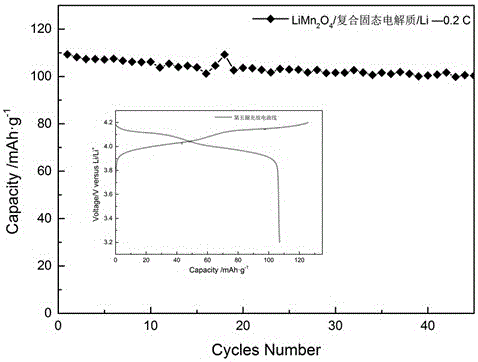
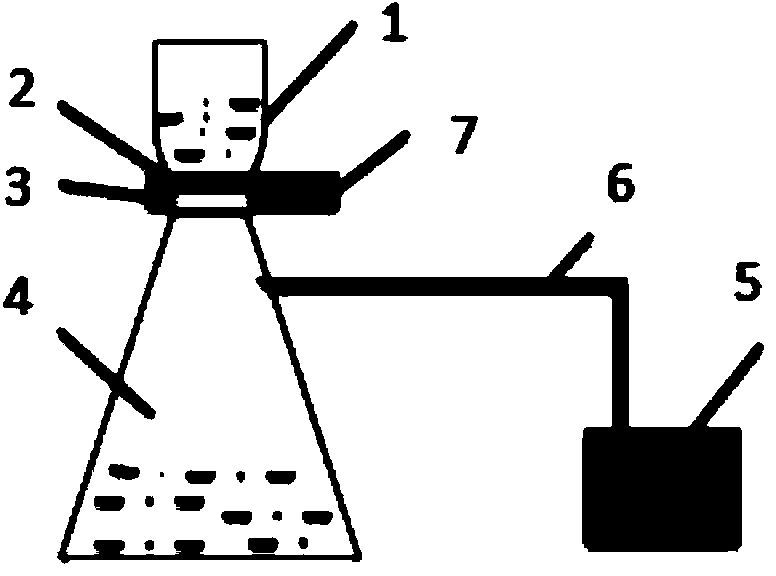
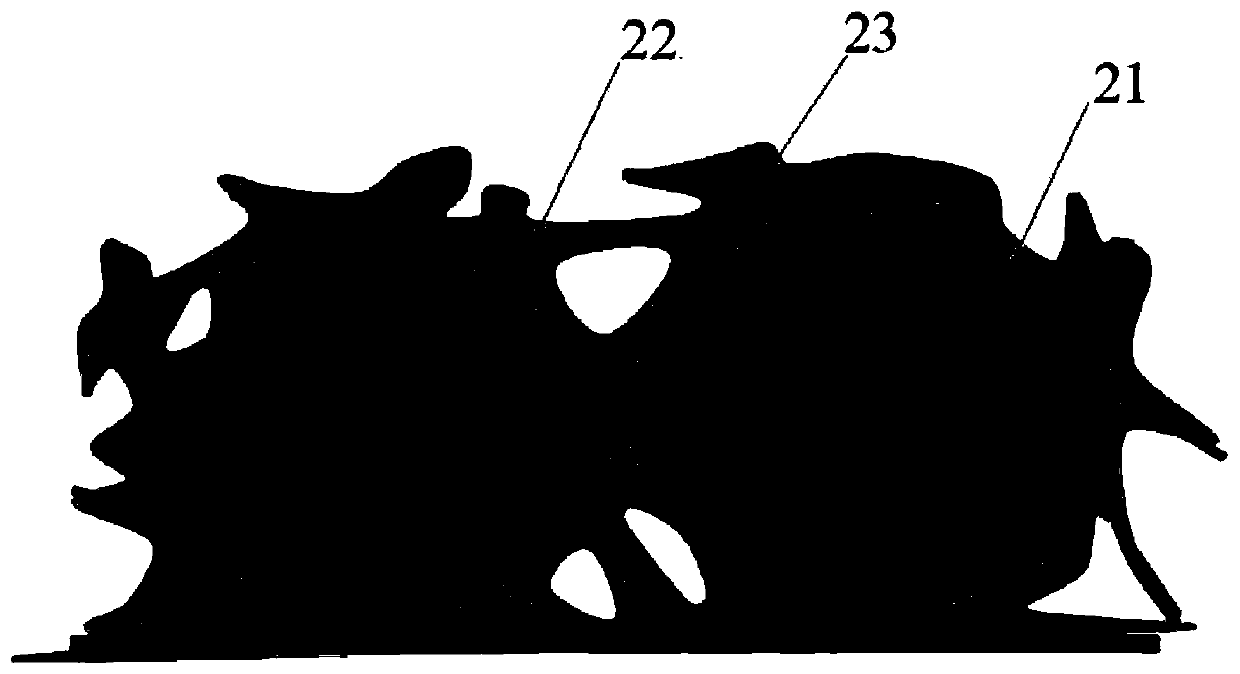
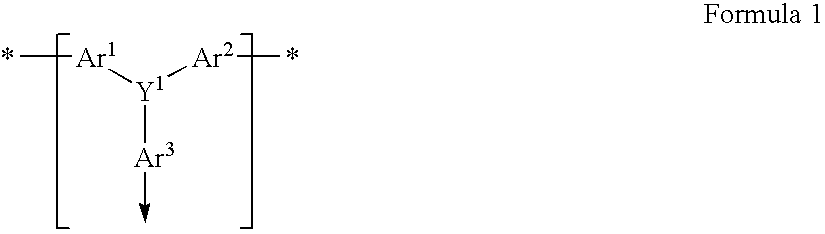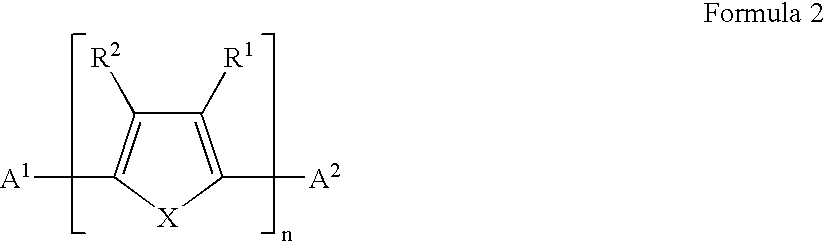Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5750 results about "Electronic conductivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
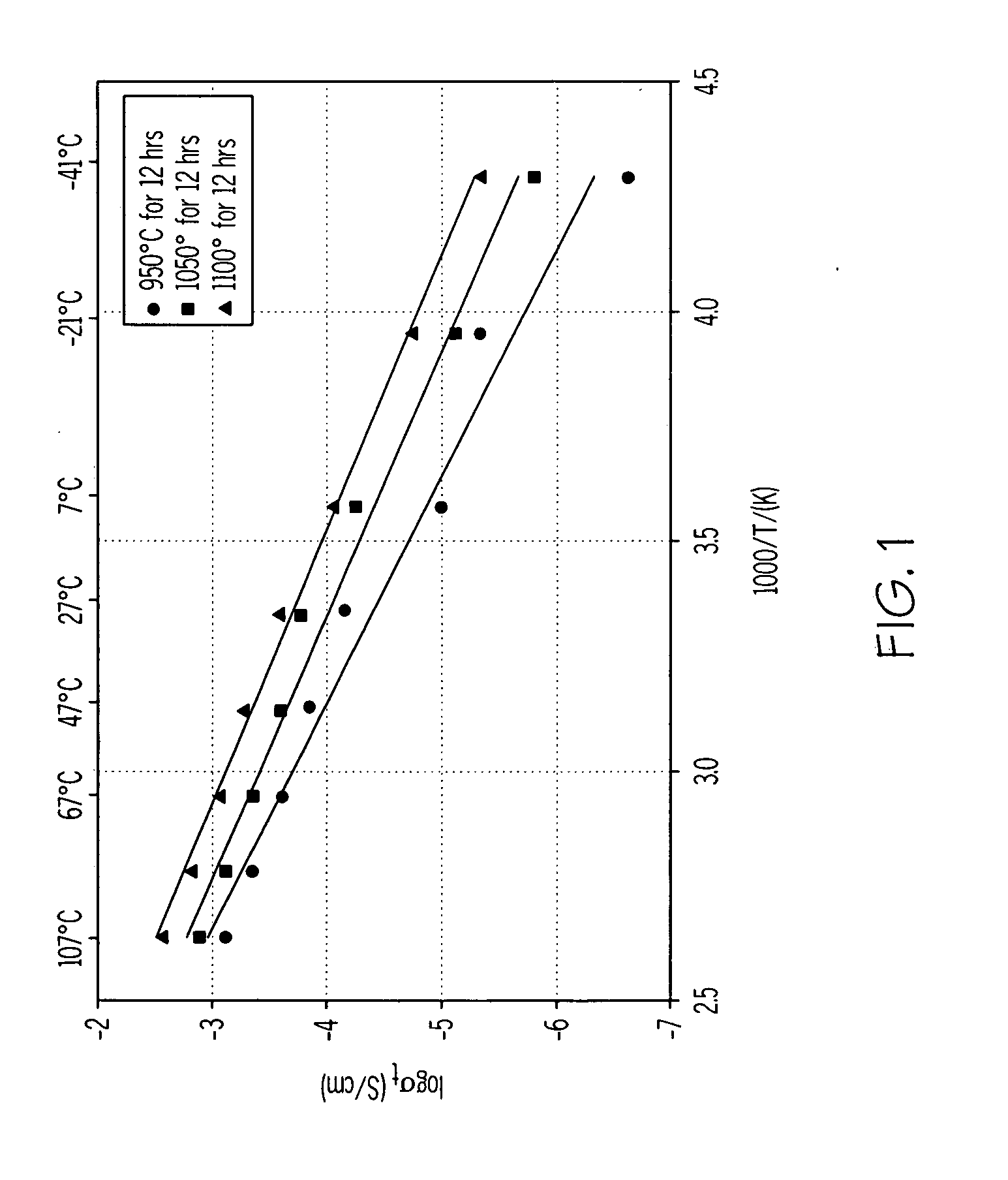
Electronic conductivity depends a lot on the material being tested. For example, in a metal, the electronic conductivity goes down with temperature and in a semiconductor. The conductivity goes up with Temperature.
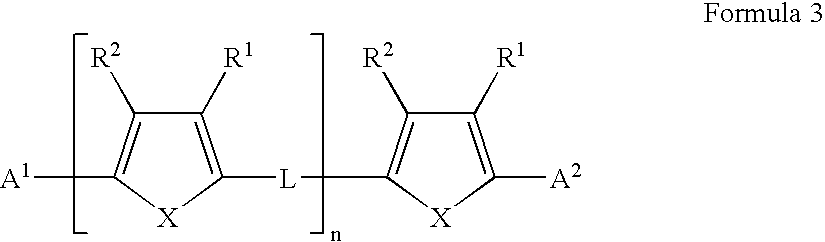
Field effect transistors and materials and methods for their manufacture
A field effect transistor in which a continuous semiconductor layer comprises:a) an organic semiconductor; and,b) an organic binder which has an inherent conductivity of less than 10−6Scm−1 and a permittivity at 1,000 Hz of less than 3.3and a process for its production comprising:coating a substrate with a liquid layer which comprises the organic semiconductor and a material capable of reacting to form the binder; and,converting the liquid layer to a solid layer comprising the semiconductor and the binder by reacting the material to form the binder.
