Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
11153 results about "Drug delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug delivery refers to approaches, formulations, technologies, and systems for transporting a pharmaceutical compound in the body as needed to safely achieve its desired therapeutic effect. It may involve scientific site-targeting within the body, or it might involve facilitating systemic pharmacokinetics; in any case, it is typically concerned with both quantity and duration of drug presence. Drug delivery is often approached via a drug's chemical formulation, but it may also involve medical devices or drug-device combination products. Drug delivery is a concept heavily integrated with dosage form and route of administration, the latter sometimes even being considered part of the definition.
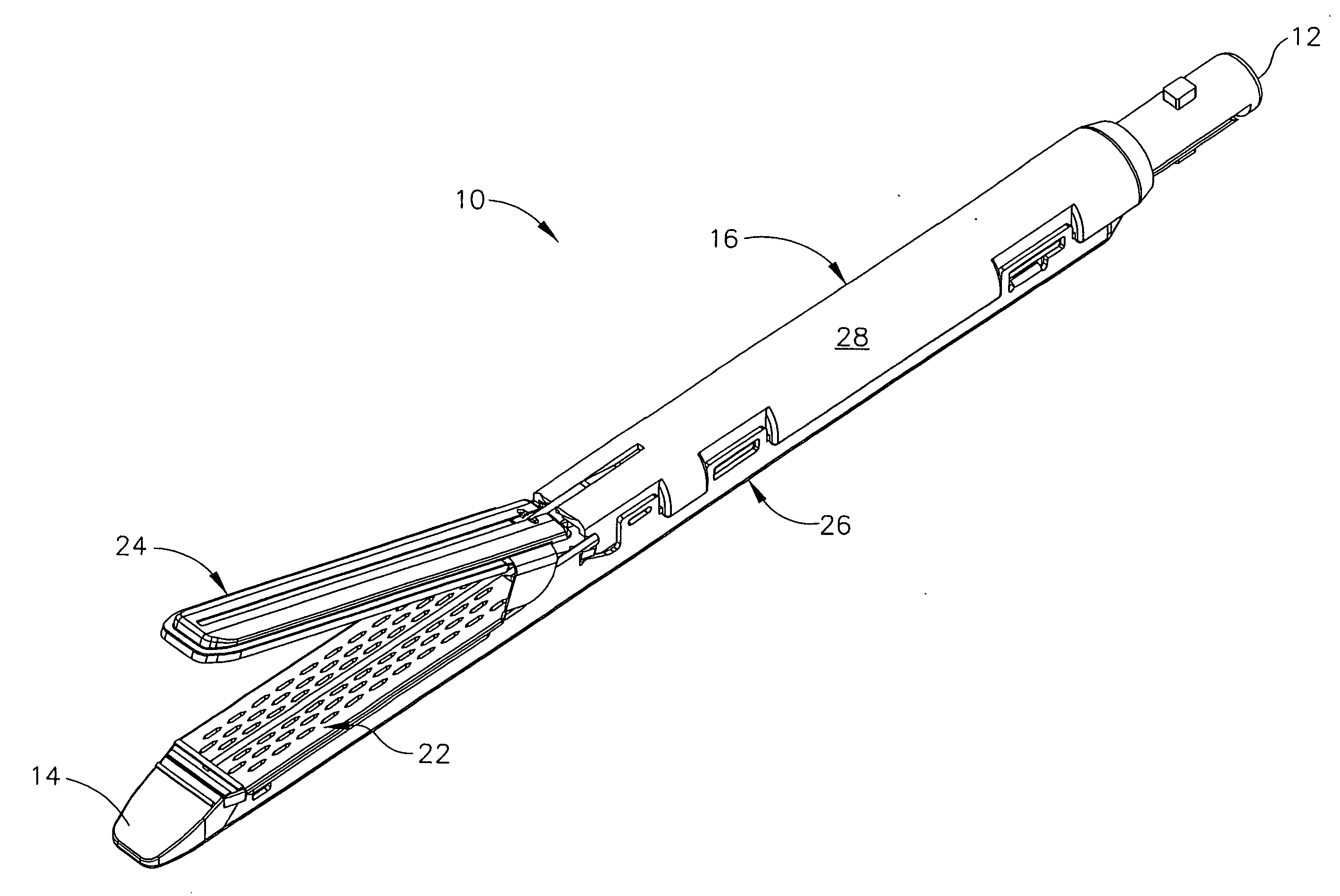
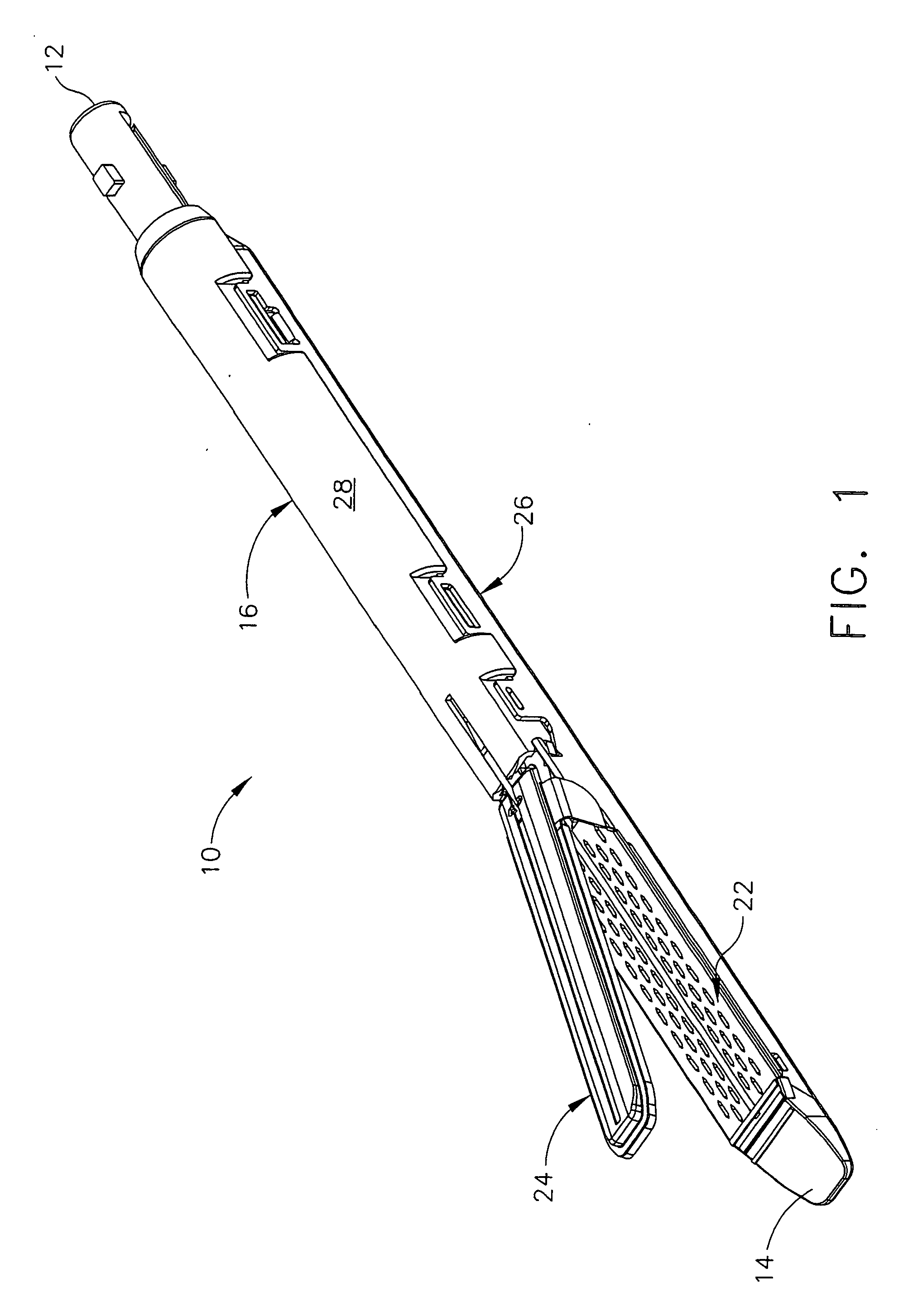
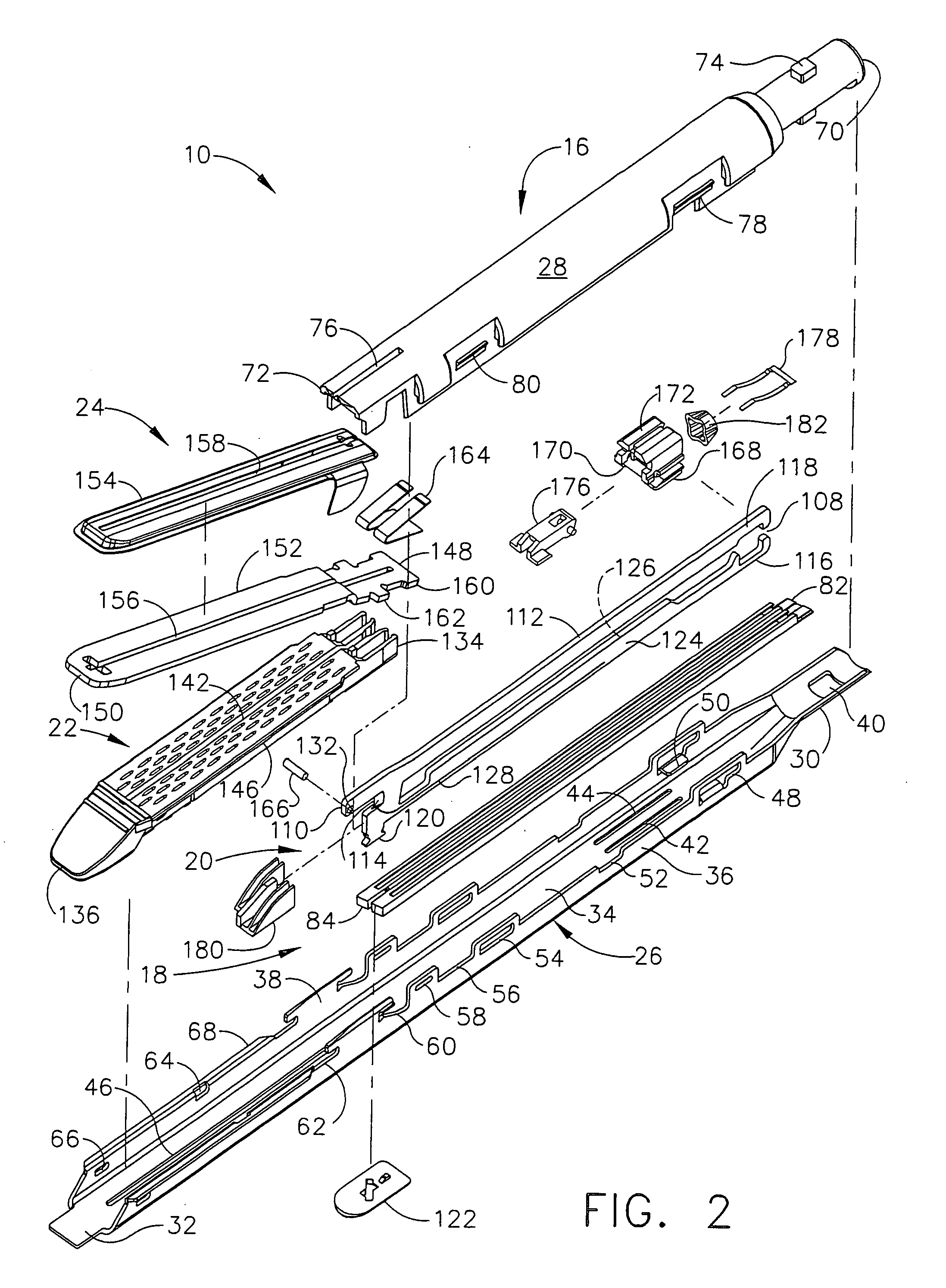
Disposable loading unit and surgical instruments including same
A disposable loading unit. The disposable loading unit includes a housing assembly, a knife assembly connected to the housing assembly, and an agent cartridge connected to the housing assembly. The agent cartridge houses a medical agent. The disposable loading unit is configured to deliver the medical agent proximate a cutting surface of the knife assembly.
Owner:CILAG GMBH INT +1
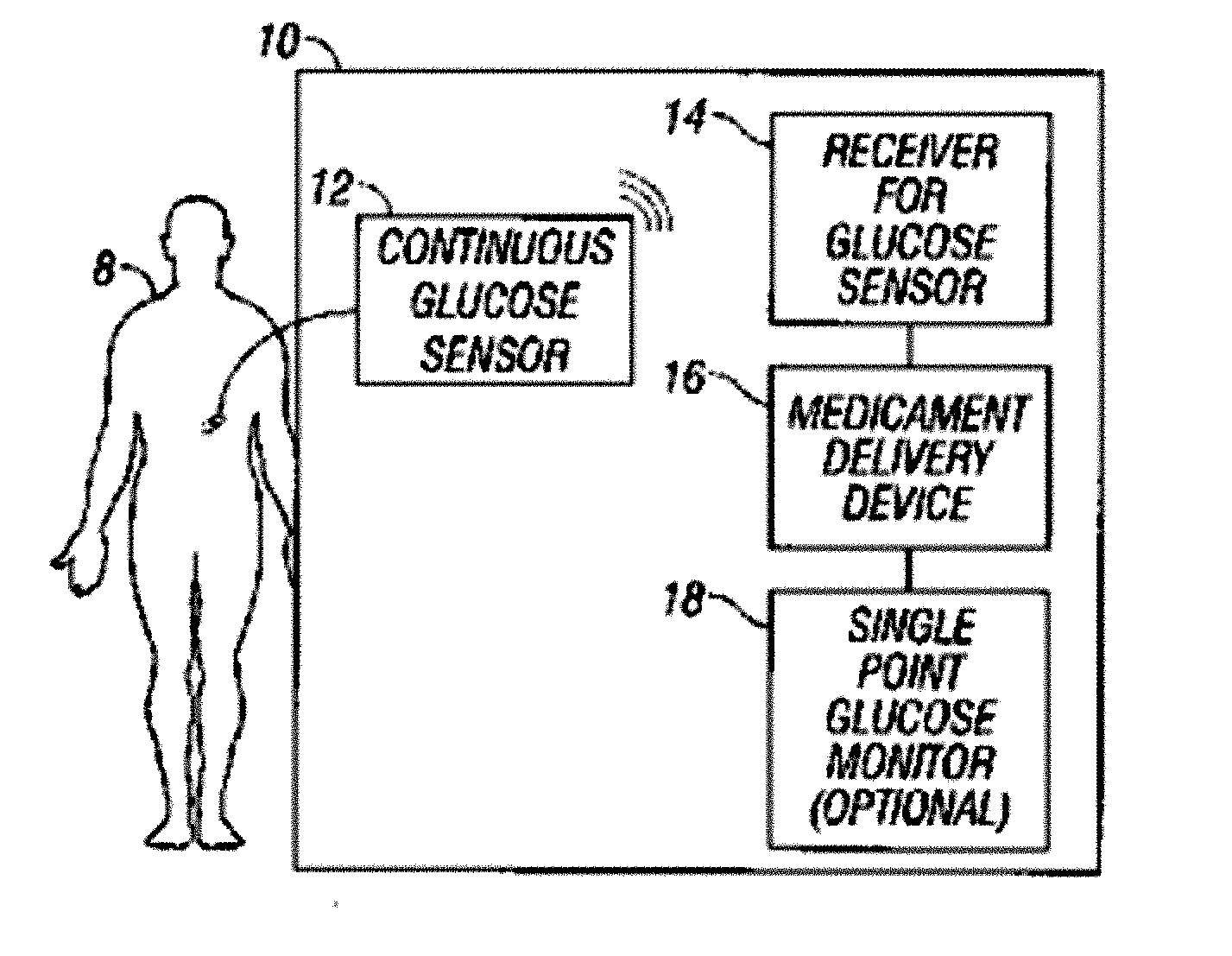
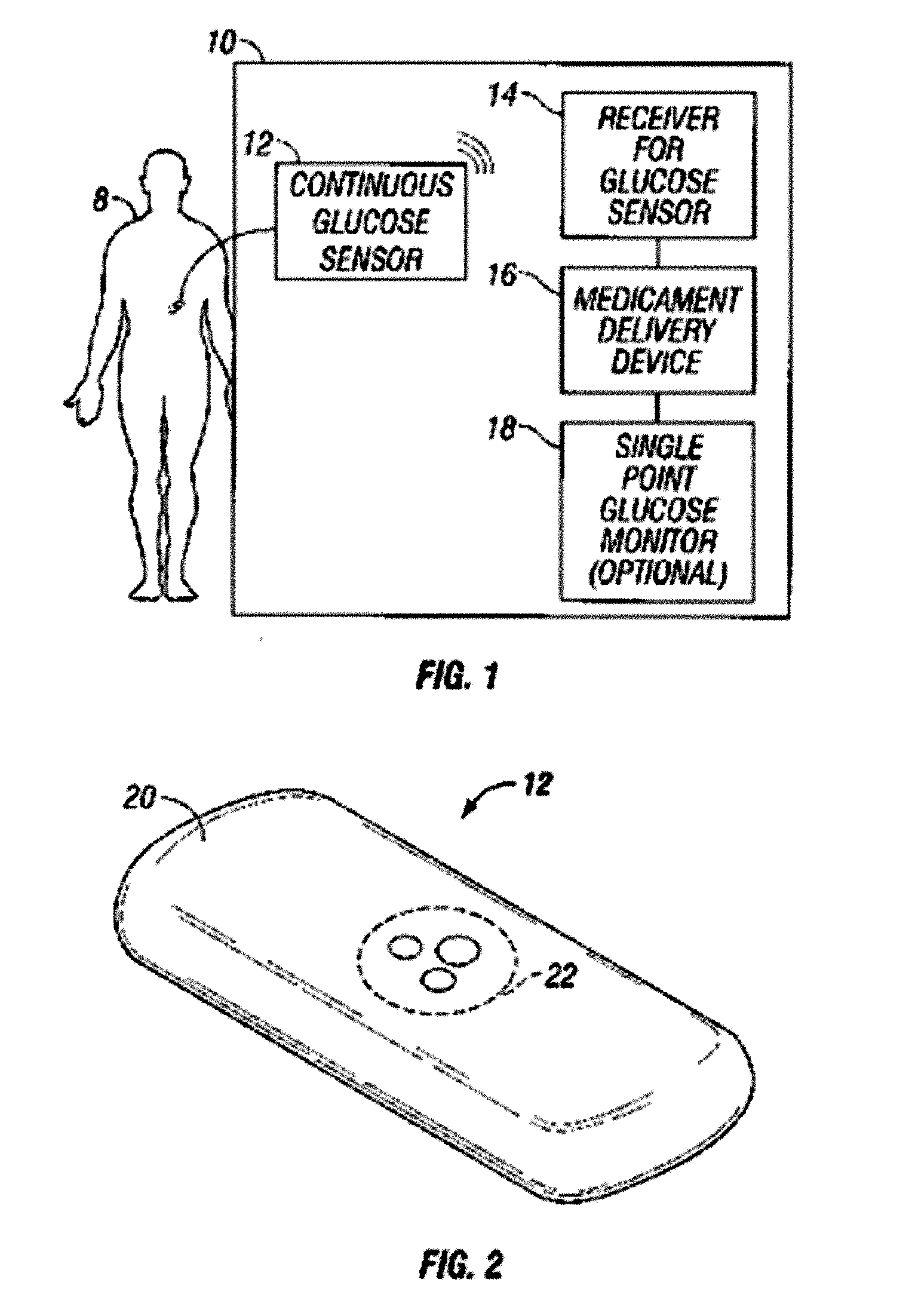
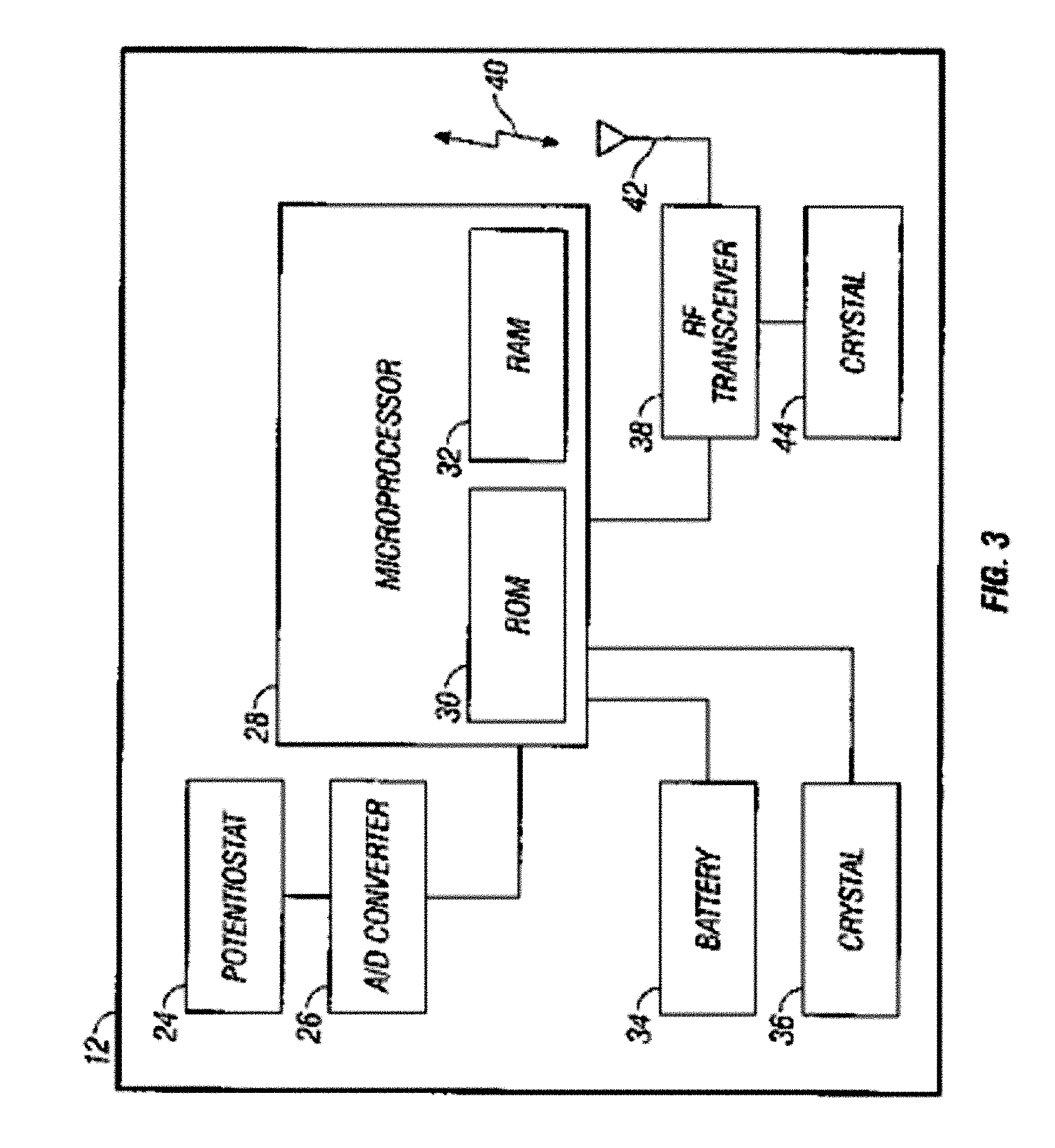
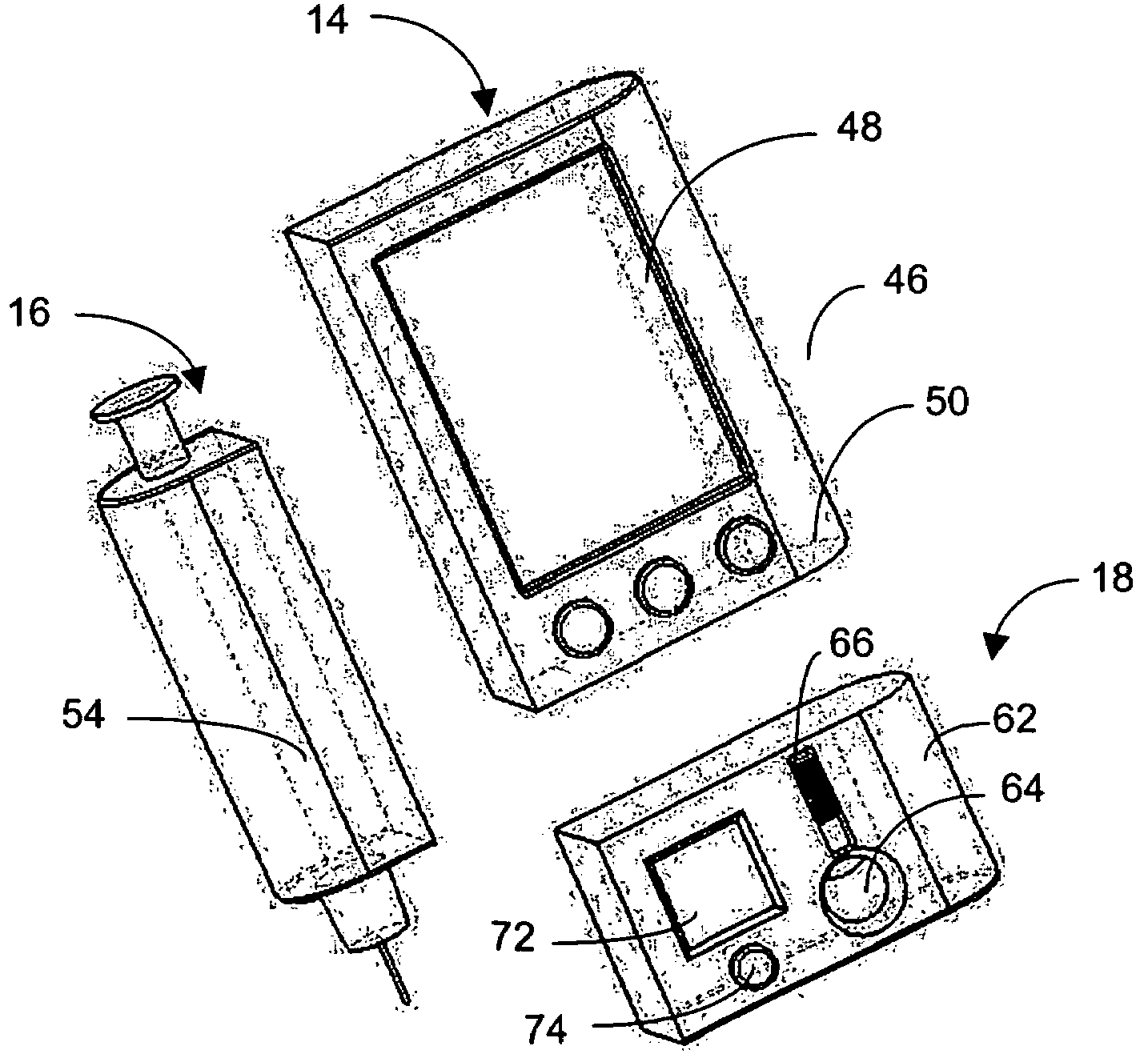
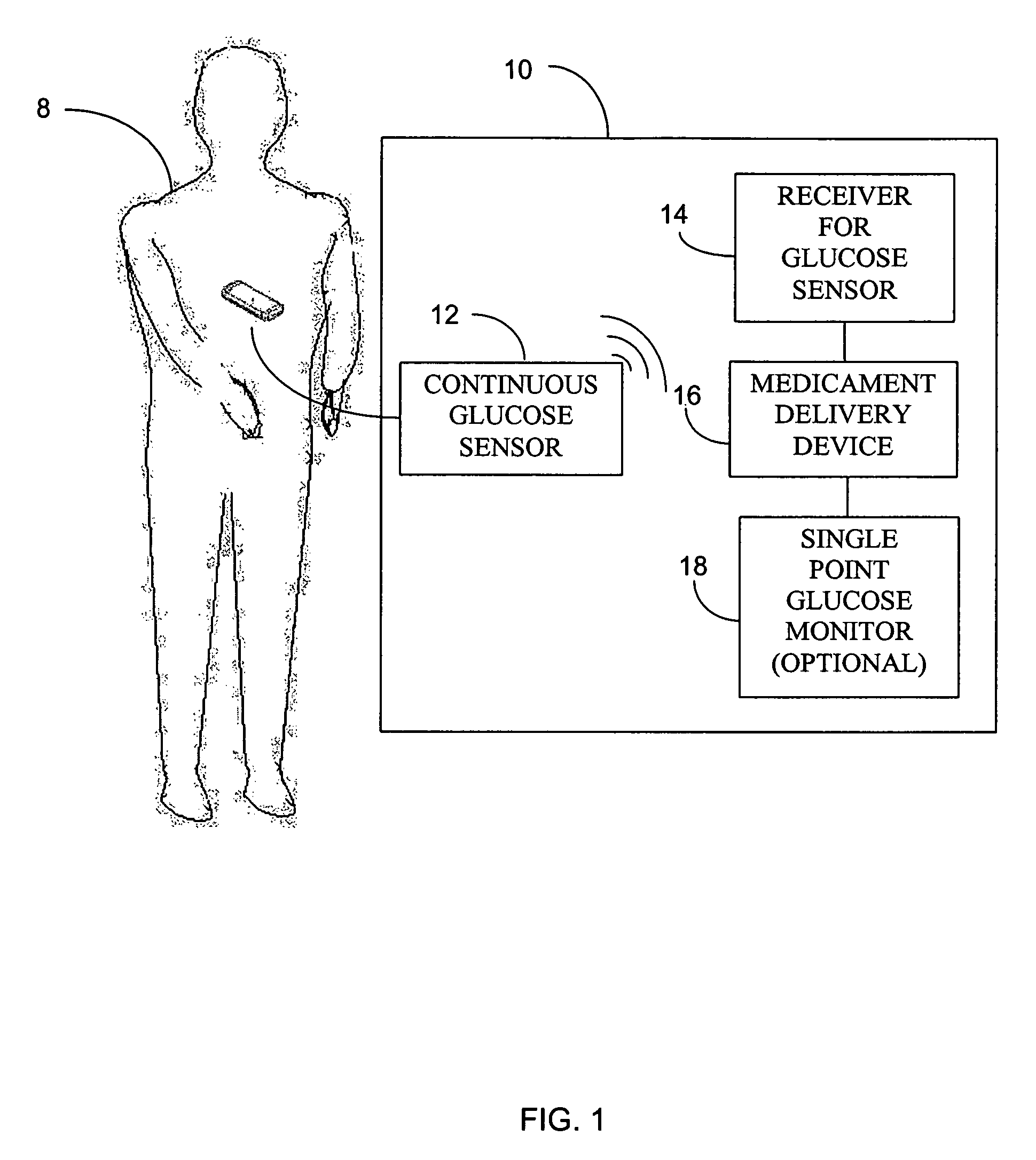
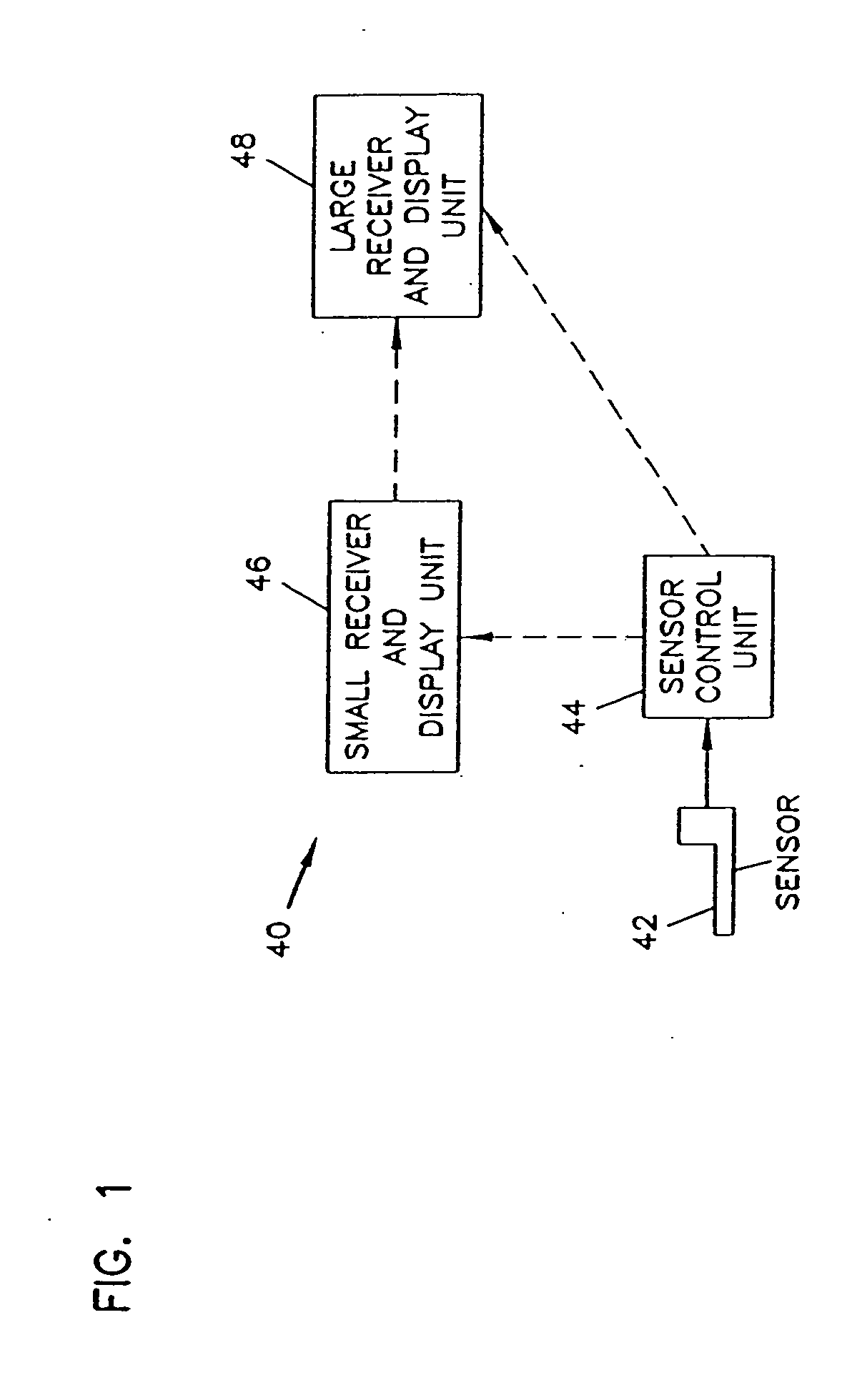
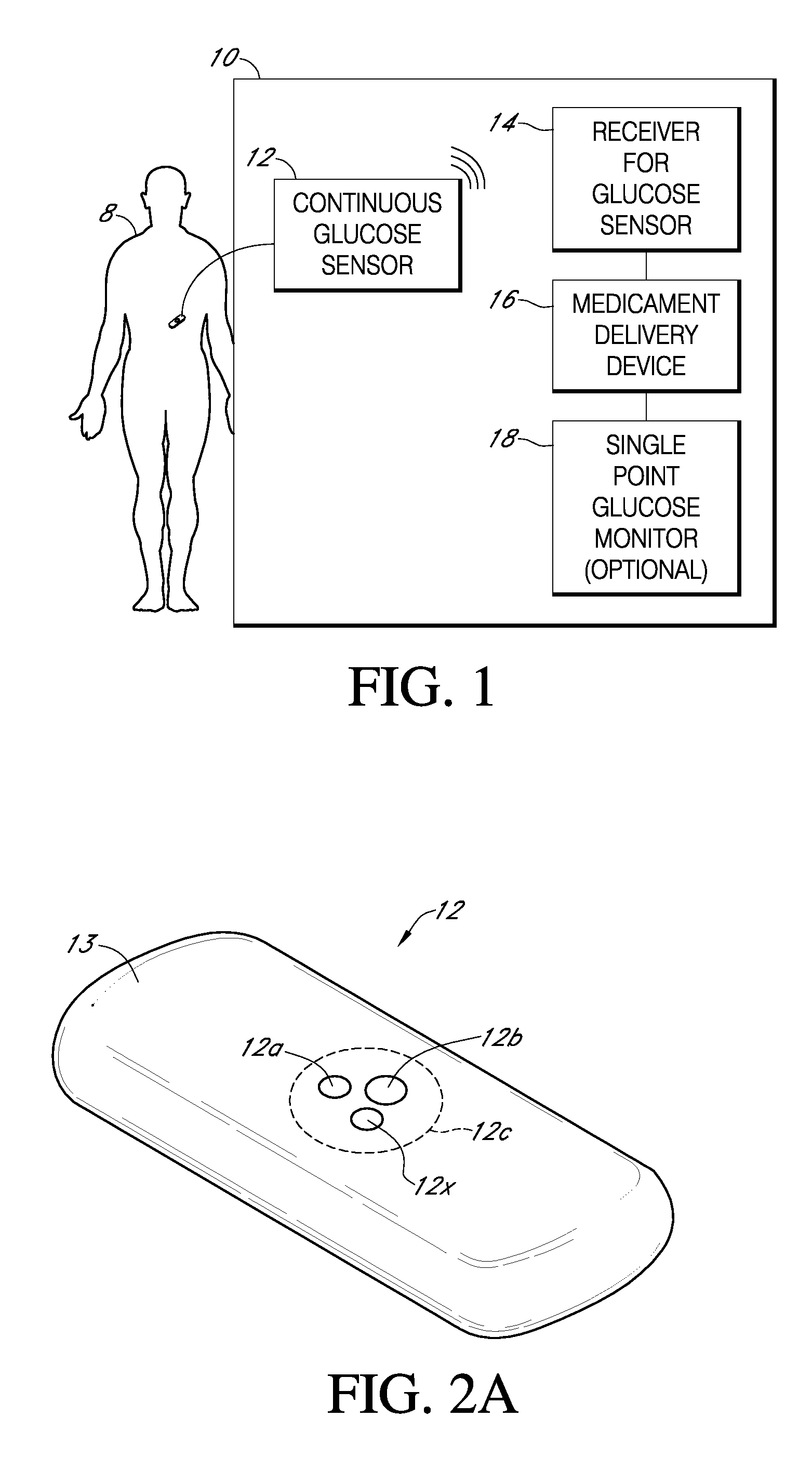
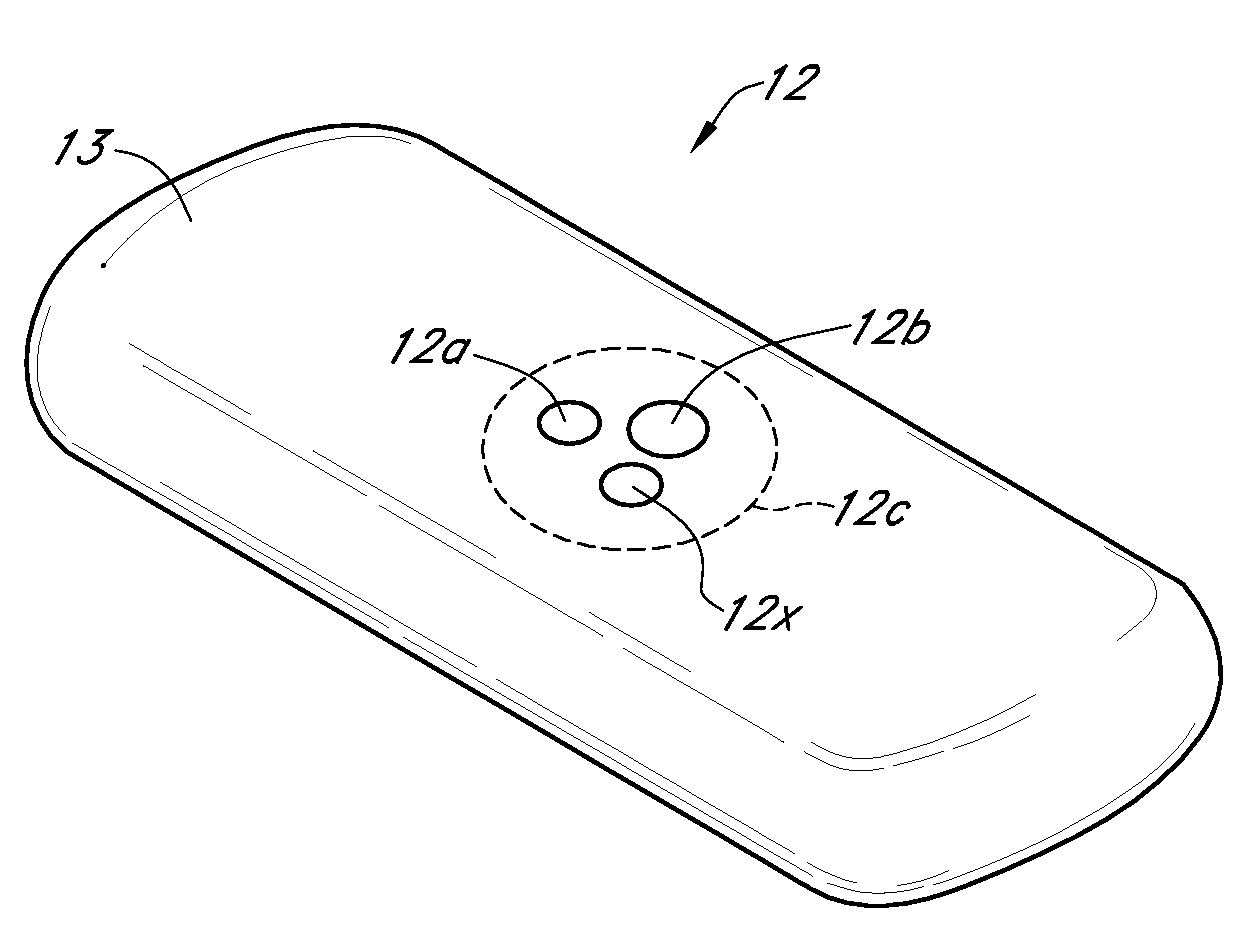
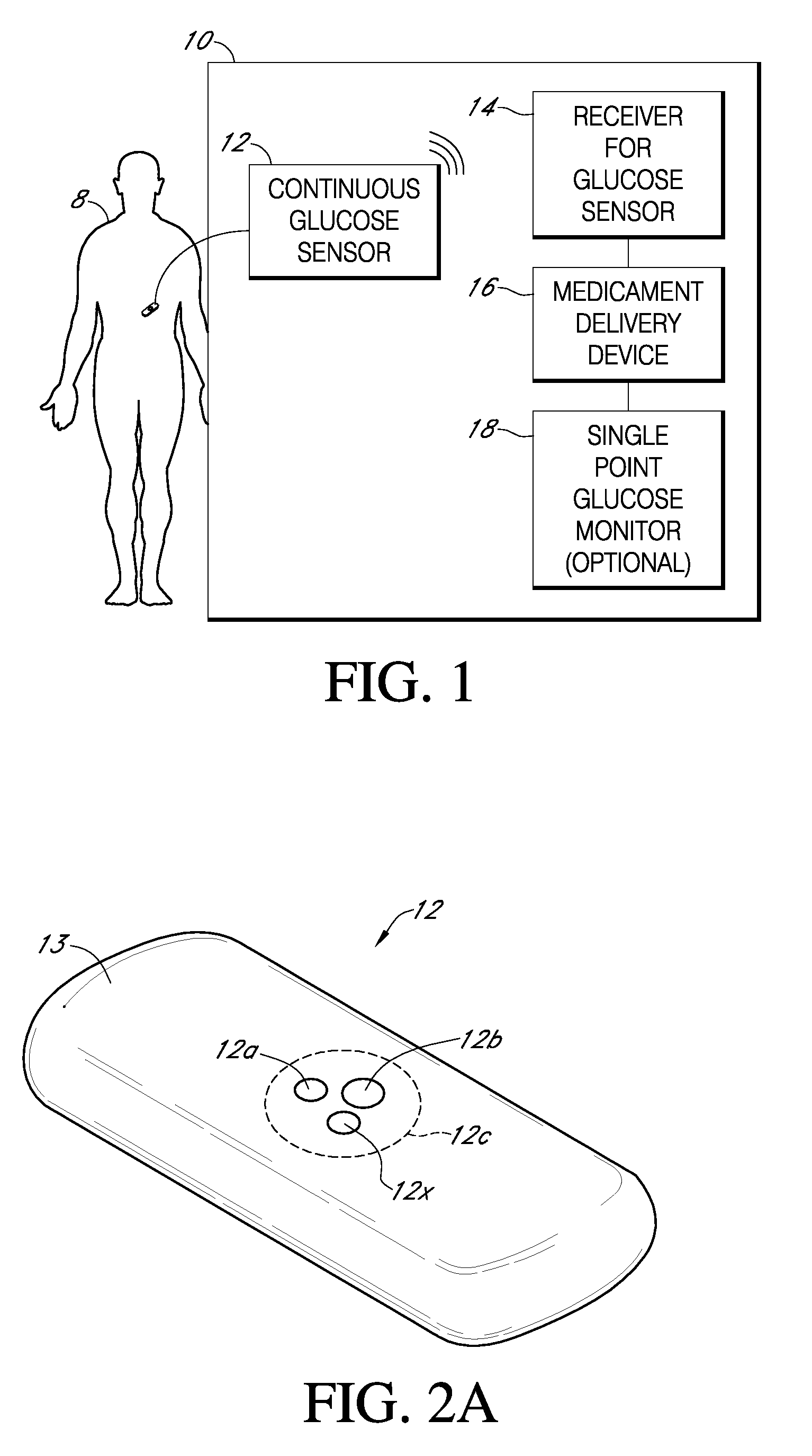
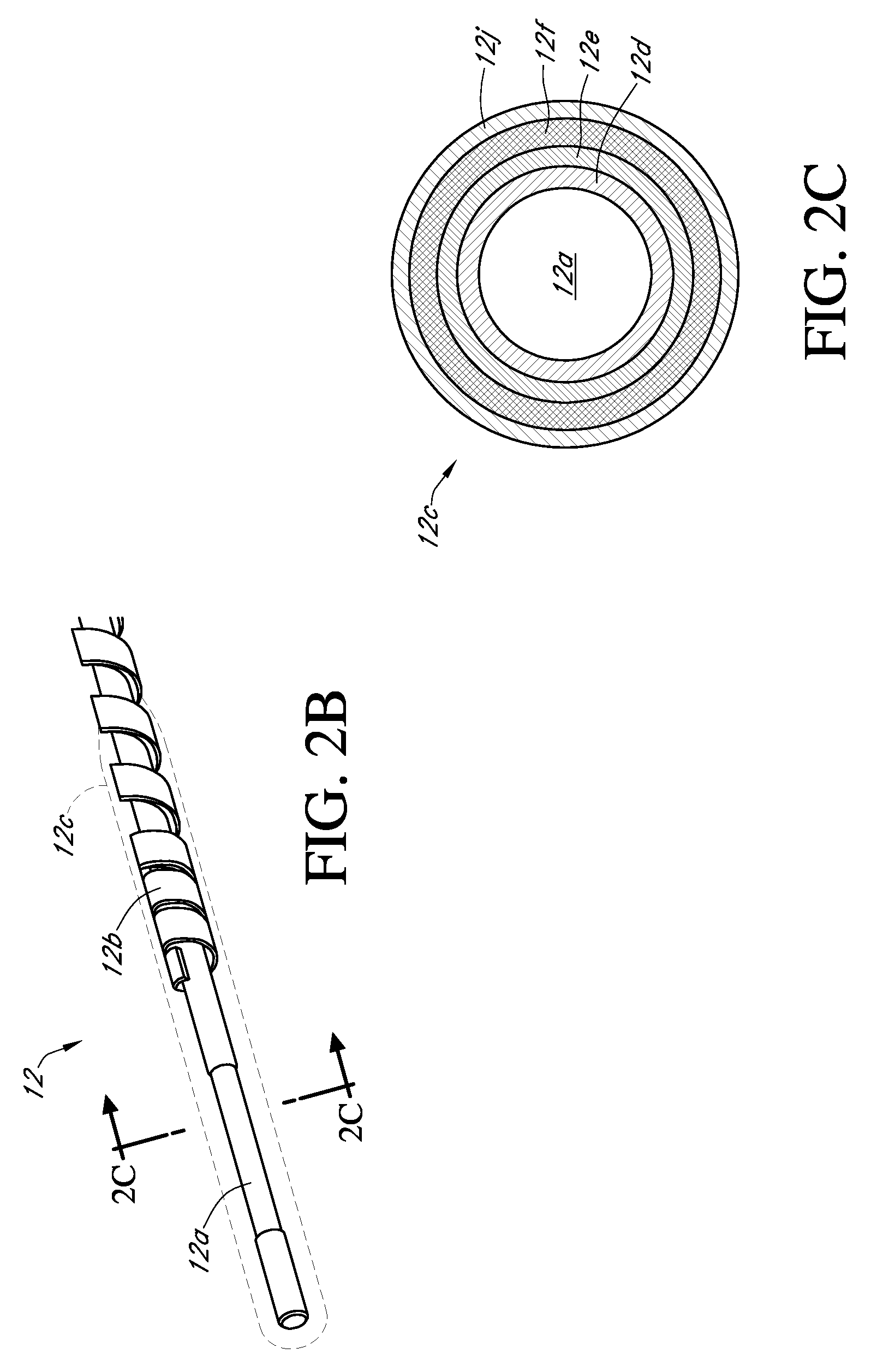
Integrated delivery device for continuous glucose sensor
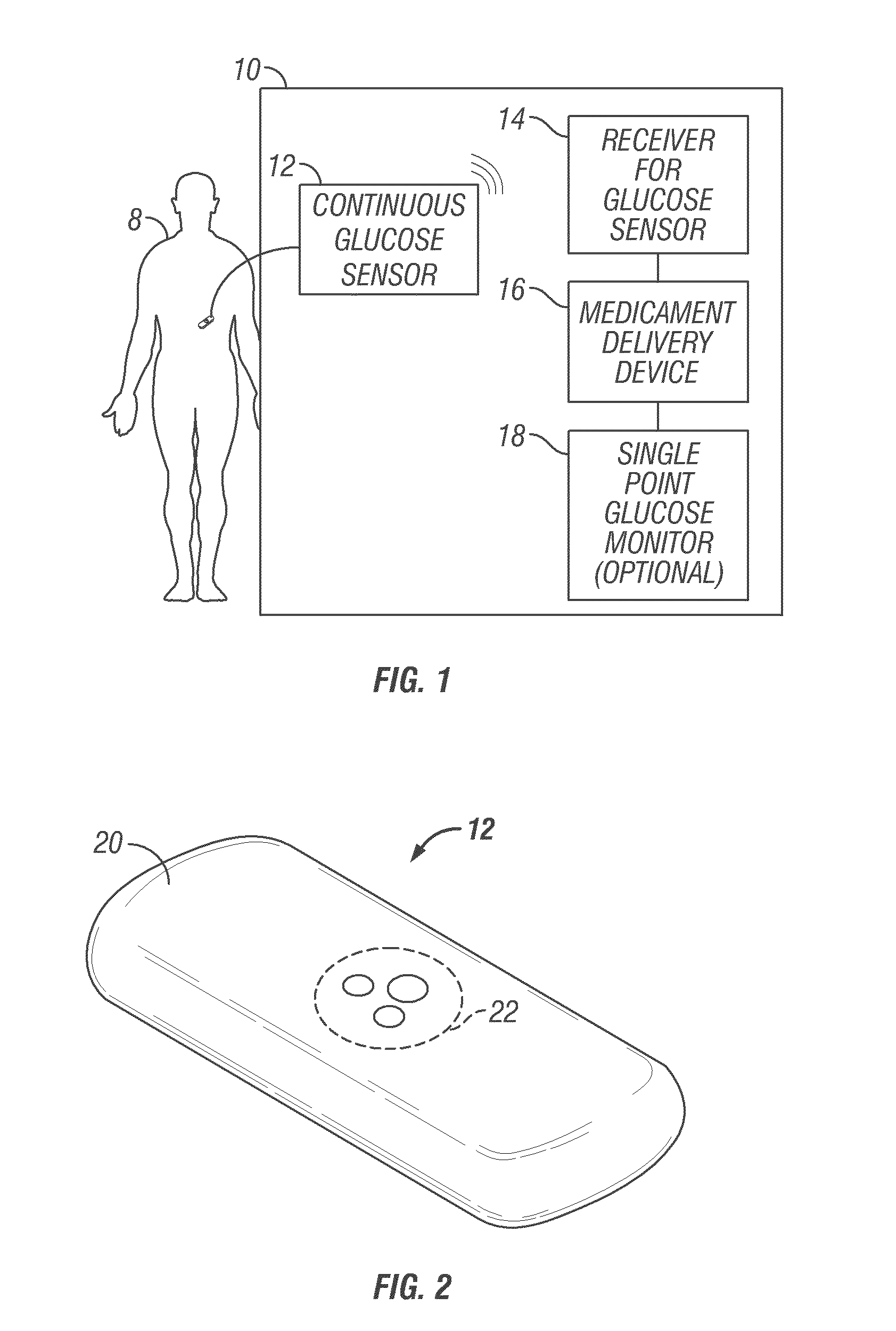
Abstract of the DisclosureSystems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM
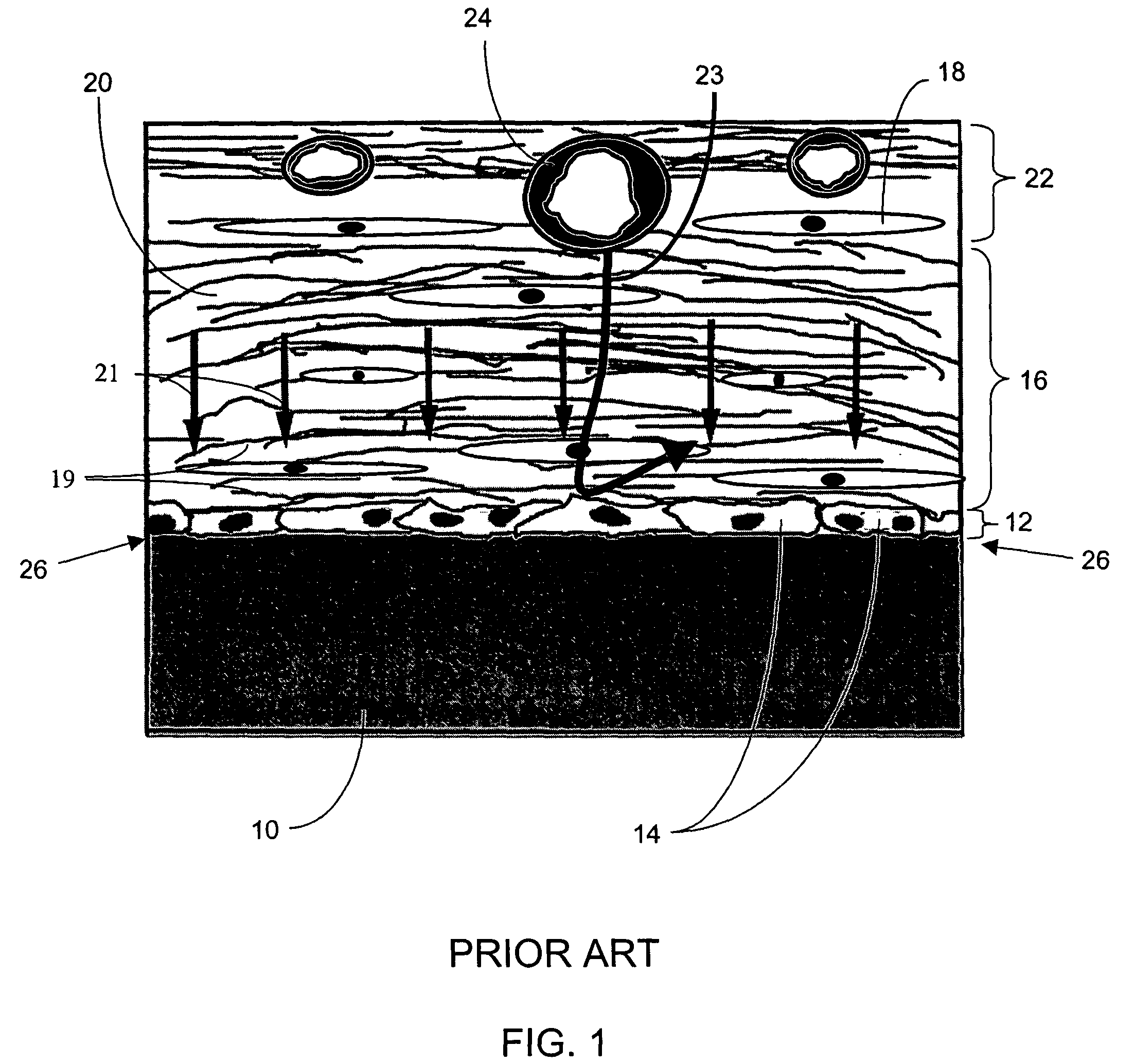
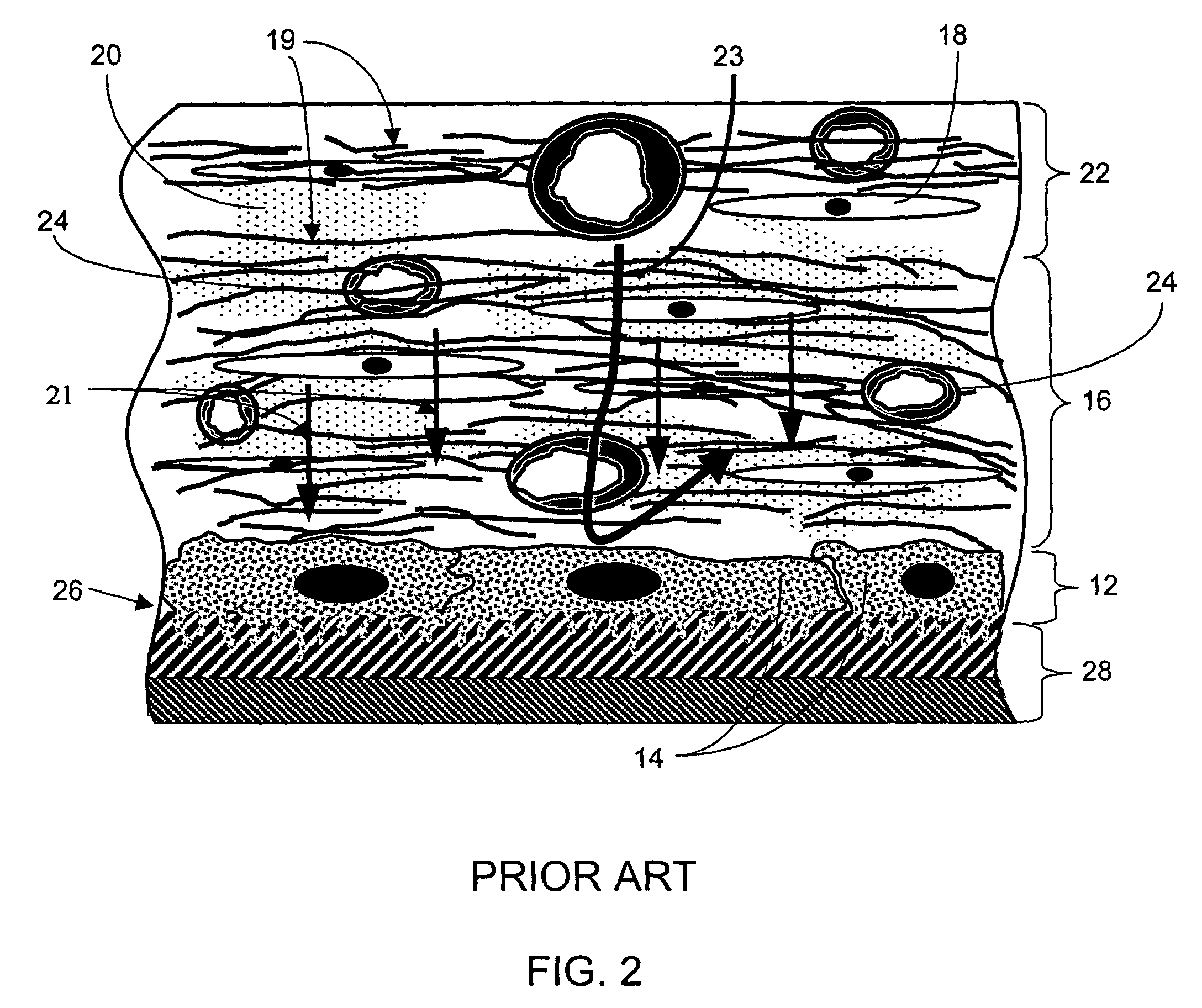
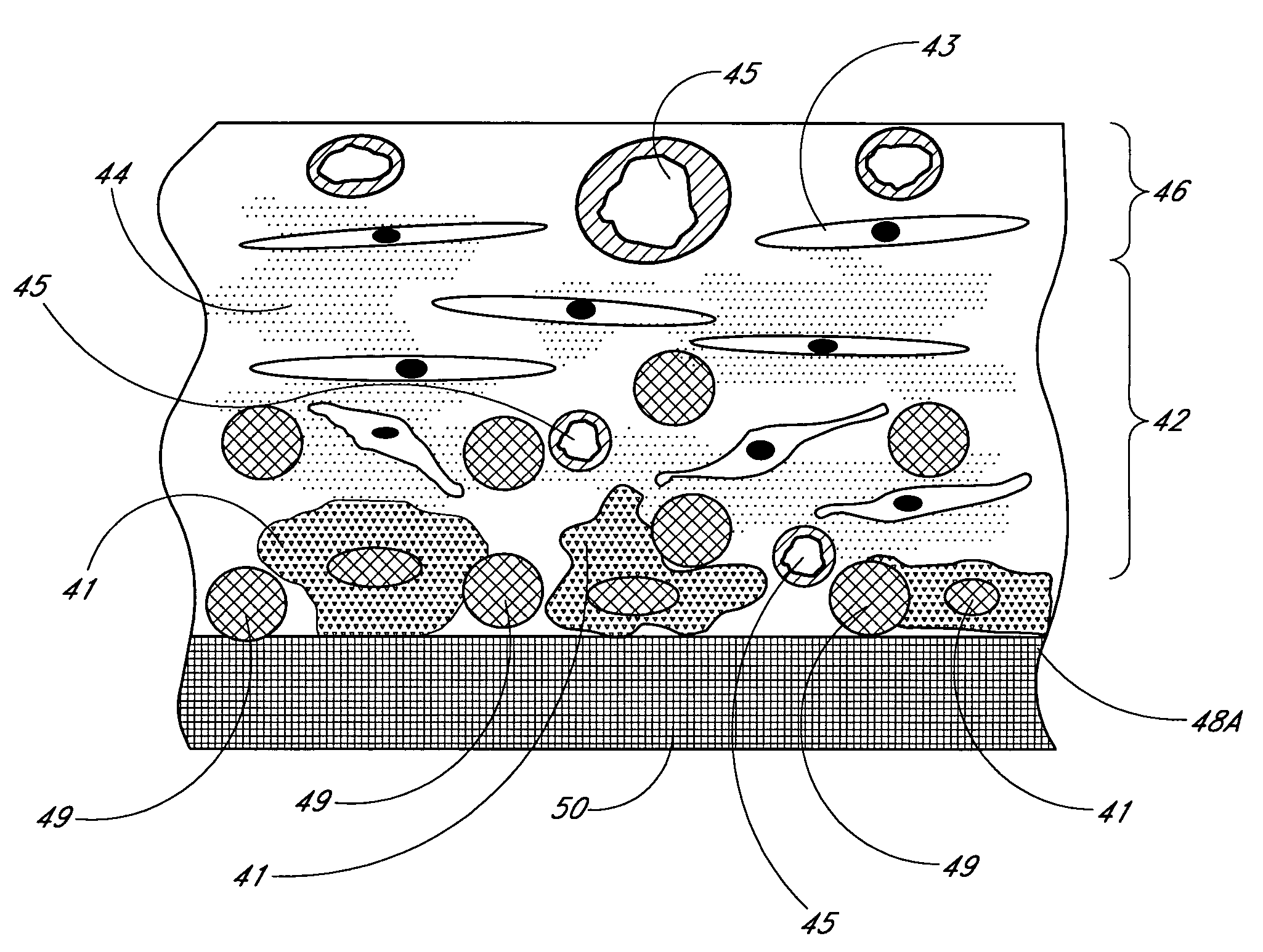
