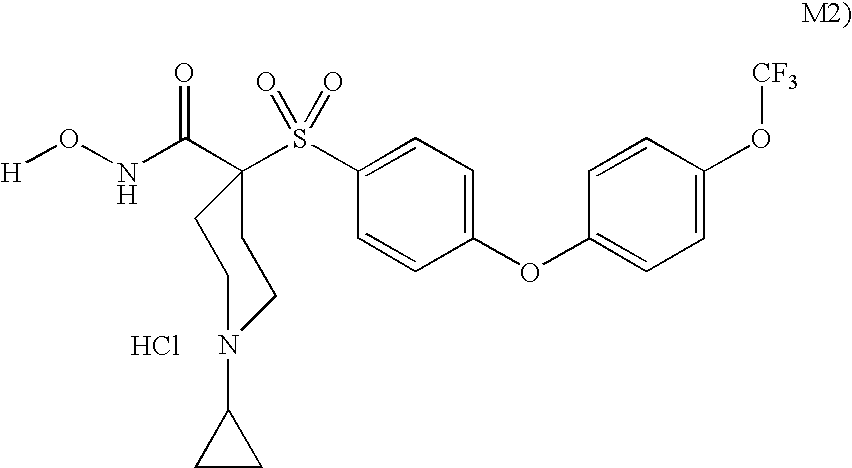
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
199 results about "Irinotecan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat cancer of the colon and rectum..
Compositions and methods for the treatment of cancer
InactiveUS20020035090A1Improve toleranceReducing and avoiding adverse effectBiocideCarbohydrate active ingredientsCancer preventionSide effect
This invention relates to compositions comprising thalidomide and another anti-cancer drug which can be used in the treatment or prevention of cancer. Preferred anti-cancer drugs are topoisomerase inhibitors. A particular composition comprises thalidomide, or a pharmaceutically acceptable salt, solvate, or clathrate thereof, and irinotecan. The invention also relates to methods of treating or preventing cancer which comprise the administration of a thalidomide and another anti-cancer drug to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of chemotherapy or radiation therapy which comprise the administration of thalidomide to a patient in need of such reduction or avoidance.
Owner:CELGENE CORP
Method of using a matrix metalloproteinase inhibitor and one or more antineoplastic agents as a combination therapy in the treatment of neoplasia
InactiveUS6858598B1Reduce morbidityReduction in severity and frequencyHeavy metal active ingredientsBiocideDNA underwindingIrinotecan
A method of using an MMP inhibitor and optionally radiation therapy, and one or more antineoplastic agents of the topoisomerase class selected from the group consisting of irinotecan and topotecan, as a combination therapy for the treatment of neoplasia is disclosed.
Owner:GD SEARLE & CO
Compositions and dosage forms for gastric delivery of irinotecan and methods of treatment that use it to inhibit cancer cell proliferation
InactiveUS6881420B2Improve oral bioavailabilityReduced bioavailabilityBiocideCapsule deliveryWhole bodyCancer cell proliferation
The present invention provides oral dosage forms and compositions for administering antineoplastic agents, such as irinotecan, etoposide, paclitaxel, doxorubicin and vincristine, whose oral effectiveness is limited by pre-systemic and systemic deactivation in the GI tract. Gelling of the gastric retention vehicle composition, and in the case of solid forms concomitant expansion of the composition, retains the antineoplastic drug in the patient's stomach, minimizing pre-systemic and / or systemic deactivation of the drug.
Owner:TEVA PHARM USA INC
Targeted liposomes
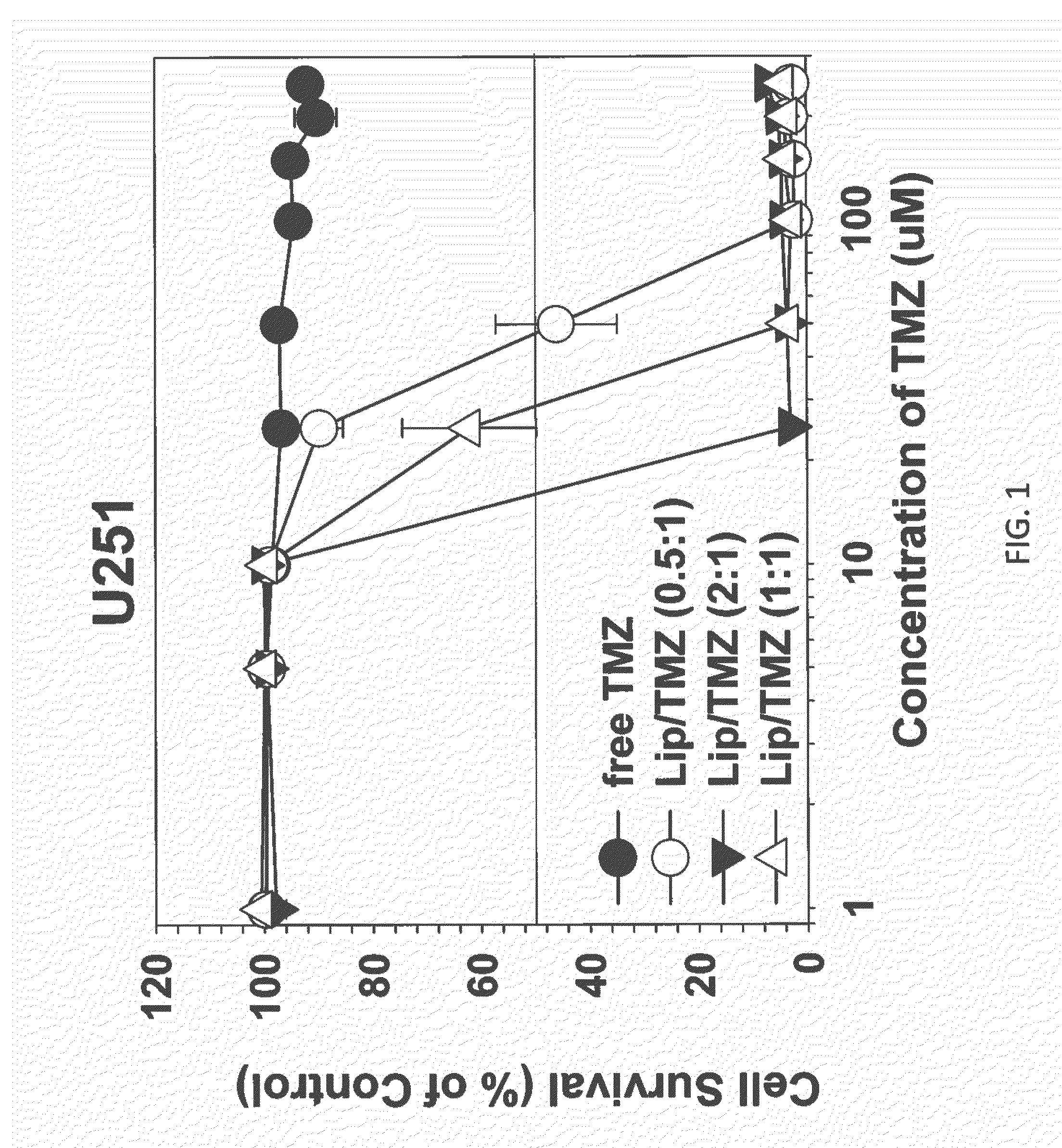
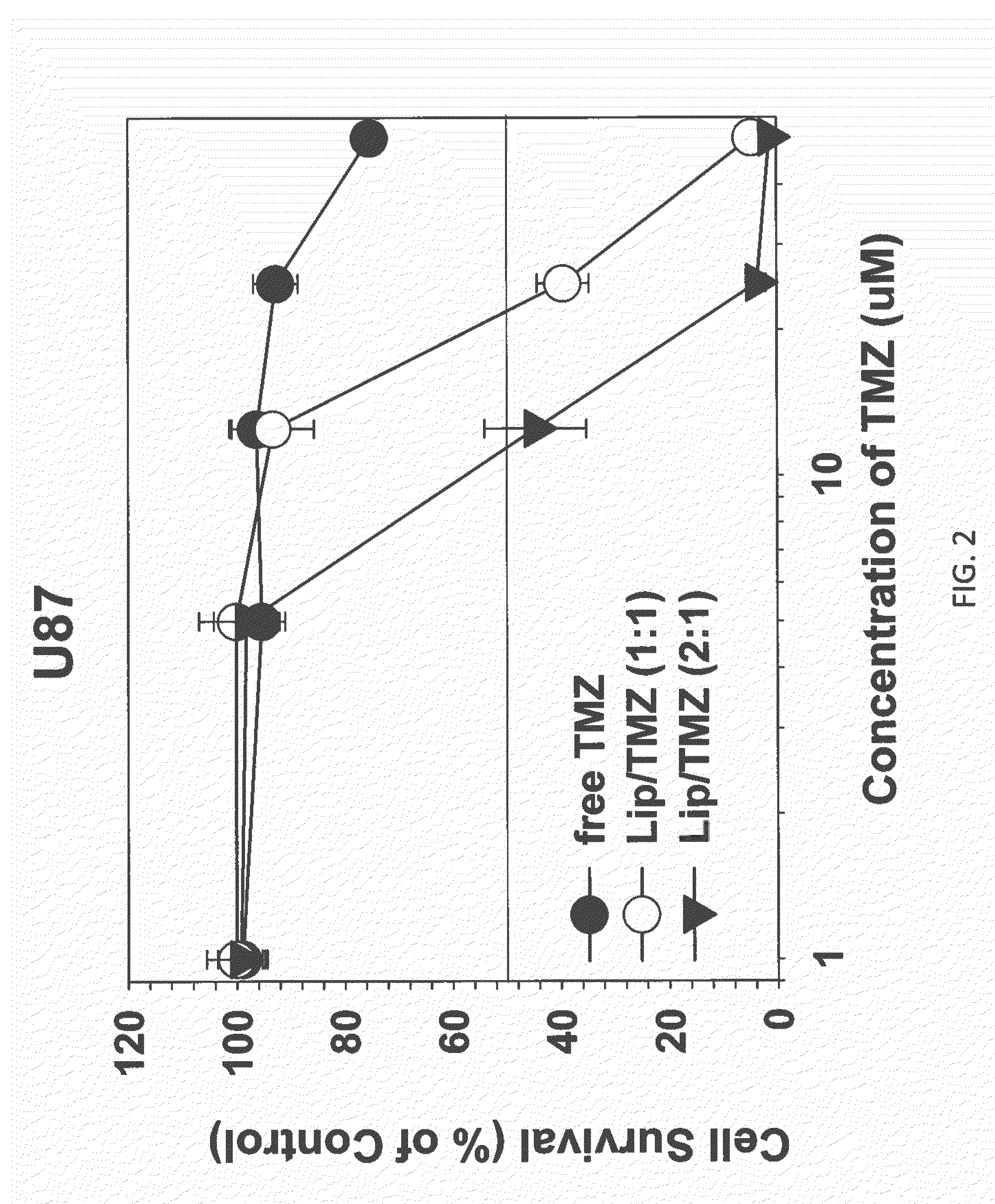
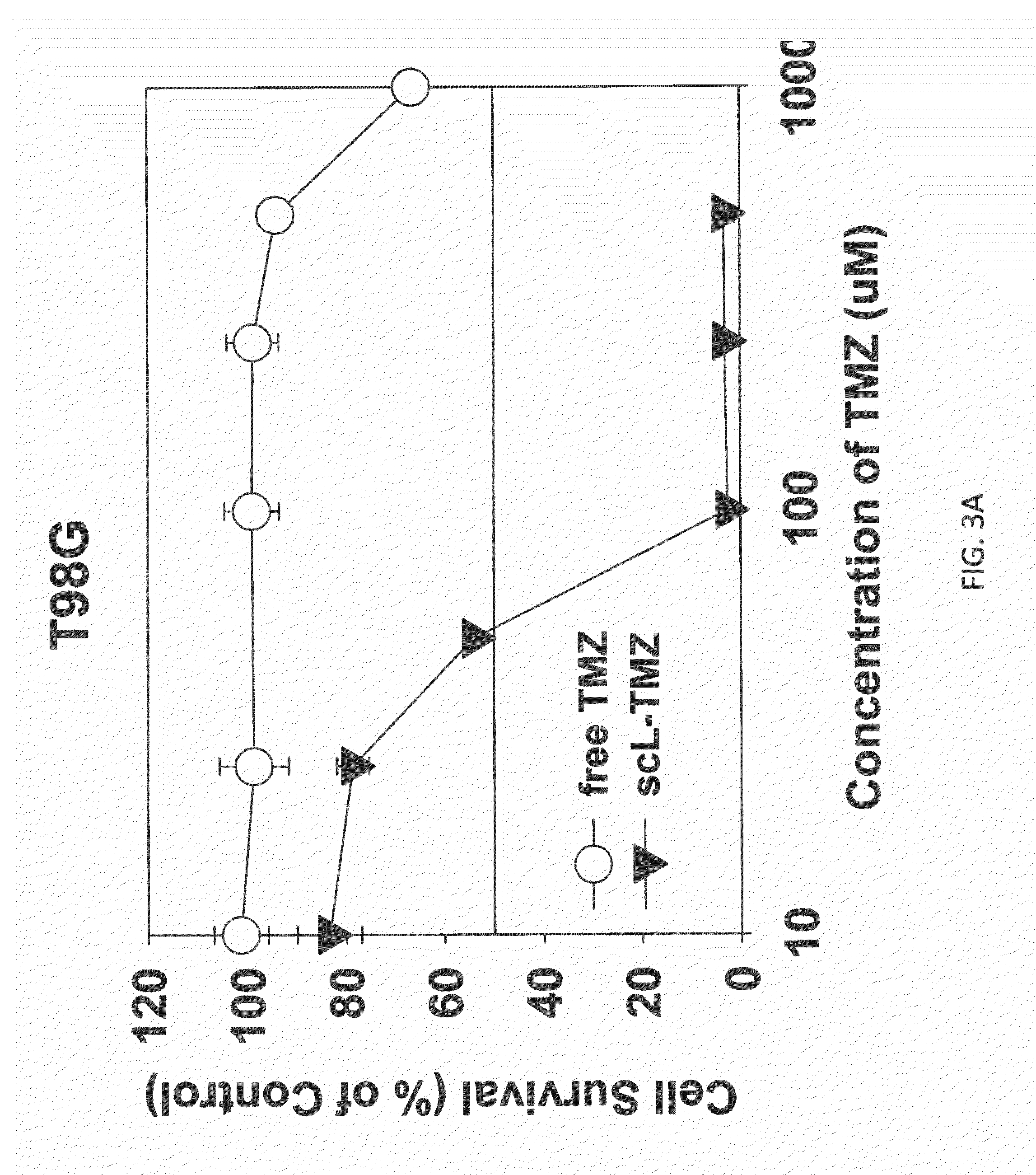
The present invention is in the field of drug delivery, and specifically, cationic liposome-based drug delivery. In embodiments, this invention provides methods of making ligand-targeted (e.g., antibody- or antibody fragment-targeted) liposomes useful for the delivery of liposomes to tumors, including brain tumors. In embodiments, the liposomes deliver temozolomide across the blood-brain barrier for treatment of primary or metastatic brain tumors. Additional cancers that can be treated with the liposomes include neuroendocrine tumors, melanoma, prostate, head and neck, ovarian, lung, liver, kidney, breast, urogenital, gastric, colorectal, cervical, vaginal, angiosarcoma, liposarcoma, rhabdomyosarcoma, choriocarcinoma, pancreatic, retinoblastoma and other types of cancer. In another embodiment the liposomes deliver melphalan for the treatment of multiple myeloma, other tumors of the blood or other solid tumors. In still other embodiments the liposomes can deliver other drugs such as pemetrexed or irinotecan for treatment of cancer or drugs including atropine for treatment of organophosphate poisoning.
