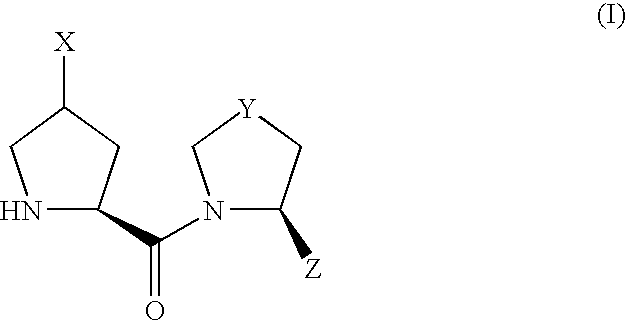
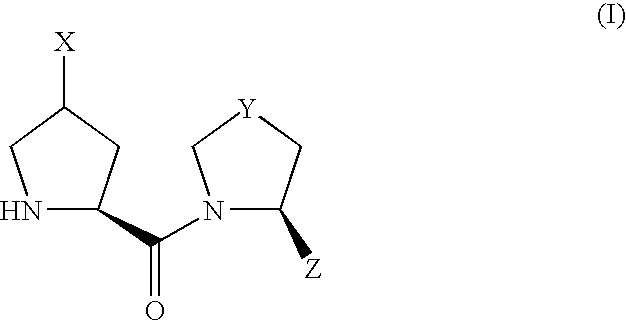
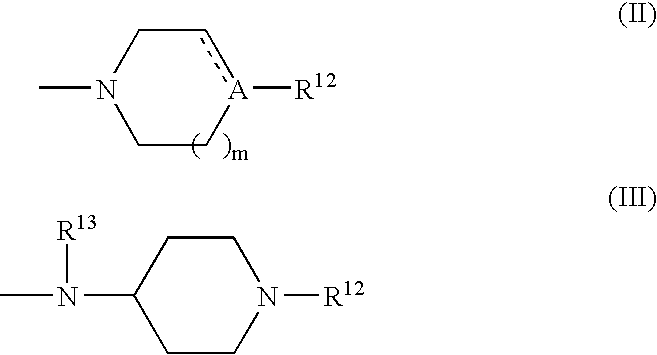
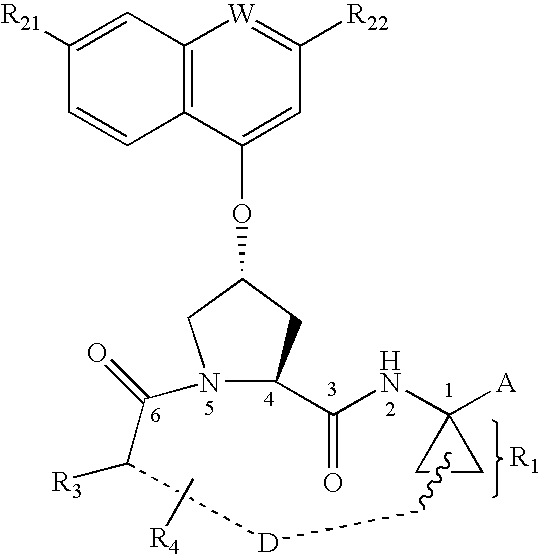
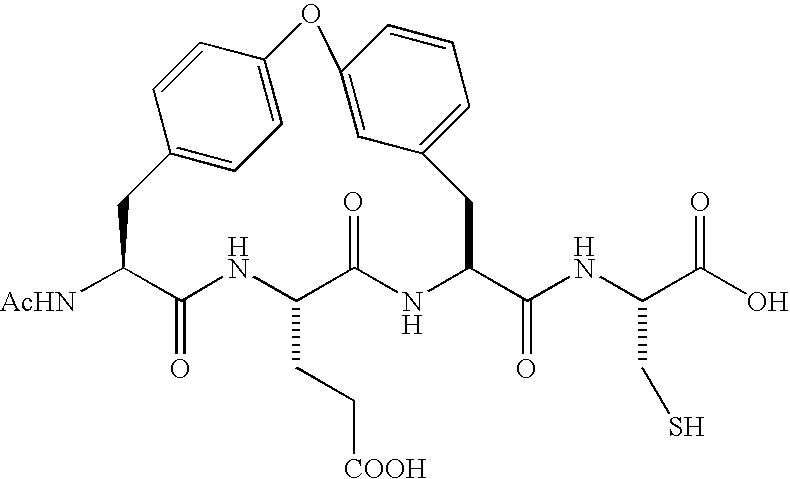
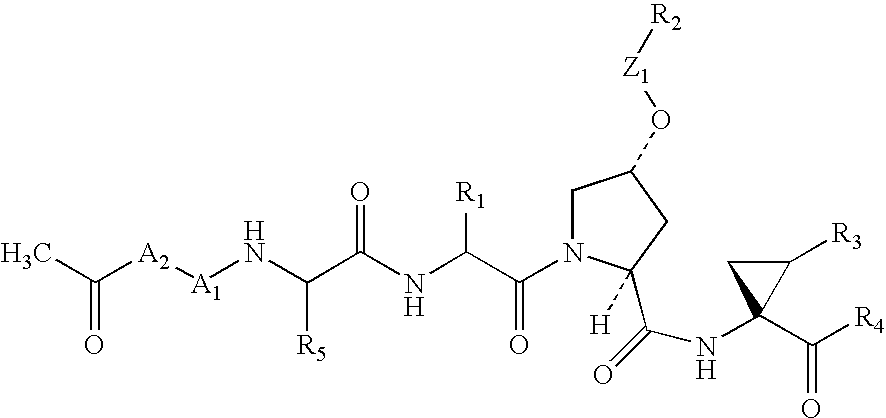
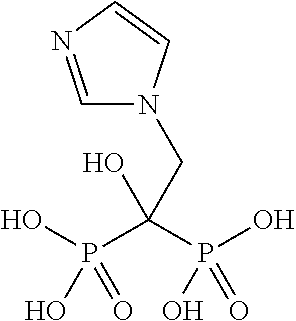
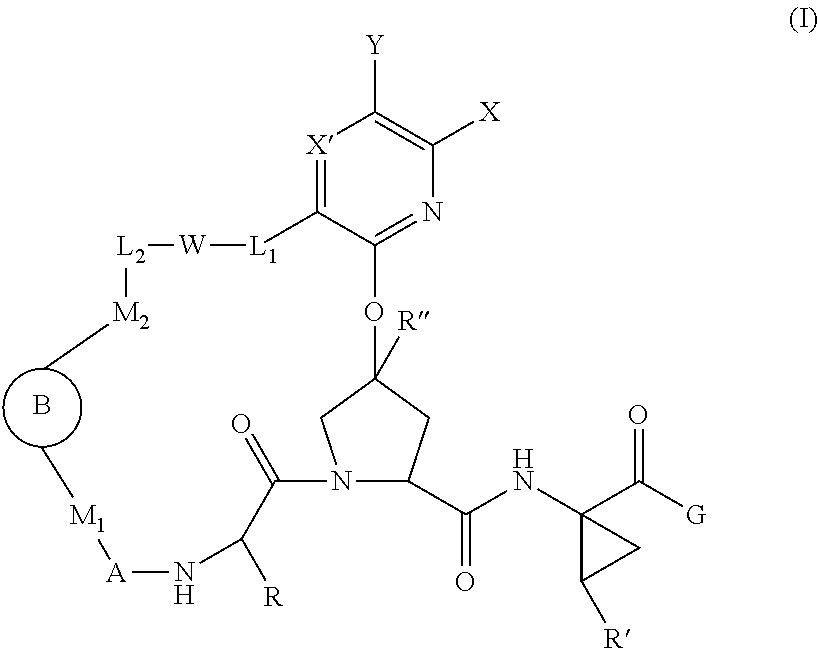
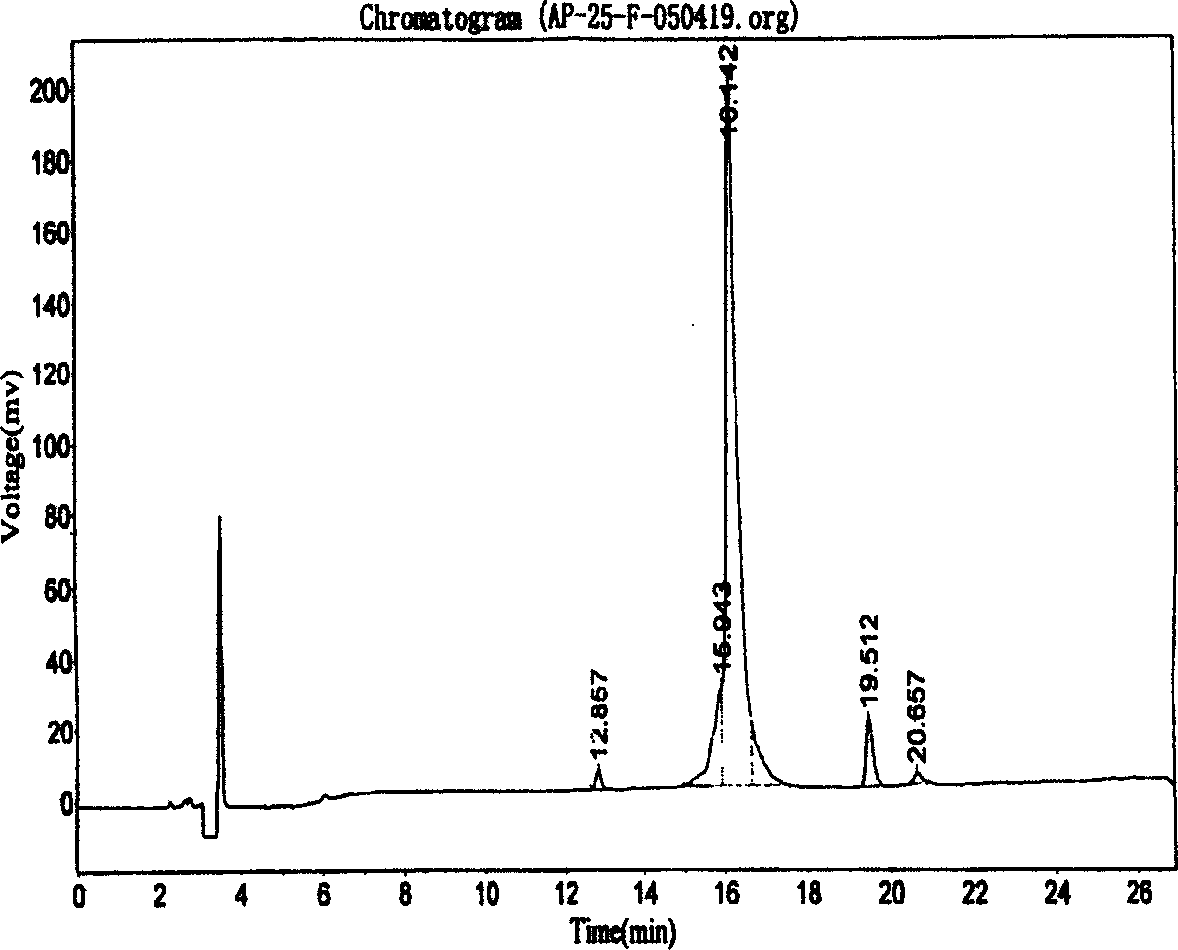
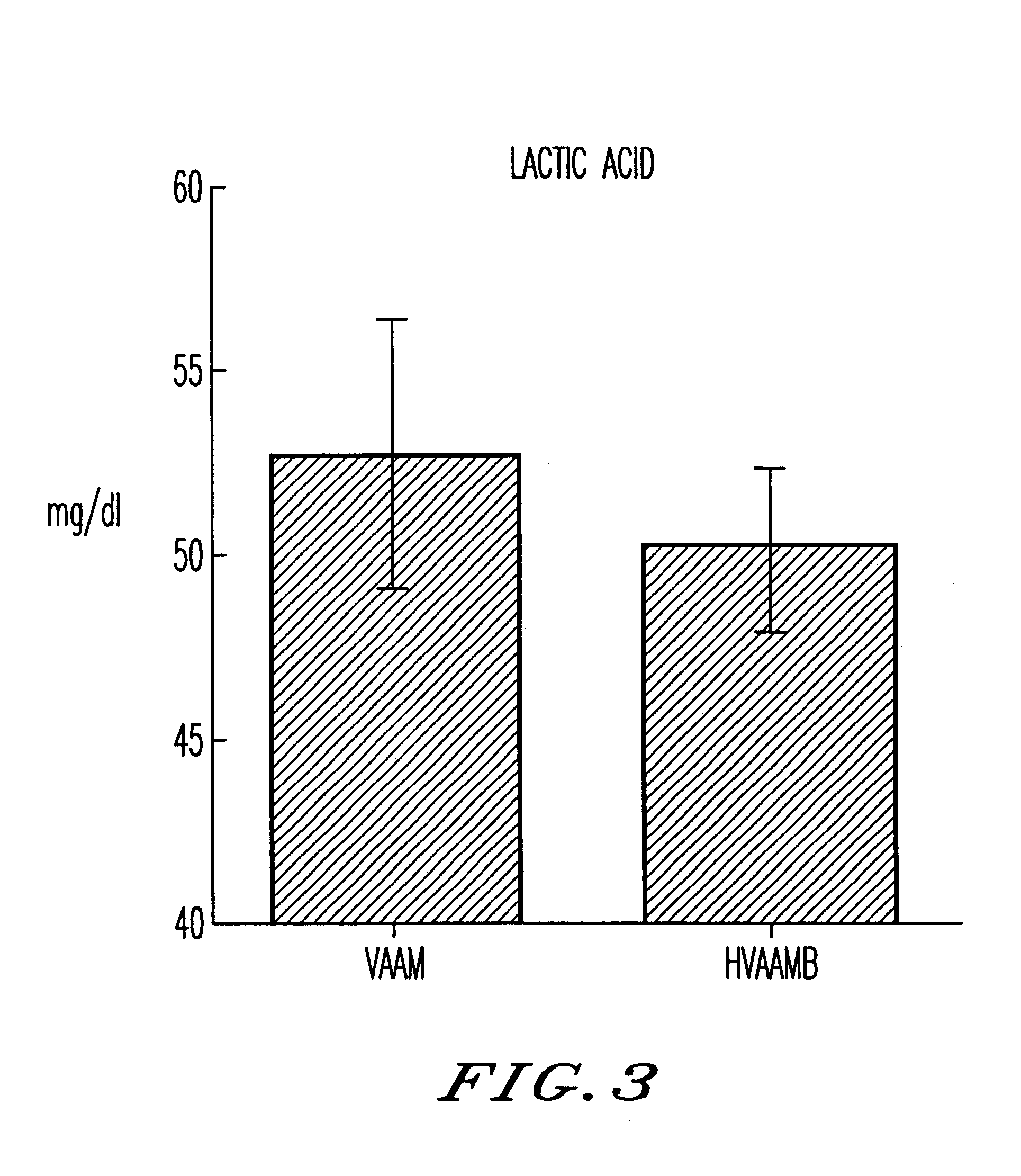
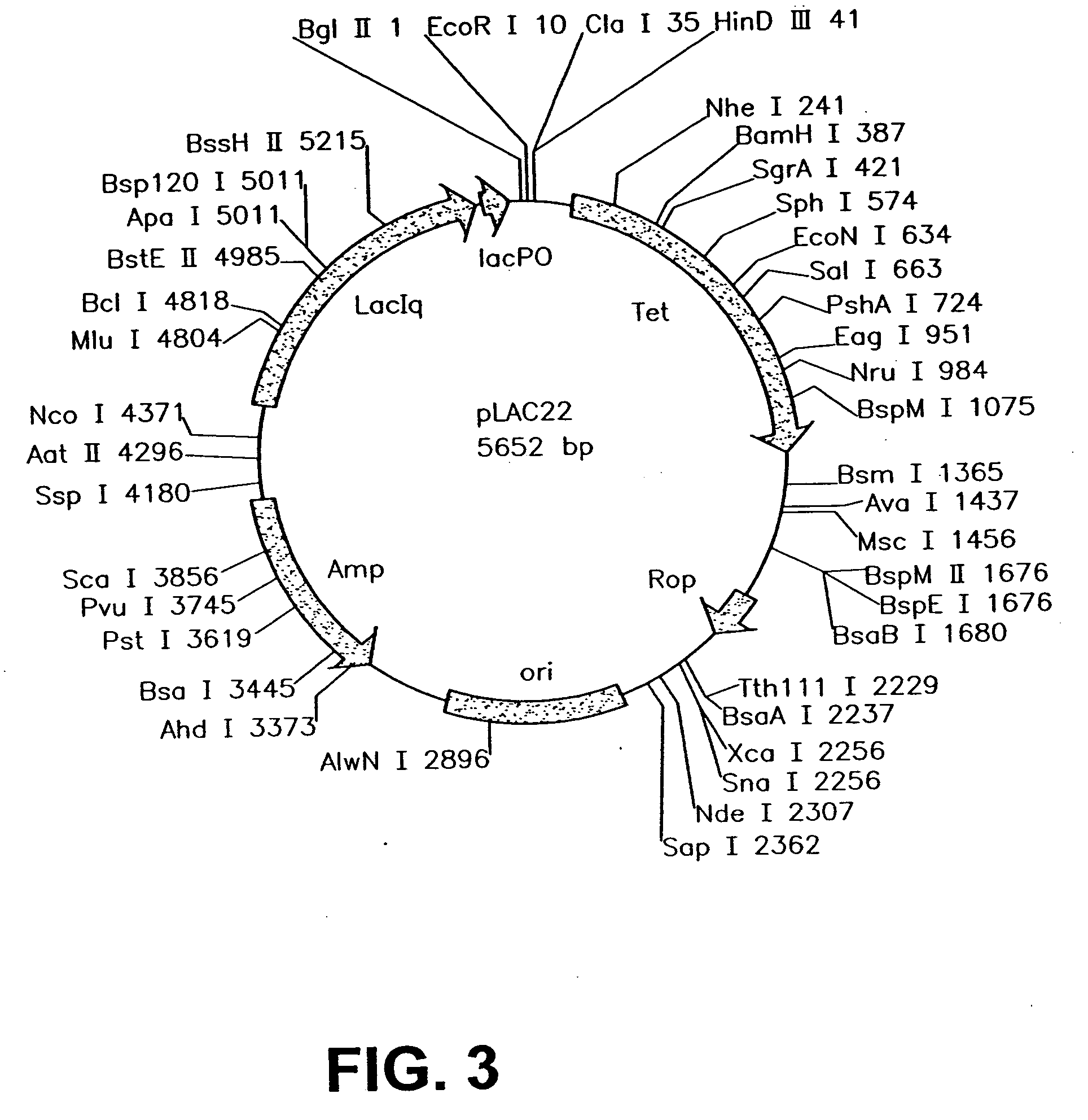
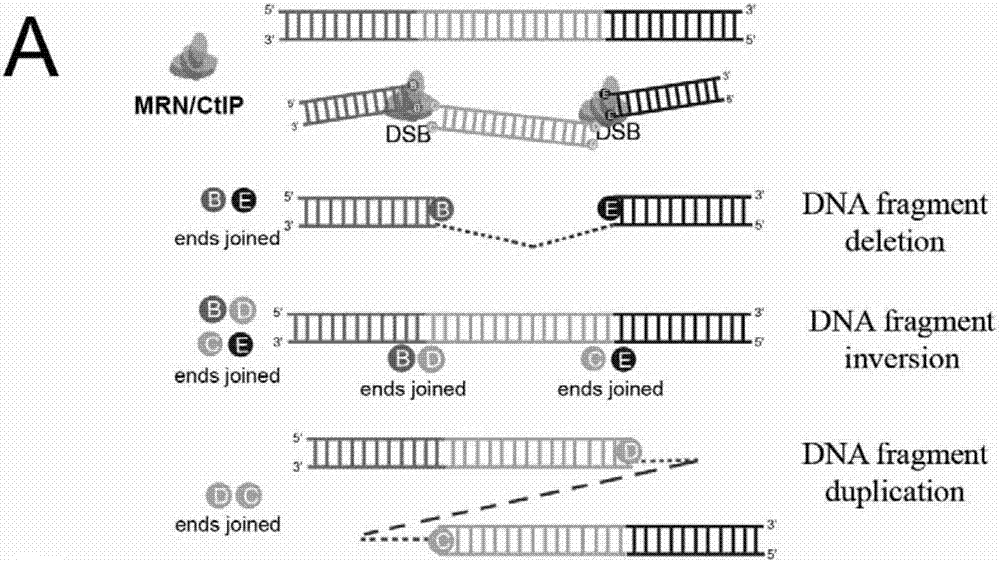
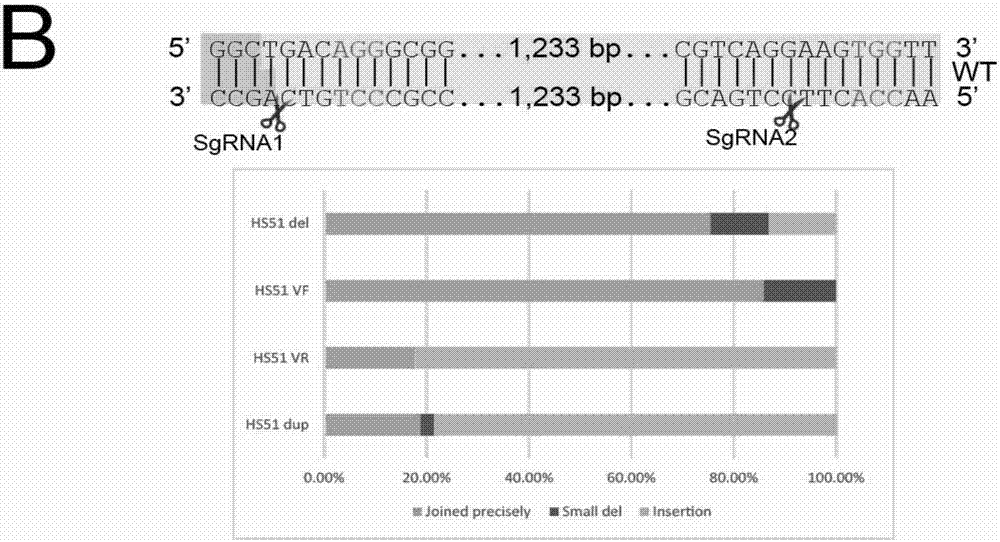
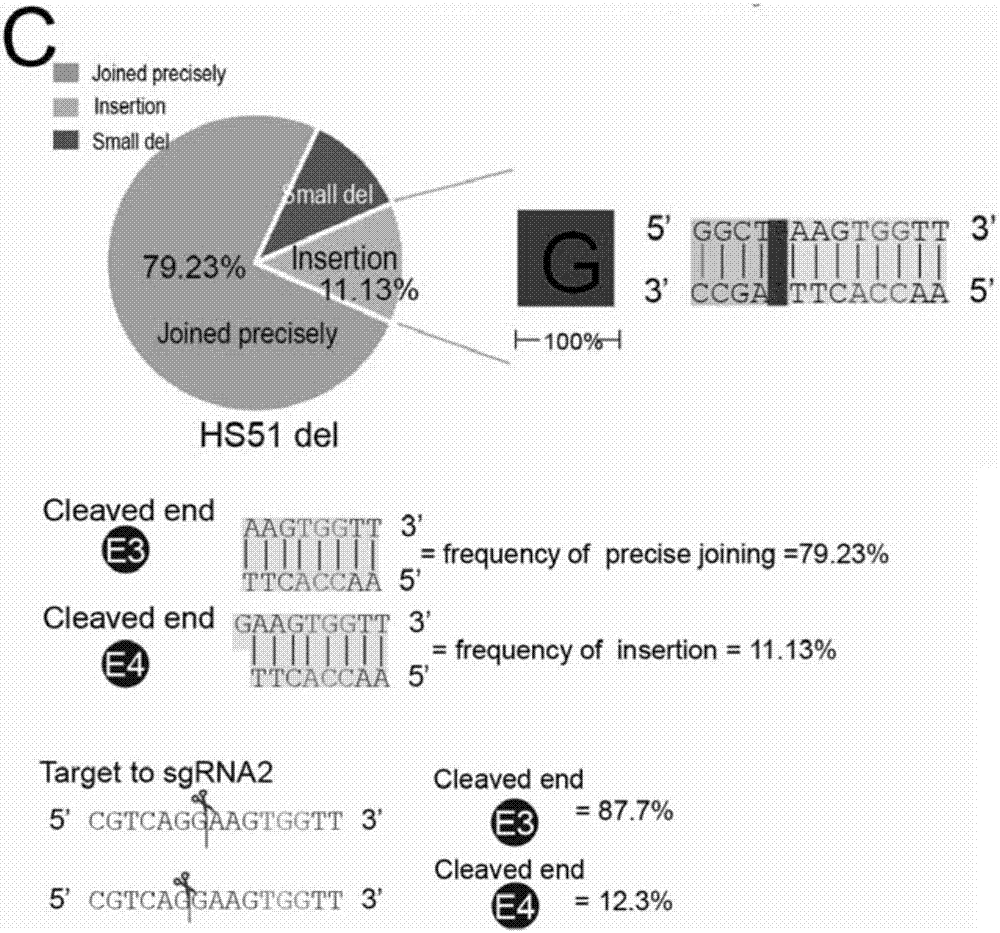
