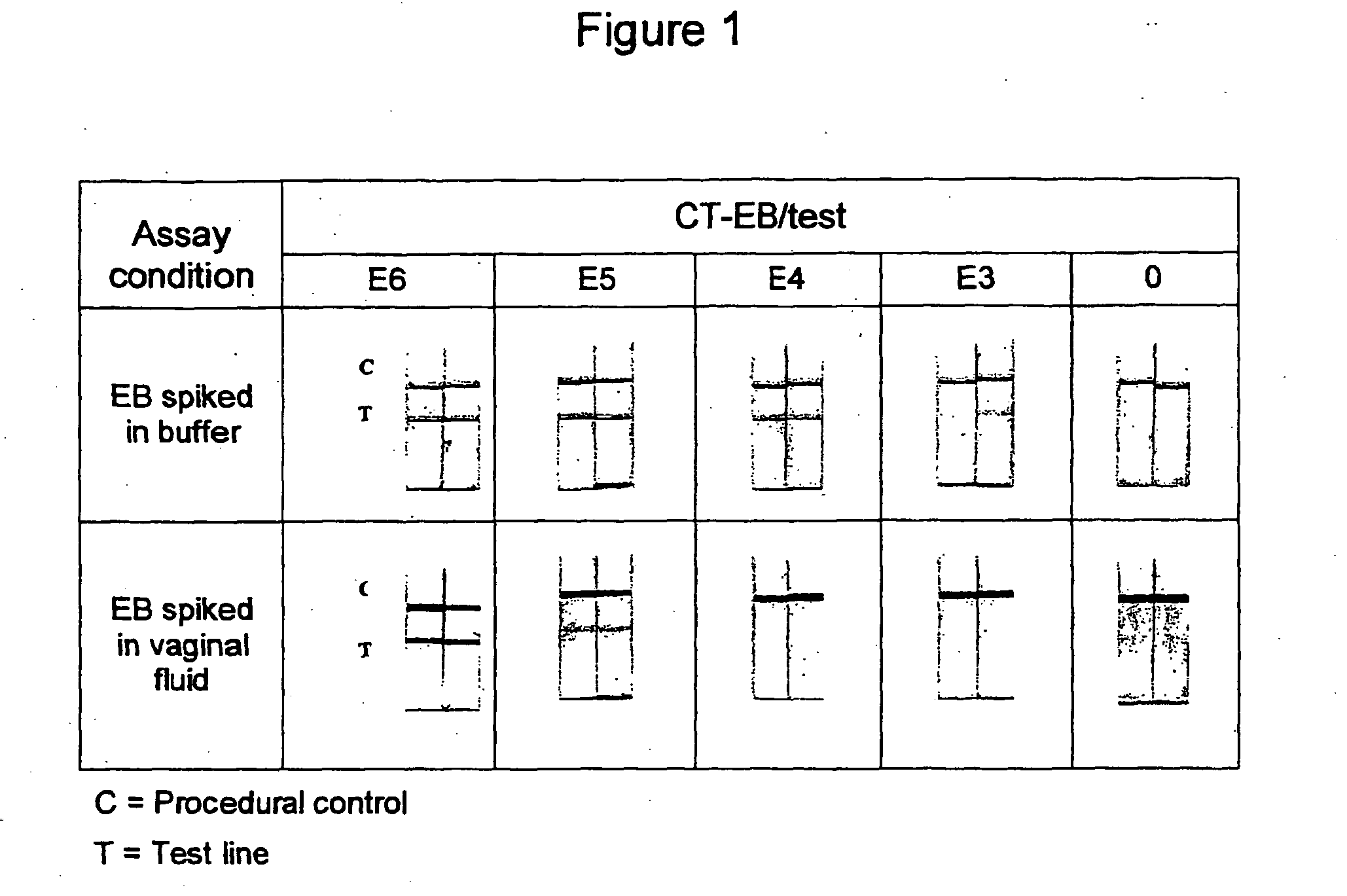
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65 results about "Vaginal fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
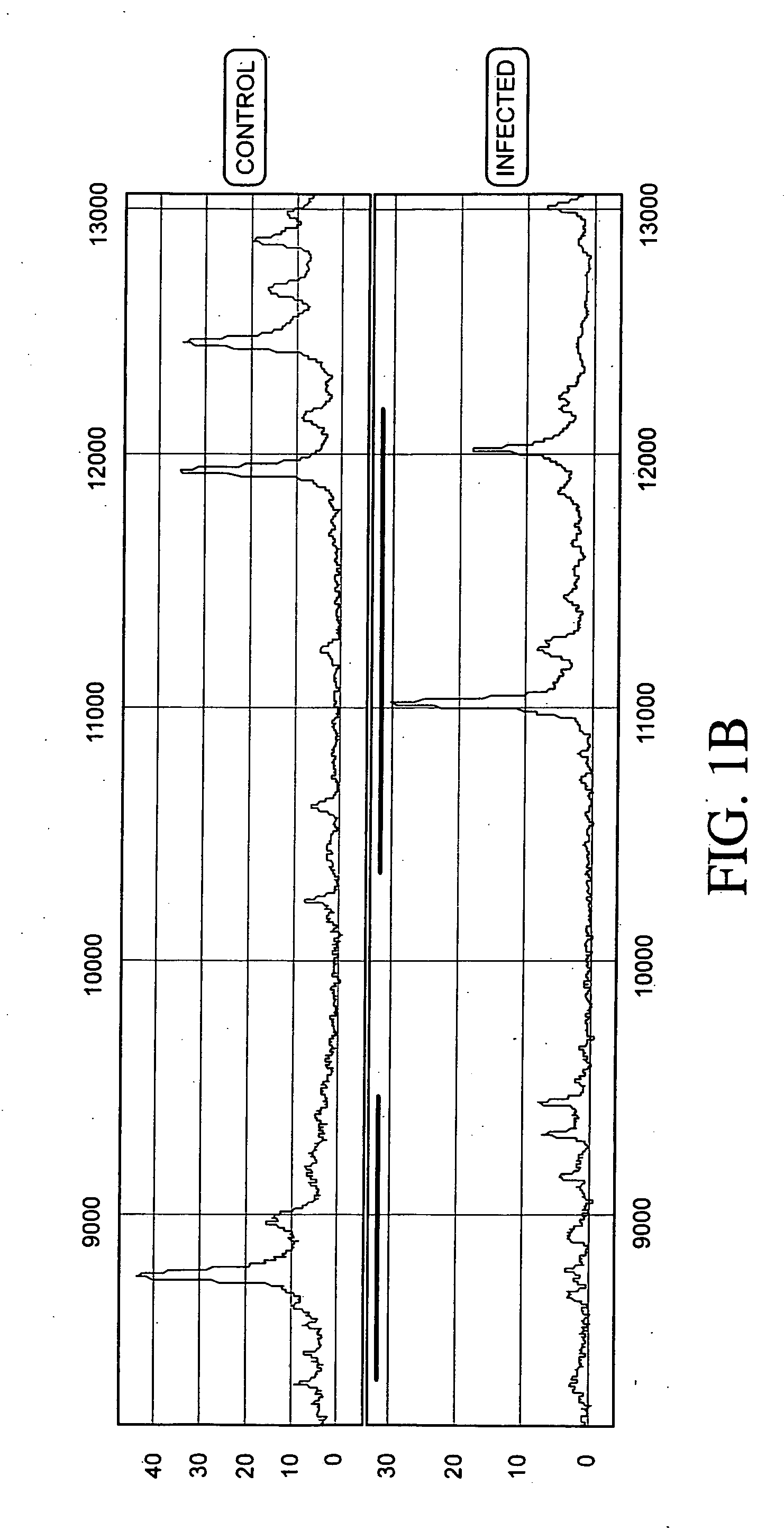
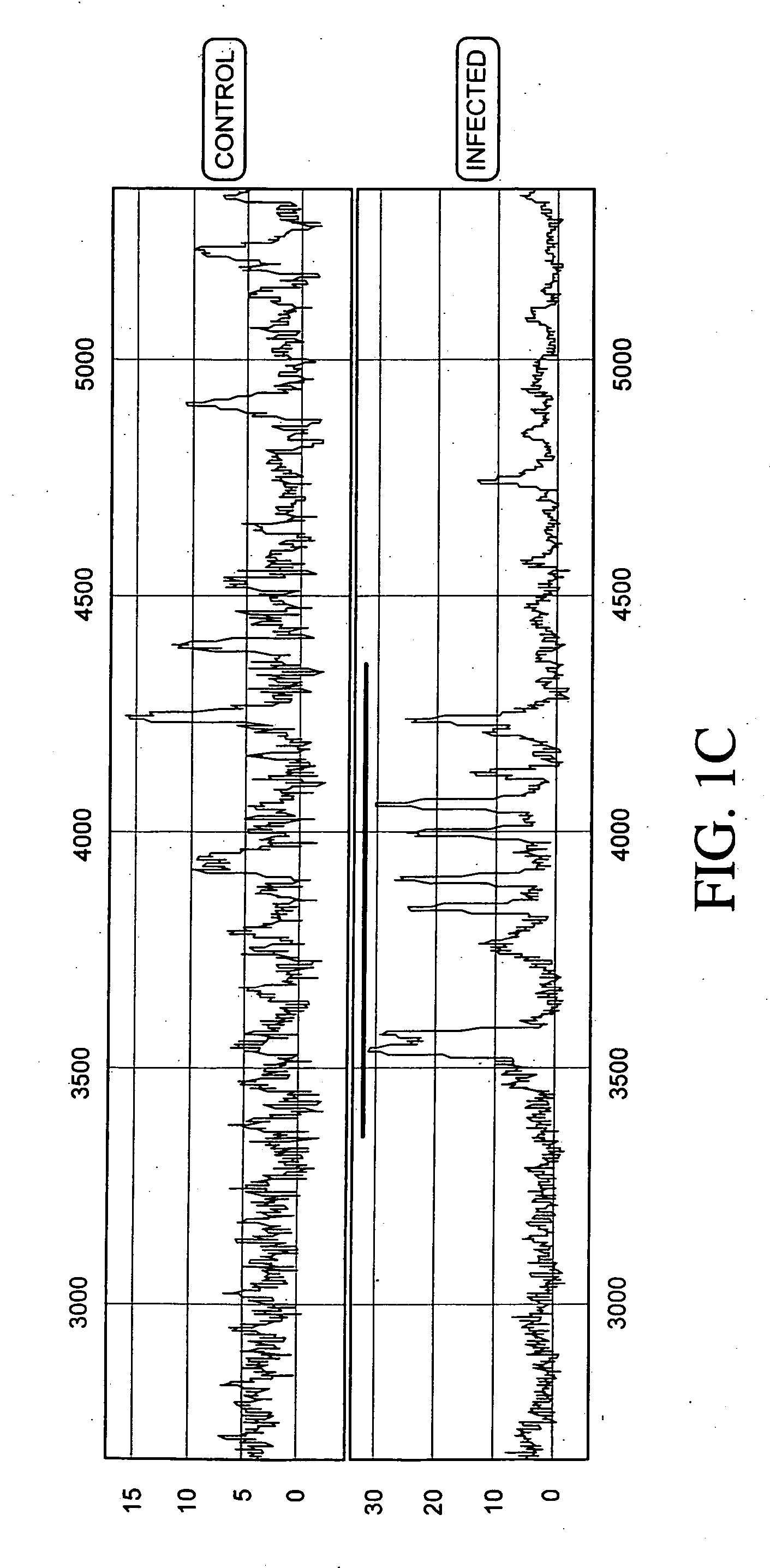
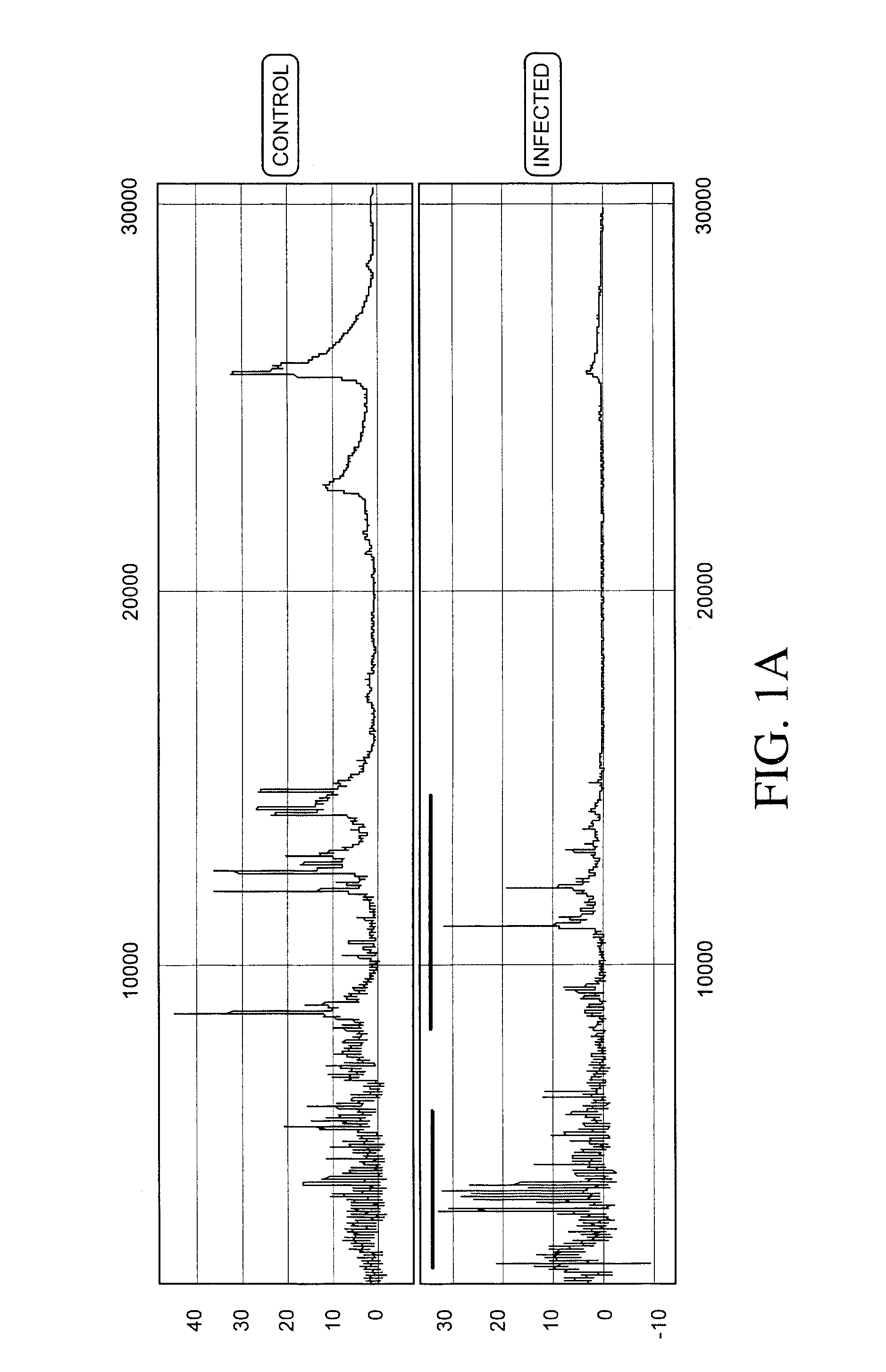
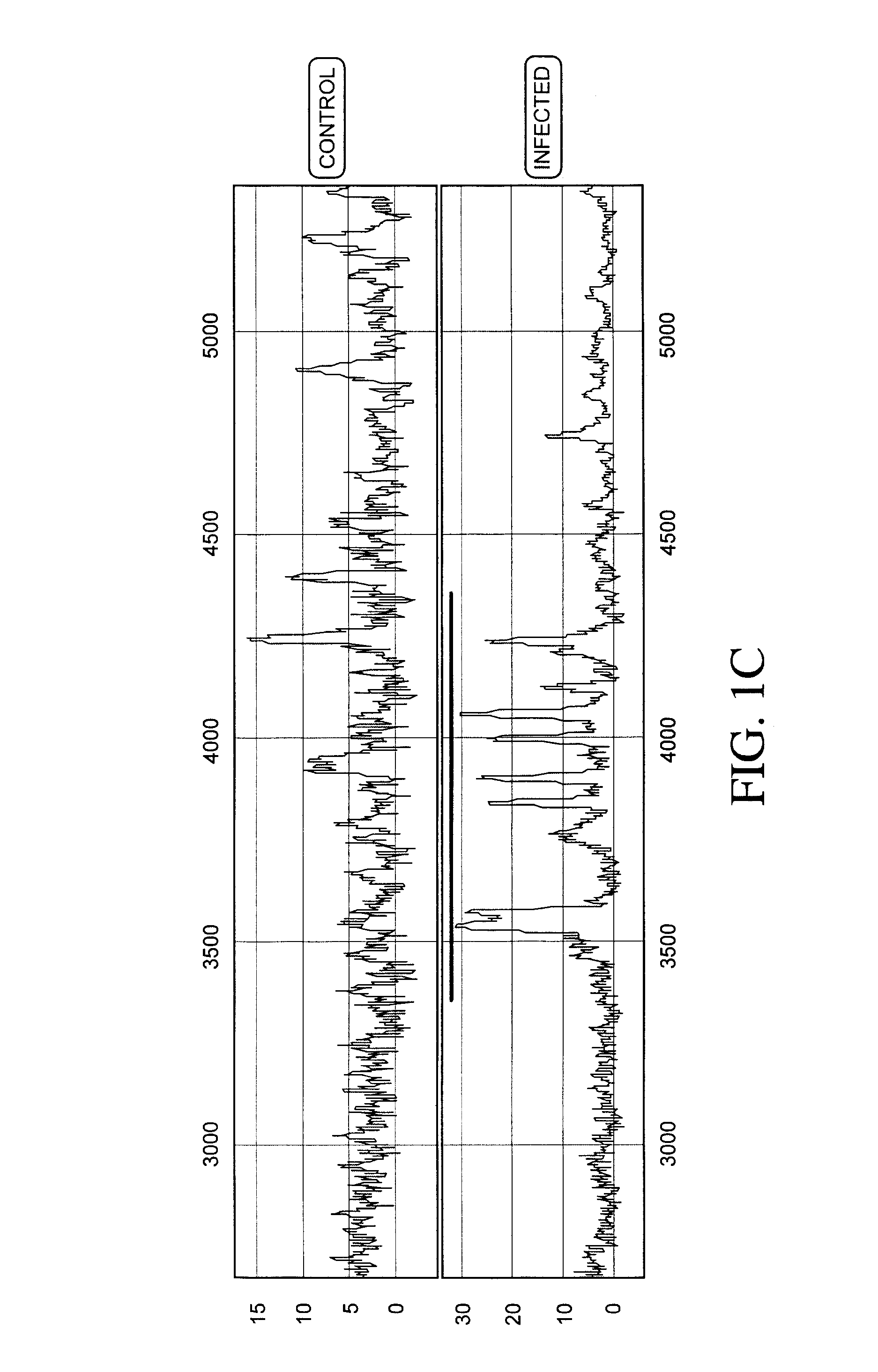
Process for discriminating between biological states based on hidden patterns from biological data
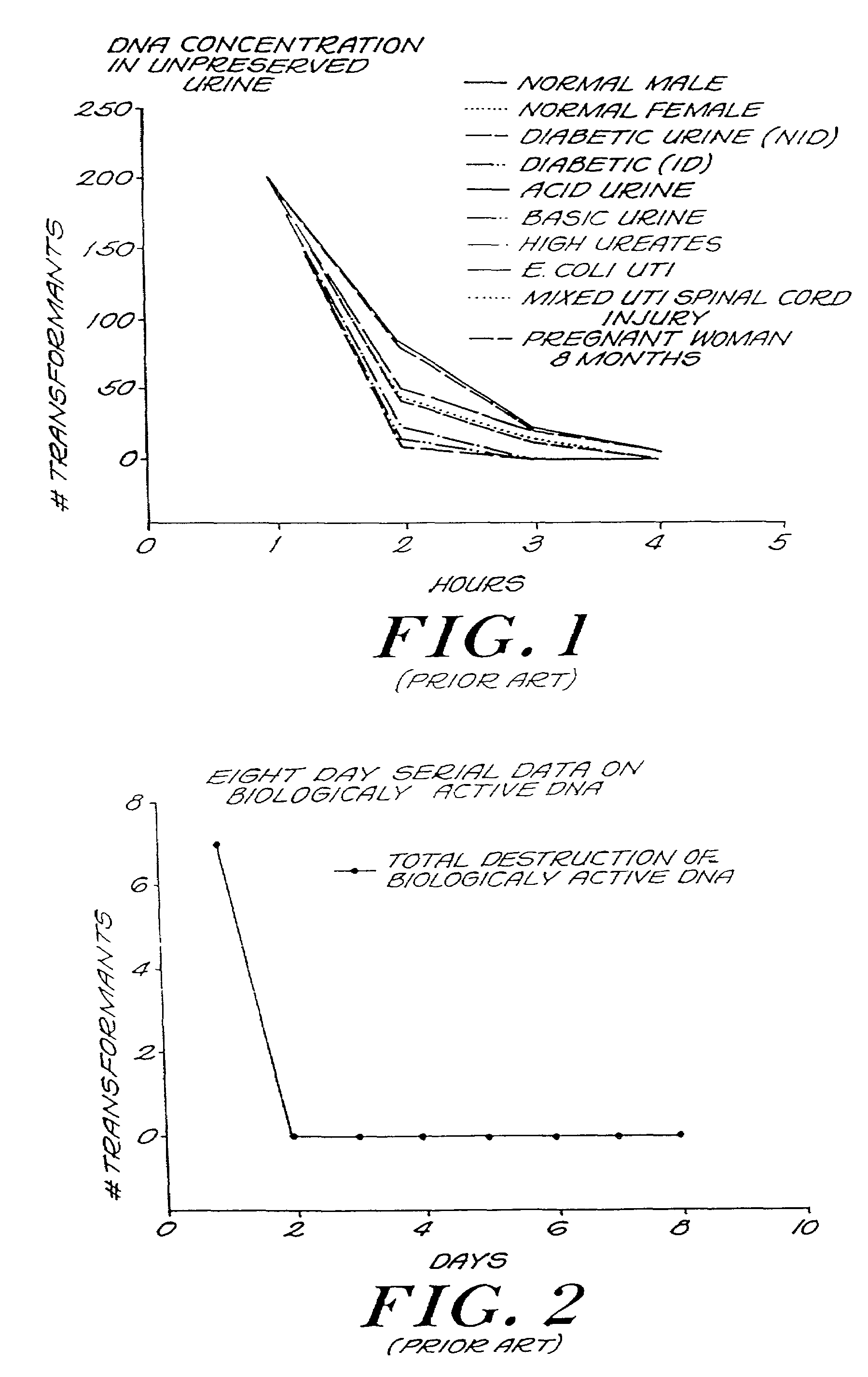
The invention describes a process for determining a biological state through the discovery and analysis of hidden or non-obvious, discriminatory biological data patterns. The biological data can be from health data, clinical data, or from a biological sample, (e.g., a biological sample from a human, e.g., serum, blood, saliva, plasma, nipple aspirants, synovial fluids, cerebrospinal fluids, sweat, urine, fecal matter, tears, bronchial lavage, swabbings, needle aspirantas, semen, vaginal fluids, pre-ejaculate.), etc. which is analyzed to determine the biological state of the donor. The biological state can be a pathologic diagnosis, toxicity state, efficacy of a drug, prognosis of a disease, etc. Specifically, the invention concerns processes that discover hidden discriminatory biological data patterns (e.g., patterns of protein expression in a serum sample that classify the biological state of an organ) that describe biological states.
