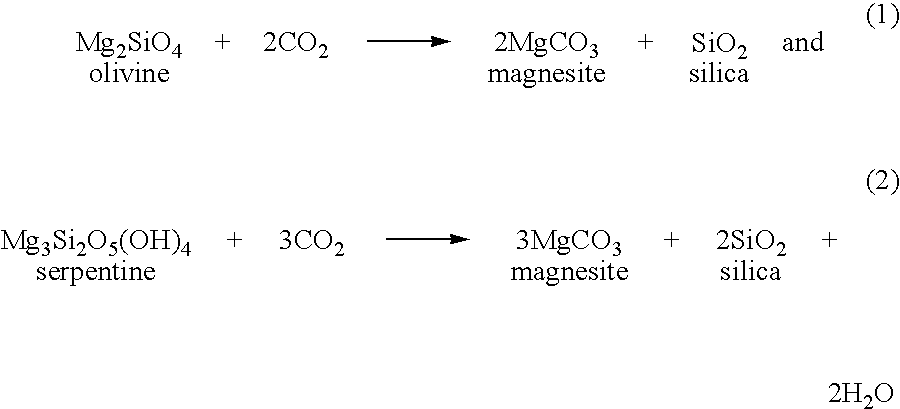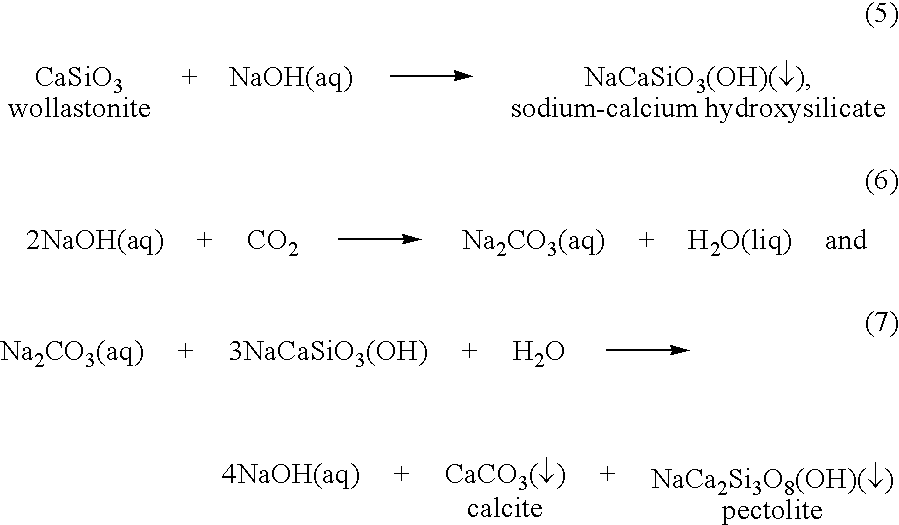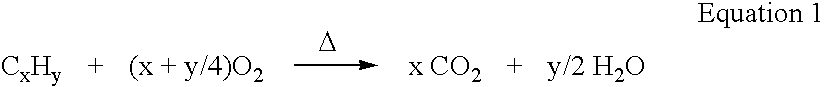Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1782 results about "Carbonation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
Hydrogen production from carbonaceous material
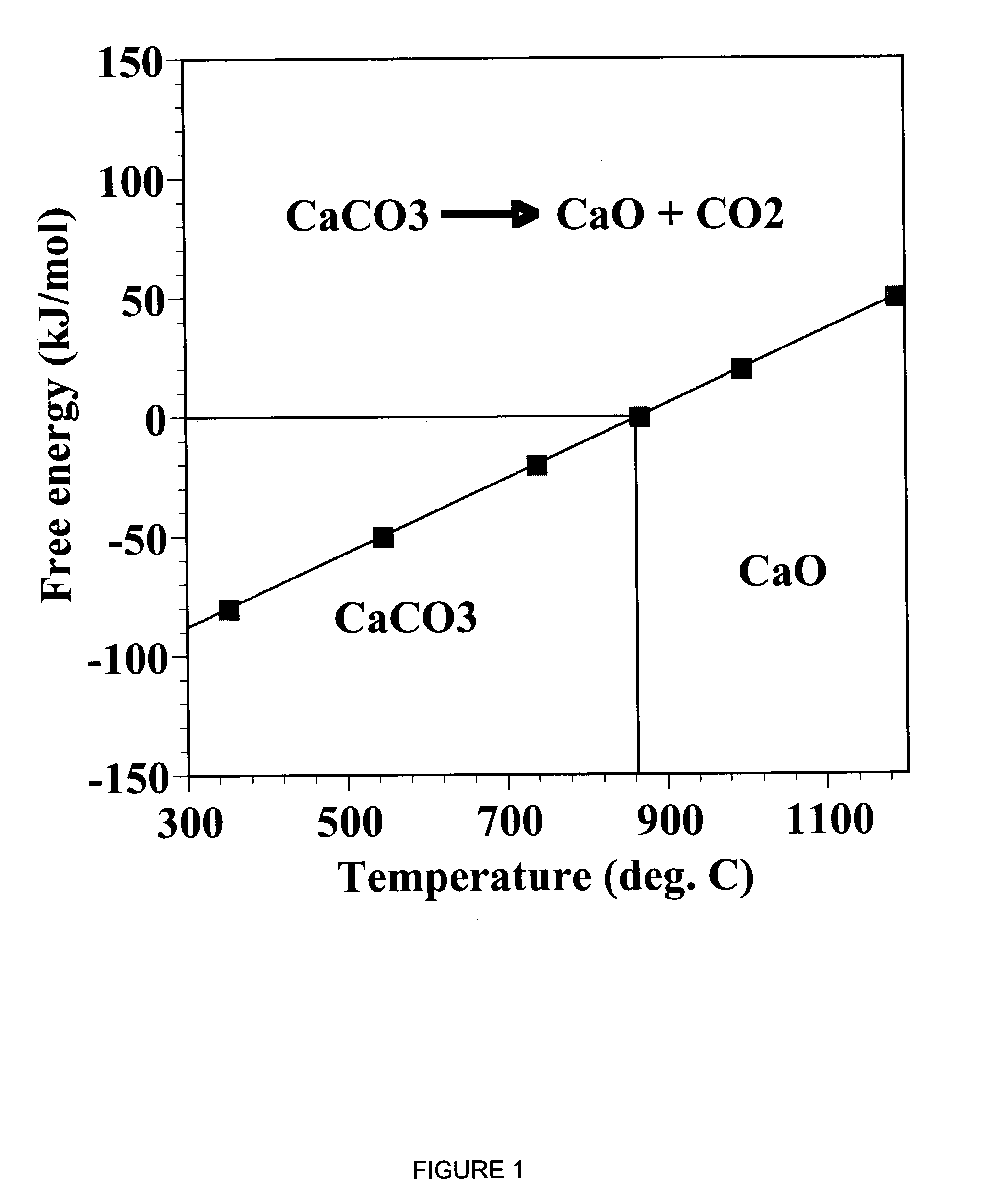
InactiveUS6790430B1Avoid the needCalcium/strontium/barium carbonatesGas turbine plantsCalcinationExothermic reaction
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Process for sequestering carbon dioxide and sulfur dioxide
A process for sequestering carbon dioxide, which includes reacting a silicate based material with an acid to form a suspension, and combining the suspension with carbon dioxide to create active carbonation of the silicate-based material, and thereafter producing a metal salt, silica and regenerating the acid in the liquid phase of the suspension.
Owner:PENN STATE RES FOUND
Protein beverage and protein beverage concentrate and methods of making the same
An improved protein beverage which may provide a relatively high protein content, ranging from about 0.01% by weight to about 15% by weight, while optionally employing a carbonation concentration between about 0.1 volumes of carbonation (per volume of liquid drink) to about 6 volumes of carbonation. Preferably the