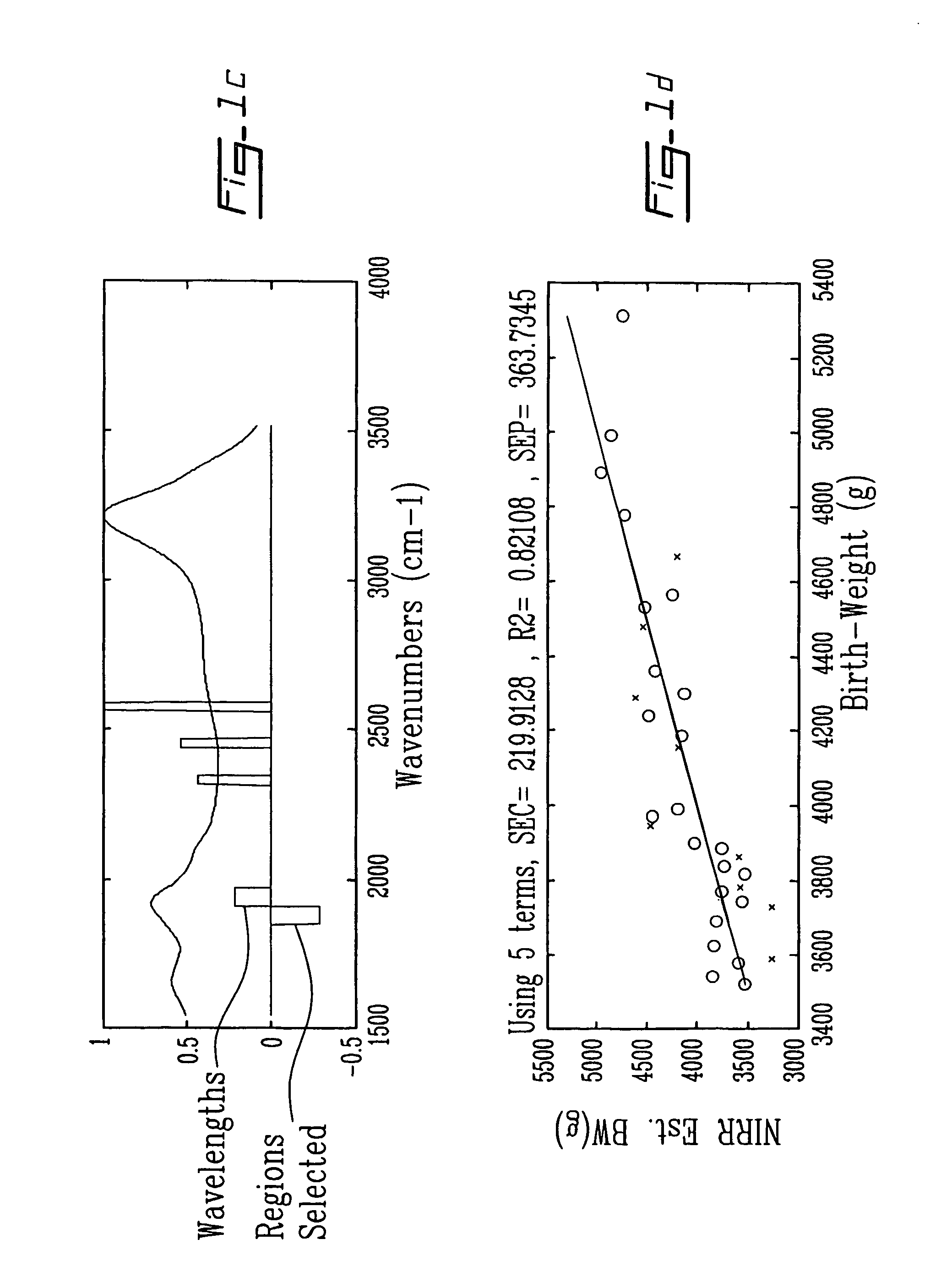
Methods and spectra for monitoring fetal growth and predicting birth weight of an infant prior to birth are provided wherein one or more selected biological markers are measured in a sample of amniotic fluid obtained from a pregnant woman. Levels of the selected biochemical markers and / or spectra correlate with one or more medical conditions, such as fetal growth and birth weight of the infant, and gestational diabetes. A measurement probe for in situ measurement can be used safely and repeatedly. Monitoring and / or treatment of maternal and fetal health is also provided.
Owner:MCGILL UNIV
Proteomic analysis of biological fluids
InactiveUS20080299594A1Eliminate needMicrobiological testing/measurementBiological testingDiseaseFetal growth
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns the identification of the proteome of amniotic fluid (multiple proteins representing the composition of amniotic fluid) and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, or chromosomal defects.
Owner:HOLOGIC INC
Diagnosis of intra-uterine infection by proteomic analysis of cervical-vaginal fluids
ActiveUS8068990B2Eliminate needMicrobiological testing/measurementAnalogue computers for chemical processesDiseaseObstetrics
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns a comprehensive proteomic analysis of human amniotic fluid (AF) and cervical vaginal fluid (CVF), and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, pre-term labor, and / or chromosomal defects. The invention further concerns the identification of biomarkers and groups of biomarkers that can be used for non-invasive diagnosis of various pregnancy-related disorders, and diagnostic assays using such biomarkers.
Owner:HOLOGIC INC
Special clinical nutritional formula for energy supply during delivery and preparation method thereof
InactiveCN107927754AIncrease consumptionSupplementary lossFood scienceFetal growthAdditive ingredient
The present invention relates to a special clinical nutritional formula for energy supply during delivery and a preparation method thereof. The special clinical nutritional formula comprises the following components in parts by weight: 1-5 parts of proteins, 0.1-1 part of lipid, 10-15 parts of carbohydrates, 0.05-1 part of dietary fiber, 0.01-0.15 part of macroelement, 0.001-0.004 part of trace element, 0.001-0.003 part of fat-soluble vitamin, 0.01-0.03 part of water-soluble vitamin, 0.01-0.05 part of dietary essence, 0.01-1 part of medicinally and edibly homogenous ingredient, 0.1-0.5 part ofa natural plant compound and 0.01-2 parts of new resource food. The present invention also relates to the preparation method of two clinical nutritional formulas. Through screening and proportioningof the components, the special clinical nutritional formula for the energy supply during the delivery fully supplements needed necessary nutrient components required for the delivery of pregnant women, is more easily digested and absorbed by the pregnant women, fully plays efficacies of the various components, quickly provides the energy required for the delivery of the pregnant women, at the sametime effectively ensures necessary nutrients for fetal growth and development, and helps the pregnant women to conduct the spontaneous delivery and shorten stages of the delivery.
Owner:上海奥医生物医药科技有限公司
Non-antibiotics functional concentrated feed for sows during gestation
ActiveCN105961863AReduce deformity rateSlow developmental delayFood processingAnimal feeding stuffBiotechnologyPhytase
The invention discloses a non-antibiotics functional concentrated feed for sows during gestation. The feed mainly comprises flaked barley, alfalfa, tofu skin, sugar beet pulp, phytase, NSP enzyme, glucose oxidase, NCG, flaxseed oil, VC, organic trace elements, a cortex eucommiae extract and yeast cell walls. By virtue of intestinal microecology nutrition and disease-resistant nutrition, intestinal digestive systems of the sows are kept healthy, the autoimmunity of the sows is improved, and the purpose of no antibiotics is achieved; in addition, by virtue of adding high-quality feed raw materials and screening placenta nutrients, the fetal growth and development can be promoted, embryo losses are reduced, and the primary uniformity and vitality of piglets are improved.