Owner:ASPIRA WOMENS HEALTH INC +1
Wetness Indicator Having Varied Hues
ActiveUS20130066289A1Component separationMaterial analysis by observing effect on chemical indicatorChange colorHue
A wetness indicator material may be used on a substrate to form a wetness sensor. The sensor may show either the presence or absence of an aqueous-based fluid or water-containing medium, such as vaginal fluid or urine in a personal hygiene article. The wetness sensor may be incorporated into the article. The wetness indicator material includes a standard colorant that does not change color when wetted. The standard colorant increases the range of hues exhibited by the wetness indicator material.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Proteomic analysis of biological fluids
ActiveUS20070161125A1Eliminate needMicrobiological testing/measurementAnalogue computers for chemical processesDiseaseGynecology
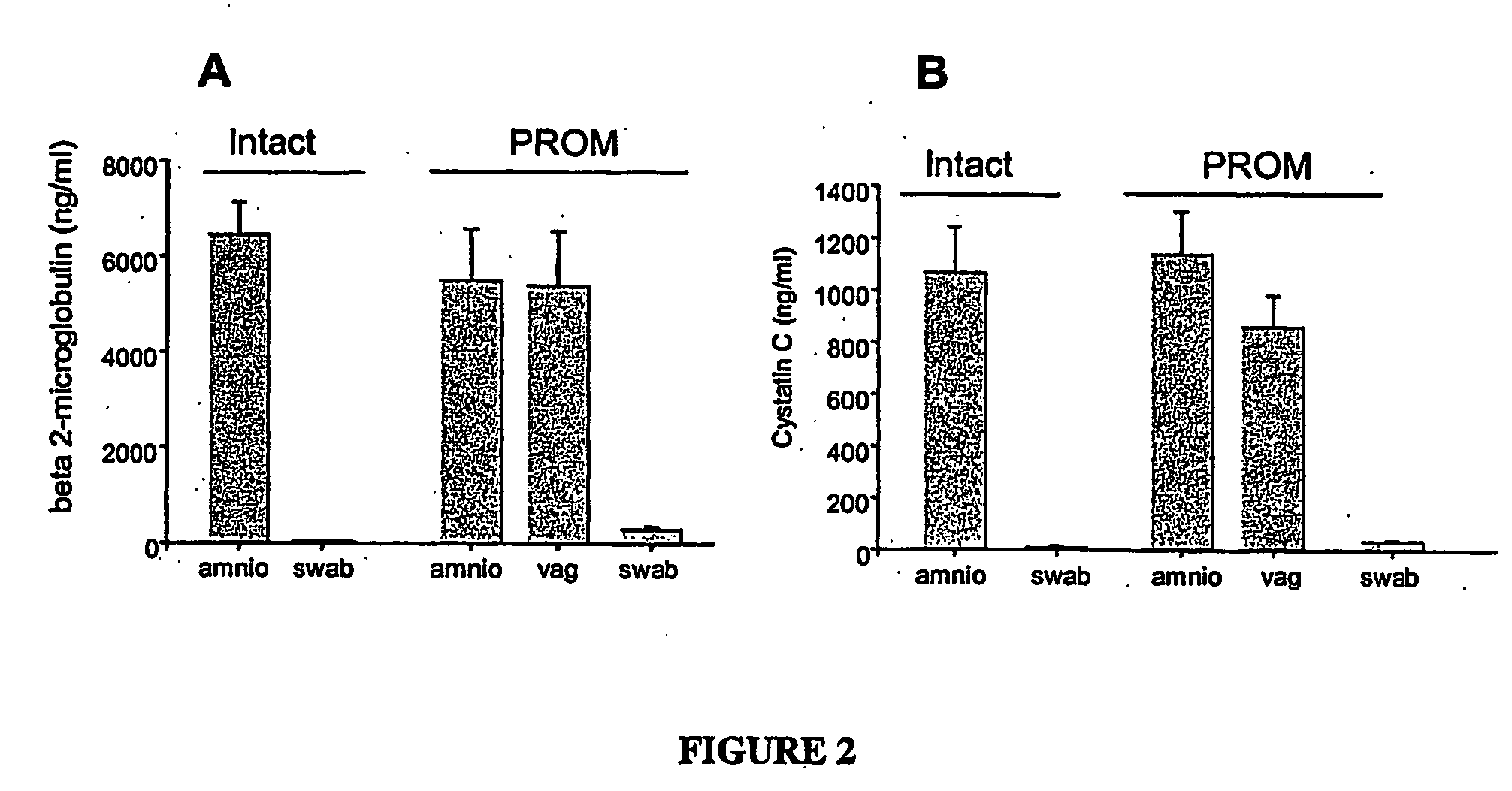
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns a comprehensive proteomic analysis of human amniotic fluid (AF) and cervical vaginal fluid (CVF), and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, pre-term labor, and / or chromosomal defects. The invention further concerns the identification of biomarkers and groups of biomarkers that can be used for non-invasive diagnosis of various pregnancy-related disorders, and diagnostic assays using such biomarkers.
