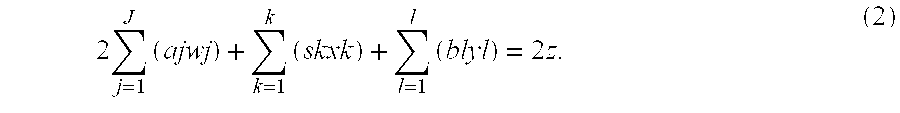Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3979 results about "Strontium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. While natural strontium is stable, the synthetic ⁹⁰Sr isotope is radioactive and is one of the most dangerous components of nuclear fallout, as strontium is absorbed by the body in a similar manner to calcium. Natural stable strontium, on the other hand, is not hazardous to health.
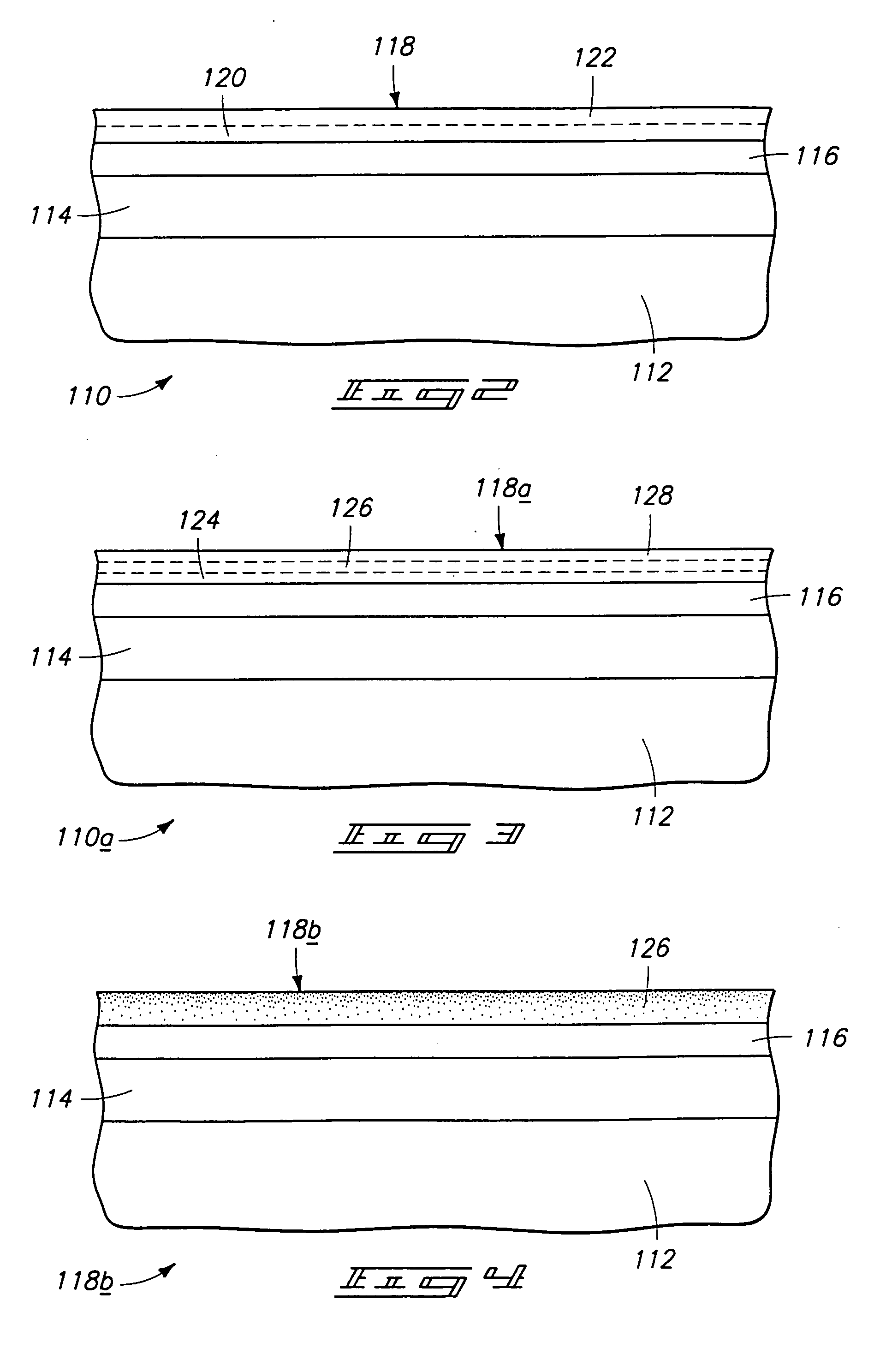
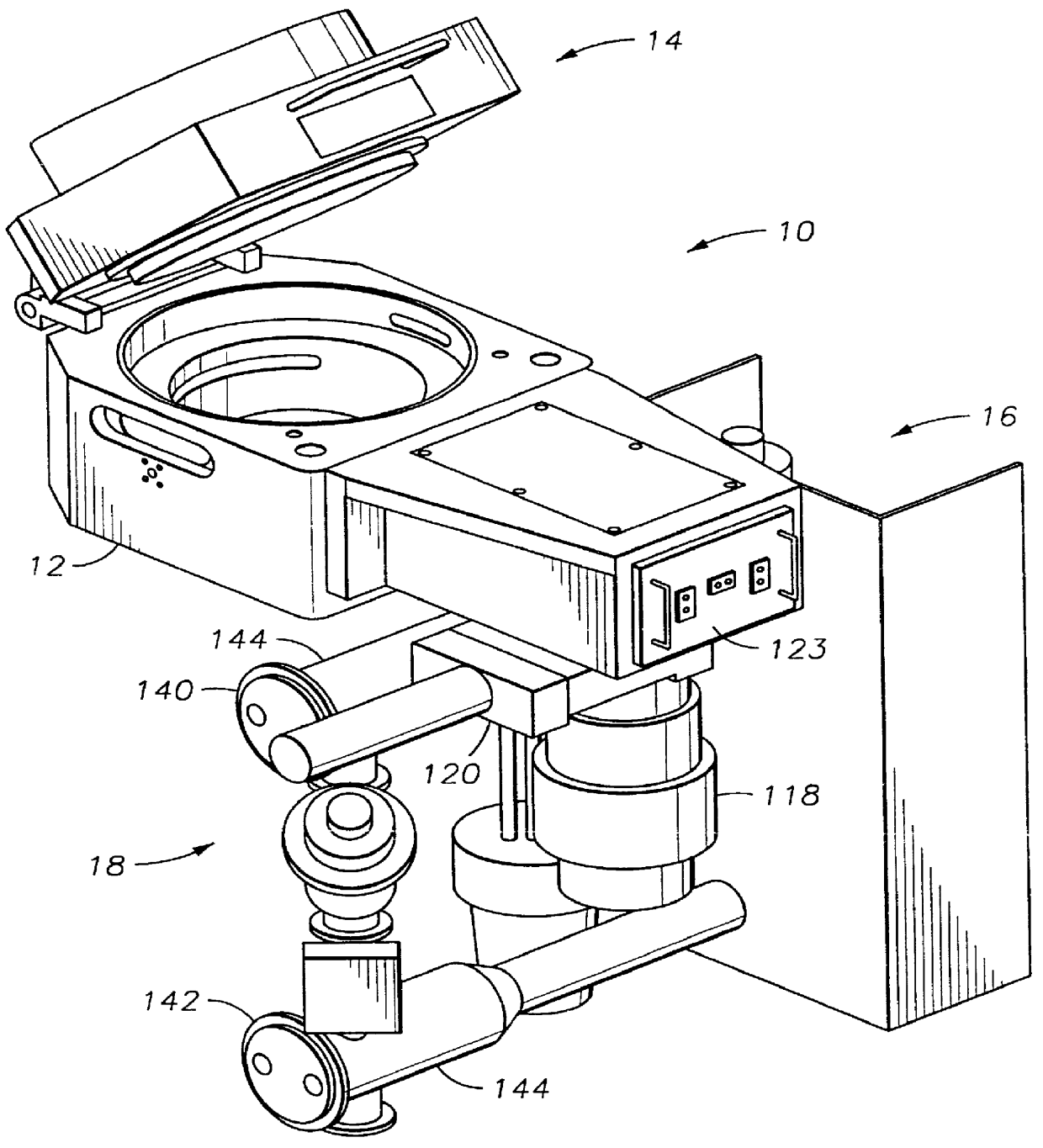
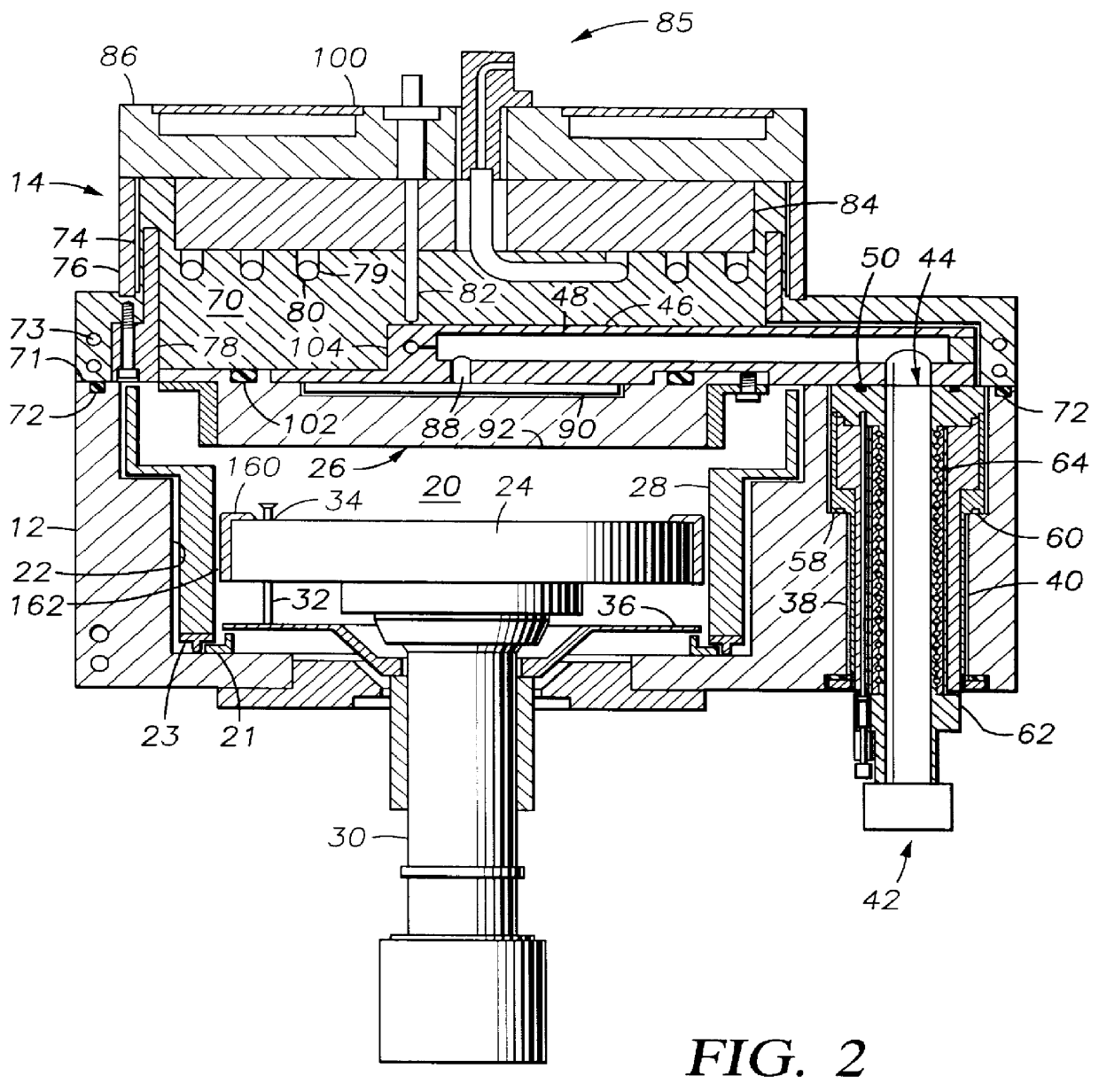
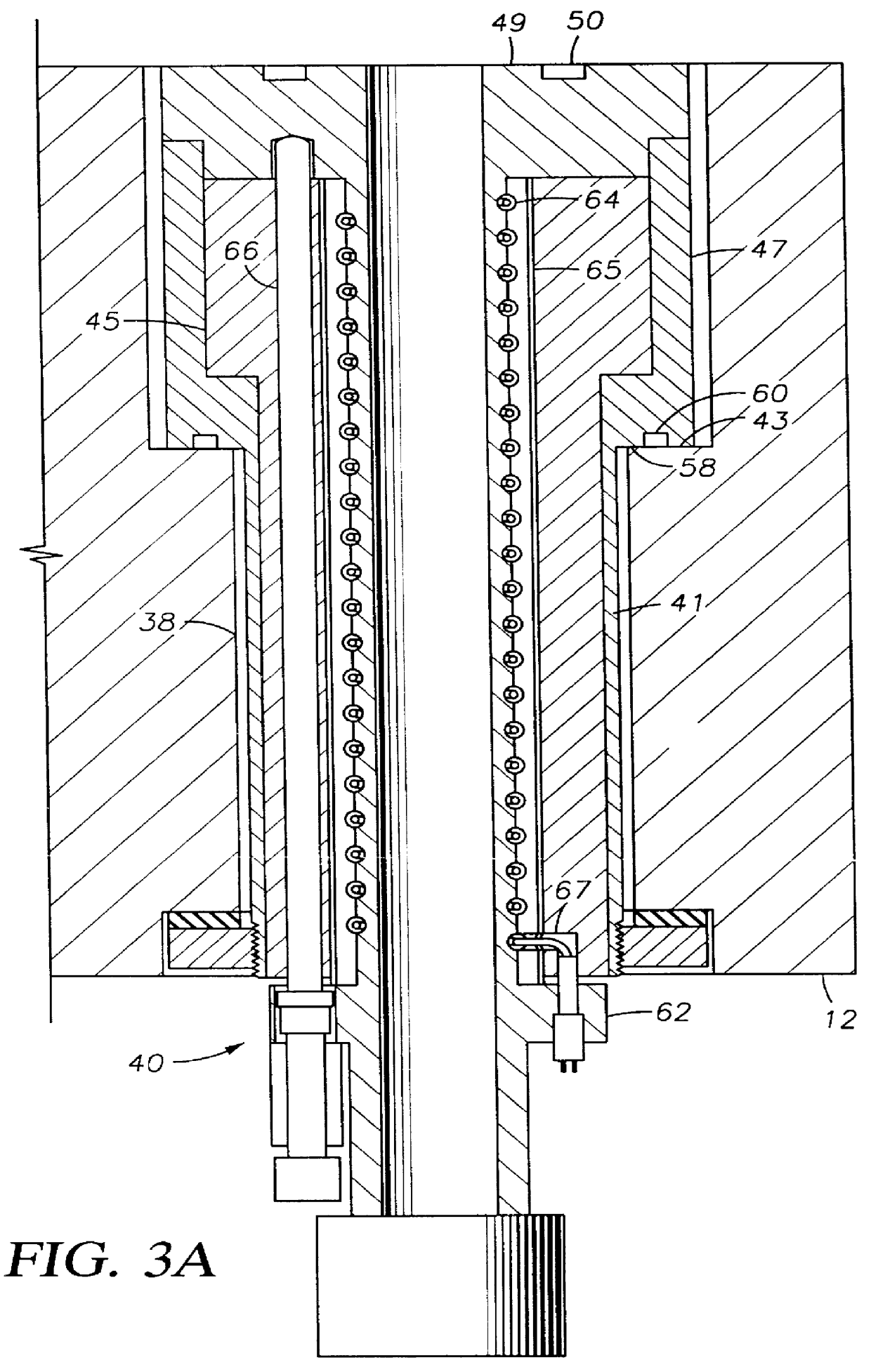
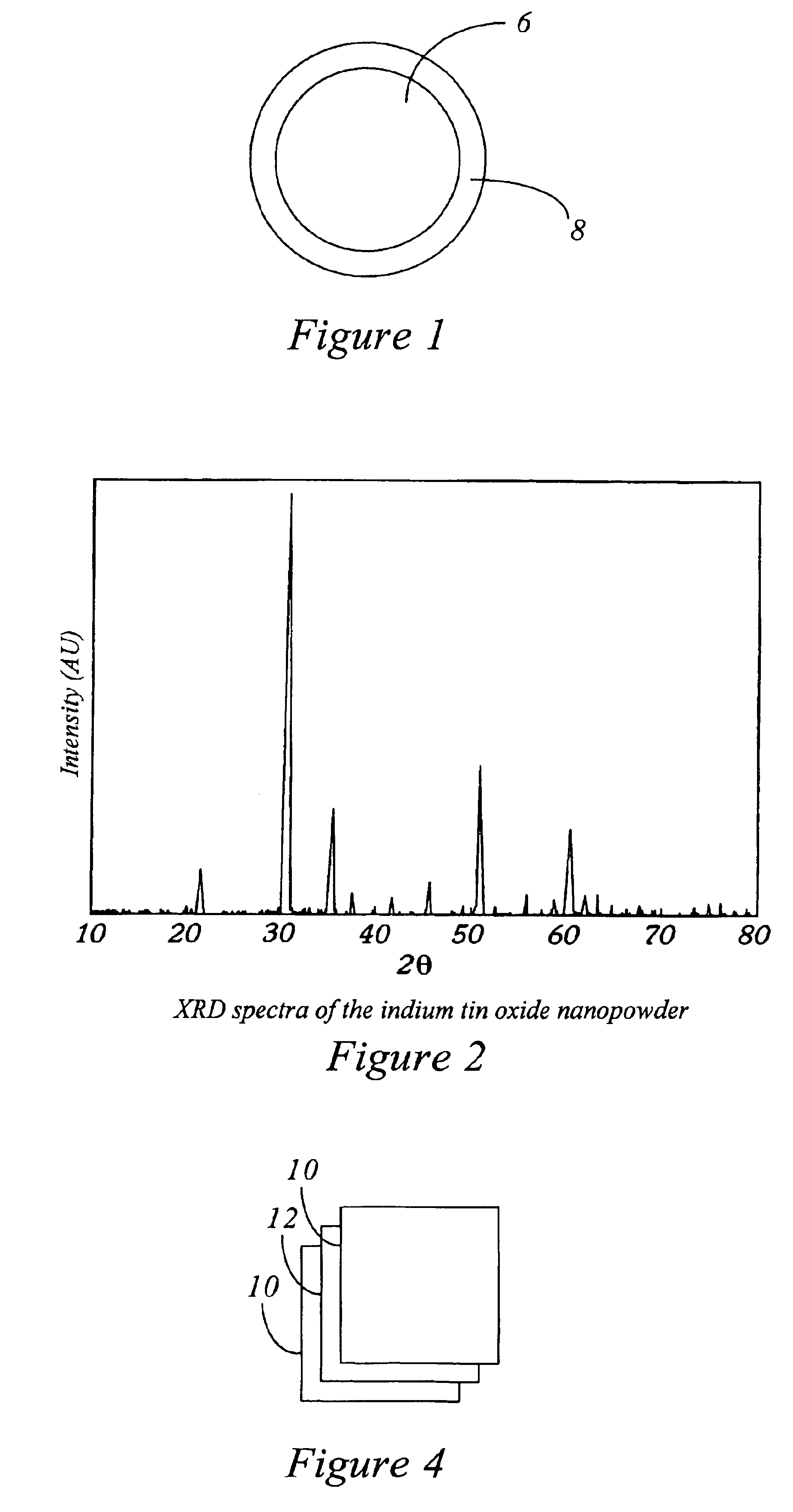
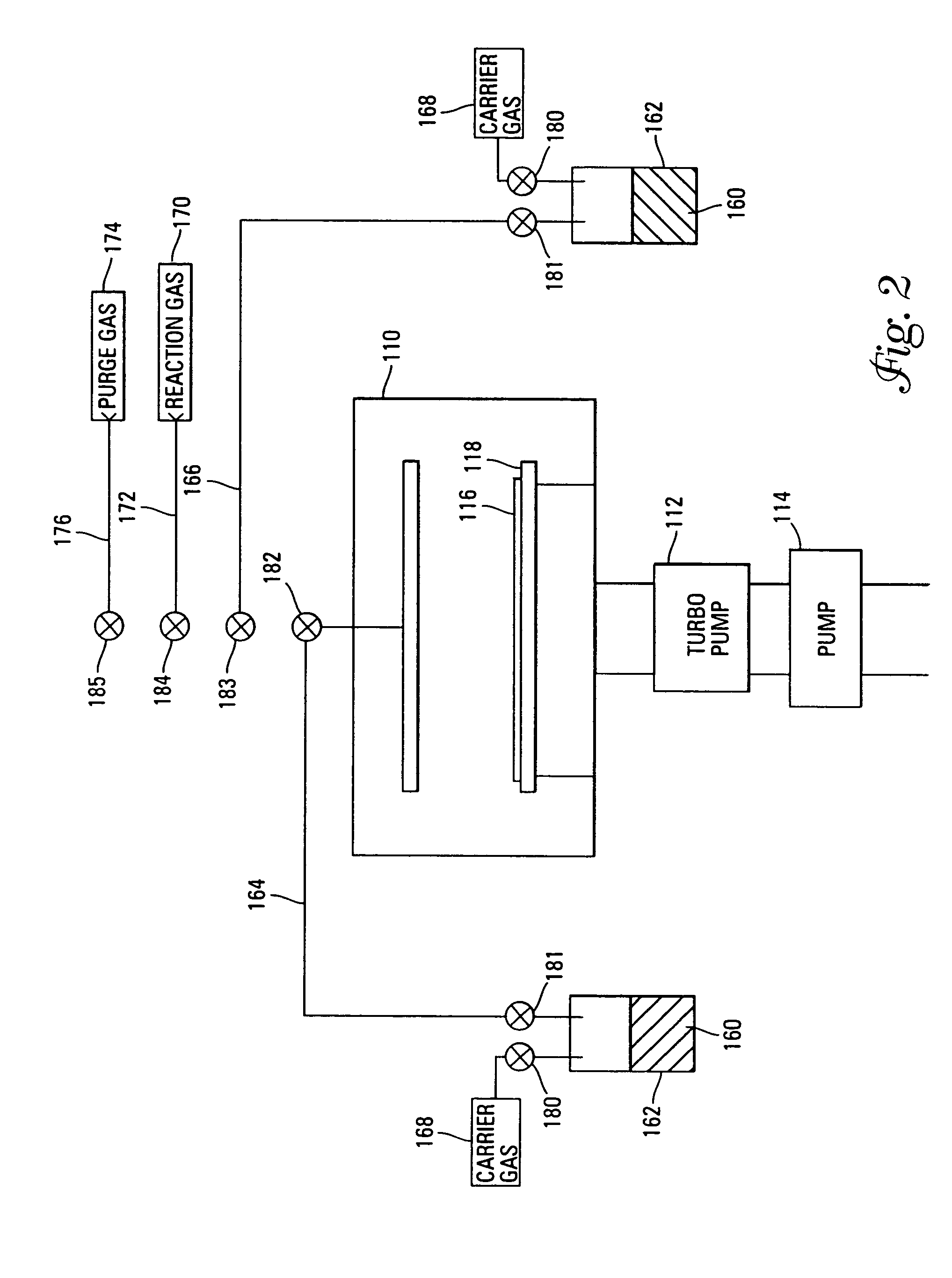
Method to protect internal components of semiconductor processing equipment using layered superlattice materials
InactiveUS20060040508A1Imparting corrosion resistanceElectric discharge tubesSemiconductor/solid-state device manufacturingStrontiumCompound (substance)
This invention relates to apparatus and a method to protect the internal components of semiconductor processing equipment such as a plasma reactor or a reactive species generator against physical and / or chemical damages during etching and / or cleaning processes. Layered superlattice materials having three or more metal elements such as strontium bismuth tantalate (SBT) are used to form a protective barrier on the surfaces of the internal components of a reaction chamber.