protein is a protein, such preferably as whey protein, or others. The protein beverage may contain juice and / or an additive which provides energy generation enhancement. The protein beverage may be heat treated to inactivate pathogenic microbes in the presence of the carbonation which may be used to provide taste and mouth feel for the drink. Typically, the treatment for pathogenic microbe inactivation is carried out in the individual package used for storage and handling of the protein drink. The protein beverage may be prepared from a protein beverage concentrate, which may be in the form of a syrup concentrate or a powder concentrate.
Owner:DAVINAS LLC
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
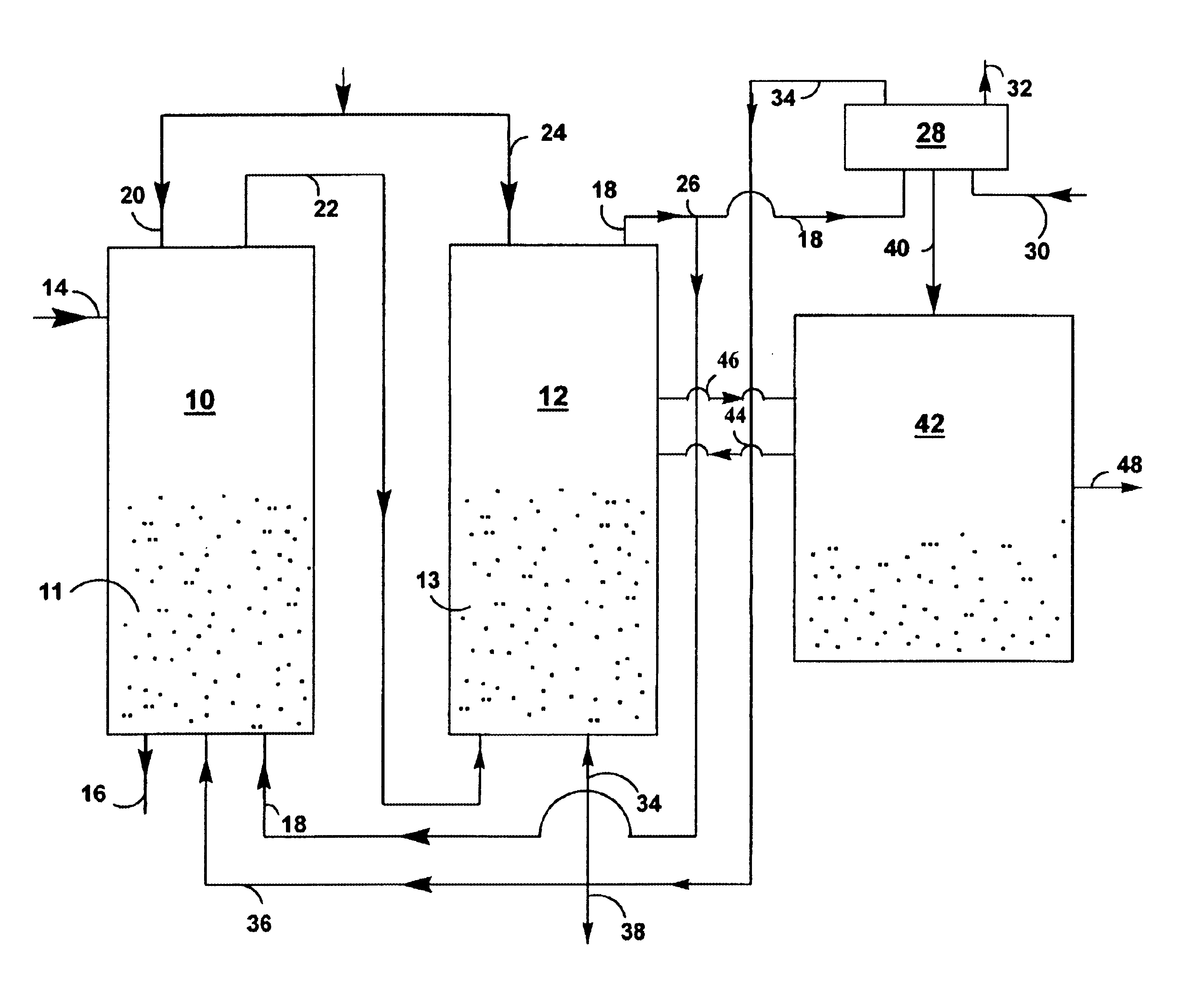
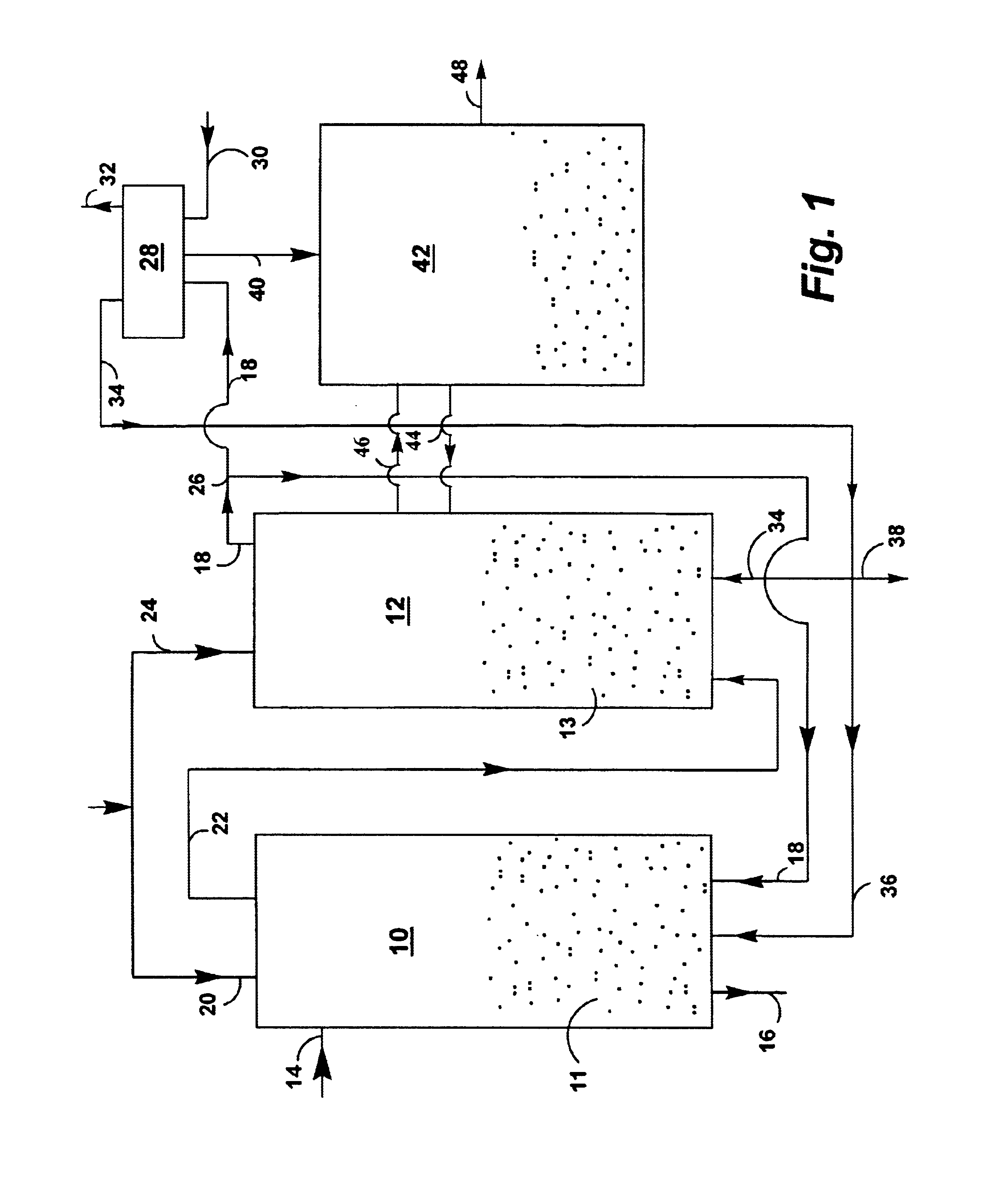
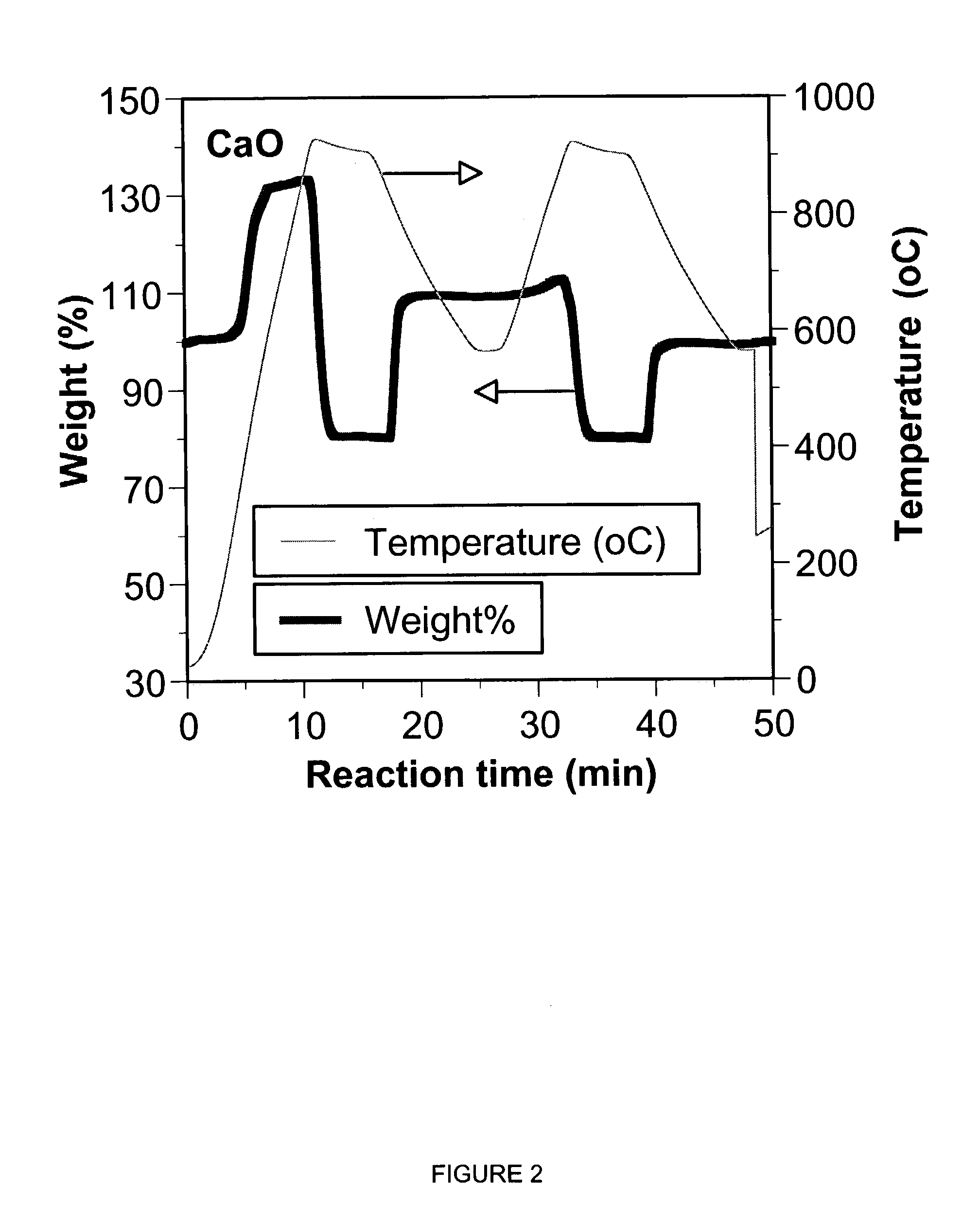
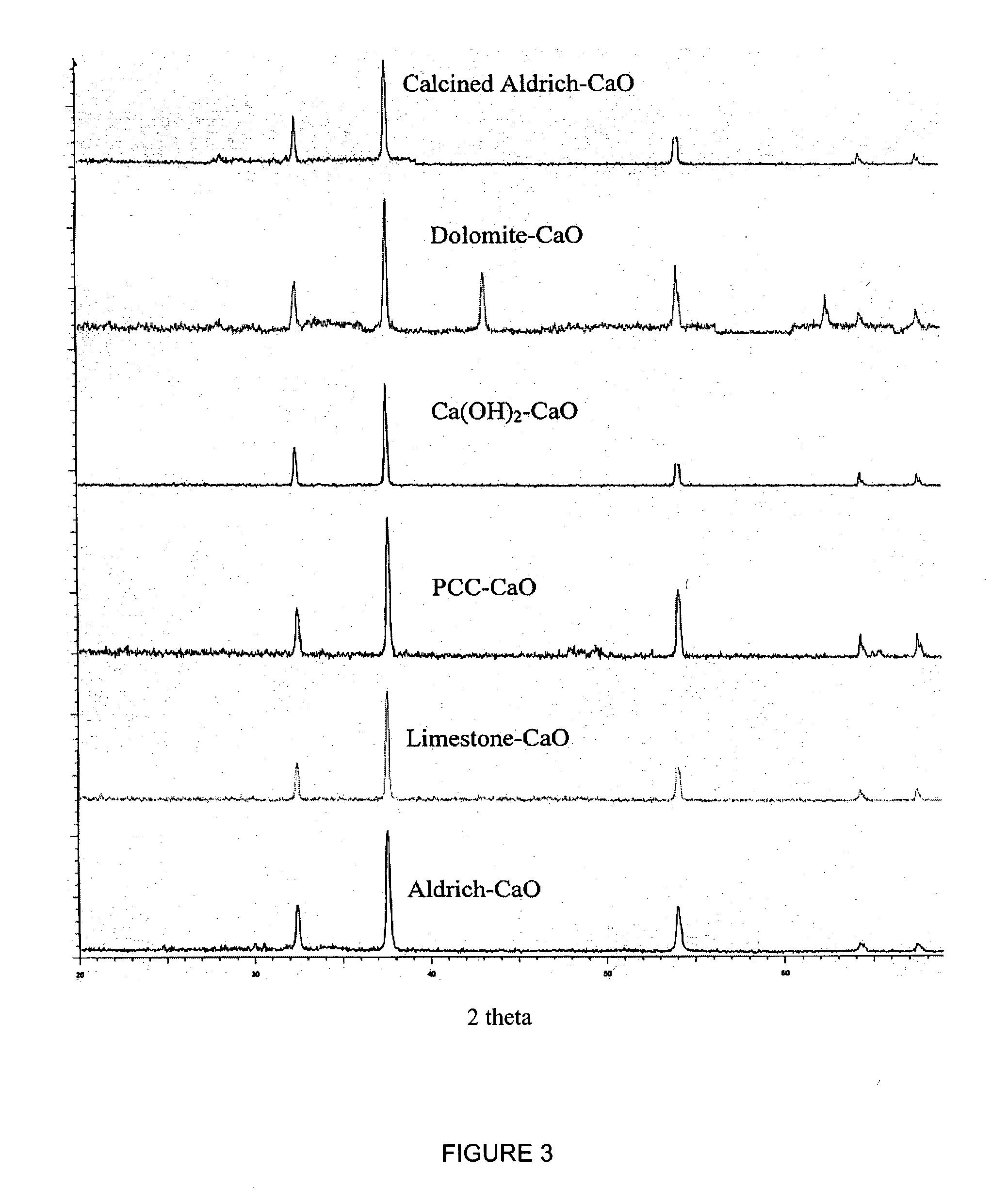
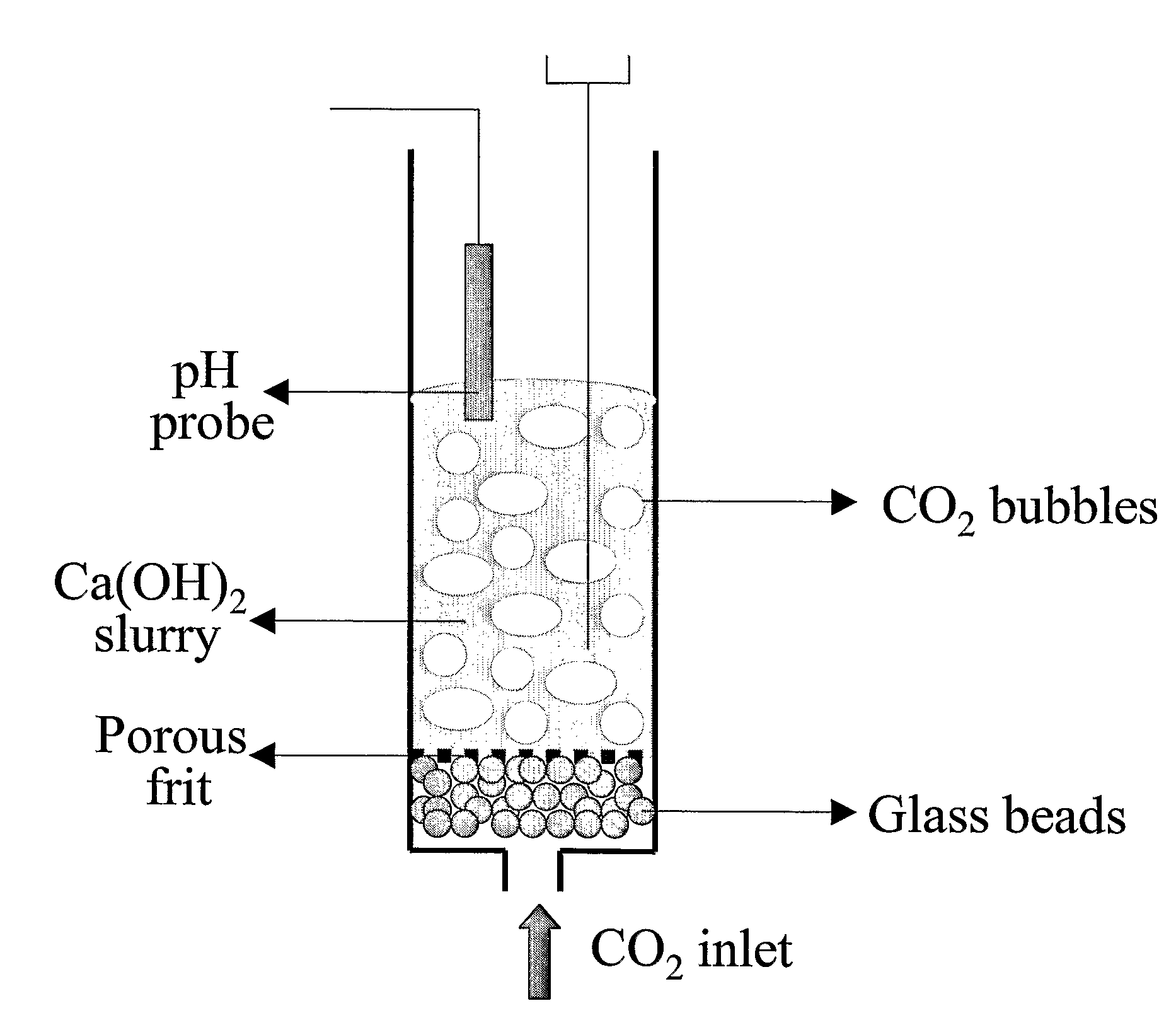
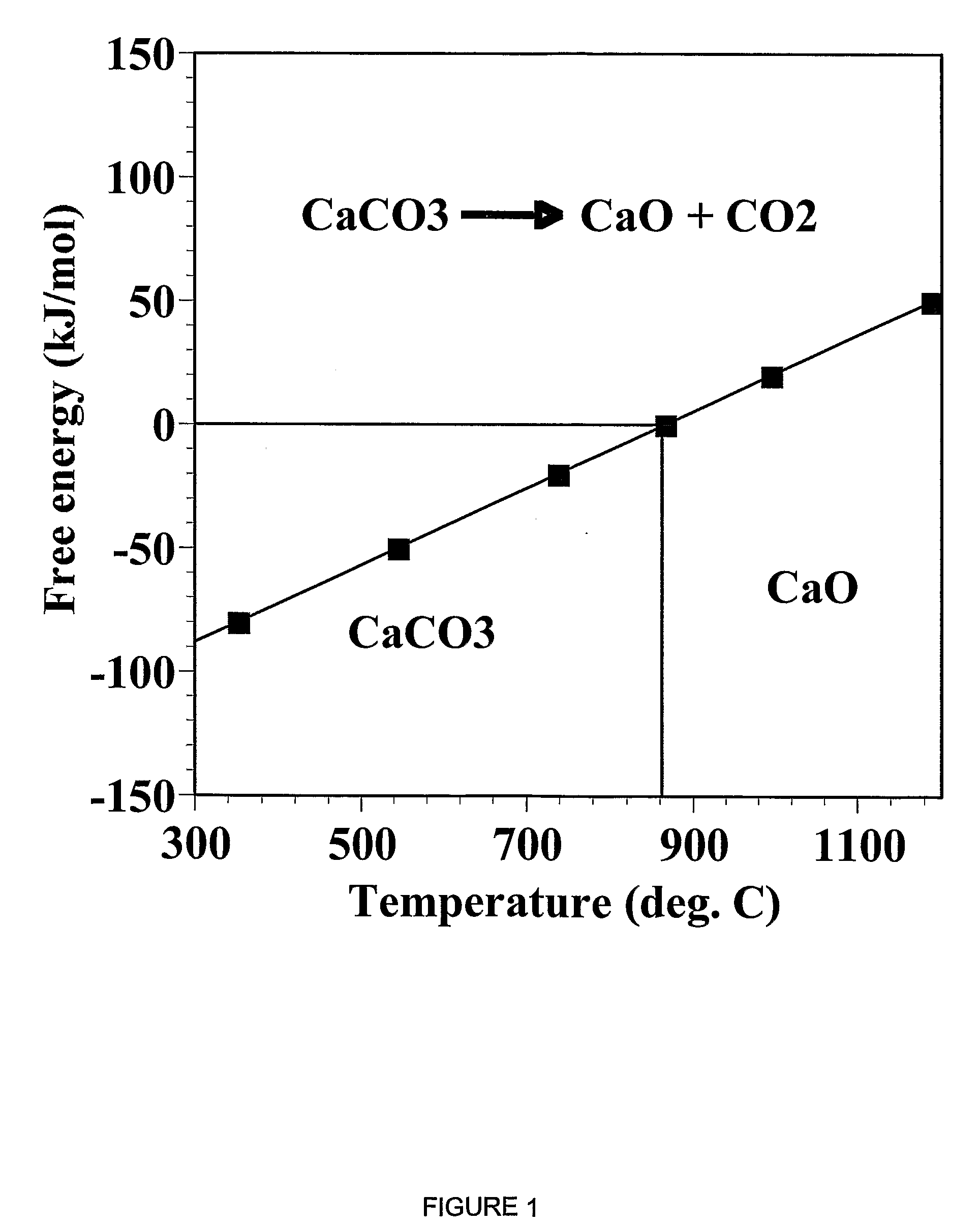
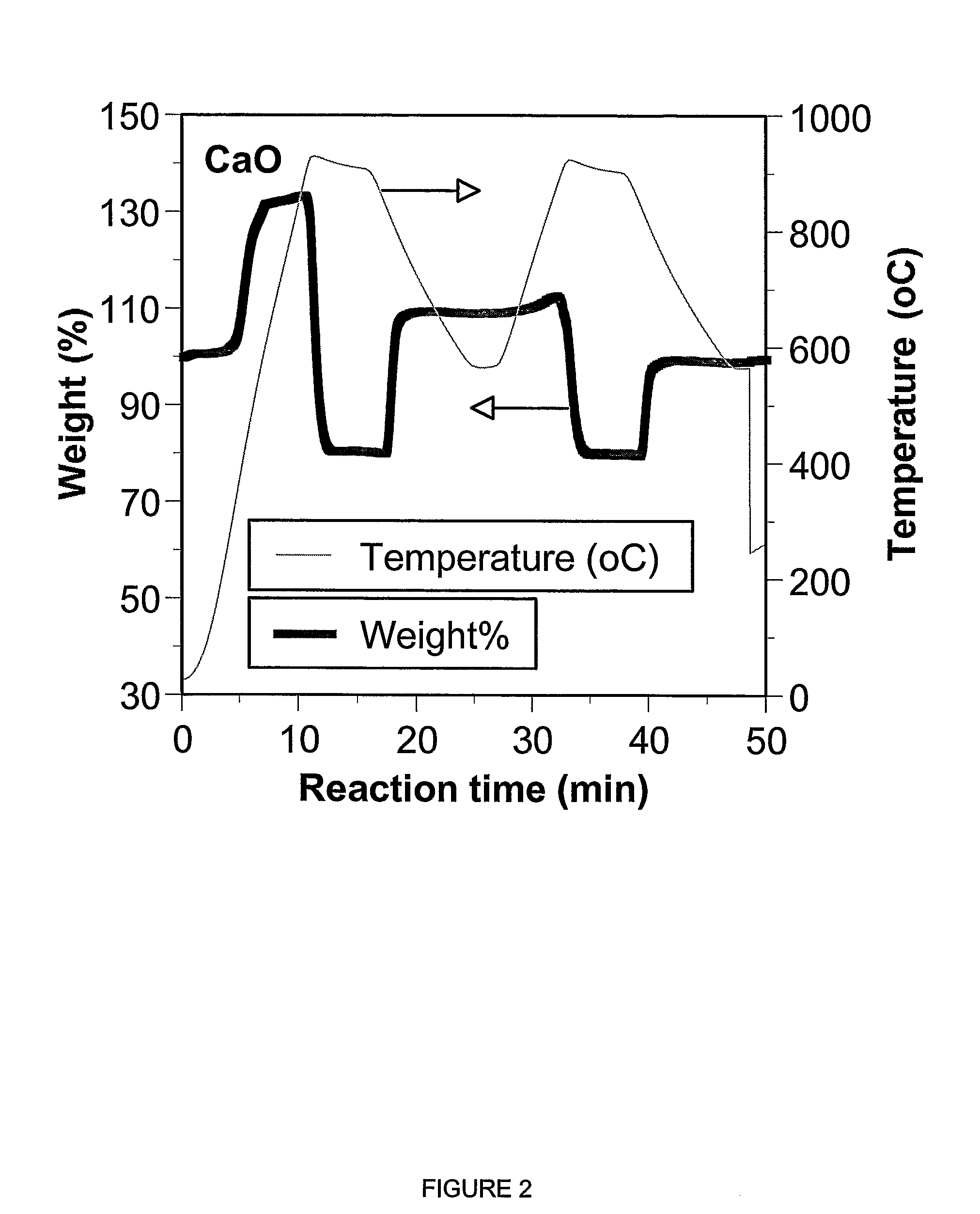
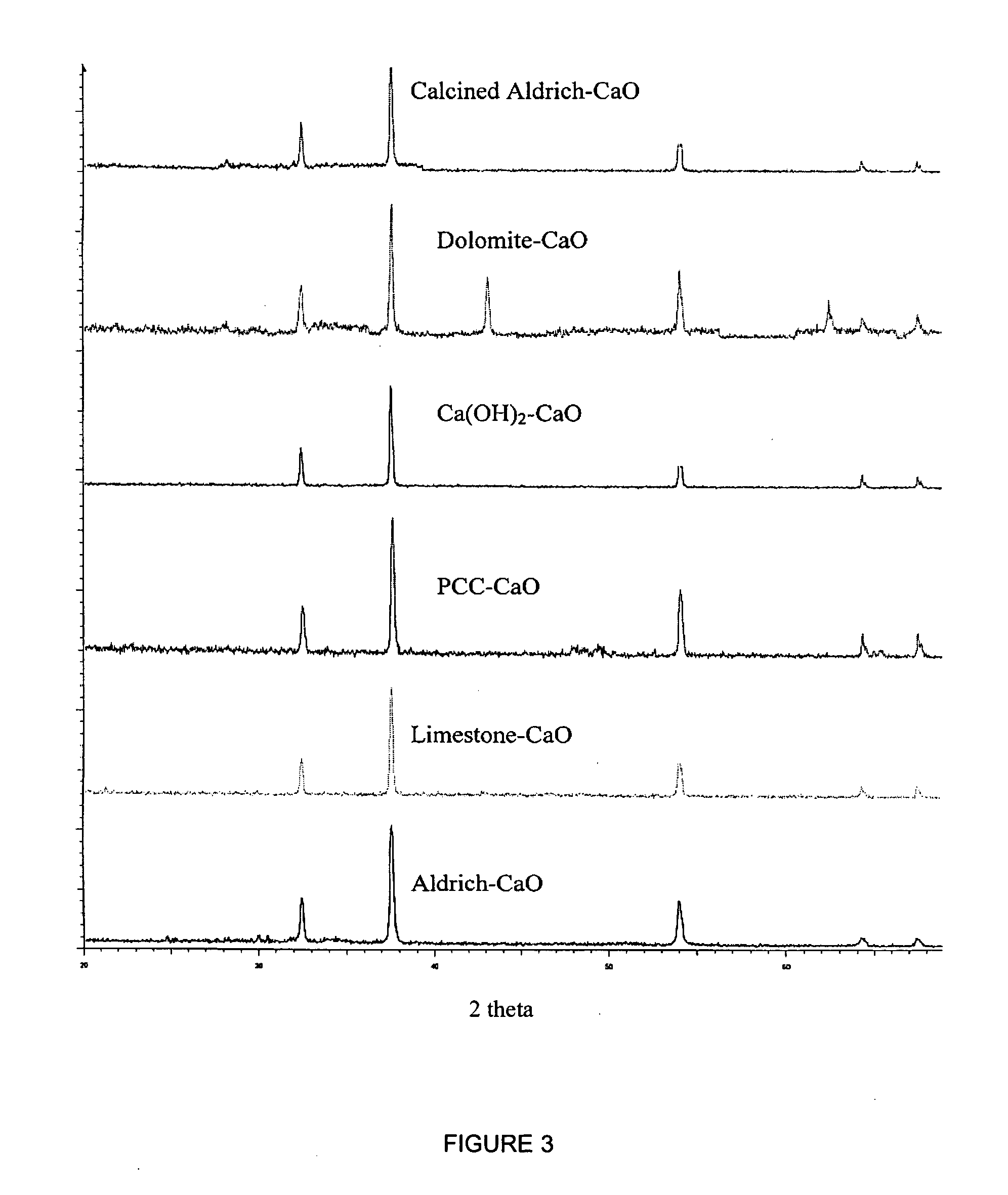
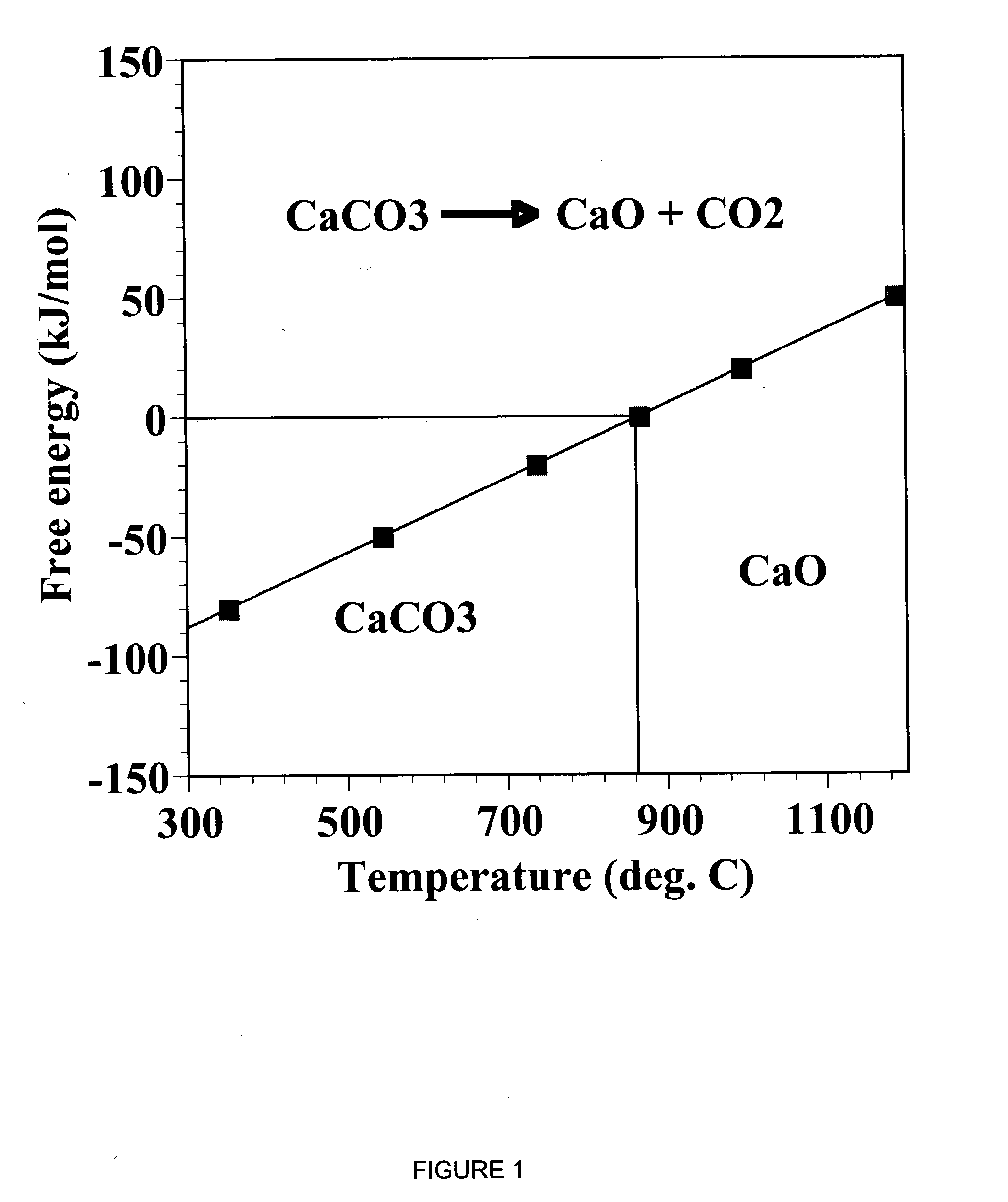
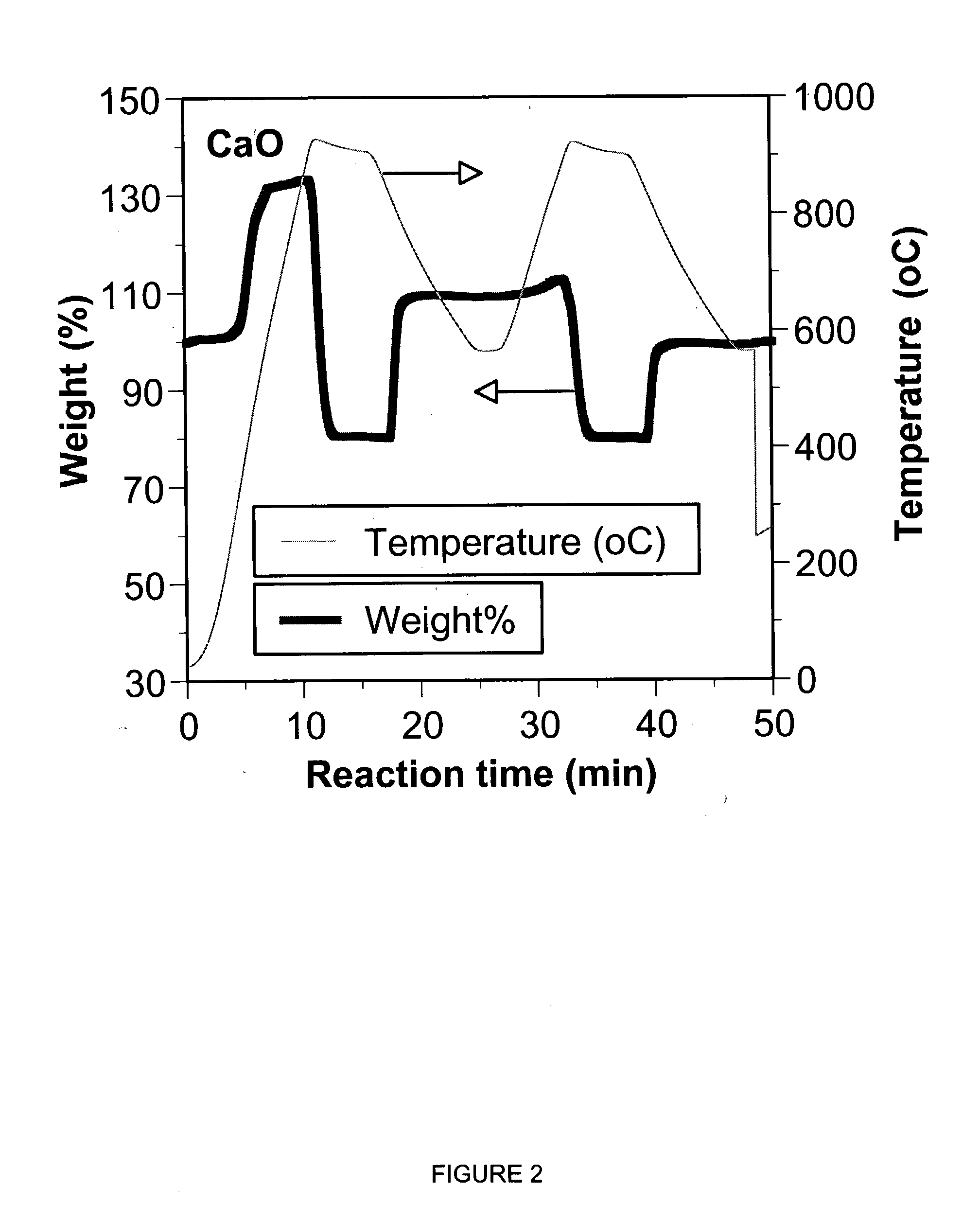
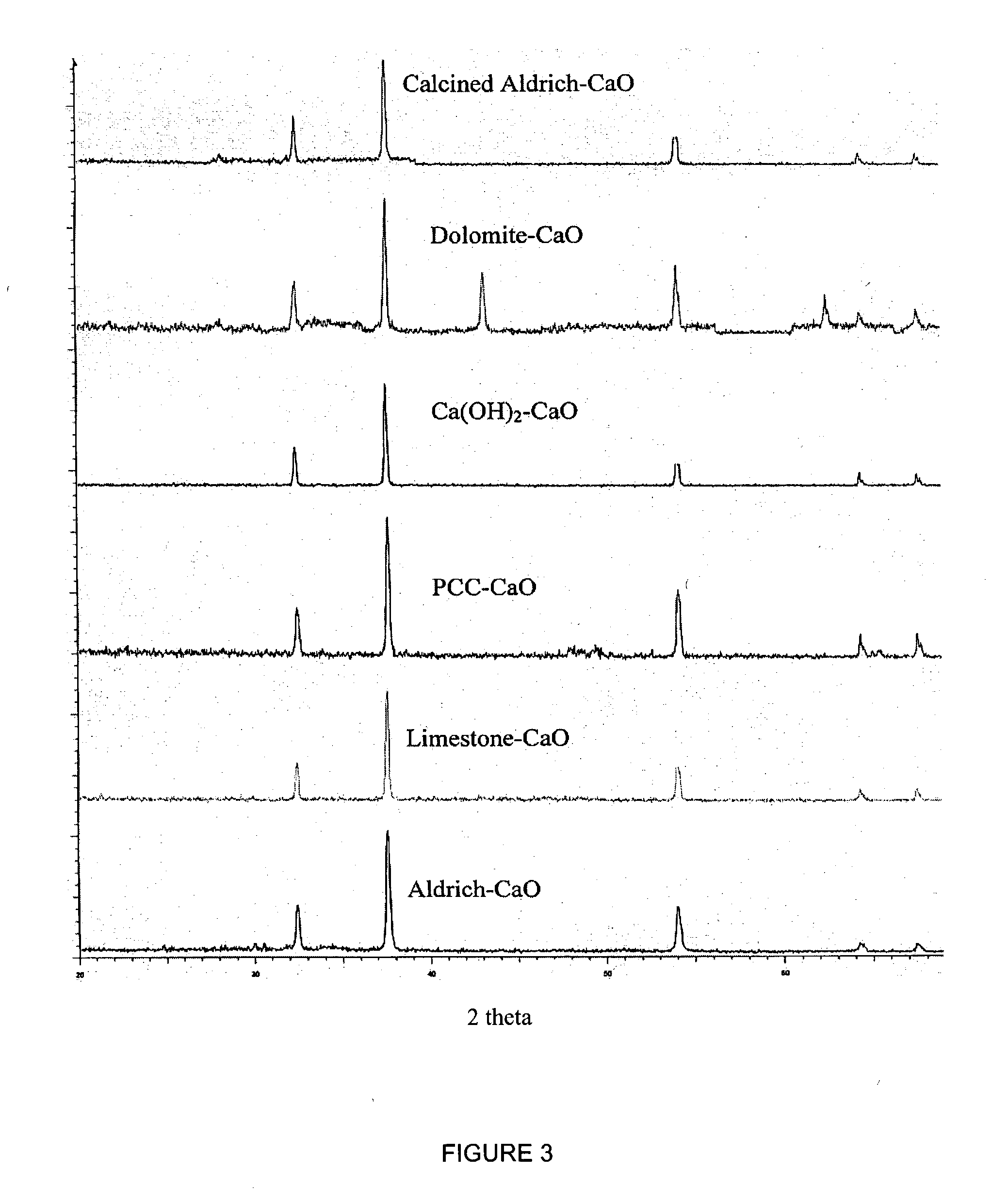
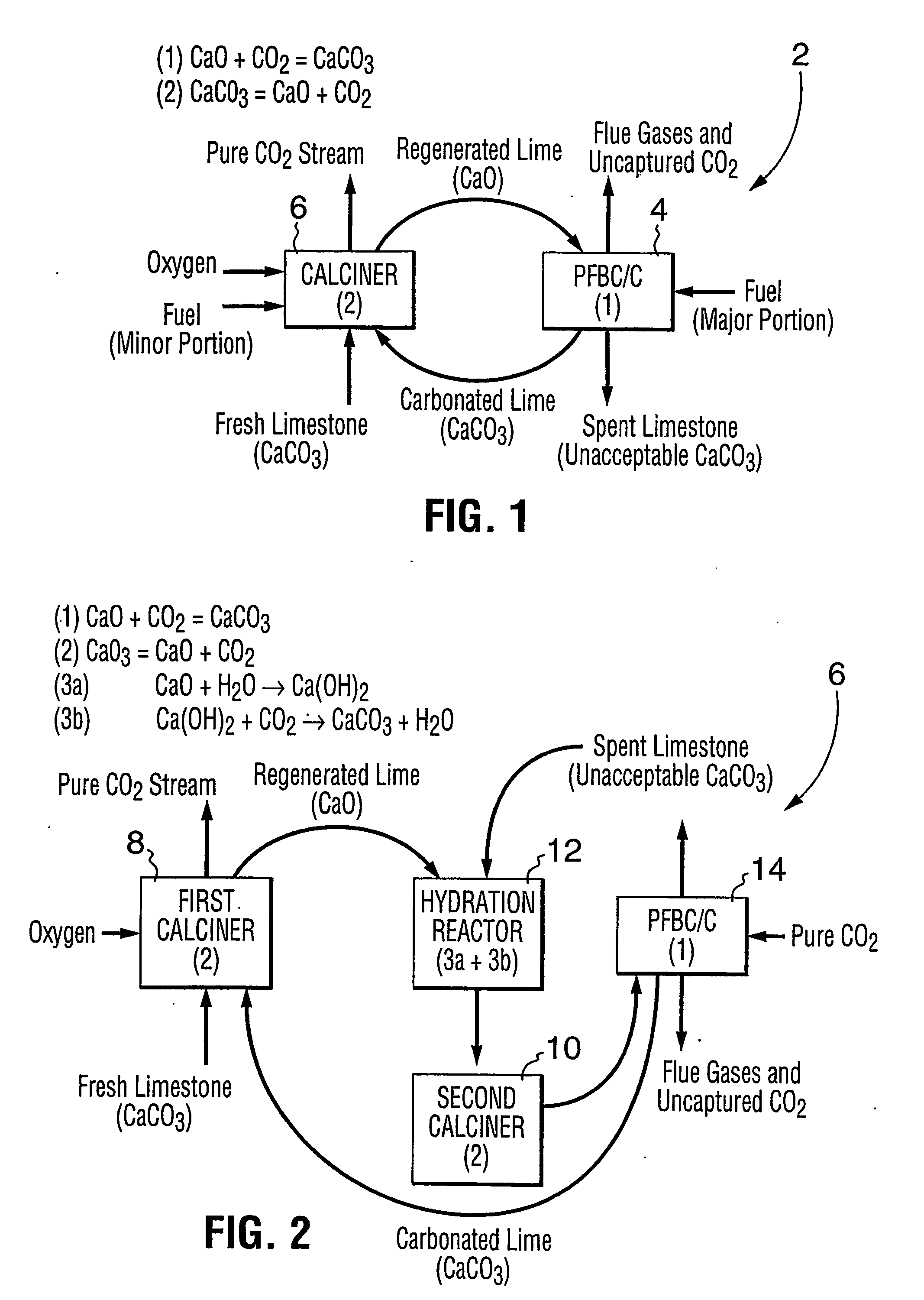
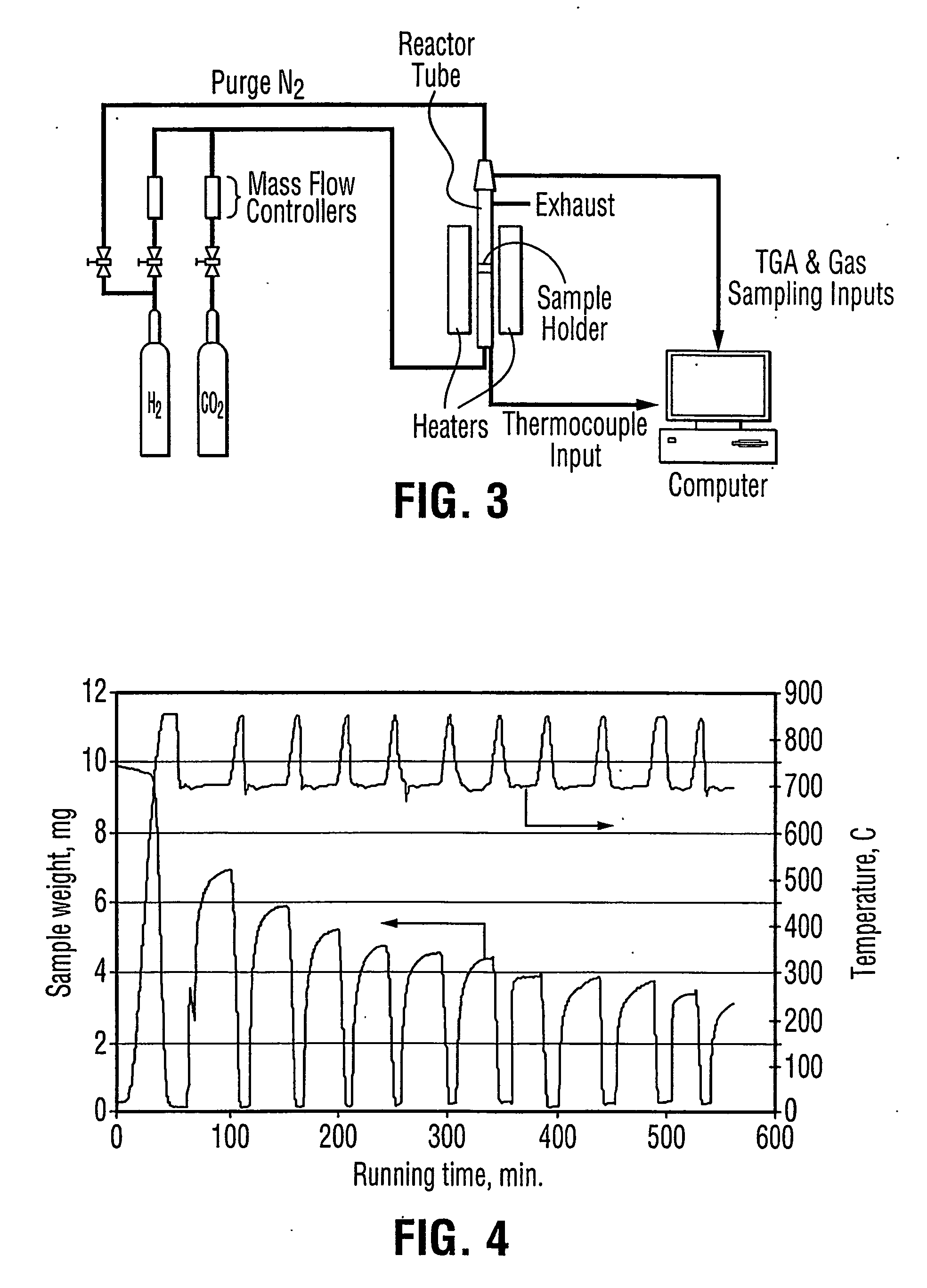
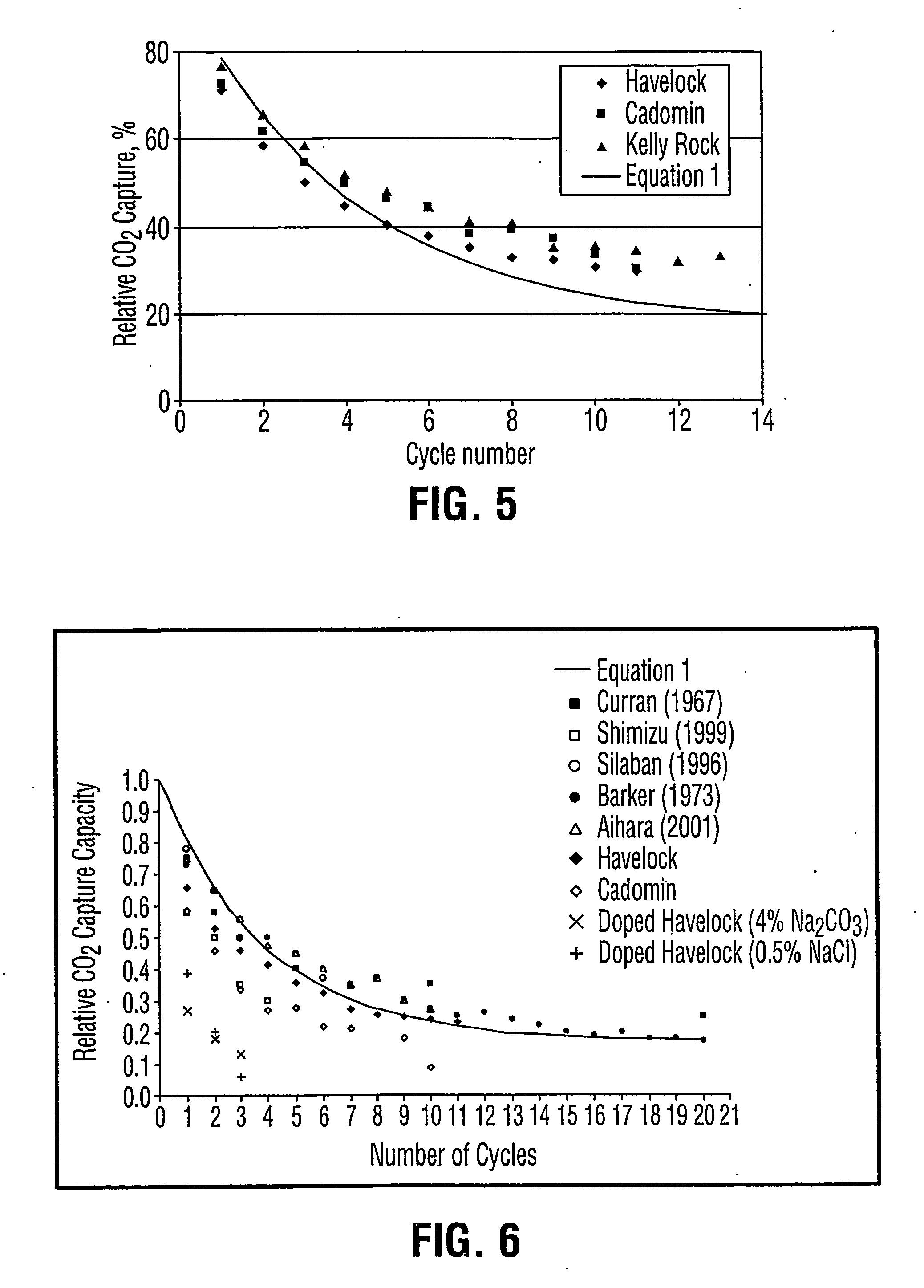
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Separation of Carbon Dioxide (Co2) From Gas Mixtures By Calcium Based Reaction Separation (Cars-Co2) Process
InactiveUS20080233029A1Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentSorbentTransformation ratio
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS—CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCOa). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS—CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Protein beverage and method of making the same
An improved protein beverage / drink composition which may provide a relatively high protein content, ranging from about 0.01% by weight to about 15% by weight, while optionally employing a carbonation concentration between about 0.1 volumes of carbonation (per volume of liquid drink solution or liquid drink suspension) to about 6 volumes of carbonation. Preferably the protein is a protein, such preferably as whey protein, or others. The protein beverage may contain juice and / or an additive which provides energy generation enhancement. The protein beverage may be heat treated to inactivate pathogenic microbes in the presence of the carbonation which may be used to provide taste and mouth feel for the drink. Typically, the treatment for pathogenic microbe inactivation is carried out in the individual package used for storage and handling of the protein drink.