Owner:GEORGETOWN UNIV
Drug delivery from embolic agents
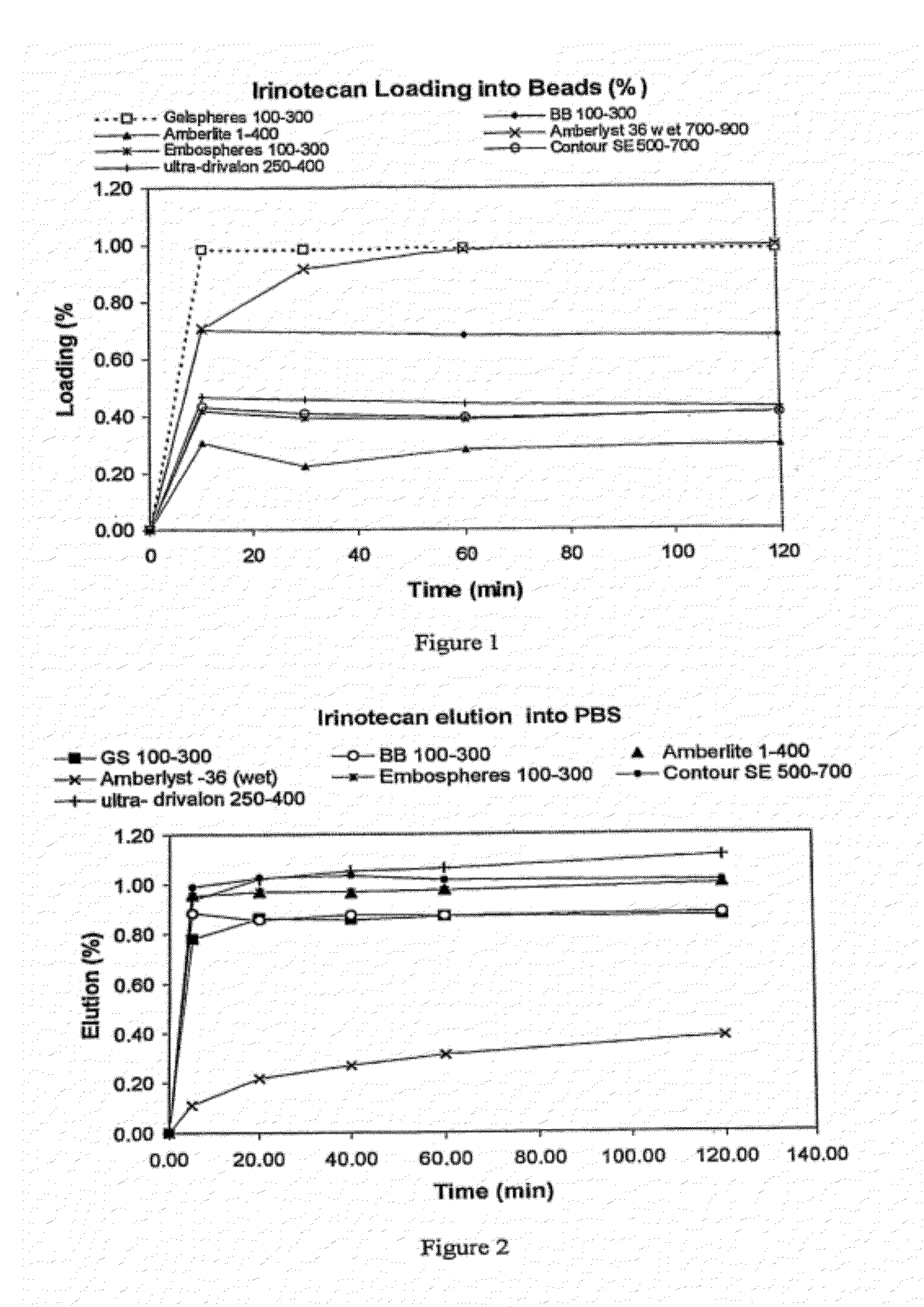
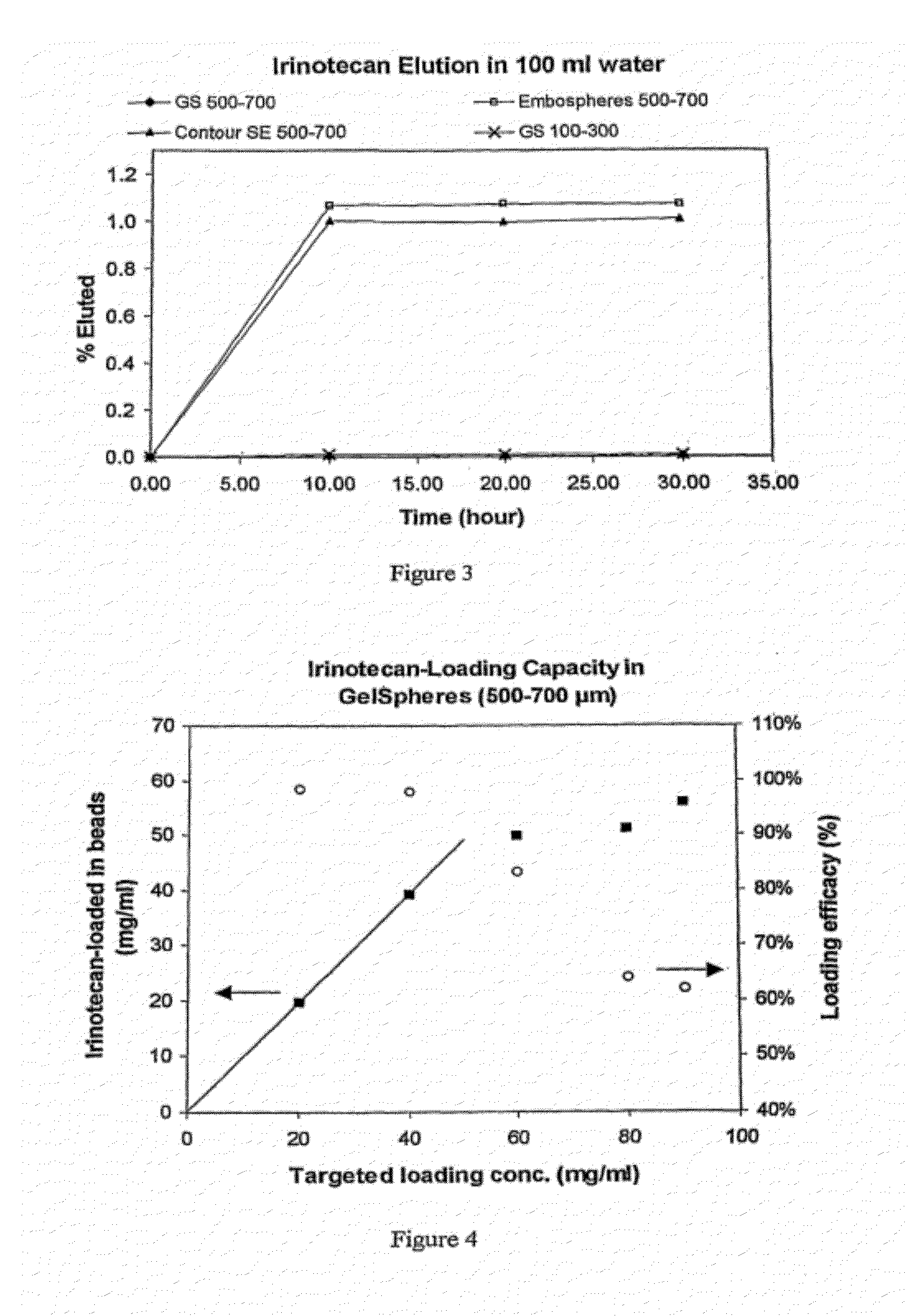
An embolic composition comprises microspheres formed of water-insoluble water-swellable anionic polymer having swollen diameter more than 100 μm and a cationic camptothecin compound, preferably irinotecan. The microspheres are preferably formed of crosslinked polyvinylalcohol, preferably of ethylenically unsaturated polyvinylalcohol macromer, crosslinked with anionic ethylenically unsaturated anionic comonomer. The compositions are used to treat hypervascular tumours for instance colorectal metastases of the liver.
Owner:BIOCOMPATIBLES UK LTD
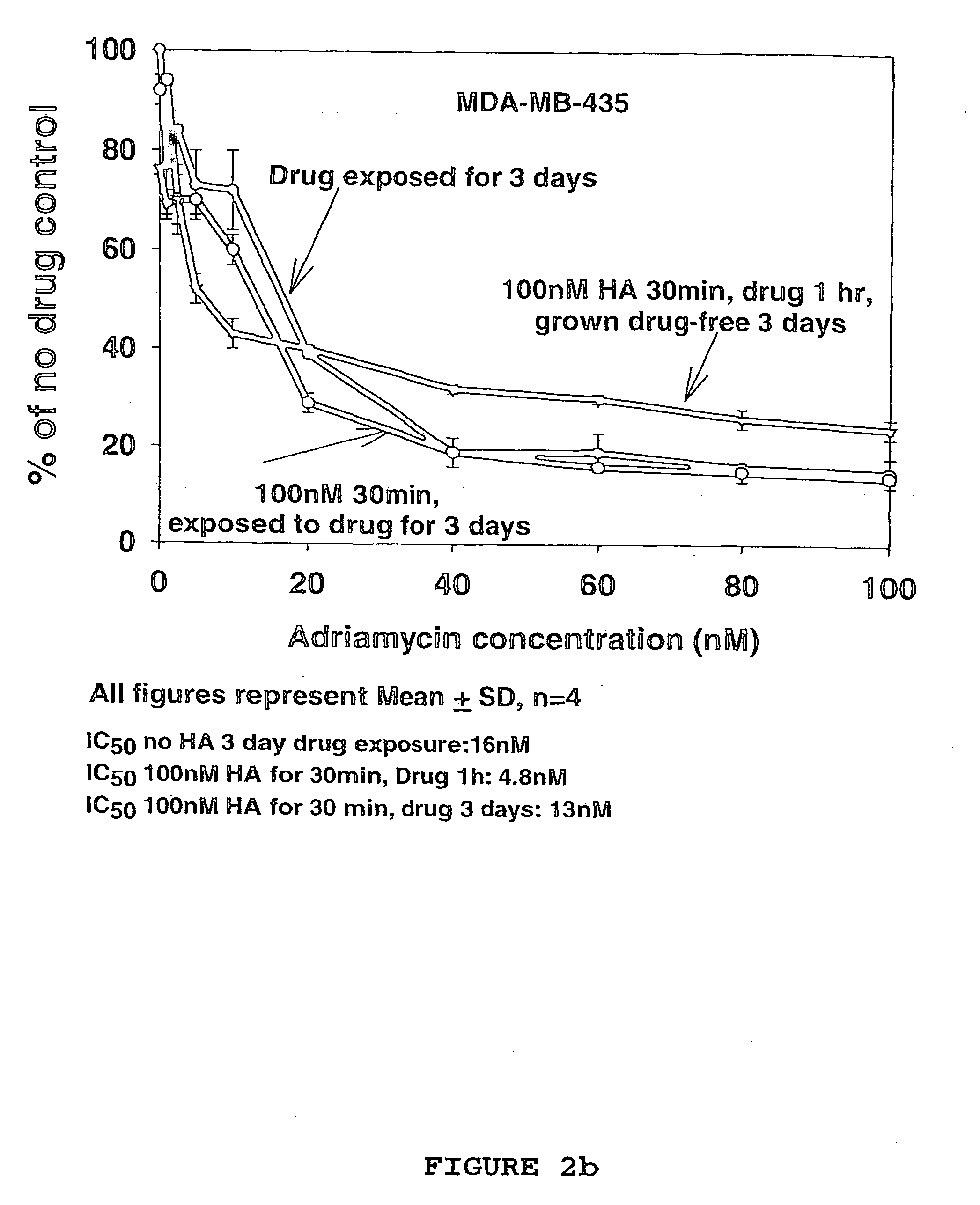
Hyaluronan as a cytotoxic agent, drug pre-sensitizer and chemo-sensitizer in the treatment of disease
InactiveUS20060178342A1Eliminate side effectsBiocideHeavy metal active ingredientsIrinotecanHyaluronic acid
Owner:ALCHEMIA ONCOLOGY PTY LTD
Camptothecin, self-emulsifying medicine precursor of derivative thereof and application thereof
InactiveCN101628919AImprove the delivery effectGood biocompatibilitySugar derivativesGroup 5/15 element organic compoundsAlcoholMatrine
The invention discloses camptothecin and a self-emulsifying medicine precursor of a derivative thereof. The self-emulsifying medicine precursor is prepared by the covalent union of medicine molecules and hydrophilic radicals, wherein the medicine molecules are camptothecin molecules or camptothecin derivative molecules, and the medicine carrying quantity of the precursor is as high as more than 50%. The invention also discloses application of the self-emulsifying medicine precursor. The self-emulsifying medicine precursor can form micelles or vesicles with nano size in water, can be used as a medicine carrier used for loading one or a plurality of other anticancer medicines, such as CPT derivatives, yew alcohol, turmeric essence, methotrexate, irinotecan, danshinolic acid, matrine, doxorubicine and the like, can form a nano medicine carrying a plurality of medicines and can realize the synergic treatment of the medicines.
Owner:ZHEJIANG UNIV
Compositions and methods for the treatment of colorectal cancer
This invention relates to compositions comprising thalidomide and irinotecan, which can be used in the treatment or prevention of colorectal cancer. The invention also relates to methods of treating or preventing colorectal cancer which comprise the administration of thalidomide and irinotecan to a patient in need of such treatment or prevention. The invention further relates to methods of reducing or avoiding adverse side effects associated with the administration of irinotecan which comprise the administration of thalidomide to a patient in need of such reduction or avoidance.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
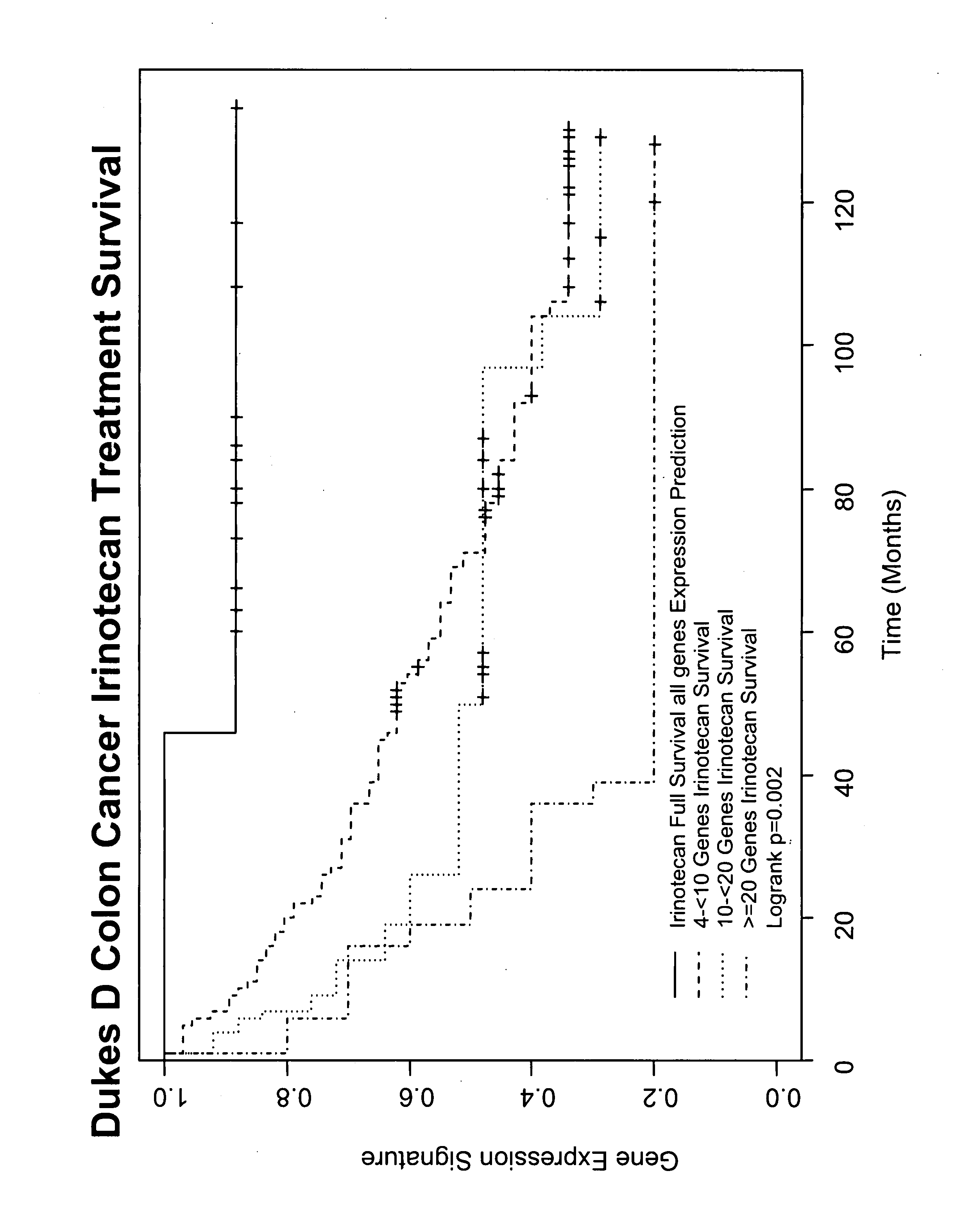
Gene and protein expression profiles associated with the therapeutic efficacy of irinotecan
InactiveUS20080076134A1Microbiological testing/measurementDisease diagnosisProtein expression profileIrinotecan
The present invention includes gene and protein expression profiles indicative of whether a cancer patient is likely to respond to treatment with irinotecan. By identifying such responsiveness, a treatment provider may determine in advance those patients who would benefit from such treatment, as well as identify alternative therapies for non-responders. The present invention further provide methods of using the gene and / or protein expression profiles and assays for identifying the presence of a gene and / or protein expression profile in a patient sample.