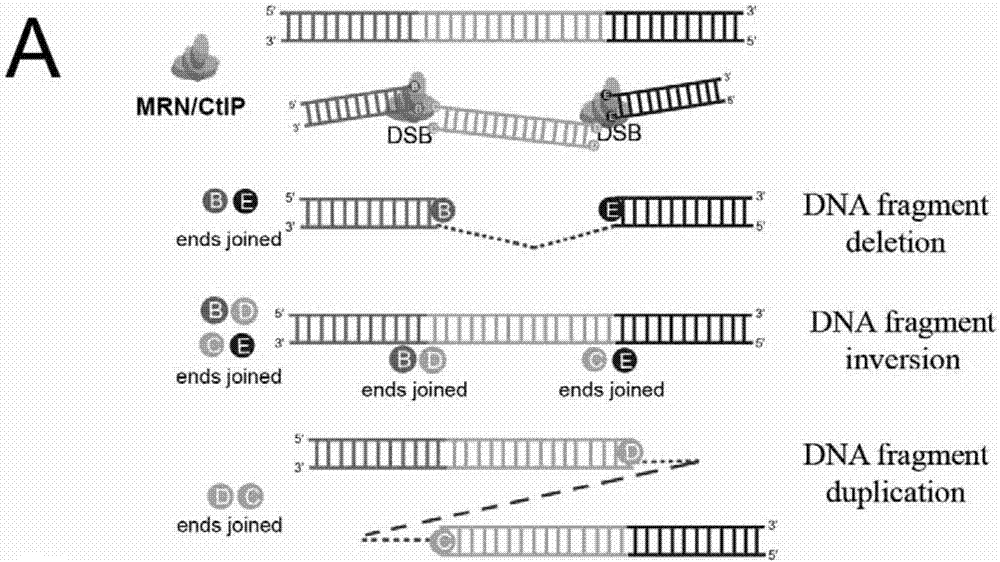
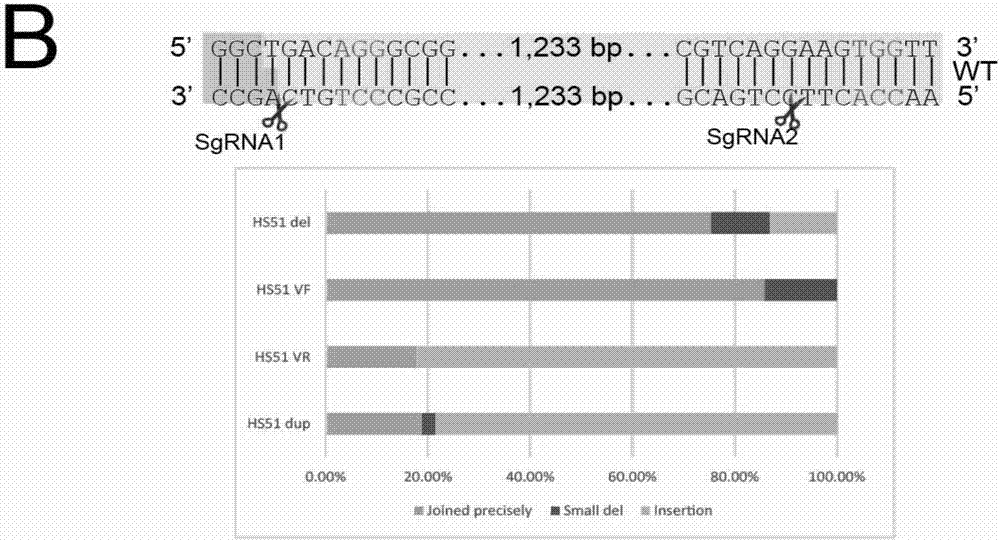
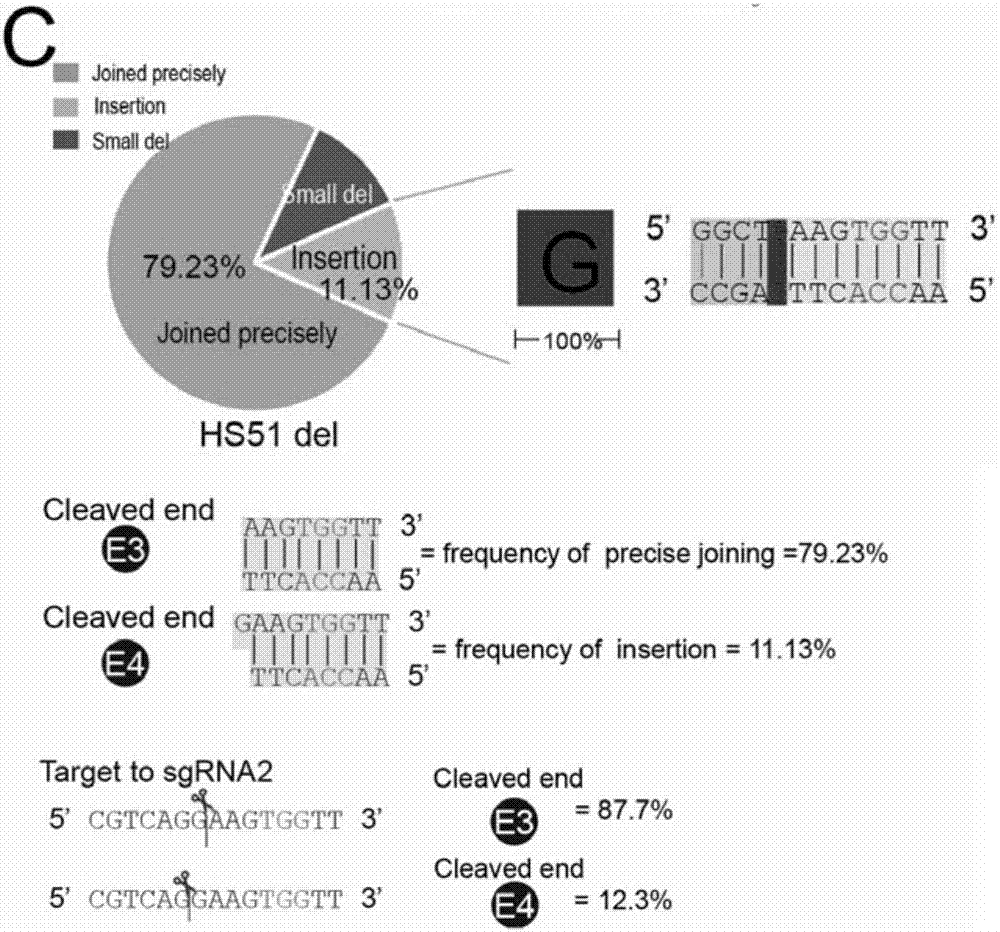
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2339 results about "Proline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Proline (symbol Pro or P) is a proteinogenic amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated NH₂⁺ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO⁻ form under biological conditions), and a side chain pyrrolidine, classifying it as a nonpolar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting CC (CCU, CCC, CCA, and CCG).
