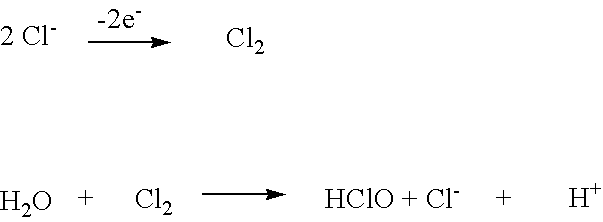
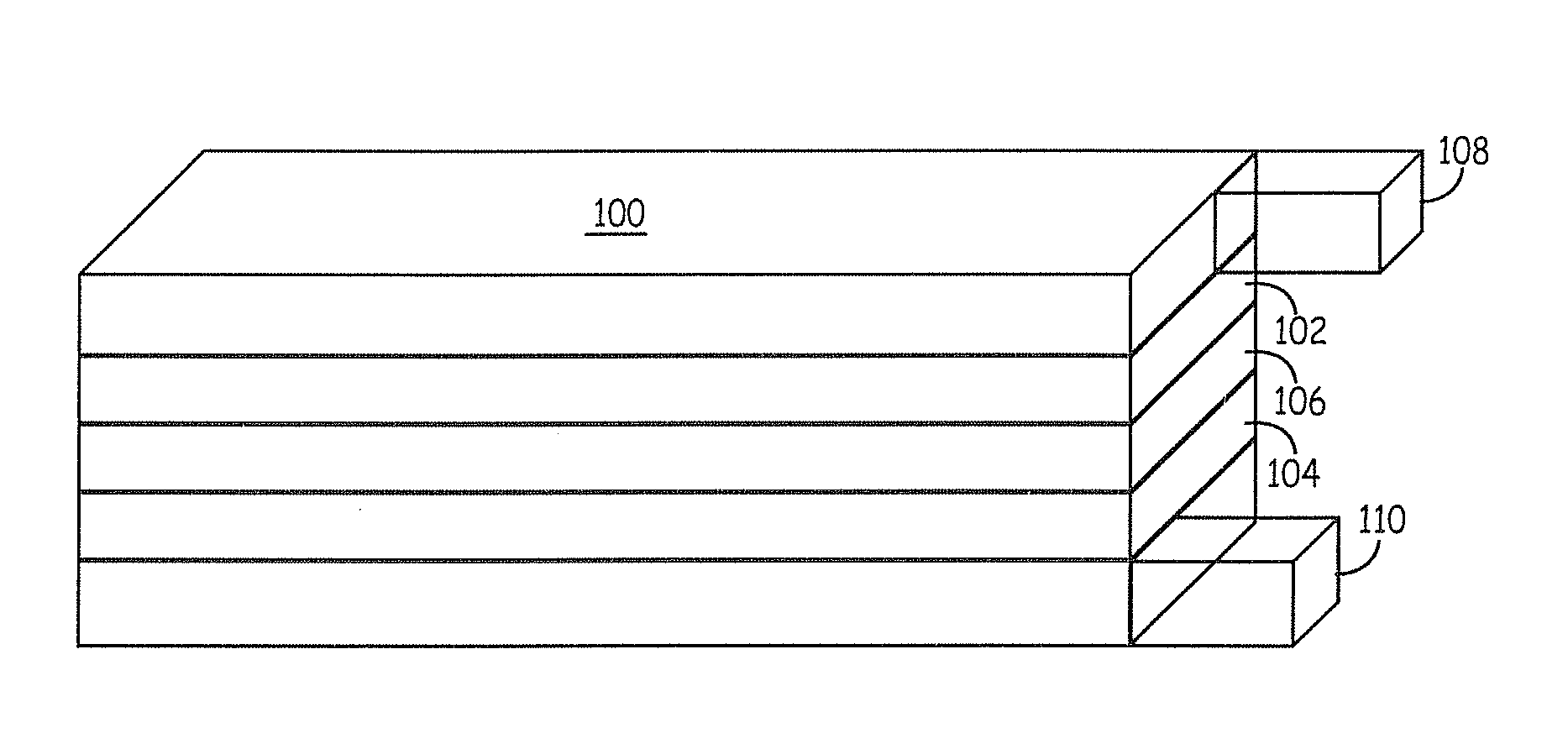
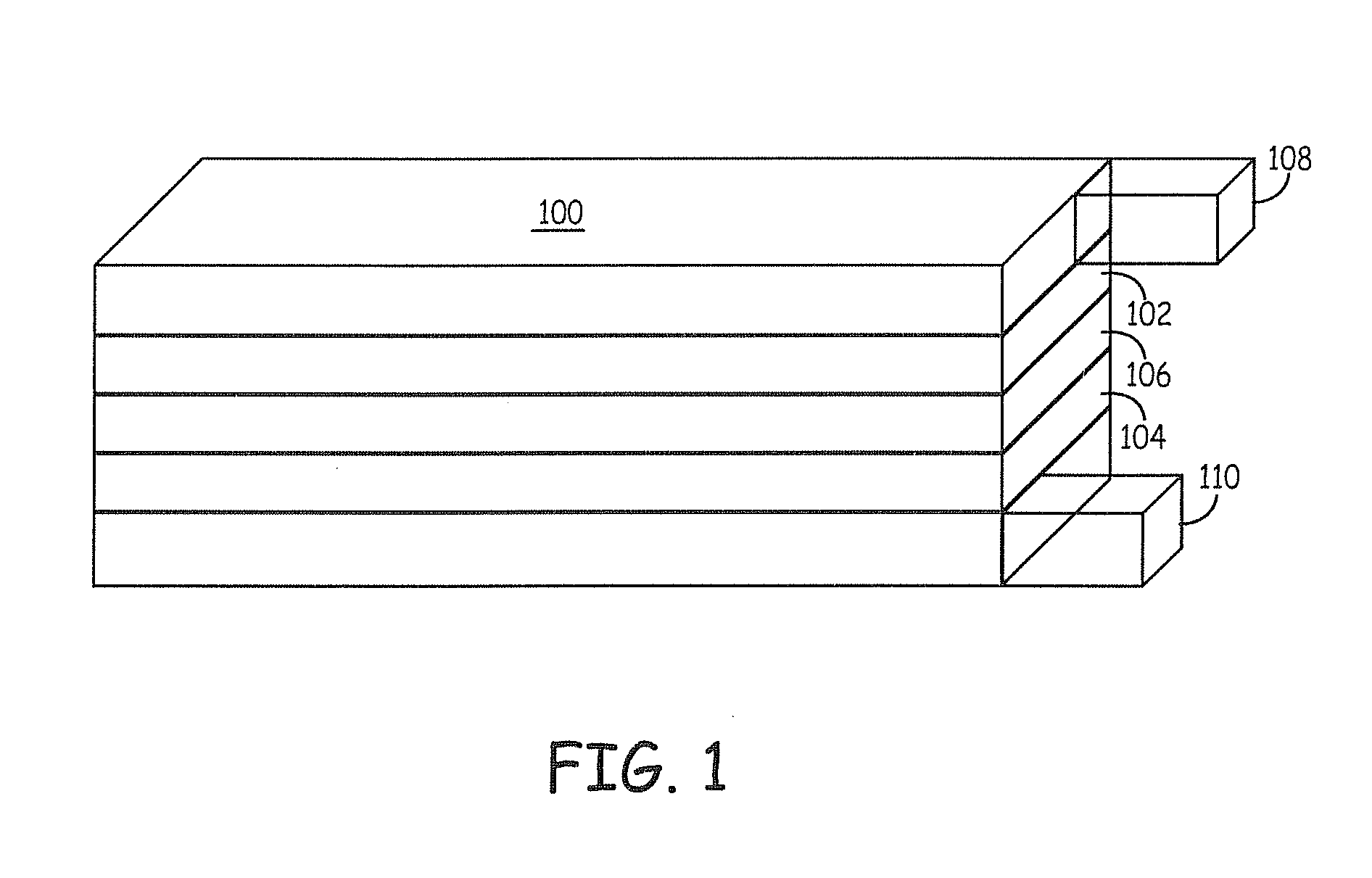
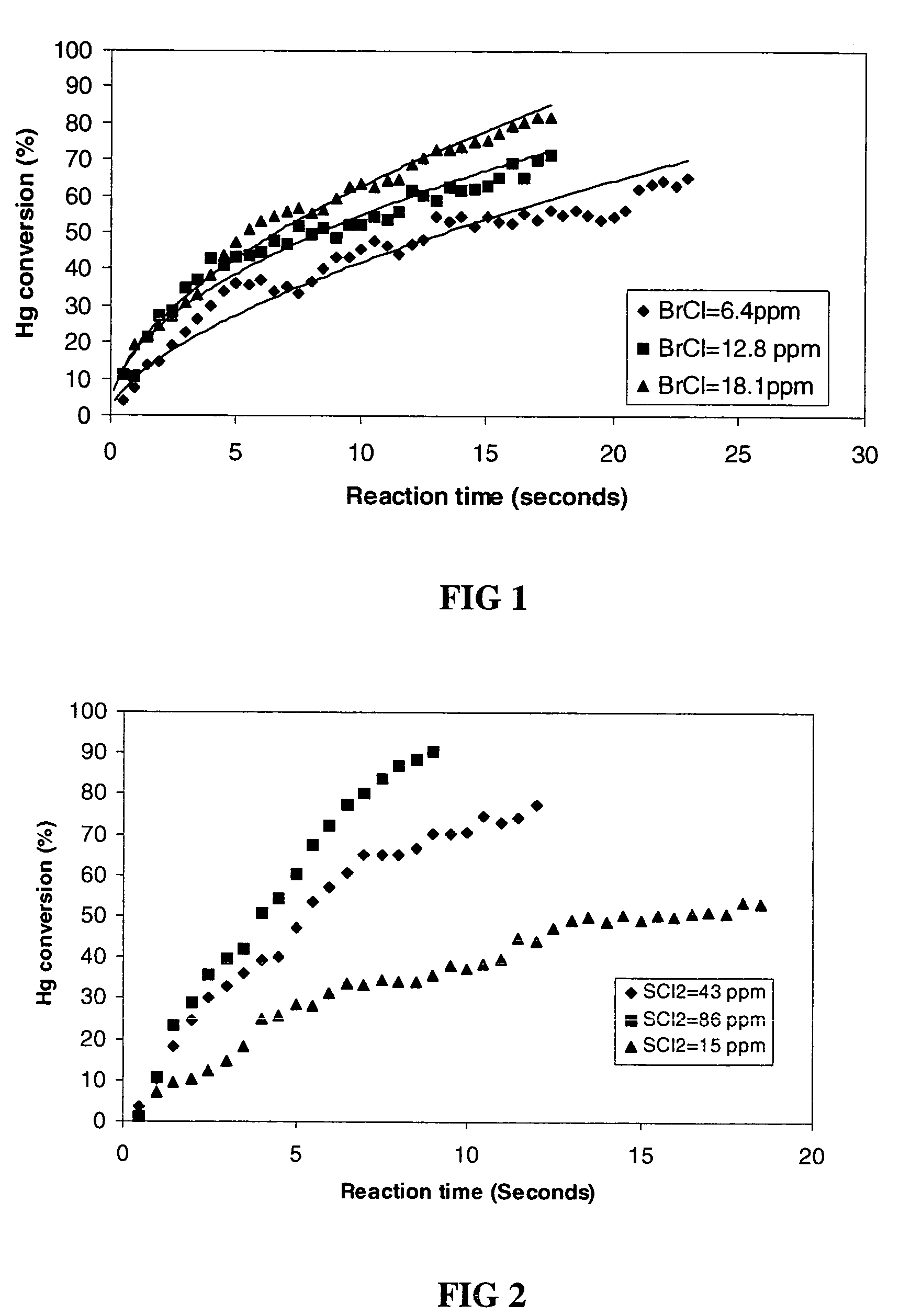
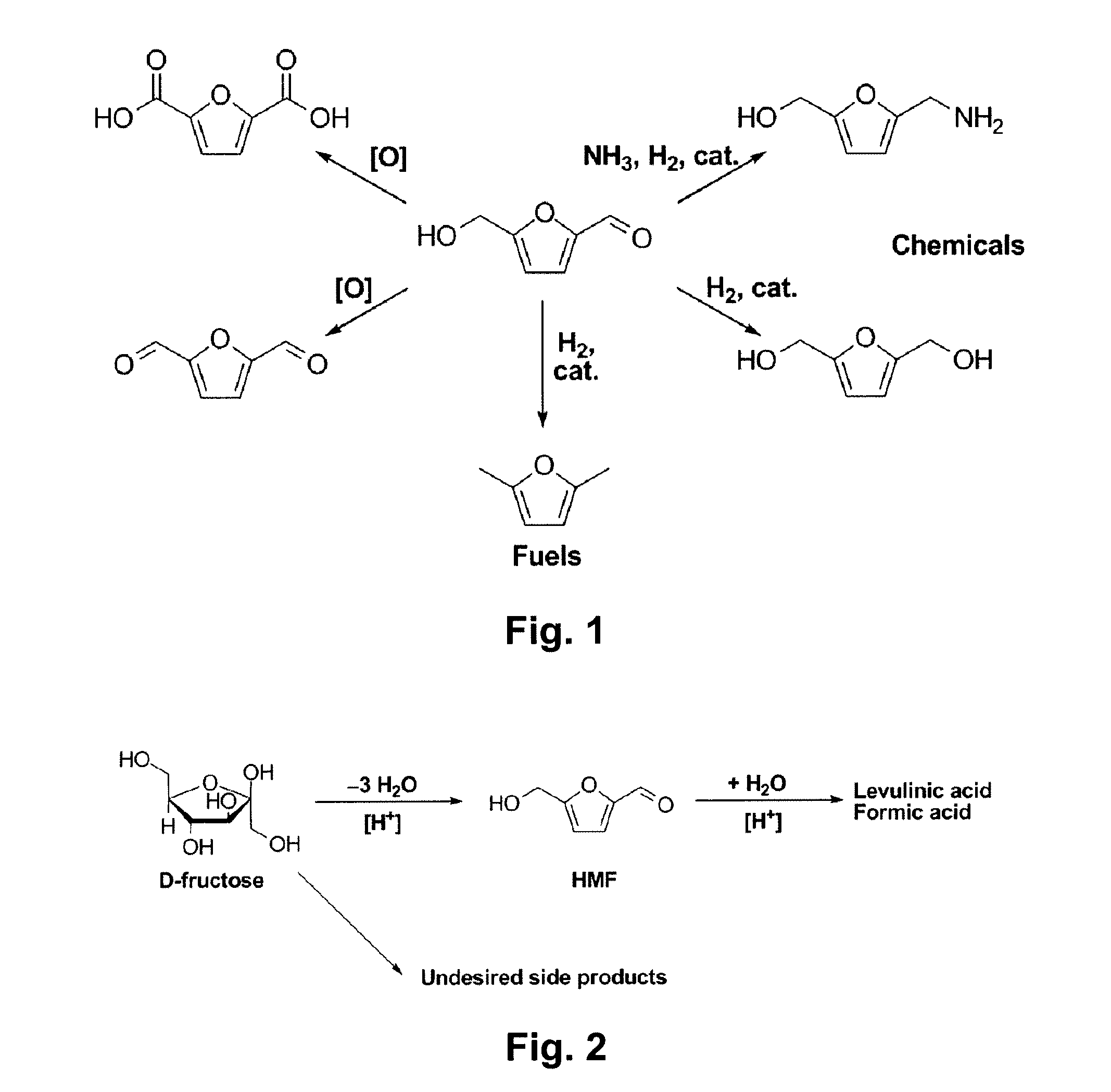
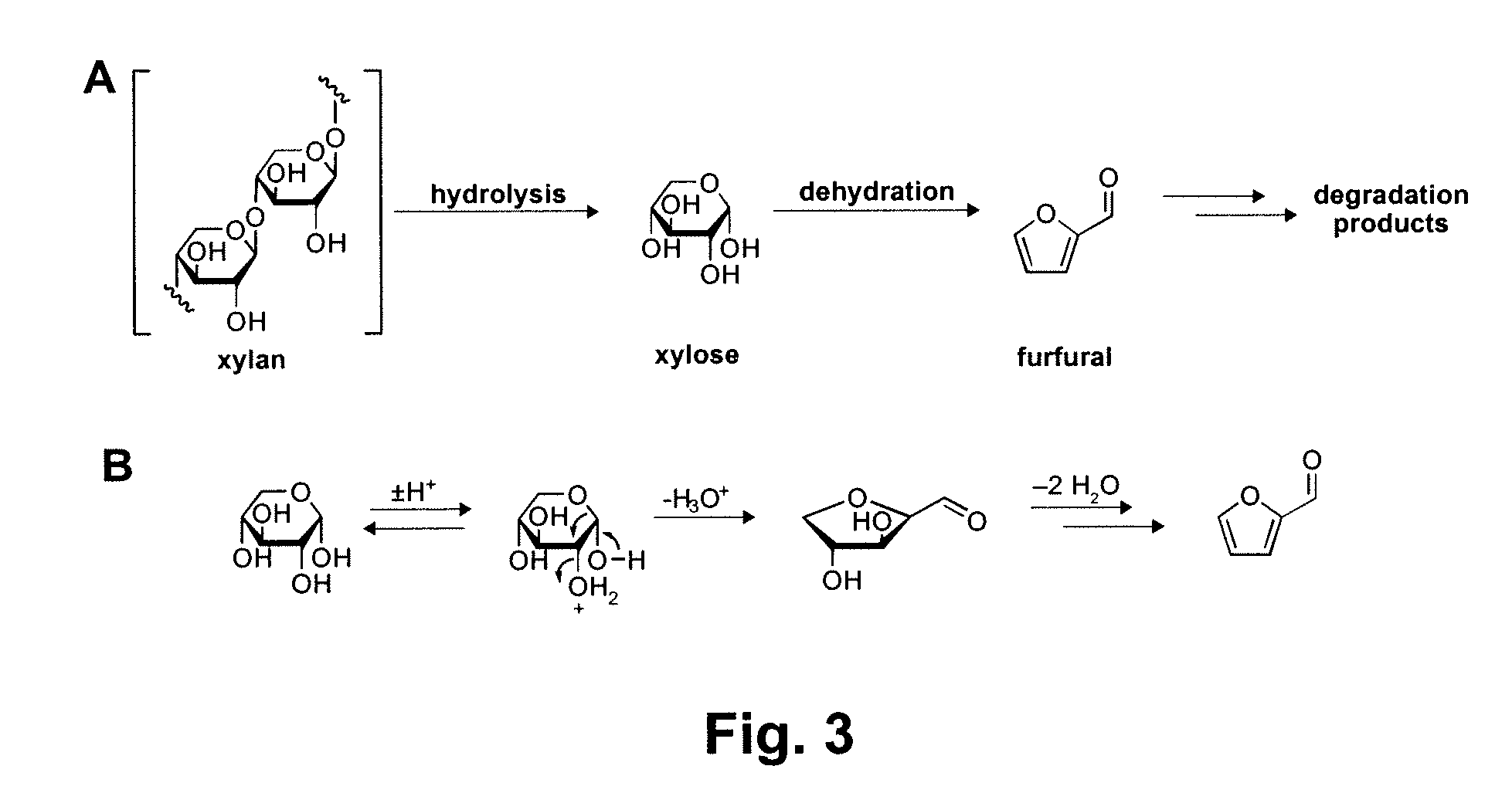
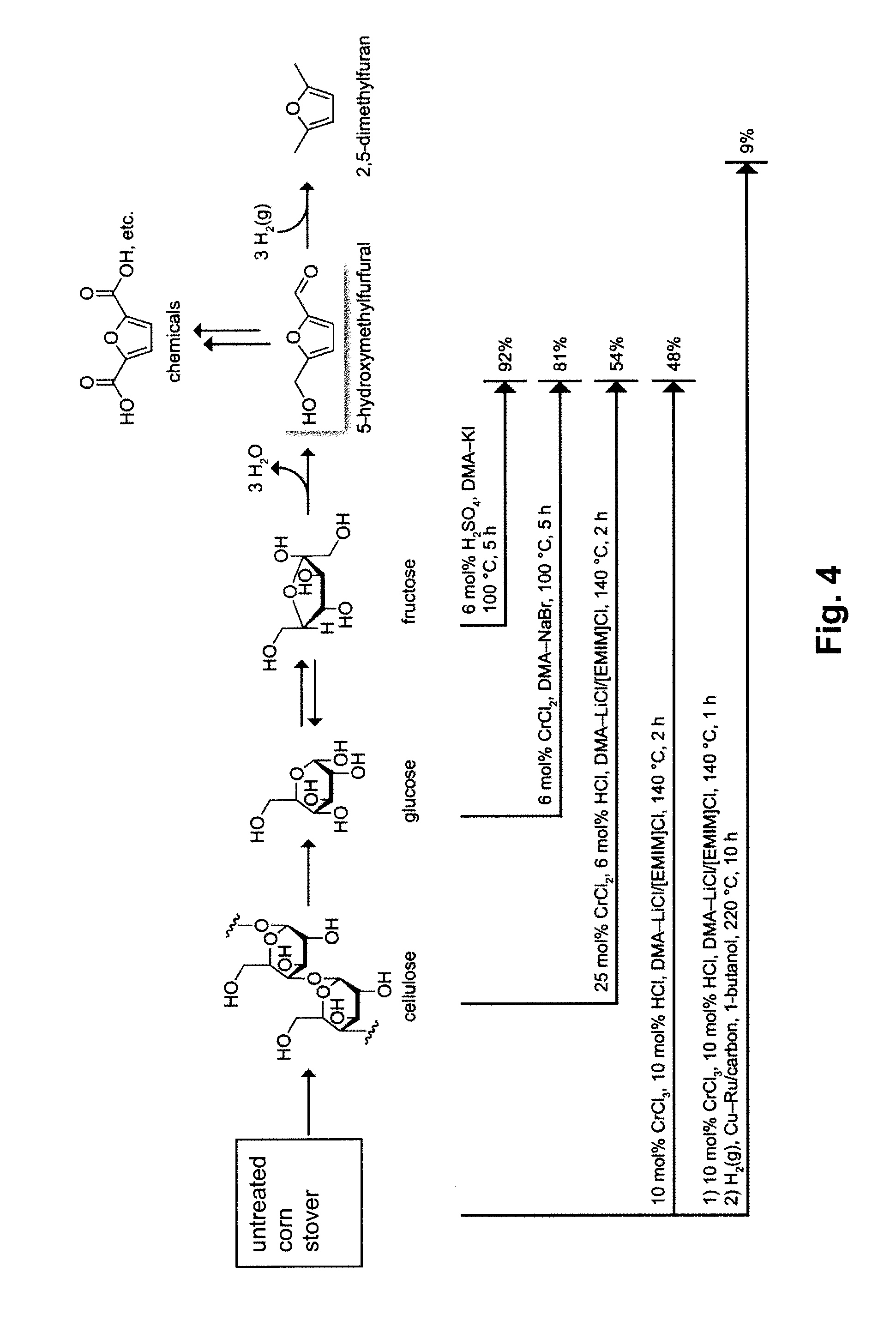
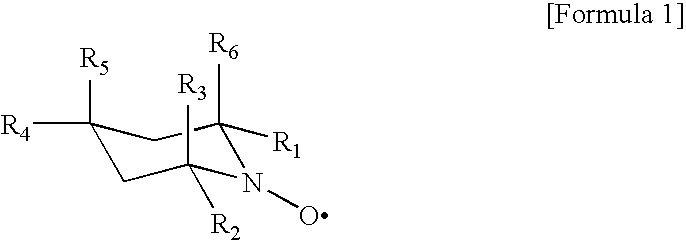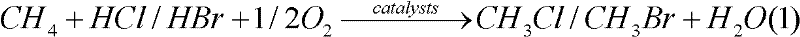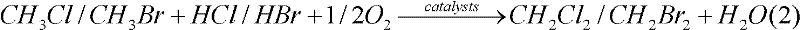Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4581 results about "Bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A bromide is a chemical compound containing a bromide ion or ligand. This is a bromine atom with an ionic charge of −1 (Br⁻); for example, in caesium bromide, caesium cations (Cs⁺) are electrically attracted to bromide anions (Br⁻) to form the electrically neutral ionic compound CsBr. The term "bromide" can also refer to a bromine atom with an oxidation number of −1 in covalent compounds such as sulfur dibromide (SBr₂).
