Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
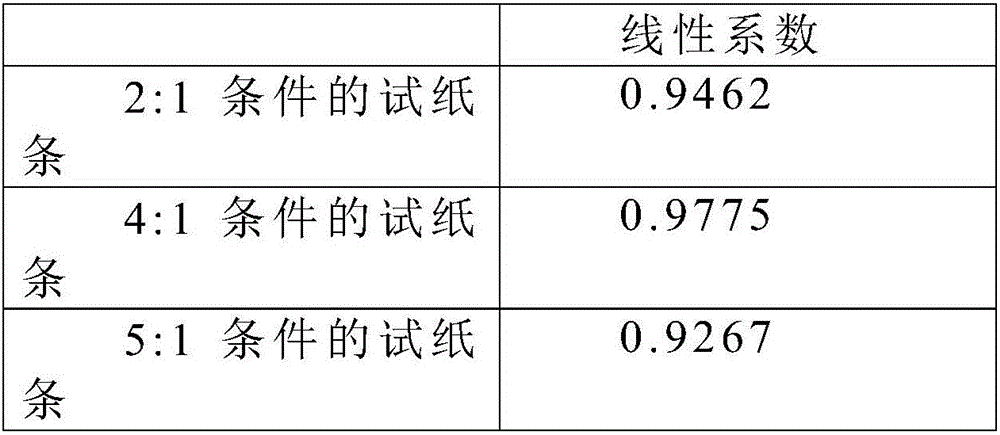
117 results about "BORATE BUFFER" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Borate buffered saline (abbreviated BBS) is a buffer used in some biochemical techniques to maintain the pH within a relatively narrow range. Borate buffers have an alkaline buffering capacity in the 8–10 range.
Detection of immobilized nucleic acid
The present invention provides methods for determining the presence of immobilized nucleic acid employing unsymmetrical cyanine dyes that are derivatives of thiazole orange, a staining solution and select fluorogenic compounds that are characterized as being essentially non-genotoxic. The methods comprise immobilizing nucleic acid, single or double stranded DNA, RNA or a combination thereof, on a solid or semi solid support, contacting the immobilized nucleic acid with an unsymmetrical cyanine dye compound and then illuminating the immobilized nucleic acid with an appropriate wavelength whereby the presence of the nucleic acid is determined. The cyanine dye compounds are typically present in an aqueous staining solution comprising the dye compound and a tris acetate or tris borate buffer wherein the solution facilitates the contact of the dye compound and the immobilized nucleic acid. Typically the solid or semi-solid support is selected from the group consisting of a polymeric gel, a membrane, an array, a glass bead, a glass slide, and a polymeric microparticle. Preferably, the polymeric gel is agarose or polyacrylamide. The methods employing the non-genotoxic compounds represent an improvement over commonly used methods employing ethidium bromide wherein the present methods retain the advantages of ethidium bromide, ease of use and low cost, but without the disadvantageous, known mutagen requiring special handling and waste procedures.
Owner:LIFE TECH CORP
Medical device, combination of coating solutions, and method for producing medical device
ActiveUS20140198294A1Maintain mechanical propertiesReduce water contentOptical articlesPharmaceutical delivery mechanismBORATE BUFFERMedical device