Proline derivatives and use thereof as drugs
InactiveUS7060722B2Inhibitory activityImprove stabilityBiocideOrganic chemistryTherapeutic effectProline
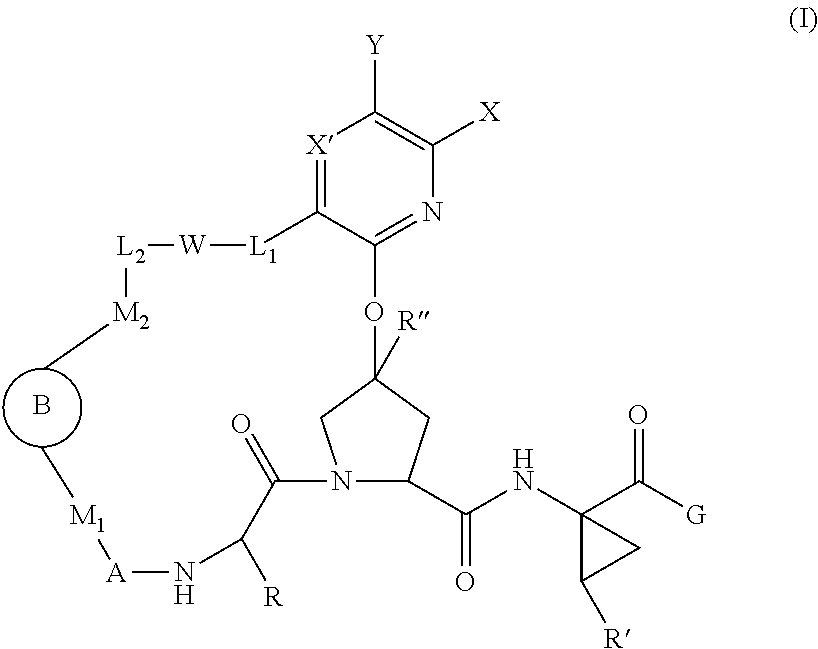
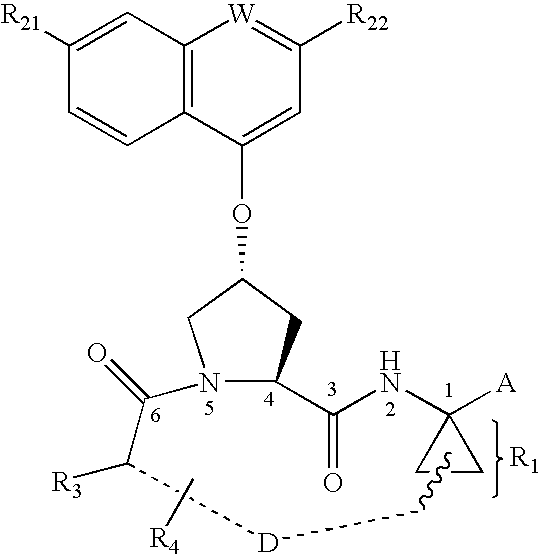
The present invention aims at providing compounds having therapeutic effects due to a DPP-IV inhibitory action, and satisfactory as pharmaceutical products.The present inventors have found that derivatives having a substituent introduced into the γ-position of proline represented by the formula (I)wherein each symbol is as defined in the specification, have a potent DPP-IV inhibitory activity, and completed the present invention by increasing the stability.
Owner:MITSUBISHI TANABE PHARMA CORP
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Stabilized antibody-containing formulations
ActiveUS20090291076A1Good cakingIncrease of the viscosity of the high-concentration antibody-containing solutionAntibody ingredientsImmunoglobulinsArginineThreonine
The present invention relates to antibody-containing lyophilized formulations free from reducing sugars, non-reducing sugars, sugar alcohols or polysaccharides as excipients and including one or more amino acid selected from the group consisting of arginine, histidine, lysine, serine, proline, glycine, alanine and threonine or a salt thereof.
Owner:CHUGAI PHARMA CO LTD
3,4-(cyclopentyl)-fused proline compounds as inhibitors of hepatitis C virus NS3 serine protease
The present invention discloses novel compounds which have HCV protease inhibitory activity as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising such compounds as well as methods of using them to treat disorders associated with the HCV protease.
Owner:SCHERING CORP
Novel oral forms of a phosphonic acid derivative
InactiveUS20120190647A1Improved aqueous solubilityIncrease ratingsBiocideOrganic active ingredientsEnprofyllineArginine
Novel solution complexes of zoledronic acid are described which give rise to improved properties of zoledronic acid. The invention includes aqueous solution and molecular complexes of zoledronic acid with and optical isomers of asparagine, histidine, arginine and proline as well as pharmaceutical complexes containing them and methods of treatment using them.
Owner:THAR PHARMA
Macrocyclic Proline Derived HCV Serine Protease Inhibitors
The present invention discloses compounds of Formula I or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Chondroitinase, process for preparing the same, and pharmaceutical composition comprising the same
InactiveUS6184023B1Avoid stickingInhibit productionBacteriaHydrolasesChondroitinase ABCConcentration gradient
A crystallizable, purified chondroitinase ABC having a molecular weight of about 100,000 dalton by the measurement of the SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the measurement by the gel permeation chromatography method, having alanine as the N-terminal amino acid and proline as the C-terminal amino acid. A process for the purification of the crystallizable purified chondroitinase ABC comprising removing nucleic acid from an surfactant solution extract obtained from cells of chondroitinase ABC-producing microorganisms and chromatographically treating by concentration gradient elution using a weak cation exchange resin or a strong cation exchange resin. A composition comprising a chondroitinase and serum albumin, gelatin, or a nonionic surfactant.
Owner:SEIKAGAKU KOGYO CO LTD
Synthetic genes for plant gums and other hydroxyproline-rich glycoproteins
InactiveUS6639050B1Enhance molecular packingEasy to identifyBacteriaAntibody mimetics/scaffoldsBiotechnologyHydroxyproline
