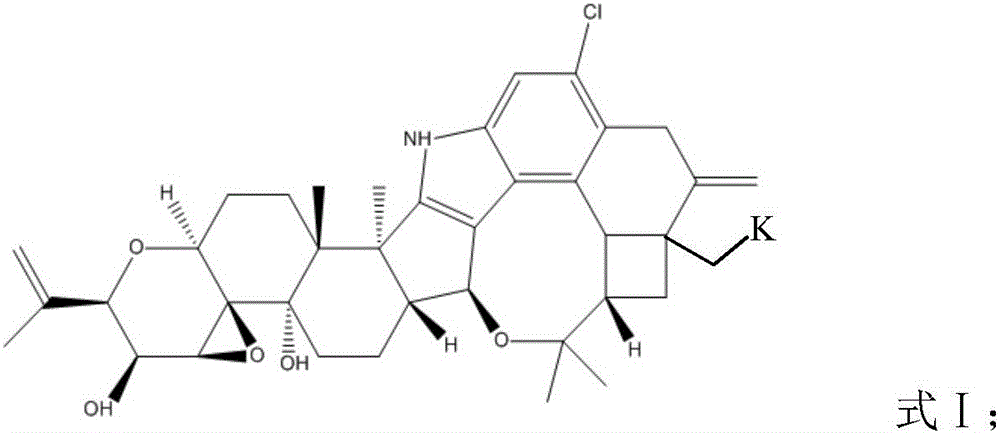
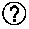Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
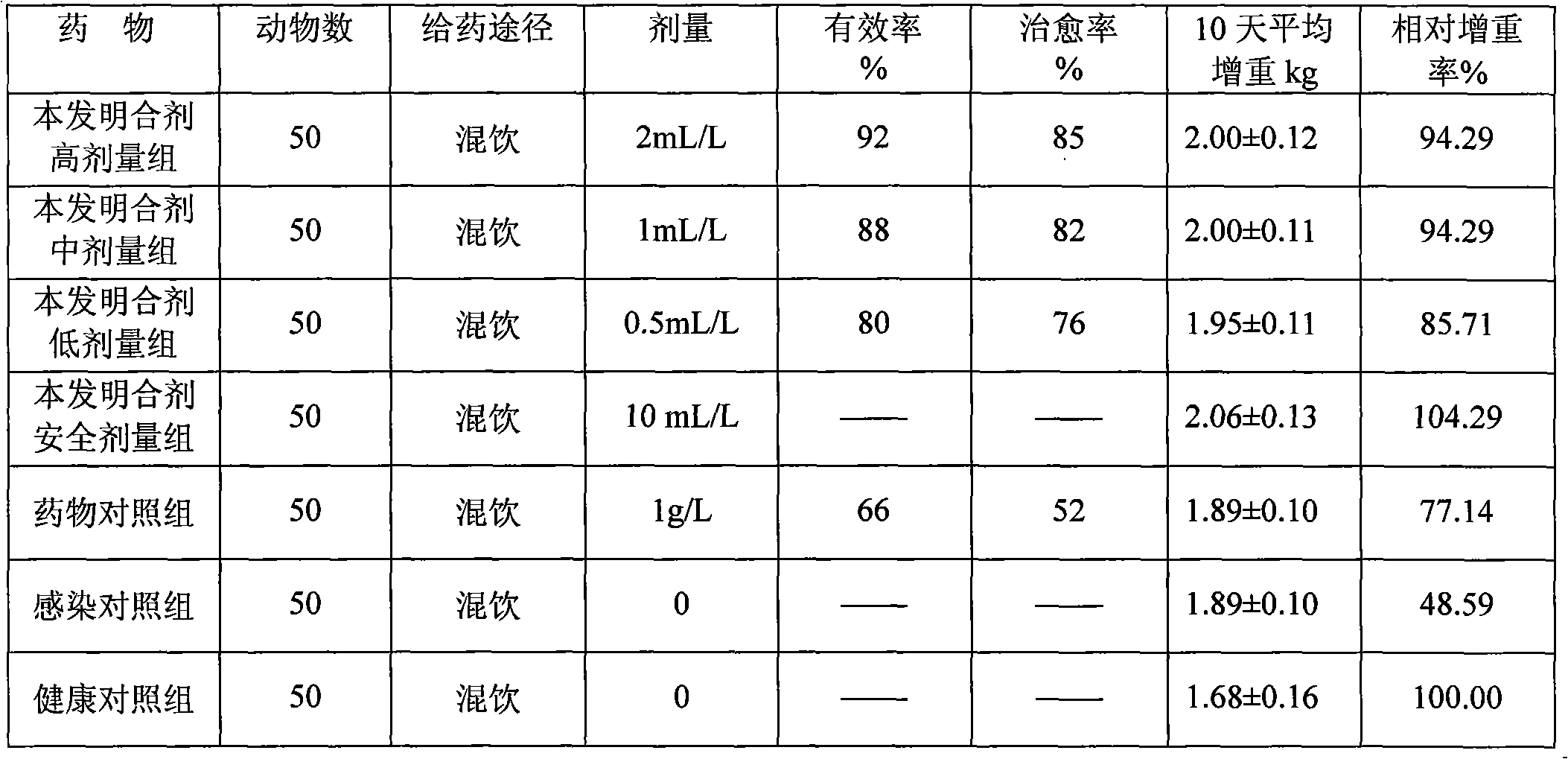
203 results about "Dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A dose is a measured quantity of a medicine, nutrient, or pathogen which is delivered as a unit. The greater the quantity delivered, the larger the dose. Doses are most commonly measured for compounds in medicine. The term is usually applied to the quantity of a drug or other agent administered for therapeutic purposes, but may be used to describe any case where a substance is introduced to the body. In nutrition, the term is usually applied to how much of a specific nutrient is in a person's diet or in a particular food, meal, or dietary supplement. For bacterial or viral agents, dose typically refers to the amount of the pathogen required to infect a host. For information on dosage of toxic substances, see Toxicology. For information on excessive intake of pharmaceutical agents, see Drug overdose.
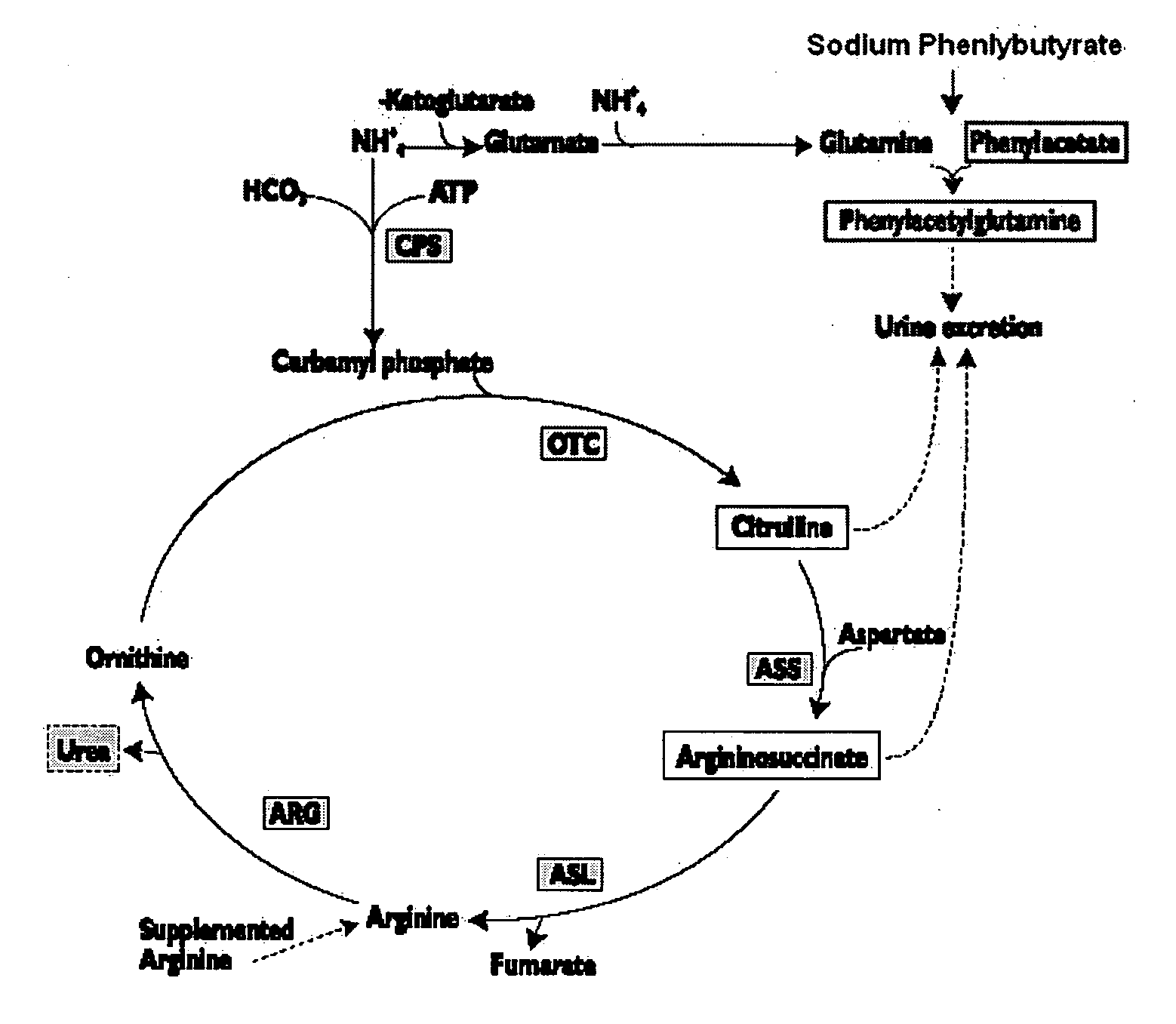
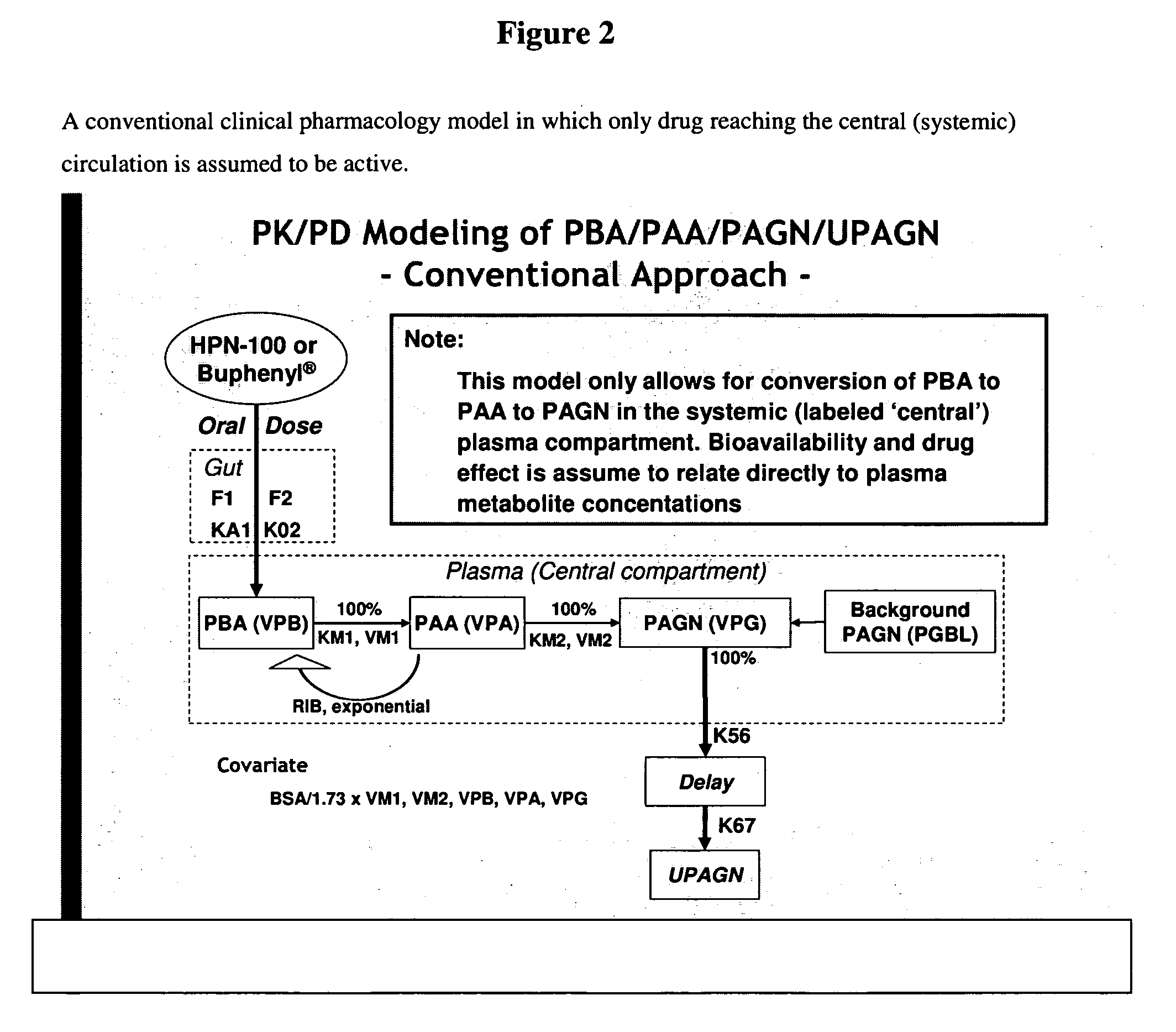
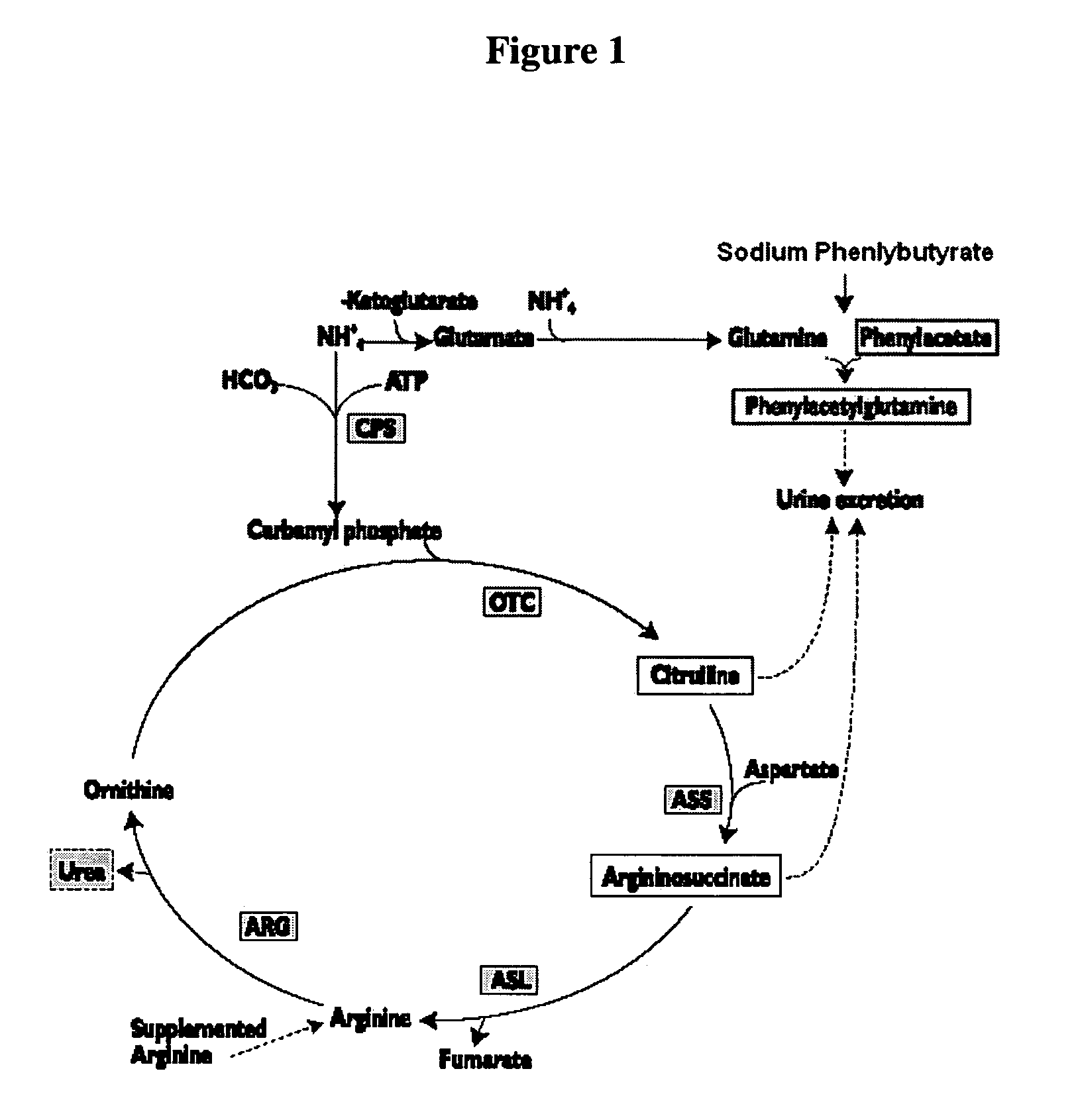
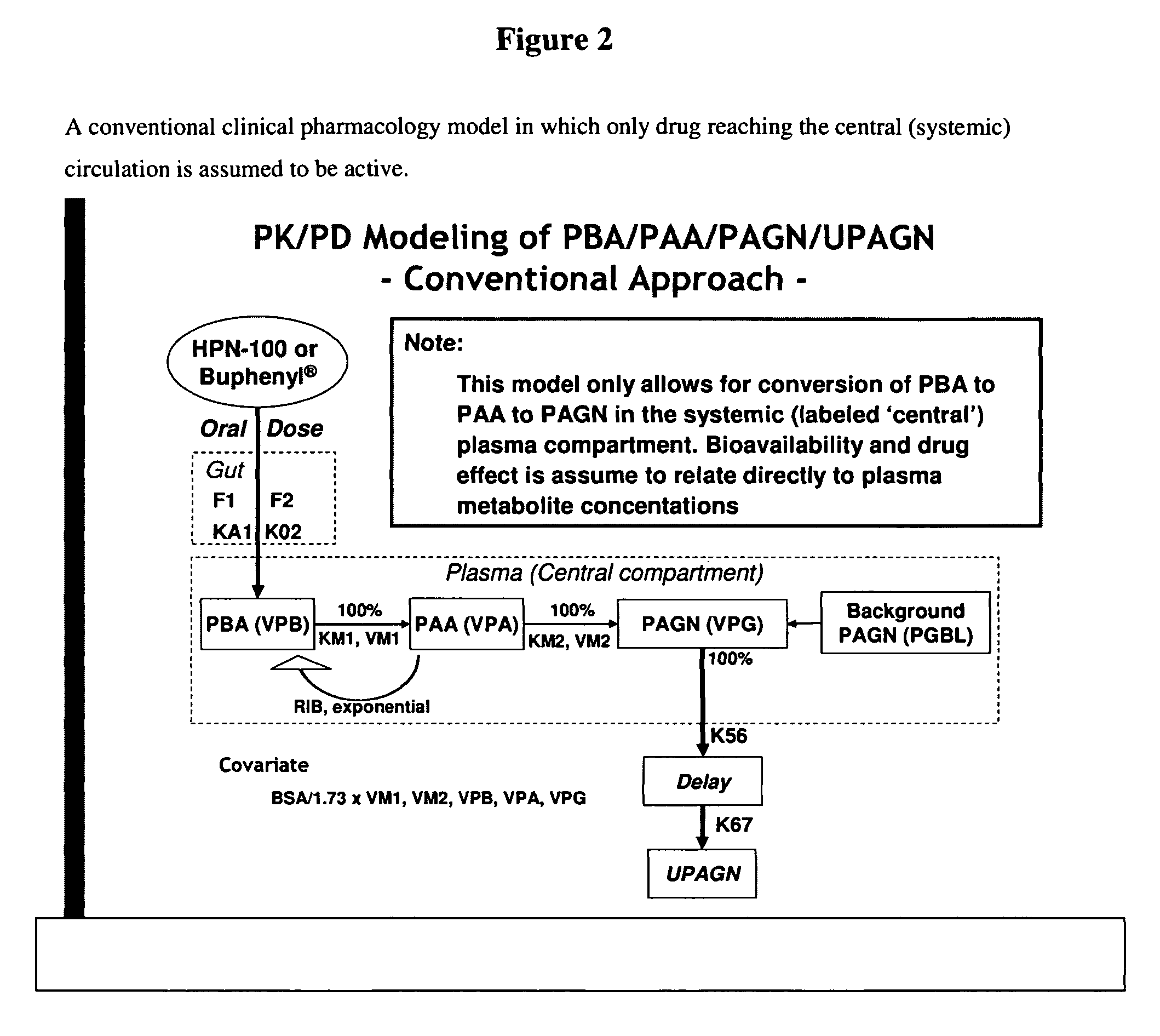
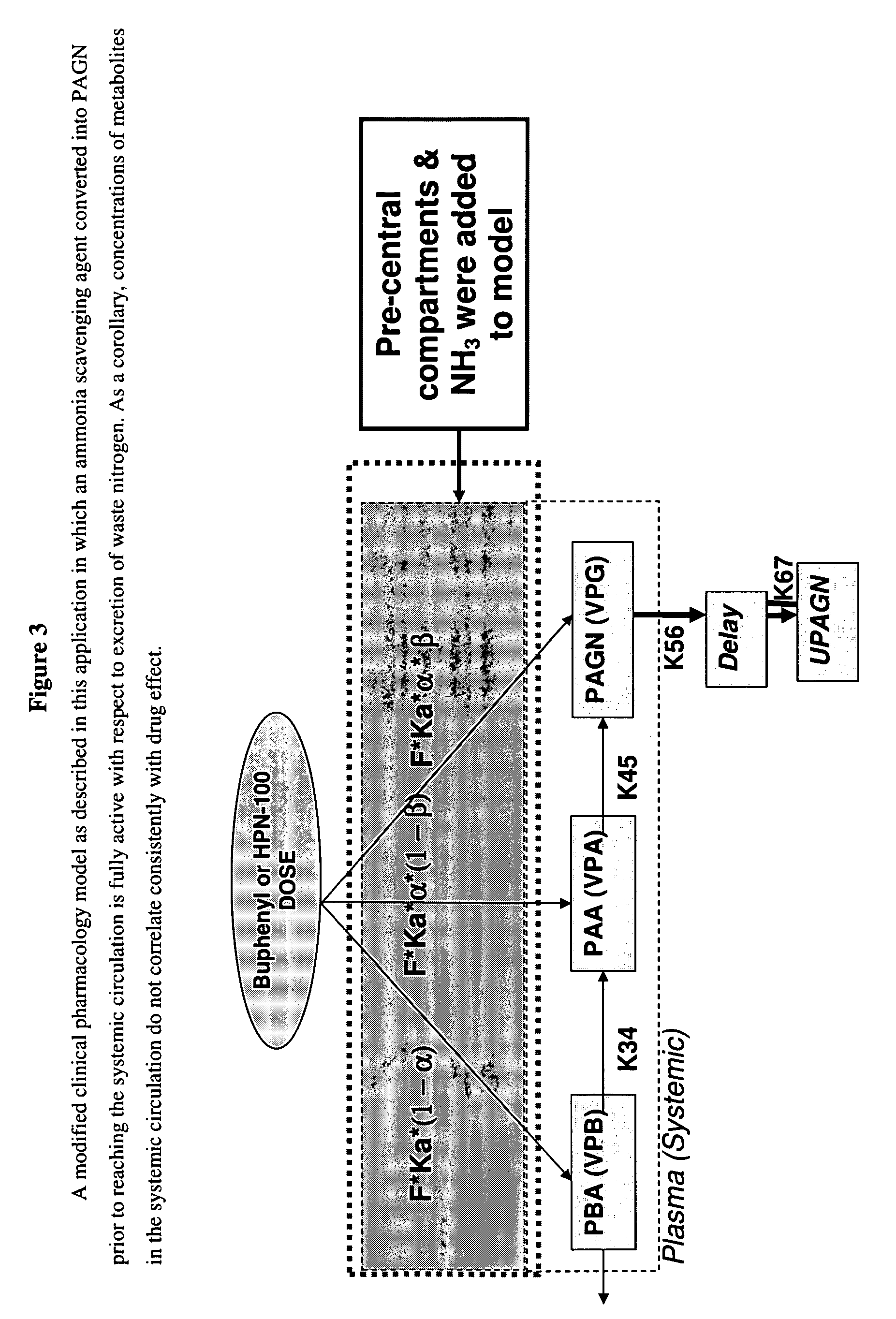
Methods of treatment using ammonia-scavenging drugs
The invention provides a method for determining a dose and schedule and making dose adjustments of PBA prodrugs used to treat nitrogen retention states, or ammonia accumulation disorders, by measuring urinary excretion of phenylacetylglutamine and / or total urinary nitrogen. The invention provides methods to select an appropriate dosage of a PBA prodrug based on the patient's dietary protein intake, or based on previous treatments administered to the patient. The methods are applicable to selecting or modifying a dosing regimen for a subject receiving an orally administered ammonia scavenging drug.
Owner:HORIZON THERAPEUTICS LLC
Methods of treatment using ammonia-scavenging drugs
ActiveUS8642012B2Mitigate issueReduce dosageBiocideMetabolism disorderDosing regimenPhenylacetylglutamine
The invention provides a method for determining a dose and schedule and making dose adjustments of PBA prodrugs used to treat nitrogen retention states, or ammonia accumulation disorders, by measuring urinary excretion of phenylacetylglutamine and / or total urinary nitrogen. The invention provides methods to select an appropriate dosage of a PBA prodrug based on the patient's dietary protein intake, or based on previous treatments administered to the patient. The methods are applicable to selecting or modifying a dosing regimen for a subject receiving an orally administered ammonia scavenging drug.
Owner:HORIZON THERAPEUTICS LLC
Anti-Diarrhea Preparations for Small Domestic Animals
