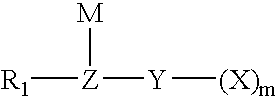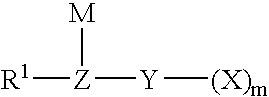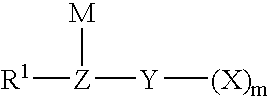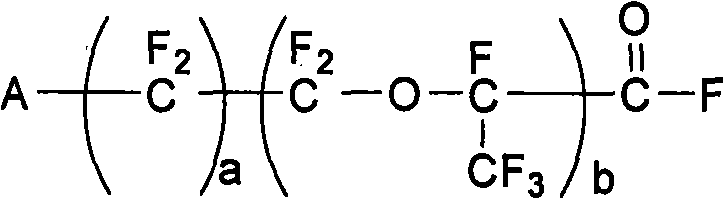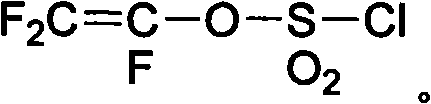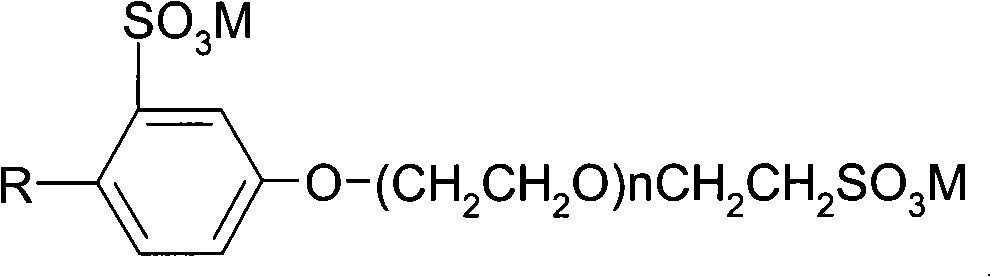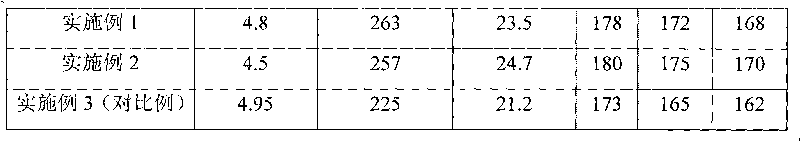Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2113results about "Sulfonic acid preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
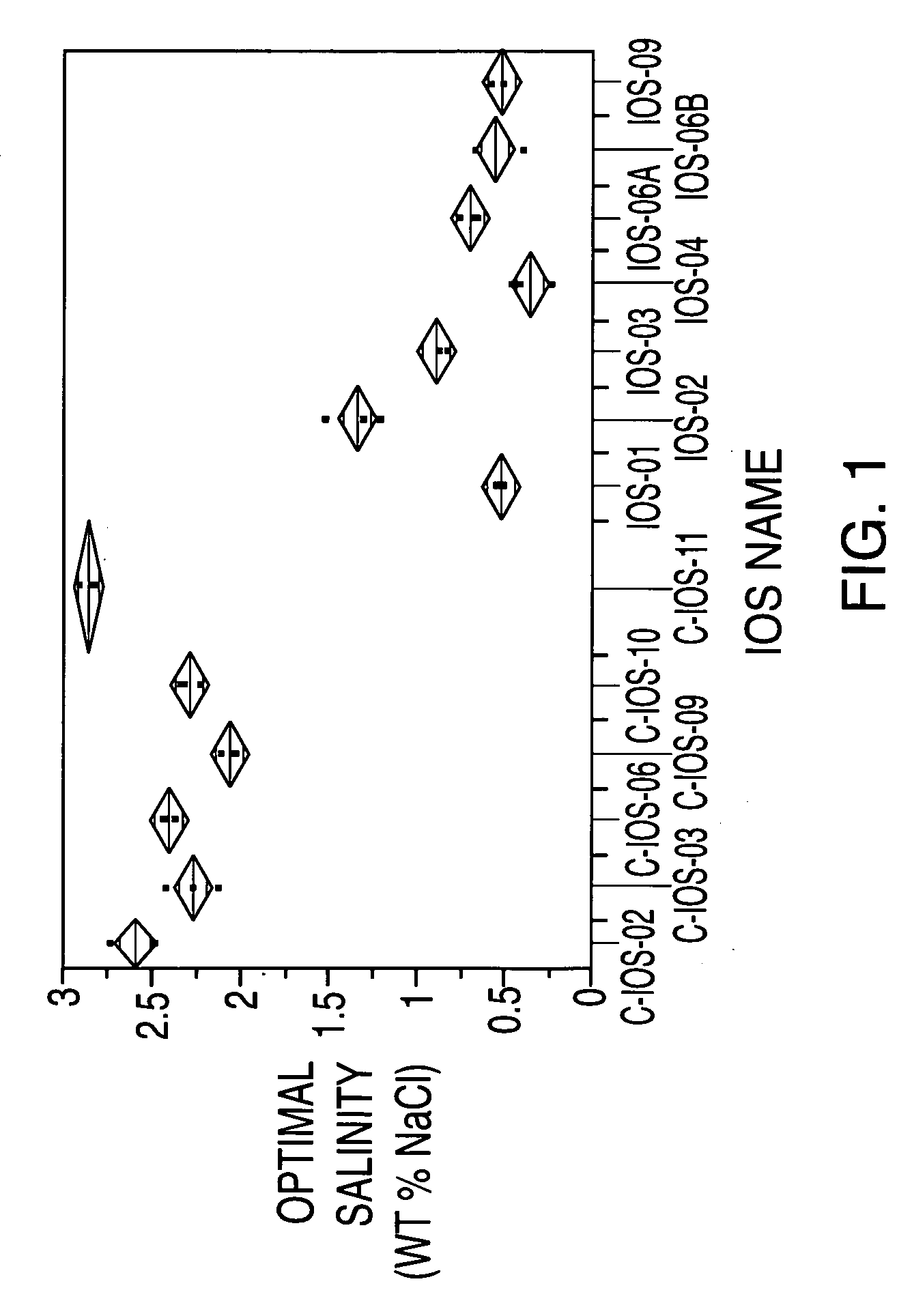
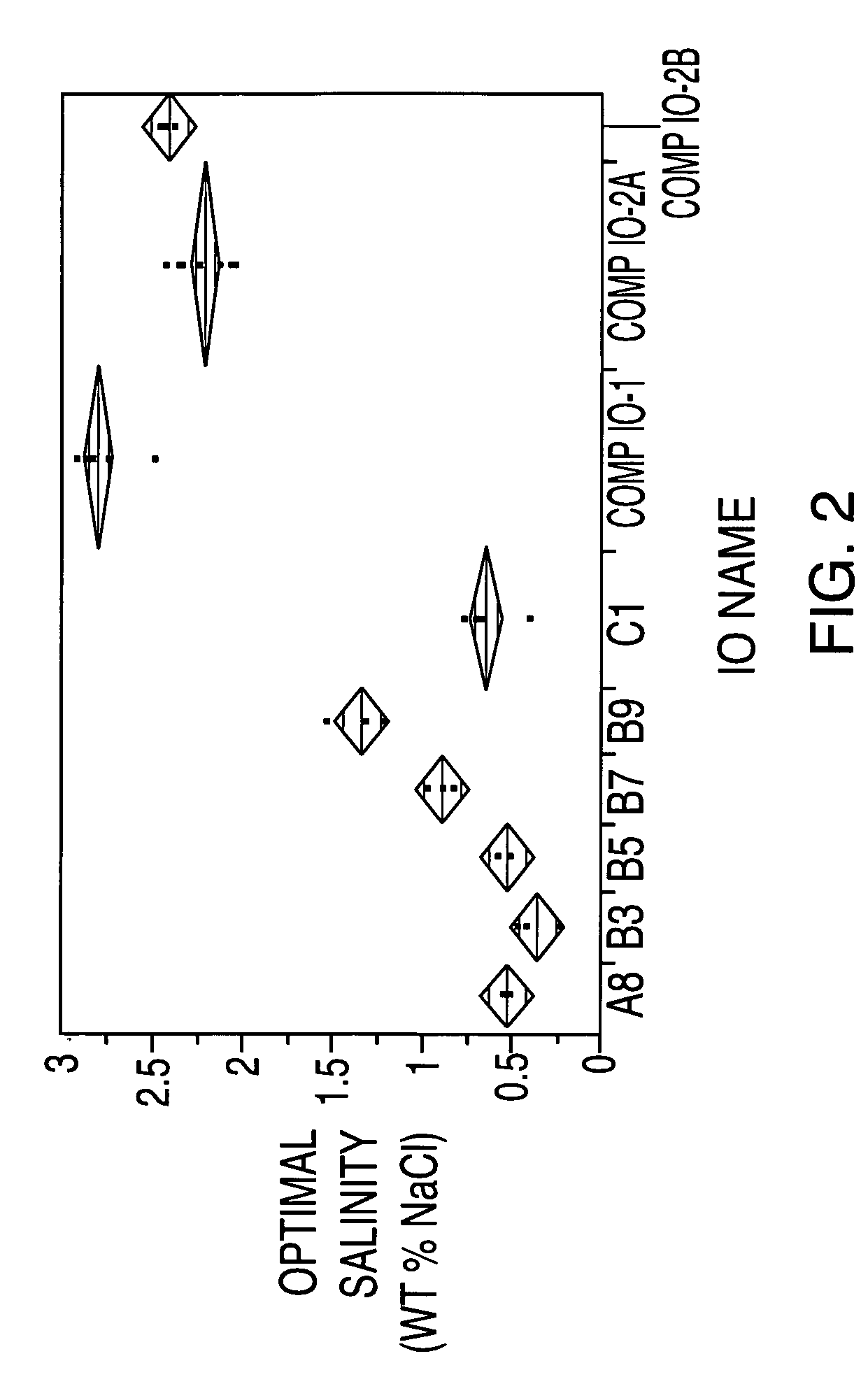
Sulfonated internal olefin surfactant for enhanced oil recovery
A process for recovering oil from an oil-bearing formation comprises introducing into said formation an aqueous composition comprising at least one sulfonated derivative of one or more internal olefins, said internal olefins being characterized by having low amounts of tri-substitution on the olefin bond, said sulfonated derivative being obtained by sulfonating a composition comprising internal olefins of the formula:R1R2C═CR3R4 wherein R1, R2, R3 and R4 are the same or different and are hydrogen or straight- or branched-chain, saturated hydrocarbyl groups and the total number of carbon atoms of R1, R2, R3 and R4 is 6 to 44 with the proviso that at least about 96 mole percent of R1 and R3 are straight- or branched-chain, saturated hydrocarbyl groups and at least about 96 mole percent of R2 and R4 are hydrogen. Further provided are compositions for use in recovering oil from an oil-bearing formation which comprise the sulfonated derivatives of one or more internal olefins having low amounts of tri-substitution on the olefin bond.
Owner:STEPAN COMPANY
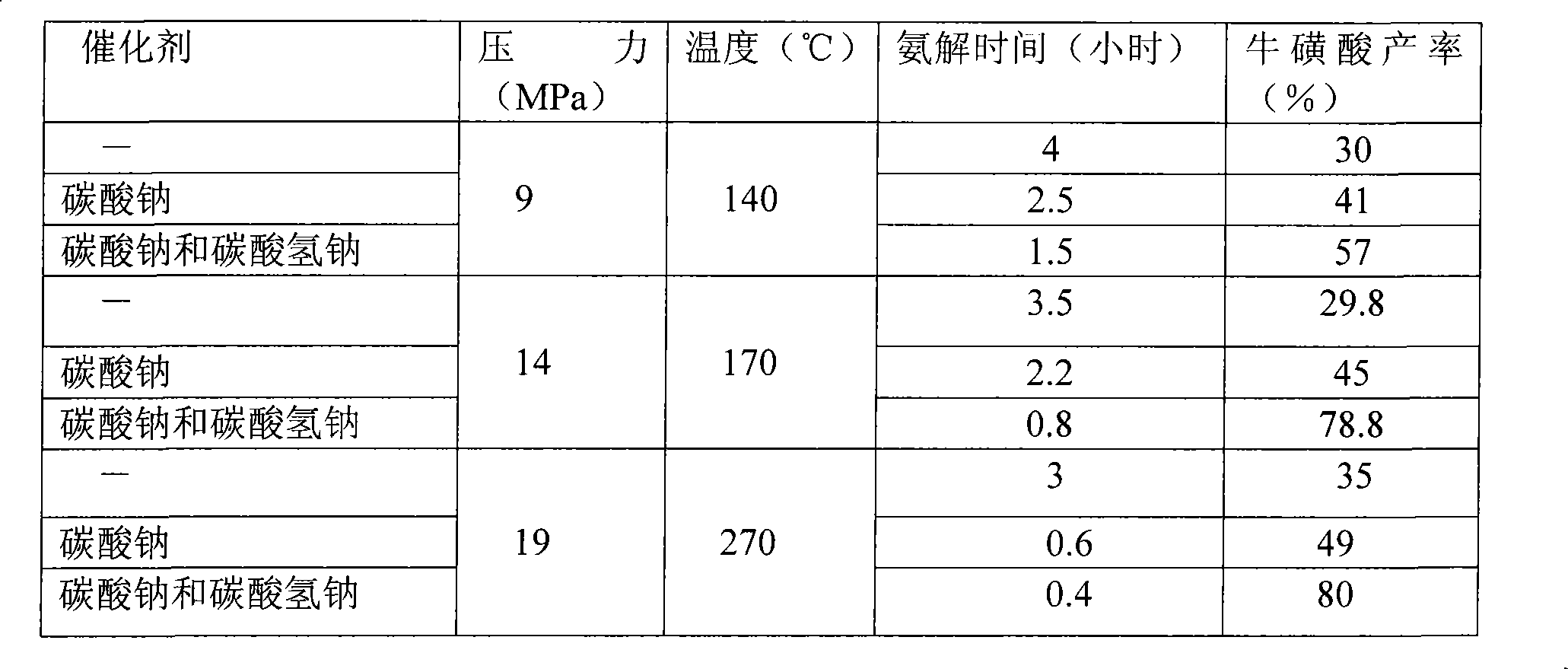
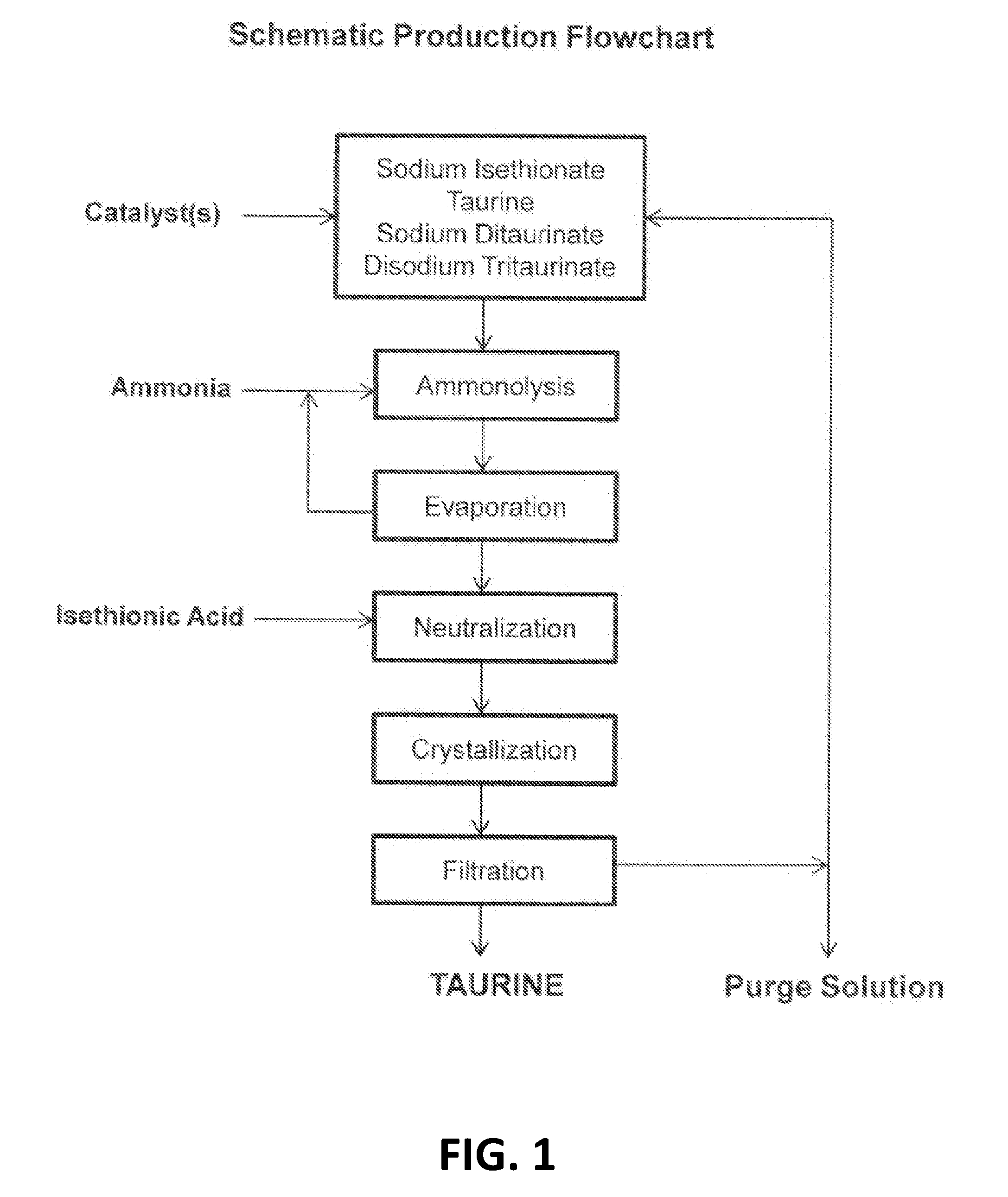
Method for synthesizing taurine
InactiveCN101486669AHigh yieldReduce chanceSulfonic acid preparationSodium IsethionateSodium bisulfite
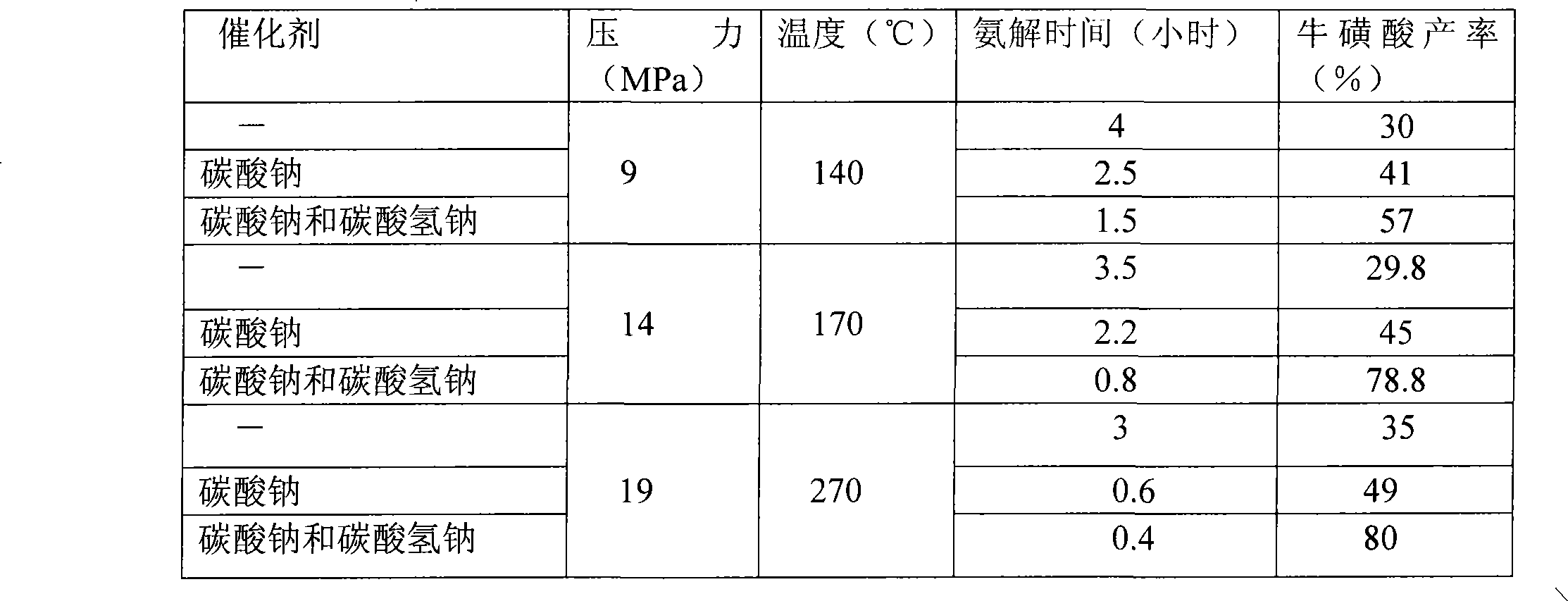
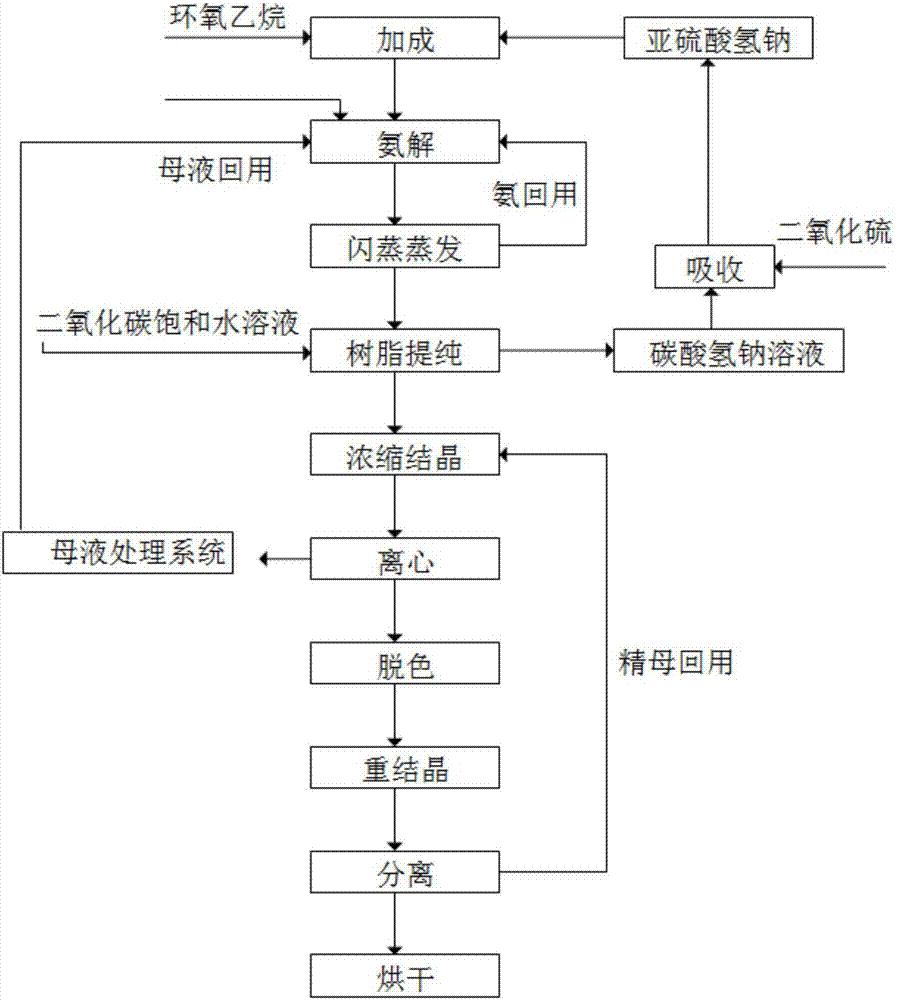
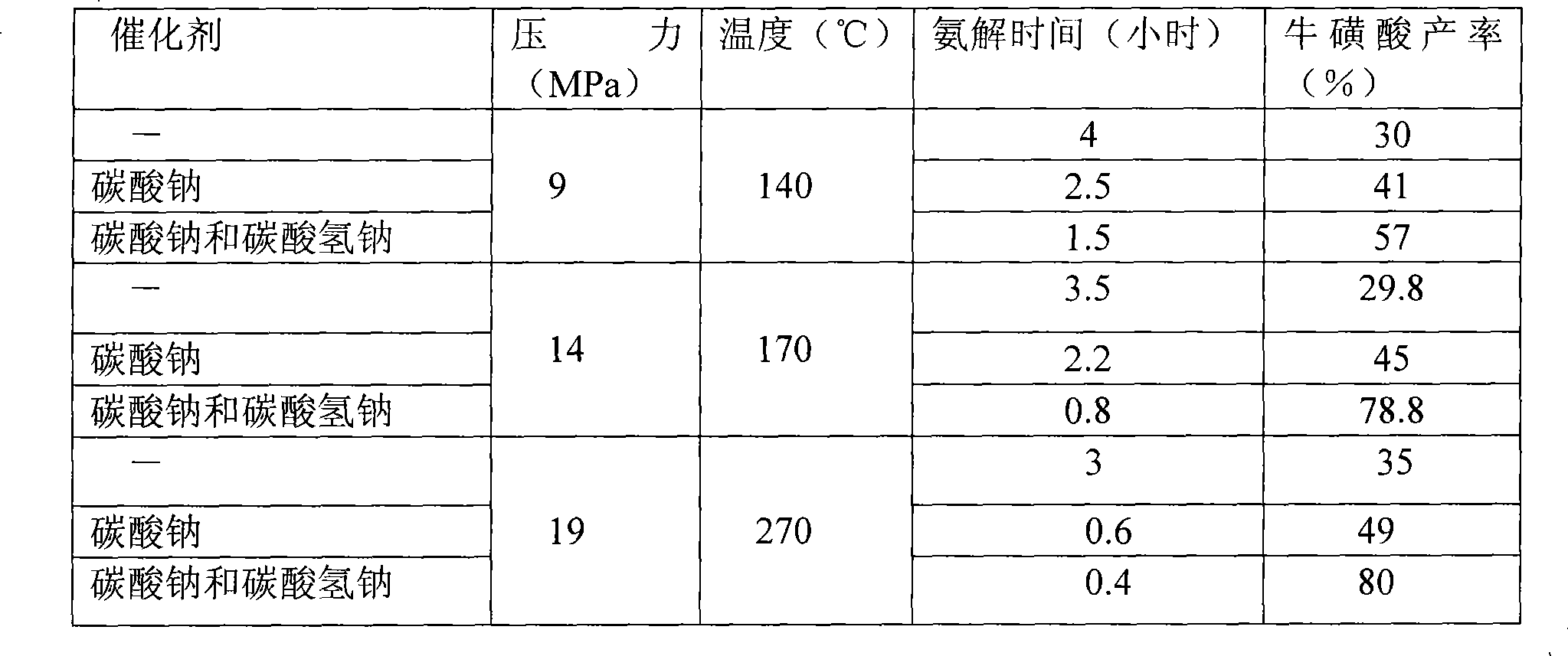
The invention relates to a method for synthesizing taurine. The production technique comprises the steps: A. sodium bisulfite, epoxyethane and sodium hydroxide are placed in a reaction kettle to produce sodium isethionate through reaction; sodium isethionate reacts with liquid ammonia at temperature of 260 DEG C and 270 DEG C and a pressure of 18.5MPa to 19MPa to synthesize and produce sodium 2-amino ethyl sulfonate; B. a sodium 2-amino ethyl sulfonate solution is added with sulphuric acid and goes through centrifugal filtration, thus obtaining the rough crystals of taurine; and C. the rough crystals of taurine are refined and dried. The method has the advantage that by changing the temperature and pressure for reaction, the yield of sodium 2-amino ethyl sulfonate (the intermediate product for synthesizing and preparing taurine) is improved; the occurrence possibility of side reaction is lowered; and the content of impurities is obviously reduced.