Owner:MERCK PATENT GMBH
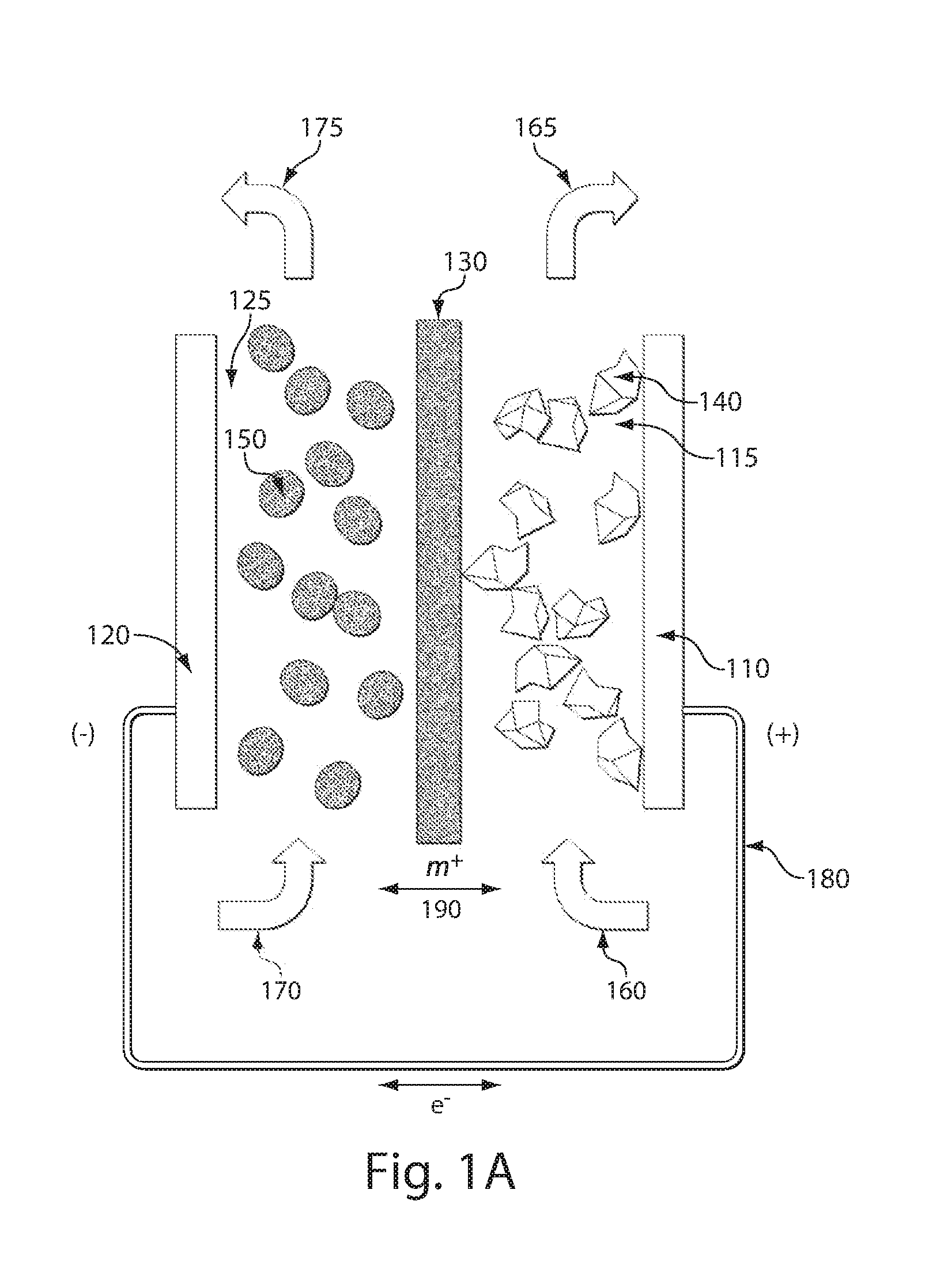
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
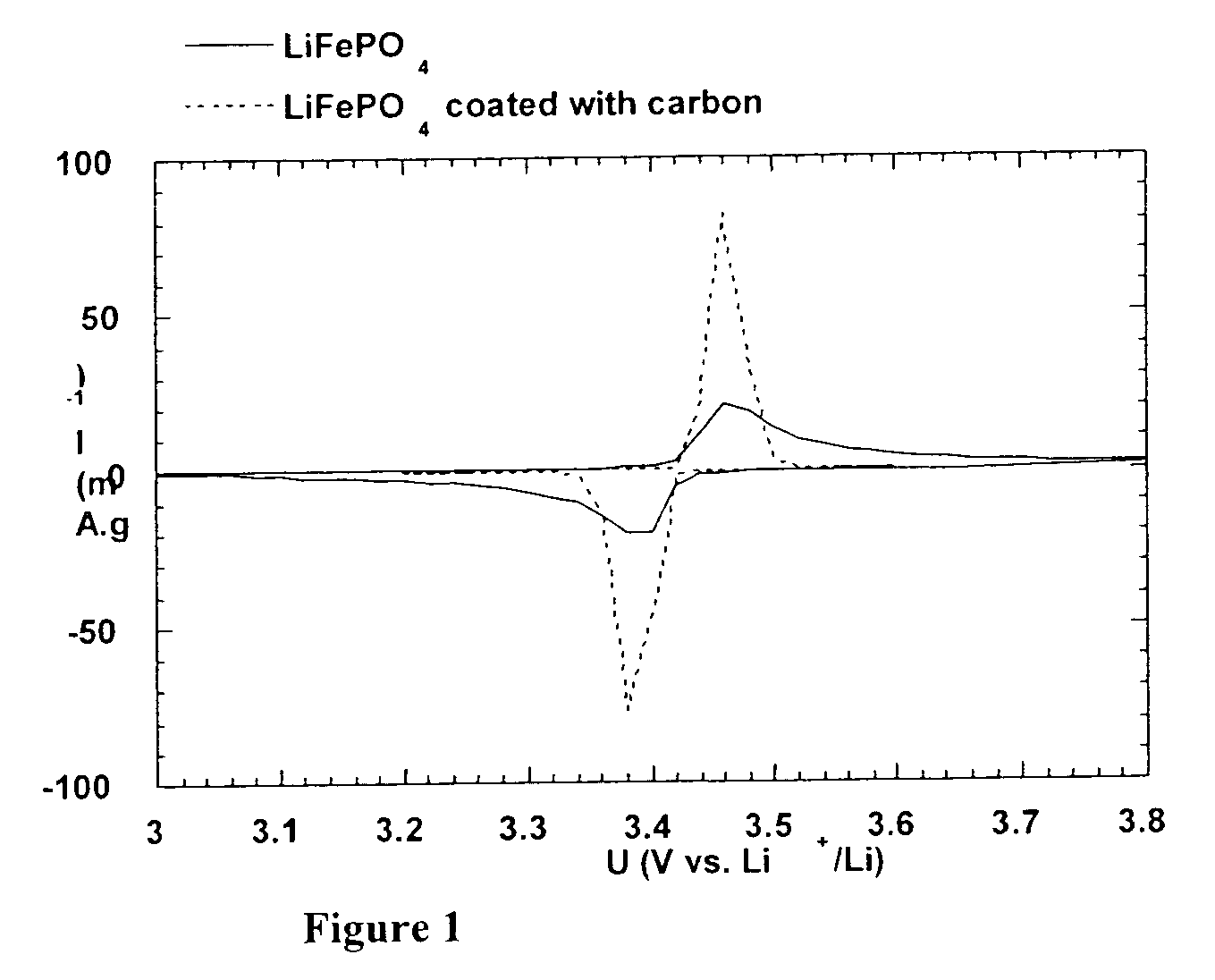
Method for synthesis of carbon-coated redox materials with controlled size
ActiveUS20040033360A1Low costReduce the numberMaterial nanotechnologyHybrid capacitorsCross-linkRedox
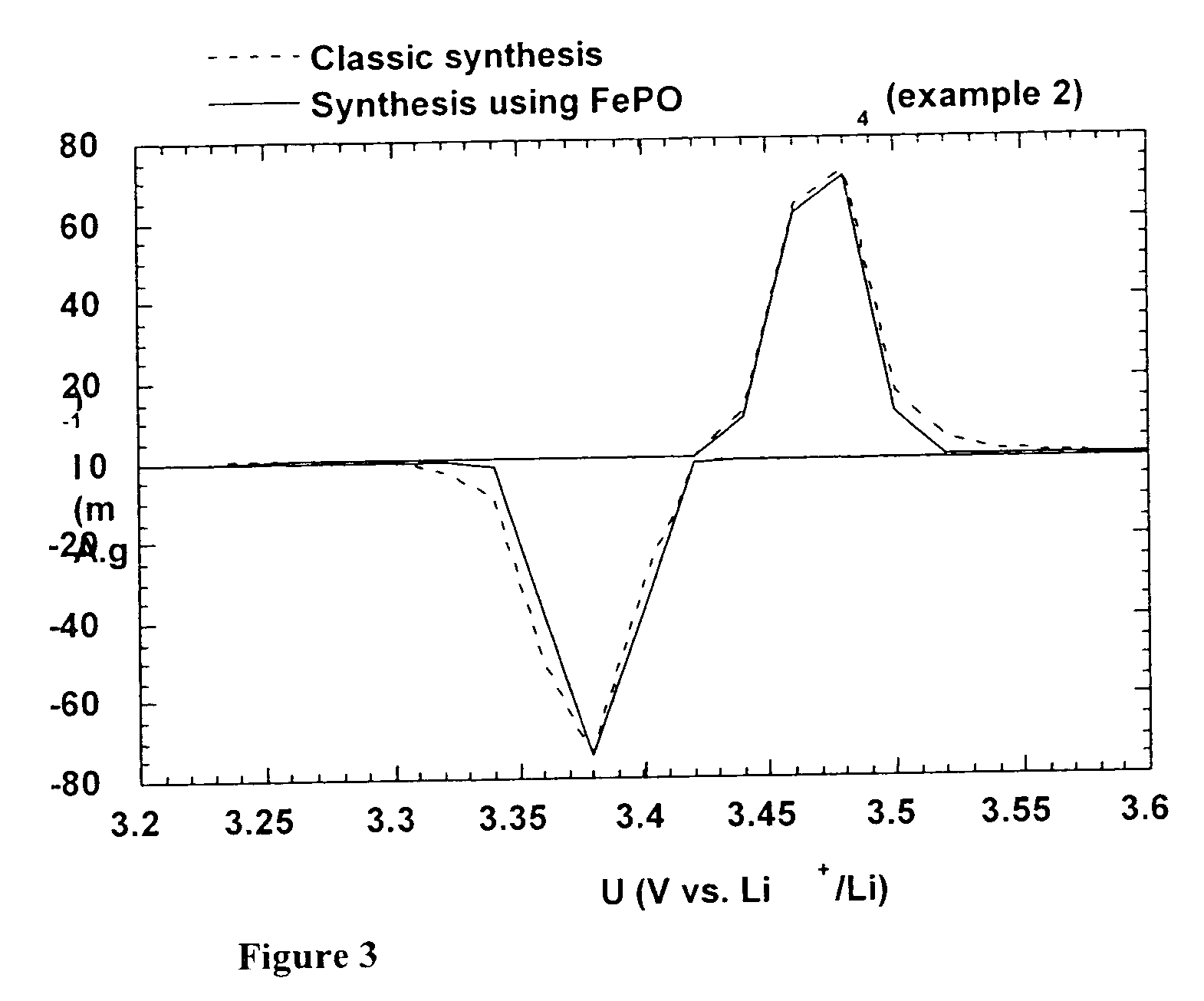
A method for the synthesis of compounds of the formula C-LixM1-yM'y(XO4)n, where C represents carbon cross-linked with the compound LixM1-yM'y(XO4)n, in which x, y and n are numbers such as 0<=x<=2, 0<=y<=0.6, and 1<=n<=1.5, M is a transition metal or a mixture of transition metals from the first period of the periodic table, M' is an element with fixed valency selected among Mg<2+>, Ca<2+>, Al<3+>, Zn<2+> or a combination of these same elements and X is chosen among S, P and Si, by bringing into equilibrium, in the required proportions, the mixture of precursors, with a gaseous atmosphere, the synthesis taking place by reaction and bringing into equilibrium, in the required proportions, the mixture of the precursors, the procedure comprising at least one pyrolysis step of the carbon source compound in such a way as to obtain a compound in which the electronic conductivity measured on a sample of powder compressed at a pressure of 3750 Kg.cm<-2 >is greater than 10<-8 >S.cm<-1>. The materials obtained have excellent electrical conductivity, as well a very improved chemical activity.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Semiconductor device comprising a contact structure based on copper and tungsten
ActiveUS20070099414A1Reduce resistanceLower resistanceTransistorSemiconductor/solid-state device detailsInter layerDevice material
By providing contact plugs having a lower plug portion, formed on the basis of well-established tungsten-based technologies, and an upper plug portion, which may comprise a highly conductive material such as copper or a copper alloy, a significant increase in conductivity of the contact structure may be achieved. For this purpose, after the deposition of a first dielectric layer of the inter-layer stack, a planarization process may be performed so as to allow the formation of the lower plug portions on the basis of tungsten, while, after the deposition of the second dielectric layer, a corresponding copper-based technology may be used for forming the upper plug portions of significantly enhanced conductivity.