Owner:AIR PROD & CHEM INC
Chemical vapor deposition methods of forming barium strontium titanate comprising dielectric layers, including such layers having a varied concentration of barium and strontium within the layer
The invention includes a chemical vapor deposition method of forming a barium strontium titanate comprising dielectric layer having a varied concentration of barium and strontium, and / or titanium, within the layer. A substrate is positioned within a chemical vapor deposition reactor. Barium and strontium are provided within the reactor by flowing at least one metal organic precursor to the reactor. Titanium is provided within the reactor. One or more oxidizers are flowed to the reactor. In one aspect, conditions are provided within the reactor to be effective to deposit a barium strontium titanate comprising dielectric layer on the substrate from the reactants.
Owner:MICRON TECH INC
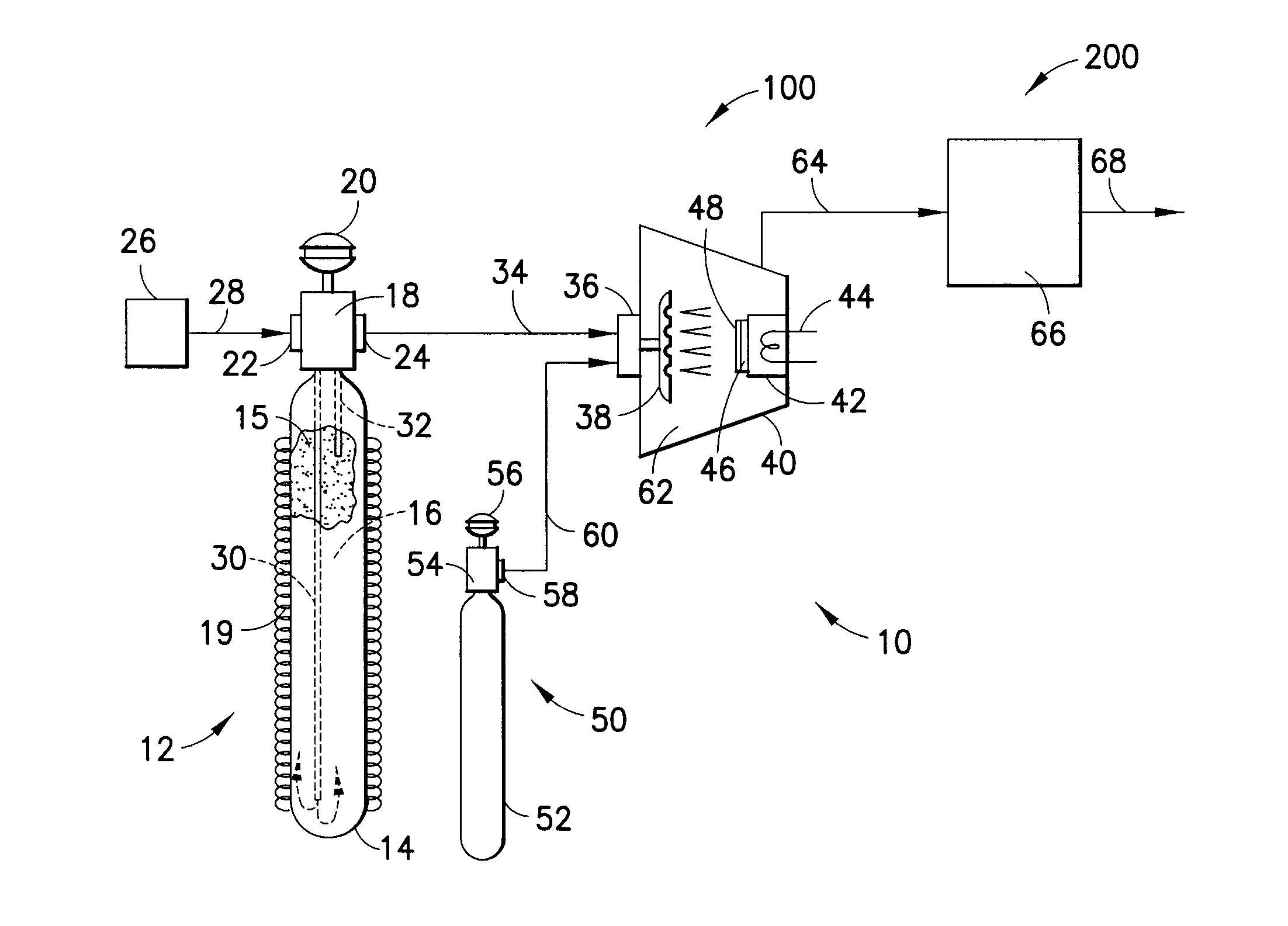
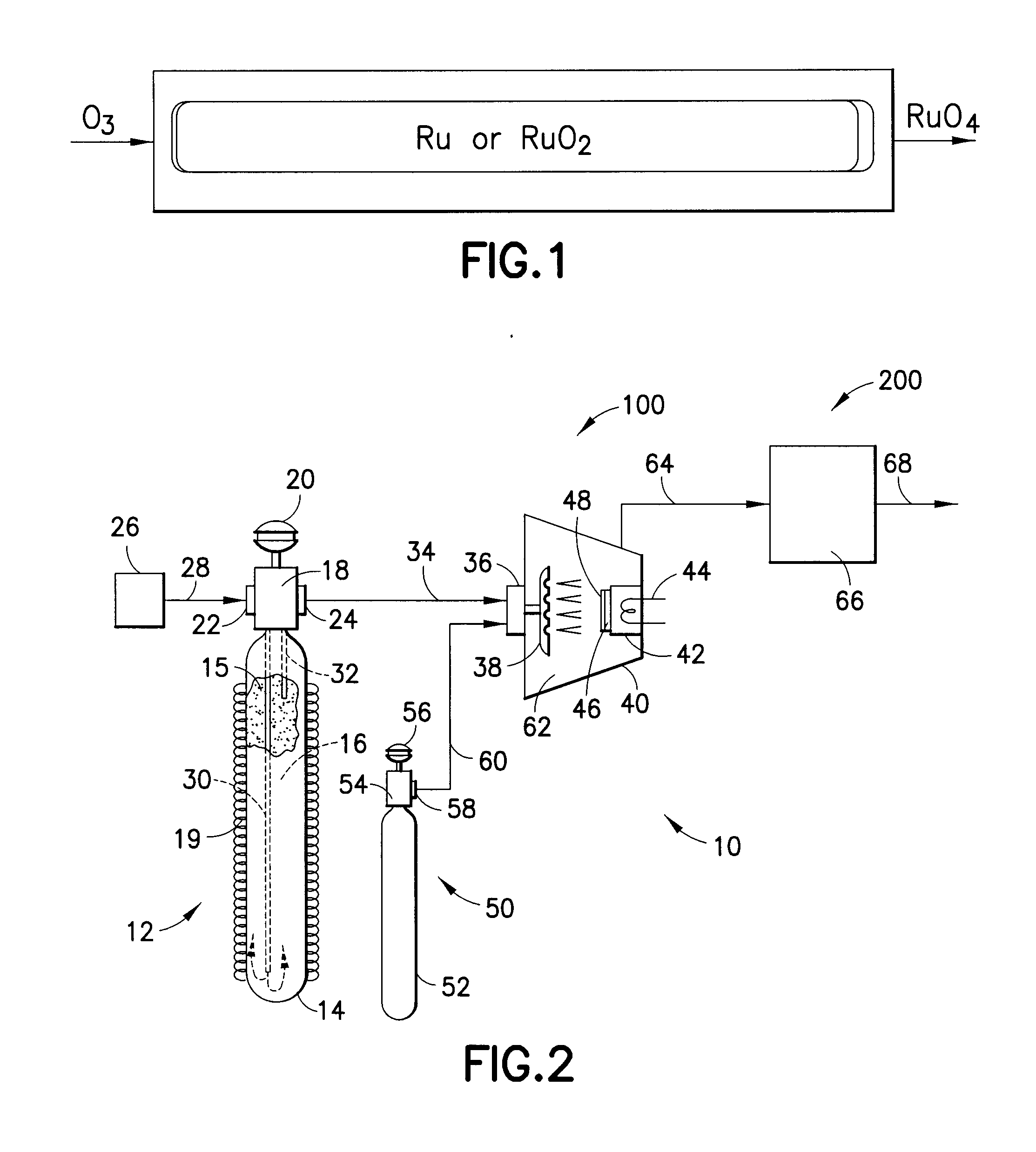
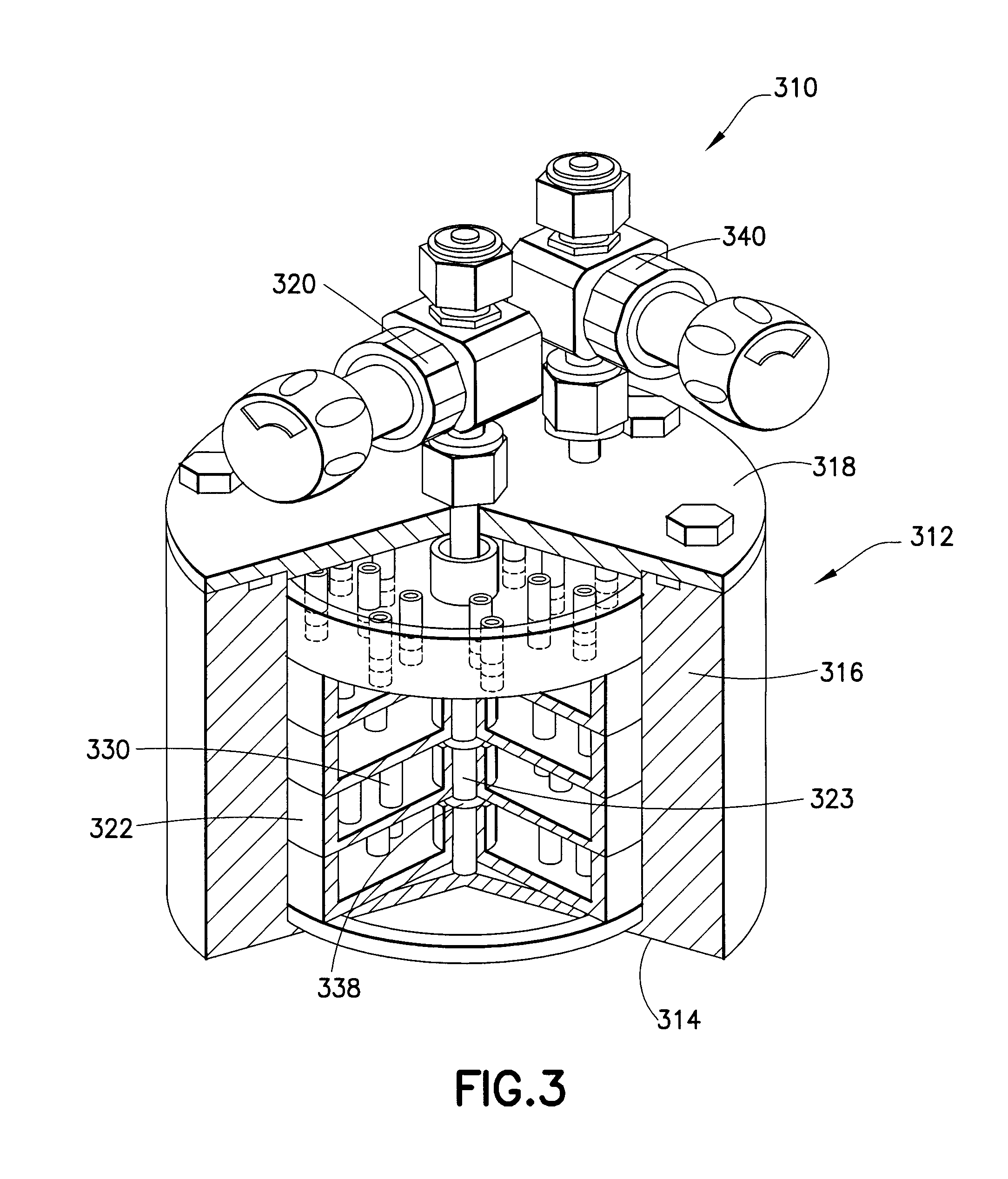
IN SITU GENERATION OF RuO4 FOR ALD OF Ru AND Ru RELATED MATERIALS
Apparatus and method for generating ruthenium tetraoxide in situ for use in vapor deposition, e.g., atomic layer deposition (ALD), of ruthenium-containing films on microelectronic device substrates. The ruthenium tetraoxide can be generated on demand by reaction of ruthenium or ruthenium dioxide with an oxic gas such as oxygen or ozone. In one implementation, ruthenium tetraoxide thus generated is utilized with a strontium organometallic precursor for atomic layer deposition of strontium ruthenate films of extremely high smoothness and purity.
Owner:ENTEGRIS INC
Temperature controlled chamber liner
InactiveUS6099651APrevent unwanted condensationPrevent decomposition and condensationSemiconductor/solid-state device manufacturingChemical vapor deposition coatingEngineeringTitanium oxide
The invention relates to an apparatus and process for the vaporization of liquid precursors and deposition of a film on a suitable substrate. Particularly contemplated is an apparatus and process for the deposition of a metal-oxide film, such as a barium, strontium, titanium oxide (BST) film, on a silicon wafer to make integrated circuit capacitors useful in high capacity dynamic memory modules.