Owner:DAVINAS LLC
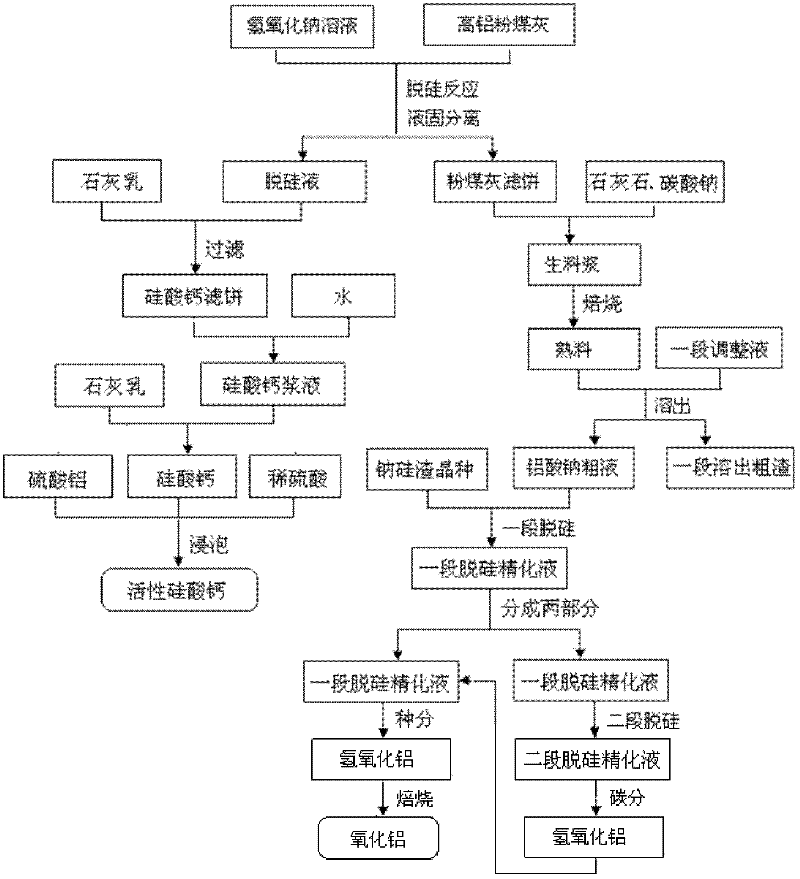
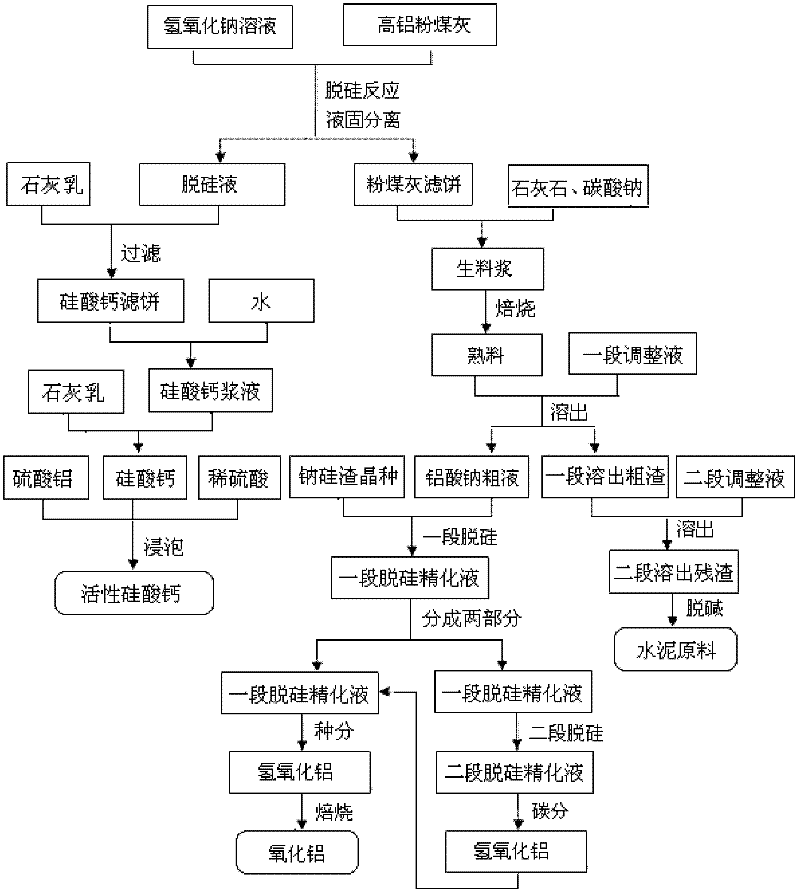
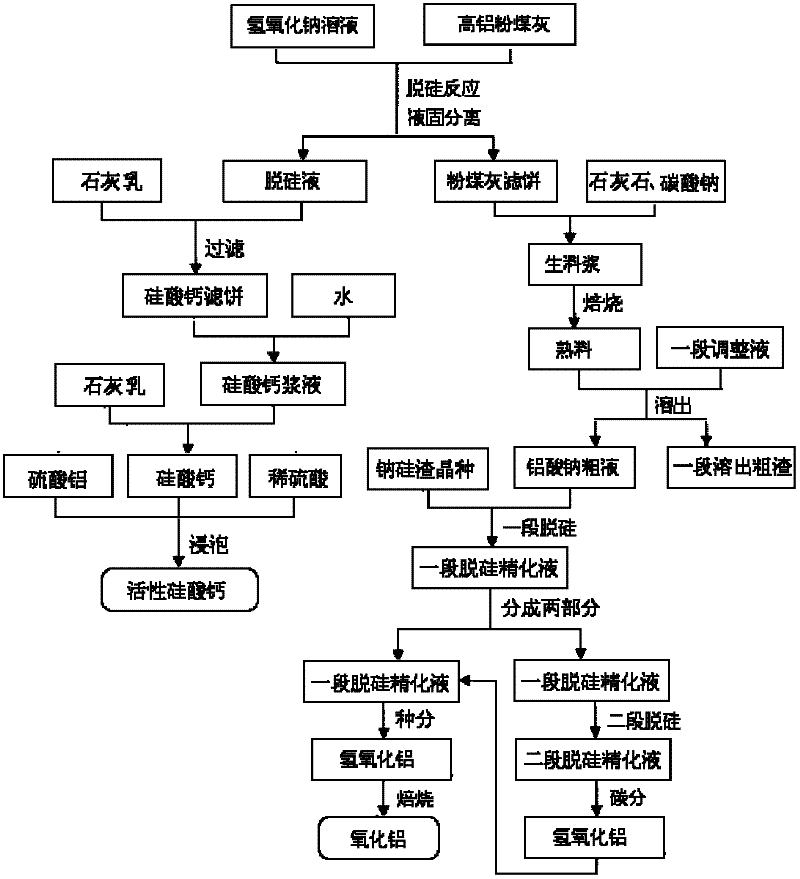
Method for producing aluminum oxide and co-producing active calcium silicate through high-alumina fly ash
ActiveCN102249253AExtraction is effective and cheapIncrease Al-Si RatioAlkaline-earth metal silicatesAluminium oxide/hydroxide preparationCalcium silicateSodium aluminate
The invention provides a method for producing aluminum oxide and co-producing active calcium silicate through high-alumina fly ash. The method comprises the following steps that: the high-alumina fly ash firstly reacts with a sodium hydroxide solution to carry out pre-desilication to obtain a liquid-phase desiliconized solution and a solid-phase desiliconized fly ash; lime cream is added to the liquid-phase desiliconized solution to carry out a causticization reaction, the resulting solid phase is active calcium silicate which is prepared through carrying out filter pressing, flash evaporation and drying to obtain the finished product; limestone and a sodium carbonate solution are added to the desiliconized fly ash to blend qualified raw slurry, then the blend qualified raw slurry is subjected to baking into the clinker, the liquid phase generated from dissolution of the clinker is a crude solution of sodium aluminate; the crude solution of the sodium aluminate is subjected to processes of first-stage deep desilication, second-stage deep desilication, carbonation, seed precipitation, baking and the like to obtain the metallurgical grade aluminum oxide meeting requirements. According to the present invention, the defects in the prior art are overcome; purposes of less material flow and small amount of slaggling are achieved; energy consumption, material consumption and production cost are relative low; extraction rate of the aluminum oxide is high; the calcium silicate with high added value is co-produced; the method provided by the present invention can be widely applicable for the field of chemical engineering.
Owner:INNER MONGOLIA DATANG INT RENEWABLE RESOURCES DEV
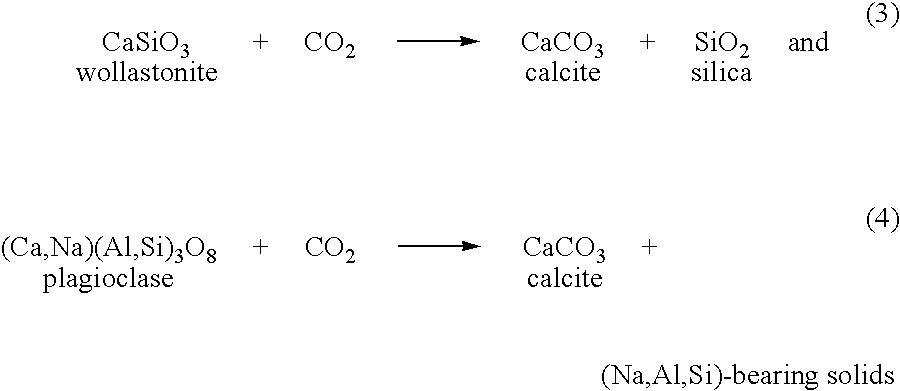
Carbonation of metal silicates for long-term co2 sequestration
In a preferred embodiment, the invention relates to a process of sequestering carbon dioxide. The process comprises the steps of: (a) reacting a metal silicate with a caustic alkali-metal hydroxide to produce a hydroxide of the metal formerly contained in the silicate; (b) reacting carbon dioxide with at least one of a caustic alkali-metal hydroxide and an alkali-metal silicate to produce at least one of an alkali-metal carbonate and an alkali-metal bicarbonate; and (c) reacting the metal hydroxide product of step (a) with at least one of the alkali-metal carbonate and the alkali-metal bicarbonate produced in step (b) to produce a carbonate of the metal formerly contained in the metal silicate of step (a).
Owner:UT BATTELLE LLC +2
Separation of carbon dioxide (CO2) from gas mixtures by calcium based reaction separation (CaRS-CO2) process
ActiveUS20060093540A1Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentReactive separationCarbonation
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS—CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS—CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Separation of carbon dioxide (CO2) from gas mixtures by calcium based reaction separation (CaRS-CO2) process
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Pre-treatment of lime-based sorbents using hydration
InactiveUS20070092427A1Increase capacityIncrease carbon dioxide capture capacityCalcium/strontium/barium carbonatesOther chemical processesCombustionSorbent
The present invention discloses a method and an apparatus for reactivating lime-based sorbents and increasing the carbon dioxide-capture capacity of the sorbent in the combustion of carbon-containing fuels. The present invention teaches the pretreatment of the lime-based sorbent using a hydration process after each process of carbon dioxide separation. The invention is useful in reducing the need to add additional sorbent to maintain the carbonation / calcination cycle. The regenerative potential of the sorbent as manifested by the present invention leads to increased carbon dioxide-capture capacity of the sorbent.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF NATURAL RESOURCES
Carbonated fortified milk-based beverage and method for suppressing bacterial growth in the beverage
InactiveUS6866877B2Increase attractivenessFast absorptionMilk preparationVitamin food ingredientsAdditive ingredientPasteurization
Dairy or non-diary based fortified carbonated beverage solutions that supply essential nutrients in the human diet. The solution contains per 354 ml, calcium, magnesium and potassium ions in the form of salts and optionally vitamins A, D, C, lutein, zeaxanthin and folic acid in specified amounts to provide dietary supplementation. Sweeteners, stabilizers, flavors and carbonation can also be added to enhance flavor, taste, mouth-feel, ingredient solubilization and product appearance. A method of making the beverages is also described. A method of using carbonation to reduce bacterial counts and reduce degradation of essential nutrients in milk-based beverages with or without pasteurization is also disclosed.
Owner:MAC FARMS
System, method and compositions for dispensing a liquid beverage concentrate
InactiveUS20050178793A1Add flavorGreat tastePower operated devicesFrozen sweetsMaillard reactionNon dairy
A beverage system for providing a beverage, methods of making the beverage and the resulting beverage are disclosed herein. The system includes a beverage-forming concentrate and an aroma or aroma-providing component separated from the concentrate; wherein the concentrate and aroma are combinable upon reconstitution for providing the beverage. One method includes delivering a fresh beverage taste to an on-premise beverage at a point of dispensation, by delivering at least one aroma or aroma-providing component in an amount sufficient to enhance the organoleptic properties of a beverage separately from a beverage concentrate prior to when the beverage is dispensed, and mixing the aroma or aroma-providing compound with a liquid and the beverage concentrate or with a mixture of a beverage concentrate and a liquid when the beverage is being dispensed. The resulting beverage may be coffee, tea, carbonation, a juice, milk, or a non-dairy creamer-based component; or a combination thereof, while the aroma or aroma-providing component is coffee aroma, tea aroma, chocolate or cocoa aroma, malt, Maillard reaction flavor, or a combination thereof.