A composition and method for treating diarrhea in small domestic animals wherein the composition includes an effective amount of kaolin, pectin, at least one probiotic, and a carrier, and the method includes the step of orally administering an effective dosage of the composition to the domestic animals.
Owner:VET SOLUTIONS
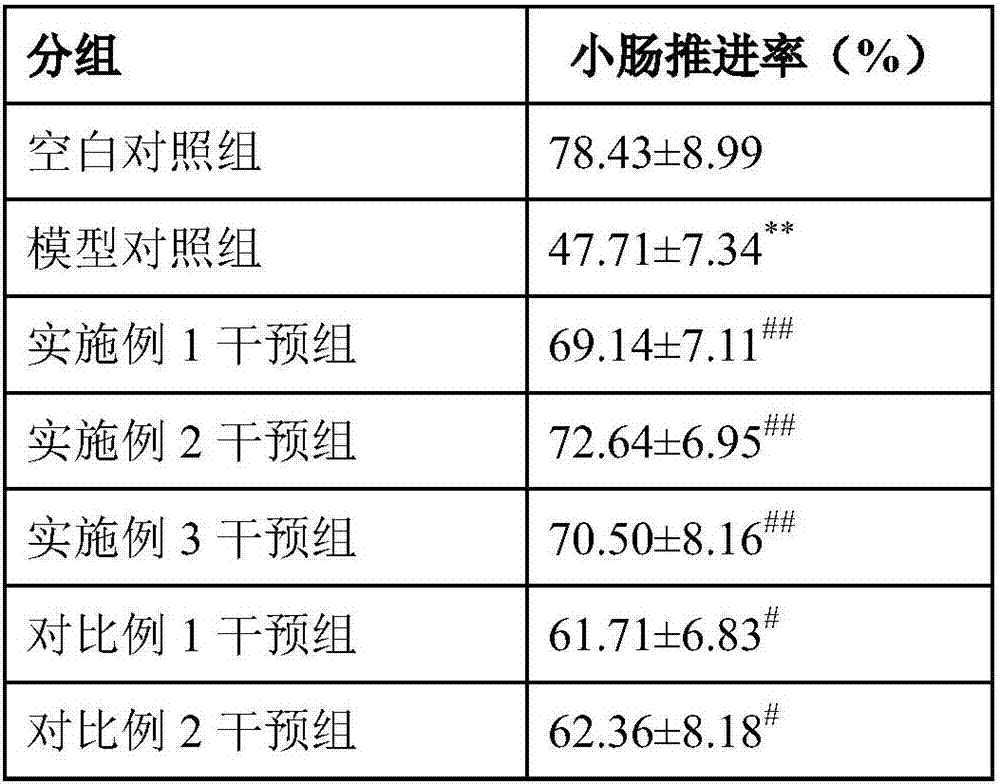
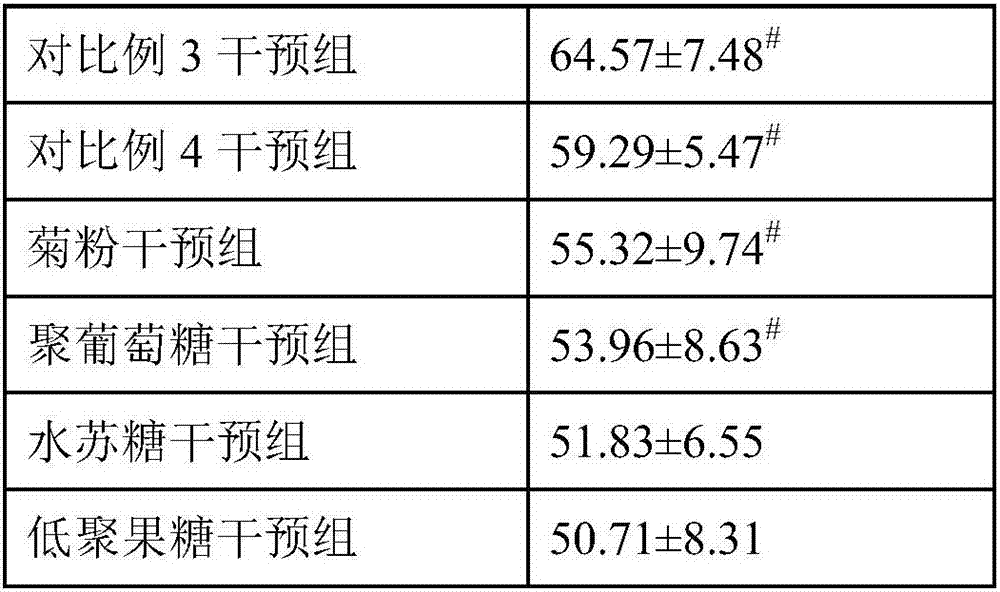
Intestine moistening and bowel relaxing compound prebiotics and preparation method and application thereof
PendingCN107495383AGood laxativeWide range of polymerizationFood ingredient functionsPolydextroseInulin
The present invention relates to intestine moistening and bowel relaxing compound prebiotics and a preparation method and an application thereof. The compound prebiotics consist of the following components in parts by weight: 30-80 parts of inulin, 20-60 parts of polydextrose, 2-10 parts of stachyose and 2-10 parts of fructooligosaccharides. The selected 4 prebiotics are reasonable in material matching and scientific in dosages, and can increase an intestinal mechanical stimulation to promote peristalsis, shorten defecation, increase satiety, reduce an intestinal pH, etc., and prevent or relieve constipation. A compounding of the various prebiotics can avoid disadvantages that an intestinal function is single, a bowel relaxing function is weak, intestinal tracts are easy to be uncomfortable, constipation tolerance and other single dietary fiber functions. The compound prebiotics can be directly used as a terminal food and can also be used as accessory materials for food or health-care food.
Owner:北京市营养源研究所有限公司
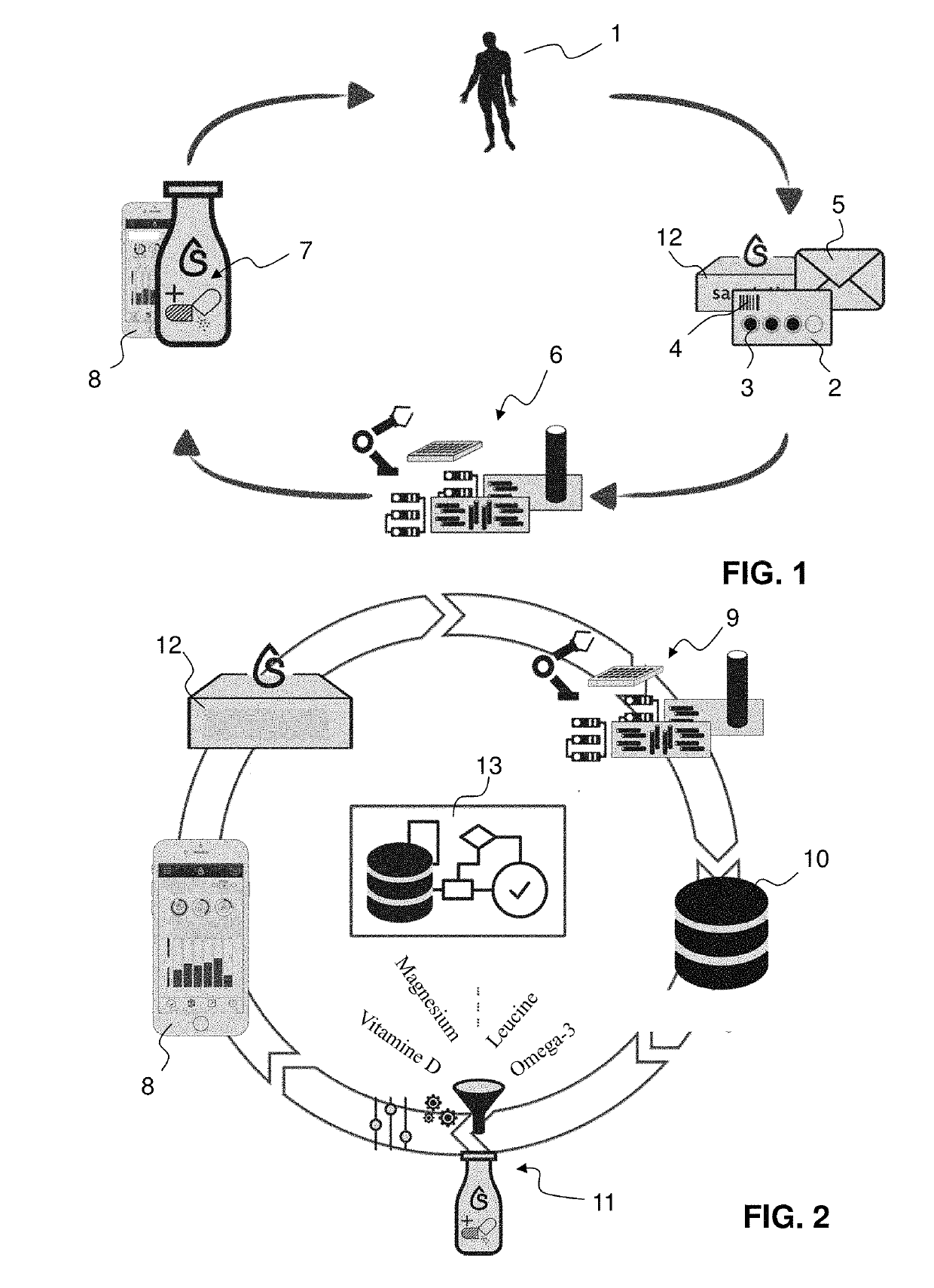
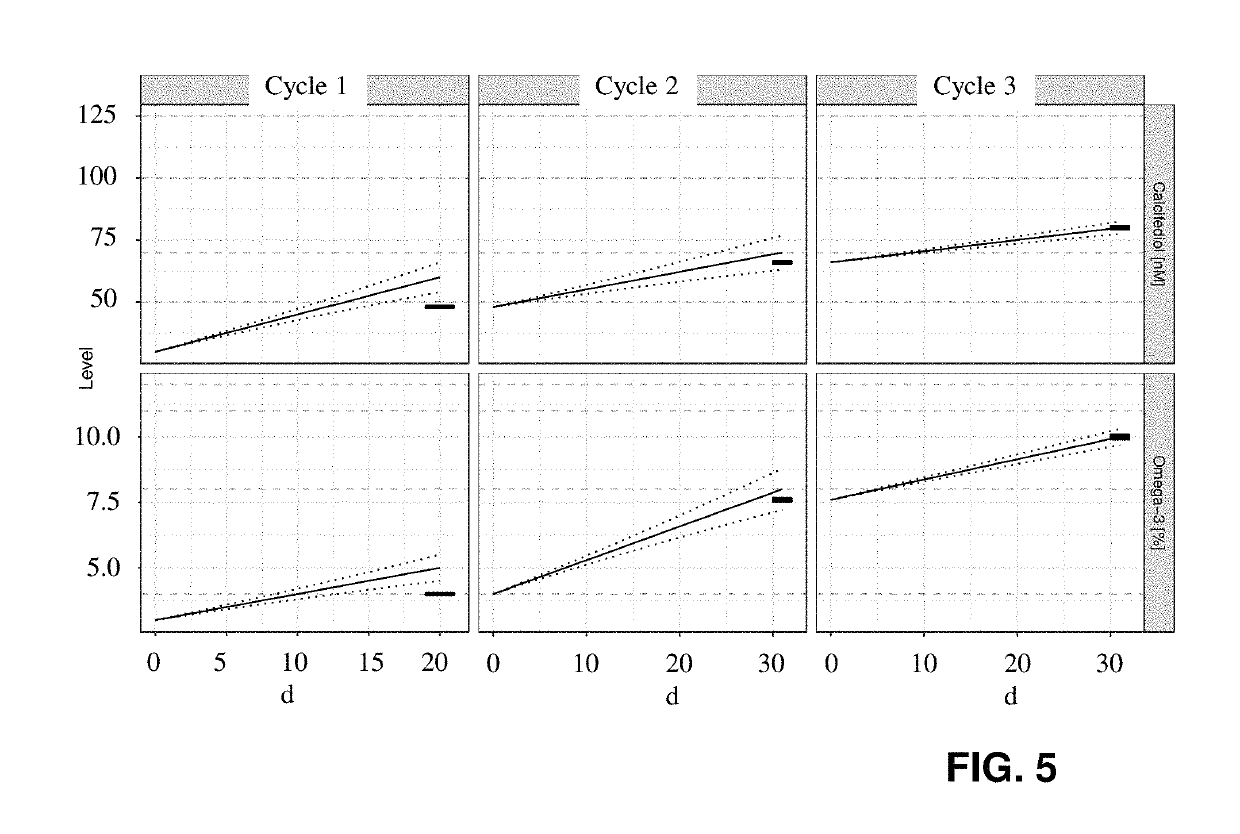
Personalised nutrient dosing with on-going feedback loop
ActiveUS20190145988A1Increase valueInhibition effectHydroxy compound active ingredientsBiostatisticsPersonalizationNutritional status
A method for providing nutritional supplement information for a subject is proposed, including a sequence of steps in given order and repeated at least once after a time span of at least 2 days or one week for adapting the provided nutritional information:A) taking a sample from the subject;B) analyzing said sample to determine the nutritional status;C) based on the results calculation of nutritional supplements to improve the nutritional status;D) providing individualized nutritional supplement information.This sequence involves the prediction of at least one initial characteristics matrix and multiplication of this matrix weighted with factors, with an initial recommendation vector for the calculation of a target profile vector after a given first time interval from the profile vector as determined in step B), and in each following cycle adaptation by adapting at least one of the characteristics matrix and the weighting factors.
