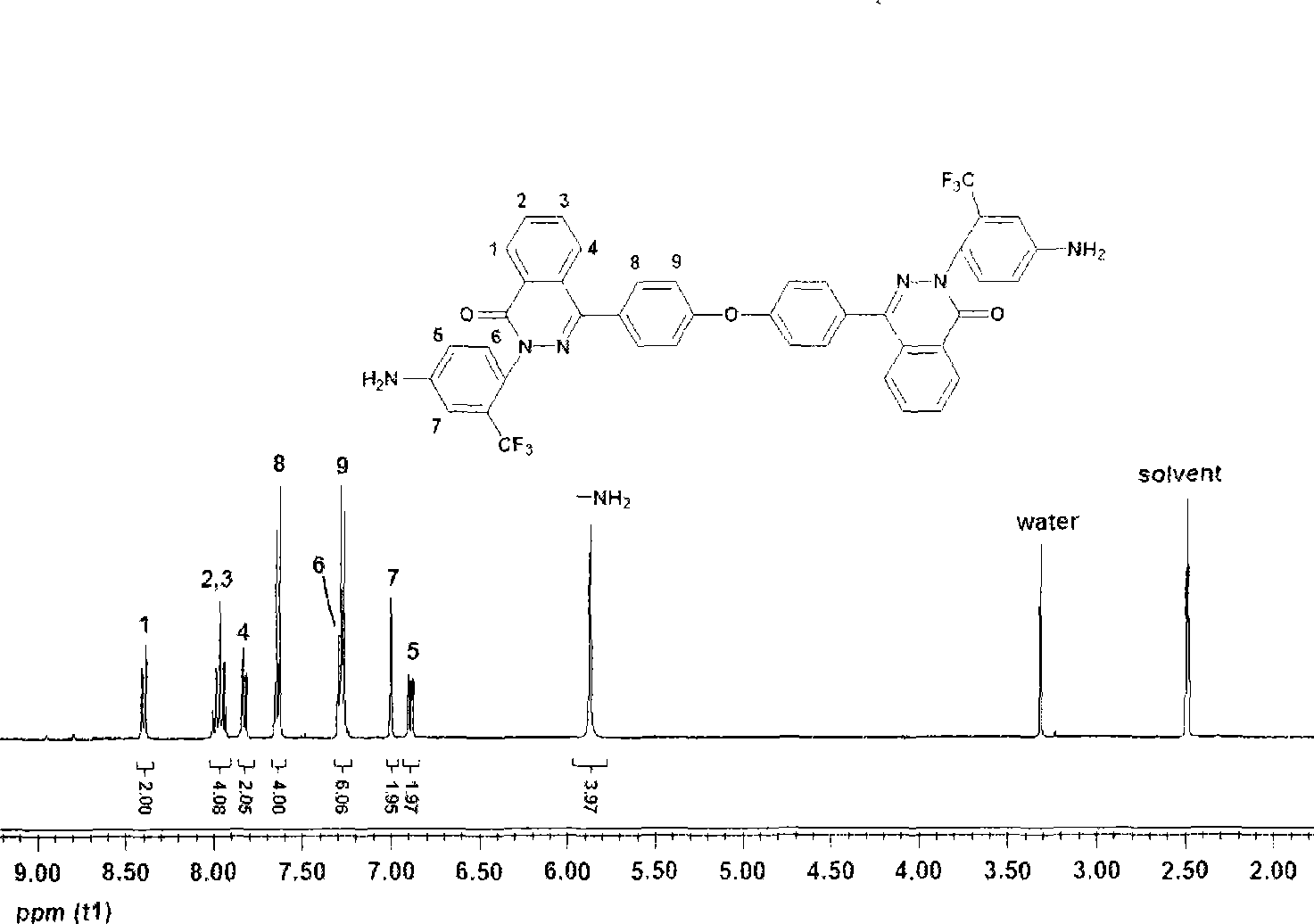
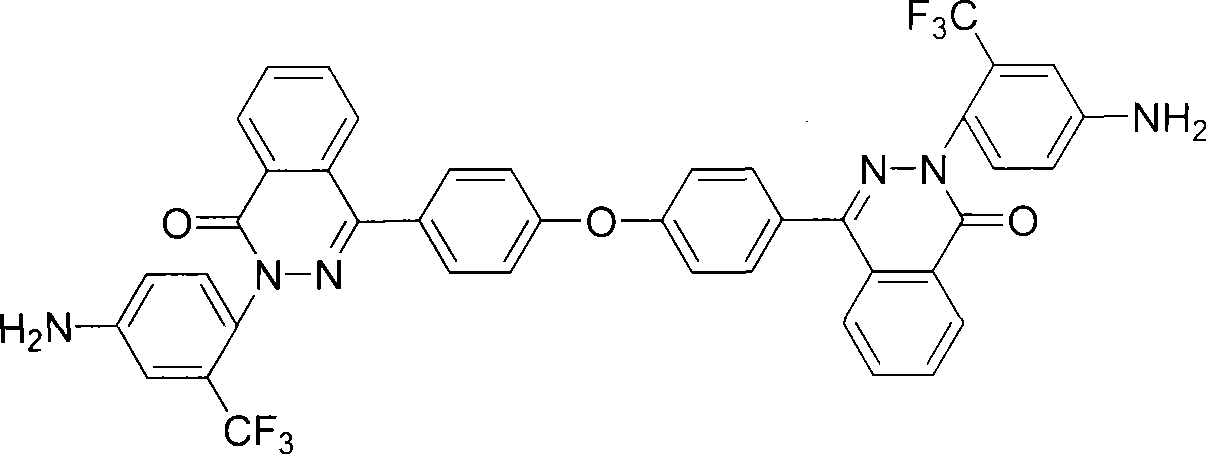
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Phthalazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phthalazine, also called benzo-orthodiazine or benzopyridazine, is a heterocyclic organic compound with the molecular formula C₈H₆N₂. It is isomeric with other naphthyridines including quinoxaline, cinnoline and quinazoline.
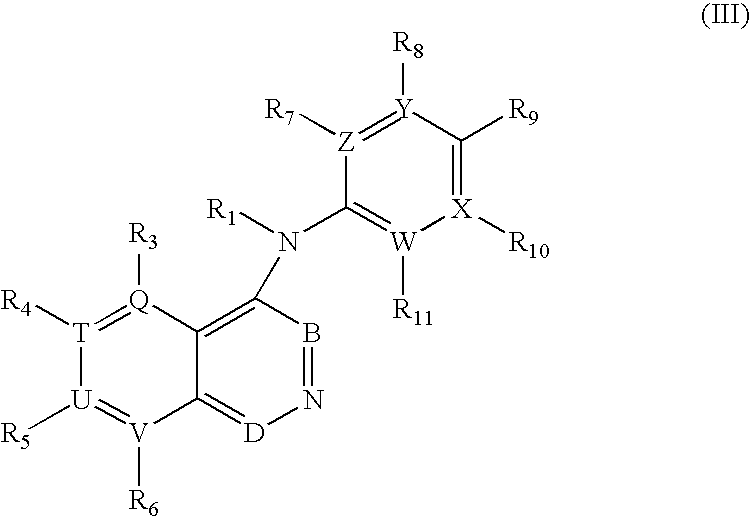
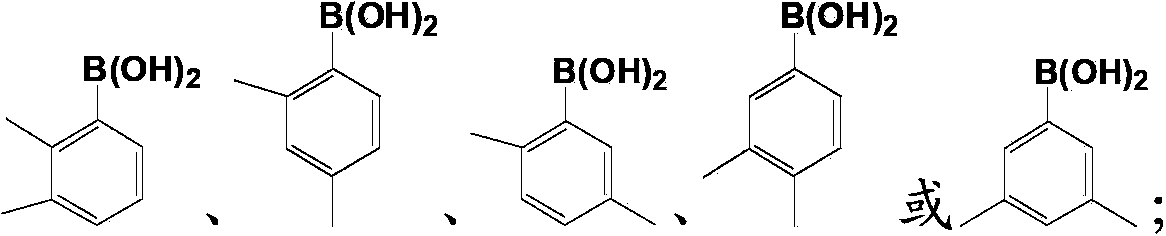
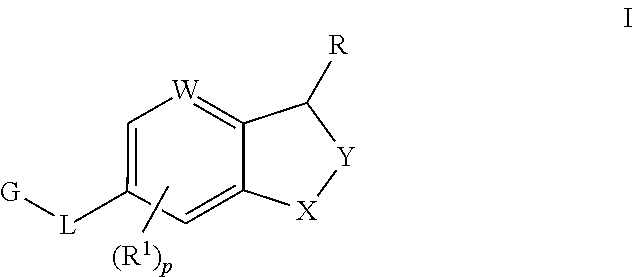
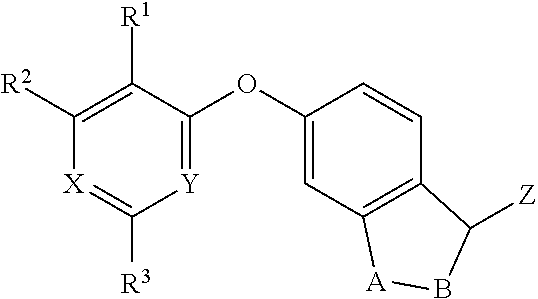
Substituted cycloocta[5,6]pyrido[4,3,2-de]phthalazines as PARP inhibitors
The present invention provides a compound of Formula (I):wherein the variables Z, n, Y and p are as defined herein, and pharmaceutically acceptable salts thereof, which can inhibit the activity of poly (ADP-ribose)polymerases, and pharmaceutical compositions comprising the same.
Owner:BEIGENE
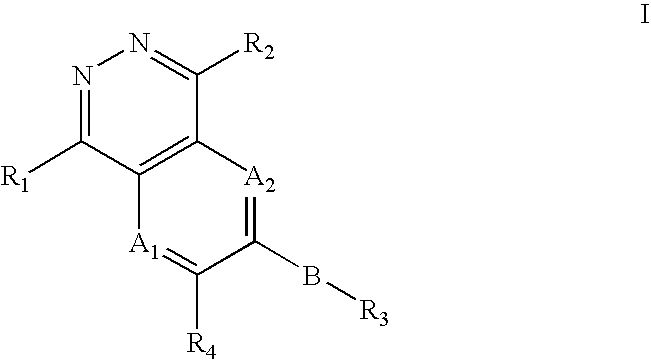
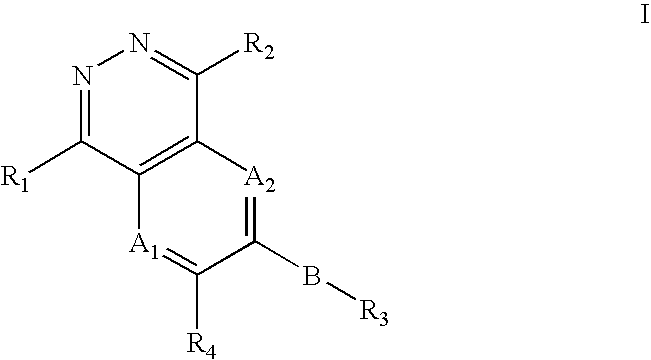
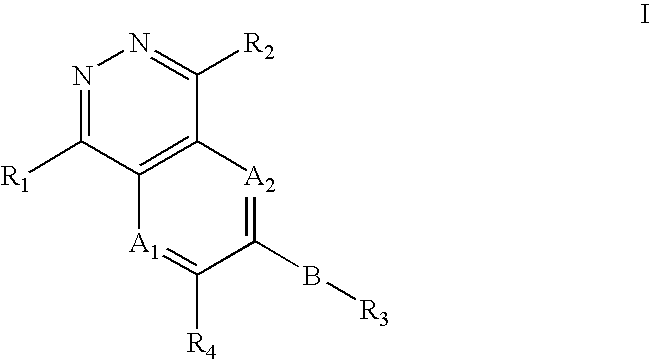
Phthalazine, aza- and diaza-phthalazine compounds and methods of use
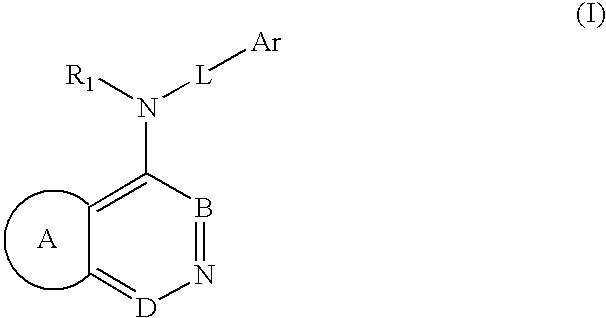
The present invention comprises a new class of compounds useful for the prophylaxis and treatment of protein kinase mediated diseases, including inflammation and related conditions. The compounds have a general Formula I wherein A1, A2, B, R1, R2, R3 and R4 are defined herein. The invention also comprises pharmaceutical compositions including one or more compounds of Formula I, uses of such compounds and compositions for treatment of kinase mediated diseases including rheumatoid arthritis, psoriasis and other inflammation disorders, as well as intermediates and processes useful for the preparation of compounds of Formula I.
