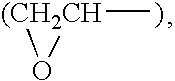Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1044 results about "Rubidium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a very soft, silvery-white metal in the alkali metal group. Rubidium metal shares similarities to potassium metal and caesium metal in physical appearance, softness and conductivity. Rubidium cannot be stored under atmospheric oxygen, as a highly exothermic reaction will ensue, sometimes even resulting in the metal catching fire.
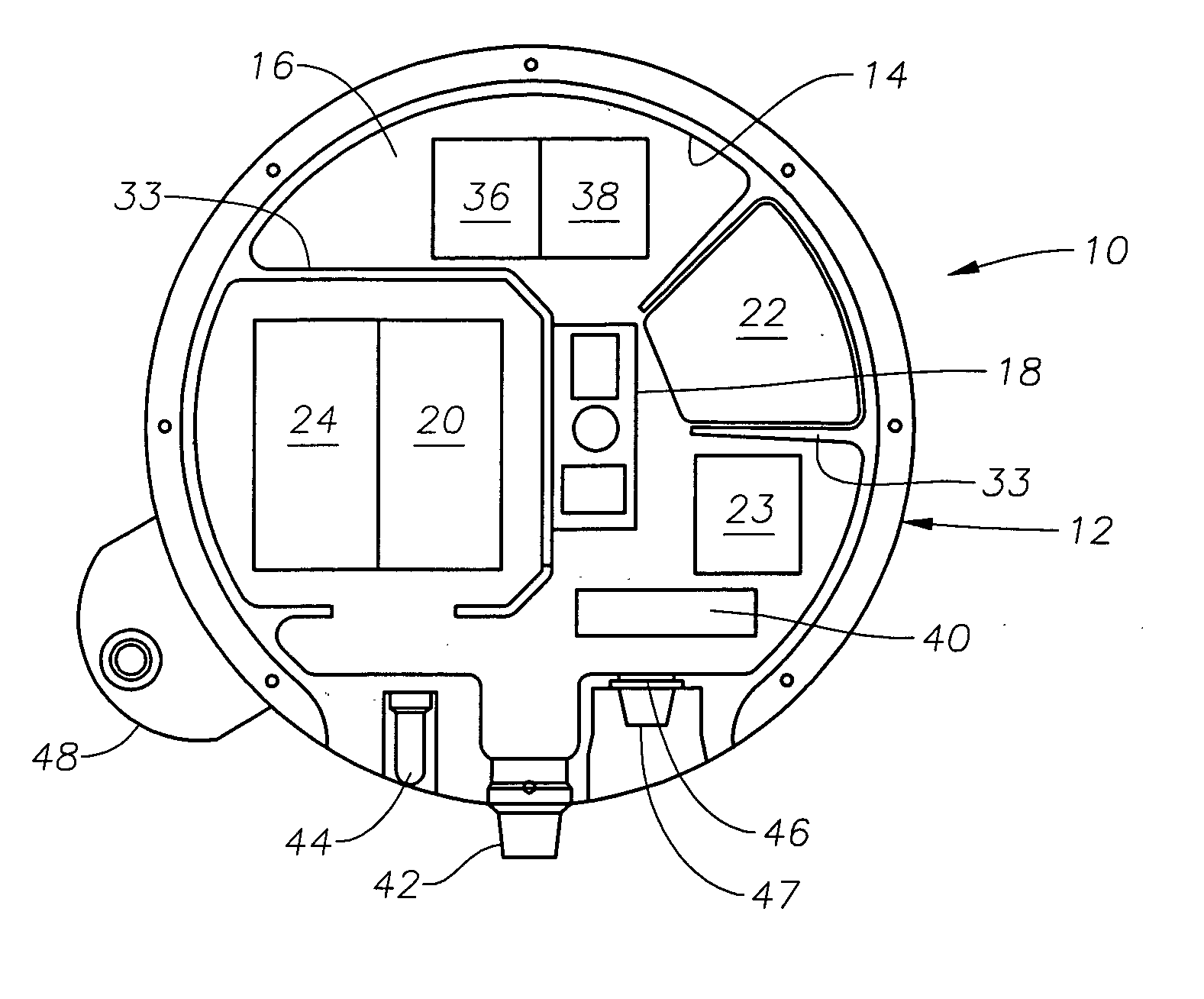
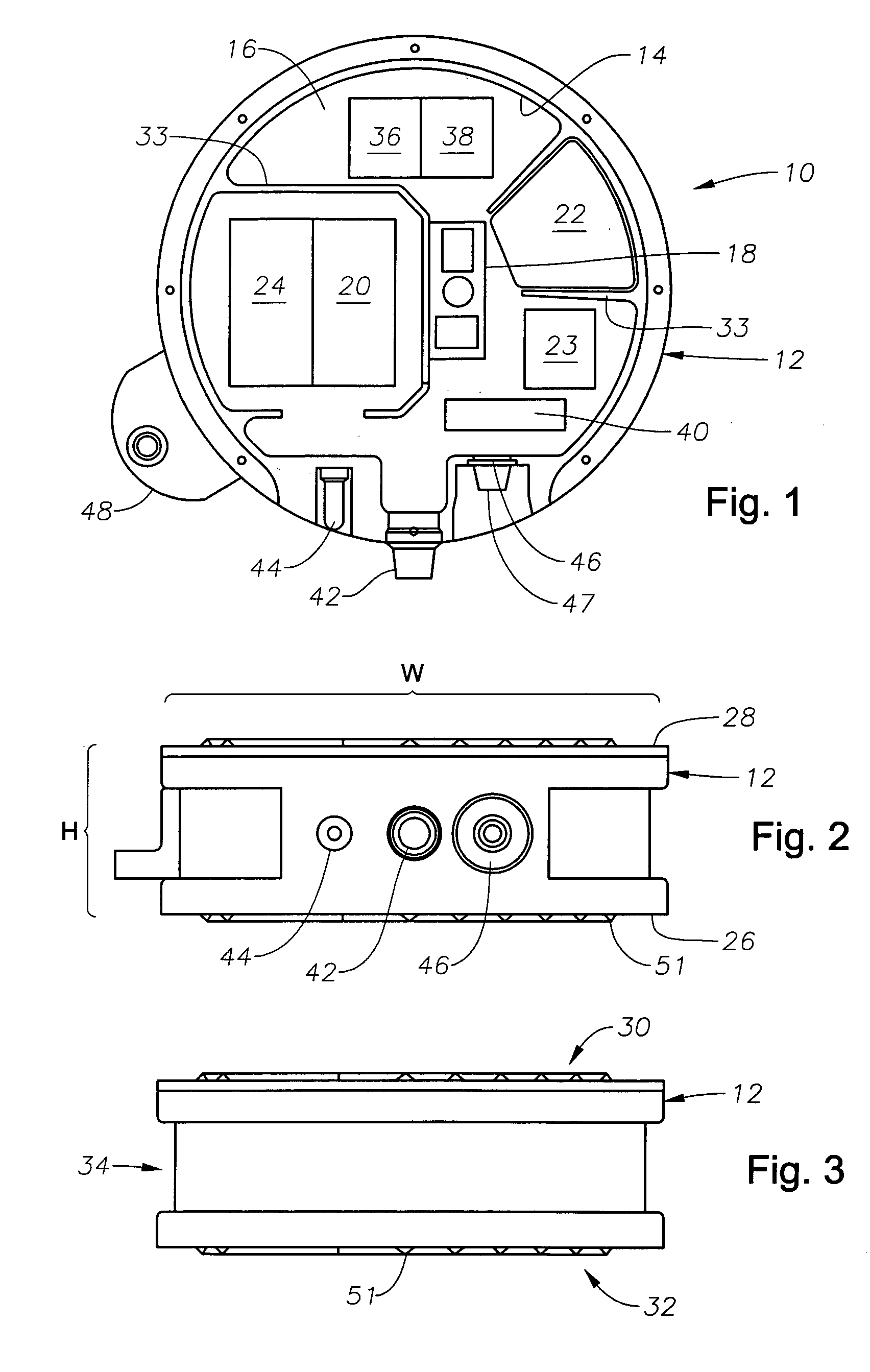
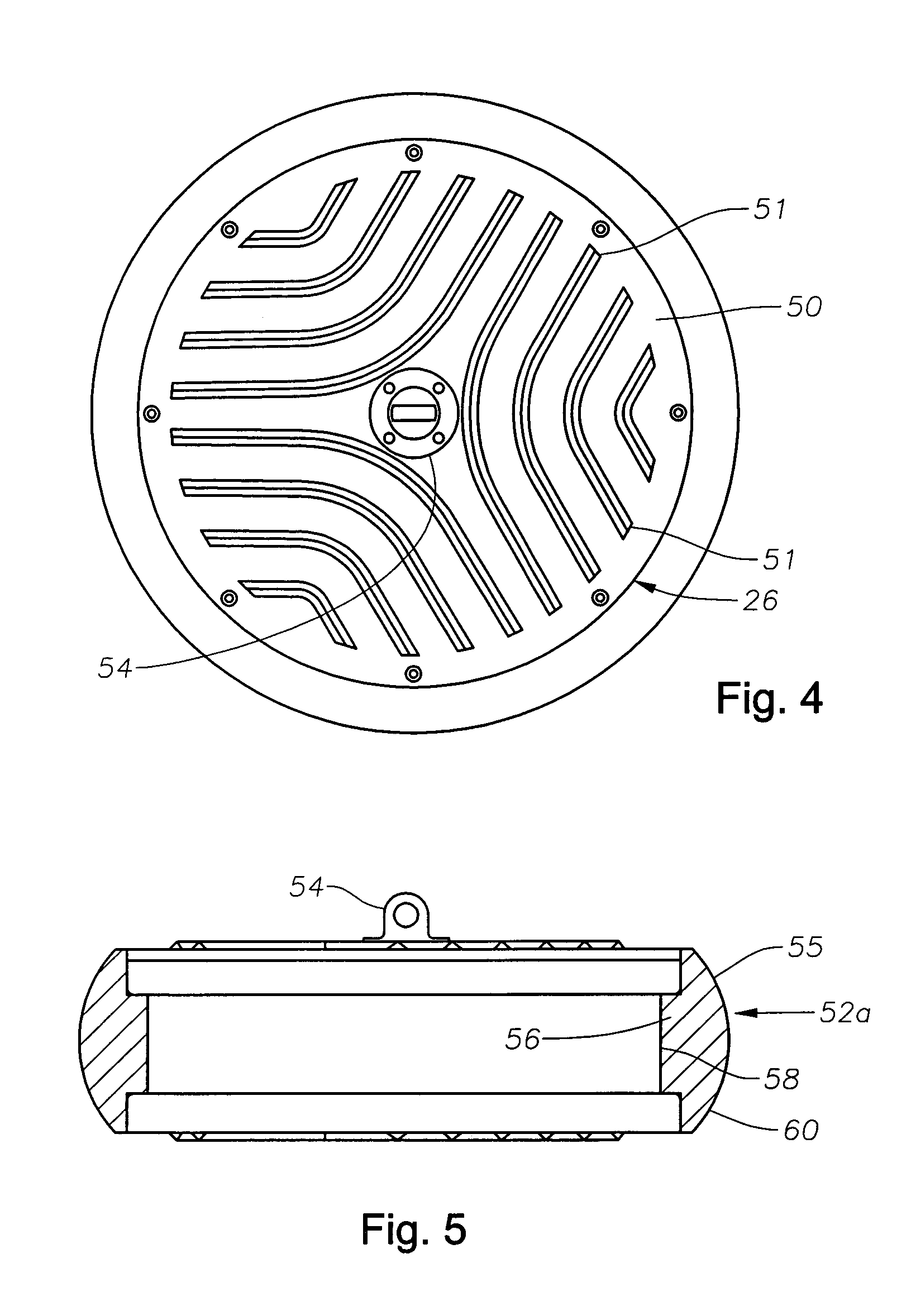
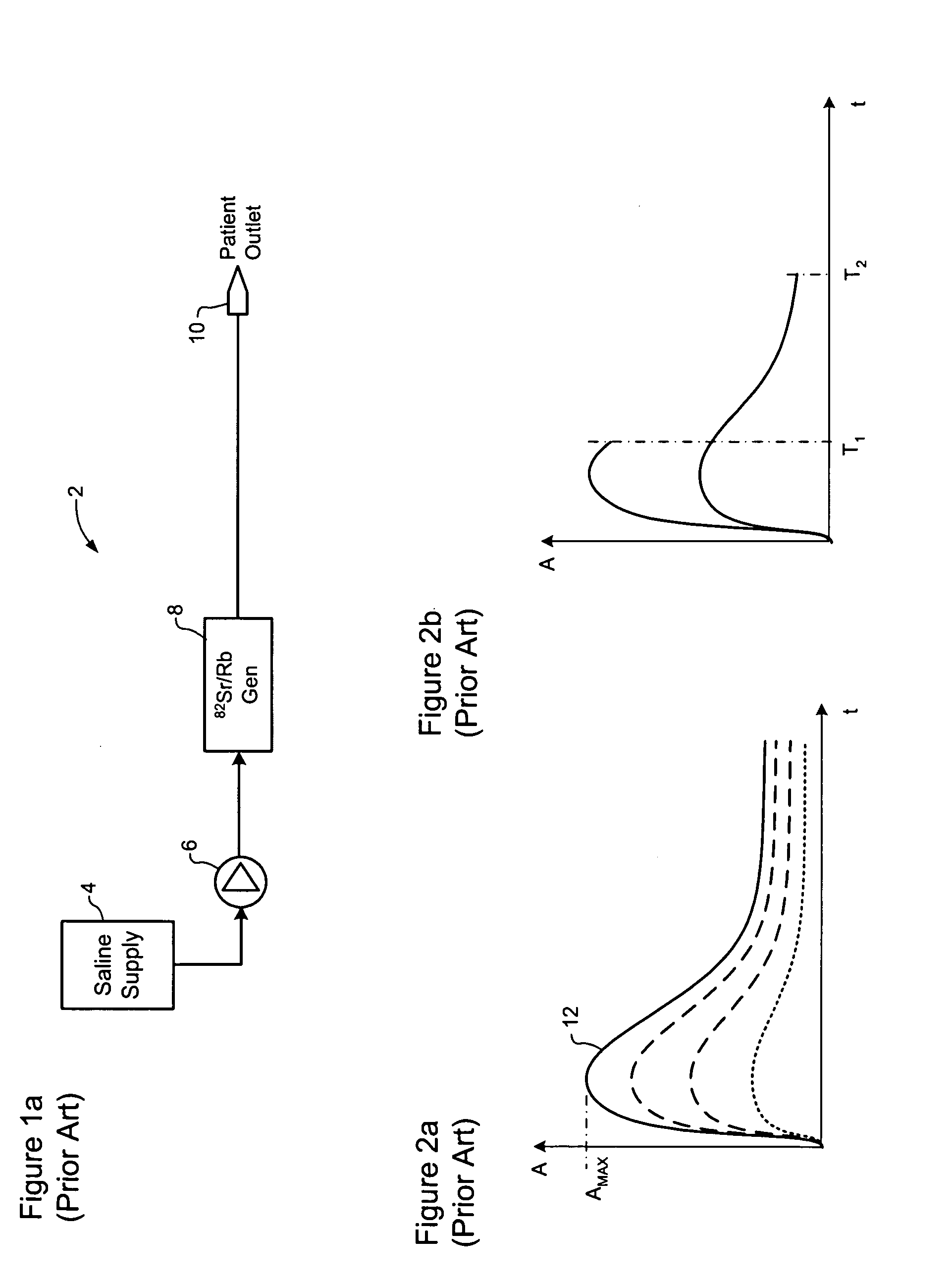
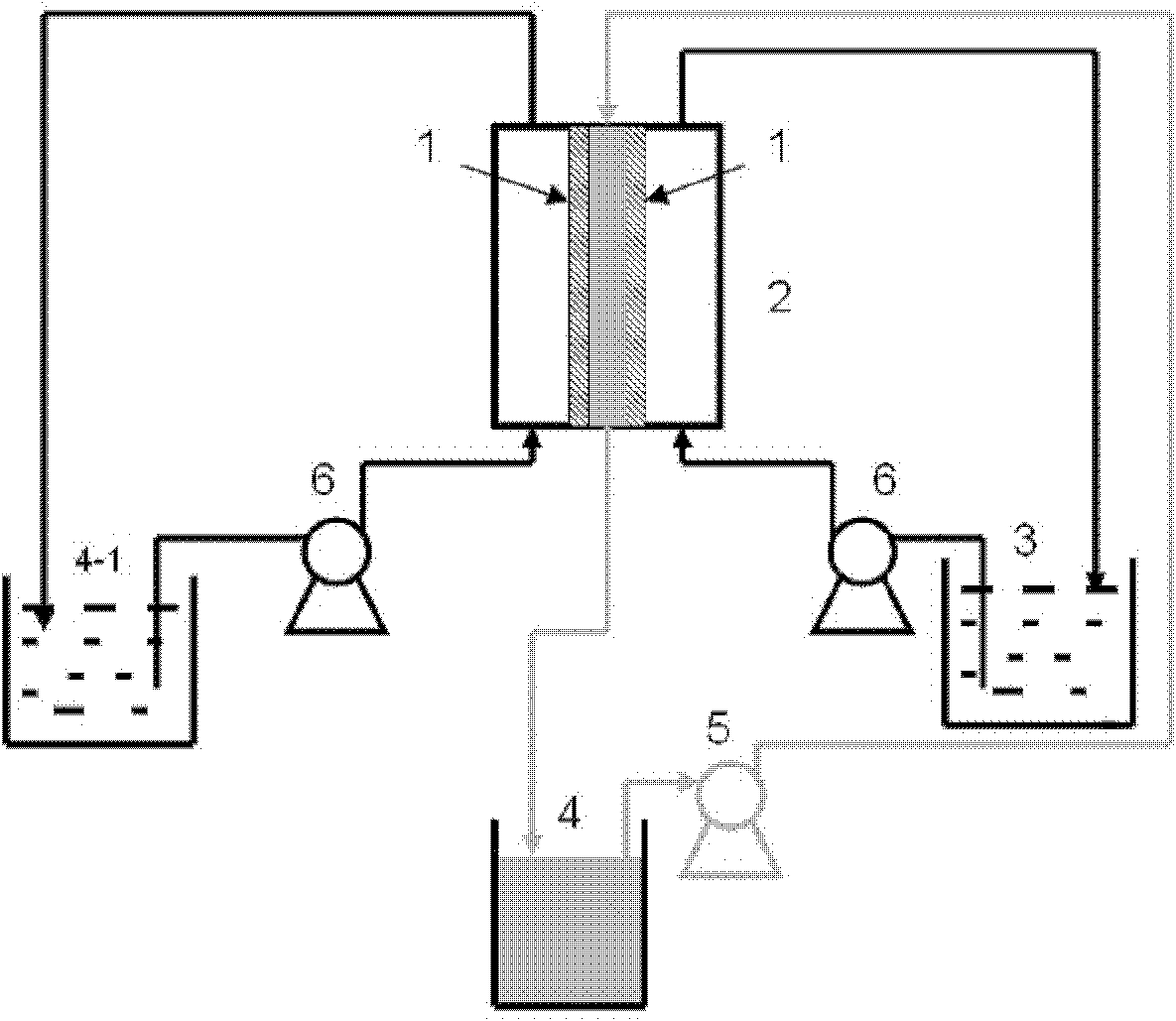
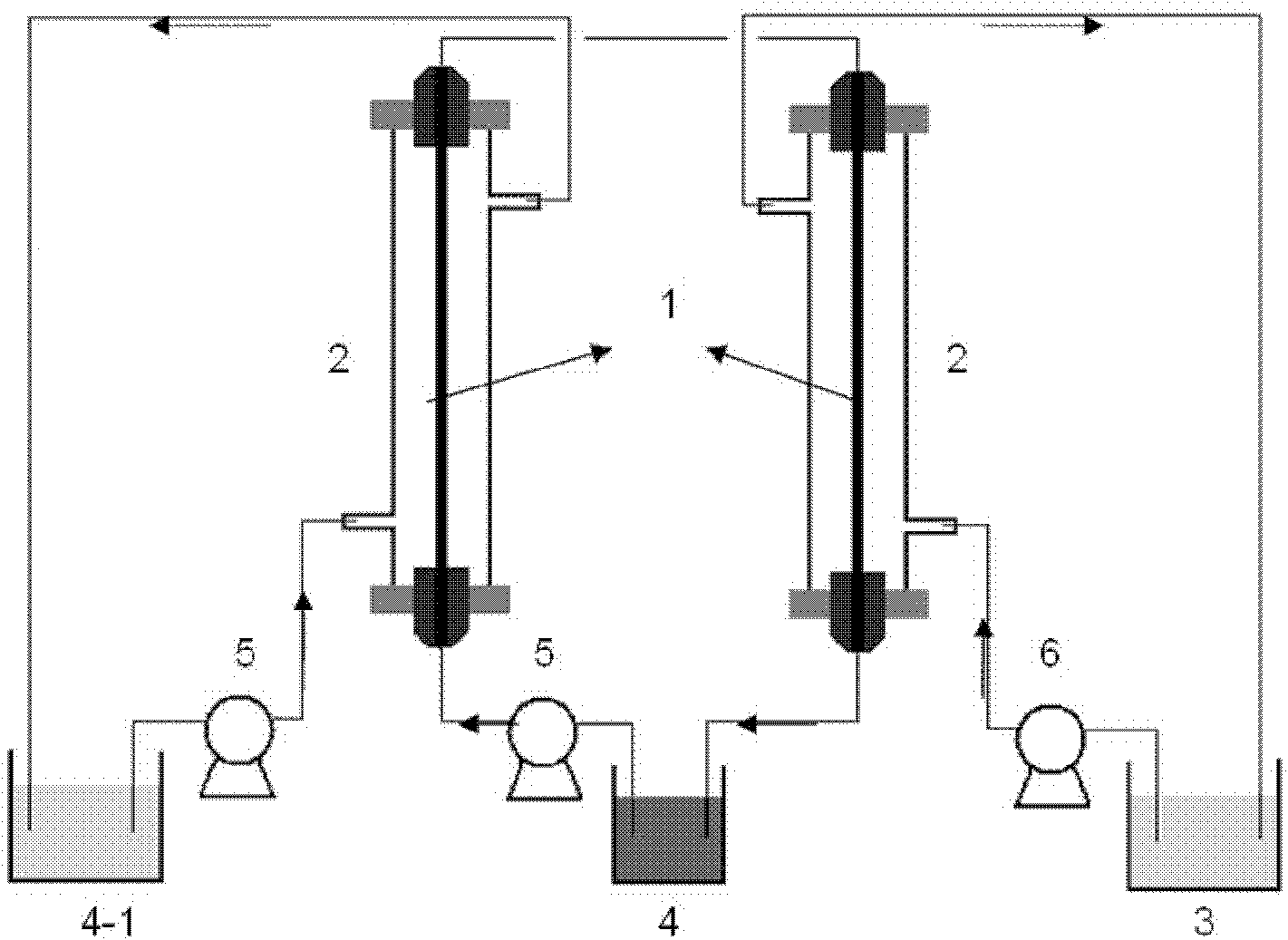
Method and apparatus for seismic data acquisition
InactiveUS20050052951A1Avoid entrapmentMinimize the possibilityTransducer detailsSeismic signal receiversOcean bottomRubidium
A marine seismic exploration method and system comprised of continuous recording, self-contained ocean bottom pods characterized by low profile casings. An external bumper is provided to promote ocean bottom coupling and prevent fishing net entrapment. Pods are tethered together with flexible, non-rigid, non-conducting cable used to control pod deployment. Pods are deployed and retrieved from a boat deck configured to have a storage system and a handling system to attach pods to cable on-the-fly. The storage system is a juke box configuration of slots wherein individual pods are randomly stored in the slots to permit data extraction, charging, testing and synchronizing without opening the pods. A pod may include an inertial navigation system to determine ocean floor location and a rubidium clock for timing. The system includes mathematical gimballing. The cable may include shear couplings designed to automatically shear apart if a certain level of cable tension is reached.
