Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
9653results about "Drying solid materials without heat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
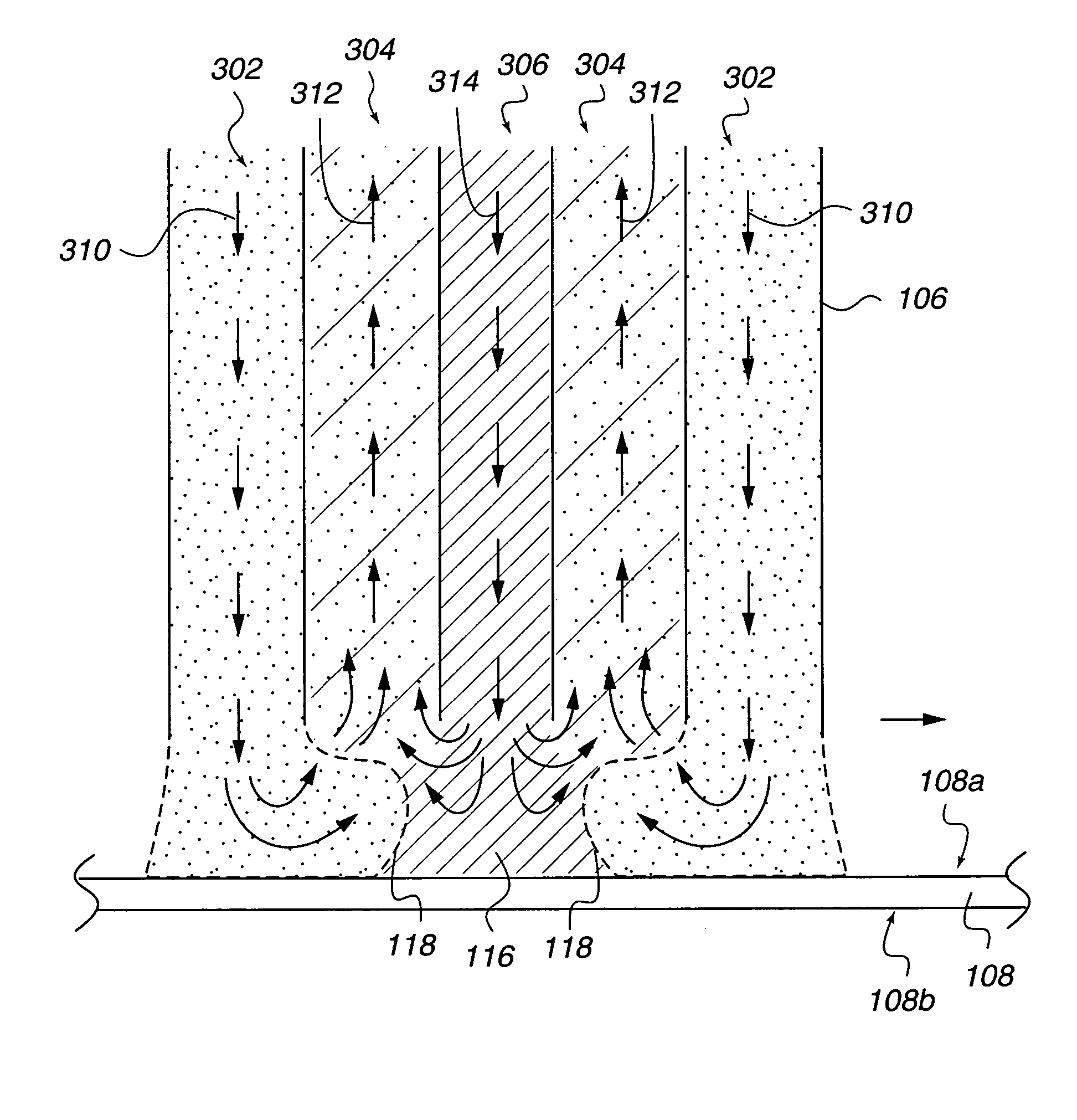
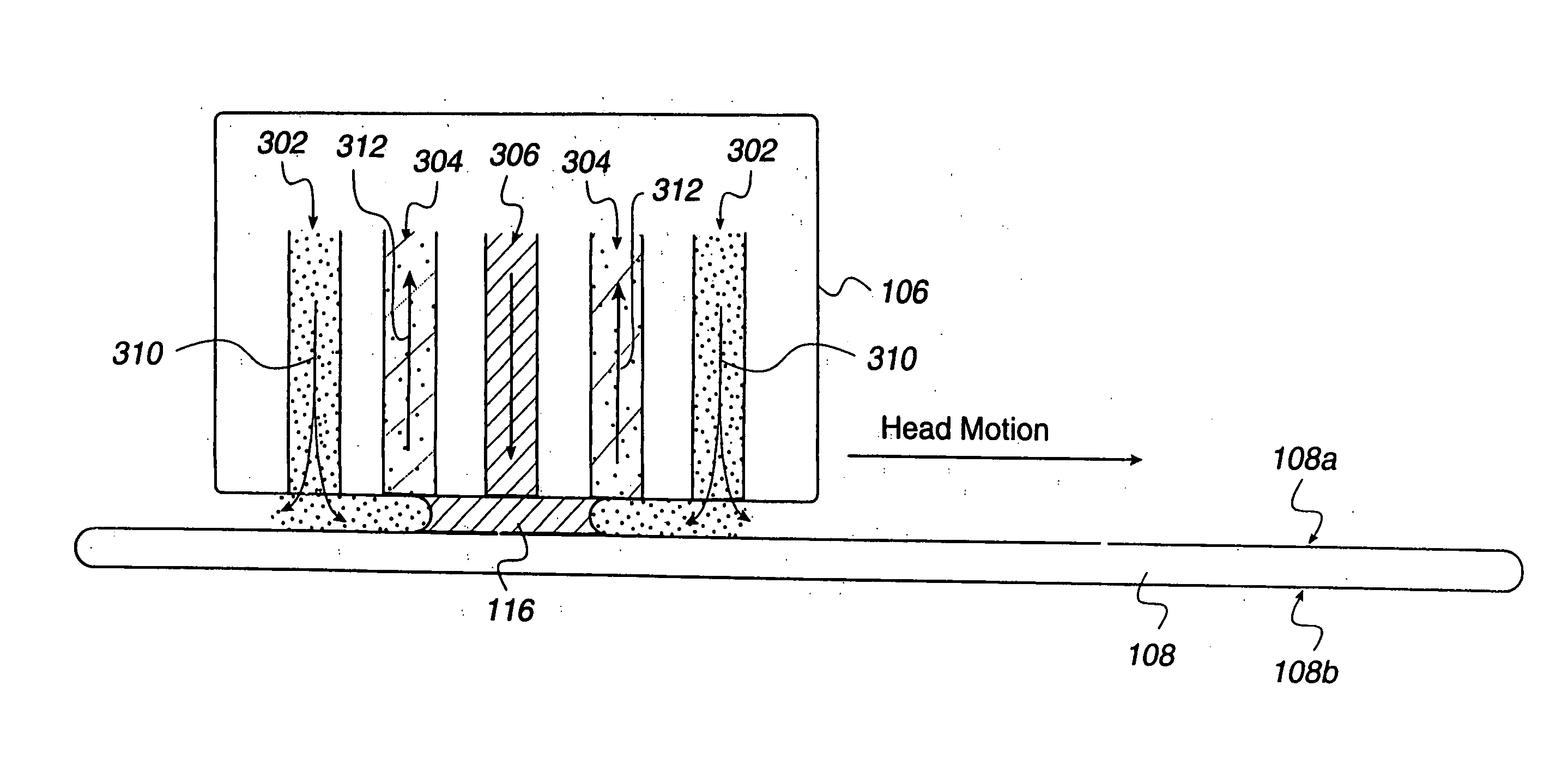
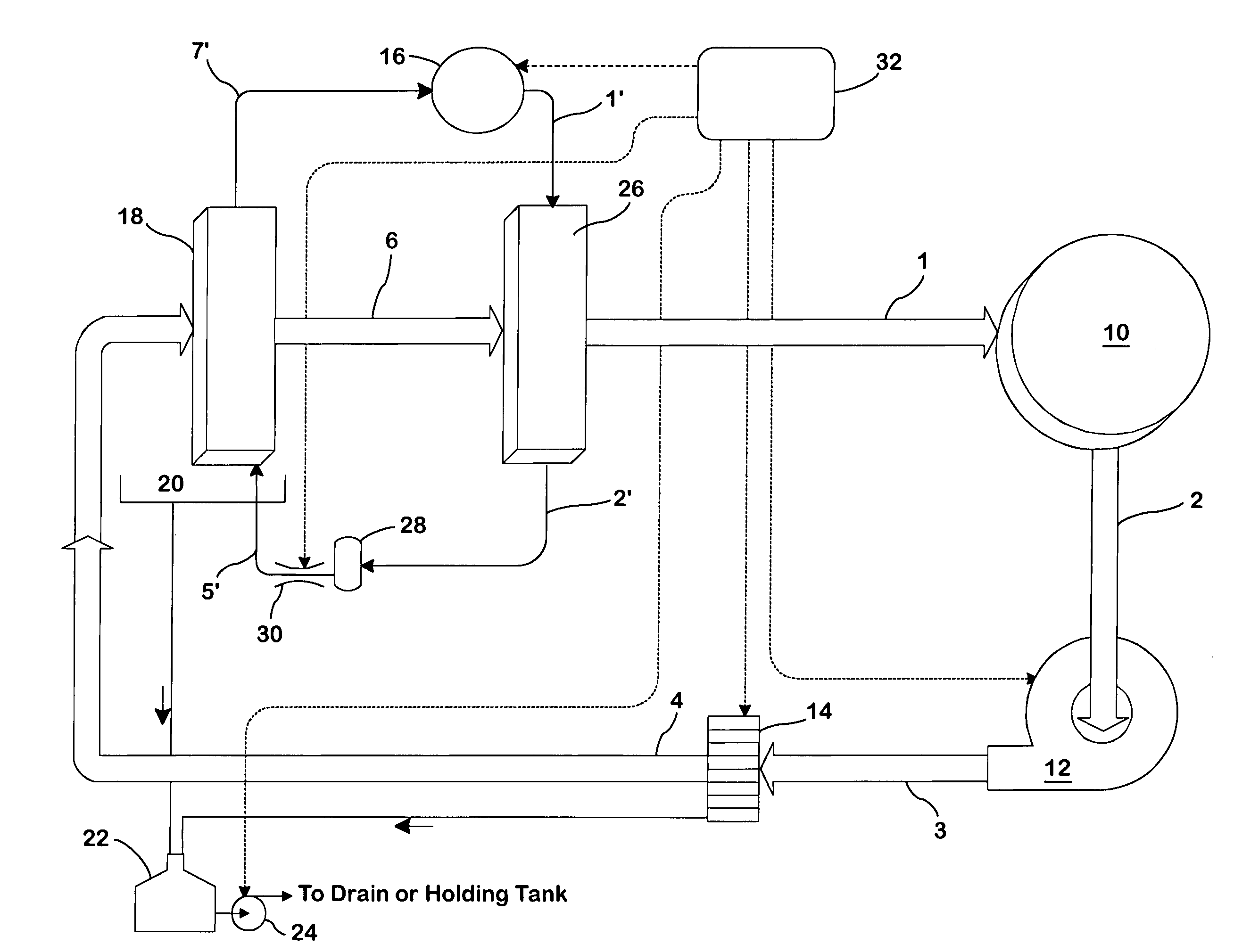
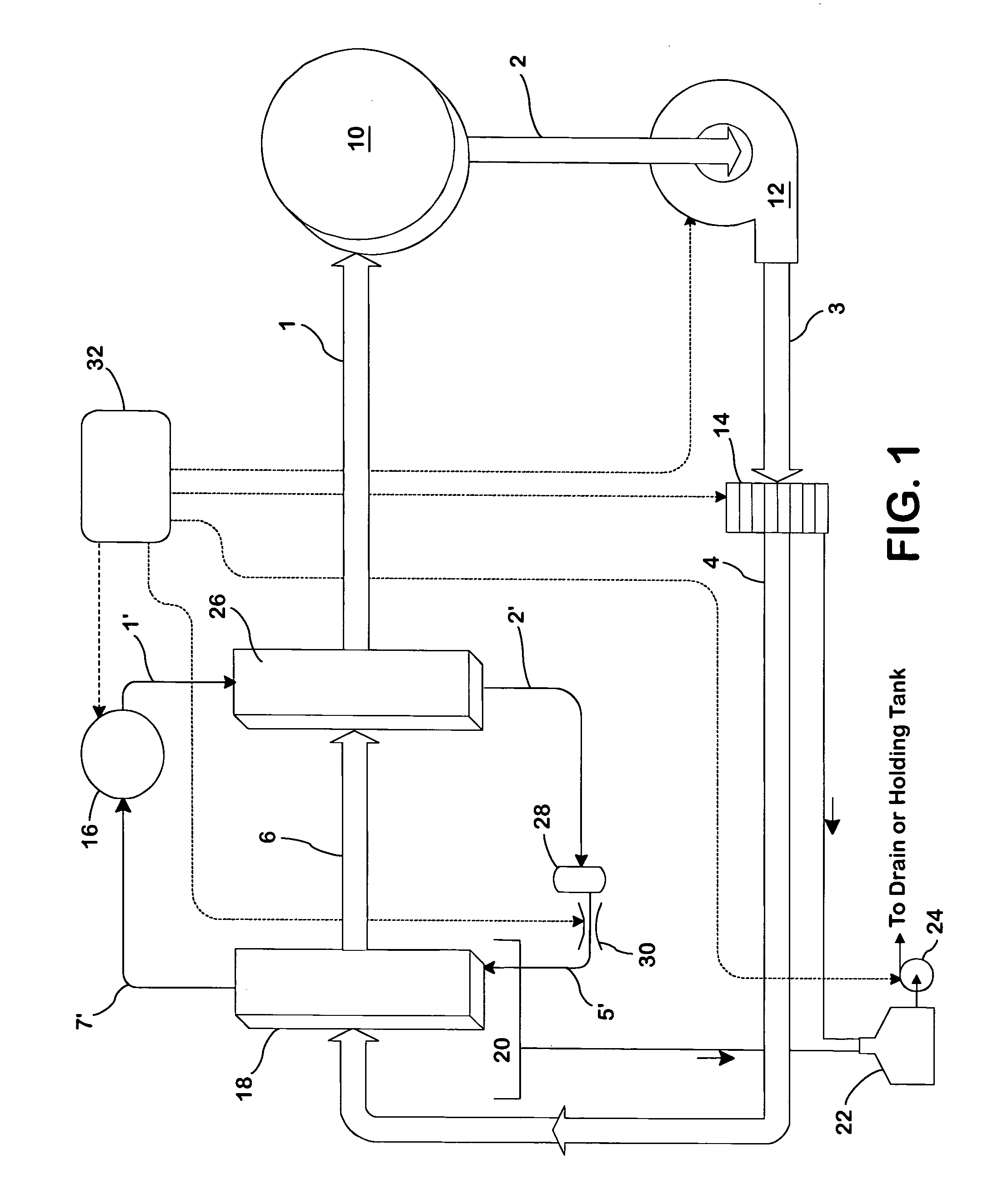
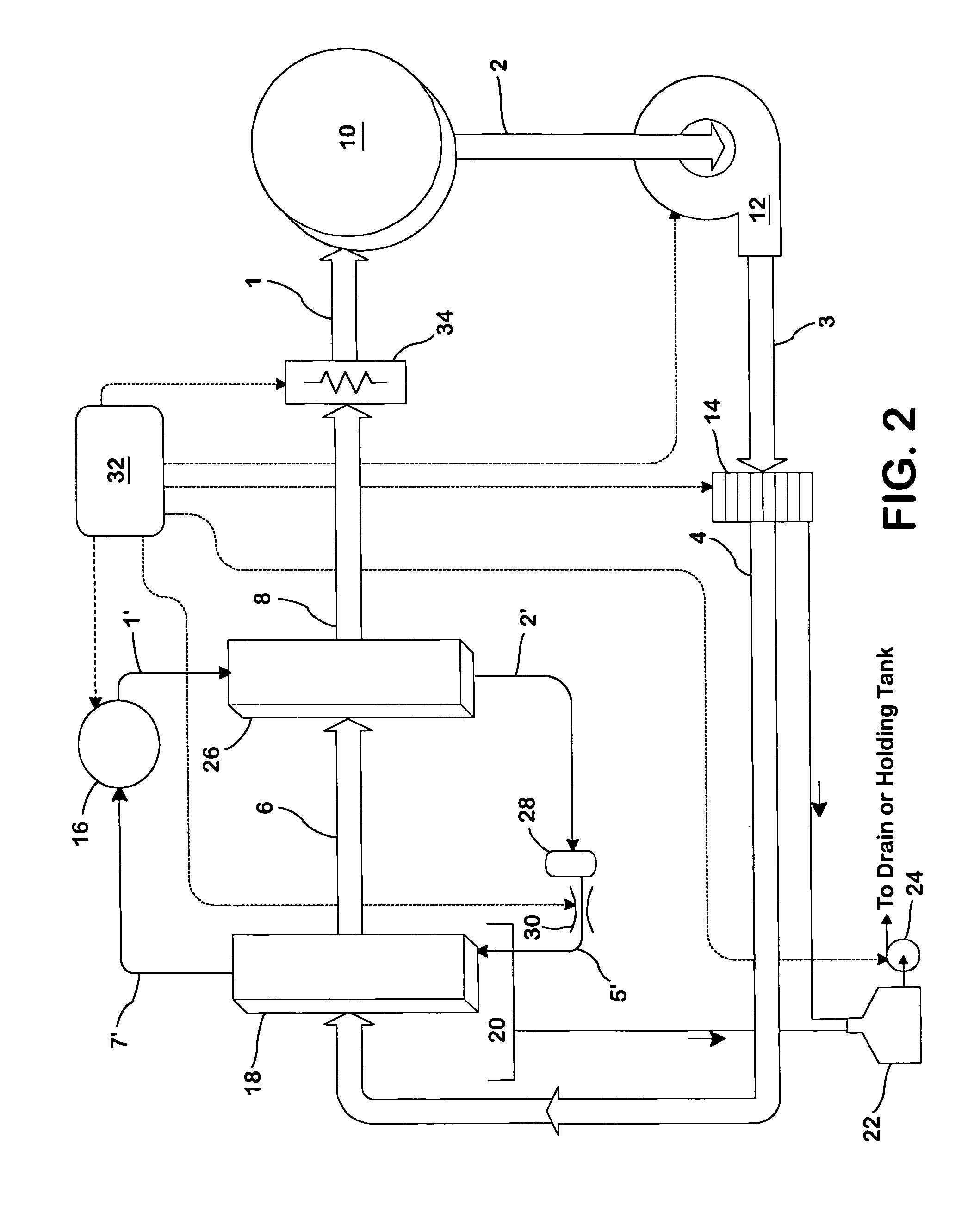
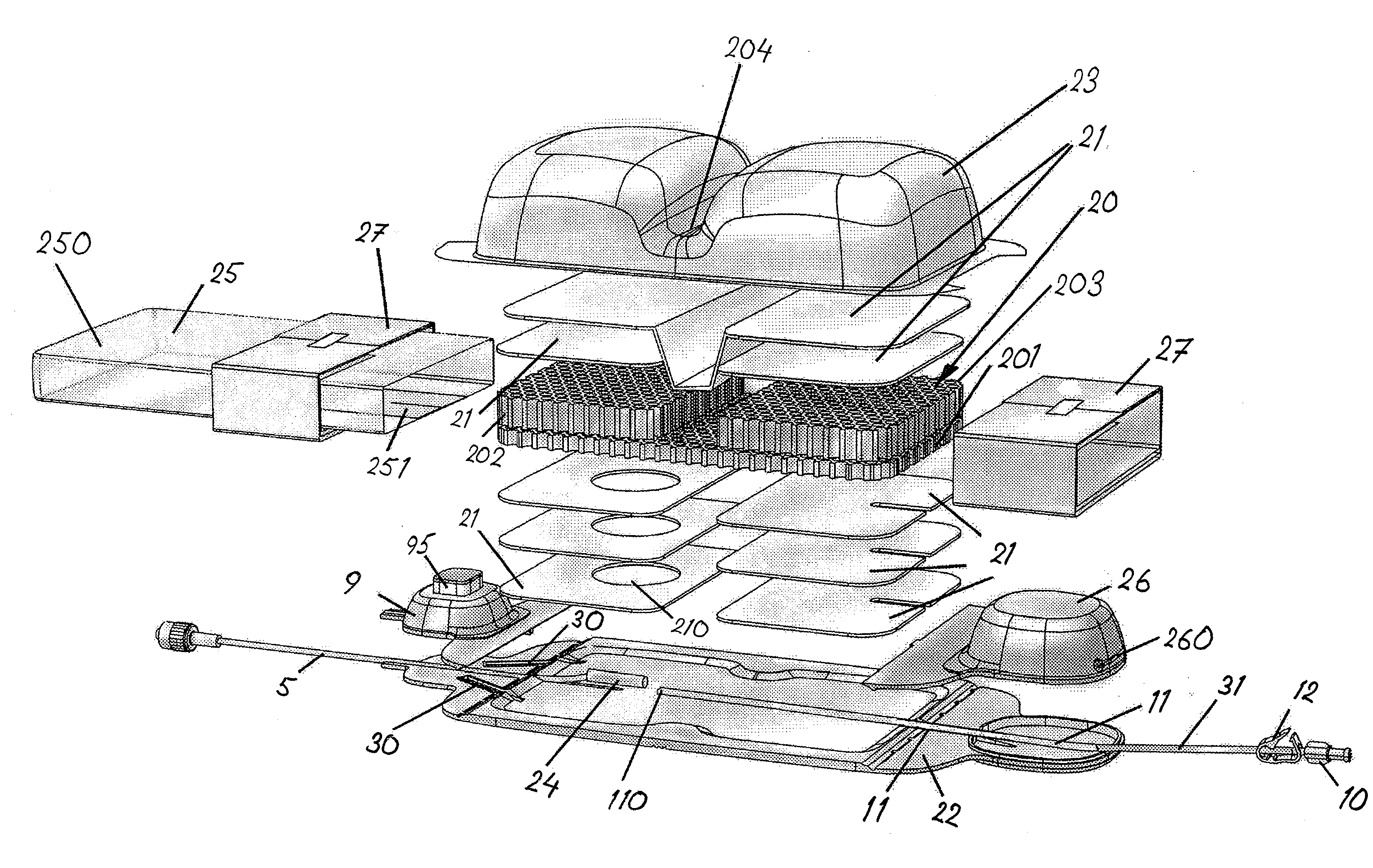
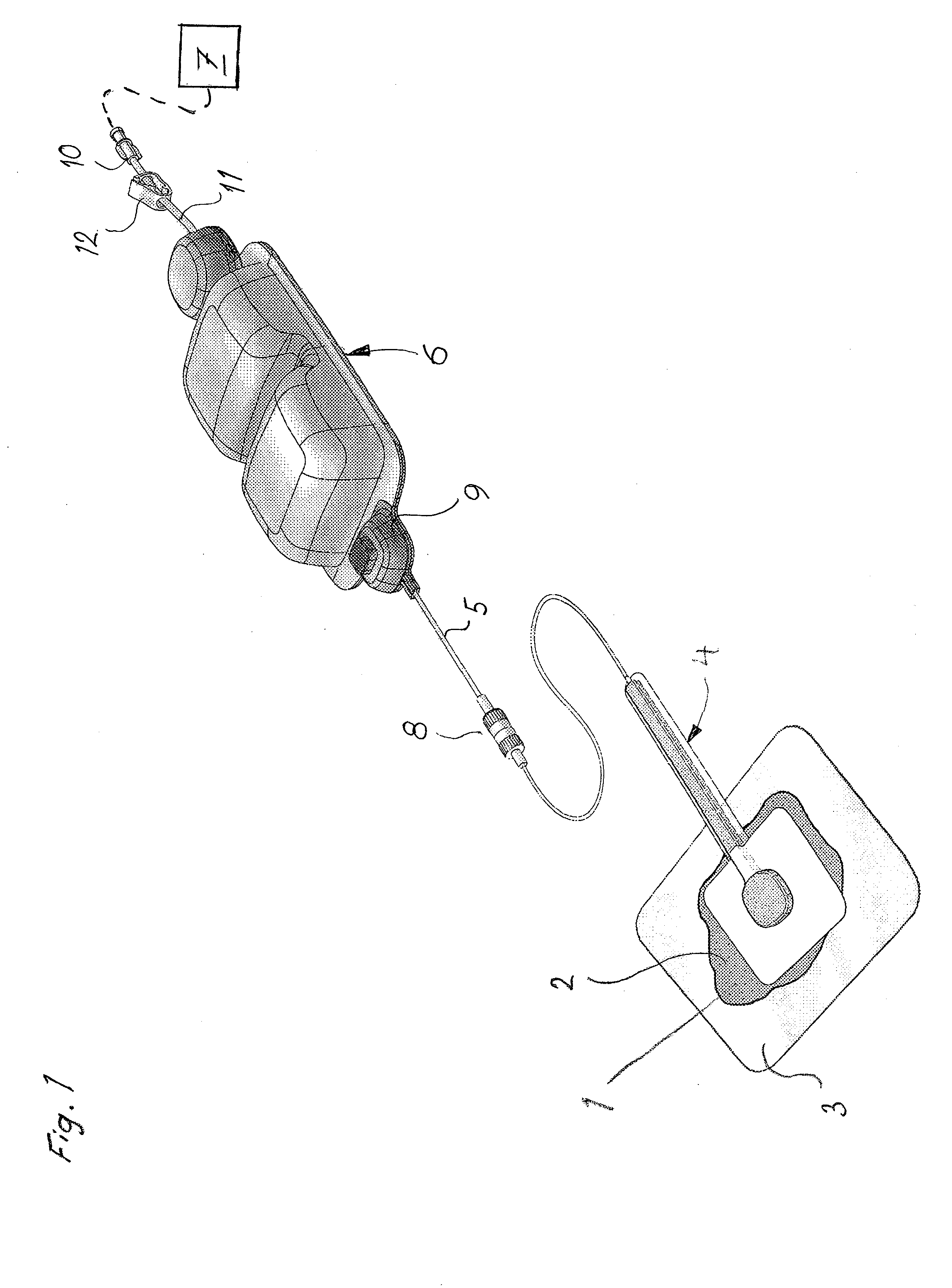
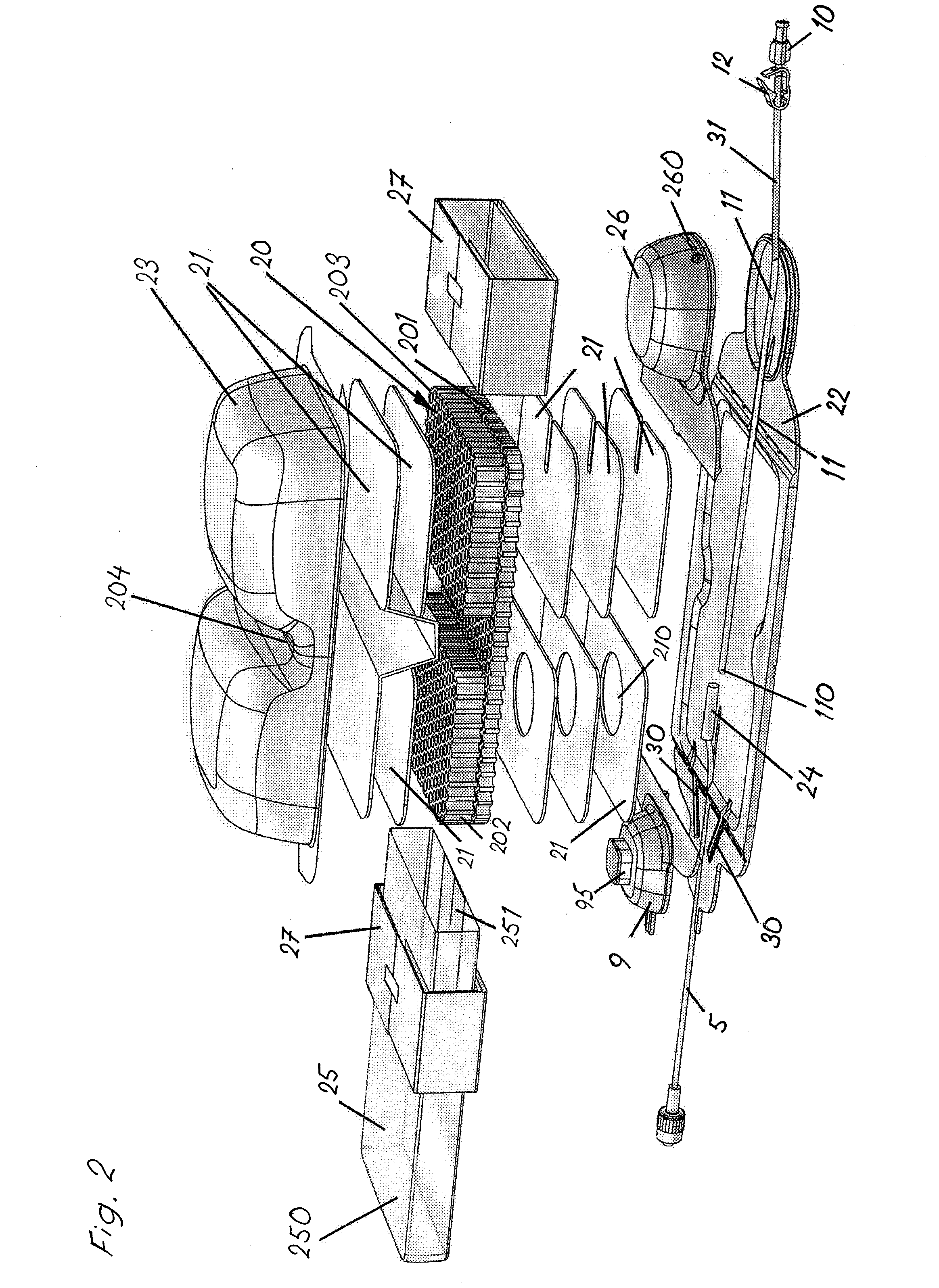
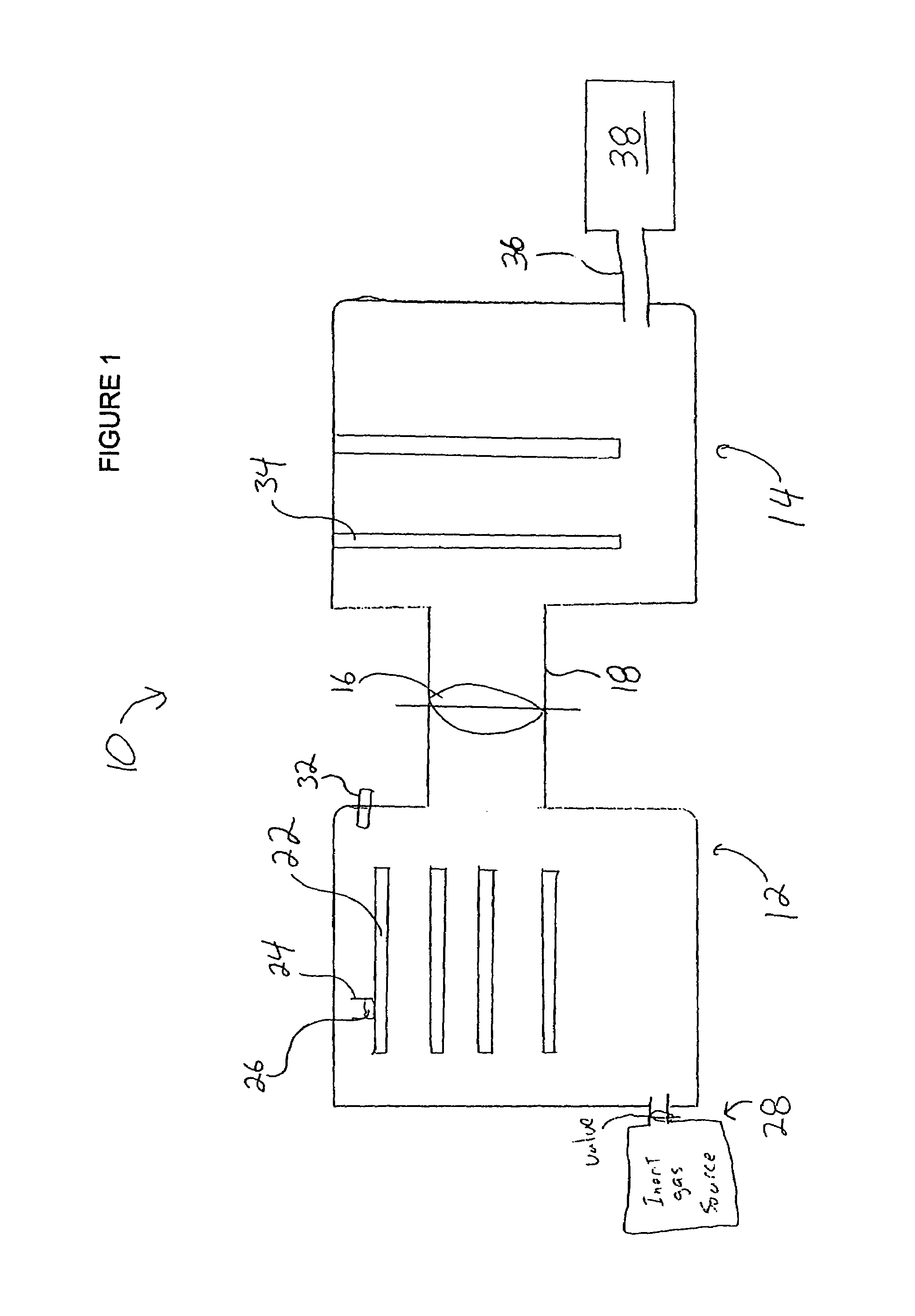
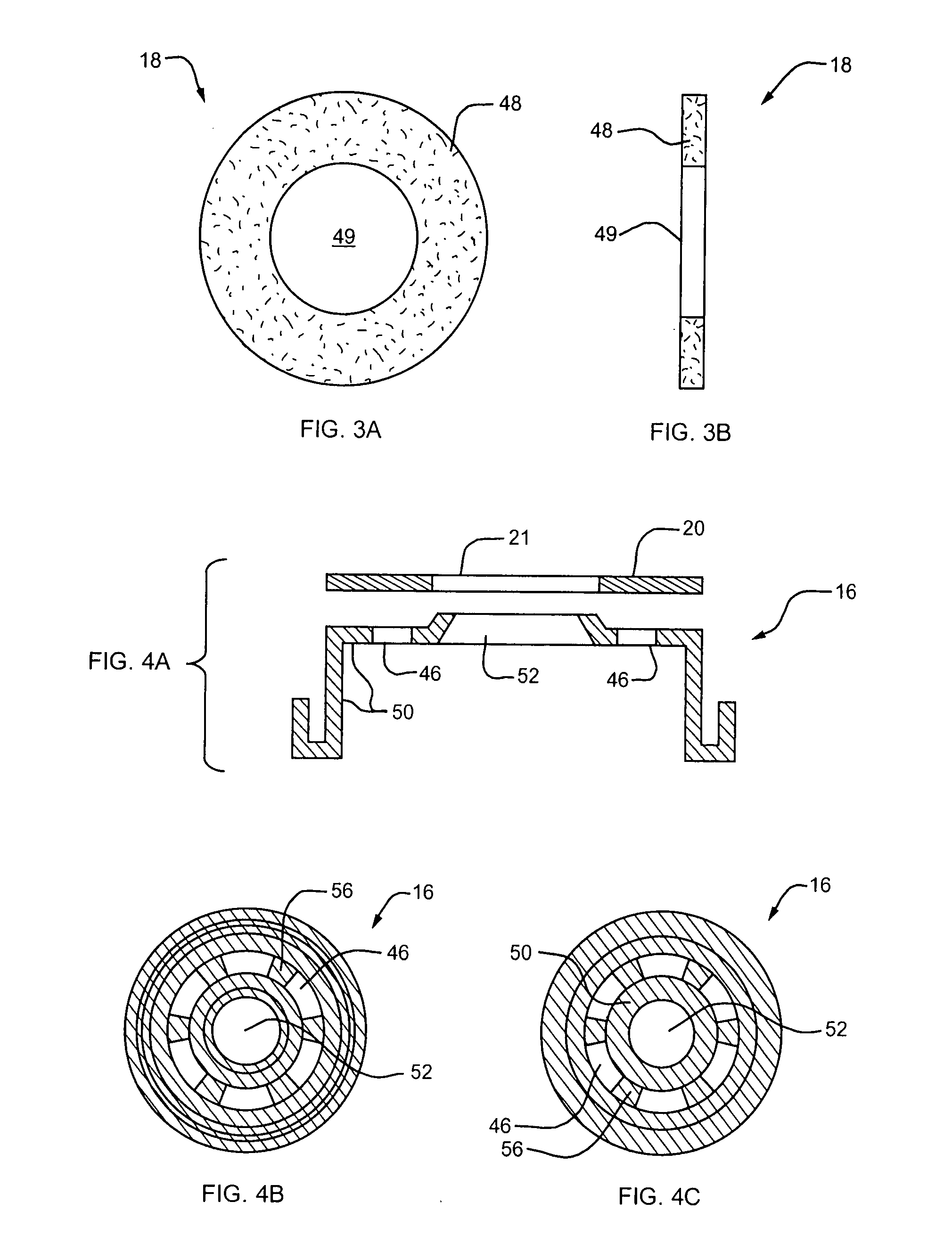
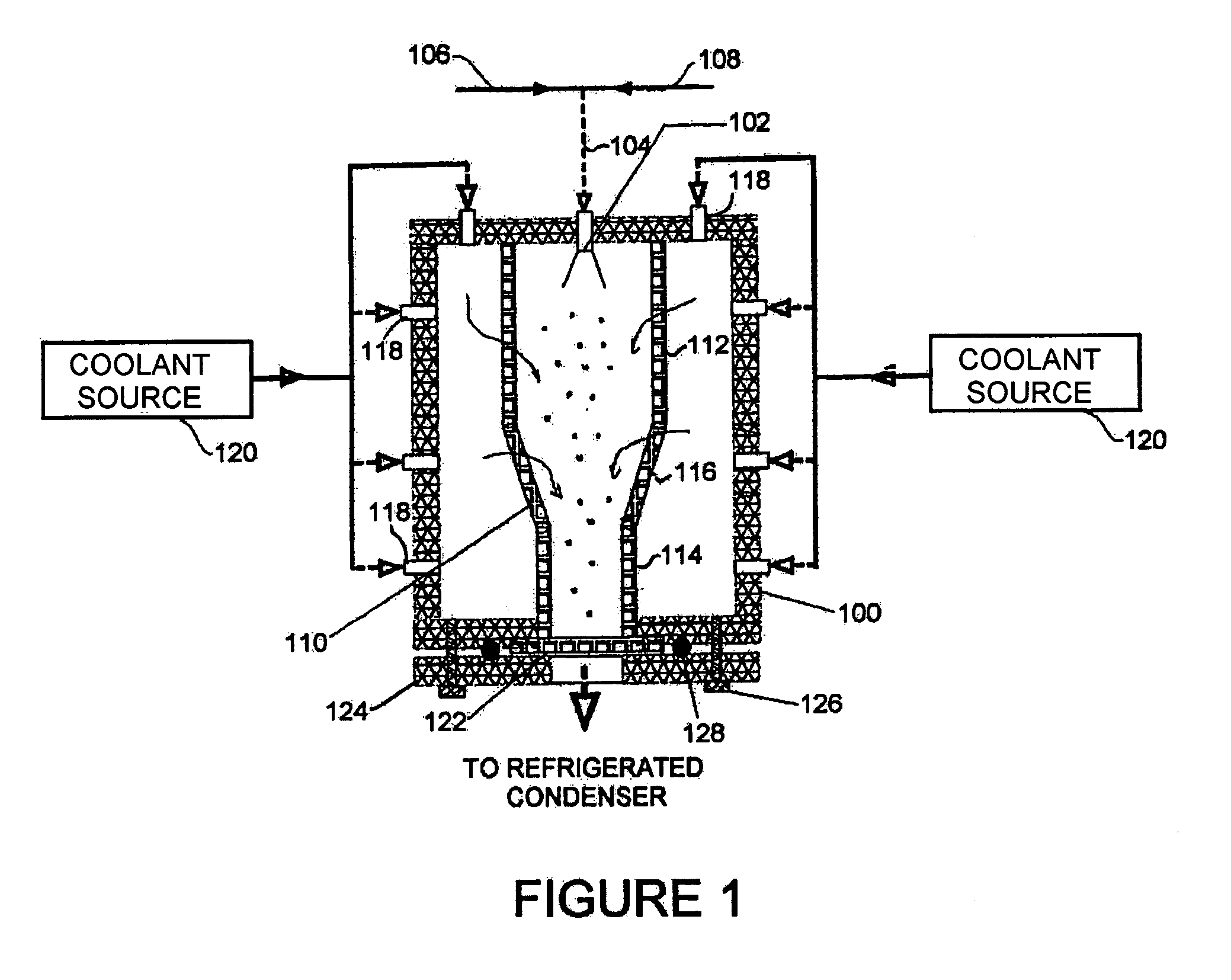
