Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Sulfotransferase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sulfotransferases EC 2.8.2.- are transferase enzymes that catalyze the transfer of a sulfo group from a donor molecule to an acceptor alcohol or amine. The most common sulfo group donor is 3'-phosphoadenosine-5'-phosphosulfate (PAPS). In the case of alcohol as acceptor, the product is a sulfate (R-OSO₃⁻), whereas an amine leads to a sulfamate (R-NH-SO₃⁻). Both reactive groups for a sulfonation via sulfotransferases may be part of a protein, lipid, carbohydrate or steroid.
Treatment of tumours
InactiveUS20050192262A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsSteroidsDiseaseAndrostane
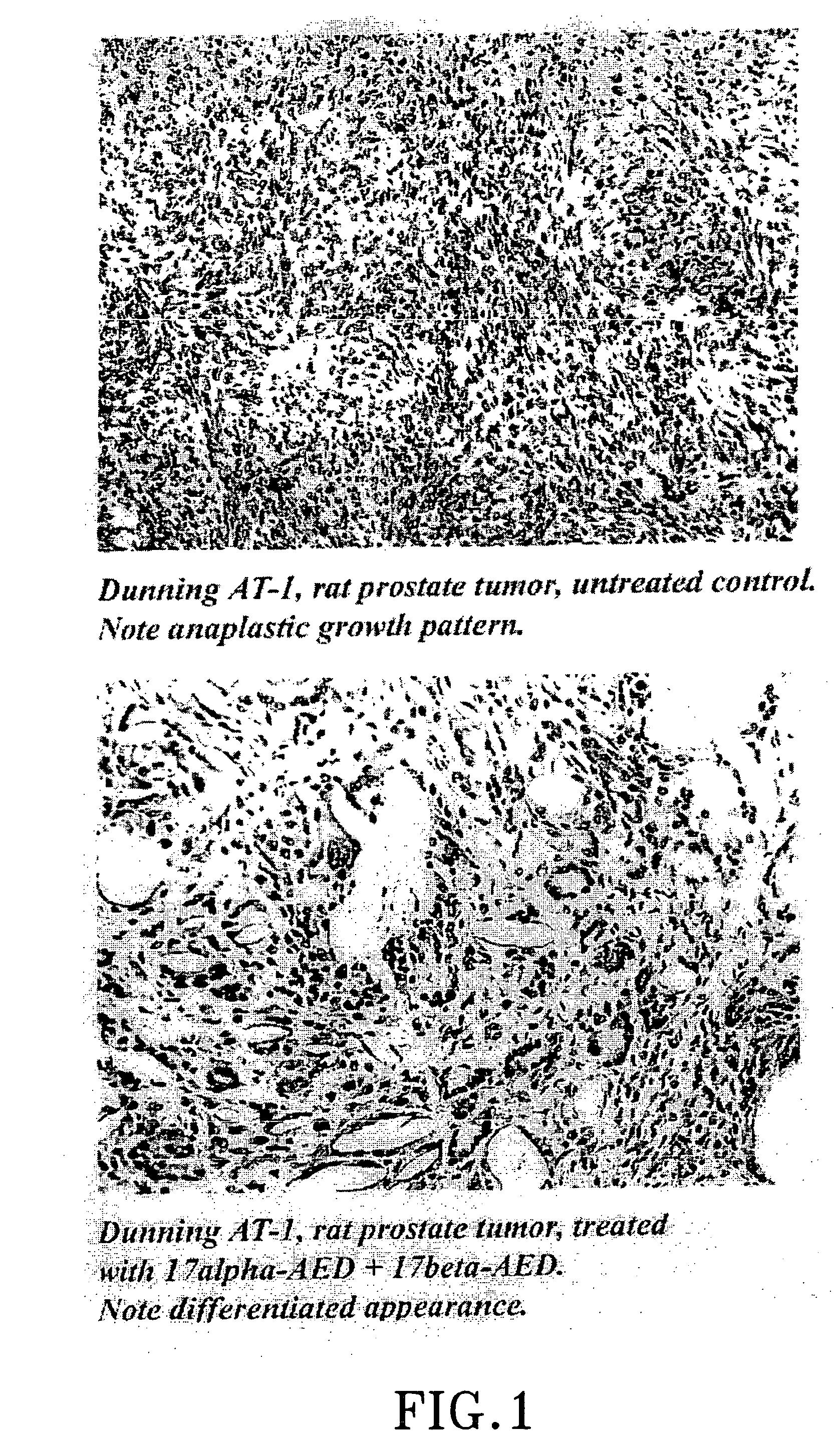
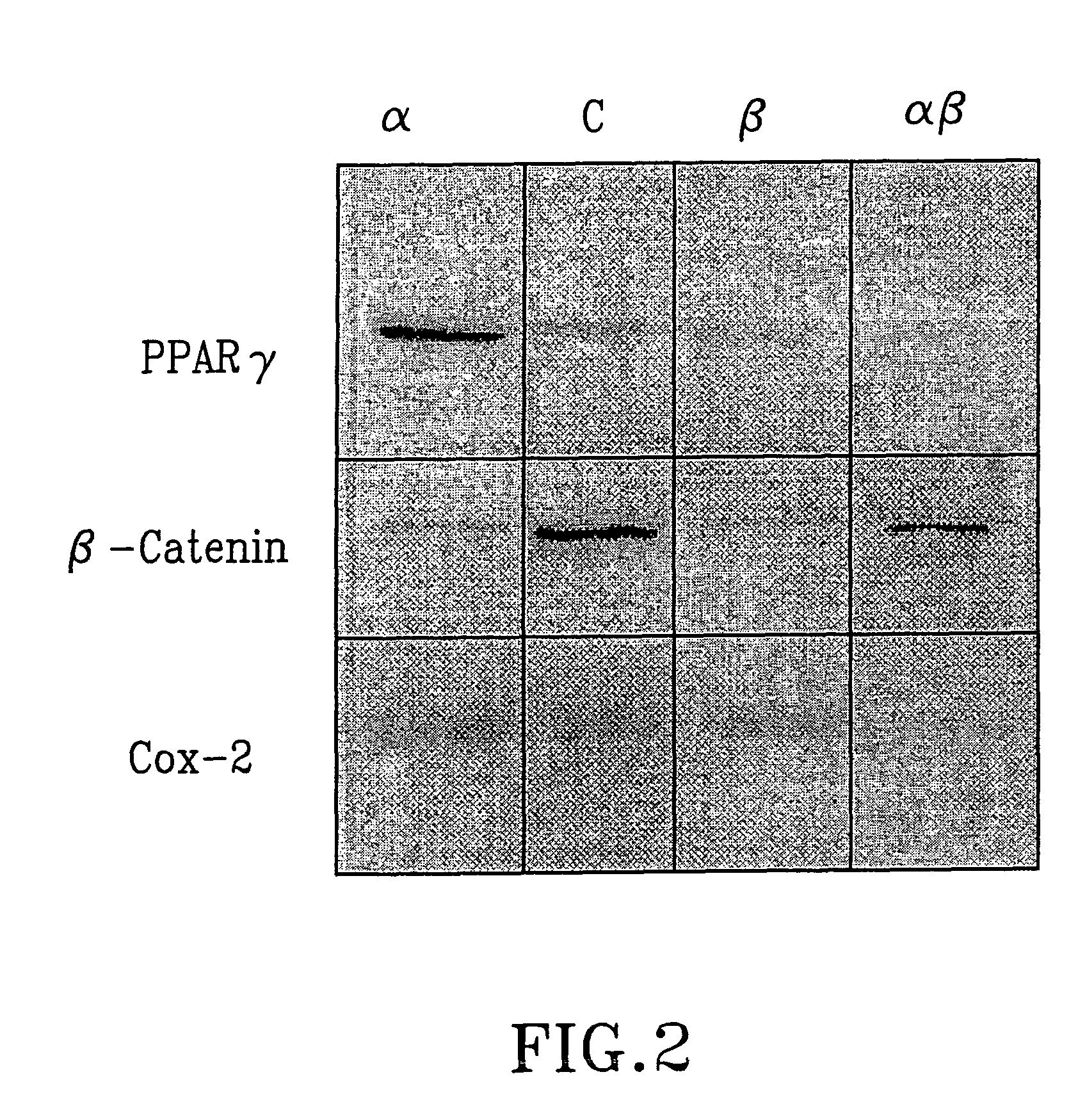
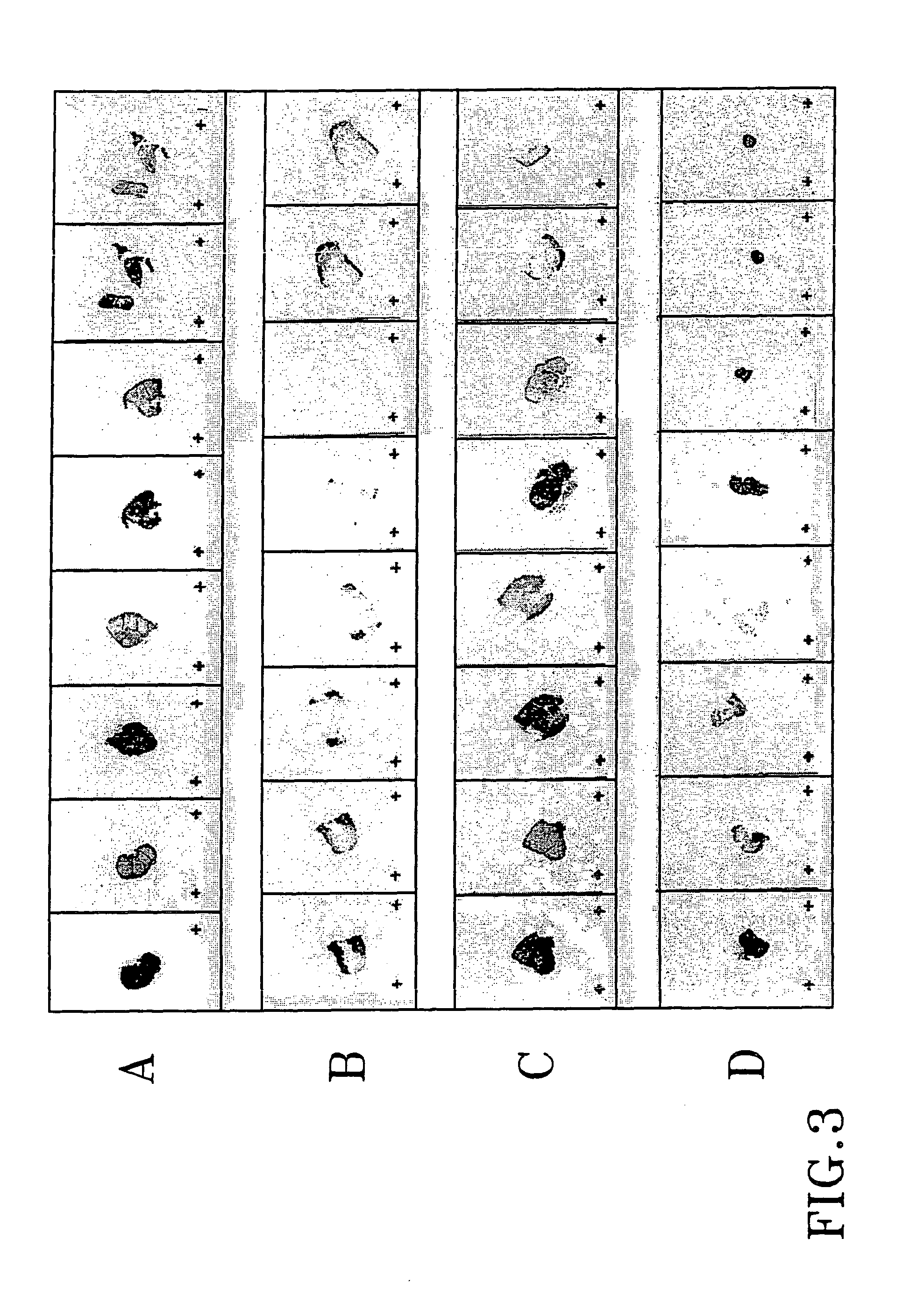
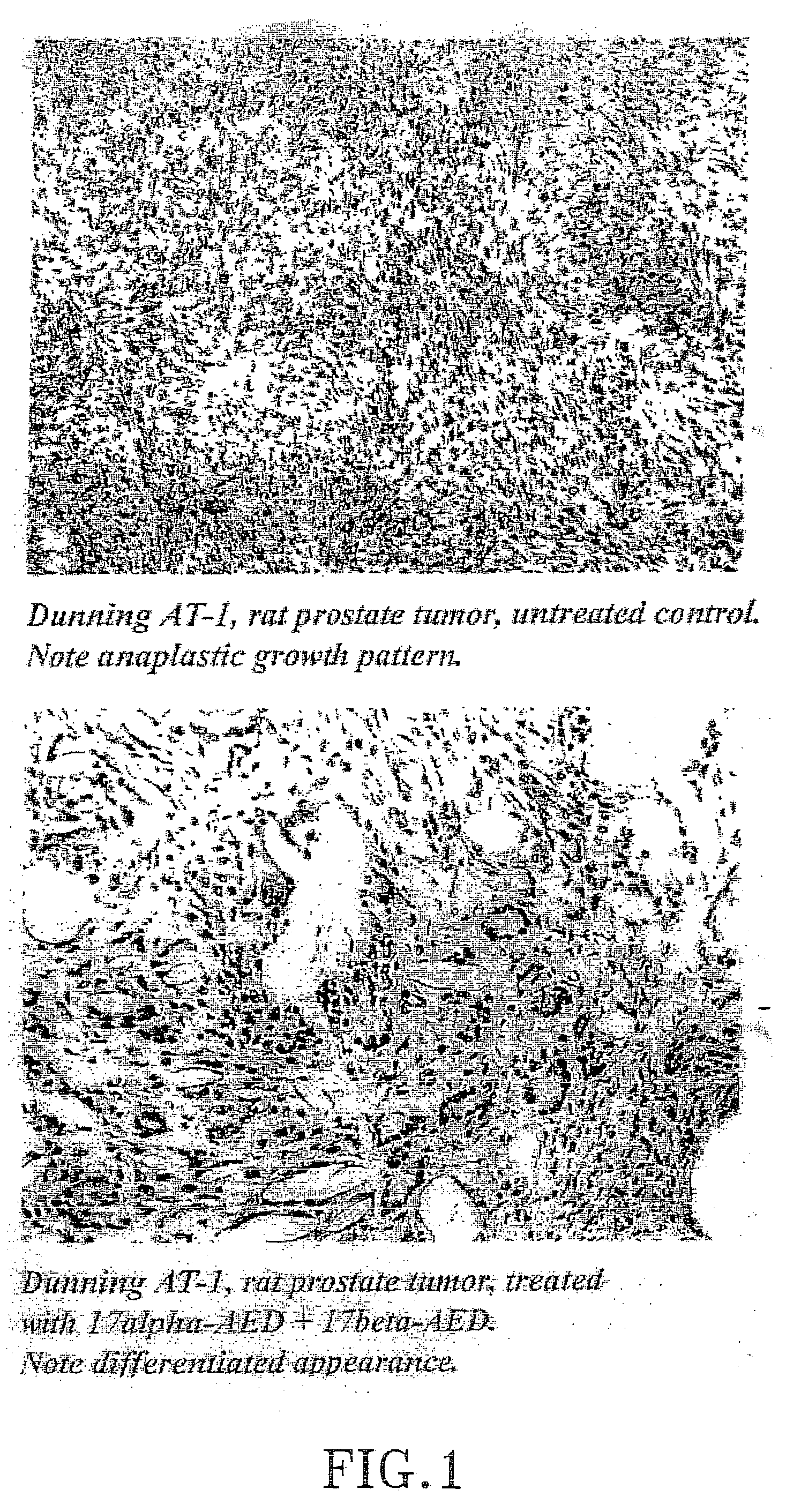
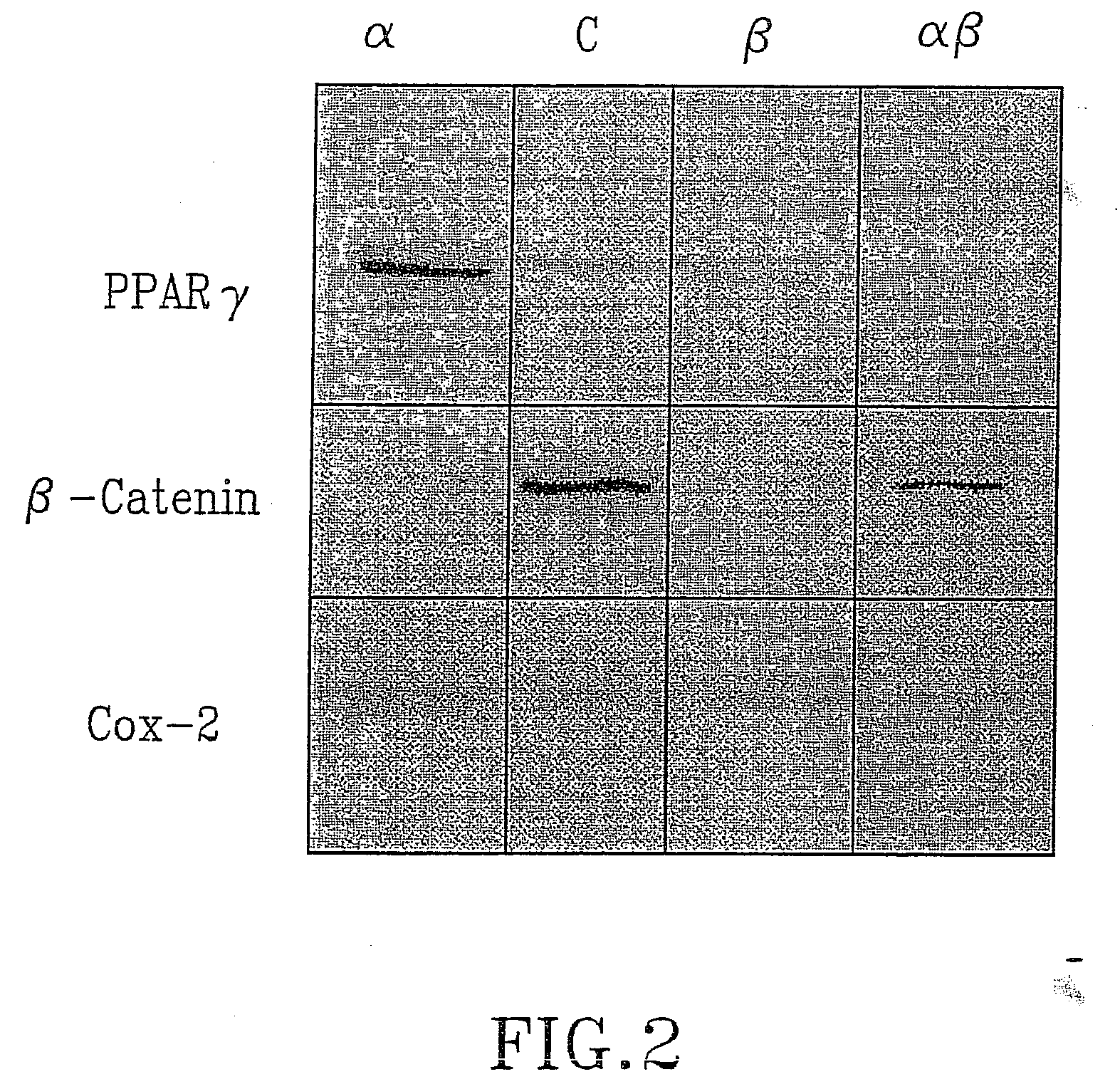
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wnt-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3β,17-diol or androstane-3β-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3β-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor-(PPAR) is produced.
