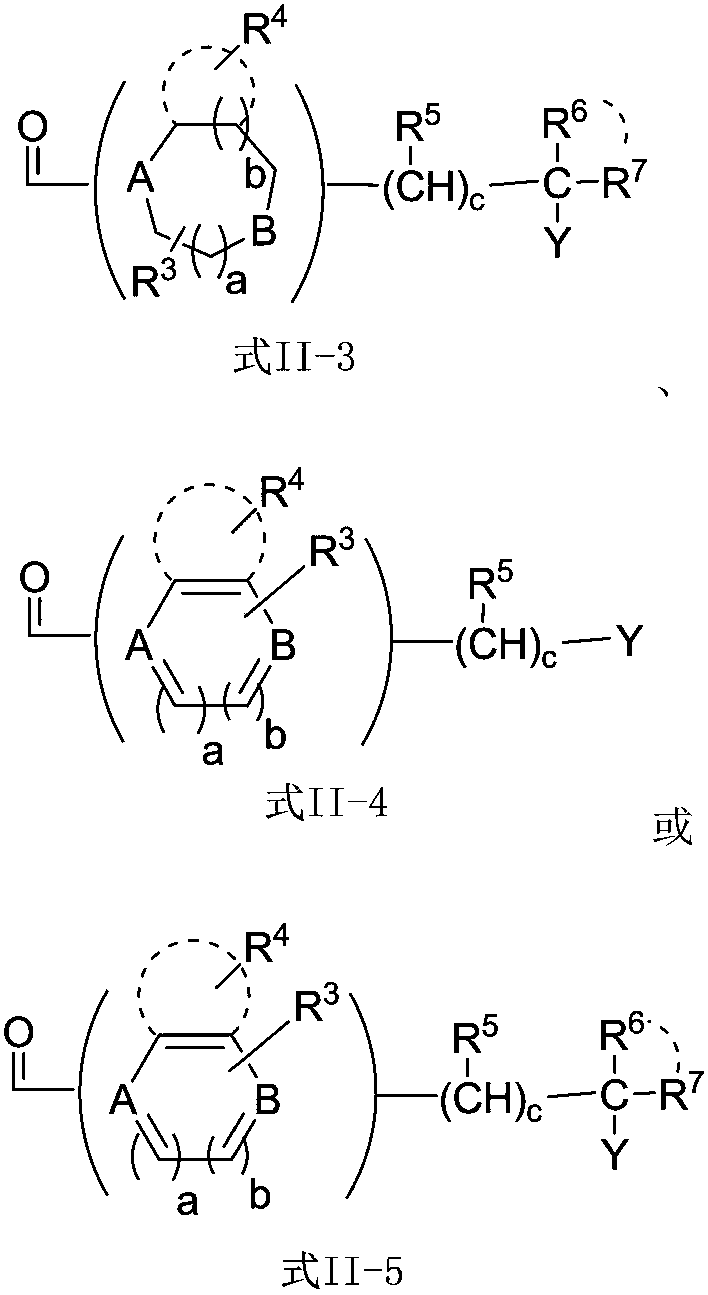
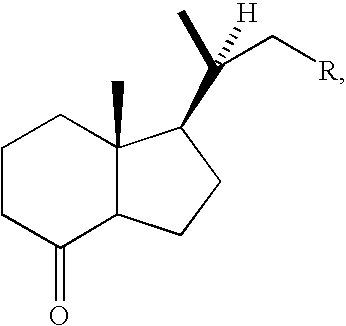
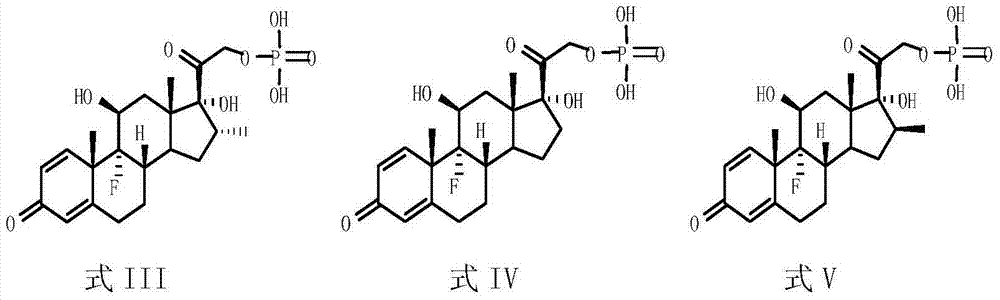
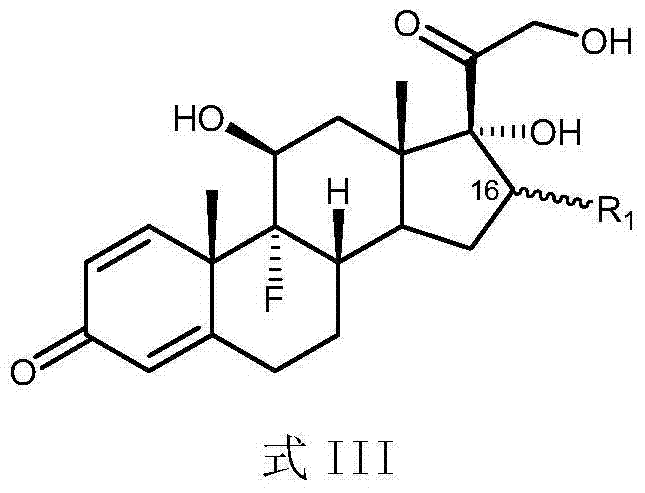
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "Pregnenolone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.
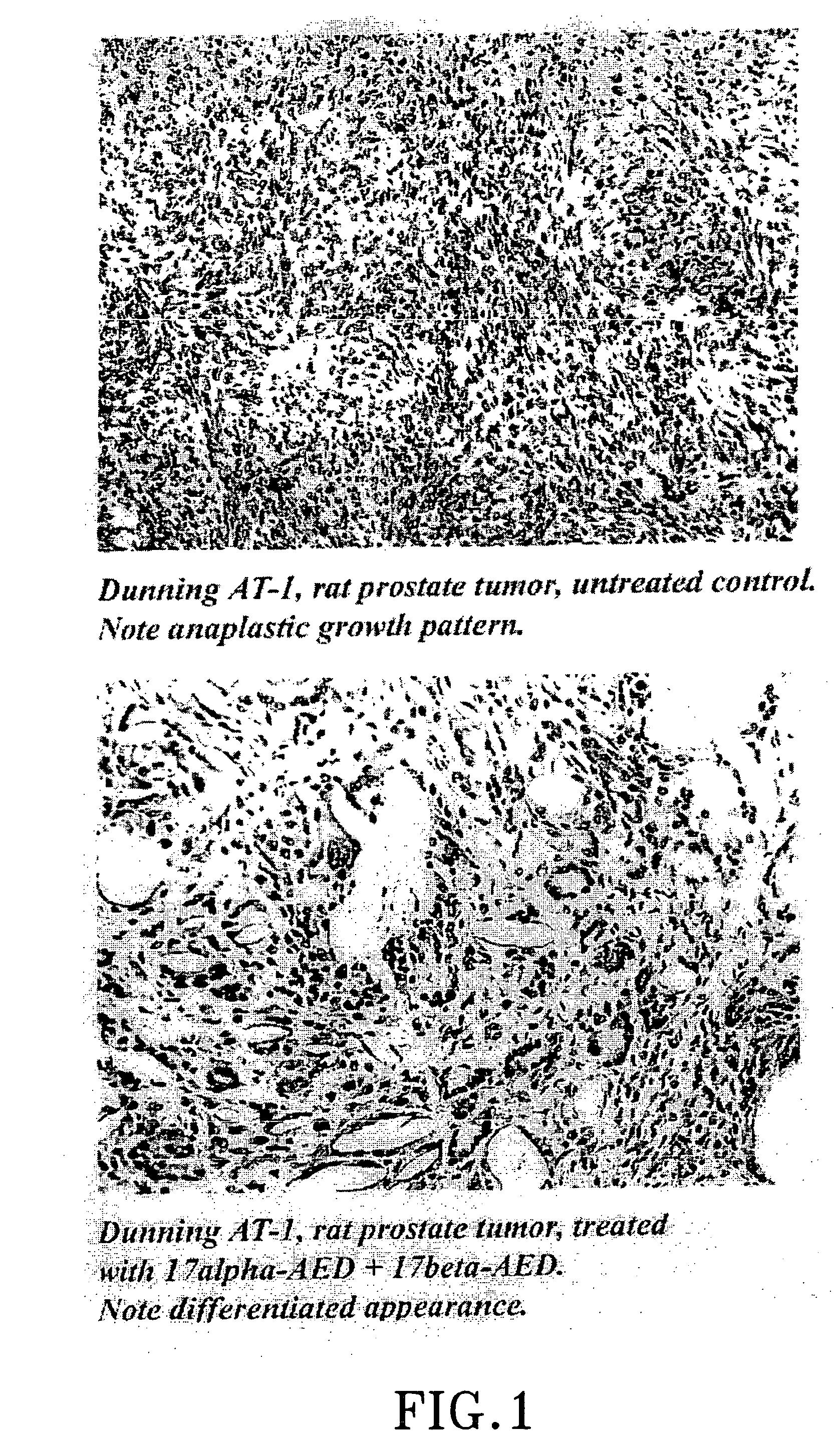
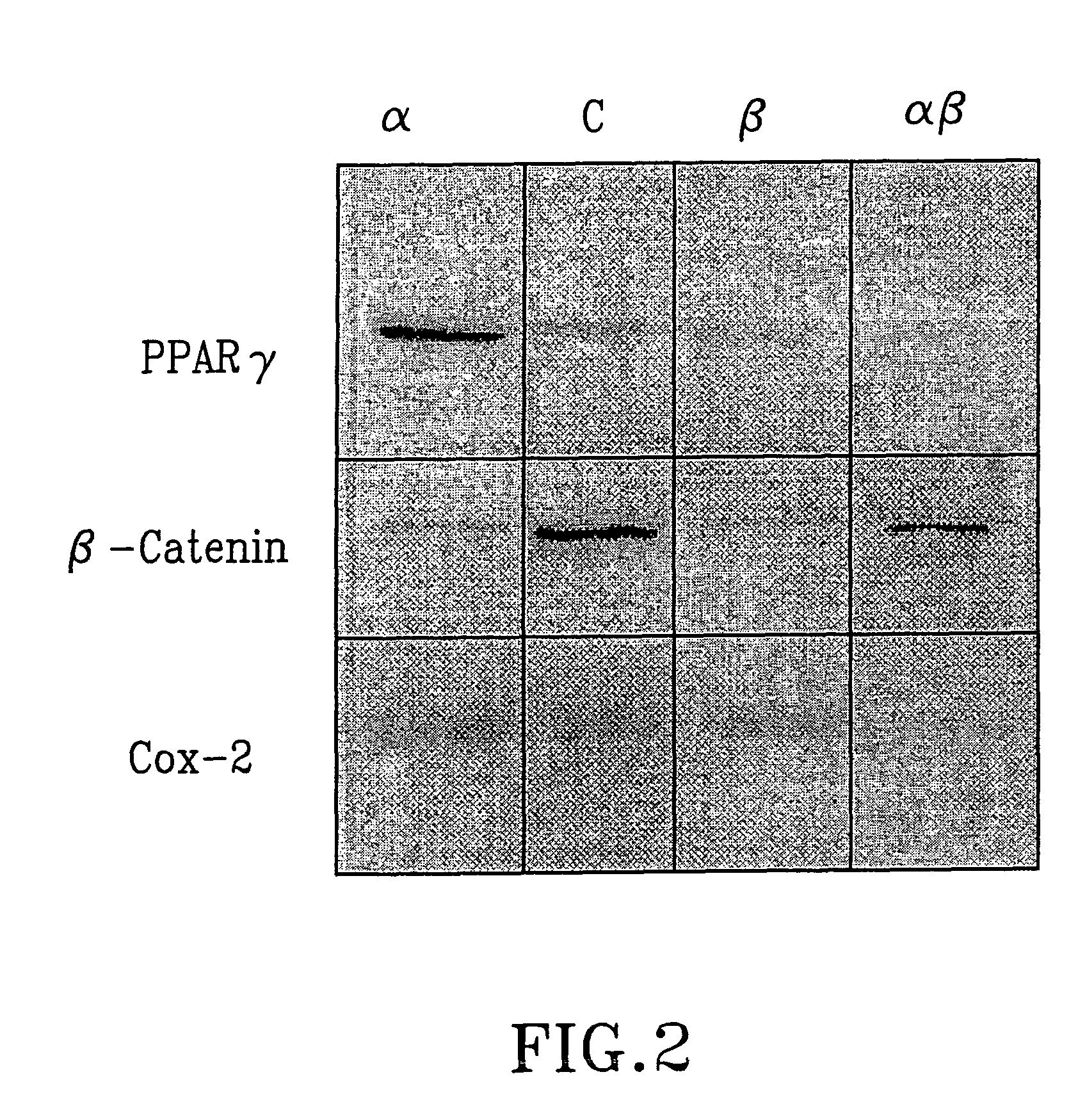
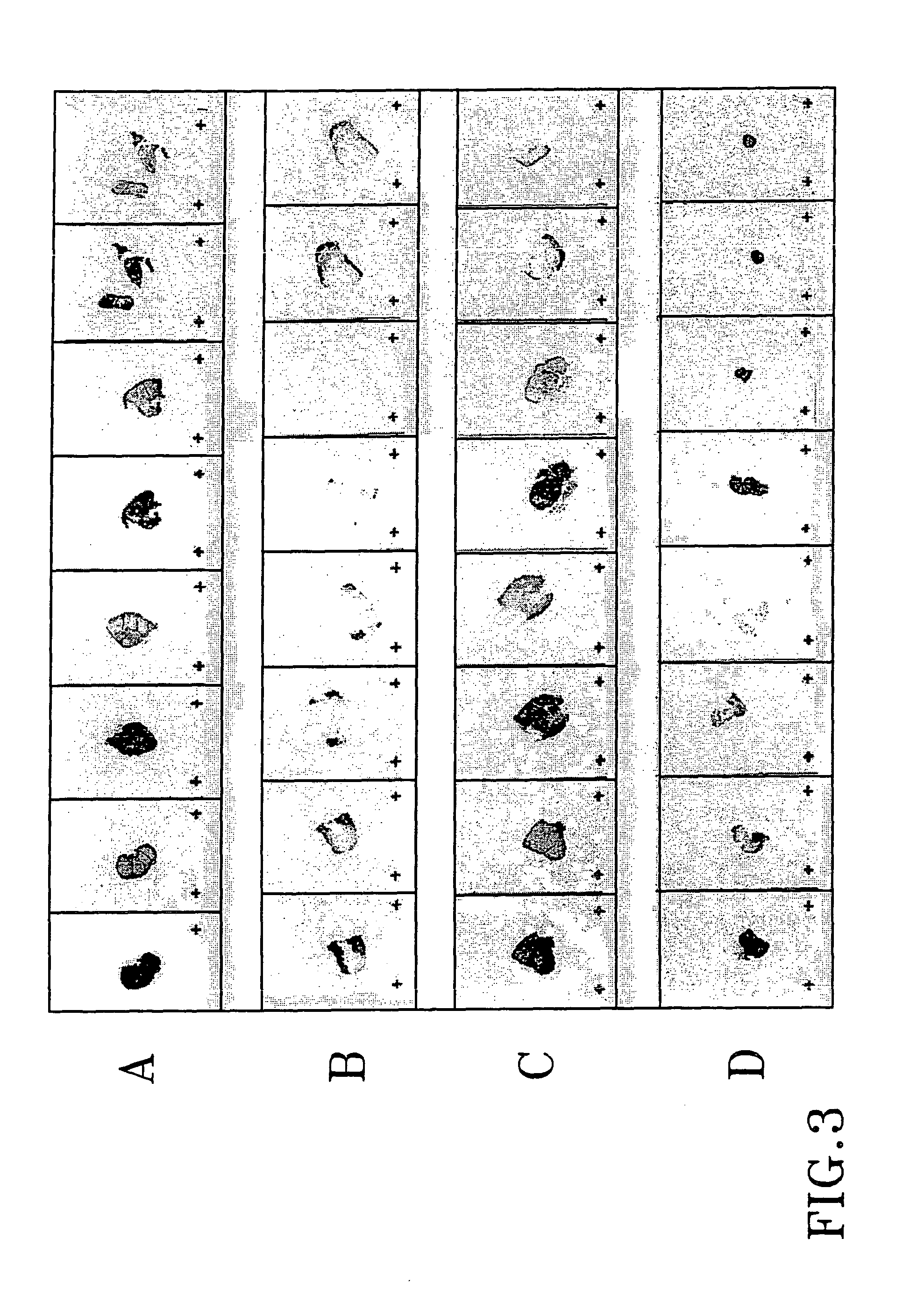
Treatment of tumours
InactiveUS20050192262A1Good curative effectSquelching unwanted PPARγ-activityOrganic active ingredientsSteroidsDiseaseAndrostane
The present invention refers to steroid derivatives for use as medicaments. More specifically, the invention also relates to the use of a steroid derivative of 5-androstene-, 5-pregnenolone or corresponding saturated derivatives (androstane- or pregnane-) in the manufacture of a medicament for the treatment of a benign and / or malignant tumour, which medicament is capable of interrupting disturbances in Wnt-signaling, such as cell-cycle arrest in G1-phase, and / or providing an angiostatic effect. Examples of such steroid derivatives are -5-androstene-17-ol, androstane-17-ol-pregnane-17-ol or pregnane-17-ol derivatives. In a further aspect, the invention relates to a method of producing a medicament for the treatment of a benign and / or malignant tumour and / or an inflammatory condition comprising the steps of contacting 5-androstane-3β,17-diol or androstane-3β-diol, an enzyme and a sulfotransferase to provide 5-androstene-17-ol-3β-sulfate or corresponding andros tane derivative (17-AEDS or 17-AADS); and mixing the 17-AEDS or 17-AADS so produced with a suitable carrier; whereby a medicament which is capable of acting as a ligand to peroxisome proliferators-activated receptor-(PPAR) is produced.