Porous membranes for use with implantable devices
A membrane for implantation in soft tissue comprising a first domain that supports tissue ingrowth, disrupts contractile forces typically found in a foreign body response, encourages vascularity, and interferes with barrier cell layer formation, and a second domain that is resistant to cellular attachment, is impermeable to cells and cell processes, and allows the passage of analytes. The membrane allows for long-term analyte transport in vivo and is suitable for use as a biointerface for implantable analyte sensors, cell transplantation devices, drug delivery devices, and / or electrical signal delivering or measuring devices. The membrane architecture, including cavity size, depth, and interconnectivity, provide long-term robust functionality of the membrane in vivo.
Owner:DEXCOM INC
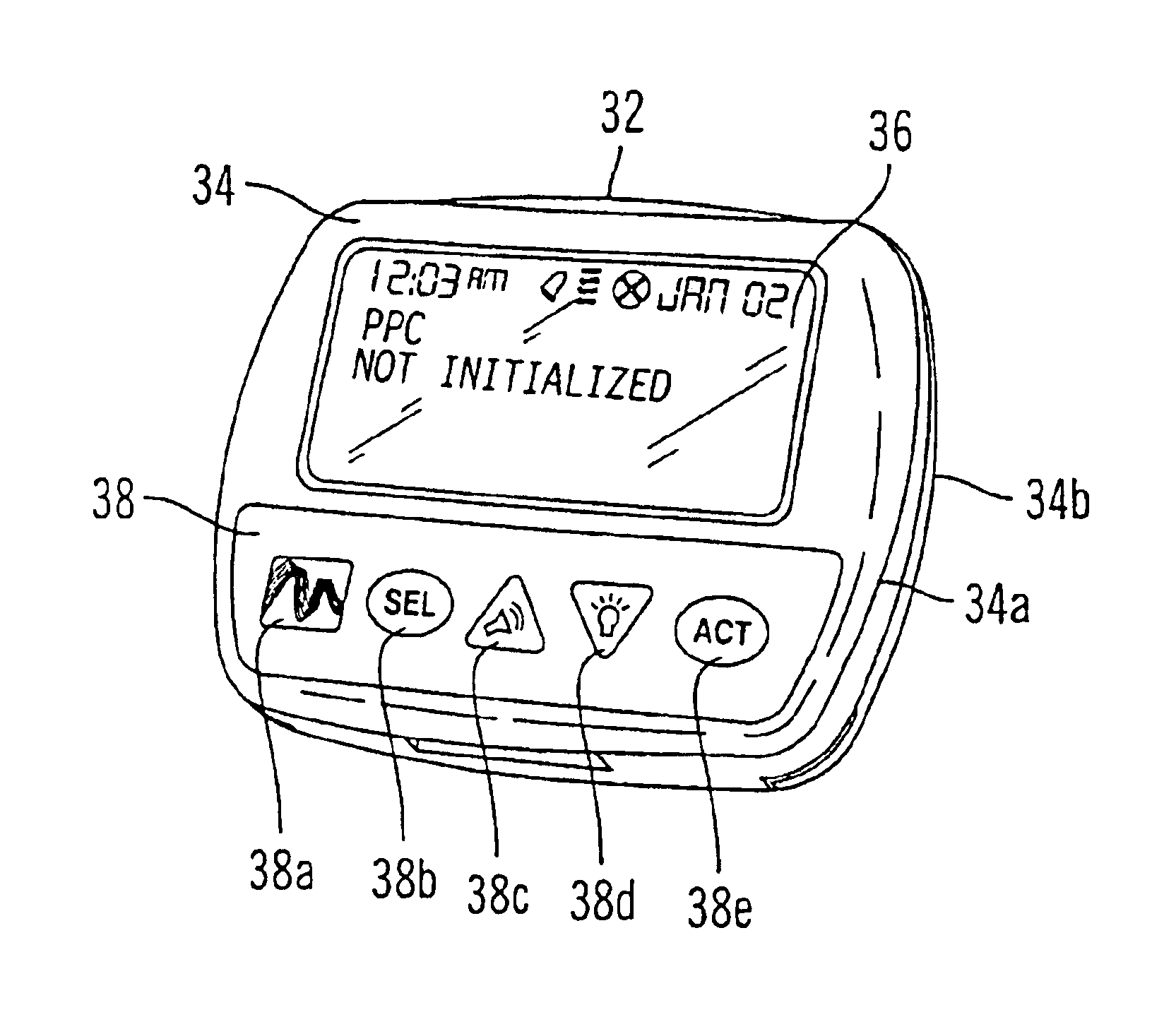
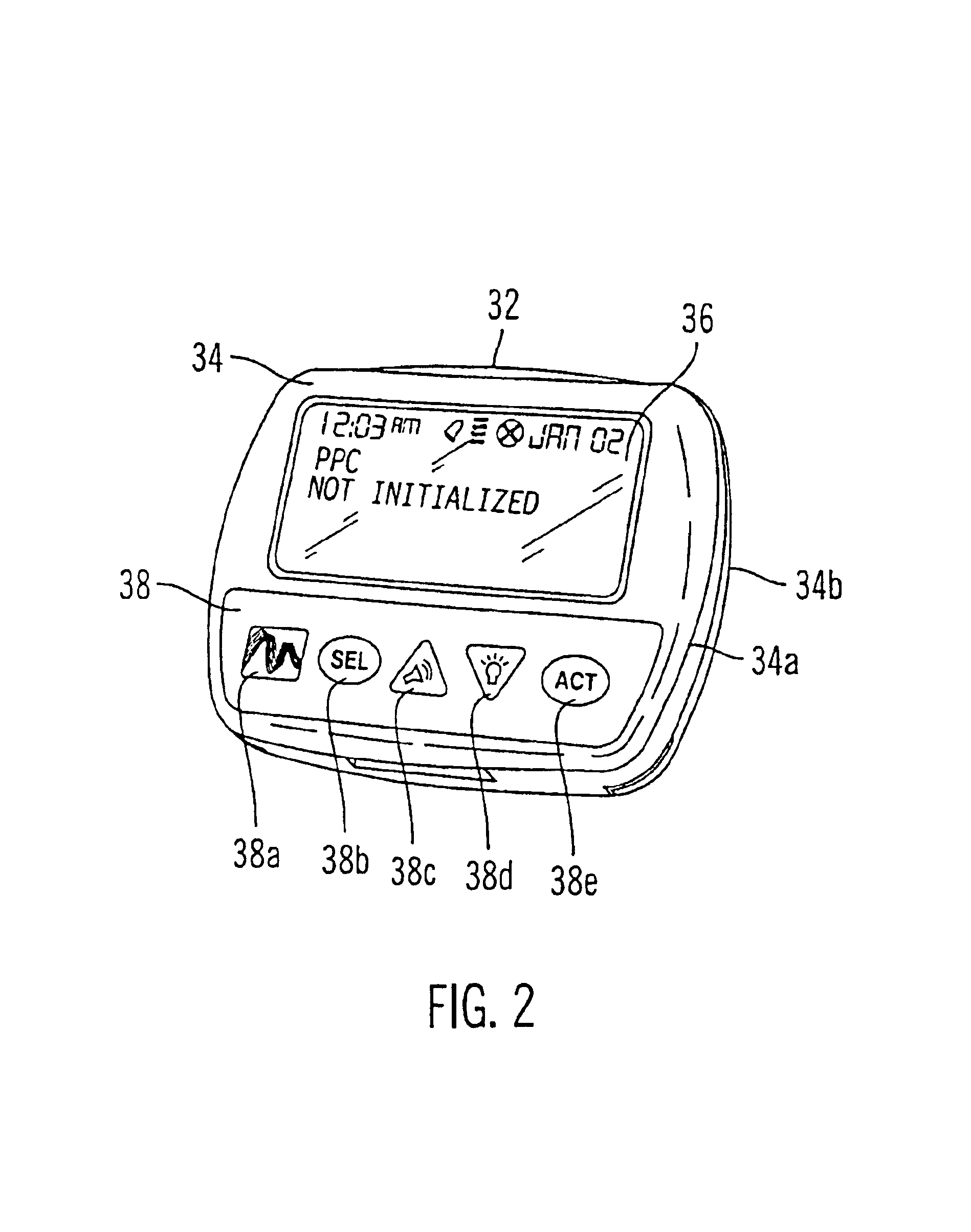
Microprocessor controlled ambulatory medical apparatus with hand held communication device
InactiveUS6873268B2Enhance user interfaceReduce system sizeEnergy efficient ICTElectrotherapyDrugs infusionHand held
An implantable infusion pump possesses operational functionality that is, at least in part, controlled by software operating in two processor ICs which are configured to perform some different and some duplicate functions. The pump exchanges messages with an external device via telemetry. Each processor controls a different part of the drug infusion mechanism such that both processors must agree on the appropriateness of drug delivery for infusion to occur. Delivery accumulators are incremented and decremented with delivery requests and with deliveries made. When accumulated amounts reach or exceed, quantized deliverable amounts, infusion is made to occur. The accumulators are capable of being incremented by two or more independent types of delivery requests. Operational modes of the infusion device are changed automatically in view of various system errors that are trapped, various system alarm conditions that are detected, and when excess periods of time lapse between pump and external device interactions.