Owner:HAGSTROM TOMAS
Stabilized bioactive peptides and methods of identification, synthesis, and use
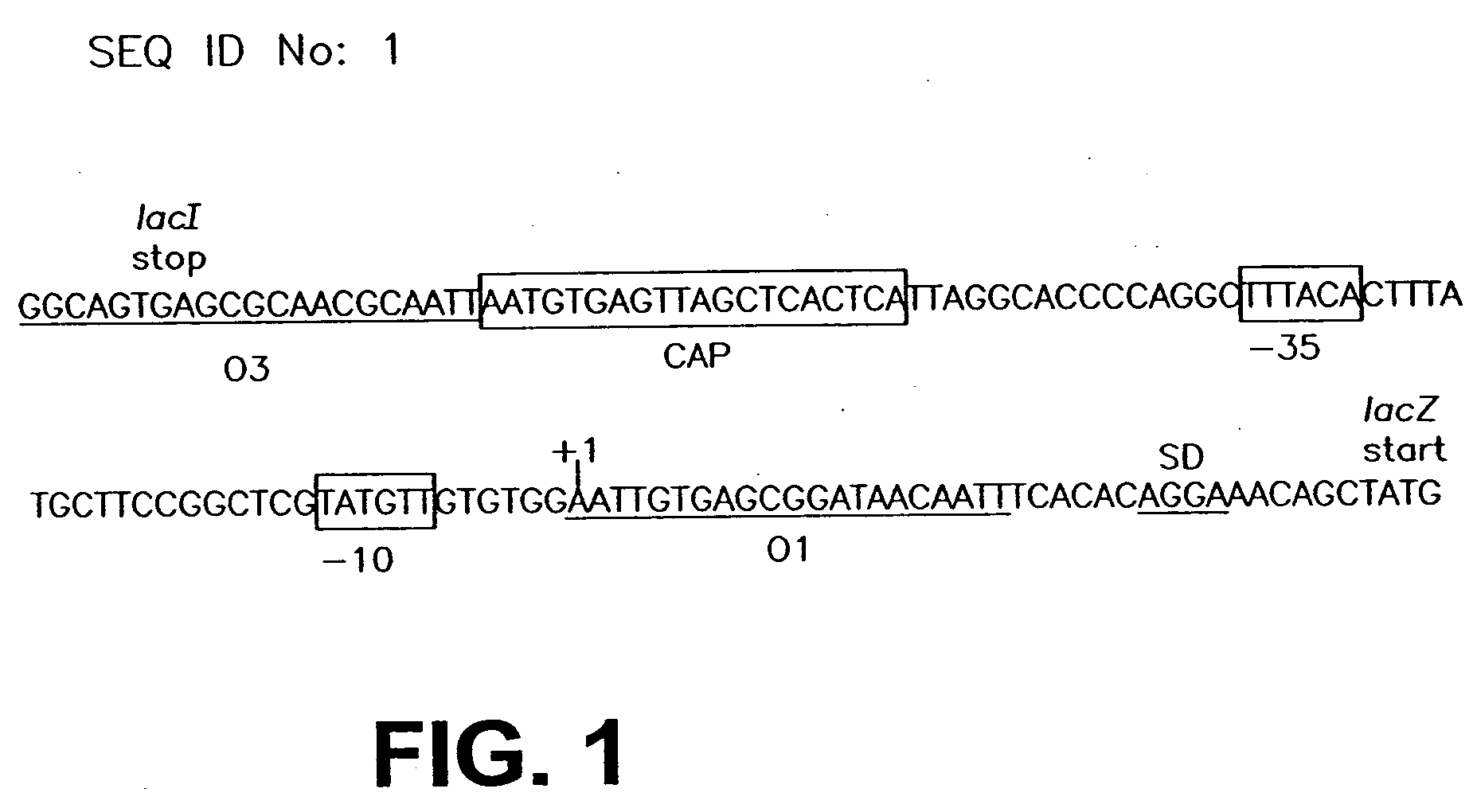
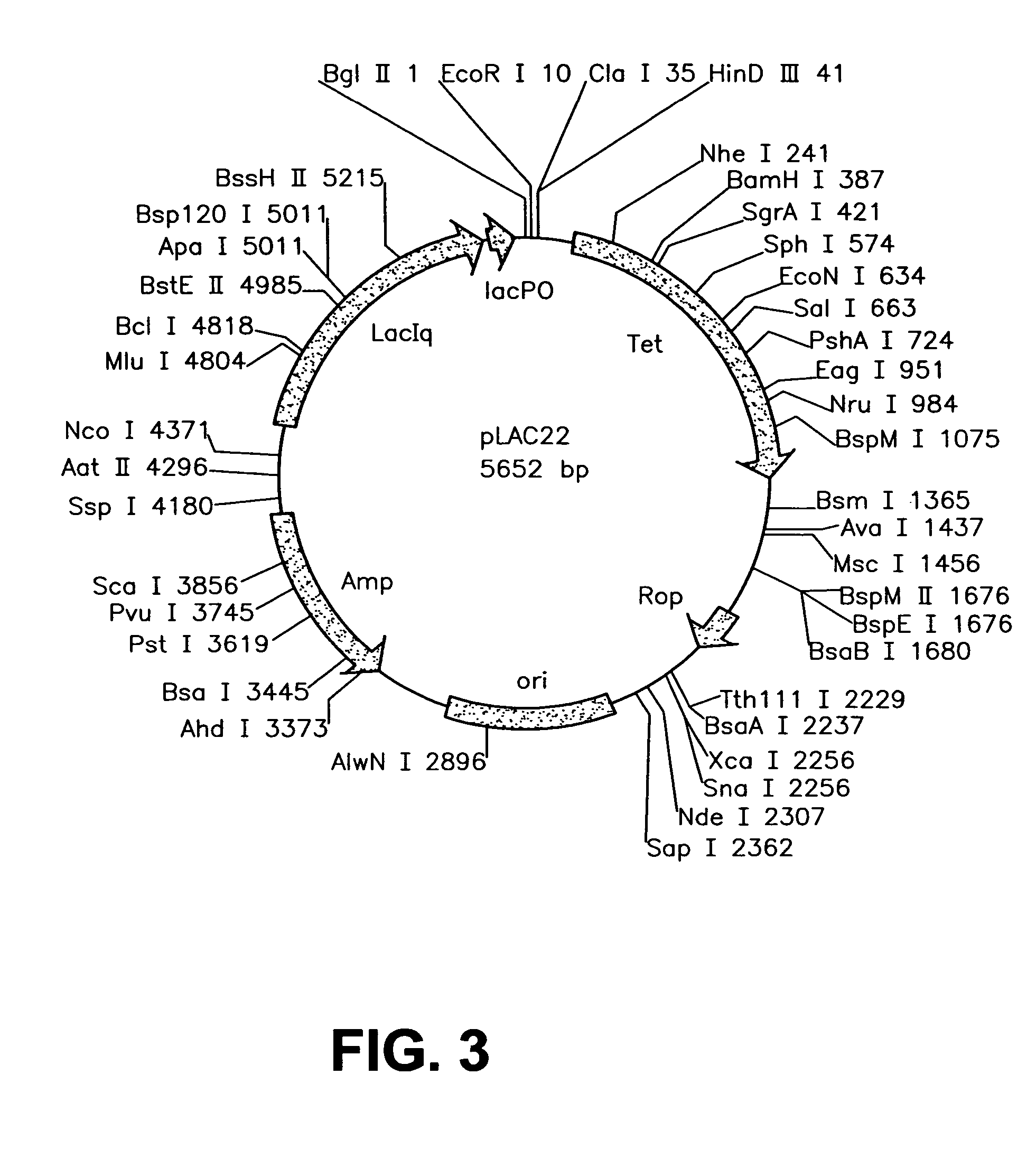
InactiveUS20060099571A1Slow down rate of intracellular degradationBacteriaPeptide/protein ingredientsLac operonΑ helical
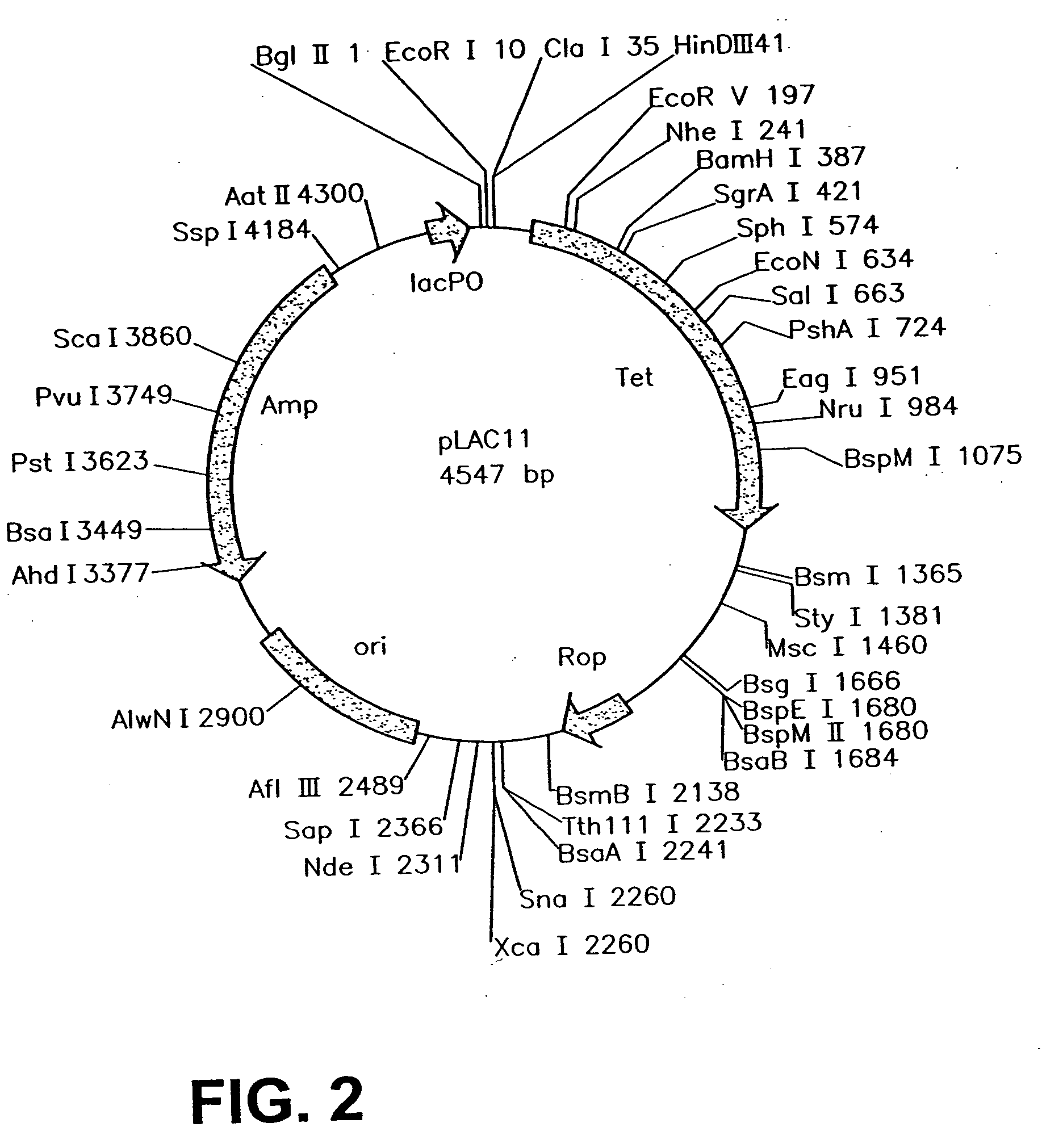
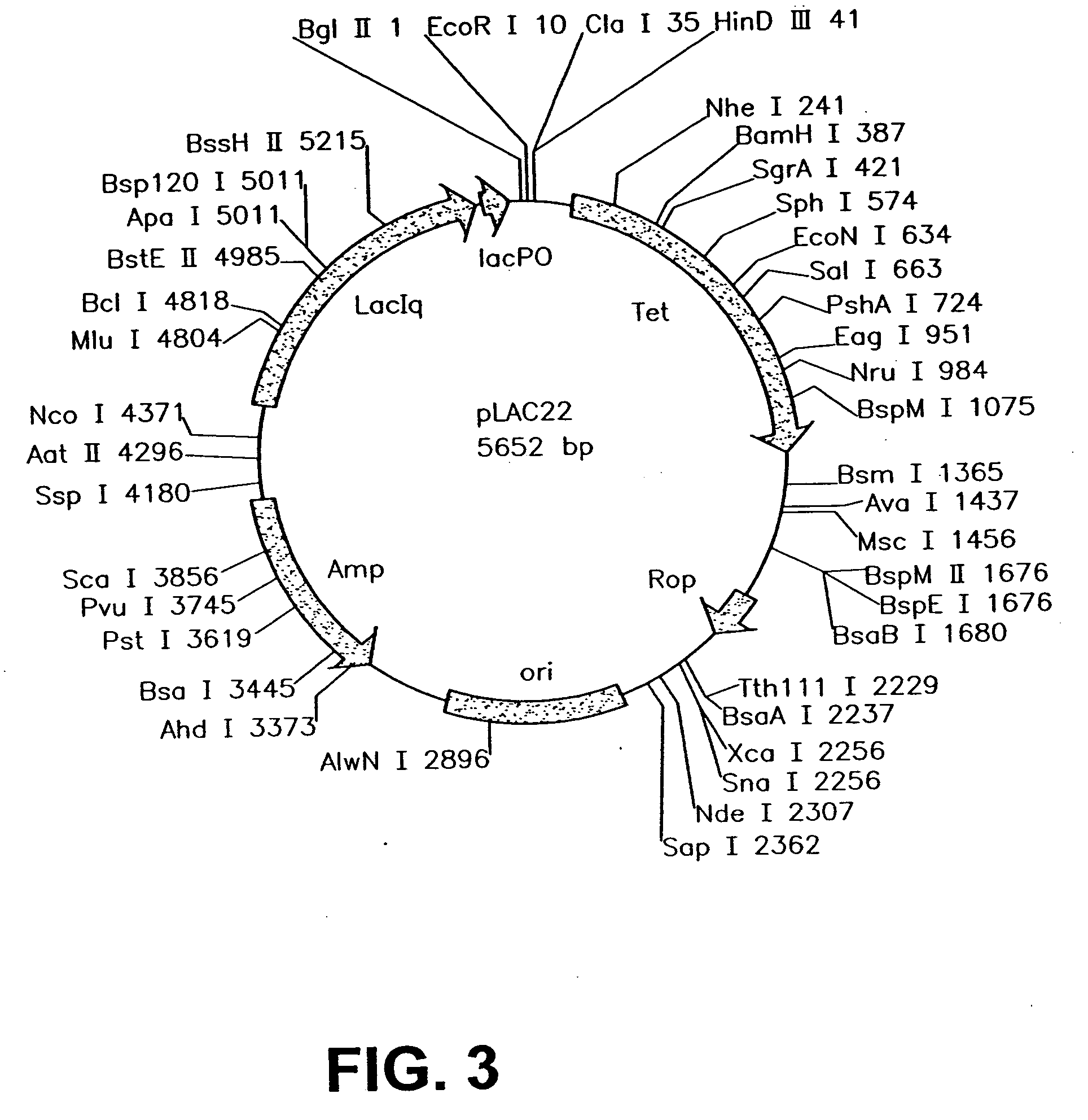
An intracellular selection system allows screening for peptide bioactivity and stability. Randomized recombinant peptides are screened for bioactivity in a tightly regulated expression system, preferably derived from the wild-type lac operon. Bioactive peptides thus identified are inherently protease- and peptidase-resistant. Also provided are bioactive peptides stabilized by a stabilizing group at the N-terminus, the C-terminus, or both. The stabilizing group can be a small stable protein, such as the Rop protein, glutathione sulfotransferase, thioredoxin, maltose binding protein, or glutathione reductase, an α-helical moiety, or one or more proline residues.
Owner:PEPTIDE BIOSCI
Stabilized bioactive peptides and methods of identification, synthesis and use
Owner:PEPTIDE BIOSCI
Stabilized bioactive peptides and methods of identification, synthesis, and use
InactiveUS7365162B2Slow down rate of intracellular degradationBacteriaPeptide/protein ingredientsLac operonWild type
An intracellular selection system allows screening for peptide bioactivity and stability. Randomized recombinant peptides are screened for bioactivity in a tightly regulated expression system, preferably derived from the wild-type lac operon. Bioactive peptides thus identified are inherently protease- and peptidase-resistant. Also provided are bioactive peptides stabilized by a stabilizing group at the N-terminus, the C-terminus, or both. The stabilizing group can be a small stable protein, such as the Rop protein, glutathione sulfotransferase, thioredoxin, maltose binding protein, or glutathione reductase, an α-helical moiety, or one or more proline residues.
