Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
551 results about "Vascular stents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
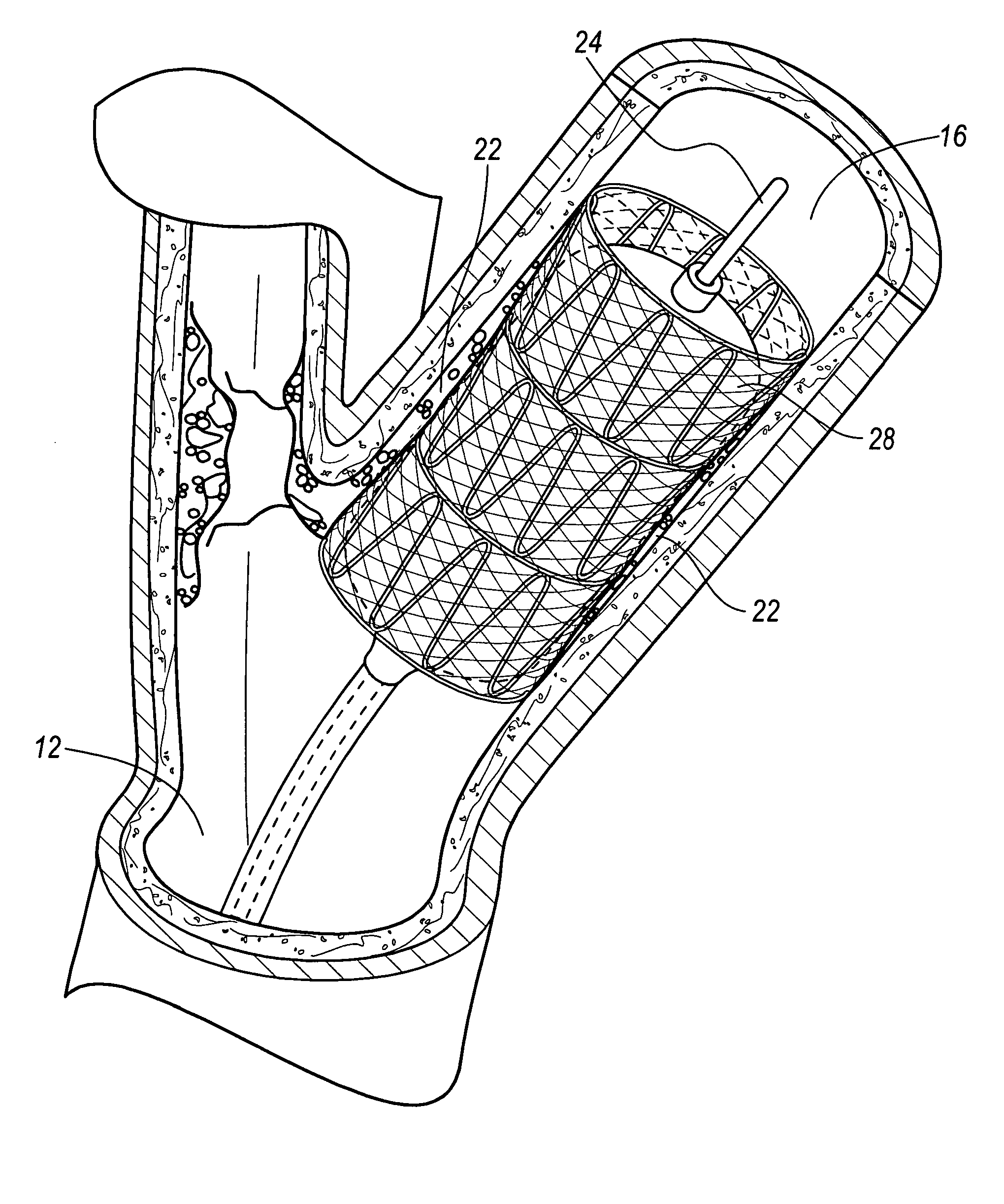
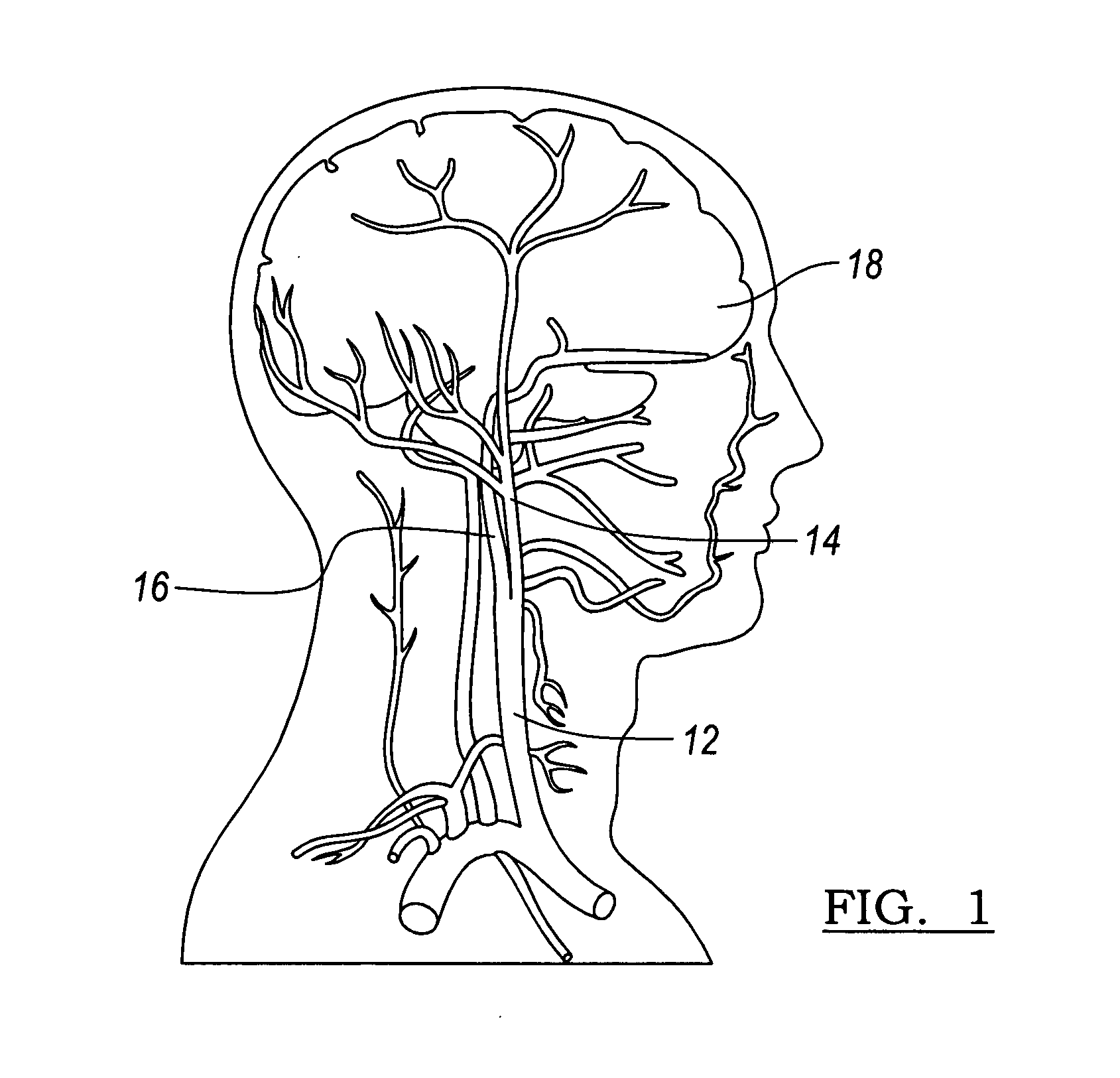
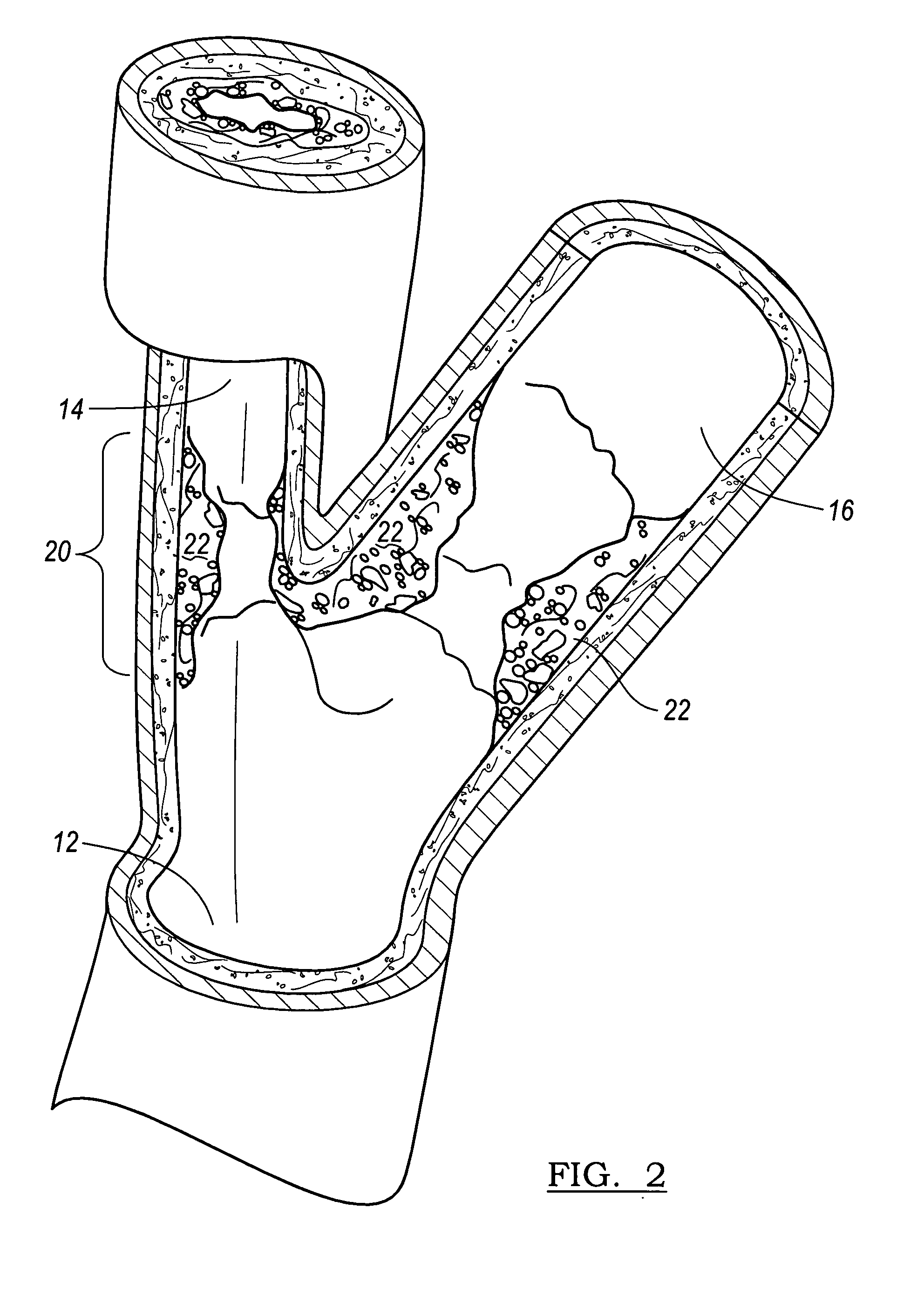
A vascular stent is a medical device designed to be inserted into a blood vessel in order to keep it open.
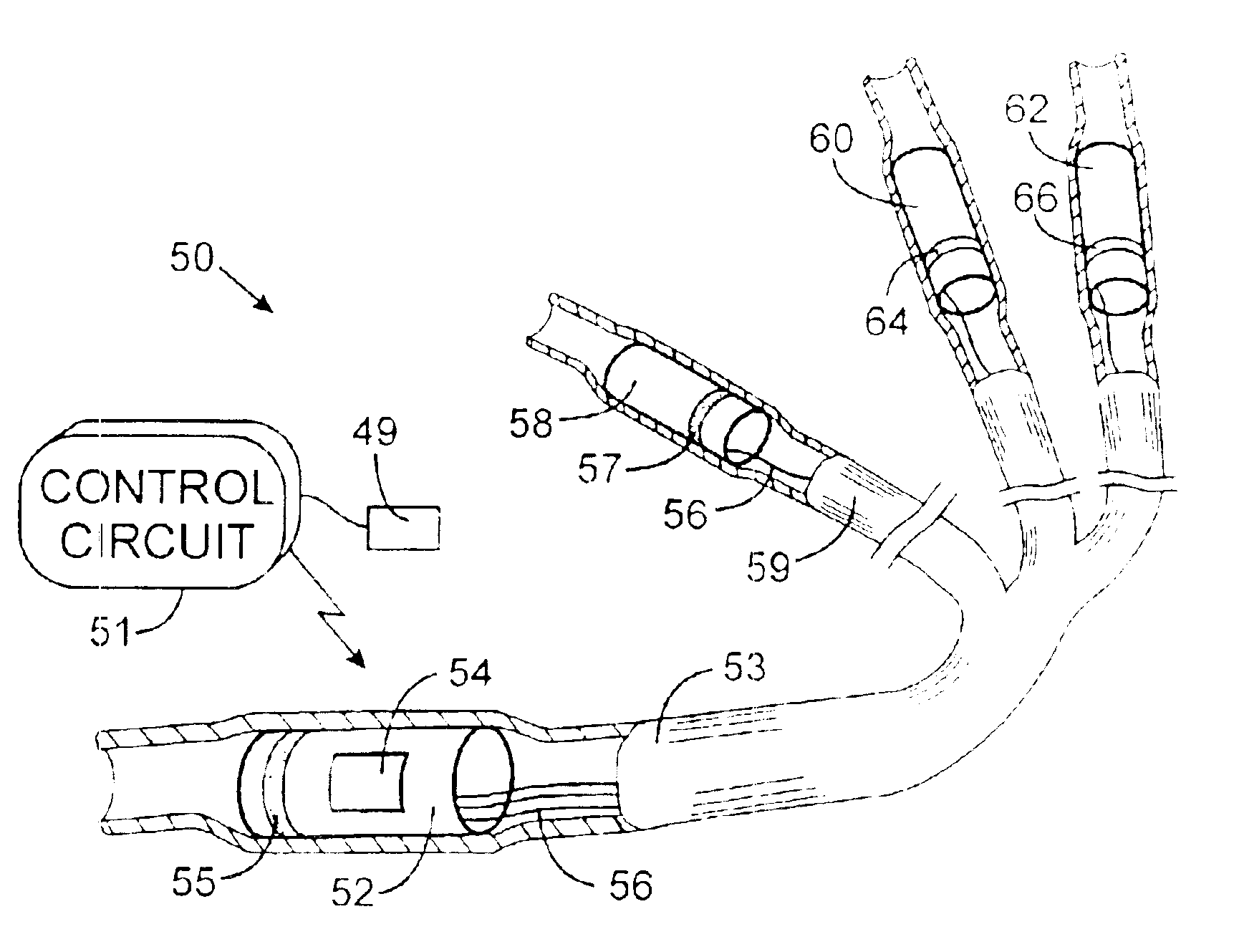
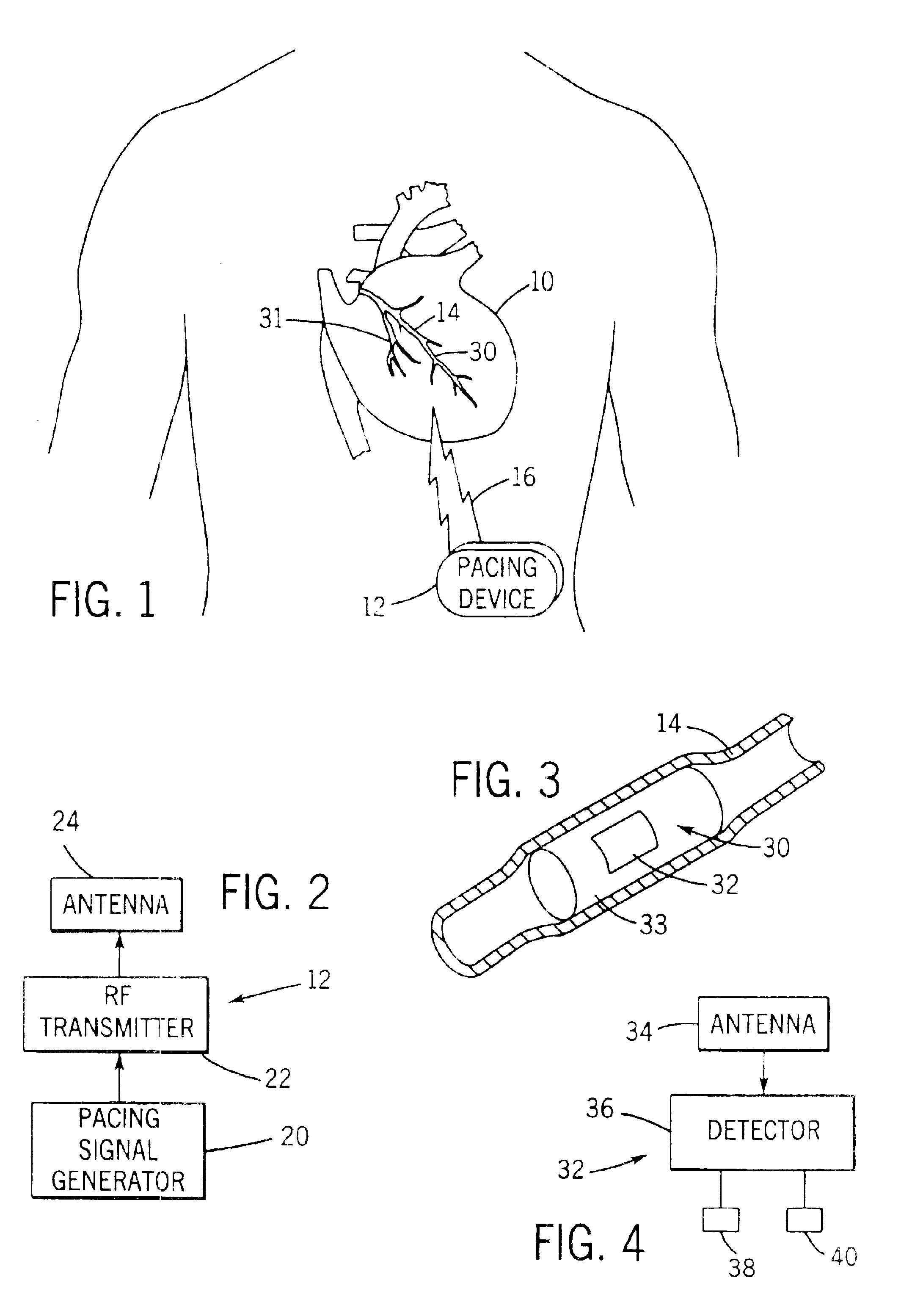
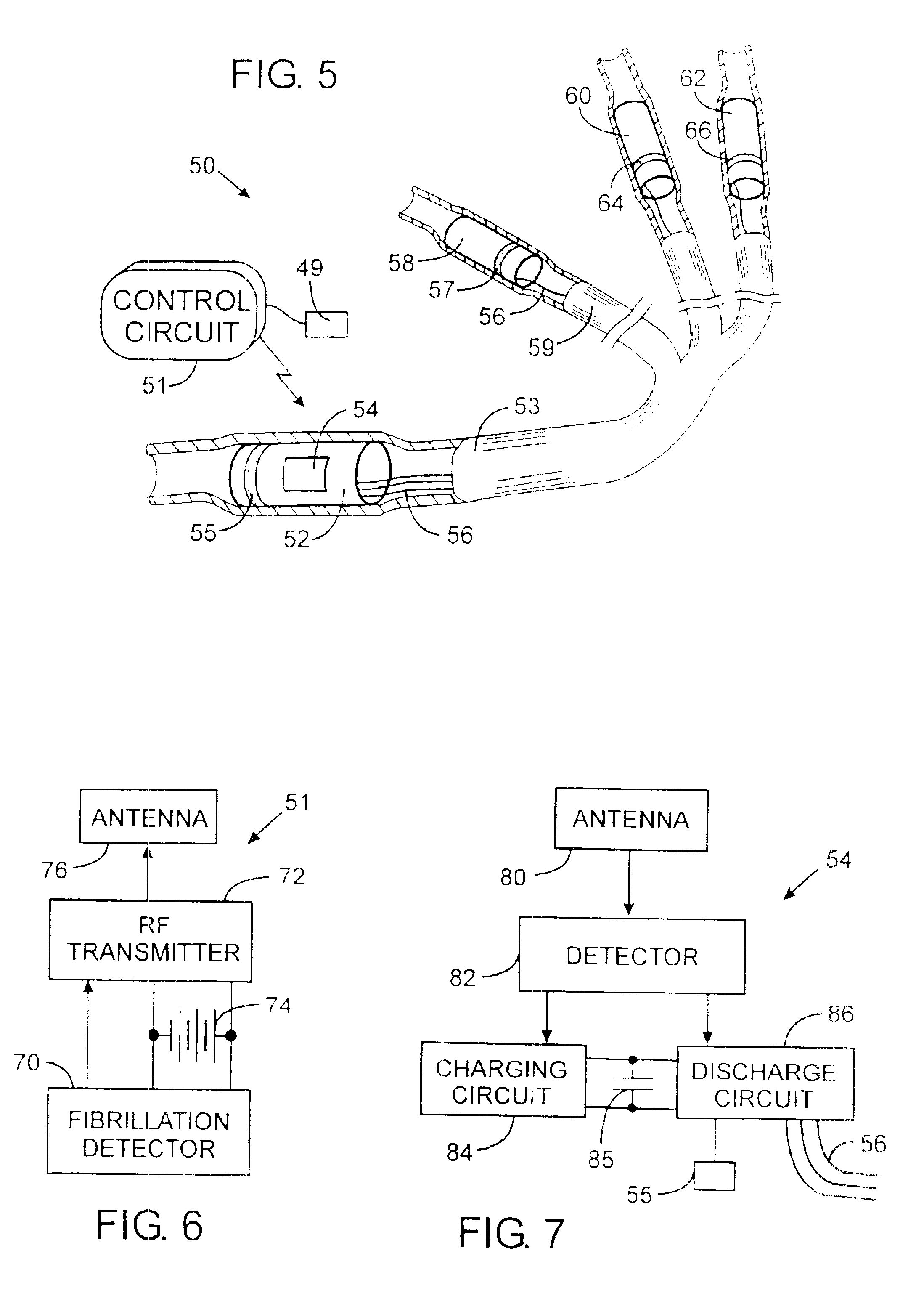
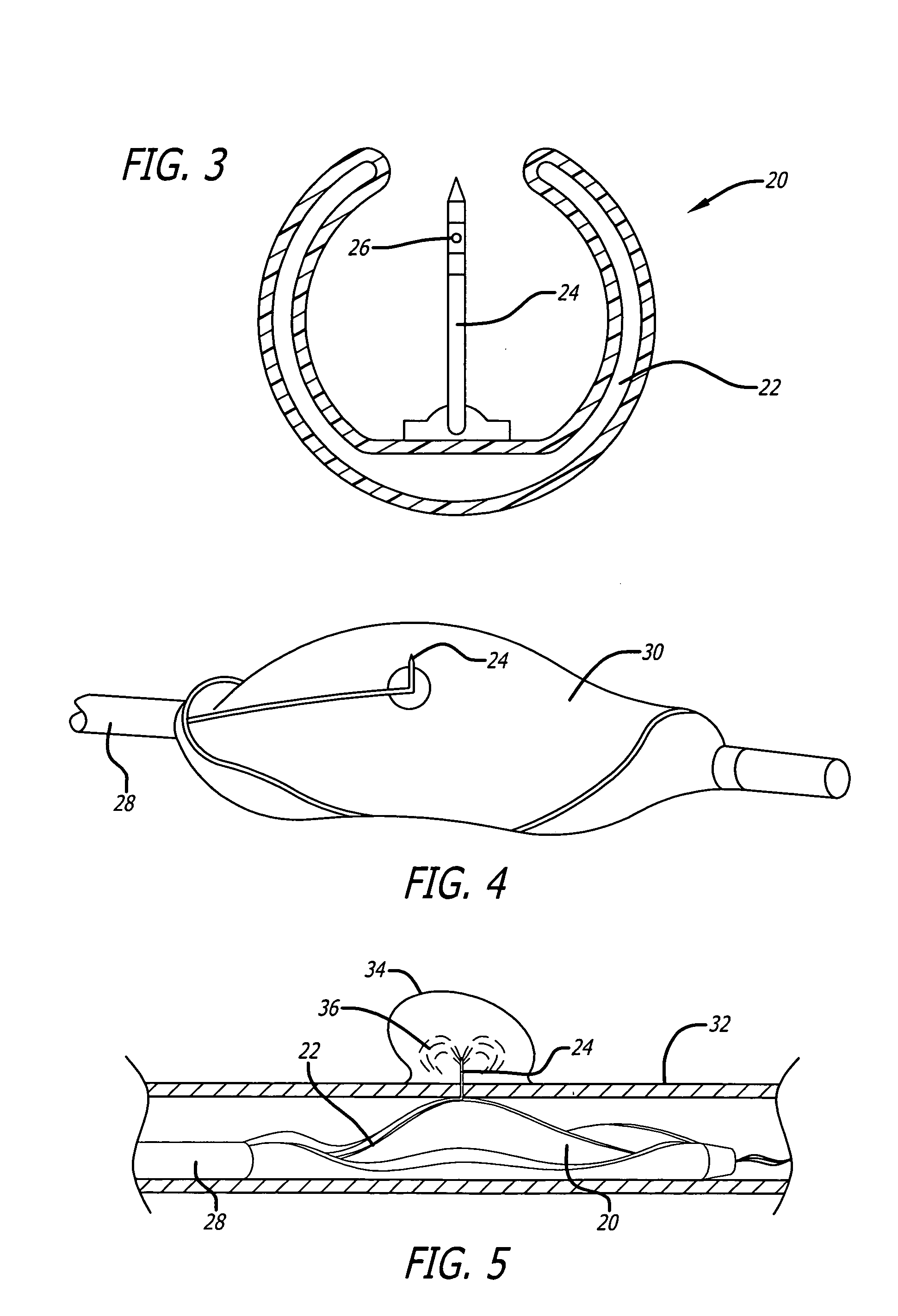
Implantable defibrillartor with wireless vascular stent electrodes
A cardiac defibrillator includes a fibrillation detector, which determines when a medical patient requires defibrillation at which time a transmitter produces a radio frequency signal. A first stent electrode is implanted into a blood vessel at a first location in the medical patient and a second stent electrode is implanted into a blood vessel at a second location. The first stent electrode contains an electronic circuit that is electrically connected to the second stent electrode. In response to receiving the radio frequency signal, the electronic circuit uses energy from that signal to apply an electric defibrillation pulse between the first and second stent electrodes.