Owner:HAGSTROM TOMAS
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
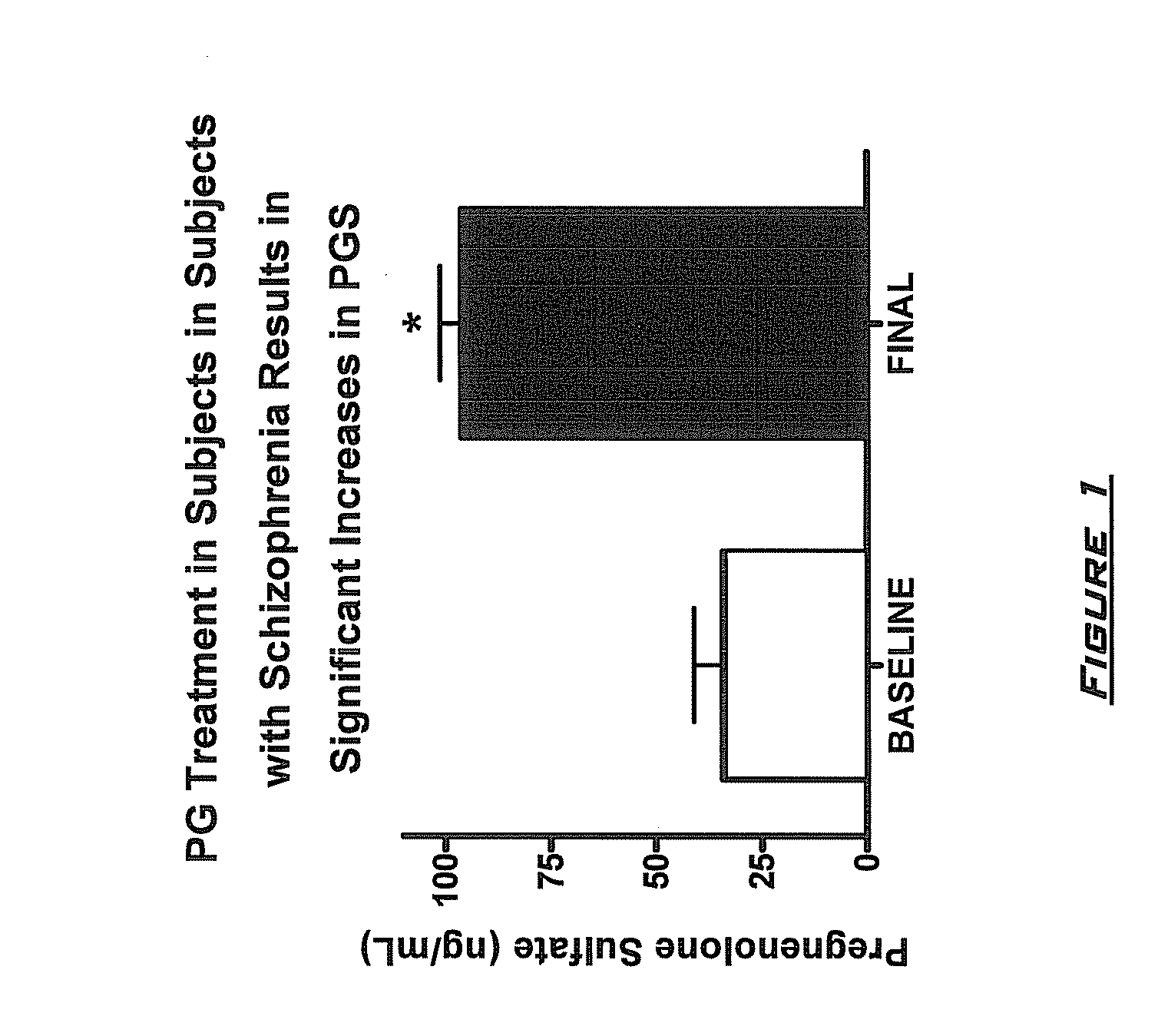
Neuroactive steroid compositions and methods of use therefor
InactiveUS20090203658A1Improve cognitive functionPromote more developedOrganic active ingredientsBiocideMetaboliteSchizo-affective type
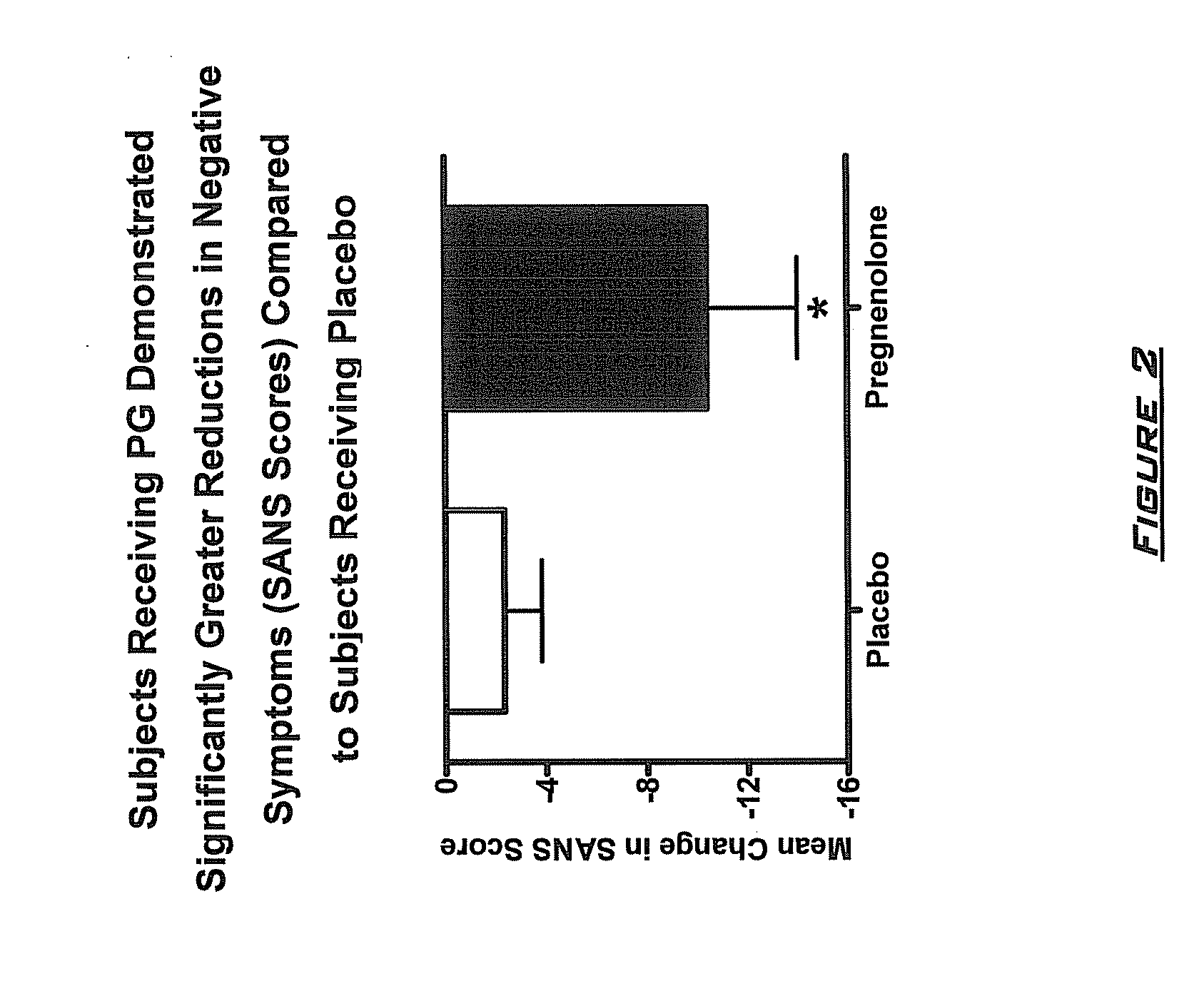
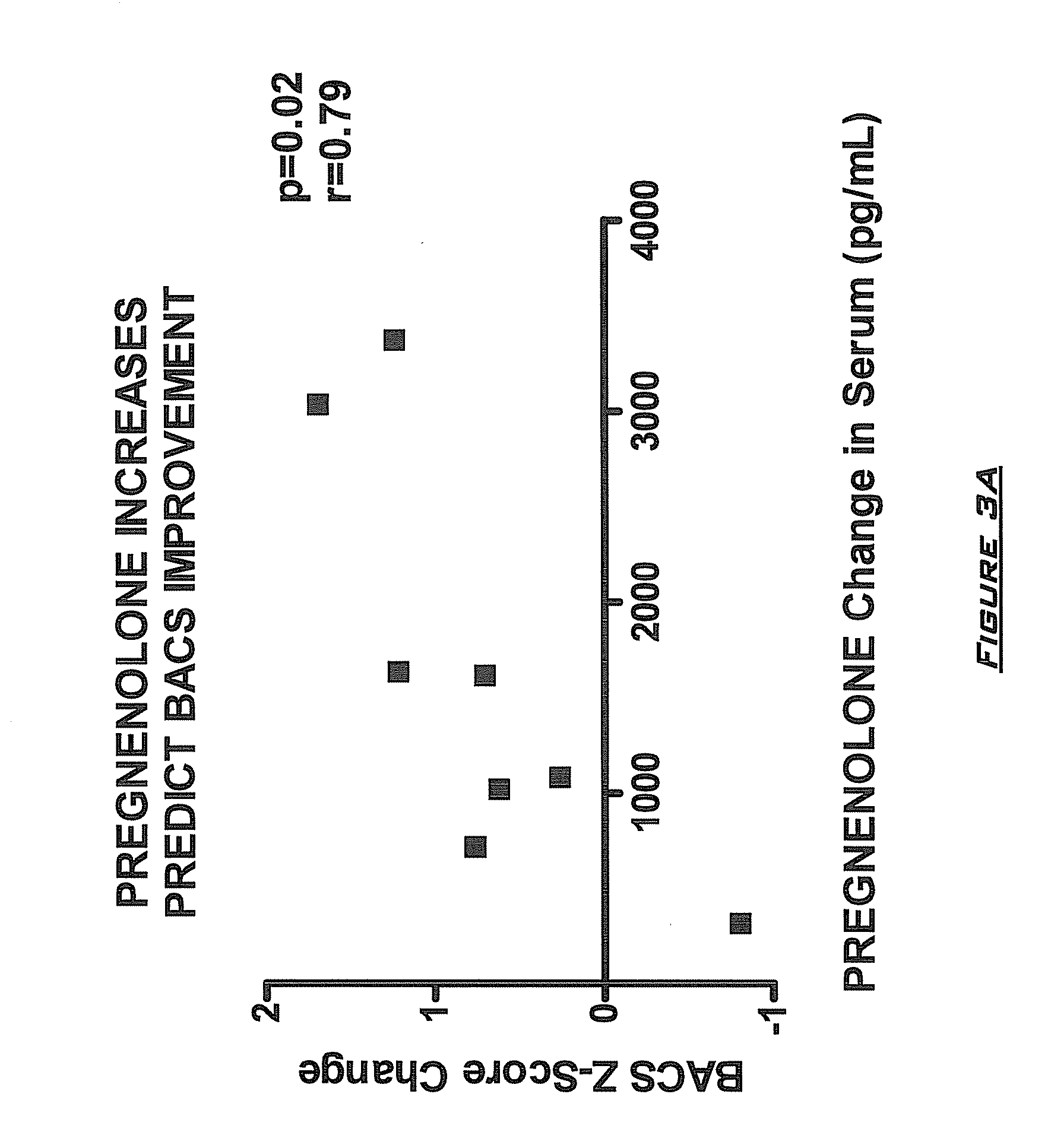
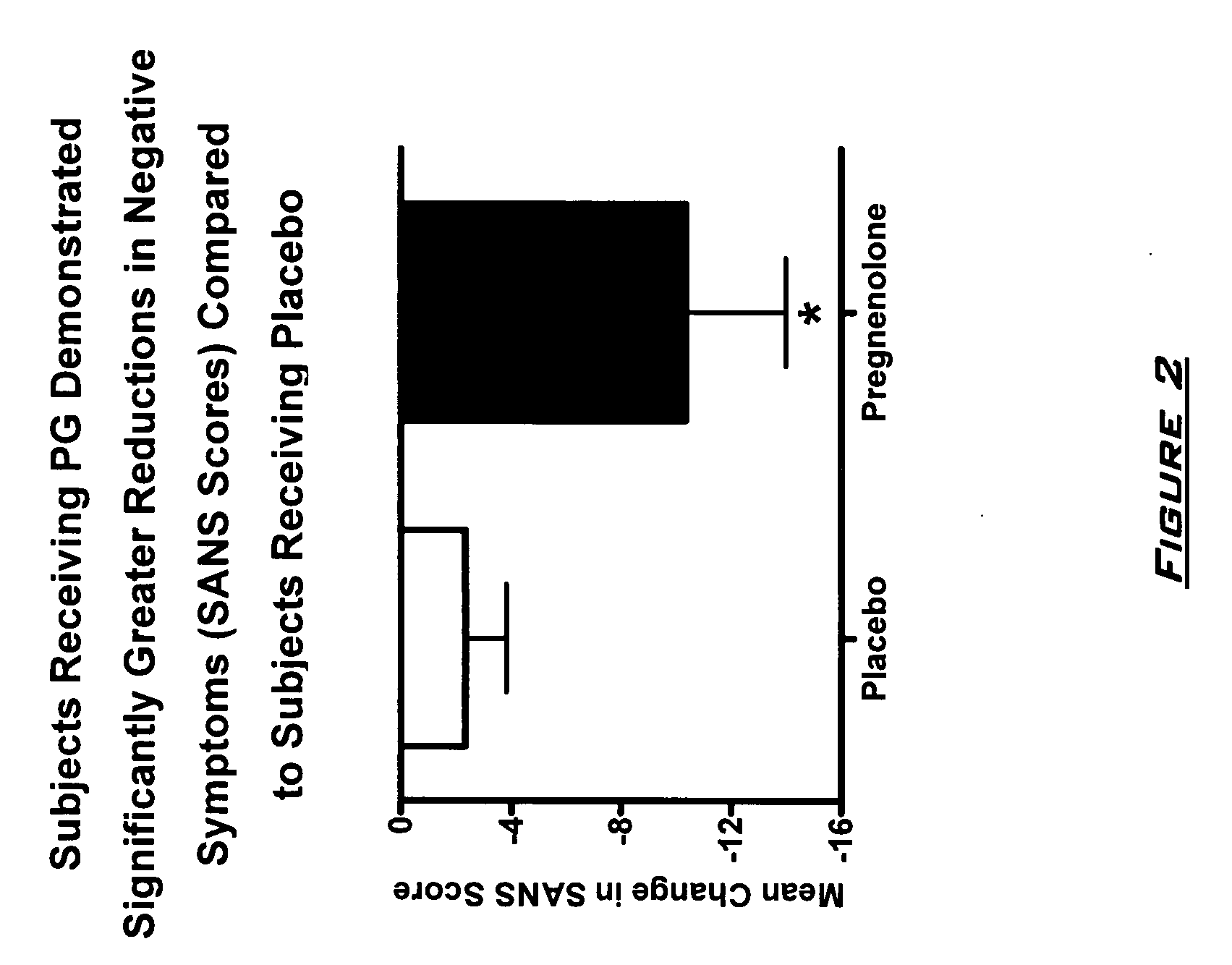
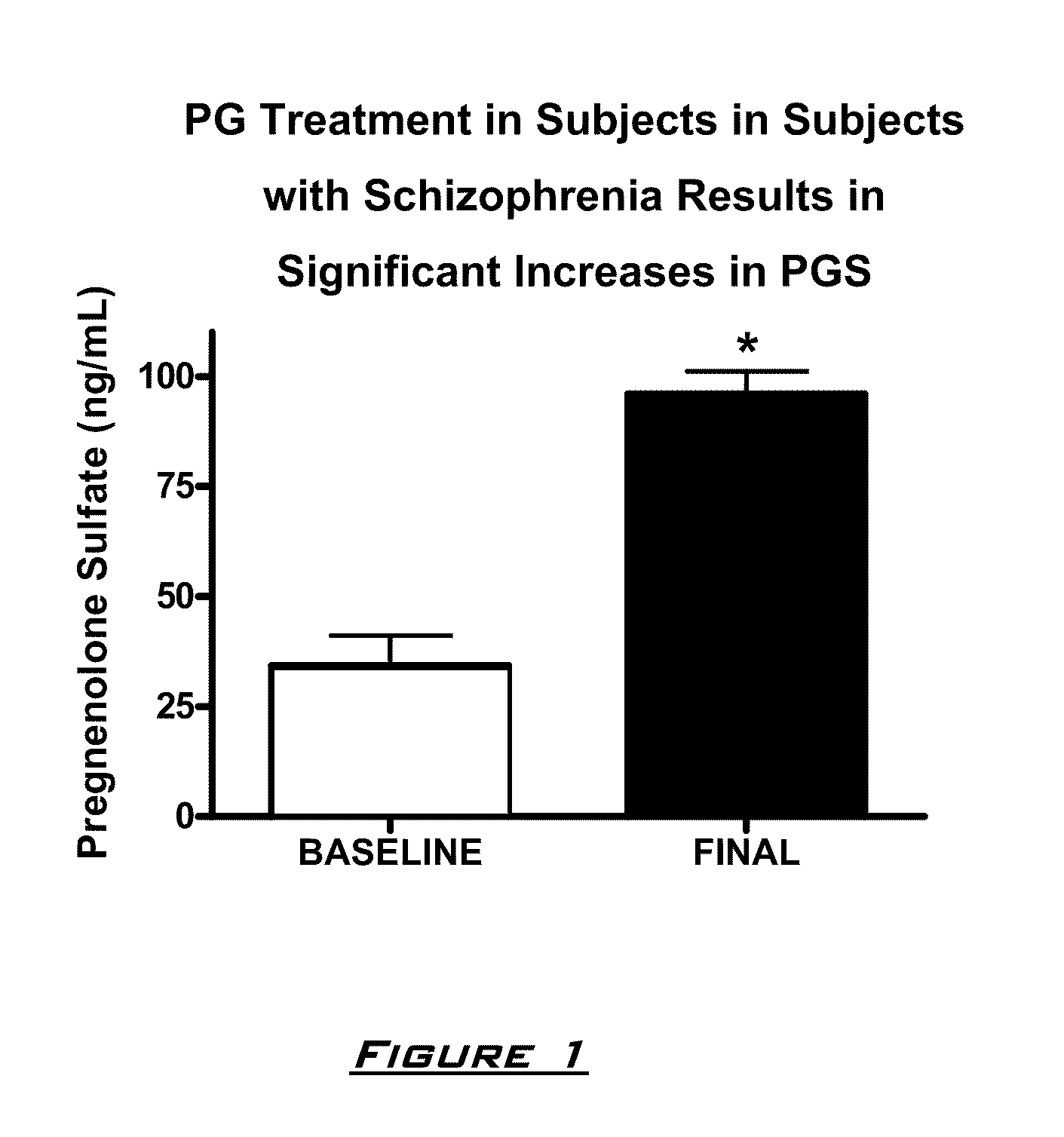
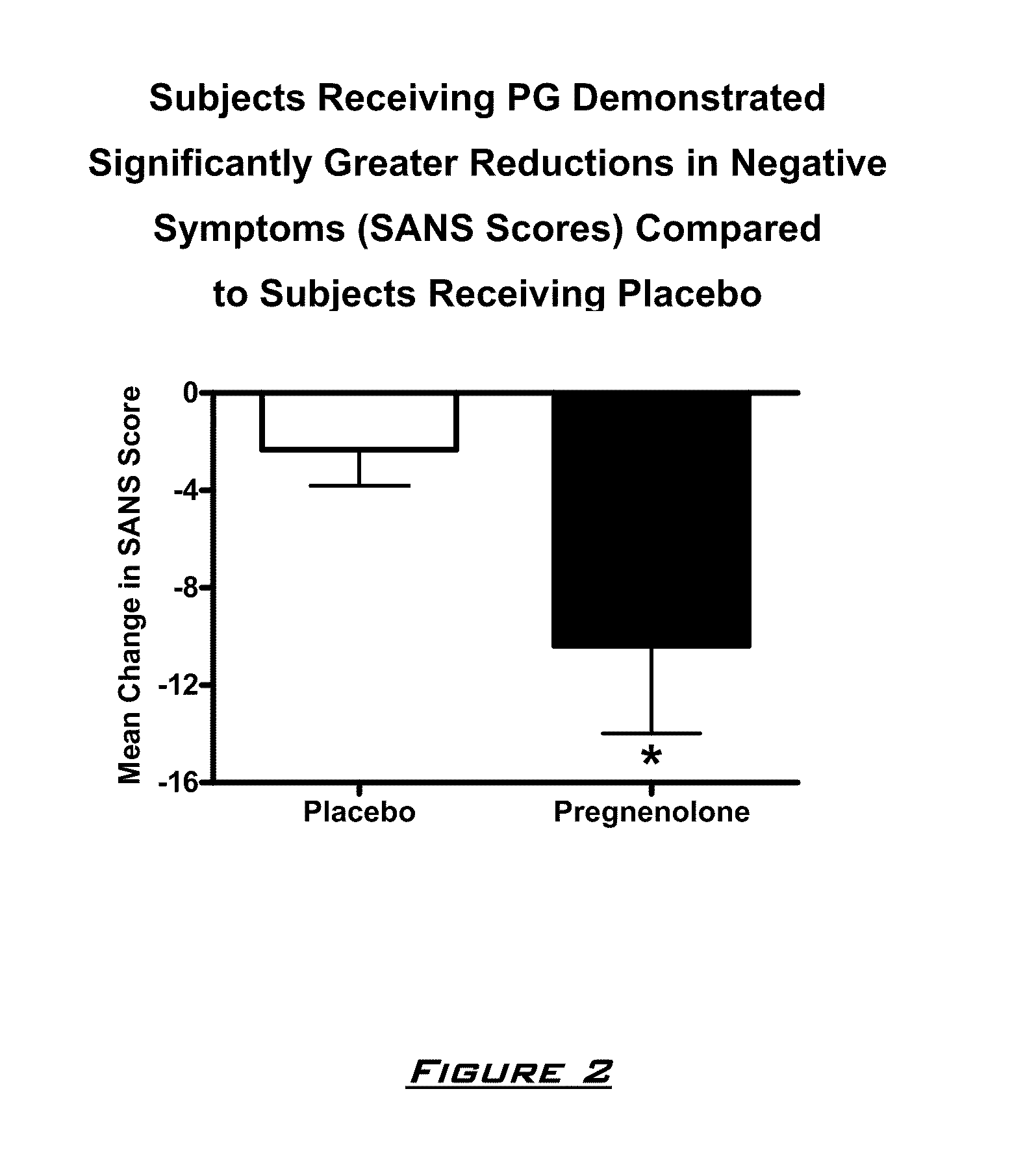
Provided are methods for ameliorating a symptom of a neuropsychiatric disorder in a subject. Also provided are methods for ameliorating at least one physical symptom or at least one psychological symptom resulting from tobacco cessation in a subject, methods for ameliorating a symptom of Alzheimer's disease or other cognitive disorder in a subject, methods for ameliorating a symptom of schizophrenia, schizoaffective disorder, or other psychotic disorder in a subject, methods for ameliorating a symptom of a depressive disorder in a subject, methods for ameliorating a symptom of bipolar disorder in a subject, methods for ameliorating a symptom of post-traumatic stress disorder or other anxiety disorder in a subject, methods for predicting a predisposition to suicide, suicidal ideation, suicidal behavior, or a combination thereof in a subject, methods for ameliorating a symptom of a pain disorder in a subject, methods for ameliorating a neurodegenerative disorder in a subject, methods for ameliorating a symptom of traumatic brain injury in a subject, methods for ameliorating a sleep disorder in a subject, and methods for improving cognitive functioning in a subject. In some embodiments, the methods include administering to a subject in need thereof an