Concentric proximity processing head
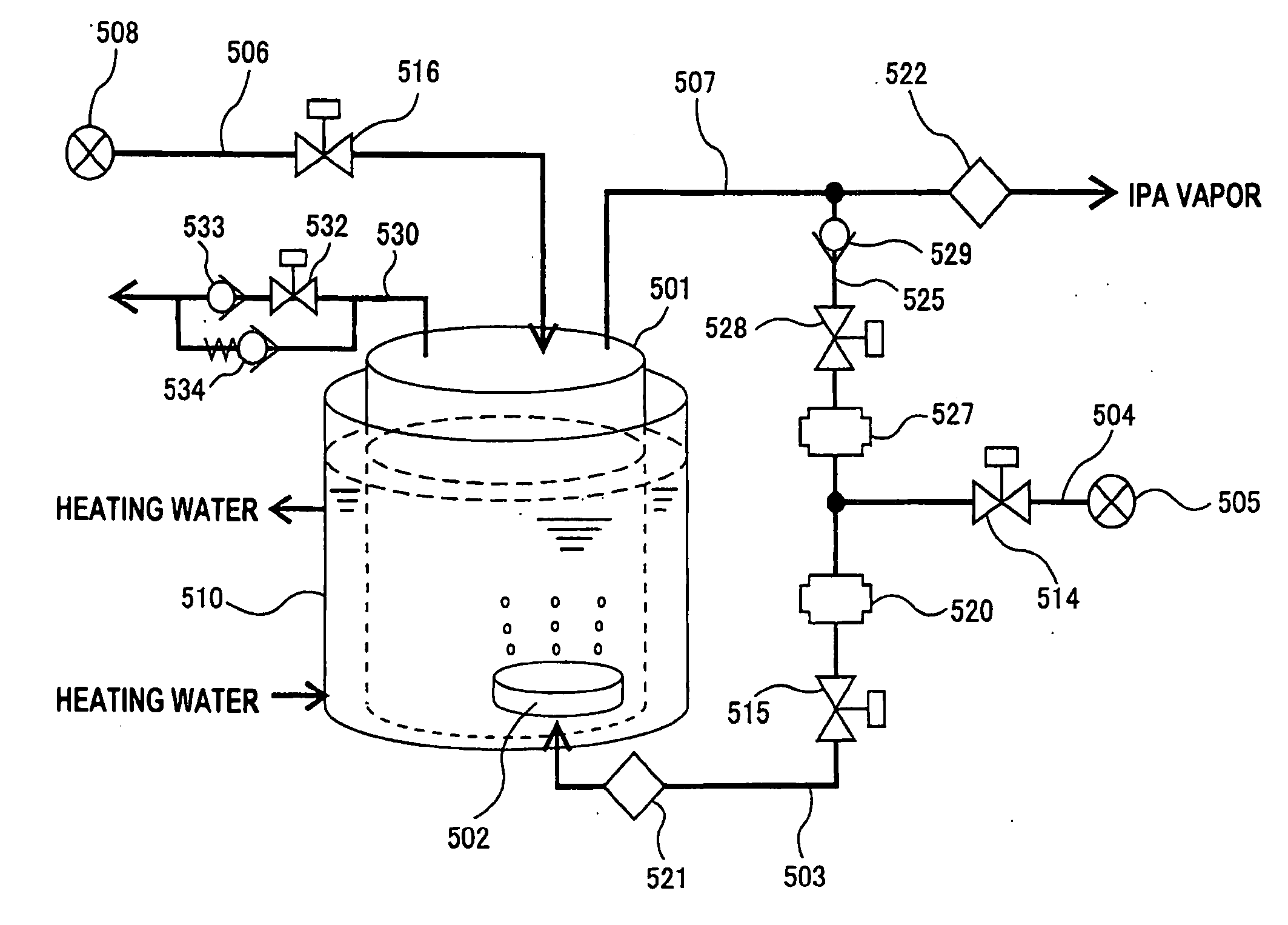
InactiveUS20050217137A1Efficient processingImprove Wafer YieldDrying using combination processesDrying solid materials without heatEngineeringMeniscus
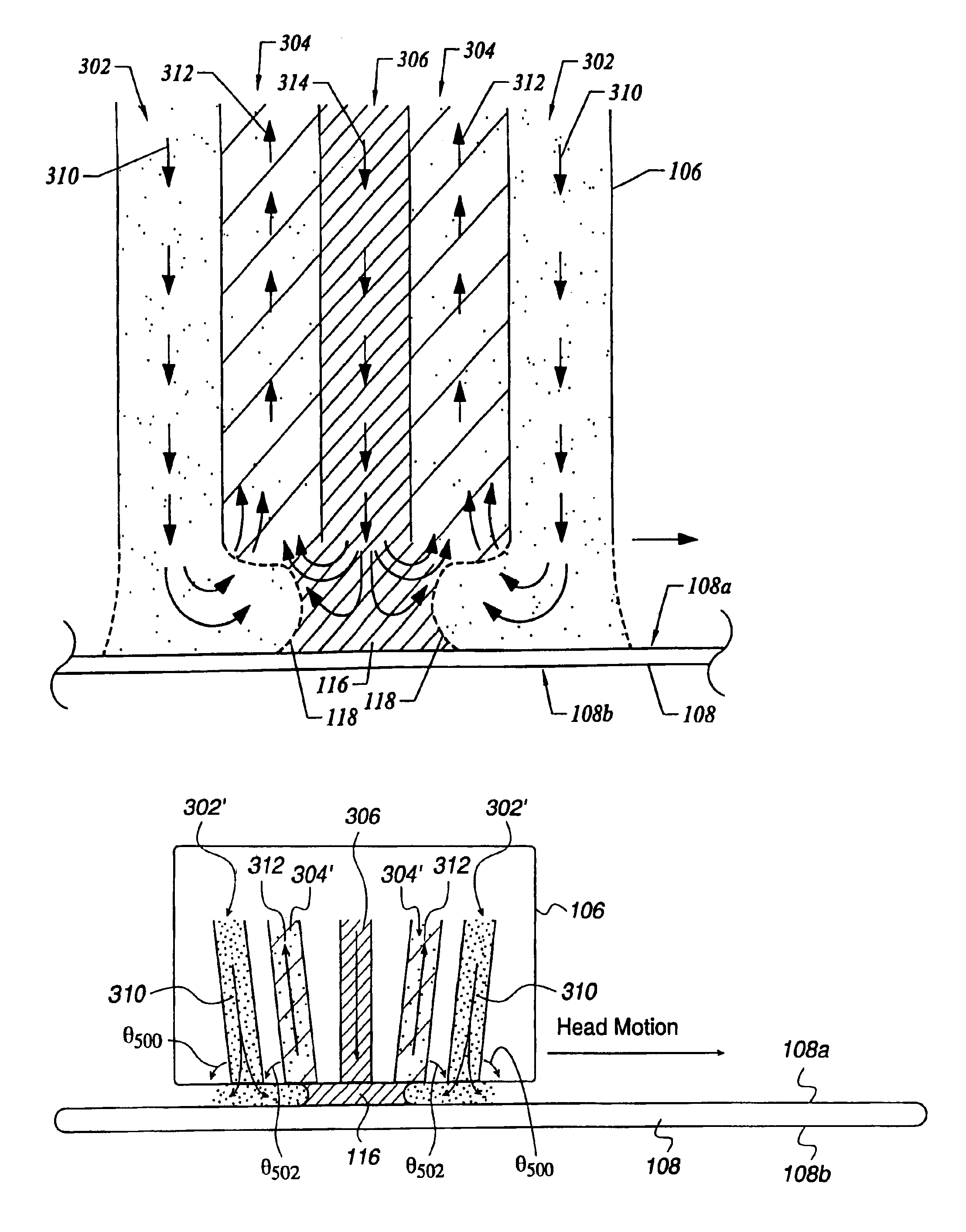
In one of the many embodiments, a method for processing a substrate is disclosed which includes generating a first fluid meniscus and a second fluid meniscus at least partially surrounding the first fluid meniscus wherein the first fluid meniscus and the second fluid meniscus are generated on a surface of the substrate.