A new approach in the field of plant gums is described which presents a new solution to the production of hydroxyproline(Hyp)-rich glycoproteins (HRGPs), repetitive proline-rich proteins (RPRPs) and arabinogalactan-proteins (AGPs). The expression of synthetic genes designed from repetitive peptide sequences of such glycoproteins, including the peptide sequences of gum arabic glycoprotein (GAGP), is taught in host cells, including plant host cells.
Owner:OHIO UNIV TECH TRANSFER OFFICE TECH & ENTERPRISE BUILDING
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
Food product comprising a proline specific protease, the preparation thereof and its use for degrading toxic or allergenic gluten peptides
InactiveUS20090304670A1Extended shelf lifeIntense and long interaction periodSpread compositionsPeptide/protein ingredientsProteinase activityWater activity
The present invention relates to a pasteurized food product having a water activity of at least 0.80, preferably at least 0.85 and containing a proline specific protease.
Owner:UPONOR INNOVATION AB +1
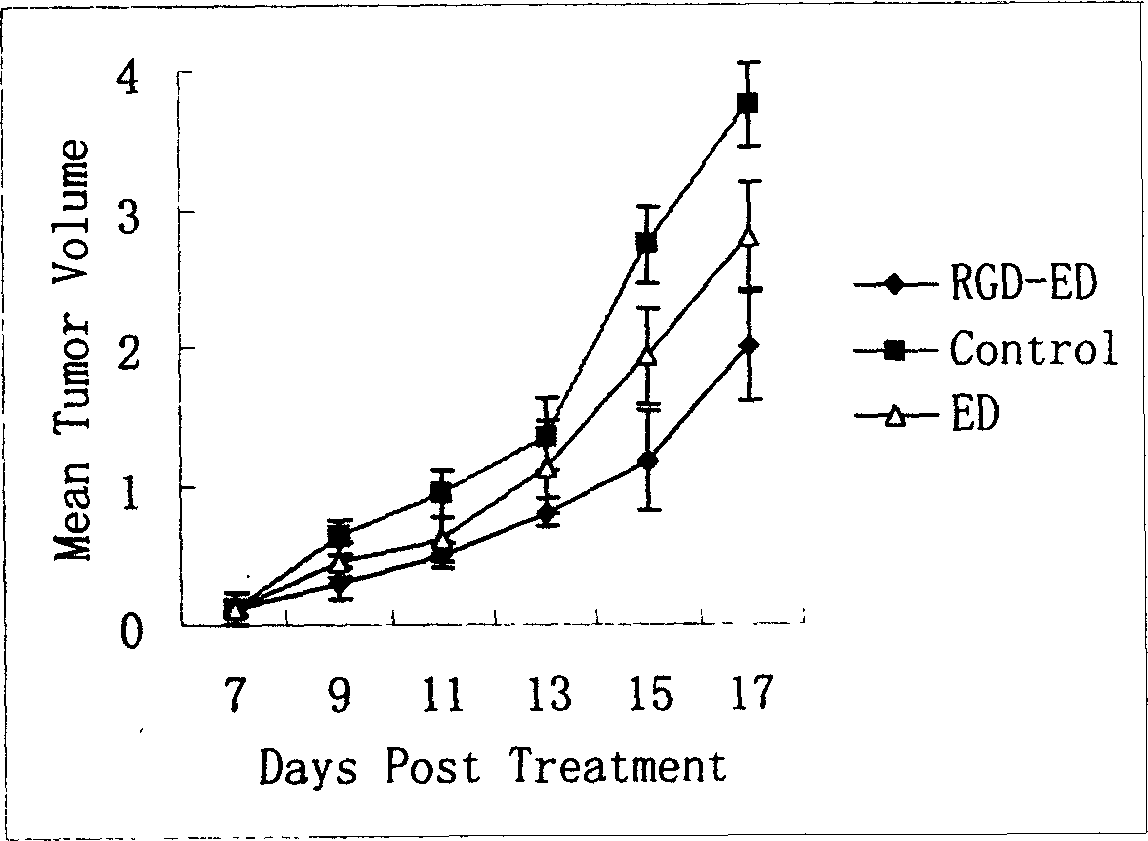
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
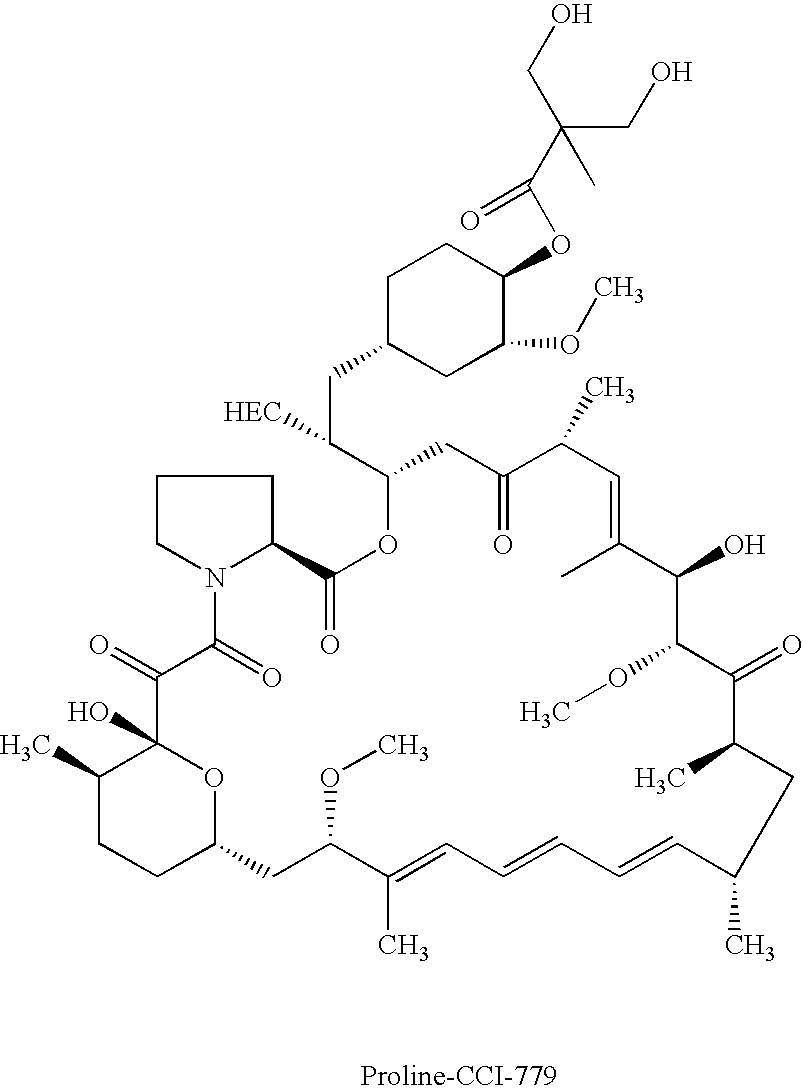
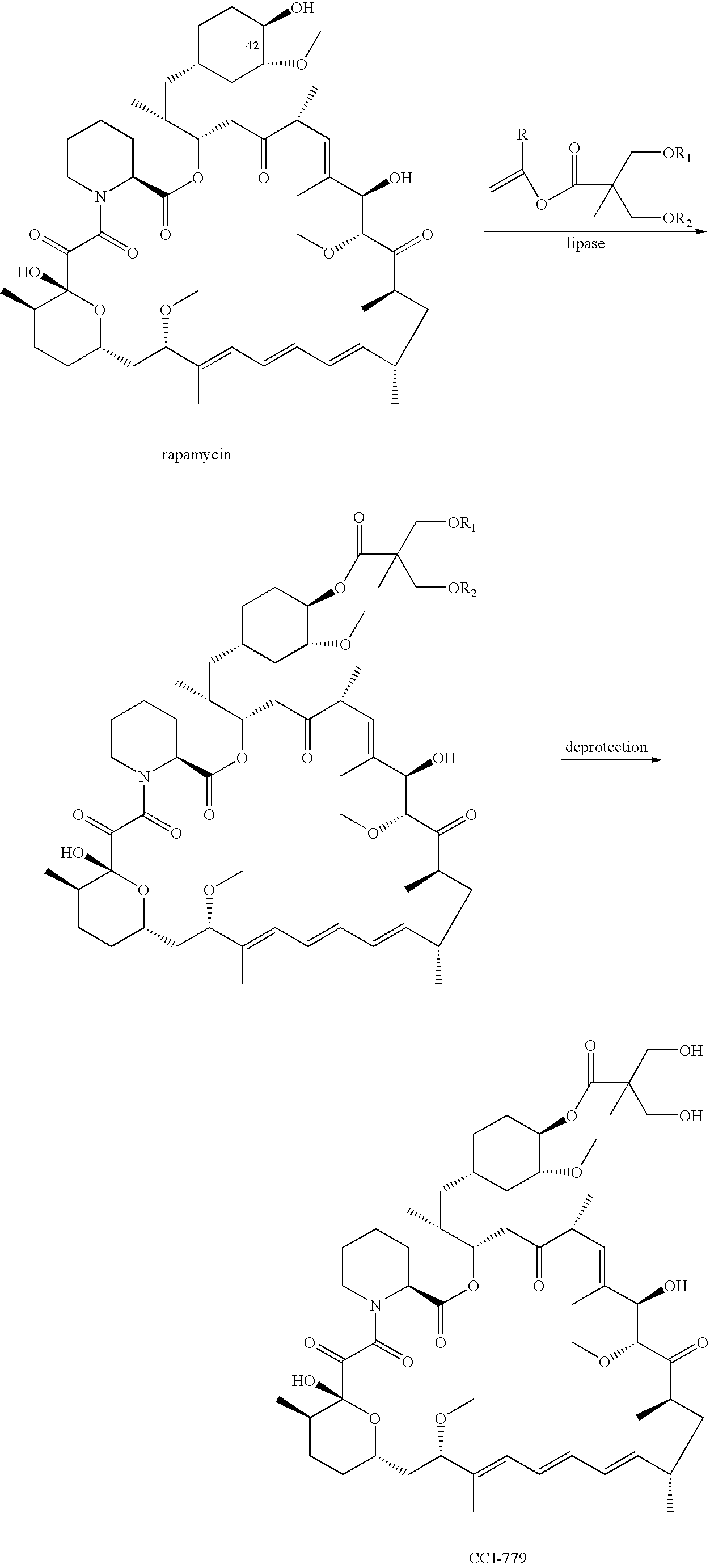
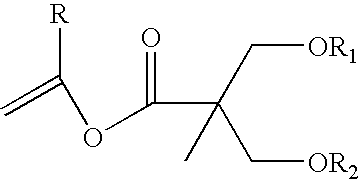
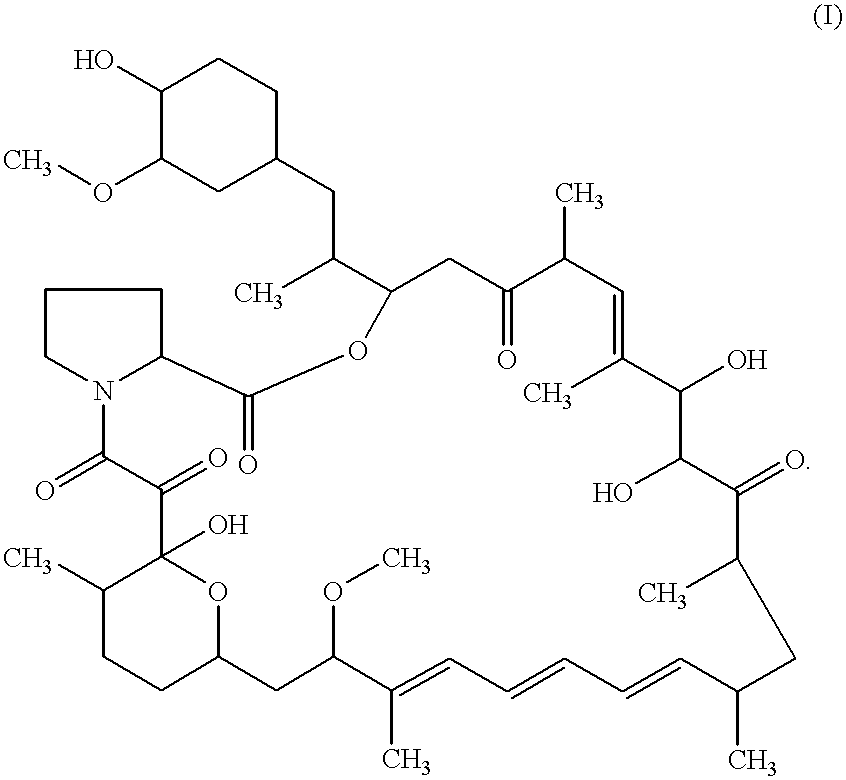
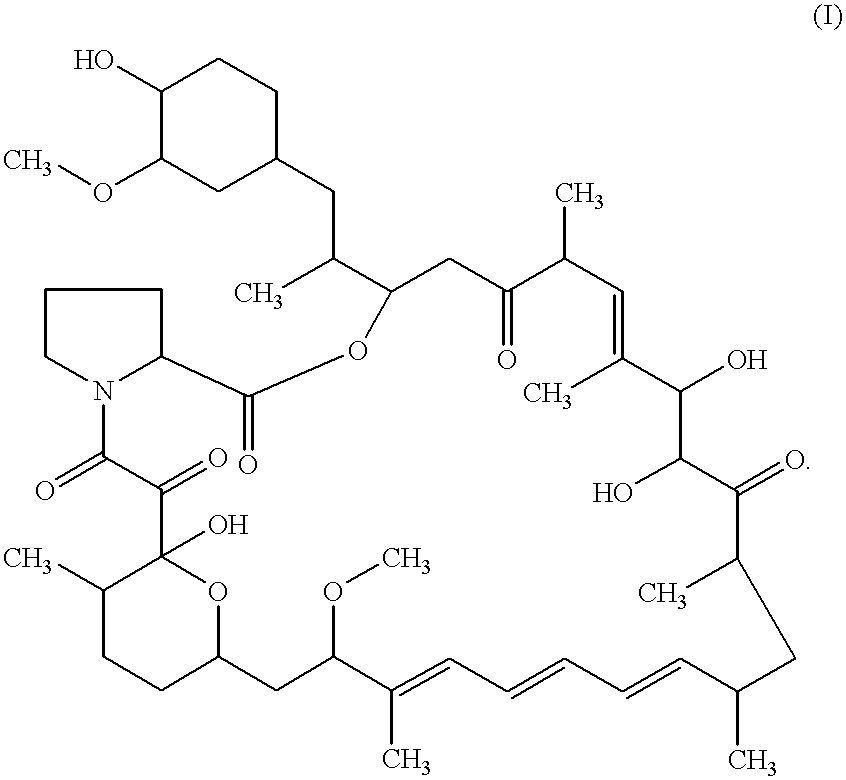
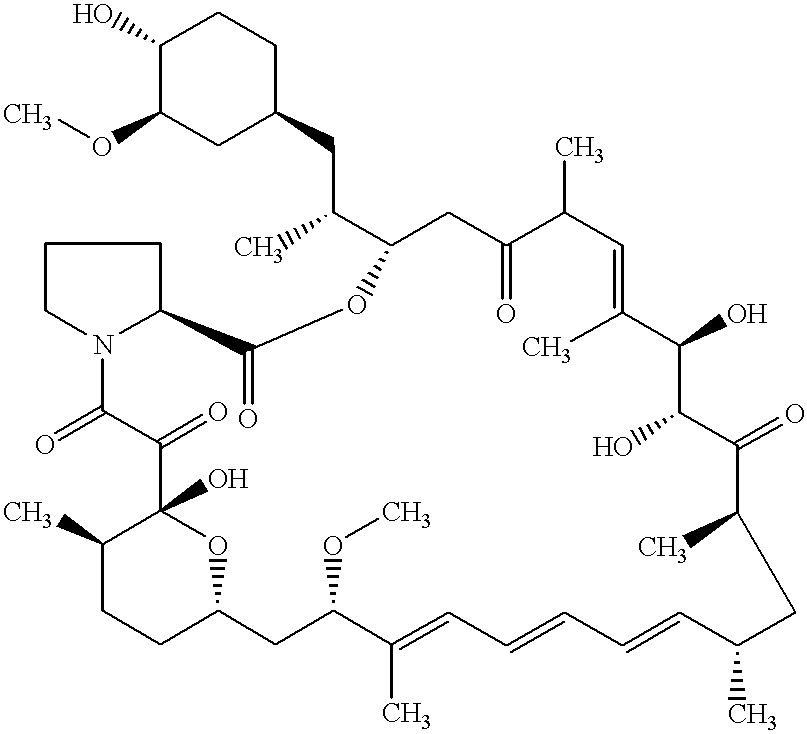
Proline CCI-779, production of and uses therefor, and two-step enzymatic synthesis of proline CCI-779 and CCI-779
Methods for the synthesis of CCI-779 and proline-CCI-779 are described, including a method involving lipase-catalyzed acetylation of 42-hydroxy of rapamycin with a vinyl ester of 2,2-bis(hydroxymethyl) propionic acid in an organic solvent followed by deprotection. Also provided are products containing proline-CCI-779 and uses thereof.
Owner:WYETH LLC
Macrocyclic lactone compounds and their production process
InactiveUS6187568B1Antibacterial agentsMicroorganism based processesMacrocyclic lactoneActinoplanes sp.
This invention provides a process for producing a macrocyclic lactone compound, which comprises cultivating Actinoplanes sp. FERM BP-3832, in the presence of L-proline, L-hydroxyproline or L-nipecotic acid, and then isolating a macrocylic lactone compound from the fermentation broth. The compounds produced by this process include a compound of the following formula:The present invention also relates to a pharmaceutical composition comprising the same, which is useful as immunosuppressive, antimycotic, antitumor agent or the like.
Owner:PFIZER INC
New effectors of dipeptidyl peptidase iv for topical use
InactiveUS20030092630A2Reduced activityOrganic active ingredientsPeptide/protein ingredientsDiseaseSide chain
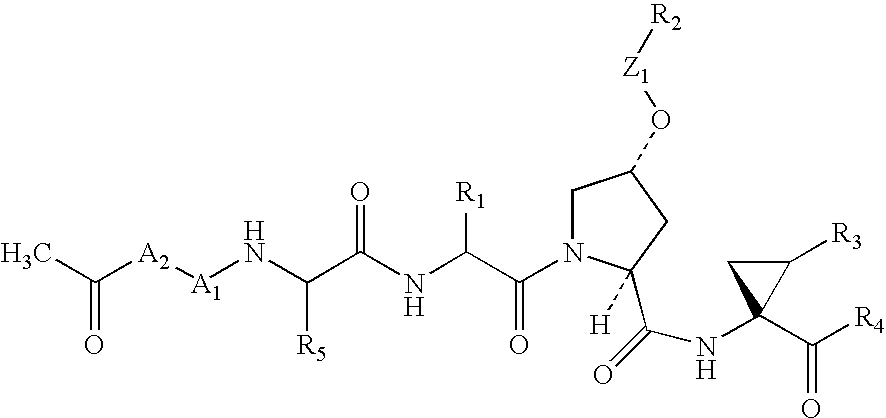
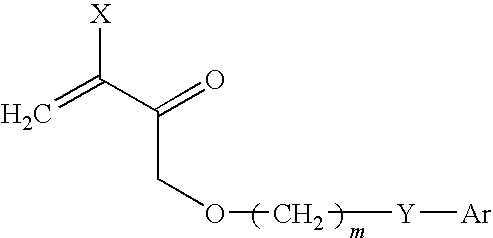
Abstract of Disclosure The invention relates to compounds for topically influencing the activity of dipeptidyl peptidase of the general formula wherein A is an amino acid having at least one functional group in the side chain;B is a chemical compound covalently bound to a functional group of the side chain of A, chosen from the group consisting of (a) oligopeptides having a chain length of up to 20 amino acids, (b) homopolymers of glycine consisting of up to 6 glycine monomers, and (c) polyethylene glycols having molar masses of up to 20 000 g / mol; and C is a group amide-bonded to A chosen from the group consisting of thiazolidine, pyrrolidine, cyanopyrrolidine, hydroxyproline, dehydroproline or piperidine. The invention further relates to the use of said compounds for targeted intervention in local immunological processes (chemotaxis, inflammatory processes, autoimmune diseases), as well as effective and targeted treatment of pathophysiological and physiological processes related thereto (psoriasis, periodontitis, arthritis, allergies, inflammation), inter alia.
