Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1732 results about "Carbon tetrachloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carbon tetrachloride, also known by many other names (the most notable being tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl₄. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It has practically no flammability at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of toxicity and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system, degenerate the liver and kidneys. Prolonged exposure can be fatal.
Process for the manufacture of 1, 1, 1, 3, 3-pentachloropropane
InactiveUS6313360B1Improve degradation rateShorten the timeOrganic chemistry methodsHalogenated hydrocarbon separation/purificationChlorideOrganic phosphates
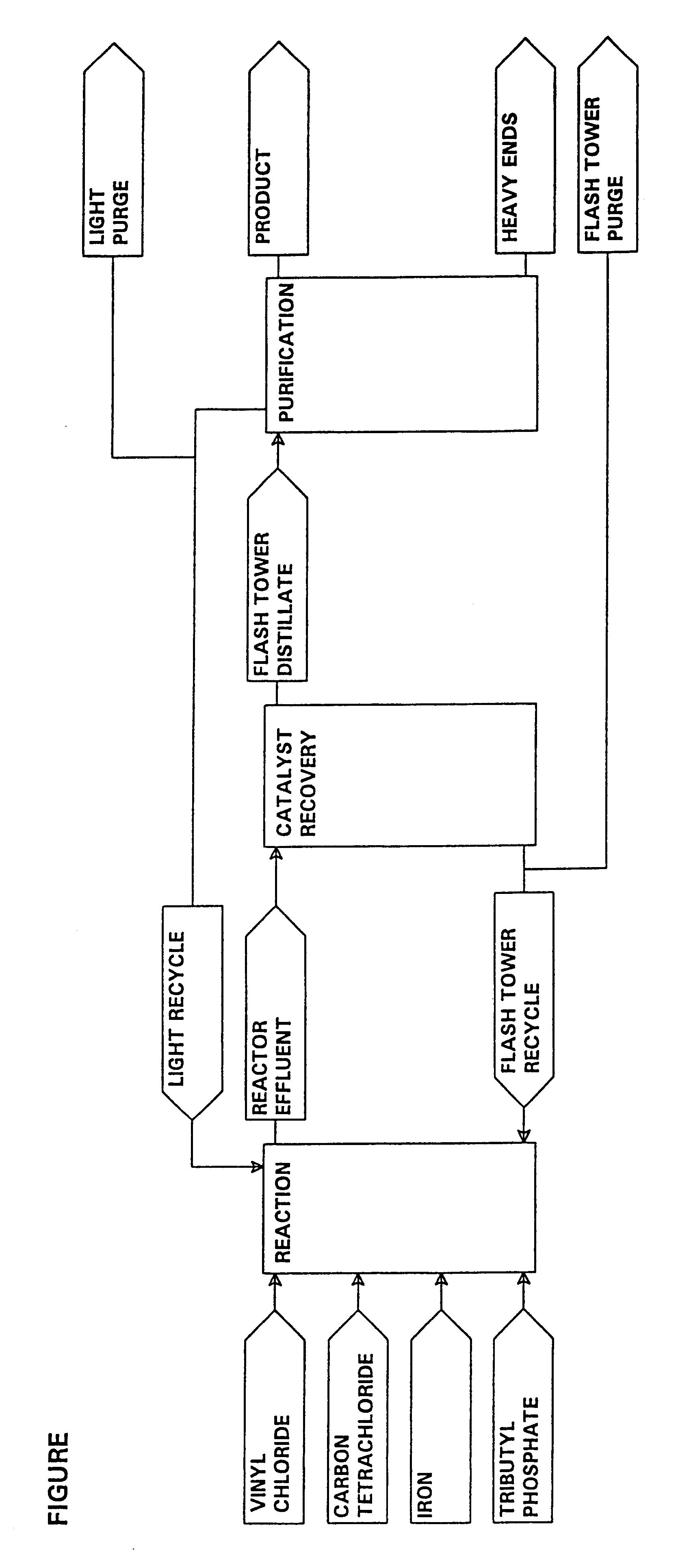
A process is provided for the production of 1,1,1,3,3-pentachloropropane. The process comprises: (a) producing a product mixture in a reactor by reacting carbon tetrachloride and vinyl chloride in the presence of a catalyst mixture comprising organophosphate solvent, iron metal and ferric chloride under conditions sufficient to produce 1,1,1,3,3-pentachloropropane; (b) subjecting the 1,1,1,3,3-pentachloropropane containing product mixture from step (a) to evaporation such that a fraction enriched in 1,1,1,3,3-pentachloropropane is separated from the product mixture and a bottoms fraction results which comprises the iron metal / ferric chloride catalyst components and heavy end by-products; and (c) recycling at least a portion of the bottoms fraction from step (b) to the reactor.
Owner:OCCIDENTAL CHEM CORP
Process for preparing halogenated alkanes
InactiveUS7094936B1High selectivityBig advantagePreparation by hydrogen halide split-offPreparation by halogen replacementAlkaneLewis acid catalysis
Methods and materials are provided for the production and purification of halogenated compounds and intermediates in the production of 1,1,1,3,3-pentafluoropropane. In a preferred embodiment, the process steps include: (1) reacting carbon tetrachloride with vinyl chloride to produce 1,1,1,3,3-pentachloropropane; (2) dehydrochlorinating the 1,1,1,3,3-pentachloropropane with a Lewis acid catalyst to produce 1,1,3,3-tetrachloropropene; (3) fluorinating the 1,1,3,3-tetrachloropropene to produce 1-chloro-3,3,3-trifluoropropene; (4) fluorinating the 1-chloro-3,3,3-trifluoropropene to produce a product mixture containing 1,1,1,3,3-pentafluoropropane; and (5) separating 1,1,1,3,3-pentafluoropropane from by-products.
Owner:EI DU PONT DE NEMOURS & CO
Method for preparing white carbon black by utilizing silicon tetrachloride
The invention discloses a method for preparing white carbon black by utilizing silicon tetrachloride. The method comprises the following steps: mixing deionized water and low molecular fatty alcohol at the molar ratio of 1:4-4:4, and adding a proper amount of surfactant; slowly adding a certain amount of the silicon tetrachloride at the room temperature; and obtaining the white carbon black through the steps of preparing reaction solution, curing the reaction solution, sedimentation and abstersion, pulpifying and spray drying, wherein the concentration of the silicon tetrachloride is controlled to be 0.2-1.0 mol.1-1, and modifiers such as 0.01-0.1mol.1-1 of Hexamethyl disilazane and the like can be added in the preparation process of the hydrophobic white carbon black. The grain diameter of the prepared white carbon black is 50-350nm and the specific surface area is 100-300m<2>.g-1; and the method has high economic value and the prepared white carbon black has good hydrophobic effect.
Owner:HENAN UNIV OF SCI & TECH
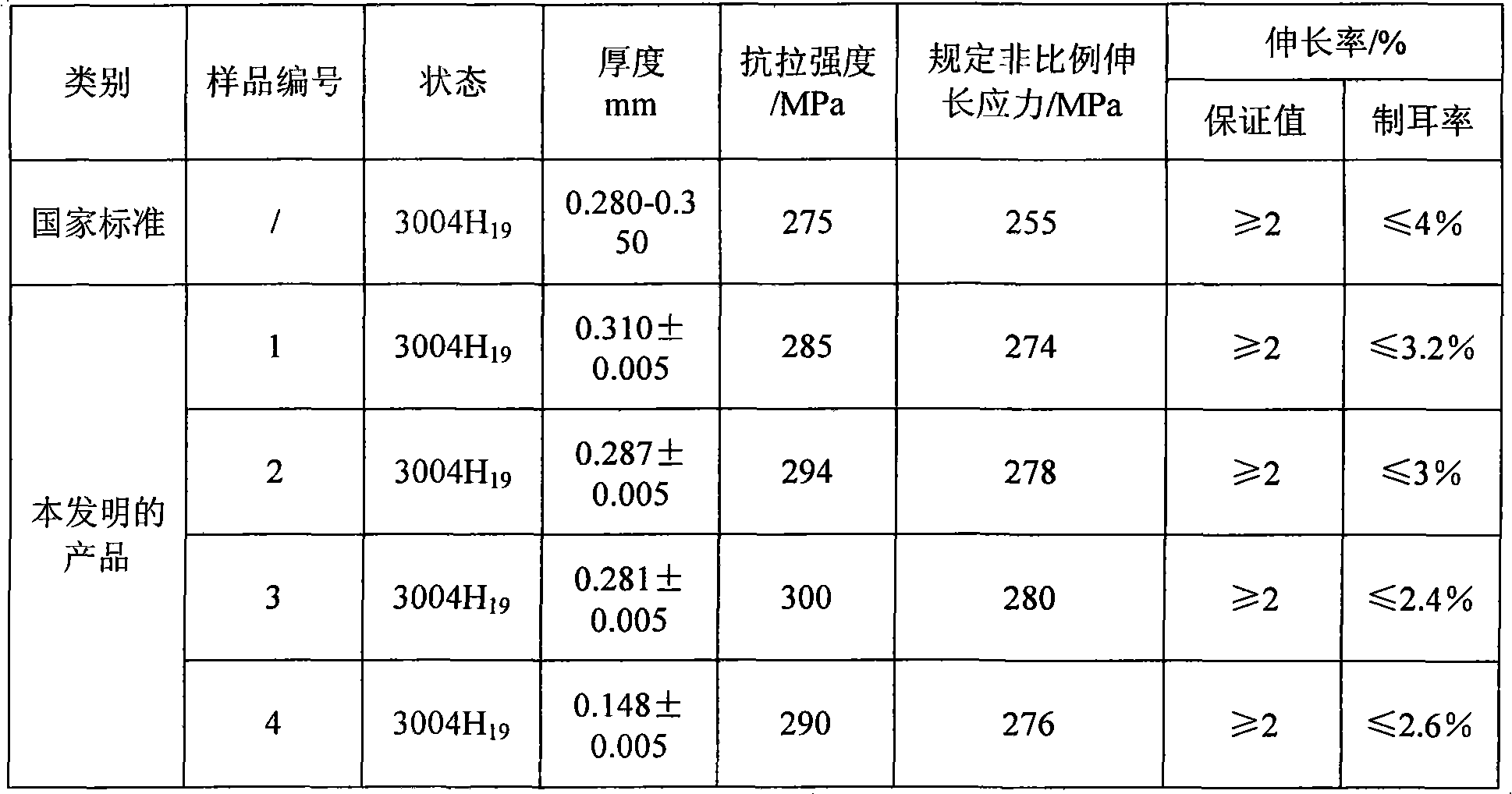
Engineering process for producing low-iron low-silicon 3004 alloy by directly cast-rolling electrolytic aluminium liquid
The invention relates to an engineering process for producing a low-iron low-silicon 3004 alloy by directly cast-rolling electrolytic aluminium liquid. The process comprises the procedures of alloy aluminium melting, ingredient adjusting, on-line refining for three times, standing, outgassing, grain refining, filtering, tilting cast-rolling, homogenizing, cold rolling, intermediate annealing, another cold rolling, full annealing, recoiling and packaging. The refining comprises powder spraying refining in a melting furnace and combined refining of on-line argon and carbon tetrachloride introducing. The annealing comprises high-temperature quick annealing, the intermediate annealing and the full annealing. During the cast-rolling, the temperature in a front box is 710-720 DEG C, and the cast-rolling speed is 600-900 mm / min. The product is the low-iron low-silicon 3004 alloy. The low-iron low-silicon 3004 alloy comprises 0.06-0.14 wt% of Si, 0.25-0.43 wt% of Fe, 0.012-0.14 wt% of Cu, 1.0-1.2 wt% of Mn, 0.8-1.0 wt% of Mg, at most 0.02 wt% of Zn, 0.015-0.03 wt% of Ti and the balance of Al. The invention has the characteristics of simple procedure, energy saving, emission reduction, goodalloy performance and low earring rate.