Owner:MEDTRONIC MIMIMED INC
Integrated delivery device for continuous glucose sensor
Systems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM
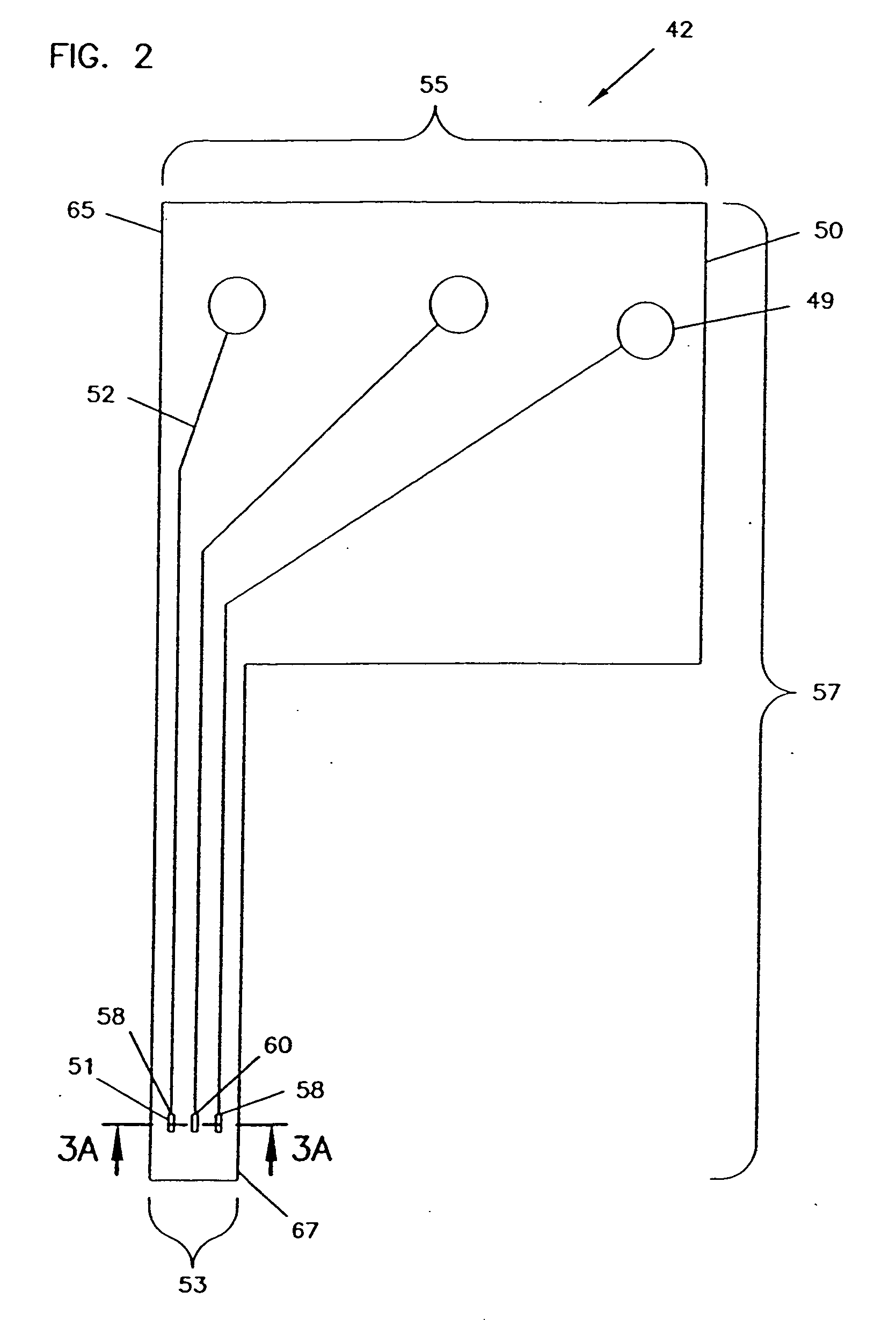
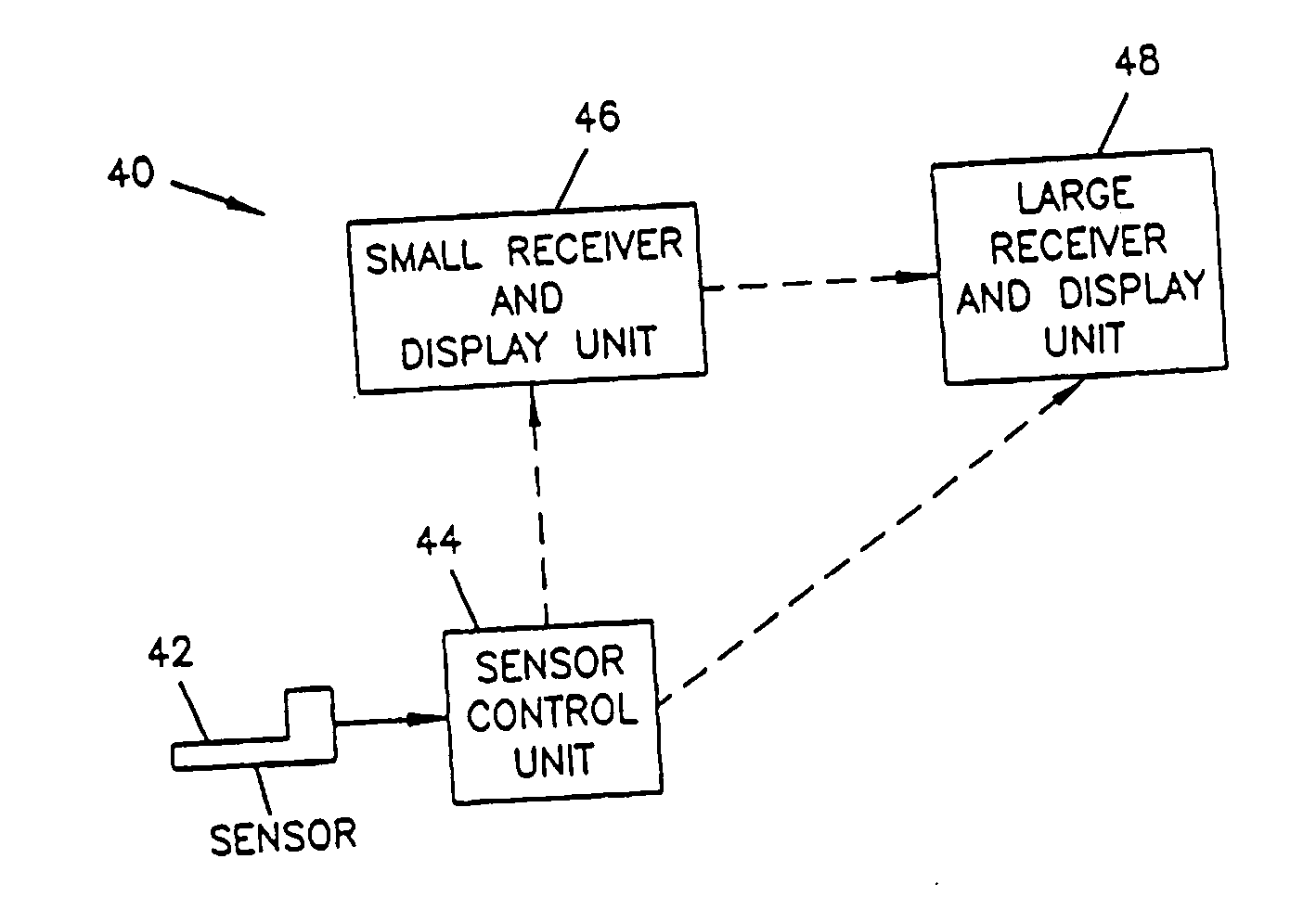
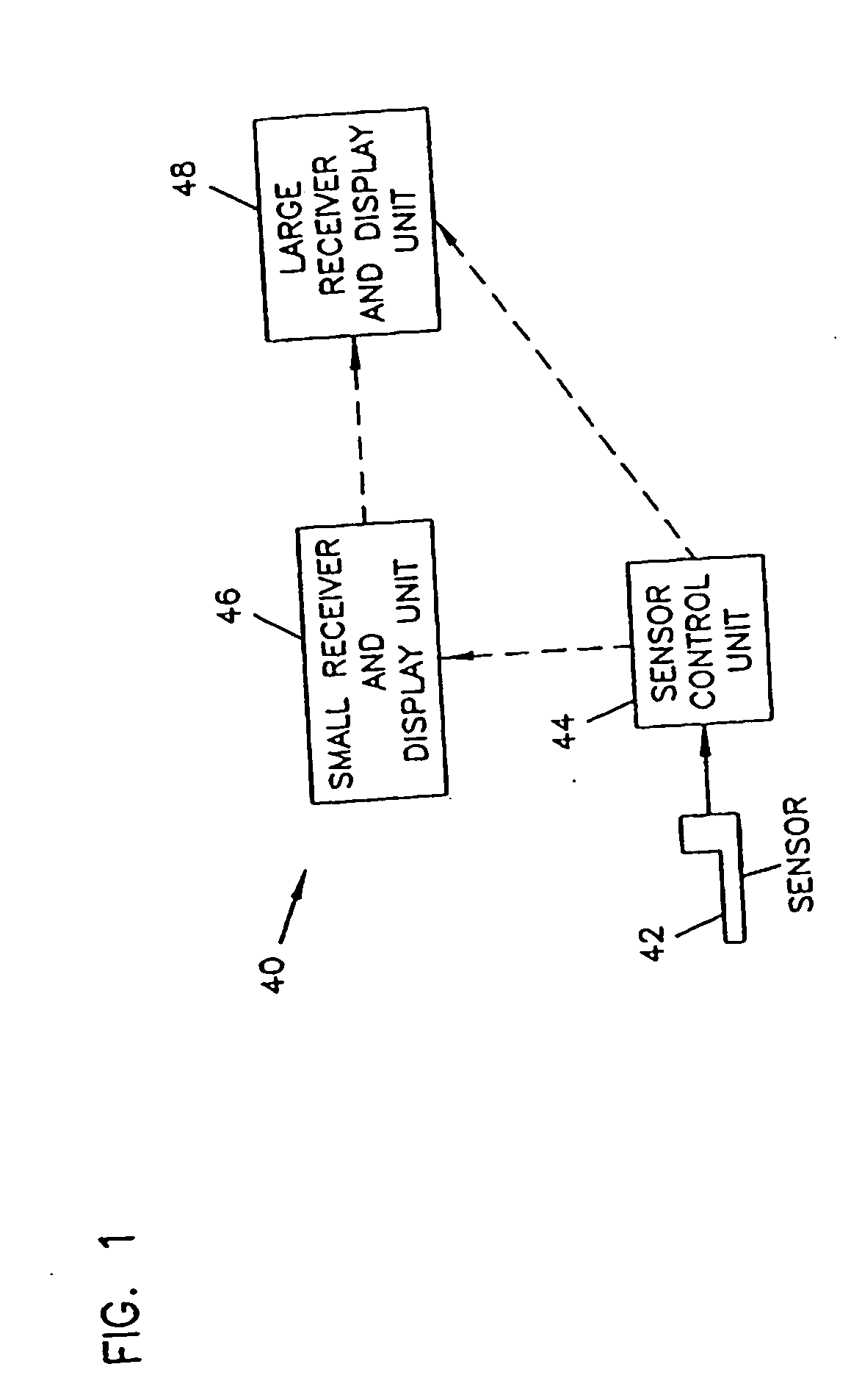
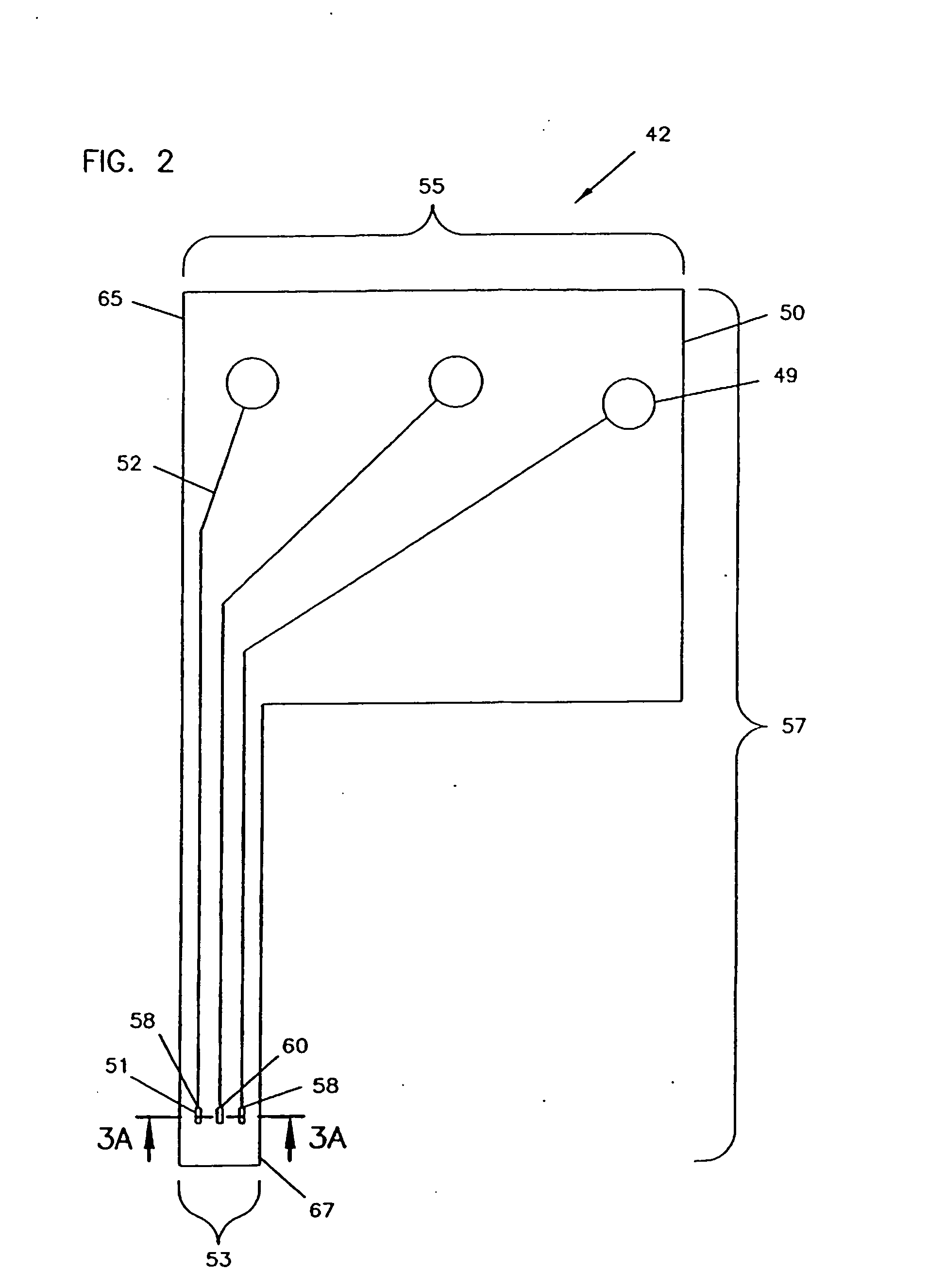
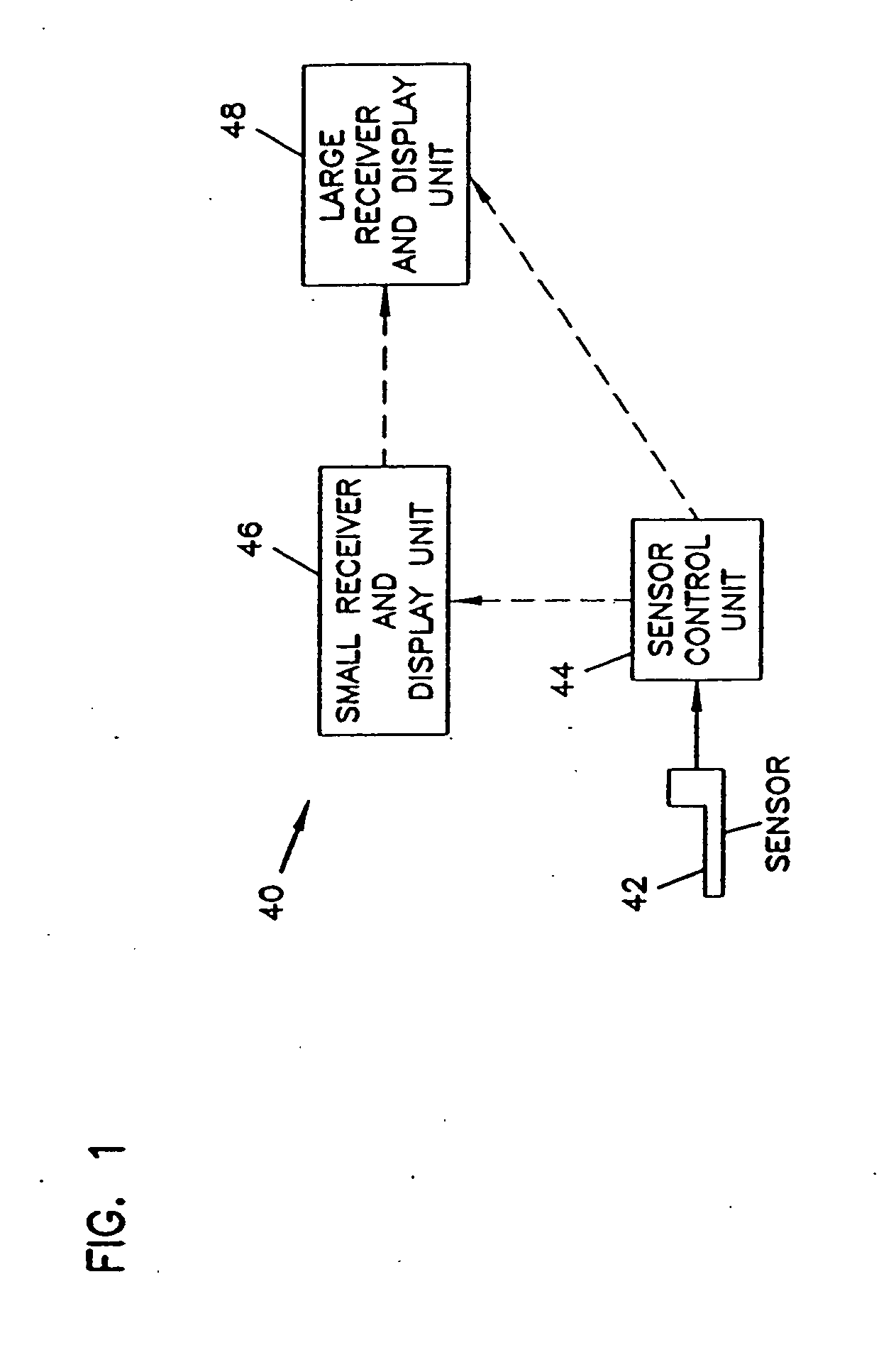
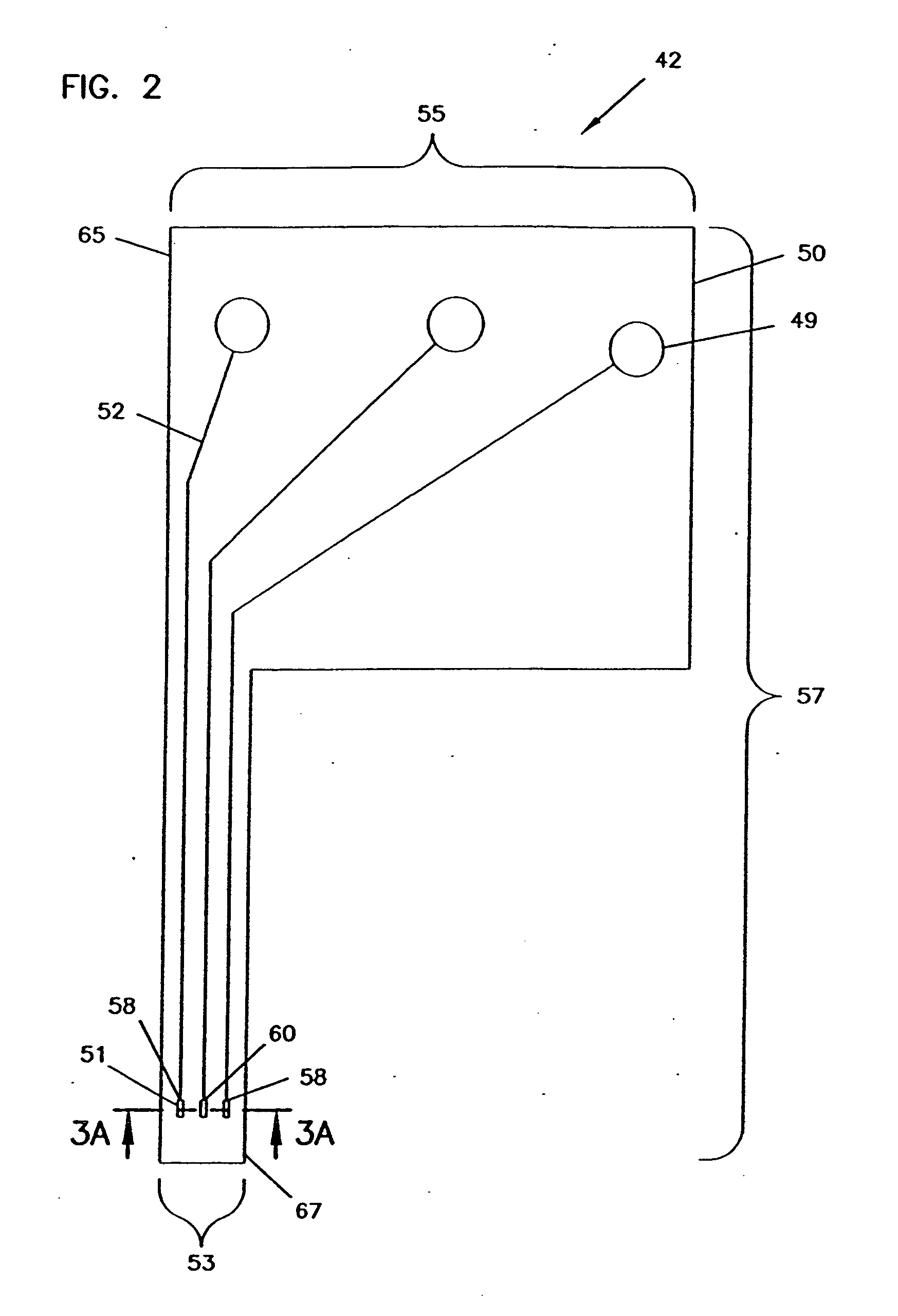
Analyte monitoring device and methods of use
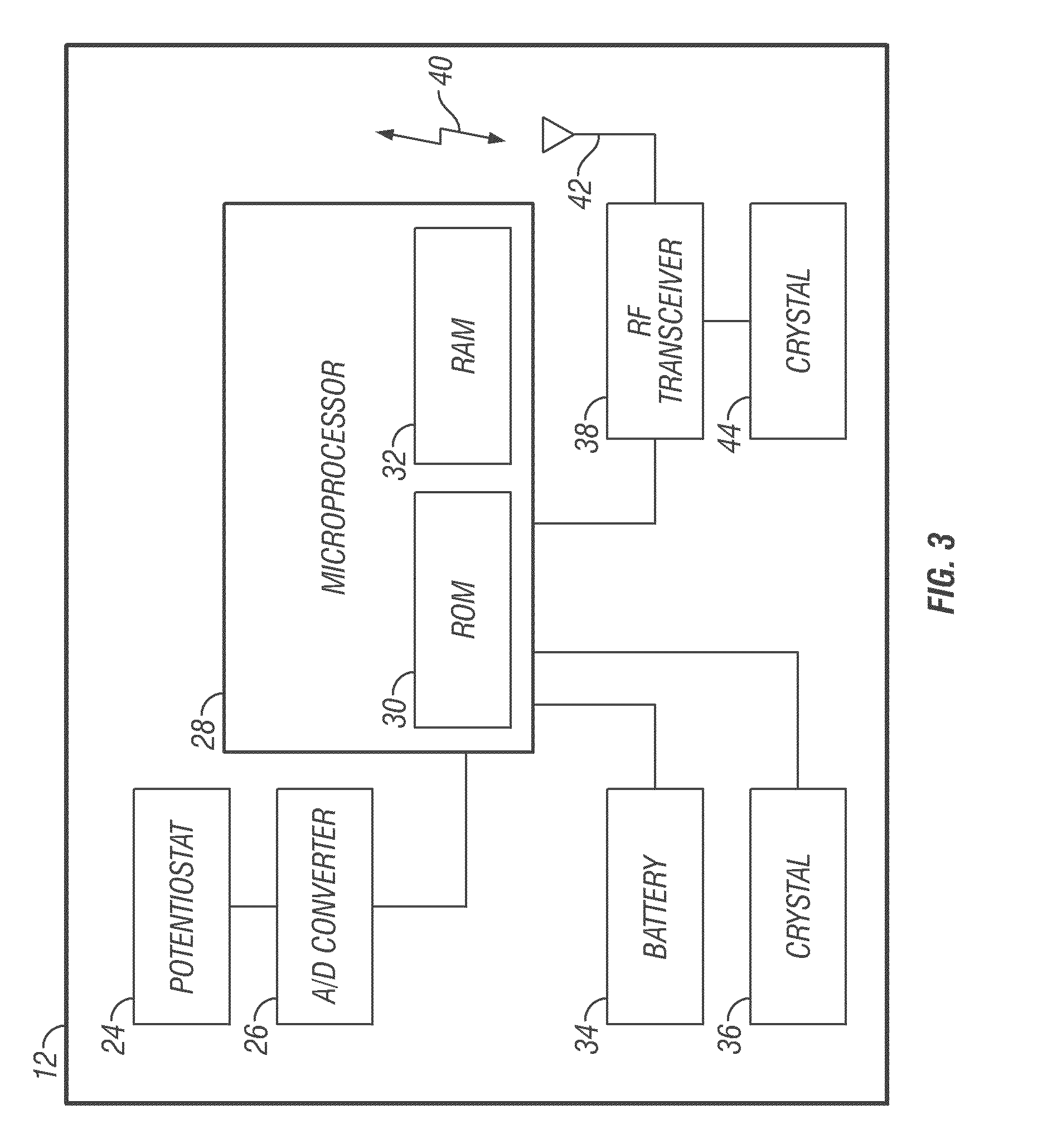
An analyte monitor includes a sensor, a sensor control unit, and a display unit. The sensor has, for example, a substrate, a recessed channel formed in the substrate, and conductive material disposed in the recessed channel to form a working electrode. The sensor control unit typically has a housing adapted for placement on skin and is adapted to receive a portion of an electrochemical sensor. The sensor control unit also includes two or more conductive contacts disposed on the housing and configured for coupling to two or more contact pads on the sensor. A transmitter is disposed in the housing and coupled to the plurality of conductive contacts for transmitting data obtained using the sensor. The display unit has a receiver for receiving data transmitted by the transmitter of the sensor control unit and a display coupled to the receiver for displaying an indication of a level of an analyte. The analyte monitor may also be part of a drug delivery system to alter the level of the analyte based on the data obtained using the sensor.
Owner:THERASENSE
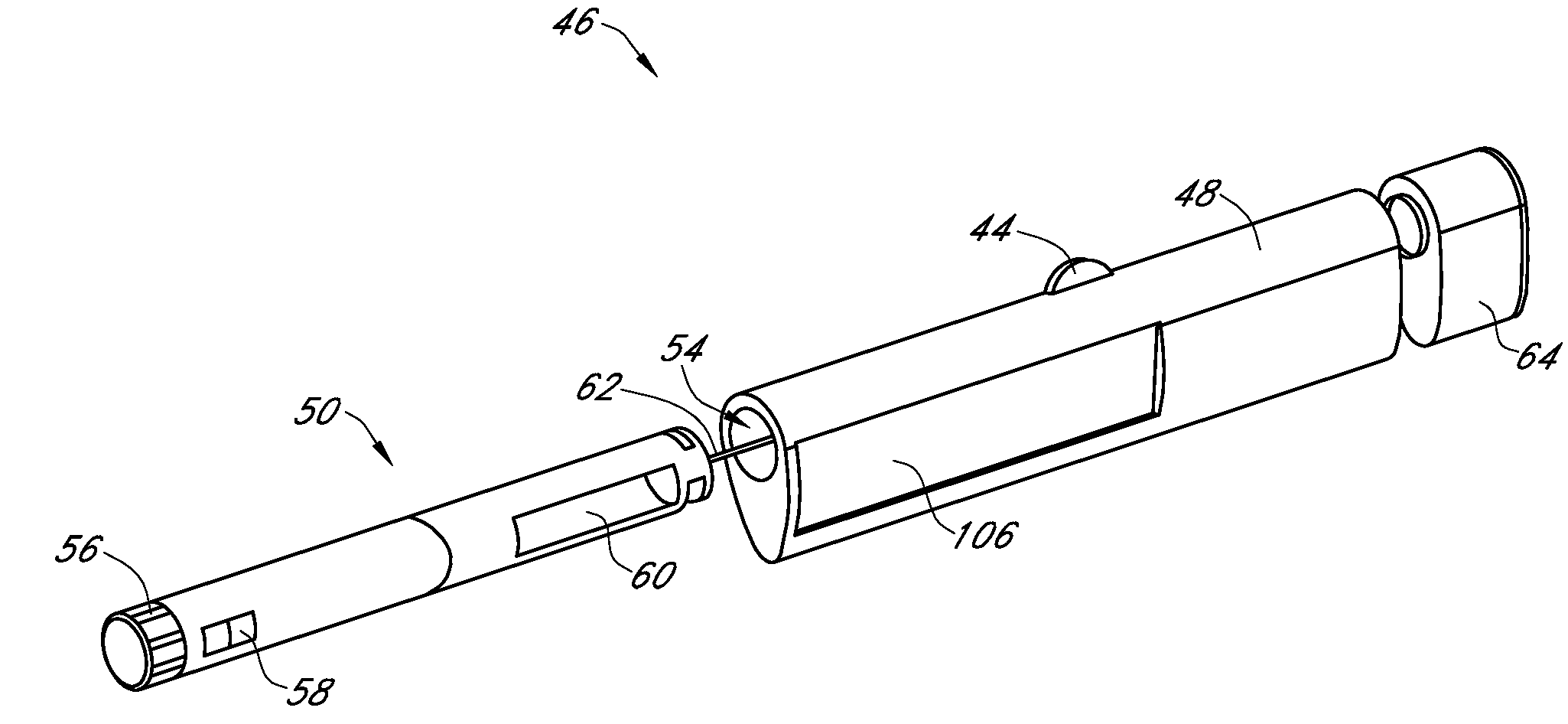
Integrated medicament delivery device for use with continuous analyte sensor
InactiveUS20080306434A1Reduce the burden onSimple processAmpoule syringes2D-image generationDiabetes mellitusMedication injection
An integrated system for the monitoring and treating diabetes is provided, including an integrated receiver / hand-held medicament injection pen, including electronics, for use with a continuous glucose sensor. In some embodiments, the receiver is configured to receive continuous glucose sensor data, to calculate a medicament therapy (e.g., via the integrated system electronics) and to automatically set a bolus dose of the integrated hand-held medicament injection pen, whereby the user can manually inject the bolus dose of medicament into the host. In some embodiments, the integrated receiver and hand-held medicament injection pen are integrally formed, while in other embodiments they are detachably connected and communicated via mutually engaging electrical contacts and / or via wireless communication.
Owner:DEXCOM
Intraocular physiological sensor
An implantable intraocular physiological sensor for measuring intraocular pressure, glucose concentration in the aqueous humor, and other physiological characteristics. The implantable intraocular physiological sensor may be at least partially powered by a fuel cell, such as an electrochemical glucose fuel cell. The implantable intraocular physiological sensor may wirelessly transmit measurements to an external device. In addition, the implantable intraocular physiological sensor may incorporate aqueous drainage and / or drug delivery features.
Owner:GLAUKOS CORP
Integrated medicament delivery device for use with continuous analyte sensor
ActiveUS20080306435A1Reduce the burden onSimple processAmpoule syringes2D-image generationMedication injectionDiabetes mellitus
An integrated system for the monitoring and treating diabetes is provided, including an integrated receiver / hand-held medicament injection pen, including electronics, for use with a continuous glucose sensor. In some embodiments, the receiver is configured to receive continuous glucose sensor data, to calculate a medicament therapy (e.g., via the integrated system electronics) and to automatically set a bolus dose of the integrated hand-held medicament injection pen, whereby the user can manually inject the bolus dose of medicament into the host. In some embodiments, the integrated receiver and hand-held medicament injection pen are integrally formed, while in other embodiments they are detachably connected and communicated via mutually engaging electrical contacts and / or via wireless communication.