Owner:LAM RES CORP
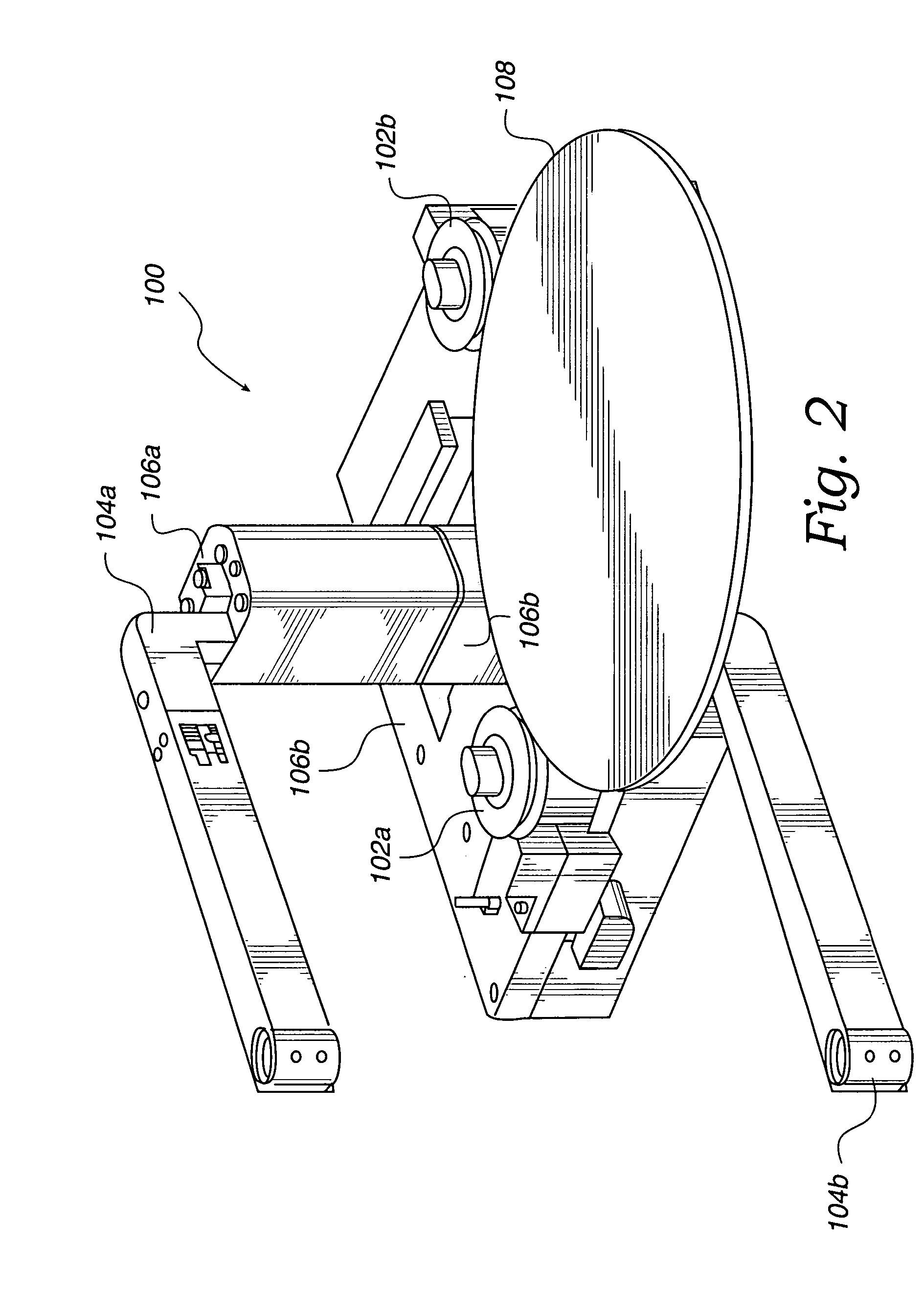
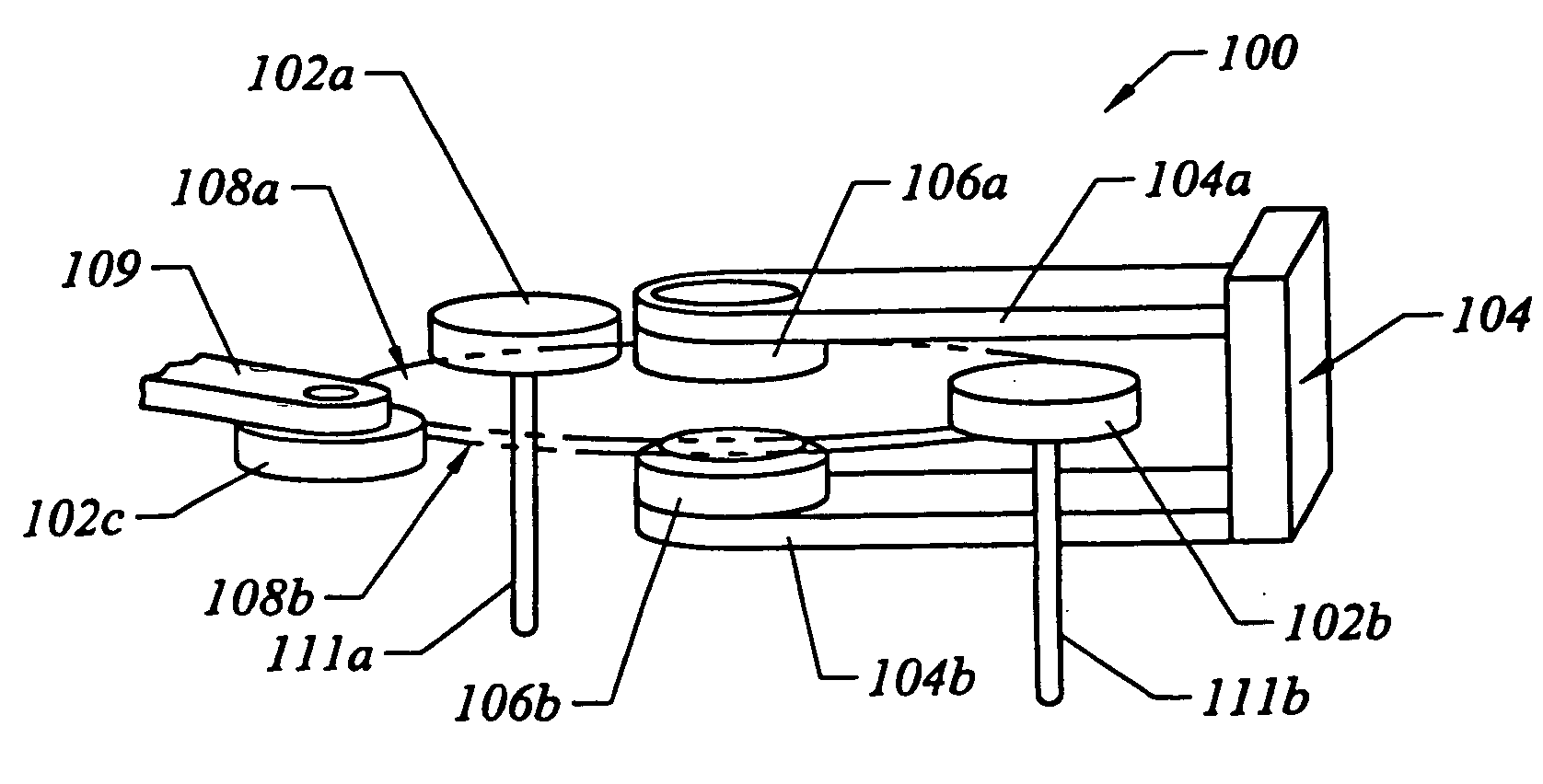
Method and apparatus for processing wafer surfaces using thin, high velocity fluid layer
InactiveUS20050145265A1Efficient processingReducing unwanted fluidElectrolysis componentsDrying solid materials without heatHigh velocityBiomedical engineering
Among the many embodiment, in one embodiment, a method for processing a substrate is disclosed which includes generating a fluid layer on a surface of the substrate, the fluid layer defining a fluid meniscus. The generating includes moving a head in proximity to the surface, applying a fluid from the head to the surface while the head is in proximity to the surface of the substrate to define the fluid layer, and removing the fluid from the surface through the proximity head by a vacuum. The fluid travels along the fluid layer between the head and the substrate at a velocity that increases as the head is in closer proximity to the surface.
Owner:LAM RES CORP
Lyophilization process and products obtained thereby
ActiveUS20070116729A1Dissolve fastSuitable for useBiocidePowder deliveryHigh concentrationFreeze-drying
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
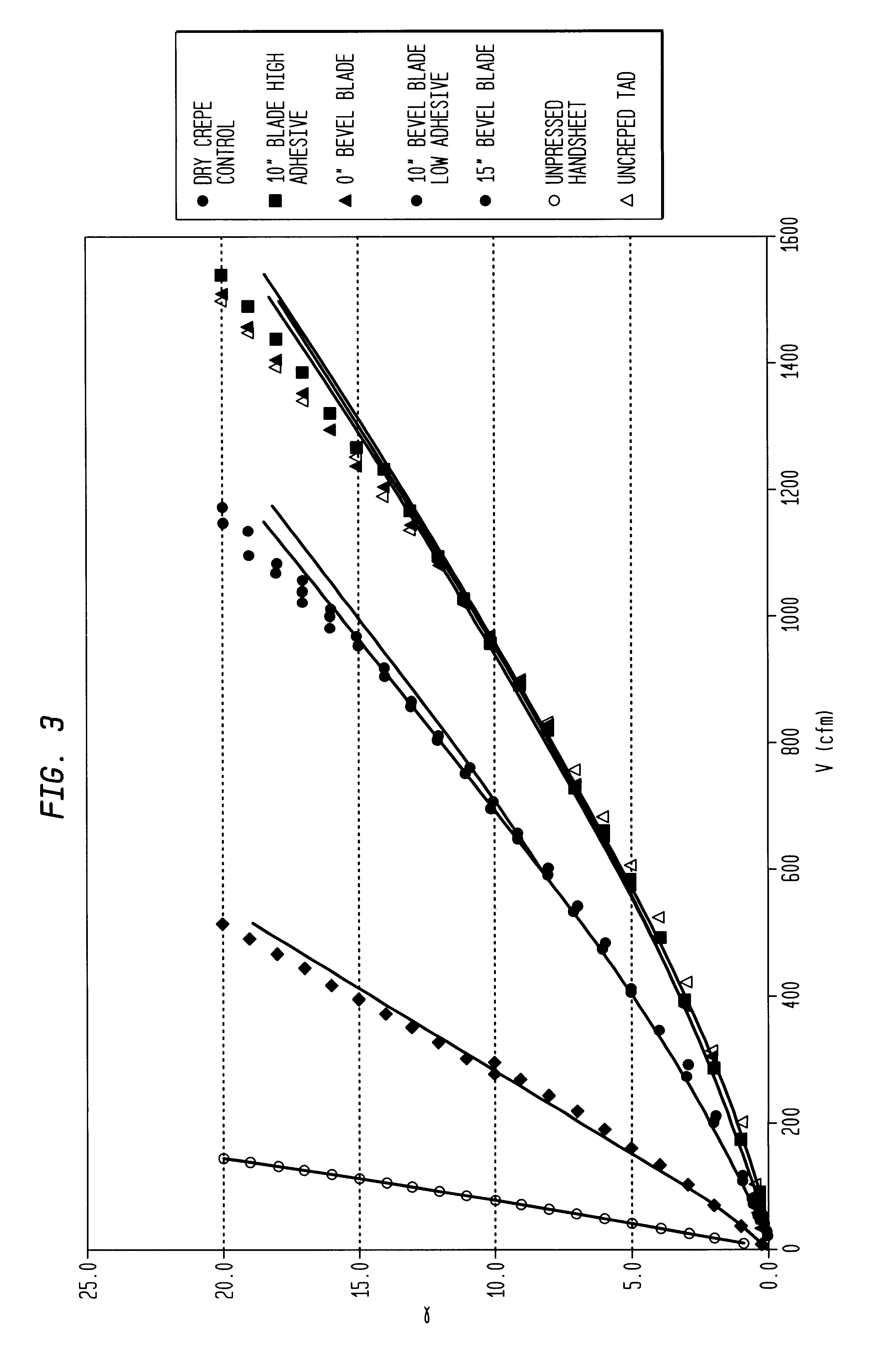
Wet crepe, impingement-air dry process for making absorbent sheet
InactiveUS6432267B1Reduce penetrationIncrease flexibilityDrying using combination processesNon-fibrous pulp additionPulp and paper industryPaper sheet
A wet crepe, impingement-air dried process for producing absorbent paper sheet is disclosed. In preferred embodiments, the process utilizes recycle furnish and the web is delaminated as it is wet-creped from a Yankee dryer. Particular embodiments include high consistency (after-crepe) wet-shaping prior to impingement air drying on a drilled vacuum roll.