effective amount of a neuroactive steroid composition comprising pregnenolone (PG), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA), progesterone (PROG), precursors thereof, metabolites thereof, pharmaceutically acceptable salts thereof, derivatives thereof, or combinations thereof.
Owner:DUKE UNIV
Controlled release delivery system for nasal application of neurotransmitters
InactiveUS20090227550A1Improve bioavailabilityEffective serum levelOrganic active ingredientsBiocideNoseProgesterones
This invention relates to a galenical gel formulation for nasal administration of neurotransmitters / neuromodulators such as dopamine, serotonin or pregnenolone and progesterone. The special lipophilic or partly lipophilic system of the invention leads to high bioavailability of the active ingredient in plasma and brain caused by sustained serum levels and / or direct or partly direct transport from nose to the brain.
Owner:MATTERN PHARMA
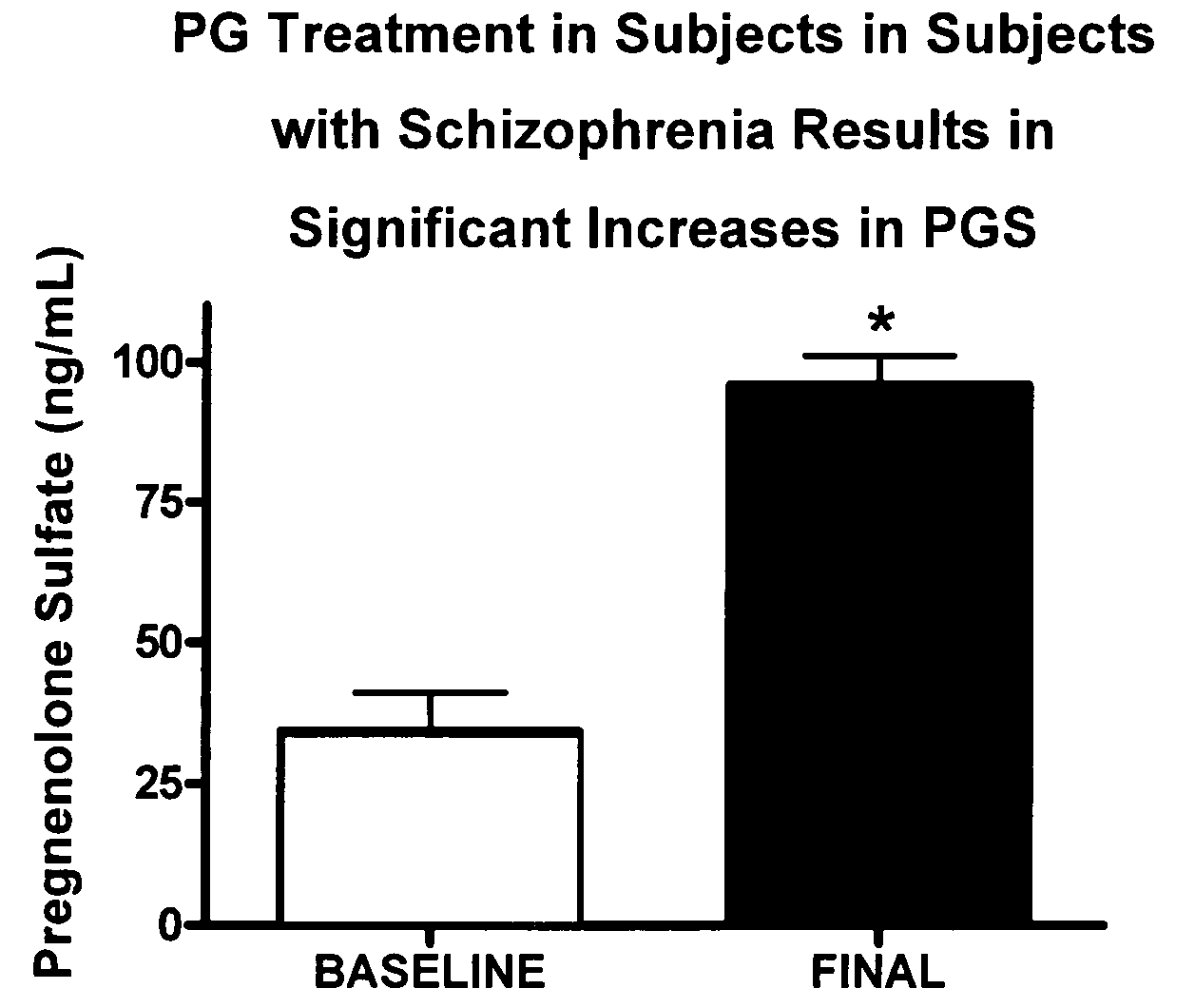
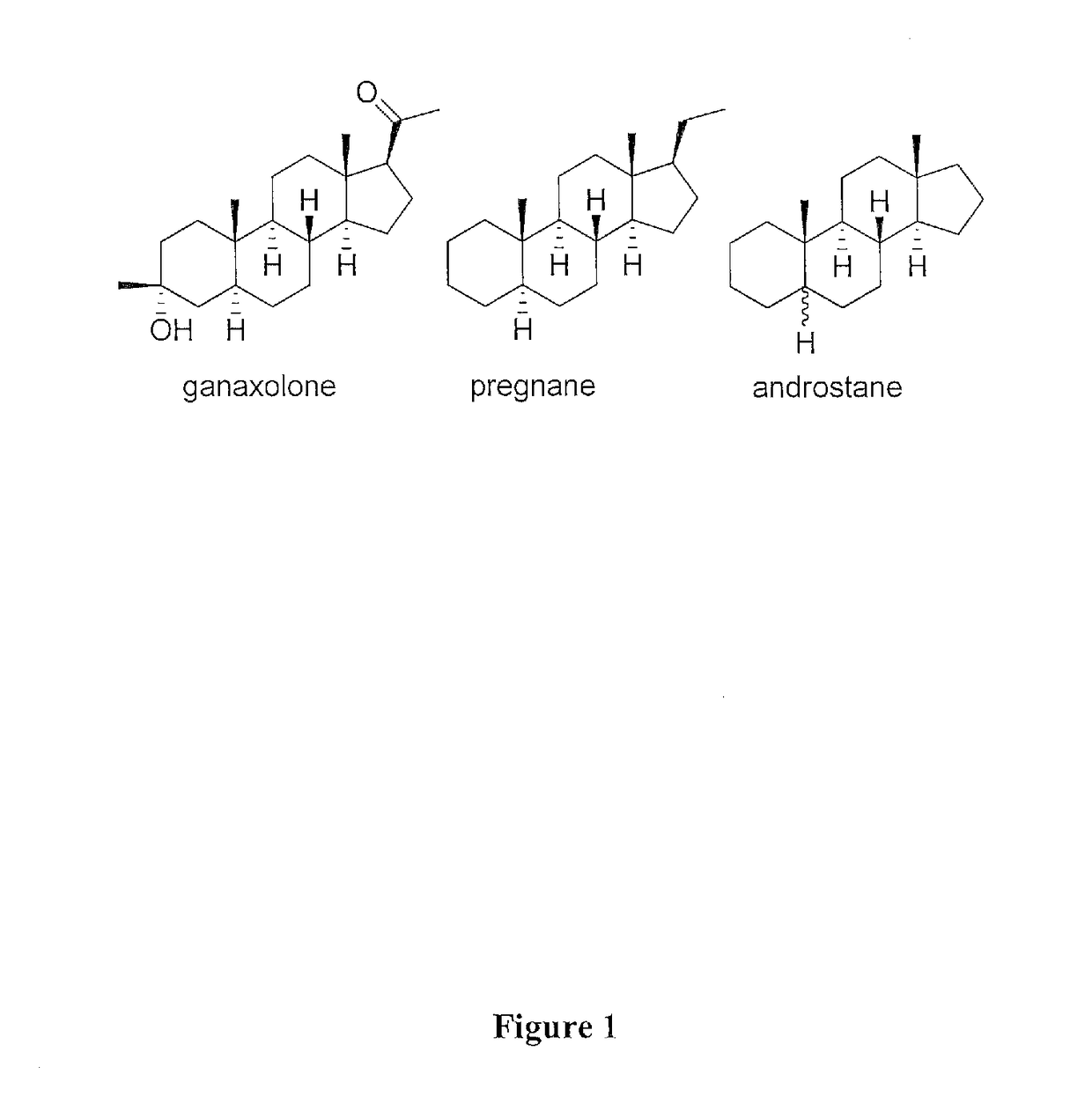
Ganaxolone for use in treating genetic epileptic disorders
InactiveUS20190160078A1Relieving conditionRelieve symptomsOrganic active ingredientsNervous disorderPregnenoloneSeizure frequency
The disclosure provides a method of treating a mammal having a genetic epileptic disorder, comprising chronically administering a pharmaceutically acceptable pregnenolone neurosteroid to a mammal having a genetic epileptic disorder in an amount effective to reduce the seizure frequency in the mammal. In certain preferred embodiments, the mammal is a human patient who has a CDKL5 genetic mutation. In certain preferred embodiments, the patient has a low endogenous level of a neurosteroid(s). In certain preferred embodiments, the pregnenolone neurosteroid is ganaxolone.