Owner:BAZE LABS LLC
Chinese medicinal composition and preparation method thereof
ActiveCN101816739AImprove immunityAnti-aging effectDigestive systemAntinoxious agentsBiotechnologyForest yam
The invention relates to the field of Chinese medicine, and in particular discloses a Chinese medicinal composition for enhancing immunity or delaying senility. The medicinal composition comprise the following raw materials in part by weight: 40 to 80 parts of astragalus, 10 to 25 parts of ginseng, 10 to 30 parts of medlar, 10 to 30 parts of glossy privet fruit, 10 to 30 parts of common yam rhizome, 10 to 30 parts of Indian buead, 10 to 25 parts of epimedium herb, 10 to 25 parts of mulberry, 5 to 20 parts of yerbadetajo herb, 10 to 25 parts of lucid ganoderma, 10 to 25 parts of tangerine peel and 5 to 20 parts of liquoric root. The Chinese medicinal composition can increase weights of immune organs of mice such as thymus and spleen, enhance the cellular immunity and ensure a dose-response relationship between the effect and the dosage, and can remarkably prolong the swimming time of the mice and keep the anti-fatigue effect in the dose-response relationship. The invention also provides a method for preparing the Chinese medicinal composition. The Chinese medicinal composition is a pure Chinese preparation, can enhance the resistance and delay the senility, and has the anti-fatigue effect and good application prospect.
Owner:KUNMING CHINESE MEDICINE FACTORY
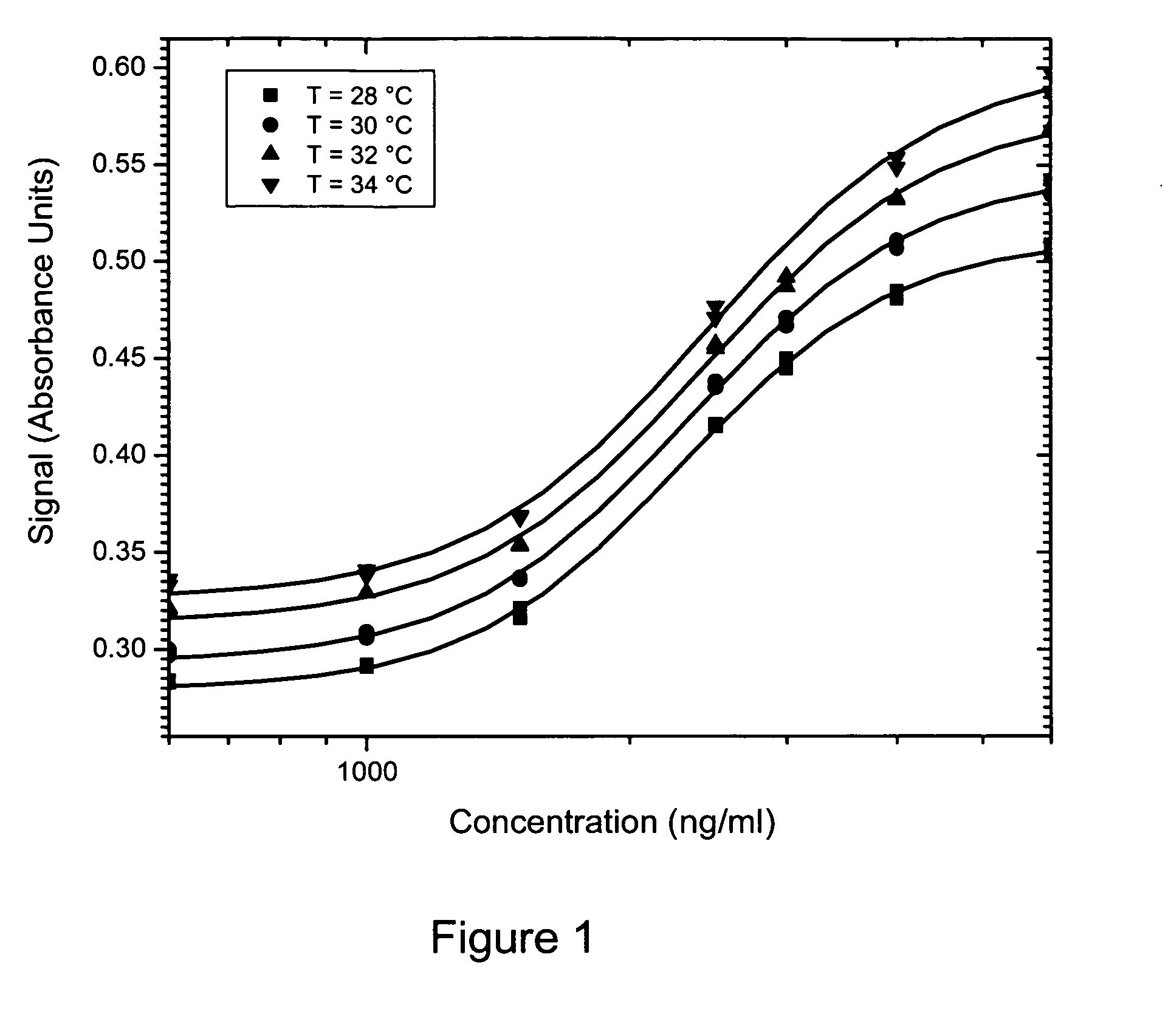
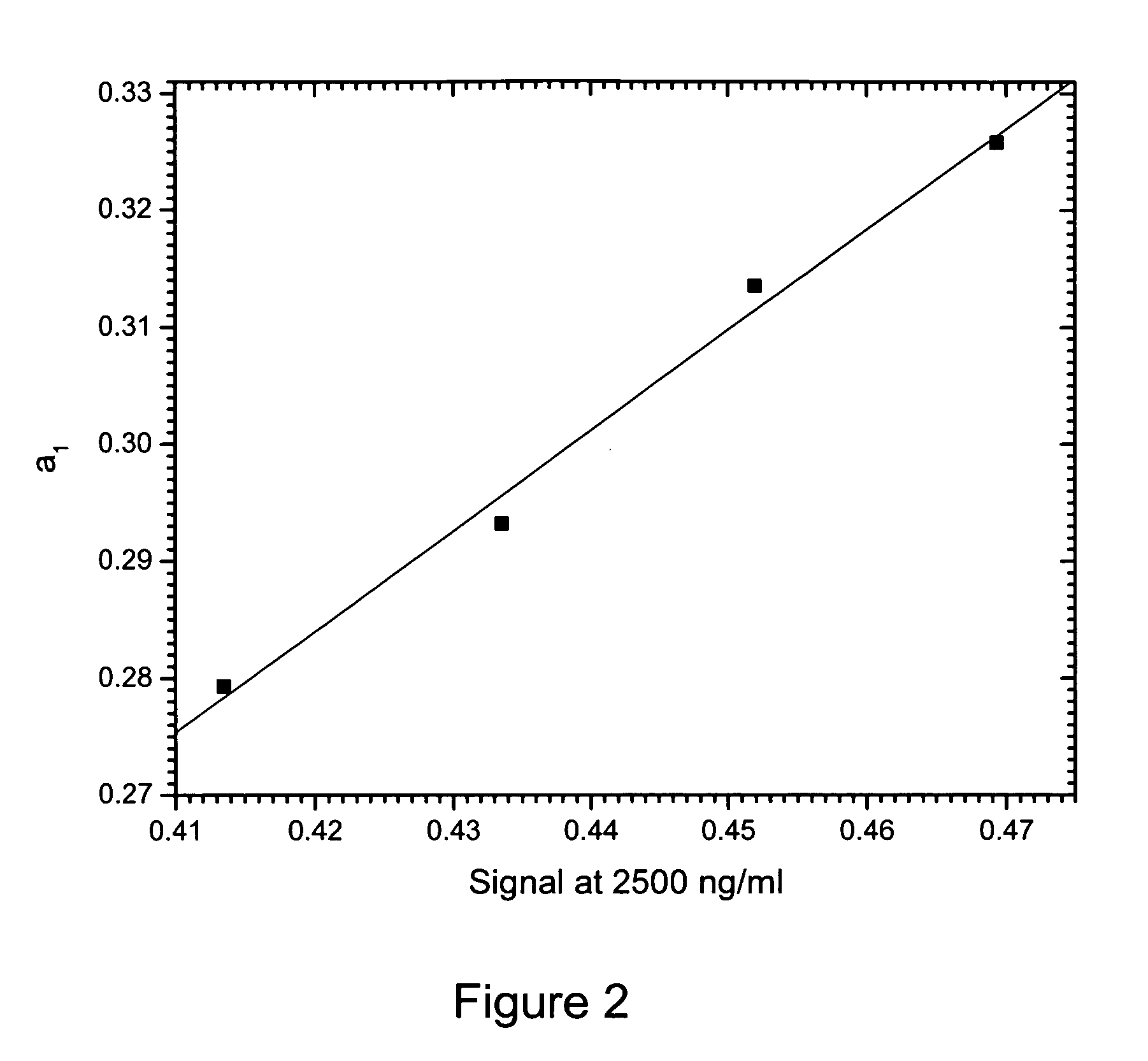
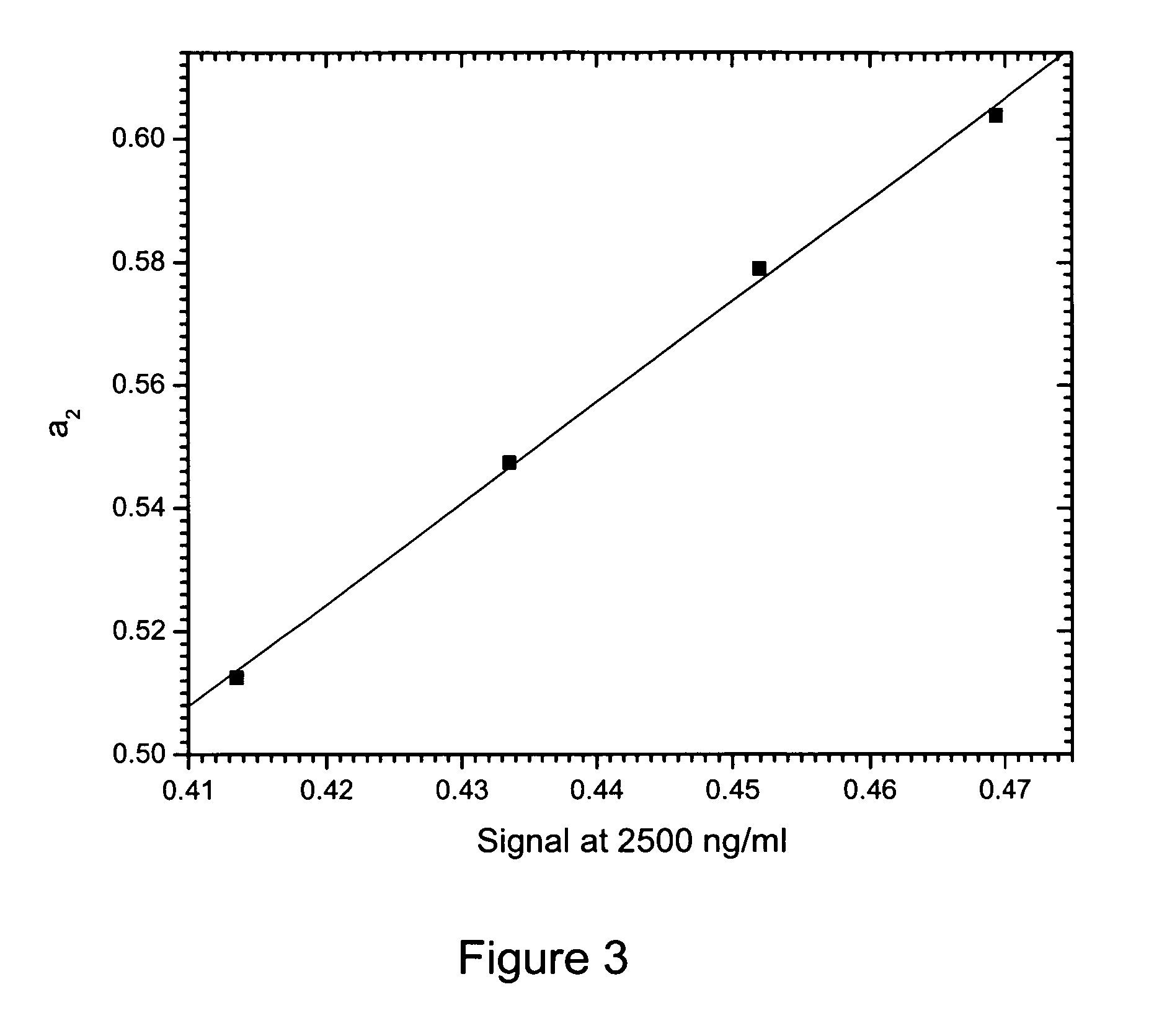
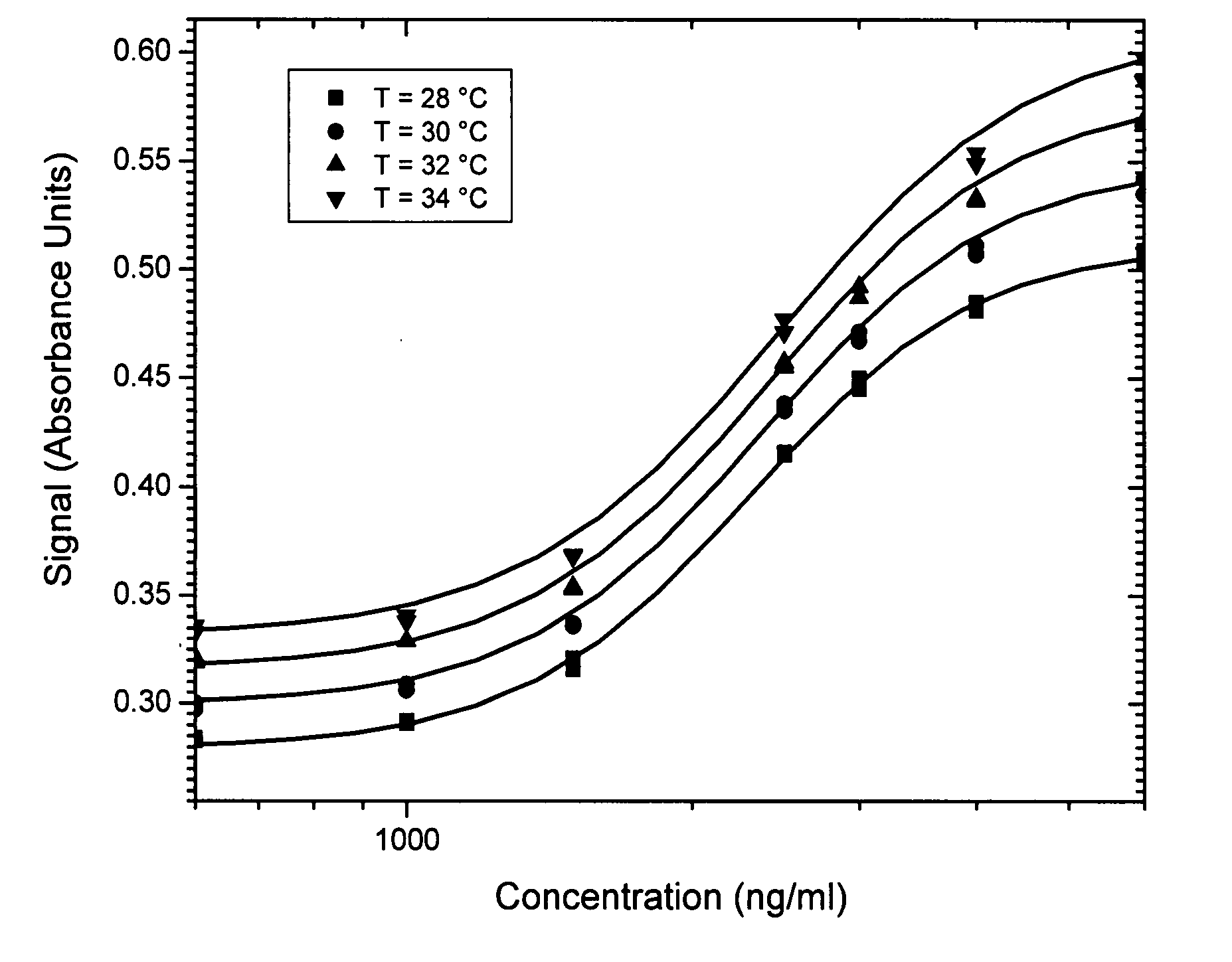
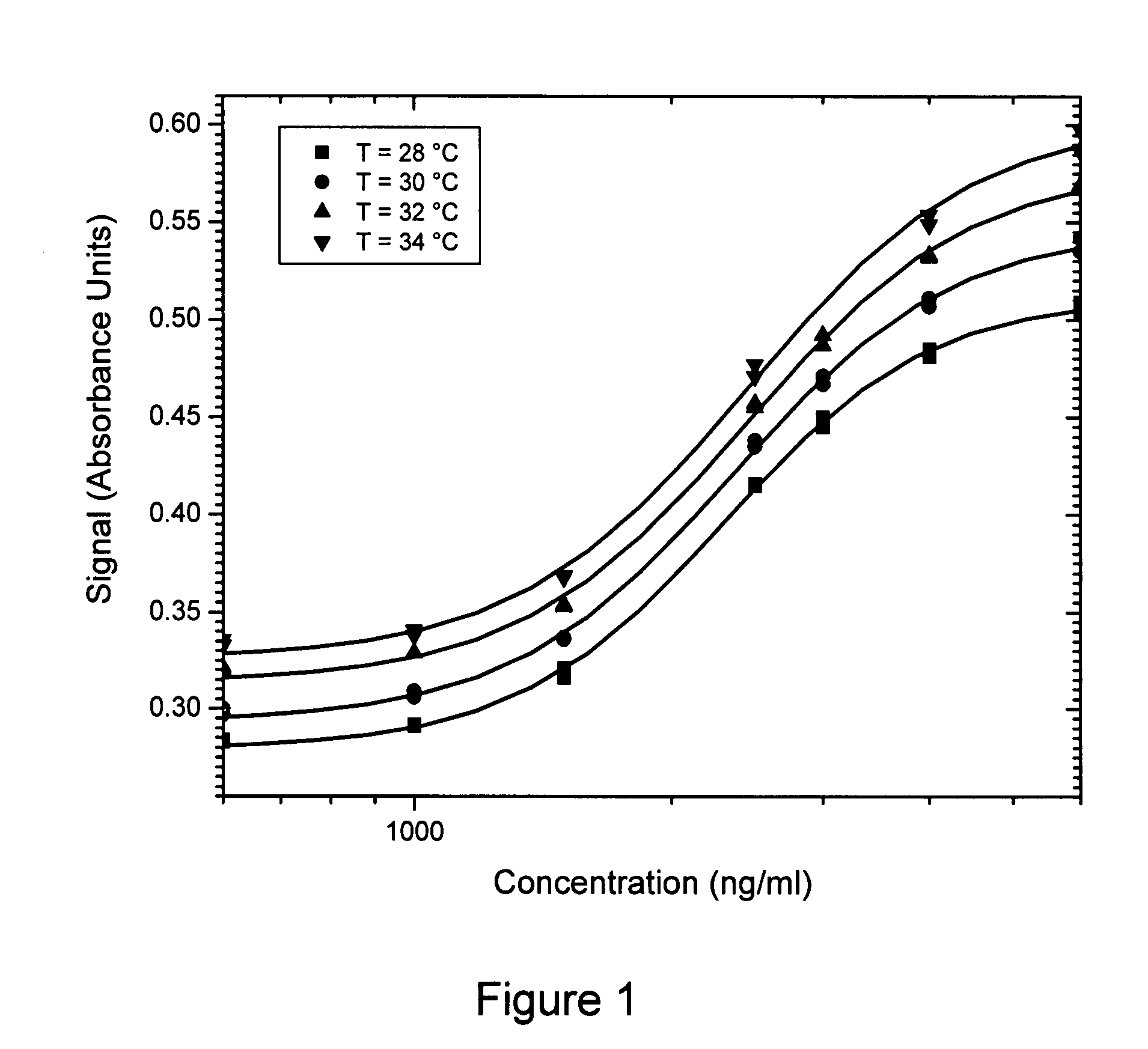
Method of compensation of dose-response curve of an assay for sensitivity to perturbing variables
InactiveUS20070166762A1Accurate measurementBiological testingSpecial data processing applicationsAnalyteTwo step
The present invention provides assays and methods of compensating for changes in the dose-response curve of an assay where such changes are due to variations in a perturbing variable such as, but not limited to temperature. This is achieved by a two-step method, the first step of which involves measurements of the dose-response curve, and thus the individual assay parameters, at many different values of the perturbing variable, spanning the expected range of the perturbing variable. In the second step, unknown samples are assayed simultaneously with a known standard at a chosen analyte concentration. During this measurement, the value of the perturbing variable is unknown and the dose-response curve is therefore also unknown. The different dose-response curves from the first step are used to determine a mathematical relationship between the assay parameters and the assay signal of the known standard. With this relationship, in which the value of the perturbing variable is implicit rather than explicit, assay parameters that are valid for the unknown value of the perturbing variable can be obtained by substituting the value of the assay signal from the known standard (measured when assaying the unknown samples) into the mathematical relationship and solving for the assay parameters. The method enables an accurate determination of the analyte concentration even when the perturbing variable is changing or fluctuating from one sample measurement to another. Once the first step is completed, the second step can be performed repeatedly to measure unknown samples with accuracy.