Owner:GLOBALFOUNDRIES US INC
Nanoscale ion storage materials
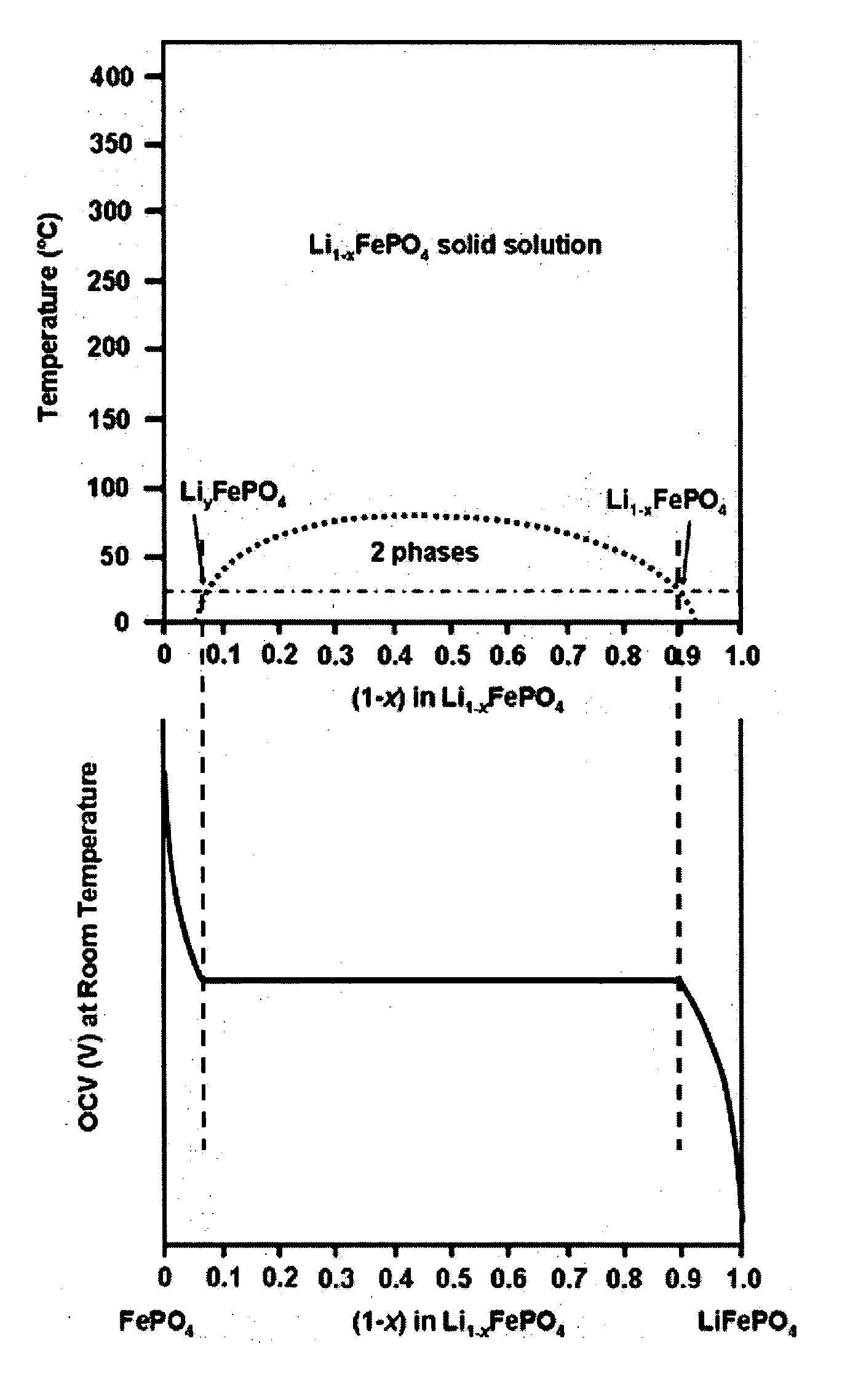
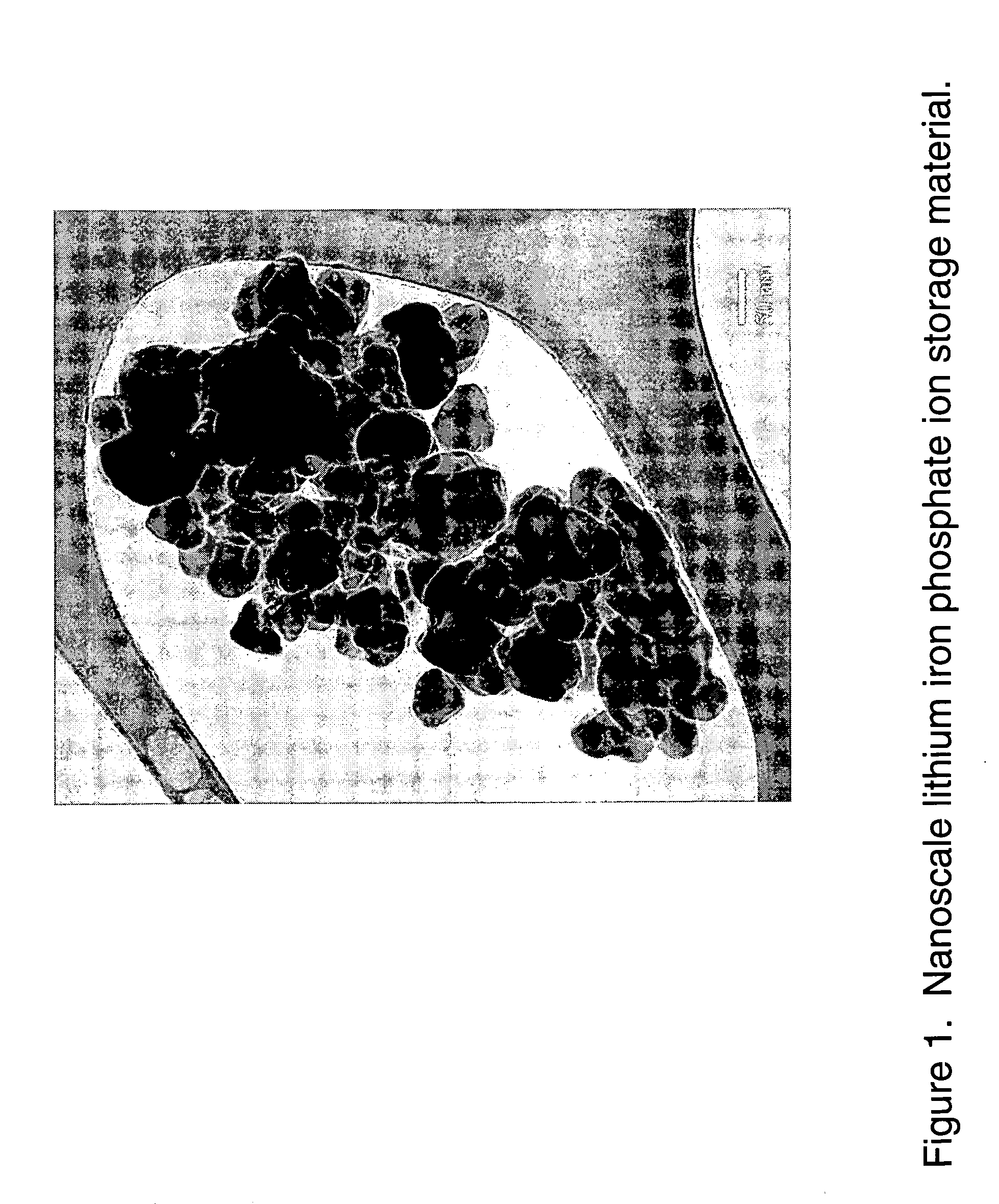
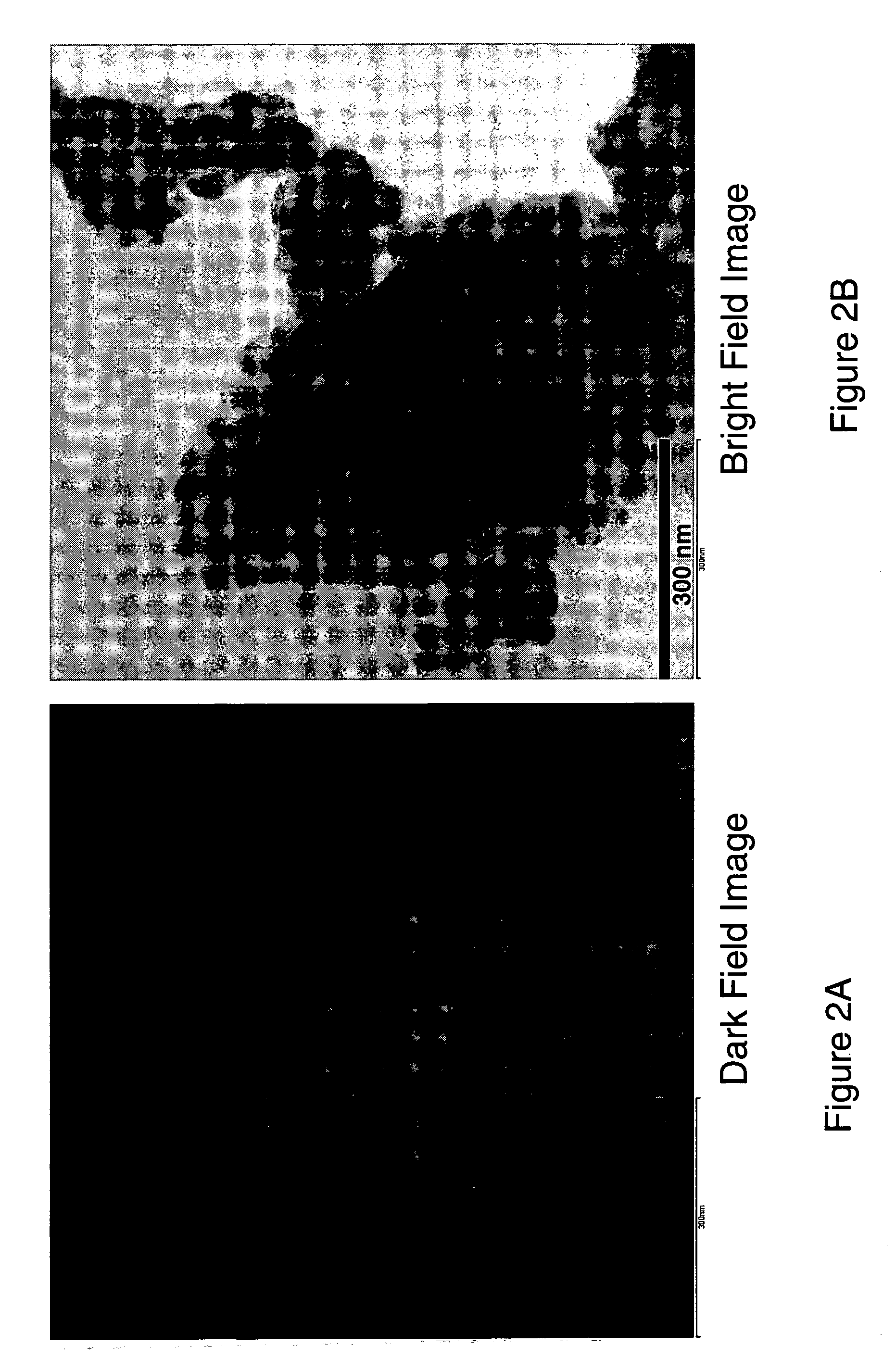
ActiveUS20070031732A1Improve electronic conductivityImproved electromechanical stabilityMaterial nanotechnologyPhosphatesHigh ratePhosphate
Nanoscale ion storage materials are provided that exhibit unique properties measurably distinct from their larger scale counterparts. For example, the nanoscale materials can exhibit increased electronic conductivity, improved electromechanical stability, increased rate of intercalation, and / or an extended range of solid solution. Useful nanoscale materials include alkaline transition metal phosphates, such as LiMPO4, where M is one or more transition metals. The nanoscale ion storage materials are useful for producing devices such as high energy and high power storage batteries, battery-capacitor hybrid devices, and high rate electrochromic devices.
Owner:RIL USA INC +1
Conductive plastic compositions and method of manufacture thereof
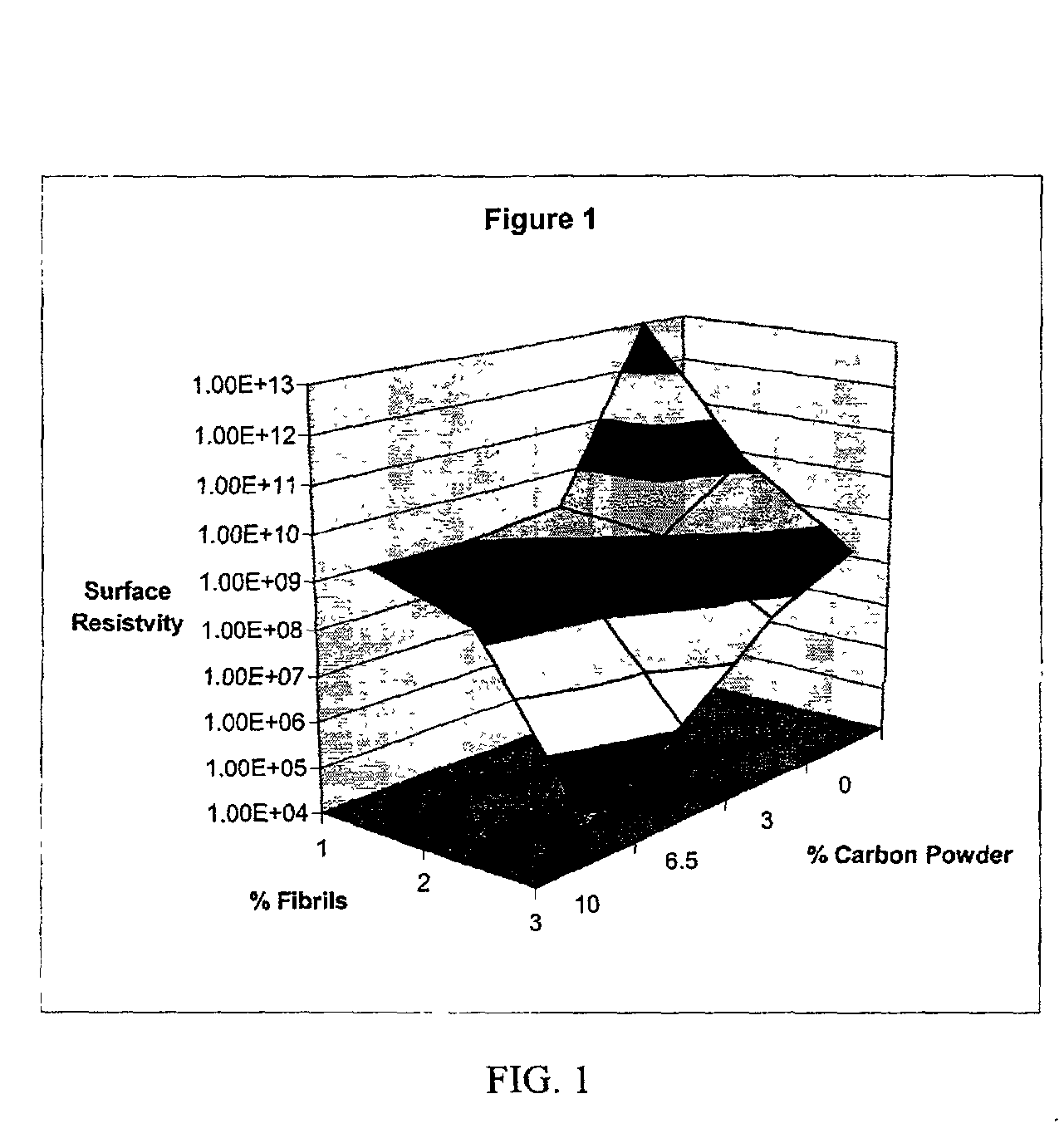
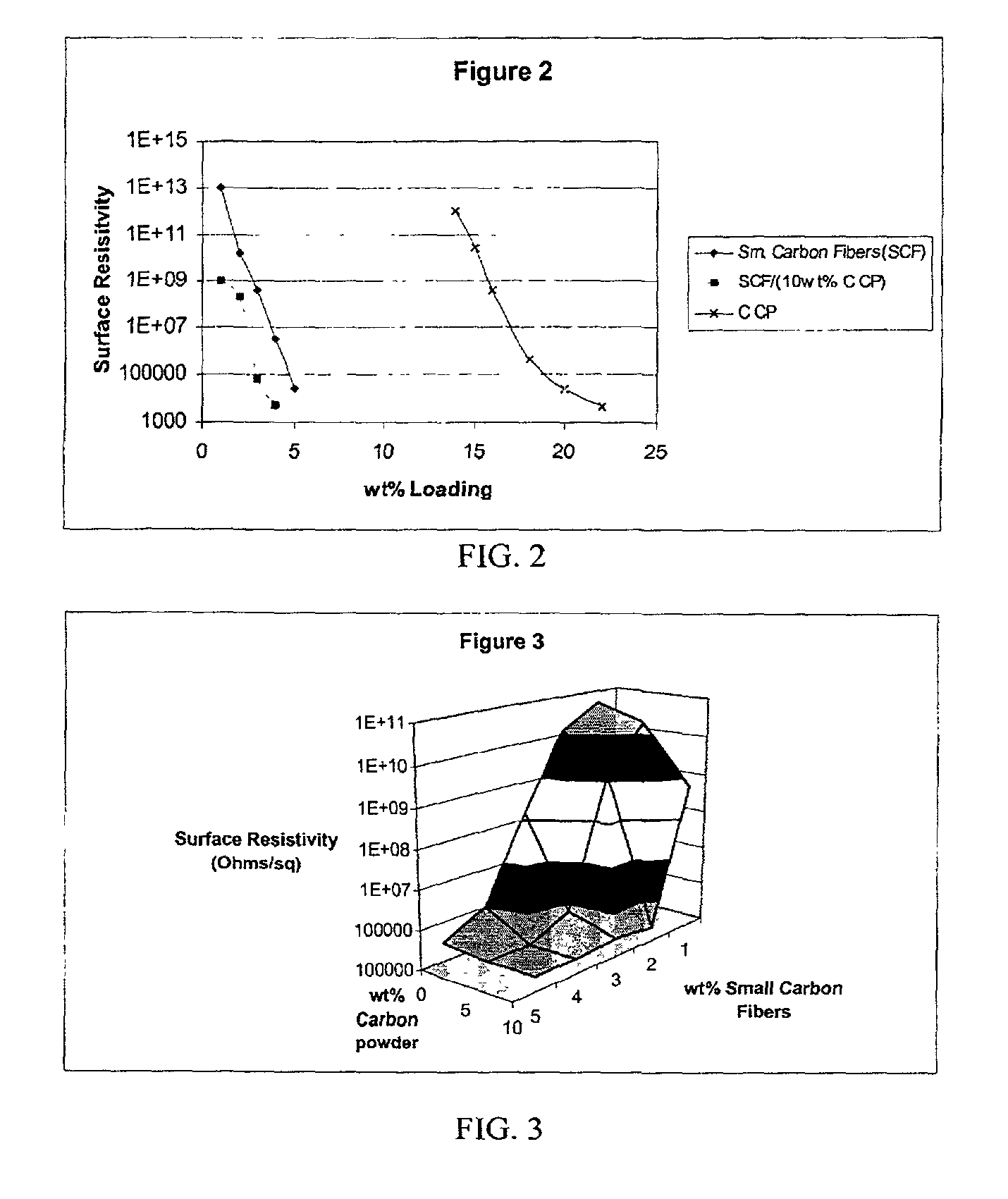
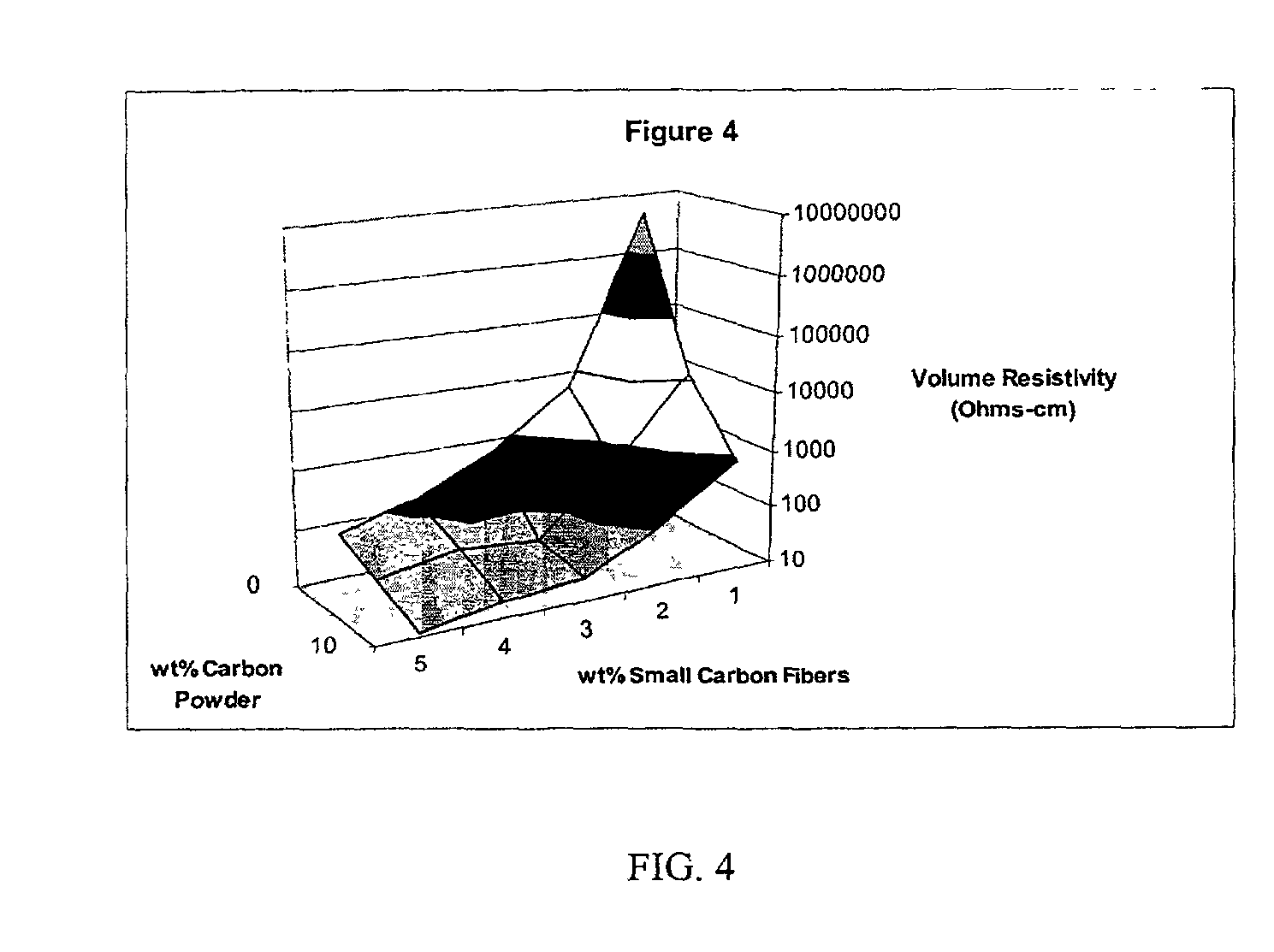
InactiveUS20020183438A1Reduce concentrationSame surface resistivitySpecial tyresNon-conductive material with dispersed conductive materialFiberCarbon fibers
An improved, conductive, polymeric composition comprises a polymeric resin; an electrically conductive filler system comprising small carbon fibers and either carbon powder or fibrous non-conductive filler or a combination of both. The amount of the conductive filler system utilized is dependent upon the desired electrical conductivity (surface and volume conductivity or resistivity) while preferably preserving intrinsic properties of the polymeric resin such as impact, flex modulus, class A finish, and the like. The conductive articles made from these compositions can therefore be used for electromagnetic shielding, electrostatic dissipation or antistatic purposes in packaging, electronic components, housings for electronic components and automotive housings.