Disclosed is a medical device having an elastic modulus of 100 kPa or more and 2,000 kPa or less, a water content of 10% by mass or less, a tensile elongation of 50% or more and 3,000% or less, and a dynamic contact angle (advancing angle) relative to a borate buffer of 80° or less. The present invention can significantly reduce or avoid a phenomenon of adhesion to a surface when contacted with a surface outside or inside the body, which has hitherto been regarded as a problem in a conventional medical device.
Owner:TORAY IND INC
Blood cell analyzer dilution as well as hemolytic agent
InactiveCN101281193AImprove working environmentReduce harmIndividual particle analysisBiological testingNon toxicityToxicant
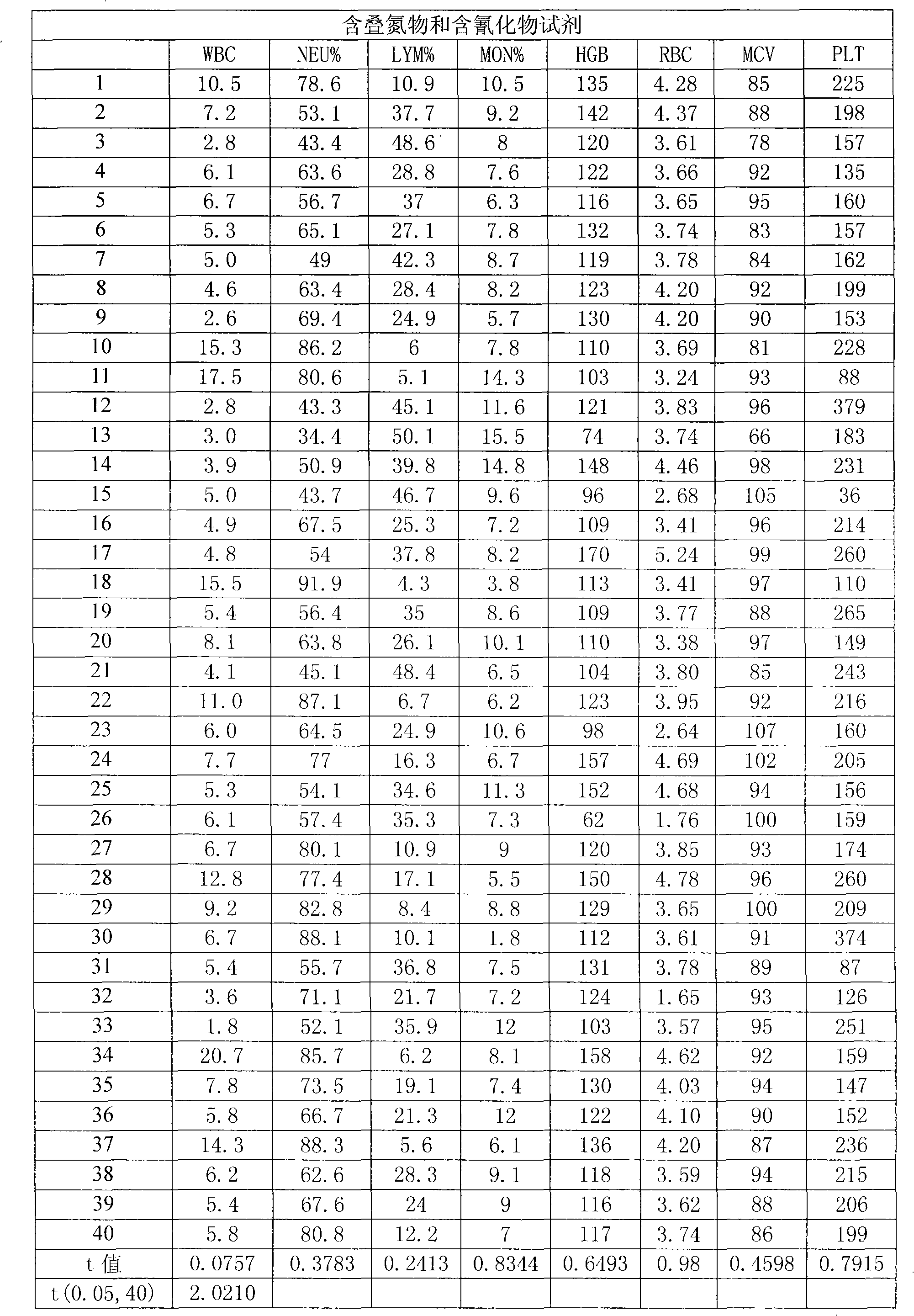
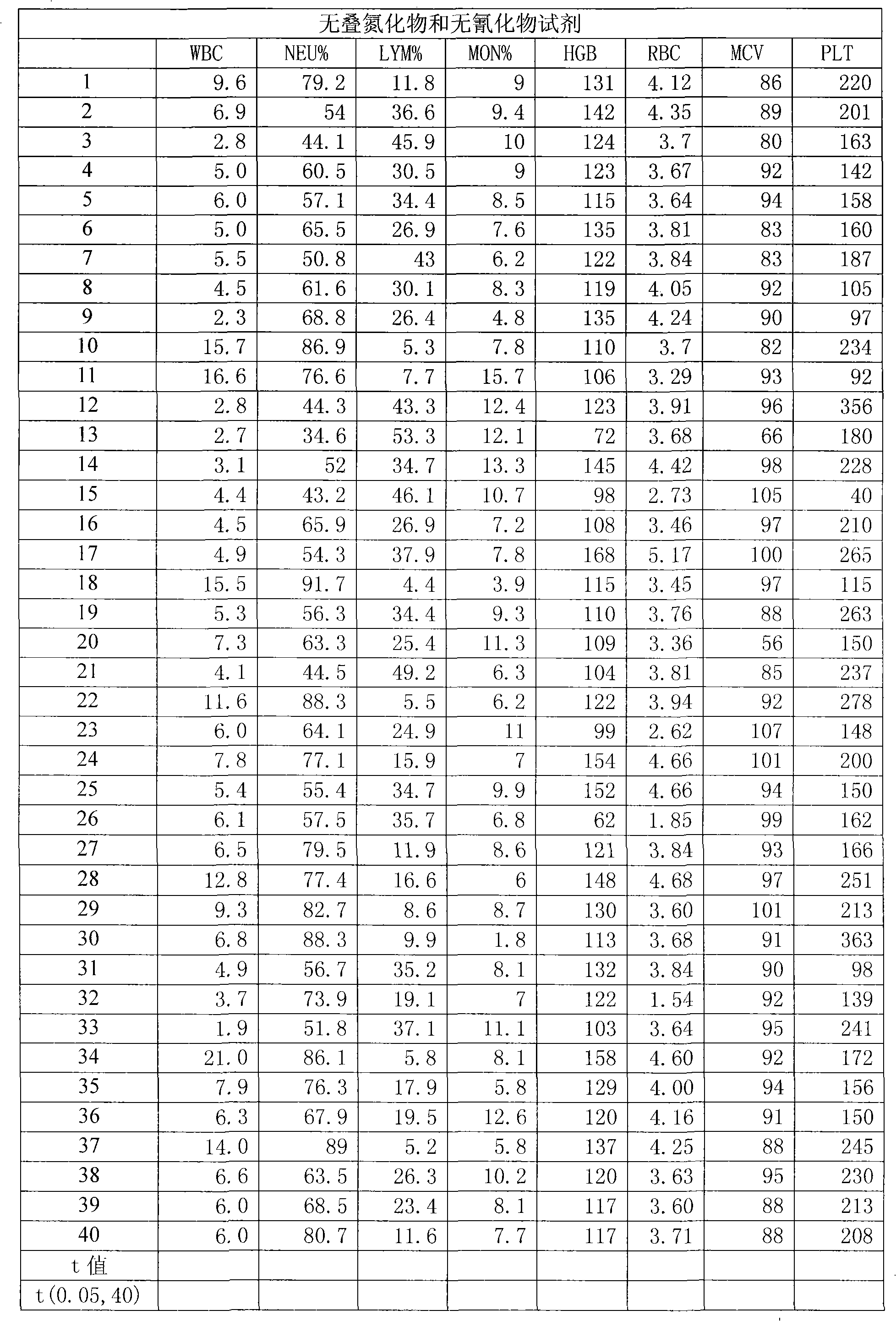
A blood cell analyzer diluent and a hemolytic agent, are characterized in that one litre diluent is provided with 12.0-4.0g of sodium chloride, 2.0-10.0g of sodium sulfate, 0.8-0.5g of 1,3,2-methylol urea, 0.2-0.5g of copper sulfite, 3.0-8.0g of EDTA-2Na, 0.2-0.7g of Piperacillin Sodium, a borate buffering liquid toning the ph value to 7.2-7.8, and the balance is water; one litre hemolytic agent is provided with 0.8-5.0g of potassium chloride, 0-60.0g of dodecyl trimethyl ammonium chloride, 14.0-0g of octadecyl trimethyl ammonium bromide, 6.0-10.0ml of isopropanol, carbonate or alcaine buffering liquid toning the ph value to 7.2-7.8, and the balance is water. The inventive reagent can form stable hemoglobin derivatives, and the absorption spectrum curves are similar when lambada is 540nm, lambada is 504nm, which can satisfy the clinical inspection requirement; the reagent does not contain cyanide, azide, and has non-toxicity, which can effective improve working atmosphere of operating staff, and can reduce harm of toxicant to personal health.
Owner:南昌百特生物高新技术股份有限公司
Fluorescent nanometer molecular imprinting biomimetic sensor, preparation method and applications thereof
InactiveCN107607498ASolve the problem of difficult mass transferOvercomes the effect of weakening hydrogen bondsFluorescence/phosphorescenceCross-linkFunctional monomer
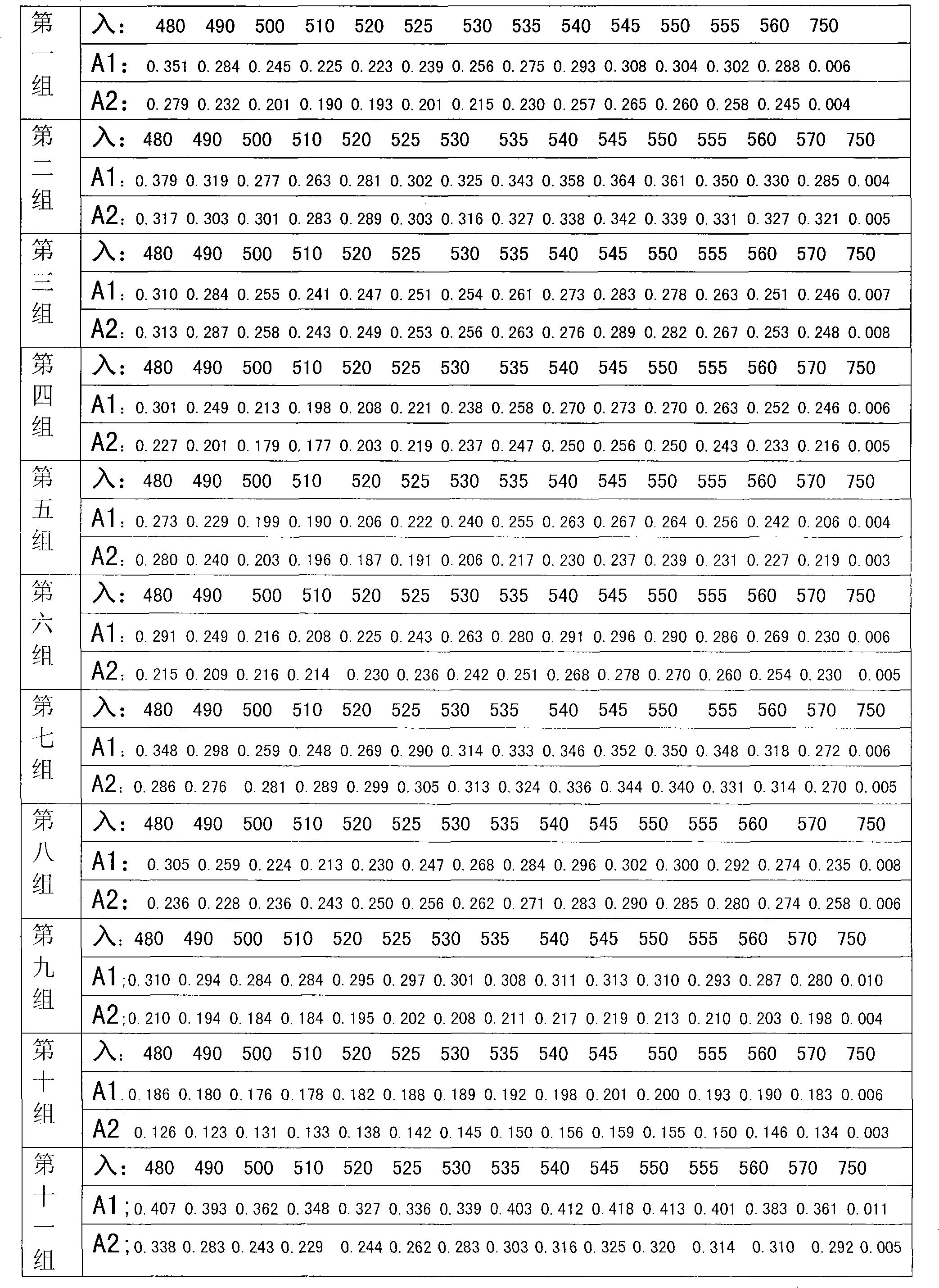
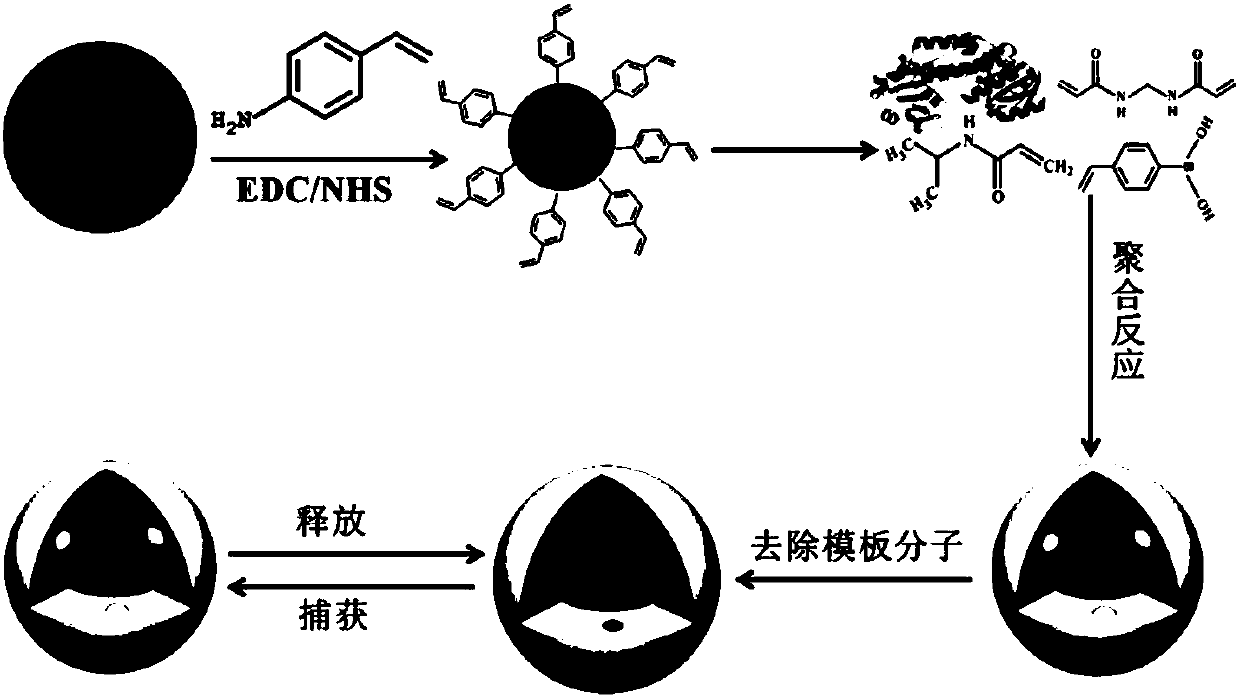