Owner:AMGEN INC
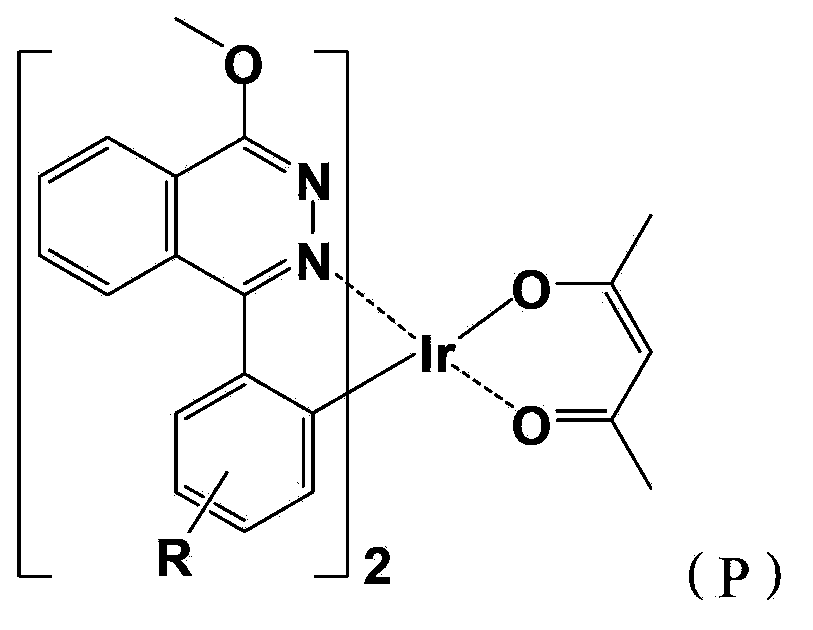
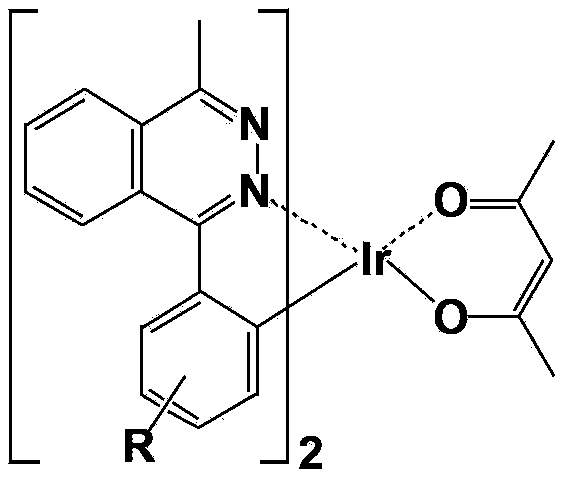
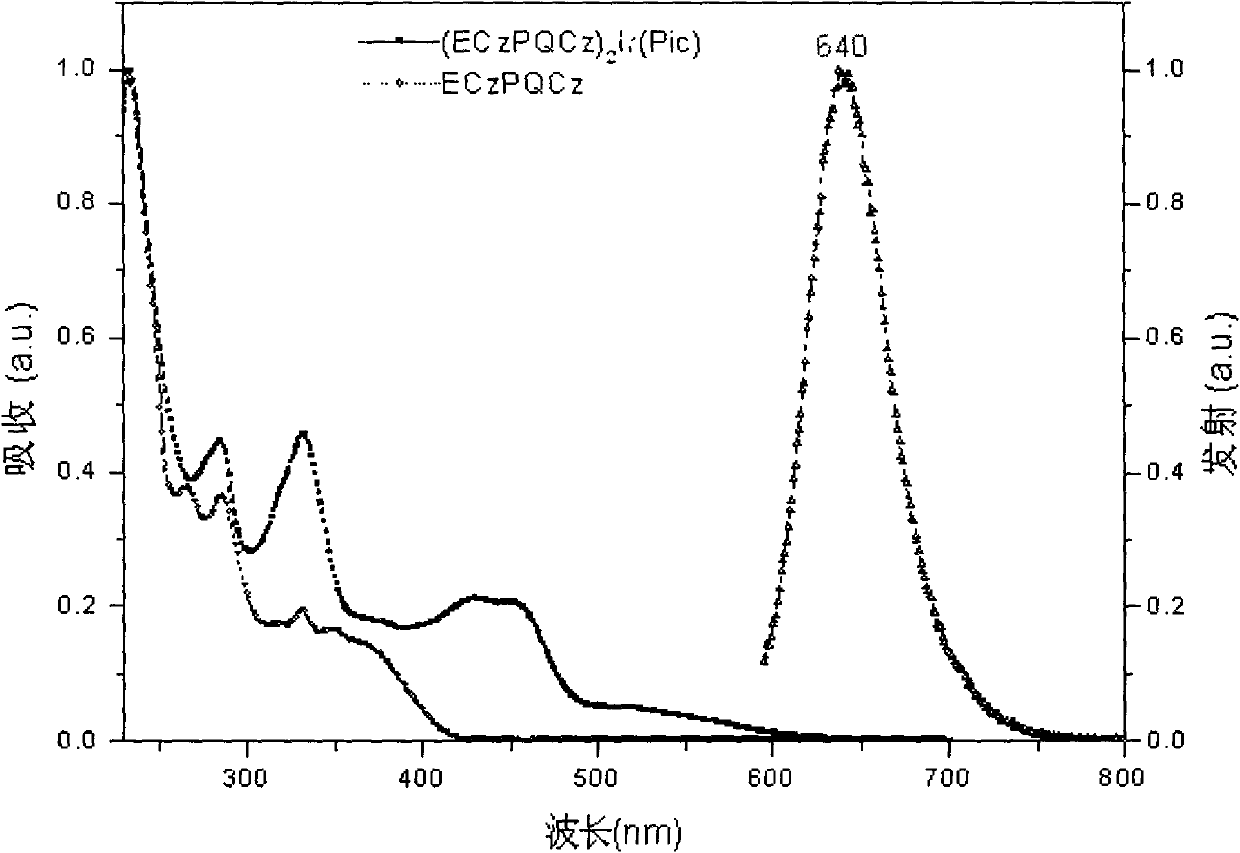
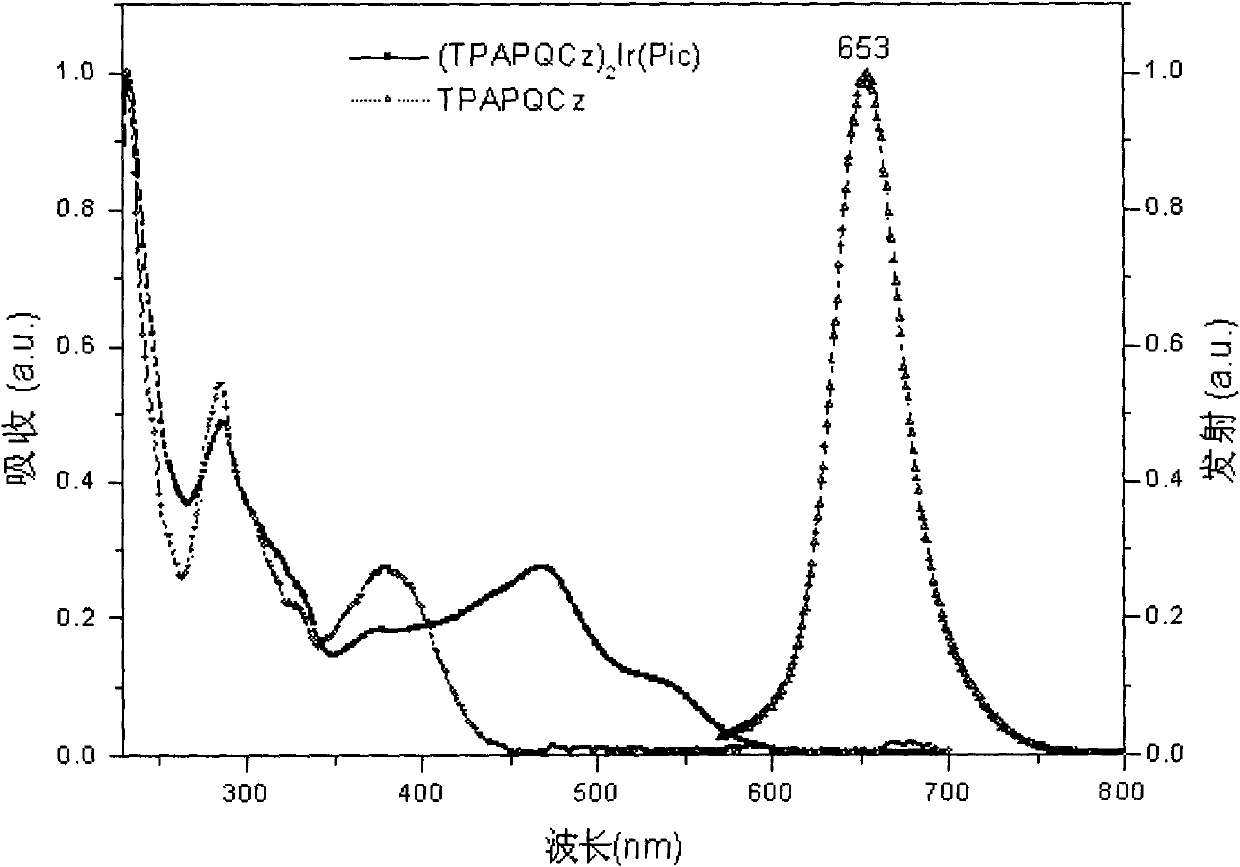
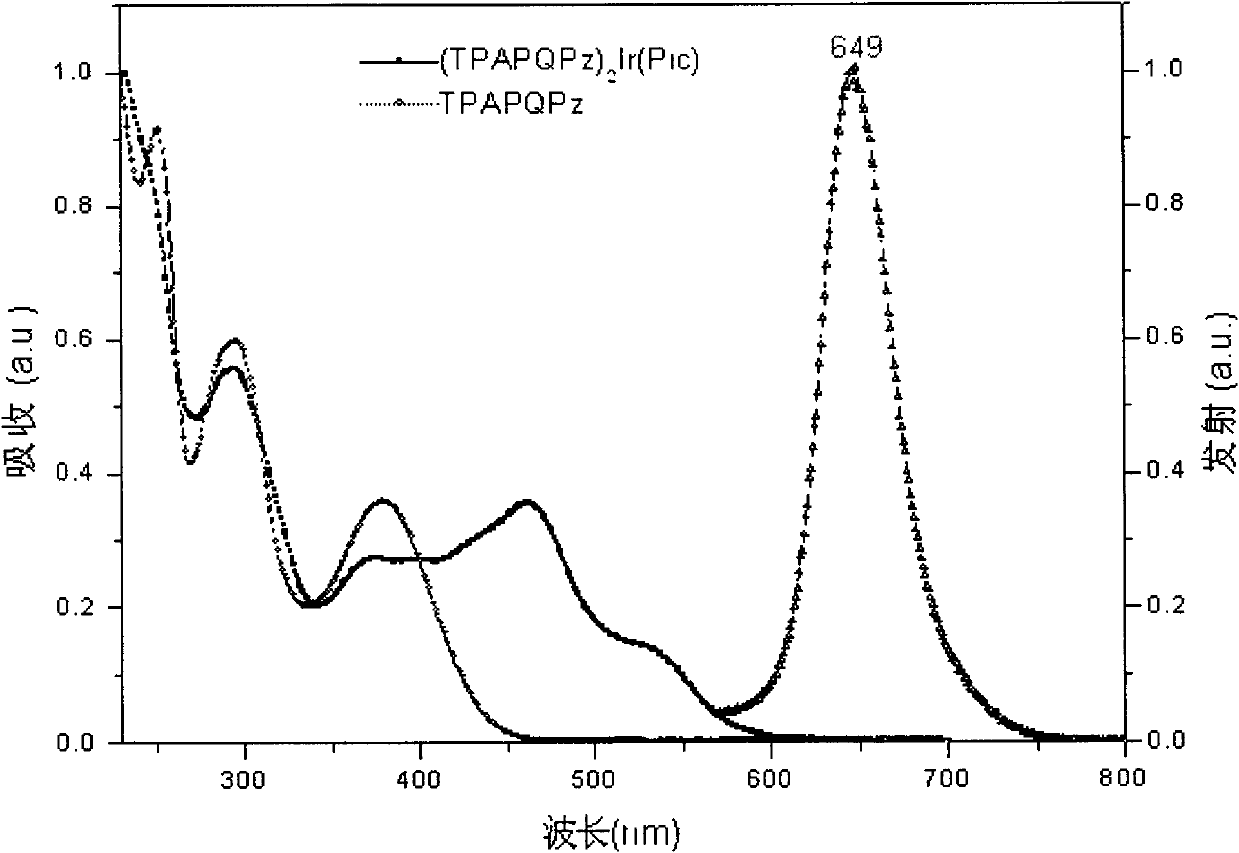
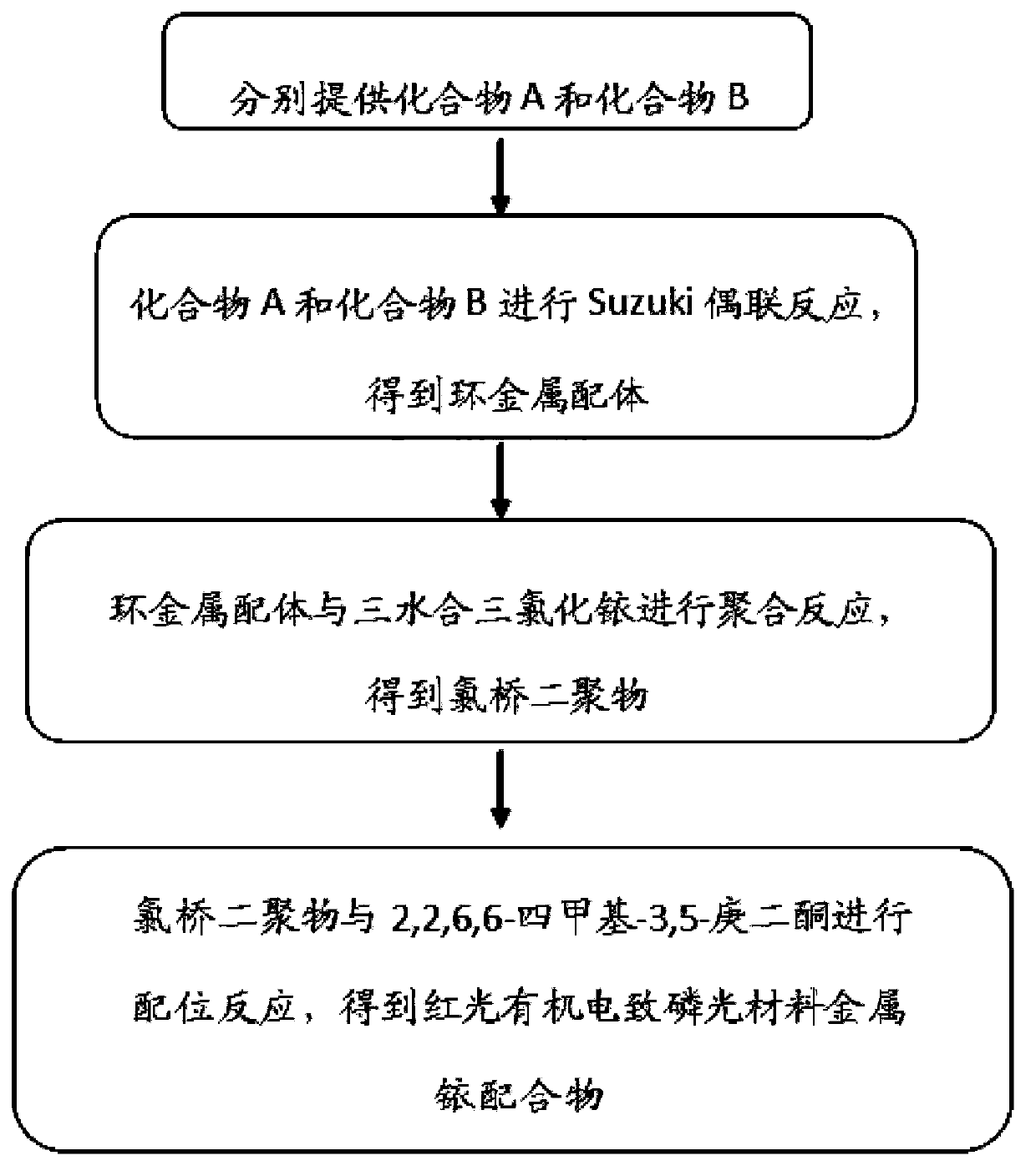
Iridium complex phosphor material taking phthalazine derivative as ligand and preparation method thereof
InactiveCN102180909AImprove solubilityImprove hole transport abilityGroup 8/9/10/18 element organic compoundsLuminescent compositionsQuantum yieldIridium
The invention discloses an iridium complex phosphor material taking a phthalazine derivative as a ligand. The structure of the material is shown as formulas (I) and (II), wherein Ar represents aryl, substituted aryl, heterocycle aryl and substituted heterocycle aryl; R can be one of a halogen atom, alkyl, substituted alkyl, alkoxyl, aryloxy, an alkylthio group, arylthio, aromatic amino, aliphatic amino or a heterocyclic substituent; L^Y can be one of N-COOH, 8-hydroxyquinoline, beta-diketone, N^NH and the like; N^N can be bipyridyl, diquinoline, 1,10-phenanthroline, a derivative of the 1,10-phenanthroline and the like; and Z can be hexafluorophosphate and perchlorate. A dicyclic or ionic iridium complex phosphorescent electroluminescent device taking the phthalazine derivative as the ligand obtained with the preparation method has high internal and external quantum yields, high luminance and high stability. A dicyclic iridium complex is shown as a formula (I), i.e., (C^N)2Ir(L^Y); and an ionic iridium complex is shown as a formula (II), i.e., (C^N)2Ir(N^N)<+>Z<->.
Owner:NANJING UNIV OF POSTS & TELECOMM
Indole, azaindole and related heterocyclic N-substituted piperazine derivatives
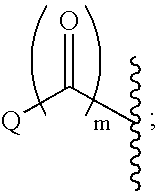
This invention provides compounds of Formula I, including pharmaceutically accceptable salts thereof, having drug and bio-affecting properties, their pharmaceutical compositions and method of use. These compounds possess unique antiviral activity, whether used alone or in combination with other antivirals, antiinfectives, immunomodulators or HIV entry inhibitors. More particularly, the present invention relates to the treatment of HIV and AIDS. The compounds of Formula I have the formula wherein: Z is Q is selected from the group consisting of m is 2; A is selected from the group consisting of cinnolinyl, napthyridinyl, quinoxalinyl, pyridinyl, pyrimidinyl, quinolinyl, isoquinolinyl, quinazolinyl, azabenzofuryl, and phthalazinyl each of which may be optionally substituted with one or two groups independently selected from methyl, methoxy, hydroxy, amino and halogen; and —W— is
Owner:VIIV HEALTHCARE UK (NO 5) LTD
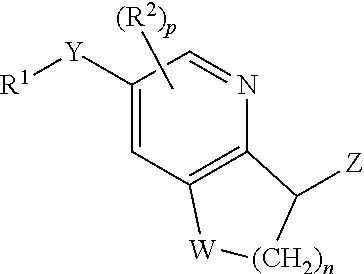
Processes of synthesizing dihydropyridophthalazinone derivatives