Owner:SABIC GLOBAL TECH BV
Solid composite electrolyte membrane and method of making
ActiveUS20070117026A1Improve conductivitySolid electrolytesSolid electrolyte cellsPorosityComposite electrolyte
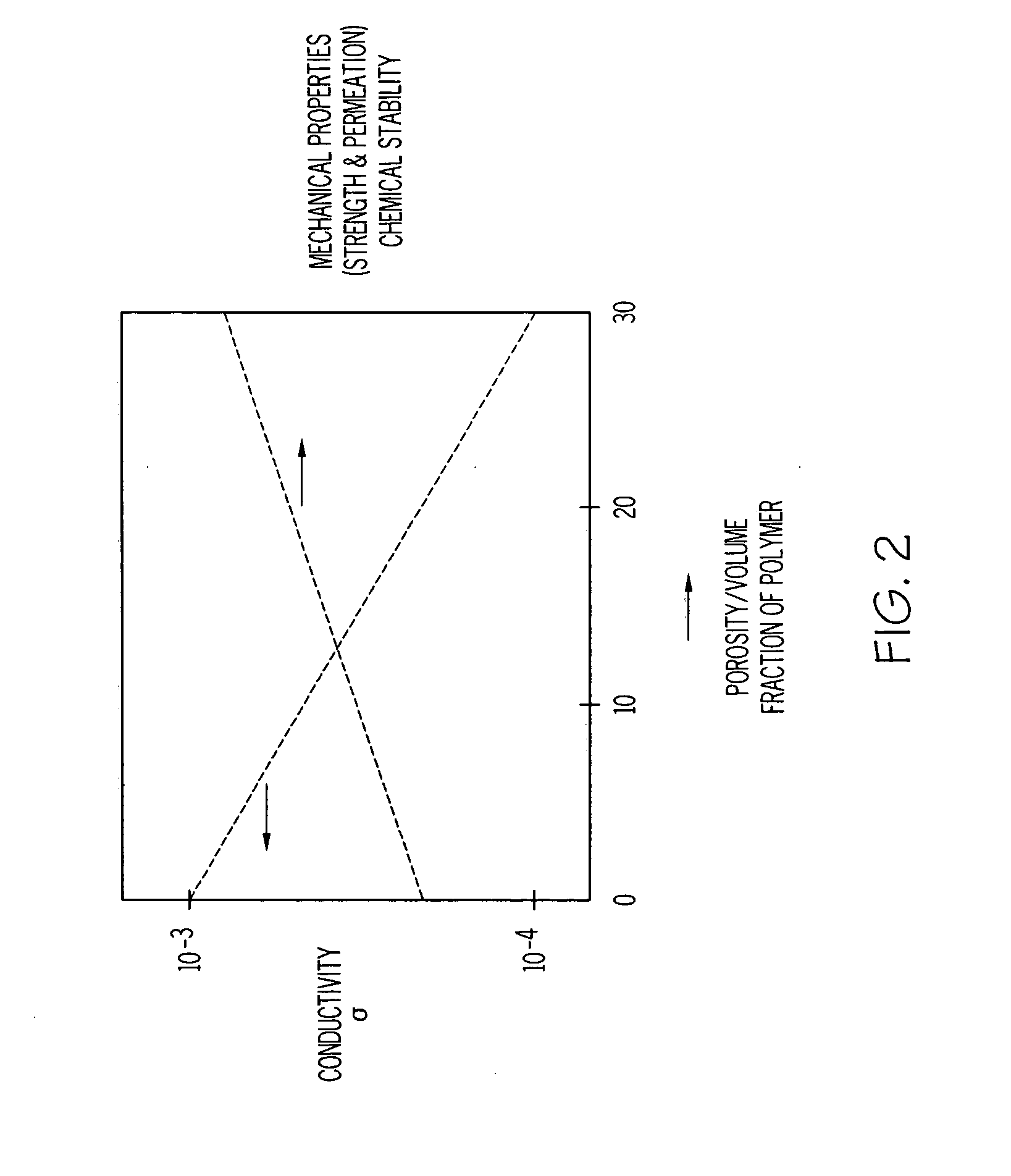
A solid composite electrolyte membrane for use in a lithium battery is provided which exhibits a conductivity ranging from about 10−4 S cm−1 to about 10−3 S cm−1 at ambient temperature. The membrane is formed by providing a glass or glass-ceramic powder formed from a mixture of lithium carbonate, alumina, titanium dioxide, and ammonium dihydrogen phosphate. The powder is mixed with a conditioning agent and at least one solvent, followed by the addition of a binder and one or more plasticizers. The resulting slurry is cast into a tape which is then subjected to a binder burn-off and sintering process to form the membrane. The resulting membrane may be a glass-ceramic composite having a porosity ranging from 0 to 50%, or the membrane may be further infiltrated with a polymer to form a water-impermeable polymeric-ceramic composite membrane.
Owner:UNIV OF DAYTON THE
Ablation devices and methods of using the same
ActiveUS20090118727A1Reduce needMinimization requirementsDiagnosticsSurgical needlesTissue volumeCorneal ablation
Devices and methods for ablating a selected tissue volume, such as for ablating tumor, are disclosed. In certain embodiments, the ablation devices include a low-conductivity, tissue-piercing tip, an adjustment mechanism for selectively adjusting the length of an exposed portion of the electrode, for producing ablation volumes of desired geometry. In other embodiment, the methods allow the adjustment of the length of the exposed electrode portion be carried out by moving an insulative sleeve along the electrode.
Owner:ANGIODYNAMICS INC
Microelectronic device and method for label-free detection and quantification of biological and chemical molecules
InactiveUS20020012937A1Wide scope of practicalWide scope of worthwhile utilizationBioreactor/fermenter combinationsBiological substance pretreatmentsCapacitanceField-effect transistor
Molecular recognition-based electronic sensor, which is gateless, depletion mode field effect transistor consisting of source and drain diffusions, a depletion-mode implant, and insulating layer chemically modified by immobilized molecular receptors that enables miniaturized label-free molecular detection amenable to high-density array formats. The conductivity of the active channel modulates current flow through the active channel when a voltage is applied between the source and drain diffusions. The conductivity of the active channel is determined by the potential of the sample solution in which the device is immersed and the device-solution interfacial capacitance. The conductivity of the active channel modulates current flow through the active channel when a voltage is applied between the source and drain diffusions. The interfacial capacitance is determined by the extent of occupancy of the immobilized receptor molecules by target molecules. Target molecules can be either charged or uncharged. Change in interfacial capacitance upon target molecule binding results in modulation of an externally supplied current through the channel.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Thin film field-effect transistor and display using the same
The present invention provides a thin film field-effect transistor comprising a substrate having thereon at least a gate electrode, a gate insulating film, an active layer, a source electrode, and a drain electrode, wherein the active layer is an oxide semiconductor layer, a resistance layer having an electric conductivity that is lower than an electric conductivity of the active layer is provided between the active layer and at least one of the source electrode or the drain electrode, and an intermediate layer comprising an oxide comprising an element having a stronger bonding force with respect to oxygen than that of the oxide semiconductor in the active layer is provided between the active layer and the resistance layer.
Owner:SAMSUNG DISPLAY CO LTD
Preparation method of silicon and carbon-coated graphene composite cathode material
ActiveCN103050666ARealize in situ restorationThe preparation process is simple, convenient and practicalMaterial nanotechnologyCell electrodesCarbon coatedStructural stability
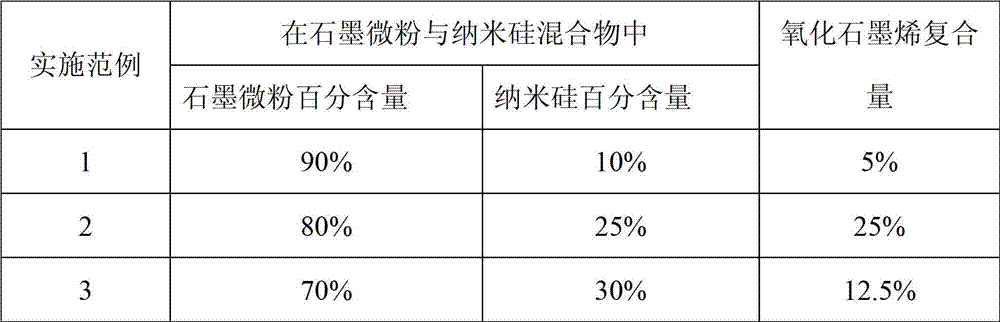
The invention discloses a preparation method of a silicon and carbon-coated graphene composite cathode material. The technical problem to be solved is to enhance the electronic conductivity of the silicon-based cathode material, buffer the volume effect produced in the process of deintercalation of the lithium in the silicon-based cathode material and enhance the structure stability in the circulation process of the material at the same time. The material is prepared by using a spray drying-thermally decomposing treatment process in the invention. The preparation method comprises the following steps of: evenly dispersing nano silicon and graphite micro powder in a dispersion solution of oxidized graphene, carrying out thermal treatment under an inert protection atmosphere after spray drying, subsequently cooling along a furnace to obtain the silicon and carbon-coated graphene composite cathode material. The extra binder does not need to add in the process of manufacturing balls in the invention and the outer oxidized graphene is thermally reduced in situ to graphene in the thermal treatment process of the composite precursor, so that the process is simple and easy to operate; and the practical degree is high. The prepared composite material has the advantages of great reversible capacity, designable capacity, good cycling performance and high-current discharging performance, high tap density and the like.
Owner:CENT SOUTH UNIV
Organic-inorganic all-solid-state composite electrolyte as well as preparation and application methods thereof
PendingCN106785009AIncrease transport activityImprove wettabilitySolid electrolytesSecondary cellsElectrical conductorComposite electrolyte
The invention relates to organic-inorganic all-solid-state composite electrolyte as well as preparation and application methods thereof, belonging to the field of lithium ion batteries. According to the organic-inorganic all-solid-state composite electrolyte, a highly-ordered three-dimensional connection network skeleton is formed by an inorganic fast lithium ion conductor, and a three-dimensional connection network is filled with a polymer and lithium salt. The organic-inorganic all-solid-state composite electrolyte which is flexible and has a controllable three-dimensional connection network structure is prepared. The electrolyte is high in lithium ion conductivity, wide in electrochemical window, good in mechanical property and stable to lithium metal. A lithium ion secondary battery assembled by a composite electrolyte membrane prepared by the method is high in capacity, stable in cycle performance, low in interface impedance and good in interface stability.
Owner:UNIV OF SCI & TECH BEIJING
Composite particle for electrode and method of making the same, electrode and method of making the same, and electrochemical device and method of making the same
InactiveUS20050058907A1Lower internal resistanceGood equipment performanceCapacitor electrodesElectrode collector coatingElectronic conductivityOxidizing agent
The composite particle for an electrode in accordance with the present invention contains an electrode active material, a conductive auxiliary agent having an electronic conductivity, and an oxidizing / reducing agent. Therefore, this composite particle can construct an effective conductive network, and effectively provide so-called oxidizing / reducing capacity due to the oxidizing / reducing agent. Hence, when the composite particle for an electrode in accordance with the present invention is used as a constituent material of an electrode in an electrochemical device, the electrochemical device can realize a higher capacity.
Owner:TDK CORPARATION
Hydrothermal synthesis method for lithium ion-cell anode material of ferric phosphate lithium
InactiveCN101117216AImprove electrochemical performanceShorten the diffusion pathCell electrodesPhosphorus compoundsExhaust valveDead volume
The invention discloses a hydrothermal synthesis method of lithium-ion battery anode material of lithium iron phosphate, relating two kinds of metal phosphate. The steps are as follows: lithium source and phosphorus source are dissolved in water or mixed with water, and added into the reaction autoclave, the quaternary cationic surfactants and the alkylphenols polyoxyethylene ethers nonionic surfactant is also added into the reaction autoclave, the air in the dead volume of the autoclave inside is purged by the inert gas, the autoclave is sealed and heated to 40-50 DEG C with stirring, a feed valve and an exhaust valve are opened, pure ferrous salting liquid is added into the autoclave, and then the autoclave is sealed for the reaction of the material at 140 to 180 DEG C for 30 to 480 minutes; the mixture ratio of the invention is set as follows: the molar ratio of Li, Fe and P is 3.0-3.15:1:1.0-1.15, and then the resultant is filtered, washed, dried and carbon-coated, thus the lithium iron phosphate is obtained. The lithium iron phosphate which is produced by the invention has the advantages that: the electrochemical performance is excellent, the particle size distribution of which the D50 is between 1.5 um to 2 um is even, the phase purity is above 99 percent and the electronic conductivity of the material is improved.