Owner:SNTO TECH GRP
Low-temperature scouring and bleaching agent and preparation method thereof and preprocessing technique
InactiveCN101037842AReduce the bleaching temperatureThe effect of the pretreatment process is obviousBleaching apparatusTextile treatment by spraying/projectingTextile printerBenzene
The present invention realtes to a low temperature scouring and bleaching agent belonging to the pretreatment process technology field of the textile printing and dyeing industry, a manufacturing method thereof and a pretreatment process. Said low temperature scouring and bleaching agent consists of promoter QR2010 consisting of carbon tetrachloride, acetic acid esters, alkyl benzenes, emulsifying agents and scouring agents; promoter QR2011 consisting of potassium hydroxide, carbonates and penetrating agents; and promoter QR2020 consisting of acyls and nitriles activating agents and builders. Said three promoters are prepared using routine methods and are deposited solely. When used, the three promoters, together with hydrogen peroxide, are mixed according to different proportions to prepare solutions. Said low temperature scouring and bleaching agent is capable of annealing, boiling and bleaching a variety of cotton as well as cotton blending textiles at any temperature ranging from 40 DEG C to 80 DEG C, and being processed at different scouring and bleaching equipments, with a whiteness of higher than 80% and a capillary effect of 10-20 cm. Compared with the process in existing, the amount used of hydrogen peroxide activating agent is 1 / 7 to 1 / 5 of that originally used, the total energy consumption is reduced, the tonnage of used water is decreased, and billet flaws such as holes bleached by oxygen and lycra folded crepes sourced and bleached are avoided.
Owner:SHANGHAI QIRUI TEXTILE CHEM
Preparation method of nitrogen-rich multimode beehive carbon-sulfur composite anode material
ActiveCN103746098ALarge specific surface areaHigh nitrogen contentPositive electrodesLi-accumulatorsArgon atmosphereOxide composite
The invention relates to a preparation method of a lithium sulphur battery composite anode material. The preparation method is as follows: preparing a mixed solution of carbon tetrachloride, a nitrogen source and carbonate, heating and flowing back to obtain a nitrogen-rich polymerization / carbonic acid salt compound; high temperature pyrolyzing in a nitrogen or argon atmosphere after drying the compound, so as to form the nitrogen-rich carbon / oxide compound; adding dilute acid to remove vestigial oxide, so as to form the nitrogen-rich multimode beehive carbon material of a multilevel porthole structure; uniformly mixing the nitrogen-rich multimode beehive carbon and sublimed sulfur, heat preserving under vacuum condition, injecting sulfur gas to the nitrogen-rich multimode beehive carbon material, so that the lithium sulphur battery composite positive material can be obtained. The composite anode material provided by the invention is alveolate, and has the advantages that portholes are abundant, sulfur content is high, sulfur particle can be distributed uniformly in the nitrogen-rich multimode beehive carbon material of the multilevel porthole structure, and the carbon sulfur particles can be combined more tightly, the material mechanical stability is high, discharge specific capacity is high, cycle performance is excellent, and technological process is simple, pollution is avoided, cost is low, and the method is liable to large scale production and application.
Owner:CENT SOUTH UNIV
Endotoxin absorbing agent for blood perfusion and its preparation method
An endotoxin adsorbent used for blood perfusion is prepared from agat through adding the aqueous solution to the liquid mixture of toluene and CCl4 to obtain spherical agar, activating by epoxy chloropropane under alkaline condition, reacting on diamine and then on glutaraldehyde to obtain high-strength agar beads used as the carrier of adsorbent, reacting on the amino contained ligand (polymyxin B), and reducing by NaBH4. Its advantages are high compatibility to blood and high effect on absorbing endotoxin from blood.