Owner:HOLOGIC INC
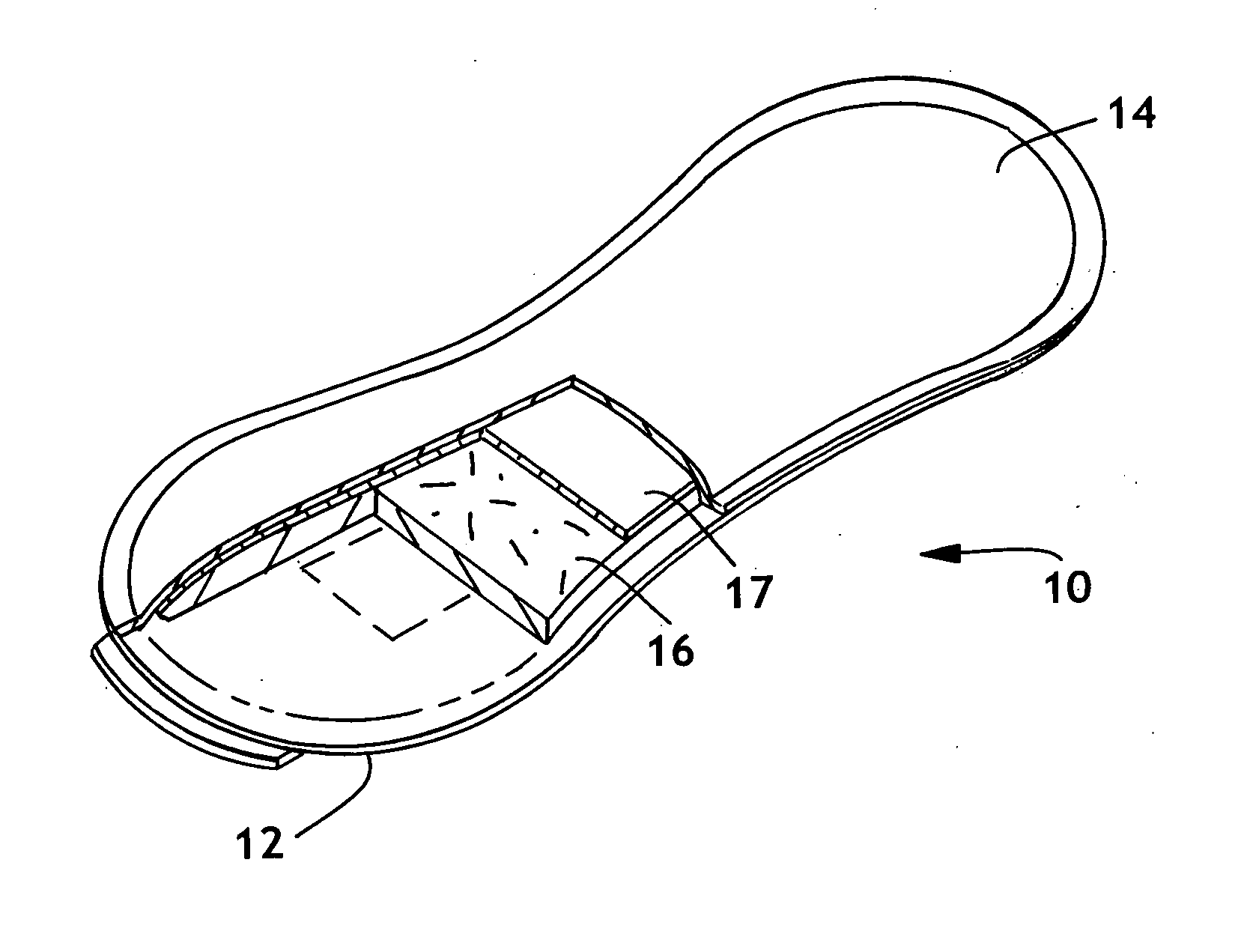
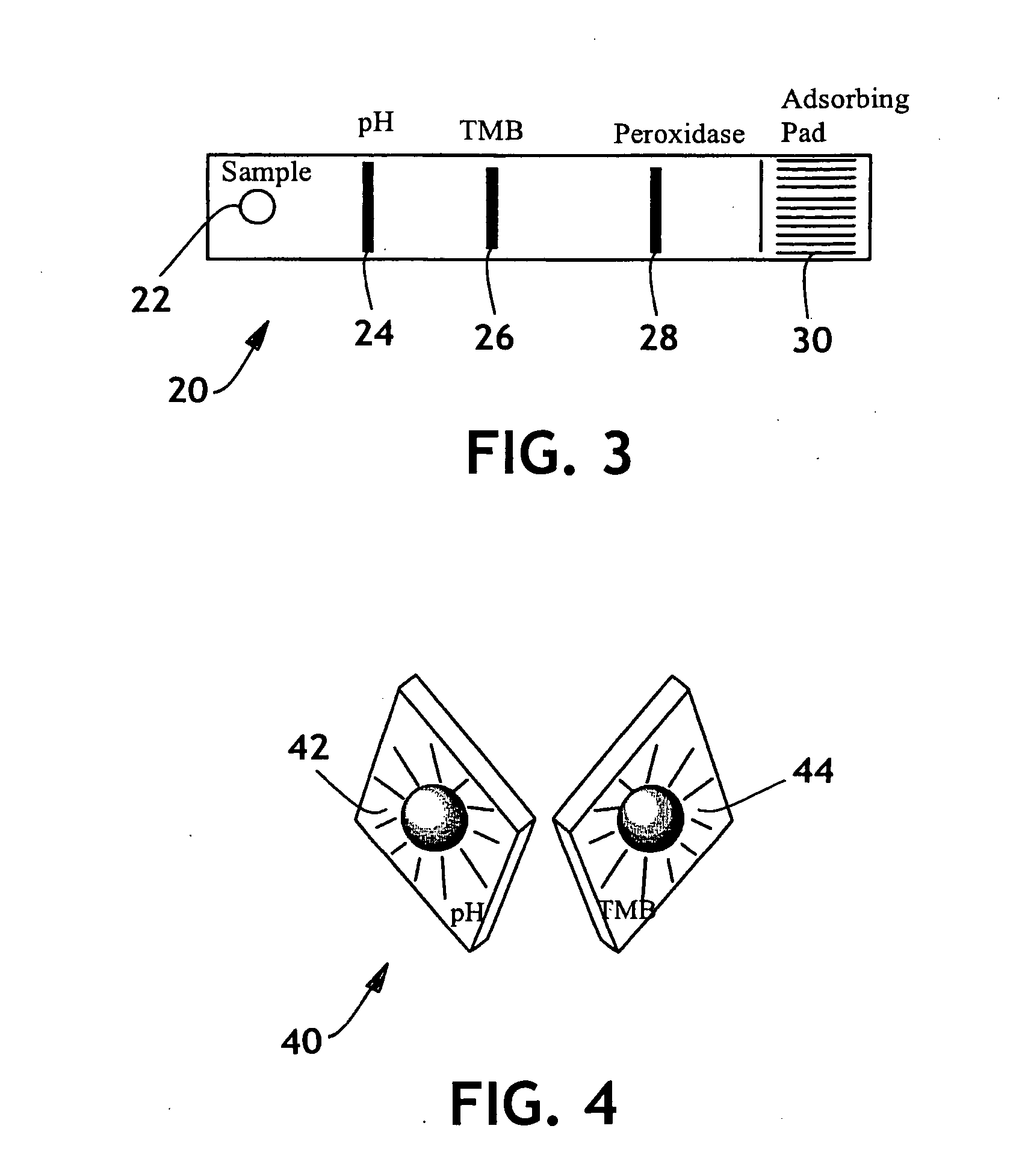
Detection of premature rupture of the amniotic membrane
The premature rupture of amniotic fluid (PROM) may be discovered through a number of inventive means. Methods of evaluating whether PROM is present include; a) through the testing of the pH of vaginal fluids using an irreversible pH test; b) through the detection of analytes (e.g. enzymes) specific to amniotic fluid in the vaginal fluids; c) though the detection of hydrogen peroxide (H2O2) in the vaginal fluid; and d) through the detection of cholesterol in vaginal fluid. While individually indicative of PROM, it is desirable to combine at least two of these techniques to yield a powerful tool of even greater reliability. Test devices and feminine hygiene pads into which the test methods may be incorporated are also included herein.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Assay devices utilizing chemichromic dyes
InactiveUS20050191704A1Microbiological testing/measurementBiological testingTest sampleColor changes
An assay device for detecting amines within a test sample (e.g., vaginal fluid) is provided. The assay device comprises a detection zone within which a chemichromic dye is contained. The chemichromic dye is capable of undergoing a color change upon exposure to one or more amines within the test sample.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Wetness sensor for use in an absorbent article
A wetness sensor for an absorbent article that is formed from an ink is provided. The ink includes a proton-accepting chromogen and a proton-donating agent (or color developer). Prior to use, the ink is generally dry and in a protonated form so that it has a visible color. However, upon contact with bodily fluids (e.g., urine, fecal matter, mucus, menses, vaginal fluid, etc.), water in the fluid can lead to deprotonation of the chromogen, thereby resulting in a shift of the absorption maxima of the chromogen towards either the red (“bathochromic shift”) or blue end of the spectrum (“hypsochromic shift”). To increase the rate of the color change during use, the proton-donating agent is an aliphatic carboxylic acid that is highly soluble in the bodily fluid (e.g., urine), and therefore results in a color change that is very rapid and may be detected within a relatively short period of time.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Wetness Sensor for Use in an Absorbent Article
A wetness sensor for an absorbent article that is formed from an ink is provided. The ink includes a proton-accepting chromogen and a proton-donating agent (or color developer). Prior to use, the ink is generally dry and in a protonated form so that it has a visible color. However, upon contact with bodily fluids (e.g., urine, fecal matter, mucus, menses, vaginal fluid, etc.), water in the fluid can lead to deprotonation of the chromogen, thereby resulting in a shift of the absorption maxima of the chromogen towards either the red (“bathochromic shift”) or blue end of the spectrum (“hypsochromic shift”). To increase the rate of the color change during use, the present inventors have discovered that a specific type of proton-donating agent may be employed. More particularly, the proton-donating agent is an aliphatic carboxylic acid that is highly soluble in the bodily fluid (e.g., urine), and therefore results in a color change that is very rapid and may be detected within a relatively short period of time. The extent of the color change is also generally sufficient to provide a “real-time” indication of wetness on the absorbent article.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Non-invasive assessment of intra-amniotic environment
InactiveUS20060240495A1Reduce oxygen free radical toxicityRaise the possibilityDisease diagnosisBiological testingProtein profilingIntra-Amniotic
Owner:VERMILLION INC
Method of predicting risk of pre-term birth
InactiveUS20120270747A1Increased riskMicrobiological testing/measurementChemiluminescene/bioluminescenceBacteriuriaObstetrics
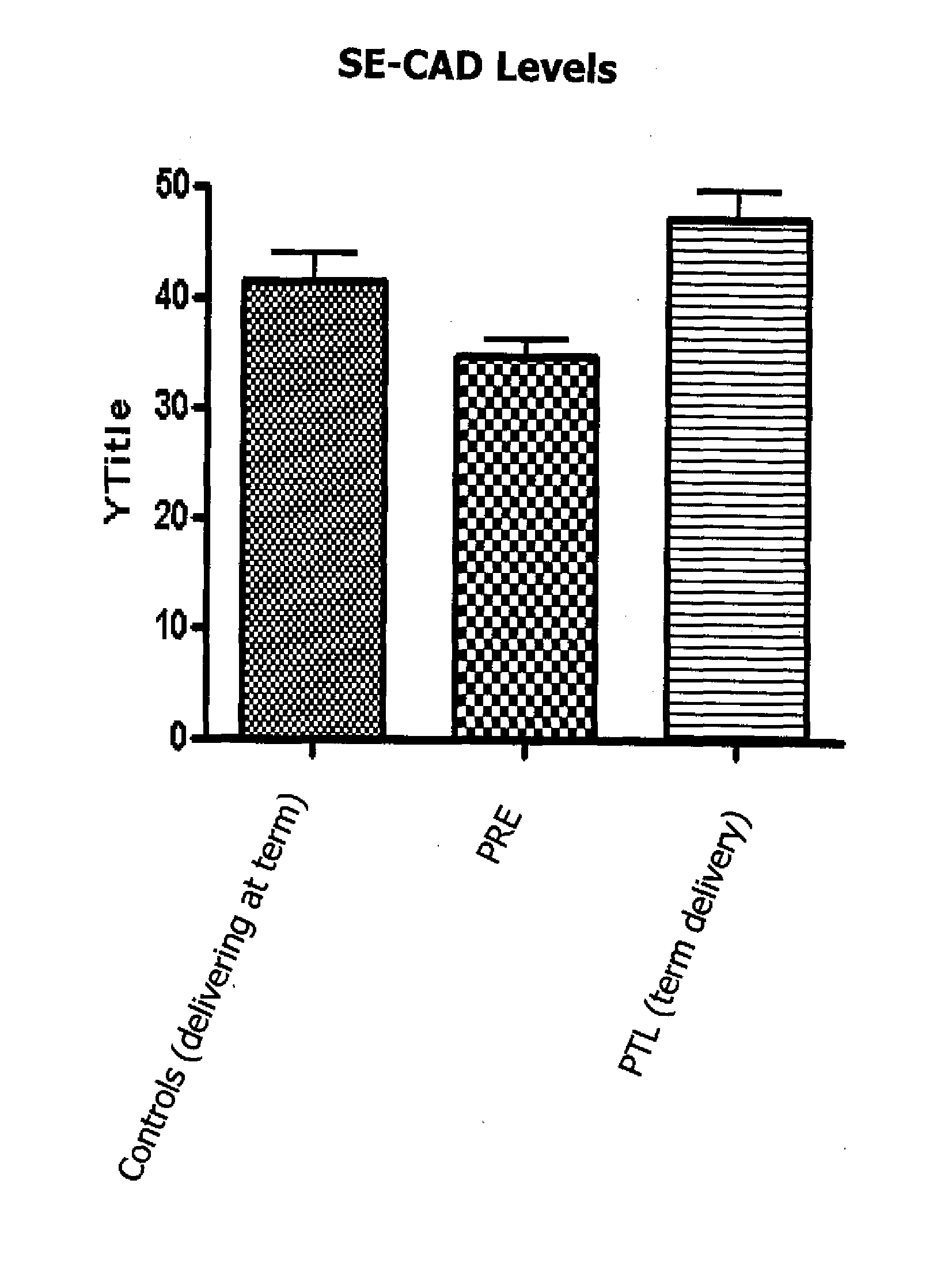
A method for diagnosing, or differentially diagnosing, an increased risk of pre-term birth (PTB) involves detecting or measuring increased expression of a biomarker Soluble E-cadherin (SE-CAD) in a biological sample from a mammalian subject, particularly in the urine, cervicovaginal fluid or blood. An increased level of expression of SE-CAD above the level of expression in the same sample of a healthy mammalian subject is an indication of a diagnosis of increased risk of PTB. Such diagnosis may further involve identify other clinical symptoms of PTB or PTL. Additionally the method may use additional biomarkers, such as fetal fibronectin.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
System and method for bacterial vaginosis testing
InactiveUS20100009336A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPhases of clinical researchComputer science
A method and system for testing fluids from a patient is provided. In one embodiment, a portable device is configured with one or more testing modules adapted perform one or more tests on a fluid sample in order to determine if a patient has bacterial vaginosis. A timer module coupled to a sample transport is configured to move a vaginal fluid sample between one or more testing modules at various stages along a predetermined testing path. In an embodiment, the timer module is configured to move the vaginal fluid sample between the testing modules along a circular testing path. The vaginal fluid sample may be processed in sequential order over predetermined time periods with respect to the processing performed by the testing module in order to detect the presence of, or lack of, Lactobacilli in the patient's vaginal flora to determine whether or not the patient has bacterial vaginosis.