Owner:NUCLEA BIOMARKERS
Liposomal formulation of irinotecan
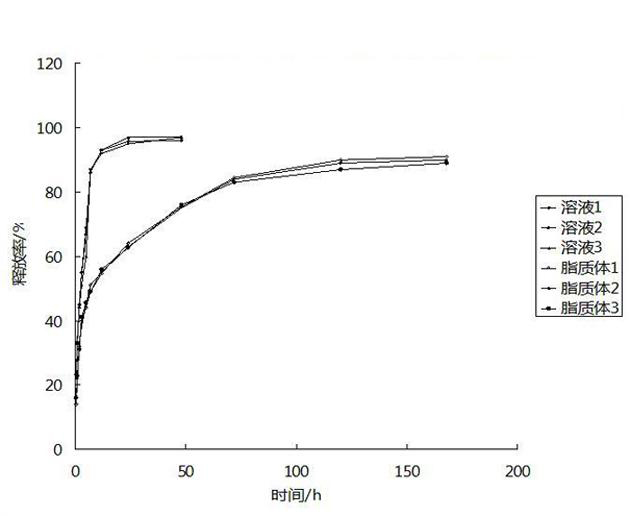
InactiveUS20050019387A1Easy to useBiocidePharmaceutical non-active ingredientsMedicineCompound (substance)
The present invention is for novel compositions and methods for treating cancer, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include liposome entrapped irinotecan in which the liposome can contain any of a variety of neutral or charged liposome-forming compounds and cardiolipin. The liposomes of the present invention can be either multilamellar vesicles and unilamellar vesicles, as desired.
Owner:NEOPHARMA INC
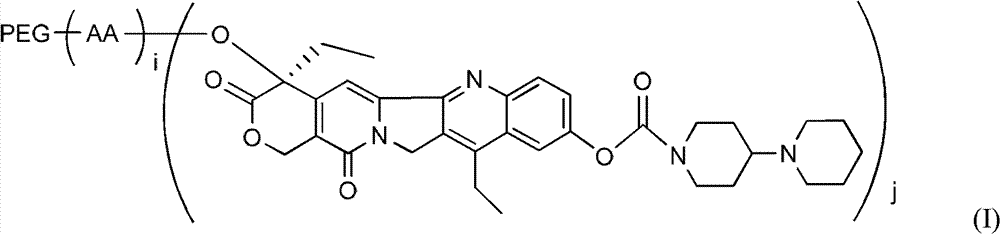
Polyethylene glycol (PEG)-amino acid oligopeptide-irinotecan combo and its medicinal composition
ActiveCN103083680APromote absorptionProlong the action timeOrganic active ingredientsPharmaceutical non-active ingredientsSide effectEnd-group
The invention relates to a PEG-amino acid oligopeptide-irinotecan combo having a structure represented by general formula (I), and a medicinal composition containing the combo. In the combo, PEG represents polyethylene glycol, and the molecular weight of PEG is 300-60,000Dalton; (AA)i represents oligopeptides, and AA represents same or different amino acids in the oligopeptides; i is an integer between 2 and 12 and represents the quantity of the amino acids in the oligopeptides; and j is an integer between 2 and 12 and represents the quantity of irinotecans connected with the oligopeptides. In the combo, each PEG end group can be connected with a plurality of irinotecans through the amino acid oligopeptides, so the medicine loading rate is substantially improved. The modification of a hydrophilic polymer can protect irinotecan, improve the medicine absorption, prolong the action time, enhance the curative effects, reduce the administration dosage and avoid toxic and side effects.
Owner:JENKEM TECH +1
Methods of treating metastatic breast cancer with 4-iodo-3-nitrobenzamide and irinotecan
Provided herein are methods, compositions and kits for the treatment of locally advanced or metastatic breast cancer or breast cancer brain metastases. The method comprises administration of 4-iodo-3-nitrobenzamide, a metabolite or salt thereof in combination with irinotecan. The method of treating locally advanced or metastatic breast cancer comprises at least one 21 day treatment cycle.
Owner:BIPAR SCI INC
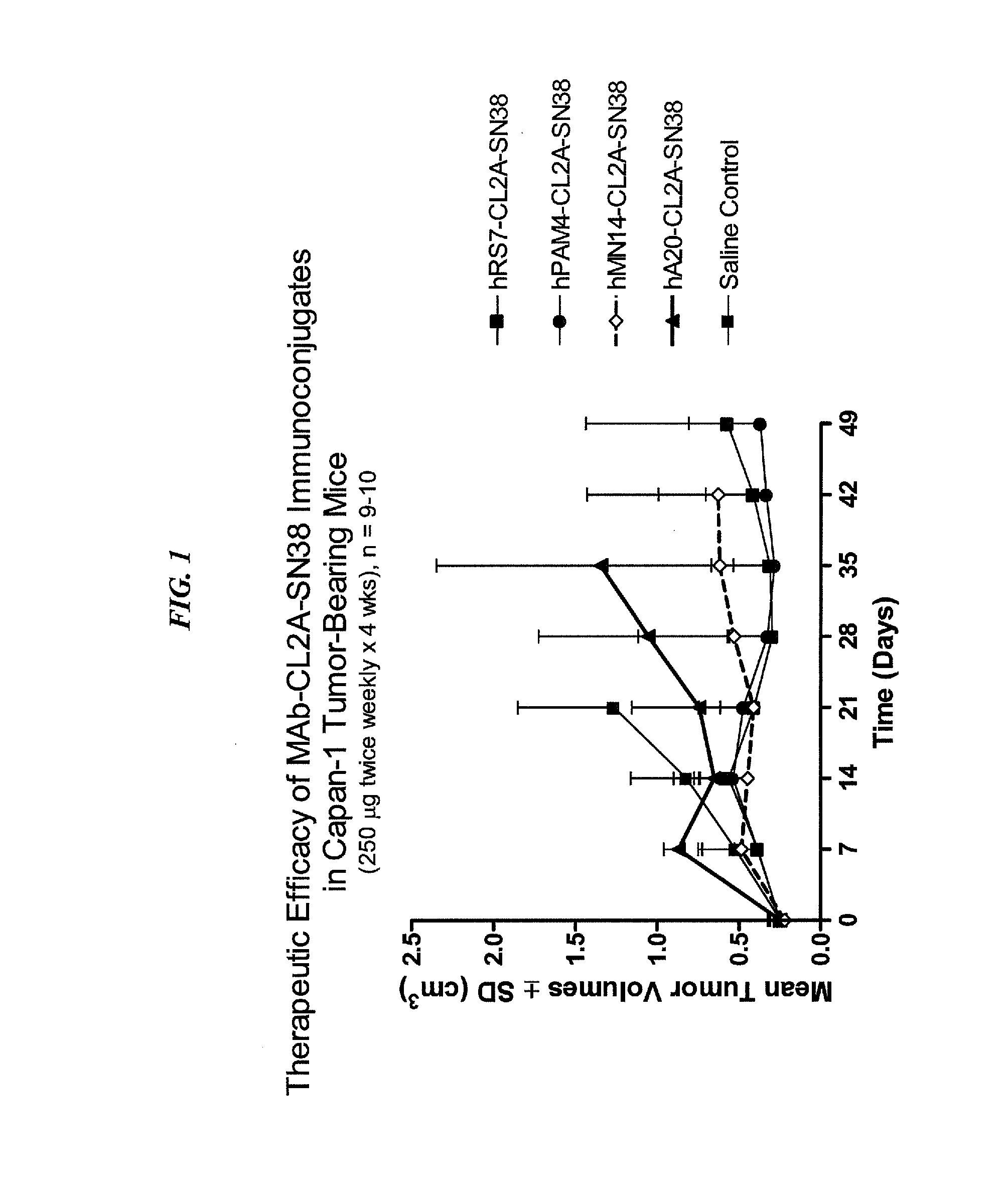
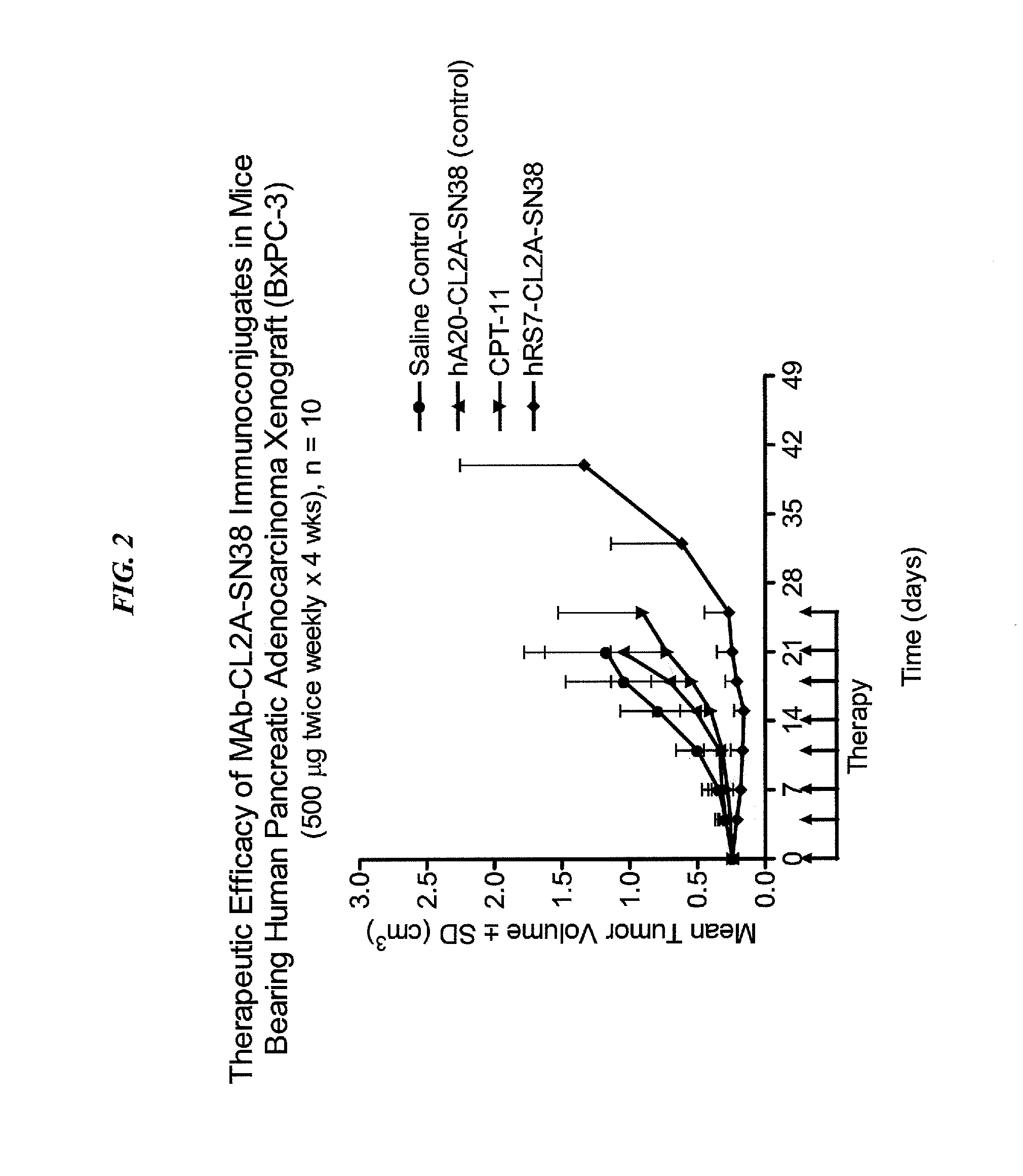
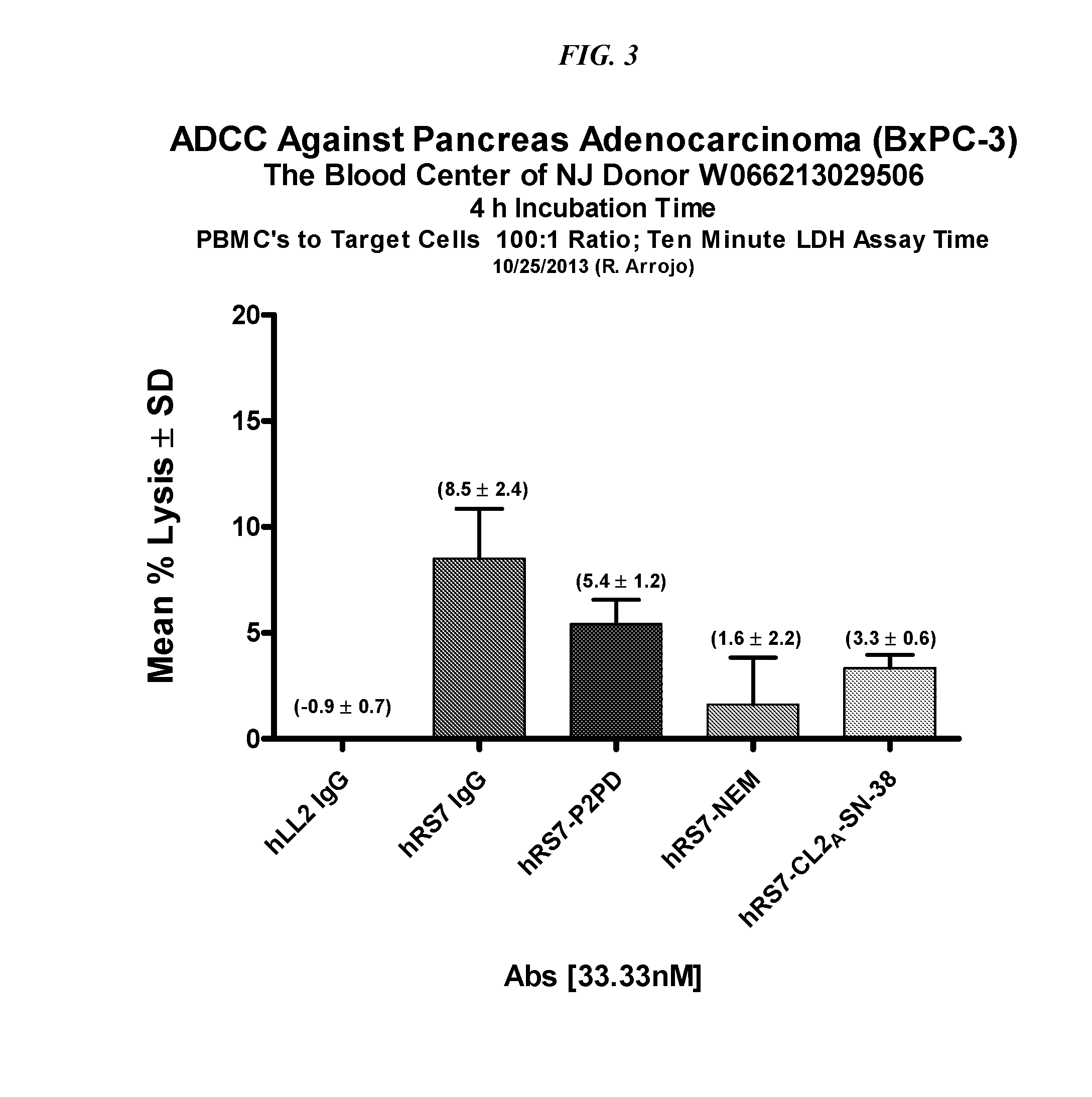
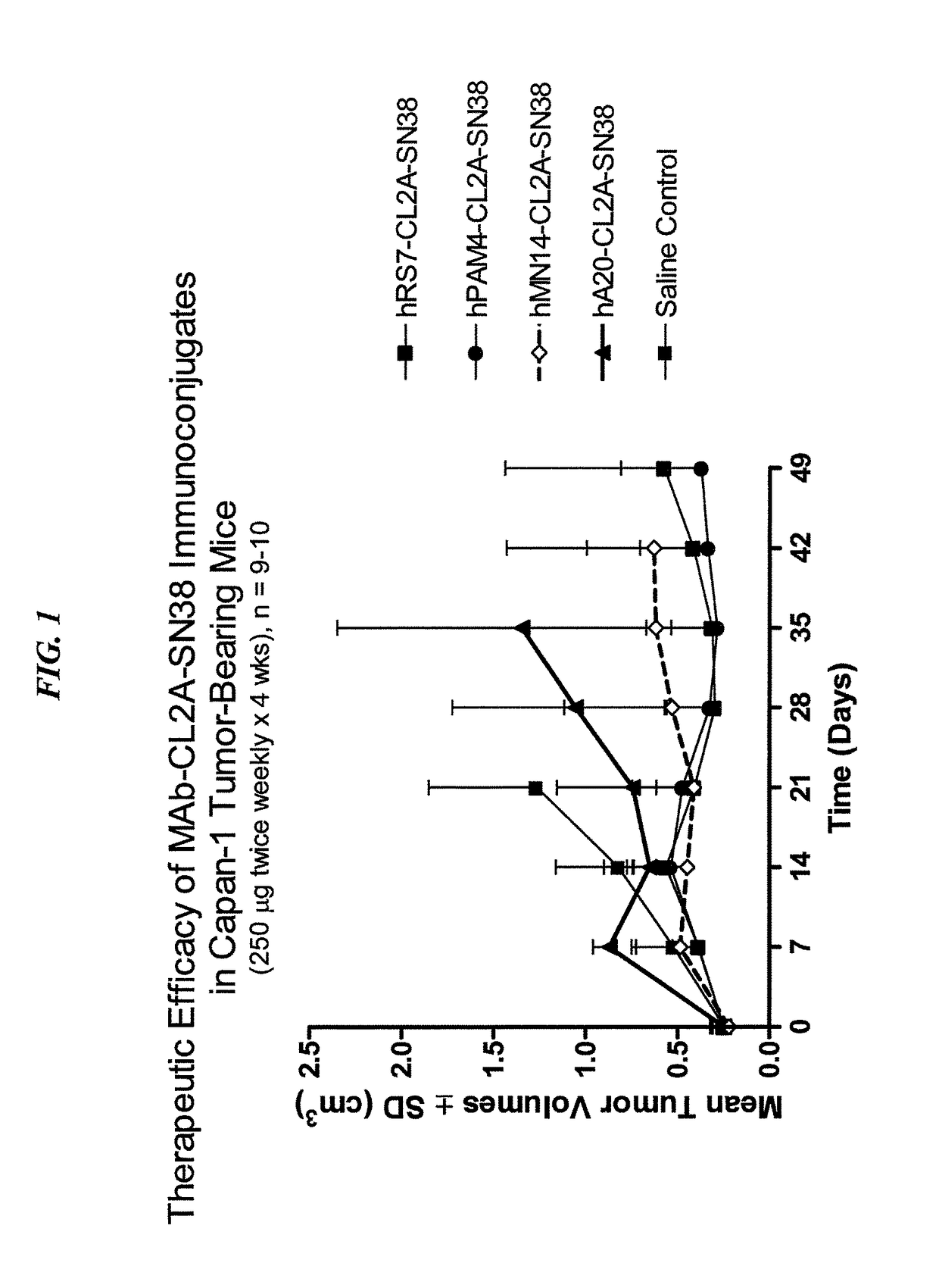
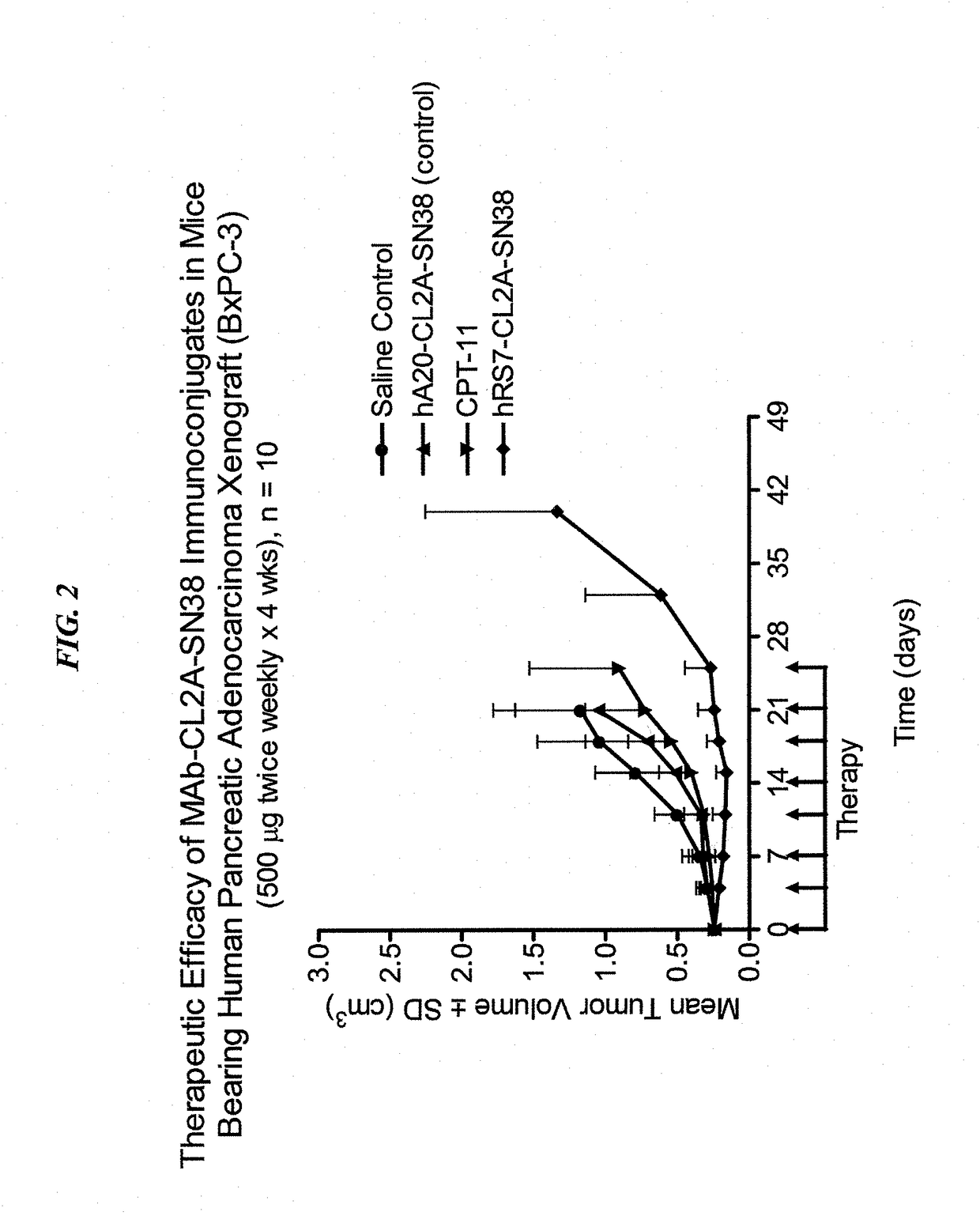