Owner:KENERGY INC
Medical device containing light-protected therapeutic agent and a method for fabricating thereof
InactiveUS20030073961A1Preservation of therapeutical propertyImprove light resistanceBiocideOrganic active ingredientsPharmaceutical drugMedical device
Light- and / or UV-radiation protective coatings for drug delivery devices, such as, for instance, drug eluting vascular stents, where the drugs being delivered via the stents are light sensitive. A method of fabricating a medical article, such as a drug eluting vascular stent, that includes the light- and / or UV-radiation protective coating.
Owner:ABBOTT CARDIOVASCULAR
Coatings on ophthalmic lenses
This invention is directed toward surface treatment of a device. The surface treatment comprises the attachment of terminal functionalized surfactants to the surface of the substrate by means of reactive functionalities of the terminal functionalized surfactant material reacting with complementary surface reactive functionalities in monomeric units along the polymer substrate. The present invention is also directed to a surface modified medical device, examples of which include contact lenses, intraocular lenses, vascular stents, phakic intraocular lenses, aphakic intraocular lenses, corneal implants, catheters, implants, and the like, comprising a surface made by such a method.
Owner:BAUSCH & LOMB INC
Medical devices with nanoporous layers and topcoats
The present invention relates generally to medical devices with therapy eluting components and methods for making same. More specifically, the invention relates to implantable medical devices having at least one porous layer, and methods for making such devices, and loading such devices with therapeutic agents. A mixture or alloy is placed on the surface of a medical device, then one component of the mixture or alloy is generally removed without generally removing the other components