Owner:VIVORYON THERAPEUTICS NV
3,4-(cyclopentyl)-fused proline compounds as inhibitors of hepatitis C virus NS3 serine protease
Owner:SCHERING CORP
Cocrystal of c-glycoside derivative and l-proline
InactiveUS20090143316A1Quality improvementGood storage stabilityBiocideSugar derivativesCompound aC-glycoside
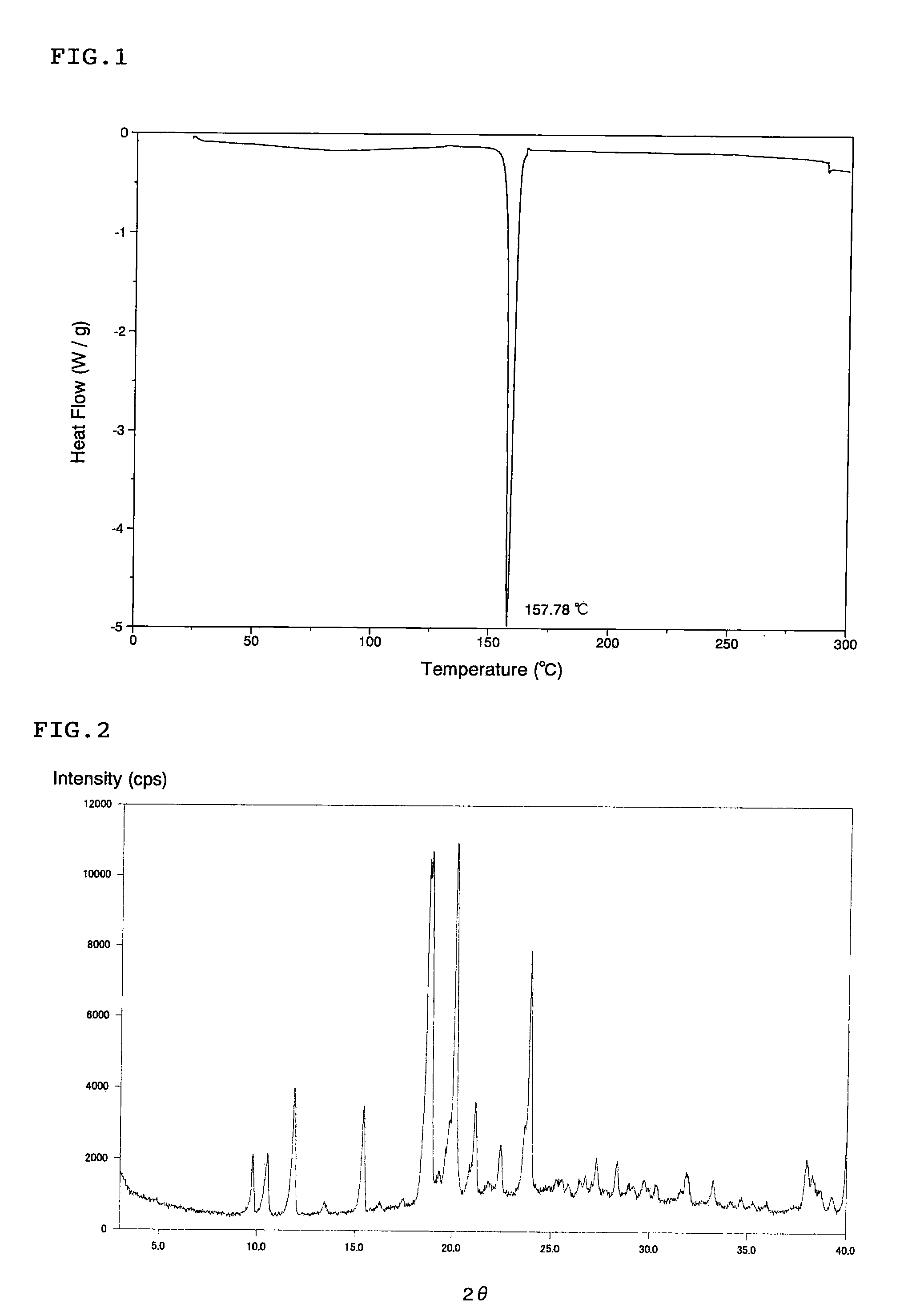
A cocrystal of (1S)-1,5-anhydro-1-[3-(1-benzothien-2-ylmethyl)-4-fluorophenyl]-D-glucitol and L-proline. It is a cocrystal of known compound A, which has a constant quality, is superior in storage stability, has no moisture absorptivity, and is suitable as a crystal of a drug substance used for preparing pharmaceuticals.
Owner:ASTELLAS PHARMA INC +1
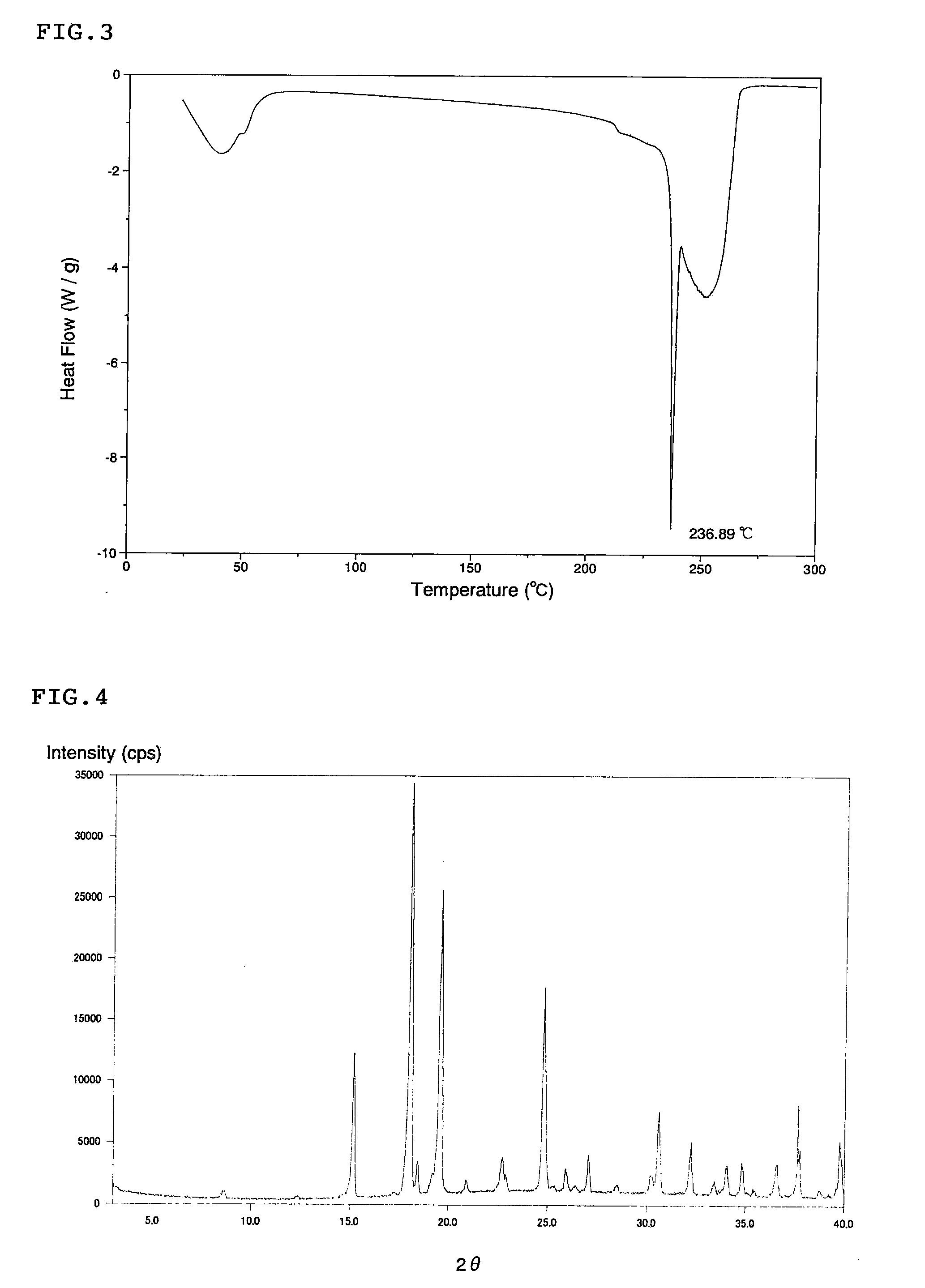
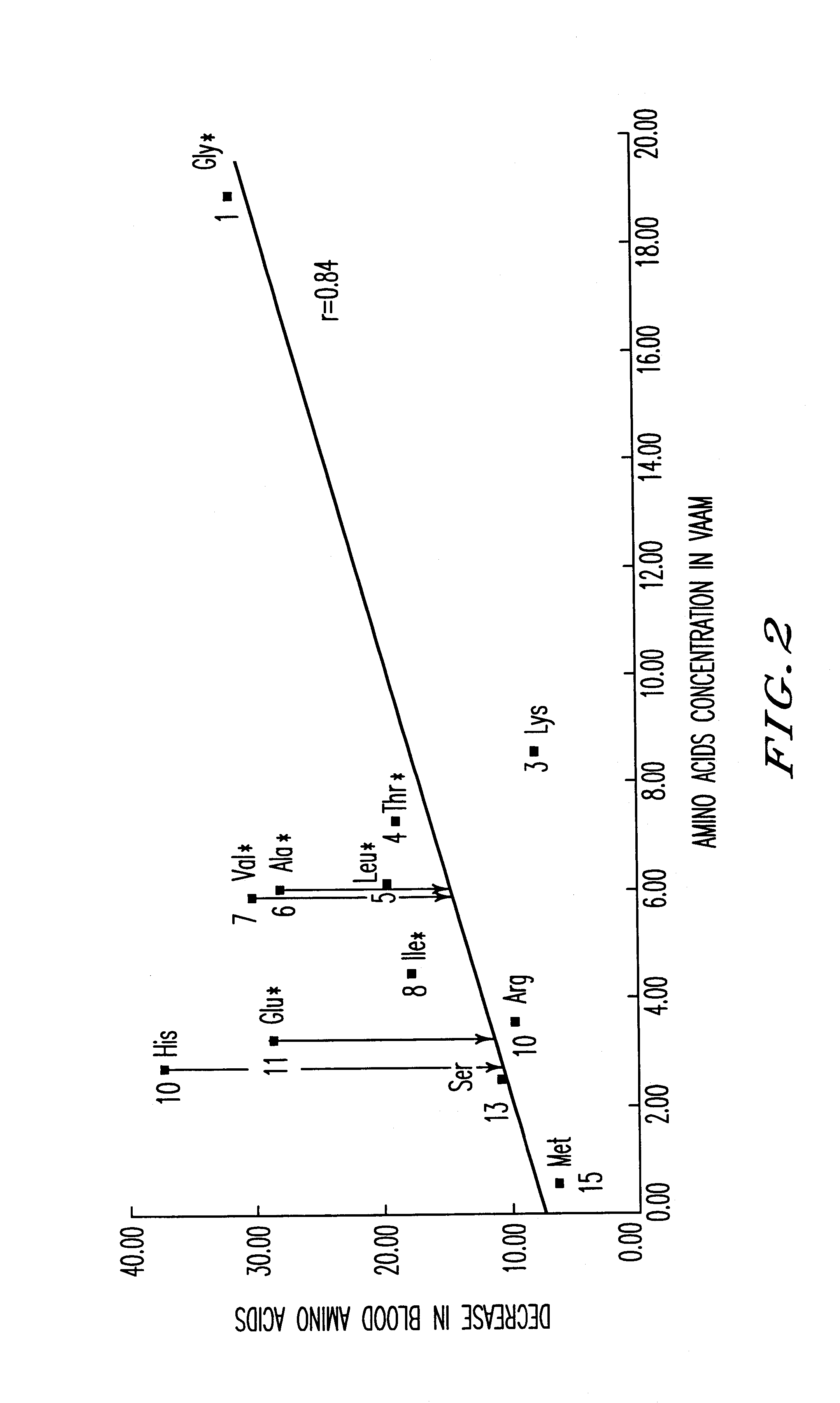
Amino acid composition
An amino acid composition comprising the following amino acids at the following molar ratio: proline (12.6 to 23.4), alanine (8.4 to 15.6), glycine (13.3 to 24.9), valine (8.2 to 15.4), threonine (5.0 to 9.4), eucine (4.3 to 8.1), histidine (1.8 to 11.9), serine (1.7 to 3.3), lysine (6.0 to 11.2), isoleucine (3.1 to 5.9), glutamic acid (2.2 to 10.4), arginine (2.4 to 4.6), phenylalanine (2.6 to 5.0), tyrosine (4.2 to 7.8) and trypsin (1.5 to 2.9). The composition supplements blood amino acids reduced during hard exercise and shows effects to improve motor function, to reduce fatigue after exercise and to help recovery from the fatigue.
Owner:THE INST OF PHYSICAL & CHEM RES WAKO +1
Chicken essence and preparation method thereof
ActiveCN103750254ALong storage timeGreat tasteFood homogenisationFood dryingMaillard reactionMonosodium glutamate
The invention relates to a preparation method of chicken essence. The method comprises the steps of weighing chicken breast and chicken skeleton, adding water, boiling at high temperature, and then, passing through a colloid mill, so as to obtain colloidal chicken juice; hydrolyzing the colloidal chicken juice, then, carrying out enzyme deactivation and filtrating, so as to obtain enzymolysis chicken juice; uniformly mixing the enzymolysis chicken juice, L-cysteine hydrochloride, ribose, L-proline, xylose, dextrose monohydrate, L-glycine, water and chicken oil, and then, adding the mixture into a reaction kettle for Maillard reaction, so as to obtain a chicken juice reactant; uniformly mixing the chicken juice reactant, salt, white granulated sugar, monosodium glutamate, modified starch, maltodextrin, cyclodextrin, water, I+G, chicken oil and chicken flavoring base, then, passing through a colloid mill, filtrating to obtain colloidal chicken cream, emulsifying the colloidal chicken cream, and then, carrying out spray drying on the emulsified colloidal chicken cream through a homogenizer, thereby obtaining the chicken essence. The invention further relates to a product of the preparation method of the chicken essence. Compared with the existing products, the chicken essence prepared by the method has the advantages that the chicken fragrance is unique, the flavor is harmonious, the taste of chicken is strong and full, and the fragrance and the flavor can be of long-term coexistence.