Volatile noble metal organometallic complexes
InactiveUS20050033075A1Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIridiumIodide
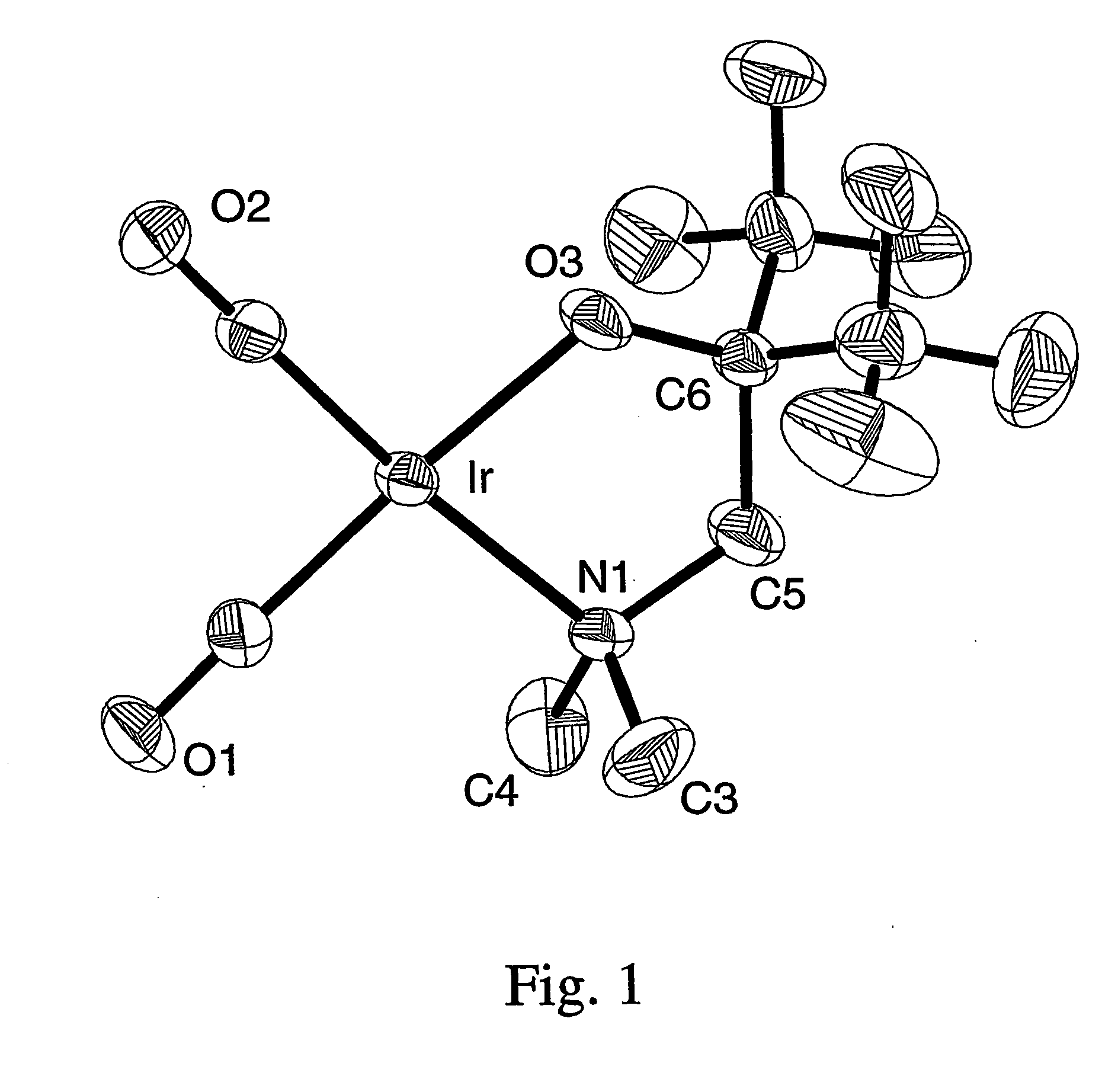
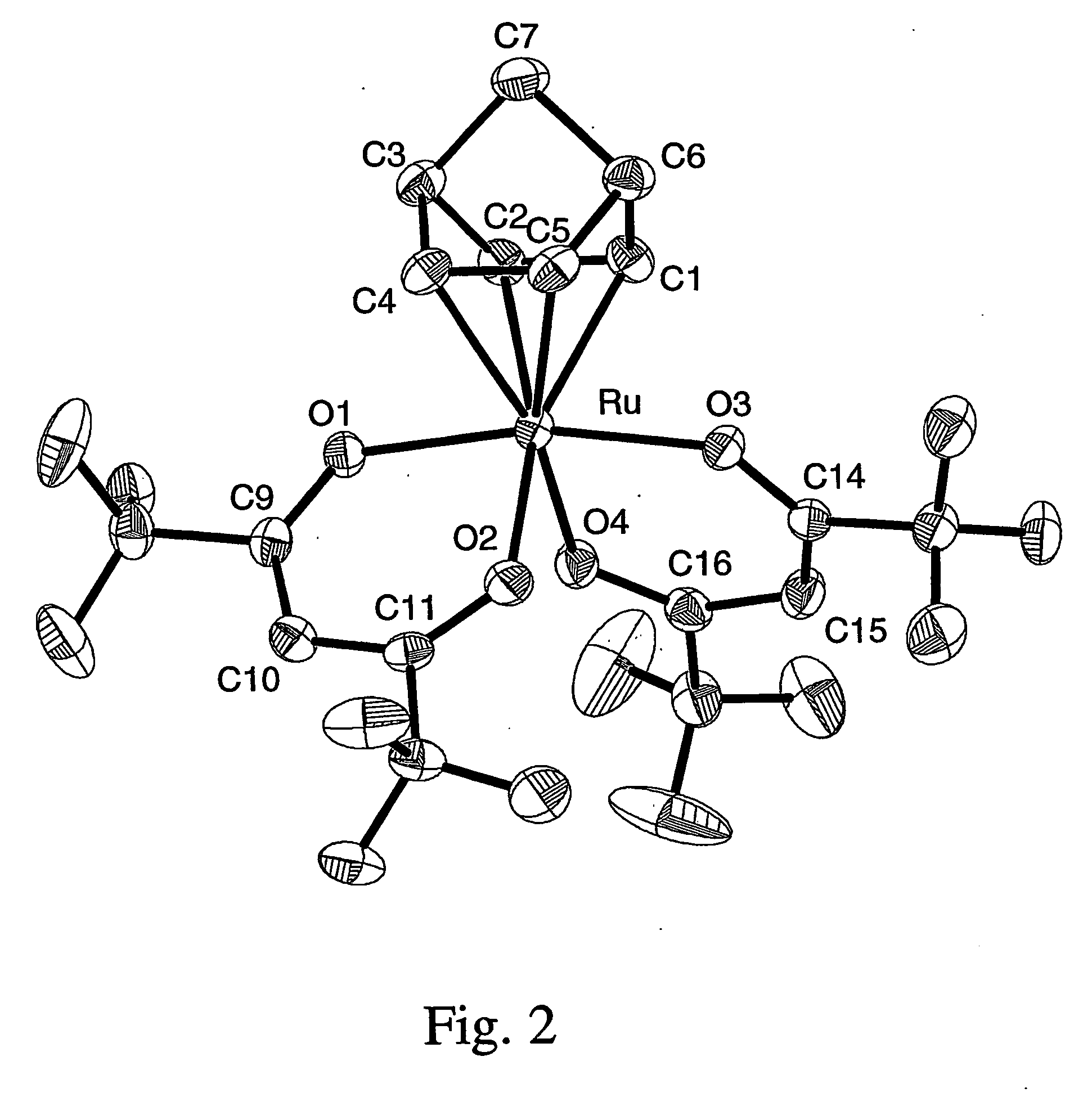
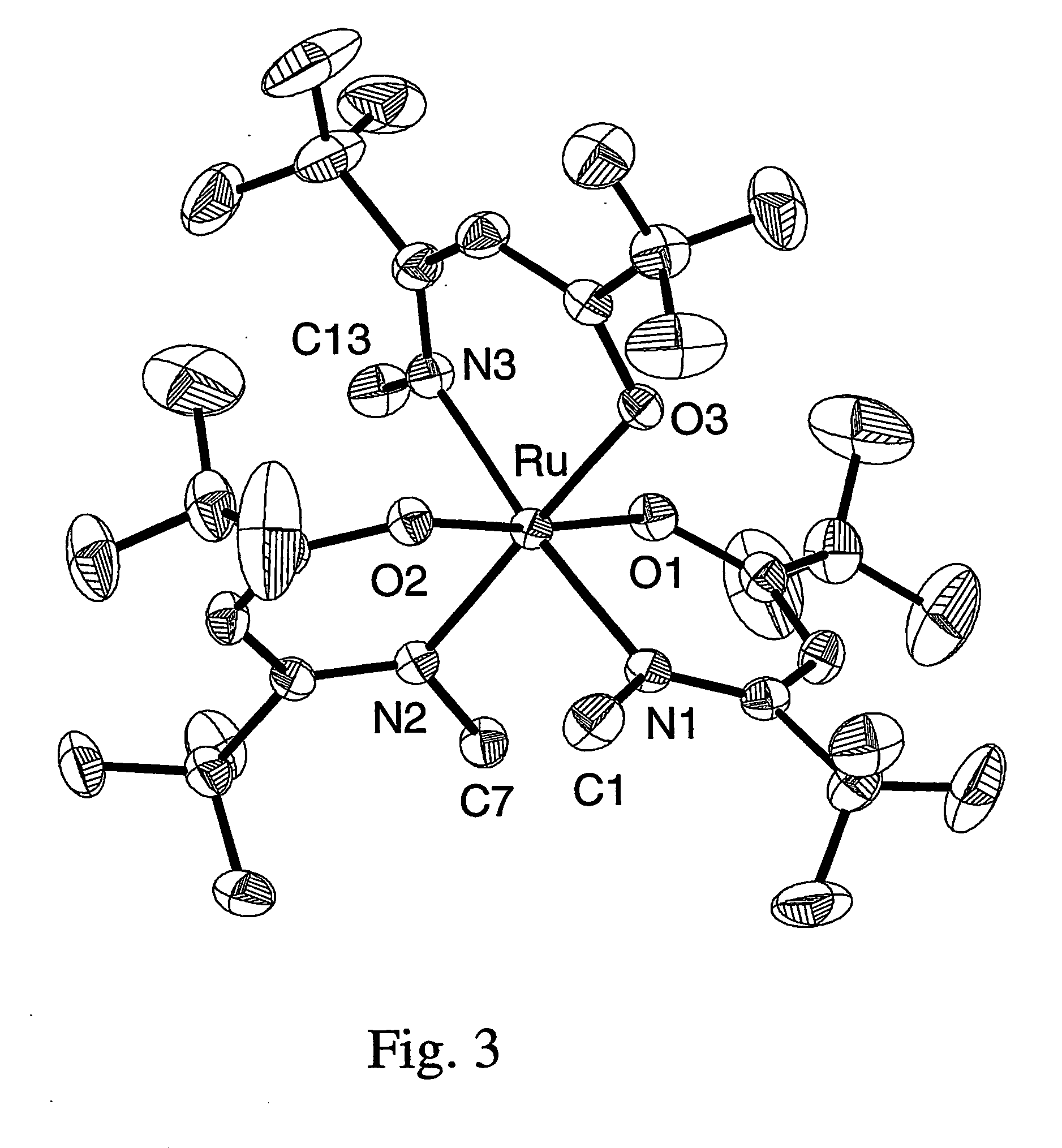
A series of noble metal organometallic complexes of the general formula (I): MLaXb(FBC)c, wherein M is a noble metal such as iridium, ruthenium or osmium, and L is a neutral ligand such as carbonyl, alkene or diene; X is an anionic ligand such as chloride, bromide, iodide and trifluoroacetate group; and FBC is a fluorinated bidentate chelate ligand such as beta diketonate, beta-ketoiminate, amino-alcoholate and amino-alcoholate ligand, wherein a is an integer of from zero (0) to three (3), b is an integer of from zero (0) to one (1) and c is an 10 integer of from one (1) to three (3). The resulting noble metal complexes possess enhanced volatility and thermal stability characteristics, and are suitable for chemical vapor deposition(CVD) applications. The corresponding noble metal complex is formed by treatment of the FBC ligand with a less volatile metal halide. Also disclosed are CVD methods for using the noble metal complexes as source reagents for deposition of noble metal-containing films such as Ir, Ru and Os, or even metal oxide film materials IrO2, OsO2 and RuO2.
Owner:NATIONAL TSING HUA UNIVERSITY +1
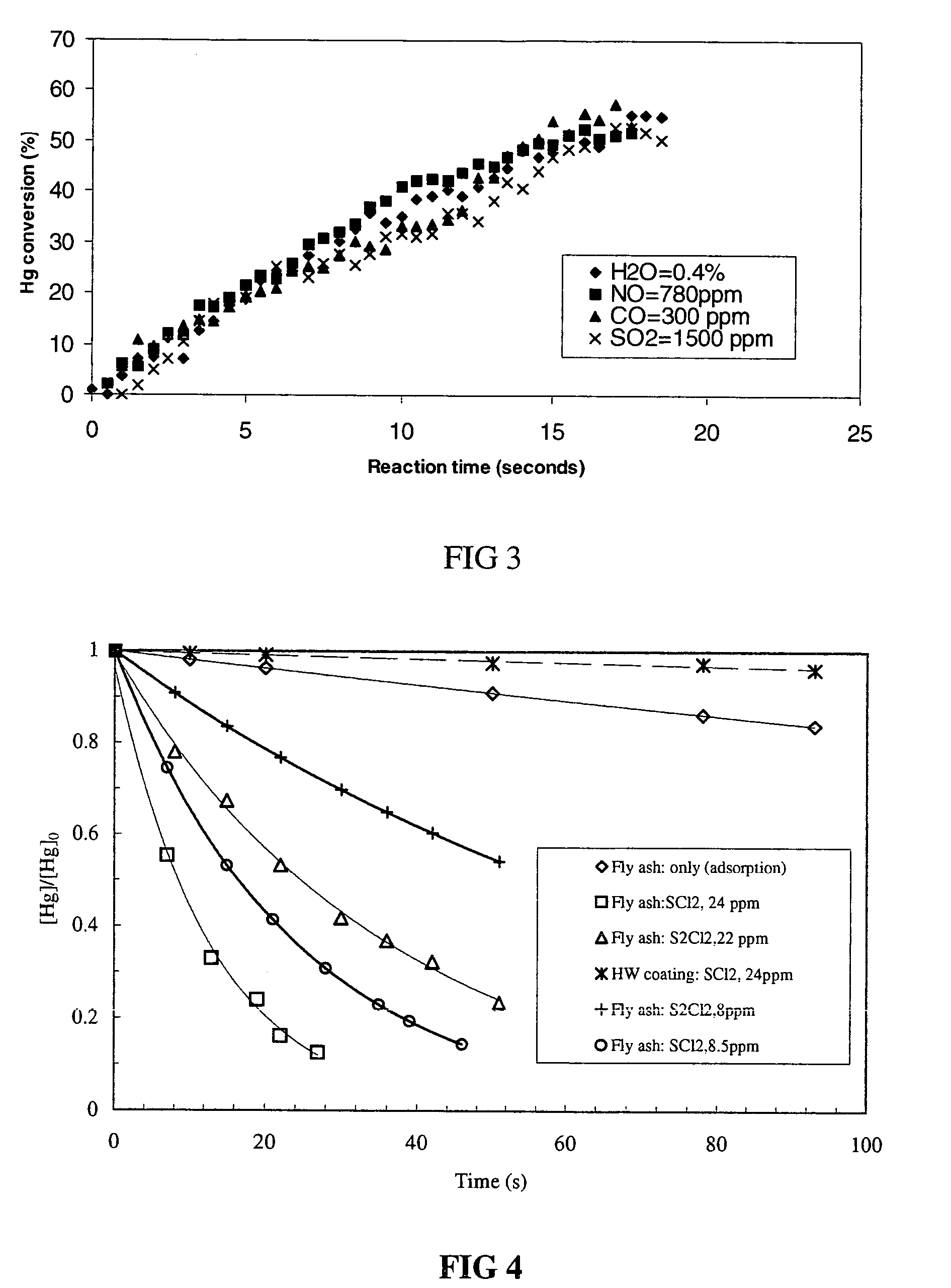
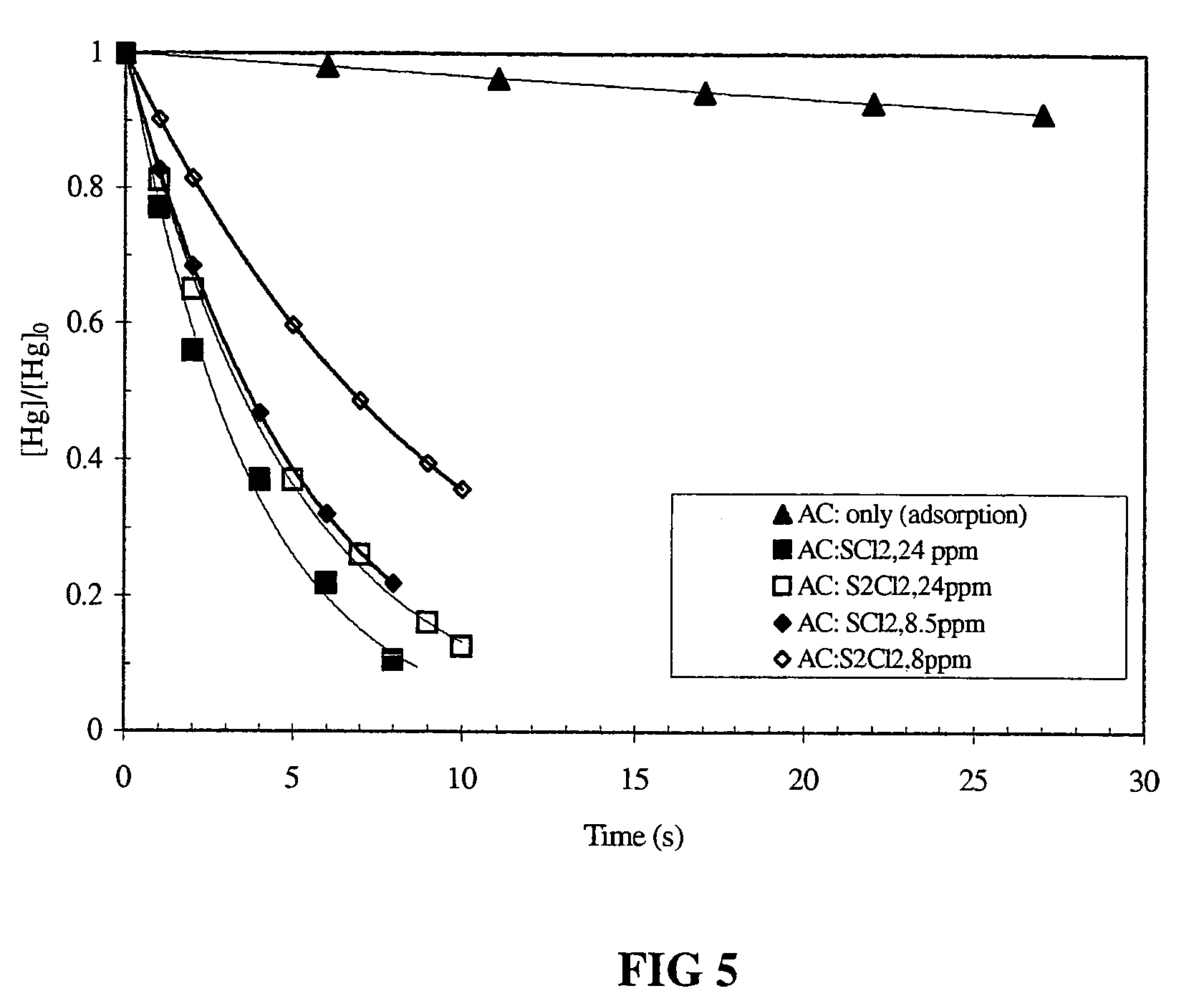
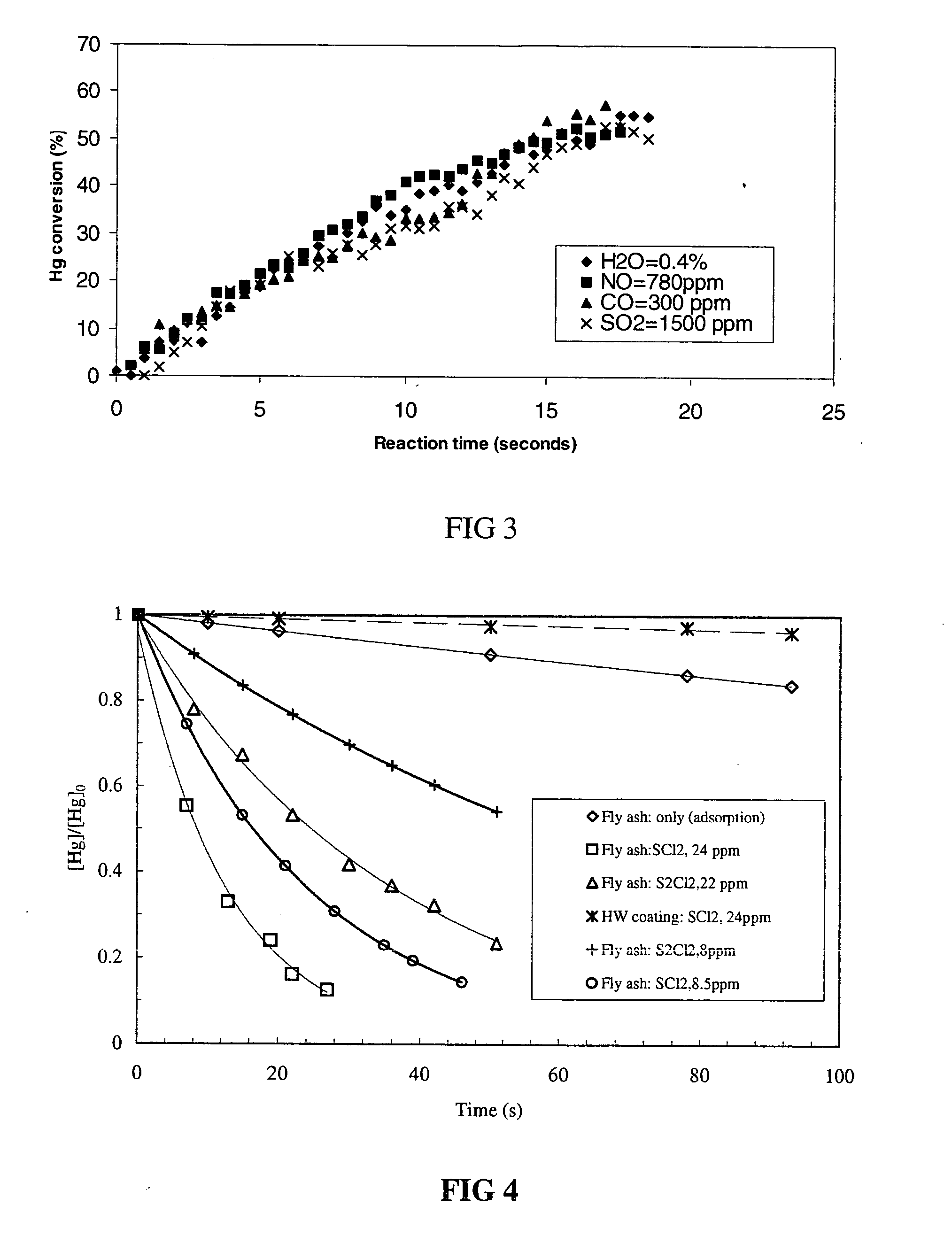
Reducing mercury emissions from the burning of coal
Processes and compositions are provided for decreasing emissions of mercury upon combustion of fuels such as coal. Various sorbent compositions are provided that contain components that reduce the level of mercury and / or sulfur emitted into the atmosphere upon burning of coal. In various embodiments, the sorbent compositions are added directly to the fuel before combustion; are added partially to the fuel before combustion and partially into the flue gas post combustion zone; or are added completely into the flue gas post combustion zone. In preferred embodiments, the sorbent compositions comprise a source of halogen and preferably a source of calcium. Among the halogens, iodine and bromine are preferred. In various embodiments, inorganic bromides make up a part of the sorbent compositions.
Owner:NOX II LTD
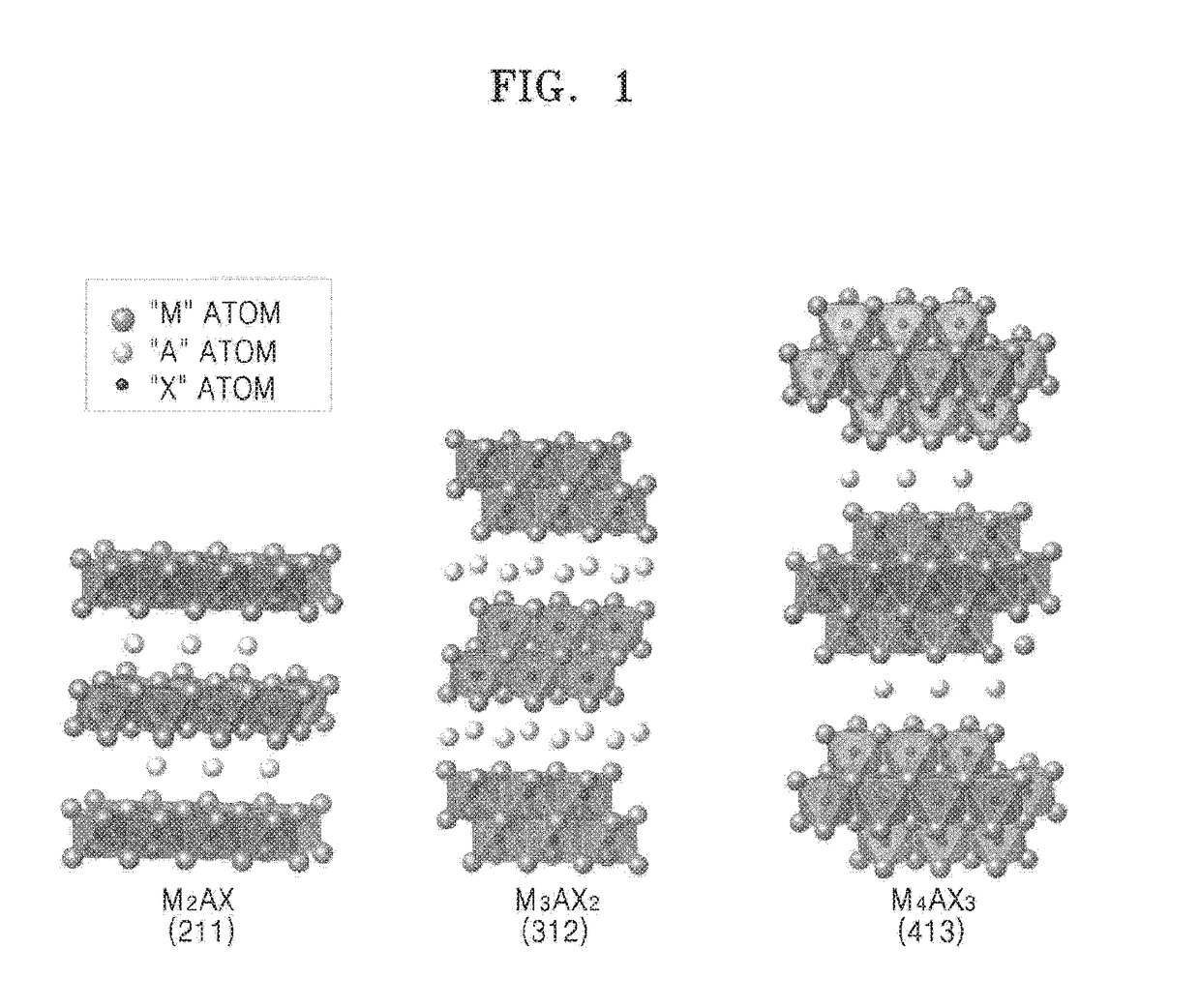
Mxene nanosheet and manufacturing method thereof
ActiveUS20170088429A1Material nanotechnologyNitrogen-metal/silicon/boron binary compoundsIodideInorganic compound
A method of manufacturing a MXene nanosheet includes removing an A atomic layer from an inorganic compound having a formula of Mn+1AXn to form a nanosheet, the nanosheet having a formula of Mn+1XnTs, and reducing the nanosheet having a formula of Mn+1XnTsto form an MXene nanosheet, the MXene nanosheet having a formula of Mn+1Xn, wherein M is at least one of Group 3 transition metal, Group 4 transition metal, Group 5 transition metal, and Group 6 transition metal, A is at least one of a Group 12 element, Group 13 element, Group 14 element, Group 15 element and Group 16 element, X is one of carbon (C), nitrogen (N) and a combination thereof, Ts is one of oxide (O), epoxide, hydroxide (OH), alkoxide having 1-5 carbon atoms, fluoride (F), chloride (Cl), bromide (Br), iodide (I), and a combination thereof, and n is one of 1, 2 and 3.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV +1
Reducing mercury emissions from the burning of coal