Owner:MARINUS PHARMA
Neuroactive steroid compositions and methods of use therefor
InactiveUS20090074677A1Improve cognitive functionPromote more developedOrganic active ingredientsBiocideSchizo-affective typePain disorder
Provided are methods for ameliorating a symptom of a neuropsychiatric disorder in a subject. Also provided are methods for ameliorating at least one physical symptom or at least one psychological symptom resulting from tobacco cessation in a subject, methods for ameliorating a symptom of Alzheimer's disease or other cognitive disorder in a subject, methods for ameliorating a symptom of schizophrenia, schizoaffective disorder, or other psychotic disorder in a subject, methods for ameliorating a symptom of a depressive disorder in a subject, methods for ameliorating a symptom of bipolar disorder in a subject, methods for ameliorating a symptom of post-traumatic stress disorder or other anxiety disorder in a subject, methods for predicting a predisposition to suicide, suicidal ideation, suicidal behavior, or a combination thereof in a subject, methods for ameliorating a symptom of a pain disorder in a subject, methods for ameliorating a neurodegenerative disorder in a subject, methods for ameliorating a symptom of traumatic brain injury in a subject, methods for ameliorating a sleep disorder in a subject, and methods for improving cognitive functioning in a subject. In some embodiments, the methods include administering to a subject in need thereof an effective amount of a neuroactive steroid composition comprising pregnenolone (PG), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA), pharmaceutically acceptable salts thereof, derivatives thereof, or combinations thereof.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Mass spectrometry assay for congenital adrenal hyperplasia
ActiveUS20100155595A1Avoid condensationSolvent is evaporatedComponent separationFuel lighters11-DesoxycortisolHydrocortisone
Methods are provided for detecting the amount of one or more CAH panel analytes (i.e., pregnenolone, 17-OH pregnenolone, progesterone, 17-OH progesterone, dehydroepiandrosterone (DHEA), androstenedione, testosterone, deoxycorticosterone, 11-deoxycortisol, and cortisol) in a sample by mass spectrometry. The methods generally involve ionizing one or more CAH panel analytes in a sample and quantifying the generated ions to determine the amount of one or more CAH panel analytes in the sample. In methods where amounts of multiple CAH panel analytes are detected, the amounts of multiple analytes are detected in the same sample injection.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
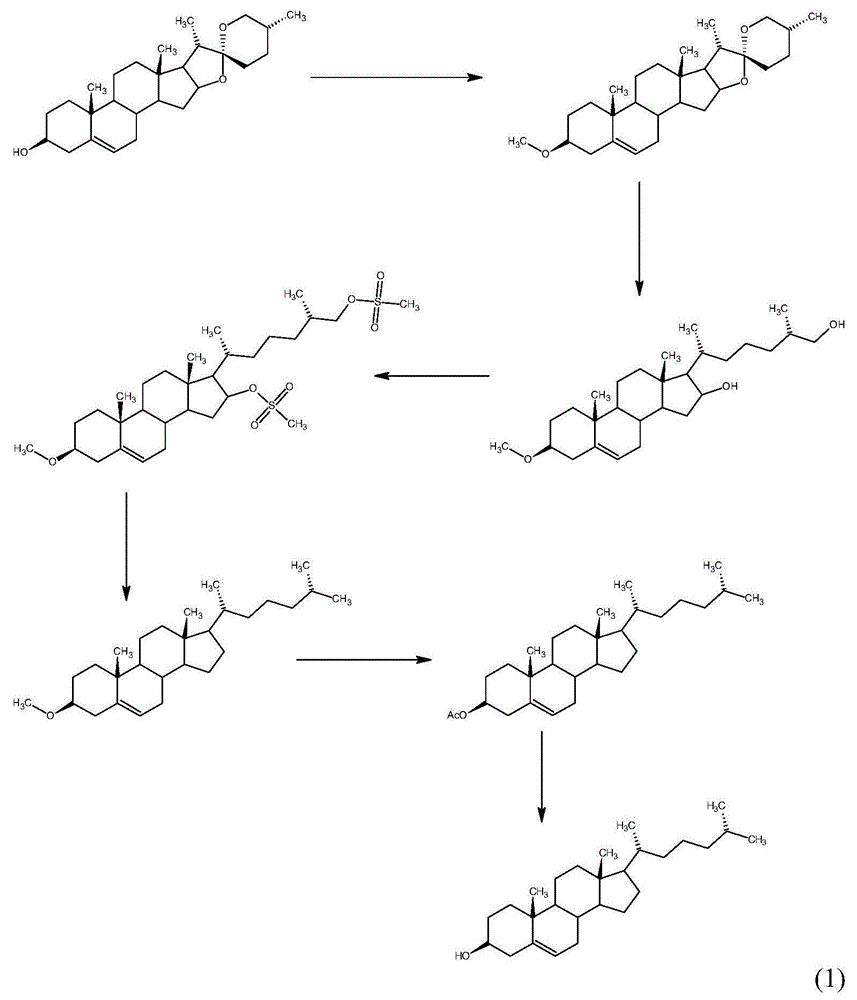
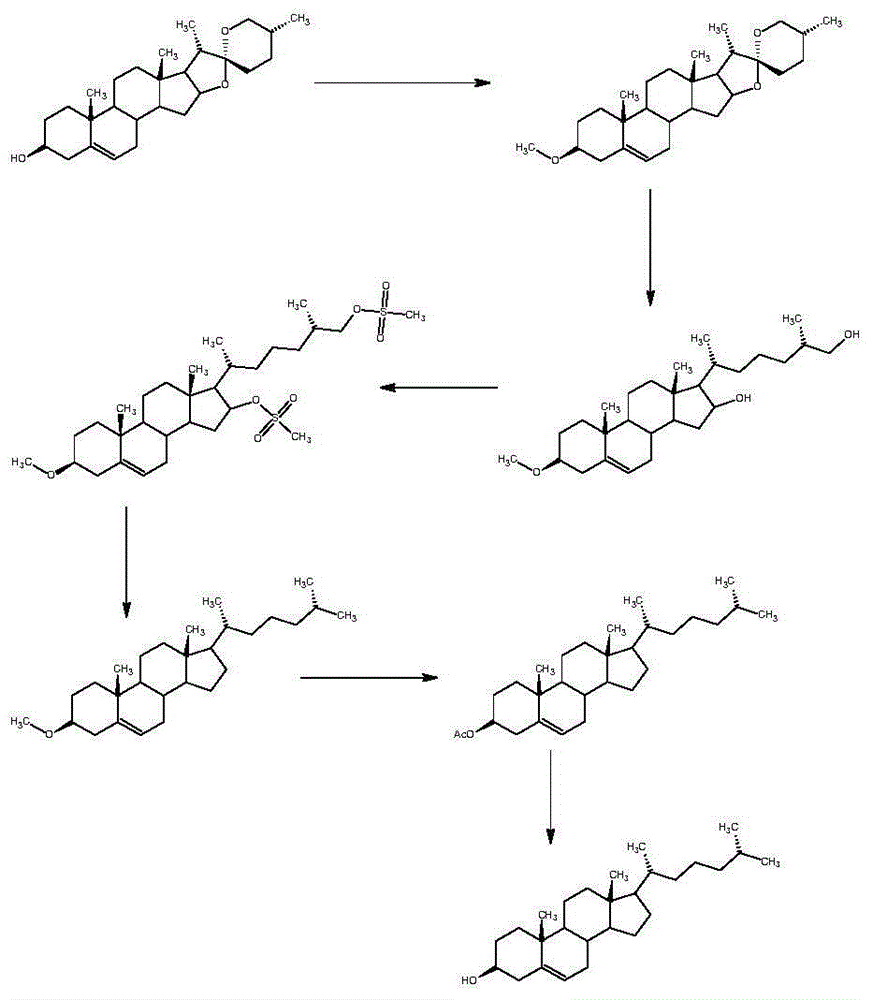
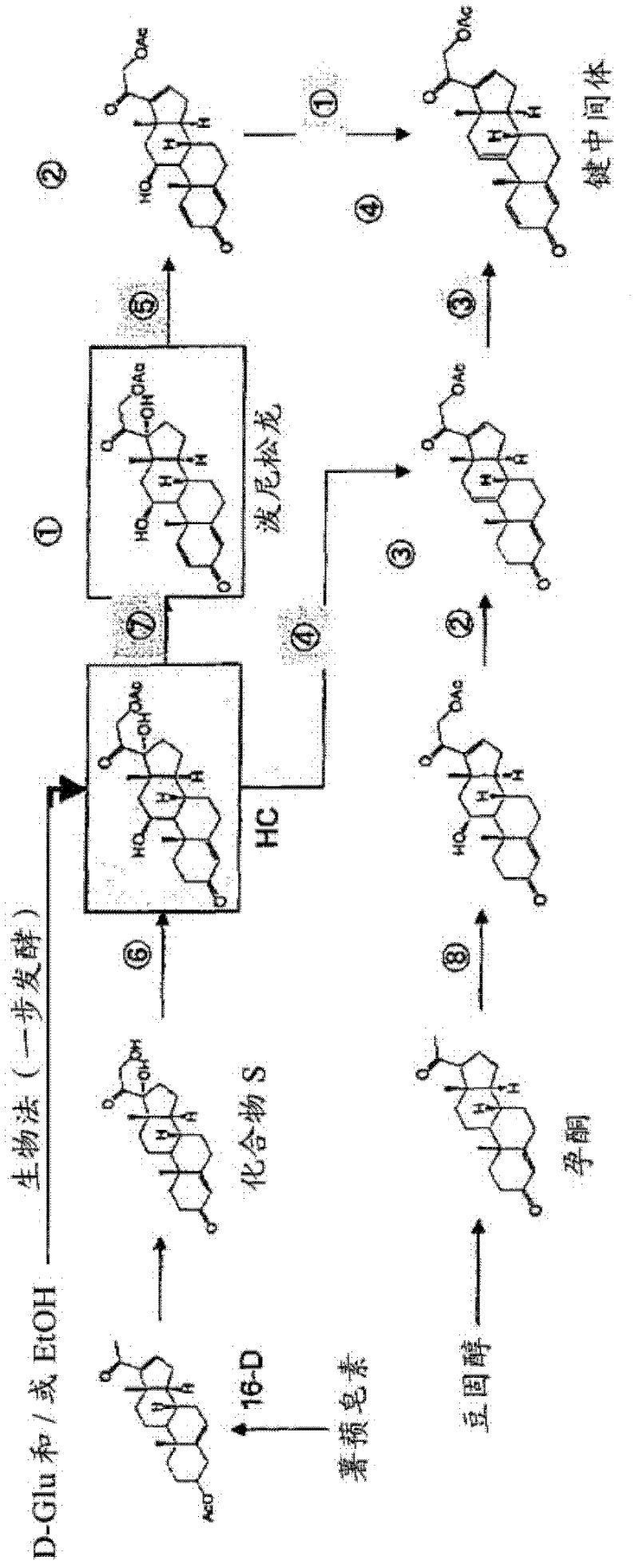
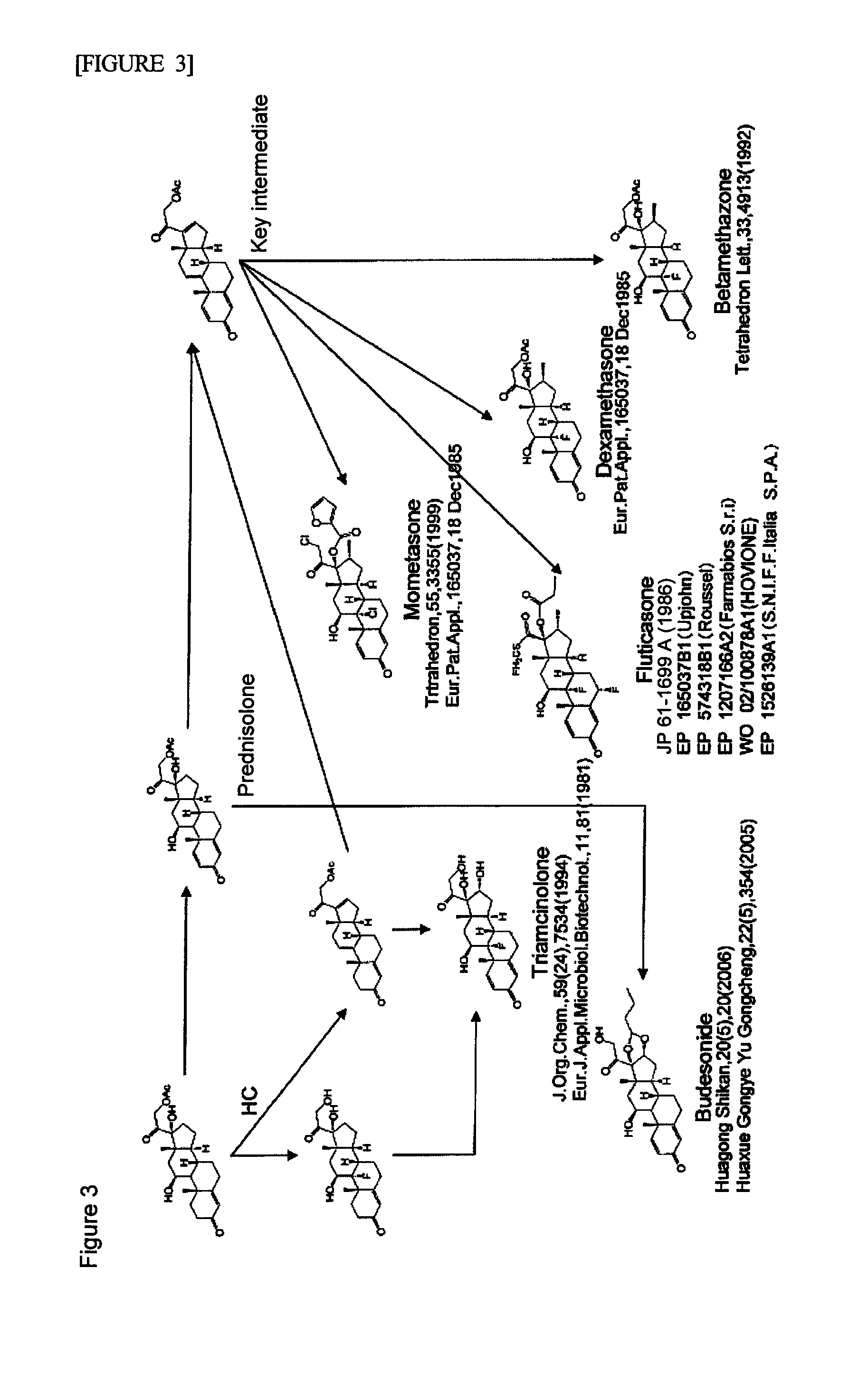
Method for synthesizing cholesterol by using pregnenolone as raw material
The invention provides a method for synthesizing cholesterol by using pregnenolone as a raw material. The method comprises the following steps: 1) adding potassium acetate into methyl alcohol, and performing a reaction on sulfonate to obtain 6-methoxyl-3,5-cyclo-5alpha-pregn-20-one; 2) performing a reaction on triphenylphosphine and 1-chloro-4-methylpentane in an aprotic solvent to obtain a 4-methylbutyltriphenyl phosphonium chloride solution; 3) adding potassium tert-butoxide into the 4-methylbutyltriphenyl phosphonium chloride solution, and performing a wittig reaction; 4) under the catalysis of a rhodium catalyst, performing an asymmetric hydrogenation reaction to obtain 6-methoxyl-3,5-cyclo-5alpha-cholestane; 5) performing a catalytic hydrolysis deprotection reaction by using sulfuric acid to obtain the cholesterol. The method provided by the invention has the advantages that six-step reactions in the conventional method are simplified into four-step reactions, and a ring-opening reaction in which a great number of hydrochloric acid and a large number of zinc powder are consumed in a route of using saponin as an initial raw material. The synthesizing method is simple in process, the consumption of the raw material and auxiliary materials is low, and the mole yield is high; the method is economical and environmentally friendly, and facilitates industrial implementation.