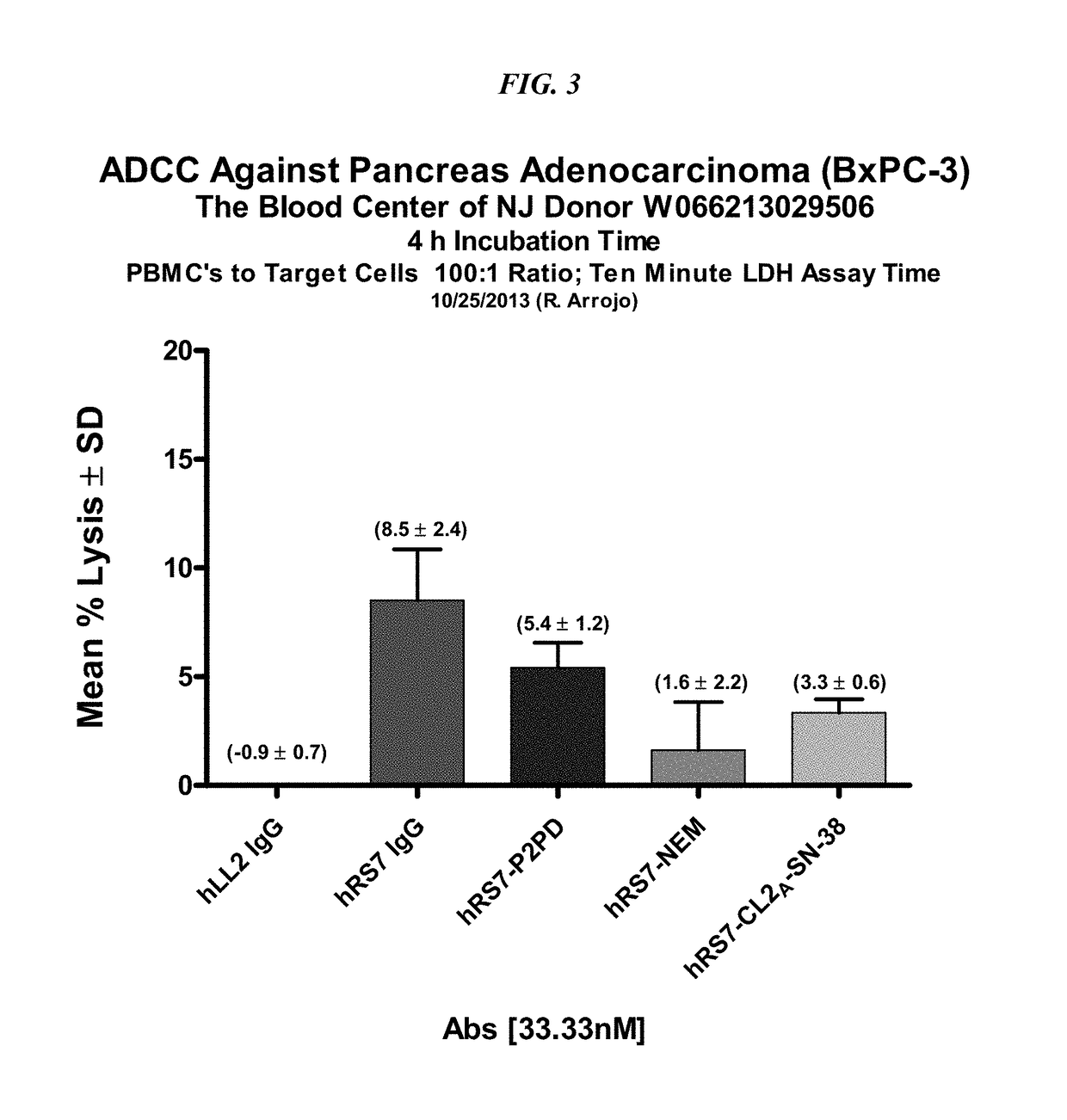
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20160193357A1Receive treatment wellGood effectOrganic active ingredientsHeavy metal active ingredientsBreast cancer metastasisAntiendomysial antibodies
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. In preferred embodiments, the antibody may be an hRS7 antibody. The methods and compostions are of use to treat Trop-2 expressing cancers in human patients, preferably in patients who are resistant to or relapsed from at least one prior anti-cancer therapy, more preferably in patients who are resistant to or relapsed from treatment with irinotecan. The immunoconjugate may be administered at a dosage of 3 mg / kg to 18 mg / kg, preferably 8 to 12 mg / kg, more preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size and reduce or eliminate metastases. Preferred tumors to treat with the subject immunoconjugates include triple-negative breast cancer, HER+, ER+, progesterone+ breast cancer, metastatic non-small-cell lung cancer, a metastatic small-cell lung cancer and metastatic pancreatic cancer.
Owner:IMMUNOMEDICS INC
Irinotecan nano circulating liposome and preparation method thereof
InactiveCN101953792ASolve uneven particle size distributionImprove in vivo and in vitro stabilityOrganic active ingredientsPharmaceutical non-active ingredientsEthanol InjectionPolyethylene glycol
The invention provides a novel Irinotecan carrier which is circulating nano liposome and a novel preparation method thereof which is an ethanol injection-ammonium sulfate active medicament-loading method. The preparation process comprises: a, dissolving a lipid material for forming the liposome in ethanol to prepare solution; b, dissolving a polyethylene glycol compound in solution of ammonium sulfate to prepare a hydration medium; c, injecting solution obtained by the step a into the hydration medium obtained by the step b with stirring in a water bath, stirring at a constant temperature for a certain period under a condition of introducing N2 to form circulating blank liposome; d, dialyzing the blank liposome obtained by the step c in physiological saline; and e, adding solution of Irinotecan into the blank liposome, regulating the pH value of an external phase, and performing incubation and medicament loading at a certain temperature to obtain the Irinotecan circulating nano liposome. The process is simple, the particle size is 80 to 150 nanometers, the coating rate is over 95 percent, the sterile preparation can be obtained easily, and an industrialization requirement is met.