Owner:MAGSEIS FF LLC
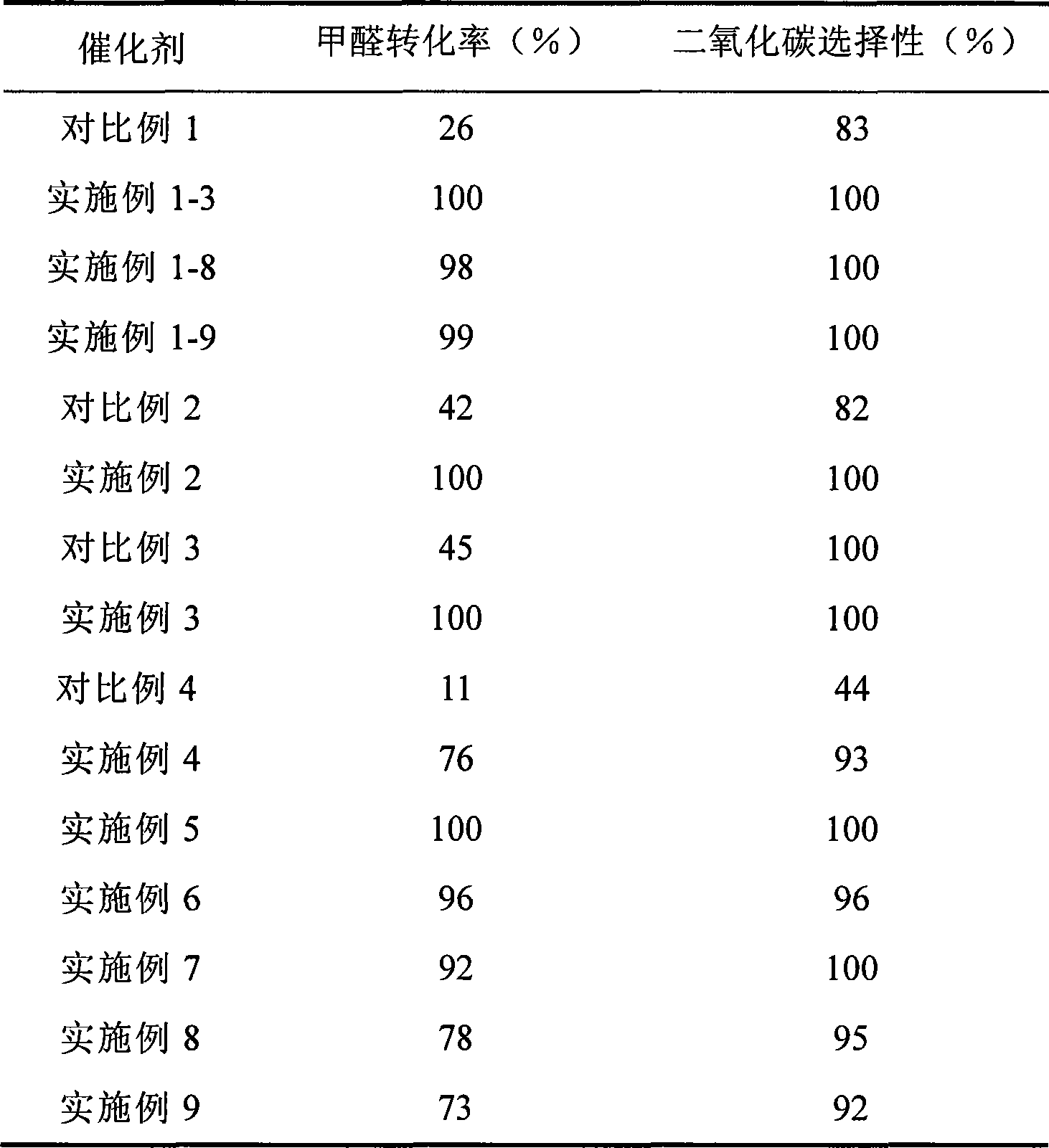
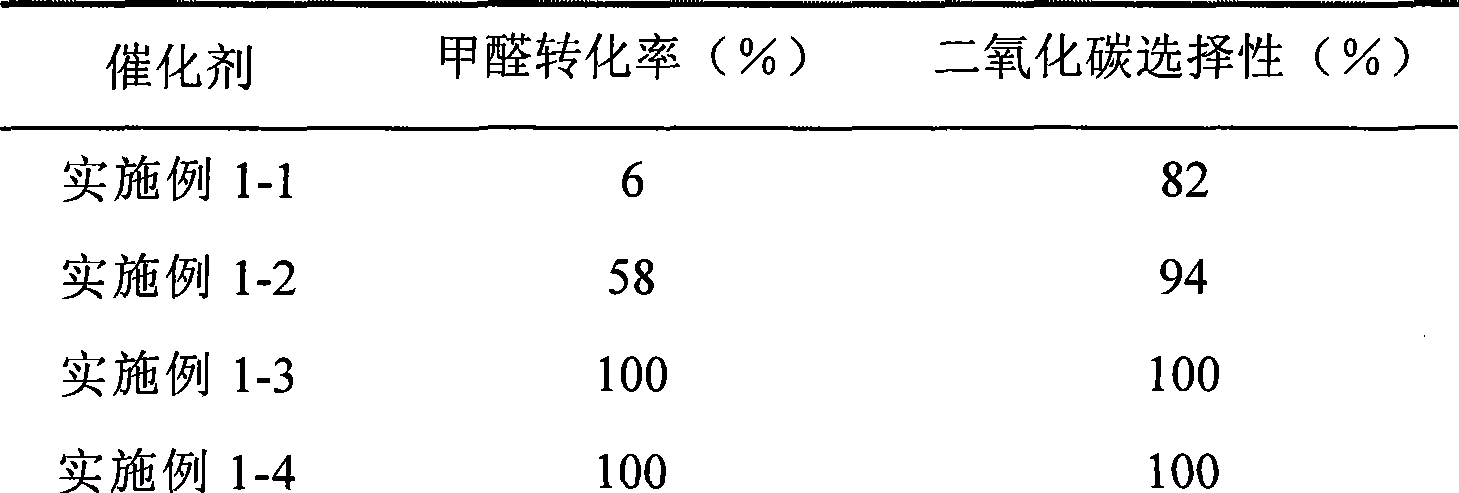
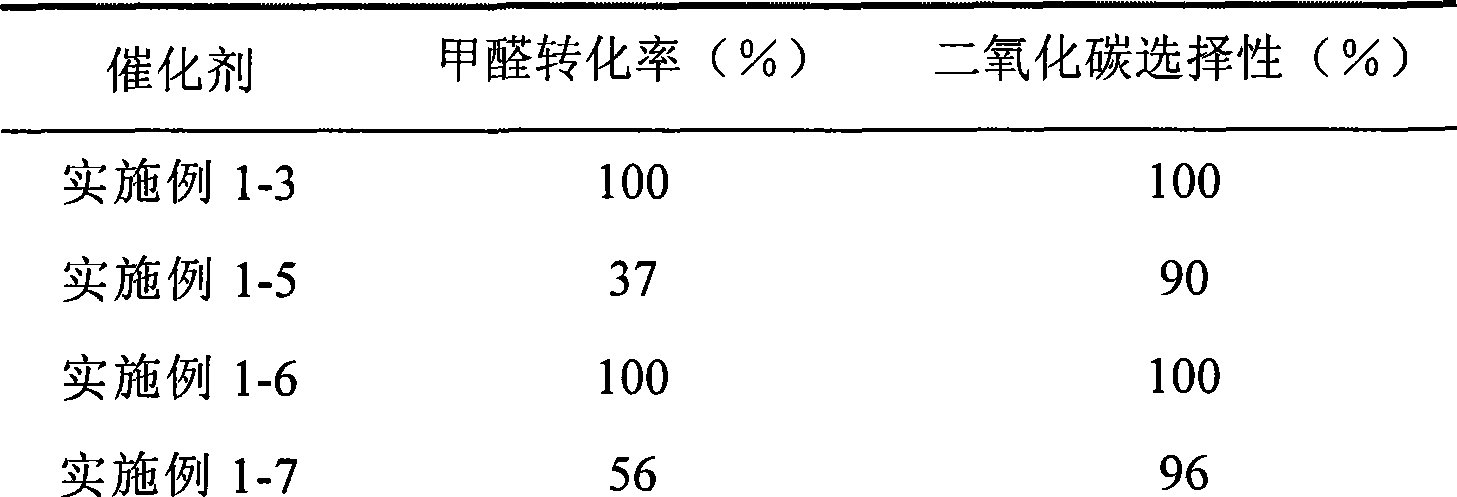
Catalyst for complete oxidation of formaldehyde at room temperature
ActiveCN101380574AEasy to makeEasy to operateDeodrantsMetal/metal-oxides/metal-hydroxide catalystsPorous carbonPt element
The invention provides a high selectivity catalyst used for catalyzing and completely oxidizing formaldehyde with low concentration at room temperature. The catalyst can catalyze formaldehyde completely so as to lead the formaldehyde to be converted into carbon dioxide and water at room temperature. In addition, the conversion rate of formaldehyde remains 100% within a long period of time, without complex auxiliary facilities such as light source, a heating oven and the like, and external conditions. The catalyst comprises three parts which are inorganic oxide carrier, noble metal component and auxiliary ingredient. Porous inorganic oxide carrier is one of cerium dioxide, zirconium dioxide, titanium dioxide, aluminium sesquioxide, tin dioxide, silicon dioxide, lanthanum sesquioxide, magnesium oxide and zinc oxide or the mixture thereof or composite oxide thereof, zeolite, sepiolite and porous carbon materials. The noble metal component of the catalyst is at least one of platinum, rhodium, palladium, gold and silver. The auxiliary ingredient is at least one of the alkali metals of lithium, sodium, kalium, rubidium and cesium. The loading of the noble metal component used in the catalyst of the invention is 0.1 to 10% according to weight converter of metal elements and the selective preference is 0.3 to 2%. The loading of the auxiliary ingredient is 0.2 to 30% according to weight converter of metal elements and the selective preference is 1 to 10%. When the loading of the auxiliary ingredient is lower than 0.2% or higher than 30%, the activity of the catalyst for catalyzing and oxidizing formaldehyde at room temperature is decreased remarkably.
Owner:广东顺德中科鸿图环境材料有限公司
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
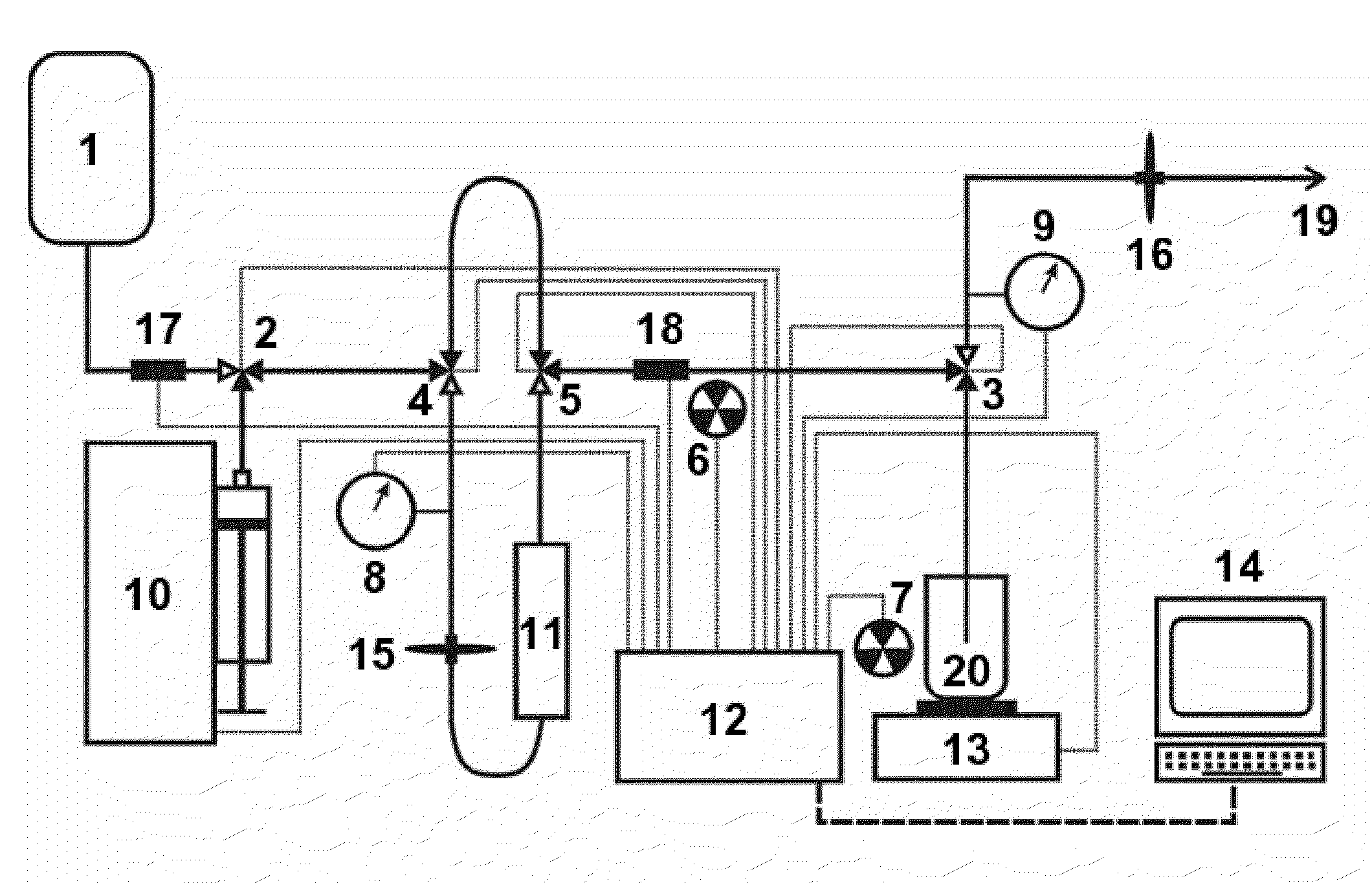
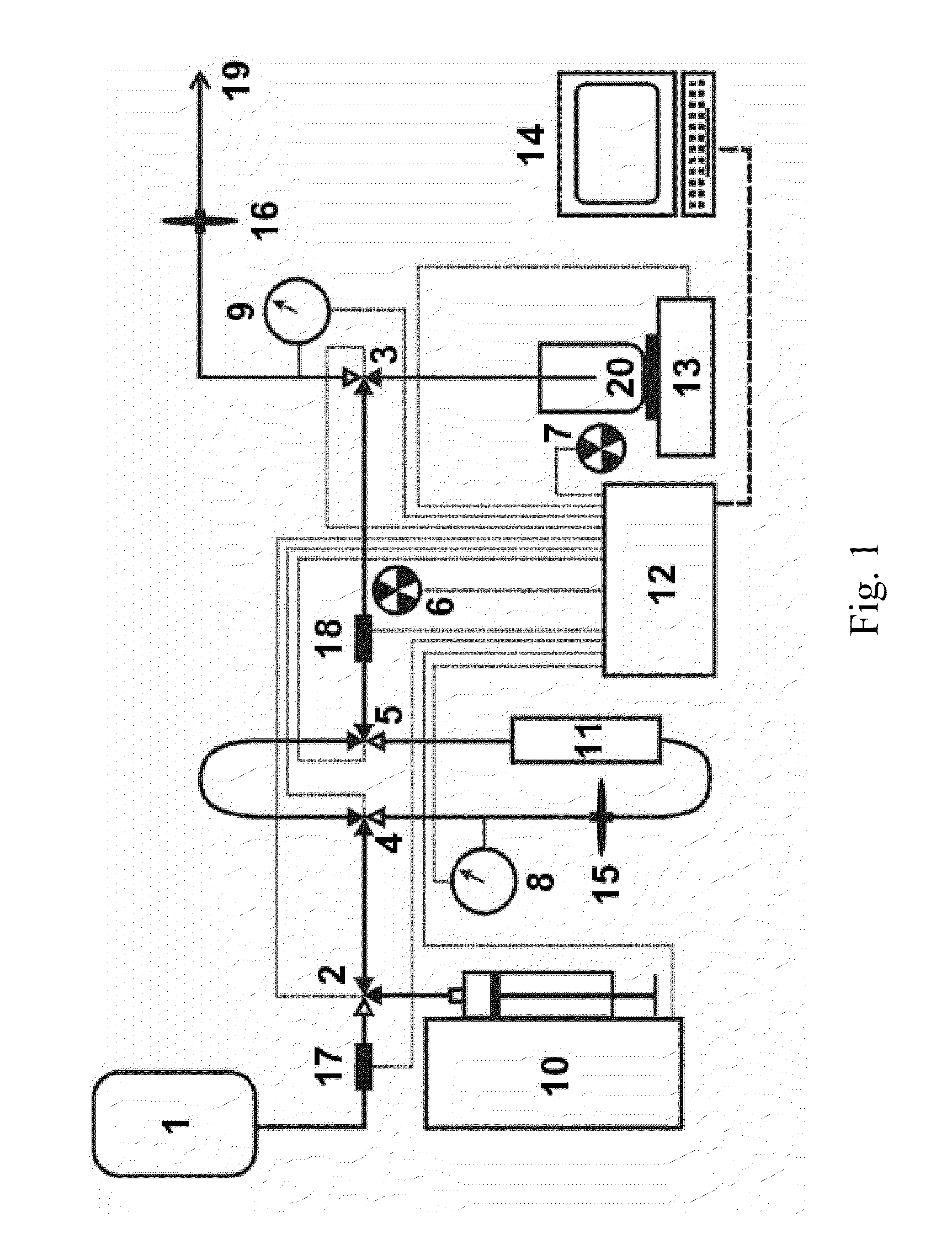
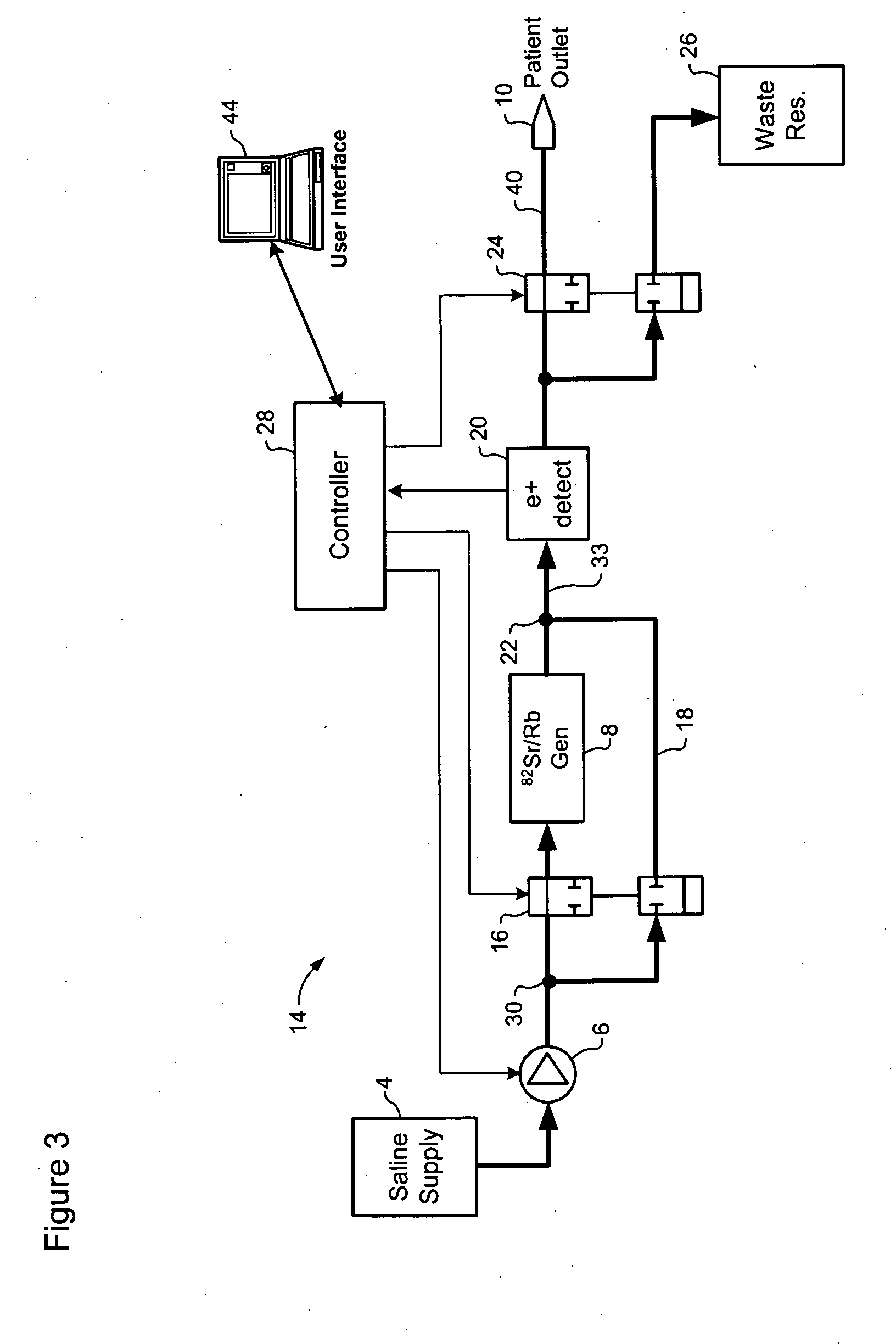
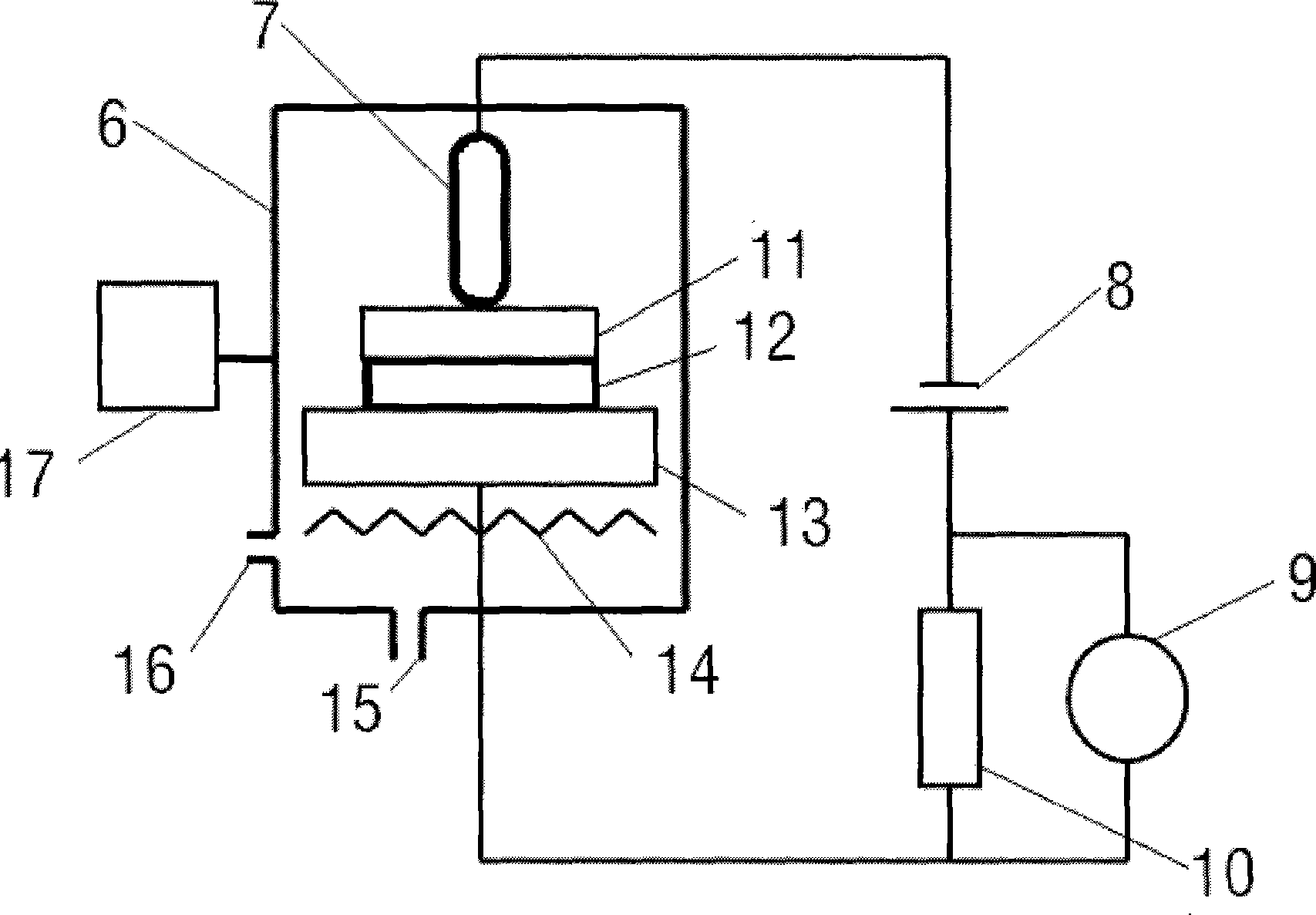
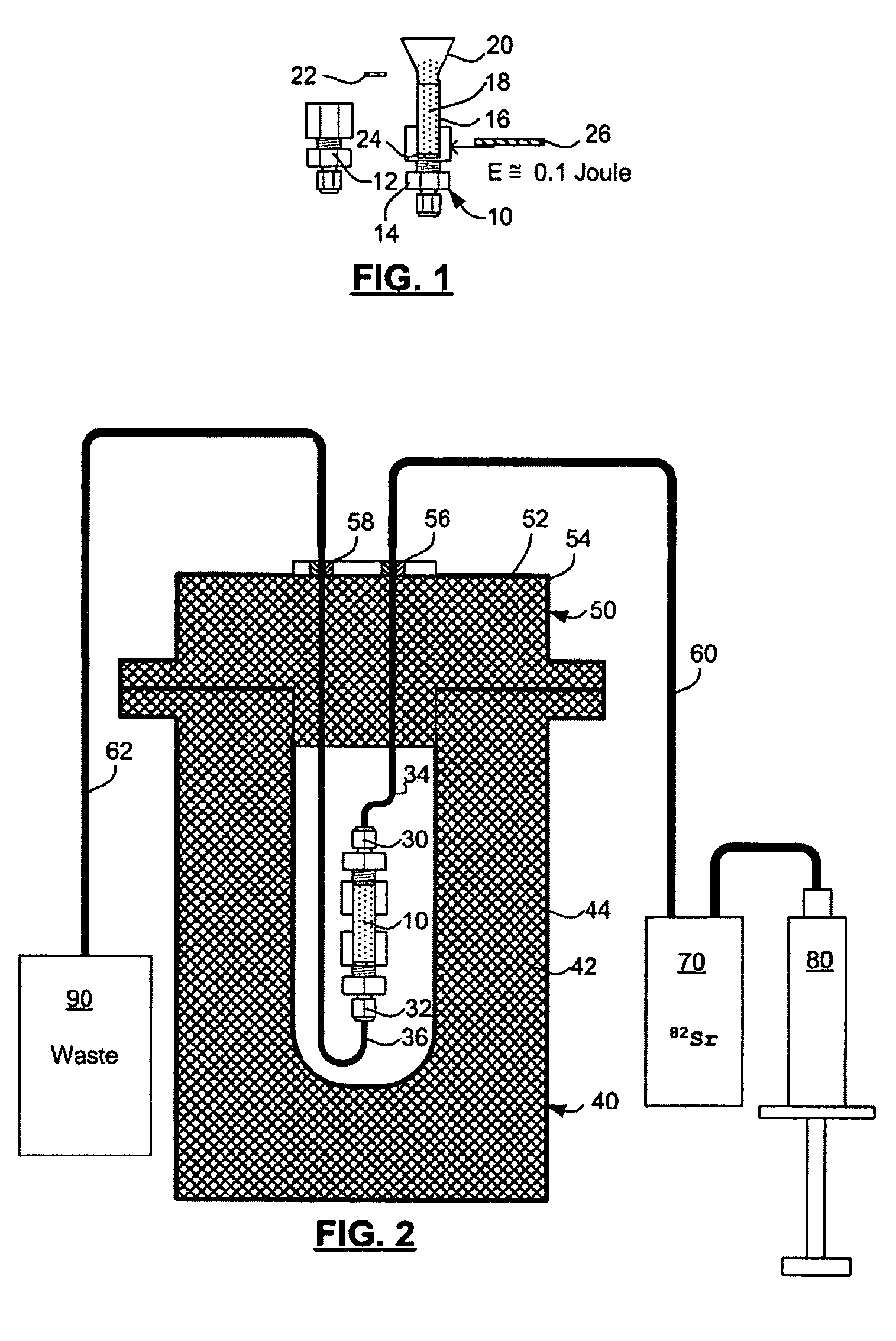
Automated strontium-rubidium infusion system
InactiveUS20090312635A1Elimination of aforesaid shortcomingImprove efficiencySolid waste disposalProtective foundationTransport systemAutomatic control
This invention relates to medical engineering, in particular, to means of automation of the process of generating a diagnostic solution from a radionuclide strontium-rubidium generator and performing of remote controlled infusion, with automatic control over the key characteristics of the process. The automated strontium-rubidium infusion system comprises a container with eluent, a strontium-rubidium generator with a filter and a pressure sensor at the input, an eluate infusion unit, which are connected by means of a transporting system provided with pipes and two three-way valves, radioactivity measuring means and a control and operating unit. Here, an eluent container is connected to a syringe pump via the first and