Owner:NESTEC SA
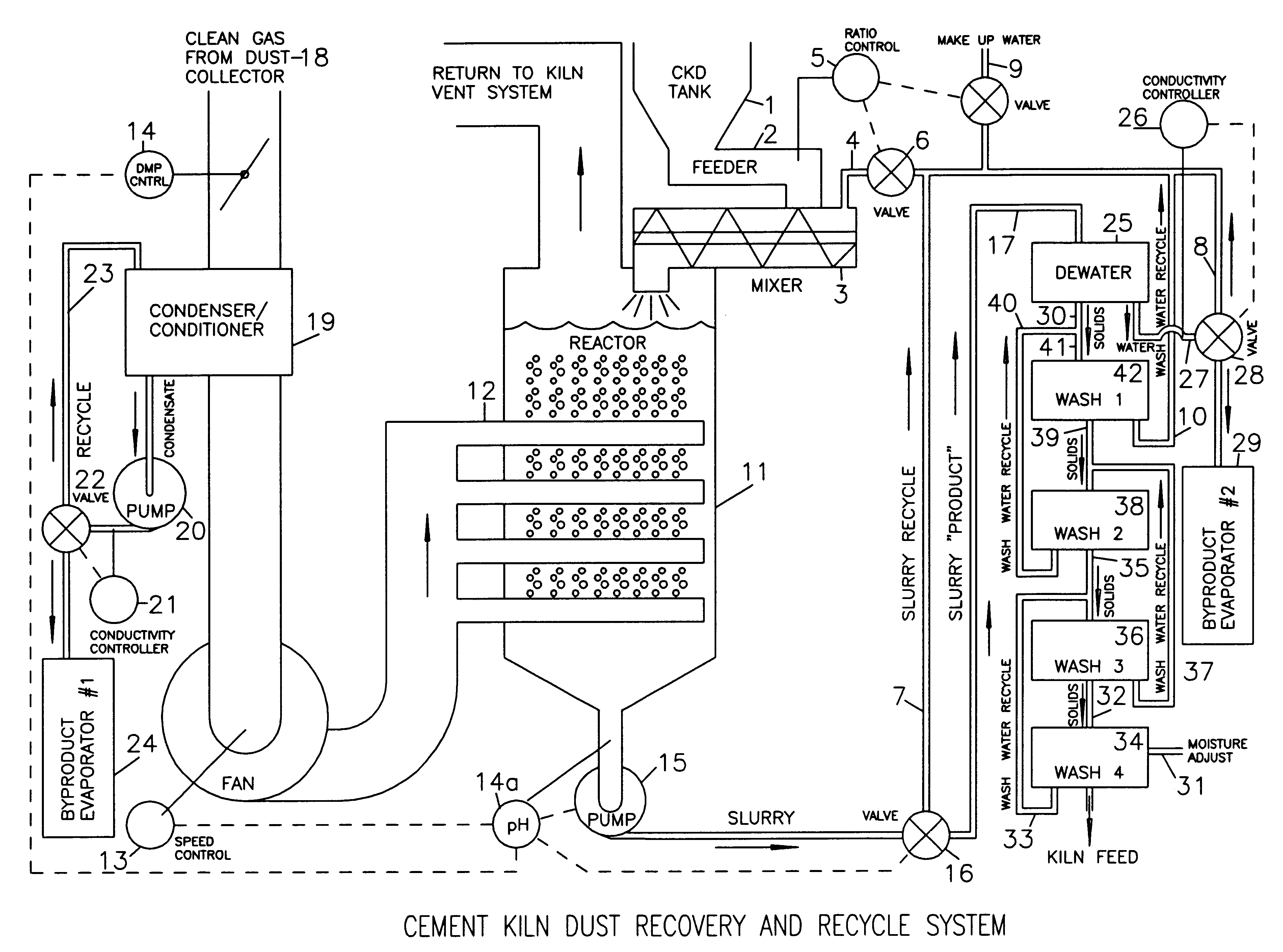
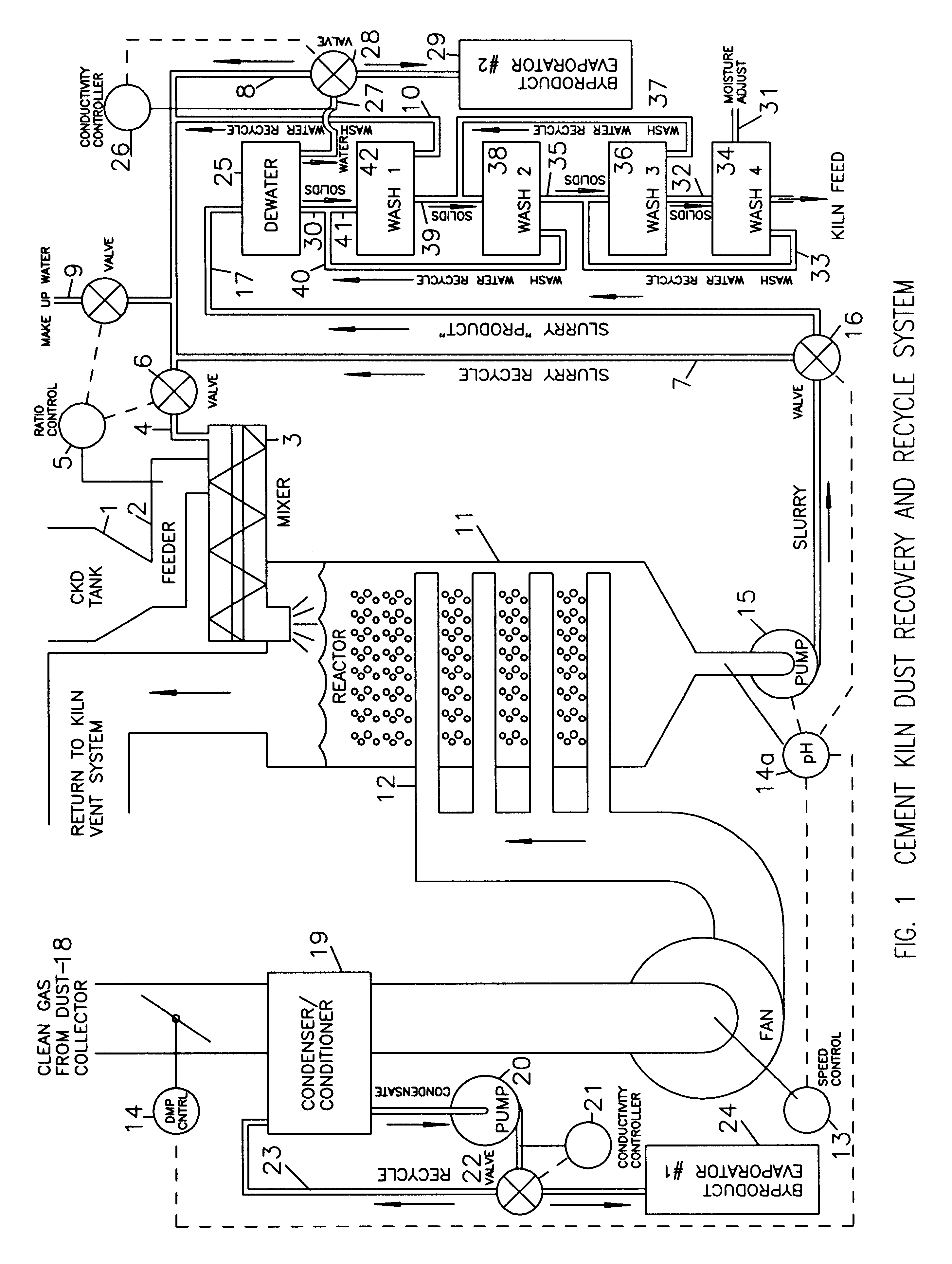
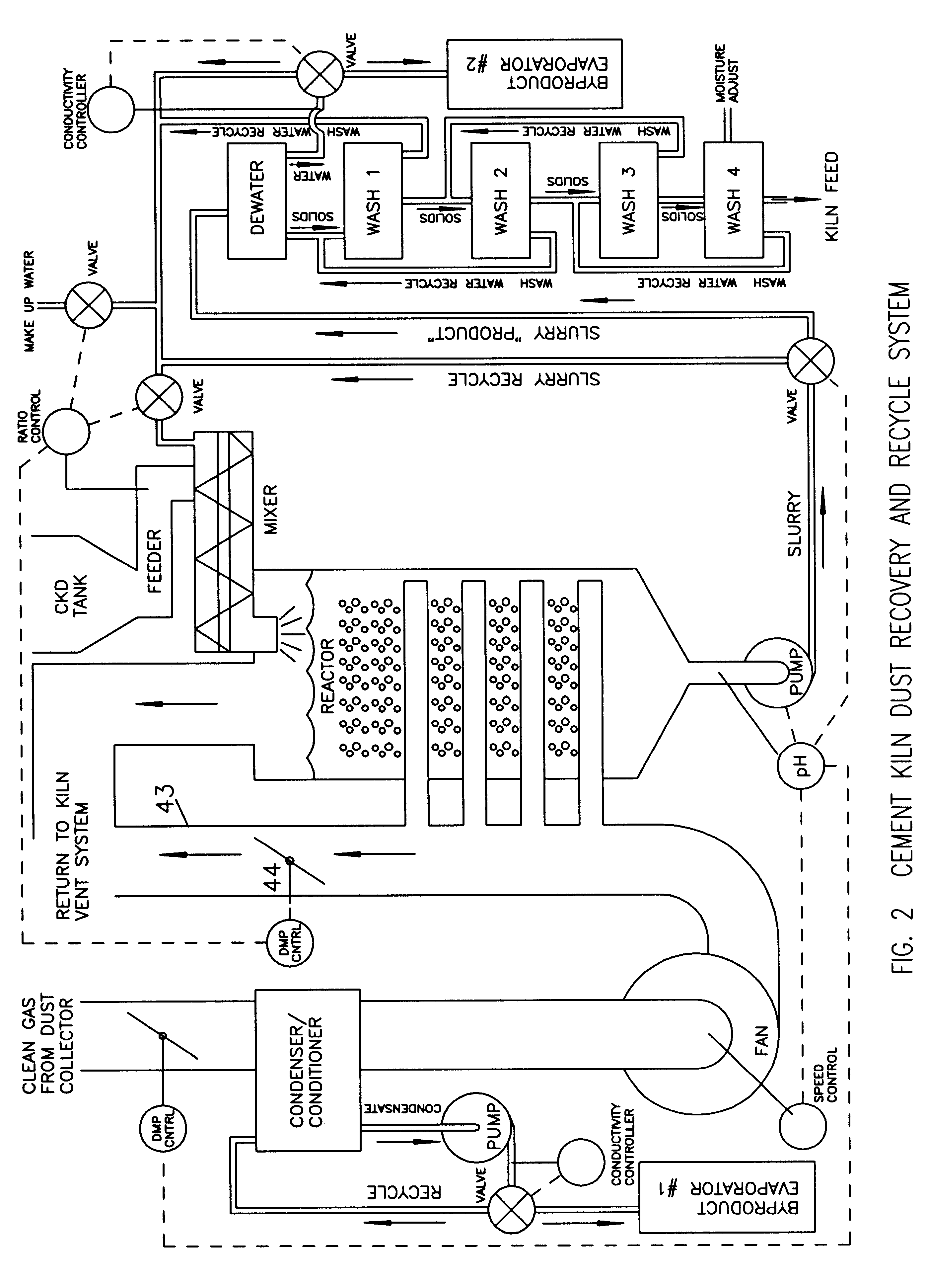
Method of treating cement kiln dust for recovery and recycle
Fresh or stockpiled cement kiln dust is moistened with sufficient water so that the amount of total free and combined water relative to dust is about 3 parts water to 1 part dust by mass, or less. The wet solids are treated with carbon dioxide to convert compounds, such as calcium hydroxide, to carbonates, such as calcium carbonate. The degree of carbonation is controlled so that the solubility of calcium becomes minimum for the dust being treated; this is also when hydroxyl and bicarbonate ions in solution are about at their minima. As the carbonation reactions occur, the water combined in hydroxides is released as free water so that the mixture becomes a slurry and the potentially soluble alkalies and sulfate (and any chlorides present) are released to the liquid phase. The solids are separated from the liquid, and the solids, which may be washed, provide a material suitable for return as feed to the kiln. The liquid, which contains the dissolved alkali compounds, is recycled to reclaim additional dust or treated to recover alkali salts when the alkali salts are sufficiently concentrated.While any source of carbon dioxide may be used, the preferred source is exit gases from the kiln. The gases are conditioned by condensation of water and removal of ammonium compounds, such as sulfate and chloride. The conditioning condensate may be treated to recover useful byproduct salts.
Owner:GEBHARDT RONALD FR
Additive for Carbonated Beverage
It is intended to provide a carbonated beverage excelling in function as thirst-quenching beverage and taste through strengthening or sustaining of carbonated feel peculiar to carbonated beverage (brisk intense stimulation and sensation at pass through the throat, brought about by carbon dioxide gas, and sensation of coolness (refreshing sensation) produced by a combination of carbon dioxide gas and flavor) which after opening, rapidly drops depending upon a combination of employed raw materials, such as fruit juice, sweetener, color additives and flavoring, and upon evaporation of carbon dioxide gas. The drop of carbonated feel brought about depending upon a combination of raw materials, such as fruit juice and sweetener, can be relieved by adding to a carbonated beverage an additive for carbonated beverage comprising spilanthol or a spilanthol-containing plant extract or plant essential oil as an active ingredient. Further, the addition would help inhibit drop of carbonated feel brought about depending upon evaporation of carbon dioxide gas after opening and would help retain the taste and function as function as thirst-quenching beverage inherently to be had by carbonated beverage even after opening.
Owner:OGAWA & CO LTD
Physical endurance drink and method of preventing cramping caused by strenuous bodily activity
InactiveUS6039987AImprove palatabilityPrevent crampsDough treatmentConfectioneryPhysical EndurancesCarbonation
A composition to prevent dehydration, supply energy and prevent cramps which contains electrolytes, carbohydrates and quinine and / or quinine salts and a method of preventing cramping caused by strenuous bodily activity. Palatability of the composition is improved by the effective use of carbohydrate, pH adjustment, flavoring and carbonation.
Owner:STRAHL ROBERT CHARLES
Carbonated fortified milk-based beverage and method for suppressing bacterial growth in the beverage
InactiveUS20030113408A1Great tasteIncrease bodyMilk preparationVitamin food ingredientsAdditive ingredientPasteurization
Dairy or non-diary based fortified carbonated beverage solutions that supply essential nutrients in the human diet. The solution contains per 354 ml, calcium, magnesium and potassium ions in the form of salts and optionally vitamins A, D, C, lutein, zeaxanthin and folic acid in specified amounts to provide dietary supplementation. Sweeteners, stabilizers, flavors and carbonation can also be added to enhance flavor, taste, mouth-feel, ingredient solubilization and product appearance. A method of making the beverages is also described. A method of using carbonation to reduce bacterial counts and reduce degradation of essential nutrients in milk-based beverages with or without pasteurization is also disclosed.
Owner:MAC FARMS
Process for producing sodium bicarbonate for flue gas desulphurization
InactiveUS20100290967A1Electrolysis componentsVolume/mass flow measurementSodium bicarbonateFlue gas
Process for producing sodium bicarbonate for purifying flue gases, according to which an aqueous solution containing sodium sulfate is subjected to electrodialysis to produce a sodium hydroxide solution and a sodium bisulfate solution, the sodium hydroxide solution being carbonated in order to obtain sodium bicarbonate.
Owner:SOLVAY SA
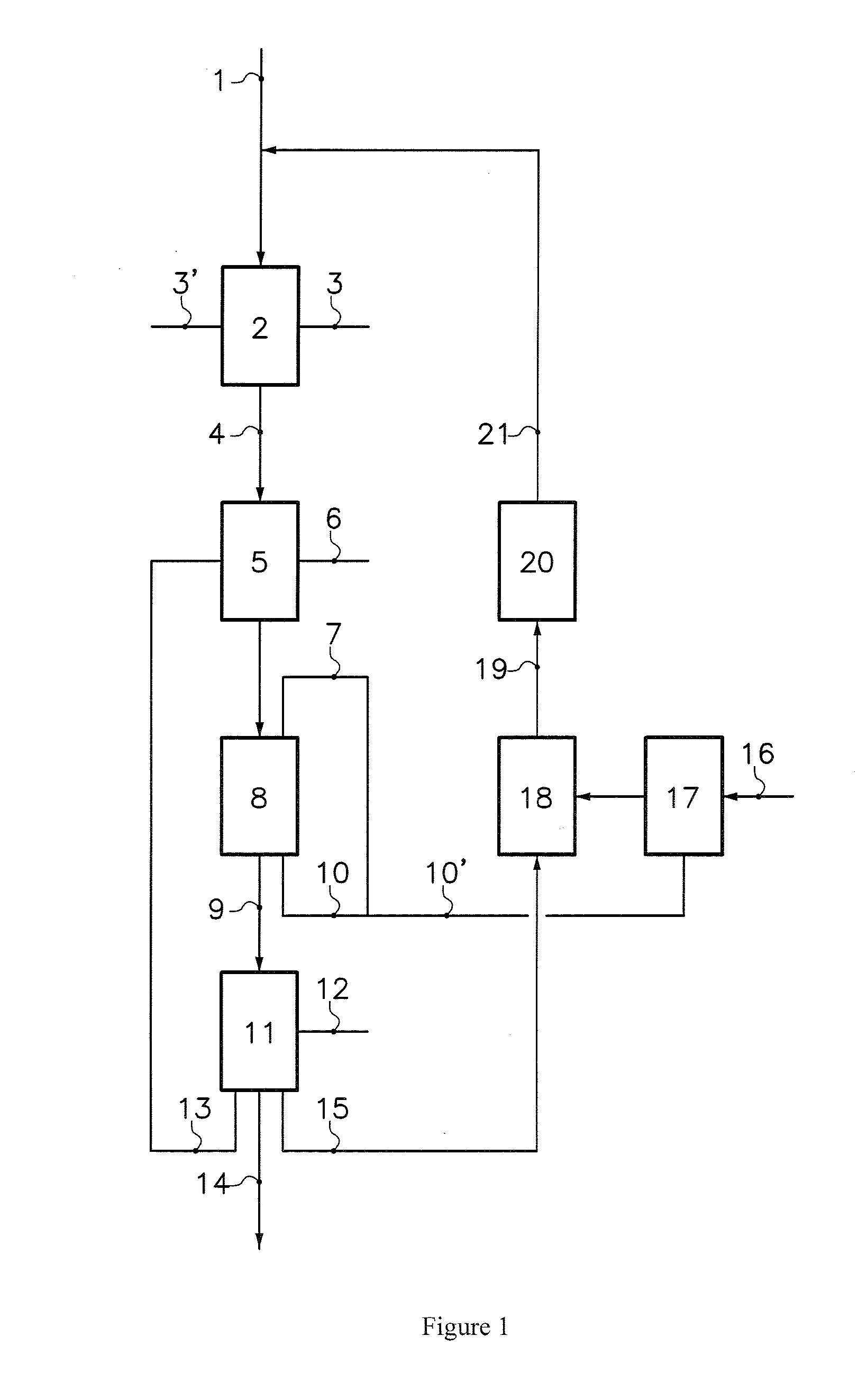
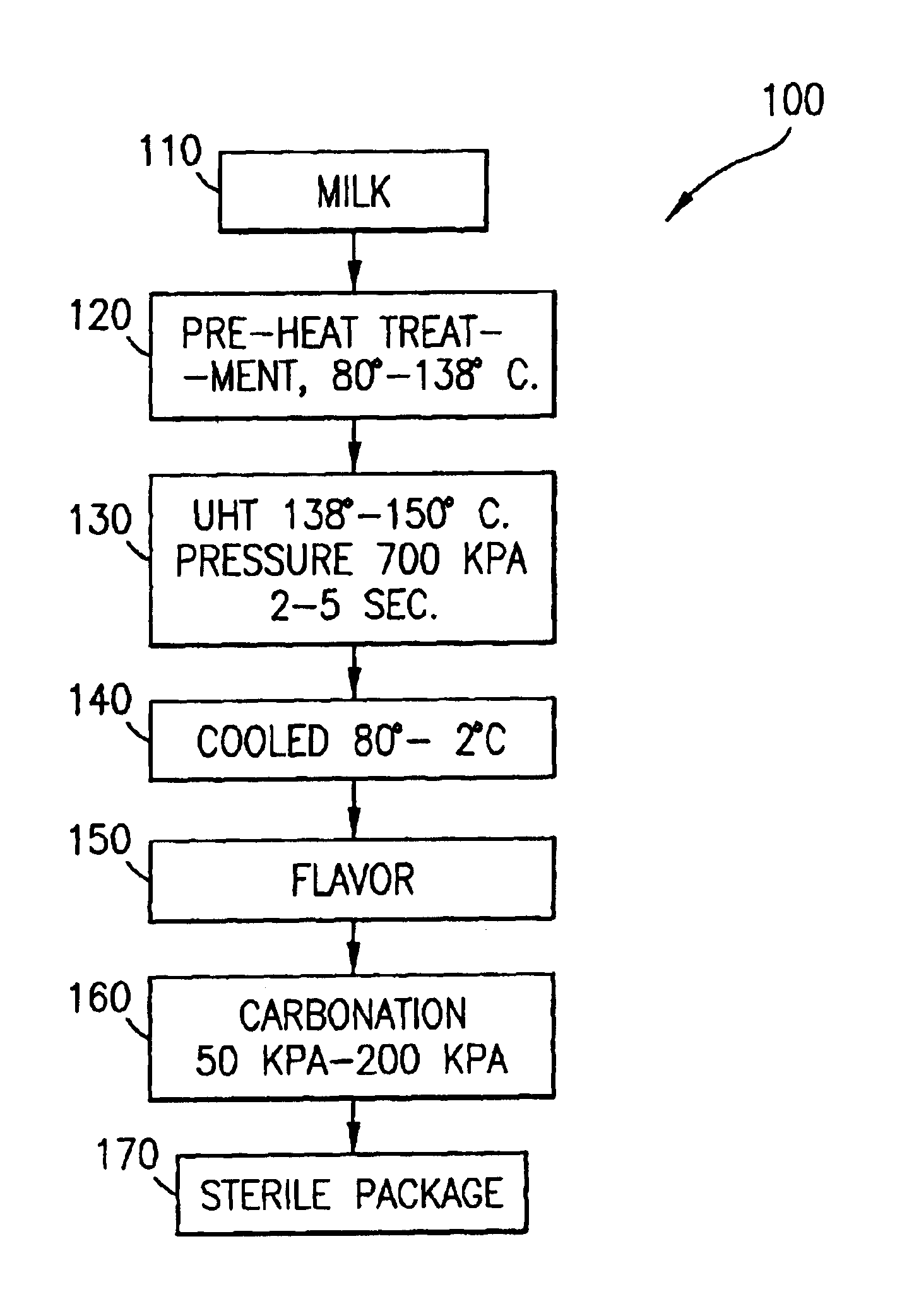
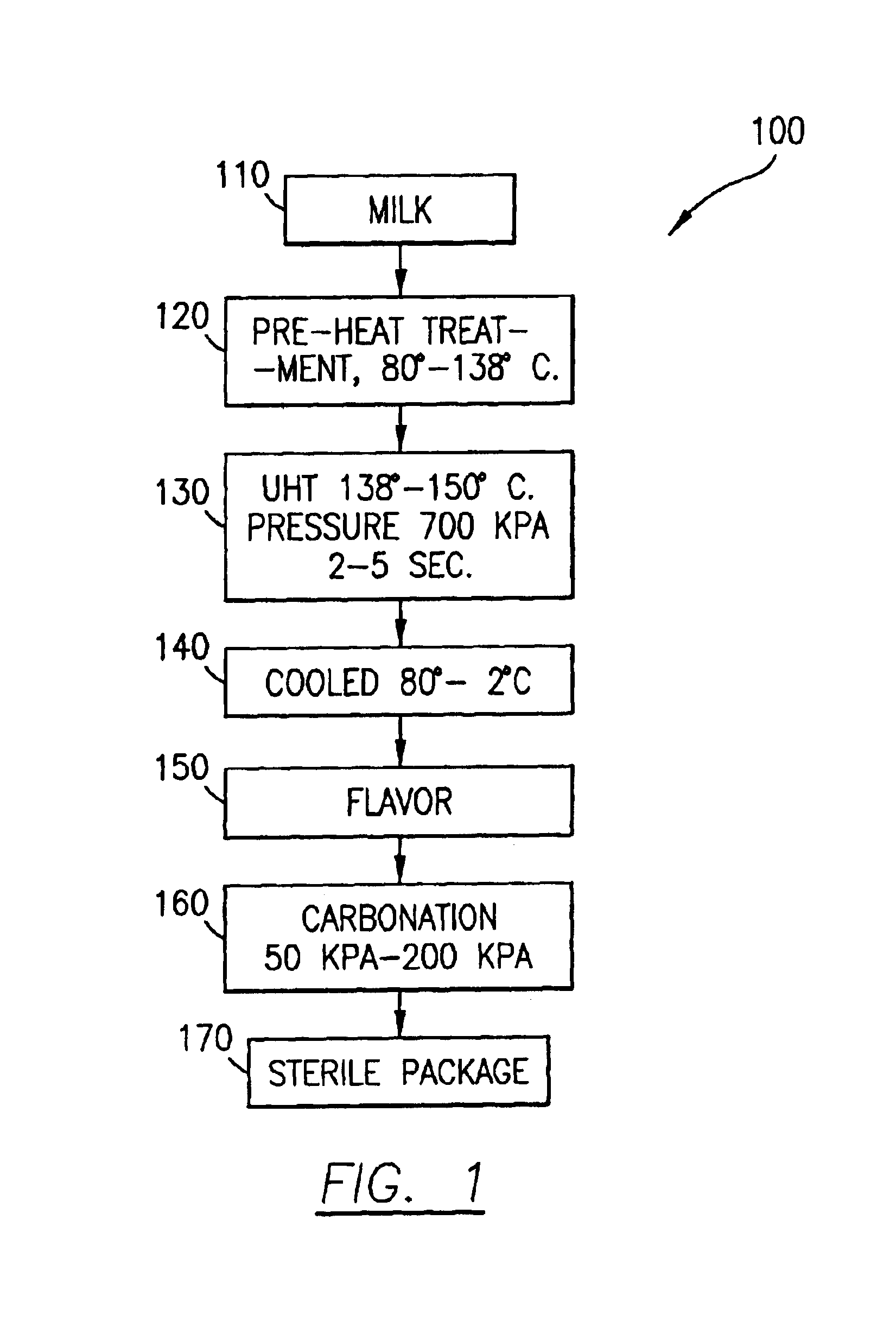
Process for making shelf-stable carbonated milk beverage
An aerated or carbonated milk product drink having a shelf stable pre-heated and pressurized ultra-heat treated milk product which has been carbonated with a gas or gases under pressure and packaged into a container. The milk product may be natural or artificial milk product including dairy products and non-dairy milk products and includes combinations of milk products with other beverages such as fruit juices. The method of producing the shelf-stable carbonated milk product of the present invention comprises injecting under pressure carbon dioxide gas or a mixture of gases into the milk product at low temperature of less than 10 degrees centigrade and high pressure of from 50 kpa to 200 kpa. The carbonated milk product remains carbonated and shelf stable in the package until opened.