Owner:SHAYANG TIANYI MEDICINE IND
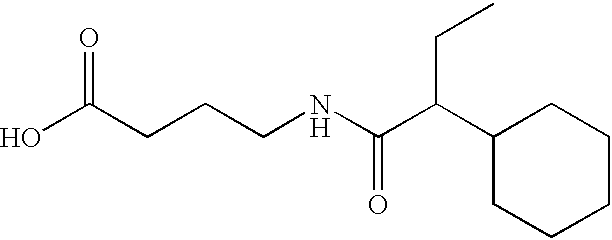
Compounds and compositions for delivering active agents
Modified amino acid compounds useful in the delivery of active agents are provided. The active agents can be peptides. Methods of administration, such as oral, subcutaneous, sublingual, and intranasal administration are provided, and methods of preparation of the modified amino acid compound are provided.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Synthesis of taurine
The invention relates to a method for synthesizing taurine, comprising the following steps: (1) according to the amount ratio of materials of 1:1 to 1:1.2, epoxy ethane and sodium bisulfite are subjected to addition reaction under 0.05 to 0.1MPa, with pH value of 6.5 to 7.5 and at a temperature between 75 and 85 DEG C to form hydroxyethyl sodium sulfonate; (2) the hydroxyethyl sodium sulfonate and liquid ammonia are subjected to ammonolysis reaction under 14 to 21MPa and at a temperature between 160 and 280 DEG C to generate sodium taurate, and the mass concentration of ammonia in the reaction liquid is 20 to 30 percent; and (3) neutralization: namely, the sodium taurate is neutralized by sulphuric acid to generate the taurine. The method for synthesizing the taurine has the advantages of short time, high yield and lower cost, and is easy for industrialized production.
Owner:王代龙 +1
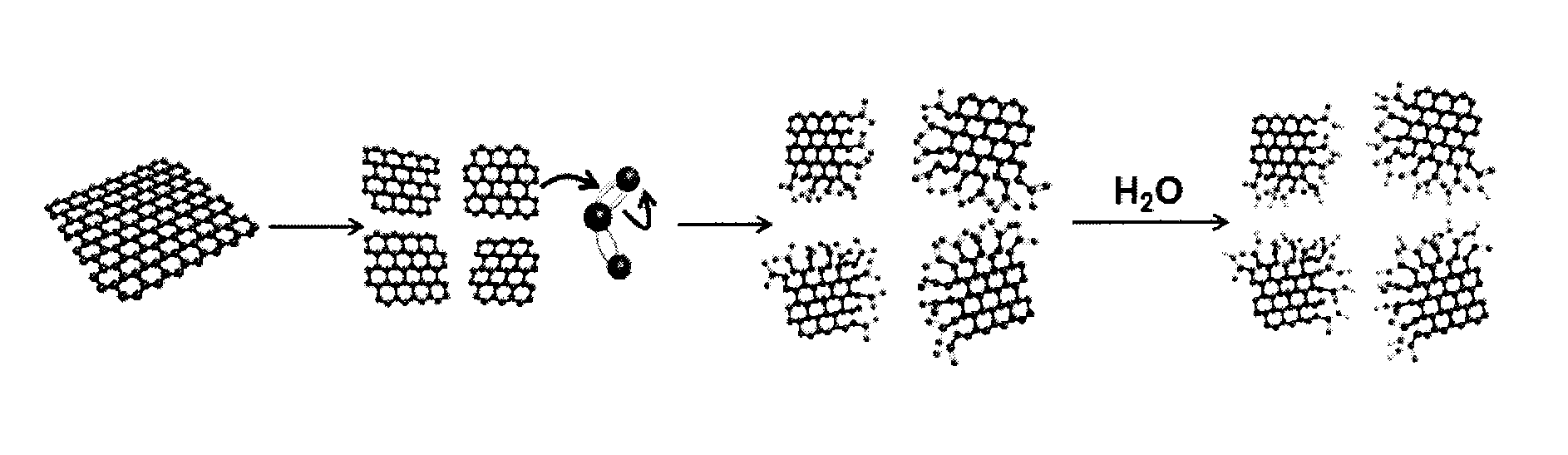
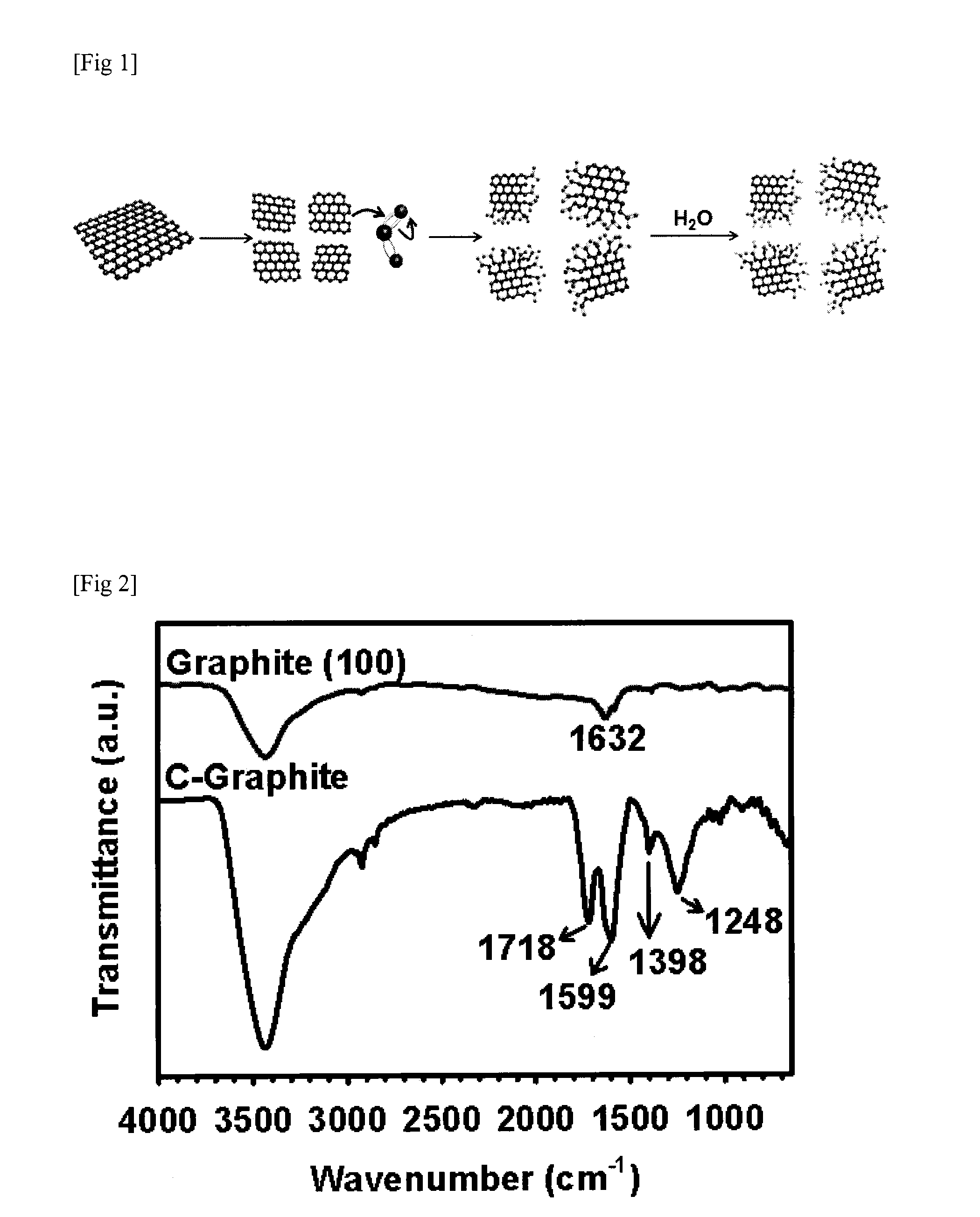
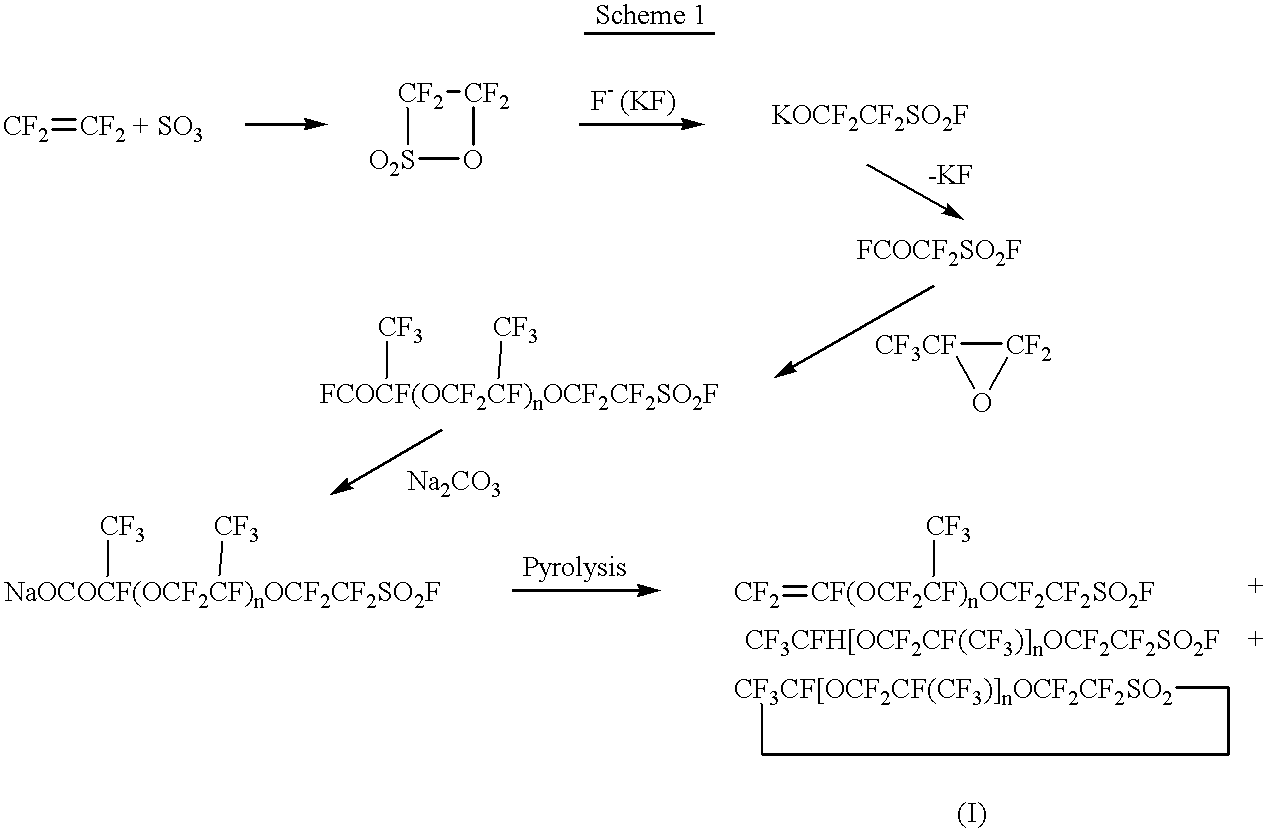
Edge-functionalized graphitic material through mechanochemical process and manufacturing method thereof
InactiveUS20130018204A1High degree of commercializationRaise the potentialMaterial nanotechnologyOrganic compound preparationGas phaseMaterials science
Disclosed is an edge-functionalized graphitic material manufactured by using a mechanochemical process. The edge-functionalized graphitic material is manufactured by pulverizing graphite in the presence of a variety of atmospheric agents in the form of gas phase, liquid phase, or solid phase. The edge-functionalized graphitic material, which is a precursor applicable into various fields, is expected to replace the prior art oxidized graphite.