Owner:GUANGZHOU TIANHUI FOOD
Biocompatible polymers and co-polymers, and uses thereof
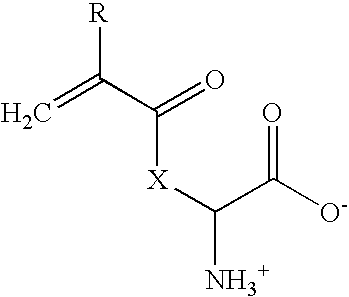
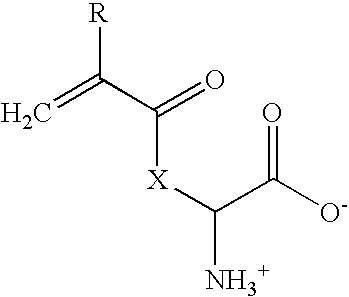
ActiveUS20090232871A1Suitable for productionReduce moistureSuture equipmentsPharmaceutical delivery mechanismFiberCross-link
The invention relates to highly biocompatible or biophilic un-cross-linked or cross-linked polymers comprising one or more side-chain active acrylic amino acids of formula Iwherein: X is —NH(CH2)4—, —O—C6H4—CH2—, —OCH2—, —O—CH(CH3)—, —S—CH2—, —O-proline, and R is H or CH3; and wherein the polymer further includes a free radical initiator and, optionally, a cross-linking agent having a plurality of polymerizable ethylenically unsaturated groups. The invention further concerns various highly biocompatible, cross-linked co-polymers comprising one or more monomers of formula I, and one or more other polymerizable monomers. Uses of such polymers and co-polymers for the production of contact lenses, intraocular lenses, implants, wound healing slabs, additives for food and cosmetics, conductive plastics, spinnable fibers, and the like are disclosed.
Owner:CIS PHARMA
Orally administered small peptides synergize statin activity
InactiveUS7148197B2Readily taken up and deliveredMany symptomOrganic active ingredientsPeptide/protein ingredientsThreonineTyrosine
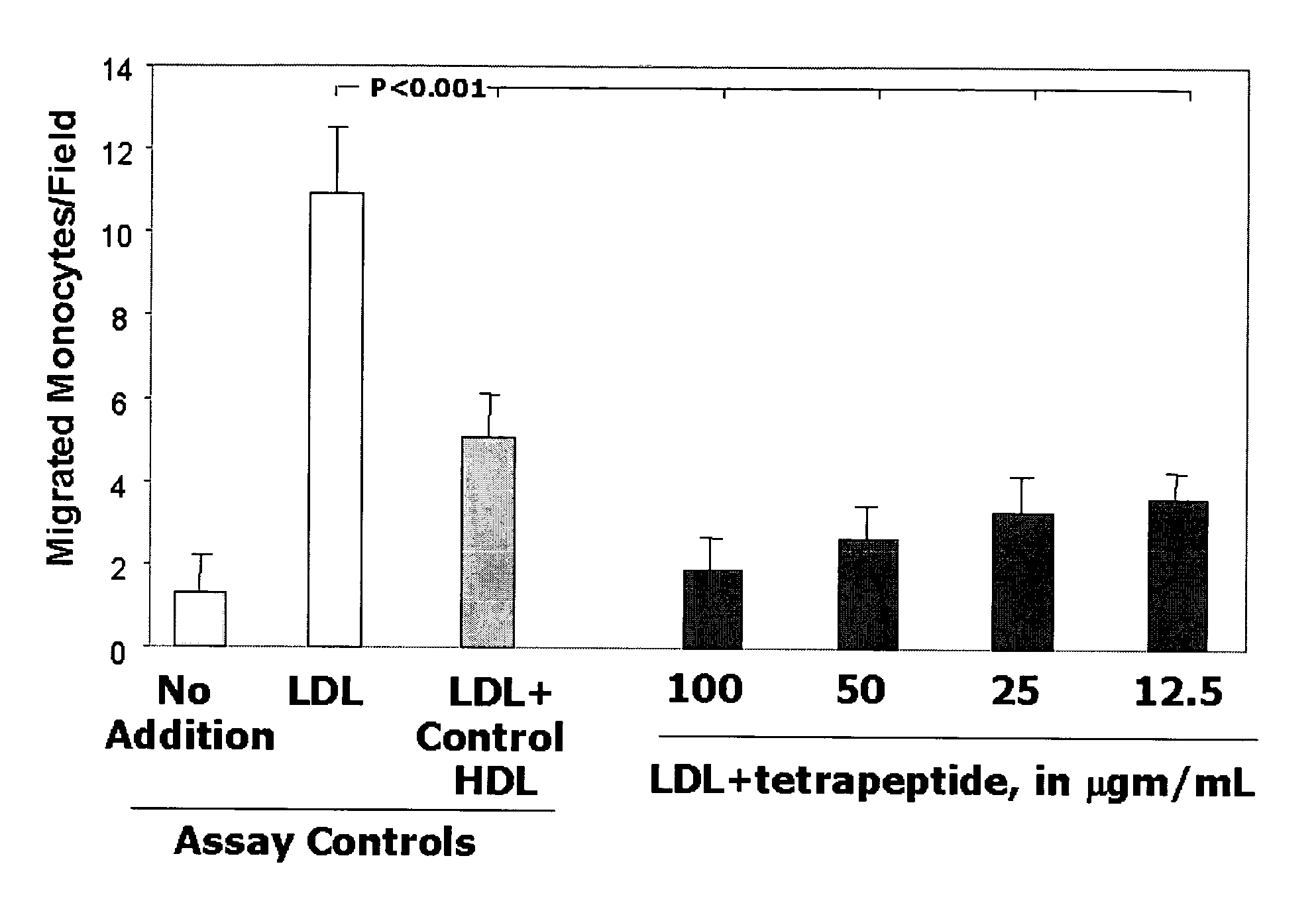
This invention provides novel peptides for the treatment of atherosclerosis. In certain embodiments the peptide is X1-X2-X3-X4 where X1 and X4 are independently selected from the group consisting of alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), tryptophan (Trp), methionine (Met), serine (Ser) bearing a hydrophobic protecting group, beta-naphthyl alanine, alpha-naphthyl alanine, norleucine, cyclohexylalanine, threonine (Thr) bearing a hydrophobic protecting group, tyrosine (Tyr) bearing a hydrophobic protecting group, lysine (Lys) bearing a hydrophobic protecting group, arginine (Arg) bearing a hydrophobic protecting group, ornithine (Orn) bearing a hydrophobic protecting group, aspartic acid (Asp) bearing a hydrophobic protecting group, cysteine (Cys) bearing a hydrophobic protecting group, and glutamic acid (Glu) bearing a hydrophobic protecting group; X2 and X3 are independently selected from the group consisting of Asp, Arg, and Glu; and the peptide converts pro-inflammatory HDL to anti-inflammatory HDL or makes anti-inflammatory HDL more anti-inflammatory.
Owner:RGT UNIV OF CALIFORNIA +1
Methods for enhancing the transport of glucose into muscle
InactiveUS20050226948A1Effective quantityLow production costBiocideUnknown materialsCysteine thiolateTryptophan
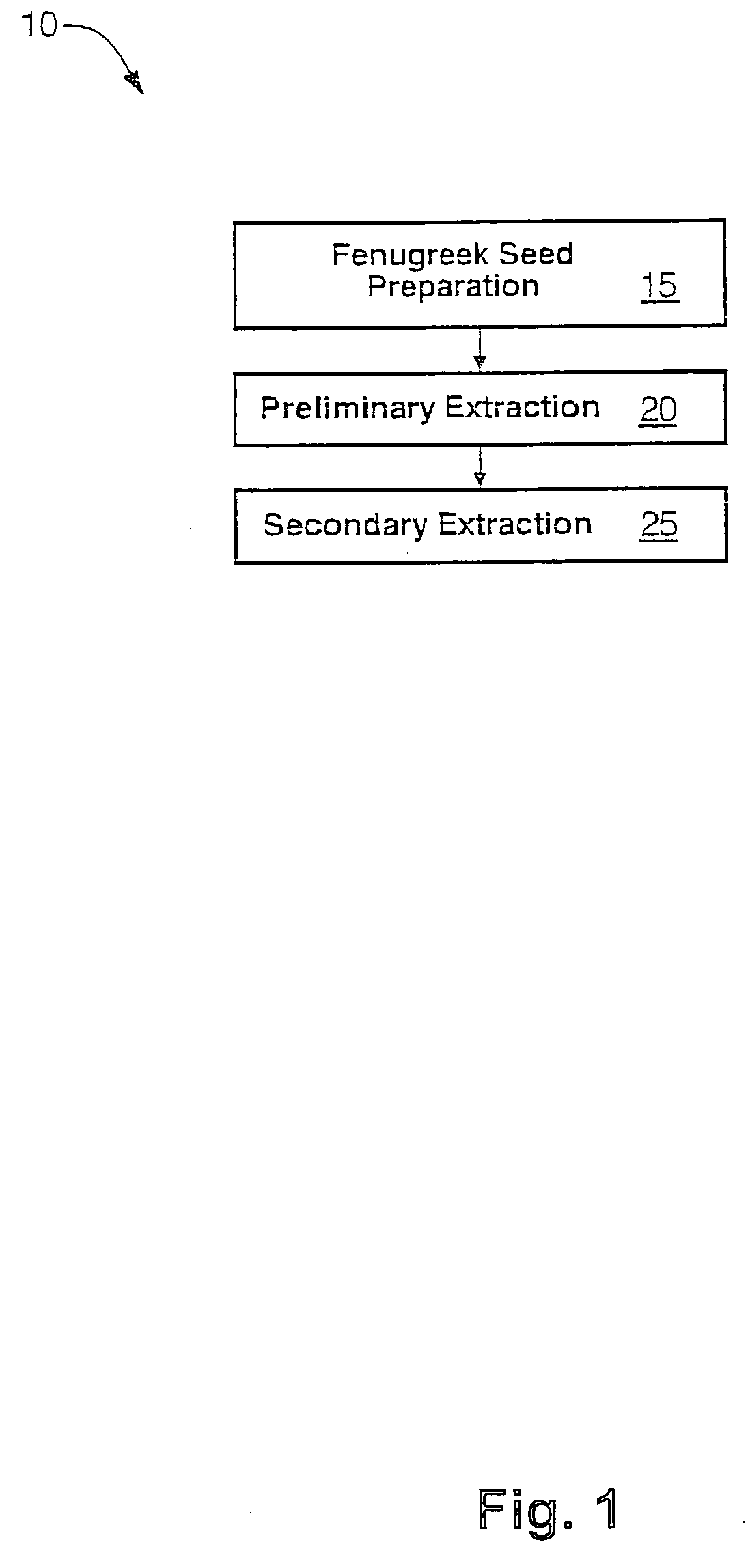
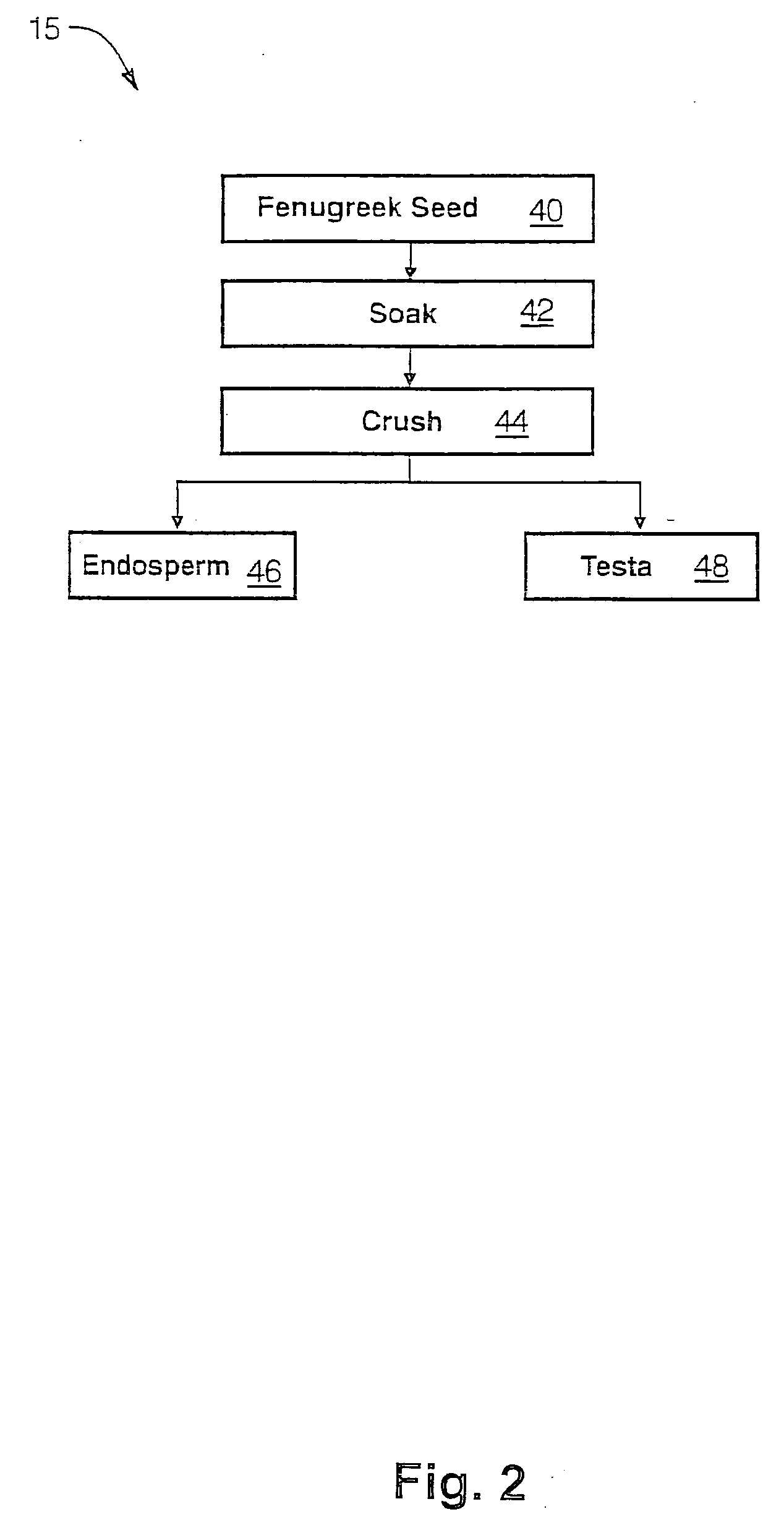
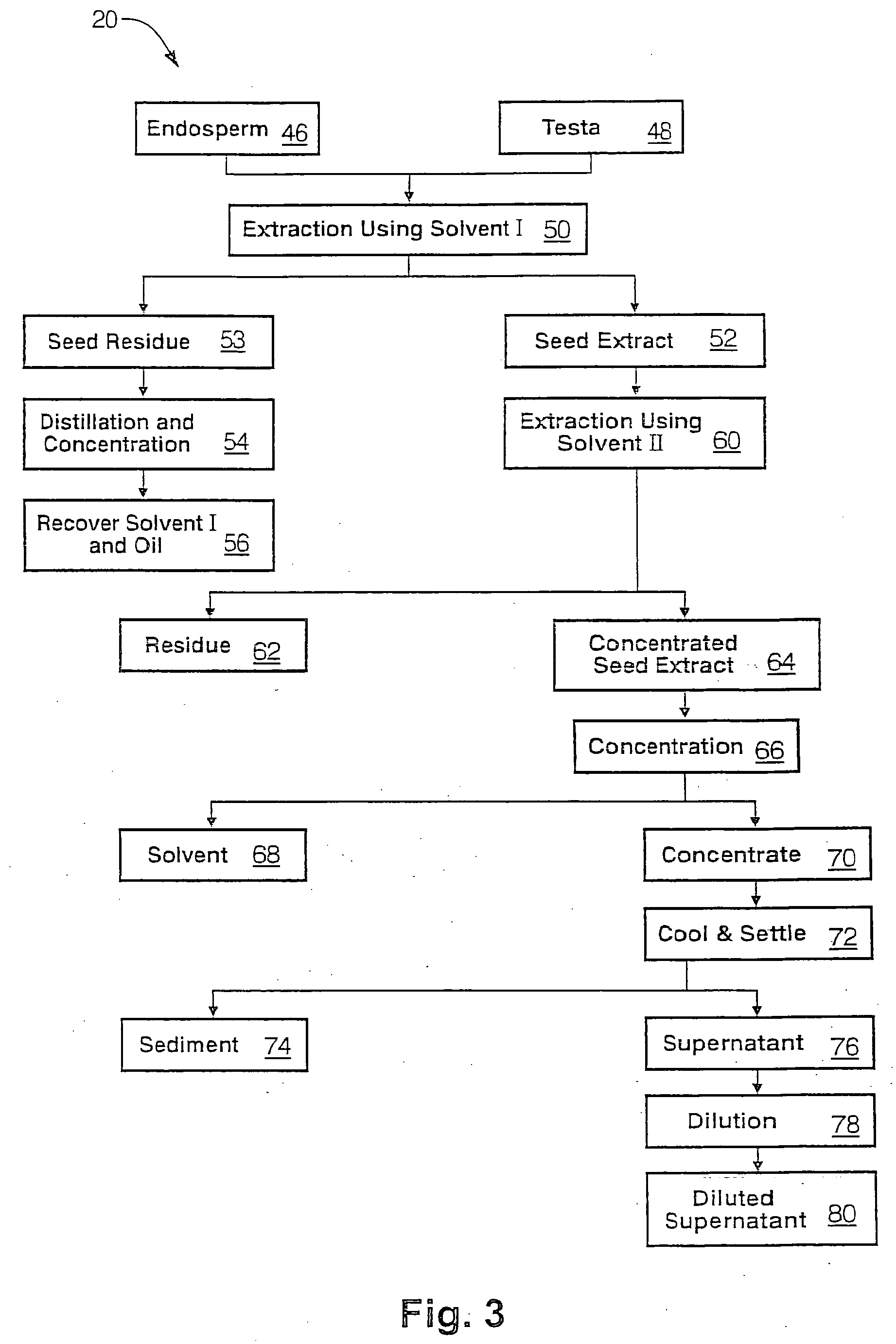
The present invention is directed to novel compositions of bio-active compounds comprising 4-hydroxyisoleucine and one or more compounds selected from the group of amino acids, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Preferably, the novel compositions of bio-active compounds include 4-hydroxyisoleucine and one or more amino acids selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, proline, lysine, histidine, and gamma-aminobutyrate. The composition of bio-active compounds preferably include between about ten percent and about seventy percent of 4-hydroxyisoleucine and between about twenty percent and about forty percent of other amino acids. The bio-active compounds of the novel composition of the present invention may be derived, isolated, and / or extracted from Fenugreek seeds. A preferred method for extracting the bio-active compounds from Fenugreek seeds includes the steps of: (1) providing a plurality of Fenugreek seeds; (2) preparing the Fenugreek seeds; and (3) extracting a novel composition of bio-active compounds from the Fenugreek seeds, which include a preliminary extraction step and a secondary extraction step. The compositions of bio-active compounds have been found to be helpful in restoring healthy energy balance in humans and animals, aiding in weight management efforts, and for balancing blood sugar levels by way of assisting the body to make more efficient use of existing (i.e., endogenous) insulin.