Owner:HUNAN JIUDING TECH GROUP
Method for diagnosing gestational diabetes, preeclampsia, and fetal growth restriction
ActiveUS8883512B1Reduce perfusionImprove concentrationElectrotherapyDisease diagnosisSerum igeVitamin A Retinol
A serum-based biomarker for diagnosing gestational diabetes, preeclampsia and fetal growth restriction (GDM-PREEC-FGR), based on retinoid profiling, and a method for diagnosing GDM-PREEC-FGR that specifically uses a blood sample to identify and measure the concentrations of retinol (vitamin A alcohol), retinyl esters and retinoic acid.
Owner:MAWSON ANTHONY R
Microencapsulated peony seed oil, compound composition and preparation method of microencapsulated peony seed oil
InactiveCN106900885AHigh yield of microencapsulationGood storage stabilityFood freezingFood shapingDiseaseAnimal science
The invention provides microencapsulated peony seed oil, compound composition and a preparation method of the microencapsulated peony seed oil. The microencapsulated peony seed oil is prepared from a peony seed oil core material and a Maillard product wall material and has good antioxidant effect. The microencapsulated peony seed oil is taken as a raw material and compounded with folic acid and xylooligosaccharide for further preparation of a product which has better antioxidant property, is high in storage stability and meets special physiological needs of pregnant women in the gestation period. The compound product has the better antioxidant property, is high in storage stability, meets physiological characteristics of the pregnant women in the gestation period and needs of growth and development of fetuses, can promote fetal brain development, guarantee normal development of fetal organs, relieve constipation of the pregnant women in the gestation period, improve organism immunity of the pregnant women and reduce blood pressure, blood fat and blood sugar, and reduces incidence rate of diseases such as hypertension, diabetes mellitus and the like in the gestation period.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
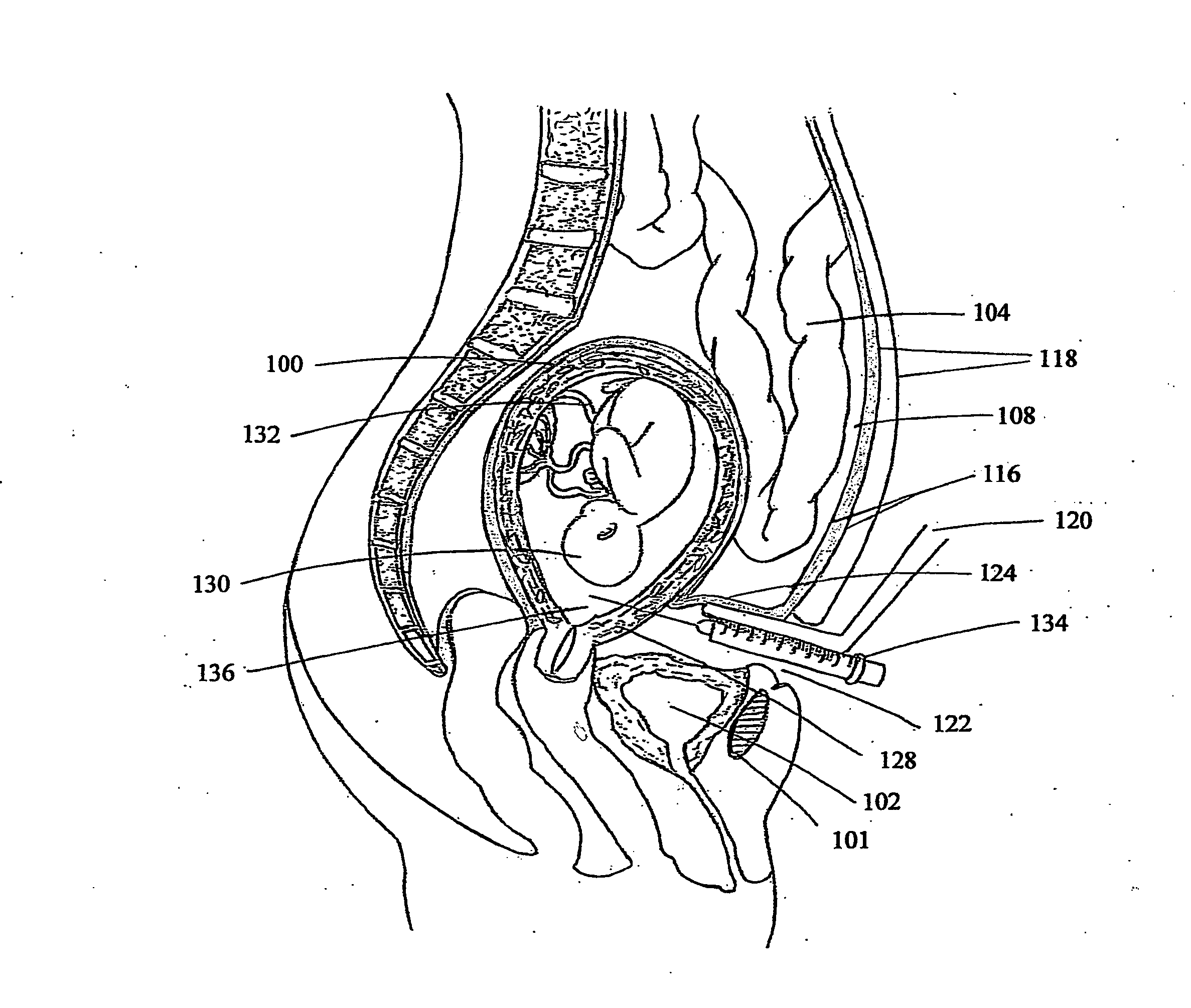
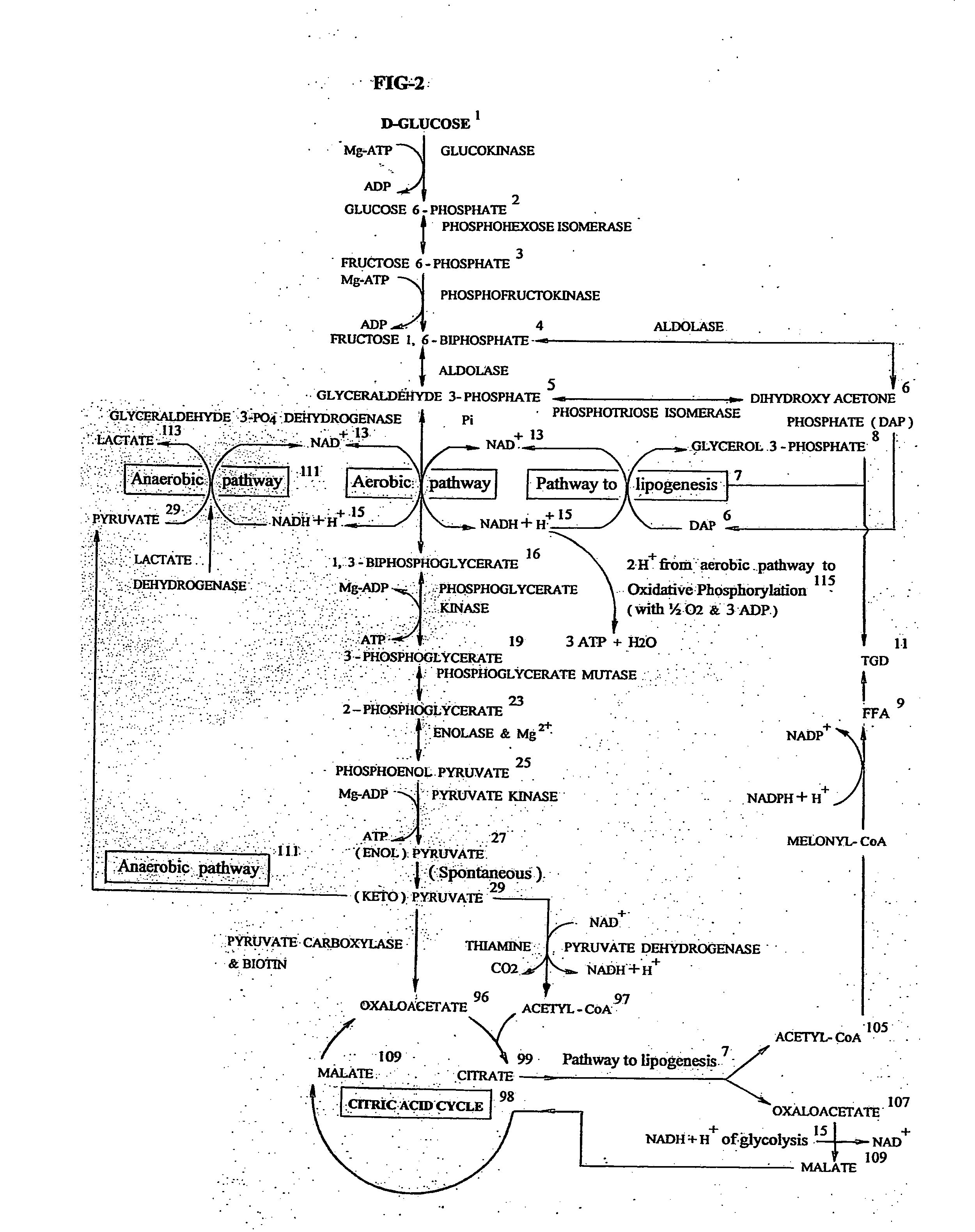
Intrauterine fetal growth restriction - The biochemical rationale of treatment modalities including extraperitoneal transamniotic fetal supplements
ActiveUS20140330246A1Decreases placental transfer of D-glucoseImproving fetal hypoglycemiaBiocideMedical devicesDiseaseMineral supplementation
Intrauterine fetal growth restriction (IUGR) is an affliction of a disparaging spectrum, placental insufficiency being the major inciting pathology. The resultant fetal hypoglycemia is alleviated by intravenous hypertonic D-glucose 25-50% maternal supplements, by improving the Vmax of placental transfer for D-glucose, in accordance with Michaelis-Menten model of substrate transfer. Fetal normoglycemia so restored in turn surprisingly improves fetal hypoxia, hypercapnia, hyperlacticemia, acidosis, hypertriglyceridemia, oliguria / hydromnios, and the fetal nutrient / mineral / vitamin acquisition. The list being phenomenal can only convince an inquiring reader by a biochemical sojourn into the aquatic world of the fetus, herein described. Maternal carbohydrate-predominant IUGR diet with maximal amounts of vitamin / mineral supplements are highly beneficial. Transamniotic isotonic D-glucose supplements via minimally invasive suprapubic extraperitonial pelvic approach and amniotomy (Sumathi Paturu's technique), with a subcutaneously implanted pregnancy port (SIPP) catheter is the additional therapy advocated.