Owner:NOVX SYST CANADA
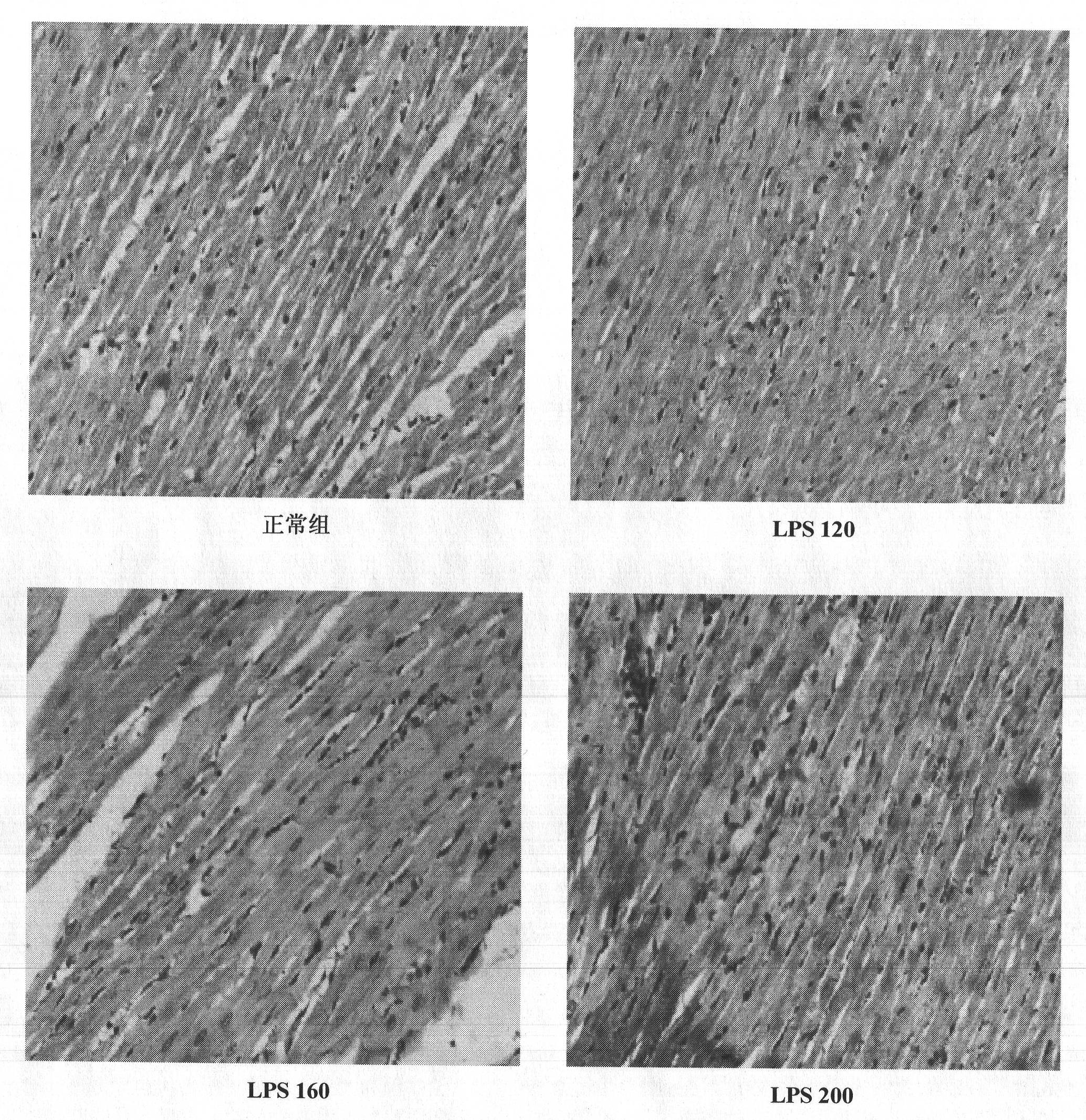
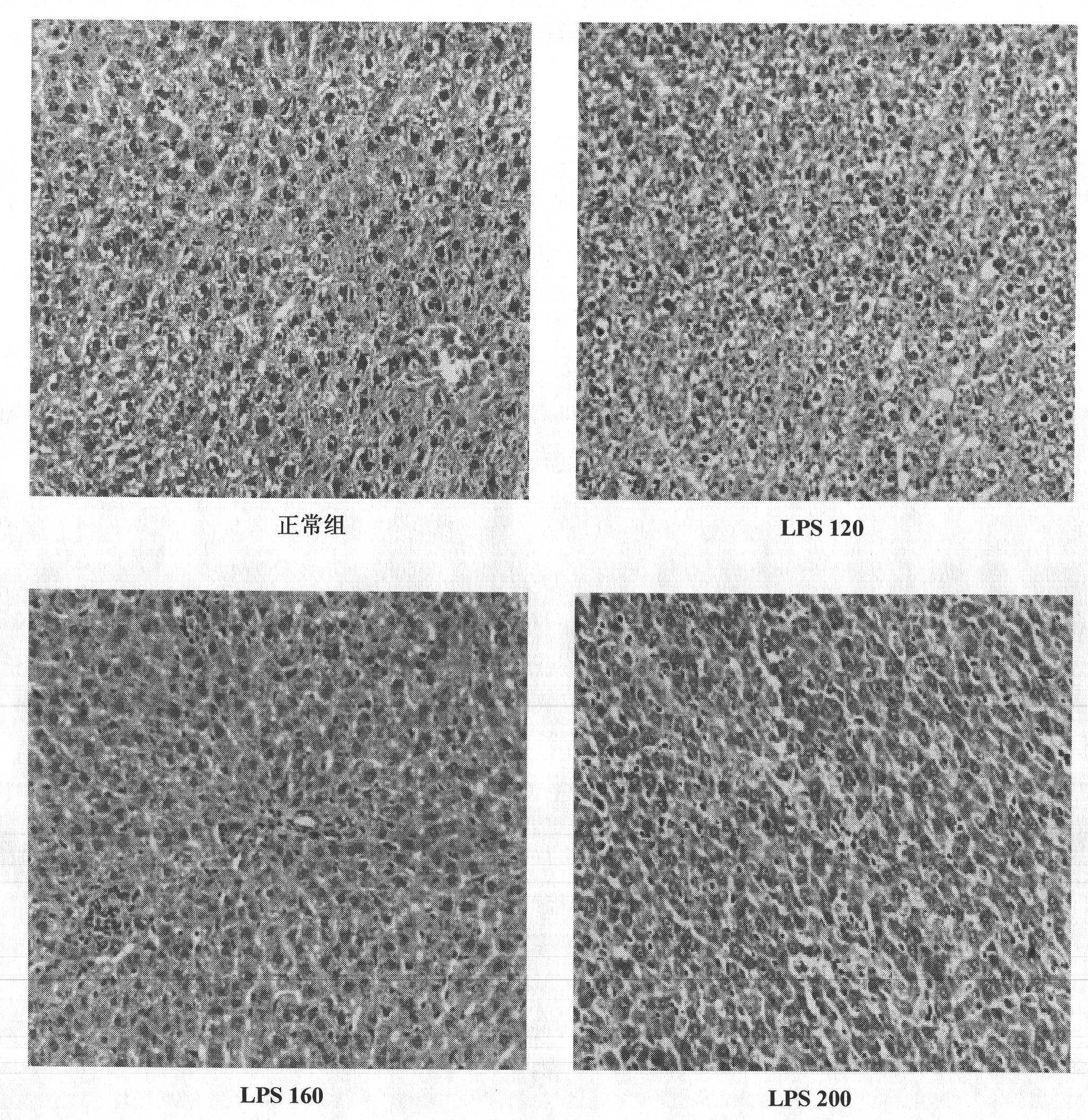
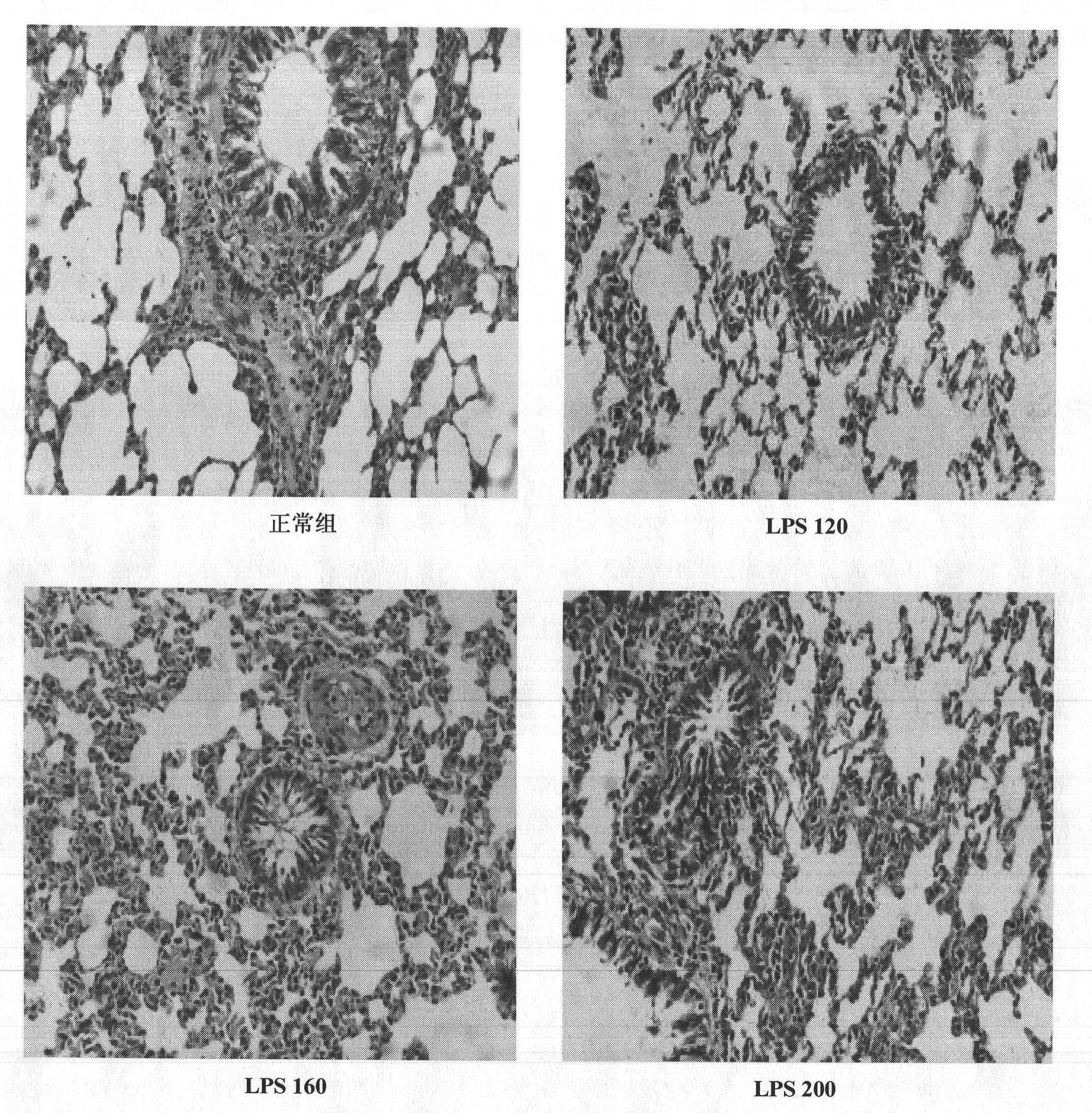
Construction method of animal model of chronic inflammation
InactiveCN102188442AOrganic active ingredientsIn-vivo testing preparationsClinico pathologicalWhite blood cell number
The invention belongs to the fields of medical evaluation, detection technology and experimental zoology, and specifically relates to a construction method of animal model of rat which is used for screening medicaments for treating chronic inflammation, and evaluating methods for treating systematic chronic inflammation. The method comprises the following step that healthy male SD rats are administered with lipopolysaccharide by tail intravenous injection, on an every first day of a week for 4-8 weeks persistently, at a dosage of 120-300 [mu]g per kg of body weight, so that pathologic changes, which meet pathological characteristics of chronic inflammation, mainly of an increased ratio of peripheral blood leucocyte count to neutrophile granulocyte, an increased serum hypersensitive C-creative protein level, infiltration of heart, liver, lung, kidney in inflammatory cells, and haemostasis and oedema of tissues are generated. The method has a good repeatability, and operation of the method is simple, easily controllable and mechanism-clear, besides indexes can be analyzed quantitatively and are convenient for statistics treatments. In addition, the constructed animal model is more ideal and more conform to clinic pathological and physiological changes of the type of disease.
Owner:广西中医学院
Cultivation method and application of cordyceps militaris
ActiveCN103477871AExcellent anti-lung cancer effectHorticultureFertilizer mixturesCordycepsCordyceps militaris
The invention provides a cultivation method and application of cordyceps militaris. The method includes the steps that a culture medium is prepared by mixing dry silkworm chrysalis meal with an intensity of 20g / L, rice meal with the intensity of an intensity of 180g / L, monopotassium phosphate with an intensity of 1.2g / L, sodium dihydrogen phosphate with an intensity of 1g / L and the balance of water, and sealing, sterilization and cooling are carried out; second, a cordyceps militaris strain is inoculated, wherein the density of the strain is 3-5pellets / mL, and the volume ratio of the culture medium to the strain is 50:1; cultivation is carried out, wherein the cultivation includes dark cultivation and light cultivation; sporocarp is harvested. The cordyceps militaris cultivated through the method is made into medicine, C57 mice inoculated with the lewiss lung carcinomas are used as experimental subjects and dosed with the medicine according to the standard that the administration dosage of the cordyceps militaris in the medicine is 5g / kg, the C57 mice are dosed with the medicine one time per day, and the suppression ratio of the lewiss lung carcinomas is 19.6% according to detection after the C57 mice are continuously dosed with the medicine for 10 days.