Owner:SULLIVAN SHANNON E
Assays for trichomonal and other hydrolases
InactiveUS7041469B2Easily hydrolyzedEasily be wettedBioreactor/fermenter combinationsBiological substance pretreatmentsParticulatesHydrolase inhibitor
The release by trichomonads of a hydrolase that hydrolyzes a narrowly defined class of substrates at a low pH without interference from hydrolases that are unrelated to trichomoniasis is the basis for a selective diagnostic assay for trichomoniasis that measures hydrolysis of any of these substrates by vaginal fluid at a low pH. Selective assays for trichomoniasis are also obtained by removing particulate matter from a sample of vaginal fluid to extract a fraction devoid of particles greater than a selected size, and where desired, combining the extracted fraction with any of certain specified hydrolase inhibitors, then testing the fraction for enzymatic hydrolase activity. These qualities of trichomoniasis are the basis for a series of diagnostic tests and test devices that produce results that are detectable by visual and other means with a high degree of accuracy.
Owner:QUIDEL
Removal of molecular assay interferences
InactiveUS7569342B2Improve trustGood signal responseBioreactor/fermenter combinationsBiological substance pretreatmentsAmniotic fluidExcretion process
Methods and systems for removing masking agents from test samples, e.g., DNA-containing samples obtained from living subjects, when they are submitted for or subjected to molecular assays. The present invention allows molecular assays of nucleic acids in bodily fluids and excretions, such as urine, blood, blood serum, amniotic fluid, spinal fluid, conjunctival fluid, salivary fluid, vaginal fluid, stool, seminal fluid, and sweat to be carried out with greater sensitivity. The masking agents are suppressed by contacting a test sample with an amount of one or more divalent metal chelators and an amount of one or more chelator enhancing components. The amounts of the divalent metal chelator(s) and the chelator enhancing component(s) are selected such that interference of a masking agent on a molecular assay of a nucleic acid-containing test sample are suppressed, and upon contact with the divalent metal chelator(s) / chelator enhancing component(s), the masking agents are suppressed.
Owner:SIERRA MOLECULAR CORP
Mucus formulation for mucosal surfaces and uses thereof
The present invention includes formulations comprising synthetic vaginal fluid, synthetic cervical mucus, and, a mixture of a synthetic cervical mucus and synthetic vaginal fluid suitable for mucosal administration of at least one therapeutic agent. The formulations of the invention are useful in the treatment, prevention and control of diseases and conditions in a subject in need thereof. The present invention also includes methods of administering the formulations. Also included are methods of using the formulations in the treatment, prevention and control of diseases or conditions affecting other mucus membranes, such as the nose, throat and gastrointestinal tract.
Owner:BIOSYN
Collection device for diagnostics of vaginal discharge
A method of analyzing vaginal fluid includes collecting vaginal fluid in a vaginal fluid collecting system and transporting the collected vaginal fluid to a location for analysis. The analysis can be stored in and retrieved from a secure cloud storage data base.
Owner:QURASENSE INC
Collection device for diagnostics of vaginal discharge
A method of analyzing vaginal fluid includes collecting vaginal fluid in a vaginal fluid collecting system and transporting the collected vaginal fluid to a location for analysis. The analysis can be stored in and retrieved from a secure cloud storage data base.
Owner:QURASENSE INC
Paper microfluidic devices for forensic serology
ActiveUS20170067881A1Low costInexpensive to fabricateMaterial analysis by observing effect on chemical indicatorLaboratory glasswaresPerspirationAnimal feces
Paper microfluidic devices for the detection of bodily fluids are provided. Such devices can be used, for example, for detection of bodily fluids from or at crime scenes, including blood, saliva, semen, urine, feces, vaginal fluids, and perspiration. Detection can be performed using colorimetric reagents that react when placed in contact with the fluid of interest. A single device can be used to test for multiple bodily fluids at the same time.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Sample preparation for the detection of infectious agents
InactiveUS20050084862A1Reduce inhibitionMicrobiological testing/measurementBiological material analysisMedicineInfectious agent
Methods for improving the quality of sub-optimal patient samples for detection of infectious agents are described. In particular, endocervical fluid samples or vaginal fluid samples are treated with DNase to improve the reliability of detection of infectious agents. Kits for carrying out the methods are also described.
Owner:DIAGNOSTICS FOR THE REAL WORLD LTD
Process for discriminating between biological states based on hidden patterns from biological data
The invention describes a process for determining a biological state through the discovery and analysis of hidden or non-obvious, discriminatory biological data patterns. The biological data can be from health data, clinical data, or from a biological sample, (e.g., a biological sample from a human, e.g., serum, blood, saliva, plasma, nipple aspirants, synovial fluids, cerebrospinal fluids, sweat, urine, fecal matter, tears, bronchial lavage, swabbings, needle aspirantas, semen, vaginal fluids, pre-ejaculate.), etc. which is analyzed to determine the biological state of the donor. The biological state can be a pathologic diagnosis, toxicity state, efficacy of a drug, prognosis of a disease, etc. Specifically, the invention concerns processes that discover hidden discriminatory biological data patterns (e.g., patterns of protein expression in a serum sample that classify the biological state of an organ) that describe biological states.
Owner:ASPIRA WOMENS HEALTH INC +1
Diagnosis of intra-uterine infection by proteomic analysis of cervical-vaginal fluids
ActiveUS8068990B2Eliminate needMicrobiological testing/measurementAnalogue computers for chemical processesDiseaseObstetrics
The invention concerns the identification of proteomes of biological fluids and their use in determining the state of maternal / fetal conditions, including maternal conditions of fetal origin, chromosomal aneuploidies, and fetal diseases associated with fetal growth and maturation. In particular, the invention concerns a comprehensive proteomic analysis of human amniotic fluid (AF) and cervical vaginal fluid (CVF), and the correlation of characteristic changes in the normal proteome with various pathologic maternal / fetal conditions, such as intra-amniotic infection, pre-term labor, and / or chromosomal defects. The invention further concerns the identification of biomarkers and groups of biomarkers that can be used for non-invasive diagnosis of various pregnancy-related disorders, and diagnostic assays using such biomarkers.
