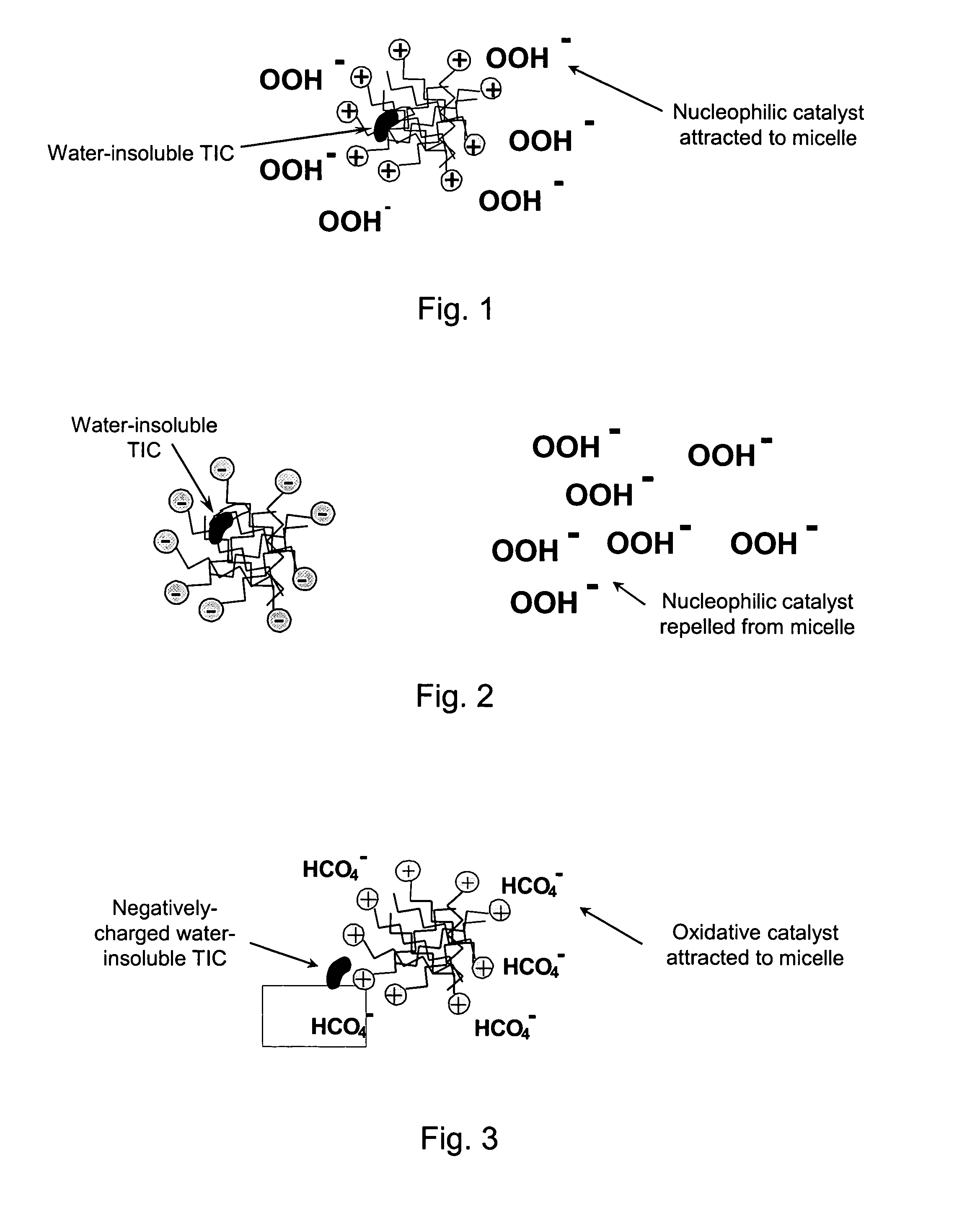
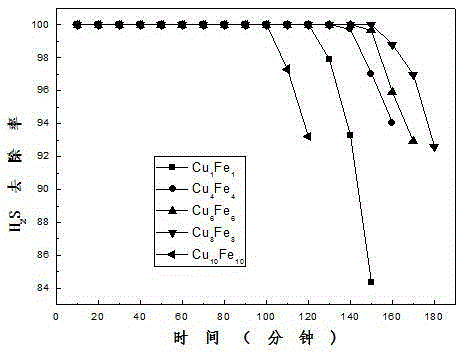
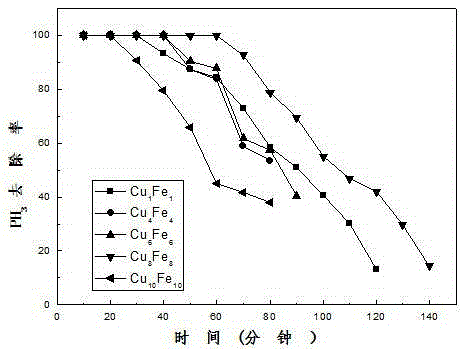
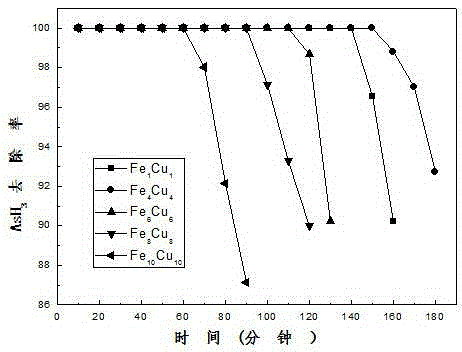
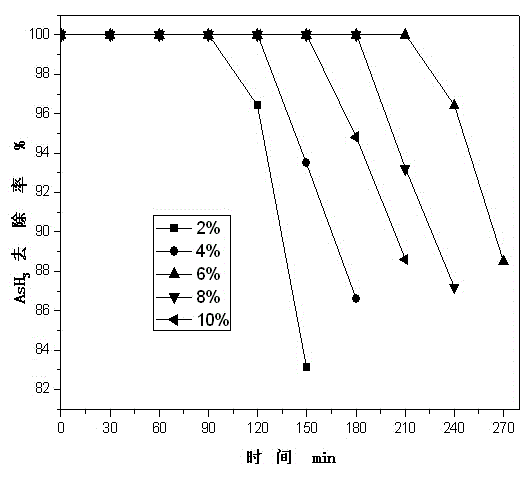
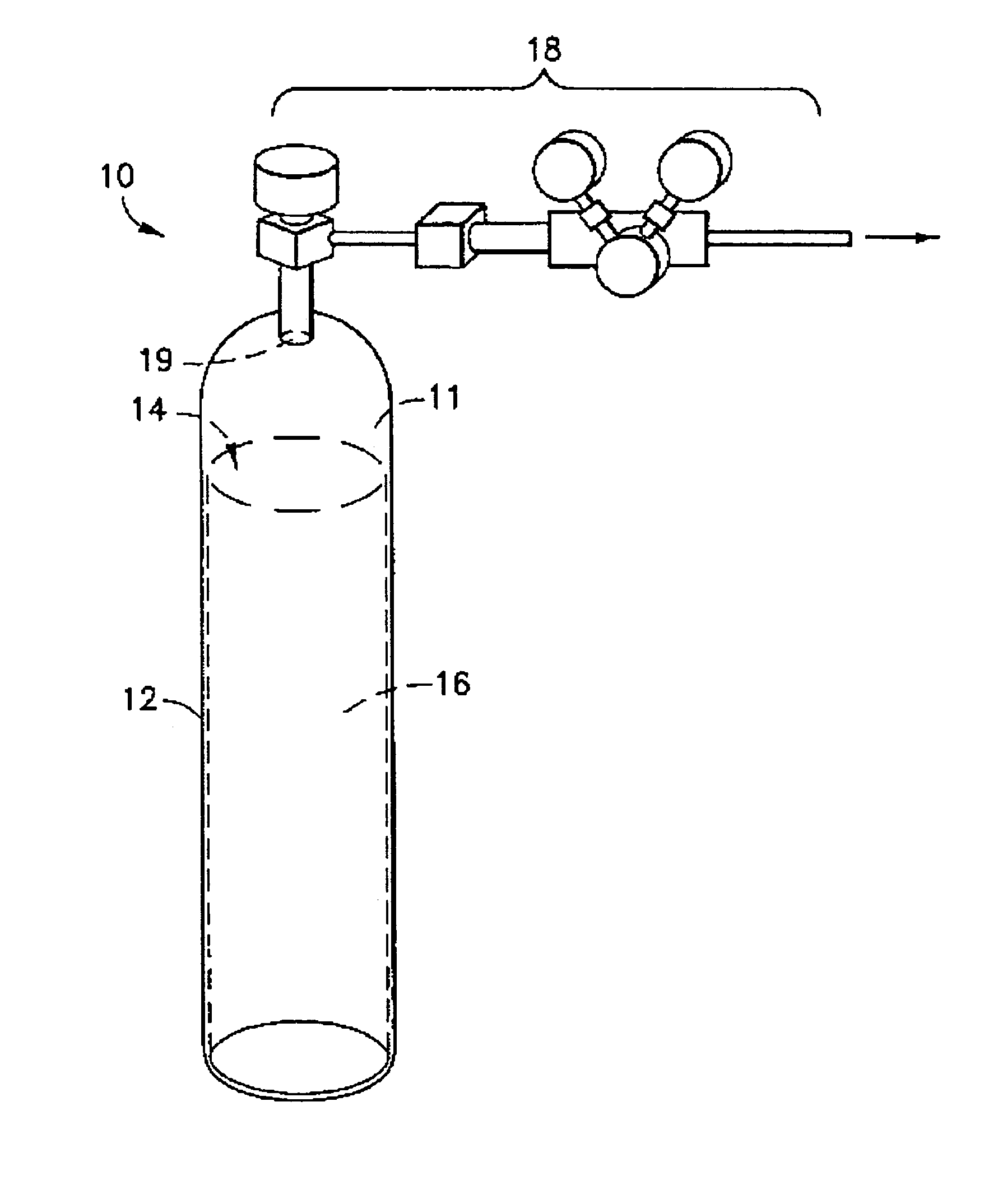
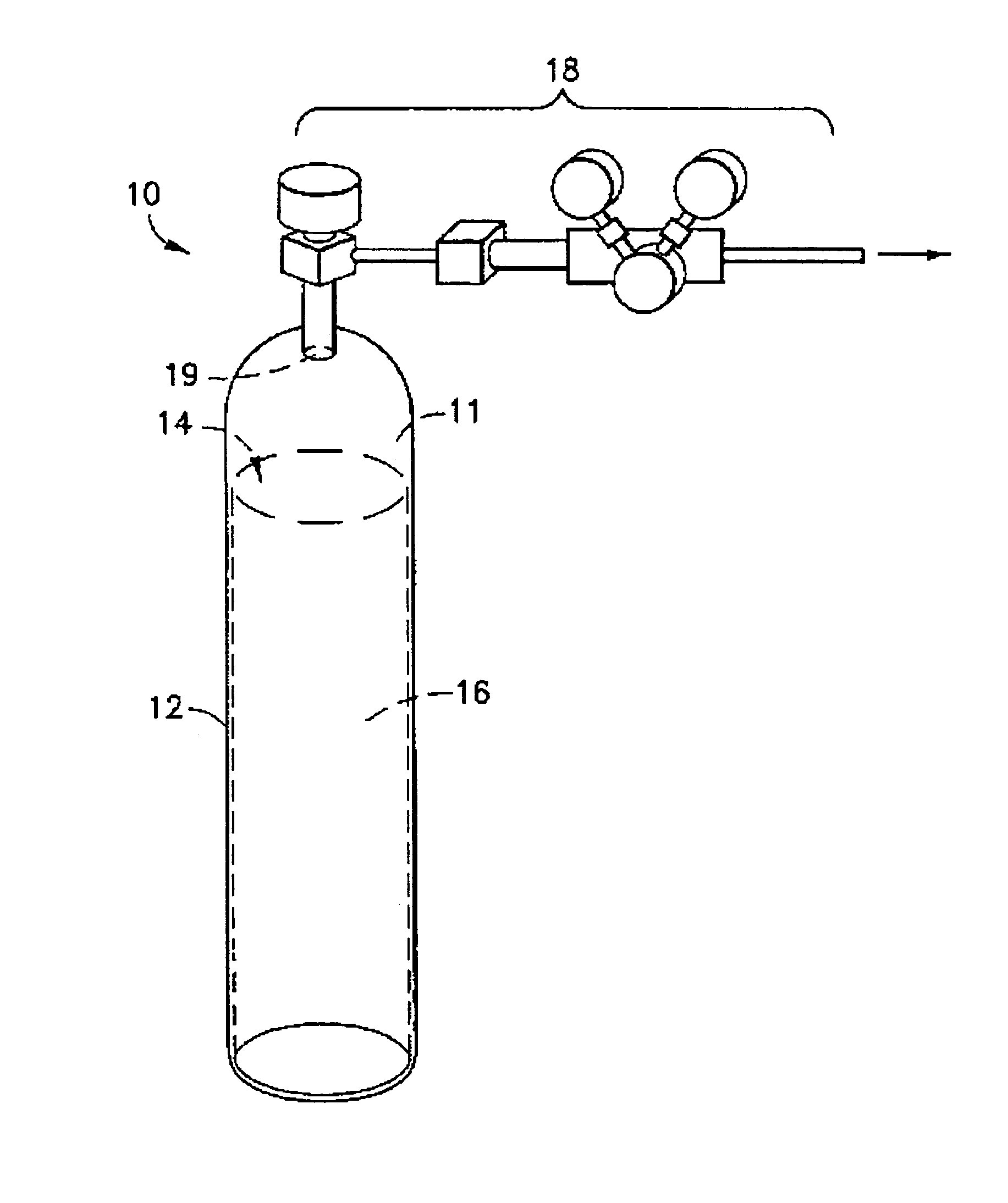
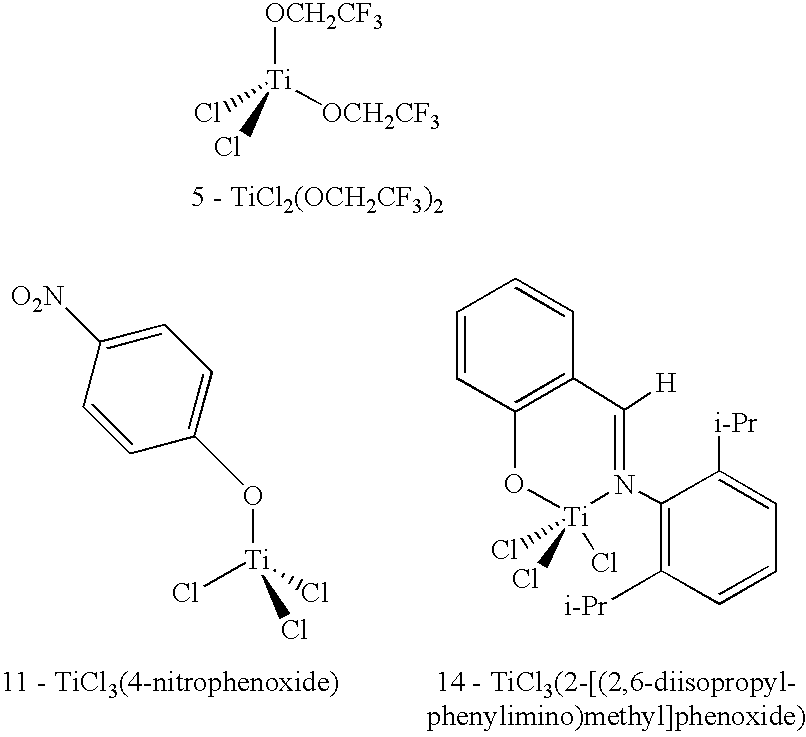
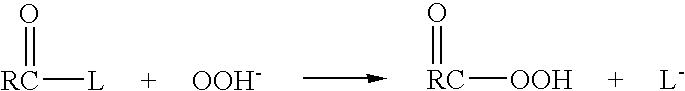Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
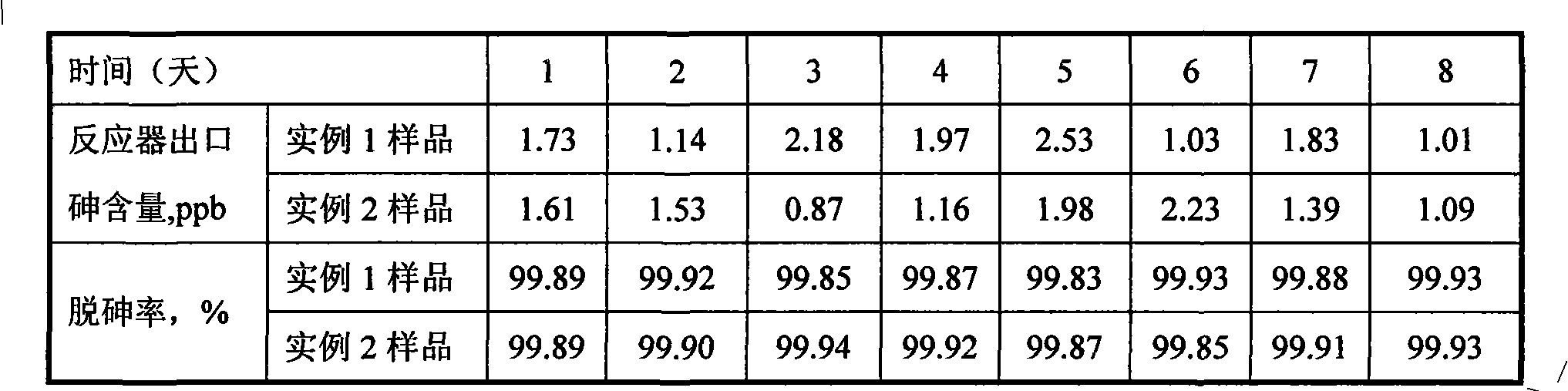
176 results about "Arsine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Arsine (IUPAC name: arsane) is an inorganic compound with the formula AsH₃. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term arsine is commonly used to describe a class of organoarsenic compounds of the formula AsH₃₋ₓRₓ, where R = aryl or alkyl. For example, As(C₆H₅)₃, called triphenylarsine, is referred to as "an arsine".