Owner:DEOKYANG ECO LTD
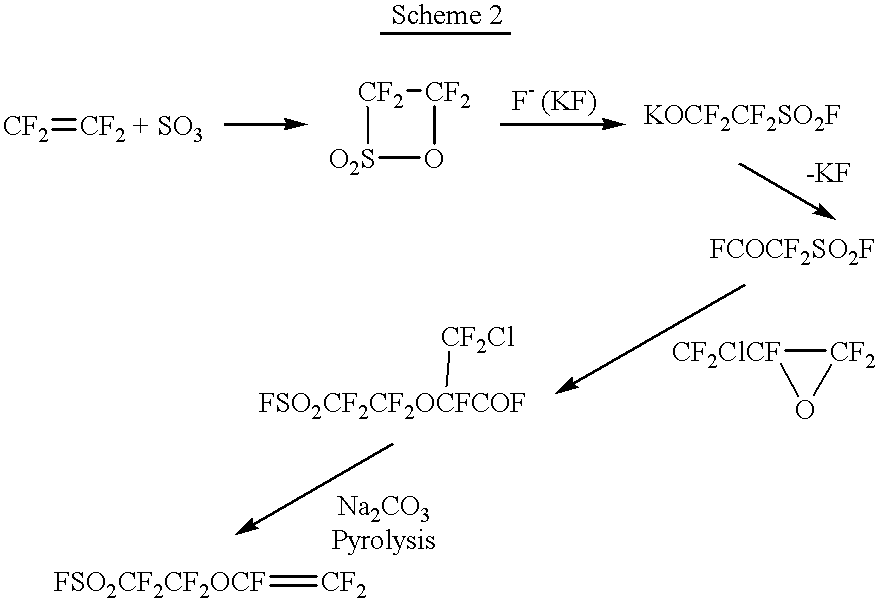
Process for the producing perfluorovinyl ethersulfonic acid derivatives and copolymer of the same
InactiveUS6274677B1Organic compound preparationSulfonic acid preparationAlkaline earth metalAcid derivative
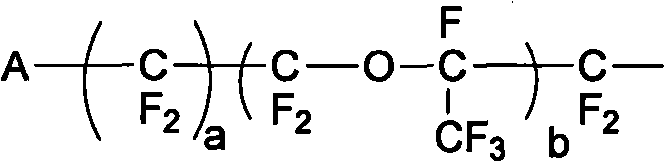
A compound of the formula (1): FSO2CFXCF2O(CFXCF2O)nCF-(CF2Y)COF in which X is a fluorine atom, a chlorine atom or a trifluoromethyl group, Y is a fluorine atom, a chlorine atom, a bromine atom or an iodine atom, and n is a number of 0 to 5, preferably 0, 1 or 2, is converted to a compound of the formula (2): MOSO2CFXCF2O(CFXCF2O)2CF(CF2Y)COOM1 in which X, Y and n are the same as defined above, and M and M1 independently represent an alkali metal atom or an alkaline earth metal atom, and then a compound of the formula (2) is pyrolyzed at a temperature of 150 to 250° C. to obtain a compound of the formula (3): ASO2CFXCF2O(CFXCF2O)nCF=CF2 in which A is MO-, a hydroxyl group or a fluorine atom, and X and n are the same as defined above.
Owner:DAIKIN IND LTD
Method for preparing taurine
The invention relates to a method for preparing taurine, comprising the following steps: epoxy ethane and sodium bisulfite are reacted under 0.05 to 0.1MPa, with pH value of 6.5 to 7.5 and at a temperature between 75 and 85 DEG C to form hydroxyethyl sodium sulfonate; the hydroxyethyl sodium sulfonate and liquid ammonia are subjected to ammonolysis reaction at under 14 to 21MPa and at a temperature between 160 and 280 DEG C to form ammonolysis solution containing sodium taurate; and the ammonolysis solution is evaporated to remove ammonia, neutralized by sulphuric acid, concentrated, crystallized, separated, pre-dried in a boiling drying device or a vibration fluidization drying device, and then added into a microwave drying device to be dried and sterilized to obtain the taurine. The method for synthesizing the taurine has the advantages of short time, high yield and lower cost, and is easy for industrialized production. The water content of a taurine wet product can be reduced to below 0.30 percent through combined application of pre-drying of the boiling drying device or the vibration fluidization drying device and microwave drying, and simultaneously the device has the function of sterilizing.
Owner:王代龙 +1
Method for cyclically producing taurine at high yield
ActiveCN107056659AReduce productionEfficient recyclingPhysical/chemical process catalystsOrganic compound preparationAfter treatmentHydrogen
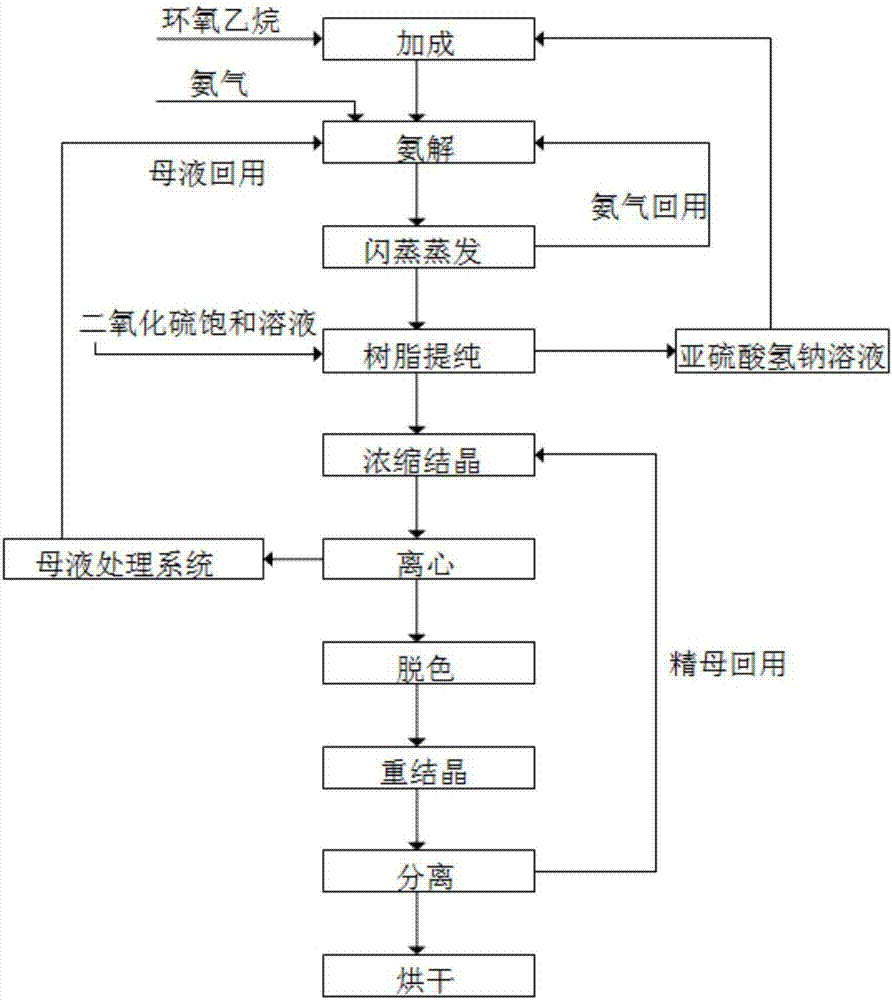
The invention relates to a method for cyclically producing taurine at a high yield. The method includes the following steps that S1, ethylene oxide reacts with a sodium hydrogen sulfite solution to generate sodium hydroxyethyl sulfonate; S2, sodium hydroxyethyl sulfonate obtained in S1 is subjected to an ammonolysis reaction in ammonium hydroxide, flashing is carried out after the reaction is completed, and ammonia gas is recycled; S3, taurine-containing feed liquid of reaction liquid obtained after flashing in S2 is collected through an acid cation exchange resin column, the resin column is regenerated with a sulfur dioxide or carbon dioxide water solution after being inactivated, and eluant obtained during regeneration can be directly reused or reused after being treated with sulfur dioxide; S4, the feed liquid collected in S3 is subjected to after-treatment, and taurine is obtained. The method has the advantages that the generation amount of waste liquid in the whole process is small, part of substances are effectively and cyclically utilized in the process, the cost is reduced, the yield of taurine can reach 90% or above, meanwhile, the production process is relatively simple, and large-scale production is easy.
Owner:QIANGJIANG YONGAN PHARMA
Method of preparing taurine
The invention relates to method for preparing taurine, comprising the following steps: (1) reacting epoxy ethane with sodium sulfite under 0.05 to 0.1MPa, with pH value of 6.5 to 7.5 and at temperature between 75 and 85 DEG C to obtain hydroxyethyl sodium sulfonate; (2) carrying out ammonolysis reaction on the hydroxyethyl sodium sulfonate and liquid ammonia under 14 to 21MPa and at temperature between 160 and 280 DEG C to obtain ammonolysis solution containing sodium taurate; (3) introducing the ammonolysis solution into a single flash evaporator for primary flash evaporating at a temperature between 160 and 200 DEG C and under 1.3 to 2.0MPa; introducing the flash evaporated liquid into a secondary flash evaporating and falling film evaporator, using the primary flash vapor as a heating medium to carry out flash evaporating and falling film evaporating on the primary flash evaporated liquid in the secondary flash evaporating and falling film evaporator at a temperature between 110 and 140 DEG C and at 0.1 to 0.6MPa; evaporating and concentrating the flash evaporated liquid subjected to secondary flash evaporating and falling film evaporating with flash vapor and steam as heating media in a multi-effect flash evaporating and falling film evaporator; and (4) neutralizing the sodium taurate by sulphuric acid to obtain the taurine. The method for preparing the taurine has the advantages of short time, high yield and low cost, and is easy for industrialized production. In addition, by primary flash evaporating and secondary flash evaporating processes, almost all the ammonia and 40% to 60% of water in the flash evaporated liquid can be removed, thus having double effects of removing ammonia and condensing.