Owner:DEXCOM
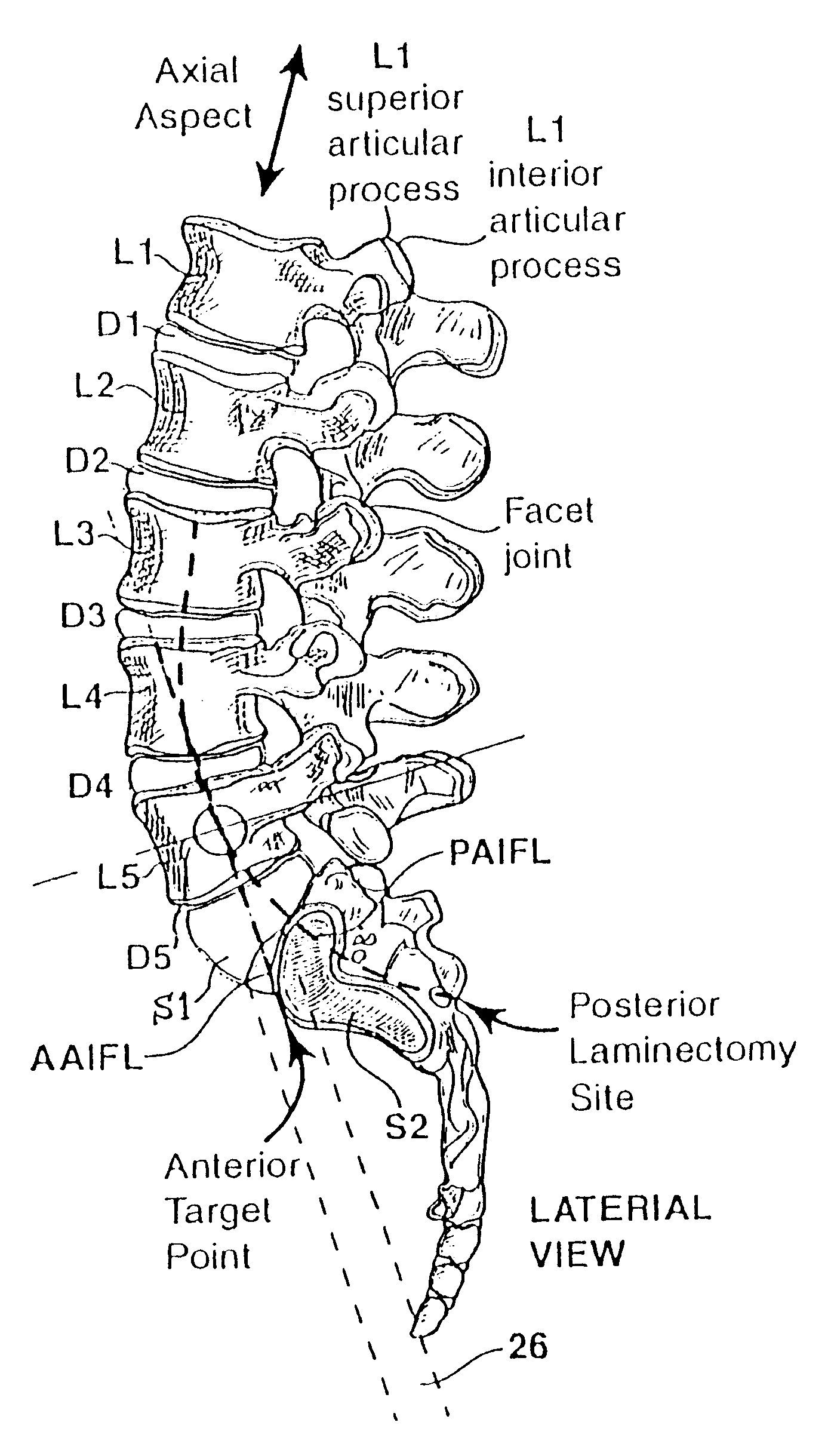
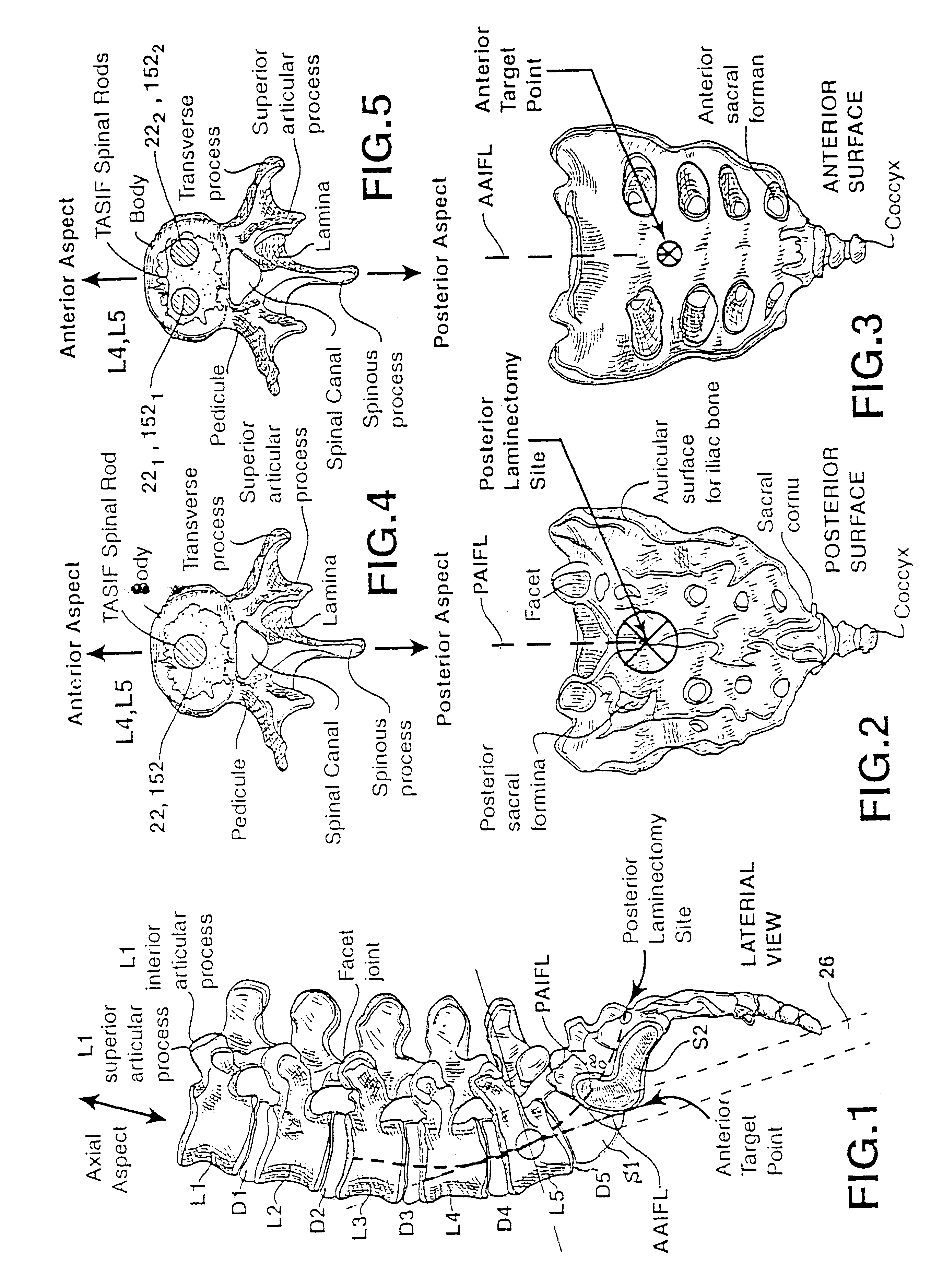
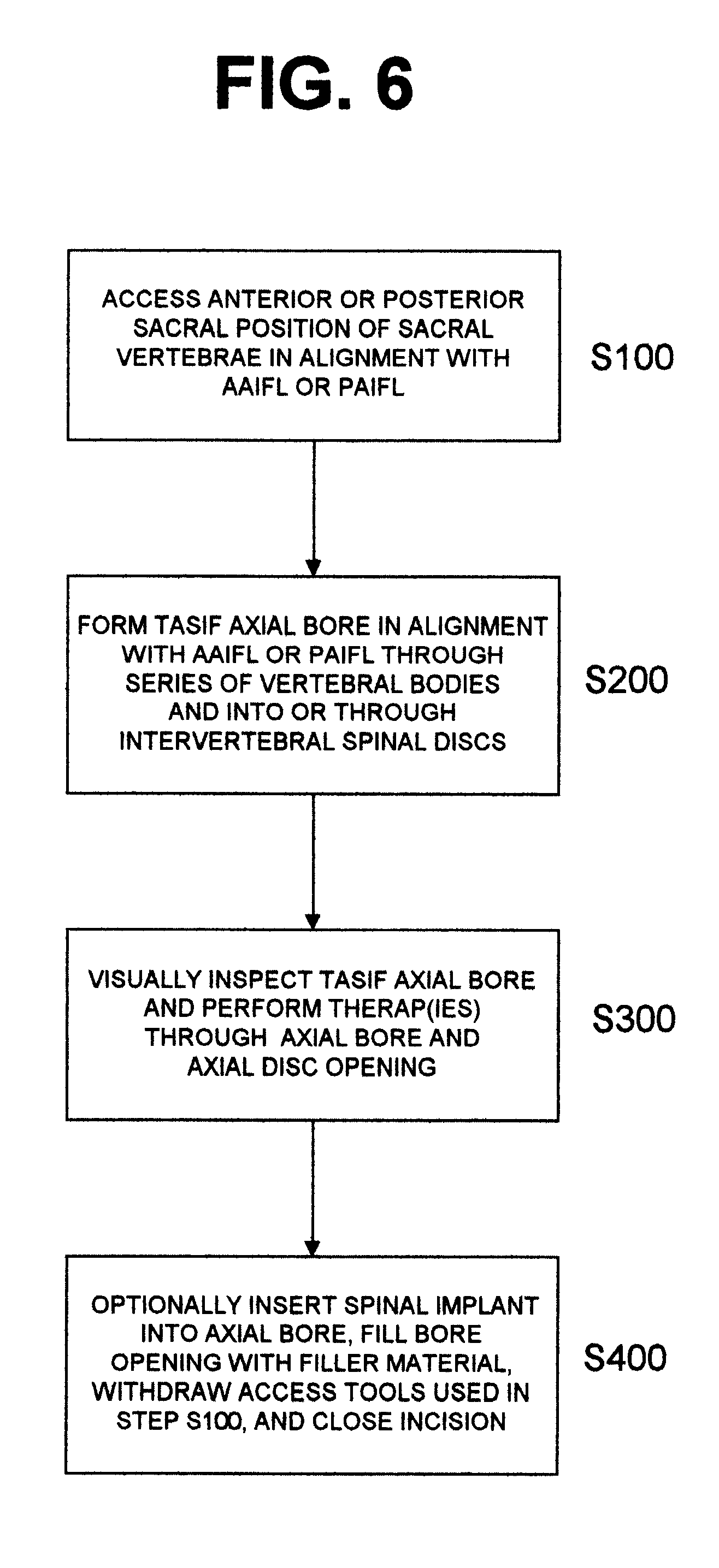
Methods and apparatus for performing therapeutic procedures in the spine
Methods and apparatus for forming one or more trans-sacral axial instrumentation / fusion (TASIF) axial bore through vertebral bodies in general alignment with a visualized, anterior or posterior axial instrumentation / fusion line (AAIFL or PAIFL) in a minimally invasive, low trauma, manner and providing a therapy to the spine employing the axial bore. Anterior or posterior starting positions aligned with the AAIFL or PAIFL are accessed through respective anterior and posterior tracts. Curved or relatively straight anterior and curved posterior TASIF axial bores are formed from the anterior and posterior starting positions. The therapies performed through the TASIF axial bores include discoscopy, full and partial discectomy, vertebroplasty, balloon-assisted vertebroplasty, drug delivery, electrical stimulation and various forms of spinal disc cavity augmentation, spinal disc replacement, fusion of spinal motion segments and implantation of radioactive seeds. Axial spinal implants and bone growth materials can be placed into single or multiple parallel or diverging TASIF axial bores to fuse two or more vertebrae, or distract or shock absorb two or more vertebrae.
Owner:MIS IP HLDG LLC
Medical devices with enhanced ultrasonic visibilty
InactiveUS20070197954A1Ultrasonic/sonic/infrasonic diagnosticsElectrotherapyTip positionSolid tissue
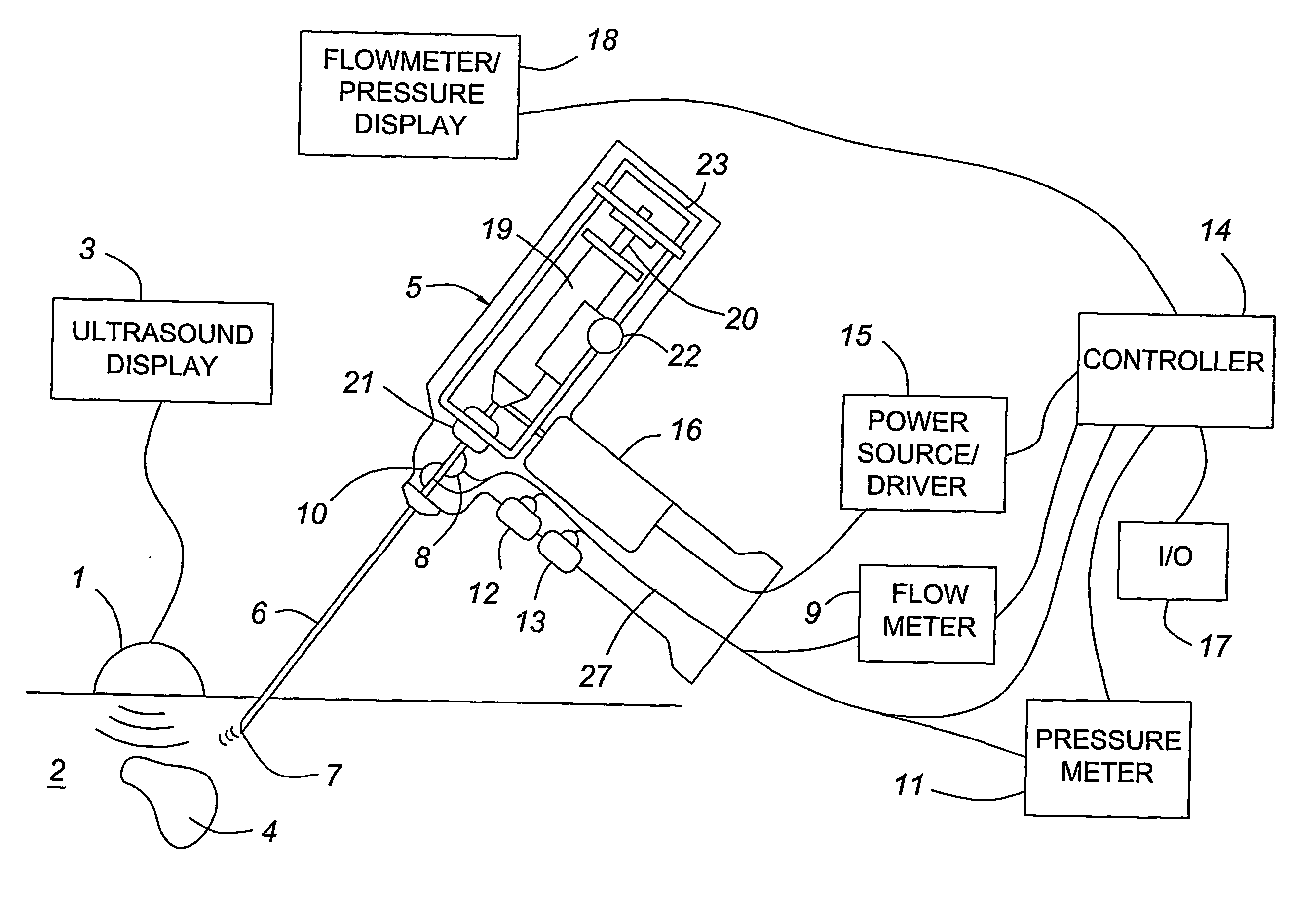
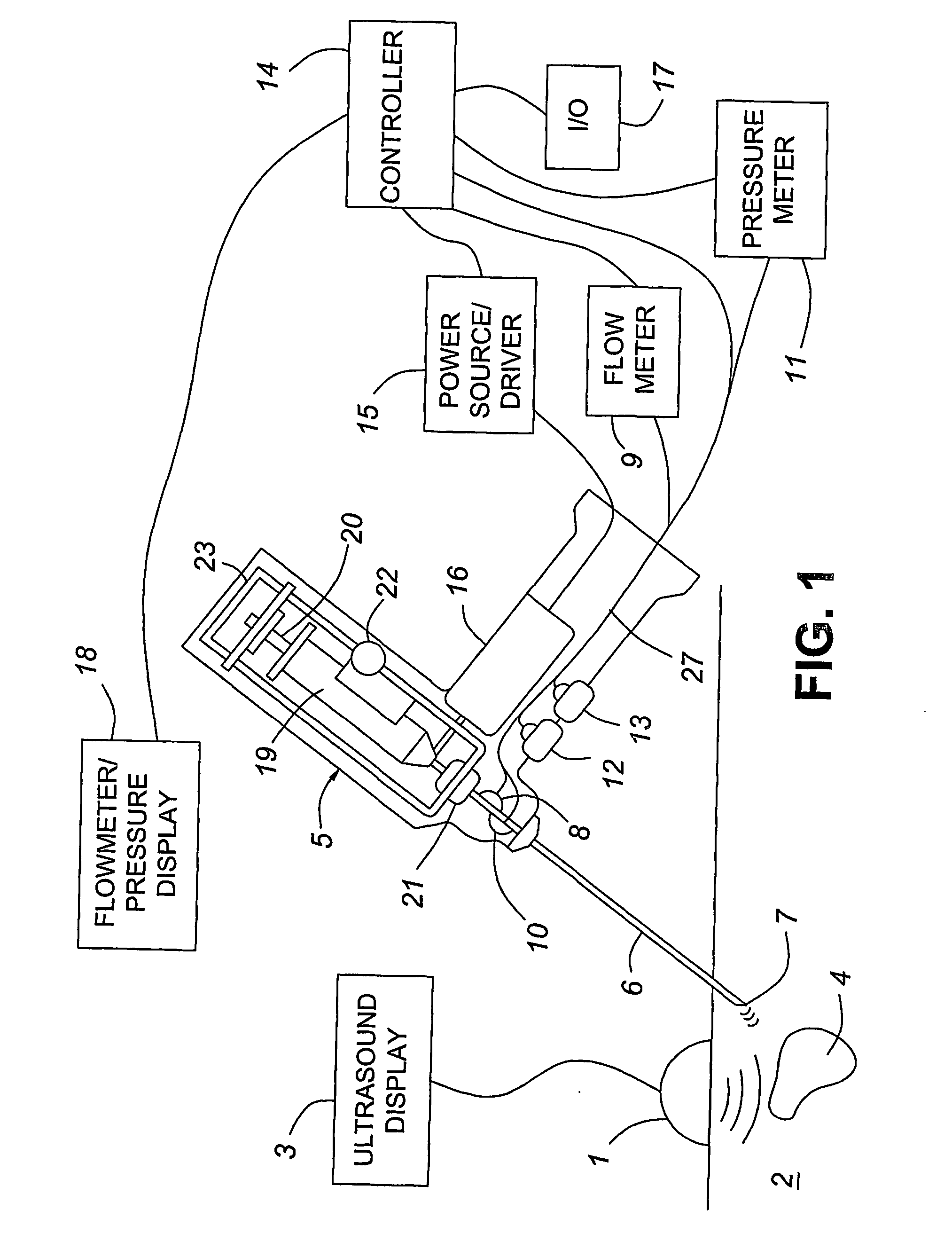
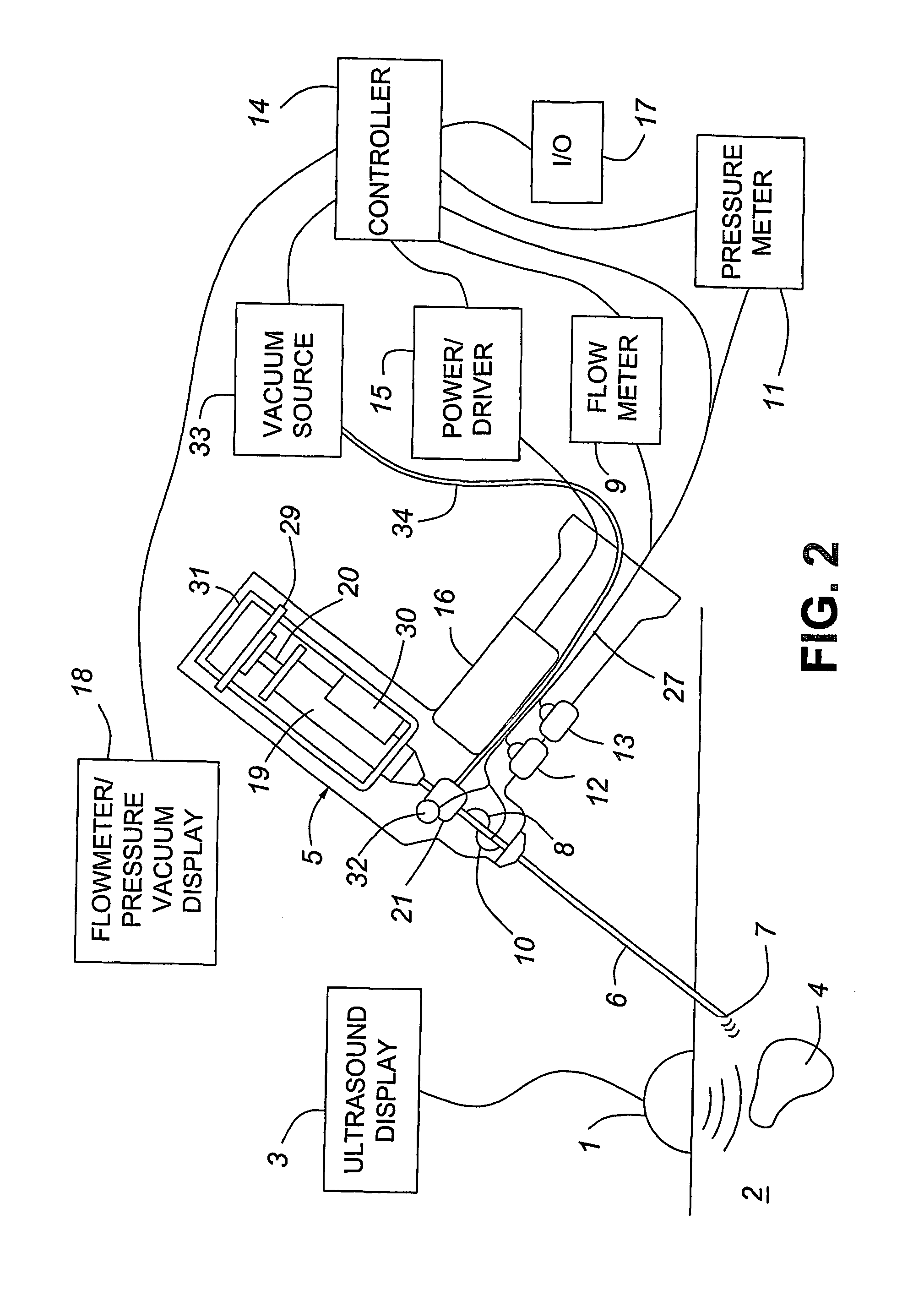
A medical device having enhanced ultrasonic visibility is provided. The device permits localized drug delivery, probe positioning, fluid drainage, biopsy, or ultrasound pulse delivery, through the real-time ultrasound monitoring of the needle tip position within a patient. The device permits controlled dispersion of a drug into solid tissue, the lodging of particles into solid tissue, and drug delivery into specific blood vessels. As a needle is inserted, a fluid that contrasts echogenically with the organ environment is injected into the patient. The fluid travels a brief distance before being slowed and stopped by the patient's tissue and this fluid flow will be detectable by ultrasound. The needle position during insertion will be monitored using ultrasound until it is at the desired point of action. A therapeutic drug is then delivered or a probe inserted
Owner:ARTENGA
Integrated delivery device for continuous glucose sensor
Systems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM INC
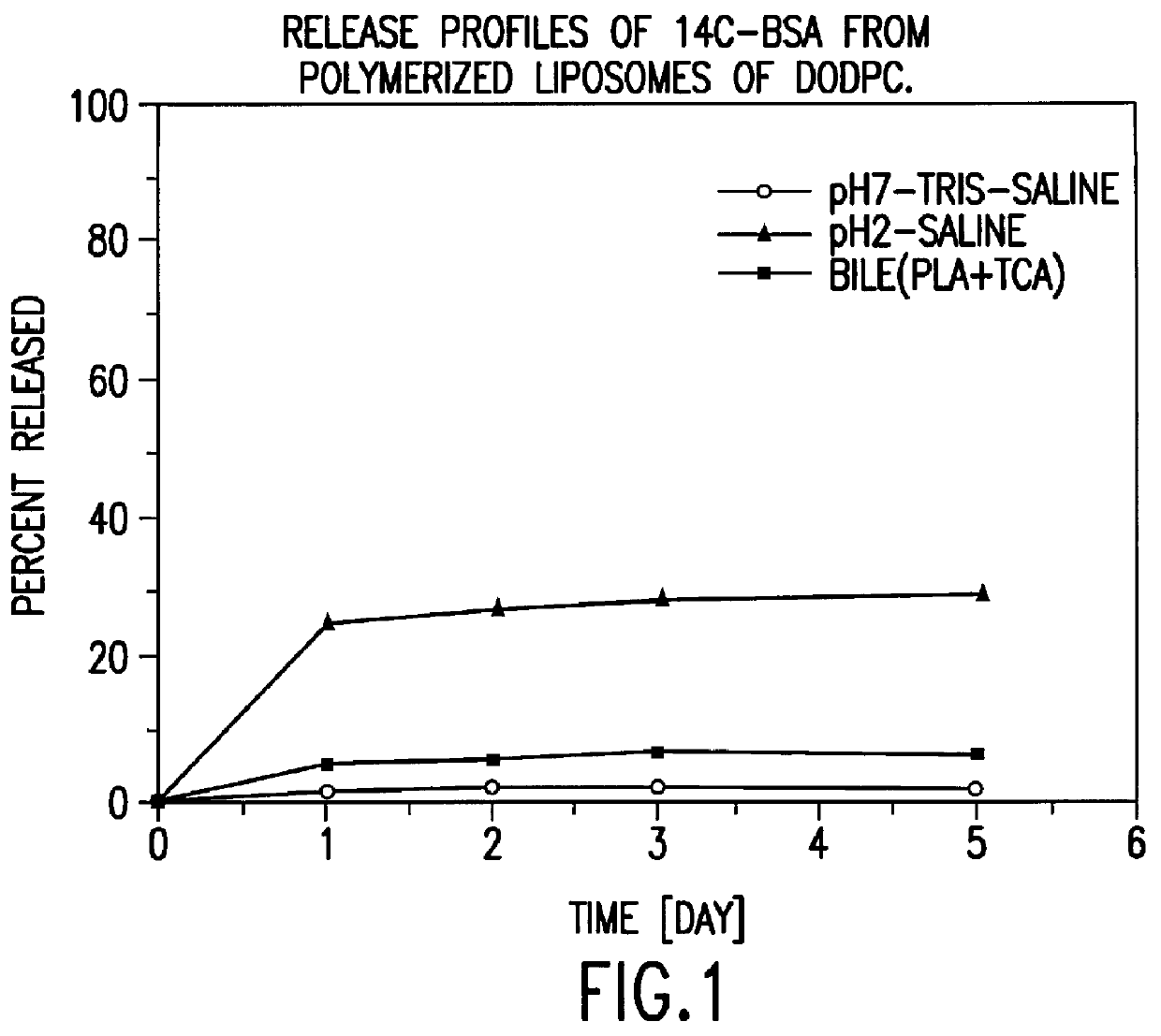
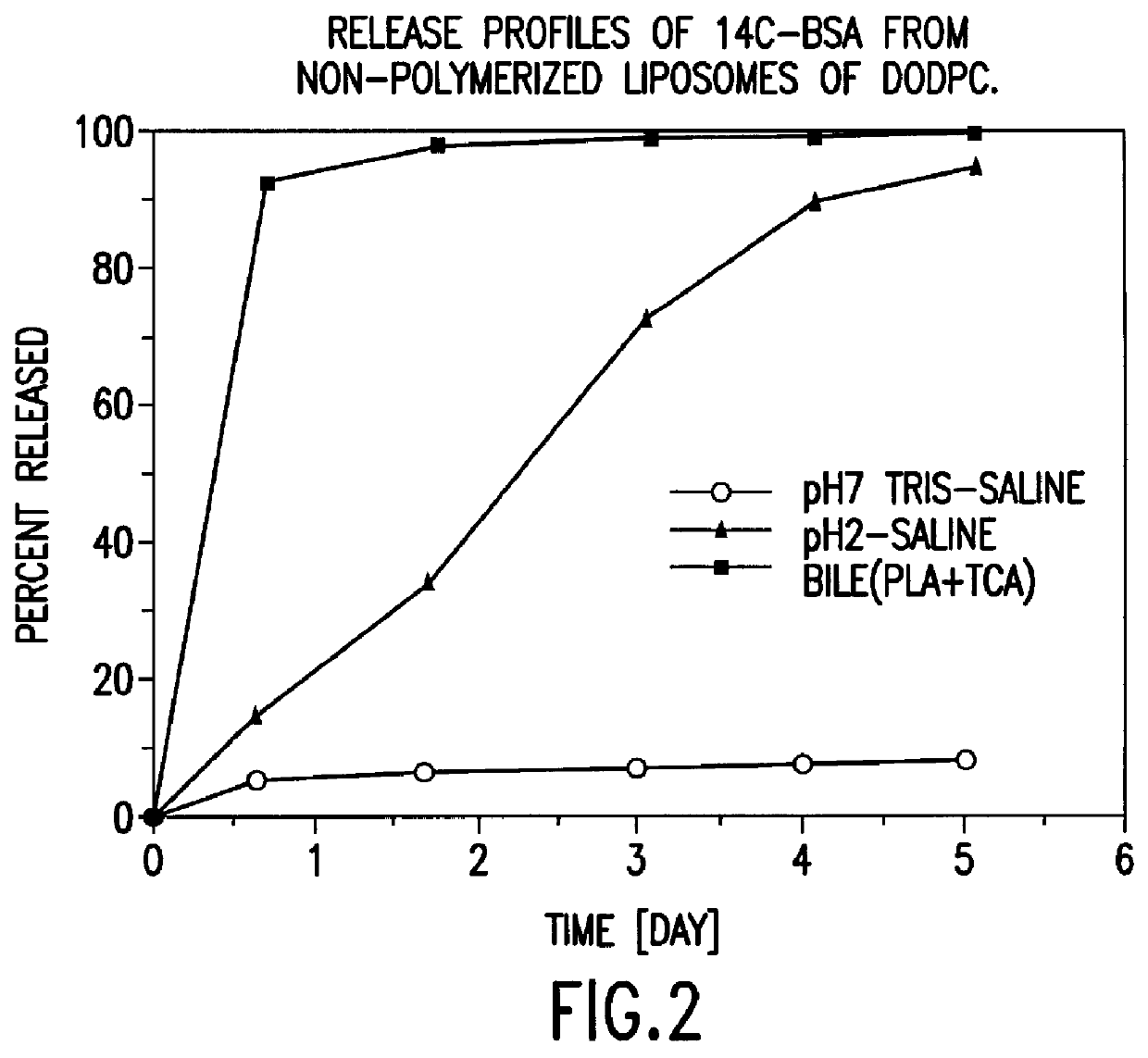
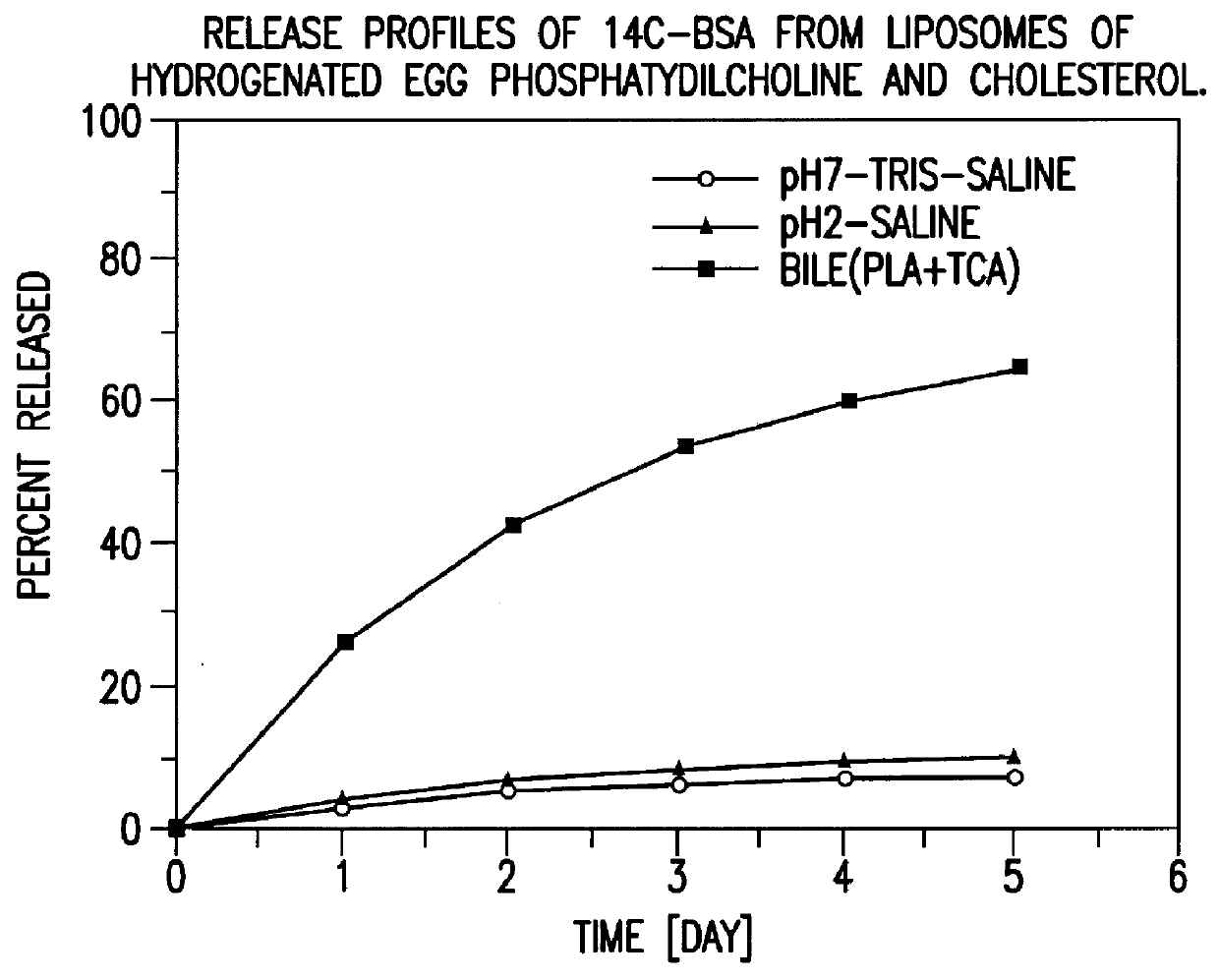
Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery
InactiveUS6060082ALessContainment leakBacterial antigen ingredientsPeptide/protein ingredientsCell typeLiposome
The present invention relates to targeted polymerized liposomes for oral and / or mucosal delivery of vaccines, allergens and therapeutics. In particular, the present invention relates to polymerized liposomes which have been modified on their surface to contain a molecule or ligand which targets the polymerized liposome to a specific site or cell type. More particularly, the invention relates to the use of polymerized liposomes modified to contain a carbohydrate or lectin on their surface.