of the mixture or alloy. In some embodiments, a porous layer is adapted for bonding non-metallic coating, including drug eluting polymeric coatings. A porous layer may have a random pore structure or an oriented or directional grain porous structure. One embodiment of the invention relates to medical devices, including vascular stents, having at least one porous layer adapted to resist stenosis or cellular proliferation without requiring elution of therapeutic agents. The invention also includes methods, devices, and specifications for loading of drugs and other therapeutic agents into nanoporous coatings.
Owner:MEDTRONIC VASCULAR INC +1
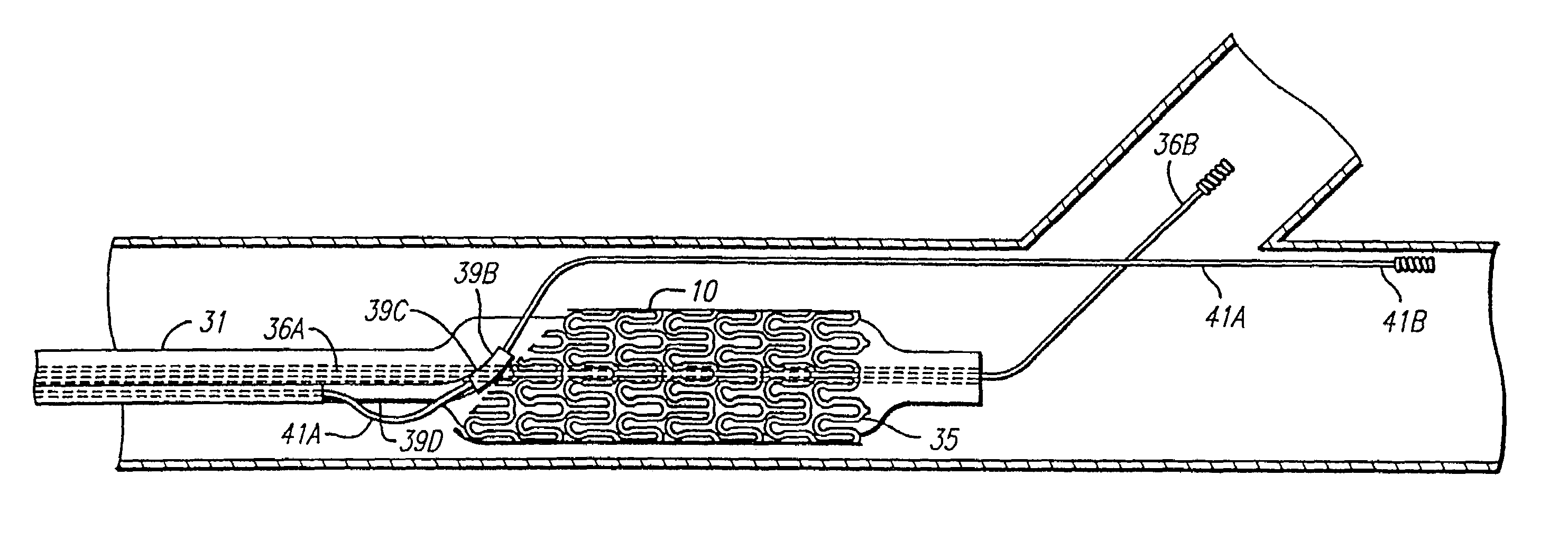
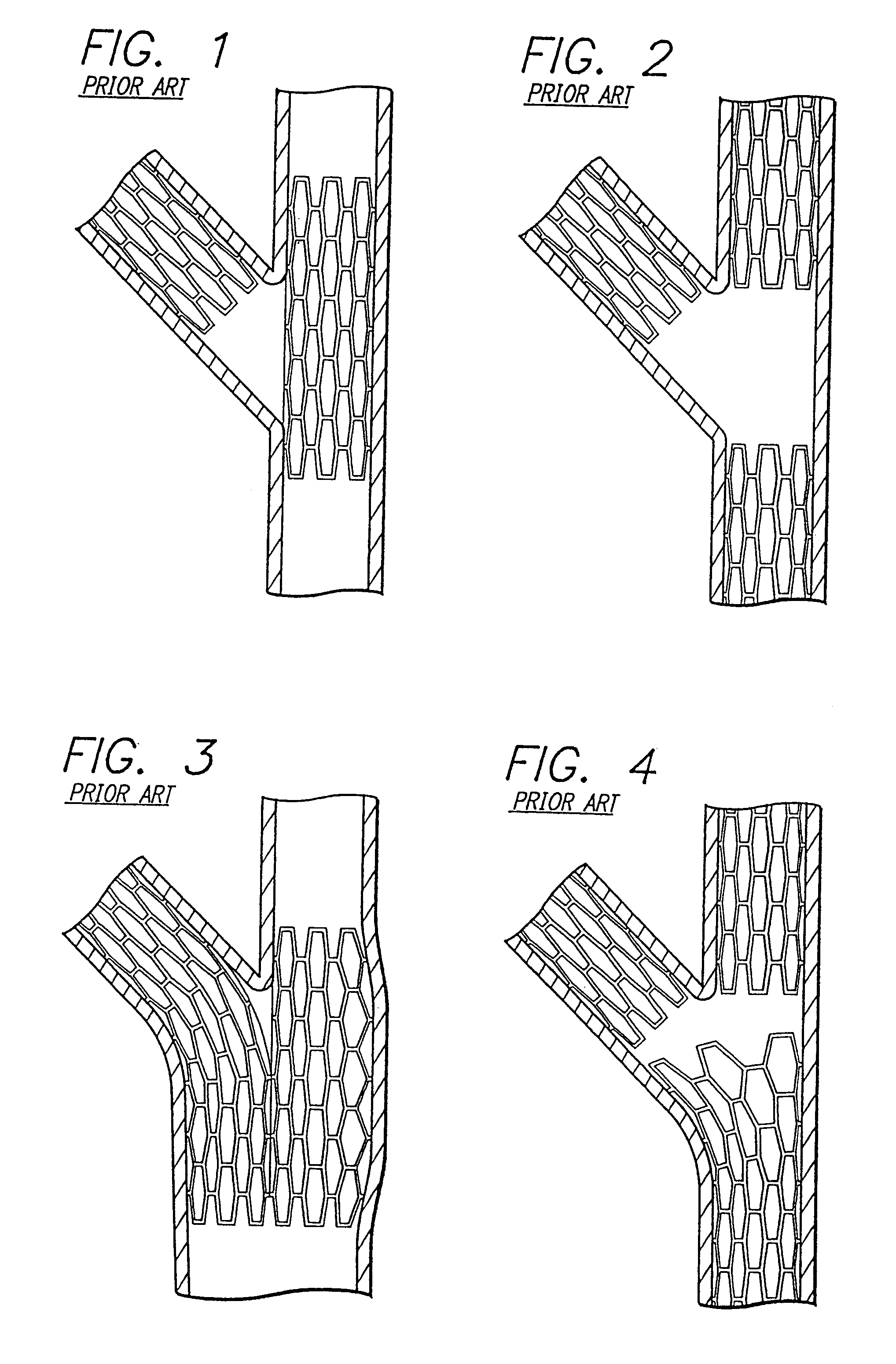
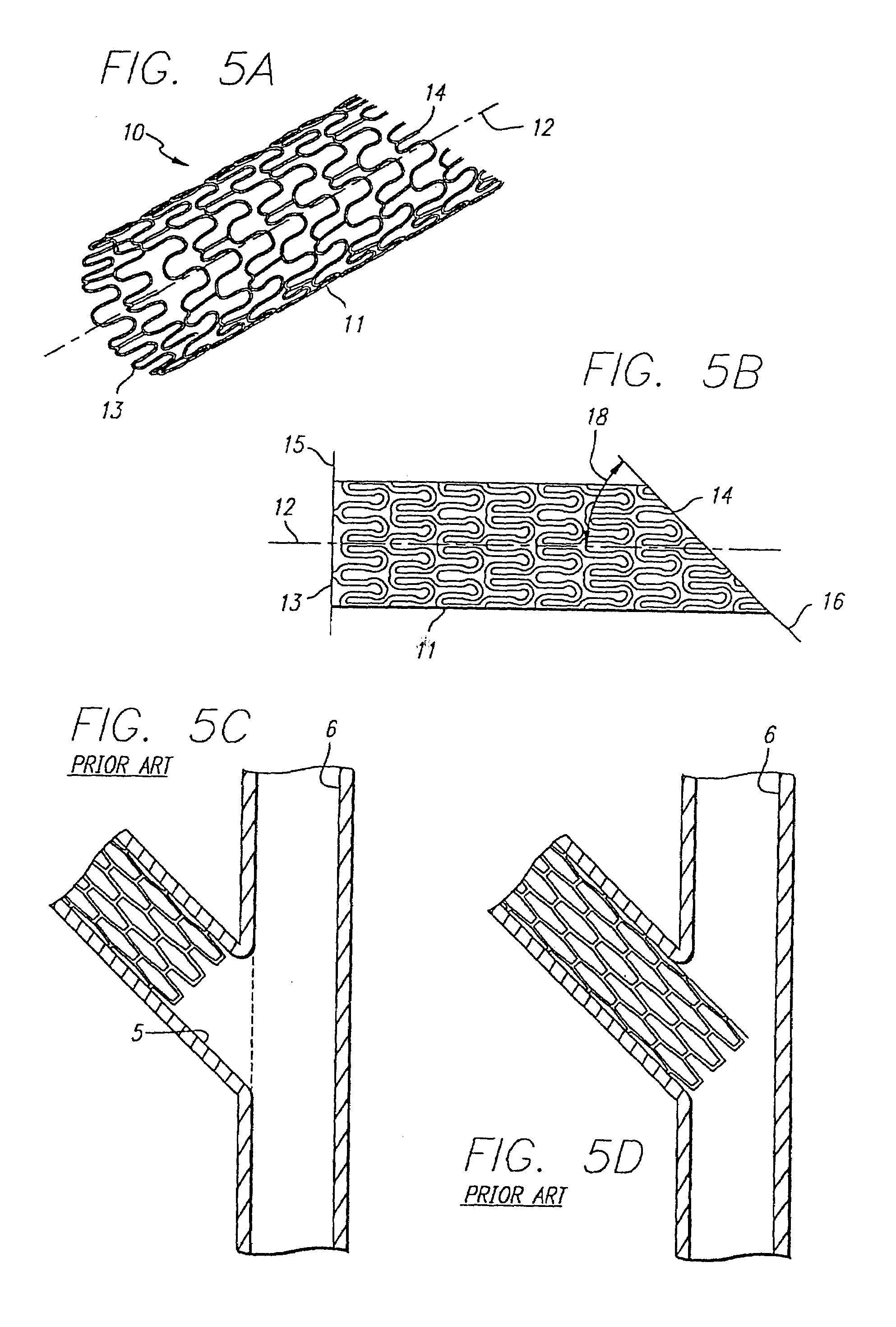
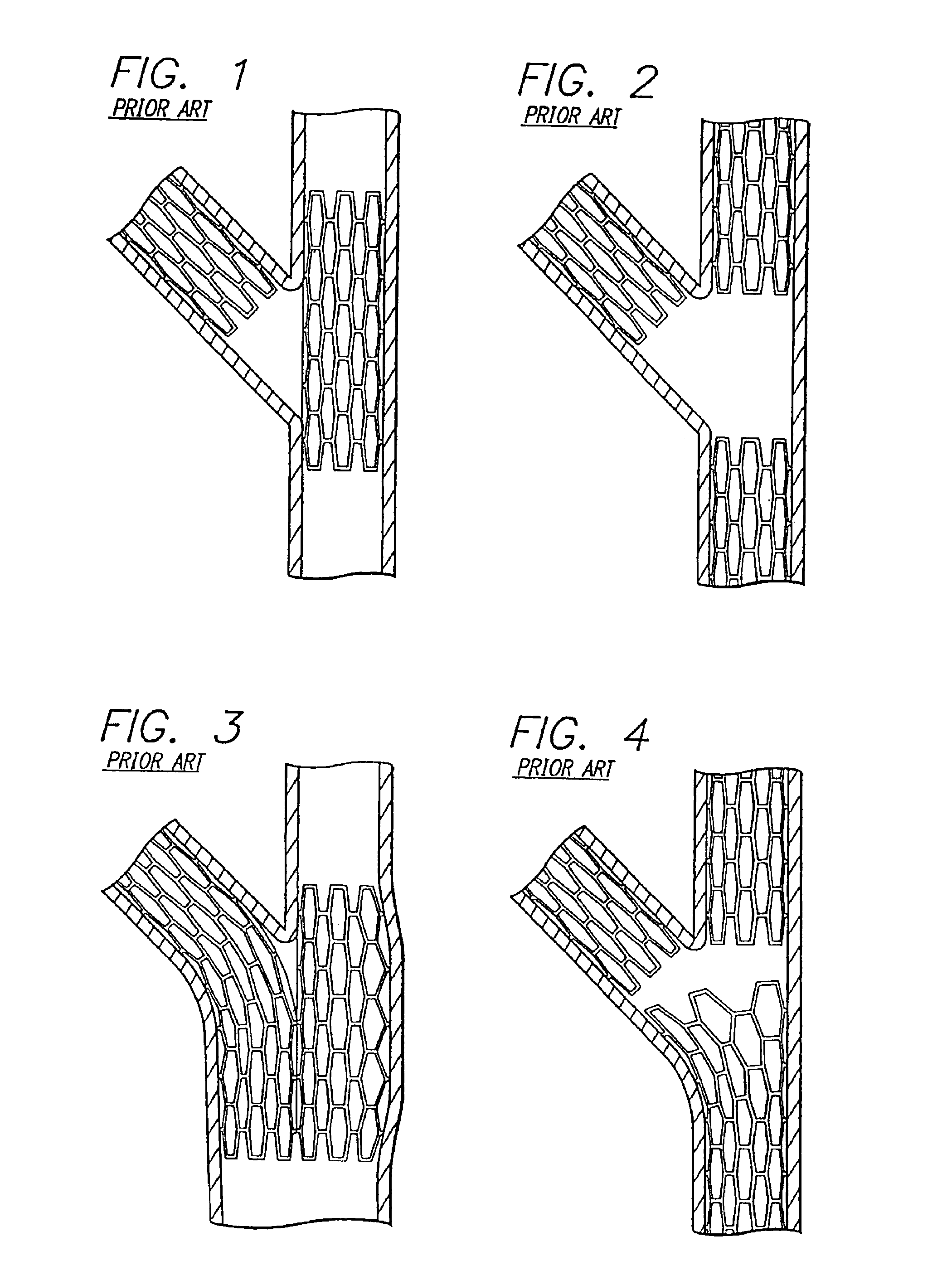
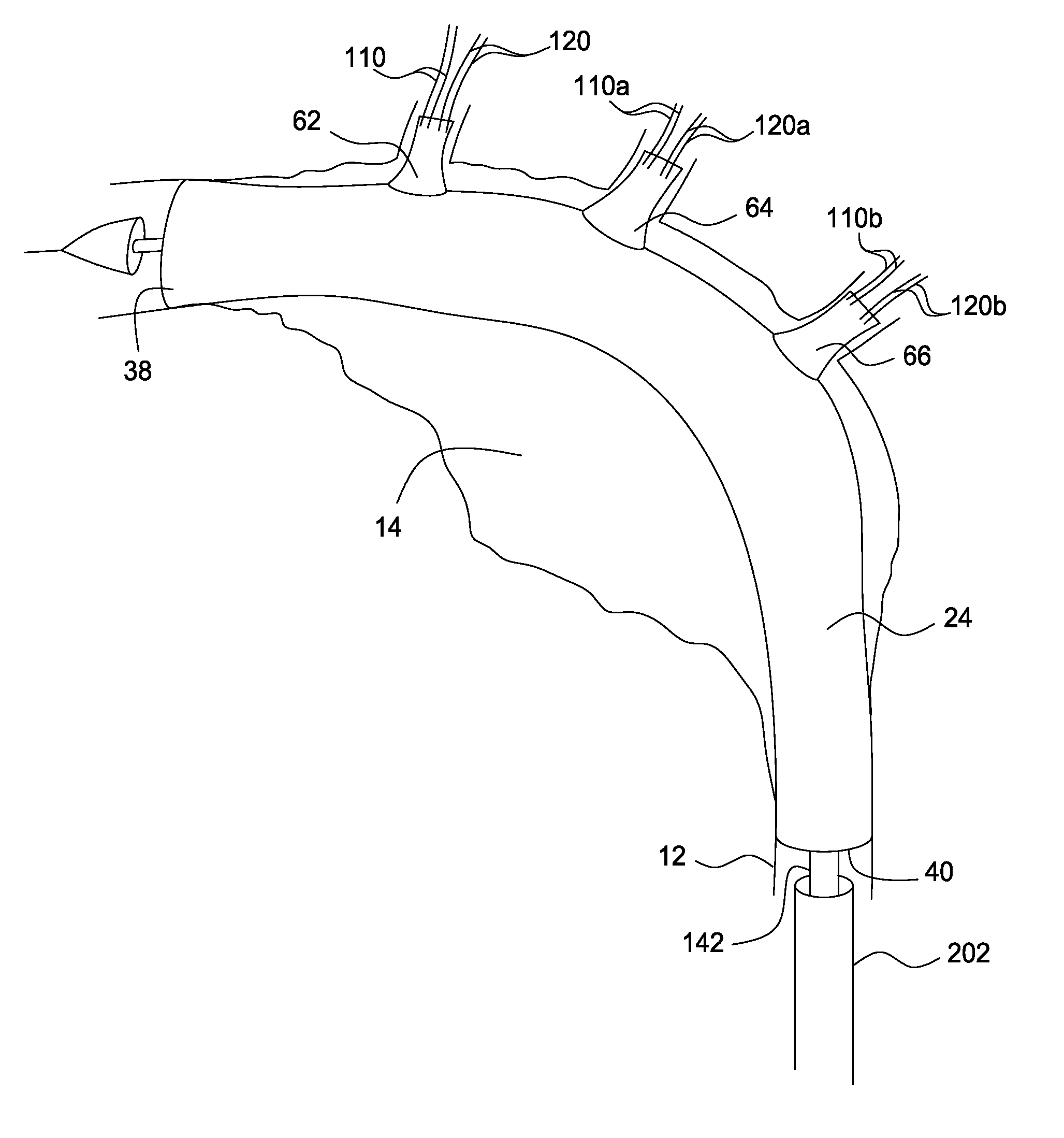
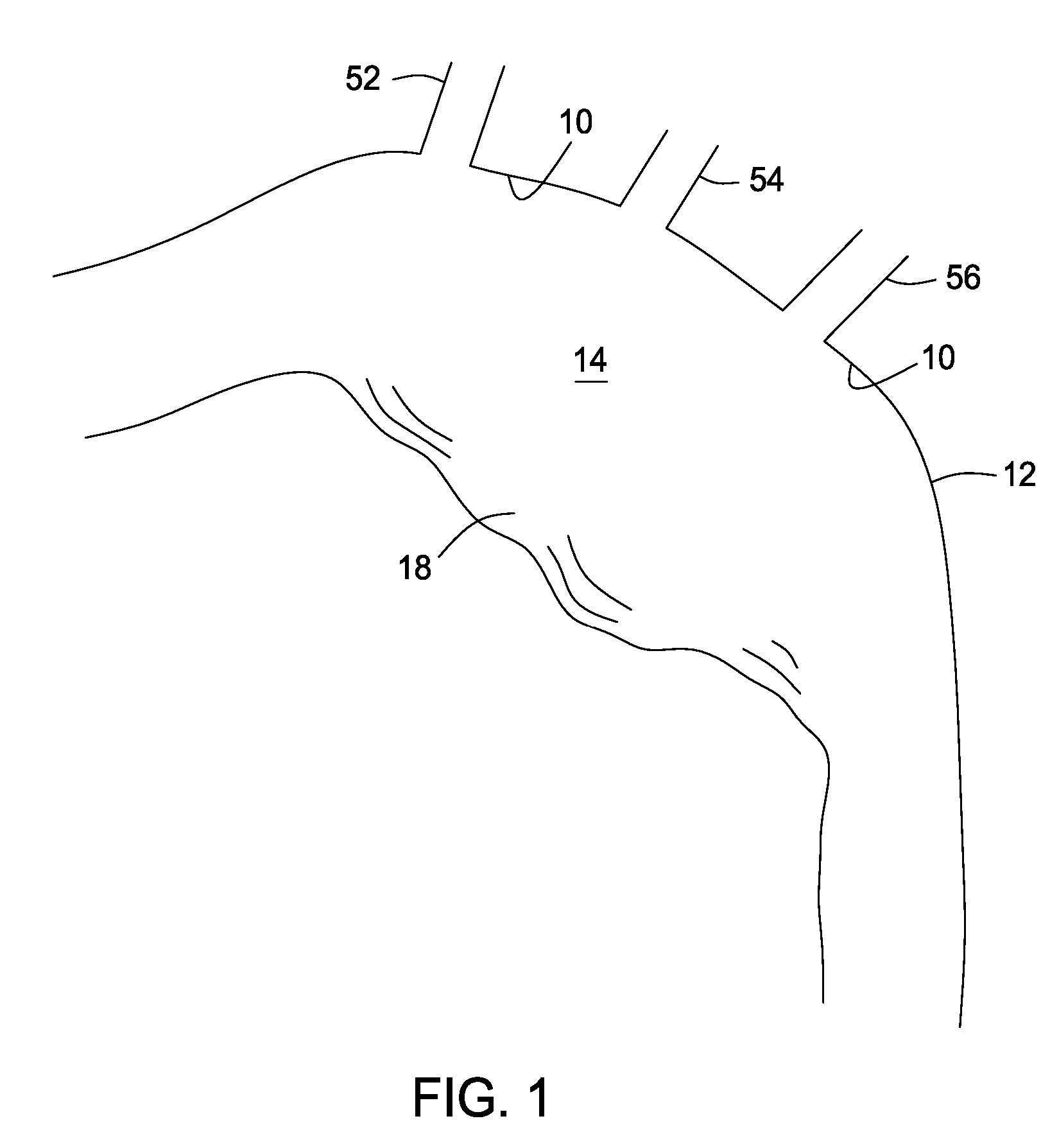
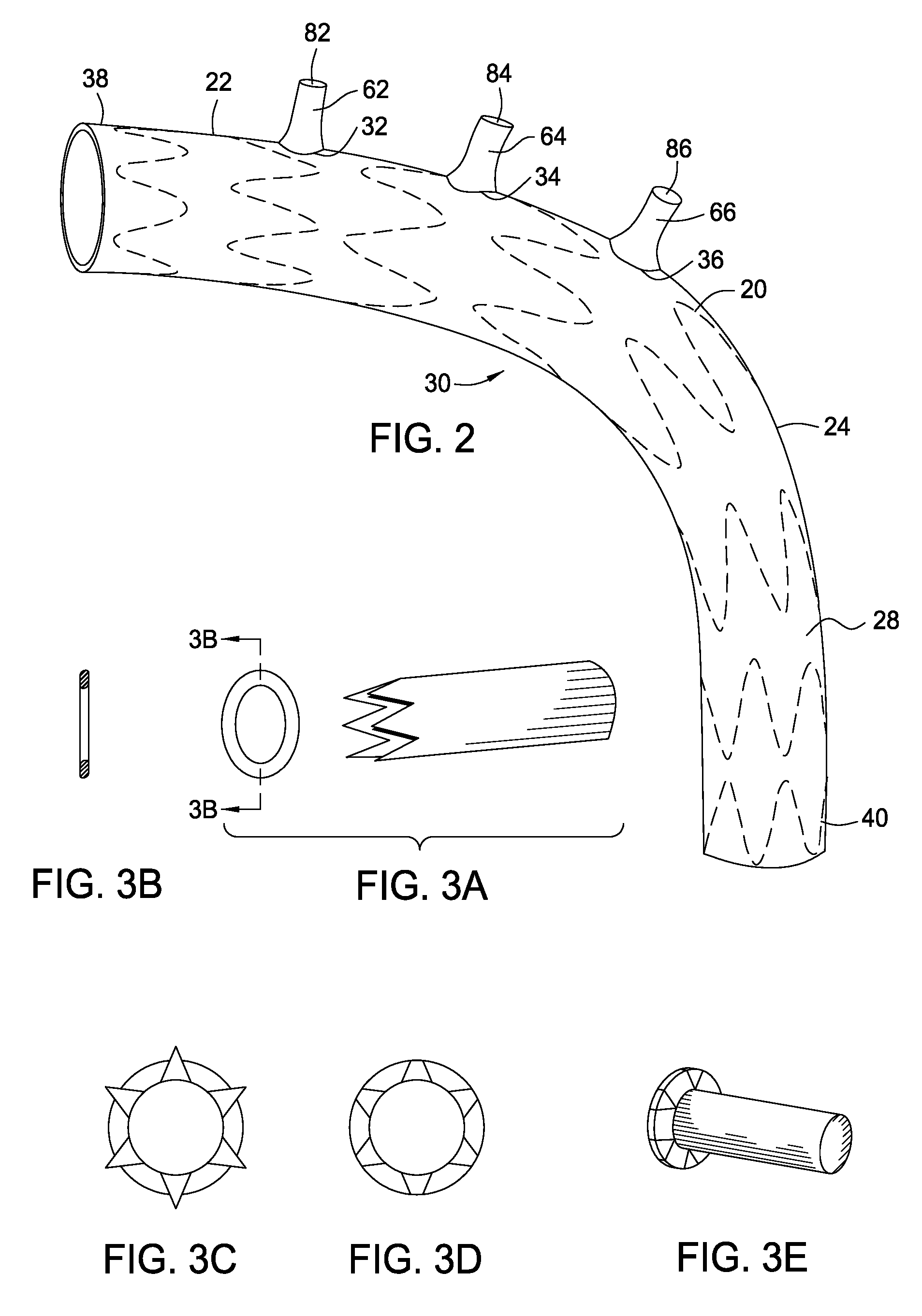
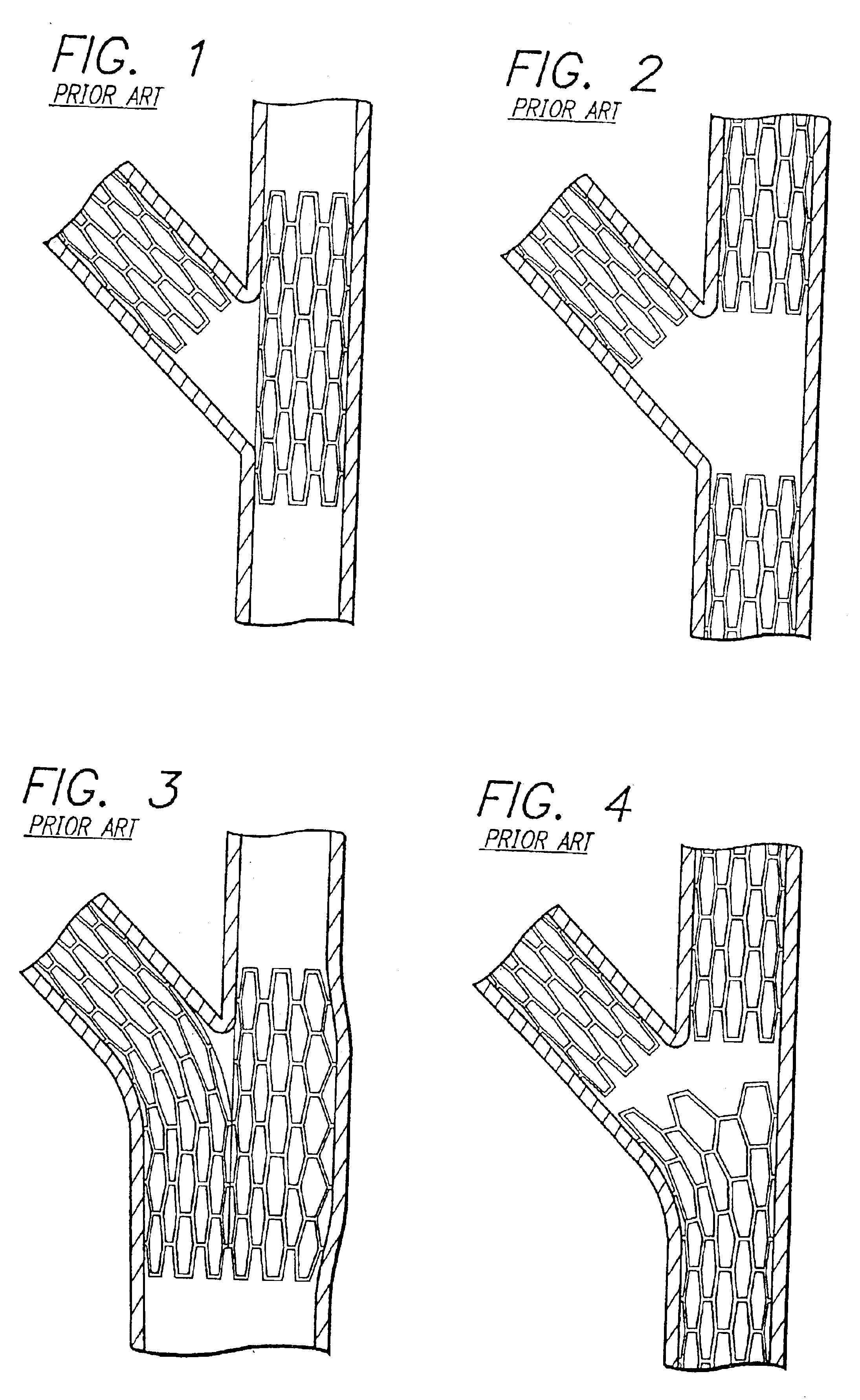
Stent and catheter assembly and method for treating bifurcations
An apparatus and method is provided for stenting bifurcated vessels. A proximal angled stent is configured for implanting in a side-branch vessel wherein the proximal angled stent has an angulated portion that corresponds to the angle formed by the intersection of the side-branch vessel and the main vessel so that all portions of the side-branch vessel at the bifurcation are covered by the proximal angled stent. A main-vessel stent is provided for implanting in the main vessel, wherein the main-vessel stent has an aperture or stent cell that aligns with the opening to the side-branch vessel to permit unobstructed blood flow between the main vessel and the side-branch vessel. Side-branch and main-vessel catheter assemblies are advanced over a pair of guide wires for delivering, appropriately orienting, and implanting the proximal angled stent and the apertured stent.