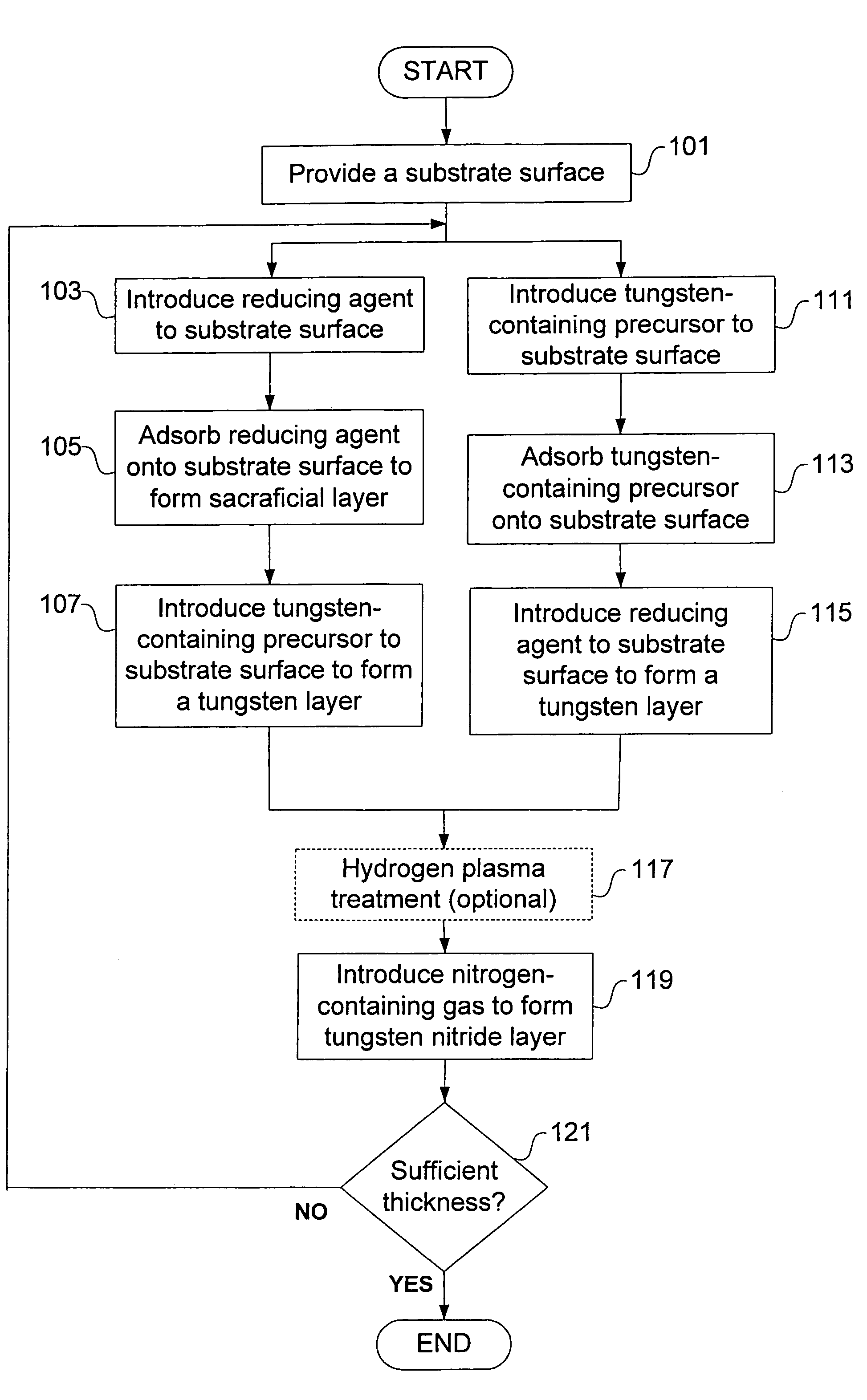
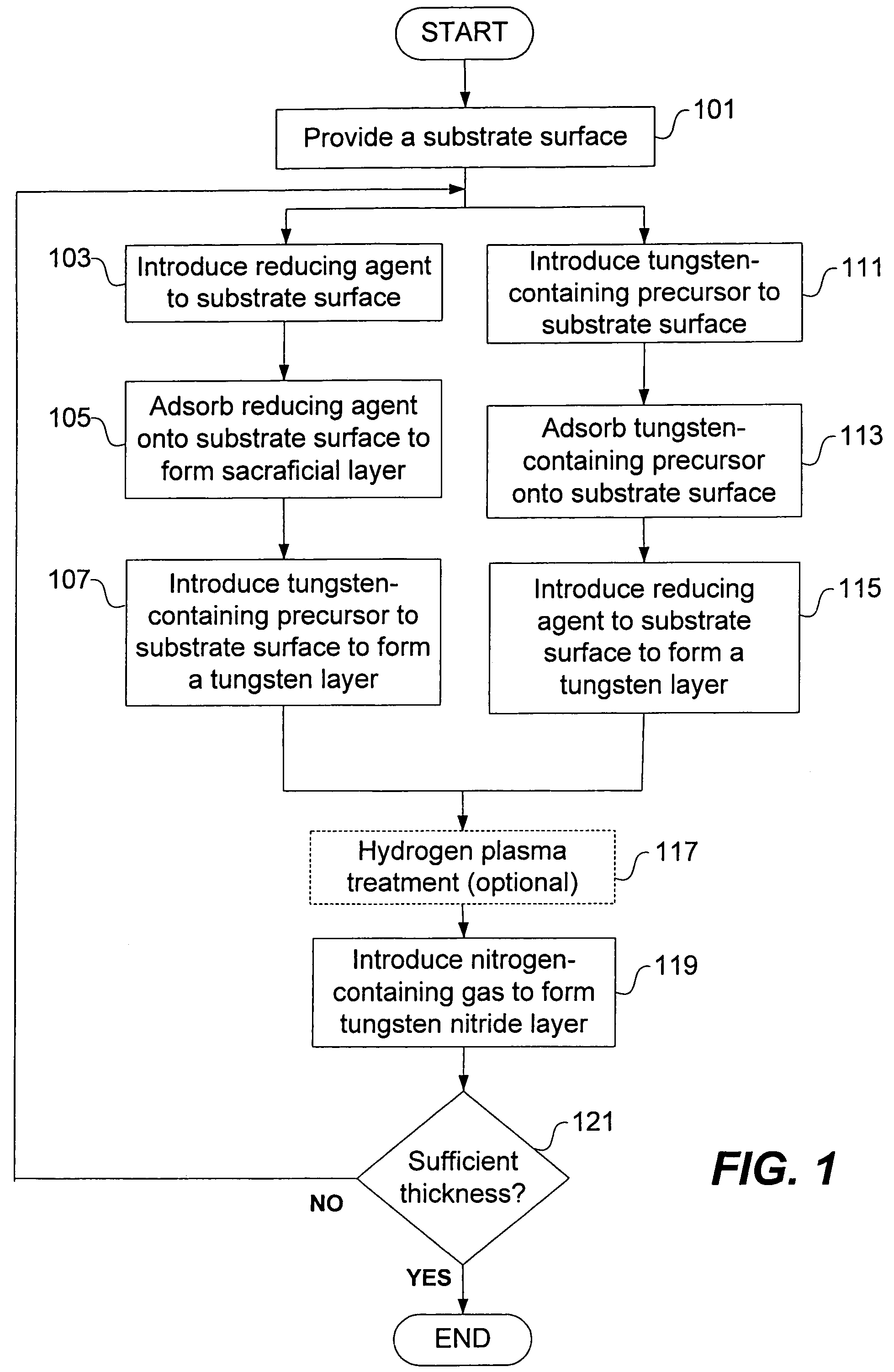
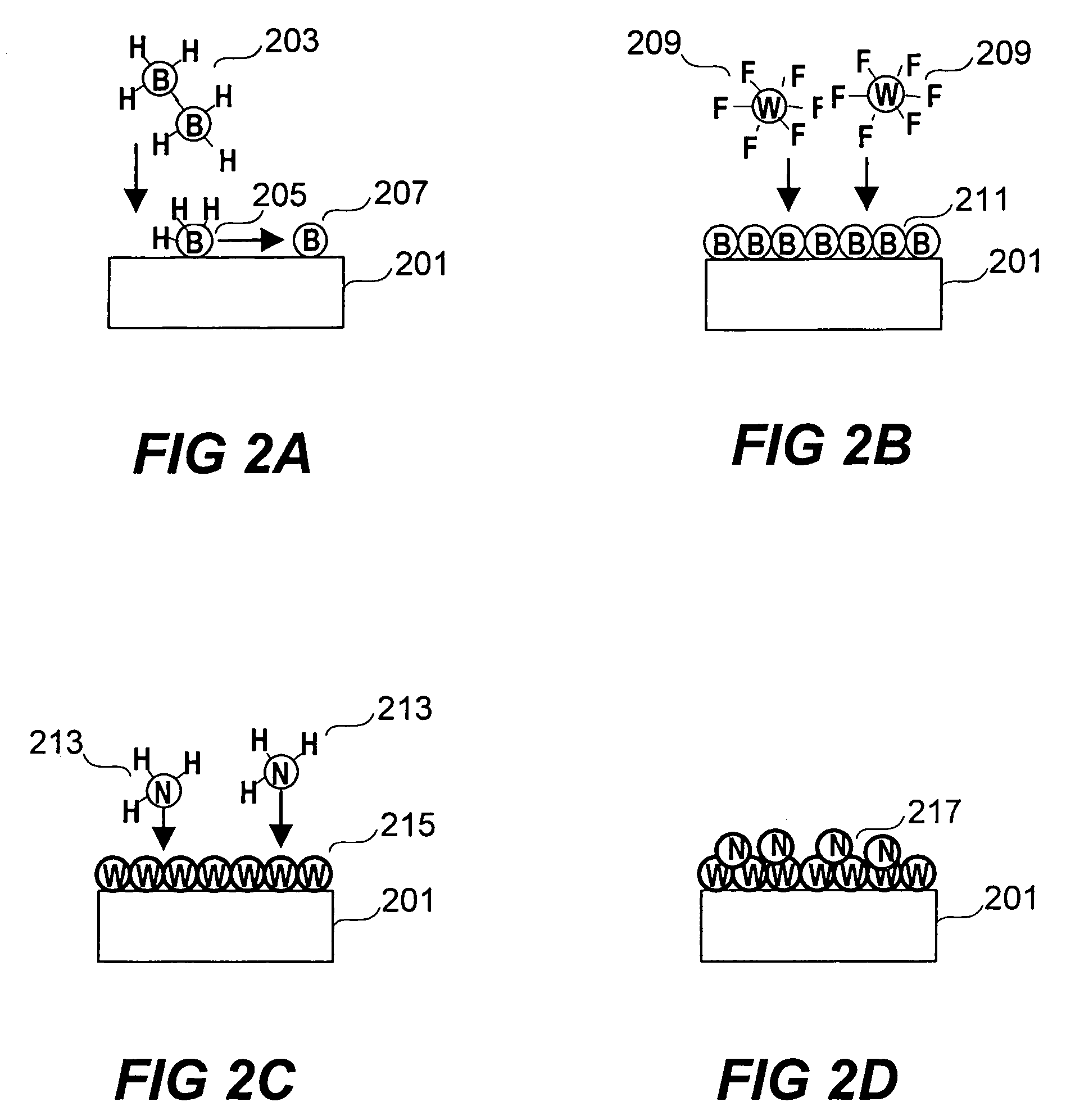
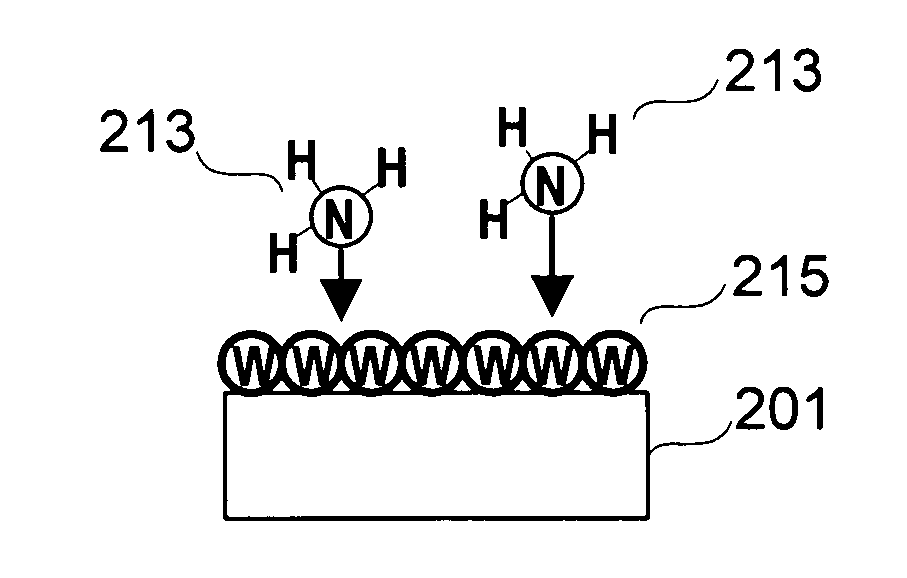
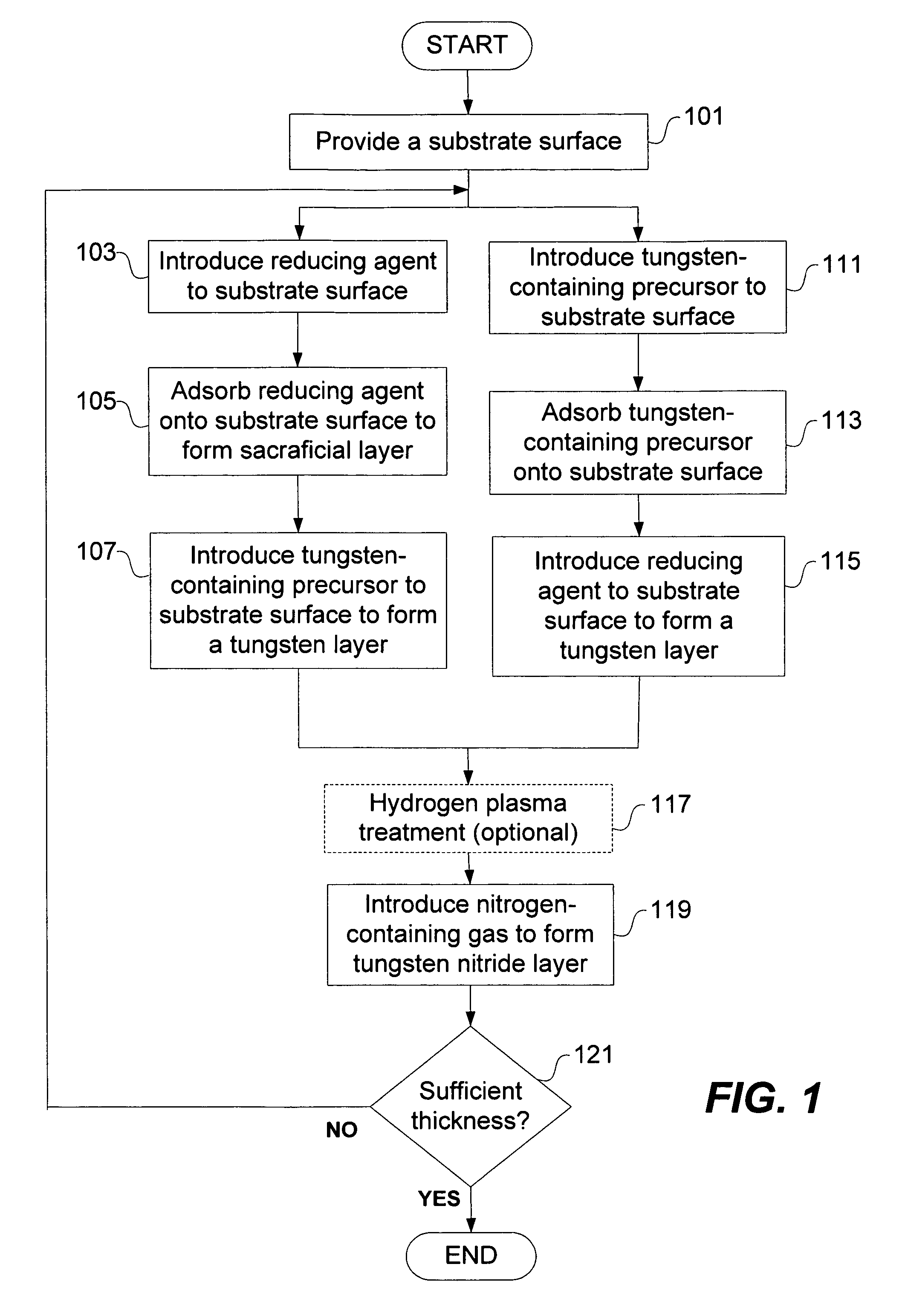
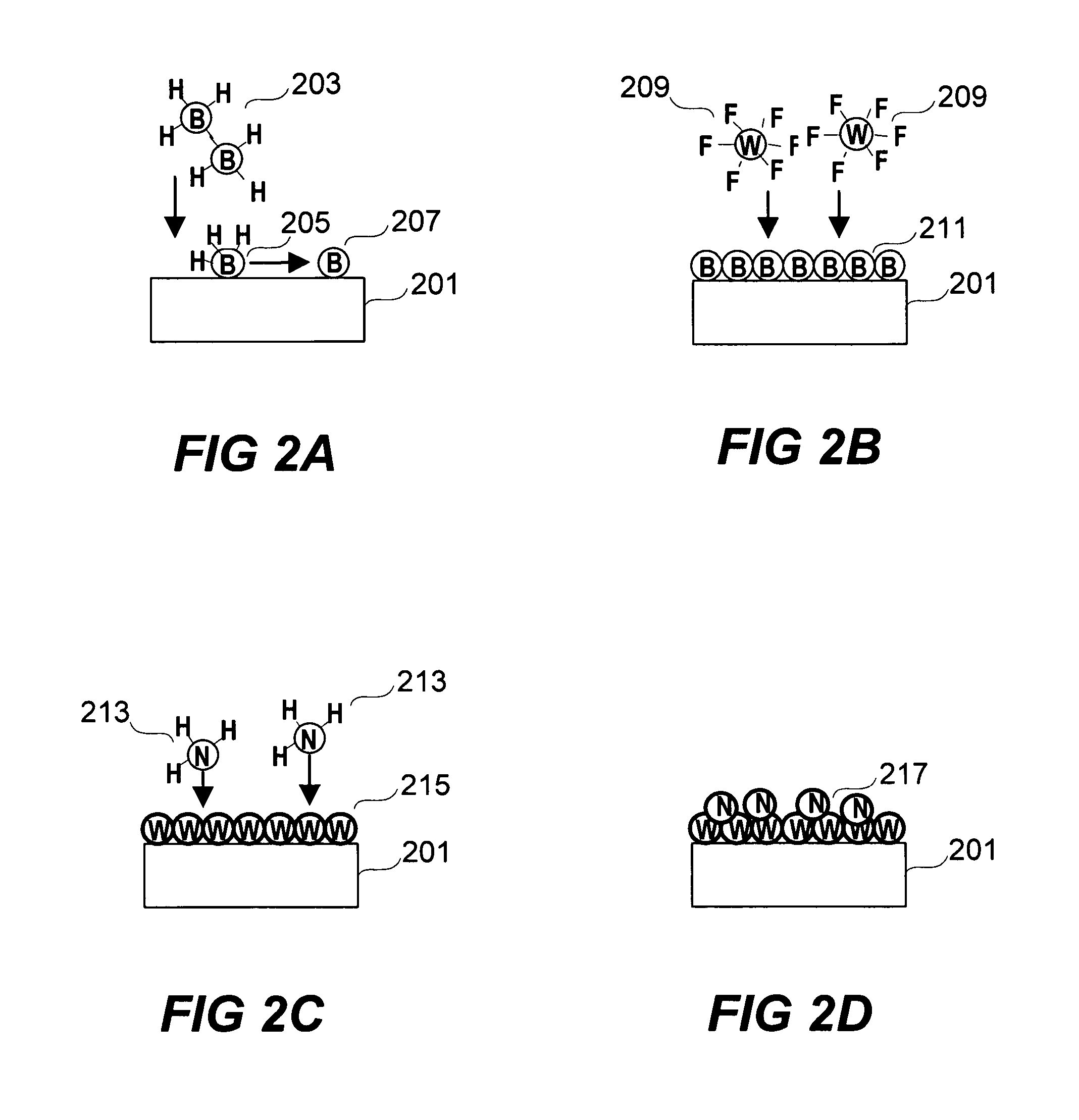
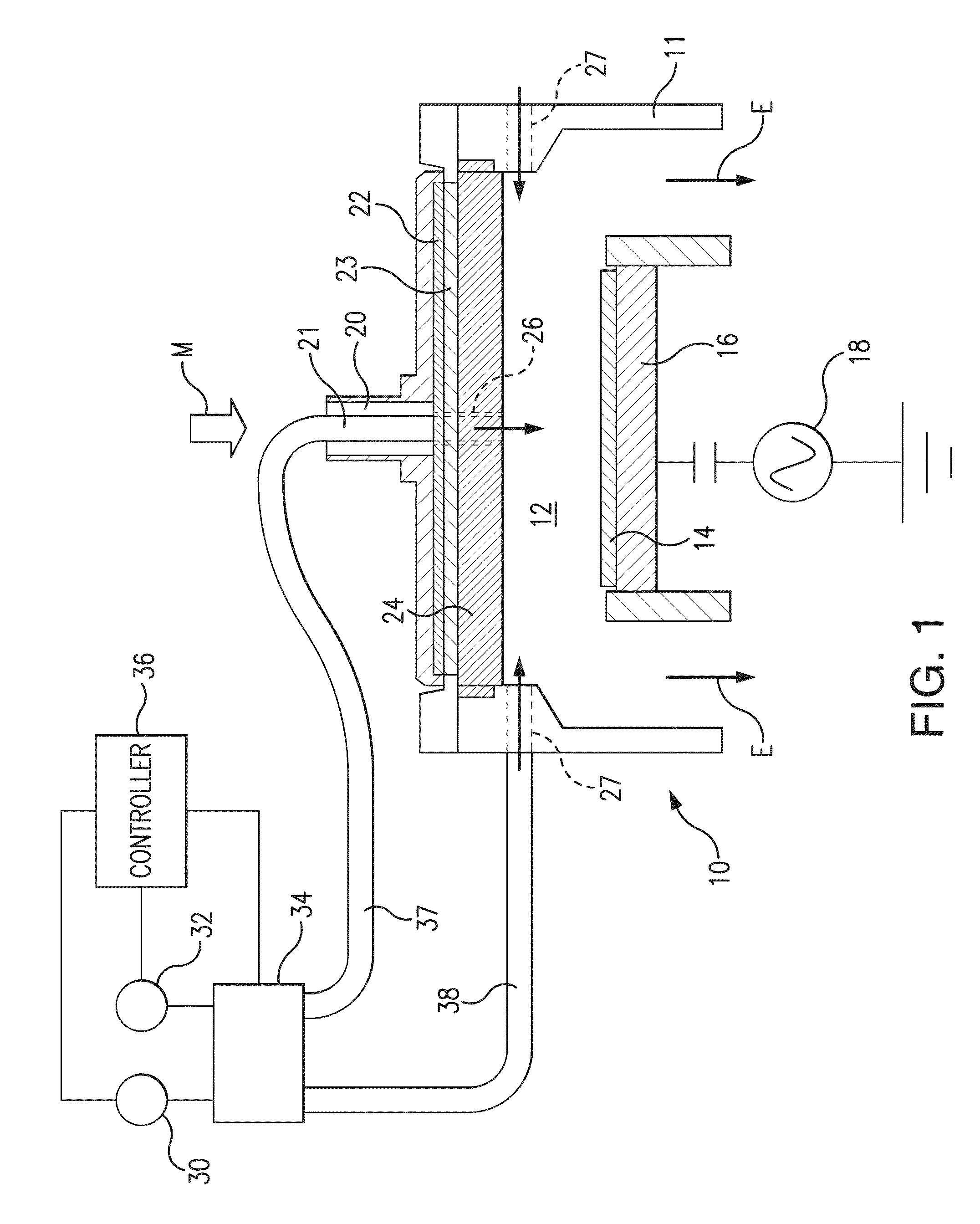
Deposition of tungsten nitride
ActiveUS7005372B2Improve adhesionDeposition thickness is limitedSemiconductor/solid-state device manufacturingChemical vapor deposition coatingArsineTungsten nitride
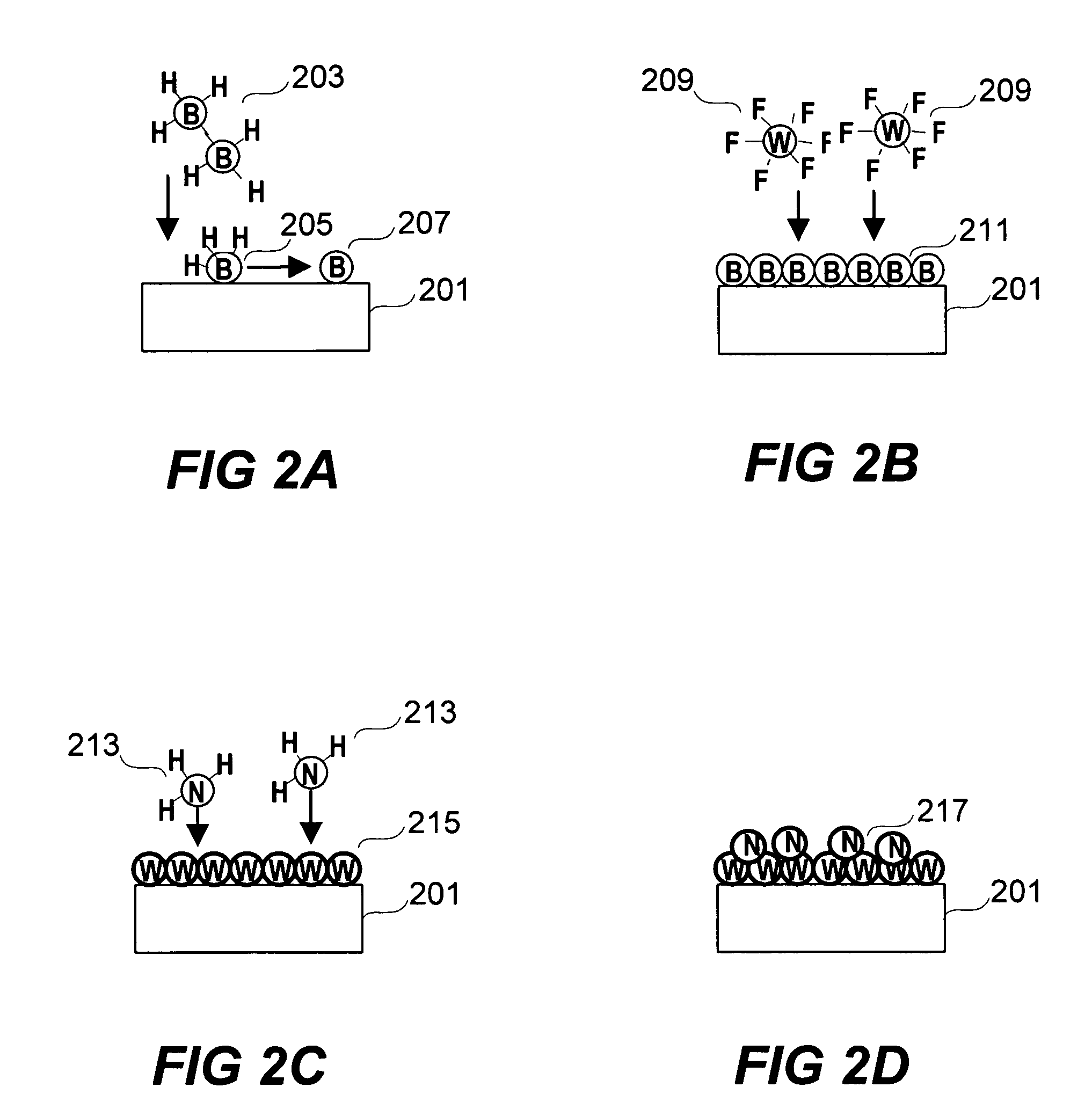
Methods for depositing a tungsten nitride layer are described. The methods form a tungsten nitride layer using a carefully controlled deposition technique such as pulsed nucleation layer (PNL). Initially, a tungsten layer is formed on a substrate surface. The tungsten layer is then exposed to a nitriding agent to form a tungsten nitride layer. Methods of forming relatively thick layers of involve repeated cycles of contact with reducing agent, tungsten precursor and nitriding agent. In some cases, the cycle may also include contact with a dopant precursor such as phosphine or arsine.
Owner:NOVELLUS SYSTEMS
Deposition of tungsten nitride
ActiveUS20060094238A1Improve adhesionControl thicknessSemiconductor/solid-state device manufacturingChemical vapor deposition coatingDopantArsine
Methods for depositing a tungsten nitride layer are described. The methods form a tungsten nitride layer using a carefully controlled deposition technique such as pulsed nucleation layer (PNL). Initially, a tungsten layer is formed on a substrate surface. The tungsten layer is then exposed to a nitriding agent to form a tungsten nitride layer. Methods of forming relatively thick layers of involve repeated cycles of contact with reducing agent, tungsten precursor and nitriding agent. In some cases, the cycle may also include contact with a dopant precursor such as phosphine or arsine.
Owner:NOVELLUS SYSTEMS
Functionalized nanoparticles and method
ActiveUS20100283014A1Material nanotechnologyPolycrystalline material growthSemiconductor materialsChemical reaction
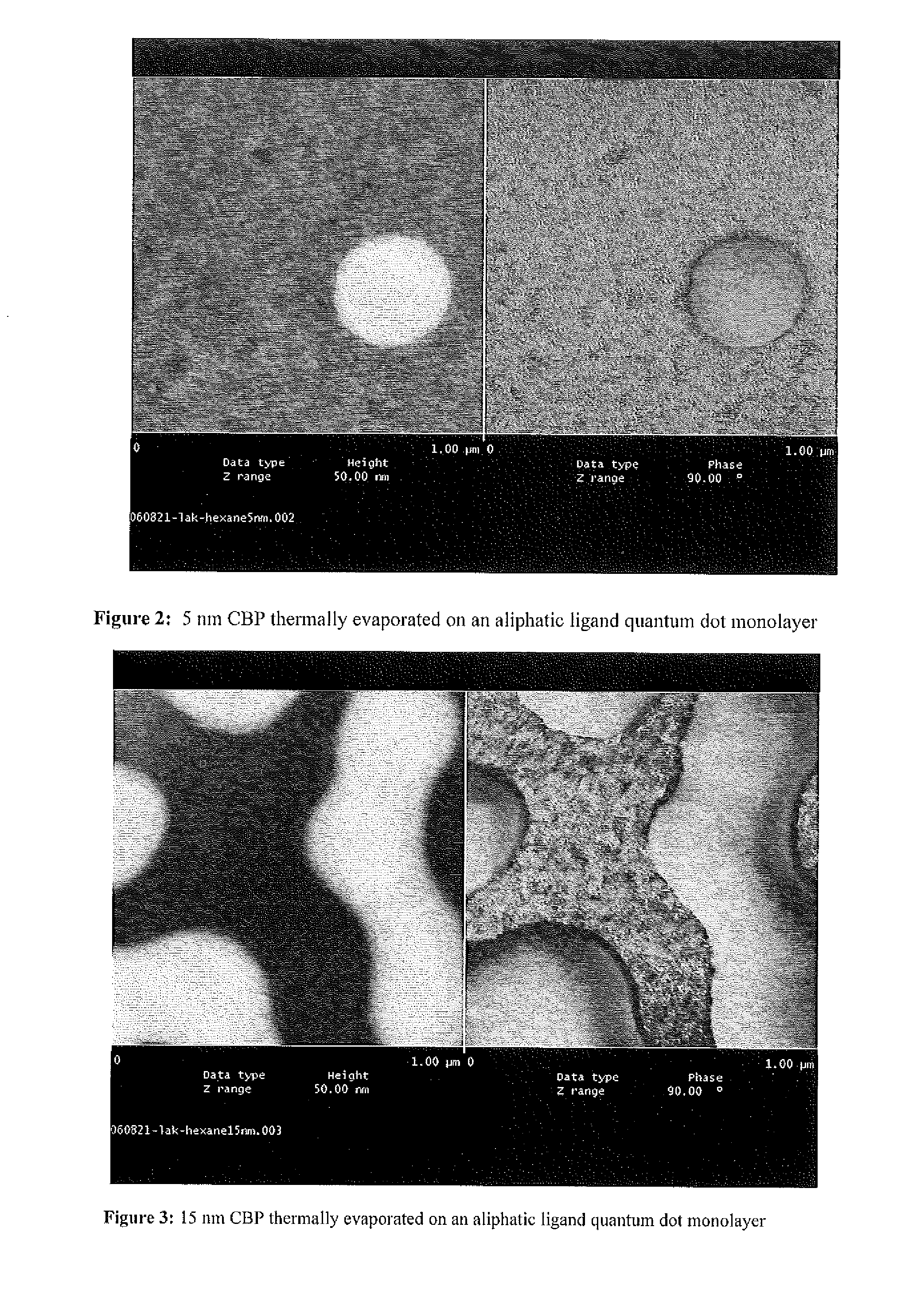