Owner:NANKAI UNIV
Production method of 1,1,1,3,3-pentachloropropane
InactiveCN101913980AEasy to transportAccurate measurementOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingReaction temperatureSilicon tetrachloride
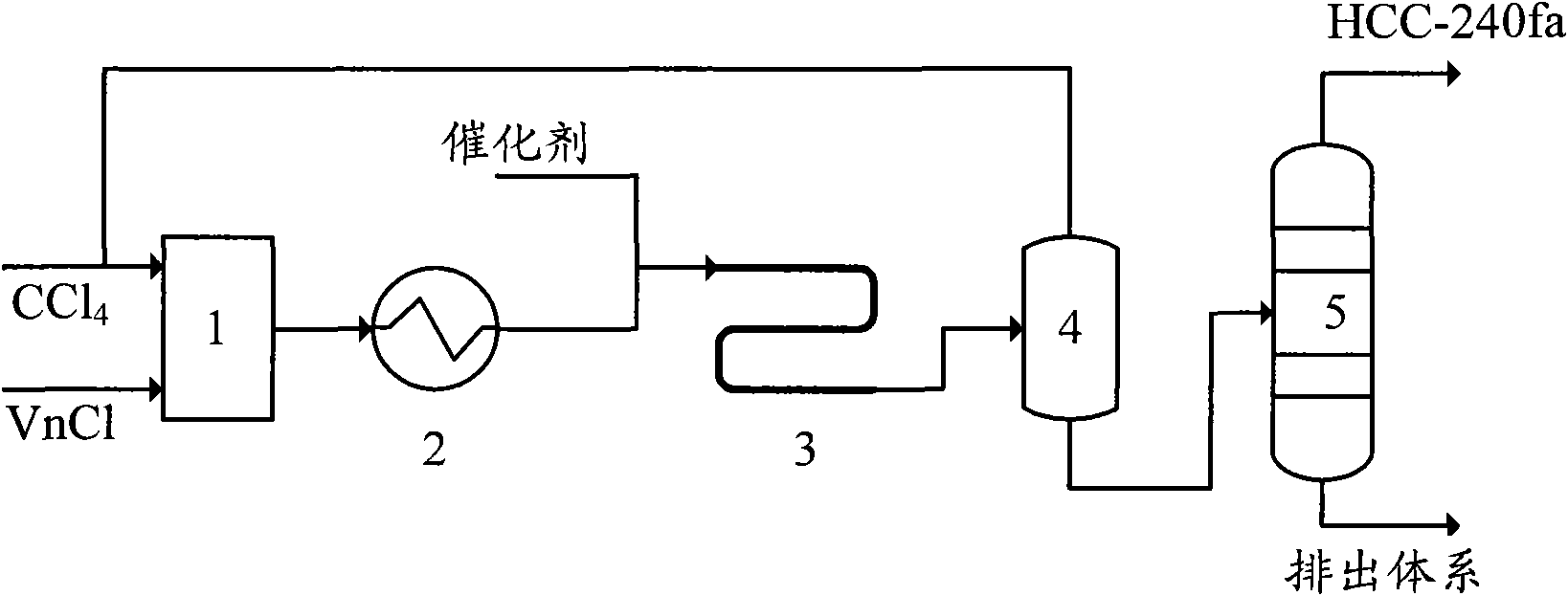
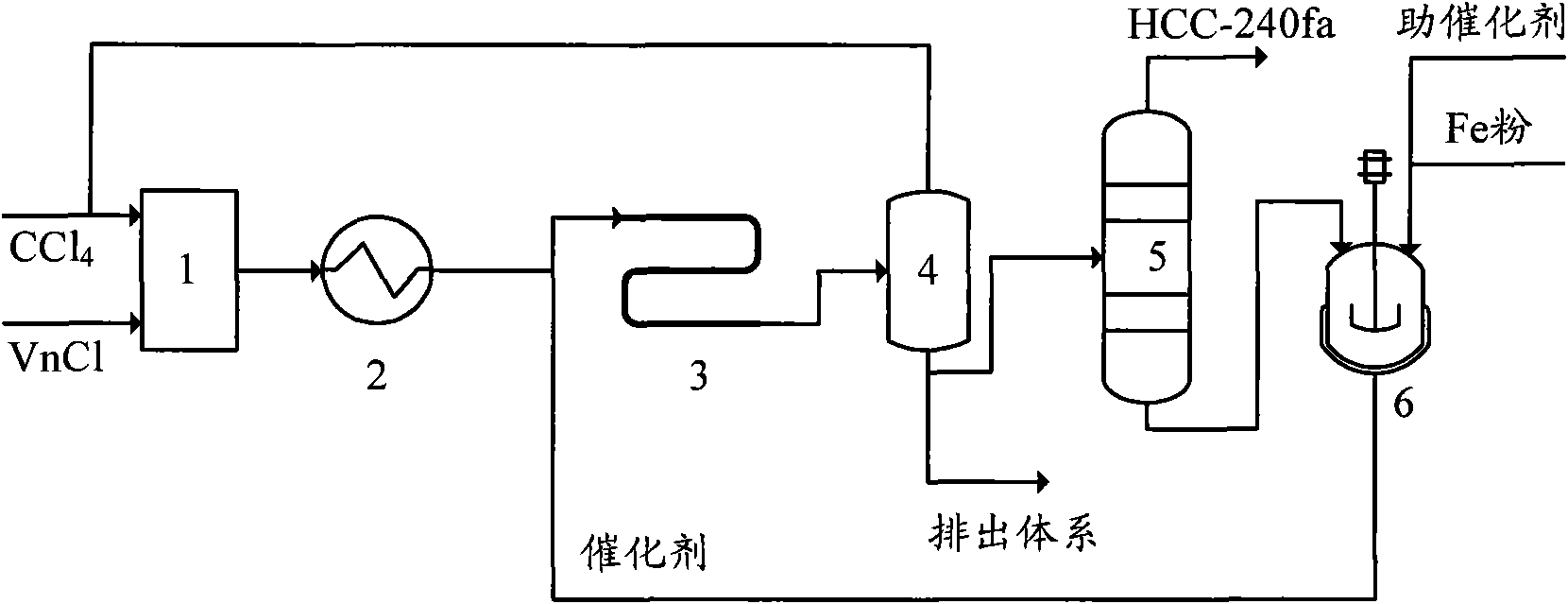
The invention provides a production method of 1,1,1,3,3-pentachloropropane (HCC-240fa). The 1,1,1,3,3-pentachloropropane is synthesized by continuously reacting the materials of carbon tetrachloride and chloroethylene in a tubular reactor. The method comprises the steps of firstly mixing the carbon tetrachloride with the chloroethylene and preheating the mixture to a reaction temperature, then introducing the mixture together with a catalyst into the tubular reactor for telomeric reaction, removing the unreacted carbon tetrachloride and chloroethylene through flash evaporation, and then further distilling to obtain the HCC-240fa, wherein the unreacted carbon tetrachloride and chloroethylene are recycled and the catalyst is recycled after being subjected to the activating treatment. The invention adopts the liquid as the activated system and uses the tubular reactor for reaction, thereby ensuring simple and convenient operation as well as continuous production.
Owner:XIAN MODERN CHEM RES INST
Reversible over-charge protective electrolyte additive of lithium ion battery and its making method
InactiveCN101145622ASolve the problem of overcharge safetyWith reversible overcharge protection functionOrganic electrolyte cellsSecondary cellsSolventLithium-ion battery
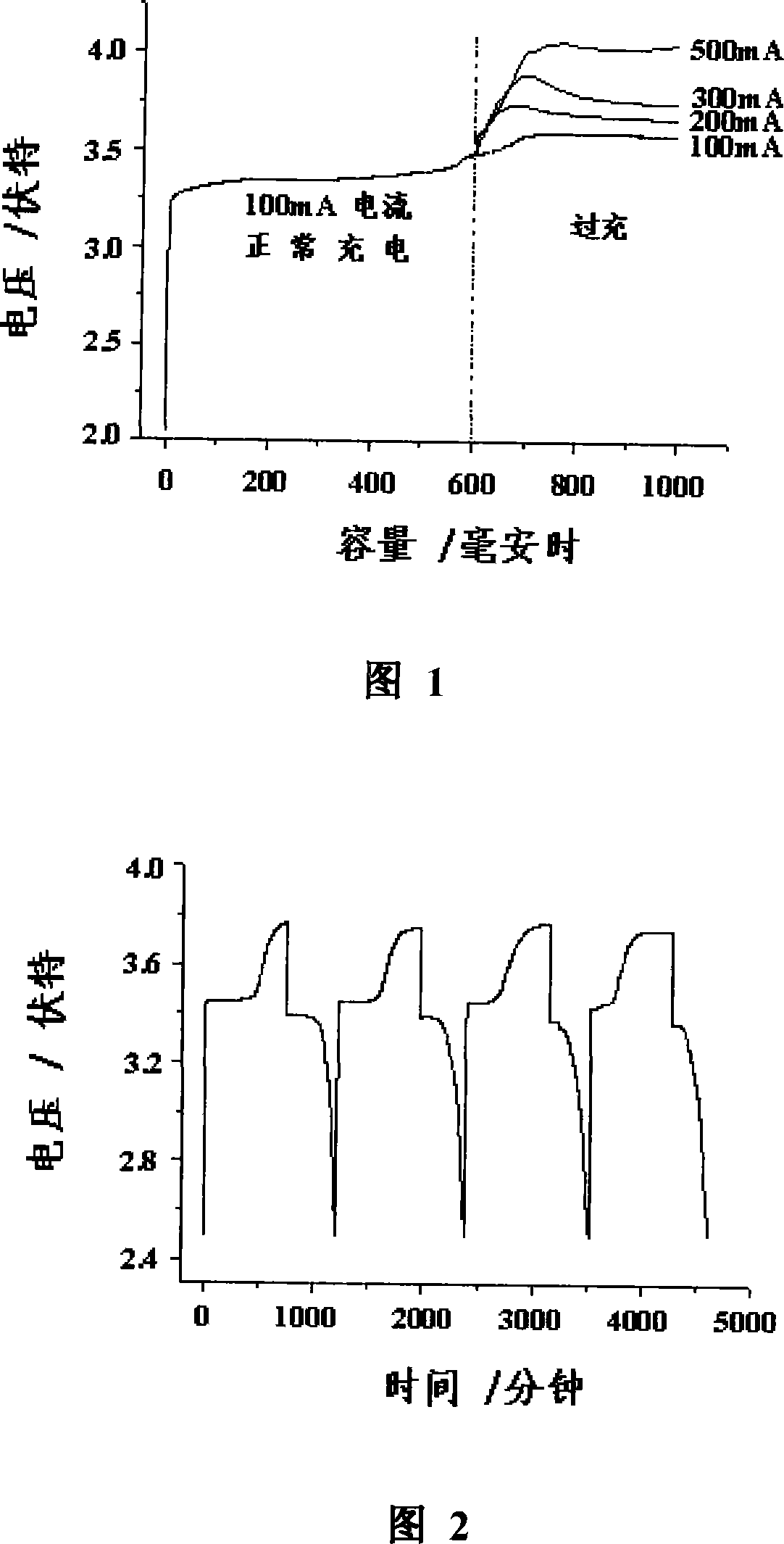
The invention provides a reversible overcharge protective electrolyte additive for a lithium battery and a preparation method thereof. The invention is characterized in that the additive contains at least one aromatic compound selected from amino-benzene, diphenylamine, triphenylamine, pyrrole, thiophene, phenylene sulfide and benzene, or at least one derivative thereof, the mass percentage of the additive in the electrolyte is in a range from 0.3 percent to 20 percent. An auxiliary solvent made of methylbenzene, dimethylbenzene, carbon tetrachloride or chloroform, in a volume less than 20 percent of the electrolyte, is added into the common electrolyte, to increase the dissolvability of the additive molecule in the electrolyte. Then the electrolyte additive is added. The additive molecule can rapidly undergo polymerization at a certain electric potential and form a polymer bridge between the anode and the cathode, and the electronic conductivity of the polymer bridge can reversibly change between insulation state and conduction state along with the voltage variation of the anode, thereby providing the battery with reversible overcharge protection.
Owner:WUHAN UNIV
Systems and methods for producing fluorocarbons
InactiveUS20050038302A1Preparation by dehalogenationPreparation by hydrogen halide split-offHydrogenFluorocarbon
Systems and methods for producing fluorocarbons are provided that include contacting a saturated halogenated fluorocarbon with hydrogen and catalyst to produce a saturated hydrofluorocarbon and an unsaturated fluorocarbon. Aspects of the present invention describe systems and methods for contacting saturated halogenated fluorocarbons such as CF3CClFCF3 and / or CF3CCl2CF3 with hydrogen and catalyst. Systems and methods of the present invention also describe contacting saturated halogenated fluorocarbons with catalysts having one or more of K, Zr, Na, Ni, Cu, Ni, Zn, Fe, Mn, Co, Ti, and Pd. Aspects of the present invention also describe contacting saturated halogenated fluorocarbons with hydrogen under pressure. Saturated hydroflourocarbons and unsaturated fluorocarbons produced in accordance with the systems and methods of the present invention can include one or more of CF3CFHCF3, CF3CH2CF3, CF3CHClCF3, CF3CF═CF2, CF3CH═CF2, and CF3CCl═CF2.
