Pyridazinone derivative, and preparation method and medical application thereof
A derivative, the technology of phthalazinone, which is applied in drug combination, antineoplastic drugs, organic chemistry, etc., can solve the problems of lack of homologous recombination repair function in cells and increase the risk of breast cancer, etc., and achieve good antitumor activity and good inhibition The effect of activity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
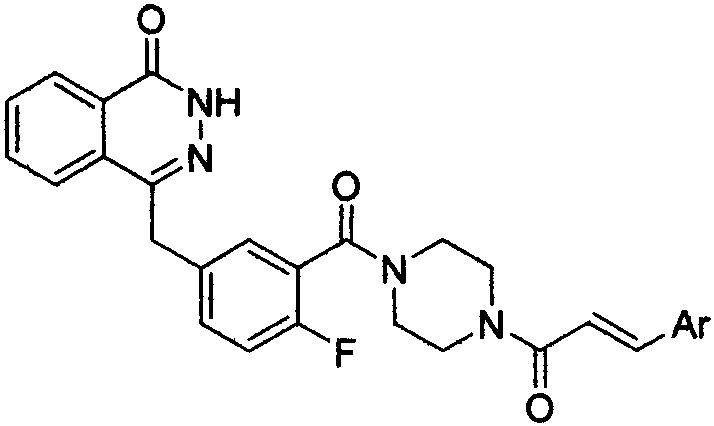
[0026] Example 1 (E)-4-{3-[4-[3-(furan-2-yl)acryloyl]piperazine-1-carbonyl]-4-fluorobenzyl}-2H-phthalazine-1- Ketone (X 1 ) preparation
[0027]
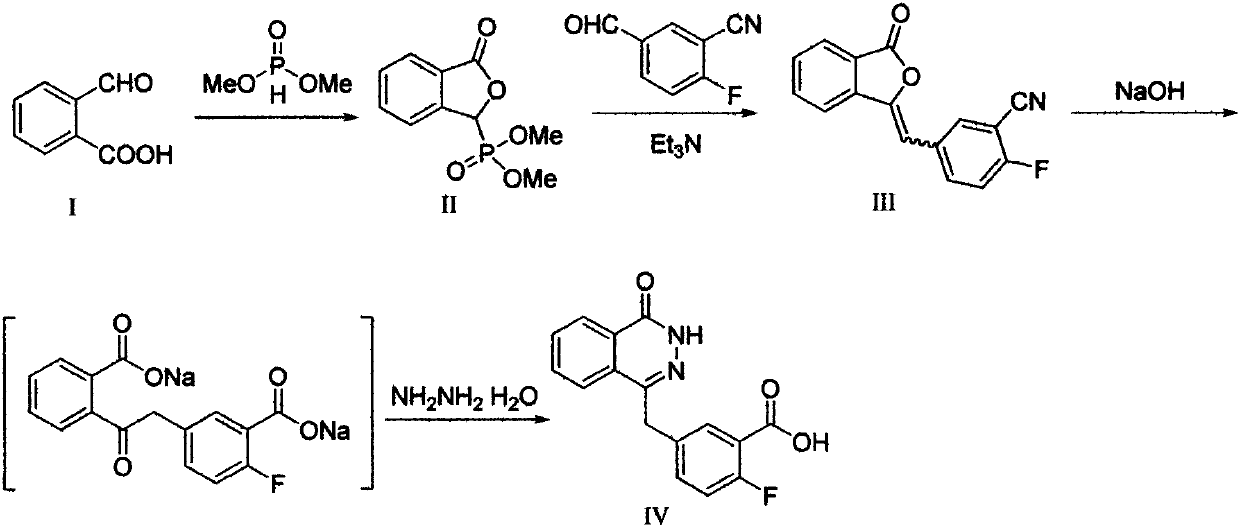
[0028] Step 1: Preparation of 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid (IV)
[0029] Add 2.00 g (0.015 mol) of o-aldehyde benzoic acid and 4.00 g (0.036 mol) of dimethyl phosphite in sequence in a 100 mL round-bottomed flask equipped with a condenser, heat the oil bath to 100 ° C, reflux for 8 h, and thin layer The reaction was monitored by chromatography (TLC) until the starting point disappeared. Stop the reaction, let it stand to cool to room temperature, pour the reaction liquid into 20 mL ice water, extract with 30 mL×3 dichloromethane, and combine the organic phases. Use a rotary evaporator to distill off excess solvent to obtain a colorless transparent oil, which is allowed to stand overnight to precipitate a colorless solid. The resulting solid was recrystallized from ethanol to obtain 1.58 g ...
