Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1679 results about "Bipyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bipyridines also known as bipyridyls, dipyridyls, and dipyridines, are a family of chemical compounds with the formula (C₅H₄N)₂, consisting of two pyridyl (C₅H₄N) rings. Pyridine is an aromatic nitrogen-containing heterocycle that forms complexes with most transition metals. It interacts with metals mainly as a σ-donating Lewis base.
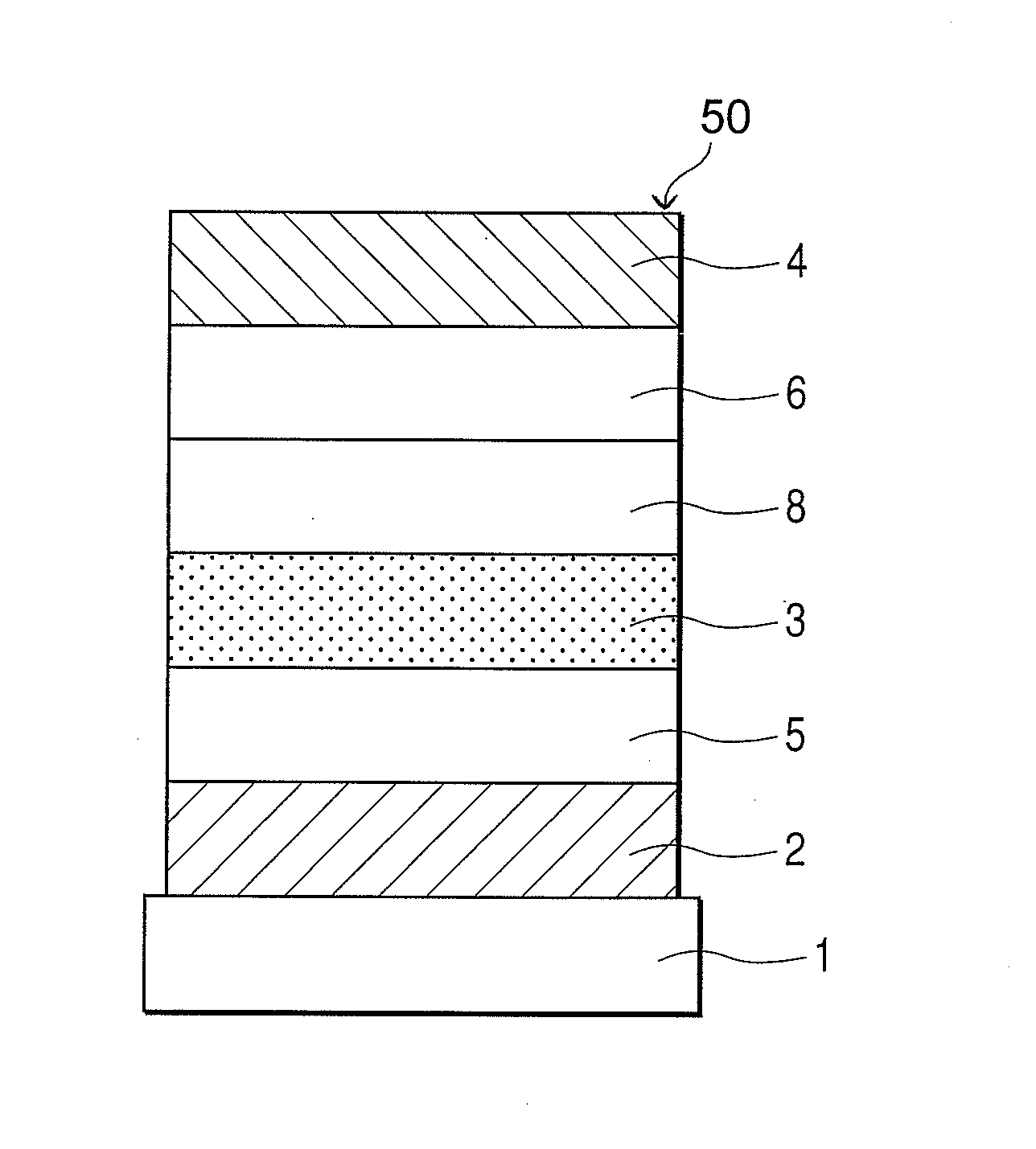
Organic electroluminescent display element, finder screen display device, finder and optical device
InactiveUS6468676B1Discharge tube luminescnet screensElectroluminescent light sourcesNitrosoAlkaline earth metal
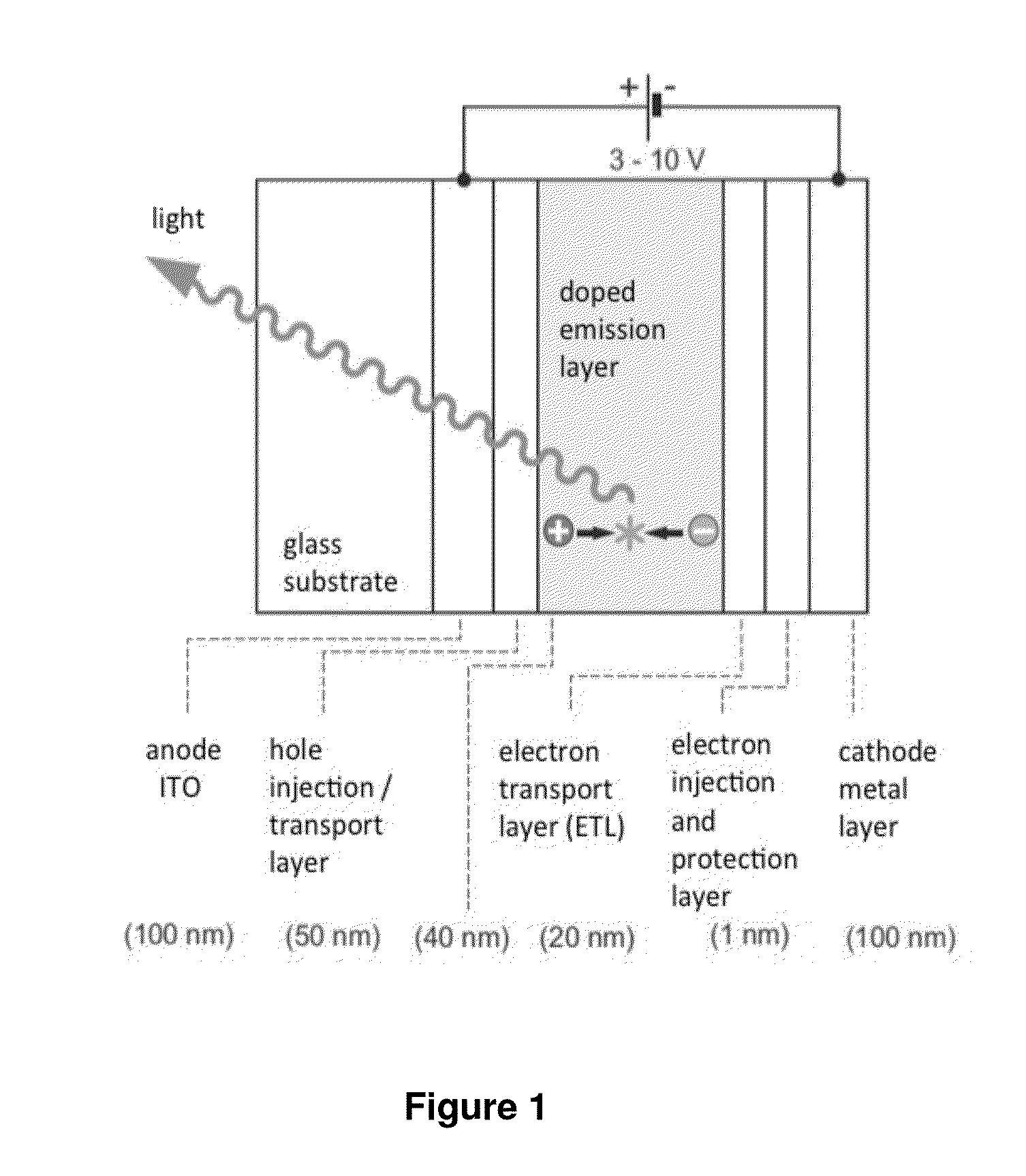
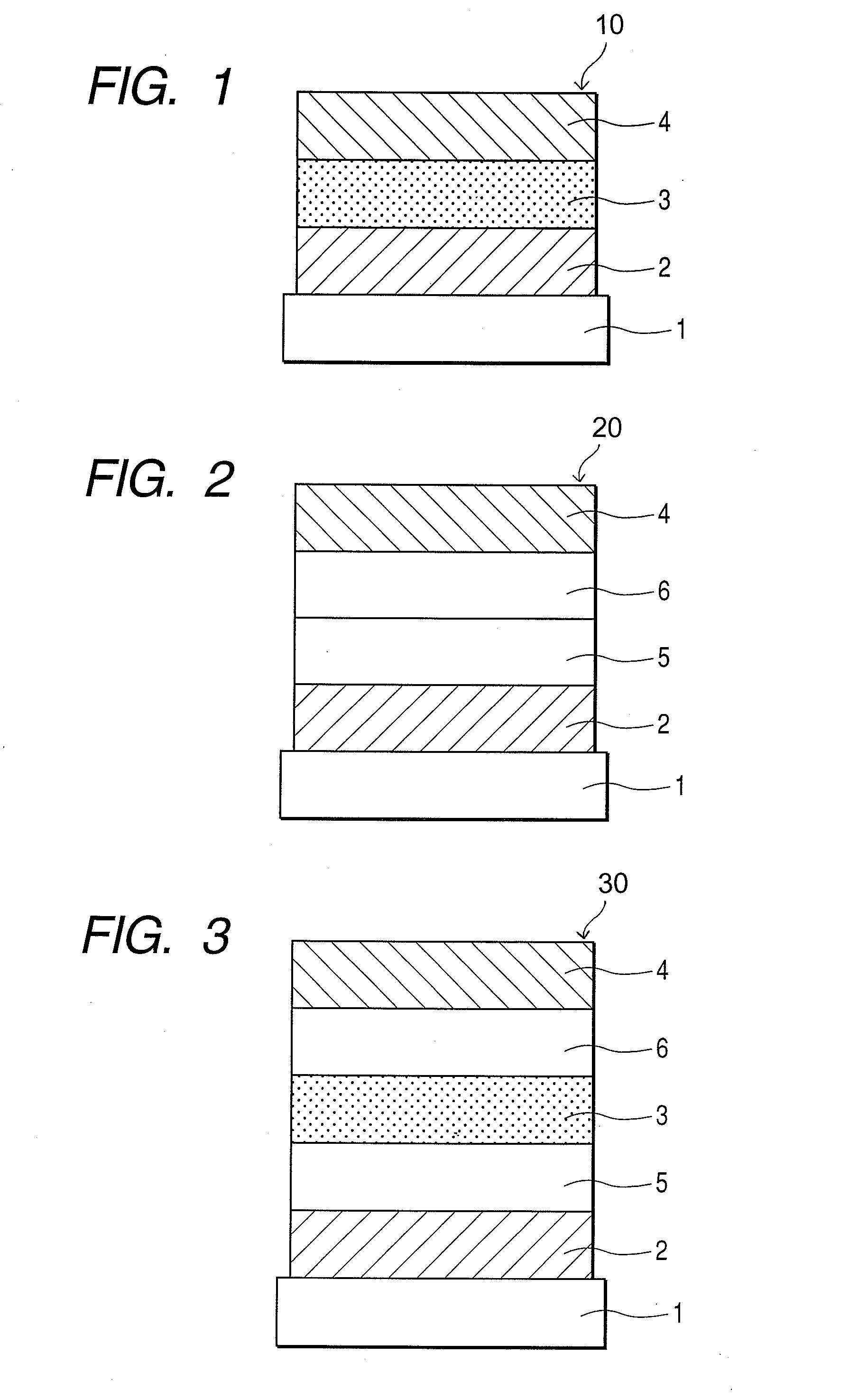
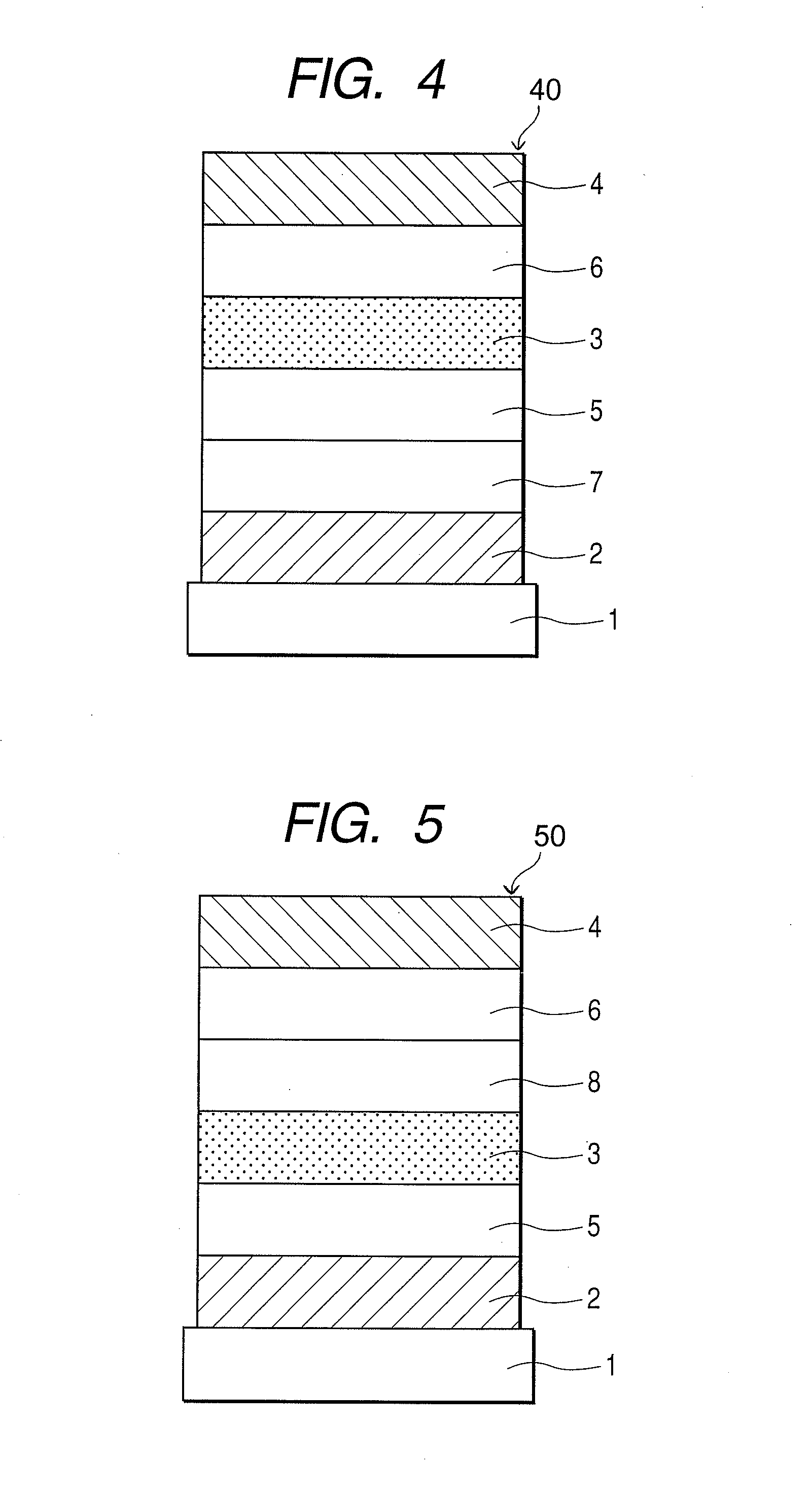
An organic electroluminescent display element has at least a positive electrode, an organic luminescent film, an electron injection layer and a negative electrode. Each of the positive and negative electrodes is formed of a transparent conductive film, the electron injection layer is formed of a thin transparent film made of a halogenide of an alkali metal or an alkaline earth metal, or an organic metal complex containing an alkali metal or an alkaline earth metal as a metal, and the organic metal complex is at least one complex selected from the group consisting of acetylacetonate complexes, alpha-nitroso-beta-naphthol complexes, salicylaldoxime complexes, cupferron complexes, benzoinoxime complexes, bipyridine complexes, phenanthroline complexes, crown complexes, proline complexes and benzoylacetone complexes.