Owner:HEBEI LITAO BATTERY MATERIAL
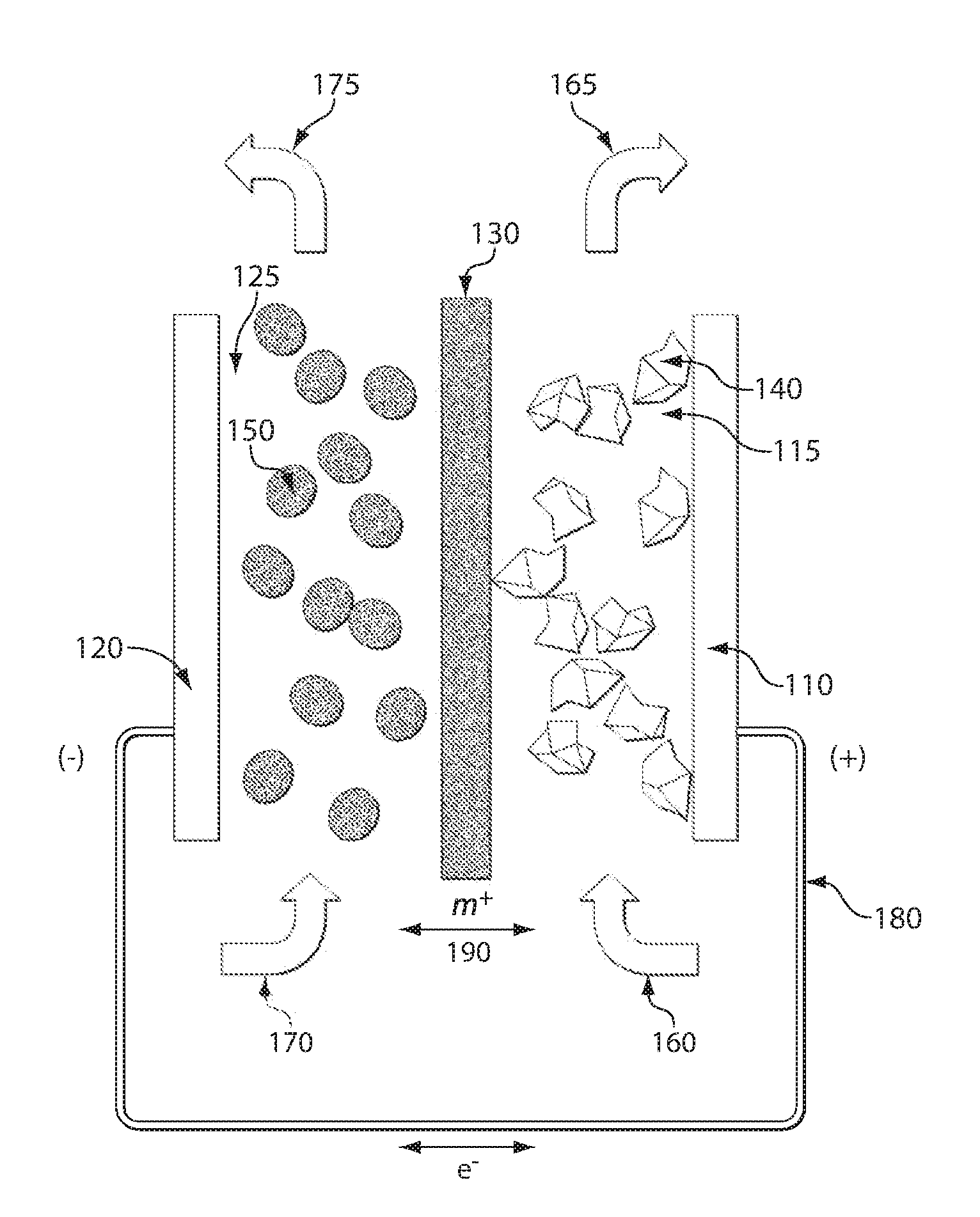
Nonaqueous secondary battery
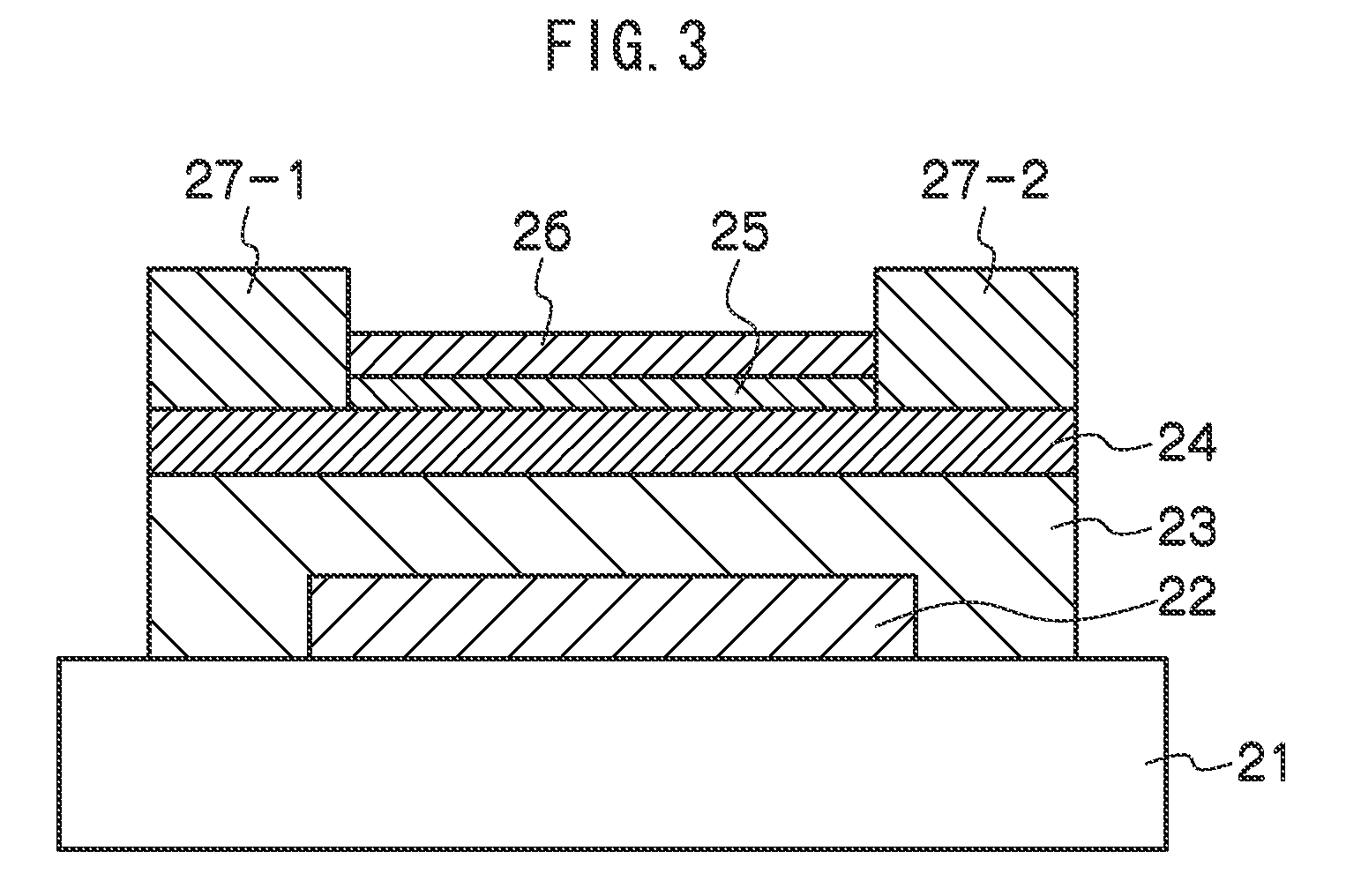
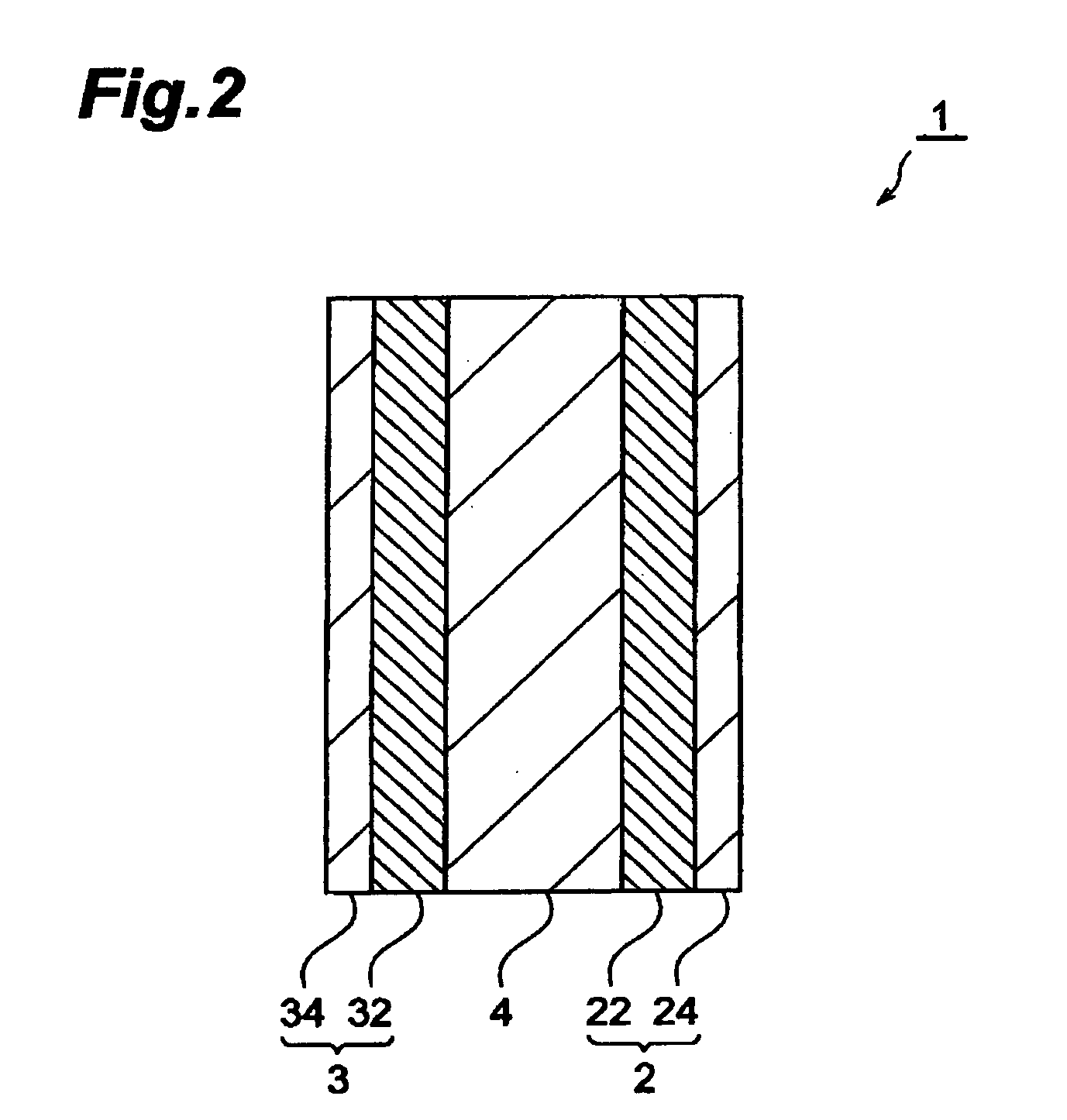
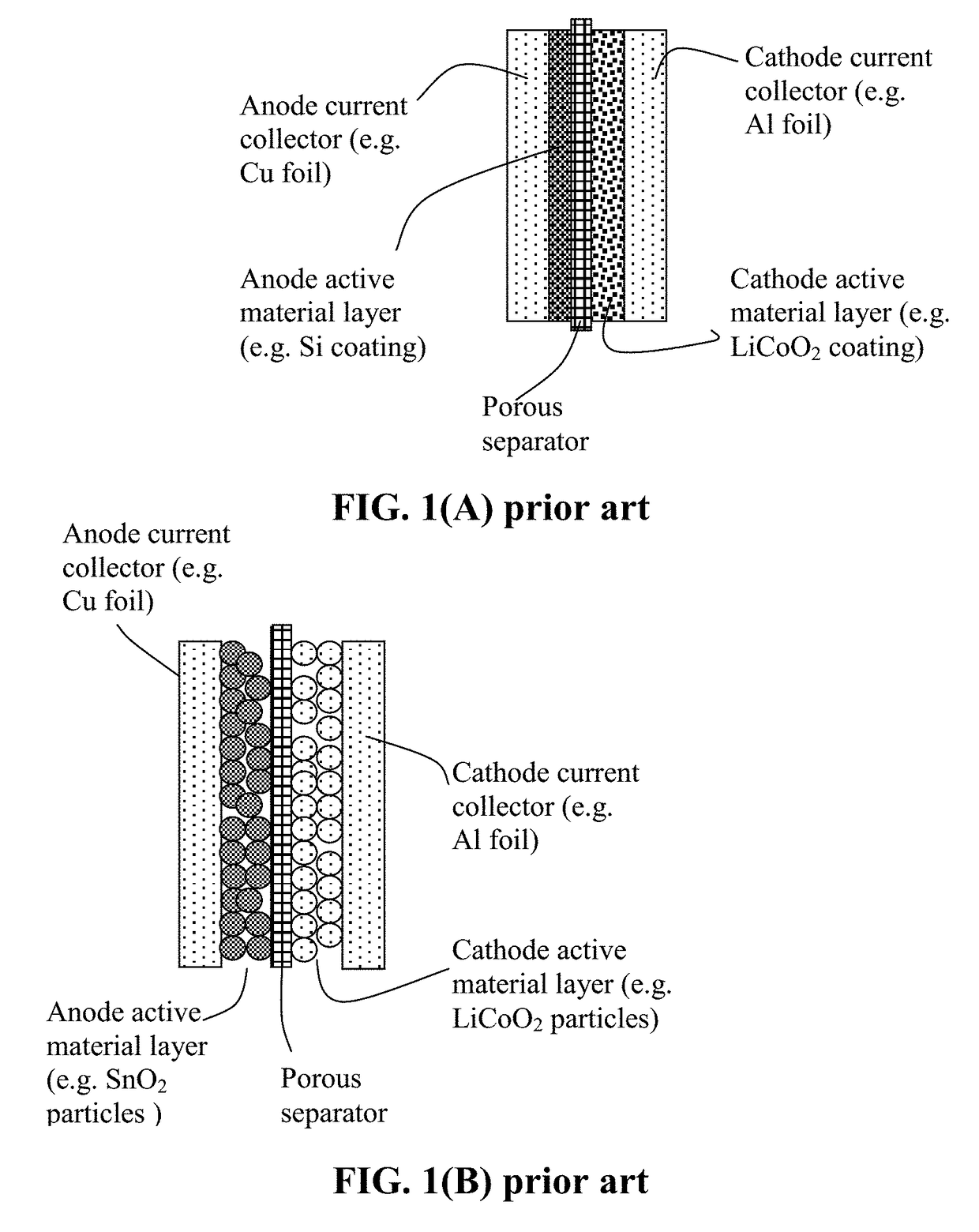
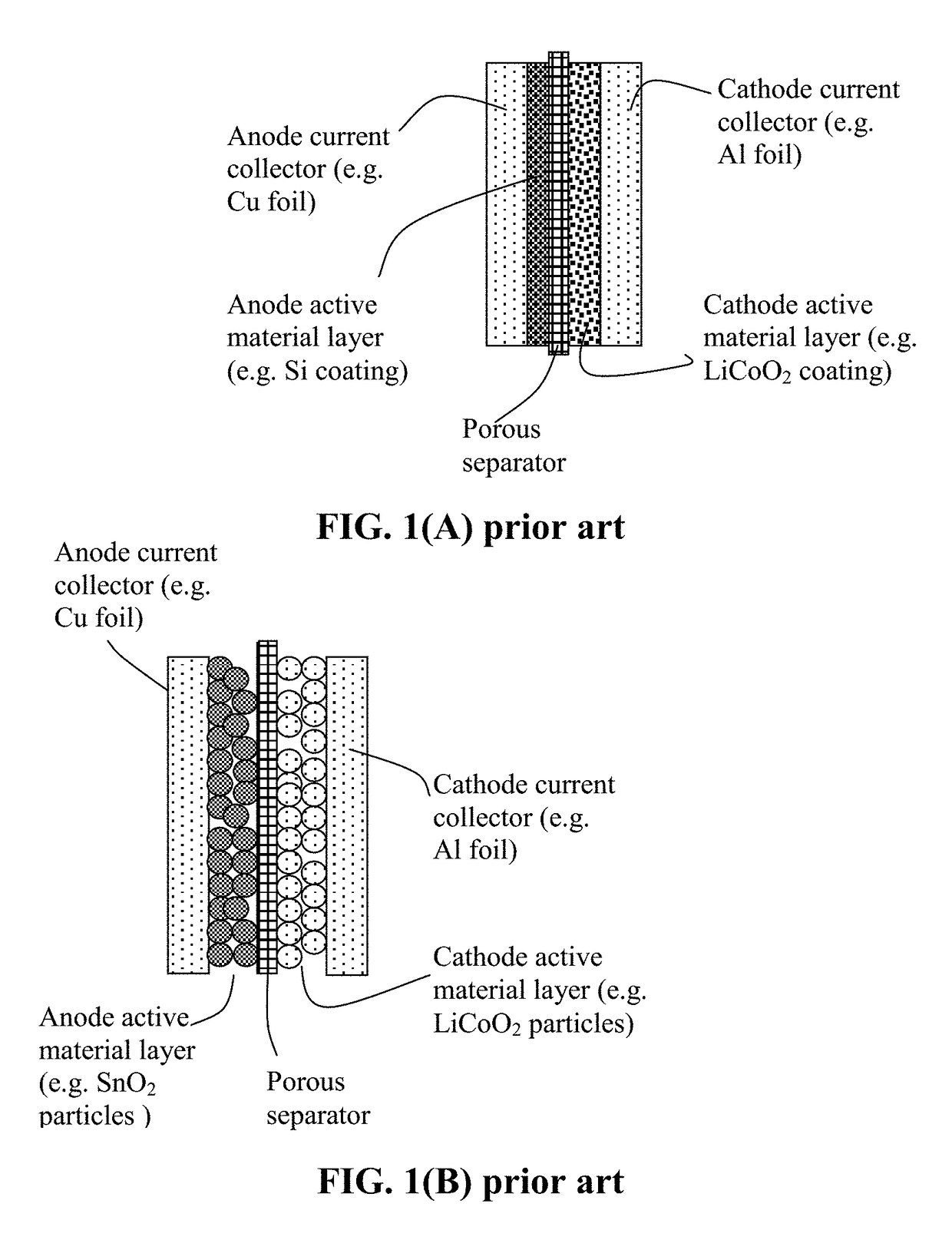
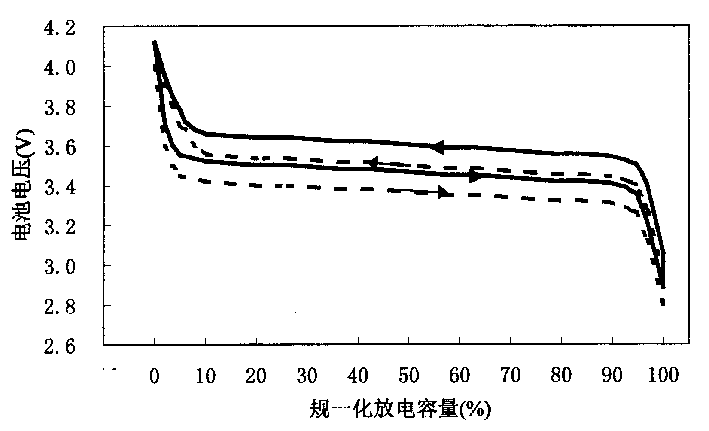
ActiveUS20070048613A1Low electronic conductivityImprove production efficiencyFinal product manufactureElectrode carriers/collectorsMetal foilElectronic conductivity
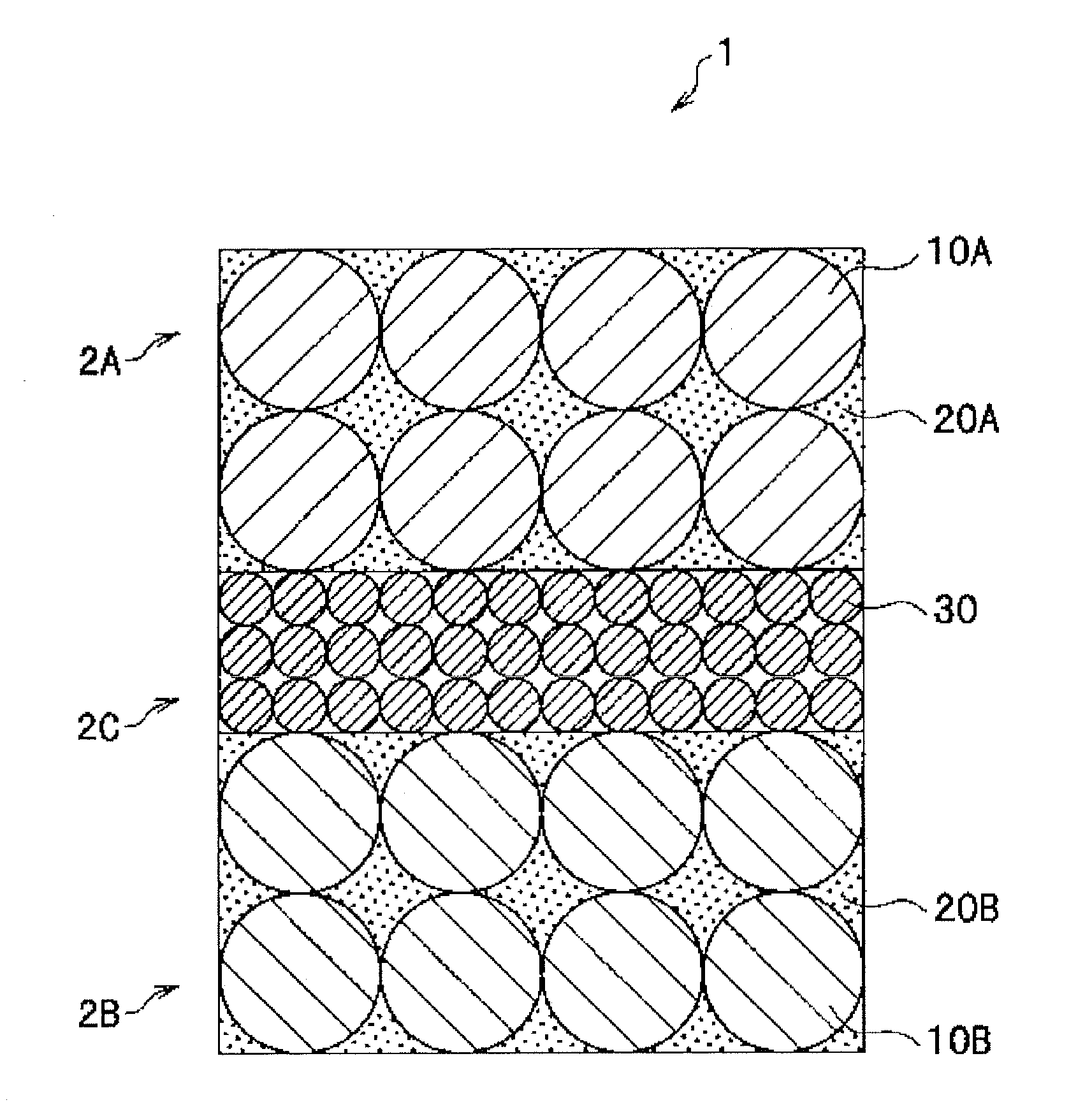
A nonaqueous secondary battery 10 of the invention has an active material compound layer 14 deposed over at least one face of a collector 12 made of metal foil and is equipped with a positive electrode 11 having a portion 13 in a part of which metal is exposed. The positive electrode 11 together with the exposed-metal portion 13 faces a negative electrode 17 through an interposed separator 23, and on that part of the exposed-metal portion 13 that faces the negative electrode 17 through the interposed separator 23 there is formed a protective layer 16 made of a material whose electronic conductivity is lower than that of the metal and which moreover is non-insulative. With such nonaqueous secondary battery 10, should part of an electrode pierce the separator and contact with the other electrode, the battery will be gently discharged, thereby averting abnormal heat generation by the battery and additionally enabling the battery's abnormality to be sensed by the equipment via the fall in battery voltage. Thus, there is provided a nonaqueous secondary battery of excellent safety that can prevent abnormal heat generation due to a short circuit caused by burr, powder or the like piercing the separator.
Owner:SANYO ELECTRIC CO LTD
Method for preparing graphite alkyne film
The invention discloses a method for preparing a graphite alkyne film. The method comprises that: a copper sheet or any one substrate the surface of which is covered with a copper film layer is used as a substrate; 6-alkynyl-benzene is subjected to coupling reaction in a solvent under the catalytic action of the copper to obtain the graphite alkyne film on the surface of the substrate. The method for preparing the graphite alkyne film, which is provided by the invention, has simple and convenient process, and can carry out large-scale preparation of the graphite alkyne film on the surface of the copper sheet or the substrate any surface of which is covered with the copper. The electrical conductivity of the graphite alkyne film is 2.516*10-4S / m. The film has uniform surface, can exist stably in the air, is a semiconductor with similar performances with silicon, and has potential application prospect in the fields of catalysis, electron, semiconductor, energy, material and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Polycarbonate all-solid-state polymer electrolyte, all-solid-state secondary lithium battery made of same and preparation and application thereof
