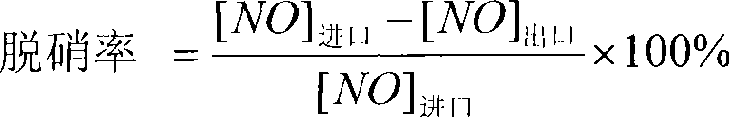Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1602 results about "Sulfur trioxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
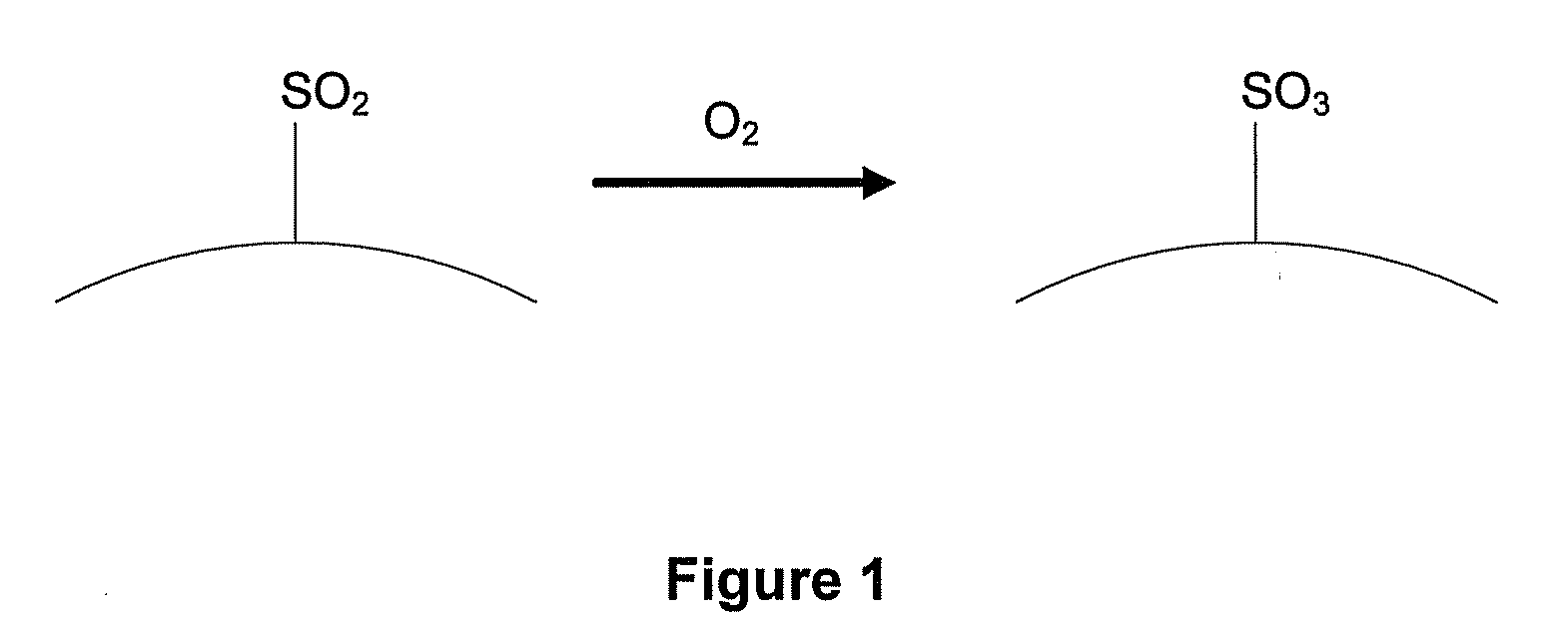
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO₃, with a relatively narrow liquid range. In the gaseous form, this species is a significant pollutant, being the primary agent in acid rain.
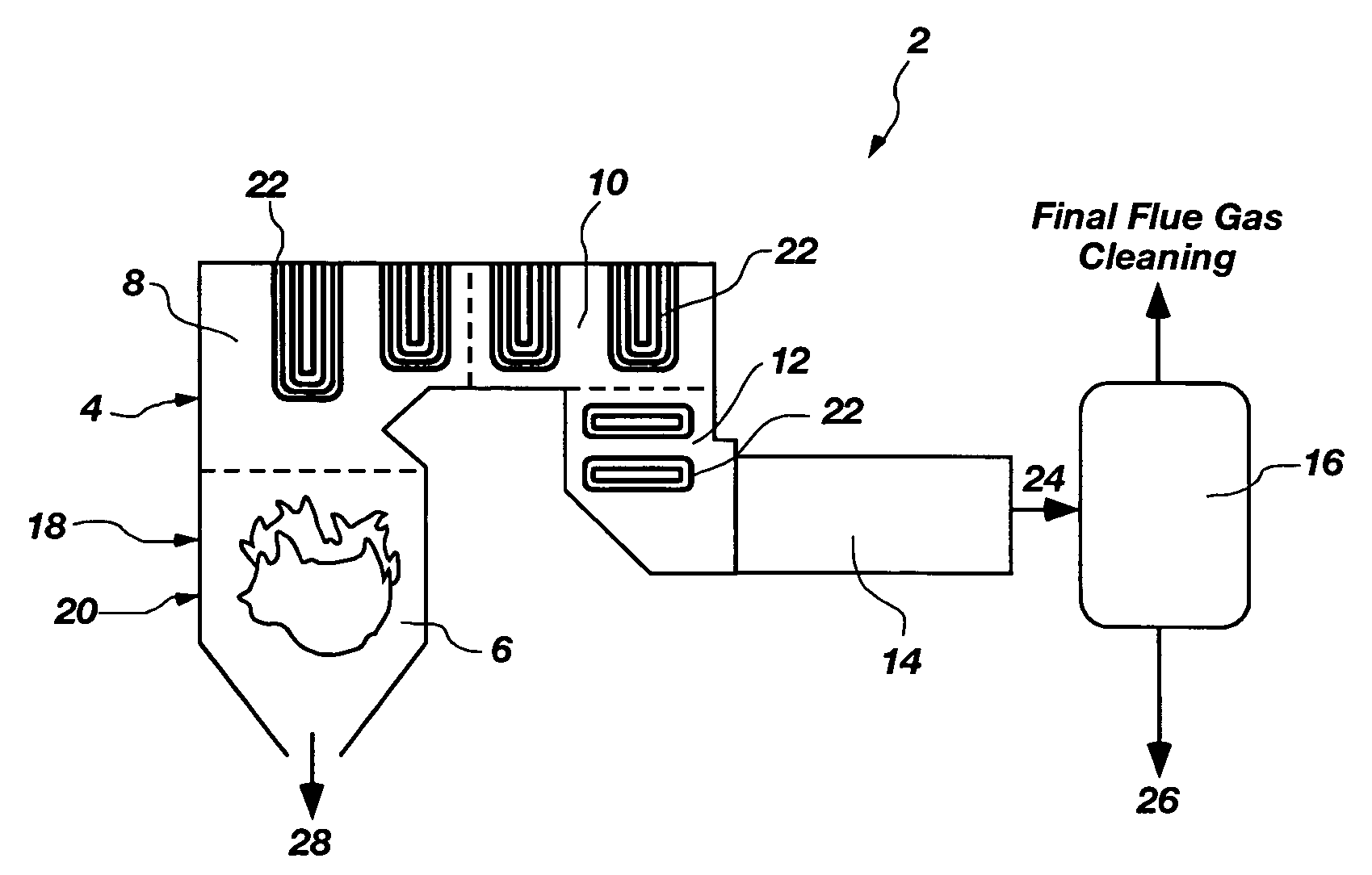
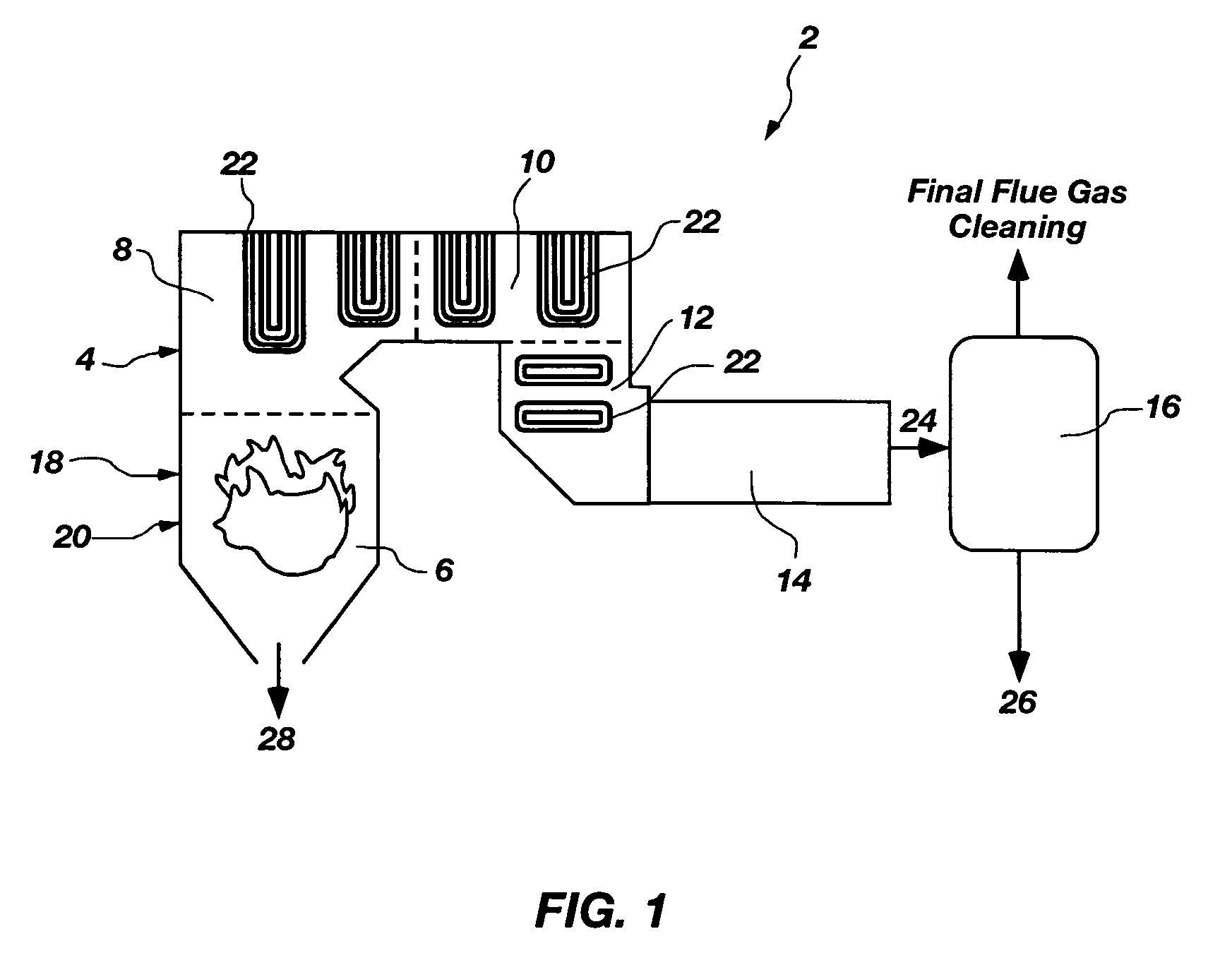
Multi-component removal in flue gas by aqua ammonia
InactiveUS7255842B1Regeneration process is less-costlyIncrease load capacityGas treatmentNitrogen compoundsNitric oxideSlurry
A new method for the removal of environmental compounds from gaseous streams, in particular, flue gas streams. The new method involves first oxidizing some or all of the acid anhydrides contained in the gas stream such as sulfur dioxide (SO2) and nitric oxide (NO) and nitrous oxide (N2O) to sulfur trioxide (SO3) and nitrogen dioxide (NO2). The gas stream is subsequently treated with aqua ammonia or ammonium hydroxide which captures the compounds via chemical absorption through acid-base or neutralization reactions. The products of the reactions can be collected as slurries, dewatered, and dried for use as fertilizers, or once the slurries have been dewatered, used directly as fertilizers. The ammonium hydroxide can be regenerated and recycled for use via thermal decomposition of ammonium bicarbonate, one of the products formed. There are alternative embodiments which entail stoichiometric scrubbing of nitrogen oxides and sulfur oxides with subsequent separate scrubbing of carbon dioxide.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
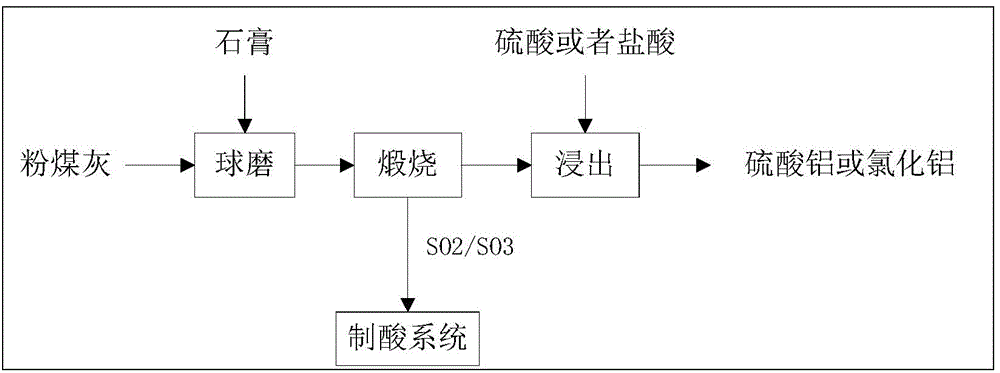
Method for cooperative activation of fly ash and decomposition of gypsum for recovery of sulfur resource
The invention provides a method for cooperative activation of fly ash and decomposition of gypsum for recovery of a sulfur resource. According to the method, solid waste, i.e., fly ash, discharged by a coal-fired power plant or coal-fired boiler is used as a raw material, a certain proportion of desulfurized gypsum discharged by the coal-fired power plant or waste phosphogypsum produced in the phosphorus chemical industry is added and mixed with the fly ash, then the obtained mixture is subjected to ball milling, and activation and calcination at a temperature of 950 to 1450 DEG C are carried out for 5 to 180 min; calcium sulfate in the gypsum are almost totally decomposed after calcination, and produced gas contains sulfur dioxide or sulfur trioxide which can be used as feed gas for preparation of sulfuric acid; and calcination enables solid fly ash to be activated, leaching with a sulfuric acid or hydrochloric acid solution is carried out at a temperature of 50 to 100 DEG C, and the leaching rate of alumina is greater than 80%. The method provided by the invention has the advantages that since all the raw materials are solid waste, the purpose of treating the waste by using the waste is achieved; elemental sulphur in the gypsum can be recovered; and the fly ash can be activated and activity of the fly ash can be improved, so a high alumina recovery rate at a low temperature can be realized. With the method, high-efficiency extraction of alumina in the fly ash is realized; the sulfur resource in the gypsum is recovered; shortage in industrial sulphur in the sulfuric acid industry in China is compensated; and the method has good economic benefits and wide industrial application prospects.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
2-acrylamide-2-methylpropanesulfonic acid and process for producing the same
ActiveUS20100274048A1Large molecular weightHigh purityOrganic compound preparationSulfonic acid preparationSulfur trioxideMethyl group
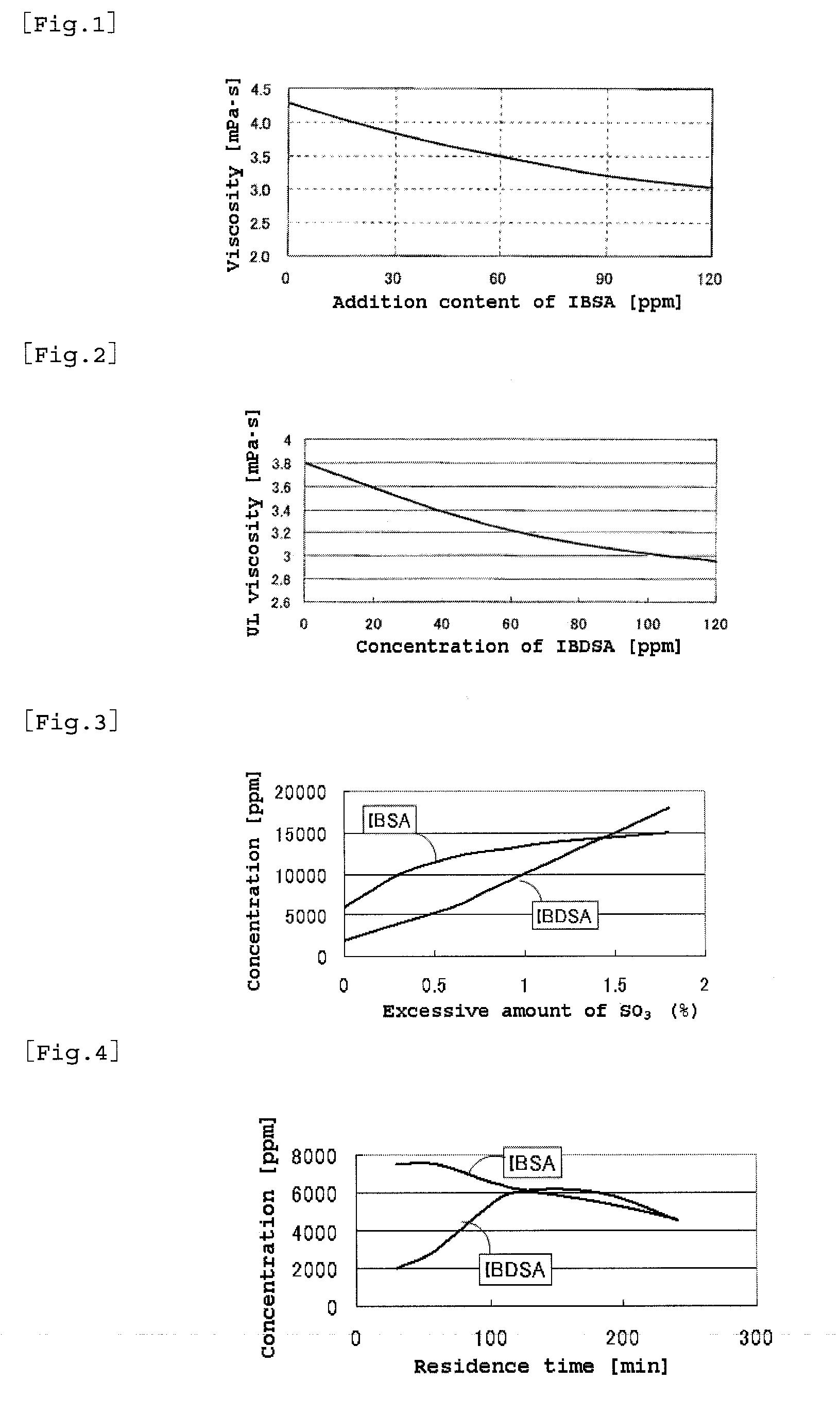
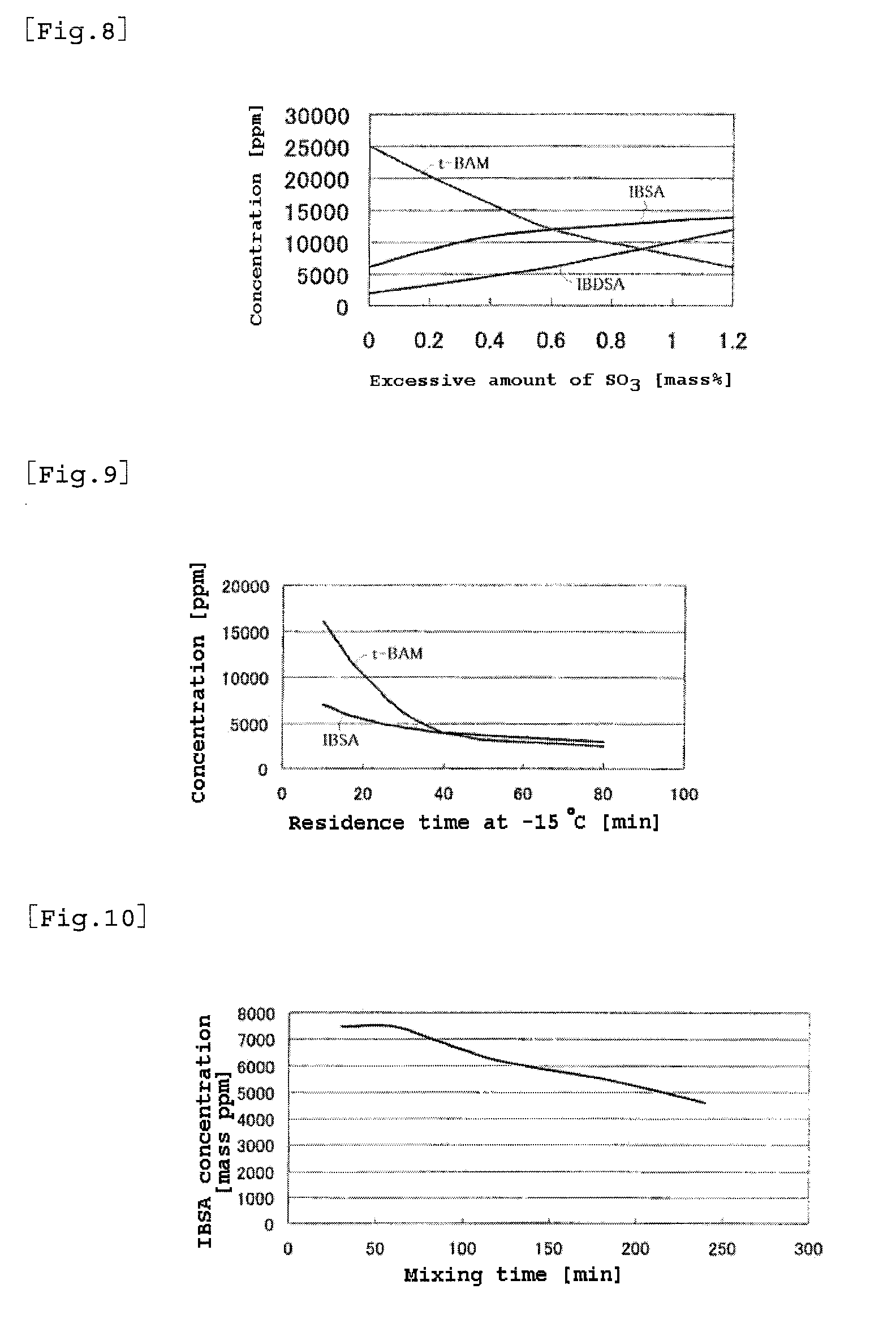
A process for producing 2-acrylamide-2-methyl propane sulfonic acid (ATBS) which comprises reacting acrylonitrile, fuming sulfuric acid, and isobutylene. During the reaction, the concentration of 2-methyl-2-propenyl-1-sulfonic acid (IBSA) and / or that of 2-methylidene-1,3-propylenedisulfonic acid (IBDSA) in the reaction system are determined. When the IBSA concentration exceeds 12,000 mass ppm and / or the IBDSA concentration exceeds 6,000 mass ppm, then the concentration of sulfur trioxide in the reaction system is reduced. Thus, ATBS having an IBSA content of 100 mass ppm or lower and an IBDSA content of 100 mass ppm or lower is produced.
