Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
124 results about "Atropine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used before eye examinations (e.g., refraction) and to treat certain eye conditions (e.g., uveitis).
Compositions for treatment or prevention of bioterrorism
InactiveUS6991779B2Improved onsetRapid disseminationPowder deliveryBacterial antigen ingredientsDiseaseAntigen
Compositions containing biologically active molecules encapsulated in self-assembling, diketopiperazine microspheres (TECHNOSPHEREs™) and methods for making and administering such compositions are described herein. The compositions can be used to immunize individuals against agents of biological warfare. The biologically active molecules include atropine, antibodies, antigens, and antibiotics. The compositions can be placed in an inhalation device for self-administration. Pulmonary delivery of TECHNOSPHERE™ encapsulated atropine, antibodies, vaccines, and antibiotics provides an accelerated onset of immunity to the targeted disease. Furthermore, the TECHNOSPHERE™ encapsulated atropine, antibodies, vaccines, and antibiotics are stable formulations, suitable for stockpiling, rapid dissemination and mass treatment.
Owner:MANNKIND CORP
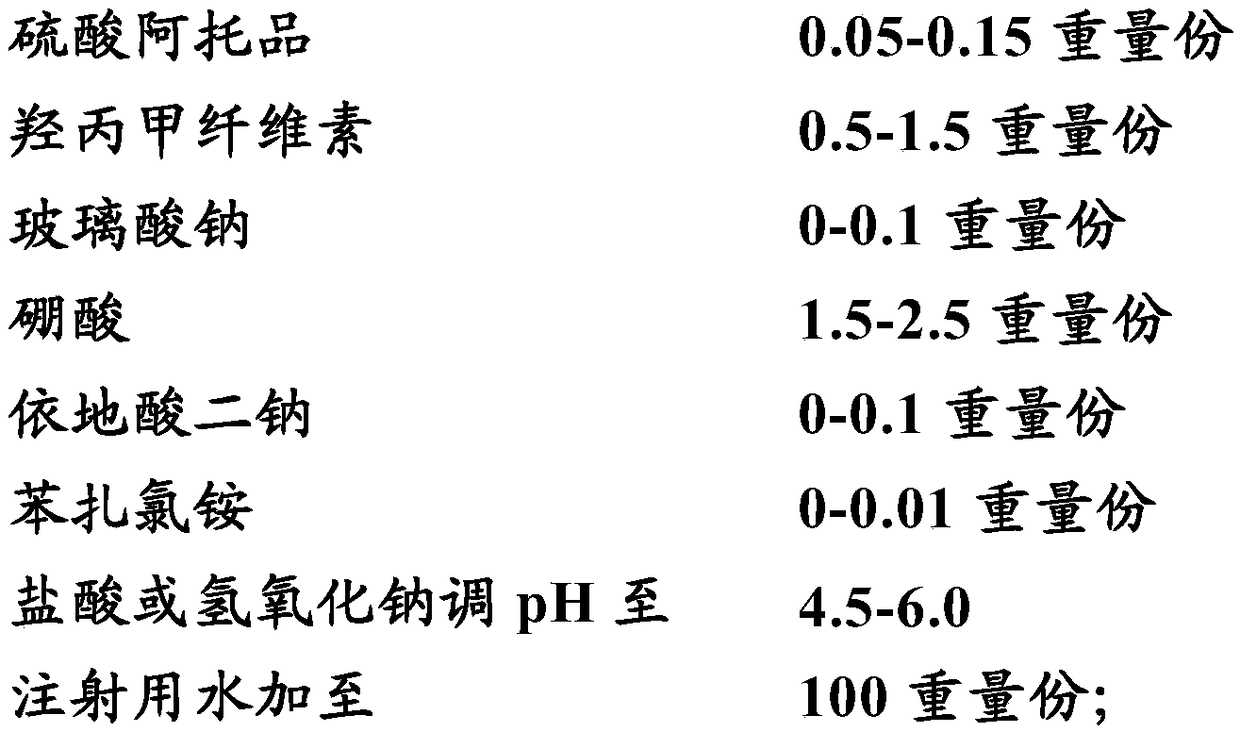
Low-concentration atropine solution for preventing myopia progression and preparing method thereof
A low-concentration atropine solution for preventing myopia progression contains an atropine concentration less than 0.1% (w / w). Preferably, the atropine concentration is 0.05% (w / w) in optimal situation. The low-concentration atropine solution in treatment causes less photophobia and systemic side-effects to patients and has excellent compliance to reduce damages from ultraviolet and hazard blue light and to avoid visual morbidities such as cataract and retinal macula lutea deterioration.
Owner:CHANG GUNG MEMORIAL HOSPITAL
Lens incorporating myopia control optics and muscarinic agents
ActiveUS20140036225A1Increase acceptanceIncreased myopia control treatment outcomeSpectales/gogglesSenses disorderAtropine sulphate monohydrateMuscarinic Agents
Ophthalmic devices, such as contact lenses, may incorporate myopia control optics in combination with therapeutic agents also known to control myopia to create a drug delivery mechanism to inhibit or arrest the progression of myopia in individuals. Any number of contact lenses incorporating myopia control optics may be combined with a therapeutic agent such as atropine, atropine sulphate monohydrate, and / or pirenzepine
Owner:JOHNSON & JOHNSON VISION CARE INC
Buccal, polar and non-polar spray containing atropine
Buccal aerosol sprays or capsules using polar and non-polar solvent have now been developed which provide atropine for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, atropine, and optional taste mask and / or flavoring agent; formulation II: aqueous polar solvent, atropine, optionally flavoring agent, and propellant; formulation III: non-polar solvent, atropine, and optional flavoring agent; and formulation IV: non-polar solvent, atropine, optional flavoring agent, and propellant; formulation V: a mixture of a polar and a non-polar solvent, atropine, and optional flavoring agent; formulation VI: a mixture of a polar and a non-polar solvent, atropine, optional flavoring agent, and propellant.
Owner:NOVADEL PHARMA
Targeted liposomes
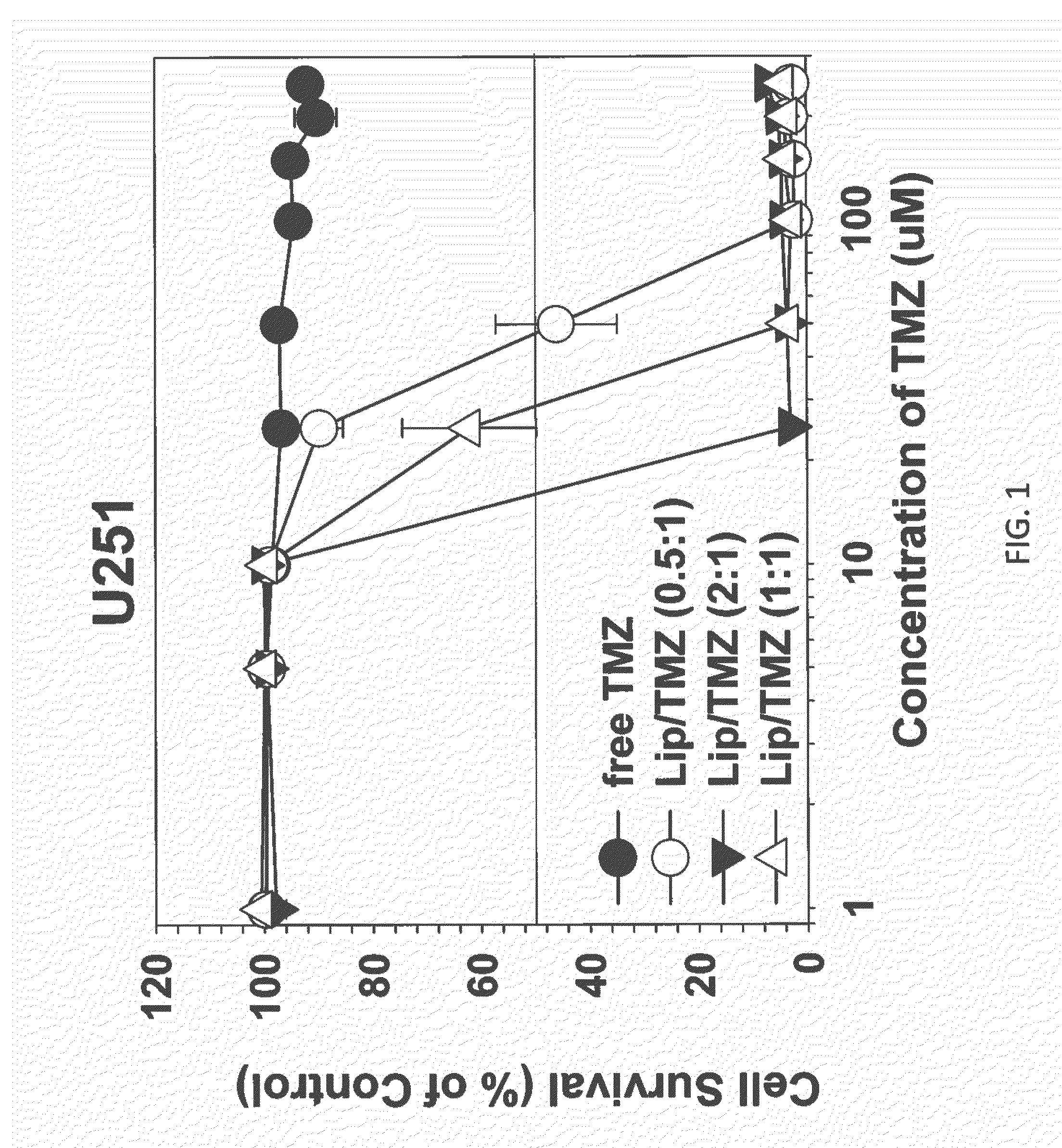
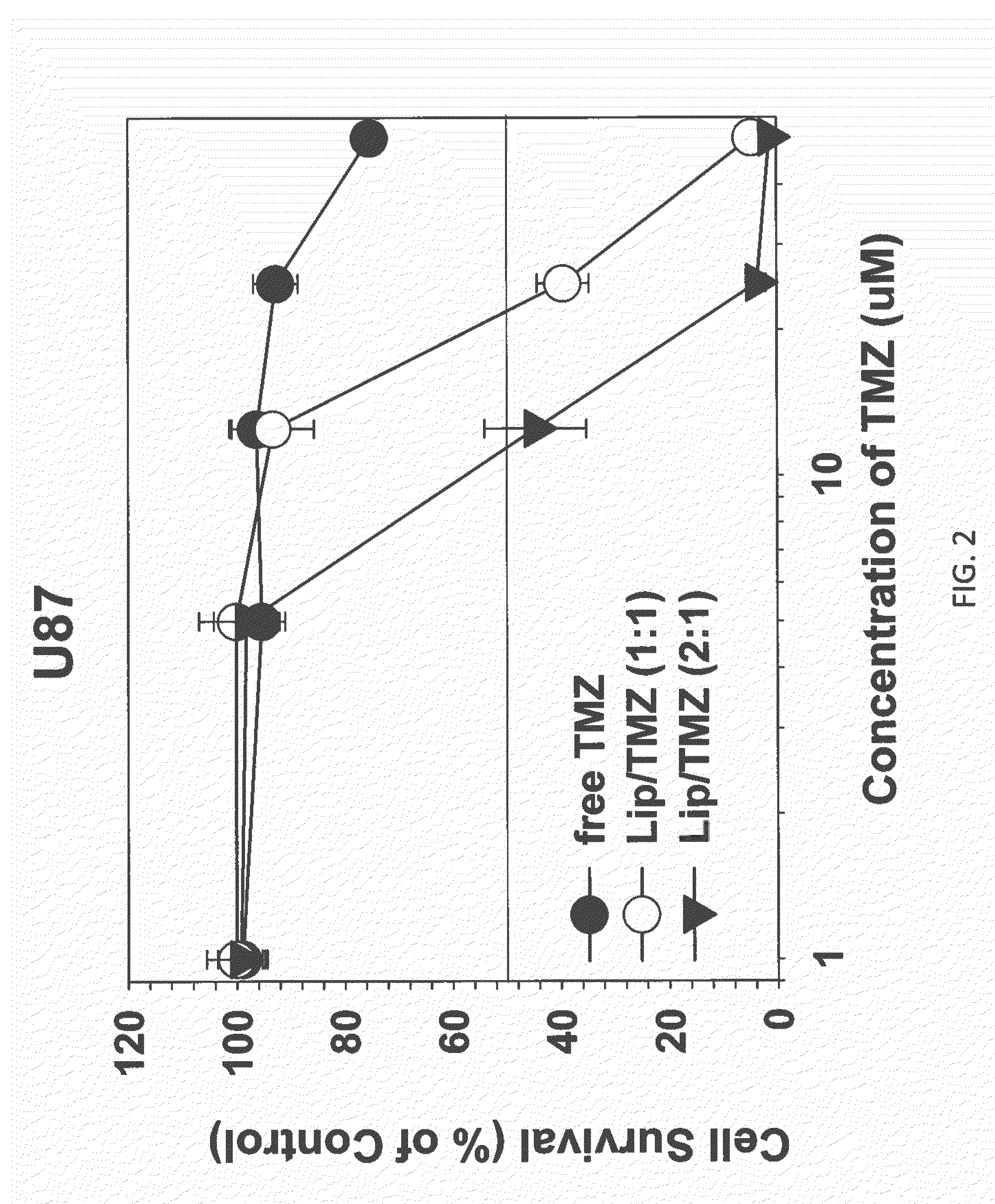
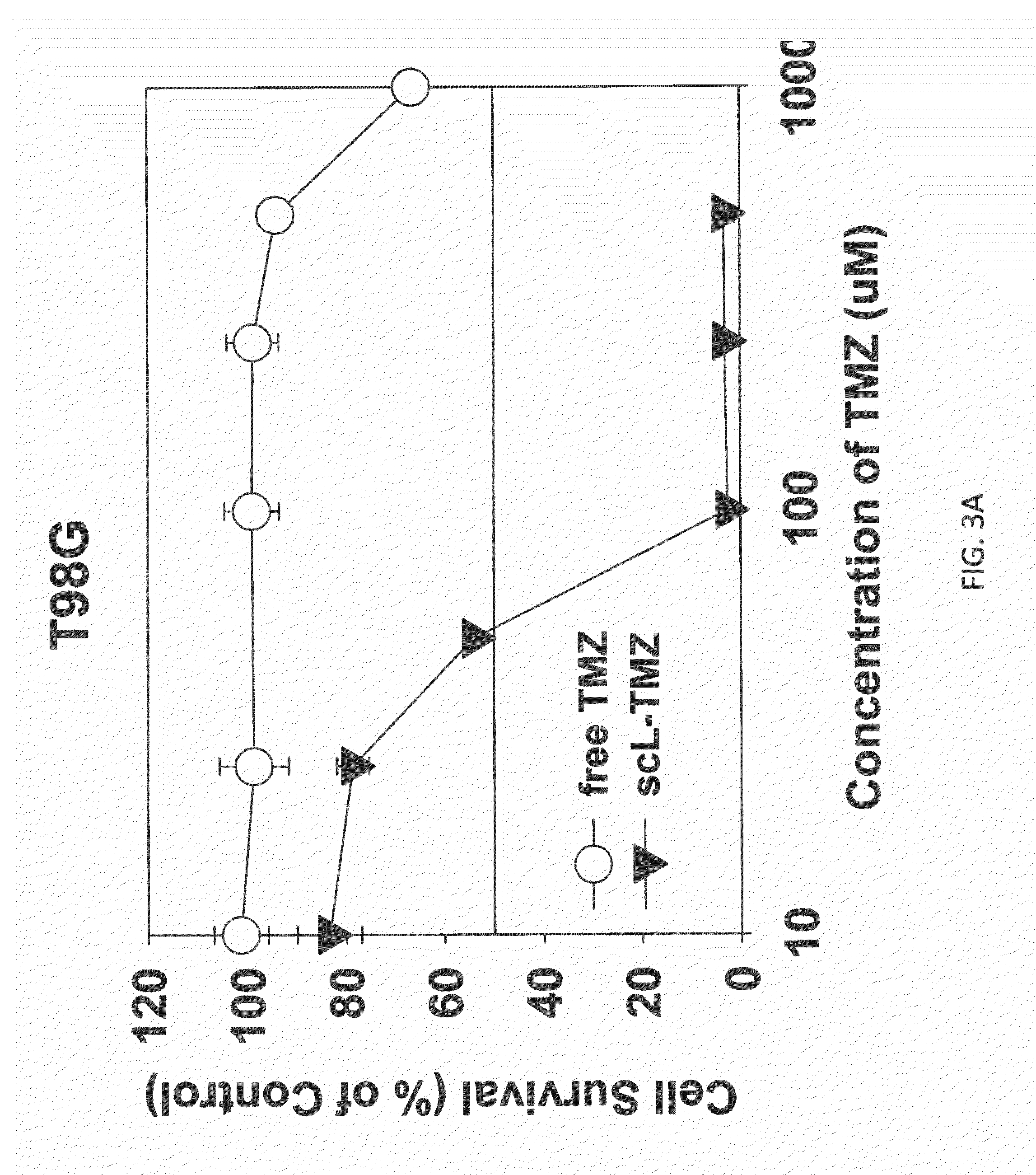
The present invention is in the field of drug delivery, and specifically, cationic liposome-based drug delivery. In embodiments, this invention provides methods of making ligand-targeted (e.g., antibody- or antibody fragment-targeted) liposomes useful for the delivery of liposomes to tumors, including brain tumors. In embodiments, the liposomes deliver temozolomide across the blood-brain barrier for treatment of primary or metastatic brain tumors. Additional cancers that can be treated with the liposomes include neuroendocrine tumors, melanoma, prostate, head and neck, ovarian, lung, liver, kidney, breast, urogenital, gastric, colorectal, cervical, vaginal, angiosarcoma, liposarcoma, rhabdomyosarcoma, choriocarcinoma, pancreatic, retinoblastoma and other types of cancer. In another embodiment the liposomes deliver melphalan for the treatment of multiple myeloma, other tumors of the blood or other solid tumors. In still other embodiments the liposomes can deliver other drugs such as pemetrexed or irinotecan for treatment of cancer or drugs including atropine for treatment of organophosphate poisoning.