The invention relates to a fluorescent nanometer molecular imprinting biomimetic sensor, a preparation method and applications thereof. The preparation method comprises: weighing carbon quantum dot powder, ultrasonically dispersing in a borate buffer solution, sequentially adding 1-ethyl-(3-(dimethylamino)propyl)carbodiimide hydrochloride and N-hydroxysuccinimide, carrying out a stirring reactionat a room temperature under a dark condition, adding 4-vinylaniline, continuously carrying out the stirring reaction, carrying out dialysis on the obtained product in water, and carrying out freeze drying to obtain surface double bond functionalized carbon quantum dots; adding a template molecule and two different functional monomers to a pore forming agent, ultrasonically dissolving, and carryingout stirring pre-polymerization at a room temperature to obtain a pre-assembly solution A; ultrasonically dispersing the surface double bond functionalized carbon quantum dots in a pore forming agentto obtain a solution B; uniformly mixing the solution A and the solution B, adding a cross-linking agent and an initiator, introducing nitrogen, stirring, carrying out centrifugation, collecting theprecipitate, and washing with distilled water; and finally carrying out elution on the template protein by using HAc-SDS, and carrying out freeze drying on the obtained product so as to obtain the fluorescent nanometer molecular imprinting biomimetic sensor.
Owner:NANJING MEDICAL UNIV
Group (VII) transition-metal complexes with multidentate aminopolycarboxylate ligands and a kit for producing them
The invention relates to novel aminocarboxylate ligands that are suitable for complexing with a radionuclide, and are useful as imaging agents for diagnostic purposes. In accordance with the present invention, a method of preparing a compound of formula (I): fac-[M(CO)3(OH2)3]+, wherein M is Mn, 99mTc, 186Re or 188Re, involves reacting a metal in permetallate form with carbon monoxide and a reducing agent, wherein a mixture of a basic borate buffer and a reducing agent soluble in water but not substantially decomposed by water is solved in a water containing solvent system containing a solution of the metal in permanganate, pertechnetate or perrhenate form in the presence of carbon monoxide. The compound of formula (I) can be reacted with a ligand Lx to form a compound of formula (II): fac-[M(CO)3(X)2L1]n, wherein M is as defined above Lx is a multidentate ligand, and n is a charge of the ligand Lx increased with one + charge. The invention also is directed to novel compounds, and kits for carrying out the disclosed methods.