Owner:KONICA MINOLTA INC
Electroless copper plating solution, method of producing the same and electroless copper plating method
ActiveUS20080223253A1Improve adhesionLower resistanceLiquid surface applicatorsAnti-corrosive paintsCopper platingHydrogen-Ion Concentrations
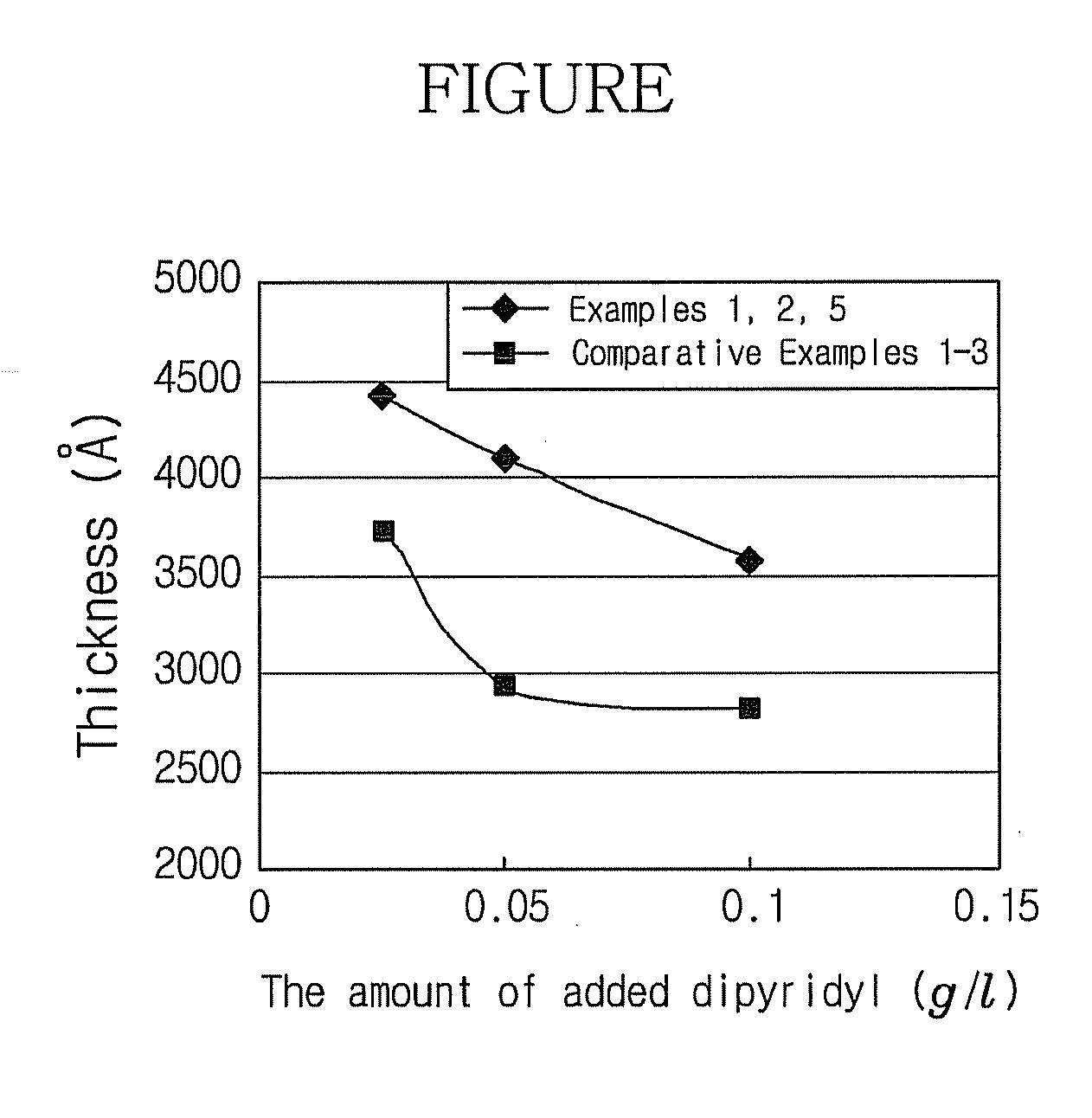
Disclosed herein is an electroless copper plating solution, including a copper salt, a completing agent, a reductant and a pH adjuster, in which the plating solution includes a 2,2-dipyridyl acid solution and the hydrogen ion concentration (pH) thereof is about 11.5 to about 13.0, a method of producing the same, and an electroless copper plating method. According to the plating solution of the present invention, an electroless copper plating film having stable and improved adhesivity and low electrical resistance can be obtained. Further, display devices including a metal pattern formed with the electroless copper plating solution can improve the reliability and price competitiveness of products prepared therefrom.
Owner:SAMSUNG DISPLAY CO LTD
Compositions And Methods For Removing Scale And Inhibiting Formation Thereof
InactiveUS20100000579A1Inhibition formationExtension of timeOrganic detergent compounding agentsScale removal and water softeningGluconic acidCarboxylic acid
Compositions for removing scale and / or inhibiting formation thereof include an alkaline agent, a primary scale inhibitor, a secondary scale inhibitor and a solvent. The primary scale inhibitor may include phosphonic acid, salts of phosphonic acids and combinations thereof. Suitable secondary scale inhibitor may include aminocarboxylic acids, salts of aminocarboxylic acids, carboxylic acids, salts of carboxylic acids, polycarboxylic acids, salts of polycarboxylic acids, gluconic acids, salts of gluconic acids, steroids, tetrapyrrols, ionophores, 2,2′-bipyridine, dimercaptopropanol, ortho-phenanthroline and combinations thereof. The compositions may be prepared as a stable concentrates that have pH values greater than or equal to 11. The compositions may also be prepared on site as a use solution. Methods of using the compositions to extend system operating times and to remove scale from and / or inhibit formation of scale on an article are also disclosed.
Owner:DELAVAL HLDG AB
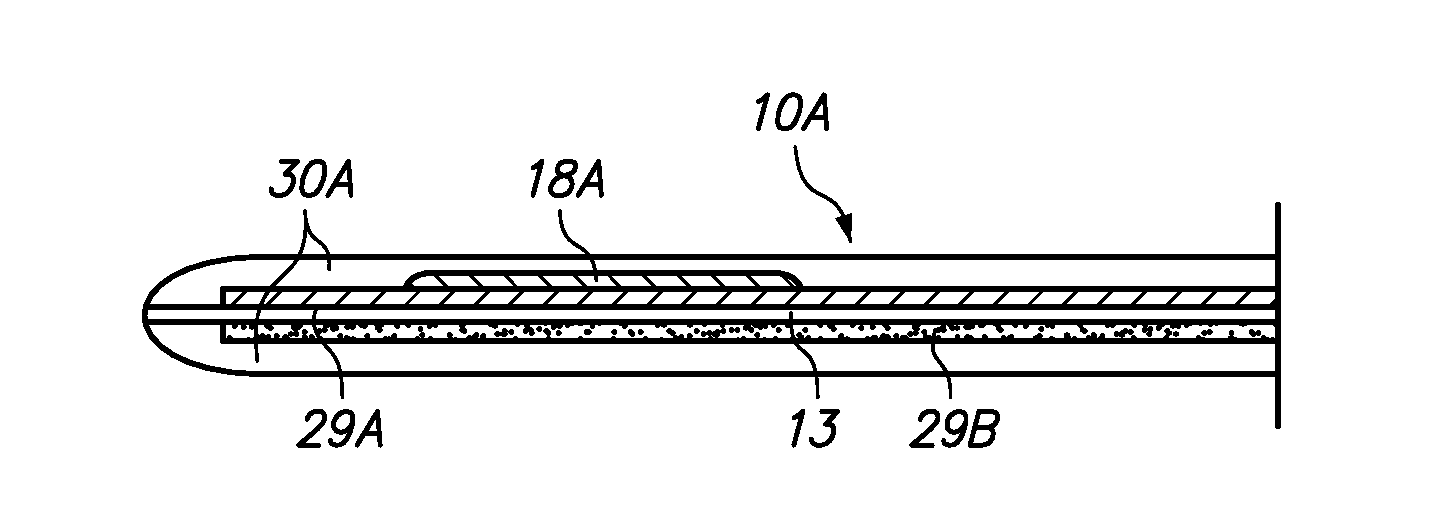
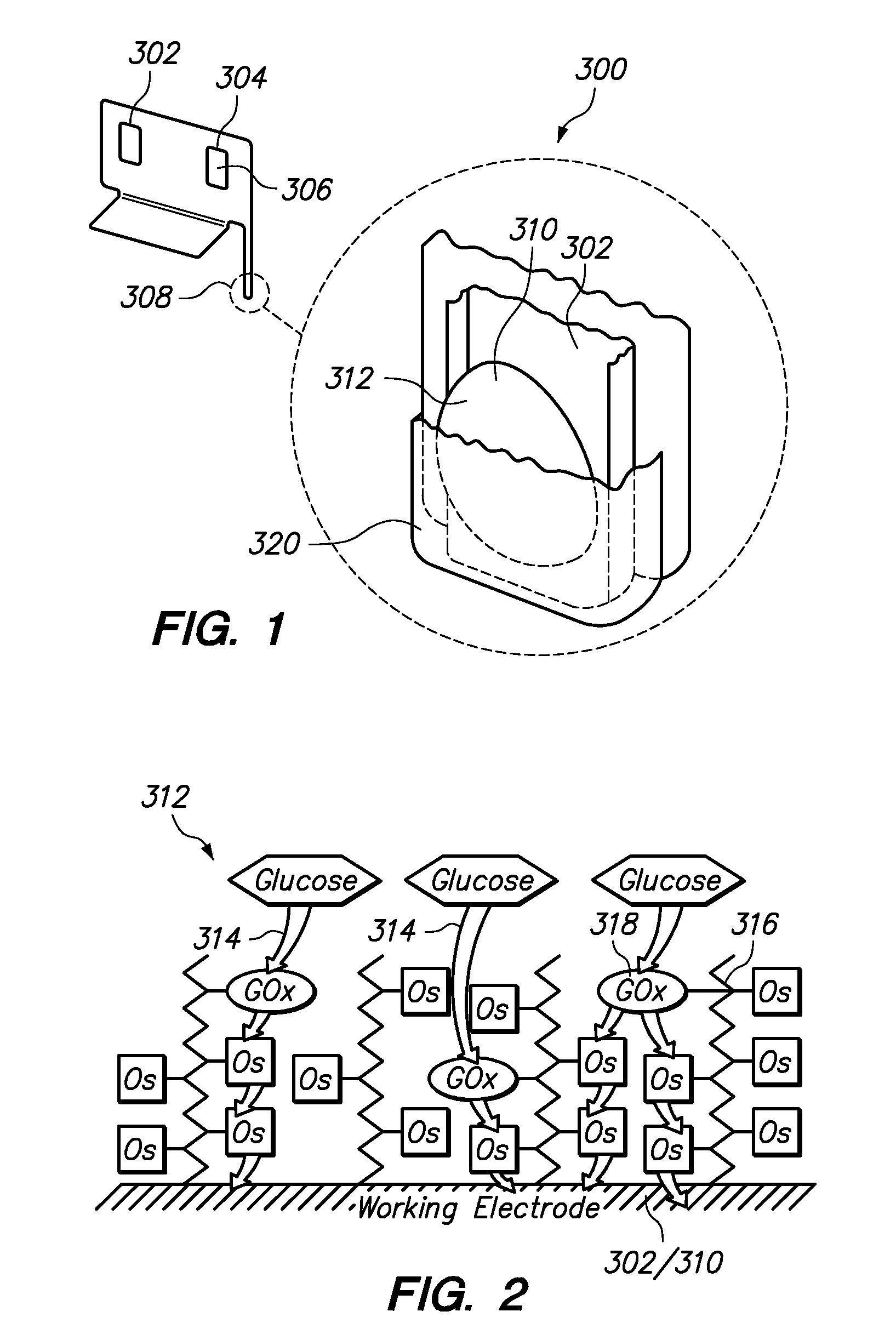
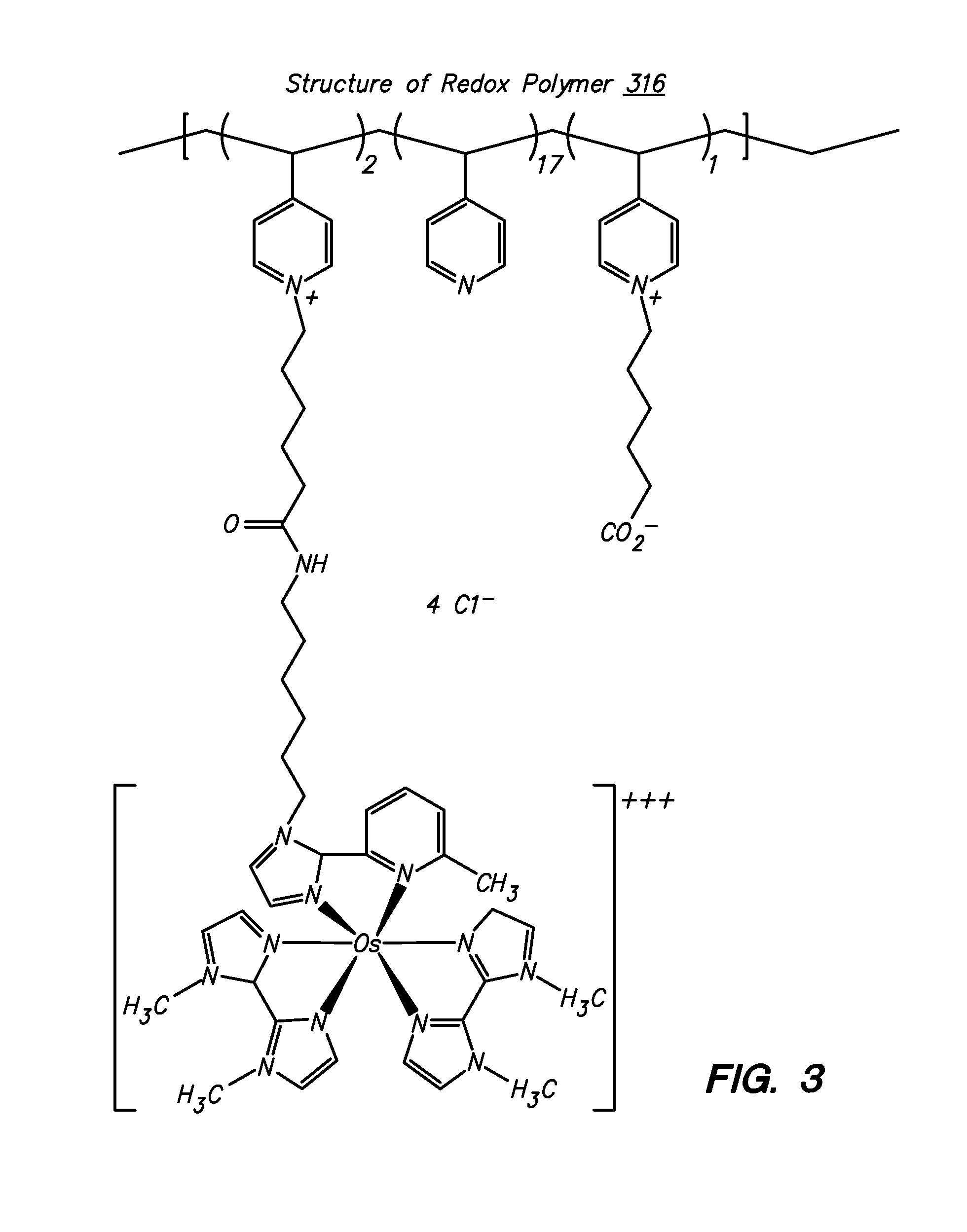
Redox polymers for use in analyte monitoring
InactiveUS8444834B2Immobilised enzymesBioreactor/fermenter combinationsHigh rateElectrochemical biosensor
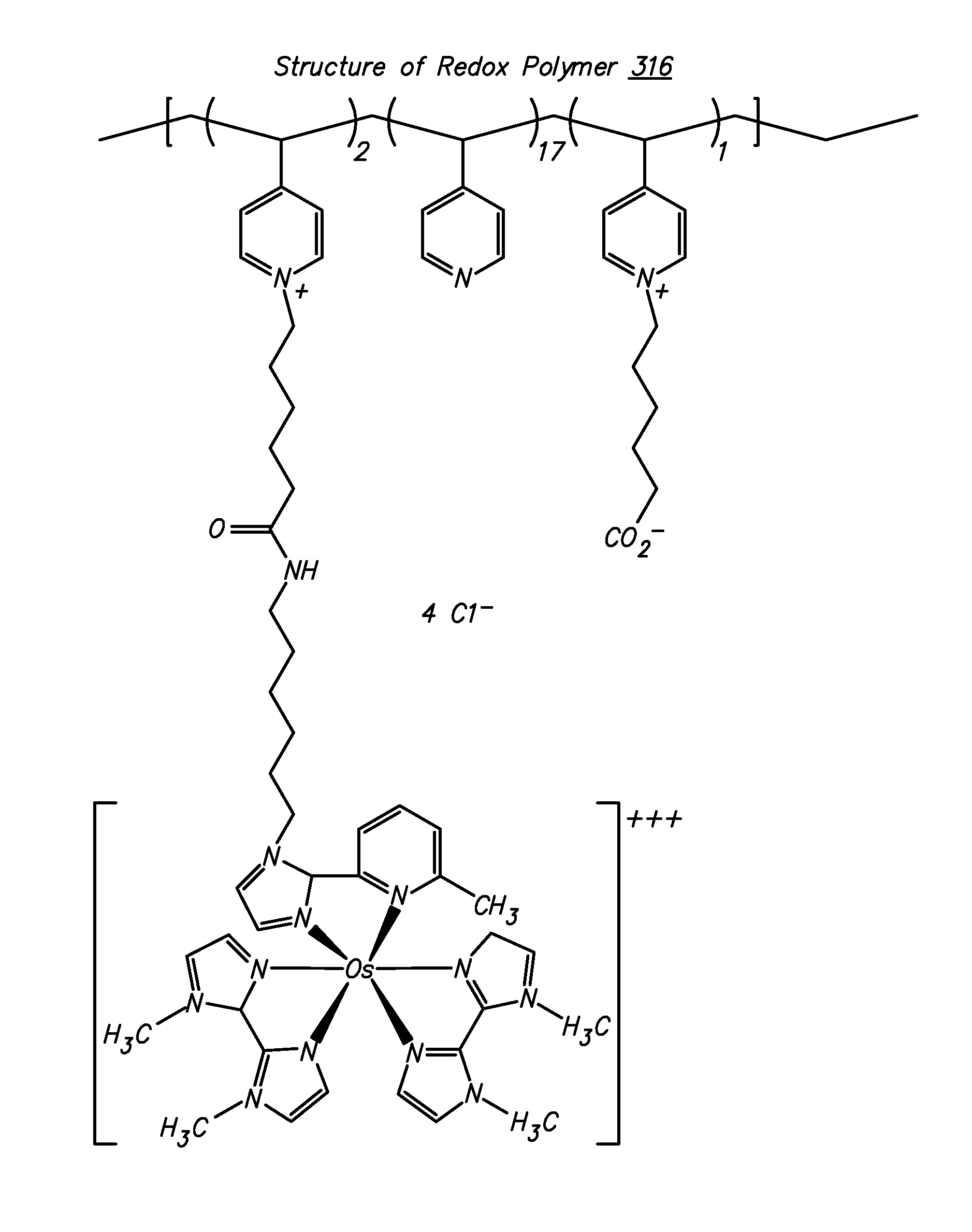
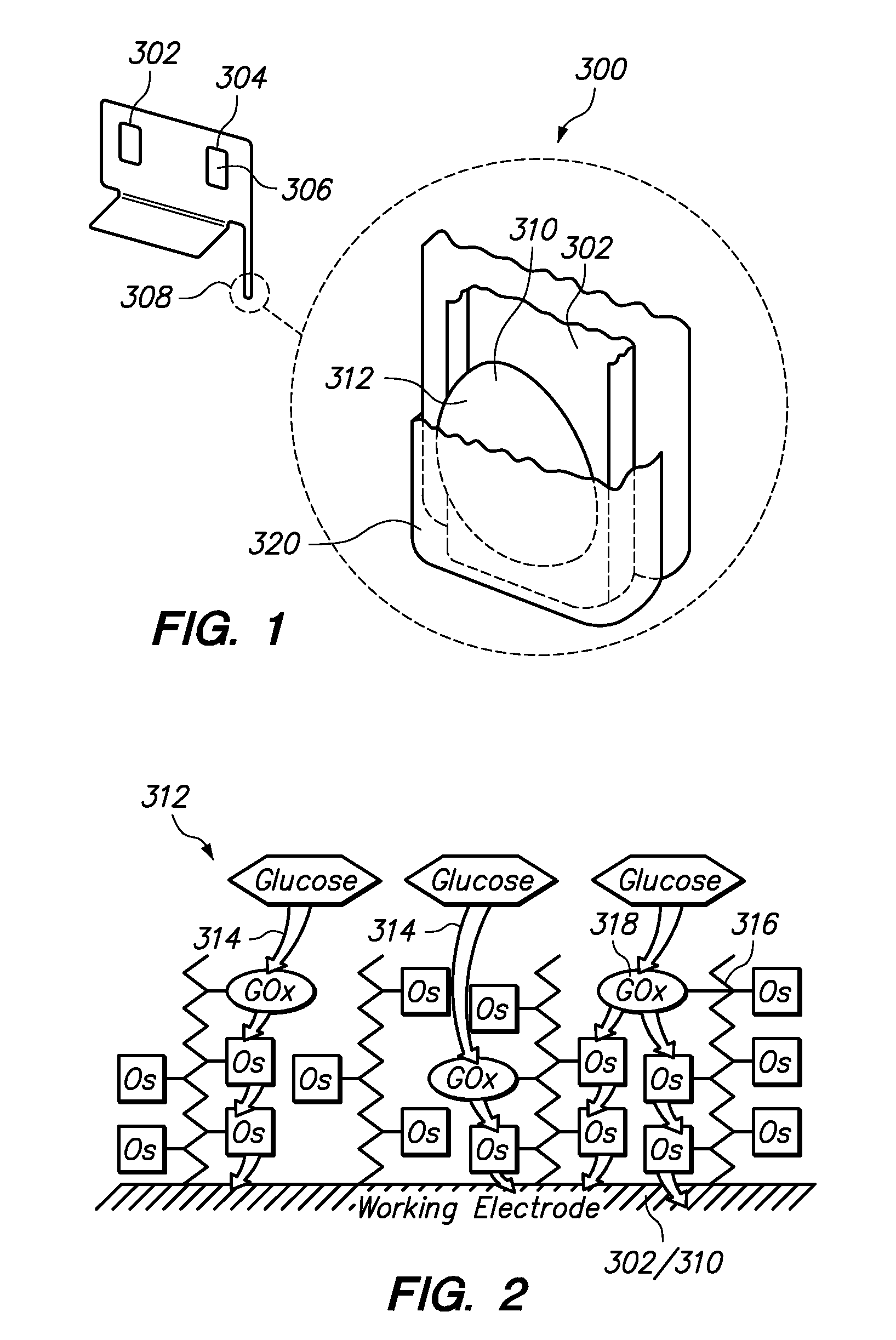
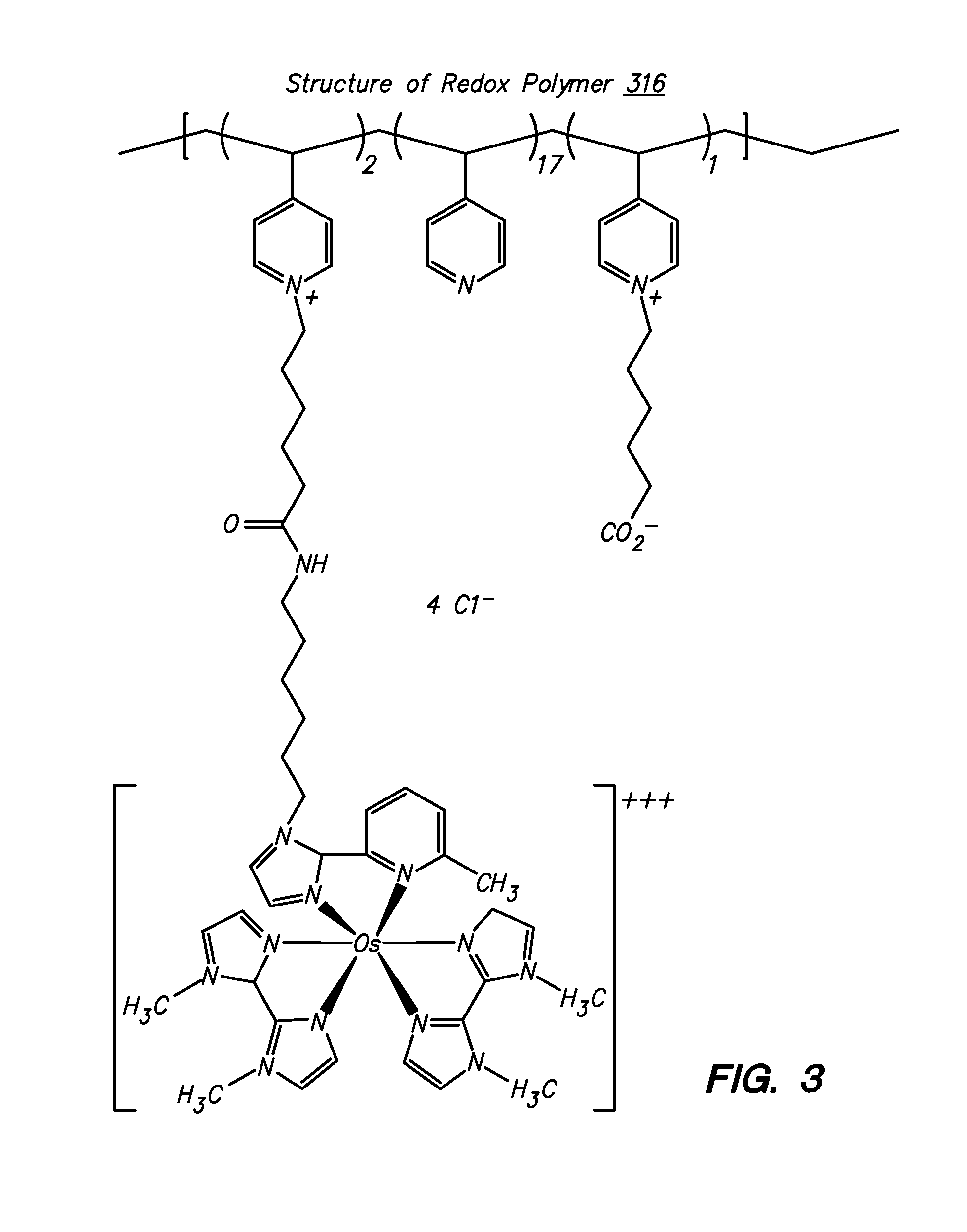