Owner:中华人民共和国卫生部肝胆肠外科研究中心 +1
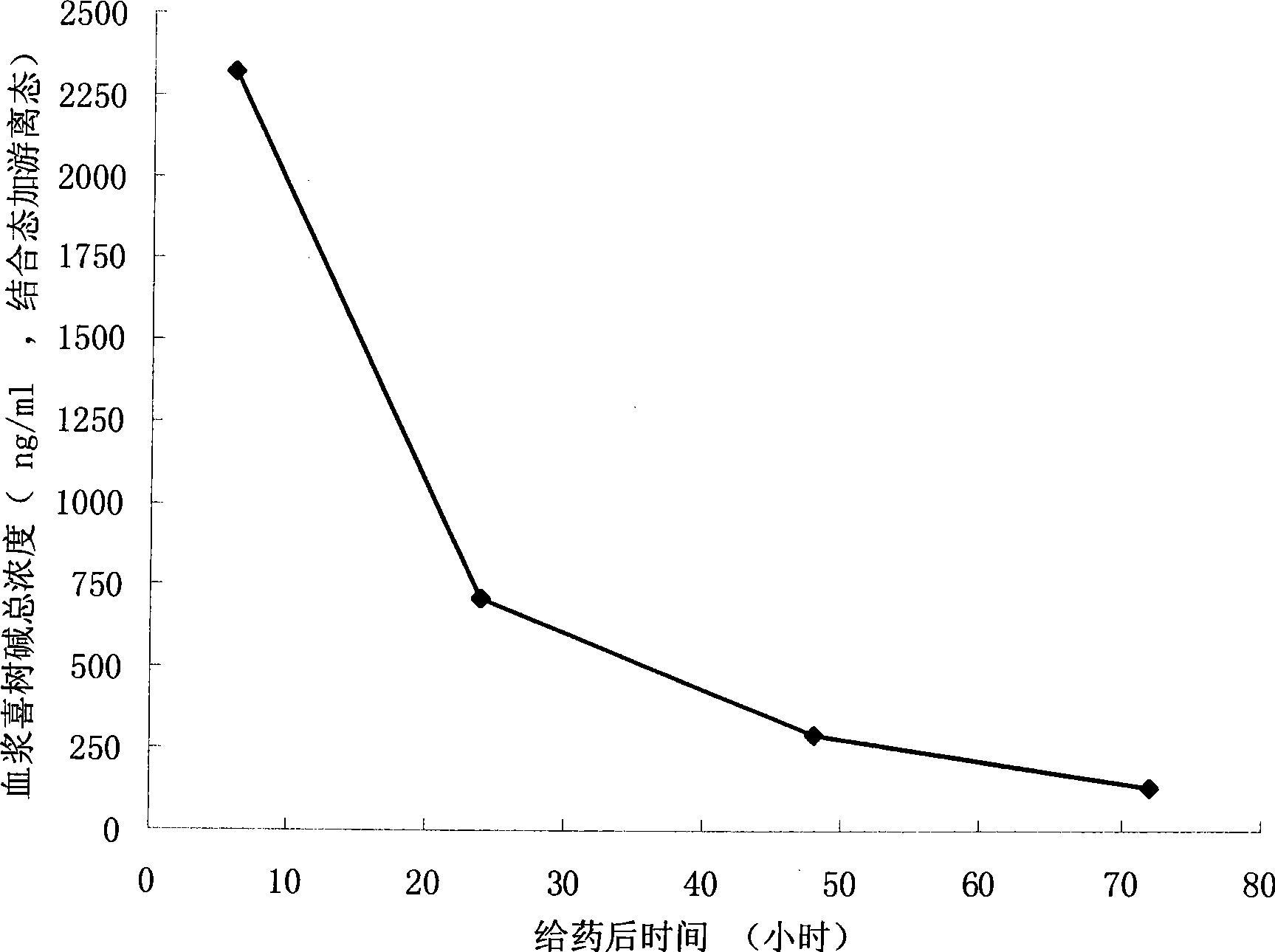
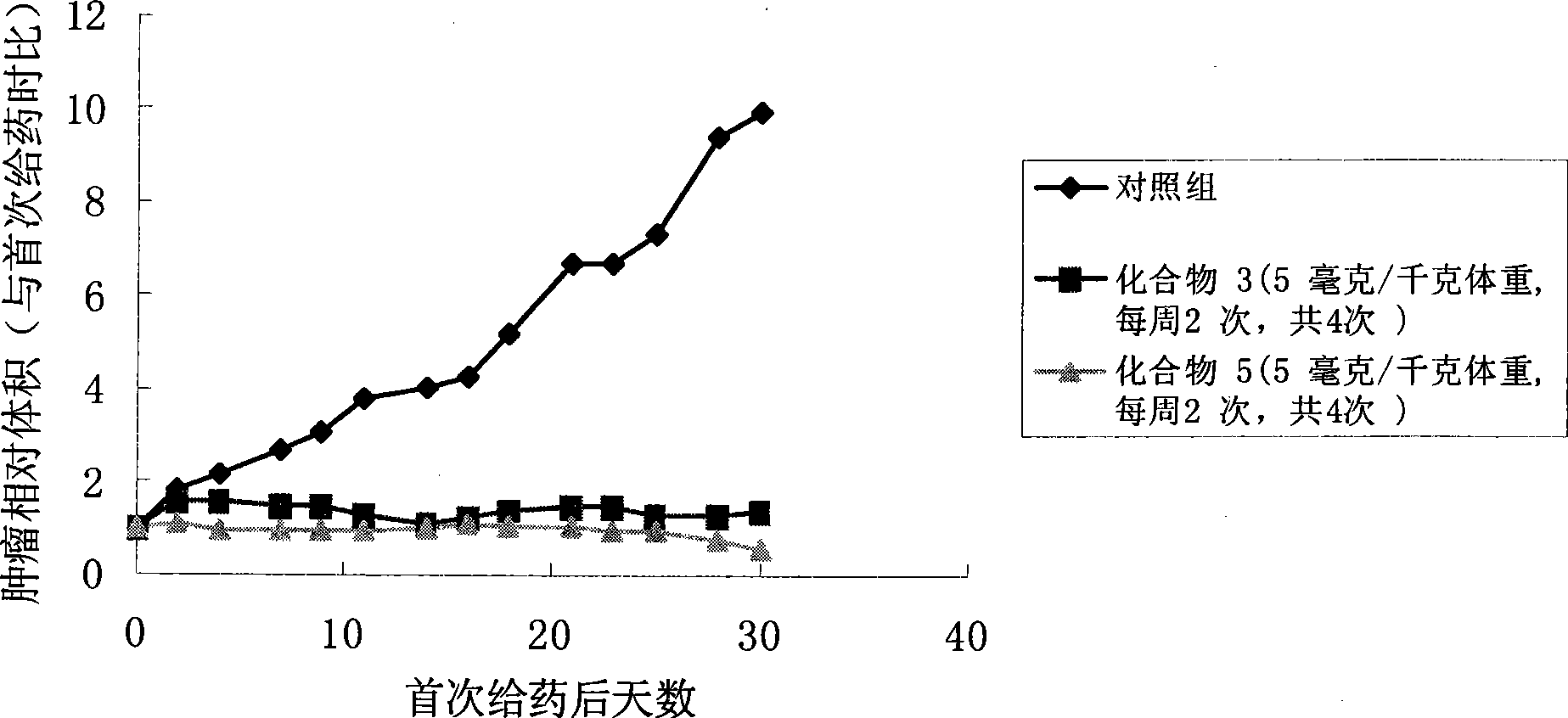
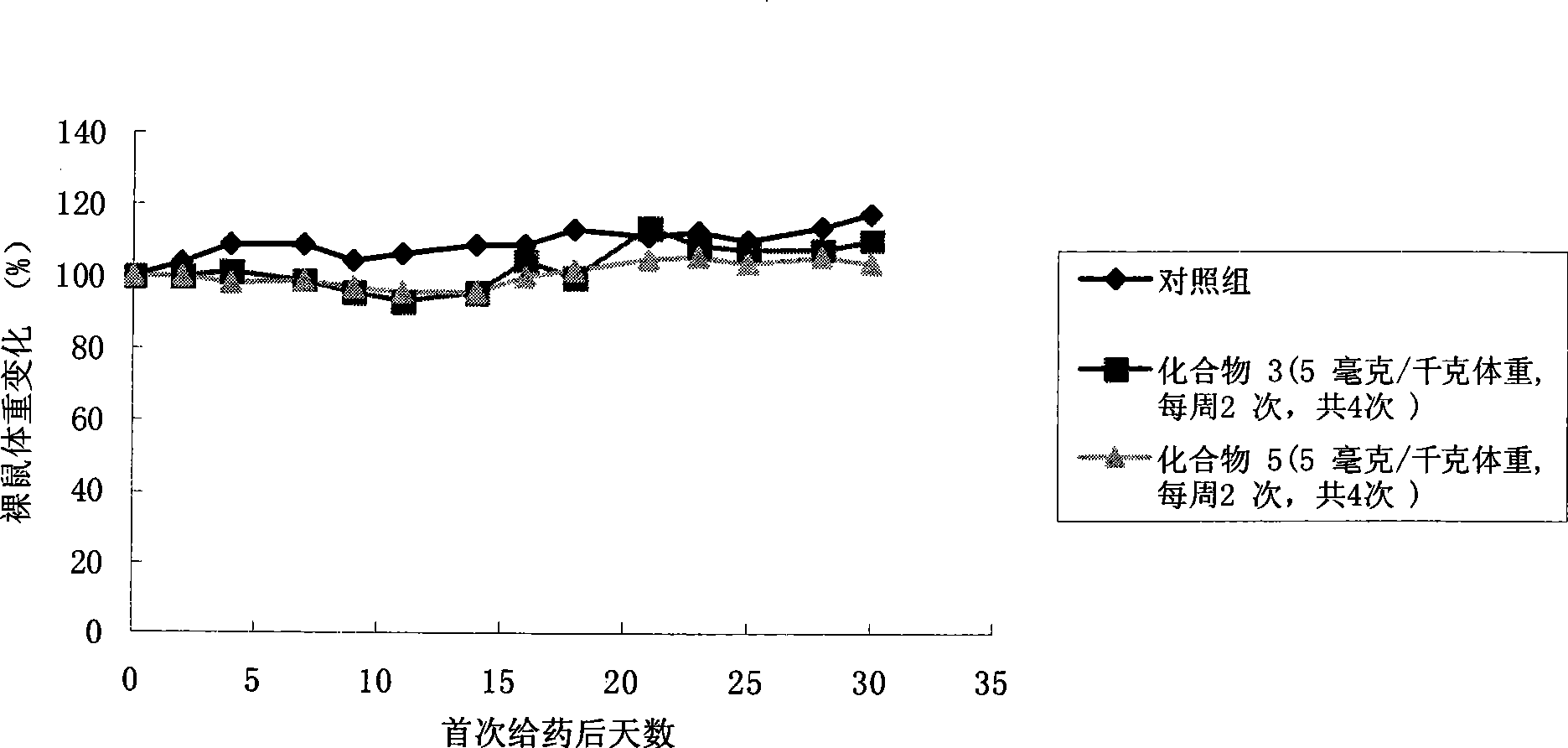
Camptothecine and non-linear polyethyleneglycol prodrug of derivative thereof
InactiveCN101385860ANo systemic toxicityReduce weightOrganic active ingredientsPharmaceutical non-active ingredientsPositive controlHCT116 Cell
The invention discloses a pro-drug as shown in general formula (I), which is formed by coupling camptothecin or a derivative of the camptothecin with non-linear configuration glycol polyethylene, wherein, the definition for varied groups is available in the specification. A physiological disposition research on nude mice discloses that: the pro-drug has a time-delay plasma concentration - time curve, and the plasma concentration can still be maintained at over 100ng / ml 72 hours after intravenous medication. Evaluation on physiological drug effect shows that the pro-drug has strong growth inhibiting effect against the LOVO HCT116 cell line transplantable tumor inoculated into the nude mice and also has obvious growth inhibitory activity against human lung carcinoma H460 cell line transportable tumor inoculated into the nude mice. The inhibition of the pro-drug is better than that of Irinotecan, a positive control drug and has no obvious systemic toxicity.
Owner:AMERICAN CAOYAOQUAN
Acid Addition Salt of Irinotecan
InactiveUS20070299099A1Good water solubilityStable and high-concentrationBiocideOrganic chemistryO-Phosphoric AcidDrugs preparations
The present invention is directed to an irinotecan acid addition salt which is formed through addition of an acid selected from the group consisting of sulfuric acid, nitric acid phosphoric acid, methanesulfonic acid, citric acid maleic acid and succinic acid to Irinotecan, to a method for producing the salt, and to a pharmaceutical composition containing the salt. The addition salt requires no heating process during drug preparation and provides an aqueous drug product in which the salt is stably dissolved.
Owner:YAKULT HONSHA KK
Tumor cell microsatellite instable state detection system
ActiveCN108374046AReduce the number of amplification cyclesReduce dosageMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorQuantitative fluorescence
The invention discloses a tumor cell microsatellite instable state detection system, and relates to quantitative fluorescence PCR (polymerase chain reaction) amplification technologies and capillary electrophoresis detection technologies. The tumor cell microsatellite instable state detection system has the advantages that six mononucleotide repeat sites NR-21, NR-24, NR-27, MONO-27, BAT-25 and BAT-26 can be amplified by the aid of the tumor cell microsatellite instable state detection system, and sites BrafV600E, UGT1A1*6 and UGT1A1*28 can be typed by the aid of the tumor cell microsatelliteinstable state detection system; reference can be provided to checking Lynch syndromes and using irinotecan medicines by detection results on the basis that MSI (microsatellite instable) states are determined. The invention further provides a detection reagent kit designed according to the tumor cell microsatellite instable state detection system.