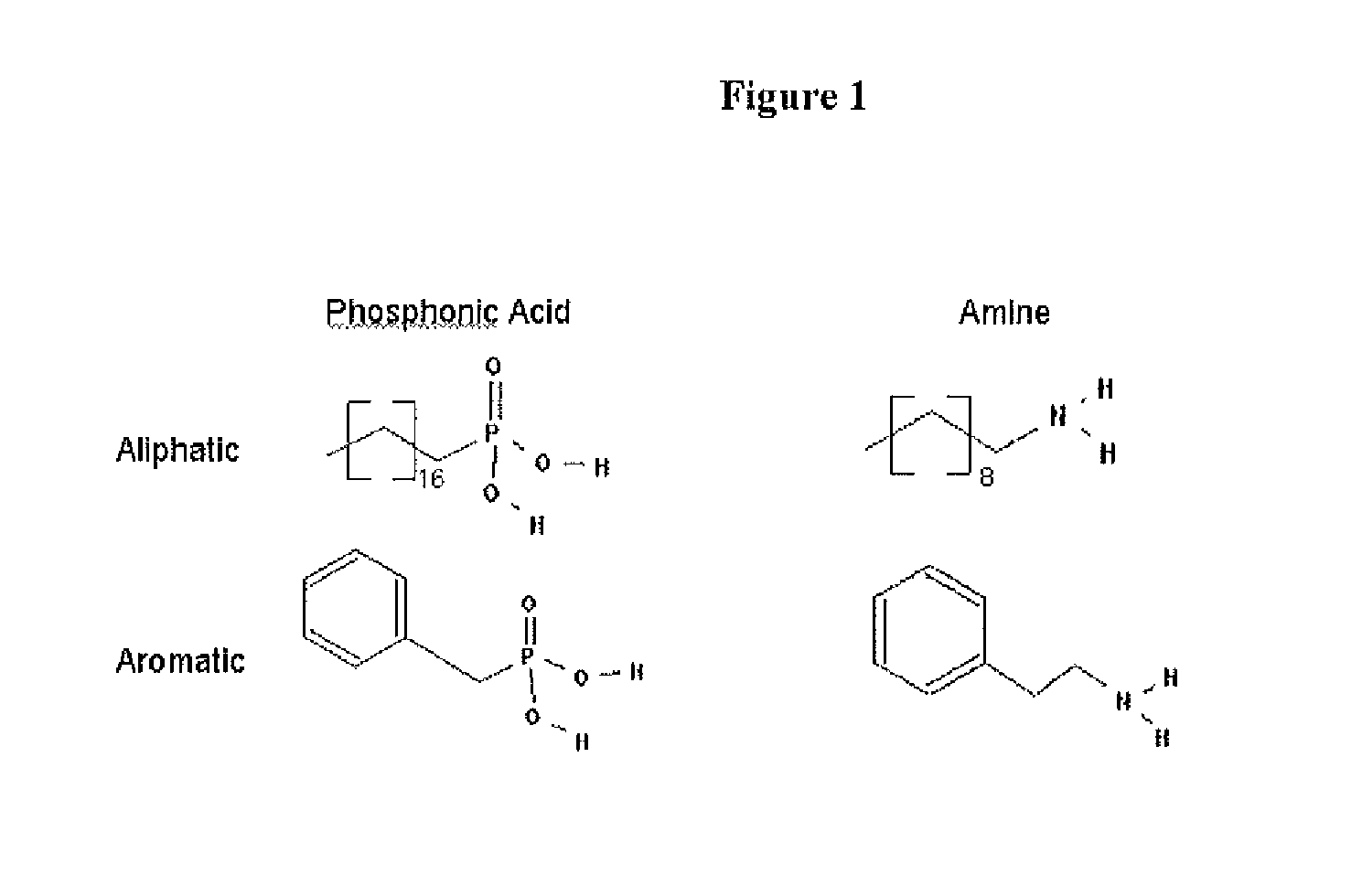
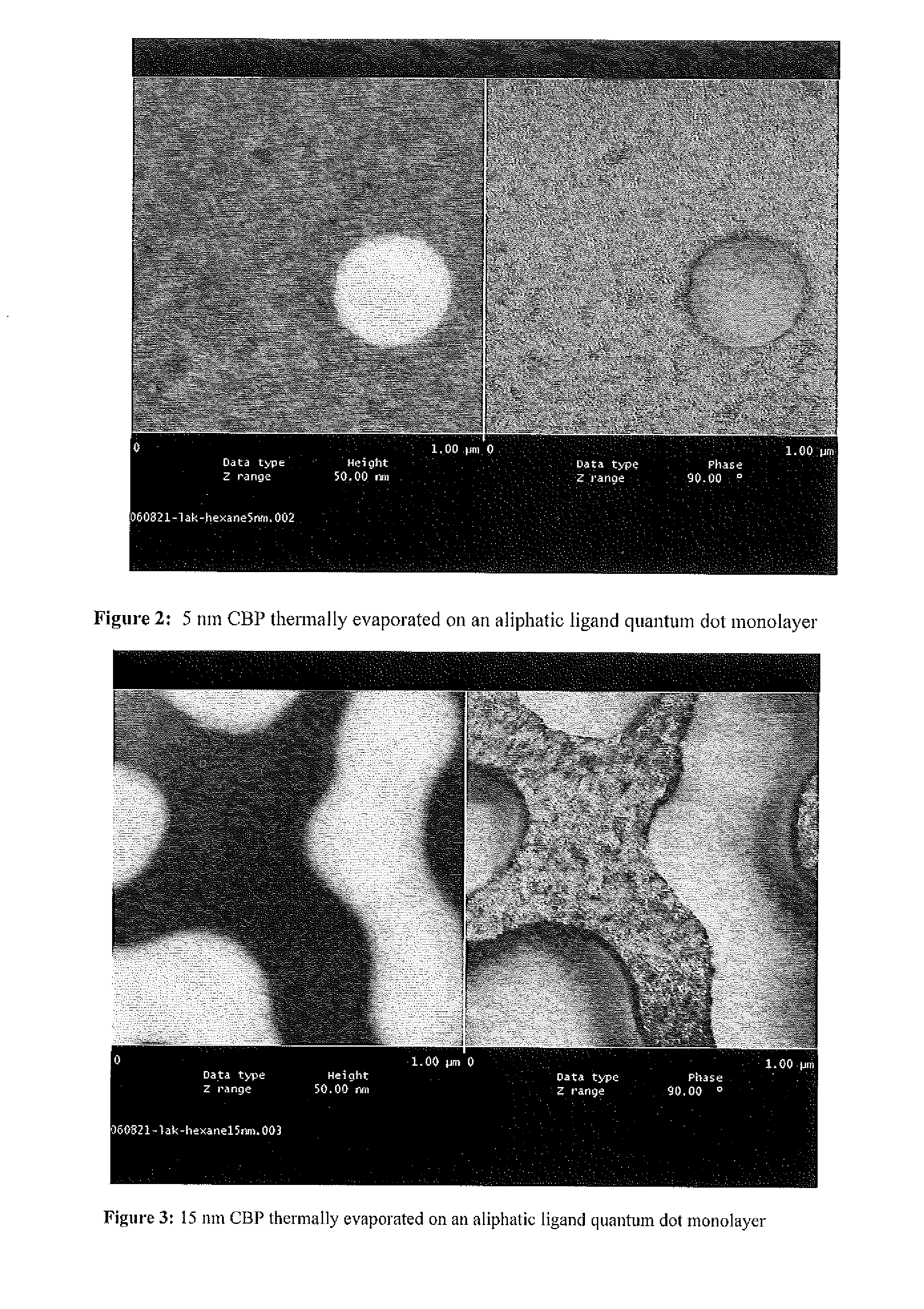
A nanoparticle including an inorganic core comprising at least one metal and / or at least one semi-conductor compound comprising at least one metal includes a coating or shell disposed over at least a portion of a surface of the core. The coating can include one or more layers. Each layer of the coating can comprise a metal and / or at least one semiconductor compound. The nanoparticle further includes a ligand attached to a surface of the coating. The ligand is represented by the formula: X-Sp-Z, wherein X represents, e.g., a primary amine group, a secondary amine group, a urea, a thiourea, an imidizole group, an amide group, a phosphonic or arsonic acid group, a phosphinic or arsinic acid group, a phosphate or arsenate group, a phosphine or arsine oxide group; Sp represents a spacer group, such as a group capable of allowing a transfer of charge or an insulating group; and Z represents: (i) reactive group capable of communicating specific chemical properties to the nanocrystal as well as provide specific chemical reactivity to the surface of the nanocrystal, and / or (ii) a group that is cyclic, halogenated, or polar a-protic. In certain embodiments, at least two chemically distinct ligands are attached to an surface of the coating, wherein the at least two ligands (I and II) are represented by the formula: X-Sp-Z. In ligand (I) X represents a phosphonic, phosphinic, or phosphategroup and in ligand (II) X represents a primary or secondary amine, or an imidizole, or an amide; In both ligands (I) and (II) Sp, which can be the same or different in the two compounds, represents a spacer group, such as a group capable of allowing a transfer of charge or an insulating group; Z, which can be the same or different in the two compounds, is a group chosen from among groups capable of communicating specific chemical properties to the nanoparticle as well as provide specific chemical reactivity to the surface of the nanoparticle. In preferred embodiments, the nanoparticle includes a core comprising a semiconductor material.