Owner:PEPTIDE BIOSCI
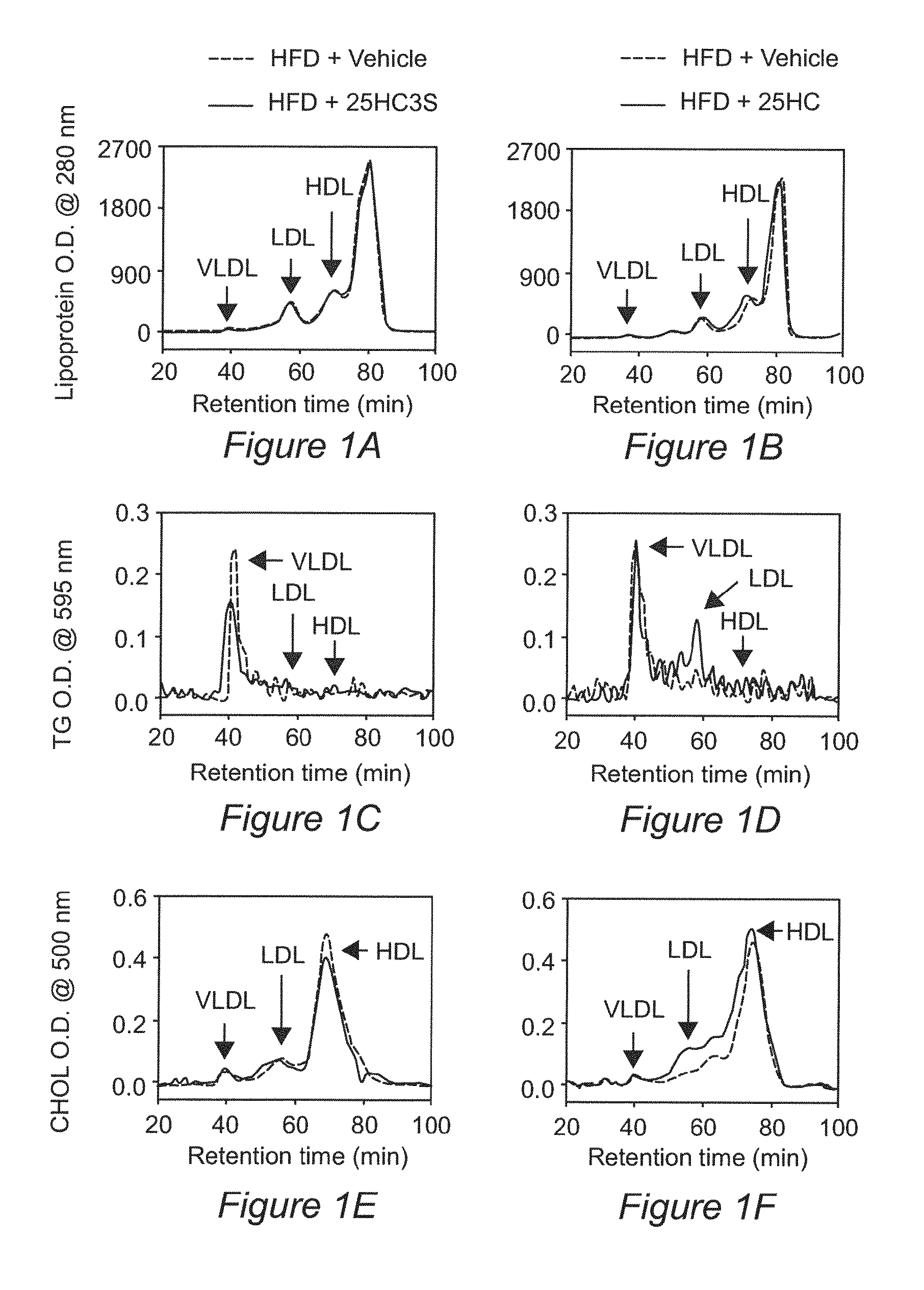
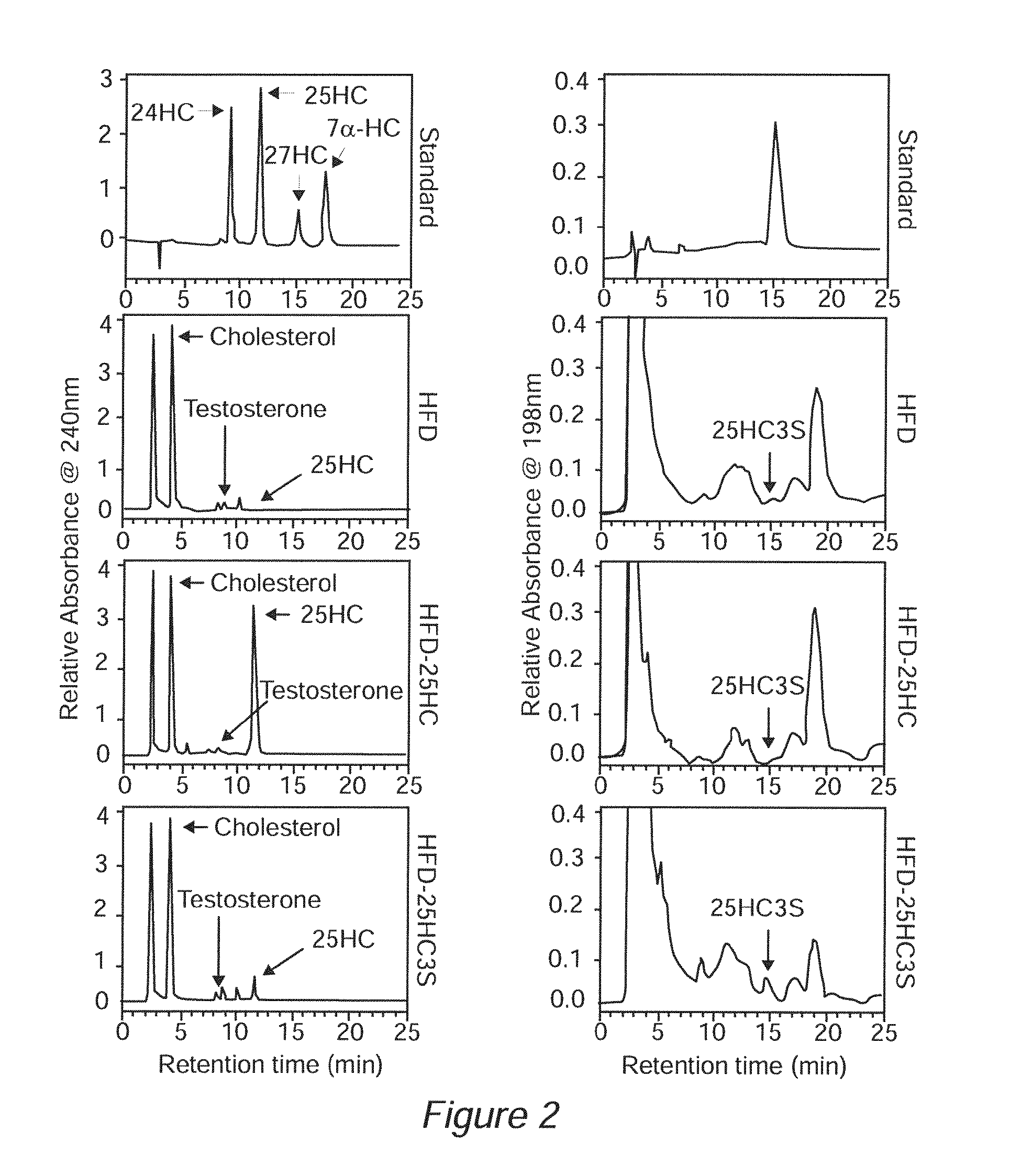
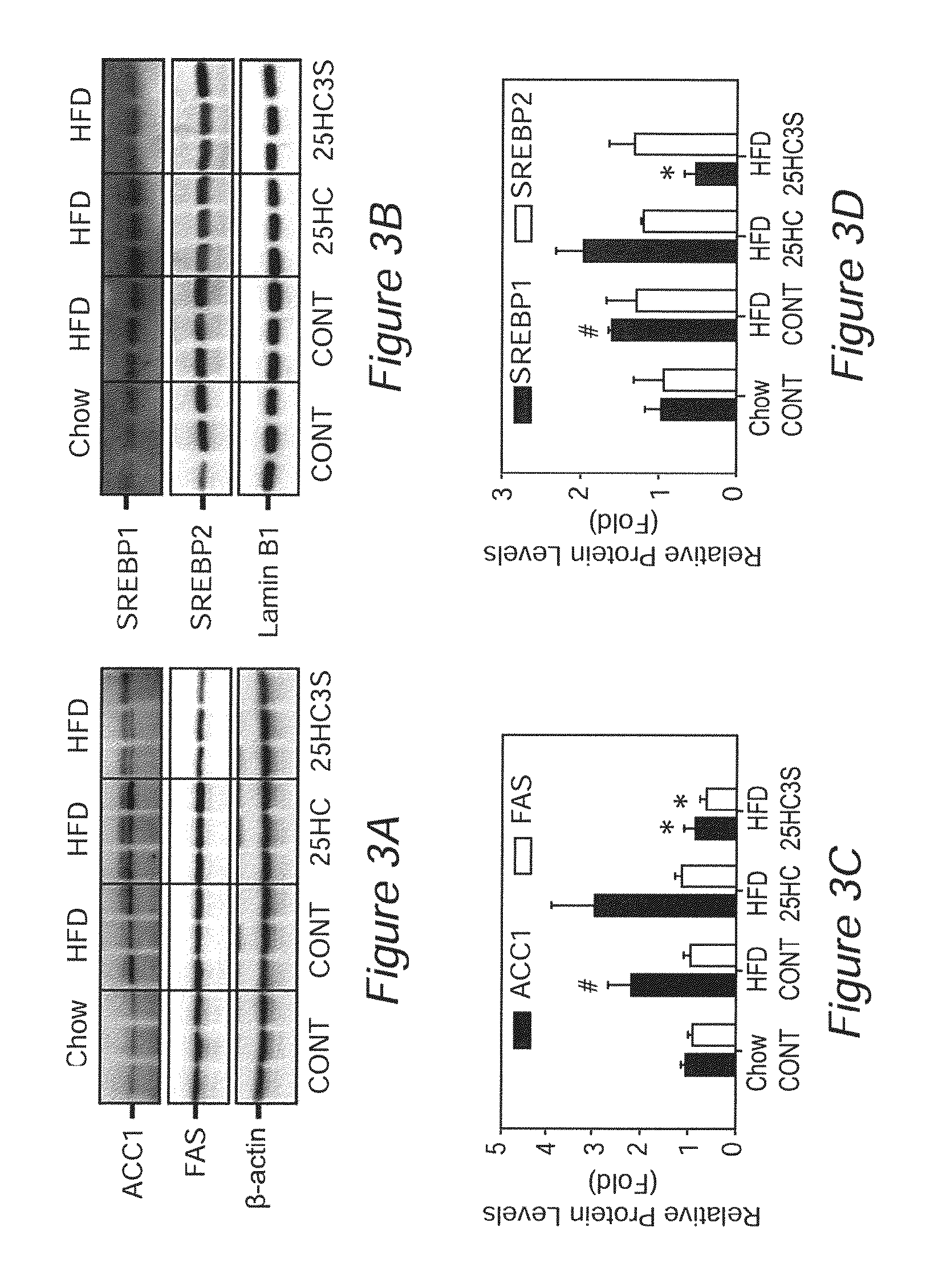
Sulfated oxysterol and oxysterol sulfation by hydroxysterol sulfotransferase promote lipid homeostasis and liver proliferation
Methods and compositions for the prevention and treatment of liver damage or disease in a subject in need thereof are provided. The methods involve providing the sulfated oxysterol 25-hydroxycholesterol-3-sulfate (25HC3S) to the subject e.g. by 1) administering 25HC3S to the subject; or 2) overexpressing, in the subject, the hydroxysterol sulfotransferase enzyme SULT2B1b, which catalyzes the sulfation of 25-hydroxycholesterol (25HC) to form 25HC3S.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
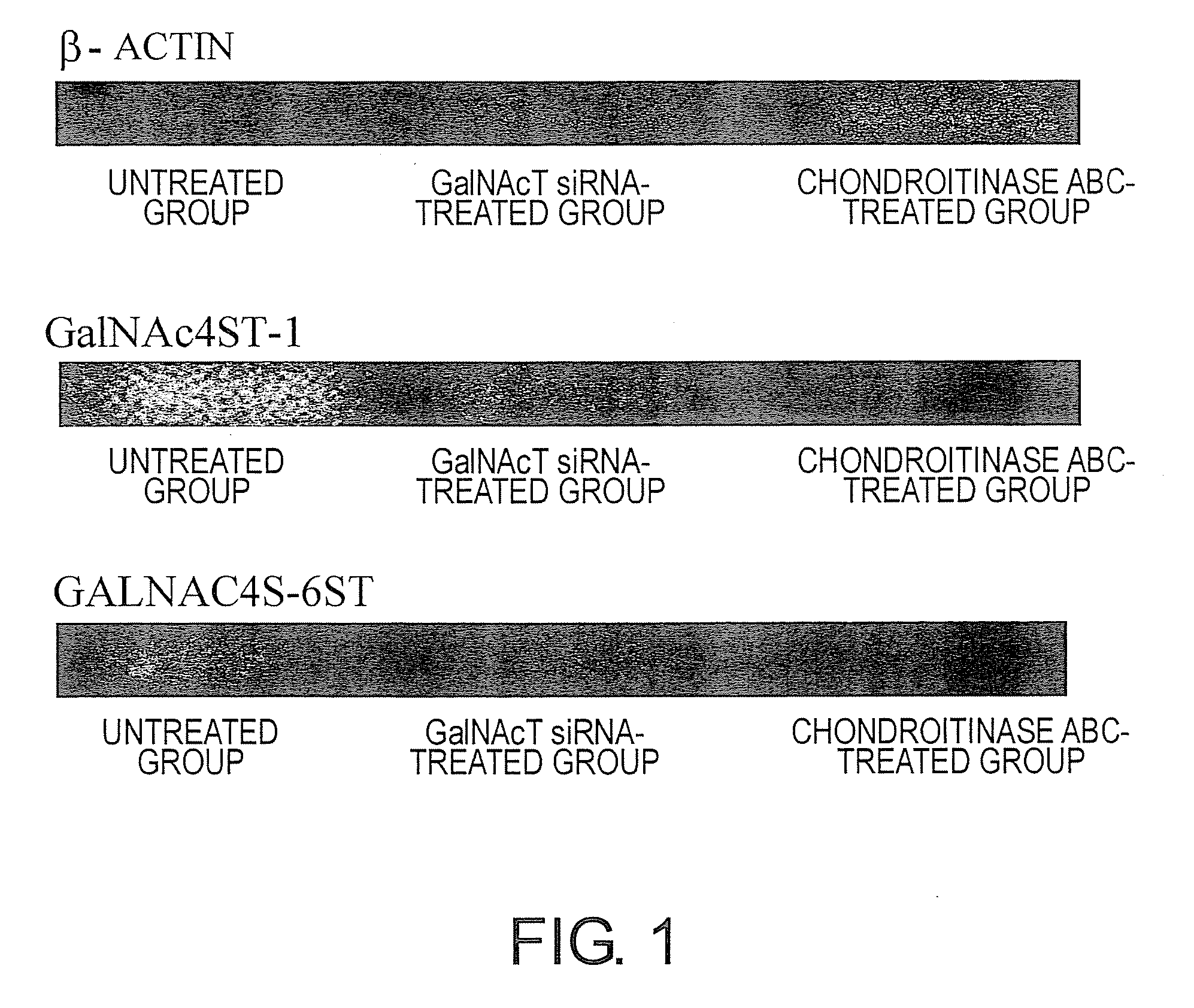
Agents for suppressing neural fibrotic degeneration
InactiveUS20090202515A1Suppress neural fibrotic degenerationGreat medical and industrial significanceOrganic active ingredientsNervous disorderSide chainNeural cell
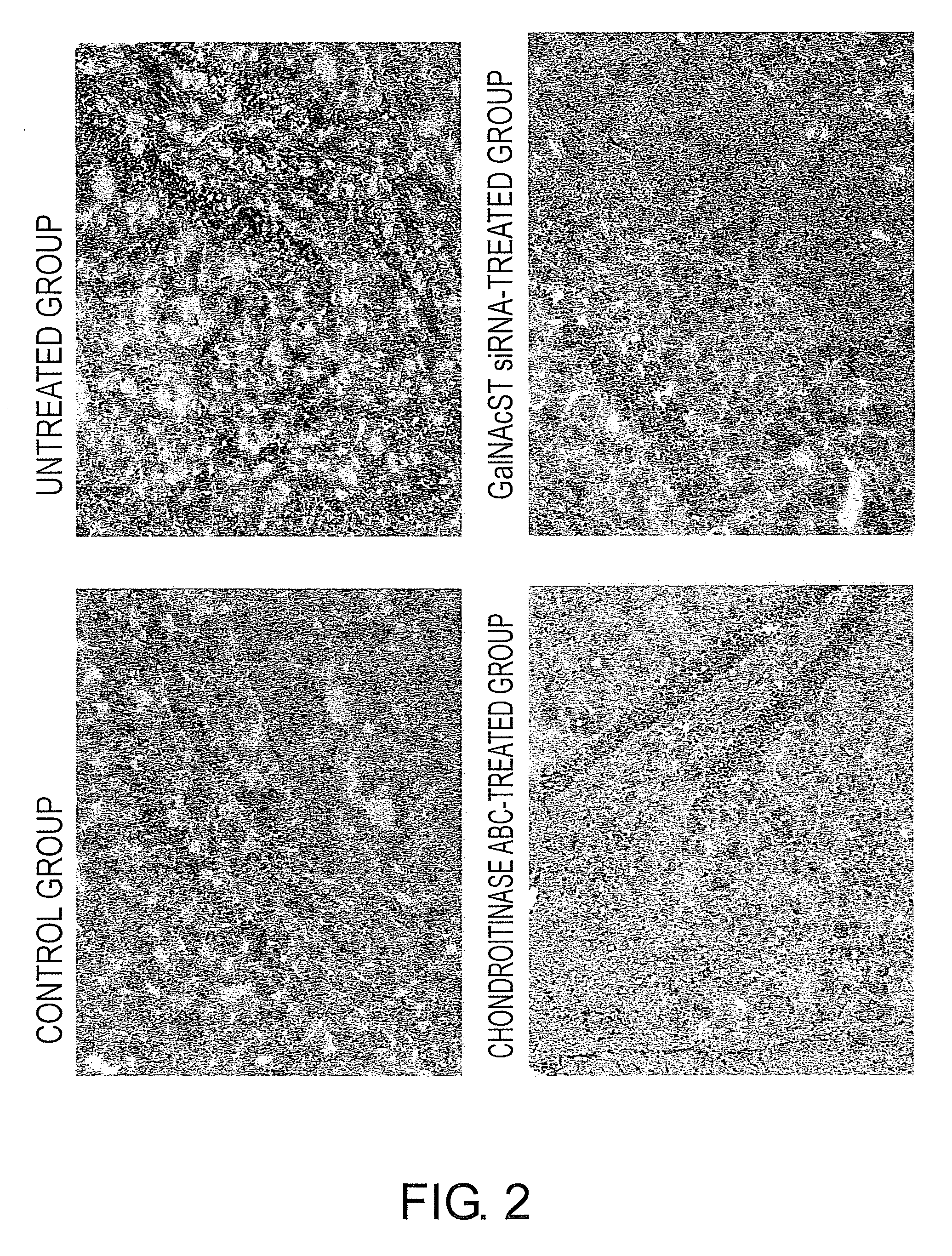
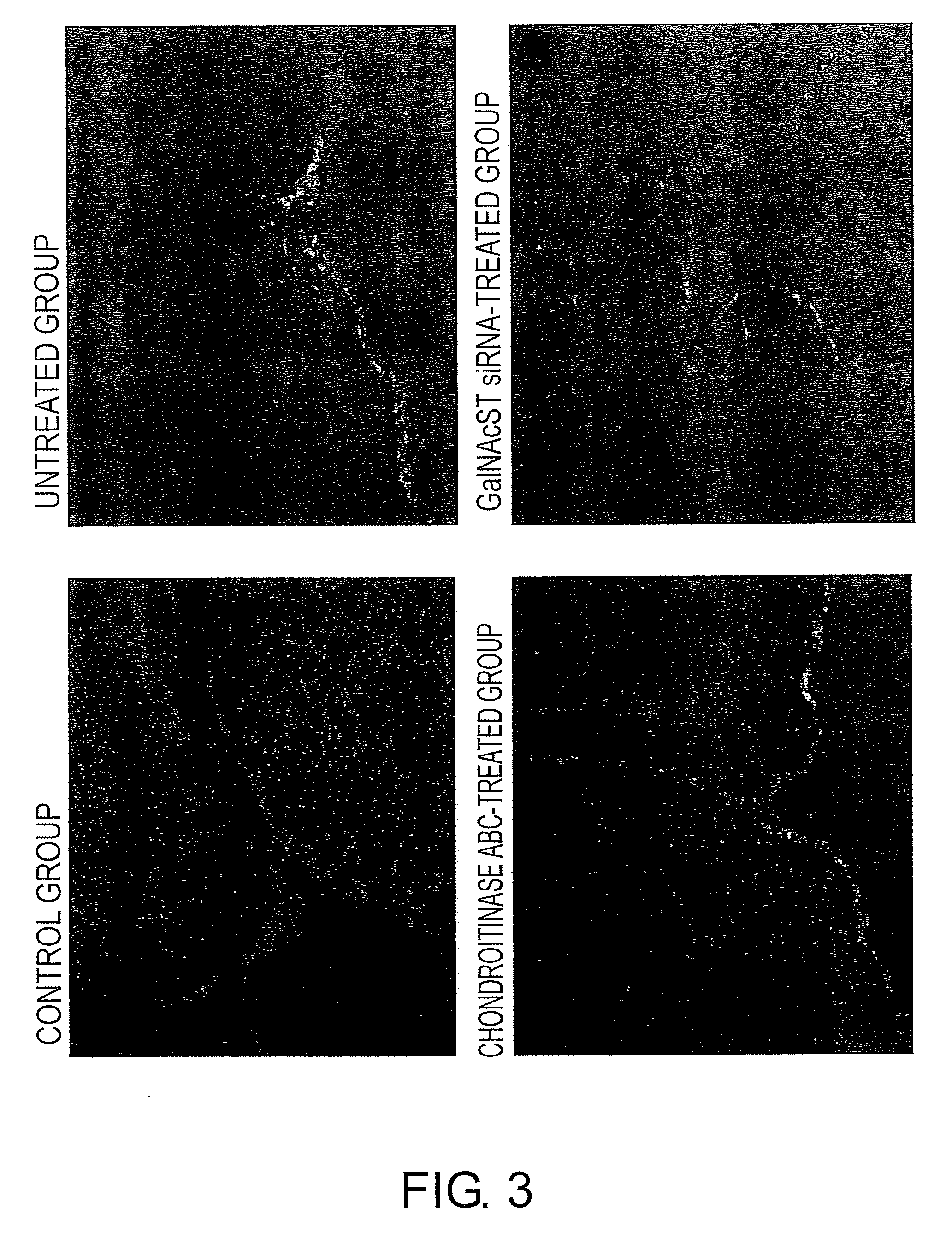