Owner:HUNAN KEREY BIOTECH
Mass spectrometry assay for congenital adrenal hyperplasia
ActiveUS8153962B2Facilitate desorptionGood removal effectComponent separationFuel lighters11-DesoxycortisolHydrocortisone
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
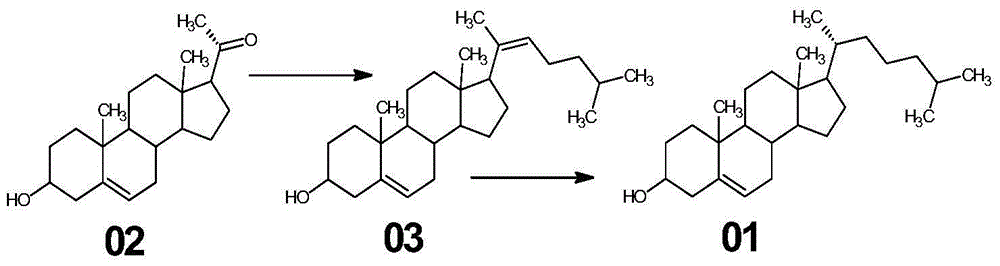
Synthetic method of cholesterol
InactiveCN104961788AReduce consumptionOmit ring opening reactionSteroidsCholesterolTriphenyl phosphonium
The invention relates to a synthetic method of cholesterol. The synthetic method of the cholesterol specifically comprises the following steps that 1, firstly, triphenylphosphine and 1-chlorine-4-methylpentane are used to react to obtain a 4-methyl pentyl triphenyl phosphonium chloride solution, then potassium tert-butoxide or sodium tert-butoxide is added to react to obtain a witting reagent, a witting reaction occurs by adding pregnenolone into the witting regent, and a compound 03 is obtained through extracting after complete reaction; 2, under the action of chirality phosphine ligand and a rhodium catalyst, an asymmetric hydrogenation reaction occurs to the compound to obtain the cholesterol. According to the synthetic method of the cholesterol, a 6-step reaction of an existing method is simplified into a 2-step reaction, and meanwhile the ring-opening reaction consuming a large amount of hydrochloric acid and zinc powder is omitted. The technology is simple, the consumption of raw materials is less, the yield is high, the cost is low, environmental protection is achieved, so that the economical effect and the environmental protection are achieved, and the industrial implementation is facilitated.
Owner:HUNAN KEREY BIOTECH
Neuroactive steroid compositions and methods of use therefor
InactiveUS20110288059A1Improve cognitive functionPromote more developedBiocideOrganic active ingredientsSchizo-affective typePain disorder
Provided are methods for ameliorating a symptom of a neuropsychiatric disorder in a subject. Also provided are methods for ameliorating at least one physical symptom or at least one psychological symptom resulting from tobacco cessation in a subject, methods for ameliorating a symptom of Alzheimer's disease or other cognitive disorder in a subject, methods for ameliorating a symptom of schizophrenia, schizoaffective disorder, or other psychotic disorder in a subject, methods for ameliorating a symptom of a depressive disorder in a subject, methods for ameliorating a symptom of bipolar disorder in a subject, methods for ameliorating a symptom of post-traumatic stress disorder or other anxiety disorder in a subject, methods for predicting a predisposition to suicide, suicidal ideation, suicidal behavior, or a combination thereof in a subject, methods for ameliorating a symptom of a pain disorder in a subject, methods for ameliorating a neurodegenerative disorder in a subject, methods for ameliorating a symptom of traumatic brain injury in a subject, methods for ameliorating a sleep disorder in a subject, and methods for improving cognitive functioning in a subject. In some embodiments, the methods include administering to a subject in need thereof an effective amount of a neuroactive steroid composition comprising pregnenolone (PG), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA), pharmaceutically acceptable salts thereof, derivatives thereof, or combinations thereof.
Owner:MARX CHRISTINE E +1
Controlled release delivery system for nasal application of neurotransmitters
ActiveUS20120009249A1Improve bioavailabilityEffective levelingBiocideOrganic active ingredientsNoseProgesterones
This invention relates to a galenical gel formulation for nasal administration of neurotransmitters / neuromodulators such as dopamine, serotonin or pregnenolone and progesterone. The special lipophilic or partly lipophilic system of the invention leads to high bioavailability of the active ingredient in plasma and brain caused by sustained serum levels and / or direct or partly direct transport from nose to the brain.
Owner:M & P PHARMA
Water-soluble pregnenolone derivative and use thereof
InactiveCN108517001AGood water solubilityFacilitate dissociationOrganic active ingredientsNervous disorderSolubilityPregnenolone
The invention relates to the technical field of biomedicines, and provides a water-soluble pregnenolone derivatives (represented by formula 1). In the formula 1, L is a bond or a linking moiety, and Wis a water-soluble group. The water-soluble pregnenolone derivative has stable chemical properties and a good water solubility, and rapidly decomposes and releases viable pregnenolone in plasma or invivo to generate pharmacological action.
Owner:JIANGSU NHWALUOKANG PHARMA RES & DEV CO LTD
Efficient Process for Preparing Steroids and Vitamin D Derivatives With the Unnatural Configuration at C20 (20 Alpha-Methyl) from Pregnenolone
InactiveUS20080171728A1High yieldHigh diastereomeric purityGroup 4/14 element organic compoundsOrganic active ingredientsPregnenoloneVitamin D+Metabolites
Disclosed herein are methods for preparing steroids and Vitamin D derivatives having the unnatural beta (usually S) configuration at C20, the methods comprising the use of compounds of the formula:wherein R is as defined herein. Also disclosed are steroids and Vitamin D derivatives made using the methods disclosed herein and pharmaceutical compositions comprising said steroids and Vitamin D derivatives.
Owner:QUATRX PHARMA
Preparation method of pregnenolone phosphate derivatives and their salts
InactiveCN104744543AImprove product qualitySolve the problem of wrapping more impuritiesSteroidsOrganic solventPhosphate
The invention relates to a method for preparing pregnenolone phosphate shown in the formula I from sodium pregnenolone phosphate as a raw material. The method utilizes a two-phase acidification crystallization method and comprises the following steps of 1, preparing an aqueous solution of a compound shown in the formula II, 2, adding a hydrophobic organic solvent into the aqueous solution to obtain a two-phase system comprising water and the organic solvent, and 3, adjusting pH of the two-phase system to less than 7 so that the compound shown in the formula I is precipitated. The product has good quality, less impurities, and solution clarification degree and color satisfying pharmacopeia requirements. The method is efficient, can remove water-soluble impurities and fat-soluble impurities by one step, improves efficiency, has simple processes, is free of reworking, realizes one-step production of the high-quality qualified product, has low energy consumption and reduces a cost.
Owner:CHONGQING HUAPONT PHARMA
Preparation method of dydrogesterone intermediate
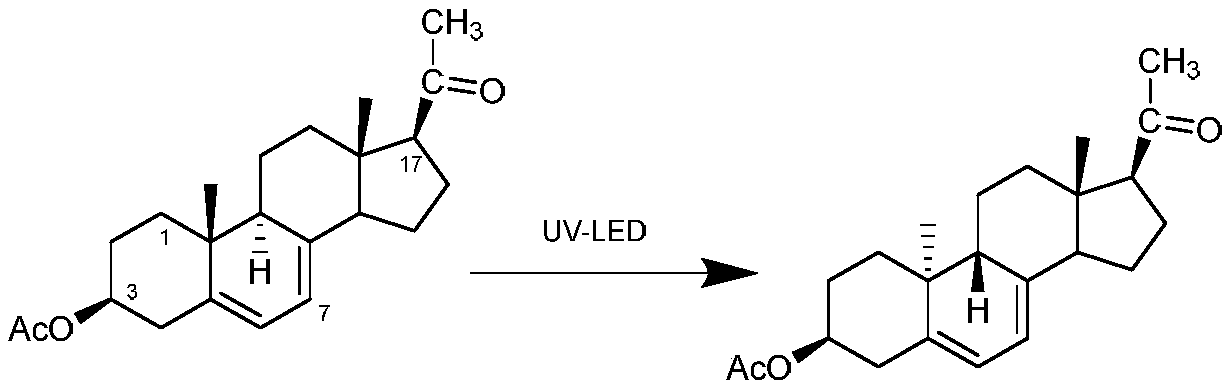
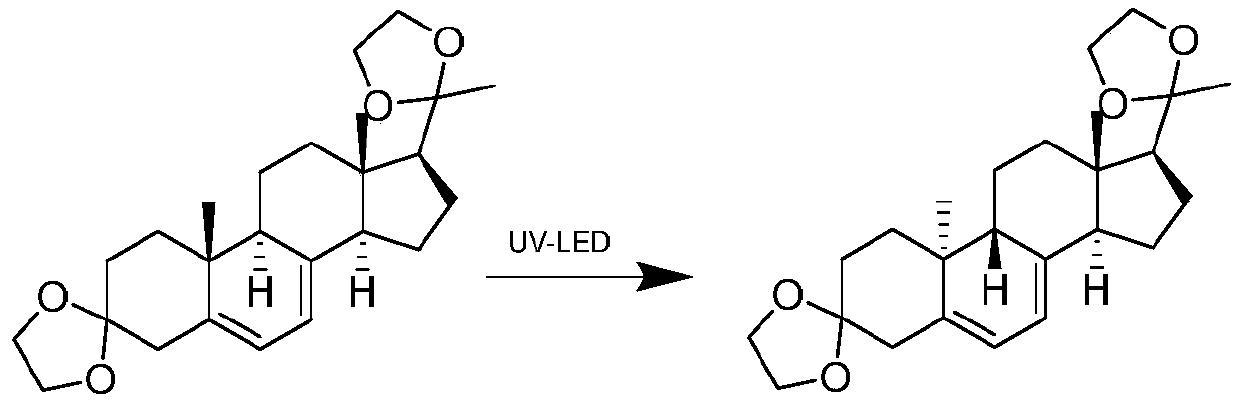
ActiveCN111171101AConcentrated luminous bandsHigh yieldChemical recyclingKetal steroidsPhotocatalytic reactionUltraviolet
The invention discloses a preparation method of a dydrogesterone intermediate. The preparation method is characterized by comprising the following steps: dissolving 5,7-diene steroid compound in an organic solvent to obtain a solution as a raw material; and carrying out a photocatalytic reaction and separating to obtain the dydrogesterone intermediate, wherein the 5,7-diene steroid compound is 7-dehydropregnenolone acetate, pregna-5,7-diene-3,20-diketodivinyl ketal, 7-dehydropregnenolone, ergosterol or pregna-5,7-diene-3,20-diketo-3-vinyl ketal, and a lamp used in the photocatalytic reaction comprises an LED ultraviolet lamp with the wavelength range of 295-335 nm. The preparation method of the dydrogesterone intermediate is high in yield, low in cost, safer in preparation and friendlier to environment.
Owner:上海璟兆实业有限公司
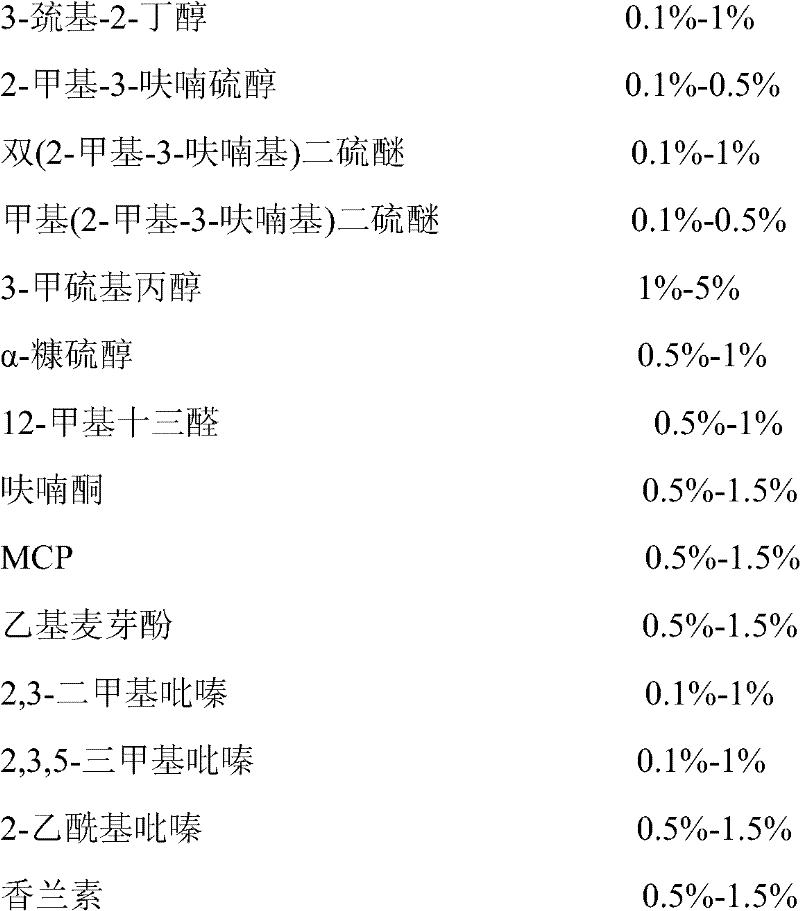
Beef oily essence and preparation method thereof
The invention relates to beef oily essence and a preparation method thereof. The essence is prepared from the following raw materials: 3-sulfydryl-2-butanol, 2-methyl-3-furanthiol, bis(2-methyl-3-furyl) disulfide, methyl(2-methyl-3-furyl) disulfide, 3-methylmercapto-propyl alcohol, alpha-furfurylmercaptan, 12-methyl tridecylic aldehyde, furanone, methylcyclopentadienyl pregnenolone (MCP), ethyl maltol, 2,3-dimethylpyrazine, 2,3,5-trimethylpyrazine, 2-acetyl pyrazine, vanillin and soybean salad oil. The invention also provides a preparation method for the essence. In an essence product prepared by the method, flavor volatile substances contained in roast beef are adopted, and a formula is formulated in a reasonable ratio, so the beef oil essence has the flavor of natural roast beef, vivid and natural fragrance, long fragrance retention and high heat stability. The beef oily essence has an irreplaceable effect in fields of seasonings and instant noodles.