Owner:APPLIED MATERIALS INC
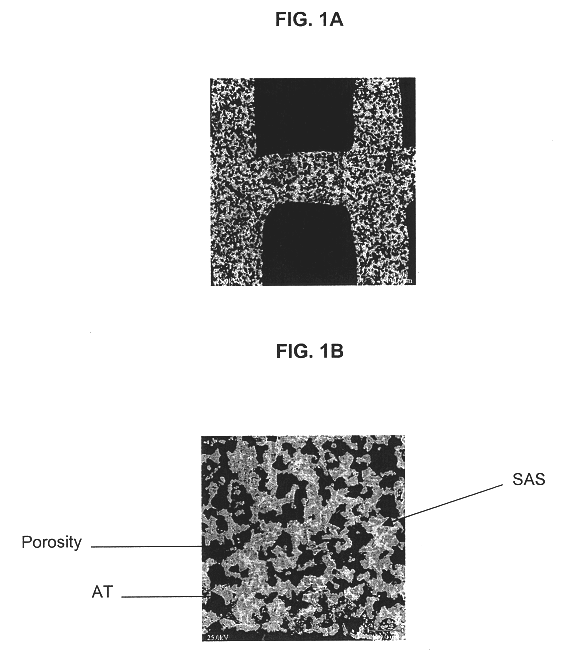
Strontium feldspar aluminum titanate for high temperature applications
InactiveUS6620751B1Improve stabilityReduce bloatInternal combustion piston enginesDispersed particle filtrationMicrometerRoom temperature
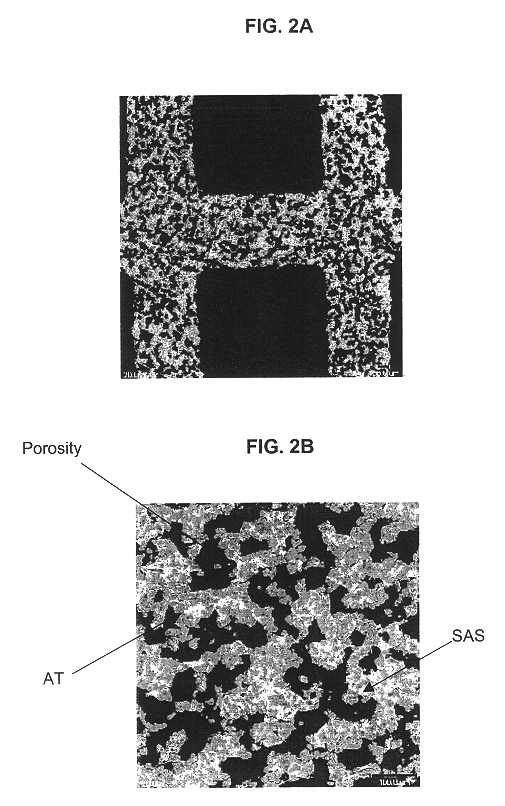
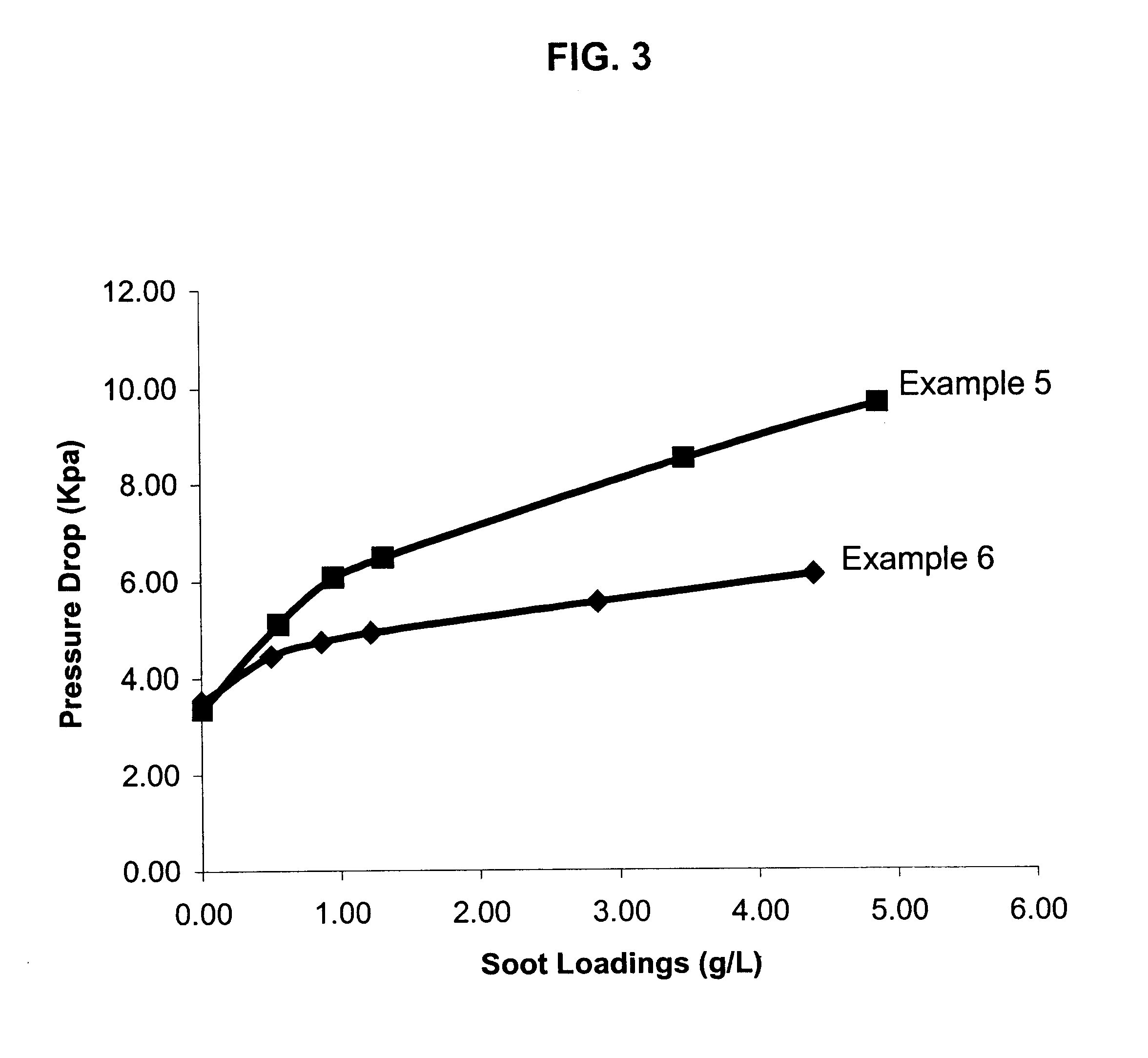
A structure for use in high temperature applications and including a porous ceramic material consisting essentially of about 50-90 percent by weight iron or magnesium stabilized aluminum titanate (AlTiO5) and about 10-50 percent by weight strontium feldspar (SrO.Al2O3.2SiO2), and having a coefficient of thermal expansion over a temperature range from room temperature to 1000° C. of about -10x10-7 / ° C. to +15x10-7 / ° C., a heat capacity at 500° C. greater than 3.2 J / cm3K, a porosity of about 15-50 percent by volume, preferably 40-50 percent by volume, and a median pore size of about 5-50 micrometers, preferably 8-15 micrometers. The structure is especially useful as a diesel exhaust particulate filter.
Owner:CORNING INC
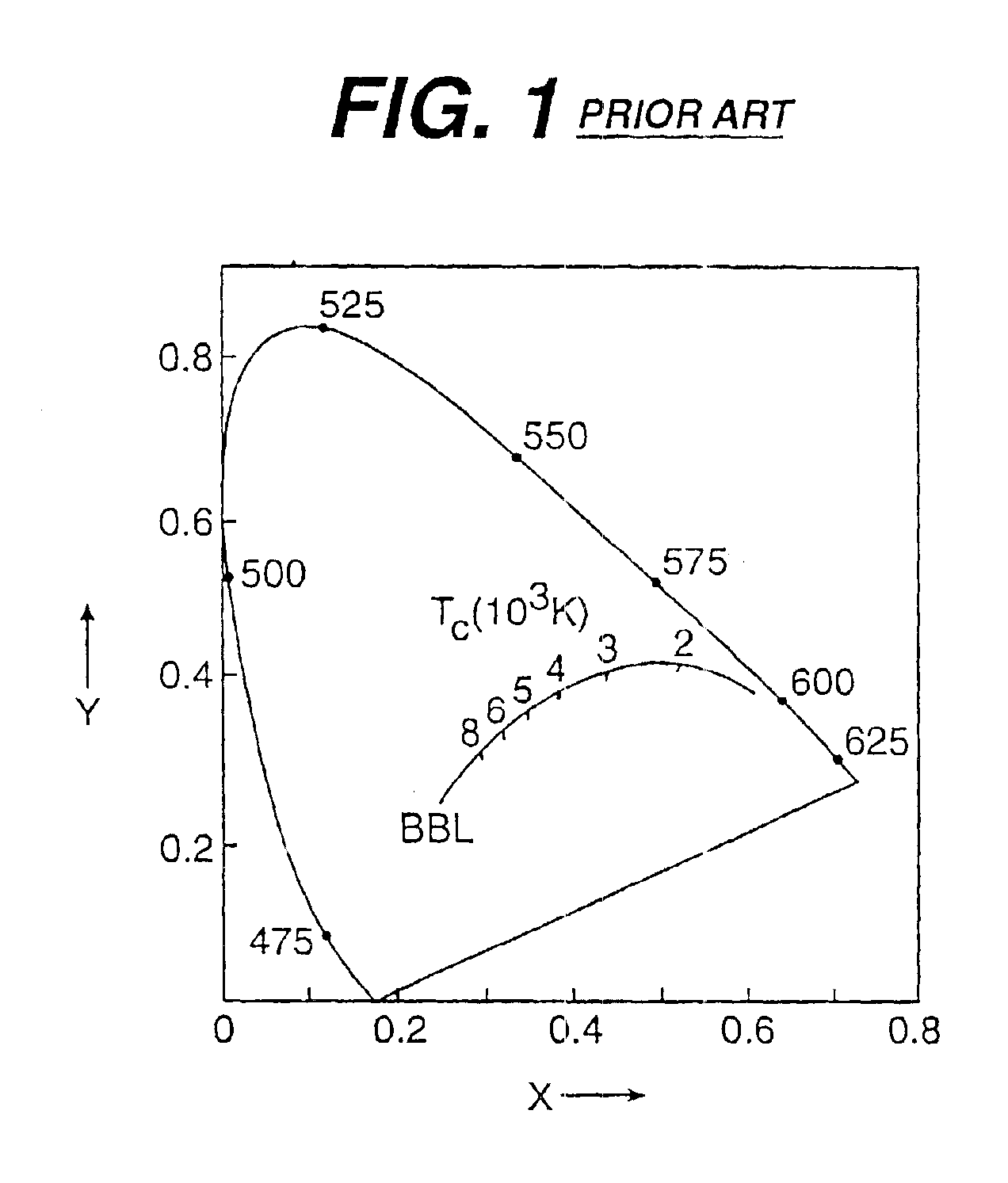
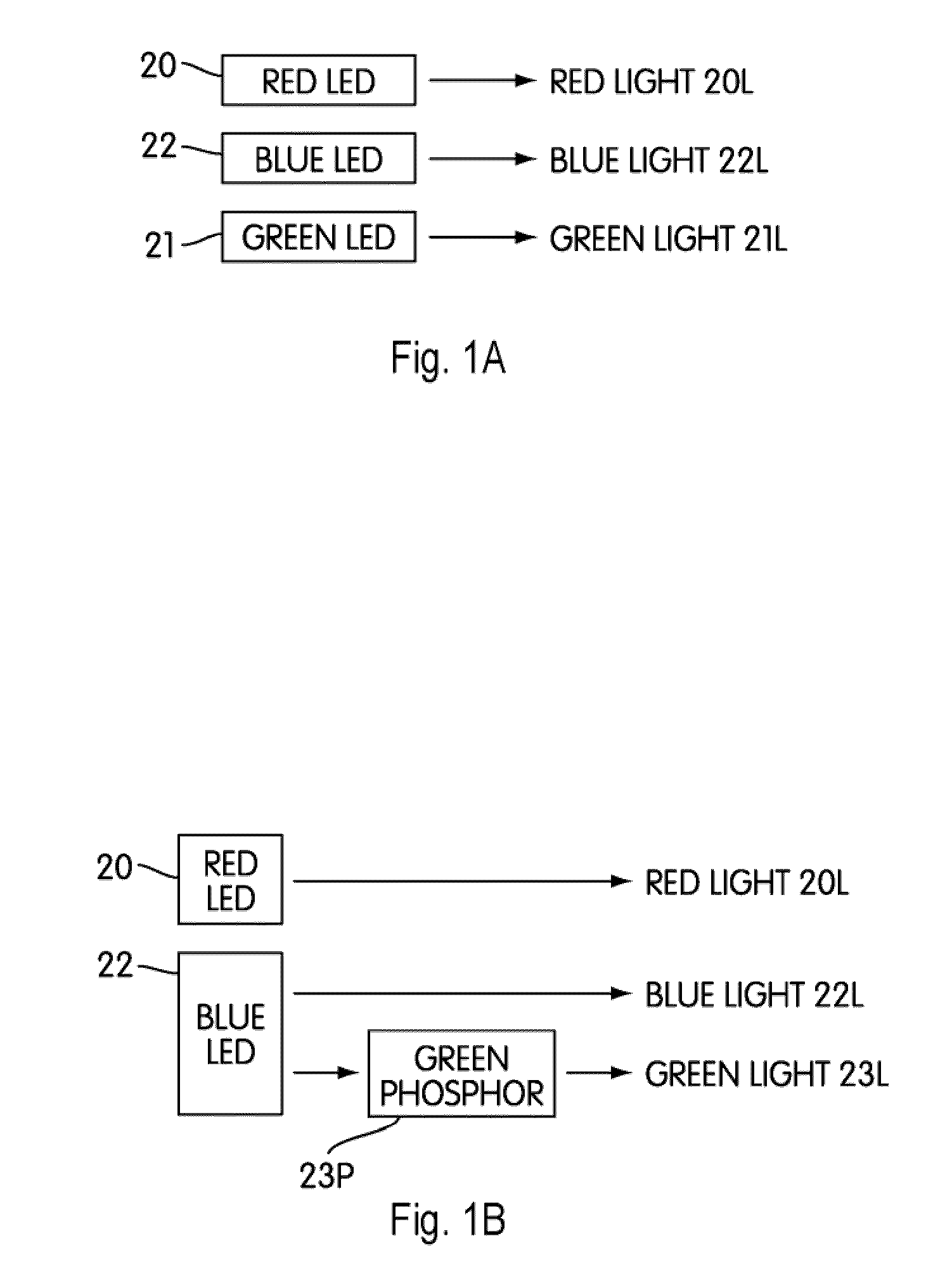
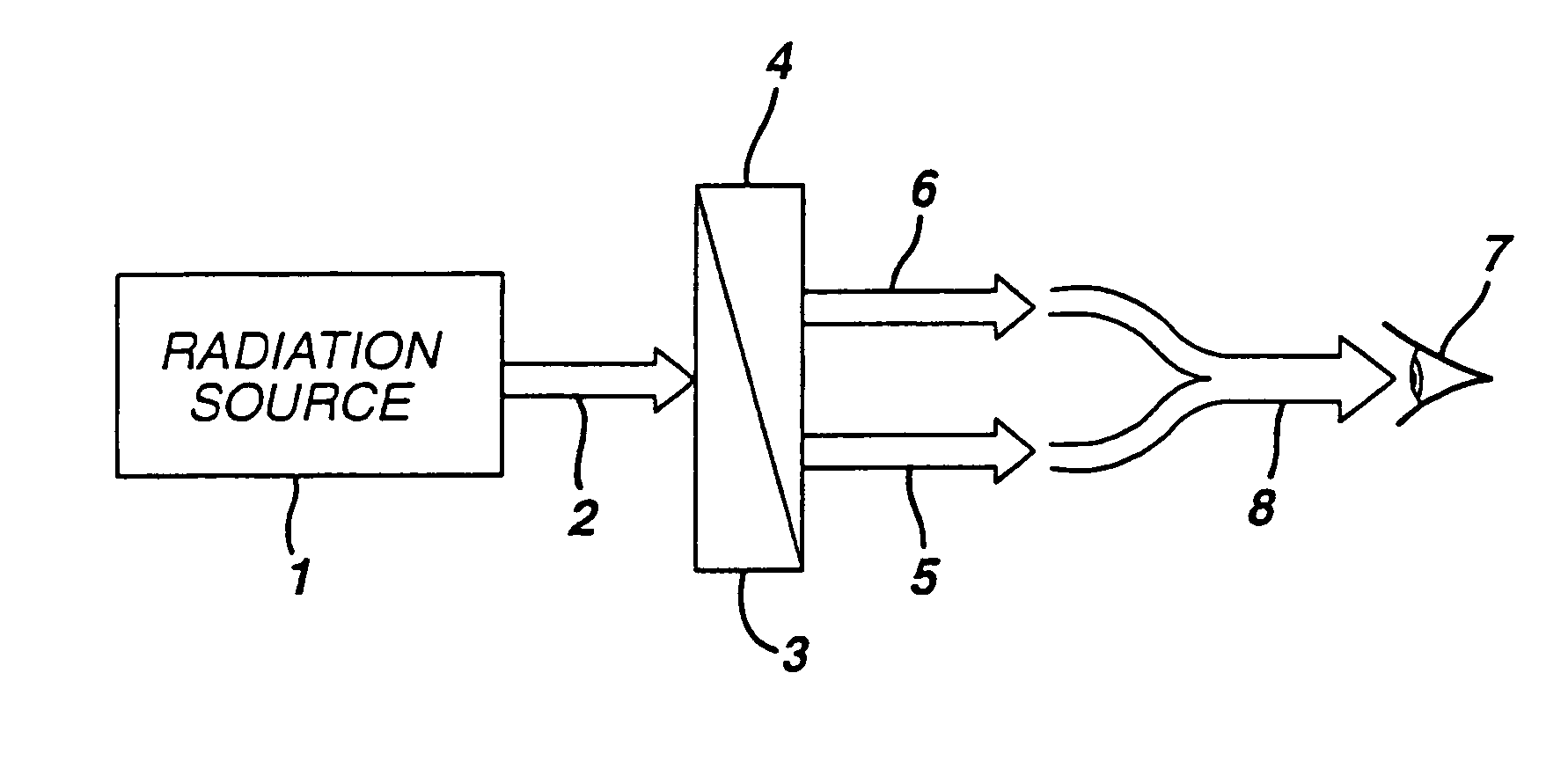
Single phosphor for creating white light with high luminosity and high CRI in a UV LED device
InactiveUS6853131B2Avoids and reduces problemGas-filled discharge tubesDischarge tube luminescnet screensX-rayUltraviolet
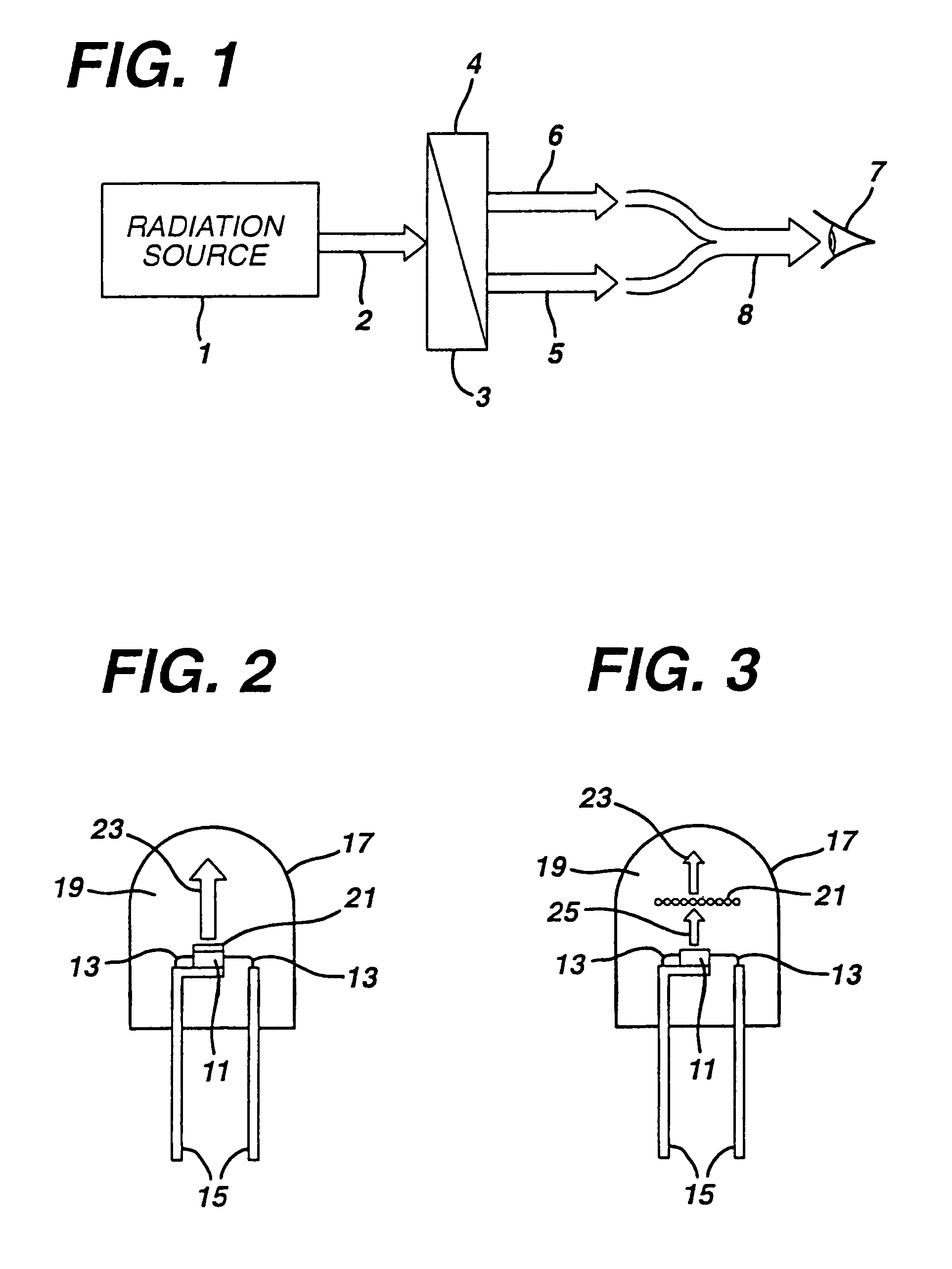
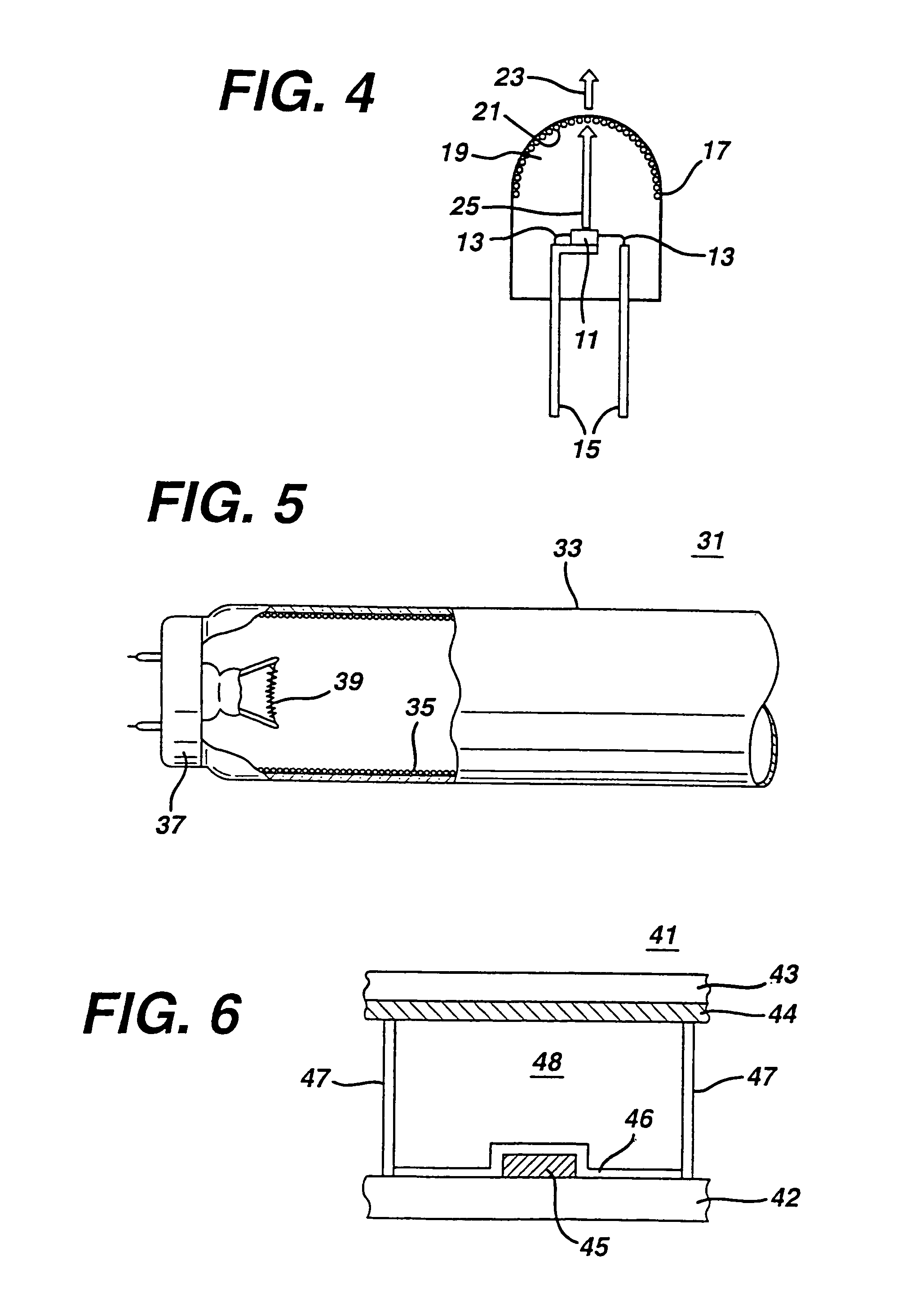
There is provided a white light illumination system. The illumination system includes a radiation source which emits either ultra-violet (UV) or x-ray radiation. The illumination system also includes a luminescent material which absorbs the UV or x-ray radiation and emits the white light. The luminescent material has composition A2−2xNa1+xExD2V3O12. A may be calcium, barium, strontium, or combinations of these three elements. E may be europium, dysprosium, samarium, thulium, or erbium, or combinations thereof. D may be magnesium or zinc, or combinations thereof. The value of x ranges from 0.01 to 0.3, inclusive.