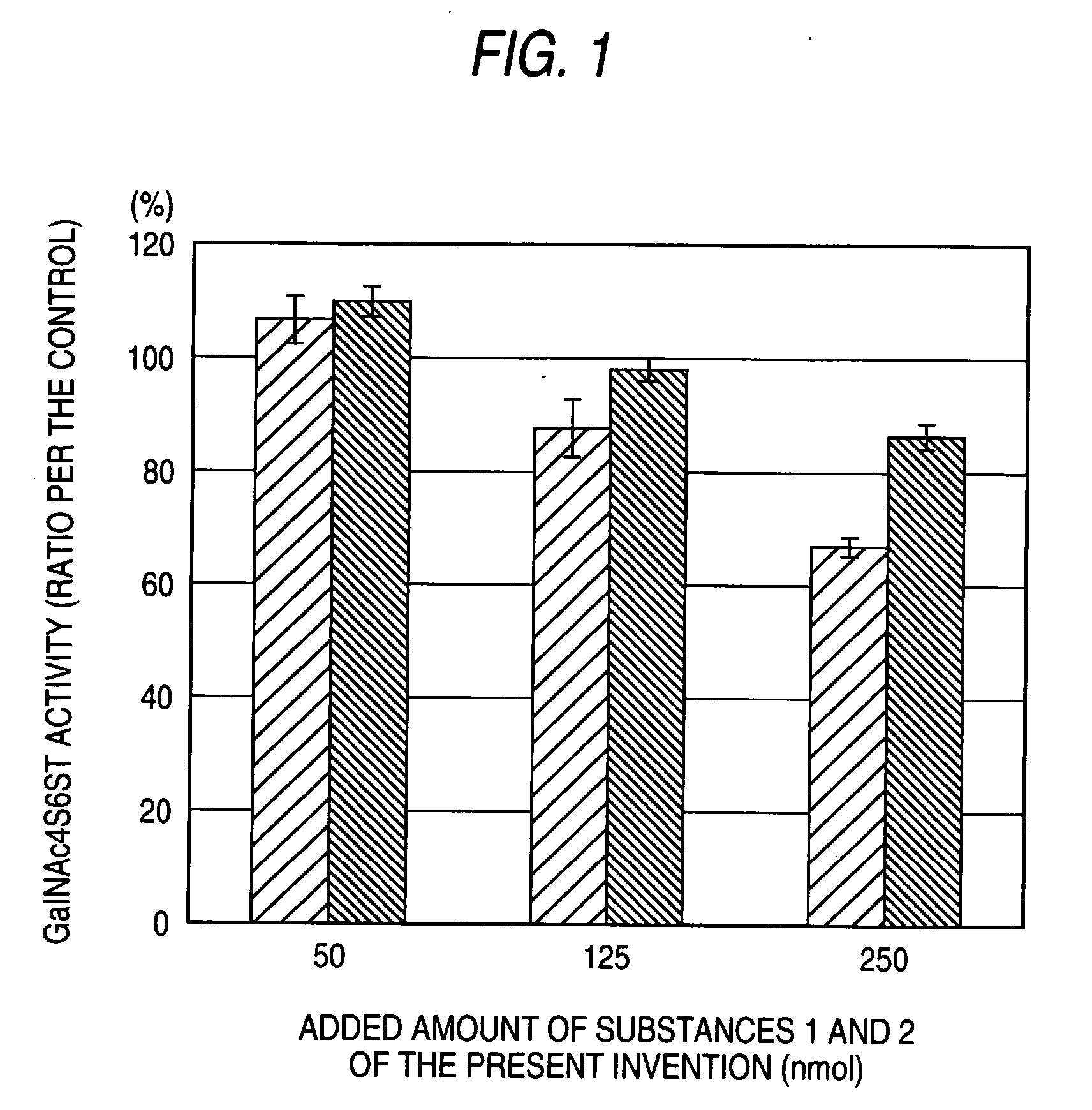
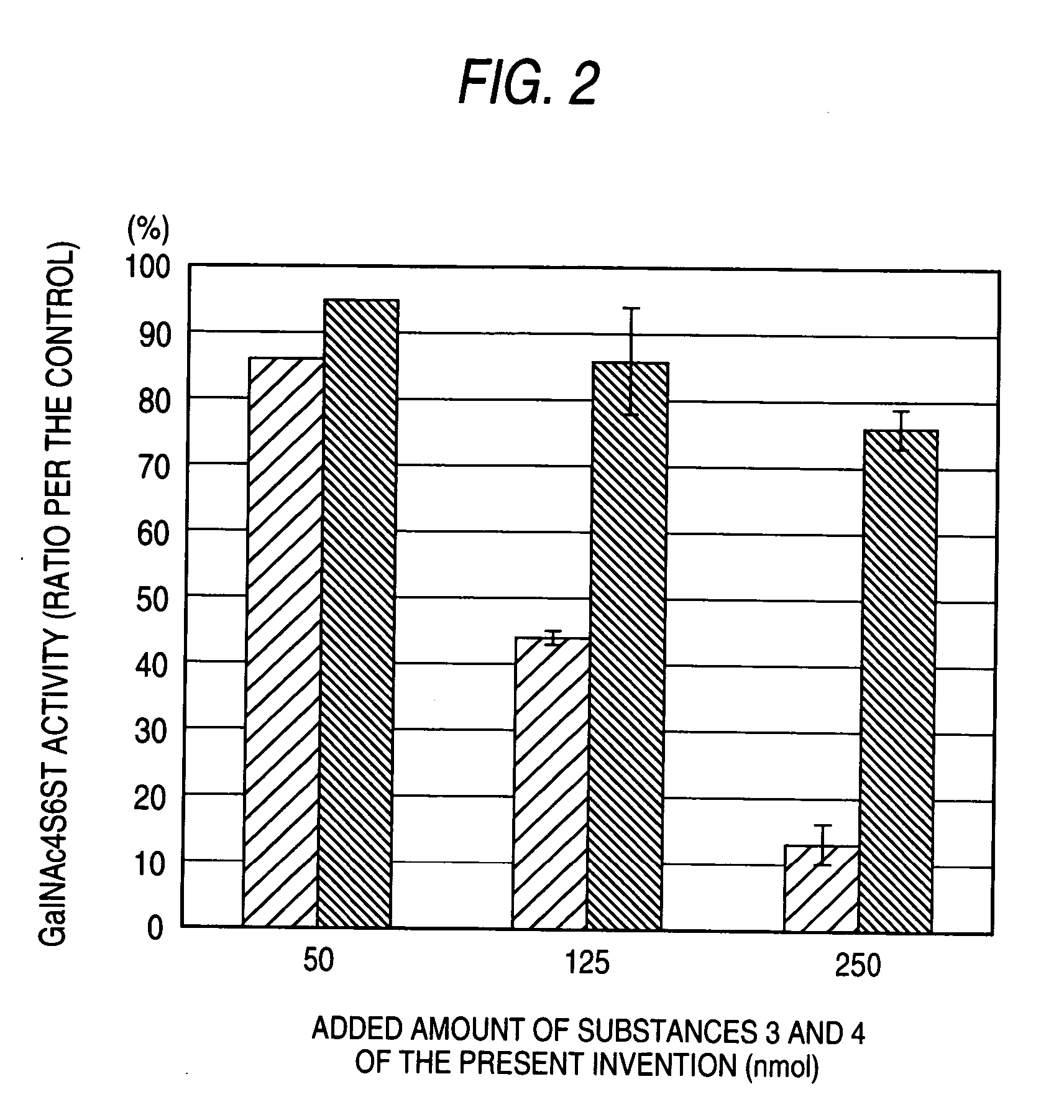
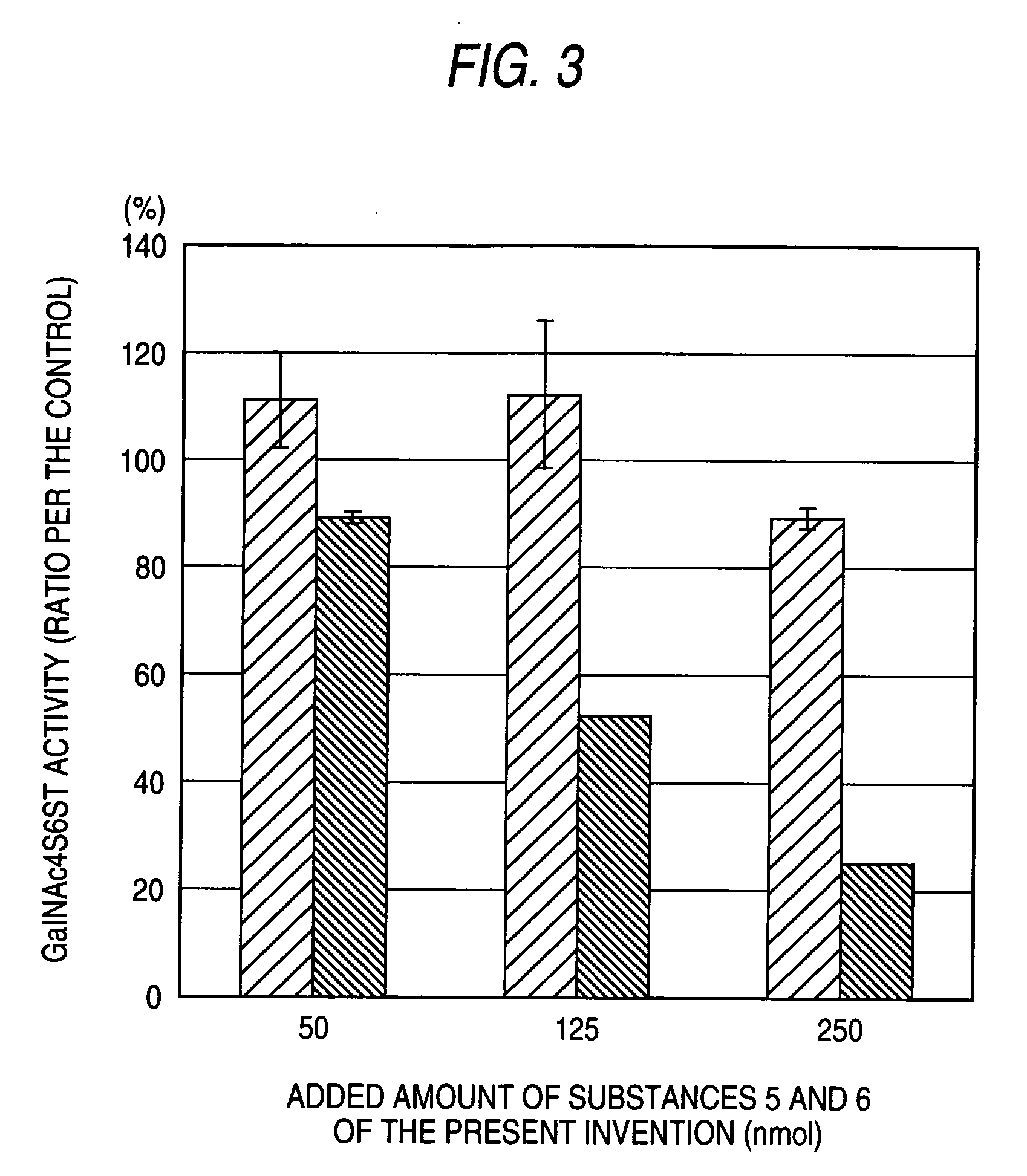
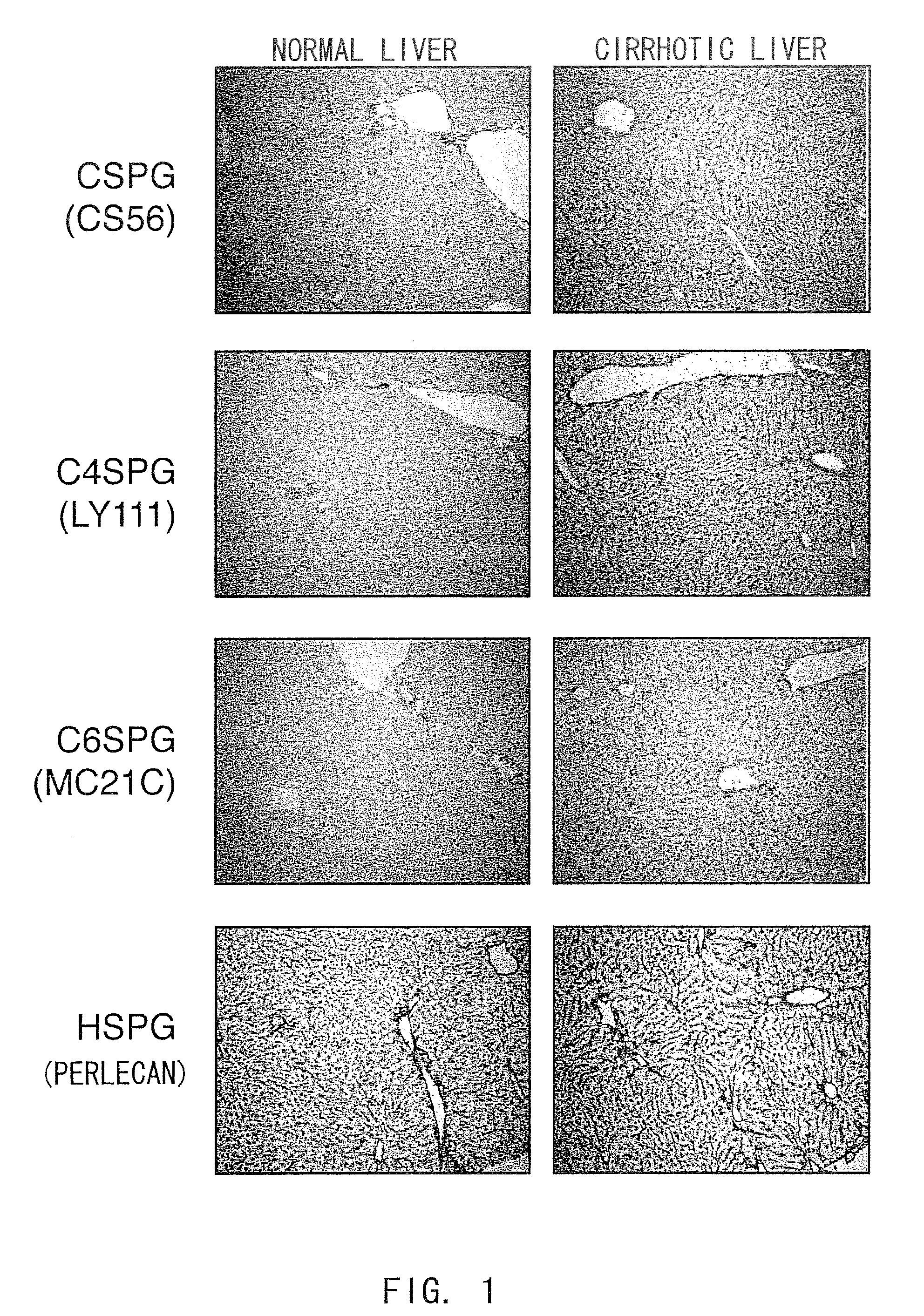
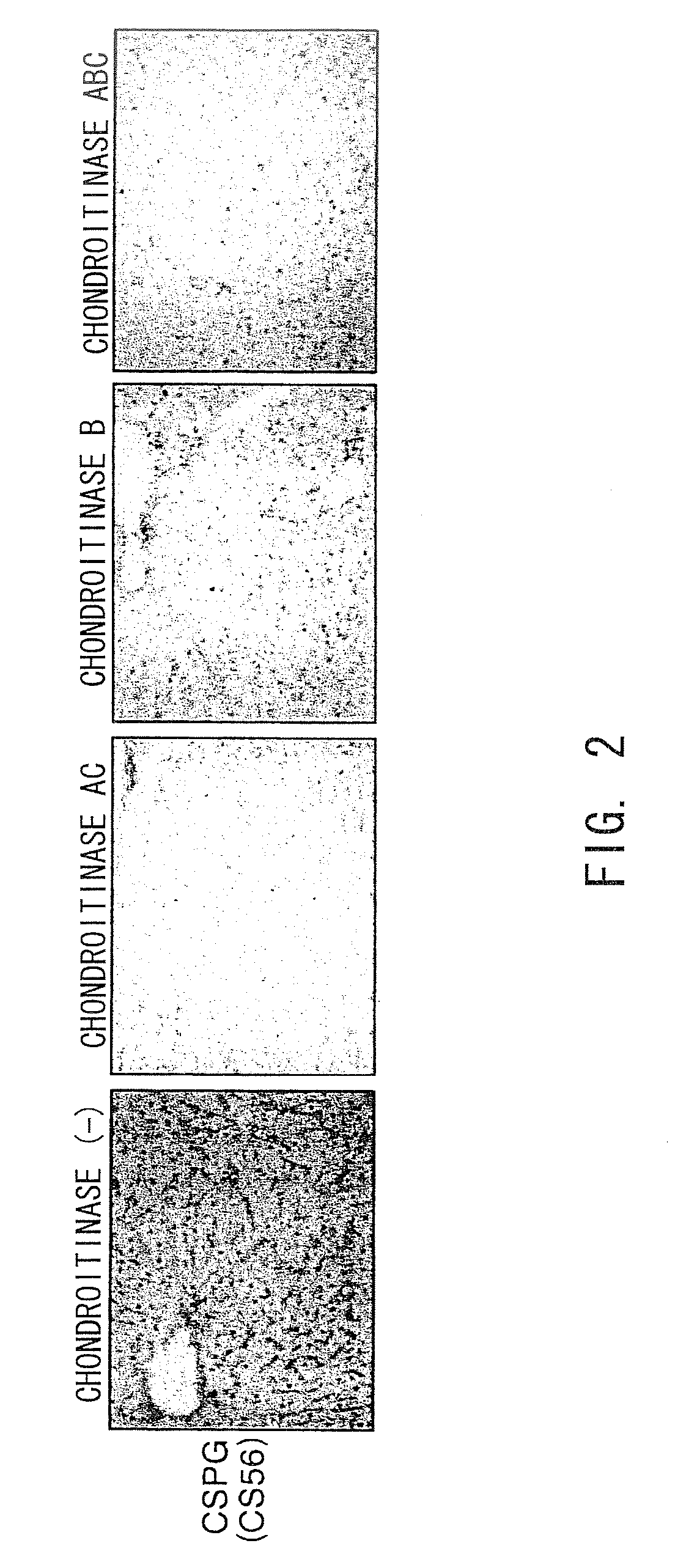
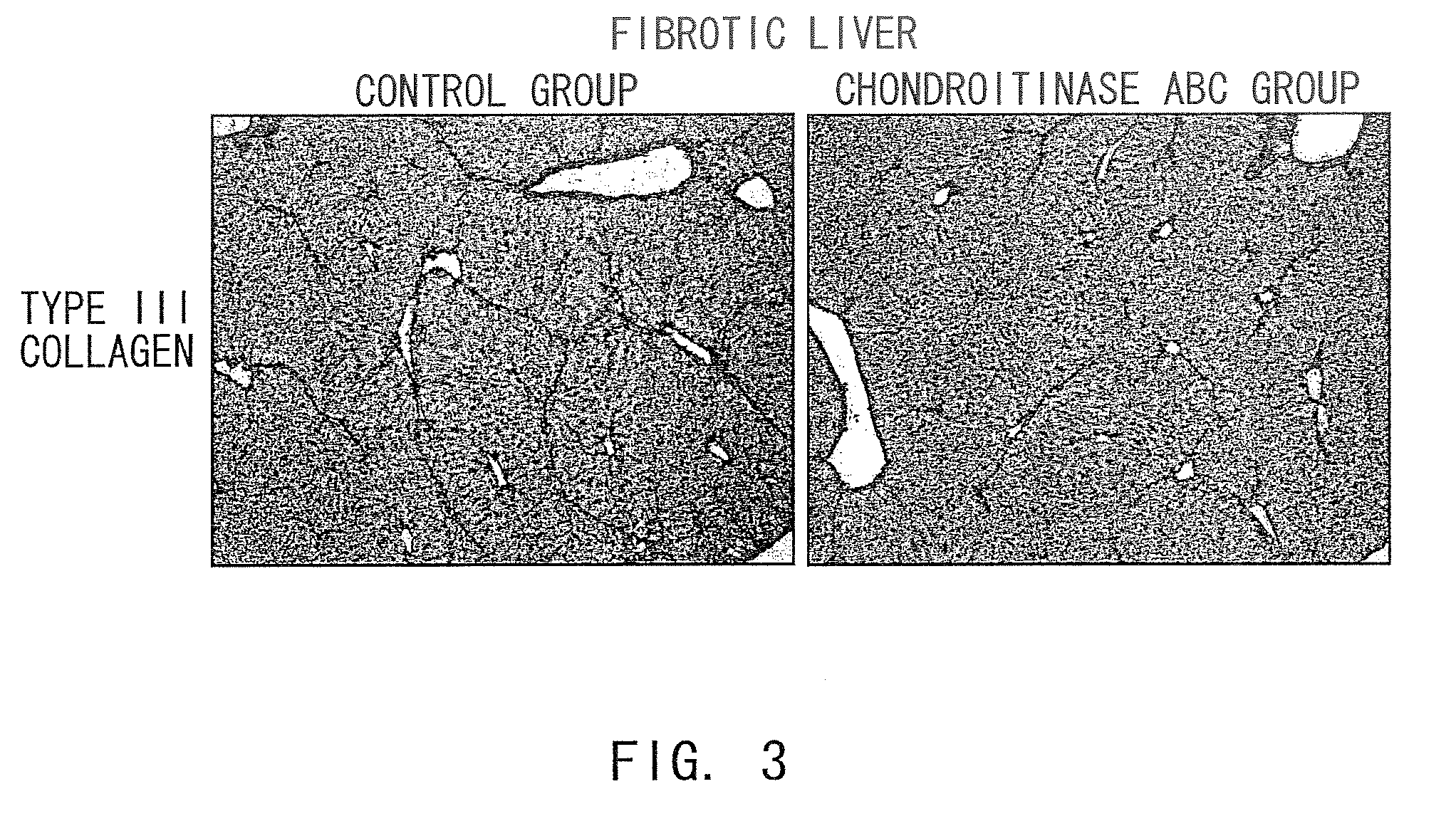
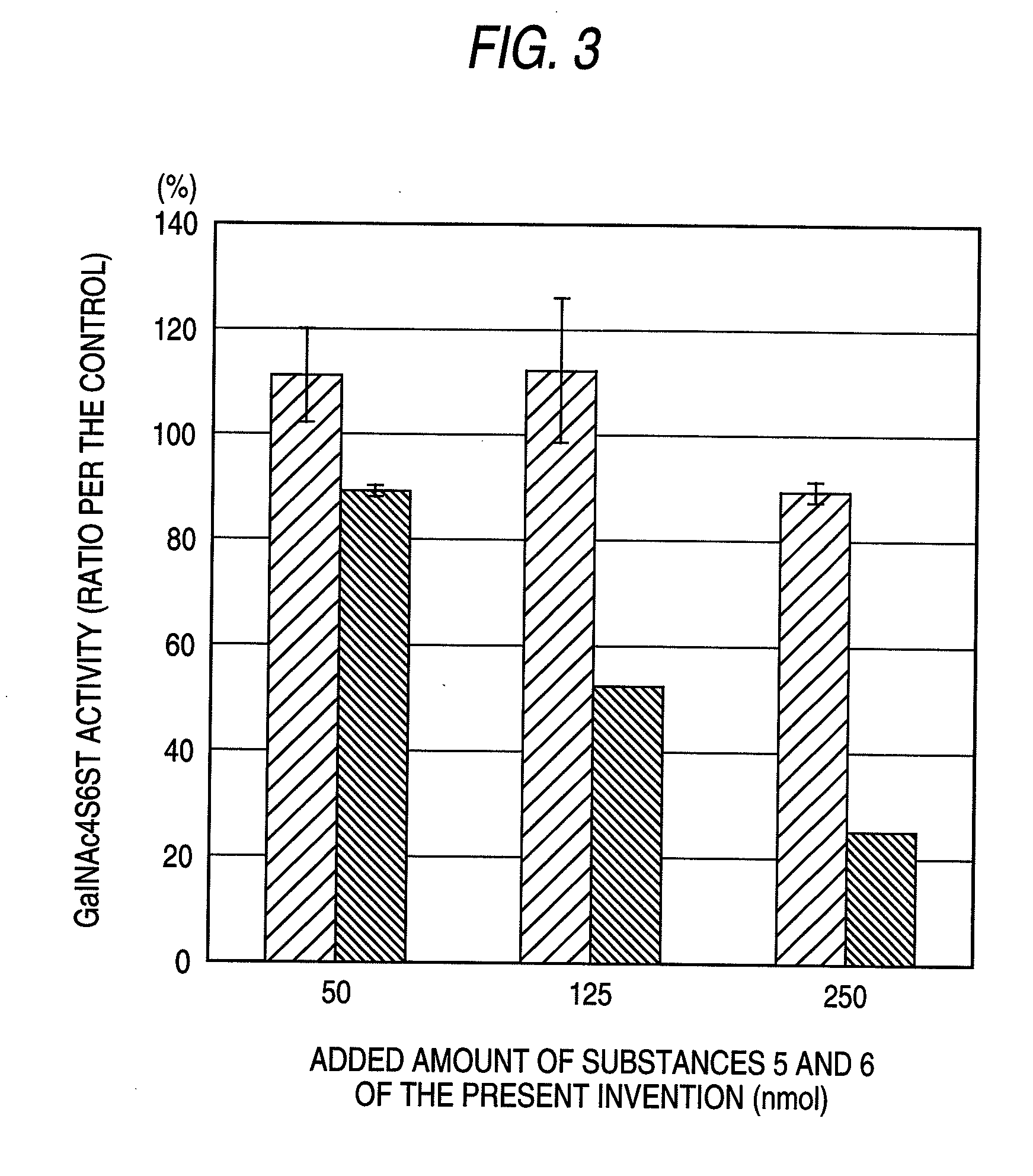
The present invention examined the accumulation of chondroitin sulfate proteoglycans (CSPGs). The present invention relates to neurodegeneration-suppressing agents that are suitable for gene therapy or prevention of neural fibrotic degenerative diseases which induce neural cell death due to an accumulation of abnormal proteins, where the therapies are based on siRNAs against N-acetylgalactosamine-4-O-sulfotransferases (N-acetylgalactosamine-4-O-sulfotransferase-1, N-acetylgalactosamine-4-O-sulfotransferase-2, and N-acetylgalactosamine-4-sulfate 6-O-sulfotransferase (GalNAc4ST-1, GalNAc4ST-2, and GALNAC4S-6ST, respectively)), which are sulfotransferases for acetylgalactosamine, a CSPG side chain, and chondroitinase ABC, an enzyme that degrades chondroitin sulfate, another CSPG side chain.