Owner:PATURU SUMATHI
Chinese medicinal composition for treating pregnancy-induced hypertension syndrome and preparation method thereof
InactiveCN102579881ADripping symptoms relievedSexual disorderFish material medical ingredientsSide effectChinese wax
The invention discloses a Chinese medicinal composition for treating pregnancy-induced hypertension syndrome. The Chinese medicinal composition consists of the following Chinese herbal medicines in part by weight: 24 parts of astragalus, 18 parts of largehead atractylodes rhizome, 15 parts of tuckahoe, 15 parts of poria peel, 10 parts of plantain seed, 10 parts of rhizoma alismatis, 15 parts of Chinese wax gourd peel, 12 parts of gastrodia elata, 15 parts of corn stigma, 15 parts of medlar, 12 parts of Himalayan teasel root, 12 parts of fried eucommia ulmoides oliv, 12 parts of fish glue, 10 parts of tangerine peel and 15 parts of raw hawthorn. The invention has the advantages that: the Chinese medicinal composition is convenient to take, wide in medicinal source and low in price, does not have toxic or side effects, does not affect fetal growth, and has an obvious curative effect; and the effective rate and the cure rate of the Chinese medicinal composition for treating the pregnancy-induced hypertension syndrome are 97 percent and 95 percent respectively.
Owner:姚淑霞 +2
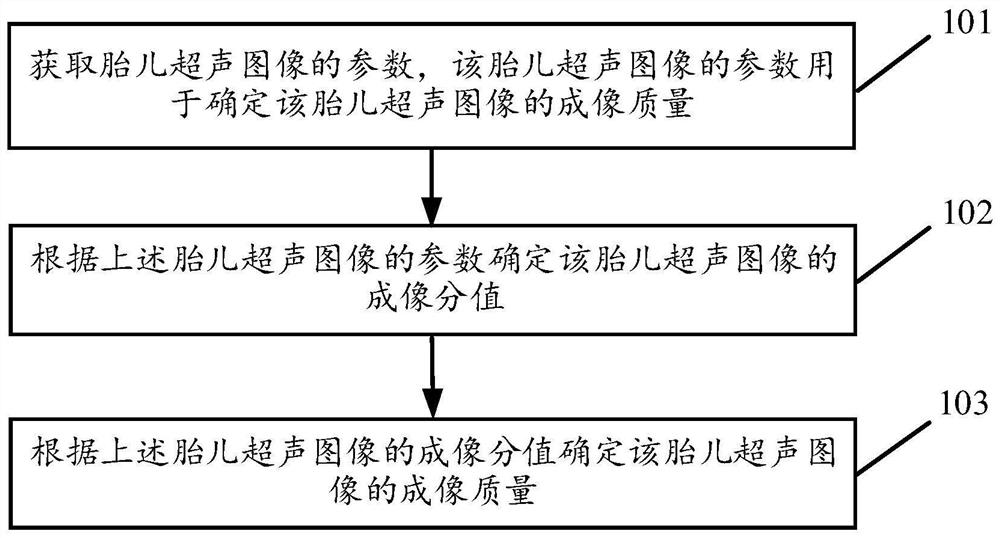
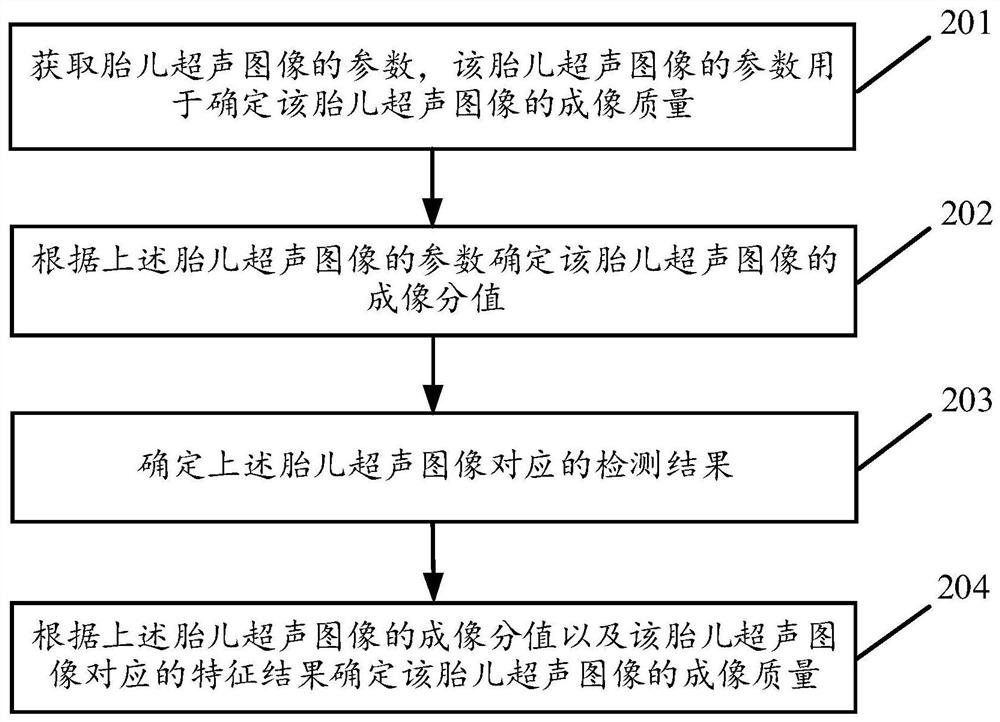
Method and device for determining imaging quality control of fetal ultrasound image
ActiveCN112155601AImaging Quality ControlDetermination of Imaging Quality ControlOrgan movement/changes detectionInfrasonic diagnosticsImaging qualityFetal growth
The invention discloses a method and a device for determining the imaging quality control of a fetal ultrasound image, and the method comprises the steps: obtaining the parameters of a fetal ultrasound image for determining the imaging quality of the fetal ultrasound image; and determining the imaging score of the fetal ultrasound image according to the parameters of the fetal ultrasound image, and determining the imaging quality of the fetal ultrasound image according to the imaging score of the fetal ultrasound image. It can be seen that, the imaging quality of the fetal ultrasonic image canbe automatically determined according to the determined imaging score of the fetal ultrasonic image, and the imaging quality of the fetal ultrasonic image can be rapidly and accurately determined, sothat the imaging quality of the fetal ultrasonic image is accurately and rapidly controlled; furthermore, a high-quality fetal ultrasound image is obtained, so that the accurate fetal growth and development condition can be obtained; and the normalization of a worker in the process of detecting the fetal ultrasound image can also be known through the determined imaging quality of the fetal ultrasound image.