Owner:GEORGETOWN UNIV
Low-concentration atropine medicine-containing eye drop and preparation method thereof
InactiveCN107456440AImprove securityImprove efficacySenses disorderHydroxy compound active ingredientsAlkalinityMicroorganism
A low-concentration atropine medicine-containing eye drop comprises the following components: (A) 0.001-1% (w / v) of an atropine medicine, (B) 0-10% (w / v) of a solute with the effects of lubricating, solubilizing and moisturizing, (C) an acidity and alkalinity regulating agent of which the amount is proper to ensure that the pH is regulated to 6.0-8.0, (D) an osmotic pressure buffering agent of which the amount is proper to ensure that the osmotic pressure is regulated to 280-380 mOsm / L, (E) a thickener of which the amount is proper to ensure that the viscosity is regulated to 6-10 mps, (F) 0.001-1% (w / v) of a solute with an atropine medicine stabilizing effect, (G) 0-0.1% (w / v) of a bacteriostatic agent, 0-0.1% (w / v) of one or more of mint, natural borneol, vitamin B6, dipotassium glycyrrhizinate or other substances with auxiliary effects which can be also optionally added under the premise that the safety is ensured and the efficacy of the main medicine is not affected, and the balance of water. The eye drop is good in stability of the main medicine and safe, is unlikely to be contaminated by microorganisms, and can effectively prevent and relieve juvenile myopia.
Owner:杭州赫尔斯科技有限公司
Atropine eyewater in low concentration for restraining increase of degree of short sight and preparing method
InactiveCN101049287AImprove pupil dilationImprove photophobia and other side effectsSenses disorderPharmaceutical delivery mechanismMedicineUltraviolet
Owner:CHANG GUNG MEMORIAL HOSPITAL
Lens incorporating myopia control optics and muscarinic agents
ActiveUS9827250B2Increase acceptanceIncreased myopia control treatment outcomeSpectales/gogglesSenses disorderAtropine sulphate monohydrateOphthalmology
Ophthalmic devices, such as contact lenses, may incorporate myopia control optics in combination with therapeutic agents also known to control myopia to create a drug delivery mechanism to inhibit or arrest the progression of myopia in individuals. Any number of contact lenses incorporating myopia control optics may be combined with a therapeutic agent such as atropine, atropine sulphate monohydrate, and / or pirenzepine.
Owner:JOHNSON & JOHNSON VISION CARE INC
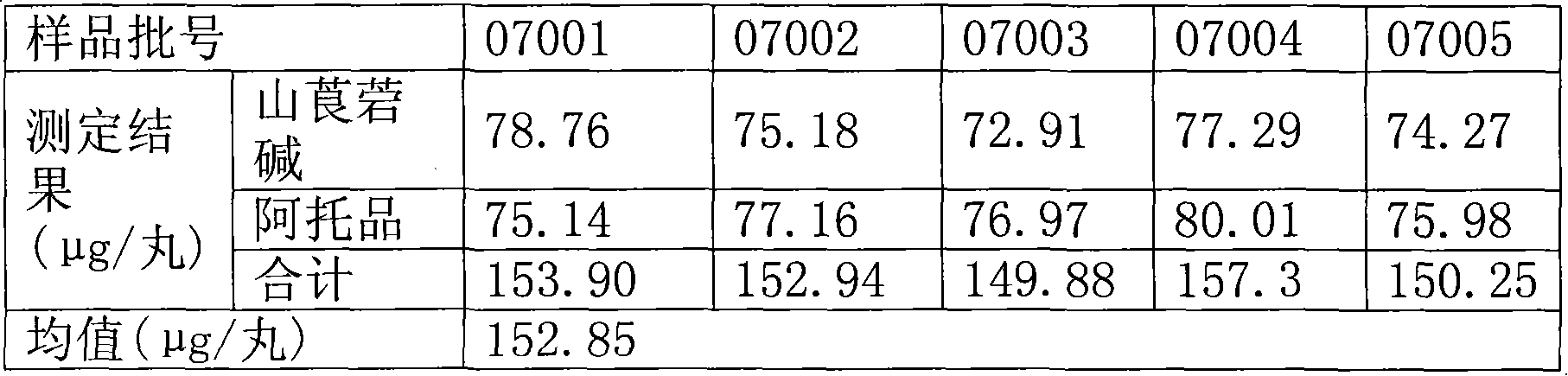
Quality standard of Sinopanax formosanus pills
InactiveCN101618119AAdd TLC DiscriminationEasy to operateComponent separationPill deliveryTechnical standardAlkaloid
The invention relates to a quality standard of Sinopanax formosanus pills. In the quality standard, the physical and chemical distinction with poor specificity in the prior standard is deleted, thin-layer identification of scopoletin is added, a method for determining the content of alkaloid by acid dye colorimetry in the prior standard is cancelled, and the contents of the scopoletin and atropine are determined by liquid chromatography. The method for determining the contents of the atropine and the scopoletin has the advantages that: the operation step is simple; the operation time is shortened compared with the prior art, and the detection time for a batch of products is generally about 2 hours; in the whole operation process, manual operation is less, the error resulted from individual difference is decreased to the lowest degree, and the data is completely viewed and processed by computer software; and the method is accurate, reliable, easy in operation and good in reproduction. The quality standard can be used in the daily quality control of the Sinopanax formosanus pills to further guarantee the inner quality of the products and have great meaning for guaranteeing the safe administration to patients.