Owner:TOAGOSEI CO LTD
Methods for removing photoresist
InactiveUS20070227556A1Affect strip performancePromote resultsPhotomechanical apparatusCleaning using liquidsLiquid layerOrganic film
In a method for removing an organic film such as photoresist from a wafer, the wafer is placed into a chamber. A liquid including an acid, such as sulfuric acid is applied to the surface of the wafer. Sulfur trioxide is supplied into the chamber. Alternatively, fuming sulfuric acid may be used. The wafer may optionally be spinning in the chamber. The liquid forms a liquid layer on the surface of the workpiece. The chemical reaction of the acid and the SO3 removes the organic film. Further improved results may be achieved in removing many organic films by heating the wafer, or the liquid, or both. Providing ozone gas into the chamber may also be helpful in some applications. In a related method, an organic film is removed from a wafer by applying an acid film or layer on to the surface of the wafer. The acid, is heated. An oxidizer is then delivered to the heated acid film. The combined action of the acid and the oxidizer removes the organic film, typically without leaving any residues. The acid may be sulfuric acid and the oxidizer may be hydrogen peroxide.
Owner:SEMITOOL INC
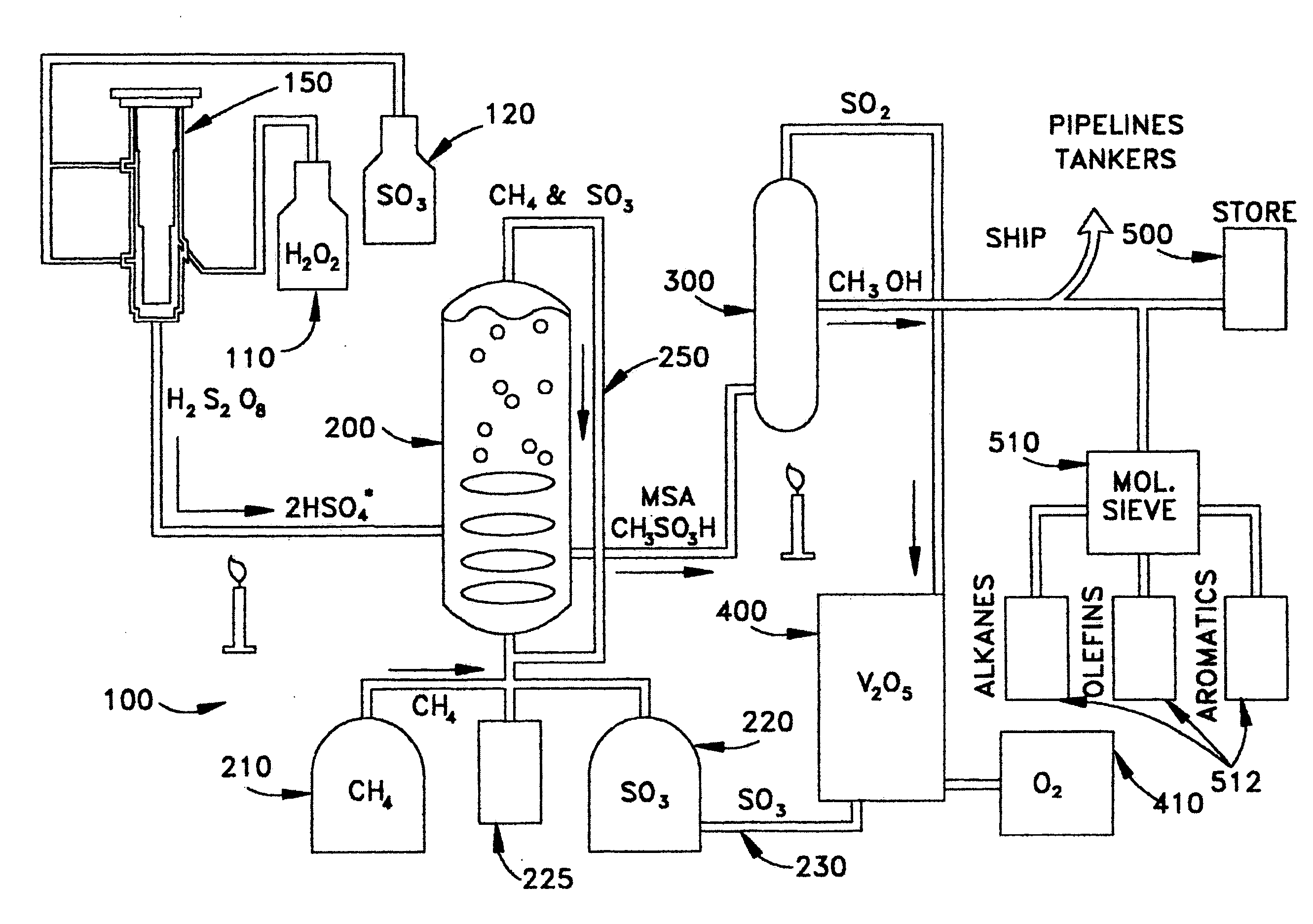
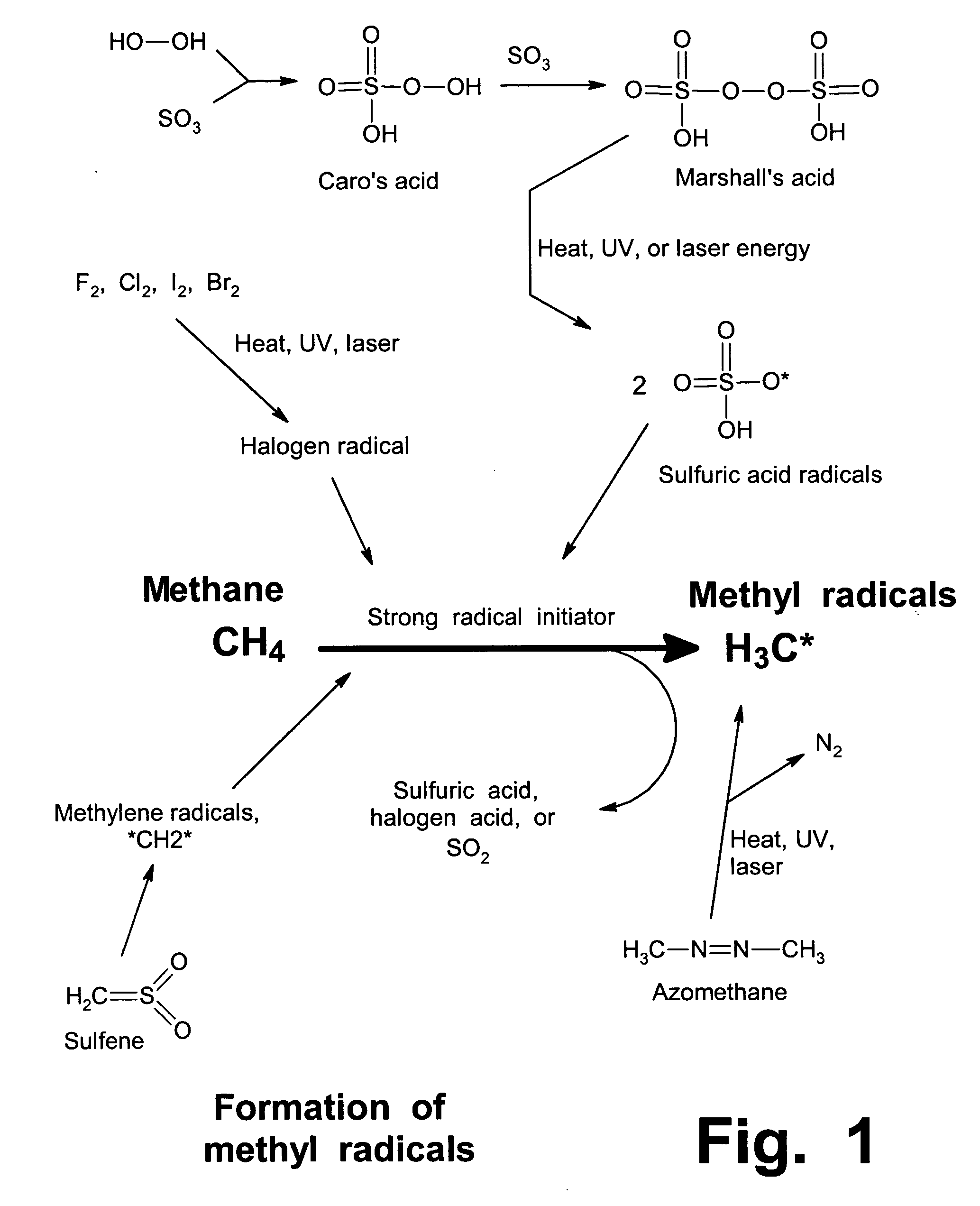
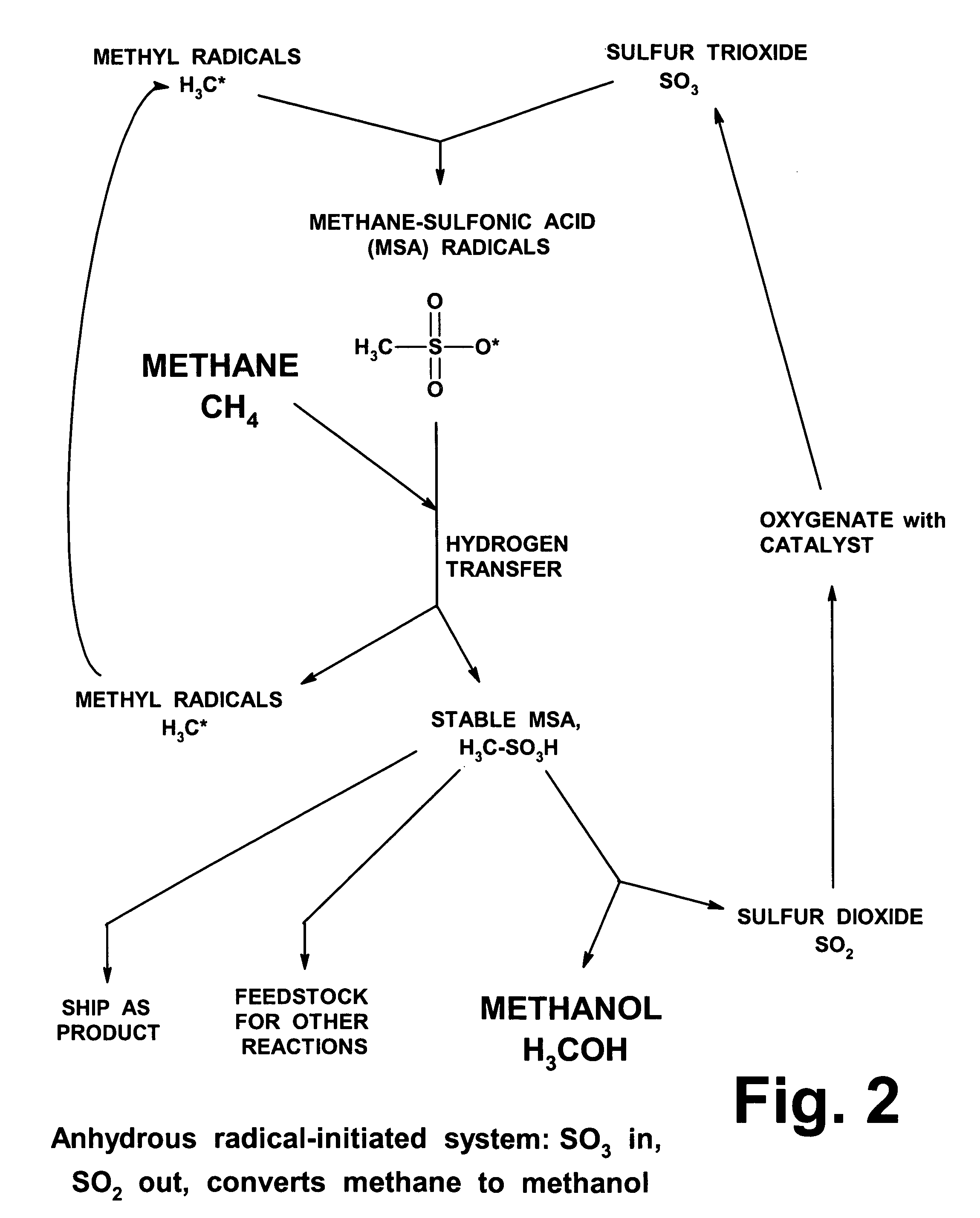
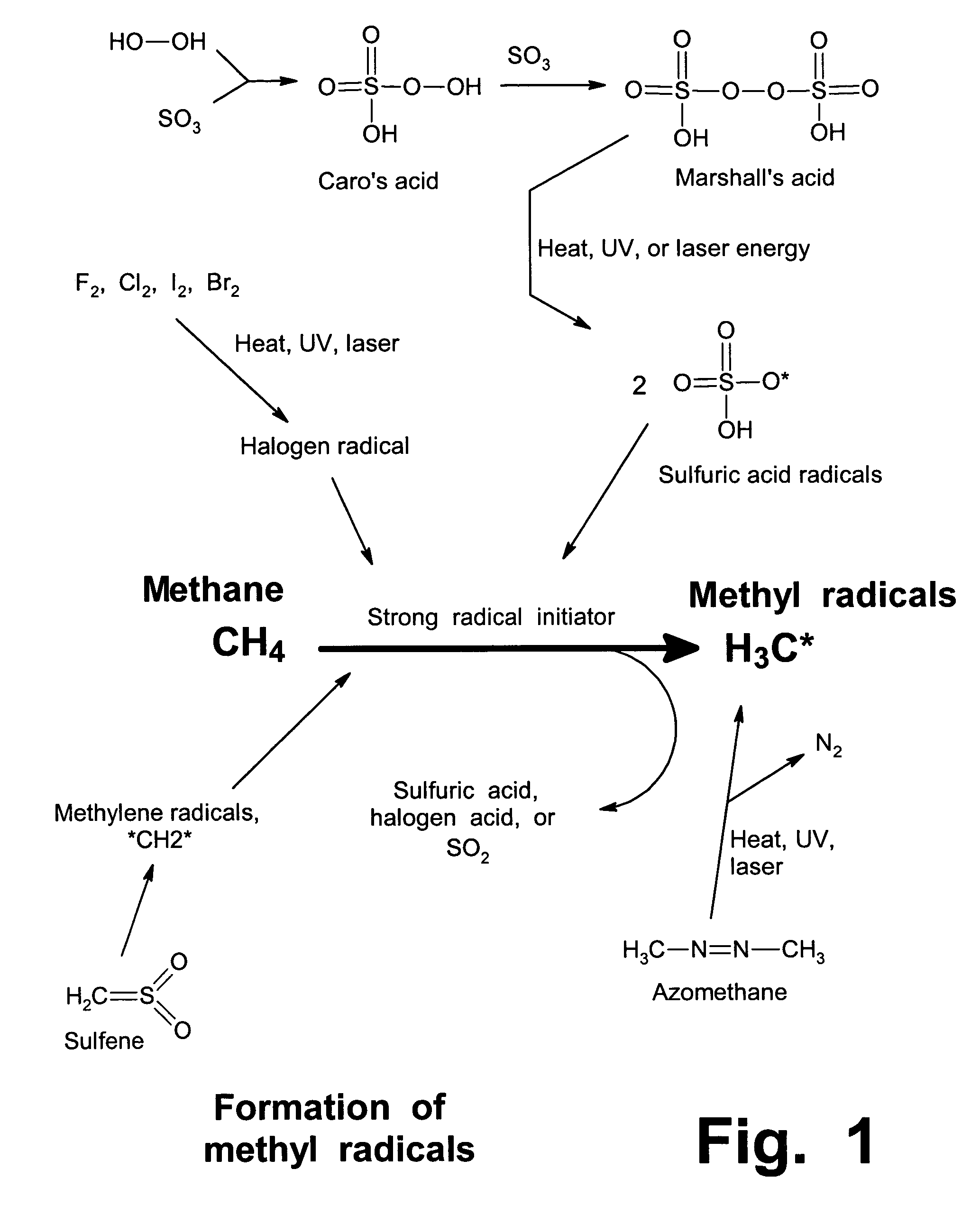
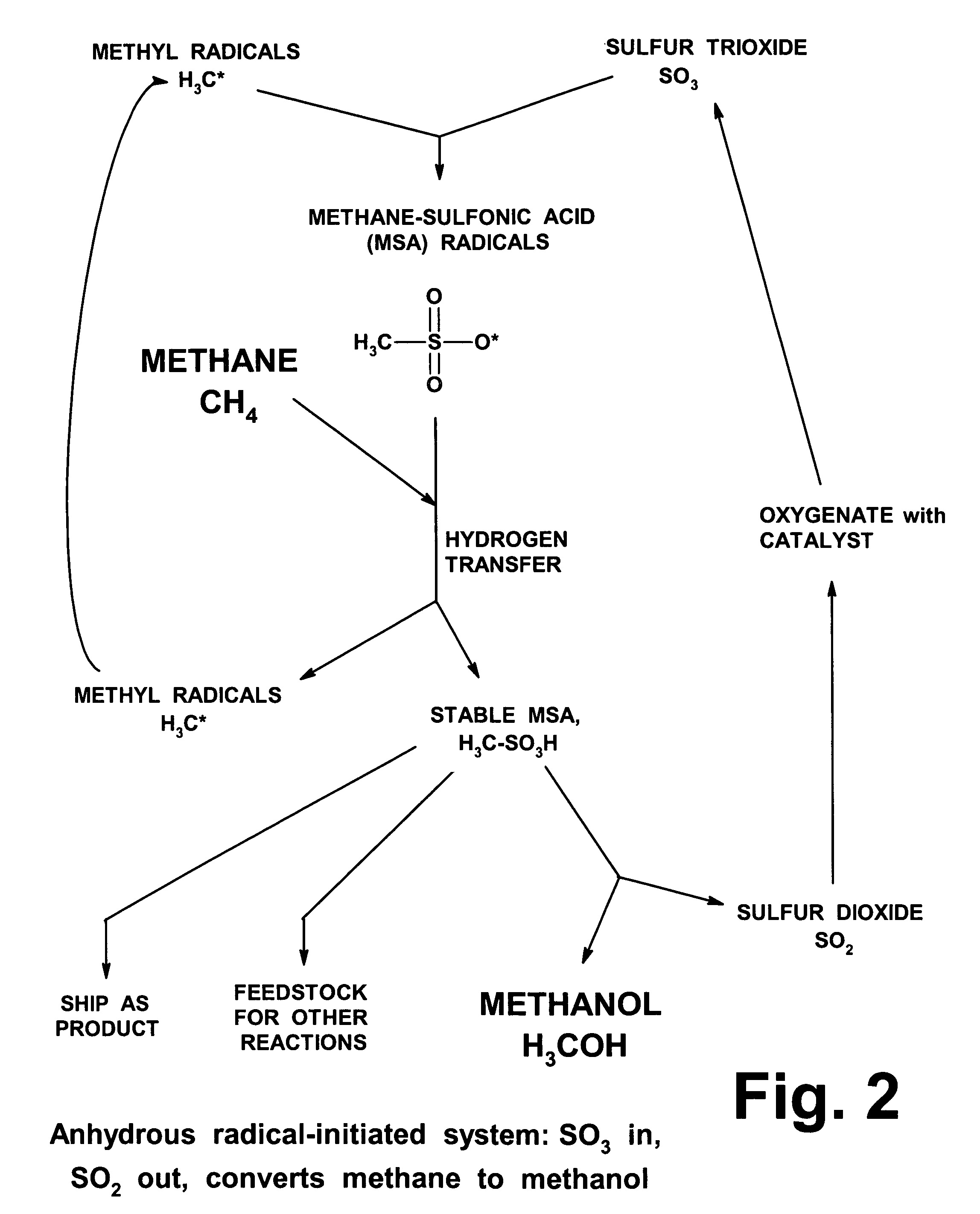
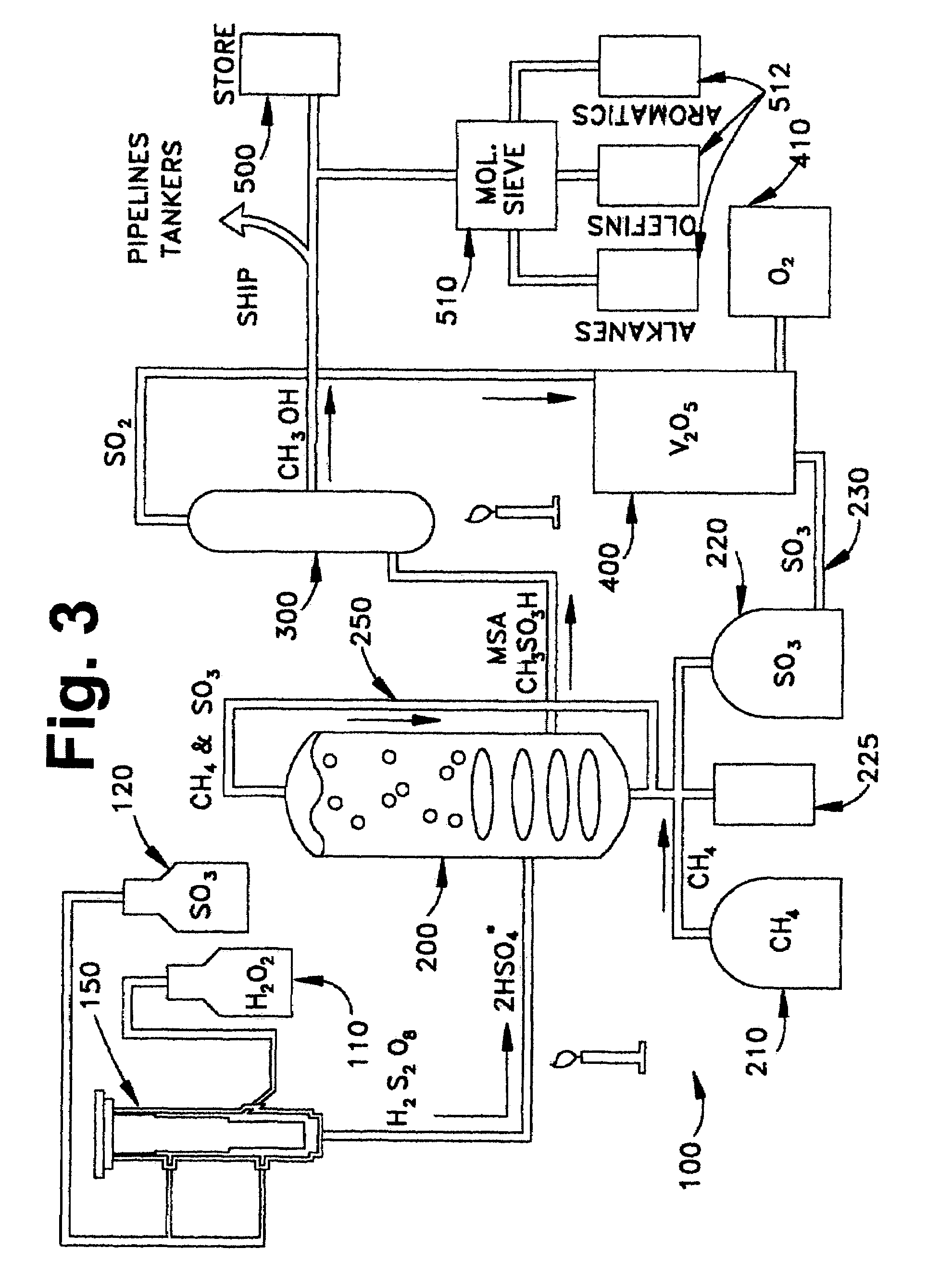
Anhydrous processing of methane into methane-sulfonic acid, methanol, and other compounds
ActiveUS20050070614A1Increases solubility and reaction rateEfficient removalHydrogenOrganic compound preparationLiquid fuelOxygen compound
Anhydrous processing to convert methane into oxygenates (such as methanol), liquid fuels, or olefins uses an initiator to create methyl radicals. These radicals combine with sulfur trioxide to form methyl-sulfonate radicals. These radicals attack fresh methane, forming stable methane-sulfonic acid (MSA) while creating new methyl radicals to sustain a chain reaction. This system avoids the use or creation of water, and liquid MSA is an amphoteric solvent that increasing the solubility and reactivity of methane and SO3. MSA from this process can be sold or used as a valuable chemical with no mercaptan or halogen impurities, or it can be heated and cracked to release methanol (a clean fuel, gasoline additive, and chemical feedstock) and sulfur dioxide (which can be oxidized to SO3 and recycled back into the reactor). MSA also can be converted into gasoline, olefins, or other valuable chemicals.
Owner:VEOLIA NORTH AMERICA REGENERATION SERVICES LLC
Anhydrous processing of methane into methane-sulfonic acid, methanol, and other compounds
ActiveUS7282603B2Increases solubility and reaction rateEfficient removalHydrogenOrganic compound preparationSolubilityLiquid fuel