Owner:KROLL DIANE +1
Method for firstly extracting silicon and secondly extracting aluminum from fly ash
ActiveCN101125656AImprove resource utilization valueIncrease Al-Si RatioAluminium oxide/hydroxide preparationSilicon oxidesSODIUM SILICATE SOLNCarbonation
Owner:PINGSHUO INDAL
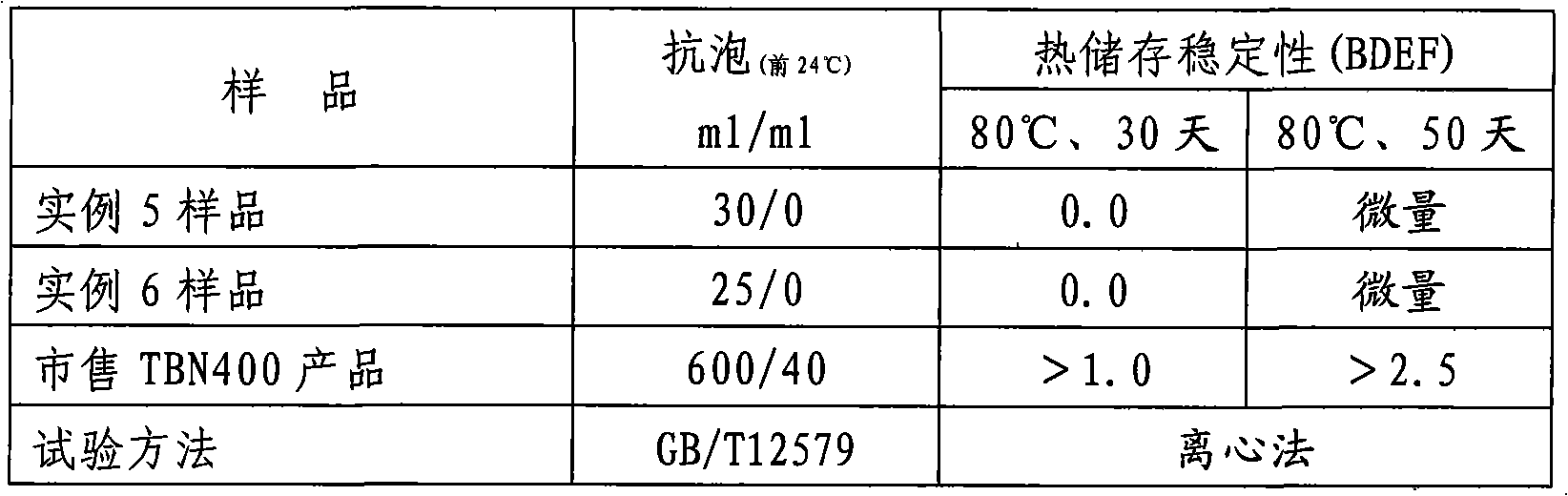
Method for preparing high-alkali value (TBN400) synthesized calcium alkyl benzene sulfonate
ActiveCN101318915AImprove cleanlinessGood dispersionAdditivesSulfonic acid preparationTotal Base NumberAlkaline earth metal
The invention provides a method for preparing high base number (TBN400) synthetic calcium alkyl-benzene sulfonate. The method comprises the following steps of: adopting a mixed acid of long-chain linear alkyl-benzene sulfonic acid and high-boiling heavy alkyl-benzene sulfonic acid, calcium oxide and / or calcium hydroxide, low-carbon alcohol, alkaline-earth metal halide or nitrate, and a mixture of alkaline-earth metal alkylphenol or alkaline-earth metal alkylphenate and polyisobutylene succinic anhydride for a neutralization reaction in the presence of a solvent and cutback oil at a temperature of between 40 and 80 DEG C; then, passing through carbon dioxide to a product of the neutralization reaction at a temperature of between 40 and 60 DEG C for a carbonation reaction; and producing high base synthetic alkyl-benzene sulfonate with a total base number (TBN) of 400mgKOH / g by adopting a process of a one-step method. The product is divided into high-base number (TBN400) synthetic alkyl-benzene sulfonate containing chlorine and high-base number (TBN400) synthetic alkyl-benzene sulfonate without the chlorine. The product produced by adopting the method with low viscosity, small turbidity, easy filtration, light color and no skin formation has the advantages of excellent high-temperature detergency, excellent anti-foaming property and excellent heat storage stability.
Owner:JINZHOU DPF TH CHEM CO LTD
Method for preparing aragonite type calcium carbonate whisker
InactiveCN1641077AReduce manufacturing costReduce qualityCalcium/strontium/barium carbonatesPolycrystalline material growthMagnesium saltDiameter ratio
The present invention relates to the preparation process of high purity aragonite type calcium carbonate whisker. The present invention prepares aragonite type calcium carbonate whisker with lime or slaked lime as main material and magnesium salt as crystal salt controlling agent and through a CO2 carbonating process. The process features the repeated use of the magnesium salt solution and the homogeneous replenishment of lime slurry during carbonating reaction. The process has low production cost, less environmental pollution and high calcium carbonate whisker yield, and the prepared calcium carbonate whisker has high purity and high length / diameter ratio. The process is suitable for industrial production of calcium carbonate whisker.
Owner:NAT UNIV OF DEFENSE TECH
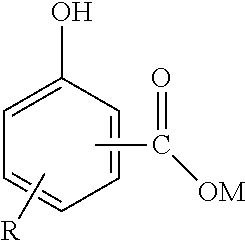
Marine engine lubrication
InactiveUS20120028522A1Easy to handlePropulsion power plantsOutboard propulsion unitsEngineeringFuel oil
Trunk piston marine engine lubrication, when the engine is fueled by heavy fuel oil, is effected by a composition comprising a major amount of an oil of lubricating viscosity containing at least 50 mass % of a basestock containing greater than or equal to 90% saturates and less than or equal to 0.03% sulphur or a mixture thereof, and respective minor amounts of an over-based metal hydrocarbyl-substituted hydroxybenzoate detergent other than such a detergent having a basicity index of less than two and a degree of carbonation of 80% or greater and at least 1 mass % of a hydrocarbyl-substituted carboxylic acid, anhydride, ester or amide thereof. Asphaltene precipitation in the lubricant, caused by the presence of contaminant heavy fuel oil, is prevented or inhibited.
Owner:INFINEUM INT LTD
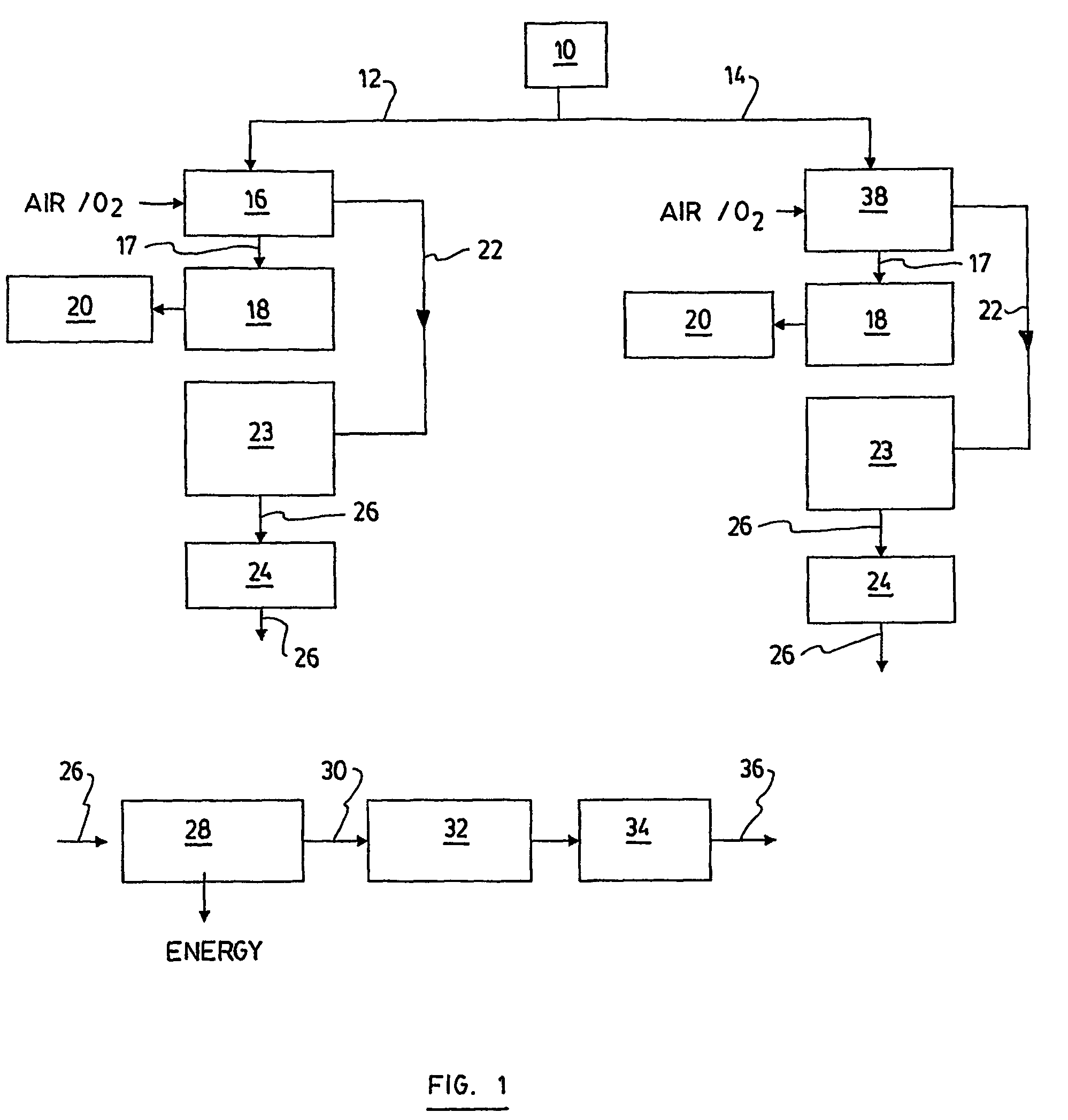
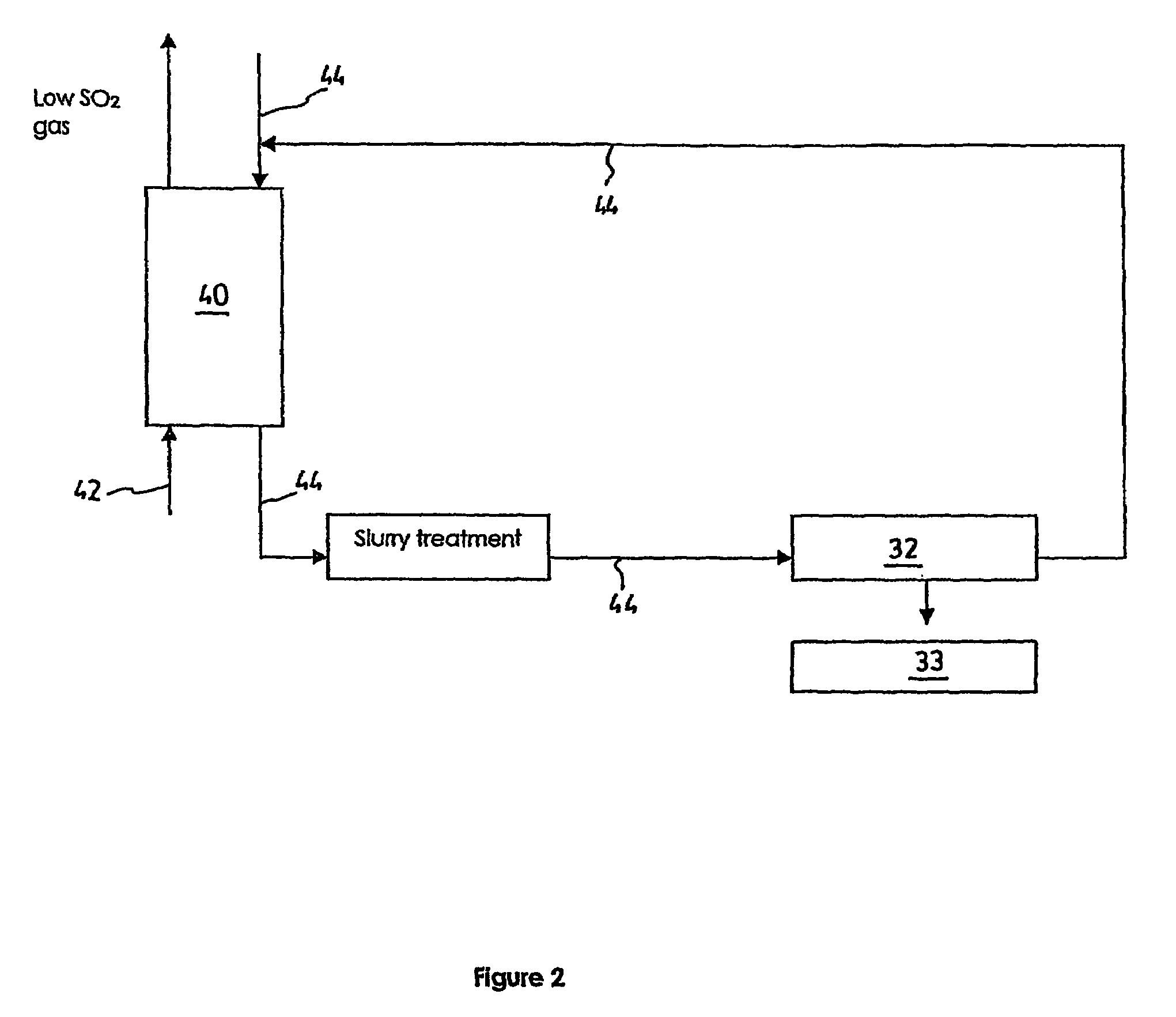
Process and a plant for recycling carbon dioxide emissions from power plants into useful carbonated species
ActiveUS7596952B2Useful carbonated speciesEnvironment safetyCalcium/strontium/barium carbonatesGas turbine plantsAtmospheric airNegative carbon dioxide emission
A process is disclosed for recycling carbon dioxide emissions from a fossil-fuel power plant into useful carbonated species The process primarily comprises the steps of: a) burning the fossil fuel, thereby generating heat and a hot exhaust gas containing CO2; and b) converting the heat into energy. The process is characterized in that it further comprises the steps of: c) cooling the exhaust gas; and d) biologically transforming the CO2 contained in the cooled exhaust gas into carbonated species, thereby obtaining a low CO2 exhaust gas and producing useful carbonated species. The low CO2 exhaust gas obtained in step d) can be released in the atmosphere without increasing the problem of greenhouse effect.