Owner:HOLOGIC INC
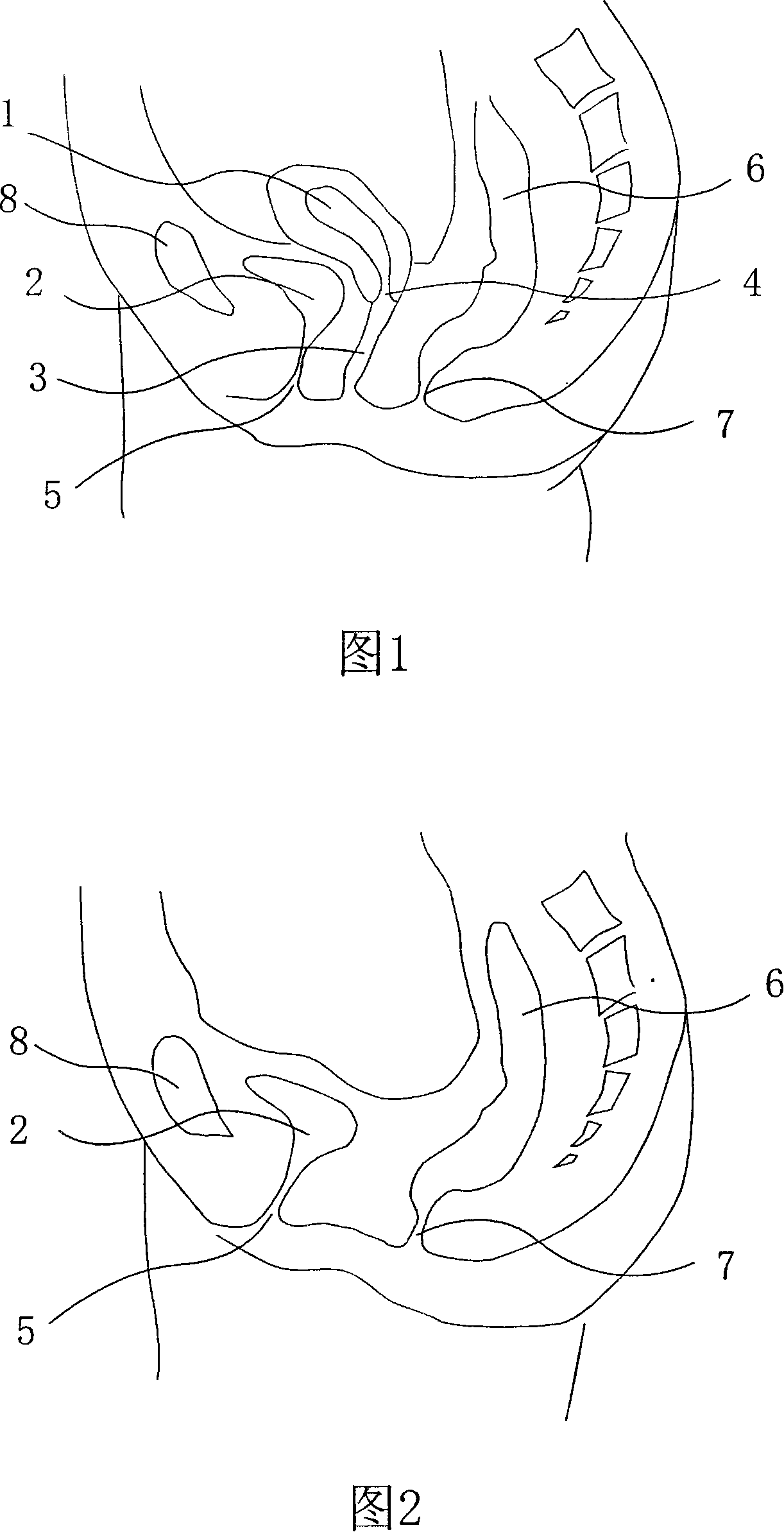
Cunt shaping expanding device
InactiveCN101152102ARelieve painReduce troubleDilatorsTubular organ implantsHuman bodyPost operative infection
The present invention discloses a vagina shaping expander that is a hollow body with a shape, size and length matching a vagina. One end of the hollow body is an open end; the hollow body is provided with holes for pouring vaginal fluid; the hollow body uses a medical denture base resin. The vagina shaping expander replaces previously articles that use non-medical materials such as wood, foam and glass tube, which fundamentally solves such problems that a post operative infection is easily caused, the glass tube is easily broken and is harmful for human body, and vaginal fluid is hard to be flowed out. The invention is characterized by not only increasing greatly success rate of an operation for reforging a vagina but also reducing troubles and pain of a patient.
Owner:李风
Joint vaginitis detection kit
InactiveCN102321730ADetection is repeatableObjective detectionMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementEcological environmentChemical reaction
The invention aims to provide a joint vaginitis detection kit which is convenient, quick and sensitive to operate, does not need any high-end instrument and equipment, and has low price. The technical scheme of the invention is to provide the joint vaginitis detection kit comprising a clamp-shaped reaction device with four dry chemical reaction blind holes, diluent and chromogenic reagent; and the four blind holes are respectively provided with a hydrogen peroxide concentration determination test reagent pad, a sialic acid glucoside enzyme activity test reagent pad, a leukocyte esterase activity determination test reagent pad and a vaginal fluid pH test reagent pad. The joint vaginitis detection kit can simultaneously test four indexes, i.e. the pH value of vaginal secretion, hydrogen peroxide, sialic acid glucoside enzyme and leukocyte esterase. Through the four indexes, the micro-ecological environment of vagina is determined, and the joint vaginitis detection kit is for identifying bacterial vaginosis of normal vaginal flora and bacteria groups.
Owner:泰普生物科学(中国)有限公司
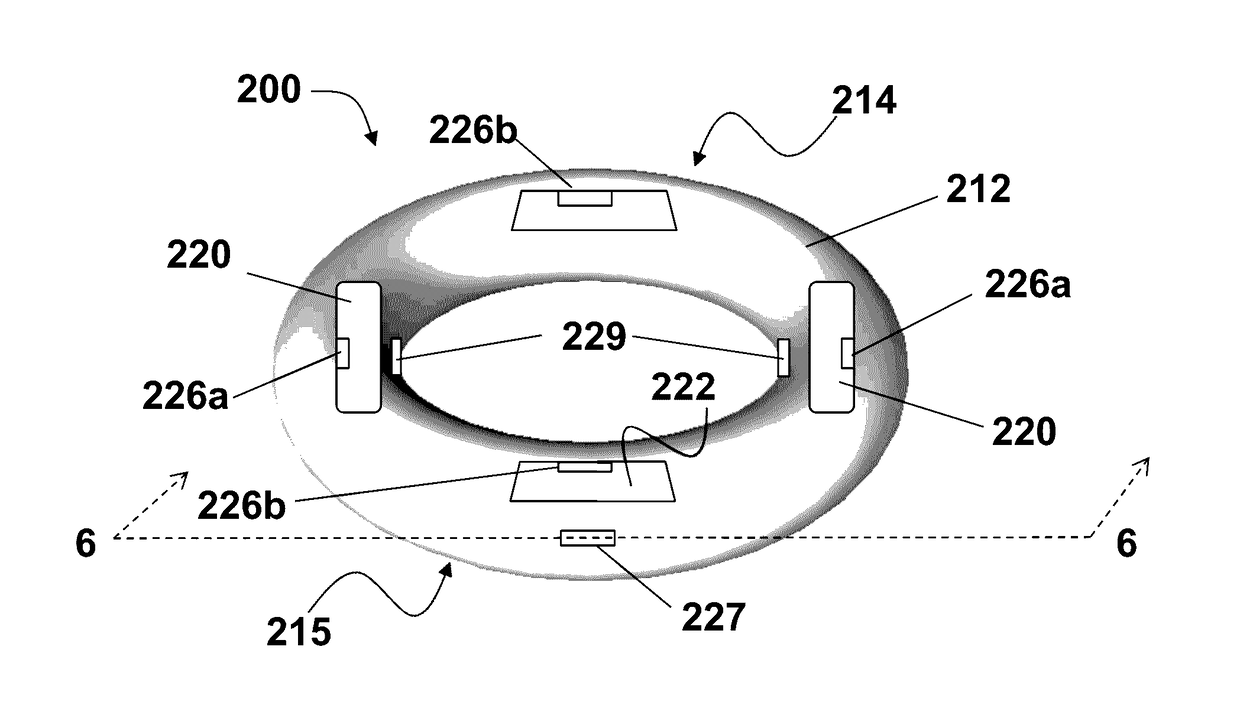
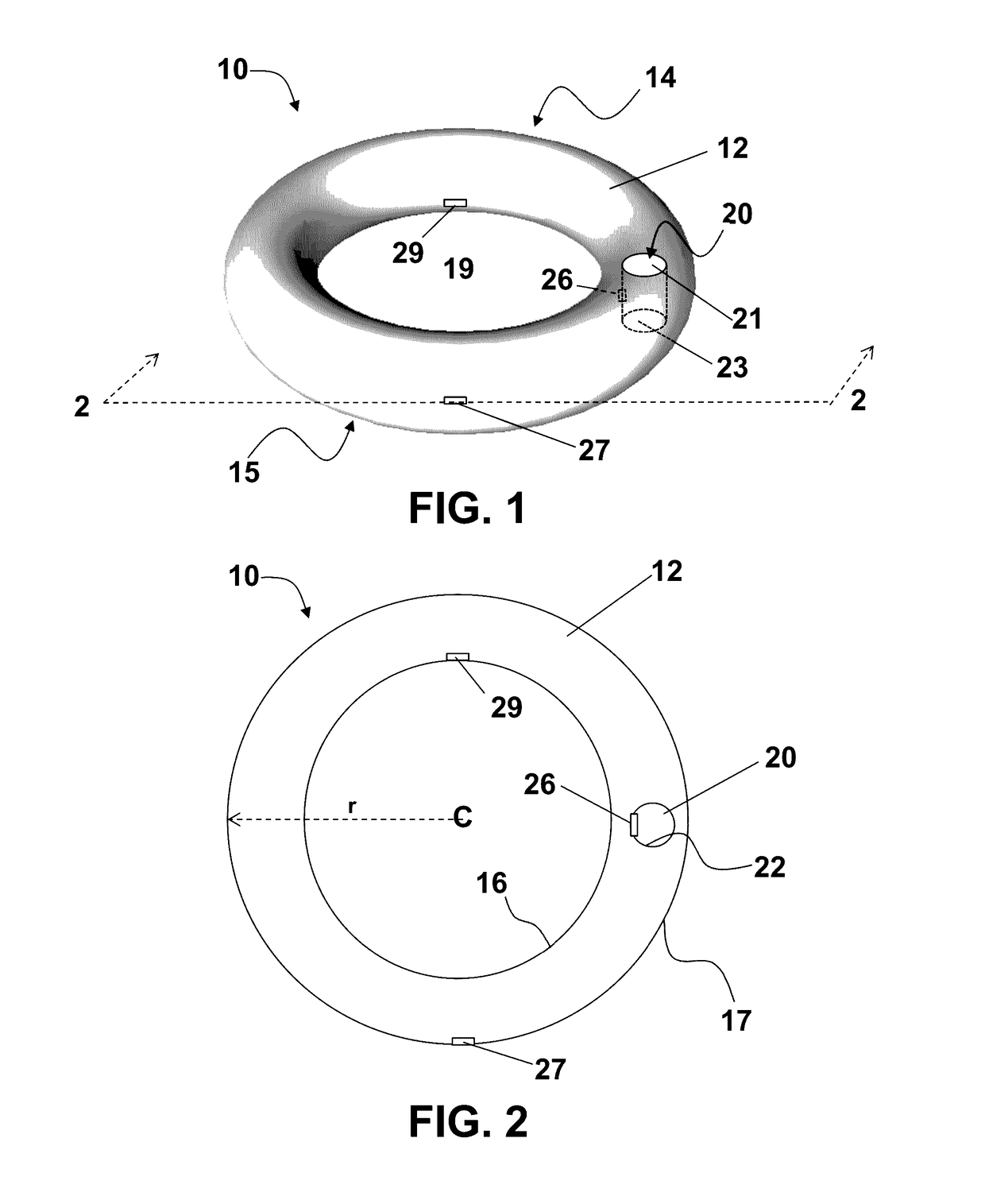
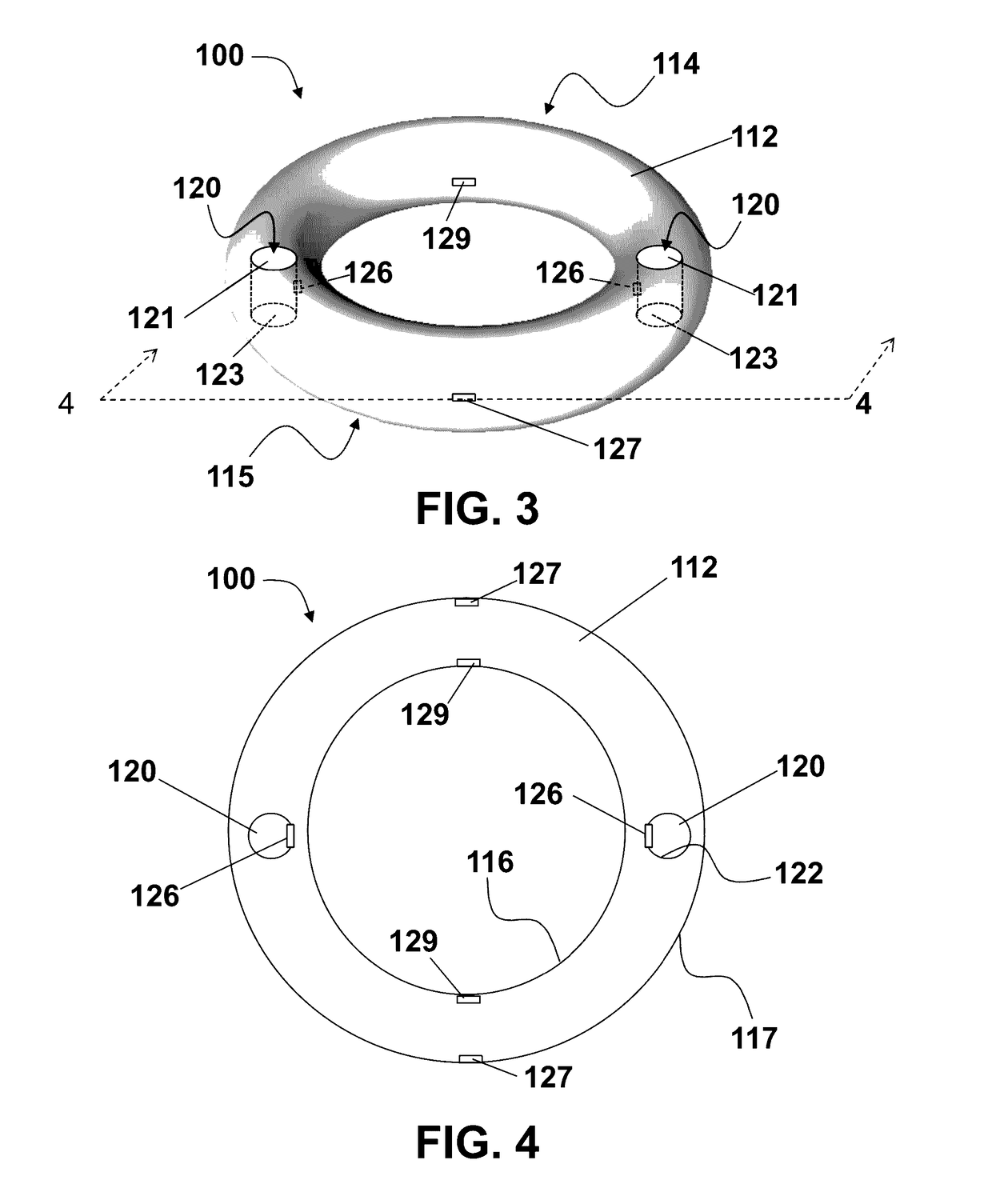
Vaginal ring sensor
A vaginal ring sensor device adapted to be placed within the vaginal vault of a user, the device including a ring body, at least one through hole that passes through the ring body, and at least one biosensor structured and arranged to sense and / or measure a parameter of vaginal fluid as such fluid passes through the at least one through hole.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Mucosal formulation
A mucosal formulation for administration to mucosal membranes, such as in the mouth, nasal passage, stomach, vagina, etc., is disclosed. The mucosal formulation contains a lipid-pharmaceutical agent complex formed from phospholipids possessing a hydrophobic moiety that orients into a hydrophobic phase and a polar head moiety that orients towards the aqueous phase (i.e., “amphipathic” lipids). When placed in an aqueous medium (e.g., vaginal fluid), the phospholipids form liposomes or other small lipid vesicles (e.g., micelles) that may then be used to deliver pharmaceutical agents into a living organism.
Owner:KIMBERLY-CLARK WORLDWIDE INC
System and method for non-invasive analysis of bodily fluids