Owner:GENERAL ELECTRIC CO
White light emitting phosphor blends for LED devices
There is provided white light illumination system including a radiation source, a first luminescent material having a peak emission wavelength of about 575 to about 620 nm, a second luminescent material having a peak emission wavelength of about 495 to about 550 nm, which is different from the first luminescent material and a third luminescent material having a peak emission wavelength of about 420 to about 480 nm, which is different from the first and second luminescent materials. The LED may be a UV LED and the luminescent materials may be a blend of three or four phosphors. The first phosphor may be an orange emitting Eu2+, Mn2+ activated strontium pyrophosphate, Sr2P2O7:Eu2+, Mn2+. The second phosphor may be a blue-green emitting Eu2+ activated barium silicate, (Ba,Sr,Ca)2SiO4:Eu2+. The third phosphor may be a blue emitting SECA phosphor, (Sr,Ba,Ca)5(PO4)3Cl:Eu2+. Optionally, the fourth phosphor may be a red emitting Mn4+ activated magnesium fluorogermanate, 3.5MgO*0.5MgF2*GeO2:Mn4+. A human observer perceives the combination of the orange, blue-green, blue and / or red phosphor emissions as white light.
Owner:GE LIGHTING SOLUTIONS LLC
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
Strontium-apatite-cement-preparations, cements formed therefrom, and uses thereof
ActiveUS20050142211A1Facilitated releaseTo promote metabolismBiocideSurgical adhesivesO-Phosphoric AcidPowder mixture
Calcium-strontium-hydroxyphosphate (strontium-apatite-) cement preparations are described, comprising a powder mixture, which contains molar quantities of the components calcium (Ca), strontium (Sr) and phosphate (P) in the mixture in the ranges 1.00<Ca / P≦1.50 and 0<Sr / P<1.5, together with an alkali salt or an ammonium salt of phosphoric acid, and with water and / or an aqueous solution. The powder mixture particularly contains, as the Ca-component, Ca3(PO4)2 (TCP), and as the Sr-component SrHPO4 and / or Sr3(PO4)2 and optionally additional SrCO3. As the aqueous mixing solution for the formation of the strontium-apatite cement, an aqueous solution of an alkali salt or an ammonium salt of the phosphoric acid is suitable.
Owner:KYPHON
Systems and methods for forming strontium- and/or barium-containing layers
InactiveUS7115166B2Easy to controlMinimizing detrimental gas phase reactionPolycrystalline material growthSolid-state devicesStrontium titanateBarium strontium titanate
Owner:MICRON TECH INC
Enhanced efficacy antiperspirant compositions containing strontium or calcium
InactiveUS6923952B2Improve efficacySimple compositionCosmetic preparationsToilet preparationsStrontiumWater soluble
Owner:THE GILLETTE CO
Enhanced efficacy antiperspirant compositions containing strontium
InactiveUS6902723B2Good curative effectEnhanced antiperspirant saltCosmetic preparationsToilet preparationsStrontiumAqueous solution
The present invention relates to enhanced efficacy antiperspirant salts containing strontium and an amino acid or a hydroxy acid and particularly to stabilized aqueous solutions of such salts. The present invention also embraces methods of making these antiperspirant salts and solutions and compositions containing same.
Owner:THE GILLETTE CO
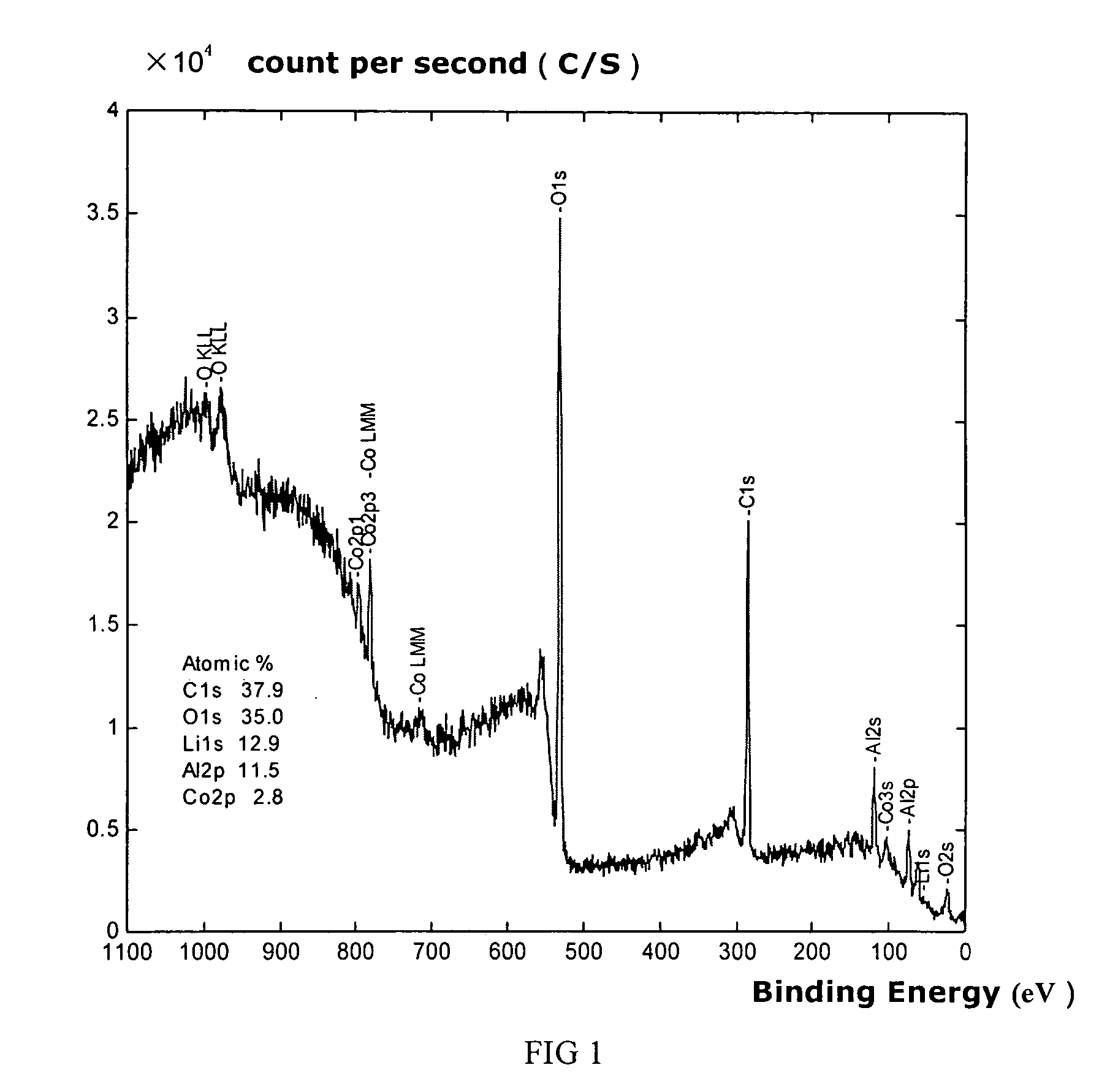
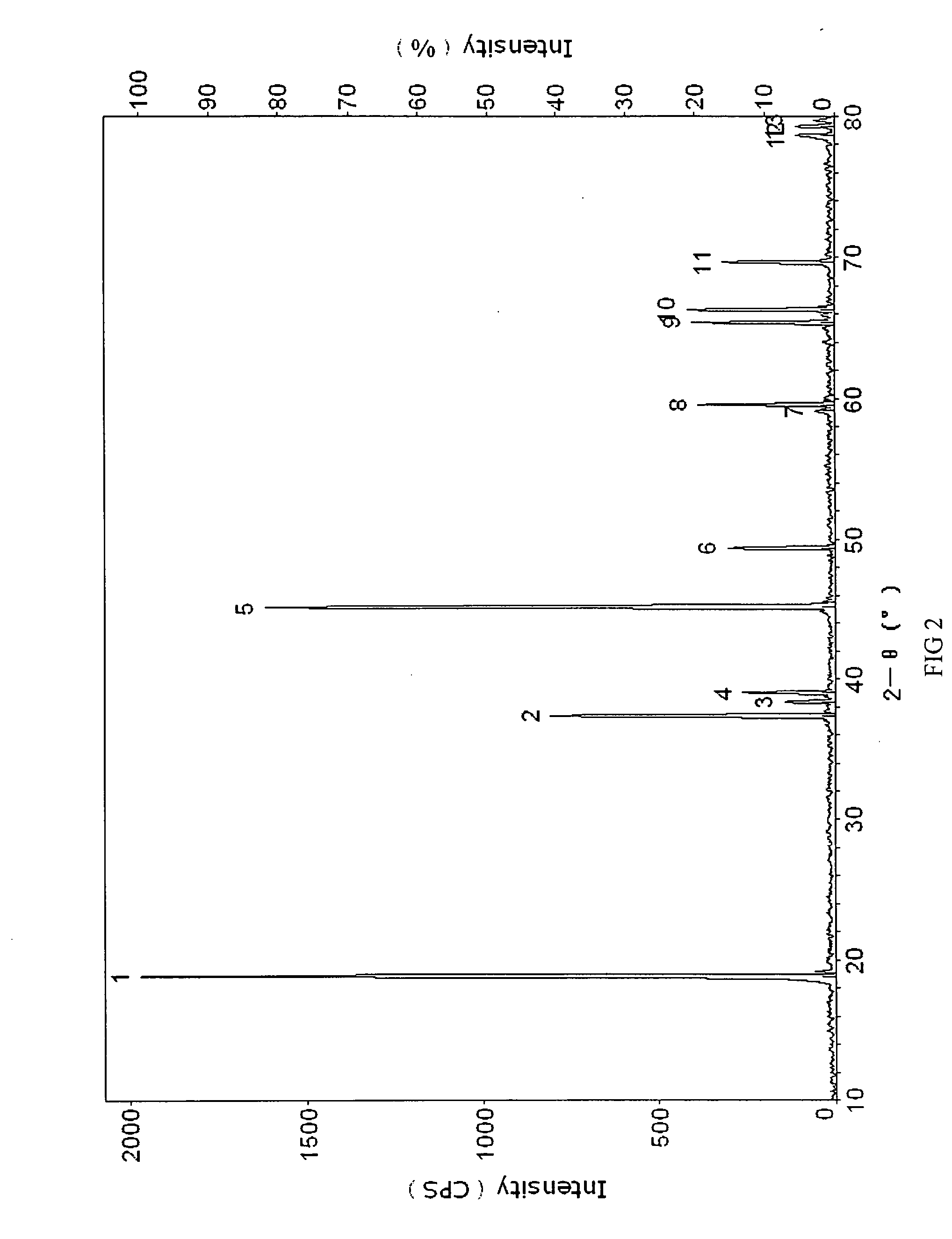
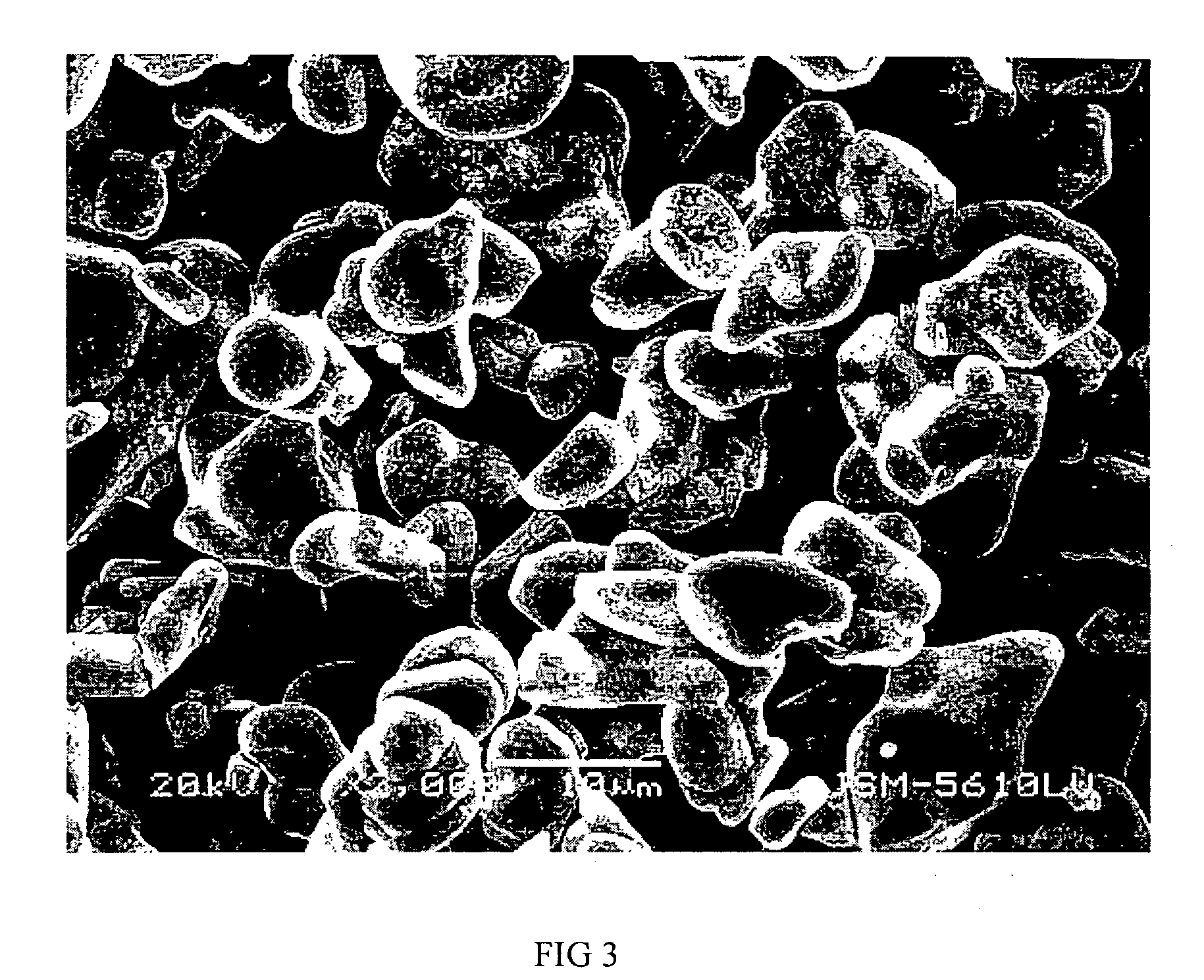
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Gradient cathode material for lithium rechargeable batteries
InactiveUS6921609B2Increase capacityImproved cyclabilityAluminium compoundsAlkaline accumulatorsManganesePotassium
A composition suitable for use as a cathode material of a lithium battery includes a core material having an empirical formula LixM′zNi1−yM″yO2. “x” is equal to or greater than about 0.1 and equal to or less than about 1.3. “y” is greater than about 0.0 and equal to or less than about 0.5. “z” is greater than about 0.0 and equal to or less than about 0.2. M′ is at least one member of the group consisting of sodium, potassium, nickel, calcium, magnesium and strontium. M″ is at least one member of the group consisting of cobalt, iron, manganese, chromium, vanadium, titanium, magnesium, silicon, boron, aluminum and gallium. A coating on the core has a greater ratio of cobalt to nickel than the core. The coating and, optionally, the core can be a material having an empirical formula Lix1Ax2Ni1−y1−z1Coy1Bz1Oa. “x1” is greater than about 0.1 a equal to or less than about 1.3. “x2,”“y1” and “z1” each is greater than about 0.0 and equal to or less than about 0.2. “a” is greater than 1.5 and less than about 2.1. “A” is at least one element selected from the group consisting of barium, magnesium, calcium and strontium. “B” is at least one element selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium.