second ports of the first three-way valve, the first port of the second three-way valve is connected with pipes via the second filter to the eluate infusion unit, and the second port is connected to a waste receptacle. The system additionally comprises the third and fourth three-way valves, the first and second air bubbles detectors are connected to the control and operating unit connected with a computer, where the third three-way valve is connected with its first and second ports via pipes to the third port of the first three-way valve and the input of the strontium-rubidium generator, respectively. The generator output is connected to the first port of the fourth three-way valve, where the third port of the third valve and the second port of the fourth valve are connected with a pipe, the first air bubbles detector is placed on the pipeline between the eluent container and the first port of the first valve, and the second air bubbles detector is placed on the pipeline between the third ports of the fourth and second valves.
Owner:OBSHCHESTVO S OGRANICHENNOY OTVETABTVENNOSTIU NAUCHNO PROIZVODSTVENNAYA FA POZITOM PRO
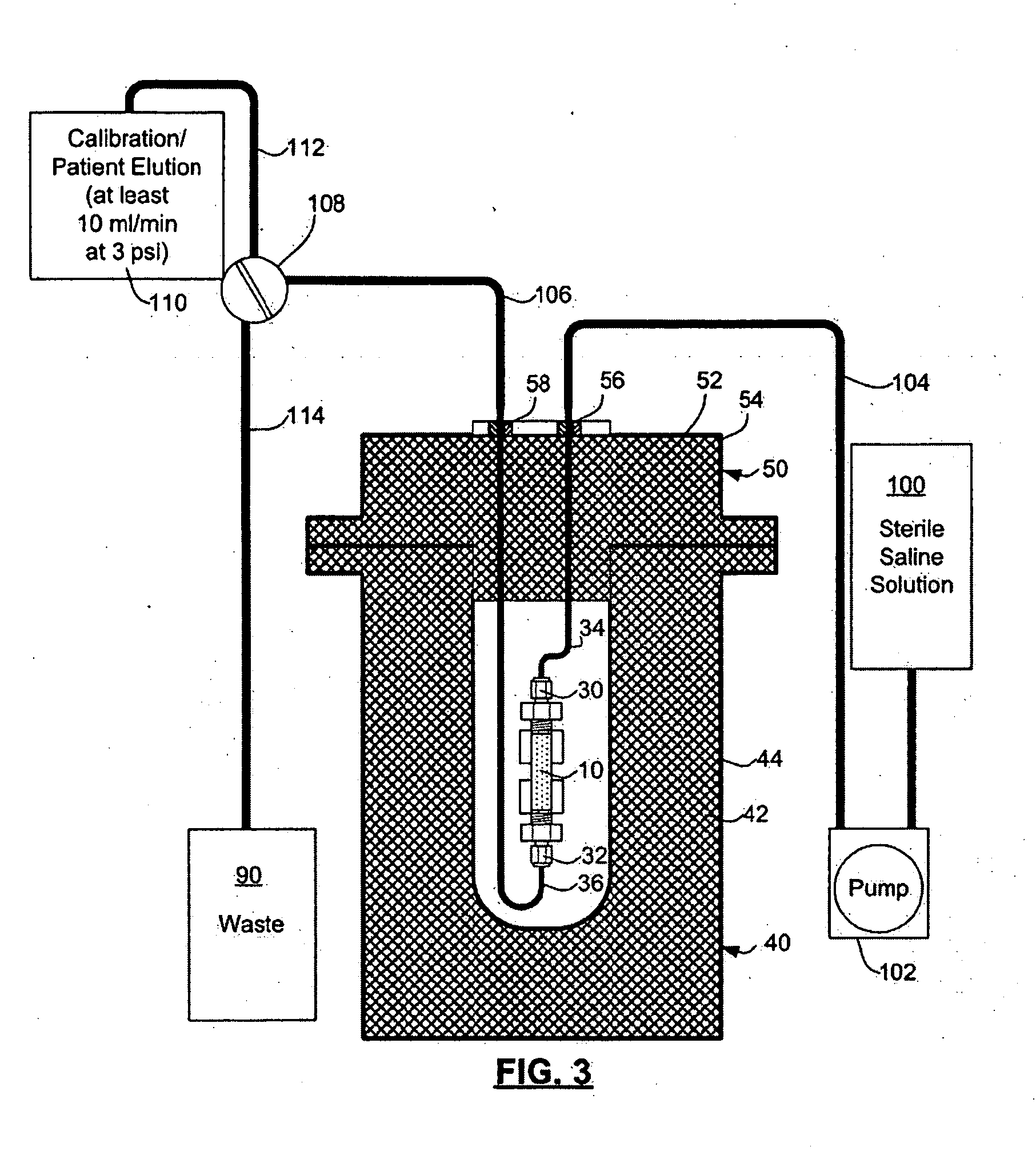
Rubidium elution system control
A method of controlling an 82Sr / 82Rb elution system having a generator valve for proportioning a flow of saline solution between an 82Sr / 82Rb generator and a bypass line coupled to an outlet of the generator such that saline solution traversing the bypass line will merge with eluted saline solution emerging from the generator to provide an active saline solution. During each elution run, a plurality of successive concentration parameter values are obtained at predetermined intervals. Each concentration parameter value is indicative of a respective instantaneous activity concentration of the active saline solution. Respective error values between each concentration parameter value and a target activity concentration value of the elution run are computed. Error data based on a plurality of the computed error values is accumulated. Between successive elution runs, at least one performance parameter of the elution system is adjusted based on the accumulated error data.
Owner:OTTAWA HEART INST RES
Silicon-containing film-forming composition, silicon-containing film, silicon-containing film-bearing substrate, and patterning method
ActiveUS20070238300A1Sufficient etching resistanceImprove accuracyPhotosensitive materialsSemiconductor/solid-state device manufacturingResistAmmonium compounds
A silicon-containing film is formed from a heat curable composition comprising (A) a silicon-containing compound obtained by effecting hydrolytic condensation of a hydrolyzable silicon compound in the presence of an acid catalyst, and substantially removing the acid catalyst from the reaction mixture, (B) a hydroxide or organic acid salt of lithium, sodium, potassium, rubidium or cesium, or a sulfonium, iodonium or ammonium compound, (C) an organic acid, and (D) an organic solvent. The silicon-containing film allows an overlying photoresist film to be patterned effectively. The composition is effective in minimizing the occurrence of pattern defects after lithography and is shelf stable.