The invention relates to solid-state electrolytes, in particular to a polycarbonate all-solid-state polymer electrolyte, an all-solid-state secondary lithium battery made of the same and preparation and application thereof. The all-solid-state polymer electrolyte is prepared from polycarbonate macromolecules, lithium salt and a porous supporting material; the thickness is 20-800 micrometers, the mechanical strength is 10-80 MPa, the room temperature ion conductivity is 2*10<-5>S / cm-1*10<-3>S / cm, and the electrochemical window is higher than 4V. The all-solid-state polymer electrolyte is easy to prepare and form, good in mechanical property, high in ion conductivity and wide in electrochemical window; meanwhile, the solid-state electrolyte effectively inhibits growth of negative electrode lithium dendrites and improves interface stability and long circulation performance.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Polymer Solid Electrolyte
InactiveUS6162563AEasy to processGood moldabilityHybrid capacitor electrolytesCell electrodesLithium metalBackbone chain
PCT No. PCT / JP97 / 02854 Sec. 371 Date Mar. 11, 1999 Sec. 102(e) Date Mar. 11, 1999 PCT Filed Aug. 19, 1997 PCT Pub. No. WO98 / 07772 PCT Pub. Date Feb. 26, 1998A polymer solid electrolyte obtained by blending (1) a polyether copolymer having a main chain derived form ethylene oxide and an oligooxyethylene side chain, (2) an electrolyte salt compound, and (3) a plasticizer of an aprotic organic solvent or a derivative or metal salt of a polyalkylene glycol having a number-average molecular weight of 200 to 5,000 or a metal salt of the derivative is superior in ionic conductivity and also superior in processability, moldability and mechanical strength to a conventional solid electrolyte. A secondary battery is constructed by using the polymer solid electrolyte in combination with a lithium metal negative electrode and a lithium cobaltate positive electrode.
Owner:OSAKA SODA CO LTD
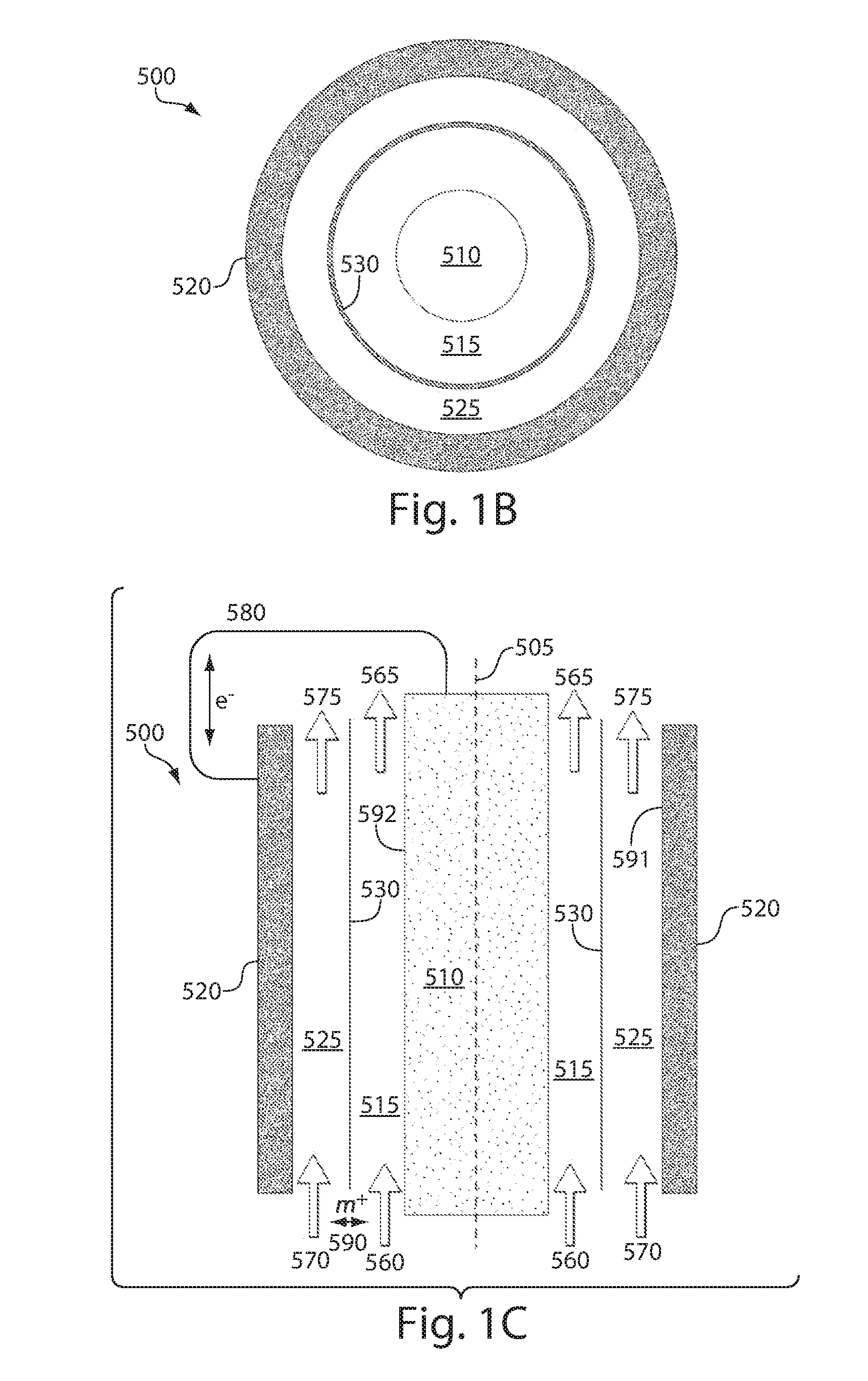
All-solid state battery, electrode for all-solid state battery, and method of manufacturing the same
InactiveUS20160233510A1Improve contact qualityImprove discharge performanceNon-aqueous electrolyte accumulatorsElectrode thermal treatmentAll solid stateEngineering
Provided are an all-solid state battery with a better quality of contact among particles of an active material and with an enhanced discharge capacity; an electrode for an all-solid state battery; and a method of manufacturing the same. The all-solid state battery is manufactured through the steps of: causing a deliquescent solid electrolyte to deliquesce, the deliquescent solid electrolyte having ionic conductivity, electronic conductivity and a deliquescent property; preparing an electrode mixture by mixing the deliquescent solid electrolyte having deliquesced and an active material together; heat-treating and shaping the electrode mixture to produce an electrode; and bonding the thus-produced electrode and a solid electrolyte layer with the solid electrolyte layer interposed between the electrode and another electrode which are paired to serve as a positive electrode and a negative electrode.