Owner:ABBOTT CARDIOVASCULAR
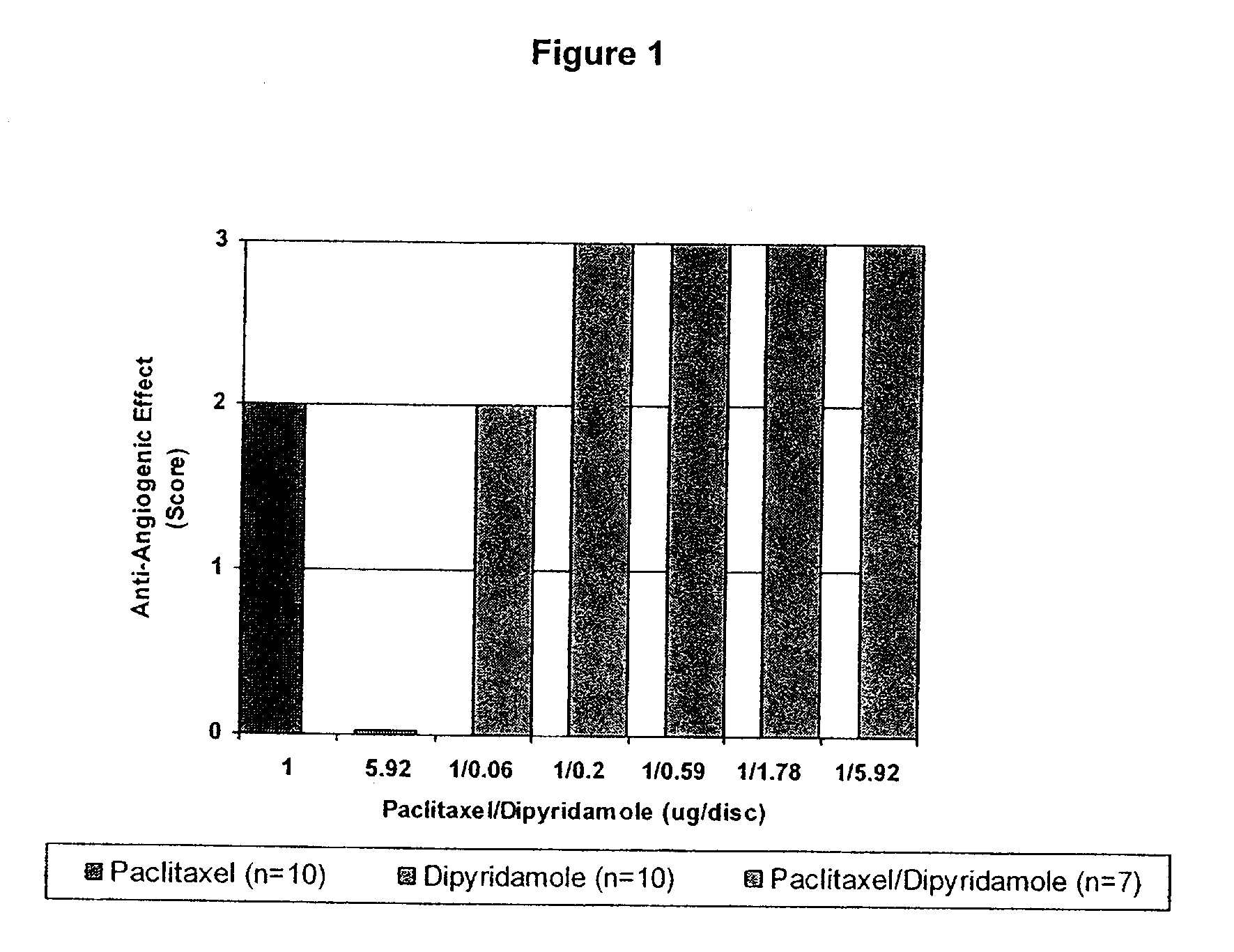
Medical implants with a combination of compounds
InactiveUS20100074934A1Minimize formationImprove biological effectOrganic active ingredientsBiocideDipyridamoleFibrosis
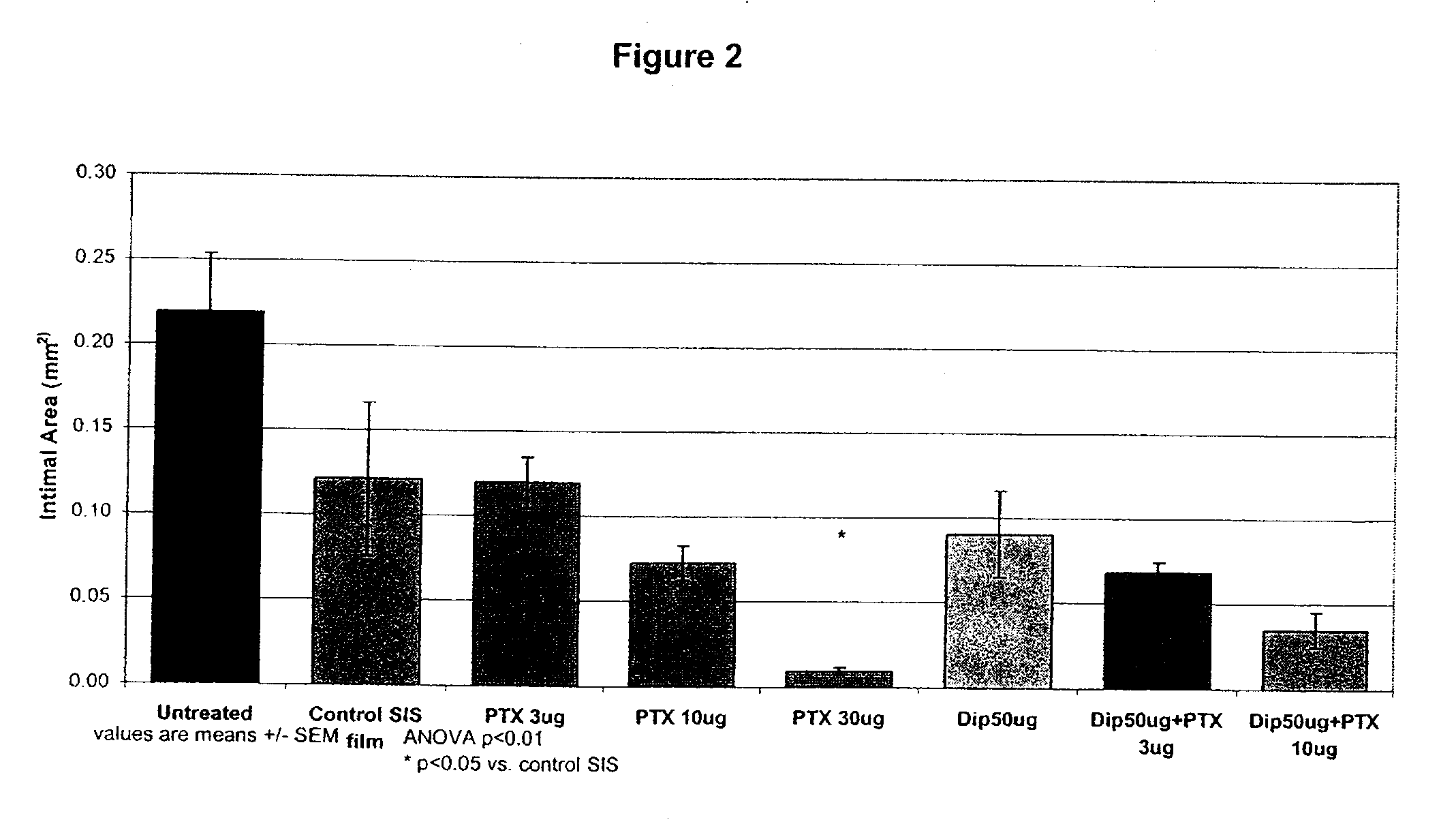
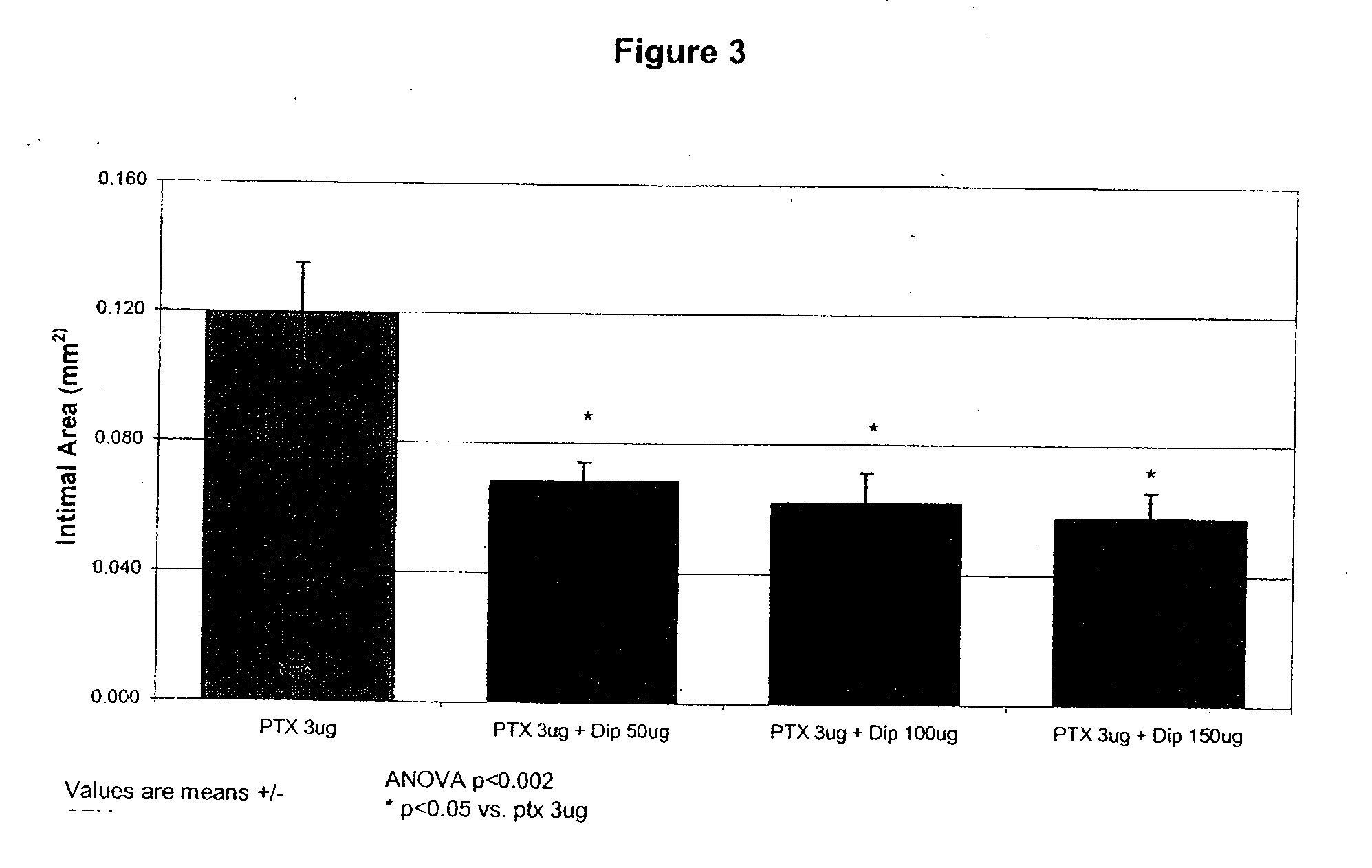
Implants are associated with a combination of paclitaxel or derivatives and dipyridamole or derivatives in order to inhibit fibrosis that may otherwise occur when the implant is placed within an animal. Exemplary implants include intravascular implants (e.g., coronary and peripheral vascular stents, catheters, balloons), non-vascular stents, pumps and sensors, vascular grafts, perivascular devices, implants for hemodialysis access, vena cava filters, implants for providing an anastomotic connection, electrical devices, intraocular implants, and soft tissue implants and fillers.
Owner:ANGIOTECH PHARMA INC
Stent and catheter assembly and method for treating bifurcations
An apparatus and method is provided for stenting bifurcated vessels. A proximal angled stent is configured for implanting in a side-branch vessel wherein the proximal angled stent has an angulated portion that corresponds to the angle formed by the intersection of the side-branch vessel and the main vessel so that all portions of the side-branch vessel at the bifurcation are covered by the proximal angled stent. A main-vessel stent is provided for implanting in the main vessel, wherein the main-vessel stent has an aperture or stent cell that aligns with the opening to the side-branch vessel to permit unobstructed blood flow between the main vessel and the side-branch vessel. Side-branch and main-vessel catheter assemblies are advanced over a pair of guide wires for delivering, appropriately orienting, and implanting the proximal angled stent and the apertured stent.
Owner:ABBOTT CARDIOVASCULAR
Elastin and elastin-based materials
It is a general object of the invention to provide a method of effecting repair or replacement or supporting a section of a body tissue. Specifically to provide an elastin or elastin-based biomaterial suitable for use as a stent, for example, a vascular stent, or as conduit replacement, as an artery, vein or a ureter replacement. The biomaterial can also be used as a stent or conduit covering or coating or lining. It is also an object of the invention to provide a method of securing an elastin or elastin-based biomaterial to an existing tissue without the use of sutures or staples.
Owner:PROVIDENCE HEALTH SYST OREGON AN OREGON NONPROFIT CORP +2
Medical devices and compositions for delivering biophosphonates to anatomical sites at risk for vascular disease
Methods, compositions and devices for inhibiting restenosis are provided. Specifically, bisphosphonate compositions and medical devices useful for the site specific delivery of bisphosphonates are disclosed. In one embodiment the medical device is a vascular stent coated with a bisphosphonate selected from the group consisting of zolendronate and pamedronate and derivatives and analogues thereof. In another embodiment an injection catheter for delivery an anti-restenotic effective amount of bisphosphonate to the adventitia is provided.
Owner:MEDTRONIC VASCULAR INC
Bioactive stents for type II diabetics and methods for use thereof
InactiveUS20060013855A1StentsImmunoglobulins against cell receptors/antigens/surface-determinantsProgenitorBlood vessel
The present invention is based on the discovery that a vascular stent or other implantable medical device can be coated with a biodegradable biocompatible polymer to which is attached a bioligand that specifically captures progenitors of endothelial cells (PECs) from the circulating blood to promote endogenous formation of healthy endothelium in Type II diabetics. In one embodiment, the bioligand is a peptide that specifically binds to an integrin receptor on PECs. The invention also provides methods for using such vascular stents and other implantable devices to promote vascular healing in Type II diabetics, for example following mechanical intervention.
Owner:MEDIVAS LLC
Bioactive stents for type II diabetics and methods for use thereof
The present invention is based on the discovery that a vascular stent or other implantable medical device can be coated with a biodegradable biocompatible polymer to which is attached a bioligand that specifically captures progenitors of endothelial cells (PECs) from the circulating blood to promote endogenous formation of healthy endothelium in Type II diabetics. In one embodiment, the bioligand is a peptide that specifically binds to an integrin receptor on PECs. The invention also provides methods for using such vascular stents and other implantable devices to promote vascular healing in Type II diabetics, for example following mechanical intervention.
Owner:MEDIVAS LLC
Intraluminal prosthesis attachment systems and methods
Owner:MEDTRONIC VASCULAR INC
Nanoporous layers using thermal dealloying
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Medical devices having nanoporous bonding layers
The present invention relates generally to medical devices with therapy eluting components and methods for making same. More specifically, the invention relates to implantable medical devices having at least one porous layer, and methods for making such devices, and loading such devices with therapeutic agents. A mixture or alloy is placed on the surface of a medical device, then one component of the mixture or alloy is generally removed without generally removing the other components of the mixture or alloy. In some embodiments, a porous layer is adapted for bonding non-metallic coating, including drug eluting polymeric coatings. A porous layer may have a random pore structure or an oriented or directional grain porous structure. One embodiment of the invention relates to medical devices, including vascular stents, having at least one porous layer adapted to resist stenosis or cellular proliferation without requiring elution of therapeutic agents. The invention also includes methods, devices, and specifications for loading of drugs and other therapeutic agents into nanoporous coatings.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Method and system for modifying the wettability characteristics of a surface of a medical device by the application of gas cluster ion beam technology and medical devices made thereby
InactiveUS20090074834A1Improve wettabilityLow wettabilityElectric discharge tubesPharmaceutical containersEtchingGas cluster ion beam
Irradiation of a surface of a material with a gas cluster ion beam modifies the wettability of the surface. The wettability may be increased or decreased dependent on the characteristics of the gas cluster ion beam. Improvements in wettability of a surface by the invention exceed those obtained by conventional plasma cleaning or etching. The improvements may be applied to surfaces of medical devices, such as vascular stents for example, and may be used to enable better wetting of medical device surfaces with liquid drugs in preparation for adhesion of the drug to the device surfaces. A mask may be used to limit processing to a portion of the surface. Medical devices formed by using the methods of the invention are disclosed.
Owner:EXOGENESIS CORP
Drug formulations for coating medical devices
The present invention relates to oil-based formulations of hydrophobic drugs for the uniform coating of medical devices such as vascular stents and balloons. Another aspect of the present invention is an intravascular medical device having an oil-based coating suitable for delivering a water-insoluble drug, comprising one or more of an anti-oxidant, an anti-inflammatory and an anti-restenotic agent.
Owner:REVA MEDICAL LLC
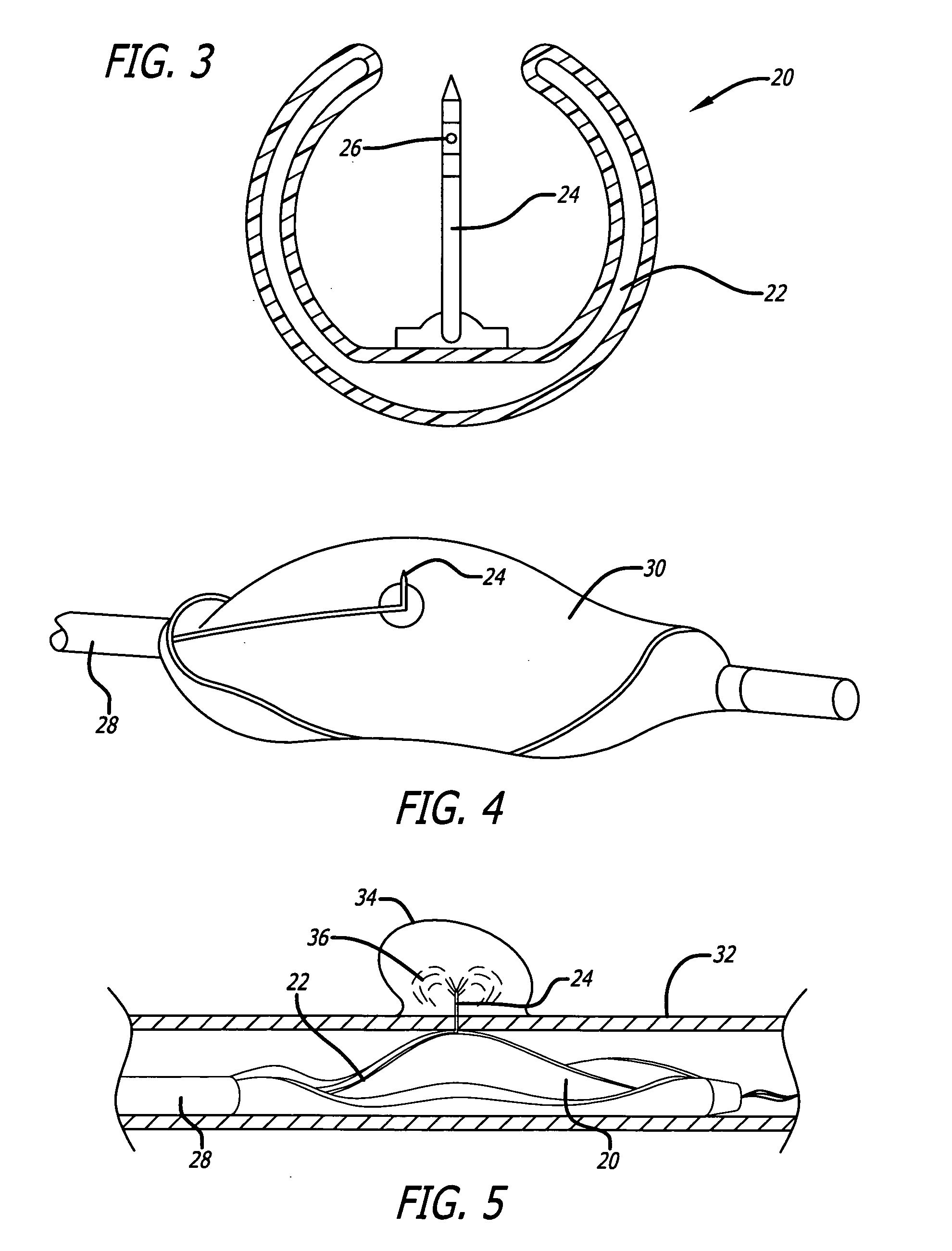
Methods and Apparatus for Treatment of Aneurysms Adjacent to Branch Arteries
InactiveUS20070208410A1Inhibit migrationExcludes regionStentsBlood vesselsStent graftingInsertion stent
Methods and apparatus for delivering and reducing the profile of a catheter for delivering a stent graft including integral branch connections. A configuration of a branch vessel stent graft, including a branch vessel connection connecting a very thin walled PTFE tubular branch to a woven polyester main body. The connection is made by using by overmolding the PTFE on a polymer ring, such as silicone. In another configuration, a main body portion includes branch portions where ends of the branch portions are connected to leads extending from a sheath of a stent graft compressed in a delivery catheter. The leads are routed into accessible branches of body lumens and act as pullwires to pull the branch portions into position in their respective branches as the delivery catheter is released to deploy the main body of the stent graft. Apertures in the main body portion are alignable with the branch lumens and an anchoring stent is separately deployed to extend and / or main the extension of the branch portion in the branch lumen and anchor it therein.