Anhydrous processing to convert methane into oxygenates (such as methanol), liquid fuels, or olefins uses an initiator to create methyl radicals. These radicals combine with sulfur trioxide to form methyl-sulfonate radicals. These radicals attack fresh methane, forming stable methane-sulfonic acid (MSA) while creating new methyl radicals to sustain a chain reaction. This system avoids the use or creation of water, and liquid MSA is an amphoteric solvent that increasing the solubility and reactivity of methane and SO3. MSA from this process can be sold or used as a valuable chemical with no mercaptan or halogen impurities, or it can be heated and cracked to release methanol (a clean fuel, gasoline additive, and chemical feedstock) and sulfur dioxide (which can be oxidized to SO3 and recycled back into the reactor). MSA also can be converted into gasoline, olefins, or other valuable chemicals.
Owner:VEOLIA NORTH AMERICA REGENERATION SERVICES LLC
Method for ethylene glycol removing SOx (X=2 or 3) in flue gas
ActiveCN101053746AImprove the degree of purificationBroad marketDispersed particle separationMicrowave methodFlue gas
A method shortened form glycol desulfurizing method for desulfurizing Sox (x=2 and / or 3) in the gas using a solution of which the main constituent is glycol which is glycol solution for short. The main constituent of glycol solution in the invention is glycol. The method of glycol desulfurization in the invention, firstly, absorbs SOx (containing: SO2 and / or SO3) in the gas using glycol solution, and then the glycol solution after absorbing Sox is generated by one or several methods in heating method, vacuum method, ultrasonic method, microwave method and radiation method. SO2 and SO3 byproduct is exhausted out, and the glycol solution after regenerated is cyclic utilized.
Owner:PEKING UNIV +2
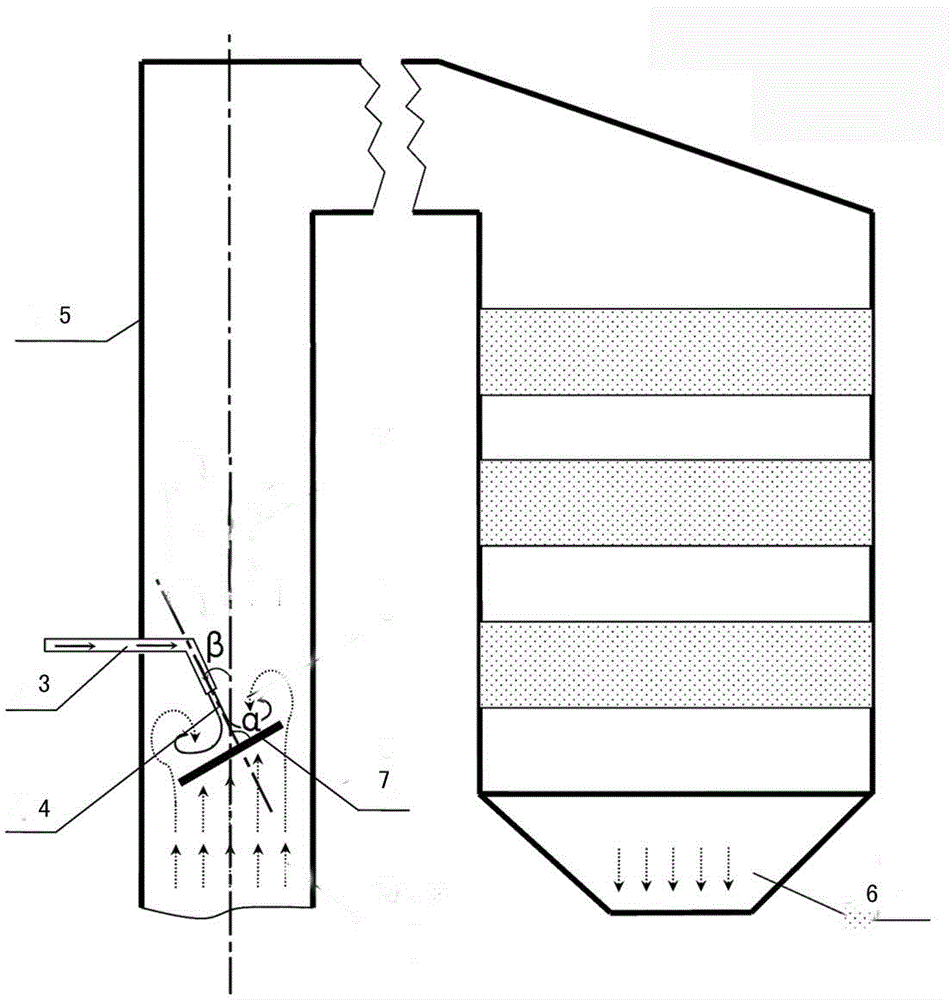
Device and method for removing sulfur trioxide from smoke
InactiveCN104857841AReduce contentReduce the impactDispersed particle separationSulfur trioxideDischarge rate
The invention discloses a device for removing sulfur trioxide from smoke. The device comprises a bin, a screw feeder, a smoke delivery pipe, a spray gun, a denitration inlet flue, a denitration reactor and a spoiler, wherein the screw feeder is provided with a feeding opening and a discharging opening, and the feeding opening is connected with the bin and used for controlling the discharging rate of an absorbent; the smoke delivery pipe is provided with a smoke inlet and a smoke outlet, and the discharging opening is connected with the smoke delivery pipe; the smoke outlet is connected with the spray gun, and the spray gun is used for spraying the absorbent for strong alkaline particles; the denitration inlet flue is used for mixing the absorbent with smoke, and the smoke outlet and the spray gun are positioned in the flue; the denitration reactor is connected with the denitration inlet flue. The device can be applied to a coal firing unit of a thermal power plant, effectively reduce the SO3 content, reduce the influence of the sulfur trioxide on the denitration device and an air pre-heater, and reduce pollutants to realize smoke clean emission.