Owner:HITACHI LTD
Single-sided lateral-field and phototransistor-based optoelectronic tweezers
ActiveUS20100101960A1Good flexibilityElectrostatic separatorsSludge treatmentDielectrophoretic forceCell culture media
Described herein are single-sided lateral-field optoelectronic tweezers (LOET) devices which use photosensitive electrode arrays to create optically-induced dielectrophoretic forces in an electric field that is parallel to the plane of the device. In addition, phototransistor-based optoelectronic tweezers (PhOET) devices are described that allow for optoelectronic tweezers (OET) operation in high-conductivity physiological buffer and cell culture media.
Owner:RGT UNIV OF CALIFORNIA
Oxide solid electrolyte material, and preparation method and application thereof
The invention discloses a lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material and a preparation method thereof. The solid electrolyte material is composed of a base material and a doping element, wherein the base material is a lithium-lanthanum-zirconium-oxygen solid electrolyte of which the chemical formula is Li7La3Zr2O12, the doping element is selected from at least one of calcium, strontium, barium and germanium, and the weight of the doping element does not exceed 15% of that of the base material. The preparation method comprises the following steps: a lithium source compound, a lanthanum source compound, a zirconium source compound and a doping element compound are evenly mixed, calcined and sintered to obtain the lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material. The lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material can be prepared under the conditions of wide sources of doping element, lower sintering temperature and shorter sintering time, and the total room-temperature ionic conductivity is greater than 1*10<-4>S / cm; and thus, the material has important application value.
Owner:TSINGHUA UNIV +1
Composite solid polymer electrolyte and preparing method and application thereof
InactiveCN106299471AHigh mechanical strengthImprove thermal stabilitySolid electrolytesSecondary cellsPolymer electrolytesElectrical battery
The invention discloses a composite solid polymer electrolyte and a preparing method and application thereof. The composite solid polymer electrolyte is prepared from a gadoleic acid ester monomer, a porous supporting matrix, lithium salt, an additive and an initiator, wherein the mass ratio of the gadoleic acid ester monomer to the additive is (1-10):1, the lithium salt accounts for 5-30% of the total mass of the gadoleic acid ester monomer and the additive, and the mass ratio of the initiator to the gadoleic acid ester monomer is (1-10):100. The composite solid polymer electrolyte prepared with the method has the advantages of being large in ion conductivity, high in mechanical strength, easy to form, stable in interface contact, high in electrochemical stability and easy to prepare and can be used for forming solid lithium ion secondary batteries, and the prepared solid lithium ion secondary batteries are high in safety, energy density and production efficiency.
Owner:HARBIN INST OF TECH +1
Combined electrode of battery and preparation method thereof
InactiveCN103730630AImprove electronic conductivityImprove ionic conductivityActive material electrodesElectrolyte layer coatingSolid state electrolyteElectrical battery
The application relates to the field of energy storage materials, and discloses a combined electrode with ultrahigh electron and ionic conductivity and a preparation method thereof. The combined electrode is formed in a manner that a battery active material is uniformly tied in a three-dimensional multi-hole network formed by carbon nano tubes which are connected in a crossing manner, and meshes and the surface of the active material are filled or coated with a solid electrolyte material. According to the combined electrode, the carbon nano tubes, which are communicated with one another, can form an ultrahigh electrical transmission network, on the one hand, a solid electrolyte can provide the ultrahigh lithium-ion transmission capacity while not influencing the connection of the carbon nano tubes and the conductive capacity of the electrode; on the other hand, the three-dimensional network formed by the carbon nano tubes is also fixed by virtue of the solid electrolyte, the formation of a solid electrolyte interface is controlled, and an active material is protected under the high charge-discharge voltage. The combined electrode has the high reversible capacity and the enhanced rate capability, and can meet the requirement of a power automobile or a mixed power automobile.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
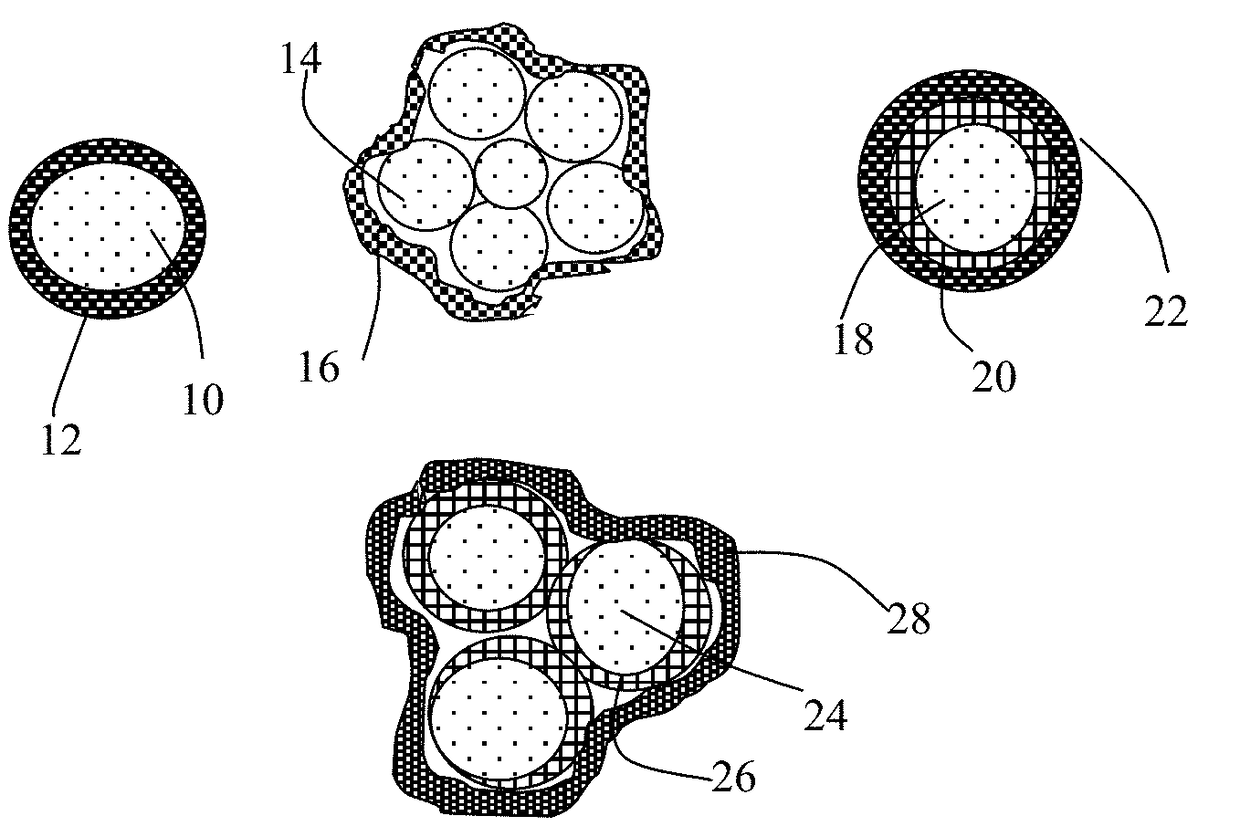
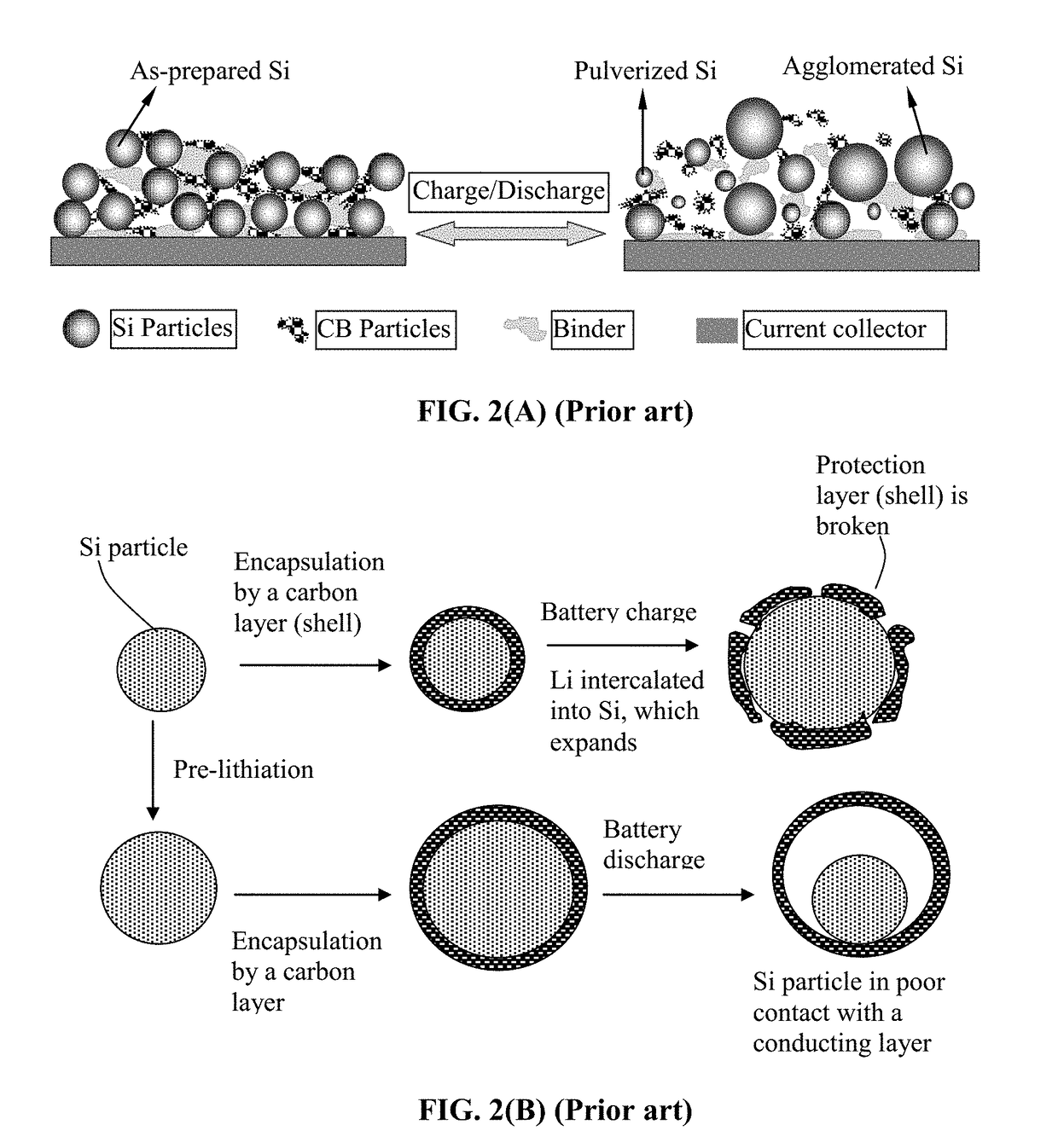
Encapsulated Anode Active Material Particles, Lithium Secondary Batteries Containing Same, and Method of Manufacturing
ActiveUS20180287142A1Improve lithium ion conductivitySolid electrolytesNegative electrodesParticulatesPolyethylene oxide
Provided is particulate of an anode active material for a lithium battery, comprising one or a plurality of anode active material particles being embraced or encapsulated by a thin layer of a high-elasticity polymer having a recoverable tensile strain no less than 5%, a lithium ion conductivity no less than 10−6 S / cm at room temperature, and a thickness from 0.5 nm to 10 μm, wherein the polymer contains an ultrahigh molecular weight (UHMW) polymer having a molecular weight from 0.5×106 to 9×106 grams / mole. The UHMW polymer is preferably selected from polyacrylonitrile, polyethylene oxide, polypropylene oxide, polyethylene glycol, polyvinyl alcohol, polyacrylamide, poly(methyl methacrylate), poly(methyl ether acrylate), a copolymer thereof, a sulfonated derivative thereof, a chemical derivative thereof, or a combination thereof.
Owner:GLOBAL GRAPHENE GRP INC
Lithium Secondary Batteries Containing Protected Particles of Anode Active Materials and Method of Manufacturing
ActiveUS20180241032A1Improve lithium ion conductivityIncrease elasticityFuel and secondary cellsSecondary cellsParticulatesTensile strain
Provided is anode active material layer for a lithium battery, comprising multiple particulates of an anode active material, wherein a particulate is composed of one or a plurality of particles of a high-capacity anode active material being embraced or encapsulated by a thin layer of a high-elasticity polymer having a recoverable tensile strain no less than 10% when measured without an additive or reinforcement, a lithium ion conductivity no less than 10-5 S / cm at room temperature, and a thickness from 0.5 nm (or a molecular monolayer) to 10 μm (preferably less than 100 nm), and wherein the high-capacity anode active material has a specific lithium storage capacity greater than 372 mAh / g (e.g. Si, Ge, Sn, SnO2, Co3O4, etc.).
Owner:GLOBAL GRAPHENE GRP INC
Lithium titanate-carbon composite nano-material, preparation method thereof and application thereof
InactiveCN101752560AHigh crystallinityImprove conductivityElectrolytic capacitorsCell electrodesCarbon compositesElectrical battery
The invention discloses a lithium titanate-carbon composite nano-material, a preparation method thereof and application thereof. The method comprises the following steps: 1) statically spinning lithium titanate sol, or lithium titanate sol doped with a conductive substance or lithium titanate sol doped with metal ions to obtain a thin film, wherein the conductive substance is conductive metal or conductive carbon; and 2) heat treating the thin film in inert atmosphere to obtain the lithium titanate-carbon composite nano-material. The lithium titanate-carbon composite nano-material provided by the invention has a standard one-dimensional morphological structure, high crystallinity, high conductivity and high safety performance, and has high lithium ion diffusion speed and high electronic conductivity when applied as the cathode material of the lithium ion battery. Moreover, the lithium titanate-carbon composite nano-material has high charge / discharge capacity, excellent high-current charge / discharge performance and stable cycling performance. The 10c charge / discharge capacity is 125mAh / g, the 40C charge / discharge capacity reaches 95mAh / g, and the retention rate of the high-current 40C charge / discharge capacity within 3000 times reaches 85 percent.