Owner:TSI INC
Stabilized bioactive peptides and methods of identification, synthesis, and use
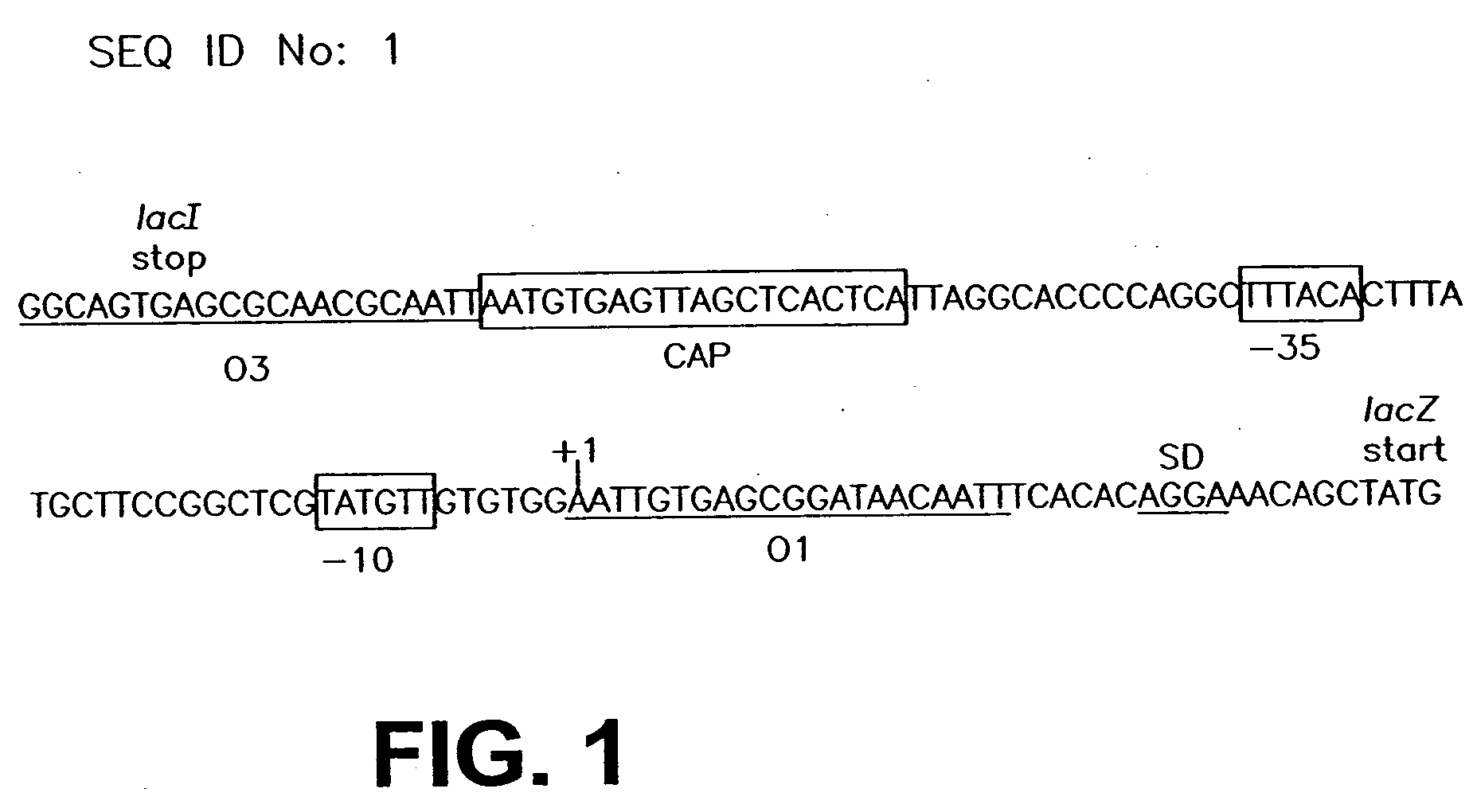
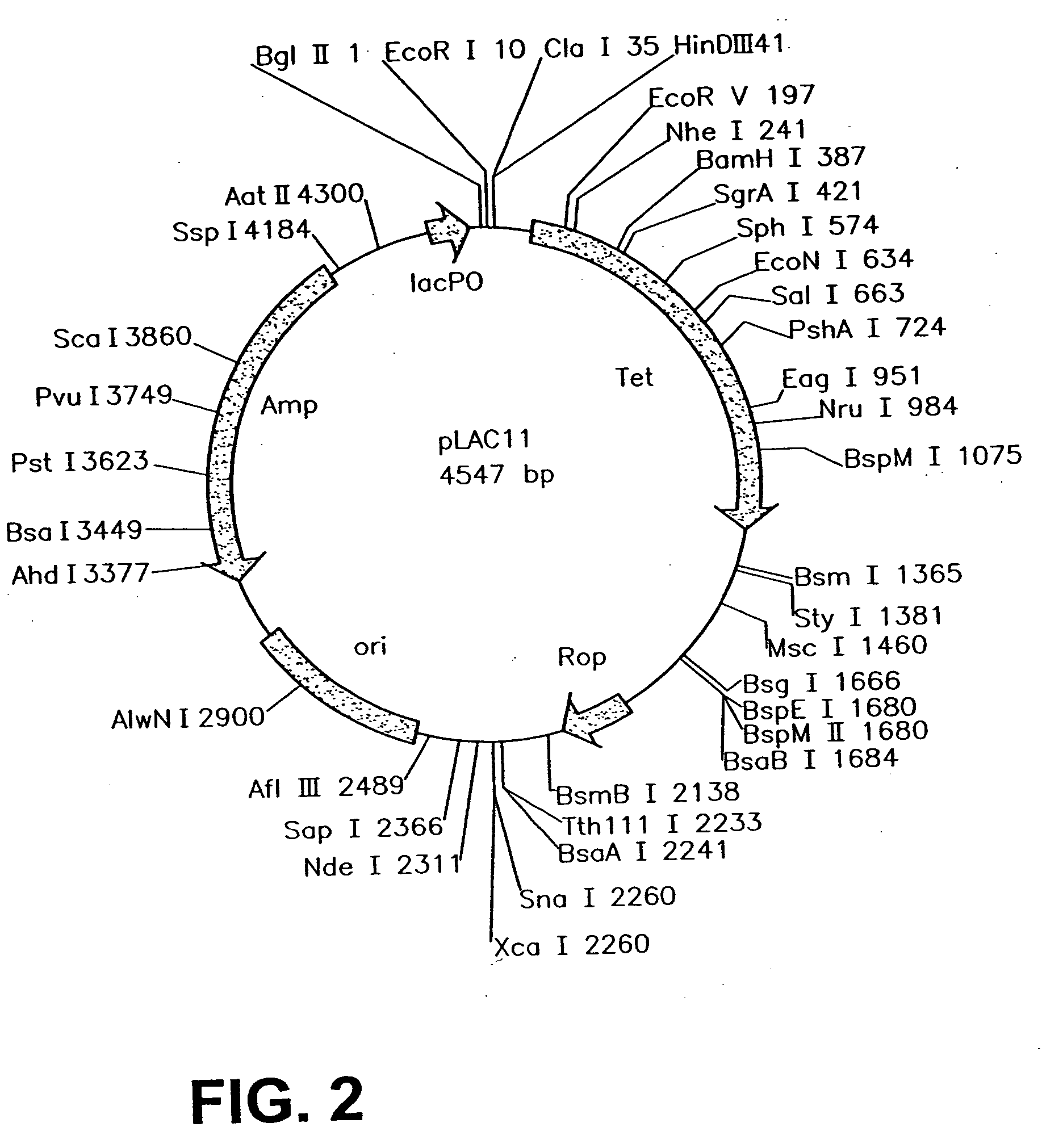
InactiveUS20060099571A1Slow down rate of intracellular degradationBacteriaPeptide/protein ingredientsLac operonΑ helical
An intracellular selection system allows screening for peptide bioactivity and stability. Randomized recombinant peptides are screened for bioactivity in a tightly regulated expression system, preferably derived from the wild-type lac operon. Bioactive peptides thus identified are inherently protease- and peptidase-resistant. Also provided are bioactive peptides stabilized by a stabilizing group at the N-terminus, the C-terminus, or both. The stabilizing group can be a small stable protein, such as the Rop protein, glutathione sulfotransferase, thioredoxin, maltose binding protein, or glutathione reductase, an α-helical moiety, or one or more proline residues.
Owner:PEPTIDE BIOSCI
Cas9 nuclease R919P and application thereof
The invention belongs to the technical field of biology, and specifically relates to Cas9 nuclease and an application. The Cas9 nuclease (Cas9-R919P) has the activity as Cas9 nuclease, fits to a CRISPR / Cas9 system, and is made by mutating the 919 location arginine of wild Cas9 nuclease to proline. A protruding fracture terminal is generated by cutting a DNA double chain with the Cas9 nuclease (Cas9-R919P), and a basic group complementary to the protruding fracture terminal is added in a filling-in manner, so that accurate addition at a specific position of the genome DNA segment can be carried out.
Owner:SHANGHAI JIAO TONG UNIV
Method for linking molecular substances
The invention relates to a method for linking two or more molecular substances, by means of adapter segments, which bring about a targeted interaction based upon the affinity of proline-rich amino acid sequences and protein domains of the type WW.
Owner:ACGT PREGENOMICS
Powders coated with specific lipoamino acid composition and cosmetics containing the same
ActiveUS20060024375A1Maintaining smooth skinRestore skin elasticityPowder deliveryBiocideHydroxyprolineSarcosine
There is provided a coated powder having a high skin care effect and a high anti-aging effect. The powder which can be used in cosmetics is coated with a mixture (lipoamino acid composition) comprising N-acyl derivatives (also including the form of a salt) of (1) at least one amino acid selected from proline and hydroxyproline, (2) at least one amino acid selected from alanine, glycine and sarcosine and (3) at least one amino acid selected from aspartic acid and glutamic acid, and at least one selected from fatty acids (and / or metal salts thereof) having a carbon number of at least 12 and at most 22.
Owner:MIYOSHI KASEI
Peptide, a method for its preparation and a pharmaceutical composition containing the peptide
A peptide of the formula Iwherein X is hydrogen, glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophan, gamma-aminobutyric acid or ζ-aminocaproic acid; A is D-gluptamic acid or D-y-glutamic acid; and Y is glycine, alanine, leucine, isoleucine, valine, N-valine, proline, tyrosine, phenylalanine, tryptophan, D-alanine, D-leucine, D-isoleucine, D-valine, D-N-valine, D-proline, D-tyrosine, D-phenylalanine, D-tryptophn, gamma-aminobutyric acid, ζ-aminocaproic acid, hydroxyl, or an amide group.