Owner:GUANGZHOU AIYUNJI INFORMATION TECH CO LTD
Method and apparatus for analyzing amniotic fluid
InactiveUS8165661B2Easy to analyzeImprove accuracyUltrasonic/sonic/infrasonic diagnosticsHealth-index calculationObstetricsFetal growth
Methods and spectra for monitoring fetal growth and predicting birth weight of an infant prior to birth are provided wherein one or more selected biological markers are measured in a sample of amniotic fluid obtained from a pregnant woman. Levels of the selected biochemical markers and / or spectra correlate with one or more medical conditions, such as fetal growth and birth weight of the infant, and gestational diabetes. A measurement probe for in situ measurement can be used safely and repeatedly. Monitoring and / or treatment of maternal and fetal health is also provided.
Owner:MCGILL UNIV
Method of treating fetal growth retardation and placental ischaemia and insufficiency
InactiveUS20070243135A1Improve angiogenesisNo adverse effectsOrganic active ingredientsBiocideObstetricsFetal growth
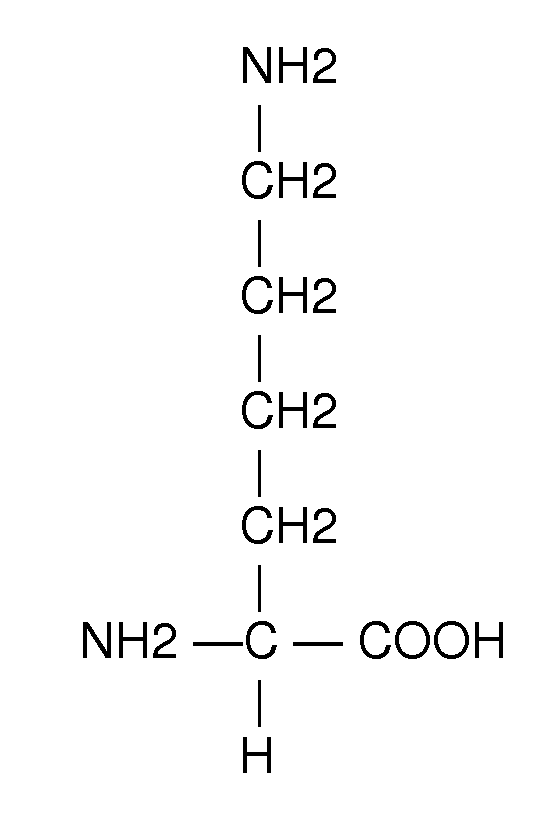
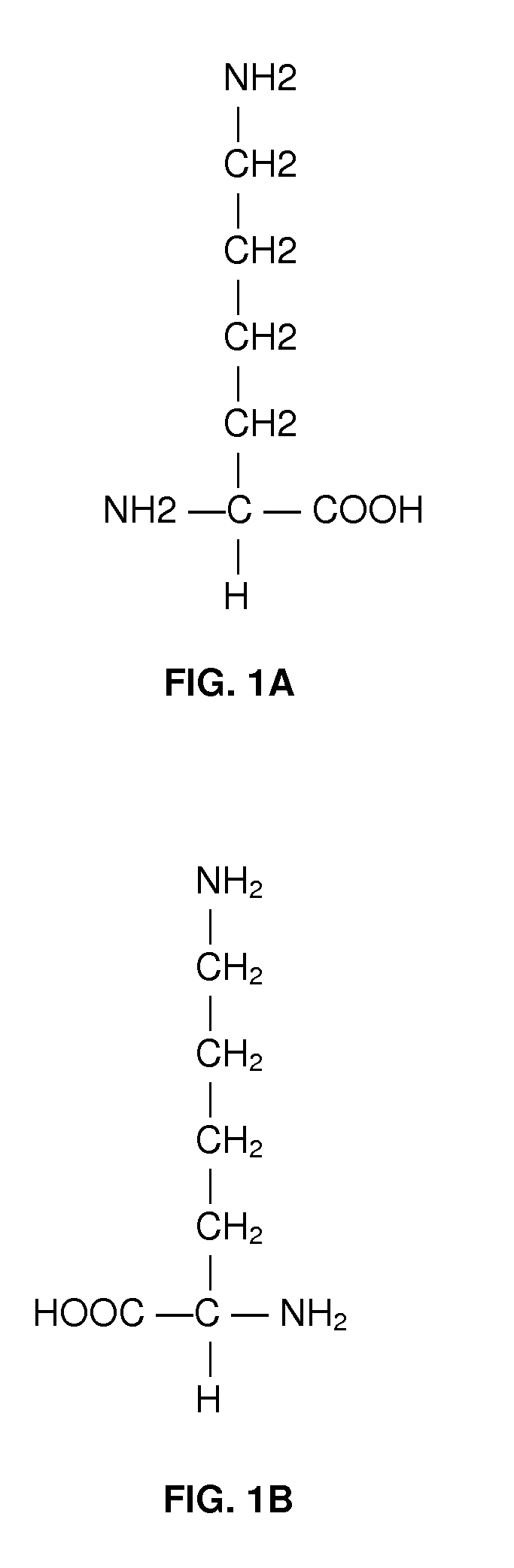
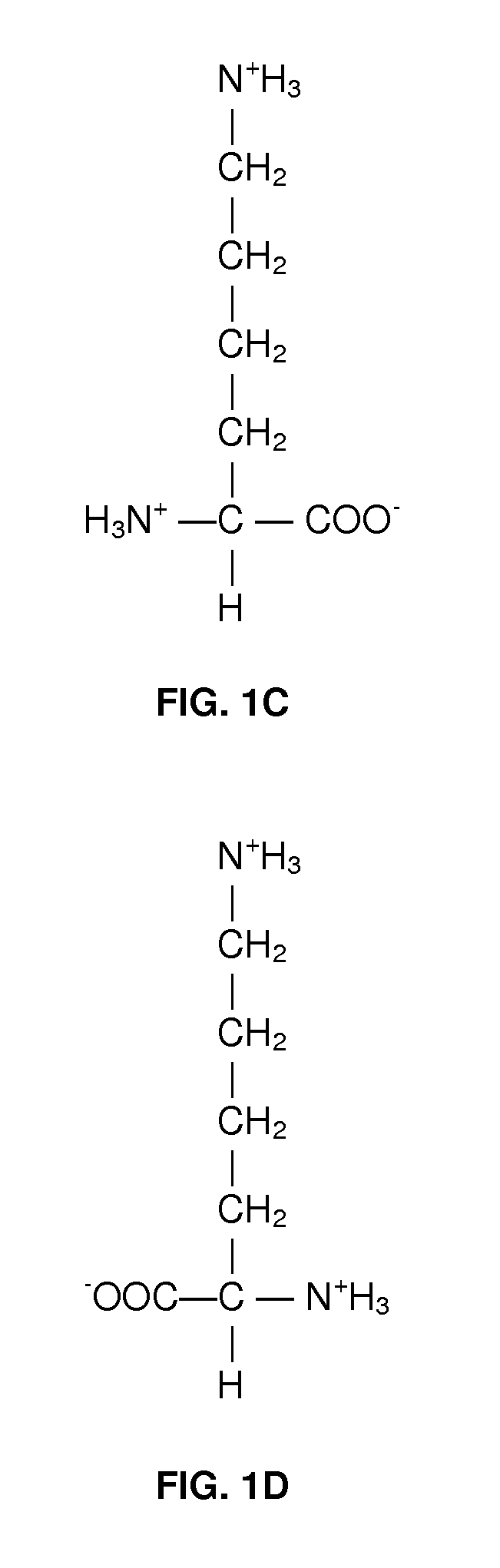
A method for treating intrauterine growth retardation in an animal, such as a human being, is disclosed comprising administration of a lysine composition.