Embodiment 2
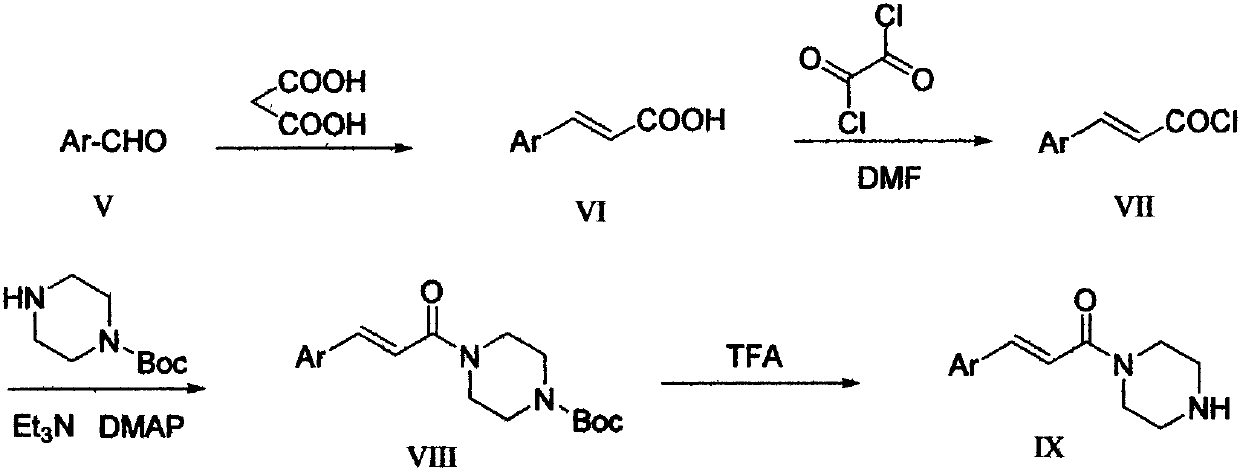
[0038] Example 2 (E)-4-{3-[4-(3-phenylacryloyl)piperazine-1-carbonyl]-4-fluorobenzyl}-2H-phthalazin-1-ketone (X 2 ) preparation
[0039]
[0040] Step 1: The preparation of 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid (IV) was prepared according to the method described in Example 1.
[0041] Step 2: phenylacryloylpiperazine (IX 2 ) preparation
[0042] Add 2.54g (0.024mol) of benzaldehyde, 4.90g (0.047mol) of malonic acid, 0.18g (0.023mol) of ammonium acetate, and 16mL of pyridine into a 50mL round-bottomed flask with a reflux condenser, and place on an oil bath Heated to 85°C and refluxed for 5 hours in the dark. Stop the reaction, let it cool down to room temperature and pour it into 50 mL of ice water, adjust the pH to about 2 with dilute hydrochloric acid, a large amount of solid precipitates, filter with suction, and wash the solid with water until there is no pyridine smell. After drying, a solid product was obtained. Recrystallized from abso...
Embodiment 3
[0048] Example 3 (E)-4-{3-[4-[3-(3,4-dimethoxyphenyl)acryloyl]piperazine-1-carbonyl]-4-fluorobenzyl}-2H-phthalein Azin-1-one (X 3 ) preparation
[0049]
[0050] Step 1: The preparation of 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid (IV) was prepared according to the method described in Example 1.
[0051] Step 2: 3,4-dimethoxyphenylacryloylpiperazine (IX 3 ) preparation
[0052] Add 3,4-dimethoxybenzaldehyde 3.98g (0.024mol) successively in the 50mL round bottom flask that has reflux condenser, malonic acid 4.90g (0.047mol), ammonium acetate 0.18g (0.023mol), Pyridine 16mL was placed on an oil bath and heated to 85°C, and refluxed for 5 hours in the dark. Stop the reaction, let it cool down to room temperature and pour it into 50 mL of ice water, adjust the pH to about 2 with dilute hydrochloric acid, a large amount of solid precipitates, filter with suction, and wash the solid with water until there is no pyridine smell. After drying, a solid pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com