Processes and compositions are provided for decreasing emissions of mercury upon combustion of fuels such as coal. Various sorbent compositions are provided that contain components that reduce the level of mercury and / or sulfur emitted into the atmosphere upon burning of coal. In various embodiments, the sorbent compositions are added directly to the fuel before combustion; are added partially to the fuel before combustion and partially into the flue gas post combustion zone; or are added completely into the flue gas post combustion zone. In preferred embodiments, the sorbent compositions comprise a source of halogen and preferably a source of calcium. Among the halogens, iodine and bromine are preferred. In various embodiments, inorganic bromides make up a part of the sorbent compositions.
Owner:NOX II LTD
Physiologically balanced, ionized, acidic solution and methodology for use in wound healing
Described herein is a physiologically-balanced, acidic solution. Typically the solution is prepared by a chemical reactions or by the electrolysis of a solution comprising a mixture of an inorganic salt to form a physiologically balanced solution. This invention also relates to methods for use of the solutions, including a specialized bandage which may be used in combination with the solutions, or optionally with other topically applied materials. A mixture of inorganic salts and, optionally minerals, is used in order to mimic the electrolyte concentration and mixture of body fluid in an isotonic state. The solution typically comprises of one halide salt of lithium, sodium, potassium, calcium, and other cations. Typically the halide is fluoride, chloride, bromide, or iodide, and most typically chloride. A typical electrolyzed solution of the present invention has a pH within the range of about 2 to about 5, an oxidation reduction potential within the range of about +600 mV to about +1200 mV, and hypohalous acid concentration in the range of about 10 ppm to about 200 ppm. The solution has bactericidal, fungicidal, and sporicidal properties. The composition of the invention is nontoxic and has antibacterial properties, and is useful in any application in which antimicrobial properties are desirable.
Owner:NOVABAY PHARM INC
High density, viscosified, aqueous compositions having superior stability under stress conditions
Owner:BJ SERVICES CO
Metal halide coatings on lithium ion battery positive electrode materials and corresponding batteries
Lithium ion battery positive electrode material are described that comprise an active composition comprising lithium metal oxide coated with an inorganic coating composition wherein the coating composition comprises a metal chloride, metal bromide, metal iodide, or combinations thereof. Desirable performance is observed for these coated materials. In particular, the non-fluoride metal halide coatings are useful for stabilizing lithium rich metal oxides.
Owner:IONBLOX INC
Recovery of common salt and marine chemicals from brine
InactiveUS6776972B2High purityLow costGeneral water supply conservationSeawater treatmentSaline waterEvaporation
A new process for recovery of common salt, potassium chloride, concentrated magnesium chloride with enriched bromide, and high purity magnesia from brine in an integrated manner, said process comprises preparation of calcium chloride by reaction of hydrochloric acid generated in the process with limestone, desulfatation of brine with calcium chloride, production of sodium chloride of superior quality in solar pans, solar evaporation of bittern thereby producing carnallite and end bittern, processing carnallite through established processes to produce potassium chloride, recovering end bittern containing highly concentrated magnesium chloride and enriched bromide and calcination of a part of the end bittern after solidification to produce high purity magnesia and hydrochloric acid utilizable in the process.
