Materials and methods for the efficient production of acetate and other products
a technology applied in the field of materials and methods for the efficient production of acetate and other products, can solve the problems of reducing the level of acetate production using these methods, increasing the cost of materials, increasing the cost of acetate purification, and waste disposal, and achieving the effects of increasing acetate production, reducing the loss of substrate carbon, and increasing acetate production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of a Homo-Acetate Fermentation Pathway in E. coli W3110
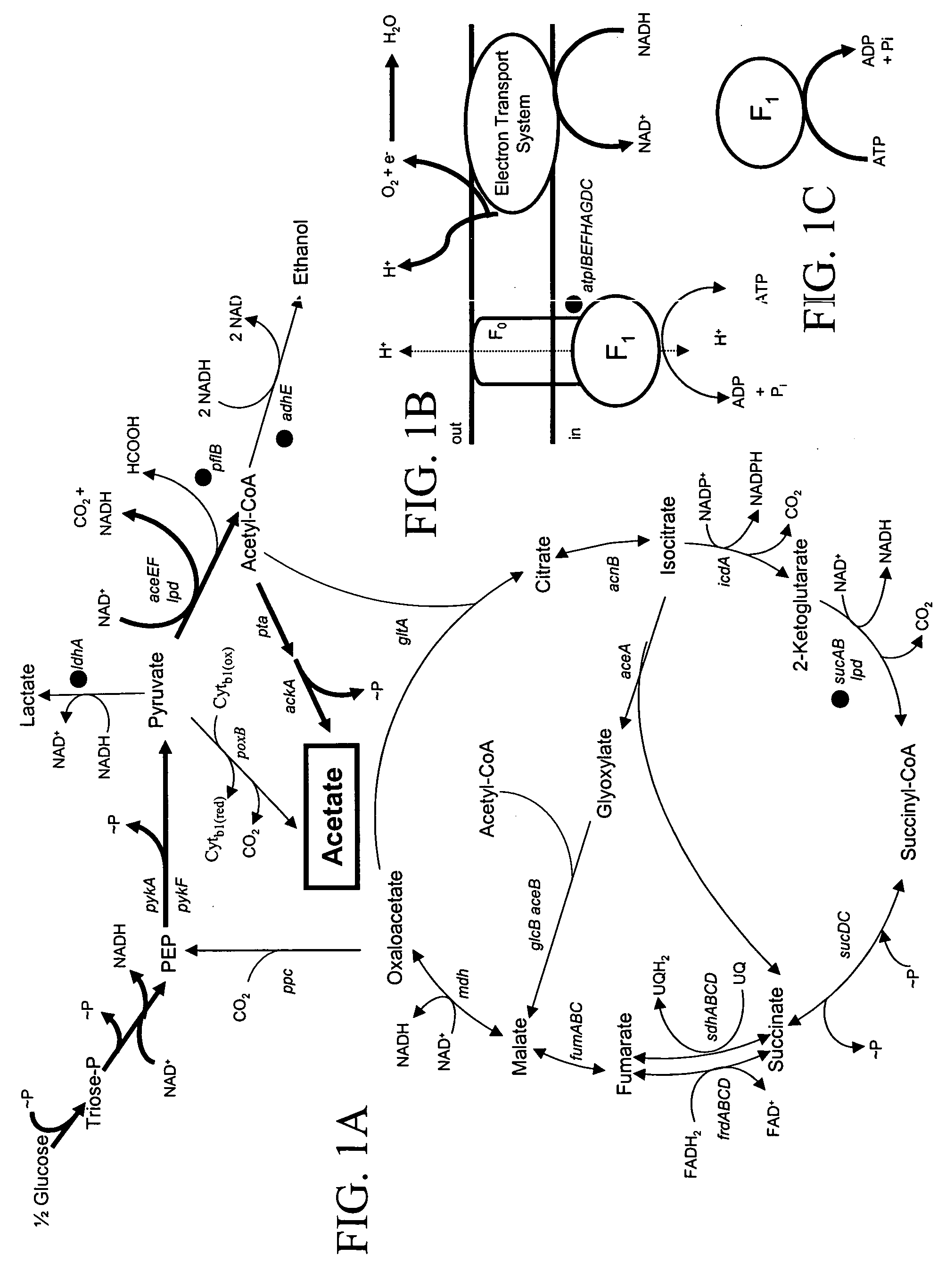
[0096] Fermentation of sugars through native pathways in E. coli produces a mixture of organic acids, ethanol, CO.sub.2 and H.sub.2 (FIG. 1). Acetate and ethanol are typically produced in approximately equimolar amounts from acetyl.about.CoA to provide redox balance (Clark, D. P., 1989 "The fermentation pathways of Escherichia coli. FEMS" Microbiol. Rev. 63:223-234; de Graef, M. R., S. Alexeeva, J. L. Snoep, and M. J. Teixiera de Mattos, 1999 "The steady-state internal redox state (NADH / NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli" J. Bacteriol. 181:2351-2357). To construct a strain for homo-acetate production, removable antibiotic resistance genes were used to sequentially inactivate chromosomal genes encoding alternative pathways.