Owner:津药达仁堂集团股份有限公司第六中药厂
Medicament for treating tumor and hemorrhoid and method for producing the same
InactiveCN101485669ANo painNo sequelaeHeavy metal active ingredientsHydroxy compound active ingredientsBone Marrow Stem CellTreatment period
The invention relates to a medicine for treating tumor and hemorrhoid and a manufacturing method thereof. The medicine aims at the disadvantages that: the prior treatment method for removing the hemorrhoid and the tumor can only kill or remove partial tumor cells, has large pain and high recurrence rate during the treatment period, has the phenomena of lassitude, hypodynamia, nausea, vomiting and hemogram reduction during the radiotherapy period, causes low immunity, damages self-immunologic function of a human body, inhibits functions of bone marrow stem cells, is easy to produce drug dependence, and is easy for recurrence. The invention prepares tannic acid, atropine, gallic acid, quinin hydrochloride, ferrous sulfate, caffeine, pure sulfuric acid, urethane, carbolic acid, sterile water and the like, into medicines for local parts of pathological changes, does not hurt normal tissues, has no pain, does not need to treat for a long time, and has the advantages of taking effect by injecting at one time, no recurrence, wide raw materials, low cost, wide clinical application, and small side effect.
Owner:秦克骏
Atropine pharmaceutical compositions
ActiveUS20190209545A1Good storage stabilitySenses disorderInorganic non-active ingredientsPreservative freePreservative
The inventive subject matter is directed to compositions and methods for sterile and storage stable low-dose atropine formulations with improved stability. Most preferably, the compositions presented herein are substantially preservative free and exhibit less than 0.35% tropic acid from degradation of atropine. Advantageously, contemplated formulations are also substantially free of preservatives.
Owner:VYLUMA INC
Compound low-concentration atropine eye drops and preparation method thereof
ActiveCN109091675ASafe and effective prevention and treatment of myopiaPrevention and treatment of myopiaSenses disorderHydroxy compound active ingredientsDiseaseSide effect
The invention discloses compound low-concentration atropine eye drops and a preparation method thereof. The eye drops are mainly prepared from low-concentration atropine, lutein or a lutein derivative, fructus lycii, taurine, liquid pearl and multiple vitamins (A, B1, B6, B12 and E) by processing in a certain ratio. Drugs used in the compound eye drops conform to Chinese pharmacopoeia and United States Pharmacopeia, by means of functional collaborative treatment synergy of various natural drug active factors and atropine for vision, dose of atropine is reduced effectively, side effects on human eyes are reduced, ciliary muscle spasm can be eliminated, eyeball nutrition can be improved, asthenopia can be relieved, the eye drops have effects of improving eyesight and clearing away heat and toxic materials and have the capacity of enhancing human eye immunity and resisting eye diseases, the effect of preventing and treating myopia of adolescents and children is enhanced effectively, and the eye drops are high in safety, long in shelf life and good in biocompatibility with eye environment and has positive promotion and application values.
Owner:上海阿尔福斯医药科技有限公司
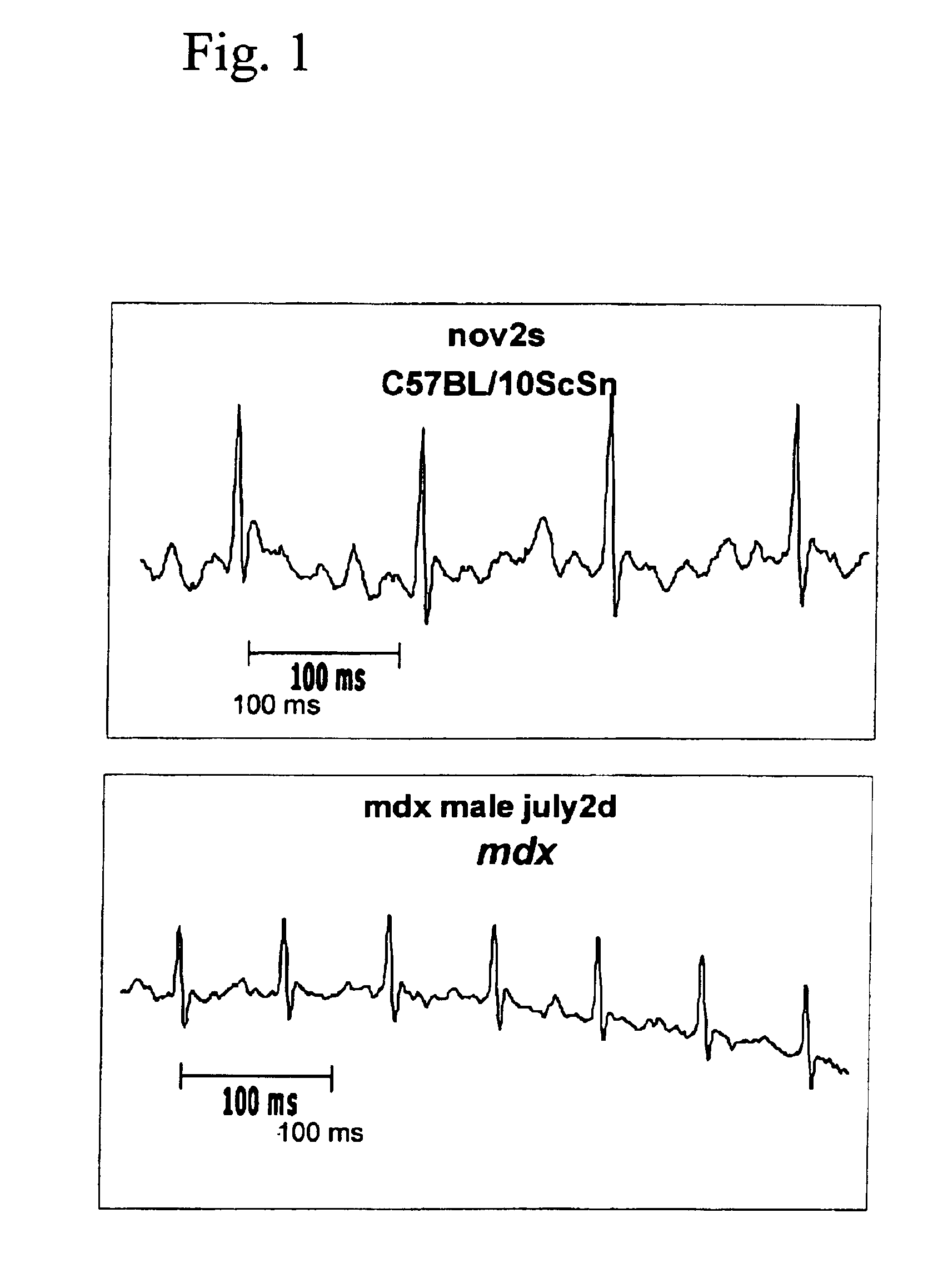
Method of early detection of Duchenne muscular dystrophy and other neuromuscular disease
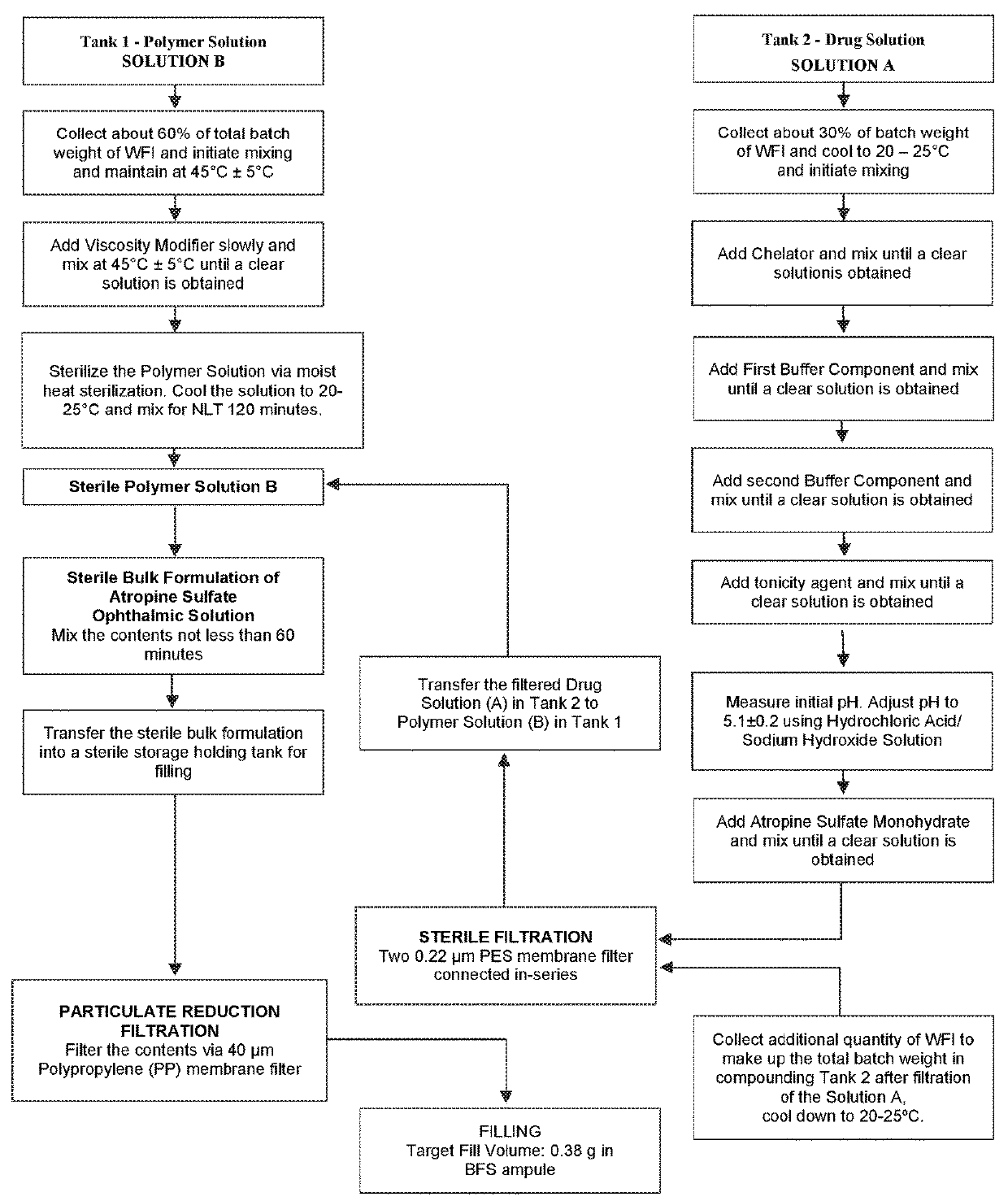
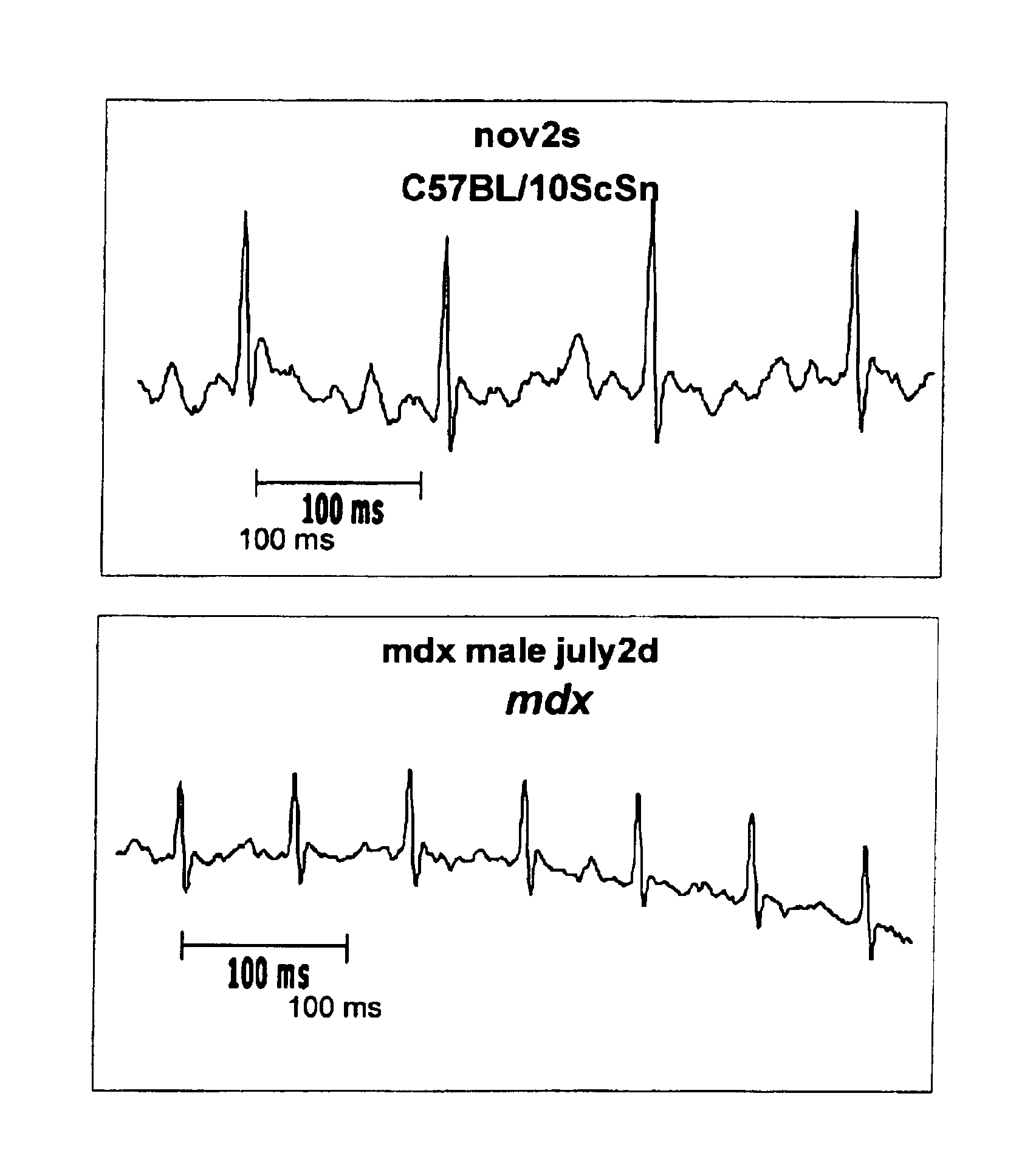
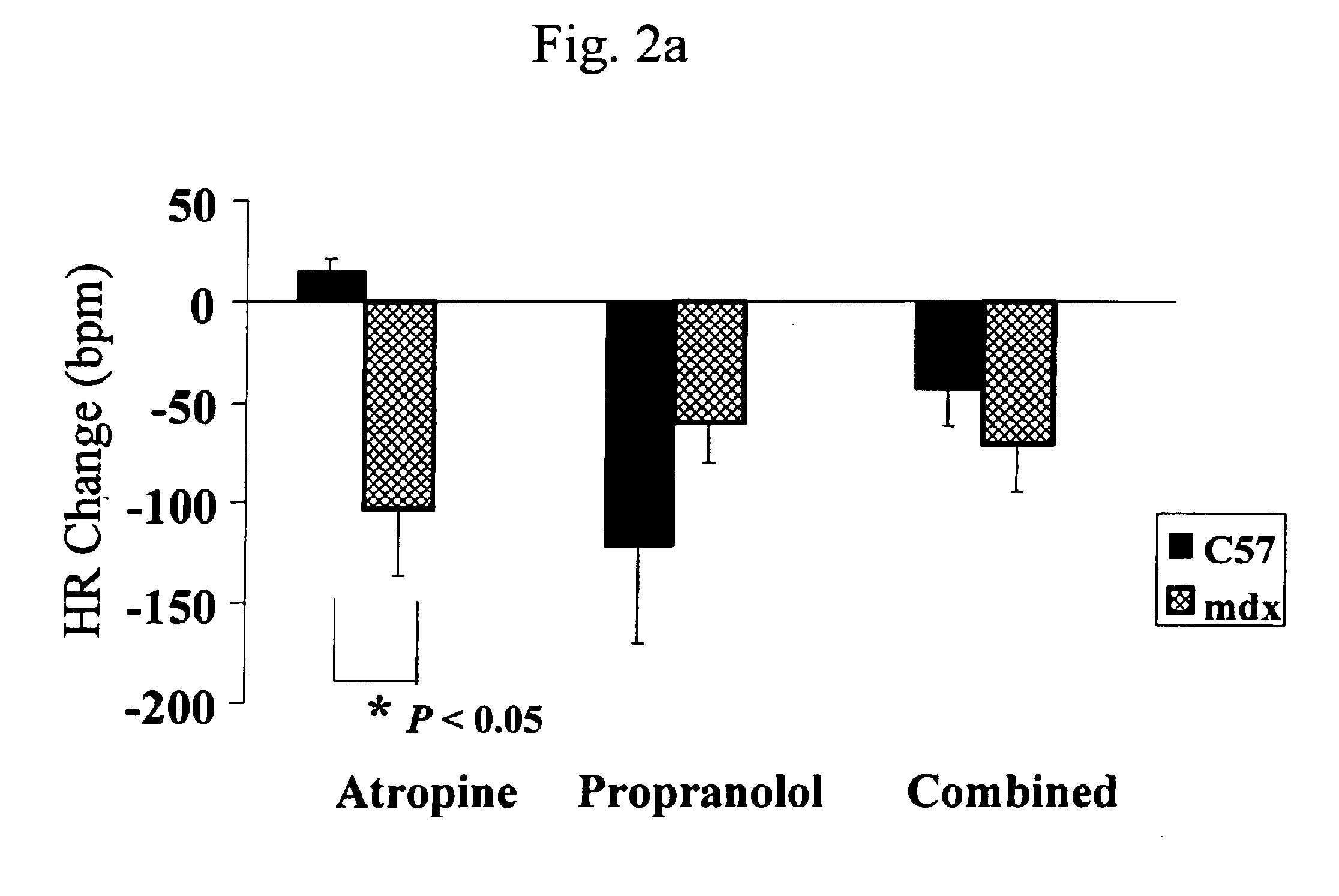
InactiveUS6875418B2Compounds screening/testingDiagnostic recording/measuringDiseaseDecreasing heart rate