Owner:MEDTRONIC VASCULAR INC
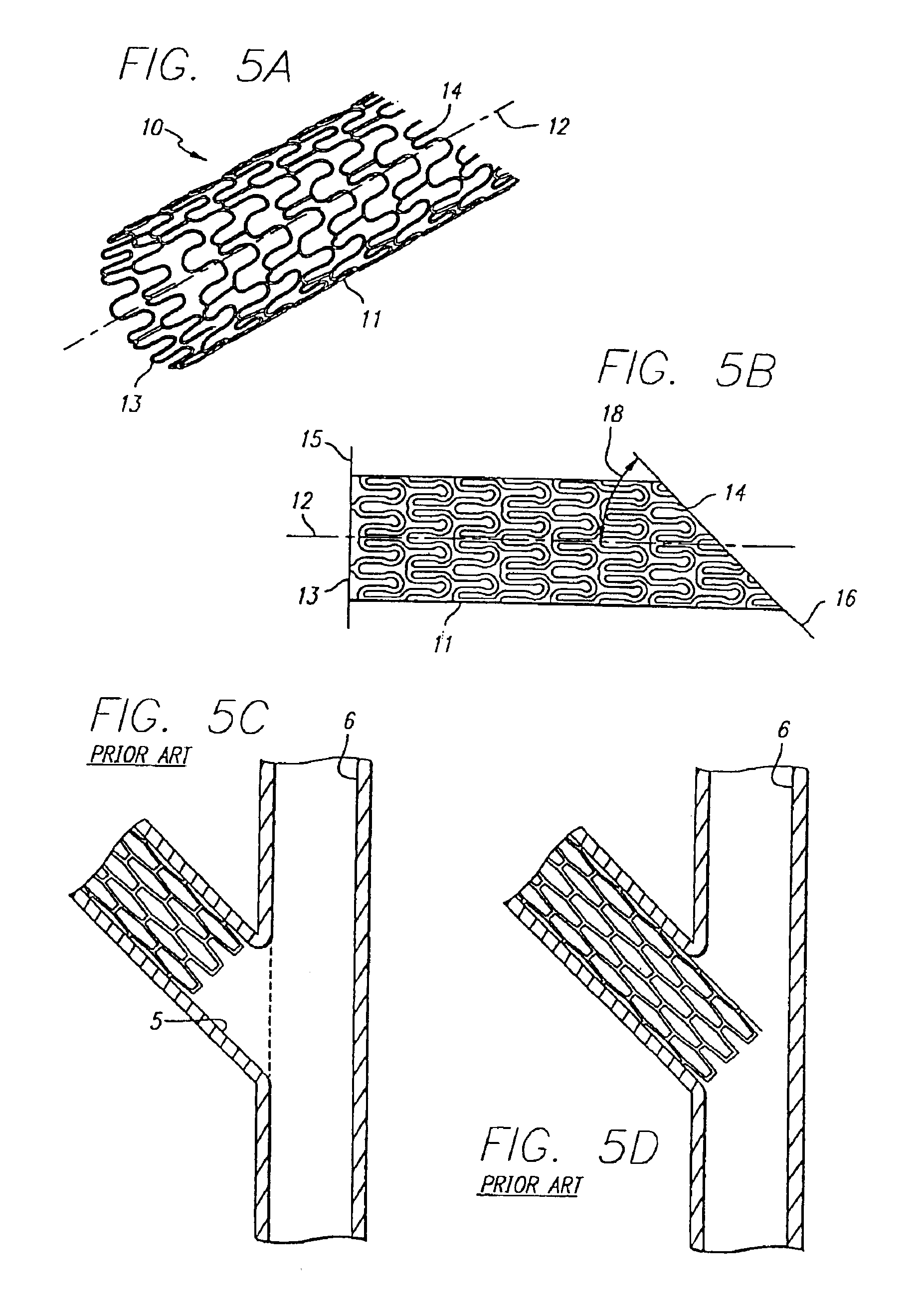
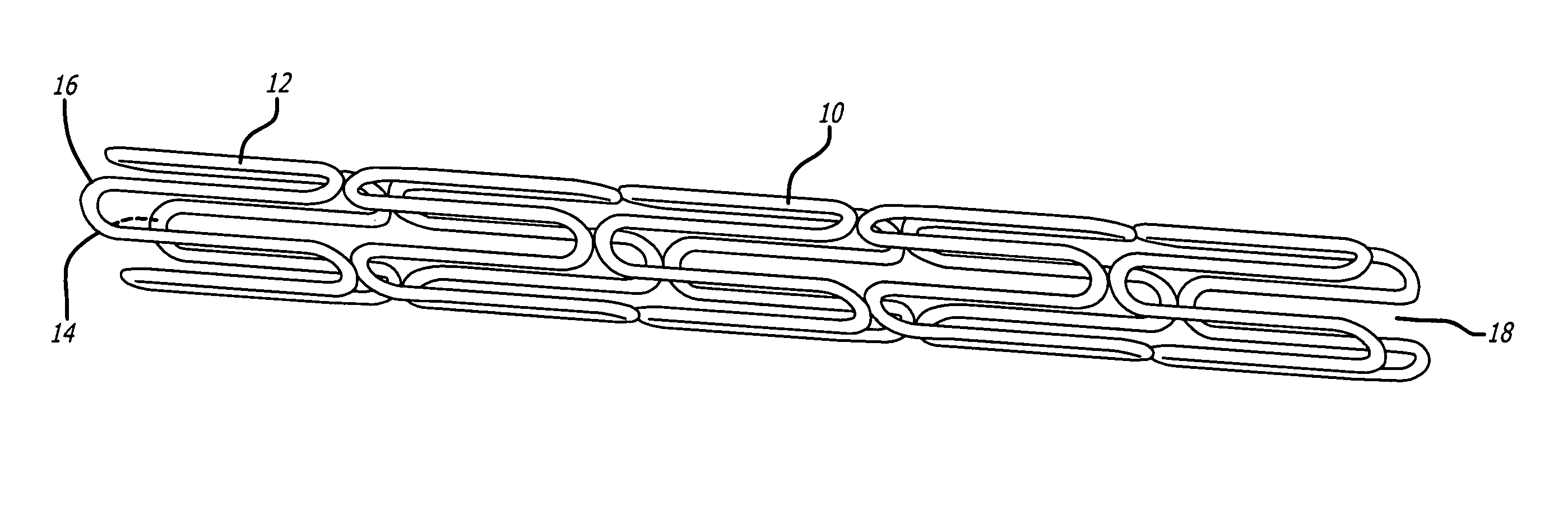
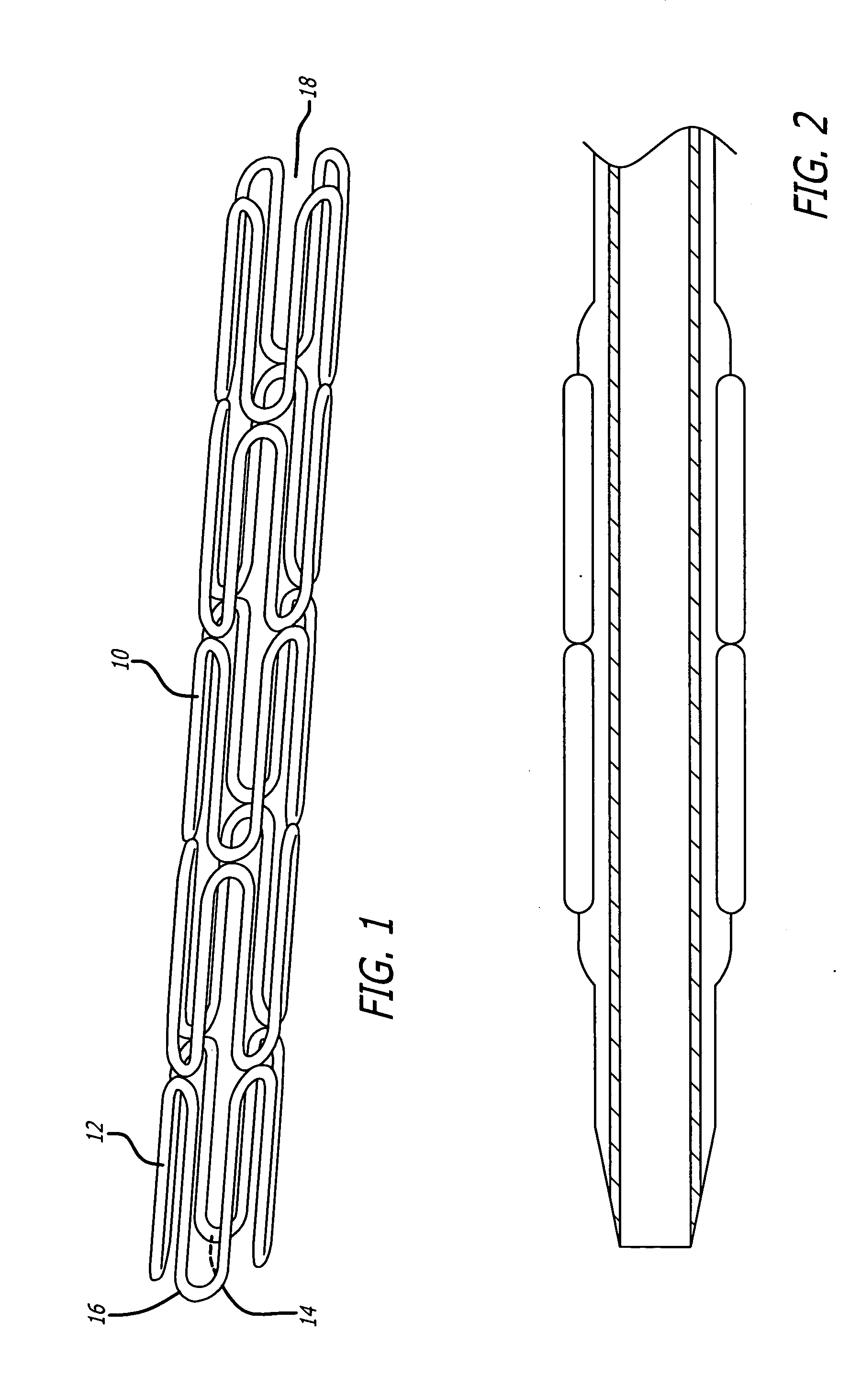
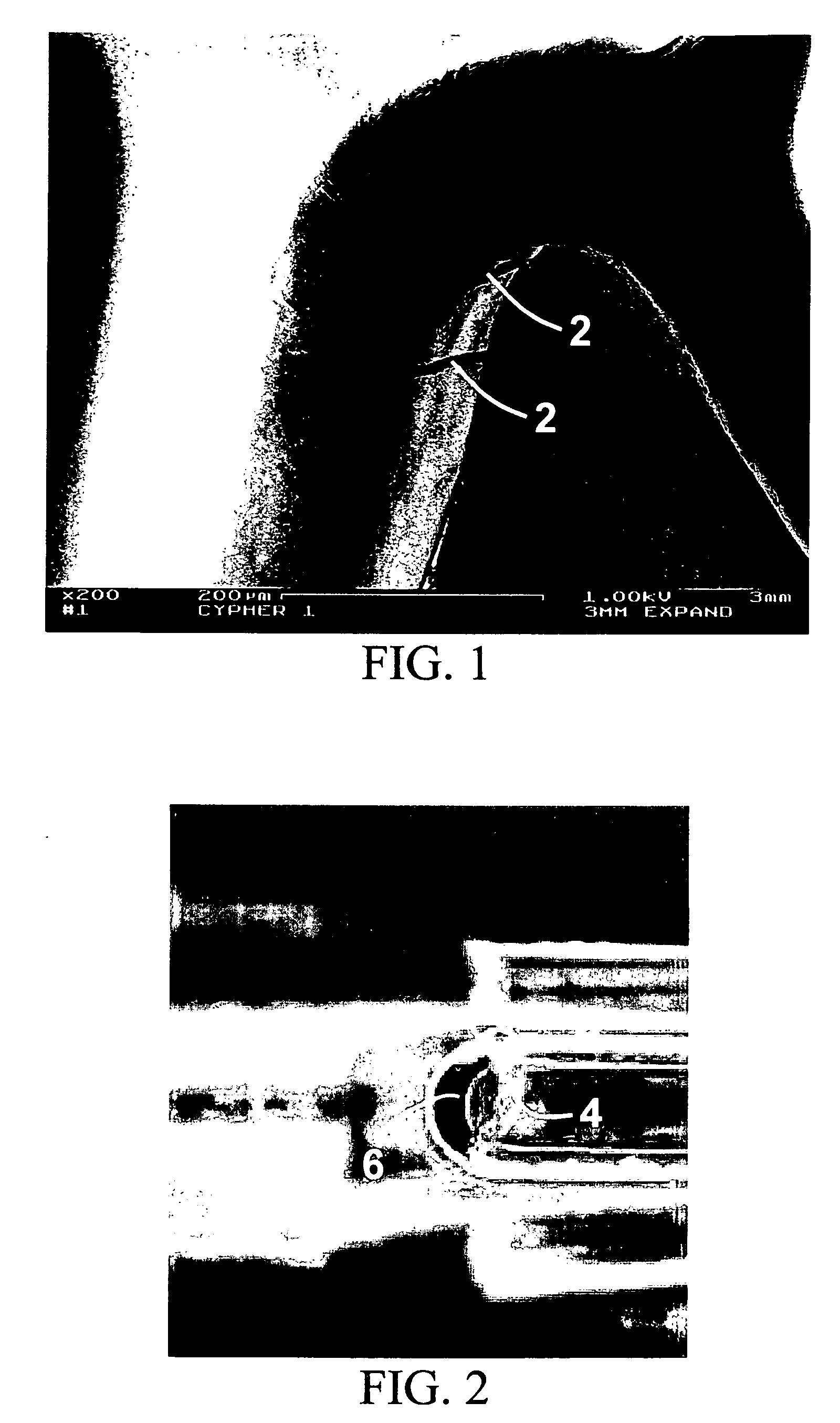
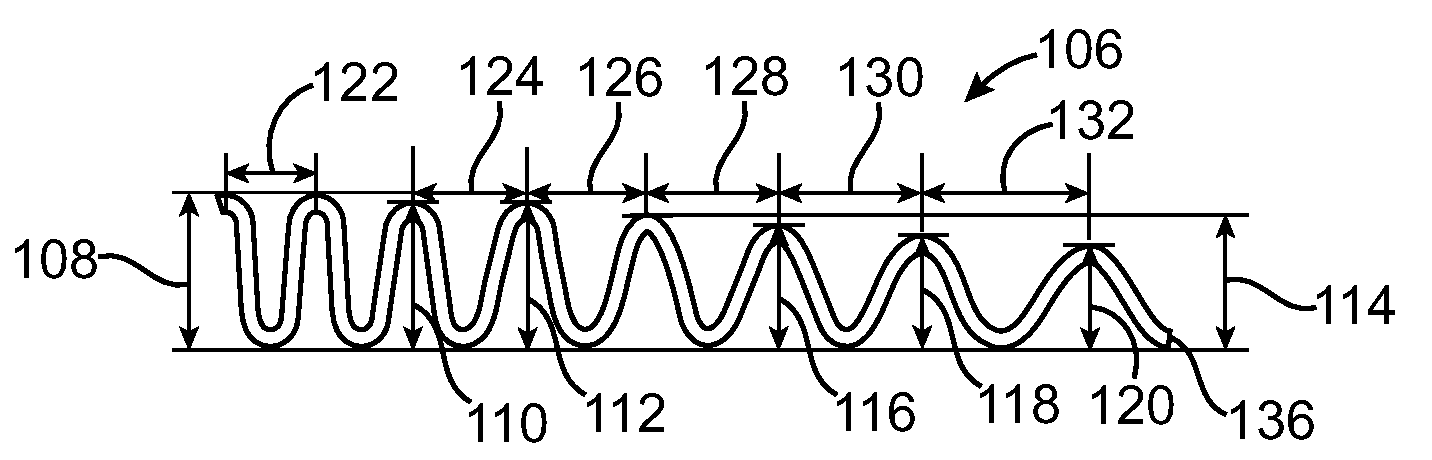
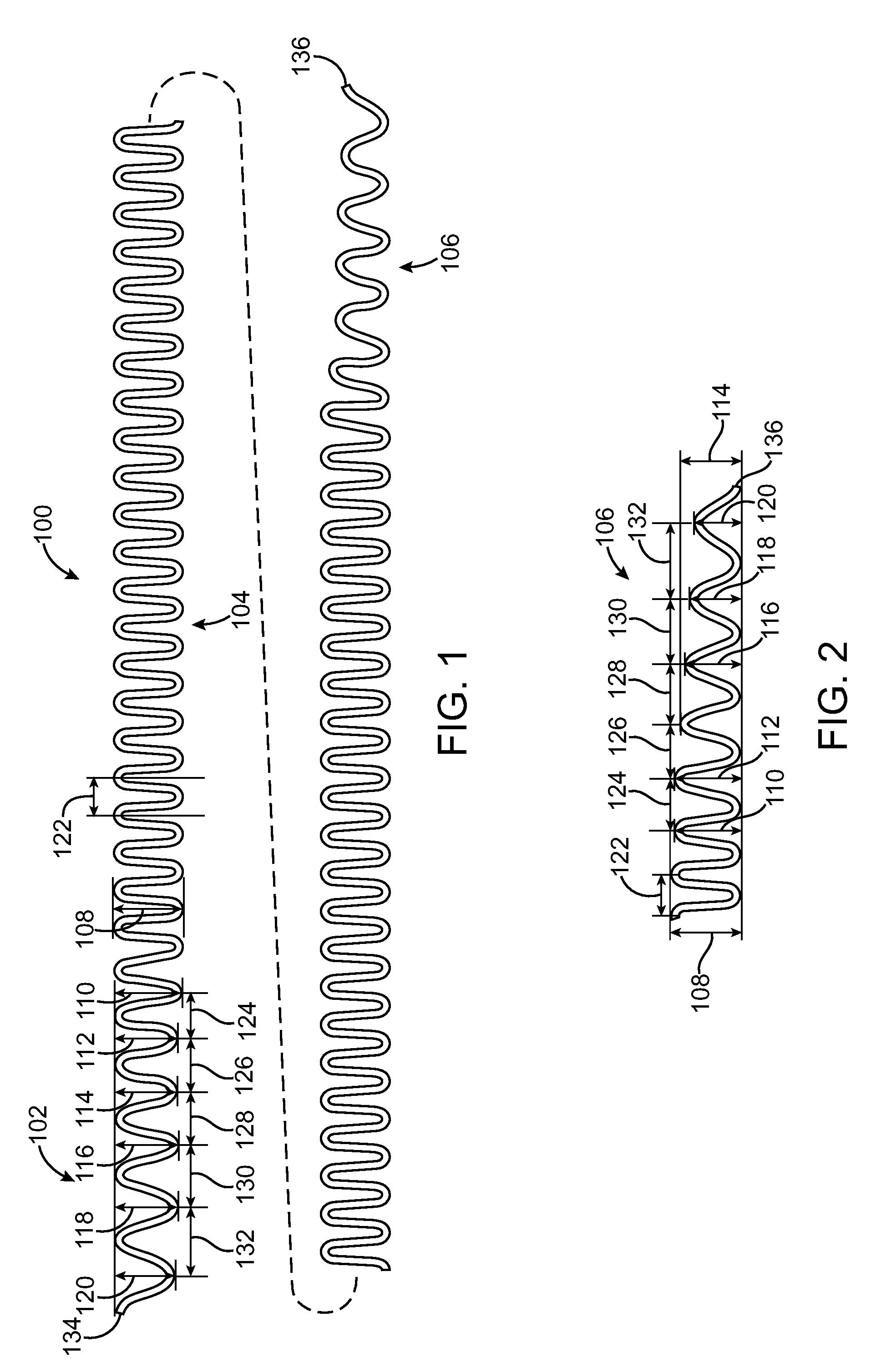
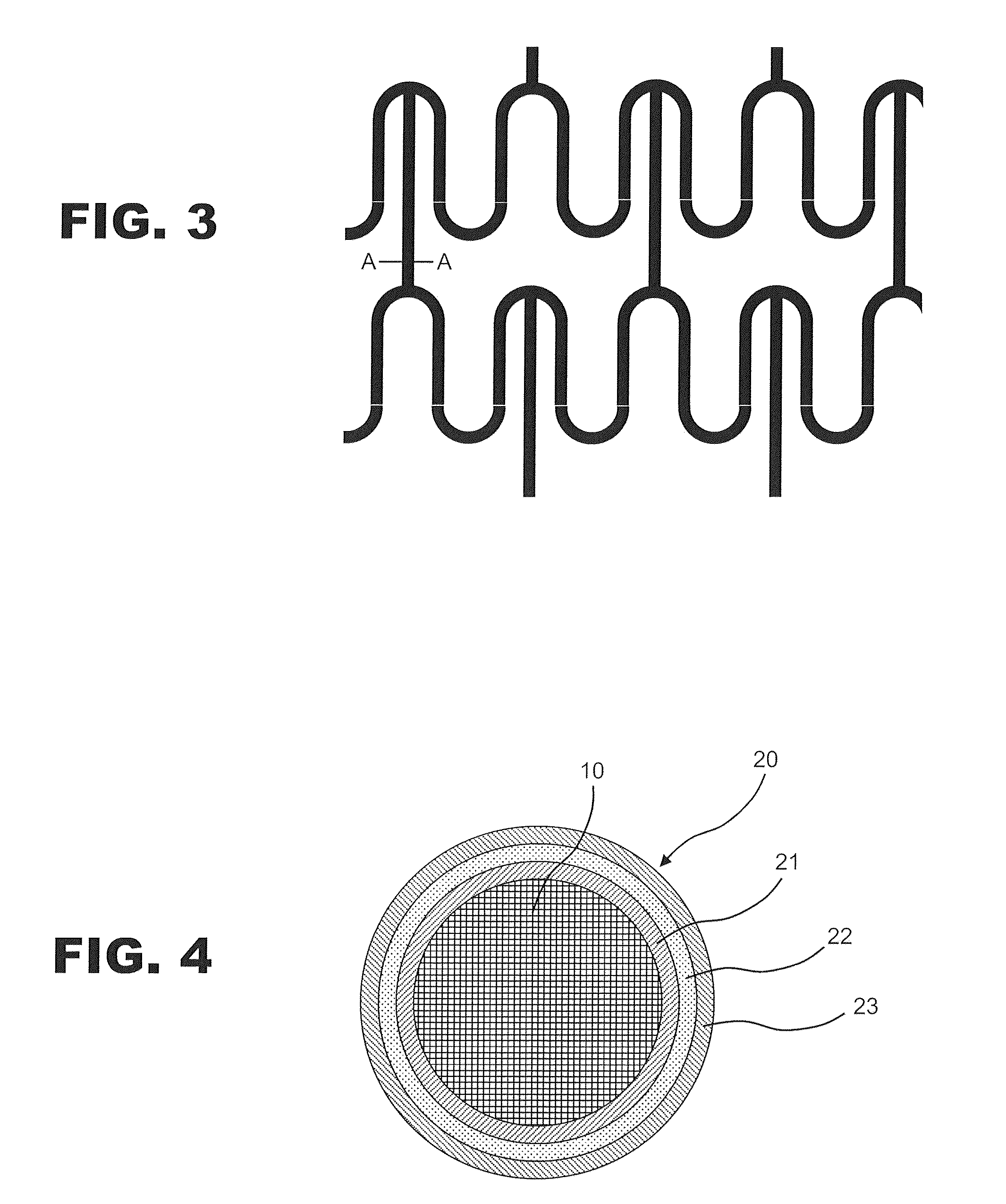
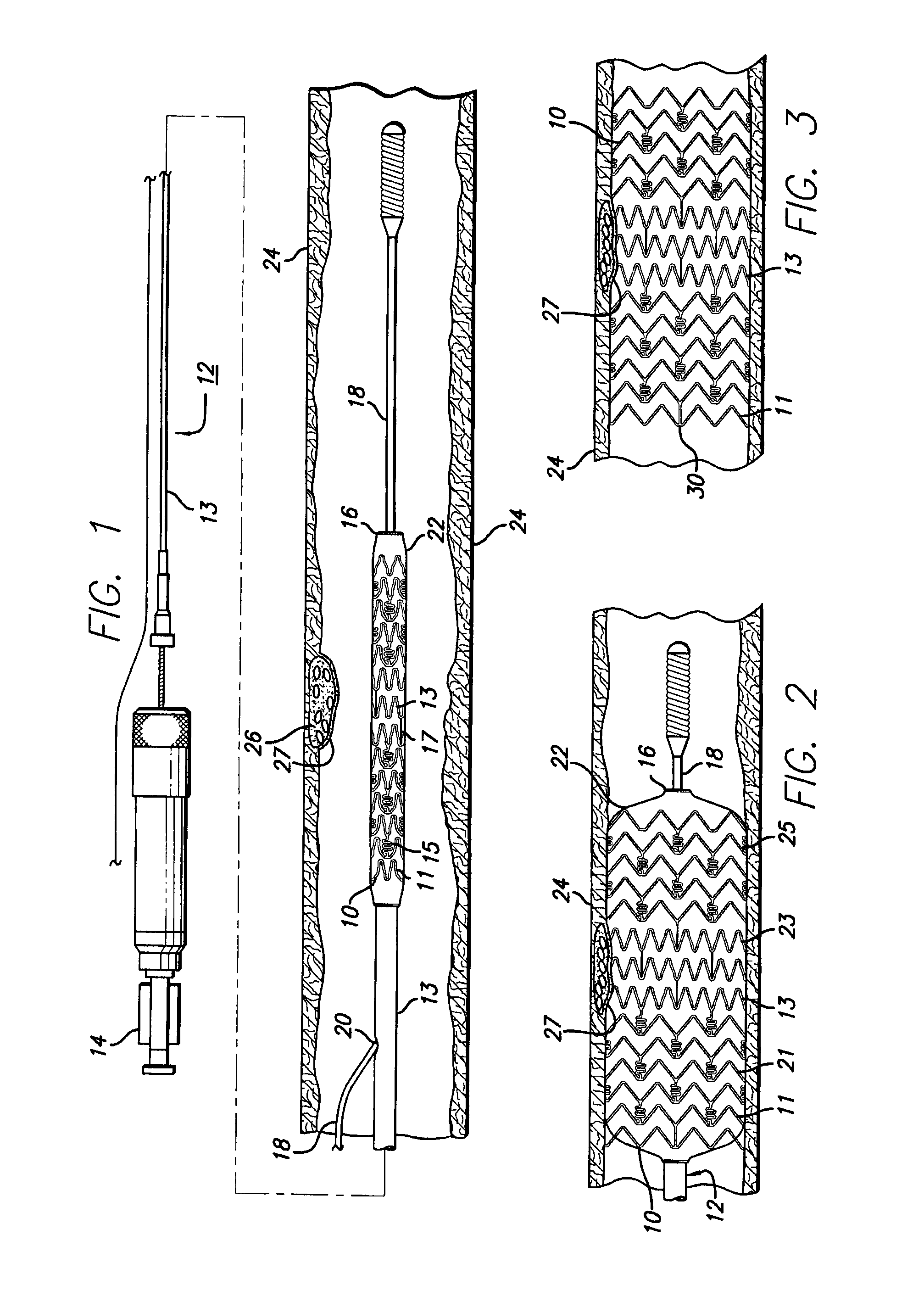
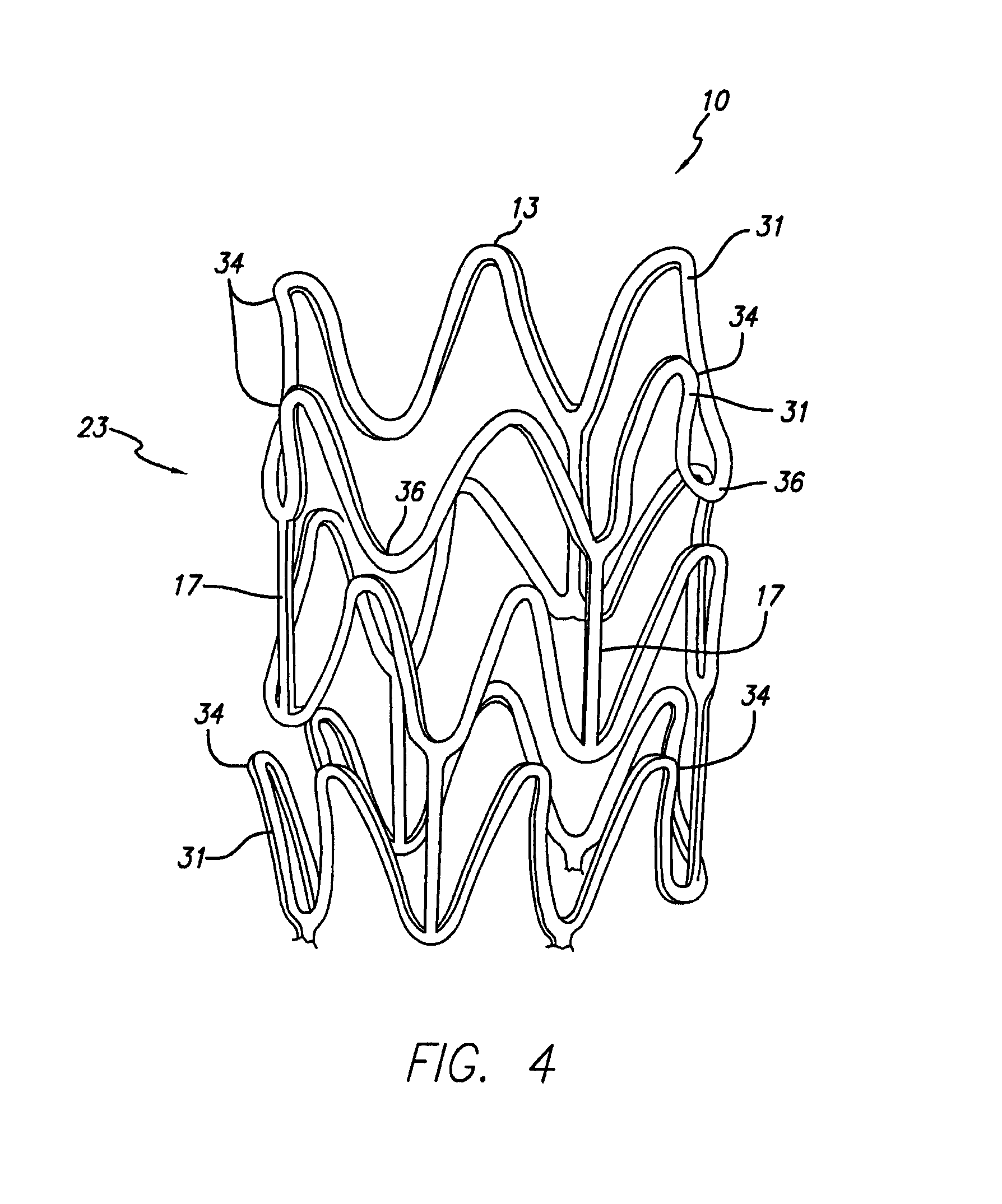
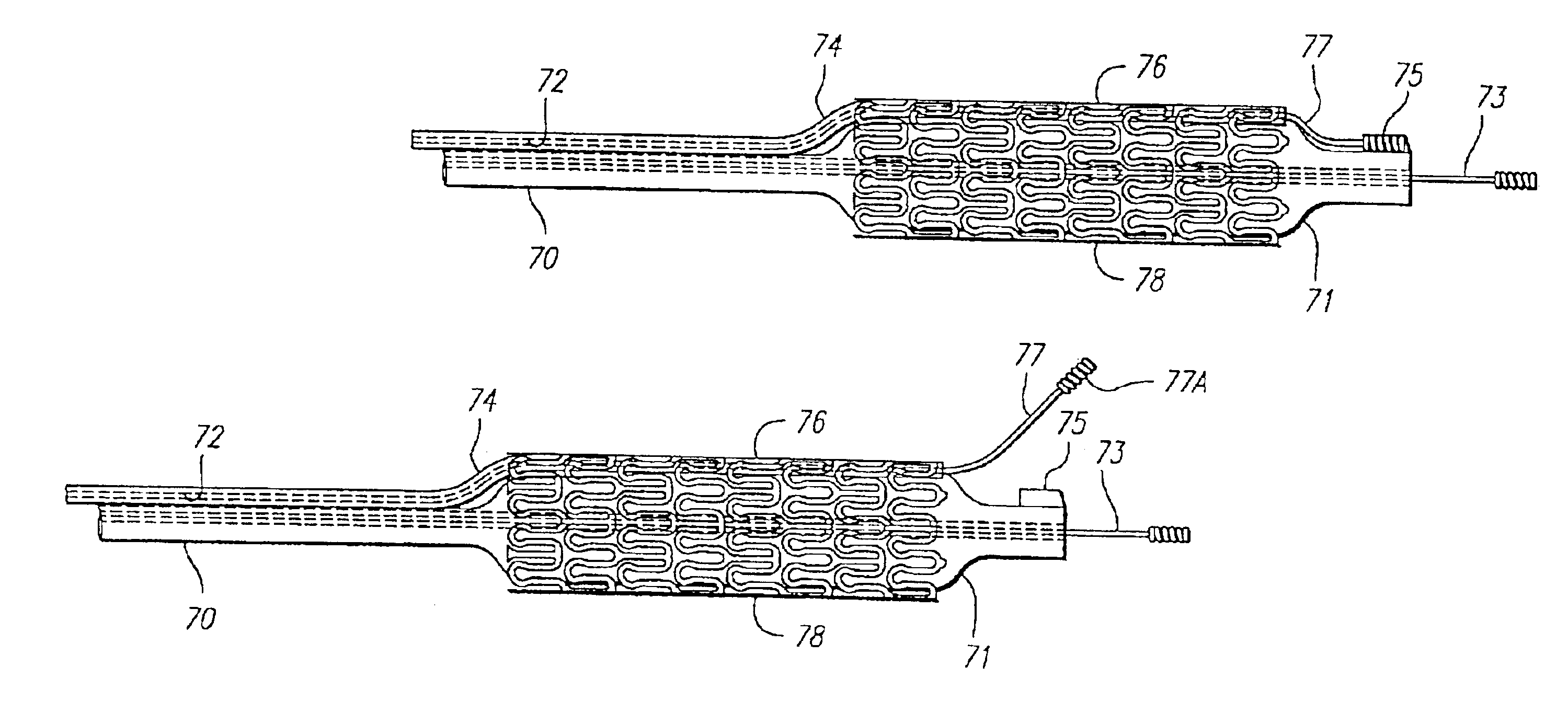
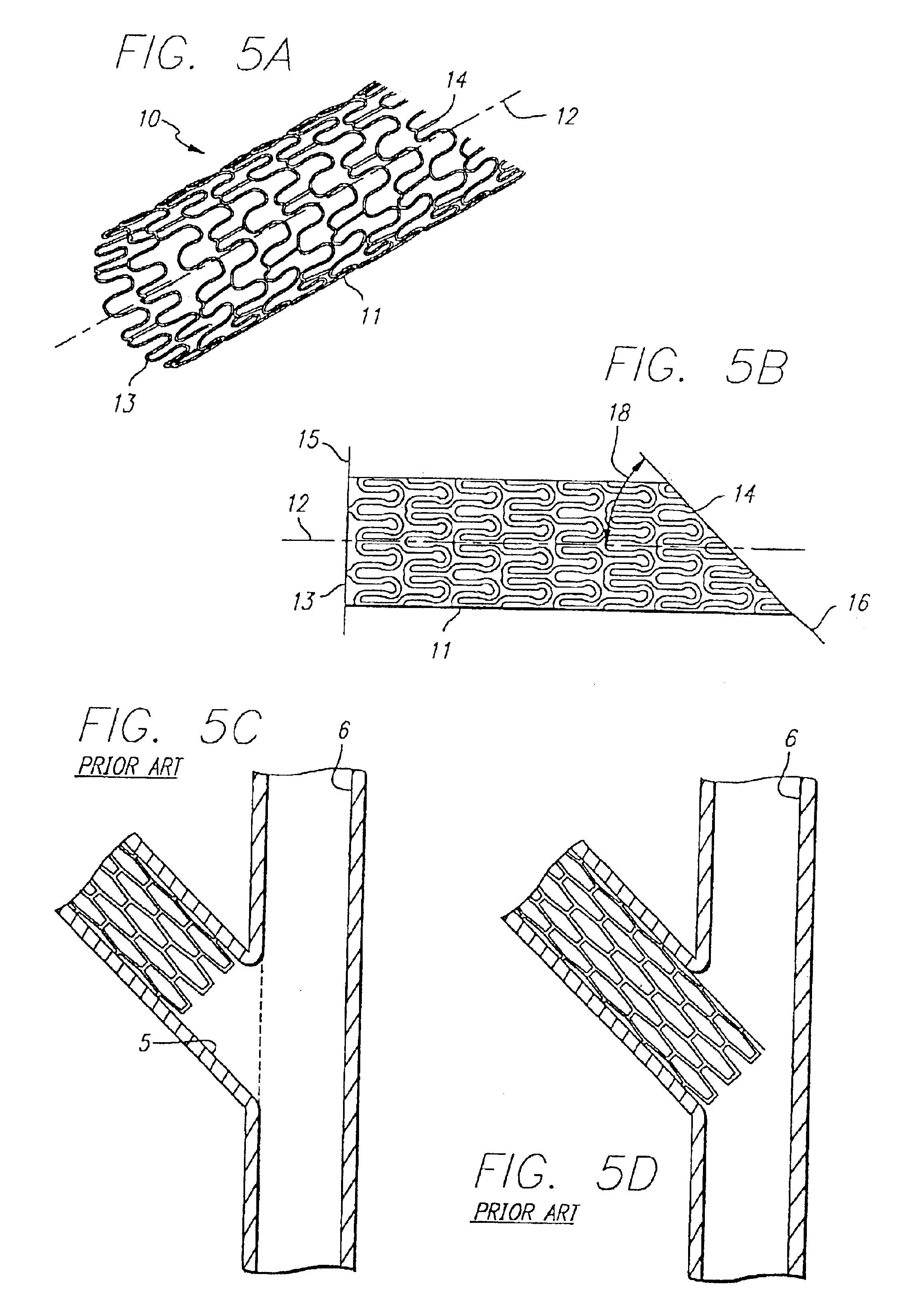
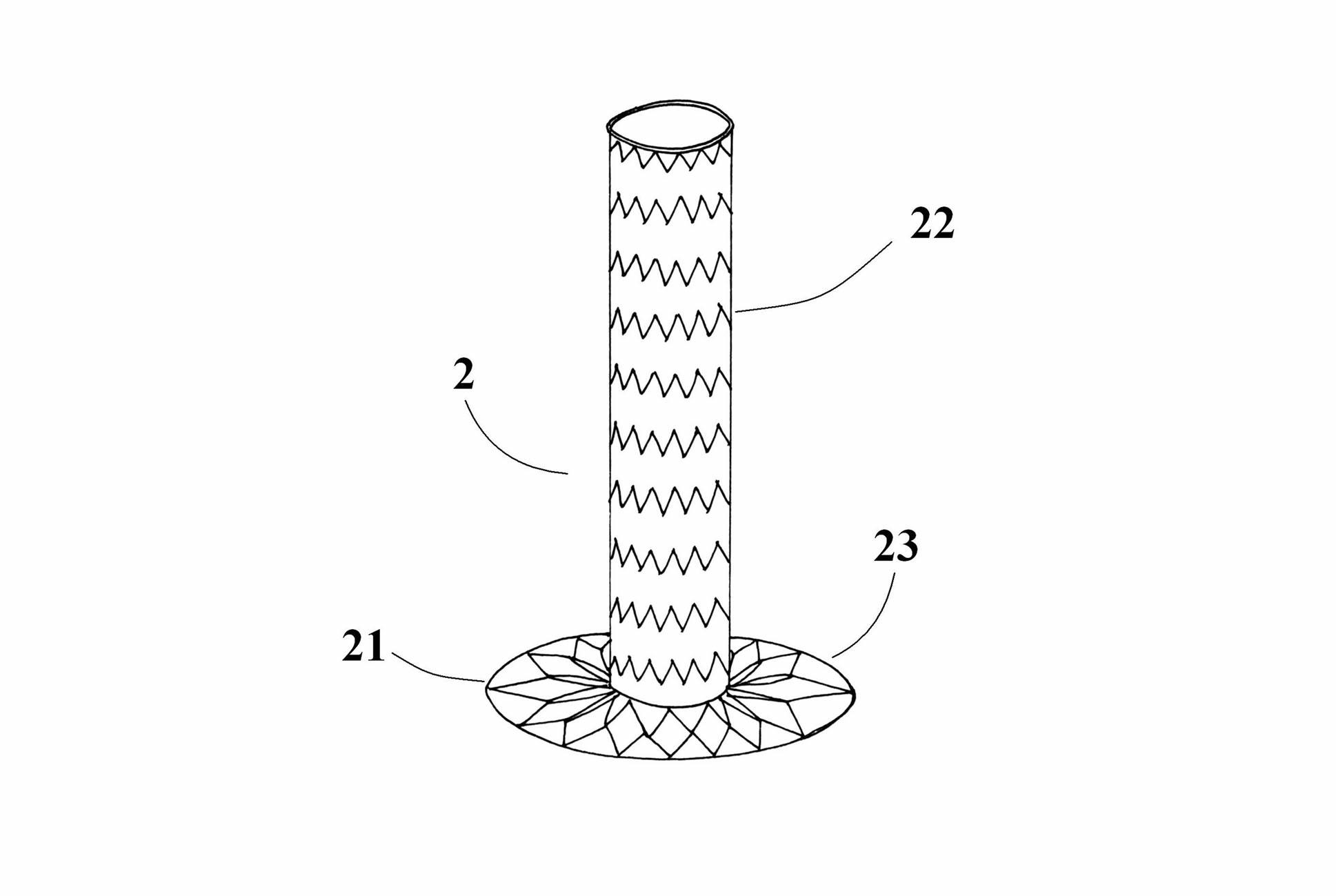
Vascular Stent and Method of Making Vascular Stent
A stent and method of forming a stent include a wire bent into a waveform spirally wrapped into a hollow cylindrical shape. The waveform includes a first end portion, a middle portion, and a second end portion. The middle portion of the waveform includes a first amplitude and a first period. The first end portion of the waveform includes a first plurality of amplitudes and a first plurality of periods, wherein the first plurality of amplitudes decrease from adjacent the middle portion to a first end of the wire and first plurality of frequencies increase from adjacent the middle portion to the first end of the wire. The waveform may also include a second end portion with a second plurality of amplitudes and a second plurality of periods, wherein the second plurality of amplitudes decrease from adjacent the middle portion to a second end of the wire and the second plurality of frequencies increase from adjacent the middle portion to the second end of the wire.
Owner:MEDTRONIC VASCULAR INC
Vascular stent
Owner:GELITA AG
Reduction of adverse inflammation
InactiveUS20050025804A1Reduce the possibilityReduction of the flux of nutrientsBiocideNervous disorderPeroxynitriteIsomerization