Owner:STELIC INST OF REGENERATIVE MEDICINE STELIC INST
Sulfotransferase inhibitors
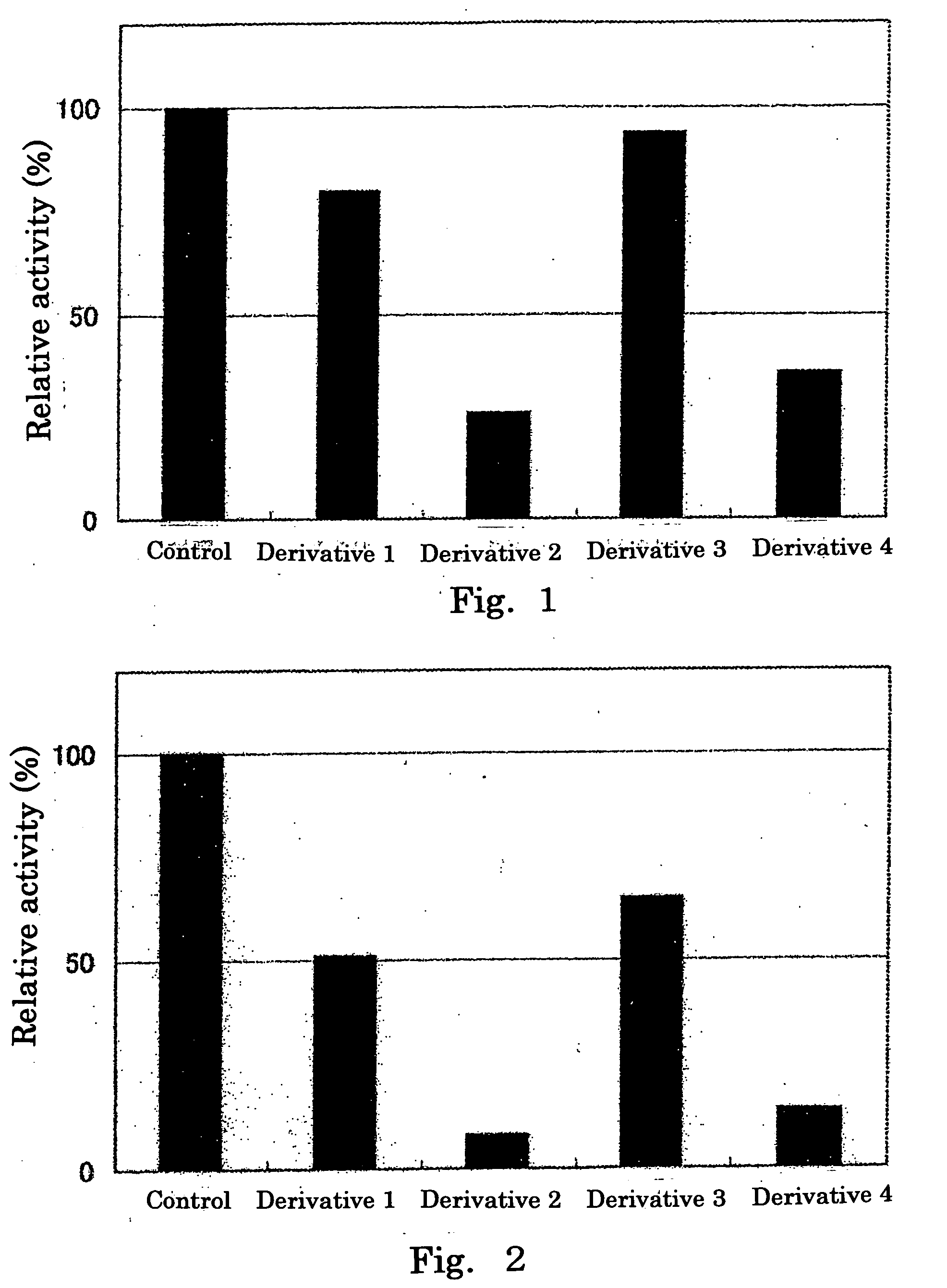
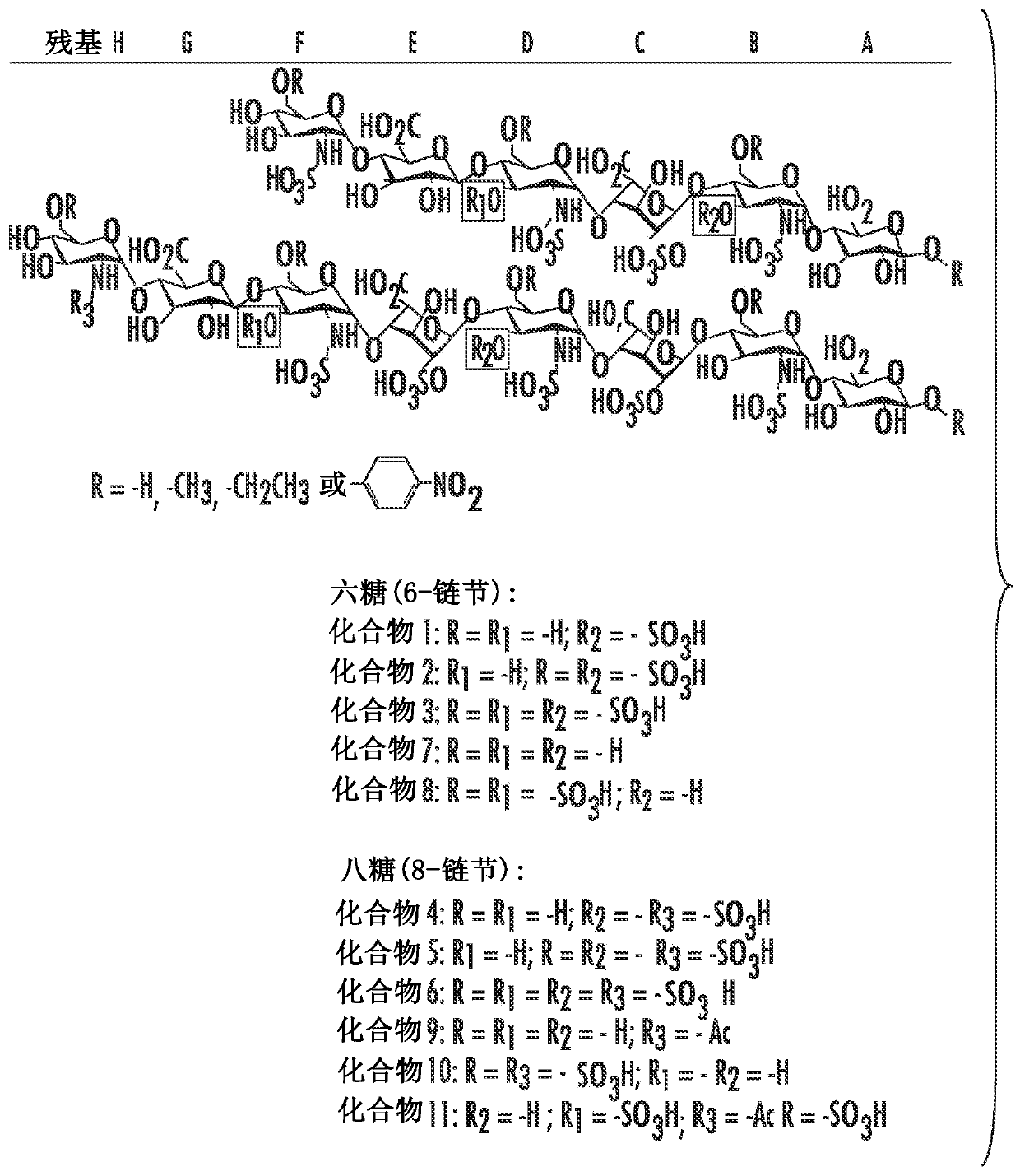
A galactosamine derivative represented by the following formula (1): wherein R1, R2 and R5 each independently represents SO3− or H, and at least one of them represents SO3−; R3 represents H, acetyl or SO3−; R4 represents H, a substituted or unsubstituted alkyl group, a substituted or unsubstituted alkenyl group, a substituted or unsubstituted alkynyl group, a substituted or unsubstituted acyl group, a substituted or unsubstituted aryl group, or a substituted or unsubstituted aralkyl group; X represents O, S, NH or CH2; and represents an α bond or a β bond, and a sulfotransferase inhibitor comprising the derivative.
Owner:SEIKAGAKU KOGYO CO LTD
Agents for suppressing hepatic fibrosis
InactiveUS20090060892A1Enhance liver tissue fibrosisEfficient degradationPeptide/protein ingredientsMetabolism disorderLiver tissueMedicine
The present invention relates to hepatic fibrosis-suppressing agents that are suitable for treating or preventing fibrotic liver diseases such as cirrhosis, which comprise as an active ingredient a substance that inhibits the production or accumulation of chondroitin sulfate proteoglycans including chondroitinase ABC and ADAMTS-4; and methods of screening for the agents.The present inventors discovered for the first time that hepatic fibrosis could be efficiently suppressed by suppressing the production or accumulation of chondroitin sulfate proteoglycans. Specifically, fibrosis of liver tissues can be suppressed by administering chondroitinase ABC, a chondroitin sulfate proteoglycan-degrading enzyme, or by using siRNA to suppress the expression of C4ST-1, C6ST-1, or C6ST-2, a sulfotransferase for chondroitin sulfate proteoglycans. Compounds such as nucleic acids that are used as siRNA can be used as effective agents for suppressing hepatic fibrosis. Furthermore, hepatic fibrosis-suppressing agents can be found by screening for compounds that suppress the production or accumulation of chondroitin sulfate proteoglycans.
Owner:STELIC INST OF REGENERATIVE MEDICINE
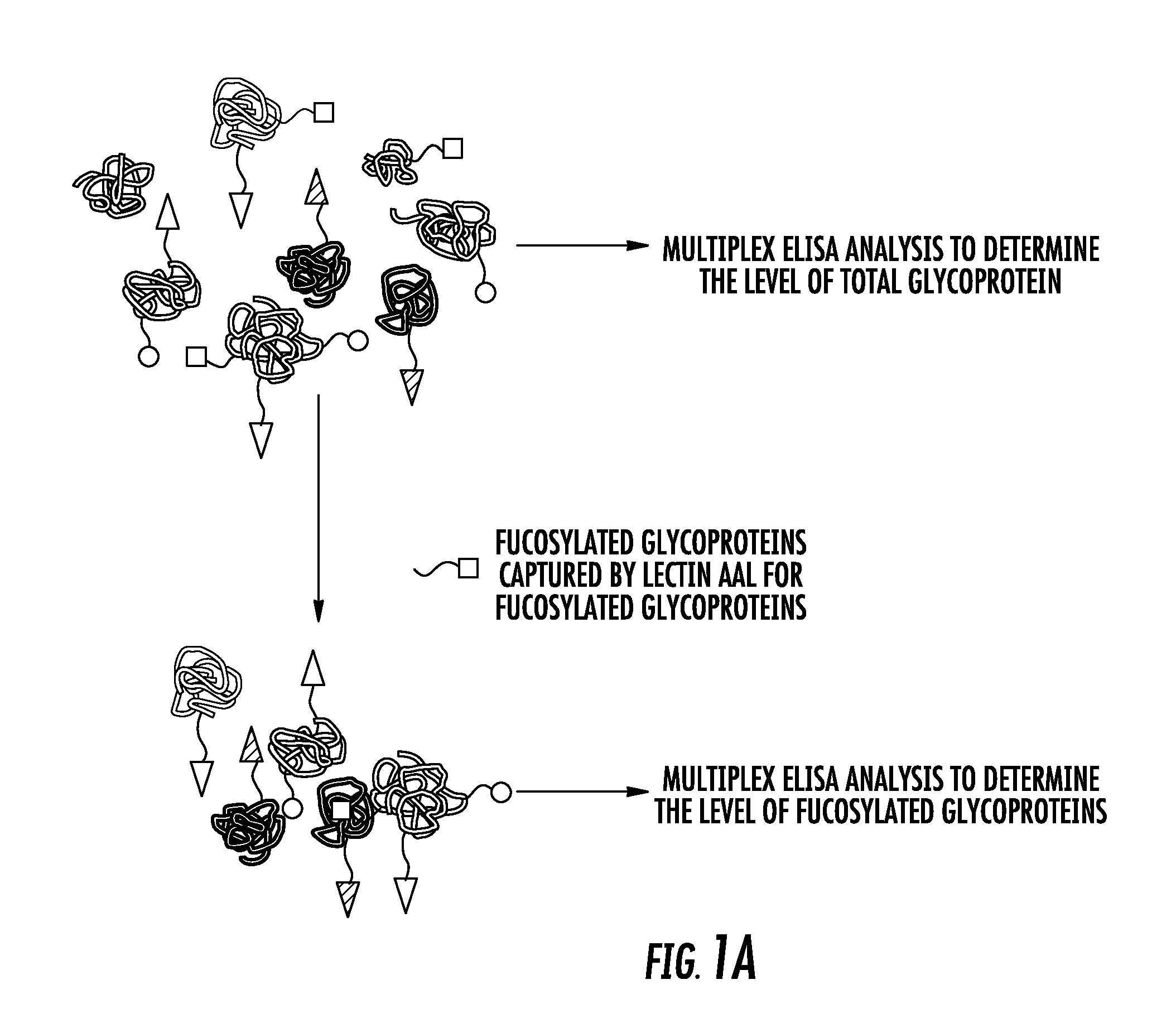
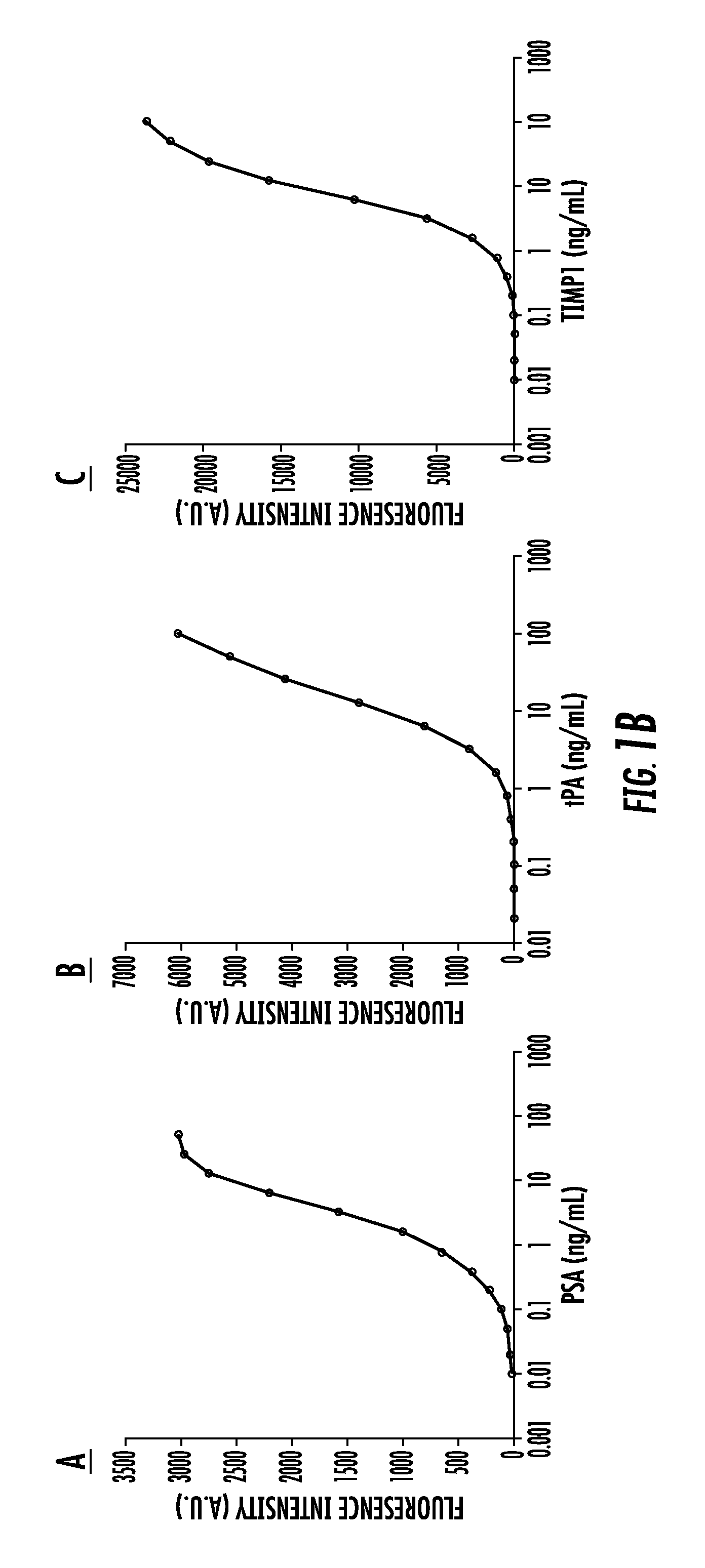
Biomarkers for distinguishing between aggressive prostate cancer and non-aggressive prostate cancer
The present invention relates to the field of biomarkers. More specifically, the present invention relates to biomarkers useful in diagnosing aggressive prostate cancer. In one embodiment, a method for identifying patients as having or likely to have aggressive prostate cancer comprises the steps of (a) performing an assay on a biological sample obtained from the patient to detect fucosylated prostate specific antigen (PSA), transmembrane prostate androgen-induced protein, kallikrein-2, lipid phosphate phosphohydrolase 3, heparan-sulfate 6-O-sulfotransferase, prostatic acid phosphatase (PAP), nuclear RNA export factor 2, and protein POF1B; and (b) identifying the patient as having or likely to have aggressive prostate cancer if there is a statistically significant difference in the levels of fucosylated PSA, transmembrane prostate androgen-induced protein, kallikrein-2, lipid phosphate phosphohydrolase 3, heparan-sulfate 6-O-sulfotransferase, PAP, nuclear RNA export factor 2, and protein POF1B as compared to corresponding levels in a control sample that correlates to non-aggressive prostate cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods of inhibition using glycosyl sulfotransferase-3
A novel human glycosylsulfotransferase expressed in high endothelial cells (GST-3) and polypeptides related thereto, as well as nucleic acid compositions encoding the same, are provided. The subject polypeptides and nucleic acid find use in a variety of applications, including research, diagnostic, and therapeutic agent screening applications. Also provided are methods of inhibiting selectin mediated binding events and methods of treating disease conditions associated therewith, particularly by administering an inhibitor of at least one of GST-3 or KSGal6ST, or homologues thereof
Owner:SYNTEX (USA) INC +1
In vivo imaging of sulfotransferases
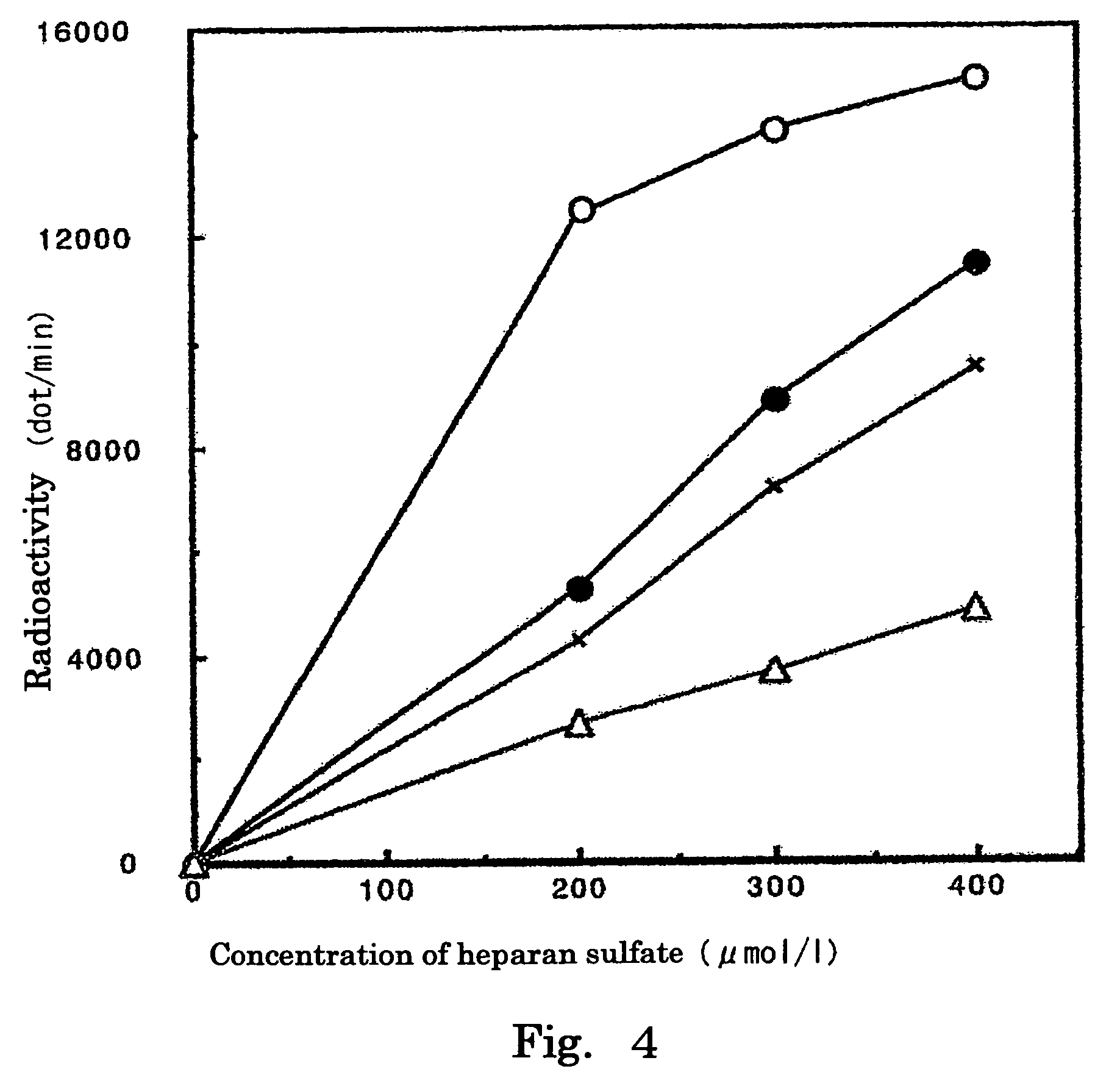
Radiolabeled tracers for sulfotransferases (SULTs), their synthesis, and their use are provided. Included are substituted phenols, naphthols, coumarins, and flavones radiolabeled with 18F, 123I, 124I, 125I, or 11C. Also provided are in vivo techniques for using these and other tracers as analytical and diagnostic tools to study sulfotransferase distribution and activity, in health and disease, and to evaluate therapeutic interventions.