Owner:SAMSUNG ELECTRONICS CO LTD
Deposition of tungsten nitride
ActiveUS7691749B2Improve adhesionControl thicknessSemiconductor/solid-state device manufacturingChemical vapor deposition coatingArsineTungsten nitride
Methods for depositing a tungsten nitride layer are described. The methods form a tungsten nitride layer using a carefully controlled deposition technique such as pulsed nucleation layer (PNL). Initially, a tungsten layer is formed on a substrate surface. The tungsten layer is then exposed to a nitriding agent to form a tungsten nitride layer. Methods of forming relatively thick layers of involve repeated cycles of contact with reducing agent, tungsten precursor and nitriding agent. In some cases, the cycle may also include contact with a dopant precursor such as phosphine or arsine.
Owner:NOVELLUS SYSTEMS
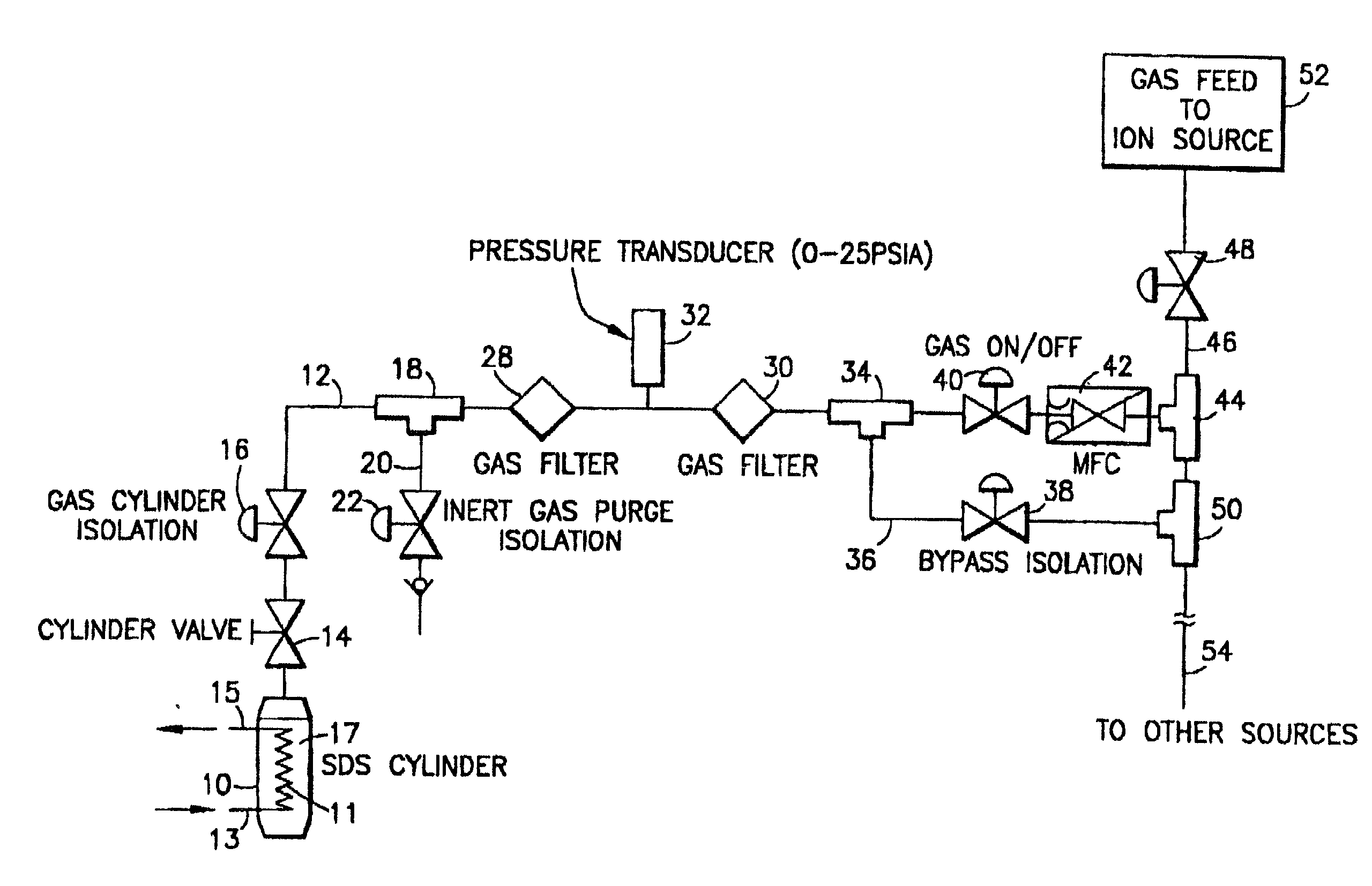
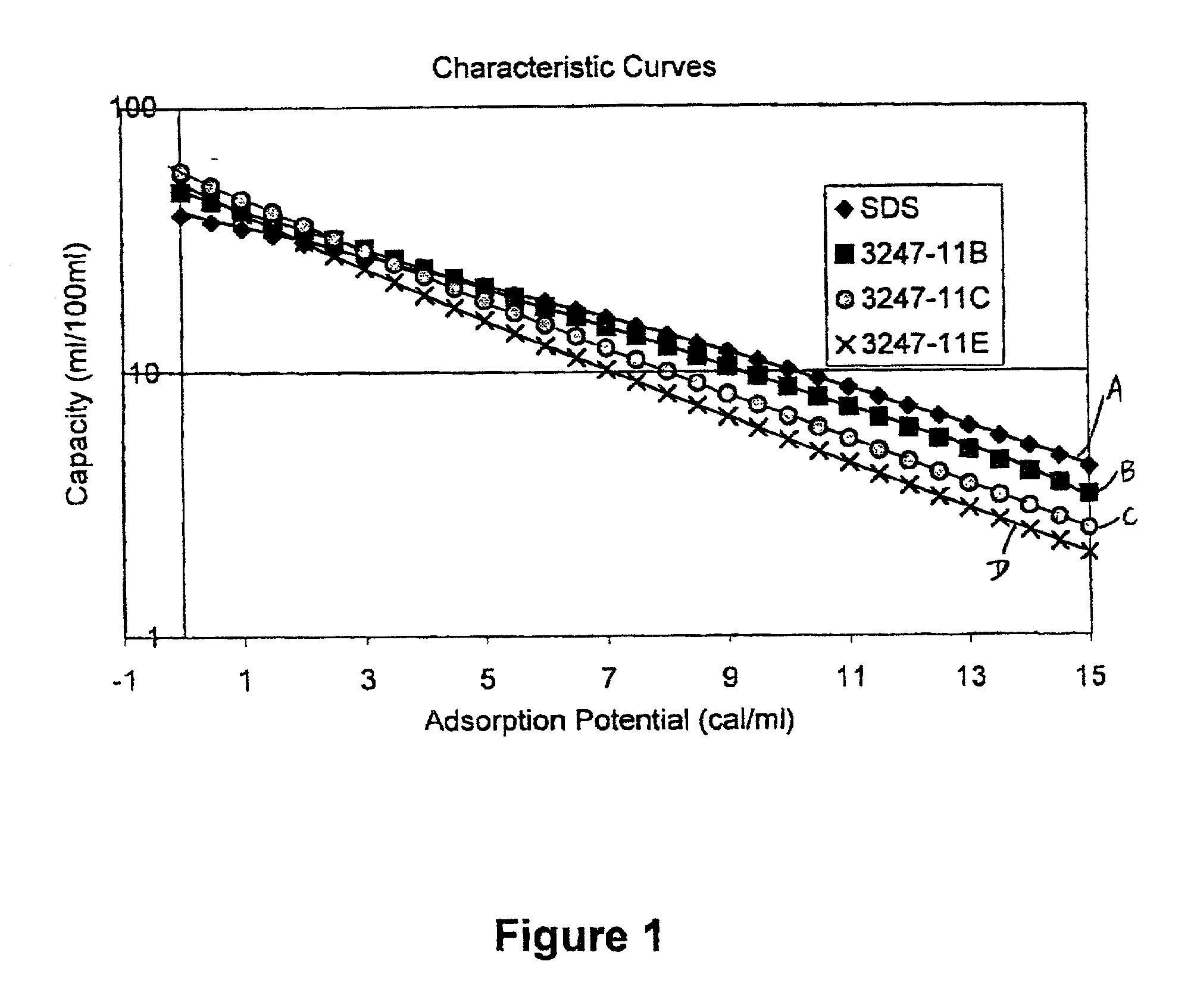
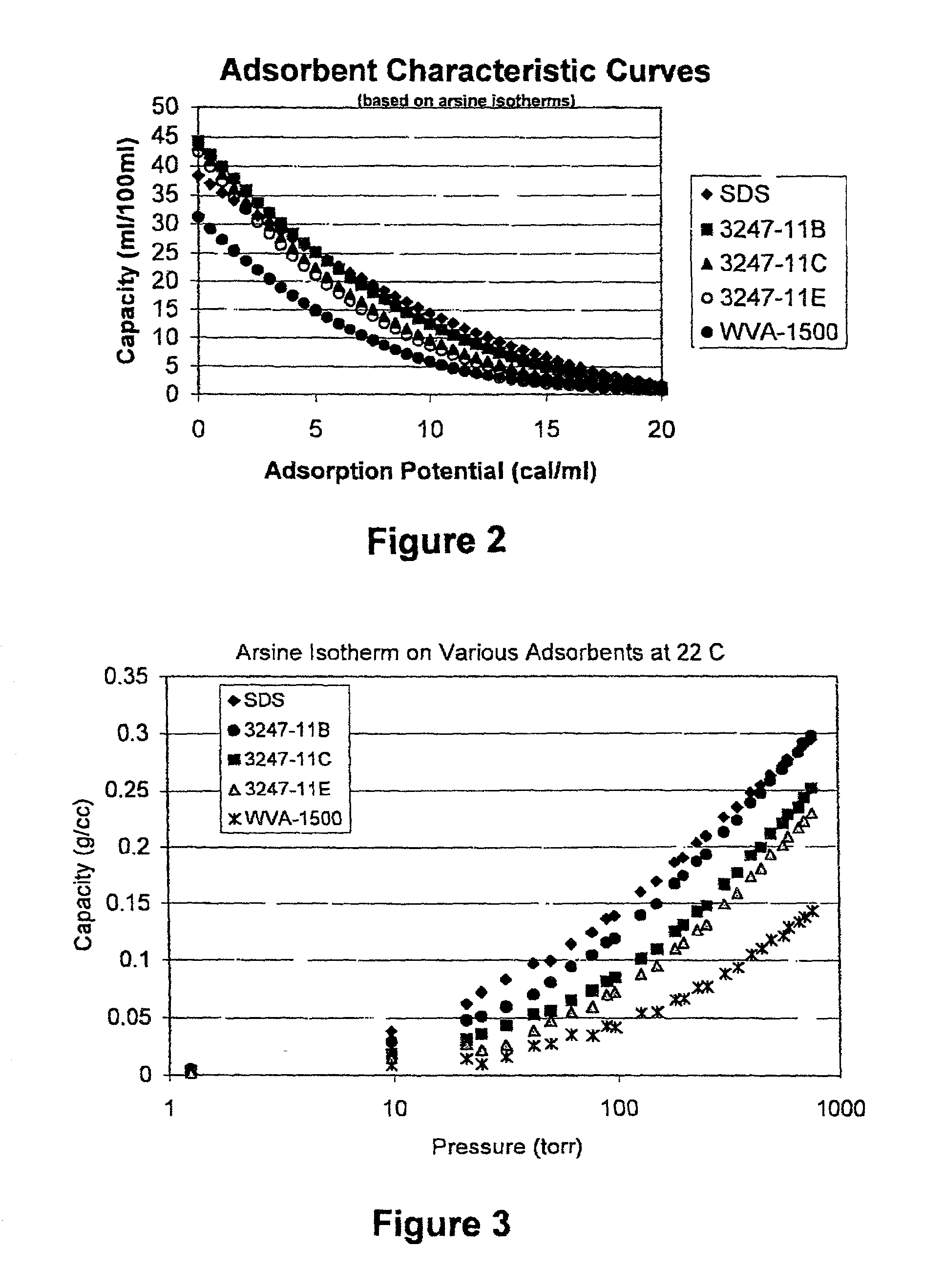
Fluid storage and delivery system utilizing low heels carbon sorbent medium
InactiveUS6592653B2Reduces sufficiencyImprove adsorption capacityGas treatmentOther chemical processesDesorptionSorbent
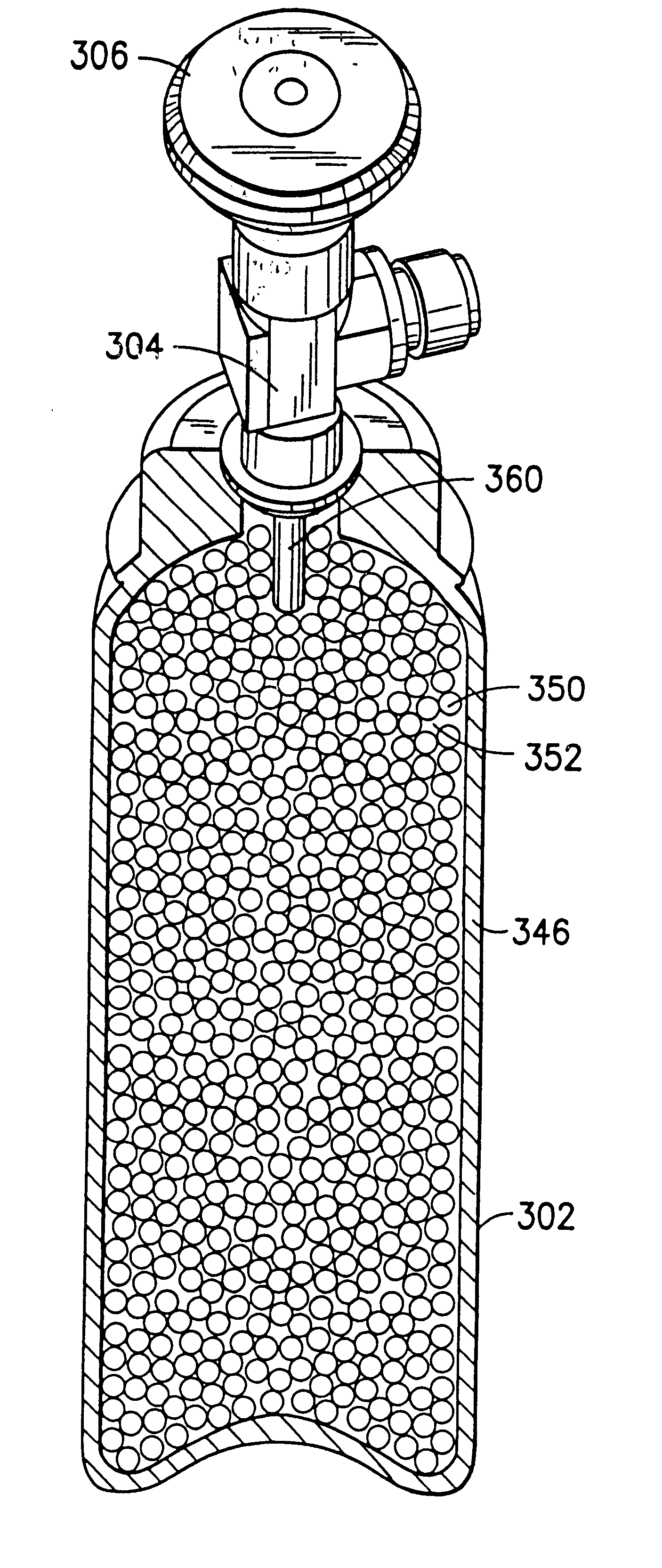
A fluid storage and dispensing system including a vessel containing a low heel carbon sorbent having fluid adsorbed thereon, with the system arranged to effect desorption of the fluid from the sorbent for dispensing of fluid on demand. The low heel carbon sorbent preferably is characterized by at least one of the following characteristics: (i) Heel, measured for gaseous arsine (AsH3) at 20° C. at 20 Torr, of not more than 50 grams AsH3 per liter of bed of the sorbent material; (ii) Heel, measured for gaseous boron trifluoride (BF3) at 20° C. at 20 Torr, of not more than 20 grams boron trifloride per liter of bed of the sorbent material; (iii) Heel, measured for gaseous germanium tetrafluoride (GeF4) at 20° C. at 20 Torr, of not more than 250 grams AsH3 per liter of bed of the sorbent material; (iv) Heel, measured for gaseous arsenic pentafluoride (AsF5) at 20° C. at 20 Torr, of not more than 700 grams AsF5 per liter of bed of the sorbent material; (v) Heel, measured for gaseous trimethyl silane (3MS) at 20° C. at 20 Torr, of not more than 160 grams 3MS per liter of bed of the sorbent material; and (vi) Heel, measured for gaseous ethane (C2H4) at 21° C. at 25 Torr, of not more than 10 grams ethane per liter of bed of the sorbent material.