Owner:杭州佗鹊堂生物科技有限公司
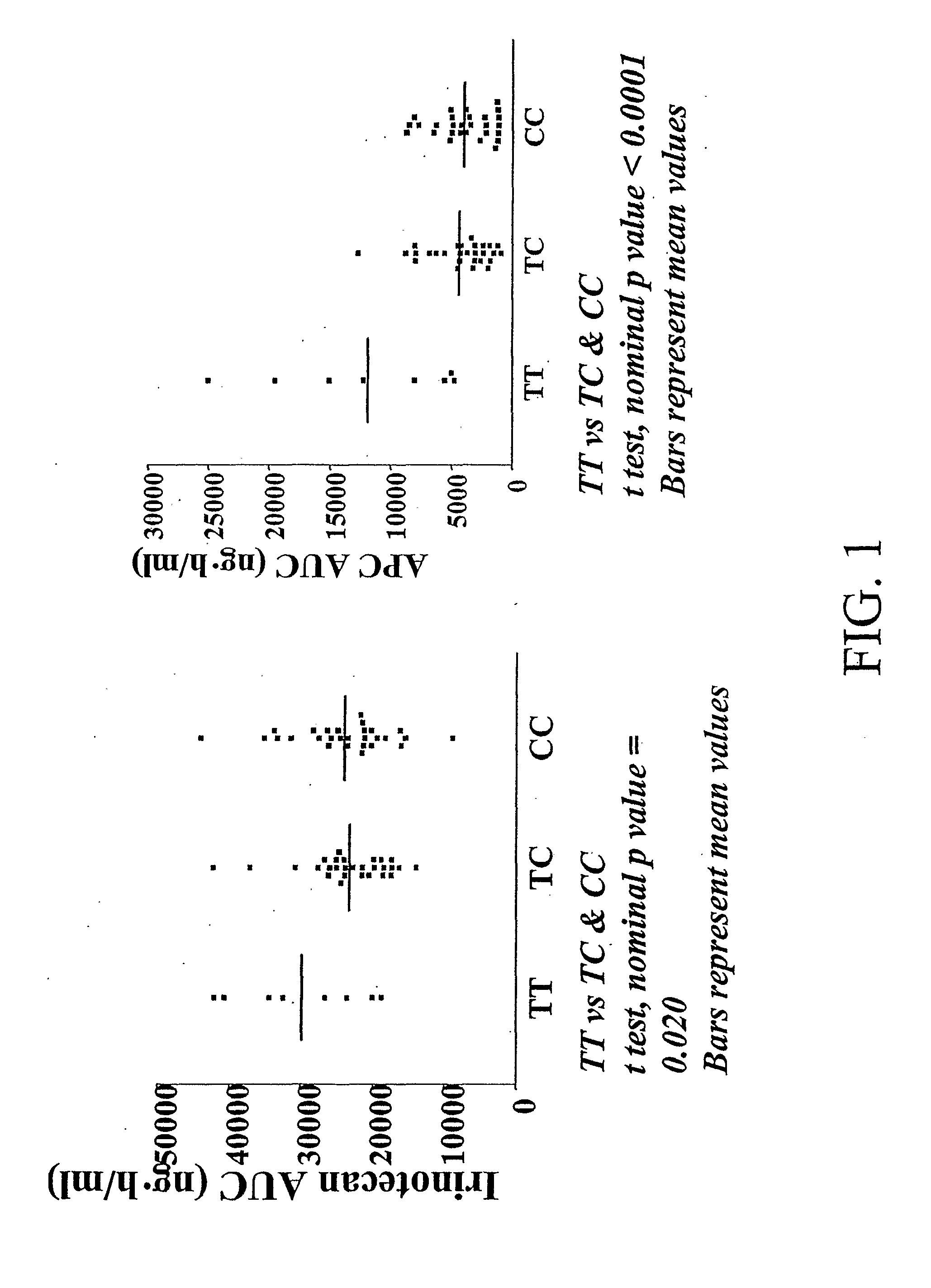
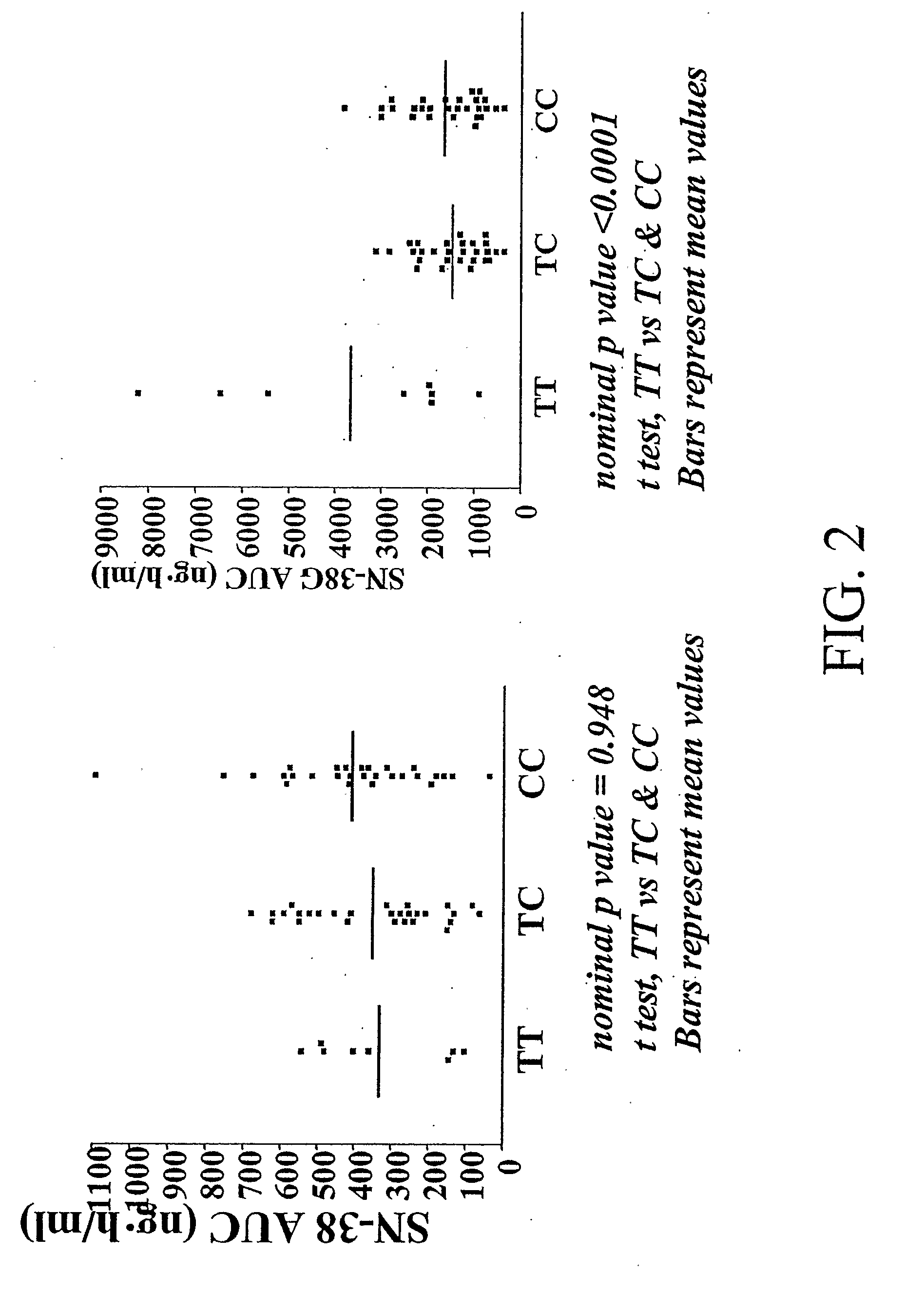
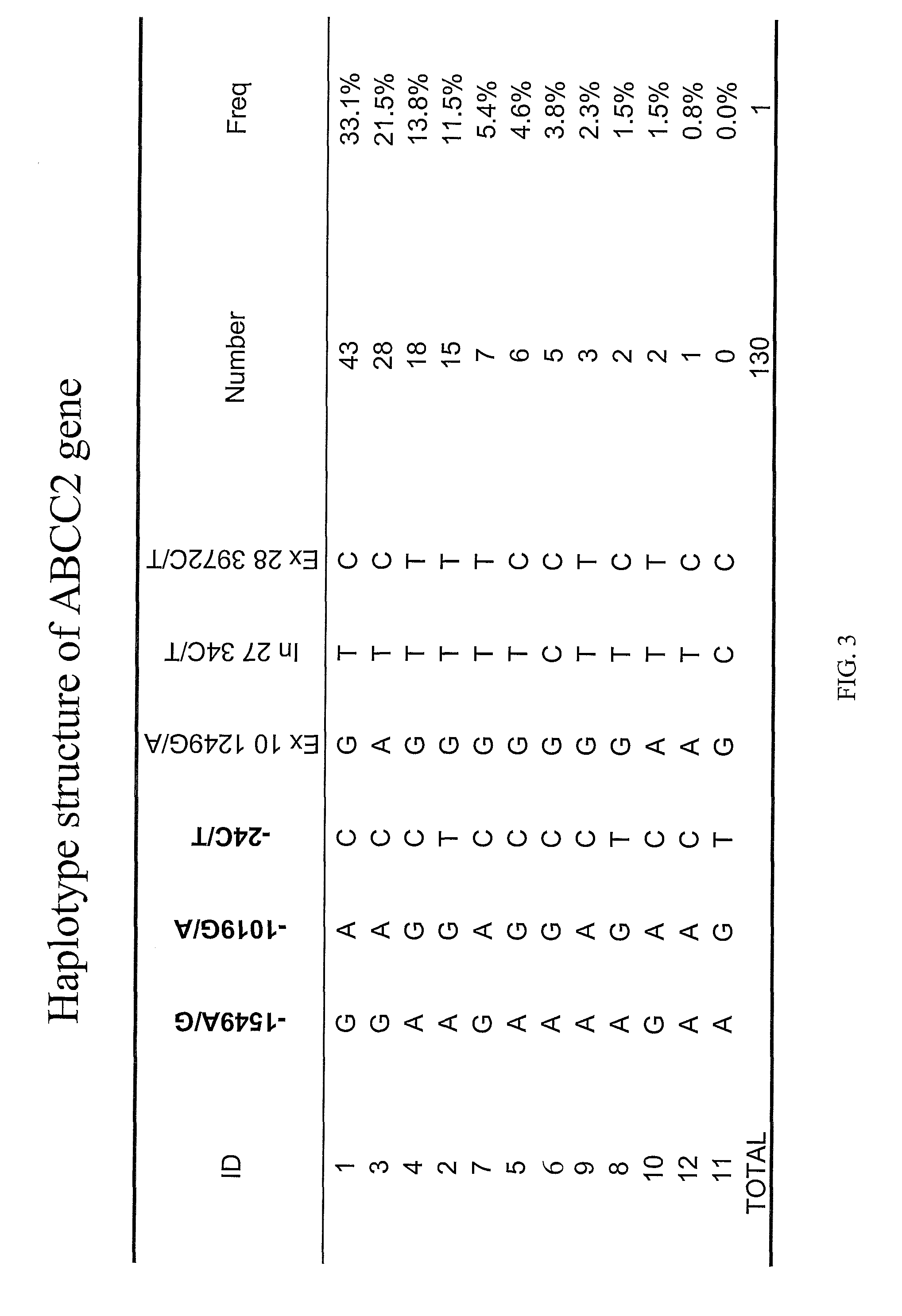
Methods and compositions relating to pharmacogenetics of different gene variants in the context of irinotecan-based therapies
InactiveUS20090247475A1Reduced cytotoxic activityIncrease and reduce chance of responseBiocideMicrobiological testing/measurementPharmacogeneticsSLCO1B1
The present invention is directed to methods and compositions for determining the presence or absence of polymorphisms within an ABCC2, UGT1A1, and / or SLCO1B1 gene and correlating these polymorphisms with activity levels of their gene products and making evaluations regarding the effect on their substrates, particularly those substrates that are drugs. In addition, there are methods and compositions of evaluating the risk of an individual for developing toxicity or adverse event(s) to an ABCC2, UGT1A1, and / or SLCO1B1 substrate. In some embodiments, the invention concerns methods and compositions for determining the presence or absence of ABCC2 3972C>T variant and predicting or anticipating the level of activity of ABCC2 and determining dosages of an ABCC2 drug substrate, such as irinotecan, in a patient. Such methods and compositions can be used to evaluate whether irinotecan-based therapy, or therapy involving other ABCC2 substrates, may pose toxicity problems if given to a particular patient or predicting their efficacy. Alterations in suggested therapy may ensue based on genotyping results.
Owner:UNIVERSITY OF CHICAGO +1
Preparations of Taxanes for Intravenous Administration and the Preparation Method Thereof
The present invention relates to the field of medical technology. More specifically, the present invention relates to a preparation of taxanes for intravenous administration, which consists of two parts: a drug solution and an emulsion. Said drug solution consists of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein said solvent for injection is an organic solvent. Said emulsion includes a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When used, the drug solution at the clinical dosage can be added and mixed homogeneously in the emulsion to perform intravenous drip directly; or the drug solution at the clinical dosage can also be firstly added into the emulsion with no less than 5 times volume of the drug solution and then a predetermined amount of normal saline or glucose solution for injection is added to perform intravenous drip. The preparation of the present invention does not contain solubilizer and has advantages of little toxicity, safety, effectiveness, stability and economy. The fat emulsion is also used as a nutritional replenishment, thus achieving a better therapeutic effect. In addition, the normal saline or glucose solution for injection can be used to replace a considerable amount of the emulsion, which makes the preparation, therefore, not only cost-efficient, but also convenient for transportation and storage in practice.
Owner:TASLY HLDG GRP CO LTD
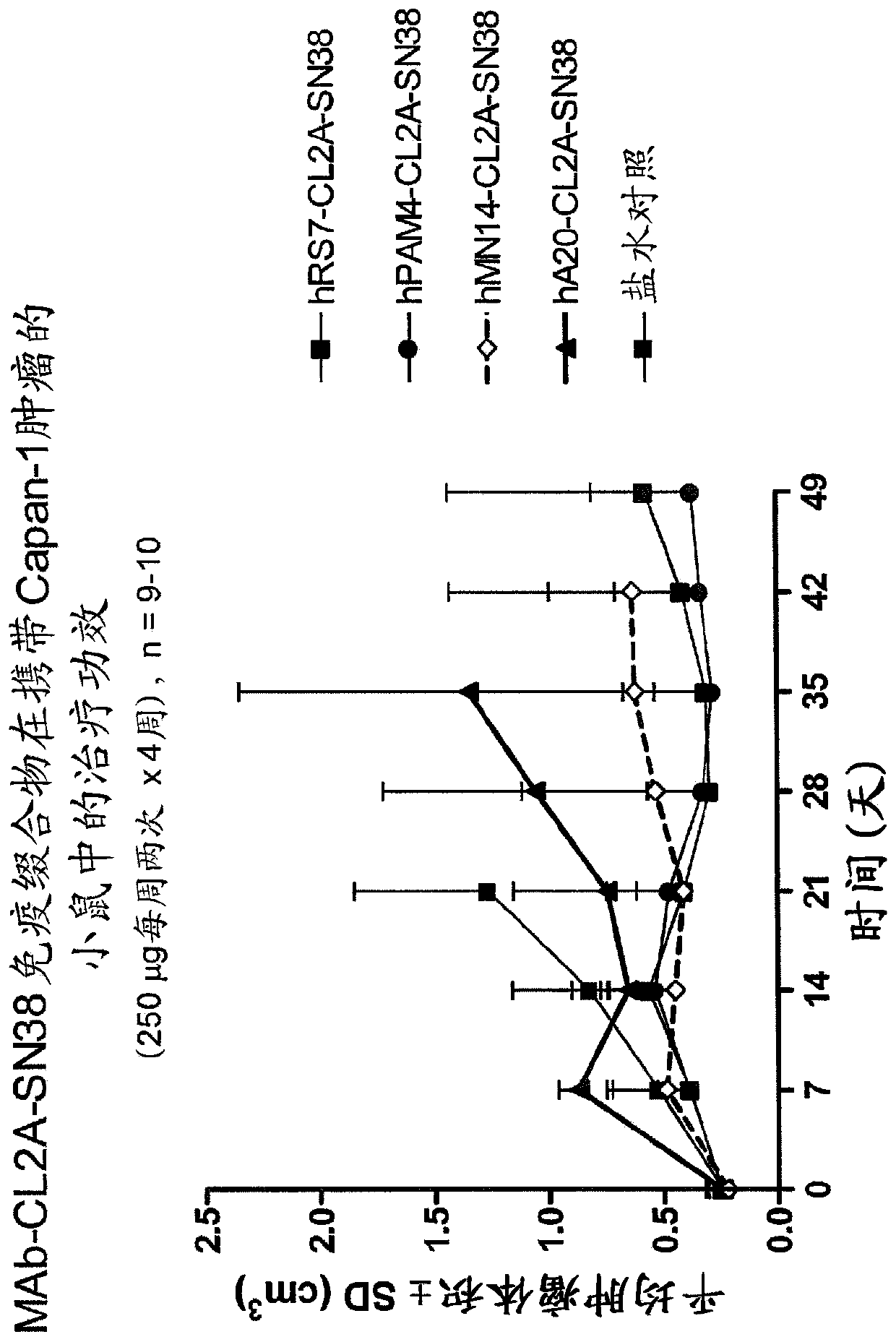
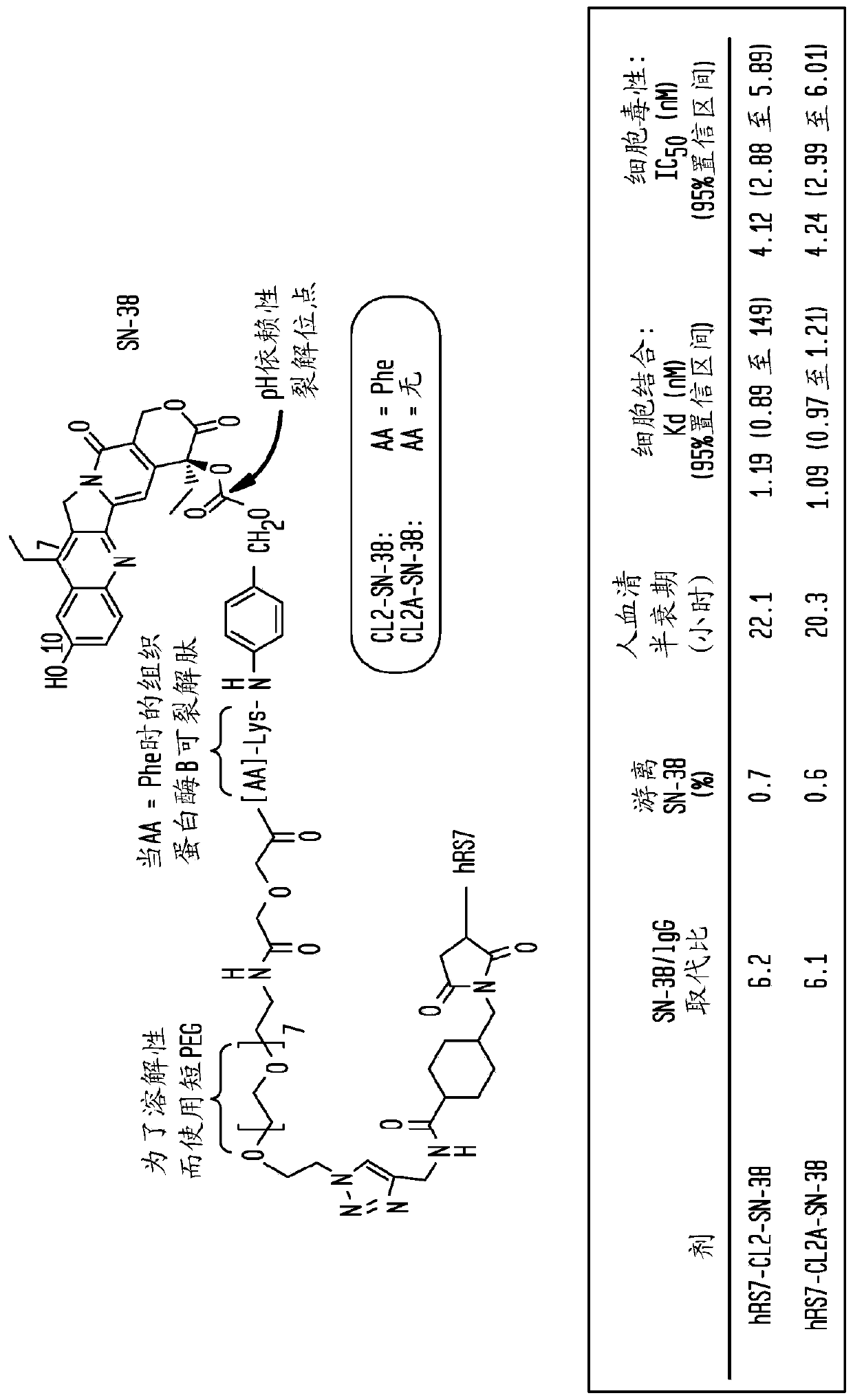
Treatment of trop-2 expressing triple negative breast cancer with sacituzumab govitecan and a rad51 inhibitor
InactiveCN110392570AInorganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesRadical radiotherapy
The present invention relates to treatment of Trop-2 postive cancers with the combination of anti-Trop-2 ADC and a Rad51 inhibitor. Preferably the drug conjugated to the antibody is SN-38, and the ADCis sacituzumab govitecan. The ADC may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg. When administered at specified dosages and schedules, the combination of ADC and Rad51 inhibitor can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the combination is effective to treat cancers that are refractory to or relapsed from irinotecan or topotecan.
