Aromatic diamines monomer with double miscellaneous naphthalenone structures and trifluoromethyl substituted and preparation thereof
A technology of trifluoromethyl and aromatic diamines, which is applied in the field of aromatic diamines and its preparation, can solve the problems of polyimide insolubility, loss, and reduction of high temperature resistance of polyimide, and achieve good film formation properties, excellent mechanical properties, and excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
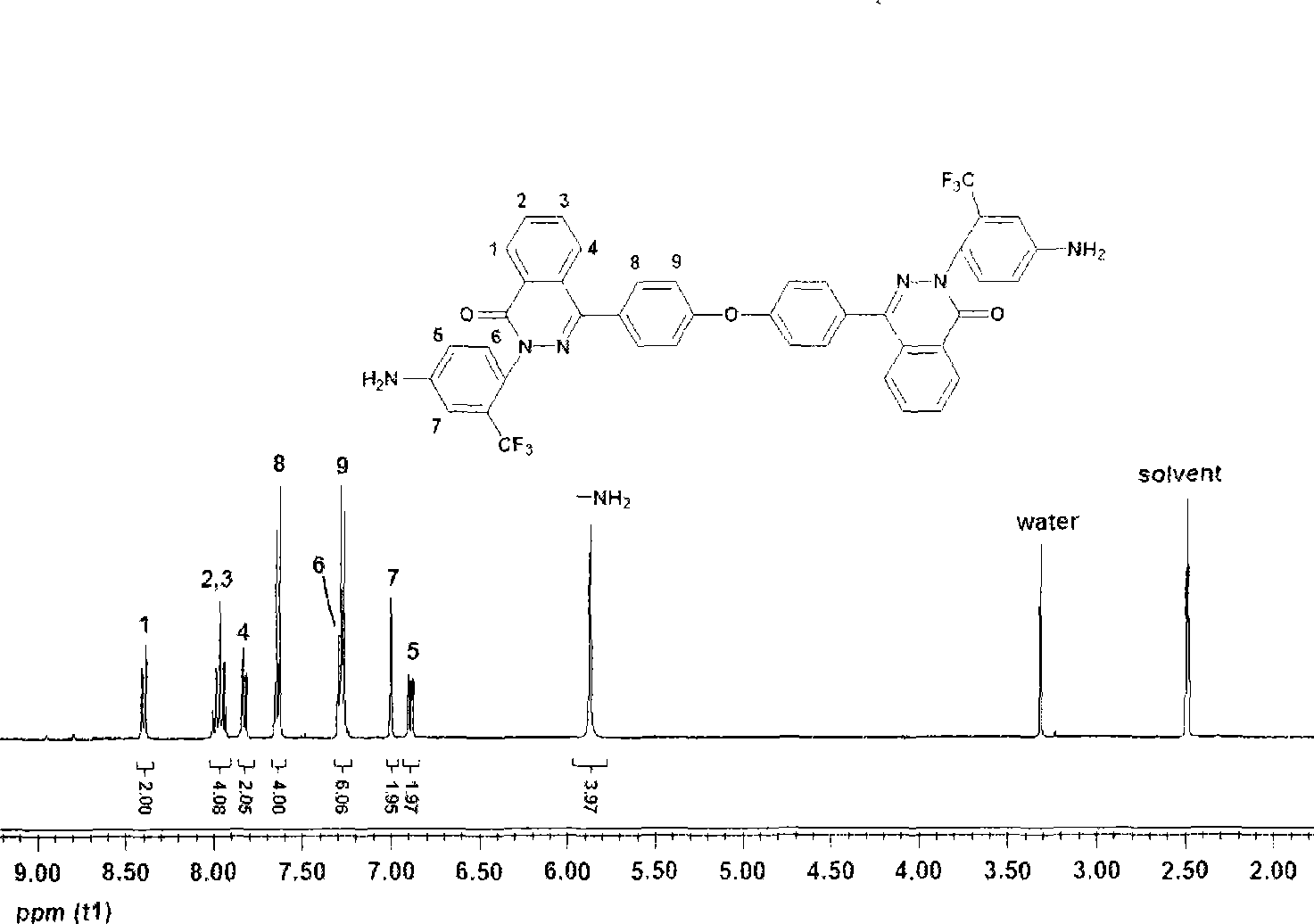
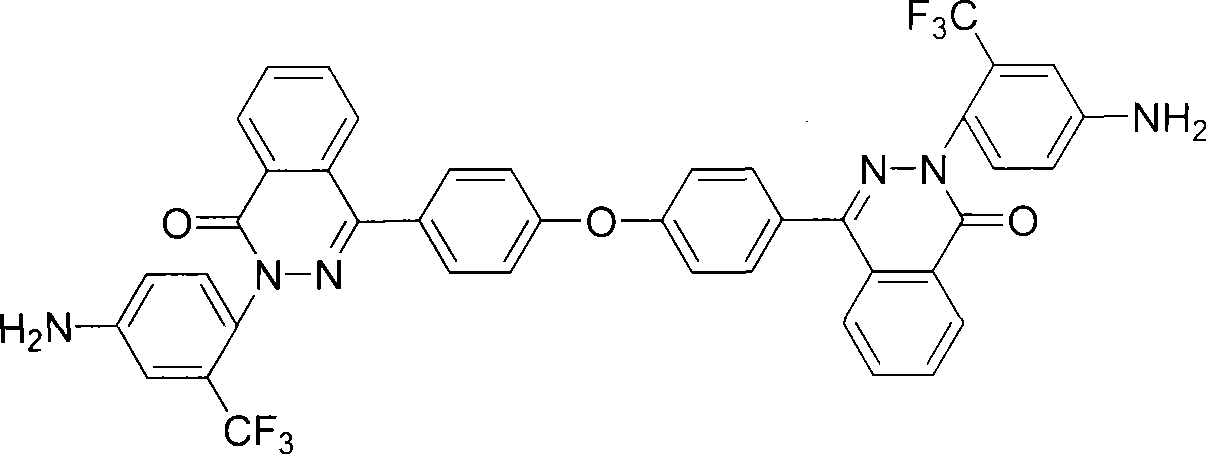
[0023] 2-(4-amino-2-trifluoromethylphenyl)-4-[4-(4-amino-2-trifluoromethylphenoxy)-naphthyl]-naphthyridine-1 - Preparation of ketones:
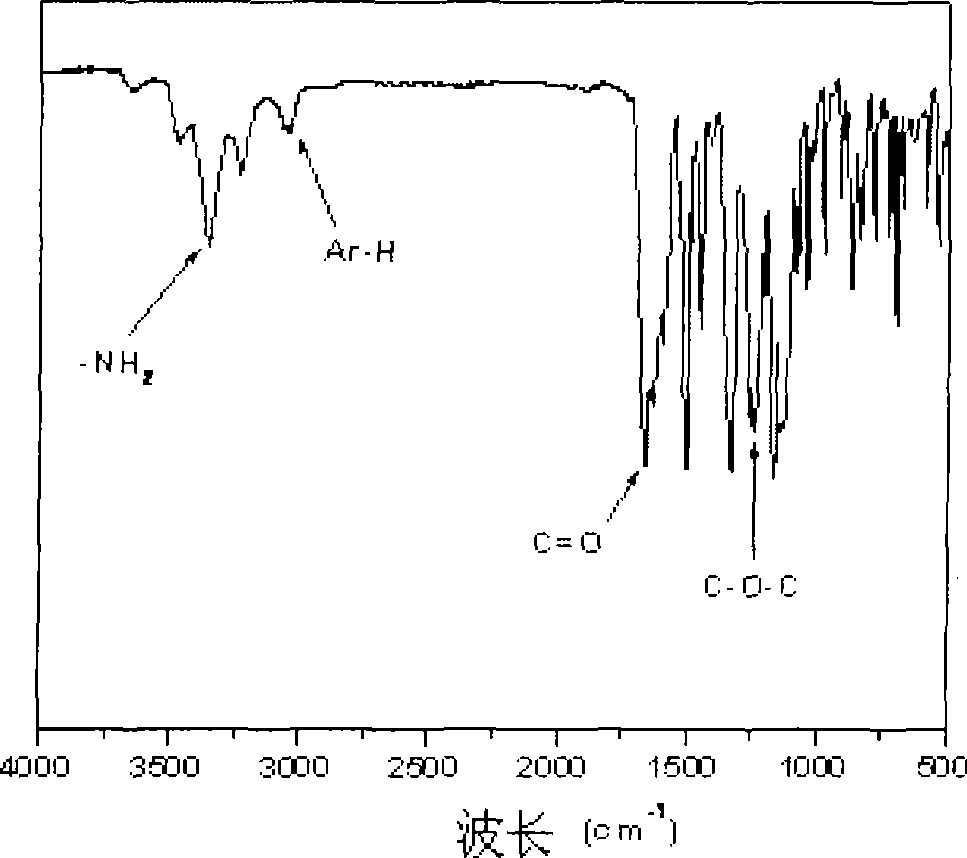
[0024] ①Under nitrogen protection, mix 22.9g (0.05mol) 4,4'-oxo-bis[(4-(1,4-phenyl)-phthalazin-1-one] and 22.6g (0.1mol) 2-Chloro-5-nitrobenzotrifluoride was added to a 500ml three-necked flask, and then 14.5g (0.105mol) of potassium carbonate and 150ml of N,N-dimethylformamide were added respectively, and the reaction was carried out at 100-150°C for 10- After 20 hours, the product was poured into 1000ml of methanol / water (1:1), fully stirred, precipitated and filtered, and the product was repeatedly washed with hot water, and further separated and purified by column chromatography to obtain a white fluorine-containing dinitro compound: 4,4'- Bis[2-(4-nitro-2-trifluoromethylphenyl)-phthalazin-1-one-yl]-diphenyl ether, the yield is about 70-80%, and the melting point is 159-161 ° C. FT -IR(KBr)v / cm -1 : 1677 (C=O), 1537, 1355 (-NO 2 ), 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com