Owner:GPCP IP HLDG LLC
Heat pump clothes dryer
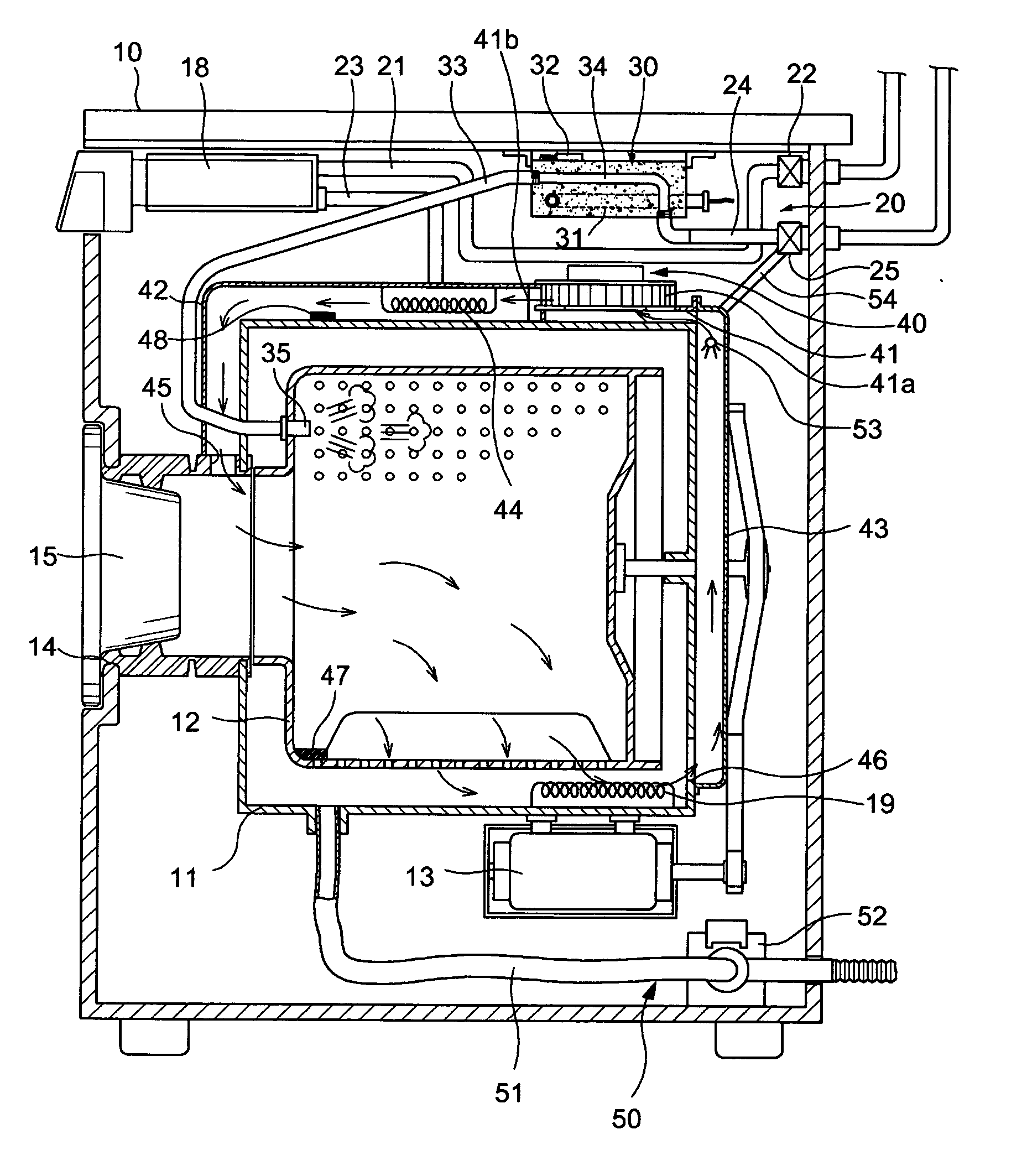
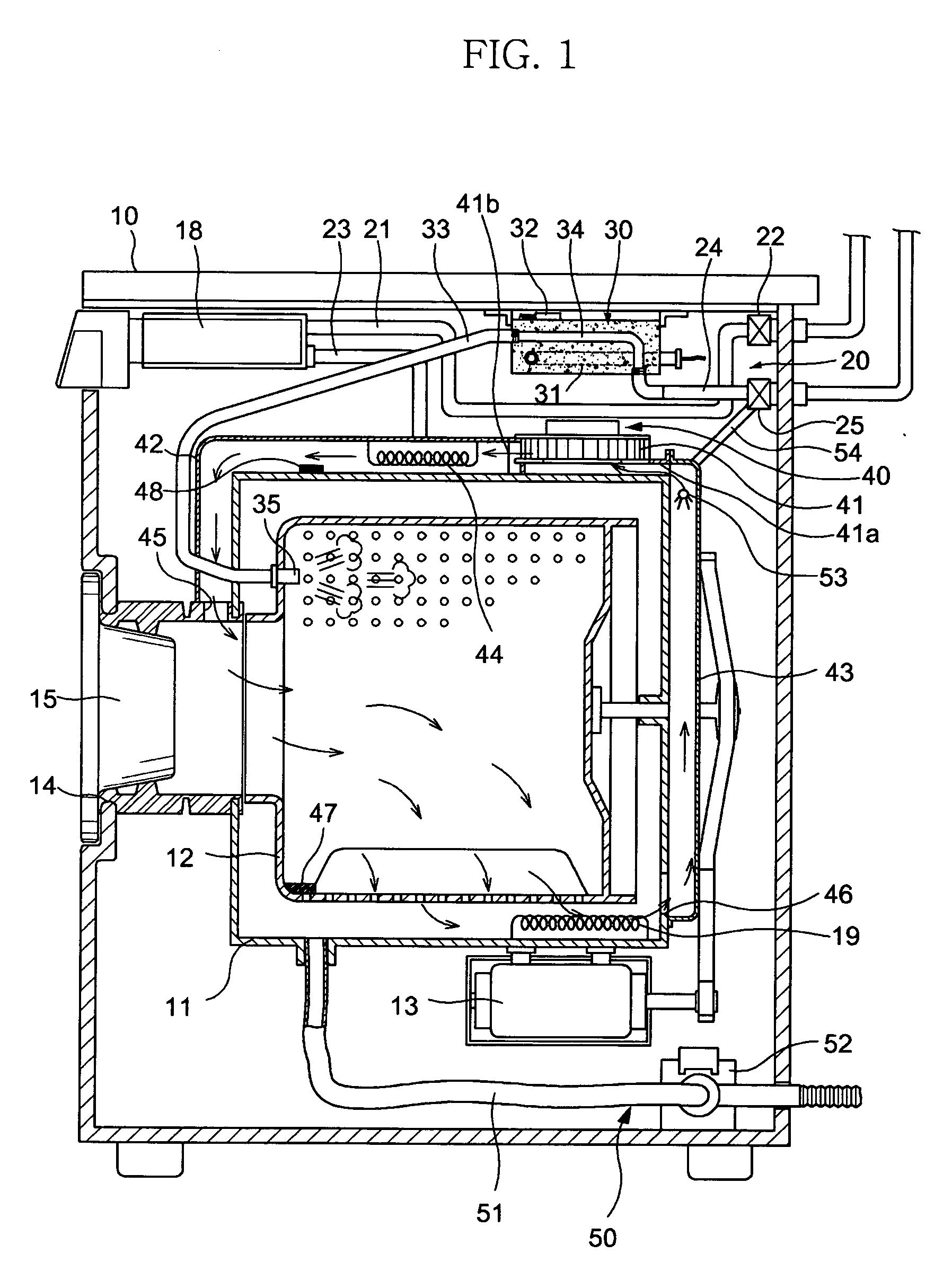
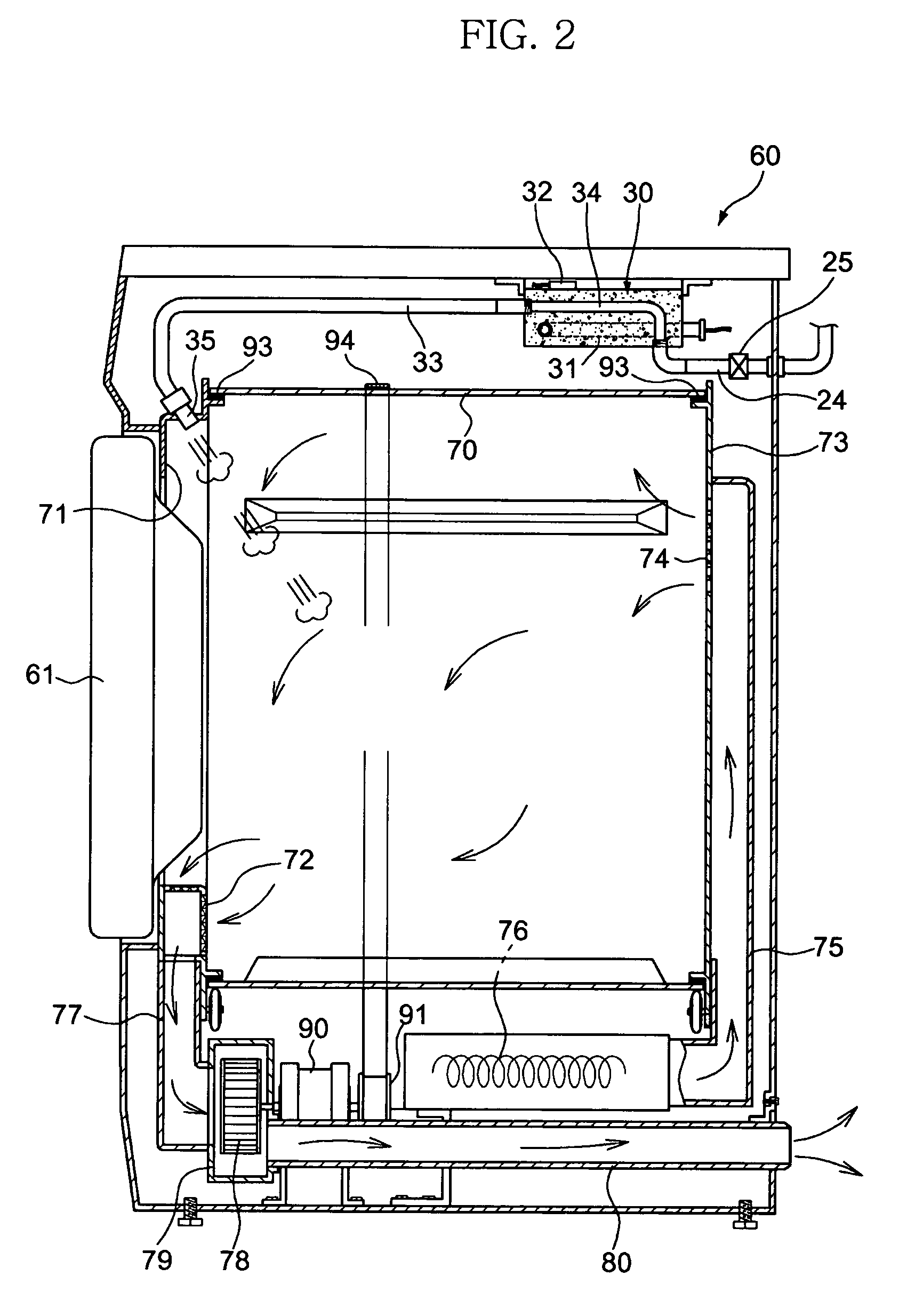
InactiveUS7055262B2Improved performance and efficiencyDrying solid materials without heatDrying gas arrangementsEngineeringRefrigerant
A drying apparatus for drying articles such as clothing is provided. The drying apparatus includes a chamber for containing articles to be dried and a system for supplying heated dry air at a first temperature to the chamber. The air supplying system comprises an air flow pathway having an evaporator for removing moisture from air exiting the chamber and for decreasing the temperature of the air to below dew point temperature. The air supply system further has a condenser for increasing the temperature of the air exiting the evaporator to the first temperature. The drying apparatus further has a heat pump system having a refrigerant loop which includes a compressor, the condenser, a TEV valve, and the evaporator.
Owner:FLI HLDG COMPANY
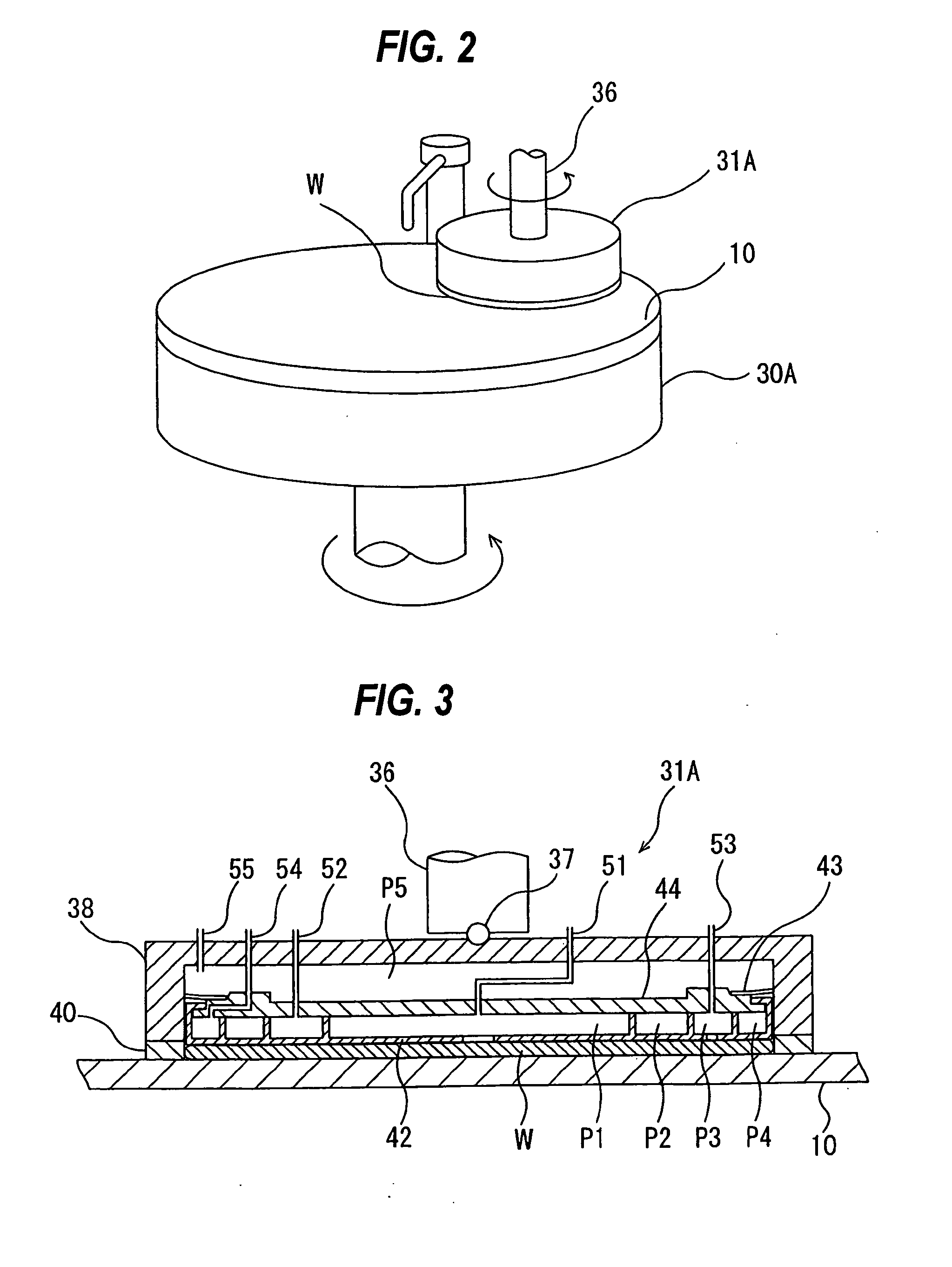
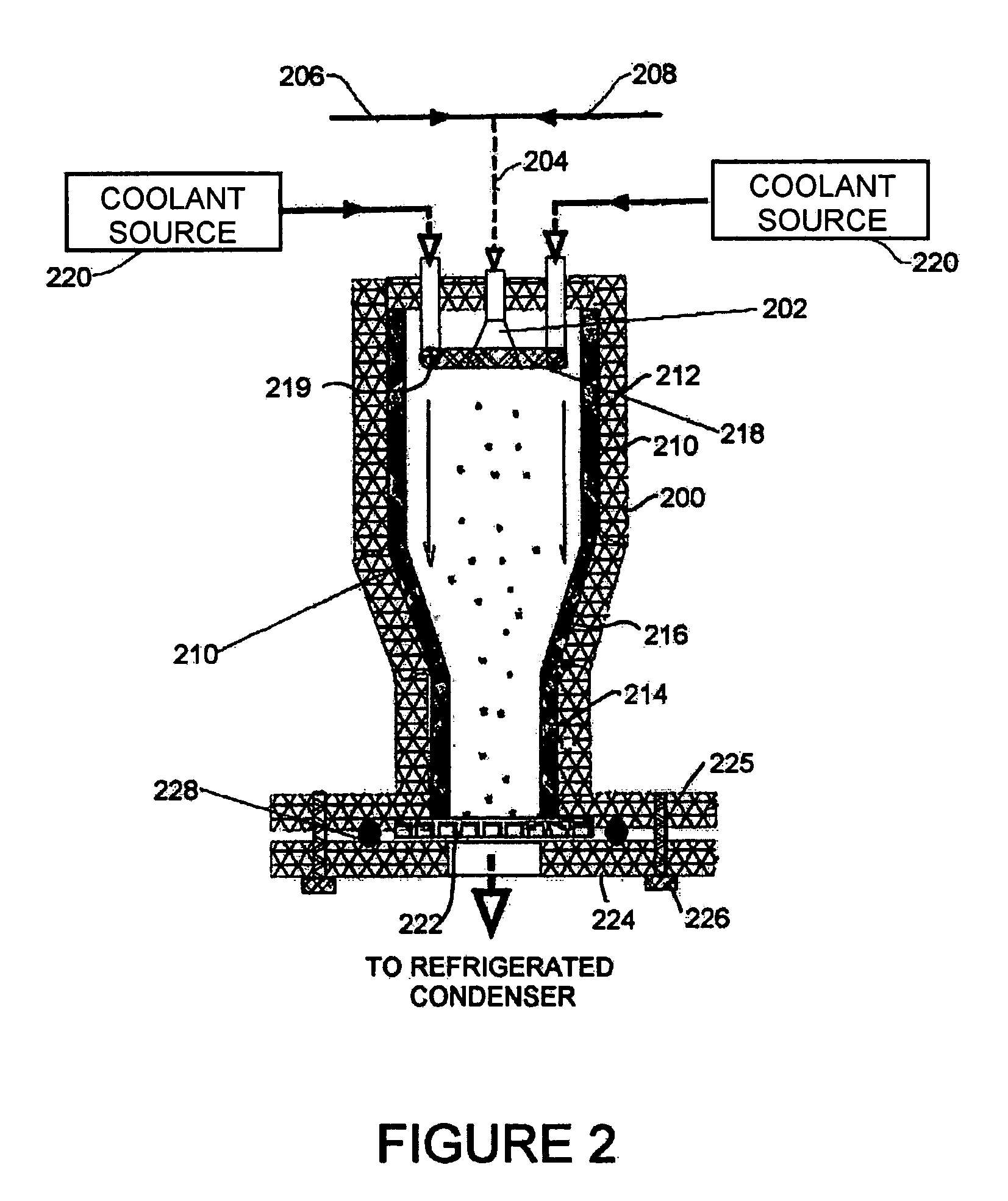
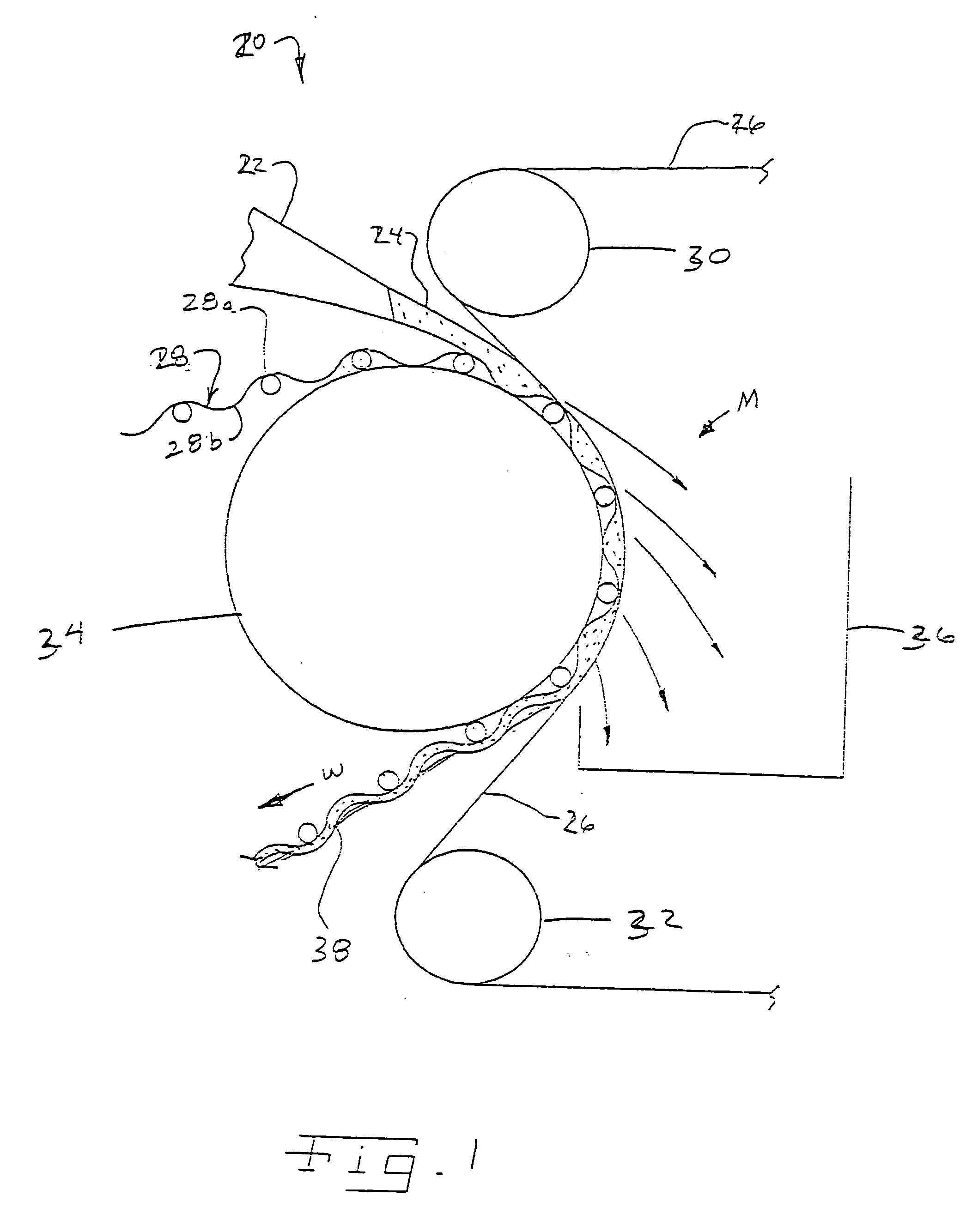
Methods and systems for processing a substrate using a dynamic liquid meniscus
InactiveUS6988327B2Increase in sizeHigh gradientDrying solid materials with heatDrying solid materials without heatEngineeringLiquid meniscus
A system and method of moving a meniscus from a first surface to a second surface includes forming a meniscus between a head and a first surface. The meniscus can be moved from the first surface to an adjacent second surface, the adjacent second surface being parallel to the first surface. The system and method of moving the meniscus can also be used to move the meniscus along an edge of a substrate.
Owner:LAM RES CORP
Fabric crepe and in fabric drying process for producing absorbent sheet
ActiveUS20050241787A1Decrease sidednessReduced areaNon-fibrous pulp additionNatural cellulose pulp/paperCellulose fiberPulp and paper industry
A method of making a cellulosic web includes: forming a nascent web from a papermaking furnish, the nascent web having a generally random distribution of papermaking fiber; b) transferring the web having a generally random distribution of papermaking fiber to a translating transfer surface moving at a first speed; drying the web to a consistency of from about 30 to about 60 percent including compactively dewatering the web prior to or concurrently with transfer to the transfer surface; fabric-creping the web from the transfer surface at a consistency of from about 30 to about 60 percent utilizing a creping fabric with a patterned creping surface, the fabric creping step occurring under pressure in a fabric creping nip defined between the transfer surface and the creping fabric wherein the fabric is traveling at a second speed slower than the speed of said transfer surface, the fabric pattern, nip parameters, velocity delta and web consistency being selected such that the web is creped from the transfer surface and redistributed on the creping fabric such that the web has a plurality of fiber-enriched regions arranged in a pattern corresponding to the patterned creping surface of the fabric, optionally drying the wet web while it is held in the creping fabric. Preferably, the formed web is characterized in that its void volume increases upon drawing.