Owner:BEIJING MICROREAD GENE TECH
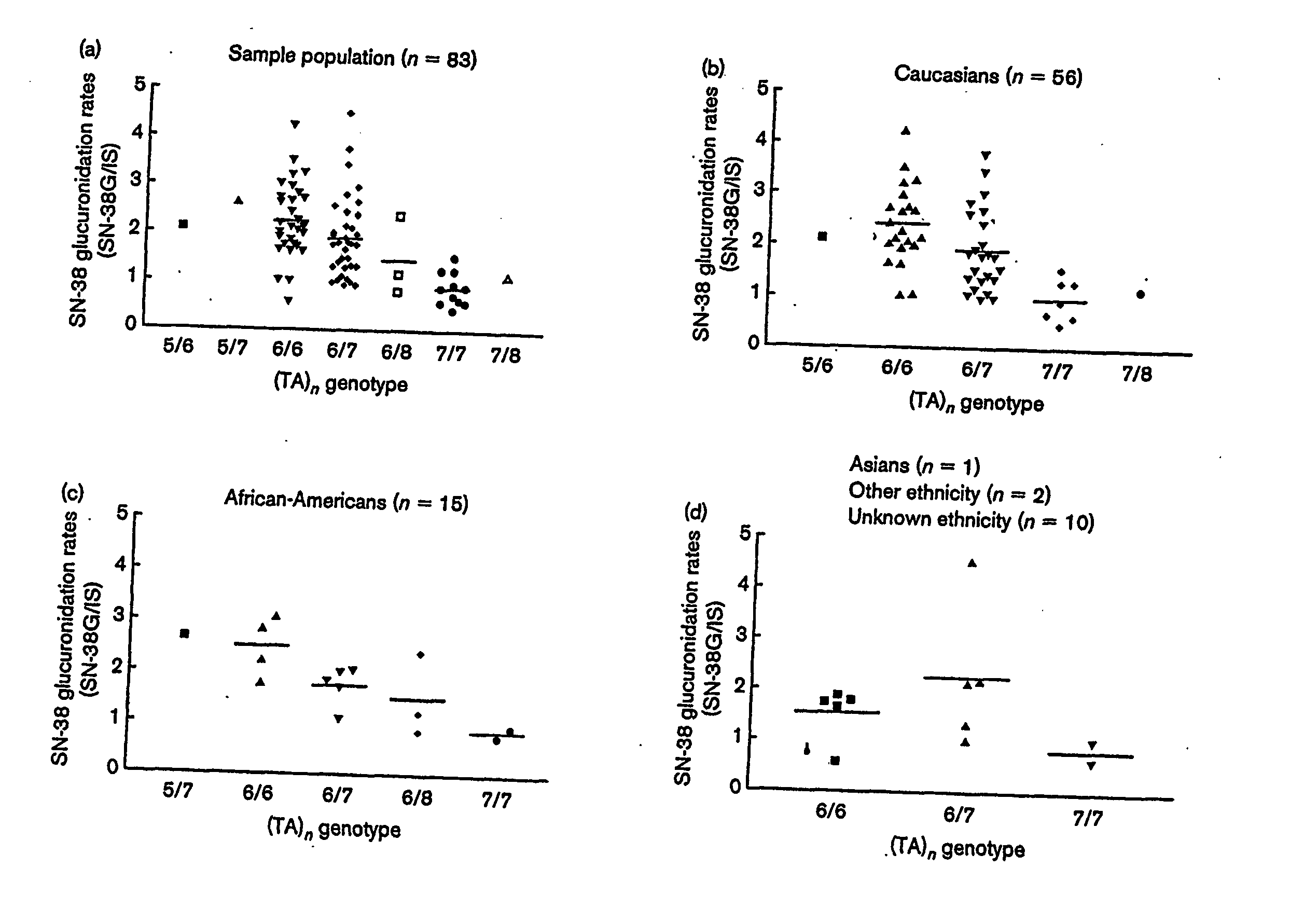
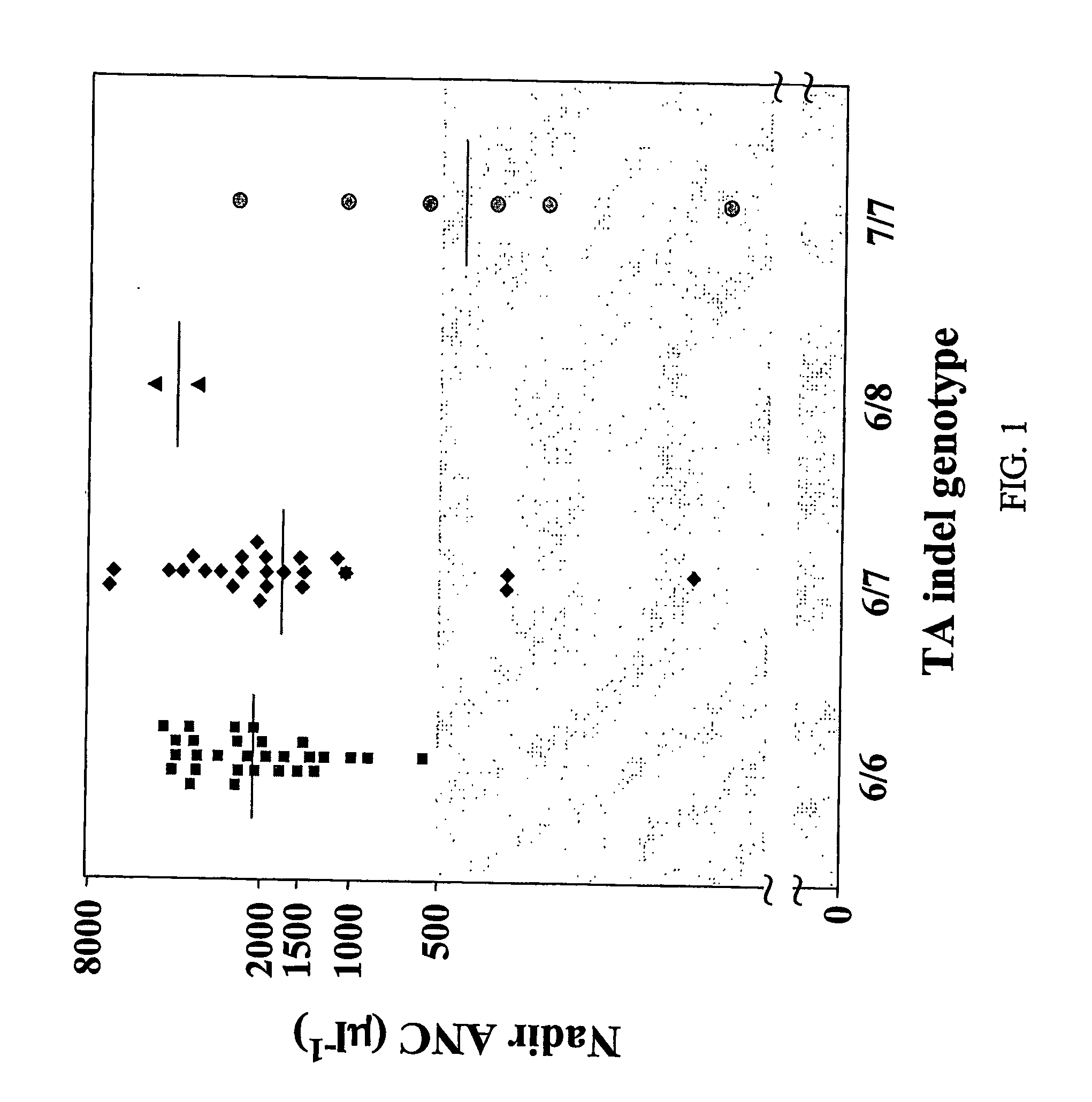
Methods and compositions relating to the pharmacogenetics of different gene variants
InactiveUS20090017452A1Reduce Toxicity RiskAddress bad outcomesMicrobiological testing/measurementPharmacogeneticsGene product
The present invention is directed to methods and compositions for determining the presence or absence of polymorphisms within an ABCC2, UGT1A1, and / or SLCO1B1 gene and correlating these polymorphisms with activity levels of their gene products and making evaluations regarding the effect on their substrates, particularly those substrates that are drugs. In addition, there are methods and compositions of evaluating the risk of an individual for developing toxicity or adverse event(s) to an ABCC2, UGT1A1, and / or SLCO1B1 substrate. In some embodiments, the invention concerns methods and compositions for determining the presence or absence of ABCC2 3972C>T variant and predicting or anticipating the level of activity of ABCC2 and determining dosages of an ABCC2 drug substrate, such as irinotecan, in a patient. Such methods and compositions can be used to evaluate whether irinotecan-based therapy, or therapy involving other ABCC2 substrates, may pose toxicity problems if given to a particular patient or predicting their efficacy. Alterations in suggested therapy may ensue based on genotyping results.
Owner:RGT UNIV OF CALIFORNIA +1
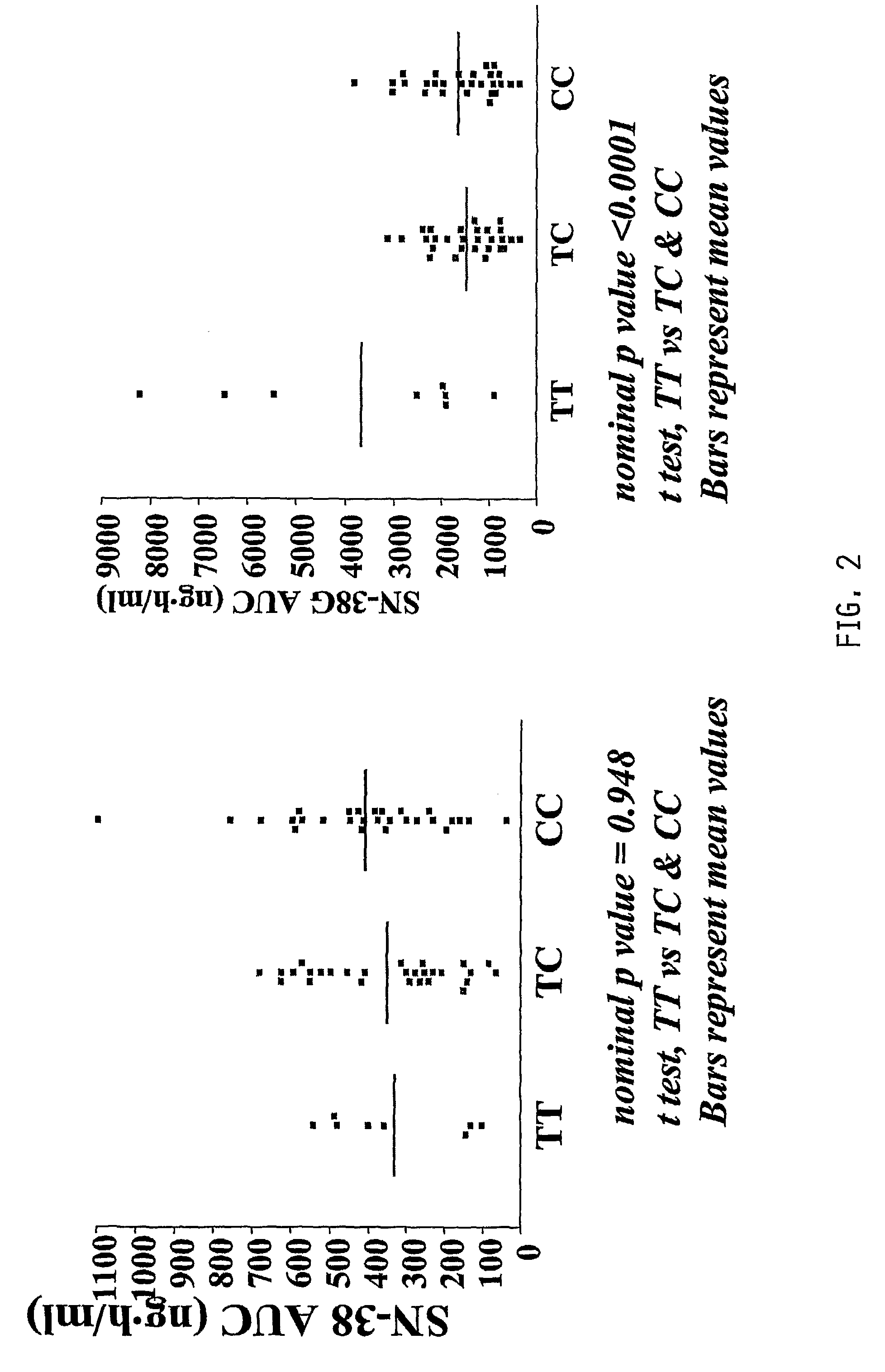
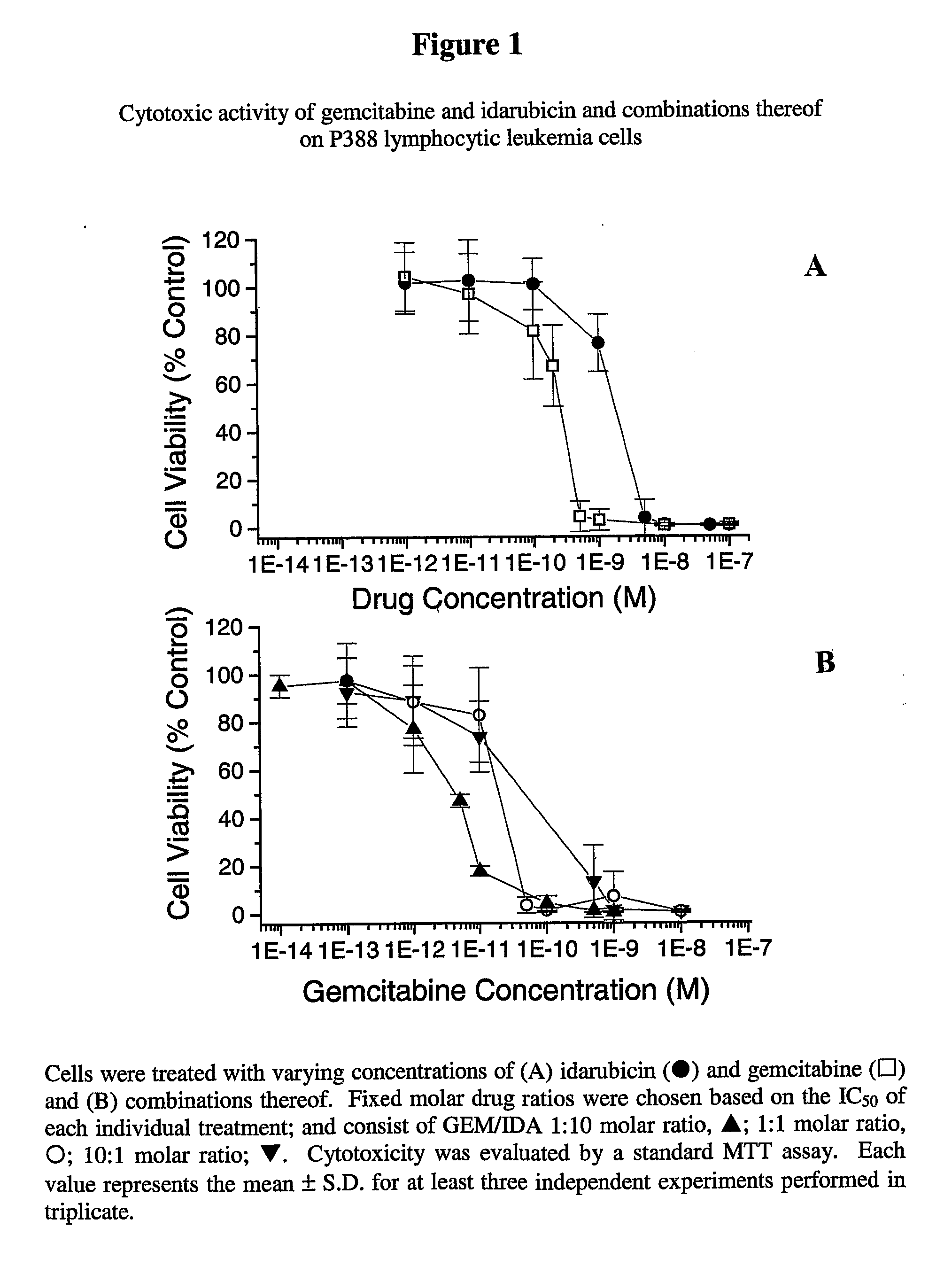
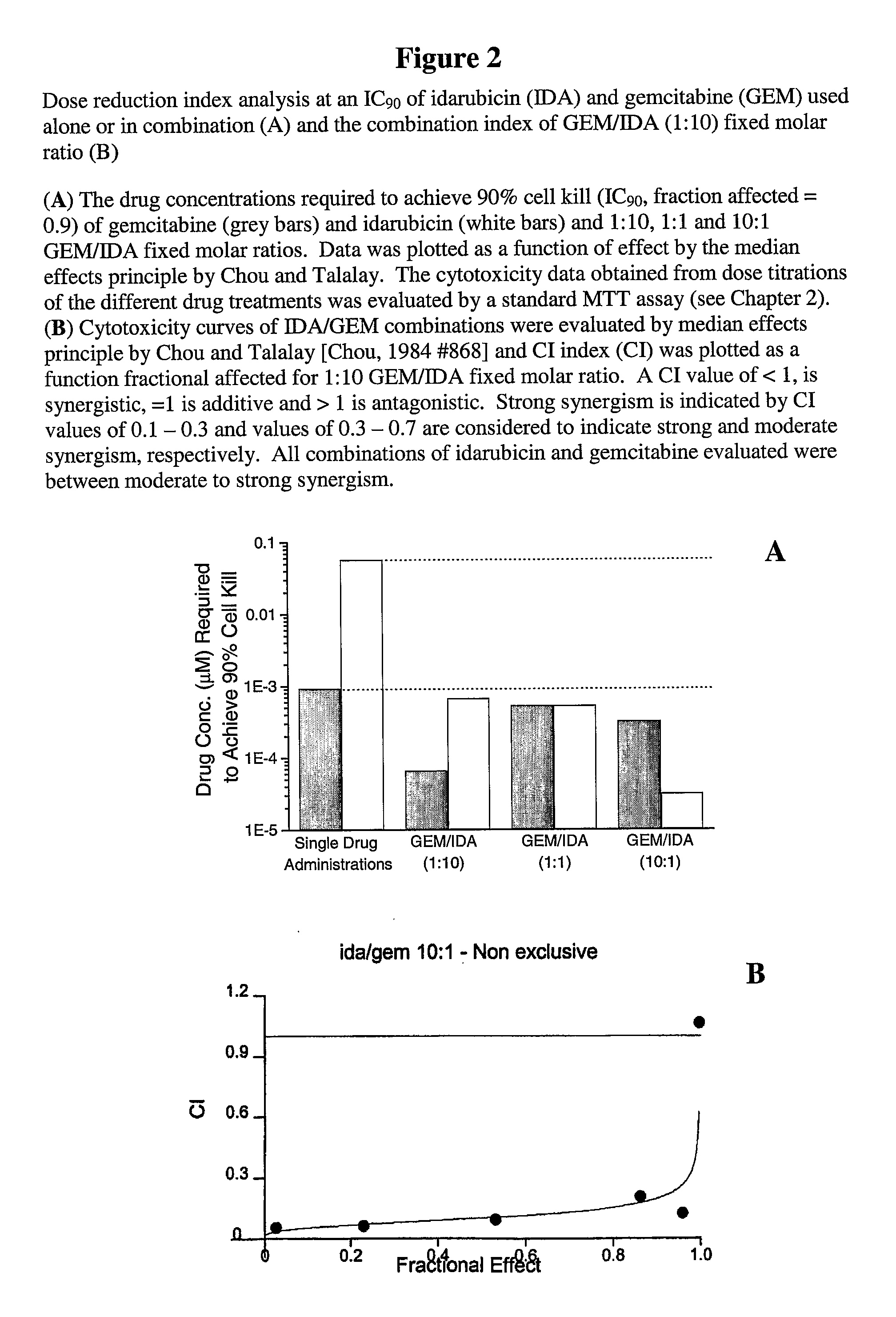
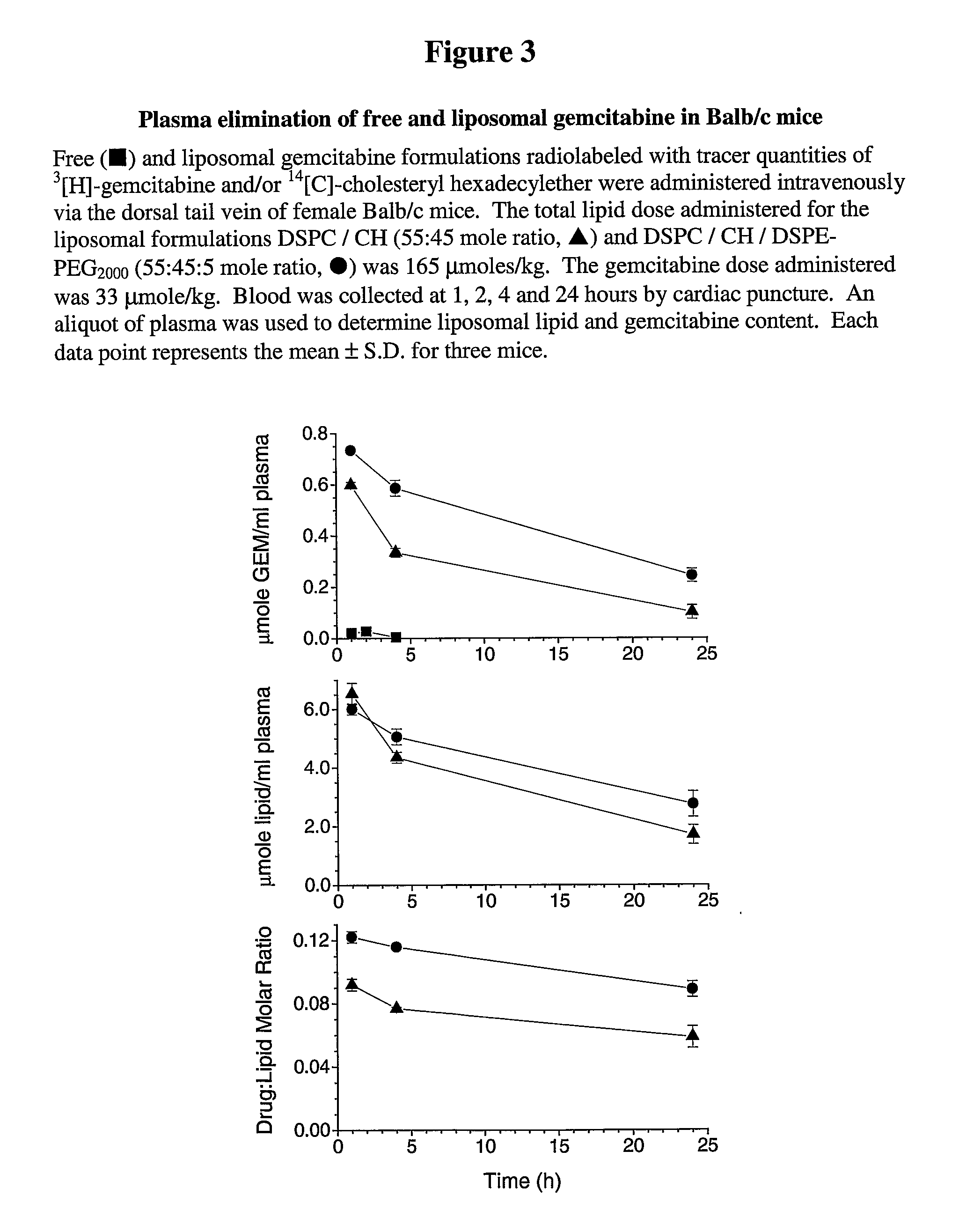
Free or Liposomal Gemcitabine Alone or in Combination with Free or Liposomal Idarubicin
InactiveUS20080213183A1Organic active ingredientsIn-vivo testing preparationsCisplatinMaximum tolerated dose
The use of the maximum tolerated dose (MTD) of individual drugs to determine appropriate administration ratios of drugs for combination therapy, wherein the ratios of drugs are fixed based on the same percentage of the MTD for each drug. Furthermore, antineoplastic compositions comprising liposomal encapsulated gemcitabine alone or in combination with free or liposomal encapsulated antineoplastic agents, such as idarubicin, irinotecan, etopside, cisplatin, cyclophosphamide, doxorubicin, or vincristine are diclosed.