A vaginal fluid monitoring device includes an absorbent layer, wherein the absorbent layer is configured to be in proximity to flow of vaginal fluid; an electrochemical detection system configured to obtain data regarding at least one analyte in a vaginal fluid, said electrochemical detection system in fluid communication with the absorbent layer; and wherein the vaginal fluid monitoring device is configured to be coupled with a feminine hygiene product.
Owner:QURASENSE INC
Wetness indicator having varied hues
ActiveUS8911681B2Bioreactor/fermenter combinationsBiological substance pretreatmentsMedicineChange color
A wetness indicator material may be used on a substrate to form a wetness sensor. The sensor may show either the presence or absence of an aqueous-based fluid or water-containing medium, such as vaginal fluid or urine in a personal hygiene article. The wetness sensor may be incorporated into the article. The wetness indicator material includes a standard colorant that does not change color when wetted. The standard colorant increases the range of hues exhibited by the wetness indicator material.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Vaginal secretion detection kit and preparation method thereof
PendingCN106399459AImprove stabilityAccurate detectionMicrobiological testing/measurementBiological material analysisBacterial vaginosisBeta-glucuronidase
The invention discloses a vaginal fluid detection kit, which comprises a reaction device and a color developing agent, wherein the reaction device comprises a reaction hole for detecting beta-glucuronidase, a reaction hole for detecting acetylglucosaminidase, a reaction hole for detecting coagulase and a reaction hole for detecting proline aminopeptidase; when the kit is used for detecting vaginal secretion, the used diluent is normal saline. The vaginal secretion detection kit has the advantage that pathogenic bacteria or pathogens of bacterial vaginal diseases and trichomonas vaginitis can be used for assisting in the fast judgment of the vaginal disease condition. The invention also discloses a preparation method of the vaginal secretion detection kit.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Multilayer lining for clothing
ActiveUS20200170309A1Inhibition releaseBreathable and reusable and leakproof multilayerNon-adhesive dressingsOrnamental textile articlesBody fluidSurgery
The present disclosure relates to a breathable, reusable, leakproof, absorbent, antimicrobial, waterproof and vapor disperser multilayer lining for clothing. A purpose of this lining is to avoid the release of body fluids such as sweat, blood, vaginal fluids, menstrual fluid, urine, breast milk or postoperative fluids.
Owner:EC BRAND COM IMP EXP DE VEST EM GERAL LTDA
Garment multilayered-lining sewing process
PendingUS20210206144A1Prevent leakageReduce in quantityBreast bandagesSanitary towelsBody fluidBiomedical engineering
This disclosure relates to a sewing process of a breathable, reusable, and leak-proof garment multilayered-lining having absorbent, antimicrobial, waterproofing, and steam dispersion functions. Such lining aims at preventing body fluids such as sweat, blood, vaginal fluids, menstrual fluid, urine, breast milk, or post-surgical fluids from leaking. Such a lining provides the absorbent, antimicrobial, waterproofing, and steam dispersion functions. The lining may be sewed or adhered to the garment piece including: men's and women's underwear, shorts, short pants, skirts, pants, bras, shirts, T-shirts, jumpsuits, body shapers, dresses, men's and women's nightwear, etc. The sewing process creates channels for the liquid and creates a non-linear U-shaped structure on the lining to prevent leakage from the sides. The disclosed lining also has a reduced number of layers on its sides due to one of its layers being smaller in width than the other layers, making the coating thinner for the wearer.