Owner:IMMUNOMEDICS INC
Method of compensation of dose-response curve of an essay for sensitivity to perturbing variables
InactiveUS20070250271A1Accurate measurementMicrobiological testing/measurementBiological testingAnalyteTwo step
The present invention provides assays and methods of compensating for changes in the dose-response curve of an assay where such changes are due to variations in a perturbing variable such as, but not limited to temperature. This is achieved by a two-step method, the first step of which involves measurements of the dose-response curve, and thus the individual assay parameters, at many different values of the perturbing variable, spanning the expected range of the perturbing variable. In the second step, unknown samples are assayed simultaneously with a known standard at a chosen analyte concentration. During this measurement, the value of the perturbing variable is unknown and the dose-response curve is therefore also unknown. The different dose-response curves from the first step are used to determine a mathematical relationship between the assay parameters and the assay signal of the known standard. With this relationship, in which the value of the perturbing variable is implicit rather than explicit, assay parameters that are valid for the unknown value of the perturbing variable can be obtained by substituting the value of the assay signal from the known standard (measured when assaying the unknown samples) into the mathematical relationship and solving for the assay parameters. The method enables an accurate determination of the analyte concentration even when the perturbing variable is changing or fluctuating from one sample measurement to another. Once the first step is completed, the second step can be performed repeatedly to measure unknown samples with accuracy.
Owner:NOVX SYST CANADA
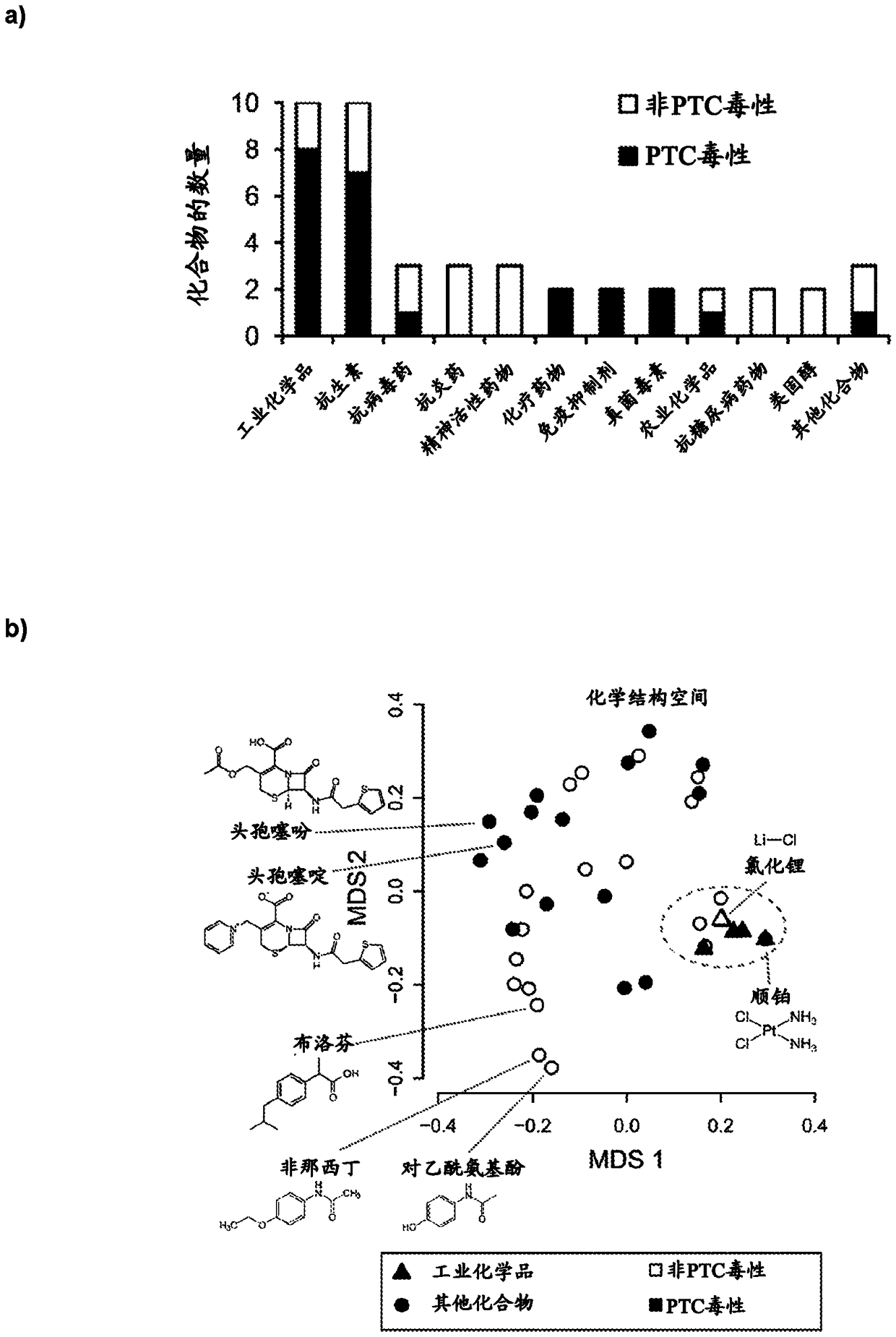
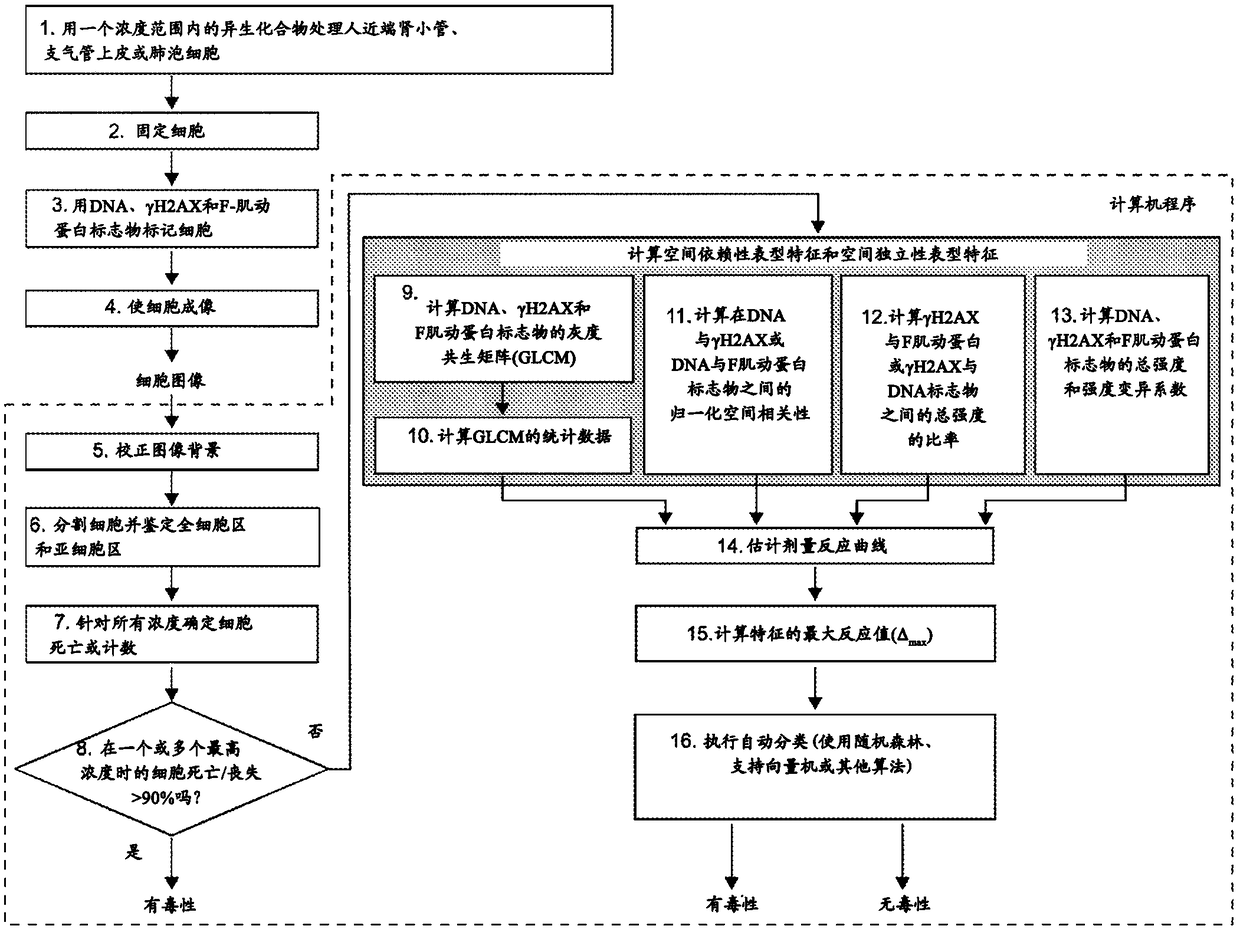
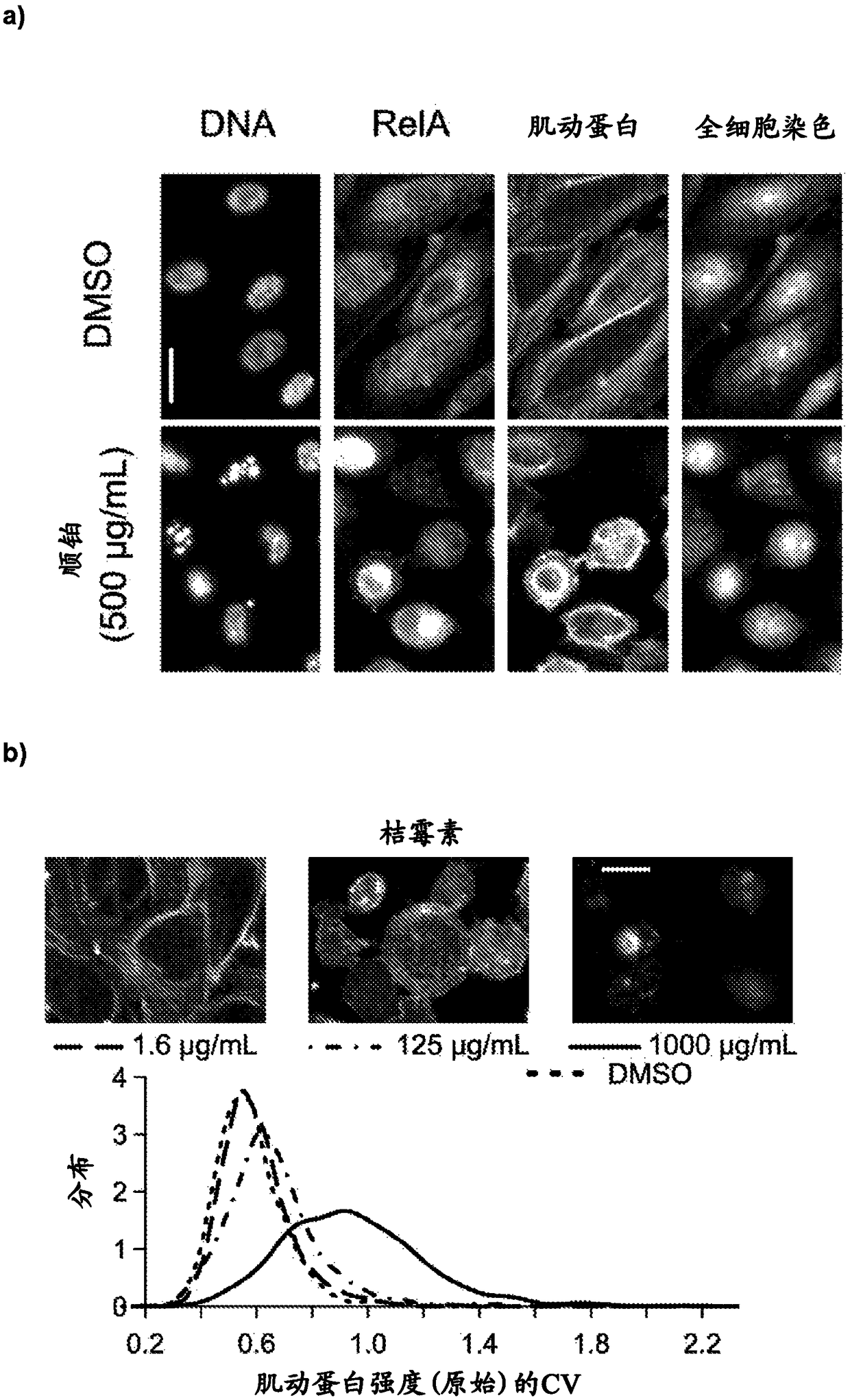
High-throughput imaging-based methods for predicting cell-type-specific toxicity of xenobiotics with diverse chemical structures
Owner:AGENCY FOR SCI TECH & RES
Medical preparations for treatment of alpha-galactosidase A deficiency
Owner:TRANSKARYOTIC THERAPIES
Optimizing mifepristone levels for cushing's patients
ActiveUS9943526B2Good curative effectOrganic active ingredientsDisease diagnosisBlood levelMifepristone
The present invention provides a method for optimizing levels of mifepristone in a patient suffering from Cushing's syndrome. The method comprises the steps of treating the patient with seven or more daily doses of mifepristone over a period of seven or more days; testing the serum levels of the patient to determine whether the blood levels of mifepristone are greater than 1631 ng / mL; and adjusting the daily dose of the patient to achieve mifepristone blood levels greater than 1631 ng / mL.
Owner:CORCEPT THERAPEUTICS INC
Compound isatis-root injection for animals and the preparing method thereof
InactiveCN101062066ASignificant effectReasonable compositionAntiviralsSolution deliveryMoroxydineMedicine
The invention discloses a compound isatic root injection for animals and preparation technology, which is characterized by the following: allocating main medicine, adjunct and dissolvent; choosing the main medicine from isatic root, linbevelin, moroxydine and amantadine hydrochloride; choosing the adjunct from anti-oxidant, metal chelating agent and dissolvent for injection. This invention possesses advanced productive technology and long shelf life.
Owner:江西百思技术咨询有限公司
Method for preparing non-human animal model with opportunistic infections and chronic wasting diseases and medicament screened by using same
InactiveCN103430903AWide range of medicinesEasy to useAntibacterial agentsMetabolism disorderBiotechnologySide effect
The invention discloses a method for establishing an animal model with opportunistic infections and chronic wasting diseases, and a traditional Chinese medicinal preparation for treating opportunistic infections and chronic wasting diseases. The traditional Chinese medicinal preparation is prepared by the following components in parts by weight: 100-180 parts of monkhood, 100-180 parts of dry ginger, and 100-160 parts of liquorice. The method has the positive effects that the method for establishing the animal model with opportunistic infections and chronic wasting diseases is found, and the model has high controllability, reliability and repeatability, and is convenient and economical to use; the model syndrome accords with the attack process of opportunistic infections and chronic wasting diseases; the model establishing factor accords with the theory of the Internal Canon of Medicine, and does not relates to causal organisms, so that the testing risks can be reduced, and the organism safety can be guaranteed; the traditional Chinese medicinal preparation obtained by screening has the characteristics of high efficiency, no toxicity, wide medicine sources and the like, is convenient to use and economical, can be used for prolonging the life of patients with opportunistic infections and chronic wasting diseases, improving the living quality of the patients and improving lesion of each tissue, and does not have toxic or side effect when a large dosage is used.