Owner:TIANJIN CHUNFA BIO TECH GRP
Use of 3-methoxy-pregnenolone for the preparation of a drug for treating depressive disorders and long-term neurological diseases
ActiveUS20090143347A1Easy to synthesizePromote growthBiocideOrganic active ingredientsDiseaseNervous system
The invention relates to the therapeutic use of pregnenolone derivatives for treating depressive disorders and long-term neurological diseases.
Owner:MAPREG
Extraction and purification method of 7 Alpha, 15Alpha-dihydroxy androstene alcohol ketone
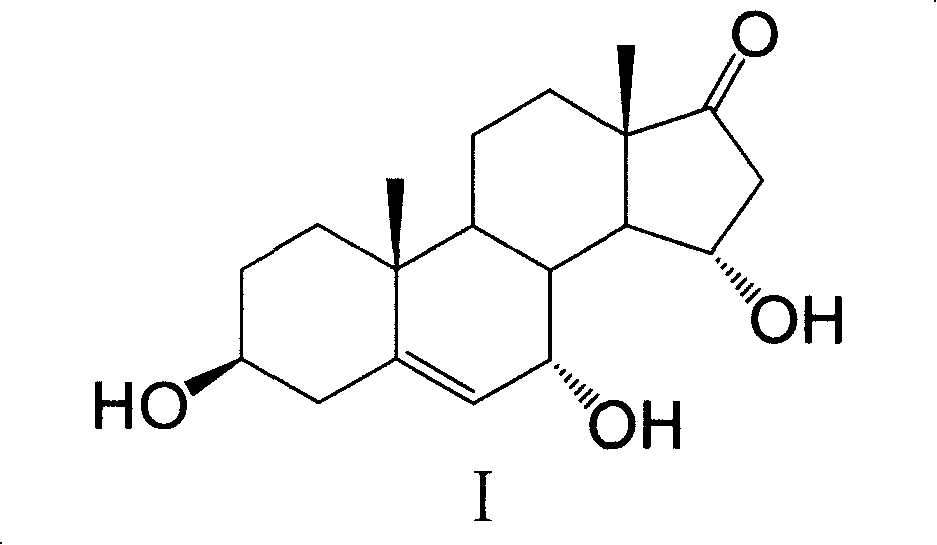
InactiveCN101182565AImprove extraction efficiencyReduce generation costMicroorganism based processesSteroidsColletotrichum liniPurification methods
The invention provides a method for extracting and purifying 7α, 15α-dihydroxyandrostenolone. The 7α, 15α-dihydroxyandrostenolone is prepared from dehydroepiandrosterone through the biological process of Colletotrichum lini AS 3.4486. obtained by transformation, after the transformation is completed, the fermentation liquid is centrifuged, and the supernatant is extracted with a non-polar organic solvent. The steps of organic solvent extraction. Using this method for extraction and purification, the yield of the product can reach nearly 90%, and the total yield of I prepared by conversion of dehydroepiandrosterone can reach 75%, thereby effectively improving the total yield of I by conversion of dehydroepiandrosterone. Yield, reducing the production cost of I.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
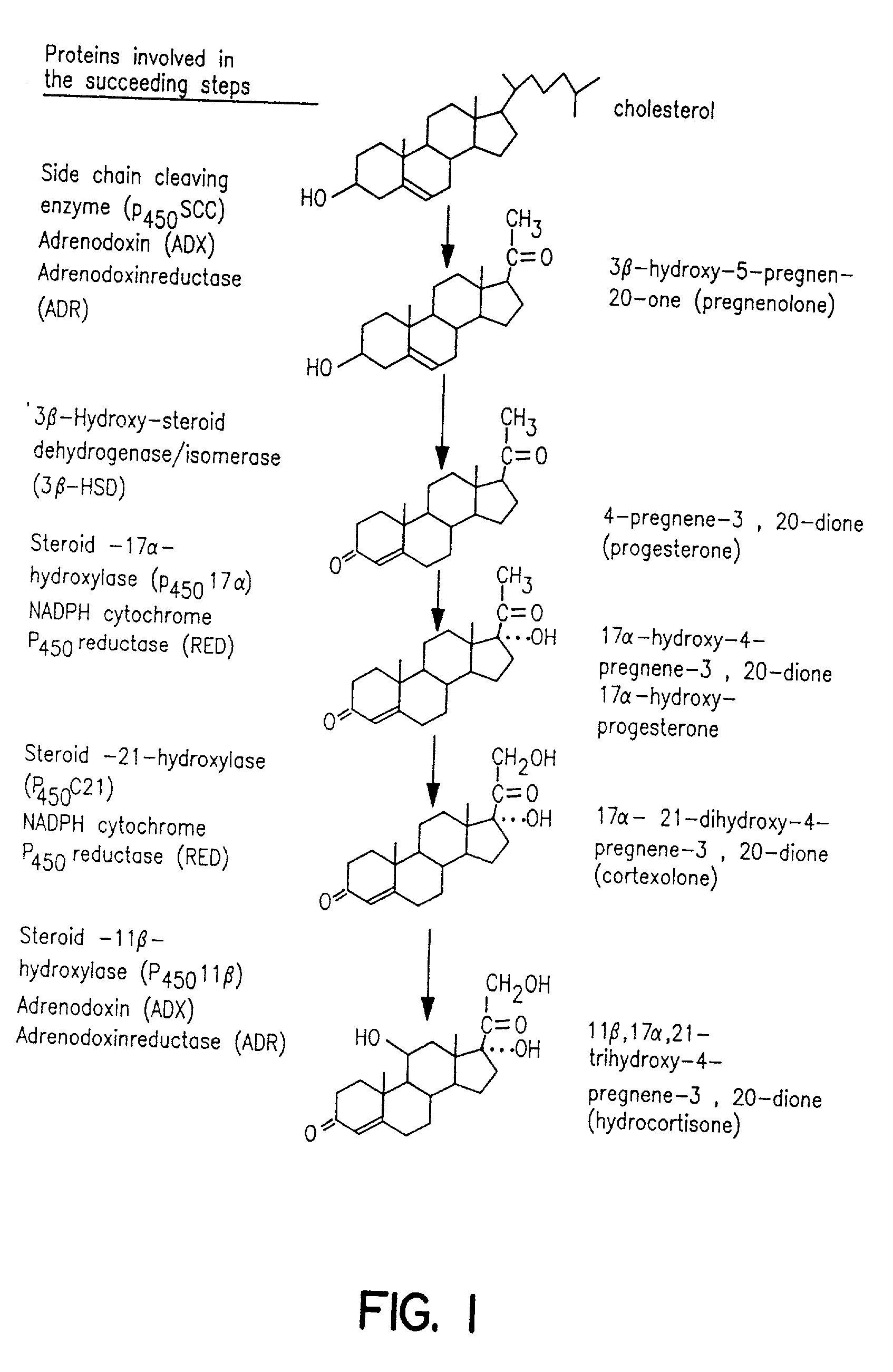
Process for oxidation of steroids and genetically engineered cells used therein
An expression cassette, operable in a recombinant host, comprising a heterologous DNA coding sequence encoding a protein, which is functional, alone or in cooperation with one or more additional proteins, of catalyzing an oxidation step in the biological pathway for conversion of cholesterol into hydrocortisone, which step is selected from the group consisting a of: the conversion of cholesterol to pregnenolone; the conversion of pregnenolone to progesterone; the conversion of progesterone to 17alpha-hydroxy-progesterone; the conversion of 17alpha-hydroxyprogesterone to cortexolone; the conversion of cortexolone to hydrocortisone, and the corresponding control sequences effective in said host.
Owner:AVENTIS PHARMA SA (US)
Method for producing pregnenolone acetic ester and 16-dehydropregnenolone acetate by utilizing infrared heating ring opening
InactiveCN102219820AGuaranteed stabilityPrecise temperature controlSteroidsTemperature controlPower flow
A method for producing pregnenolone acetic ester and 16-dehydropregnenolone acetate by utilizing infrared heating ring opening comprises the steps of ring-opening cracking, extraction and refining, wherein infrared heating is carried out on a reaction kettle during ring-opening cracking process. In the invention, as a silicon carbide far infrared heating and radiation heating device and a tank body are assembled together, the reaction kettle is heated by controlling the voltage and current of a heating device, so that temperature and voltage can be automatically controlled, power can be automatically adjusted and randomly set, the temperature control is precise, heat buffer is small, personal errors are reduced, the stability of infrared heating ring-opening reaction is ensured, and further the reaction is more sufficient.
Owner:湖北民生生物医药有限公司
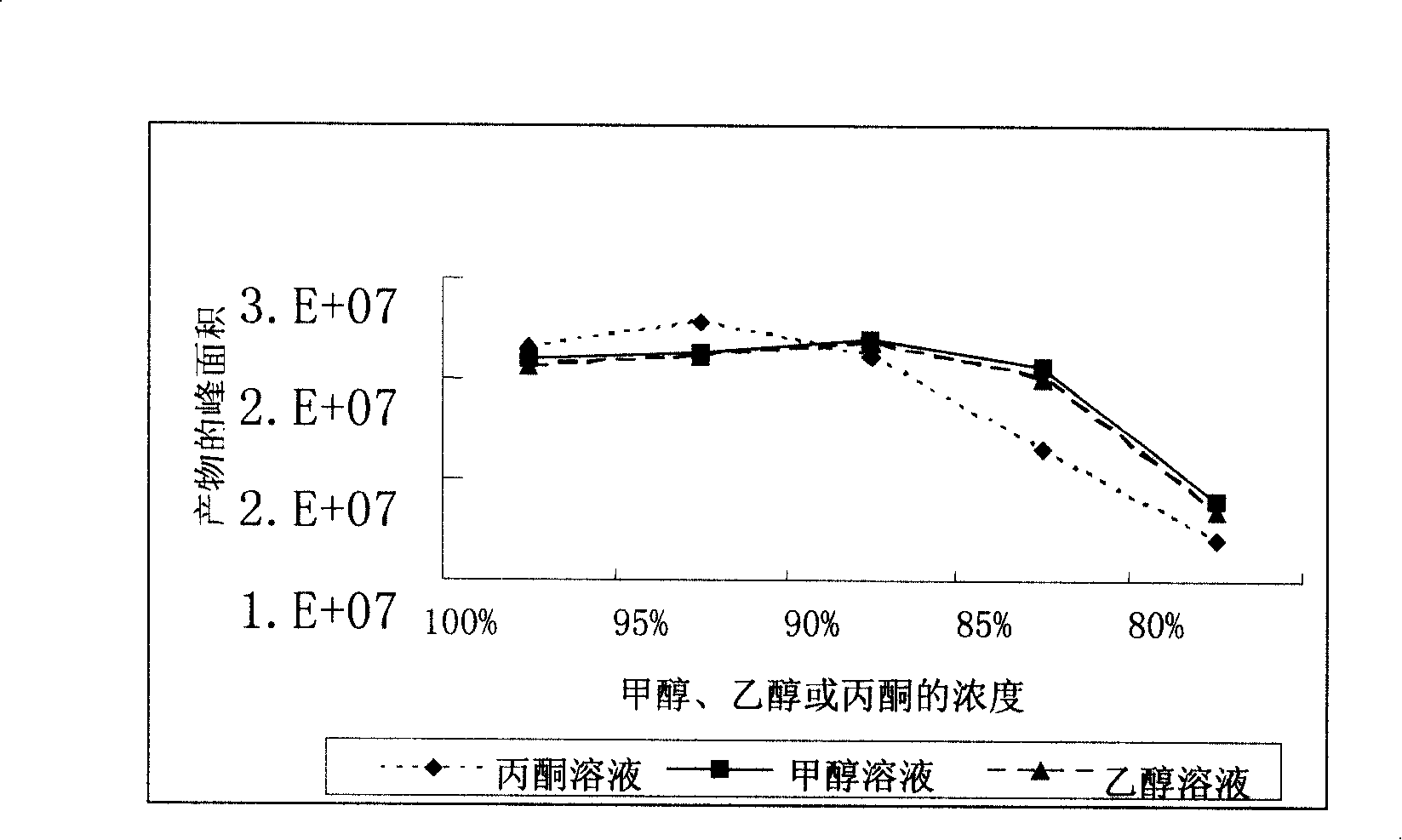
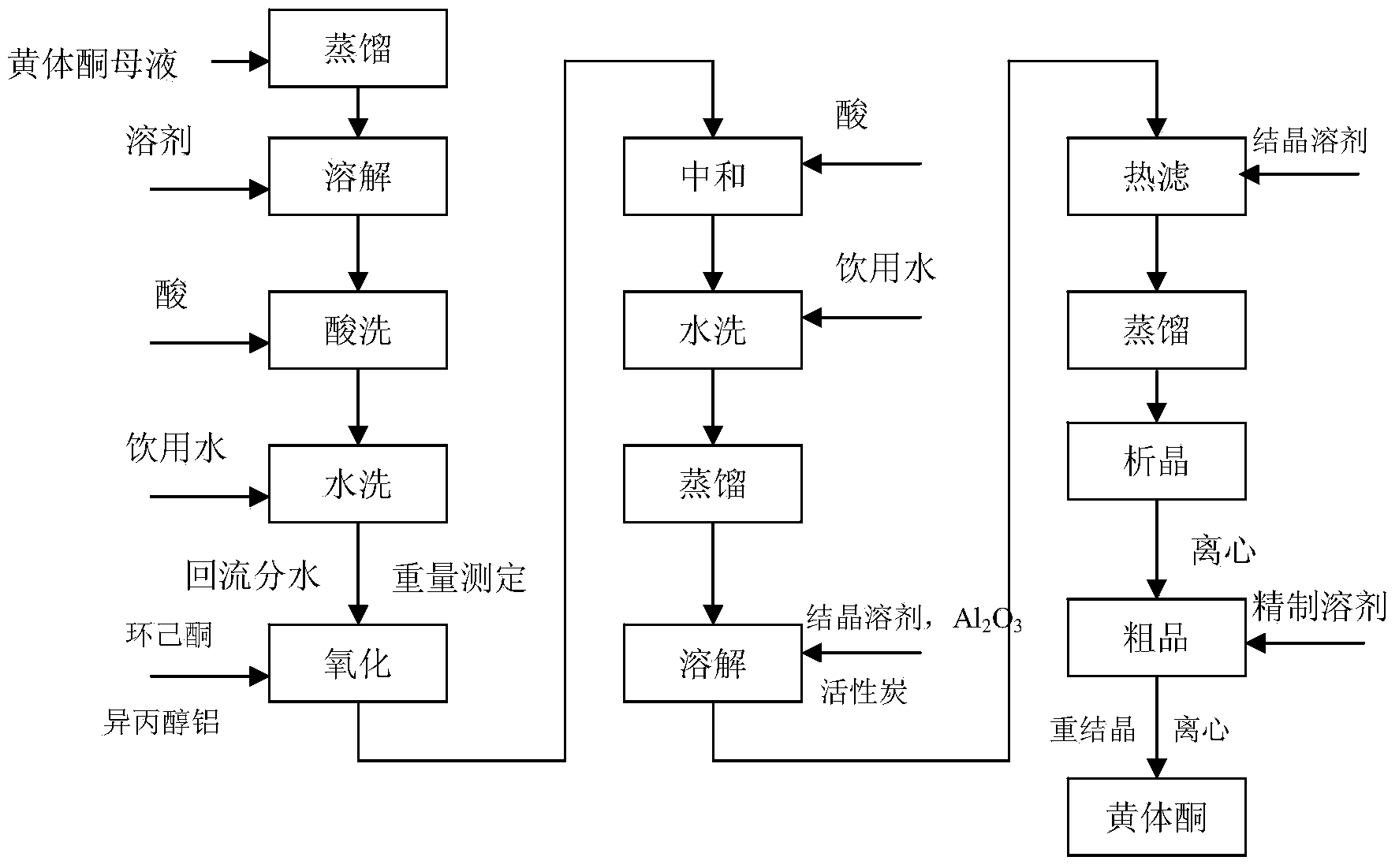
Method for recovering progesterone from progesterone production mother liquor
The invention relates to a method for recovering progesterone from a progesterone production mother liquor. The progesterone production mother liquor can be a crude product mother liquor generated in a process for producing progesterone by oxidizing pregnenolone and a refined mother liquor. The method comprises the following steps: steaming out a solvent in the mother liquor, dissolving mother liquor residues obtained after distillation in a new solvent, washing with a dilute acid, washing with water to neutrality, carrying out refluxing water diversion, detecting the weight of each of main components comprising progesterone and pregnenolone after the water diversion, oxidizing pregnenolone by cyclohexanone and aluminum isopropoxide, adding an acid to neutralize, washing with water, steaming out the new solvent, adding a crystallization solvent for crystallizing to obtain crude progesterone, and refining the crude progesterone to obtain progesterone. The method changing the progesterone mother liquor to valuables has the following advantages: progesterone in the mother liquor is recovered, so the total yield of progesterone is improved by about 4.0%, thereby the economic benefit of progesterone is improved; and the emission of dangerous wastes (containing hormone compounds) is reduced, so the pollution to the environment is reduced.