Owner:COUNCIL OF SCI & IND RES
Method for scavenging mercury
Owner:RGT UNIV OF CALIFORNIA
Processes for producing cellulose nanofibers, cellulose oxidation catalysts and methods for oxidizing cellulose
The present invention aims to provide a process for producing cellulose nanofibers using a 4-hydroxy TEMPO derivative less expensive than TEMPO and a process capable of rapidly producing homogeneous cellulose nanofibers. Wood cellulose can be efficiently converted into nanofibers by a process for producing cellulose nanofibers comprising treating a cellulosic material with an oxidizing agent in water in the presence of a cellulose oxidation catalyst comprising an N-oxyl compound represented by formula 1 below:wherein R1 and R2 are each independently hydrogen or a C1-C6 straight or branched alkyl group; and(i) one of R4 or R5 is —OR, —OCOR or —OSO2R wherein R is a straight or branched carbon chain having 4 or less carbon atoms, and the other of R4 or R5 is hydrogen, and R3 and R6 are methyl, or(ii) R4 is hydrogen, and R5, R3 and R6 are taken together with a piperidine ring to form an aza-adamantane compound having formula 2 below:or a mixture thereof, and a compound selected from the group consisting of bromides, iodides and mixtures thereof to prepare oxidized cellulose, and microfibrillating the oxidized cellulose to convert it into nanofibers.
Owner:NIPPON PAPER IND CO LTD
Chemical Transformation of Lignocellulosic Biomass into Fuels and Chemicals
A method for converting a carbohydrate to a furan in a polar aprotic solvent in the presence of a chloride, bromide, or iodide salt or a mixture thereof and optionally in the presence of an acid catalyst, a metal halide catalyst and / or an ionic liquid (up to 40 wt %). The method can be employed in particular to produce furfural or 5-hydroxymethylfurfural.
Owner:WISCONSIN ALUMNI RES FOUND
Reactive formulations for a neutralization of toxic industrial chemicals
InactiveUS7125497B1Efficiently neutralizedHydrogen peroxideLiquid degasificationBoron trichlorideMalathion
Decontamination formulations for neutralization of toxic industrial chemicals, and methods of making and using same. The formulations are effective for neutralizing malathion, hydrogen cyanide, sodium cyanide, butyl isocyanate, carbon disulfide, phosgene gas, capsaicin in commercial pepper spray, chlorine gas, anhydrous ammonia gas; and may be effective at neutralizing hydrogen sulfide, sulfur dioxide, formaldehyde, ethylene oxide, methyl bromide, boron trichloride, fluorine, tetraethyl pyrophosphate, phosphorous trichloride, arsine, and tungsten hexafluoride.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Process for preparing 2,3,5,6-Tetrafluoro-para-xylyl alcohol
InactiveCN1458137AOrganic compound preparationHydroxy compound preparationSulfolanePotassium fluoride
The preparation process of 2, 3, 5, 6-tetrafluoro-para-xylyl alcohol with 2, 3, 5, 6-tetrachloro-p-phenyl diformyl chloride as material includes fluorination with potassium fluoride, etc. as fluorination agent, sulfolane, etc. as solvent and calixarene, etc. as catalyst and at 40-230 deg.c; esterification of ester with fatty alcohol or aromatic alcohol as esterifying agent and at 20-120 deg.c; reduction of ester with sodium borohydride, etc. as reductant and sulfolane, etc. as solvent, and at -10 deg.c to 60 deg.c; bromination with HBr acid as brominating agent and dichloromethane as solvent and at 30-160 deg.c; and reduction of bromide with magnesium powder, etc as reductant and fatty alcohol, water, chloride, etc as solvent, and at -10 deg.c to 50 deg.c. The present invention has the advantages of simple process, low production cost, high product yield and purity, and is suitable for industrial production.
Owner:TIANJIN UNIV
Recovery of common salt and marine chemicals from brine
InactiveUS20030080066A1High purityLow costGeneral water supply conservationSeawater treatmentSaline waterEvaporation
A new process for recovery of common salt, potassium chloride, concentrated magnesium chloride with enriched bromide, and high purity magnesia from brine in an integrated manner, said process comprises preparation of calcium chloride by reaction of hydrochloric acid generated in the process with limestone, desulfatation of brine with calcium chloride, production of sodium chloride of superior quality in solar pans, solar evaporation of bittern thereby producing carnallite and end bittern, processing carnallite through established processes to produce potassium chloride, recovering end bittern containing highly concentrated magnesium chloride and enriched bromide and calcination of a part of the end bittern after solidification to produce high purity magnesia and hydrochloric acid utilizable in the process.
Owner:COUNCIL OF SCI & IND RES
Acid-cleaning inhibiter for cleaning boiler and use method thereof
ActiveCN101634030ASlow down the dissolution rateIncrease activation energyBoiler cleaning apparatusThioureaPhosphoric acid
The invention discloses an acid-cleaning inhibiter for cleaning a boiler and a use method thereof. The acid-cleaning inhibiter comprises the following components by weight percent: 2.0%-10.0% of acid liquor, 0.08-0.25% of urotropine, 0.04-0.15% of dimethylbenzene thiourea or thiourea, 0.01-0.05% of thiocyanate, 0.03-0.12% of dodecyl-cetyl phenmethyl chloride or bromide, 0.05-0.12% of a surface active agent and the balance of water. Acids suitable for the acid-cleaning inhibiter include hydrochloric acid, citric acid, sulphuric acid, hydrofluoric acid, aminosulfonic acid and phosphoric acid, which are suitable for boiler steel, mild steel, alloy steel and steam steel. The inhibition effect of the invention is over 98% with less toxicity under the boiler chemical cleaning condition, the value of LD50 (orally taken by mice) is 5.74 g / kg. The invention can be stored for 2 years, resistant Fe<3+> ion is 800 mg / kg in the acid cleaning. The preparation method has simple steps, easily obtained raw materials and convention operation.
Owner:湖南省湘电试验研究院有限公司 +2
Induction plasma synthesis of nanopowders
ActiveUS20070029291A1Tight controlEasy to controlMaterial nanotechnologyOxygen/ozone/oxide/hydroxideIodideInduction plasma technology
A process and apparatus for synthesizing a nanopowder is presented. In particular, a process for the synthesis of nanopowders of various materials such as metals, alloys, ceramics and composites by induction plasma technology, using organometallic compounds, chlorides, bromides, fluorides, iodides, nitrites, nitrates, oxalates and carbonates as precursors is disclosed. The process comprises feeding a reactant material into a plasma torch in which is generated a plasma flow having a temperature sufficiently high to yield a superheated vapour of the material; transporting said vapour by means of the plasma flow into a quenching zone; injecting a cold quench gas into the plasma flow in the quenching zone to form a renewable gaseous cold front; and forming a nanopowder at the interface between the renewable gaseous cold front and the plasma flow.
Owner:TEKNA PLASMA SYST INC
Method for quickly synthesizing small-crystallite titanium-silicon molecular sieve in cheap system
ActiveCN101913620ALower synthesis costFast crystallization rateCrystalline aluminosilicate zeolitesCyclohexanoneHigh activity
The invention provides a method for preparing a small-crystallite titanium-silicon molecular sieve in a cheap system by changing the adding mode of seed crystals and by a hydrothermal process. The titanium-silicon molecular sieve is prepared by using silicon sol as a silicon source, titanium tetrachloride or tetrabutyl titanate as a titanium source, tetrapropylammonium bromide ( TPABr) as a template agent, organic amine as an alkali source and unseparated nanoscale TS-1 mother liquor as seed crystals directly and by the hydrothermal process. The grain size of the titanium-silicon molecular sieve is less than 1 micrometer, and the titanium-silicon molecular sieve has high activity for selective oxidization reactions using hydrogen peroxide as an oxidant, such as epoxidation of olefins, hydroxylation of phenol, ammoxidation of cyclohexanone and the like. The method simplifies the synthesis process of small crystallite TS-1 in the cheap system and reduces synthesis time and cost.
Owner:DALIAN UNIV OF TECH
Titanium silicalite TS-1 catalyst preparation method
InactiveCN101767036AHigh activityLow costMolecular sieve catalystsCatalyst activation/preparationMolecular sieveMicrometer
The invention discloses a titanium silicalite TS-1 catalyst preparation method. Cheap inorganic Titanium silicalite is used as raw material, low quantity of tetrapropylammonium hydroxide or tetrapropylammonium bromide is adopted as template agent, and inorganic alkali such as ammonia water is used as alkali source so that the raw materials for preparation have low price and the production cost isgreatly reduced. The precursor preparation process is simple and easy to control, seed crystal is added to reduce the crystallization time and the repeatability is good. As secondary crystallization is adopted, the non-framework titanium is further reduced, and the acid site of the molecular sieve is reduced, the grain size is controllable within certain range, the molecular sieve channel becomessmoother to facilitate direct membrane separation of micrometer crystalline grains, and the obtained Titanium silicalite TS-1 catalyst has large grains, high activity, stable catalytic performance and broad industrial application prospect.
Owner:XIANGTAN UNIV
Base metal catalysts for the oxidation of carbon monoxide and volatile organic compounds
A method for oxidizing carbon monoxide (CO) and volatile organic compounds (VOCS) comprises contacting a gas containing water vapor and said CO and VOCs with a catalyst composition comprising at least one base metal promoter and at least one base metal catalyst supported on an oxide support material comprising one or more of alumina, silica, zirconia, ceria, and titania, wherein the VOCs comprise one or more of methyl acetate, methane, methyl bromide, benzene, methanol, methyl ethyl ketone, butane, and butene.
Owner:JOHNSON MATTHEY PLC
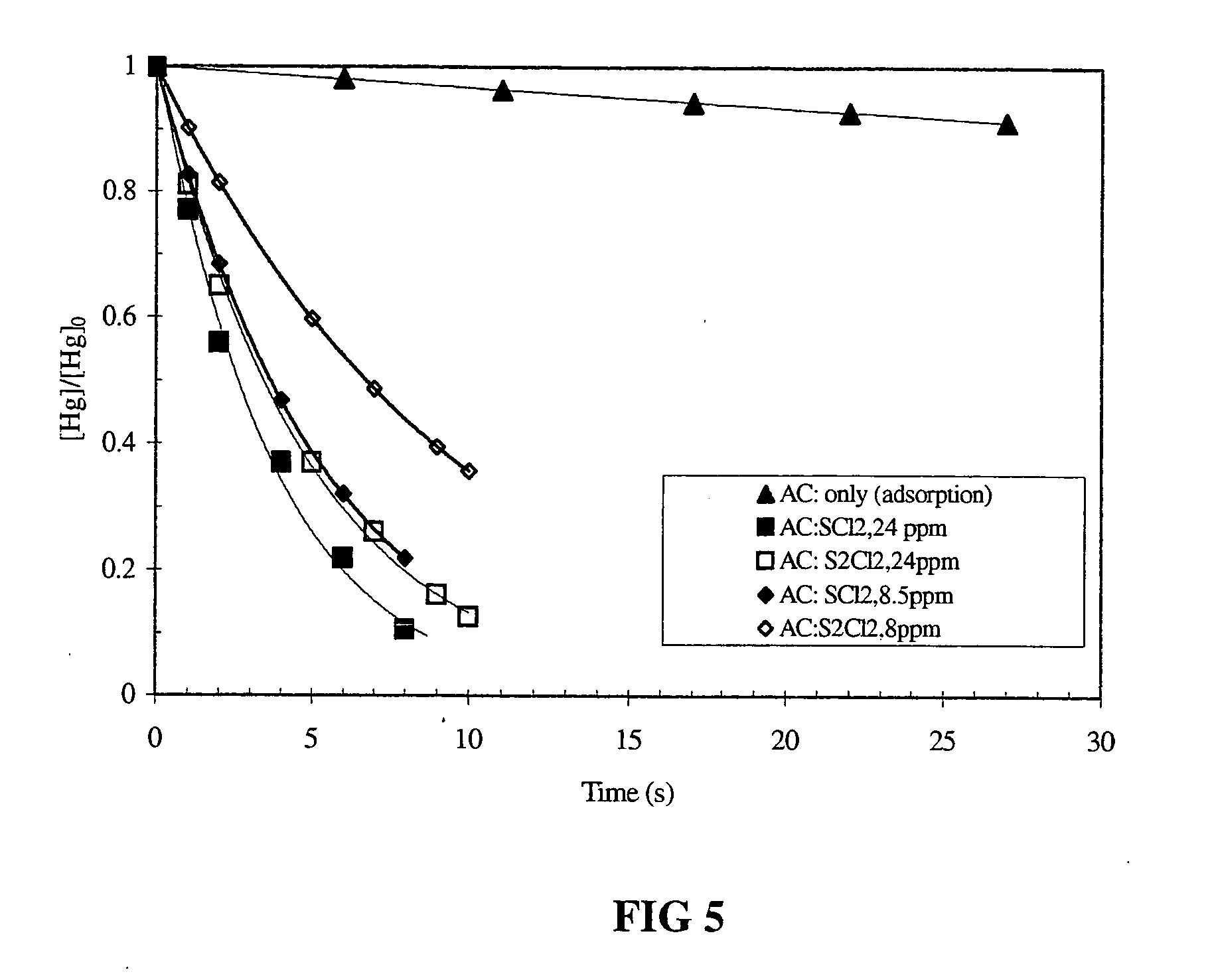
Method for scavenging mercury
Disclosed herein is a method for removing mercury from a gas stream comprising contacting the gas stream with a getter composition comprising bromine, bromochloride, sulphur bromide, sulphur dichloride or sulphur monochloride and mixtures thereof. In one preferred embodiment the getter composition is adsorbed onto a sorbent. The sorbent may be selected from the group consisting of flyash, limestone, lime, calcium sulphate, calcium sulfite, activated carbon, charcoal, silicate, alumina and mixtures thereof. Preferred is flyash, activated carbon and silica.