Owner:GREAT LAKES CHEM CORP
Preparation method of fucosan sulphate oligosaccharide
A process for preparing the oligose of fucosan sulfate includes such steps as preparing the aqueous solution of fucosan sulfate, adding oxidant chosen from hydrogen peroxide, hypochlorous acid, nitrous acid, or their salts, heating, membrane ultrafilter, vacuum concentrating and freeze drying. Its advantages are high medical effect to protect liver and take care of health, and low cost.
Owner:OCEAN UNIV OF CHINA
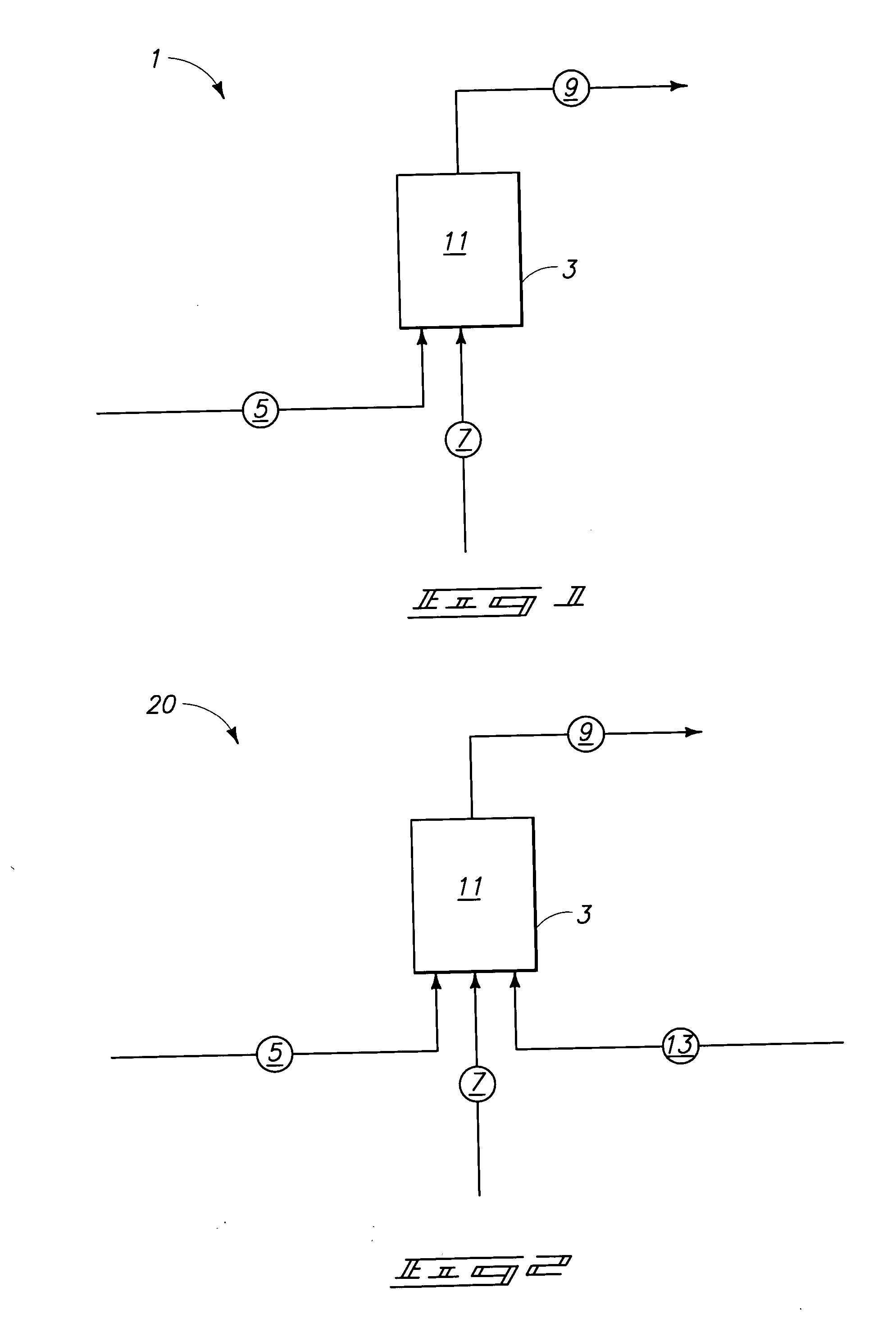
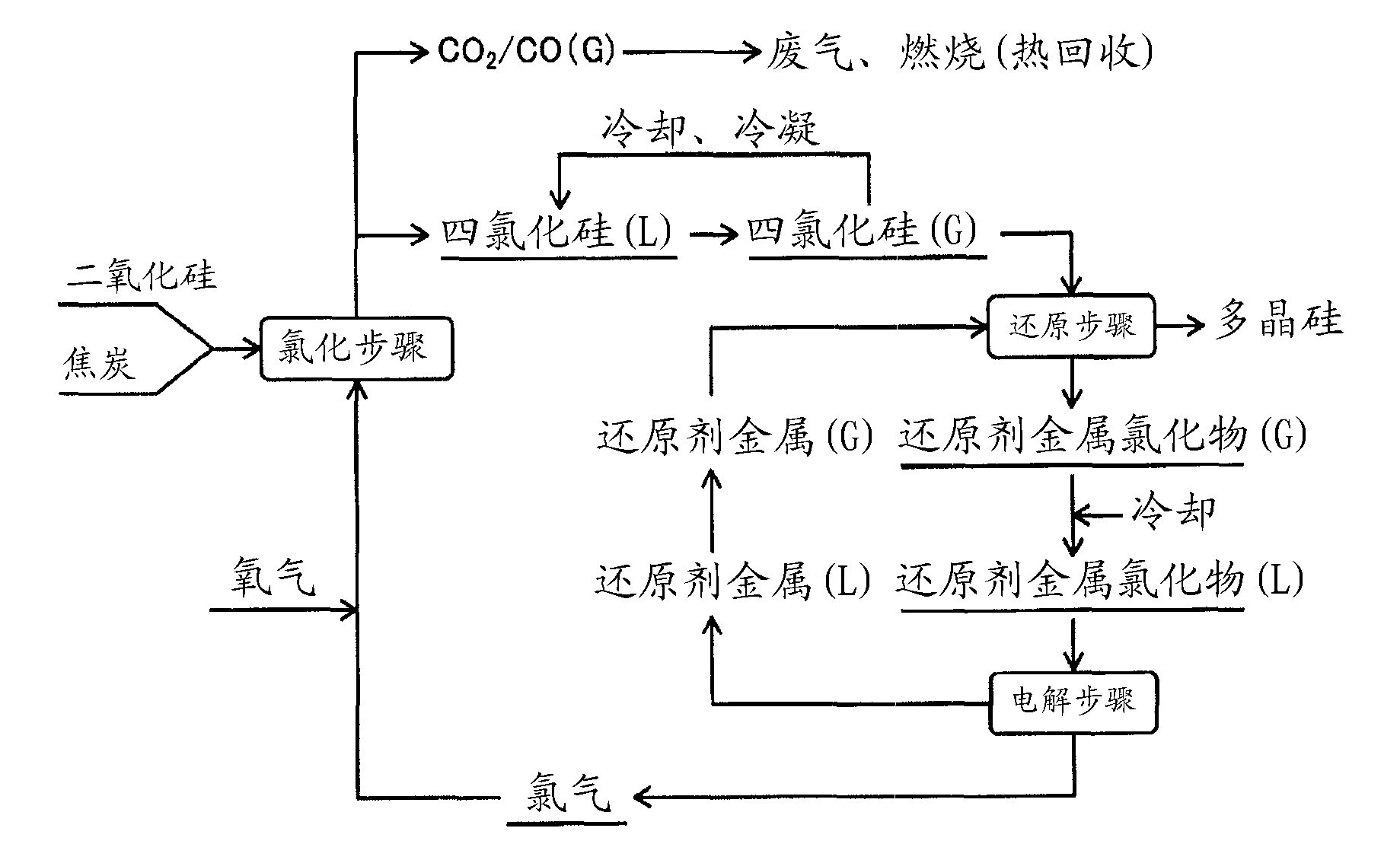
Method for manufacturing polysilicon and method for manufacturing silicon tetrachloride
InactiveCN102686514AEfficient use ofCheap manufacturingSiliconHalogenated silanesElectrolysisSilicon dioxide
Provided is a method for manufacturing polysilicon and silicon tetrachloride. The polysilicon manufacturing process used in said method is energy-efficient and can use an inexpensive raw material, the supply of which is stable. The chlorination reaction in said process proceeds smoothly and post-chlorination impurities are minimized. The provided method comprises: a chlorination step which generates silicon tetrachloride by chlorinating granules comprising silicon dioxide and a carbon-containing substance; a reduction step which generates polysilicon by using a reducing metal to reduce the silicon tetrachloride; and an electrolysis step which generates chlorine gas and a reducing metal by performing molten-salt electrolysis on a reducing metal chloride generated as a by-product from the reduction step. In the chlorination step, chlorine gas is supplied to the silicon dioxide and the carbon-containing substance in the presence of oxygen gas in order to react said substances. The reducing metal generated in the electrolysis step is reused as the reductant for the silicon tetrachloride in the reduction step, and the chlorine gas generated in the electrolysis step is reused in the chlorination step.