Owner:DATTA DEBATOSH
Treatment or prevention of hypertensive disorders of pregnancy or fetal growth retardation
The present invention relates to the use of a steroid in the manufacture of a pharmaceutical composition for use in the therapeutic or prophylactic treatment of a hypertensive disorder of pregnancy (HDP) or fetal growth retardation, said treatment comprising administering to a female mammal a steroid selected from the group consisting of: substances represented by the following formula (formula I) in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1 - 5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors of such substances; and mixtures of one or more of the aforementioned substances and / or precursors.
Owner:PANTARHEI BIOSCI
Diagnosis of gluten sensitive enteropathy and other autoimmunopathies
InactiveUS7781169B1Increased riskReduce the overall heightDisease diagnosisBiological testingInsulin dependentRheumatism
Method for diagnosis of autoimmune diseases of the GSE-type or associated with gluten sensitive enteropathy comprising taking a sample and testing the sample for antibodies against human tissue transglutaminase, tissue-specific transglutaminases, or other transglutaminases. It was found that autoimmune diseases other than celiac disease can be diagnosed and distinguished in this way, notably, dermatitis herpetiformis Duhring, Crohn's disease, Addison's disease, AI hemolytic anemia, AI thrombocytopenic purpura, AI thyroid diseases, atrophic gastritis—pernicious anemia, IgA nephropathy or IgA glomerulonephritis, myasthenia gravis, partial lipodystrophy, polymyositis, primary biliary cirrhosis, primary sclerosing cholangitis, recurrent pericarditis, relapsing polychondritis, rheumatoid arthritis, rheumatism, sarcoidosis, Sjögren's syndrome, SLE, splenic atrophy, type I (insulin-dependent) diabetes mellitus, diabetes mellitus of other types, ulcerative colitis, vasculitis (both systemic and cutaneous), vitiligo as well as autoimmune diseases associated with infertility, increased risk of abortion, or reduced fetal growth.
Owner:PAULSSON MATS +5
Intrauterine fetal growth restriction—the treatment modalities for clinical research, and the biochemical rationale
Owner:PATURU SUMATHI
Serum micro ribonucleic acid marker related to human fetal growth restriction and application thereof
ActiveCN103194542AHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationSerum igeFetal growth
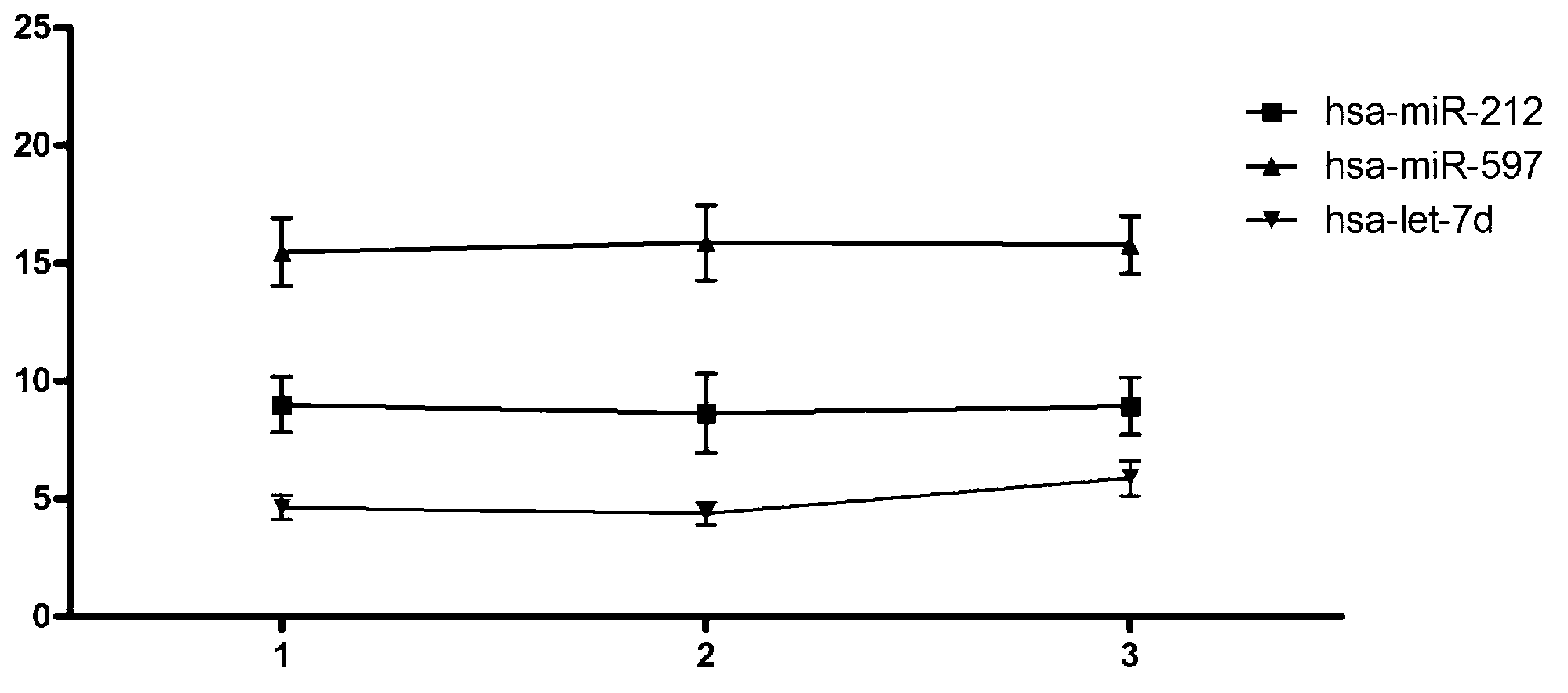
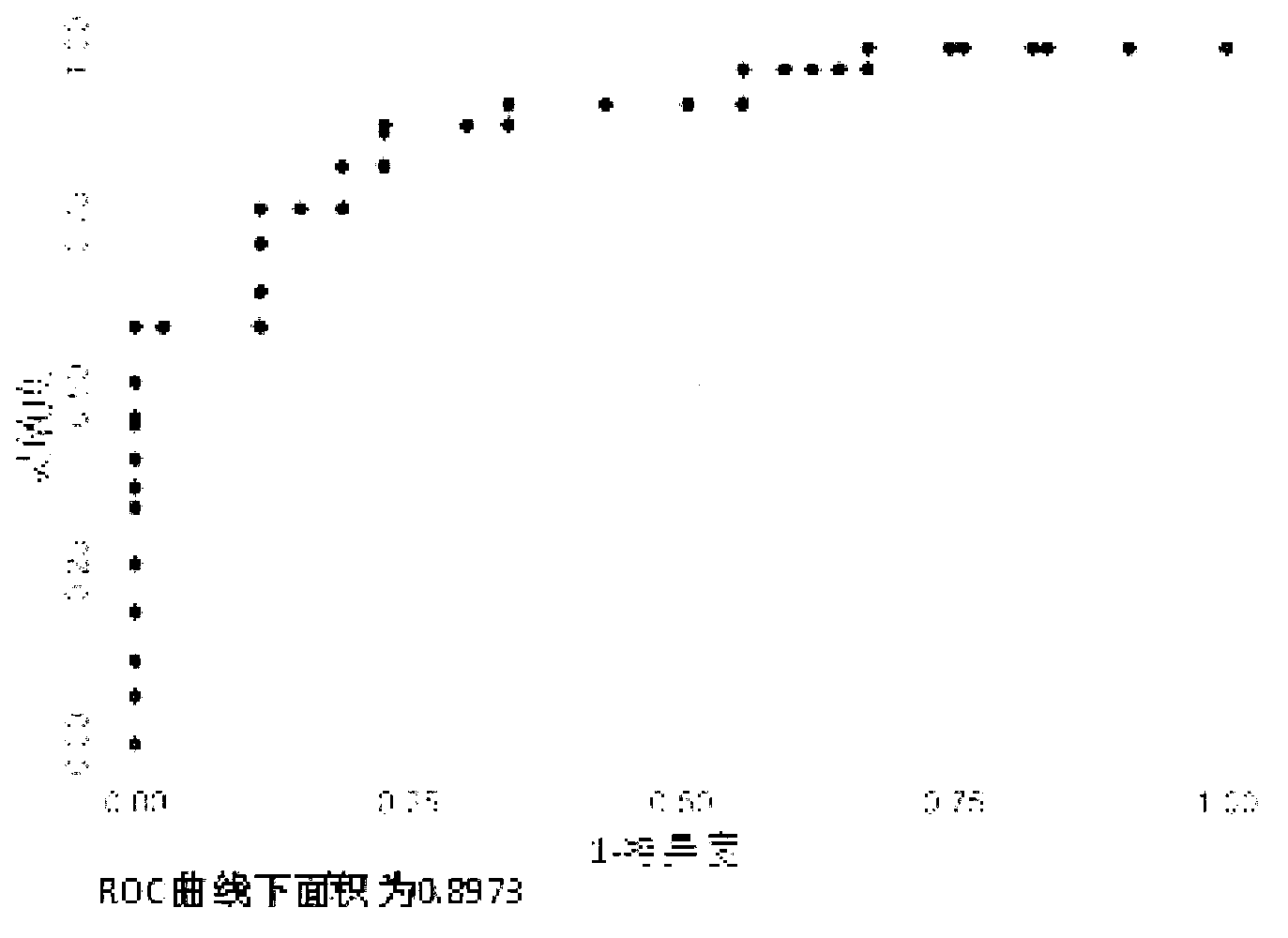