Owner:IMMUNOTECH DEV
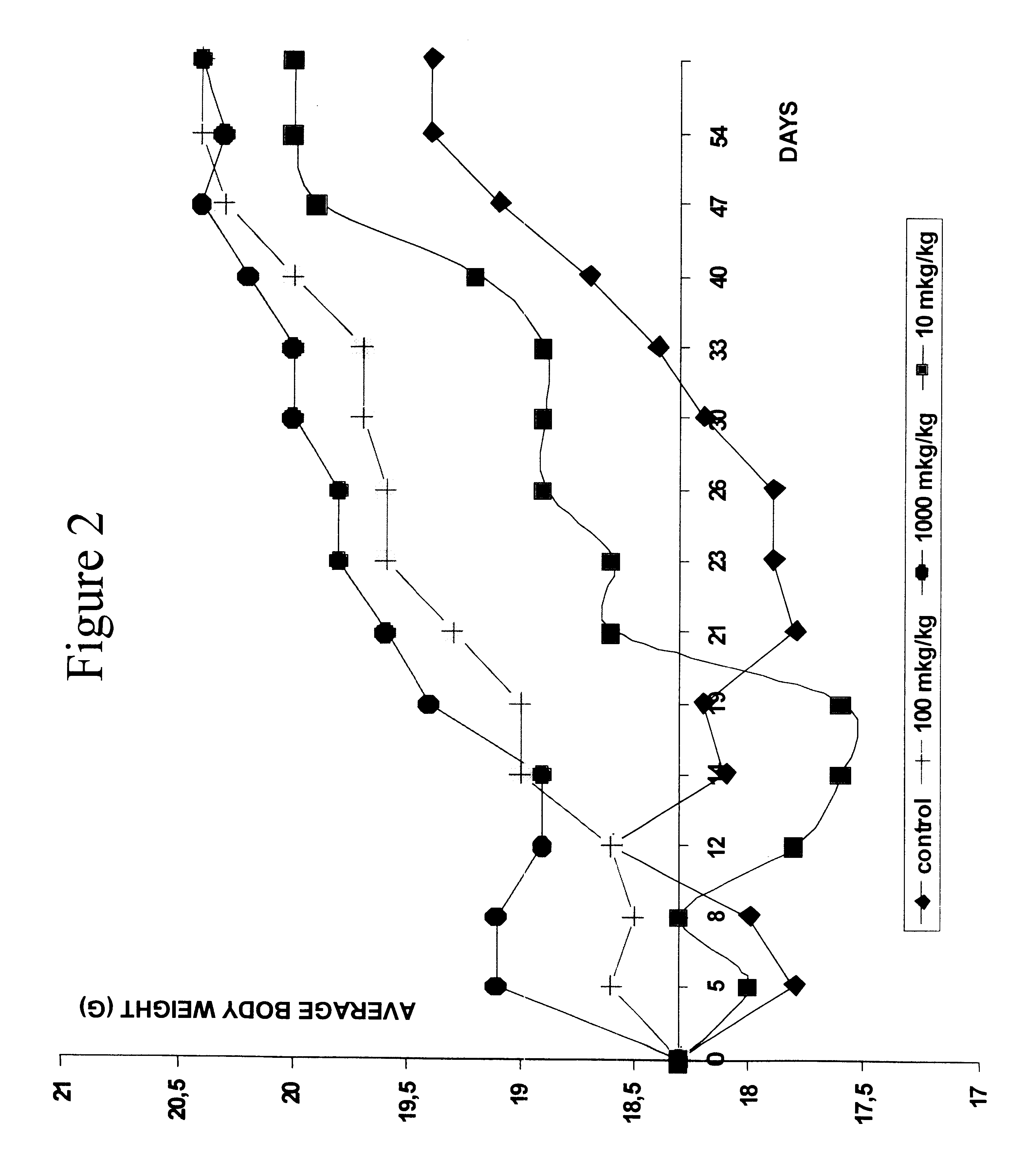
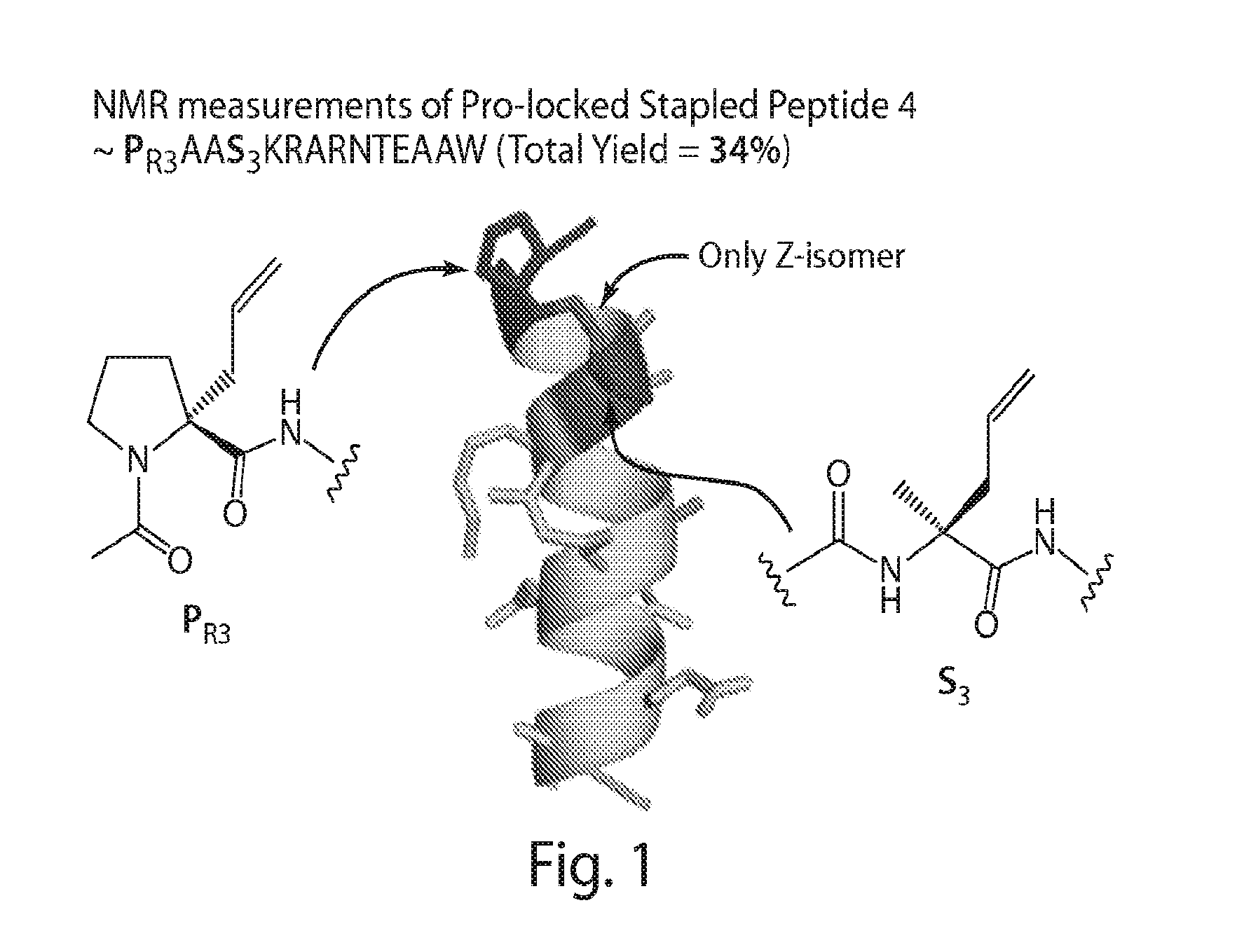
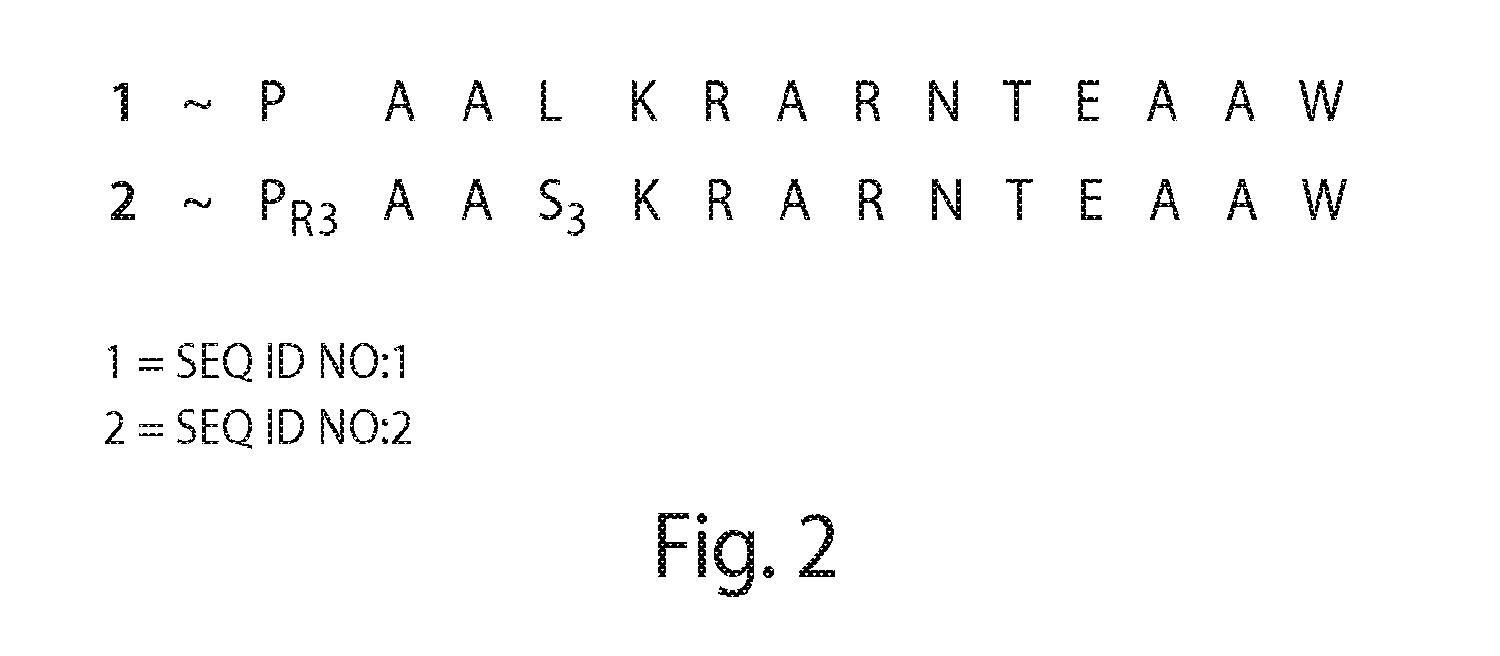
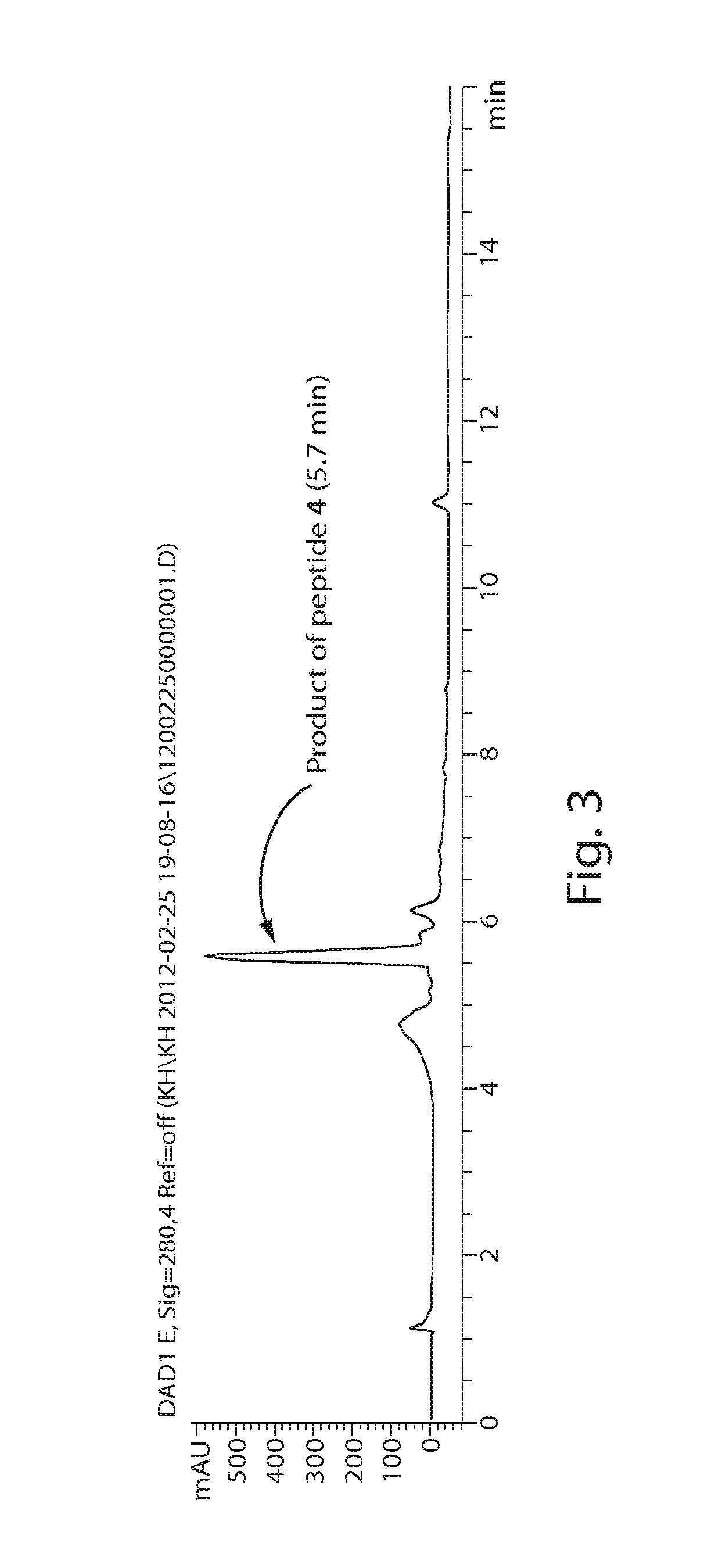
Proline-locked stapled peptides and uses thereof
ActiveUS20150239937A1Oral bioavailabilityImprove stabilitySenses disorderNervous disorderCross-linkSide chain
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Cas9 (CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) associated protein 9) nuclease Q920P and application thereof
The invention belongs to the technical field of biology and in particular relates to Cas9 (CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) associated protein 9) nuclease and application thereof. The Cas9 nuclease (Cas9-Q920P) has Cas9 nuclease activity and is applicable to a CRISPR / Cas9 system; the Cas9 nuclease (Cas9-Q920P) is obtained by mutating a 920th glutamine of wild type Cas9 nuclease into proline. The Cas9 nuclease (Cas9-Q920P) is used for cutting DNA (Deoxyribonucleic Acid) double strand to generate an protruded broken terminal and an alkaline group, which is complementary with the protruded broken terminal, can be added in a filling-in connecting manner, so that accurate editing of a specific position of a DNA segment of a genome can be realized.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com