Owner:RGT UNIV OF CALIFORNIA
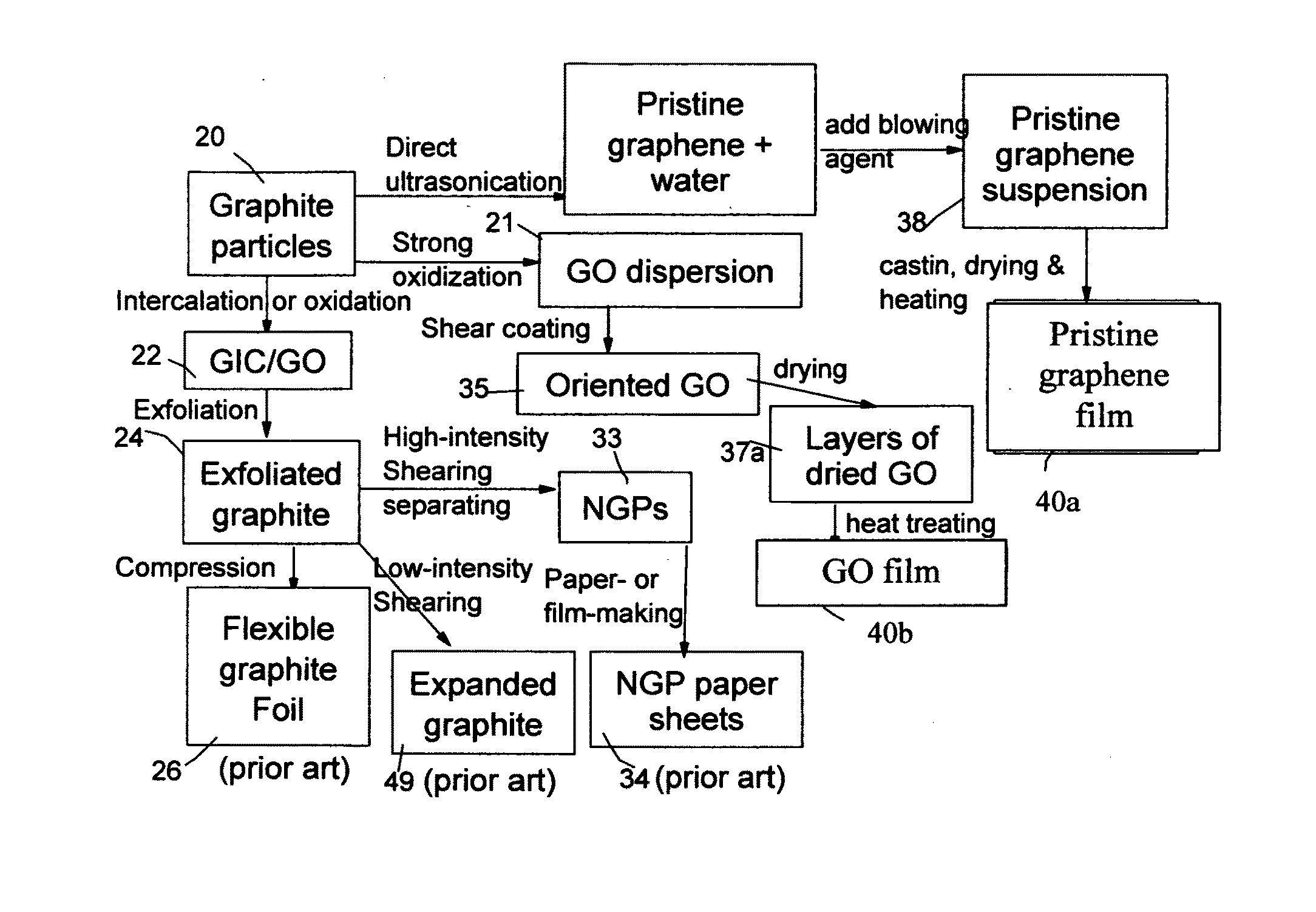
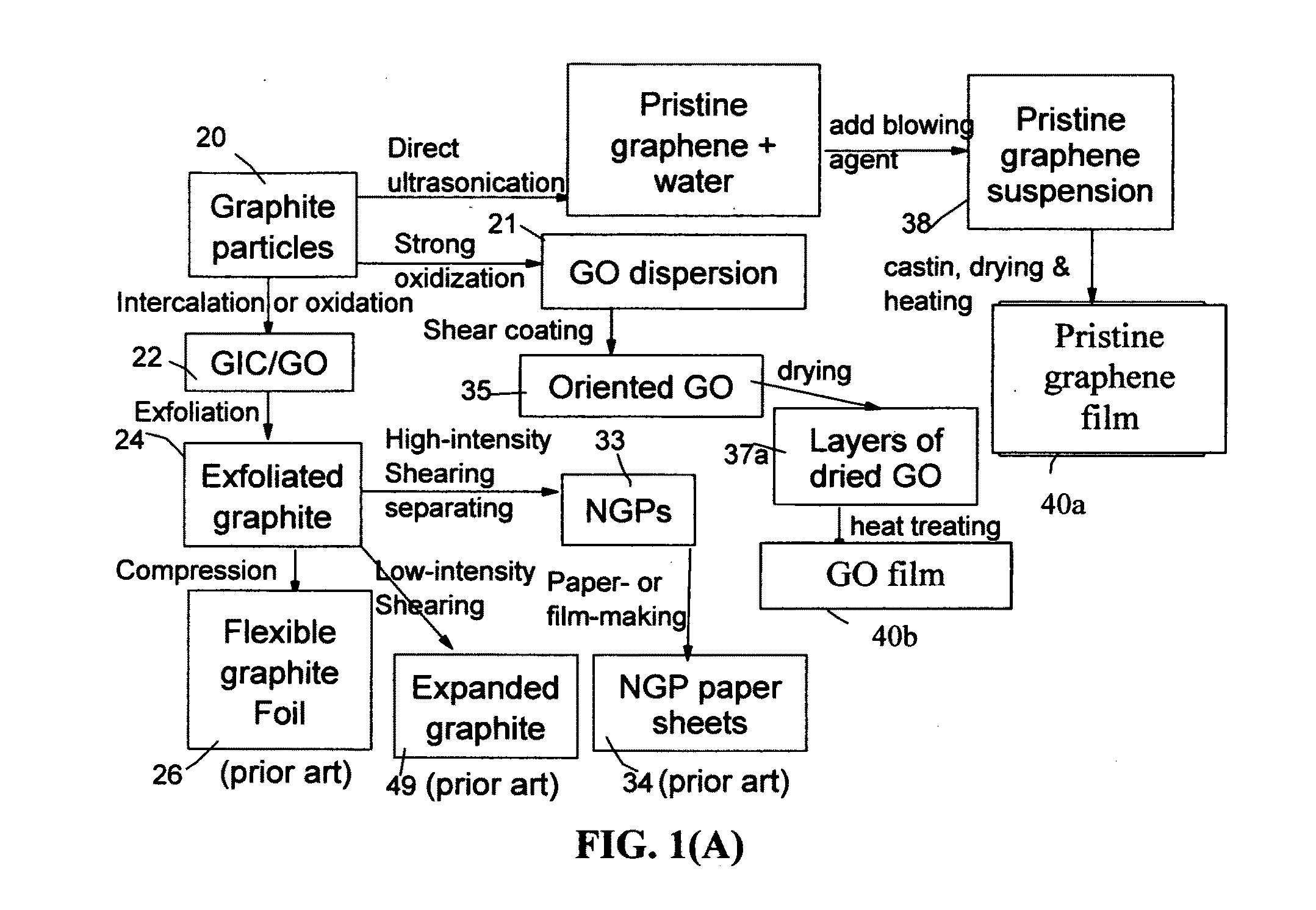
Graphene Electrode Based Ceramic Capacitor
ActiveUS20160079001A1Improve crystal structureGood physical propertiesFixed capacitor electrodesFixed capacitor dielectricDoped grapheneIodide
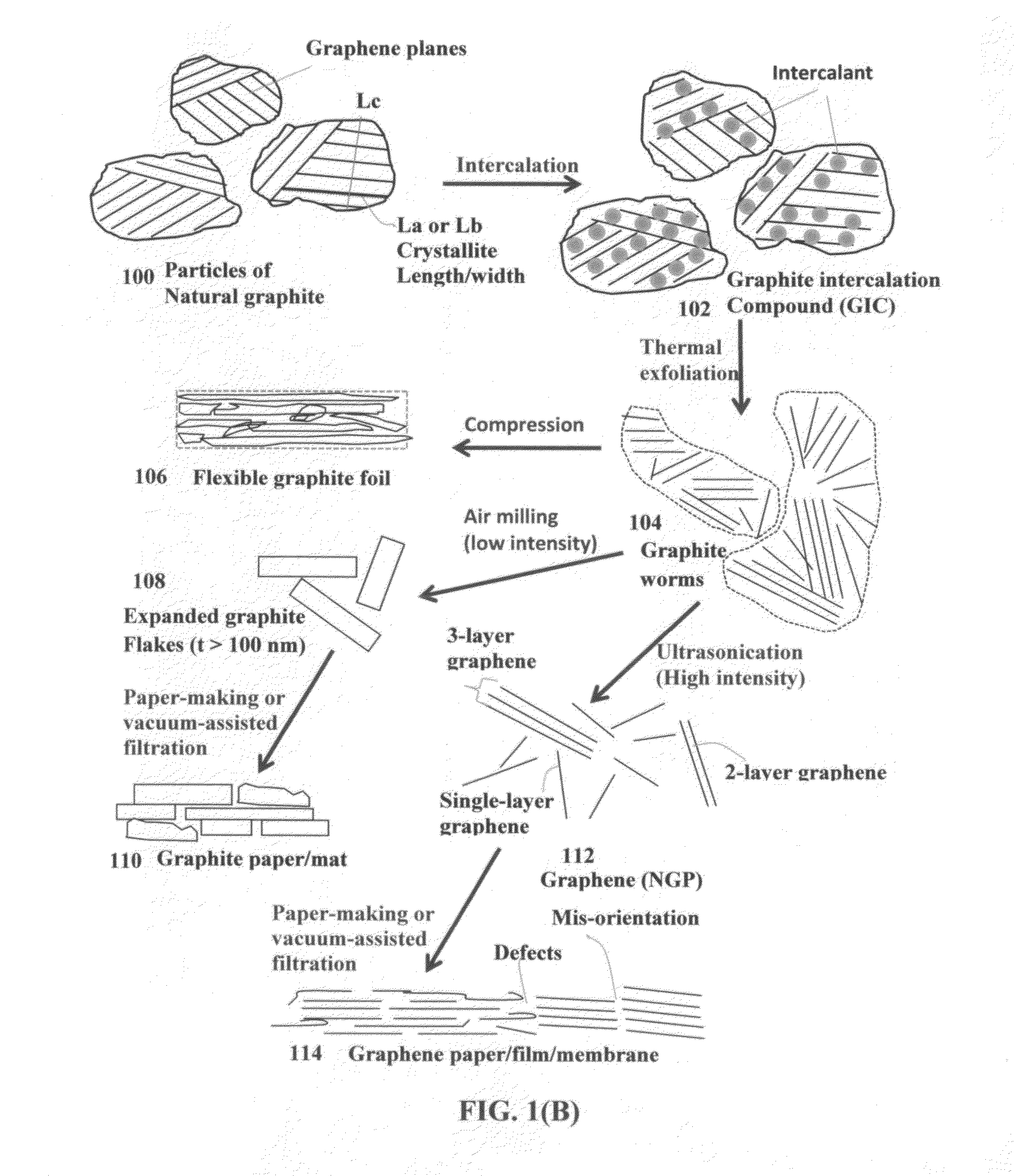
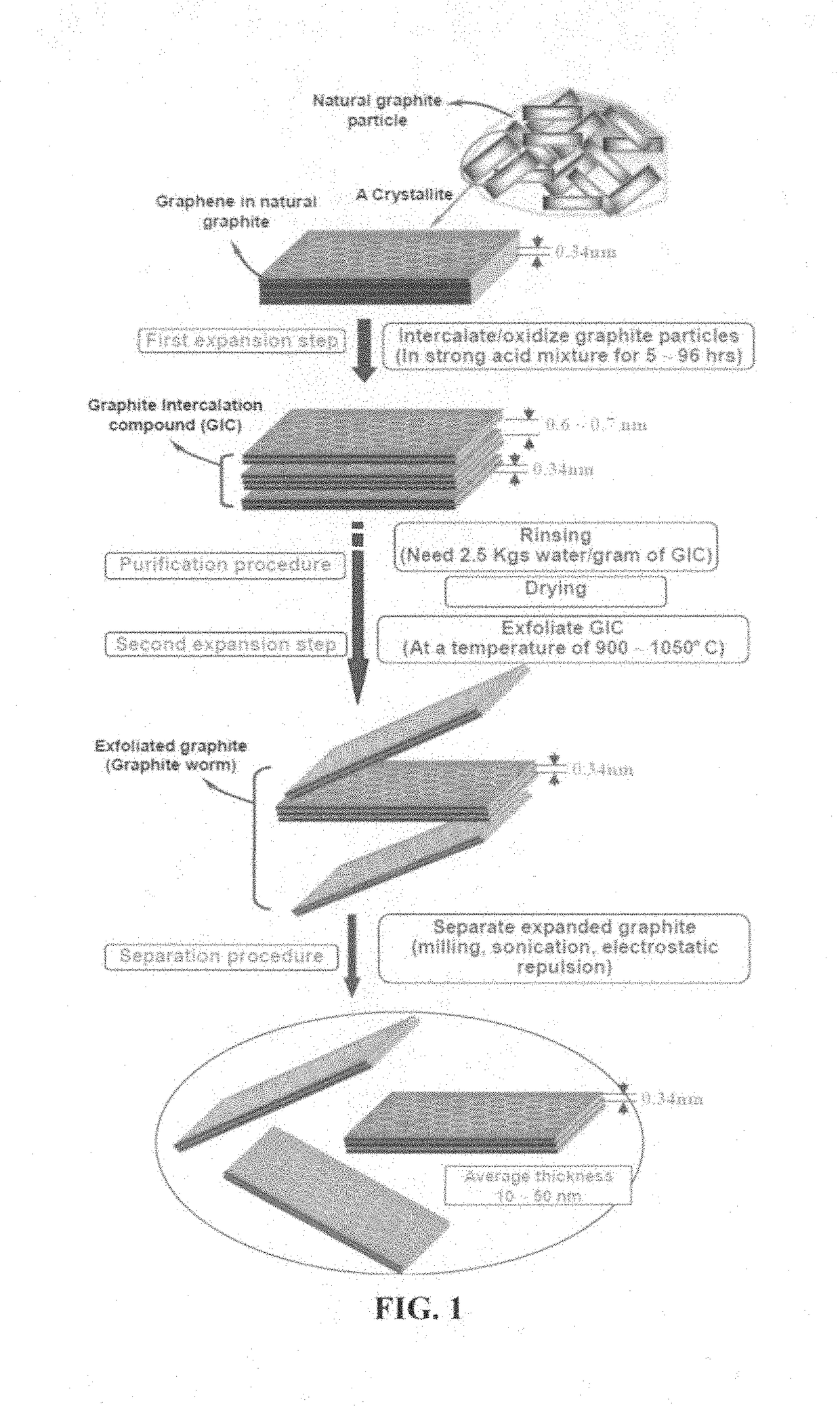
A ceramic capacitor comprising at least a dielectric ceramic layer and at least a graphene electrode layer deposited on the ceramic layer, wherein the graphene electrode layer has a thickness no less than 2 nm and consists of a graphene material or a graphene composite material containing at least 0.1% by weight of a graphene material dispersed in a matrix material or bonded by a binder material, wherein the graphene material is selected from (a) a plurality of single-layer or multi-layer pristine graphene sheets having less than 0.01% by weight of non-carbon elements, or (b) one or a plurality of a non-pristine graphene material having at least 0.01% by weight of non-carbon elements, wherein the non-pristine graphene is selected from graphene oxide, reduced graphene oxide, graphene fluoride, graphene chloride, graphene bromide, graphene iodide, hydrogenated graphene, nitrogenated graphene, doped graphene, chemically functionalized graphene, or a combination thereof.