Owner:SHIN ETSU CHEM IND CO LTD
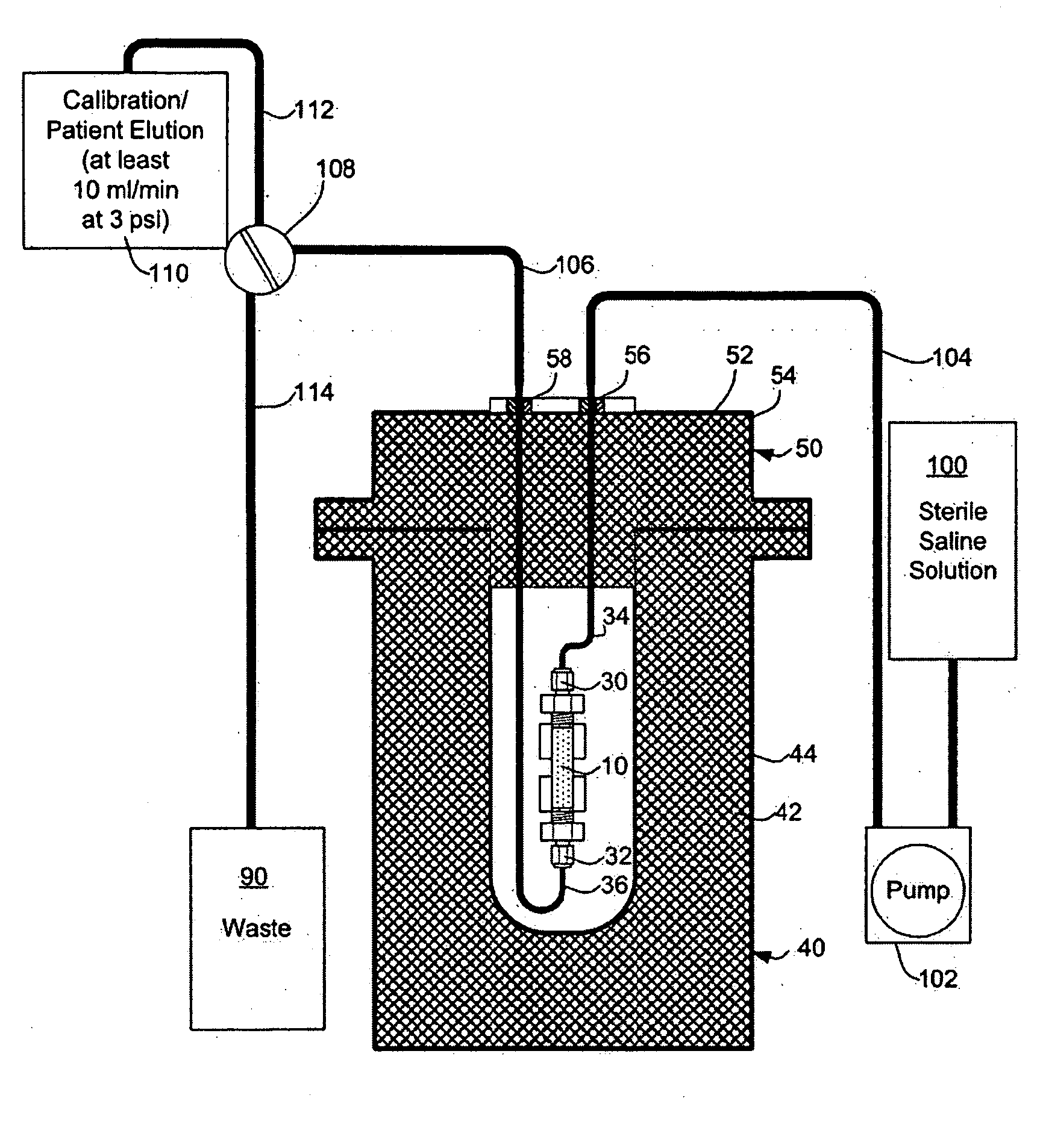
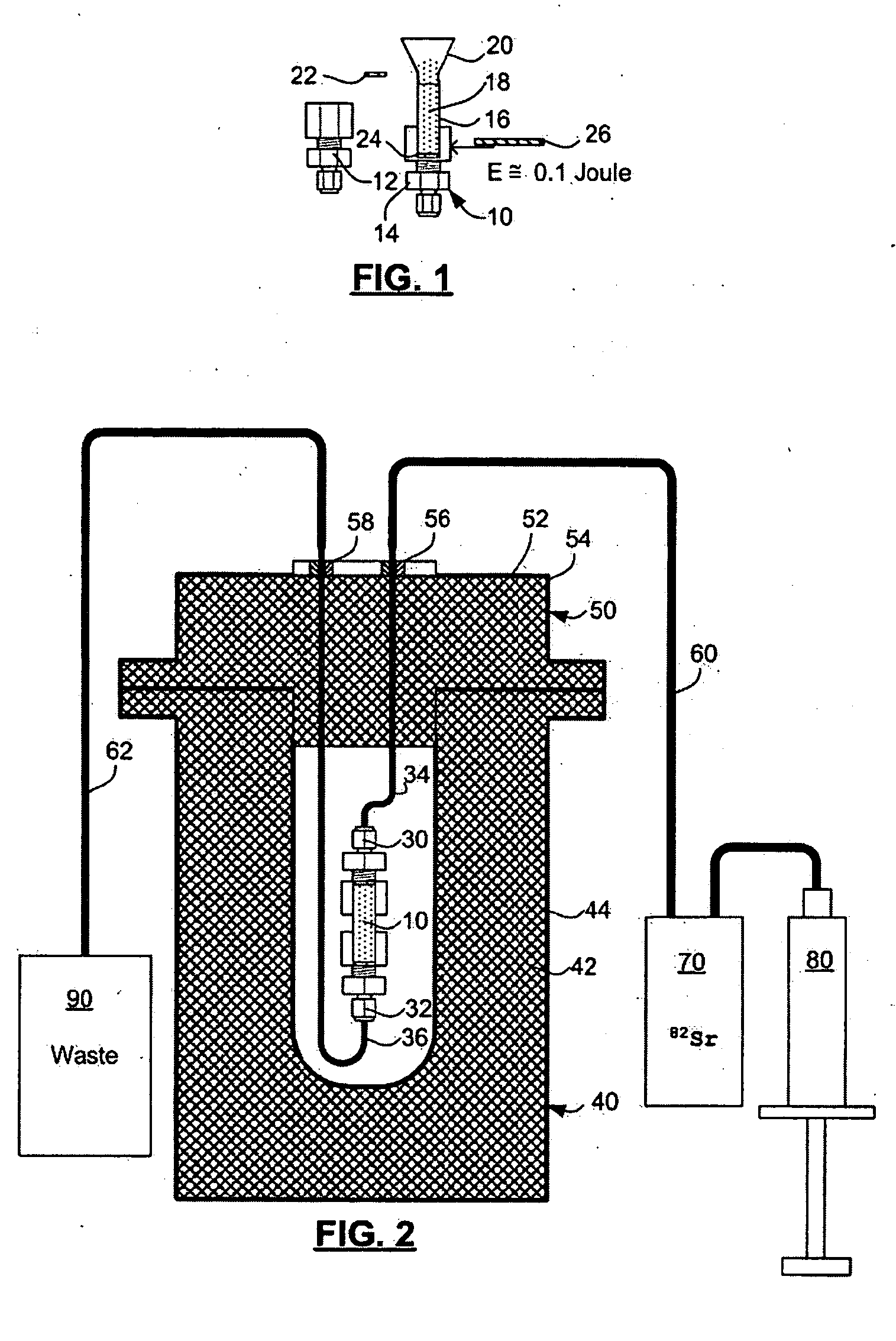
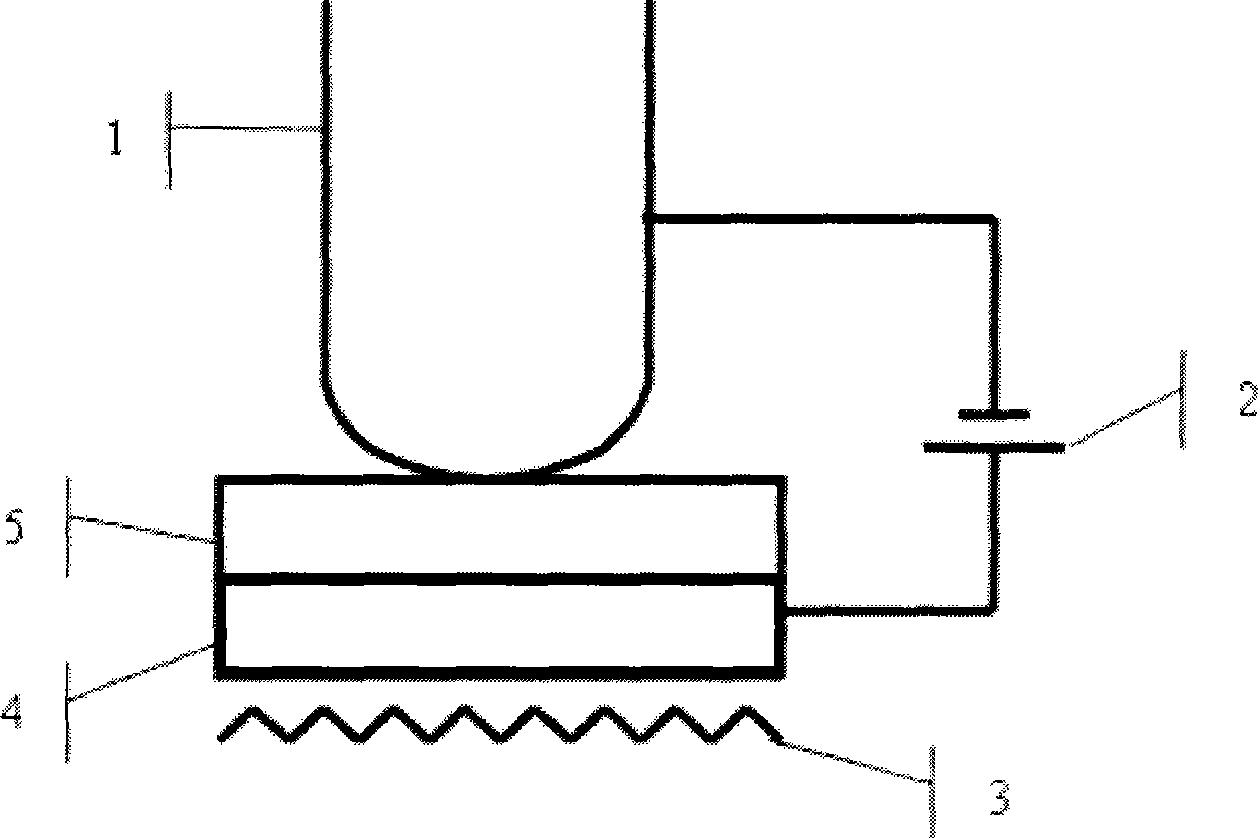
Rubidium generator for cardiac perfusion imaging and method of making and maintaining same
ActiveUS20070140958A1Low pressure elutionFacilitates precision flow controlIn-vivo radioactive preparationsConversion outside reactor/acceleratorsFractographyRubidium
An 82Sr / 82Rb generator column is made using a fluid impervious cylindrical container having a cover for closing the container in a fluid tight seal, and further having an inlet for connection of a conduit for delivering a fluid into the container and an outlet for connection of a conduit for conducting the fluid from the container. An ion exchange material fills the container, the ion exchange material being compacted within the container to a density that permits, the ion exchange material to be eluted at a rate of at least 5 ml / min at a fluid pressure of 1.5 pounds per square inch (10 kPa). The generator column can be repeatedly recharged with 82Sr. The generator column is compatible with either three-dimensional or two-dimensional positron emission tomography systems.
Owner:OTTAWA HEART INST RES
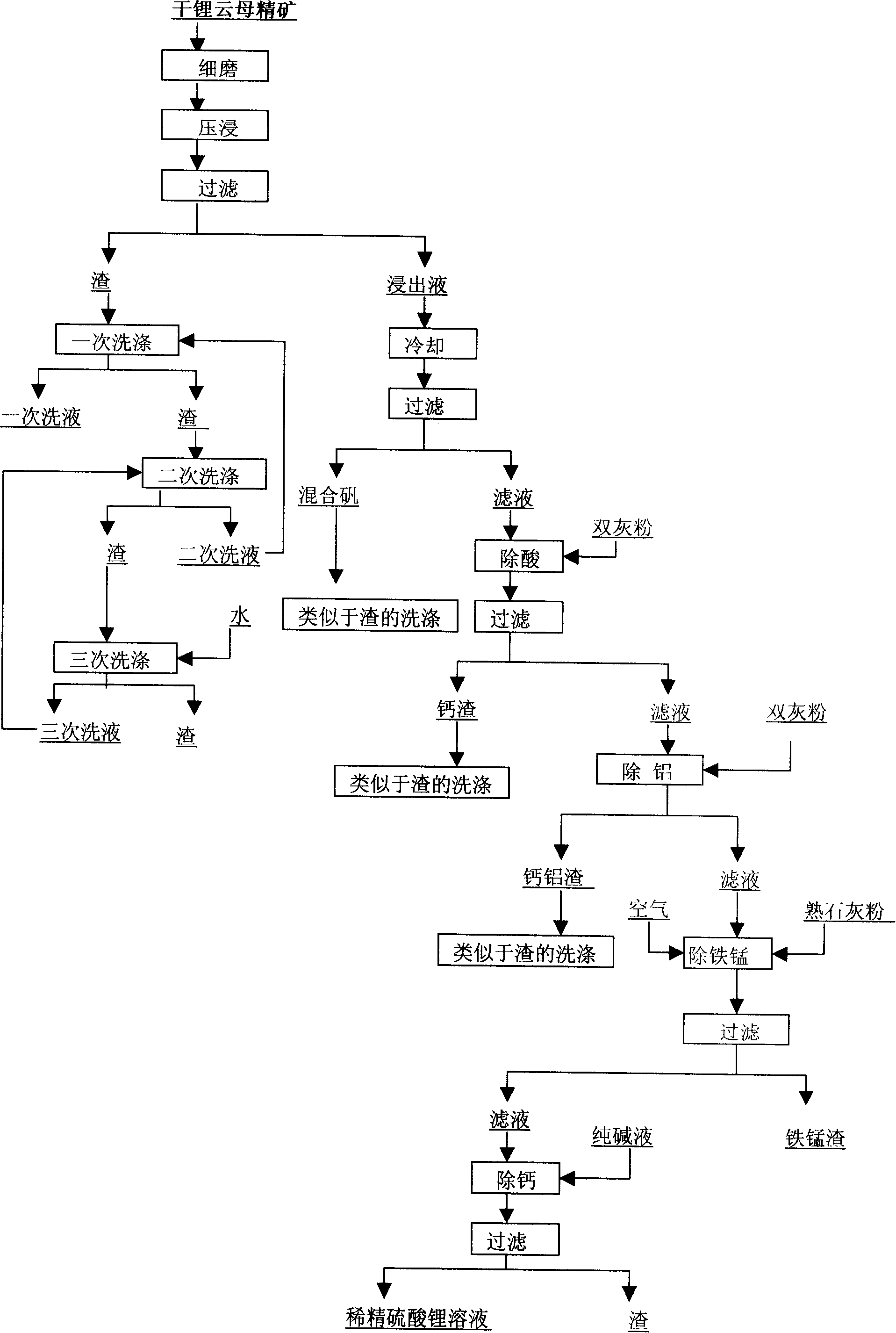
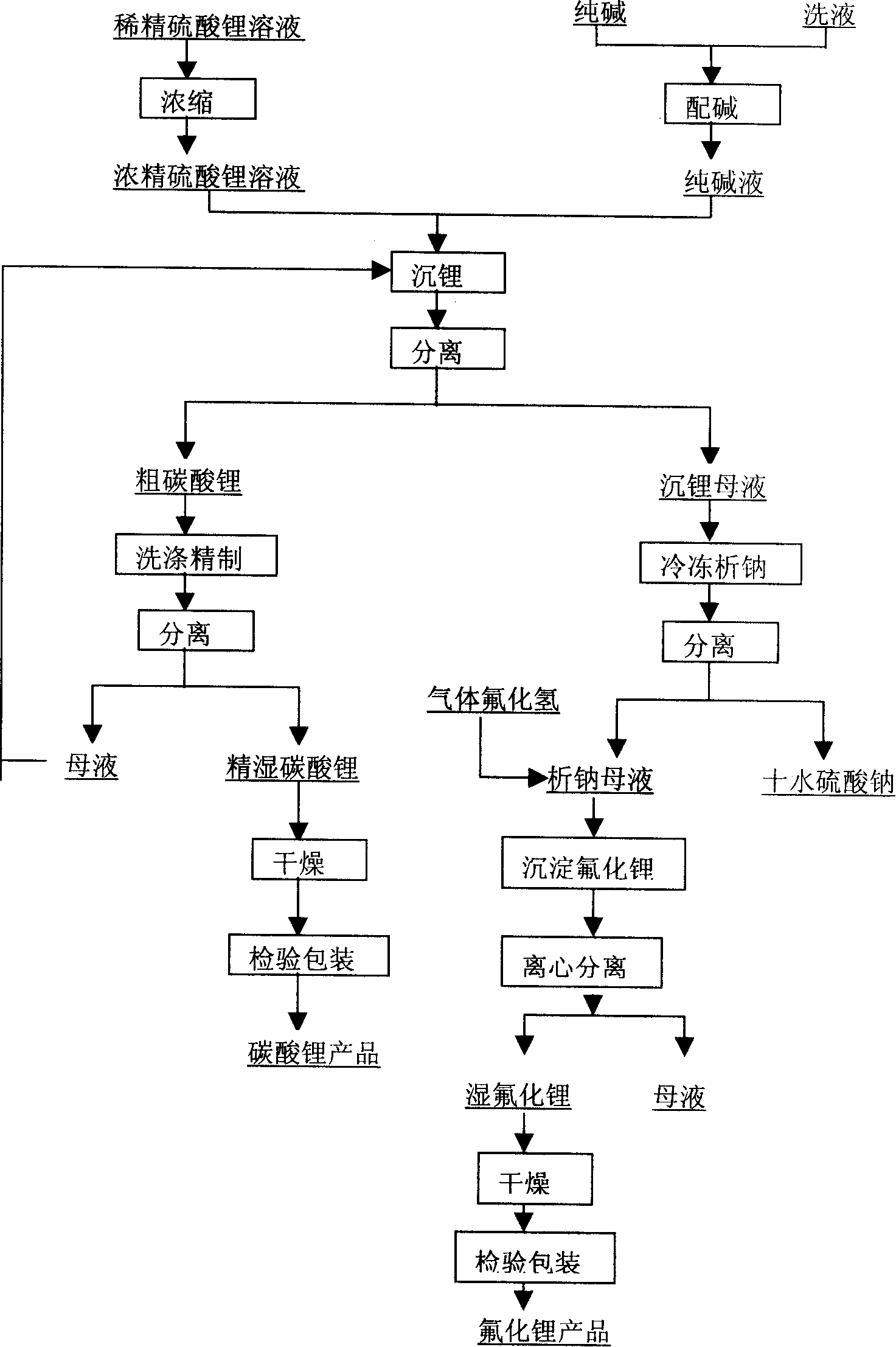
Method for producing refined lithium sulfate solution used in lepidolite lithium-extracting technique by sulfuric acid process
Provided is a process for producing refined lithium sulfate solution of lepidolite lithium extracting technology with sulfuric acid process, which takes lepidolite clean ore as raw material and sequentially includes the following steps, including leaching, alum cooling and decanting, acid removing, aluminum removing, decontaminating and deliming, thereby achieving refined lithium sulfate solution. The alum cooling and decanting process of the invention can precipitate kalium, rubidium and caesium in alum form, thereby the separation of lithium and kalium, rubidium and caesium is easily achieved, and the achieved alum dregs of kalium, rubidium and caesium are blend alum with high purity, which creates perfect condition for comprehensive utilization and simultaneously reduces the burdens of the separation of lithium and aluminum. The aluminum removing process can easily achieve the separation of lithium and aluminum. The process of the invention has the advantages that the energy consumption is relatively low, and the lithium yield is relatively high, most of the residues can be used and the process is favorable for comprehensive utilization. The invention further provides a process for producing lithium carbonate and lithium fluoride with the achieved refined lithium sulfate solution.
Owner:GANFENG LITHIUM CO LTD
Aromatization of alkanes using a germanium-zeolite catalyst
ActiveUS20080255398A1Molecular sieve catalystsRefining to change hydrocarbon structural skeletonAlkaneAlkaline earth metal
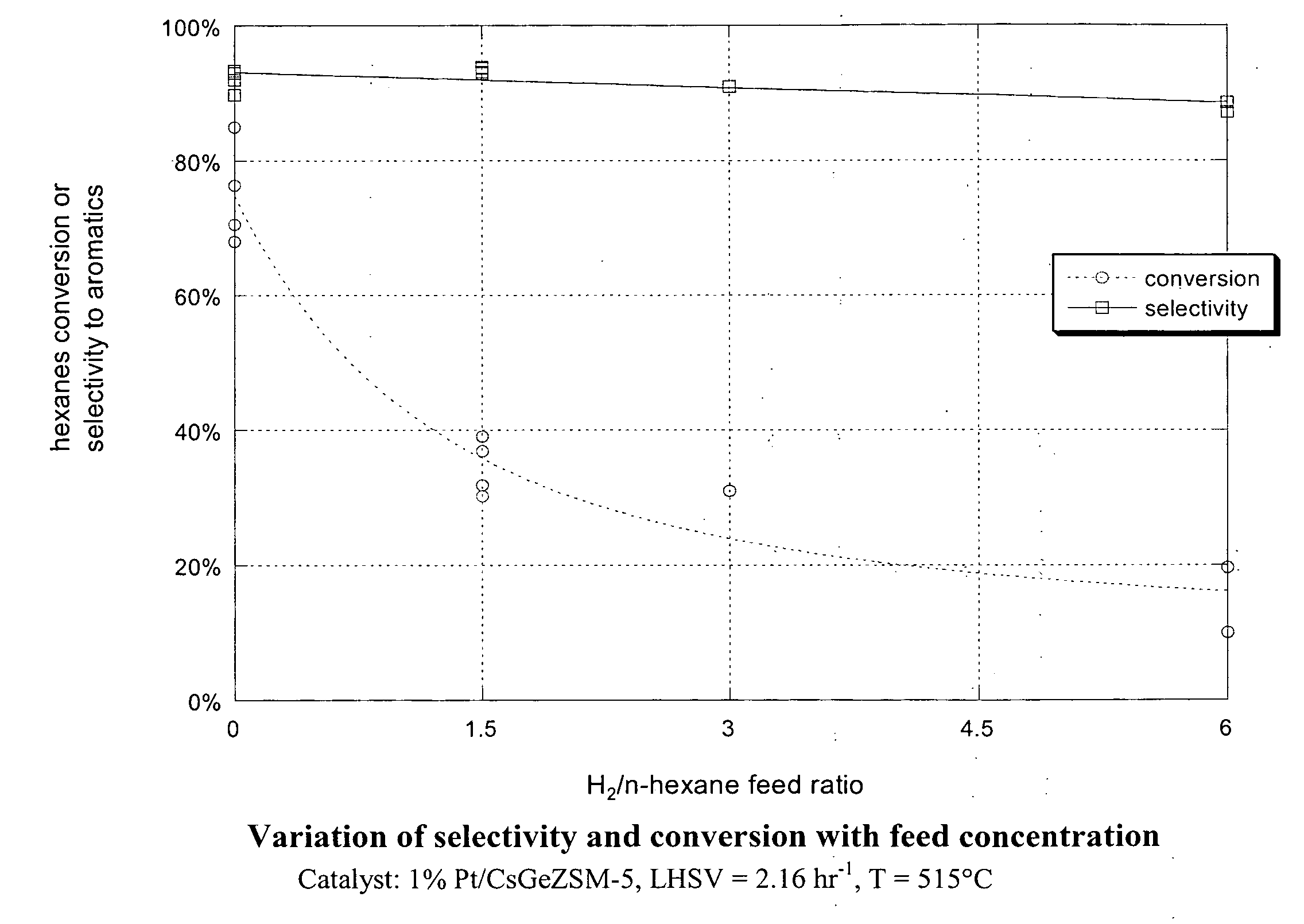
This invention relates to a process for the aromatization of C6 to C12 alkanes, such as hexane, heptane and octane, to aromatics, such as benzene, ethyl benzene, toluene and xylenes, with a germanium-containing zeolite catalyst. The catalyst is a non-acidic aluminum-silicon-germanium zeolite on which a noble metal, such as platinum, has been deposited. The zeolite structure may be of MFI, BEA, MOR, LTL or MTT. The zeolite is made non-acidic by being base-exchanged with an alkali metal or alkaline earth metal, such as cesium, potassium, sodium, rubidium, barium, calcium, magnesium and mixtures thereof, to reduce acidity. The catalyst is sulfur tolerant and may be pretreated with a sulfur compound, i.e., sulfided. The hydrocarbon feed may contain sulfur up to 1000 ppm. The present invention could be applicable to a feedstream which is predominantly paraffinic and / or low in naphthenes. Lowering the hydrogen to hydrocarbon ratio increases conversion and aromatics selectivity.