Owner:MALLINCKRODT INC
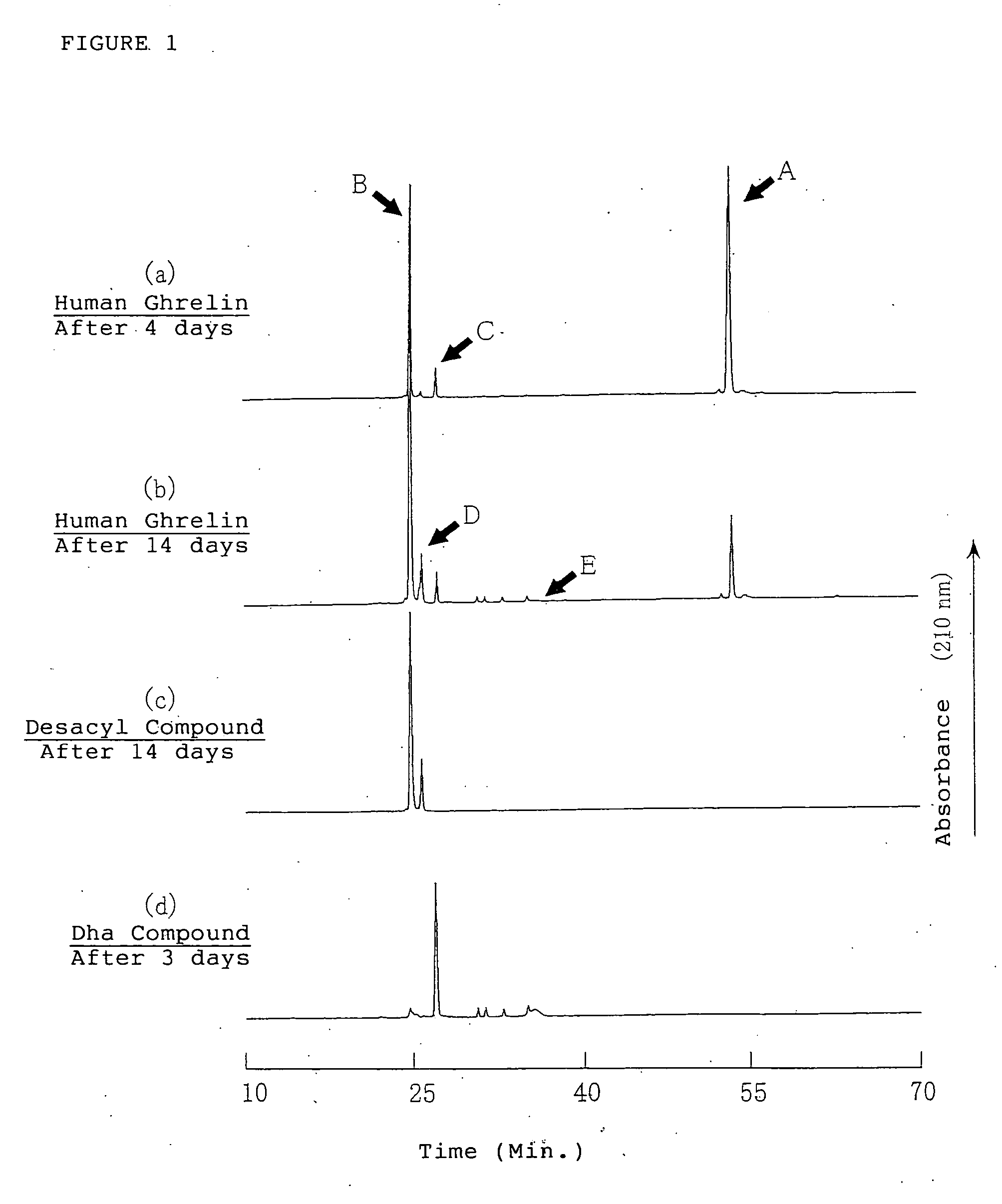
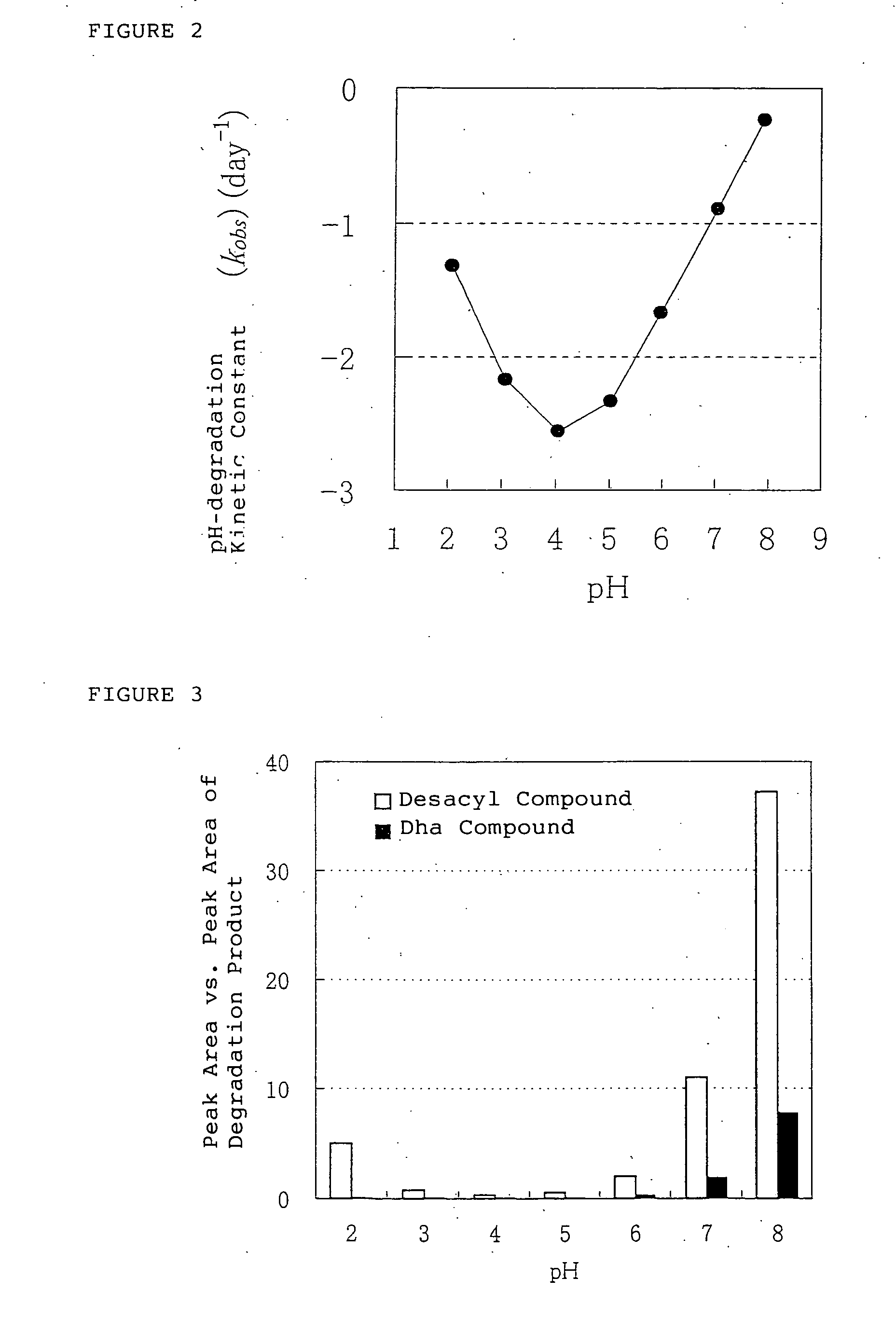
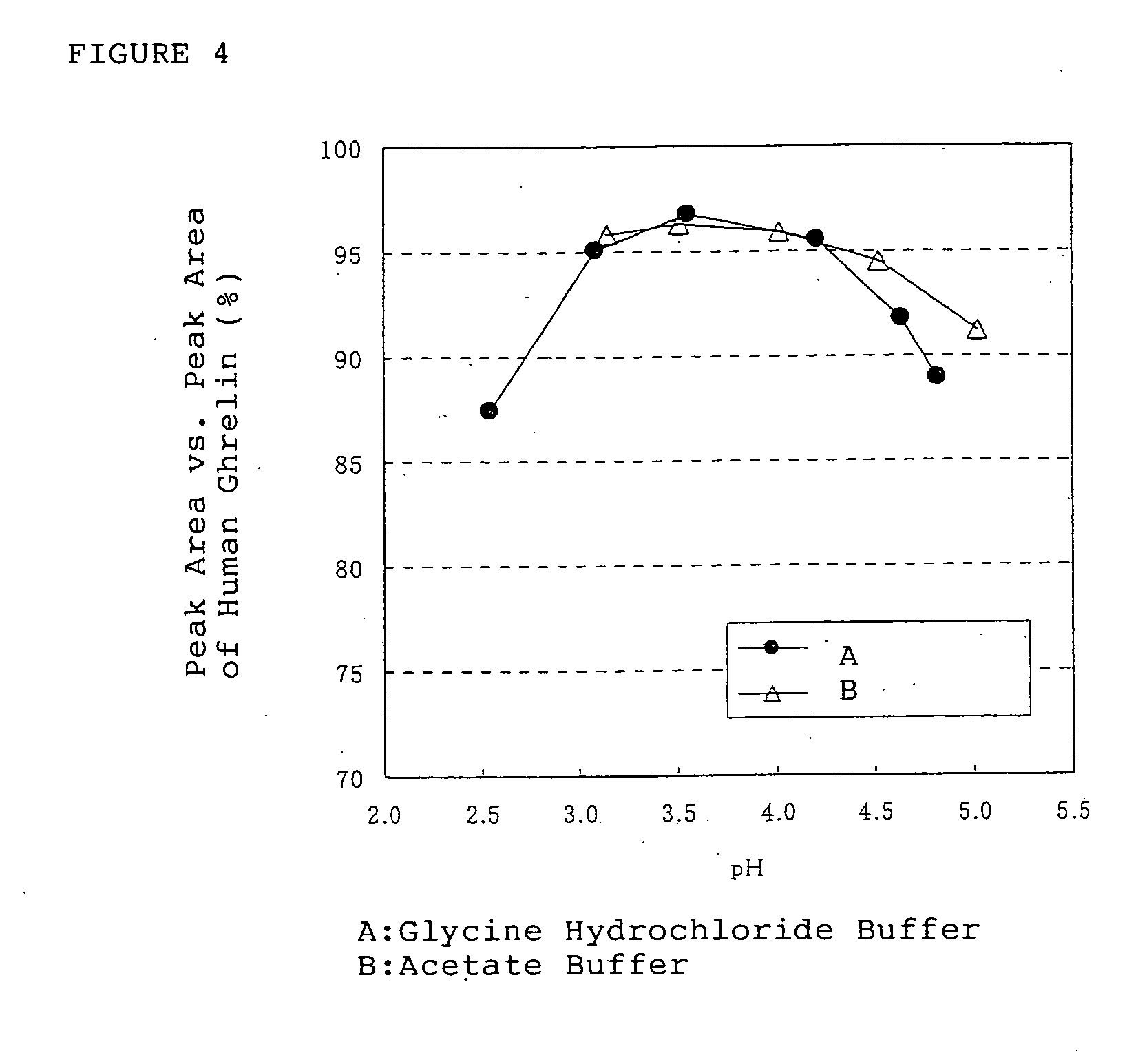
Medical compositions containing ghrelin
It is provided a pharmaceutical composition stably containing ghrelin or its derivative, which is an endogenous growth hormone secretagogue (GHS) to a growth hormone secretagogue-receptor (GHS-R), comprising a aqueous solution containing the ghrelins having pH range of 2 to 7, wherein the aqueous solution having pH range of 2 to 7 is a buffer solution, especially, glycine hydrochloride buffer, acetate buffer, citrate buffer, lactate buffer, phosphate buffer, citric acid-phosphate buffer, phosphate-acetate-borate buffer or phthalate buffer, and the concentration of the ghrelins in the solution is from 0.03 nmol / mL to 61 μmol / mL.
Owner:ASUBIO PHARMA +1
Quantum dot-antibody fluorescent probe, preparation method, probe and test paper strip
InactiveCN106526192AHigh fluorescence recovery rateImprove detection accuracyBiological testingFluorescenceN-Hydroxysuccinimide
The invention provides a quantum dot-antibody fluorescent probe preparation method, a quantum dot-antibody fluorescent probe, and an immunochromatography test paper strip and a preparation method thereof. The quantum dot-antibody fluorescent probe preparation method comprises: (1) adding quantum dots to a borate buffer solution to prepare a quantum dot solution; (2) adding an activator to the quantum dot solution, and activating, wherein the activator is 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide or a hydrochloride thereof and N-hydroxysuccinimide or a sulfide thereof, a molar ratio of the activator to the quantum dots is 5-9:1, and a molar ratio of the 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide or the hydrochloride thereof to the N-hydroxysuccinimide or the sulfide thereof is 2-3:4-5; and 3) adding antibody to the activated quantum dot solution, and coupling to obtain the quantum dot-antibody fluorescent probe, wherein a molar ratio of the antibody to the quantum dots is 4-2:1.