Polymers for use as redox mediators in electrochemical biosensors are described. The transition metal complexes attached to polymeric backbones can be used as redox mediators in enzyme based electrochemical sensors. In such instances, transition metal complexes accept electrons from, or transfer electrons to, enzymes at a high rate and also exchange electrons rapidly with the sensor. The transition metal complexes include at least one substituted or unsubstituted biimidazole ligand and may further include a second substituted or unsubstituted biimidazole ligand or a substituted or unsubstituted bipyridine or pyridylimidazole ligand.
Owner:ABBOTT DIABETES CARE INC
Iridium complex phosphor material taking phthalazine derivative as ligand and preparation method thereof
InactiveCN102180909AImprove solubilityImprove hole transport abilityGroup 8/9/10/18 element organic compoundsLuminescent compositionsQuantum yieldIridium
The invention discloses an iridium complex phosphor material taking a phthalazine derivative as a ligand. The structure of the material is shown as formulas (I) and (II), wherein Ar represents aryl, substituted aryl, heterocycle aryl and substituted heterocycle aryl; R can be one of a halogen atom, alkyl, substituted alkyl, alkoxyl, aryloxy, an alkylthio group, arylthio, aromatic amino, aliphatic amino or a heterocyclic substituent; L^Y can be one of N-COOH, 8-hydroxyquinoline, beta-diketone, N^NH and the like; N^N can be bipyridyl, diquinoline, 1,10-phenanthroline, a derivative of the 1,10-phenanthroline and the like; and Z can be hexafluorophosphate and perchlorate. A dicyclic or ionic iridium complex phosphorescent electroluminescent device taking the phthalazine derivative as the ligand obtained with the preparation method has high internal and external quantum yields, high luminance and high stability. A dicyclic iridium complex is shown as a formula (I), i.e., (C^N)2Ir(L^Y); and an ionic iridium complex is shown as a formula (II), i.e., (C^N)2Ir(N^N)<+>Z<->.
Owner:NANJING UNIV OF POSTS & TELECOMM
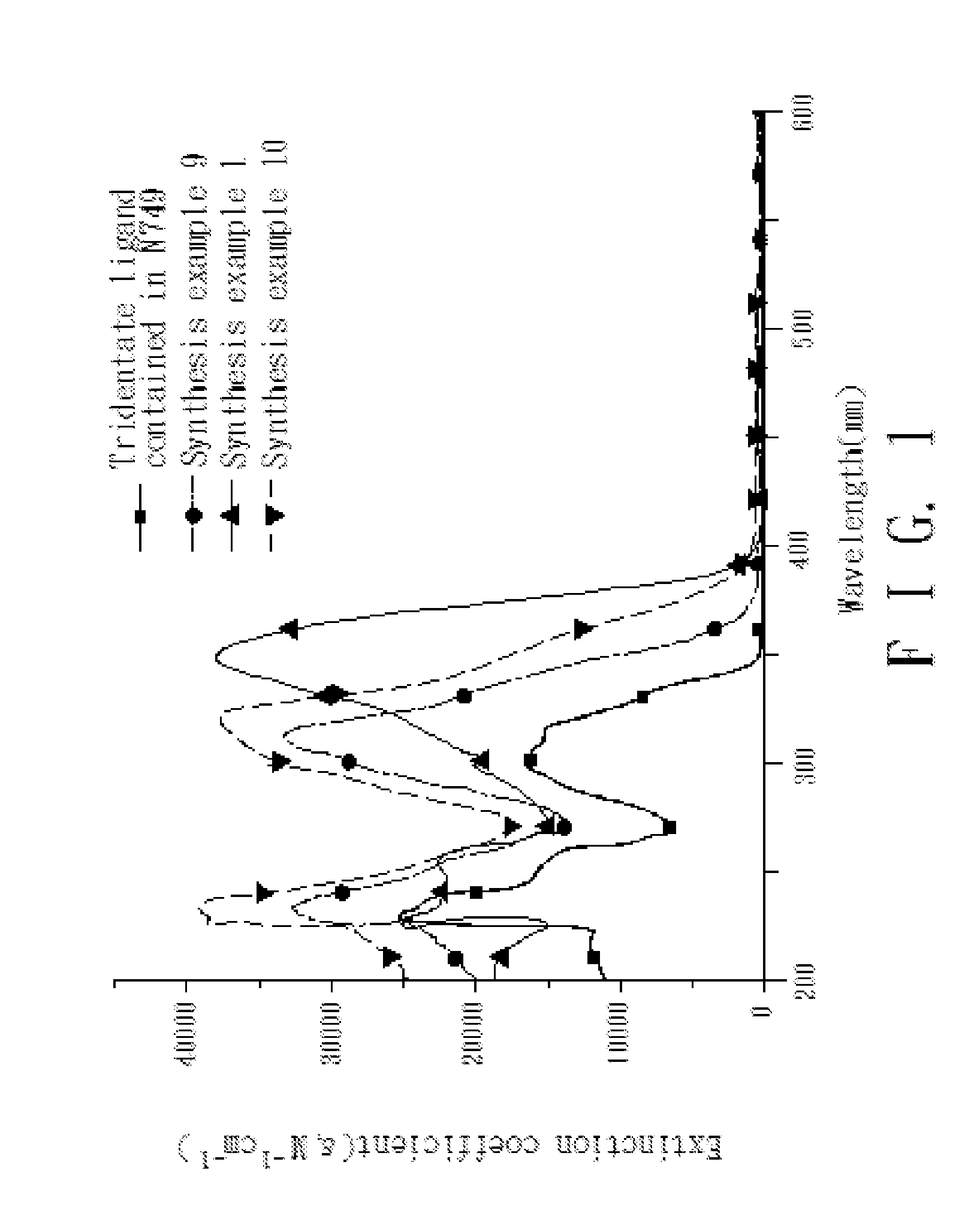
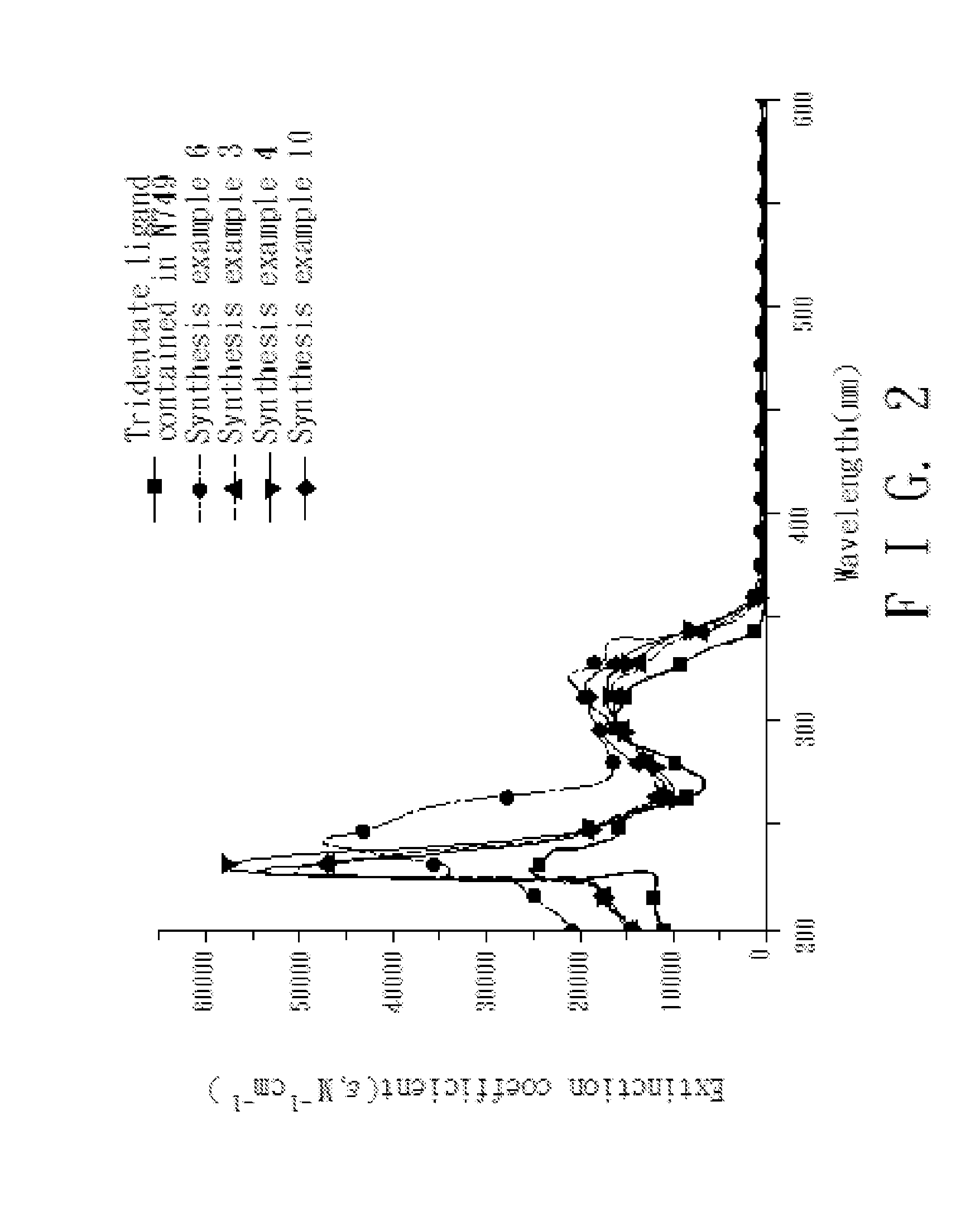
4,4'-dicarboxy-2,2'-bipyridine derived tridentate ligand, metal complex containing the same, and application thereof
InactiveUS20120247561A1Improve bindingImprove the overall coefficientRuthenium organic compoundsElectrolytic capacitorsBipyridinePyridine
Disclosed is a 4,4′-dicarboxy-2,2′-bipyridine derived tridentate ligand represented by formula (I):wherein definitions of Y1, Y2, and R are the same as those defined in the specification.Also disclosed are a metal complex containing the aforesaid tridentate ligand and a dye-sensitized solar cell containing the metal complex.