Owner:BEIJING GUODIAN LONGYUAN ENVIRONMENTAL ENG
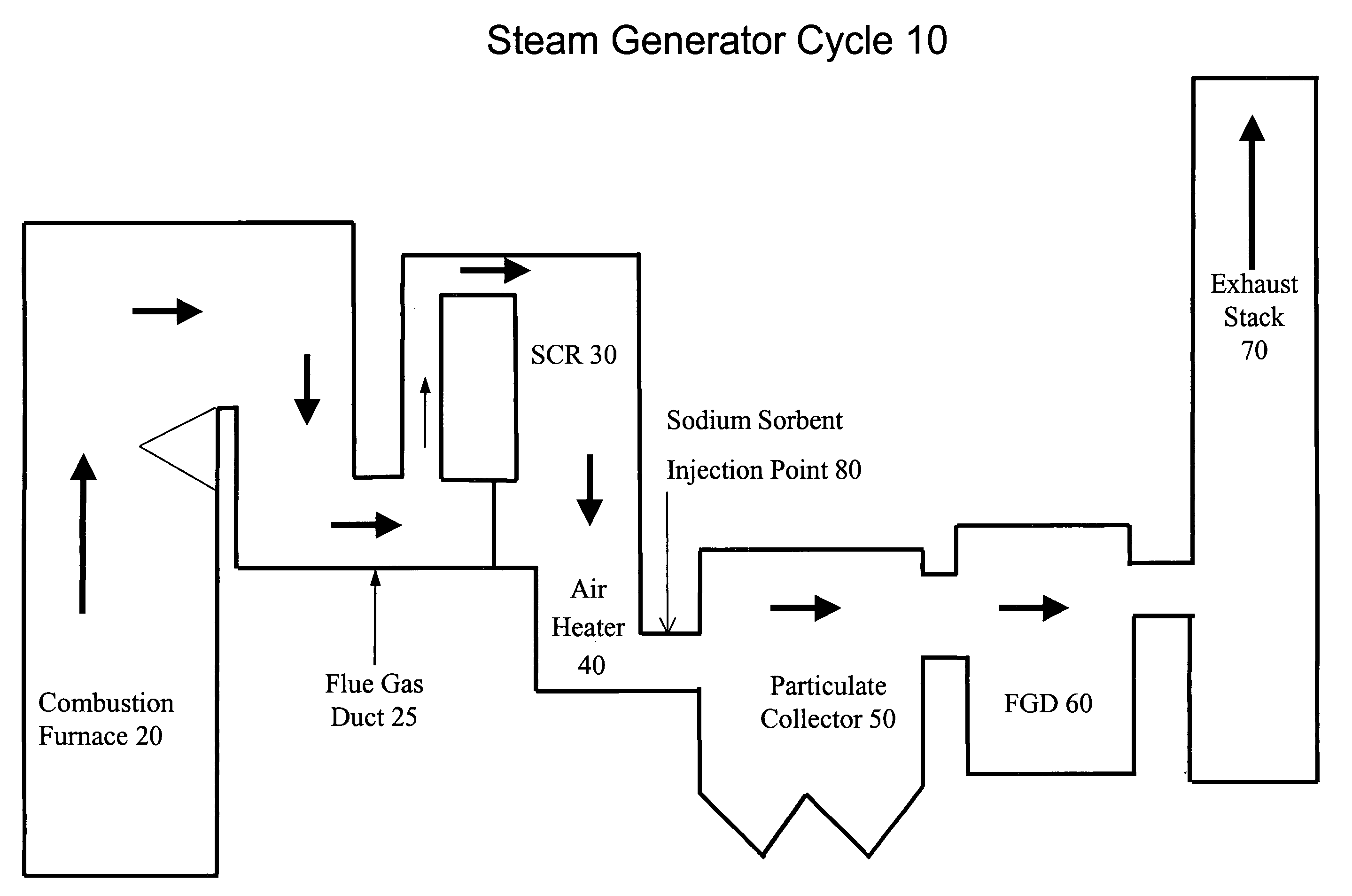
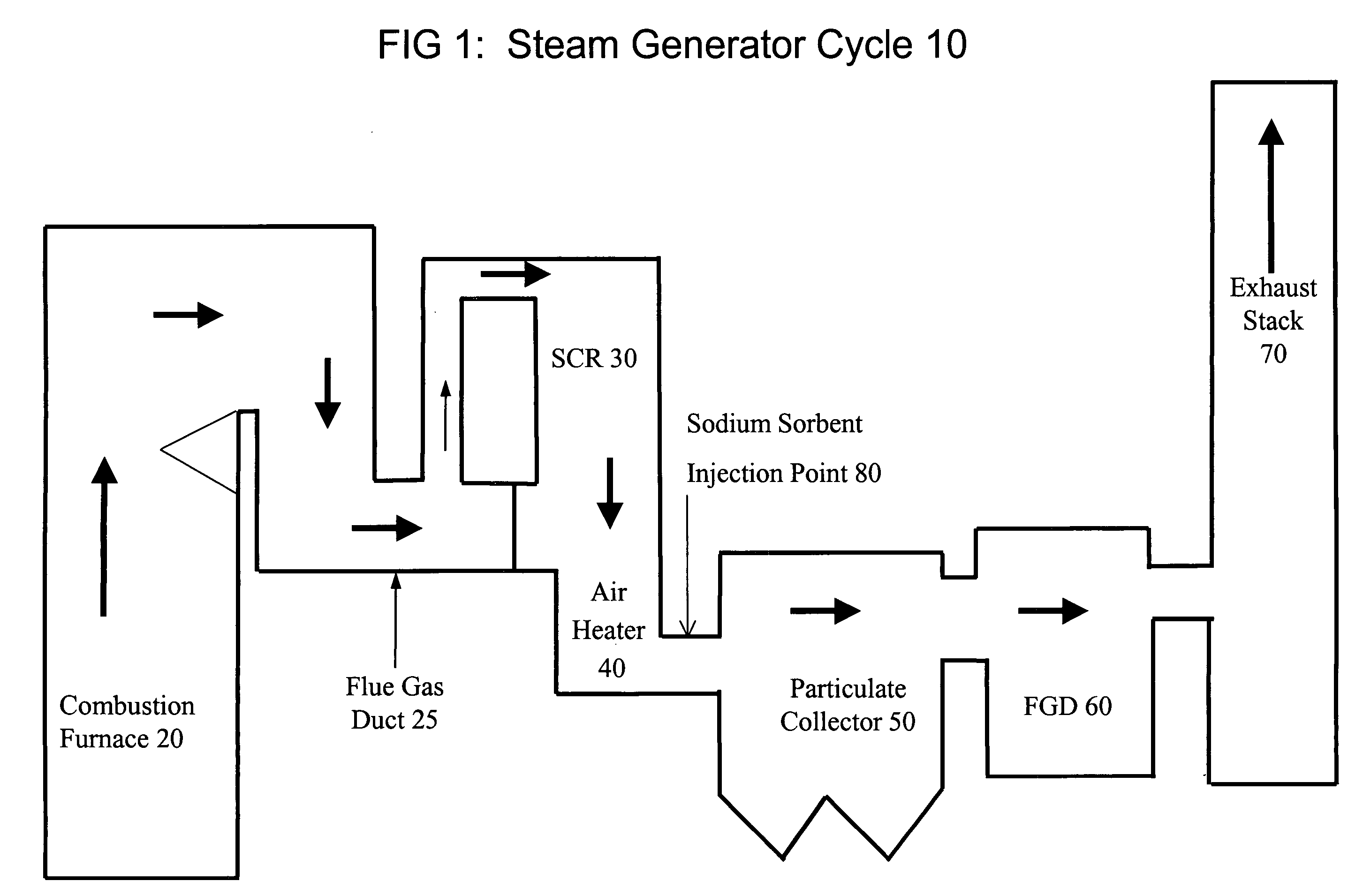
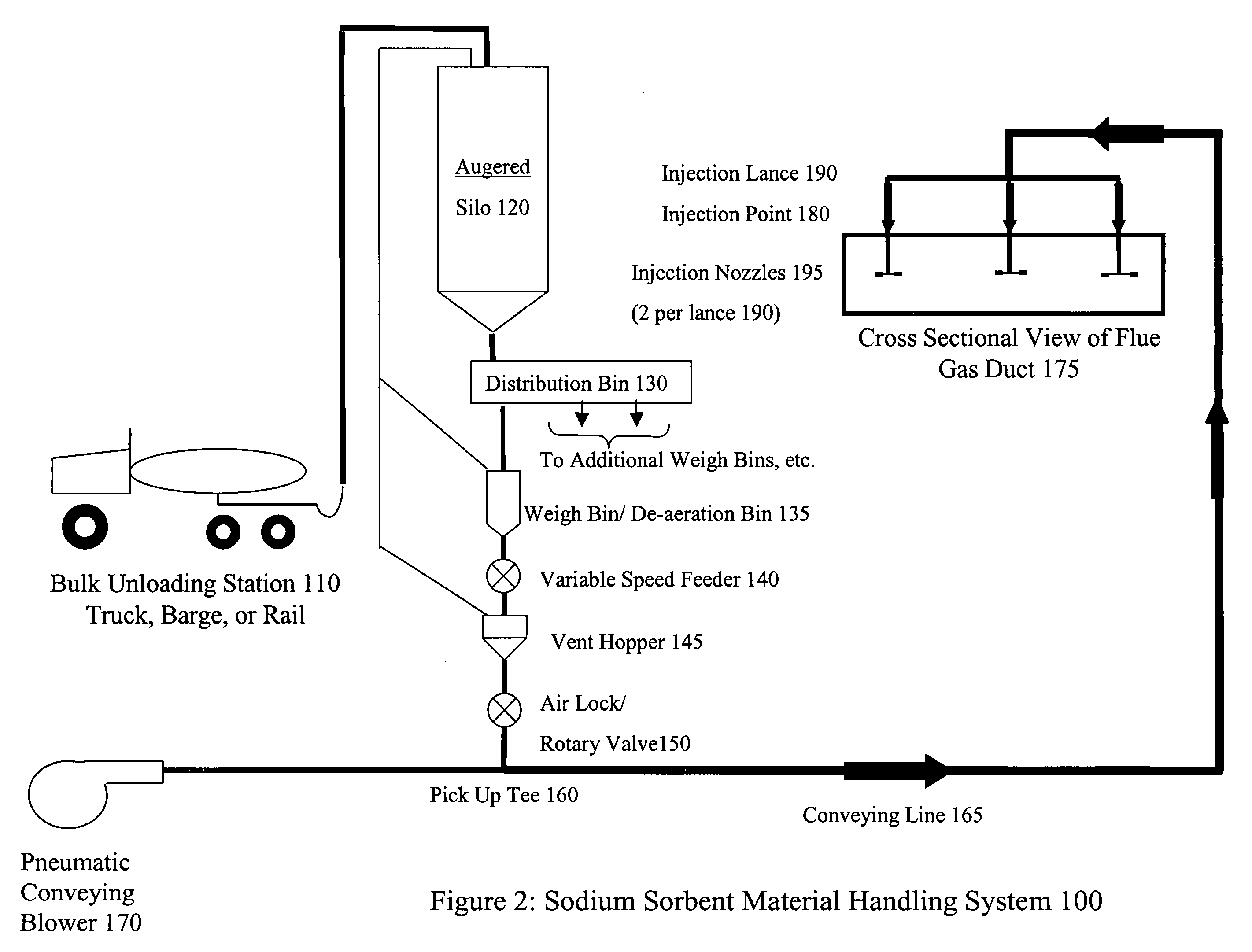
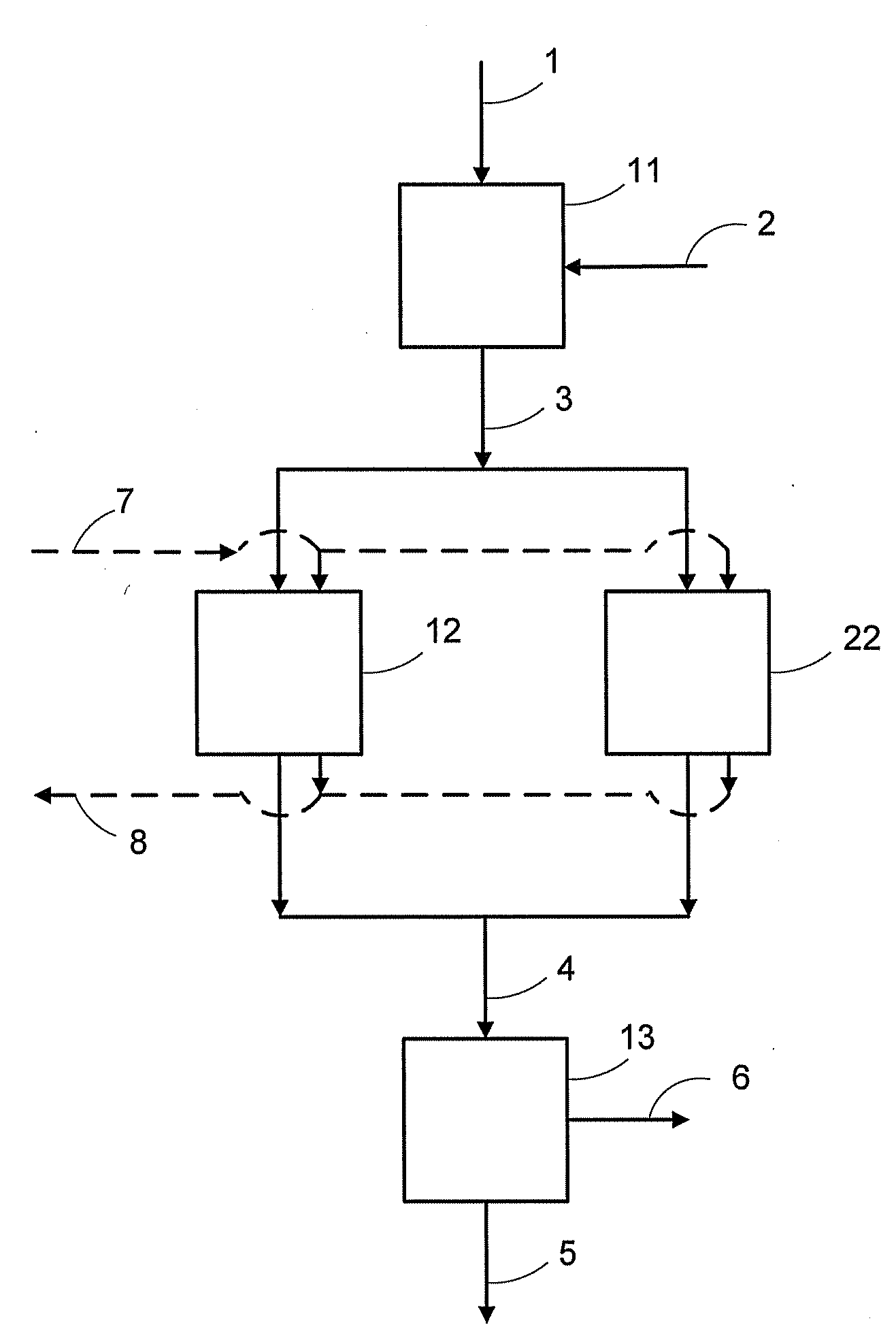
System and method for treating a flue gas stream
PendingUS20050201914A1Lower volume resistivityEfficient removalCombination devicesGas treatmentParticulatesDicarbonate
The present invention is a system and method for treating a flue gas stream to remove strong acid compounds selected from the group consisting of hydrofluoric acid (HF), hydrochloric acid (HCl), sulfuric acid (H2SO4), and sulfur trioxide (SO3) by injecting a sodium sorbent selected from the group consisting of sodium sesquicarbonate, sodium carbonate-bicarbonate, trona ore, mechanically refined trona ore, and trona into the flue gas stream, calcining substantially all of the sodium sorbent in the presence of the flue gas stream to form a soda ash, reducing the concentration of the at least one strong acid compound in the flue gas stream by reacting the at least one strong acid compound with the soda ash to form a sodium based by-product; and changing the chemistry of the flue gas stream to reduce the overall average resistivity of the particulate matter.