The mdx mouse is a model of Duchenne muscular dystrophy. The present invention describes that mdx mice exhibited clinically relevant cardiac phenotypes. A non-invasive method of recording electrocardiograms (ECGs) was used to a study mdx mice (n=15) and control mice (n=15). The mdx mice had significant tachycardia, consistent with observations in patients with muscular dystrophy. Heart-rate was nearly 15% faster in mdx mice than control mice (P<0.01). ECGs revealed significant shortening of the rate-corrected QT interval duration (QTc) in mdx mice compared to control mice (P<0.05). PR interval duration were shorter at baseline in mdx compared to control mice (P<0.05). The muscarinic antagonist atropine significantly increased heart-rate and decreased PR interval duration in C57 mice. Paradoxically, atropine significantly decreased heart-rate and increased PR interval duration in all mdx mice. Pharmacological autonomic blockade and baroreflex sensitivity testing demonstrated an imbalance in autonomic nervous system modulation of heart-rate, with decreased parasympathetic activity and increased sympathetic activity in mdx mice. These electrocardiographic findings in dystrophin-deficient mice provide new bases for diagnosing, understanding, and treating patients with Duchenne muscular dystrophy.
Owner:MOUSE SPECIFICS
Preparation method of belladonna extract
The invention relates to a preparation method of a traditional Chinese medicine extract, and in particular relates to a preparation method of a belladonna extract. The method comprises the following steps: carrying out reflux extraction on coarse belladonna powder thrice by taking a sulfuric acid aqueous solution as a solvent, concentrating an extracting solution, adding an inorganic base and 95% ethyl alcohol and standing to obtain a supernatant; neutralizing the supernatant with a dilute acid, carrying out reduced pressure concentration on the supernatant until forming a thick paste, thereby obtaining the belladonna extract. The method is simple in process, less in time consumption and low in cost, and ensures that the extraction ratio of alkaloid in belladonna is high and the loss of the alkaloid in an alcohol precipitation process is less. The content of atropine in the prepared belladonna extract is more than 30% higher than that of the atropine prepared in the prior art.
Owner:HUNAN UNIV OF SCI & ENG +1
Atropine Pharmaceutical Compositions
ActiveUS20190175579A1Improve stabilityGood storage stabilitySenses disorderInorganic non-active ingredientsPreservative freePreservative
The inventive subject matter is directed to compositions and methods for sterile and storage stable low-dose atropine formulations with improved stability. Most preferably, the compositions presented herein are substantially preservative free and exhibit less than 0.35% tropic acid from degradation of atropine. Advantageously, contemplated formulations are also substantially free of preservatives.
Owner:VYLUMA INC
Frostbite and chilblain treating ointment
InactiveCN1706381AIt has the function of windproof, waterproof and antifreezeRelieve spasmsHeterocyclic compound active ingredientsSide effectWhite petrolatum
The ointment for treating frostbite and chilblain is prepared with anticholine drug powder and vaseline and through homogeneous mixing. Pharmacological test proves the effects of resisting peripheral M choline receptor, relieving acetylcholine caused smooth muscle spasm, improving circulation, etc of anticholine drug. Vaseline has the effects of preventing wind, preventing water and preventing frostbite. Therefore, the ointment with combined anticholine drug and vaseline has obvious frostbite and chilblain treating effect and no side effect when it is applied to the affected parts.