[0097] Inspection of native pathways in E. coli (FIG. 1) indicated that the production of acetate and CO.sub.2 as sole metabolic ...
example 2
Effects of Gene Disruptions on Growth and Glycolytic Flux
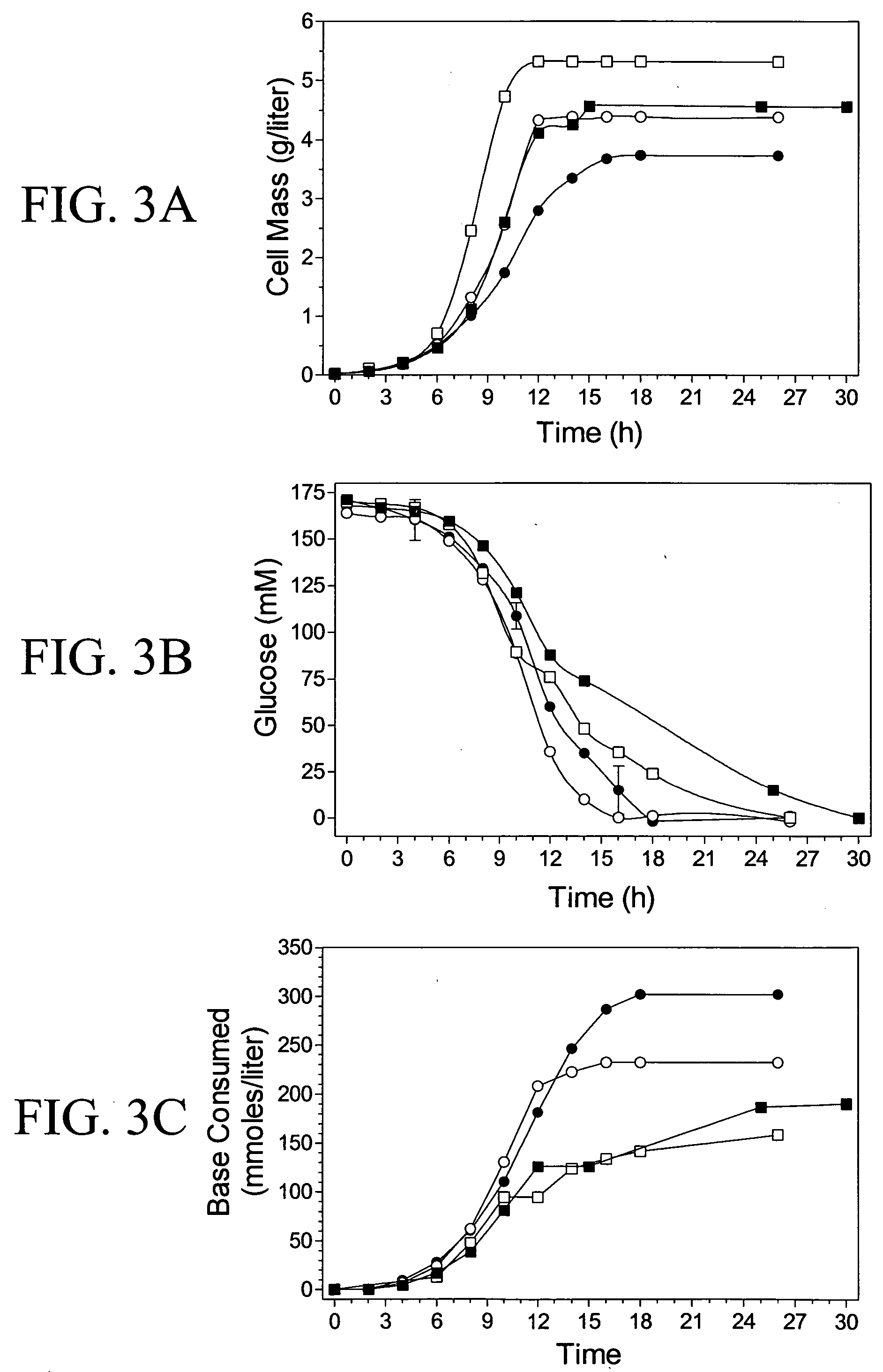
[0106] TC36 was genetically engineered for the production of acetate from carbohydrates such as glucose. Batch fermentations with pH control were used to compare the performance of this strain with W3110 (wild type) and two intermediate strains used for construction, SZ47(.DELTA.pflB,.DELTA.f-rdCD,.DELTA.ldhA) and TC24(.DELTA.pflB,.DELTA.frdCD,.DELTA.ldhA .DELTA.atpFH). Under 5% oxygen saturation and 3% glucose (37.degree. C.) test conditions, the broth pH was maintained at neutrality to minimize toxicity from undissociated acids (Chotani, G., T. Dodge, A. Hsu, M. Kumar, R. LaDuca, D. Trimbur, W. Weyler, and K. Sanford, 2000 "The commercial production of chemicals using pathway engineering" Biochim. Biophys. Acta 1543:434-455).
[0107] Disruption of oxidative phosphorylation and the cyclic function of the tricarboxylic acid cycle, elimination of the primary fermentation pathways, and the production of acetate as the primary end-...
example 3