Owner:王代龙 +1
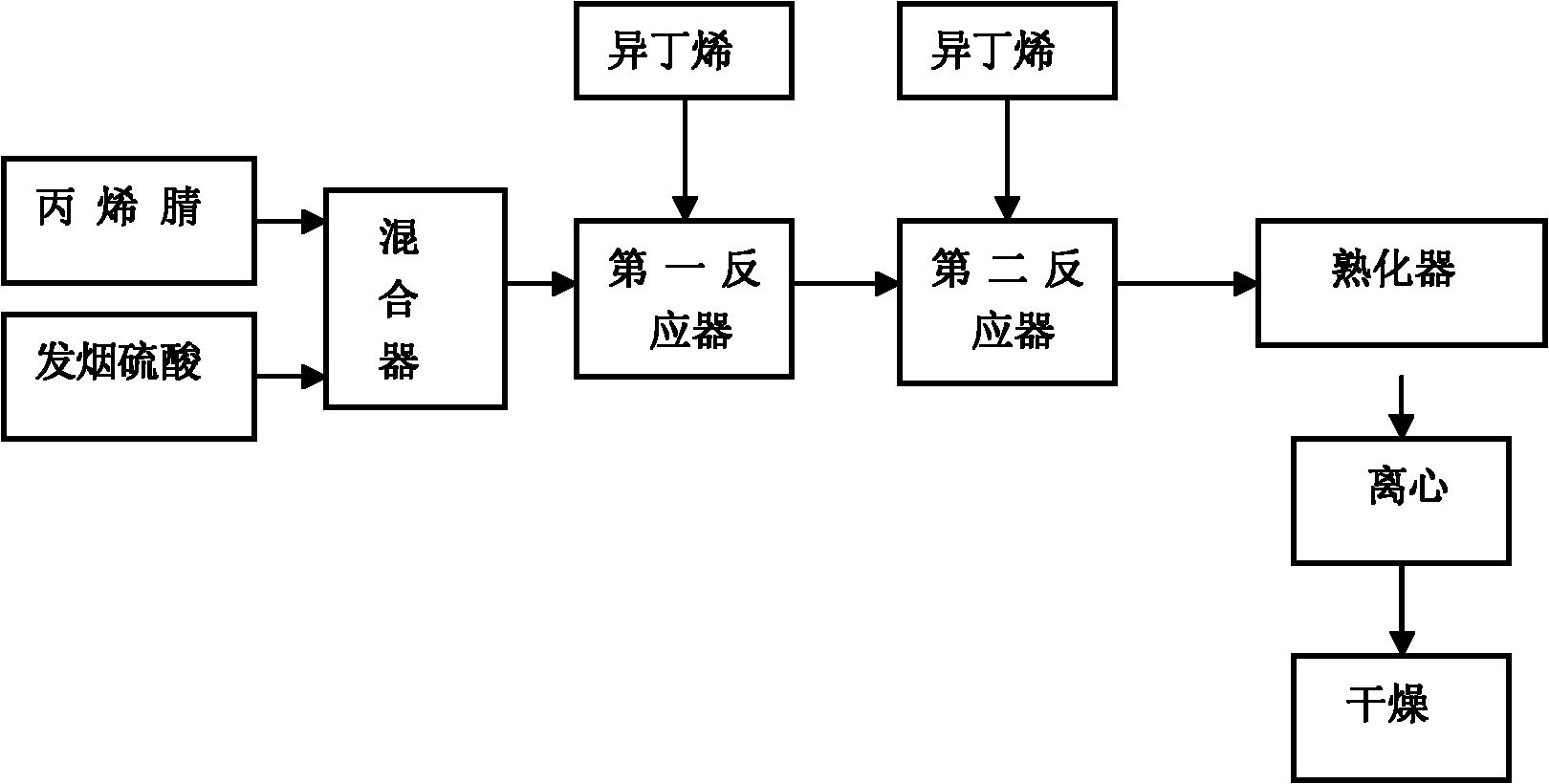
Synthesis process for 2-acrylamido-2-methyl propane sulfonic acid through continuous method
InactiveCN102351744AStable ratioStrong reaction stabilitySulfonic acid preparationFirst-order reactionAcrylonitrile
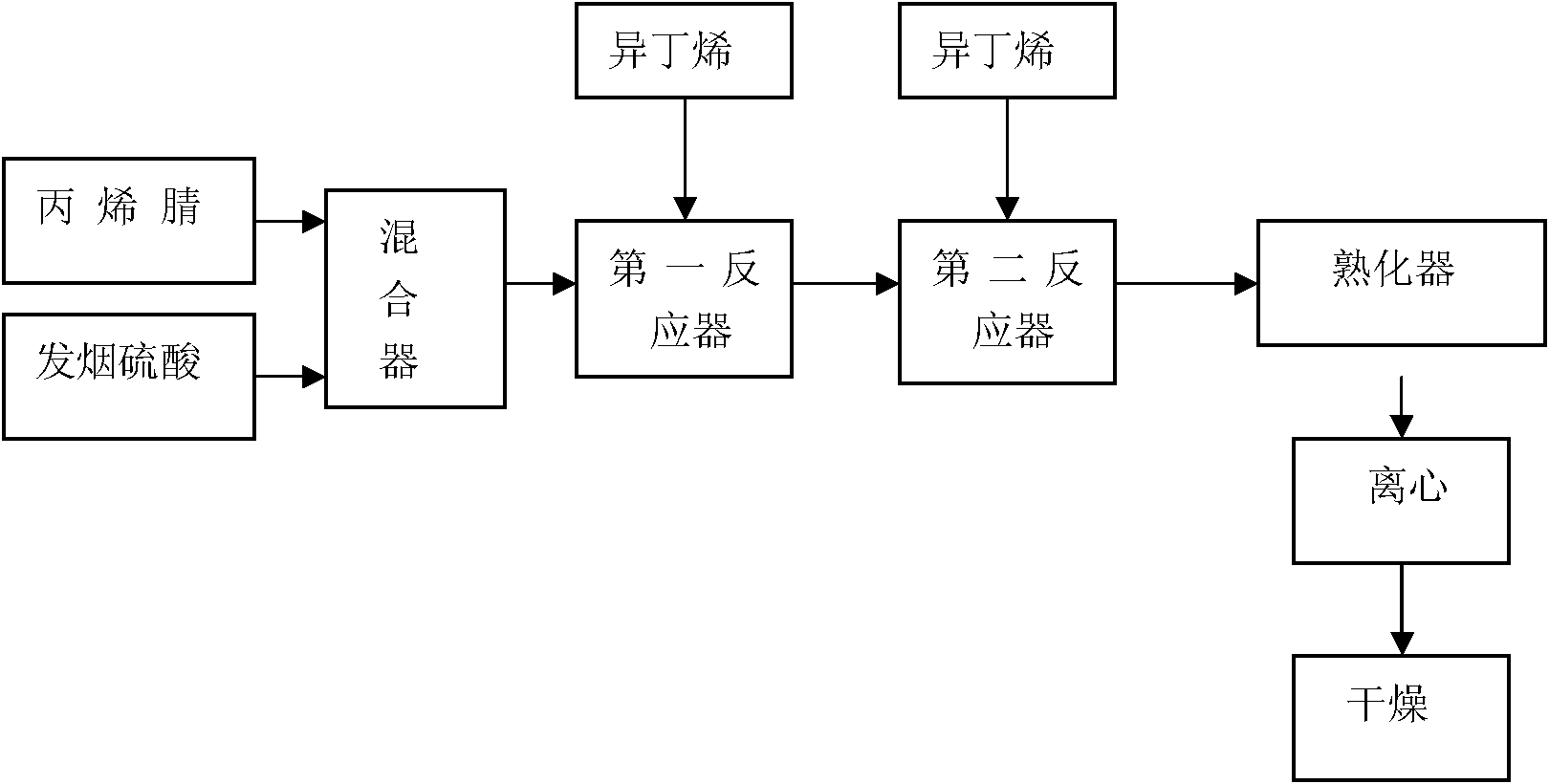
The invention discloses a synthesis process for 2-acrylamido-2-methyl propane sulfonic acid through a continuous method. The synthesis process comprises the following steps of: continuously and uniformly mixing acrylonitrile and fuming sulfuric acid at the temperature of 10-12 DEG C; performing first order reaction and second order reaction at the temperature of 20-45 DEG C under a condition of introducing vaporized isobutene; and slaking, centrifuging and drying to continuously produce the 2-acrylamido-2-methyl propane sulfonic acid product. By designing unique first order reaction, second order reaction and slaking flow, completeness degree of reaction of the material is guaranteed, the product yield and the quality stability are improved, and the reaction period is greatly shortened. The synthesis process has the advantages of scientific and rational flow design, practicability, product yield of over 96 percent and liquid phase detection purity of over 96 percent.
Owner:WEIFANG QUANXIN CHEM
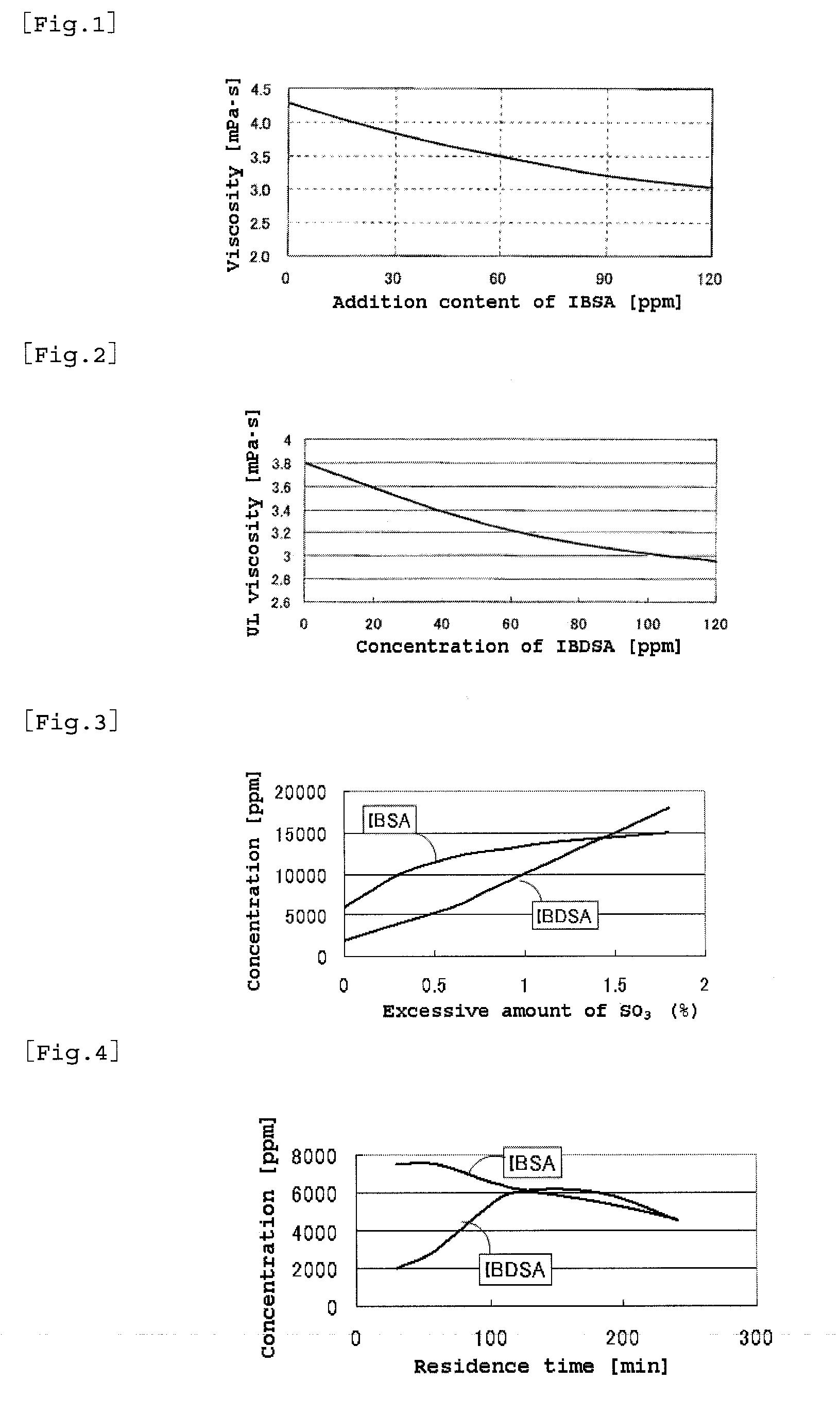
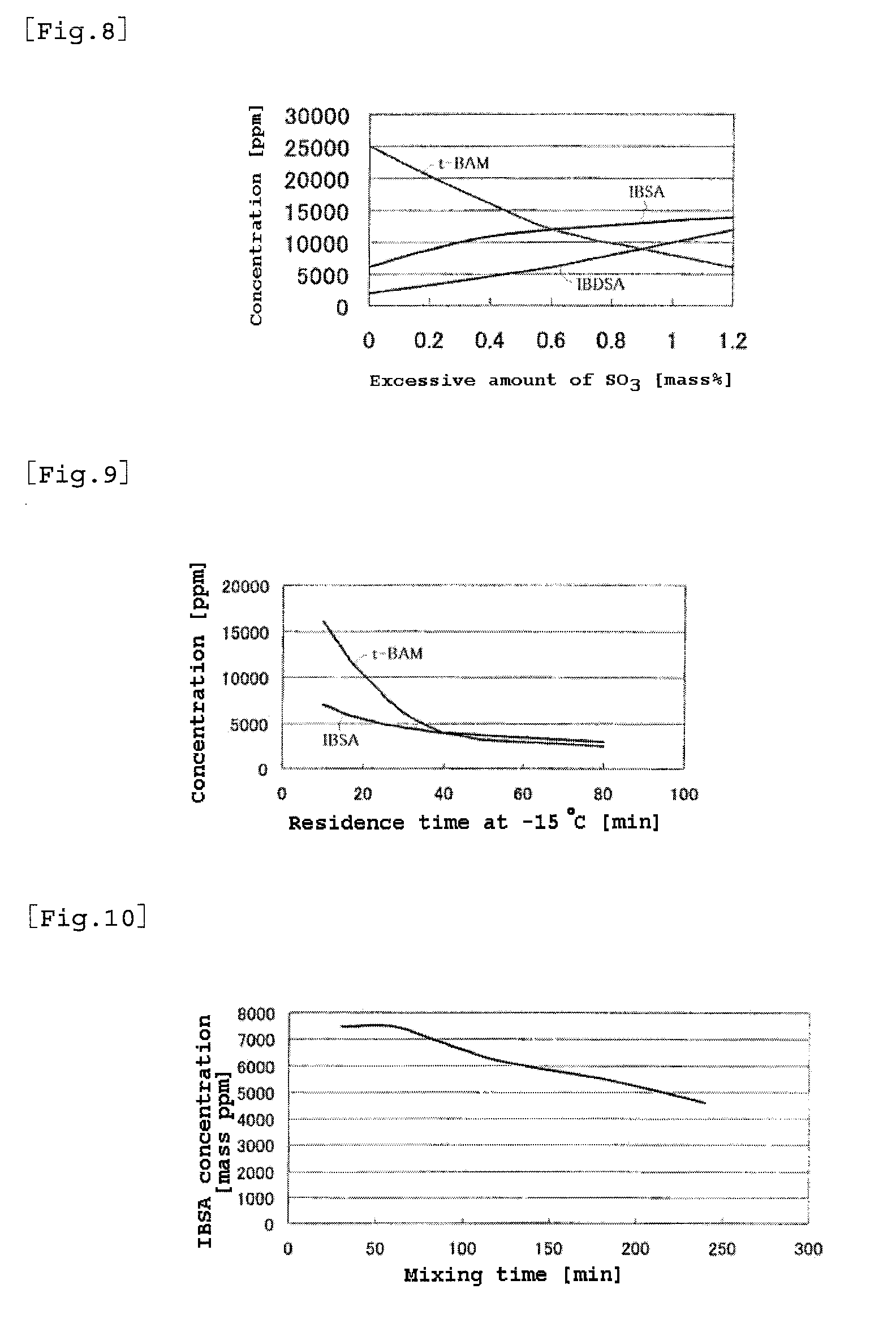
2-acrylamide-2-methylpropanesulfonic acid and process for producing the same
ActiveUS20100274048A1Large molecular weightHigh purityOrganic compound preparationSulfonic acid preparationSulfur trioxideMethyl group
A process for producing 2-acrylamide-2-methyl propane sulfonic acid (ATBS) which comprises reacting acrylonitrile, fuming sulfuric acid, and isobutylene. During the reaction, the concentration of 2-methyl-2-propenyl-1-sulfonic acid (IBSA) and / or that of 2-methylidene-1,3-propylenedisulfonic acid (IBDSA) in the reaction system are determined. When the IBSA concentration exceeds 12,000 mass ppm and / or the IBDSA concentration exceeds 6,000 mass ppm, then the concentration of sulfur trioxide in the reaction system is reduced. Thus, ATBS having an IBSA content of 100 mass ppm or lower and an IBDSA content of 100 mass ppm or lower is produced.
Owner:TOAGOSEI CO LTD
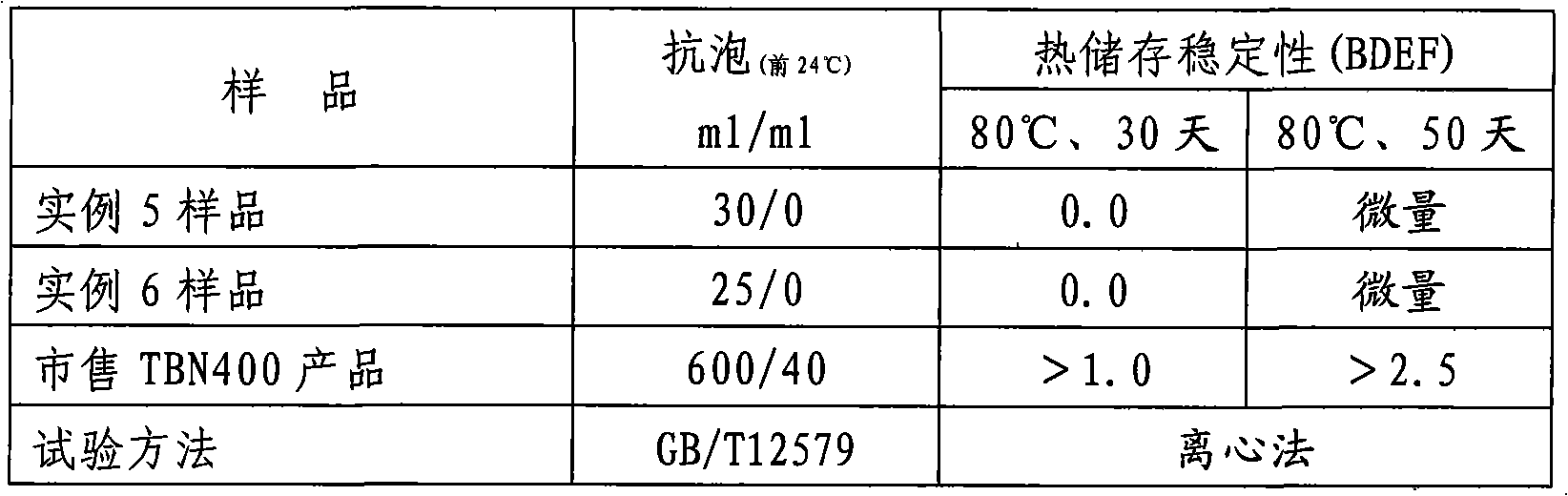
Method for preparing high-alkali value (TBN400) synthesized calcium alkyl benzene sulfonate
ActiveCN101318915AImprove cleanlinessGood dispersionAdditivesSulfonic acid preparationTotal Base NumberAlkaline earth metal
The invention provides a method for preparing high base number (TBN400) synthetic calcium alkyl-benzene sulfonate. The method comprises the following steps of: adopting a mixed acid of long-chain linear alkyl-benzene sulfonic acid and high-boiling heavy alkyl-benzene sulfonic acid, calcium oxide and / or calcium hydroxide, low-carbon alcohol, alkaline-earth metal halide or nitrate, and a mixture of alkaline-earth metal alkylphenol or alkaline-earth metal alkylphenate and polyisobutylene succinic anhydride for a neutralization reaction in the presence of a solvent and cutback oil at a temperature of between 40 and 80 DEG C; then, passing through carbon dioxide to a product of the neutralization reaction at a temperature of between 40 and 60 DEG C for a carbonation reaction; and producing high base synthetic alkyl-benzene sulfonate with a total base number (TBN) of 400mgKOH / g by adopting a process of a one-step method. The product is divided into high-base number (TBN400) synthetic alkyl-benzene sulfonate containing chlorine and high-base number (TBN400) synthetic alkyl-benzene sulfonate without the chlorine. The product produced by adopting the method with low viscosity, small turbidity, easy filtration, light color and no skin formation has the advantages of excellent high-temperature detergency, excellent anti-foaming property and excellent heat storage stability.
