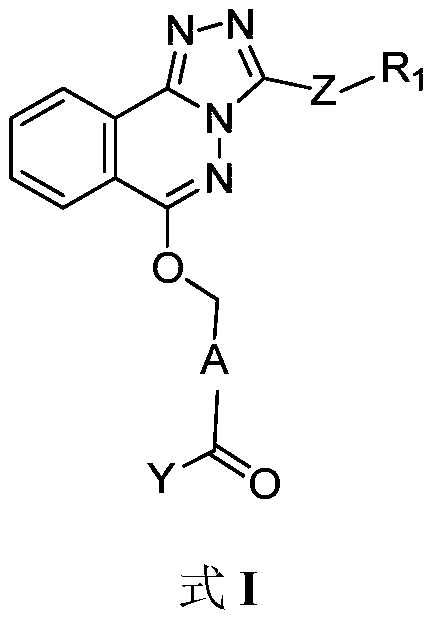
Phthalazine isoxazole alkoxy derivative, preparation method, pharmaceutical composition and uses thereof
An alkoxy and alkyl technology, which can be used in drug combinations, antipyretics, pharmaceutical formulations, etc., and can solve the problem of single inverse agonist structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0483]
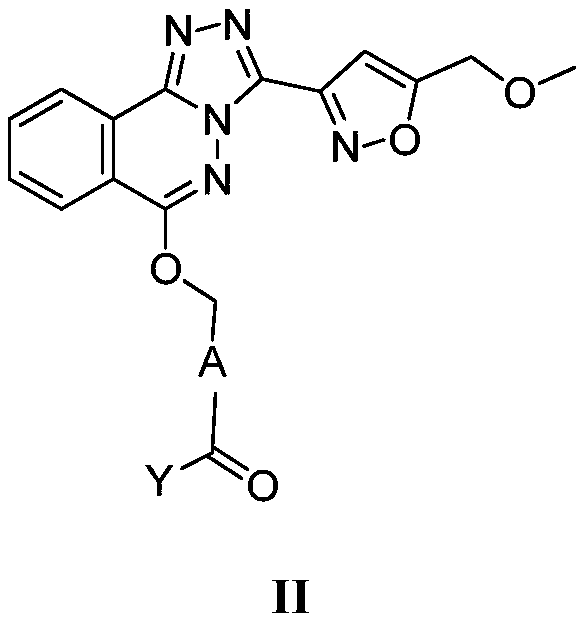
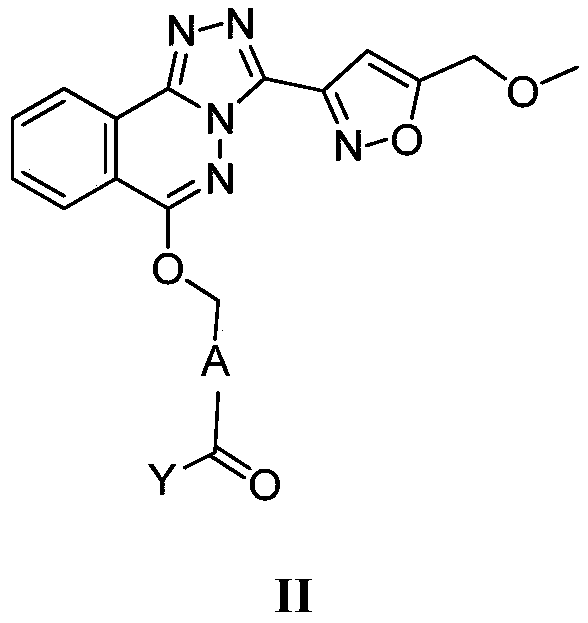
[0484] 3-(6-Chloro-[1,2,4]triazolo[3,4-a]phthalazin-3-yl)-5-(methoxymethyl)isoxazole (B-2)
[0485] Mix B-1 (0.8 g, 4 mmol) (CAS: 4752-10-7) and A-1 (0.75 g CAS: 625120-12-9) in DMF (10 mL), and stir at 90° C. for 1 hour. TLC showed that the starting material was completely reacted, and the mixture was cooled to room temperature and poured into ice water (100 mL). The resulting precipitate was collected by filtration, washed with water three times, and dried to obtain product B-2 (1 g crude) as a pale yellow solid.
preparation example 2
[0487] 6-[3-(5-Methoxymethyl-isoxazol-3-yl)-[1,2,4]triazolo[3,4-a]phthalazin-6-yloxymethyl ]-Nicotinic acid methyl ester (B-3)
[0488]Mix B-2 (1g crude), A-2 (CAS: 56026-36-9) (0.53g, 3.2mmol) and cesium carbonate (2.1g, 6.4mmol) in DMF (20mL), stir at room temperature for 16 Hour. TLC showed that the reaction of the starting material was complete, and the mixture was poured into ice water (200 mL). The resulting precipitate was collected by filtration, washed with water three times, and dried to obtain the light brown solid product B-3 (0.93 gcrude).
Embodiment 1
[0490] 6-[3-(5-Methoxymethyl-isoxazol-3-yl)-[1,2,4]triazolo[3,4-a]phthalazin-6-yloxymethyl ]-Niacin (01)
[0491] Lithium hydroxide (4.96g, 95.29mmol) was dissolved in 60ml of water, then added to a suspension of B-3 (8.5g, 19.06mmol) in methanol (150mL), and stirred for 3 hours. The reaction solution was poured into water, the pH was adjusted to 4, and a yellow solid was obtained by suction filtration, which was then slurried with ethanol and then with methyl tert-butyl ether, and dried by an oil pump to obtain a light yellow powder (5.3 g, 64%). 1 H NMR (400MHz, DMSO-d 6 ):δ13.42(s,1H),9.10(s,1H),8.58(d,J=8.0Hz,1H),8.38(d,J=8.4Hz,1H),8.34-8.32(m,1H) ,8.15-8.11(m,1H),8.00-7.98(m,1H),7.87(d,J=8.0Hz,1H),7.15(s,1H),5.80(s,2H),4.71(s,2H ), 3.39(s,3H).LCMS: Rt=3.059min, [M+H] + =432.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com