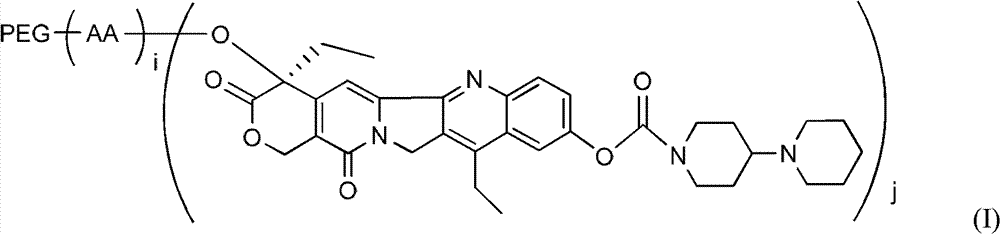
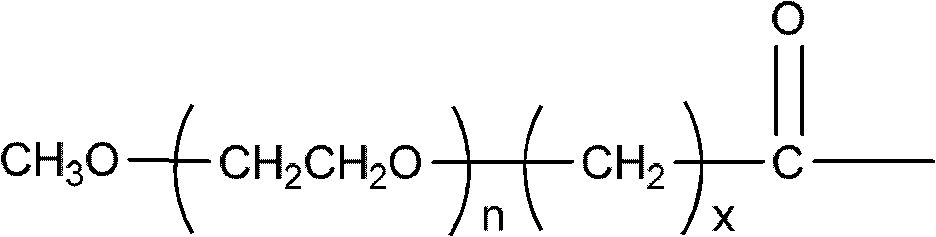
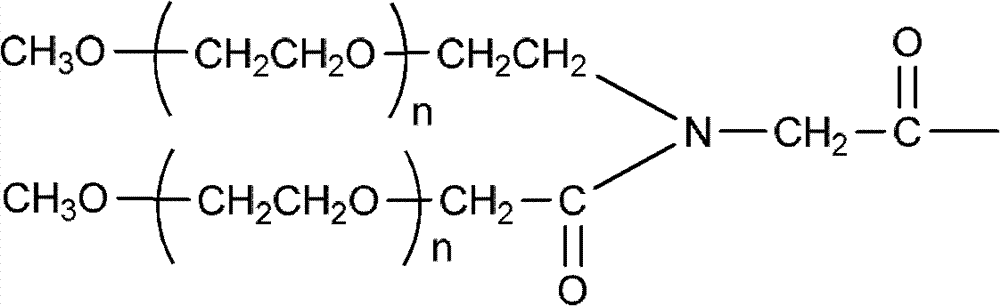
Polyethylene glycol (PEG)-amino acid oligopeptide-irinotecan combo and its medicinal composition
A technology of polyethylene glycol and conjugates, which can be used in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., and can solve problems such as drug loading rate limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Preparation of linear polyethylene glycol (20k)-amino acid pentapeptide-Inotecan conjugate (CPT-1)
[0070] 29.4 g of L(+)-glutamic acid, 40 g of p-toluenesulfonic acid, and 80 mL of benzyl alcohol were dissolved in 500 mL of toluene, and 11 mL of water was separated under reflux under nitrogen protection. The reflux was continued for 3 hours, and 150 mL was evaporated. After cooling to 50°C, the reaction solution was poured into a beaker containing 600 mL petroleum ether, stirred for 1 hour, and the precipitate was collected by filtration. After the filter cake was heated and dissolved with 280 mL of 95% ethanol, the heating was stopped and the cake was cooled overnight. The precipitate was collected by filtration and dried under vacuum to obtain 61 g of L(+)-glutamic acid dibenzyl ester p-toluenesulfonate.
[0071] 30g of dibenzyl glutamate p-toluenesulfonate was dissolved in 500mL of dichloromethane, 20.86g of tert-butoxycarbonyl-L-glutamate-5-benzyl ester was...
Embodiment 2
[0076] Example 2: Preparation of linear polyethylene glycol acetic acid (20k)-amino acid hepeptide-Inotecan conjugate
[0077] 29.4 g of L(+)-glutamic acid, 40 g of p-toluenesulfonic acid, and 80 mL of benzyl alcohol were dissolved in 500 mL of toluene, and 11 mL of water was separated under reflux under nitrogen protection. The reflux was continued for 3 hours, and 150 mL was evaporated. After cooling to 50°C, the reaction solution was poured into a beaker containing 600 mL petroleum ether, stirred for 1 hour, and the precipitate was collected by filtration. After the filter cake was heated and dissolved with 280 mL of 95% ethanol, the heating was stopped and the cake was cooled overnight. The precipitate was collected by filtration and dried under vacuum to obtain 61 g of L(+)-glutamic acid dibenzyl ester p-toluenesulfonate.
[0078] 30g of dibenzyl glutamate p-toluenesulfonate was dissolved in 500mL of dichloromethane, 20.86g of tert-butoxycarbonyl-L-glutamate-5-benzyl ester wa...
Embodiment 3
[0082] Example 3: Preparation of Y-type polyethylene glycol (40k)-amino acid pentapeptide-Inotecan conjugate
[0083] N-tert-butoxycarbonyl glutamate benzyl ester dipeptide (Boc-Glu(obzl)-Glu(obzl)-obzl) 0.776g (Example 1) was dissolved in 7mL dichloromethane, and 3mL trifluoroacetic acid was added at room temperature Reaction for 2h. Remove the solvent, add 100 mL of dichloromethane, use 5% sodium bicarbonate solution (NaHCO 3 ) Adjust pH=7-8. Extract and separate the liquids, and use 5% sodium bicarbonate solution (NaHCO 3 ) Wash twice and dry with anhydrous sodium sulfate. Filter, add the filtrate directly to the reaction flask, under the protection of nitrogen, add Y-type polyethylene glycol carboxylic acid (molecular weight is 40,000, structure such as (V)) 40.0g, 4-dimethylaminopyridine (DMAP) 245mg, 1 -Hydroxybenzotriazole (HOBT) 135mg. After all was dissolved, 412 mg of dicyclohexylcarbodiimide (DCC) was added. The reaction was stirred overnight at room temperature. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com