Provided herein are processes for synthesizing dihydropyridophthalazinone derivatives, such as for example, 5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one and its stereoisomers, which are potent poly(ADP-ribose)polymerase (PARP) inhibitors as well as novel synthetic intermediate compounds.
Owner:MEDIVATION TECH INC
Modulation of cell fates and activities by diketo phthalazines
Phthalazine diones that function as intracellular redox modulators and buffers are used to treat stressed cells in various disease states in which the intracellular redox status is impaired. By optimal buffering of aberrant redox states, phthalazine diones enhance the cellular processes essential for survival and augment the conventional or other external therapies necessary for treatment. The phthalazine diones of the invention thus regulate cell growth, differentiation, or death to serve as essential adjunctive therapy for the stressed host in various disease states.
Owner:BACH PHARMA
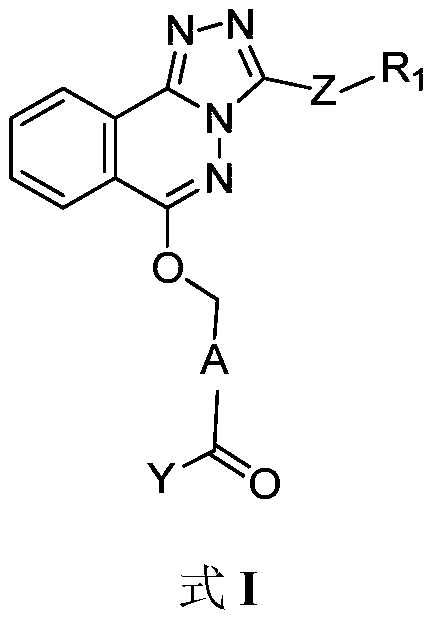
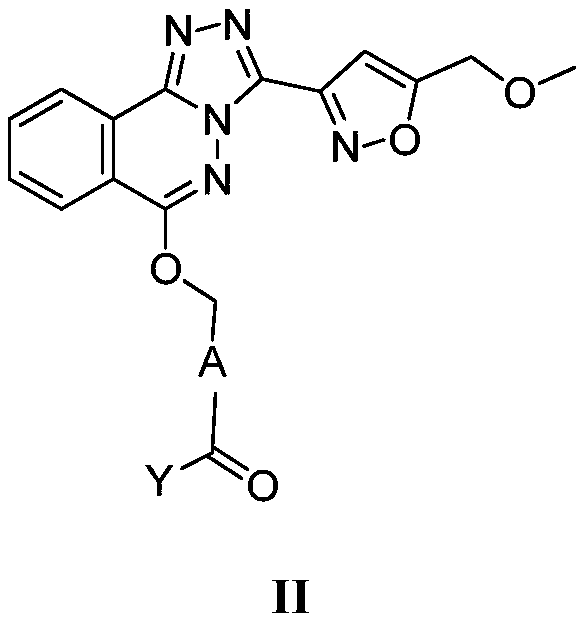
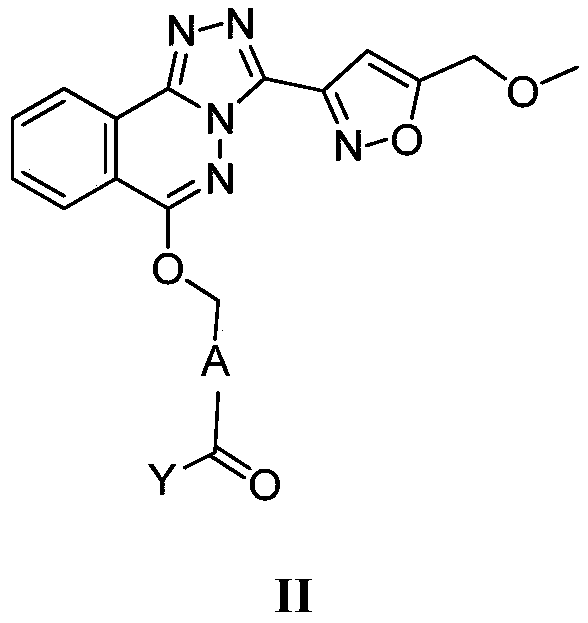
Phthalazine isoxazole alkoxy derivative, preparation method, pharmaceutical composition and uses thereof
ActiveCN110256440AGood inverse agonismOrganic active ingredientsNervous disorderEnantiomerDiastereomer
The present invention discloses a phthalazine isoxazole alkoxy derivative, a preparation method, a pharmaceutical composition and uses thereof, and provides a compound represented by a formula I, a cis-trans isomer, an enantiomer, a diastereomer, a racemate, a solvate, a hydrate, a pharmaceutically acceptable salt or a prodrug thereof. According to the present invention, the compound has good inverse agonistic effect on alpga5-GABAA.
Owner:SHANGHAI SIMR BIOTECHNOLOGY CO LTD +1
Novel block anion exchange membrane and preparation method thereof
InactiveCN105906812AHigh conductivity free volume increaseIncreased free volumeFuel cellsPolymer scienceFuel cells
The invention discloses a novel block anion exchange membrane. A block main chain of the anion exchange membrane contains a bending unit (biphenyl fluorene) and a twisting unit (phthalazinone); according to the structure, a non-coplanar effect can be enhanced. A preparation method of the anion exchange membrane comprises the following steps of performing polycondensation on decafluorobiphenyl (slightly excessive) and bisphenol fluorine to prepare an oligomer 1; using the decafluorobiphenyl and 4-(4-hydroxyphenyl)-2,3-phthalazine-1-one to prepare an oligomer 2; polymerizing the oligomer 1 with the oligomer 2 to obtain a polymer main chain, and performing ionization treatment, membrane casting and alkali treatment on a chloromethylation main chain to obtain the anion exchange membrane. The anion exchange membrane has better chemical stability, and higher electric conductivity and resistance to swelling, and is suitable for being applied in the aspect of alkaline fuel cells.