Owner:JIANGSU LIANGDIAN TECH CO LTD
Ophthalmic Compositions with Hyaluronic Acid
Owner:BAUSCH & LOMB INC
Analysis of Amino Compounds by Precolumn Derivatization with Dimethoxybenzenesulfonyl Chloride
InactiveCN102288707ASuitable for analysisDerivatization reaction conditions are mildComponent separationIce waterReaction temperature
The invention discloses a pre-column derivatization method for high-performance liquid chromatography for analyzing amino compounds, and belongs to the technical field of analytical chemistry. The process is as follows: prepare the sample solution with a borax buffer solution with a pH of 8.0 to 11.5 and a concentration of 0.05 to 0.35M for the primary or secondary or primary and secondary amino compound samples, and add dimethoxybenzenesulfonyl chloride Methanol or ethanol or acetonitrile solution, mix well, conduct derivatization reaction at 25-45°C for 10-40 minutes, cool in ice-water bath, filter and inject samples into full-performance liquid chromatography, use ultraviolet detector to detect the derivatization of amino compounds Derivatives were analyzed quantitatively. The invention has the advantages of simple derivation reaction operation, short reaction time, high sensitivity, simultaneous derivation of primary and secondary amine compounds, no need to remove excess reagents after derivation, stable derivatized products, low derivatization reaction temperature, and is especially suitable for the analysis of biological samples.
Owner:NANJING TECH UNIV
Preparation and application of polymer-coated magnetic nanoparticle contrast agent
InactiveCN101474414AImprove image contrastGood effectNMR/MRI constrast preparationsEmulsion deliveryPotassium borohydridePotassium cyanide
The invention relates to the field of medical image diagnosis and provides a preparation method and the application of macromolecule encapsulation magnetic nano-particle contrast agent. The current preparation method of superparamagnetic contrast agent is complex. The preparation of the macromolecule encapsulation magnetic nano-particle contrast agent comprises the steps as follows: iron chloride, iron dichloride and glucan are dissolved in secondary distilled water; strong ammonia is added slowly at the temperature of 15 DEG C to 40 DEG C to be constantly stirred for 130 to 150 minutes; centrifugal separation and deposition are carried out; potassium periodate solution is added; the processed solution is put into borate buffer solution with pH of 8.0; mouse anti-human CA19-9 antibody is added to be reacted at the temperature of 4 DEG C over a night; potassium borohydride is added to be reacted for 5 to 8 hours; and the mixture is purified and separated to be dispersed in buffer solution with pH of 7.4 to obtain the macromolecule encapsulation magnetic nano-particle contrast agent. The invention has the advantages that the invention improves the tumor magnetic resonance imaging contrast with good effect, has simple preparation method and is safe and convenient for use.
Owner:SHANGHAI NORMAL UNIVERSITY
Method for efficiently separating and detecting theanine enantiomer by capillary electrophoresis
InactiveCN102095775AEasy to separateNo pollution in the processMaterial analysis by electric/magnetic meansElectrophoresesCapillary electrophoresis
The invention discloses a method for efficiently separating and detecting theanine enantiomer by capillary electrophoresis, belonging to the technical field of separation and detection of enantiomers. The method determines the chiral separation environment required by separation of theanine, a borate buffer liquid system with pH value of 10 to 12 is selected, and a chiral selecting reagent is added in the buffer liquid. A theanine sample is prepared, the concentration is 0.1 to 2.0mg / mL, and the theanine enantiomer is separated on baseline in a condition of 15-30kV separation voltage of capillary electrophoresis. The method has good separating efficiency, no pollution and low cost, and is applicable to analysis and detection of theanine enantiomer.
Owner:JIANGNAN UNIV
Aqueous solution based on an azo dye, process for its manufacture and use thereof
InactiveUS6777242B1Determine accurately and selectivelyAvoid lostMonoazo dyesAnalysis using chemical indicatorsChlorine dioxideAqueous solution
The present invention relates to a stable aqueous solution (A) comprising an azo dye, a borate buffer and one or more masking agents, wherein the azo dye changes its coloration or coloration intensity in the presence of chlorine dioxide. The present invention further relates to a process for manufacturing the aqueous azo-dye solution (A), and to its use for the determination of residual chlorine dioxide in water.
Owner:ELF ATOCHEM SA
Immunization grouping analysis and detection method based on quantum point
InactiveCN101551387AOmit operations such as transparencyShorten the timeChemiluminescene/bioluminescencePhosphoric acidFluorescence microscope
An immunization grouping analysis and detection method based on quantum point belongs to the nano-material field, comprises the following steps: (1) dispersing quantum points into borate buffer solution, adding sulfurated diimine and second antibody, mixing, reacting, centrifugating, washing, and obtaining second antibody compound with quantum point marks; wherein, the mole ratio of the quantum points, the sulfurated diimine and the second antibody is: 60: 15: 1; or (2) dispersing the quantum points into phosphoric acid buffer solution containing 1.25 % of glutaraldehyde by volume fraction, dispersing, reacting, adding second antibody, reacting, centrifugating, washing, and obtaining second antibody compound with quantum point marks; wherein, the mole ratio of the quantum points, the glutaraldehyde and the second antibody is: 60: 15: 1; then performing immunization reaction and observation according to the standard immunization grouping detection method. The method in the invention performs observation and judgement directly by fluorescence microscope, is simple and easy, can increase the fluorescence stability, and improve analysis accuracy and detection sensitivity.
Owner:SHANGHAI JIAO TONG UNIV
Product made of native silk fibres
ActiveCN104822867ARobust preparation methodSuitable for industrial scale productionSuture equipmentsWeft knittingFiberAqueous ethanol
Disclosed is a method for the production of two- or three-dimensional silk products, wherein native silk fibres are formed into a two- or three-dimensional silk product and the silk product formed is subjected to a degumming step wherein a borate buffer is used as degumming agent and wherein the silk product formed is preferably contacted with an aqueous ethanol buffer prior to the degumming step.
Owner:MORPHOMED GMBH
Synthesizing method of 3-N-ethyl gentamicin C1a
InactiveCN107652334AHigh purityHigh yieldSugar derivativesSugar derivatives preparationPotassium borohydridePotassium
The present invention relates to a kind of synthetic method of 3-N-ethyl gentamicin C1a, the method comprises the following steps: ethylene glycol dimethyl ether, hexamethyldisilazane, concentrated sulfuric acid, 2 ", 6" ‑N,N‑Diacetylgentamycin C1a was put into a round-bottomed flask and refluxed. After the reactants were dissolved, part of the solvent was distilled off. Dichloromethane was added and stirred at 10°C, and acetaldehyde was added dropwise. After the reaction was completed, potassium borohydride and boric acid buffer were added to stir the reaction, and part of the solvent was distilled off under normal pressure. Add 10%-20% sodium hydroxide solution, heat to reflux, cool down to room temperature, and concentrate the reaction system. Desalting, the target compound 3-N-ethyl gentamycin C1a was obtained by separation.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
Detection of immobilized nucleic acid
The present invention provides methods for determining the presence of immobilized nucleic acid employing unsymmetrical cyanine dyes that are derivatives of thiazole orange, a staining solution and select fluorogenic compounds that are characterized as being essentially non-genotoxic. The methods comprise immobilizing nucleic acid, single or double stranded DNA, RNA or a combination thereof, on a solid or semi solid support, contacting the immobilized nucleic acid with an unsymmetrical cyanine dye compound and then illuminating the immobilized nucleic acid with an appropriate wavelength whereby the presence of the nucleic acid is determined. The cyanine dye compounds are typically present in an aqueous staining solution comprising the dye compound and a tris acetate or tris borate buffer wherein the solution facilitates the contact of the dye compound and the immobilized nucleic acid. Typically the solid or semi-solid support is selected from the group consisting of a polymeric gel, a membrane, an array, a glass bead, a glass slide, and a polymeric microparticle. Preferably, the polymeric gel is agarose or polyacrylamide. The methods employing the non-genotoxic compounds represent an improvement over commonly used methods employing ethidium bromide wherein the present methods retain the advantages of ethidium bromide, ease of use and low cost, but without the disadvantageous, known mutagen requiring special handling and waste procedures.
Owner:LIFE TECH CORP
Method for preparing carbon quantum dots by using Enteromorpha
InactiveCN107311143AUniform particle sizeExcellent fluorescence propertiesNanotechnologyNano-carbonBiological imagingImpurity
The invention belongs to the technical field of preparation of carbon nanometer materials and new marine materials, and relates to a method for preparing carbon quantum dots by using Enteromorpha in sea, and applications of the prepared carbon quantum dots in metal ion detection, biological imaging and other fields. The method comprises: rinsing enteromorpha by using deionized water, placing into a 150 ml polytetrafluoroethylene reaction kettle, adding a borate buffer solution with the pH value of 10.0, placing the reaction kettle into a vacuum oven, carrying out a reaction for 6 h at a temperature of 180 DEG C, taking out the reaction kettle, cooling to a room temperature to obtain a carbon quantum dot solution, carrying out centrifugation on the obtained carbon quantum dot solution for 20 min through a centrifuge under a condition of 11900 rpm, removing solid impurities and precipitate, and taking the supernatant so as to obtain the carbon quantum dots. According to the present invention, the method has characteristics of simple preparation process, convenient operation, easily available raw materials and low preparation cost, and the prepared carbon quantum dots have advantages of no toxicity, uniform particle size, excellent fluorescence property, and good stability.