Owner:JINZHOU DPF TH CHEM CO LTD
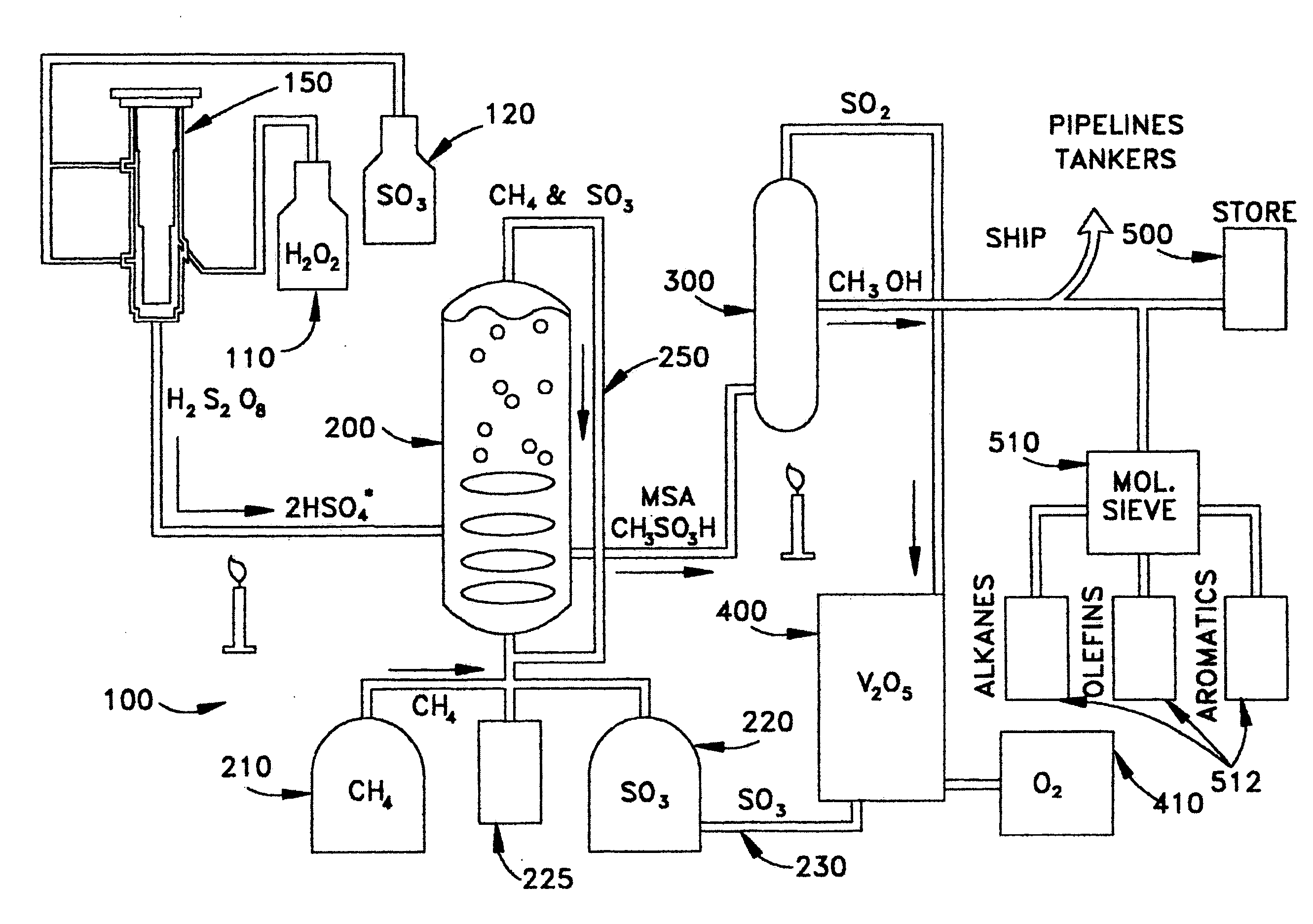
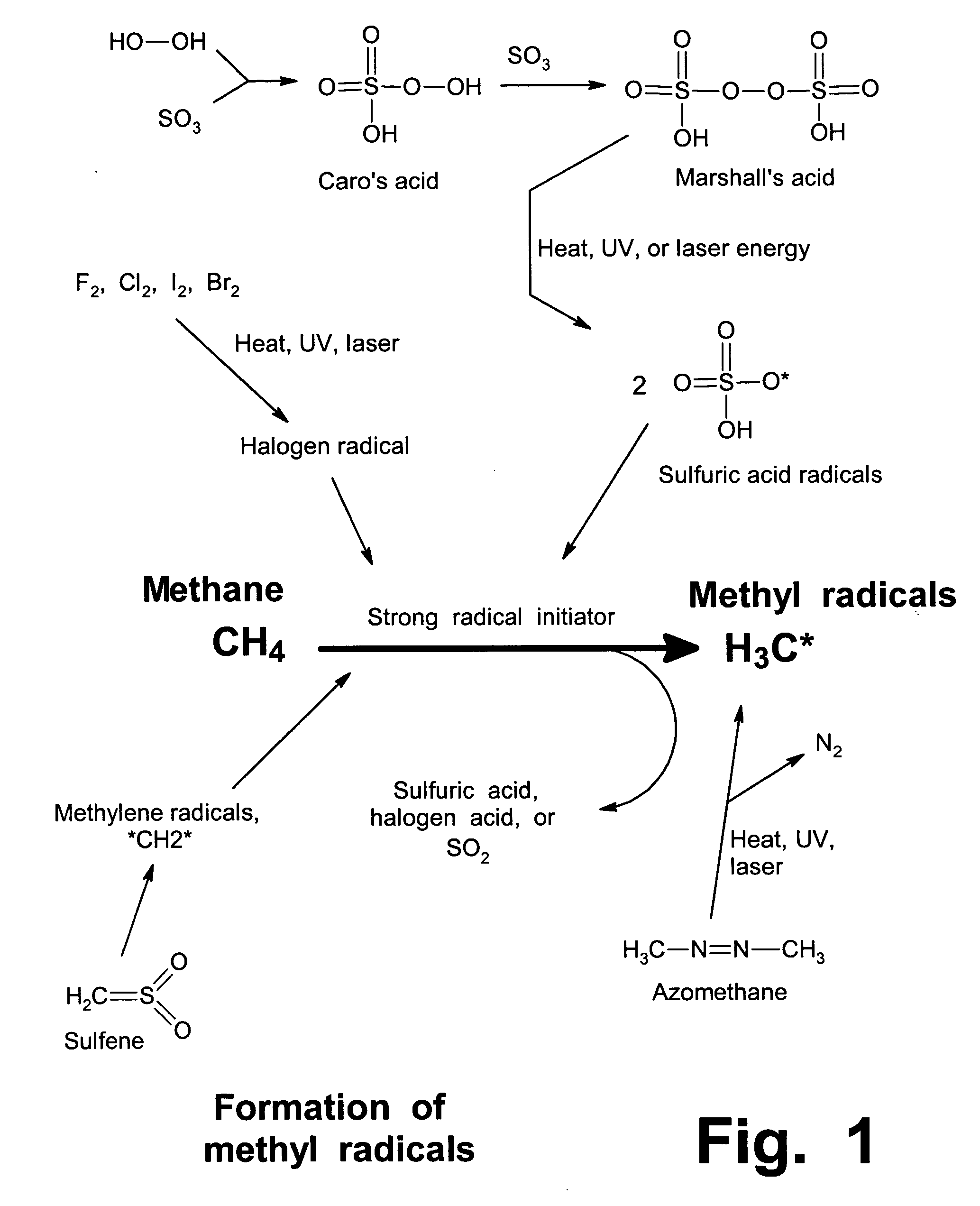
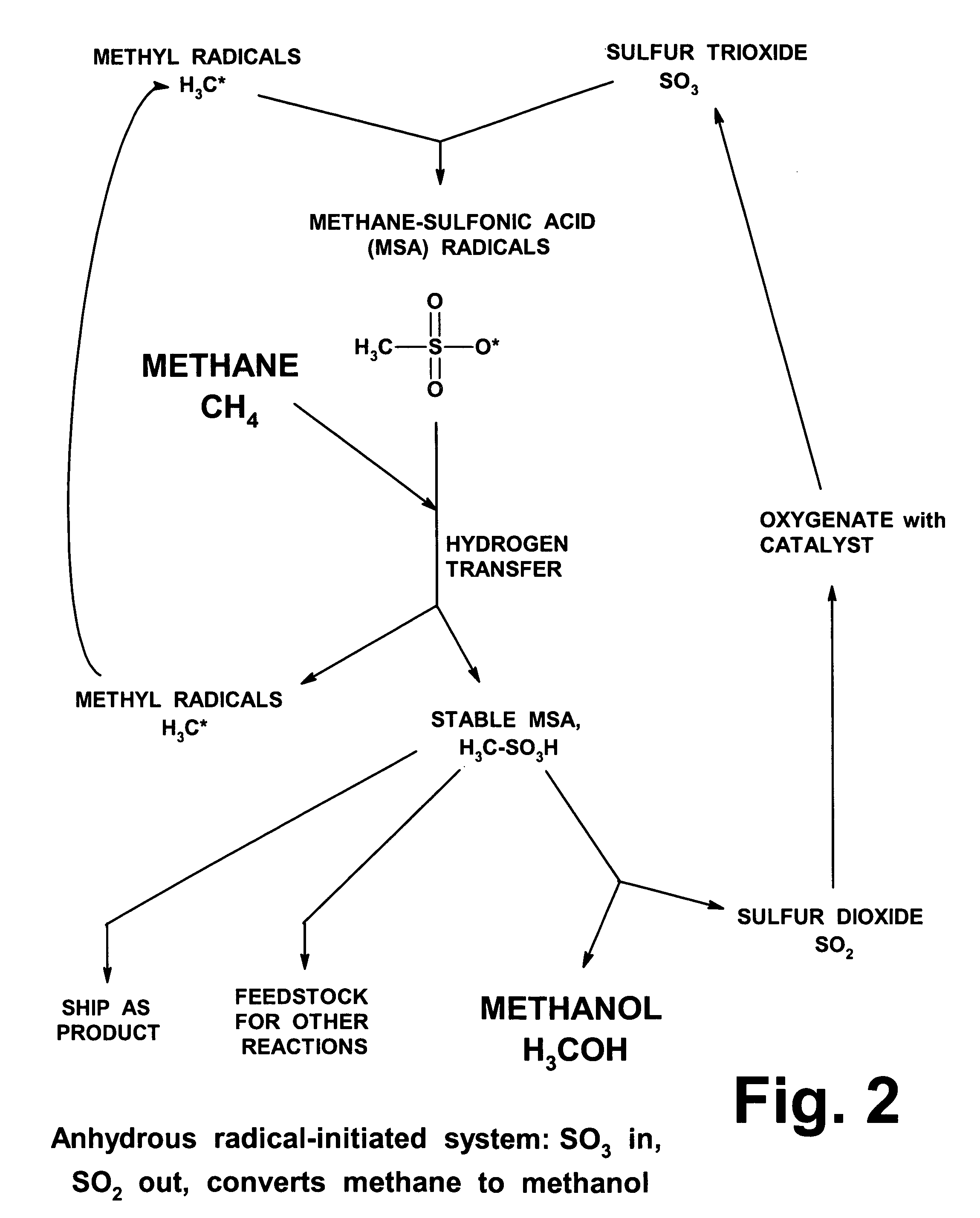
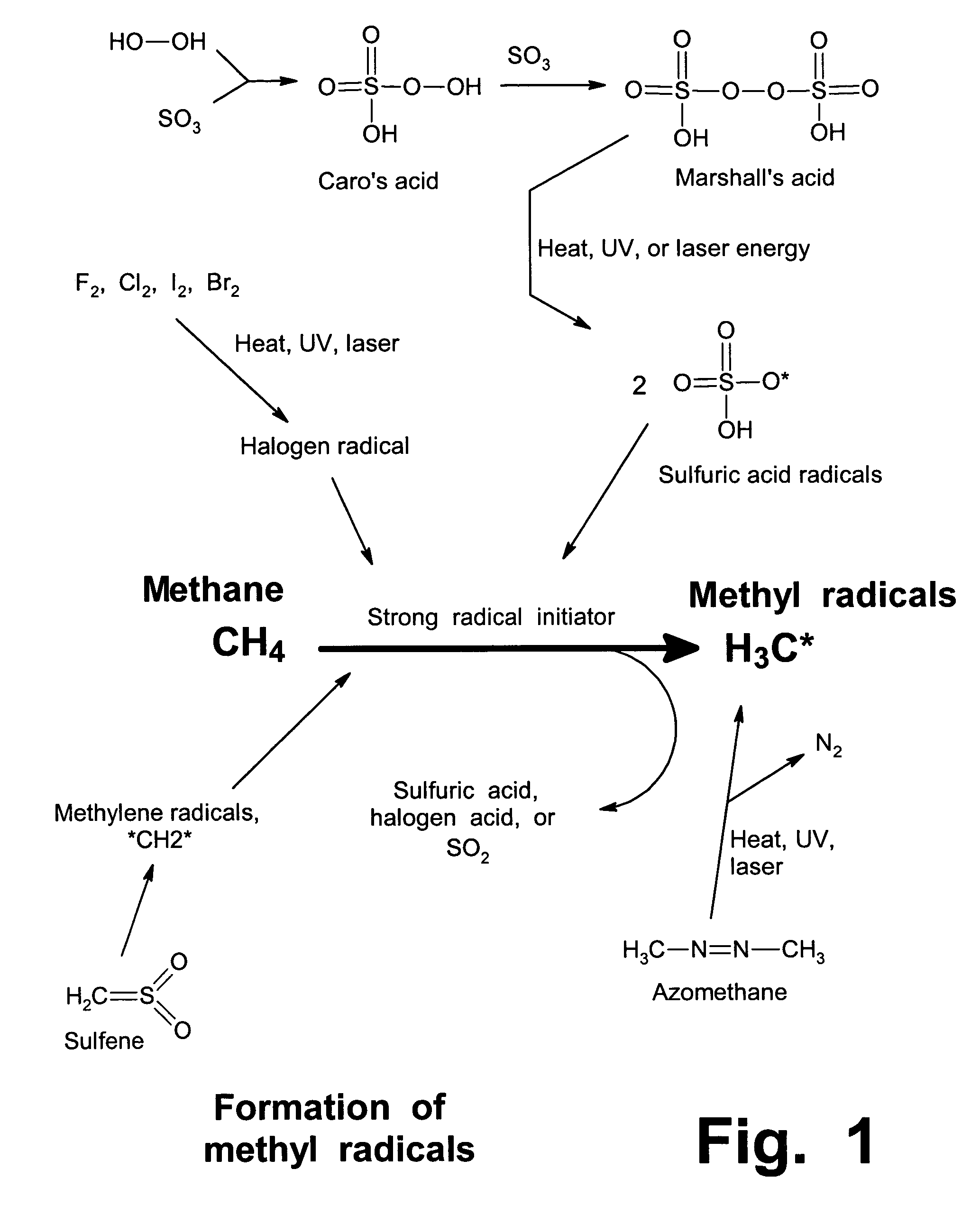
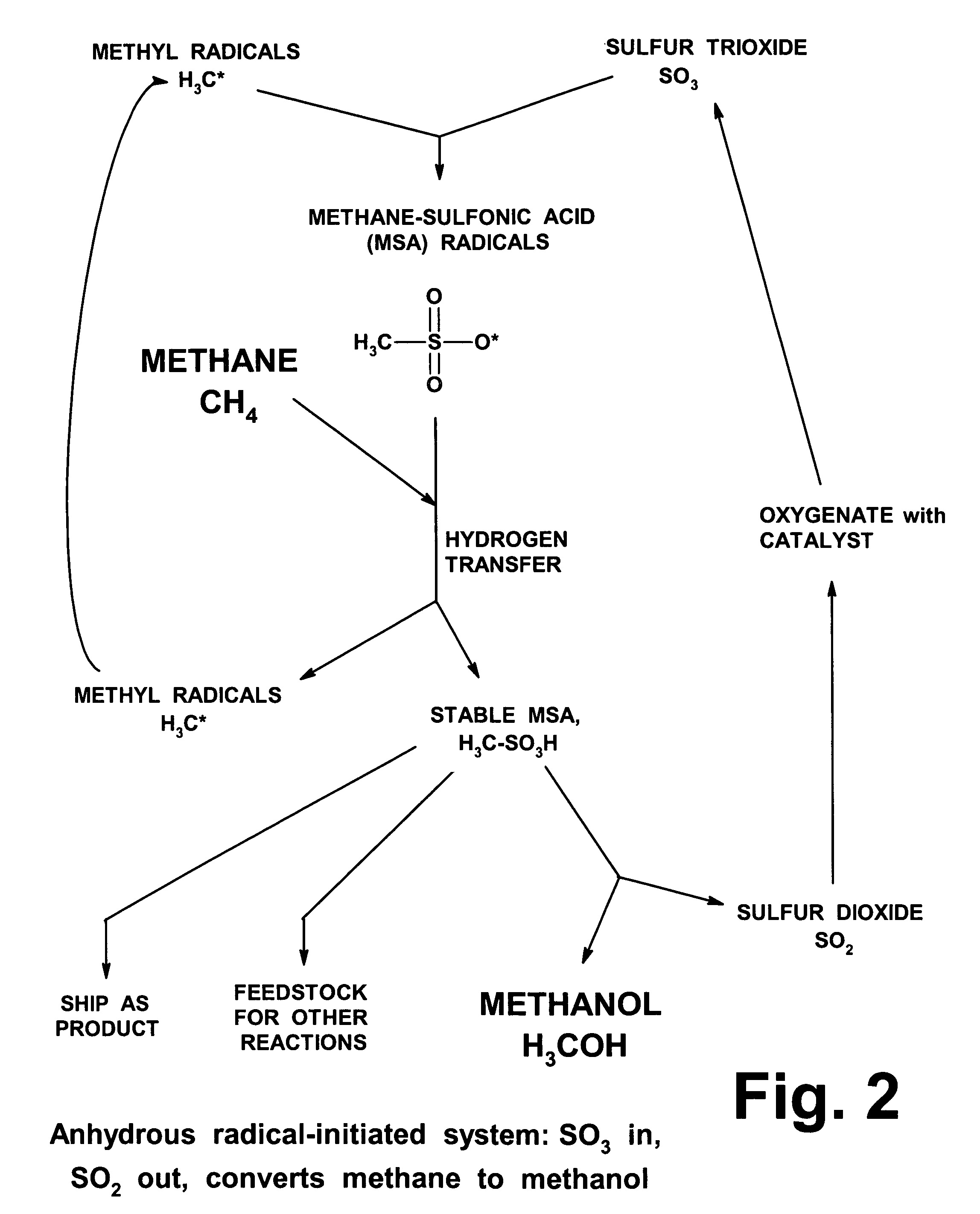
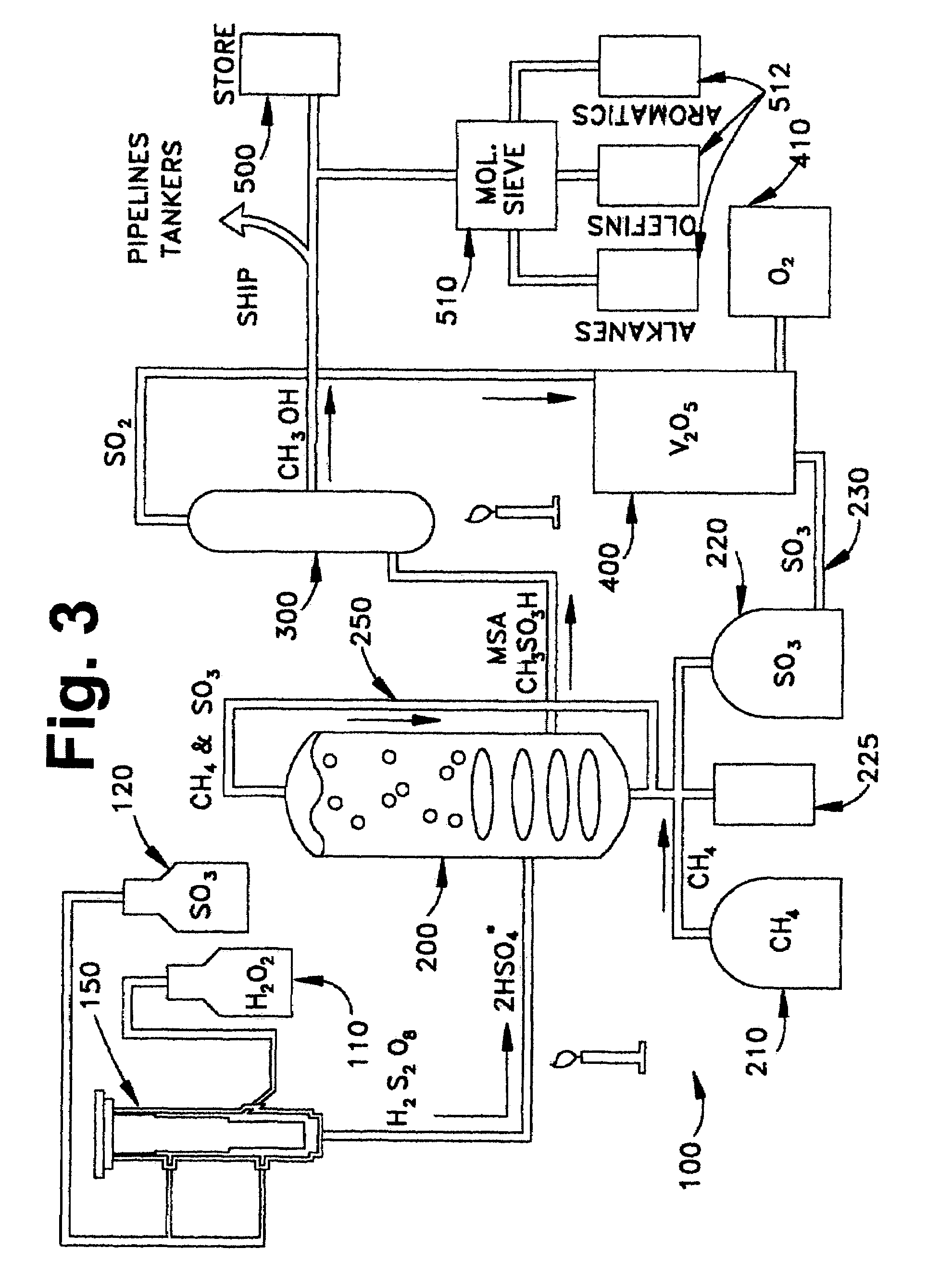
Anhydrous processing of methane into methane-sulfonic acid, methanol, and other compounds
ActiveUS20050070614A1Increases solubility and reaction rateEfficient removalHydrogenOrganic compound preparationLiquid fuelOxygen compound
Anhydrous processing to convert methane into oxygenates (such as methanol), liquid fuels, or olefins uses an initiator to create methyl radicals. These radicals combine with sulfur trioxide to form methyl-sulfonate radicals. These radicals attack fresh methane, forming stable methane-sulfonic acid (MSA) while creating new methyl radicals to sustain a chain reaction. This system avoids the use or creation of water, and liquid MSA is an amphoteric solvent that increasing the solubility and reactivity of methane and SO3. MSA from this process can be sold or used as a valuable chemical with no mercaptan or halogen impurities, or it can be heated and cracked to release methanol (a clean fuel, gasoline additive, and chemical feedstock) and sulfur dioxide (which can be oxidized to SO3 and recycled back into the reactor). MSA also can be converted into gasoline, olefins, or other valuable chemicals.