Owner:ENTEGRIS INC
Reactive formulations for a neutralization of toxic industrial chemicals
InactiveUS7125497B1Efficiently neutralizedHydrogen peroxideLiquid degasificationBoron trichlorideMalathion
Decontamination formulations for neutralization of toxic industrial chemicals, and methods of making and using same. The formulations are effective for neutralizing malathion, hydrogen cyanide, sodium cyanide, butyl isocyanate, carbon disulfide, phosgene gas, capsaicin in commercial pepper spray, chlorine gas, anhydrous ammonia gas; and may be effective at neutralizing hydrogen sulfide, sulfur dioxide, formaldehyde, ethylene oxide, methyl bromide, boron trichloride, fluorine, tetraethyl pyrophosphate, phosphorous trichloride, arsine, and tungsten hexafluoride.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
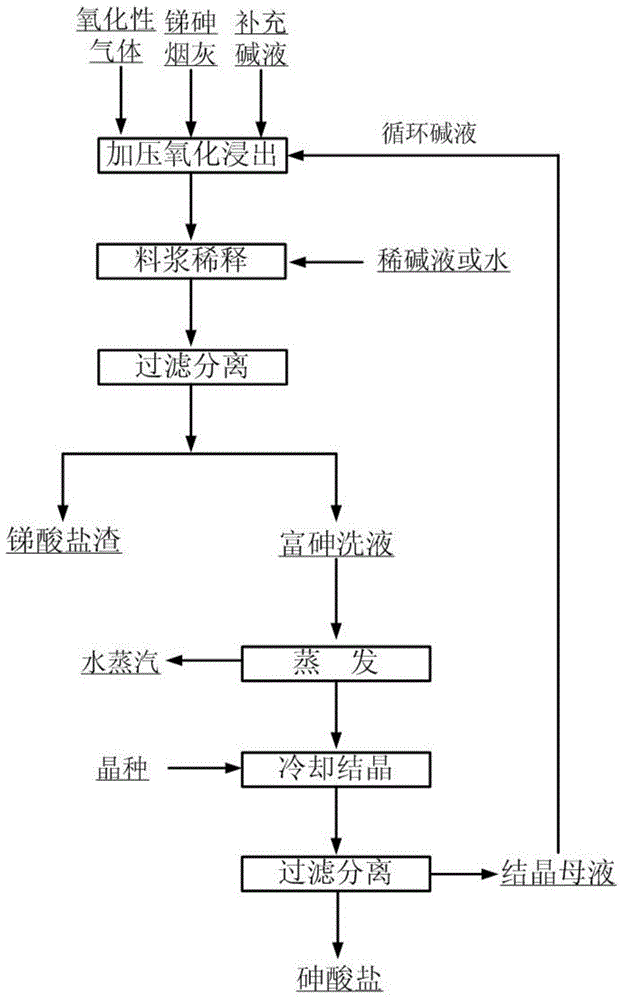
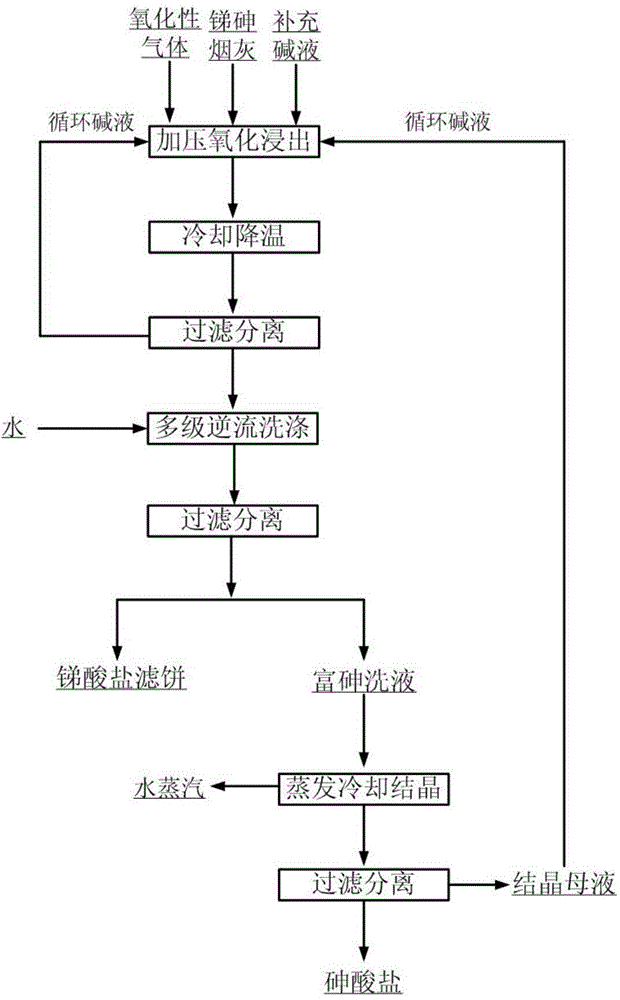
Method for separating antimony from arsenic in antimony-arsenic soot
The invention relates to a method for separating antimony from arsenic in antimony-arsenic soot through oxidation alkaline leaching. The method specifically includes the steps that the antimony-arsenic soot and an alkali solution of a certain concentration are mixed and placed in an alkali-resisting and pressure-resisting reaction still, a gas oxidizing agent of certain pressure is introduced, and a reaction is carried out at a certain temperature; after the reaction, the alkali concentration is adjusted to be below 150 g / L with water or dilute alkali, liquid and solid are separated, an arsenic-rich leaching solution and antimonate slags are obtained, and the antimonate slags can be used for preparation of antimonious oxide powder as antimony concentrate; the arsenic-rich leaching solution is evaporated and added with little seed crystals for cooling crystallization, arsenate crystal products are obtained, and crystallization mother liquor returns to the leaching process of the antimony-arsenic soot. The method is simple in process, capable of separating antimony and arsenic thoroughly and short in flow path; and the method can also avoid arsenic volatilization of the roasting process and harm caused by arsenic hydride gas of the acid leaching process, thereby being environmentally friendly.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Method for separating antimony and arsenic from antimony and arsenic-containing ash through oxidation and alkaline leaching
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Catalyst for acid and halogen gas treatment and production method thereof
InactiveCN101549300ADispersed particle separationPerfluorocarbons/hydrofluorocarbons captureActivated carbonArsine
The invention provides a catalyst capable of treating acid gas and halogen gas and a production method thereof. The acid gas and halogen gas are gas such as hydrogen chloride (HCl), hydrogen fluoride (HF), hydrogen bromide (HBr), hydrogen iodide (HI), fluorine gas (F2), chlorine gas (Cl2), bromine (Br2), iodine (I2), chlorine trifluoride (ClF3), phosphine (PH3), phosphorus trichloride (PCl3), phosphorus pentachloride (PCl5), phosphorus oxychloride (POCl3), phosphorus pentoxide (P2O5), arsine (AsH3), silicane (SiH4), silicane tetrafluoride (SiF4), silicane tetrachloride (SiCl4), silicane trichloride (SiHCl3), silicane dichloride (SiH2Cl2), boron trifluoride (BF3), boron trichloride (BCl3), germanium tetrachloride (GeCl4), single-Germane (GeH4), nitric oxide (NO), nitrogen dioxide (NO2), sulfur dioxide (SO2), sulfur trioxide (SO3 ), sulfur hexafluoride (SF6), and the like. The catalyst comprises one or more of the following carrier materials: activated carbon, gumbrine, diatomite, cement, silica sand, ceramic materials, and the like; and the catalyst also comprises one or more of the following metal compounds: hydroxides, oxides, carbonates and supercarbonate of alkali metals, hydroxides, oxides, carbonates and supercarbonate of alkaline-earth metals, oxides of IIIA-group metals, oxides of IVA-group metals, as well as oxides, oxide hydrate, sulphates and carbonates of transition metals.
Owner:刘宝珠
Preparation method of adsorbent for purifying hydrogen sulfide, hydrogen phosphide and arsenic hydride simultaneously
ActiveCN105233806ALarge specific surface areaGood hydrothermal stabilityOther chemical processesDispersed particle separationDesorptionSorbent
The invention relates to a preparation method of an adsorbent for purifying hydrogen sulfide, hydrogen phosphide and arsenic hydride simultaneously, and belongs to the technical field of adsorbent preparation. The method comprises the steps that P123 or F127 is dissolved in a dilute acid solution and stirred till being dissolved, a certain number of pore-enlarging agents are added, stirring and even mixing are carried out, ethyl orthosilicate is added, the mixture is poured into a reaction kettle after being stirred for 1-6 h, powder obtained after crystallization is washed with distilled water till the color of test paper is not changed, the dry powder obtained after drying is placed into a roaster to be roasted, temperature is naturally decreased to indoor temperature, an adsorbent carrier is obtained, the obtained carrier is dissolved in water, a certain amount of metal salt is added, filtering, drying and roasting are carried out, and then the large-aperture adsorbent is obtained. The preparation method is easy to implement, the metal salt impregnant is low in cost and easy to obtain, the desorption effect is good even at low temperature, and a wide development prospect is provided for simultaneous desulfurization, dephosphorization and dearsenization through a dry method.