Owner:JNC CORP +2
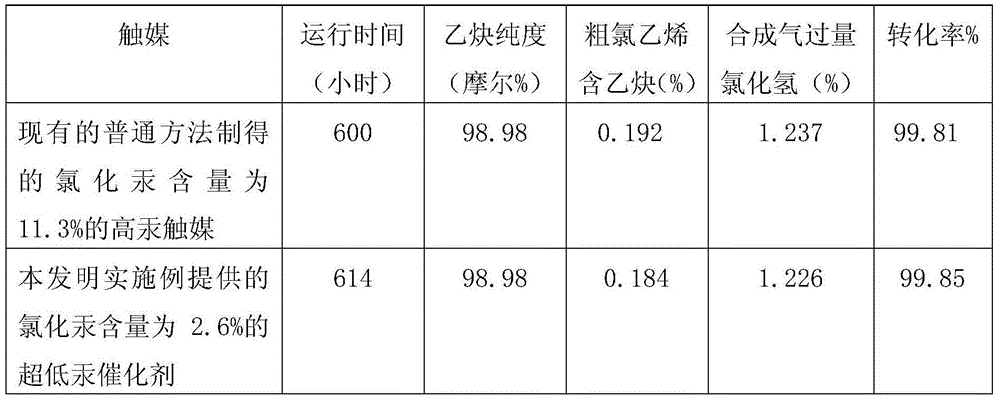
Ultralow-mercury catalyst and production process thereof
InactiveCN103551139AReduce manufacturing costLow in Mercury ChlorideCatalyst carriersPreparation by halogen halide additionVoid ratioPotassium
Owner:SHIJIAZHUANG KECHUANG ADDITIVES CO LTD
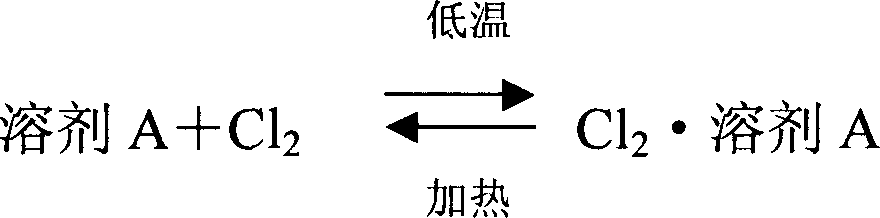
Separation and purification method of mixed waste gas containing chlorine and hydrogen chloride
InactiveCN1865127ARealize recycling of resourcesHigh recovery rateChlorine/hydrogen-chloride purificationPurification methodsGas phase
This invention relates to a method for separating and purifying mixing exhaust gas of chlorine and hydrochloride, comprising: absorb the chlorine in the mixing exhaust gas using solvent A to separate from gas phase hydrochloride, the chlorine can be obtained after solvent A desorption, the hydrochloride can be obtained by removing solvent A in the gas phage hydrochloride using solvent B, wherein: solvent A is one of benzene, silicon tetrachloride, nonyl hydride, sulfur monochloride, carbon tetrachloride and chlorosulfonic acid, and solvent B is one of cyclopentadiene and hexachlorobutadiene. By washing with concentrated hydrochloric acid to remove trace amount of organic compounds, and chemical washing to remove heavy metal elements, the hydrochloride is absorbed by water to make hydrochloric acid. This invention is characterized of efficient separation of chlorine and hydrochloride, high efficiency of separation, low cost, high-usage of resources, high purity of chlorine up to 99%, recycling use, and the purity of hydrochloride meets the requirement of producing industrial hydrochloride and hydrochloride regent .
Owner:ZHEJIANG WEIHUA CHEMICAL CO LTD
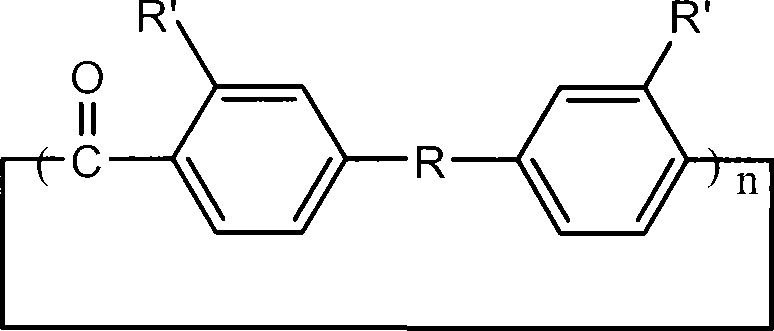
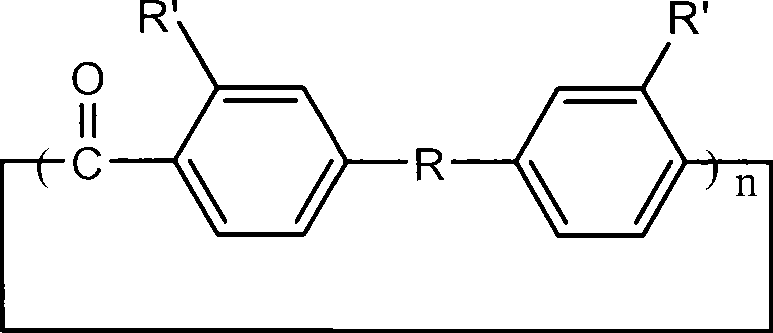
Aromatic ring-shaped polyether-ketone oligomer and preparation method thereof
The invention relates to an aromatic ring-shaped polyether-ketone oligomer and a preparation method thereof. Under 'pseudo-high dilution' condition, Friedel-Crafts-Karrer electrophilic reaction is utilized to open up a new route of taking carbon tetrachloride and substituted aromatic hydrocarbon monomers as basic raw materials to synthesize commercial, helicrystalline macrocylic oligomers of high-performance PEEK and PEK, namely the aromatic ring-shaped polyether-ketone oligomer with high yield. The aromatic ring-shaped oligomer can be melted and subjected to ring opening polymerization under the initiation of an anion initiator to obtain high-performance linear polyaryletherketone by using a cyclic structure and low melt viscosity peculiar to the aromatic ring-shaped oligomer. By the combination of low cost and high performance, the aromatic ring-shaped polyether-ketone oligomer and the synthetic route thereof has important application in the field of preparation of high-performance composite materials.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Production method of 1,1,1,3,3-pentachlorobutane
InactiveCN101913979ASuitable for continuous productionEasy to useOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingReaction temperatureTelomerization
The invention provides a production method of 1,1,1,3,3-pentachlorobutane (HCC-360jfa), in which carbon tetrachloride and 2-chloropropene are taken as raw materials for continuous reaction in a tubular reactor to synthesize the 1,1,1,3,3-pentachlorobutane. In the method, the carbon tetrachloride and 2-chloropropene are firstly mixed and preheated to reaction temperature; the carbon tetrachloride and 2-chloropropene enter the tubular reactor with a catalyst for telomerization; a product undergoes flash evaporation to remove the un-reacted tetrachloride and 2-chloropropene and then is further distilled to obtain the HCC-360jfa product; the un-reacted tetrachloride and 2-chloropropene are recycled; and the catalyst undergoes activating treatment for recycle. The catalyst used by the invention is liquid, no solid exists in the reaction product, and the tubular reactor is used for reaction, so that the invention is convenient for operation and easy for continuous production.
Owner:XIAN MODERN CHEM RES INST
Phosphorus/silicon-containing reactive high-molecular flame retardant, and preparation method and application thereof
The invention provides a phosphorus / silicon-containing reactive high-molecular flame retardant, and a preparation method and application thereof, relating to flame retardants. The preparation method comprises the following steps: reacting methacryloyl chloride and p-phenylenediamine in the presence of a solvent and an acid-binding agent, carrying out vacuum filtration to remove the acid-binding agent salt generated by the reaction, and purifying the crude product by water washing, rotary evaporation and drying to obtain 4-methacrylamidoaniline; reacting hydroxyethyl methacrylate and 9,10-dihydro-9-oxa-10-phosphaphenanthryl-10-oxide in the presence of carbon tetrachloride, a solvent and an acid-binding agent, carrying out vacuum filtration to remove the acid-binding agent salt generated by the reaction, and purifying the crude product by water washing, rotary evaporation and drying to obtain a phosphorus-containing monomer; and carrying out free-radical polymerization on the 4-methacrylamidoaniline, phosphorus-containing monomer and methylacryloyloxypropyl heptaisobutyl POSS (polyhedral oligomeric silsesquioxane) under the initiation conditions of a solvent and an initiator to obtain the phosphorus / silicon-containing reactive high-molecular flame retardant. The phosphorus / silicon-containing reactive high-molecular flame retardant can be used for preparing a flame-retardant epoxy resin.