Owner:AMERICAN ELECTRIC POWER CO INC
Effective removal of acidic sulfide gas using ammonia-based desulfurization
InactiveUS20150352489A1Efficient removalPromote recoveryGas treatmentDispersed particle separationChemical industryPretreatment method
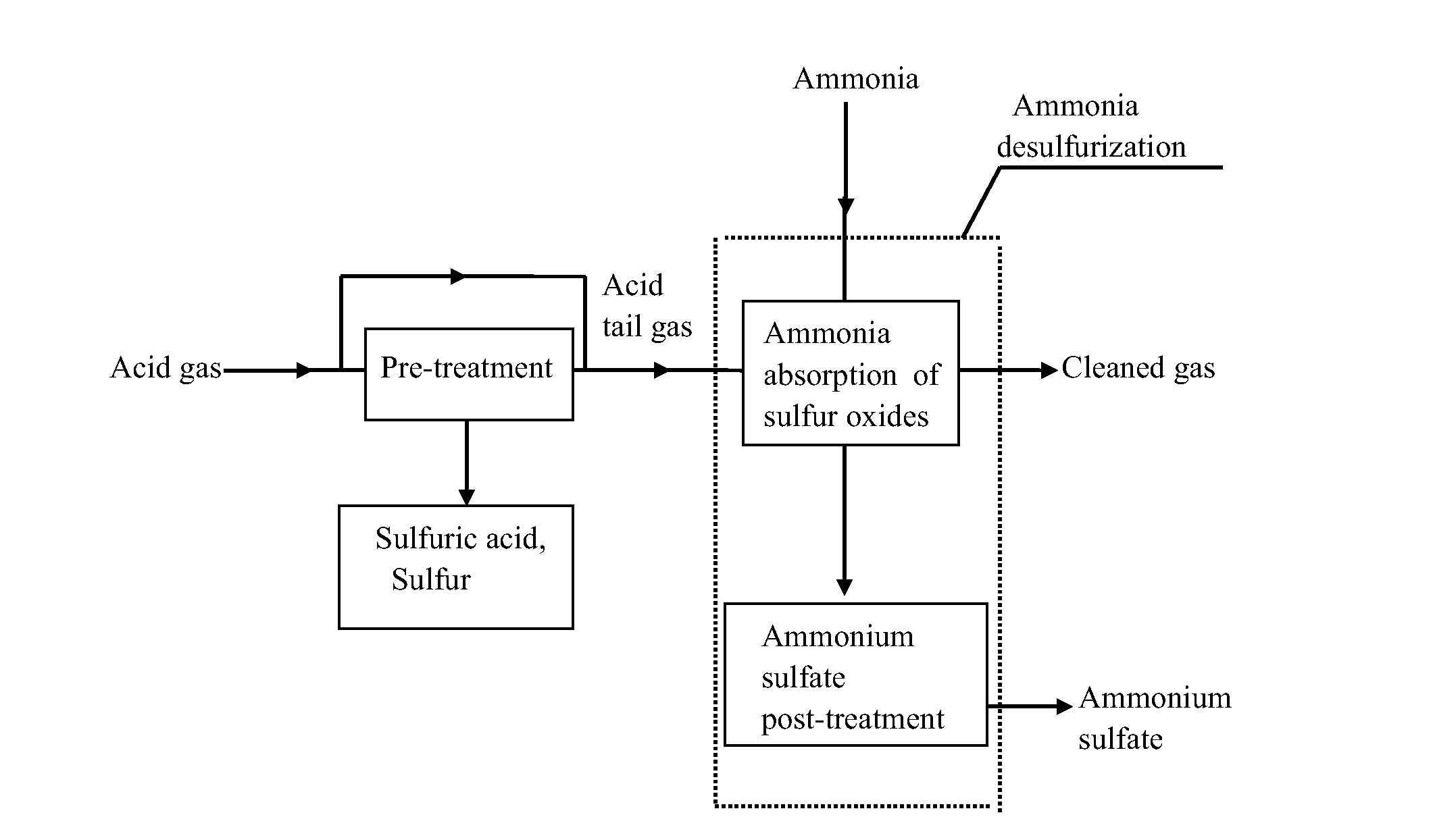
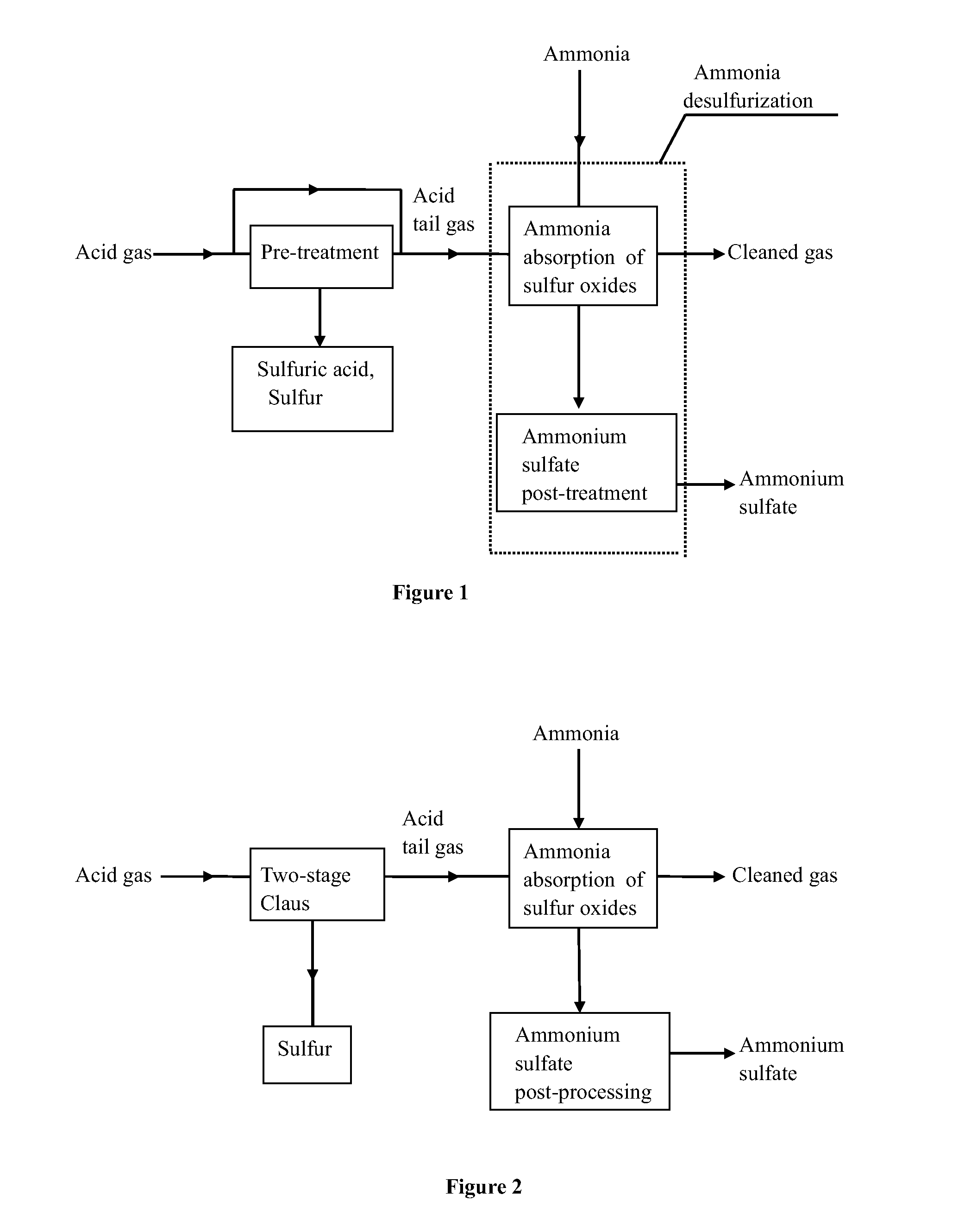
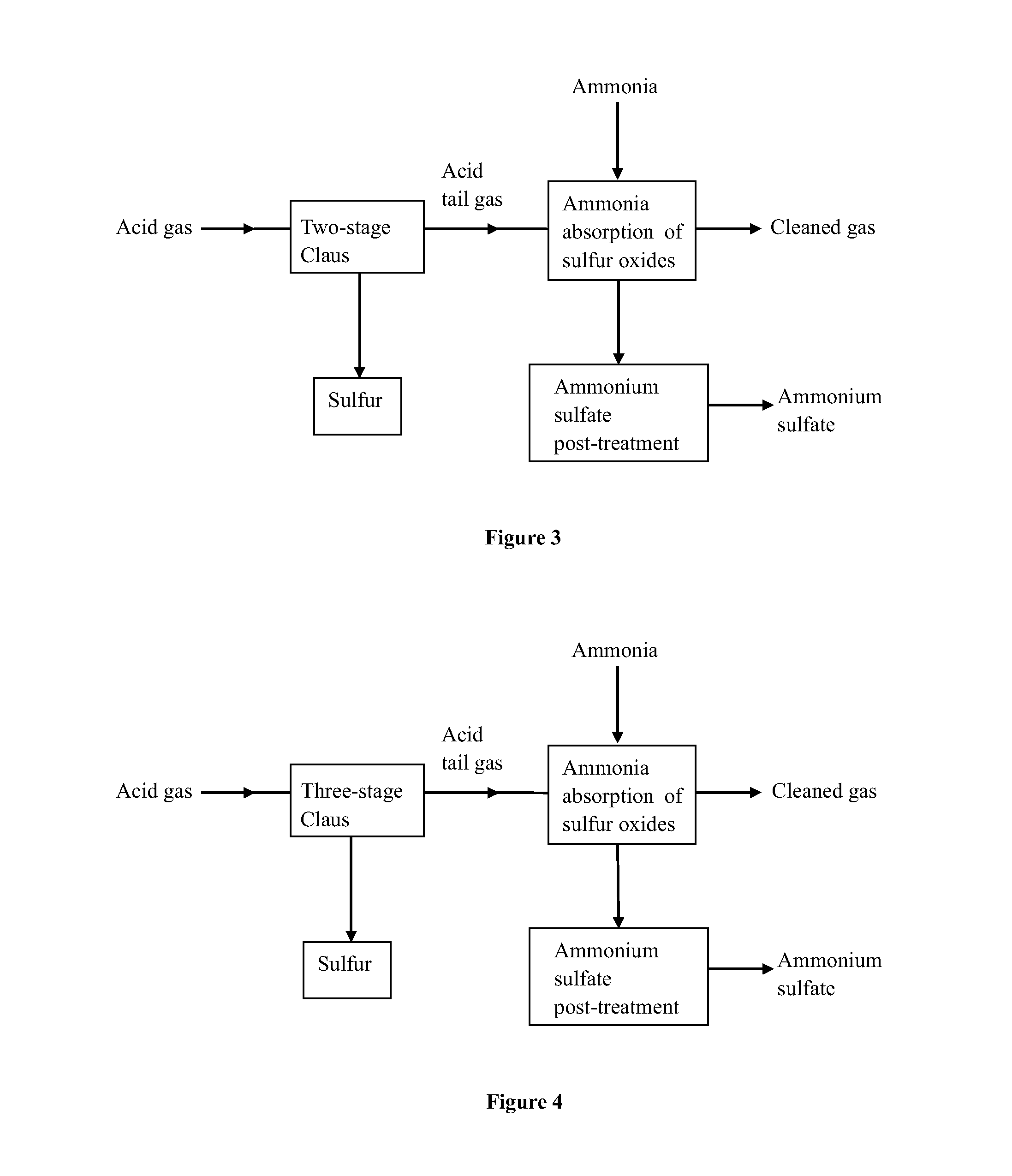
A method for effectively removing acidic sulfide gas using ammonia-based desulfurization includes the following steps of: 1) pre-treatment, wherein sulfide in acid gas undergoes through pre-treatment methods of sulfur recovery, acid making or / and incineration to convert remaining sulfur in the acid gas into sulfur oxides, and the acid tail gas with sulfur oxides is obtained; and the acid gas is derived from petrochemical industry, natural gas chemical industry, coal chemical industry, etc.; 2) ammonia absorption of sulfur oxides, wherein the acid tail gas with sulfur oxides is allowed to flow into an ammonia absorption apparatus, and a cyclic absorption solution is used to absorb sulfur oxides; and 3) post-treatment of ammonium sulfate, wherein a saturated or nearly saturated absorption solution undergoes concentration, crystallization, solid-liquid separation, and drying to obtain a solid product of ammonium sulfate. Sulfur oxides (including sulfur dioxide, sulfur trioxide and hydrates thereof) in the acid tail gas are removed and sulfuric acid, sulfur and ammonium sulfate are byproduced, and the cleaned gas is discharged upon meeting the emission standard.
Owner:JIANGSU NEW CENTURY JIANGNAN ENVIRONMENTAL PROTECTION
Exhaust gas treating method and apparatus
ActiveUS20100071348A1Easy to adjustReduce concentrationCombination devicesNitrogen compoundsSorbentAcid corrosion
After adjusting an exhaust gas temperature at an exit of a heat recovery unit (11) of an exhaust gas treating apparatus to not more than a dew point temperature of sulfur trioxide (SO3), a heavy metal adsorbent is supplied from a heavy metal adsorbent supply unit (16) disposed in an exhaust gas at an entrance of a precipitator (4) or an intermediate position within the precipitator (4), and the exhaust gas containing the heavy metal adsorbent is supplied into the precipitator (4). Preferably at this stage, the heavy metal adsorbent is supplied into the exhaust gas at the entrance of the precipitator (4) 0.1 seconds after the exhaust gas temperature at the exit of the heat recovery unit (11) has been adjusted to not more than the dew point temperature of SO3. Further preferably, in order to prevent acid corrosion of equipment, the heavy metal adsorbent is supplied after spraying an alkali into the exhaust gas at the entrance or exit of the heat recovery unit (11) and adjusting the exhaust gas temperature at the exit of the heat recovery unit to not more than the dew point temperature of SO3. Accordingly, even when coal with a high sulfur content is used as fuel, heavy metals in the exhaust gas can be removed effectively.
Owner:MITSUBISHI POWER LTD
Method for preparing naphthalene sulphonic acid by sulfonating sulfur trioxide in microreactor
InactiveCN101607925ALarge specific surface areaImprove heat transfer performanceChemical/physical/physico-chemical processesSulfonic acid preparationReaction temperatureNitromethane
The invention relates to a method for preparing naphthalene sulphonic acid by sulfonating sulfur trioxide in a microreactor, belonging to methods for preparing dye intermediates in the field of fine chemical engineering. The method comprises the following steps: taking alkyl halide and nitromethane as an organic solvent, naphthalene and derivative thereof as the raw material and organic solution of liquid sulfur trioxide as a sulfonating agent, preparing a solution according to the mol ratio of the organic solvent, the raw material and the sulfonating agent of 19-74:1:1-3 in the microreactor of with channel with the diameter of 10-50 microns, and sulfonating the reaction solution to prepare the naphthalene sulphonic acid by controlling the sulfonation reaction temperature to be between 17DEG C below zero and90 DEG C. The method adopts a continuous flow reactor, solves the problem of impossible transient mixing in the conventional reactor, prevents secondary reaction caused by local excess, and is especially suitable for strong exothermic reaction, fast reaction and flammable and explosive reaction. Compared with the preparation technology in the traditional batch reactor, the invention has the advantages of no generation of waste water and waste acid, clean and environment-friendly technology, consumption of sulphonic acid close to the theoretical quantity, fast reaction speed, low sulfonation temperature, high product yield, good repeatability, high labor productivity and low equipment cost.
Owner:SPECIAL CHEM CO LTD DALIAN FIRSTAR
Acesulfame potassium cyclization continuous production method
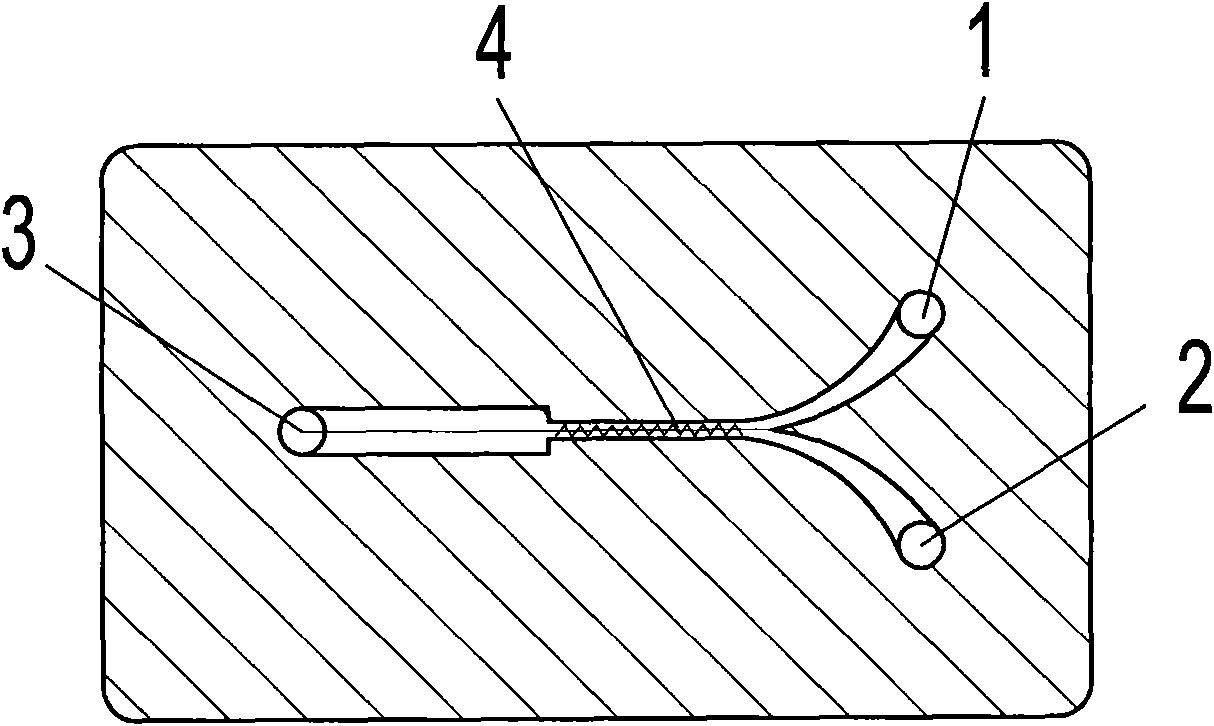
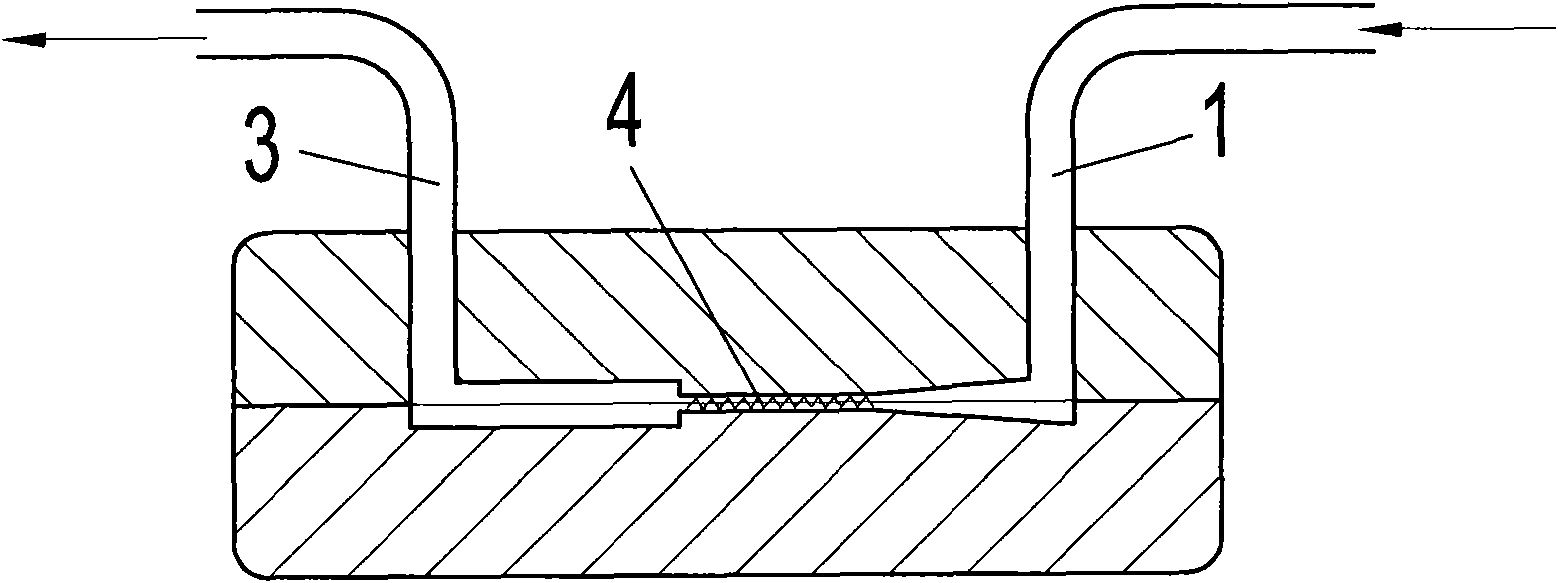
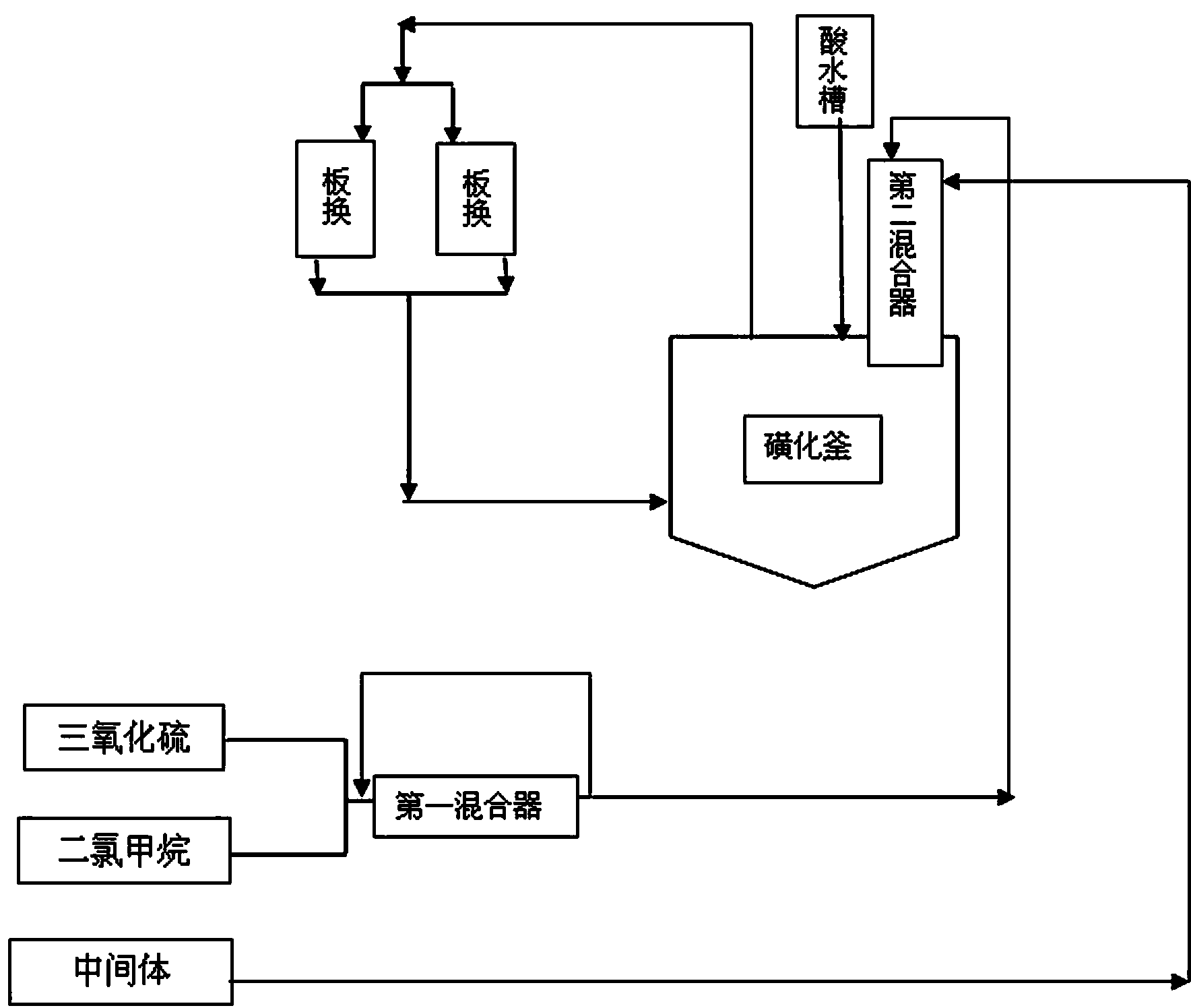
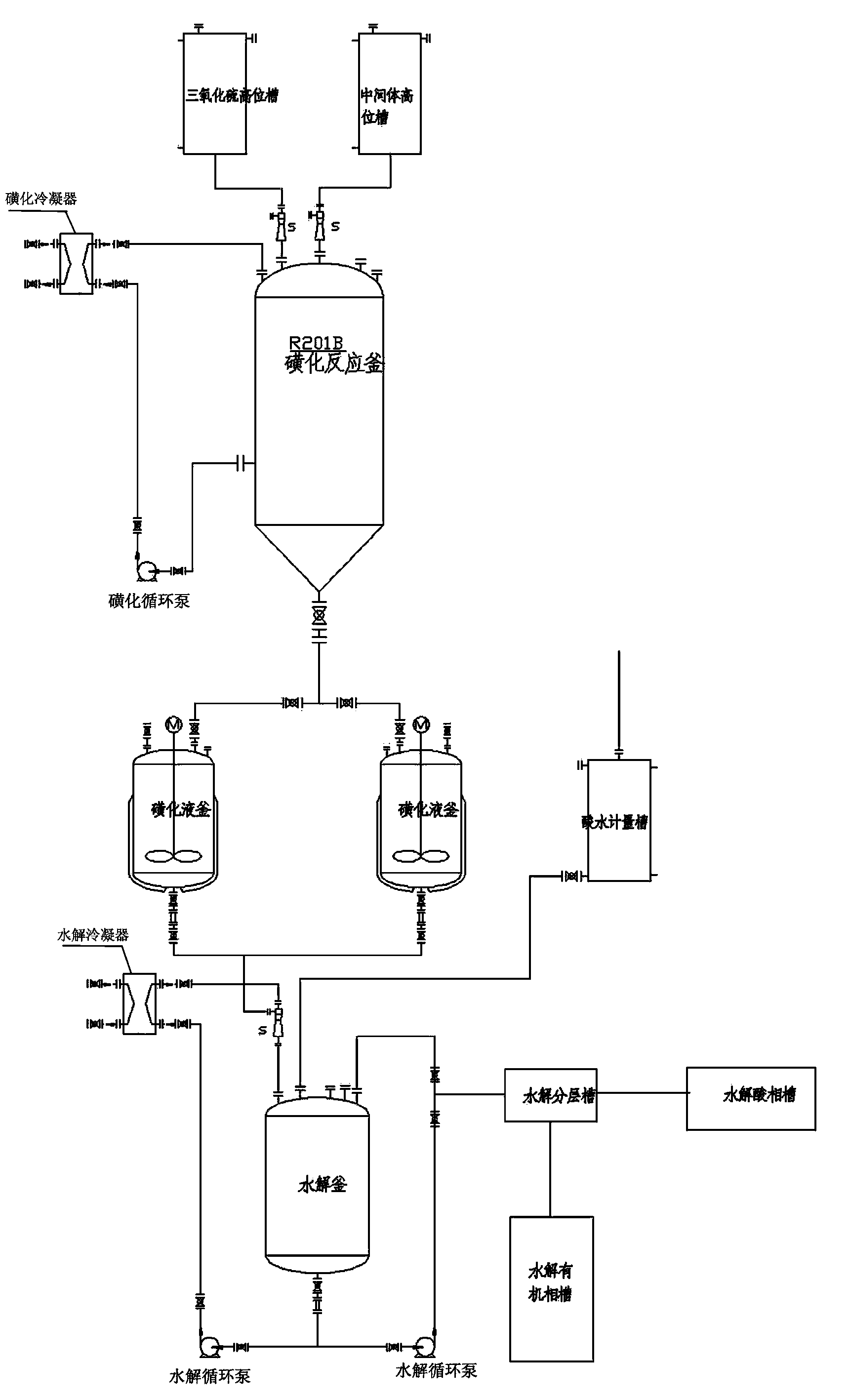
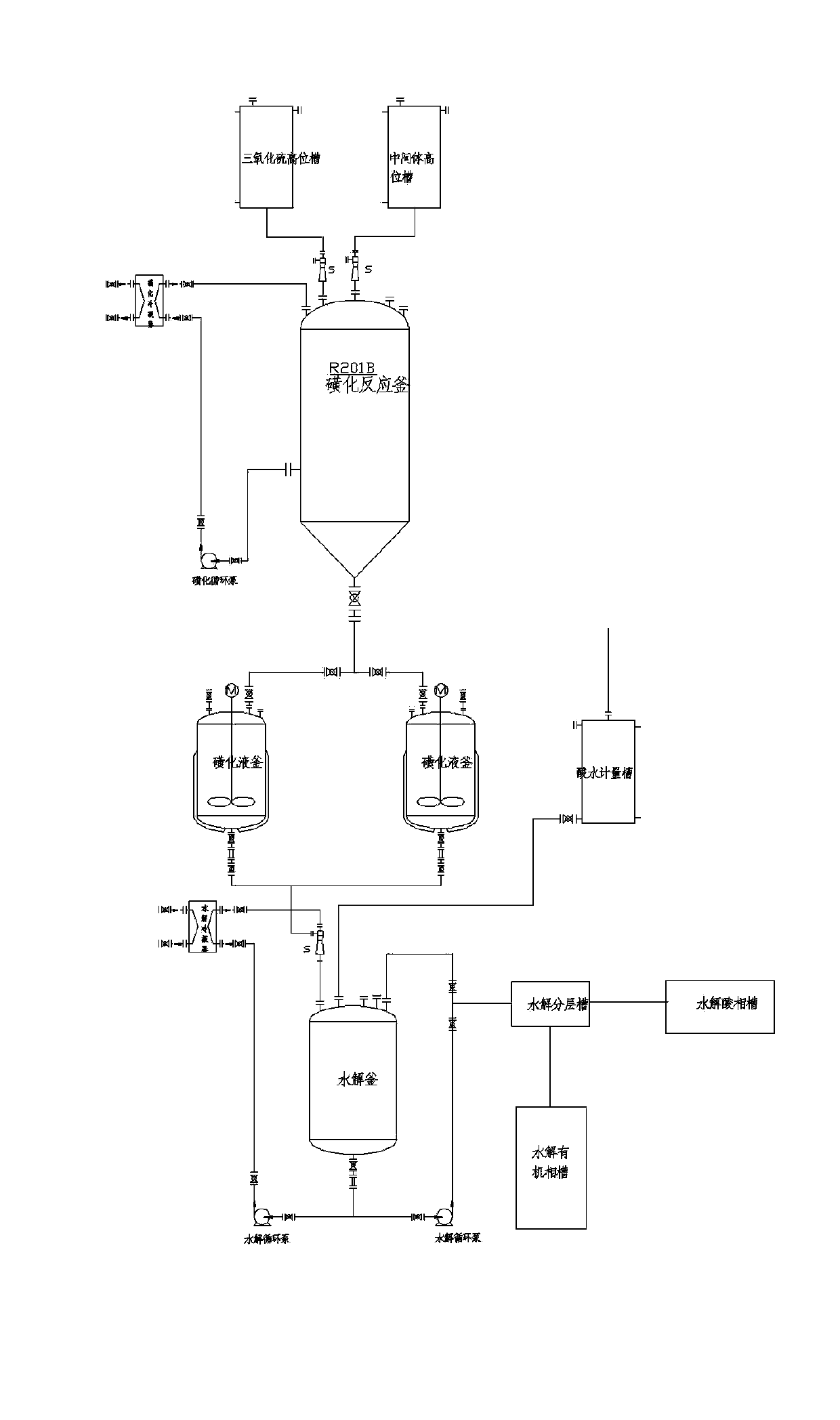
ActiveCN103613566AImprove continuityReduce labor intensityOrganic chemistryReaction temperatureSulfur trioxide