Owner:GPCP IP HLDG LLC
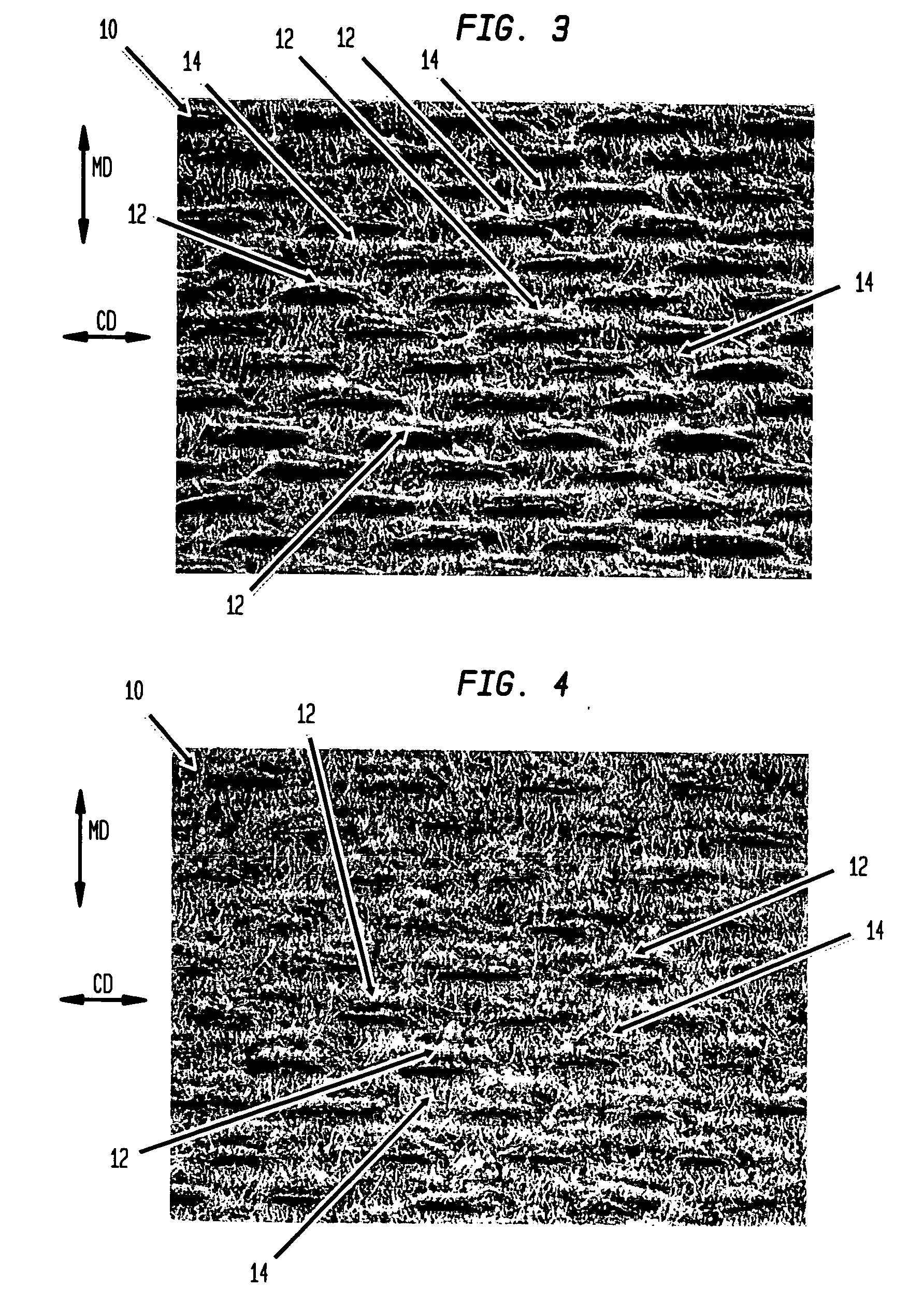
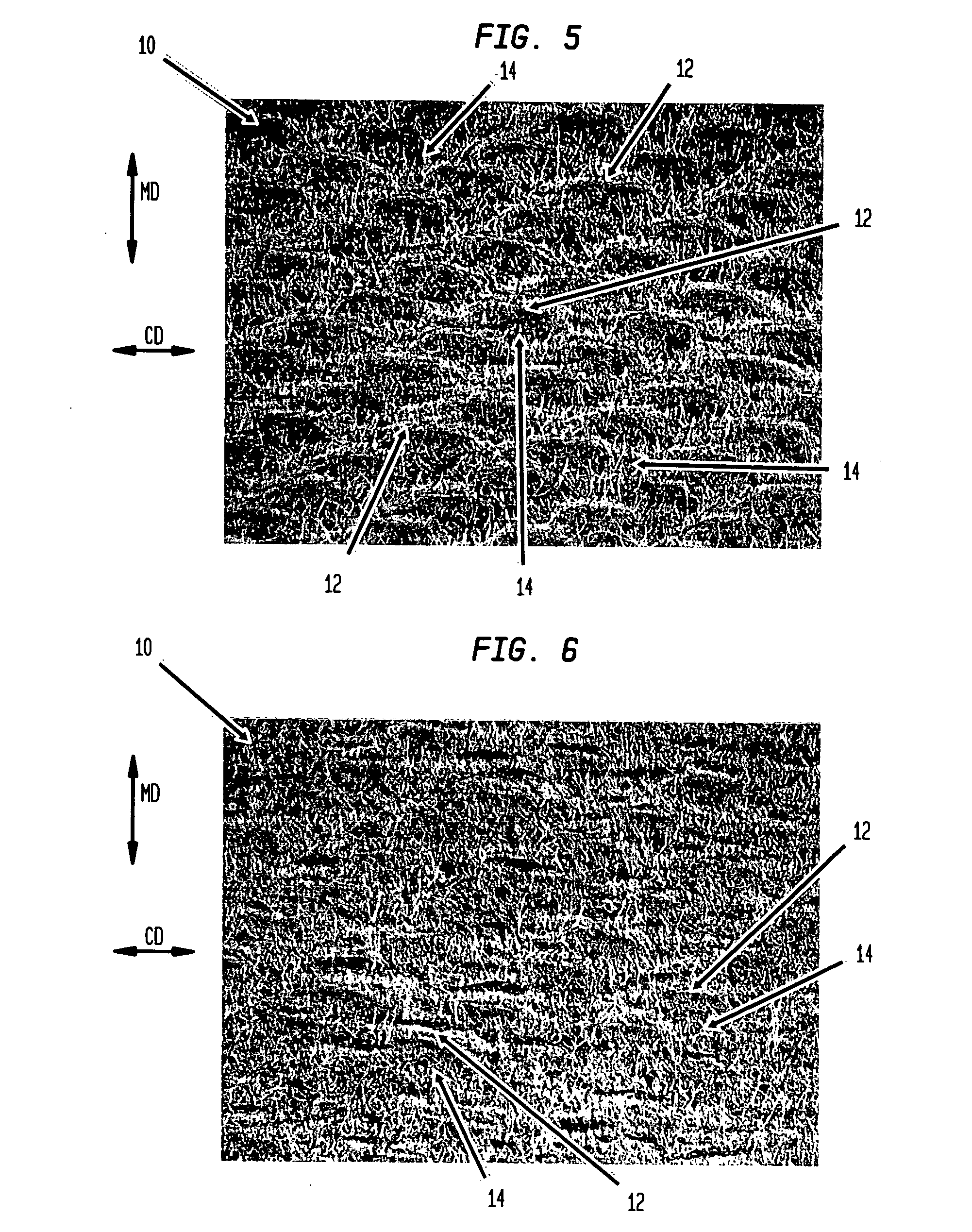
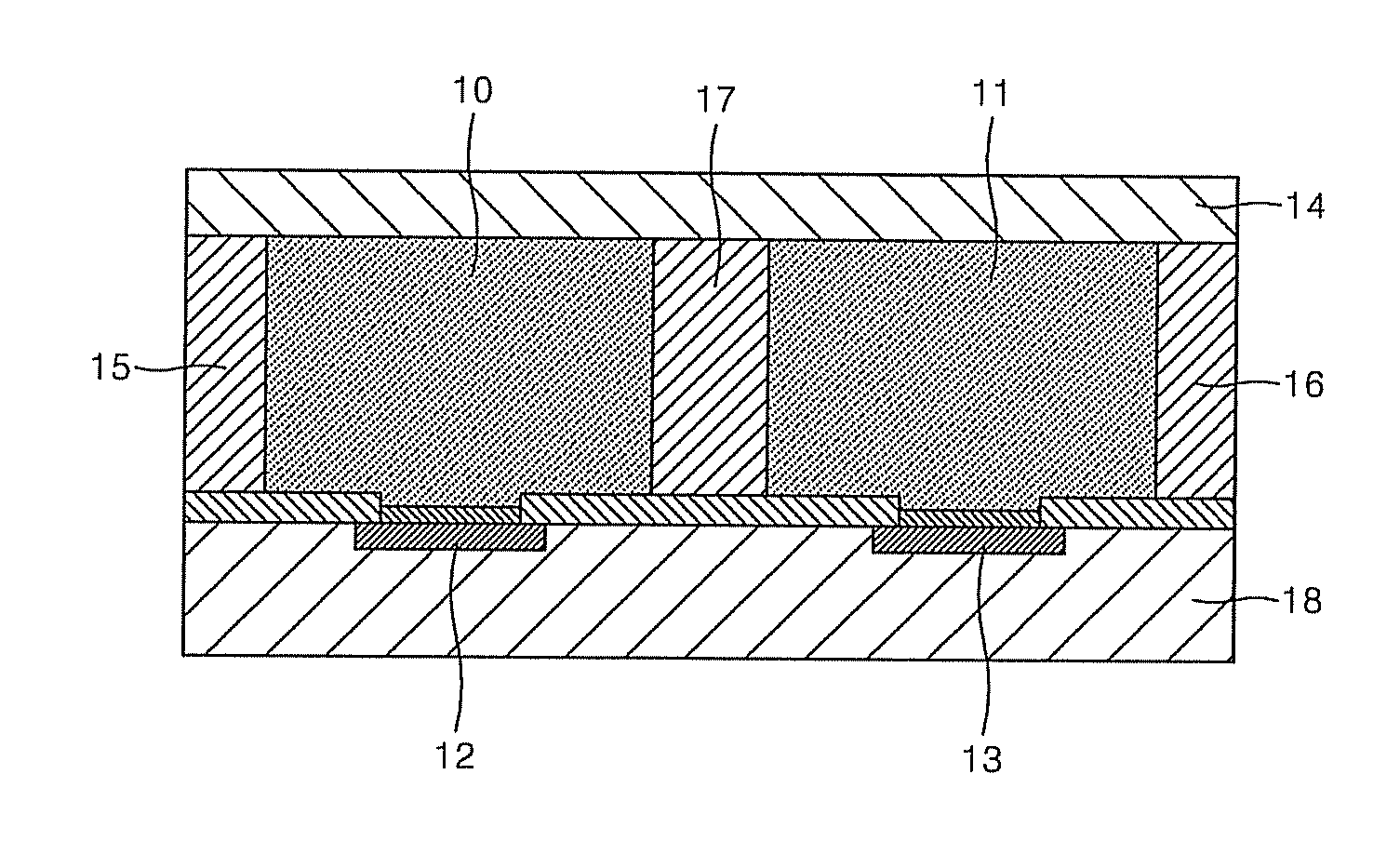
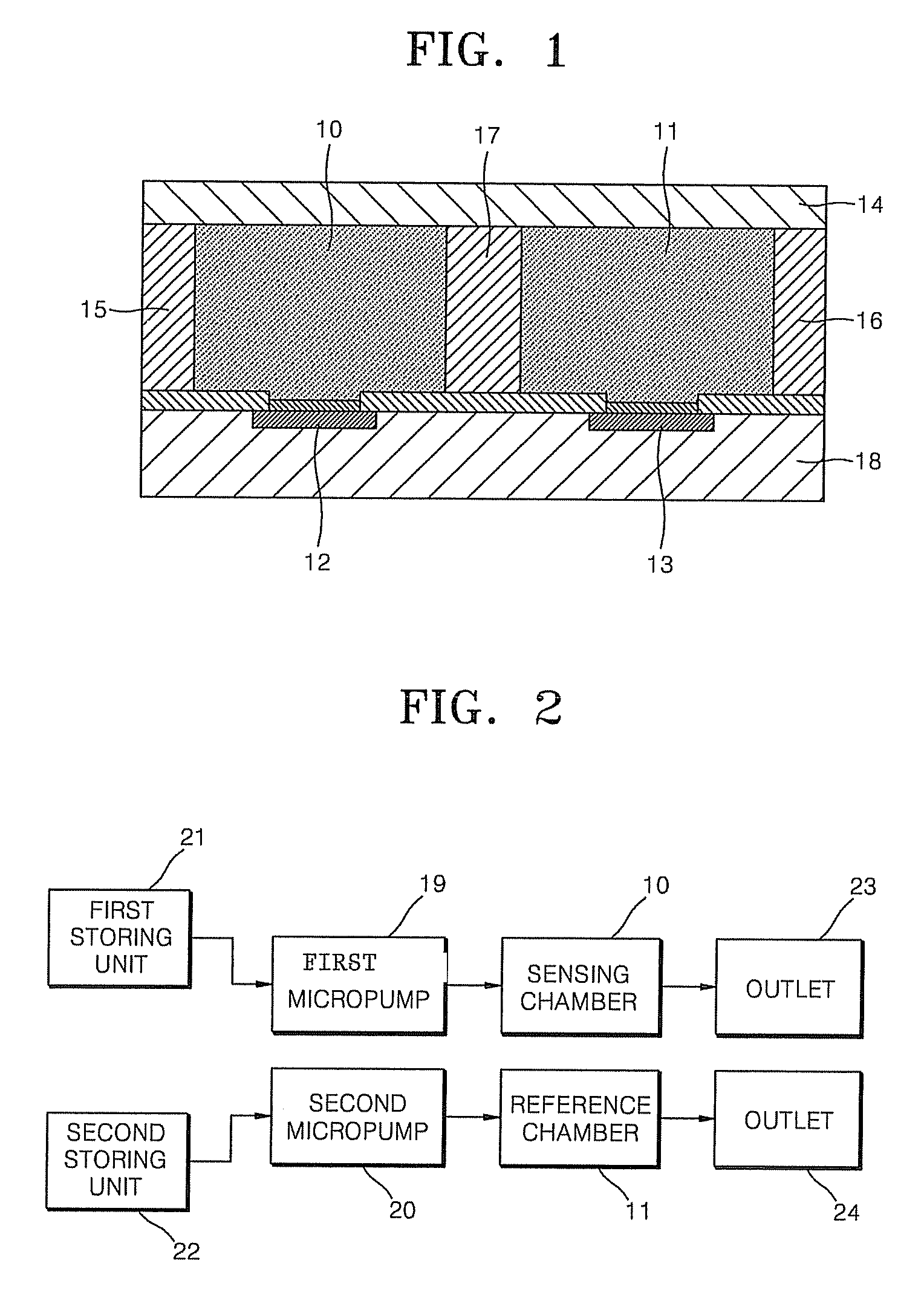
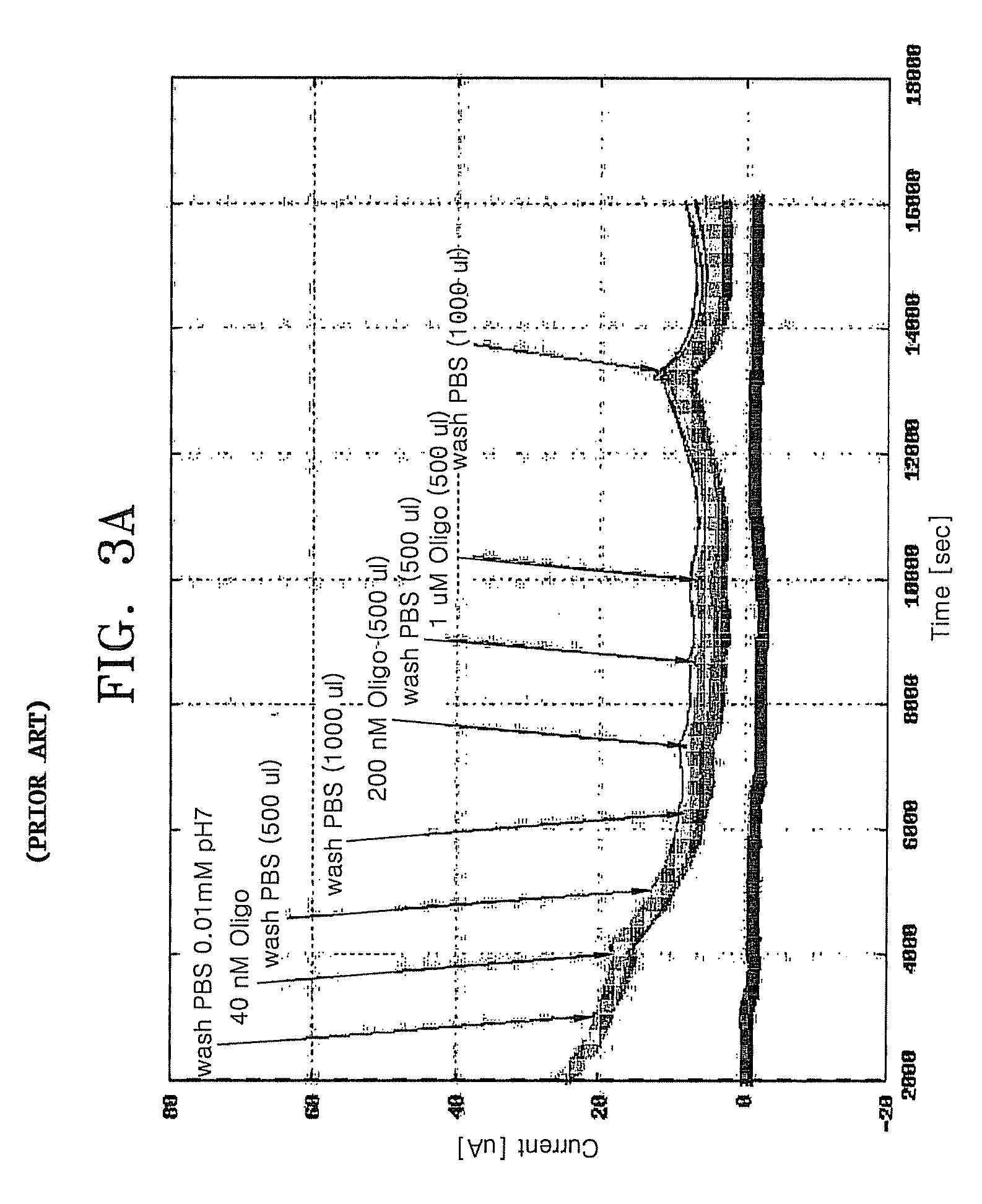
FET-based sensor for detecting ionic material, ionic material detecting device using the FET-based sensor, and method of detecting ionic material using the FET-based sensor
ActiveUS7859029B2Reduce concentrationHigh sensitivityDrying solid materials without heatSolid-state devicesEngineeringElectric current
Provided are a FET-based sensor for detecting an ionic material, an ionic material detecting device including the FET-based sensor, and a method of detecting an ionic material using the FET-based sensor. The FET-based sensor includes: a sensing chamber including a reference electrode and a plurality of sensing FETs; and a reference chamber including a reference electrode and a plurality of reference FETs. The method includes: flowing a first solution into and out of the sensing chamber and the reference chamber of the FET-based sensor; flowing a second solution expected to contain an ionic material into and out of the sensing chamber while continuously flowing the first solution into and out of the reference chamber; measuring a current in a channel region between the source and drain of each of the sensing and reference FETs; and correcting the current of the sensing FETs.