Owner:XIAMEN UNIV
Nanometer multilayer mesoporous metal nitride/graphene composite materials for super capacitor and preparation method thereof
ActiveCN103021662AIncrease capacityImprove conductivityElectrolytic capacitorsEthylenediamineCapacitance
The invention relates to multilayer metal nitride / graphene electrochemical super capacitor composite electrode materials and a preparation method thereof. The preparation method of the multilayer metal nitride / graphene electrochemical super capacitor composite electrode materials comprises (1) preparing graphene oxide; (2) self-assembling silicon source on the surface of the oxidized graphene to obtain layered graphene oxide-mesoporous silica composite materials GO-SiO2 under an alkaline condition with a cationic surface active agent as a template agent; (3) roasting carbon tetrachloride and ethylenediamine in inert gas after polymerization with the GO-SiO2 as a template and then removing the SiO2 in the template agent to obtain layered graphene-mesoporous carbon nitride composite materials G-C3N4 and (4) cross-linking a metal precursor on the template agent G-C3N4 and then roasting in inert atmosphere to obtain the multilayer metal nitride / graphene electrochemical super capacitor composite electrode materials. The electrode materials prepared from the method have the advantages of being up to 876 farads per gram in specific capacitance, being capable of remaining over 95% in specific capacitance after charge-discharge cycles for 2000 times, being long in service life and being good in cycling stability.
Owner:泰州市海通资产管理有限公司
Method for synthesizing carbide nano powder by solid-phase reaction
InactiveCN101891192AControl morphologyRaw materials are easy to getTungsten/molybdenum carbideReaction temperatureMixed materials
The invention relates to a method for synthesizing carbide nano powder by a solid-phase reaction, which comprises the following steps of: mixing a silicon source or a metal source, a carbon source, magnesium powder and elementary iodine according to a mol ratio of 1: (0.05-2): (1-25): (1.25-12), sealing the mixed material in an autoclave, and placing the autoclave in a drying box to perform a reaction for 6 to 48 hours at the temperature of between 100 and 500 DEG C; and washing, separating and drying the product to obtain the carbide nano powder. In the invention, glucose or maltose which universally exists in the natural world is used as the carbon source to avoid using a chlorine-containing carbon source such as carbon tetrachloride and tetrachloroethylene which have bad influences on an operation environment, and the reaction temperature and the reaction pressure are relatively mild, so that the method for synthesizing carbide nano powder by the solid-phase reaction has a potential for mass production.
Owner:SHANDONG UNIV
Fire retardant in use for cellulose and fabricating method
InactiveCN1563160AImprove flame retardant performanceMaintain flame retardant functionCelluloseEthylenediamine
The molecular formula of designed fire retardant for cellulose is C12H26O4P2S2N2 named as DDPSN. The preparing method includes reacting on trichloro-thiophosphorus with neophenyl dialcohol for 1.5-3 h. under dissolving of benzene or pyridine at 45-75 deg.C temperature, washing and distillating to remove benzene, forming DDSP by benzinum recrystallization and by drying for 24h. with airflow, reacting on drid DDSp with ethylene diamine for 3-5 hr. at 10-30 deg.C temp under dissolving of chloroform or carbon terochloride and obtaining DDPSN by ethyl ether recrystallization through 24 hr. drying with airflow.
Owner:TIANJIN POLYTECHNIC UNIV
Positive temperature coefficient material and thermistor containing the material and preparation method therefore
ActiveCN101465185AImprove stabilityGood resistance stability at room temperaturePositive temperature coefficient thermistorsRoom temperatureHeat sensitive
Disclosed are a positive temperature coefficient material, a preparation method thereof, a positive temperature coefficient thermistor including the positive temperature coefficient material and a preparation method thereof. The material is a product formed by a mixture through melting; the mixture comprises resin, conductive filling, non-conductive filling, promoter and cross-linking agent, wherein, the mixture also comprises additives; and the additives are selected from silicon dichloride, sodium fluoride and carbon tetrachloride. The positive temperature coefficient material is provided with good stability and the output power of the positive temperature coefficient material is slowly attenuated along with the increase of the switching movement times, so that the thermistor prepared with the positive temperature coefficient material is provided with good room temperature resistance stability and PTC intensity stability after the thermistor is frequently used.
Owner:BYD CO LTD
Chloration method for phenoxyacetic acid and derivatives thereof
InactiveCN102336654AEasy to industrializeOrganic compound preparationCarboxylic compound preparationChlorobenzeneIndustrial waste water
The invention provides a chloration method for phenoxyacetic acid and derivatives thereof, which comprises the following steps that: raw materials of the phenoxyacetic acid or the derivatives of the phenoxyacetic acid and chlorinating agents take reaction at a certain temperature in organic solvents under the effect of catalysts, wherein the chlorinating agents can be chlorine gas, sodium hypochlorite, calcium hypochlorite and sulfuryl chloride, the catalysts can be lewis acid and sulfurated substances, and solvents can be dichloromethane, dichloroethane, trichloromethane, carbon tetrachloride, formic acid, ethyl acetate, benzene, toluene, dimethylbenzene, chlorobenzene and o-dichlorobenzene. The method has the advantages that the operation can be carried out under the waterless condition, the generation of a large amount of industrial waste water is avoided, the used catalysts are safe and are easy to obtain, the solvents can be cyclically used, and the industrialization is easy.
Owner:DALIAN RES & DESIGN INST OF CHEM IND
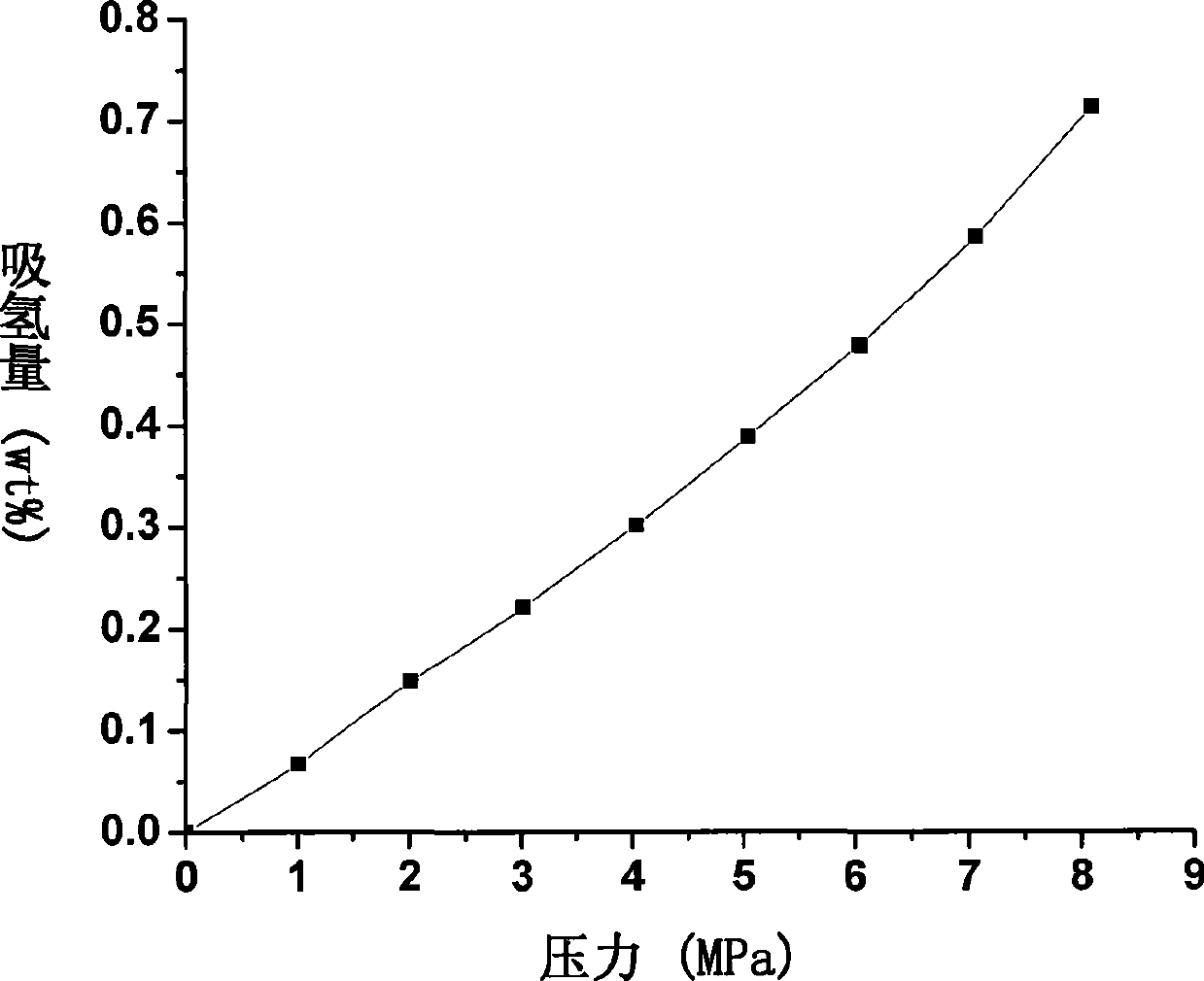
Carbon nitride polyporous material and use thereof for hydrogen storage
The invention relates to a carbon-nitrogen porous material and a hydrogen storage application thereof, which belong to the field of inorganic nano material chemistry and new energy resources-related technology. An ordered meso-porous carbon-nitrogen compound is prepared by a meso-porous silicon dioxide hard template method, wherein, ethylene diamine is taken as a precursor and carbon tetrachloride is taken as a solution; the ethylene diamine and the carbon tetrachloride are mixed and stirred evenly for reflux and immersion; the sample obtained is carbonized at high temperature and detemplated to obtain an ordered meso-porous carbon-nitrogen material; the ordered meso-porous carbon-nitrogen material is treated and activated by carbon dioxide at high temperature, wherein, air flow, activation temperature and activation time are controlled; and then a nitrogen-doped porous carbon material with special appearance and pore structure is obtained. The material can be taken as a high-performance hydrogen storage medium at room temperature, and can absorb 2.21wt% hydrogen in a reversible manner under the condition of room temperature and 80Mpa. Compared with the conventional hydrogen alloy, the carbon material has the advantages of low cost, good cycle performance and higher hydrogen storage capacity.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Catalyst and preparing method thereof, and use in hydrogenation-dechlorination of carbon tetrachloride
ActiveCN101015803AAchieving zero emissionsHigh activityHalogenated hydrocarbon preparationMetal/metal-oxides/metal-hydroxide catalystsPt elementTitanium oxide
The invention relates to a novel catalyst for hydrotreating and dechlorinating high-level chloromethane as carbon tetrachloride to prepare low-level chloromethane. The catalyst uses one or several of palladium, platinum and nickel as active components, and uses at least two of zircite, titanium oxide and alumina as catalyst carrier. With said catalyst, the carbon tetrachloride is hydrotreated into chloroform which is mainly transferred into carrene, while the produced hydrochloride is reacted with carbinol to obtain methyl chloride.