Owner:MASSACHUSETTS INST OF TECH
Analyte monitoring device and methods of use
An analyte monitor includes a sensor, a sensor control unit, and a display unit. The sensor has, for example, a substrate, a recessed channel formed in the substrate, and conductive material disposed in the recessed channel to form a working electrode. The sensor control unit typically has a housing adapted for placement on skin and is adapted to receive a portion of an electrochemical sensor. The sensor control unit also includes two or more conductive contacts disposed on the housing and configured for coupling to two or more contact pads on the sensor. A transmitter is disposed in the housing and coupled to the plurality of conductive contacts for transmitting data obtained using the sensor. The display unit has a receiver for receiving data transmitted by the transmitter of the sensor control unit and a display coupled to the receiver for displaying an indication of a level of an analyte. The analyte monitor may also be part of a drug delivery system to alter the level of the analyte based on the data obtained using the sensor.
Owner:ABBOTT DIABETES CARE INC
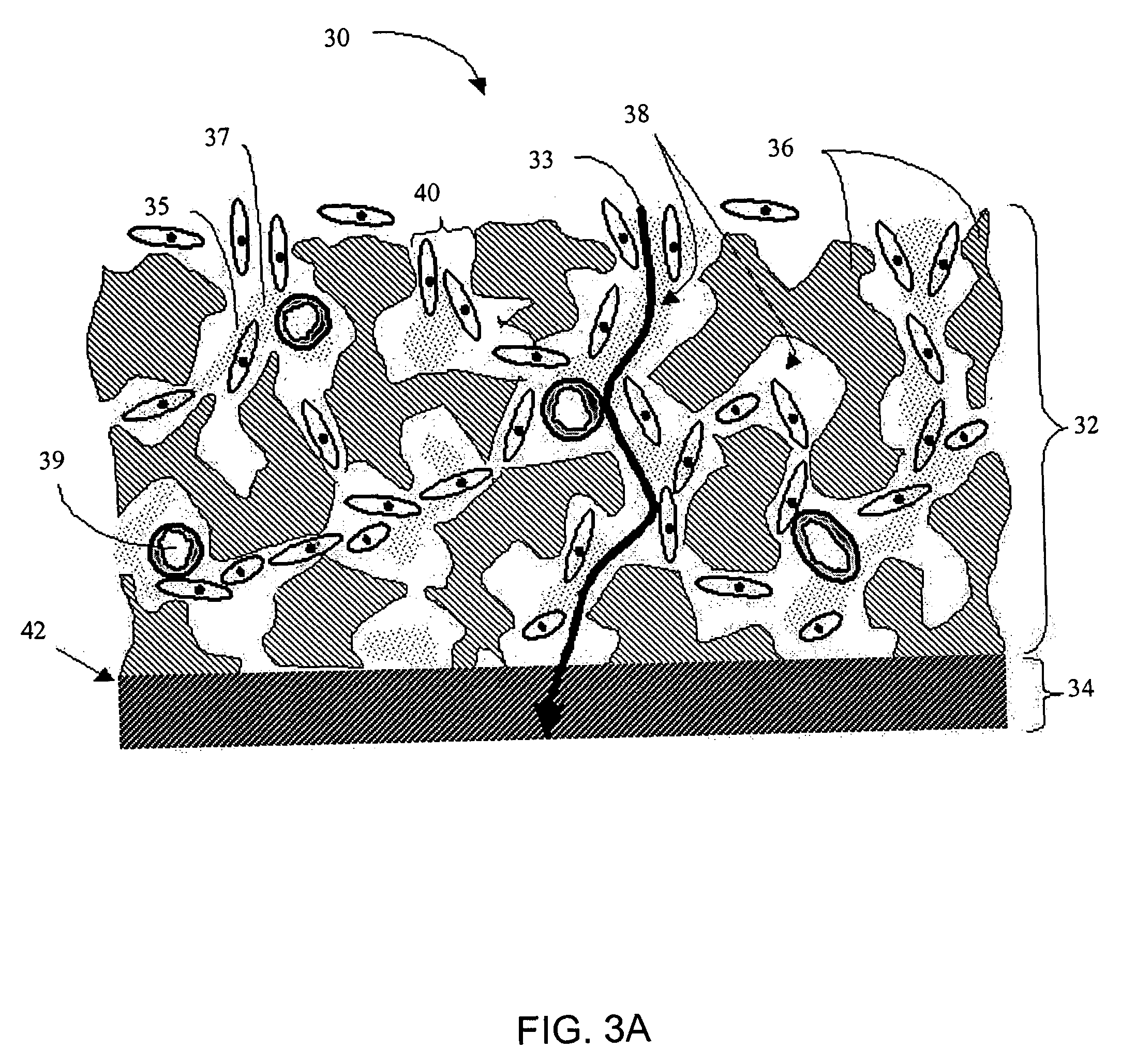
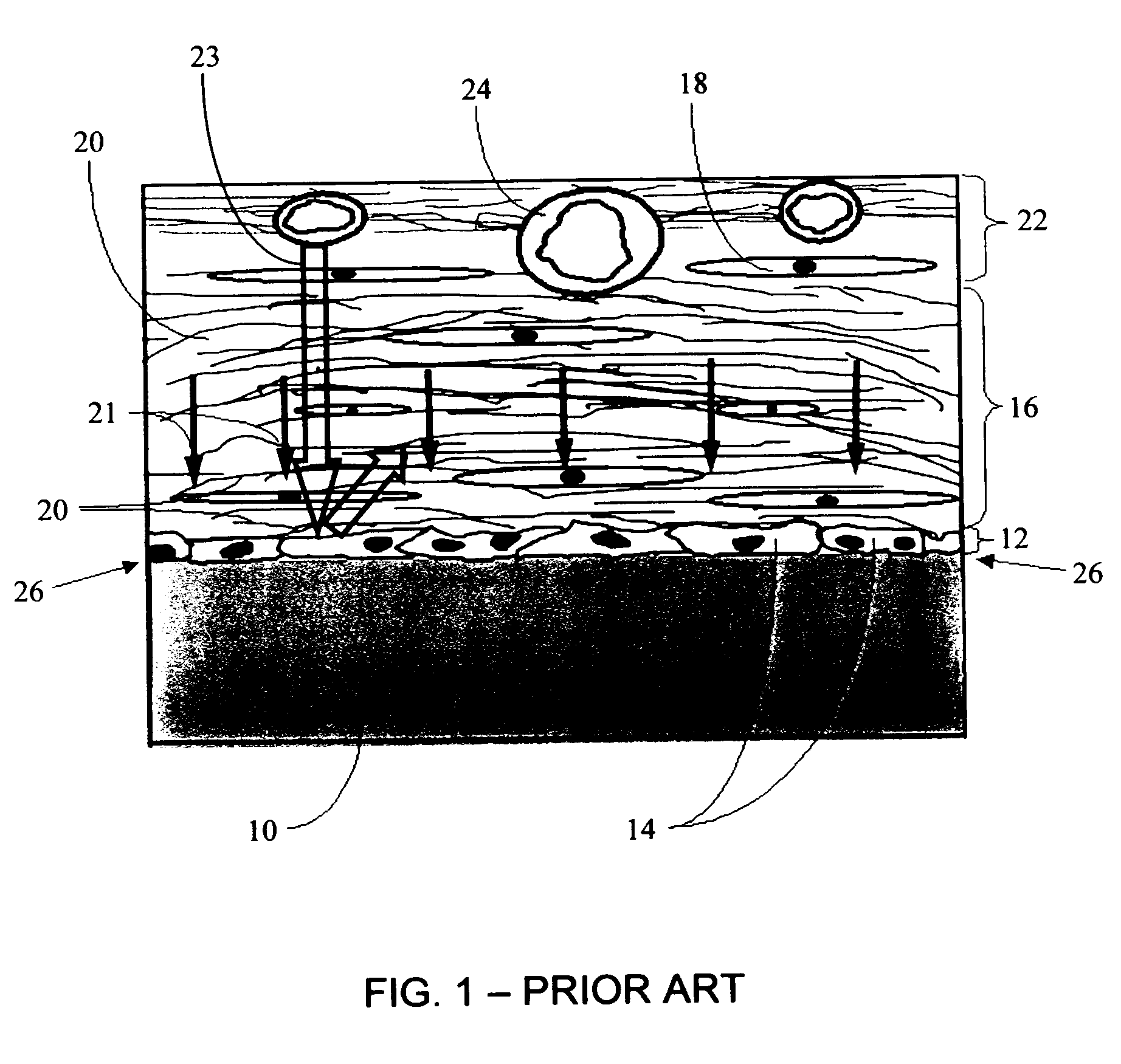
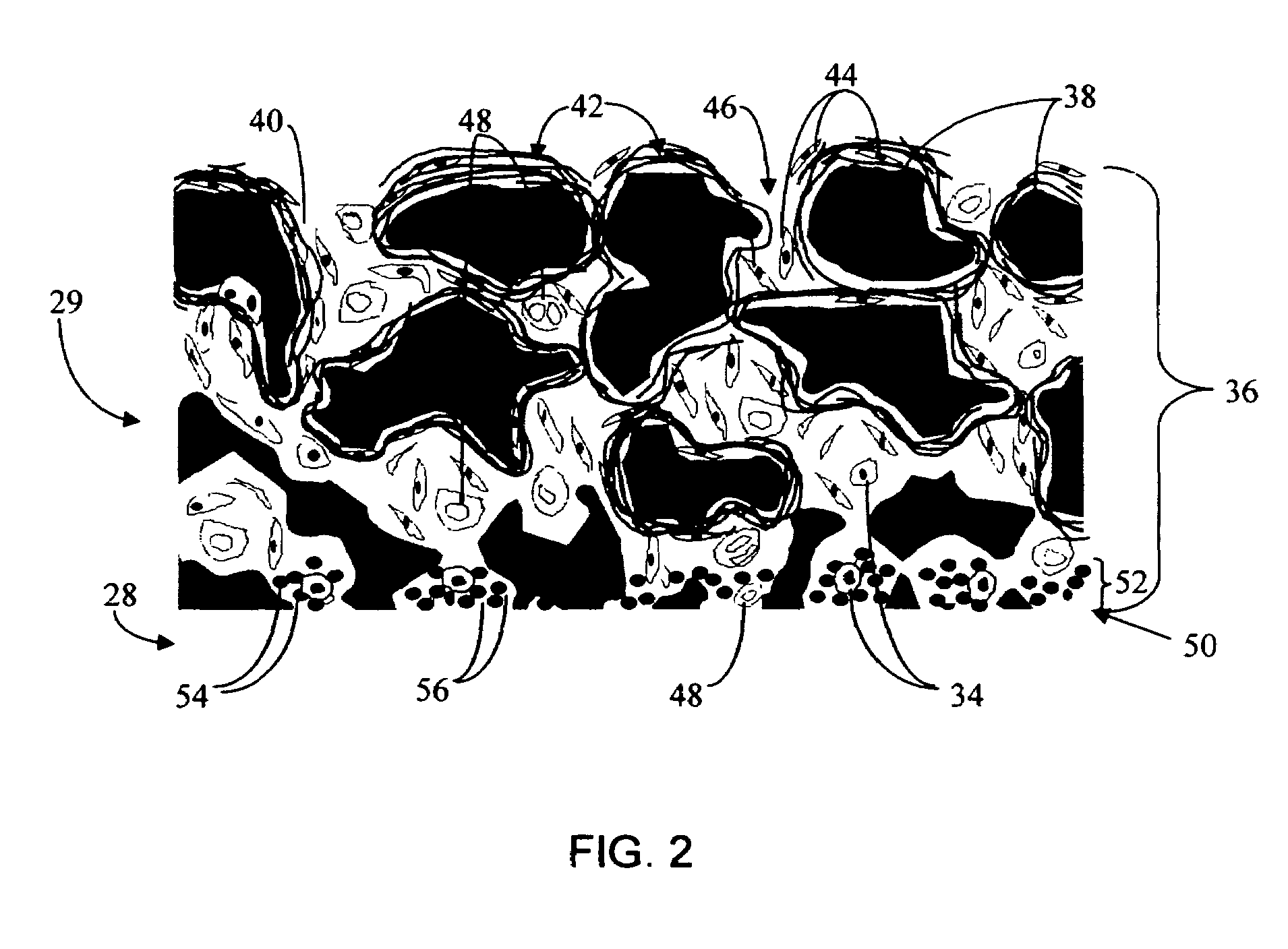
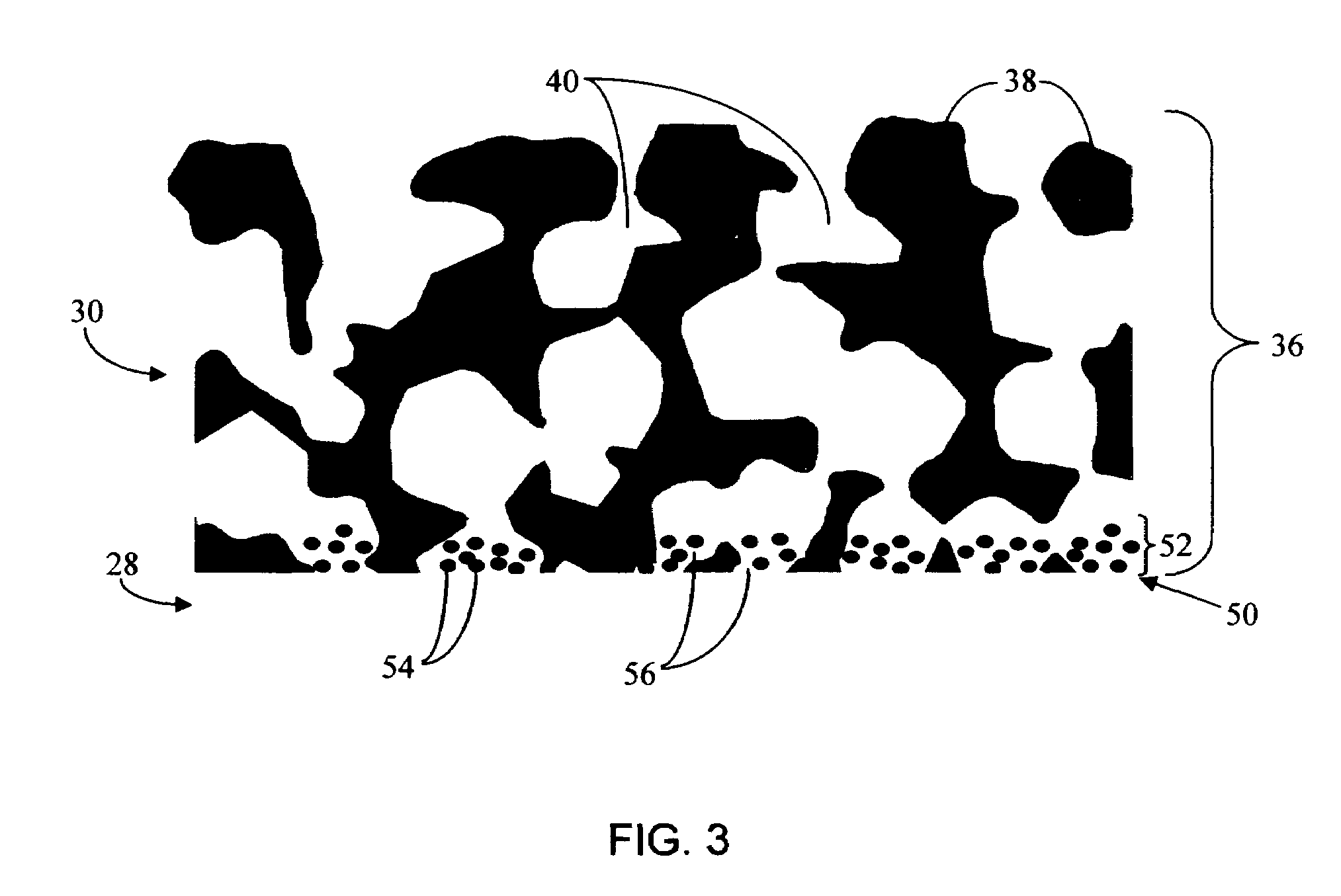
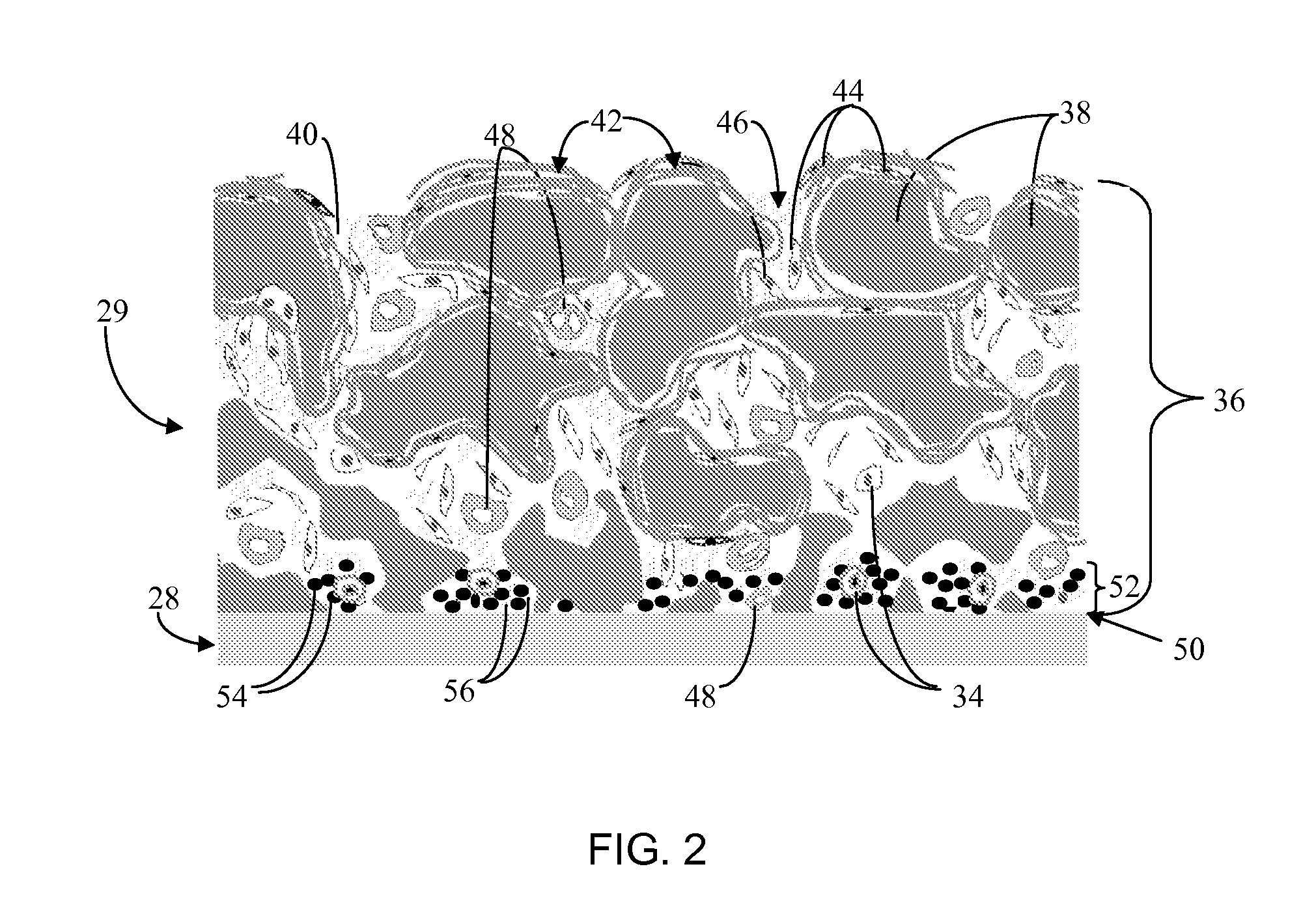
Biointerface membrane with macro-and micro-architecture
Disclosed herein are biointerface membranes including a macro-architecture and a micro-architecture co-continuous with and bonded to and / or located within at least a portion of the macro-architecture. The macro- and micro-architectures work together to manage and manipulate the high-level tissue organization and the low-level cellular organization of the foreign body response in vivo, thereby increasing neovascularization close to a device-tissue interface, interfering with barrier cell layer formation, and providing good tissue anchoring, while reducing the effects of motion artifact, and disrupting the organization and / or contracture of the FBC. The biointerface membranes of the preferred embodiments can be utilized with implantable devices such as devices for the detection of analyte concentrations in a biological sample (for example, from a body), cell transplantation devices, drug delivery devices, electrical signal delivering or measuring devices, and / or combinations thereof.
Owner:DEXCOM
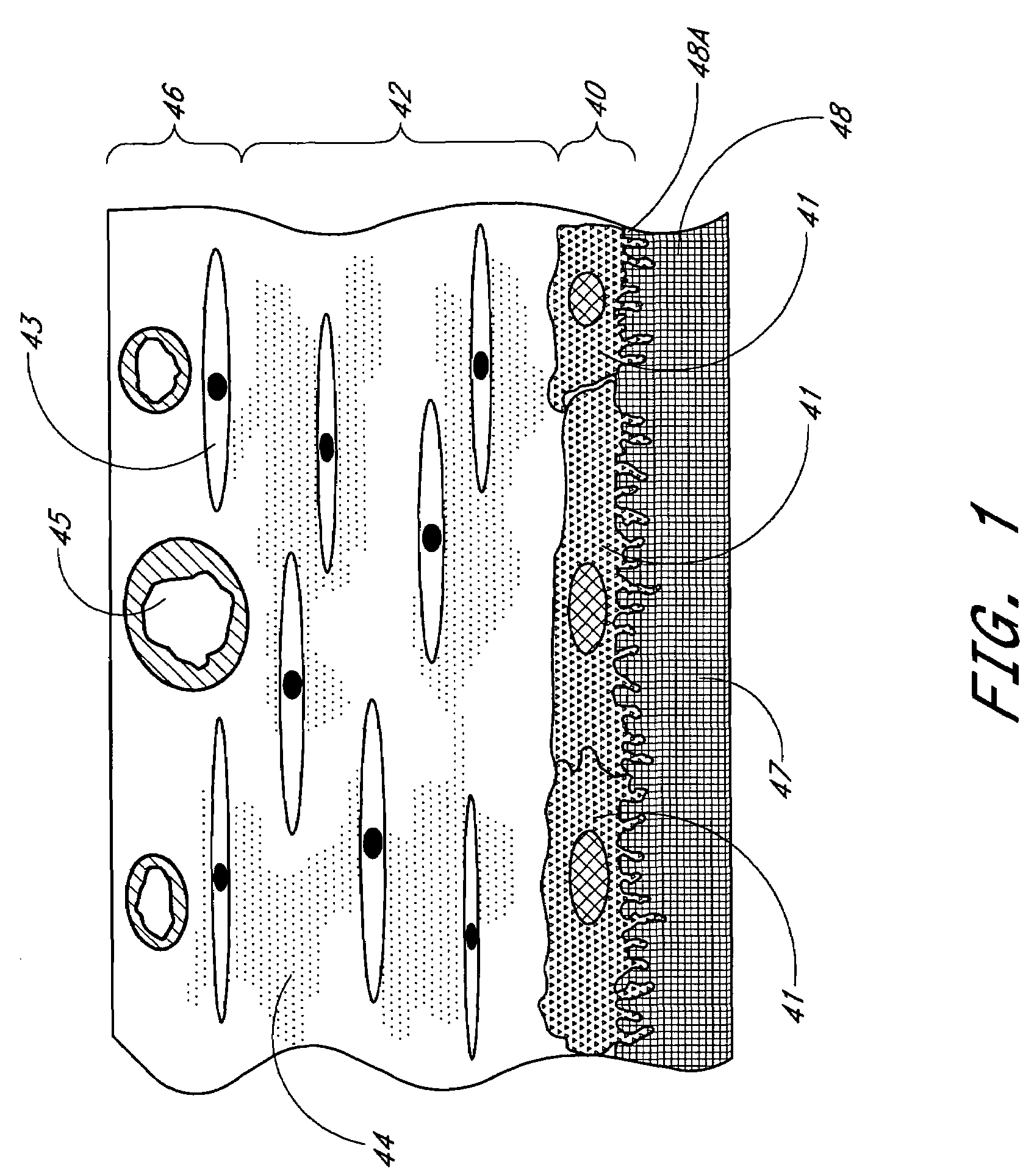
Membrane for use with implantable devices
The present invention provides a biointerface membrane for use with an implantable device that interferes with the formation of a barrier cell layer including; a first domain distal to the implantable device wherein the first domain supports tissue attachment and interferes with barrier cell layer formation and a second domain proximal to the implantable device wherein the second domain is resistant to cellular attachment and is impermeable to cells. In addition, the present invention provides sensors including the biointerface membrane, implantable devices including these sensors or biointerface membranes, and methods of monitoring glucose levels in a host utilizing the analyte detection implantable device of the invention. Other implantable devices which include the biointerface membrane of the present invention, such as devices for cell transplantation, drug delivery devices, and electrical signal delivery or measuring devices are also provided.
Owner:DEXCOM
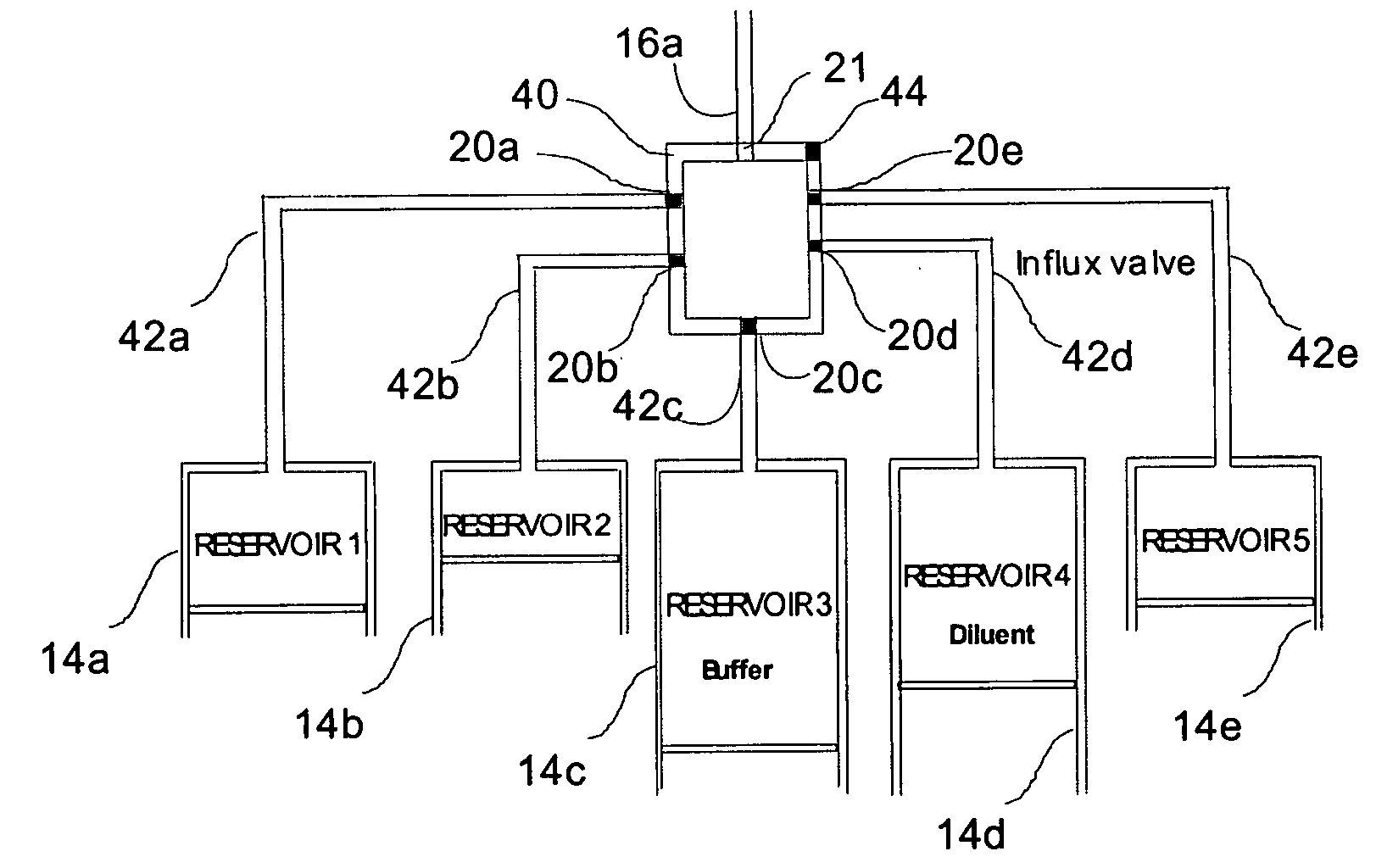
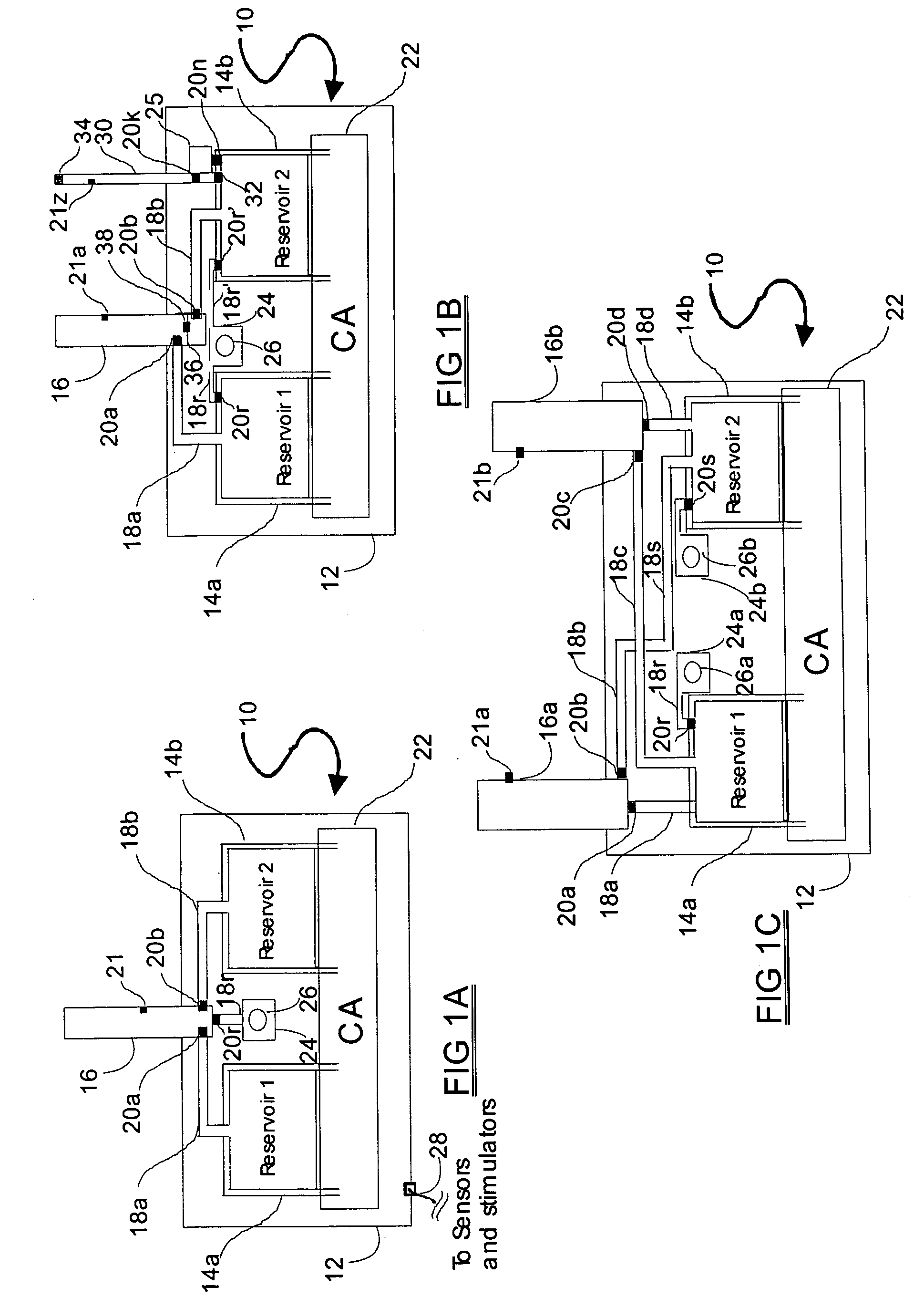
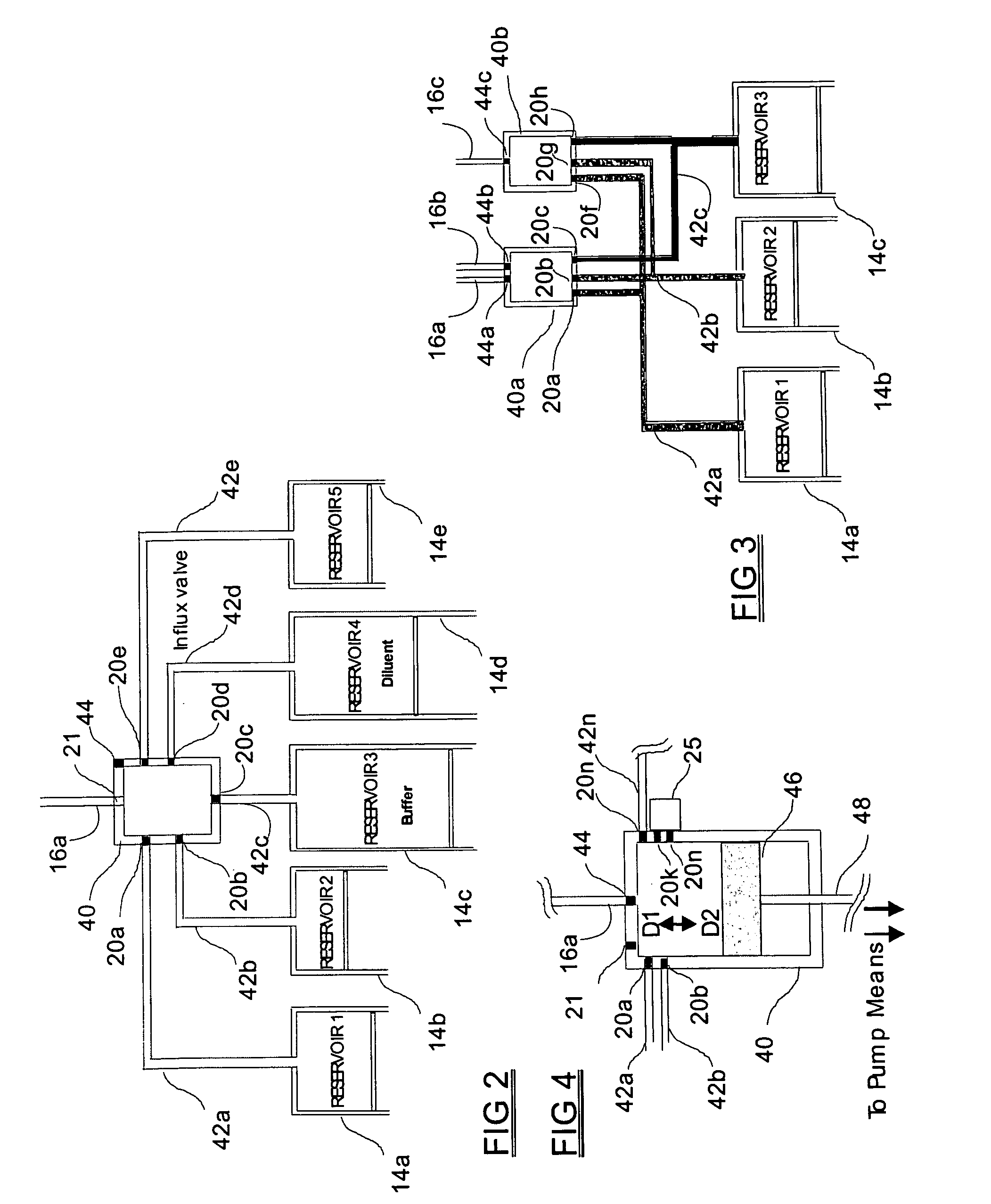
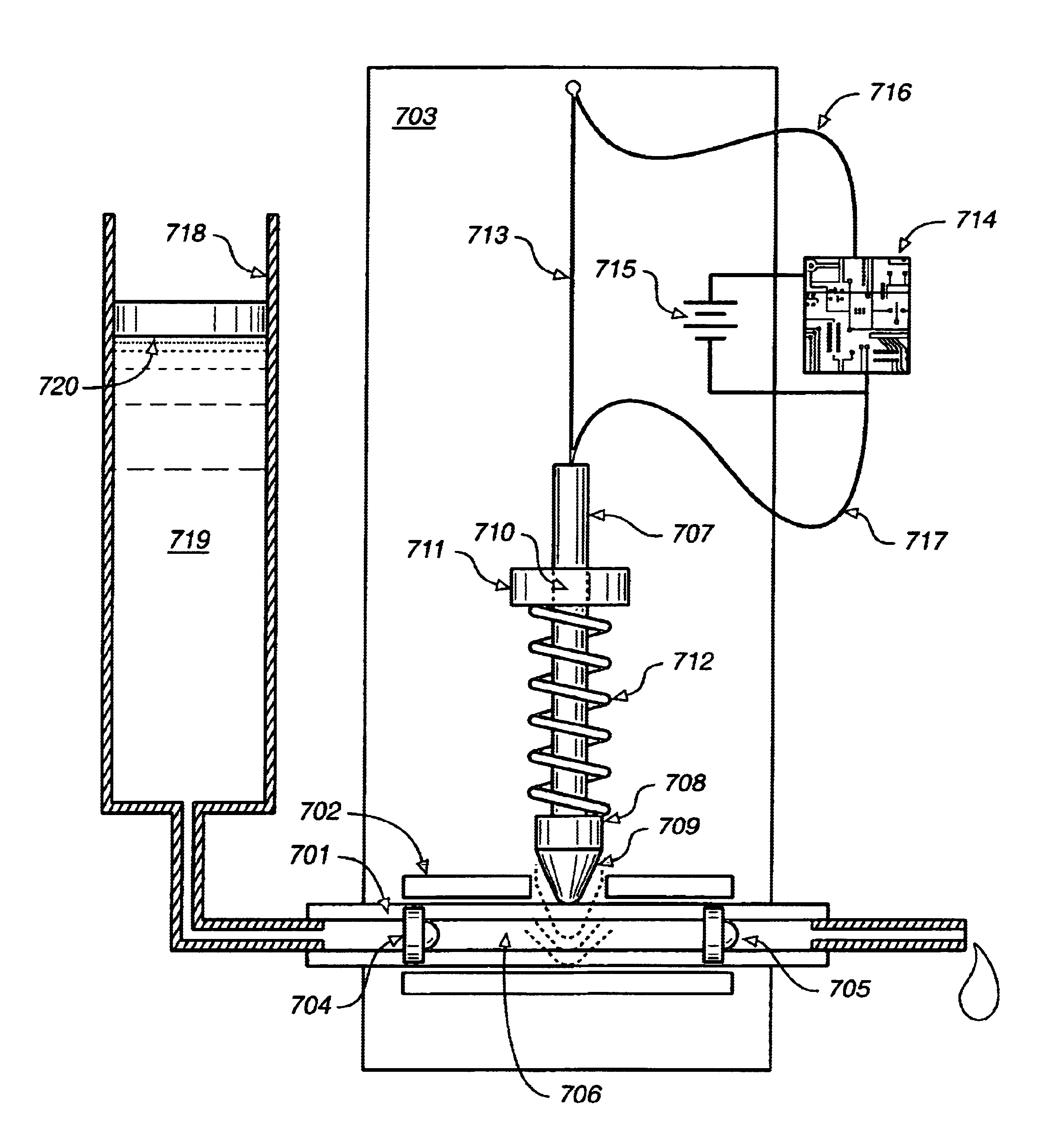
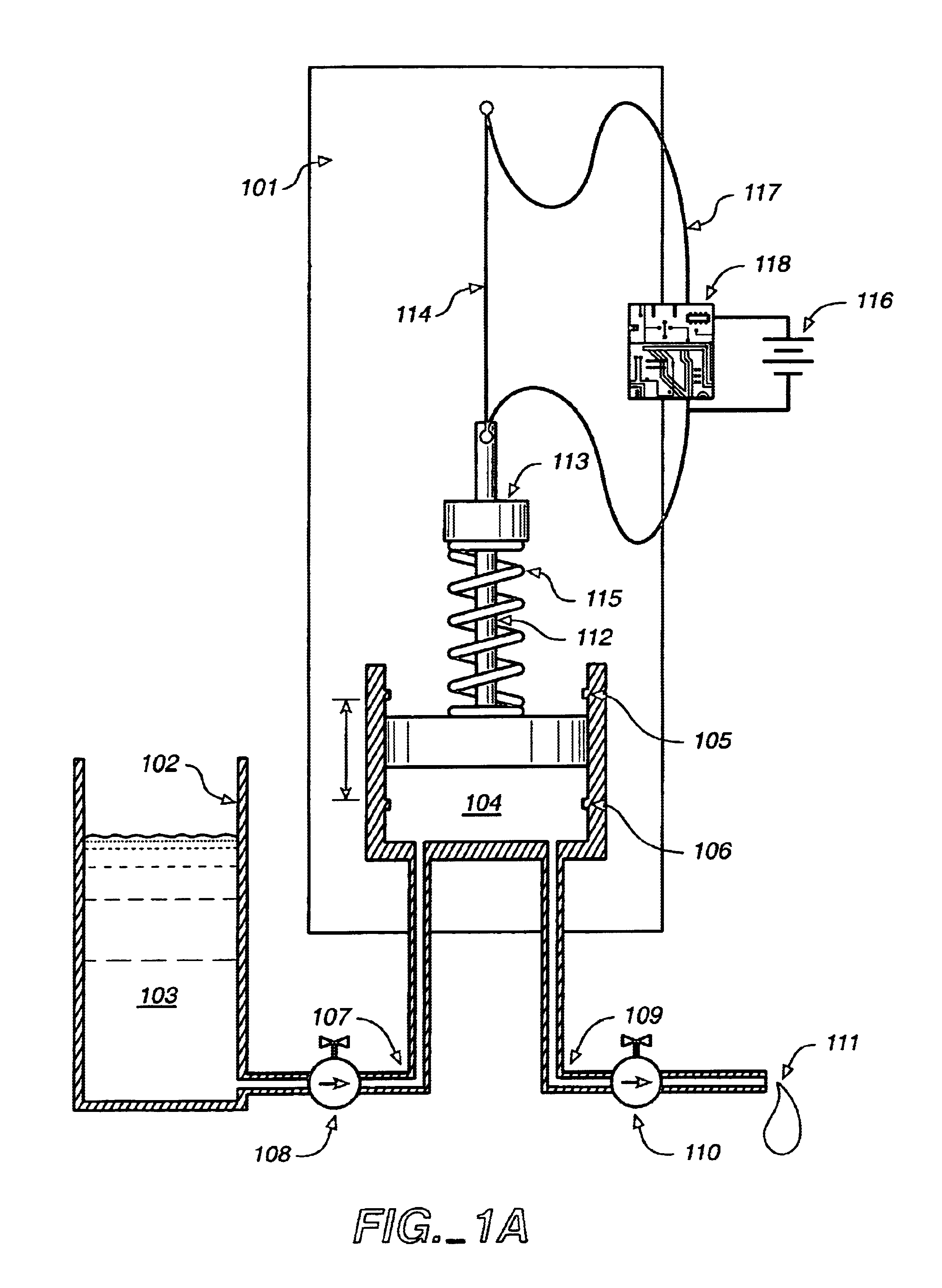
Programmable medical drug delivery systems and methods for delivery of multiple fluids and concentrations
InactiveUS20050277912A1Extension of timeEasy to fillMulti-lumen catheterDrug and medicationsControl mannerDelivery system
A drug delivery system provides for mixing various drugs in an optimally controlled manner, for using flow controllers to guide multiple drugs into a single or into multiple catheters, for enabling a single lumen catheter to treat a specific region with several drugs, for allowing for dilution of a concentrated drug in order to both increase the time between refilling and also for providing any concentration of a drug that might be desired, for using a buffer fluid to deliver exact amounts of several drugs from the same catheter or to separate several drugs within a single catheter, for using external fluid present in the human body either as a diluent or buffer fluid, and for providing for a drug testing / filler apparatus to be used prior to implant to ensure proper function and easy means of filling multiple reservoirs with different fluids, and also after implant for refilling operations. The drug delivery system (DDS) can perform both bolus and continuous delivery of substances, and enable the measured delivery of any one of several drugs to one or more distal locations at independently programmable rates. New types of catheter systems and uses therefore are also described. Catheter hub assemblies allowing for easy replacement of drug delivery systems offer advantages when replacing drug delivery systems. New methods for using the DDS in the promotion of healthy pregnancy and treatment of a developing fetus are also possible.