Owner:RGT UNIV OF CALIFORNIA
Recombinant bacterium for producing sulfo-recombinant hirudin as well as preparation method and application of recombinant bacterium
ActiveCN113699086ARealize the sulfolation reactionHigh activityBacteriaAntibody mimetics/scaffoldsEscherichia coliEnzyme Gene
The invention discloses a recombinant bacterium for producing sulfo-recombinant hirudin as well as a preparation method and application of the recombinant bacterium, and belongs to the technical field of genetic engineering. In order to improve the synthesis yield of the sulfated recombinant hirudin and reduce the production cost, the invention provides the recombinant bacterium for producing the sulfo-recombinant hirudin, wherein the recombinant bacterium overexpresses a sulfotransferase gene and a hirudin gene, and an original strain is escherichia coli; and the nucleotide sequence of the hirudin gene is one of SEQ ID No.1 to SEQ ID No.3, and the nucleotide sequence of the sulfotransferase gene is as shown in SEQ ID No.4. According to the method, the activity of the recombinant hirudin is remarkably improved, a one-step purification method of the hirudin is realized, a recombinant hirudin product with relatively high purity can be obtained at one time, and the pressure of subsequent purification is greatly reduced.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Method for detecting gynecologic cancer
InactiveUS20110306049A1Increase chanceImprove the detection rateMicrobiological testing/measurementBiological material analysisGynecologyGynecologic cancer
The object of the present invention is to provide a method for detecting gynecologic cancer and a kit for detecting the same.The object can be solved by a method for detecting gynecologic cancer characterized in that β1,3-galactosyltransferase-5 and / or β1,3-galactosyltransferase-4, and GlcNAc 6-O-sulfotransferase-2 are analyzed. According to the detecting method, ovarian cancer and endometrial cancer patients can be detected at an early stage i.e. stages I and II, wherein patients have no subjective symptoms.
Owner:LSIP
Method of examining colon cancer and colon adenoma
InactiveUS20070196874A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsNon cancerIsozyme
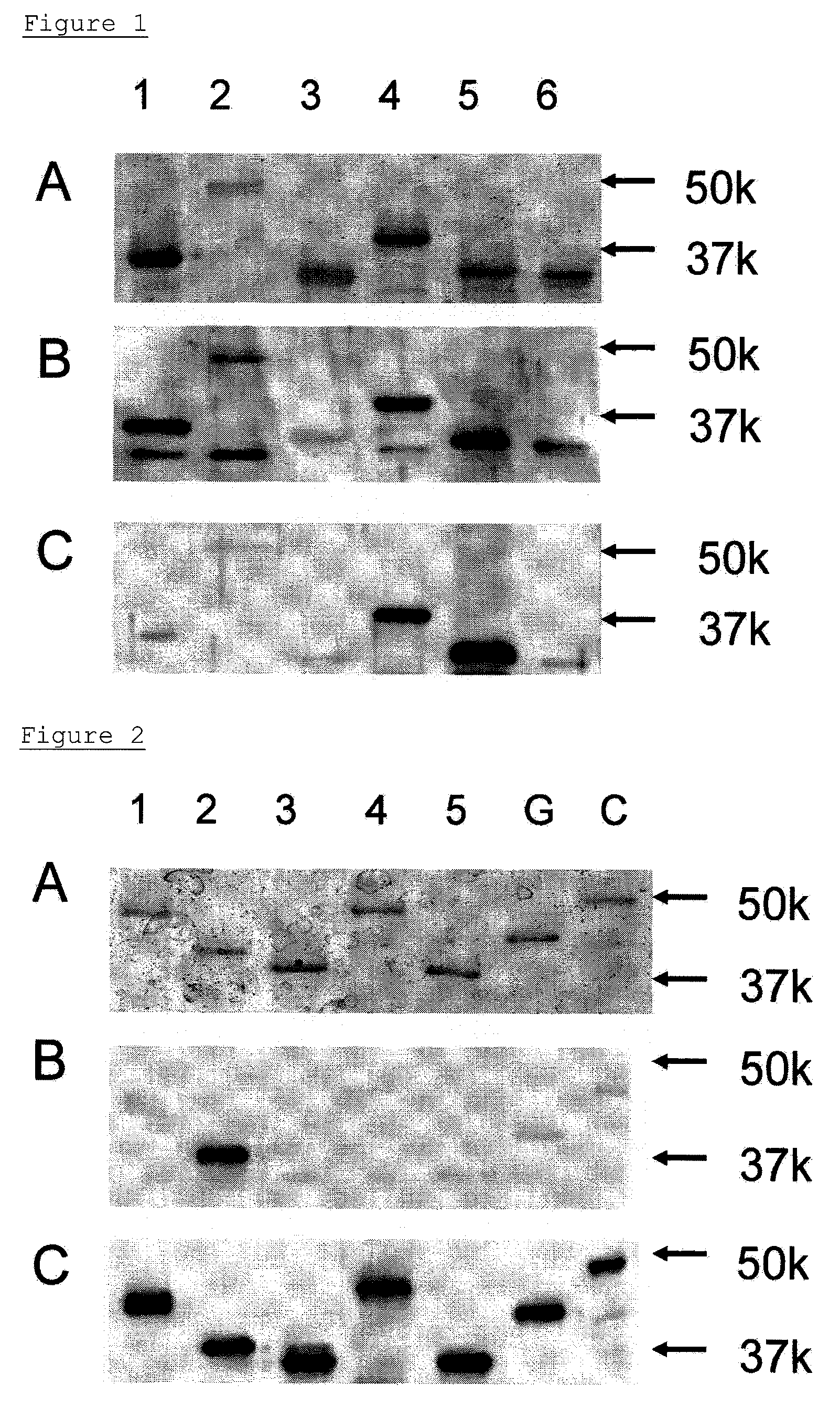
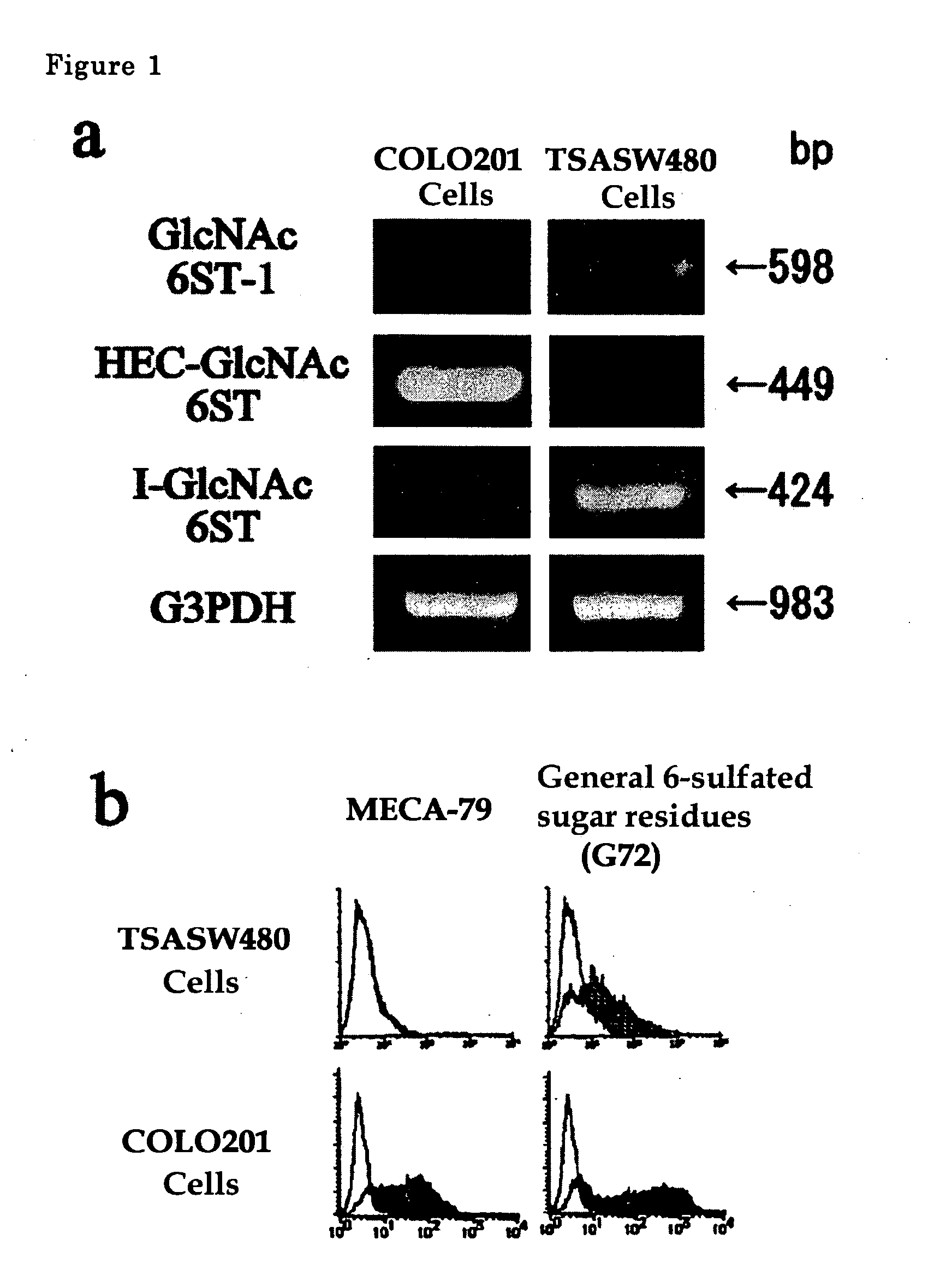
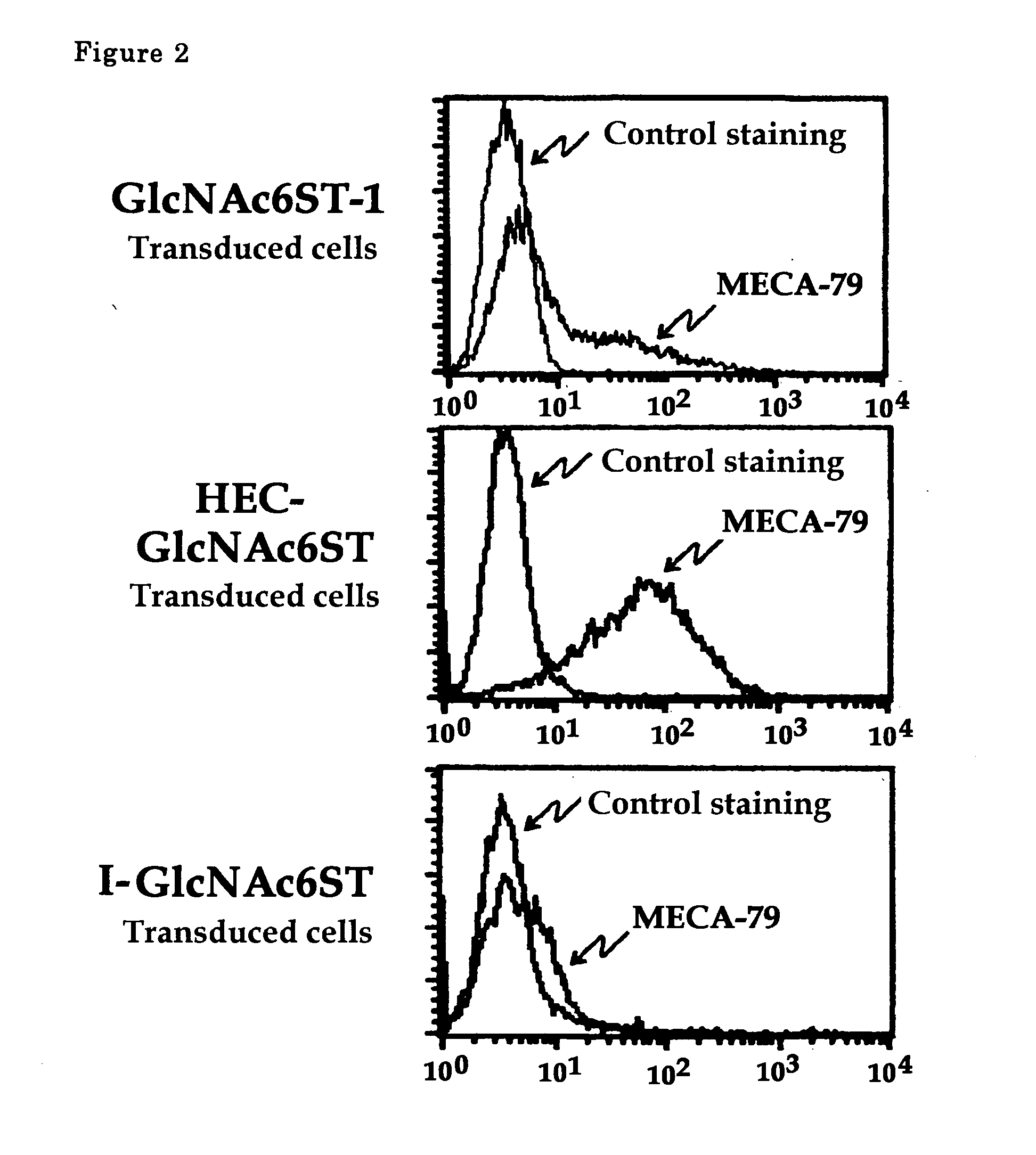
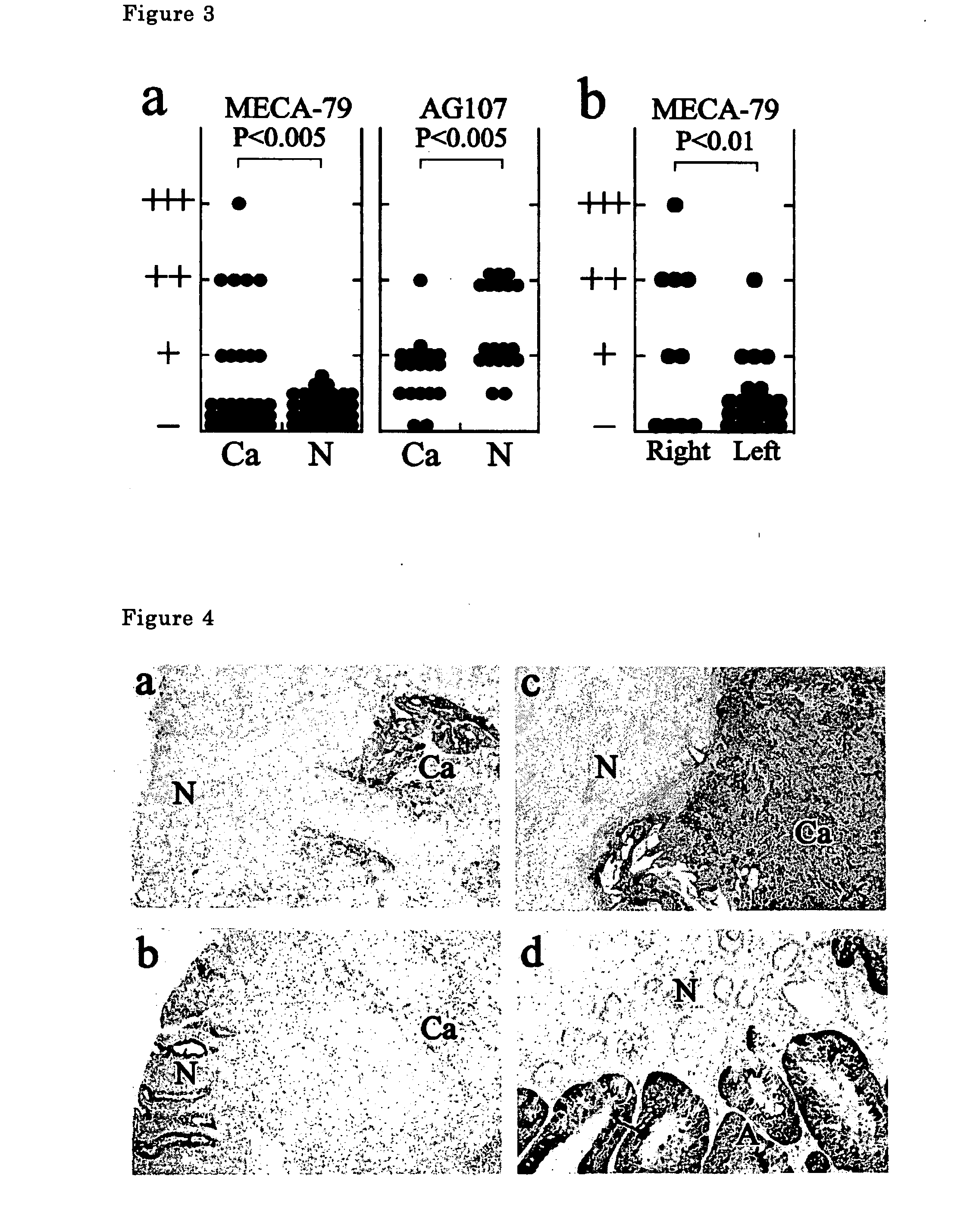
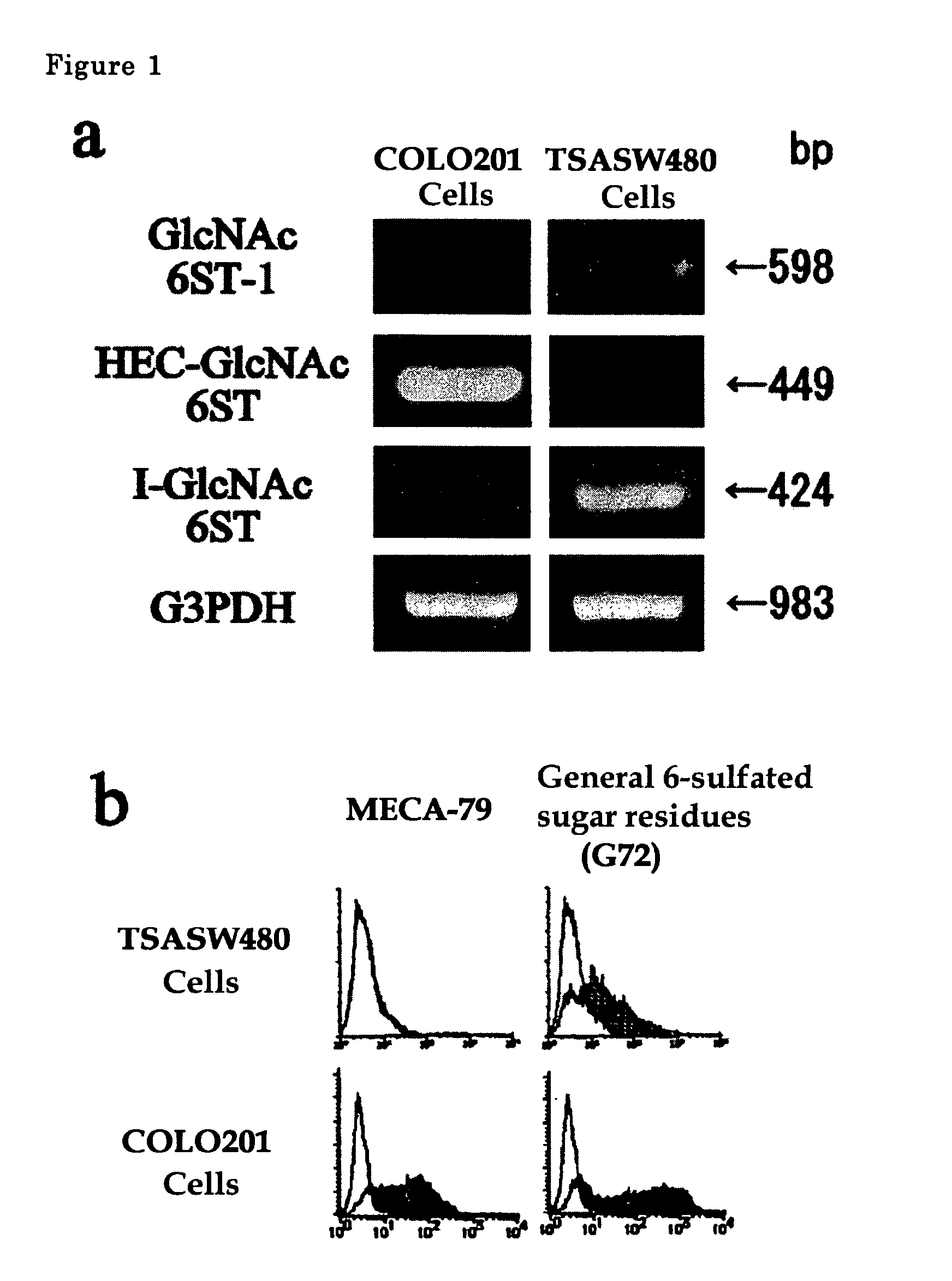
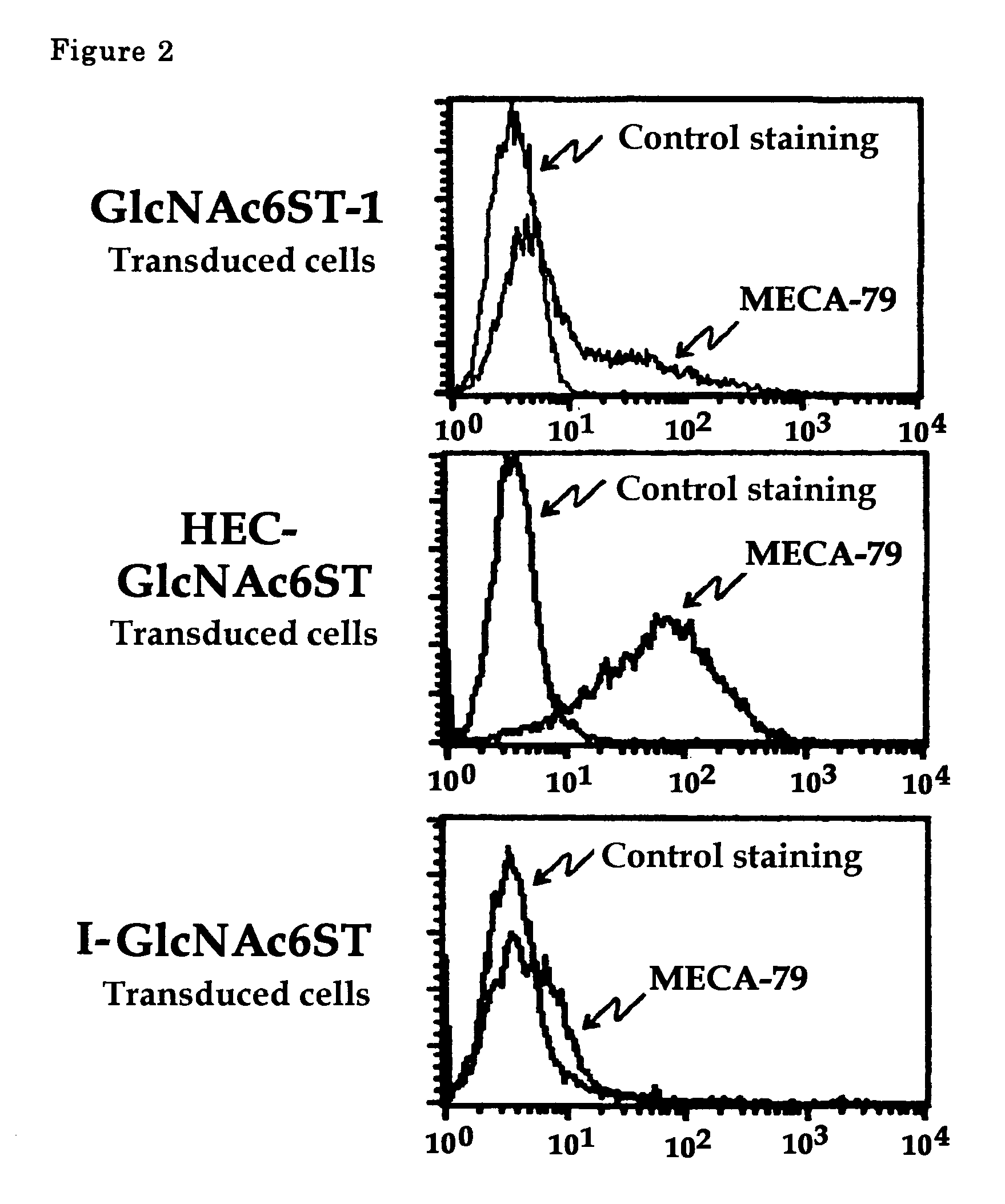
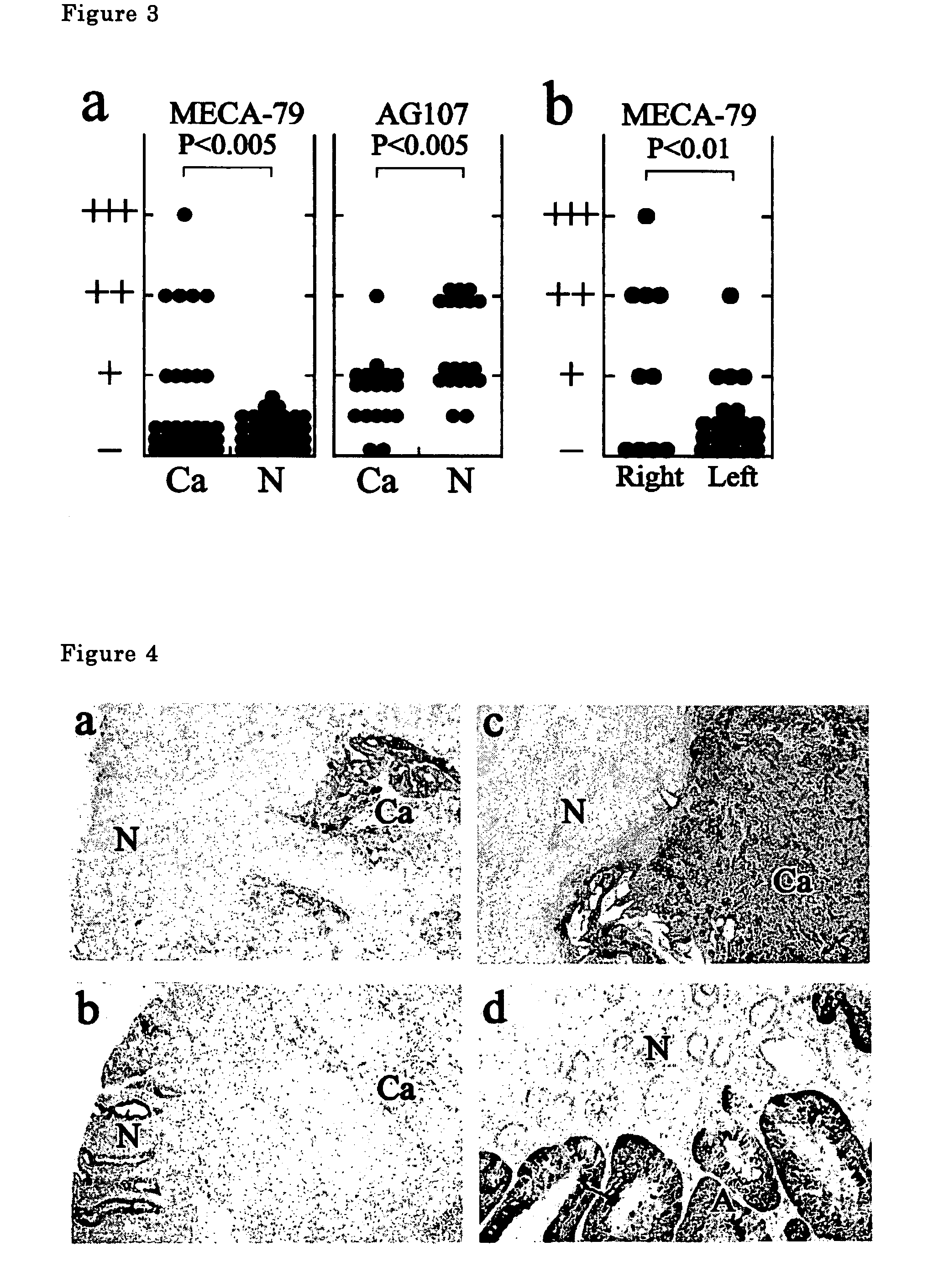
The present invention provides a method for examining colorectal cancer and colorectal adenoma, which enables to detect colorectal cancer patients and patients at high risk of colorectal cancer at a high probability and is useful for diagnosis of colorectal cancer and colorectal adenoma, and provides the examination reagents thereof. The present inventors discovered that there are significant differences in the distribution of GlcNAc-6-sulfotransferase isozymes, sulfation enzymes of sugar residues, among non-cancer colorectal tissues, colorectal cancer tissues and colorectal adenoma tissues. Furthermore the inventors applied the discovery to diagnosis and found that colorectal cancers and adenomas are detected specifically by assaying a definite range of GlcNAc-6-sulfated sugar residues in tissues from patients or feces samples. MECA-79 antibody (Pharmingen, catalog No. 09961D, Distributor: Becton Dickinson), reacting with GlcNAc-6-sulfated sugar residues, which are produced specifically by the enzyme present in colorectal cancer and colorectal adenoma tissues could be used for the examination of colorectal cancers and colorectal adenomas.
Owner:JAPAN SCI & TECH CORP +2
Method of examining colon cancer and colon adenoma