Owner:QINGDAO UNIV
High performance liquid phase detection method of homopiperazine
ActiveCN110596293AHigh precisionImprove stabilityComponent separationIon chromatographyUltraviolet absorption
The invention discloses a high performance liquid phase detection method of homopiperazine. The high performance liquid phase detection method comprises the steps of: diluting a fasudil hydrochlorideinjection with water and adding homopiperazine into the diluted fasudil hydrochloride injection; adding a borate buffered saline and a derivatization reagent into a dry derivatization tube; sealing the derivatization tube with a paraffin film, heating the derivatization tube, and cooling the derivatization tube to obtain a derivative sample; and acquiring a chromatogram of the derivative sample byadopting a liquid chromatograph, and measuring the content of homopiperazine. On the basis of a reversed-phase high-performance liquid phase chromatographic pre-column derivatization method-AccQ.Tagmethod developed by the Waters company, the derivatization reagent is used for adding a fluorophore onto an amino group of homopiperazine; the modified homopiperazine derivative has ultraviolet absorption, and qualitative and quantitative analysis is carried out by using high performance liquid phase chromatography. The high performance liquid phase detection method disclosed by the invention is high in precision, good in stability and good in reproducibility, enriches the method for detecting the homopiperazine content, and fills the blank that only ion chromatography can be used for liquid phase detection of the homopiperazine content.
Owner:SHANDONG WEIGAO PHARM CO LTD
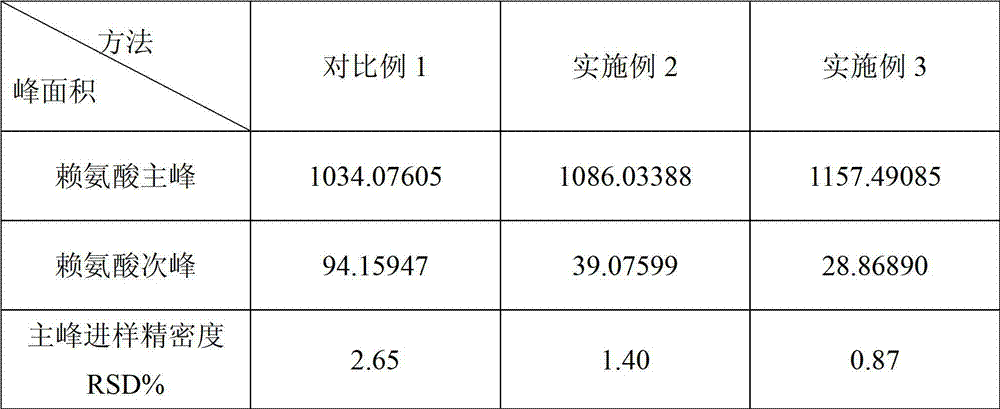
Content detection method for lysine of compound ketoacid tablet
InactiveCN103048407ASolve the double peak problemImprove injection precisionComponent separationBimodalityBORATE BUFFER
The invention relates to a content detection method for lysine of a compound ketoacid tablet, which comprises the following steps: preparing an o-phthalaldehyde reaction liquid (OPA reagent) with the concentration of 10 mg / ml, wherein 2-3 percent (v / v) of 3-thiohydracrylic acid is contained; afterwards, mixing lysine and the reaction liquid at ratio of 1:1(v / v) in a borate buffer system with a pH valve of 10.2 plus or minus 0.1 through an automatic pre-column derivation method, and then carrying out ambient temperature reaction; and finally, adopting a high performance liquid chromatography to measure the content of the lysine at 338 nm. The method provided by the invention can effectively solve the problem that bimodality of the lysine occurs in the current OPA pre-column derivation method, improves the sample introduction precision of the lysine in the detection through the automatic pre-column derivation method, greatly shortens the detection time, and is particularly suitable for detecting the content of the lysine of amino acid samples in a laboratory.
Owner:SHANGHAI JINGFENG PHARMA
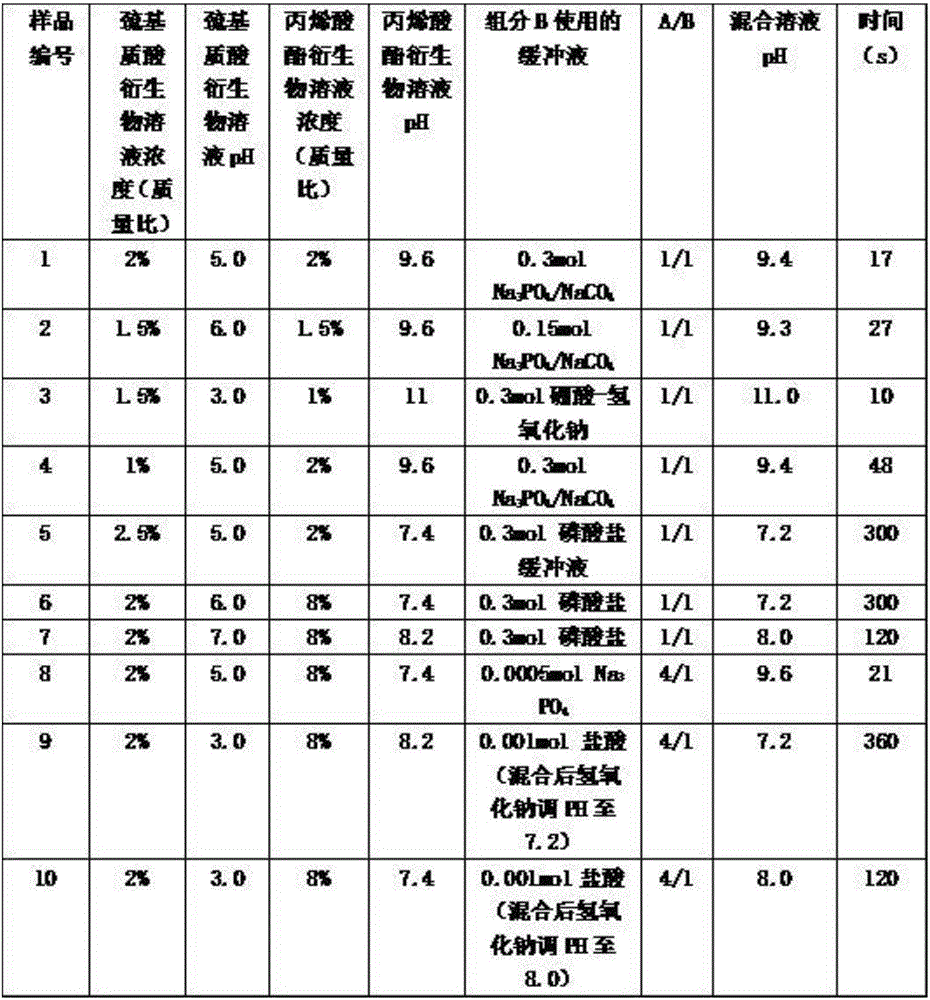
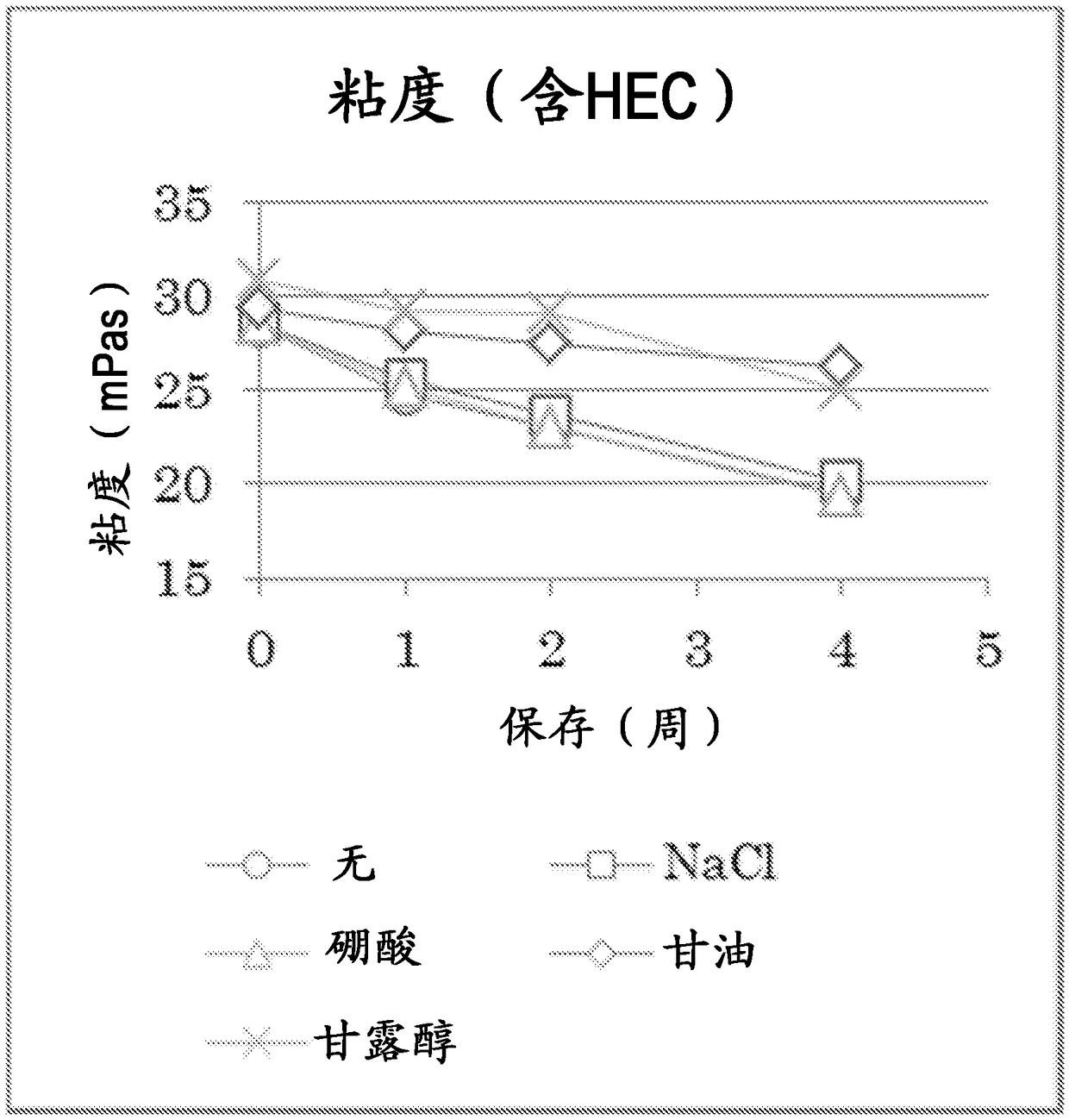
Method for preparing liquid bandage for war wounds
The invention discloses a method for preparing a liquid bandage for war wounds. The method is characterized by comprising the following steps: 1, dissolving a mercapto-hyaluronic acid derivative, a tackifier, a humectant and a preservative into de-ionized water to form a component A; 2, dissolving an acrylic ester derivative and the preservative into de-ionized water, and adding a borate buffer solution to form a component B; 3, spraying the component A onto the surface of a wound, then spraying the component B to the surface of the wound, and fast generating cross-linking within 10 seconds to form the liquid bandage for the war wounds. The prepared liquid bandage for the war wounds is high in biocompatibility, free of skin irritation and cytotoxicity and applicable to relatively small war wounds such as gunshot wounds, burns and cuts. The preparation method is simple and fast, and has high practical value.
Owner:南京盛骏医疗科技有限公司
A detection method for African swine fever based on quantum dot technology
InactiveCN102279265ASave dehydrationOmit operabilityMaterial analysis by observing effect on chemical indicatorQuantum technologyAfrican swine fever
An immunohistochemical analysis and detection method based on quantum dots in the field of nanomaterials, comprising the following steps: (1) first dispersing the quantum dots in a borate buffer solution, then adding diimide sulfide and a secondary antibody, mixing , react, centrifuge, and wash to obtain the African swine fever monoclonal antibody complex labeled with quantum dots; wherein, quantum dots: diimine sulfide: the molar ratio of the secondary antibody is 60: 15: 1; or (2) first the quantum dots Disperse in a phosphate buffer solution containing 1.25% glutaraldehyde by volume, disperse, react, add a secondary antibody, react, centrifuge, and wash to obtain a quantum dot-labeled African swine fever monoclonal antibody complex; wherein, the quantum dot The molar ratio of :glutaraldehyde:secondary antibody was 60:15:1; after that, immunoreaction and observation were carried out according to the standard immunohistochemical detection method. The method of the invention directly observes with a fluorescent microscope, is simple and easy to implement, increases the stability of the fluorescent light, and improves the accuracy of analysis and the sensitivity of detection.