The invention relates to an acesulfame potassium cyclization continuous production method. The method is characterized in that the sulfonation reaction and hydrolysis reaction comprise the following steps: step a, separately pumping an intermediate generated during a synthesis reaction and sulfur trioxide into a sulfonation reactor in a certain speed, carrying out sulfonation reactions in the sulfonation reactor; step b, gasifying dichloromethane when the concentration of the reactants in the sulfonation reactor reach a certain level, spraying the sulfonation liquid into a hydrolysis reactor; step c, dropwise adding acidic water into the hydrolysis reactor to carry out hydrolysis reactions. The method has the advantages that an cyclization one-step reaction technology is adopted, thus the continuity of production operation is realized, and the work strength of workers is reduced; compared to the conventional intermittent production technology, the one-step reaction method has a higher stability, improves the service life of the reactor, shortens the reaction time, and reduces the side reactions. Furthermore, in the method, the sulfonation reaction temperature is raised, then dichloromethane gasification is utilized to reduce the reaction temperature, so that low temperature production is avoided, and a deep cooling ice machine is stopped, so the production efficient is greatly improved, and the energy consumption is largely reduced.
Owner:ANHUI JINGHE IND
Hollow glass microballoons and production method thereof
InactiveCN101638295AGood chemical stabilityIncrease Young's modulusGlass shaping apparatusSilicon dioxideQuenching
The invention relates to hollow glass microballoons and a production method thereof. The hollow glass microballoons are characterized by comprising the following raw materials in portion by weight: 75to 80 portions of silicon dioxide, 7 to 15 portions of boron oxide, 1 to 4 portions of aluminium oxide, 0.5 to 1.5 portions of calcium oxide, 0.8 to 1.2 portions of magnesium oxide, 5 to 6 portions of sodium oxide, 0.0 to 0.3 portion of sulfur trioxide and 0 to 0.05 portion of ferric oxide. The production method of the hollow glass microballoons comprises the following steps: compounding, melting, water quenching, drying, crushing, grading, hollow balling, collection and air separation, and is characterized in that the hollow balling comprises the following steps: ejecting a glass raw material, gas and combustion-supporting gas upwards from the bottom of a balling furnace for combustion, and cooling the glass microballoons upwards by a cooling device after melting under the action of a draft fan. The hollow glass microballoons have the advantages of good surface tension, high compression strength and chemical stability. The production method can better ensure material balling, and hassimple process, less equipment and good effect as much as possible.
Owner:CHINA TRIUMPH INT ENG +1
Single-wall carbon nanotube film having high modulus and conductivity and process for making the same
The invention relates to a film comprising greater than 80 wt % single-wall carbon nanotubes wherein the tensile modulus is at least about 6 GPa at 0.2% strain and the conductivity of the film is at least about 70,000 S / m. The tensile modulus is typically about 8 GPa at 0.2% strain. The method for making the film comprises preparing a solution of single-wall carbon nanotubes in a superacid, such as oleum containing approximately 20 to 30% sulfur trioxide, under a dry, oxygen-free atmosphere. The solution is placed on a surface in a moisture-containing atmosphere, wherein the solution absorbs moisture and acid leaches out. The film is washed to further remove acid, dried, and, optionally, subjected to a heat treatment. Besides free-standing films, coatings of single-wall carbon nanotubes can be made on a variety of surfaces including polymers, glass, metals, and ceramics. The surfaces can be flat planes, fibers or contour shapes.
Owner:GEORGIA TECH RES CORP
Method for oil shale pollutant sorption/NOx reburning multi-pollutant control
A method of decreasing pollutants produced in a combustion process. The method comprises combusting coal in a combustion chamber to produce at least one pollutant selected from the group consisting of a nitrogen-containing pollutant, sulfuric acid, sulfur trioxide, carbonyl sulfide, carbon disulfide, chlorine, hydroiodic acid, iodine, hydrofluoric acid, fluorine, hydrobromic acid, bromine, phosphoric acid, phosphorous pentaoxide, elemental mercury, and mercuric chloride. Oil shale particles are introduced into the combustion chamber and are combusted to produce sorbent particulates and a reductant. The at least one pollutant is contacted with at least one of the sorbent particulates and the reductant to decrease an amount of the at least one pollutant in the combustion chamber. The reductant may chemically reduce the at least one pollutant to a benign species. The sorbent particulates may adsorb or absorb the at least one pollutant. A combustion chamber that produces decreased pollutants in a combustion process is also disclosed.
Owner:BATTELLE ENERGY ALLIANCE LLC
Isomerized alpha olefin sulfonate and method of making the same
The present invention is directed to an isomerized alpha olefin sulfonate and a method of making the same wherein the isomerized alpha olefin sulfonate is derived from sulfonating an isomerized alpha olefin with sulfur trioxide in the presence of air thereby producing an isomerized alpha olefin sulfonic acid, wherein the isomerized alpha olefin is derived from the isomerization of C12-C40 normal alpha olefins; and neutralizing the isomerized alpha olefin sulfonic acid with a source of a mono-valent cation.