Owner:VEOLIA NORTH AMERICA REGENERATION SERVICES LLC
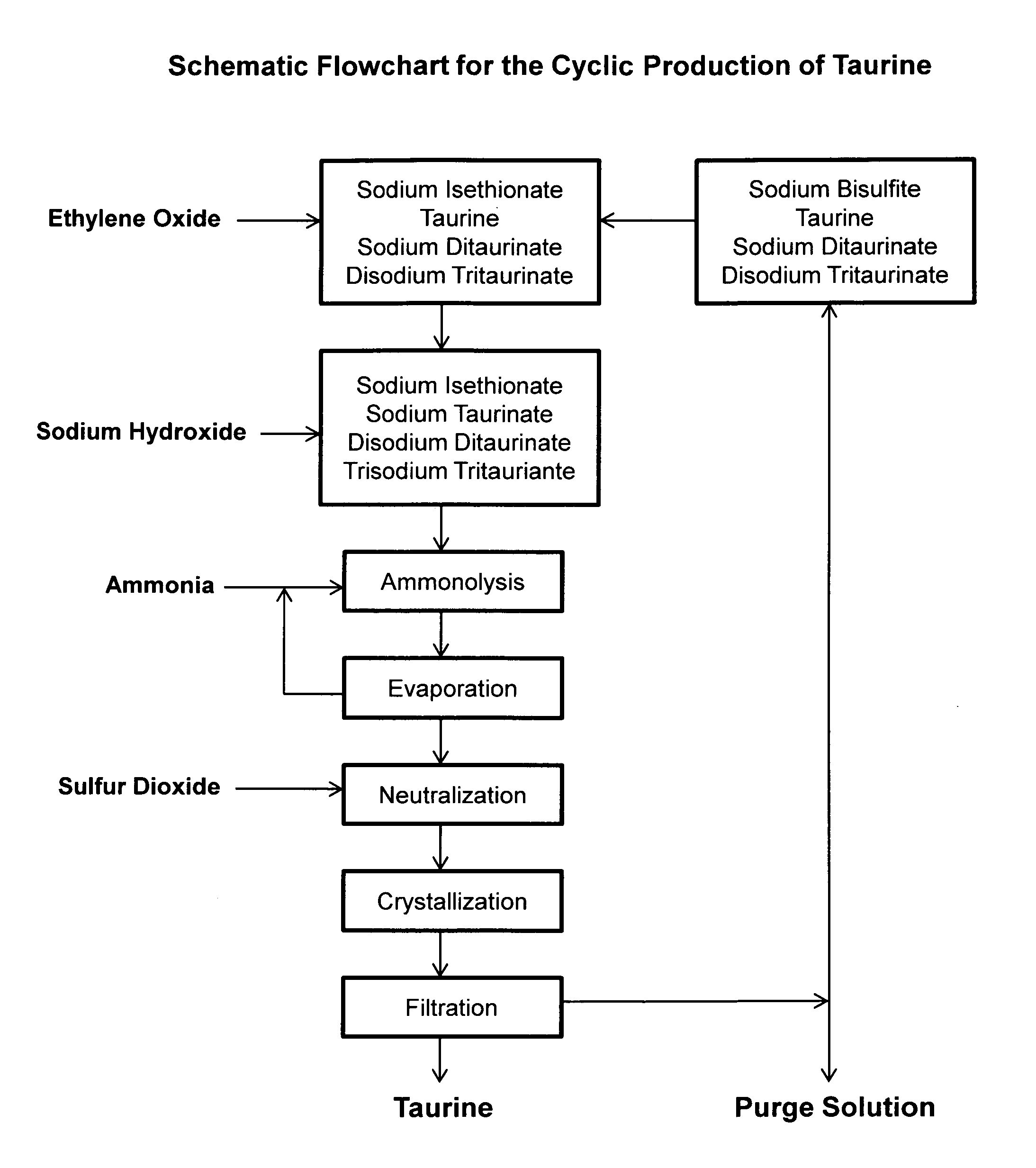
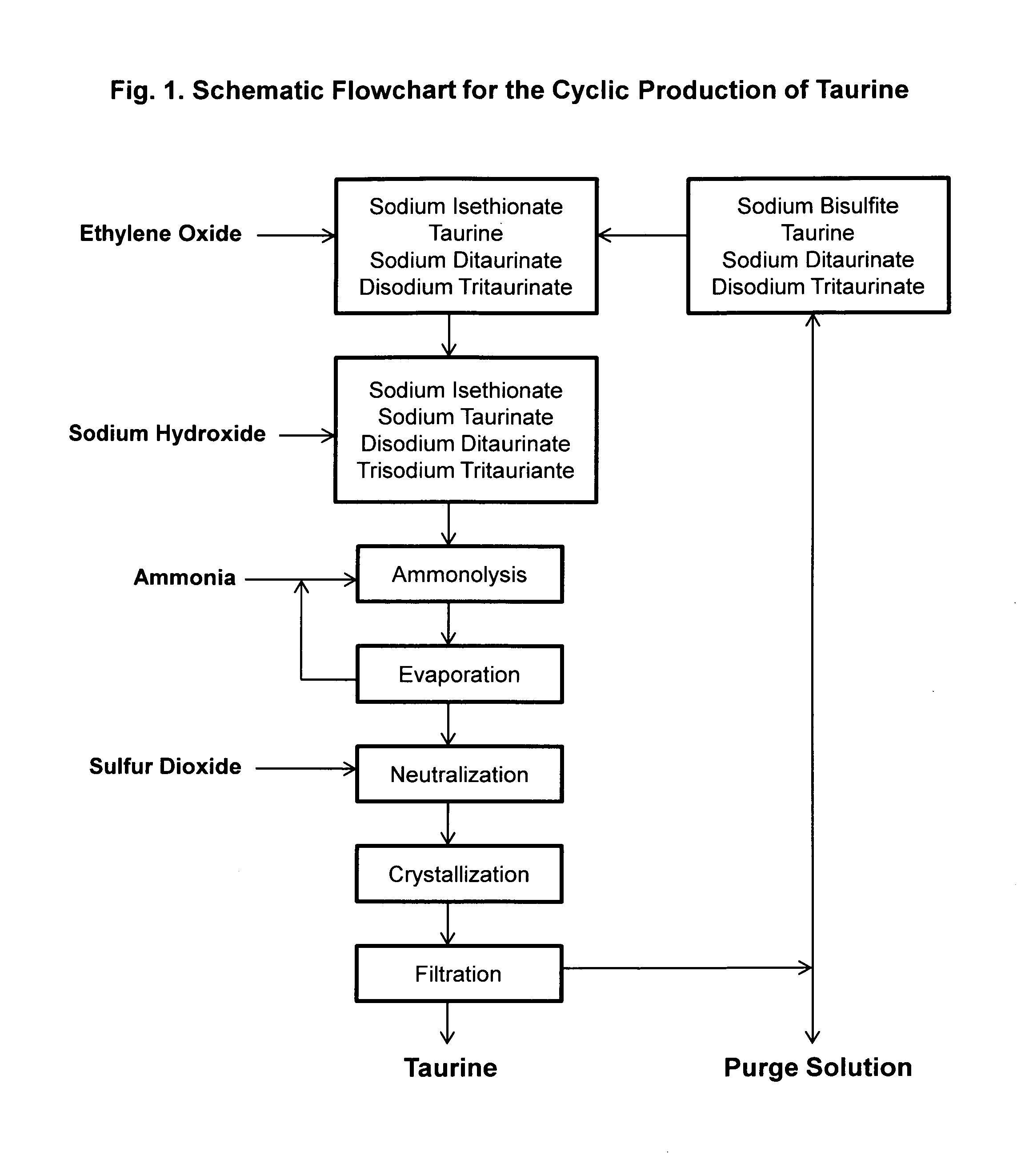
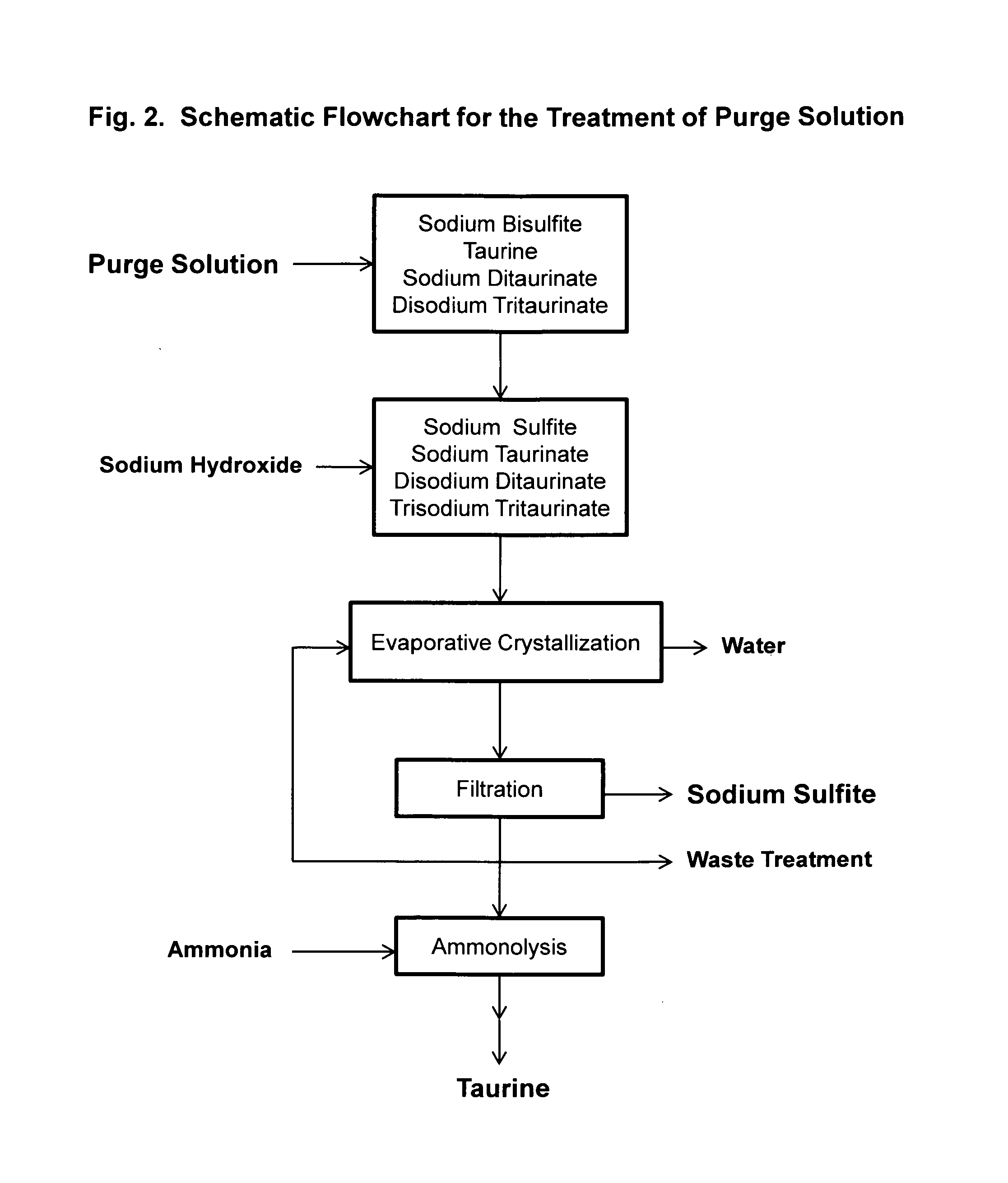
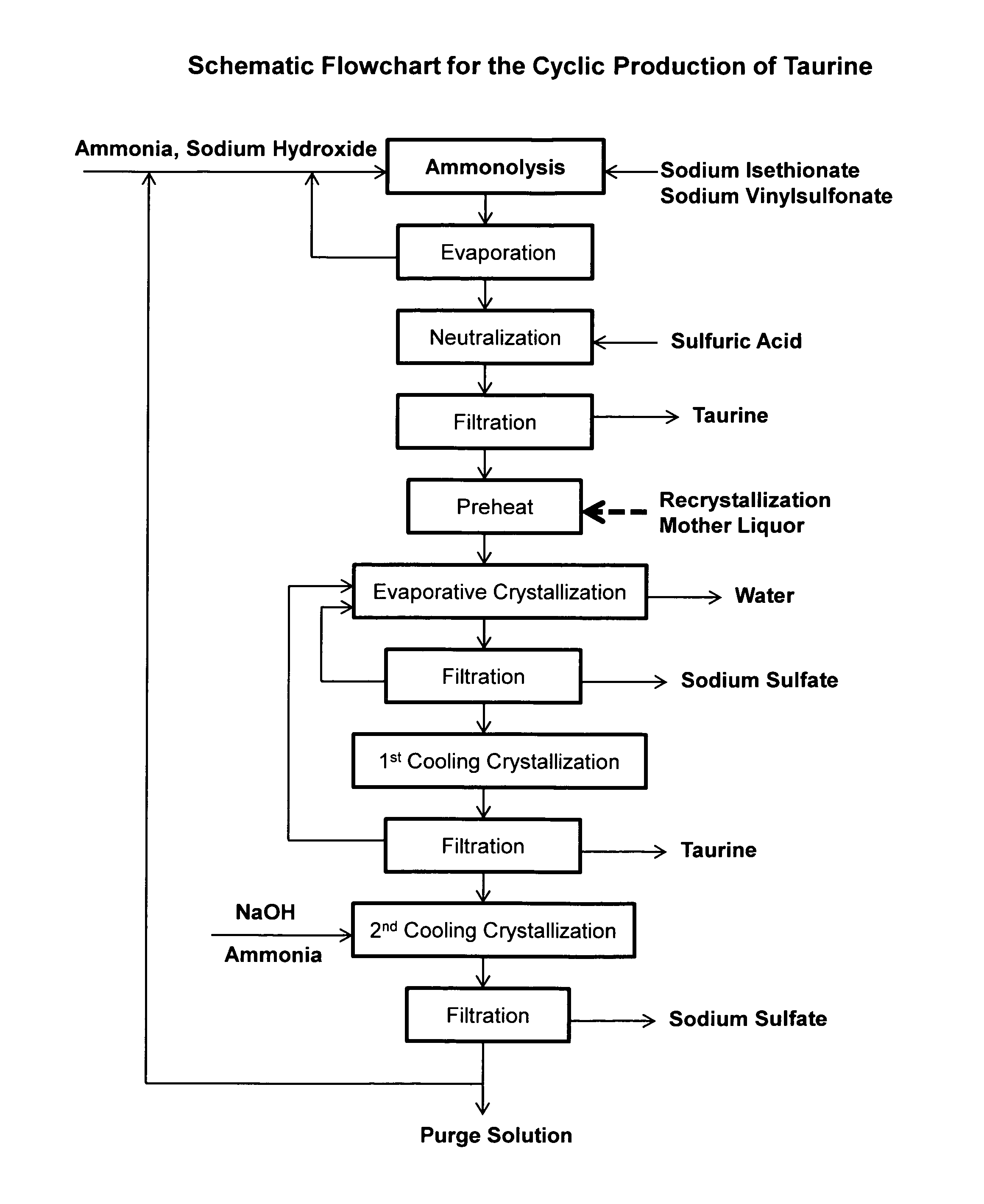
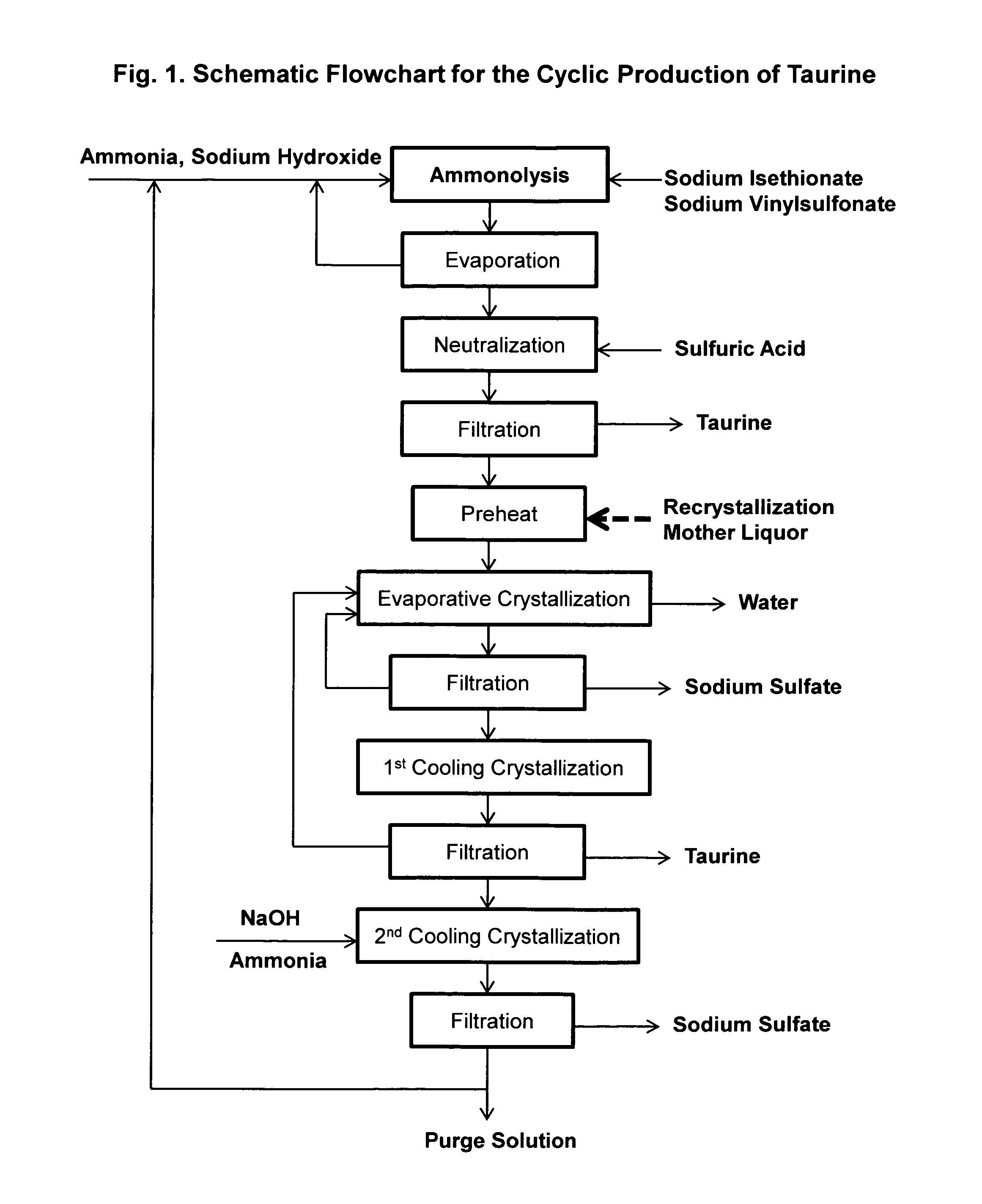
Cyclic process for the production of taurine from ethylene oxide
ActiveUS9061976B1Organic compound preparationSulfonic acids salts preparationCyclic processSodium dithionite
The present invention discloses a cyclic process for the production of taurine from ethylene oxide in a high yield of greater than 95% by continuously converting the byproducts of the ammonolysis reaction, sodium ditaurinate and sodium tritaurinate, to sodium taurinate. The cyclic process is completed by using sulfur dioxide or sulfurous acid to neutralize sodium taurinates to recover taurine and to regenerate sodium bisulfite, which is then reacted with ethylene oxide.
Owner:VITAWORKS IP LLC
Anhydrous processing of methane into methane-sulfonic acid, methanol, and other compounds
ActiveUS7282603B2Increases solubility and reaction rateEfficient removalHydrogenOrganic compound preparationSolubilityLiquid fuel
Anhydrous processing to convert methane into oxygenates (such as methanol), liquid fuels, or olefins uses an initiator to create methyl radicals. These radicals combine with sulfur trioxide to form methyl-sulfonate radicals. These radicals attack fresh methane, forming stable methane-sulfonic acid (MSA) while creating new methyl radicals to sustain a chain reaction. This system avoids the use or creation of water, and liquid MSA is an amphoteric solvent that increasing the solubility and reactivity of methane and SO3. MSA from this process can be sold or used as a valuable chemical with no mercaptan or halogen impurities, or it can be heated and cracked to release methanol (a clean fuel, gasoline additive, and chemical feedstock) and sulfur dioxide (which can be oxidized to SO3 and recycled back into the reactor). MSA also can be converted into gasoline, olefins, or other valuable chemicals.
Owner:VEOLIA NORTH AMERICA REGENERATION SERVICES LLC
Process for producing taurine from alkali taurinates
Owner:VITAWORKS IP LLC
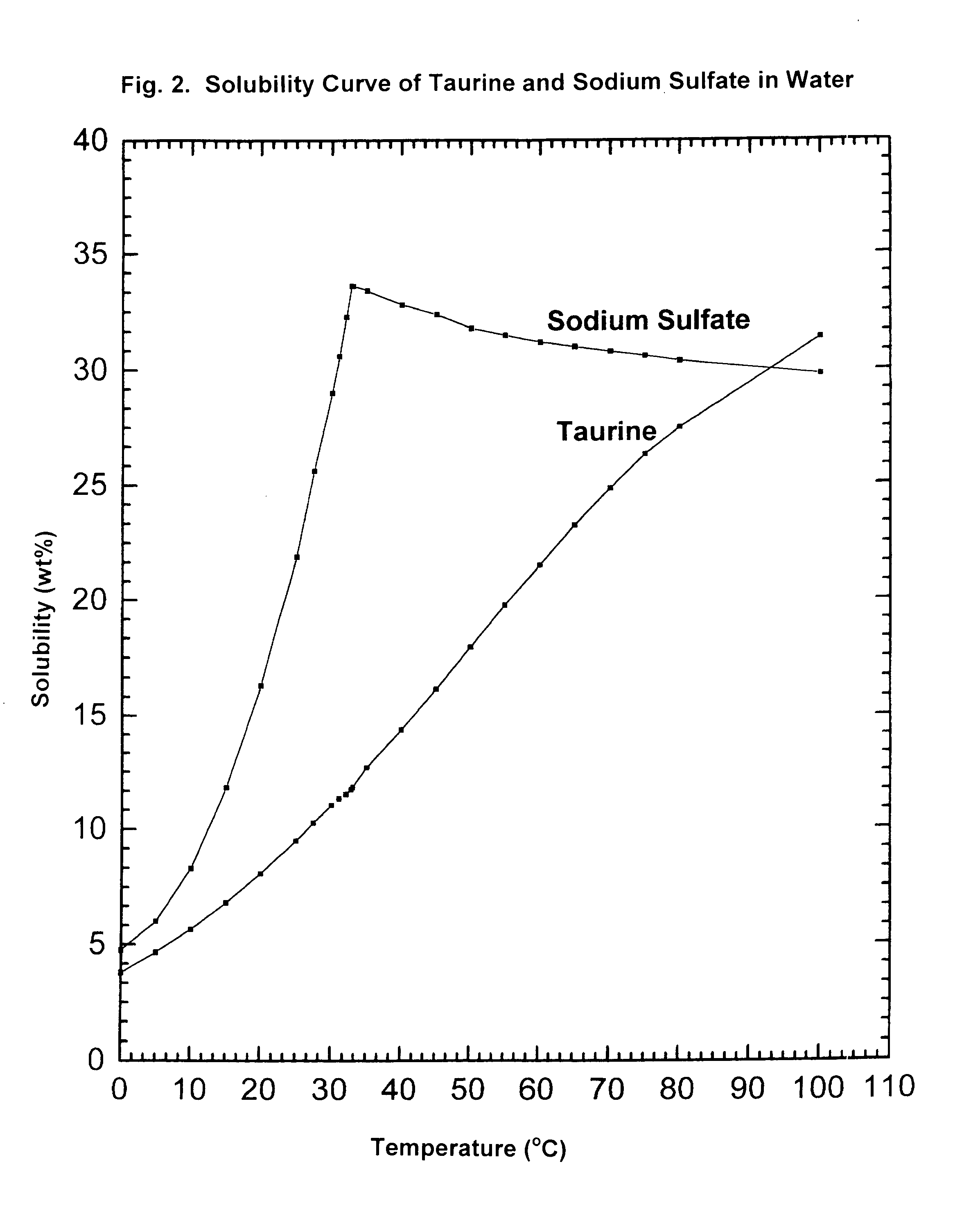
Cyclic process for the production of taurine from alkali isethionate
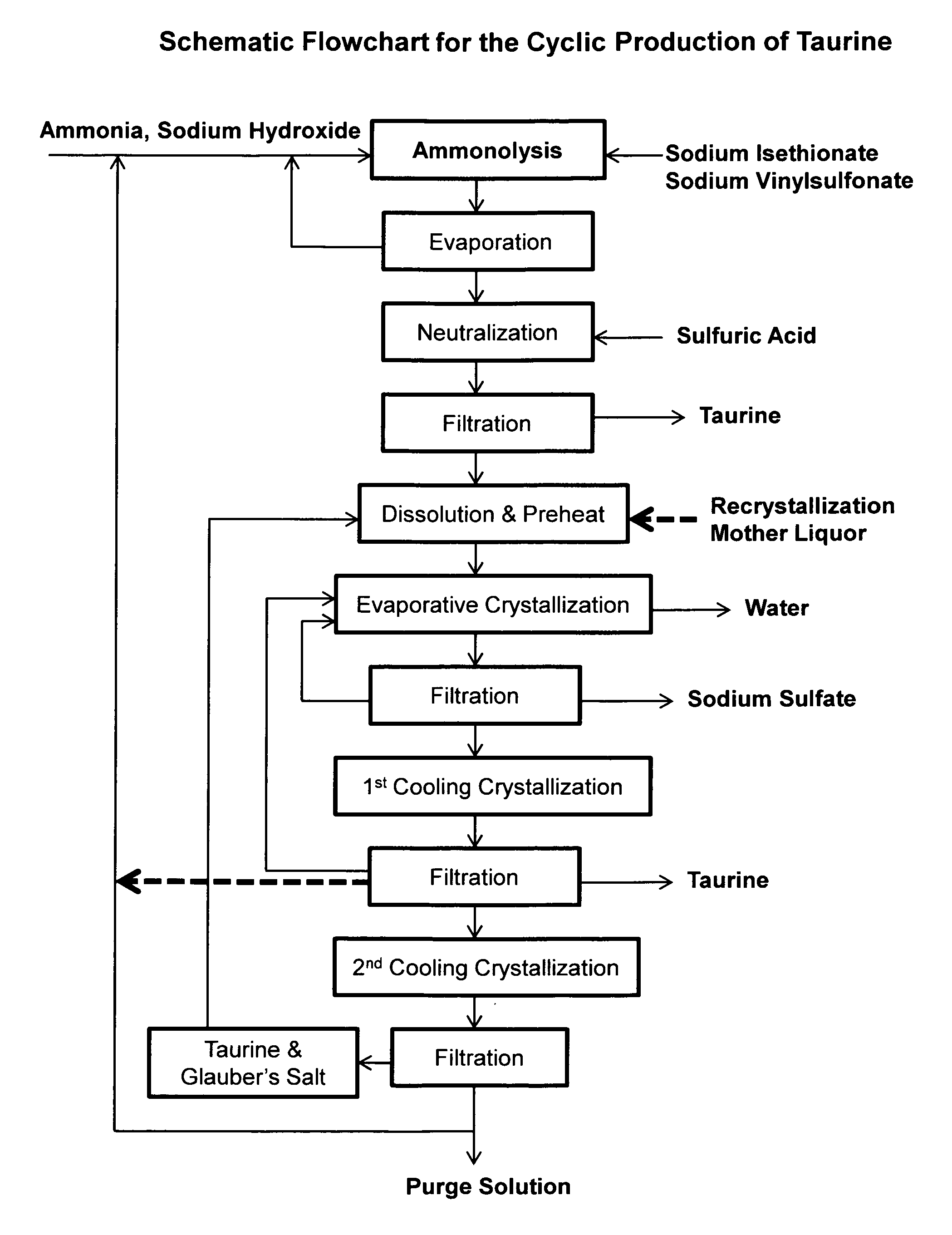
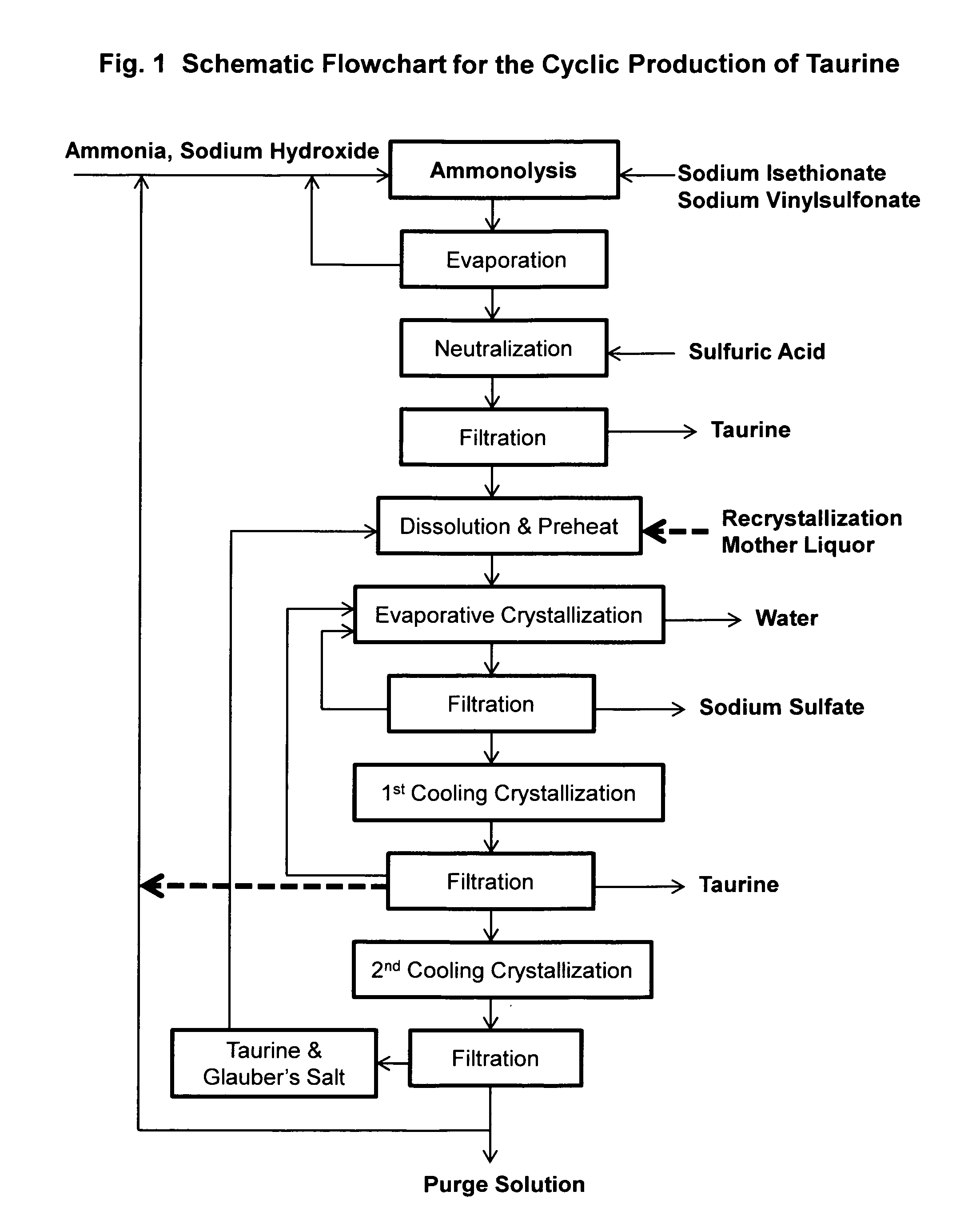
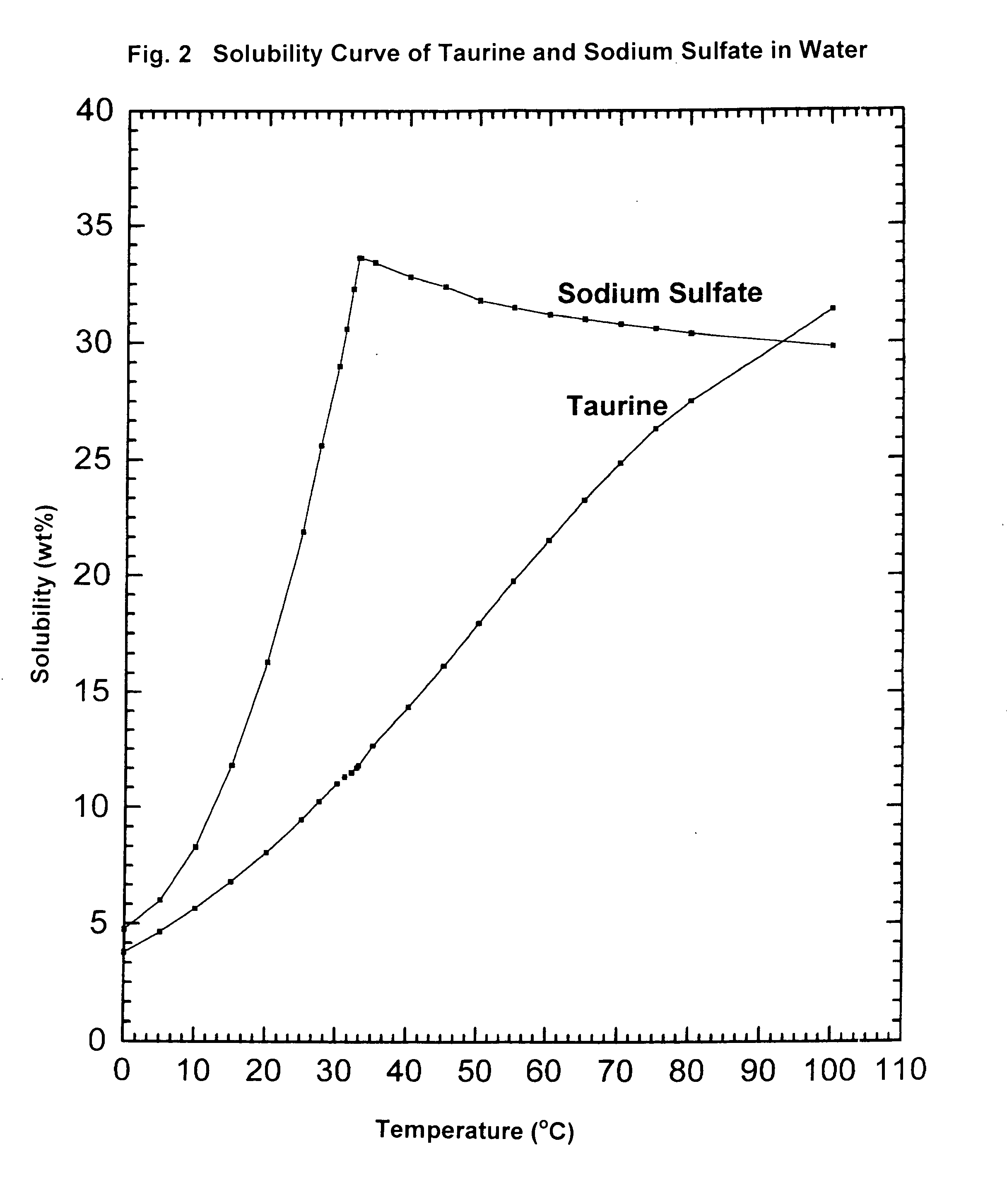
ActiveUS9428451B2Facilitate cooling crystallizationOrganic compound preparationSulfonic acids salts preparationCyclic processTaurine
A cyclic process is disclosed for the production of taurine from alkali isethionate in a high overall yield by continuously converting the byproducts of the ammonolysis reaction, sodium ditaurinate and sodium tritaurinate, to sodium taurinate. Sodium sulfate and residual taurine in the crystallization mother liquor are efficiently separated by converting taurine into a highly soluble form of sodium taurinate or ammonium taurinate while selectively crystallizing sodium sulfate.
Owner:VITAWORKS IP LLC
Method for preparing naphthalene sulphonic acid by sulfonating sulfur trioxide in microreactor
InactiveCN101607925ALarge specific surface areaImprove heat transfer performanceChemical/physical/physico-chemical processesSulfonic acid preparationReaction temperatureNitromethane
The invention relates to a method for preparing naphthalene sulphonic acid by sulfonating sulfur trioxide in a microreactor, belonging to methods for preparing dye intermediates in the field of fine chemical engineering. The method comprises the following steps: taking alkyl halide and nitromethane as an organic solvent, naphthalene and derivative thereof as the raw material and organic solution of liquid sulfur trioxide as a sulfonating agent, preparing a solution according to the mol ratio of the organic solvent, the raw material and the sulfonating agent of 19-74:1:1-3 in the microreactor of with channel with the diameter of 10-50 microns, and sulfonating the reaction solution to prepare the naphthalene sulphonic acid by controlling the sulfonation reaction temperature to be between 17DEG C below zero and90 DEG C. The method adopts a continuous flow reactor, solves the problem of impossible transient mixing in the conventional reactor, prevents secondary reaction caused by local excess, and is especially suitable for strong exothermic reaction, fast reaction and flammable and explosive reaction. Compared with the preparation technology in the traditional batch reactor, the invention has the advantages of no generation of waste water and waste acid, clean and environment-friendly technology, consumption of sulphonic acid close to the theoretical quantity, fast reaction speed, low sulfonation temperature, high product yield, good repeatability, high labor productivity and low equipment cost.
Owner:SPECIAL CHEM CO LTD DALIAN FIRSTAR
Methylsulfonic acid preparing process
InactiveCN1810780ANo pollution in the processRaw materials are easy to getSulfonic acid preparationSulfate radicalsWater insoluble
The environment friendly methyl sulfonic acid preparing process includes the following steps: the reaction of the water solution or solid of ammonium sulfite or mixture of ammonium sulfite and ammonium bisulfite with dimethyl sulfate at high temperature to produce ammonium methyl sulfonate; treating the reacted solution containing ammonium methyl sulfonate and ammonium sulfate with calcium hydroxide or other compound capable of producing precipitate with sulfate radical ion to produce water soluble calcium methyl sulfonate, water insoluble calcium sulfate and ammonium hydroxide; treating obtained calcium methyl sulfonate with strong acid capable of forming precipitate with calcium ion; and final decompression distilling to obtain methyl sulfonic acid. The present invention has facile material, simple technological process, high product quality, low cost and no environmental pollution, and is suitable for industrial production.