Owner:GUILIN MEDICAL UNIVERSITY
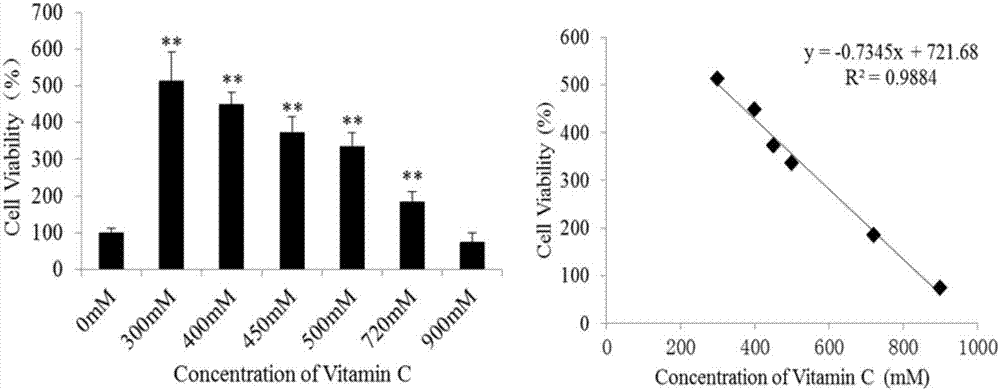
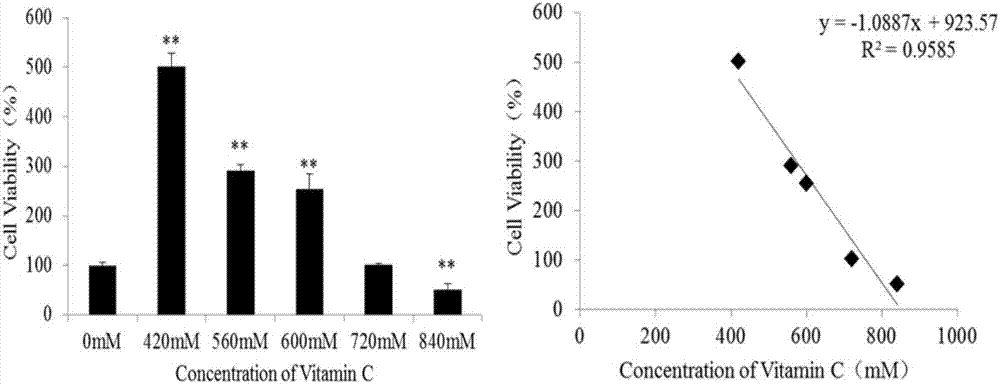
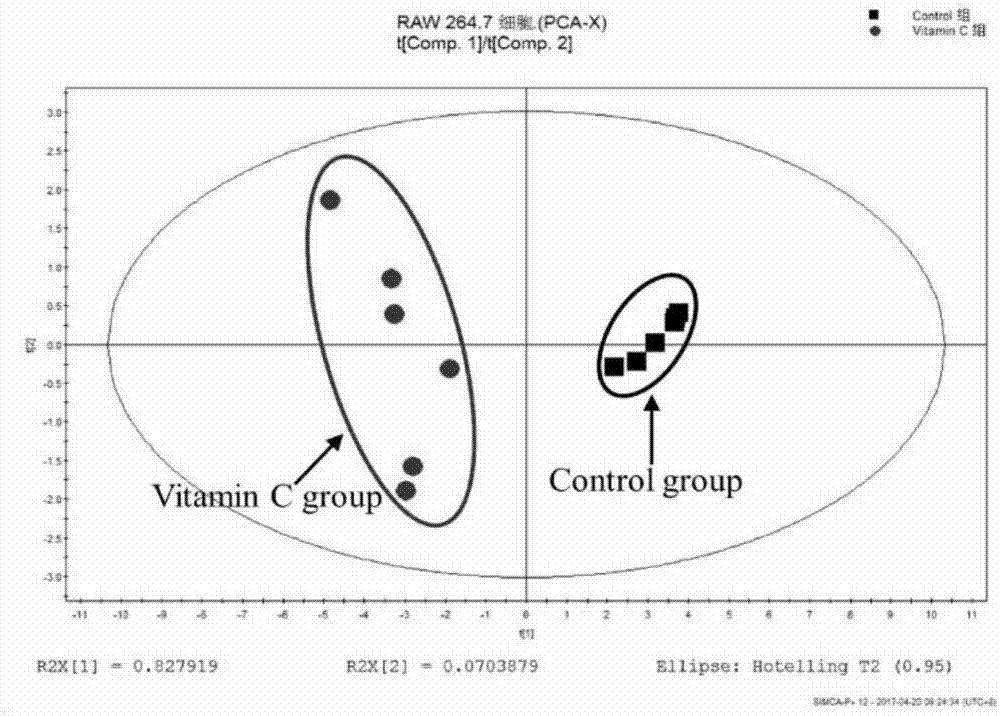
Method for identifying differential marker of IC50 dose of vitamin C for RAW264.7 and K562 cells
ActiveCN107167617AEasy to operateReliable and fast data preprocessingPreparing sample for investigationOmicsNMR - Nuclear magnetic resonanceMetabolite
The invention discloses a method for identifying a differential marker of IC50 dose of vitamin C for RAW264.7 and K562 cells by applying 1H NMR technology and metabonomic methodology. The method comprises the following steps: (1) determining the IC50 dosage of vitamin C for RAW264.7 or K562 cells; (2) preparing a cell extract; (3) performing nuclear magnetic resonance pretreatment; (4) performing 1H nuclear magnetic resonance detection; (5) screening differential metabolites; and (6) performing metabolism pathway analysis. The method studies influence of different cell differential metabolites and metabolic pathway by applying H-nuclear magnetic resonance technology in combination with metabonomic methodology, and the basic research provides theoretical basis for studying the method for analyzing sub-toxicity of vitamin C for RAW264.7 or K562 cells.
Owner:SHANDONG NORMAL UNIV
Use of immunological stimulant compound(ISCOMs)in fish immunity by oral administration and dipping bath method
InactiveCN1879880AImprove the ability of proliferation and transformationHigh antibody titerImmunological disordersAntibody medical ingredientsStimulantT lymphocyte
The invention relates to a bath or oral application of immune activate compound in the fish immunity, wherein the invention has the advantages that: said compound ISCOMs is used to bath immunity to improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection; when in the same dosage, the antibody level is higher than non-adjuvant group and the ISM1312 group; and the ISCOMs used in oral can improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI +3
Health product with functions of invigorating spleen to promote digestion and eliminate stagnation
ActiveCN104256592ACompatibility is simpleGood health effectOrganic active ingredientsDigestive systemBiotechnologySide effect
The invention provides a health product with the functions of invigorating spleen to promote digestion and eliminate stagnation, aiming at solving the problems that products on the market contain diversified and miscellaneous components and is single in function, can be taken only for infected population, have certain side effect and cannot be taken as daily prevention and health products for eating of common people. The health product provided by the invention is characterized in that each dosage of the health product contains the health and treatment ingredients according to the proportions: 38-42 parts of hericium erinaceus polysaccharide, 8-12 parts of lentinan, 8-12 parts of fermented curcuma powder, 8-12 parts of poria cocos extract, 8-12 parts of Chinese hawthorn extract, 3-7 parts of Chinese yam extract and 3-7 parts of fructus amomi powder. The finally formed dosage forms of the health product are powder, tablets or capsules. The health product is simple in compatibility, good in health effect, high in safety and reliability, free of toxic or side effect, light in flavor, good in mouthfeel and convenient to carry and is capable of being taken as a daily health product to be eaten by common people.
Owner:河南佳禾康生物食品科技有限公司
Construction method of antibacterial cefquinome PK/PD model for livestock and application
PendingCN110607344ADelay drug resistanceMolecular designMicrobiological testing/measurementAntibacterial actionDrug administration
The invention discloses a construction method of an antibacterial cefquinome PK / PD model for livestock and an application. The specific contents of the construction method comprise detecting the drugsusceptibility of antibacterial cefquinome to haemophilus parasuis so as to obtain an MIC distribution range; determining the concentrations of free drugs in plasma samples of healthy model animals and disease model animals at different time points after drug administration to obtain drug-time curves by a liquid chromatography detection method; performing fitting on pharmacokinetics parameters ofdrugs by pharmacokinetics software to obtain PK parameters; researching the antibiotic action of the antibacterial on pathogenic bacteria under the condition of in vitro and half in vivo, and performing fitting on the time limitation relationship of the antibacterial to the pathogenic bacteria under the condition of in vitro and half in vivo to obtain PD parameters; establishing a half in vivo PK-PD model according to a Sigmoid Emax equation; and obtaining drug administration schemes under different drug administration objectives through a dosage calculating formula and Mlxplore software. According to the construction method disclosed by the invention, an optimization method is provided for a drug resistance resistant medication scheme of the cefquinome clinically, and production and propagation of bacterial drug resistance are relieved.
Owner:HUAZHONG AGRI UNIV
Medical composition and mixture for treating chicken infectious bursal disease and preparation method thereof
ActiveCN101856495AStrong heat-clearing and detoxifying abilityIncrease appetitePeptide/protein ingredientsAntiviralsVitamin CTherapeutic effect
The invention relates to a medical composition and a mixture for treating chicken infectious bursal disease and a preparation method thereof. The medical composition (100 grams) comprises: 1 to 10 grams of astragalus polysaccharides, 3 to 12 grams of taurine, 4 to 15 grams of aminopyrine, 2 to 10 grams of vitamin C, 0.04 to 0.2 gram of interferon inducer, 5 to 20 grams of alcohol, 0.1 to 0.4 gram of sodium benzoate, and the balance of water for injection. The product is characterized by reasonable formula composition, stable physical and chemical properties, accurate dose, quick effect, long-lasting medicament effect, high bioavailability, safe use and exact treatment effect.
Owner:HENAN HUITONG TIANXIA BIO ENG
Constructing method of antibiosis medicament ceftiofur pharmacokinetic-pharmacodynamic synchronous model and application thereof
InactiveCN110223734ADelay drug resistanceMedical simulationComputational theoretical chemistryBlood plasmaPK/PD models