Owner:CHEVRON ORONITE CO LLC
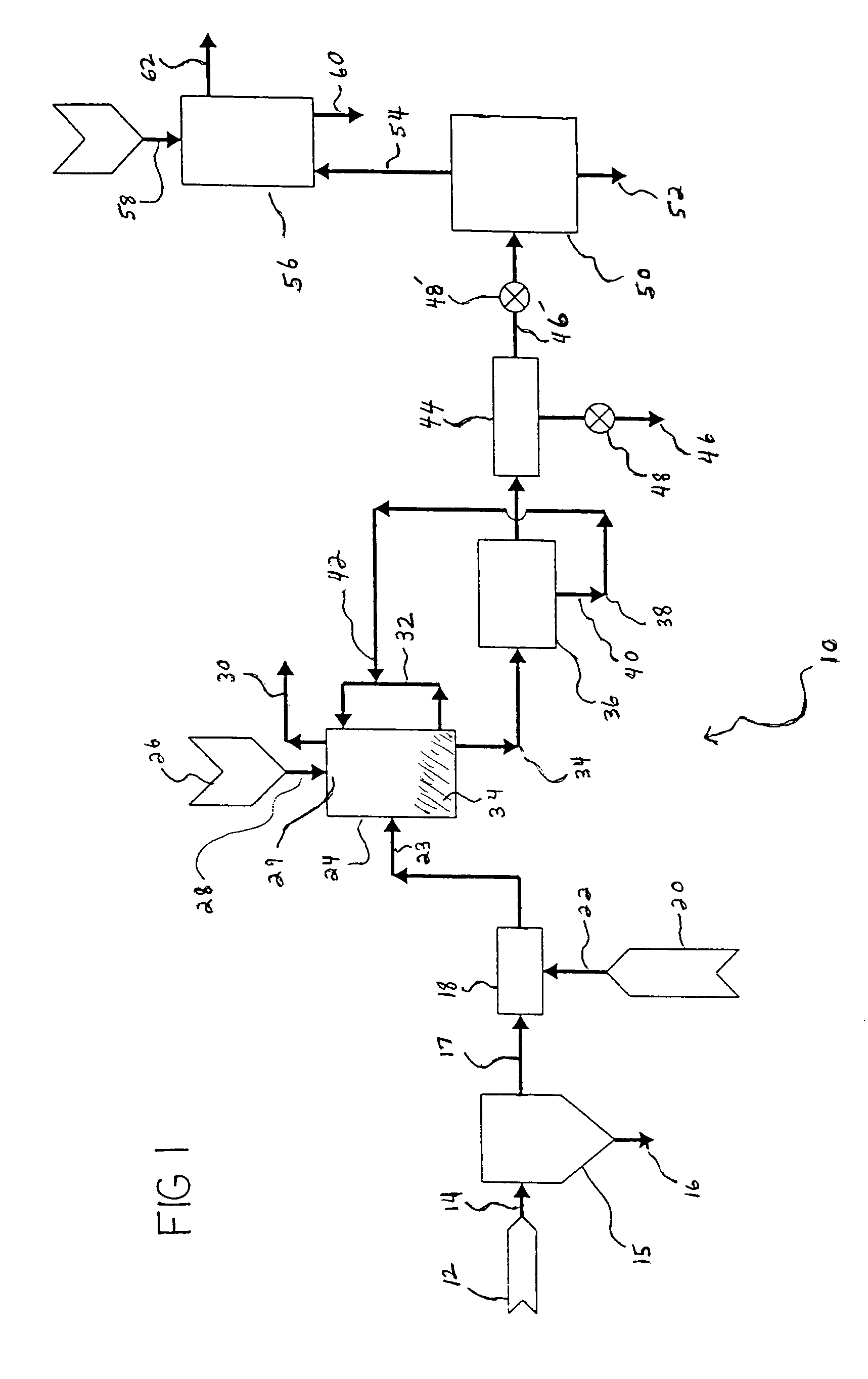
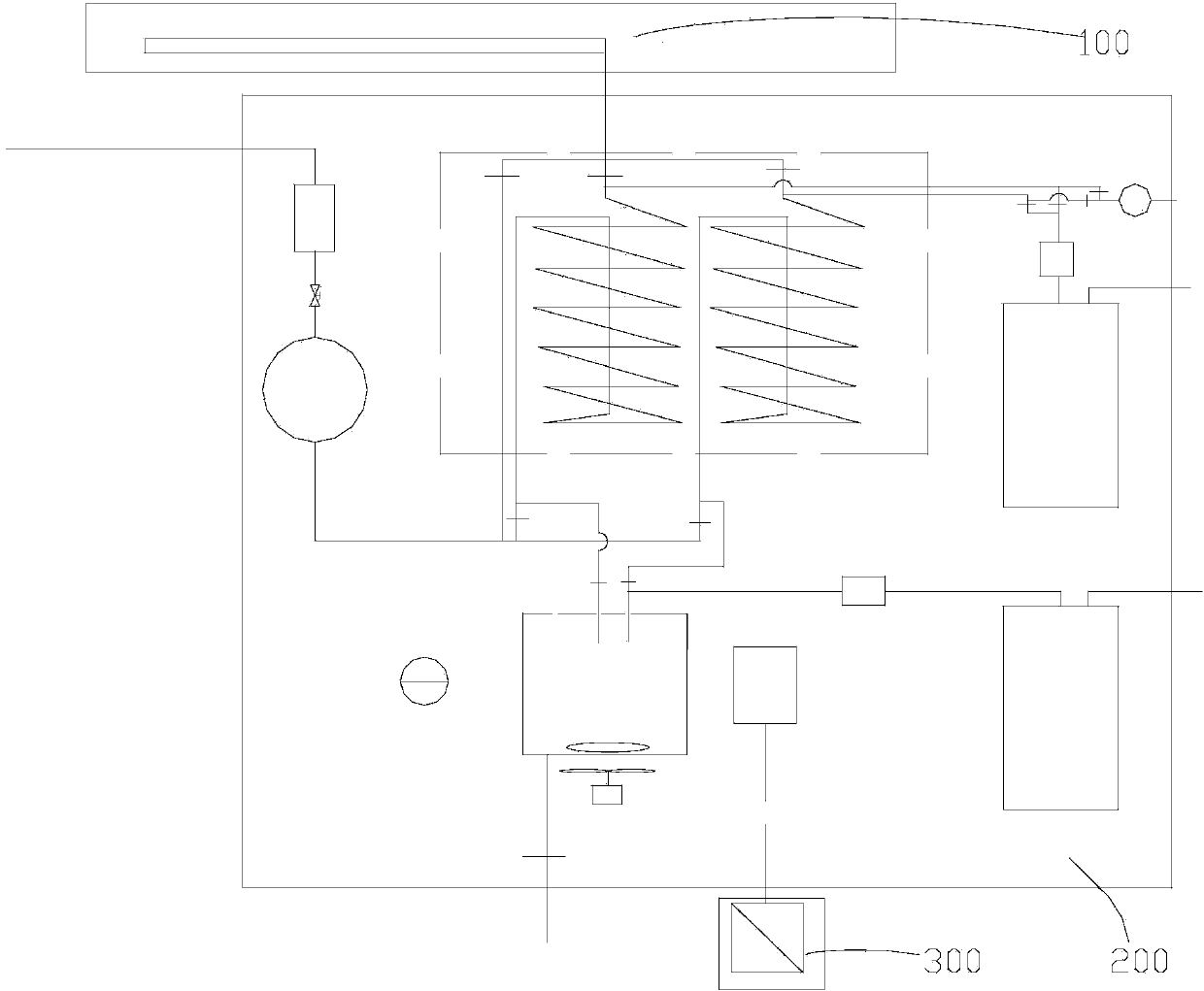
Cluster tool architecture for sulfur trioxide processing
A cluster tool architecture and method are provided for processing substrates by exposure to a process environment, including a reactive gas, such as sulfur trioxide, as well as prior and subsequent treatments thereto. The cluster tool architecture comprises: (a) an atmospheric processing area, maintained at atmospheric pressure or higher; (b) cassette means for introducing a plurality of the substrates into the atmospheric processing area; (c) at least one process station in the atmospheric processing area; (d) an enclosed vacuum processing area, maintained at a vacuum pressure; (e) a first buffer station between the atmospheric processing area and the enclosed vacuum processing area; (f) at least one process station in the enclosed vacuum processing area isolated from the enclosed vacuum processing area by an isolation valve for exposing the substrates to the process environment; (g) a second buffer station between the atmospheric processing area and the enclosed vacuum processing area; (h) an atmospheric transfer arm in the atmospheric processing area for transferring the substrates from the cassette means between one of the buffer stations and at least one process station in the atmospheric processing area and then to the cassette means; and (i) a vacuum transfer arm in the enclosed vacuum processing area for transferring the substrates from one of the buffer stations to one of the vacuum process stations in the enclosed vacuum processing area and from that vacuum process station in the enclosed vacuum processing area to the buffer station, wherein both buffer stations are equally accessible to both the atmospheric transfer arm and the vacuum transfer arm. The cluster tool architecture integrates atmospheric or high pressure processing with vacuum processing. Since integration allows random access, there is a freedom of programming process flow. The architecture allows re-entry of substrates, so that process steps can be repeated at any time, and it allows substrates to be replaced back into original cassette after process is complete.
Owner:BEST LABEL
Process and catalyst for desulfurization of hydrocarbonaceous oil stream
ActiveUS20090148374A1Organic-compounds/hydrides/coordination-complexes catalystsRefining with oxygen compoundsSolid carbonNitrogen dioxide
The invention relates to a process and catalyst for the oxidative desulfurization of hydrocarbonaceous oil. In one aspect, solid carbon materials are provided having stable sulfur trioxide and nitrogen dioxide oxidative species on the surface thereof. Such materials are useful in the production of low sulfur hydrocarbon feedstocks and in the removal of refractory sulfur compounds.
Owner:SAUDI ARABIAN OIL CO
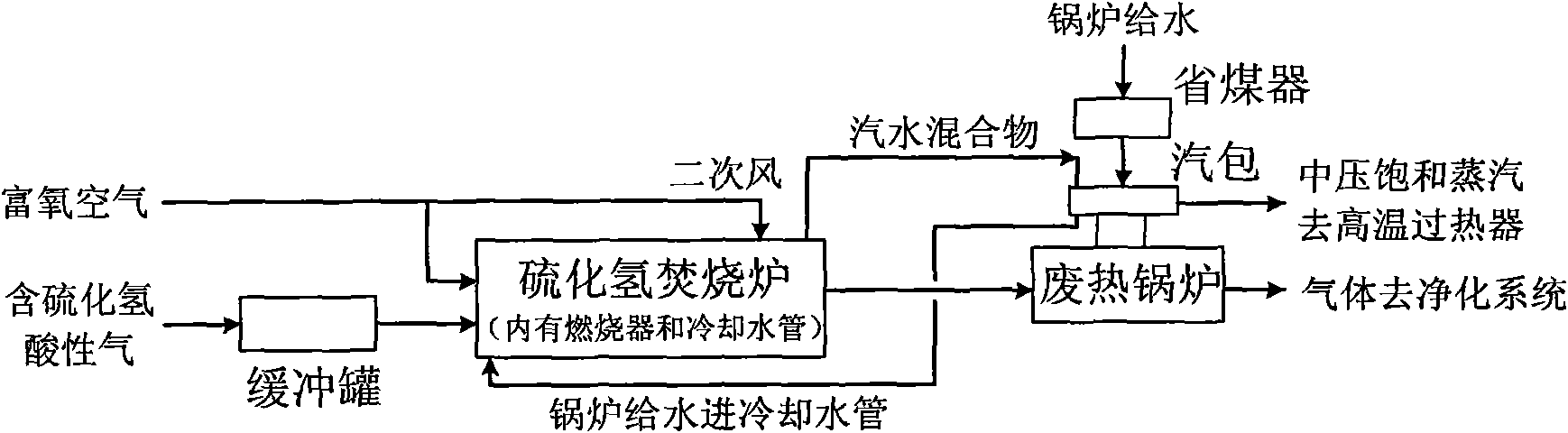
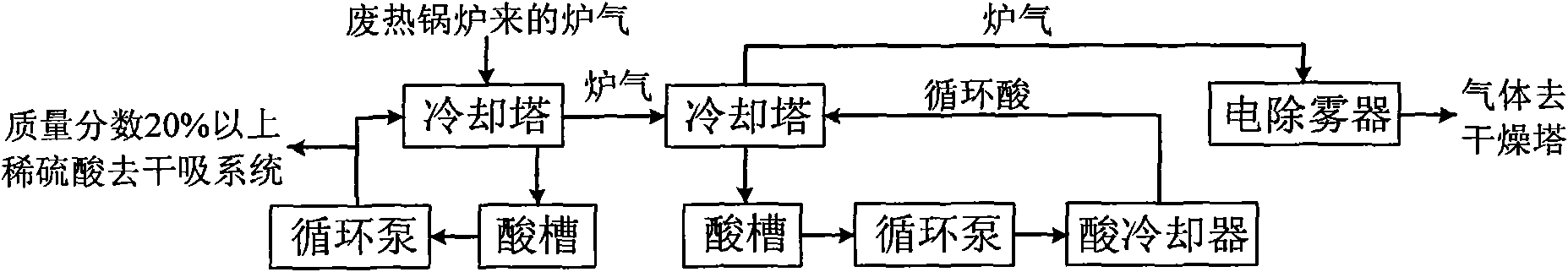
Method for producing sulfuric acid by oxygen-enriched air incineration of acid gas containing hydrogen sulfide
InactiveCN102371108AIncinerate completelyImprove waste heat recovery efficiencyDispersed particle separationSulfur compoundsThermal energyPollution
A method for producing sulfuric acid by oxygen-enriched air incineration of acid gas containing hydrogen sulfide belongs to the technical field of environment protection technology and comprises the following steps of: sending the acid gas containing hydrogen sulfide and oxygen-enriched air into a hydrogen sulfide incinerator for incineration, discharging the furnace gas after incineration, and recovering high-temperature furnace gas heat energy of an outlet; adding water into the furnace, and heating by the utilization of heat released from incineration; using dilute sulfuric acid discharged from a purification system as addendum water; demisting and drying the cooled furnace gas, entering into a conversion system for heat exchange preheating so as to generate sulfur trioxide, carrying out heat exchange on the discharged conversion gas, and entering into an absorbing tower for absorption. According to the invention, high and medium grade heat energy generated during the sulfuric acid production process is fully utilized, and dilute sulfuric acid is fully recovered and used, thus realizing less investment, low cost, high recovery rate of waste heat, clean production and no pollution in the production of sulfuric acid.
Owner:CHINA PETROCHEMICAL CORP +1
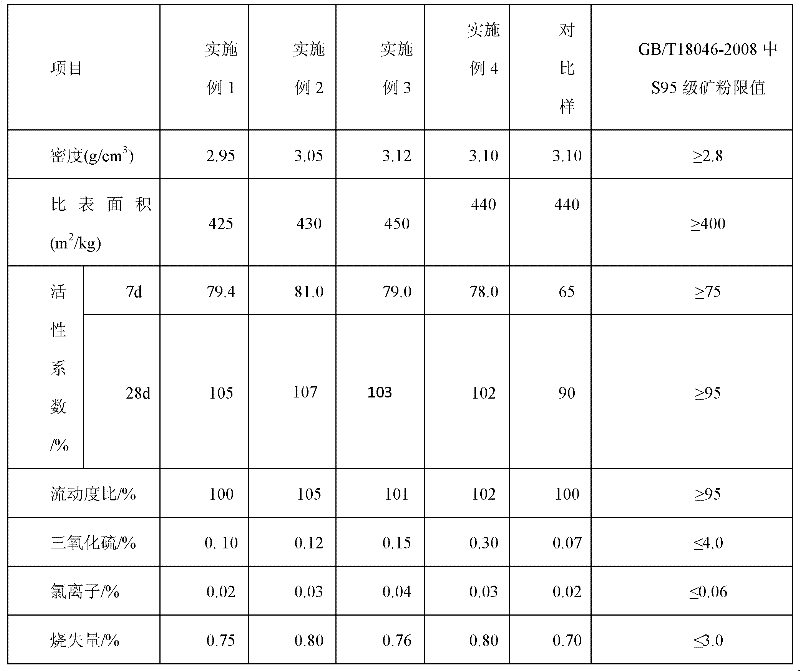
Modified steel slag composite admixture and preparation method thereof
InactiveCN102491664AOvercoming stability issuesImprove early activitySolid waste managementWork performanceSlag
The invention discloses a modified steel slag composite admixture and a preparation method thereof. The modified steel slag composite admixture comprises 55-75wt% of micro mineral slag powder, 20-40wt% of micro steel slag powder, and 1-5wt% of sintering desulphurization slag. Concrete prepared by mixing the modified steel slag composite admixture of the invention with cement has the following characteristics: the seven day active index is greater than 75%, the twenty-eight day active index is greater than 100%, the specific surface area is equal to or greater than 400m<2> / kg, the sulfur trioxide content is equal to or less than 4.0%, the chloride ion content is equal to or less than 0.06%, the ignition loss amount is equal to or less than 3.0%, and the autoclaving stability is qualified. The modified steel slag composite admixture which allows a low early strength disadvantage of steel slag composite admixtures to be overcome, working performances of concrete to be effectively improved, the strength and the endurance of concrete to be improved, and energy consumption required by slag grinding to be effectively reduced and is in favor of the low carbon economy realization, is a resource use type concrete admixture.
Owner:SHANGHAI BAOTIAN NOVEL BUILDING MATERIALS
Single-wall carbon nanotube film having high modulus and conductivity and process for making the same
The invention relates to a film comprising greater than 80 wt % single-wall carbon nanotubes wherein the tensile modulus is at least about 6 GPa at 0.2% strain and the conductivity of the film is at least about 70,000 S / m. The tensile modulus is typically about 8 GPa at 0.2% strain. The method for making the film comprises preparing a solution of single-wall carbon nanotubes in a superacid, such as oleum containing approximately 20 to 30% sulfur trioxide, under a dry, oxygen-free atmosphere. The solution is placed on a surface in a moisture-containing atmosphere, wherein the solution absorbs moisture and acid leaches out. The film is washed to further remove acid, dried, and, optionally, subjected to a heat treatment. Besides free-standing films, coatings of single-wall carbon nanotubes can be made on a variety of surfaces including polymers, glass, metals, and ceramics. The surfaces can be flat planes, fibers or contour shapes.
Owner:GEORGIA TECH RES CORP
Universal rock and soil curing agent and preparation method thereof
ActiveCN102503328ASolidified rock and soil quality is goodLow costOrganic fertilisersSoil conditioning compositionsPowder mixtureFirming agent
The invention relates to a universal rock and soil curing agent and a preparation method of the universal rock and soil curing agent and aims at solving the problem of poor curing quality of the existing curing agent. The universal rock and soil curing agent comprises a powdery mixture formed by mixing the following raw materials: calcium oxide, silicon dioxide, aluminium oxide, sulfur trioxide, magnesium oxide and ferric oxide. The universal rock and soil curing agent is characterized in that raw materials also comprise viscous polymers and materials with cinerite activity or powdery materials with viscosity after sintering and water meeting, wherein the viscous polymers account for 2 to 8 percent of the total weight of the powdery mixture, the materials with cinerite activity or the powdery materials with viscosity after sintering and water meeting account for 5 to 20 percent of the total weight of the powder mixture, the raw materials also comprise compound body additional adding agents, the compound body additional adding agents account for 1 to 3 percent of the total weight of the powdery mixture and are formed by mixing the following raw materials in percentage by weight or are the following raw materials in percentage by weight: 0 to 100 percent of NaOH and 100 to 0 percent of water reducers. The preparation method comprises the following steps that: the raw materials according to the weight mixing ratio are put into a powder body mixing machine, the pre-mixing is firstly carried out, then, the major mixing is carried out, and the materials are sufficiently and uniformly mixed. The universal rock and soil curing agent and the preparation method have the advantages that the rock and soil curing quality is good, the cost is low, the application range is wide, various inorganic solid waste materials can be cured, waste materials are changed into useful materials, the cost performance is high, economic benefits and environment benefits are obvious, and popularization and application prospects are wide.