Owner:SAUDI BASIC IND CORP SA
Method for extracting alkali metal from salt lake brine and seawater through membrane extraction-back extraction
InactiveCN102312110AEfficient extractionEfficient enrichmentLiquid solutions solvent extractionRubidiumIon-exchange membranes
The invention discloses a method for extracting high-value alkali metal from salt lake brine or seawater through membrane extraction-back extraction. The method is implemented through continuous operation and comprises the following steps of: fixing an ion exchange blend membrane in a membrane component, allowing an organic solution containing an extracting agent to contact salt lake brine or seawater which contains alkali metal ions by a first ion exchange membrane, and allowing alkali metal ions to pass through the ion exchange membrane and be combined with the organic solution containing the extracting agent to obtain metal complex; then transmitting an organic solution of the metal complex to a second ion exchange membrane and allowing the organic solution of the metal complex to contact a back extraction solution by the second ion exchange membrane, and allowing the metal ions to pass through the ion exchange membrane to enter the back extraction solution; during membrane extraction-back extraction, and circulating feed liquid, the back extraction solution and the organic solution containing the extracting agent on one side of the first ion exchange membrane, on one side of the second ion exchange membrane and between the first and second ion exchange membranes; and performing back extraction until a certain concentration of the back extraction solution is reached, and separating lithium, rubidium or caesium precipitates to obtain the final product. The invention provides a high-efficiency, low-cost and feasible route for industrial production of alkali metal salts.
Owner:何涛 +1
Method and apparatus for seismic data acquisition
InactiveUS20060120216A1Avoid entrapmentMinimize the possibilitySeismic signal receiversSeismology for water-covered areasOcean bottomRubidium
A marine seismic exploration method and system comprised of continuous recording, self-contained ocean bottom pods characterized by low profile casings. An external bumper is provided to promote ocean bottom coupling and prevent fishing net entrapment. Pods are tethered together with flexible, non-rigid, non-conducting cable used to control pod deployment. Pods are deployed and retrieved from a boat deck configured to have a storage system and a handling system to attach pods to cable on-the-fly. The storage system is a juke box configuration of slots wherein individual pods are randomly stored in the slots to permit data extraction, charging, testing and synchronizing without opening the pods. A pod may include an inertial navigation system to determine ocean floor location and a rubidium clock for timing. The system includes mathematical gimballing. The cable may include shear couplings designed to automatically shear apart if a certain level of cable tension is reached.
Owner:MAGSEIS FF LLC
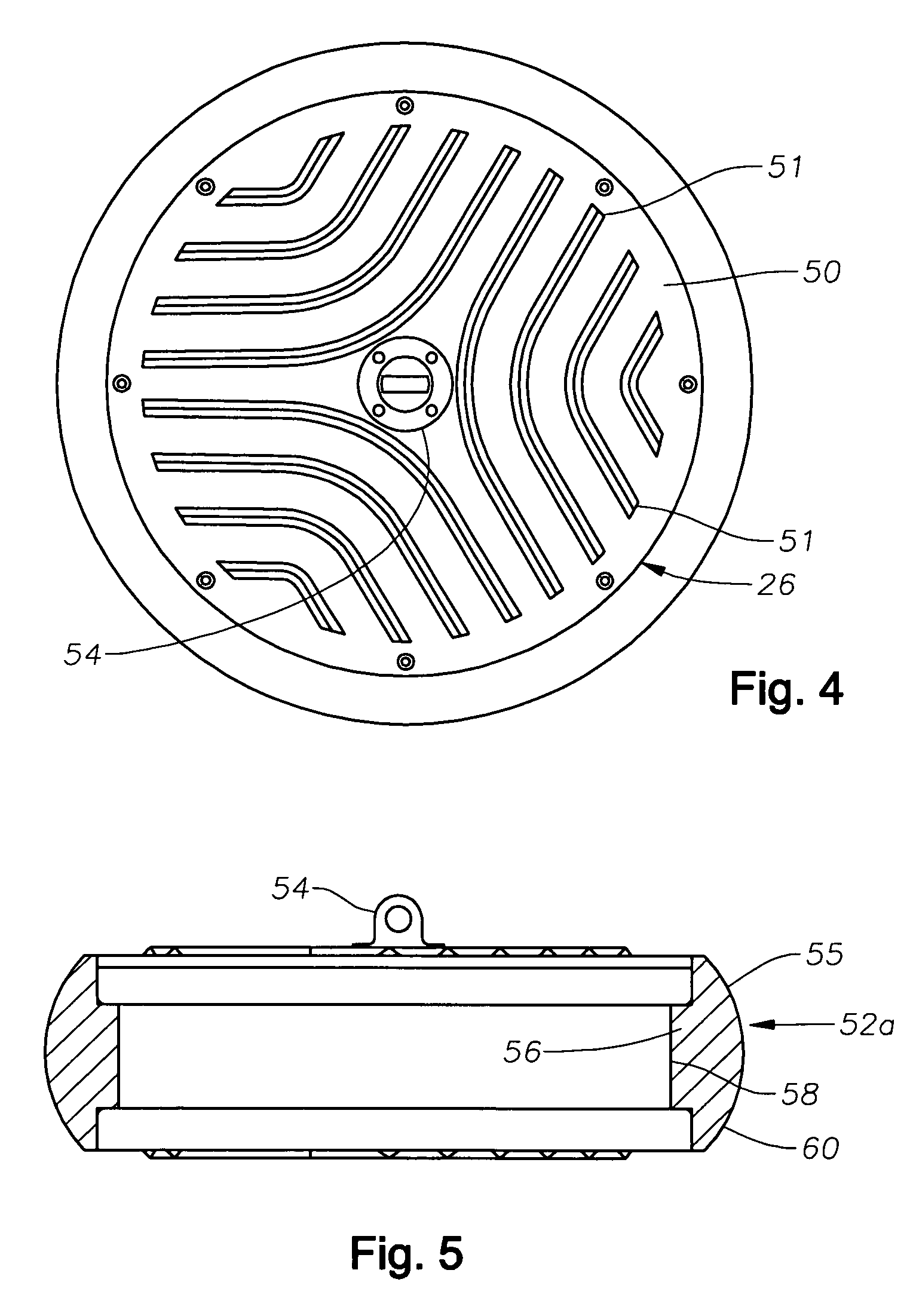
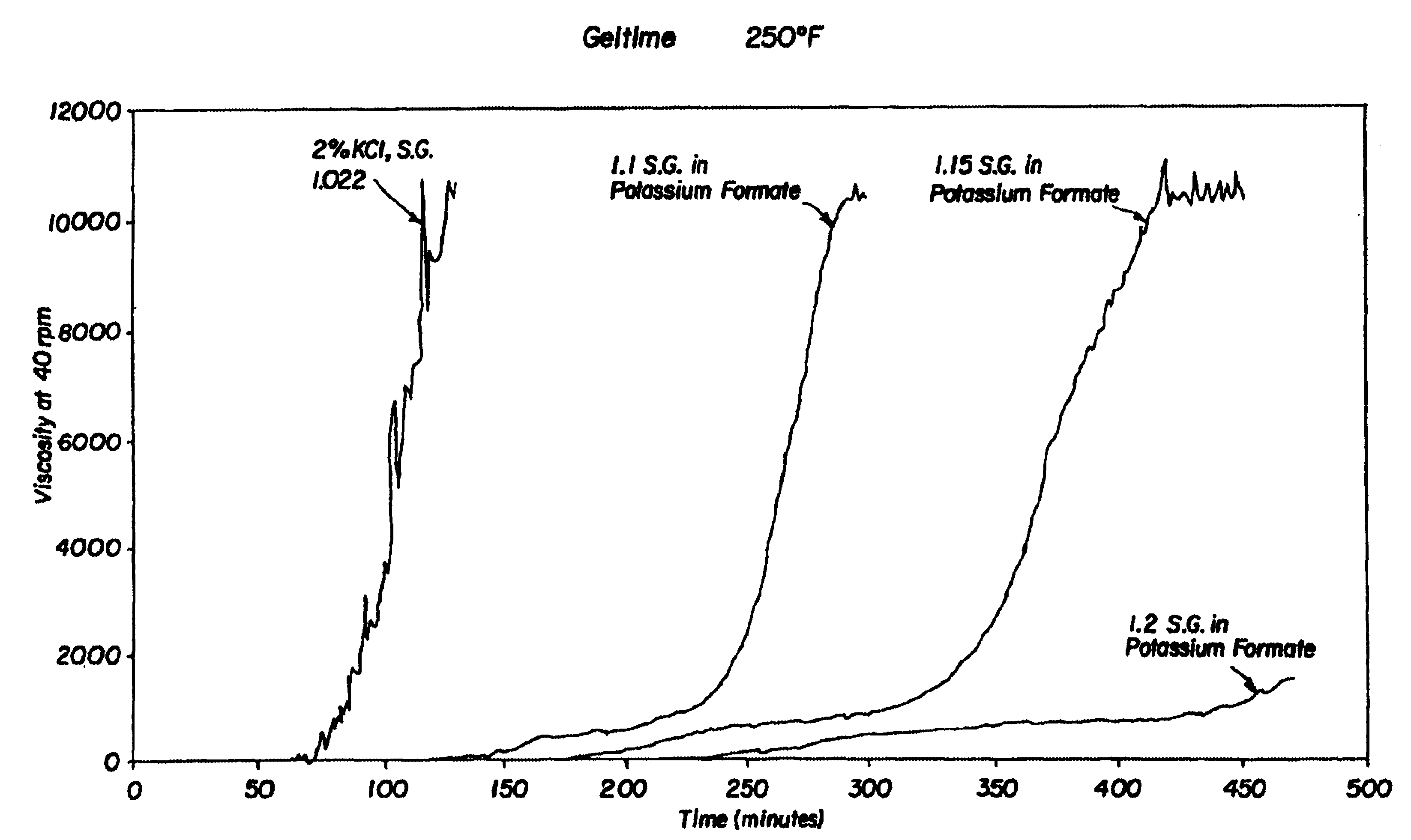
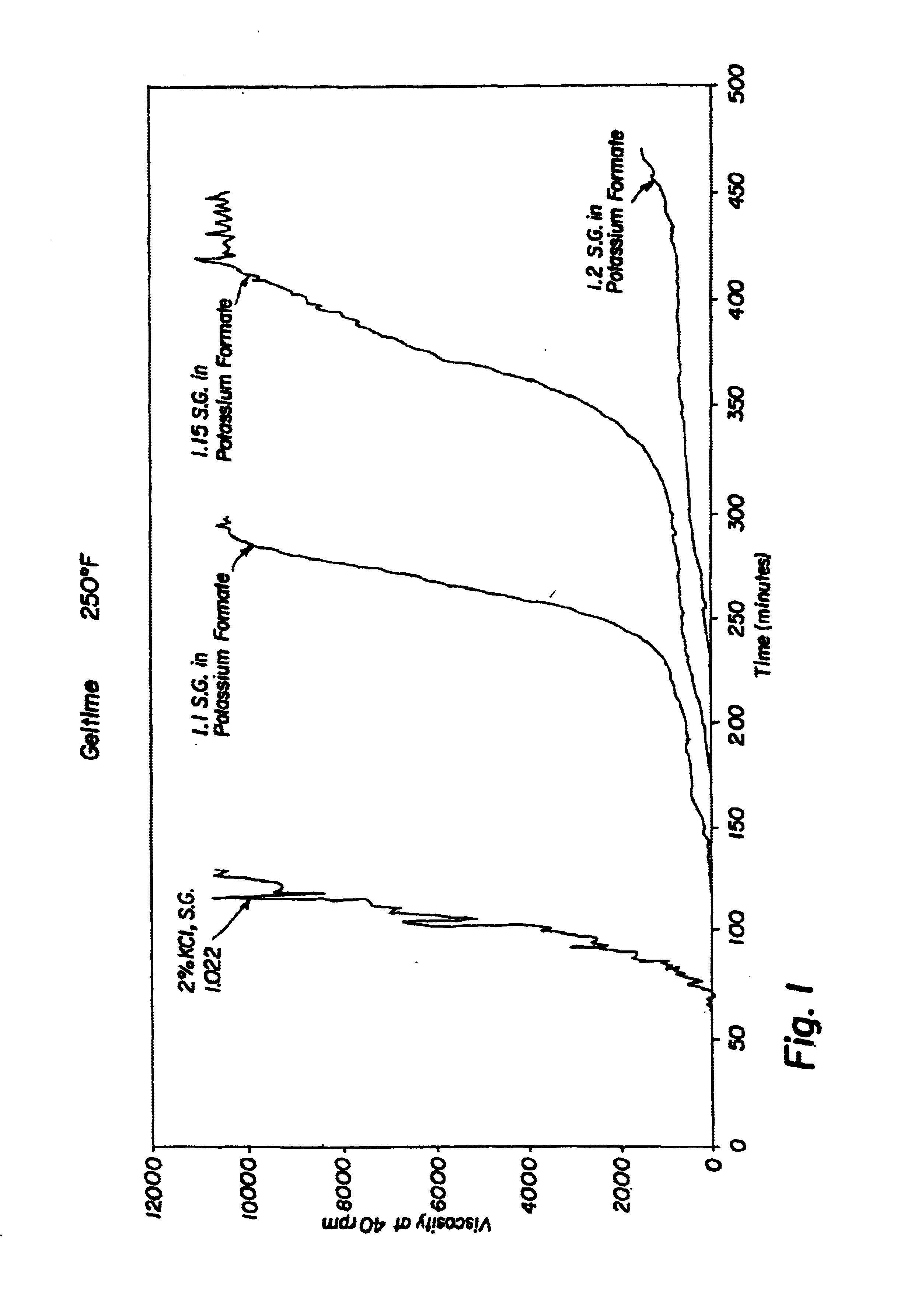
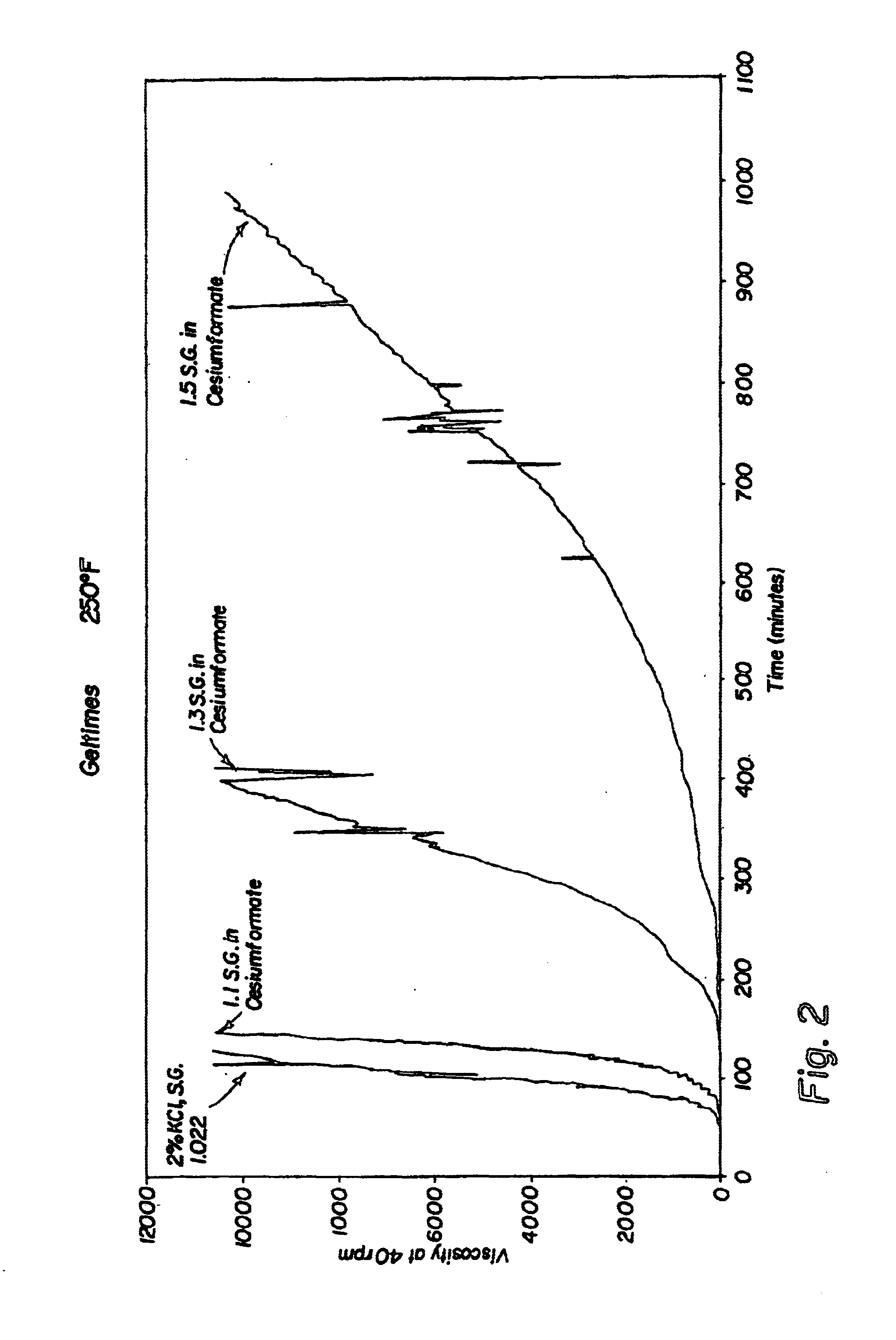
Compositions and methods including formate brines for conformance control
Compositions and methods are provided for reducing the permeability of subterranean zones. More particularly, water-soluble polymeric compositions which form cross-linked gels in the zones. In general, the composition comprises (a) at least one water-soluble polymer; (b) at least one organic gelling agent capable of cross-linking the water-soluble polymer; and (c) at least one water-soluble formate. More preferably, the water-soluble polymer is a copolymer of (i) at least one non-acidic ethylenically unsaturated polar monomer, and (ii) at least one copolymerisable ethylenically unsaturated ester. The gelling agent is preferably selected from the group consisting of a polyalkyleneimine, polyfunctional aliphatic amine, an aralkylamine, and a heteroaralkylamine. The preferred water-soluble formate is selected from the group consisting of ammonium formate, lithium formate, sodium formate, potassium formate, rubidium formate, cesium formate, and francium formate. Water is used to make an aqueous composition prior to use in a subterranean formation. The methods of this invention for reducing the permeability of a subterranean zone are comprised of the steps of introducing an aqueous composition according to the invention into a subterranean zone, and then allowing the aqueous composition to form a cross-linked gel in the zone. Preferably, the method includes the step of subsequently producing hydrocarbons from the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
Multilayer composite metal oxide catalyst and preparation method thereof
ActiveCN102247862AHigh reactivityHigh selectivityOrganic compound preparationCarbonyl compound preparationAlkaline earth metalCerium
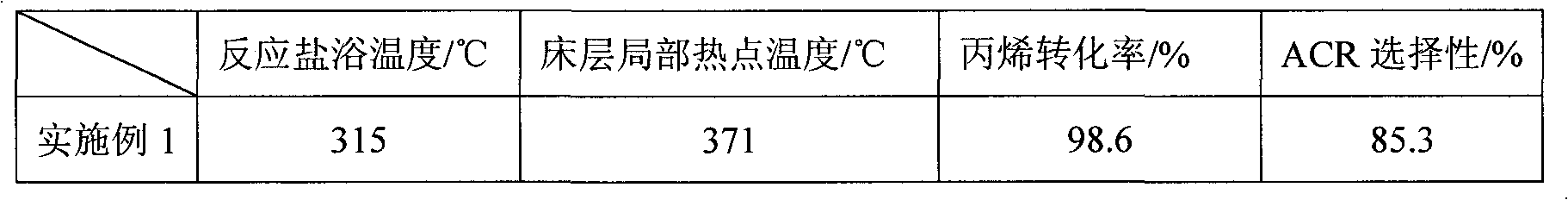
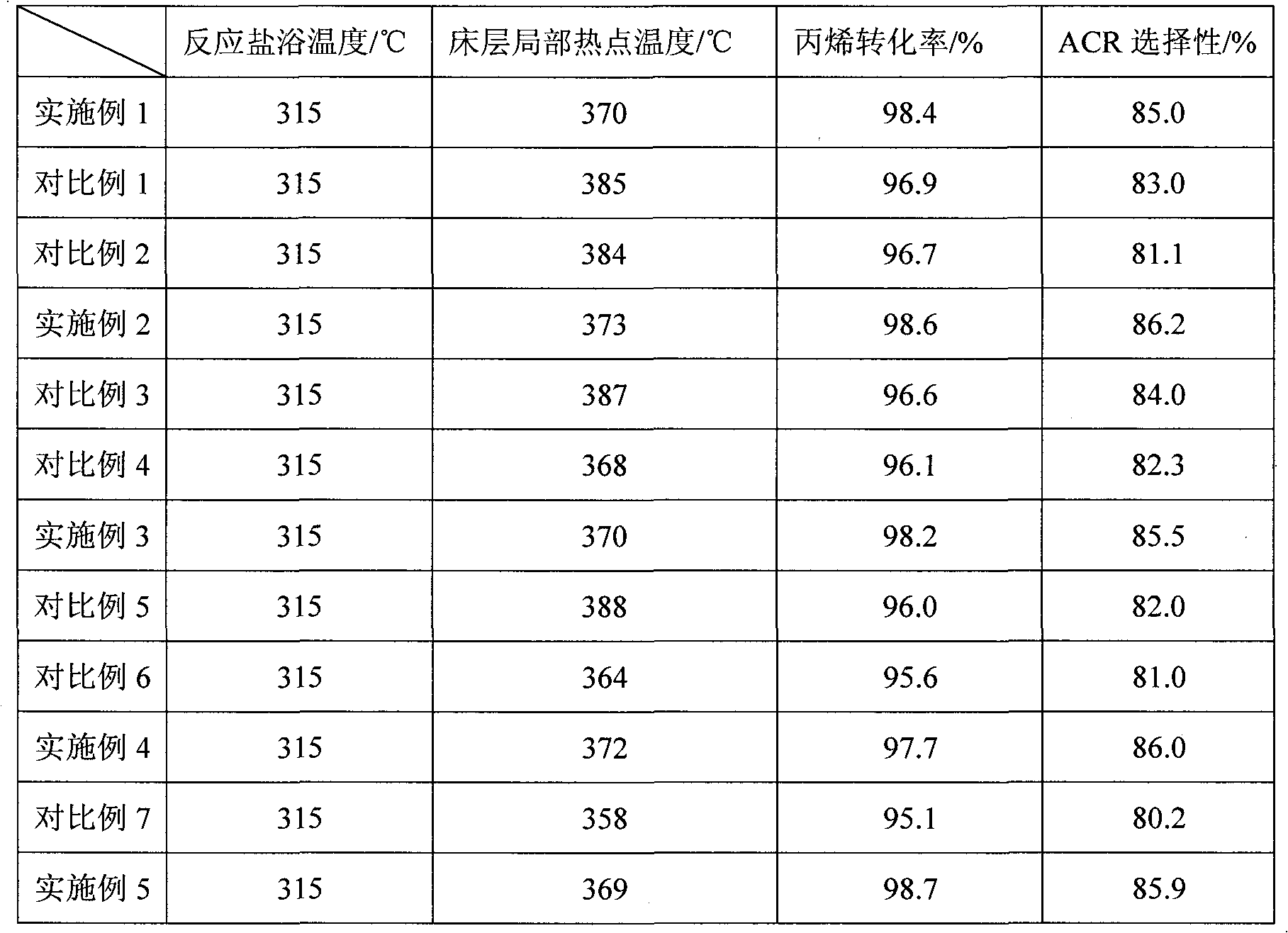
The invention relates to a multilayer composite metal oxide catalyst. The multilayer composite metal oxide catalyst has the general formula as follows: Mo[a]Bi[b]Ni[c]Cs[d]Cu[e]Ti[f]A[g]B[h]C[i]O[j], wherein Mo is molybdenum, Bi is bismuth, Ni is nickel, Cs is cesium, Cu is copper, and Ti is titanium; A is at least one element selected from arsenic, tellurium, manganese, cerium, niobium, zirconium, rubidium, cadmium and germanium; B is at least one element selected from cobalt, boron, strontium, tantalum, alkali metal and alkaline earth metal; C is at least one element selected from vanadium, stannum, gallium, zinc, ferrum, tungsten and stibium; and O is oxygen. The composite metal oxide catalyst has a multilayer structure in which the concentrations of all the elements are reduced progressively from a matrix at the inner layer to the outer layer. The catalyst provided by the invention can effectively reduce the local heat accumulation of single pipe reactors and inhibit the formation of hot spots, and has the characteristics of high reactivity and selectivity and long service life.