Owner:陕西汉江药业集团股份有限公司
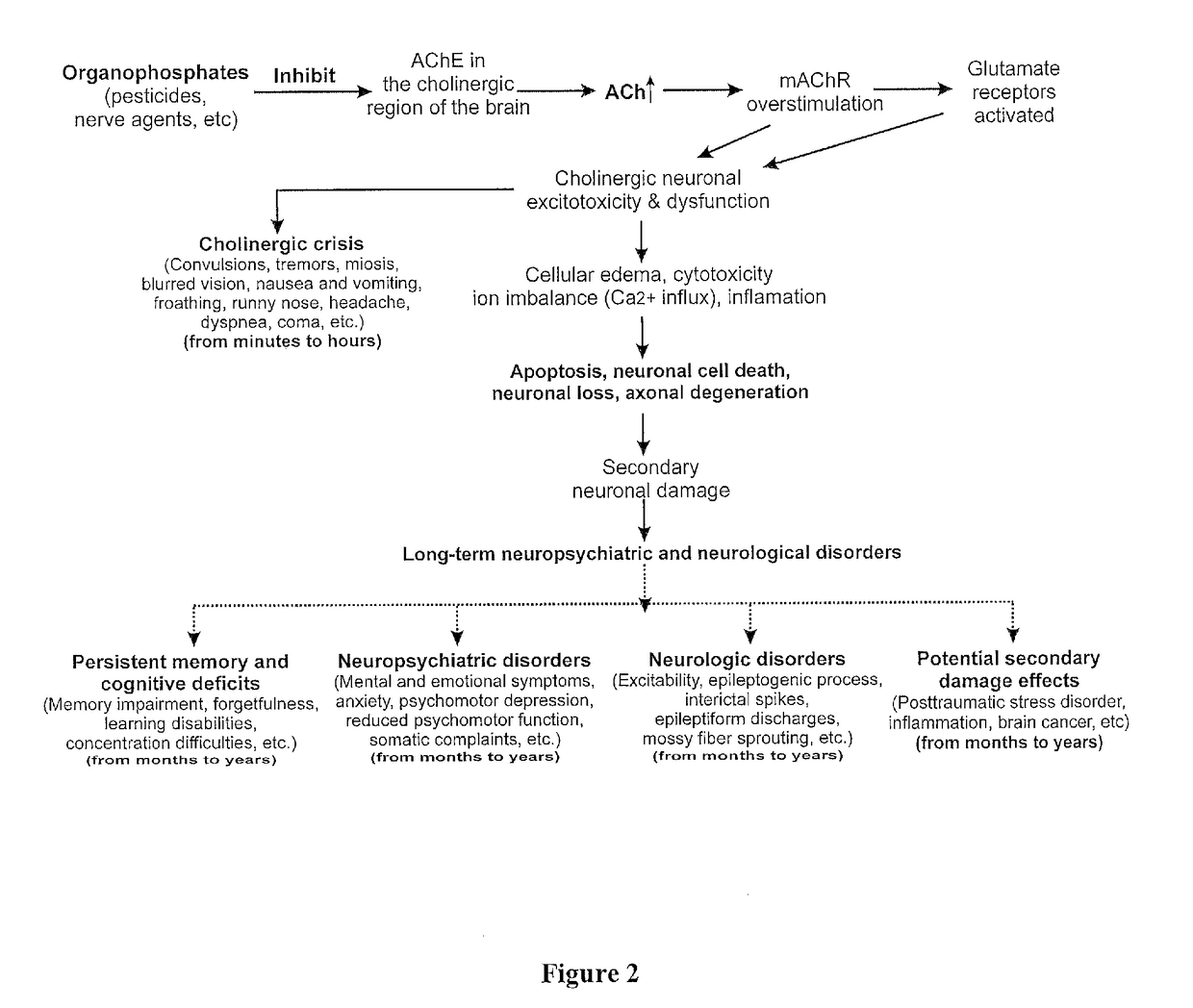
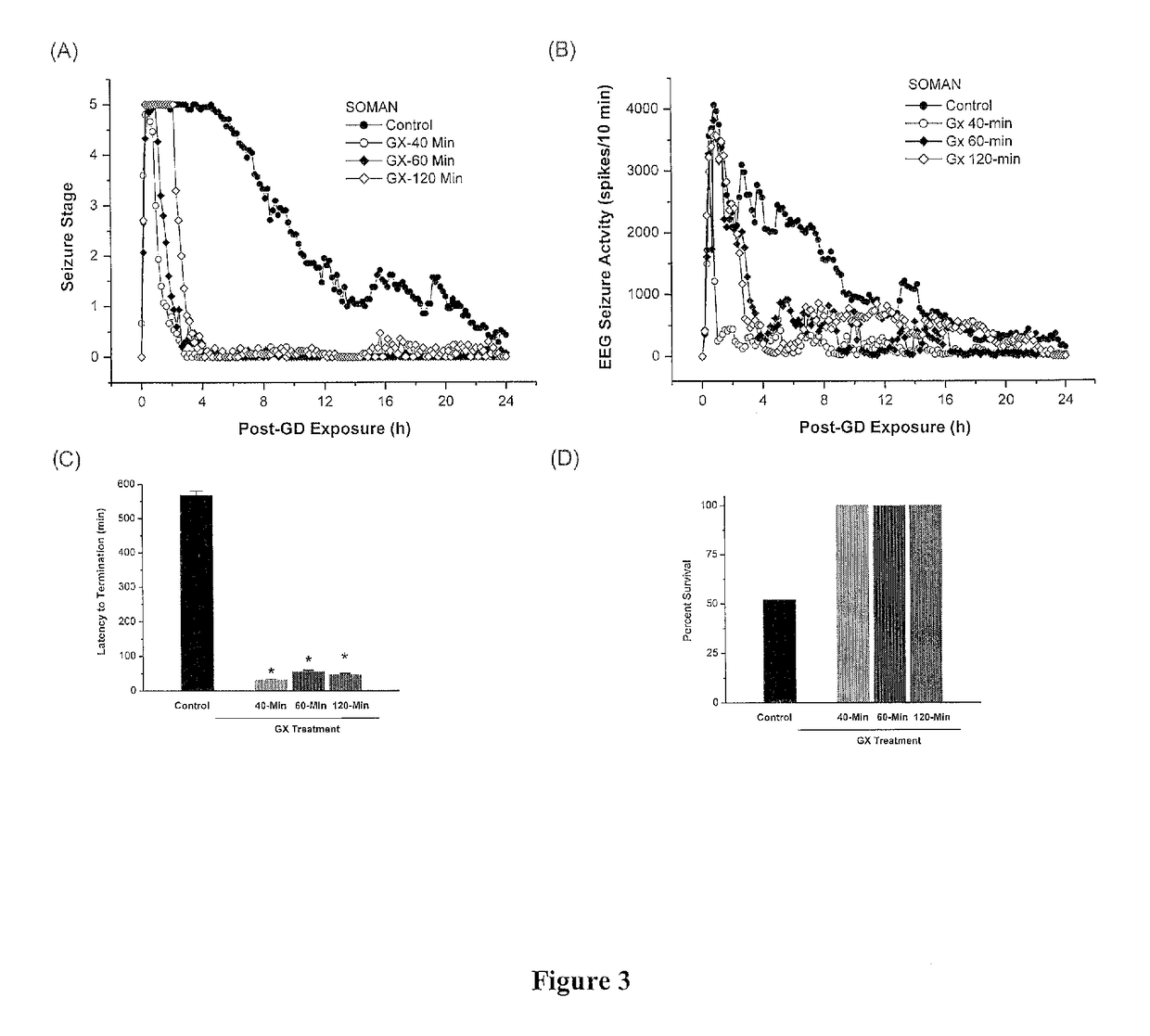
Method of treating organophosphate intoxication
ActiveUS20170246188A1Effective post-exposure treatmentPrevents subsequent neuronal injuryOrganic active ingredientsNervous disorderBenzodiazepineAntidote
The present invention provides new compositions and methods for treating and / or reversing organophosphate intoxication, manifested by both cholinergic and non-cholinergic crisis, in a mammal resulting from exposure to organophosphate compounds. The neurosteroidal compounds of this invention are those having the general structural formula of pregnane, androstane, 19-norandrostanes, and norpregnane with further moieties as defined herein. These compounds include, but are not limited to, ganaxolone, pregnanolone, and androstanediol and their analogs, salts and prodrugs. The present invention further relates to combining a therapeutically effective amount of a neurosteroidal compound with a standard organophosphate antidote (e.g. atropine, pralidoxime). The data suggests that neurosteroids are effective or more effective than benzodiazepines, whether given earlier or later than 40-min (up to several hours) after organophosphate compound exposure. Neurosteroids are effective to attenuate long-term neuropsychiatric deficits caused by organophosphate exposure.
Owner:TEXAS A&M UNIVERSITY
Enzyme protein for cutting sterol side chain and its application
Owner:MITSUBISHI RAYON CO LTD
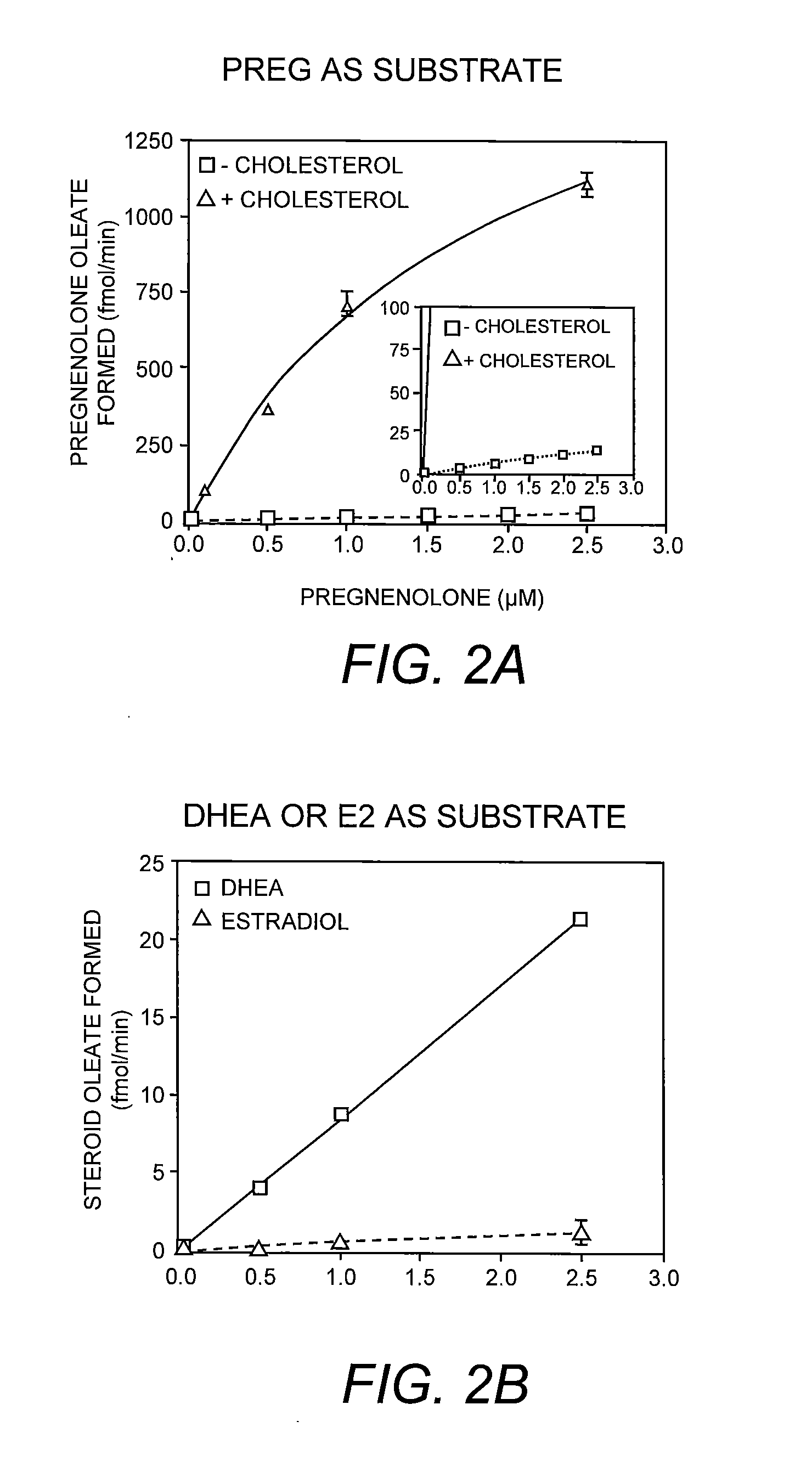
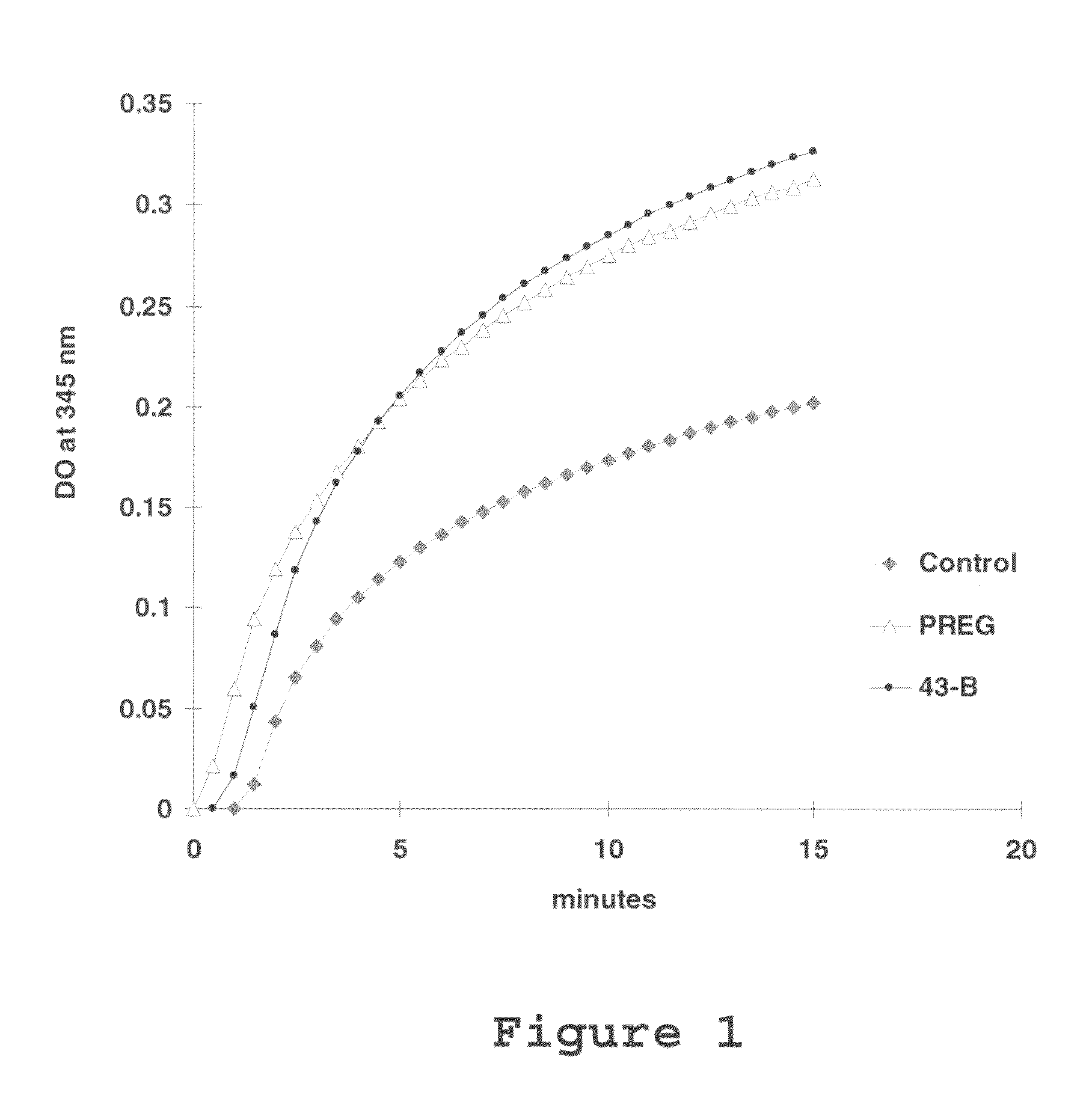
Methods for Identifying Allosteric and Other Novel Acyl-Coenzyme A:Cholesterol Acyltransferase Inhibitors
InactiveUS20130040327A1High throughput screeningCompound screeningApoptosis detectionPregnenoloneCholesterol
The present invention is a method for identifying compounds that are allosteric and / or other novel ACAT inhibitors that is based on the novel finding that pregnenolone is a substrate for ACAT; esterification of pregnenolone by ACAT is dramatically activated when cholesterol is present in the assay. The method comprises measuring the esterification of pregnenolone by ACAT under two different conditions: with cholesterol, or without cholesterol. This method can be used to test and categorize various candidate ACAT inhibitors as allosteric or other novel ACAT inhibitors, or it can be used in high-throughput screening for identifying such ACAT inhibitors.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Use of 3-methoxy-pregnenolone for the preparation of a drug for treating depressive disorders and long-term neurological diseases
ActiveUS8334278B2Faster and more long-lasting crossingCrosses the blood-brain barrier betterBiocideOrganic active ingredientsDiseaseNervous system
The invention relates to the therapeutic use of pregnenolone derivatives for treating depressive disorders and long-term neurological diseases.