Owner:陈文刚
Atropine-containing aqueous composition
ActiveCN109310687AMaintain initial viscosityInhibition of length elongationSenses disorderInorganic non-active ingredientsPhosphateCarboxylic acid
Disclosed herein is an aqueous composition comprising 0.001 - 0.1 % (w / v) atropine or a salt thereof, a water-soluble polymer, and buffer (I), which is at a pH range of 6 or lower, wherein the buffer(I) is at least one selected from the group consisting of a phosphate buffer, an aminocarboxylate buffer, a carbonate buffer, an acetate buffer, a tartrate buffer, a borate buffer, and trometamol.
Owner:SINGAPORE HEALTH SERVICES PTE +2
Bensulfuron-methyl artificial immunogen BE-BSA, preparation thereof and application thereof
InactiveCN101717443AStrong specificityHigh potencySerum albuminPeptide preparation methodsProtein solutionBovine serum albumin
The invention relates to bensulfuron-methyl artificial immunogen BE-BSA, a preparation thereof and application thereof. The preparation for a structural formula comprises the following steps: (1) dissolving bensulfuron-methyl in solution of NaOH for reaction, extracting and separating the solution by chloroform, acidifying an aqueous layer, filtering the solution under reduced pressure, and drying the filtrated solution in vacuum to obtain a purified product; and (2) dissolving bensulfuron-methyl hapten, N-hydroxy succinimide (NHS) and N,N'-dicyclohexyl carbimide (DCC) in an organic solvent, stirring and reacting the mixture, and centrifugating the solution and taking supernatant to obtain active ester; and dissolving bovine serum albumin (BSA) in borate buffer solution (pH 8), adding the active ester into solution of protein under strong stirring, dialyzing the solution after reacting, packing the dialyzed product, and preserving the product at the temperature of 20 DEG C below zero. The preparation is simple and convenient, has low cost, and is easy for industrialized production; and the bensulfuron-methyl artificial immunogen BE-BSA is prepared into a bensulfuron-methyl specific antibody through immune animals for detecting trace bensulfuron-methyl in water body and soil on site.
Owner:NANJING UNIV OF TECH
Extraction method of RNA (ribonucleic acid) of rape seeds
The invention belongs to the technical field of gene engineering, and particularly relates to an extraction method of an RNA (ribonucleic acid) of rape seeds. The method comprises the following steps of: preheating a hot borate buffer solution to 65 DEG C; quickly freezing the rape seeds with liquid nitrogen, grinding into powder, adding the preheated buffer solution, and continuously grinding to form homogenate; incubating, precipitating proteins with potassium chloride, and performing centrifugation, impurity removal and other steps to obtain general total RNA. The total RNA of the rape seeds extracted by the method has complete quality and no remarkable degrading phenomenon, the OD260 / OD280 and OD260 / OD230 are at about 2.0, other impurity pollution, such as proteins and salt ions, does not exist in the sample, and the product production rate is more than 40mu g / 100mg, which is in accordance with the requirement of cDNA library construction.
Owner:YANGZHOU UNIV
Eye drops capable of preventing asthenopia
InactiveCN102793786AProduce dependenceRelieve eye fatigueSenses disorderPharmaceutical delivery mechanismEye strainingSemen
The invention discloses eye drops capable of preventing asthenopia, containing the following components: 2-4g of bear gall, 5-8g of semen cassiae, 2-5g of golden cypress, 8-10g of white chrysanthemum, 2-4g of Gentiana scabra Bunge, 4-6g of honeysuckle and 1-2g of Cordyceps sinensis. The eye drops are prepared by adopting the following method: taking cleaned processed medicinal materials with impurity removed according to weight, adding a proper amount of water for soaking for two hours, heating and decocting for two times, with each time lasting for 30-50 minutes, then cooling, filtering and concentrating to 100ml, and adding a pH value modifier to adjust the pH value to 4-9; and the pH value modifier can be phosphate buffer agent or borate buffer agent. The eye drops are prepared by adopting pure nature Traditional Chinese medicine materials through deep processing, can effectively relieve asthenopia, and can avoid dependence of eye on the eye drops even after being used for a long time.