Owner:DALIAN UNIV OF TECH
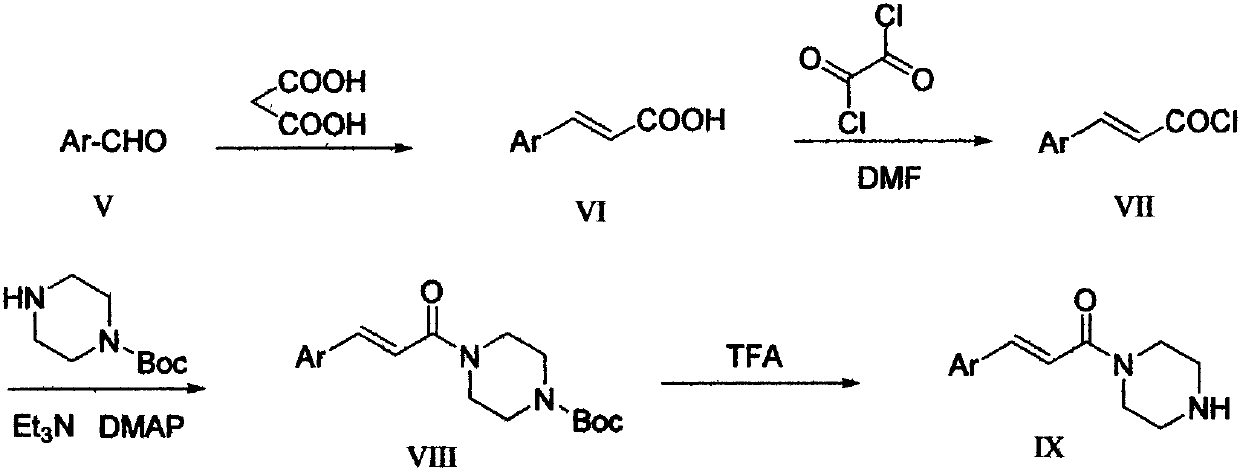
Synthetic technology of azelastine hydrochloride
The invention discloses a synthetic technology of azelastine hydrochloride, which is characterized in that acylhydrazone is formed by N-Methylhexahydroazepin-4-one hydrochloride and benzoyl hydrazine and then is reduced with potassium borohydride, and finally is condensed with 2-(para chlorobenzene acetyl) benzoic acid into 4-( chlorine benzyl)-2-piperazidine-1- methyl-1H-azelastine-4- radical)-1-(2H)-phthalazine hydrochloride. The synthetic technology has the advantages of simple process and higher yield.
Owner:GUIZHOU YUNFENG PHARMA
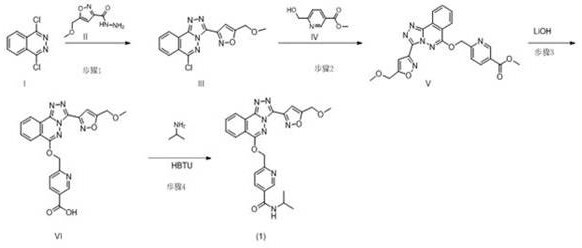
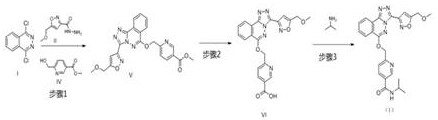
Process for preparation of substituted nicotinamides
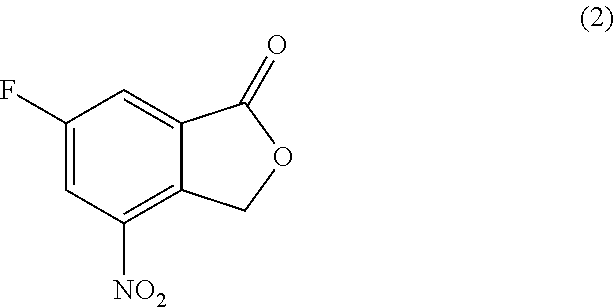
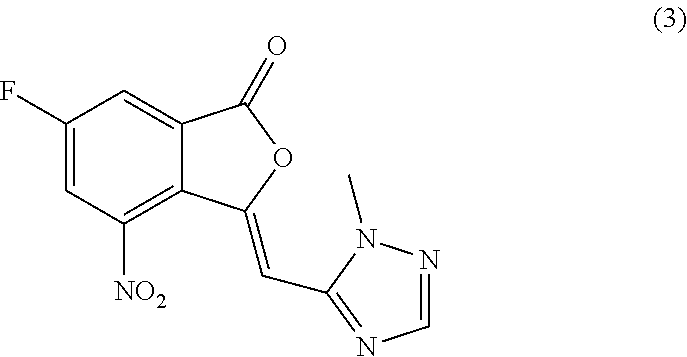
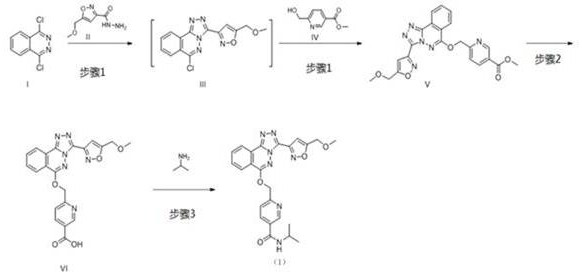
The invention relates to a method for preparing substituted nicotinamide of a formula (1), which comprises the following steps: step 1): 1, 4-dichlorophthalazine of a formula (I), a compound of a formula (II) and a compound of a formula (IV) are used as starting raw materials to react, and a compound of a formula (V) is obtained by a one-pot method; the preparation method comprises the following steps of 1, carrying out a saponification reaction on a compound shown in a formula (V) to obtain a compound shown in a formula (VI), and 2, carrying out a saponification reaction on the compound shown in the formula (V) to obtain a compound shown in a formula (VI), and 3, carrying out a reaction on the compound shown in the formula (VI) and isopropylamine in the presence of a condensing agent to obtain N-isopropyl-6-(((3-(5-(methoxymethyl) isoxazole-3-yl)-[1, 2, 4] triazole [3, 4-a] phthalazin-6-yl) oxygen) methyl) nicotinamide shown in a formula (1).
Owner:SHANGHAI SIMR BIOTECHNOLOGY CO LTD
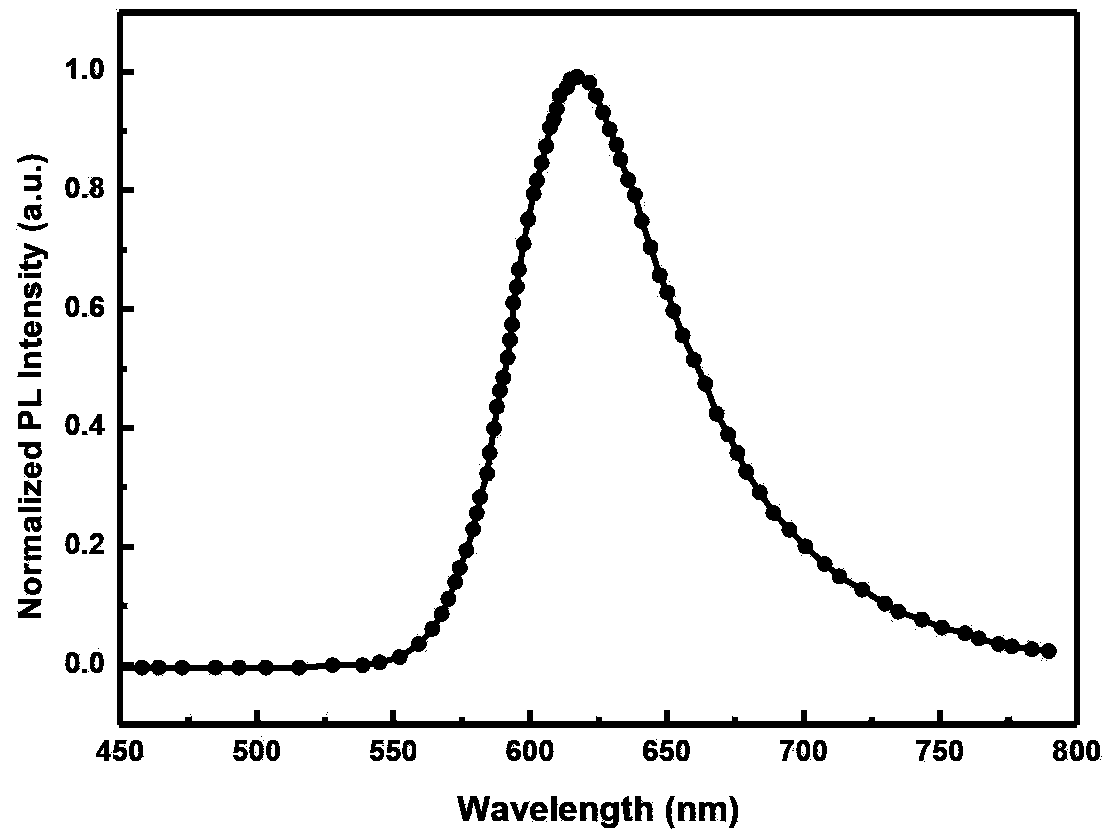
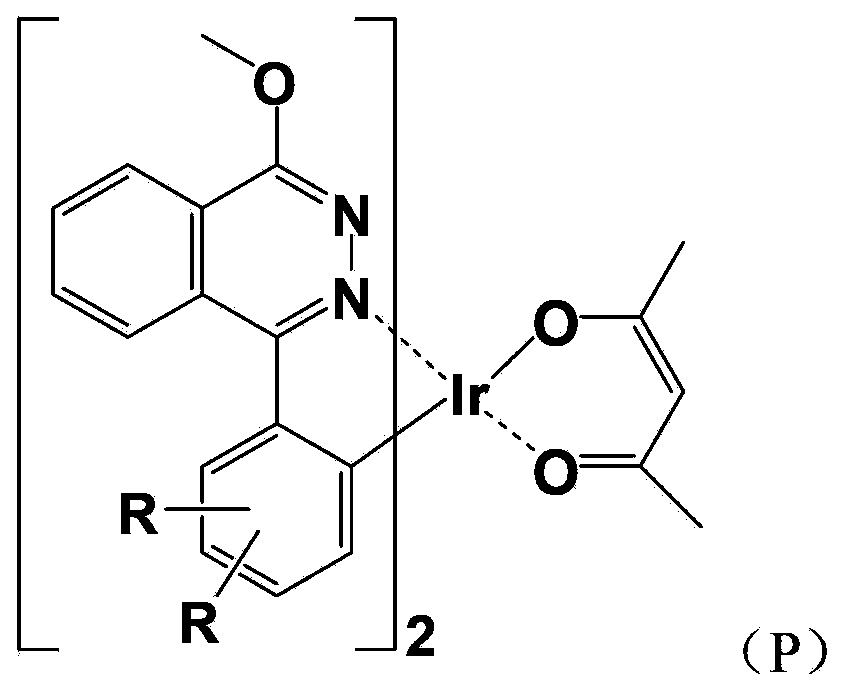
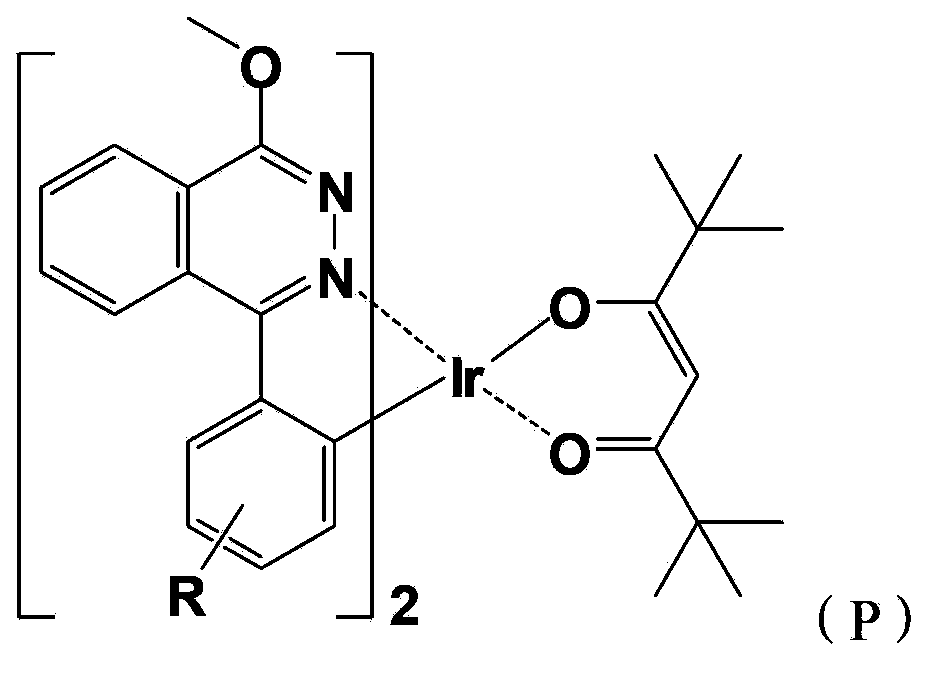
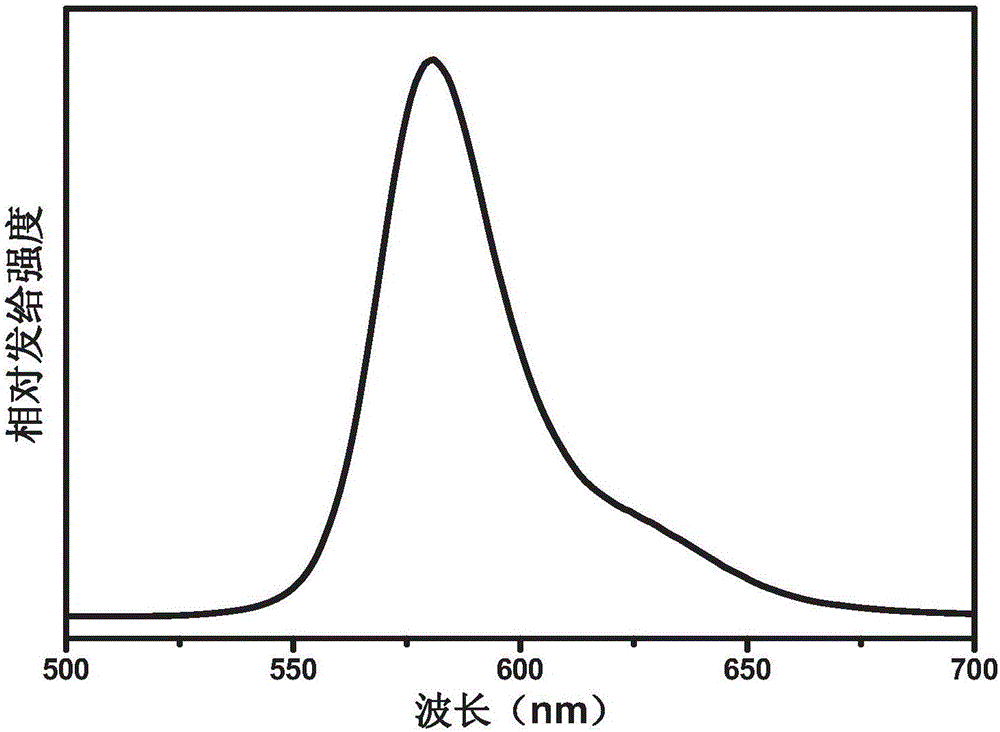
Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
InactiveCN103965261AProperly adjust the emission wavelengthImprove luminous performanceGroup 8/9/10/18 element organic compoundsSolid-state devicesIridiumCoordination type
The present invention discloses a red light organic electrophosphorescence material metal iridium coordination compound having a structural formula represented by a formula (P), wherein R is methyl. The present invention further provides an organic electroluminescent device containing the metal iridium coordination compound. According to the present invention, 1-methoxy-4-phenyl phthalazine is adopted as the ring metal ligand main structure, and acetylacetone is adopted as the heterotypic auxiliary ligand to synthesize the heterotypic coordination type metal iridium coordination compound having high red luminescence color purity so as to reduce the synthesis difficulty and the purification difficulty of the metal iridium coordination compound; and the chemical modification method that the two methyl groups are introduced on different C sites of the benzene ring in the ring metal ligand is adopted to achieve adjustment on the material luminescence color so as to make the metal iridium coordination compound phosphorescence emission spectrum produce red shift.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Copolyaramide containing phthalazine biphenyl structure and its preparation method
InactiveCN1513898AEasy to withstand high temperature and long-term use requirementsMeet the requirements of high temperature resistance and long-term useSolubilityAlkaline earth metal
A refractory and soluble copolyarylamide is prepared from the moldifier which is the mixture of a novel diamine and the diamine H2N-Ar-X-Ar-NH2, and poly-p-phenyl diformyl p-pheyldiamine (PPTA) through directly condensation or low-temp solution polymerizing in the solution of the salt of alkali metal or alkali-earth metal. Its advantages are high vitrification temp and inherent viscosity, and better solubility, mechanical performance and thermal stability.