Owner:BRITISH COLUMBIA CANCER AGENCY
Combination of a chemotherapeutic agent and an inhibitor of the TGF-beta system
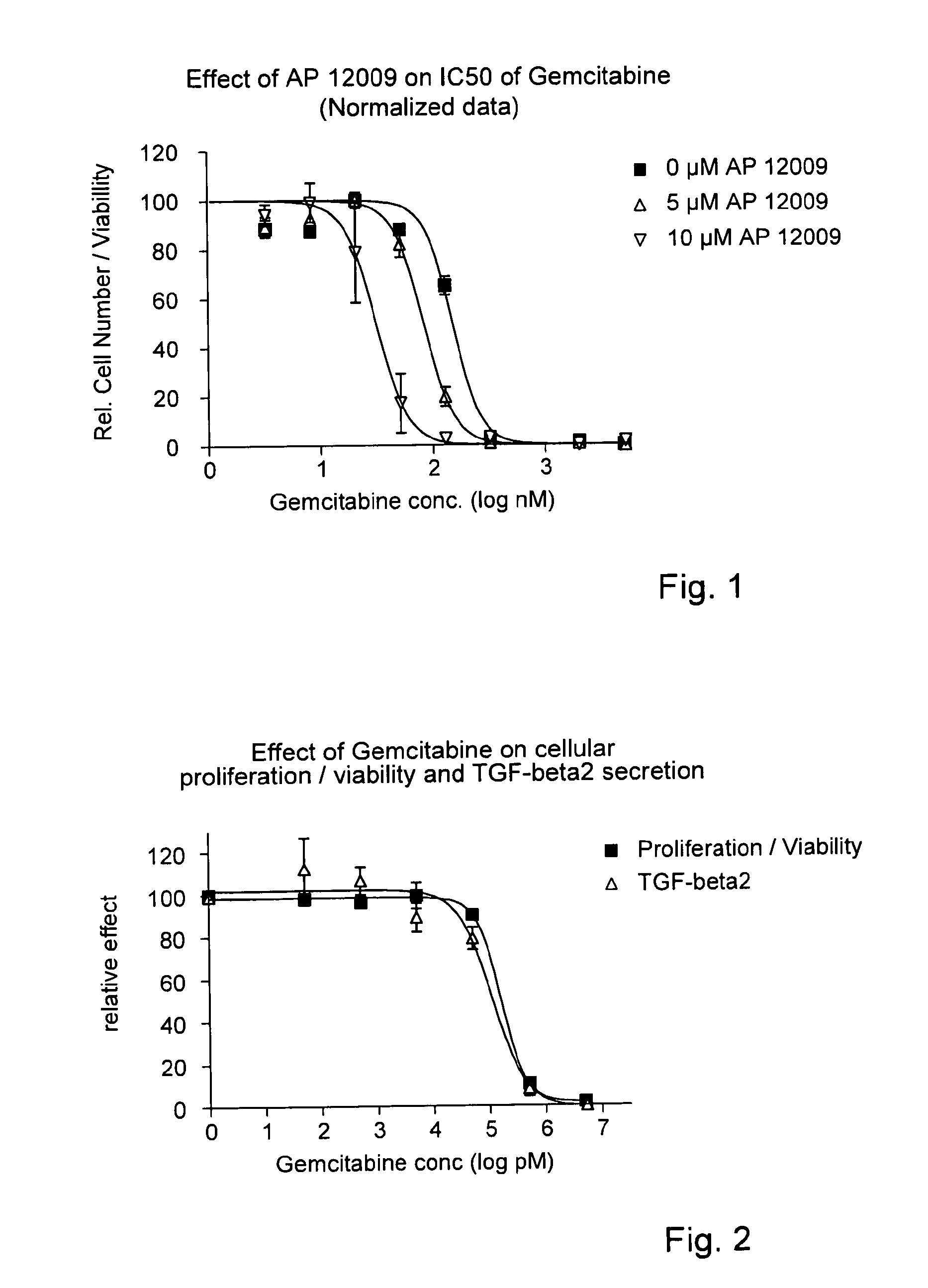
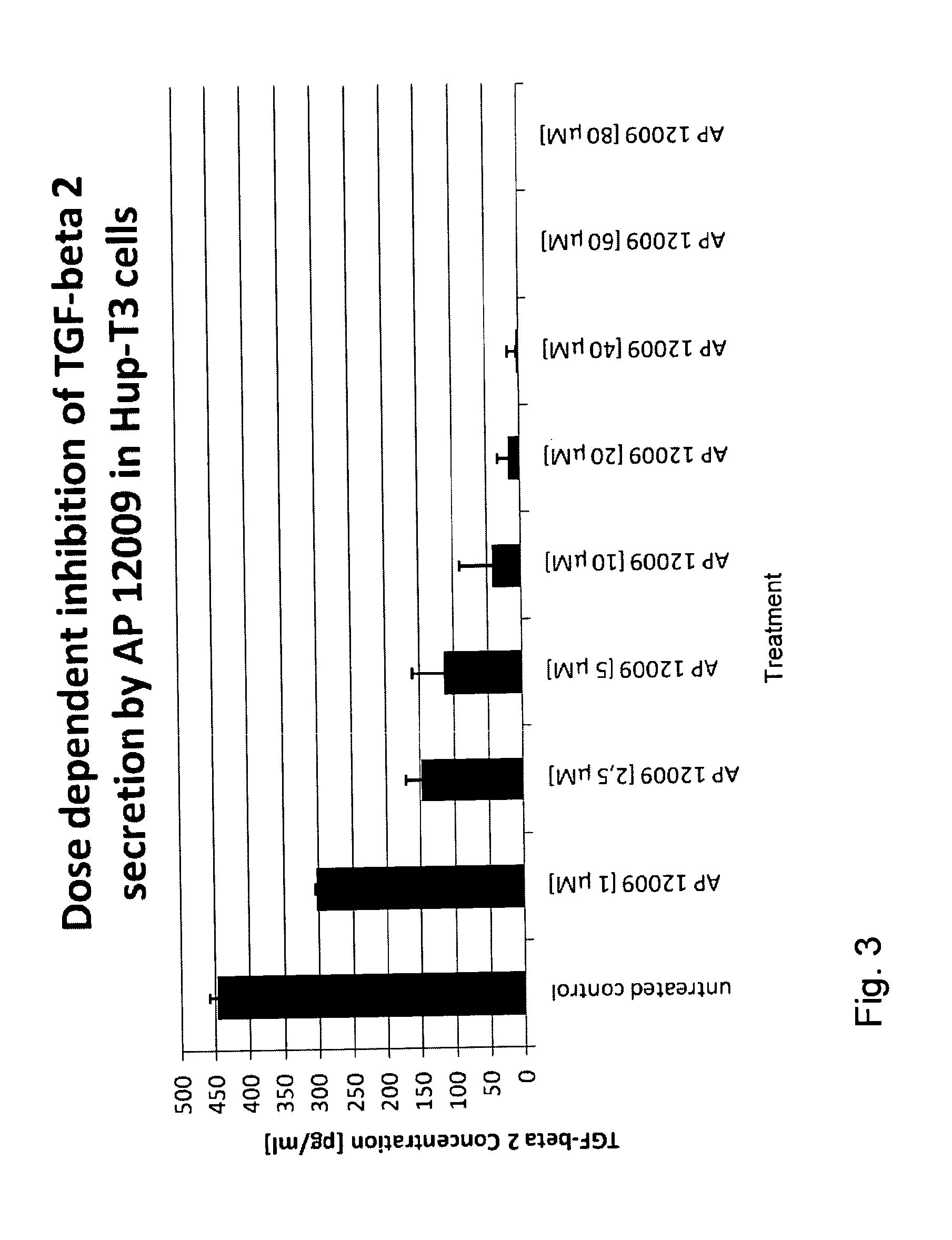
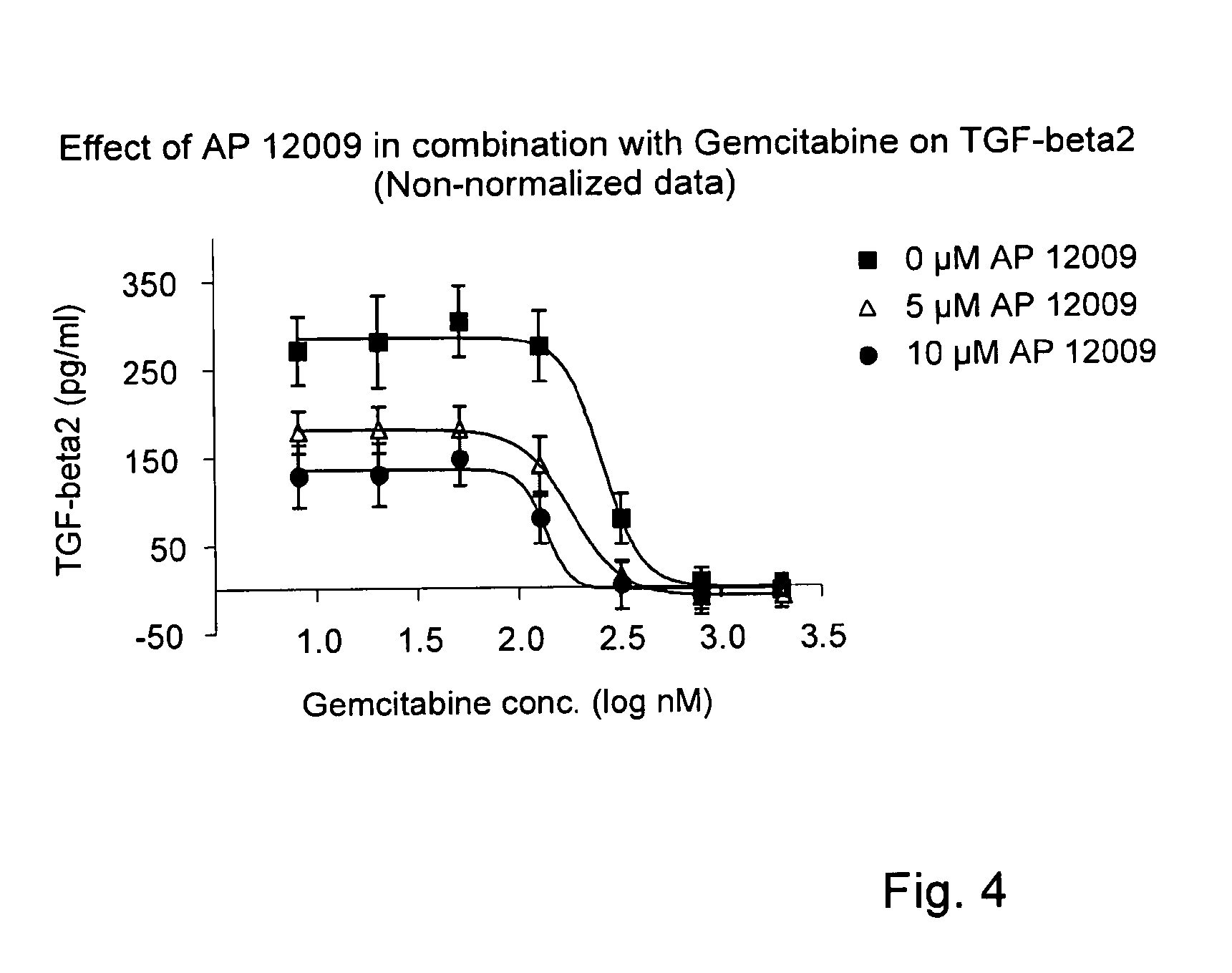
ActiveUS8476246B2Reduce IC50Improve efficiencyBiocideOrganic active ingredientsDocetaxel-PNPDocetaxel
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
ActiveUS10137196B2Heavy metal active ingredientsOrganic active ingredientsAntibody fragmentsAntigen binding
Owner:IMMUNOMEDICS INC
Hyaluronan as a cytotoxic agent, drug pre-sensitizer and chemo-sensitizer in the treatment of disease
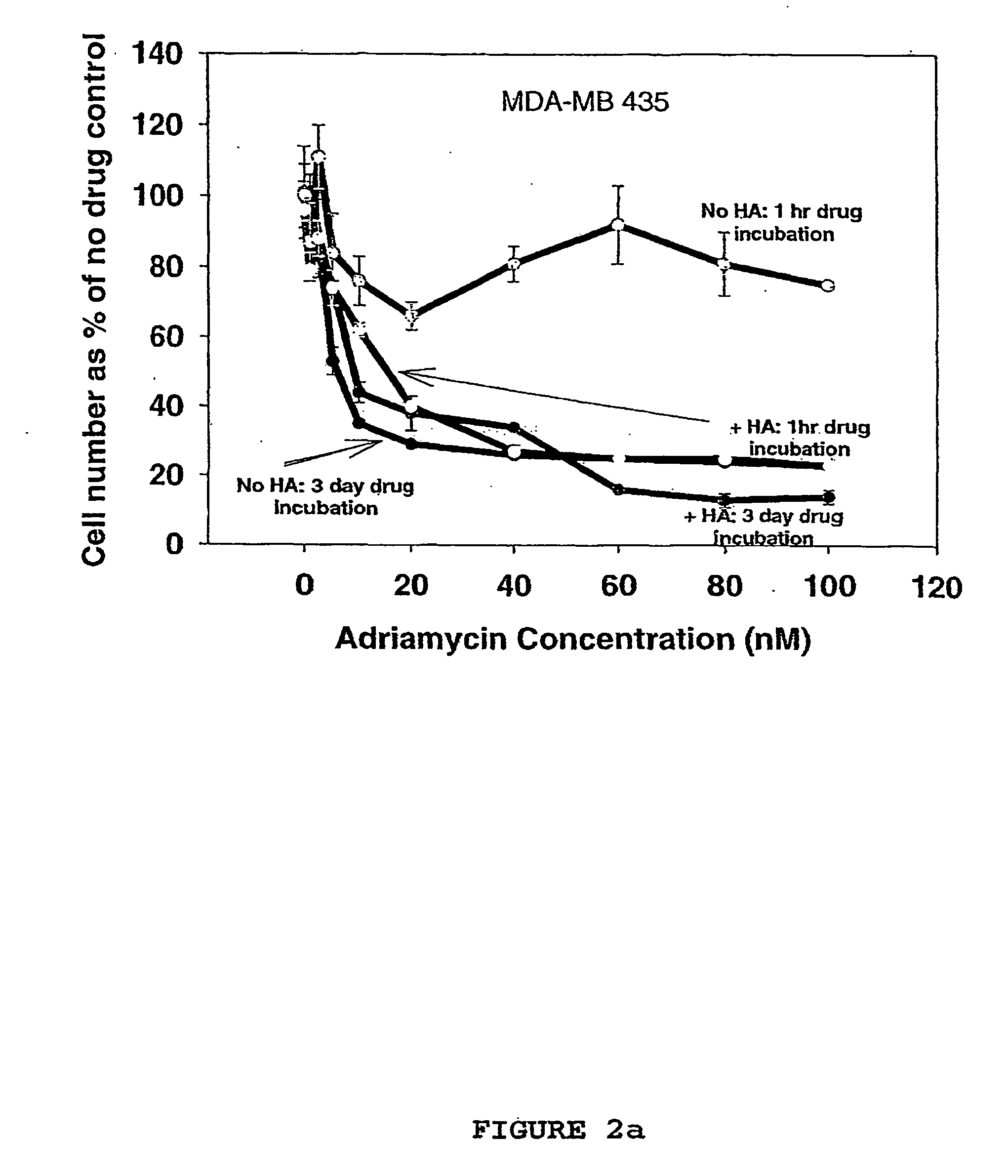
This application provides methods and compositions for the treatment of cancer. The application provides compositions comprising hyaluronic acid and a chemotherapeutic agent such as irinotecan that are useful in the treatment of cancer.