Reduction of the likelihood of adverse inflammatory reaction to an implant or a transplant is achieved through several mechanisms including the catalysis of isomerization of peroxynitrite by a hydrogel-bound peroxynitrite isomerization catalysts. A second mechanism controls acceptable and unacceptable dimensions of surface features of implants, such as vascular stents. A third mechanism fabricates implants from materials which are substantially free from alloys transition metals which produce ions of which catalyze cell killing radical formation.
Owner:HELLER ADAM
Stent for treating vulnerable plaque
InactiveUS7273492B2Reducing cap stressReduce stressStentsBlood vesselsCoronary arteriesVulnerable plaque
An intravascular stent assembly for implantation in a body lumen, such as a coronary artery, is designed to treat a lesion with vulnerable plaque by reducing the fibrous cap stresses. The stent includes distal, proximal, and center sections where the center section is configured to treat the vulnerable plaque. The stent consists of radially expandable cylindrical rings generally aligned on a common longitudinal stent axis and either directly connected or interconnected by one or more interconnecting links placed so that the stent is flexible in the longitudinal direction while providing high degrees of radial strength and vessel scaffolding.
Owner:ABBOTT CARDIOVASCULAR
Medical devices and compositions useful for treating or inhibiting restenosis
InactiveUS20060099235A1Reduce needPrevent restenosisBiocideGenetic material ingredientsPercent Diameter StenosisMedical device
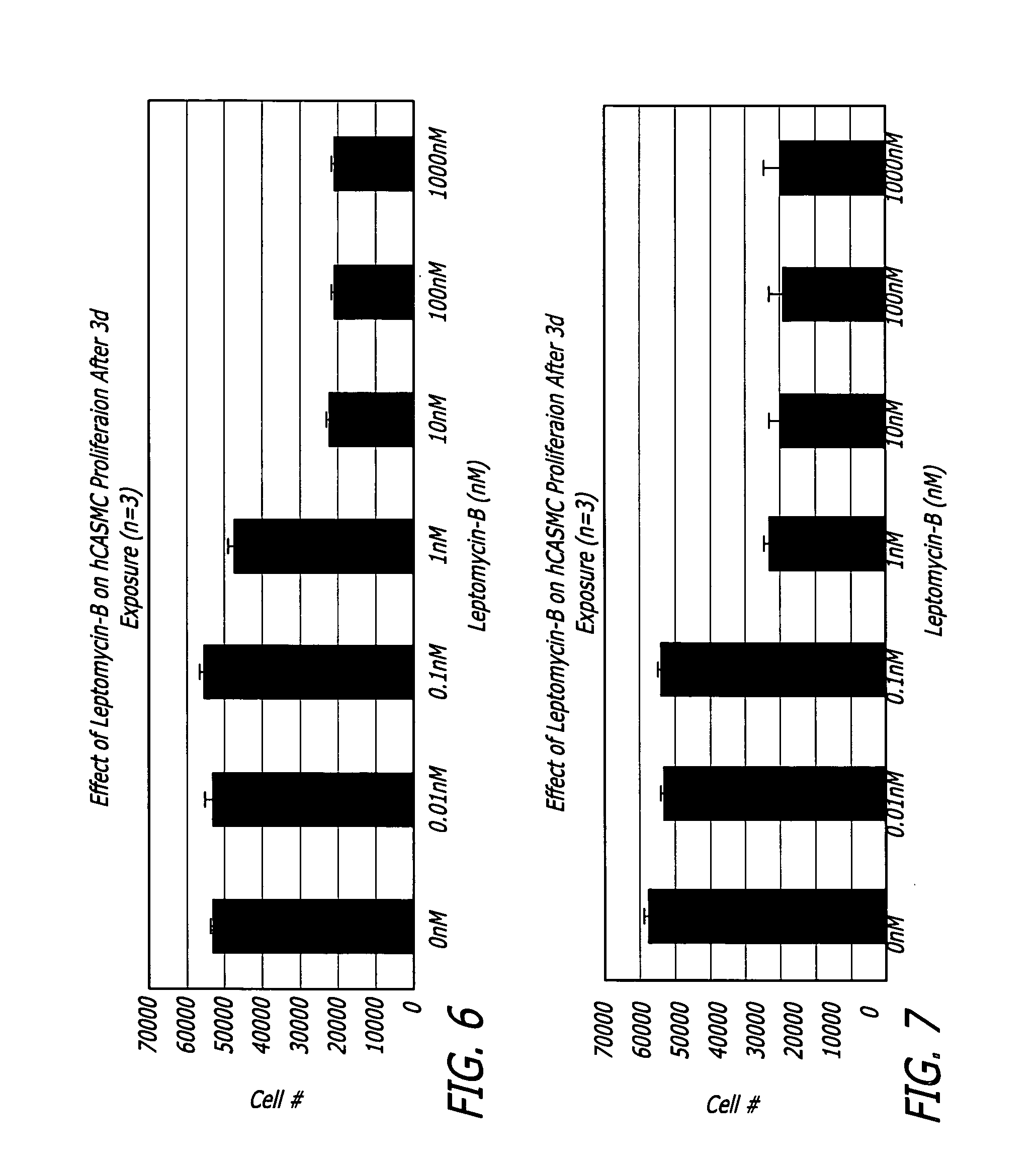
Medical devices and related methods for making and using same suitable for treating or inhibiting restenosis are proved. Specifically, compositions and methods for I kappa B alpha (IkBα)nuclear factor kβ (NFkβ) complex breakdown inhibition are provided. One embodiment includes a CRM-1 protein binding composition such as leptomycin B. Another embodiment includes a combination of a CRM-1 protein binding composition and a nucleic acid encoding for mammalian IkBα. Medical devices disclosed include catheters and vascular stents.