Owner:DALIAN UNIV OF TECH +2
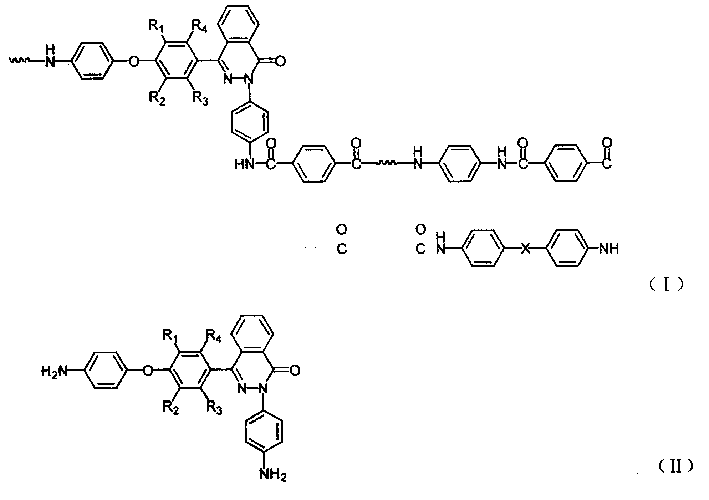
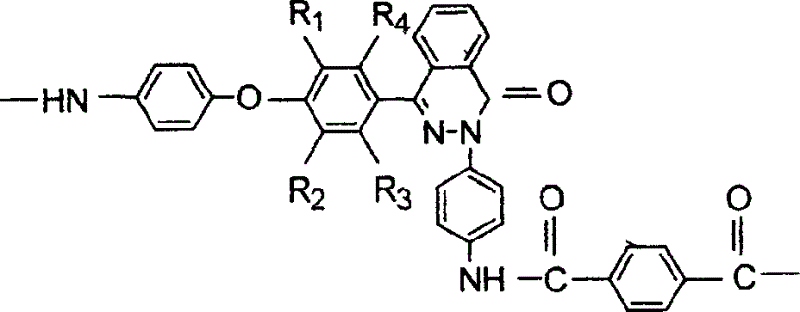
Method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone
ActiveCN104876932AIncrease acidityHigh catalytic activityOrganic chemistryFiltrationSynthesis methods
The invention discloses a method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone, and belongs to the technical field of ionic liquid catalysis. The molar ratio of aromatic aldehyde, phthalylhydrazine and 5,5-dimethyl-1,3-cyclohexanedione in synthesis reaction is 1 to 1 to 1; the molar quantity of an acidic ionic liquid catalyst is 3%-7% of used phthalylhydrazine; the volume dose of a reaction solvent based on milliliter is 3-5 times of the molar quantity of the phthalylhydrazine based on millimole; and the method comprises the following steps: carrying out reflux reaction for 8-30 minutes; ending reaction, cooling to a room temperature, and separating out a lot of solids; carrying out suction filtration, washing residues with ethanol, and drying in vacuum to obtain a product. Compared with a synthesis method employing other acidic ionic liquid catalysts, the method has the characteristics of being high in catalytic activity of the catalyst, good in biodegradability and relatively in low preparation cost; the overall synthesis process is simple and convenient to operate; and industrialized large-scale application is facilitated.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Phthalazine-containing antidiabetic compounds
This invention provides for certain phthalazine-containing compounds of the formula (I) or a pharmaceutically acceptable salt, ester or solvate thereof, wherein G is an optionally substituted N—N containing heteroaryl group and the variables are defined herein; the inventive compounds are agonists of the G-protein coupled receptor 40 (GPR40, also known as free fatty acid receptor FFAR). This invention further relates to pharmaceutical compositions containing these compounds, and the use of these compounds to regulate insulin levels in a mammal. The compounds may be used, for example in the prevention and treatment of Type 2 diabetes mellitus and in the prevention and treatment of conditions related to Type 2 diabetes mellitus, such as insulin resistance, obesity and lipid disorders.
Owner:MERCK SHARP & DOHME CORP
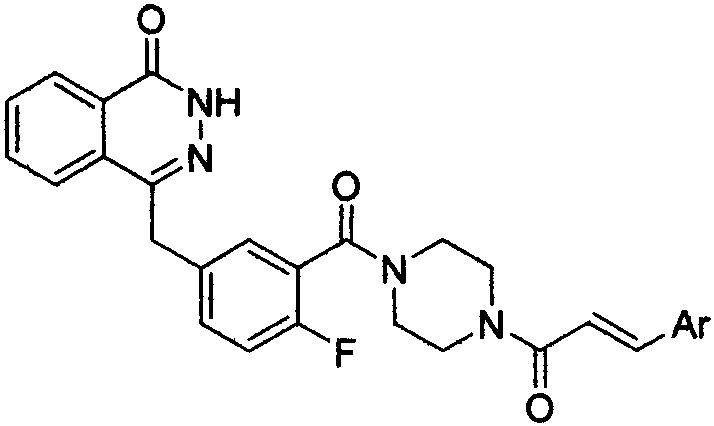
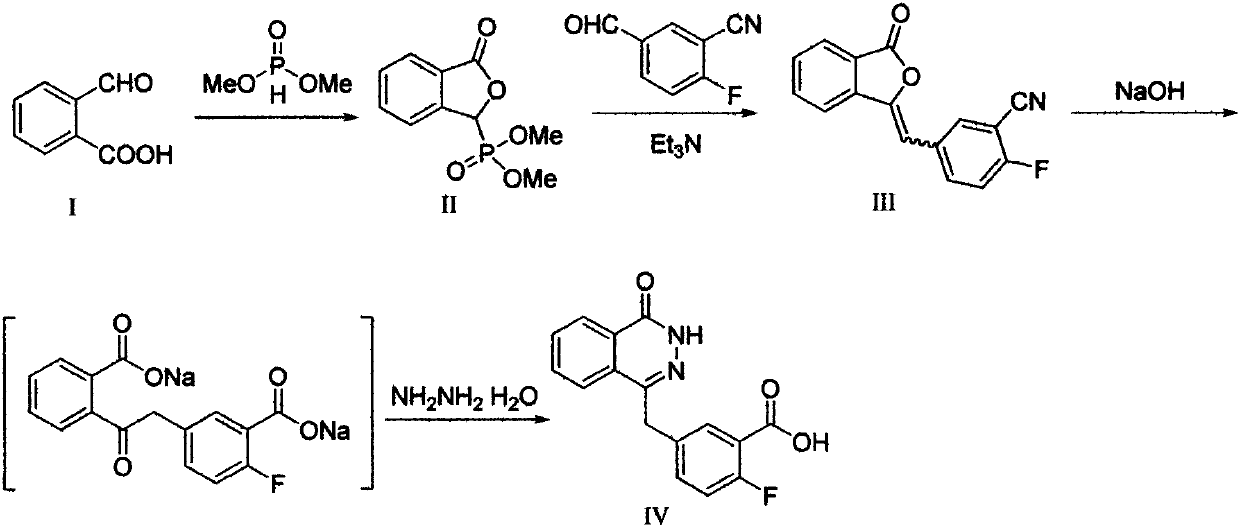
Pyridazinone derivative, and preparation method and medical application thereof
InactiveCN110272412AHigh activityEnhanced inhibitory effectOrganic chemistryAntineoplastic agentsBenzoic acidBenzaldehyde
The invention provides a pyridazinone derivative, and a preparation method and a medical application thereof. O-formylbenzoic acid used as a raw material reacts with dimethyl phosphite to obtain dimethyl (3-oxo-1,3-dihydroisobenzofuran-1-yl)phosphonate, the dimethyl (3-oxo-1,3-dihydroisobenzofuran-1-yl)phosphonate reacts with 3-cyano-4-fluorobenzaldehyde in the presence of triethylamine to prepare (Z,E)-2-fluoro-5-[(3-oxoisobenzofuran-1(3H)-ylidene)methyl]benzonitrile, and the (Z,E)-2-fluoro-5-[(3-oxoisobenzofuran-1(3H)-ylidene)methyl]benzonitrile is reduced by hydrazine hydrate to prepare 2-fluoro-5-[(4-oxo-3,4-dihydropyridazin-1-yl)methyl]benzoic acid; and benzaldehyde or substituted aromatic formaldehyde or furfural used as a raw material and malonic acid undergo a Knoevenagel reaction to obtain cinnamic acid or substituted cinnamic acid or furan-2-acrylic acid, the cinnamic acid or substituted cinnamic acid or furan-2-acrylic acid and 1-tert-butoxycarbonylpiperazine undergo an amidation reaction, a tert-butoxycarbonyl group is removed from the obtained amidation product in the presence of trifluoroacetic acid, and the obtained product and the 2-fluoro-5-[(4-oxo-3,4-dihydropyridazin-1-yl)methyl]benzoic acid undergo the amidation reaction to obtain a series of (E)-4-{3-[4-[(3-substituted aryl)acryloyl]piperazin-1-carbonyl]-4-fluorobenzyl}-2H-pyridazin-1-one derivatives. Results of preliminary pharmacological activity screening show that the compound represented by a general formula shown in the present invention has a certain in-vitro PARP-1 inhibition ability and a certain in-vitro tumor cell proliferation resisting activity. The structural general formula of compound is shown in the description; and in the general formula, Ar is selected from two formulas also shown in the description, and R1, R2, R3, R3, R4 and R5 can be the hydrogen atom, the fluorine atom, the chlorine atom, the bromine atom, a methyl group, a methoxy group, a tetrafluoromethyl group and a nitro group.