Owner:SAMSUNG ELECTRONICS CO LTD
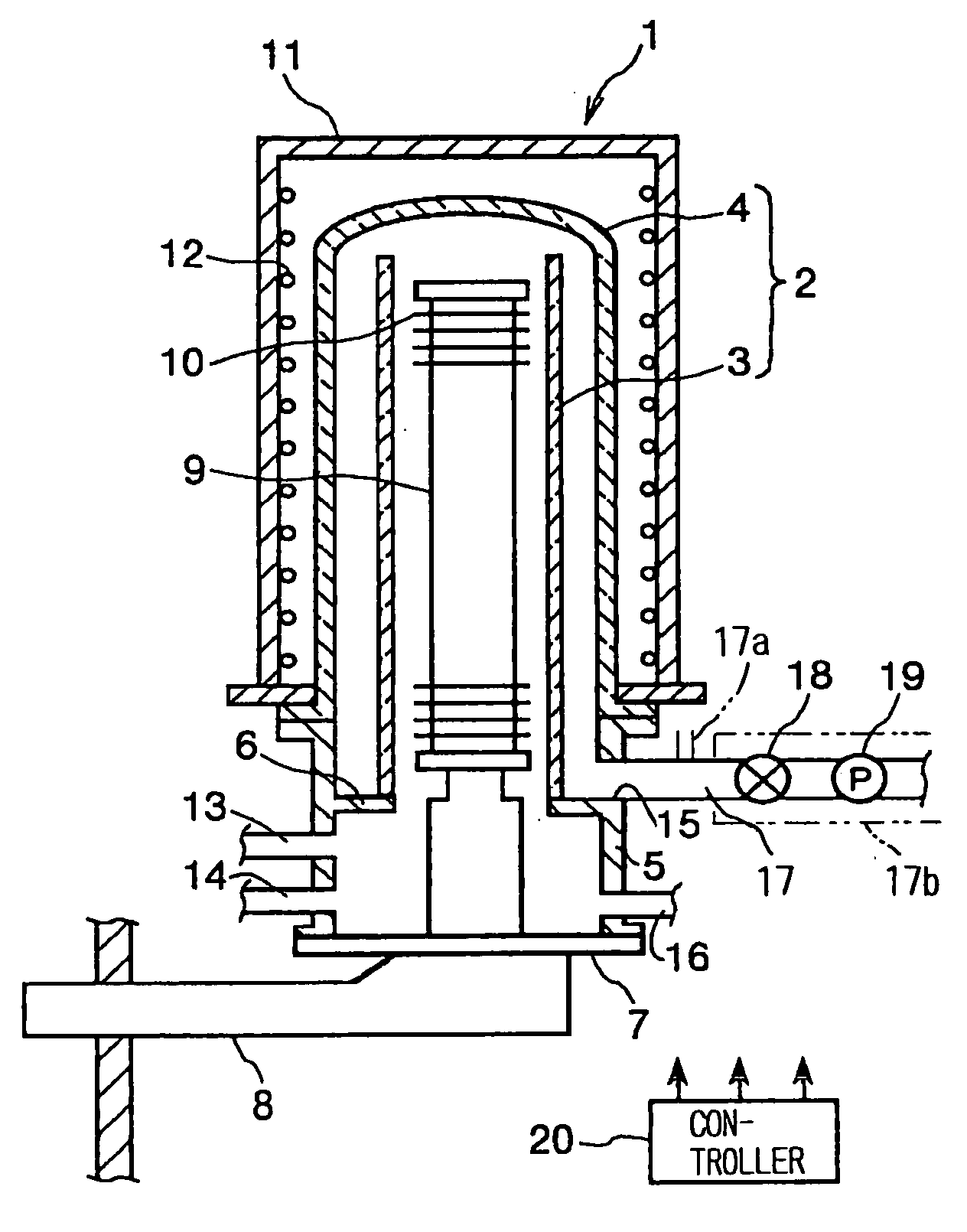
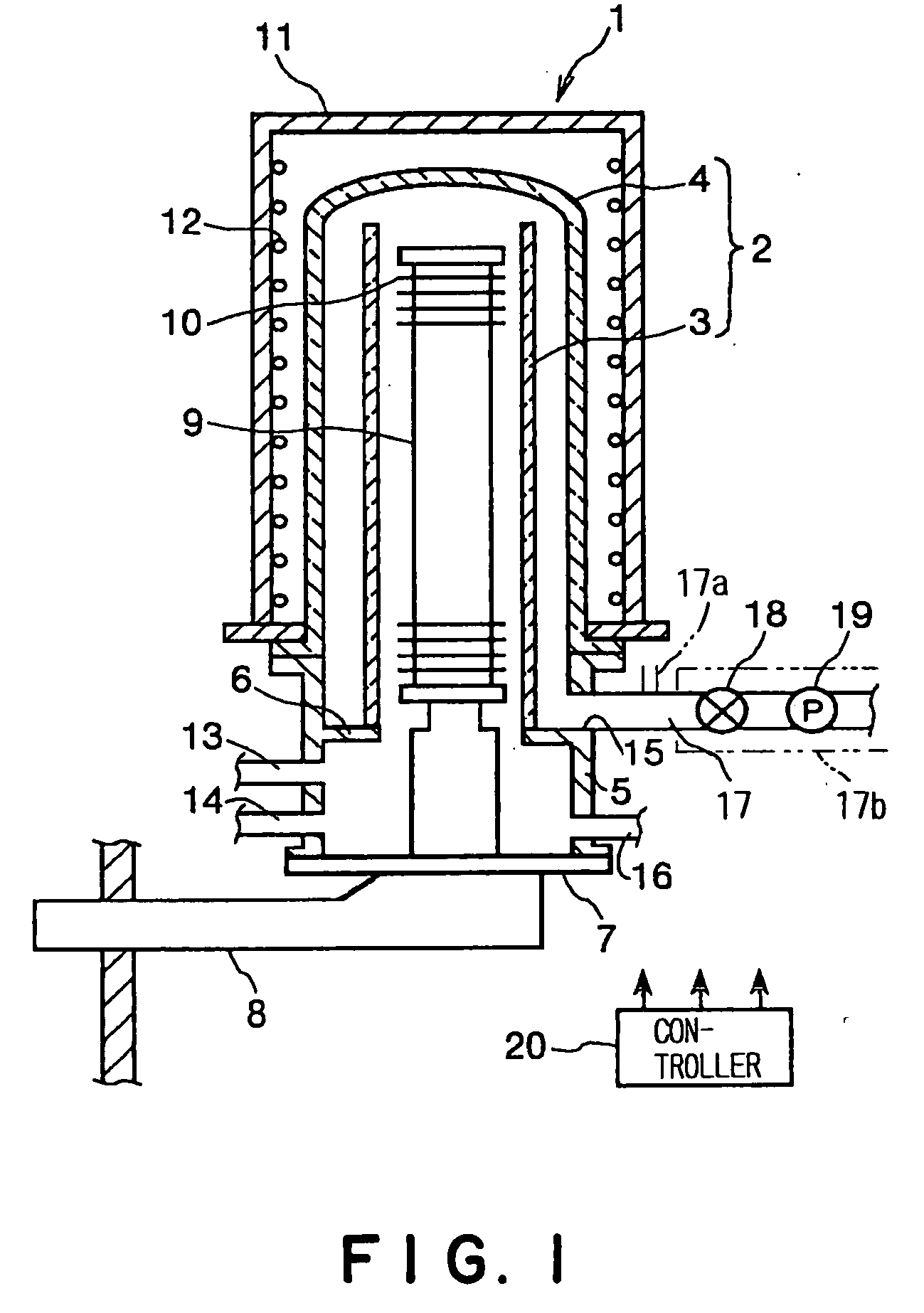
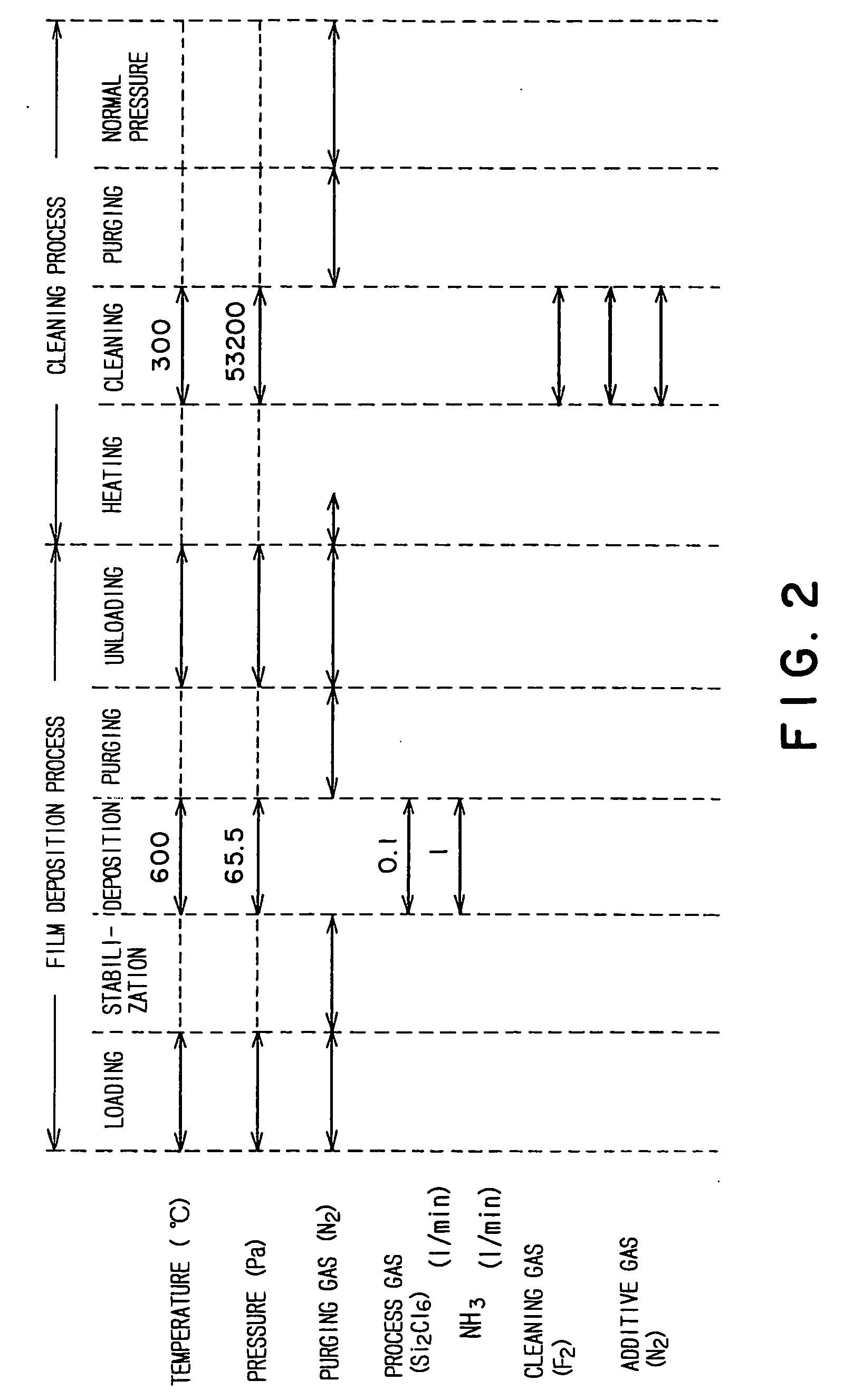
Thin film forming apparatus cleaning method
InactiveUS20050090123A1Inhibit deteriorationIncrease etch rateDrying solid materials without heatSemiconductor/solid-state device manufacturingCleaning methodsNitrogen gas
A cleaning process for cleaning a thermal processing apparatus includes: a heating step of heating an interior of a reaction tube at 300° C., and a cleaning step of removing deposits deposited in the thermal processing apparatus. In the cleaning step, a cleaning gas containing fluorine gas, chlorine gas and nitrogen gas is supplied into the interior of the reaction tube heated at 300° C. to remove silicon nitride so to clean an interior of the thermal processing apparatus.
Owner:TOKYO ELECTRON LTD
High solids fabric crepe process for producing absorbent sheet with in-fabric drying
ActiveUS20050279471A1Furnish toleranceAbsorbency, bulk and stretch is improvedNon-fibrous pulp additionNatural cellulose pulp/paperCellulosePolymer science
A method of making a fabric-creped absorbent cellulosic sheet is provided which includes dewatering a papermaking furnish and partially drying the web without wet-pressing before applying it to a translating transfer surface moving at a first speed. The process further includes fabric-creping the web from the transfer surface at a consistency of from about 30 to about 60 percent utilizing a creping fabric, the creping step occurring under pressure in a creping nip defined between the transfer surface and the creping fabric wherein the fabric is traveling at a second speed slower than the speed of said transfer surface, the fabric pattern, nip parameters, velocity delta and web consistency being selected such that the web is creped from the surface and redistributed on the creping fabric. After creping, the web is dried, preferably with a plurality of can dryers to a consistency of at least about 90 percent while it is held in the creping fabric.
Owner:GPCP IP HLDG LLC
Process for production of nanoparticles and microparticles by spray freezing into liquid
InactiveUS6862890B2Improve efficiencyImprove efficacyPowder deliveryNervous disorderPorosityNanoparticle
The present invention provides a system and a method for the production of microparticles and nanoparticles of materials that can be dissolved. The system and method of the present invention provide quicker freezing times, which in turn produces a more uniform distribution of particle sizes, smaller particles, particles with increased porosity and a more intimate mixing of the particle components. The system and method of the present invention also produce particles with greater surface area than conventional methods. One form of the present invention provides a method for the preparation of particles. An effective ingredient is mixed with water, one or more solvents, or a combination thereof, and the resulting mixture is sprayed through an insulating nozzle located at or below the level of a cryogenic liquid. The spray generates frozen particles.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for processing and preserving collagen-based tissues for transplantation
InactiveUS20060210960A1Minimize surface tension damageAugment selective preservationSsRNA viruses positive-senseViral antigen ingredientsOrganismBiology
A method for processing and preserving an acellular collagen-based tissue matrix for transplantation is disclosed. The method includes the steps of processing biological tissues with a stabilizing solution to reduce procurement damage, treatment with a processing solution to remove cells, treatment with a cryoprotectant solution followed by freezing, drying, storage and rehydration under conditions that preclude functionally significant damage and reconstitution with viable cells.
Owner:LIFECELL
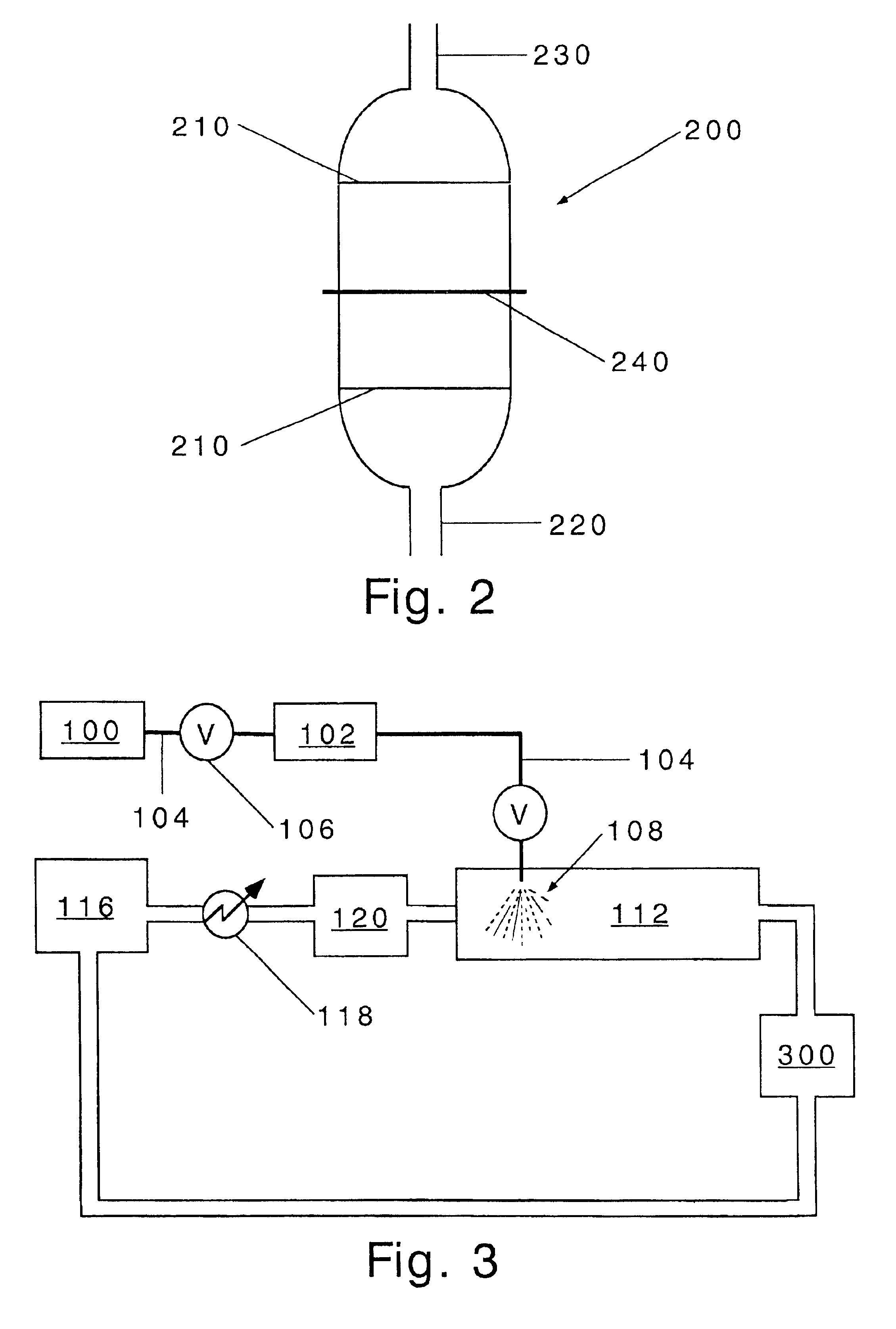
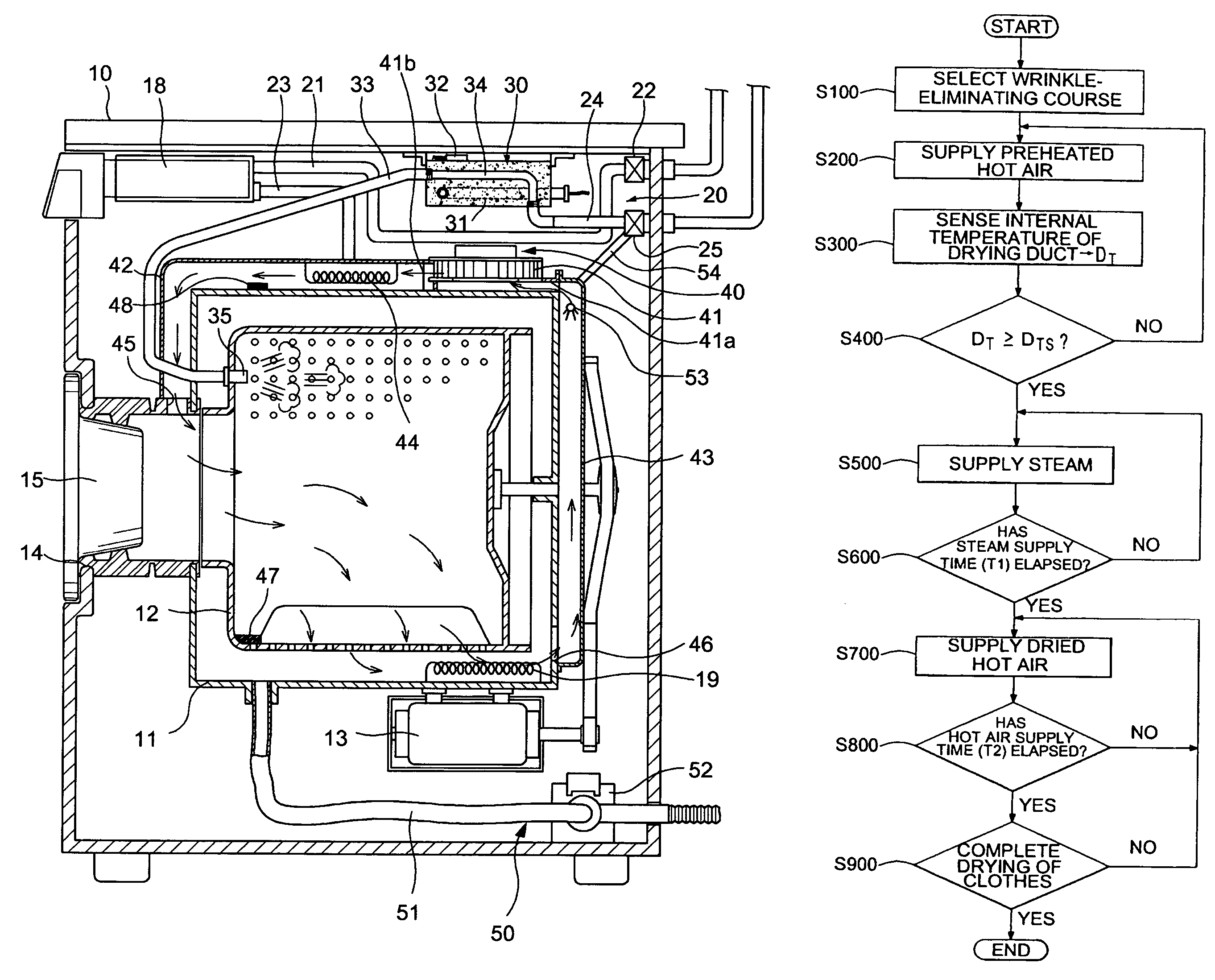
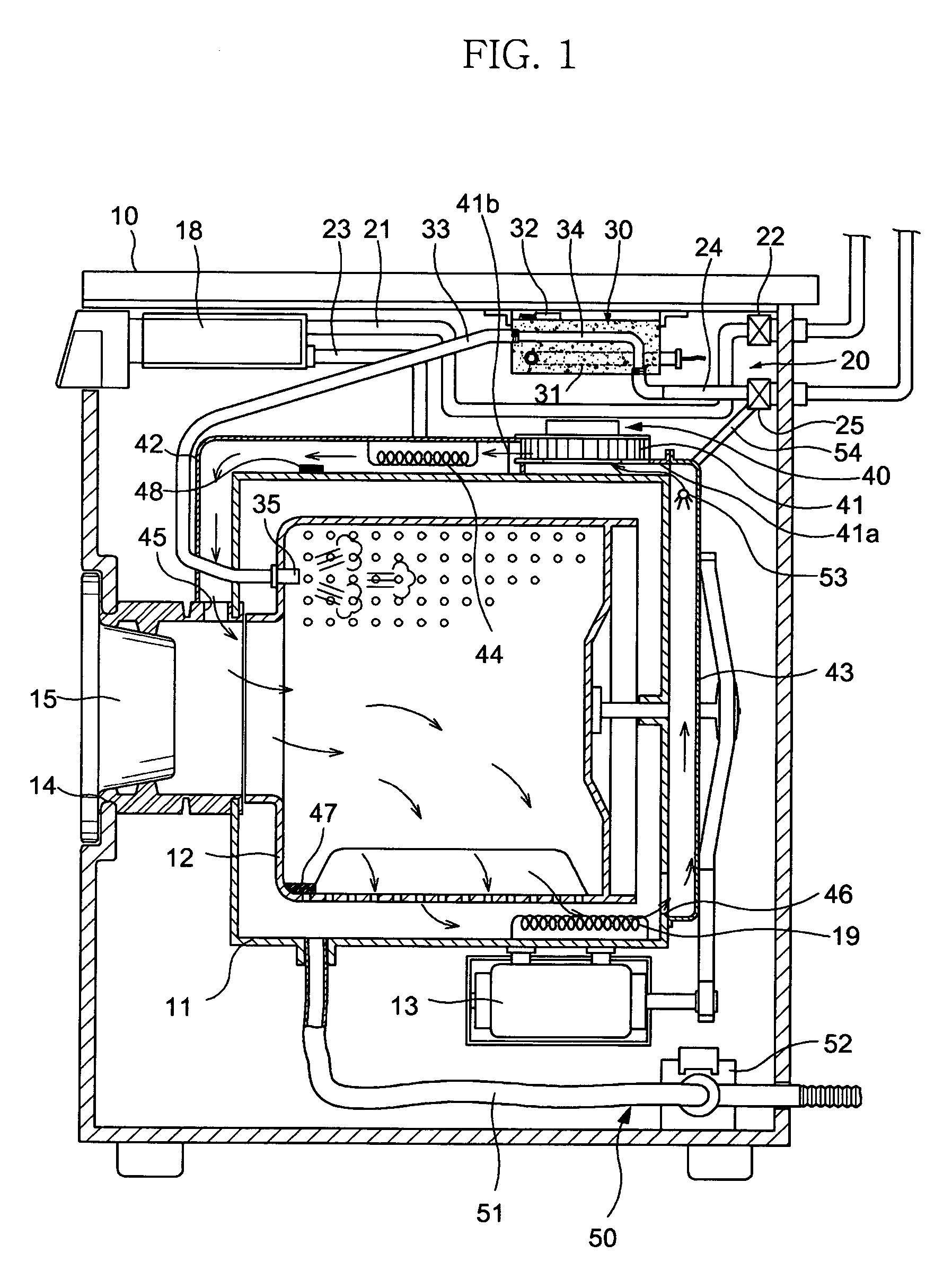
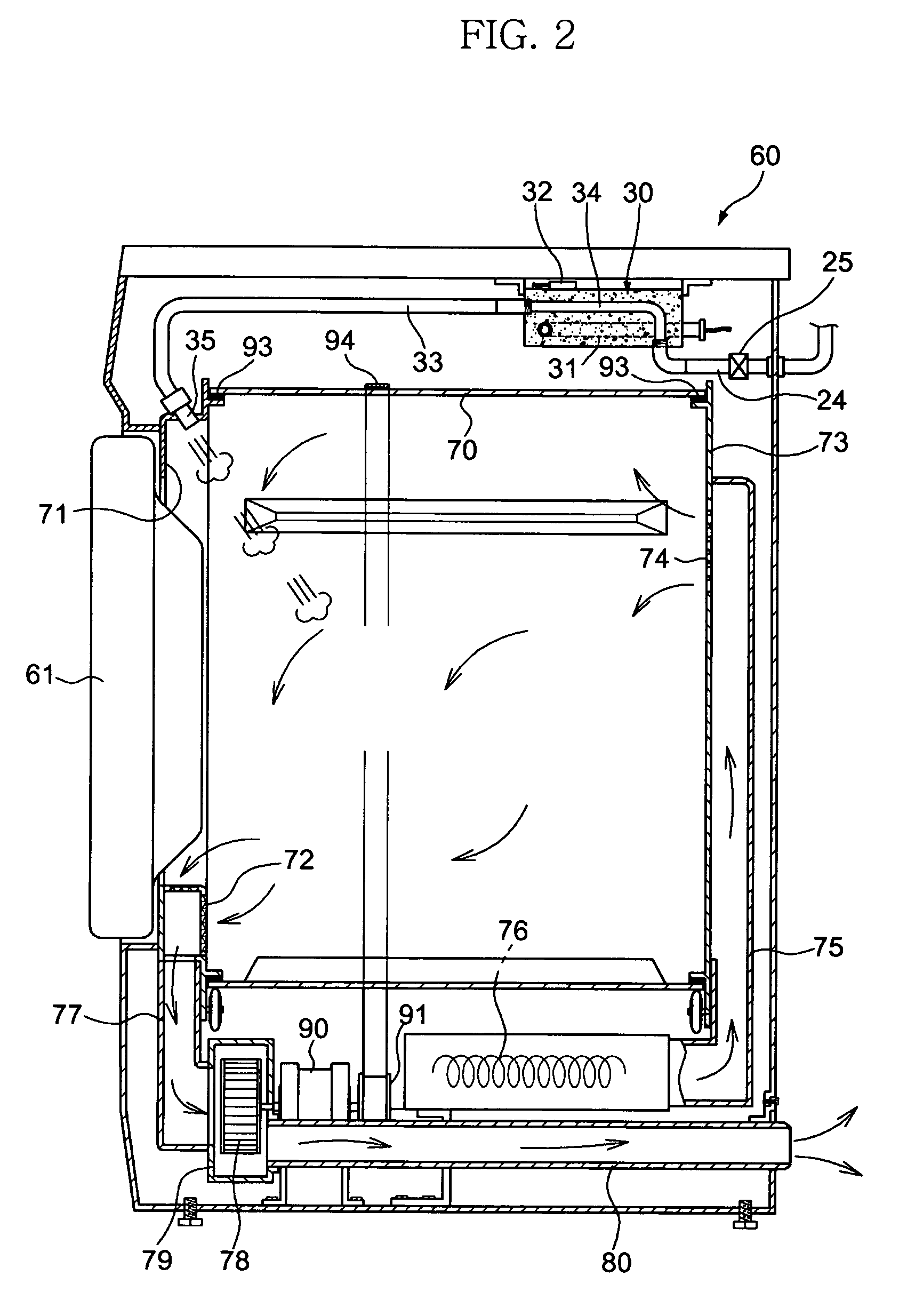
Apparatus and method for eliminating wrinkles in clothes
ActiveUS7325330B2Eliminate wrinklesEffectively eliminating the wrinkles in the clothesDrying solid materials without heatHollow article cleaningWrinkle skinEngineering
Apparatus and method for eliminating wrinkles in clothes in a washing or drying machine using steam to eliminate the wrinkles in clothes, in a state in which the clothes are worn by a user or stored, as well as in a state in which washing of the clothes is terminated. The method includes determining whether or not a wrinkle-eliminating course is selected; supplying hot air to the clothes to eliminate dust from the clothes when it is determined that the wrinkle-eliminating course is selected; and supplying steam to the clothes, from which the dust was eliminated, to eliminate the wrinkles in the clothes.