Owner:李爱华
Atropine eye drop having eye posterior targeting function
The invention relates to an atropine eye drop having an eye posterior targeting function, solving the problem that general eye drops are easy to be diluted by tear and remains in eyes in short time. The atropine eye drop has the advantages that bioavailability of medicines in eyes is increased, medicine distribution in eyes is improved, concentration of medicine in eye posterior sclera and retinais increased, and the eye posterior targeting function is achieved.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
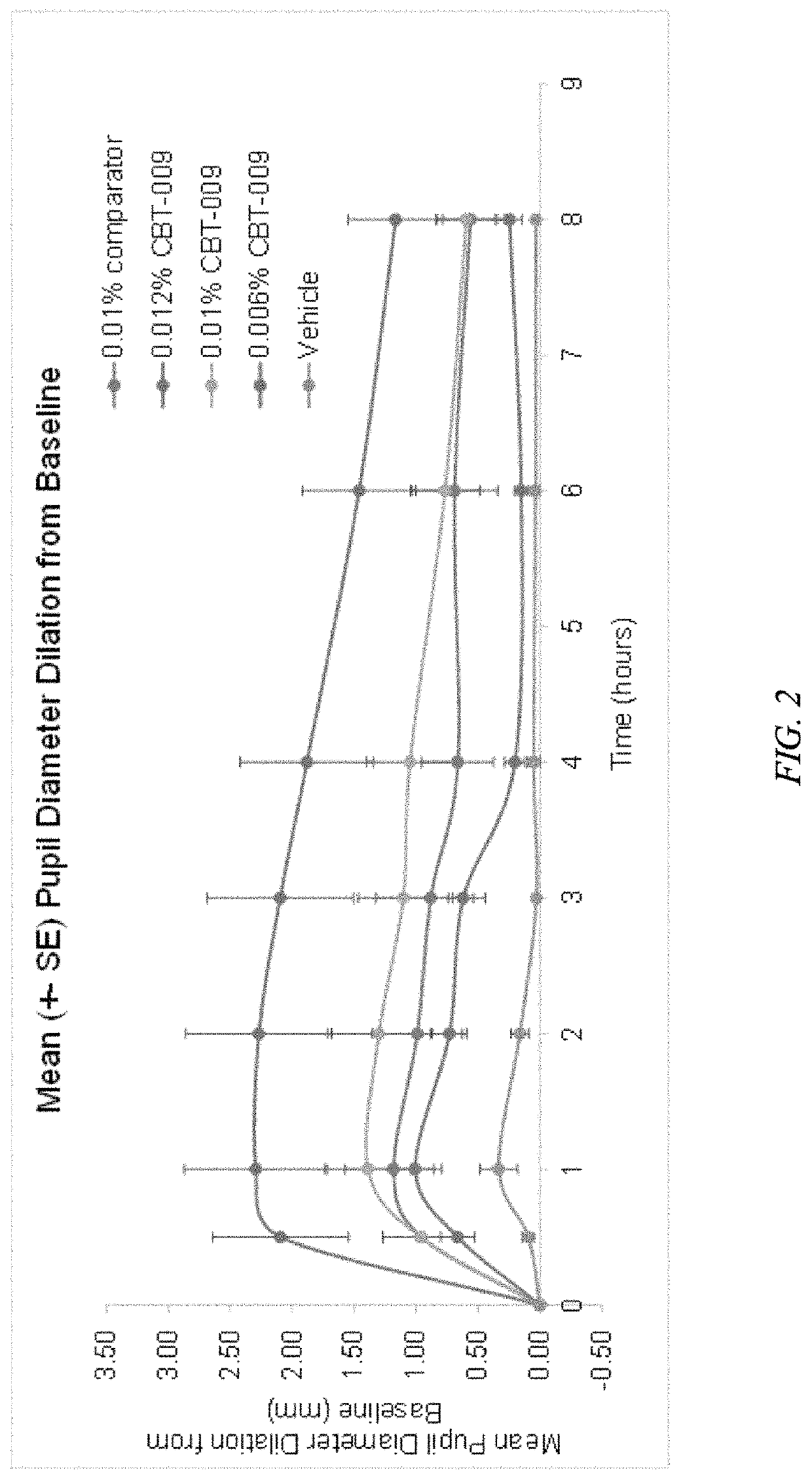
Medical composition for preventing and treating NITM and pharmaceutical application of medical composition
ActiveCN109157503AGood treatment effectReduce mydriasis side effectsSenses disorderInorganic non-active ingredientsPh regulationBoronic acid
The invention belongs to the field of medicines, and relates to a medical composition for preventing and treating NITM and a pharmaceutical application of the medical composition. Specifically, the medical composition is a medical composition for eyes, such as a preparation for eyes. Specifically, the medical composition disclosed by the invention comprises 0.001%-0.2% of atropine or pharmaceutical salt thereof, and one or more auxiliary materials acceptable in pharmacy, wherein the pH value of the medical composition is 4.0-6.5, the medical composition comprises 0.5%-5% of a pH regulation agent, and the pH regulation agent is selected from any one or more of sodium dihydrogen phosphate, disodium hydrogen phosphate, citric acid, citrate, boric acid and borate. The medical composition disclosed by the invention can effectively treat and / or prevent NITM, and has favorable application prospects.
Owner:SHENYANG XINGQI PHARM CO LTD
Topical ophthalmological atropine free base compositions
ActiveUS11191751B1Minimal irritationSenses disorderOrganic non-active ingredientsAlkanePharmaceutical Substances
A topical ophthalmological composition includes a muscarinic receptor antagonist as an active pharmaceutical ingredient; and a semifluorinated alkane, as a liquid vehicle. The topical ophthalmological composition treats an ocular disease.
Owner:ADS THERAPEUTICS LLC
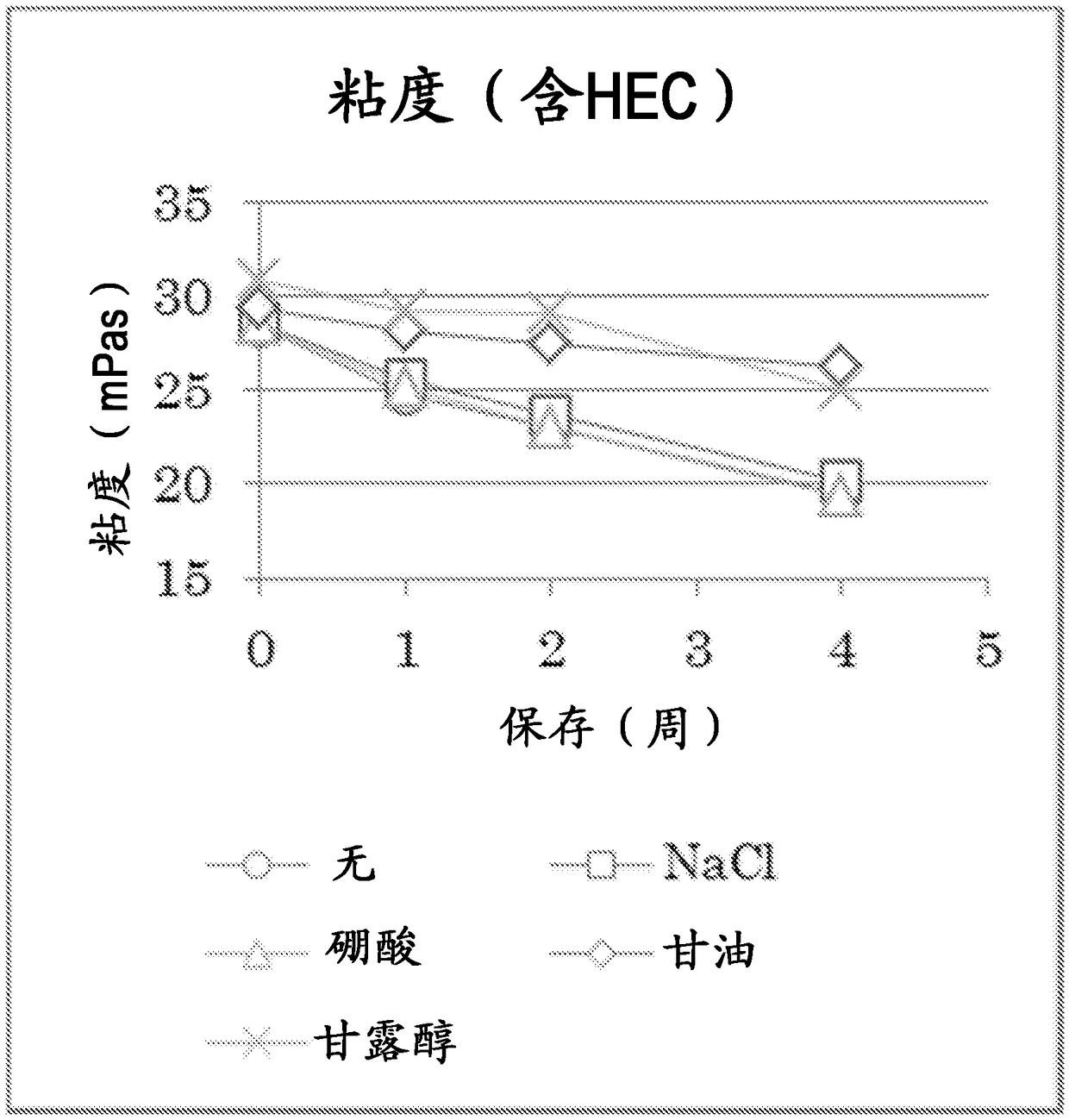
Atropine-containing aqueous composition
ActiveCN109310687AMaintain initial viscosityInhibition of length elongationSenses disorderInorganic non-active ingredientsPhosphateCarboxylic acid
Disclosed herein is an aqueous composition comprising 0.001 - 0.1 % (w / v) atropine or a salt thereof, a water-soluble polymer, and buffer (I), which is at a pH range of 6 or lower, wherein the buffer(I) is at least one selected from the group consisting of a phosphate buffer, an aminocarboxylate buffer, a carbonate buffer, an acetate buffer, a tartrate buffer, a borate buffer, and trometamol.
Owner:SINGAPORE HEALTH SERVICES PTE +2
Medicine for treating slow arrhythmia sick sinus syndromes
ActiveCN101822729AImprove heart functionImprove the quality of lifeCardiovascular disorderPlant ingredientsTherapeutic effectAtropine
The invention discloses a medicine for treating slow arrhythmia sick sinus syndromes and a preparation method thereof. The active components of the medicine for treating slow arrhythmia sick sinus syndrome are prepared from the following raw materials in weight proportion: 1 to 90g of red ginseng, 1 to 90g of processed Radix Aconiti Lateralis, 1 to 90g of Radix Glycyrrhizae Preparata, 1 to 90g of cassia twig, 1 to 90g of Panax pseudo-ginseng and 1 to 90g of Poria cocos. The medicine is obviously superior to atropine in the aspect of improving clinical symptoms, can improve the heart functions of patients and improve the quality of life of the patients; the medicine is superior to the atropine in the aspects of increasing the heart rate and therapeutic effect indexes of Holter, electrocardiogram and the like, improving EF and CO values, an electrocardiogram ST segment and the like in the ultrasonic heart function indexes of hearts; obvious adverse effects are not generated in treatment groups in the research process, and the medicine is clinically safe and valid. Therefore, the medicine has huge popularization and application values in the field of treating the slow arrhythmia sick sinus syndromes.
Owner:吉林康乃尔药业有限公司 +1
Anticholinergic pharmaceutical composition
ActiveCN103553996AEfficient removalImprove stabilityOrganic chemistryDigestive systemAnticholinergic DrugsDuodenal ulcer
The invention relates to an anticholinergic pharmaceutical composition. Specifically, the invention relates to a medicament capable of being used in pharmacy, wherein the,medicament comprises a compound expressed by the following formula. The invention further provides a preparation of the medicament, and the medicament and the preparation are used as an anticholinergic medicament and can be clinically used for anesthesia, peptic ulcer, salivation, etc. The raw material medicament or preparation has the function of inhibiting gastric secretion and regulating gastrointestinal peristalsis; the raw material medicament or preparation has relatively strong salivary secretion resistance than atropine while having no central anticholinergic activity. The anticholinergic pharmaceutical composition is generally used for clinically treating such symptoms as gastric and duodenal ulcer, chronic gastritis and excessive secretion of gastric acid.