Owner:TIAX LLC
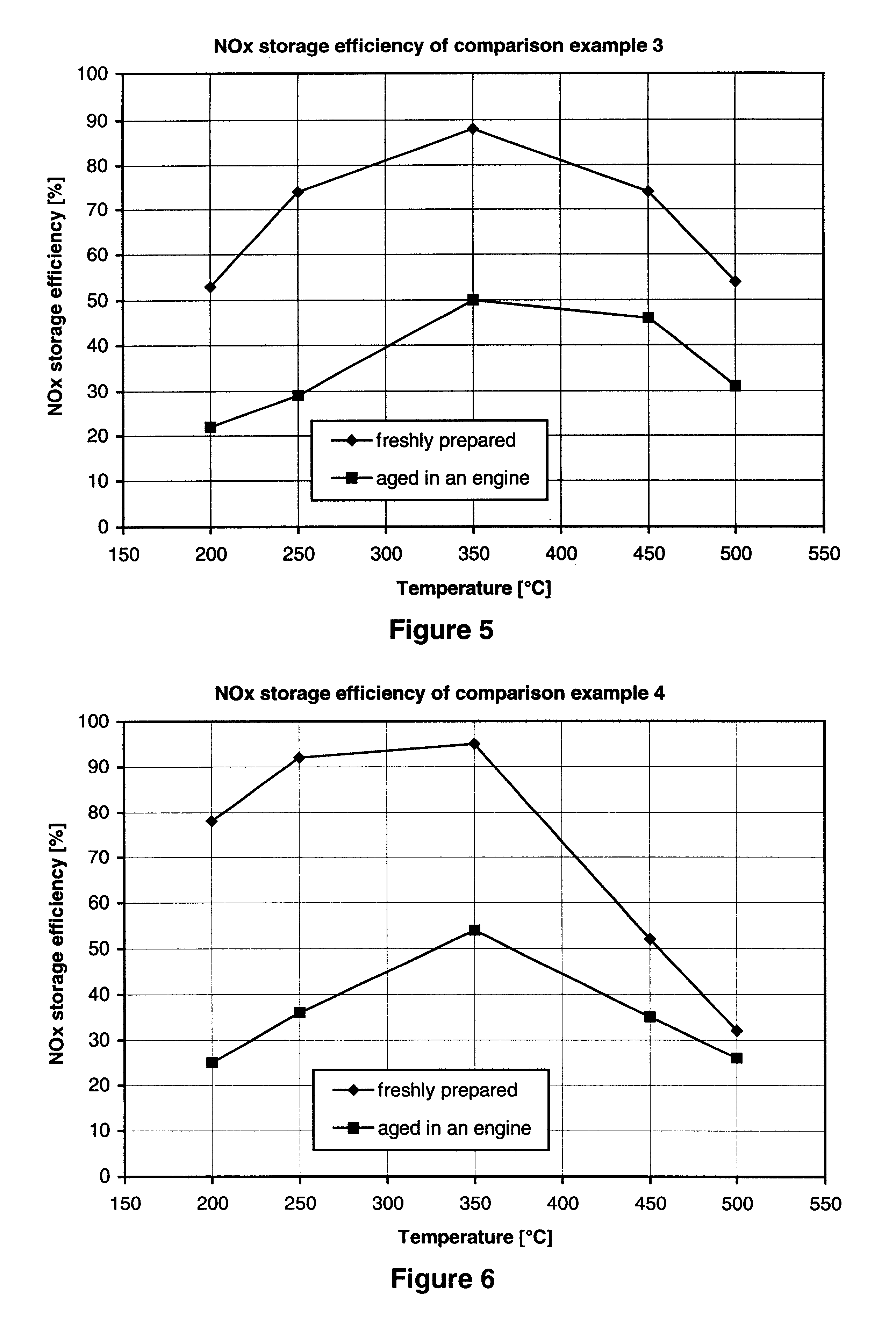
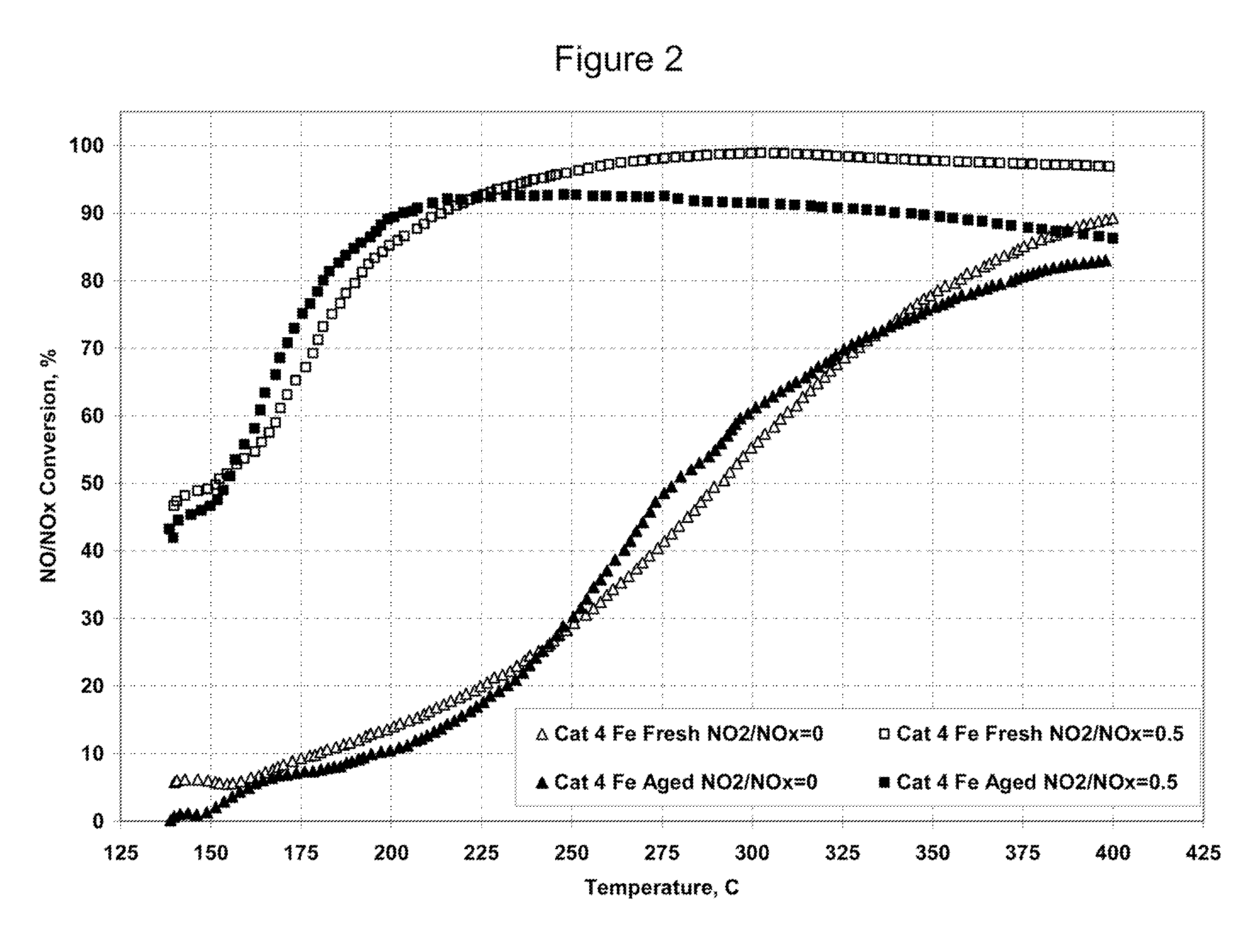
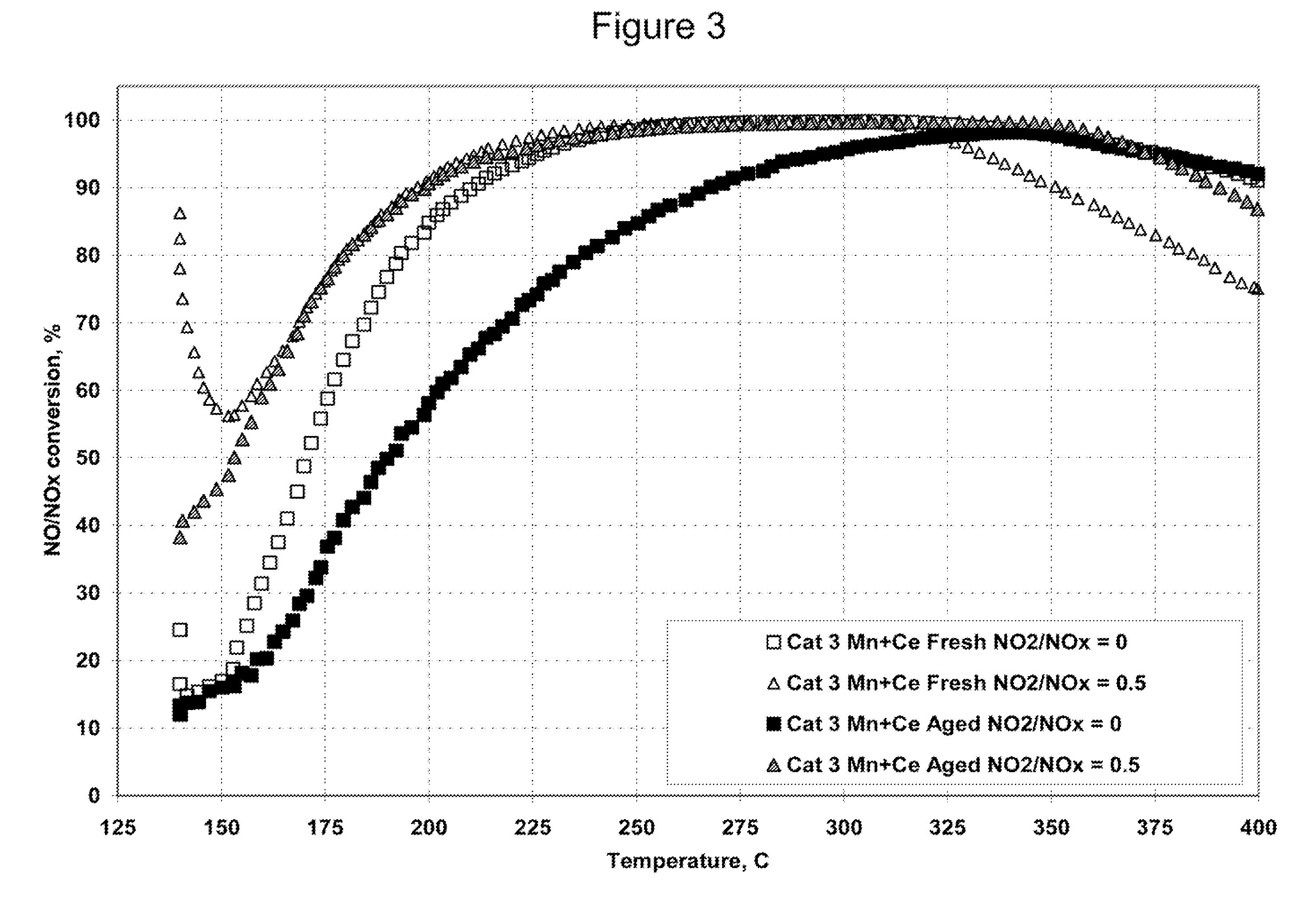
Nitrogen oxide storage material and nitrogen oxide storing catalyst prepared therefrom
InactiveUS6350421B1Determine efficiencyNitrogen compoundsExhaust apparatusAlkaline earth metalCuprate
A nitrogen oxide storage material is disclosed which contains at least one storage component for nitrogen oxides in the form of an oxide, mixed oxide, carbonate or hydroxide of the alkaline earth metals magnesium, calcium, strontium and barium and the alkali metals potassium and caesium on a high surface area support material. The support material can be doped cerium oxide, cerium / zirconium mixed oxide, calcium titanate, strontium titanate, barium titanate, barium stannate, barium zirconate, magnesium oxide, lanthanum oxide, praseodymium oxide, samarium oxide, neodymium oxide, yttrium oxide, zirconium silicate, yttrium barium cuprate, lead titanate, tin titanate, bismuth titanate, lanthanum cobaltate, lanthanum manganate and barium cuprate or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
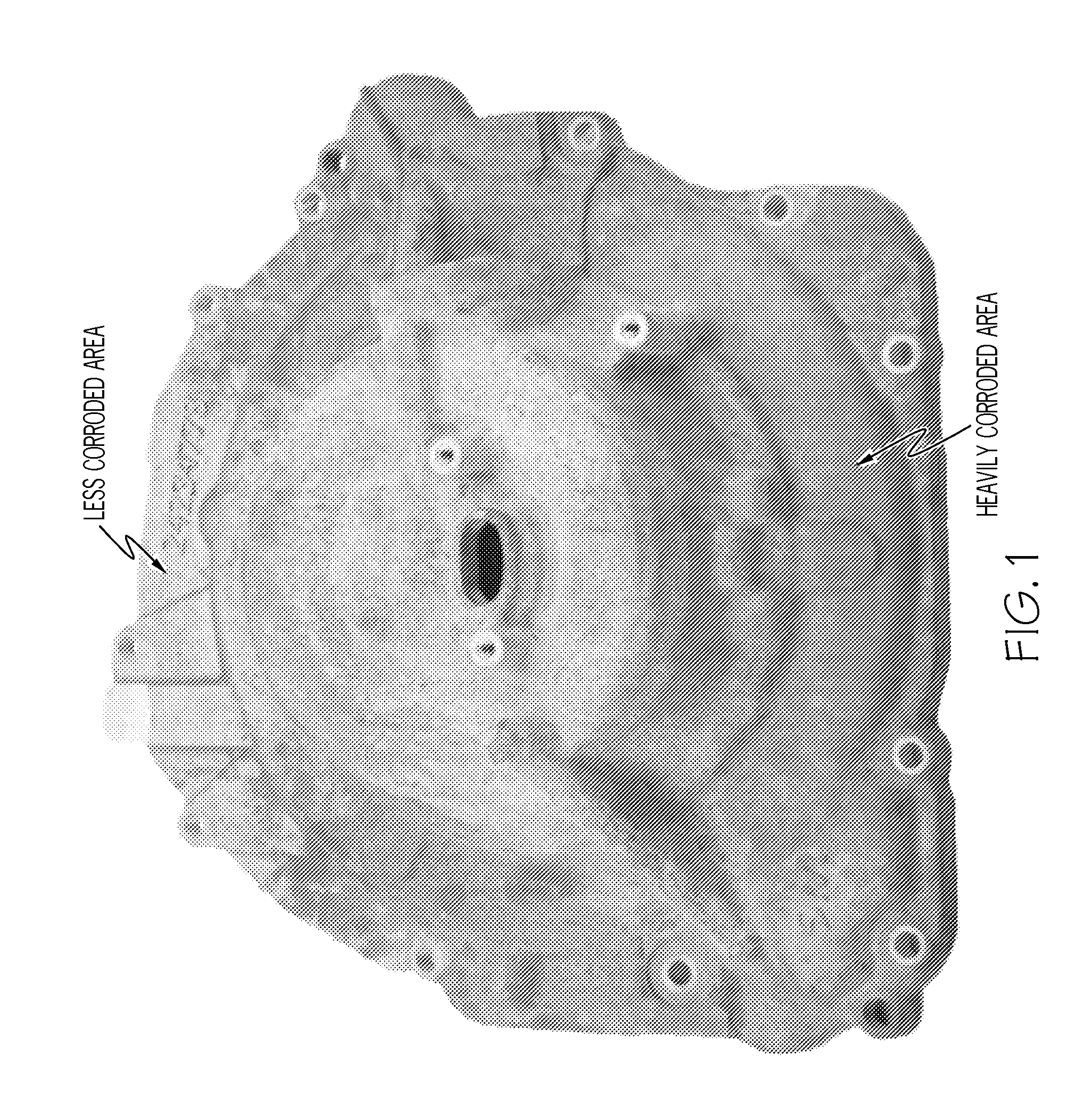
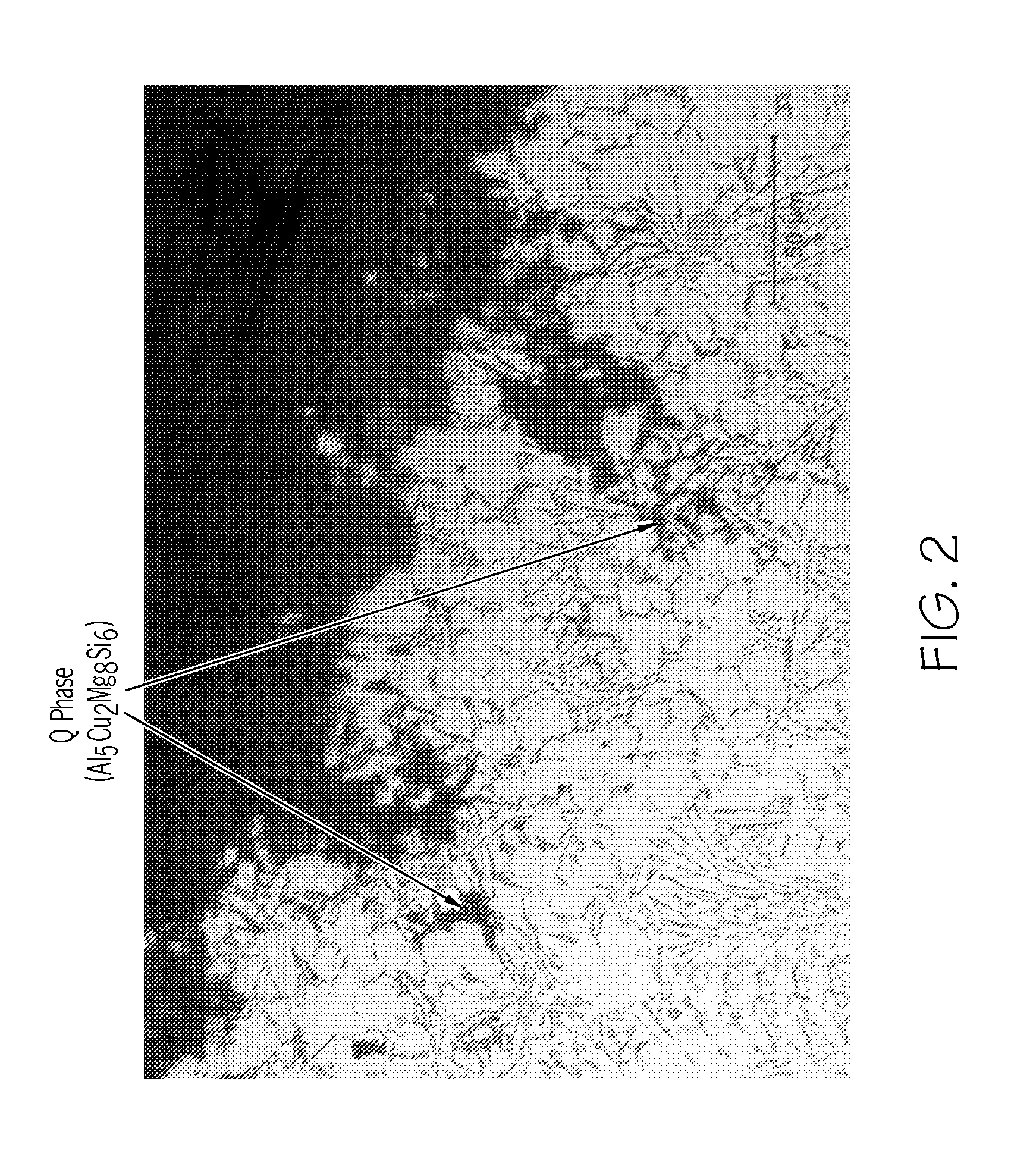
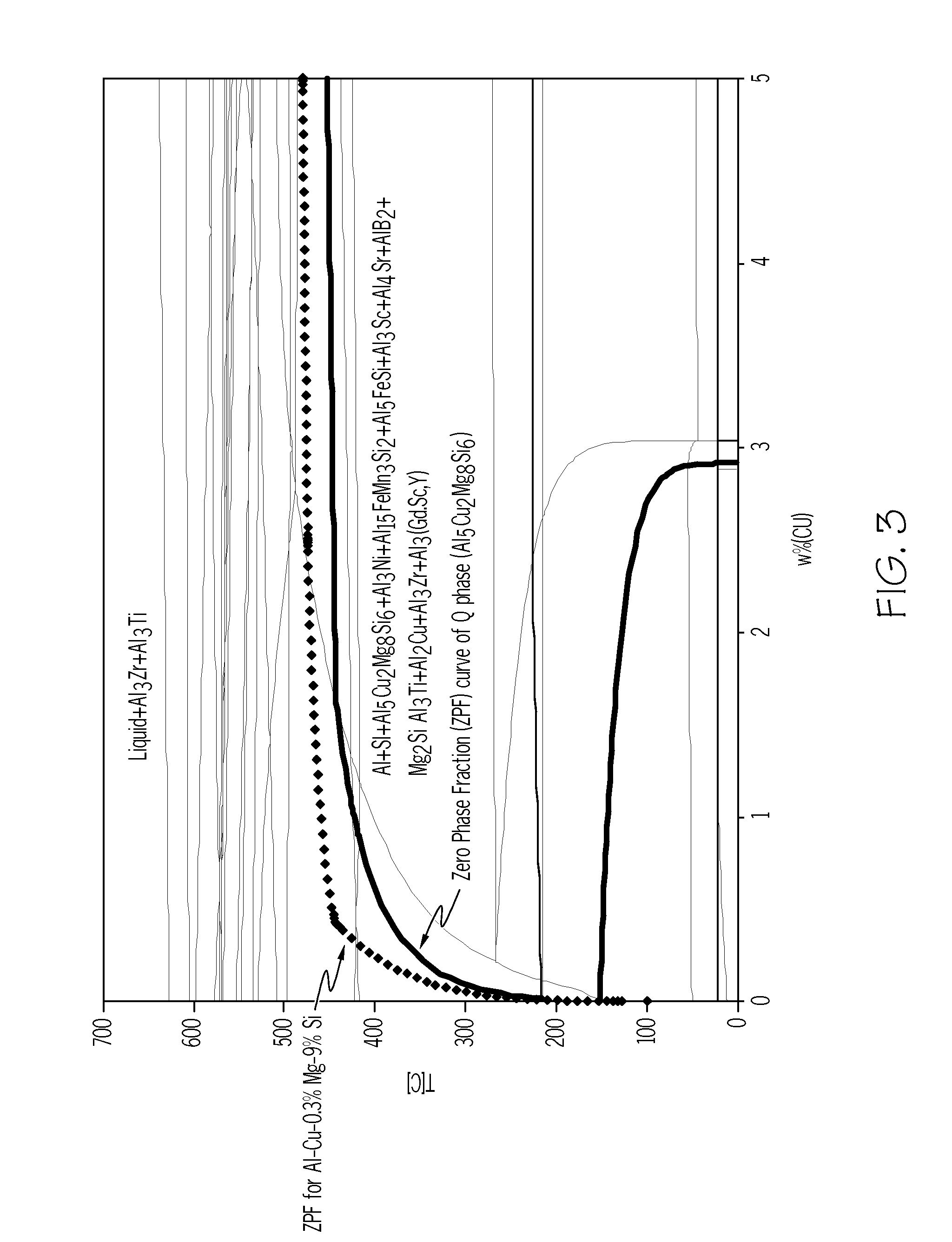
Cast aluminum alloys
ActiveUS20120000578A1Improve mechanical propertiesImprove corrosion resistanceAngiosperms/flowering plantsRare-earth elementIndium
Aluminum alloys having improved properties are provided. The alloy includes about 0 to 2 wt % rare earth elements, about 0.5 to about 14 wt % silicon, about 0.25 to about 2.0 wt % copper, about 0.1 to about 3.0 wt % nickel, approximately 0.1 to 1.0% iron, about 0.1 to about 2.0 wt % zinc, about 0.1 to about 1.0 wt % magnesium, 0 to about 1.0 wt % silver, about 0.01 to about 0.2 wt % strontium, 0 to about 1.0 wt % scandium, 0 to about 1.0 wt % manganese, 0 to about 0.5 wt % calcium, 0 to about 0.5 wt % germanium, 0 to about 0.5 wt % tin, 0 to about 0.5 wt % cobalt, 0 to about 0.2 wt % titanium, 0 to about 0.1 wt % boron, 0 to about 0.2 wt % zirconium, 0 to 0.5% yttrium, 0 to about 0.3 wt % cadmium, 0 to about 0.3 wt % chromium, 0 to about 0.5 wt % indium, and the balance aluminum. Methods of making cast aluminum parts are also described.