Owner:JOHN SASHA
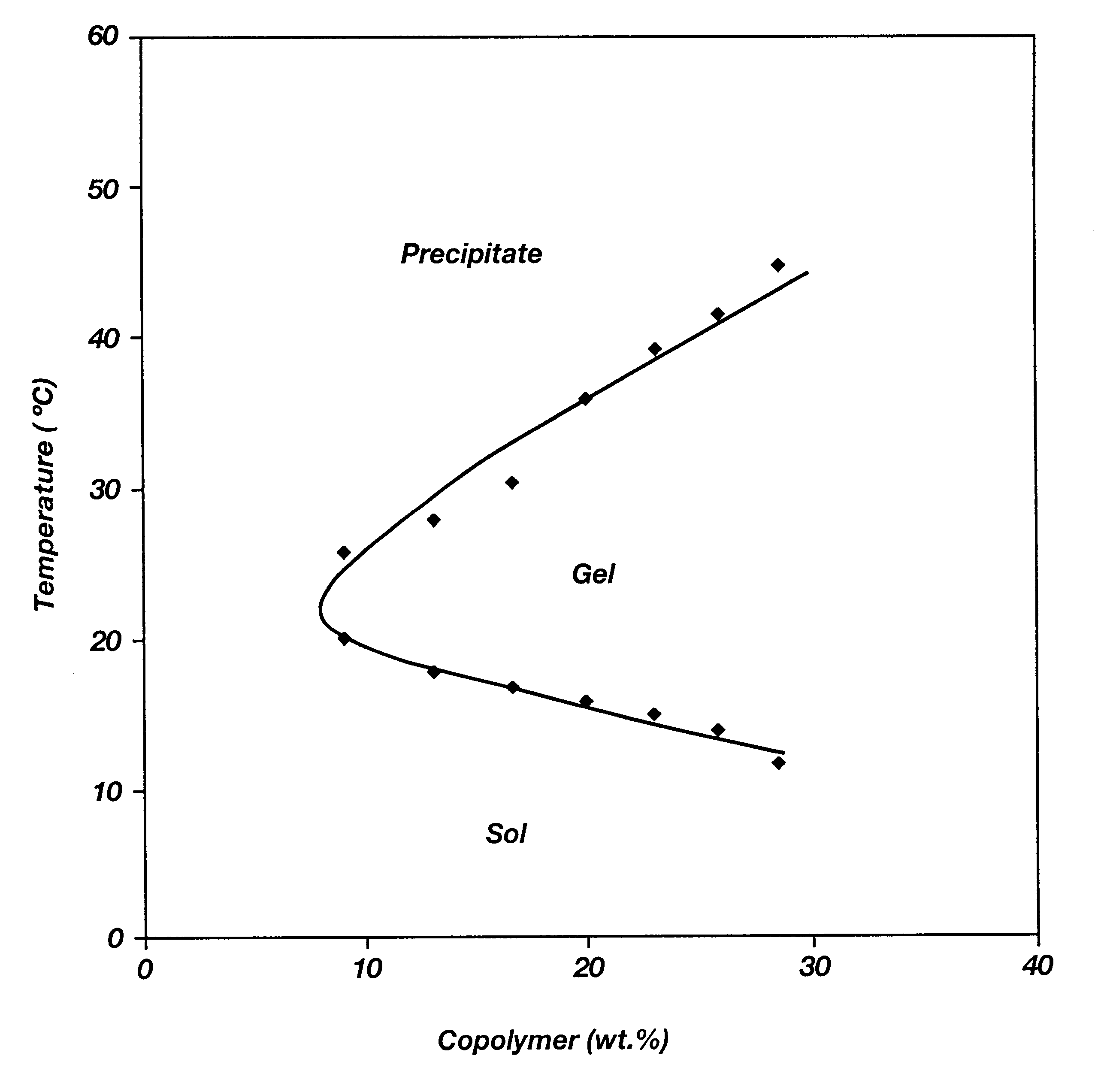
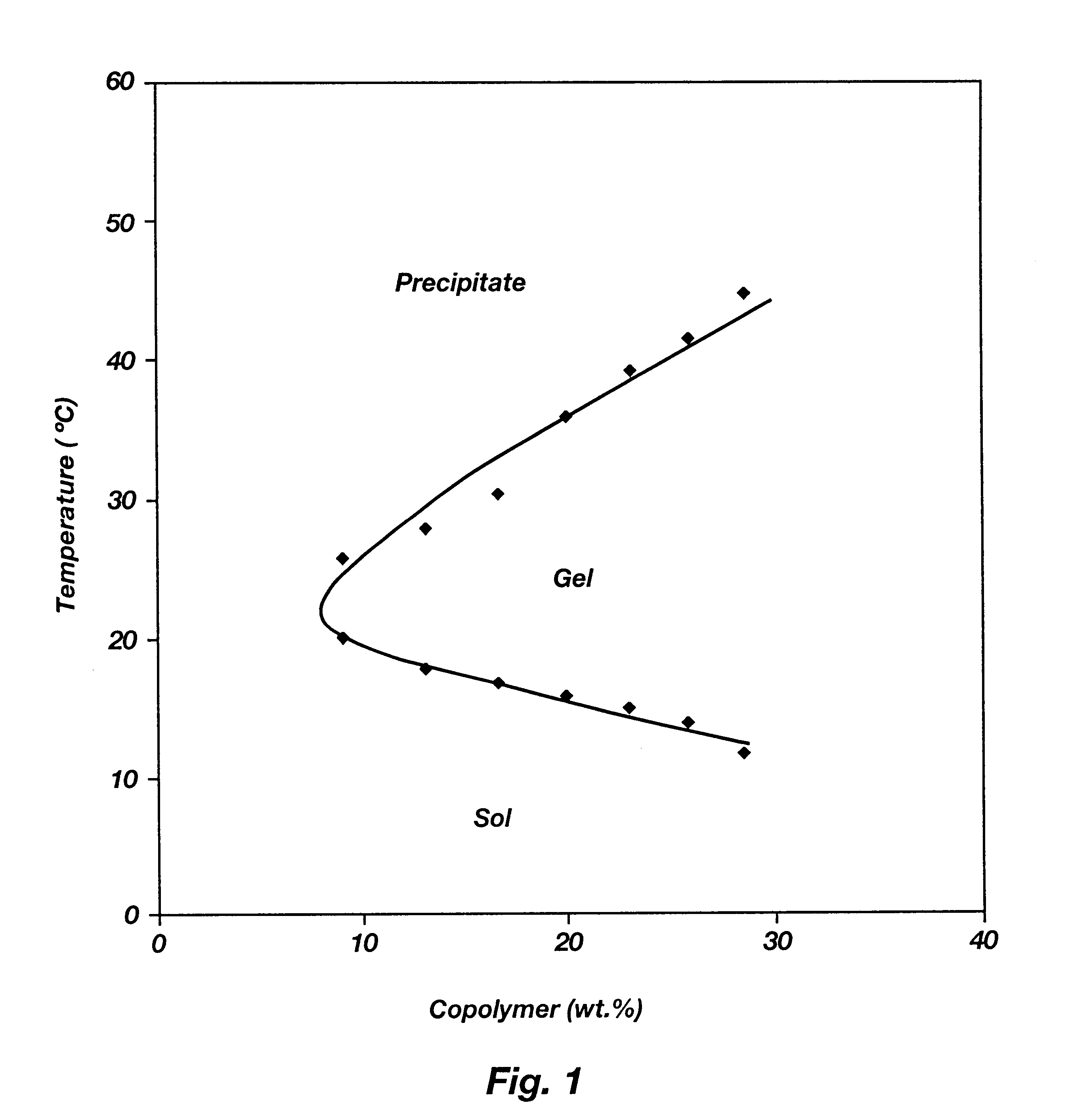
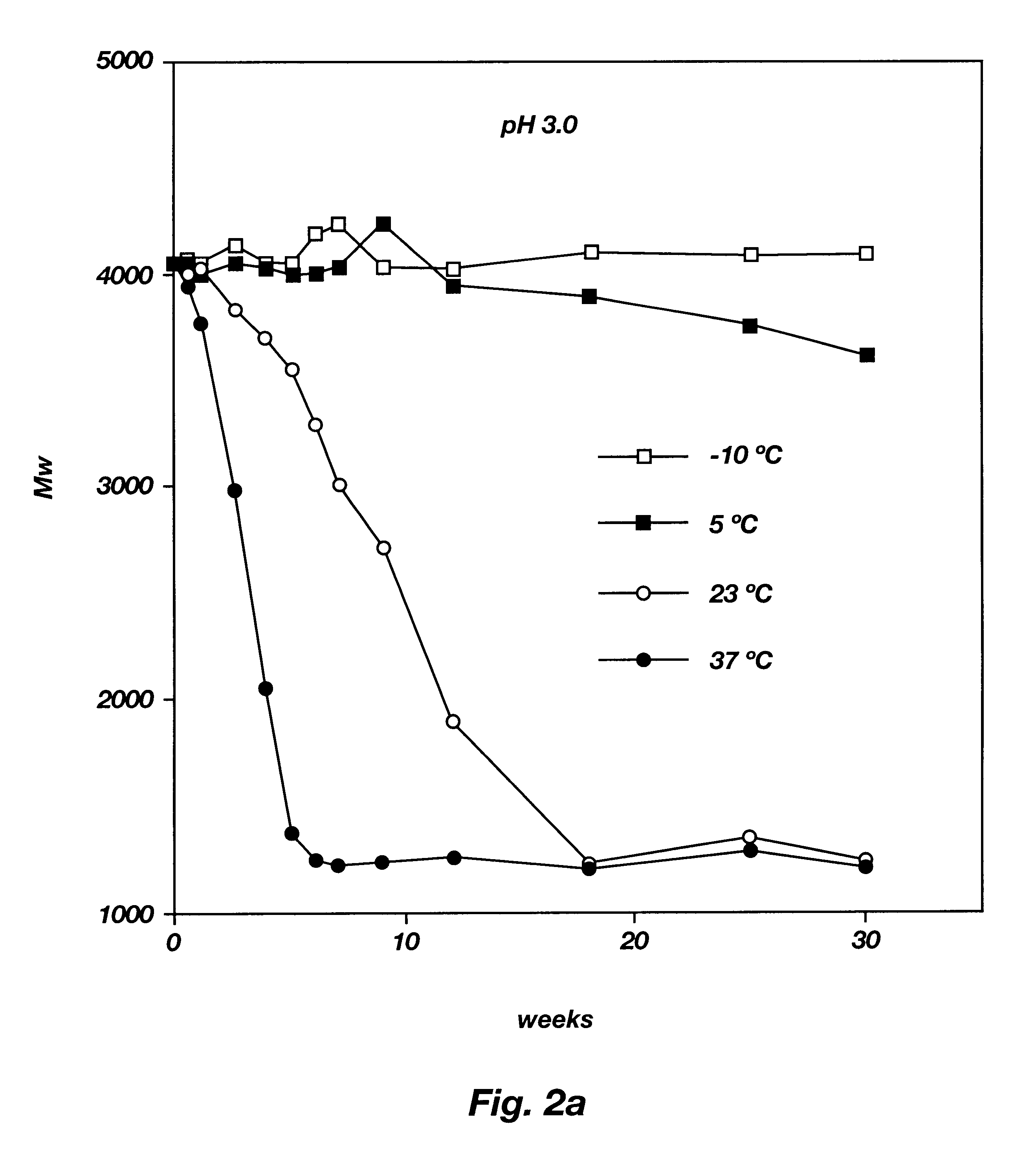
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
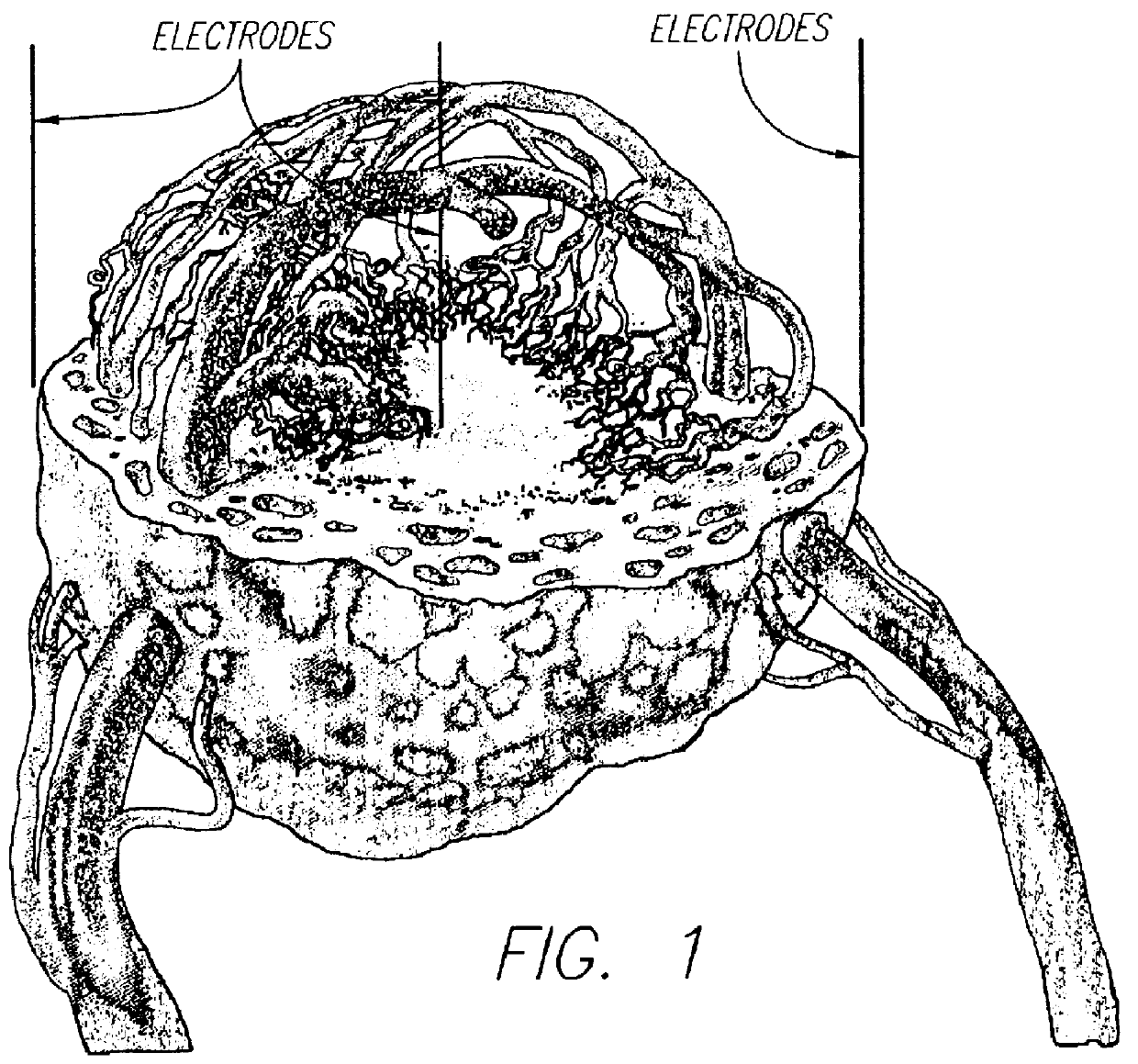
Tissue electroperforation for enhanced drug delivery
The present invention relates to a method and a device for transporting a molecule through a mammalian barrier membrane of at least one layer of cells comprising the steps of: ablating the membrane with an electric current from a treatment electrode; and utilizing a driving force to move the molecule through the perforated membrane.
Owner:LIFESCAN INC
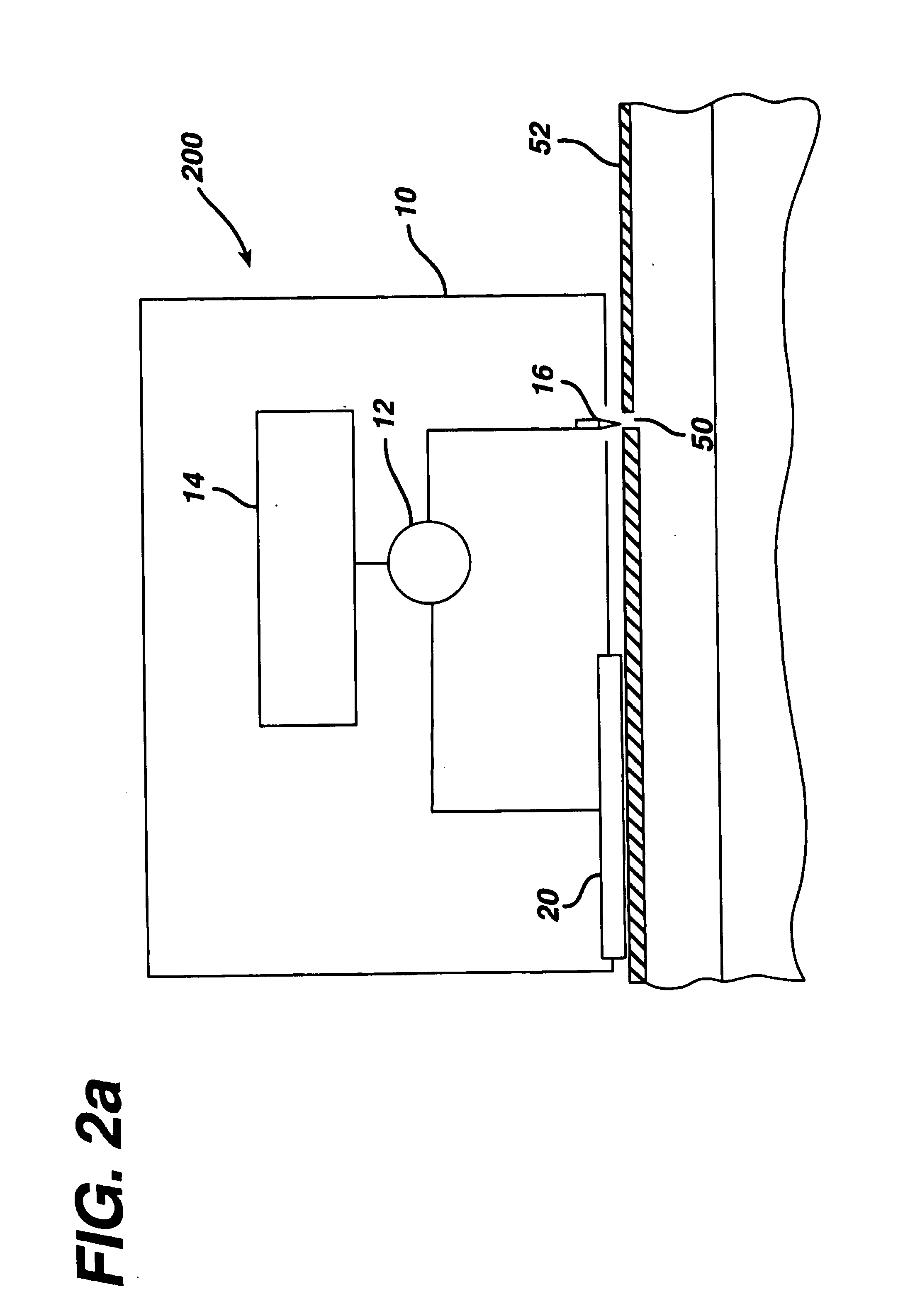
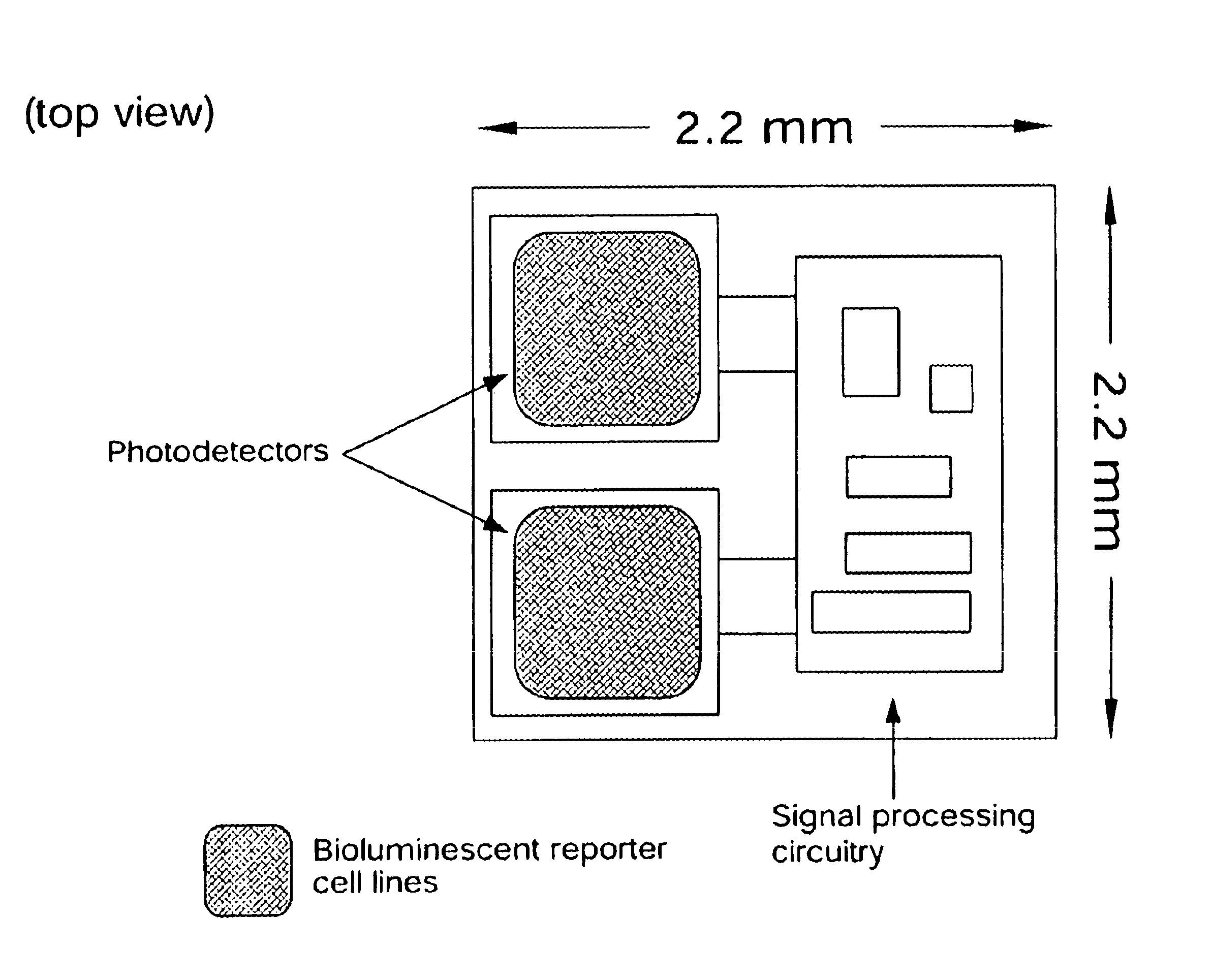
In vivo biosensor apparatus and method of use
InactiveUS6673596B1Less can be administeredCost-effective administration of drugBioreactor/fermenter combinationsBiological substance pretreatmentsIn vivoGenetically engineered
Disclosed are bioluminescent bioreporter integrated circuit devices that detect selected analytes in fluids when implanted in the body of an animal. The device comprises a bioreporter that has been genetically engineered to contain a nucleic acid segment that comprises a cis-activating response element that is responsive to the selected substance operably linked to a gene encoding a bioluminescent reporter polypeptide. In preferred embodiments, the target analyte is glucose, glucagons, or insulin. Exposure of the bioreporter to the target substance causes the response element to up-regulate the nucleic acid sequence encoding the reporter polypeptide to produce a luminescent response that is detected and quantitated. In illustrative embodiments, the bioreporter device is encapsulated on an integrated circuit that is capable of detecting the emitted light, processing the resultant signal, and then remotely reporting the results. Also disclosed are controlled drug delivery systems capable of being directly or indirectly controlled by the detection device that provide drugs such as insulin to the animal in reponse to the amount of target analyte present in the body fluids.