The present invention provides a method for examining colorectal cancer and colorectal adenoma, which enables to detect colorectal cancer patients and patients at high risk of colorectal cancer at a high probability and is useful for diagnosis of colorectal cancer and colorectal adenoma, and provides the examination reagents thereof. The present inventors discovered that there are significant differences in the distribution of GlcNAc-6-sulfotransferase isozymes, sulfation enzymes of sugar residues, among non-cancer colorectal tissues, colorectal cancer tissues and colorectal adenoma tissues. Furthermore the inventors applied the discovery to diagnosis and found that colorectal cancers and adenomas are detected specifically by assaying a definite range of GlcNAc-6-sulfated sugar residues in tissues from patients or feces samples. MECA-79 antibody (Pharmingen, catalog No. 09961D, Distributor: Becton Dickinson), reacting with GlcNAc-6-sulfated sugar residues, which are produced specifically by the enzyme present in colorectal cancer and colorectal adenoma tissues could be used for the examination of colorectal cancers and colorectal adenomas.
Owner:JAPAN SCI & TECH CORP +2
Sulfotransferase inhibitor
ActiveUS20070066564A1Highly specific inhibitory activitySafety managementBiocideSugar derivativesGlycosideHeparan sulfate sulfotransferase
The hexuronic acid derivative represented by the following formula 1 or a salt thereof is used as an active ingredient of a heparin / heparan sulfate sulfotransferase inhibitor. In the formula, each of R1, R2, and R3 independently represent(s) SO3− or H which may have a substituent, provided that at least one thereof represents SO3−; X represents OR4, SR4, N(R4)2, or C(R4)3, R4 independently represents H, alkyl, alkenyl, alkynyl, acyl, aryl, or aralkyl group; one of R5 and R6 represents COOH while the other represents H; and the wavy line represents α-glycosidic bond or β-glycosidic bond.
Owner:SEIKAGAKU KOGYO CO LTD
Biomarker and composition for diagnosis of preeclampsia and method for using the same
InactiveUS20110269136A1Microbiological testing/measurementSulfateHeparan sulfate 6-O-sulfotransferase
The present invention relates to a biomarker and a composition for diagnosis of preeclampsia. In accordance with one aspect of the present invention, there is provided a biomarker for diagnosis of preeclampsia using an enzyme selected from the group consisting of placental chondroitin 4-O-sulfotransferase 1 (C4ST), chondroitin 6-sulfotransferase (C6S), heparan sulfate 6-O-sulfotransferase 1 (HS6S), and dermatan / chondroitin sulfate 2-sulfotransferase (CS-2OST), or uronic acid-2-sulfate (UA2S).
Owner:INJE UNIV IND ACADEMIC COOP FOUND
Sulfotransferase inhibitor
ActiveUS7396818B2Excellent heparin/heparan sulfate sulfotransferase inhibitory activityBiocideSugar derivativesArylTransferase inhibitor
Owner:SEIKAGAKU KOGYO CO LTD
Methods of treating macular corneal dystrophy
InactiveUS20020061562A1Increase expression and activityIncrease transcriptionSugar derivativesPeptide/protein ingredientsMacular corneal dystrophy Type IMacular corneal dystrophy
The invention provides an isolated polypeptide encoding a corneal N-acetylglucosamine-6-sulfotransferase (GlcNAc6ST) or active fragment thereof, where the GlcNAc6ST or active fragment thereof catalyzes sulfation of keratan sulfate. The present invention also provides a method of treating a subject with macular corneal dystrophy. The method includes the steps of administering to the subject an effective amount of an agent that increases expression or activity of a GlcNAc6ST, whereby the amount of sulfated keratan sulfate in the cornea of the subject is elevated. A method of the invention can be used to treat macular corneal dystrophy type I or type II.