Owner:PETROCHINA CO LTD
A kind of preparation method of mems atomic vapor chamber and atomic vapor chamber
ActiveCN102259825AEasy to makeIncrease contrastPrecision positioning equipmentDecorative surface effectsWater vaporRubidium
The invention relates to a preparation method for a micro-electro-mechanical system (MEMS) atomic vapor chamber and the atomic vapor chamber. The chamber is prepared by bonding a Pyrex glass sheet, a silicon wafer and a Pyrex glass sheet by an anodic bonding technology; the Pyrex glass sheet is taken as a window of the chamber; a chamber space is formed by etching or corroding the silicon wafer; paraffin packaged alkali metal such as rubidium (Rb) or cesium (Cs) is put into the chamber, and buffer gas with appropriate pressure is introduced simultaneously; paraffin is taken as a packaging material of the alkali metal, so that active alkali metal is isolated from oxidants such as oxygen, water vapor and the like in an environment; the paraffin is also used as a plating material of the chamber, so that collision between Rb or Cs atoms and a chamber wall is slowed down; and a CO2 laser is used for melting the paraffin to release the alkali metal, so that a uniform paraffin plating is formed on the chamber wall. The problem of long-term drift caused by reaction residues generated by a field preparation mode is solved, the collision between the Rb or Cs atoms and the chamber wall is slowed down, and the contrast of atomic resonance line width of the alkali metal is improved.
Owner:江苏智能微系统工业技术股份有限公司
Miniature atomic air chamber encapsulation apparatus and technology method
InactiveCN101439843AImplement encapsulationAchieve bondingPrecision positioning equipmentSoldering apparatusChemical reactionRubidium
The invention discloses packaging equipment for a micro atomic gas chamber and a process technology method thereof. The equipment comprises a sample chamber, a pressure bar, a sample wafer, a sample stage, a heating wire, a temperature measurement probe, a vacuum pump connector, an inflation inlet, a direct-current high-voltage power supply, a voltmeter, a resistor, and other measurement and control devices. The method comprises: step 1, the selection of materials; step 2, the processing of the materials; step 3, the washing of the sample wafer; step 4, the bonding of a first surface; step 5, the bonding of a second surface; and step 6, the detection of a sample, and is a method which closes metal rubidium generated by adopting an in-situ chemical reaction method in a micro gas chamber. The method has the advantages that the special equipment is a common high vacuum system which is based on an anode bonding technology principle and adopts a relatively cheap mechanical pump, namely molecular pump air-bleed set; at the same time, inert gas is used to take measures such as the repeated inflation to the vacuum system to clean, the high temperature baking to the local sample wafer to remove gas and so on, to lighten the influence of residual gas and adsorbed gas as far as possible, particularly lighten the oxidation of rubidium.
Owner:PEKING UNIV
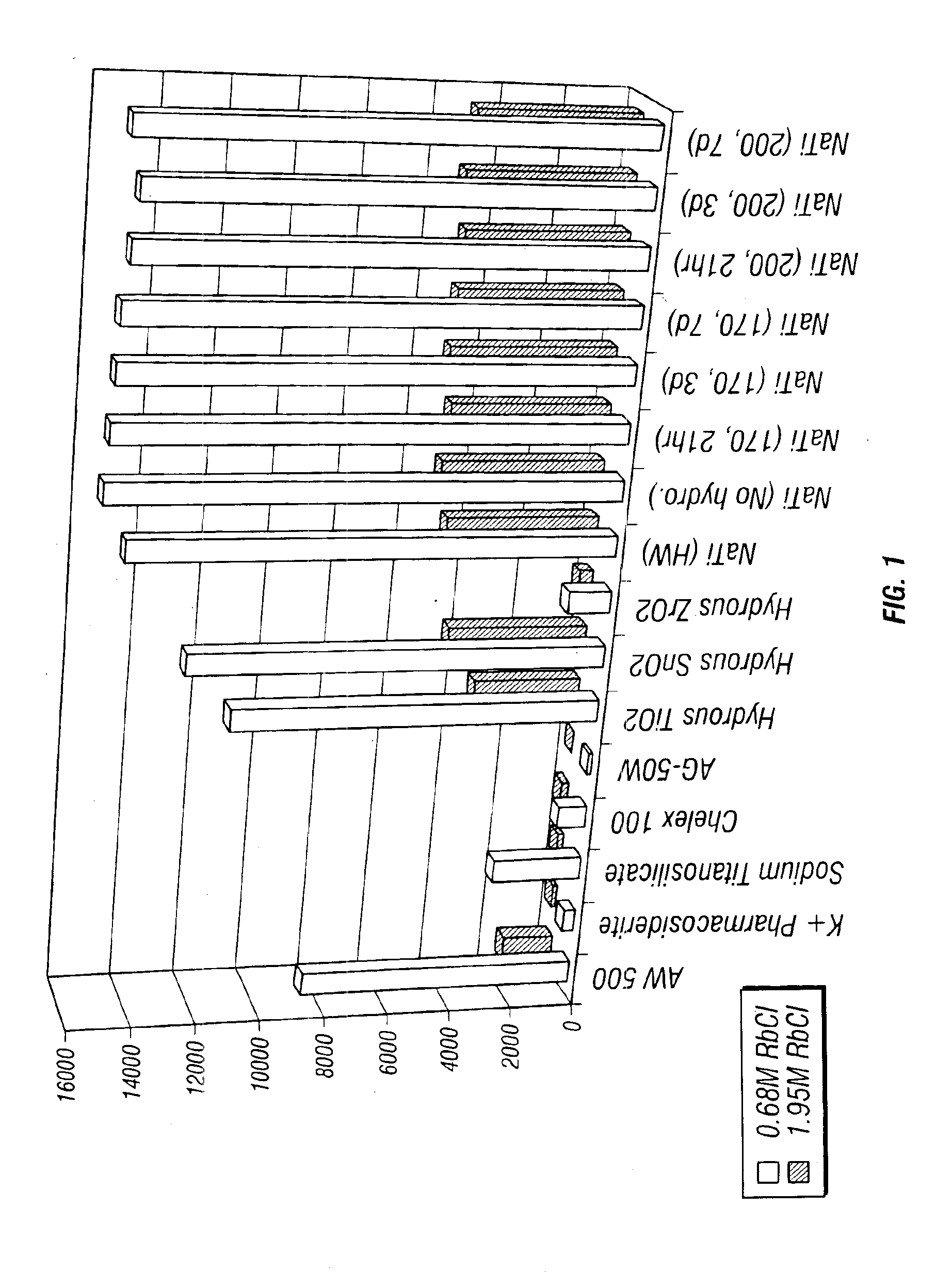
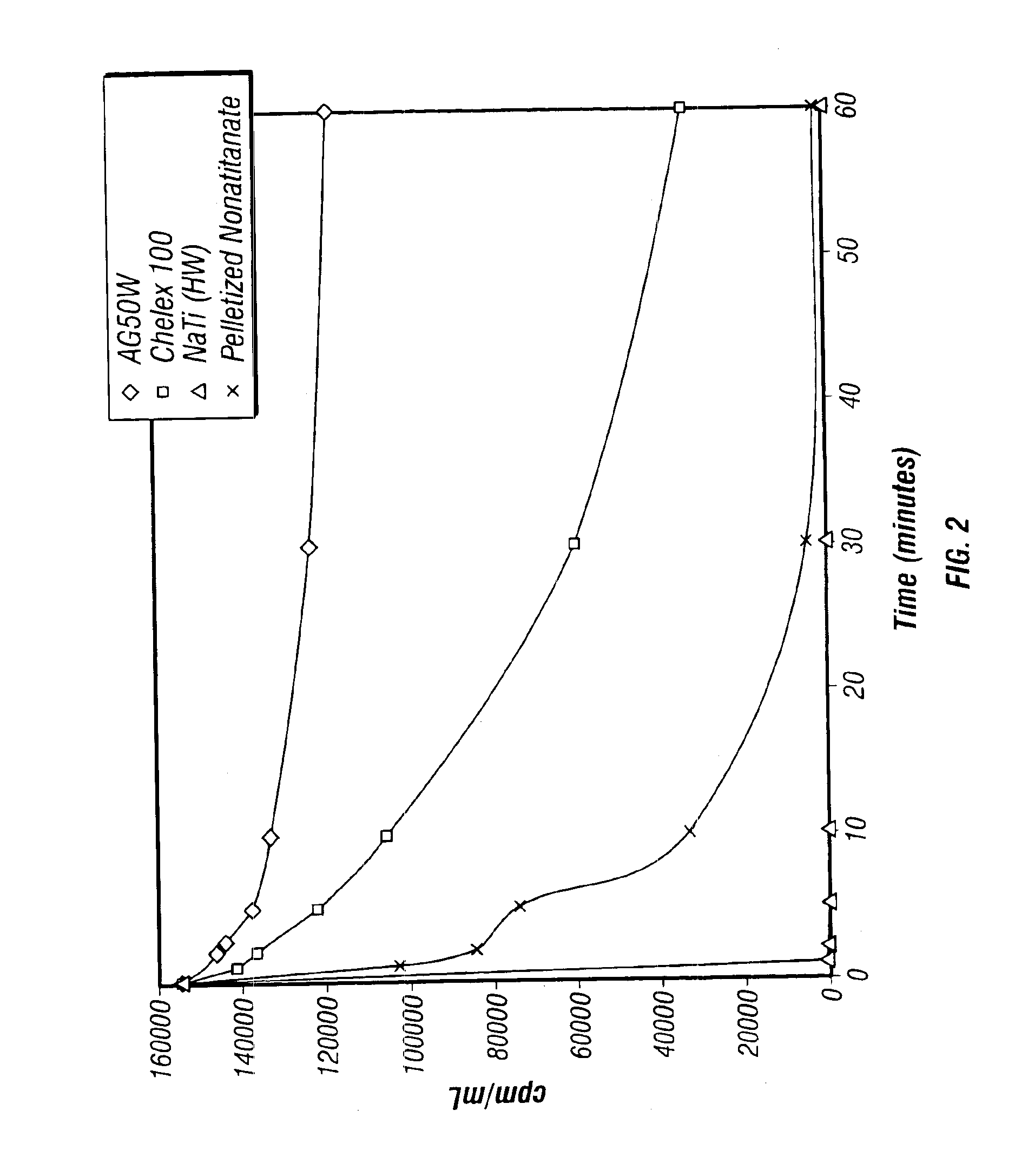
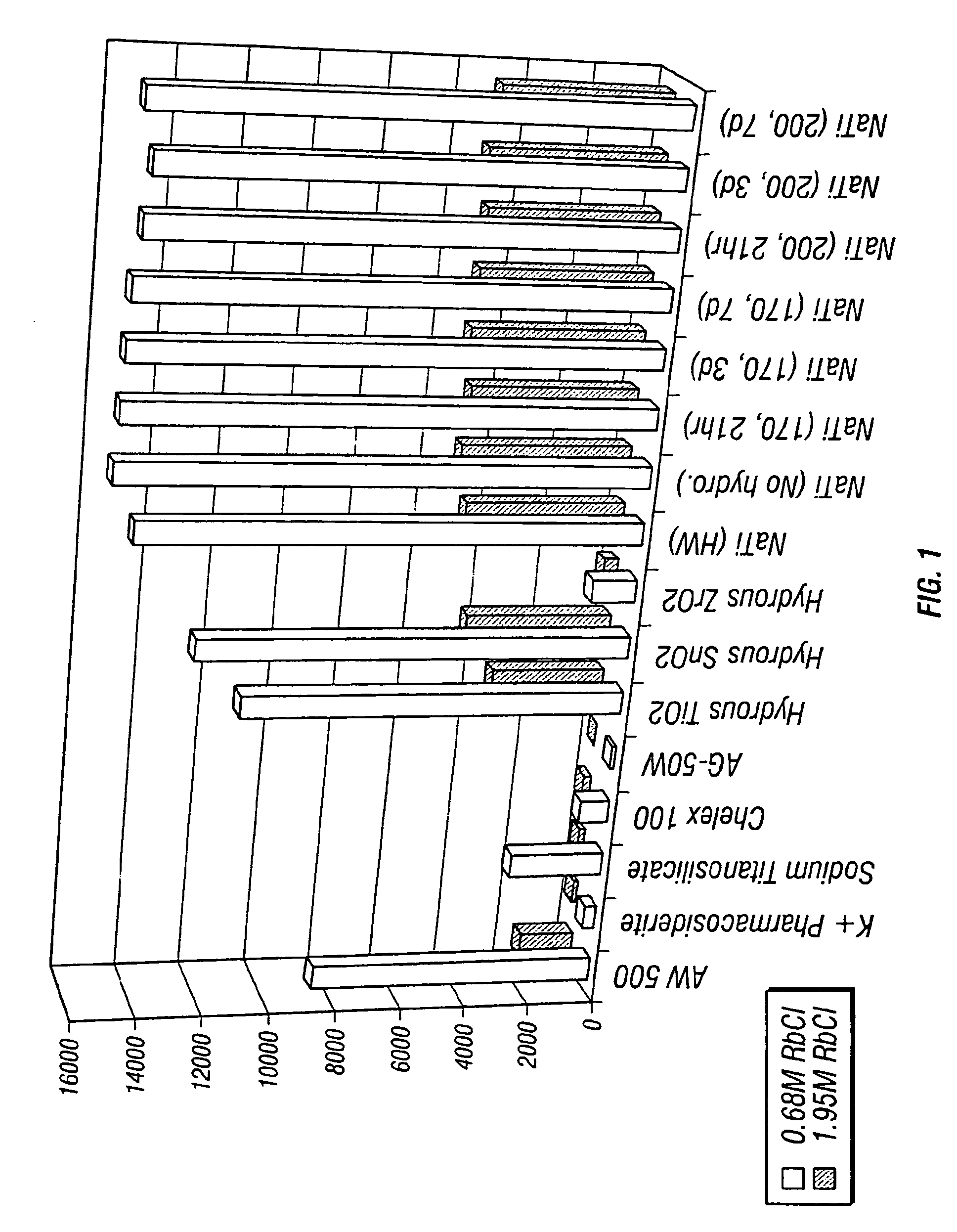
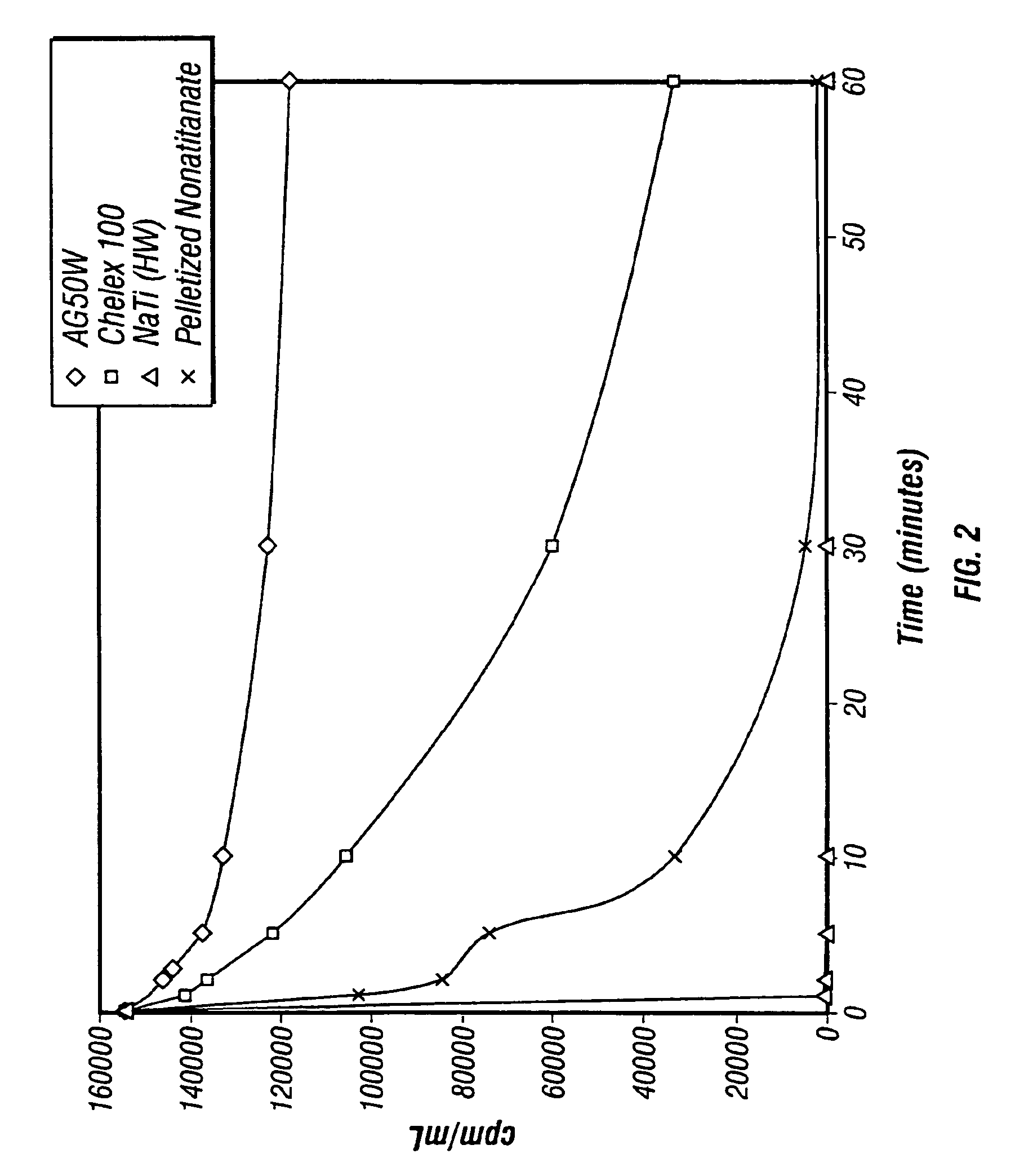
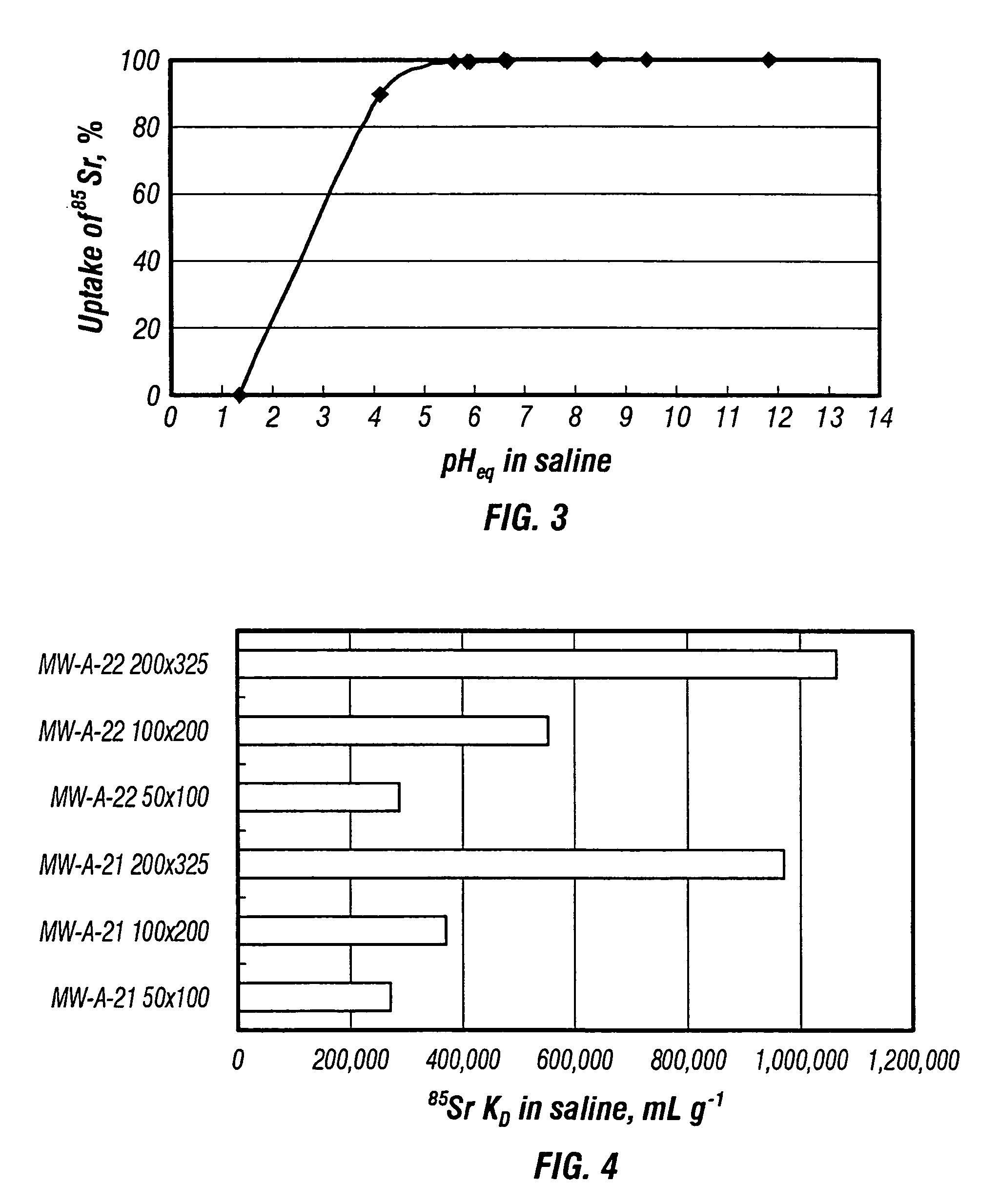
Rubidlum-82 generator based on sodium nonatitanate support, and improved separation methods for the recovery of strontium-82 from irradiated targets
InactiveUS6908598B2Effective recoveryOther chemical processesTransuranic element compoundsLow affinityRubidium
Sodium nonatitanate compositions, a method using the composition for recovery of 82Sr from irradiated targets, and a method using the composition for generating 82Rb. The sodium nonatitanate materials of the invention are highly selective at separating strontium from solutions derived from the dissolution of irradiated target materials, thus reducing target processing times. The compositions also have a very low affinity for rubidium, making it an ideal material for use as a 82Rb generator. Sodium nonatitanate materials of this type both improve the recovery of 82Sr and provide a safer, more effective 82Rb generator system.
Owner:LYNNTECH
Purification of hydride gases
Purification material for removing a contaminant from an impure hydride gas comprising an adsorbent comprising a reduced metal oxide on a porous support and a desiccant. The porous support may be selected from the group consisting of activated carbon, alumina, silica, zeolite, silica alumina, titania, zirconia, and combinations thereof. The reduced metal oxide may comprise one or more metals selected from the group consisting of Group I alkali metals (lithium, sodium, potassium, rubidium, and cesium), Group II alkaline earth metals (magnesium, calcium, strontium, and barium), and transition metals (manganese, nickel, zinc, iron, molybdenum, tungsten, titanium, vanadium, cobalt, and rhodium). The desiccant may be selected from the group consisting of hygroscopic metal salts, zeolites, single metal oxides, mixed metal oxides, and combinations thereof.
Owner:VERSUM MATERIALS US LLC
Compositions of oak bark extract related synthetic compositions and method of using same
InactiveUS7014870B1Highly useful propertyReduce concentrationBiocideHeavy metal active ingredientsHigh concentrationMedicine
Higher concentrations of oak bark ash extract, i.e., greater than 20% by weight, are useful for the treatment of skin cancers. Lower concentrations of oak bark extract possess additional therapeutic properties not heretofore recognized. For example, preparations containing 40–80% oak bark extract are useful in the treatment of acute cancerous skin ulcers. In addition, synthetic mixtures containing potassium ions, zinc ions, calcium ions provide many of the same advantageous properties of oak bark extract. The inclusion of rubidium ions and sulfur is also advantageous for some applications.
Owner:GREYSTONE MEDICAL GROUP
Method of supplementing the diet and ameliorating oxidative stress
A method of supplementing a diet and ameliorating oxidative stress in a mammal includes administering a pharmaceutically effective amount of an active compound having the chemical structure:where n=1-4 and X is selected from the group consisting of hydrogen, lithium sodium, potassium, rubidium, cesium and francium.
Owner:EMERAMED LTD
Rubidium generator for cardiac perfusion imaging and method of making and maintaining same