Owner:WUHAN UNIV OF SCI & TECH
Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
InactiveCN103965259AIncreased steric hindranceThe synthesis process is simpleGroup 8/9/10/18 element organic compoundsSolid-state devicesIridiumTriplet state
The present invention discloses a red light organic electrophosphorescence material metal iridium coordination compound having a structural formula represented by a formula (P), wherein R is hydrogen atom or methyl. The present invention further provides a preparation method for the red light organic electrophosphorescence material metal iridium coordination compound, and an organic electroluminescent device containing the red light organic electrophosphorescence material metal iridium coordination compound. According to the present invention, 1-methoxy-4-phenyl phthalazine is adopted as the main ligand, and 2,2,6,6-tetramethyl-3,5-heptanedione is added to the ring main ligand as the auxiliary ligand so as to increase the steric hindrance effect of the red light organic electrophosphorescence material metal iridium coordination compound, such that the direction effect between the metal atoms is reduced, the self-quenching phenomenon of the triplet state excitons is reduced, and the luminescence efficiency of the organic electroluminescent device is increased.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Iridium complexes, preparation method thereof, and electroluminescent devices using the iridium complexes
InactiveCN106496277AImprove luminous efficiencyHigh electron mobilityIndium organic compoundsSolid-state devicesIridiumLuminous intensity
The invention relates to iridium complexes, wherein 2-(5-phenyl-1,3,4-oxadiazole-2-)phenol or 2-(5-(3-pyridine)-yl-1,3,4-oxadiazole-2-)phenol is emplyoed as an auxiliary ligand; and a derivative of any one of 2-(4,6-ditrifluoromethylpyridine-3-)quinoline, 2-(4,6-ditrifluoromethylpyridine-4-)quinoline, 2-(4,6-ditrifluoromethylpyridine-3-)isoquinoline, 2-(4,6-ditrifluoromethylpyridine-4-)isoquinoline, 2-(4,6-ditrifluoromethylpyridine-3-)quinazoline, 2-(4,6-ditrifluoromethylpyridine-4-)quinazoline, 2-(4,6-ditrifluoromethylpyridine-3-)phthalazine and 2-(4,6-ditrifluoromethylpyridine-4-)phthalazine is employed as a main ligand. The luminous intensity and efficiency of the iridium complexes, within the wavelength range of red light, can be regulated.
Owner:AAC MICROTECH CHANGZHOU +1
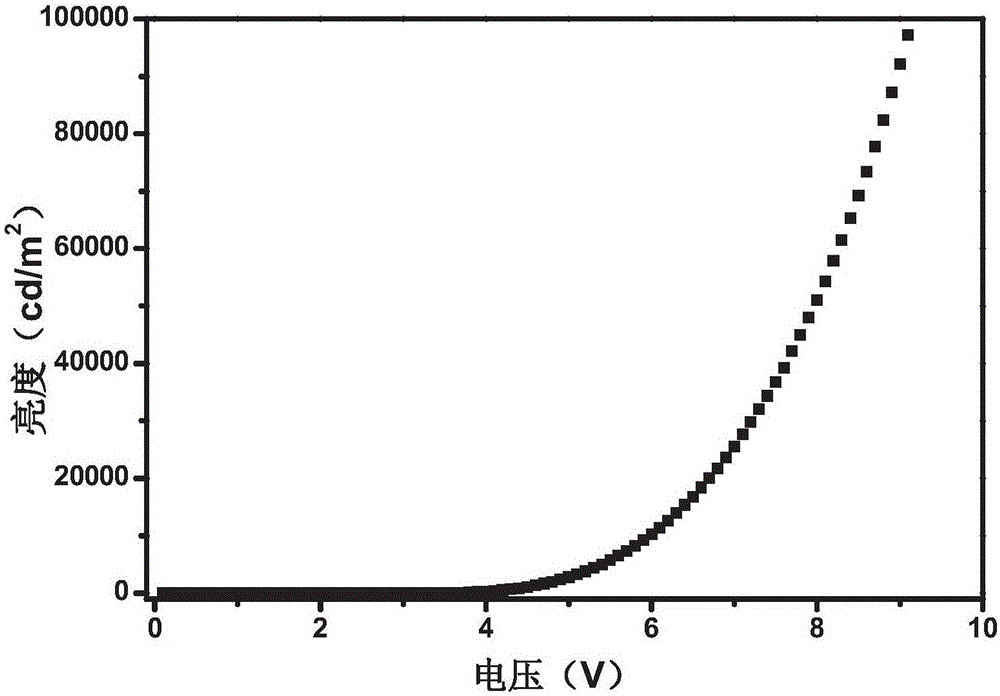
Application of compound 6-(4-chlorophenoxy)-tetrazolo [5,1-a] phthalazine to preparation of medicine for treating depression
The invention relates to the use of a compound (shown in formula I) as a medicine for treating depression.
Owner:JILIN YINGLIAN SHANGDE SCI & TECH DEV
Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound
InactiveCN105461723ACan induce apoptosisEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryApoptosisHuman gastric carcinoma
The invention discloses a phthalizine [1,2,b] quinazoline-8-ketone compound, which is characterized in that the structural general formula of the compound is shown in the description, and phthalizine [1,2,b] quinazoline-8-ketone derivatives disclosed by the invention have very strong inhibiting effect on common cancer cells such as human gastric carcinoma cells (MGC-803), human lung cancer cells (NCI-H460), human hepatoma cells (HepG-2), cervical carcinoma cells (Hela), human bladder cancer cells (T-24) and the like, can induce apoptosis of the cancer cells, hence the phthalizine [1,2,b] quinazoline-8-ketone and derivatives thereof have potential application in preparation of antitumor drugs.
Owner:GUANGXI NORMAL UNIV
Phthalazinopyrrole compound and preparation method thereof
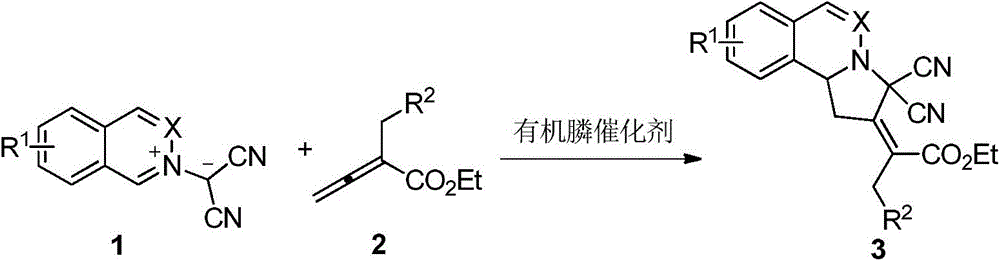
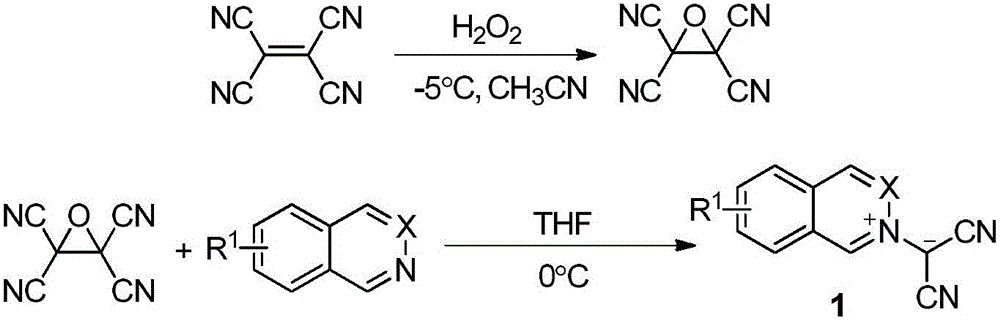
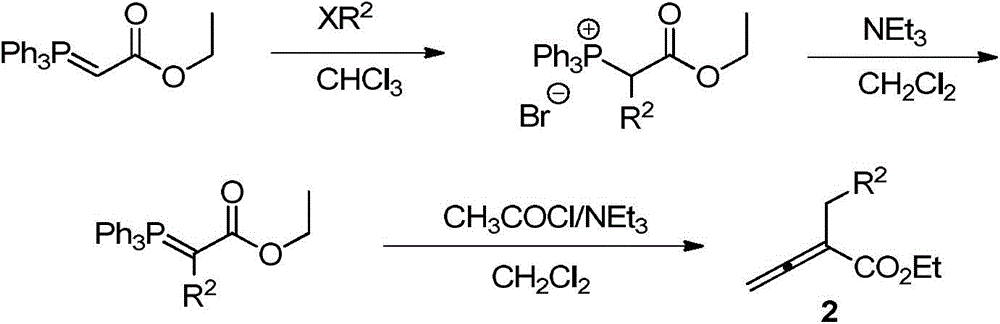
ActiveCN105669678AAtom economy reactionDoes not involve transition metal catalysisOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventNitrogen
The invention discloses a phthalazinopyrrole compound and a preparation method thereof. The phthalazinopyrrole compound has potential antibacterial, anti-inflammatory and anti-tumour activities. The preparation method of the compound comprises the following steps: under the nitrogen protection condition, dissolving phthalazine derived 1,3-dipole and allene ester into an organic solvent, adding an organic phosphine catalyst, stirring, and carrying out cycloaddition reaction, so that the phthalazinopyrrole compound can be obtained.
Owner:CHINA AGRI UNIV
Aromatic diamines monomer with double miscellaneous naphthalenone structures and trifluoromethyl substituted and preparation thereof
The invention relates to an aromatic diamines monomer with double miscellaneous naphthalenone structures and trifluoromethyl substituted and a preparation method thereof. A diamine structure is prepared according to the following steps: (1), 4,4'-oxygen-bis(4-(1,4-phenyl)-phthalazine-1-ketone) reacts with 2-chlorine-5-nitro benzotrifluoride under an alkaline condition according to a molar ratio of 1 to 2, and then a dinitro compound is acquired; and (2), the dinitro compound is reduced in organic solvent by palladium-charcoal and hydrazine hydrate, and then the monomer is acquired; and the monomer can be used to prepare polyimide and aromatic polyamides. The prepared aromatic diamines with double miscellaneous naphthalenone structures and trifluoromethyl substituted has high purity, and is steady at normal temperature; and the polyimide and the aromatic polyamides prepared by the aromatic diamines monomer have excellent comprehensive properties such as good solubility and film forming capability, strong high temperature resistance and excellent mechanical properties, high optical transparency, low dielectric constant and the like.