Owner:MEDTRONIC VASCULAR INC
Hydrogel entrapping therapeutic agent and stent with coating comprising this
A hydrogel forming system which comprises a hydrophobic macromer with unsaturated group terminated ends and a hydrophilic polymer which is a polysaccharide containing hydroxy groups which are reacted with unsaturated group introducing compound, is convertible by free radical polymerization to form a hydrogel containing a three dimensional crosslinked polymer network containing hydrophobic and hydrophilic components. Agent can be entrapped in the polymer network, e.g., drugs, macromolecules or synthetic or natural polymers, for controlled release therefrom. In one embodiment, a vascular stent is coated with hydrogel with therapeutic agent entrapped therein.
Owner:CORNELL RES FOUNDATION INC
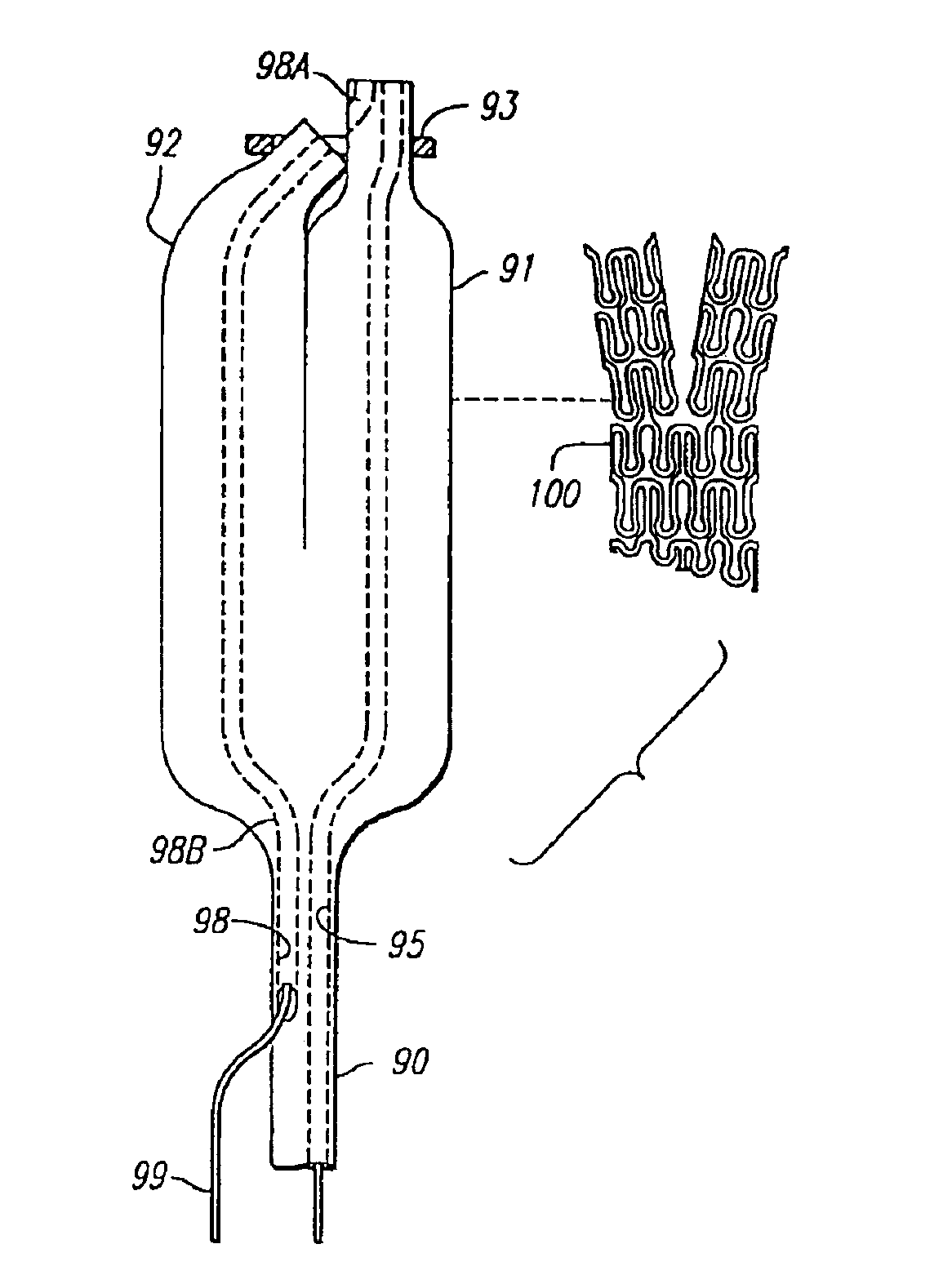
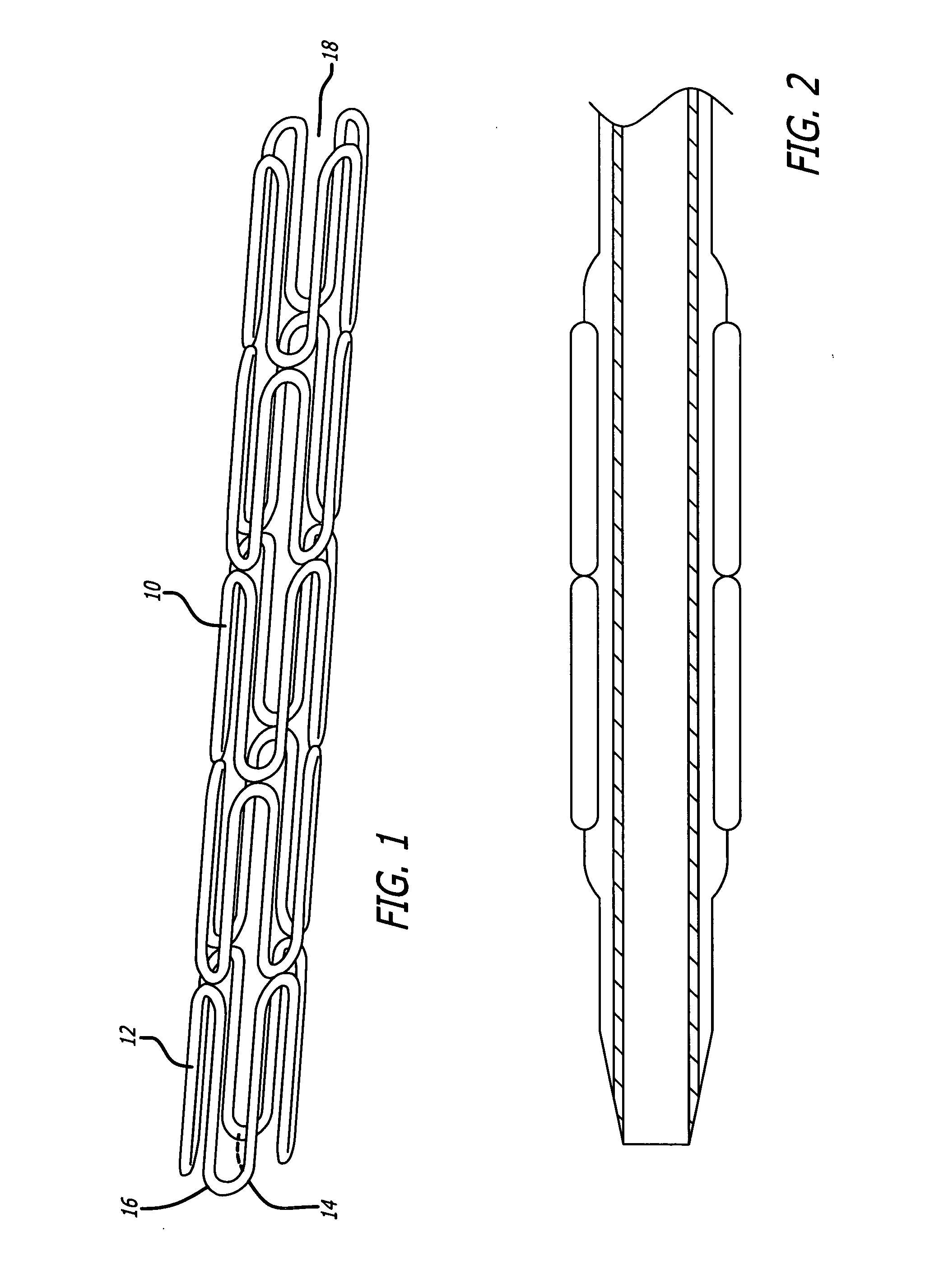
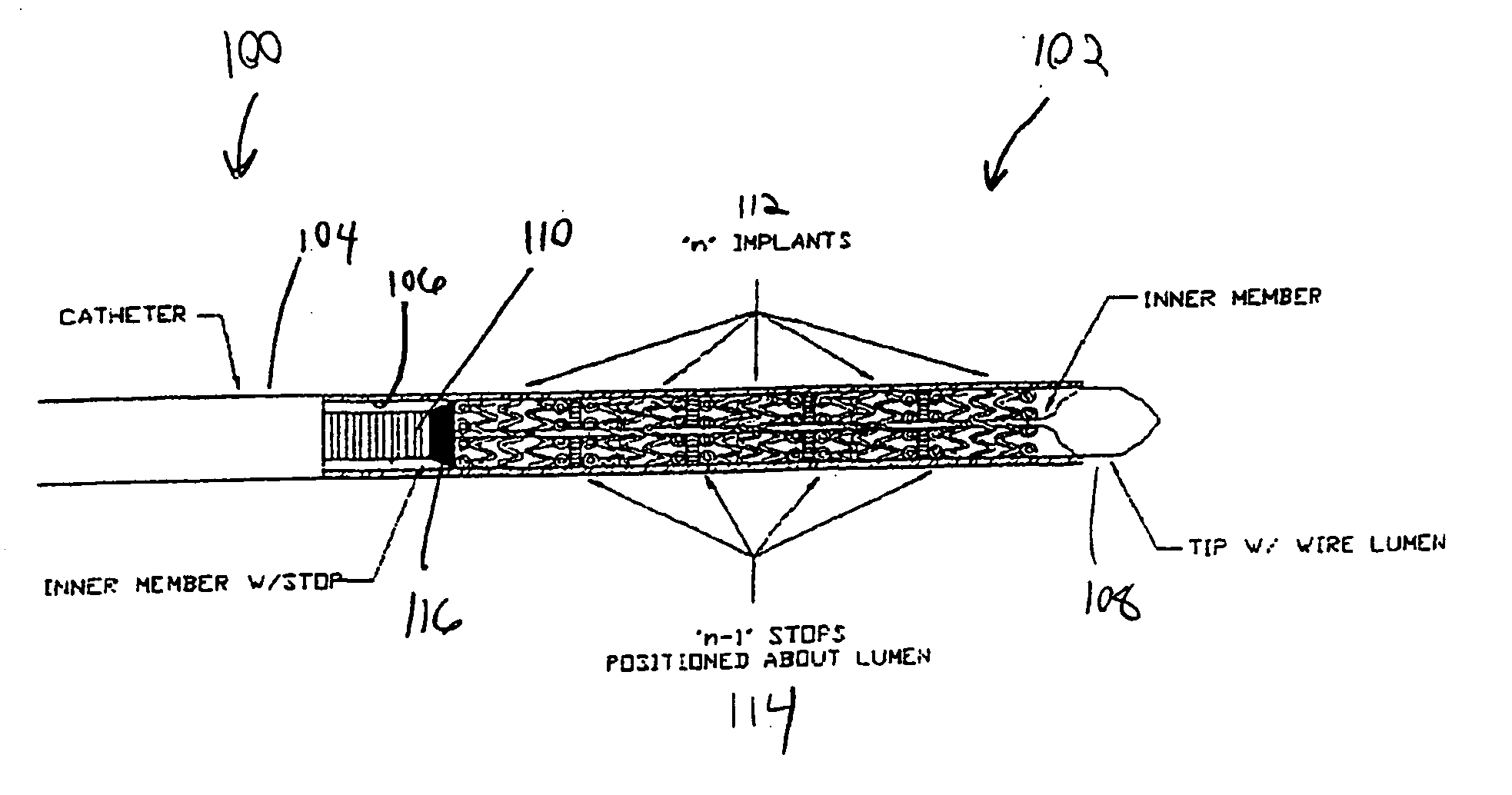
Multiple in vivo implant delivery device
A delivery catheter for providing the percutaneous delivery of a plurality of vascular stents. One or more stops are provided in the delivery catheter between each of the plurality of stents. The stops be radiopaque to assist in deploying the stents at desired locations within the vasculature of a patient.
Owner:CORDIS CORP
Stent and catheter assembly and method for treating bifurcations
An apparatus and method is provided for stenting bifurcated vessels. A proximal angled stent is configured for implanting in a side-branch vessel wherein the proximal angled stent has an angulated portion that corresponds to the angle formed by the intersection of the side-branch vessel and the main vessel so that all portions of the side-branch vessel at the bifurcation are covered by the proximal angled stent. A main-vessel stent is provided for implanting in the main vessel, wherein the main-vessel stent has an aperture or stent cell that aligns with the opening to the side-branch vessel to permit unobstructed blood flow between the main vessel and the side-branch vessel. Side-branch and main-vessel catheter assemblies are advanced over a pair of guide wires for delivering, appropriately orienting, and implanting the proximal angled stent and the apertured stent.
Owner:ABBOTT CARDIOVASCULAR
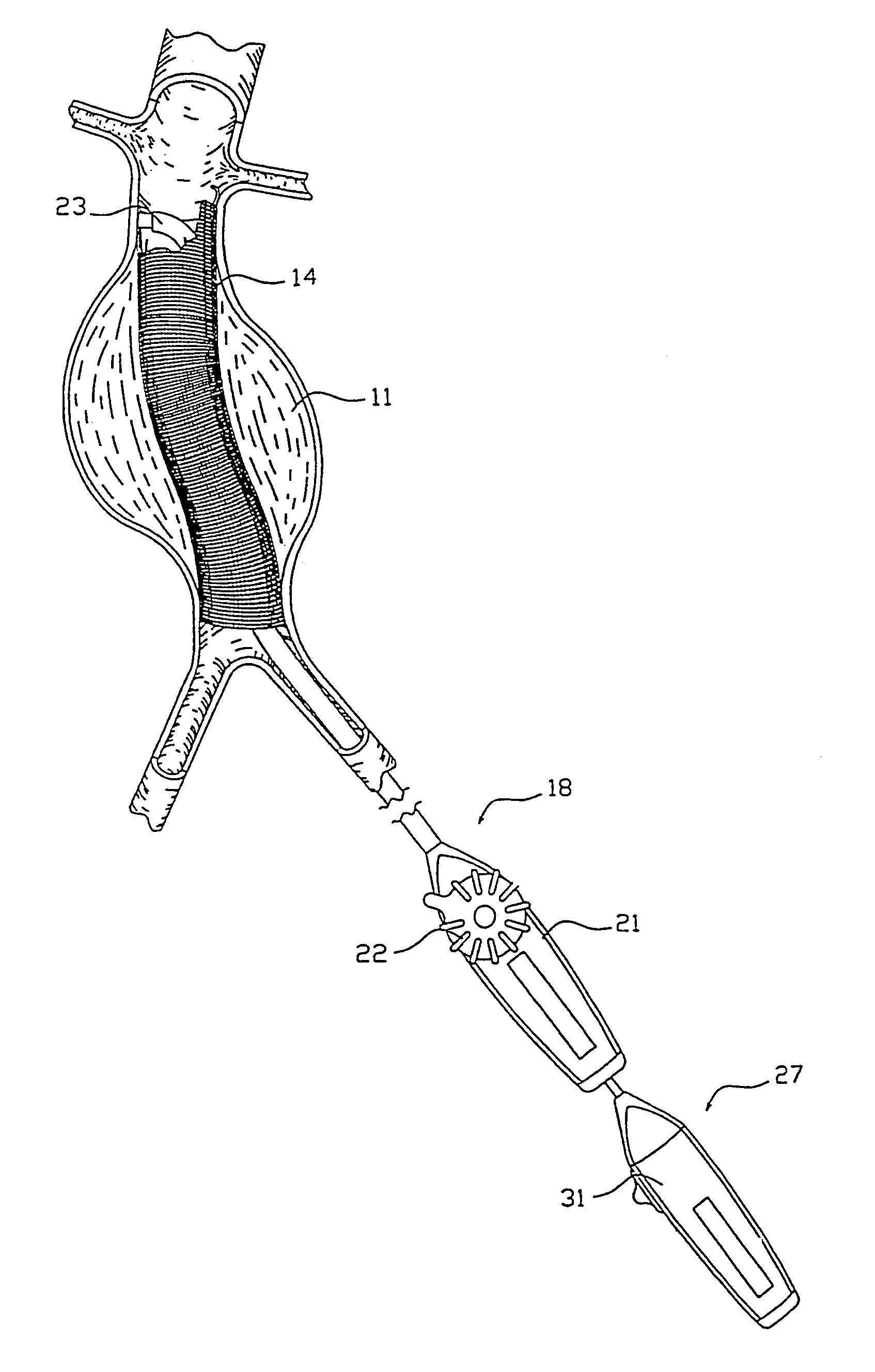
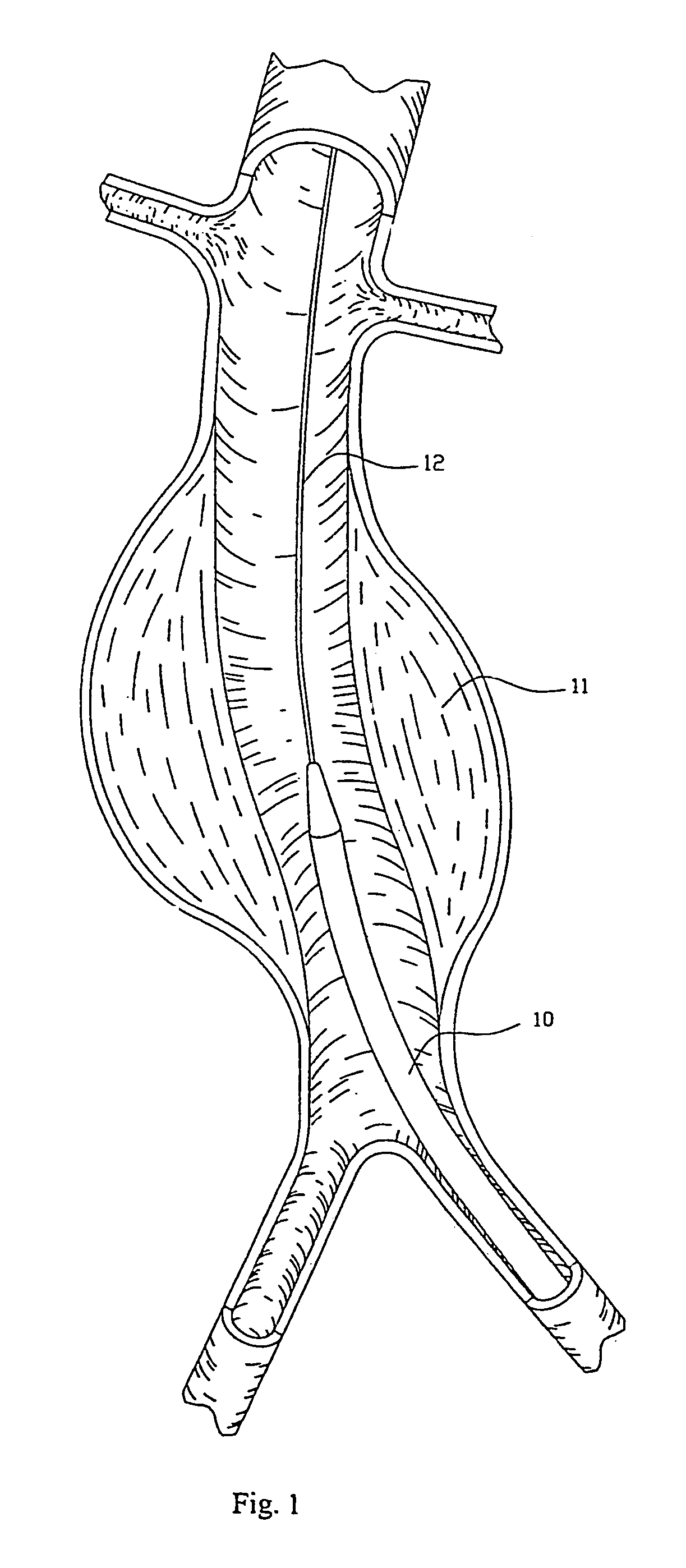
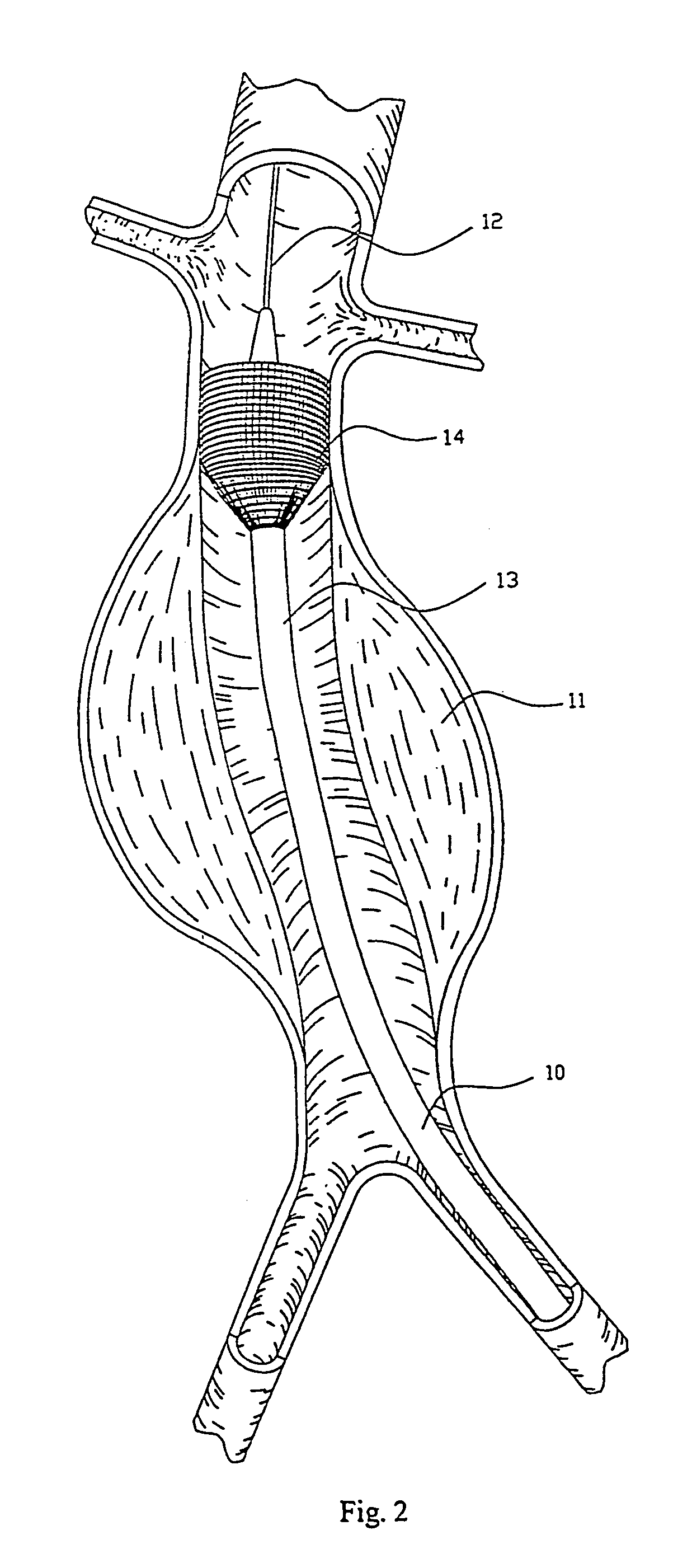
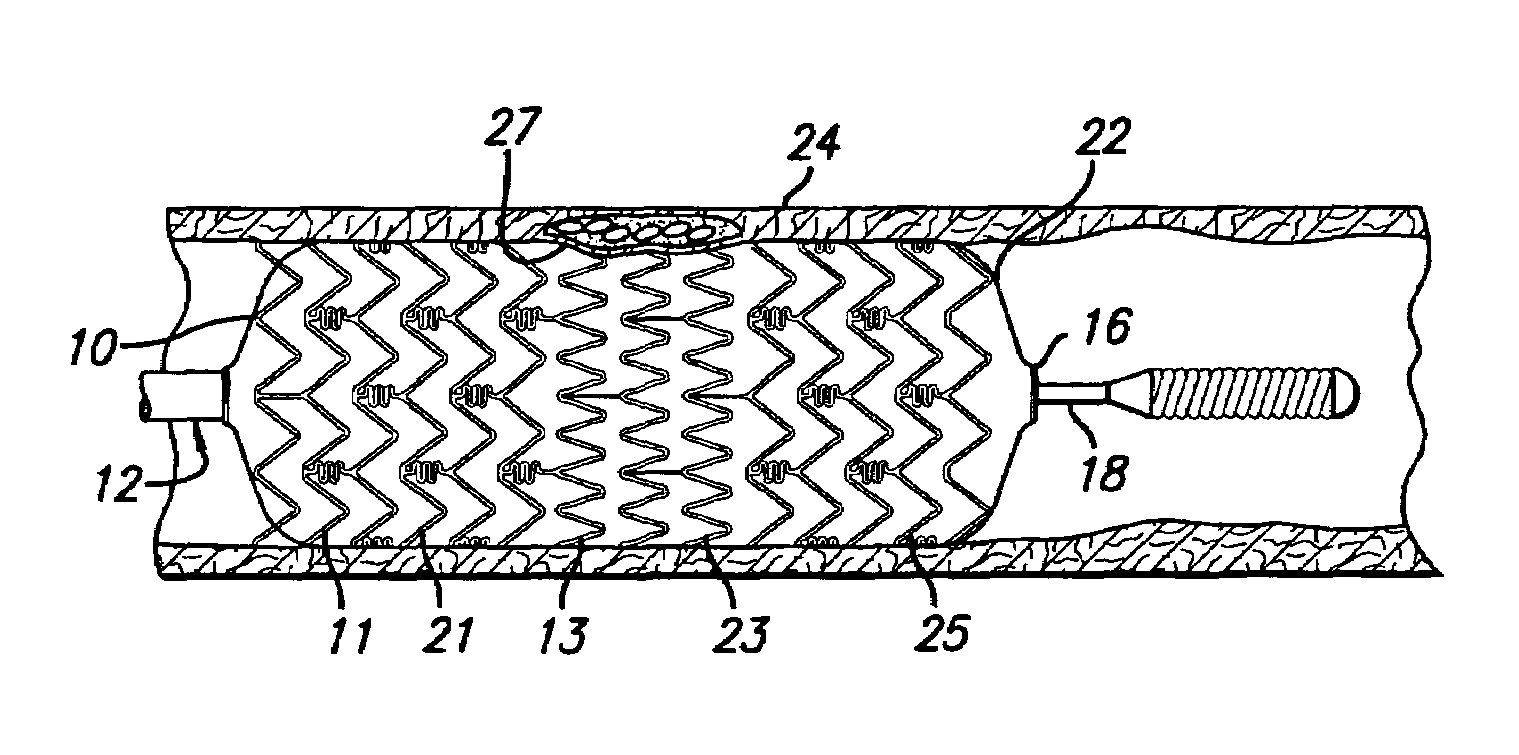
Pulsating Stent Graft
A tubular vascular stent graft with passively pulsating midsection where the difference between the cross-sectional areas of the lumen under the systolic and diastolic pressures after the implantation is 10% or more. The pulsating stent graft accumulates blood during the systolic pressure wave thus lowering the peak value of the tugging force at the proximal attachment site.
Owner:ARKUSZ MIKE +1
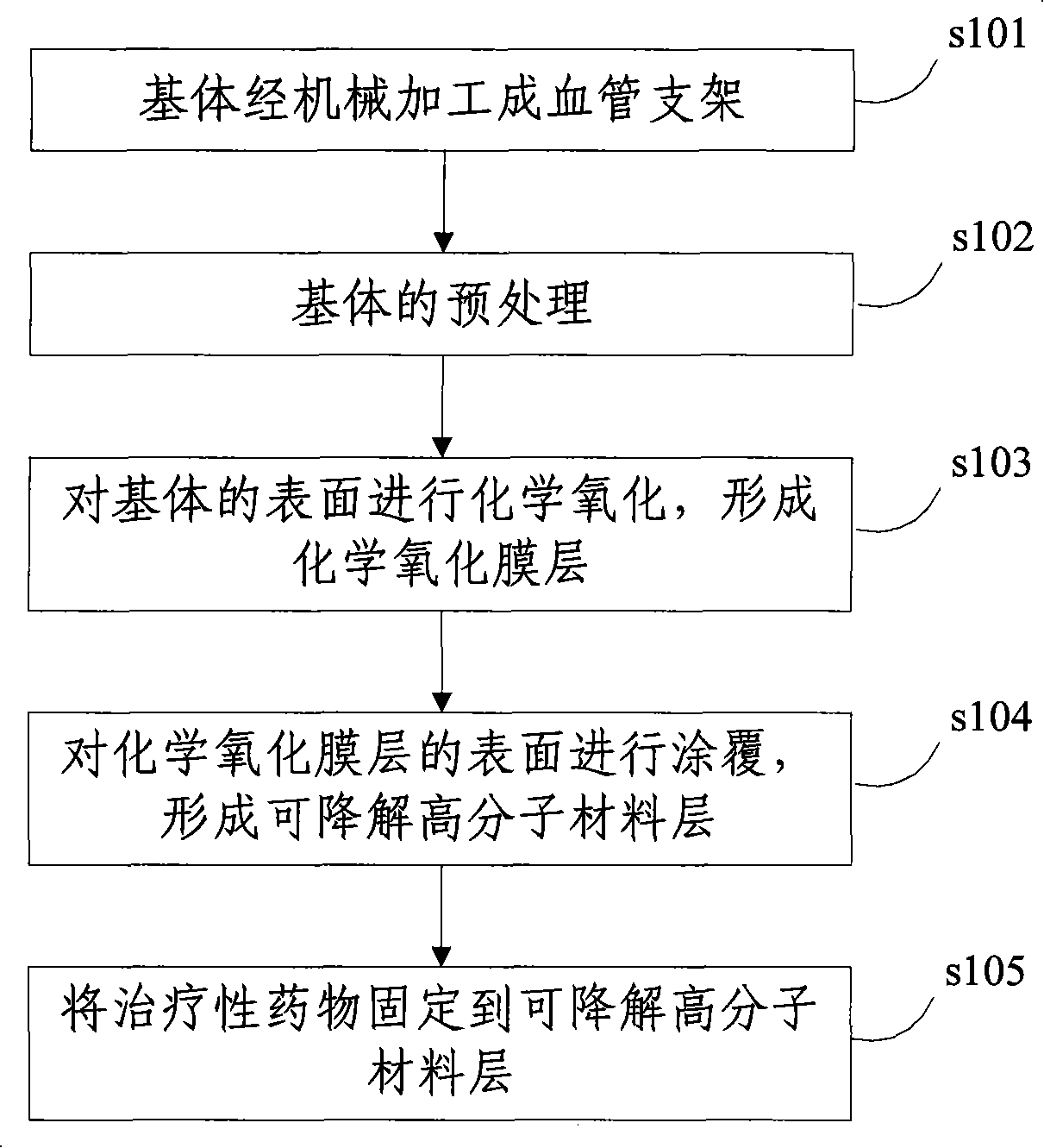
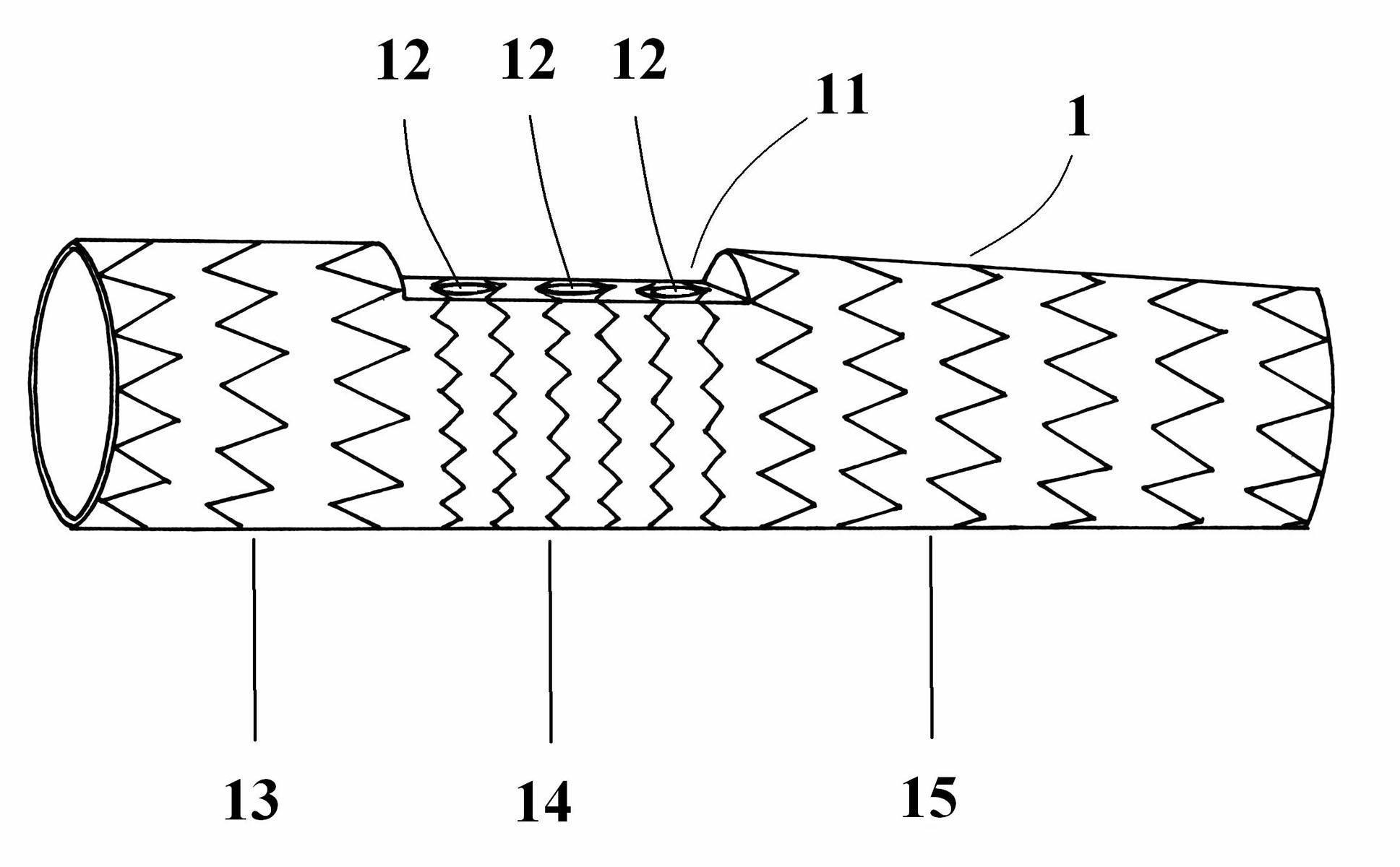
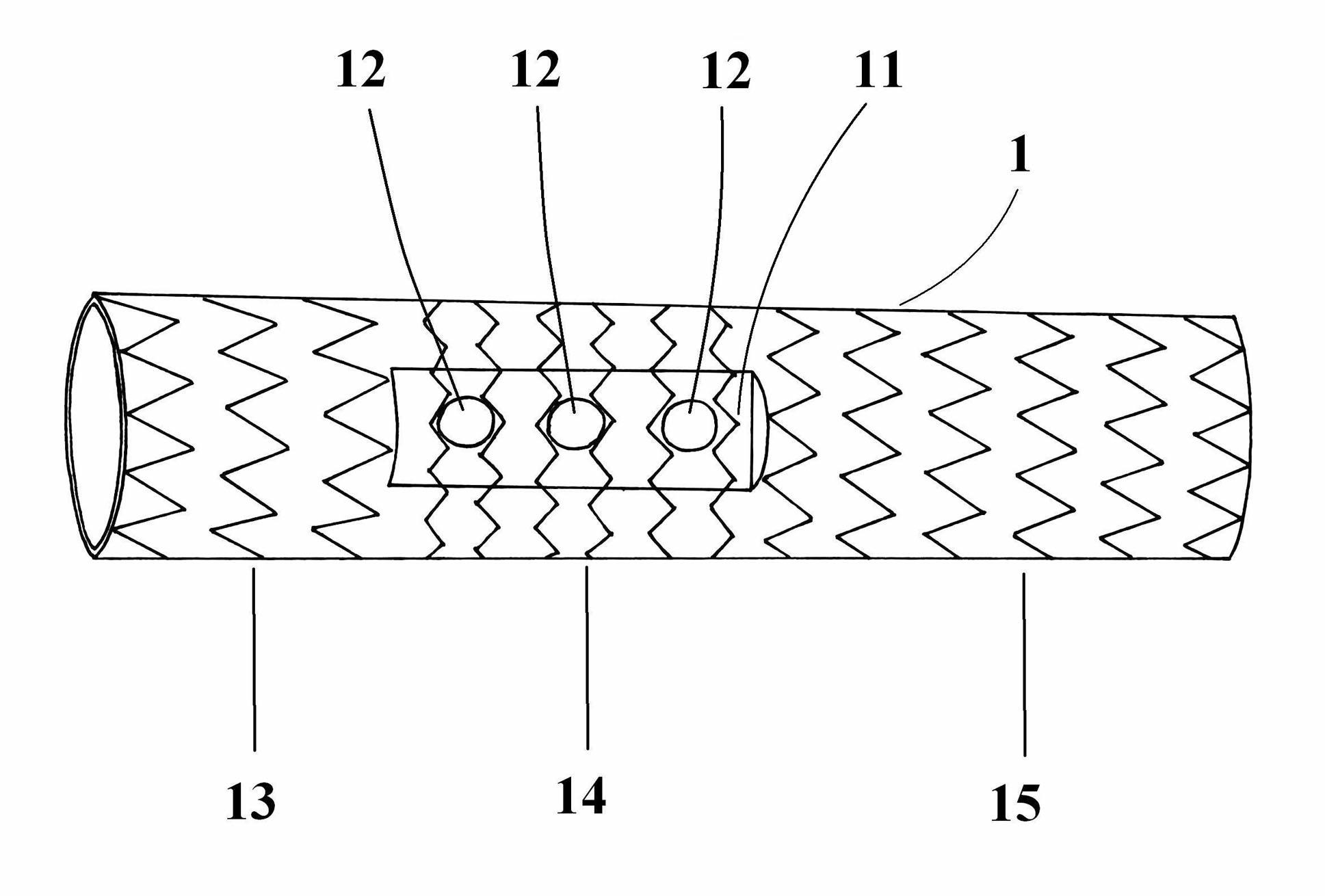
Degradable chemical bitter earth alloy bracket and method of preparing the same
ActiveCN101249286AImprove mechanical propertiesExcellent pharmacological propertiesSuture equipmentsSurgical adhesivesInsertion stentPolymer chemistry
The invention discloses a degradable vascular stent with a composite coating and a preparation method thereof, and comprises a matrix made from pure magnesium or magnesium alloy material; a chemical oxide film layer that is arranged on the surface of the matrix and is used for decreasing the corrosion rate of the matrix and prolonging the service life of the matrix; a degradable polymer material layer that is located at the surface of the chemical oxide film layer and therapeutic drug that are fixed at the degradable polymer material layer. The degradable vascular stent with a composite coating of the invention has excellent mechanical and pharmacological properties, otherwise, the degradation rate of the magnesium material is reduced, and the service life of the stent is prolonged.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Branching type aortal vascular stent system