Owner:溧阳常大技术转移中心有限公司
Method for preparing-ester by using solid chlorine substitution of phosgene
ActiveCN1660868AHigh selectivityHigh activityEsterified saccharide compoundsSugar derivativesSucroseEthyl acetate
A process for preparing trichlorosucrose-6-ester by solid-state phosgene chlorinating includes dissolving solid phosgene in inertial solvent, slowly dropping it in the solution of sucrose-6-ester in dimethyl formamide, stirring, heating while reaction, alkali solution neutralizing, distilling to remove solvent, dissolving in water, decoloring by activated carbon, extracting in organic solvent and crystallizing.
Owner:JK SUCRALOSE
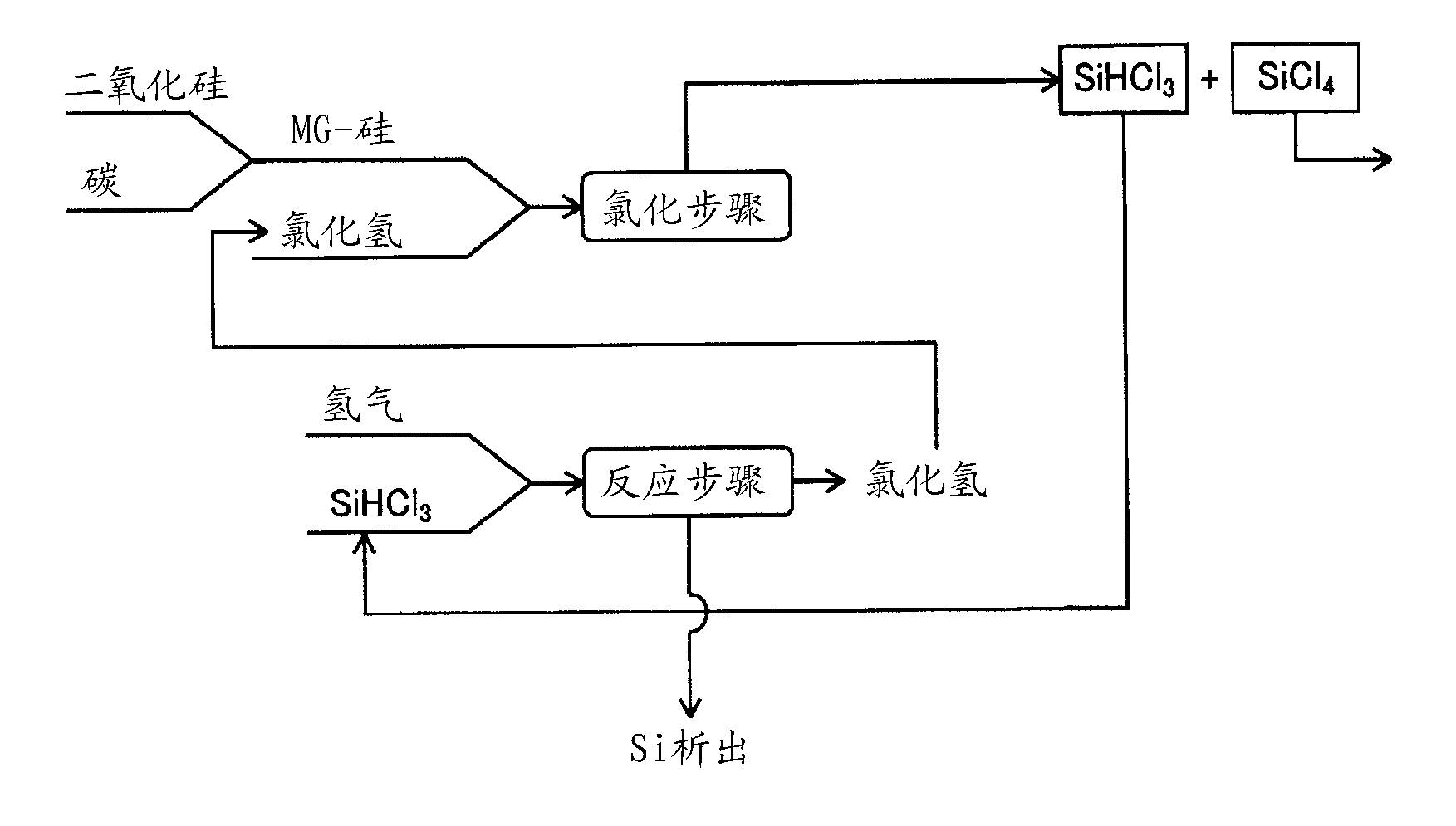
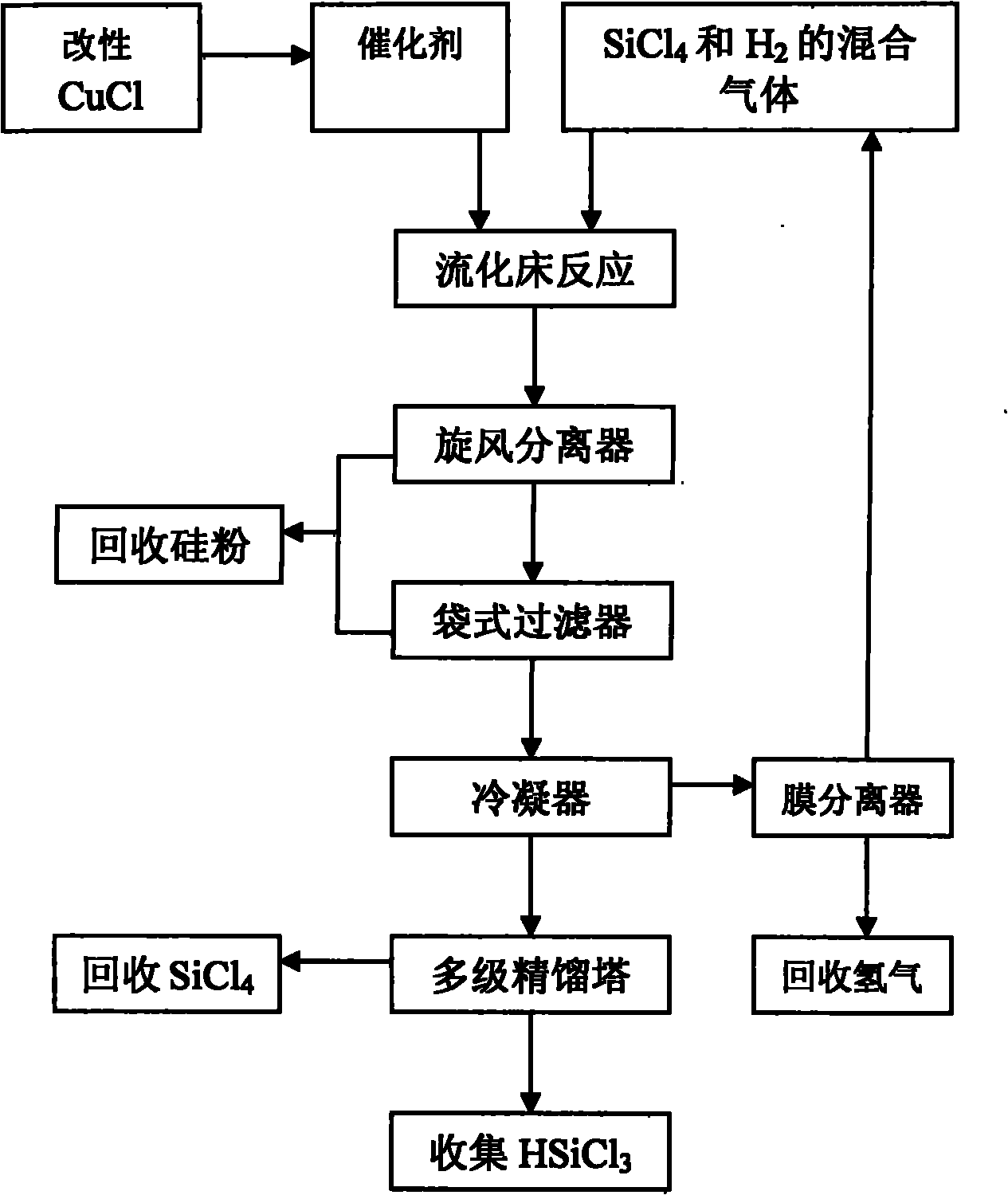
Preparation method and application of catalyst used in hydrogenation of silicon tetrachloride
InactiveCN101816946AHigh catalytic efficiencyHigh yieldSilicon organic compoundsPhysical/chemical process catalystsFiltrationShielding gas
The invention provides a preparation method of a catalyst used in hydrogenation of silicon tetrachloride, which comprises the following steps of: mixing cuprous chloride after pretreatment and silicon powder in an agitated bed reactor, and performing heated reaction on the mixture under a hydrogen condition, wherein the pretreatment refers to putting the cuprous chloride into the silicon tetrachloride, stirring and heating the mixture, and drying the mixture in a shielding gas after filtration. The invention also discloses a method for applying the catalyst in the hydrogenation of the silicon tetrachloride, which comprises the following steps of: premixing the silicon tetrachloride and hydrogen gas to form a gas mixture; adding the gas mixture and the catalyst into a fluidized bed reactor for reaction; and separating a product of trichlorosilane from a reaction tail gas. The catalyst prepared by the method has high catalytic efficiency and greatly improves the yield of the trichlorosilane.