Owner:GM GLOBAL TECH OPERATIONS LLC
Platinum group metal-free catalysts for reducing the ignition temperature of particulates on a diesel particulate filter
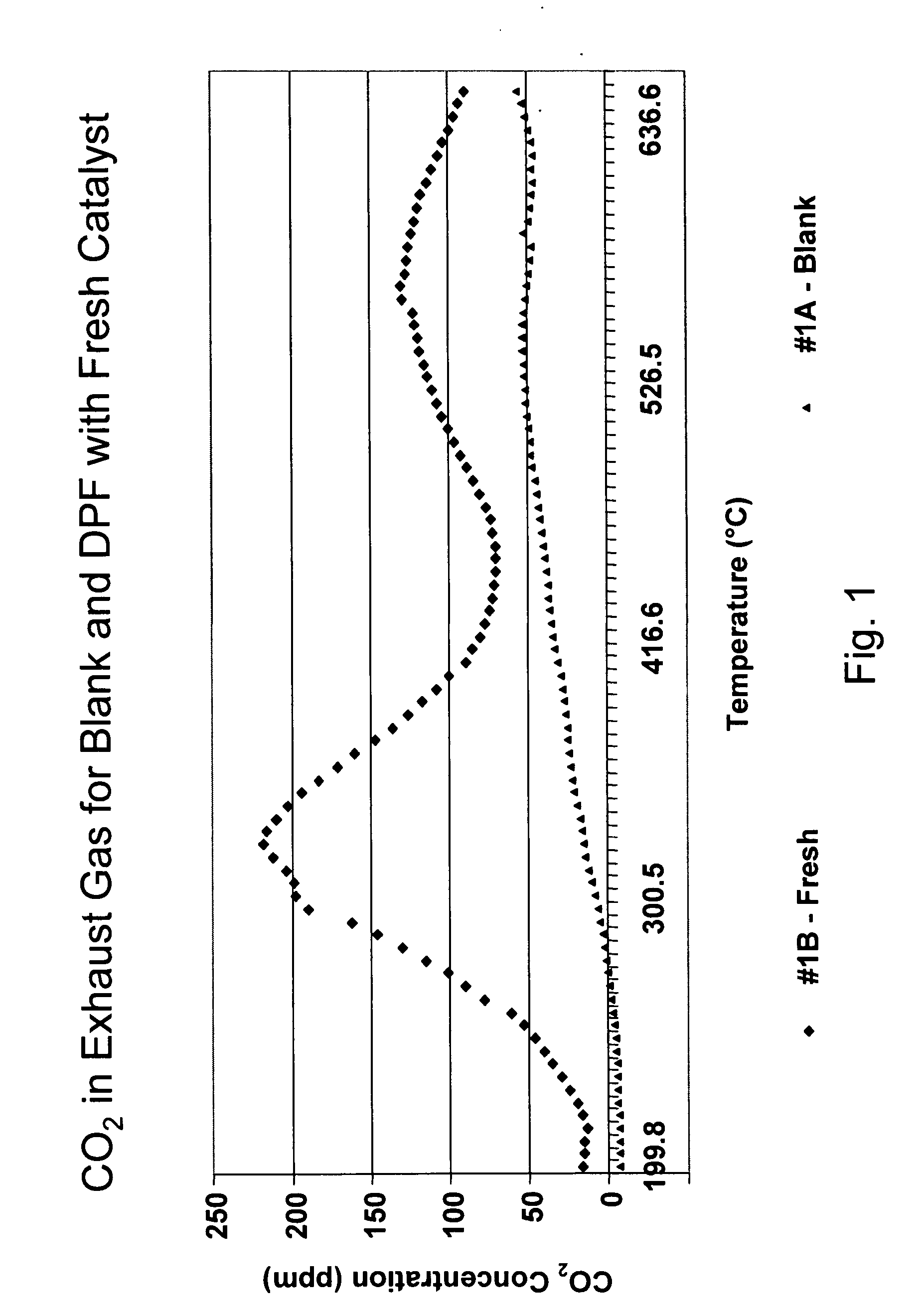
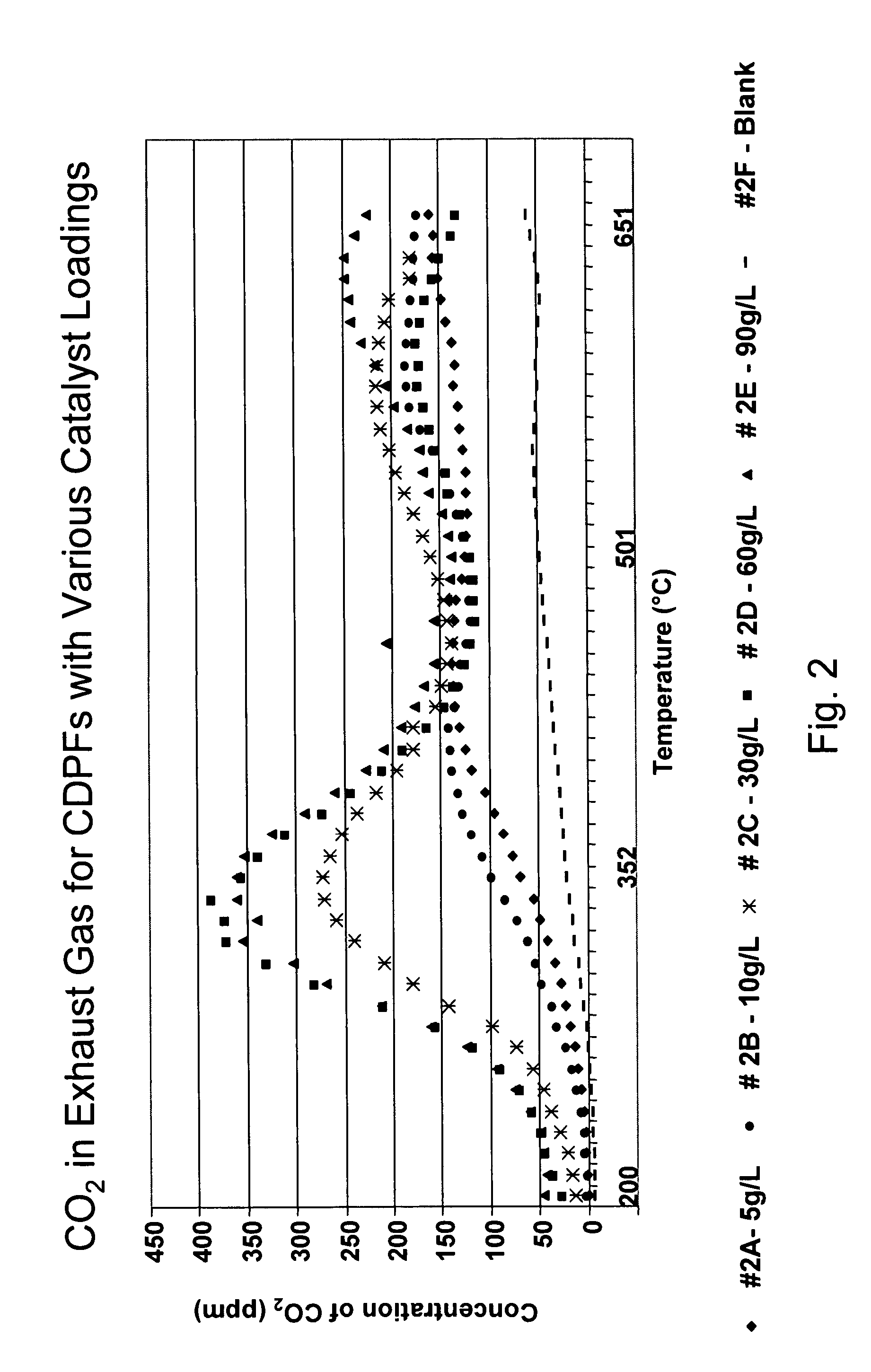
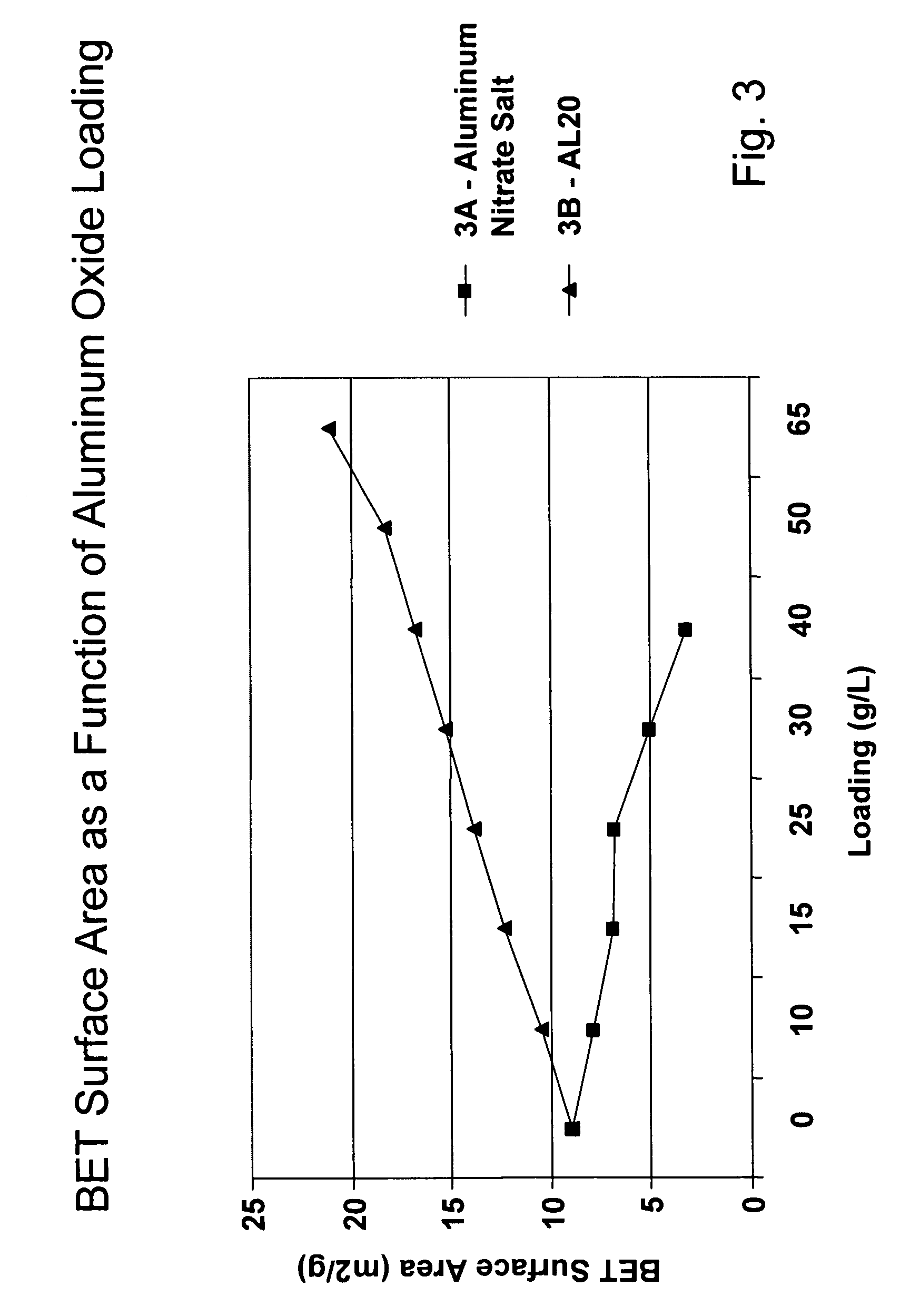
A catalyzed diesel particulate filter (CDPF) and a method for filtering particulates from diesel engine exhaust are provided, where the catalyzed diesel particulate filter includes a substrate and a catalyst composition, where the catalyst composition contains at least one first component, at least one second component, and at least one third component, where the first component is at least one first component selected from the group consisting of cerium and a lanthanide and mixtures thereof, the at least one second component is selected from the group consisting of cobalt, copper, manganese and mixtures thereof; and the third component comprises strontium, where the first component, the second component, and the third component are in an oxide form after calcination. The catalyst on the catalyzed diesel particulate filter lowers the temperature at which particulates are removed from the CDPF by oxidizing the particulates on the filter. The catalyzed diesel particulate filter may also include a washcoat. Washcoats prepared from colloidal aluminum oxide may have higher surface areas and pore volumes loadings than washcoats containing aluminum oxide prepared from aluminum nitrate.
Owner:CATALYTIC SOLUTIONS INC
High performance lithium ion battery anode material lithium manganate and preparation method thereof
The invention provides a high performance lithium ion battery anode material lithium manganate and a preparation method of the material. The lithium manganate is a doped lithium manganate LiMn2-yXy04 which is doped with one kind or a plurality of other metal elements X, wherein X element is at least one kind selected form the group of aluminium, lithium, fluorine, silver, copper, chromium, zinc, titanium, bismuth, germanium, gallium, zirconium, stannum, silicon, cobalt, nickel, vanadium, magnesium, calcium, strontium, barium and rare earth elements lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium, and y is larger than 0 but less than or equal to 0.11. The lithium ion battery anode material lithium manganate provided in the invention has extraordinary charge and discharge cycle performance both in the environments of normal temperature and high temperature. According to the invention, the preparation method of the material is a solid phase method, the operation is simple and controllable and the cost is low so that it is easy to realize large-scale productions.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Ammonia scr catalyst and method of using the catalyst
ActiveUS20090304566A1Enhanced Surface AcidityCombination devicesOrganic chemistryParticulatesNiobium
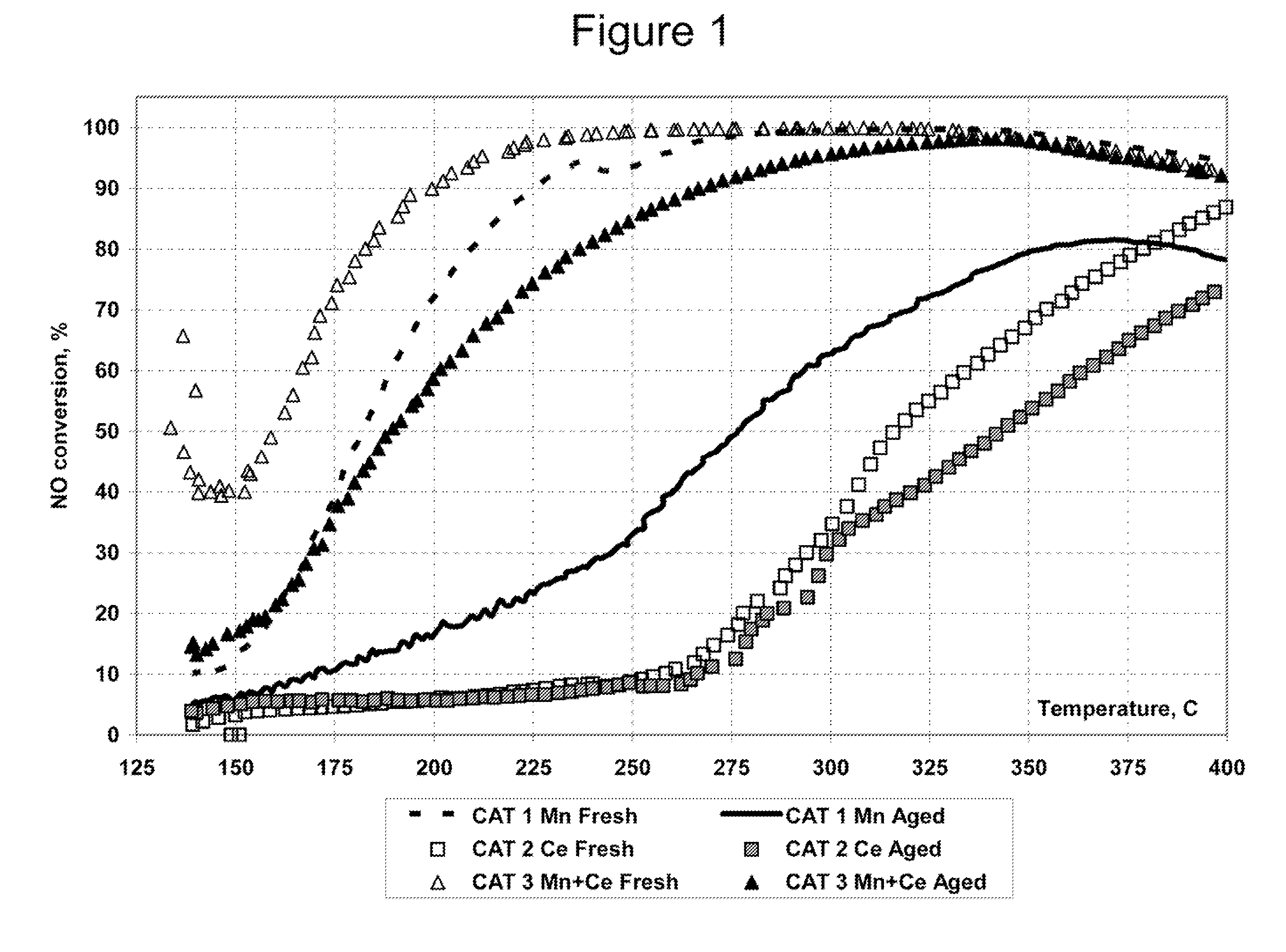
A DPF with an SCR catalyst and a method for selectively reducing nitrogen oxides with ammonia, filtering particulates, and reducing the ignition temperature of soot on a DPF are provided. The catalyst includes a first component of copper, chromium, cobalt, nickel, manganese, iron, niobium, or mixtures thereof, a second component of cerium, a lanthanide, a mixture of lanthanides, or mixtures thereof, and a component characterized by increased surface acidity. The catalyst may also include strontium as an additional second component. The catalyst selectively reduces nitrogen oxides to nitrogen with ammonia and oxidizes soot at low temperatures. The catalyst has high hydrothermal stability.