Owner:DONGHUA UNIV
Heat developing emulsion and material contg. 2,3-phthalazine compound
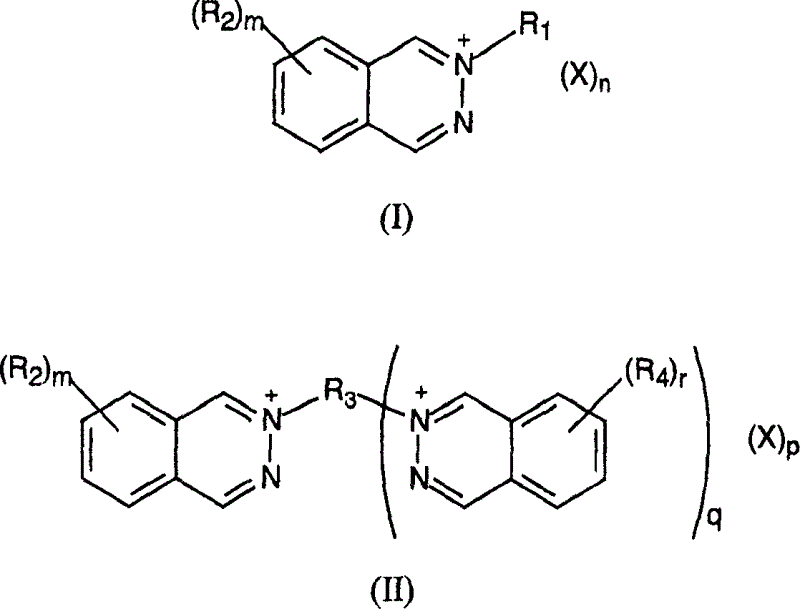
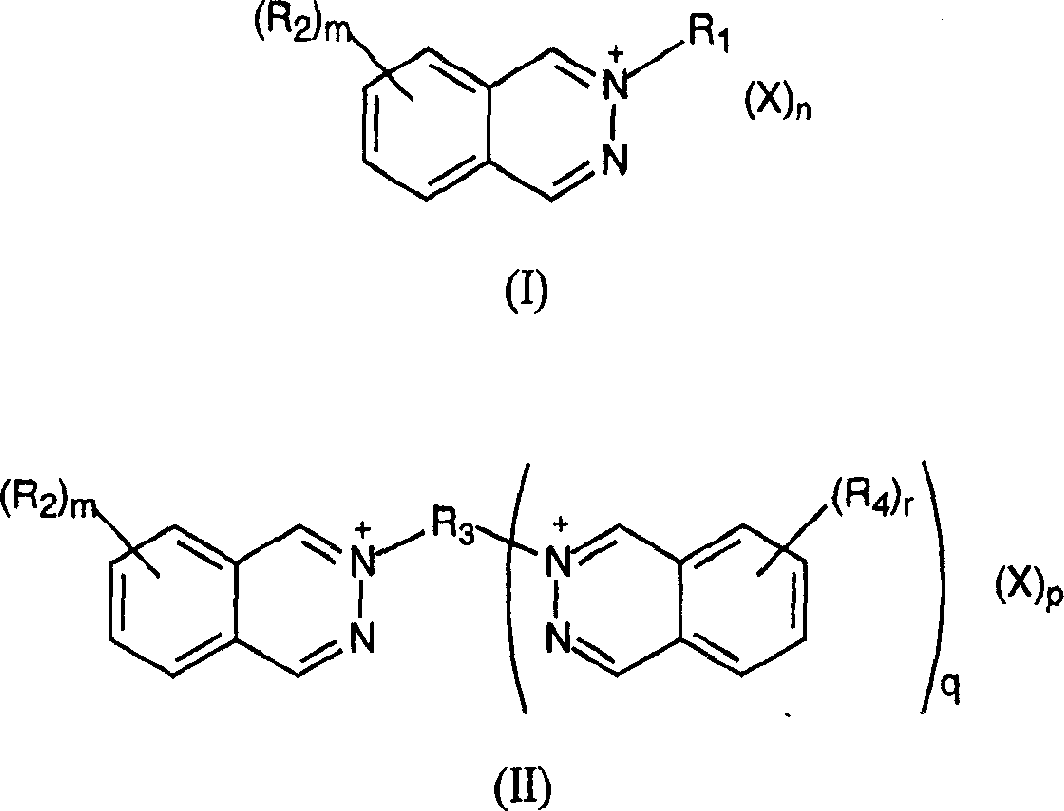
This invention discloses thermally developable compositions such as thermal and photothermographic emulsions which include certain quaternary phthalazine compounds. These emulsions are useful in thermally developable materials, such as thermosensitive materials and photothermographic materials, whereby improved sensitometric and postprocessing properties can be obtained. Such materials can have imaging layers on one or both sides of the support.
Owner:EASTMAN KODAK CO
Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
InactiveCN103965260AReduce the difficulty of synthesisHigh red luminous saturationGroup 8/9/10/18 element organic compoundsSolid-state devicesIridiumHydrogen atom
The present invention discloses a red light organic electrophosphorescence material metal iridium coordination compound having a structural formula represented by a formula (P), wherein R is hydrogen atom or methyl. The present invention further provides a preparation method for the coordination compound, and an organic electroluminescent device containing the coordination compound. According to the present invention, 1-methoxy-4-phenyl phthalazine having large plane rigidity is adopted as the main ligand so as to balance charge transmission of the organic electroluminescent device, and acetylacetone is added to the ring metal ligand as the heterotypic auxiliary ligand so as to reduce the synthesis difficulty of the metal iridium coordination compound, and easily synthesize and purify the product.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
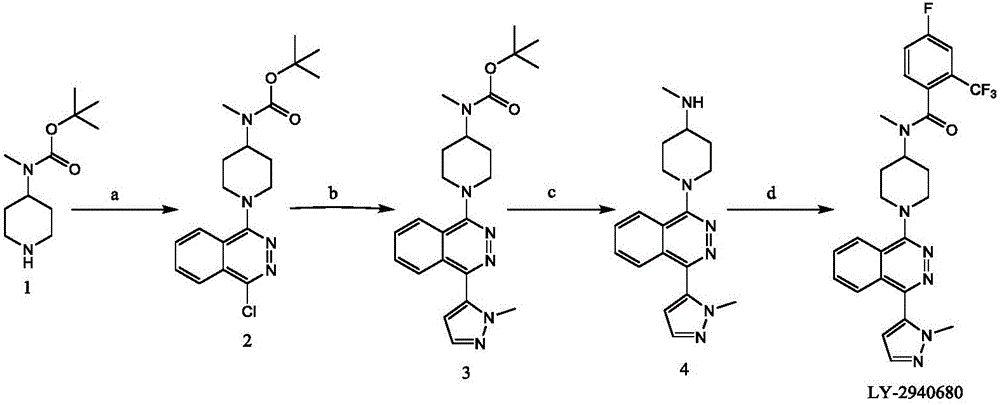
Synthetic method of Taladegib
The invention discloses a synthetic method of Taladegib. The synthetic method is characterized by comprising the following steps that reductive amination is carried out on N-benzyl-4-piperidone to obtain a compound in formula VI; in the presence of an organic base, an acylation reaction is carried out on the compound in the formula VI and an acylation reagent to obtain a compound in the formula VIII; the acylation reagent is 4-fluoro-2-(trifluoromethyl)benzoyl chloride; in the presence of a hydrogen source and a palladium catalyst, debenzylation is carried out on the compound in formula VIII to obtain a compound in the formula IX; in the presence of an inorganic base, the compound in the formula IX is reacted with 1,4-dichloro phthalazine to obtain a compound in the formula X; in the presence of the inorganic base and the palladium catalyst, an SUZUKI coupling reaction is carried out on the compound in the formula X and 1-methyl-1H-pyrazole-5-boronic acid pinacol ester to obtain Taladegib. According to the invention, the process route is improved, cheap N-benzyl-4-piperidone is taken as a starting material, the processes of Boc protection and deprotection are removed to reduce the synthesis steps and reduce the production cost.
Owner:SOUTHEAST UNIV
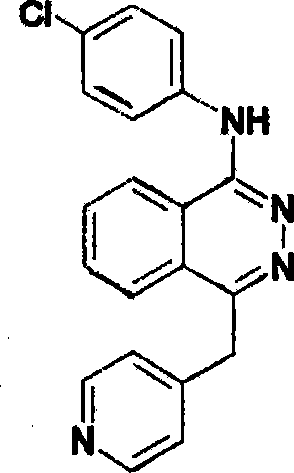
Application of compound 1-( 4-chloroaniline )-4-( 4-picoline )-2, 3-naphthyridine
ActiveCN101116664AAvoid formingImprove the quality of lifeOrganic active ingredientsAntineoplastic agentsDiseaseChlorobenzene
The invention relates to the use of compound 1-(4-chlorobenzene amidocyanogen)-4-(4-methylpyridine)-2, 3-phthalazine in preparing medicine used for preventing tumor diseases, wherein the medicine contains 1-(4-chlorobenzene amidocyanogen)-4-(4-methylpyridine)-2, 3-phthalazine and is used through administration method via or outside intestines and stomach. The compound can at least prolong or avoid occurrence or formation of tumor; moreover, tumor patient adopting medicine containing the compound for prevention obtains higher survival rate as compared with the patient who does not adopt the prevention method.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
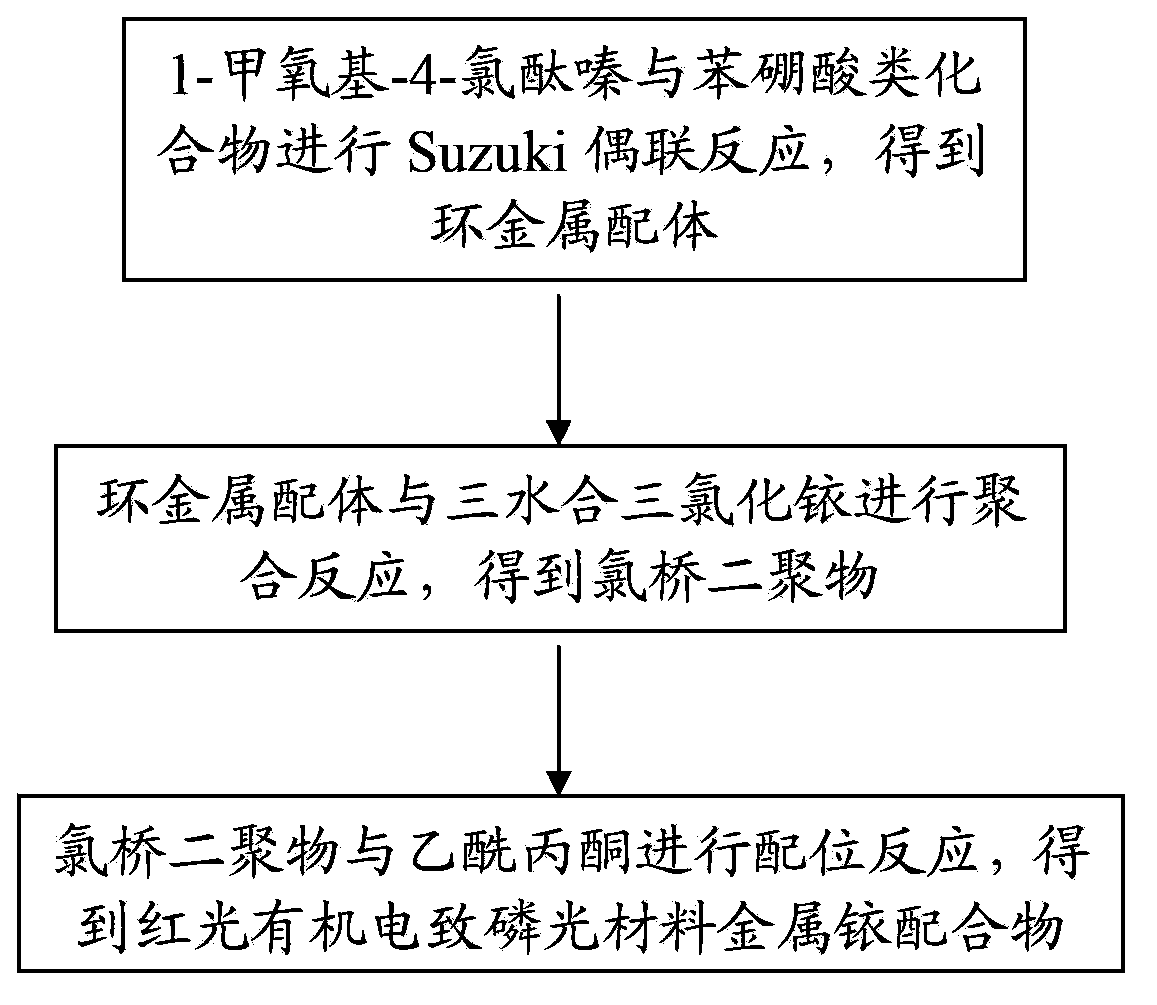
Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
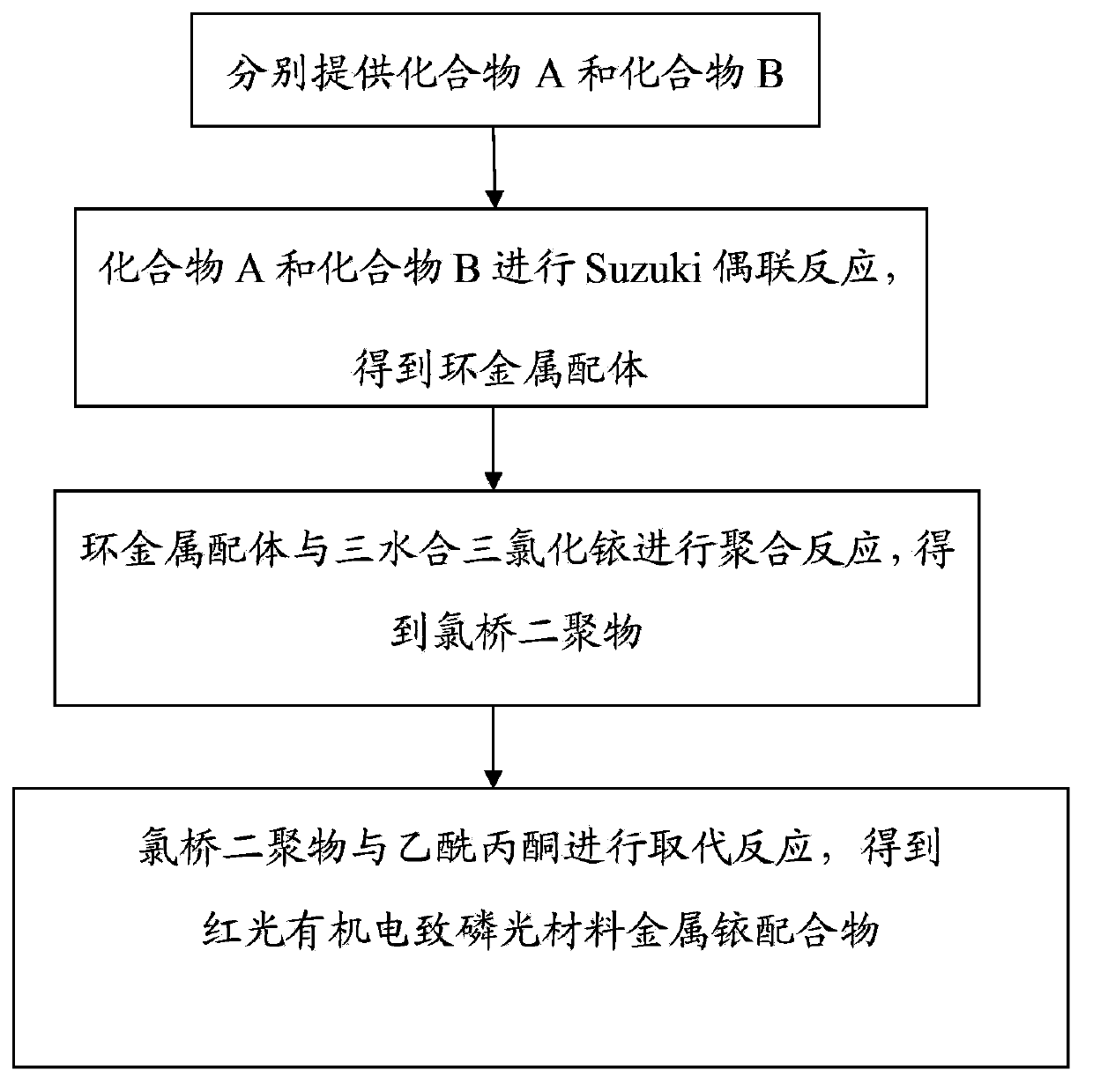
InactiveCN103965888AEase of industrial productionThe preparation process is simple and controllableGroup 8/9/10/18 element organic compoundsSolid-state devicesIridiumStructural formula
The present invention provides a red light organic electrophosphorescence material metal iridium coordination compound, which adopts 1-methyl-4-phenyl phthalazine or a derivative as a ring metal main ligand, adopts acetylacetone as an auxiliary ligand, and has a structural formula represented by a formula (1), wherein R is hydrogen or methyl. The preparation method comprises that: a Suzuki coupling reaction is performed to prepare a ring metal main ligand, the ring metal main ligand and IrCl3.3H2O are subjected to polymerization in a 2-ethoxyethanol and water mixed solvent to obtain a chloro-bridge dimer, and the chloro-bridge dimer and acetylacetone are subjected to a ligand exchange reaction to obtain the red light organic electrophosphorescence material metal iridium coordination compound. According to the present invention, 1-methyl-4-phenyl phthalazine or a derivative thereof is adopted as the ring metal main ligand, acetylacetone is adopted as the auxiliary ligand, and the emission wavelength can be adjusted so as to obtain the red light near the standard red color; and the organic electroluminescent device prepared from the material can emit high purity red light, the luminescence efficiency is high, and the preparation method is simple and is suitable for industrial production.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Preparation method of ethyl benzoylacetate
ActiveCN113200855AImprove corrosion resistanceNo corrosionSemi-permeable membranesOrganic compound preparationEpoxySodium bicarbonate
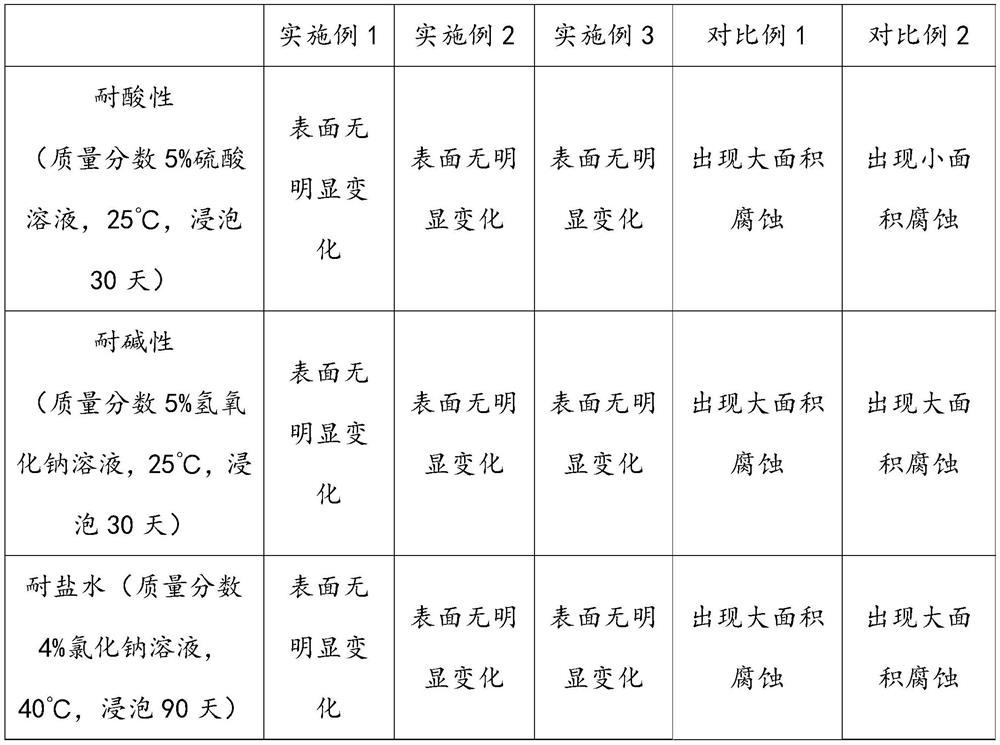
The invention discloses a preparation method of ethyl benzoylacetate, and belongs to the technical field of chemical synthesis. The preparation method comprises the following steps of: adding sodium bicarbonate into carbon tetrachloride, slowly adding ethyl acetoacetate, dropwise adding benzoyl chloride for reaction, adding sodium hydroxide for reaction, and filtering after the reaction is finished to obtain ethyl benzoylacetate. A microporous filter membrane is used in the process of preparing ethyl benzoylacetate. A reinforcing filler is prepared in the process of preparing the microporous filter membrane, and nano silicon dioxide and aniline are treated by the reinforcing filler, so that polyaniline is grafted on the surface of the nano silicon dioxide. Then, the nano silicon dioxide and modified graphene are subjected to ultrasonic treatment to obtain a reinforced substrate. 4-(4-hydroxyphenyl)-2, 3-phthalazine-1-one and epichlorohydrin are subjected to a reaction to prepare epoxy resin, then the epoxy resin and amino groups on the reinforced substrate are cured to prepare a reinforcing filler, the reinforcing filler can enhance the corrosion resistance of the microporous filter membrane, and the microporous filter membrane cannot be corroded after being used for a long time.