Owner:GLOBAL GRAPHENE GRP INC
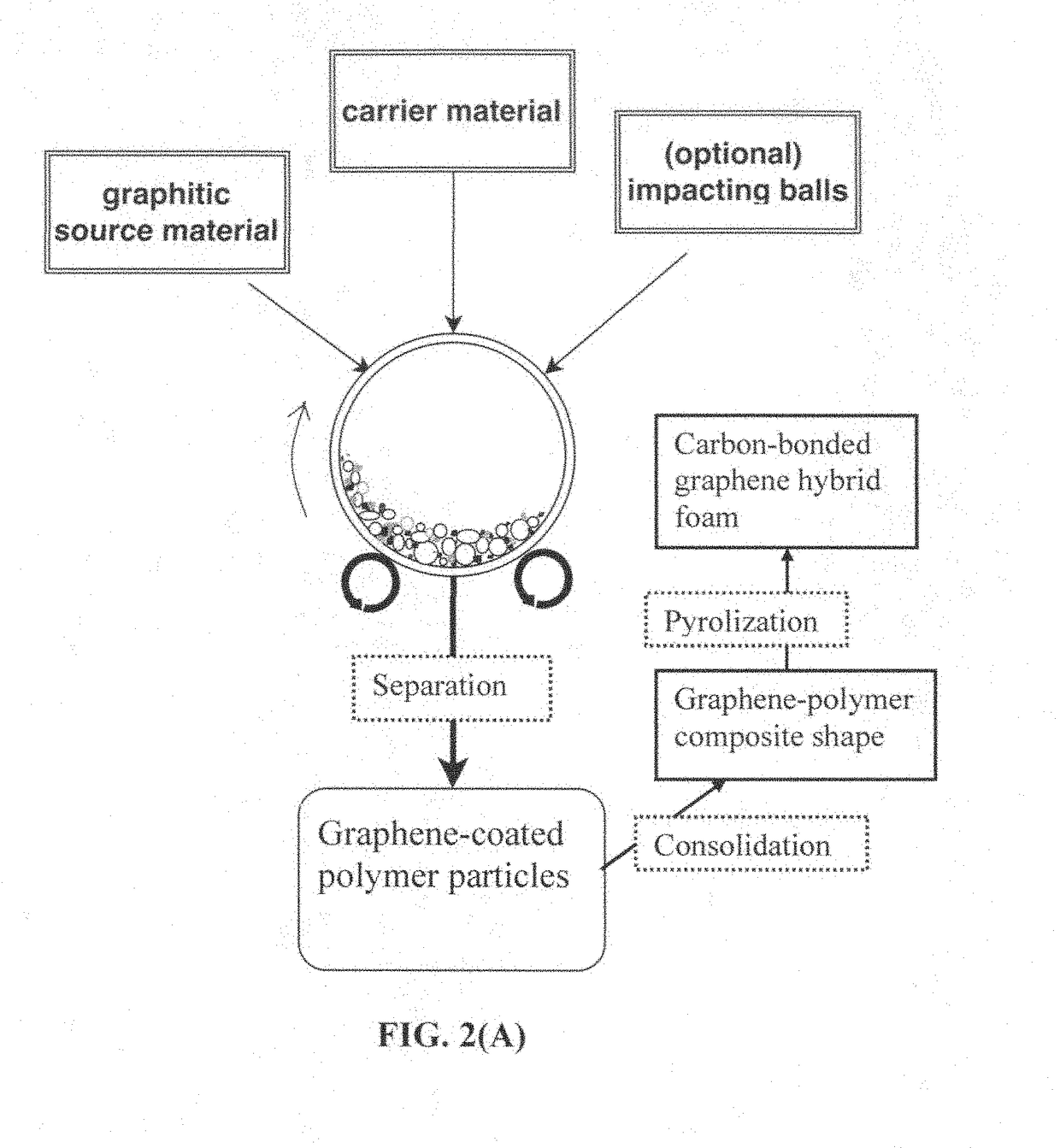
Integral 3D graphene-carbon hybrid foam and devices containing same
ActiveUS20170182474A1Increase heightReduce yieldIon-exchange process apparatusOther chemical processesDoped grapheneIodide
Provided is an integral 3D graphene-carbon hybrid foam composed of multiple pores and pore walls, wherein the pore walls contain single-layer or few-layer graphene sheets chemically bonded by a carbon material having a carbon material-to-graphene weight ratio from 1 / 100 to 1 / 2, wherein the few-layer graphene sheets have 2-10 layers of stacked graphene planes having an inter-plane spacing d002 from 0.3354 nm to 0.40 nm and the graphene sheets contain a pristine graphene material having essentially zero % of non-carbon elements, or a non-pristine graphene material having 0.01% to 25% by weight of non-carbon elements wherein said non-pristine graphene is selected from graphene oxide, reduced graphene oxide, graphene fluoride, graphene chloride, graphene bromide, graphene iodide, hydrogenated graphene, nitrogenated graphene, doped graphene, chemically functionalized graphene, or a combination thereof. Also provided are a process for producing the hybrid form, products containing the hybrid foam, and its applications.
Owner:GLOBAL GRAPHENE GRP INC
Synthetic technology for pyraclostrobin
ActiveCN104211641AFormation reaction is easy to controlSmooth responseOrganic chemistryMethylanilineChlorobenzene
The invention concretely relates to a synthetic technology for pyraclostrobin. The synthetic technology comprises: firstly performing cyclization to obtain 1-(4-chlorophenyl)-pyrazol-3-one, oxidizing the pyrazol ring under the effect of an oxidant to generate 1-(4-chlorophenyl)-3-hydroxypyrazole, then using 2-nitrobenzyl bromide to performing etherification to generate 1-(4-chlorophenyl)-3-[2-(nitrophenyl)methoxy]-1H-pyrazole, then using a reducing agent to perform nitro reducing, so as to generate N-hydroxyl-2-[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]aniline, then using ClCO2CH3 to perform N-acylation reaction to generate methyl N-hydroxyl-N-2-{[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]phenyl}formate, and finally performing hydroxyl methylation under an alkaline condition to generate pyraclostrobin. The technology enables all operations in the pyraclostrobin preparation process to be relatively controllable, helps to improve the stability of the preparation process and improve the product yield, successfully employs low-cost reagents and substantially reduces production cost, and also the employed reagents are relatively small in toxicity, is relatively beneficial for environment protection, and has no corrosivity on plastic pipes, so that the production safety is improved.
Owner:SHANDONG KANGQIAO BIO TECH CO LTD
Synthesis of mesoporous zeolite
Silicic and aluminum sources as raw materials of zeolite are mixed with carbon aerogel. The mixture is subjected to hydrothermal reaction for synthesis of zeolite, while an aqueous solution, which contains sodium hydroxide and tetrapropylammonium bromide, is being dropped to the mixture. After completion of the hydrothermal reaction, the zeolite-supporting aerogel is washed, dried and then heat-treated for oxidative extrusion of the carbon aerogel. Since the carbon aerogel is used as a template, the synthesized zeolite has the morphology that traces of spheroidal particles of the carbon aerogel remain as mesopores.
Owner:JAPAN SCI & TECH CORP
Molten nuclear fuel salts and related systems and methods
InactiveUS20160189813A1Improve power densityImprove the level ofFuel elementsNuclear energy generationPresent methodChloride
This disclosure describes nuclear fuel salts usable in certain molten salt reactor designs and related systems and methods. Binary, ternary and quaternary chloride fuel salts of uranium, as well as other fissionable elements, are described. In addition, fuel salts of UClxFy are disclosed as well as bromide fuel salts. This disclosure also presents methods and systems for manufacturing such fuel salts, for creating salts that reduce corrosion of the reactor components and for creating fuel salts that are not suitable for weapons applications.
Owner:TERRAPOWER
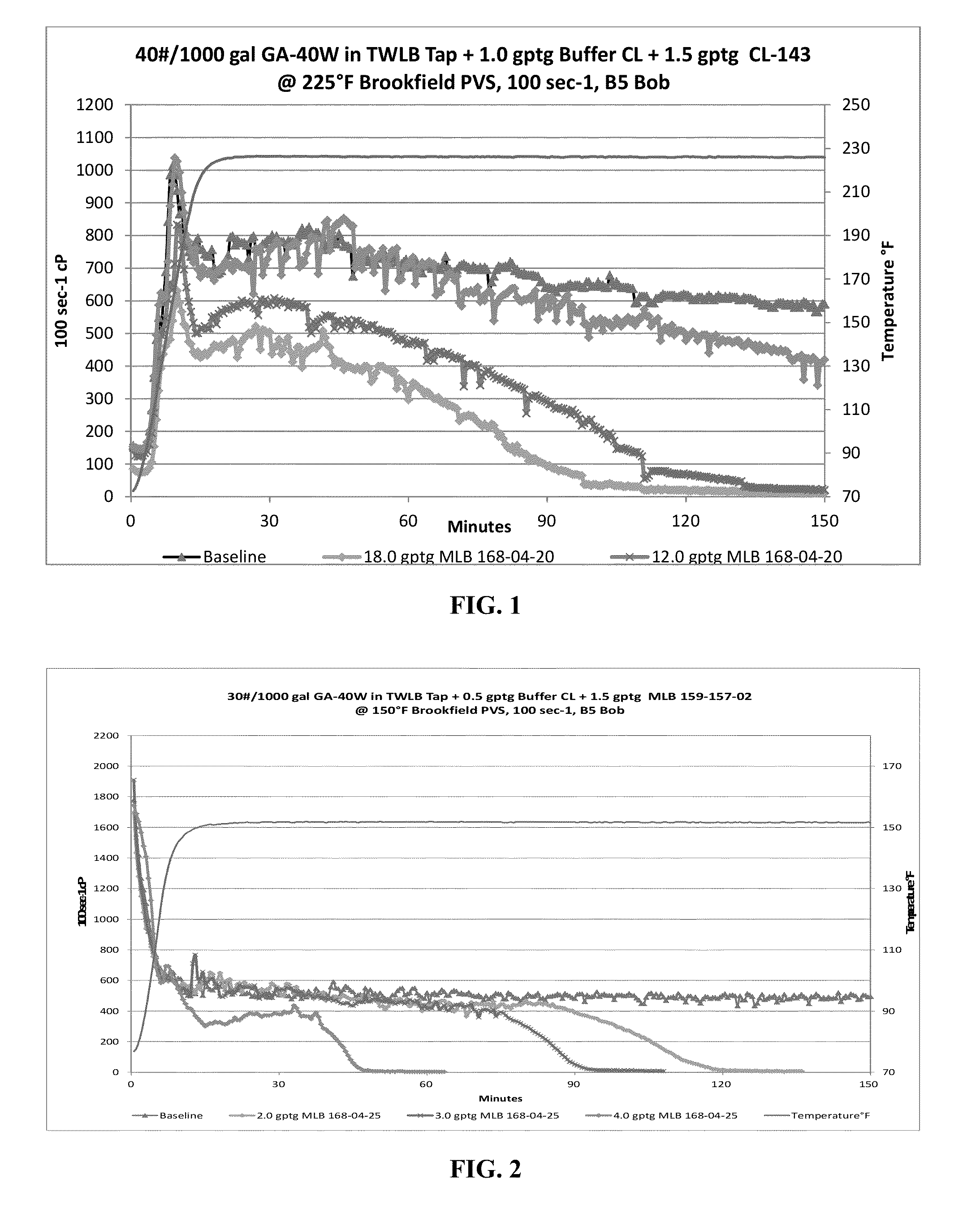
Oxidative breakers in a silicone based suspension
An oxidative breaker system for use in reducing the viscosity of a guar-based suspension includes a silicone oil carrier fluid, an oxidizer and a suspension aid. The suspension aid is preferably fumed silica. The oxidizer may be selected from the group consisting of alkali metal peroxide, transition metal peroxide, persulfate, bromide and bromate. In highly preferred embodiments, the oxidizer is magnesium peroxide or calcium peroxide. Also disclosed is a method for breaking a guar-based suspension with the inventive oxidative breaker system.
Owner:PNC BANK NAT ASSOC
Use of an ammonia storage device in production of energy
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power.