Owner:NATIONAL TSING HUA UNIVERSITY
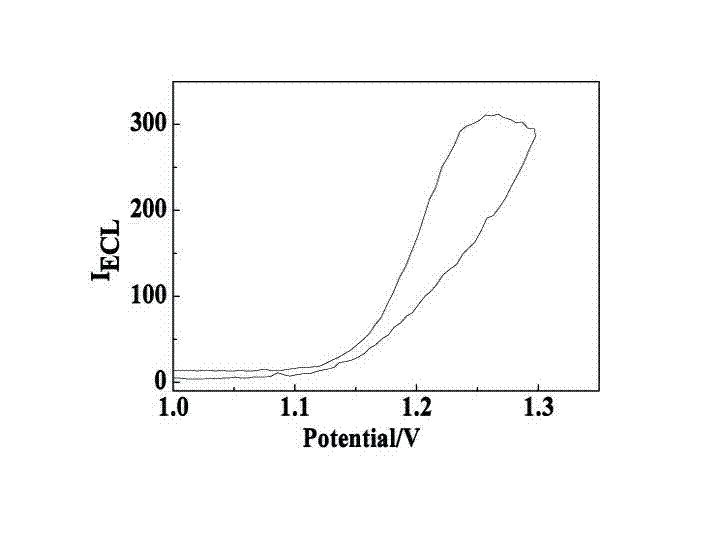
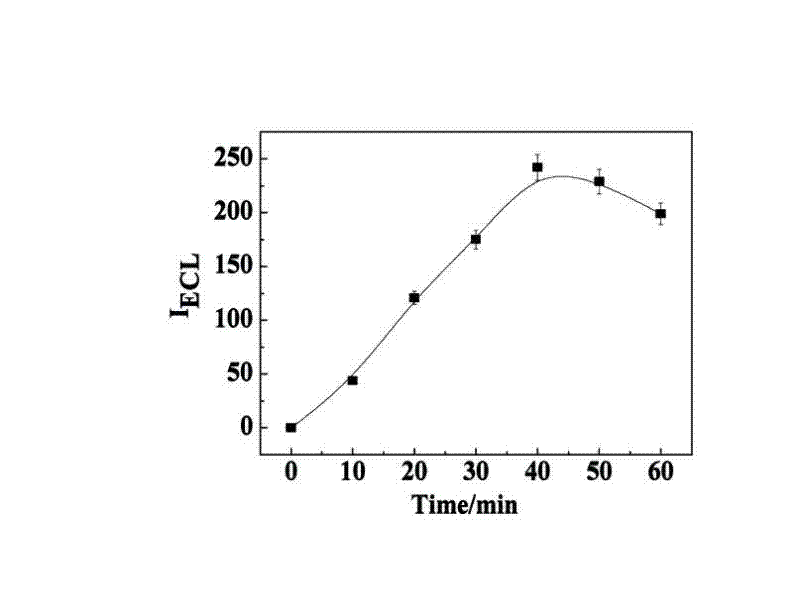
Manufacturing method and application of electrochemiluminescence sensor for detecting thrombin
ActiveCN102507689AHigh sensitivityHigh luminous intensityChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansSingle strandElectrochemiluminescence
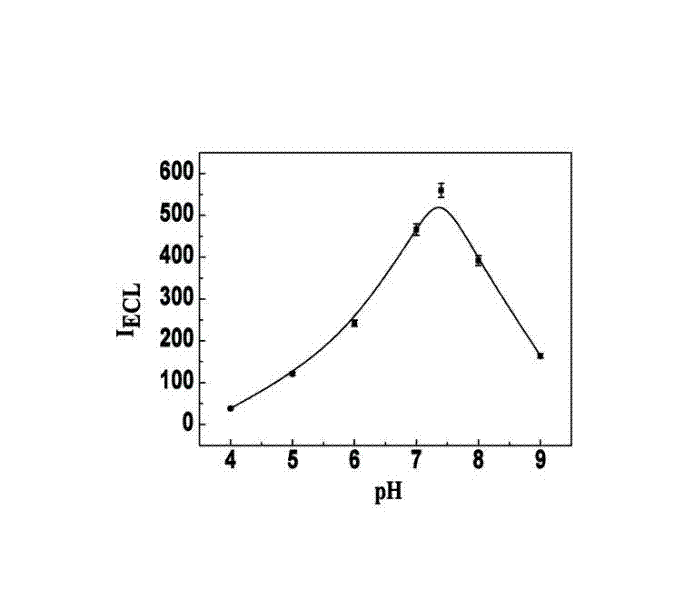
The invention relates to a manufacturing method and application of an electrochemiluminescence sensor for detecting thrombin. Ruthenium bipyridine with active group is modified to the gold nanoparticle surface to form an electrochemiluminescence nanoparticle probe, which plays the role of amplifying electrochemiluminescence, and the upper part DNA single strand is modified on the working electrode surface to constitute an electrochemiluminescence sensor. The target analyte of thrombin is subjected to reaction with partial DNA double strands and then with DNA polymerase, Nb.BbvCI nicking enzyme and partial DNA single strand to obtain DNA hybrid solution which is added into the sensor, so as to increase electrochemiluminescence nanoparticle probes on the electrode surface and further lead to an enhanced electrochemiluminescence signal, and accordingly thrombin determination is realized. The inventive sensor has high selectivity and detection sensitivity.
Owner:武汉菲思特医学检验实验室有限公司
Non-cyanide silver plating bath composition
InactiveUS20050183961A1Chemically stableIncrease brightnessChemical vapor deposition coatingCyanideSilver plate
Disclosed is an electroplating solution for the deposition of silver; said solution containing silver in the form of a complex of silver with hydantoin or a substituted hydantoin compound; said solution also containing an excess of the hydantoin or substituted hydantoin compound employed, together with an effective quantity of a nonprecipitating electrolyte salt, and also an effective quantity of 2,2′ dipyridyl for the purpose of obtaining a mirror-bright to brilliant deposit.
Owner:TECHNIC INC
Copper (i) complexes for optoelectronic devices
ActiveUS20130150581A1Short possible emission decay timeDiminished roll-off behaviorFinal product manufactureGroup 5/15 element organic compoundsSolubilityChlorobenzene
The invention relates to neutral mononuclear copper (I) complexes for emitting light and with a structure according to formula (A) in which: M represents: Cu(I); L∩L represents: a single, negatively charged, bidentate ligand; N∩N represents: a diimine ligand (substituted with R and FG), in particular a substituted 2,2′-bipyridine derivative (bpy) or a substituted 1,10-phenanthroline derivative (phen); R represents: at least one sterically demanding substituent for preventing the planarisation of the complex in the excited state; FG=functional group, and represents: at least one second substituent for increasing solubility in organic solvents. The substituent can also be used for electron transport or alternatively for hole transport, said functional group being bound to the diimine ligands either directly or by means of suitable bridges; and the copper (I) complex: having a ΔE(S1−T1) value of less than 2500 cm−1 between the lowest excited singlet state (S1) and the triplet state (T1) which lies below; having an emission lifespan of at most 20 μs; having an emission quantum yield of greater than 40%, and a solubility of at least 1 g / L in organic solvents, in particular polar organic hydrocarbons such as acetone, methyl ethyl ketone, benzene, toluene, chlorobenzene, dichlorobenzene, dichloromethane, chloroform, dichloroethane, tetrachloroethylene, alcohols, acetonitrile or water.
Owner:SAMSUNG DISPLAY CO LTD
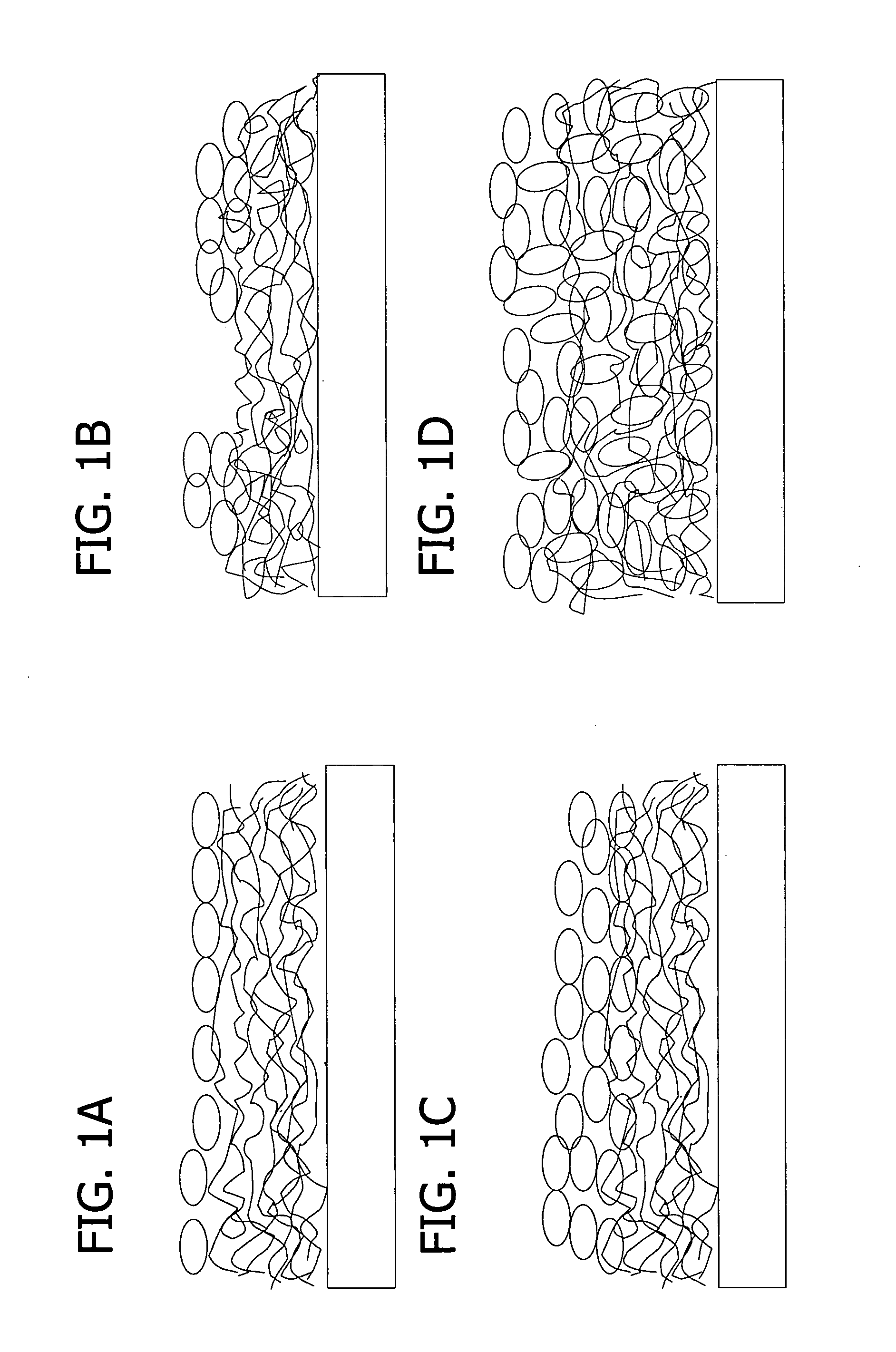
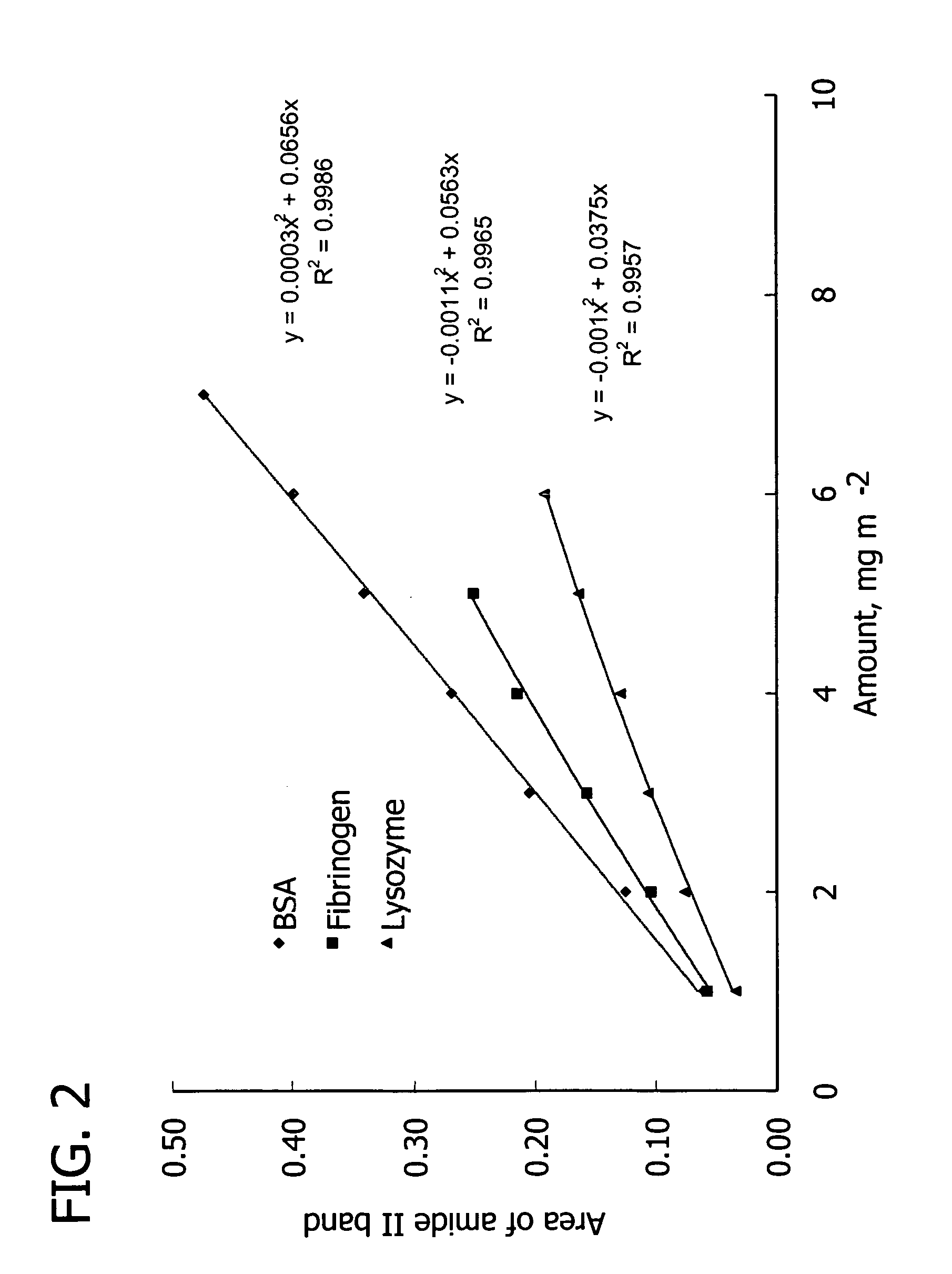
Thin films for controlled protein interaction
A medium for isolating or releasing an electrostatically charged component from or into an aqueous composition. The medium has a polyelectrolyte film on at least one surface of an article wherein the polyelectrolyte film is characterized by an interpenetrating network of a predominantly positively charged polymer and a predominantly negatively charged polymer. The predominantly positively charged polymer, the predominantly negatively charged polymer or both contain (i) a pH sensitive imidazole repeat unit having a pKa between 3 and 9, or (ii) a redox sensitive repeat unit selected from the group consisting of quaternized bipyridine repeat units, coordinated metal repeat units, pyrrole repeat units, aniline repeat units, thiophene repeat units and combinations thereof having a redox potential between +1.2 volts and −1.2 volts versus a standard hydrogen electrode.