Owner:EC BRAND COM IMP EXP DE VEST EM GERAL LTDA
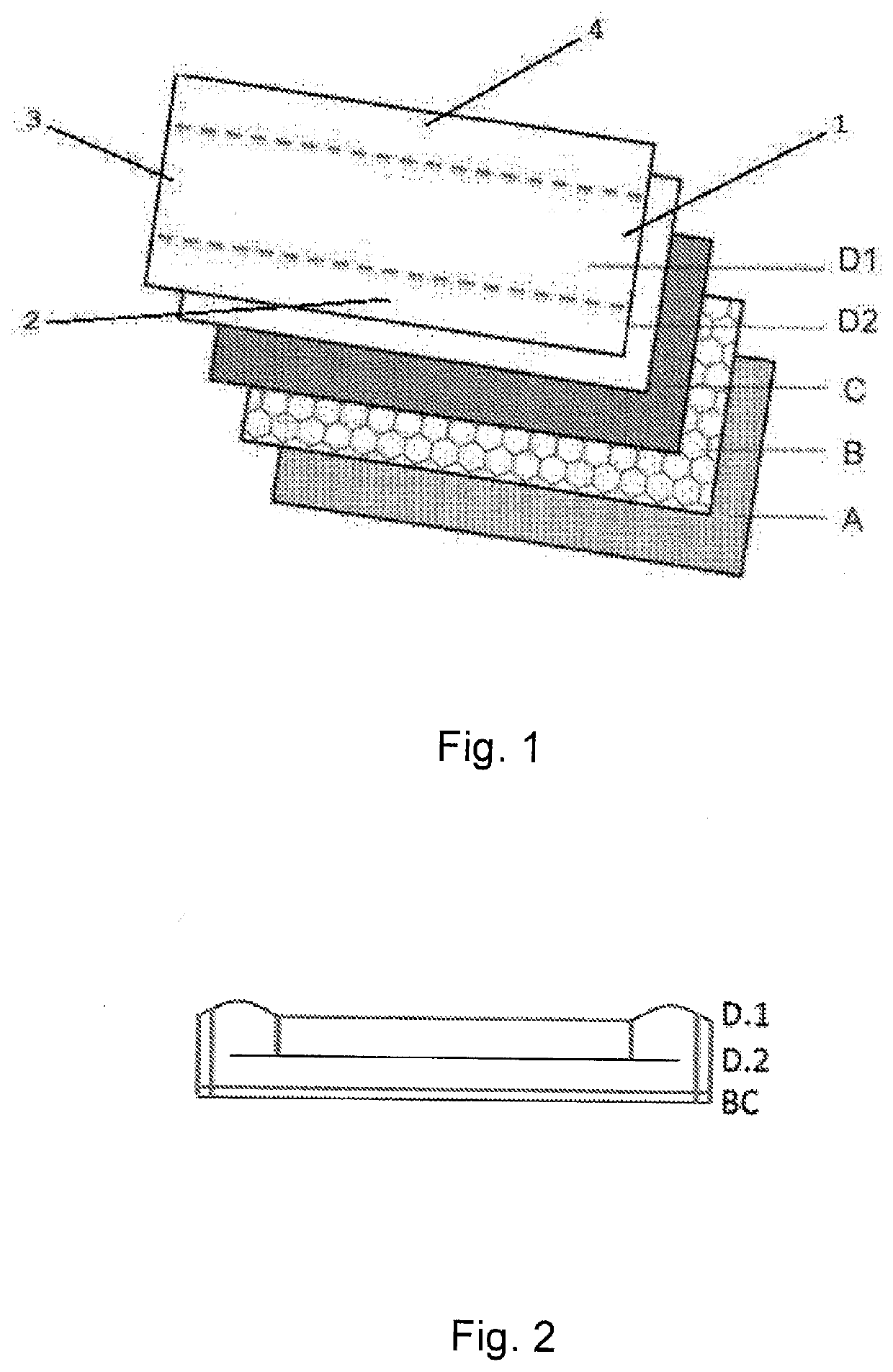
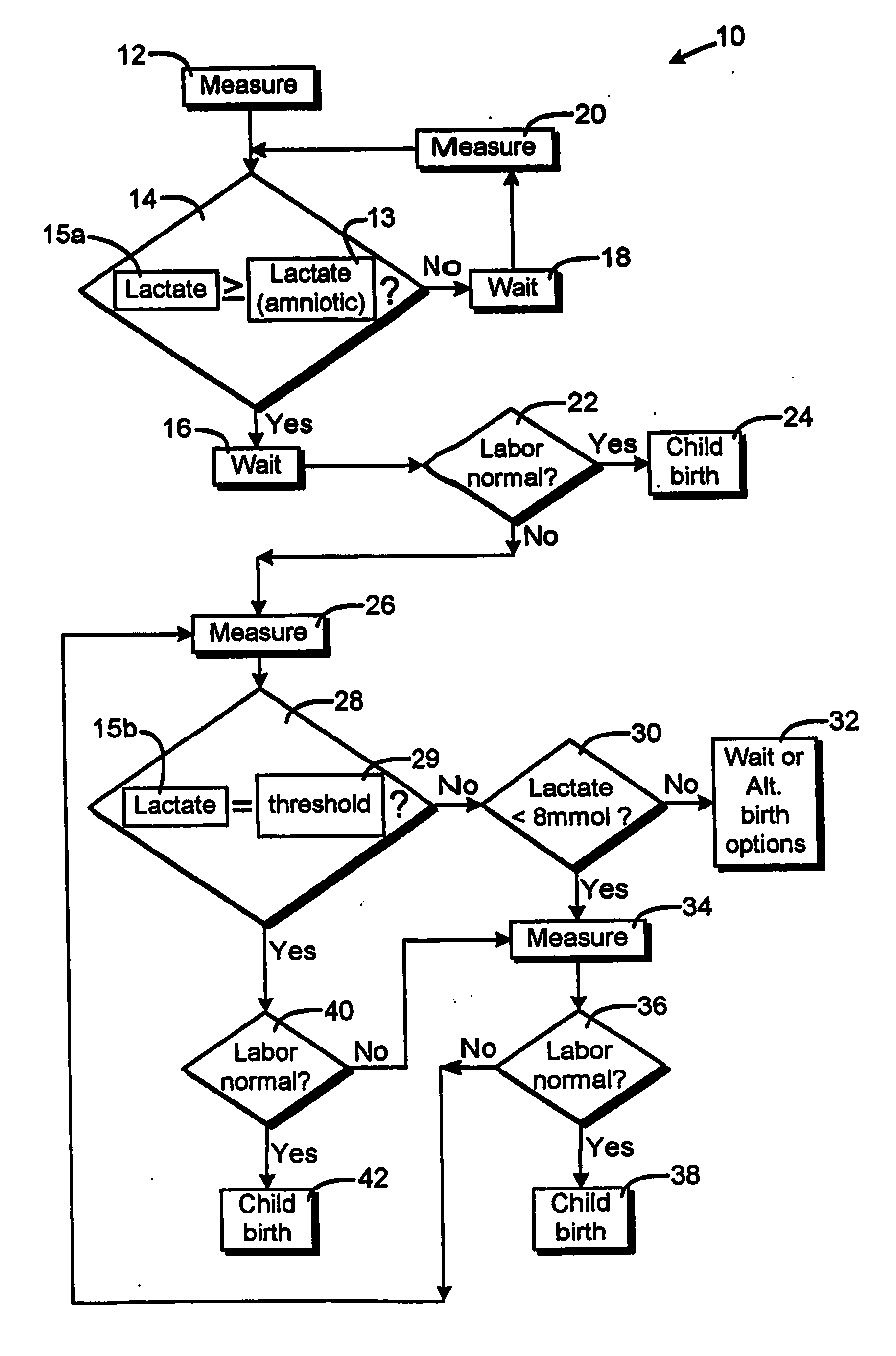
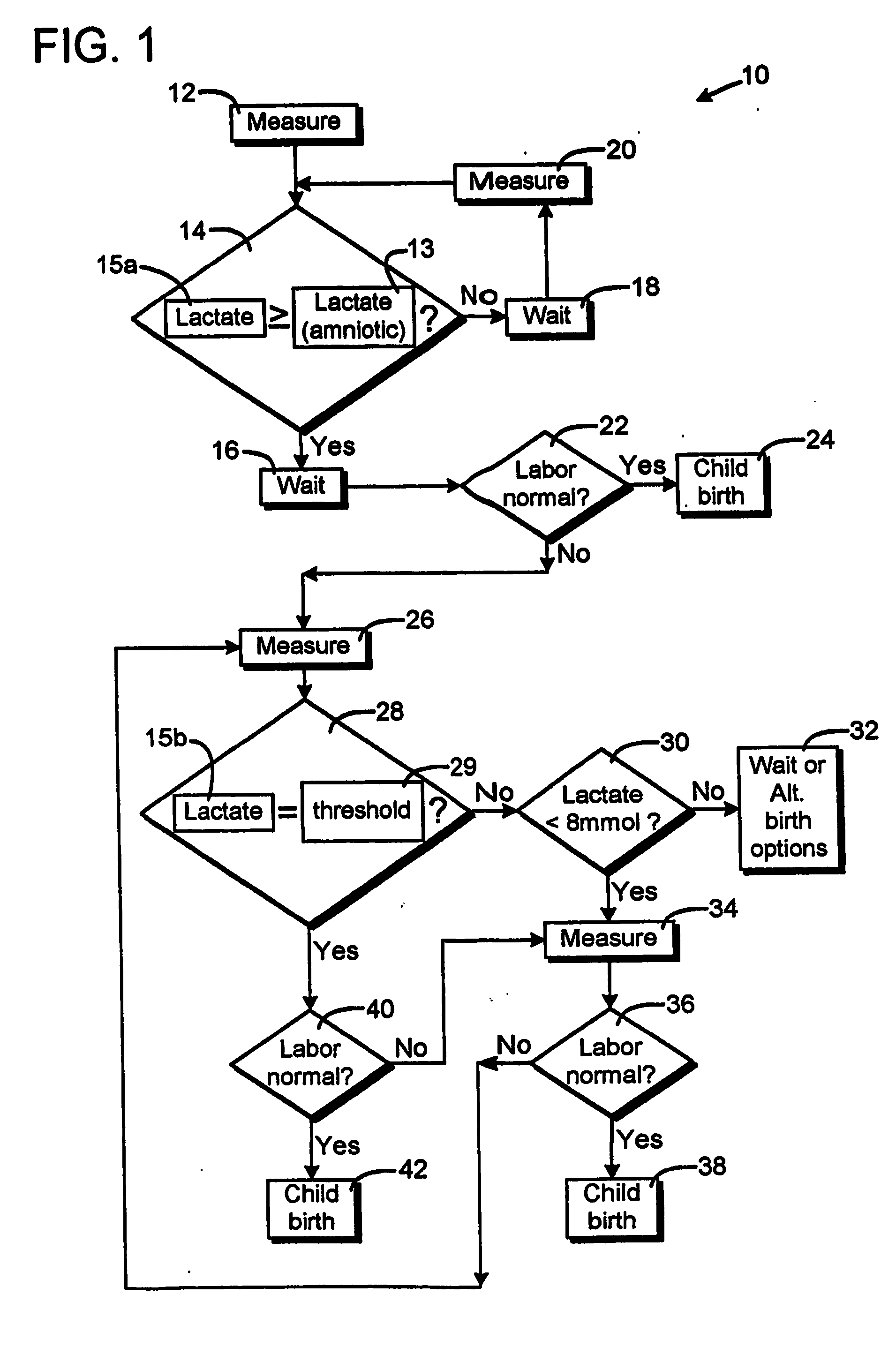
Method for monitoring a childbrith process
ActiveUS20060180161A1Prevent unnecessary agonizing and drawn out effortsSurgeryPerson identificationObstetricsAmniotic fluid
The method is for monitoring a childbirth process of a pregnant woman. In a measuring step, a lactate concentration of vaginal fluids is measured. In a comparison step, it is determined if the measured lactate concentration is greater than a predetermined lactate concentration that indicates that amniotic fluid has passed from an amnion of the pregnant woman. In a measuring step, the lactate concentration is measured. In a comparison step, it is determined if the measured lactate concentration is greater than a lactate threshold interval. When the lactate concentration is less than the lactate threshold interval the pregnant woman is stimulated in a stimulating step to give birth.
Owner:OBSTECARE AB
Intravaginal ring devices
PendingUS20210275347A1Less waterHeavy metal active ingredientsPharmaceutical non-active ingredientsSexually transmitted diseaseVaginal ring
Disclosed herein are intravaginal ring (IVR) devices comprising barriers that may be metal, polymeric or combinations of metal and polymeric. The IVRs are useful for contraception, treating and / or preventing sexually transmitted diseases, and treating and / or preventing bacterial infections. The IVR's are resistant to distortion due to absorption of vaginal fluids. This allows the IVRs to perform better over time as compared to other intravaginal rings made with copolymeric silicone.
Owner:DARE BIOSCIENCE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com