Owner:SAIPEM SPA
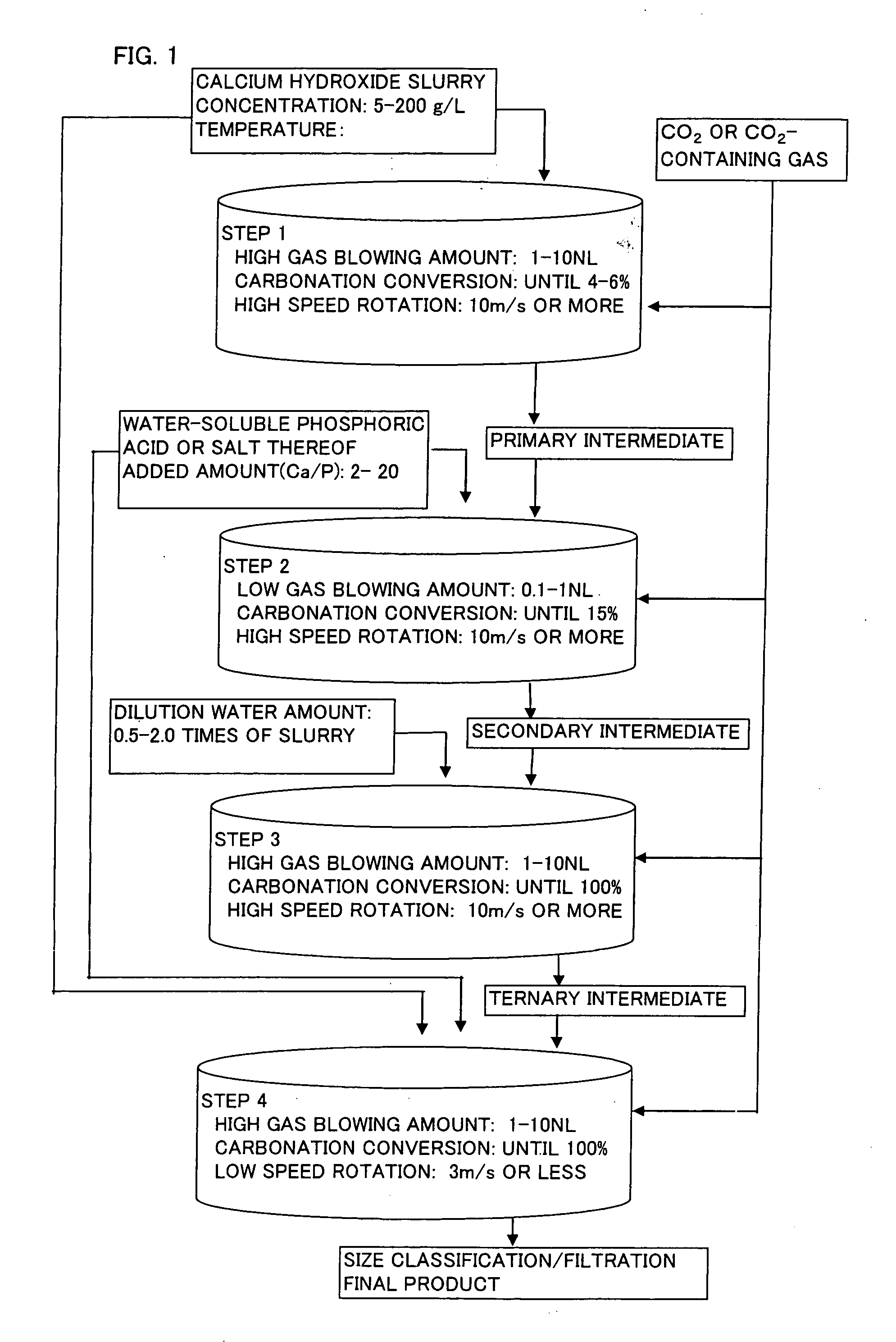
Spherical calcium carbonate and method for producing thereof
InactiveUS20060165583A1Inferior physical propertyLow blowing rateCalcium/strontium/barium carbonatesCosmetic preparationsCalcium hydroxideO-Phosphoric Acid
When spherical calcium carbonate is produced by blowing a carbon dioxide gas or a carbon dioxide-containing gas into an aqueous suspension containing calcium hydroxide to react them, after start of the reaction, an aqueous solution or suspension of a water-soluble phosphoric acid compound or a water-soluble salt thereof is added to the reaction mixture when carbonation ratio reaches 2 to 10%, and the reaction is further allowed to continue at a low gas blowing rate of 1.0 NL / minute or lower (step (a)). Subsequently, an aqueous suspension containing calcium hydroxide and an aqueous solution or suspension of a water-soluble phosphoric acid compound or a water-soluble salt thereof are added to the reaction mixture, and a carbon dioxide gas or a carbon dioxide-containing gas is introduced to allow to react and thereby produce spherical calcium carbonate having a mean particle diameter of 10 μm or larger. This production method is performed under high velocity revolution from the start of the reaction to the end of the step (a) This method provides calcite type spherical calcium carbonate showing high brightness and small friction coefficient, and having a shape comparatively close to a true sphere and a mean particle diameter of 10 μm or larger.
Owner:OKUTAMA IND
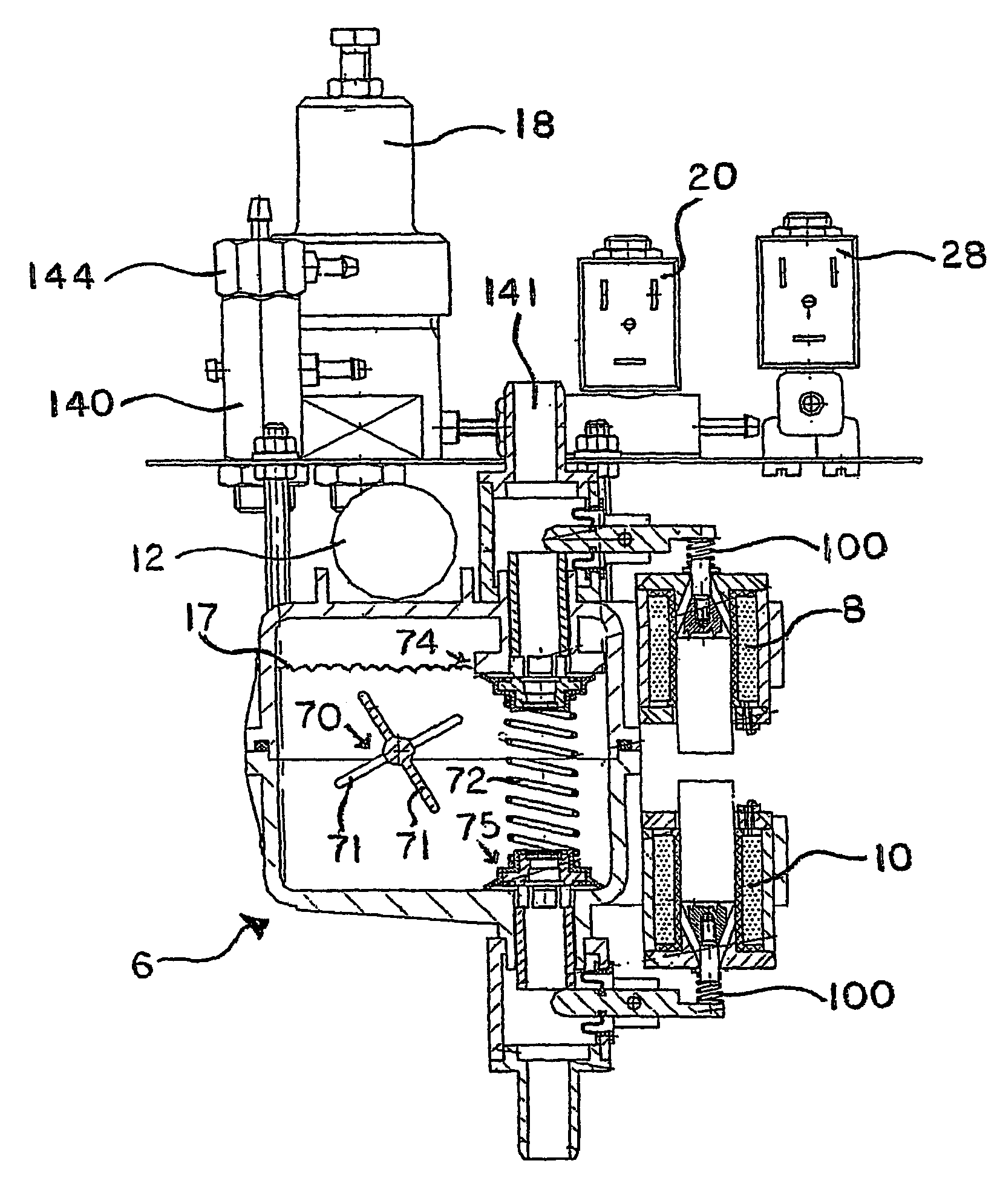
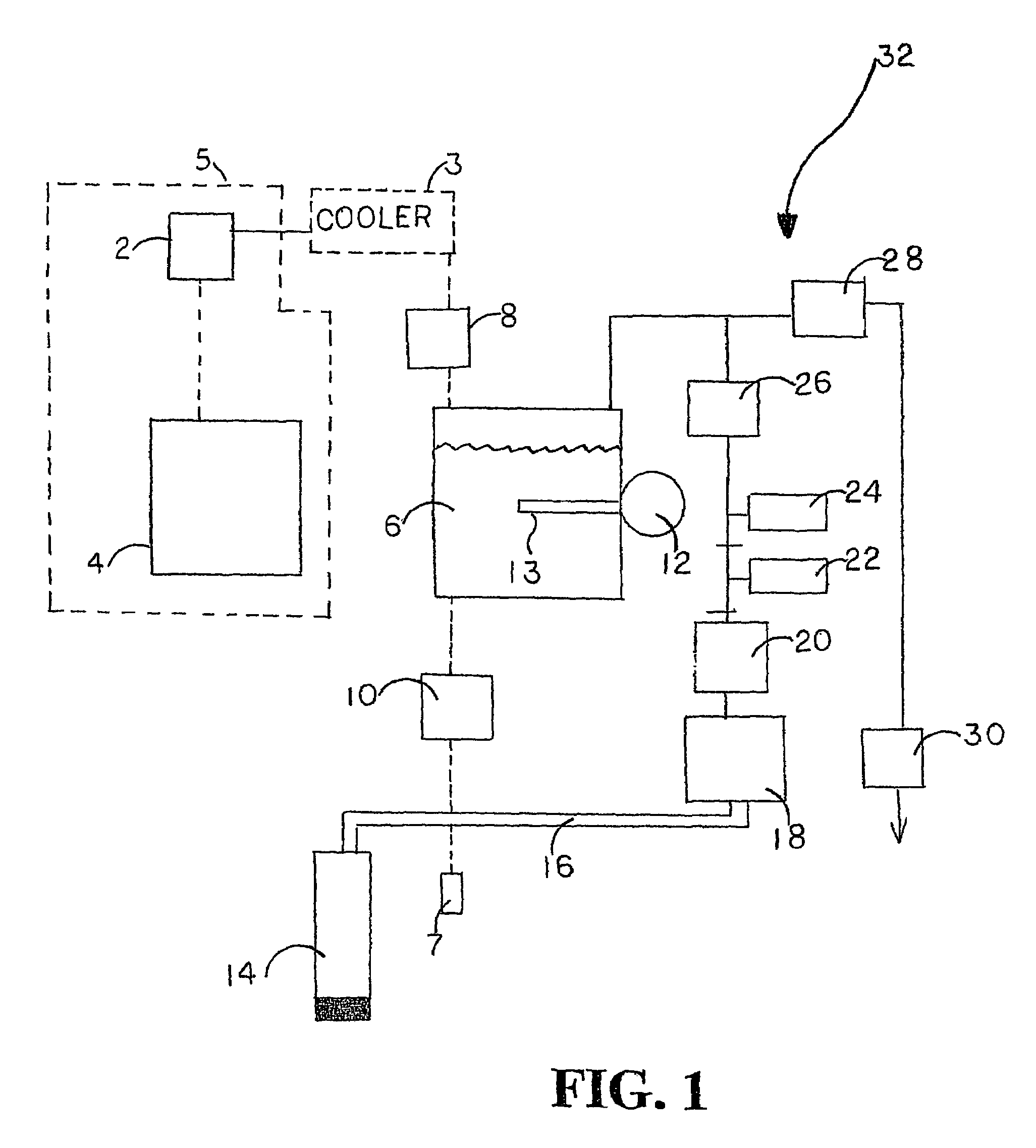
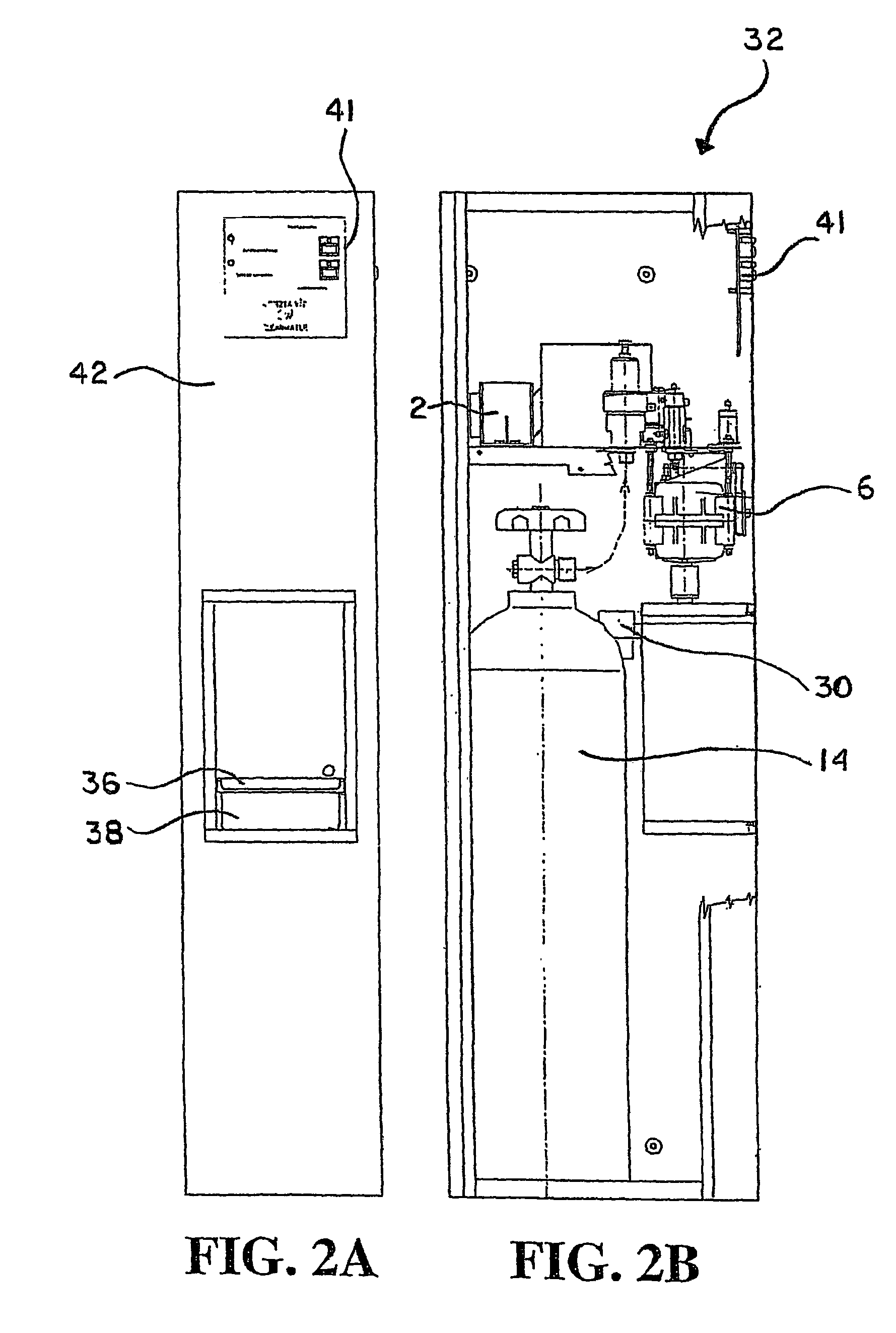
Carbonation system and method
The water carbonation method and apparatus of the present invention consists of a square mixer within a carbonated chamber. The mixer is partially filled with water. Carbon dioxide is then added above the level of water. A rotating member attached to the mixing motor then mixes the water and carbon dioxide to from a carbonated solution. Varying the time for which the carbonation operation is carried on may vary the degree of carbonation. After the specified carbonation cycle, excess carbon dioxide is then relieved through an exhaust solenoid and the remaining carbonated solution is released through the dispensing solenoid into a cup.
Owner:PRIMO WATER HLDG INC
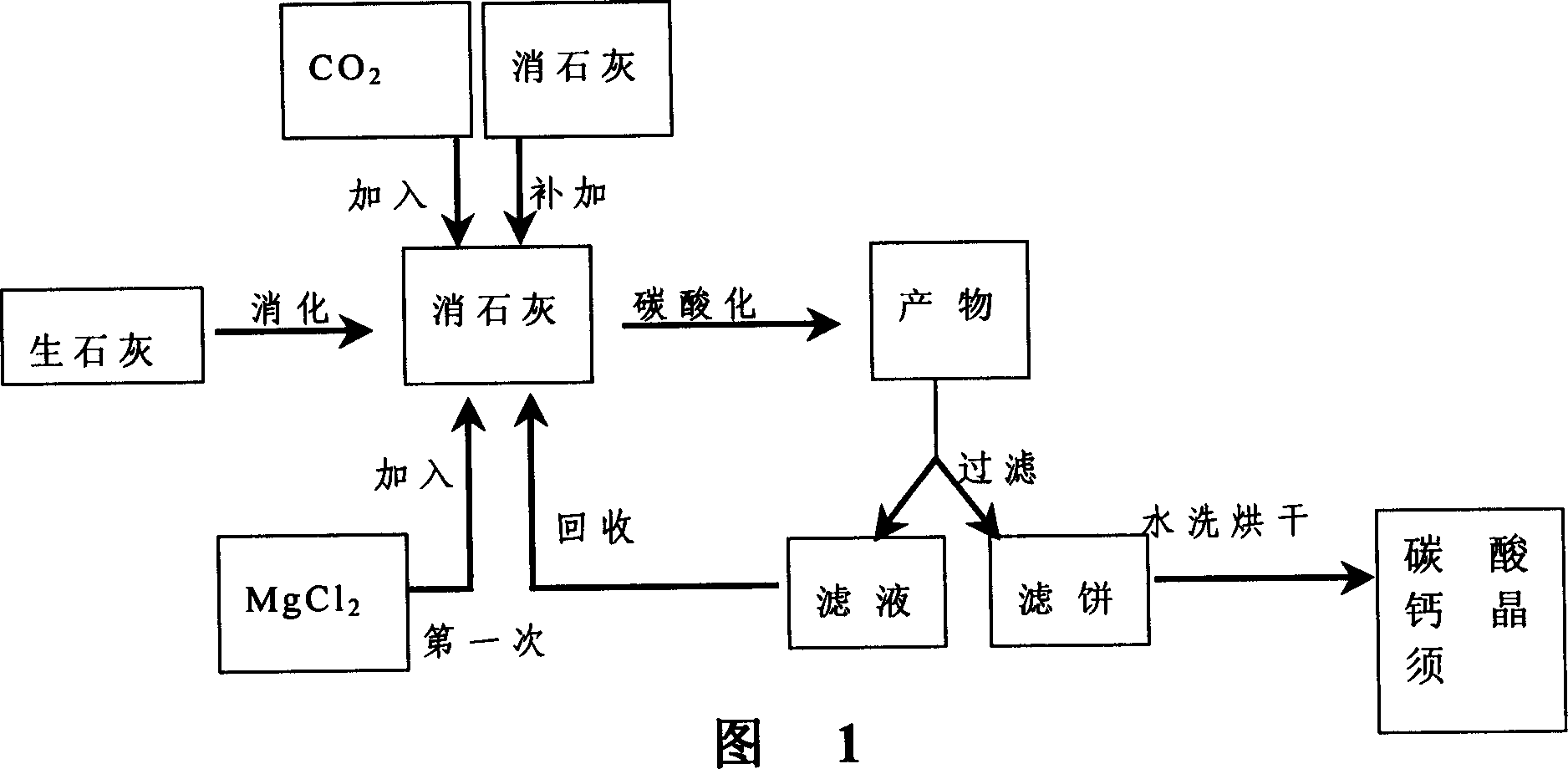
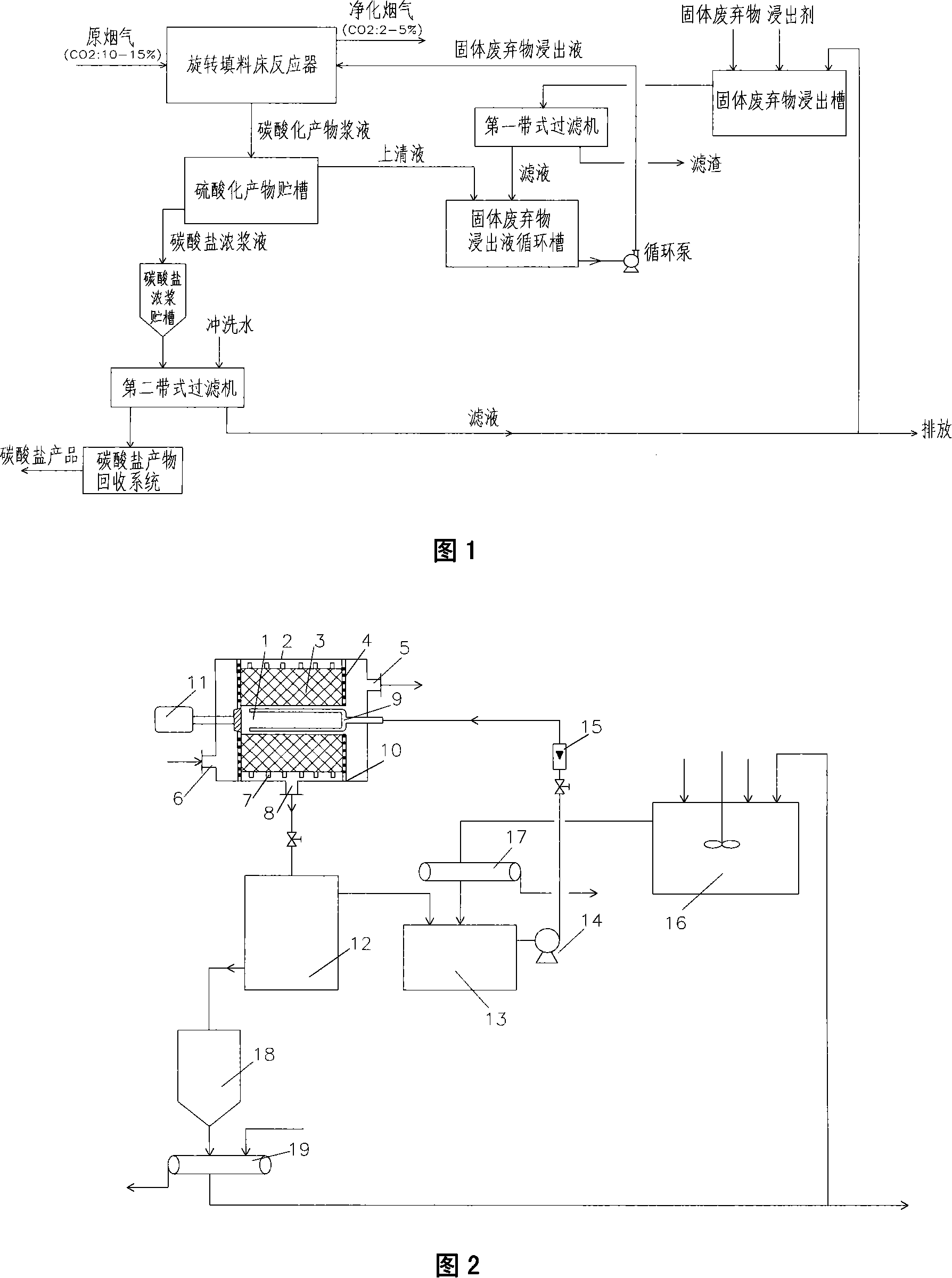
Method and device for fixing CO2 in stack gas by solid castoff carbonatation
InactiveCN101219330AIncrease mass transfer absorption rateIncrease carbon sequestration capacityDispersed particle separationBy chemical separationProcess integrationCentrifugal force
The invention provides a technique method for integrating coupling in-situ CO2 through utilizing a plurality of processes such as CO2 absorbing separation, carbonation fixation and solid wastes recovery in stack gas and a device thereof. A supergravity rotating packing bed reactor is used for intensifying transmission and multi-phase reacting process through utilizing powerful centrifugal force generated by the high-speed rotary packing bed, which is a breakthrough technology and can strengthen order of magnitude to the transmitting process of liquidoid-controlled CO2, etc., thus greatly improving mass transfer absorption rate of the CO2.