Owner:PEKING UNIV
Lithium cell positive electrode materials and preparing method thereof
InactiveCN1457111AImprove conductivityHigh conductivity at room temperatureElectrode thermal treatmentPositive electrodesNano structuringElectrical battery
The chemical general formula of the material is expressed as follows: LixM1-xFePO4, where M is selected from Mg2+, Ca2+...P5+ etc. With conduction adulterant added, reaction at 500-900 deg.C for 10 hr. by using metal oxide, phoshpate, fluoride etc. and non saturated crystal of Li-Fe phoshpate through nonstoichiometric method obtains the crystal of Li-Fe phosphate with high conductivity, which can be expressed as LiFePO4-y. The formula of material prepared by using method of pressurized type substitution ion is LixM1-xFezM'1-z. The formula of material of solid power prepared by using method of solid phase reaction is as LixM1-xFezMn1-zPO4. The formula of anode material in nano structure prepared by using method of vacuum sputter deposition is LixFePO4-y, whose conductivity and discharge capacity can reach 10 to the power -2 S / cm and 240 Ah / g.
Owner:徐瑞松
Solid state electrochemical composite
InactiveUS20050214612A1Improve power densitySolve the lack of activityFinal product manufactureCell electrodesElectrolysisConductive materials
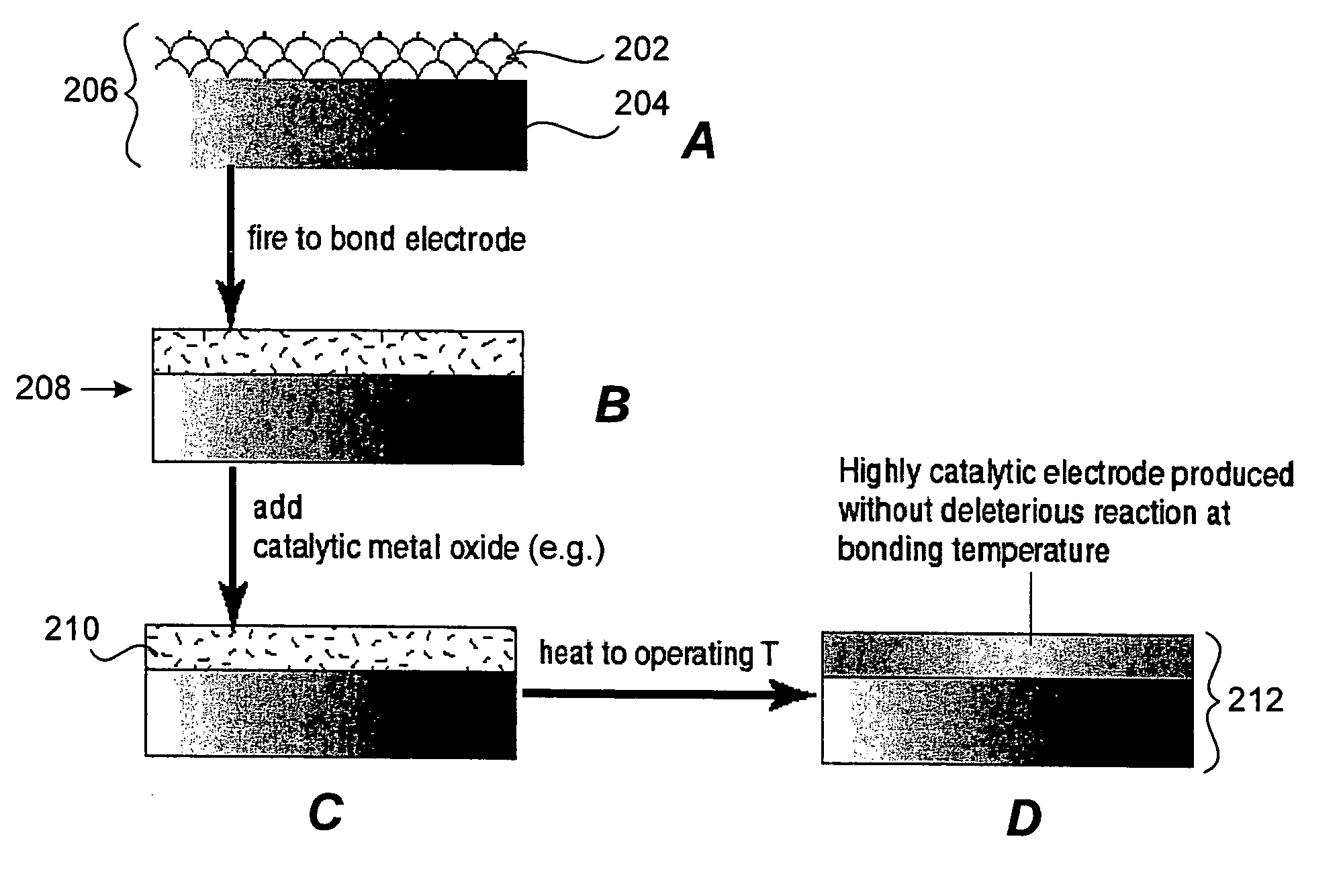
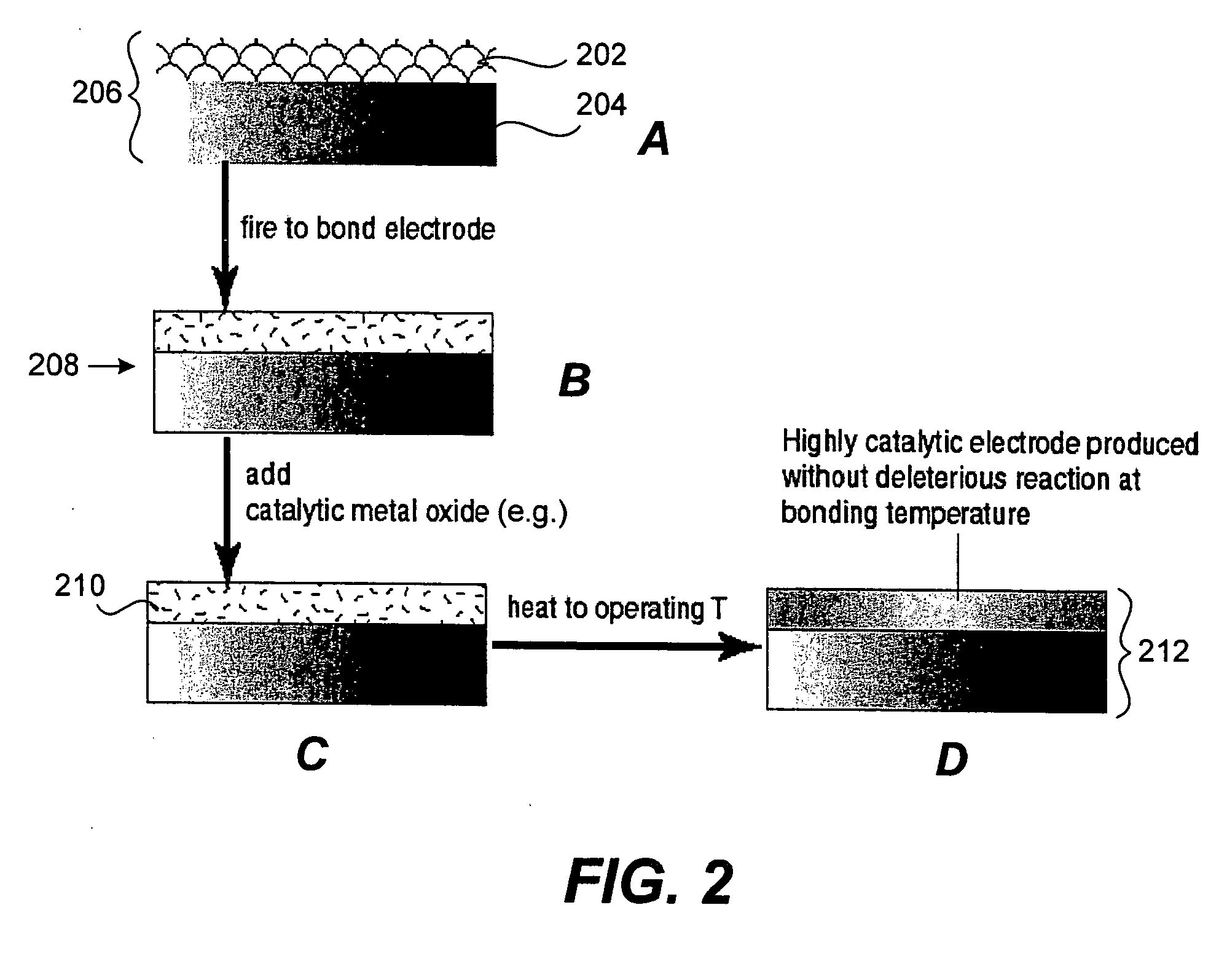
Provided is a composite electrochemical device fabricated from highly electronically conductive materials such as metals, metal alloys, or electronically conductive ceramics. The electronic conductivity of the electrode substrate is maximized. The invention allows for an electrode with high electronic conductivity and sufficient catalytic activity to achieve high power density in ionic (electrochemical) devices such as fuel cells and electrolytic gas separation systems including oxygen generation system.
Owner:RGT UNIV OF CALIFORNIA
Alkali Metal-Sulfur Secondary Battery Containing a Protected Sulfur Cathode and Manufacturing Method
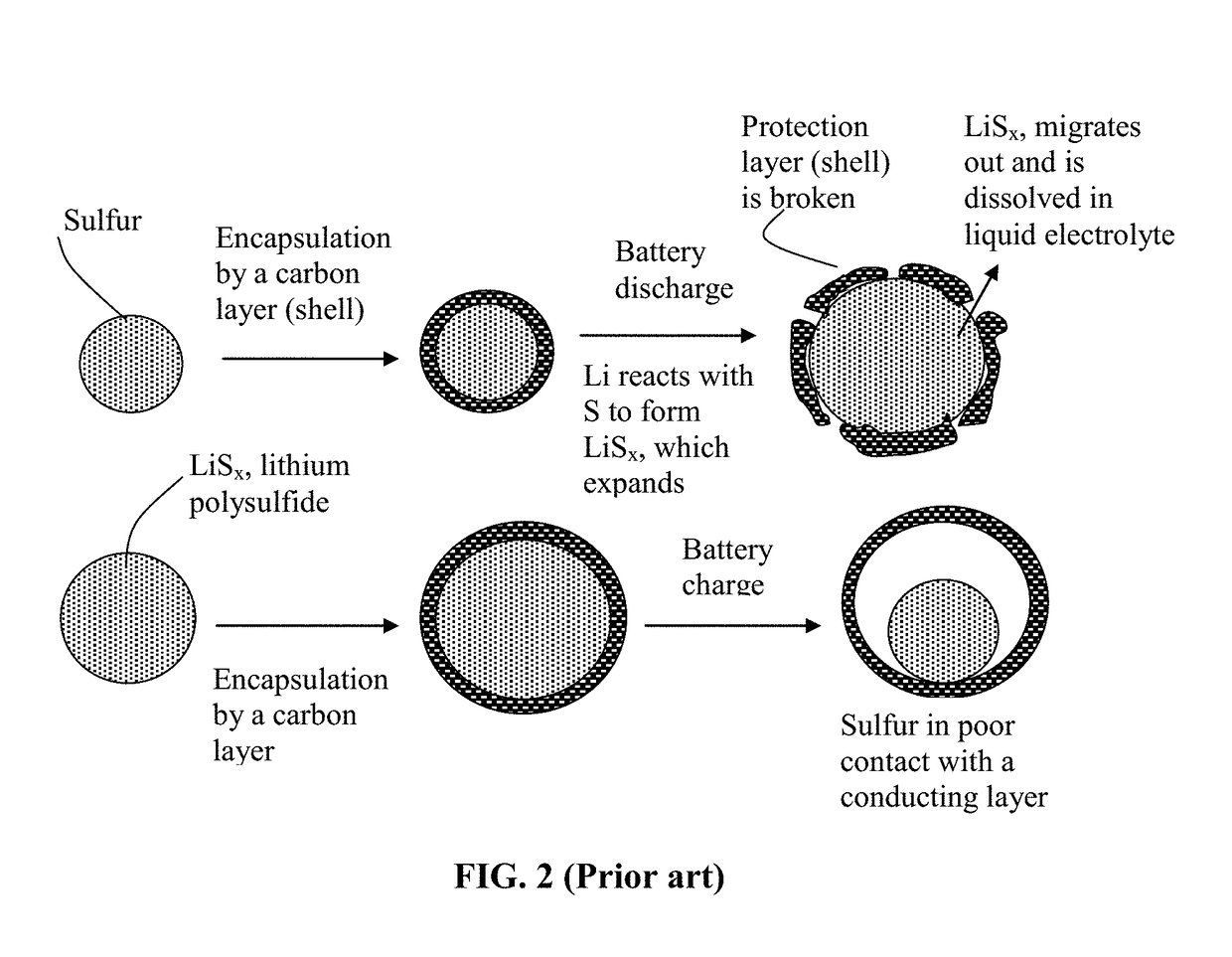
ActiveUS20180241031A1Inhibited DiffusionReduces and eliminates shuttling effectFinal product manufacturePositive electrodesElectrical batteryConductive polymer
Provided is a rechargeable alkali metal-sulfur cell comprising an anode active material layer, an electrolyte, and a cathode active material layer containing multiple particulates of a sulfur-containing material selected from a sulfur-carbon hybrid, sulfur-graphite hybrid, sulfur-graphene hybrid, conducting polymer-sulfur hybrid, metal sulfide, sulfur compound, or a combination thereof and wherein at least one of the particulates is composed of one or a plurality of sulfur-containing material particles being embraced or encapsulated by a thin layer of a high-elasticity polymer having a recoverable tensile strain no less than 10% when measured without an additive or reinforcement, a lithium ion conductivity no less than 10−5 S / cm at room temperature, and a thickness from 0.5 nm to 10 μm. This battery exhibits an excellent combination of high sulfur content, high sulfur utilization efficiency, high energy density, and long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
High performance electrodes
InactiveUS20110070488A1Improve electronic conductivityImprove thermal conductivityActive material electrodesElectrode collector coatingCarbon nanotubeSlurry
Techniques, arrangements and compositions are provided to incorporate nanostructured materials into electrodes for energy storage devices. Materials such as, for example, carbon nanotubes, silicon nanowires, silicon carbide nanowires, zinc nanowires, and other materials may be used to modify electrode properties such as electronic conductivity, thermal conductivity, or durability, for example. In some embodiments, nanostructured materials may be added to electrode formulations such as, for example, slurries or powders. Nanostructured materials may be deposited directly onto active material particles or electrode components. In some embodiments, coatings may be used to assist in deposition.
Owner:G4 SYNERGETICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com