Owner:AMMINEX
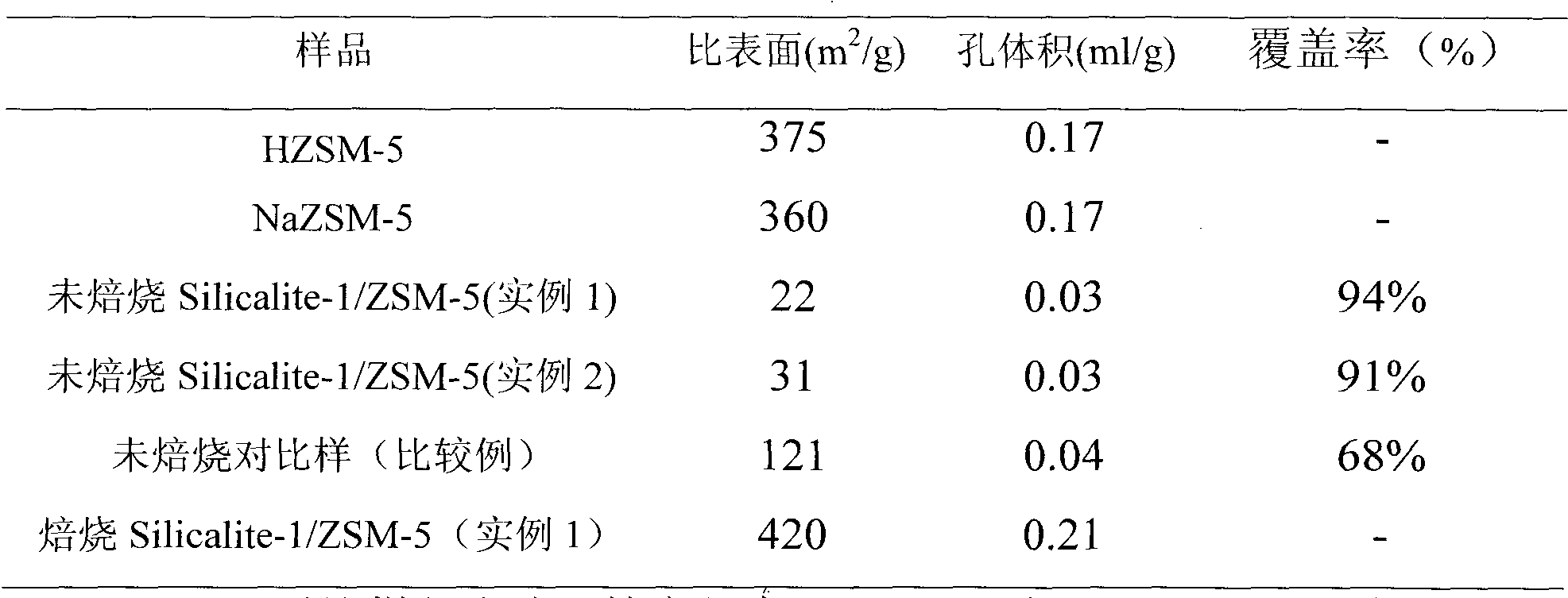
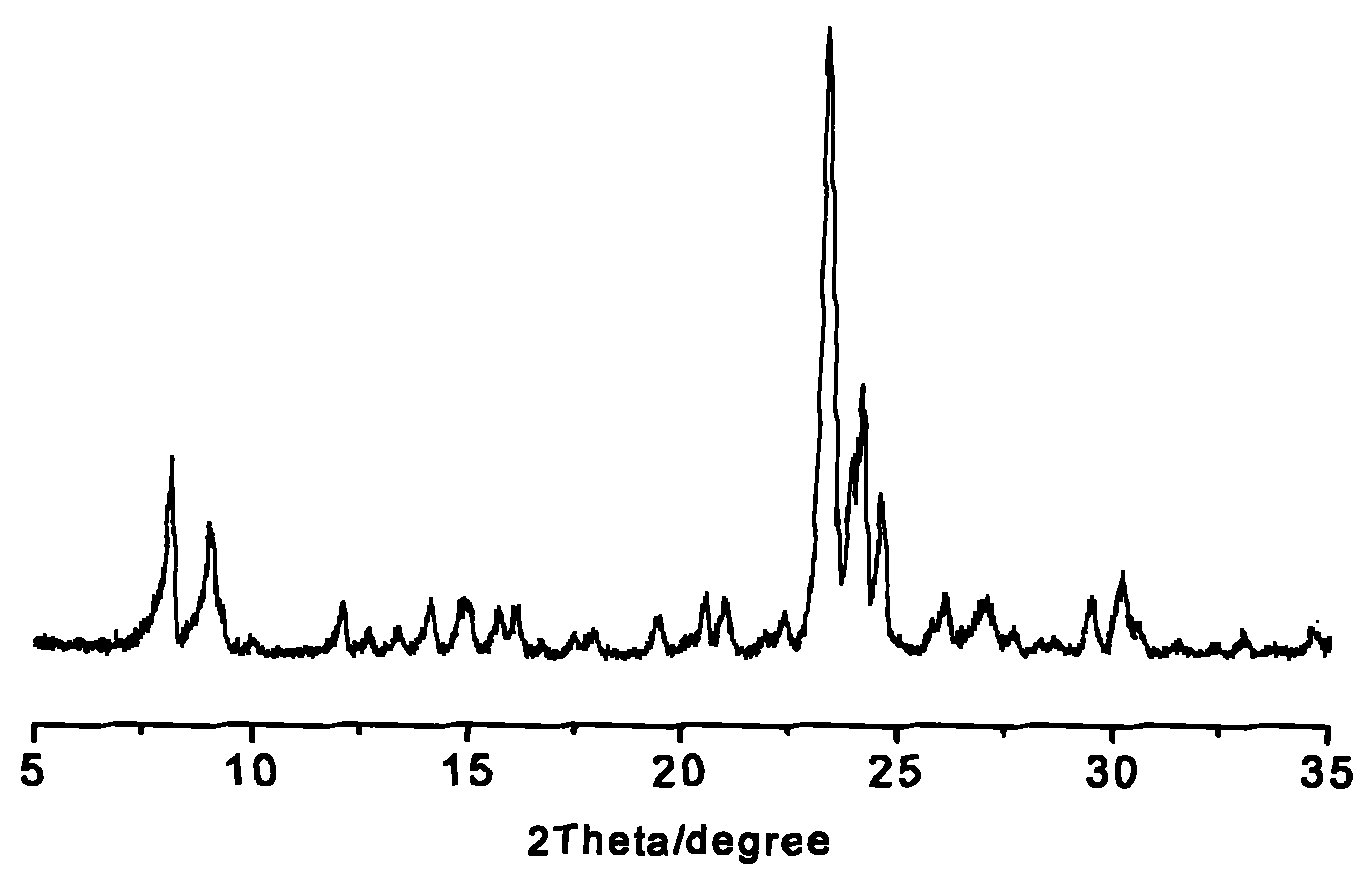
Method for preparing Silicalite-1/ZSM-5 composite molecular sieve
ActiveCN102311124AIncrease coverageEase of industrial applicationCrystalline aluminosilicate zeolitesCore shellSilicon
The invention discloses a method for preparing a Silicalite-1 / ZSM-5 composite molecular sieve. The method comprises the following steps of: crystallizing a mixture containing a silicon source, a ZSM-5 molecular sieve, tetrapropylammonium bromide, sodium hydroxide and water, and drying and roasting a crystallized product to obtain the core-shell structured Silicalite-1 / ZSM-5 composite molecular sieve taking the ZSM-5 molecular sieve as a core and Silicalite-1 molecular sieve as a shell. In the method, cheap template agent and silicon source are adopted and the production cost if greatly reduced; and the prepared Silicalite-1 / ZSM-5 composite molecular sieve has the advantages of high coverage rate, uniform coverage and the like.
Owner:CHINA PETROLEUM & CHEM CORP +1
Application of cerium-based catalyst in methane oxyhalogenation methods used for preparing halogenated methane
InactiveCN102344339ARich reservesEasy to prepareCarbon monoxideHalogenated hydrocarbon preparationBromineCerium
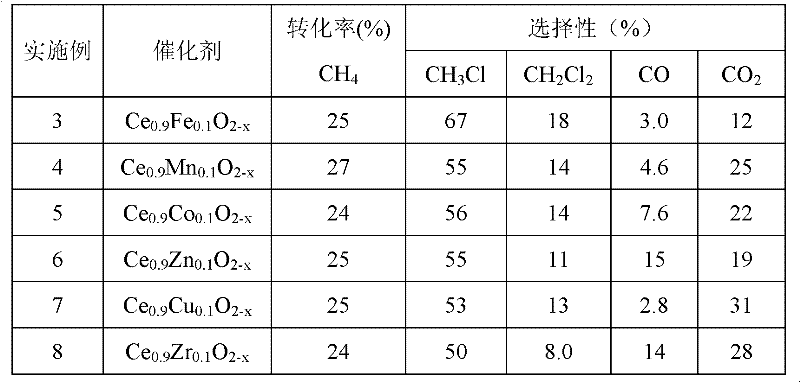
The invention relates to a cerium-based catalyst, and relates to an application of the cerium-based catalyst in methane oxyhalogenation methods used for preparing halogenated methane. The methane oxyhalogenation methods used for preparing halogenated methane comprise a methane chlorine oxidation method used for preparing methyl chloride and a methane bromine oxidation method used for preparing methyl bromide. The cerium-based catalyst is suitable to be used in methane oxyhalogenation reactions. The cerium-based catalyst can be a CeO2 catalyst, a cerium-based bi-component composite oxide catalyst, a load-type cerium-based oxide catalyst, or the like. With the cerium-based catalyst, the methane oxyhalogenation reactions including a chlorine oxidation reaction and a bromine oxidation reaction can be effectively and stably catalyzed, and methyl chloride and methyl bromide can be prepared. With the cerium-based catalyst, reactants such as CH4, HCl and O2 can be catalytically converted with high efficiency for carrying out a chlorine oxidation reaction, such that products CH3Cl and CH2Cl2 are produced. Also, with the cerium-based catalyst, reactants such as CH4, HBr(H2O), and O2 can be converted with high efficiency for carrying out a bromine oxidation reaction, such that products CH3Br and CH2Br2 are produced.
Owner:XIAMEN UNIV
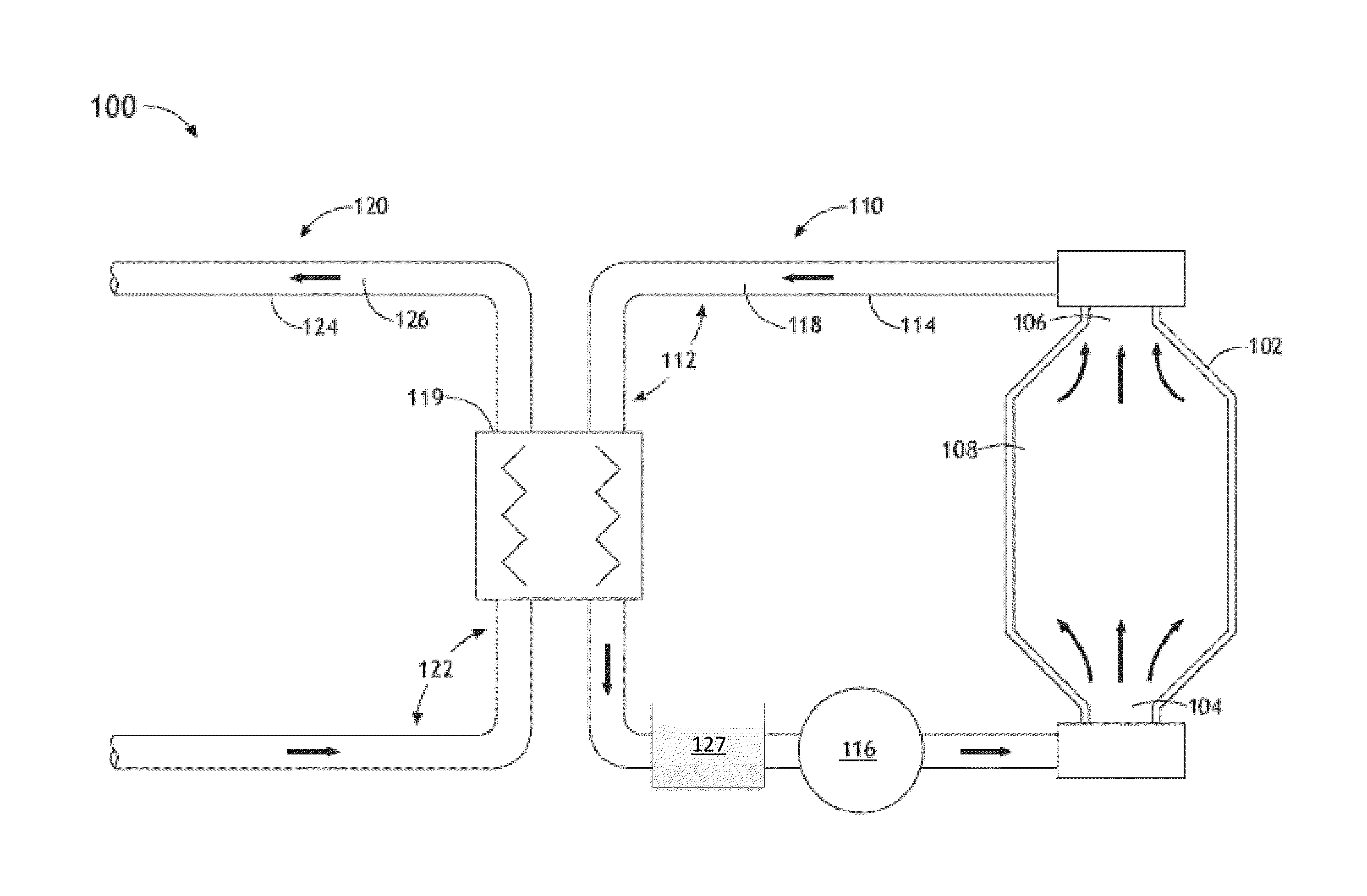
Structural fumigation process and apparatus
InactiveUS6047496AReduce the amount requiredNot extending current fumigant exposure timeFood preservationFumigatorsCombustible gasLiquid carbon
Processes and devices for producing a heated non-flammable gaseous fumigant for structural fumigation use by the application of a carrier gas, such as carbon dioxide (102), to a heater (103) either prior or subsequent to mixing the gas with a toxic agent gas, such as methyl bromide (213), such as by means of a mixer (214) and applying the mixture of gasses as a structural fumigant to eradicate target pests within a structure (106). In the preferred embodiment, the gaseous carbon dioxide is formed by flashing liquid carbon dioxide (614) directly to the gaseous state in a heater (630) prior to being mixed with methyl bromide (654) in a mixer (648) and applying the mixture of gasses as a structural fumigant within a structure (668).
Owner:LEITNER KENNETH D +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com