Owner:FLORIDA STATE UNIV RES FOUND INC
Non-cyanide silver plating bath composition
Owner:TECHNIC INC
Preparation method of copper-coated tungsten composite powder
InactiveCN101537491AHigh sintering activityUniform thicknessLiquid/solution decomposition chemical coatingWater bathsBipyridine
The invention discloses a preparation method of copper-coated tungsten composite powder, belonging to the technical field of powder metallurgy. Corresponding tungsten powder and blue vitriod are employed according to the weight ratio of composite powder to be prepared and the tungsten powder is pre-treated; copper sulphate and seignette sol are dissolved into solution; bipyridine is added and NaOH is used to adjust pH value to 12-14; an appropriate amount of formaldehyde solution and the tungsten powder are added; the mixture is heated and then stirred continuously in constant-temperature bathing at 30-60 DEG C until the tungsten powder is red; the tungsten powder is washed, dried, reduced and annealed, thereby obtaining copper-coated tungsten composite powder. The preparation method of copper-coated tungsten composite powder has wide application range and is applicable to tungsten-powder coating coppers with different finenesses and shapes. The preparation method of copper-coated tungsten composite powder prepares copper-coated tungsten composite powder in different content ratios according to different requirements. The copper-coated tungsten composite powder obtained by the method of the invention has excellent sintering activity, thereby improving the combination property of tungsten copper alloy.
Owner:UNIV OF SCI & TECH BEIJING
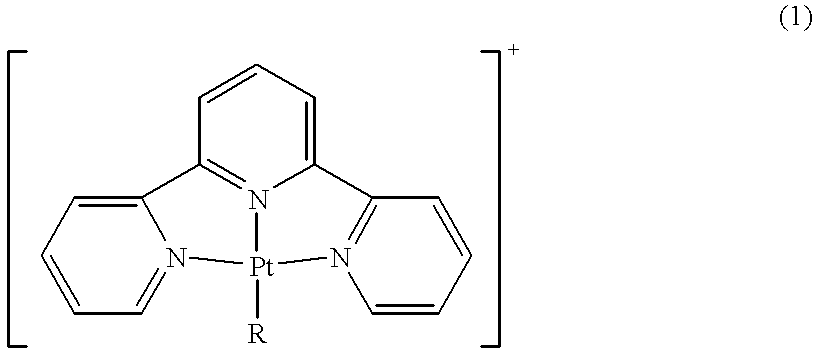
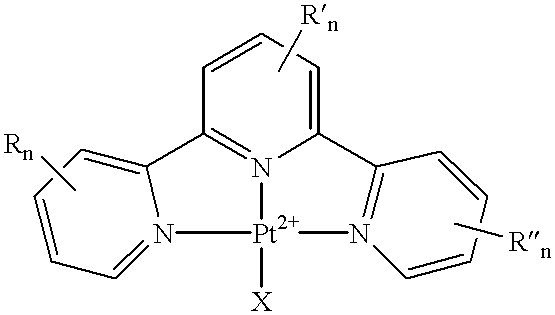
Terpyridine-platinum(II) complexes
InactiveUS20020013306A1Fast displacementImprove purification effectBiocideHeavy metal active ingredientsAntiparasiticChemical compound
A new class of 2,2':6',2''-terpyridine-platinum (II) and substituted 2,2':6',2''-terpyridine-platinum (II) complexes in which an N- or O- or halo nucleophile is the fourth ligand to platinum. The compounds are potent intercalators of DNA. Some have antitumour activity. Some have anti-parasitic activity. A new method of preparing the complexes involves reacting a Pt complex of 1,5-cyclooctadiene with a 2,2':6',2''-terpyridine.
Owner:ISIS INNOVATION LTD
Transition metal complex based on semirigid bipyridine bisamide organic ligand and terephthalic acid as well as synthetic method and application of transition metal complex
InactiveCN103724365AIncrease the coordination pointImprove the coordination effectFluorescence/phosphorescenceLuminescent compositionsSolvent moleculeFluorescence
The invention discloses a transition metal complex based on a semirigid bipyridine bisamide organic ligand and terephthalic acid as well as a synthetic method and application of the transition metal complex. The molecular formula of the transition metal complex is as follows: [Zn(3-bpah)(1,4-BDC)).H2O; [Cd(3-bpah)(1,4-BDC)(H2O)], wherein 3-bpah is N,N'-bi(3-pyridine acylamino)-1 2-cyclohexane. The method comprises the steps of mixing Zb<2+> nitrate or Cd<2+> chloride, the semirigid bipyridine bisamide ligand, terephthalic acid and deionized water, adjusting the pH value of the mixture, pouring the mixture into a high-pressure reaction kettle, performing heat preservation in hydrothermal condition, cooling the mixture to room temperature, and airing the mixture, so as to obtain the target complex. The transition metal complex as well as the synthetic method and application of the transition metal complex have the advantages that the synthetic method is simple, the synthetic yield is high, the synthesized complex has high fluorescence-emission property, fluorescent selectivity to different solvent molecules, and fluorescence identification and detection properties for environmental pollutant nitrobenzene, and can be used as fluorescent material.
Owner:BOHAI UNIV
Alkaline ionic liquid and its prepn process and application
InactiveCN1931845AEasy to separateAvoid formation of solid viscousOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsInorganic saltsIon exchange
The present invention is one kind of alkaline ionic liquid and its preparation process and application in Pd catalyzed C-C and C-N coupling reaction, and belongs to the field of new material and fine chemical technology. The alkaline ionic liquid consists of two parts, cation A+ and anion B-. The cation A+ precursor is imidazole, pyridine, etc; and the anion B- is alkaline group X- or non-alkaline group Y-. The alkaline ionic liquid is prepared through the first quaternizing with alkyl halide as the quaternizing reagent to obtain ion type halide; and the subsequent ion exchange to obtain the target alkaline ionic liquid. The alkaline ionic liquid of the present invention may act as acid-binding agent and can avoid formation of sticky inorganic salt to facilitate reuse.
Owner:EAST CHINA NORMAL UNIVERSITY
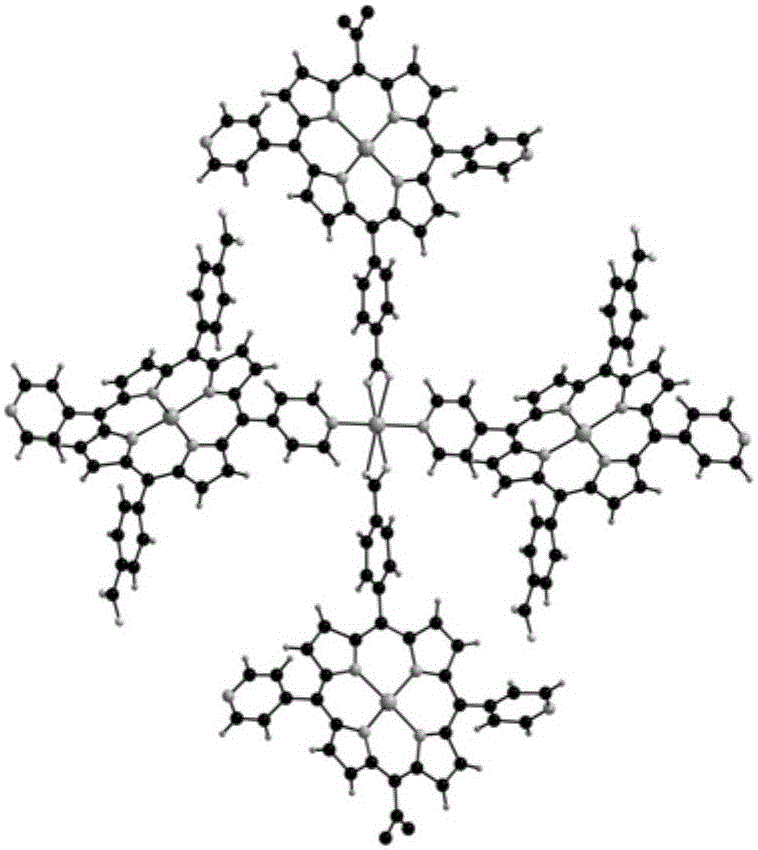
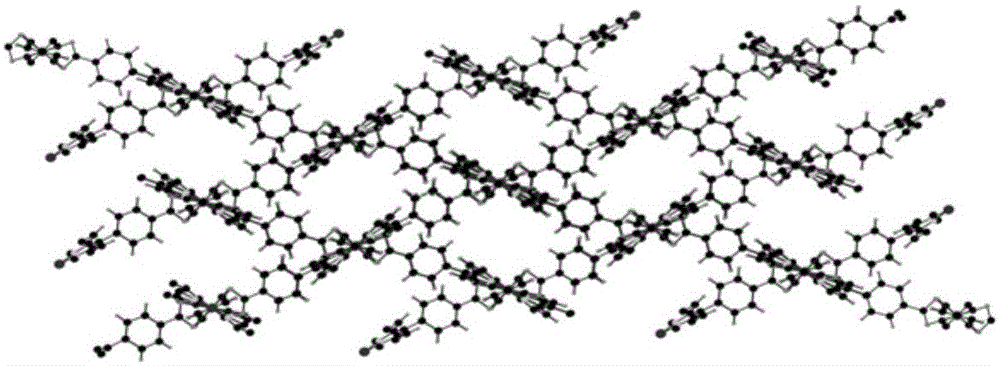
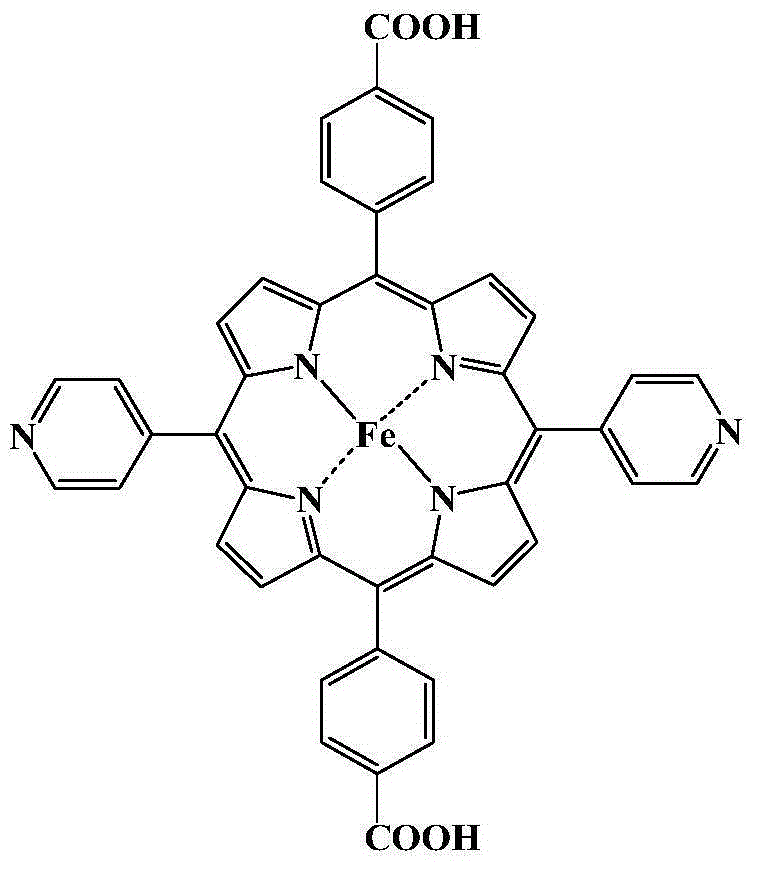
Metal organic framework material of Fe porphyrin ligand, preparation method therefor and application thereof
InactiveCN105061776ANovel structureImprove catalytic performancePreparation by oxidation reactionsOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidN dimethylformamide
The invention discloses a metal organic framework material of a Fe porphyrin ligand, a preparation method therefor and an application thereof, and belongs to the technical field of crystalline materials. A chemical formula of the Fe porphyrin ligand is FeL, wherein L is am organic ligand, namely 5, 15-bipyridine-10 and 20-dicarboxyphenyl ironporphyrin. In a closing condition, a crystal of the metal organic framework material is obtained by the organic ligand, namely 5, 15-bipyridine-10, 20-dicarboxyphenyl ironporphyrin and iron nitrate nonahydrate by virtue of thermal reaction in a solution of N,N-dimethylformamide and acetic acid. The metal organic framework material has an iron porphyrin catalytic property, and can be used for selective catalysis of cycloalkane.
Owner:BEIJING UNIV OF TECH
Carbazole compound and organic light-emitting device using same
InactiveUS20080166591A1Improve efficiencyEasy to produceElectroluminescent light sourcesSolid-state devicesCarbazoleOrganic light emitting device
There are provided a novel carbazole compound and an organic light-emitting device having an optical output with extremely high efficiency and luminance and having extremely high durability. Specifically, there are provided a novel carbazole compound represented by the general formula (1):wherein Ar represents a substituted or unsubstituted bipyridine group, a substituted or unsubstituted terpyridine group, or a substituted or unsubstituted 4,5-diazafluorene group and is bonded to any of the eight available carbon atoms on the carbazole skeleton, and an organic light-emitting device using the carbazole compound.