Owner:江苏巨莱生物医药有限公司
Iridium complexes, preparation method thereof and electroluminescent device using iridium complexes
InactiveCN106432347AImprove luminous efficiencyHigh electron mobilityIndium organic compoundsSolid-state devicesIridiumLuminous intensity
The invention relates to three-element co-ligand iridium complexes with novel ligands. The ligands in molecules of the iridium complexes in the series are 2-(4,6-bis(trifluoromethyl)pyridine-3-)quinoline, 2-(4,6-bis(trifluoromethyl)pyridine-4-)quinoline, 2-(4,6-bis(trifluoromethyl)pyridine-3-)isoquinoline, 2-(4,6-bis(trifluoromethyl)pyridine-4-)isoquinoline, 2-(4,6-bis(trifluoromethyl)pyridine-3-)quinazoline, 2-(4,6-bis(trifluoromethyl)pyridine-4-)quinazoline, 2-(4,6-bis(trifluoromethyl)pyridine-3-)phthalazine and 2-(4,6-bis(trifluoromethyl)pyridine-4-)phthalazine derivatives. The novel iridium complexes have the advantages that the luminous efficiency is high, the chemical property is stable, sublimation purification is easy and the like, and besides, the performance of a device is good. The luminous intensity and efficiency of the ligands can be adjusted in the wavelength coverage of red light through a molecular structure modifying the ligands, which brings convenience to design and production of organic electroluminescent displays and lighting sources.
Owner:AAC MICROTECH CHANGZHOU +1
Pharmaceutical compounds as activators of caspases and inducers of apoptosis and the use thereof
Disclosed are 1-arylamino-phthalazines, 4-arylamino-benzo[d][1,2,3]triazines, and analogs thereof effective as activators of caspases and inducers of apoptosis. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC +1
Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
InactiveCN103965262AEase of industrial productionThe preparation process is simple and controllableGroup 8/9/10/18 element organic compoundsSolid-state devicesIridiumSolvent
The present invention provides a red light organic electrophosphorescence material metal iridium coordination compound, which has a structure formula represented by a formula (1), wherein R is methyl. The preparation method comprises that: a Suzuki coupling reaction is performed to obtain a ring metal ligand, the ring metal ligand and IrCl3.3H2O are subjected to a polymerization reaction in a 2-ethoxyethanol and water mixed solvent to obtain a chloro-bridge dimer, and the chloro-bridge dimer and the ring metal ligand are subjected to a ligand exchange reaction to obtain the red light organic electrophosphorescence material metal iridium coordination compound. According to the present invention, 1-phenoxy-4-(substituted phenyl)phthalazine is adopted as the ring metal ligand to synthesize to obtain the material, wherein the emission wavelength can be adjusted so as to obtain the red light near the standard red color; and the organic electroluminescent device prepared from the material can emit high purity red light, the luminescence efficiency is high, and the preparation method is simple and is suitable for industrial production.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted cycloocta[5,6]pyrido[4,3,2-de]phthalazines as PARP inhibitors Substituted cycloocta[5,6]pyrido[4,3,2-de]phthalazines as PARP inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08582902-7082-4a5d-b5ce-85145ca1d42d/US09328111-20160503-C00001.PNG)
![Substituted cycloocta[5,6]pyrido[4,3,2-de]phthalazines as PARP inhibitors Substituted cycloocta[5,6]pyrido[4,3,2-de]phthalazines as PARP inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08582902-7082-4a5d-b5ce-85145ca1d42d/US09328111-20160503-C00002.PNG)
![Substituted cycloocta[5,6]pyrido[4,3,2-de]phthalazines as PARP inhibitors Substituted cycloocta[5,6]pyrido[4,3,2-de]phthalazines as PARP inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08582902-7082-4a5d-b5ce-85145ca1d42d/US09328111-20160503-C00003.PNG)



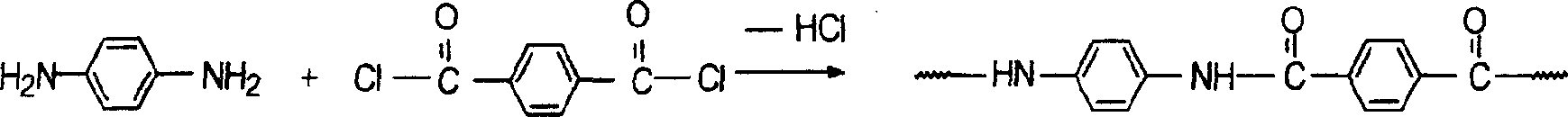
![Method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone Method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ccc83d27-d745-44ff-95b0-2e9c38f52fb7/BDA0000715058310000031.PNG)
![Method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone Method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ccc83d27-d745-44ff-95b0-2e9c38f52fb7/BDA0000715058310000032.PNG)
![Method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone Method for efficient catalytic synthesis of 2H-indole [2,1-b] phthalazine-1,6,11(13H) triketone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ccc83d27-d745-44ff-95b0-2e9c38f52fb7/FDA0000715058300000011.PNG)


![Application of compound 6-(4-chlorophenoxy)-tetrazolo [5,1-a] phthalazine to preparation of medicine for treating depression Application of compound 6-(4-chlorophenoxy)-tetrazolo [5,1-a] phthalazine to preparation of medicine for treating depression](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e91c953d-e1ff-4e79-99b2-aea45c877e95/HSA00000745037300011.PNG)
![Application of compound 6-(4-chlorophenoxy)-tetrazolo [5,1-a] phthalazine to preparation of medicine for treating depression Application of compound 6-(4-chlorophenoxy)-tetrazolo [5,1-a] phthalazine to preparation of medicine for treating depression](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e91c953d-e1ff-4e79-99b2-aea45c877e95/HSA00000745037300012.PNG)
![Application of compound 6-(4-chlorophenoxy)-tetrazolo [5,1-a] phthalazine to preparation of medicine for treating depression Application of compound 6-(4-chlorophenoxy)-tetrazolo [5,1-a] phthalazine to preparation of medicine for treating depression](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e91c953d-e1ff-4e79-99b2-aea45c877e95/HSA00000745037300021.PNG)
![Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff86e77d-5670-414a-8b2a-a33221d46931/BDA0000886530140000011.PNG)
![Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff86e77d-5670-414a-8b2a-a33221d46931/BDA0000886530140000021.PNG)
![Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ff86e77d-5670-414a-8b2a-a33221d46931/BDA0000886530140000041.PNG)