Atropine-containing aqueous composition
A technology of water-based composition and water-soluble polymer, which is applied in the direction of drug combination, active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, etc., which can solve problems such as glare, hindrance of daily activities, and reduction of lens adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
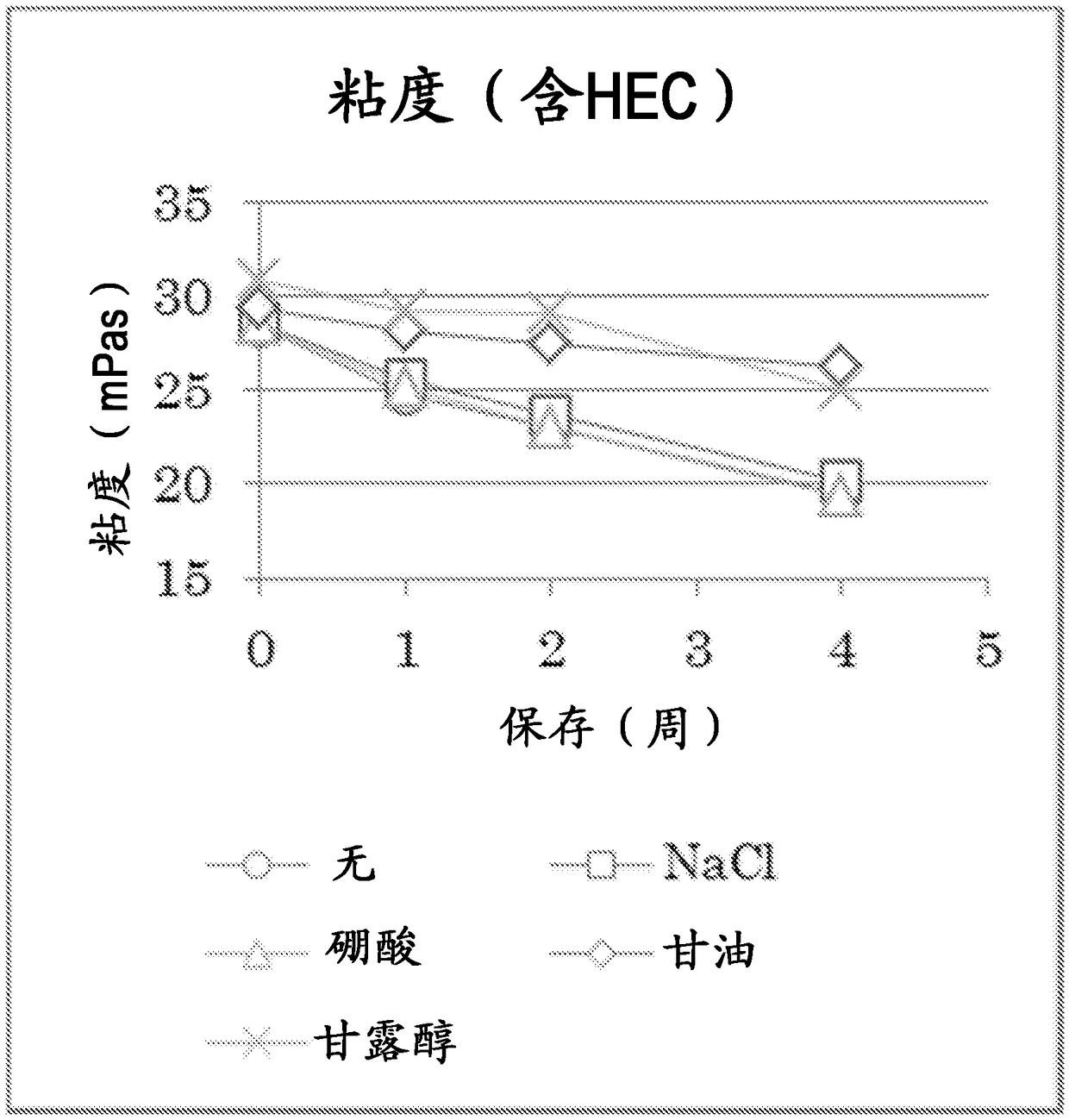
Image
Examples
Embodiment 1
[0168] The aqueous composition in Example 1 was prepared according to the formulation shown in Table 1. Specifically, 0.01 g of atropine sulfate hydrate, 0.32 g of hydroxyethylcellulose, 0.1 g of sodium dihydrogen phosphate, and 2.4 g of concentrated glycerin were dissolved in purified water. To the thus-obtained solution, appropriate amounts of hydrochloric acid and sodium hydroxide were added to adjust the solution to pH 5, and the total volume was adjusted to 100 ml.
Embodiment 2、3、 and comparative example 1~3
[0170] According to the formulation shown in Table 1, it carried out similarly to Example 1, and prepared the aqueous composition in Examples 2, 3, and Comparative Examples 1-3.
[0171]
[0172] (experiment method)
[0173] A single dose (50 μl volume) of each aqueous composition was instilled into one eye of the rabbits (4 eyes from 4 rabbits, or 6 eyes from 6 rabbits for each aqueous composition). The images of rabbit pupils before instillation and 1 hour after instillation were obtained by optical coherence tomography (OCT), and then analyzed by image analysis software to calculate the pupil area and pupil dilation ratio of rabbits. The pupil dilation ratio was calculated using the following formula:
[0174] Pupil expansion rate (%)=((b-a) / a)×100,
[0175] Wherein, a is the average value (mm 2 ), a is 16.8(mm 2 ), b is the value of the pupil area 1 hour after instillation.
[0176] (test results)
[0177] Table 2 shows the results in Examples 1-3 and Comparative ...
Embodiment 4~11
[0190] The aqueous compositions in Examples 4-11 were prepared according to the formulations shown in Table 3.
[0191] (experiment method)
[0192] A single dose (50 μl volume) of each aqueous composition was instilled into one eye of the rabbits (4 eyes from 4 rabbits, or 6 eyes from 6 rabbits for each aqueous composition). The image of the rabbit pupil 1 hour after instillation was obtained by optical coherence tomography (OCT), and then analyzed by image analysis software to calculate the pupil area of the rabbit.
[0193] (test results)
[0194] Table 3 shows the results in Examples 4 to 11. In Table 3, each value is an average value of data from 4 cases or 6 cases.
[0195] The pupil dilating effect of each aqueous composition was evaluated based on the following criteria.
[0196] A: Pupil area is less than 30.0mm 1 hour after instillation 2 Case.
[0197] B: Pupil area is 30.0mm 1 hour after instillation 2 More than and less than 35.0mm 2 Case.
[0198] C: P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com