Owner:CANON KK
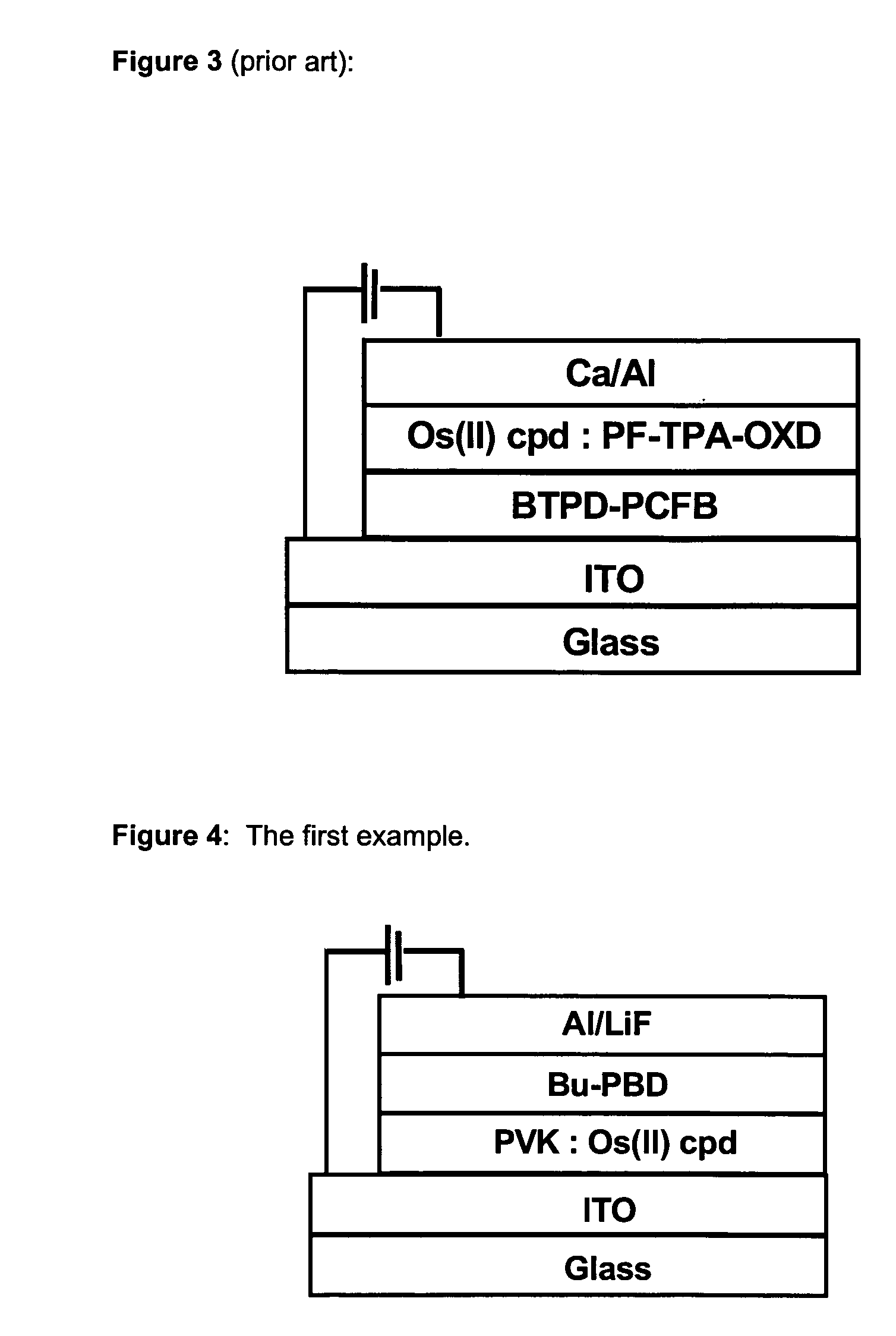
Osmium complexes and related organic light-emitting devices
InactiveUS7416791B1Discharge tube luminescnet screensGroup 8/9/10/18 element organic compoundsOrganic light emitting devicePhenanthroline
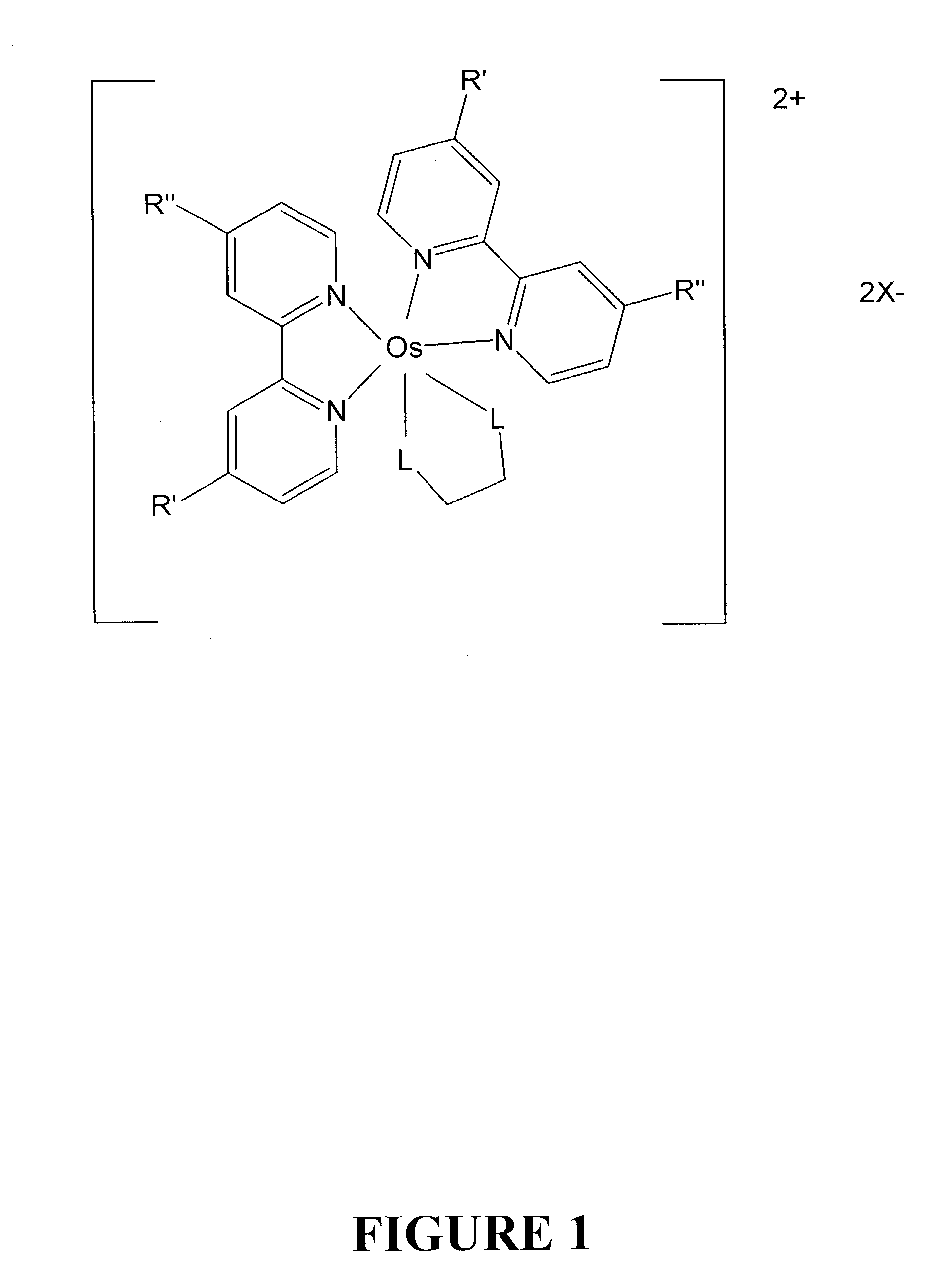
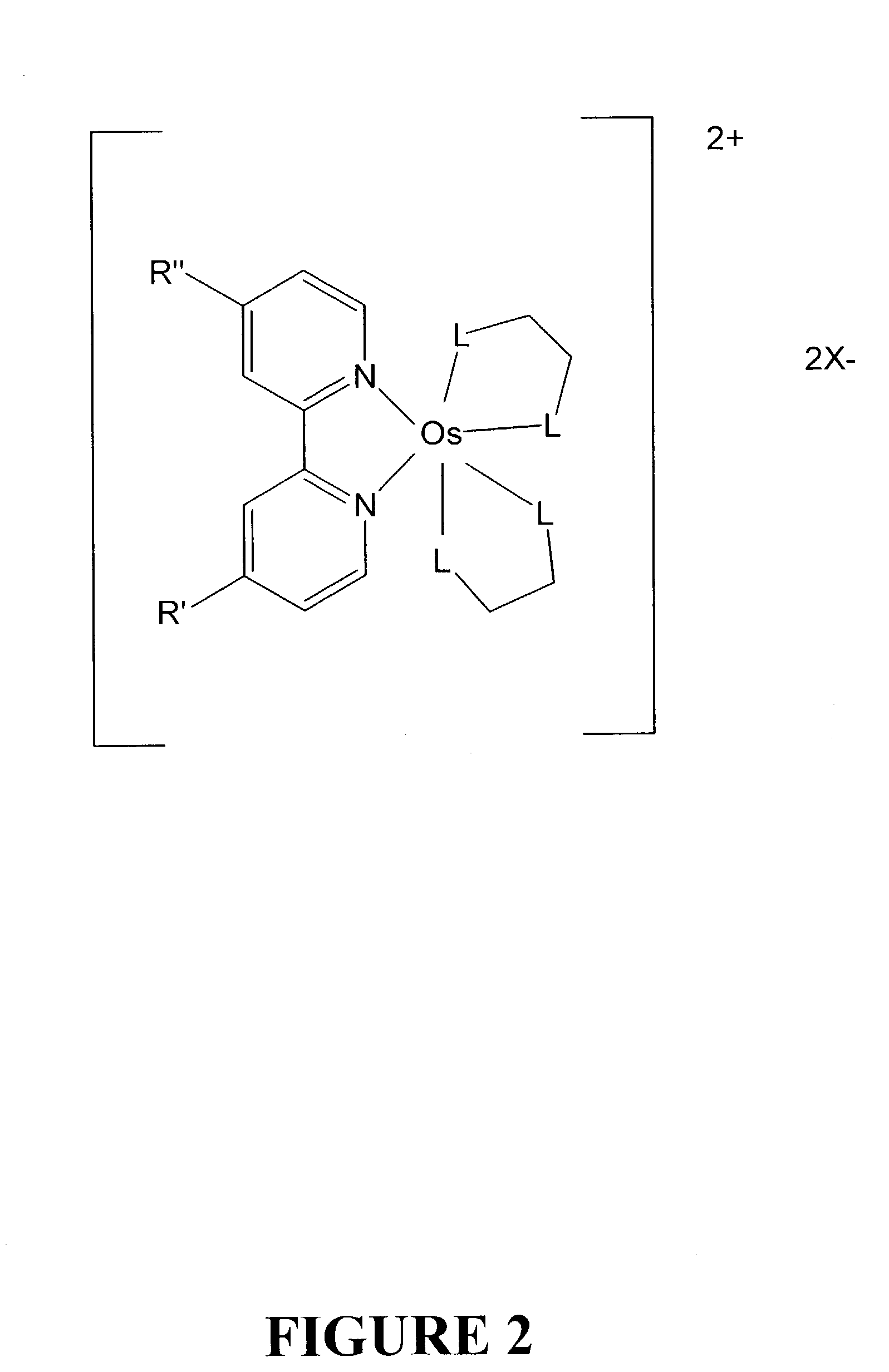
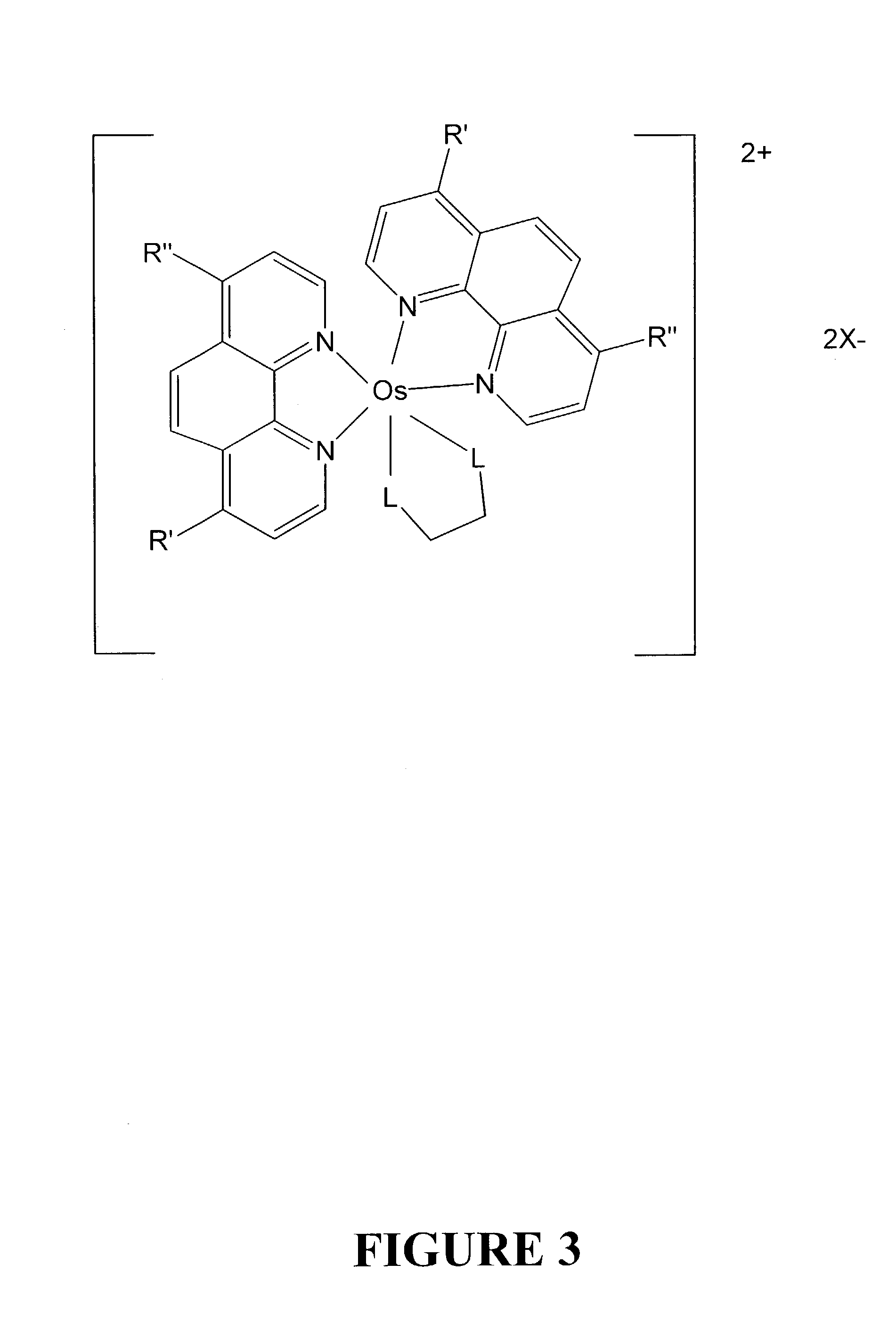
Osmium complexes having the formula [Os(II) (N—N)2L—L]2+ 2A− (or A2−), or [Os(II) N—N(L—L)2]2+ 2A− (or A2−), where N—N is a bipyridine or phenanthroline ligand, L—L is a π-acid bidentate ligand, and A is counter ion.
Owner:UNIV OF WASHINGTON
Dye for photoelectric device and photoelectric device comprising the dye
InactiveUS20080110496A1Light absorption efficiency can be improvedHigh photosensitivity to sunlightRuthenium organic compoundsElectrolytic capacitorsPhosphoric acidPhotochemistry
Disclosed herein are a novel dye for a photoelectric device and a photoelectric device comprising the dye. More particularly, the dye for a photoelectric device incorporates different quaternary ammoniums into a carboxyl or phosphoric acid-substituted bipyridyl ligand of the dye, and a photoelectric device comprising the same. The dye for a photoelectric device as disclosed herein exhibits improved photosensitivity and light absorbing characteristics, thereby making it possible to fabricate a highly efficient photoelectric device when the dye is included in the device.
Owner:SAMSUNG SDI CO LTD
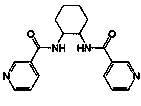
1',3'-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2h,1'h-[1,4']bipyridinyl-2'-ones
The present invention relates to novel compounds, in particular novel pyridinone derivatives according to Formula (I)wherein all radicals are as defined in the application and claims. The compounds according to the invention are positive allosteric modulators of metabotropic receptors-subtype 2 (“mGluR2”) which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. In particular, such diseases are central nervous system disorders selected from the group of anxiety, schizophrenia, migraine, depression, and epilepsy. The invention is also directed to pharmaceutical compositions and processes to prepare such compounds and compositions, as well as to the use of such compounds for the prevention and treatment of such diseases in which mGluR2 is involved.
Owner:JANSSEN PHARMA INC +1
Method for preparing temperature-sensitive polyvinylidene fluoride intelligent membrane material and its product
This invention relates to a method for prparing temperature-sensitive polyvinylidene flourine intelligent membrane. The method is: NIPA as grafted monomer, PVDF as macromelocular initiator, cuprous chloride as catalyst,4,4 demethyl 2,2 bipyridine as ligand, using atom transfer free radical process, preparation of temperature-sensitive polyvinylidene flourine intelligent membrane comprising: in reaction kettle, adding 7-13% PVDF(WT%) and solvent N-methyl-2-pyrrolidone,heating to 50 deg. C to make it solve completely; then adding grafted monomer NIPA, its mol ratio with cuprous chloride is 2000:1-2500:1,filling nitrogen gas 30mins. at stirring, and separately adding 0.20-025g ligand 4,4-dimethyl 2,2-bipyridine and 0.04-0.06g catalyst cupous chloride, stirring and heating to 80-120deg.C,constant reaction 19-25 hrs; then washing reaction product using pure water to obtain light brown solid, filtering and 80 C degree drying to obtain finish product.
Owner:TIANJIN POLYTECHNIC UNIV
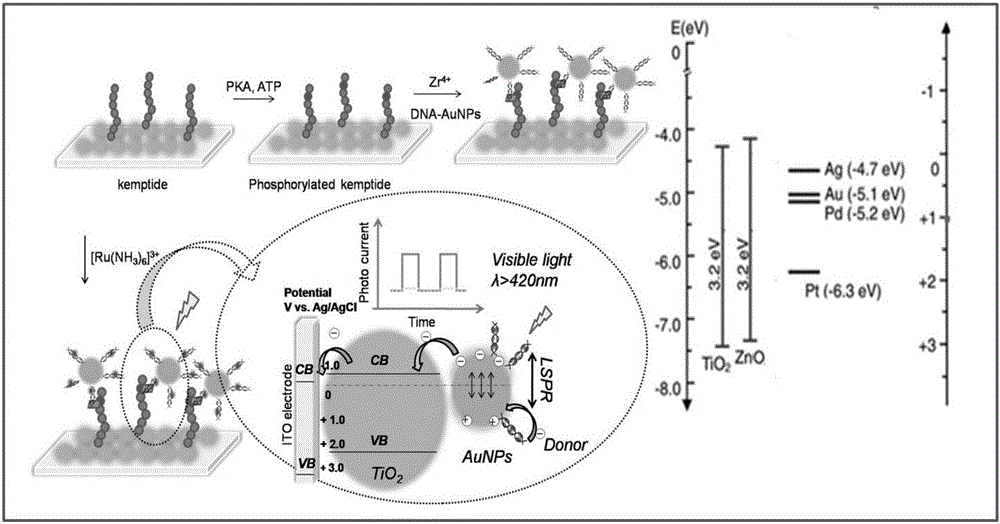
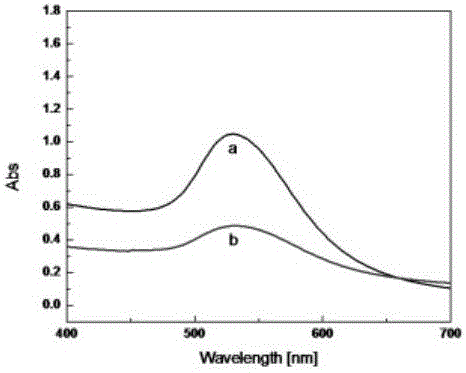
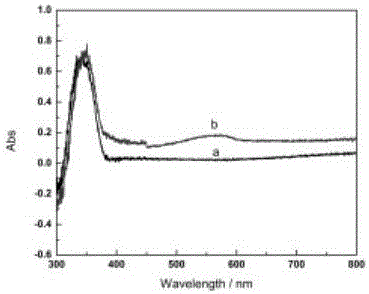
Photoelectric sensor for detection of kinase activity on the basis of local area surface plasma resonance
ActiveCN105021575AGood light stabilityHigh sensitivityMaterial analysis by optical meansSemiconductor materialsEnzyme inhibition
The present invention discloses a photoelectric sensor for detection of kinase activity on the basis of local area surface plasma resonance. The biosensor is prepared as follows: a semiconductor material metal oxide is modified onto an indium tin oxide electrode (ITO), then Kemptide, PKA, and a probe are assembled onto the electrode layer by layer, since the probe contains noble metal nanoparticles and photosensitizer tris (bipyridine) ruthenium, under visible light irradiation, the photosensitizer tris (bipyridine) ruthenium captures more photons, local area surface plasma resonance effect of the noble metal nanoparticles occurs under photon excitation, so that more electrons jump onto a semiconductor metal oxide conduction band to produce photocurrent. The biosensor quantitative detection is based on that different concentrations of PKA cause different extents of phosphorylation of Kemptide, so that the amount of the probe connected to the modified electrode is also different, and photocurrent changes are caused. The method has high sensitivity and selectivity, enzyme inhibition experiments also show that the method achieves the purpose of efficient and sensitive detection of the kinase PKA activity.