ActiveUS8071959B2Low pressure elutionFacilitates precision flow controlIn-vivo radioactive preparationsConversion outside reactor/acceleratorsFractographyRubidium
An 82Sr / 82Rb generator column is made using a fluid impervious cylindrical container having a cover for closing the container in a fluid tight seal, and further having an inlet for connection of a conduit for delivering a fluid into the container and an outlet for connection of a conduit for conducting the fluid from the container. An ion exchange material fills the container, the ion exchange material being compacted within the container to a density that permits the ion exchange material to be eluted at a rate of at least 5 ml / min at a fluid pressure of 1.5 pounds per square inch (10 kPa). The generator column can be repeatedly recharged with 82Sr. The generator column is compatible with either three-dimensional or two-dimensional positron emission tomography systems.
Owner:OTTAWA HEART INST RES CORP
Fuel compositions exhibiting improved fuel stability
InactiveUS6652608B1Enhanced combustion structureImprove stabilityNon-fuel substance addition to fuelCombustion enginesIndiumCobalt
A fuel composition of the present invention exhibits minimized hydrolysis and increased fuel stability, even after extended storage at 65° F. for 6-9 months. The composition, which is preferably not strongly alkaline (3.0 to 10.5), is more preferably weakly alkaline to mildly acidic (4.5 to 8.5) and most preferably slightly acidic (6.3 to 6.8), includes a lower dialkyl carbonate, a combustion improving amount of at least one high heating combustible compound containing at least one element selected from the group consisting of aluminum, boron, bromine, bismuth, beryllium, calcium, cesium, chromium, cobalt, copper, francium, gallium, germanium, iodine, iron, indium, lithium, magnesium, manganese, molybdenum, nickel, niobium, nitrogen, phosphorus, potassium, palladium, rubidium, sodium, tin, zinc, praseodymium, rhenium, silicon, vanadium, or mixture, and a hydrocarbon base fuel.
Owner:OCTANE INT
Method for extracting lithium from lepidolite
The invention relates to a method for extracting lithium from lepidolite, which comprises the steps of removing fluorine, pressing and soaking, separating, removing impurity, separating out natrium, analyzing kalium, separating, depositing lithium, separating, and separating crude wet lithium carbonate and lithium depositing mother liquid after depositing lithium. The invention is characterized in that the concentration of Rb+ contained by the lithium depositing mother liquid is detected, the circulation trend of the lithium depositing mother liquid is decided by the consistency, when the consistency is low, the product quality is not influenced, the lithium depositing mother liquid which is acidulated returns to the step of removing impurity before analyzing natrium, and when the concentration of the Rb+ is high and influences the product quality, the lithium depositing mother liquid is separated in an Rb and Cs product manufacturing procedure. The invention can avoid increasing the load of a pressing and soaking kettle when the lithium depositing mother liquid returns to the pressing and soaking step, and the content of natrium and kalium in the lithium depositing mother liquid which is finally educed is reduced to enable the subsequent separation of Rb and Cs to be easier, thereby the cost of whole technique comprising the processing cost of lithium carbonate, rubidium and cesium is greatly reduced.
Owner:GANFENG LITHIUM CO LTD
Rubidium-82 generator based on sodium nonatitanate support, and improved separation methods for the recovery of strontium-82 from irradiated targets
InactiveUS7476377B2Effective recoveryTransuranic element compoundsOther chemical processesLow affinityRubidium
Sodium nonatitanate compositions, a method using the composition for recovery of 82Sr from irradiated targets, and a method using the composition for generating 82Rb. The sodium nonatitanate materials of the invention are highly selective at separating strontium from solutions derived from the dissolution of irradiated target materials, thus reducing target processing times. The compositions also have a very low affinity for rubidium, making it an ideal material for use as a 82Rb generator. Sodium nonatitanate materials of this type both improve the recovery of 82Sr and provide a safer, more effective 82Rb generator system.
Owner:LYNNTECH
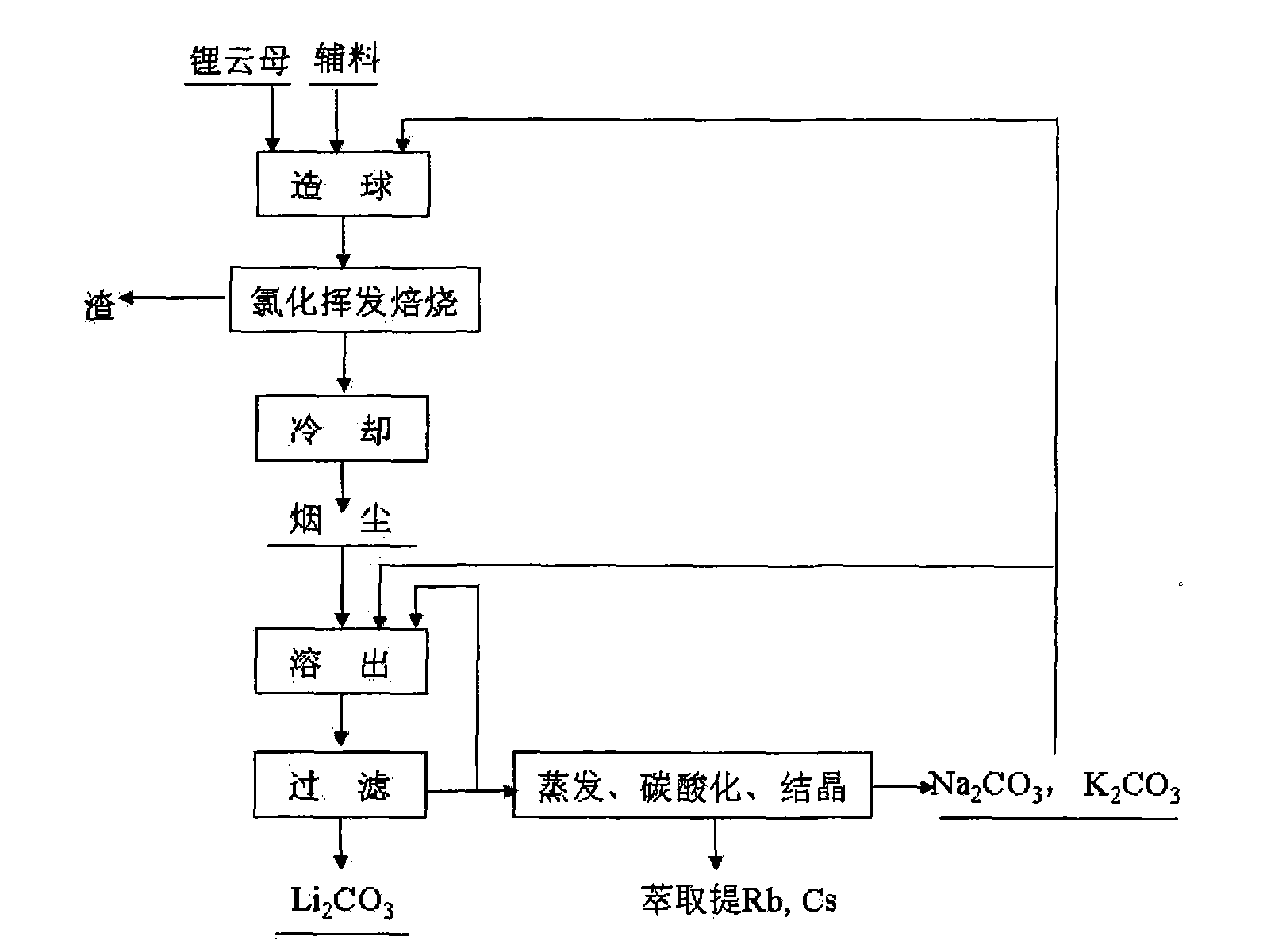
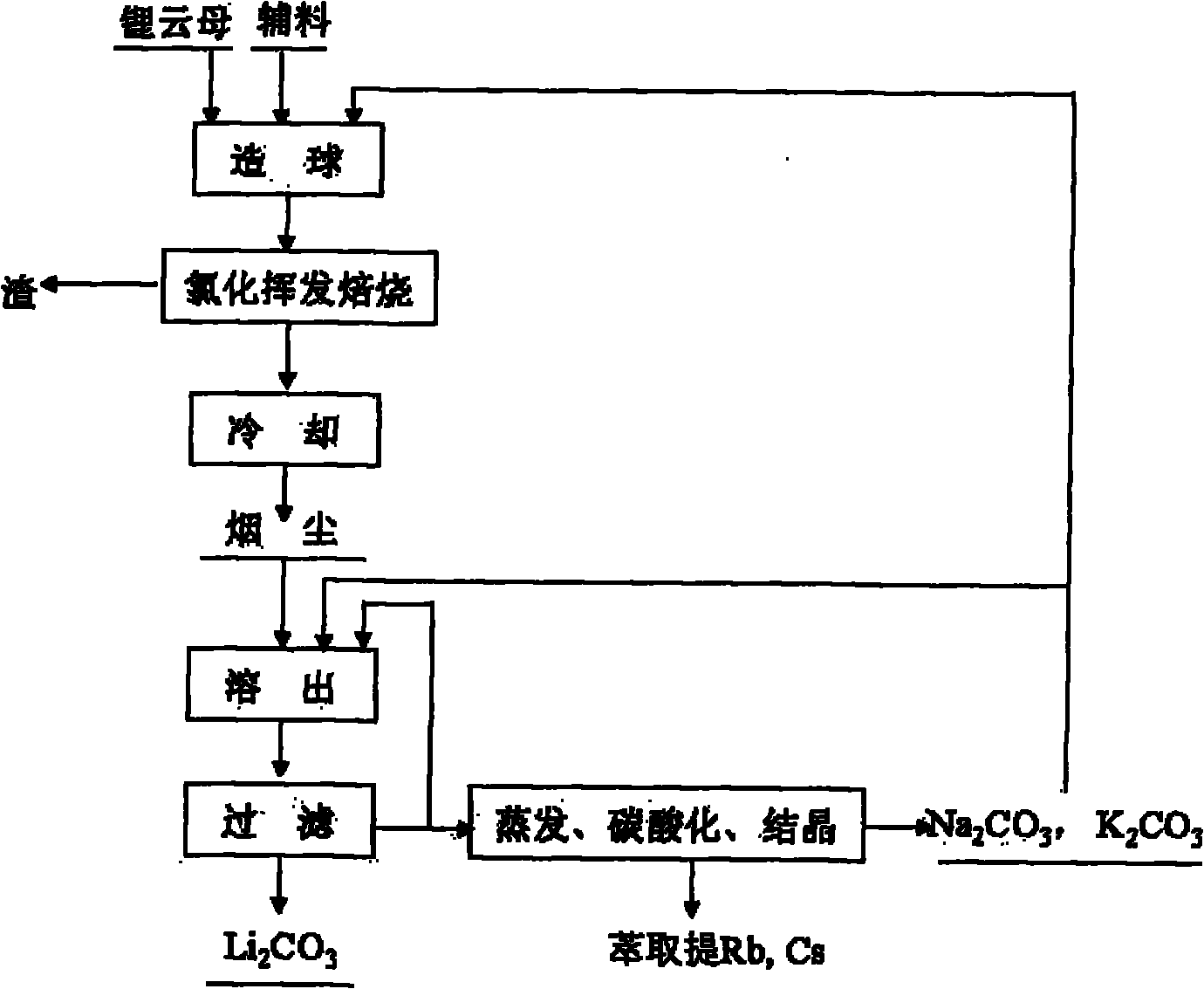
Method and device for extracting lithium from lapidolite by chloridizing roasting method
The invention discloses a method and a device for extracting lithium, which is used for preparing lithium carbonate, from a lapidolite ore by a chloridizing roasting method. The method comprises the following steps of: firstly, mixing the lapidolite ore, calcium chloride and sodium hydroxide with a compound bonding agent for pellet fabrication; secondly, performing chloridizing roasting in a square-frame shaped track type roasting furnace; thirdly, leaching out soot dust by using solution containing sodium carbonate and potassium carbonate to ensure that potassium, sodium, rubidium and cesium enter the solution and convert the lithium into lithium carbonate; fourthly, filtering the mixture to obtain a lithium carbonate solid, and circularly using the filtrated mother liquor to leach out the soot dust; fifthly, when an alkali metal salt is close to be saturated, indirectly heating the filtrated mother liquor by using the residual heat of the gas in the roasting furnace to evaporate part of water; sixthly, passing CO2 into the filtrated mother liquor to perform carbonation; and seventhly, performing cooling crystallization to separate out a mixed salt of the sodium carbonate and the potassium carbonate, returning part of the mixed salt which is used as an auxiliary material mixed and roasted with lapidolite for cyclic utilization, using another part of the mixed salt as a carbonate reagent needed in dissolution, and using the rest part of the mixed salt as byproducts of the sodium carbonate and the potassium carbonate. The method has the advantages of high lithium recovery rate, good material comprehensive utilization, large equipment productivity, high production efficiency, small water consumption in the process and less wastewater discharge.