Owner:张加宾
Angiotensin converting enzyme detection kit with good repeatability
InactiveCN104792779ASolve the problem of poor repeatabilityReduce surface tensionMaterial analysis by observing effect on chemical indicatorAngiotensin-converting enzymeBiochemistry
The invention provides an angiotensin converting enzyme detection kit with good repeatability. The detection kit comprises the following components: 0.5 mmol / L of FAPGG, 300 mmol / L of NaCl, 80 mM of a borate buffer solution with the PH value of 8.2 and 0.1-1% of AEO-9. The detection kit provided by the invention overcomes the defect of poor repeatability in the prior art in detecting angiotensin converting enzyme.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Preparation and application of polymer-coated magnetic nanoparticle contrast agent
InactiveCN101474414BImprove image contrastGood effectNMR/MRI constrast preparationsEmulsion deliveryPotassium borohydrideMagnetite Nanoparticles
Owner:SHANGHAI NORMAL UNIVERSITY
Method for performing bio-enzyme immobilization through rice bran
ActiveCN104651341AWide variety of sourcesReduce manufacturing costOn/in organic carrierBiological water/sewage treatmentSewageSewage treatment
The invention discloses a bio-enzyme immobilization method in the field of sewage treatment process, and specifically discloses a method for performing bio-enzyme immobilization through utilizing rice processing product rice bran. The method comprises the following steps: processing bio-enzyme, namely, adding hydrogen peroxide to an alkaline solution, mixing bio-enzyme with a NaOH water solution at the temperature of 30 to 35 DEG C, and then collecting supernate; adding a borate buffer solution to dissolve and precipitate; filtering to obtain alkaline protein modified enzyme liquid; mixing the alkaline protein modified enzyme liquid with rice bran in a proportion; spraying the alkaline protein modified enzyme liquid on the rice bran; and slowly drying so as to obtain the rice bran immobilization bio-enzyme. The method has the advantages of low production cost, wide raw material source and good sewage treatment effect.
Owner:HANGZHOU CHENGJIE ENVIRONMENTAL PROTECTION
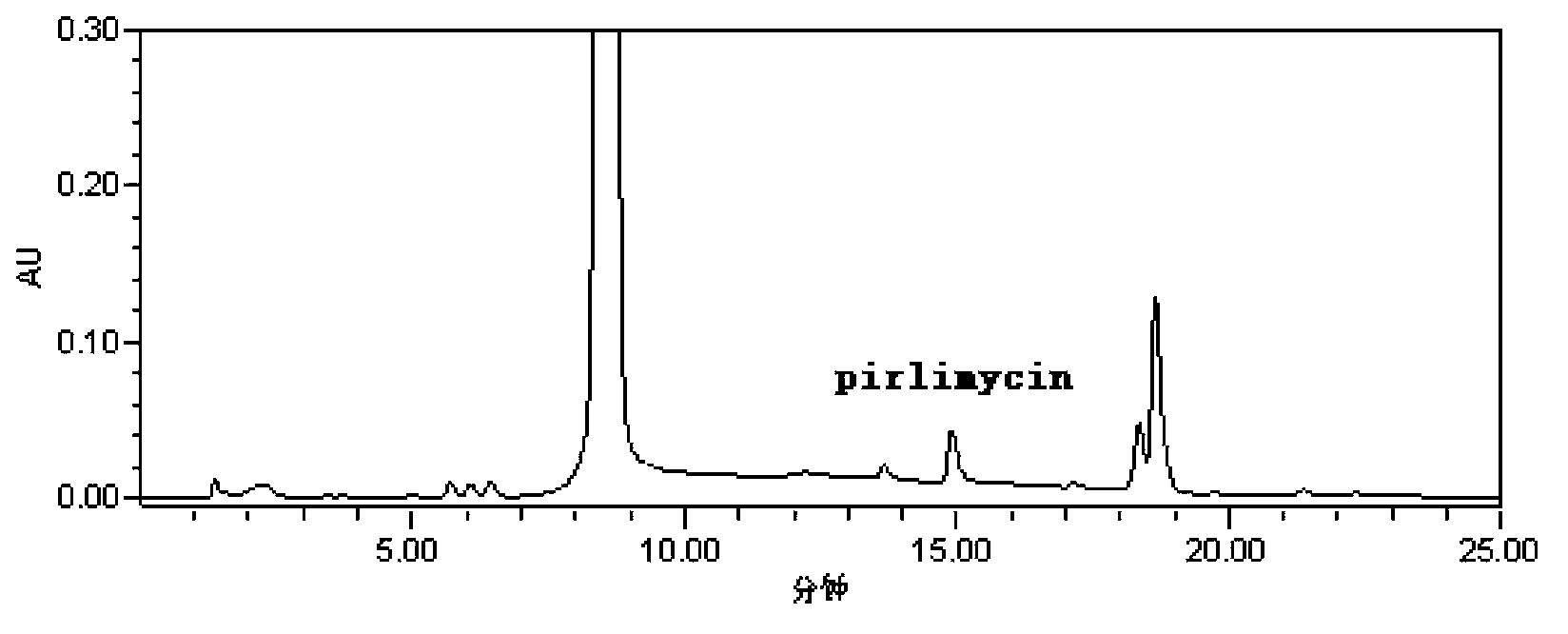
Pirlimycin residue analysis method
InactiveCN103424478AHigh fluorescence activityHigh UV absorption strengthComponent separationUltravioletChloroformic acid
The invention belongs to the field of veterinary drug residue analysis and relates to a pirlimycin residue analysis method. According to the invention, a high-performance liquid chromatograph pre-column derivatization reaction analysis is adopted for a hydrogen substitution reaction of a secondary amine compound. At the room temperature, the concentration of a derivatization reagent, chloroformic acid-9-fluorenylmethyl ester, is 10-2 mol / L, a pH value of a borate buffer solution of 0.1 mol / L is regulated to 9, the reaction is an instant reaction, as only one secondary amine is used as a single reaction site, no reaction terminator is required to be added to achieve the purpose of derivatization, and the derivation product is stable. The derivation product is subject to high performance liquid chromatograph detection, has the maximum ultraviolet strength when the ultraviolet detection wavelength is 266 nm, has the minimum detectable amount of 5 mug / L, is in linear response to the peak area within the range of 20-4000 [mu]g / L, is good in reproducibility of results, and has three concentrations, and the variable coefficient of 3 concentrations and 15 repetitions is smaller than 8%.
Owner:HUAZHONG AGRI UNIV
Kit for rapidly detecting ischemia modified albumin in blood
ActiveCN105973888AUniform physical and chemical propertiesMulti-buffer mixing spaceMaterial analysis by observing effect on chemical indicatorBORATE BUFFERCobalt
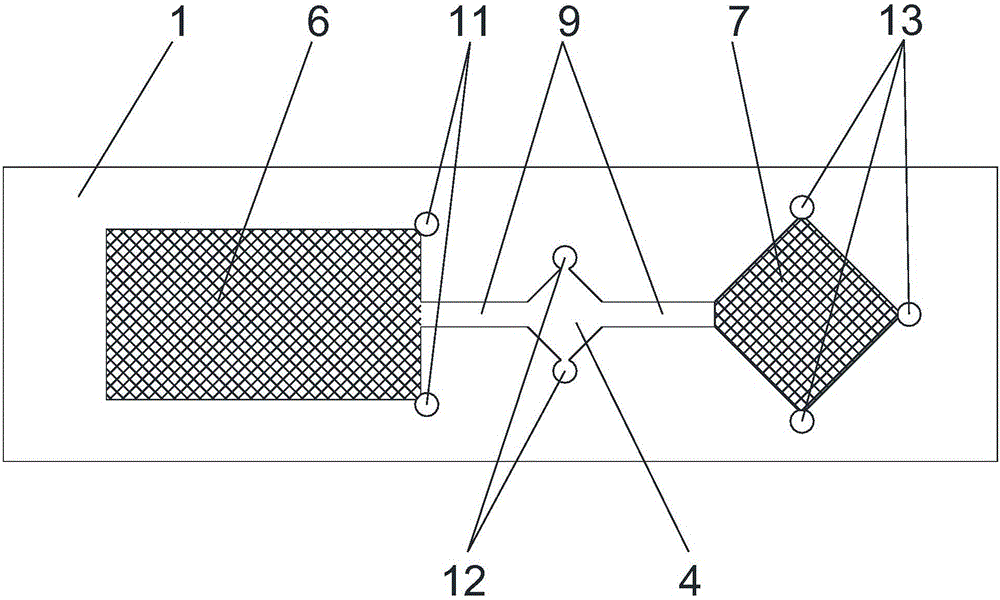
The invention discloses a kit for rapidly detecting ischemia modified albumin in blood. The kit comprises a detection chip and a reagent bottle filled with detection reagents, wherein the detection chip comprises a non-absorbent bottom plate and a non-absorbent cover plate; a sample cell, a mixing cell and a detection cell are sequentially arranged on the bottom plate; micro pipelines are formed between the sample cell and the mixing cell and between the mixing cell and the detection cell; a sample pad is filled in the sample cell; a cobalt standard substance is coated on the sample pad; a detection pad is filled in the detection cell; a cobalt indicator is coated on the detection pad; the cover plate is fixedly arranged above the bottom plate; a sample injection hole is formed in a position, which is positioned above the sample cell, of the cover plate; and the detection reagents refer to phosphate buffer, borate buffer or Tris-HCl buffer. According to the kit disclosed by the invention, based on the principle of an albumin cobalt binding test, sample separation and detection analysis processes are integrated into a device, so that IMA detection is more convenient.
Owner:西安良升生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com