Owner:SOUTHEAST UNIV
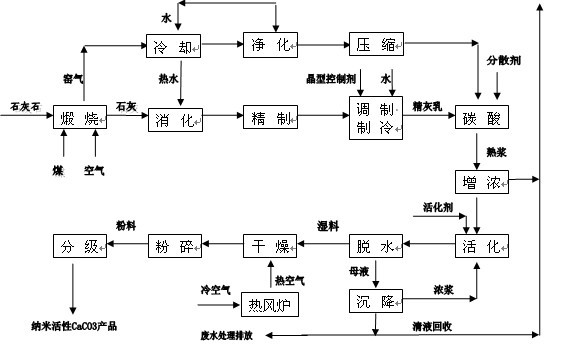
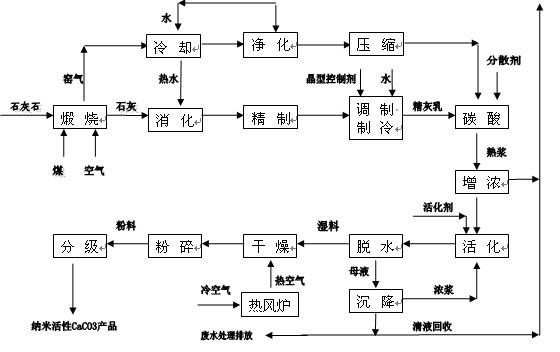
Preparation method of nano-calcium carbonate SCC-2 special for silicone sealant
InactiveCN102491397AProcessability can be optimizedEasy to processCalcium/strontium/barium carbonatesNanotechnologyDispersitySlurry
The invention discloses a preparation method of nano-calcium carbonate SCC-2 special for a silicone sealant, belonging to the technical field of inorganic chemical industry. According to an adopted technical scheme, the method comprises the following steps of: calcining limestone, crushing to obtain CaO; digesting, refining and aging the CaO in a hot state in the ratio of 1:4.8 of CaO to H2O; adding 1 percent of white granulated sugar crystal form control agent for undergoing a carbonation reaction to obtain CaCO3 slurry; performing activation surface treatment; and dehydrating, drying, crushing and degrading to obtain a nano-active CaCO3 product of 60-100 nanometers. The crystal form, size, regularity, oil absorption value and pH value of CaCO3 are controlled, so that 'hard aggregation' among particles is avoided from a surface modification production process, and the product has low oil absorption value and high bulk specific weight and has high dispersity in polymers.
Owner:广东嘉维化工实业有限公司
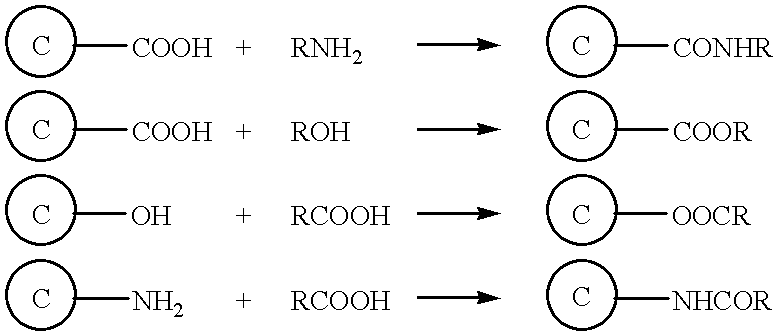
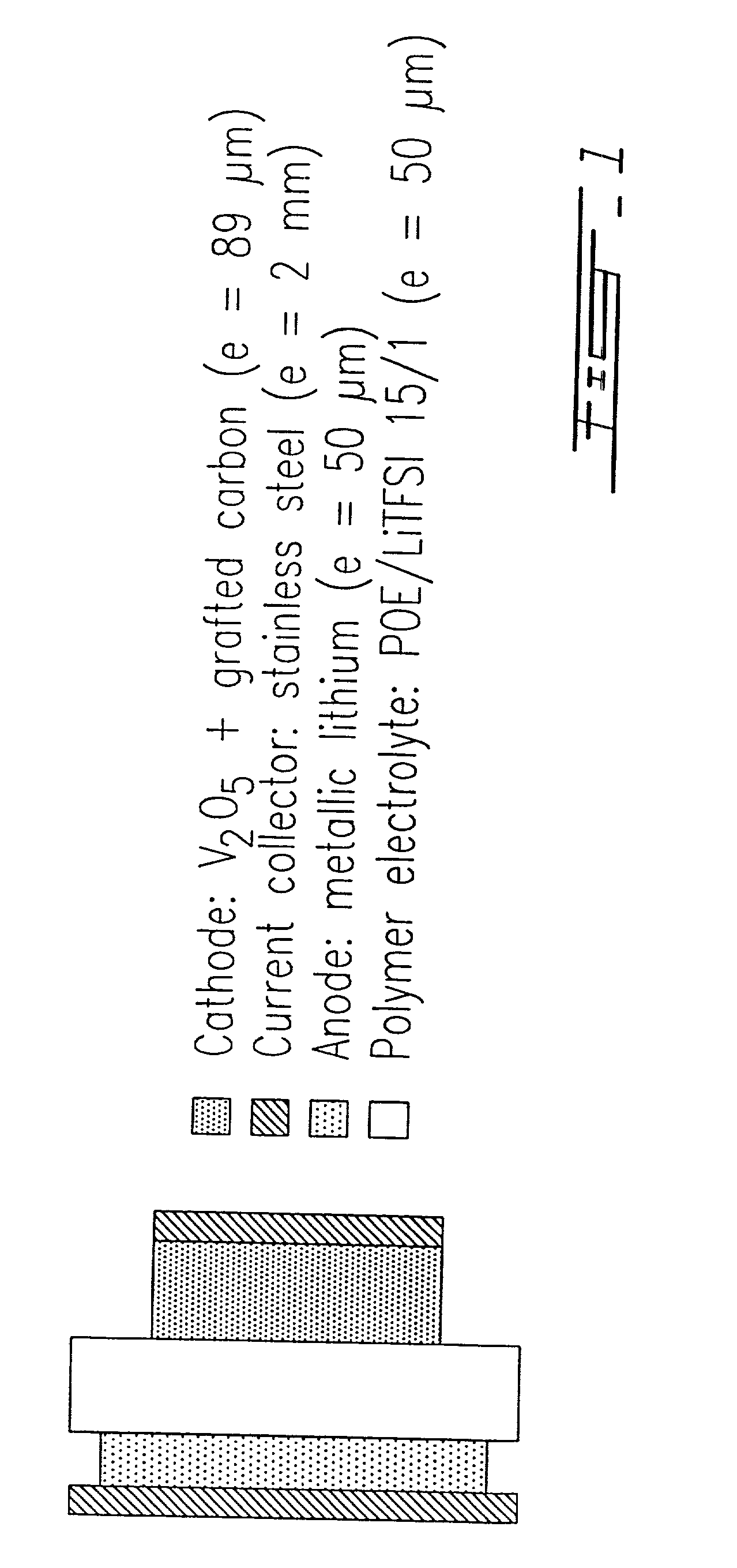
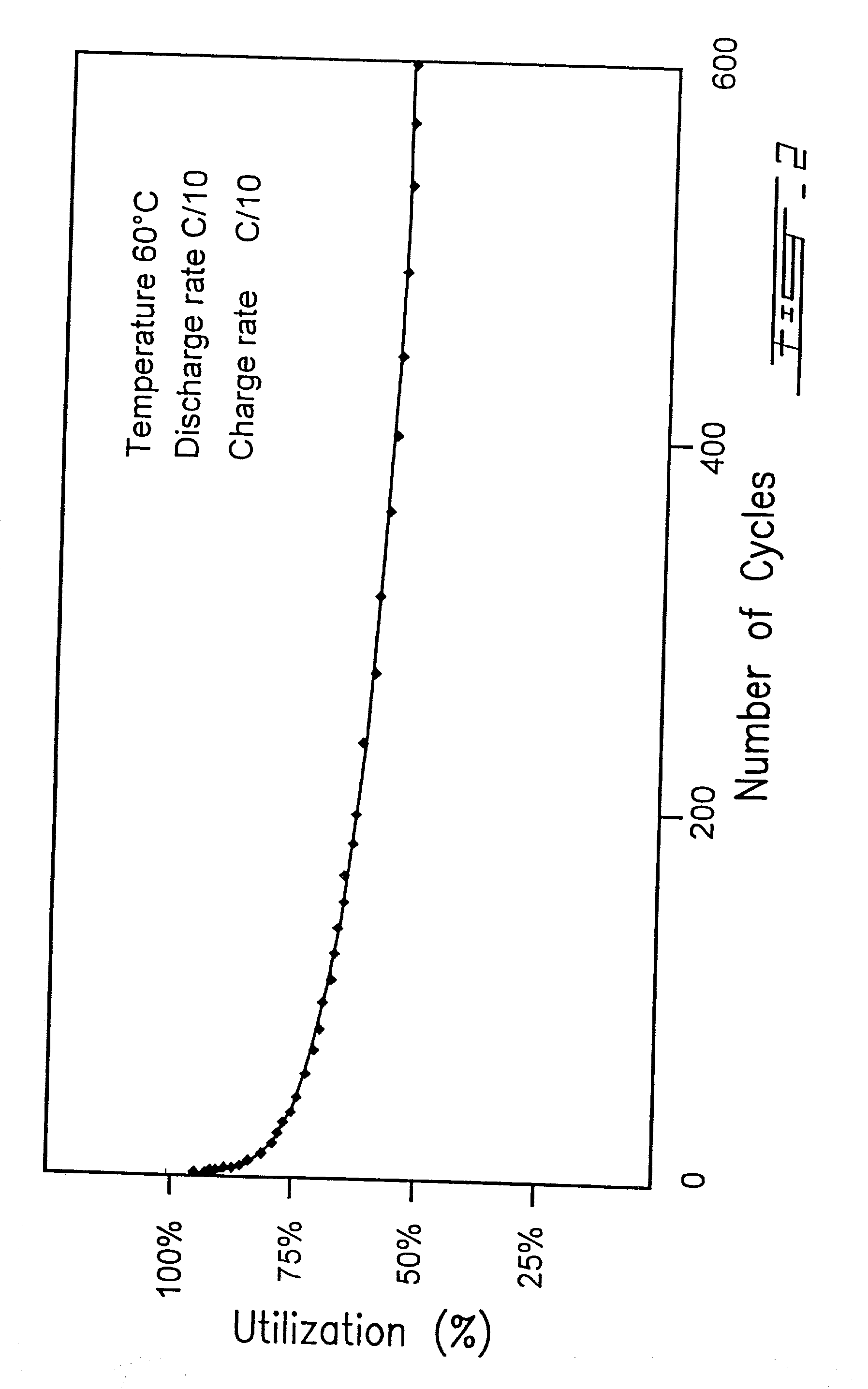
Surface modified carbonaceous materials
Grafting a polymer at the surface of a carbonated material containing carboxyl, amine and / or hydroxyl functions at its surface. This material is suspended in a solution comprising the polymer to be grafted, which includes a carboxyl, amine and / or hydroxyl function, the solution also comprising a solvent of the polymer. This is followed by a treatment causing dehydration into a carboxyl function, an amide and / or hydroxyl function and the polymer is thus grafted on the carbonated material by means of ester or amide bonds. Utilization in the cathode or anode of an electrochemical generator, in a polymer material with low polarity, in an ink, and as a conductive deposit on flexible plastic used as electrical contact, electromagnetic protection and antistatic protection.
Owner:HYDRO QUEBEC CORP +1
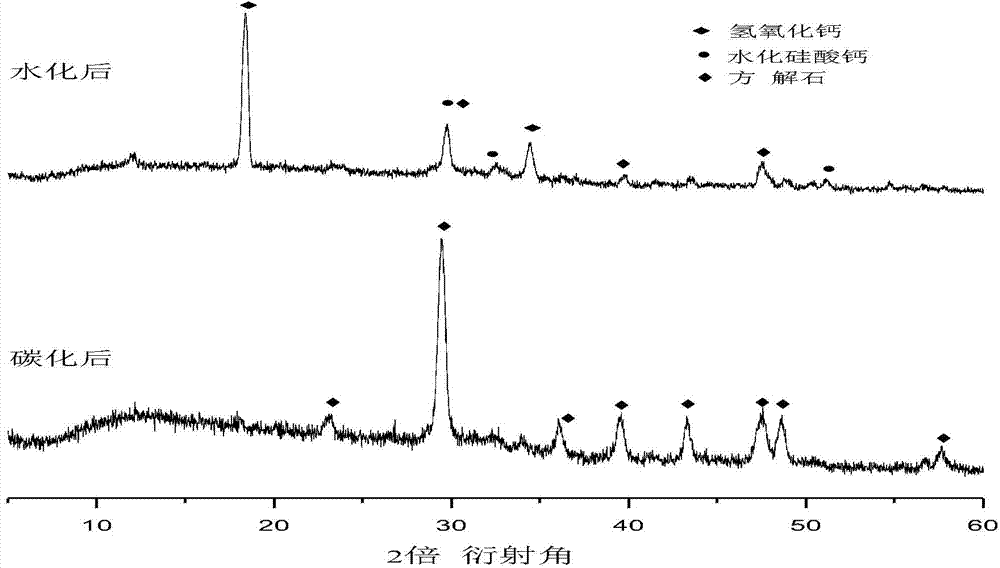
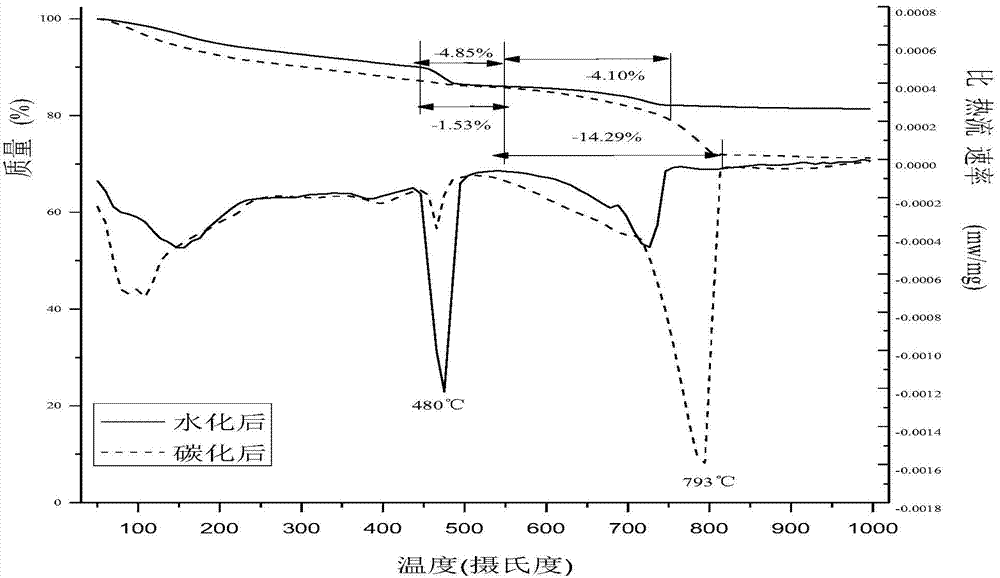
Method for preparing building material products through hydration-carbonation coupling technique
The invention belongs to the technical field of building materials, and provides a method for preparing building material products through processing industrial solid waste by hydration-carbonation coupling technique. The method comprises steps of uniformly mixing the industrial waste comprising at least one of calcium oxide, calcium hydroxide, dicalcium silicate, tricalcium silicate and tobermorite with alkaline excitation material and proper amount of water so as to prepare the blank of the building material product, wherein the industrial waste comprises steel slag, mineral waste residue, furnace slag, coal ash or coal gangue, the alkaline excitation material comprises carbide slag, lime, Portland cement or waste cement; maintaining for a period through hydration, then maintaining through carbonation so as to obtain the carbonate-based building material product. The coupling technique can effectively use the industrial solid wastes such as steel slag, mineral waste residue, furnace slag, coal ash, coal gangue, carbide slag and the like, so that emission of greenhouse gases is reduced, the greenhouse effect is relieved, furthermore, the method can be used for producing the building material products with good properties, effectively uses the waste and is environmentally friendly.
Owner:DALIAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com