Owner:CATALYTIC SOLUTIONS INC
Nitride-based, red-emitting phosphors
ActiveUS8274215B2Discharge tube luminescnet screensElectroluminescent light sourcesDisplay deviceEuropium
Embodiments of the present invention are directed to nitride-based, red-emitting phosphors in red, green, and blue (RGB) lighting systems, which in turn may be used in backlighting displays and warm white-light applications. In particular embodiments, the red-emitting phosphor is based on CaAlSiN3 type compounds activated with divalent europium. In one embodiment, the nitride-based, red emitting compound contains a solid solution of calcium and strontium compounds (Ca,Sr)AlSiN3:Eu2+, wherein the impurity oxygen content is less than about 2 percent by weight. In another embodiment, the (Ca,Sr)AlSiN3:Eu2+ compounds further contains a halogen in an amount ranging from about zero to about 2 atomic percent, where the halogen may be fluorine (F), chlorine (Cl), or any combination thereof. In one embodiment at least half of the halogen is distributed on 2-fold coordinated nitrogen (N2) sites relative to 3-fold coordinated nitrogen (N3) sites.
Owner:INTEMATIX
White light emitting phosphor blend for LED devices
There is provided a white light illumination system including a radiation source, a first luminescent material having a peak emission wavelength of about 570 to about 620 nm, and a second luminescent material having a peak emission wavelength of about 480 to about 500 nm, which is different from the first luminescent material. The LED may be a UV LED and the luminescent materials may be a blend of two phosphors. The first phosphor may be an orange emitting Eu2+, Mn2+ doped strontium pyrophosphate, (Sr0.8Eu0.1Mn0.1)2P2O7. The second phosphor may be a blue-green emitting Eu2+ doped SAE, (Sr0.90-0.99 Eu0.01-0.1)4Al14O25. A human observer perceives the combination of the orange and the blue-green phosphor emissions as white light.
Owner:GE LIGHTING SOLUTIONS LLC
Aluminum titanate ceramic articles and methods of making same
ActiveUS7259120B2Reduced strengthLower firing temperatureInternal combustion piston enginesSilencing apparatusAlkaline earth metalRare earth
An aluminum titanate ceramic article having a predominant crystal phase of aluminum titanate and a material composition including aluminum, titanium, silica, an alkaline earth metal (e.g., at least one selected from the group of strontium, calcium, barium, or combinations), and a rare earth metal (e.g., at least one selected from the group consisting of yttrium, lanthanum, and combinations) and methods of making such aluminum titanate bodies are described. An oxide of yttrium metal or lanthanide metals is preferably used as a sintering aid in combination with the other compositional components to enable firing of the resulting green body at a lower heating temperature of less than 1500° C., and more preferably between 1400°-1450° C., with a preferable hold time of less than 8 hours, more preferably of 6 to 8 hours.
Owner:CORNING INC
Automated strontium-rubidium infusion system
InactiveUS20090312635A1Elimination of aforesaid shortcomingImprove efficiencySolid waste disposalProtective foundationTransport systemAutomatic control
This invention relates to medical engineering, in particular, to means of automation of the process of generating a diagnostic solution from a radionuclide strontium-rubidium generator and performing of remote controlled infusion, with automatic control over the key characteristics of the process. The automated strontium-rubidium infusion system comprises a container with eluent, a strontium-rubidium generator with a filter and a pressure sensor at the input, an eluate infusion unit, which are connected by means of a transporting system provided with pipes and two three-way valves, radioactivity measuring means and a control and operating unit. Here, an eluent container is connected to a syringe pump via the first and second ports of the first three-way valve, the first port of the second three-way valve is connected with pipes via the second filter to the eluate infusion unit, and the second port is connected to a waste receptacle. The system additionally comprises the third and fourth three-way valves, the first and second air bubbles detectors are connected to the control and operating unit connected with a computer, where the third three-way valve is connected with its first and second ports via pipes to the third port of the first three-way valve and the input of the strontium-rubidium generator, respectively. The generator output is connected to the first port of the fourth three-way valve, where the third port of the third valve and the second port of the fourth valve are connected with a pipe, the first air bubbles detector is placed on the pipeline between the eluent container and the first port of the first valve, and the second air bubbles detector is placed on the pipeline between the third ports of the fourth and second valves.
Owner:OBSHCHESTVO S OGRANICHENNOY OTVETABTVENNOSTIU NAUCHNO PROIZVODSTVENNAYA FA POZITOM PRO
Betaine with Calcium and/or Strontium Antiperspirants
InactiveUS20070020211A1Good curative effectImprove skinCosmetic preparationsToilet preparationsAntiperspirantsBetaine
Aluminum and aluminum-zirconium antiperspirant compositions comprising basic aluminum chlorides that have a particular molecular size distribution defined by having an SEC-HPLC Band III / II ratio of at least 0.5, having SEC-HPLC Band III plus Band II area of at least 70% of the total area and having SEC-HPLC Band I content no more than 5% and containing betaine (trimethylglycine), calcium and / or strontium are disclosed. Also disclosed are the methods of making these compositions and the use thereof in consumer acceptable antiperspirant vehicles such as aerosols, gels, roll-on, sticks and soft solids.
Owner:SUMMIT RES LAB
Use of alkaline earth metal containing small pore non-zeolitic molecular sieve catalysts in oxygenate conversion
A method for converting starting material to olefins comprising contacting the starting material with a small pore non-zeolitic molecular sieve catalyst under effective conditions to produce olefins, wherein the non-zeolitic molecular sieve has been prepared in-situ or modified after synthesis by incorporation using an alkaline earth metal compound, wherein the alkaline earth metal ion is selected from the group consisting of strontium, calcium, barium, and mixtures thereof.
Owner:EXXON CHEM PAT INC
Strontium silicate-based phosphor, fabrication method thereof, and LED using the phosphor
ActiveUS7045826B2Wide wavelength spectrumLuminous properties are stableSolid-state devicesSemiconductor/solid-state device manufacturingPhosphorUltraviolet
A strontium silicate-based phosphor, a fabrication method thereof, and an LED using the strontium silicate-based phosphor are provided. The phosphor is applied to a long wavelength ultraviolet LED, an active luminous LCD, etc., to enable an improvement in the color purity and to enhance the luminous efficiency. The strontium silicate-based phosphor is expressed by a chemical formula: Sr3-xSiO5Eu2+x wherein x is 0<x≦1. The LED using the phosphor has a wide wavelength spectrum, shows a superior color purity characteristic, and can have a very high luminous efficiency as applied in the backlight source of an LED panel or an active luminous LCD.
Owner:KOREA RES INST OF CHEM TECH
High strength aluminum alloy for high temperature applications
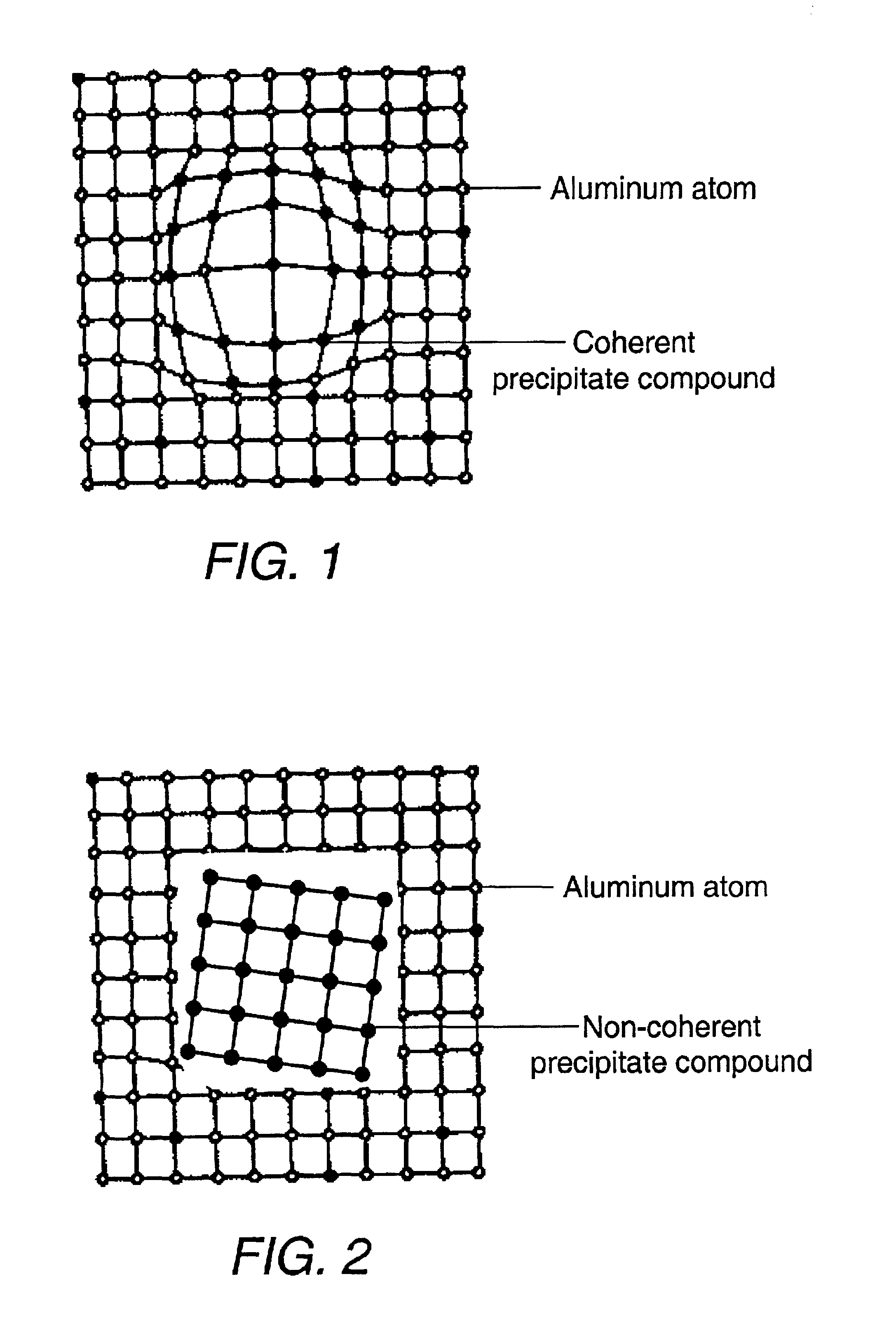
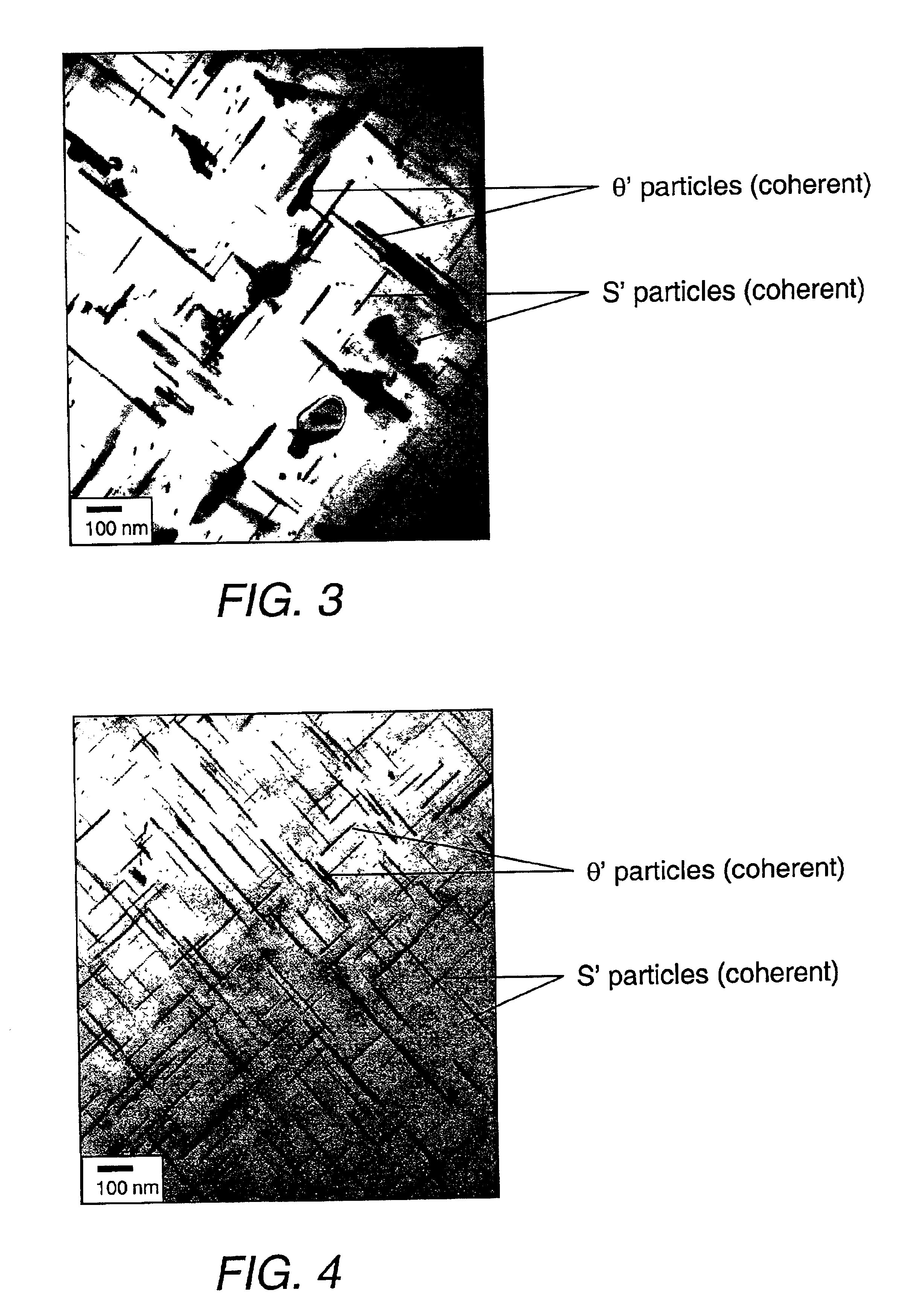
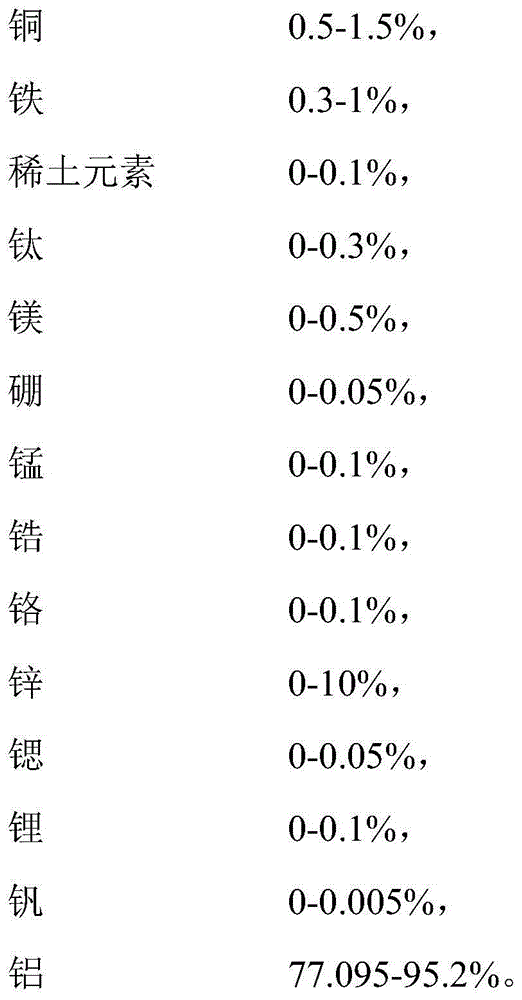
A cast article from an aluminum alloy has improved mechanical properties at elevated temperatures. The cast article has the following composition in weight percent: Silicon 6.0-25.0, Copper 5.0-8.0, Iron 0.05-1.2, Magnesium 0.5-1.5, Nickel 0.05-0.9, Manganese 0.05-1.2, Titanium 0.05-1.2, Zirconium 0.05-1.2, Vanadium 0.05-1.2, Zinc 0.05-0.9, Strontium 0.001-0.1, Phosphorus 0.001-0.1, and the balance is Aluminum, wherein the silicon-to-magnesium ratio is 10-25, and the copper-to-magnesium ratio is 4-15. The aluminum alloy contains a simultaneous dispersion of three types of Al3X compound particles (X=Ti, V, Zr) having a L12 crystal structure, and their lattice parameters are coherent to the aluminum matrix lattice. A process for producing this cast article is also disclosed, as well as a metal matrix composite, which includes the aluminum alloy serving as a matrix containing up to about 60% by volume of a secondary filler material.
Owner:NASA
Strontium-apatite-cement-preparations, cements formed therefrom, and uses thereof
ActiveUS7273523B2Facilitated releaseTo promote metabolismBiocideSurgical adhesivesPowder mixturePhosphate
Calcium-strontium-hydroxyphosphate (strontium-apatite-) cement preparations are described, comprising a powder mixture, which contains molar quantities of the components calcium (Ca), strontium (Sr) and phosphate (P) in the mixture in the ranges 1.00<Ca / P≦1.50 and 0<Sr / P<1.5, together with an alkali salt or an ammonium salt of phosphoric acid, and with water and / or an aqueous solution. The powder mixture particularly contains, as the Ca-component, Ca3(PO4)2 (TCP), and as the Sr-component SrHPO4 and / or Sr3(PO4)2 and optionally additional SrCO3. As the aqueous mixing solution for the formation of the strontium-apatite cement, an aqueous solution of an alkali salt or an ammonium salt of the phosphoric acid is suitable.
Owner:KYPHON
Aluminum alloy and preparation method and application thereof
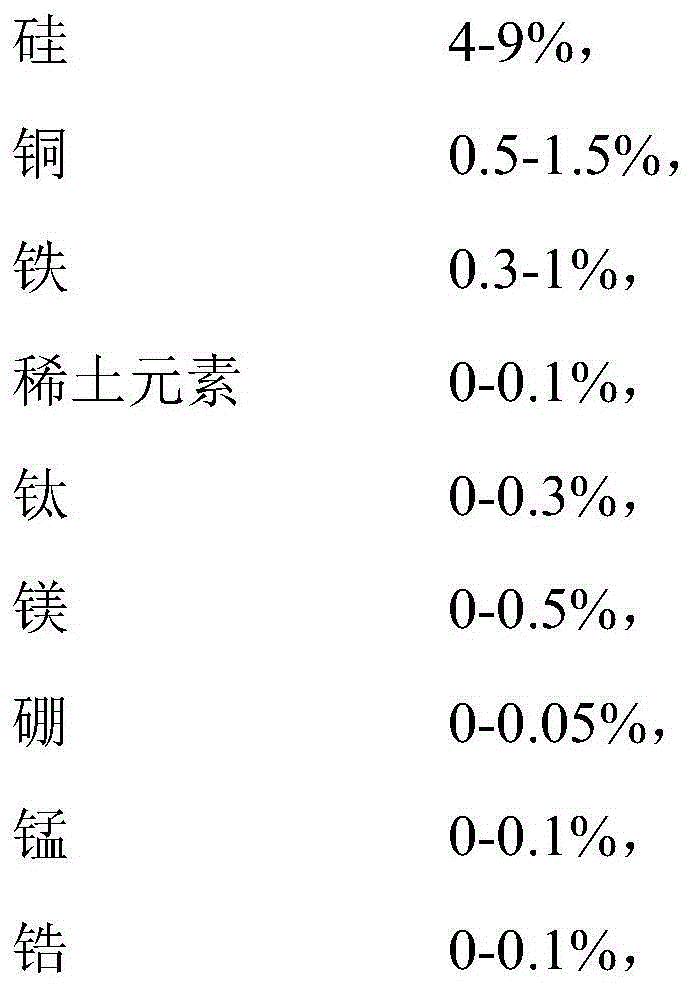
The invention discloses an aluminum alloy and a preparation method and application thereof. The aluminum alloy comprises the following components in percentage by weight: 4-9% of silicon, 0.5-1.5% of copper, 0.3-1% of iron, 0-0.1% of rare earth elements, 0-0.3% of titanium, 0-0.5% of magnesium, 0-0.05% of boron, 0-0.1% of manganese, 0-0.1% of zirconium, 0-0.1% of chromium, 0-10% of zinc, 0-0.05% of strontium, 0-0.1% of lithium, 0-0.005% of vanadium, and 77.095-95.2% of aluminum. The aluminum alloy, provided by the invention, is excellent in comprehensive mechanical performance, higher in strength and hardness and higher in ductility, and can be machined to structural elements having various shapes and thicknesses. Above all, the aluminum alloy, provided by the invention, is excellent in heat conductivity. The aluminum alloy, provided by the invention, is suitable for serving as a structural material having higher requirements on the heat conducting performance, and in particular, is suitable for serving as a structural part of an electronic product.