Owner:UNIV OF TENNESSEE RES FOUND +1
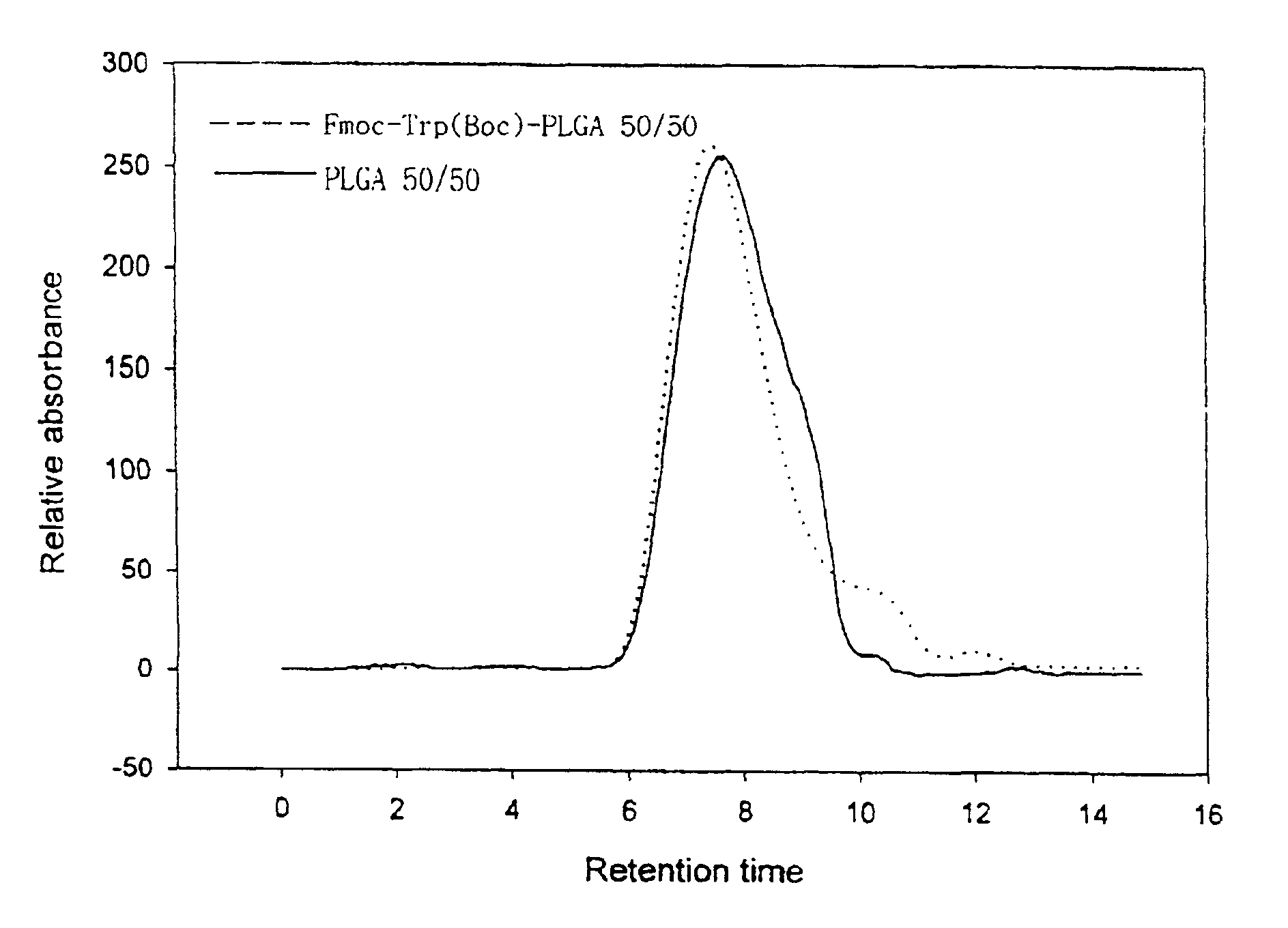
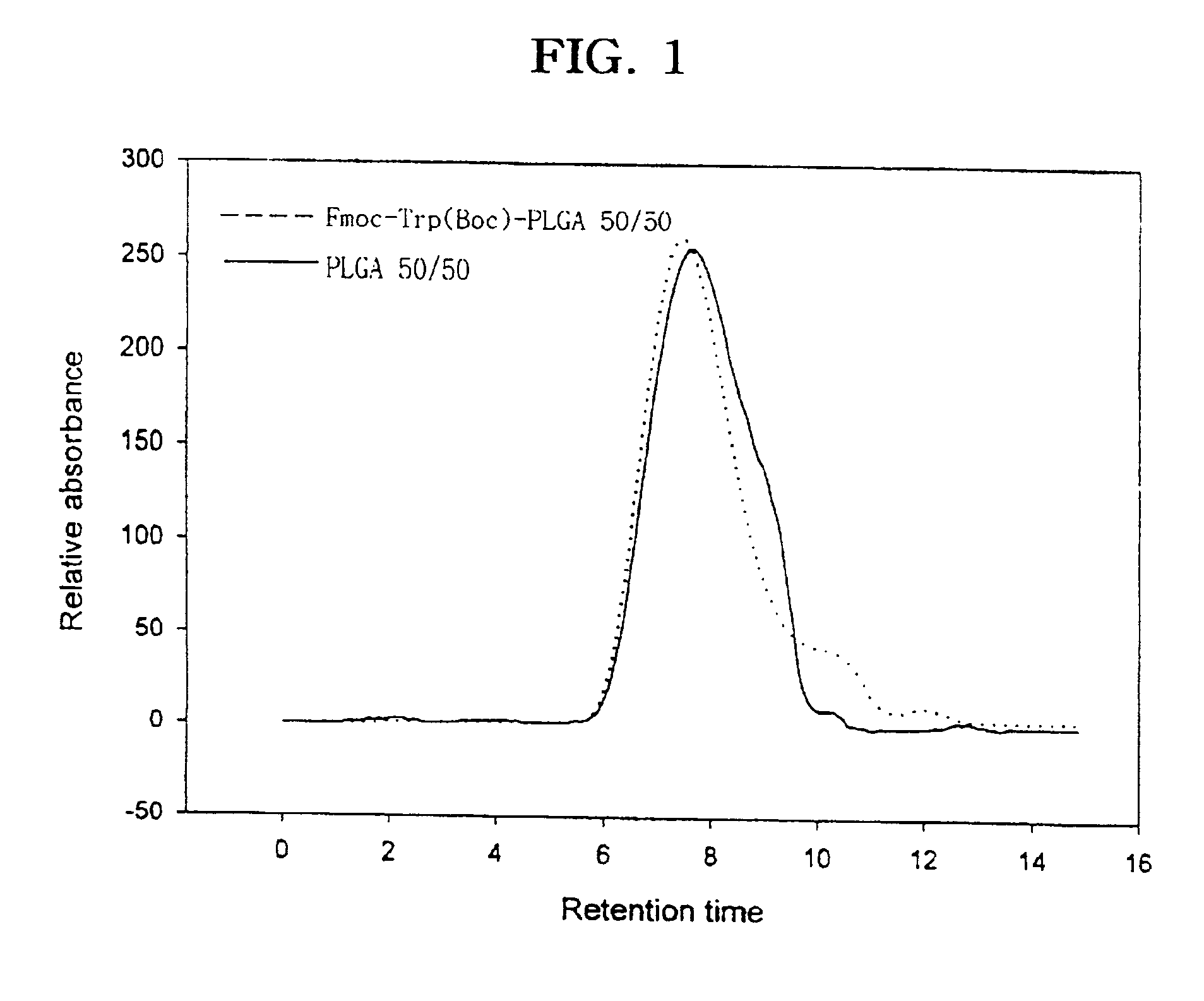
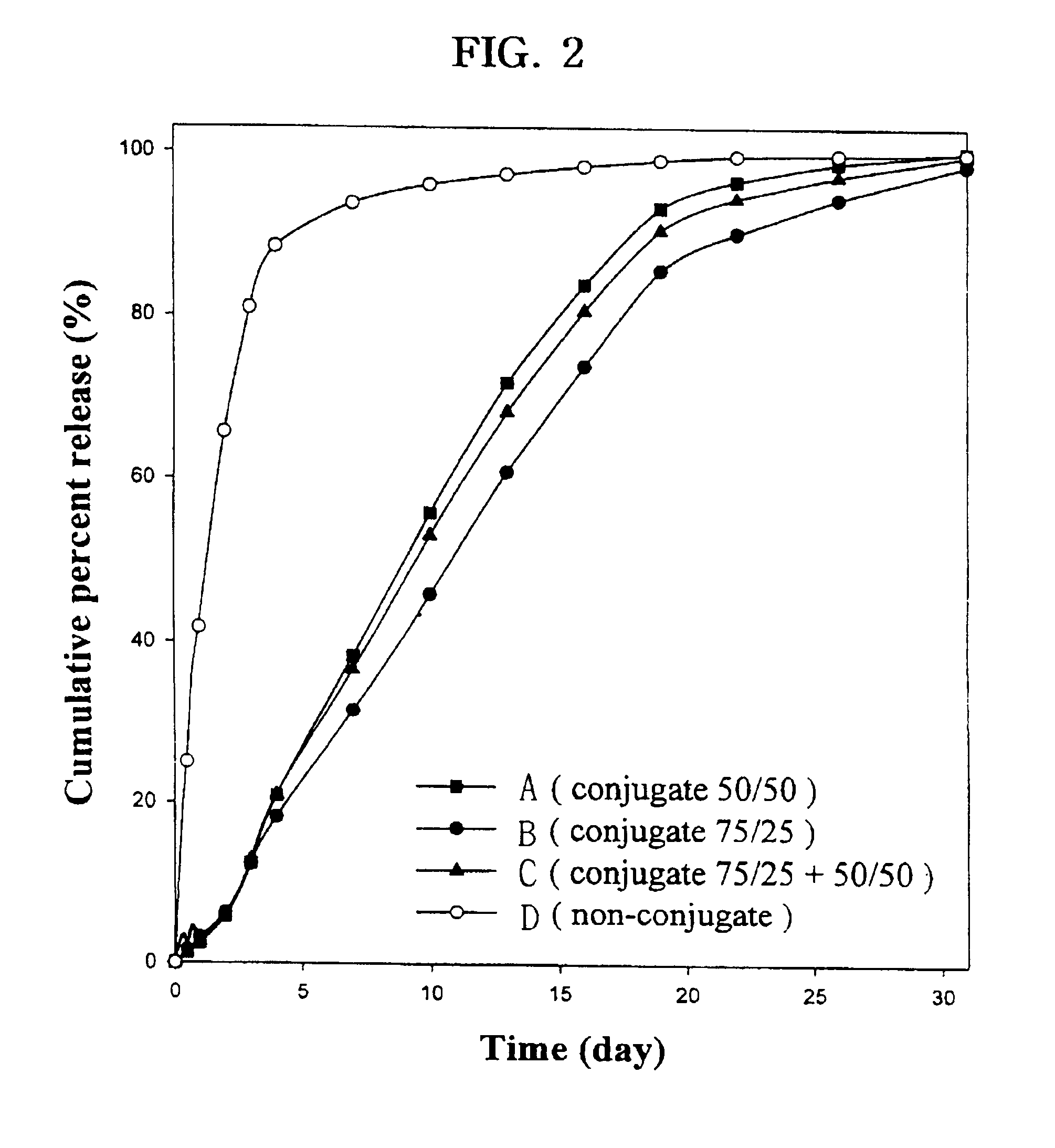
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
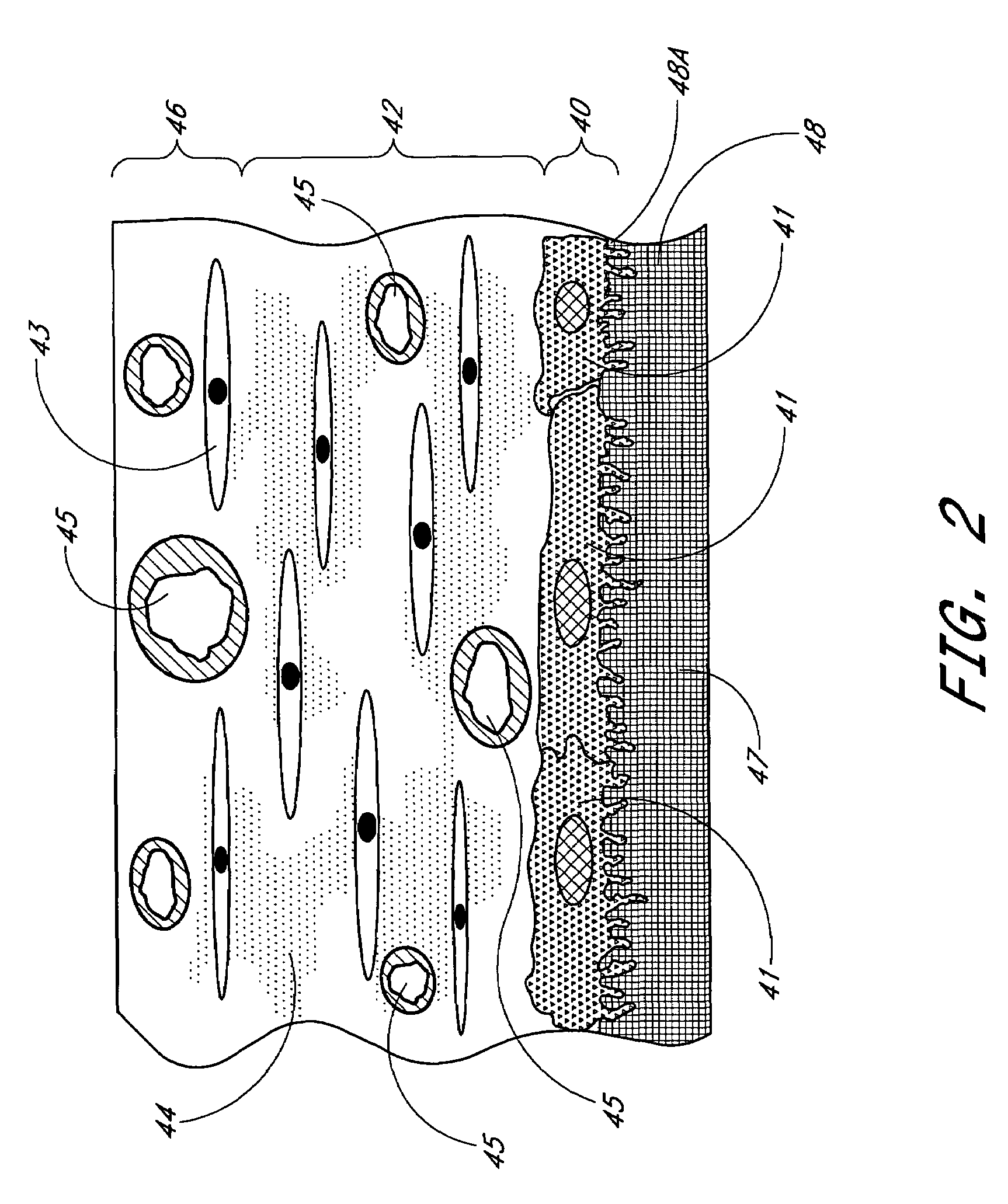
Membrane for use with implantable devices
ActiveUS20100087724A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteBiointerface
The present invention provides a biointerface membrane for use with an implantable device that interferes with the formation of a barrier cell layer including; a first domain distal to the implantable device wherein the first domain supports tissue attachment and interferes with barrier cell layer formation and a second domain proximal to the implantable device wherein the second domain is resistant to cellular attachment and is impermeable to cells. In addition, the present invention provides sensors including the biointerface membrane, implantable devices including these sensors or biointerface membranes, and methods of monitoring glucose levels in a host utilizing the analyte detection implantable device of the invention. Other implantable devices which include the biointerface membrane of the present invention, such as devices for cell transplantation, drug delivery devices, and electrical signal delivery or measuring devices are also provided.
Owner:DEXCOM INC
Biointerface with macro- and micro-architecture
Owner:DEXCOM INC
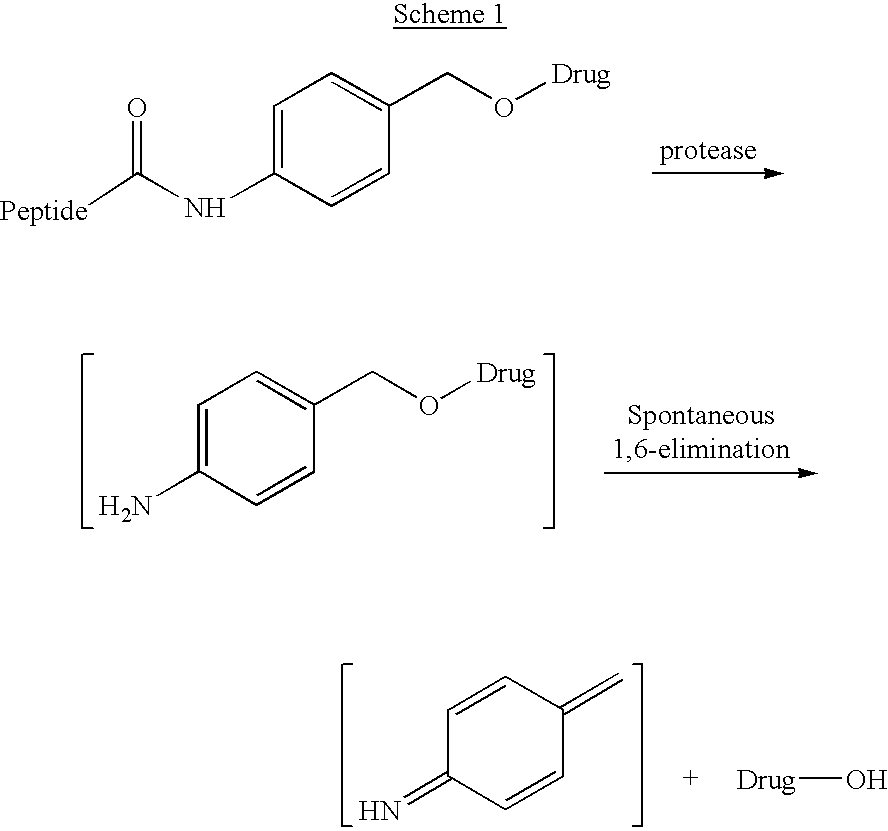
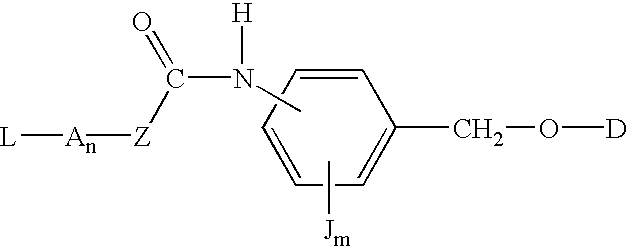
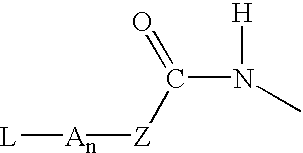
P-amidobenzylethers in drug delivery agents
InactiveUS20030130189A1Decrease in drug activityImprove stabilityTetrapeptide ingredientsTripeptide ingredientsEtherDiluent
Compounds of the formulas LAn-Z-X-WwD and BZ-X-WwD wherein: D is a drug moiety; L is a ligand; B is a blocking group; A is an optional acyl unit; Z is an amino acid or a peptide; X is an aminobenzyl ether self-immolative spacer group; W is an optional second self-immolative group; n is an integer of 0 or 1; and w is an integer of 0 or 1, and compositions of said compounds with pharmaceutically acceptable carrier, diluent and / or excipient, and methods of delivery the drug D via the compounds.
Owner:SEAGEN INC
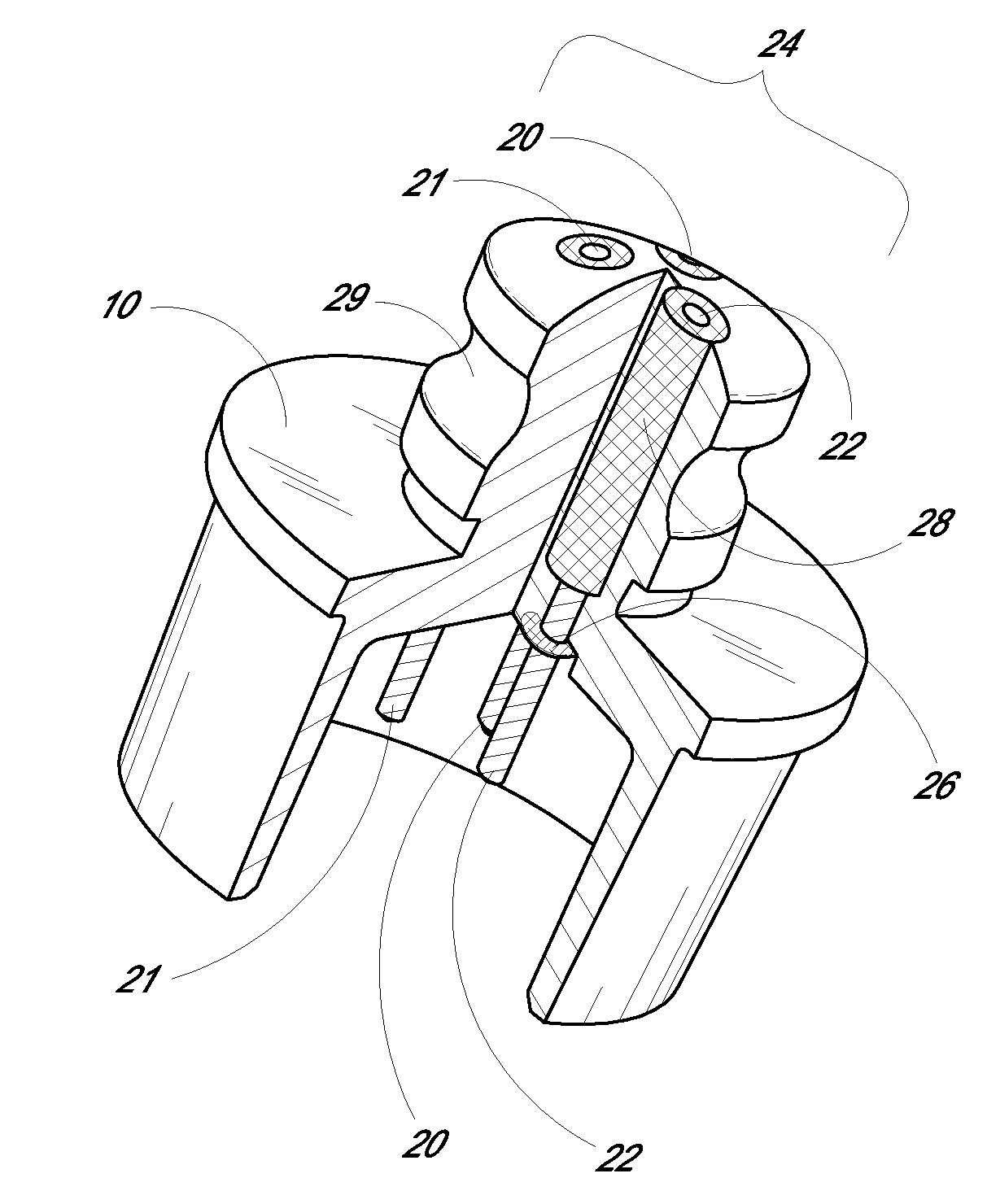
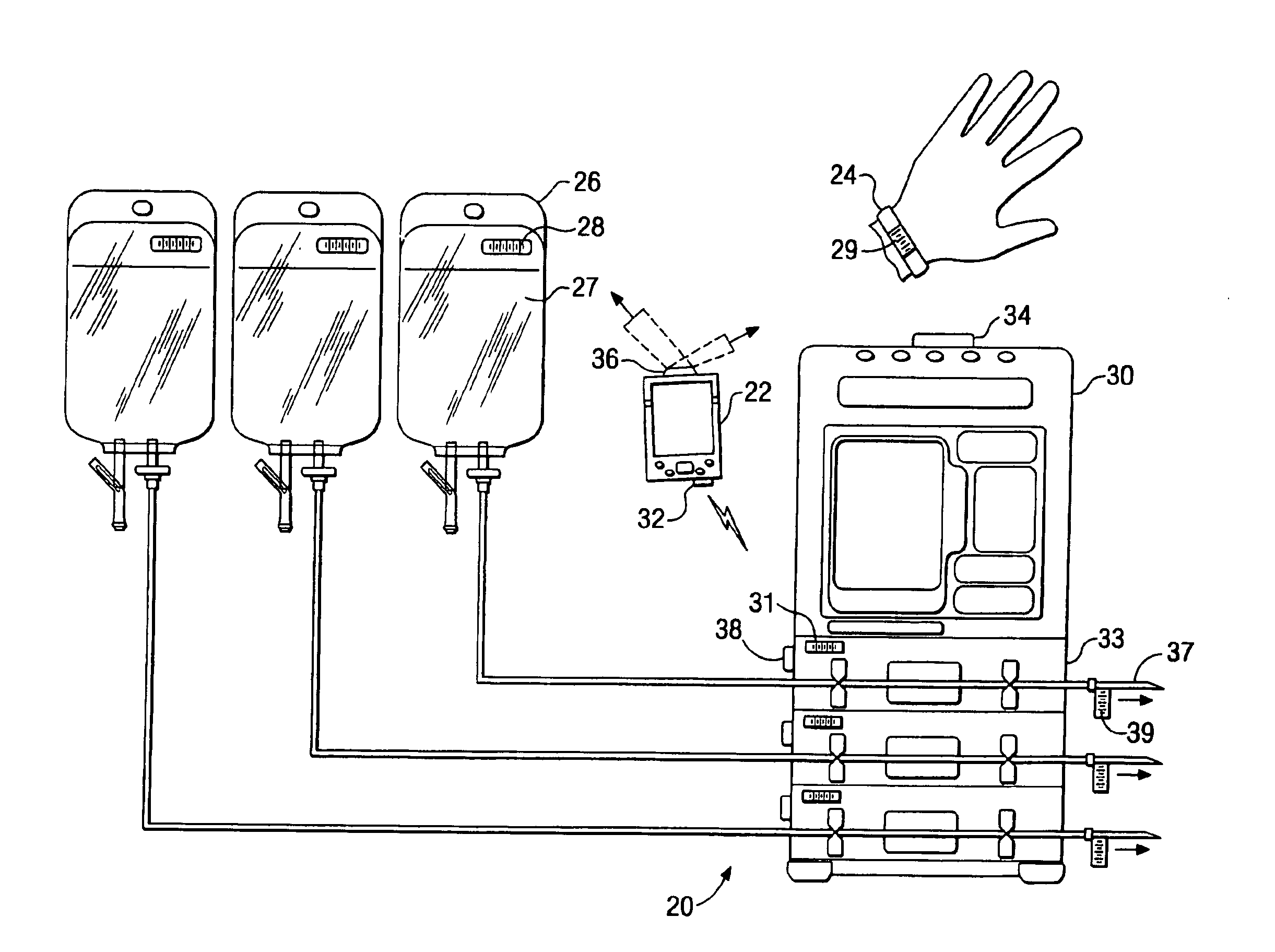
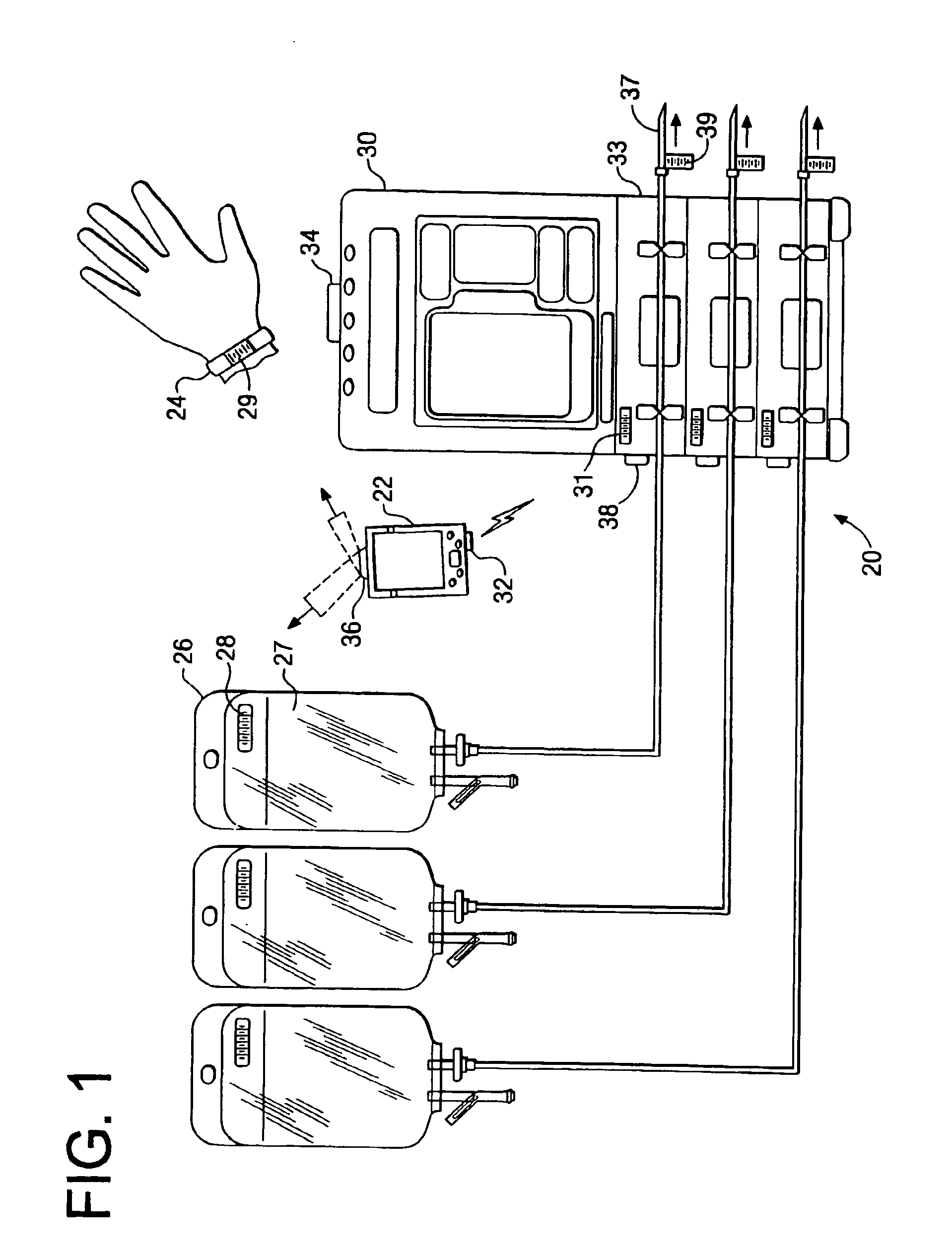
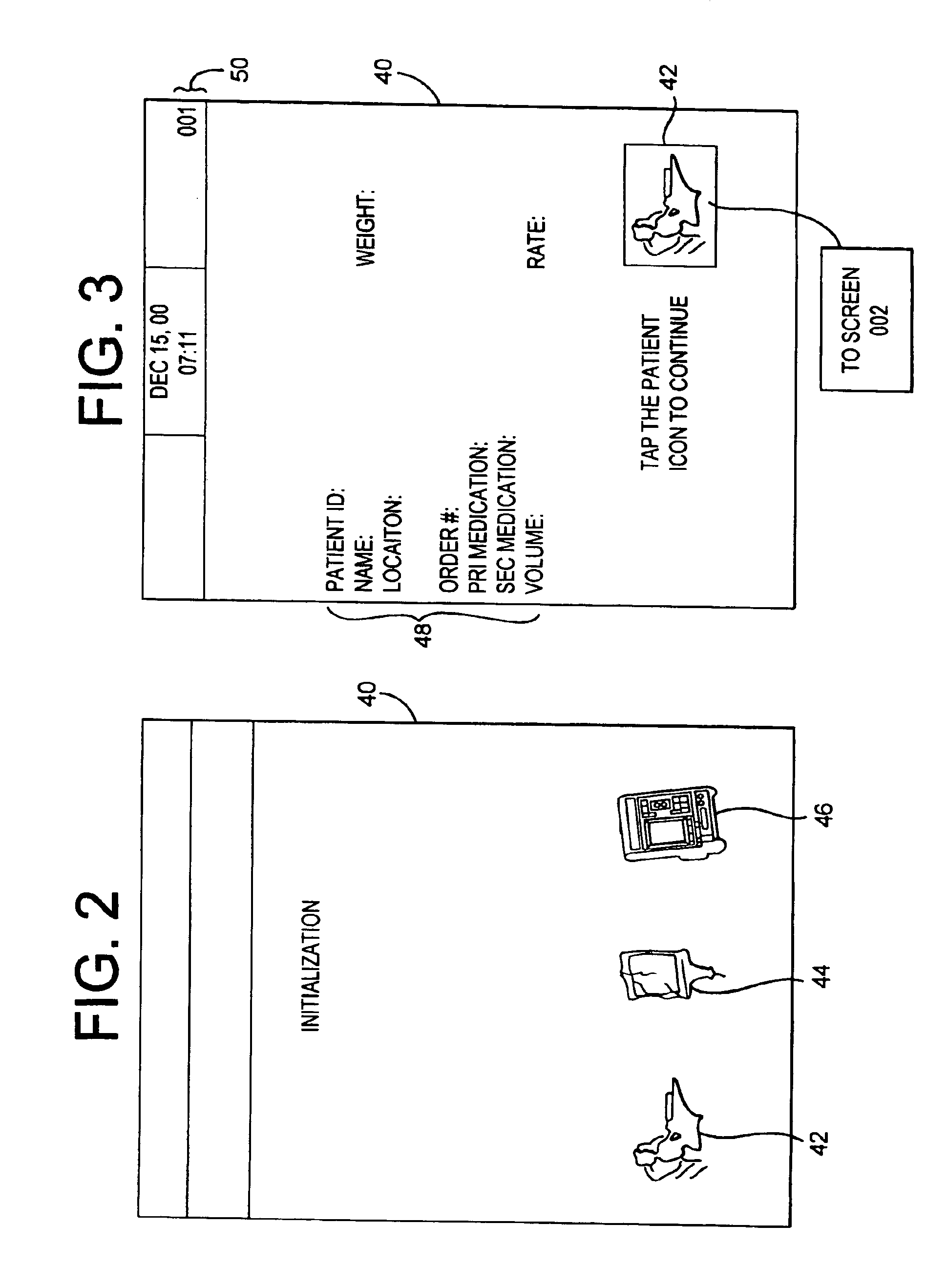
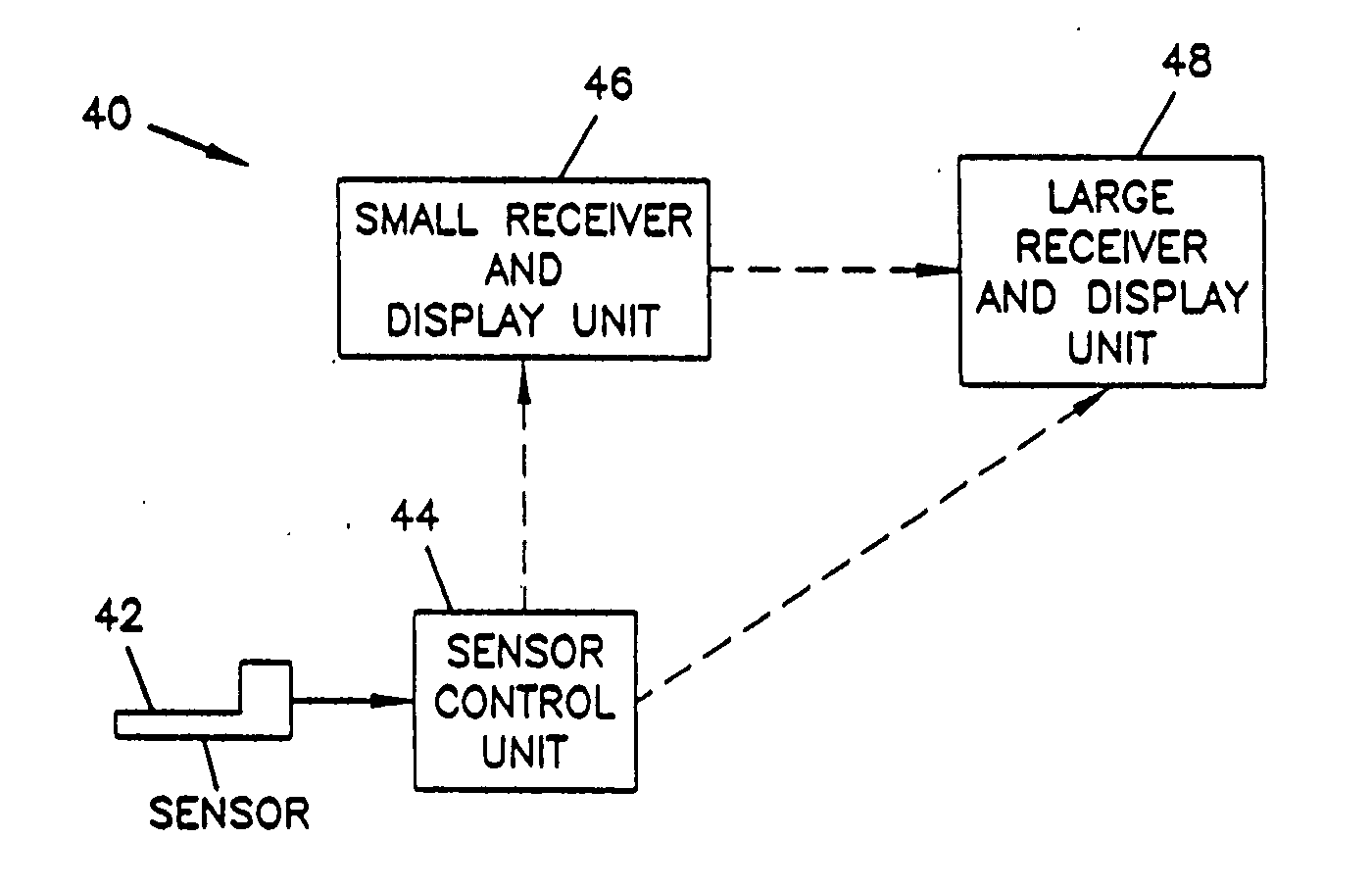
Medication delivery system
InactiveUS6985870B2Reduces potential medication errorDegrade some medicationHand manipulated computer devicesDrug and medicationsEmergency medicinePatient data
A medication delivery system (20) having features of the present invention comprises a medical container (26) holding a prescribed medication (27) to be delivered to a patient, a tag 24 adapted to be worn by the patient, a handheld computing device (22), and an electronic medication delivery device (30). Data on the medication (27) is contained in a first label (28) on the medication container (27). The first label (28) also contains the instruction on how the medication is delivered to the patient, including the appropriate settings for an electronic medication delivery device for delivering the medication to the patient. Patient data is contained in a second label (29) on the tag (24) worn by the patient. The medication data, medication delivery instruction, and patient data are provided in machine readable formats. The handheld computing device (22) reads the medication data and the medication delivery instruction on the medication container (26) and the patient data on the patient tag (24). The handheld computing device (22) stores the information obtained and performs a matching check to confirm that the medication data matches with the patient data. Upon a confirmed match, it transmits the medication delivery instruction to the electronic medication delivery device (30), which downloads the instruction, programs the delivery device 30, and prompts an operator to begin delivering the medication (27) to the patient according to the downloaded instruction.