Owner:BYD CO LTD
Catalyst used for preparing tetrachloroethylene and its preparation method and use
InactiveCN101143328AHalogenated hydrocarbon preparationMetal/metal-oxides/metal-hydroxide catalystsGas phaseCobalt
The invention relates to novel catalyst to prepare tetrachloroethylene in carbon tetrachloride gas-phase catalysis. The invention is characterized in that the catalyst carrier is prepared from soluble salt solution or mixed solution with ammonia in coprecipitation method. And then one or two of the mixtures such as the compounds of platinum, palladium and nickel, etc, together with one or a plurality of the mixtures such as the compounds of silver, cobalt, tin, zinc, copper or iron is / are loaded on the catalyst carrier in conventional equivalent impregnation method or excessive impregnation method. One or two of the composite oxides of alumina, zirconia, silica and titania is / are used as the carrier or carriers of the catalyst. One or two of platinum, palladium or nickel and one or two of silver, cobalt, tin, zinc, copper and iron are used as the active components to form multi-metal catalyst. The catalyst prepared in the method of the invention maintains high transformation rate of carbon tetrachloride, at the same time reduces the production of by-products such as methane, maintains high selectivity of tetrachloroethylene, and maintains a long service life of the catalyst.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
Hydrophobic high oil absorption soft polyurethane foam material and preparation method thereof
The invention relates to a hydrophobic high oil absorption soft polyurethane foam material and a preparation method and an application thereof, and belongs to the fields of synthesis of macromolecular oil absorption materials and environmental friendliness. The material is characterized by being prepared from 20 to 40 parts of isocyanate, 0 to 20 parts of polyethylene glycol / triol, 30 to 95 parts of polypropylene glycol / triol, 5 to 50 parts of polytetramethylene ether glycol / triol, 1.1 to 4.8 parts of cross linker, 1.0 to 4.0 parts of surfactant, 1 to 15 parts of foaming agent, 3 to 20 parts of flame retardant and 0.5 to 5 parts of catalyst by one-step foaming. The material can quickly absorb oil, and in 3 minutes, the oil absorption rate of nitrobenzene is 83g.g<-1>, the oil absorption rate of carbon tetrachloride is 80g.g<-1>, the oil absorption rate of acetone is 69g.g<-1>, the oil absorption rate of dimethyl benzene is 67g.g<-1>, the oil absorption rate of toluene is 62g.g<-1>, the oil absorption rate of kerosene is 52g.g<-1>, and the oil absorption rate of diesel oil is 47g.g<-1>; and the material almost does not absorb water when being reused in an oscillation mixed oil-water system. The material can be effectively and quickly used for the recovery of leaked oil or organic reagents on the dynamic water surface, the ground or the surfaces of other objects and treatment of oil leakage accidents.
Owner:NANJING UNIV OF TECH
Eco-friendly method of preparation of high purity tetrabromobisphenol-A
InactiveUS6365786B1High yieldMost efficientOrganic chemistryOrganic compound preparationTetrabromobisphenol AOrganic layer
A highly pure and colorless tetrabromobisphenol-A (TBBPA) possessing melting point in the range of 178-182° C. is prepared in yields of 50-70% in first batch and 90-100% when the spent organic layer is recycled. In this method, the corrosive liquid bromine is displaced by sodium bromide / hydrobromic acid as brominating agent. Further, sodium bromate is used as an oxidizing as well as brominating agent to utilize the hydrobromic acid that is produced during the bromination of bisphenol-A (BPA). The reaction is conducted at 10-15° C. in a mixture of methylene chloride-water or carbon tetrachloride-water in the presence of hydrochloric acid and sodium lauryl sulfate. The crystalline product settled at the bottom of the reaction vessel is filtered, washed, dried and weighed. The spent organic layer is recycled in subsequent batches to maximize the overall yield of product recovered directly as solid and to minimize generation of organic effluent.
Owner:COUNCIL OF SCI & IND RES
Method for producing silicon tetrachloride
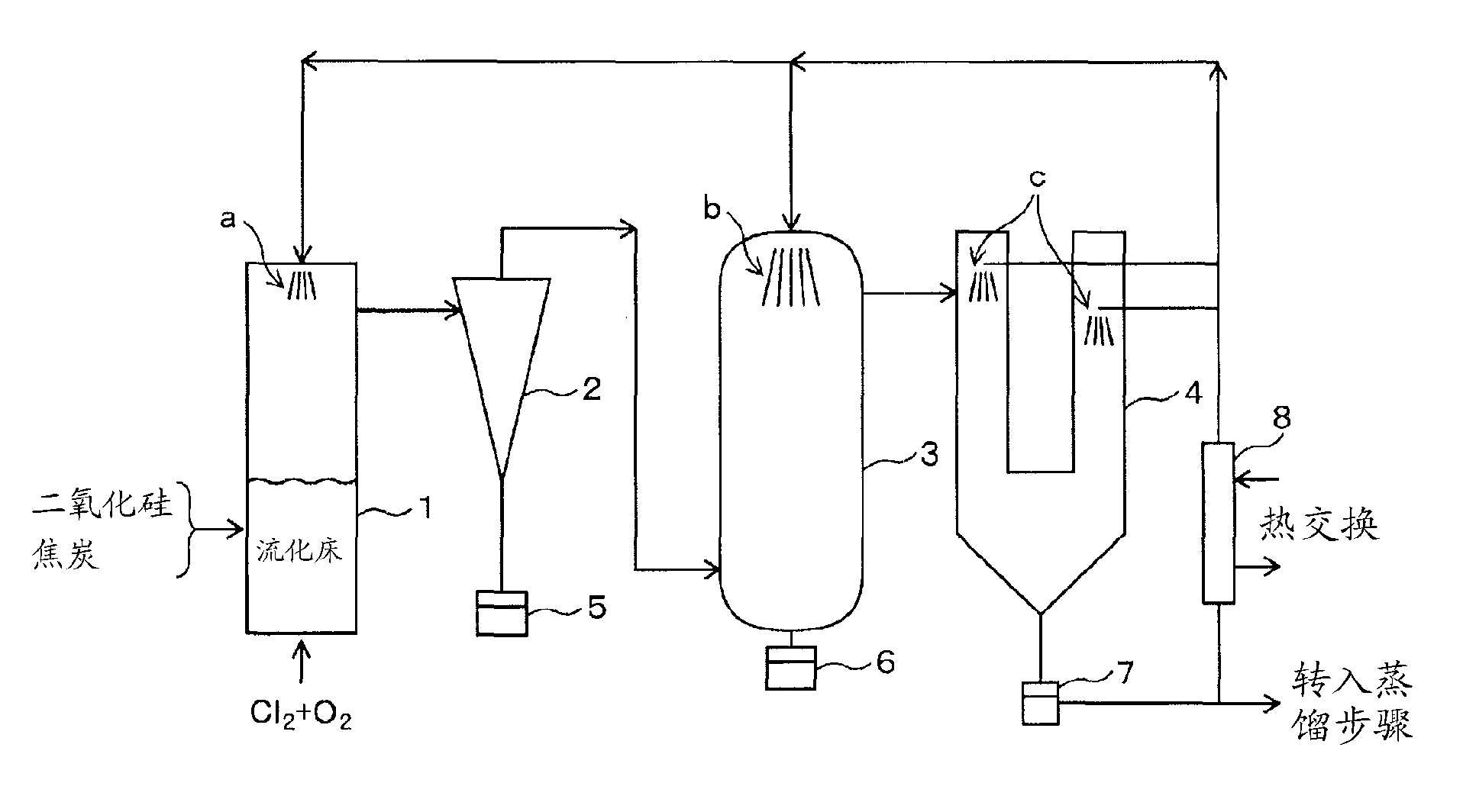
The invention concerns a method for the manufacture of silicon tetrachloride by conversion of a mixture of finely divided and / or amorphous silicon dioxide, carbon and an energy donator with chlorine. Energy donators are metallic or silicon alloys such as silicon, ferrosilicon or calcium suicide. The addition of the donors effects a self-sustaining, exothermic reaction on one hand and a significant lowering of the reaction starting temperature on the other hand. As finely divided and / or amorphous silicon dioxide ashes containing silicon dioxide are primarily used. These are produced by the incineration of silicon-containing plant skeletal structures such as rice husks or straw. Other sources include silicas from the digestion of alkaline earth silicates with hydrochloric acid and filtered particulate from the electrochemical manufacture of silicon, as well as naturally occurring products containing silicon dioxide, such as diatomaceous earth kieselguhr).
Owner:NORSK HYDRO ASA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com