Owner:QINGDAO UNIV
Diode-addressed color display with lanthanoid phosphors
InactiveUS6165631AImprove quantum efficiencyImprovement factorDischarge tube luminescnet screensCathode ray tubes/electron beam tubesEthylenediamineDisplay device
A diode-addressed color display comprising an UV-diode and a phosphor of the general formula LnL3X2, wherein Ln=Eu3+, Tb3+, Tm3+, Dys3+, Sm3+, L=4-R-4'-benzophenone carboxylic acid, wherein R=phenyl, benzyl, CH3, CF3, C2H5, F, Cl, OCH3, CH3CO; 4-R-4'-benzophenone acetylacetonate, wherein R=phenyl, benzyl, CH3, CF3, C2H5, F, Cl, OCH3, CH3CO; 4-acetophenone carboxylic acid, 4-trifluoroacetophenone carboxylic acid, 4-acetophenone acetylacetonate or 4-trifluoroacetophenone acetylacetonate and X=+E,fra 1 / 2+EE phenanthroline, +E,fra 1 / 2+EE diphenyl phenanthroline, +E,fra 1 / 2+EE 4-Cl-phenanthroline, +E,fra 1 / 2+EE bipyridine, +E,fra 1 / 2+EE ethylenediamine, triphenyl phosphineoxide, trimethyl phosphineoxide, triethyl phosphineoxide, +E,fra 1 / 2+EE diethylene glycol-dimethylether (diglyme) or ethanol.
Owner:US PHILIPS CORP
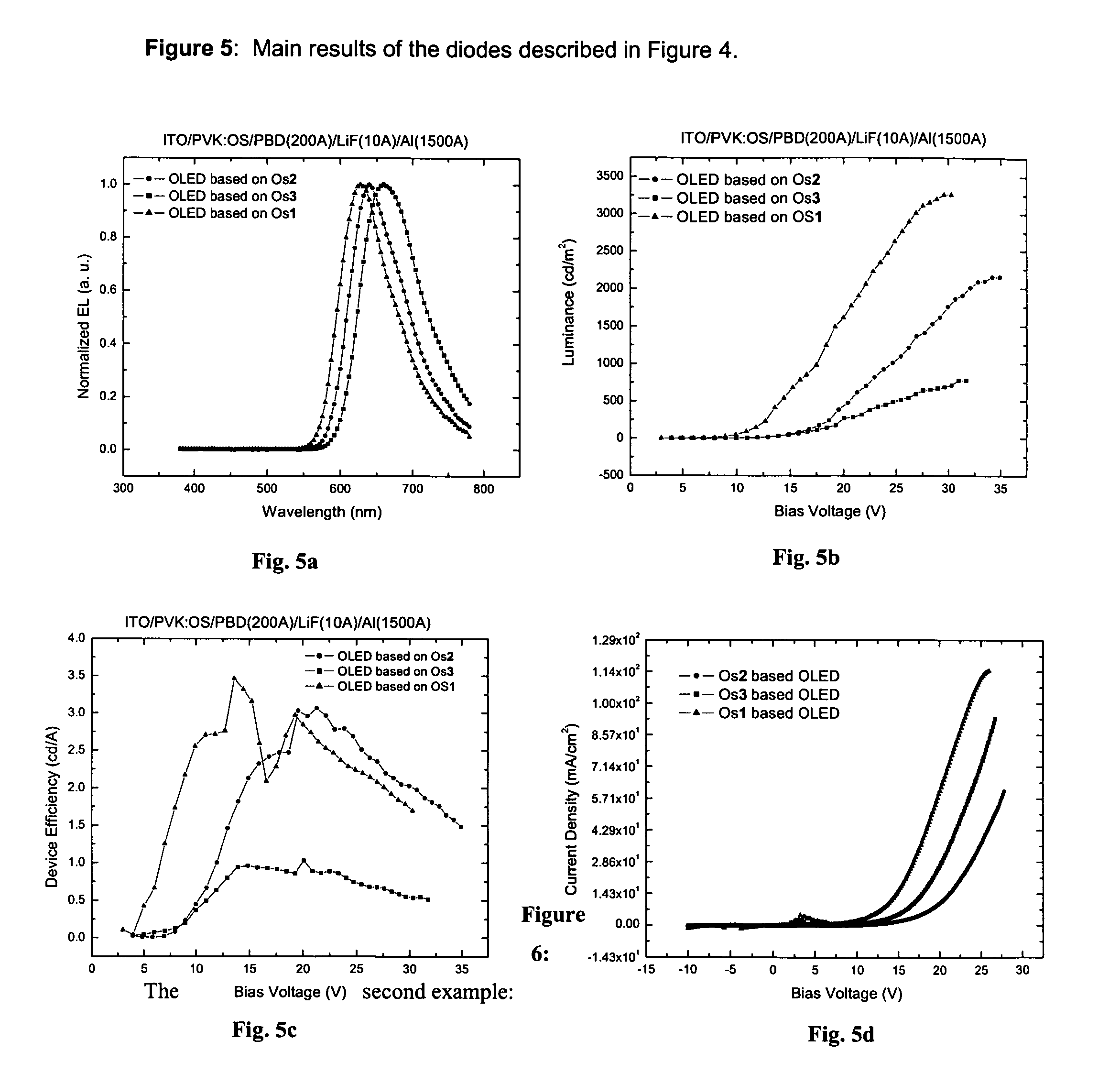
Phosphorescent Osmium (II) complexes and uses thereof
InactiveUS20070001166A1Discharge tube luminescnet screensElectroluminescent light sourcesPhenanthrolineCarbene
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds. Compounds of interest include: wherein A is Os or Ru The anionic chelating chromophores NˆN, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage. L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene. L can occupy either cis or trans orientation. When L occupies the trans position, the preferred structure contains both the hexagonal fragment of NˆN as well as its pentagonal fragment located at the trans position respect to their counterparts of the second NˆN chromophore. When L occupies the cis position, the preferred structure consists of the pentagonal unit of NˆN chromophores residing opposite to the L. X,1 X2 and X3 independently are C or N; when X2 is N, R1 is omitted, when X3 is N, R2 is omitted, R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl, R2 is H, F or cyano substituent, X4 is either C or N; X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4, R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:TAO YE +3
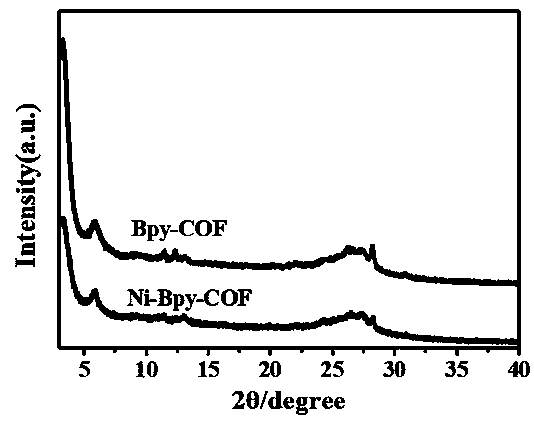
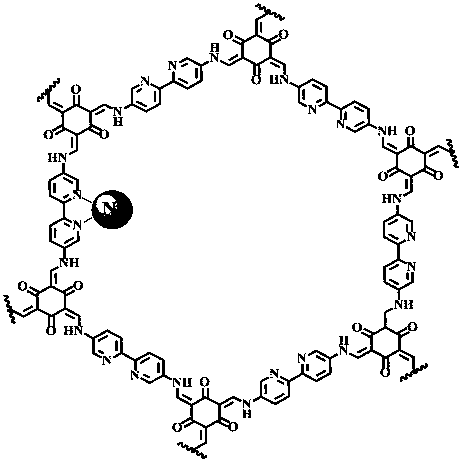
Preparation method of nickel ion modified covalent organic framework material and application thereof
ActiveCN108794756AHigh selectivityFair priceOrganic-compounds/hydrides/coordination-complexes catalystsCarbon monoxideMaterials preparationBipyridine
The invention discloses a preparation method of a nickel ion modified covalent organic framework material and an application thereof, and belongs to the field of material preparation and environment.The preparation method includes utilizing 5,5'-diamido-2,2'-bipyridine and 2,4,6-trihydroxy-1,3,5-benzenetrialdehyde to synthesize a covalent organic framework Bpy-COF by Schiff base condensation; conducting nickel ion post modification to obtain the nickel ion modified covalent organic framework material Ni-Bpy-COF. The principle and a method of the coordination chemistry are utilized to combinethe advantages of a metal complex molecular catalyst in high-selectivity photocatalytic conversion of CO2 with COFs having semiconductor properties, and the obtained Ni-Bpy-COF photocatalytic materialexhibits photocatalytic activity and has high efficiency and high selectivity photocatalytic reduction of CO2 to produce CO under visible light, the selectivity to CO2 photocatalytic reduction reaches 94%, and the problem of low selectivity of products in the existing photocatalytic reduction of CO2 of a semiconductor is solved.
Owner:FUZHOU UNIV
Rare earth metal complexes that excite in the long UV wavelength range
ActiveUS20090000509A1Good fluorescence stabilityImprove solubilityDuplicating/marking methodsInksArylDopant
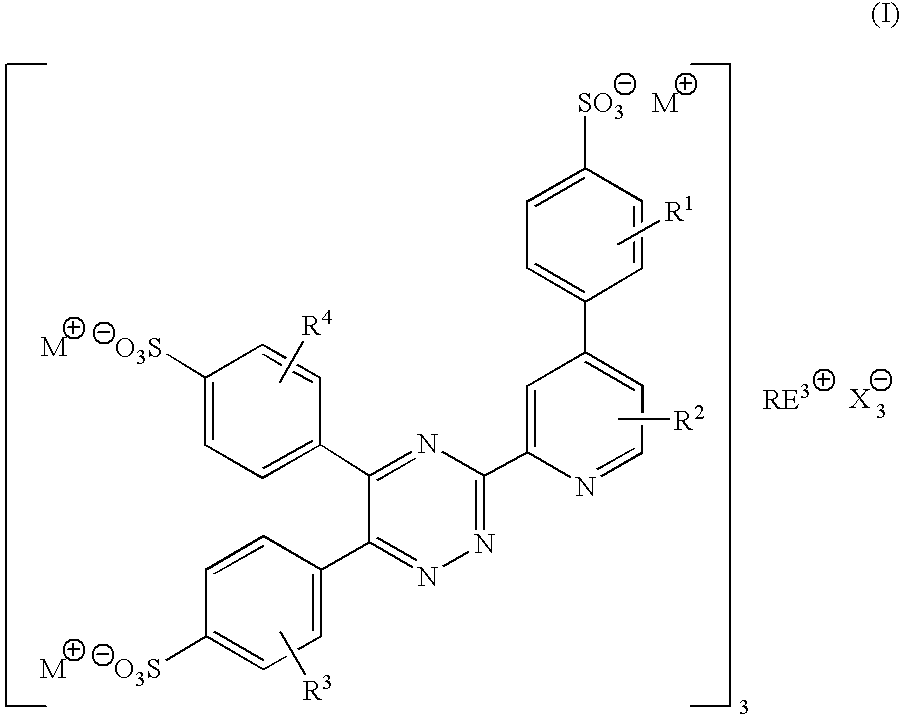
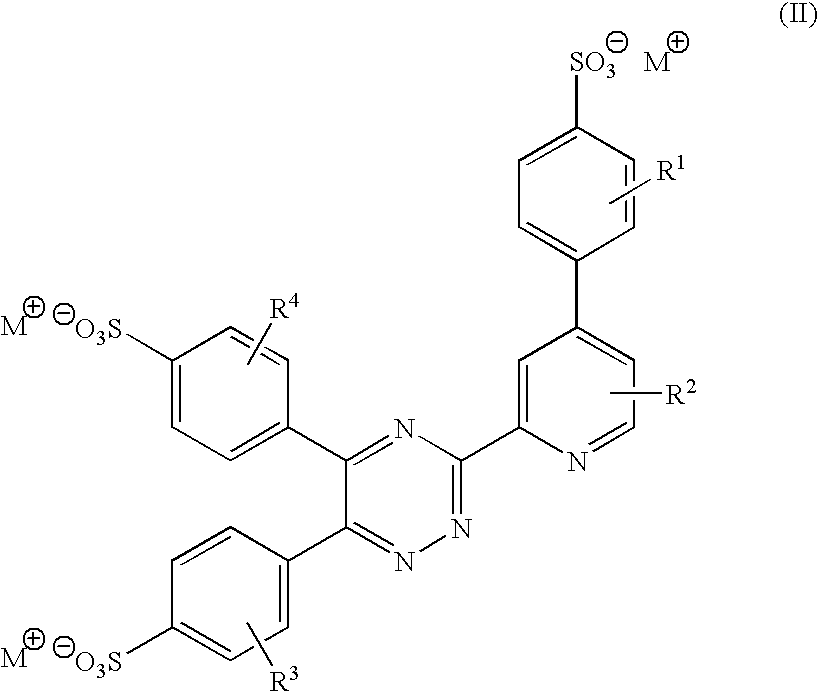
There is provided rare earth metal complexes of formula (I):wherein M is a cation, RE is a rare earth metal; X is a counter anion; and R1, R2, R3, and R4 are each independently selected from hydrogen, alkyl of 1-8 carbon atoms, aryl, halo, and alkoxy. Also provided is a composition of europium bis(2,2′-bipyridine-N,N′)-trinitrate with a diketonate dopant. The disclosure also provides processes for preparing the rare earth metal complexes. A mark comprising the rare earth metal complexes, and a method of applying the mark, are disclosed. The rare earth metal complexes may be used in printing systems, and for security applications.
Owner:HONEYWELL INT INC
Redox polymers for use in analyte monitoring
InactiveUS20120132525A1Immobilised enzymesBioreactor/fermenter combinationsElectrochemical biosensorHigh rate
Polymers for use as redox mediators in electrochemical biosensors are described. The transition metal complexes attached to polymeric backbones can be used as redox mediators in enzyme based electrochemical sensors. In such instances, transition metal complexes accept electrons from, or transfer electrons to, enzymes at a high rate and also exchange electrons rapidly with the sensor. The transition metal complexes include at least one substituted or unsubstituted biimidazole ligand and may further include a second substituted or unsubstituted biimidazole ligand or a substituted or unsubstituted bipyridine or pyridylimidazole ligand.