The invention belongs to the field of gene engineering and clinical medicine, and discloses a serum micro ribonucleic acid marker related to human fetal growth restriction and application thereof. The marker is more of hsa-miR-212, hsa-miR-597 and hsa-let-7d. The marker is specific and sensitive to fetal growth restriction, and can be used for preparing diagnostic or monitor reagents for fetal growth restriction.
Owner:夏彦恺
Tiny serum ribonucleic acid marker related to human fetal growth restriction and application thereof
ActiveCN103757017AHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationFetal growthHuman fetal
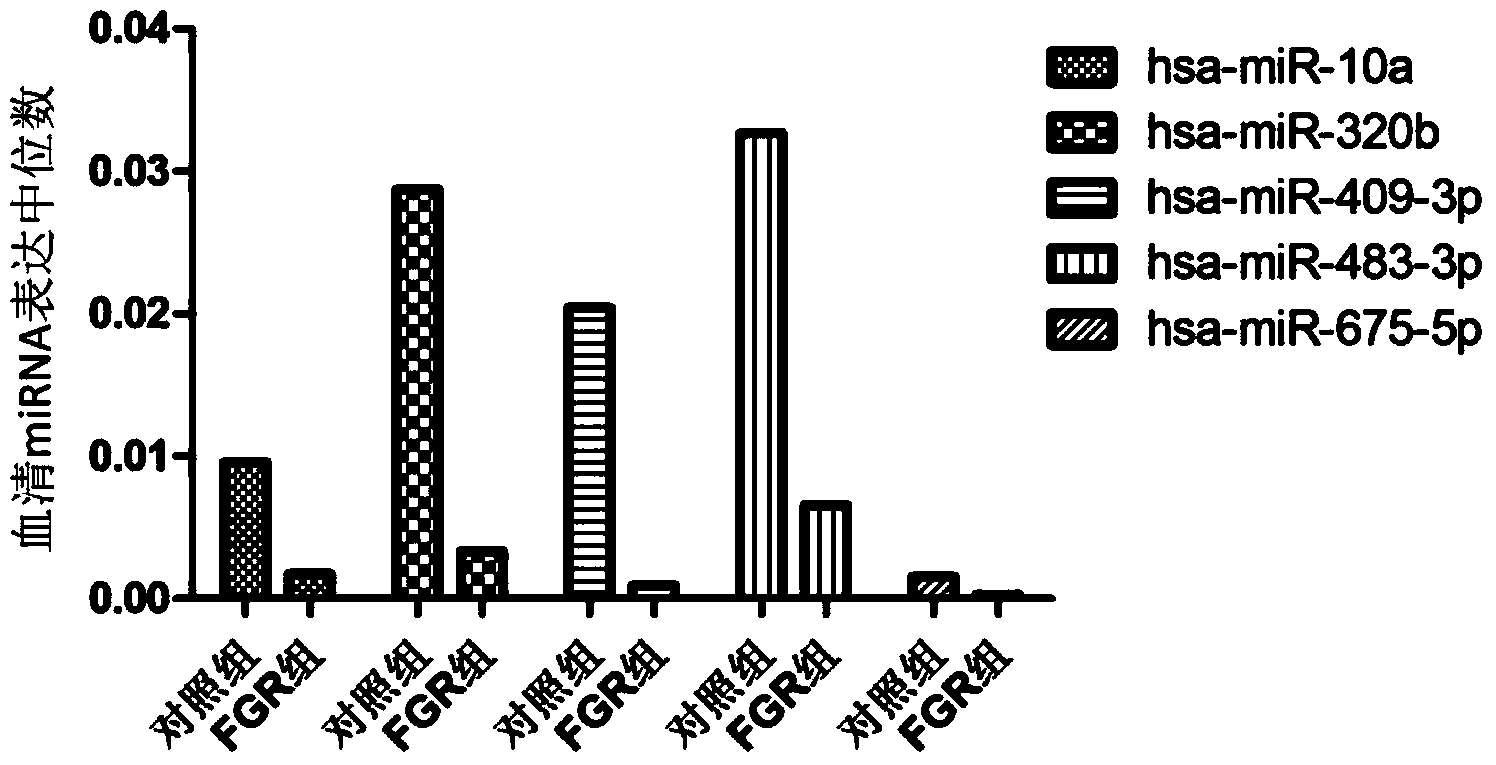
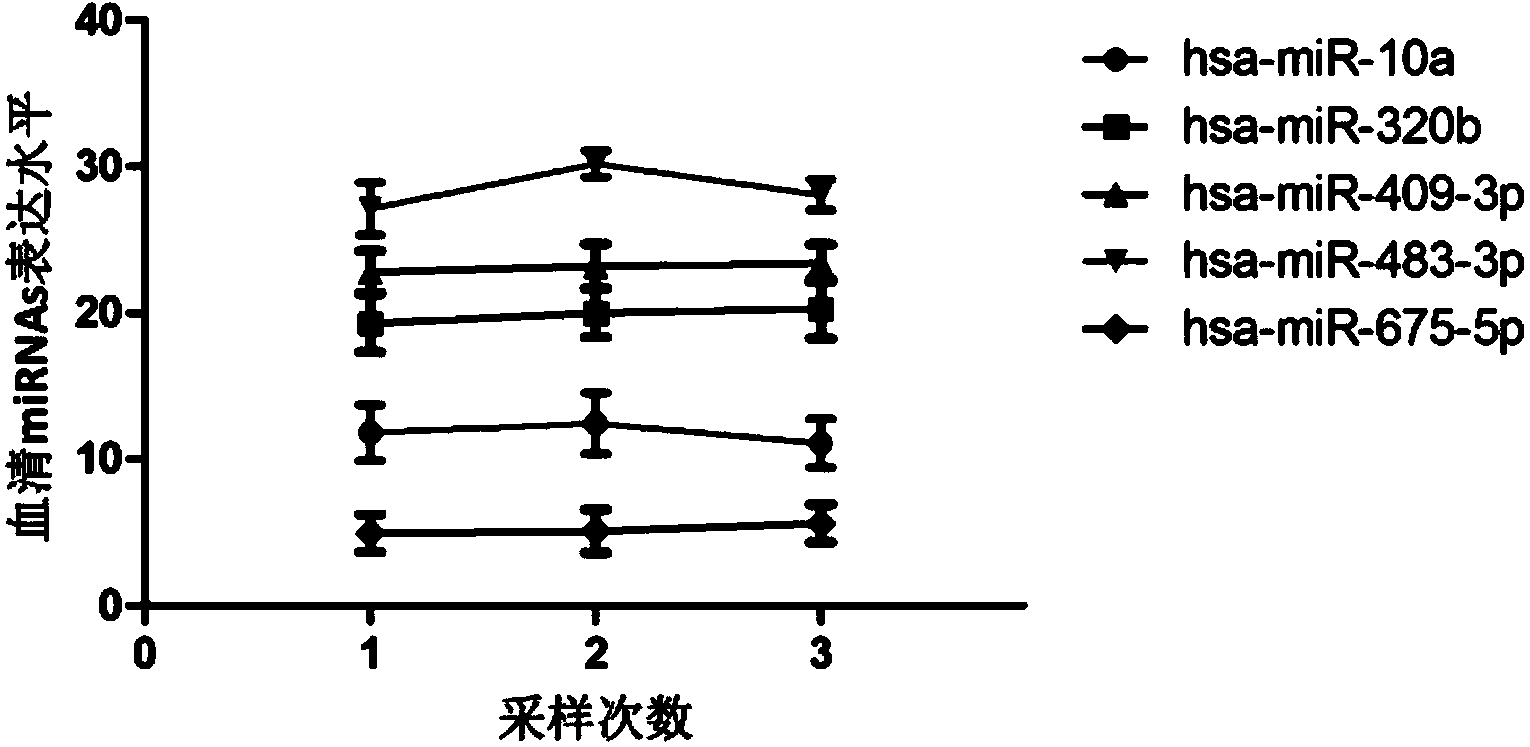
The invention belongs to the fields of genetic engineering and reproductive medicine, and discloses a tiny serum ribonucleic acid marker related to human fetal growth restriction and application thereof. The marker is a plurality of hsa-miR-10a, hsa-miR-320b, hsa-miR-409-3p, hsa-miR-483-3p and hsa-miR-675-5p. The marker has specificity and sensibility on the fetal growth restriction, can be applied to preparation of the kit for diagnosing and monitoring the fetal growth restriction, can repeatedly detect, and is easy to dynamically monitor the fetal growth restriction degree.
Owner:NANJING MEDICAL UNIV
A compound premixed feed for sows suitable for late pregnancy
ActiveCN103621793BIncrease production capacityPromote growthAnimal feeding stuffBiotechnologyPregnancy
The invention discloses a sow compound premix feed suitable for sow in later stage of pregnancy. The sow compound premix feed is composed of the following components in parts by weight: 200-300 parts of expanded soybean meal, 80-200 parts of fish meal, 180-220 parts of bean pulp, 200-300 parts of beer yeast, 120-150 parts of calcium hydrogen phosphate, 100-200 parts of stone powder, 20-30 parts of lysine, 10-30 parts of methionine, 15-35 parts of threonine, 60-100 parts of compound trace elements, 12-20 parts of decavitamin, 100-150 parts of oligopeptide, 0.5-1 part of enzyme preparation, 1-2 parts of probiotics, 1-2 parts of feed preservative, 1-2 parts of magnesium sulfate, 4-5 parts of zinc methionine and 3-4 parts of chromium methionine. The sow compound premix feed suitable for sow in the later stage of pregnancy disclosed by the invention can be adapted to the metabolic mode of sow in the later stage of pregnancy, improve the sow endocrine, promote rise of blood sugar and ketone body levels and enhance the placental function to ensure that a fetus can obtain enough nutrients from the female parent so as to ensure the normal development of the fetus and promote the fetal growth.
Owner:山东和美华农牧科技股份有限公司
Preparation method of hand-washing agent for maternity underpants
InactiveCN106854502AReduce staticReduce frictionOrganic detergent compounding agentsSurface-active detergent compositionsFiberFetal growth