Owner:HUNAN DONGTING PHARMA
Method of treating organophosphate intoxication
ActiveUS20170246188A1Effective post-exposure treatmentPrevents subsequent neuronal injuryOrganic active ingredientsNervous disorderBenzodiazepineAntidote
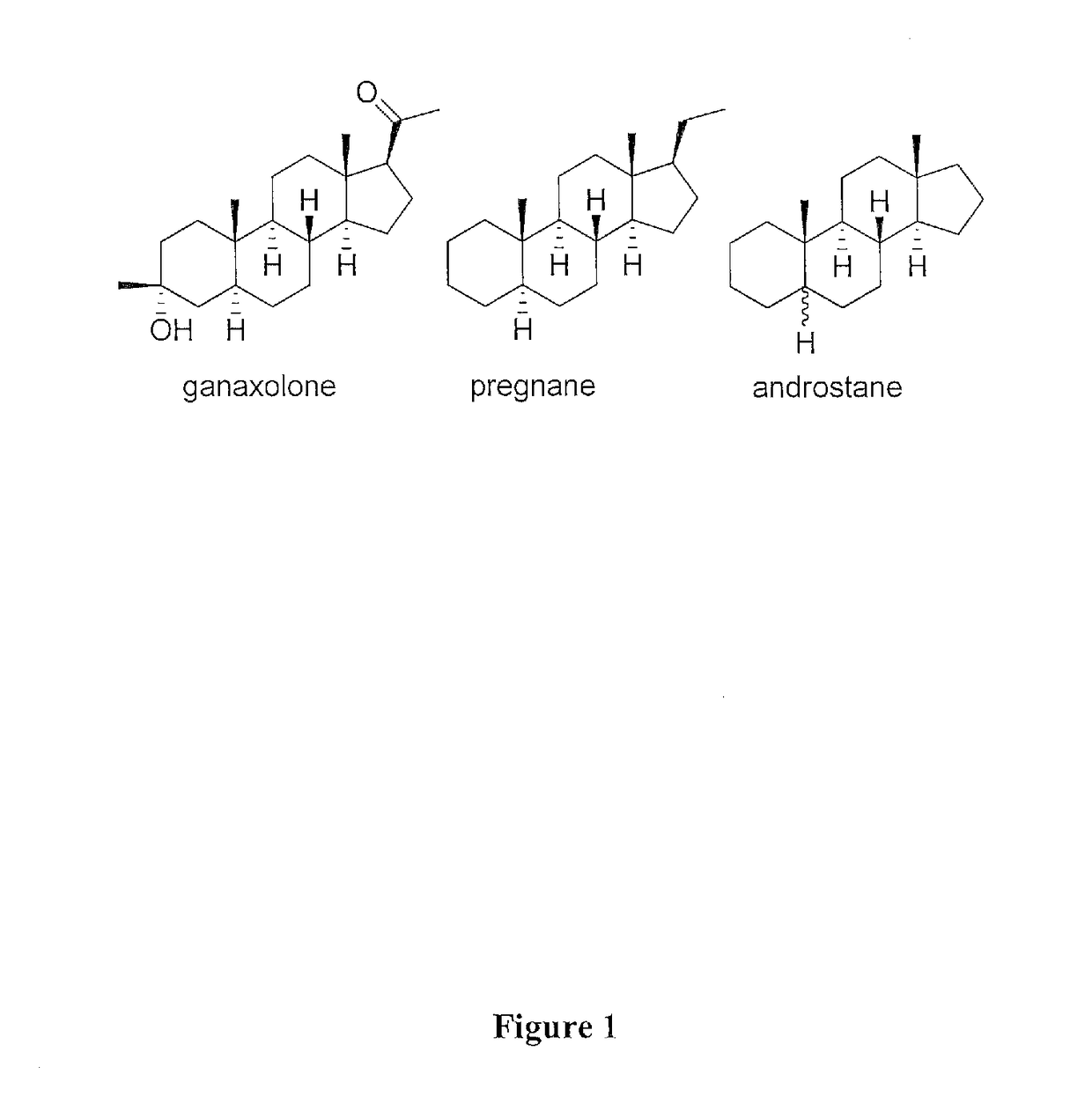
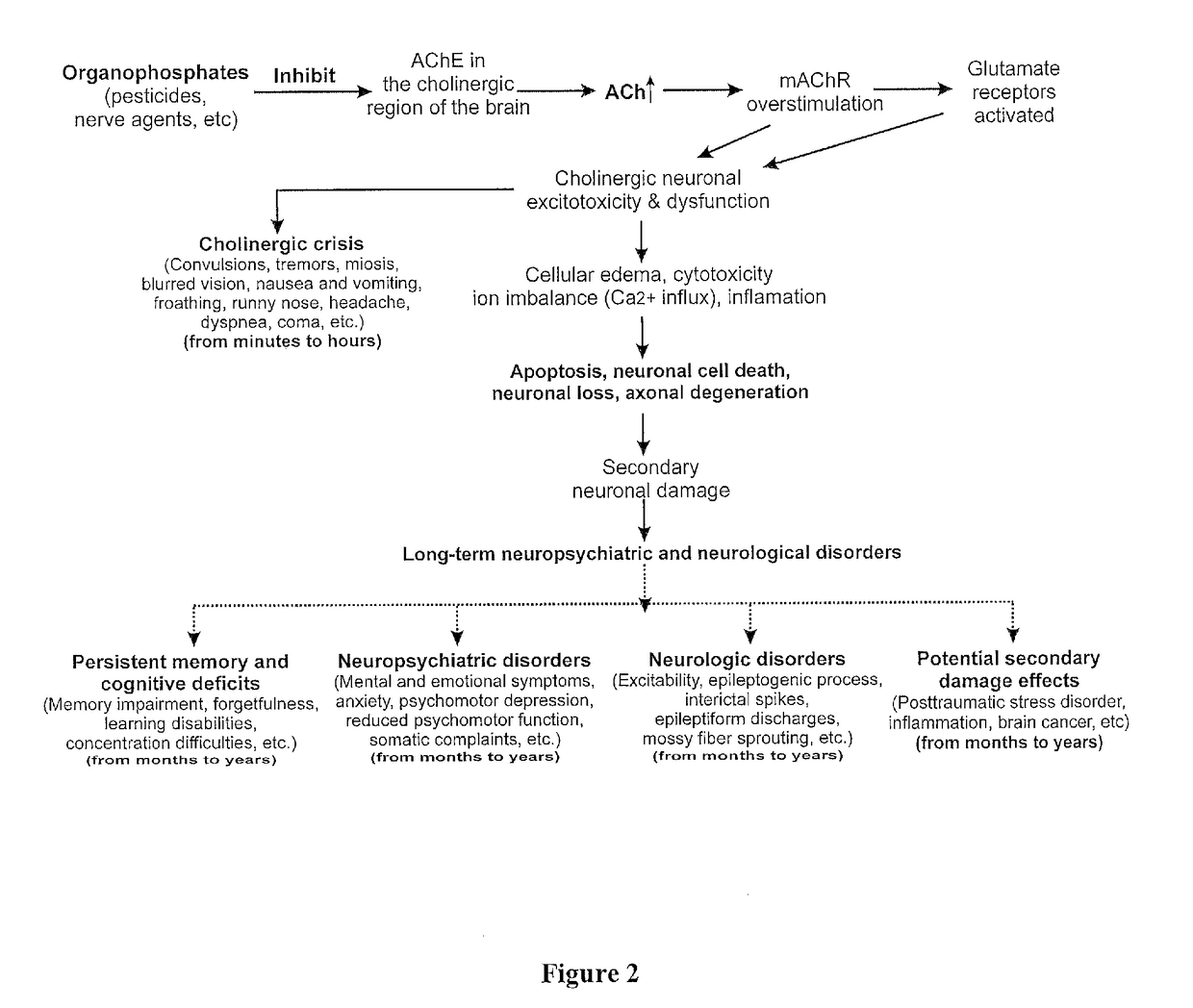
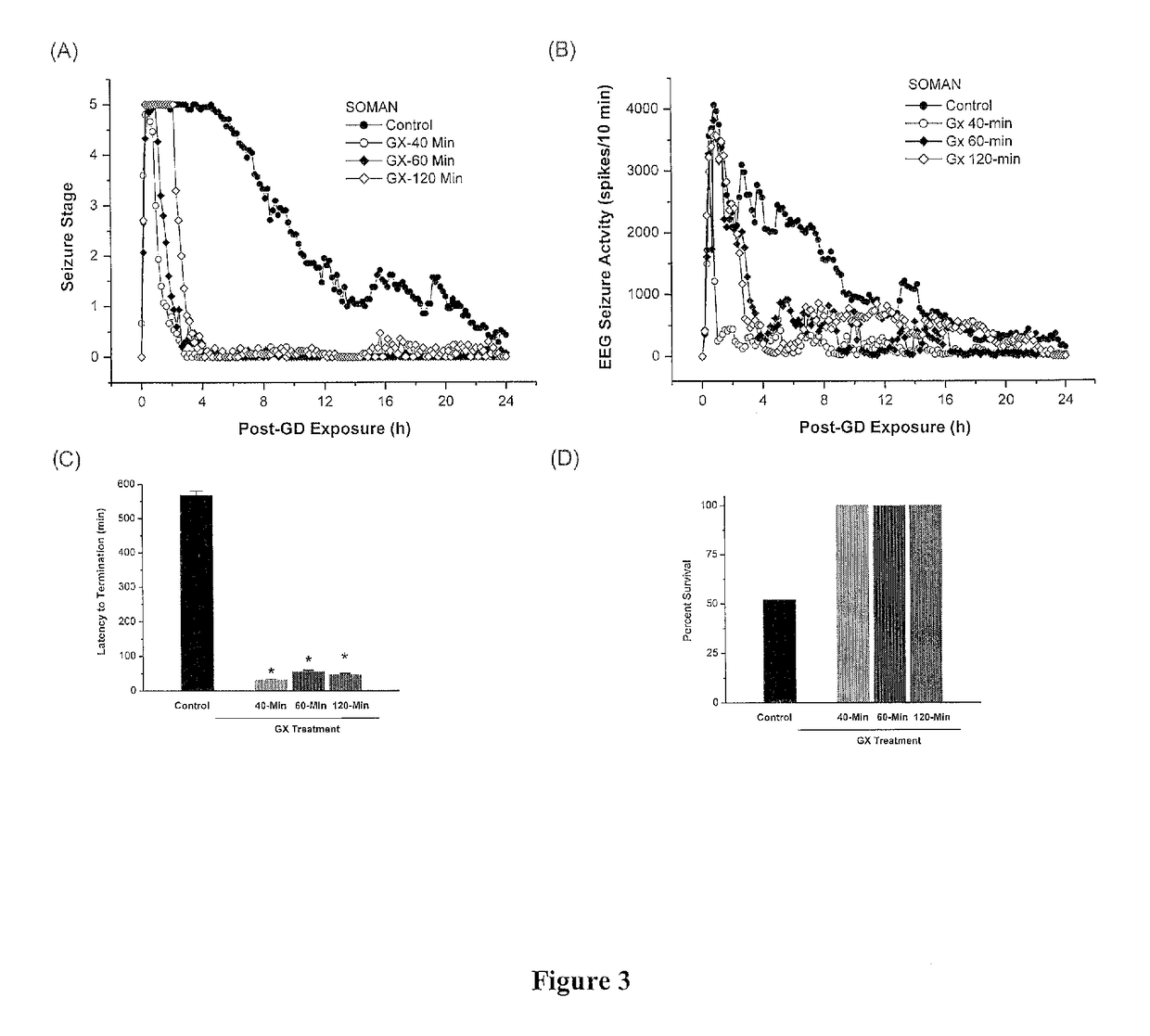
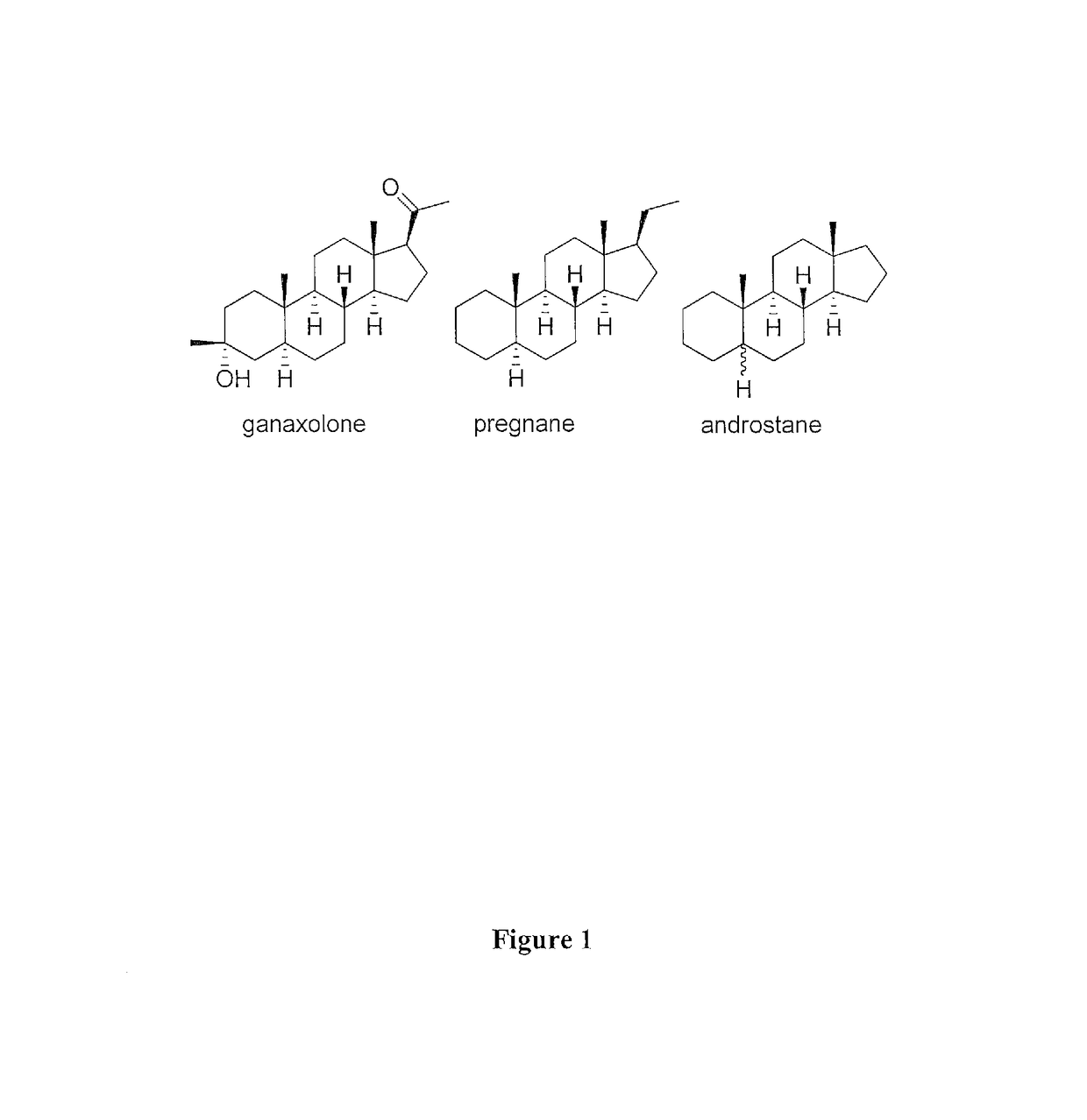
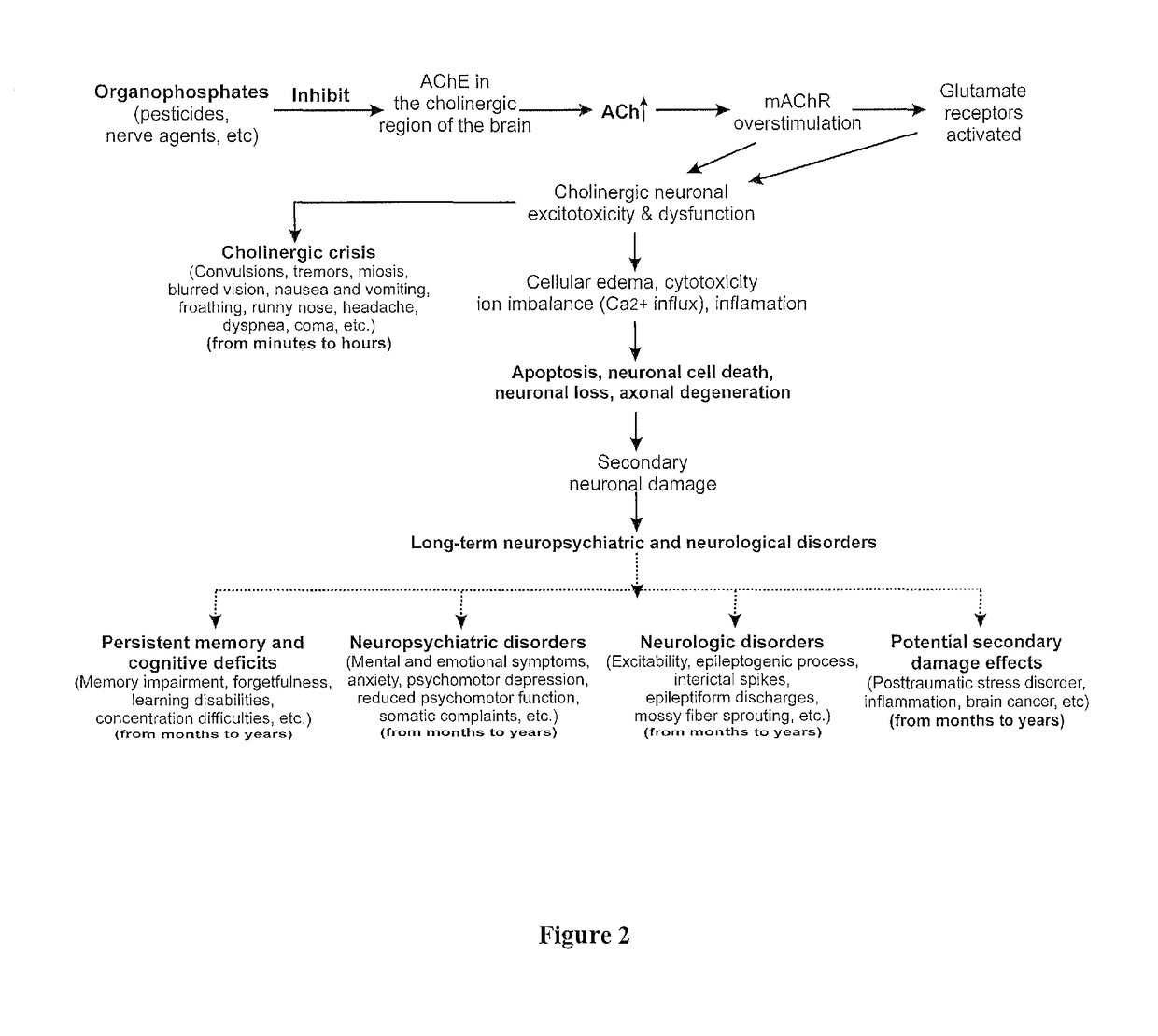
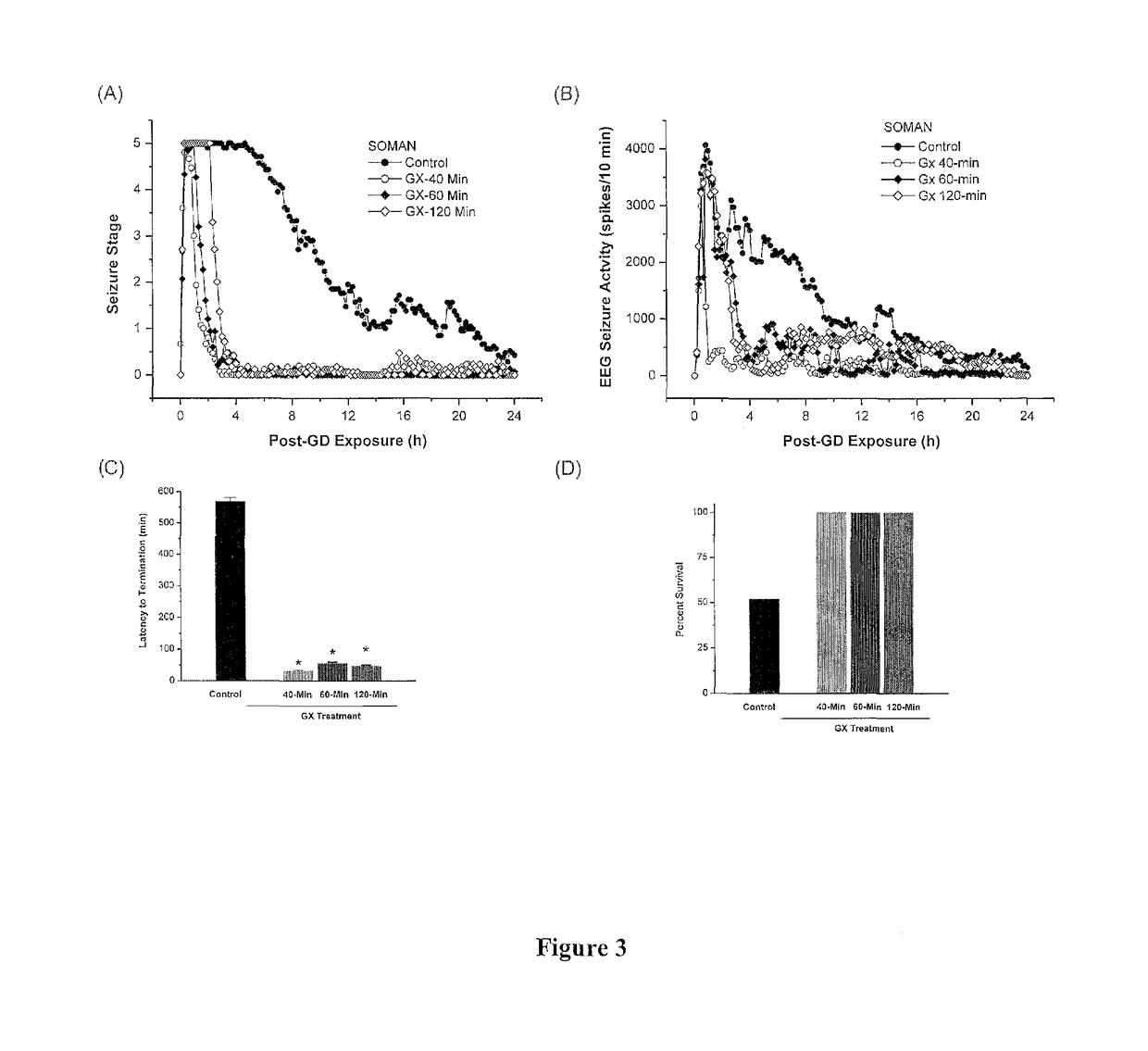
The present invention provides new compositions and methods for treating and / or reversing organophosphate intoxication, manifested by both cholinergic and non-cholinergic crisis, in a mammal resulting from exposure to organophosphate compounds. The neurosteroidal compounds of this invention are those having the general structural formula of pregnane, androstane, 19-norandrostanes, and norpregnane with further moieties as defined herein. These compounds include, but are not limited to, ganaxolone, pregnanolone, and androstanediol and their analogs, salts and prodrugs. The present invention further relates to combining a therapeutically effective amount of a neurosteroidal compound with a standard organophosphate antidote (e.g. atropine, pralidoxime). The data suggests that neurosteroids are effective or more effective than benzodiazepines, whether given earlier or later than 40-min (up to several hours) after organophosphate compound exposure. Neurosteroids are effective to attenuate long-term neuropsychiatric deficits caused by organophosphate exposure.