Owner:HEBEI YANUO CHEM IND
Novel sulfonate and its derivative, photosensitive acid generator, and resist composition and patterning process using the same
ActiveUS20100209827A1Improve solubilityImprove stabilityOther chemical processesOrganic compound preparationResistSolubility
There is disclosed a sulfonate shown by the following general formula (2).R1—COOC(CF3)2—CH2SO3−M+ (2)(In the formula, R1 represents a linear, a branched, or a cyclic monovalent hydrocarbon group having 1 to 50 carbon atoms optionally containing a hetero atom. M+ represents a cation.) There can be provided: a novel sulfonate which is effective for a chemically amplified resist composition having a sufficiently high solubility (compatibility) in a resist solvent and a resin, a good storage stability, a PED stability, a further wider depth of focus, a good sensitivity, in particular a high resolution and a good pattern profile form; a photosensitive acid generator; a resist composition using this; a photomask blank, and a patterning process.
Owner:SHIN ETSU CHEM IND CO LTD
Alkylated aromatic compositions, zeolite catalyst compositions and processes for making the same
ActiveUS6977319B2Prolong lifeReduce inactivation rateMolecular sieve catalystsOrganic chemistry methodsAlkyl transferMordenite
Owner:CHEVRON ORONITE CO LLC +1
Method of preparing alpha-sulphonic acid fatty acid ester
InactiveCN101508660AImprove securityRelaxed reaction conditionsSulfonic acid preparationState of artReaction temperature
The invention relates to a method for preparing alpha-sulfonic fatty acid ester by sulfonating fatty acid ester, comprising the following steps: injecting the fatty acid ester and a sulfonating agent into a micro-structural reactor (5) and a reactor (6) inside diameter of which is a micro passage respectively, mixing and sulfonating the fatty acid ester and the sulfonating agent, controlling the reaction temperature between 50 and 100 DEG C, and controlling the reaction materials to stay for 1ms to 1s in the micro-structural reactor (5) and stay for 0.5 to 30 minutes in the micro-passage reactor (6); and then injecting the reaction materials into a micro-passage reactor (7), aging the reaction materials at a reaction temperature between 50 and 100 DEG C, and controlling the reaction materials to stay for 0.5 to 30 minutes in the micro-passage reactor to obtain the alpha-sulfonic fatty acid ester. In the method, the novel micro-structural reactor is adopted, the defect of difficult control due to strong reaction heat release in the prior art is overcome, the materials can be mixed evenly, the released reaction heat can be removed in time, the reaction time can be controlled accurately, and the method has the advantages of good sulfonating efficiency, simple and safe operation and good product quality.
Owner:NANJING UNIV OF TECH
Method for fluorinating a compound comprising a halosulphonyl or dihalophosphonyl group
The invention relates to a fluorination process for producing fluorinated compounds.The process consists in reacting a compound (I) corresponding to the formulawith an ionic fluoride of a monovalent cation. M represents H, an alkali metal, a quaternary phosphonium group or a quaternary ammonium group. Y represents SO2 and m is 1, or else Y is PO and m is 2. Z represents CR2, N or P. R1 represents an electron-withdrawing group which has a Hammet σP parameter of greater than 0.4. R2 represents a carbonaceous and / or electron-withdrawing group. X represents a halogen other than a fluorine.The fluorinated compounds obtained are of use in particular as electrolytes in lithium batteries.
Owner:HYDRO QUEBEC CORP
Method for preparing functional fluorine-bearing monomer by vertical-tube type catalytic reaction
ActiveCN101712639AHigh purityFast addition reactionGroup 5/15 element organic compoundsSulfonic acid preparationLiquid productVertical tube
The invention discloses a preparation method of a functional fluorine-containing monomer. The method comprises the following steps of: uniformly mixing a perfluoro-linear chain or branched chain acyl fluoride compound, trifluoroethylene sulfuric ester and anhydrous hydrogen fluoride liquid in the mole ratio of 1:1:(1-20); passing from bottom to top through a vertical-tube type reactor which is filled with a catalyst; finishing addition reaction in a short time under the action of the high specific surface area catalyst inside the reactor; and setting apart a reaction liquid product from the upper part of the vertical-tube type reactor to form a product with a general formula in formula (I). In the invention, the functional fluorine-containing monomer can be prepared continuously and efficiently with easily operated reaction equipment, conveniently controlled reaction temperature and no use of any organic solvent, and a high-purity monomer can be obtained through simple rectification. The catalyst filling in the tubular reactor is one or a mixture of active carbon, macro-porousmolecular sieve and porous aluminum oxide. The reaction can be carried out continuously inside the reactor and satisfies industrialization requirements. CF2=CF-O-Rf(I).
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Alkyl phenol sulfonic polyoxyethylene ether sulfonate and preparation thereof
ActiveCN101279937AEnhanced overall recoveryImprove technical effectDrilling compositionSulfonic acid preparationSulfonateAlkylphenol