Owner:SAMSUNG ELECTRONICS CO LTD
Gas hydrate production device and gas hydrate dehydrating device
InactiveUS20050107648A1Easy to implementSmall sizeOrganic chemistryDrying solid materials without heatAmbient pressureSlurry
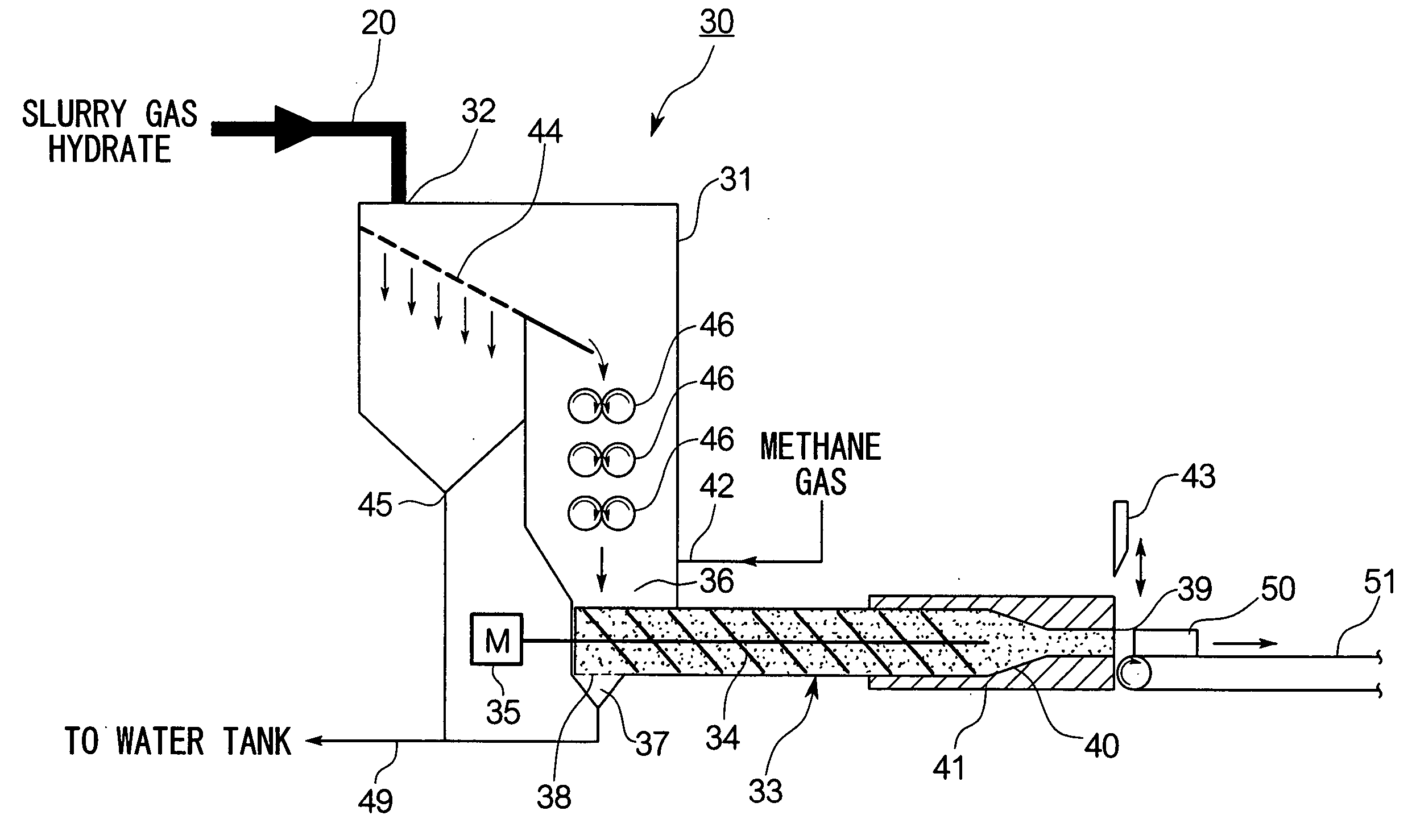
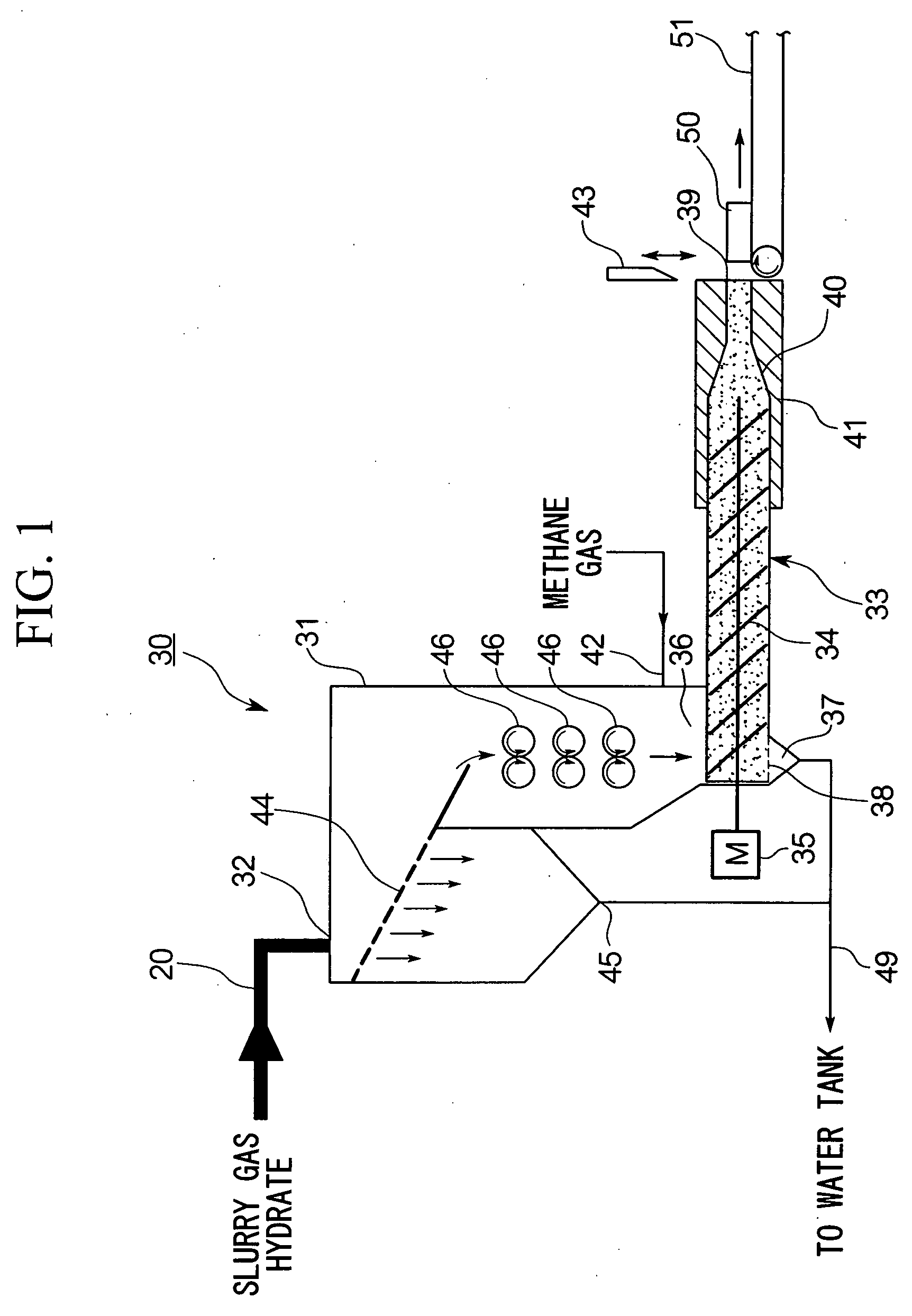
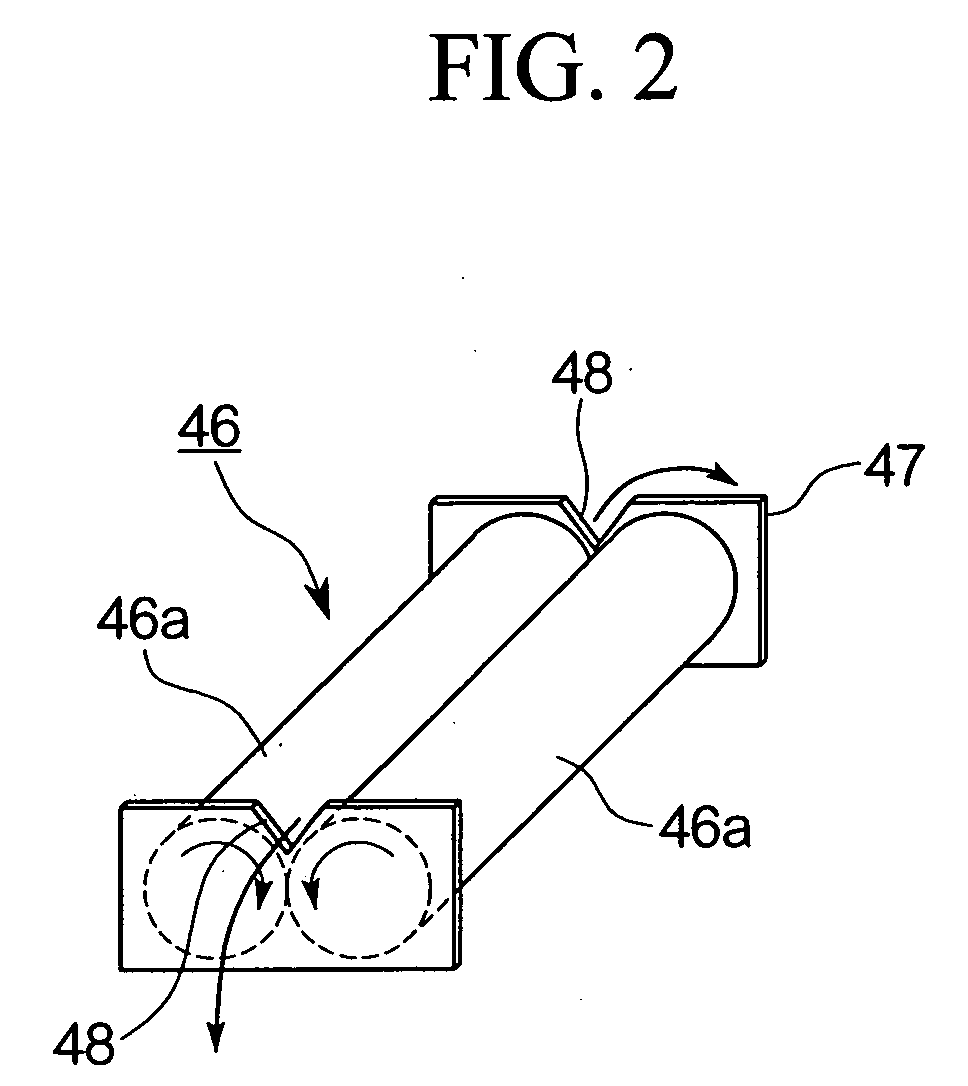
The invention relates to a gas hydrate dewatering cooling and outputting apparatus which dewaters and solidifies gas hydrate slurry and takes out the solidified gas hydrate under ambient pressure. The apparatus comprises an output apparatus body 31 having a supply port 32 for the gas hydrate slurry on an upper part of a pressure vessel, a screw extruder 33 provided at a lower part inside the output apparatus body 31 and having a drain 37 and an outlet sealing device, and a cooling device 41 which cools the vicinity of the outlet 39 of the screw extruder 33. According to such a construction, slurry gas hydrate can be efficiently and continuously dewatered, cooled and solidified, and gas hydrate powder can be consolidated into blocks and taken out to the atmosphere.
Owner:MITSUBISHI HEAVY IND LTD
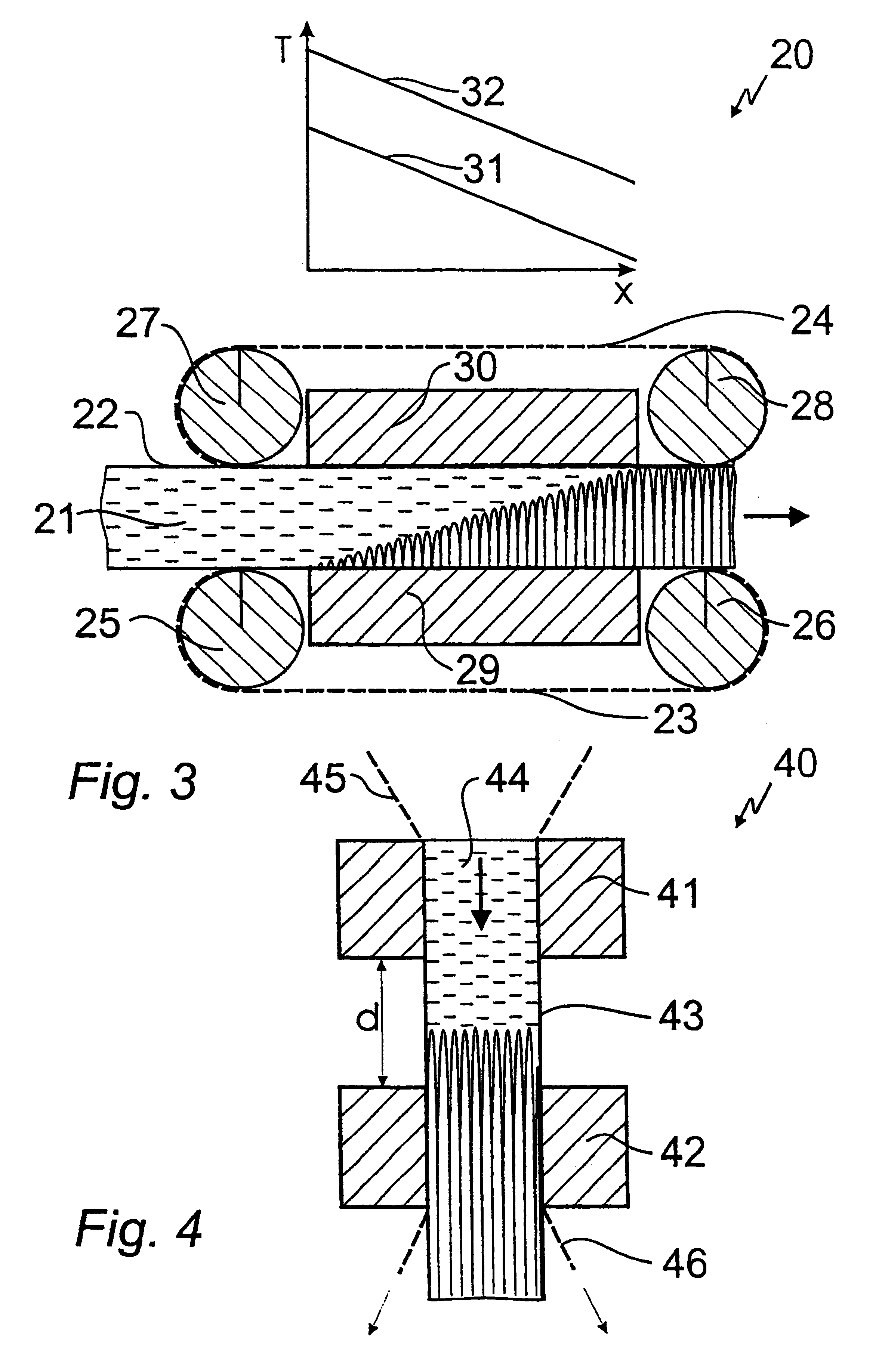
Method and apparatus for making crystalline PET pellets
InactiveUS20050110182A1Reduce dwell timeIncrease speedMouldsDrying solid materials without heatSlurryCrystallinity
A method and apparatus for underwater pelletizing and subsequent drying of polyethylene terephthalate (PET) polymers and other high temperature crystallizing polymeric materials to crystallize the polymer pellets without subsequent heating. High velocity air or other inert gas is injected into the water and pellet slurry line to the dryer near the pelletizer exit. Air is injected into the slurry line at a velocity of from about 100 to about 175 m3 / hour, or more. Such high-speed air movement forms a vapor mist with the water and significantly increases the speed of the pellets into and out of the dryer such that the PET polymer pellets leave the dryer at a temperature sufficient to self-initiate crystallization within the pellets. A valve mechanism in the slurry line after the gas injection further regulates the pellet residence time and a vibrating conveyor after the dryer helps the pellets to achieve the desired level of crystallinity and to avoid agglomeration.
Owner:GALA INDUSTRIES INC
Method of dewatering a fibrous web with a press belt
ActiveUS7351307B2Efficient removalThin dewatering fabricNon-fibrous pulp additionNatural cellulose pulp/paperFiberPaper machine
A method of dewatering a fibrous web in a paper machine including the steps of carrying the fibrous web on a side of a first fabric; contacting the fibrous web with a side of a second fabric, the fibrous web being between the first fabric and the second fabric; and passing air successively through the first fabric, the fibrous web and the second fabric.
Owner:VOITH PATENT GMBH
Apparatus and method for eliminating wrinkles in clothes
ActiveUS20060117596A1Eliminate wrinklesEffectively eliminating the wrinkles in the clothesDrying solid materials without heatHollow article cleaningWrinkle skinEngineering
Apparatus and method for eliminating wrinkles in clothes in a washing or drying machine using steam to eliminate the wrinkles in clothes, in a state in which the clothes are worn by a user or stored, as well as in a state in which washing of the clothes is terminated. The method includes determining whether or not a wrinkle-eliminating course is selected; supplying hot air to the clothes to eliminate dust from the clothes when it is determined that the wrinkle-eliminating course is selected; and supplying steam to the clothes, from which the dust was eliminated, to eliminate the wrinkles in the clothes.
Owner:SAMSUNG ELECTRONICS CO LTD
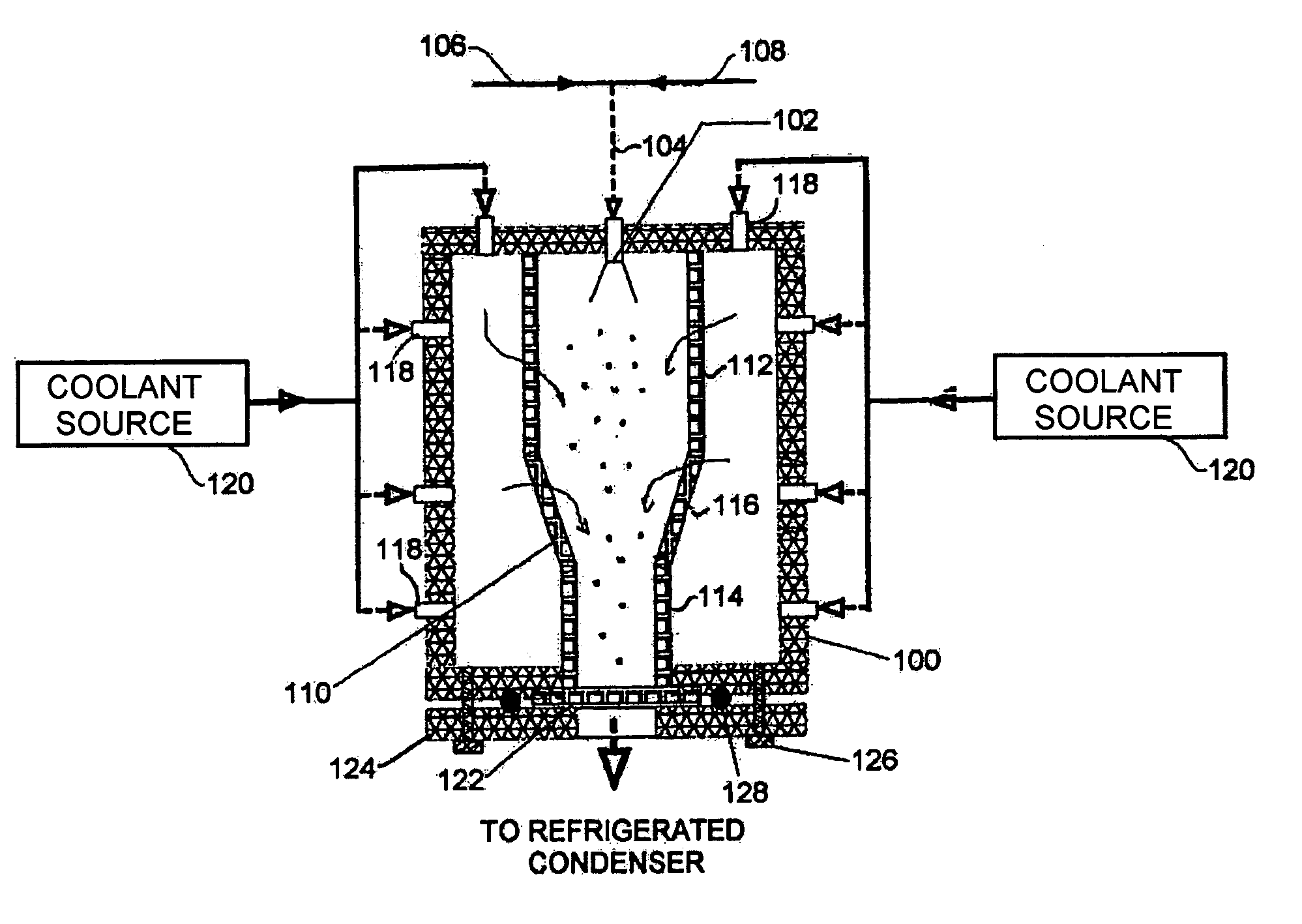
Preservation by Vaporization
ActiveUS20080229609A1Easy to controlImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
Device for treatment of wounds and a method for manufacturing of wound pads
ActiveUS20110028919A1Adjustable pressureExcellent impregnationElectrotherapyNon-adhesive dressingsSilicone GelsMedicine
A device for treatment of wounds using reduced pressure, said device comprisinga pumpa reservoir connected to said pump for collecting exudate from a wound (1),an inlet to the reservoir for connecting the reservoir with the wound so as to allow the pump to expose the wound to the reduced pressure, a wound pad to be arranged in the wound cavity and a sealing covering the wound and said wound pad.The wound pad consist of an open-cell polyurethane foam which is fully impregnated with a soft hydrophobic silicone gel, and that the foam has a hardness of 1.0-6.0 kPa measured according to ISO 3386-1 at 40% compression. The invention also concerns such a wound pad to be used with an absorbent member. In addition the invention concerns a method for manufacturing the wound pad.