Owner:ALCHEMIA ONCOLOGY PTY LTD
Methods of treating colorectal cancer
InactiveUS20120020989A1Improve clinical symptomsOrganic active ingredientsImmunoglobulins against growth factorsSurgical treatmentExisting Treatment
The present invention provides a pharmaceutical composition and a treating method for colorectal cancer which is difficult for a surgical treatment, comprising an anti-A33 human antibody and a chemotherapy agent in combination. Based on the present invention, a pharmaceutical composition and a treating method comprising the combination of anti-A33 human antibody with CPT-11 (irinotecan) can exhibit a certain degree of antitumor effect on a patient with colorectal cancer who is found recurrence after previously-initiated therapy and / or a patient with colorectal cancer who is difficult to be cured by existing treatments for colorectal cancer.
Owner:KYOWA HAKKO KIRIN CO LTD
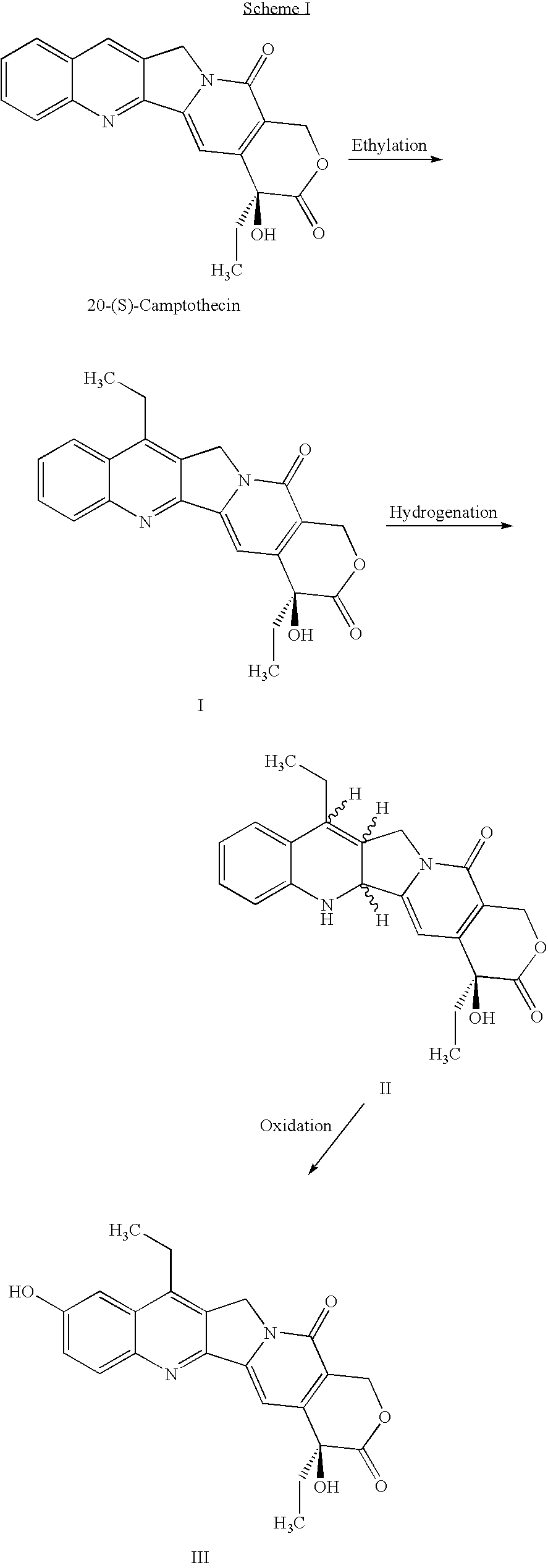
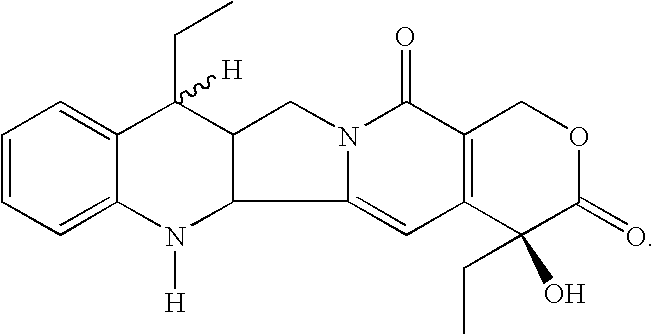
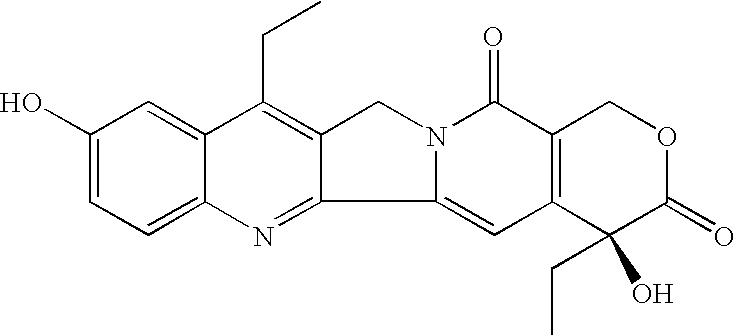
Process for the preparation of 7-alkyl-10-hydroxy-20(S)-camptothecin
The key intermediate in any synthesis of Irinotecan is 7-ethyl-10-hydroxy-20(S)-camptothecin. A process for the efficient synthesis of this intermediate is demonstrated proceeding through readily available 20(S)-camptothecin. Various other tecan compounds may be made by use of corresponding 7-alkyl-10-hydroxy-20(S)-camptothecin intermediates.
Owner:SCINOPHARM TAIWAN LTD
Compositions and methods for the treatment of colorectal cancer
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
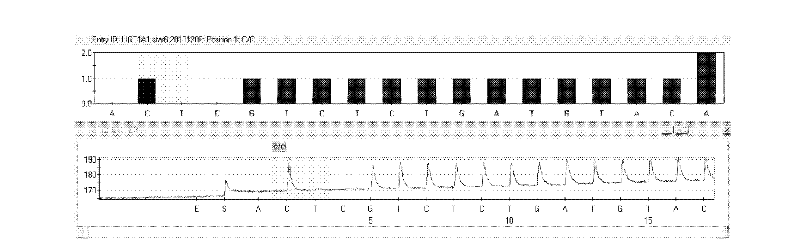
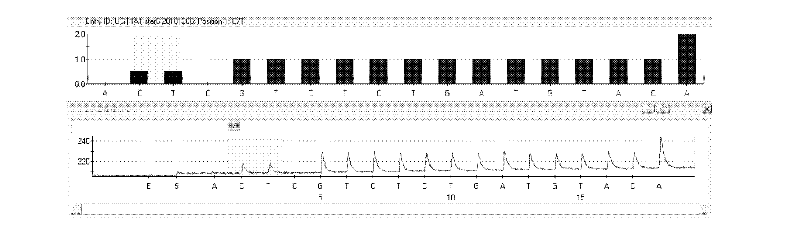
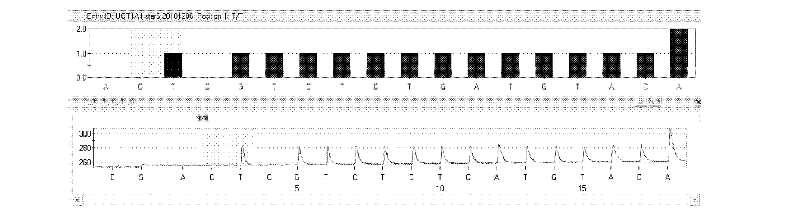
Kit and method for detecting gene polymorphism of irinotecan personalized medicine by pyrophosphoric acid sequencing method
InactiveCN102643906AQuick analysisAccurate analysisMicrobiological testing/measurementIrinotecanPyrophosphoric acid
The invention discloses a kit and method for detecting gene polymorphism of an irinotecan personalized medicine by a pyrophosphoric acid sequencing method. The kit is used for parting an irinotecan medicine gene, particularly single nucleotide polymorphism of UGT1A1*6 (rs4148323) and UGT1A1*28 (rs3064744). The kit comprises primers shown as SEQ ID NO. 3-8. According to the kit, accurate, quick and high-flux detection of the UGT1A1*6 (rs4148323) and the UGT1A1*28 (rs3064744) can be realized, so that individual administration for realizing safe, rational and effective irinotecan medicine is achieved.
Owner:周宏灏
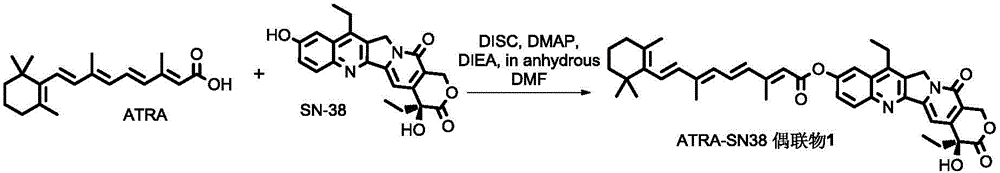
All-trans retinoic acid-camptothecin anticancer drug conjugate as well as preparation method and application thereof
ActiveCN104478890AImprove solubilityExpand the scope of clinical applicationOrganic active ingredientsOrganic chemistryPolyoxyethylene castor oilSolubility
The invention discloses an all-trans retinoic acid-camptothecin anticancer drug conjugate as well as a preparation method and application thereof. The structural formula of the all-trans retinoic acid-camptothecin anticancer drug conjugate is shown in a formula (I), (II), (III), (IV), (V) or (VI). The all-trans retinoic acid-camptothecin anticancer drug conjugate has good solubility in Tween, polyoxyethylene castor oil, a Poly(ethylene adipate)-polylactic acid copolymer and a Poly(ethylene adipate)-poly (lactic acid-glycollic acid) copolymer, can be self assembled into nanometer grains in water, can be directly injected or taken orally or processed into other dosage forms. According to the all-trans retinoic acid-camptothecin anticancer drug conjugate disclosed by the invention, as all-trans retinoic acid and SN-38 or camptothecin take synergistic effect, compared with a conjugate only containing one of irinotecan, SN-38 and all-trans retinoic acid, the all-trans retinoic acid-camptothecin anticancer drug conjugate has good tumor suppression effect.
Owner:ZHEJIANG UNIV
Combination of a chemotherapeutic agent and an inhibitor of the tgf-beta system
ActiveUS20120027873A1Reduce IC50Improve efficiencyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Pharmaceutical composition comprising a chemotherapeutic agent and a TGF-beta antisense oligonucleotide, wherein the antisense oligonucleotide reduces the sensitivity and IC50, respectively, of the cytotoxicity of the chemotherapeutic agent. Preferably, the antisense oligonucleotide is a TGF-beta 1, 2, and / or 3 antisense oligonucleotide and the chemotherapeutic agent is preferably gemcitabine, 5-fluorouracil, temozolomide, dacarbacine, docetaxel, cisplatin, oxaliplatin, tamoxifen, or irinotecan.
Owner:ANTISENSE PHARMA GMBH
Irinotecan nano lipid binding preparation and preparation method thereof
ActiveCN103735504AUniform particle size distributionLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetTumor targeting
The invention relates to an irinotecan nano lipid binding preparation and a preparation method thereof. The irinotecan nano lipid binding preparation contains phospholipid, polyethylene glycol-dodecahydroxycy stearate, glycerol, ethanol and irinotecan, as well as an aqueous solvent for injection. The irinotecan nano lipid binding preparation is even in grain size, and as the lipid binding preparation, capable of reducing the toxicity of the irinotecan with long circulation effect and enhancing the tumor targeted enrichment effect and improving the compliance of a patient, and the preparation method is simple in process, high in repeatability and applicable to industrial production.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Methods and compositions for predicting irinotecan toxicity
The present invention concerns the methods and compositions for evaluating the risk of ironotecan toxicity in a cancer patient based on the genotype of the patient at position −3156 of the UGT1A1 gene or at any position in linkage disequilibrium with the −3156 variant.
Owner:UNIVERSITY OF CHICAGO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com