The invention discloses a constructing method of an antibiosis medicament ceftiofur pharmacokinetic-pharmacodynamic synchronous model and application thereof. The method comprises the steps of detecting drug susceptibility of the antibiosis medicament ceftiofur to actinobacillus pleuropneumoniae, and obtaining an MIC distribution range; measuring free drug concentrations which are obtained in blood plasma samples which are obtained after drug application to healthy and disease model animals, and obtaining a drug concentration-time curve; fitting the pharmacokinetic parameter of the drug for obtaining a PK parameter; researching an antibiosis function of an antibiosis drug to pathogenic bacteria in in-vitro and semi-in vivo conditions, performing fitting for obtaining a PD parameter; establishing a semi-in vivo PK-PD model; and by means of a dosage calculating formula and Mlxplore software, obtaining a reasonable drug taking plan. The invention provides an optimizing method for an anti-drug-resistance drug taking plan of clinical ceftiofur, thereby alleviating generation and propagation of bacteria drug resistnace, and protecting and sustaining effectiveness of ceftiofur in clinic treatment.
Owner:HUAZHONG AGRI UNIV
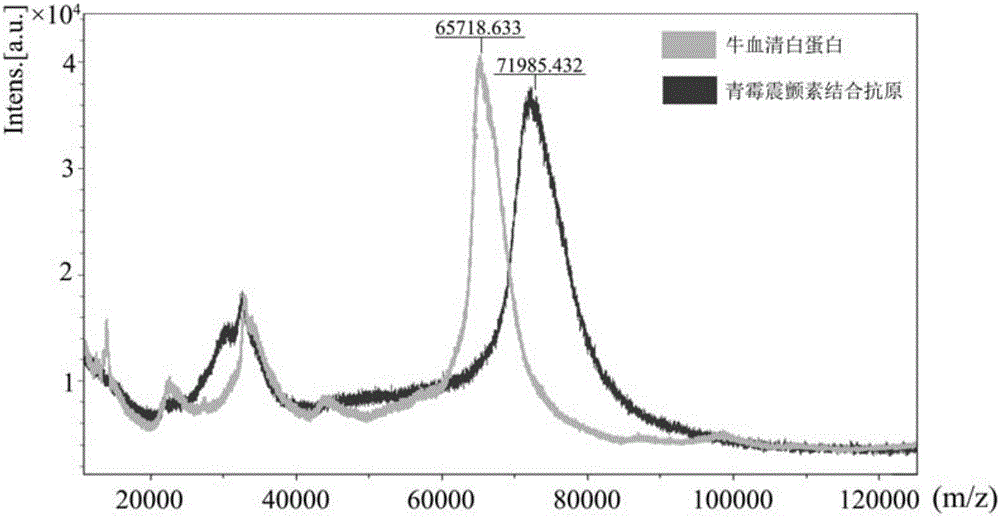
Penitrem A combined antigen as well as preparation and application of antibody of antigen
The invention discloses a penitrem A combined antigen as well as preparation and an application of an antibody of the antigen. The median inhibitory dose (IC50), detected by a penitrem A enzyme-linked immunosorbent assay kit, of penitrem A for a reaction between a specific antibody of the penitrem A and penitrem A coating antigens is 100 ng / mL, the lowest detection limit of penitrem A is 30 ng / mL, and the cross reaction rate of penitrem A and an aflatoxin B1 sample, the cross reaction rate of penitrem A and a cyclopiazonic acid sample, the cross reaction rate of penitrem A and a vomitoxin and the cross reaction rate of penitrem A and a citreoviridin sample are all smaller than 0.1%. The specific antibody of penitrem A in the penitrem A enzyme-linked immunosorbent assay kit has the characteristics of high potency, high sensitivity and high specificity and can be used for quickly, sensitively and easily detecting the residual amount of penitrem A in animal-origin food.
Owner:CHINA AGRI UNIV
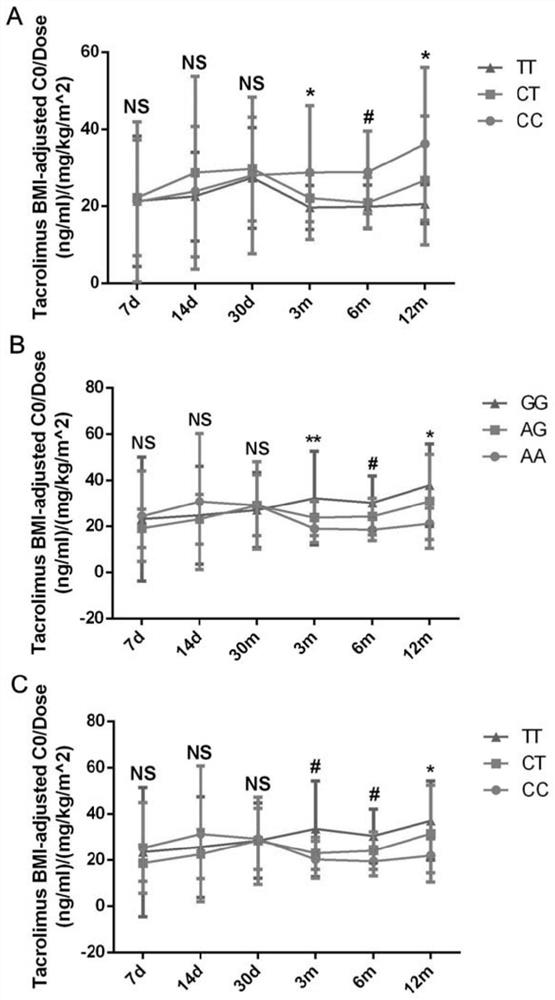
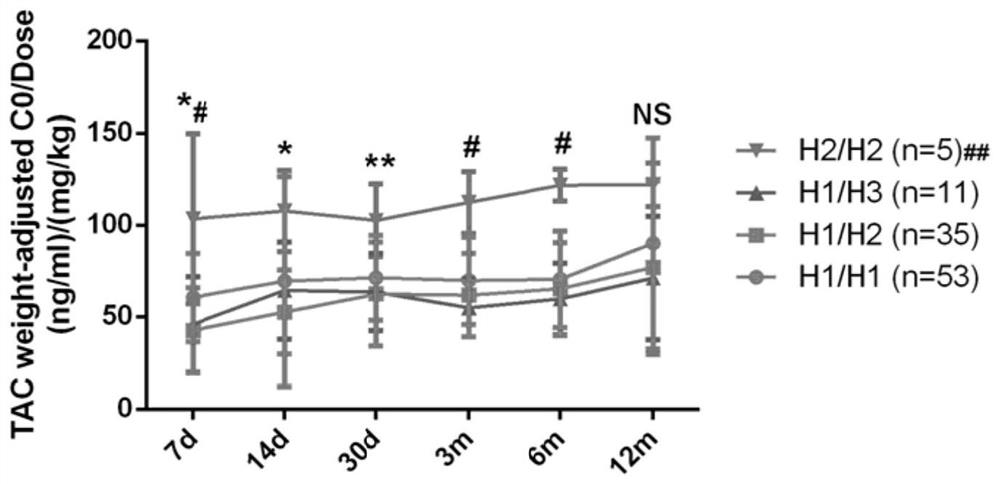
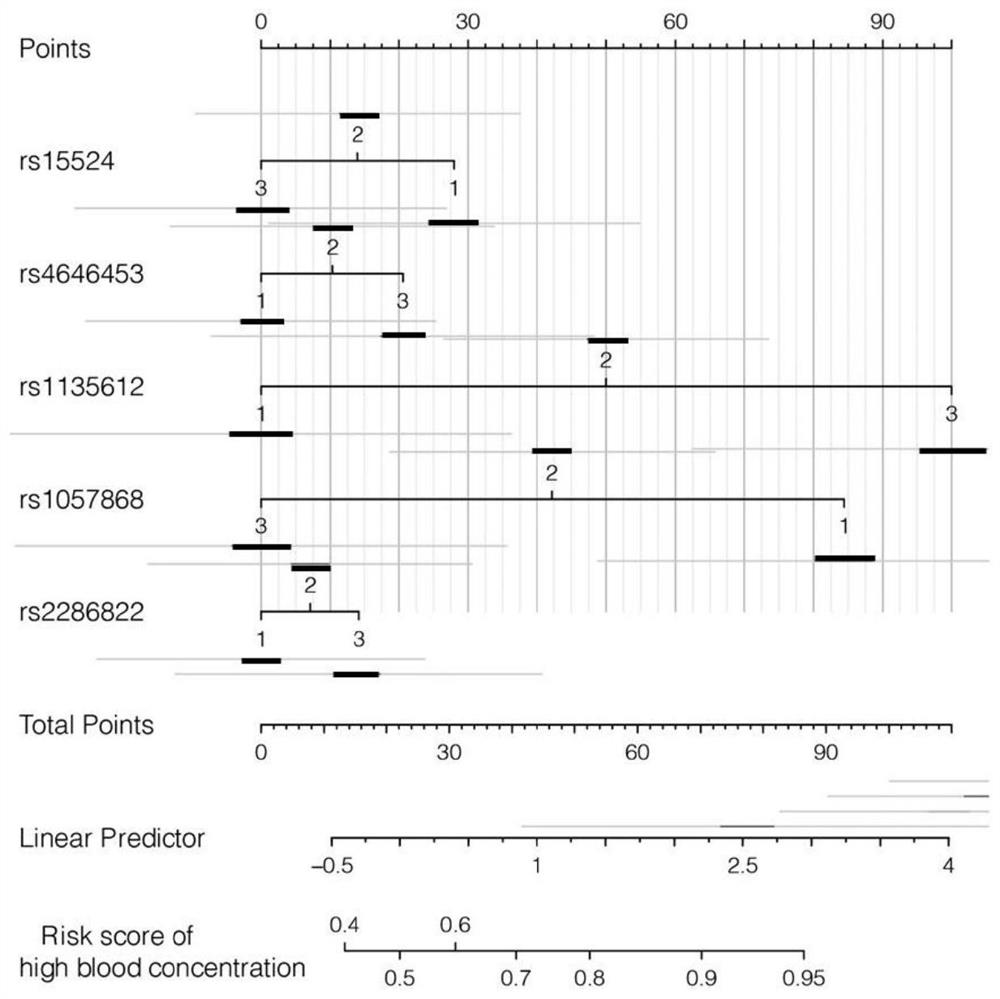
Primer set, kit and evaluation method for evaluating tacrolimus metabolism
ActiveCN112322723APredict metabolic stateAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesPharmaceutical drugGenotype
The invention belongs to the field of serum drug concentration monitoring after renal transplantation, and discloses a primer set, a kit and an evaluation method for evaluating tacrolimus metabolism.Specifically, genotypes of five SNP loci (CYP3A5 rs15524, CYP3A5 rs4646453, POR rs2286822, POR rs1135612 and POR rs1057868) on two important genes CYP3A5 and POR related to tacrolimus metabolism are detected to predict individual metabolic conditions of tacrolimus in a patient, so as to provide medication guidance for individualized design of an initial dosage of tacrolimus.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV
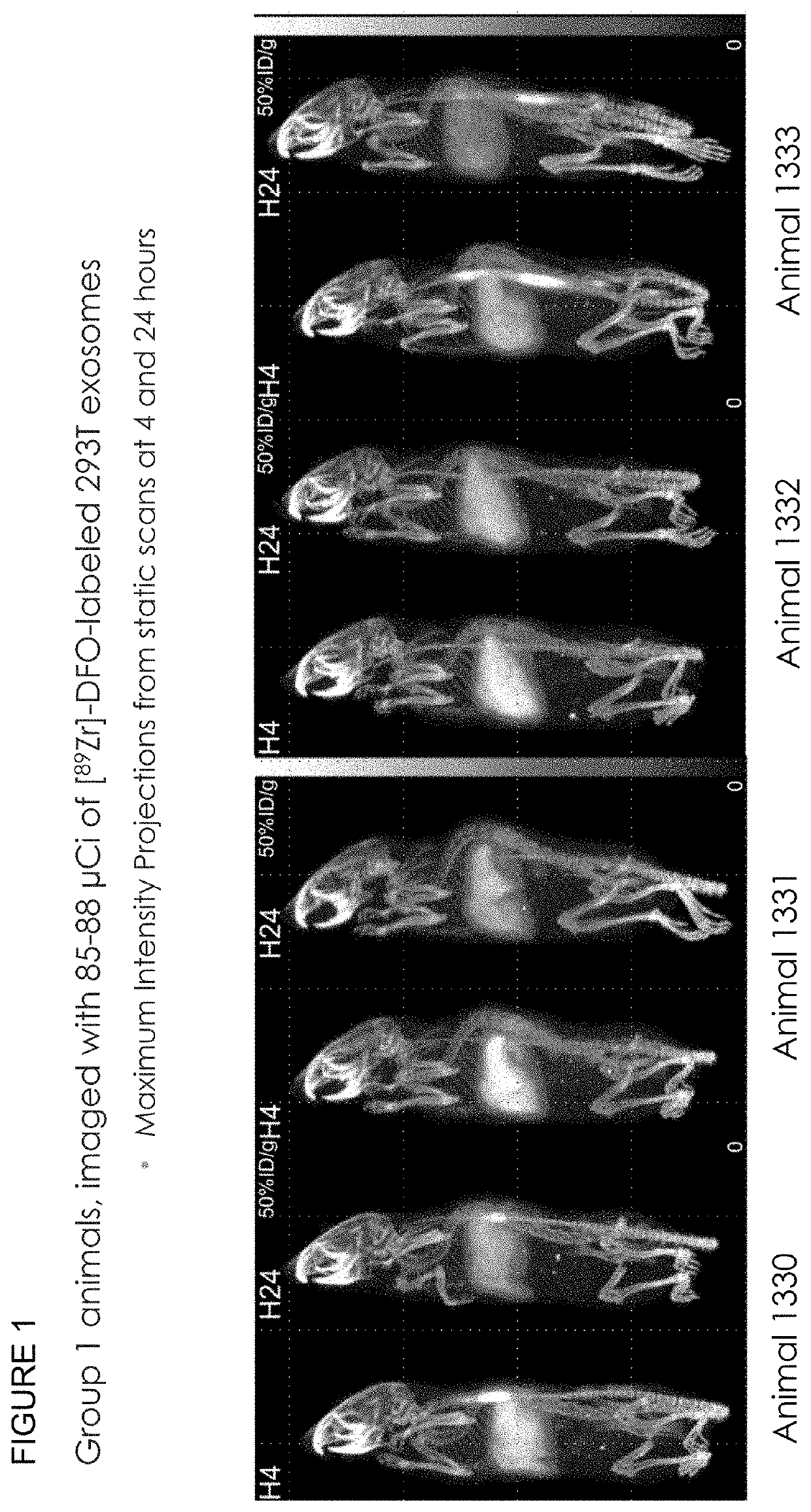
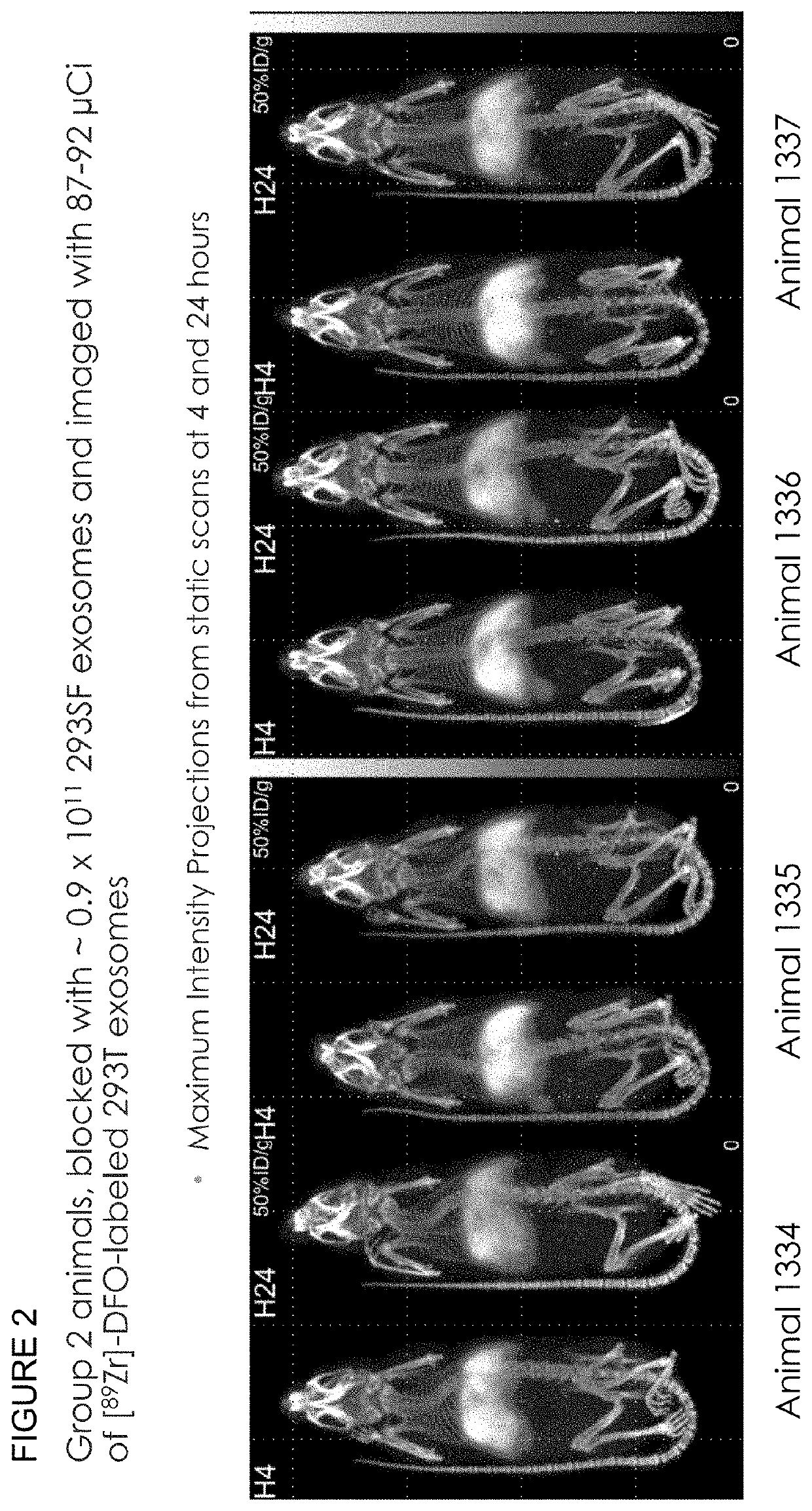
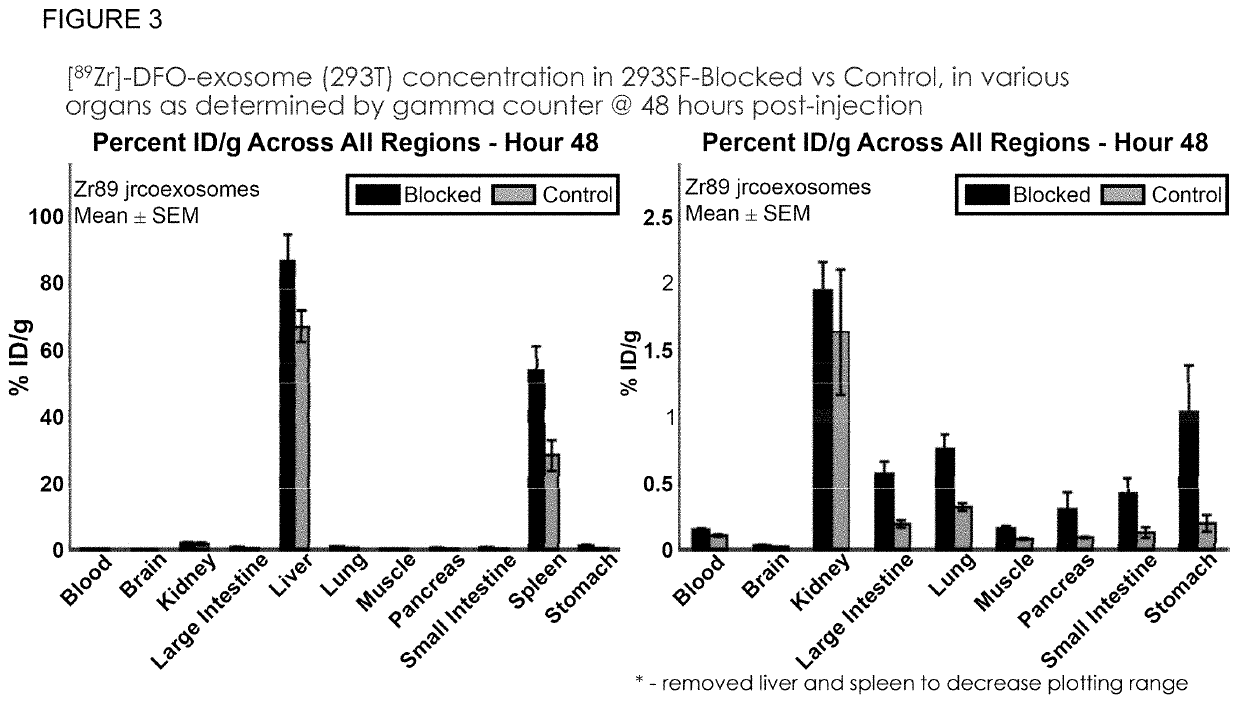
Methods of suppressing delivery of exosomes to liver and spleen
PendingUS20210290556A1Reduce deliveryAvoid phagocytosisOrganic active ingredientsSpecial deliveryImmune clearancePancreas
The instant application describes improved methods and compositions for the systemic delivery of therapeutic exosomes to a subject in need thereof. In certain embodiments, the current invention reduces the amount of exosomes delivered to liver, spleen and combinations thereof to allow greater distribution to other areas of the body such as, but not limited to, the brain, pancreas, lung, kidney, muscle. In certain embodiments, the methods involve the injection of one or multiple doses of non-therapeutic exosomes prior to the injection of a suitable therapeutic dose of exosomes with a therapeutic payload. Also included are methods to improve immune clearance of exosomes in subjects by inhibiting phagocytosis.
Owner:LONZA SALES AG
Application of proanthocyanidins in preparing diet food or drug
InactiveCN103829254AReduce concentrationNo side effectsOrganic active ingredientsDispersion deliveryCholesterolEfficacy
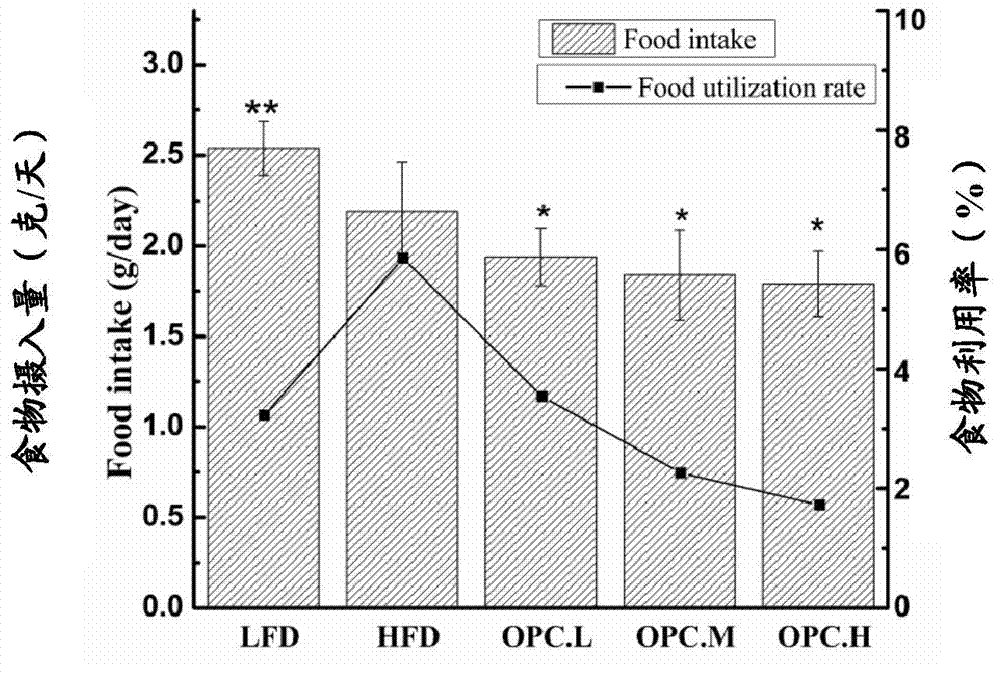
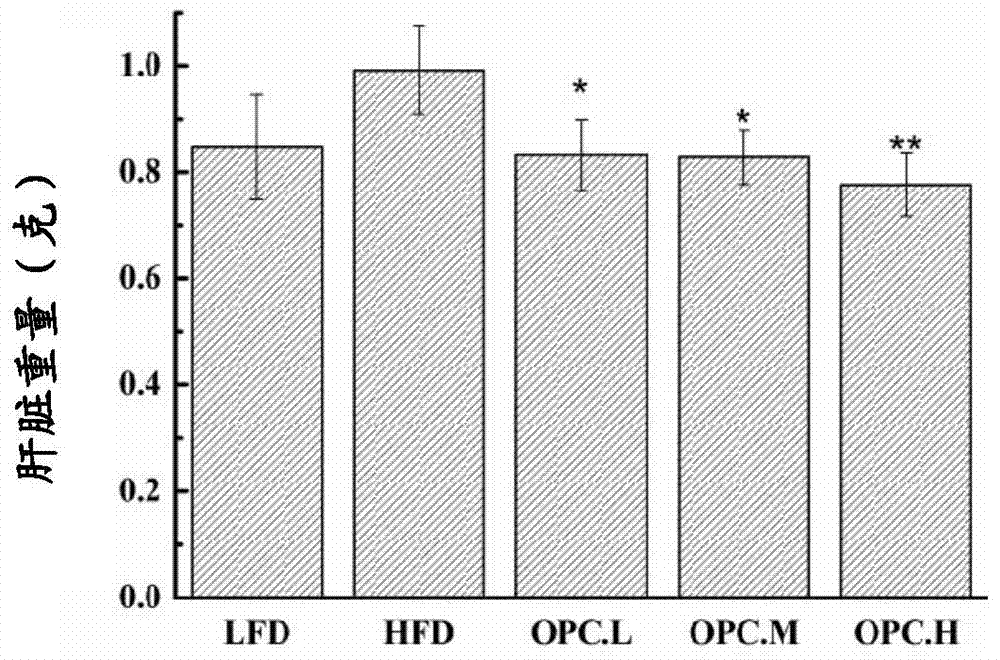
The invention discloses an application of proanthocyanidins in preparing diet food or drug, and discloses an application of a drug composition with proanthocyanidins as an active component and pharmaceutically acceptable additives in preparing the diet food or drug. Mice experiments demonstrate that proanthocyanidins has obvious reduction effects for growth rate of mice body weight, food utilization rate and body fat and presents a dose-dependent relationship, can effectively reduce blood glucose level and serum cholesterol level of the mice, and can increase serum free fatty acid level and oxidation resistant level. Novel medical application and novel application field are developed for proanthocyanidins. Experiments show that the concentration of proanthocyanidins required for treating obesity is relatively low; proanthocyanidins has no side or toxic effect and has the functions of protecting human body blood circulation, enhancing immunity and protecting eyesight, thereby guaranteeing effectiveness of weight reduction and playing a promotion effect for body health.
Owner:ZHEJIANG UNIV
Bacterium inhibiting feed and preparation method and application thereof
The invention relates to a bacterium inhibiting feed and a preparation method and application thereof. The bacterium inhibiting feed comprises a basic ration and yeast essence, wherein the content ofbeta-glucan in the yeast essence is 25-50%, and the relative molecular weight of 99% or above of the beta-glucan is 3.0*10<5>-1.0*10<6>; and the content of mannan in the yeast essence is greater thanor equal to 20%, and the relative molecular weight of 99% or above of the mannan is 8.0*10<5>-2.0*10<6>. The yeast essence related in the feed disclosed by the invention is used as a single substanceto be added in the feed, the recommendation dosage is stable, and after in vitro bacterium inhibiting zone simulation and breeding animal in vivo experiment verification, the bacterium inhibiting feedshows good antibiotic replacement effects; an efficient feasible antibiotic replacement scheme can be provided for the animal breeding trade, besides, the defects that antibiotics have drug toleranceand are remained, and current antibiotic replacement products are bad in effects and lack stability are overcome, and the bacterium inhibiting feed has considerable future prospects.
Owner:安琪酵母(柳州)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com