Owner:KUNMING UNIV OF SCI & TECH
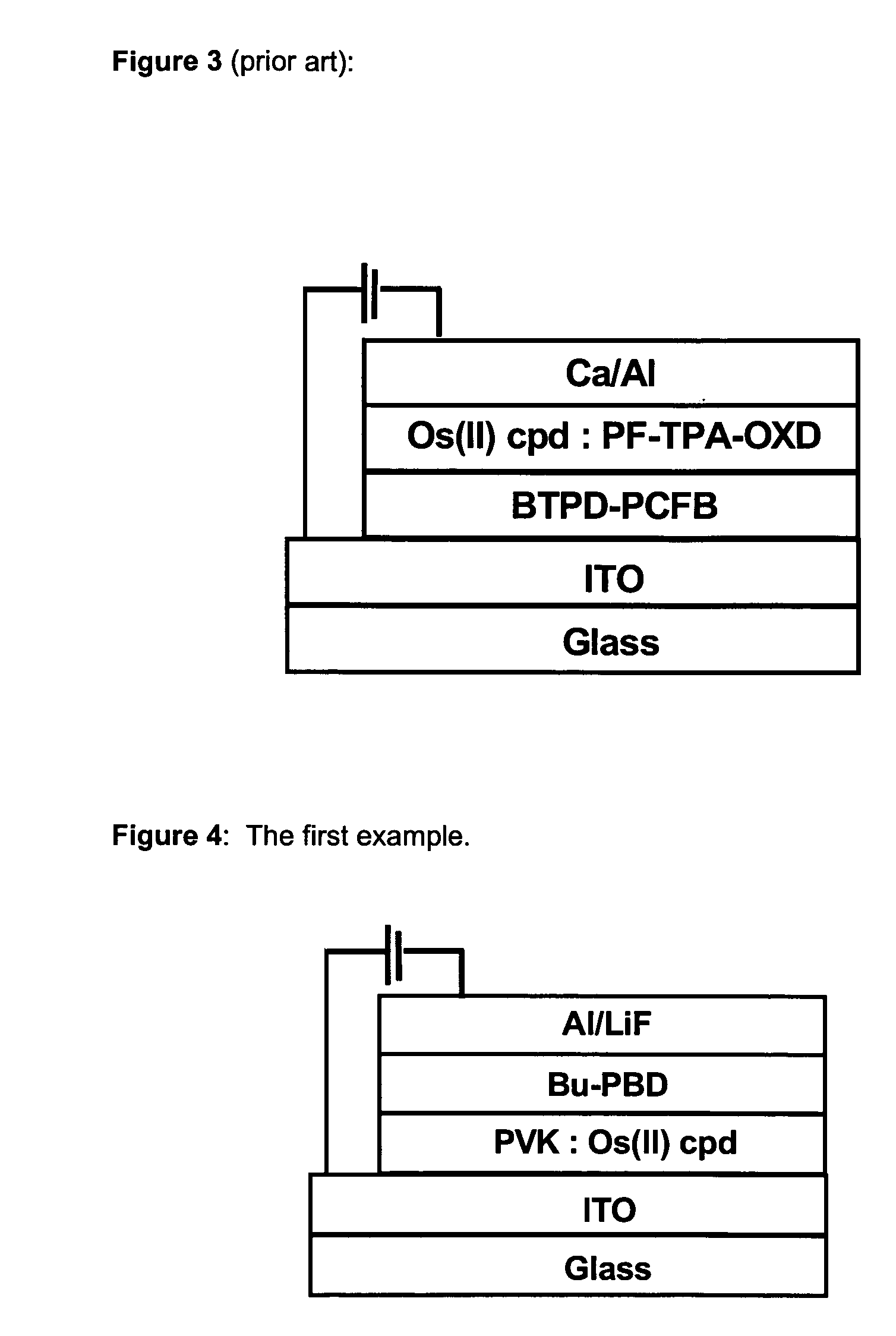
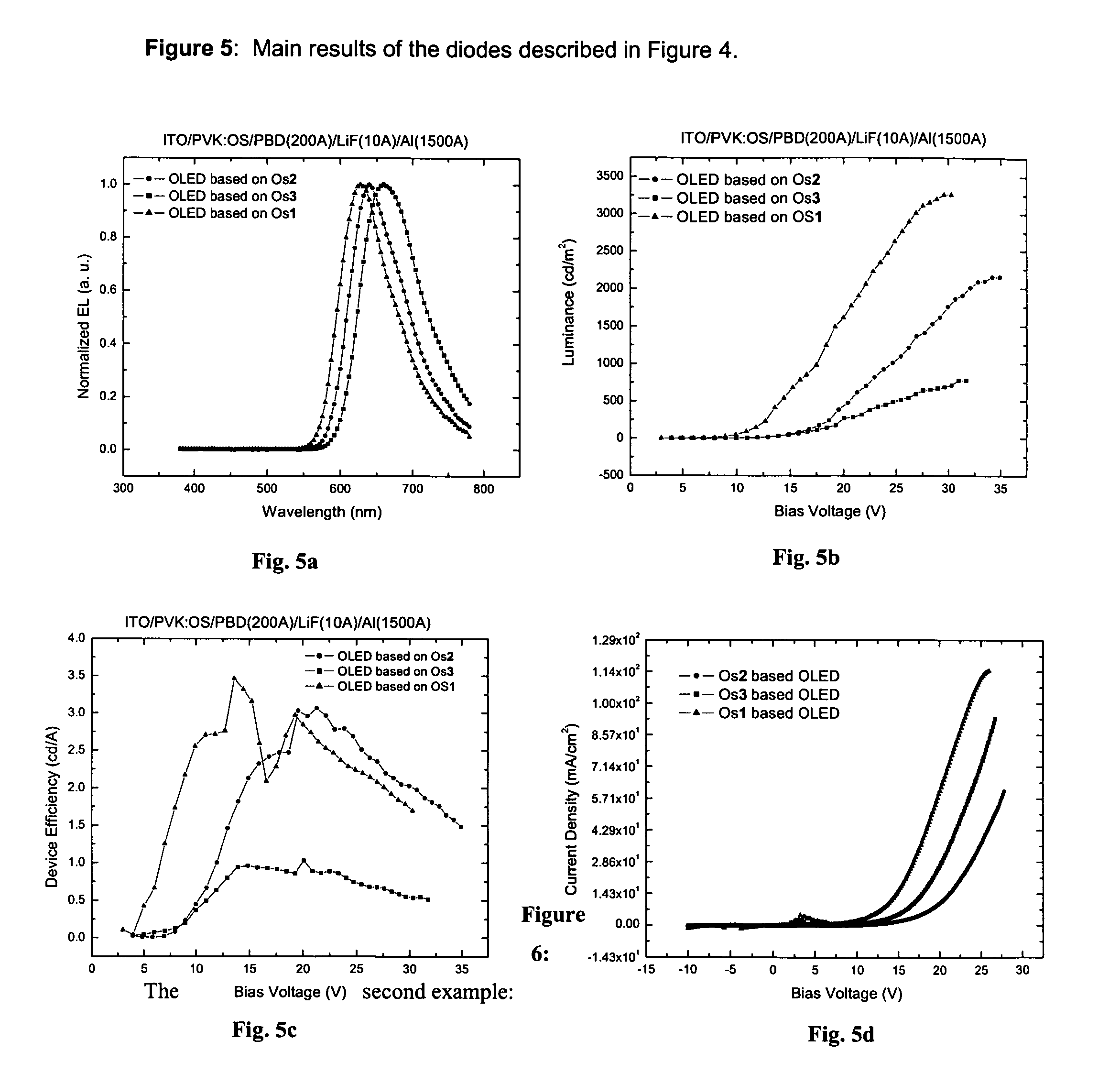
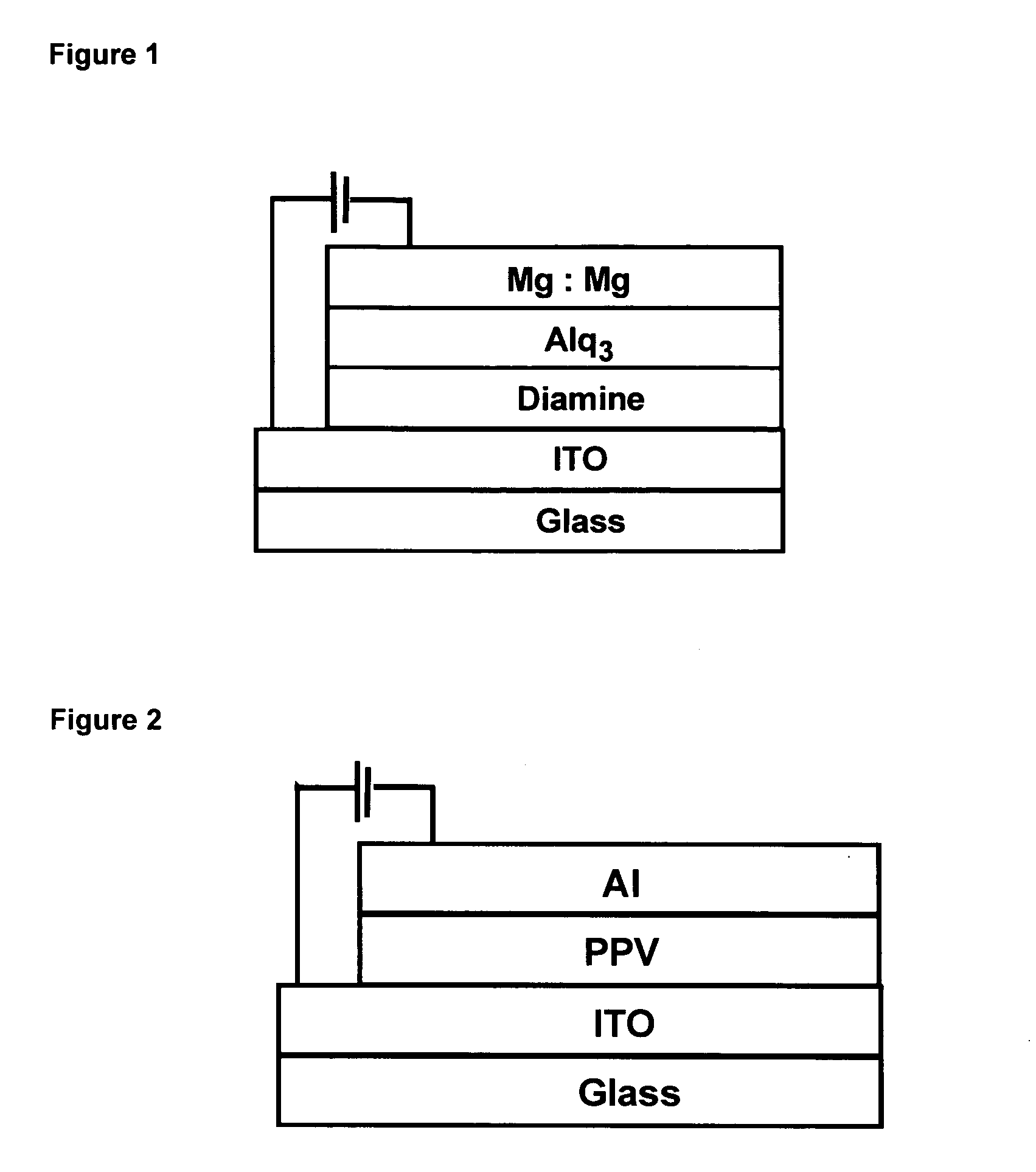
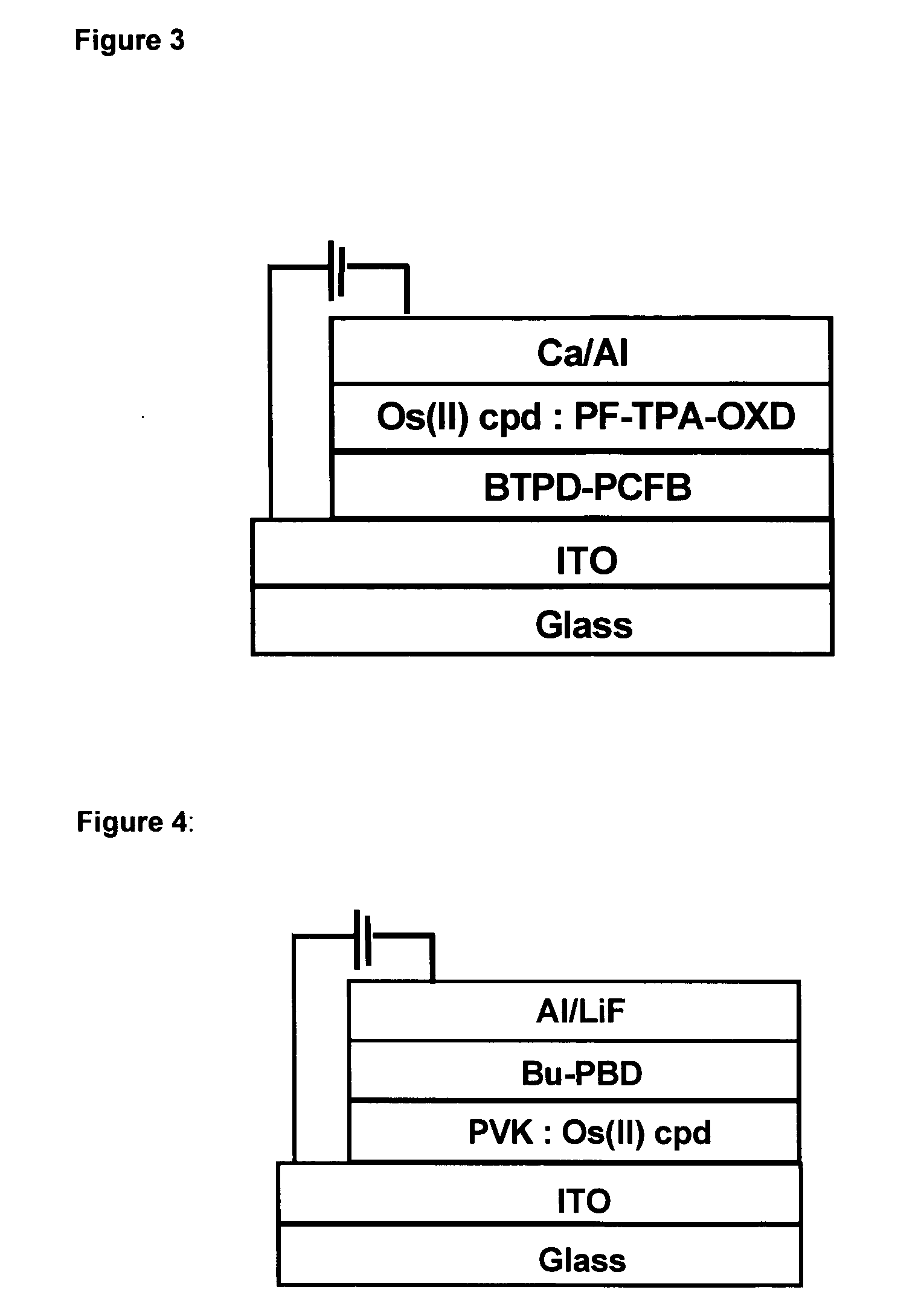
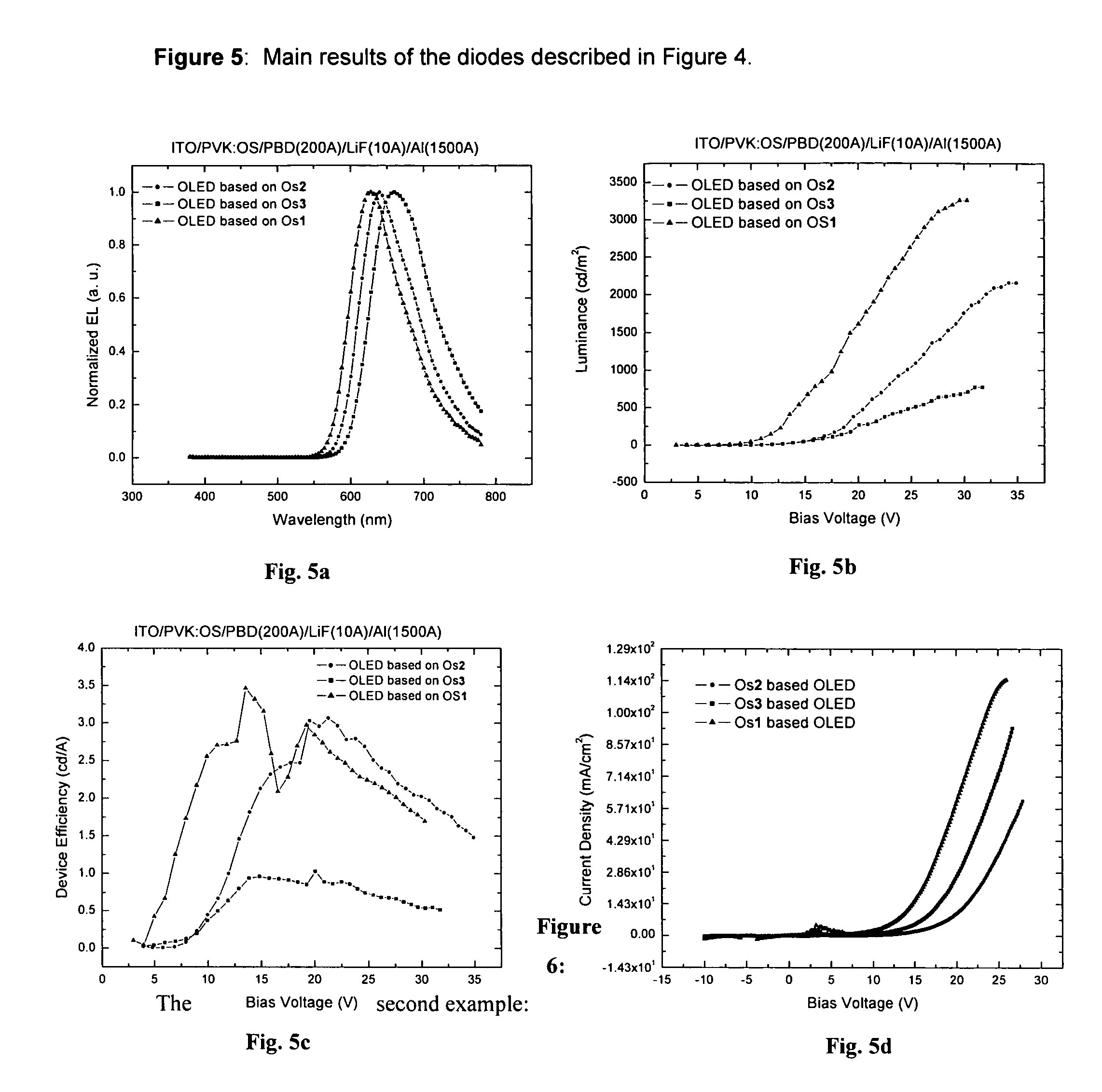
Phosphorescent Osmium (II) complexes and uses thereof
InactiveUS20070001166A1Discharge tube luminescnet screensElectroluminescent light sourcesPhenanthrolineCarbene
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds. Compounds of interest include: wherein A is Os or Ru The anionic chelating chromophores NˆN, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage. L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene. L can occupy either cis or trans orientation. When L occupies the trans position, the preferred structure contains both the hexagonal fragment of NˆN as well as its pentagonal fragment located at the trans position respect to their counterparts of the second NˆN chromophore. When L occupies the cis position, the preferred structure consists of the pentagonal unit of NˆN chromophores residing opposite to the L. X,1 X2 and X3 independently are C or N; when X2 is N, R1 is omitted, when X3 is N, R2 is omitted, R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl, R2 is H, F or cyano substituent, X4 is either C or N; X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4, R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:TAO YE +3
Liquid media containing Lewis acidic reactive compounds for storage and delivery of Lewis basic gases
InactiveUS20050276733A1High binding affinityHigh gas (or working) capacityLiquid degasificationPhysical/chemical process catalystsAlkalinityLiquid medium
This invention relates to an improvement in a low-pressure storage and delivery system for gases having Lewis basicity, particularly hazardous specialty gases such as phosphine and arsine, which are utilized in the electronics industry. The improvement resides in storing the gases in a liquid incorporating a reactive compound having Lewis acidity capable of effecting a reversible reaction between a gas having Lewis basicity. The reactive compound comprises a reactive species that is dissolved, suspended, dispersed, or otherwise mixed with a nonvolatile liquid.
Owner:VERSUM MATERIALS US LLC
Peroxo-containing metal complexes having amine oxide, phosphine oxide, arsine oxide, pyridine N-oxide or pyridine ligands as epoxidation catalysts
InactiveUS6054407AOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsPyridine-N-oxideOxygen
PCT No. PCT / EP96 / 03888 Sec. 371 Date Mar. 11, 1998 Sec. 102(e) Date Mar. 11, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 10054 PCT Pub. Date Mar. 20, 1997Olefins can be epoxidized using catalysts I where M is a metal of the 4th to 7th transition group of the Periodic Table of the Elements, L1 is an amine oxide, phosphine oxide, arsine oxide, pyridine N-oxide or pyridine ligand of the formula II, III, VII or VIII, L2 is a customary auxiliary ligand or a further ligand L1 or a free coordination site, X is oxo oxygen or an imido ligand, m is 1 or 2, and n is 1, 2 or 3.
Owner:BASF AG
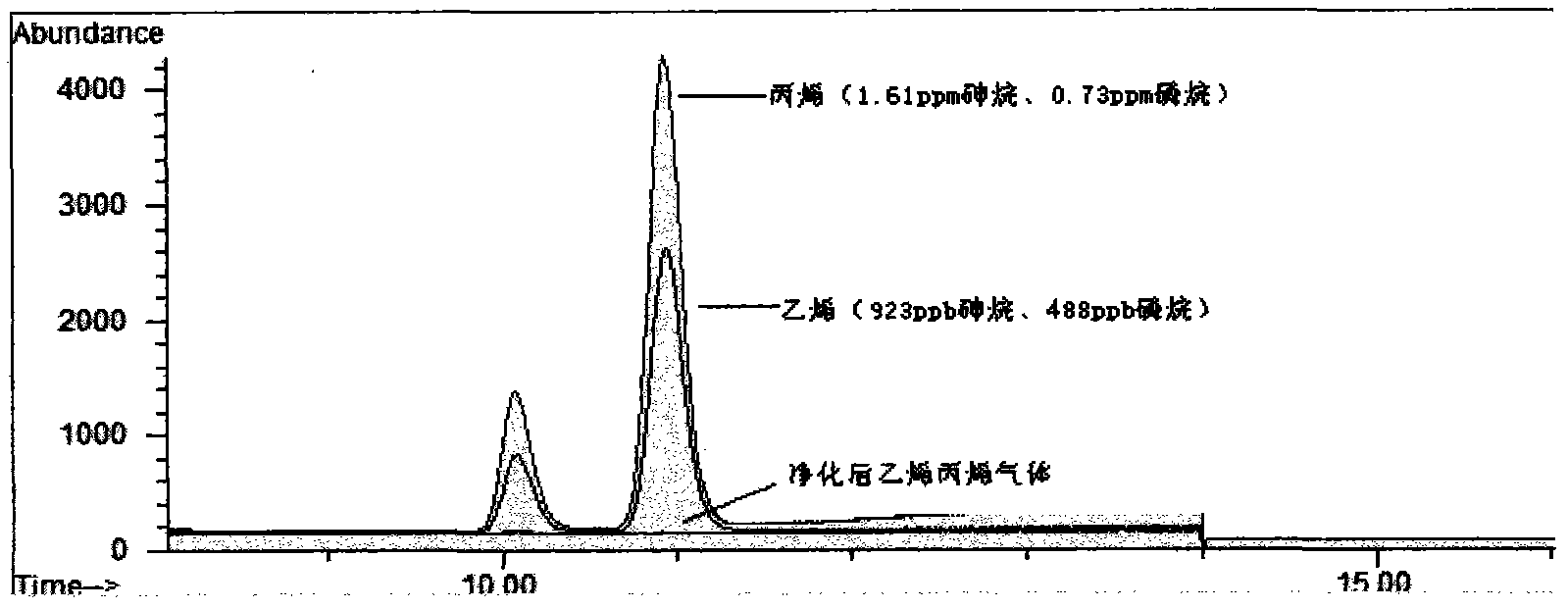
Purificant used for desulfuration of propylene and ethylene light hydrocarbon materials and preparation method thereof
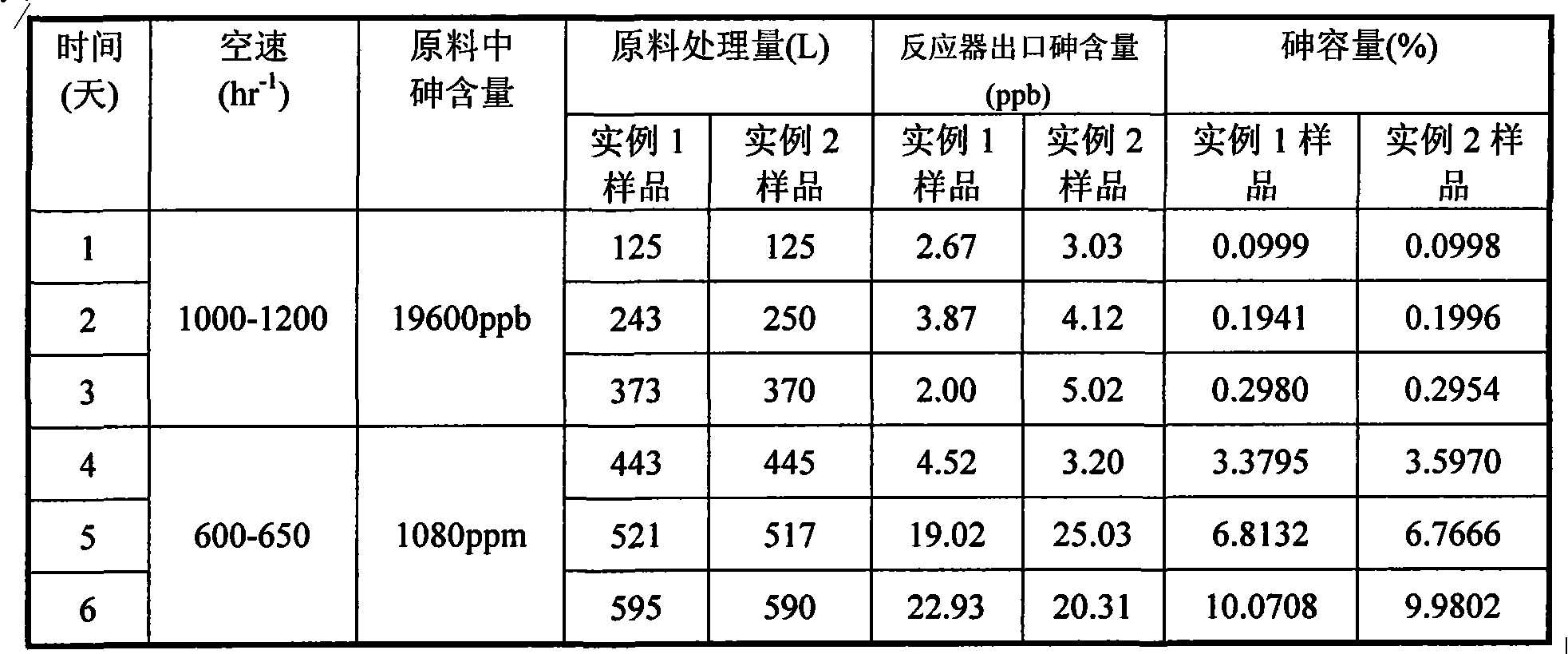
InactiveCN101602642AHigh precision of arsenic removalLarge arsenic capacityOther chemical processesAdsorption purification/separationHigh concentrationArsine
The invention relates to a purificant used for the desulfuration of propylene and ethylene light hydrocarbon materials. The purificant used for the desulfuration of propylene and ethylene materials takes alumina as a carrier, takes copper oxide as an active component, takes zinc oxide as an auxiliary agent and contains the following components according to the weight percent: 32-35% of CuO, 33-40% of ZnO and the balance of alumina. The purificant used for desulfuration does not need any preprocessing and can be directly used in the production technology of the desulfuration of the propylene and ethylene light hydrocarbon materials with high airspeed and high concentration of hydrogen arsenide. The purificant can be used at normal temperature, normal pressure and airspeed of 1200-2100hr-1, equivalent to the condition of the liquid airspeed of 4.5-8.0hr-1, and has the characteristics of high desulfuration precision and large arsenic capacity. The content of arsenic in raw materials can be removed from 1000ppm to less than 5ppb at normal temperature and normal pressure, the capacity of saturated arsenic reaches more than 30%, and the capacity of industrial effective arsenic is greater than 15%. Moreover, the purificant has a stronger desulfuration function.