Owner:TEXAS A&M UNIVERSITY
Anti-neoplastic compositions comprising extracts of black cohosh
InactiveUS20090075919A1Treating or preventing neoplasia in a subjectTreat and prevent neoplasiaBiocideSugar derivativesAcetic acidBlack Cohosh Extract
The present invention provides a composition for use in treating or preventing neoplasia, comprising an effective actein. The present invention also provides a composition for use in treating or preventing neoplasia, comprising an effective anti-neoplastic amount of an ethyl acetate extract of black cohosh. The present invention further provides a combination of anti-neoplastic agents, comprising an effective anti-neoplastic amount of an ethyl acetate extract of black cohosh and an effective anti-neoplastic amount of at least one additional chemopreventive or chemotherapeutic agent. Methods for treating and preventing neoplasia are also provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Compound medicine for preventing and treating enteritis of poultry and preparation method thereof
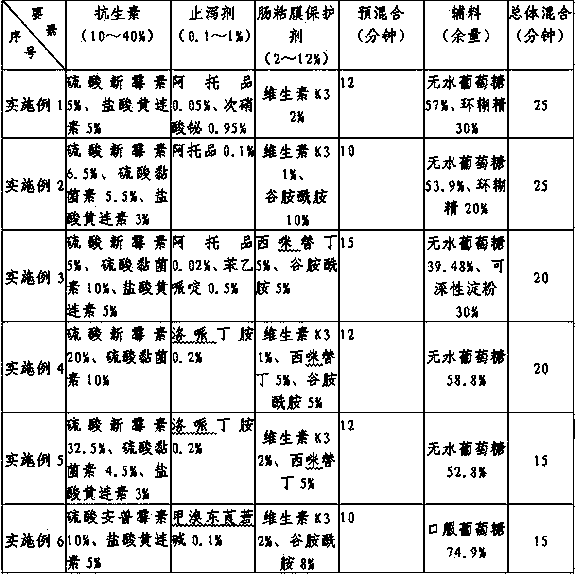
InactiveCN103638524AEasy to combineReasonable combinationAntibacterial agentsDigestive systemOral glucoseVitamin K3
The invention discloses a compound medicine for preventing and treating enteritis of poultry, which contains the following constituents by mass percent: 10-40% of antibiotics, 0.1-1% of obstruent, 2-12% of intestinal mucosa protector, and the balance of auxiliary material. The antibiotics constituent in the compound medicine is one or more of aminoglycosides, polymyxins and berberine hydrochloride. The obstruent is one of scopolamine methobr, atropine, diphenoxylate, loperamide and bismuth subnitrate, or combinations of more. The intestinal mucosa protector is one of vitamin K3, cimetidine and glutamine, or combination of more. The auxiliary material is one of oral glucose, anhydrous glucose, soluble starch, dextrin and cyclodextrin, or combination of more. The invention further discloses a preparation method of the compound medicine for preventing and treating enteritis of poultry.
Owner:JIANGSU WEITAILONG BIOTECH
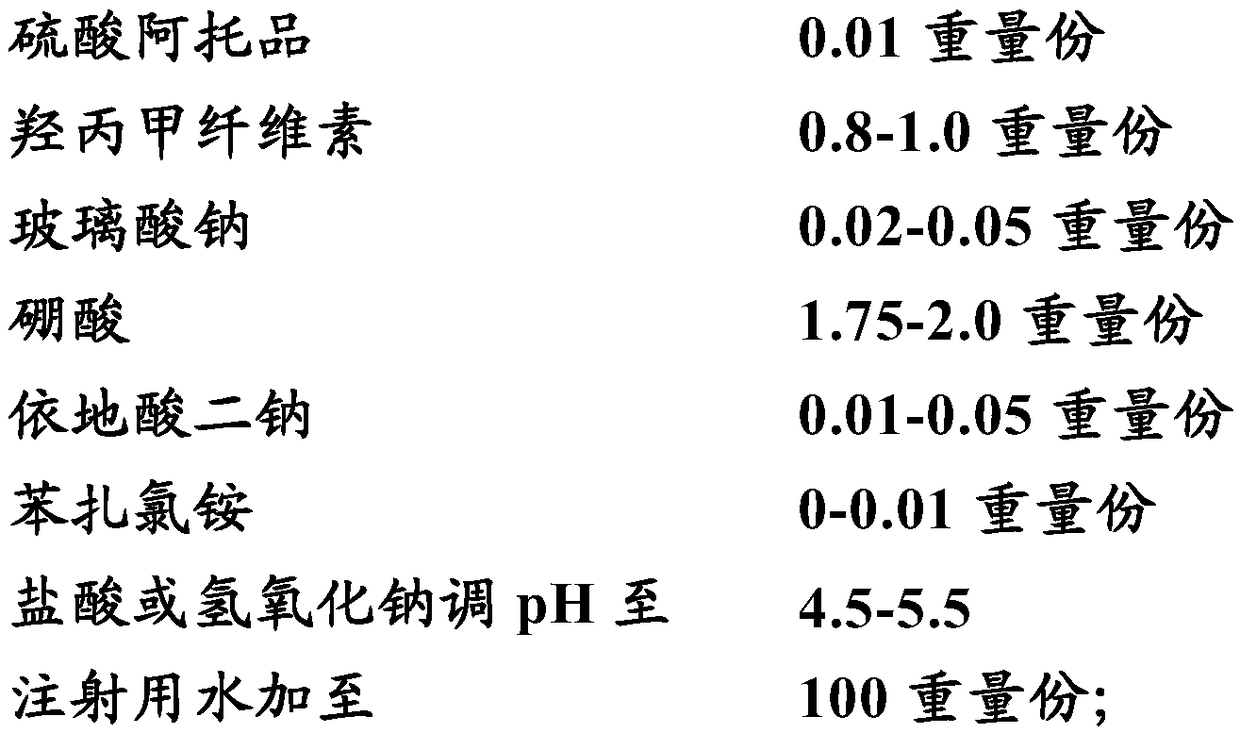
Sodium hyaluronate eye drops containing 0.01% of atropine and preparation method of sodium hyaluronate eye drops
PendingCN111803441AAvoid side effectsAvoid potential dangerSenses disorderInorganic non-active ingredientsAllergic reactionSodium hyaluronate
The invention discloses sodium hyaluronate eye drops containing 0.01% of atropine. The eye drops comprise sodium hyaluronate, atropine, sodium hydroxide, hydrogen chloride, sodium chloride and water for injection. The mass concentration of sodium hyaluronate is 0.1-10 g / L, the mass concentration of atropine is 0.1 g / L, sodium hydroxide and hydrogen chloride are pH regulators, and sodium chloride is an osmotic pressure regulator. The eye drops contain no traditional Chinese medicinal components, bacteriostatic agent, preservative, cosolvent, thicken agent, complexing agent or the like, the components are simple and are normal substances of human bodies except for atropine, so that corneal damage caused by damage of the microenvironment on the surfaces of eyeballs is avoid, harm to the humanbodies and growth and development due to long-term excessive use of components such as traditional Chinese medicine is also avoided, and the incidence of allergic reaction is effectively reduced. Inaddition, the eye drops have pH and osmotic pressure adjusted, use comfort is improved, small-dose independent packages are also produced based on aseptic filling and a hot pressing sterilization process, and biological safety of the product is ensured.
Owner:SECOND AFFILIATED HOSPITAL OF COLLEGE OF MEDICINEOF XIAN JIAOTONG UNIV
Compound, hydroxycholine M receptor antagonist, composition and application of saturated heterocyclic nitrogen containing caffeic acid derivative in preparing toadin M receptor antagonist
The invention discloses a saturated heterocyclic nitrogen containing caffeic acid derivative separated from nightshades, or pharmacological acceptable salt of the caffeic acid derivative. The compound is a hydroxycholine M receptor antagonist and can be used for treating diseases of spasm, stenocardia, cholecystalgia, renal colic, visceral spasm and the like. An unmarked cell-targeting pharmacological technique is adopted, research on dose-effect relationship displays DMR response signals caused by antagonistic acetylcholine with compound dose dependency and shows that the compound has high hydroxycholine M receptor antagonistic activity. Compared with known M receptor antagonist atropine, scopolamine and the like, the compound is large in structural difference, and selectivity on muscle glands and the nervous system can be expectedly improved; current research shows that a hydroxycholine M receptor is related to diseases of spasm, analgesia, calm, schizophrenia and the like, and an efficient new ligand definite in action target can be provided for the related diseases.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Prepn process of isocollidine for eye
InactiveCN1739508AEasy to useGood curative effectOrganic active ingredientsSenses disorderSide effectOrganic acid formation
The present invention relates to preparation process of isocollidine preparation for eye. Through mixing isocollidine and inorganic acid or organic acid to form soluble isocollidine, dissolving in water, adding osmotic pressure regulator to osmotic pressure 280-320 mOsm / kg, adding hydrochloric acid or sodium hydroxide solution to regulate pH value to 3.0-7.0, adding water via stirring and sterilizing, solution for eye is obtained; or, gel as excipient is further added to obtain gel for eye. The isocollidine preparation for eye may be used to replace atropine, which has serious toxic side effect, for mydriasis, may be used also in regulating eyesight, treating pseudomyopia of teenage and eliminate eye fatigue, and is safe, effective and controllable.
Owner:郭曙平
Method of treating organophosphate intoxication by administration of neurosteroids
ActiveUS10172870B2Effective post-exposure treatmentReverses organophosphate intoxication more effectivelyOrganic active ingredientsNervous disorderBenzodiazepineAntidote
The present invention provides new compositions and methods for treating and / or reversing organophosphate intoxication, manifested by both cholinergic and non-cholinergic crisis, in a mammal resulting from exposure to organophosphate compounds. The neurosteroidal compounds of this invention are those having the general structural formula of pregnane, androstane, 19-norandrostanes, and norpregnane with further moieties as defined herein. These compounds include, but are not limited to, ganaxolone, pregnanolone, and androstanediol and their analogs, salts and prodrugs. The present invention further relates to combining a therapeutically effective amount of a neurosteroidal compound with a standard organophosphate antidote (e.g. atropine, pralidoxime). The data suggests that neurosteroids are effective or more effective than benzodiazepines, whether given earlier or later than 40-min (up to several hours) after organophosphate compound exposure. Neurosteroids are effective to attenuate long-term neuropsychiatric deficits caused by organophosphate exposure.
Owner:TEXAS A&M UNIVERSITY
Combined medicine used for treating pain, and preparations and preparation method thereof
ActiveCN105311622AAvoid side effectsSimple preparation processNervous disorderPeptide/protein ingredientsSide effectOil emulsion
The invention provides a combined medicine used for treating pain, and preparations and a preparation method thereof. The combined medicine is prepared from active ingredients cobratoxin and atropine, and pharmaceutic adjuvants; excellent synergistic effects are achieved; and effect is treating pain is excellent. The invention also discloses a controlled-release injection microemulsion used for treating pain. According to the controlled-release injection microemulsion, oil for injection is taken as a menstruum, medicine active ingredients are uniformly dispersed in the oil menstruum via emulsion process so as to obtain a water-in-oil emulsion, slow and stable release of the controlled-release injection microemulsion is realized, pain treatment effect is better, and toxic and side effect is avoided. A preparation of the controlled-release injection microemulsion is simple; cost is low; and the preparation method is suitable for industrialized production.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com