ActiveCN102641164AWon't blockAvoid the risk of ischemiaStentsBlood vesselsAscending aortaAortic arch
The invention discloses a branching type aortal vascular stent system comprising an aortal vascular stent and three branch arterial vascular stents, wherein a concave part is formed in the middle section of the aortal vascular stent, and provided with three side holes; and the three branch arterial vascular stents are separately mounted in the three side holes. The branching type aortal vascular stent system is capable of isolating aortal lesion involving the aortic arch and the ascending aorta, reestablishing the blood flow of the branch artery and avoiding the customization of the stent; and the branching type aortal vascular stent system is suitable for batch production and can be used for emergency operations.
Owner:舒畅 +1
Vascular stent for embolic protection
InactiveUS20060259132A1Increase the diameterIncreasing of regionStentsBlood vesselsInsertion stentThree vessels
A method and device to repair a stenosis in a blood vessel is provided. The medical device has a tubular member and a frame. The frame may be expanded or contracted while maintaining its generally cylindrical configuration. The medical device is retained in a contracted state inside an introducer sheath. The introducer sheath is guided through the stenosis such that a first end of the medical device is located distal the stenosis. The introducer sheath is retracted relative to the medical device, such that the first end of the stent expands to engage the blood vessel distal the stenosis. A mid-portion of the medical device engages the plaque of the stenosis trapping any emboli against the wall of the vessel. The second end expands to engage the blood vessel proximal to stenosis.
Owner:COOK INC
Recyclable vascular stent and recycling method thereof
The invention discloses a recyclable vascular stent and a recycling method thereof. The vascular stent comprises a tubular netted framework woven by silks, the end part of the stent is provided with a connecting structure, and the connecting structure forms separable connection with a stent conveyor; the stent can be arranged in the stent conveyor, and can be restored to the original shape after being released; and net holes on the part of the connecting structure are more loosen than other grids, and are used for repeatedly positioning and / or recycling the stent in a vascular cavity. The netholes positioned on the connecting structure on the end part of the stent are easier to stretch and deform than other net holes at other positions, the connecting structure is folded and pulled into a sheath catheter of the stent conveyor, and the rest part of the stent is also collected in the sheath catheter of the stent conveyor, so an operator is greatly convenient to adjust the form of the stent and adjust the proper position of the stent in the vascular cavity and even recycle the stent from the vascular cavity, the repeated positioning and smooth recycling of the vascular stent are realized, and the technical requirement of PTA therapy is better met.
Owner:LIFETECH SCIENTIFIC (SHENZHEN) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com