Owner:LIAONING PROVINCE PETROLEUM CHEM IND PLANNING&DESIGNING INST
Cleansing agent for chemical absorbing hydrid gas and method for cleansing noxious gas
InactiveCN1565706AImprove adsorption capacityLow weight percentageOther chemical processesDispersed particle separationArsineCompound (substance)
The invention relates to a cleaning agent for chemical adsorption of hydride gas, specially a cleaning agent with titanium dioxide as carrier. The cleaning agent is made by coprecipitation process and BET specific surface area is no less than 60m#+[2] / g. The cleaning agent can remove harmful hydride component from the semiconductor factory and the photoelectricity factory by chemical adsorption.
Owner:IND TECH RES INST
Functionalized nanoparticles and method
ActiveUS8845927B2Material nanotechnologyPolycrystalline material growthThioureaSpecific chemical reaction
A nanoparticle has a semiconductor nanocrystal capable of emitting light. The nanoparticle further includes a ligand attached to a surface of the coating. The ligand is represented by the formula: X-Sp-Z, wherein X represents, e.g., a primary amine group, a secondary amine group, a urea, a thiourea, an imidizole group, an amide group, a phosphonic or arsonic acid group, a phosphinic or arsinic acid group, a phosphate or arsenate group, a phosphine or arsine oxide group; Sp represents a spacer group, such as a group capable of allowing a transfer of charge or an insulating group; and Z represents: (i) reactive group capable of communicating specific chemical properties to the nanocrystal as well as provide specific chemical reactivity to the surface of the nanocrystal, and / or (ii) a group that is cyclic, halogenated, or polar a-protic.
Owner:SAMSUNG ELECTRONICS CO LTD
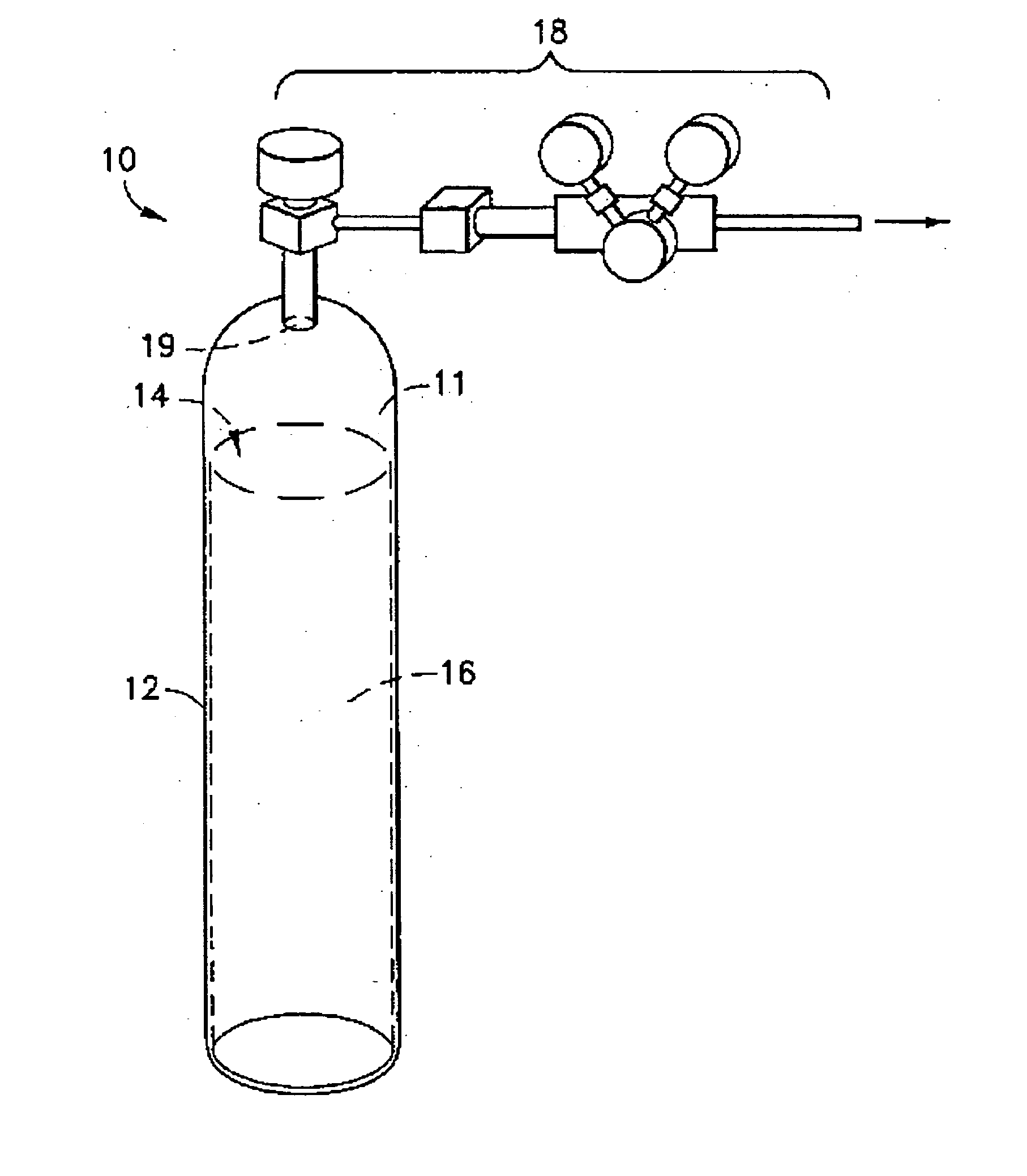
Steel cylinder filling device and method for high-purity arsine
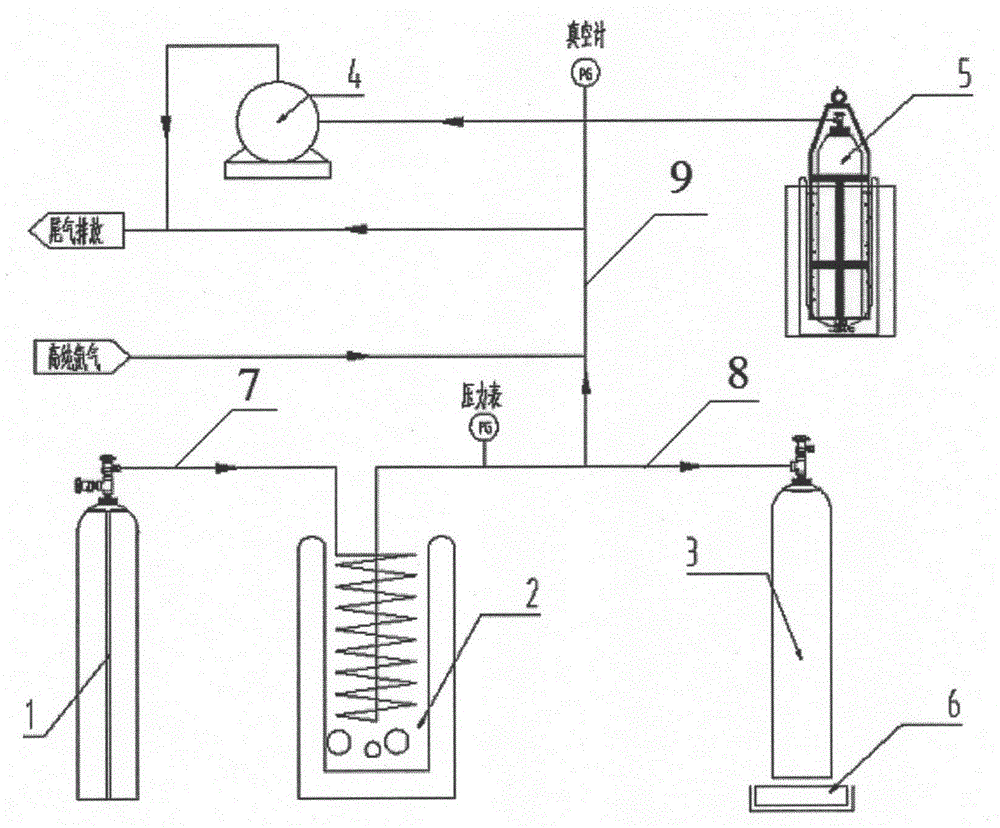
InactiveCN104930342AImprove cleanlinessQuality is not affectedContainer discharging methodsContainer filling under pressureArsineLiquid state
The invention provides a steel cylinder filling device and method for high-purity arsine. The steel cylinder filling device for the high-purity arsine is characterized by comprising a first pipeline, one end of the first pipeline is used for being connected with a source gas steel cylinder, the other end of the first pipeline is connected with the upper end of a cooling coil, the cooling coil is placed in a cooling tank, the lower end of the cooling coil is connected with one end of a second pipeline, the other end of the second pipeline is used for being connected with a to-be-filled steel cylinder, a main pipeline is formed by the first pipeline, the cooling coil and the second pipeline, the second pipeline is connected with a branch pipeline, the branch pipeline is connected with a cold trap collecting steel cylinder and a vacuum pump, and the branch pipeline is further connected with a high-purity helium source and an exhaust gas discharging outlet. The arsine is filled in a liquid state, a filling system is needed to be maintained cleaning in the filling process, and the quality of the arsine cannot be influenced by the pipelines and the steel cylinders.
Owner:SHANGHAI ZHENGFAN TECH +1
Fluid storage and delivery system utilizing low heels carbon sorbent medium
InactiveUS20030106429A1Reduces sufficiencyImprove adsorption capacityGas treatmentOther chemical processesDesorptionSorbent
A fluid storage and dispensing system including a vessel containing a low heel carbon sorbent having fluid adsorbed thereon, with the system arranged to effect desorption of the fluid from the sorbent for dispensing of fluid on demand. The low heel carbon sorbent preferably is characterized by at least one of the following characteristics: (i) Heel, measured for gaseous arsine (AsH3) at 20° C. at 20 Torr, of not more than 50 grams AsH3 per liter of bed of the sorbent material; (ii) Heel, measured for gaseous boron trifluoride (BF3) at 20° C at 20 Torr, of not more than 20 grams boron trifluoride per liter of bed of the sorbent material; (iii) Heel, measured for gaseous germanium tetrafluoride (GeF4) at 20° C. at 20 Torr, of not more than 250 grams AsH3 per liter of bed of the sorbent material; (iv) Heel, measured for gaseous arsenic pentafluoride (AsF5) at 20° C. at 20 Torr, of not more than 700 grams AsF5 per liter of bed of the sorbent material; (v) Heel, measured for gaseous trimethyl silane (3MS) at 20° C. at 20 Torr, of not more than 160 grams 3MS per liter of bed of the sorbent material; and (vi) Heel, measured for gaseous ethane (C2H4) at 21° C. at 25 Torr, of not more than 10 grams ethane per liter of bed of the sorbent material.
Owner:ENTEGRIS INC
Arsenic analysis
InactiveUS6696300B1Increase ratingsSimple methodWithdrawing sample devicesTesting waterArsineImproved method
An improved method for the analysis of arsenic in an aqueous sample, is disclosed. In accordance with the inventive method, arsenic is reduced to arsine gas in an acidic aqueous reaction environment and in the presence of an effective amount of an agent for increasing the rate of arsine gas production. Beneficially, metal cations are used as rate-increasing agents.
Owner:IND TEST SYST
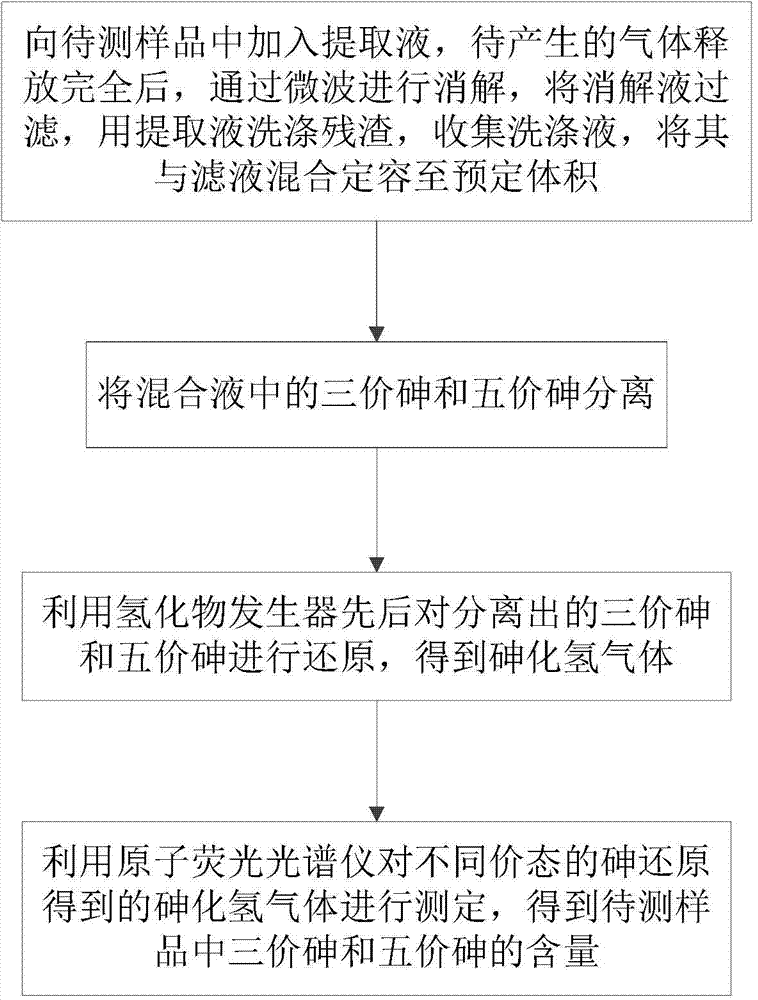
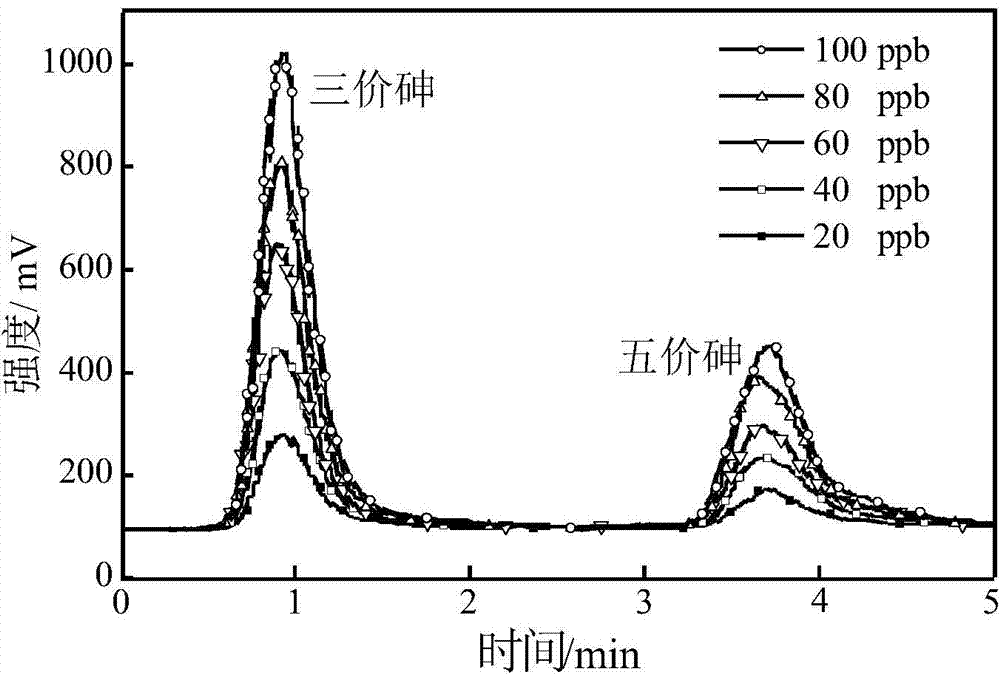
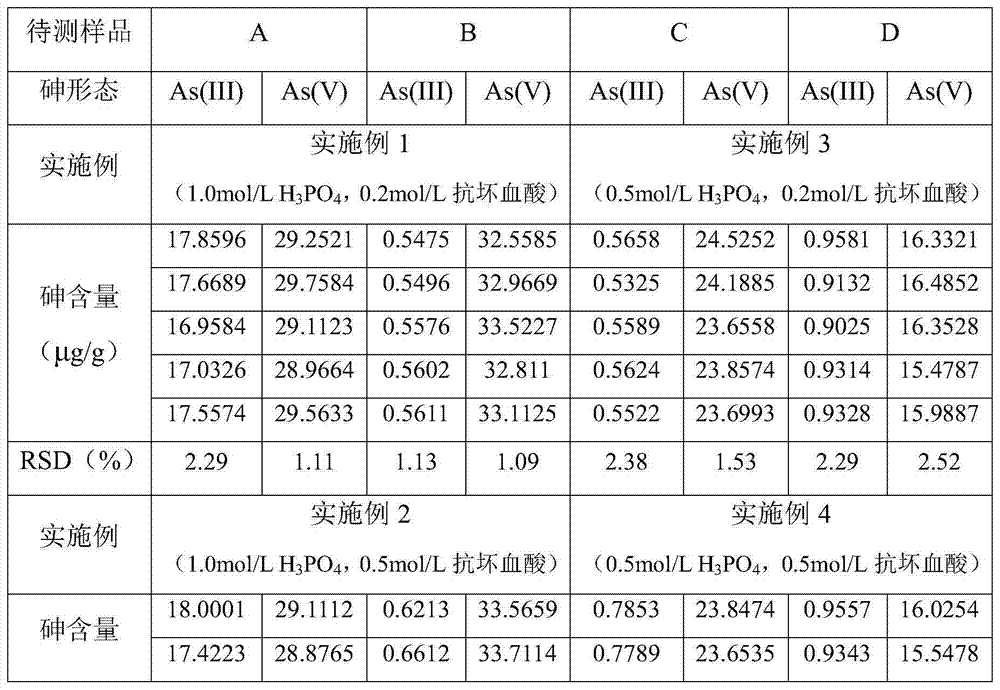
Determination method of arsenic valence
The invention discloses a determination method of arsenic valence. The method comprises the steps of: (1) adding an extracting solution which is a mixed solution of H3PO4 and ascorbic acid into a sample to be measured, which is coal or household garbage incineration ash, soil or sludge, completely releasing the produced gas, digesting by microwave, filtering a digestion liquid, washing the residue by an extracting solution, collecting the washing liquid, mixing the extracting solution with the filtrate, and making up to volume; (2) separating trivalent and pentavalent arsenic; (3) reducing the separated trivalent and pentavalent arsenic to obtain arsenic hydride gas; and (4) determining the arsenic hydride gas obtained by reduction of arsenic in different valence, so as to obtain the content of trivalent and pentavalent arsenic in the sample to be measured. The method can achieve nondestructive extraction and fast and effective separation of trivalent and pentavalent arsenic, has the advantages of high accuracy of the determination results, simple operation, low cost on determination and equipment maintenance, and is applicable to analysis and determination of arsenic form in solid wastes such as fuel burning ash, soil and sludge.