Owner:CENT SOUTH UNIV
Method for extracting lithium and other alkali metal elements from lepidolite mineral
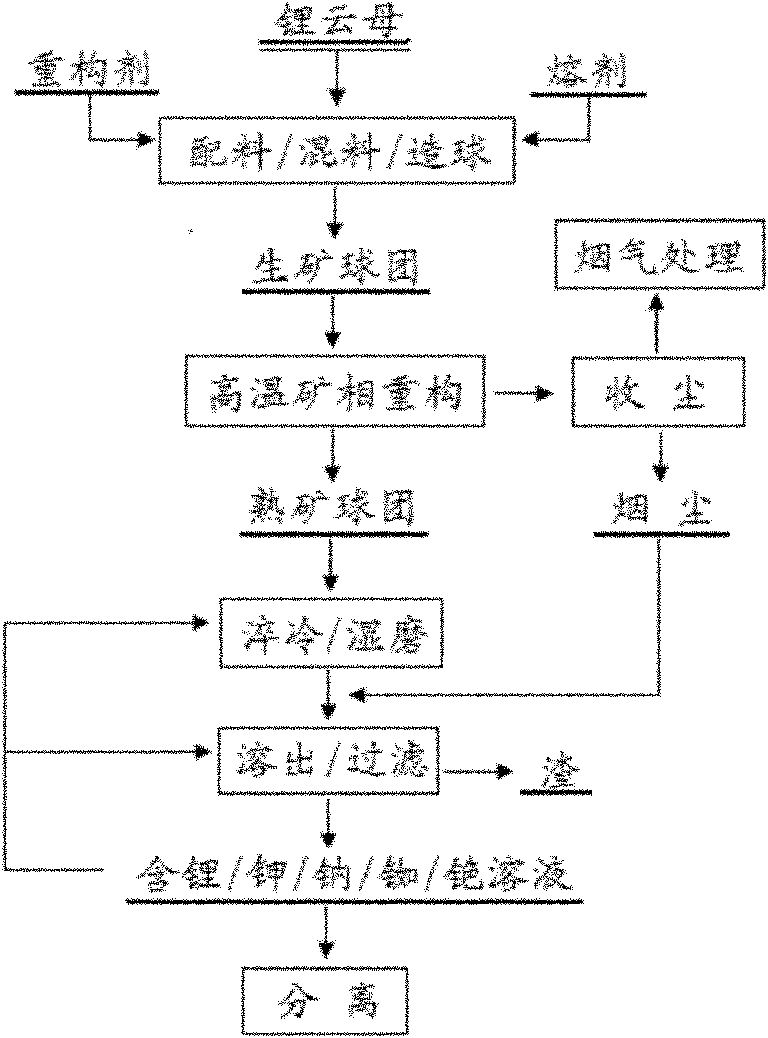
InactiveCN101974678AEfficient extractionEasy to optimizeProcess efficiency improvementRubidiumPotassium
The invention discloses a high-temperature mineral phase reconstruction method for extracting lithium and other alkali metal elements from lepidolite mineral, comprising the following steps: mixing raw materials, pelletizing, calcining at high temperature, water quenching, ball milling, dissolving out, producing compounds and the like. The invention teaks raw mineral component composition to design target reconstruction mineral and composition to obtain the purpose of optimizing processes, lowering energy consumption and cost of treatment process and efficiently extracting lithium, potassium, rubidium, caesium and the like. Silicon and aluminum in lepidolite can enter anorthite type mineral phase (CaO.Al2O3.2SiO2, (Ca, Na)O. (Al, Si)2O3.2SiO2) and calcium ash quarry phase (CaO.SiO2) after mineral phase reconstruction, and do not dissolve in water and aqueous solution. After mineral phase reconstruction reaction, fluorine in lepidolite enters calcium fluoride mineral phase and does not dissolve in water and aqueous solution. Lithium and other alkali metal elements in lepidolite enter salt (chloride, sulfate) or alkali (hydroxide) phase of lithium and other alkali metal elements after mineral phase reconstruction reaction and can be dissolved in water and aqueous solution.
Owner:CENT SOUTH UNIV
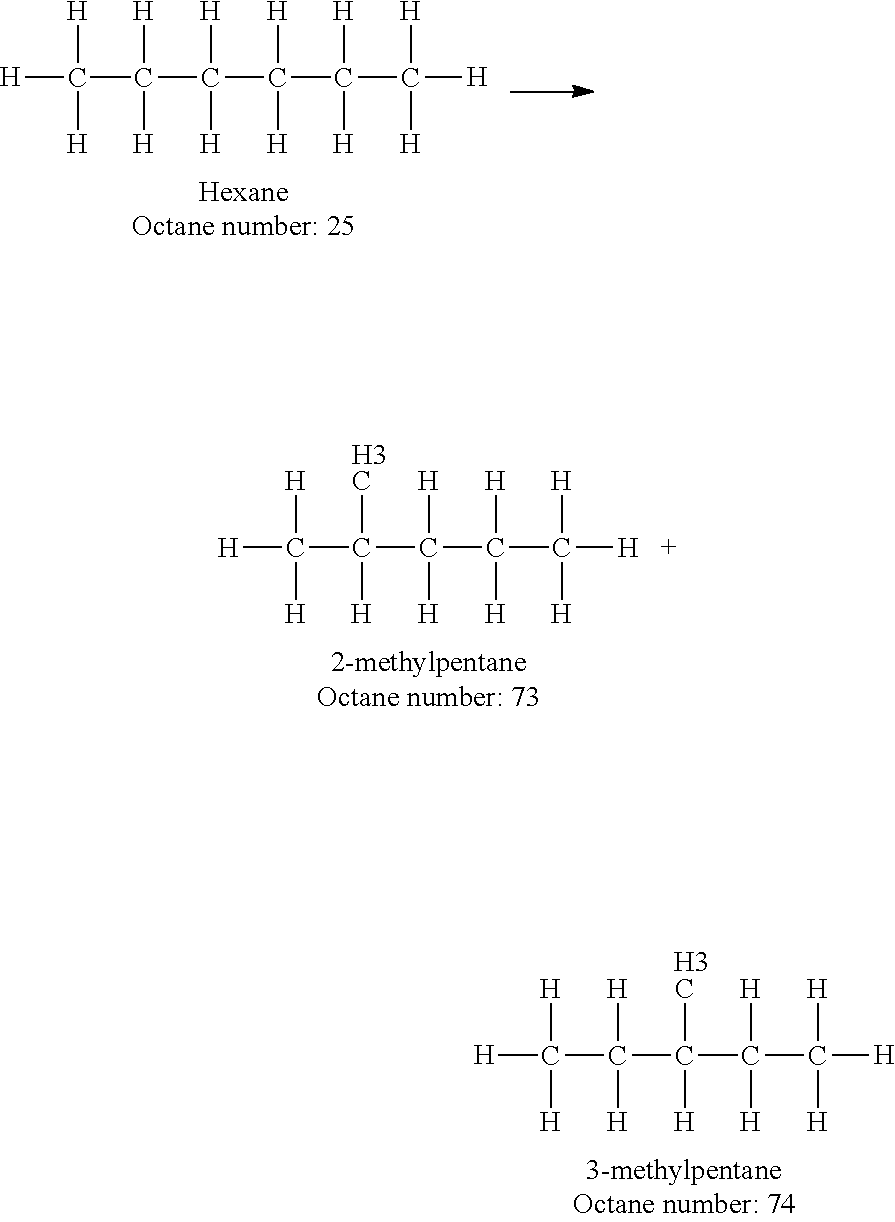
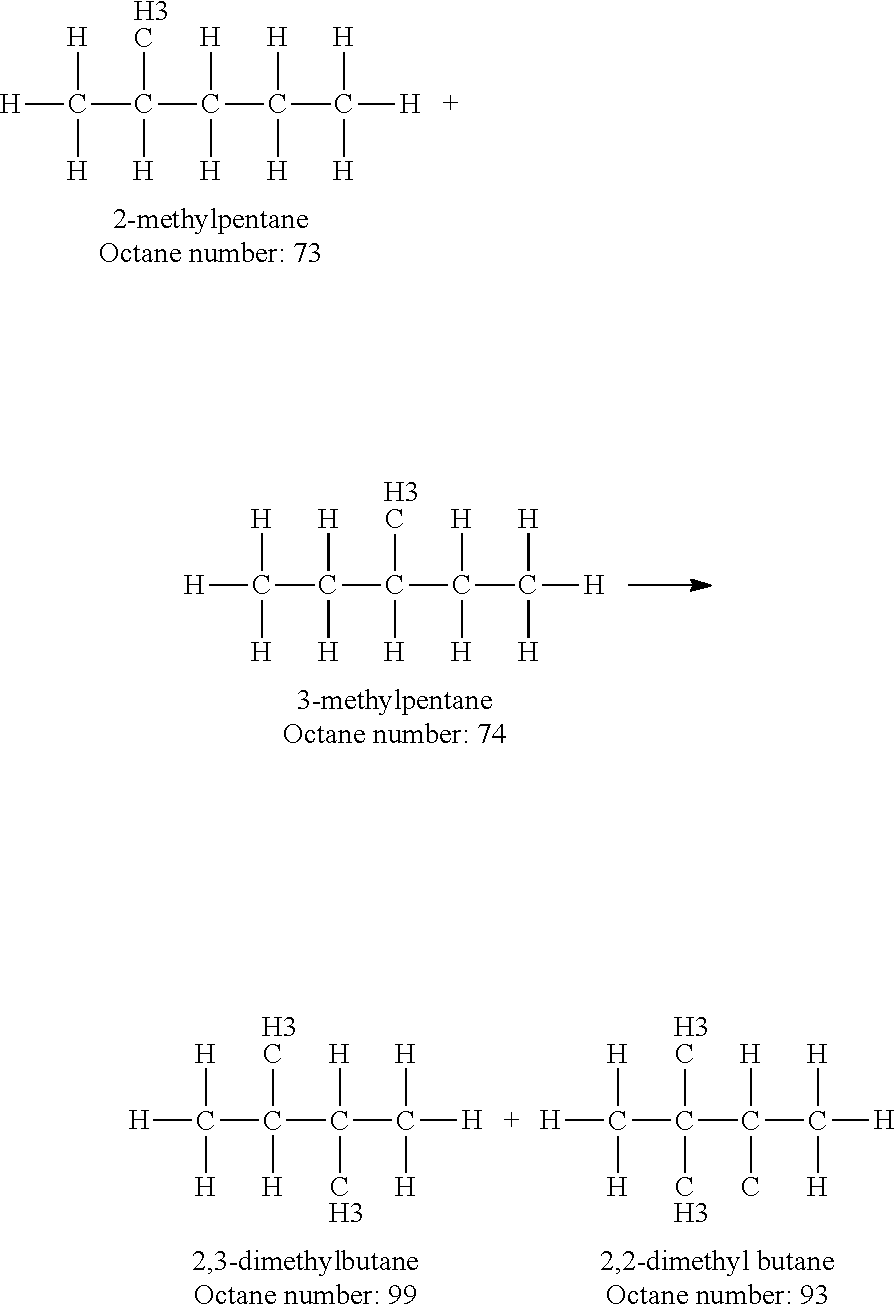
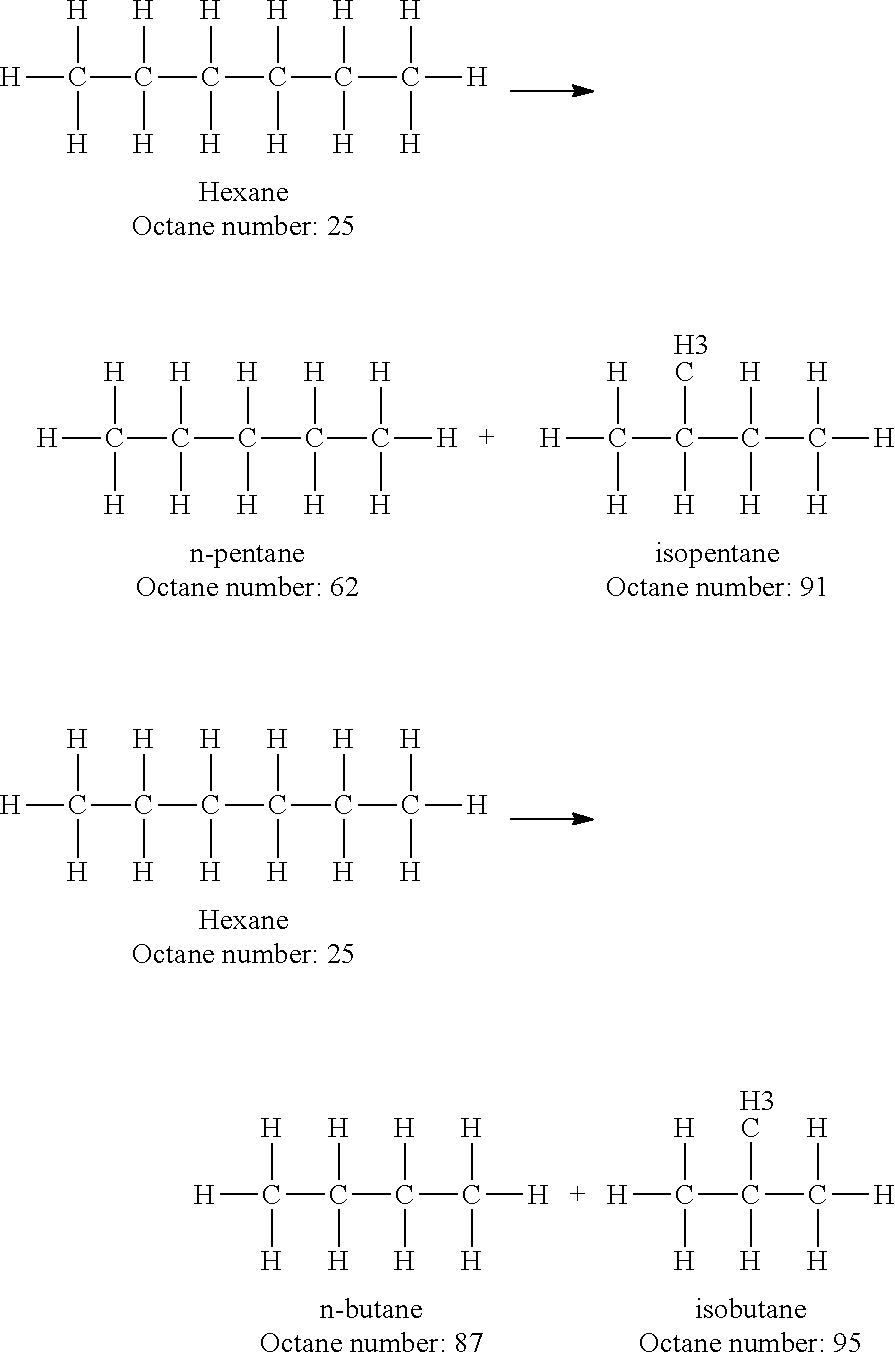
Increasing octane number of light naphtha using a germanium-zeolite catalyst
InactiveUS20110132804A1Boost octaneMolecular sieve catalystsHydrocarbon by hydrogenationAlkaneAlkaline earth metal
This invention relates to a process for the increasing the octane number of a naphtha hydrocarbon feed having a predominantly paraffin content with a germanium-containing zeolite catalyst. The catalyst is a non-acidic germanium zeolite on which a noble metal, such as platinum, has been deposited. The zeolite structure may be of MTW, MWW, MEL, TON, MRE, FER, MFI, BEA, MOR, LTL or MTT. The zeolite is made non-acidic by being base-exchanged with an alkali metal or alkaline earth metal, such as cesium, potassium, sodium, rubidium, barium, calcium, magnesium and mixtures thereof, to reduce acidity. The catalyst is sulfur tolerant. The hydrocarbon feed may contain sulfur up to 1000 ppm. The present invention could be applicable to a feedstream which is predominantly naphthenes and paraffins.
Owner:SAUDI BASIC IND CORP SA
Coproduction of hydrofluoroolefins
InactiveUS7687670B2Preparation by hydrogen halide split-offHalogenated hydrocarbon separation/purificationCeriumCobalt
Disclosed is a process for the co-manufacture of the hydrofluoroolefins HFC-1225ye and HFC-1234yf. The process comprises contacting a blend of 1,1,1,2,3,3-hexafluoropropane and 1,1,1,2,3-pentafluoropropane at a temperature of from about 200° C. to about 500° C. with a catalyst, optionally in the presence of an inert gas. The catalyst includes, but is not limited to, aluminum fluoride; fluorided alumina; metals on aluminum fluoride; metals on fluorided alumina; oxides, fluorides, and oxyfluorides of magnesium, zinc and mixtures of magnesium and zinc and / or aluminum; lanthanum oxide and fluorided lanthanum oxide; chromium oxides, fluorided chromium oxides, and cubic chromium trifluoride; carbon, acid-washed carbon, activated carbon, three dimensional matrix carbonaceous materials; and metal compounds supported on carbon. The metal compounds are oxides, fluorides, and oxyfluorides of at least one metal selected from the group consisting of sodium, potassium, rubidium, cesium, yttrium, lanthanum, cerium, praseodymium, neodymium, samarium, chromium, iron, cobalt, rhodium, nickel, copper, zinc, and mixtures thereof. The product hydrofluoroolefins are separated from unreacted hydrofluorocarbons and hydrogen fluoride. In another embodiment, the unreacted hydrofluorocarbons optionally may be recirculated back through the process.
Owner:EI DU PONT DE NEMOURS & CO
Method for extracting rubidium salt and cesium salt
ActiveCN103787375ASpeed up extractionImprove working environmentLiquid solutions solvent extractionRubidium/caesium/francium compoundsRubidiumSalinity
The invention relates to a method for extracting rubidium salt and cesium salt. The method comprises the steps of 1) regulating a high-salinity solution to obtain an alkaline solution which has a pH value of 11-14; 2) extracting rubidium ions and cesium ions from the alkaline solution obtained from the step 1) in a centrifugal extractor through an organic extracting agent, thus obtaining a loaded organic phase (I) and raffinate; 3) washing the organic phase (I) obtained from the step 2) through washing water to obtain an organic phase (II) and a washing solution; 4) implementing reverse extraction to the loaded organic phase (II) through reverse extraction acid (I) to obtain a rubidium salt reverse extraction solution and an organic phase loaded with cesium ion; and 5) implementing reverse extraction to the organic phase loaded with the cesium salt obtained from the step 4) through reverse extraction acid (II) to obtain a cesium salt reverse extraction solution and a blank organic phase. The extraction method disclosed by the invention is simple and easy to implement, and can be matched with comprehensive development and utilization of a high-salinity medium to produce a rubidium salt and cesium salt product of preset type.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Catalyst for producing unsaturated nitrile
InactiveUS6479691B1High selectivityGood physical propertiesCarboxylic acid nitrile preparationOrganic compound preparationAtomic groupCerium
A catalyst composition represented by the following empirical formula which is useful in production of unsaturated nitrites by ammoxidation:wherein F represents at least one element selected from the group consisting of zirconium, lanthanum and cerium, G represents at least one element selected from the group consisting of magnesium, cobalt, manganese and zinc, H represents at least one element selected from the group consisting of vanadium, niobium, tantalum and tungsten, x represents at least one element selected from the group consisting of phosphorus, boron, and tellurium, Y represents at least one element selected from the group consisting of lithium, sodium, rubidium and cesium, the suffixes a-k, x and y represent a ratio of atoms or atomic groups, and a=0.1-3, b=0.3-15, c=0-20, d=3-8, e=0.2-2, f=0.05-1, e / f>1, g=0-5, h=0-3, k=0.1-1, x=0-3, y=0-1, i is the number of oxygen produced by bonding of the above respective components, and j=0-100.
Owner:MITSUBISHI CHEM CORP
Measurement method and measurement device for intensity of microwave electric field
The invention discloses a measurement method and measurement device for the intensity of a microwave electric field. The measurement method includes the steps of splitting a probe light generated by a first laser into two beams of identical probe light, one of which enters a rubidium cell and the other of which enters vacuum equipment; coupling light, which is generated by a second laser, entering the rubidium cell, coherently exciting hot atoms in the rubidium cell from a ground state to a rydberg state by the coupling light and the probe light, and realizing electromagnetically induced transparency in an atomic vapor chamber; applying a microwave electric field generated by a microwave source onto the hot atoms, and coupling another adjacent rydberg state onto a three-level EIT system to form a four-level system; and respectively probing the two ways of transmission light emitted from the rubidium cell and the vacuum equipment, determining the time difference of the two ways of transmission light by analyzing a dispersion relation between the two ways of transmission light, and thus obtaining the intensity of the microwave electric field.
Owner:清远市天之衡量子科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com