Owner:BAXTER INT INC
Analyte monitoring device and methods of use
An analyte monitor includes a sensor, a sensor control unit, and a display unit. The sensor has, for example, a substrate, a recessed channel formed in the substrate, and conductive material disposed in the recessed channel to form a working electrode. The sensor control unit typically has a housing adapted for placement on skin and is adapted to receive a portion of an electrochemical sensor. The sensor control unit also includes two or more conductive contacts disposed on the housing and configured for coupling to two or more contact pads on the sensor. A transmitter is disposed in the housing and coupled to the plurality of conductive contacts for transmitting data obtained using the sensor. The display unit has a receiver for receiving data transmitted by the transmitter of the sensor control unit and a display coupled to the receiver for displaying an indication of a level of an analyte. The analyte monitor may also be part of a drug delivery system to alter the level of the analyte based on the data obtained using the sensor.
Owner:ABBOTT DIABETES CARE INC
Device and method employing shape memory alloy
InactiveUS6916159B2Low costSmall size and weightTesting/calibration apparatusVolume/mass flow measurementShape-memory alloyEngineering
Owner:THERASENSE
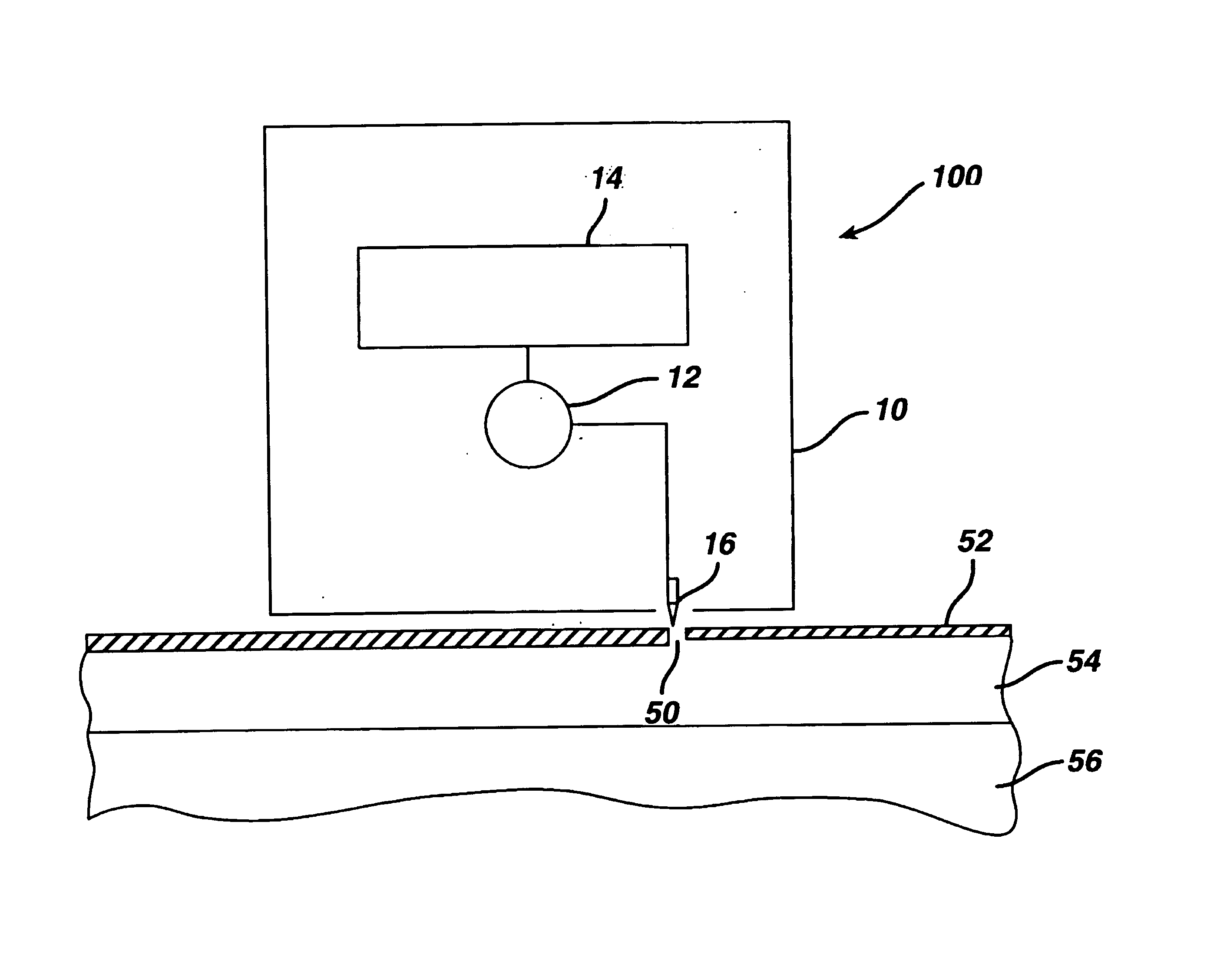
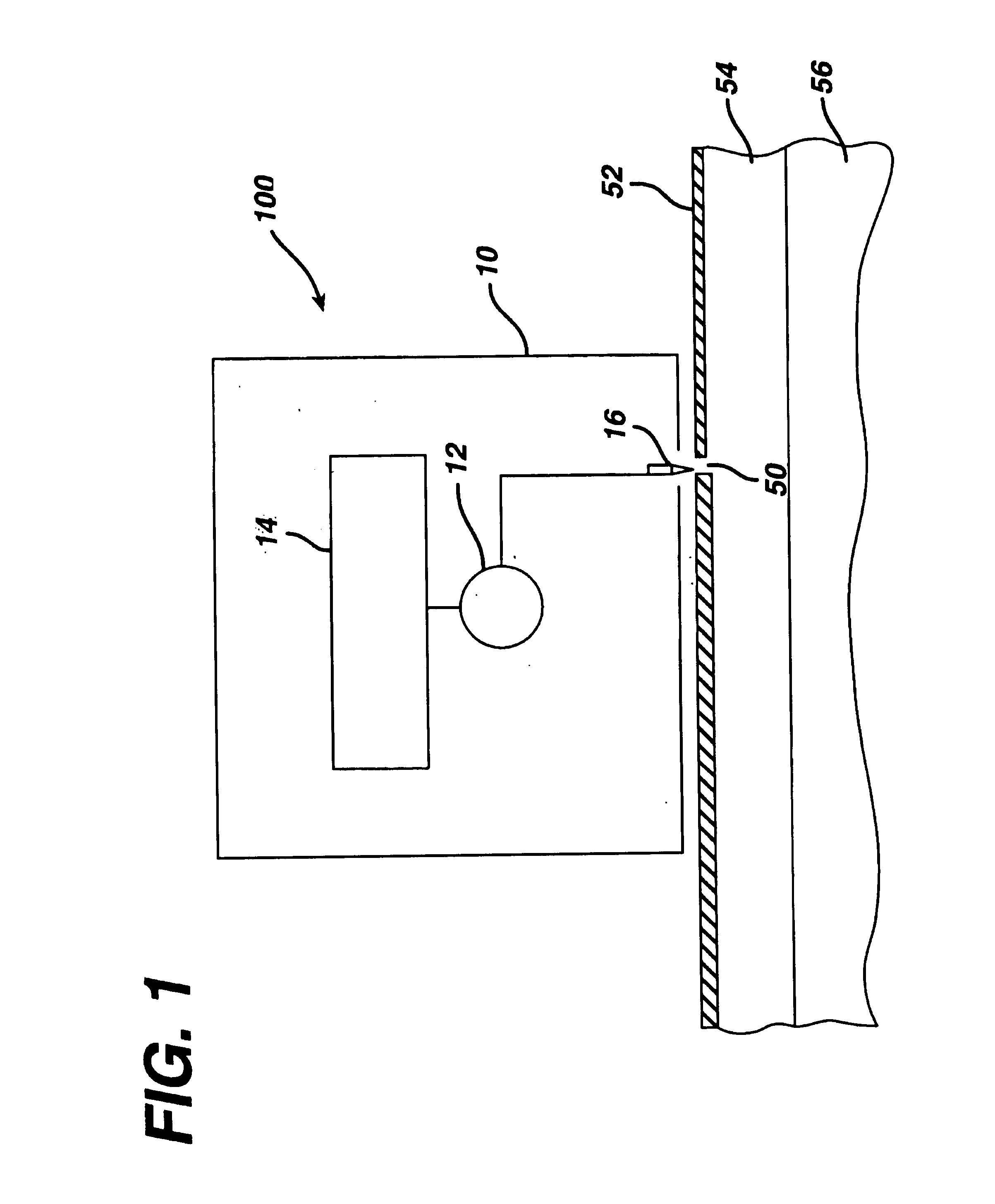
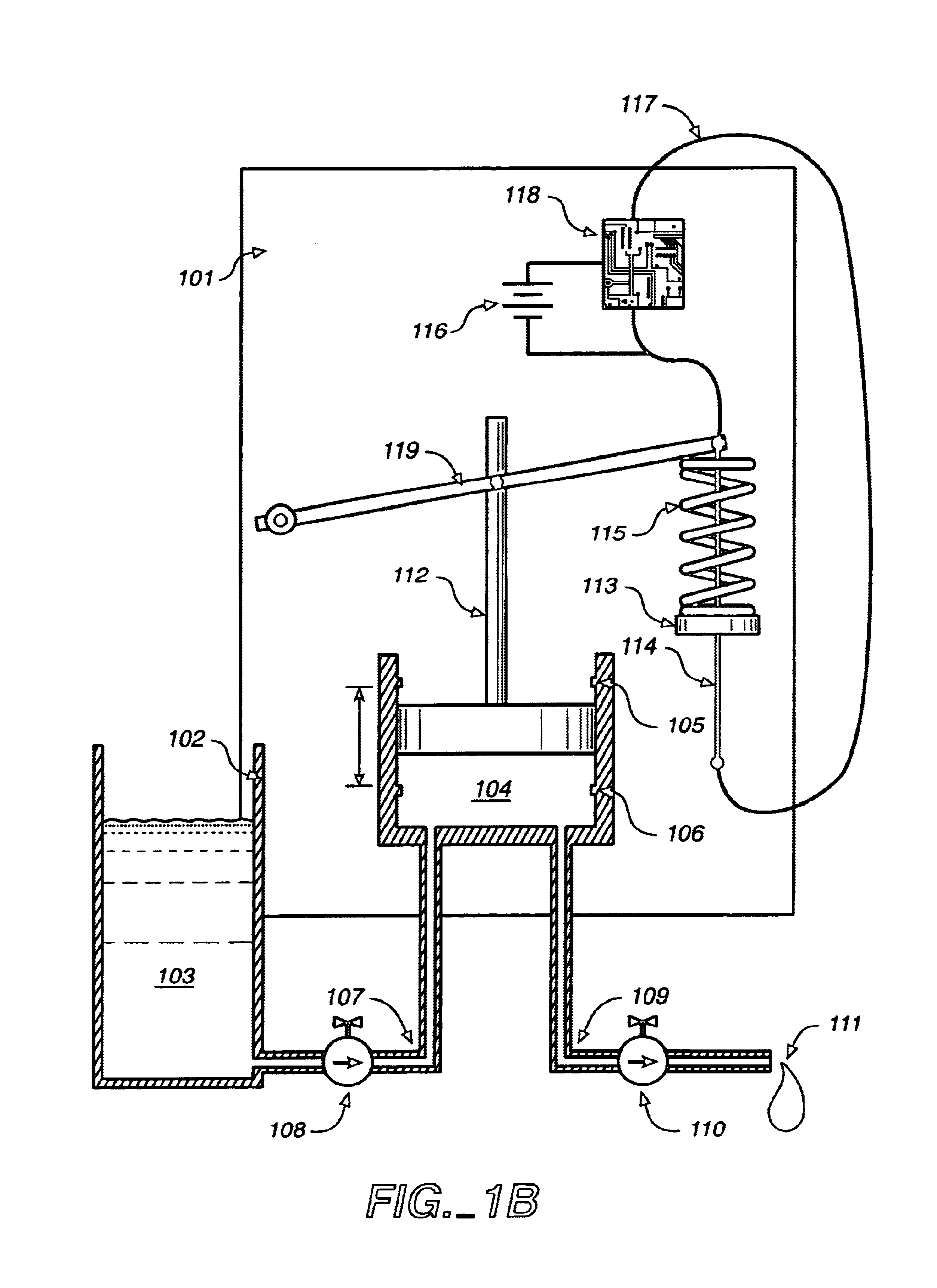
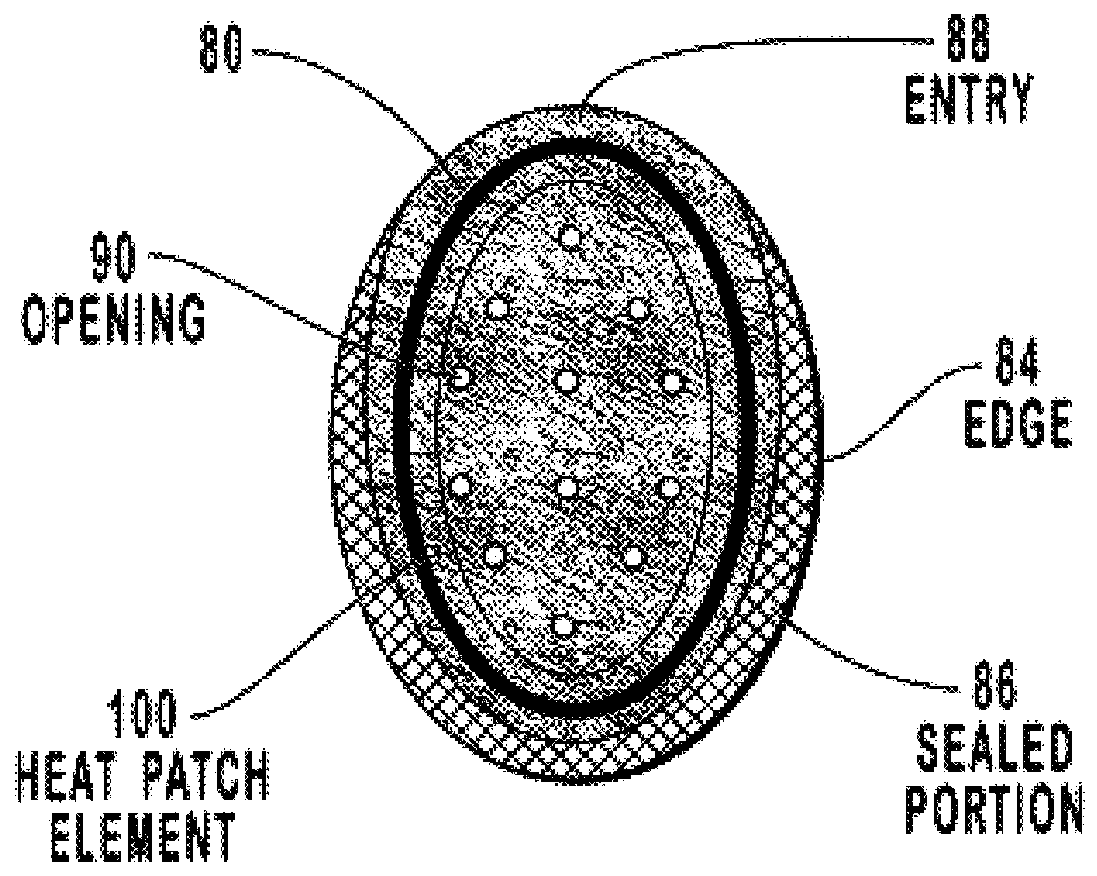
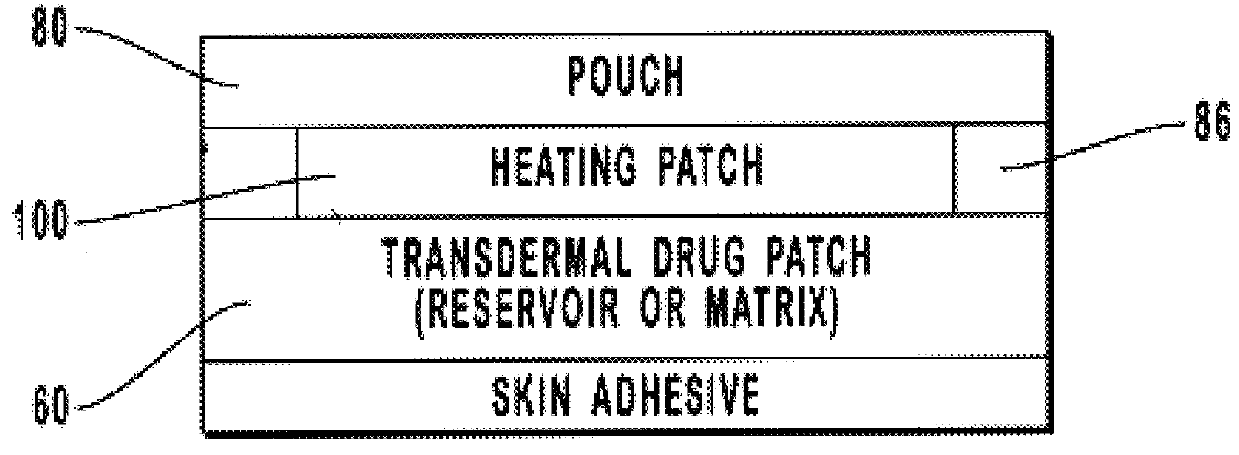
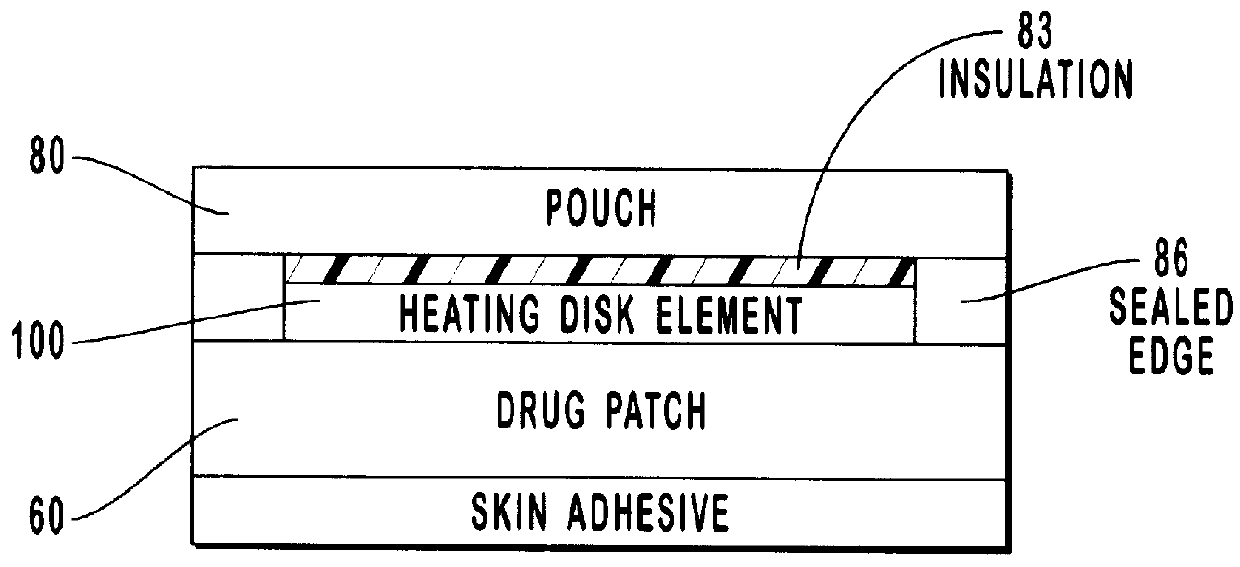
Transdermal drug patch with attached pocket for controlled heating device
InactiveUS6261595B1Shorten the timeEasy to replaceElectrotherapyMedical devicesTransdermal patchDrug administration
The present invention relates to a transdermal drug delivery system comprising a dermal drug delivery patch and a heating element compartment securable to the dermal drug delivery patch. A freely transferrable heating element is securable within the heating element compartment. A drug can be administered transdermally using the present invention by placing the dermal drug delivery patch upon a patient's skin at an administration site. A heating element compartment is secured to the dermal drug delivery patch and a freely transferrable heating element is placed within the heating element compartment. The heating element provides controlled heat to the dermal drug patch and the patient's skin aid thereby improves dermal drug administration.
Owner:ZARS INC
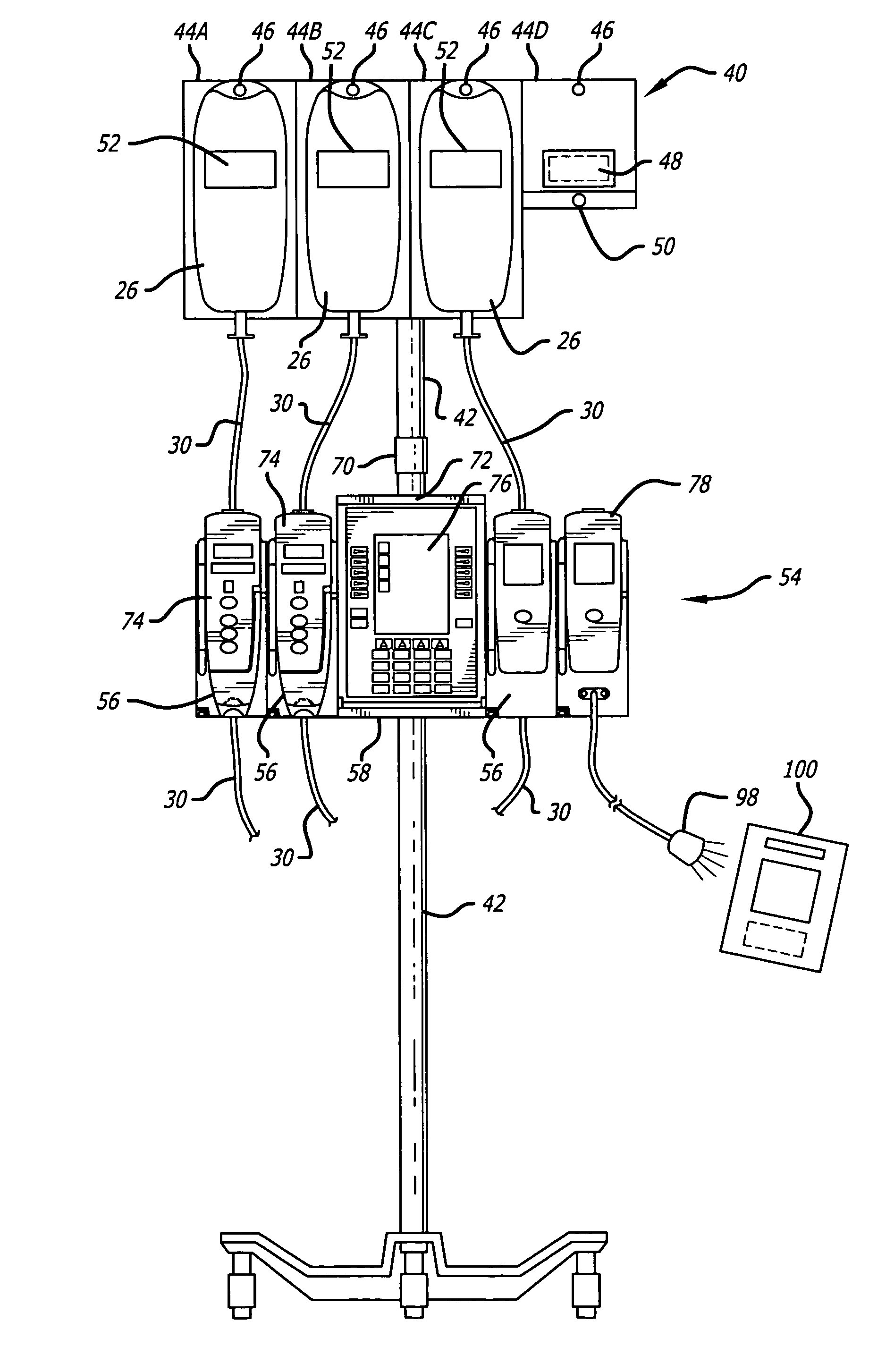
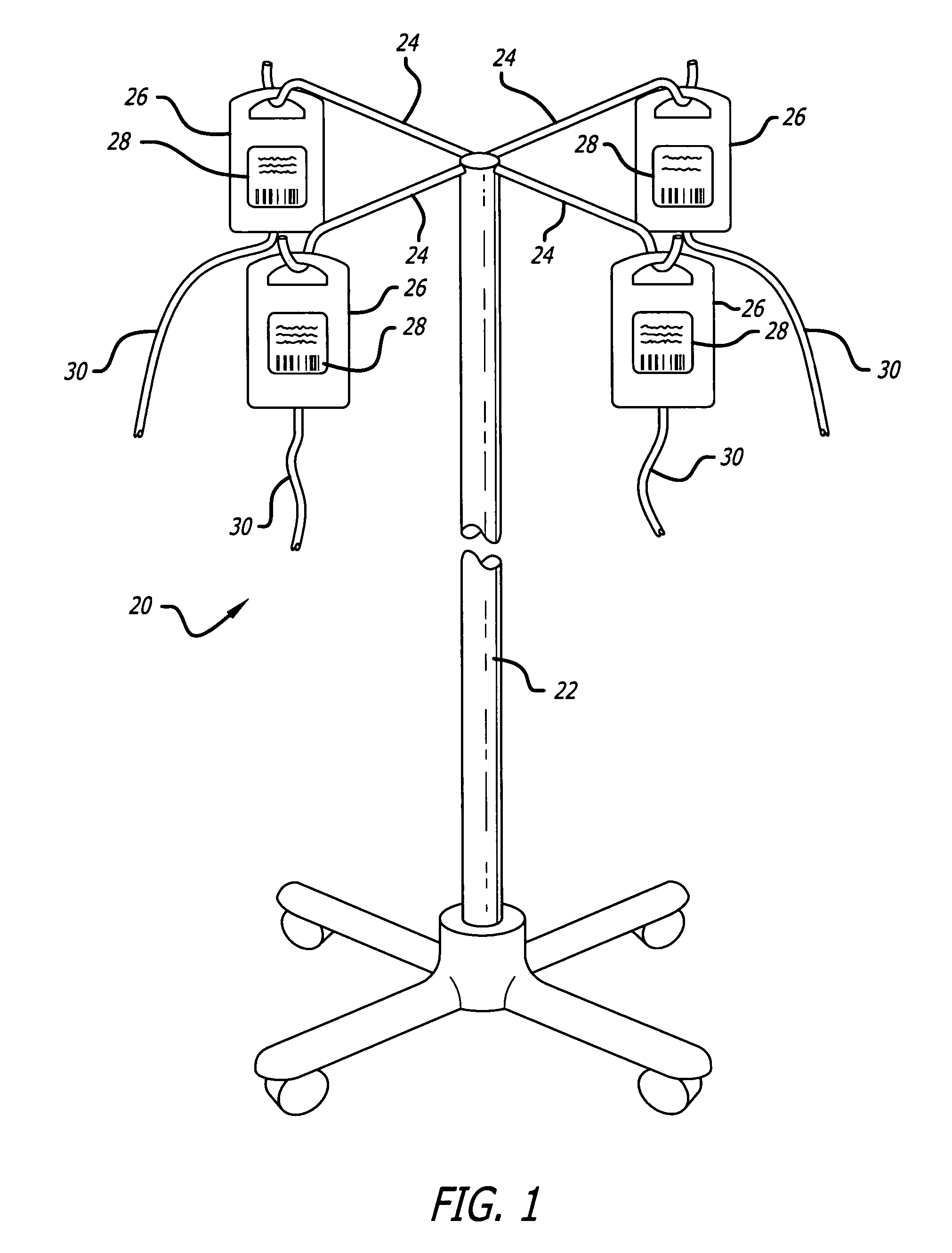
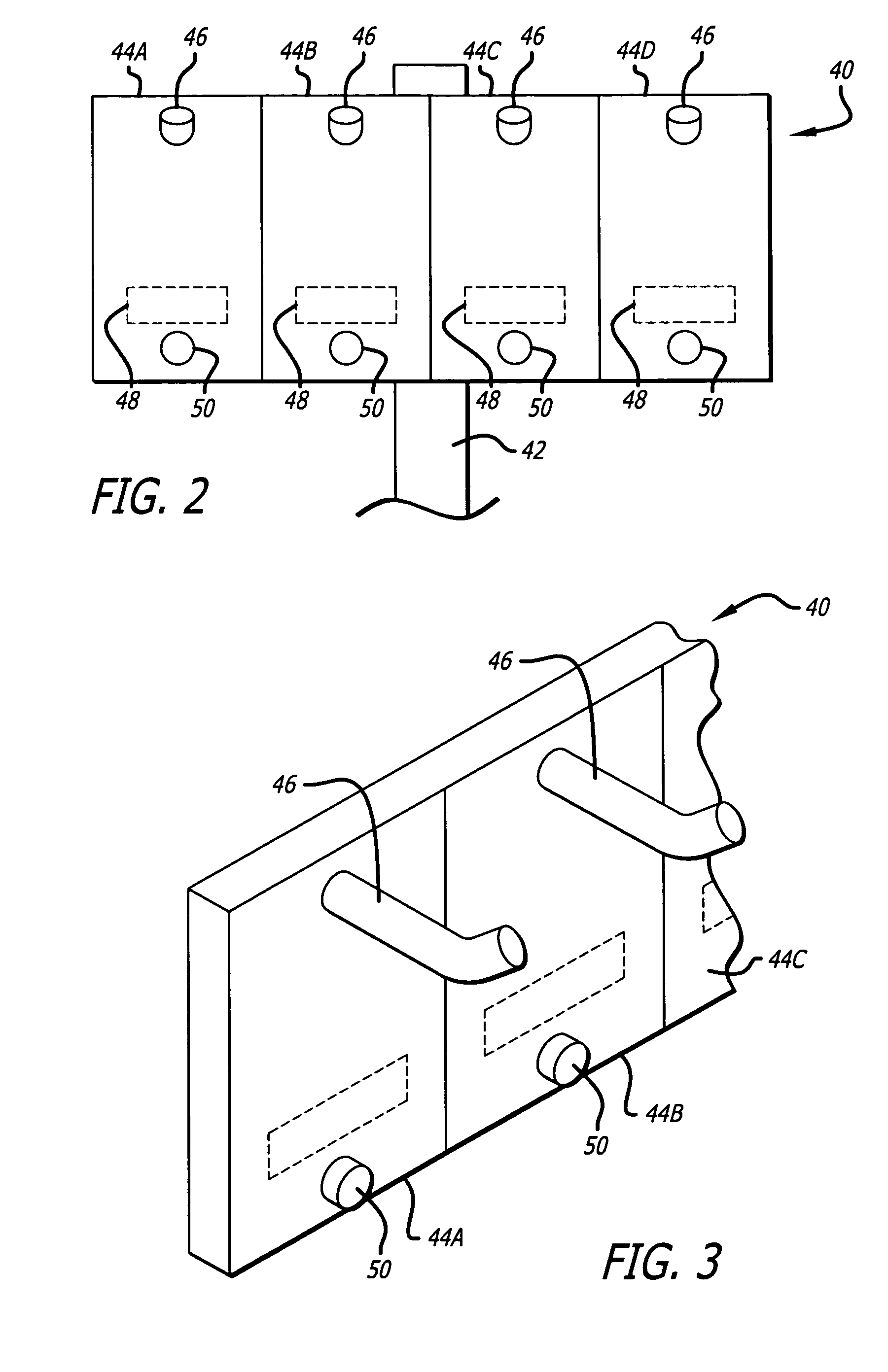
Medication safety system featuring a multiplexed RFID interrogator panel
A medication safety system includes a panel mounted to an IV pole above a multi-channel infusion pump also mounted to the IV pole. The panel includes multiple RFID readers for reading the RFID tags placed on each of the medication containers mounted to the panel. The pump includes a controller that communicates with the RFID readers at the panel to receive the information read by the RFID readers and automatically program the respective pump channel. A verification program verifies that the medication delivery information from the containers matches the patient identified for the pump and the programming parameters of the pump fall within acceptable ranges. In another aspect, the panel contains multiple vibration devices to impart vibrations to the medication of each of the containers mounted to the panel. Those vibrations are sensed by the particular pumping channel to confirm that the correct channel has been programmed for that medication. When the clinician opens the door of the pumping channel, the pump may request information from the RFID reader at the panel for that medication and may wait for receipt of the vibrations in the medication of the tubing mounted into the channel.
Owner:CAREFUSION 303 INC
Drug delivery system and method
InactiveUS6041252AFacilitated releaseFast wayBioreactor/fermenter combinationsElectrotherapyLiposomeBiomedical engineering
A method for delivering a therapeutic agent to a predetermined location in a host is disclosed, wherein a liposome-encapsulated therapeutic agent is administered to the host, and an electrical field which encompasses a predetermined region within the host is established, such that as the liposome-encapsulated agent is exposed to the electrical field the release of the agent from the liposome to the predetermined region is enhanced.
Owner:ICHOR MEDICAL SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com