Owner:MOLNLYCKE HEALTH CARE AB
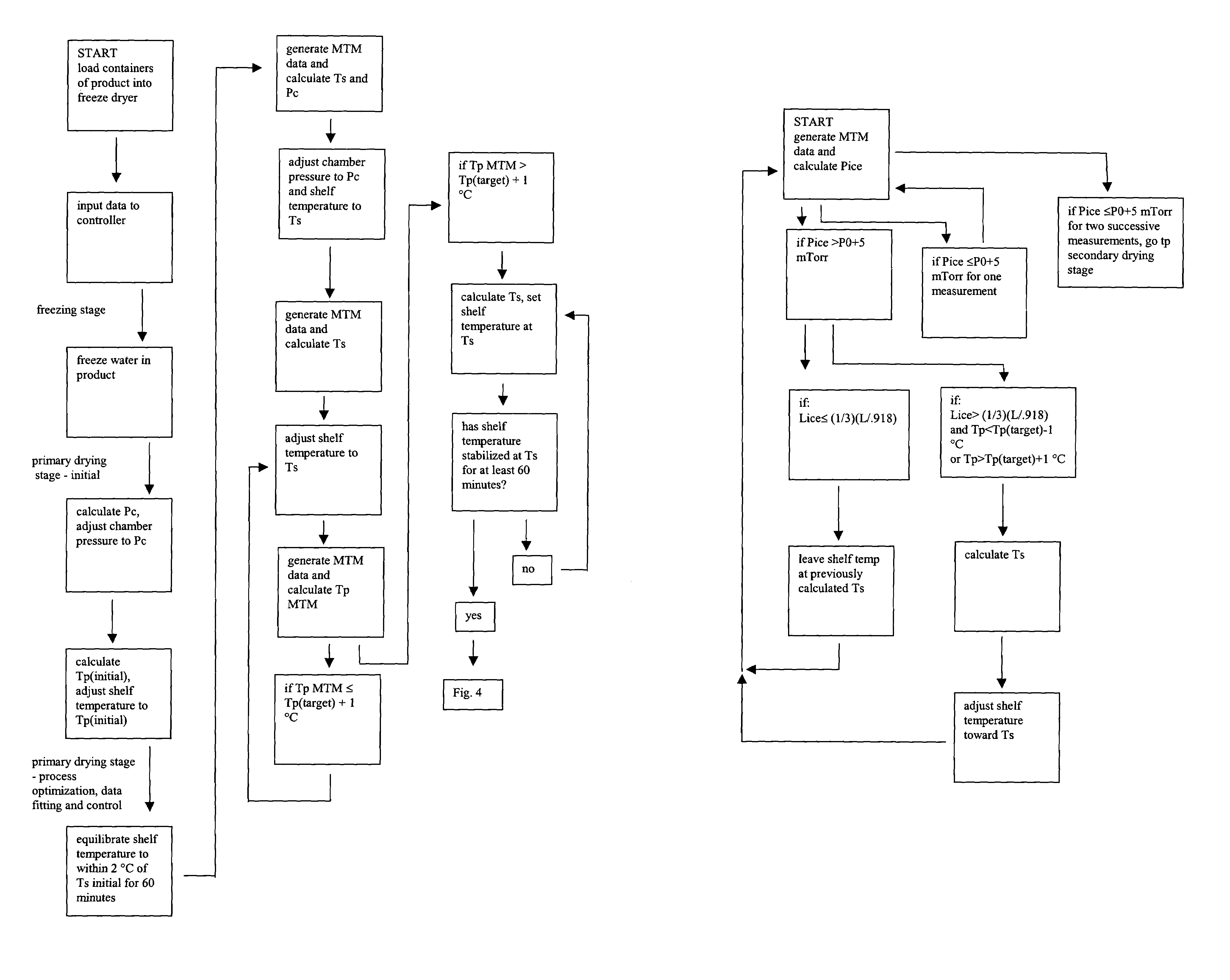
Automated process control using manometric temperature measurement
ActiveUS6971187B1Simple processDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingControl system
Owner:PURDUE RES FOUND INC +1
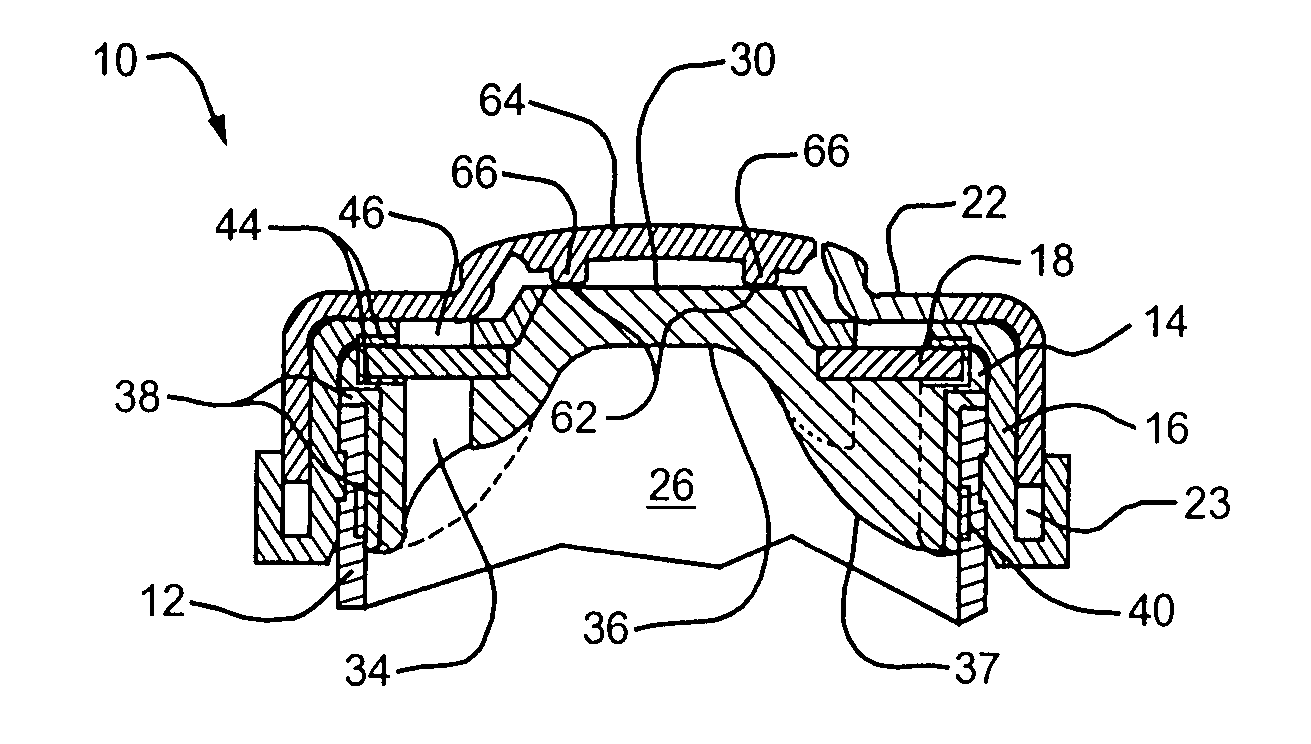
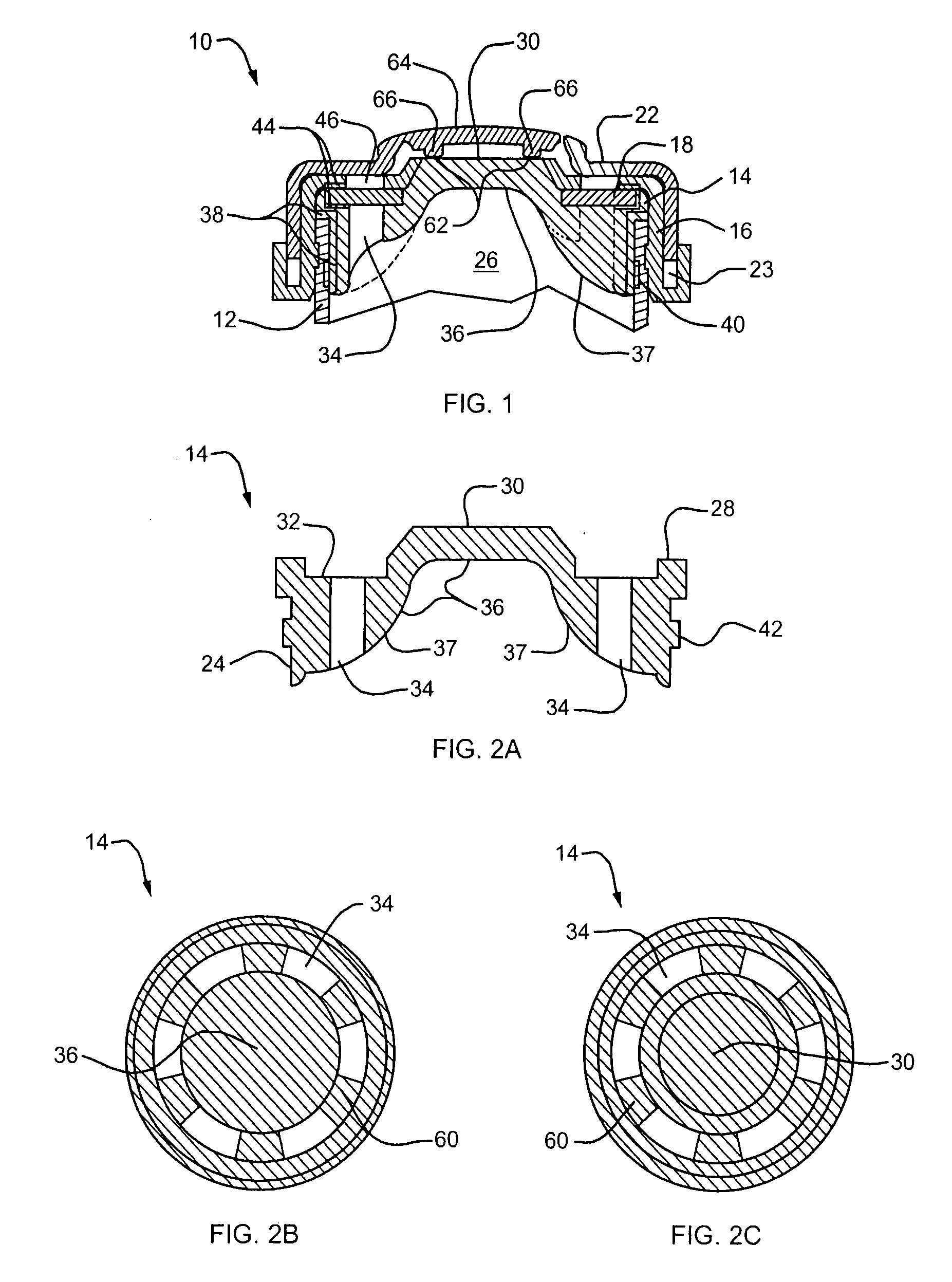
Needle penetrable and laser resealable lyophilization device and related method
InactiveUS20080039773A1Improve abilitiesGuaranteed growthDrying solid materials without heatSuction devicesBiomedical engineeringLaser source
A device and related method are provided for lyophilizing a substance within the device and storing therein the lyophilized substance. The device is penetrable by a needle for filling the device with the substance to be lyophilized, and a resulting needle hole in the device is laser resealable by transmitting thereon laser radiation from a laser source. The device defines a chamber for receiving therein the substance to be lyophilized. A needle penetrable and laser resealable portion of the device is pierceable with a needle to form a needle aperture therethrough to fill the chamber with the substance to be lyophilized through the needle, and is laser resealable to hermetically seal the needle aperture by applying laser radiation thereto. A filter is connectable in fluid communication between an interior and exterior of the chamber for permitting fluid to flow therethrough in a direction from the interior to the exterior of the chamber, and for substantially preventing contaminants from flowing therethrough in a direction from the exterior to the interior of the chamber.
Owner:MEDINSTILL DEV
Methods and systems for processing a substrate using a dynamic Liquid meniscus
InactiveUS20040060195A1Drying solid materials with heatDrying solid materials without heatEngineeringLiquid meniscus
A system and method of moving a meniscus from a first surface to a second surface includes forming a meniscus between a head and a first surface. The meniscus can be moved from the first surface to an adjacent second surface, the adjacent second surface being parallel to the first surface. The system and method of moving the meniscus can also be used to move the meniscus along an edge of a substrate.
Owner:LAM RES CORP
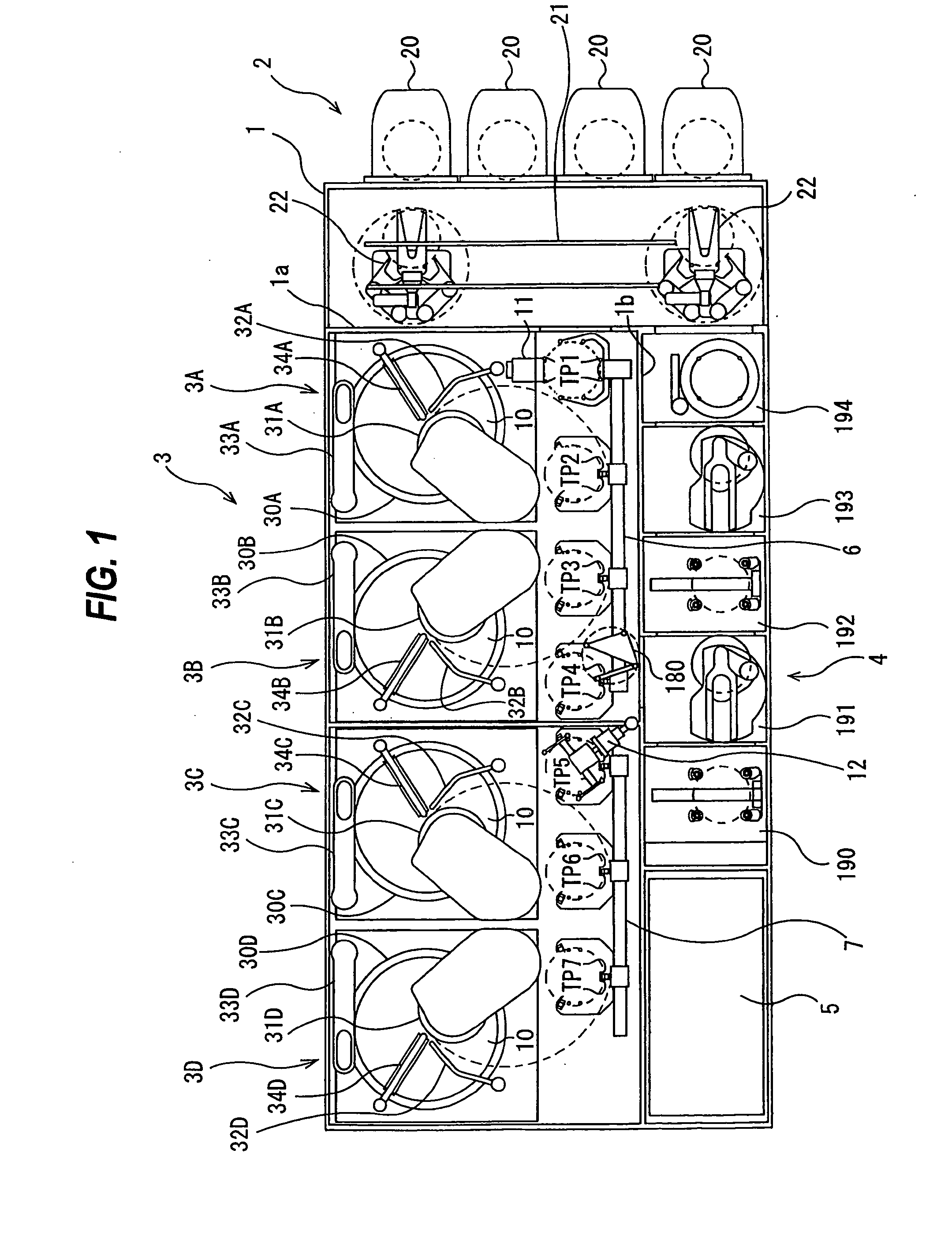
Substrate processing apparatus, substrate processing method, substrate holding mechanism, and substrate holding method
ActiveUS20090305612A1Easy maintenanceImprove throughputServomotor componentsDrying solid materials without heatTransfer mechanismElectrical and Electronics engineering
An apparatus for processing a substrate is disclosed. The apparatus includes a polishing section configured to polish a substrate, a transfer mechanism configured to transfer the substrate, and a cleaning section configured to clean and dry the polished substrate. The cleaning section has plural cleaning lines for cleaning plural substrates. The plural cleaning lines have plural cleaning modules and plural transfer robots for transferring the substrates.
Owner:EBARA CORP
Powder formation by atmospheric spray-freeze drying
ActiveUS7007406B2High emitted doseEasy to storeDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingAtomizer nozzle
A method of manufacturing heat-sensitive pharmaceutical powder is disclosed. The original pharmaceutical substances are dissolved in a solution or suspended in a suspension, which is sprayed through an atomizing nozzle and frozen in a cold gas phase or liquid nitrogen atomized directly in the spray-freeze chamber or gas jacket at the same time (for cooling purposes). The particles are freeze-dried at roughly atmospheric pressure in a down-stream fluid flow with exit filter thereby to remove moisture entrapped on or inside the frozen particles. The system has applicability for forming other powders.
Owner:AEROSOL THERAPEUTICS
Device and method for producing a material web
InactiveUS8382956B2Increase contentLow compressibilityDrying solid materials with heatDrying solid materials without heatTwo bandPulp and paper industry
A device for drainage of a pulp web, particularly a tissue web, having a first pressing zone having a first pressing zone length through which the pulp web is fed horizontally between a circulating, permeable band and a circulating, permeable support band. The first pressing zone is designed such that a fluid can flow through the band, the pulp web and the support band at least on one part of the first pressing zone length. In addition, the device has a subsequent second pressing zone having a second pressing zone length. The pulp web is guided through the second pressing zone between two bands having differing compressibility.
Owner:VOITH PATENT GMBH
Centrifugal pellet dryer with plastic wall panels
InactiveUS7024794B1Reduce noise transmissionEasy constructionWater/sewage treatment by centrifugal separationDrying solid materials without heatMetal frameworkEngineering
A centrifugal pellet dryer including a stationary cylindrical screen, a driven elevating rotor in said screen elevating wet pellets inside the screen and imparting radial forces to the wet pellets to impact them against the interior of the screen to enable moisture on the pellets to be separated and discharged through the screen, a housing enclosing the screen and rotor and including an inlet for a slurry of pellets, an outlet for dried pellets and an outlet for water removed from the pellets. The side walls of the housing are constructed of a plurality of relatively large, flat panels made of plastic sheet material are supported in a metal framework to attenuate noise of the dryer produced by rotation of the drier rotor and impact of the wet pellets against the screen. A dewaterer in advance of the dryer also has walls made of plastic sheet material.
Owner:GALA INDUSTRIES INC
Paper machine dewatering system
ActiveUS20050167061A1Efficient removalHigh suppression characteristicsNon-fibrous pulp additionNatural cellulose pulp/paperFiberPaper machine
A method of dewatering a fibrous web in a paper machine including the steps of carrying the fibrous web on a side of a first fabric; contacting the fibrous web with a side of a second fabric, the fibrous web being between the first fabric and the second fabric; and passing air successively through the first fabric, the fibrous web and the second fabric.
Owner:VOITH PATENT GMBH
Method for producing porous structures
InactiveUS6447701B1Homogeneous structureBioreactor/fermenter combinationsBiological substance pretreatmentsFreeze-dryingMaterials science
Owner:HESCHEL INGO +1
Acellular dermal matrix and method of use thereof for grafting
InactiveUS8067149B2Reduce harmSsRNA viruses positive-senseViral antigen ingredientsMedicineAcellular Dermis
A method for processing and preserving an acellular collagen-based tissue matrix for transplantation is disclosed. The method includes the steps of processing biological tissues with a stabilizing solution to reduce procurement damage, treatment with a processing solution to remove cells, treatment with a cryoprotectant solution followed by freezing, drying, storage and rehydration under conditions that preclude functionally significant damage and reconstitution with viable cells.
Owner:LIFECELL
Method and apparatus for making crystalline PET pellets
InactiveUS7157032B2Reduce dwell timeImprove heating conditionsMouldsDrying solid materials without heatPolyethylene terephthalateSlurry
Owner:GALA INDUSTRIES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com