Owner:北京旷世达资源环境工程发展中心
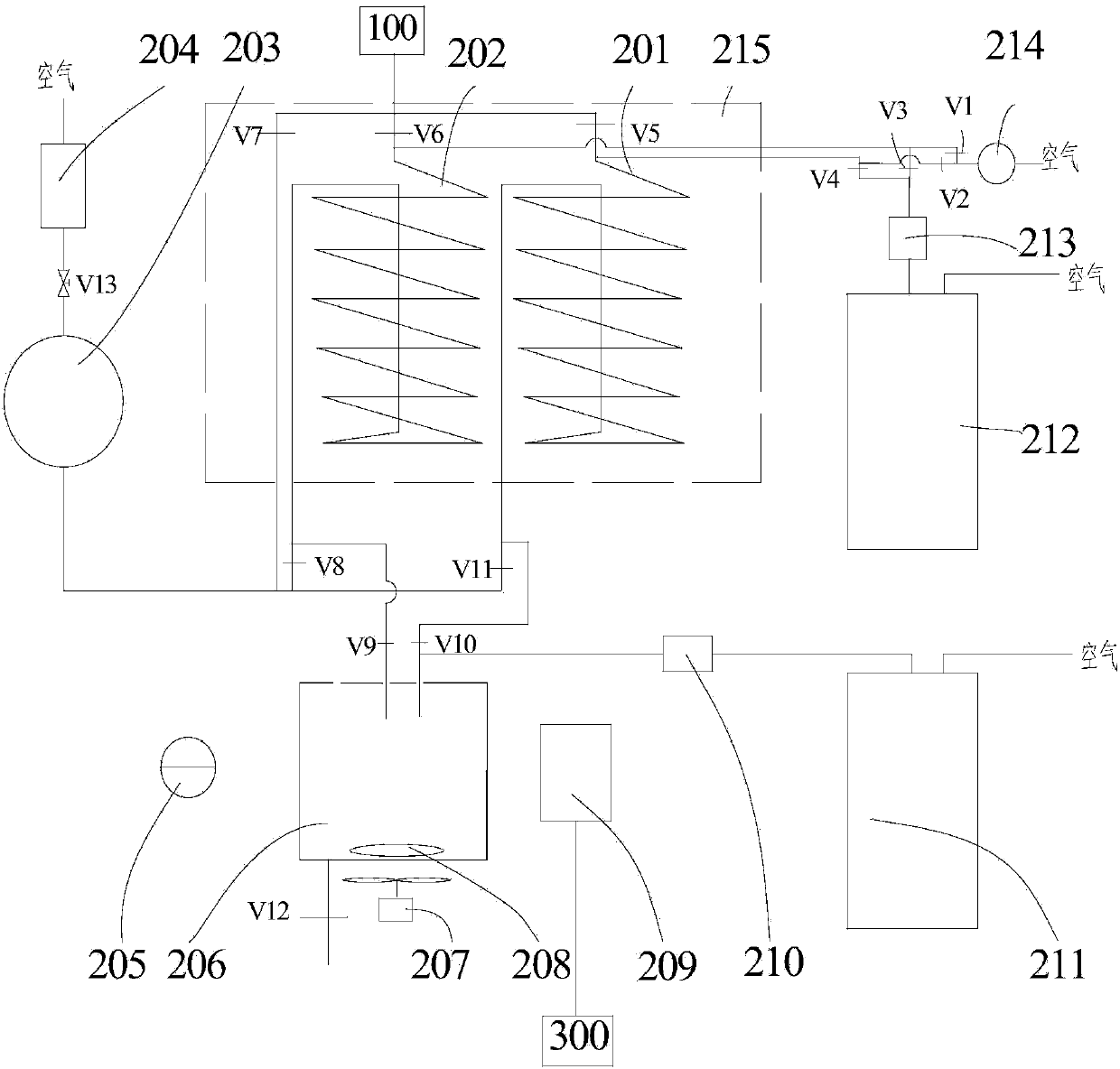
Device and method for detecting sulfur trioxide in flue gas on line
ActiveCN103472061ARealize online measurementTake convenienceMaterial analysis by observing effect on chemical indicatorWithdrawing sample devicesGas phaseEngineering
The invention discloses a device and a method for detecting sulfur trioxide (SO3) in flue gas on line. The device comprises a flue gas collecting unit, a gas liquid separator, a gas phase detection unit, a first solution tank, a liquid phase detection unit and a digital control unit, wherein the gas liquid separator is used for condensing SO3 in the flue gas from a flue gas sampling unit into sulfuric acid liquid drops and separating the sulfuric acid liquid dropsfrom gas phase; the gas phase detection unit is used for measuring the flow of the gas phase from the gas liquid separator; the first solution tank is used for providing an absorption liquid to the gas liquid separator and mixing the absorption liquid with the sulfuric acid liquid drops to form mixed liquid; the liquid phase detection unit is used for measuring the mass of the transformed SO3 in the mixed liquid from the gas liquid separator; and the digital control unit is used for calculating the SO3 concentration of the flue gas according to the detection results of the gas phase detection unit and the liquid phase detection unit. According to the device and the method, on-line measurement of the SO3 of the flue gas is realized, the SO3 of the flue gas is sampled and the SO3 content is analyzed, so that a coal-fired power plant can take corresponding measures conveniently, the SO3 content of the flue gas is controlled, the corrosion to a flue and equipment is reduced, and the heat loss due to exhaust smoke of a boiler is reduced to the maximum degree.
Owner:ZHEJIANG UNIV
Stable and compatible polymer blends
The present invention pertains to products and processes relating to compatible polymer blends comprising at least one sulfonated polymer and at least one non-sulfonated polymer. The sulfonated polymers may be produced using a number of sulfonating agents including a coordination complex of sulfur trioxide. The polymeric blended materials described herein are useful in a variety of applications, including as coatings for medical devices, protective clothing and fabric, laboratory equipment, vascular stents and shunts, absorbent materials and separation membranes, three-dimensional constructs, devices, and other uses.
Owner:AEGIS BIOSCI +1
Acesulfame cyclization continuous production method
ActiveCN103130743AImprove continuityReduce labor intensityOrganic chemistryBiochemical engineeringSulfur trioxide
The invention relates to an acesulfame cyclization continuous production method. The acesulfame cyclization continuous production method is characterized in that sulfonation reaction and hydrolysis reaction comprise the following steps: respectively dripping intermediates and sulfur trioxide generated in synthesis reaction into a sulfonation reaction kettle by a certain flow rate, conducting cooling in the sulfonation reaction kettle, and conducting primary reaction; reactants in the sulfonation reaction kettle automatically overflowing into a sulfonation liquid kettle after reaching a certain amount, and conducting spontaneous reaction; putting sulfonation liquid into a hydrolysis kettle after the spontaneous reaction, and meanwhile dripping acid water to conduct hydrolysis reaction. The acesulfame cyclization continuous production method has the advantages that cyclization continuous reaction process is adopted, continuity of production operation is achieved, and labor intensity of workers is reduced, besides, compared with traditional batch production process, stability of cyclization continuous reaction is high, service life of the reaction kettles is prolonged, cryogenic load is lightened, and production efficiency is greatly improved.
Owner:ANHUI JINGHE IND
Power plant flue-gas SCR denitration catalyst capable of preventing sulfur trioxide poisoning and preparation method thereof
InactiveCN101396656ASimple preparation processEasy to operateDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsAmmonium paratungstateSlurry
The invention belongs to the chemical material field, in particular to an SO3 poisoning resisting power plant flue gas SCR denitrification catalyst and a preparation method thereof. Tungsten doped titanium pigment which is synthesized from domestic metatitanic acid slurry and ammonium paratungstate oxalic acid solution is adopted by the catalyst as a carrier. Vanadium is used as a main catalyst, and oxide of germanium or zinc is added as additive for lowering the oxidization of the vanadium on SO2. Through drying, calcining and sieving, the catalyst is obtained. The catalyst comprises 80-95wt percent of TiO2, 3-15wt percent of WO3, 1-8wt percent of V2O5 and 1-8wt percent of GeO2 or ZnO2. The preparation method has the advantages of simple preparation process, easy operation, low cost and good denitrification effect. The SCR catalyst prepared by the method is tested to prove high denitrification efficiency and good SO3 poisoning resistance. Added SO2 has almost no influence on the activity of the catalyst.
Owner:TSINGHUA UNIV +1
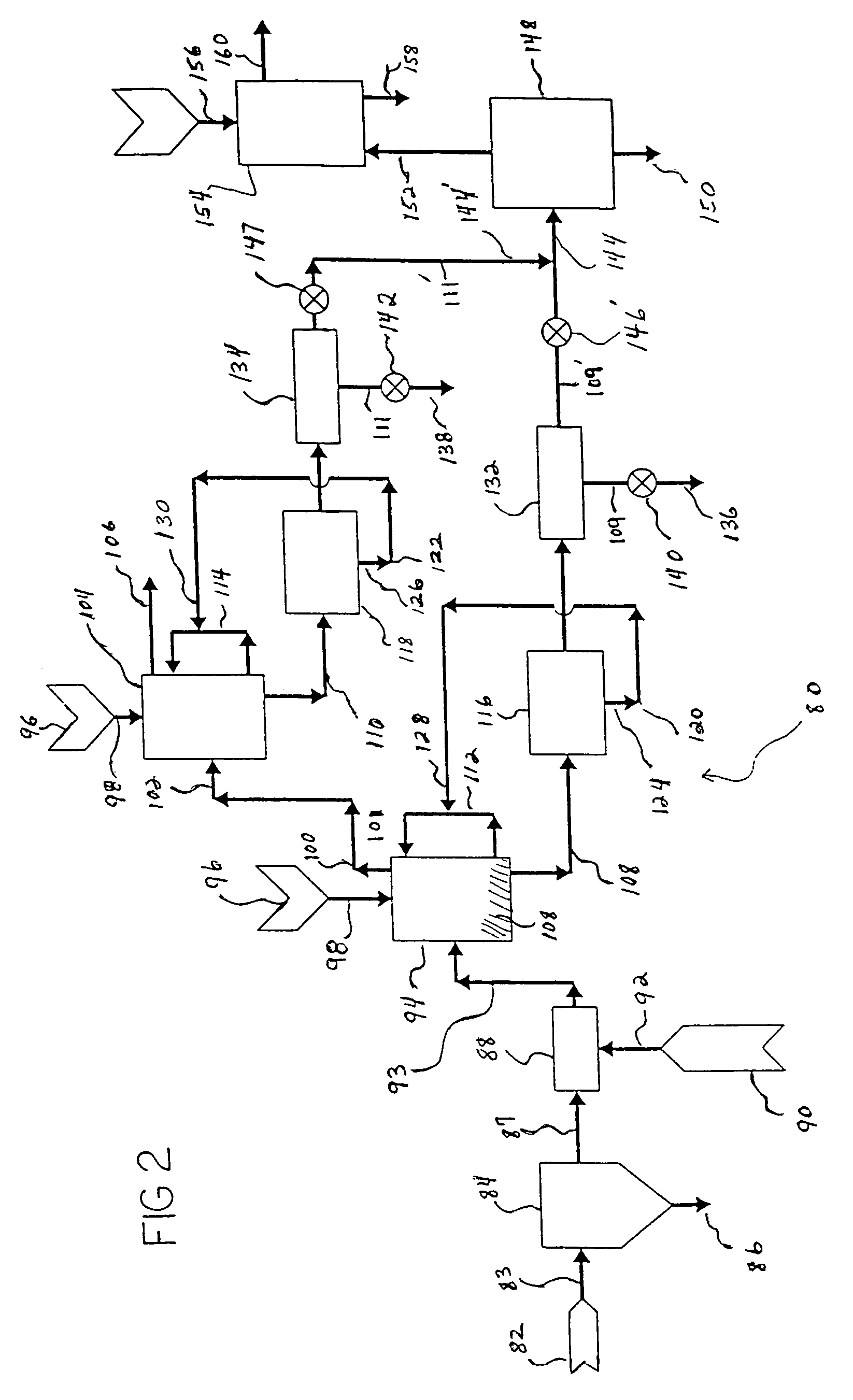
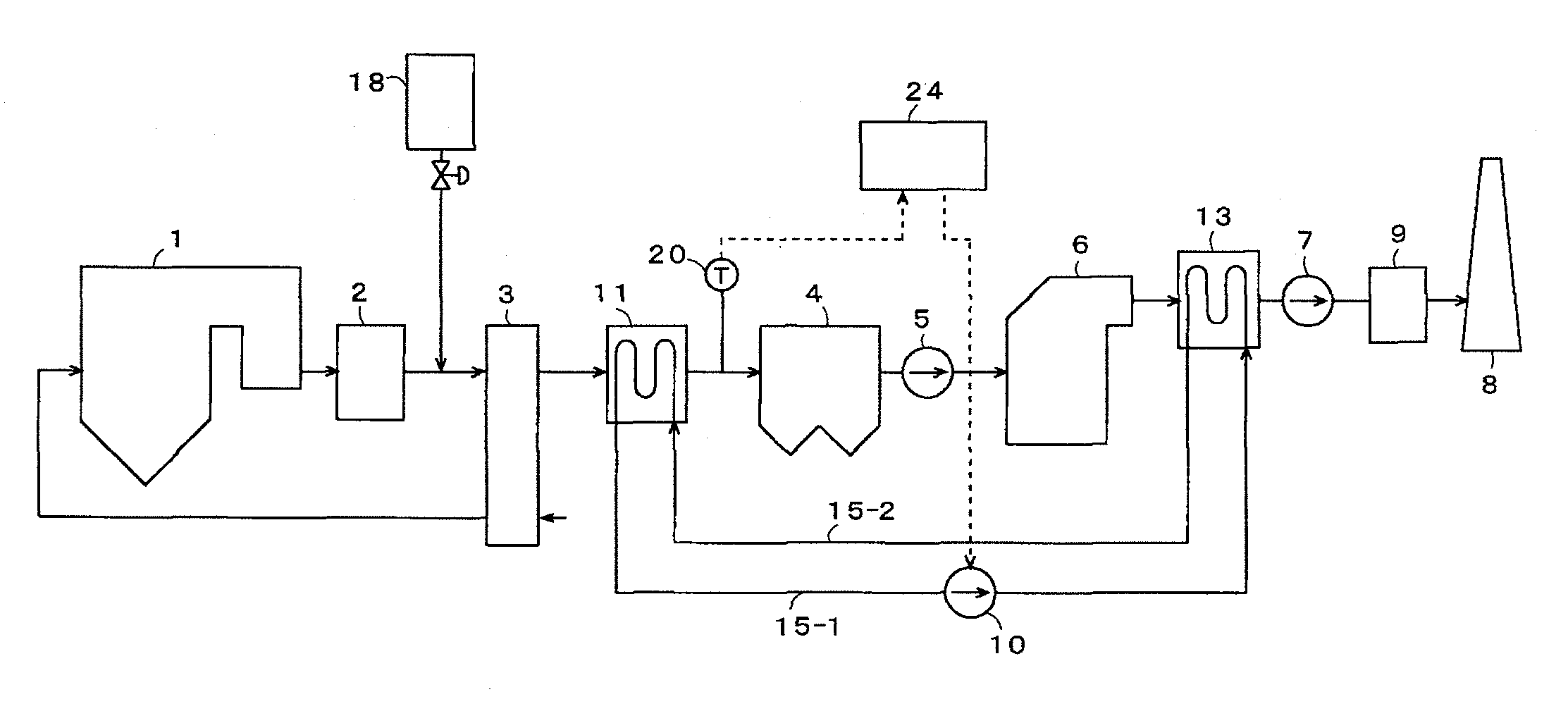
Method and apparatus for treating discharge gas
ActiveUS20100074817A1Reduce concentrationImprove adsorption capacityCombination devicesNitrogen compoundsAir preheaterSorbent
The following devices are successively disposed in the following order from an upstream side to a downstream side in an exhaust gas duct of a combustion apparatus: an air preheater, preheating combustion air for use in an exhaust gas treating apparatus; a heat recovery unit, recovering exhaust gas heat at an exit of the air preheater; a precipitator, collecting soot / dust contained in an exhaust gas at an exit of the heat recovery unit; a wet flue gas desulfurizer, removing sulfur oxides contained in the exhaust gas at the exit of the precipitator; and a reheater, heating the exhaust gas at the exit of the wet flue gas desulfurizer. Each of the heat recovery unit and the reheater has a heat exchanger tube, and a circulation line is disposed to connect the heat exchanger tubes. A sulfur trioxide (SO3) removing agent is supplied to the upstream side of the heat recovery unit, and the temperature of the exhaust gas at the exit of the heat recovery unit is adjusted to not more than a dew point of sulfur trioxide. As the sulfur trioxide removing agent, use is preferably made of at least one among a sulfur trioxide adsorbent, a sulfur trioxide reducing agent, and a sulfur trioxide neutralizing agent. Thus, even when coal with a high sulfur content is used as fuel, heavy metals contained in the exhaust gas can be removed effectively from the exhaust gas.
Owner:MITSUBISHI POWER LTD
Sorbent formulation for removal of mercury from flue gas
Methods and systems for reducing mercury emissions from fluid streams having a high concentration of sulfur oxide species are provided herein. In embodiments, mercury is removed from flue gas streams containing sulfur trioxide (SO3) by injecting a dry admixture of a porous mercury adsorptive material and at least one dry agent into the flue gas stream.
Owner:CALGON CARBON
Device and process for effectively removing sulfur trioxide in smoke through natural alkali
InactiveCN103055684ASmall pressure lossReduce resistanceDispersed particle separationAir preheaterAlkalinity
The invention relates to a device and a process for effectively removing sulfur trioxide in smoke through a natural alkali. The device comprises a smoke inlet and a smoke outlet, wherein a bent transitional flue is arranged between the smoke inlet and the smoke outlet; a nozzle system is arranged on the bent transitional flue; a wear-proof plate is arranged on the smoke incoming direction of the nozzle system; and the nozzle system is connected with a natural alkali slurry preparation system. The smoke from the outlet of a selective catalyst reduction (SCR) reactor is sprayed and washed through the natural alkali slurry to completely absorb sulfur trioxide (SO3), and then enters an air preheater. The device for effectively removing sulfur trioxide in the smoke through the natural alkali disclosed by the invention, through the nozzle system on the flue between the tail part of the SCR reactor and the air preheater and by spraying the natural alkali slurry into the flue, can absorb and remove SO3 with a removal efficiency of 80-90% through the strong alkalinity of the natural alkali; through setting the position, quantity, angle and outlet flow velocity of the nozzle, uniform mixing of the absorbent and the smoke is guaranteed; and through the wear-proof plate, abrasion of the smoke on the nozzle is reduced, and optimization and adjustment are carried, so as to reduce the resistance of the flue to the greatest extent.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com