Owner:MAPREG
Sterol side chain-cleaving enzyme protein and use thereof
InactiveUS20120178124A1Efficient productionEasy to getFungiSugar derivativesSterolCarbon–carbon bond
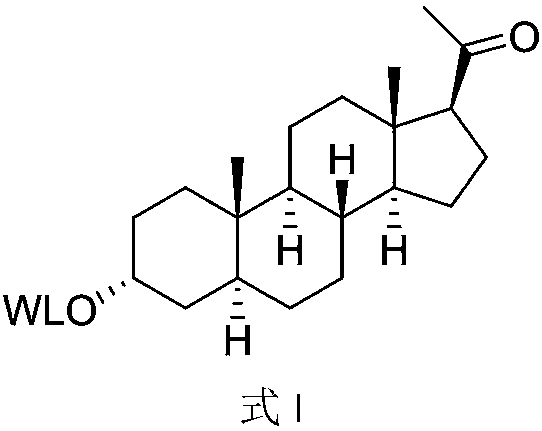
It is an object of the present invention to obtain highly active P450scc enzyme protein which is an important enzyme protein that catalyzes the first step of the biosynthesis of industrially useful steroid hormone. The present invention provides a sterol side chain cleavage enzyme protein having the following physicochemical properties:(1) action: the enzyme acts on sterol represented by formula (I) as defined in the specification and cleaves the carbon-carbon bond between positions 20 and 22 of a sterol side chain portion by its activity of cleaving the bonds, so as to generate a compound represented by formula (II) as defined in the specification;(2) substrate specificity: when microorganisms that produce the enzyme protein are allowed to react with an aqueous solution containing 100 μg / ml 4-cholesten-3-one or cholesterol at 28° C. for 5 hours, the conversion reaction rate from 4-cholesten-3-one to progesterone is 10% or more, and the conversion rate from cholesterol to pregnenolone is 10% or more;
Owner:MITSUBISHI CHEM CORP
Pyrazolyl steroid derivatives and preparation method and application thereof
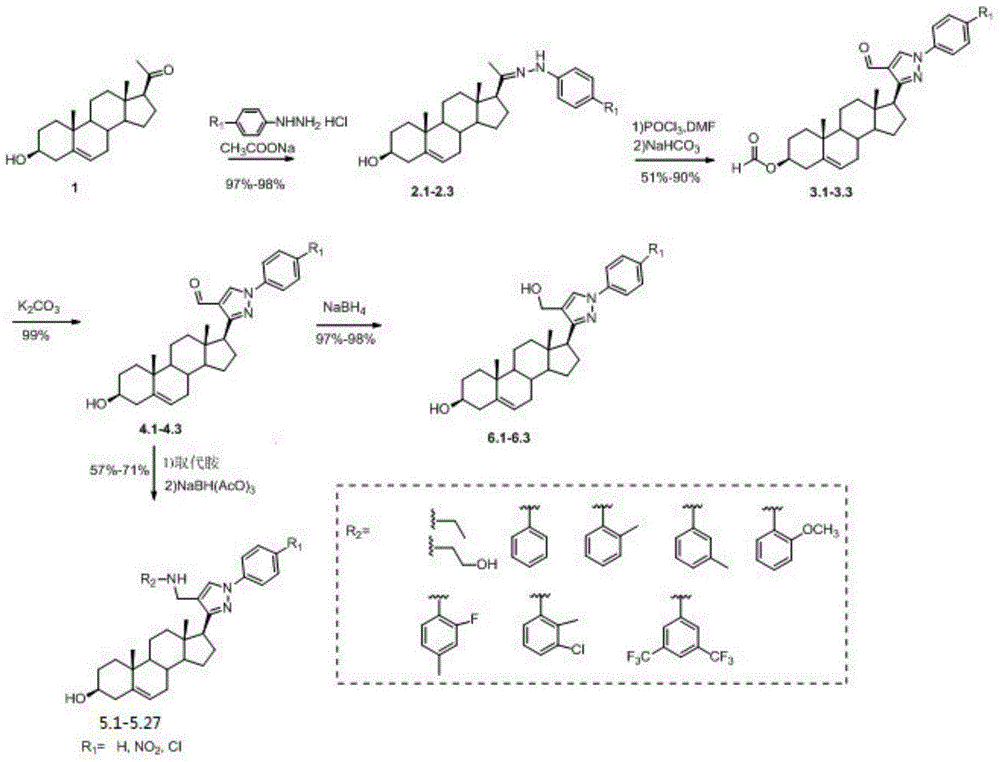
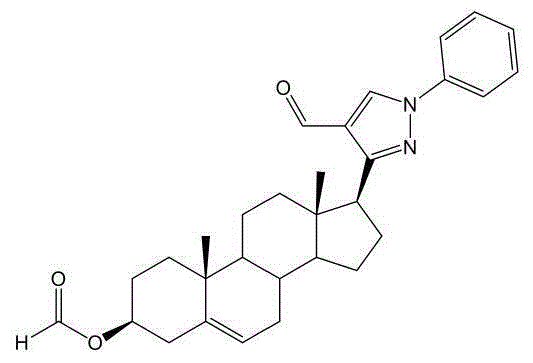
The invention discloses pyrazolyl steroid derivatives having a structure shown in the formula (I) in the specification, wherein substituent R1 can be H, nitryl or halogen, substituent R2 can be C1-C6 alkyl, hydroxy-substituted C1-C6 alkyl or monosubstituted or polysubstituted aryl. The synthesis route of the pyrazolyl steroid derivatives takes pregnenolone as a raw material; the method is used for synthesizing the pyrazolyl steroid derivatives for the first time and has the advantages of high yield, easy separation and the like. The pyrazolyl steroid derivatives show inhibitory activity for human lung adenocarcinoma cell, cervical carcinoma cell line and hepatoma carcinoma cell and have a prospect of being developed into anti-cancer drug or medicine compositions.
Owner:PESTICIDE INST XIBEI AGRI & FORESTRY TECHUNIV
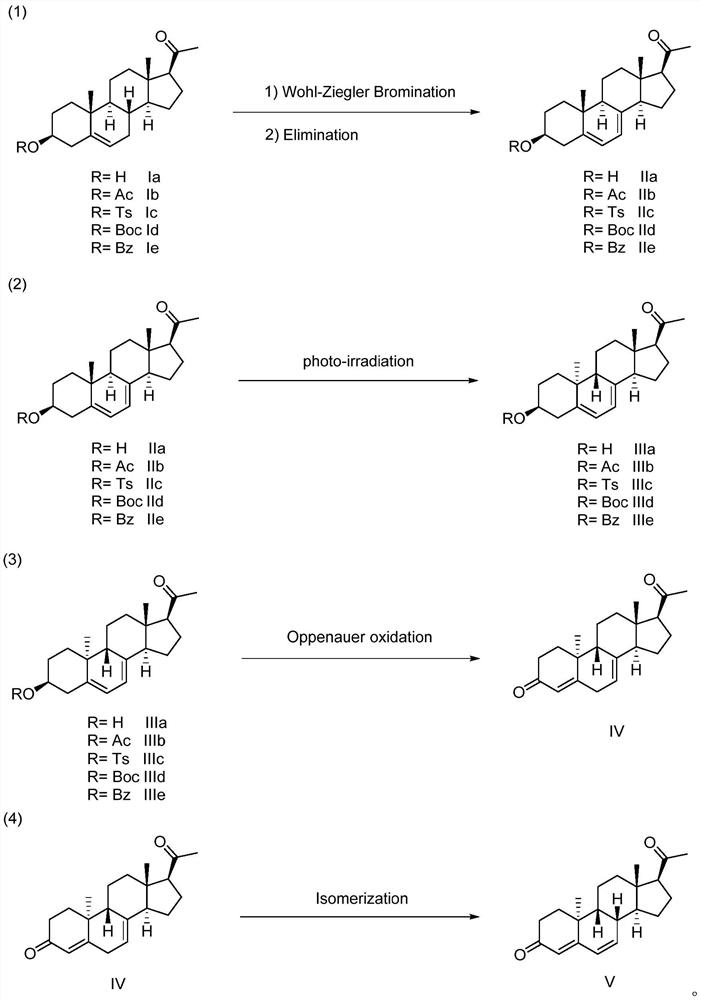
Method for synthesizing dydrogesterone
The invention relates to a method for synthesizing a steroid drug dydrogesterone (CAS: 152-62-5). The method comprises the following steps: starting from pregnenolone, carrying out allylic halogenation and elimination to obtain a Pregna-5, 7-dien-3-ol-20-one intermediate; carrying out illumination isomerization to obtain a key intermediate 9 beta, 10 alpha-Pregna-5, 7-dien-3-ol-20-one; then carrying out Oppenauer oxidation (Oppenauer oxidation) to obtain 7-Dehydro-9beta, 10 alpha-progesterone, and finally, carrying out olefin shift isomerization under the action of acid to obtain the final product 6-Dehydro-9beta, 10 alpha-progesterone (a crude drug of dydrogesterone). The method is economical in steps and comprises four conversion steps; the total yield is as high as 16.4%; the raw materials are cheap and easy to obtain, the whole process route is green and safe, the bulk drugs are high in quality, the purity can reach 99.5% or above, and the method is suitable for industrial production.
Owner:江苏诺维尔医药科技有限公司
Steroid hormone detection method
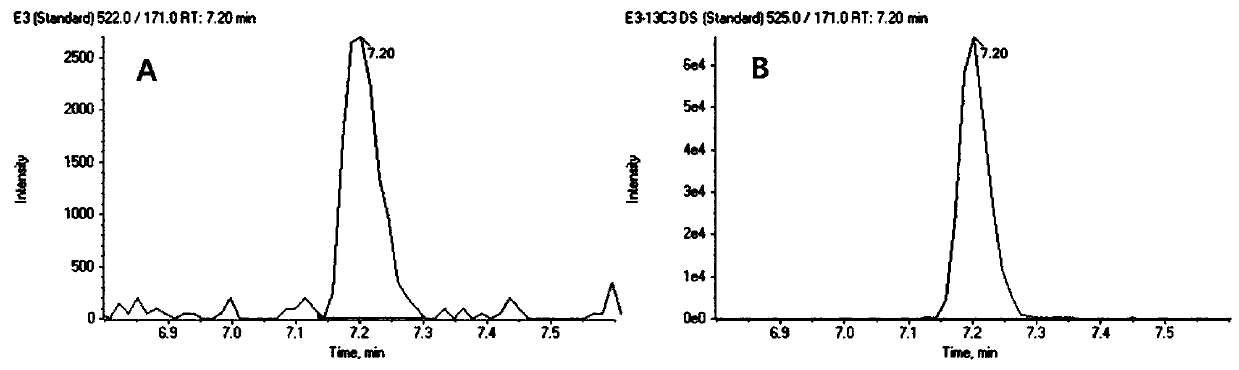
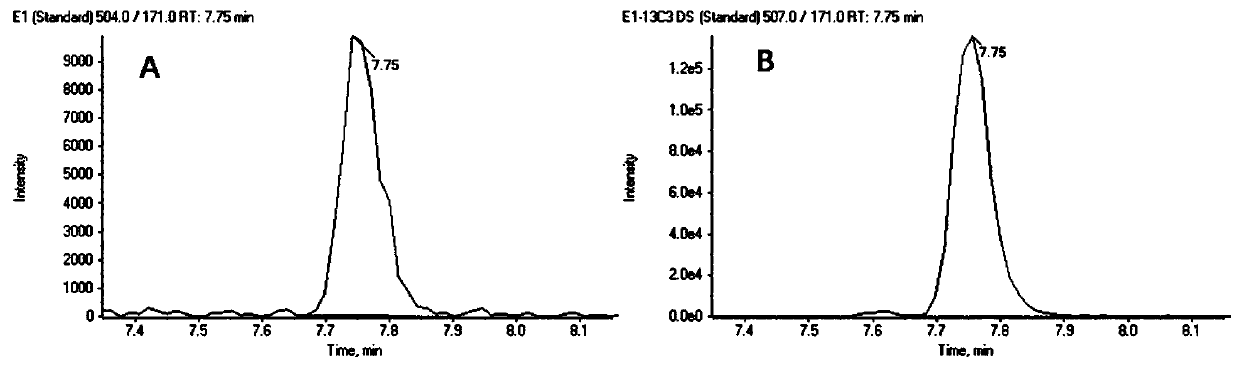
The invention discloses a steroid hormone detection method that comprises the following steps: adding an internal standard substance into a sample to be detected; carrying out a derivatization reaction; detecting an analyte by using chromatography tandem mass spectrometry; the analyte comprises a first steroid hormone and a second steroid hormone at the same time; the first steroid hormone is selected from at least one of the following steroid hormones: estradiol and estriol; and the second steroid hormone is selected from at least one of the following steroid hormones: dehydroepiandrosterone,dehydroepiandrosterone sulfate, testosterone, dihydrotestosterone, androstenedione, cortisol, cortisone, 11-deoxycortisol, 17alpha-hydroxyprogesterone, 17alpha-hydroxypregnenolone, aldosterone, cortisol, deoxycortisol, progesterone and pregnenolone. The detecting method has the advantages of few operation steps and short detection time, and can specifically, sensitively, accurately and quantitatively analyze the analyte substance.
Owner:杭州度安医学检验实验室有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com