The invention relates to a preparation method of a hand-washing agent for maternity underpants. Improper choosing of washing products also can make a pregnant woman to be allergic, and further can become a potential carcinogenic factor to harm the health of an expectant mother and further indirectly affect the fetal growth. The hand-washing agent for the maternity underpants, disclosed by the invention, is prepared by mixing a cactus extract, a chaga extract, a pueraria extract, a pine bark extract, Blumea oil, a ginseng extracting solution, AES, probiotic flora, paeonol, chitosan, citric acid, a sophora flavescens extracting solution, a chamomile extracting solution, a magnolin extracting solution, a honeysuckle extracting solution, chitin, phytosterol, acetic acid, essence and water. The hand-washing agent for the maternity underpants, disclosed by the invention, aims at decomposing common stains during the pregnancy and puerperium, deeply penetrates into clothes fibers to play antibacterial and antimicrobial effects, washes off various stubborn stains, reduces static electricity and friction and makes the underpants cleaner, softer and brighter, quietly elegant and faintly scented and comfortable and soft.
Owner:CHANGSHA XIEHAOJI BIOENG CO LTD
Feed additive used for pregnant ewes in late period
InactiveCN106858085AAbundant raw materialsNutritional balanceFood processingAnimal feeding stuffDiseaseEggshell
The present invention mainly relates to the technical field of breeding and discloses a feed additive used for pregnant ewes in a late period. The feed additive is prepared from the following raw materials: egg shells, walnuts, green papayas, folium ginkgo, camphor tree leaves, flos sophorae immaturus, semen pruni, herb of virgate lespedeza, sea buckthorn seed oil, fructooligosaccharides, compound proteins, compound minerals, compound vitamins and grape wine yeasts. The feed additive is rich in raw materials and balanced in nutrition, protects gastrointestinal functions of the ewes, is easy to digest and absorb, reduces sickness rate of the ewes by 4.7%, promotes the growth and development of fetuses, decreases miscarriage rate by 3.2%, significantly improves the breeding efficiency, and increases economic incomes of farmers by 9.8%. The eggshells, walnuts and green papayas are subjected to primary fermentation with the grape wine yeasts, fragrance components are increased, palatability is improved, the feed additive is balanced in nutrition, promotes digestion and absorption of the ewes, improves feed utilization and disease resistance of the ewes, speeds up the secretion of milk, is conducive to fetal growth, and increases the survival rate of lambs by 3.6%.