Owner:BURNHAM INST THE
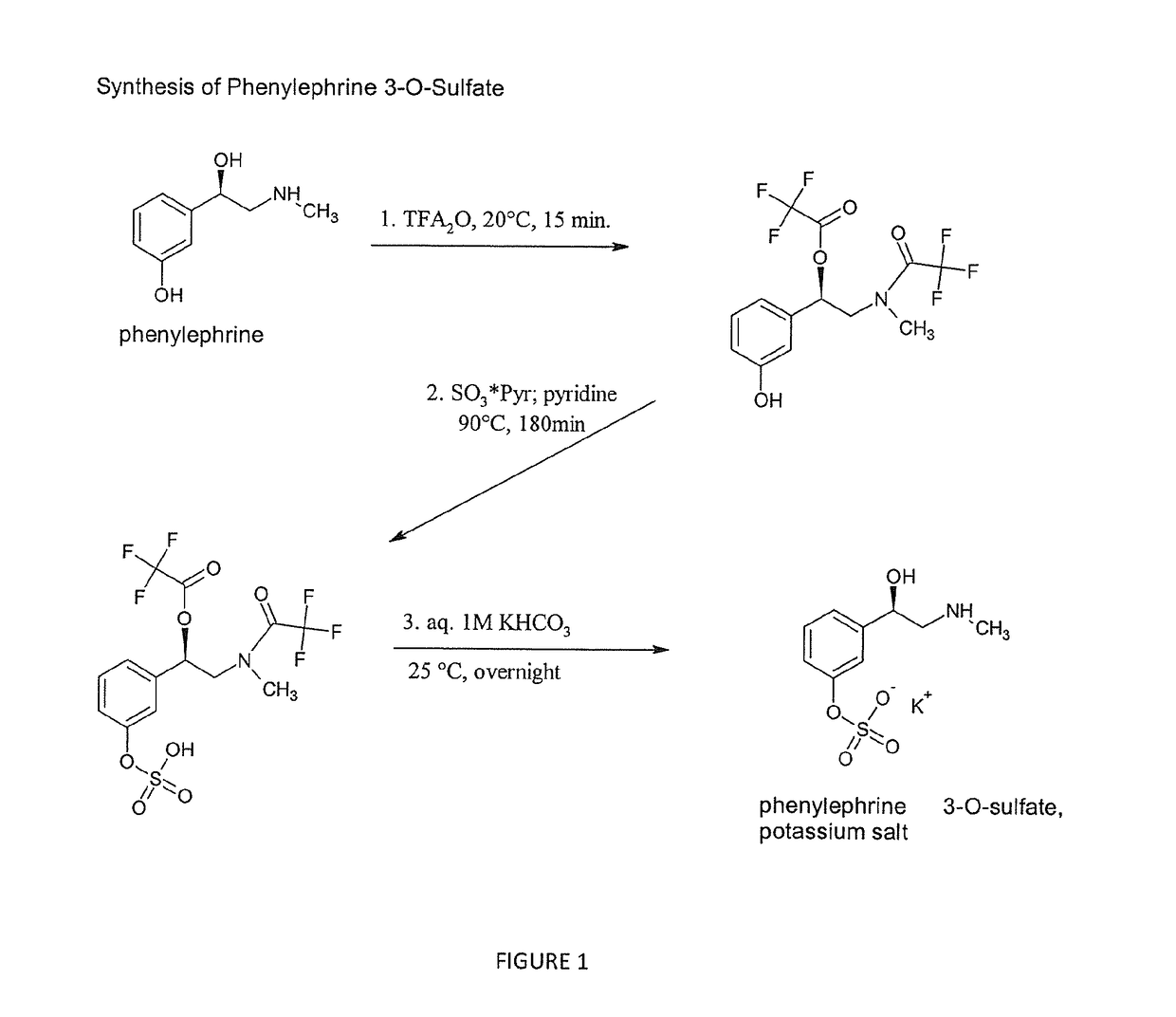
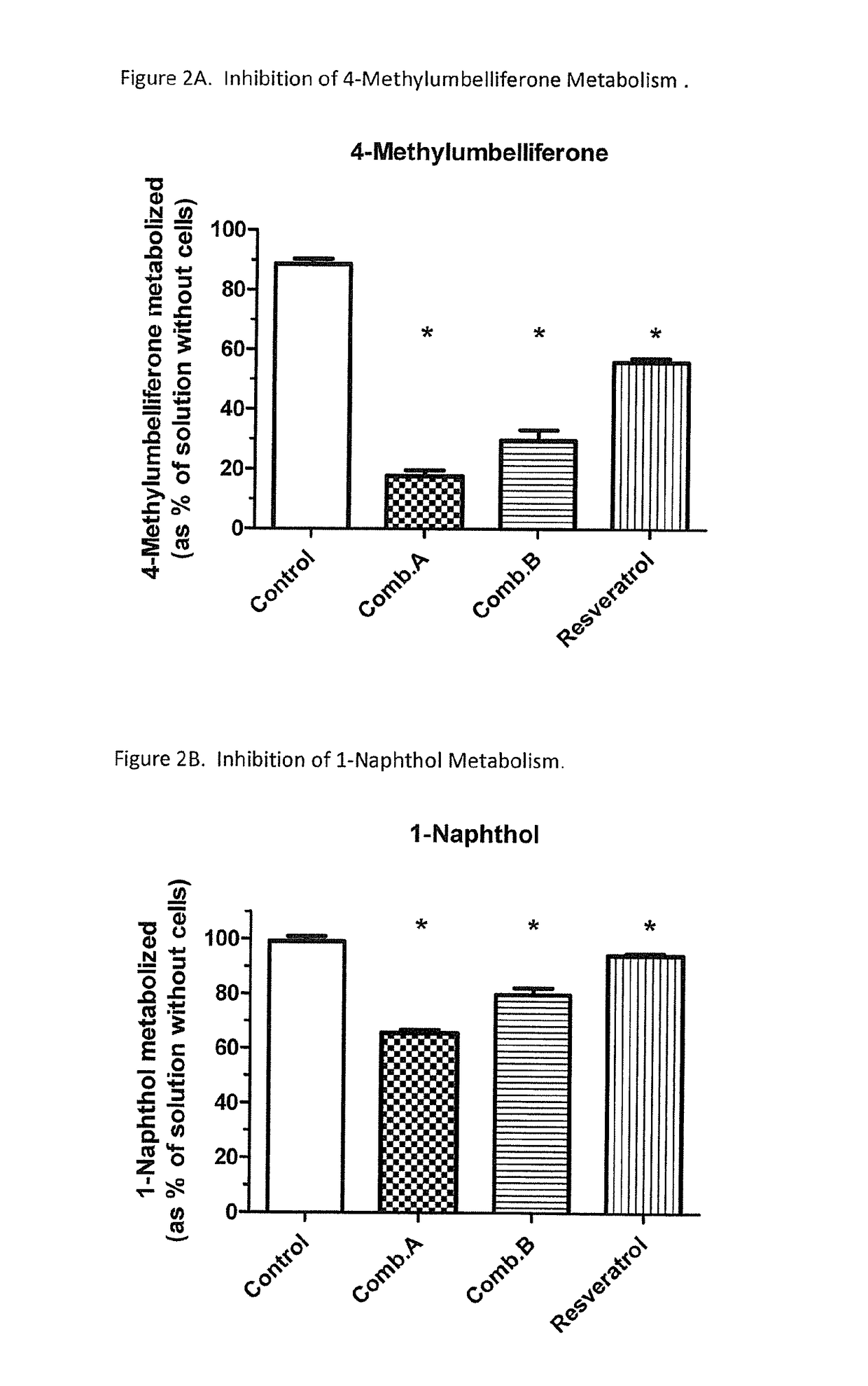
Selective metabolic approach to increasing oral bioavailability of phenylephrine and other phenolic bioactivities
ActiveUS9616033B2Promote absorptionReduced bioavailabilityHydroxy compound active ingredientsMetabolism disorderIntestinal structureUridine diphosphate
Presystemic metabolism in intestine of bioactives such as phenylephrine is avoided by administering a subject (human or animal) the bioactive (e.g., phenylephrine) in combination with one or more inhibitors of sulfation (e.g., sulfotransferase enzymes aka SULTs). This can also be enhanced be co-administering inhibitors of monoamine oxidases aka, MAOs, and uridine diphosphate glucoronysl transferases, aka UGTs. Preferably the inhibitors are GRAS compounds. The one or more inhibitor compounds inhibit the enzymes responsible for rapid presystemic metabolism, thus allowing the bioactives (e.g., phenylephrine) to be more readily absorbed intact into the circulatory system.
Owner:VIRGINIA COMMONWEALTH UNIV
Recombinant strains expressing chondroitin 4-sulfotransferase gene and application of recombinant strain
ActiveCN109897812AIncrease valueBacteriaMicroorganism based processesEscherichia coliBacillus subtilis
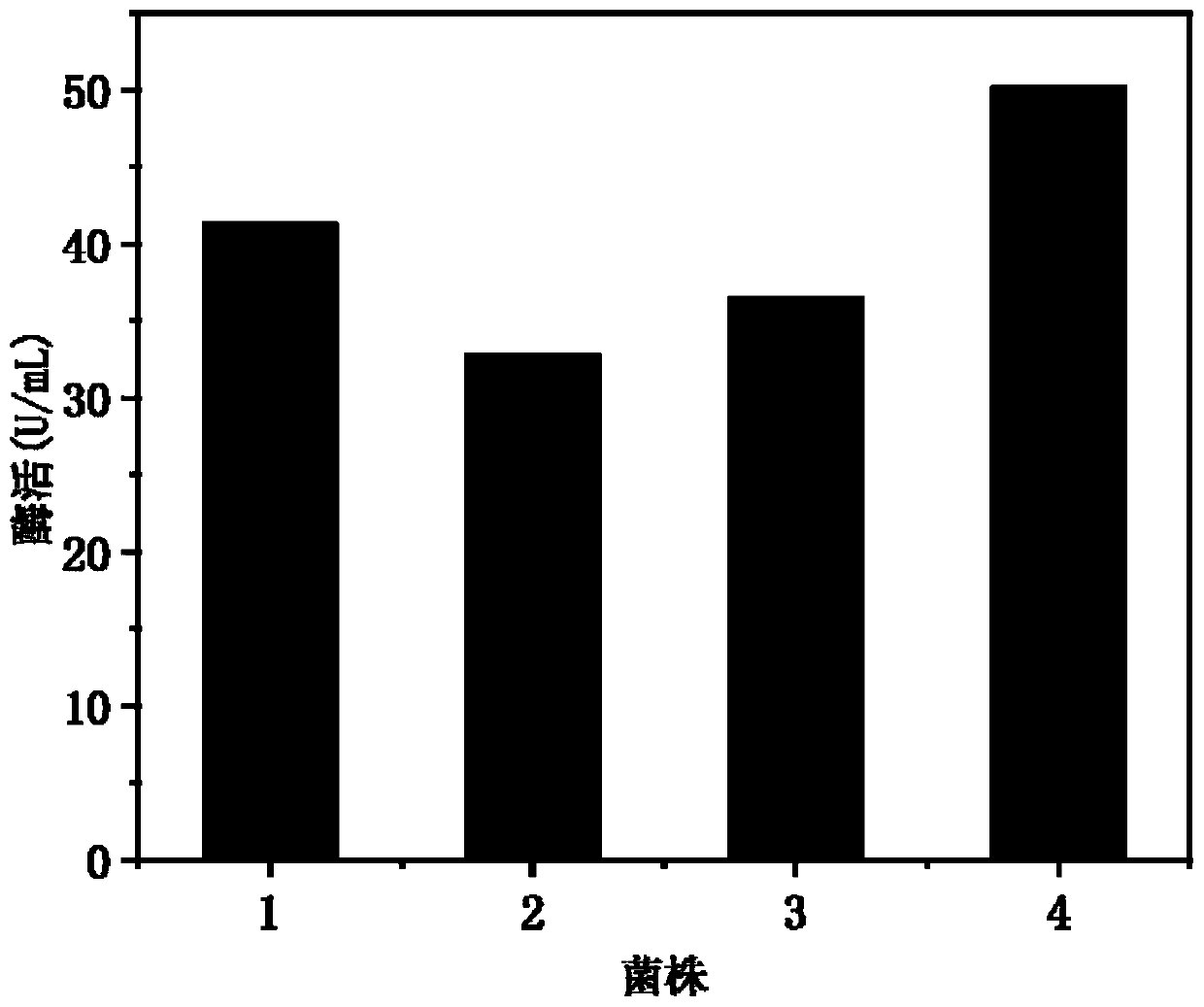
The invention discloses recombinant strains expressing a chondroitin 4-sulfotransferase gene and an applicationof the recombinant strain, and relates to the technical field of bioengineering. Chondroitin 4-sulfotransferase gene with the amino acid sequence shown in SEQ ID NO.1 is expressed with Escherichia coli or bacillus subtilis as a host, four recombinant strains are constituted, the high-efficiency expression of chondroitin 4-sulforansferase gene is realized, and final enzyme activity respectively reaches 41.3 U / mL, 32.8 U / mL, 36.5 U / mL and 50.2 U / mL. By using the enzyme as a catalyst, chondroitin sulfate A is catalyzed and synthesis in one step with chondroitin as a substrate, the final conversion efficiency reaches 80%. The method has potential and quite wide values in industrial synthesis of chondroitin sulfate A.
Owner:JIANGNAN UNIV +1
Method for efficiently producing chondroitin sulfate A by artificial enzymatic method
ActiveCN111621533AUniform structureNo potential pathogenic factorFungiAntibody mimetics/scaffoldsHeterologousArylsulfotransferase
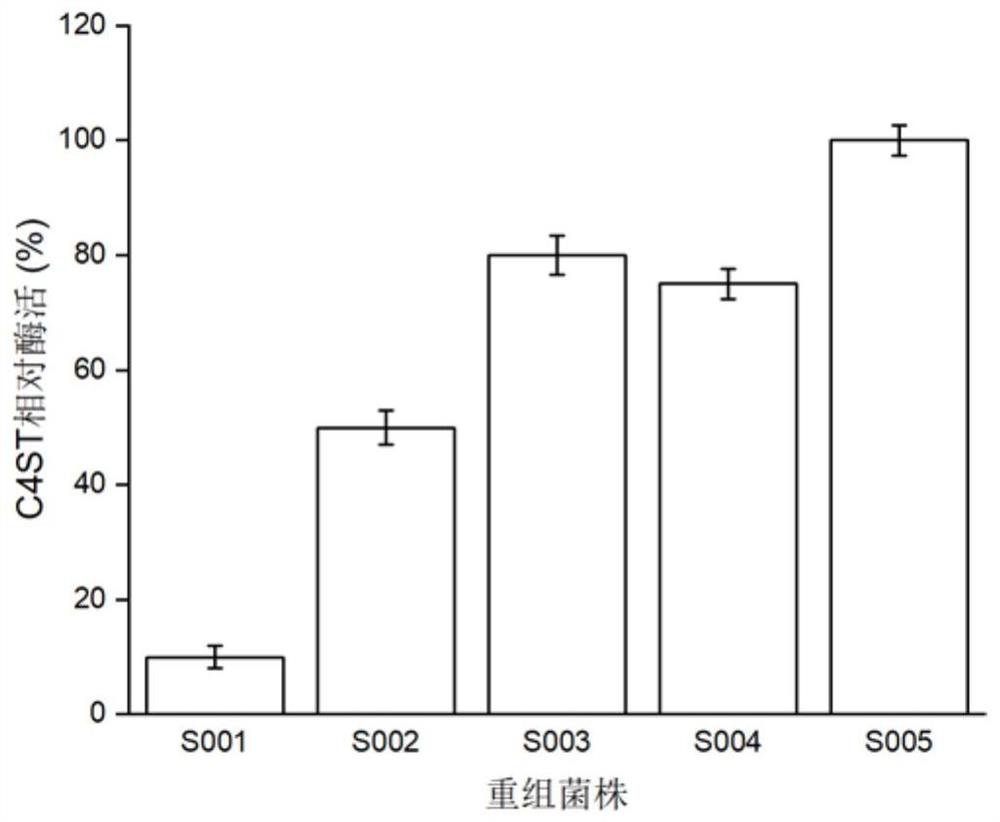
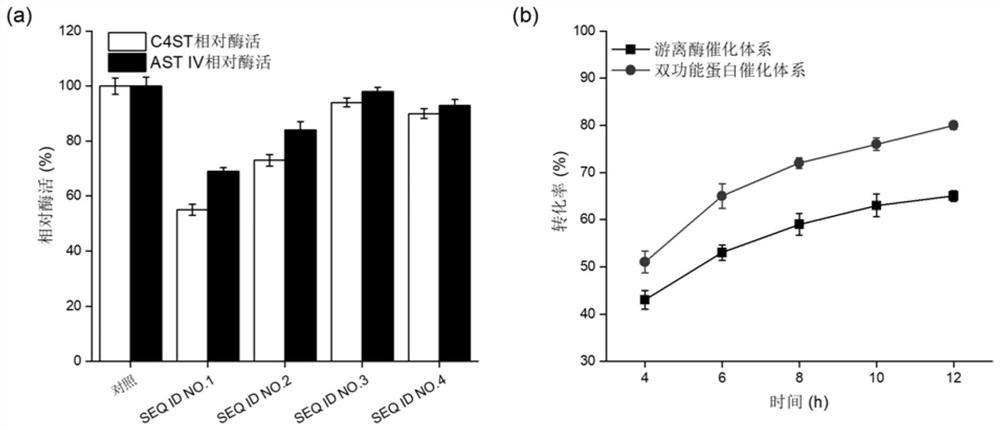
The present invention discloses a method for efficiently producing chondroitin sulfate A by an artificial enzymatic method and belongs to the technical field of bioengineering. In the method, a bifunctional protein chondroitin 4-O-sulfotransferase C4ST and an arylsulfotransferase AST IV are heterologously expressed by microorganisms, and are used to catalyze synthesis of the chondroitin sulfate Afrom chondroitin, and site-directed mutation and optimization of reaction system components are combined to accelerate synthesis efficiency of the chondroitin sulfate.
Owner:JIANGNAN UNIV
Polypeptide of N-acetylglucosamine-6-O-sulfotransferase and DNA encoding the same
A polypeptide of N-acetylglucosamine-6-O-sulfotransferase and a DNA encoding the peptide are provided. The polypeptide is (a) or (b) below: (a) a polypeptide consisting of an amino acid sequence represented by SEQ ID NO: 2; or (b) a polypeptide which comprises an amino acid sequence including substitution, deletion, insertion or transposition of one or few amino acids in the amino acid sequence of (a) and which has an enzymatic activity to transfer a sulfate group from a sulfate group donor to a hydroxyl group at 6 position of an N-acetylglucosamine residue located at a non-reducing end of an oligosaccharide represented by the formula I: GlcNAcβ1-3Gal β1-4GlcNAc (I) wherein GlcNAc represents an N-acetylglucosamine residue, Gal represents a galactose residue, β1-3 represents a β1-3 glycosidic linkage, and β1-4 represents a β1-4 glycosidic linkage.
Owner:AICHI PREFECTURE
Treatment of tumours
InactiveUS20070111973A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsBiocideDiseaseAndrostane
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wut-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3B,17-dio 1 or androstane-3B-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3B-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor—(PPAR) is produced.
Owner:HAGSTROM TOMAS +2
Antibody that reacts with 6-sulfated sialyl Lewis X
A polypeptide of N-acetylglucosamine-6-O-sulfotransferase and a DNA encoding the peptide are provided. The polypeptide is (a) or (b) below:(a) a polypeptide consisting of an amino acid sequence represented by SEQ ID NO: 2; or(b) a polypeptide which comprises an amino acid sequence including substitution, deletion, insertion or transposition of one or few amino acids in the amino acid sequence of (a) and which has an enzymatic activity to transfer a sulfate group from a sulfate group donor to a hydroxyl group at 6 position of an N-acetylglucosamine residue located at a non-reducing end of an oligosaccharide represented by the formula I:GlcNAcβ1-3Galβ1-4GlcNAc (I)wherein GlcNAc represents an N-acetylglucosamine residue, Gal represents a galactose residue, β1-3 represents a β1-3 glycosidic linkage, and β1-4 represents a β1-4 glycosidic linkage.
Owner:AICHI PREFECTURE
Vector expressing n-deacetylase/n-sulfotransferase 2
An object of the present invention is to provide an expression vector that allows for stable production of N-deacetylase / N-sulfotransferase 2 in large amounts and a process for production of N-deacetylase / N-sulfotransferase 2 using the same. The present invention provides a recombinant baculovirus expression vector obtained by incorporating into baculovirus DNA, a DNA fragment having lobster L21 DNA, DNA encoding gp67 signal peptide and DNA encoding the 79th to 883rd amino acids of human N-deacetylase / N-sulfotransferase 2 in this order in the 5′ to 3′ direction.
Owner:RIKEN +1
Leech tyrosine sulfonate transferase gene and application thereof
ActiveCN113789335AHigh anticoagulant activityStrong substrate preferenceFungiBacteriaTyrosineAcyl group
The invention discloses a leech tyrosine sulphonate transferase gene and application thereof, and belongs to the field of biomedicine. The leech tyrosine sulphonate transferase gene is characterized in that on the basis of a hirudo nipponia transcriptome database, a leech tyrosine sulphonate transferase cDNA sequence is excavated and identified through a bioinformatics means, and on this basis, the leech tyrosine sulfotransferase is synthesized through codon optimization, gene synthesis and microbial cell expression, and the recombinant hirudin is catalyzed by the leech tyrosine sulfotransferase to be subjected to sulfonation modification to obtain the natural hirudin. The catalytic ability of the transferase is greatly improved compared with that of acyl sulfonate transferase, human-derived tyrosine sulfonate transferase and bovine-derived tyrosine sulfonate transferase. Compared with other tyrosine sulfonic acid transferase, the leech tyrosine sulfonic acid transferase obtained in the invention has stronger substrate preference for recombinant hirudin, is suitable for large-scale preparation of natural hirudin, and provides a new way for synthesis of natural hirudin.
Owner:INST OF BOTANY JIANGSU PROVINCE & CHINESE ACADEMY OF SCI
Sulfotransferase Inhibitors
A galactosamine derivative represented by the following formula (1):wherein R1, R2 and R5 each independently represents SO3− or H, and at least one of them represents SO3−; R3 represents H, acetyl or SO3−; R4 represents H, a substituted or unsubstituted alkyl group, a substituted or unsubstituted alkenyl group, a substituted or unsubstituted alkynyl group, a substituted or unsubstituted acyl group, a substituted or unsubstituted aryl group, or a substituted or unsubstituted aralkyl group; X represents O, S, NH or CH2; and represents an α bond or a β bond, and a sulfotransferase inhibitor comprising the derivative.
Owner:SEIKAGAKU KOGYO CO LTD
Short-acting heparin-based anticoagulant compounds and methods
PendingCN110446511AOrganic active ingredientsCation exchanger materialsChemo enzymaticAnticoagulant activity
Heparin compounds and synthetic heparin analogues having short acting anticoagulant activity are provided. Methods of synthesizing such heparin compounds, including chemoenzymatic pathways using sulfotransferase enzymes are provided. Methods of treating subjects in need of anticoagulant activity are provided.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Porcine sulfotransferase 2A1 polynucleotide sequence, protein, and methods of use for same
InactiveUS20060024708A1Improve stabilityHigh affinityCompound screeningApoptosis detectionBoar taintGene product
The invention pertains to the identification and isolation of a gene involved in the boar taint phenotype. A porcine form of a gene, designated SULT2A1, is identified. The invention additionally provides methods of inhibiting or reducing boar taint using the novel SULT2A1 gene or gene product. Methods for reducing or inhibiting boar taint and methods for screening compounds to modulate SULT2A1 are also disclosed.
Owner:UNIVERSITY OF GUELPH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com