Owner:ABBOTT DIABETES CARE INC
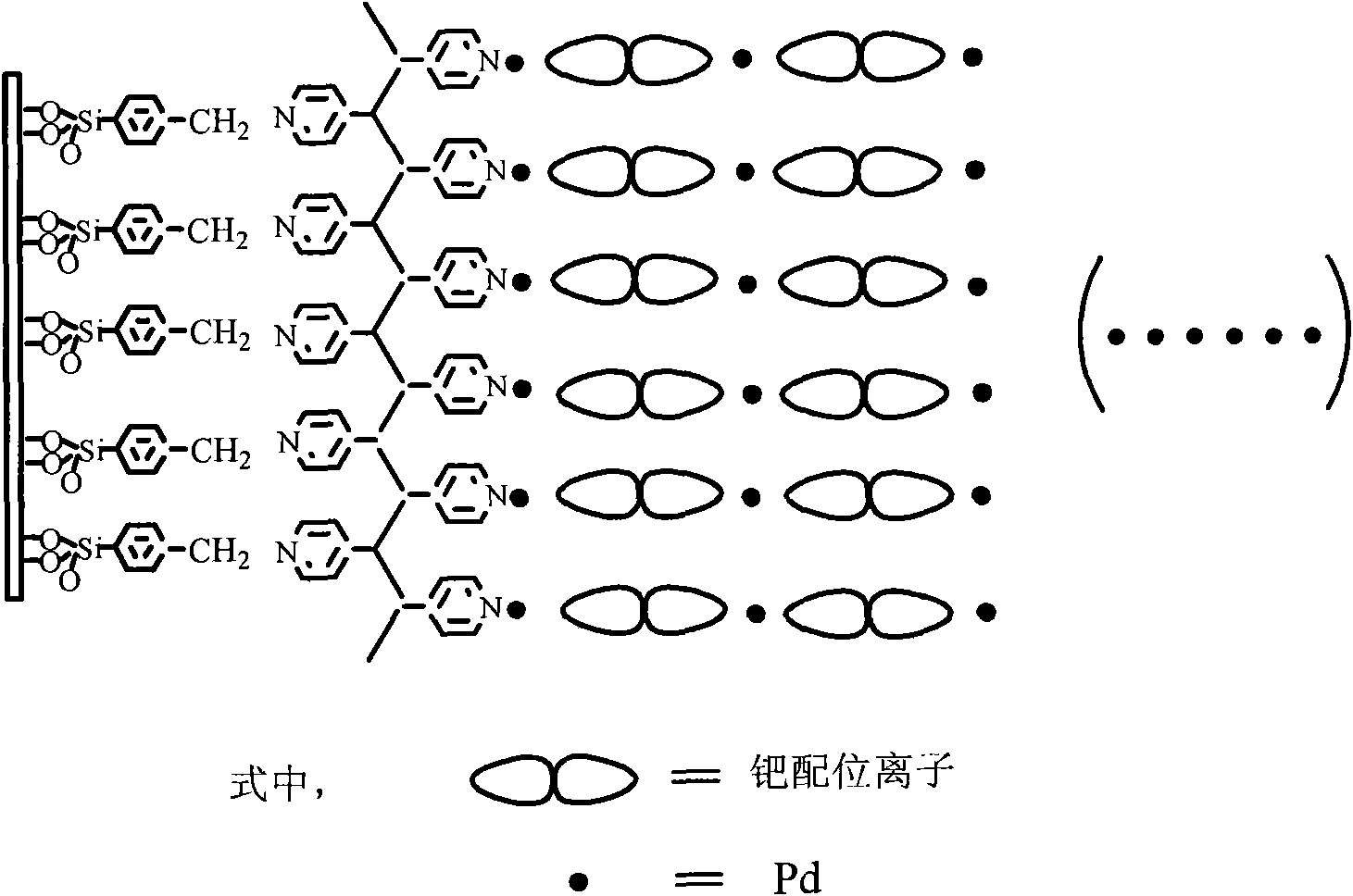
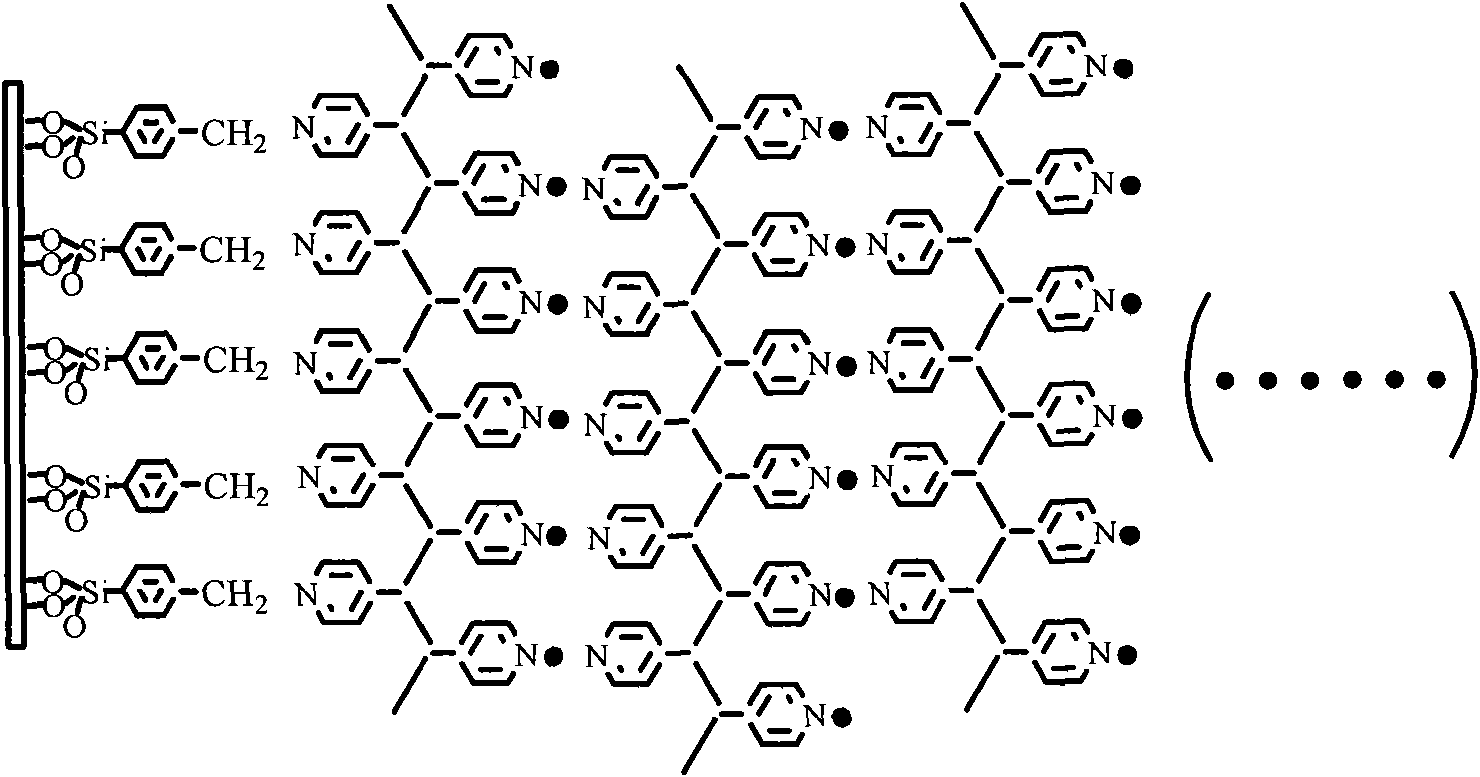
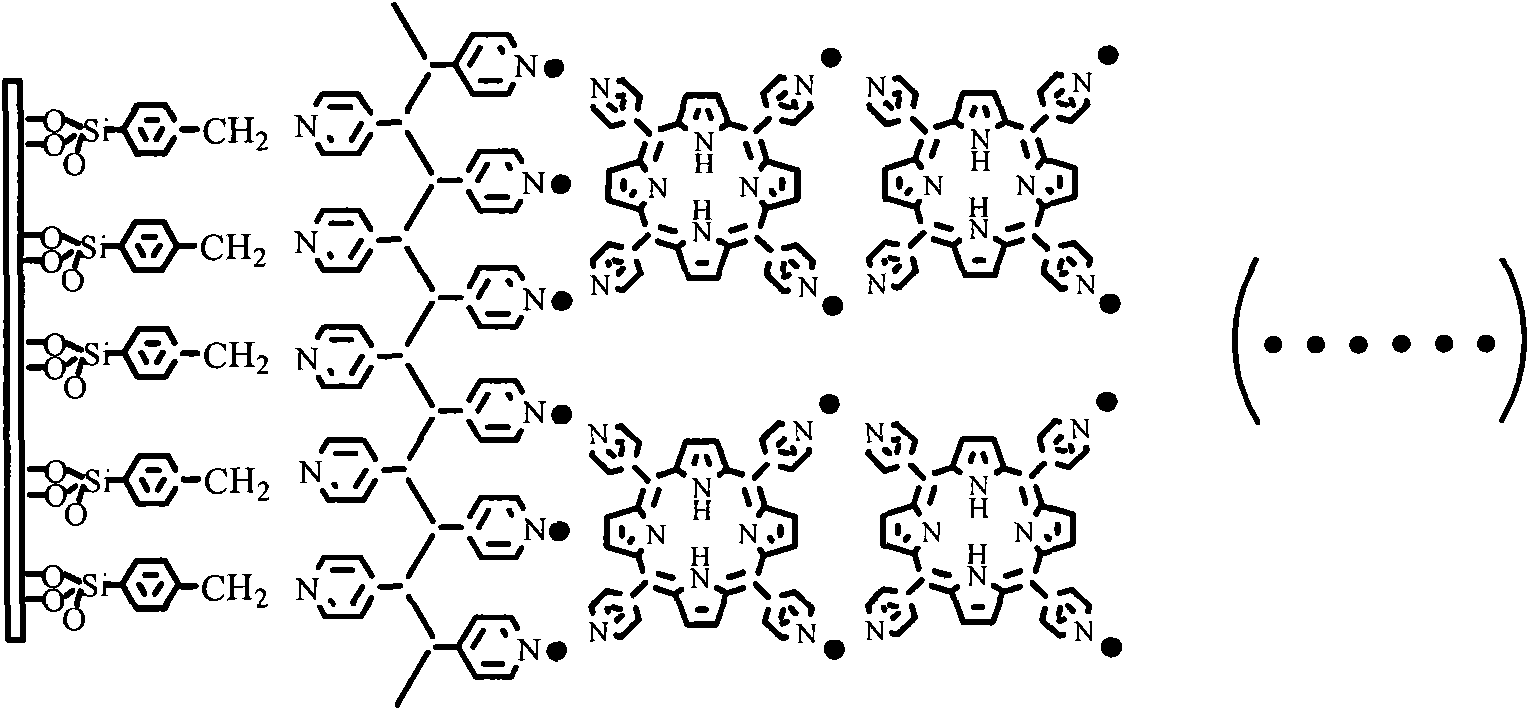
Palladium coordination polymer molecule aggregate catalysis material as well as preparation method and application thereof
InactiveCN101607213AEasy to makeEasy to separateOrganic reductionOrganic compound preparationPorphyrinSolid substrate
The invention relates to a palladium coordination polymer molecule aggregate catalysis material as well as a preparation method and an application thereof. The catalysis material is a coordination polymer molecule aggregate catalysis material formed by self-assembling on the surface of a solid substrate, the precursor of the catalysis material comprises palladium coordination ion as well as pyridine containing multidentate ligand or high molecular ligand, wherein the palladium coordination ion is selected from one of tetrachloro combined palladium acid radical, dichloro-2, 2'-dipyridine palladous, and dichloroacetamide palladous, and the pyridine containing multidentate ligand or high molecular ligand is selected from one of 4, 4'-combined pyridine, 2, 4, 6- collidine triazine, metal or hollow tetra pyridyl porphyrin, trans-1, 2- collidine ethylene, combined dipyridylzinc thiocyanate and polyethylene pyridine. LB film preparation technology or layered assembly method is adopted for organism selective ethylenic bond organic hydrogenation reaction, thus having high catalysis efficiency, easy realization in separation of catalyst and reactant and product, convenient operation and low cost.
Owner:FUDAN UNIV
Three-dimensional bipyridine functionalized covalent organic frame material synthesis method
ActiveCN104761488AHigh catalytic activityApparent shape selectivityCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsMethane
The invention discloses a three-dimensional bipyridine functionalized covalent organic frame material (COF-LZU301) synthesis method which comprises following steps: (1) uniformly mixing tetra(4-anilino)methane in a tetrahedral configuration and linear 6-6'-diformyl-3,3'-bipyridine in an organic solvent; and (2) adding an acetic acid water solution as an acidic catalyst, and performing heating crystallization, centrifugal washing and heat drying to obtain the three-dimensional bipyridine functionalized covalent organic frame material (COF-LZU301). The COF-LZU301 has a three-dimensional diamond topological structure, regular and ordered porous and uniformly-distributed pyridine groups, so that the COF-LZU301 has excellent catalytic activity and shape selectivity for catalyzing a Knoevenagel reaction process. The COF-LZU301 achieves shape-selective catalysis of a typical alkali catalytic reaction.
Owner:LANZHOU UNIVERSITY
Selective oxidative light catalyst and preparing method thereof
InactiveCN1552524AShort lifeLower reaction costOrganic-compounds/hydrides/coordination-complexes catalystsPhotocatalytic reactionBipyridine
A photocatalyst for selective oxidization is prepared from the inorganic carrier including NaX and NaY molecular sieves, ZSM-5 molecular sieve, mesopore molecular sieve, SO2, and Al2O3, the transition metal ions chosen from Fe, Mn, Ni and Co and the organic ligand chosen from alpha, alpha'-bipyridine, 1-(2-pyridineazo)-2-naphthol (PAN), schiff base, etc through complexing.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



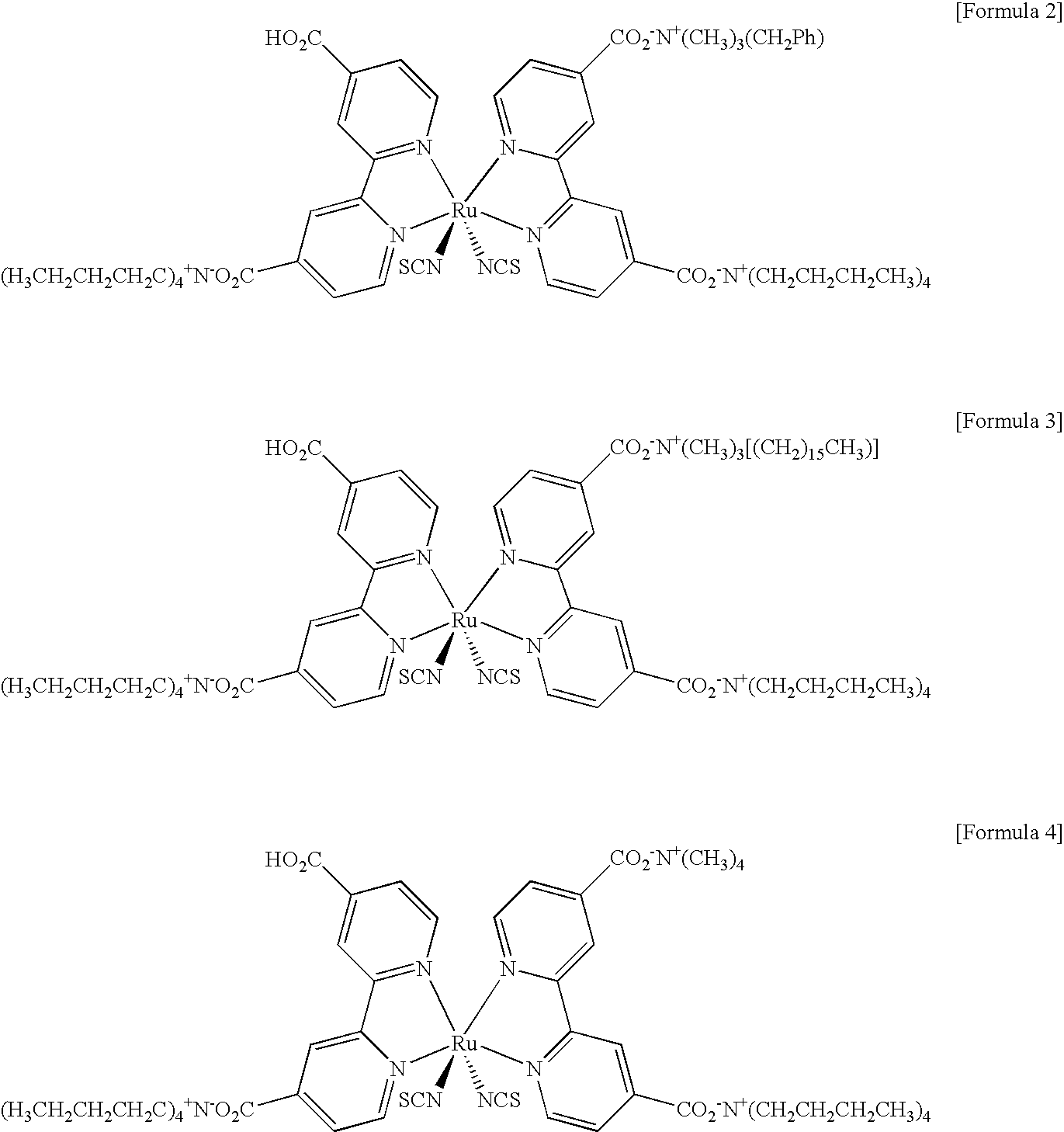
![1',3'-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2h,1'h-[1,4']bipyridinyl-2'-ones 1',3'-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2h,1'h-[1,4']bipyridinyl-2'-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/52ce5e06-4d47-4b17-88ae-c360f8e2b2f5/US20130197019A1-20130801-C00001.png)
![1',3'-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2h,1'h-[1,4']bipyridinyl-2'-ones 1',3'-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2h,1'h-[1,4']bipyridinyl-2'-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/52ce5e06-4d47-4b17-88ae-c360f8e2b2f5/US20130197019A1-20130801-C00002.png)
![1',3'-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2h,1'h-[1,4']bipyridinyl-2'-ones 1',3'-disubstituted-4-phenyl-3,4,5,6-tetrahydro-2h,1'h-[1,4']bipyridinyl-2'-ones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/52ce5e06-4d47-4b17-88ae-c360f8e2b2f5/US20130197019A1-20130801-C00003.png)