Production of Other Organic Acids
[0110] A substantial portion of glucose carbon was not recovered in the carbon balance (Table 3) for W3110 (40%) and SZ47 (80%). This loss is attributed to the production of volatile products by high flux through the tricarboxylic acid cycle (CO.sub.2) but may also include the reduction of acetyl.about.CoA to acetaldehyde and ethanol (FIG. 1).
3TABLE 3 Summary of fermentation products. Cell Carbon Yield Fermentation Products.sup.a (mM) Recovery.sup.c Strain Conditions (g / liter) Acetate 2-ketoglutarate Fumarate Lactate Pyruvate Succinate Yield.sup.b(%) (% substrate C) W3110 3% glucose 4.5 30 39 0.8 33 <1 5 9 60 5% DO SZ47 3% glucose 5.3 6 11 0.9 <1 1 3 2 20 5% DO TC24 3% glucose 4.4 156 1 1.0 <1 <1 2 47 66 5% DO TC36 3% glucose 3.5 224 16 0.4 <1 0 4 68 89 5% DO _0.2 _14 _6 _0.1 _0.5 _1 TC36 3% glucose 3.2 190 24 <1 <1 <1 3 57 88 15% DO TC36 3% Glucose 2.5 220 31 <1 <1 <1 10 66 95 5% DO N-limited TC36 3 + 3% glucose 3.8 523 21 <1 3 14 2 78 95 5% DO TC36...
PUM
| Property | Measurement | Unit |
|---|---|---|
| working volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| dry cell weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com