Owner:安徽省微科生物科技有限公司
Molecular marker for diagnosis and treatment of intrauterine fetal growth restriction
ActiveCN104928394AInnovativeSensitiveMetabolism disorderMicrobiological testing/measurementDiseaseFetal growth
The present invention discloses application of a hypomethylation gene PIK3R1. On the one hand, the hypomethylation gene PIK3R1 can be used to the preparation of diagnostic products for intrauterine fetal growth restriction, and on the other hand the hypomethylation gene PIK3R1 can be used to prepare the medicament for treating intrauterine fetal growth restriction. The experiments of the present invention demonstrate that the patients with intrauterine fetal growth restriction have PIK3R1 gene hypomethylation in blood; the findings provide strong for molecular biology basis for the diagnosis and treatment of patients with intrauterine fetal growth restriction, and have far-reaching clinical significance and generalization value.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
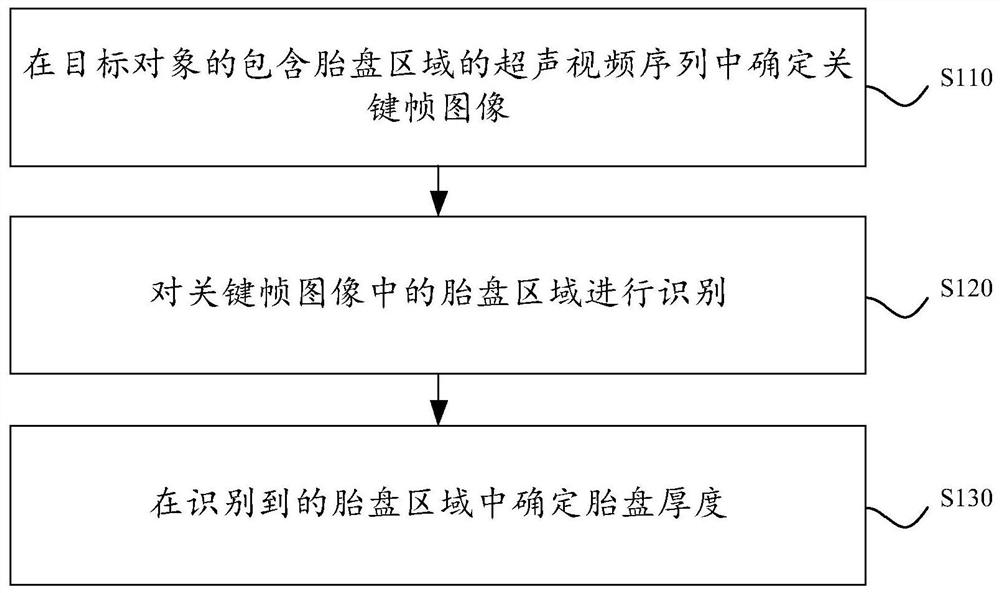
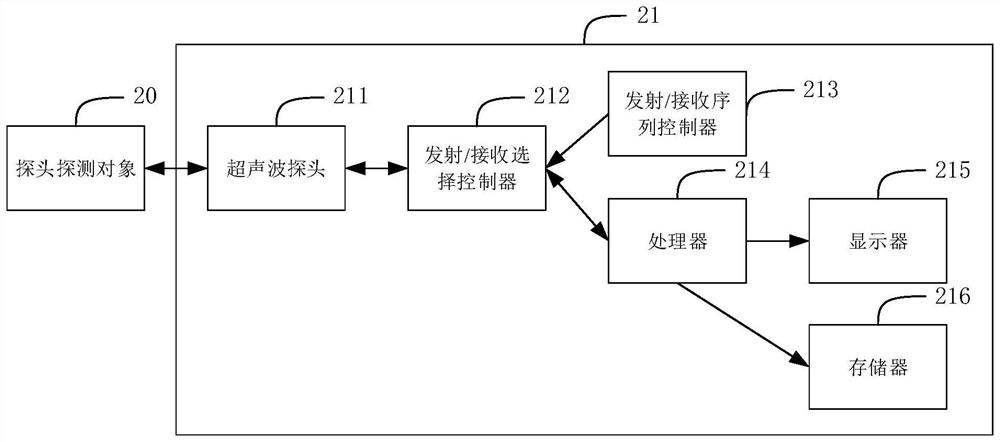
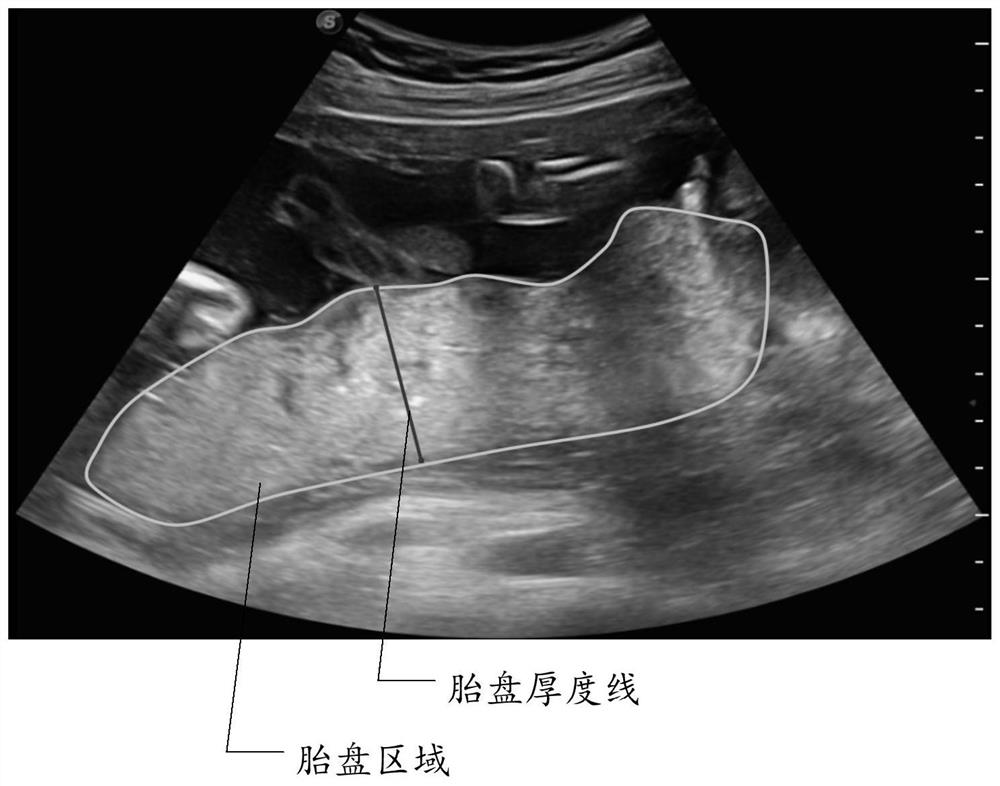
Placenta thickness determination method, device and equipment and storage medium
PendingCN112426170AImprove measurement efficiencyGuaranteed accuracyOrgan movement/changes detectionInfrasonic diagnosticsFetal growthVideo sequence
The invention discloses a placenta thickness determination method. The method comprises the following steps: determining a key frame image in an ultrasonic video sequence containing a placenta regionof a target object; identifying a placenta region in the key frame image; determining the placenta thickness in the identified placenta region. By applying the technical scheme provided by the invention, the determination of the key frame image, the identification of the placenta region, the determination of the placenta thickness and the like can be automatically completed, the user operation canbe simplified, the placenta thickness measurement efficiency can be improved, excessive dependence on user experience and subjective judgment can be avoided, and the placenta thickness determinationaccuracy can be effectively ensured; and data support is provided for subsequent fetal growth and development and maternal health assessment. The invention further discloses a placenta thickness determination device and equipment and a storage medium, and the corresponding technical effects are achieved.
Owner:SONOSCAPE MEDICAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com