The invention relates to alkylphenol sulfonic polyoxyethylene ether sulfonate and the preparation method thereof, mainly aiming at solving the problems that current oil displacement agent containing surfactant is poor in oil displacement efficiency under high temperature with the existence of high salt and that the oil displacement agent contains alkali and causes damages to the stratum and the oil field and erodes the equipment and the pipes, which results in high production cost. The invention adopts alkylphenol sulfonic polyoxyethylene ether sulfonate with general molecular formula (I)to solve the problem; wherein M is alkali metal; R is alkyl from C1-C22; n is any integer from 1 to 20. The alkylphenol sulfonic polyoxyethylene ether sulfonate can be applied to the production of tertiary oil recovery in oil fields.
Owner:CHINA PETROLEUM & CHEM CORP +1
Cyclic process for producing taurine
ActiveUS20160355470A1Reduce wasteImprove production yieldOrganic compound preparationSulfonic acids salts preparationAlkali saltSulfite
There is disclosed a process for producing taurine by the ammonolysis of alkali isethionate in the presence of alkali ditaurinate or alkali tritaurinate, or their mixture, to inhibit the formation of byproducts and to continuously convert the byproducts of the ammonolysis reaction to alkali taurinate. Alkali taurinate is neutralized with isethionic acid to obtain taurine and to regenerate alkali isethionate. The production yield is increased to from 90% to nearly quantitative. The ammonolysis reaction is catalyzed by alkali salts of hydroxide, sulfate, sulfite, phosphate, or carbonate.
Owner:VITAWORKS IP LLC
Method for preparing naphthalene series water reducing agent
ActiveCN101723860AFeed lessReduce pollution factorSulfonic acid preparationCalcium hydroxideHydrolysis
The invention provides a method for preparing a naphthalene series water reducing agent. The method comprises the following steps of naphthalene fusion, sulphonation reaction, steam hydrolysis naphthalene removal, recovery and reuse of naphthalene, condensation reaction and neutral reaction. Compared with the conventional production technology, the process of the invention has the advantages that: the consumption of sulfuric acid is reduced by adjusting a material charging ratio of sulfuric acid to material naphthalene at the step of sulphonation reaction; the step of the recovery and reuse of naphthalene is added, so that the raw materials are fully utilized; and in the following step of neutral reaction, only a small amount of alkali solution is added and the addition of calcium hydroxide is unnecessary, so that the filter step is eliminated, and deposition loss and entrainment loss of the products are avoided.
Owner:ZHEJIANG JISHENG CHEM BUILDING MATERIALS
Fatty acid esters of hydroxyalkyl sulfonate salts and process for producing same
InactiveUS6069262ANegligible changeMaintain good propertiesOrganic compound preparationSurface-active detergent compositionsPersonal careHair care
Compositions of matter comprising fatty acid esters of hydroxyalkyl sulfonate salts, in particular sodium cocoyl isethionate (SCI) and process for preparing same. The esters are useful for personal care cleansing products, such as bar and liquid soaps, skin and hair care products.
Owner:INNOSPEC ACTIVE CHEM
Olefin conversion process and olefin recovery process
InactiveUS20070225536A1Increase difference boiling pointEasy to separatePreparation by oxo-reaction and reductionMolecular sieve catalystBoiling pointAlkene
The present invention provides a process for converting olefins from a mixture of olefins and non-olefinic organic compounds of comparable boiling point to olefin products with a larger difference in boiling point from the boiling point of the non-olefinic organic compounds. Additional steps may be performed to recover the olefin product including separating the olefin product from the mixture produced in the conversion step.
Owner:LUTZ EUGENE FREDERICK
Method for preparing active carbon carrying precious metal catalyst
InactiveCN102658133AReduce usageAvoid acid and alkaliSulfonic acid preparationMetal/metal-oxides/metal-hydroxide catalystsEthylene diamineActivated carbon
The invention discloses a method for preparing an active carbon carrying precious metal catalyst. The method comprises the following steps of: immersing an active carbon carrier into an ethylene diamine tetraacetic acid disodium salt (EDTA disodium salt for short) aqueous solution for pretreatment; then mixing the pretreated active carbon carrier and water to prepare carbon slurry; adding a nitrate solution or chlorate aqueous solution which contains precious metal into the carbon slurry, and stirring at the temperature between 25 and 90 DEG C for 2 to 6 hours; adding an alkaline aqueous solution under the condition of the temperature between 25 and 80 DEG C, adjusting the pH value of the reaction solution until the pH value is 6 to 9; stirring at the constant temperature between 25 and 80 DEG C for 0.5 to 4 hours, thus obtaining slurry; and post-treating the slurry, thus obtaining the active carbon carrying precious metal catalyst. According to the method, acid and alkaline are not used during pretreatment of the active carbon carrier, so that environment friendliness is realized; during preparation of the catalyst, any auxiliary catalyst is not added, so that the cost is saved, and the process is simplified; the preparation process is simple in condition; the requirement on equipment is low; and industrialization is facilitated.
Owner:杭州凯大催化金属材料股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com