Owner:HUAZHONG UNIV OF SCI & TECH
Purifier for adsorbing arsenic hydride and hydrogen phosphide in olefin tail gas and preparation method thereof
ActiveCN102806065AReduce contentSimple ingredientsOther chemical processesDispersed particle separationSimple componentAtmospheric pollution
The invention relates to a normal-temperature purifier for adsorbing arsenic hydride and hydrogen phosphide in an olefin tail gas, belonging to the atmospheric pollution purification field. The purifier comprises following components in percentages by mass: 0.5-5% of potassium permanganate, 1-10% of soluble copper salt, 1-10% of soluble zinc salt and 75-97.5% of active carbon; the active carbon is a carrier; the potassium permanganate, and the soluble copper salt and the soluble zinc salt are active components. The preparation method of the purifier comprises following steps: (1), preparing a mixed water solution of the potassium permanganate, the soluble copper salt and the soluble zinc salt; (2), loading the potassium permanganate, the soluble copper salt and the soluble zinc salt on the active carbon carrier by a dipping method to obtain a carrier loaded with active metals; and (3), drying the carrier loaded with active metals in a drying medium to obtain the finished product purifier. The purifier disclosed by the invention has the advantages of simple component, low cost and low usage cost; after the purification, the content of arsenic hydride and hydrogen phosphide is less than 10ppb (volume percentage).
Owner:CHINA PETROLEUM & CHEM CORP +1
Organic molybdenum complex compound, preparation method and application thereof
InactiveCN101659679AGood chemical stabilityLower reaction conditionsGroup 6/16 element organic compoundsWater vaporPhosphine oxide
The invention discloses an organic molybdenum complex compound; organic ligand containing nitrogen group elements can be adopted to be matched with molybdenum to form metal organic compound, wherein the organic ligand includes triphenyl phosphine or alkyl-substituted derivative of the triphenyl phosphine, triphenyl phosphine oxide or alkyl-substituted derivative of the triphenyl phosphine oxide, triphenyl amine or alkyl-substituted derivative of the triphenyl amine, triphenyl amine oxide or alkyl-substituted derivative of the triphenyl amine oxide, triphenyl arsine or alkyl-substituted derivative of the triphenyl arsine, and triphenyl arsine oxide or alkyl-substituted derivative of the triphenyl arsine oxide. Meanwhile, the invention further discloses a preparation method of the organic molybdenum complex compound and the application of taking the organic molybdenum complex compound as catalyst in synthesizing poly-dicyclo-pentadiene. The organic molybdenum complex compound has higherchemical stability, is not sensitive for air and water vapor, can be taken as the catalyst to be applied in synthesizing the poly-dicyclo-pentadiene with higher catalytic activity, reduces the reaction conditions required by synthesizing the poly-dicyclo-pentadiene due to the chemical stability, and has good popularization and application prospects.
Owner:HENAN UNIV OF SCI & TECH
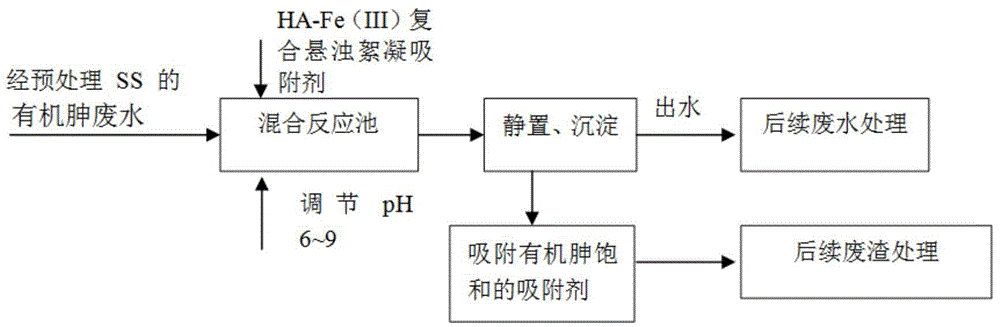
High-efficiency flocculation-adsorption treatment method for waste water containing low-concentration organic arsine preparations
InactiveCN104355381ALarge particle sizeReduce consumptionWater contaminantsWater/sewage treatment by flocculation/precipitationFlocculationArsine
The invention discloses a high-efficiency flocculation-adsorption treatment method for waste water containing low-concentration organic arsine preparations, and belongs to the technical field of sewage treatment. The method disclosed by the invention comprises the following steps: step 1, preparing a suspended flocculation-adsorption agent of humic acid-iron (III) composite substance; step 2, adjusting the pH of the low-concentration organic arsine waste water of pretreated solid suspended substance (SS), controlling the range of the pH during 5-9, and according to the content of organic arsine in the waste water, putting and adding the suspended flocculation-adsorption agent of the humic acid-iron (III) composite substance, which is obtained in the step 1; step 3, quickly stirring a flocculation-adsorption reaction system in the step 2 for 10 minutes at the rotating speed of 300 rpm, slowly stirring and adsorbing the flocculation-adsorption reaction system at a slow speed for 60-120 minutes at the rotating speed of 100 rpm, standing and precipitating the flocculation-adsorption reaction system which is stirred and adsorbed for 30-60 minutes, and then performing solid-liquid separation. Through adoption of the method disclosed by the invention, waste water containing the low-concentration organic arsine preparations (the content of the organic arsine preparations is not larger than 100mg / L) can be treated in the manners of high efficiency and low consumption, and the method is convenient to operate and easy to control.
Owner:NANJING NORMAL UNIVERSITY
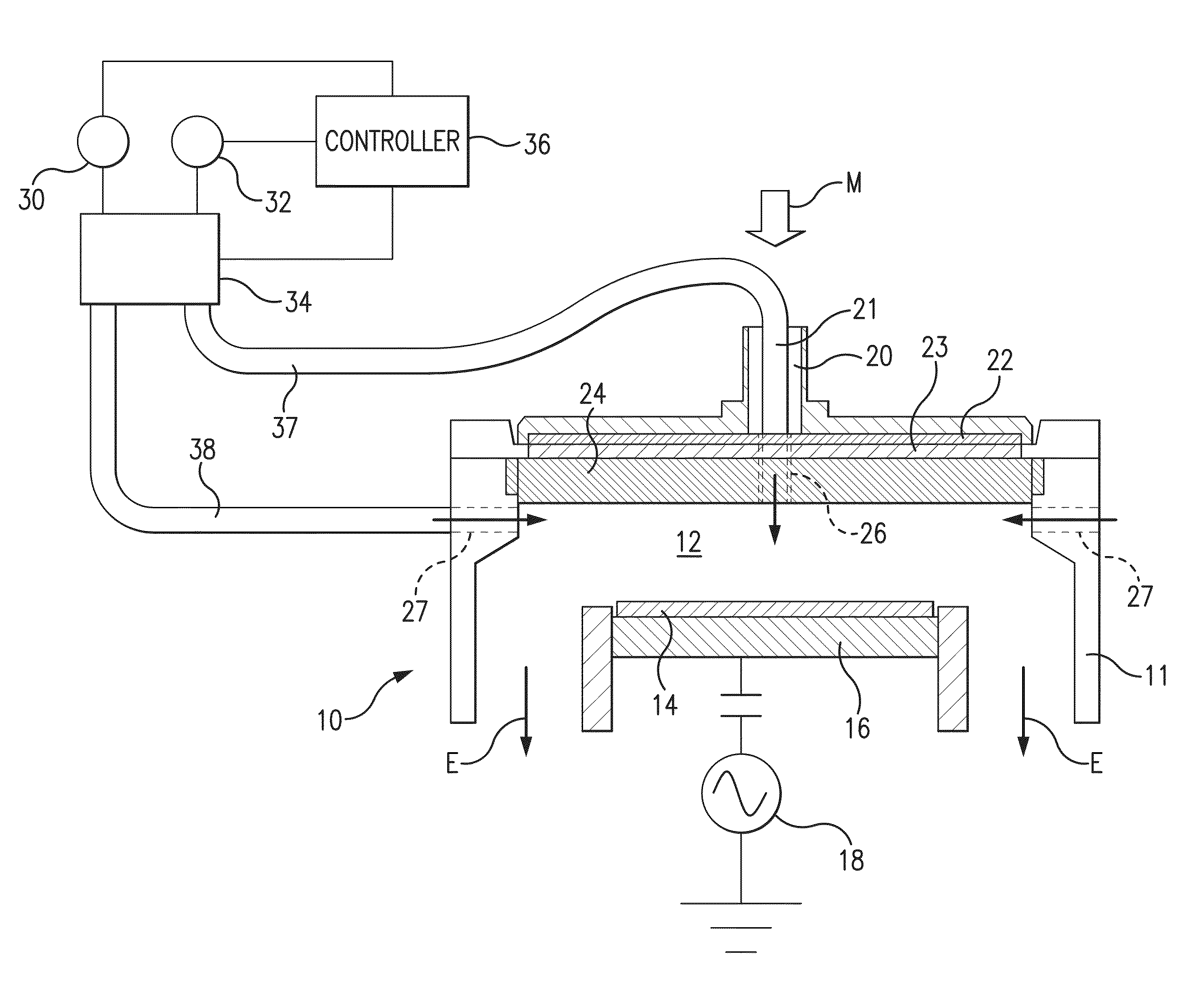
Pulsed gas plasma doping method and apparatus
ActiveUS20140302666A1Prevents and reduces possibility of ion induced damageLow plasma potentialElectric discharge tubesSemiconductor/solid-state device manufacturingDopantInjection port
A method and apparatus for doping a surface of a substrate with a dopant, with the dopant being for example phosphine or arsine. The doping is performed with a plasma formed primarily of an inert gas such as helium or argon, with a low concentration of the dopant. To provide conformal doping, preferably to form a monolayer of the dopant, the gas flow introduction location is switched during the doping process, with the gas mixture primarily introduced through a center top port in the process chamber during a first period of time followed by introduction of the gas mixture primarily through peripheral or edge injection ports for a second period of time, with the switching continuing in an alternating fashion as the plasma process.
Owner:TOKYO ELECTRON LTD
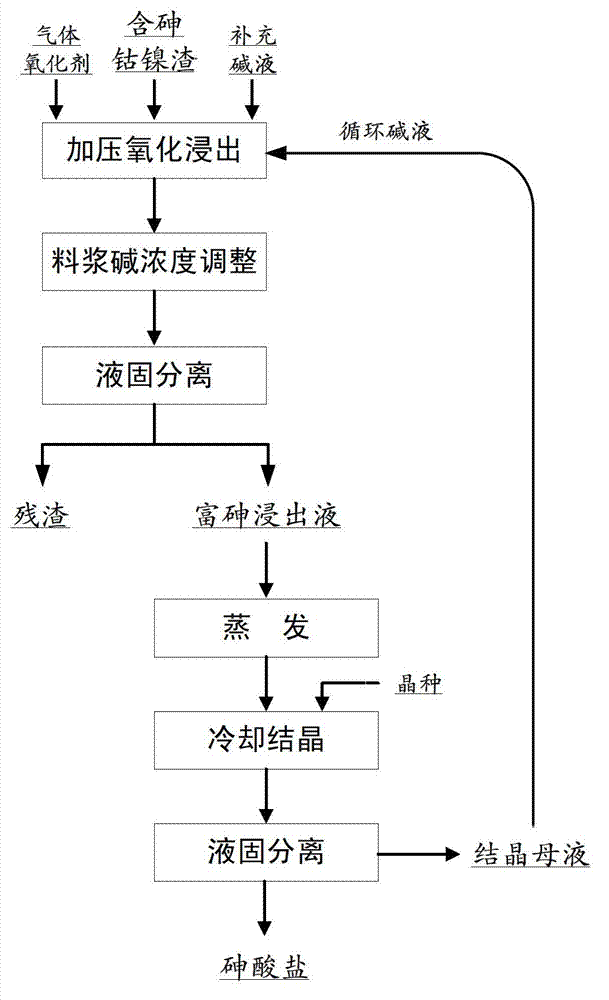
Method using wet alkaline process of cobalt-nickel (Co-Ni) residue containing arsenic to prepare arsenate
InactiveCN102925701ACrystallize fastAchieve separationArsenites/arsenatesProcess efficiency improvementPotassium hydroxideSlurry
The invention relates to a method using wet alkaline process of cobalt-nickel (Co-Ni) residue containing arsenic to prepare arsenate, and then preparing an arsenate product by using evaporative cooling and crystallizing method. The method comprises the steps of evenly mixing the Co-Ni residue containing the arsenic with sodium hydroxide or potassium hydroxide solution of certain density, placing mixture in a alkali-resistant high-pressure reaction kettle, introducing oxygen or oxygen-enriched gas of certain pressure as an oxidant, and reacting at a certain temperature, adjusting alkali density of slurry after reaction to less than 150g / L with water or low-density alkali liquid when the reaction is over, carrying out liquid-solid separation, obtaining arsenic-enriched liquid, evaporating the arsenic-enriched liquid, adding little seed crystal to cool and crystallize, and obtaining qualified arsenate crystal products after the liquid-solid separation. Crystallized mother liquid can be returned to be used for material distribution of the Co-Ni residue containing the arsenic. Recycling rate of the arsenic is over 99%, the method can prevent the arsenic from volatilizing during baking process and arsenic hydride gas from being generated during acid leaching. The method is simple in process, and can achieve recycle and reuse of the arsenic of the Co-Ni residue containing the arsenic.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for synthesizing high-purity arsine
The invention relates to a method for synthesizing high-purity arsine, which belongs to the technical field of electron gas synthesization and purification. The method includes the following steps: (1) crude arsine synthesization process: first producing arsenic powder and zinc powder into zinc arsenide, which then reacts with dilute sulphuric acid to produce crude arsine; and (2) high-purity arsine purification process: carrying out liquid nitrogen cold trap low-temperature vacuum separation, molecular sieve adsorption and drying and gallium-indium alloy deep absorption for dehydration and deoxidation on the crude arsine. The method can deeply remove oxygen, water, carbon dioxide and other harmful impurities in electronic material, which normally can reach less than 0.01 to 0.1ppm; and gallium-indium alloy as the main body of purifying agent can be reused, and theoretically has a long service life except slight loss in chemical treatment.
Owner:武峰
Method for purifying arsenic hydride gas in industrial waste gas
InactiveCN101402020ALow costLow releasabilityMolecular sieve catalystsDispersed particle separationFixed bedCalcination
The invention relates to a method for purifying hydrogen arsenide in industrial waste gas, which comprises the following steps: firstly, a mixed modified solution of a cobalt phthalocyanine solution and one ion or a plurality of ions of Fe<3+>, Mn<2+> and Ni<2+> is prepared; secondly, molecular sieve is placed into the modified solution, and modified molecular sieve catalyst is obtained after impregnation, drying and calcination; and thirdly, the modified molecular sieve catalyst is placed into a fixed bed reactor, and the industrial waste gas, including 80 to 90 volume percent of CO, less than 2 volume percent of O2 and 500 to 1600 milligrams per cubic meter of AsH3, undergoes solid catalysis, oxidation and purification, so that the content of the hydrogen arsenide in the industrial waste gas is reduced to below 0.3 milligram per cubic meter. The modified molecular sieve catalyst has high low-temperature activity, can be used in complex industrial gas atmosphere containing CO, H2S, SO2, COS, PH3 and so on, and has good selectivity on AsH3. The method can be applied in factories such as large-scale coke-oven plants, yellow phosphorus manufacturing plants, calcium carbide plants, and provides a feasible path for air pollution control and monocarbide chemical process development of China.
Owner:KUNMING UNIV OF SCI & TECH
Phosphorescent Osmium (II) complexes and uses thereof
InactiveUS20050137400A1Discharge tube luminescnet screensElectroluminescent light sourcesCyanide compoundPhenanthroline
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds. Compounds of interest include: wherein A is Os or Ru The anionic chelating chromophores N{circumflex over ( )}N, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage. L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene. L can occupy either cis or trans orientation. When L occupies the trans position, the preferred structure contains both the hexagonal fragment of NAN as well as its pentagonal fragment located at the trans position respect to their counterparts of the second NAN chromophore. When L occupies the cis position, the preferred structure consists of the pentagonal unit of N{circumflex over ( )}N chromophores residing opposite to the L. X1, X2 and X3 independently are C or N; when X2 is N, R, is omitted, when X3 is N, R2 is omitted, R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl, R2 is H, F or cyano substituent, X4 is either C or N; X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4, R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:NATIONAL TSING HUA UNIVERSITY +1
Method for preparing adsorbent capable of simultaneously removing hydrogen sulfide, hydrogen phosphide and arsenic hydride
ActiveCN104888692AEasy to operateEase of industrial applicationOther chemical processesDispersed particle separationArsineSorbent
The invention discloses a method for preparing an adsorbent capable of simultaneously removing hydrogen sulfide, hydrogen phosphide and arsenic hydride and belongs to the adsorbent preparation field. The method comprises the steps of grinding, washing and drying the diatomaceous earth, adding the dried diatomaceous earth in the HCl solution, stirring and standing the solution, washing the solution by utilizing distilled water till the pH value of the solution to be neutral, filtering the solution and drying the filtrate; adding the dried diatomaceous earth in a metal salt solution for ultrasonic impregnation, standing the impregnated diatomaceous earth at the normal temperature, drying and calcinating the diatomaceous earth, adding the calcined diatomaceous earth in an acidic solution for ultrasonic immersion, and drying the impregnated diatomaceous earth to obtain a mesoporous silica-based adsorbent. According to the technical scheme of the invention, the preparation method is simple in operation, wherein the HCl solution and the metal salt impregnant are cheap and readily available. Therefore, the method is suitable for industrial application. At a low temperature, the removal effect is still good. In this way, the method can be widely applied to the simultaneous removal of hydrogen sulfide, hydrogen phosphide and arsenic hydride for all kinds of gas sources.
Owner:KUNMING UNIV OF SCI & TECH
Liquid media containing Lewis acidic reactive compounds for storage and delivery of Lewis basic gases
InactiveUS7303607B2Reliable sourceLiquid degasificationSemiconductor/solid-state device detailsArsineLiquid medium
This invention relates to an improvement in a low-pressure storage and delivery system for gases having Lewis basicity, particularly hazardous specialty gases such as phosphine and arsine, which are utilized in the electronics industry. The improvement resides in storing the gases in a liquid incorporating a reactive compound having Lewis acidity capable of effecting a reversible reaction between a gas having Lewis basicity. The reactive compound comprises a reactive species that is dissolved, suspended, dispersed, or otherwise mixed with a nonvolatile liquid.
Owner:VERSUM MATERIALS US LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com