Owner:BYD CO LTD
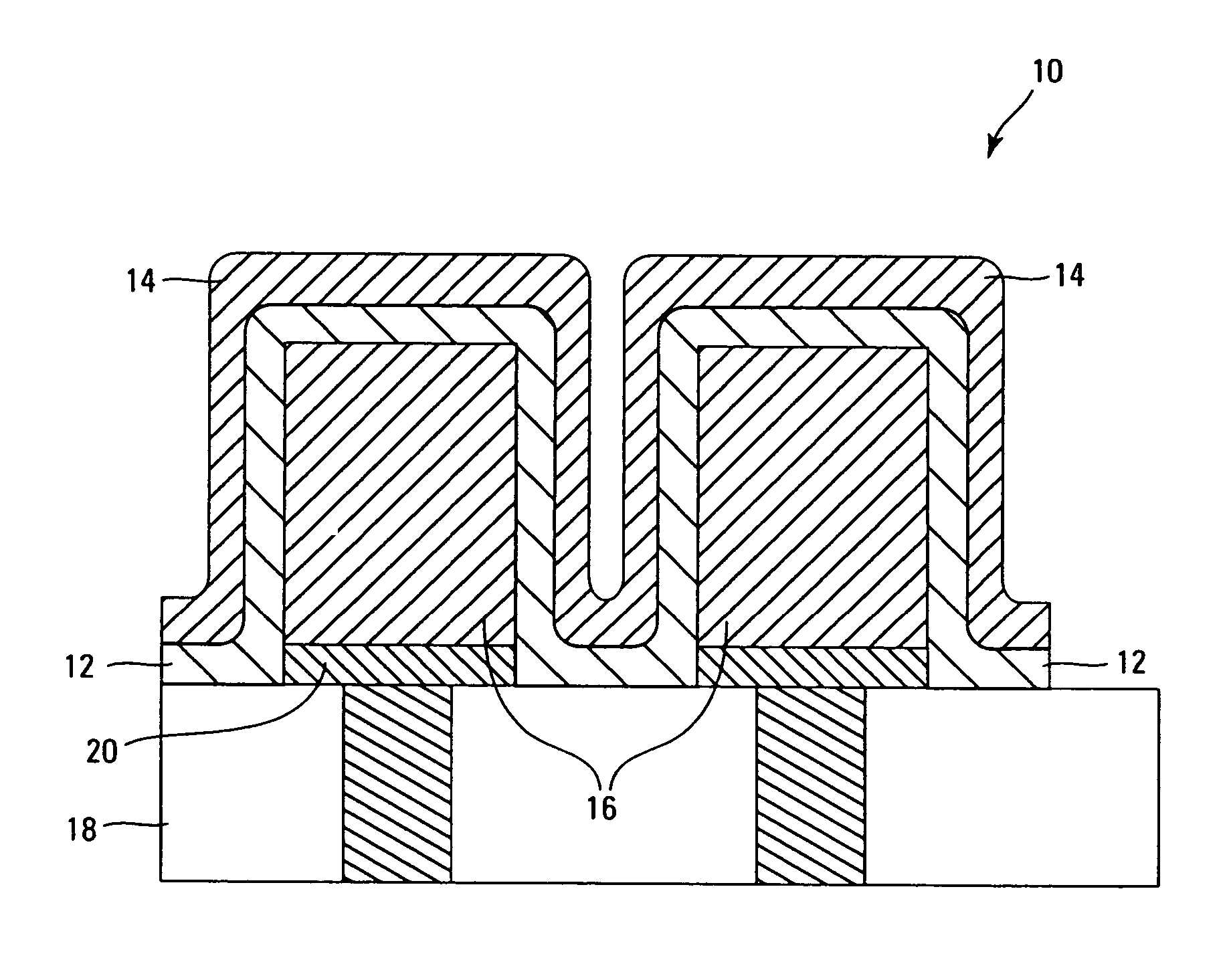
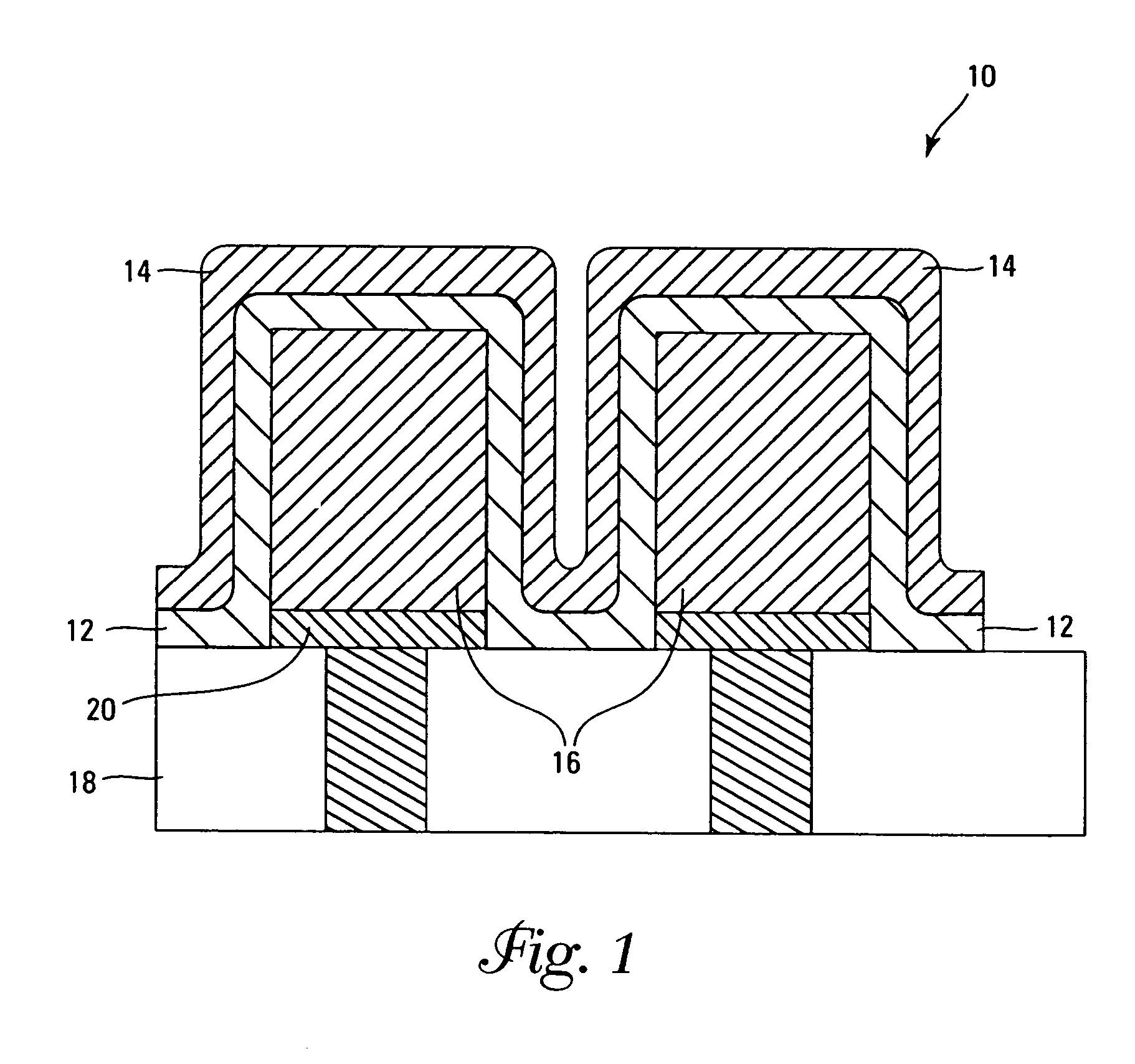
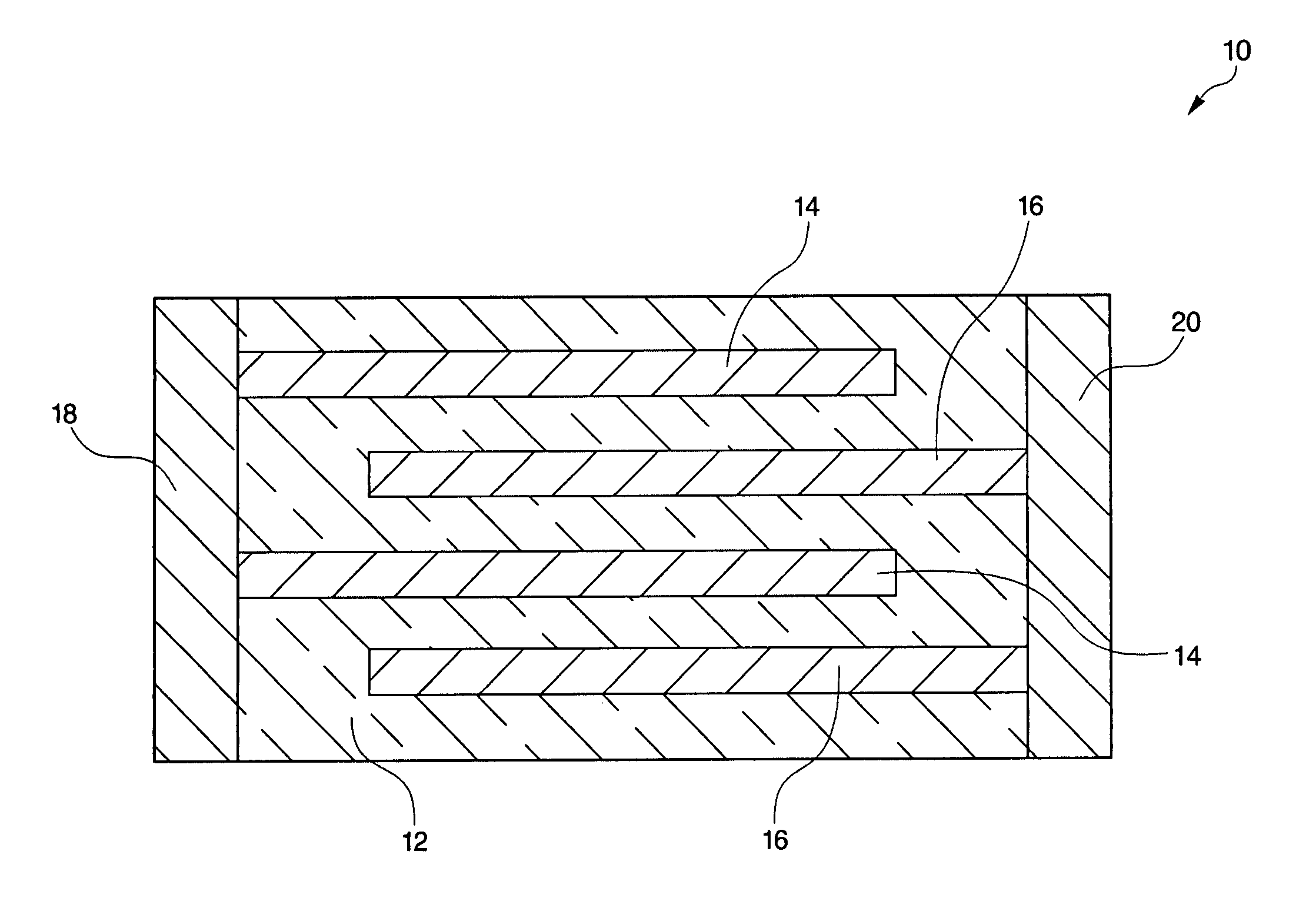
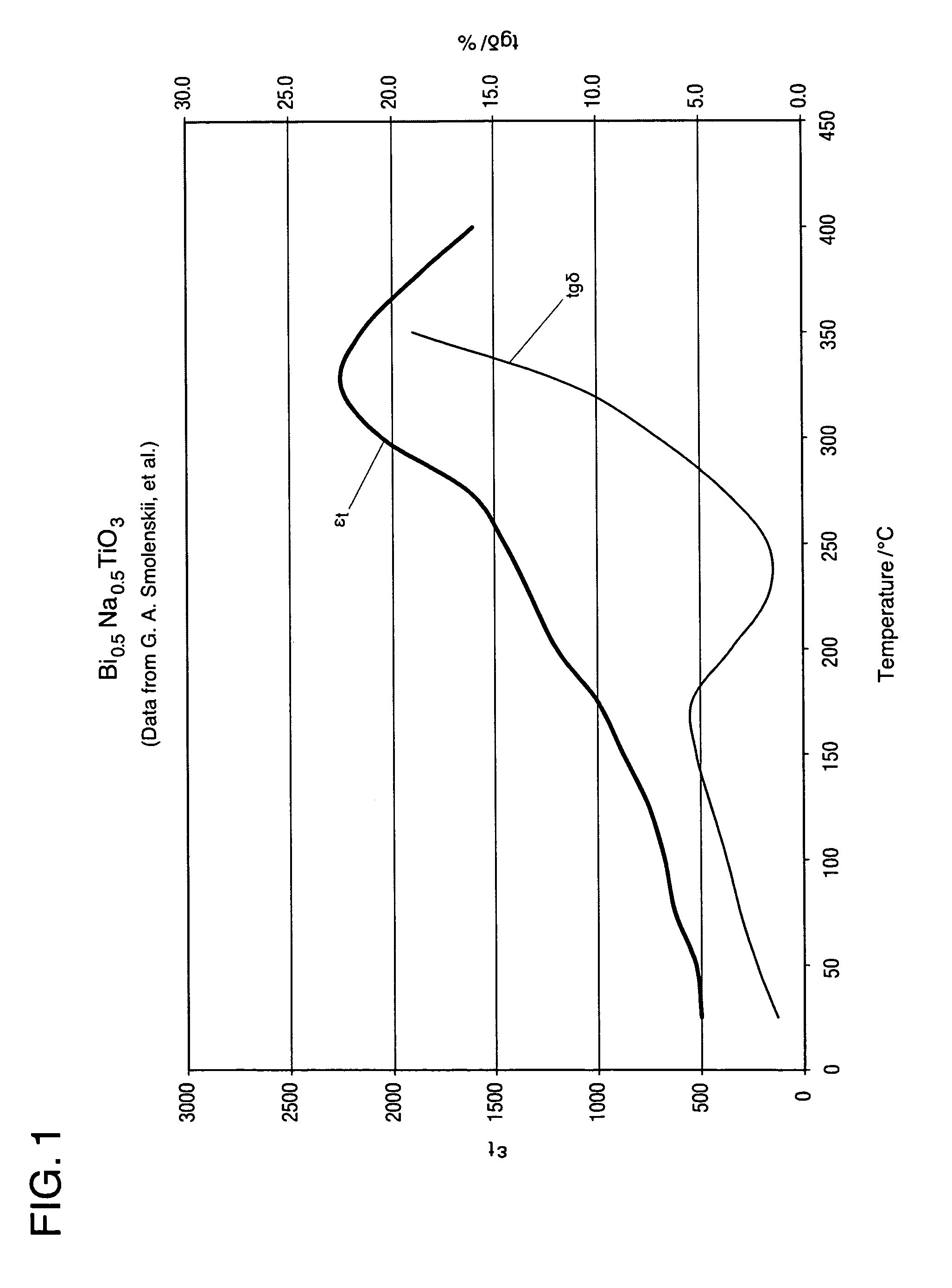
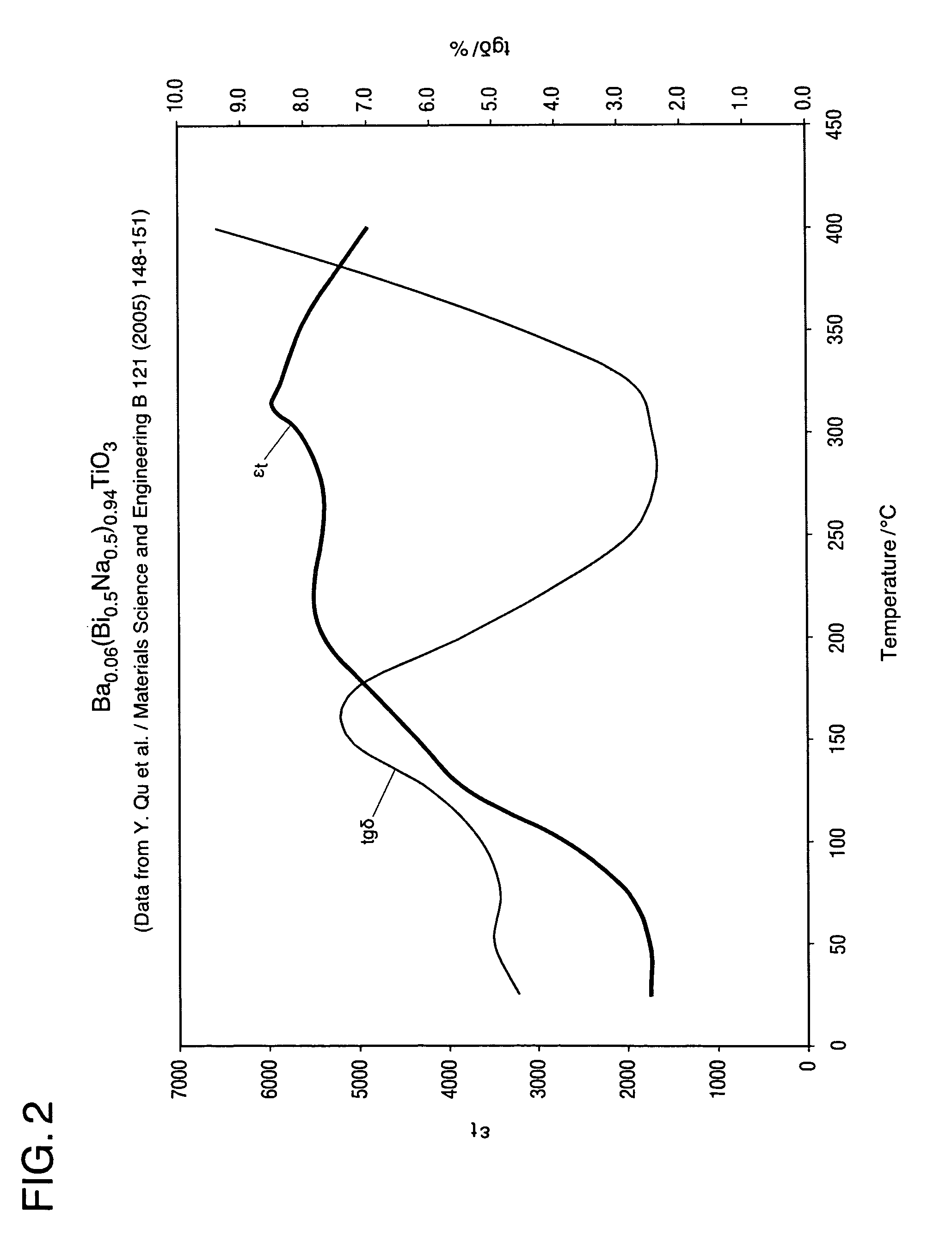
High temperature ceramic dielectric composition and capacitors made from the composition
ActiveUS8076257B1Highly desirable propertyExcellent dielectric constant/voltage characteristicFixed capacitor dielectricStacked capacitorsCapacitanceDielectric
A bismuth sodium titanate (Bi0.5Na0.5TiO3) base material is modified by the partial substitution of aliovalent A-site cations such as barium (as BaO) or strontium (as SrO), as well as certain b-site donor / acceptor dopants and sintering aids to form a multi-phase system, much like known “core / shell” X7R dielectrics based solely on BaTiO3. The resulting ceramic dielectric composition is particularly suitable for producing a multilayer ceramic capacitor (10) that maintains high dielectric constant (and thus the capability of maintaining high capacitance) over a broad temperature range of from about 150° C. to about 300° C. Such capacitors (10) are appropriate for high temperature power electronics applications in fields such as down-hole oil and gas well drilling.
Owner:FERRO CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com